Pathologic Fractures
bone predisposes the patient for failure during normal activity or
after minor trauma. Failure (pathologic fracture) of bone under these
circumstances should alert the orthopaedic surgeon to the presence of
an underlying condition. Successful management of the patient requires
recognition, diagnosis, and treatment of the condition affecting the
bone. The management of the fracture may be dramatically altered by the
associated pathologic condition, and failure to recognize a condition
such as osteoporosis or metastatic bone disease may be detrimental to
the patient’s life or limb.
pathologic fracture and systemic, nonneoplastic skeletal disease, it is
best to separate the underlying problem into correctable and
uncorrectable conditions. Correctable conditions include renal
osteodystrophy, hyperparathyroidism, osteomalacia, and disuse
osteoporosis. Uncorrectable conditions include osteogenesis imperfecta,
polyostotic fibrous dysplasia, postmenopausal osteoporosis, Paget’s
disease, and osteopetrosis. All of these disorders involve bones that
are weak and predisposed to fracture or plastic deformation. The
fracture callus may not form normally, and healing often occurs slowly.
Many of these patients have an increased incidence of fracture, delayed
union, and nonunion.
treatment should be initiated. If the underlying process cannot be
corrected, the condition of the remainder of the skeleton must be
considered when planning treatment of the fracture. In the management
of patients with systemic skeletal disease, it is important to try and
prevent disuse osteoporosis, which may lead to additional pathologic
fractures.
with pathologic fractures, and the management of patients with this
condition may only require minor modifications of typical fracture
care. In contrast, the treatment of patients with metastatic bone
disease who have actual or impending pathologic fractures necessitates
a multidisciplinary approach with different principles applied to
fracture fixation.
treatment of patients with metastatic bone disease and actual or
impending pathologic fractures. It will briefly cover the management of
pathologic fractures in patients with primary benign or malignant bone
tumors. Treatment of patients with metabolic abnormalities and
decreased bone density unrelated to malignancy will be addressed in a
less comprehensive fashion. The majority of patients with pathologic
fractures are treated by general orthopaedic surgeons. It is important
that all orthopaedic surgeons have a basic understanding of the
principles involved in the care of these patients so that appropriate
treatment is initiated.
osteoporosis, while another 34 million have osteomalacia and are at
risk for developing osteoporosis.31
It is a major public health concern for 55% of people who are 50 years
or older. Eighty percent of those affected by osteoporosis are women.
Approximately 2 million people sustain a pathologic fracture related to
osteoporosis each year.31 Of patients over 50 years of age, 24% who sustain a hip fracture die within 1 year.31 One of every two women will have an osteoporosis-related fracture in her lifetime.16
Other skeletal conditions such as Paget disease affect an estimated 1
million people in the United States, while approximately 20,000 to
50,000 Americans have osteogenesis imperfecta.31
new cancer cases will be diagnosed in 2009, and nearly 50% of these
tumors can metastasize to the skeleton.48
With improved medical treatment of many cancers, especially those
originating in the breast and prostate, patients are living longer.
There is an increased prevalence of bone metastasis in this population,
which increases the chances that these patients will develop a
pathologic fracture. The vast majority of bone metastasis originate
from cancers of the breast, lung, and prostate, thyroid, and kidney.83 The most common sites of metastasis in the skeleton include the spine, pelvis, ribs, skull, and proximal long bones.94
A thorough history must be obtained to understand the circumstances
surrounding the current injury. Certain symptoms should alert the
orthopaedic surgeon to the possibility of an associated pathologic
process (Table 20-2). The degree of trauma
required to cause the fracture and presence of pain before the injury
may provide information about the underlying bone strength. Pain is the
most common presenting symptom before fracture, ranging from a dull
constant ache to an intense pain exacerbated by weight bearing.
Patients must be asked specifically about previously diagnosed or
treated cancer; otherwise, they may consider themselves cured and not
volunteer this information. A history of radiation is important.
Standard review of systems questions about recent weight loss, fevers,
night sweats, and fatigue are important. Questions about relevant risk
factors such as smoking, dietary habits, and toxic exposures should be
asked.
|
TABLE 20-1 Comprehensive Evaluation of a Patient with a Lytic Bone Lesion
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
evaluation of the affected skeletal region. Palpation of a mass,
identification of an obvious deformity, and a detailed neurologic
examination of the extremities are essential. All extremities and the
entire spine should be evaluated for additional lesions or
lymphadenopathy, as patients can have multiple sites of involvement
with bone metastasis, lymphoma, multiple myeloma, or osteoporosis. A
physical examination should include careful evaluation of all possible
primary sites (breast, prostate, lung, thyroid) and a stool guiac test.94
overall patient evaluation. A baseline laboratory profile should
include a complete blood count with manual differential, erythrocyte
sedimentation rate, serum chemistries, blood urea nitrogen (BUN), serum
glucose, liver function tests, protein, albumin, calcium, phosphorus,
and alkaline phosphatase. Patients with widespread bone metastasis may
exhibit anemia of chronic disease, hypercalcemia, and increased
alkaline phosphatase. The hemoglobin is also often low in patients with
multiple myeloma. A standard urinalysis is necessary to look for
microscopic hematuria which suggests renal cell carcinoma, and a
24-hour urine collection is necessary for a complete metabolic
evaluation. Serum and urine protein electrophoreses are important to
exclude multiple myeloma. Thyroid function tests, carcinoembryonic
antigen (CEA), CA125, and prostate specific antigen (PSA) are serum
markers for specific tumors. N-telopeptide and C-telopeptide are new
biomechanical markers of bone collagen breakdown that can be measured
in the serum and urine. These markers are used to confirm increased
destruction caused by bone metastasis, measure the overall extent of
bone involvement, and assess the response of the bone to
bisphosphonates.20
|
TABLE 20-2 Factors Suggesting a Pathologic Fracture
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
|
TABLE 20-3 Disorders Producing Osteopenia
|
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||
aforementioned laboratory tests, whereas patients with osteomalacia
have low serum calcium and phosphorus, high serum alkaline phosphatase,
high urinary phosphorus, and high urinary hydroxyproline values (Table 20-3).
Patients with primary hyperparathyroidism have high serum calcium,
alkaline phosphatase, and parathyroid hormone with low serum
phosphorus. They also have high urinary calcium, phosphorus, and
hydroxyproline. Patients with renal osteodystrophy have low serum
calcium with high serum phosphorus, alkaline phosphatase, and BUN. When
secondary hyperparathyroidism develops in these patients, the serum
calcium increases to normal or elevated values with elevated
parathyroid hormone levels. Urine values are difficult to assess in
patients with secondary hyperparathyroidism caused by abnormal
glomerular filtration. Patients with Paget disease have normal values
for serum calcium and phosphorus but markedly elevated levels of
alkaline phosphatase and urinary hydroxyproline. Prostate specific
antigen is a sensitive measurement of prostate cancer. A value less
than 10 ng/mL essentially excludes the presence of bone metastasis.
Remember that serum calcium is a measurement of unbound calcium in the
serum and, therefore, determination of serum protein is necessary to
interpret the calcium level. If the serum protein is lower than normal,
the normal range of serum calcium is lowered.
metastatic bone disease are substantial. Patients often have marked
pain or pathologic fractures that leave them unable to ambulate or
perform their activities of daily living (ADLs). Patients with spinal
fractures may develop neurologic deficits that lead to paralysis.
Patients with impending or actual extremity fractures may be forced to
remain at bedrest for prolonged periods of time, predisposing them to
hypercalcemia. Anemia is a common hematologic abnormality in these
patients. The most encompassing and tragic concern of patients with
pathologic fractures from metastatic disease is the general loss in
their quality of life.
diagnosed in the United States each year are related to hypercalcemia
of malignancy, most commonly associated with cancers of the lung,
breast, kidney, and genitourinary tract.74
The remainder is caused by primary hyperparathyroidism. Rarely, the two
causes occur simultaneously. The orthopaedic surgeon managing a patient
with metastatic carcinoma to bone must be aware of the risks, symptoms,
and management of hypercalcemia as it can be lethal if untreated (Table 20-4).
malignancy, but it portends a poor prognosis for the patient. As many
as 60% of patients with hypercalcemia will survive less than 3 months,
and only 20% will be alive at 1 year. Often the symptoms
are
nonspecific, so it is easiest to diagnose the problem by measuring the
serum calcium. There is not a reliable correlation between the severity
of the hypercalcemia and the degree of metastatic bone disease.
Patients with lung cancer often develop hypercalcemia without obvious
bone metastases, whereas hypercalcemia in multiple myeloma or breast
carcinoma correlates with the extent of bone metastases.74
Diffuse osteoclastic activity associated with clinical hypercalcemia
can be seen histologically without the presence of metastasis in the
bone.
|
TABLE 20-4 Signs and Symptoms of Hypercalcemia
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
often requires inpatient care. Vigorous volume repletion is a
temporizing measure, so treatment must focus on reducing the degree of
bone resorption. This can be accomplished by treating the primary tumor
directly or by using bisphosphonates to reduce osteoclastic activity.64 Correction of any electrolyte imbalance or hypercalcemia should ideally be done before surgery.
study used to evaluate a patient with a destructive bone lesion or
pathologic fracture is a plain radiograph in two planes.94
The radiographs should be carefully reviewed with attention to specific
lesions and overall bone quality. Specifically they should be examined
for diagnostic clues such as generalized osteopenia, periosteal
reaction, cortical thinning, Looser’s lines, and abnormal soft tissue
shadows. A series of questions to assist in determining the underlying
process was popularized by W. Enneking, M.D., and can be reviewed in Table 20-5.
The entire affected bone should be imaged to identify all possible
lesions, and it must be remembered that referred pain to distal sites
may be caused by a more proximal lesion.
radiographic term used to indicate inadequate bone (osteoporosis) or
inadequately mineralized bone (osteomalacia). These two disorders
cannot be definitively distinguished on plain radiographs, but there
are some suggestive differential clues. Looser’s lines
(compression-side radiolucent lines), calcification of small vessels,
and phalangeal periosteal reaction are features of osteomalacia or
hyperparathyroidism. Thin cortices and loss of the normal trabecular
pattern without other abnormalities are more suggestive of osteoporosis.
|
TABLE 20-5 Evaluation of Plain Radiographs
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
otherwise normal bone, the process is most likely neoplastic. It is
important to determine whether the lesion is inactive, active, or
aggressive. Small osteolytic lesions surrounded by a rim of reactive
bone without endosteal or periosteal reaction are usually inactive or
minimally active benign bone tumors. Lesions that erode the cortex but
are contained by periosteum are usually active benign or low-grade
malignant bone tumors. Large lesions that destroy the cortex are
usually aggressive, malignant lesions that can be primary or
metastatic. A permeative or “moth-eaten” pattern of cortical
destruction is highly suggestive of malignancy. Most destructive bone
lesions in patients over 40 years of age are caused by metastatic
carcinoma followed by multiple myeloma and lymphoma; however, a
solitary bone lesion should be fully evaluated to rule out a primary
bone tumor such as a chondrosarcoma, malignant fibrous histiocytoma, or
osteosarcoma.94
osteolytic, osteoblastic, or mixed. Osteolytic destruction is most
common and occurs in metastases from cancers of the lung, thyroid,
kidney, and colon (Fig. 20-1). An osteoblastic
appearance with sclerosis of the bone is common in metastatic prostate
cancer. Metastatic breast cancer often has a mixed osteolytic and
osteoblastic appearance in the bone (Fig. 20-2).
The radiographic appearance is determined by the balance of bone
destruction by osteoclasts and bone production by osteoblasts. Tumor
cells secrete factors that interact with host cells in the bone
microenvironment and affect the cycle of normal bone turnover.21,75,90,93
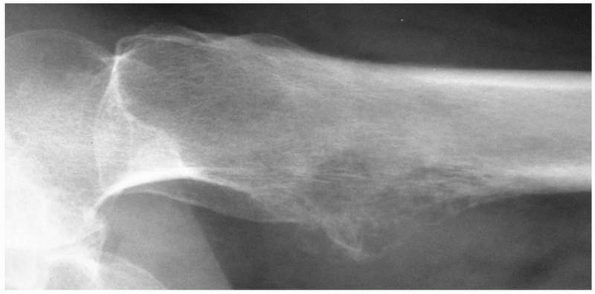
An isolated avulsion of the lesser trochanter is almost always
pathologic, and this specific injury should arouse suspicion of occult
metastatic disease and an imminent femoral neck fracture (Fig. 20-3).8 A cortical lesion in an adult is usually a metastasis, most commonly from lung cancer.34
of disease. Technetium bone scintigraphy is helpful in determining the
extent of metastatic disease to the skeleton, as it detects
osteoblastic activity and is quite sensitive. Multiple myeloma is
falsely negative on a bone scan as are occasional cases of metastatic
renal cell carcinoma because of the decreased osteoblastic response to
the tumor. More recently, positron emission tomography (PET) scanning
has been available but the indications are not clear for staging
patients with metastatic bone disease.70 It has been useful in staging patients with lymphoma and monitoring response to lymphoma treatment.54
In a recent study, PET/ computed tomography (CT) scanning had higher
sensitivity and specificity than PET scanning alone for detection of
malignant bone lesions.26
 |
|
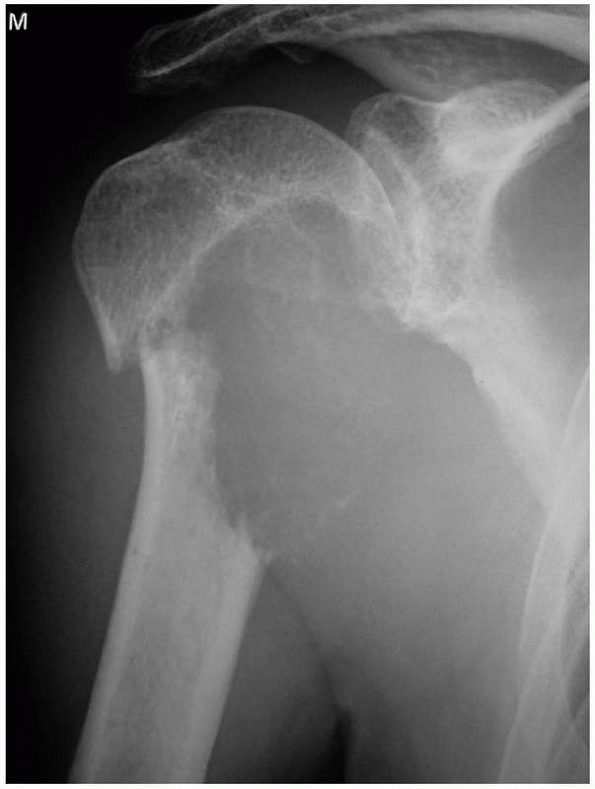
FIGURE 20-1
Anteroposterior radiograph of the right shoulder in a 55-year-old man with metastatic renal cell carcinoma. He had pain for 6 weeks before sustaining a minor injury to the right shoulder. Note the purely lytic lesion with a pathologic fracture through the surgical neck of the humerus. |
 |
|
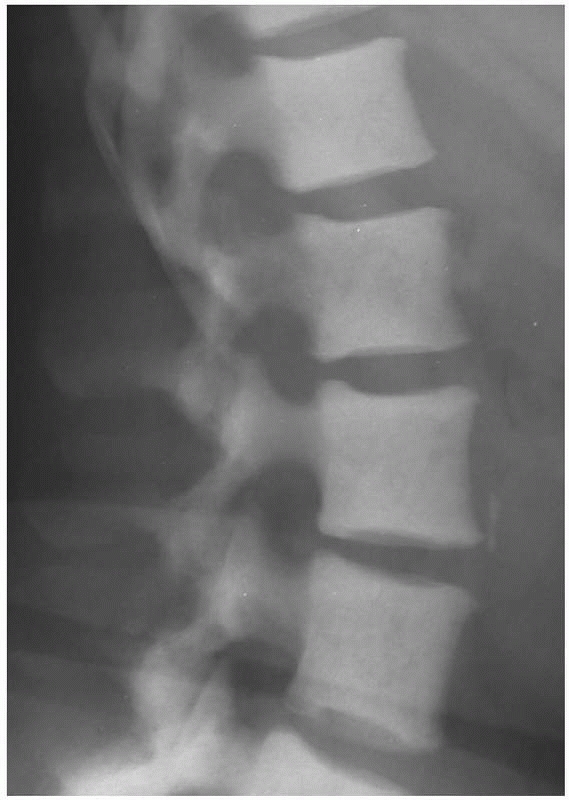
FIGURE 20-2
Lateral radiograph of the thoracic vertebral bodies in a 56-year-old woman with metastatic breast cancer. Note their osteoblastic appearance caused by an imbalance in bone production over bone destruction. |
 |
|
FIGURE 20-3
Lateral radiograph of the left hip reveals an osteolytic lesion in the lesser trochanter. This is a classic worrisome sign that suggests an impending fracture of the femoral neck. |
The recommended radiographic staging study is a CT scan of the chest,
abdomen, and pelvis. A mammogram should also be done if breast cancer
is suspected. If multiple myeloma is considered, a skeletal survey
including skull films is recommended.
to evaluate metastatic lesions in the extremity, but it is useful in
the evaluation of patients with spinal metastasis to define the
relationship of tumor to the underlying neurologic structures. A
standard angiogram is still useful when embolizing feeding tumor
vessels in vascular lesions such as metastatic renal cell carcinoma or
multiple myeloma as definitive treatment or before surgery.
appropriate imaging studies often leads to the correct diagnosis,
particularly in the case of widespread metastatic bone disease.
However, a solitary bone lesion in a patient with or without a history
of cancer should be biopsied to obtain an accurate diagnosis. Presuming
a solitary lesion is a bone metastasis in an older patient may lead you
to perform the wrong operation, thereby potentially compromising the
life and limb of the patient if the lesion is actually a primary
sarcoma of bone.
performed. Either a needle or open incisional biopsy is reasonable
depending on the availability of expert musculoskeletal radiologists
and pathologists.91 A needle biopsy is usually definitive when
differentiating a carcinoma from a sarcoma. Specific
immunohistochemical staining may allow determination of the primary
site of origin of a carcinoma, most commonly from the lung, breast,
thyroid, or prostate. When there is a pathologic fracture through a
lytic lesion, the biopsy can be complicated due to bleeding and early
fracture callus. The fracture should be stabilized initially with
traction or a cast to allow preliminary staging studies to be
completed, which may allow the diagnosis to be made on imaging alone,
or there may be a different lesion more amenable to biopsy.
done, a careful incisional biopsy should be performed using oncologic
principles so as not to preclude subsequent surgical treatment.65
When possible, the tissue should be obtained from a site near but
unaffected by the fracture. The biopsy should be as small as possible,
in a longitudinal fashion in line with the extremity, and with
excellent hemostasis. Tissues contaminated by a postbiopsy hematoma
must be considered contaminated by tumor cells. Cultures should always
be sent at the time of biopsy to rule out infection, which can be
confused radiographically with a tumor. If a definitive diagnosis of
metastatic disease can be made on an intraoperative frozen section,
surgical treatment of the pathologic fracture can be performed at the
same operative setting. If the frozen section is not diagnostic, it is
best to wait for the permanent sections before definitively treating
the tumor and fracture.
fracture. Treatment options for known skeletal metastasis include (a)
prophylactic surgical stabilization before radiation therapy or (b)
radiation and/or chemotherapy without prophylactic fixation.47,94 The term impending fracture
is used throughout the literature on metastatic disease, but there are
no clear guidelines supported by prospective clinical studies to define
this term. Retrospective studies have formed the basis to guide the
indications for prophylactic fixation, but they are often limited by
the use of plain radiographs, subjective patient information, and an
inadequate understanding of the biomechanical factors involved in the
bone affected by a neoplastic process.28,68,82
Although experienced orthopaedic oncologists may have an intuitive
sense for which lesions are at high risk for fracture, there is
considerable controversy about what constitutes an impending fracture
and little reliable data to guide treatment.
|
TABLE 20-6 Mirels Criteria for Risk of Fracture
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
include the radiographic appearance of the lesion and the patient’s
symptoms. Fidler28 assessed
preoperative and postoperative pain in patients with impending
fractures and found that, among patients with 50% to 75% cortical
involvement, all had moderate to severe pain preoperatively and none or
minimal pain after prophylactic internal fixation. Commonly, a lesion
is considered to be at risk for fracture if it is painful and larger
than 2.5 cm and involves over 50% of the cortex.82 In an attempt to quantify this risk, Mirels67
developed a scoring system based on the presence or absence of pain and
the size, location, and radiographic appearance of the lesion. Each of
the four variables is assigned from 1 to 3 points (Table 20-6).
Mirels analyzed 78 lesions previously irradiated without prophylactic
surgical fixation. Over a 6-month period, 27 lesions (35 %) fractured
and 51 remained stable. A mean score of 7 in the nonfracture group and
10 in the fracture group was calculated. The author concluded that
lesions scoring 7 or lower can be safely irradiated, while lesions
scoring 8 or higher require prophylactic internal fixation before
radiation.67
the risk of pathologic fracture in patients with metastatic bone
disease. Fracture risk is defined as the
load-bearing requirement of the bone divided by its load-bearing
capacity. The load-bearing requirement depends on the patient’s age,
weight, activity level, and ability to protect the site. The
load-bearing capacity depends on the amount of bone loss, modulus of
the remaining bone, and location of the defect with respect to the type
of load applied.68 A biomechanical
study of simulated lytic defects in whale vertebral bodies demonstrated
that relative fracture risk in vivo could be predicted by a structural
rigidity analysis using cross-sectional imaging data.44
Although this system provides a comprehensive method to determine the
risk of pathologic fracture, it is not yet routinely used in the
clinical setting.
impending fracture versus those treated after an actual fracture have
the following outcomes: shorter hospitalization (average 2 days),
discharge
to home more likely (40%), more immediate pain relief, faster and less
complicated surgery, less blood loss, quicker return to premorbid
function, improved survival, and fewer hardware complications.12,51
Elective stabilization also allows the medical oncologist and surgeon
to coordinate operative treatment and systemic chemotherapy. One
critical caveat when treating patients with impending pathologic
fractures is that fracture risk is greatest during the surgical
positioning, preparation, and draping. When patients are anesthetized,
they cannot protect the affected extremity and must rely on the
surgical team to proceed carefully. Low energy fractures will occur
after very minor trauma or a twisting movement. If a pathologic
fracture occurs, damage to the surrounding soft tissues is minimal
compared to traumatic fractures in healthy bone.
impending pathologic fracture are to alleviate pain, reduce narcotic
utilization, restore skeletal stability, and regain functional
independence.47,94
However, the decision to proceed with operative intervention is
multifactorial and must be individualized. Factors included in the
decision making are (a) life expectancy of the patient, (b) patient
comorbidities, (c) extent of the disease, (d) tumor histology, (e)
anticipated future oncologic treatments, and (f) degree of pain.
Patients with a life expectancy of less than 6 weeks may not gain
significant benefit from major reconstructive surgery. However, an
accurate prognosis is not always possible, and the decision of whether
to proceed with surgery should be discussed with the multidisciplinary
team, the patient, and the patient’s family.
pathologic fracture is caused by osteoporosis. In most situations,
these fractures should be managed in a standard fashion as recommended
in the accompanying chapters of this text. Modifications such as the
addition of methylmethacrylate or locking plate fixation may be
necessary because of the weakened bone.5
Pathologic fractures caused by metastatic bone disease demand special
considerations, which will be discussed in further detail.
are living with bone metastasis. Because of the advances in systemic
treatment, pain control, and local modalities including radiation and
surgery, the philosophy has changed from one of palliation for
immediate demise to aggressive care to improve the quality of remaining
life. The local bone lesion can be treated with nonsurgical management
(radiation, functional bracing, and bisphosphonates) or surgical
stabilization with or without resection. Medical treatment with
bisphosphonates has decreased the incidence of pathologic fractures
because of inhibition of osteoclast-mediated bone destruction.40,61,62
Patients with small bone lesions, especially in non-weight-bearing
bones, are often candidates for radiation therapy rather than surgical
stabilization. Surgical intervention is usually employed for large
lytic lesions at risk for fracture or for existing pathologic
fractures. Postoperatively, external beam radiation is used as an
adjuvant local treatment for the entire operative field and implant
unless the metastatic lesion is completely resected.88,94
often medically debilitated and require multidisciplinary care. In
addition to an orthopaedic surgeon, the comprehensive team includes
medical oncologists, radiation oncologists, endocrinologists,
radiologists, pathologists, pain specialists, nutritionists, physical
therapists, and psychologist/psychiatrists. Nutrition is of particular
concern; serum prealbumin should be measured and improved if it is low.
This may require the addition of enteral or parenteral
hyperalimentation perioperatively. Patients may have relative bone
marrow suppression and will require adequate replacement of blood
products. Perioperative antibiotic coverage, prophylaxis for embolic
events, aggressive postoperative pulmonary toilet, and early
mobilization are all instituted as standard treatment.
indicated if the patient is not a surgical candidate. Nonsurgical
candidates are those with limited life expectancies, severe
comorbidities, small lesions, or radiosensitive tumors.94
The use of a fracture brace works well for lesions in the upper
extremity. Patients should limit weight bearing on the affected
extremity. A braced lesion may heal with or without radiation therapy.
Lesions most amenable to bracing are those in the humeral diaphysis,
forearm, and occasionally the tibia. Patients with proximal humeral
lesions can be treated with a sling, and those with distal humeral
lesions can be immobilized in a posterior elbow splint with or without
a hinge. If a patient has multiple lesions requiring the use of all
extremities to ambulate, surgical stabilization will provide better
support than a brace.
or may not heal. The factors that influence whether healing will occur
include location of the lesion, extent of bony destruction, tumor
histology, type of treatment, and length of patient survival. Gainor et
al.30 determined the most important
factor affecting union was length of patient survival. Of 129
pathologic long bone fractures, the overall rate of fracture healing
was 34%; however, it was 74% in the group of patients who survived
greater than 6 months. Among different tumor histologies, fractures
secondary to multiple myeloma were most likely to heal.30
most current internal fixation devices and prosthetic replacements. The
ideal reconstruction allows immediate weight bearing and is durable
enough to last for the increased total life span of patients with
metastatic bone disease.47,94
It should be assumed that the fixation device used will be
load-bearing, as only 30% to 40% of pathologic fractures unite even
after radiation treatment.11,30
likelihood of tumor progression, standard internal fixation may be
contraindicated. An intramedullary device or modular prosthesis
provides more definitive stability. Polymethylmethacrylate (PMMA) is
often used to increase the strength of the fixation, but it should not
be used alone to replace a segment of bone. PMMA improves the bending
strength of a fixation construct and the outcome of fixation in both
animal and human studies.39,81 It does not affect the use of therapeutic radiation, nor are the properties of the PMMA affected adversely by the radiation.25 Autogenous bone graft is not generally used in the treatment
of extremity fractures from metastatic bone disease. Segmental
allografts are also rarely indicated, as they require a prolonged
healing time.
complication or failure should be used for patients with metastatic
bone disease. In the vast majority of cases, this requires metal and
PMMA. When a prosthesis is used to replace a joint affected by a
metastatic lesion or a pathologic fracture, it should be cemented into
the host bone. The goal is to have the patient allowed to bear weight
as tolerated after the surgical procedure. Another guideline when
treating patients with metastatic disease is to prophylactically
stabilize as much of the affected bone as possible. When an
intramedullary device is indicated, the entire femur, humerus or tibia
should be treated with a statically locked nail.96,101
For femoral lesions, a reconstruction nail is used to stabilize the
femoral neck even if no lesion is present there at the time of surgery.
Patients with metastatic disease often develop subsequent lesions and
the reconstruction nail is helpful in preventing a future pathologic
femoral neck fracture.
and radiation therapy when they spread to the skeleton. Renal cell
carcinoma (RCC) is a notable example. Surgical treatment is often
indicated for even small RCC lesions, as they tend to progress despite
standard medical treatment and external beam radiation.49,84
Depending on the patient’s expected lifespan and location of the
lesion, open treatment with thorough curettage of metastatic RCC
followed by intramedullary fixation and PMMA will decrease the tumor
burden.60 Postoperative radiation is often used to prevent growth of the residual microscopic disease.88
When complete resection and joint replacement is performed for
metastatic disease, the chances of progressive bone destruction from
recurrent tumor are decreased.49,84
life-threatening intraoperative hemorrhage without adequate
precautions. Metastatic RCC is the most likely lesion to cause
excessive blood loss, but metastatic thyroid cancer and multiple
myeloma are also hypervascular. When possible, a tourniquet should be
used during surgery. However, most metastasis occur in the proximal
extremities, precluding use of a tourniquet. Excessive blood loss can
often be avoided if preoperative embolization is performed by an
interventional radiologist within 36 hours of the surgical procedure.14
Patients with metastatic RCC often have only one functioning kidney, so
a careful evaluation of their renal status should be performed before
injecting nephrotoxic dye for angiography.
extremity with approximately 50% occurring in the humerus. Upper
extremity metastases can result in substantial functional impairment by
hindering personal hygiene, independent ambulation, meal management,
ability to use external aids, and general ADLs.94
When making decisions about treatment of upper extremity metastasis,
the benefits to quality of life should outweigh the risks of potential
surgery. Contractures of the shoulder and elbow are common with or
without surgical treatment, and these joints should be kept moving.
Gentle pendulum exercises can maintain motion in the shoulder and, with
appropriate precautions against using torsion, are safe for most
proximal and mid-humeral impending fractures. Gravity-assisted elbow
flexion and extension exercises can also be performed safely by most
patients.
scapula are generally treated nonoperatively with shoulder
immobilization, radiation, and/or medical management. Occasionally a
large, destructive metastasis will occur in the inferior body or
articular portion (glenoid) of the scapula. As pain dictates, these
areas of the scapula can be resected.
humeral head or neck are treated with a proximal humeral replacement or
intramedullary fixation. If enough bone is available in the proximal
humerus, an intramedullary locked device with multiple proximal screws
is acceptable and maintains shoulder range of motion.101
PMMA may be required to supplement the fixation. When there is
extensive destruction of the proximal humerus or a fracture leaving
minimal bone for adequate fixation, resection of the lesion and
reconstruction with a cemented proximal humeral endoprosthesis are
indicated.52 This modular construct
replaces a variable amount of proximal humerus and has a long cemented
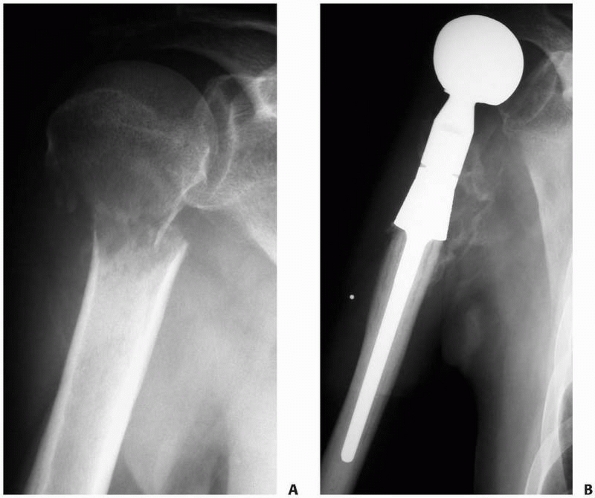
stem to protect the remainder of the bone (Fig. 20-4).
In the face of distal disease progression, it can be modified to a
total humeral prosthesis. Involvement of the glenoid is rare, so
replacement of this articular surface is generally not necessary. The
goal of a proximal humeral replacement is pain relief and local control
of the tumor; shoulder range of motion and stability are often
compromised because of poor soft tissue attachments to the metal
construct. A synthetic vascular graft or mesh sutured to the glenoid
labrum and around the prosthetic humeral head can offer some stability.
Postoperative radiation therapy is used for patients when intralesional
treatment is performed.
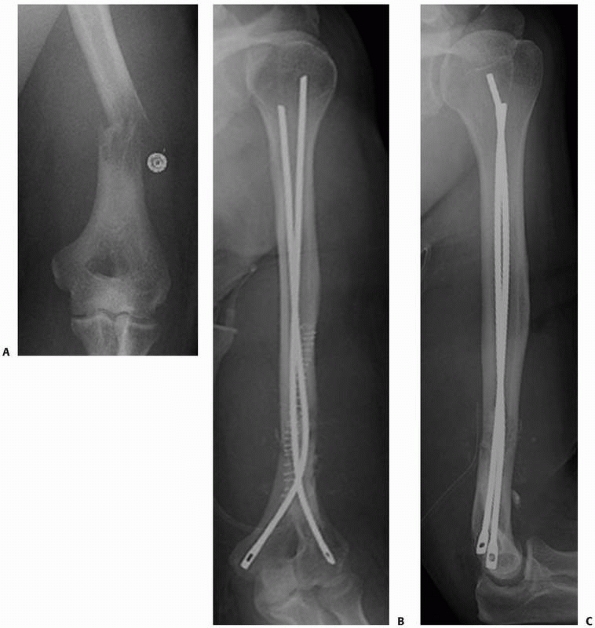
fractures can be surgically treated with locked intramedullary fixation
or an intercalary metal spacer.17,18,101 Locked intramedullary humeral nails span the entire humerus and provide mechanical and rotational stability (Fig. 20-5).
As previously mentioned, PMMA improves implant stability and
supplements poor bone quality when used with surgical stabilization.39 Intercalary spacers offer a modular reconstructive option after resection of large diaphyseal lesions.18
They are used in segmental defects and cases of failed fixation caused
by progressive disease. Intercalary spacers can be used for
reconstruction after complete resection of a metastatic lesion in the
humeral diaphysis, minimizing blood loss in hypervascular lesions and
often alleviating the need for postoperative radiation. Damron et al.
reported that intercalary spacers provide immediate stable fixation,
excellent pain relief, and early return of function.18,42
Plate fixation produces good to excellent functional results in
nonpathologic humeral fractures; however, drawbacks for their use in
metastatic disease include the need for extensive exposure of the
humerus and the inability to protect the entire bone. With disease
progression, there is risk of hardware failure when plate fixation is
used.
treated with flexible intramedullary nails, bicondylar plate fixation,
or resection with modular distal humeral reconstruction. Flexible
nails,
inserted in a retrograde manner through small medial and lateral
incisions, offer ease of insertion, the ability to span the entire
humerus, excellent functional recovery, and preservation of the native
elbow joint. Curettage of the distal humeral lesion allows an open
reduction in the case of a fracture and the opportunity to use PMMA in
the lesion to gain rotational stability (Fig. 20-6).
Orthogonal plate fixation is similar to nonpathologic fracture care
but, when combined with PMMA, it can provide a stable construct about
the elbow. This method of fixation does not protect the proximal
humerus against a future metastatic lesion or fracture. A distal
humeral resection and modular endoprosthetic reconstruction of the
elbow is the best option for massive bone loss involving the condyles.95
 |
|
FIGURE 20-4 A.
Anteroposterior radiograph of the right proximal humerus in a 54-year-old man with multiple myeloma. He has a displaced fracture through the humeral neck with a large lytic lesion filling the proximal humerus. B. Postoperative radiograph after resection of the proximal humerus and modular prosthetic replacement. The stem is cemented into the native humerus. Excellent pain control was achieved with this reconstruction. |
 |
|
FIGURE 20-5 A.
Anteroposterior radiograph of the left humerus in a 58-year-old man with multiple myeloma. This minimally displaced fracture was the presenting feature of his disease. B. Postoperative radiograph 6 months after closed intramedullary humeral nail placement. With systemic chemotherapy and external beam radiation to the left humerus, the fracture and lesion have healed. |
Metastatic lesions to the radius and ulna can be treated with flexible
rods or rigid plate fixation. Pathologic fractures of the radial head
can be treated with resection. Intralesional surgery is preferred for
hand metastasis with curettage, internal fixation, and cementation. If
the lesion is distal or extensive, amputation may be the best option.
pelvis do not affect weight-bearing functions; consequently, they do
not require surgical intervention. Lesions of the iliac wing,
superior/inferior pubic rami, or sacroiliac region fit into this
category. Insufficiency fractures caused by osteoporosis frequently
occur in these locations and are managed with protected weightbearing
until the pain diminishes followed by assessment of bone density and
appropriate medical treatment.10,69
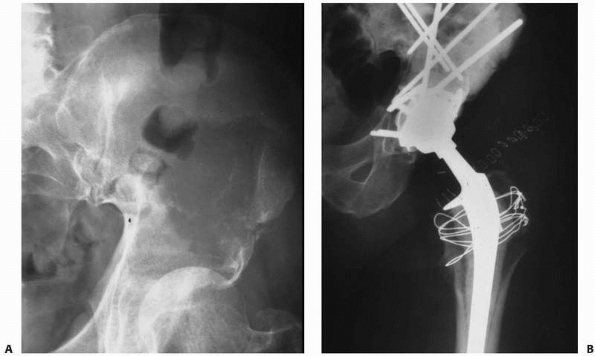
femoral head through a pathologic acetabular fracture (Fig. 20-7).
All pathologic fractures or defects in this location should be assessed
with CT scans with three-dimensional reconstruction. There are several
classification systems for acetabular defects, but various
modifications of the Harrington classification are used for assessing
metastatic disease. This system classifies the location and extent of
the defect and guides the technical considerations of fixation.38
The modification often used describes Class I lesions as minor
acetabular defects with maintenance of the lateral cortices, superior
and medial walls. A conventional cemented acetabular component provides
sufficient support. Class II lesions are major acetabular defects with
a deficient medial wall and superior dome. An antiprotrusion device
and/or medial mesh is necessary (Fig. 20-7).
Class III lesions are massive defects with deficient lateral cortices
and superior dome. There is no substantial peripheral rim for fixation
of a metal component; therefore, weight-bearing stresses must be
transmitted from the acetabular component into bone unaffected by the
tumor, usually near the sacroiliac joint. An acetabular cage should be
used with long screw fixation into any remaining pubis, ischium, and
ilium. The massive bony defect is filled with PMMA to provide immediate
stability after long screws and threaded 5/16-inch Steinman pins anchor
the construct. A polyethylene cup is then cemented into the acetabular
cage in the correct orientation (Fig. 20-8).
Class IV lesions involve pelvic discontinuity and can be treated
expediently with resection and reconstruction using a saddle prosthesis
or a resection arthroplasty depending on patient factors and expected
life span.1 With these techniques,
satisfactory pain relief and function can be achieved in 70% to 75% of
patients. Complications are common and occur in 20% to 30% of cases.1,2,38,53,66,85
Extensive blood loss can be anticipated with massive lytic defects.
This demanding surgery is best done by surgeons with extensive
experience treating this type of lesion. The trabecular metal tantalum
provides new options for acetabular fixation by allowing early bone
ingrowth. It can be used in combination with a cemented acetabular cage.76
 |
|
FIGURE 20-6 A.
Anteroposterior radiograph of the left distal humerus in a 53-year-old-man with metastatic thyroid carcinoma. The osteolytic lesion was considered too distal for stabilization with an anterograde intramedullary nail. Internal fixation with dual plates is an option but would leave the remainder of the proximal humerus unprotected if future lesions occur. Anteroposterior (B) and lateral (C) postoperative radiographs demonstrate the reconstruction with flexible pins extending the length of the humerus. Curettage of the lesion was performed via a posterior approach, the humerus was intentionally shortened slightly for increased cortical contact, and methylmethacrylate was used to fill the defect after the fixation was placed. |
 |
|
FIGURE 20-7 A.
Anteroposterior radiograph of the left hip in a 61-year-old woman with metastatic breast cancer. Note the femoral head protrusion into the pelvis through a pathologic acetabular fracture. It is important to try and identify metastatic lesions before they fracture so that prophylactic fixation can be performed. Note the extensive bone loss in the superior dome and medial wall. This would be categorized as a Class II lesion. B. Postoperative radiograph after acetabular reconstruction with an antiprotrusion cage, multiple screws, and PMMA. |
 |
|
FIGURE 20-8 A.
Anteroposterior radiograph of the left acetabulum in a 49-year-old woman with metastatic renal cell carcinoma and a Class III acetabular lesion. Note the massive bone destruction of the periacetabular region, precluding peripheral rim fixation. Arterial embolization of the tumor to decrease intraoperative bleeding is recommended. B. Postoperative radiograph after reconstruction using a cemented acetabular cage with long Steinman pins transmitting stress into the sacroiliac region. |
The proximal third is involved in 50% of cases, with the
intertrochanteric region accounting for 20% of cases. Metastatic
disease to the femur is the most painful of the bone metastasis, likely
because of the high weight-bearing stresses through the proximal
region. Pathologic fractures of the femur suddenly alter the quality of
a patient’s life and threaten an individual’s level of independence.
Without proper surgical attention, the patient with a pathologic
fracture of the femur will be confined to bed, a situation that is
medically and psychologically devastating.
be prophylactically stabilized whenever possible because of the high
incidence of subsequent fracture and the comparative ease of the
operation. The development of bone metastasis is a continuous process,
so it is important to stabilize as much of the femur as possible to
avoid future peri-implant failure.96
At a minimum, it is recommended that the tip of the chosen fixation
device should bypass a given lesion by at least twice the diameter of
the femur.
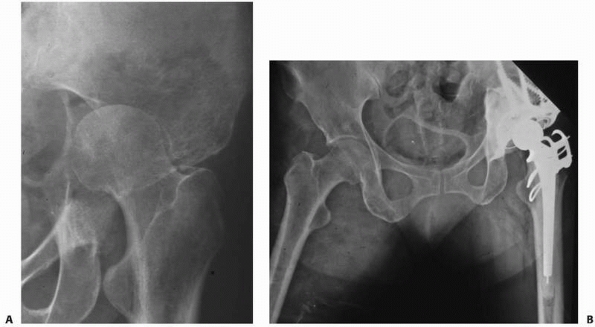
Accordingly, there is a high incidence of failure if traditional
fracture fixation devices are used. The procedure of choice for
patients with metastatic disease to the femoral head or neck is a
cemented replacement prosthesis56,71 (Fig. 20-9).
The decision to use a hemiarthroplasty versus a total hip replacement
depends on the presence of acetabular involvement. This must be
carefully scrutinized as acetabular disease can go unrecognized. All
tumor tissue should be curetted from the femoral canal before
implanting the prosthesis. When there are adjacent lesions in the
subtrochanteric region or proximal diaphysis, a long-stemmed cemented
femoral component should be used for prophylactic fixation distally,
avoiding a future pathologic fracture through a distal lesion and
allowing full weight bearing postoperatively. When there are no
additional lesions in the femur, the length of the cemented femoral
stem is controversial. The risk of cardiopulmonary complications from
monomer embolization after pressurizing the extra cement and long stem
within the canal must be weighed against the potential risk of future
metastasis distal to the tip of the prosthesis if a shorter stem is
used.94 If long-stemmed femoral
components are used, it is important to inject the cement into the
canal while still in a fairly liquid state.6,15
 |
|
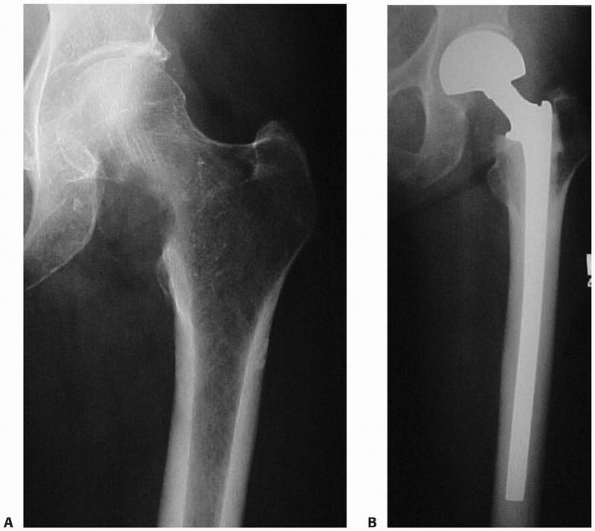
FIGURE 20-9 A.
Anteroposterior radiograph of the left hip in a 52-year-old woman with metastatic lung carcinoma. There is an impending fracture caused by an osteolytic lesion in the medial aspect of the femoral neck. B. Postoperative radiograph after placement of a cemented medium-length, calcar-replacing, bipolar hemiarthroplasty. |
intertrochanteric fracture with screw and side-plate fixation has a
high rate of failure when used in the setting of metastatic bone
disease, even when supplemented with adjuvant PMMA and postoperative
radiation. The standard of care is intramedullary fixation or
prosthetic replacement.96 The choice
of fixation in this region of the femur depends on the extent of the
lesion and whether it is radiosensitive. If bone with sufficient
strength remains in the femoral head and neck and local control is
likely to be achieved with postoperative external beam radiation, an
intramedullary reconstruction device is recommended, which will allow
the highest level of function. A cephalomedullary nail protects the
femoral neck and is used for all metastatic lesions or pathologic
fractures of the femur when an intramedullary device is indicated. If
the destruction is more extensive, a cemented calcar-replacing
prosthesis is required (Fig. 20-9). The same issues arise related to the length of the femoral stem as discussed in the previous section.
fixation for subtrochanteric fractures in patients with metastatic bone
disease will usually end in failure. This region of the femur is
subjected to forces of up to four to six times body weight. Statically
locked intramedullary fixation with or without PMMA will stabilize the
area and provide weight-bearing support.97
Even impending fractures should be statically locked as the lesion can
fracture later causing shortening of the femur. A modular proximal
femoral prosthesis is reserved for cases with extensive bone
destruction or used as a salvage device for failed internal fixation (Fig. 20-10).71
It can also be used when a wide resection is necessary for a pathologic
fracture through a primary bone sarcoma. There is an increased risk of
dislocation and abductor mechanism weakness with a megaprosthesis, but
this should not prevent its use in patients with radioresistant or
locally aggressive tumors. A bipolar head is used to provide more
stability if the acetabulum is not involved with metastatic disease.
Excellent pain relief and local tumor control can be obtained after
tumor resection and prosthetic reconstruction.
diaphysis are treated most effectively with a statically locked
cephalomedullary nail, with or without PMMA96,101 (Fig. 20-11).
Plate fixation, although more rigid, will not protect a large enough
segment of bone and is prone to failure with disease progression.
Cephalomedullary nail fixation protects the entire bone and is
technically simple, especially when performed prophylactically. A
trochanteric or piriformis entry point can be used, and the canal is
slowly overreamed 1.0 to 1.5 mm to avoid high impaction forces during
rod placement.6 Because the device
will be load bearing if the fracture does not unite, a nail with the
largest possible diameter should be used. The fields for postoperative
radiation should encompass the entire implant.
 |
|
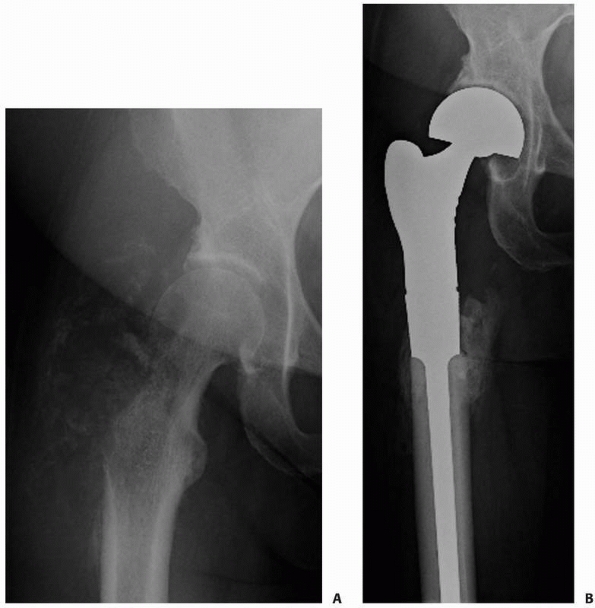
FIGURE 20-10 A.
Anteroposterior radiograph of the right proximal femur in a 52-year-old woman with metastatic adrenocortical carcinoma affecting the right greater trochanter and femoral neck. There is a visible lateral soft tissue mass. B. Postoperative radiograph after resection of the proximal femur and reconstruction with a cemented, modular proximal femoral replacement and bipolar cup. |
pathologic supracondylar femur fractures depends on the extent of local
bone destruction and the presence of additional lesions in the proximal
femur. The distal lesions can be a treatment challenge caused by
frequent comminution and poor bone stock, especially in older patients.
Options include lateral locking plate fixation supplemented with PMMA
or a modular distal femoral prosthesis.91
A retrograde nail has the drawback of potentially seeding the knee
joint with tumor while failing to provide fixation to the femoral neck
region. The locking plate provides stable fixation after curettage and
cementation of the metastatic lesion. The modular endoprosthesis is the
optimal choice for local control when there is massive destruction of
the femoral condyles, as it allows the lesion to be resected en bloc.24
for proximal tibial lesions, similar principles should be used as for
lesions in the supracondylar femoral area. A locking plate with PMMA
after thorough curettage of the lesion is generally sufficient.
Extensive lesions may require a modular proximal tibial prosthesis.
Tibial diaphyseal lesions and fractures should be managed with a locked
intramedullary device. Various techniques can be employed for
pathologic fractures of the distal
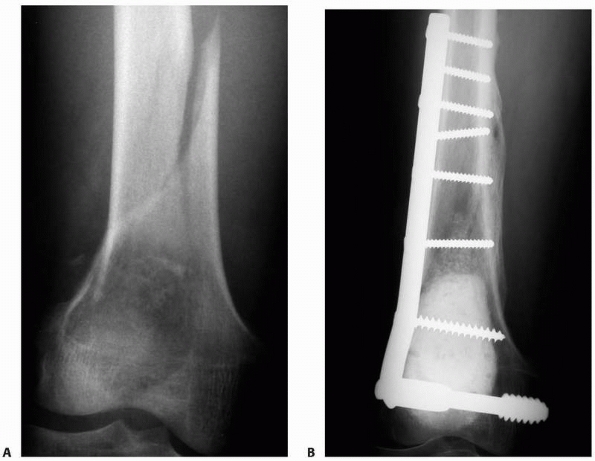
tibia, but standard internal fixation methods are generally advised with generous use of PMMA to augment the construct.19,57 The treatment of foot and ankle lesions must be individualized to maintain maximal function.41
 |
|
FIGURE 20-11 A.
Anteroposterior radiograph of the left femur in a 65-year-old man with metastatic renal cell carcinoma. He has a pathologic fracture through a small, osteolytic lesion in a high-stress area. B. Postoperative radiograph after stabilization of the fracture with a statically locked cephalomedullary femoral nail. Preoperative embolization was performed to minimize blood loss. Open curettage of the lesion was not performed because of its small size and the limited (less than 3 months) life span of the patient. |
carcinoma will have microscopic disease in their spine. The metastases
most commonly involve the vertebral body rather than the posterior
elements. The majority of these patients will not have clinically
significant spine disease during their lifetime and will not need
treatment specific to this location. The lesions are often discovered
incidentally on a bone scan during a routine metastatic workup in a
patient with known cancer. However, if the disease progresses, it can
cause moderate to severe pain persisting for months before the onset of
focal neurologic deficits. Occasionally, the onset of pain is sudden
following a pathologic compression fracture.
must be decided whether a compression fracture is secondary to
osteoporosis or a bone metastasis. If the patient has a history of
cancer or if the patient’s current symptoms, physical examination,
laboratory studies, or imaging suggests a primary carcinoma or myeloma,
the patient should be evaluated for a compression fracture caused by
metastatic disease. It is imperative to consider spinal metastasis in
any cancer patient with back pain. A delay in diagnosis can allow
progression and possible neurologic compromise, leading to permanent
functional deficits. Patients with a suspected malignancy should have a
biopsy, but others should be treated symptomatically. If a patient
treated for an osteoporotic compression fracture does not respond to
the treatment or if there is progressive destruction of bone, a biopsy
should be performed. Percutaneous CT-guided needle biopsy of vertebral
lesions can be performed with local anesthesia and intravenous sedation.
involvement of the spine is loss of a pedicle on an anteroposterior
view. MRI can be used to differentiate an osteoporotic compression
fracture from one caused by a malignant lesion.102
When there is complete replacement of the vertebral segment, multiple
vertebral body lesions, pedicle involvement, and an intact
intervertebral disk, metastatic disease is most likely (Fig. 20-12).
Some patients with myeloma, lymphoma, or leukemia may present with
osteopenia of the vertebra. To determine if the patient has a
hematologic malignancy, a bone marrow aspirate should be considered.
Most of these patients will have systemic findings (e.g., weight loss,
fatigue, fever). If a metastatic lesion in the spine is identified, the
patient is at risk of having additional skeletal lesions.
 |
|
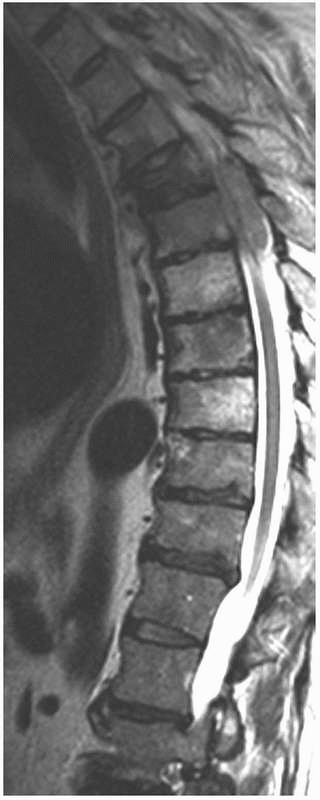
FIGURE 20-12
This sagittal T2-weighted MR image without fat suppression in a 57-year-old man with multiple myeloma shows a thoracic vertebral compression fracture with marrow replacement in multiple vertebral bodies and an associated large epidural lesion. |
metastatic disease to the spine include nonoperative management with
radiation, corticosteroids, and/or bracing; minimally invasive
techniques such as kyphoplasty and vertebroplasty; and surgical
treatment with adjuvant radiation.3,22,27,35,37,63,80
Scoring systems for the evaluation of patients with vertebral
metastasis have been reported, but no system has been universally
adopted to guide treatment.58,86
Quality of life must be considered as these are painful lesions, but
surgical treatment is often a major undertaking that may require a
prolonged recovery.45
fracture caused by osteoporosis are minor and can be successfully
controlled with temporarily decreased activity or bracing. If the
patient has asymptomatic vertebral metastases that are not at risk for
pathologic fracture, systemic treatment can be used to address the
primary and metastatic disease. Regular imaging of the spine should be
performed to ensure that any disease progression is identified quickly.
Often, early recognition of a spinal metastasis allows pain relief with
nonoperative management. If the patient has pain but no neurologic
compromise or risk of impending fracture, radiation treatment is
indicated. Radiation is also used for radiation-sensitive tumors such
as lymphoma or myeloma even when they present with neurologic
compromise. The tumor response is usually sufficiently rapid such that
the risk of permanent neurologic loss is no higher than that seen after
surgical decompression. When there is minimal or no bone destruction
but cord compression is caused by tumor extension, emergent radiation
is recommended.73 The patient should
also be treated with a short course of high-dose corticosteroids to
reduce edema surrounding the tumor that contributes to compression and
neurologic damage. Other indications for radiation of vertebral
metastasis include patients with medical comorbidities precluding
surgery, patients with 6 weeks or less to live, and those with
multilevel disease. Radiation should be added preoperatively or
postoperatively to improve local disease control when patients are
treated with surgery.88 More
recently, cyberknife radiosurgery has provided effective pain relief in
patients with spinal metastases. It can be used in patients who have
had prior external beam radiation as it focuses small beams of
radiation into the tumor from many different directions via a robotic
arm. This minimizes radiation exposure to the surrounding tissues.
Cyberknife is a computer-assisted, minimally invasive procedure that
can be performed as an outpatient in only one to three sessions and
serves as another alternative to major surgery.32
metastasis include progression of disease after radiation, neurologic
compromise caused by bony impingement or radioresistant tumor within
the spinal canal, an impending fracture, or spinal instability caused
by a pathologic fracture or progressive deformity. The goals of surgery
are to maintain or restore neurologic function and spinal stability.
compression of the spinal cord, decompression and stabilization are
required. Before surgery, MRI is used to verify the level of the lesion
and rule out the possibility of compression at additional levels. A
preoperative angiogram with embolization of feeder vessels should be
considered in patients with highly vascular metastasis, such as RCC, to
reduce intraoperative blood loss.14 Relief of symptoms can often be accomplished via a posterior decompression and fusion using instrumentation.3
When there is anterior collapse of the vertebrae and anterior
compression of the spinal cord resulting in kyphosis, the patient is
also treated with an anterior decompression and stabilization.27,37,50,63
When the posterior elements are involved with tumor and the cord is
compressed anteriorly, the patient should have an anterior
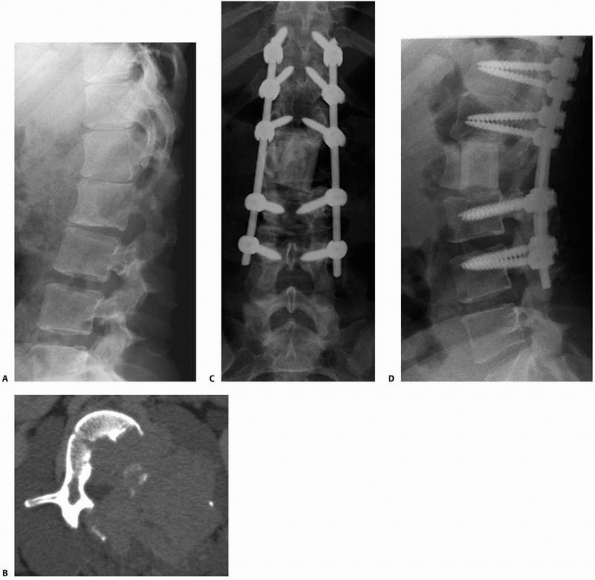
decompression with posterior stabilization and fusion (Fig. 20-13).86
Internal fixation is indicated to provide immediate stability for all
but the most limited decompressions. In recent years, there has been
considerable improvement in the available implants to manage structural
deficiency of the spine, including pedicle screws, cages, and more
sophisticated plates and rods. Specific techniques for anterior and
posterior decompression and stabilization, including the use of modern
instrumentation systems, are described in the literature.27,86
Surgical implants made of titanium allow easier assessment of recurrent
disease on MRI. As patients live longer with their metastatic disease,
aggressive surgical treatment of spinal lesions can enhance quality of
life. However, the magnitude of the operative procedure should not
exceed the patient’s chance of surviving the surgery or the surgeon’s
level of competence.
treatments for metastatic disease to the spine have been used to
control pain in patients who have developed compression fractures.22,80 Vertebroplasty
or kyphoplasty can be used for pathologic vertebral body fractures
caused by osteoporosis, metastatic carcinoma, or multiple myeloma. The
literature suggests that the results are similar in patients with
malignancy versus osteoporosis, although these procedures have not been
directly compared. Indications include patients with stable compression
fractures who have normal neurologic function but persistent pain. One
technique, vertebroplasty, involves percutaneous direct injection of
PMMA through the pedicle to maintain vertebral height. Kyphoplasty is a
way of regaining vertebral body height by expanding the compression
fracture with a balloon before injecting the PMMA (Fig. 20-14).
Reported complications include extrusion of cement around the
neurologic structures, so this procedure should only be performed after
careful consideration of the risks.
 |
|
FIGURE 20-13 A.
Lateral lumbar spine radiograph in a 27-year-old woman with metastatic thyroid cancer reveals destruction of the posterior portion of the L2 vertebral body. B. Axial CT scan through L2 reveals extensive destruction of the bone with soft tissue extension posterolaterally into the spinal canal. The patient presented with L2 nerve root symptoms. Anteroposterior (C) and lateral (D) postoperative radiographs after L2 anterior corpectomy, posterior spinal decompression, and segmental instrumentation and fusion from T11 to L4. She also had anterior reconstruction and fusion from L1 to L3 using humeral allograft. |
older with multiple associated medical problems, the chance of
perioperative complications is increased. These patients have the same
risks as those with nonpathologic fractures when they consent to
surgical treatment, but some complications are more likely in patients
with widespread cancer. Two of the most concerning problems are tumor
progression with resultant hardware failure and cardiopulmonary
compromise.
 |
|
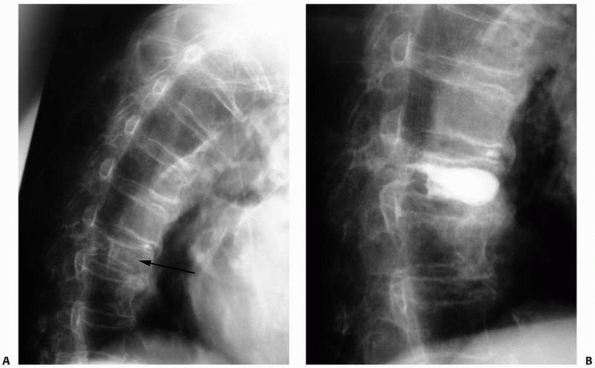
FIGURE 20-14 A. Lateral radiograph of a patient with senile osteoporosis and a thoracic compression fracture. B.
Lateral radiograph after treatment with kyphoplasty to regain vertebral height and relieve pain. Note that the methylmethacrylate is relatively well contained. The fracture reduction is minimal but she had complete pain relief 2 weeks after the procedure. |
and radiation will continue to destroy bone so that the existing
hardware or prosthesis is load bearing rather than load sharing. Using
the principles of surgical treatment outlined in this chapter will
minimize the risk of hardware failure, but inevitably some constructs
will fail. The salvage of failed reconstructions must be
individualized, but modular endoprostheses can frequently be used to
salvage failed intramedullary fixation.47 Again, the patient’s life span and general health must be favorable before they are indicated for a prolonged procedure.
with bone metastasis. First, many of these patients have pulmonary
metastasis or primary lung tumors that make ventilation more difficult.
Some patients will have a surgical procedure to stabilize a pathologic
fracture and fail postoperative attempts at extubation, remaining in an
intensive care setting for a prolonged time. Second, the placement of
long-stemmed cemented femoral prostheses or prophylactic femoral or
humeral nails must be done carefully to avoid embolic events. Careful
suction of the canal and slow reaming are tips to decrease this
complication.6,15
It is unclear from the available literature whether the actual
incidence of fat emboli is increased during placement of intramedullary
rods or long cemented femoral stems in patients with malignancy
compared to those without cancer. However, patients with cancer are
more hypercoagulable and are likely less able to compensate for fat
emboli to the lung than are patients without cancer, especially if they
have primary or metastatic pulmonary disease.
to bone metastases, halt the progression of bony destruction, and allow
healing of an impending pathologic fracture. It is a reasonable
alternative to surgical treatment for certain lesions. When the
endpoint is pain relief, local radiation therapy typically results in
partial relief in over 80% of patients with bone metastasis and
complete pain relief in 50% to 60% of cases.94 Variability in response rates depends on multiple factors including the histology and location of the lesion.99
The onset of symptomatic relief usually occurs in the first 1 to 2
weeks, but maximal relief may take several months. Radiation is used in
the postoperative setting to increase local tumor control after
surgical stabilization. Retrospective data have shown that
postoperative radiation improves limb function and decreases the rates
of second orthopedic procedures.88
The majority of patients in this study had the entire prosthesis or
internal fixation device included in the treatment field. Radiation can
usually begin 2 weeks after the surgical procedure if there are no
wound complications.
boneseeking agents provides palliation of bone pain. It may be
appropriate for widespread bone metastases when more traditional forms
of radiation have reached their limit or when standard radiation
techniques are not feasible because of surrounding normal tissue
tolerances. Strontium-89 is used clinically and preferentially taken up
at sites of active bone mineral turnover, similar to bisphosphonates.
There is a greater uptake of the radionucleotides in metastatic lesions
than in normal bone. A systematic review of the published literature on
palliation of painful bone metastasis with radiopharmaceuticals
revealed better pain relief with fewer sites of disease using
strontium-89 compared to placebo or local radiation therapy.7
resorption cavities at osteoclastic binding sites, inhibit osteoclastic
function, alter the morphology of the osteoclast ruffled border, and
inhibit maturation and recruitment of osteoclasts from the
monocyte/macrophage cell line. They promote osteoclast apoptosis, and
there are some data to suggest direct effects on tumor cells.
Intravenous bisphosphonates have been used with success to treat bone
pain and hypercalcemia in breast cancer, and they are most commonly
used as an adjunct to other systemic therapies.62
document a decrease in the time to skeletal-related events as well as a
decrease in the rate of these events in patients with bone metastasis
treated with various bisphosphonates.40,61,62
patients with metastatic bone disease are (a) the ideal length of a
cemented femoral stem in patients with metastatic disease about the hip
and (b) the specific characteristics that define an impending fracture.
These topics were discussed previously.
treatment of patients with a solitary metastasis. There is literature
to suggest that wide resection of a solitary RCC metastasis leads to
increased survival.49,84
However, it has not been shown that these data are applicable to
metastatic disease from other primary sites. The study recommending
resection of solitary RCC metastasis was conducted before widespread
use of PET scanning, which allows discovery of smaller foci of active
disease. It is likely that many patients presumed to have solitary
metastasis would have additional sites of disease if screened with PET
imaging. However, a patient with a solitary metastasis from any origin
who has been tumor free for several years should be theoretically
considered a candidate for a resection. RCC and follicular thyroid
carcinoma are the two tumor types most likely to produce isolated bone
metastasis years after treatment of the primary tumor.
with metastatic bone disease of the spine and extremities will likely
include continued use and new applications for trabecular metal.59
The tantalum acetabular components allow excellent bone ingrowth and
are being used more routinely in revision joint arthroplasty to
reconstruct large acetabular defects.47,76
Further advances in this type of metal fixation may allow improvement
in the attachment of soft tissues to megaprostheses after tumor
resection. Endoprostheses made of porous tantalum have been used in
limb-sparing surgery in patients with lower extremity sarcomas with
short-term follow-up.43
with orthopaedic surgeons to manage patients with bone metastasis.
Radiofrequency ablation (RFA) and cryotherapy are now being used
routinely for palliative treatment of painful metastatic lesions. These
techniques provide an alternative to external beam radiation or surgery.33
A recent study of patients with pelvic and sacral metastasis treated
with RFA showed a clinical benefit with significant pain relief in 95%
of patients.33 Most of these
patients had failed to respond to prior treatment or were considered to
be poor candidates for narcotic medication or radiation. Another new
procedure termed acetabuloplasty is similar to vertebroplasty in that
PMMA is injected percutaneously into an acetabular defect to provide
pain relief and possibly avoid a major surgical reconstruction.47,98
young adults. Most tumors gradually enlarge until the patient reaches
skeletal maturity and then resolve or become inactive. Inactive lesions
do not require surgical treatment. Active or aggressive benign lesions
often require intralesional curettage with or without bone grafting to
remove the tumor and allow healing of the underlying bone. A pathologic
fracture through a benign bone tumor may change the course of
treatment. Because of the age and activity level of patients who have
benign bone tumors, pathologic fractures are not uncommon.
through a benign bone lesion depends on the activity of the underlying
lesion. Most can be treated nonoperatively in a cast until the fracture
heals. At that time, treatment of the benign tumor can be addressed.
Indications for surgical treatment of the fracture include unacceptable
deformity in a cast, open fracture, fracture nonunion, or an
association with active or aggressive lesions such as giant cell tumor
or aneurysmal bone cyst. The treatment of pathologic fractures in the
context of specific benign bone tumors is discussed next. The reader is
referred to comprehensive musculoskeletal oncology textbooks to learn
more about the diagnosis and treatment of individual tumors.
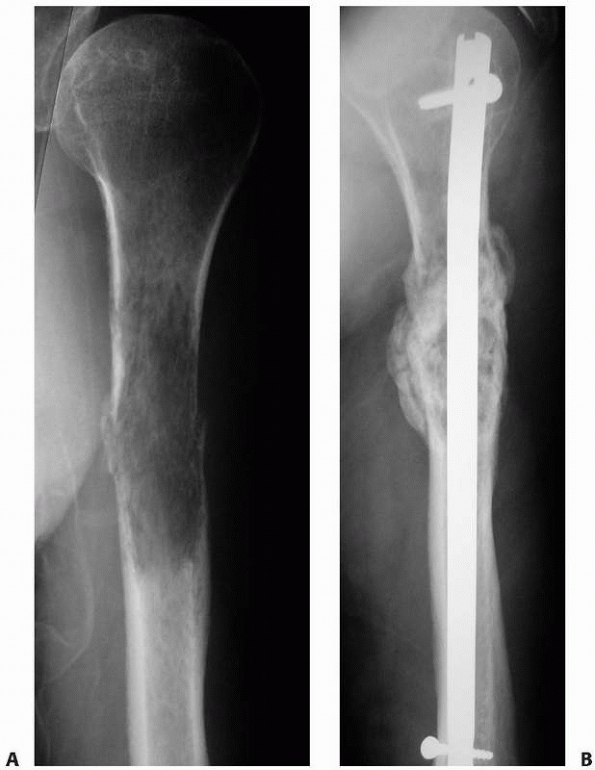
A humeral fracture should be allowed to heal in a satisfactory position
as the fracture occasionally stimulates healing of the cyst. If the
cyst does not heal spontaneously after the fracture callus remodels,
corticosteroid injection into the cyst is recommended. A displaced
fracture through a proximal femoral UBC in a child usually requires
open reduction, bone grafting of the cyst, and internal fixation due to
weight-bearing requirements.
that can grow rapidly in the metaphysis of a young patient, simulating
a malignancy.13 Despite its
occasional aggressive growth pattern, pathologic fractures are
uncommon. Approximately 15% to 20% of lesions occur in the posterior
elements of the spine and can cause neurologic compromise. The standard
treatment of an ABC with or without a fracture is intralesional
curettage and bone grafting. Depending on the age of the patient and
location of the ABC, a pathologic fracture might require internal
fixation at the time of curettage.
spectrum of disease known as Langerhans cell histiocytosis. It is a
benign bone tumor, and affected patients present with pain. This tumor
can
cause collapse of a vertebral body (vertebra plana) and neurologic
symptoms. Patients with symptomatic vertebra plana are braced, and
eventually the vertebral height is restored without surgery.72
For extremity lesions that do not spontaneously resolve, the standard
of care is an intralesional corticosteroid injection. Open curettage is
reserved for selected lesions that fail to respond or are unsuitable
for steroid injection because of the size, location, or aggressiveness
of the lesion.100 A pathologic
fracture should be allowed to heal before performing a needle biopsy
and injection, so the fracture callus does not confuse the histologic
picture.
 |
|
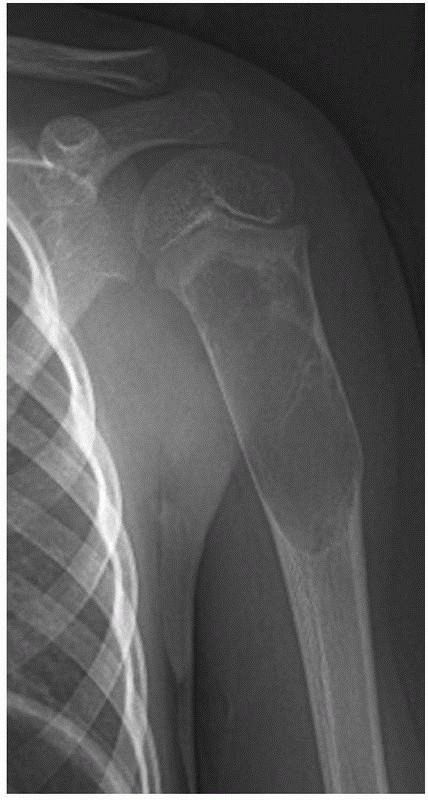
FIGURE 20-15
Anteroposterior radiograph of the left proximal humerus in a 5-year-old girl with a unicameral bone cyst. Note the centrally located, osteolytic lesion. She previously had a pathologic fracture through the lesion and subsequently had an intralesional steroid injection. The cyst does not affect the proximal humeral growth plate. |
in young patients. They spontaneously resolve after skeletal maturity.
They are asymptomatic, but large lesions can fracture. Common
pathologic fracture locations include the distal tibia, distal femur,
and proximal tibia (Fig. 20-16). Patients with
multiple lesions have a higher risk of fracture. Pathologic fractures
can be treated successfully in the majority of cases with closed
reduction and cast immobilization.23
If the lesion persists after fracture consolidation, curettage and bone
grafting can be performed if necessary. If a fracture is unstable and
cannot be reduced in a closed fashion, curettage and bone grafting is
combined with internal fixation.
 |
|
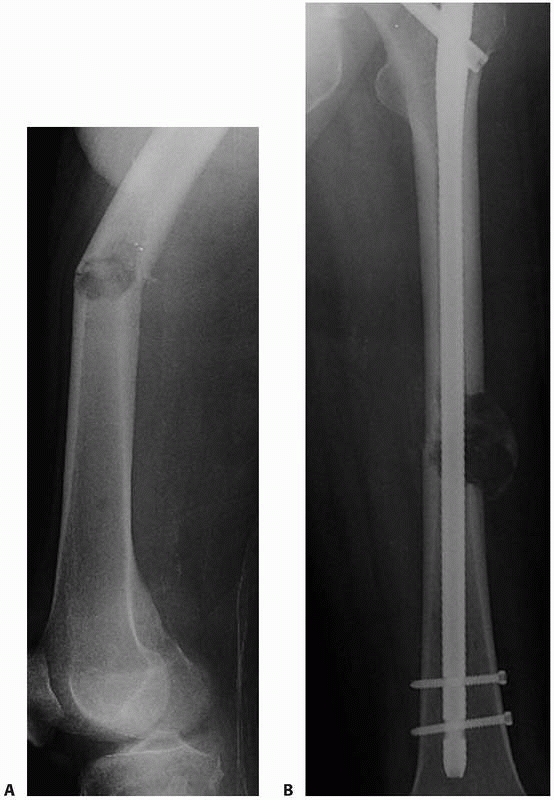
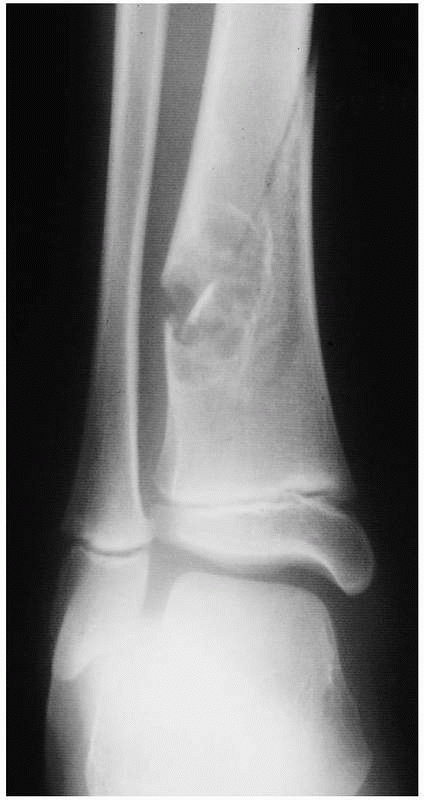
FIGURE 20-16
Anteroposterior radiograph of the distal tibia of a 10-year-old boy. The well-developed reactive rim of bone around the eccentric, metaphyseal, radiolucent lesion is virtually diagnostic of a nonossifying fibroma (NOF). The patient had no symptoms until he slid into second base and caught his foot, twisting his lower leg. He heard a crack and had acute pain. The fracture was treated in a cast and healed, but the NOF remained 2 more years before healing completely. |
The lesions in long bones rarely fracture. Those most prone to
pathologic fractures and pain occur in the small bones of the hand (Fig. 20-17).
Some advocate nonsurgical treatment of these lesions, as the fracture
occasionally stimulates resolution of the enchondroma. Most agree that
surgical intervention, if performed, should be delayed until the
fracture has healed.87 Surgical
treatment of the enchondroma eliminates the future risk of pathologic
fracture and avoids progressive deformity. Whether to perform a bone
graft to the defect after curettage remains controversial. Multiple
enchondromas with frequent hand fractures and deformities occur in
Ollier disease and Mafucci syndrome.
abnormality rather than a true neoplasm and occurs in both monostotic
and polyostotic forms.36 Most
solitary lesions of fibrous dysplasia are asymptomatic, but patients
can present with a painful pathologic fracture or bowed extremity. In
the polyostotic form, lesions involve multiple areas of a single bone
or multiple bones in one extremity, and fractures occur in 85% of these
patients. The structural bone strength is decreased in fibrous
dysplasia, and sequential fractures can result in progressive deformity
producing
the classic Shepherd’s crook varus appearance of the proximal femur. The fractures are rarely displaced and heal well.
 |
|
FIGURE 20-17
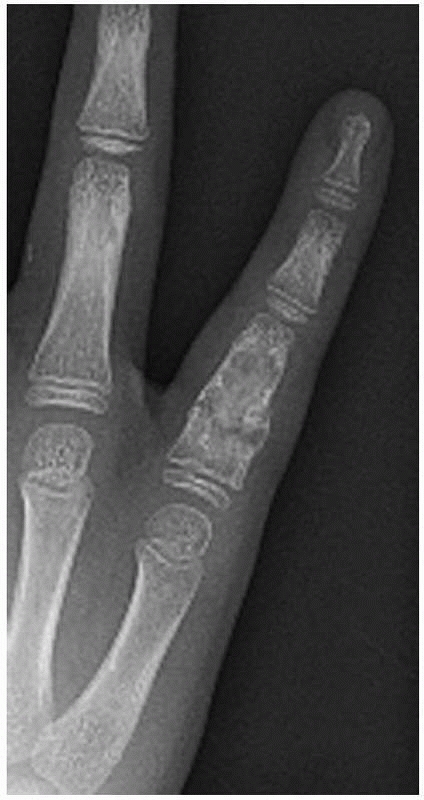
Anteroposterior radiograph of the right fifth finger in a 9-year-old boy with a proximal phalanx pathologic fracture. The fracture is through an enchondroma and is minimally displaced. It healed with immbolization and no treatment was necessary for the asymptomatic benign bone lesion. |
extremity and spine can be treated nonoperatively, whereas lower
extremity fractures usually require internal fixation.36
Extensive areas of fibrous dysplasia in high-stress weight-bearing
areas are treated with prophylactic internal fixation. The lesion
should be biopsied at the time of surgery to confirm the diagnosis
before proceeding with intramedullary fixation to stabilize long bones.
The goal is to strengthen and straighten the bone, not to resect the
lesion. If bone graft is used, it should be allograft, as autograft has
the same genetic abnormality as the dysplastic bone and may not heal
properly. Internal fixation does not alter the disease process but
provides mechanical support and pain relief. Another option is medical
treatment with bisphosphonates alone or in combination with surgery.55
 |
|
FIGURE 20-18 A.
Anteroposterior radiograph of a 37-year-old man with a giant cell tumor in the right distal femur (lateral femoral condyle). Note the lack of a sclerotic rim and extension to the subchondral bone. There is a spiral pathologic displaced fracture through the lesion. B. Postoperative radiograph after curettage and cementation of the giant cell tumor. The fracture was reduced and internally fixed and healed uneventfully. |
tumor that occurs in young adults. Ten percent of patients present with
a pathologic fracture. In patients whose adjacent joint can be
preserved, the GCT should be treated with thorough curettage and bone
grafting or cementation.89 Internal fixation is often necessary after a pathologic fracture as there is usually extensive bone loss and deformity (Fig. 20-18).
Adjuvant treatment with phenol or cryosurgery should be used with
caution in patients when a pathologic fracture exposes adjacent soft
tissues. Primary wide resection and reconstruction is only necessary
when the associated joint is destroyed.
combination of surgery, chemotherapy, and/or radiation. Multiple
myeloma is a primary malignant bone tumor with a systemic presentation
that occurs in older patients. Pathologic fractures in patients with
myeloma, lymphoma, and metastatic carcinoma can be treated with
fixation through the tumor as they are systemic diseases treated
primarily with chemotherapy and radiation. The overall survival of
these patients is not compromised by palliative surgical stabilization.
Ewing’s sarcoma, and chondrosarcoma are treated much differently than
systemic neoplastic disease.92 These
tumors grow initially in the bone and can metastasize to the lungs.
Local control of the primary lesion is achieved by complete surgical
resection. A pathologic fracture through the lesion theoretically
decreases the chance of local control, because tumor cells spread
throughout the hematoma. Amputation should be discussed as a potential
surgical option for patients with a pathologic fracture through primary
malignant bone tumors. The literature is controversial on whether a
pathologic fracture through an osteosarcoma compromises the chance for
local control. To some degree this depends on the amount of fracture
displacement, histology of the tumor, and response to chemotherapy.
pathologic fracture through a presumed primary bone sarcoma, the
patient should be staged and a biopsy performed. An appropriate staging
workup includes a CT scan of the chest for all patients, and a bone
marrow biopsy when Ewing sarcoma is suspected. The biopsy of a presumed
bone sarcoma is especially difficult in the setting of an associated
pathologic fracture. The fracture hematoma and healing process alters
the histology and may confuse the pathologist. Whenever possible, the
biopsy should be performed away from the fracture. When there is an
extraosseous soft tissue mass associated with the tumor, an
image-guided needle biopsy is usually adequate. When the lesion is
intraosseous and fracture callus is present, an open biopsy may be
necessary. The surgeon who will eventually perform the definitive
surgical treatment of the lesion should be the one who orders or
performs the diagnostic biopsy.
primary sarcoma may compromise the limb and life of the patient. If the
patient will be treated with preoperative chemotherapy, cast
immobilization of the fracture is preferred. The fracture usually heals
during systemic treatment, and a cast avoids potential pin tract
infections in neutropenic patients stabilized with an external fixator.
malignancy of bone require a coordinated multidisciplinary team that
includes a medical oncologist, radiation oncologist, musculoskeletal
pathologist, radiologist, physical therapist, and orthopaedic
oncologist; only with the full complement of care can these patients
achieve the best quality of life and maximum overall survival.
tumors in children. Approximately 10% of patients present with a
pathologic fracture. Closed treatment of the fracture in a cast is
indicated after a needle or open biopsy is performed. When staging is
complete, preoperative chemotherapy is used for patients with
osteosarcoma or Ewing sarcoma. After 3 to 4 months of systemic therapy,
a decision is made about local control of the primary tumor. For
patients with osteosarcoma, surgical resection is indicated. If
patients have a clinical and radiographic response to chemotherapy, a
limb salvage procedure is generally preferred. Articles have shown no
difference in overall survival for patients with osteosarcoma and a
pathologic fracture that are treated with limb salvage compared to
amputation.4,79 Close follow-up is necessary to identify a possible local recurrence.
surgical resection, radiation, or both. In reconstructable sites, most
patients are treated with limb salvage surgery to remove all
chemotherapy-resistant clones and avoid the risks of radiation in a
growing child. However, in patients with a pathologic fracture treated
with surgical resection, consideration should be given to adding
radiation as a postoperative adjuvant to improve the chance of local
control and avoid amputation.29
The pelvis is a common site, but displaced pathologic fractures are
rare in this location. The most common location of a pathologic
fracture through a chondrosarcoma is in the proximal femur (Fig. 20-19).
A serious mistake is to assume the fracture is secondary to metastatic
carcinoma and place an intramedullary rod through the lesion. This act
generally precludes any safe limb salvage option for the patient. An
older patient with a solitary lytic lesion should be staged
appropriately with a biopsy to confirm the diagnosis before any
surgical treatment.
through a chondrosarcoma is wide resection by an orthopaedic
oncologist. Chemotherapy and radiation are not effective for this
tumor. A pathologic fracture greatly compromises the local area, as any
stray tumor cells not resected will likely grow into a locally
recurrent lesion. A displaced fracture through a chondrosarcoma is a
reason to consider amputation, especially if wide resection cannot be
achieved with a limb salvage procedure.
-
Any process that reduces bone strength
predisposes a patient to a pathologic fracture during normal activity
or after minimal trauma. It must be recognized as a pathologic fracture
if the patient is to be treated properly. -
The most common cause for a pathologic fracture is osteoporosis or osteomalacia.
-
Patients with osteoporosis or osteomalacia require evaluation and management of the underlying disorder.
-
Patients over 40 years of age with a
pathologic fracture through a discrete bone lesion are much more likely
to have metastatic bone disease than a primary bone tumor. -
The prognosis for patients with
metastatic bone disease is improving because of early recognition and
better adjuvant treatment; therefore, many patients will live longer
than 2 years. -
Do not immediately assume that a lytic
lesion or pathologic fracture is from metastatic disease. A thorough
workup and possible biopsy are required. -
Prophylactic fixation for impending
(versus actual) fractures from metastatic disease is technically easier
for the surgeon and allows a quicker patient recovery. -
The Mirels scoring system is available to guide the treatment of an impending fracture from metastatic bone disease.
-
Femoral neck fractures caused by
metastatic bone disease require a cemented hip prosthesis, as internal
fixation has a high rate of failure with disease progression. -
When surgery is required for metastatic
disease to the spine, decompression and stabilization with internal
fixation are generally necessary. -
Surgical reconstruction for pathologic
fractures should be durable enough to allow immediate weight bearing
and last the patient’s expected lifespan. -
A pathologic fracture through a primary
malignant bone tumor is treated much differently than a fracture
through a metastatic lesion. -
Treatment of patients with pathologic
fractures requires the presence of a multidisciplinary team comprised
of orthopaedic surgeons, medical oncologists, radiation oncologists,
endocrinologists, radiologists, pathologists, pain specialists,
nutritionists, physical therapists, and psychologist/ psychiatrists.
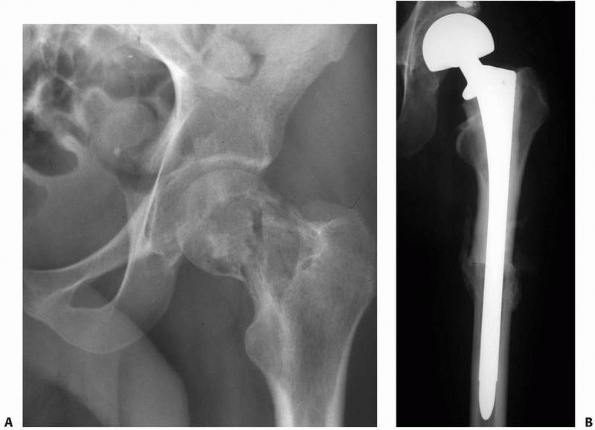 |
|
FIGURE 20-19 A.
Anteroposterior radiograph of the left hip in a 24-year-old man with a pathologic fracture of the femoral neck. He had pain for 6 months prior sustaining the fracture after minimal trauma. This history in a young healthy patient should raise suspicion of an underlying process in the bone. There is matrix production within the lesion, and an open biopsy revealed a grade 2 chondrosarcoma. B. Postoperative radiograph after resection of the proximal femur and hip capsule and reconstruction with an allograft-prosthetic composite. The prosthesis is cemented into the allograft and press-fit into the host bone. The patient was free of local recurrence when he died of metastatic disease 2 years later. |
AJ, Buch R, Mathews J, et al. Reconstruction using the saddle
prosthesis following excision of primary and metastatic periacetabular
tumors. Clin Orthop 1995; May:203-213.
A, Grimer RJ, Cannon SR, et al. Reconstruction of the hemipelvis after
the excision of malignant tumors. Complications and functional outcome
of prostheses. J Bone Joint Surg Br 1997;79:773-779.
EW, McCutcheon IE. Single-stage posterior vertebrectomy and replacement
combined with posterior instrumentation for spinal metastasis. J
Neurosurg 1996;85: 211-220.
G, Ferrari S, Longhi A, et al. Nonmetastatic osteosarcoma of the
extremity with pathologic fracture at presentation: local and systemic
control by amputation or limb salvage after preoperative chemotherapy.
Acta Orthop Scand 2003;74:449-454.
EJ, Gonzalez MH, Cooperman DR, et al. The effect of adjunctive
methylmethacrylate on failures of fixation and function in patients
with intertrochanteric fractures and osteoporosis. J Bone Joint Surg
1985;67:1094-1107.
SA, Wilson JL, Molnar RR, et al. The incidence of acute
cardiorespiratory and vascular dysfunction following intramedullary
nail fixation of femoral metastasis. Acta Orthop Scand 2000;71:147-152.
G, Charette M, Reid R, et al. Radiopharmaceuticals for the palliation
of painful bone metastasis: a systemic review. Radiother Oncol
2005;75:258-270.
KC, Horstman J, Coleman SS. Isolated fractures of the lesser trochanter
in adults: an initial manifestation of metastatic malignant disease. J
Bone Joint Surg 1984;66: 770-773.
SK, Cervilla V, Vinct V, et al. Magnetic resonance appearance of sacral
insufficiency fractures. Skeletal Radiol 1990;19:489-493.
RK, Pelker RR, Friedlaender GE, et al. Postfracture radiation effects
on the biomechanical and histologic parameters of fracture healing. J
Orthop Res 1991;9: 876-882.
RW, Boublik M, Blevins FT, et al. Functional outcome of pathologic
fracture secondary to malignant diseases in a rehabilitation hospital.
Cancer 1992;69:98-102.
AN, Johnson ME, Penumaticos SG, et al. Preoperative embolization of
bone metastases from renal cell carcinoma. Eur J Radiol 2000;10:593-596.
DL, Incavo SJ, Uroskie JA, et al. Femoral stem insertion generates high
bone cement pressurization. Clin Orthop 2001;Dec:335-344.
TA, Rock MG, Choudhury SN, et al. Biomechanical analysis of
prophylactic fixation for middle third humeral impending pathologic
fractures. Clin Orthop 1999; 363:240-248.
TA, Sim FH, Shives TC, et al. Intercalary spacers in the treatment of
segmentally destructive diaphyseal humeral lesions in disseminated
malignancies. Clin Orthop 1996;324:233-243.
S, Lieberman IH, Reinhardt MK, et al. Kyphoplasty in the treatment of
osteolytic vertebral compression fractures as a result of multiple
myeloma. J Clin Oncol 2002;20:2382-2387.
ME, Kneisl JS. Pathologic fractures through nonossifying fibromas: is
prophylactic treatment warranted? J Pediatr Orthop 1997;17:808-813.
NS, Thurston CW. Effect of radiation on acrylic cement with special
reference to fixation of pathological fractures. J Biomech 1975;8:53-56.
E, Metser U, Flusser G, et al. Assessment of malignant skeletal
disease: initial experience with 18F-fluoride PET/CT and comparison
between 18F-Fluoride PET and 18F-fluoride PET/CT. J Nucl Med
2004;45:272-278.
I, Rhines LD, Weinberg JS. The role of surgery in the management of
metastatic spinal tumors. Semin Oncol 2008;35:108-117.
MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided
radiofrequency ablation of painful metastases involving bone: a
multicenter study. J Clin Oncol 2004;22:300-306.
A, Norman A. Osteolytic cortical destruction: an unusual pattern of
skeletal metastases. Skeletal Radiol 1988;17:402-406.
DH, Schirp S, Gevargez A. Image-guided radiofrequency ablation of
spinal tumors: preliminary experience with an expandable array
electrode. Cancer J 2002;8: 33-39.
JT, Jumar SJ, MacEwin GD. Fibrous dysplasia of the proximal part of the
femur. Long-term results of curettage and bone-grafting and mechanical
realignment. J Bone Joint Surg 1998;80:648-658.
KD. Anterior decompression and stabilization of the spine as a
treatment for vertebral collapse and spinal cord compression from
metastatic malignancy. Clin Orthop 1988;233:177-197.
KD. The management of acetabular insufficiency secondary to metastatic
malignant disease. J Bone Joint Surg 1981;63:653-664.
KD, Sim FH, Enis JE, et al. Methylmethacrylate as an adjunct in
internal fixation of pathologic fractures. J Bone Joint Surg
1976;58:1047-1055.
HT, Lin SJ, Smith MR, et al. Zoledronic acid and skeletal complications
in patients with solid tumors and bone metastases: analysis of a
national medical claims database. Cancer 2008;113:1438-1445.
JC, Damron TA, Weiner MM, et al. Biomechanical analysis of humeral
diaphyseal segmental defect fixation. Clin Orthop 2002:231-239.
GE, Christie MJ, Schwartz HS. Trabecular Metal Endoprosthetic Limb
Salvage Reconstruction of the Lower Limb. J Arthroplasty 2008;Sept 30
(epub ahead of print).
J, Cabe GD, Tedrow JR, et al. Failure of trabecular bone with simulated
lytic defects can be predicted noninvasively by structural analysis. J
Orthop Res 2004;22: 479-486.
A, Crockard A, Antonietti P, et al. Does spinal surgery improve the
quality of life for those with extradural (spinal) osseous metastases?
An international multicenter prospective observational study of 223
patients. Invited submission from the Joint Section Meeting on
Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg
Spine 2008;8:271-278.
A, Heijink A, Leerapun T, et al. Successful canine patellar tendon
reattachment to porous tantalum. Clin Orthop Relat Res 2007;463:202-207.
DJ, Papagelopoulos PJ, Sim FH. Advances and challenges in the surgical
treatment of metastatic bone disease. Clin Orthop 2003;(415
suppl):S14-S18.
ST, Ghert MA, Harrelson JM, et al. Treatment of osseous metastases in
patients with renal cell carcinoma. Clin Orthop 2003;Apr:223-231.
M, Ng JT, Cunningham BW, et al. Biomechanical analysis of anterior
versus circumferential spinal reconstruction for various anatomic
stages of tumor lesions. Spine 1999;24:445-450.
D, Grimer RJ, Abudu A, et al. Endoprosthetic replacement of the
proximal humerus. Long-term results. J Bone Joint Surg Br
2003;85:717-722.
T, Choong PF. Major reconstruction for periacetabular metastasis: early
complications and outcome following surgical treatment in 40 hips. Acta
Orthop Scand 2000;71:585-590.
JM, Sculco TP, Zolan S. Treatment of pathological fractures of the hip
by endoprosthetic replacement. J Bone Joint Surg 1980;62:954-959.
A, Radl R, Gruber G, et al. Predictive value of seven preoperative
prognostic scoring systems for spinal metastases. Eur Spine J
2008;17:1488-1495.
BR, Sporer S, Poggie RA, et al. Experimental and clinical performance
of porous tantalum in orthopaedic surgery. Biomaterials
2006;27:4671-4681.
PP, Mirza AN, Lewis VO, et al. Patient survival after surgery for
osseous metastases from renal cell carcinoma. J Bone Joint Surg Am
2007;89:1794-1801.
A. Efficacy and safety of intravenous bisphosphonates in patients with
bone metastases caused by metastatic breast cancer. Clin Breast Cancer
2007;7(suppl 1): S14-S20.
JK, Apfelbau RI, Chiles BW III, et al. Cervical spinal metastasis:
anterior reconstruction and stabilization techniques after tumor
resection. Neurosurg Focus 2003;15:E2.
P, Lortholary A, Hon J, et al. Zoledronic acid is superior to
pamidronate in the treatment of hypercalcemia of malignancy: a pooled
analysis of two randomized, controlled clinical trials. J Clin Oncol
2001;19:558-567.
HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members
of the Musculoskeletal Tumor Society. J Bone Joint Surg 1996;78:656-663.
RA, Sheth DS, Boland PJ, et al. Functional and oncological outcome of
acetabular reconstruction for the treatment of metastatic disease. J
Bone Joint Surg 2000;82: 642-651.
H. Metastatic disease in long bones: a proposed scoring system for
diagnosing impending pathological fractures. Clin Orthop
1989;249:256-265.
A, von Stechow D, Zurakowski D, et al. Bone volume fraction explains
the variation in strength and stiffness of cancellous bone affected by
metastatic cancer and osteoporosis. Calcif Tissue Int 2008;Oct 23.
M, Tokuda Y, Suzuki Y, et al. Whole body PET for the evaluation for
bony metastases in patients with breast cancer: comparison with
99Tcm-MDP bone scintigraphy. Nucl Med Commun 2001;22:875-879.
PJ, Galanis EC, Greipp PR, et al. Prosthetic hip replacement for
pathologic or impending pathologic fractures in myeloma. Clin Orthop
1997:192-205.
P, Hohmann F, Kuhl J. et al. Vertebral remodeling in eosinophilic
granuloma of the spine. A long-term follow-up. Spine 1998;23:1351-1354.
D, Blach M, Nerreter V, et al. Metastatic spinal cord compression.
Influence of time between onset of motoric deficits and start of
radiation on therapeutic effect. Strahlenther Onkol 1999;175:378-381.
S, Fogelman I, Gardner MD, et al. Hypercalcemia and metastatic bone
disease: is there a causal link? Lancet 1982;2:903-905.
PS, Halasy M, Trousdale RT, et al. Preliminary results of tantalum
acetabular components for THA after pelvic radiation. Clin Orthop Relat
Res 2006;453:195-198.
BT. Evaluation of the patient with carcinoma of unknown primary origin
metastatic to bone. Clin Orthop Relat Res 2003;415:S105-S109.
SP, Ghert MA, Zurakowski D, et al. Pathologic fracture in osteosarcoma:
prognostic importance and treatment implications. J Bone Joint Surg Am
2002;84A:49-57.
K, Lieberman IH: Vertebral augmentation in osteoporosis and bone
metastasis. Curr Opin Support Palliat Care 2007;1:323-327.
FH, Daugherty TW, Ivins JC. The adjunctive use of methylmethacrylate in
fixation of pathological fractures. J Bone Joint Surg 1974;56:40-48.
H, Yamada K, Sugiura T, et al. Predictors of survival in patients with
bone metastasis of lung cancer. Clin Orthop Relat Res 2008;466:729-736.
RM, Myers GJ, Abudu AT, et al. The three-pin modified “Harrington”
procedure for advanced metastatic destruction of the acetabulum. J Bone
Joint Surg Br 2008; 90B:84-87.
P, Lugnegard H. Is the treatment of enchondroma in the hand by simple
curettage a rewarding method? J Hand Surg 1990;15B:331-334.
P, Smalley S, Cozad S. Role of postoperative radiation therapy after
stabilization of fractures caused by metastatic disease. Int J Radiat
Oncol Biol Phys 1995;31:43.
RE, Wunder JS, Isler MH, et al. Giant cell tumor of long bone: a
Canadian Sarcoma Group study. Clin Orthop Relat Res 2002:248-258.
MS, Petrigliano FA, Liu NQ, et al. Influence of simultaneous targeting
of the bone morphogenetic protein pathway and RANK/RANKL axis in
osteolytic prostate cancer lesion in bone. Bone 2008;Sept 26.
KL, Lewis VO, Randall L, et al. An approach to the management of the
patient with metastatic bone disease. Instr Course Lect Ser
2004;53:663-676.
DR, Schwartz HS. Intramedullary nailing for impending pathological
subtrochanteric fractures. J Bone Joint Surg Br 1991;73B:668-670.
A, Kobaiter H, Chiras J: Acetabulum malignancies: technique and impact
on pain of percutaneous injection of acrylic surgical cement. Eur
Radiol 1998;8:123-129.
J, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation
radiotherapy trials for the palliation of painful bone metastases. Int
J Radiat Oncol Biol Phys 2003; 55:594.
AW, Fanning CV, Ayala AG, et al. Percutaneous techniques for the
diagnosis and treatment of localized Langerhans-cell histiocytosis
(eosinophilic granuloma of bone). J Bone Joint Surg 1998;80:219-228.
Y, Frassica FJ, Chao EY, et al. Metastatic bone disease: a study of the
surgical treatment of 166 pathologic humeral and femoral fractures.
Clin Orthop 1990;251: 213-219.
WTC, Zacharck CK, Barloon TJ, et al. Vertebral compression fractures:
distinction between benign and malignant causes with MR imaging.
Radiology 1989;172:215-218.
