Embryology
Editors: Tornetta, Paul; Einhorn, Thomas A.; Cramer, Kathryn E.; Scherl, Susan A.
Title: Pediatrics, 1st Edition
Copyright ©2004 Lippincott Williams & Wilkins
> Table of Contents > Section III: – Specialty Clinics > 31 – Embryology
31
Embryology
William A. Phillips
Susan A. Scherl
Knowledge of basic embryology, particularly that of the
musculoskeletal and nervous systems, is necessary in the practice of
pediatric orthopaedics. Many congenital pediatric orthopaedic
conditions are a direct result of problems in early fetal development.
The birth of a fully formed child 9 months after conception is the
culmination of an intricate series of interactions exquisitely
choreographed by the expression of many genes at different times and
places, beginning with the fertilized ovum. The events of greatest
interest to orthopaedists—the development of the musculoskeletal
system—occur primarily during the embryonic period, that is, the third
through eighth weeks after conception.
musculoskeletal and nervous systems, is necessary in the practice of
pediatric orthopaedics. Many congenital pediatric orthopaedic
conditions are a direct result of problems in early fetal development.
The birth of a fully formed child 9 months after conception is the
culmination of an intricate series of interactions exquisitely
choreographed by the expression of many genes at different times and
places, beginning with the fertilized ovum. The events of greatest
interest to orthopaedists—the development of the musculoskeletal
system—occur primarily during the embryonic period, that is, the third
through eighth weeks after conception.
Ultimate control of embryonic development is through the
expression of the genes contained on the chromosomes. Some of the
mechanisms used in development include cell proliferation, cell
differentiation, cell migration, and cell death. These cellular events
occur not only within cells but also take place as interactions between
adjacent cells and tissues. The location of cells in the embryo and
their interactions with their local environment play a major role in
development. Cells can express genes that up- or down-regulate the
expression of other genes. This can occur by direct cell-to-cell
contact or by messenger molecules.
expression of the genes contained on the chromosomes. Some of the
mechanisms used in development include cell proliferation, cell
differentiation, cell migration, and cell death. These cellular events
occur not only within cells but also take place as interactions between
adjacent cells and tissues. The location of cells in the embryo and
their interactions with their local environment play a major role in
development. Cells can express genes that up- or down-regulate the
expression of other genes. This can occur by direct cell-to-cell
contact or by messenger molecules.
DEFINITIONS
-
Zygote: a fertilized egg and sperm
-
Morula: a solid ball of cells formed by division of the zygote
-
Blastocyst: the morula develops a fluid-filled cyst, and becomes a blastocyst
-
Germ layers: the three germ layers—ectoderm, mesoderm, and endoderm—give rise to all the structures of the embryo.
-
Ectoderm:
-
□ Central nervous system
-
□ Peripheral nervous system
-
□ Sensory epithelia of eye, ear, and nose
-
□ Epidermis, hair, and nails
-
□ Mammary glands
-
□ Pituitary gland
-
□ Tooth enamel
-
□ Neural crest cells
-
□ Spinal, cranial, and autonomic ganglia
-
□ Ensheathing cells of the peripheral nervous system
-
□ Pigment cells of the dermis
-
□ Tissues of branchial arch origin
-
□ Adrenal medulla
-
□ Leptomeninges
-
-
-
Mesoderm:
-
□ Cartilage, bone, and connective tissue
-
□ Smooth and striated muscle
-
□ Heart
-
□ Blood and lymph vessels and cells
-
□ Gonads
-
□ Pericardium, pleura, and peritoneum
-
□ Adrenal cortex
-
□ Spleen
-
-
Endoderm:
-
□ Gastrointestinal, respiratory, genitourinary, and auditory epithelium
-
□ Tonsils
-
□ Thyroid and parathyroids
-
□ Thymus and pancreas
-
□ Liver
-
-
Mesenchyme: mesodermal embryonic connective tissue
-
□ Can differentiate into fibroblasts, chondroblasts, or osteoblasts
-
-
Intramembranous ossification: direct transformation of mesenchyme into bone
-
□ Mesenchymal cells condense and differentiate into osteoblasts, which lay down osteoid to become mineralized into bone.
-
□ Occurs in the skull and face
-
-
Endochondral ossification: ossification of a preexisting cartilaginous bone precursor (anlage)
-
□ A primary ossification center forms in the diaphyseal region of the anlage, and a secondary center forms in the epiphysis.
-
□ Mesenchymal cells condense and
differentiate into chondroblasts, which secrete collagen and ground
substance to form the extracellular matrix. -
□ Cartilage begins to appear in the fifth week.
-
□ A primary ossification center forms in
the diaphyseal region of the anlage by week 12, and a secondary center
forms in the epiphysis, at various times after birth, depending on the
bone. -
□ Occurs in long bones.
P.340 -
-
Notochord: embryonic structure around which the vertebral column develops
-
□ Also induces the overlying ectoderm to form the neural plate, the precursor to the central nervous system
-
-
Neural tube: the neural plate invaginates centrally to form the neural groove, bounded on either side by the neural folds
-
□ The folds fuse to form the neural tube.
-
□ The cranial end of the neural tube
becomes the brain, the remainder becomes the spinal cord, and the lumen
becomes the ventricles of the brain and central canal of the spinal
cord (Fig. 31-1). -
□ The neural tube is induced to form different regions along its length by retinoic acid.
-
□ Retinoids activate genes in the Hox (homeobox) family.
-
□ Inhibition of bone morphogenic proteins also appears to play an important role in this process.
-
-
Somites: paired cuboidal bodies derived from the mesoderm running paraxially alongside the neural tube
-
□ Give rise to the axial skeleton, trunk musculature, and dermis
-
□ Each somite differentiates into a ventromedial sclerotome, which will form the vertebrae and ribs, and a dorsolateral dermomyotome, which separates into the myotome, which will form the muscles and the dermatome, which will form the skin.
-
□ Some of the molecular events associated with somite formation include the expression of Notch pathway and Hox genes.
-
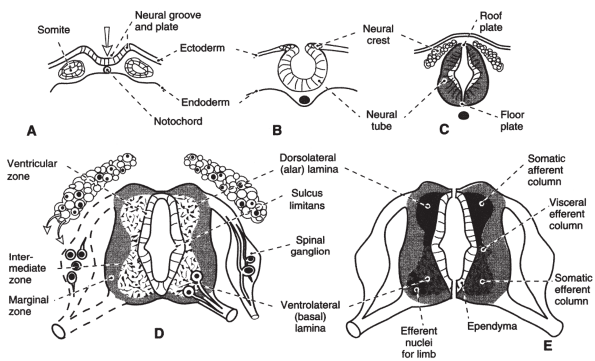 |
|
Figure 31-1 Development of the spinal cord. (A-C) Formation of the neural tube and neural crest in early somite embryos. (D) Cellular differentiation within the cord. (E)
Development of cell columns in the alar and basal laminae shown on one side for a segment that innervates the limbs and on the other for a segment that contains the visceral efferent column. (From Rosse C, Gaddum-Rosse P. The vertebral canal, spinal cord, spinal nerves, and segmental innervation. In: Hollinshead’s textbook of anatomy, 5th ed. Philadelphia: Lippincott-Raven, 1997:152.) |
EARLY FETAL DEVELOPMENT
-
Day 1: fertilization, formation of zygote
-
Day 2: morula stage
-
Day 4: blastocyst stage
-
Day 6 to 10: uterine implantation
-
Day 11: placental circulation established
-
Day 15: first period missed
-
Day 16 to 18: notochord and neural plate form
-
Day 20: somites formP.341
-
□ Early in the third week, formation of the primitive streak orients the embryo in all three planes—longitudinal (cranial-caudal), sagittal (dorsal-ventral), and coronal (left-right).
-
□ Cells migrating from the deep surface
of the primitive streak move between the dorsally placed ectoderm and
ventrally placed endoderm to become the mesoderm.
-
-
Week 4: neural tube forms, heart beats, arm and leg buds appear
-
Week 5: mouth, eyes, and hands develop
-
Week 6: feet and fingers develop
-
Week 7: toes develop
In general, if there is a congenital abnormality in one
organ system, it is imperative to rule out abnormalities in all organ
systems developing at the same time. For example, since the elements of
the spine and heart develop simultaneously, children with congenital
scoliosis must be evaluated for heart defects.
organ system, it is imperative to rule out abnormalities in all organ
systems developing at the same time. For example, since the elements of
the spine and heart develop simultaneously, children with congenital
scoliosis must be evaluated for heart defects.
ARTICULAR AND SKELETAL DEVELOPMENT
-
The skeleton derives from mesodermal mesenchyme.
-
Regulators of bone development include bone morphogenic proteins (BMP), transforming growth factor-β (TGF-β), and homeobox (Hox) genes.
-
In long bones, the mesenchyme develops into a cartilaginous anlage, which undergoes endochondral ossification to form bone.
-
In the skull, the mesenchyme transforms directly into bone through intramembranous ossification.
-
The vertebrae and ribs develop from the fusion of the upper and lower halves of adjacent somites.
-
Joints develop from the interzonal
mesenchyme between the bony anlage. Interzonal mesenchyme can
differentiate into fibrous or cartilaginous tissue to form a joint, and
cavitates to form synovial spaces. -
Malformations of the axial skeleton:
-
□ Klippel-Feil syndrome: failure of segmentation of cervical vertebrae
-
□ Spina bifida occulta: failure of fusion of the halves of the vertebral arch
-
□ Hemivertebrae: failure of appearance of
one of the two ossification centers forming a vertebral body, leading
to failure of formation of half of the vertebra -
□ Rachischisis: failure of fusion of the neural folds
-
□ Achondroplasia: disruption of endochondral ossification.
-
LIMB DEVELOPMENT
-
Limb buds appear at the end of the fourth week of gestation.
-
The upper limb buds develop slightly ahead of the lower.
-
The limb buds are formed from somatic mesoderm and ectoderm.
-
Limb buds start forming on the ventrolateral surface of the body wall.
-
Mesenchymal cells in the lateral mesoderm are activated beneath a thickened band of ectoderm.
-
□ This thickening is the apical ectodermal ridge (AER).
-
□ The AER interacts with limb bud mesenchyme to promote growth of the limb from proximal to distal.
-
□ Fibroblast growth factors are expressed in the AER.
-
□ At the posterior margin of the limb bud, mesenchymal cells form the zone of polarizing activity,
which controls limb development in the anteroposterior axis (first vs.
fifth digit sides of limb) through the expression of the sonic hedgehog (Shh) gene. -
□ The dorsoventral axis (flexor vs. extensor sides of the limb) is determined by Wnt7 from the dorsal epidermis and engrailed-1 (EN-1) from the ventral side.
-
-
Homeobox (Hox) genes play an important role in limb development.
-
□ This gene family was first described in the fruit fly (Drosophila). Hox genes are highly conserved and regulate vertebra development in a similar manner as they do in Drosophila.
-
□ They code for transcription factors
that regulate DNA transcription of other genes. In a sense, Hox genes
are the switches by which other genes are activated during
embryogenesis. -
□ The order of Hox
genes along the chromosome determines the order in which they are
expressed. Genes at the 3′ end of a sequence are expressed earlier and
more anteriorly on the body.
-
-
At the beginning of the seventh week, the
limbs extend ventrally with the palmar surface of the hands and the
plantar surface of the feet facing each other. The thumbs and great
toes are both on the cephalad, preaxial border of their respective
limbs. -
During the eighth week, the upper limbs
rotate laterally, pointing the elbows caudally. The elbows flex so the
hands meet across the chest. The forearm flexor muscles now lie on the
medial side of the arm and the extensors lie laterally. At the same
time, the lower limbs rotate medially. This places the soles of the
feet in a plantar position. The leg flexor muscles lie dorsally and the
extensors lie ventrally. -
Limb development is complete by the eighth week of gestation (Fig. 31-2).
By the end of the sixth week, the rays of the hands have formed,
followed in a few days by the foot rays. By the eighth week the
mesenchyme between the rays breaks down, separating the digits. This
occurs through apoptosis (programmed cell death) and is regulated by bone morphogenic proteins. -
Limb malformations are usually genetic,
but environmental and teratogenic factors have also been implicated in
some cases, and causation may be multifactorial. -
Often, limb malformations are relatively
minor in and of themselves, but they may be associated with other more
serious congenital problems or syndromes. -
Limb malformations:
-
□ Cleft hand and foot—failure of formation of central ray
-
□ Radial club hand—partial or complete
absence of the radius. May present with thrombocytopenia (TAR
(thrombocytopenia-absent radius] syndrome) or as part of VATER syndrome
(vertebral anomalies, anal atresia, tracheoesophageal fistula, renal
and radial anomalies) -
□ Brachydactyly—short fingers or toes, often inherited.
-
□ Polydactyly—extra fingers or toes
-
□ Postaxial (medial on the upper extremity, lateral on the lower) polydactyly is autosomal dominant
-
□ Preaxial is rarer and more likely to be a sporadic event.
-
-
□ Amelia—complete absence of the limbs,
caused by early suppression of limb development. Thalidomide, an
anti-nausea drug used during pregnancies between 1957 and 1962, can
cause amelia. -
□ Meromelia—partial absence of the limbs, caused by suppression of limb development later in gestation
P.342 -
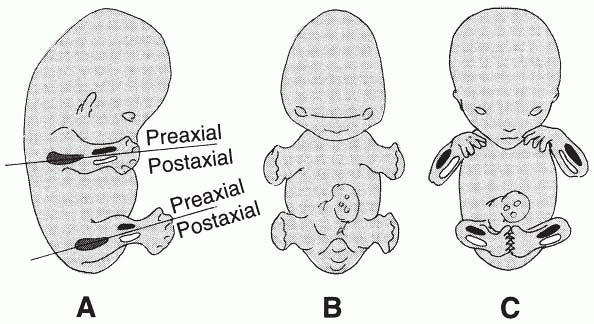 |
|
Figure 31-2 (A)
Development of the bones in the free limbs in relation to the limb axis. The bone primordium of the proximal segment is dark gray, the preaxial bone of the intermediate segment is black, and the postaxial bone is white. (B and C) Successive stages of limb development from a ventral view before the limbs undergo rotation. The position of the preaxial and postaxial bone in the upper and lower limbs is still symmetric. (From Rosse C, Gaddum-Rosse P. Basic structural plan of the limbs. In: Hollinshead’s textbook of anatomy, 5th ed. Philadelphia: Lippincott-Raven, 1997:183.) |
MUSCLE DEVELOPMENT
-
Most skeletal muscle is derived from the ventral dermomyotomal portion of the somites.
-
Head and neck muscle is derived from branchial arch mesoderm.
-
Limb muscles develop from mesenchyme derived from somatic mesoderm.
-
Muscle development is initiated by Shh from the notochord and Wnts from the neural tube, inducing Pax-3 and Myf-5 in the somites.
-
Pax-3 in turn regulates c-met expression, which directs migration of the myogenic precursor cells into the limb bud.
-
In each limb the cells condense into two masses, dorsal and ventral to the axial mesenchyme.
-
□ The dorsal muscle mass becomes most of
the extensors and supinators of the upper limb and the extensors and
abductors of the lower limb. -
□ The ventral muscle mass becomes the flexors and pronators of the upper limb and the flexors and adductors of the lower limb.
-
-
Cardiac and smooth muscles develop from splanchnic mesoderm.
-
There is wide developmental variation in musculature.
-
Absences or variations is size, shape, and position of muscles is common, and generally not of clinical significance.
-
The most common significant congenital
muscle lesion is torticollis, an intrauterine scarring and fibrosis of
the sternocleidomastoid muscle, which causes a fixed rotation and
tilting of the head.
NERVOUS SYSTEM DEVELOPMENT
-
In the peripheral nervous system, all sensory cells are derived from the neural crest.
-
□ Neural crest cells in the region of the brain form the sensory ganglia of cranial nerves V and VII through X.
-
□ The autonomic ganglia are also derived from the neural crest.
-
□ Motor nerve fibers develop from cells in the fetal spinal cord.
-
□ Growing limb buds are supplied with segmental motor and sensory innervation, which grow into the mesenchyme.
-
□ The central nervous system derives from ectodermal tissue on the dorsal aspect of the fetus known as the neural plate.
-
□ The neural plate infolds to form the neural groove and neural folds.
-
□ The neural folds fuse to form the neural tube.
-
□ The neural crest is a layer of cells between the neural tube and the surface ectoderm.
-
□ The cranial end of the neural tube
becomes the brain and the remainder becomes the spinal cord, while the
lumen becomes the ventricles of the brain and central canal of the
spinal cord.
-
-
Problems during this process are common,
and lead to various congenital brain and spinal cord abnormalities,
including spina bifida and anencephaly. -
Since this process occurs very early in
gestation (at about 3 weeks), typically before a woman knows she is
pregnant, preventive measures such as adequate (400 to 800 µg) intake
of folate should begin prior to attempts at conception.
SUGGESTED READING
Buckwalter
JA, Einhorn TA Simon, SR. Orthopaedic basic science, 2nd ed. Rosemont,
IL: American Academy of Orthopaedic Surgeons, 2000.
JA, Einhorn TA Simon, SR. Orthopaedic basic science, 2nd ed. Rosemont,
IL: American Academy of Orthopaedic Surgeons, 2000.
P.343
Dietz
FR, Morcuende JA. Embryology and development of the musculoskeletal
system. In: Morrissey RT, Weinstein SL, eds. Lovell and Winter’s
pediatric orthopaedics, 5th ed. Philadelphia: Lippincott Williams &
Wilkins, 2001.
FR, Morcuende JA. Embryology and development of the musculoskeletal
system. In: Morrissey RT, Weinstein SL, eds. Lovell and Winter’s
pediatric orthopaedics, 5th ed. Philadelphia: Lippincott Williams &
Wilkins, 2001.
Larsen WJ. Human embryology, 2nd ed. New York: Churchill Livingstone, 1997.
Moore KL. The developing human, 3rd ed. Philadelphia: WB Saunders, 1982.
Moore KL, Persaud TVN. The developing human, 7th ed. Philadelphia: Elsevier, 2003.
O’Rahilly RO, Müller F. Human embryology and teratology, 2nd ed. New York: Wiley-Liss, 1996.
Shapiro F. Pediatric orthopedic deformities. San Diego: Academic Press, 2001.
Sweeney LJ. Basic concepts in embryology. New York: McGraw-Hill, 1998.
