DEGENERATIVE DISC DISEASE
VIII – THE SPINE > Disc Injury and Degeneration > CHAPTER 145 –
DEGENERATIVE DISC DISEASE
(DDD) has been used to describe a wide variety of morphologic and
radiographic changes in the adult lumbosacral spine. The North American
Spine Society Consensus Committee on Nomenclature (16) defined disc degeneration as follows:
desiccation, fibrosis, or cleft formation in the nucleus; fissuring or
mucinous degeneration of the annulus; defects and sclerosis of the
endplates; and/or osteophytes at the vertebral apophysis.
does not imply an etiology. Depending on age, degree, and type of
change … the changes of disc degeneration may be clinically
insignificant.” Degenerative disc disease,
on the other hand, is broadly defined as “a clinical syndrome
characterized by manifestations of disc degeneration and symptoms
thought to be related to those changes.” The authors point out that
“causal connections between degenerative changes and clinical symptoms
are often difficult clinical distinctions.”
refers to a continuum of nonradicular, mechanical pain disorders of
presumably degenerative origin. Specifically excluded are those disc
pathologies with neurologic impingement, such as disc displacement,
spinal stenosis, and deforming degenerative conditions, such as
degenerative scoliosis and spondylolisthesis.
-
A confusing and contradictory nomenclature
-
The similarity of “pathologic” changes to those of normal aging
-
The difficulty in accurately identifying the source of pain
-
Our limited understanding of the etiology and natural history of this process
-
Historically low success rates from surgical intervention for patients with DDD
pain cannot be overestimated. With a lifetime incidence of 60% to 80%,
low back pain (LBP) generates at least 15 million office visits per
year (23). In fact, low back complaints are the
leading compensable cause of injury in the workplace. In the United
States, $24 billion a year is spent on the evaluation and direct
management of patients with back pain. Indirect costs include work and
productivity losses, and they account for an additional $27 billion
annually (28).
months of the onset of symptoms, 95% of patients return to their
previous employment. The 5% of patients with residual symptoms after 3
months, however, incur 85% of the costs. Moreover, the probability of
returning to work falls with increasing duration of disability. After 2
years off work, less than 2% will return. Therefore, the early
detection of those patients in whom LBP is more likely to become
chronic would be of tremendous clinical and social benefit.
-
Referred back pain
-
LBP with radiculopathy or myelopathy
-
LBP with deformity, such as scoliosis, kyphosis, or spondylolisthesis
-
LBP in the context of fractures, tumors, or infections
-
Mechanical LBP without the features already noted
consider other potential sources of referred pain. Included in the
first LBP group are intra-abdominal and retroperitoneal pathologies
such as abdominal aortic aneurysm and endometriosis. In groups 2
through 4, symptoms more readily correlate with evident spinal
pathology; as a result, treatment of these patients is satisfying and,
overall, associated with good results.
includes several benign conditions, most likely representing
ligamentous and muscular strain, mechanical stress from poor posture,
or facet joint irritation. More chronic and disabling degenerative disc
conditions, however, are included as well. In these patients, uncertain
identification of the pain generator is associated with vague
diagnostic groupings and failed surgical management. Included among
these is DDD.
incomplete understanding of the pathophysiology and natural history of
DDD. Moreover, because the “pathoanatomic” changes noted in DDD do not
qualitatively differ from those of normal aging, the appropriateness of
the appellation disease is intensely debated.
Historically, while the lumbar facets, posterior longitudinal ligament,
dura, dorsal root ganglion, and myofascial structures of the lumbar
spine have been recognized as pain-generating structures, the disc was
felt to be aneural. More recently, however, nociceptive fibers have
been identified in the outer annulus (22,30,47).
used a progressive local anesthesic technique to gauge pain response in
different tissues in 193 consecutive laminectomies. Stimulation via
blunt probe or unipolar electrocautery on the facet cartilage and
synovium never caused pain. Also, no pain followed stimulation of the
lamina, spinous process, ligamentum flavum, lumbar fascia, and
uncompressed roots. Stimulation of the facet capsule, however, was
associated with sharp, localized pain in 30%. This pain did not match
the patient’s preoperative symptoms. Stimulation of a compressed root
resulted in sciatic pain in 79%. Finally, LBP similar to preoperative
symptoms was noted in 70% of patients after stimulation of the
posterior annulus or posterior longitudinal ligament (PLL). Local
anesthetic injection obliterated the pain.
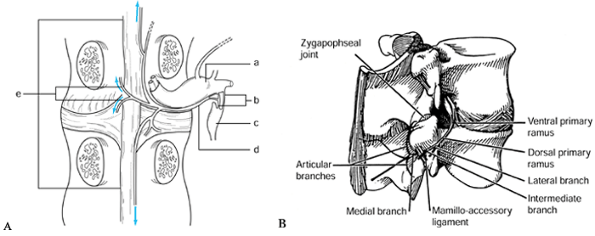
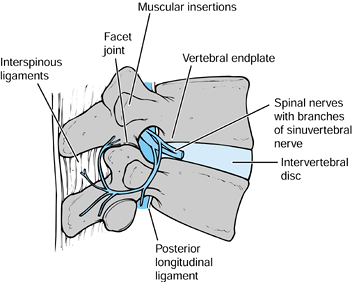
sinuvertebral nerve most likely carry these impulses. The sinuvertebral
nerve, first described by Von Luschka, consists of a postganglionic
derivative of rami communicantes that branches into segments. These
segments ascend and descend into one or more adjacent levels (30,78) (Fig. 145.1).
The branches accompany the venous plexus into the vertebral endplates
and terminate in nociceptive free nerve endings in both the PLL and
outer lamina of the annulus (51). In
histometric studies, the outer third of the annulus has been found to
contain nerve endings with nociceptive neurotransmitters [substance P,
calcitonin, vasoactive intestinal peptide (VIP)] (54). Further, nociceptive fibers may grow into diseased discs (22). Theoretically, a small tear of the outer annulus may cause pain, even with a normal nucleus pulposus.
 |
|
Figure 145.1. The course of the sinuvertebral nerve (24,37). A: The sinuvertebral nerve shown on a cutaway drawing. B: The branches of the invertebral nerve shown on a lateral view of an intact spine. a: Dorsal root ganglion. b: Rami communicantes. c: Autonomic ganglion. d: Sinuvertebral nerve. e: Terminal branches of the nerve (may ascend or descend one or two vertebral levels).
|
receptors elsewhere. Disc height collapse may produce nociceptive
signals from the facet mechanoreceptors by abnormal loading. Such
mechanical derangement has been associated with abnormal intervertebral
motion and has been termed lumbar segmental instability.
extrusion of nuclear material, a source of neural irritation and
inflammation (54). The potential sources of pain in the lumbar spine are illustrated in Figure 145.2.
 |
|
Figure 145.2. Various potential pain generators in the lumbar spine.
|
composition changes greatly. Given that certain degenerative phenomena
may lead to pain, it is necessary to have a clear understanding of what
changes constitute normal degeneration.
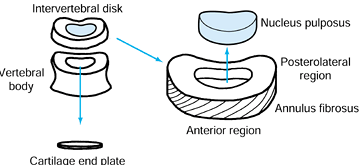
(NP) and 50% annulus. The notochordal cells of the NP are gradually
replaced by chondrocytes throughout the early teenage years. This
replacement is associated with annular thickening (Fig. 145.3). The demarcation between
the annulus and the nucleus becomes less distinct. The older NP has a
higher collagen content with more structured fibers. In these fibers,
the ratio of type II to type I collagen increases (51).
 |
|
Figure 145.3. Schematic of basic intervertebral disc anatomy.
|
Chondroitin-4-sulfate and chondroitin-6-sulfate concentrations
decrease, and the ratio of keratin sulfate to chondroitin sulfate
increases. Keratin sulfate has a smaller hydrophilic potential and a
reduced tendency to form stable aggregates with hyaluronic acid.
Dehydration, in turn, decreases the disc’s resistance to axial loading (20).
originate in the central portion of the dehydrated NP. One hypothesis
holds that these clefts eventually migrate toward the peripheral
annulus and endplate and cause tears. Annular tears are classified by
Vernon-Roberts (20) as peripheral,
circumferential, and radiating. Circumferential and radiating tears are
associated with degenerative changes in the endplate and NP.
obliterated. In adults, the disc is the largest avascular structure in
the body. Thereafter, disc nutrition requires diffusion through the
vertebral endplates (80%) and outer annulus (20%) (25).
from pathological degeneration. One study found that altered collagen
staining patterns and increases in lipofuscin and amyloid can be used
to differentiate aged from degenerated discs (20).
Further, this cycle of degeneration may be self-promoting, that is,
small annular tears lead to further nuclear degeneration in animal
models (47,54). Vascular ingrowth may also mark degenerated and herniated discal tissues (41).
aging changes and disc degeneration. While some authors claim a
quantitative if not qualitative difference between aged and degenerated
discs, all discs degenerate with age. Miller et al. (55) reported evidence of disc degeneration by the age of 50 in 90% of 600 autopsy specimens. Holt (39) found evidence of disc degeneration on the plain radiographs of 80% of adults studied, although 53% had no history of LBP.
degenerate with age, the degree and rate of this degeneration vary
significantly from individual to individual. The underlying reasons for
this variability are only partly known.
insufficient nutrition, impaired waste transport, and traumatic
mechanical factors combine with a genetic and hormonal proclivity to
cause desiccation and annular tearing. Severe degeneration is
associated with increased lactate metabolism, decreased pH,
accumulation of proteolytic enzymes, and chondrocyte necrosis (Fig. 145.4) (37).
 |
|
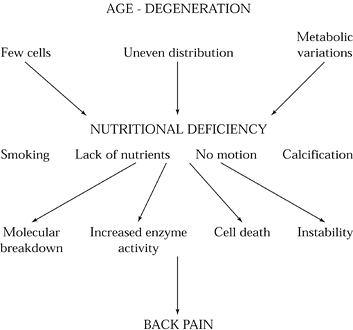
Figure 145.4.
The role of nutritional deficiency in the etiology of discogenic back pain. With advancing age, disc cells diminish in number and distribution and undergo metabolic variations. As a consequence, disc nutrition diminishes. These changes are accelerated by systemic factors such as overall nutritional status, smoking, motion, and endplate or disc calcification. Ultimately, cell death, increased enzyme activity, molecular breakdown, and instability ensue. |
precocious degeneration. Smoking and a familial tendency toward
degeneration have long been established as contributing factors (68). Twins demonstrate similar degeneration patterns (3). In one study of 15-year-olds, LBP, decreased activity, and decreased spinal range of motion predicted later DDD (66). Anatomically, no association between DDD and facet tropism can be demonstrated (6). The endplate irregularities of thoracolumbar Scheuermann’s disease, however, may be related to DDD (35).
phenomenon influenced by heredity and environment, it is incumbent upon
the clinician to ascertain when these changes constitute a disease.
Benign age-related phenomena have been differentiated from pathologic
phenomena on the basis of three factors: impaired function, structural
changes, and an association with pain.
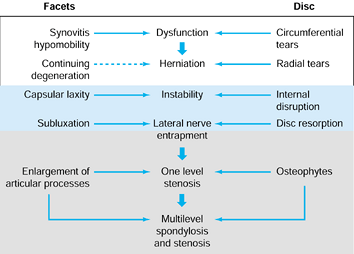
and the two facet joints posteriorly. They theorized that benign
microscopic alterations progress to pathologic degeneration in stages.
In this way, circumferential annular tears progress to radial tears.
Radial tears, in turn, engender further disc degeneration or frank disc
herniation. The ensuing loss of disc height alters facet joint
mechanics, and facet cartilage disruption or destruction may take
place. Coincidentally, the decrease in the intervertebral height causes
buckling of the ligamentum flavum and facet overriding or enlargement.
These changes may, singly or in combination, cause narrowing of the
neuroforaminal and central canals. So, while disc degeneration
manifests as mechanical LBP in some patients, others will experience
neurologic claudication or radiculopathy from frank disc herniation (Fig. 145.5, Fig. 145.6).
 |
|
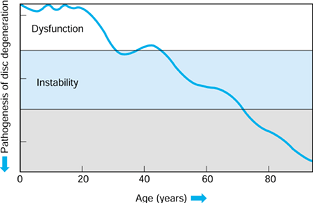
Figure 145.5. The Kirkaldy-Willis (86)
states of lumbar degeneration, including (left side) the events that occur in the facets and (right side) intervertebral discs and associated syndromes. |
 |
|
Figure 145.6.
The natural history of lumbar degeneration (Yong-Hing K, and Kirkaldy-Willis WH: The Pathophysiology of Disc degeneration of the Lumbar Spine. Ortho Clin North Am 1983;14:59). |
-
Microscopic alterations of disc consistent with aging
-
Increased spinal mobility
-
Stabilization of the functional spinal unit (discs anterior and facets posteriorly)
many of these changes are present in nearly all middle-aged people and
are not necessarily associated with back pain. Thus, the issue of when
degenerative changes represent a disease remains unresolved. While data
are lacking, it has been suggested that younger patients with
relatively precocious disc degeneration do not tolerate these changes
as well as older adults. Whether this perceived difference stems from
higher functional demands or from a subtle difference in disc mechanics
is not known. It is reasonable, however, to identify DDD as a chronic,
mechanical LBP syndrome associated with changes in the structural and functional integrity of one or more intervertebral discs.
While some authors use these terms to refer to the same global
discogenic pain syndrome, others view them as a means to differentiate
among subgroups of patients. The divisiveness and misapplication of
nomenclature further confuses any evidence-based appraisal of DDD, and
its incidence, pathophysiology, and natural history.
 |
|
Table 145.1. Named Discogenic Disorders
|
(IDD) refers to a painful annular tear in the absence of bony changes
or disc height loss. As such, IDD must not be confused with disc
protrusions, which are normal hydrodynamic findings (5).
and osteophyte formation. LS is the most frequently described form of
DDD, and it is the entity most authors are describing when they use the
more global term DDD.
(LSI), which represents a progressive relaxation of facet capsules and
ligamentous restraints, occurs in the context of chronically
compromised disc biomechanics. White and Panjabi (83)
define lumbar instability as more than 4.5 mm of translation, 15° to
25° of angular motion between adjacent segments on flexion–extension
radiographs, or both (Fig. 145.7). It is not
clear, however, that the chronic increase in intervertebral motion seen
in degenerative lumbar diseases may be mechanically equated with
traumatic spinal instability. Therefore, some authors classify this
abnormality with degenerative spondylolisthesis and degenerative
scoliosis, which have a similar pathogenesis. Others feel that all the
subtypes of DDD represent a painful “microinstability” of the motion
segment (48).
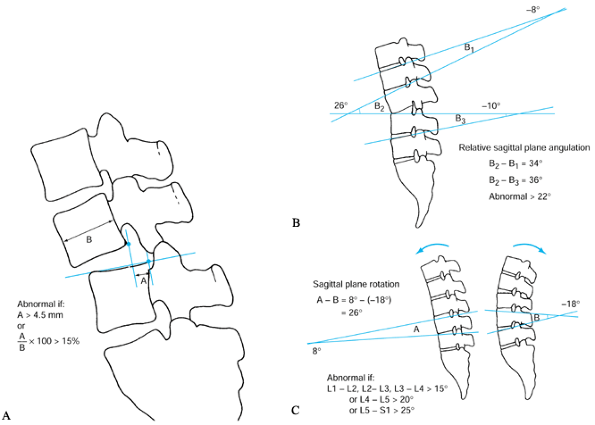 |
|
Figure 145.7. A: The White and Panjabi (83)
method for measuring sagittal plane translation. Greater than 4.5 mm (or 15% of vertebral body) of motion is considered abnormal. B: Method of measuring static sagittal plane angulation. Greater than 22° of relative angulation (i.e., angulation greater than that seen in the levels above and below) is considered abnormal. C: Method of measuring sagittal plane rotation on flexion–extension radiographs. Cobb measurements taken in extension are subtracted from those in extension. Abnormal values are greater than 15° at L1-2, L2-3, and L3-4, and greater than 20° at L4-5 and 25° at L5–S1. |
asymptomatic remains to be answered. Even in those patients with
degenerative changes, severe back pain, and positive discography, pain
may spontaneously improve. In one series, 25 patients who had not had
surgery were positive on a single-level discogram. When they were
evaluated after an average of 4.9 years, 68% had improvement without
surgery (70). Of the 32% who were unimproved,
66.7% had an underlying psychiatric diagnosis. In the absence of a more
complete understanding of the natural history of this disorder,
appropriate evaluation of surgical outcomes is extremely difficult.
of the aging spine continues to grow, surprisingly little is known
about disc degeneration as a disease process (Table 145.2) (78).
 |
|
Table 145.2. What We Do Not Know about Disc Degeneration as a Disease
|
a diagnosis of exclusion. A thorough history and physical examination
are mandatory.
age, nonmechanical pain, constitutional symptoms, and trauma, perform a
thorough radiologic and serologic evaluation for infection, tumor, and
fractures (Table 145.3). Other important
considerations include intra-abdominal and intrapelvic pathology.
Posterior penetrating ulcers, pancreatitis, renal disease, abdominal
aortic aneurysm, and endometriosis are all known causes of severe,
referred pain to the back.
 |
|
Table 145.3. Historical Elements Requiring Thorough Evaluation (“Red Flags”)
|
significant social and psychological issues. Ask specific questions
regarding drug and alcohol intake, mood, sleep disturbance, pending
litigation, and job satisfaction.
exclude those with radicular or myelopathic signs and symptoms.
Similarly, the evaluation of patients with significant thoracolumbar
deformities such as scoliosis, hyperlordosis, and kyphosis is
considered elsewhere (see Chapter 153, Chapter 155, Chapter 156, Chapter 159, Chapter 160 and Chapter 161).
For example, spondylolisthesis, another disorder of genetic and
environmental stress, is the most common structural abnormality in the
adult spine (see Chapter 162). Spondylolisthesis is related to LBP in 5% of the population (21).
isolated, mechanical LBP is challenging. The difficulty in identifying
a specific pain generator accounts for the fact that only 15% of
patients are given a definitive diagnosis. Physically and
radiographically abnormal structures may not cause symptoms. On the
other hand, grossly and radiographically normal structures may be
associated with severe pain in certain individuals.
patients reporting acute LBP. Patients with myofascial pain are
especially difficult to distinguish from those with “discogenic pain”
when radiographic signs of disc degeneration are present. However,
myofascial pain tends to have an acute onset and a relatively brief
duration. The pain is localized to a specific paraspinal area, and
muscle spasm is evident. In most cases, while some DDD patients
identify a specific, traumatic event (such as bending, lifting, or
twisting) with symptom onset, their pain is midline and does not
resolve but rather worsens over time.
that it is aggravated by activity, particularly flexion. Relative rest
temporarily ameliorates symptoms. Patients with lumbar segmental
instability may complain of a catch with flexion and extension (Fig. 145.8).
DDD patients may also report having difficulty when getting up from a
chair. In an attempt to splint the back, some will use upper extremity
leverage, pressing their arms against their thighs, when arising.
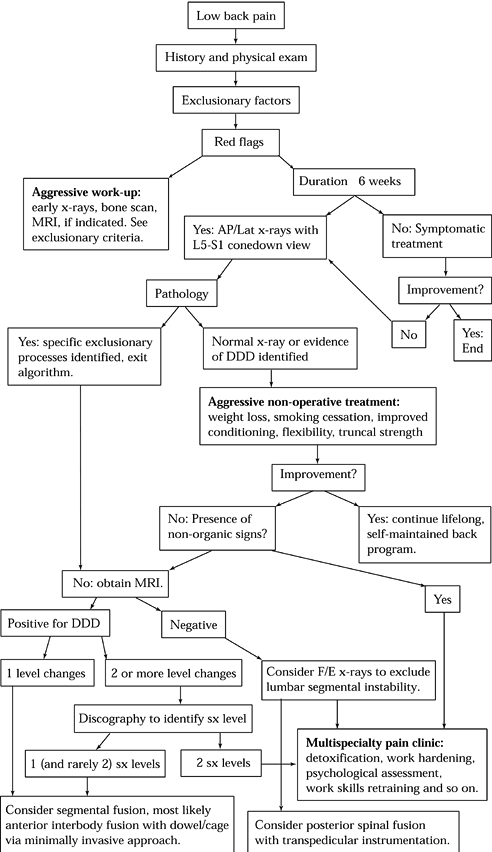 |
|
Figure 145.8. A suggested algorithm for the evaluation of discogenic back pain. DDD, degenerative disc disease; F/E, Flexion/Extension; SX, symptomatic.
|
of sciatica. Often, DDD patients report having had prior discectomy or
chymopapain injections (20). Further, the disc collapse associated with LS may result in foraminal
stenosis and mild radiculopathies (48).
Other patients followed for axial spinal pain will later present with
acute radicular complaints and imaging studies consistent with disc
herniation. Diagnosis and treatment for these patients is discussed in Chapter 144.
sclerotomal pain to the buttocks and posterior thigh. However, it is
very difficult or impossible to localize the symptomatic disc level on
the basis of history or physical exam alone.
nonspecific. These patients exhibit no point tenderness or paraspinal
spasms. They often report pain or difficulty with flexion and rotation
maneuvers of the spine, however. Normal neurologic findings including
sensory, motor, and reflex exams are expected. Particular attention
must be paid to Waddell’s signs (Table 145.4) (79), which suggest psychological overlay.
 |
|
Table 145.4. Waddell’s Nonorganic Physical Signs
|
such as anal sphincter laxity or major muscle weakness should be
construed as red flags and investigated accordingly.
plain radiography in DDD. In the absence of red flags, radiographs are
indicated only after a trial of symptomatic treatment. In patients
failing to improve with these modalities, begin radiologic assessment
with plain anteroposterior (AP) and lateral views of the lumbosacral
spine. Oblique views and a lateral L5–S1 cone-down view are often
helpful. The principal purpose of these studies in the early management
of mechanical back pain is to exclude spondylolisthesis and the less
benign entities mentioned previously.
plain radiographic changes. The cardinal findings of LS are endplate
sclerosis and loss of disc space. Radiographs may also show a loss of
lordosis, subluxations, vacuum phenomenon, and osteophytes (Fig. 145.9). Radiography, however, can be misleading: Frymoyer et al. (24) showed that signs suggestive of disc degeneration were present in 90% of adults studied, whereas 53% had no history of LBP (Table 145.5).
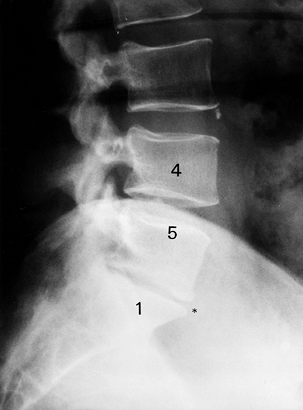 |
|
Figure 145.9. Plain radiographic findings of DDD: disc space loss, endplate sclerosis, and osteophyte formation.
|
 |
|
Table 145.5. Plain Radiographic Findings of DDD
|
bubbles (the vacuum phenomenon) in degenerative discs probably excludes
the diagnosis of discitis, as infection by gas-forming organisms are
exceedingly rare.
obtain flexion–extension radiographs to exclude subtle instability
patterns. Relatively subtly increased intervertebral motion can be
associated with pain. In these patients, discography may be useful to
establish a pain generator. Some authors assign no clinical importance
to lumbar segmental motion (24,32).
evaluation of disc degeneration by excluding other suspected pathologic
processes, such as tumor, infection, or spondylolysis. Computed
tomography (CT) may demonstrate degenerative changes in the lumbar
spine, but is not particularly useful in the evaluation of patients
with DDD.
(MRI) is the most commonly employed imaging modality. In that MRI can
directly measure water content of the disc, it is the only imaging
technique that can detect biochemical changes in the nucleus (56,61).
The normal, hydrated NP has an increased proton signal on T2 images.
With increasing desiccation, this signal blends with that of the
surrounding annulus. With further degeneration, a dark, isointense
signal may be seen on T2 (Fig. 145.10, Fig. 145.11).
An increased T2 signal may be noted in some areas, where it is thought
to represent free fluid in annular tears and fissures (87). Aprill and Bogduk (1)
described a high-intensity zone (HIZ) representing a tear of the outer
annulus. In their study, these HIZ lesions were associated with
painful, concordant discography. Subsequent reports as to the
significance of these lesions have been mixed (64).
 |
|
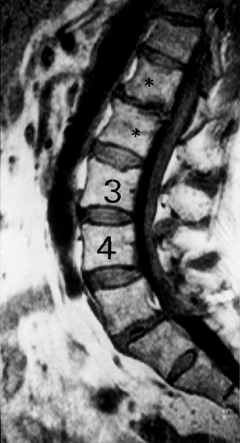
Figure 145.10. Sagittal T1-weighted MRI depicting disc degeneration at L1–L2 with endplate sclerosis (asterisk).
|
 |
|
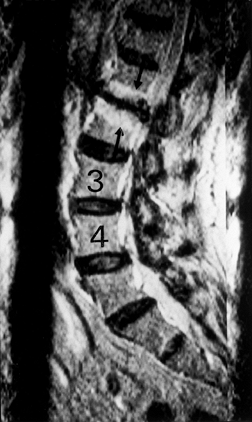
Figure 145.11. Sagittal T2-weighted MRI depicting black disc at L1–L2 with typical endplate changes (arrow).
|
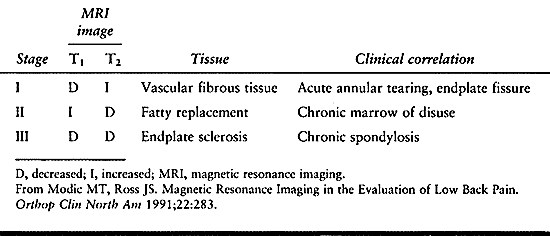
and can be divided into three types. Type I reflects an acute
disruption and fissuring of endplates, which leads to ingrowth of
vascularized fibrous tissue into the adjacent vertebral body marrow.
This tissue exhibits a diminished signal on T1 images and increased
signal on T2 images.
peridiscal marrow undergoes fatty degeneration. Here, a type II pattern
is exhibited with an increased T1 and an isointense or slightly
hyperintense T2 signal. While type II changes tend to remain stable,
type I changes have been shown to develop into type II. Type III
changes probably reflect extrinsic bone sclerosis as seen on plain
radiographs. Dense bone in the vertebral endplates yields a hypointense
signal on both T1 and T2 images (Table 145.6).
 |
|
Table 145.6. Modic Changes
|
likely to be a normal part of aging, the MRI findings associated with
the changes described by Modic and Ross (56) frequently do not correlate with LBP. In 1990, Boden et al. (5)
found that MRI evidence of degenerative discs was present in 34% of
patients 20–29 years old, and in 93% of patients 60–80 years old.
only provocative method available to assess patients with a possible
discogenic pain generator. In theory, fluid injected into the disc
increases endplate pressures. These transferred pressures may cause
pain (34). Abnormal pressure transmission may
account for the small subset of patients with normal MRI findings and
positive discography. Properly performed, discograms may
be able to directly identify a cause-and-effect relationship between
radiographic signs of degenerated discs and clinical symptoms of lumbar
pain.
Abnormal radiographic findings with leakage of dye were seen in 37% of
an asymptomatic population, so the study is positive only if the
radiographic changes correlate with a concordant pain response. A
concordant response requires replication of the patient’s usual pain
with injection at the degenerated level, and no pain at adjacent,
control levels (Table 145.8).
 |
|
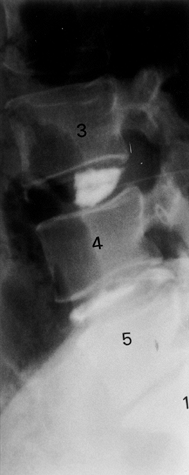
Figure 145.12.
Discography at the L3-4, L4-5, and L5–S1 interspaces. Note the normal appearance of the control L3-4 level and the abnormal morphology below. |
 |
|
Table 145.7. Information Available from Discography
|
 |
|
Table 145.8. Key Elements of Discographic Technique
|
positive discography, considered the test unreliable. This study was later criticized by Simmons et al. (69),
who noted that injections without fluoroscopic guidance often
pressurize the sensitive annulus rather than the nucleus. Further, the
contrast material used, diatrizoate meglumine, has been found to be
irritating and painful. Later, the Holt study was repeated using modern
techniques by Walsh et al. (80), who injected a
nonionic, water-soluble contrast agent under fluoroscopic guidance. The
study was considered positive only if the disc was radiographically
abnormal and a concordant pain response reported. A false-positive rate
of 0% with a specificity of 100% was noted in 10 asymptomatic
volunteers.
89% of 137 patients with positive provocative discography had
significant and sustained benefit from fusion at the indicated level.
Of 20 patients fused without a positive discogram, only 52% enjoyed
postoperative pain relief.
it can be expected that recommendations regarding its role in patient
assessment also vary. Some report that a negative MRI may miss
clinically significant DDD (7). Others report that positive discography in the context of a negative MRI is associated with inferior results after fusion (27).
high correlation between MRI and discographic findings. They reported
on 101 disc levels in 36 patients with LBP of longer than 2 months’
duration. In each patient, both MRI and discography were performed and
blindly reviewed by a neuroradiologist. MRI was accurate in predicting
discographic disc morphology 99% of the time. Only one disc level with
a normal MRI signal had an abnormal discogram. Of 49 levels with
decreased signal on MRI, only two were normal on discogram. The authors
found that concordant pain with discography was helpful in the
assessment of abnormal discs identified by MRI, but they felt that
discography was not indicated in the presence of a normal MRI. Simmons
et al. (69), on the other hand, found only a
55% correlation between the tests. They wrote that discography is the
only dynamic test available for disc evaluation and thus the only study
that can determine which abnormal discs are truly symptomatic.
that, in many cases, MRI could not reliably predict or replace
discography. They divided the MRI signal of lumbar discs into dark,
white, and speckled patterns, and they characterized the posterior
annulus as flat, bulged, or torn. Most dark or torn discs demonstrated
positive discography, whereas white or flat discs were very likely to
be negative on discography. The intermediate MRI patterns had uncertain
correlation with discographic findings, however.
only as a preoperative test in psychologically normal patients with
positive MRI findings, and after aggressive,
nonsurgical
measures have failed. Discography is probably not warranted in patients
with single-level changes. In patients with multilevel or equivocal MRI
findings, we use a discogram to detect the symptomatic level.
The underlying hypothesis is that bracing will simulate effects of
fusion by restricting segmental motion. In general, such bracing is not
justified, in that the braces most commonly recommended are unreliable
in restricting lumbar spinal motion (17,65). Adequate immobilization of the lower lumbosacral spine requires a pantaloon brace.
pain, the perception of which is quite variable. Depression and anxiety
are quite common in DDD patients and are associated with heightened
pain perception (77). Historically, it has been
difficult to establish whether the pain preceded the psychological
disturbance. However, in a series of 200 patients with chronic back
pain, 77% met the American Psychiatric Association’s Diagnostic and
Statistical Manual of mental disorders (DSM III-R) lifetime criteria
for psychiatric illness, and 59% met criteria for current, active
psychiatric illness (63). In this study, the
authors concluded that psychiatric illness (particularly anxiety and
substance abuse disorders) often preceded the onset of back pain.
Multiphasic Personality Inventory (MMPI) include abnormal elevations of
the hypochondriasis and hysteria scales, above that of the depression
scale. On this inventory, somatic fixation tends to be predictive of
poor surgical outcomes (84). Patients with low
hypochondriasis and hysteria scores had 90% good to excellent results 1
year after surgery, while patients with higher scores had only a 10%
rate of good to excellent results (84). Southwick and White (71) reported that patients in the latter category were more likely to have positive discograms at nondegenerated levels.
disc degeneration, there are no findings pathognomonic for DDD. As in
any degenerative condition of the spine, begin the evaluation with a
complete history and physical exam. Pursue atypical pain and other red
flags vigorously. Then, assuming limited findings on physical exam,
commence further management with a rigorous nonoperative management
regimen.
trial require plain radiographic evaluation. While the changes on plain
radiographs associated with disc degeneration do not necessarily confer
a diagnosis, use plain radiography to exclude other potentially serious
causes of pain. If these radiographs are negative, obtain
flexion–extension lateral radiographs to rule out subtle instability.
factors. A long period of evaluation and nonoperative management
affords the surgeon an opportunity to get to know the patient well.
Obtain an MMPI if there are any doubts as to the psychological profile.
A motivated, professionally satisfied patient is the ideal candidate
for further evaluation and possible surgical treatment.
intervertebral disc. In the context of unresponsive mechanical pain in
a psychologically normal individual, single-level degenerative changes
may warrant consideration of operative treatment. Perform multilevel
discography if several levels are involved or MRI findings are
equivocal. Consider surgery only for those patients demonstrating one
(possibly two) levels of concordant pain with no pain at control
levels. Discography is at present not indicated in patients with a
normal MRI.
Begin treatment at the initial patient encounter and, should the pain
fail to improve, continue it through the protracted evaluation period.
During this period, optimize the patient’s physiologic status through
cessation of smoking, increasing spinal flexibility, and increasing
aerobic exercise tolerance. Consider only patients actively
participating in a surgeon-directed rehabilitation program for invasive
presurgical testing, such as discography.
therapy including strengthening and stretching. Some have found that
flexion exercises exacerbate discogenic pain. Specific abdominal
strengthening and trunk-stabilizing exercises, such as abdominal
crunches with flexed hips and knees, however, can be performed with
limited lumbar flexion. Extension exercises and low-impact aerobic
exercises, such as swimming and cycling, are often recommended and well
tolerated.
begin conditioning and strengthening exercises, start a course of
acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). Muscle
relaxants may be useful in the setting of acute LBP but are not
recommended for patients with chronic difficulties (5).
of acute LBP episodes, although no convincing evidence of efficacy in
the context of chronic LBP is available. Further, as in acute back
pain, extended periods of bed rest have no role in the management of
DDD patients. Several clinical trials have studied the role of bracing.
The Quebec Task Force on Spinal Disorders found insufficient scientific
evidence to support the efficacy of a lumbar corset or support (5,14,89).
and acupuncture for chronic, mechanical LBP are not supported by the
scientific literature (89).
who fail to adequately participate in a nonoperative regimen. In such a
setting, detoxification, psychological assessment, work hardening, work
skills retraining, tricyclic antidepressant medication, and other
modalities may be effectively applied to improve the patient’s pain and
functional status.
whom operative results would represent an improvement over the natural
history of DDD. Since the natural history of DDD remains to be
elucidated, balance any recommendations for surgery carefully against
surgical risks and the significant possibility of failure to obtain
clinical improvement.
reported for the various operative modalities in DDD. There are no data
to suggest that the various subtypes of DDD require different
approaches to surgical management.
to quantify acceptable surgical results. Often, failures result from
unrealistic expectations on the part of both physician and patient. One
factor is “fusion disease,” described by Zdeblick (89).
The stripping and retraction required for posterior spinal fusion
procedures may cause permanent fibrosis and ischemic injury of the
extensor musculature. Long periods of intense manual labor may be
impossible even in the presence of a solid bony union.
single-level (and occasionally double-level) fusions for DDD may be
considered if the following prerequisites have been met (36):
-
Pain and disability are present for at least 1 year.
-
There is failure of aggressive physical conditioning and conservative treatment of more than 4 months duration.
-
There is single-level disc degeneration on MRI with concordant pain response on discography.
-
There is absence of psychiatric or secondary gain issues.
limiting mechanical stimuli across the painful motion segment. The
least controversial procedures achieve this goal by solid arthrodesis.
Several methods currently advocated to promote such a fusion will be
described next.
The advantage of the posterior approach is that fusion can be performed
in the absence of the posterior element; the risk of injury to the
neural elements is low; and because the graft is placed away from
midline, there is less risk of iatrogenic spinal stenosis.
reported 89% good results, and they achieved an 80% fusion rate by
radiographic criteria; there was a high correlation between successful
fusion and the clinical result. Dawson et al. (11)
reported a 92% rate of solid fusion, with a 70% to 90% clinical success
rate. Those undergoing a fusion above the lumbosacral motion segment
were found to have a 45% pseudarthrosis rate, however.
performed a posterolateral fusion for DDD in 20 patients with
concordant discography. While 11 felt the operation was worthwhile,
only six had a good outcome as measured by impairment, disability, and
work status. Parker et al. (62) reported a
prospective, consecutive series of patients with discogenic LBP
undergoing posterolateral fusion. They observed only 39% good to
excellent results, with 13% fair and 48% poor results. Poor results
were associated with workers’ compensation status, pseudarthrosis, and
being out of work longer than 3 months Greenough et al. (29)
concluded that posterolateral fusion was an acceptable treatment for
discogenic back pain only in very carefully selected patients.
posterolateral fusion procedures in an attempt to decrease
pseudarthrosis rates (Fig. 145.13, Fig. 145.14).
Data from fusion procedures for degenerative spondylolisthesis suggest
increased fusion rates but no effect on clinical outcome (19).
Instrumentation is associated with higher costs and complication rates.
One recent series noted 10% of patients had instrumentation-related
problems. Yet, biomechanical studies suggest that pedicle screw
constructs are superior in stabilizing the nonosteoporotic spine (31).
These constructs may confer immediate stability to motion segments and
allow an expedited postoperative recovery. This added stiffness and
faster recovery interval may be more important in the younger
population with DDD than in the older patient with degenerative
spondylolisthesis.
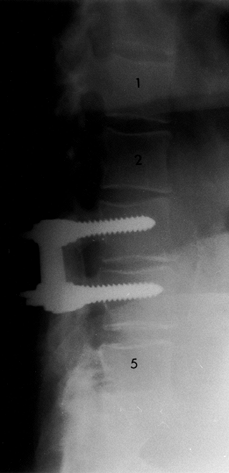 |
|
Figure 145.14. Postoperative lateral radiograph depicting fusion with instrumentation at L3-4.
|
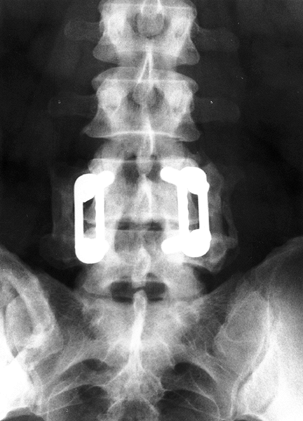 |
|
Figure 145.13. Postoperative AP radiograph after single-level L4-5 posterior fusion with instrumentation.
|
from 35% to 68%. Instrumented fusions have pseudarthrosis rates
reported from 0% to 33%. Increased rates of 75% to 95% significant
clinical improvement are also reported in patients undergoing
instrumented fusions, versus 59% to 70% in those fused without
instrumentation (3,88). In patients undergoing fusion for discogenic pain, solid fusion is associated with increased return-to-work rates as well (11).
Posterolateral fusions undertaken to treat DDD should probably be
undertaken with rigid, transpedicular instrumentation, particularly in
the revision situation (85).
interbody fusion (ALIF). The first, the open retroperitoneal approach,
employs a 5–10 cm incision to directly access the anterior spine. A
complete discectomy may then be undertaken and a variety of implants,
including allograft rings and threaded cages, placed into the disc
space. Various endoscopic methods of threaded cage placement have been
described as well. These approaches use relatively straightforward
techniques for access, but they do not include a complete discectomy.
Moreover, the threaded cage techniques require violation of the
vertebral endplate. While these newer approaches may be less invasive,
long-term data regarding fusion rates and implant stability are not
available.
More recently, increased ease of access and concerns over extensor
muscle retraction in a relatively young patient population have renewed
interest in this approach. Moreover, some authors, citing the disc as
the primary source of pain, recommend its complete extirpation (27,29,43,58,59,72,82,89,90).
-
Complete excision of disc material
-
Placement of the graft under compression
-
Availability of a large surface area for graft incorporation
-
Availability of a virgin operative site if there has been a prior posterior spinal fusion
-
Avoidance of extensor muscle injury (“fusion disease”)
arthrodesis, flexion may occur through the fusion mass. This slight
movement may cause continuing pain in the intervening degenerated
discs. Weatherly et al. (81) reported complete
relief of pain after ALIF in four patients with solid posterolateral
fusion and concordant discograms at the previously fused level. Because
ALIF places the fusion mass at the center of motion of the spine, it
more rigidly immobilizes the spine once it is solid (33,90).
However, the pseudarthrosis rate is generally felt to be lower than
that for one-level posterolateral fusions or posterior lumbar interbody
fusions (76,85).
Similar variability in rates of pain relief and return to work have
been reported. Ostensibly, this variability is caused by differences in
surgical indications and techniques.
Traditionally, corticocancellous bone from the iliac crest was placed
in the disc space and maintained in position with a screw and washer.
This approach was sometimes associated with graft collapse and
pseudarthrosis. Allograft rings were then recommended and,
subsequently, threaded cage techniques.
structural support for the anterior column, indirect decompression of
the foramina and nerve roots by distraction of the disc space, the
potential for bony ingrowth through the cage, and the possibility of
minimally invasive implantation (90). While clinical results are preliminary, early series demonstrate good mechanical stability with these constructs (52). Figure 145.15 shows sample cases.
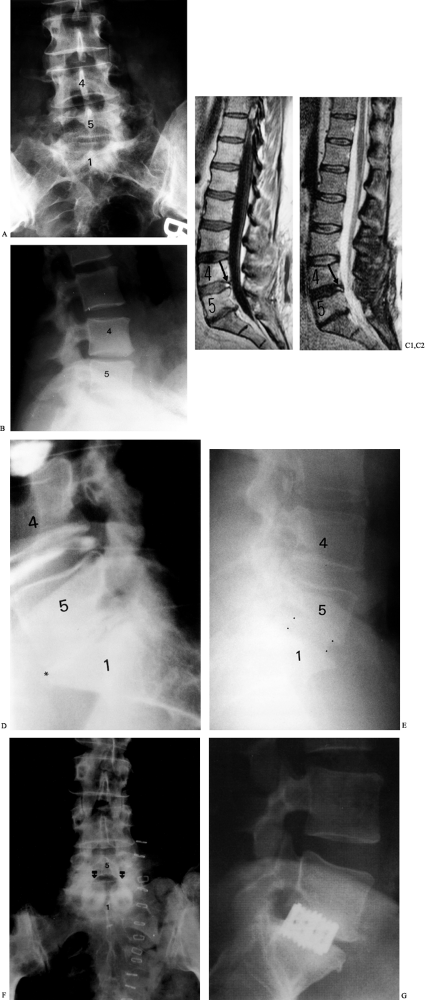 |
|
Figure 145.15. Case example. A: Preoperative AP radiograph of the lumbar spine depicting loss of disc space height and L5–S1. B: Lateral radiograph showing disc degeneration at L5–S1. C1: Sagittal T1-weighted MRI demonstrating disc degeneration at L4-5 and L5–S1 with an annular tear evident at L4-5 (arrow). C2: Sagittal T2-weighted MRI in the same patient. D: Discography was performed at the L3-4, L4-5, and L5–S1 levels. Concordant pain was noted at the L5–S1 level (asterisk). While the L4-5 annular tear is again appreciated on discography, this level was not painful to dye injection. E: Lateral radiograph demonstrating corticocancellous autograft ALIF at L5–S1 (dots outline the anterior and posterior extent of the graft). F: An immediately postoperative AP radiograph of another patient with allograft dowel placement via a mini-open ALIF approach. G: Lateral radiograph of another patient with BAK cage placement.
|
methods of ALIF, it is important to remember that they represent only a
new technique, not a new operation. Therefore, operative indications
remain the same. There are proposed benefits of an endoscopic approach,
however. Preliminary studies suggest shorter hospital stays, decreased
morbidity, and earlier return to work with minimally invasive
techniques (52,53).
The transperitoneal approach with gas insufflation serves as a direct
extension of conventional laparoscopic surgery (90).
This technique allows direct access to L5–S1, L4–L5, and occasionally
L3–L4. Proposed advantages include ease of organ retraction, more rapid
exposure of the spine, increased working space, and decreased bleeding.
Disadvantages include the requirement for expensive trocars with
diaphragms and other special instruments, as well as the potential for
air leakage and a carbon dioxide venous embolism.
space is maintained by lifting the anterior abdominal wall with a
fan-like retractor. This approach allows the use of conventional
instruments and avoids carbon dioxide effects and expensive valves. The
procedure is associated with increased time for exposure, limited
lateral vision, and an overall more technically difficult approach.
Therefore, an intermediate, combined insufflated and gasless technique
was devised, in which insufflation is used for the initial spine
exposure. Retractors and Steinmann pins are placed, and then conversion
to gasless technique is undertaken.
space between the spine and the abdominal cavity, make a 2.5 cm flank
incision by splitting the anterior lateral abdominal muscles. After an
initial finger dissection, enlarge the space with a retroperitoneal
balloon. Again, the laparoscopic retractor with a hydraulic arm is
employed to create a tent-like effect. Further peritoneal reflection
can then be carried out under direct vision. This procedure provides
access from T-12 to S-1. Further, conventional instruments may be used
and the procedure can be converted from a pure percutaneous endoscopic
to an endoscopic-assisted anterior approach should the degree of
difficulty be increased. Some authors report difficulty obtaining a
direct frontal approach to the disc space with this technique, however.
reported a series of 69 patients with global fusions for DDD. Fusion
levels were determined by provocative discograms. With one- and
two-level procedures, 90% fusion rates were achieved. Three-level and
greater procedures were associated with 77% fusion rates. Overall, 80%
had acceptable clinical results. O’Brien et al. (59)
described a global fusion procedure for 150 patients with severe
disability due to back pain or with previous failed operations. With
posterior instrumentation, they noted an 86% success rate.
principal justification for the added surgical morbidity of these
combined procedures (44,76).
More recently, concerns regarding the effectiveness of threaded cages
as stand-alone devices has intensified the debate over the need for
posterior stabilization.
posterior stabilization in a single surgical approach is the posterior
lumbar interbody fusion (PLIF). A wide posterior decompression is
performed, allowing retraction of the dural sleeve and nerve roots for
complete disc excision and anterior column fusion.
He stated that PLIF was indicated for “the treatment of low back pain
with or without sciatica due to lumbar disc disease.” This procedure
has also been recommended for spondylolisthesis, lumbar scoliosis,
osteomyelitis, lumbar kyphosis, and to increase posterior fusion rates
in high-risk patients (e.g., smokers and diabetics).
graft may enhance the fusion rate, stabilize the construct, and protect
the posterior spinal implant by load sharing. In patients with
deformity, the PLIF may aid in correction by partial anterior release.
Most commonly, however, PLIF procedures are performed for discogenic
back pain.
as able to address “all sources of pathologic change of the motion
segment in one operation, through one incision.” Yet, after initial
enthusiasm, use of the PLIF declined due to high rates of
pseudarthrosis and graft dislodgement. More recently, the advent of
transpedicular instrumentation led to a resurgence of interest in PLIF.
Steffee and Sitkowski (74) reported 104 fusions performed without graft dislocation, pseudarthrosis, or infection.
excision, restoration of disc height and normal sagittal contour, root
decompression, solid mechanical arthrodesis, immediate load-sharing
with structural support, large surface area for fusion between the
vertebral endplates, fusion under compression, and avoidance of an
additional anterior approach.
pseudarthrosis, anterior and posterior destabilization, increased
bleeding, dural tears, risk to nerve roots, and risk of epidural
fibrosis from root retraction (36).
PLIFs for back or leg pain, spondylolisthesis, or failed back syndrome.
There was an 11% reoperation rate: six for pseudarthrosis, three for
bone graft extrusion, one bone graft fracture, and one hematoma. Others
have reported good to excellent results in 89%, with a fusion rates of
73% to 95% and an 82% return-to-work rate (49,76).
from wide retraction. Epidural fibrosis may develop into a chronic
radiculopathy for which there is presently no satisfactory solution.
Harms et al. (33) recently popularized a
variant of the PLIF procedure that avoids significant retraction on the
thecal sac. This posterolateral approach to the disc space relies on
facet excision and has been termed the transforaminal lumbar interbody
fusion (TLIF). While transpedicular instrumentation is recommended for
the midline laminotomy version of the PLIF, it is mandatory here.
anterior and posterior ligamentous complex, thereby maintaining a
tension band for compression of the graft and prevention of
retropulsion. While long-term outcome reports are not yet available,
some authors noted the proximity of the dorsal root ganglion and the
potential for chronic, neurogenic pain after even minor trauma to this
structure.
patients with preexisting, significant epidural fibrosis and those with
significant osteopenia. Aside from the more typical complications such
as bleeding, infection, and pseudarthrosis (mentioned later), the
unique complications possible with PLIF deserve special mention here.
With standard PLIF procedures, damage to nerve roots remains a
principal concern. The surgeon must be careful about overdistraction,
particularly in patients with nerve root anomalies. New or increased
deficits occur in 0.5% to 4% of patients after PLIF (48).
The upper (exiting) root traverses the interspace just out of direct
view in the lateral recess and can be damaged when grafts are inserted
in the disc space.
“a worse outcome than failure of any other fusion procedure.” They
report that exploration of patients with a post-PLIF chronic
radiculopathy reveals epidural fibrosis for which there is no
satisfactory salvage. Careful attention to the tension placed on the
cauda and nerve roots may diminish the incidence of these problems.
bone graft migration. The subtotal discectomy required for a PLIF
procedure risks penetration of the anterior annulus with attendant
anterior vessel damage, a potentially catastrophic complication.
noninstrumented PLIF may be converted to an instrumented PLIF. A failed
instrumented PLIF is most often revised with an attempted anterior
fusion (82).
treatment of lumbar disc disease, the role of PLIF or TLIF is not
entirely clear. For example, the importance of “fusion disease” in
these relatively young patients remains to be established (42). Certain criteria, however, may reasonably aid in the selection between ALIF and PLIF (Table 145.9).
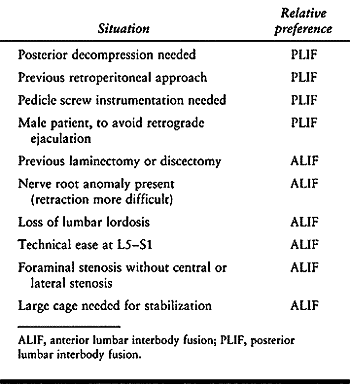 |
|
Table 145.9. Soft Selection Criteria to Decide Between ALIF and PLIF
|
revise, present recommendations for PLIF include lumbar disc disease
with sciatica (10) and certain revision situations in which an anterior/posterior fusion through a single incision is desirable.
the gold standard for the rare patient with true lumbar segmental
instability. Those patients with painful lumbar spondylosis LS are best
served with a disc ablative procedure such as ALIF and PLIF.
Circumferential fusions should be reserved for patients with
significant canal pathologies, where revision is required, and other
situations in which additional stabilization is required.
PLIF and its potential for nerve root injury, epidural fibrosis, and
“fusion disease.” The advent of transforaminal interbody fusion
procedures may decrease the risk of the PLIF technique, but firm
evidence is not available. As more information regarding the long-term
results of interbody cage placement becomes available, treatment
recommendations will no doubt be revised.
in its various forms are discussed here: posterior lumbar fusion (with
and without instrumentation) and PLIF. ALIFs, either through
traditional open or laparoscopic techniques, are increasingly favored
for patients with these disorders and are covered in Chapter 146.
carefully review your patient selection criteria. Be sure the patient
is well informed as to the risks and the potential for an
unsatisfactory clinical outcome. Pay careful attention to patient
physiology by the following measures:
-
Discontinue aspirin and NSAIDs.
-
Ensure a good nutritional status.
-
Have the patient stop smoking and other tobacco use.
-
Recommend preoperative blood donation and institute prophylactic antibiotics.
positioning. Choose a frame or bolsters that allow the abdominal
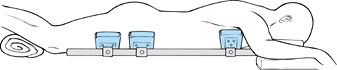
contents to be free of compression and maintain proper lumbar lordosis (Fig. 145.16).
 |
|
Figure 145.16.
Proper positioning on a frame that decompresses the abdomen and avoids pressure points is critical to the success of any lumbar fusion procedure. |
-
Freedom of abdominal contents will decrease epidural venous engorgement and blood loss.
-
A Foley catheter similarly decreases intra-abdominal pressure by preventing bladder distention.
-
Carefully pad all bony prominences and avoid pressure over the eyes.
-
Use a radiolucent turning frame in procedures in which multiplanar image intensification is anticipated.
-
Maintain the patient’s core body temperature with ventral and lower-extremity air-circulating devices.
the effectiveness of the exposure. Intraoperative neural monitoring may
be used to stimulate pedicle screws during placement to detect
pedicular penetration.
significant blood loss. Intraoperative blood salvage is occasionally
useful. More important, anticipate and control bleeder sites as you
encounter them. Three fairly constant bleeding points include the pars
interarticularis artery, the artery of Macnab (transverse process
artery), and the sacral arteries.
-
The pars artery is encountered during the
initial exposure. Emerging from a recess inferior to the facet, it
wraps around the pars. -
A curved bayonet may help control the
artery of Macnab, which lies on the upper aspect of the junction of the
transverse process. -
The sacral arteries protrude from the
posterior sacral foramina and are difficult to control without
inserting bipolar cautery or forceps into the bony recess. Often,
temporary packing with Gelfoam will provide adequate hemostasis. -
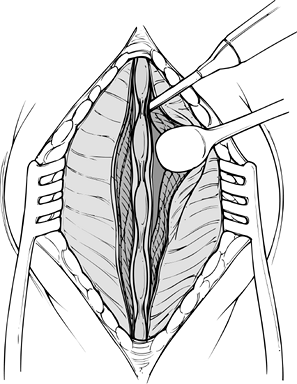
Begin the exposure by centering a 6–10 cm midline skin incision just cranial to the involved level (Fig. 145.17).
![]() Figure 145.17. Initiation of a midline subperiosteal approach.
Figure 145.17. Initiation of a midline subperiosteal approach. -
Inject a dilute epinephrine solution into the skin and subcutaneous tissue to decrease bleeding.
-
Use electrocautery to proceed through the subcutaneous tissue to the fascia.
-
Expose a 0.5 cm portion of the fascia on
either side of the midline to aid subsequent closure. Avoid extensive
fascial stripping, which increases dead space. -
Enter the deep space over the spinous processes in a subperiosteal fashion. Use the Cobb elevator for countertraction.
-
Carry the subperiosteal dissection over
the laminae to the facets with the electrocautery. Do not violate the
facet capsules at this point. -
Insert a Penfield #4 elevator (Fig. 145.18)
under a lamina and obtain a radiograph. Confirm operative levels and
mark the superior level by resecting a portion of the spinous process
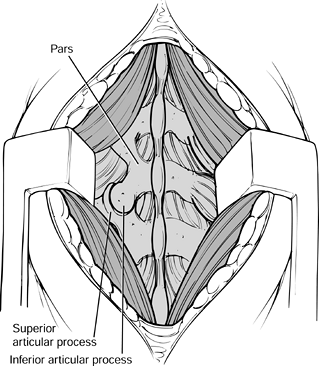
with a rongeur.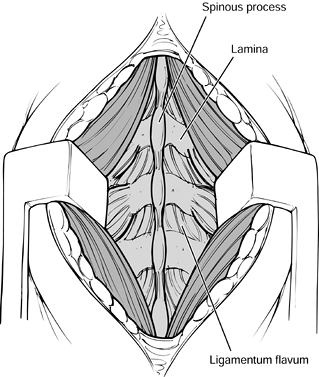 Figure 145.18. Exposure of the facets.
Figure 145.18. Exposure of the facets. -
Bluntly dissect over the facets with a Cobb elevator and a sponge at the operative level.
-
Then expose the lateral pars
interarticularis and transverse processes with electrocautery. The
transverse processes lie immediately adjacent to the facets. Be careful
not to penetrate the intertransverse membrane (Fig. 145.19).![]() Figure 145.19. Exposure of the transverse processes and pars interarticularis.
Figure 145.19. Exposure of the transverse processes and pars interarticularis.
bilateral Wiltse (paramedian) approach. A larger midline skin incision
and subcutaneous dissection is required. Make the fascial incisions two
finger breadths lateral to the midline bilaterally. Then bluntly
dissect down to the lateral facets between the multifidus and
longissimus muscles. This approach is covered more extensively in Chapter 138.
-
Remove all soft tissue from the posterior
aspect of the transverse processes, outer facets, and pars. If the
fusion includes S-1, clear the sacral ala. Complete soft-tissue removal
will double the area available for bone graft. Preparation of the graft
bed is the most important part of any fusion procedure. -
Decorticate the lateral pars, the transverse process, and the lateral wall of the facets.
-
Lay autologous cancellous bone into place
and impact gently. Then add corticocancellous strips and compress
gently into position. -
For each level to be included in the fusion, remove the entire facet joint capsule and denude the cartilage from the facet.
-
Close the wound in layers to create a watertight fascial closure. Place a drain in the deep space.
-
Periodically release the retractor to reestablish muscle vascularity.
-
Handle muscle carefully to minimize devascularization and necrosis.
-
Make the exposure generous to minimize the required retractor tension.
-
Use Gelfoam or bipolar cautery over the pars, transverse processes, neural foramen, and dorsal sacral foramen.
-
Prevent overcauterization to minimize injury to nearby neural structures.
fusion. Do not insert pedicle screws prior to complete exposure of the
posterior elements. Decortication of the transverse processes and bone
graft insertion are more easily and completely accomplished prior to
hardware insertion. Many surgeons, however, prefer to decorticate after
instrumentation to diminish blood loss.
and pedicle-screw placement have been described. Thorough knowledge of
pedicular anatomy is critical. Various radiographic techniques of
localization are now available, but none replaces a firm grasp of the
relationships of the posterior elements to one another.
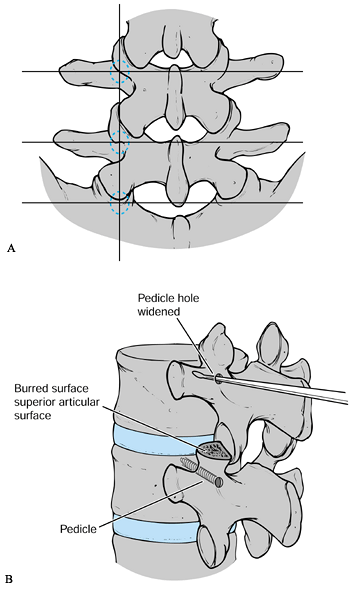
transverse process. The outer border of the facets describes a line
roughly conforming to the lateral border of the pedicle. The lateral
border of the pars defines the medial pedicular border. The accessory
process is often identified at the junction of the facet and the
transverse process. This process serves as a useful entry point (Fig. 145.20A).
 |
|
Figure 145.20. A: Anatomy of pedicle location. See text for details. B: Sounding the pedicle with a probe or curret.
|
for DDD, instrumentation in these cases is placed at each level in an
effort to reduce micromotion. How much segmental stiffness is required
to achieve fusion and eliminate pain is not established (45); however, given that
micromotion has been hypothesized as a cause of pain in four screw micromotion-segment constructs (81),
maximizing points of fixation is recommended. Screw placement is
abandoned if preoperative imaging demonstrates thin pedicles.
Similarly, if difficulties with cortical breech are noted
intraoperatively, abandonment of that point of fixation is recommended.
-
Carefully review the preoperative MRI or
CT scan to confirm the extent of convergence of the pedicle at each
level, as well as the length and size of screw necessary. -
In general, screws should converge by 5° at the thoracolumbar junction. This convergence increases to 10° at L-2 and 15° at L-5.
-
Use the localization lateral radiograph to define the proper attitude of the pedicle in the cranial and caudad planes.
-
Most often, an L-3 pedicle screw is
inserted perpendicular to the floor. Angle superior screws
progressively more cranially. Angle inferior screws progressively more
caudally. -
Decorticate the pedicle entry site with a burr.
-
Enter the pedicle with a curet or pedicle probe. Gently work this device anteriorly into the vertebral body (Fig. 145.20B).
-
Check for cortical penetration with a
ball-tip probe. If a midline decompression has been performed, the
pedicle may also be palpated from within the canal. If necessary, place
markers and confirm radiographically. -
Tap the hole and insert the screw (Fig. 145.20B).
The optimal depth of screw placement has not been determined, but
pullout strength increases linearly with depth of penetration (46).
We estimate 75% penetration using lateral fluoroscopy to increase
purchase while minimizing risk to anterior vascular structures. -
Once the screws and bone graft have been placed, affix a plate or rod according to the manufacturer’s instructions.
-
Be careful to maintain proper lumbar lordosis.
-
Undertake closure over a drain with
watertight fascial closure, and follow with a layered closure of the
subcutaneous tissues and skin.
Large constructs extending to the sacrum require additional fixation.
The combination of large forces transmitted to the sacrum and the size
and bone content of the sacral pedicle may render S-1 screws inadequate
as the sole point of inferior fixation. In the degenerative conditions
described in this chapter, however, smaller, single-level constructs
are recommended. In these cases, simple transpedicular instrumentation
is usually successful. The sacral pedicle requires larger screws.
-
Locate the S-1 pedicle at the
intersection of a horizontal line connecting the inferior aspect of the
lumbosacral facets, and a vertical line tangential to the lateral
border of the superior facet. -
Insert the screws above and in line with
the first sacral foramen. Converge the screws and incline them
superiorly, parallel with the lumbosacral disc space.
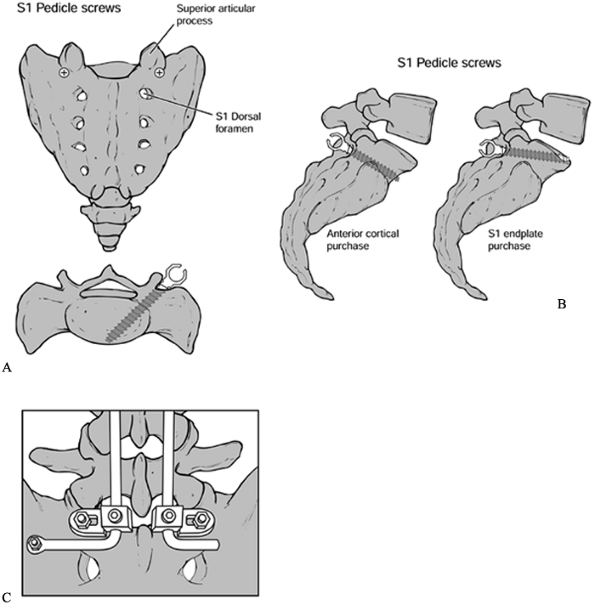
fixation strength, although iliac crest overhang may limit optimal
screw trajectory. In the case of tenuous fixation, additional cortical
purchase is recommended to prevent “windshield-wipering.” Options
include careful perforation of the anterior cortex or of the L5–S1 disc
space (Fig. 145.21).
 |
|
Figure 145.21. A: Sacral screw placement. B: Lateral view of sacral screw placement. C: Iliac bolts.
|
anatomic variability (13).
Differences in sacral bony anatomy are associated with differences in
vascular tree branching patterns and in the location of the neural
foramina. Assess the morphology and choose an optimal trajectory from
preoperative CT or MRI scans.
penetrate the anterior cortex. More lateral sacral screws may hit the
lumbosacral plexus, which is affixed to the bone here. Lateral
trajectories also risk injury to the iliac veins, particularly on the
left.
interspace include S-2 pedicle screws, sacroiliac bolts, and sacral
rods. S-2 screws are easily inserted along the intermediate sacral
crest midway between the first and second dorsal foramina, but they are
considered biomechanically weak. Cortical penetration at this level
risks injury to the sigmoid colon.
genuinely effective bracing requires a pantaloon extension. Sacral
fixation techniques are further discussed in Chapter 156, Chapter 159 and Chapter 160.
in the same manner as posterolateral fusion. Careful positioning is
critical. Lumbar lordosis and abdominal decompression are important.
Some authors feel the knee–chest position adequately maintains lordosis
while maximizing hip and
knee
flexion. Hip and knee flexion ensure decreased tension on the nerve
roots, which may allow greater thecal sac retraction for PLIF surgery.
instrumentation, this is not recommended. The wide posterior exposure
necessary for a safe PLIF produces increased instability with increased
rates of pseudarthrosis and graft dislodgement.
-
Perform a standard 10 cm midline exposure
of the posterior elements (as described previously) with a wide
laminotomy at the level of interest. -
Preserve the superior portion of the
superior lamina and the inferior portion of the inferior lamina with
portions of the spinous processes. Muscular attachment sites and
interspinous ligaments to levels above and below operative level are
thereby preserved. -
Remove the inferior third of the inferior
facet, and the medial two thirds of the superior facet to the level of
the pedicle. Visualize the lateral half of the intervertebral disc as
well as the cranial and caudal nerve roots. -
The medial facet resection can be more aggressive if a combined posterior fusion and instrumentation is planned (Fig. 145.22).
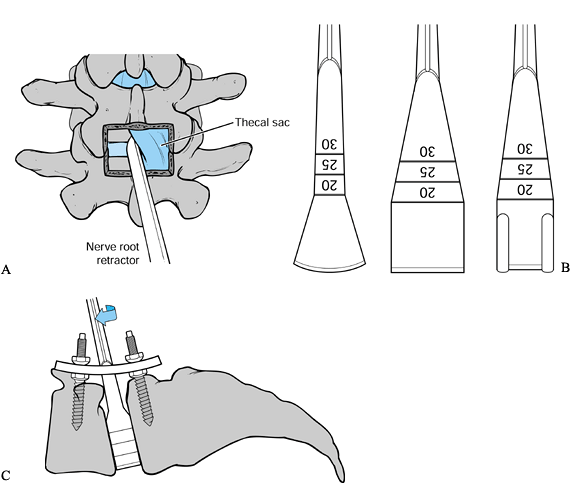 Figure 145.22. A: Midline PLIF exposure. B:
Figure 145.22. A: Midline PLIF exposure. B:
PLIG instruments. These instruments represent a sampling of those
available for the purposes of complete discectomy and disc space
distraction. C: Obtaining distraction through the disc space. -
Next, insert pedicle screws as described previously to allow placement of a working plate or rod.
-
Distract the disc space with a lamina
spreader and hold the distraction with the working plate. Minimize the
force placed through the screws themselves. -
Retract and protect the dural sac and
nerve roots. Take great care to prevent overdistraction of the neural
structures. Ensure adequate mobilization of the thecal sac to the
midline from either side. -
Meticulously cauterize the epidural venous plexus over the posterior annulus with bipolar cautery.
-
Make box annulotomies with a knife, and aggressively clean the disc space on both sides of the thecal sac.
-
Curet the endplates to remove the annulus and endplate cartilage.
disc space. Specialized instruments are particularly useful. If they
are not available, curets, pituitary rongeurs, and osteotomes may be
used. The goal is to clear 80% to 90% of the disc space. Better
preparation of the graft site yields a larger area of bony contact with
the graft and increases the chance of successful fusion.
-
Rotate the shaper to allow its side
cutting flutes to remove disc material and cartilaginous endplate.
Repeat this step on the opposite side. -
Increase the shaper size by increments of
1 mm. Although these instruments are graduated, the disc space depth
varies from 25 to 35 mm and it is necessary to pay careful attention to
the depth of insertion at all times. -
Avoid the anterior portion of the disc
space beneath the anterior longitudinal ligament to minimize the
chances of a catastrophic vascular injury.
With disc space distraction maintained, various graft materials may be
inserted. These include dowels, corticocancellous ramps, titanium
plugs, and metal or carbon fiber cages of the vertical (e.g., the Harms
cage, DePuy-Acromed, Raynham, MA) or the horizontal variety (e.g., the
BAK, Sulzer-Spinetech Minneapolis, MN) (12).
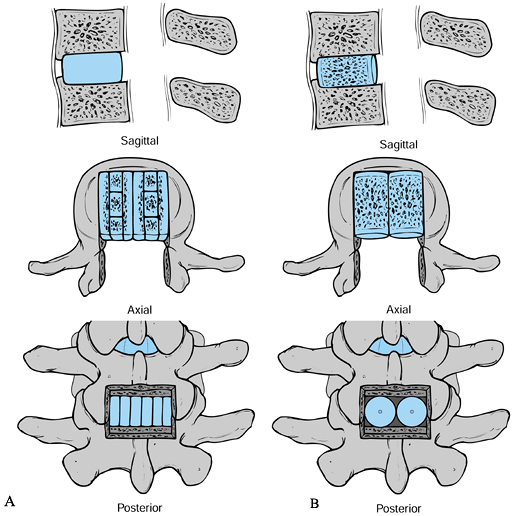 |
|
Figure 145.23. Bone grafting options in PLIF. A: Sagittal, axial, and posterior views (from top to bottom) of Cloward’s (9) tricortical rectangular graft technique. B: Similar views of threaded bone dowels used to obtain a PLIF.
|
providing a structural support to the interspace while the cancellous
graft material becomes incorporated. The use of morcelized cancellous
bone rather than corticocancellous pieces may minimize donor site
morbidity. Specialized instrumentation has been developed for the
posterior insertion of threaded interbody cages. While the specific
instruments vary by the system used, the concepts are the same. The
larger size of these implants requires a larger laminotomy or complete
laminectomy and facetectomy. Base implant sizing on preoperative
templating. Furthermore, the larger size of the instrumentation
warrants a heightened awareness of the position of and tension on the
dura and nerve roots.
-
In some systems (e.g., the BAK), a drill
tube is available to dock onto the disc space posteriorly. This allows
insertion of the remainder of the instrumentation in a relatively safe
fashion. The instrumentation includes distraction plugs or other
devices to open the disc space, reamers to remove disc and endplate
tissue, taps to prepare the threaded cage path, and cage inserters. -
When using this instrumentation, avoid long periods of traction on the dural sleeve with the tube in place.
-
Remember to tightly pack the cage with autograft.
-
Insert remaining autograft around the
cage in the disc space. If individual bone pieces are used, pack the
anterior disc space first, then pack medially under the thecal sac,
then laterally. -
In all cases, countersink the graft material 3–5 mm to prevent canal encroachment.
-
Some authors recommend placing a fat graft anteriorly between the dura and the grafts.
-
Once maximal fill of the disc space has
been achieved, remove the distractive forces to allow compression of
the graft. With transpedicular instrumentation, further compression is
achieved across the screws before final tightening. In all cases, make
sure that appropriate lordosis is maintained.
-
First remove the inferior facet of the
superior vertebra with an osteotome. Then remove the superior facet of
the inferior vertebra (Fig. 145.24).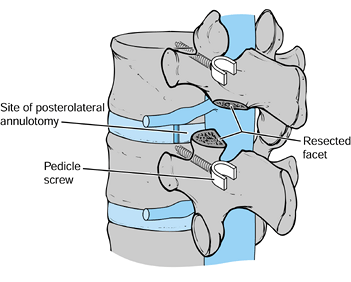 Figure 145.24.
Figure 145.24.
Facet resection and interspace distraction through pedicle screws
allows access to the disc without significant retraction of the thecal
sac. -
Identify and protect the exiting and traversing nerve roots.
-
After the pedicles have been identified,
insert pedicle screws. Distract the disc space with an intervertebral
spreader and maintain the distraction with working plates or rods
affixed to the screws. -
Perform an annulotomy by creating a medially based annular flap. This flap may aid in thecal sac protection.
-
Perform a complete discectomy, as described previously, and increase disc space distraction after the annulotomy.
-
Tamp loose, morcelized, autogenous graft into the anterior disc space.
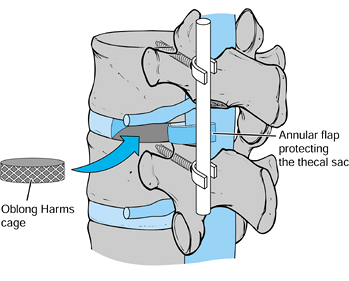
-
Insert additional graft into an oblong 10–12 mm Harms cage (Fig. 145.25). Tamp the cage across the disc space to the contralateral side with either a straight or an angled impactor.
![]() Figure 145.25.
Figure 145.25.
Once a subtotal discectomy has been performed through a posterolateral
annulotomy, bone graft is packed into the disc space anteriorly. Next,
oblong, autogenous bone-graft-filled Harms cages are tamped across the
disc space. -
Insert a second cage ipsilaterally.
-
When packing extra bone graft around the insertion site, be sure that the nerve root is protected.
-
Once grafting is completed, compress the posterior instrumentation.
-
Mobilize patients to a chair the evening after surgery. No braces are employed.
-
Request physical therapy assistance for
ambulation and transfer techniques in the early postoperative period.
Most patients can comfortably ambulate by the third day after surgery.
Typically, young DDD patients rarely require assistive devices. -
Early postoperative restrictions include
limitations on bending, lifting, and twisting, but other gentle
activities are encouraged. -
After posterior fusion, remove drains and Foley catheters on the first postoperative day.
-
At present, we use 24–48 hours of postoperative antibiotics.
postoperative day. See them for a wound check and staple removal at
7–14 days after surgery. Subsequent visits include 6- and 12-week
checks. Obtained radiographs at these intervals to assess the status of
the fusion.
is individualized on the basis of clinical status, radiographic union,
and the nature of the activities involved. Early return with limited
duty is preferred to lengthy sick leave.
spinal flexibility, and truncal strength and stability are important,
life-long aspects of the patient’s personal fitness regime. Further
specific physical therapy is not usually needed, however.
demanding occupations. Early intervention with skills retraining and a
realistic outlook are crucial to optimal functional recovery.
potential pitfalls before selecting one of these procedures.
Perisurgical problems include general surgical complications and
specific procedure-related complications. The latter are detailed in
their respective sections. Also, see Chapter 147.
preoperative, intraoperative, and postoperative problems. Preoperative
problems include the following:
-
Wrong patient. Patient selection cannot be overemphasized.
-
Wrong level. Correct identification of a
pain generator amenable to surgical treatment is fraught with
difficulty and remains the biggest hurdle in the treatment of patients
with disc degeneration. -
Wrong surgery. Fusion procedures,
particularly disc ablative procedures, are the only acceptable surgical
modalities for DDD patients. -
Wrong doctor. Anterior and posterior
spinal fusion procedures are technically demanding and should be
practiced only by surgeons with special training.
Anterior and posterior approaches have a unique complement of attendant
problems.
in young, active patients with stiff spinal segments. While risks and
pathomechanics are not entirely understood, some patients will require
extension of fusion in the future for painful degeneration above or
below the index fusion.
have been discussed. In general, grafts under compression have lower
pseudarthrosis rates. But anterior column applications require
structural graft or cage support. Autogenous structural grafts have
higher harvest morbidity. Allografts may have higher collapse
potential. Further, autogenous bone harvest is recommended in each of
these procedures. The morbidity of bone graft harvest, covered in Chapter 9, should not be underestimated.
related to poor patient selection or misidentification of the pain
generator. In some cases, a solid posterior fusion may allow painful
micromotion of the painful disc anteriorly (as described in the section
on ALIF).
cauda equina syndrome and other postsurgical causes must be excluded.
Further, indwelling catheters or repeated instrumentation of the
genitourinary tract may lead to urinary tract infection and subsequent
sepsis.
is more common after transperitoneal approaches or those
retroperitoneal approaches that violate the peritoneum.
common than after hip and knee procedures, have rates similar to those
in most general surgery procedures. Use of intermittent pneumatic
compression stockings after surgery is recommended as a mechanical
prophylaxis against this potentially fatal complication.
of back pain via nocireceptors and mechanoreceptors in the annulus and
chemical irritants from the NP. The changes ascribed to DDD, however,
are similar to the changes of normal aging. Moreover, a clear
understanding of the pathophysiology and natural history of this pain
complex is lacking. Given the ubiquity of back pain in society at
large, isolating those symptom patterns and imaging findings consistent
with a surgically treatable pain syndrome has been fraught with
failure. A range of painful degenerative lumbar conditions exists.
These entities have been grouped as degenerative disc disease or
subclassified with names such as internal disc disruption, lumbar
segmental instability, and lumbar spondylosis. These distinctions
are largely conjectural and almost nothing is known about their natural histories.
pain. Begin investigation with a thorough history and physical
examination. In the absence of red flags, a long trial of nonoperative
treatment is indicated. Should symptoms continue, a progressive
preoperative evaluation including MRI and psychosocial assessment is
mandatory. Based on these studies, discography may be indicated to
identify concordant pain at abnormal levels seen on MRI.
fulfilled, may a patient be considered for surgery. Lumbar fusion
remains the treatment of choice. In any operative procedure, the known
benefits must outweigh the risks. As the benefits of fusion for DDD
have not been clearly established, a great deal of further study is
required before routine operative intervention can be recommended for
these patients.
justified. However, results of multilevel fusion for painful disc
degeneration are abysmal, and it is best avoided. Multidisciplinary
pain clinics remain a viable alternative.
evolving. Presently, minimally invasive anterior approaches with
threaded cages appear promising, but significant long-term data are
lacking. PLIF techniques are also frequently employed. A transforaminal
posterior interbody fusion may be a sensible approach in certain
patients. As with any major posterior lumbar procedure, however, the
long-term effects of “fusion disease” in an otherwise active patient
population must be considered. With reasonable results reported for
each of the various anterior and posterior procedures, the surgeon
should ultimately choose that technique with which she is most
comfortable. The most important factor in clinical success with this
group of patients remains patient selection.
scheme: * classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
MC, Haynor DR, Fisher LD, et al. Similarities in Degenerative Findings
on Magnetic Resonance Images of the Lumbar Spines of Identical Twins. J Bone Joint Surg [Am] 1995;77:1662.
M, Swatz D, Clothiaux P, et al. Posterolateral Lumbar and Lumbosacral
Fusion With and Without Pedicle Screw Internal Fixation. Clin Orthop 1992;284:109.
SJ. A Literature-based Review as a Guide for Generating Recommendations
to Patients Acutely Limited by Low Back Symptoms. In: American
Association of Orthopaedic Surgeons, eds. Orthopaedic Knowledge Update: Spine. Rosemont, IL: AAOS, 1997:A15.
SD, Davis DO, Dina TS, et al. Abnormal Magnetic Resonance Scans of the
Lumbar Spine in Asymptomatic Subjects. A Prospective Investigation. J Bone Joint Surg [Am] 1990;72:403.
TC, Pile N, Eichelberger RP, Whitman M Jr. Normal Magnetic Resonance
Imaging and Abnormal Discography in Lumbar Disc Disruption. Spine 1994;19:1075.
DF, Herzog RJ, Mink JH, et al. Nomenclature of Lumbar Disc Disorders.
In: In: American Association of Orthopaedic Surgeons, eds. Orthopaedic Knowledge Update: Spine. Rosemont, IL: AAOS, 1997:A3.
JS, MacKay M, Herkowitz HN, et al. Degenerative Lumbar
Spondylolisthesis with Spinal Stenosis. A Prospective, Randomized Study
Comparing Decompressive Laminectomy and Arthrodesis With and Without
Instrumentation. Spine 1997;22:2807.
RD, Bleasel JF, Moskowitz RW. Spinal Degeneration: Pathogenesis and
Medical Management. In: Frymoyer JW, Ducker TB, Hadler NM, et al., eds.
The Adult Spine: Principles and Practice, 2nd ed. New York: Lippincott-Raven, 1997.
A, Crock H, Bedbrook G. The Results of 150 Anterior Lumbar Interbody
Fusion Operations Performed by Two Surgeons in Australia. Clin Orthop 1982;165:164.
J, Jeszensky D, Stoltze D, Bohm H. True Spondylolisthesis Reduction and
Monosegmental Fusion in Spondylolisthesis. In: Bridwell KH, DeWald RL,
eds. Textbook of Spinal Surgery, 2nd ed. Philadelphia: Lippincott-Raven, 1997:1337.
HN, Sidhu KS. Lumbar Spine Fusion in the Treatment of Degenerative
Conditions. Current Indications and Recommendations. J Am Assoc Orthop Surg 1995;3:123.
J, O’Brien J. Simultaneous Combined Anterior and Posterior Fusion. An
Independent Analysis of a Treatment for the Disabled Low Back Pain
Patient. Spine 1990;15:322.
MH, Beynnon BD, Decoster TA, Pope MH. Depth of Insertion of
Transpedicular Vertebral Screws into Human Vertebrae: Effect upon
Screw–Vertebra Interface Strength. J Spinal Disord 1988;1(4):287.
HH, Evans MT, Molligan HJ, Long BH. Laparoscopic Discectomy with
Anterior Lumbar Interbody Fusion. A Preliminary Review. Spine 1995;20:1797.
PC, Regan JR, Zdeblick T, et al. The Incidence of Complications in
Endoscopic Anterior Thoracolumbar Spinal Reconstructive Surgery. A
Prospective Multicenter Study Comprising the First 100 Cases. Spine 1995;20:1624.
RF, Wimpee MW, Hudkins P, et al. The Inflammatory Effect of Nucleus
Pulposus. A Possible Element in the Pathogenesis of Low Back Pain. Spine 1987;12:760.
MT, Steinberg PM, Ross JS, et al. Degenerative Disc Disease. Assessment
of Changes in Vertebral Body Marrow with MR Imaging. Radiology 1988;166:193.
LM, Murrell SE, Boden SC, Horton WC. The Outcome of Posterolateral
Fusion in Highly Selected Patients with Discogenic Low Back Pain. Spine 1996;21:1909.
PB, Kinney RK, Gatchel RJ, et al. Psychiatric Illness and Chronic Low
Back Pain. The Mind and the Spine. Which Goes First? Spine 1993;18:66.
R, Manninen H, Battie MC, et al. Observer Variability in the Assessment
of Disc Degeneration on Magnetic Resonance Images of the Lumbar and
Thoracic Spine. Spine 1995;20:1029.
JJ, Erkintalo M, Laine M, Pentti J. Low Back Pain in the Young. A
Prospective Three-year Follow-up Study of Subjects With and Without Low
Back Pain. Spine 1995;20:2101.
G, Flannigan B, Kingston S, et al. Magnetic resonance Imaging in the
Diagnosis of Disc Degeneration. Correlation with Discography. Spine 1987;12:276.
L, Rocchio P. Preoperative Psychological Tests as Predictors of Success
of Chemonucleolysis in the Treatment of Low Back Pain Syndrome. J Bone Joint Surg [Am] 1975;57:478.
GW, Boyd RJ, Carothers TA, et al. The Effect of Pedicle Screw/Plate
Fixation on Lumbar/Lumbosacral Autogenous Bone Graft Fusions in
Patients with Degenerative Disc Disease. Spine 1995;20:819.