Systemic Complications
in the stress response, which is initiated at the time of injury. The
early resuscitation of injured patients, aiming primarily to save life,
then to save limb and thirdly to restore function, has been covered in Chapter 9.
This chapter aims to describe the stress response and its later
consequences in greater detail and to address the associated issues of
rhabdomyolysis, venous thromboembolism, and postoperative pyrexia.
injury and is often well advanced by the time a patient is brought to
the emergency department. The response not only continues to evolve
throughout the process of resuscitation and definitive management, but
is influenced by this process. Where the response is excessive, a cycle
of events develops leading to multiple organ dysfunction and death. Our
current understanding of this complex process is incomplete, and this
is an area of considerable controversy, interest, and exciting
potential. In order to influence this process effectively in the future
we need to:
-
Understand more fully the natural history of the systemic response to major trauma.
-
Be able to identify soon after presentation those patients who are at risk of developing complications later in their management.
-
Develop an intervention, targeted at this
group of patients, which will allow this excessive response to be
attenuated or avoided.
pathophysiologic processes including fat embolism, inflammatory
hyperstimulation, coagulation activation, and neuroendocrine
stimulation (Fig. 22-1).
but only a small proportion of these patients will go on to develop the
clinical features of fat embolus syndrome (FES). Fat emboli arise from
intravasation of fat and bone marrow from the medullary cavity of bone.
There are at least 130 mL of liquid fat in the adult human tibia or
femur113 and the sudden pressure wave at the time of fracture
forces fat out of the medullary cavity and into the venous circulation.
Bone fracture is not necessarily required: several cases of fatal fat
embolism have been reported after bone contusion.8
Fat emboli also form from the destabilization of serum lipids (under
the influence of C-reactive protein), and from the formation of fat de
novo from depot precursors (following neuroendocrine activation).6,118 Continued movement of unstabilized fractures and surgical instrumentation of the medullary canal causes further intravasation.152
Fat travels through the right side of the heart and embolizes in the
lungs, which act as a filter. Neutral fats from the bone marrow are
chemically innocuous, but over the course of 12 to 72 hours undergo
hydrolysis in the lungs to form fatty acids.8,42,64
These are exceptionally toxic to pulmonary tissue, causing disruption
of the alveolar capillary membrane and the development of hemorrhagic
pulmonary edema, accompanied by reduced surfactant activity.
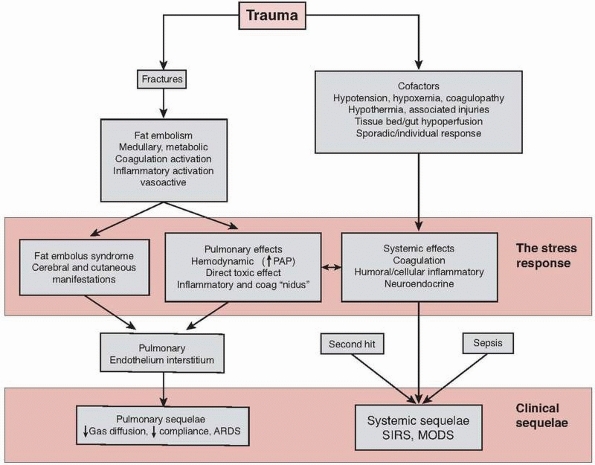 |
|
FIGURE 22-1 Flow chart to show schematic representation of the stress response to trauma.
|
40 billion microemboli, 10 µm in diameter, which would be sufficient to
block every capillary in the lung8
were it not for the opening up of arteriovenous connections. This
“shunting” allows fat to enter the left-sided (systemic) circulation,
where it eventually embolizes in peripheral vascular beds including the
brain, skin, and kidney. However, shunting also results in deoxygenated
blood passing directly back into the right-sided circulation, resulting
in hypoxemia.
are widely quoted and represent an early attempt to understand and
describe this process. The criteria are largely empirical, and
alternatives have been proposed.43,63,76 Clinically, most patients spontaneously improve from Fat Embolism Syndrome and return to normal after about 5 days,118 but there is an associated mortality of between 10%43,124 and 20%.42
mechanistic interpretation of fat embolism and posttraumatic
respiratory failure is insufficient to explain the range of responses
encountered after trauma, and the inflammatory system is implicated as
an important and complex component of the response. The fracture itself
causes a localized inflammatory reaction: monocytes, macrophages,
neutrophils, and endothelial cells all release cytokines at the
fracture site,91 and these enter the venous circulation along with the fat and are carried to the lungs and then to the systemic circulation.
anti-inflammatory, and certain cytokines such as interleukin-6 (Il-6)
have been shown to exhibit pleiotropism, displaying either
proinflammatory or anti-inflammatory effects depending upon
the circumstances.71
Proinflammatory cytokines include tumour necrosis factor α (TNFα),
Il-6, and Il-8, which serve to initiate inflammatory activity and the
release of other cytokines, and to stimulate the hepatic acute phase
response. Anti-inflammatory cytokines (including Il-4, Il-10, and
Il-13) suppress this inflammatory activity.
|
TABLE 22-1 Fat Embolus Syndrome: Gurd and Wilson’s Diagnostic Criteria
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
defined sequence of neutrophil chemotaxis and recruitment. This process
requires the expression of specific adhesion molecules by both the
neutrophil (CD11-b) and endothelial cell (intercellular adhesion
molecules [ICAM] and selectins) and is stimulated by the local
secretion of Il-8.50,97
Endothelial permeability is increased, and the neutrophil gains access
to the interstitial space by diapedesis, and then degranulates with the
release of further cytokines, reactive oxygen metabolites, and
proteases.20 These substances cause
the local disruption of cell membranes and connective tissue. In the
lung, the parenchyma becomes edematous, reducing oxygen diffusion from
the alveoli, impairing respiratory compliance, and generating the
typical radiographic appearance of acute lung injury: diffuse pulmonary
infiltration (Table 22-2).
|
TABLE
22-2 Criteria Stipulated by the American-European Consensus Document for the Diagnosis of Adult Respiratory Distress Syndrome10,11 |
||||||
|---|---|---|---|---|---|---|
|
the stress response to trauma. The extrinsic pathway of the coagulation
system is activated after injury by tissue hypoxia24
and the exposure of fat and subendothelial tissue factor, resulting in
thrombin and fibrin formation. This activation is further promoted by
the inflammatory response in two ways: (i) circulating TNFα, Il-1, and
Il-6 stimulate the expression of tissue factor and upregulate
fibrinogen production, resulting in a procoagulant response, and (ii)
the same cytokines also stimulate increased levels of plasminogen
activator inhibitor (PAI-1), which leads to inhibition of fibrinolysis,
resulting in an antifibrinolytic response.70,127
After injury, this locally serves to promote hemostasis. However,
systemic activation causes a shift in the dynamic equilibrium between
the stimulation and suppression of coagulation, and results in net
systemic coagulation (demonstrated by elevated prothrombin fragment and
fibrinogen levels), platelet activation (elevated β-thromboglobulin
levels), and fibrinolysis (elevated D-dimers).121
embolize in distal vascular beds (causing tissue ischemia and cell
death), and the consumption of clotting factors and platelets results
in a prolonged prothrombin time or even disseminated intravascular
coagulation.47 With the
transendothelial exudation of edema fluid, coagulation factors, and
cytokines, fibrin and fibrinogen deposition also occurs in the
extravascular space. As a result, platelets levels fall over the 24
hours after injury, while fibrinogen levels and prothrombin time rise
gradually over the first 4 days.47
Such coagulopathy during this period increases the risk of hemorrhage
and is an independent predictor of mortality after trauma.81
animal studies and the clinical implications are unclear, the
perception (or anticipation) of nociceptive stimuli is likely to be an
important component of the stress response.152.
However, ameliorating pain (aside from a clear humanitarian
requirement) probably also contributes to the control of the stress
response.
The clinical signs of hypoxemia are well recognized: agitated or
obtunded sensorium, tachypnea, tachycardia, and cyanosis. These signs
are easily confirmed by measurements of hemoglobin saturation and
arterial oxygen tension, which should be routinely monitored after
major trauma. A discrete underlying cause may be apparent on clinical
assessment (Table 22-3).
respiratory failure develops, which is refractory to oxygen therapy and
is not due solely to these treatable causes. The resulting hypoxemic
state has been variously termed FES, shock lung, neurogenic pulmonary
edema,122 acute lung injury, and
acute respiratory distress syndrome (ARDS). These terms have been
applied inconsistently and often interchangeably, and in some instances
probably reflect recognition of the same pathologic and clinical
process by those in disparate branches of medicine. The preferred
definitions
for this hypoxic state are provided by the American-European Consensus
Criteria, which distinguish acute lung injury and ARDS according to
degree of severity (see Table 22-2).
Management of ARDS, once developed, is currently entirely supportive
with use of high-inspired oxygen fractions and ventilator settings to
counter the poor compliance and reduced diffusion capabilities of the
stiff, heavy lung tissue26; however,
a number of treatment modalities have been tried (and rejected) in the
past. The administration of steroids (methylprednisolone) as “membrane
stabilizers” was initially promising, but sepsis proved to be a
significant complication, and subsequent modern studies have not
supported its use.1,12,75,86,138 Heparin transiently enjoyed widespread clinical use,8,123 despite the dangers of hemorrhage and rapid lipolysis, but its current role remains to be defined.74
Ethanol, which decreases lipolysis, and dextrose, which decreases free
fatty acid mobilization, have also been used empirically.53,133,138
|
TABLE 22-3 Causes of Hypoxemia after Trauma
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
immunology of sepsis, have suggested more focussed possible treatments,
including (in animal models) specific receptor antagonists to Il-1,95 antibodies to adhesion proteins CD 11 and ICAM, and cyclo-oxygenase inhibitors.30 Tissue plasminogen activator and antithrombin III also reduce lung injury in animal models of ARDS.7
Activated protein C (APC) has been shown to reduce mortality in septic
human patients, an effect which may be due to its promotion of
fibrinolysis or its direct inhibition of Il-1, Il-6, and TNFα
expression.64 Although APC increased
the risk of significant hemorrhage (which limits its applicability in
trauma), the success of this immunologic therapy raises the prospect of
more specific drug treatments in the future.
aim of the orthopaedic traumatologist should be to prevent the
development of ARDS wherever possible, by identifying patients who are
at risk, monitoring them closely, and ensuring that unstable fractures
of the pelvis and femur are stabilized promptly. The issues of how to
identify these patients accurately, and what constitutes their optimal
initial management, remain unclear.
Analysis of the Il-8 concentrations within bronchioalveolar lavage
fluid taken at the time of presentation has been shown to be indicative
of the risk of the later development of ARDS, but is invasive and
requires additional expertise in the emergency department.38
Urinary albumin excretion rate 8 hours after admission following trauma
has been shown to offer a high positive and negative predictive value
for the later development of ARDS and respiratory insufficiency, but is
not so discriminatory at the time of admission.97,98
It has been proposed that laboratory measurements of humoral
inflammatory and coagulation markers at the time of admission may allow
more convenient and precise identification of patients at increased
risk.48,120
circulation after trauma, tending to peak between 7 and 24 hours,
usually returning to normal after 3 to 4 days.51,93,110 The degree of elevation of Il-6 is associated with the severity of trauma:108,110 There is no increase in Il-6 after ankle fracture compared with normal levels (around 10 pg/mL-1), but levels increase to 50 pg/mL-1 after an isolated femoral fracture and nearly 600 pg mL-1 after multiple trauma with femoral fracture.108 Although Il-6 levels as high as 700 pg/mL-1 are observed in patients not suffering complications,108
there is accumulating circumstantial evidence that the degree of
elevation of these cytokines after injury correlates with the
likelihood of subsequent adverse outcome.93
Serum levels of Il-6, Il-8, and elastase are significantly higher among
those developing multiple organ dysfunction syndrome (MODS), and levels
are higher again in those dying of MODS93: Nast-Kolb93 reported that trauma patients developing MODS had a mean Il-6 at admission of over 700 pg/mL-1, compared with a mean level of less than 200 pg/mL-1 at admission for those patients not developing complications. Pape107,109 reported that Il-6 concentrations in excess of 500 pg/mL-1 were associated with the development of MODS.
levels are all raised at the time of admission. Increases in markers of
coagulation system activation are also reported after trauma, and the
degree of elevation is also associated with the development of
complications.46,64,93,120
Patients developing respiratory insufficiency have been shown to have
significantly greater perturbations in coagulation times, platelet
consumption, and the levels of β-thromboglobulins, prothrombin
fragments, and D-dimers than those that do not.120
activation are therefore attractive as surrogate outcome measurements
after trauma, and the identification of a discrete test or tests that
would correlate with clinical outcome would be highly desirable.48
However, the sensitivity, specificity, and relationship with clinical
complications remains undefined for analyses of this highly complex
system of cascades. Most importantly, cytokine levels have not been
shown to be of independent (or even additional) predictive importance
beyond standard clinical data at the time of admission. There is
insufficient experience with these techniques to base judgements or
management decisions on plasma cytokine assays, and these measurements
remain research tools at present.
suffer exaggerated systemic responses to injury, and a genetic basis
for such sporadic complications has been proposed.51
A few polymorphisms have been identified as important after injury. For
example, a single base-pair (4G/5G) polymorphism exists for the gene
that governs plasma concentrations of PAI-1 and influences
inflammation. In a striking study of 61 comparable trauma patients, it
was shown that the homozygous 4G/4G genotype resulted in increased
levels of PAI-1, with a mortality of 51%, whereas the mortality of the
heterozygous 4G/5G genotype was only 28% and of the 5G/5G genotype,
only 15%.88
and several proinflammatory polymorphisms have been identified in
relation to autoimmune, inflammatory, and neoplastic disease; these may
also have an influence in the posttraumatic state.49,116,135,154
trauma patients have resulted in a marked fall in the incidence of
respiratory insufficiency from as much as 22% of trauma admissions in
the 1960s and 1970s, when much of the published work on FES was
produced,119 to below 5% in recent
studies. Many factors are likely to be of importance in this falling
incidence, including better prehospital care, more rapid (and
aggressive) resuscitation protocols, and improved intensive medical
supportive therapy. Major advances in fracture management have also
occurred over the past 4 decades, from a conservative approach
involving traction and plaster cast immobilization, to an
interventional strategy involving operative internal stabilization and
early patient rehabilitation.
stabilization of long bone (principally femoral) fractures reduces
mortality. Early and delayed stabilization were directly compared in
several retrospective reviews9,18,28,33,68,86,115,123,131,141,143 and a small prospective study.78
The risk of ARDS was demonstrated to be around five times higher in
patients who had stabilization delayed beyond 24 hours. The influential
prospective randomized study by Bone et al.17
confirmed a decreased risk of ARDS, FES, pulmonary emboli, and
pneumonia in those patients with multiple injuries undergoing
stabilization of all long bone fractures within 24 hours of injury, and
the concept of early surgical stabilization of major fractures has now
been widely accepted. There is strong biologic support for this
concept: delayed stabilization of fractures results in prolonged
activation of the coagulation and complement responses, which then
rapidly decrease towards normal following surgical stabilization.130
is termed the “first hit.” In addition to this direct response, the
first hit also results in neutrophil “priming.” A primed neutrophil
generates a more intense reaction to a subsequent stimulus,20,21,97,111,157 termed the “second hit.” This priming response may persist for more than 24 hours after the first hit.21
is a potent potential second hit and intensifies the inflammatory
response.51,109 The nailing of femoral fractures in particular causes an increase in circulating levels of elastase and Il-6.51 The magnitude of this secondary response is related to the extent of the first hit109 and thus is more marked in severely injured patients and those with persisting physiologic instability.59
In comparisons of surgical strategies in physiologically stable
patients, immediate intramedullary nailing raised Il-6 levels from 55
to 250 pg/mL-1, while primary external fixation resulted in no such increase.105
reduces the incidence of posttraumatic respiratory insufficiency,
nailing also theoretically provides the circumstances for the
exacerbation of the stress response and lung injury. Refinements in the
technique of nailing have been sought in order to minimize this second
hit. Animal and surrogate end-point studies have suggested that the use
of unreamed intramedullary nails,51,107 altered reamer design,102 faster reamer revolutions with slower introduction of the reamer,92 and venting of the distal fragment83
may result in less severe pulmonary injury. However, no advantage has
been substantiated by clinical studies, and much interest centers on
whether there is an association between this second hit response
detected using surrogate outcome measurements and clinically relevant
respiratory insufficiency, and whether such complications can be
diminished by pharmacologic therapy or by reducing the severity of the
second hit.
remain physiologically unstable despite initial resuscitation are
unsuitable for prolonged or extensive immediate surgery. Scalea and his
coauthors128 first proposed that
these patients should undergo “damage control orthopaedic surgery”
(DCO): a rapid, minimally invasive stabilization procedure
(effectively, the external fixation of femoral shaft fractures and
mechanically unstable pelvic fractures) followed by a period of
resuscitation and physiologic stabilization in the intensive therapy
unit, before undergoing later conversion to internal fixation. These
proposed patients include those with head and thoracic injuries, and
the “lethal triad” of hypoxemia, hypothermia, and coagulopathy.105,106,114
It has not been possible to establish clear, evidenced-based criteria
for the use of damage control techniques. Moreover, there are a number
of potential disadvantages, including the requirement for two operative
procedures, and the morbidity often resulting from definitive external
fixation of femoral fractures in those who do not undergo conversion.
Proposed applications of DCO include the following:
-
Physiologic instability:
An empirical categorization of patients into four categories has been
proposed: stable, borderline, unstable, and in extremis. The precise
definition of each category, particularly that of the borderline
patient, has tended to vary in the literature (Table 22-4).
A detailed description based on the grades of shock identified by
advanced trauma life support and elements of the lethal triad measured
in the resuscitation room has been proposed (Table 22-5).104 This paradigm proposes the use of standard
P.595internal fixation for stable patients and the use of DCO techniques
(external fixation) for patients who are unstable or are in extremis.
The management of the “borderline” patient is with standard techniques
if the patient is stable on review during resuscitation or with DCO
techniques if their response is uncertain. A retrospective study has
suggested that use of these techniques may result in improved outcomes,
but this has not been confirmed elsewhere and the issue remains
contentious.85,100,106 -
Thoracic injury:
Of particular interest has been the potential application of DCO
techniques in the management of patients with femoral fractures in
association with significant thoracic injuries. A direct thoracic
injury is three times more likely to be associated with respiratory
failure than is a long bone fracture.120 After a femoral fracture, an additional thoracic injury imparts a greater risk of pulmonary complications.101,103
Interest has centered on the converse question: whether a patient with
thoracic injury is at any additional risk when this injury is
accompanied by a femoral fracture.101
Contused, atelectatic, or collapsed regions of lung are hemorrhagic and
edematous and have a reduced capillary bed perfusion. Therefore, in the
presence of lung injury, the pulmonary blood flow may be directed to a
smaller volume of parenchyma, delivering a greater concentration of
fat, thrombus, and inflammatory cytokines, and thus a long bone injury
might be expected to exacerbate a thoracic injury. However, the
majority of studies15,17,19,22,32,145,146 and a meta-analysis120
report that the additional femoral fracture (however treated) is
inconsequential in precipitating respiratory insufficiency, and it is
the thoracic injury rather than the femoral fracture which determines
overall risk. -
Head injury:
Head injuries represent a similar potential exacerbating injury. The
concept of secondary brain injury as a result of systemic hypotension
or hypoxia is well established, and these patients might be expected to
benefit from the application of DCO principles. However, the available
evidence does not confirm this concept. Again, it appears that the head
injury itself defines prognosis, and that the addition of a femoral
fracture (however treated) does not influence outcome.14,84
|
TABLE 22-4 Characteristics of the Borderline Trauma Patient104
|
|||||
|---|---|---|---|---|---|
|
|
TABLE 22-5 Clinical Parameters Influencing Management after Major Trauma
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
commences with the release of fat, thrombus, and cytokines from the
site of injury to the systemic circulation and a neuroendocrine
response. Respiratory insufficiency is an early and prognostically
important consequence. It is largely accepted that multiply injured
patients should undergo rapid resuscitation followed by early
immobilization of major injuries, particularly pelvic and femoral
fractures, within 12 to 24 hours. Although DCO is an attractive,
rational concept and is probably as safe as definitive femoral nailing,142
there is as yet no clinical evidence that it confers any advantage in
terms of survival or outcome, and there are several inherent
disadvantages. There remains a requirement for a large-scale
prospective randomized clinical trial.
orthopaedic trauma surgeon in a facility equipped and familiar with
contemporary orthopaedic trauma management. The majority of injuries
should be treated in the first 24 hours after injury, with long bone
fractures of the lower limbs being stabilized with locked
intramedullary nails. Where there is on-going physiologic instability
during resuscitation, as measured by persistent grade III shock,
coagulopathy, and hypothermia (the lethal triad), consideration should
be given to rapid stabilization of femoral fractures and unstable
pelvic fractures with an external fixator (DCO) to allow for further
resuscitation in the intensive care setting. Definitive fixation is
then provided once these elements of instability have been resolved.
crush injury, compartment syndrome, vascular occlusion, or unprotected
immobilization resulting in prolonged muscle compression. These
factors, and injury sustained from reperfusion of ischemic tissue, may
result in “crush syndrome,” a systemic complication characterized by
tense, edematous, painful muscles, dark-tea-colored urine, shock, and
acidosis.30 The history and a
positive urine dipstick test, indicating the presence of hemoglobin in
the absence of red blood cells on microscopy, should raise the
suspicion of irreversible muscle injury (rhabdomyolysis), which can be
confirmed by elevated plasma creatine kinase (CK) levels. Muscle can
tolerate short periods (up to 2 hours) of complete ischemia, but after
longer periods rhabdomyolysis occurs.58
Partial ischemia may be more injurious and produce more severe systemic
complications due to the constant leakage of metabolic by-products of
injured myocytes. In a limb with total vascular occlusion, the systemic
injury occurs at the time of reperfusion. After 3 hours of ischemic
time, reperfusion results in a localized inflammatory response and
edema due to increased capillary permeability. Calcium-mediated local
cellular injury results in the release of myoglobin and potassium into
the systemic circulation. Patients with rhabdomyolysis are at risk of
acute renal failure (ARF) for the following reasons: intravascular
hypovolemia as a result of localized intramuscular sequestration of
plasma, precipitation of myoglobin in glomerular filtrate resulting in
tubular obstruction, and renal ischemia due to the release of potent
vasoconstrictors.
develop renal failure. Prediction of patients at risk of ARF as a
result of rhabdomyolysis is difficult. Admission serum CK
concentrations cannot be used to predict who will develop renal failure
after crush injury. Despite evidence that the mean CK level in this
group is significantly higher, no suitable cut-off has been identified36;
however, laboratory measurement of serum bicarbonate levels has been
shown to be accurate in predicting who will develop renal failure
especially in the presence of myoglobinuria. If the admission
bicarbonate level is less than 17 mml/L, then the risk of developing
renal failure is high.93
prevention of muscle injury and prevention of ARF. Restoration of blood
flow to muscle compartments by treatment of the causative factor must
be prompt (ischemia of longer than 6 hours is associated with a less
favorable outcome).89 Direct
vascular injury may require emergency repair or bypass, and skeletal
injuries must be stabilized. Fasciotomy may be indicated for
compartment syndrome, although there is debate concerning the wisdom of
reperfusion following prolonged ischemia.13,45
Amputation may be indicated to protect the patient from the systemic
injury of reperfusion. The Mangled Extremity Severity Score may help
identify nonsalvageable extremities.67
injury to prevent ARF is effective fluid resuscitation to correct the
hypotensive renal ischemia. After significant crush injury, serum
myoglobin levels fall exponentially following removal of the causative
factor even in the absence of renal function or hemodialysis.149
High-volume diuresis by crystalloid infusion is indicated in these
patients and central venous pressure recording may be required in those
with cardiac impairment to avoid overload. An initial bolus of 1 L of
normal saline followed by an infusion of normal saline at 200 to 700
mL/hr has been proposed.125 Other measures include the use of mannitol to create an osmotic diuresis78
and alkalinization of the urine with sodium bicarbonate to prevent
tubular cast formation; however, the value of these additional
interventions has been questioned, with no apparent benefit seen in one
retrospective review of intensive care unit patients.27
High-volume crystalloid infusion alone may suffice. If renal failure
develops, renal dialysis or hemofiltration may be required. Mortality
from crush syndrome is threefold higher (59%) in those who develop ARF.36
the triad of factors responsible for venous thrombosis in 1856: venous
stasis, vascular damage, and hypercoagulability. All three are
frequently encountered following trauma, especially following
high-energy injuries (Fig. 22-2).
Orthopaedic trauma patients, in particular those requiring hospital
admission, are often at risk of venous thrombosis and pulmonary
embolism.5
The estimate of that risk varies considerably and is dependent on the
definition, mode of identification, and the nature of the study
population’s injuries. Most studies employ the outcome measure of deep
vein thrombosis (DVT) identified on venography, duplex ultrasound,
radioisotope uptake, or plethysmography as a surrogate for clinical
DVT, placing the incidence between 6% and 60%. The incidence of
symptomatic venous thromboembolism (VTE) following hip fracture from
recent studies using chemical prophylaxis is 1% to 2%.4,40
Fatal pulmonary embolism occurs in approximately 0.3% of hip fracture
patients undergoing modern treatment including early mobilization and
chemical thromboprophylaxis.
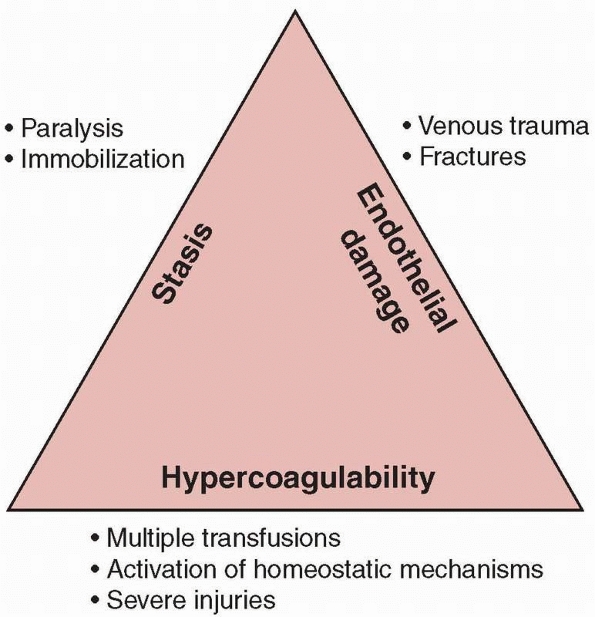 |
|
FIGURE 22-2 Virchow triad.
|
developing VTE is increased in those with spinal cord injury, fractures
of the femur or tibia, those requiring surgery or a blood transfusion,
and with increasing age.48 Immobilization for more than 3 days has also been shown to increase the risk of VTE,72
but it is not clear whether the factors outlined above are independent
of this. Inheritance of a genetic predisposition to thrombus formation
(thrombophilia) may increase the risk for an individual but
cost-benefit analysis does not support trauma population screening.25
history of venous thrombosis and consider postoperative anticoagulation
for those with a positive history, although the latter has been shown
to be a poor predictor of the presence of thrombophilia.129
Approximately 10% of patients with hip fractures will have evidence of
asymptomatic DVT using surrogate measures on admission to hospital. If
admission is delayed by more than 48 hours from the time of the injury,
then the incidence of DVT has been reported to be as high as 55%.61
Importantly, the incidence of DVT for hospitalized patients with a
delay greater than 48 hours between injury and surgery is associated
with a similarly high (62%) incidence of DVT on ascending venography
despite chemical prophylaxis with unfractionated heparin initiated on
admission.156
using a combination of history, clinical signs, clinical probability
assessment, and imaging techniques. Assessment tools such as the Wells
scoring system rely on factors including calf or thigh swelling,
localized tenderness along the distribution of the deep venous system,
unilateral pitting edema, recent history of surgery or immobilization,
and personal or family history of VTE.151
Fibrin D-dimer testing has been employed to exclude the diagnosis of
VTE in ambulatory patients in whom the diagnosis is suspected; however,
in the seriously injured patient or postoperative period, the
sensitivity and specificity have been shown to be too low to guide
diagnosis.148
which is regarded as the criterion standard, in diagnosis of DVT
proximal to the popliteal region and has the advantage of being
noninvasive and repeatable.73,144 Computed tomography (CT) venography has been shown to be less accurate than ultrasonography for the diagnosis of DVT.
chest pain and hemoptysis, with right-sided heart failure, syncope, and
hypotension if the embolus is very large. Electrocardiogram findings
are abnormal but nonspecific in 70% of patients with PE, the most
common finding being a sinus tachycardia. Chest radiographs and blood
gas analysis help to exclude alternative causes of dyspnea. Pulmonary
angiography is the criterion standard in the diagnosis of PE. CT
pulmonary angiography has been shown to be superior to
ventilation-perfusion lung scanning2
and is now the most widely used imaging modality with a specificity of
96% and sensitivity of 83%, which can be increased by the addition of
venous phase imaging.138 There are concerns about the clinical significance of some smaller lesions identified on modern high-resolution scanners.
patients surviving only 20 minutes from the onset of symptoms, which is
why efforts are aimed at thromboembolic prophylaxis.
mobilization, avoiding hemoconcentration by maintaining adequate
hydration, and chemical and physical prophylaxis. Numerous
investigators have studied the effectiveness of chemical prophylaxis at
reducing the incidence of VTE following hip fracture surgery using
surrogate asymptomatic DVT as the primary outcome measure but the
picture remains unclear. Level 1 placebo-controlled studies have shown
the use of low-molecular-weight heparin (LMWH) to be effective in
reducing the surrogate DVT rate up to 9 days following surgery.69
Fondaparinux sodium has been found to be more effective than LMWH in
reducing the asymptomatic VTE rate but at the cost of higher
significant postoperative bleeding.40 The addition of mechanical prophylaxis may reduce the rate of DVT further for those on LMWH.136
A large randomized study has demonstrated significant reduction in the
incidence of symptomatic VTE with the use of aspirin for
chemoprophylaxis when compared to placebo.4
Concerns have been raised about this study, in particular over the use
of other chemoprophylactic agents for some patients enrolled in the
trial. No study has yet shown evidence of a significant reduction in
fatal PE with any prophylactic regime, and in many studies there are no
significant differences in symptomatic VTE events.
prophylaxis is that the end-point is selected during the initial
inpatient stay, typically day 7 to 10, whereas there is some evidence
that the risk to the patient may be greatest in the period between
discharge and 3 months. The concept of a rebound thrombophilia on
cessation of chemoprophylaxis at discharge has prompted investigators
to look at the effect of extended prophylaxis. One level 1 study has
shown significant reduction in asymptomatic and symptomatic VTE events
with fondaparinux sodium continued up to 23 days compared to only
inpatient fondaparinux prophylaxis41
after hip fracture surgery. Again, there was a trend toward an increase
in major bleeding. Mechanical devices, including foot or calf
compression devices and inferior vena cava filters, avoid the risk of
anticoagulation and can be used when chemical prophylaxis is
contraindicated (e.g., patients with head injury or abdominal injury).
Few studies demonstrate any significant differences in VTE rate with
mechanical prophylaxis compared to chemical prophylaxis, but they may
be associated with a significant reduction in asymptomatic events when
used in combination.46 Inferior vena cava filters do not eliminate the risk of PE and may increase the risk of recurrent DVT and VTE37;
however, no pulmonary embolic events were reported in a retrospective
study of 56 orthopaedic patients with removable caval filters.140 Graded venous compression stockings have not been shown to reduce the DVT rate when used as an adjunct to chemical prophylaxis,34 and
patient compliance is poor. Most clinicians treat DVT and PE with oral
adjusted dose warfarin for a minimum of 3 months; alternatively, DVT
can be treated with appropriate doses of LMWH.
patients with ankle fractures with cast immobilization) varies widely.
Recent evidence suggests that the risk is low, with 5 of 100 patients
with stable ankle fractures treated in below-knee plaster cast having
DVT diagnosed on color Doppler duplex ultrasound.132
Two were proximal to the calf and none were symptomatic. Chemical
prophylaxis has not been shown to reduce the risk of DVT in this
ambulatory group.74
evidence and institutions’ recommendations vary considerably. In
attempts to harmonize practice, many groups have published guidelines
based on scientific critique of the literature. Unfortunately, some of
these recommendations have added to the controversy.
remains contentious. While guidelines help to standardize management
and improve prescribing compliance, each patient should be assessed
individually. This assessment should include consideration of the
individual’s preinjury risk factors (increased risk with increasing
age, obesity, oral contraceptive pill, malignancy), injury sustained
(increased risk with spinal cord injury, femur/tibial fracture, the
need for surgery or transfusion), and postoperative rehabilitation
(increased risk with immobilization greater than 3 days). Equal weight
should be given to the risk to the patient of chemical
thromboprophylaxis. Patients with an increased bleeding risk should be
managed with mechanical prophylaxis devices. Those patients considered
to have a high risk of venous thrombosis and a low risk of bleeding
should be managed with mechanical prophylaxis and chemical prophylaxis
which should be continued until 4 weeks after surgery or until the
patient is ambulant, whichever is longer.
should be investigated with duplex ultrasound for DVT or CT pulmonary
angiography with venous phase imaging for PE depending on the clinical
picture. Where venous thrombosis is demonstrated, the patient should be
commenced on therapeutic warfarin for a minimum of 3 months.
(+/- 0.4°C). There is diurnal variation in body temperature with a peak
in the late afternoon and trough in the early morning.80
Fever, which is typically defined as a core body temperature greater
than 38.0°C, is a normal physiologic adaptation in response to cytokine
and prostaglandin-mediated stimuli. The hypothalamus is responsible for
thermoregulation, controlling heat loss in response to metabolic heat
production, and the surrounding ambient environment. It has no contact
with circulating pathogens or inflammatory factors because of the blood
brain barrier, but receives prostaglandin E2 mediated
signals from the circumventricular organ system that is in contact with
the systemic circulation. While fever is clearly an adaptive response
that presumably affords some survival advantage, there is uncertainty
as to what these benefits might be. Indirect evidence suggests that it
may enhance the immunologic response to infection or impede replication
of some microorganisms.39 Fever is
most commonly triggered in response to an infectious pathogen or its
toxins; however, in the perioperative period, there are a number of
other factors that may produce a rise in core body temperature.
and include blood transfusion, hematoma, and pulmonary atelectasis.
Administration of antipyrogens for patient comfort should suffice, and
the fever will usually settle within a few days. Fever developing
around day 5 is often as a result of infectious pathogen.
Catheter-associated urinary tract infection is the most common cause,
responsible for over 40% of all acquired infections in the
postoperative phase. Infecting organisms are introduced at the time of
catheter insertion or ascend from the perineum adjacent to the
catheter, and most originate from the patient’s own cutaneous flora or
from the hands of healthcare workers. Avoidance or prompt removal of
in-dwelling urinary and vascular catheters can prevent nosocomial
infection. Pyrexia developing 5 to 7 days after surgery may suggest a
surgical site infection. A mandatory survey of surgical site infection
in England carried out between October 1997 and December 2003 found
that the rate of infection following hip hemiarthroplasty was 4.9% (95%
confidence interval [CI] 4.6 to 5.3) and following open reduction of a
fracture was 3.8% (95% CI 3.3 to 4.3).60
management of a patient with postoperative pyrexia. A thorough clinical
examination,
including
chest auscultation, may suggest a cause. Plain chest radiography,
culture of urinary specimens, and blood cultures may aid the diagnosis
and identify the infecting organism to guide treatment. In addition,
elevated serum levels of C-reactive protein and the erythrocyte
sedimentation rate may support the diagnosis or at least provide a
baseline to monitor progress. There is some evidence that persistently
elevated serum Il-644
levels may be more specific for infection in the early postoperative
phase, but this test is not widely available. The value of wound swabs
when surgical site infection is suspected has been questioned (see Chapter 24).23 In noncatheterized patients, a count of 105
organisms per mL of urine is used as the criterion for the diagnosis of
a urinary tract infection. In catheterized patients, it has been shown
that a count of 103 microorganisms per mL is a sensitive cut-off.137
|
TABLE 22-6 Causes of Perioperative Pyrexia
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
cause of postoperative pyrexia. Adults at greatest risk are those over
65 years, those with serious comorbidities, immunosuppression,
depressed sensorium, malnutrition, and those subject to
thoracoabdominal trauma or surgery. Those receiving mechanical
ventilation make up a small proportion of patients with nosocomial
pneumonia but are at high risk, and the development of
ventilator-associated pneumonia is a poor prognostic marker with
mortality rates up to 10 times greater for this group than ventilated
patients without pneumonia.117
Ventilator-associated pneumonia also typically occurs 4 to 5 days after
intubation. Most bacterial infections occur secondary to aspiration of
pathogens that colonise the oropharynx or upper gastrointestinal tract
and are frequently polymicrobial with Gram-negative bacilli as the
predominant organism. The diagnosis is made on the basis of pyrexia,
cough, and purulent sputum with the presence of radiographic evidence
of new or progressive pulmonary infiltrate. Sputum cultures, tracheal
aspirate, pleural aspiration, and blood cultures may help to identify
the responsible organisms to guide treatment. Preventative measures
include decreasing aspiration by the patient, hand washing by
healthcare workers, appropriate decontamination of ventilatory devices,
and use of available vaccines.
preinjury level of function. Patients are often restricted in their
level of mechanical or social function, cognitive ability, and ability
to care for and provide for themselves and their families. With the
more frequent use of patient-centered outcome measurements, it has
become possible to measure the long-term sequelae of major trauma more
easily.
implications for subsequent physical, cognitive, psychologic, and
social function, and the specialty of rehabilitation medicine has
developed to provide for the requirements of this group of patients.95
More generally, survivors of critical illness, particularly ARDS, are
also known to suffer from depression and psychologic morbidity.35
However, it is also clear that secondary brain injury resulting from
purely orthopaedic injuries can have substantial long-term cognitive
consequences, particularly where these deficits are sought. Recent case
reports and retrospective reviews have detailed deficits in working
memory, executive functioning, verbal fluency, and mental processing
speed, along with anxiety, depression, and posttraumatic stress
disorder occurring after trauma in the absence of head injury.55,66
local injuries to nerve or muscle, a profound and persisting weakness
can occur after trauma, termed critical illness polyneurop-athy. This
condition may operate at the level of the nerve, motor end plate, or
muscle, with proposed etiologies including compression, disuse atrophy,
inflammation, and pharmacologic neuromuscular blockade, and may persist
indefinitely despite extensive rehabilitation, resulting in loss of
function and independence.3,62
DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary
angiography vs. ventilation-perfusion lung scanning in patients with
suspected pulmonary embolism: a randomized controlled trial. JAMA
2007;298:2743-2753.
MJ, Bril V, Shannon P, et al. Neuromuscular function in survivors of
the acute respiratory distress syndrome. Can J Neurol Sci
2007;34:427-432.
Prevention of pulmonary embolism and deep vein thrombosis with low-dose
aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000;355:
1295-1302.
Risk of and prophylaxis for venous thromboembolism in hospital
patients. Thromboembolic Risk Factors (THRIFT) Consensus Group. BMJ
1992;305:567-574.
PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and
arterial hypoxia in patients with fat embolism. J Trauma
1971;11:1026-1030.
RA, Jacobs RF, Tryka AF, et al. Effects of ibuprofen on neutrophil
function and acute lung injury in canine endotoxin shock. Crit Care Med
1988;16:1121-1127.
SW, Fabian TC, Kudsk KA, et al. Improved outcome with femur fractures:
early vs. delayed fixation. J Trauma 1990;30:792-797.
GR, Artigas A, Brigham KL. The American-European Consensus Conference
on ARDS; definitions, mechanisms, relevant outcomes, and clinical trial
coordination. Am J Resp Crit Care Med 1994;149:818-824.
GR, Artigas A, Brigham KL, et al. Report of the American-European
Consensus conference on acute respiratory distress syndrome:
definitions, mechanisms, relevant outcomes, and clinical trial
coordination. Consensus Committee. J Crit Care 1994;9: 72-81.
GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients
with the adult respiratory distress syndrome. N Engl J Med
1987;317:1565-1570.
OS, Stein JH. Early management of shock and prophylaxis of acute renal
failure in traumatic rhabdomyolysis. N Engl J Med 1990;322:825-829.
M, Guyatt GH, Khera V, et al. Operative management of lower extremity
fractures in patients with head injuries. Clin Orthop Relat Res
2003;407:187-198.
LB, Anders MJ, Rohrbacher BJ. Treatment of femoral fractures in the
multiply injured patient with thoracic injury. Clin Orthop Relat Res
1998;347:57-61.
LB, Babikian G, Stegemann PM. Femoral canal reaming in the polytrauma
patient with chest injury. A clinical perspective. Clin Orthop Relat
Res 1995;318:91-94.
LB, Johnson KD, Weigelt J, et al. Early versus delayed stabilization of
femoral fractures. A prospective randomized study. J Bone Joint Surg Am
1989;71:336-340.
MJ, MacKenzie EJ, Riemer BL, et al. Adult respiratory distress
syndrome, pneumonia, and mortality following thoracic injury and a
femoral fracture treated either with intramedullary nailing with
reaming or with a plate. A comparative study. J Bone Joint Surg Am
1997;79:799-809.
AJ, Moore FA, Moore EE, et al. Early neutrophil sequestration after
injury: a pathogenic mechanism for multiple organ failure. J Trauma
1995;39:411-417.
AJ, Moore FA, Moore EE, et al. Postinjury neutrophil priming and
activation: an early vulnerable window. Surgery 1995;118:358-364.
BR, Stephen D, Brenneman FD. Thoracic trauma and early intramedullary
nailing of femur fractures: are we doing harm? J Trauma 1997;43:24-28.
PG, Duerden BI, Armstrong DG. Wound microbiology and associated
approaches to wound management. Clin Microbiol Rev 2001;14:244-269.
K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism,
identification, and effect. Curr Opin Crit Care 2007;13:680-685.
JL, Veeger NJ, Kluin-Nelemans HC, et al. The pathogenesis of venous
thromboembolism: evidence for multiple interrelated causes. Ann Intern
Med 2006;145: 807-815.
RG, Lanken PN, MacIntyre N, et al. Higher-versus lower-positive
end-expiratory pressures in patients with the acute respiratory
distress syndrome. N Engl J Med 2004;351:327-336.
CV, Rhee P, Chan L, et al. Preventing renal failure in patients with
rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma
2004;56:1191-1196.
SI, McGhan R, Jurkovich GJ, et al. Timing of femur fracture fixation:
effect on outcome in patients with thoracic and head injuries. J Trauma
2002;52:299-307.
KE, Selig WM, Beeler DA, et al. Effect of heparin on increased
pulmonary microvascular permeability after bone marrow embolism in
awake sheep. Am Rev Respir Dis 1987;136:134-141.
EG, Delory GE, Rimington C, et al. Myohaemoglobin in the urine of air
raid casualties with crushing injury. Biochem J 1941;35:1164-1168.
PD, Leeper-Woodford SK, Walsh CJ, et al. Delayed cyclo-oxygenase
blockade reduces the neutrophil respiratory burst and plasma tumor
necrosis factor levels in sepsis-induced acute lung injury. J Trauma
1991;31:733-740.
DW, Rodman GH Jr, Kaehr D, et al. Femur fractures in chest-injured
patients: is reaming contraindicated? J Orthop Trauma 1998;12:164-168.
WE, Fabian TC, Croce MA. Delayed surgical fixation of femur fractures
is a risk factor for pulmonary failure independent of thoracic trauma.
J Trauma 1994;37: 667-672.
AT, Skinner JA, Warwick D, et al. The use of graduated compression
stockings in association with fondaparinux in surgery of the hip. A
multicenter, multinational, randomized, open-label, parallel-group
comparative study. J Bone Joint Surg Br 2007; 89:887-892.
TA, Caldwell ES, Curtis JR, et al. Reduced quality of life in survivors
of acute respiratory distress syndrome compared with critically ill
control patients. JAMA 1999; 281:354-360.
Meijer AR, Fikkers BG, de Keijzer MH., et al. Serum creatine kinase as
predictor of clinical course in rhabdomyolysis: a 5-year intensive care
survey. Intensive Care Med 2003;29:1121-1125.
H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval
filters in the prevention of pulmonary embolism in patients with
proximal deep-vein thrombosis. Prevention du Risque d’Embolie
Pulmonaire par Interruption Cave Study Group. N Engl J Med
1998;338:409-415.
SC, Strieter RM, Kunkel SL, et al. Interleukin-8 and development of
adult respiratory distress syndrome in at-risk patient groups. Lancet
1993;341:643-647.
BI, Bauer KA, Lassen MR, et al. Fondaparinux compared with enoxaparin
for the prevention of venous thromboembolism after hip-fracture
surgery. N Engl J Med 2001;345:1298-1304.
BI, Lassen MR. Duration of prophylaxis against venous thromboembolism
with fondaparinux after hip fracture surgery: a multicenter,
randomized, placebo-controlled, double-blind study. Arch Intern Med
2003;163:1337-1342.
TC, Hoots AV, Stanford DS, et al. Fat embolism syndrome: prospective
evaluation in 92 fracture patients. Crit Care Med 1990;18:42-46.
K, Pargger H, Muller W, et al. Interleukin-6 and acute-phase protein
concentrations in surgical intensive care unit patients: diagnostic
signs in nosocomial infection. Crit Care Med 1993;21:1175-1180.
S, Heyse T, Rudofsky G, et al. Continuous passive motion in the
prevention of deep-vein thrombosis: a randomized comparison in trauma
patients. J Bone Joint Surg Br 2005;87:1117-1122.
WH, Code KI, Jay RM, et al. A prospective study of venous
thromboembolism after major trauma. N Engl J Med 1994;331:1601-1606.
PV, Hildebrand F, Pape HC. Inflammatory serum markers in patients with
multiple trauma. Can they predict outcome? J Bone Joint Surg Br
2004;86:313-323.
PV, Smith RM, Bellamy MC, et al. Stimulation of the inflammatory system
by reamed and unreamed nailing of femoral fractures. An analysis of the
second hit. J Bone Joint Surg Br 1999;81:356-361.
PV, Smith RM, Windsor AC, et al. Monocyte human leukocyte antigen-DR
expression correlates with intrapulmonary shunting after major trauma.
Am J Surg 1999;177:454-459.
RJ, Gimbrere JS, van Niekerk JL, et al. Early osteosynthesis and
prophylactic mechanical ventilation in the multitrauma patient. J
Trauma 1982;22:895-903.
AC, Torrens L, White TO, et al. The cognitive effects of fat embolus
syndrome following an isolated femoral shaft fracture. A case report. J
Bone Joint Surg Am 2007; 89:1092-1096.
PJ, Giannoudis PV, van Griensven M, et al. Alterations in the systemic
inflammatory response after early total care and damage control
procedures for femoral shaft fracture in severely injured patients. J
Trauma 2005;58:446-452.
FG Jr, Nelson CL, Puskarich-May CL. Effect of delayed admission to the
hospital on the preoperative prevalence of deep-vein thrombosis
associated with fractures about the hip. J Bone Joint Surg Am
1996;78:581-583.
MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the
acute respiratory distress syndrome. N Engl J Med 2003;348:683-693.
JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of
medical intensive care unit patients. Crit Care Med 2003;31:1226-1234.
K, Daines M, Howey T, et al. Objective criteria accurately predict
amputation following lower extremity trauma. J Trauma 1990;30:568-572.
KD, Cadambi A, Seibert GB. Incidence of adult respiratory distress
syndrome in patients with multiple musculoskeletal injuries: effect of
early operative stabilization of fractures. J Trauma 1985;25:375-384.
PS, Knudsen JB, Broeng L, et al. The thromboprophylactic effect of a
lowmolecular-weight heparin (Fragmin) in hip fracture surgery. A
placebo-controlled study. Clin Orthop Relat Res 1992;278:95-100.
LJ, de Bri E, Ponzer S, et al. High sensitivity with color duplex
sonography in thrombosis screening after ankle fracture surgery. J
Thromb Haemost 2006;4:807-812.
LJ, Ponzer S, Elvin A, et al. Prolonged thromboprophylaxis with
Dalteparin during immobilization after ankle fracture surgery: a
randomized, placebo-controlled, double-blind study. Acta Orthop
2007;78:528-35.
BG, Schoeman HS, Dommisse GF, et al. Fat embolism and the fat embolism
syndrome. A double-blind therapeutic study. J Bone Joint Surg Br
1987;69:128-131.
J, Deno DC, Feustel PJ, et al. Pulmonary and cardiovascular
consequences of immediate fixation or conservative management of
long-bone fractures. Arch Surg 1986;121:992-999.
PA, Wasserman SS, Levine MM. A critical appraisal of 98.6°F, the upper
limit of the normal body temperature, and other legacies of Carl
Reinhold August Wunderlich. JAMA 1992;268:1578-1580.
K, Futami S, Nishida M, et al. Effects of trauma and sepsis on soluble
L-selectin and cell surface expression of L-selectin and CD11b. J
Trauma 1998;44: 460-468.
R, Leighton RK, Petrie D, et al. Effect of proximal and distal venting
during intramedullary nailing. Clin Orthop Relat Res 1996;332:80-89.
MD, Schemitsch EH, Vincent LO, et al. The effect of a femoral fracture
on concomitant closed head injury in patients with multiple injuries. J
Trauma 1997;42: 1041-1045.
RN, Vivoda EE, Pirani S. Comparison of mortality of patients with
multiple injuries according to type of fracture treatment—a
retrospective age- and injurymatched series. Injury 1986;17:2-4.
T, Hermans PW, Little SG, et al. Plasminogen-activator-inhibitor-1
4G/5G promoter polymorphism and prognosis of severely injured patients.
Lancet 2001;357: 1096-1097.
BR, Boyd DW, Andring RE. Clinically inapparent hypoxemia after skeletal
injury. The use of the pulse oximeter as a screening method. Clin
Orthop Relat Res 1993; 293:269-273.
JR, Smith RM, Pape HC, et al. Stimulation of the local femoral
inflammatory response to fracture and intramedullary reaming: a
preliminary study of the source of the second-hit phenomenon. J Bone
Joint Surg Br 2008;90:393-399.
M, David R, Schwendenwein I, et al. Influence of controlled reaming on
fat intravasation after femoral osteotomy in sheep. Clin Orthop Relat
Res 2002;394: 263-270.
DJ, Moodley M, Naidu AG, et al. Prediction of acute renal failure
following softt-issue injury using the venous bicarbonate
concentration. J Trauma 1992;33:813-817.
D, Waydhas C, Gippner-Steppert C, et al. Indicators of the
posttraumatic inflammatory response correlate with organ failure in
patients with multiple injuries. J Trauma 1997;42:446-454.
K, Thu A, Turner-Stokes L. Complex specialized rehabilitation following
severe brain injury: a UK perspective. J Head Trauma Rehabil
2007;22:239-247.
K, Bjork P, Bergenfeldt M, et al Interleukin-1 receptor antagonist
reduces mortality from endotoxin shock. Nature 1990;348:550-552.
I, Dent C, Topley N. Increased neutrophil migratory activity after
major trauma: a factor in the etiology of acute respiratory distress
syndrome? Crit Care Med 2002; 30:1717-1721.
I, Dent C, Wise CC, et al. Early posttraumatic acute respiratory
distress syndrome and albumin excretion rate: a prospective evaluation
of a “point-of-care” predictive test. Injury 2001;32:177-181.
I, Gosling P, Alpar K, et al. Prediction of posttraumatic adult
respiratory distress syndrome by albumin excretion rate 8 hours after
admission. J Trauma 1997;42: 1056-1061.
HC. John Border Memorial Lecture controversy comment. Fracture
lines—the newsletter of the Orthopaedic Trauma Association. 2006;2.
HC, Auf’m’Kolk M, Paffrath T, et al. Primary intramedullary femur
fixation in multiple trauma patients with associated lung contusion—a
cause of posttraumatic ARDS? J Trauma 1993;34:540-547.
HC, Dwenger A, Grotz M, et al. Does the reamer type influence the
degree of lung dysfunction after femoral nailing following severe
trauma? An animal study. J Orthop Trauma 1994;8:300-309.
HC, Giannoudis PV, Krettek C, et al. Timing of fixation of major
fractures in blunt polytrauma: role of conventional indicators in
clinical decision making. J Orthop Trauma 2005;19:551-562.
HC, Giannoudis P, Krettek C. The timing of fracture treatment in
polytrauma patients: relevance of damage-control orthopedic surgery. Am
J Surg 2002;183: 622-629.
HC, Grimme K, van Griensven M, et al. Impact of intramedullary
instrumentation versus damage control for femoral fractures on
immunoinflammatory parameters: prospective randomized analysis by the
EPOFF Study Group. J Trauma 2003;55:7-13.
HC, Hildebrand F, Pertschy S, et al. Changes in the management of
femoral shaft fractures in polytrauma patients: from early total care
to damage-control orthopedic surgery. J Trauma 2002;53:452-461.
HC, Regel G, Dwenger A, et al. Influences of different methods of
intramedullary femoral nailing on lung function in patients with
multiple trauma. J Trauma 1993;35: 709-716.
HC, Remmers D, Grotz M, et al. Levels of antibodies to endotoxin and
cytokine release in patients with severe trauma: does posttraumatic
dysergy contribute to organ failure? J Trauma 1999;46:907-913.
HC, Schmidt RE, Rice J, et al. Biochemical changes after trauma and
skeletal surgery of the lower extremity: quantification of the
operative burden. Crit Care Med 2000;28:3441-3448.
HC, van Griensven M, Rice J, et al. Major secondary surgery in blunt
trauma patients and perioperative cytokine liberation: determination of
the clinical relevance of biochemical markers. J Trauma
2001;50:989-1000.
DA, Moore FA, Moore EE, et al. Barney Resident Research Award winner.
The inflammatory profile of interleukin-6, interleukin-8, and soluble
intercellular adhesion molecule-1 in postinjury multiple organ failure.
Am J Surg 1996;172:425-429.
S, Gandhi J, Curzon I, et al Incidence of deep-vein thrombosis in
patients with fractures of the ankle treated in a plaster cast. J Bone
Joint Surg Br 2007;89:1340-1343.
TF, Contreras DM. Timing of operative treatment of fractures in
patients who have multiple injuries. J Bone Joint Surg Am
1990;72:784-788.
SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral
fractures: does timing of nailing influence incidence? Injury
1998;29:131-133.
TE, Hallett JW Jr, Metzger RL, et al. Genetic risk factors in
inflammatory abdominal aortic aneurysms: polymorphic residue 70 in the
HLA-DR B1 gene as a key genetic element. J Vasc Surg 1997;25:356-364.
J, Paiva JA, Baraibar J, et al. International Conference for the
Development of Consensus on the Diagnosis and Treatment of
Ventilator-Associated Pneumonia. Chest 2001;120:955-970.
EJ, Herndon JH. Alterations in pulmonary function, coagulation, and fat
metabolism in patients with fractures of the lower limbs. Clin Orthop
Relat Res 1976; 115:248-267.
EB, von Bonsdorff H, Hakkinen S, et al. Prevention of fat embolism by
early internal fixation of fractures in patients with multiple
injuries. Injury 1976;8:110-116.
CM, Ludlam CA, Ray DC, et al. The coagulative and cardiorespiratory
responses to reamed intramedullary nailing of isolated fractures. J
Bone Joint Surg Br 2001;83:963-973.
FB, Shackford SR, Trevisani GT, et al. Neurogenic pulmonary edema in
fatal and nonfatal head injuries. J Trauma 1995;39:860-866.
FB, Shackford SR, Vane DW, et al. Prompt fixation of isolated femur
fractures in a rural trauma center: a study examining the timing of
fixation and resource allocation. J Trauma 1994;36:774-777.
P, Lahdensuu M, Kataja J, et al. The syndrome of fat embolism: analysis
of 30 consecutive cases compared to trauma patients with similar
injuries. J Trauma 1970; 10:299-306.
D, Taitelman U, Michaelson M, et al. Prevention of acute renal failure
in traumatic rhabdomyolysis. Arch Intern Med 1984;144:277-280.
RM, Hendriks T, van der Ven-Jongekrijg J, et al. Cytokine patterns in
patients after major vascular surgery, hemorrhagic shock, and severe
blunt trauma. Relation with subsequent adult respiratory distress
syndrome and multiple organ failure. Ann Surg 1993;218:769-776.
TM, Boswell SA, Scott JD, et al. External fixation as a bridge to
intramedullary nailing for patients with multiple injuries and with
femur fractures: damage-control orthopedics. J Trauma 2000;48:613-621.
CM, Schwender S, Haubitz I, et al. Selective screening for the Factor V
Leiden mutation: is it advisable prior to the prescription of oral
contraceptives? Thromb Haemost 1997;78:1480-1483.
R, LaDuca J, Hassett JM, et al. Blunt multiple trauma (ISS 36), femur
traction, and the pulmonary failure-septic state. Ann Surg
1985;202:283-295.
T, van’t Hooft FM, Kallin B, et al. A common functional polymorphism
(C->A substitution at position -863) in the promoter region of the
tumour necrosis factoralpha (TNF-alpha) gene associated with reduced
circulating levels of TNF-alpha. Hum Mol Genet 1999;8:1443-1449.
JP, Lopez B, Volgas DA, et al. Prophylaxis against deep-vein thrombosis
following trauma: a prospective, randomized comparison of mechanical
and pharmacologic prophylaxis. J Bone Joint Surg Am 2006;88:261-266.
RP, Maki DG. Bacteriuria in the catheterized patient. What quantitative
level of bacteriuria is relevant? N Engl J Med 1984;311:560-564.
PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for
acute pulmonary embolism. N Engl J Med 2006;354:2317-2327.
JJ, Gustilo RB. The use of methylprednisolone and hypertonic glucose in
the prophylaxis of fat embolism syndrome. Clin Orthop Relat Res
1979;143:211-221.
EJ, Egol KA, Alaia M, et al. The use of retrievable inferior vena cava
filters in orthopaedic patients. J Bone Joint Surg Br 2008;90:662-667.
S, Nesse O, Finsen V, et al. Prevention of fat embolism syndrome in
patients with femoral fractures—immediate or delayed operative
fixation? Ann Chir Gynaecol 1987;76:163-166.
G, Ruchholtz S, Waydhas C, et al. Damage-control orthopedics in
patients with multiple injuries is effective, time saving, and safe. J
Trauma 2005;59:409-416.
RC, Manning J, Lampard S, et al. Early intramedullary nailing of
femoral shaft fractures: a cause of fat embolism syndrome. Am J Surg
1983;146:107-111.
M, Ozaki T, Sato T. Diagnosis of deep vein thrombosis after operation
for fracture of the proximal femur: comparative study of
ultrasonography and venography. J Orthop Sci 2006;11:146-153.
der Made WJ, Smit EJ, van Luyt PA, et al. Intramedullary femoral
osteosynthesis: an additional cause of ARDS in multiply injured
patients? Injury 1996;27:391-393.
Os JP, Roumen RM, Schoots FJ, et al. Is early osteosynthesis safe in
multiple trauma patients with severe thoracic trauma and pulmonary
contusion? J Trauma 1994;36: 495-498.
WL, Ahrns KS, Zajkowski PJ, et al. Normal D-dimer levels do not exclude
thrombotic complications in trauma patients. Surgery 2003;134:529-532.
Y, Kikuno T, Ohwada T, et al. Rapid fall in blood myoglobin in massive
rhabdomyolysis and acute renal failure. Intensive Care Med
1994;20:109-112.
C, Nast-Kolb D, Trupka A, et al. Posttraumatic inflammatory response,
secondary operations, and late multiple organ failure. J Trauma
1996;40:624-630.
PS, Hirsh J, Anderson DR, et al. A simple clinical model for the
diagnosis of deep-vein thrombosis combined with impedance
plethysmography: potential for an improvement in the diagnostic
process. J Intern Med 1998;243:15-23.
TO, Clutton RE, Salter D, et al. The early response to major trauma and
intramedullary nailing. J Bone Joint Surg Br 2006;88:823-827.
TO, Jenkins PJ, Smith RD, et al. The epidemiology of posttraumatic
adult respiratory distress syndrome. J Bone Joint Surg Am
2004;86-A:2366-2376.
SM, Hirsch NP, Smith GB. Anaesthesia and Intensive Care A-Z: An
Encyclopaedia of Principles and Practice. Philadelphia:
Butterworth-Heinemann, 2000.
HR, Skinner JA, Porteous MJ. The preoperative prevalence of deep vein
thrombosis in patients with femoral neck fractures and delayed
operation. Injury 1999;30: 605-607.
G, Moore EE, Johnson JL, et al. Circulating postinjury neutrophils are
primed for the release of proinflammatory cytokines. J Trauma
1999;46:42-48.
