LUMBAR DISC HERNIATION
VIII – THE SPINE > Disc Injury and Degeneration > CHAPTER 144 –
LUMBAR DISC HERNIATION
the patient with low back pain or sciatica, or both, that results from
a herniation of a lumbar disc. The gold standard technique using open
lumbar discectomy with loupe magnification is presented. In addition,
the technical options for removing a lateral extraforaminal disc
herniation are also discussed, as are pitfalls and complications. The
most important concern regarding the management of lumbar disc
herniation is selection of the appropriate patient for surgery than it
is the technique for removal, assuming that a competent surgeon
performs an adequate decompression.
understand the precise cause for lumbar disc herniation in any given
patient. Why is it that one person develops a lumbar disc herniation
and another person does not? Patients with lumbar disc herniations are
commonly seen in clinical practice. Fortunately, the majority of these
patients respond to nonoperative management and do not require surgical
removal of the disc. The reason for this improvement remains elusive,
although recent work by Haro et al. (6)
suggests that a local inflammatory process in the epidural space may
stimulate host macrophages to resorb the displaced disc tissue.
has shown that lumbar disc herniation may be reflective of high
stresses at the posterolateral region of the disc secondary to torsion.
These high loads cause fatigue failure of the annulus fibrosis that
enables the inner nucleus pulposus to penetrate the laminations of the
annulus gradually until a herniation occurs (4).
Because the region of the disc with the highest torsional stresses is
adjacent to the nerve root, these posterolateral herniations nearly
always affect the exiting root or the central thecal sac. Less
commonly, the disc may protrude into the extraforaminal area and
produce compromise of the more proximal exiting root (e.g., L-4 for a
lateral L4–L5 herniation).
The factors that seem to emerge as possibly being predictive include
tall men, heavy women, individuals with a small spinal canal, and those
who work in an environment with considerable vibration, such as that of
airplane pilots and heavy equipment operators (2).
randomized 126 patients between surgical and nonsurgical treatments. At
1 year, those in the surgical group had fewer pain complaints. At 4
years, there was no statistically significant difference between the
two groups. Weber was not a surgeon, and he had no bias toward the
efficacy of surgery. One can conclude that surgical intervention should
be limited to patients with a significant neurologic deficit or to
those patients who are unable to engage in the lifestyle they desire
because of sciatica. Weber (20) also demonstrated that less than 2% of the patients in both groups remained symptomatic at the end of 10 years.
general history and physical examination. If no findings are identified
that suggest another disease process, and the history and physical exam
are consistent with a lumbar disc herniation without major neurologic
compromise, initiate nonoperative treatment without imaging studies.
Many patients improve rapidly and do not require further diagnostic
testing. Those patients who do not improve within 30 days and who wish
a prompt resolution to their problem warrant a thorough assessment.
Plain radiographs with an anteroposterior (AP) and lateral
flexion-extension views are useful to document hypermobility patterns
or any spinal deformity. Obtain an magnetic resonance imaging (MRI)
scan to characterize any disc pathology, localize any herniation, and
exclude other conditions such as a spinal cord tumor or tethered cord.
If the MRI reveals herniations at numerous disc levels, or if the
patient has a contraindication to MRI (e.g., claustrophobia,
intracranial vascular clips), obtain a computed tomography (CT) scan or
myelogram.
to evaluate the psychological characteristics of the patient. The pain
drawing has been shown to correlate well with more formalized
psychological testing (13). Psychological factors have been shown to be important predictors of outcome and, therefore, are of value to the surgeon (19).
If a patient has less than 50 points on this system, I recommend
nonoperative management. More recently, we have found that even
patients who have lumbar disc herniation with more than 50 points on
the OPES will not necessarily achieve a “good outcome,” as we
previously reported (11). If the patient has
engaged an attorney to assist with a compensation claim or if the
patient has an attorney who is involved in a civil claim, such as for a
car accident, the likelihood for a good clinical outcome drops
approximately 30% (11).
 |
|
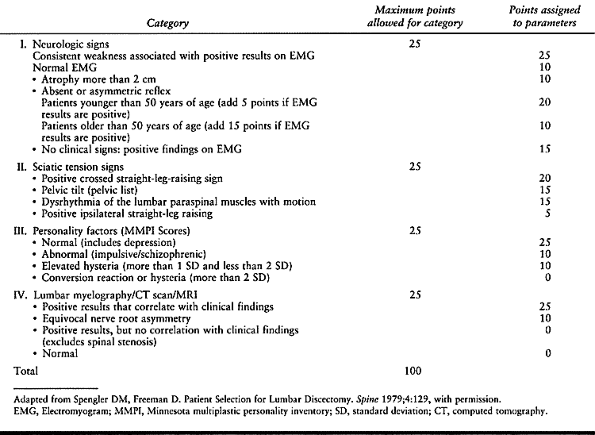
Table 144.1. Objective Patient Evaluation System
|
such as complete foot drop or cauda equina syndrome, require prompt
imaging studies and early surgical decompression. These patients will
not be discussed in this chapter because the focus here is on elective
lumbar discectomy.
is imperative to formulate a good nonoperative treatment plan. The
initial goal is to control symptoms. Once symptoms are controlled,
start an activation program. Such a program should include both an
aerobic conditioning component and trunk muscle strengthening. In the
majority of patients, symptoms can be controlled with nonsteroidal
anti-inflammatory drugs (NSAIDs), acetaminophen (Tylenol), or
salicylates. Narcotic medications are occasionally necessary in the
first few days of the acute phase, but do not use narcotics for
long-term pain control. Steroid dose packs have not been shown to be
effective in a controlled study (5). Bed rest
may be useful for 1 to 2 days but no longer. As the patient improves,
implement a self-guided or supervised physical therapy program to
enhance aerobic activity and to improve trunk strength. I prefer
resistive exercise equipment such as Cybex, Nautilus, and therabands to
strengthen both trunk flexors and extensors. Limit range of motion to
30° of flexion and 30° of extension if resistive equipment is used. If
the patient demonstrates good progress, he or she can resume full
activities at approximately 3 months after onset, assuming that there
is full compliance with an activation program.
patients with back or radicular pain, I avoid these injections in a
patient with lumbar disc herniation, especially if a neural deficit is
present. No prospective study has demonstrated success with the use of
epidural injections in patients with a lumbar disc herniation.
I recommend an elective discectomy only if the patient has failed to
respond to a minimum of 30 days of nonoperative management. The patient
must demonstrate that his or her quality of life is sufficiently
impaired to warrant an elective spinal procedure. Finally, the patient
must have a minimum of 50 points on the OPES (19).
The technique that I select for surgery has nothing to do with the
indications. Once I am comfortable that the patient understands the
diagnosis and alternate treatment options, I select the optimal
procedure for the patient based on the preoperative imaging studies as
well as my knowledge and experience.
patient management remains open laminotomy with discectomy using loupe
magnification (7,16). I
perform the approach unilaterally if the symptoms are unilateral. If
the patient has a central herniation with bilateral symptoms, I use a
bilateral approach with bilateral removal of the disc nucleus. If a
massive extruded disc is apparent on imaging studies, a hemilaminectomy
may be necessary to provide sufficient longitudinal decompression to be
able to safely mobilize the affected nerve root or thecal sac.
extraforaminal disc herniation, there are two options. An intervening
hemilaminectomy may be performed so that the lateral herniation can be
clearly visualized between the exiting nerve root above and the exiting
nerve root below (18). Thus, for a far lateral
disc herniation at L4–L5, expose both the L-4 and the L-5 nerve roots.
Some surgeons prefer an extraforaminal approach (21,22).
In this approach, make an incision lateral to the midline in the region
of the transverse processes and facet joints. Carry the dissection
through the intertransverse muscles and ligament to expose the lateral
disc and exiting nerve root. Gain adequate exposure to understand the
pathologic process clearly because the nerve root may be displaced from
the normal course (3).
who have written an entire text dealing with the nuances of this
technique. Although lumbar microdiscectomy remains an appropriate
technique to remove a lumbar disc herniation, I continue to prefer the
open laminectomy approach with loupe magnification. Potential benefits
of lumbar microdiscectomy would include a smaller incision, more
focused lighting, and not wearing loupes and a fiberoptic headlight.
Disadvantages include a higher incidence of dural tears and a more
limited exposure, which might contribute
to
overlooking foraminal stenosis. Length of stay, blood loss, and time to
full recovery are not different between the two procedures. I have no
objection to these techniques but prefer the open-loupe technique. Our
patients do not require blood transfusions. Our average hospital stay
has been reduced to a 23-hour admission for the majority of patients
with primary lumbar disc herniations.
interest and deserve our continued study to determine their usefulness
once more clinical outcome studies are reported by noninventors. Kambin
and Zhou (8) have demonstrated the clinical
feasibility of a percutaneous approach by being able to remove the
herniated portion of the disc and visually freeing the nerve root. This
was a clear advance over the original automated percutaneous approach,
which removed the central disc without addressing the posterolateral
herniation. Revel et al. (14) demonstrated
automated percutaneous lumbar discectomy to be ineffective in a
randomized clinical trial. Treatment was considered successful in 61%
of the 72 patients in the chymopapain group, with 44% of the 69
patients in the percutaneous discectomy group. At 1 year, overall
success rates were 66% in the chemonucleolysis group and 37% in the
automated percutaneous group. Within 6 months of treatment, 7% of the
patients in the chemonucleolysis group and 33% in the discectomy group
underwent subsequent open surgery. Although complication rates were
low, 42% of the chemonucleolysis group continued to complain of
significant low back pain.
have described the MicroEndoscopic Discectomy (MED) system, which seems
appropriately designed for patients with a soft herniation in close
relationship to the nerve root. The visualization and lighting are
superb. Before widespread endorsement, however, further clinical study
is required to ascertain the indications, contraindications, and
complications.
factors: patient size, size and location of the lumbar herniation, and
patient age. For a patient who is in excellent health and good physical
condition, I recommend a unilateral approach. Place a small vertical
incision over the appropriate vertebral interspace and perform only
unilateral paraspinous stripping. Another option is to strip the
paraspinal muscles bilaterally but to perform only a unilateral
laminotomy. An older patient with significant degenerative changes may
require bilateral stripping of the paraspinal muscles in addition to
partial resection of the adjacent spinous processes. A patient with a
large herniation and a neurologic deficit may require bilateral
paraspinous stripping plus a partial or complete laminectomy to gain
sufficient visualization to safely remove the herniation without
incurring additional neurologic injury. No study has shown any adverse
outcomes with a more generous exposure, so I believe that it is always
better to err on the side of too generous rather than too small an
approach. Greater retraction may be required when attempting to remove
a large disc herniation through a small exposure. The technique that is
illustrated describes a more generous exposure to illustrate better the
anatomic details for an axillary lumbar disc herniation. A range of
options exists regarding the extent of the exposure, depending on the
above-mentioned parameters.
sparingly in various centers around the world, but few centers employ
this technique in the United States. Although the concept makes some
sense, the enzyme uses the central nucleus as the primary substrate
without necessarily affecting the protruded portion of the disc. In
addition, an enzymatic reaction is difficult to standardize with a set
dosage because the composition of the substrate varies widely among
patients. For example, the ratio of collagen to nucleus pulposus varies
considerably among different age groups. Finally, as noted in the Rand (14) study, only 66% of the patients improved using chymopapain as compared with 85% to 90% in open discectomy (19).
sparingly in various centers around the world, but few centers employ
this technique in the United States. Although the concept makes some
sense, the enzyme uses the central nucleus as the primary substrate
without necessarily affecting the protruded portion of the disc. In
addition, an enzymatic reaction is difficult to standardize with a set
dosage because the composition of the substrate varies widely among
patients. For example, the ratio of collagen to nucleus pulposus varies
considerably among different age groups. Finally, as noted in the Rand (14) study, only 66% of the patients improved using chymopapain as compared with 85% to 90% in open discectomy (19).
inhalation anesthesia. Once anesthesia has been administered, place the
patient prone on a Jackson table with slight hip flexion. This table
enhances intraoperative positioning by accommodating a wide range of
patient sizes. The abdominal viscera hang free without pressure on the
venous plexus. The knees are well supported, and the lumbar spine can
be flexed adequately. Transient paresthesias in the lateral femoral
cutaneous nerve distribution are common postoperatively. In addition,
some patients complain of mild postoperative discomfort over the
greater trochanter. To prevent irritation of the brachial plexus, the
arms must not be elevated excessively in relation to the trunk.
-
Once the patient is properly positioned
and carefully checked for pressure areas, especially the eyes, prepare
and drape the lower back in sterile fashion. -
Palpate the iliac crests to locate the suspected L4–L5 level.
-
Compare the clinically observed level of
the iliac crests with preoperative radiographs, and label the level
with a marking pencil. -
Make a vertical midline incision over the appropriate spinous processes.
-
After the skin incision is made, complete the remaining portion of the dissection to the bony lamina using electrocautery.
-
Extend the dissection to the lumbodorsal
fascia. Identify the spinous processes of L-4 and L-5, and use the
cautery unit to incise the fascia directly over the spinous processes.
Use pickups to apply tension or a Cobb elevator as a gentle retractor. -
Dissect the fascia away from the spinous
processes of L4–L5 and the inferior portion of L-3. By using Cobb
elevators, carefully strip the paraspinous muscles from the spinous
process to the lamina. -
Take care to maintain a subperiosteal approach. Blood loss is markedly reduced by avoiding the muscle envelope.
-
Once the periosteum is stripped, insert a
self-retaining retractor. Irrigate the wound and clearly identify the
interlaminar space of L4–L5. -
Place a towel clip through a spinous process, and obtain a lateral radiograph to confirm the level.
-
Once you are satisfied that you are
operating at the proper level, continue dissection by partially
resecting the inferior portion of the L-4 spinous process and the
superior portion of the L-5 spinous process: -
Examine the interlaminar space. With a
Penfield 1 elevator, strip the ligamentum flavum from the inferior and
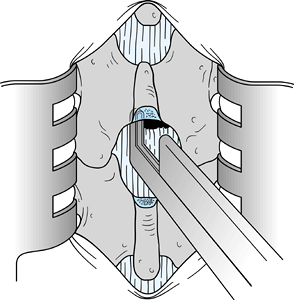
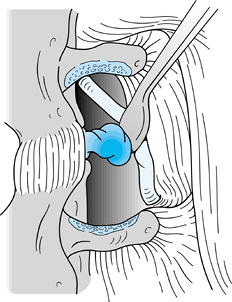
anterior surface of the lamina of L-4 (Fig. 144.1).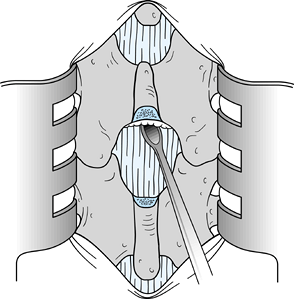 Figure 144.1. Use a Penfield 1 elevator or curved curet to remove the ligamentum flavum from the anteroinferior surface of the lamina of L4.
Figure 144.1. Use a Penfield 1 elevator or curved curet to remove the ligamentum flavum from the anteroinferior surface of the lamina of L4. -
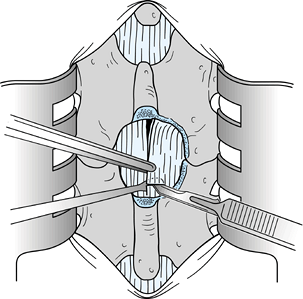
Use a Harper 45 rongeur to excise part of the middle portion of the L-4 lamina (Fig. 144.2).
![]() Figure 144.2.
Figure 144.2.
Use an upbiting rongeur with a 45° angle to resect additional bone in
the midline from the inferior portion of the L-4 lamina. Resect a
section of bone that is approximately 2 to 3 mm in length superior to
the midline raphe of the ligamentum flavum. -
Carry the dissection cephalad until the midline raphe of the ligamentum flavum can be identified.
-
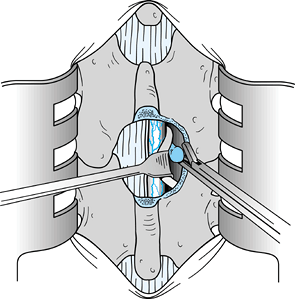
Insert a Penfield 3 elevator in the midline from proximal to distal immediately anterior to the ligamentum flavum (Fig. 144.3). Using a #15 blade knife, divide the ligamentum flavum in the midline.
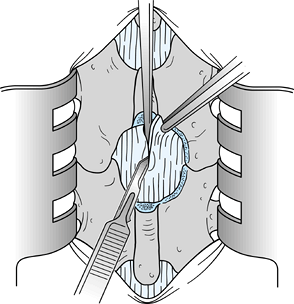 Figure 144.3.
Figure 144.3.
Pass a Penfield 3 elevator from proximal to distal, and divide the
ligamentum flavum on top of the elevator to minimize the possibility of
injury to the dura. -
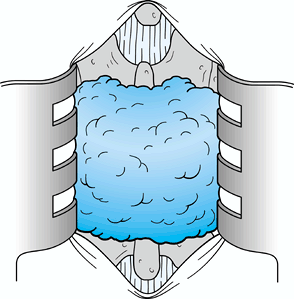
Carry the dissection laterally toward the
lateral recess, again using the Penfield 1 or Penfield 3 elevator to
protect the underlying dura (Fig. 144.4). Dissection can be accomplished using either a scalpel, curets, or a rongeur with a 45° angle.![]() Figure 144.4.
Figure 144.4.
After the midline division of the ligamentum flavum, use a Penfield 1
or 3 elevator to protect the contents of the spinal canal while the
flavum is resected further using a #15 blade scalpel. Other options are
to use a small curet or to elevate the flavum and remove it with an
upbiting rongeur with a 45° angle. -
Once the bulk of the ligamentum flavum
has been removed, examine the epidural fat and dura to determine the
degree of lateral wall dissection necessary to identify and retract the
displaced nerve root safely. -
In this chapter, I have chosen to depict a less common form of disc herniation to emphasize the pitfalls of root
P.3770
injury (Fig. 144.5).
When the disc herniation is medial to the root, the lateral portion of
the dura may be misinterpreted as being the lateral border of the nerve
root. Such a misinterpretation can result in division of the nerve root
or at least injury to the nerve root. Take extreme care to identify the
nerve root.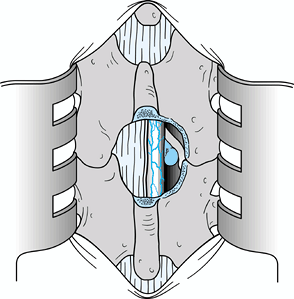 Figure 144.5.
Figure 144.5.
Expose the contents of the vertebral canal after resection of the
ligamentum flavum from the right side and after lateral wall resection
using an upbiting rongeur with a 45° angle. A disc herniation medial to
the nerve root was illustrated intentionally to emphasize the point
that the surgeon can mistake the lateral border of the dura for the
lateral border of the nerve root. The nerve root must be clearly
visualized. -
Resection of the lateral wall of the spinal canal using a rongeur with a 45° angle is necessary in most cases.
-
Once the nerve root is identified and the
disc herniation is visualized, remove a portion of the herniation. Then
medially displace the root and excise the remaining portion of the
displaced disc (Fig. 144.6).![]() Figure 144.6.
Figure 144.6.
Gently elevate the nerve root with a Freer elevator and a Penfield 4
elevator, and retract it medially over the displaced disc tissue. A
Love root retractor can be used as illustrated to displace the root
dura medially. Then remove the extruded disc with a small pituitary
rongeur. -
A disc herniation is categorized as being
extruded when the annulus is disrupted, and the herniated portion of
the disc is categorized as being protruded when the annulus is intact
but eccentrically displaced. The term sequestered is applied to a free
fragment of intervertebral disc lying in the spinal canal with no
defect evident in the annulus fibrosus. Seventy-five percent of lumbar
disc herniations are of the extruded variety, whereas sequestered disc
fragments are far less common (less than 5%). -
Dissect from the medial portion of the
dura laterally, using a Penfield 4 elevator in the dominant hand and a
Freer elevator in the other hand. Take extreme care to identify the
displaced nerve root. Use of surgical loupes with 2.5 magnification and
a fiberoptic headlight is essential for optimal visualization in this
operation. Once
P.3771
the
nerve can be displaced medially, insert a Love root retractor to
displace the nerve root and dura gently medially, so that the extruded
disc herniation can be excised (Fig. 144.6).
A sharp incision into the annulus is seldom necessary because the
pseudomembrane overlying an extruded disc can nearly always be
dissected using a Penfield 4 elevator. In protruded disc herniation,
however, the annulus must be incised. -
Once the disc has been removed, free the
nerve root of tension and carefully inspect the neural foramina. The
nerve root should be able to be displaced at least 1 cm medially (Fig. 144.7).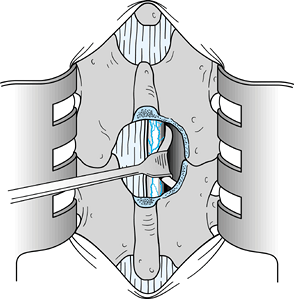 Figure 144.7.
Figure 144.7.
After disc removal, it should be easy to displace the involved nerve
root 1 cm medially. Should the root remain fixed, additional
exploration of the foramina and the vertebral canal is necessary. -
Take care to ensure that no disc material is present between the annulus and the posterior longitudinal ligament.
-
Once the disc has been excised, irrigate
the wound and place an interposition membrane of Gelfoam or fat over
the laminotomy. At present, I prefer to use Gelfoam (Fig. 144.8).![]() Figure 144.8. An interposition membrane of Gelfoam; many surgeons prefer fat grafts.
Figure 144.8. An interposition membrane of Gelfoam; many surgeons prefer fat grafts. -
Then close the wound in layers. Use
figure-8 sutures to achieve watertight closure of the fascia; I prefer
subcuticular stitches for the skin. -
Apply a dry, sterile bandage.
-
Gently straighten and rotate the patient
onto the stretcher for extubation. Following extubation, take the
patient to the recovery room. Blood transfusion is seldom necessary for
a primary lumbar discectomy because blood loss usually averages less
than 100 ml.
years with no incidence of recurrent disc herniation over that reported
by others (2%).
taking down the entire facet is one alternative advocated by some (1), the extraforaminal approach has been advocated by Wiltse (21,22).
The proponents of going from the midline through the facets suggest
that instability is not a subsequent problem and believe the nerve root
can be more easily identified. Furthermore, they believe the surgeon
can have greater confidence of complete decompression. However, many do
not share this view.
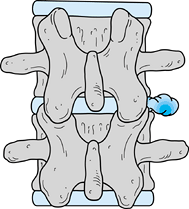 |
|
Figure 144.9. Far lateral disc lesion. Note the relationship of the disc to the transverse processes.
|
-
Make the incision 5 cm from the midline, followed by blunt dissection of the paraspinal muscles (Fig. 144.10).
![]() Figure 144.10. The annulus is incised, the nerve root is retracted laterally, and the disc is removed with a pituitary rongeur.
Figure 144.10. The annulus is incised, the nerve root is retracted laterally, and the disc is removed with a pituitary rongeur. -
At this point, take radiographs to verify the level and clear the transverse processes of soft tissues.
-
Enter the intertransverse ligaments and
fascia with a knife or curet, then remove those structures between the
transverse processes. -
Identify the nerve, which is usually 2 to 4 mm anterior to the fascia and directed at a 45° angle (Fig. 144.11).
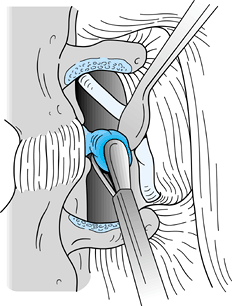 Figure 144.11. Nerve identified and retracted at a 45° angle.
Figure 144.11. Nerve identified and retracted at a 45° angle. -
Follow the nerve medially and identify the disc.
-
If a free fragment is present, remove it.
If only a bulge is present, incise the annulus and remove easily
identifiable fragments. When the nerve root is easily mobile,
sufficient disc has been removed. -
If the lesion is intraforaminal, take
down a portion of the facet laterally to expose the nerve root canal.
McCulloch and Young (12) point out, however, that this procedure is rarely necessary because of the the usual anatomic location of these lesions. -
Closure in both instances is routine, using a free fat graft or Gelfoam to cover the nerve.
are improved when they awaken in the recovery room. On the evening of
surgery, the patient is permitted to stand. By the first postoperative
day, the patient is allowed to ambulate. If a dural leak occurs,
ambulation is delayed by 24 to 48 hours. An abdominal binder is used
for 4 weeks as partial protection for the patient. Rehabilitation is
initiated 4 weeks after surgery and continued indefinitely. Patients
are advised to maintain ideal weight and
to
develop good abdominal and trunk extensor muscle tone. Aerobic activity
is encouraged and is initiated 4 weeks following surgery. Patients are
discharged from follow-up approximately 12 months after surgery.
appropriate patients for surgical repair of lumbar disc herniations.
The use of an objective method to accomplish this, as described in this
chapter, will lessen the incidence of negative explorations (19),
which is less than 2% in my experience. Although technical errors
infrequently account for persistent pain after surgery, such errors
invariably compromise the outcome. In a patient with established disc
herniation, exploration at the wrong intervertebral level will not
benefit the patient (10). Avoid this mistake by
taking an intraoperative radiograph to confirm the appropriate level.
If a disc herniation is not found, consider exploring additional levels
after an intraoperative radiograph to confirm the proper level.
Explorations at the wrong level are more common in patients who have
transitional lumbar vertebrae.
excessive traction of neural tissue can cause irreversible nerve
damage, use loupe magnification and fiberoptic lighting. These
technologies have done much to minimize injury to neural tissue.
Likewise, the use of an interpositional membrane (either free fat
grafts or Gelfoam) at the completion of surgical exploration may
minimize long-term perineural scar formation. Presently, newer
antiadhesion barriers are being investigated in the United States.
Experience in Europe has suggested that such barriers may lessen
scarring within the spinal canal and perhaps even enhance clinical
outcome. Additional evaluations are necessary to support these early
but exciting assertions scientifically.
to detect a fragment of disc tissue that has migrated from the level of
herniation, either cephalad in the canal or laterally into the
extraforaminal area, trapping nerve roots (10).
Because the only patients who should undergo surgery are those with
objective findings, explore the canal thoroughly if no specific
pathologic changes are encountered at the suspected level. Because the
nerve root should be able to be displaced easily for a distance of
approximately 1 cm medially, a nerve root that is tight in the canal
and cannot be displaced suggests either a sequestered fragment along
the nerve root or a stenotic lateral recess (10).
Gently palpate the cauda equina to ensure that an intradural disc
herniation is not present. Although a neoplasm involving the neural
elements of the lumbar spine is always a possibility, it is distinctly
uncommon. The most likely neoplastic lesion encountered in the lumbar
vertebral canal is a metastatic extradural lesion.
proper technique, blood loss should be minimal, rarely exceeding 150
ml. Generally, epidural bleeding is easily controlled with bipolar
coagulation, packing, or both.
straightfoward unilateral lumbar disc herniation rarely requires a
complete laminectomy; however, when the surgical findings do not
explain the preoperative symptoms, more open exposure is necessary.
the dura and the nerve root, and expose the lateral wall adequately
before examining the nerve root. Attempting to extract a large,
extruded fragment through a small incision may traumatize the involved
nerve root unnecessarily.
patient who presents with a cauda equina syndrome or a large central
herniation without neurologic involvement. In these situations, remove
the ligamentum flavum on both sides of the spine. Because the dura
mater will be displaced posteriorly, a careful dissection is essential
to avoid injury. A laminectomy may be the most appropriate exposure to
ensure adequate visualization of the thecal sac and the exiting nerve
roots on both sides. Often, the most appropriate beginning may be to
perform an annulotomy and discectomy on the side with least herniation
to debulk the herniation and to provide easier access to the root under
maximal tension.
the neural canal must include a careful assessment of the lateral
recess to interpret pathologic changes accurately. Performing a
discectomy in a patient who also has lateral entrapment of the nerve
root will not reduce the patient’s symptoms. Careful probing of the
neural foramina at the completion of the lumbar discectomy will locate
the narrowing of the neural foramina. If stenosis of the lateral recess
is the only manifestation, lumbar discectomy may not be necessary.
complications that might occur would be exhaustive. Dural tears may
occur during a lumbar discectomy. Such tears are more common in
patients who undergo surgery for spinal stenosis or revision
procedures. I recommend primary repair of dural tears with 5-0
Dermalon. Injuries to the iliac arteries and veins and viscera,
including any structure from the appendix to the ureter, have been
reported in association with a lumbar discectomy (15).
Errors in diagnosis may occur. The patient may have symptoms suggestive
of a lumbar disc herniation, which may, in fact, be related to
intra-abdominal pathologic processes such as an aneurysm or a
malignancy. Minimize these unusual complications by performing a
thorough diagnostic
assessment
and decision analysis before a decision to operate on the spine. With
proper patient selection and careful operative technique, lumbar disc
surgery is highly successful and yields gratifying results for both the
patient and the surgeon.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
AF, Wolber PG, Warfield JR, Gunadi IK. Surgical Management of Extreme
Lateral Lumbar Disc Herniations: Review of 138 Cases. Neurosurgery 1988;22:648.
Presented at the 6th Annual Meeting of the International Society for
the Study of the Lumbar Spine, New Orleans Louisiana, 1980.
J, McCarty E, Spengler D. Results in Elective Lumbar Discectomy for
Patients Involved in the Workers’ Compensation System. J Spinal Disord 1998;II:277.