Complications of Total Hip Arthroplasty: Prevention and Management
– HIP > Part C – Operative Treatment Methods > 13 – Complications
of Total Hip Arthroplasty: Prevention and Management
the restoration of function and relief of pain resulting from arthritic
conditions of the hip. However, complications can and do occur after
THA. This chapter reviews the major complications associated with THA,
which include (but are not limited to) the following: infection,
neurovascular injury, thromboembolism, instability, heterotopic
ossification, leg-length discrepancy, component fracture or failure,
and the possibility of systemic complications. Each of these
complications will be reviewed and methods of prevention and management
will be discussed.
patient satisfaction, morbidity, and mortality and places a large
financial burden on the healthcare system. The incidence of infection
after THA has remained relatively constant, 1% to 2%, for primary THA
and 3% to 5% for revision THA. The development of a periprosthetic
infection depends on the number and virulence of the bacteria, the
status of the wound environment, and the host’s ability to eliminate
the bacteria. Staphylococcus aureus and Staphylococcus epidermidis
account for >50% of the pathogens in patients with a THA infection;
Gram-negative aerobic and facultative organisms for 11% of pathogens,
and anaerobic bacteria 12% of pathogens. Pathogens that cause infection
originate from the patient’s skin (most frequent), blood, or the
operating room environment. The wound environment is important and
often suboptimal in patients who have advanced vascular disease, a
history of multiple operative procedures, extensive scarring, or a
history of wound infection. Increased risks of infection have been
shown to occur in patients with rheumatoid or psoriatic arthritis,
those on immunosuppressive medication, or those with diabetes mellitus,
hemophilia, obesity, and malnourishment.
(type I) may be caused by wound colonization, infected hematomas, or
superficial infections spreading to the periprosthetic space and
usually present during the first postoperative month. Late chronic infections
(type II) originate at the time of surgery, but owing to either a small
inoculum or low virulence of the organisms, onset of presentation is
often delayed to between 1 and 24 months. Acute hematogenous infections
(type III) are the least common and characterized by deterioration in a
previously well-functioning joint; these may be associated with a
history of an acute illness or recent dental work. Positive intraoperative cultures
(type IV) was added as a fourth type to include patients with two out
of five positive intraoperative cultures without any other features of
obvious infection.
periprosthetic THA infection include proper identification of risk
factors, surgical technique, and operating room environment.
Prophylactic antibiotic use is the most important factor for reducing
the incidence of deep periprosthetic
infection.
The optimal time for its administration is just before the skin is
incised, and current recommendations are that systemic antimicrobial
prophylaxis be given 30 to 60 minutes before the skin incision is made.
Prolonged procedures require an additional intraoperative dose of
antibiotic. Other measures designed to reduce the incidence of
infection include the use of body exhaust suits, laminar flow (vertical
laminar airflow units generally reduce airborne contamination better
than horizontal units), ultraviolet lights (which destroy airborne
bacteria), proper sterilization of instruments, careful preparation of
the operative site, the use of double gloves, and reduction of traffic
flow in the operating room. Characteristics of the prosthesis have also
been found to predispose a patient to infection: cobalt-chromium
surfaces have been found to be more conducive to infection than
titanium surfaces; porous surfaces have been found to be more conducive
to infection than polished surfaces.
|
TABLE 13-1 Classification of Infected Total Joint Replacements
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
approximately 7% to 14% of patients. No correlation has been found,
however, between bacteria isolated from urine and those isolated from
deep infections. Late hematogenous (type III) infections have been
reported following dental, gynecologic, urologic, and gastroenterologic
procedures. Streptococcus viridans is the
predominant bacteria in the human oral flora but accounts for a low
percentage of late infection around prosthetic joints. Dental procedure
prophylaxis (associated with gingival hemorrhage) includes amoxicillin
2 g PO (or clindamycin 300 mg PO) administered 1 hour prior to
procedure. The American Society of Colon and Rectal Surgeons and the
American Society for Gastrointestinal Endoscopy do not recommend
prophylactic antibiotics for colonoscopy, sigmoidoscopy, or endoscopy.
erythematous, swollen wound with persistent drainage, unremitting pain,
and an irritable range of motion. Night or rest pain is also worrisome.
Radiographic evidence of early failure should also raise concern for
septic loosening. Investigations to rule out infection include
erythrocyte sedimentation rate (ESR), C-reactive protein (CRP),
aspiration, frozen section, and intraoperative cultures.
suppressive antibiotics, irrigation and debridement with prosthetic
retention, prosthetic exchange (one- or two-stage), resection
arthroplasty, arthrodesis, and very rarely amputation. Management is
ultimately dictated by timing of the diagnosis, medical presentation,
and patient comorbidities. The goals of treatment are eradication of
infection and restoration of function of the affected limb. Antibiotic
therapy and operative debridement remain the mainstays of treatment.
Cephalosporins are the most commonly used antibiotics in the setting of
THA infection and have a broad spectrum of activity against the most
common pathogens involved in THA infection. They also have low toxicity
to patients and high soft tissue and bone concentrations. Since the
late 1990s, however, several strains of resistant bacterial flora have
emerged.
eradication of infection without operative debridement almost
impossible. In addition, bacteria can adhere to the surface of a
biomaterial and form a biofilm, or glycocalyx, that protects the
bacteria from antibiotics and host defenses. Surgical debridement
should include excision of all infected and necrotic tissue and the
removal of cement, wires, cables, plates, screws, nonabsorbable
sutures, and prostheses. Patients who present with an acute (type I or
III) infection can be treated with surgical debridement, polyethylene
liner exchange, and component retention (if well fixed) followed by
intravenous antibiotics. The optimal treatment for patients with
chronic infections (type II) is surgical debridement, removal of
components, insertion of an antibiotic spacer, and administration of
intravenous antibiotics under the direction of an infectious disease
specialist (usually about 6 to 8 weeks). The use of a PROSTALAC
(prosthesis of antibiotic-loaded acrylic cement) offers the advantages
of better mobilization, control over limb-length discrepancy, and
antibiotic delivery.
healing, antibiotic effectiveness, soft tissue and bone quality, and
potential for rehabilitation. Antibiotic suppression is reserved for
those patients who are too sick or refuse surgery, when the organism is
identifiable and sensitive to an appropriate oral antibiotic, the
prosthesis is well fixed, and there are no signs of systemic sepsis.
Long-term suppression is reported to have about a 30% success rate,
with
the
outcome being retained implants. In select patients who may not be able
to tolerate a second surgery, a one-stage exchange can be used with the
advantages of a single hospitalization and avoidance of interim
instability, disuse atrophy, and limb shortening. Two-stage exchange
with the use of antibiotic-loaded cement has the lowest overall
reinfection risk and a success rate approaching 90%. Resection
arthroplasty is an uncommon salvage procedure for an infection around a
THA and is most indicated for patients who are not candidates for
staged reimplantation or who are unable to comply with postoperative
rehabilitation protocols. Arthrodesis is recommended in young patients
with unilateral hip infections. Disarticulation of the hip is performed
only in the face of life-threatening infection, severe loss of soft
tissue and bone stock, and vascular injury.
distressing complications of THA for both patient and surgeon. The
prevalence of nerve palsy after THA has been reported as between 0.6%
and 3%; this incidence increases up to around 5% for revision THA or
for THA done for congenital dysplasia. The sciatic, femoral, obturator,
and gluteal nerves can be injured. The prevalence of vascular injury is
extremely rare, ranging from 0.2% to 0.3%. Nonetheless, vascular
injuries to the iliac, femoral, obturator, and gluteal arteries have
been described. The proposed causes of nerve or vascular injury include
direct trauma, traction or pressure from retractors, extremity
positioning, excessive tensioning (often from lengthening the
extremity), ischemia, thermal injury from cement, constriction by wire
or suture, or dislocation of the components. The placement of
acetabular screws into major intra-abdominal vascular cavities has also
been reported with catastrophic results.
about the pelvis helps the surgeon to avoid injury to these vital
structures. Extreme care must be undertaken during surgical dissection,
retractor placement, insertion of acetabular screws, and the passing of
cerclage wires. Preoperative angiography may be indicated for high-risk
situations, such as those involving intrapelvic migration of a failed
acetabular component or intrapelvic extravasation of cement. Anatomic
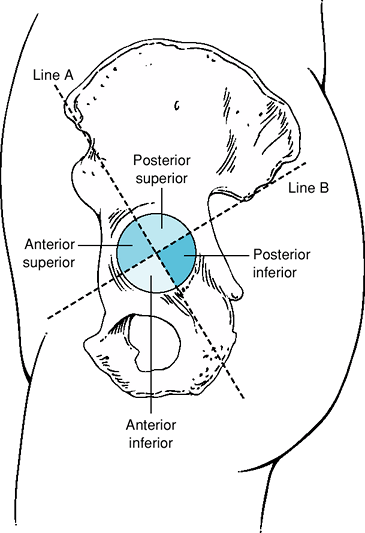
studies have defined four acetabular quadrants created by the
intersecting lines from the anterior and posterior iliac spines (Fig. 13-1).
The posterior-superior quadrant has been shown to be the safest area
for the placement of acetabular screws. Placement of screws in the
anterior-superior or anterior-inferior quadrants should be avoided
because of risk of vascular injury to the iliac vessels.
anesthetist and nursing staff should be immediately informed so as to
have appropriate blood and instruments (vascular clips) available. All
vascular injuries should be treated with prompt identification,
application of pressure, proximal and distal control of the vessel, and
hemostasis with direct repair, shunting, clipping, or ligation of the
vessel. A vascular surgeon may be required for major vascular injuries.
In instances of postoperative neurovascular compromise, a surgical
exploration is warranted if there has been passage of cerclage wires
around the femur, excessive lengthening of the extremity has occurred,
or a large postoperative hematoma is diagnosed or suspected.
femoral nerve injury to prevent knee buckling. Patients with sciatic or
peroneal nerve injury should have the foot splinted postoperatively to
prevent equinus deformity.
 |
|
Figure 13-1
Diagram of the four acetabular quadrants created by two intersecting lines from the anterior and posterior superior iliac spines. (Reproduced with permission from Wasielewski
RC, Cooperstein LA, Kruger MP, et al. Acetabular anatomy and the transacetabular fixation of screws in total hip arthroplasty. J Bone Joint Surg Am. 1990;2[4]:501–508. ) |
following THA and is the leading cause of postoperative morbidity.
Abnormalities in coagulation that occur following THA can be related to
the Virchow triad of venous stasis, endothelial damage, and
hypercoagulable state. Venous stasis occurs as a result of leg
positioning during the
procedure,
localized postoperative swelling, and postoperative immobility. Venous
endothelial injury occurs with local dissection, thermal injury from
cautery or bone cement, and during limb positioning for component
insertion. Hypercoagulability results from the intraoperative stimulus
of the clotting cascade and because blood loss can result in reduction
in antithrombin III and inhibition of the fibrinolytic system. The
presence of a factor V Leiden mutation (activated protein-C
resistance), antiphospholipid antibody syndrome, protein C and S
deficiency, and genetic abnormalities related to antithrombin III
increase the risk of TE. Previous TE and active malignancy are the
other most potent risk factors for postoperative TE. Use of hormone
replacement or oral contraceptive therapy, pregnancy, advanced age,
obesity, smoking, poor mobilization, and lengthy duration of surgery
are less potent risk factors for TE.
asymptomatic deep vein thrombosis (DVT) will develop after 40% to 60%
of THAs, proximal DVT will develop after 15% to 25% of THAs, and a
fatal pulmonary embolism (PE) will develop after 1% to 3% of THAs.
Prophylaxis of TE disease was recommended by the National Institutes of
Health (NIH) consensus in 1986. The American College of Chest
Physicians (ACCP) currently recommends the use of fractionated heparin,
warfarin (target INR [international normalized ratio] 2.0 to 3.0), or
fondaparinux (Table 13-2). This prophylaxis
should occur even if the patient has been discharged. The ACCP also
recommends that patients at high risk of TE (active malignancy,
obesity, bilateral surgery) should receive extended prophylaxis for 28
to 35 days. The ACCP recommends against the use of acetylsalicylic acid
(ASA), dextran, low-dose unfractionated heparin, graduated compression
stockings, intermittent compression stockings, or venous foot pumps as
the only method of prophylaxis. Under the
influence of current prophylaxis, 85% to 90% of all DVTs following THA
occur in the calf, and 17% to 23% of these distal thrombi extend to
more proximal veins in the thigh. After 7 to 10 days of prophylaxis,
the frequency of symptomatic TE within 80 days of surgery is between 2%
and 3%, symptomatic nonfatal PE is 0.6%, and fatal PE is 0.06%.
|
TABLE 13-2 ACCP Grade 1A Recommendations for Thromboembolism Prophylaxis in Total HIP Arthroplasty
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||
the occurrence of DVT by 40% to 50%. The proposed mechanism is probably
related to a sympathetic blockade resulting in increased lower
extremity blood flow mitigating the effects of stasis. The use of
short-acting unfractionated heparin given intravenously following
component insertion has also been found to significantly inhibit fibrin
formation. Patients who receive autologous blood have demonstrated
lower rates of DVT (9%) and PE (0.3%) compared with those patients who
received banked blood (DVT of 13.5% and PE of 0.7%).
of TE disease following THA and exerts its anticoagulant effect by
inhibiting the hepatic production of vitamin K–dependent clotting
factors II, VII, IX, and X. Warfarin is administered orally but
requires regular monitoring of the INR. Unfractionated heparin exerts
its anticoagulant effect through a high binding affinity for
antithrombin III, thereby accelerating the inhibition of thrombin,
factor IX, and Xa. Fractionated low-molecular-weight heparins (LMWH)
differ in their molecular weights and exert their anticoagulant effect
through the inhibition of factor Xa. These offer several advantages
over unfractionated heparin because they have better bioavailability,
prolonged circulating half-life, and a lower frequency of development
of thrombocytopenia. However, in 1997 the United States Food and Drug
Administration (FDA) issued a public health advisory on the use of
fractionated heparin with epidural or spinal anesthesia. This was owing
to reports of epidural and spinal hematomas causing permanent
neurologic injury following the use of neuraxial anesthesia and LMWH.
Fondaparinux is a synthetic pentasaccharide that acts as a specific
inhibitor of factor Xa with no direct inhibition of thrombin. Although
an effective prophylactic agent, it causes an irreversible change to
the binding site for factor Xa and has been associated with an
increased risk of a major bleeding episode if
administered
within 6 hours of surgery. Aspirin limits platelet aggregation by
inhibiting thromboxane A2 and offers the advantages of low cost, ease
of administration without monitoring, and few bleeding complications.
However, the PEP (Pulmonary Embolism Prevention) trial found that
aspirin did not reduce the risk of symptomatic DVT following THA, and
therefore, it is not recommended as the only means of prophylaxis.
venous return, decrease stasis, and enhance endothelial-derived
fibrinolysis without bleeding risk. Calf and thigh sleeves have been
associated with a reduction in distal calf DVT but a greater prevalence
of high-risk proximal DVT after THA compared with warfarin. EPCDs alone
have not been shown to be more effective than pharmacologic prophylaxis
after THA but may offer an advantage when used in combination.
presence of calf tenderness (the Homan sign), low-grade fever, fatigue,
tachycardia, and diaphoresis may or may not be present. Patients with
proximal DVT may have pain or swelling in their thigh. The classic
presentation of PE, consisting of shortness of breath, pleuritic chest
pain, mental status changes, tachycardia, and tachypnea, is rarely
present. Ascending contrast venography is the most reliable and
sensitive method for detection of asymptomatic and nonocclusive venous
thrombi in the THA patient; however, this is expensive, invasive, and
has been associated with complications including contrast-induced
nephropathy, limb edema, and contrast allergy. Doppler ultrasound is a
noninvasive technique that allows visualization of venous channels but
is not sufficiently sensitive for routine postoperative surveillance of
the THA patient. The ACCP guidelines recommend against the routine use
of Doppler ultrasound screening at the time of hospital discharge in
asymptomatic patients following THA. A chest radiograph in conjunction
with an electrocardiogram and ventilation-perfusion (V/Q) scan can help
in the diagnosis of PE; however, PE can be more accurately diagnosed
with spiral CT, MR, or pulmonary angiography.
|
TABLE 13-3 Risk Factors Considered to Relate to Dislocations after Total HIP Arthroplasty
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||
high-dose fractioned LMWH followed by oral anticoagulation with
warfarin for 3 months is recommended for isolated cases of proximal DVT
and 6 months in cases of PE. Anticoagulation prevents further thrombus
formation while allowing the fibrinolytic system to dissolve clots that
have already formed. An inferior vena cava filter is reserved for
circumstances where full anticoagulation is absolutely contraindicated
or with recurrent PE despite therapeutic anticoagulation.
complications for a patient following THA. The incidence of dislocation
after THA varies widely (0.3% to 9%) with most large series averaging
2% to 3% for primary THA. Many variables can predispose to dislocation,
including disease and patient, surgical, and rehabilitation factors (Table 13-3).
identified; however, not all studies support all risk factors. Disease
conditions such as developmental dysplasia of the hip, prior hip
surgery, and nonhealed fracture and disease states such as rheumatoid
arthritis and prior sepsis have been identified as risk factors for
dislocation following THA. Gender has also been recognized to influence
the likelihood of dislocation, with dislocation reported to occur twice
as frequently in females as in males in some studies. Age was not found
to be important as a risk factor for THA instability in many studies;
however, two studies have shown that older patients have higher
dislocation rates. Factors that may decrease the patient’s ability to
control the hip, such as alcoholism or
neuromuscular disease, also have been shown to increase dislocation rates.
rate of dislocation, with the lateral and anterolateral approaches
showing lower dislocation rates than the posterior approach. More
recently, however, enhanced soft tissue closure after posterior
approach has shown significantly reduced dislocation rates. The
orientation of the acetabular component has also been related to
dislocation, with retroverted components predisposing to posterior
dislocation and excessively anteverted components predisposing to
anterior dislocation. Vertical orientation of the acetabular component
(>55 degrees) has also been considered a risk factor for dislocation
(Figs. 13-2 and 13-3).
Femoral head size and the avoidance of re-enforcement at the base (a
“skirt”) have also been studied as possible factors in dislocation,
and it is believed that enlarging the size of the femoral head and
avoiding the use of a skirt improves hip stability. When head size is
increased and skirts are avoided, the head-to-neck ratio is increased,
the range of motion required for intra-articular impingement increases,
and dislocation decreases. A similar benefit can be gained by
decreasing the femoral neck diameter for a given head diameter or by
using a neck trunnion with a narrow anterior-posterior profile. The use
of acetabular outside diameters >62 mm have also been shown to have
greater dislocation rates than when components <60 mm were used.
Elevated rim and lipped liners have also been shown to improve
stability, provided they are used to maximize restoration of femoral
offset and avoid impingement. Finally, surgeon experience influences
the dislocation rate, with less experienced surgeons having a higher
dislocation rate than experienced surgeons.
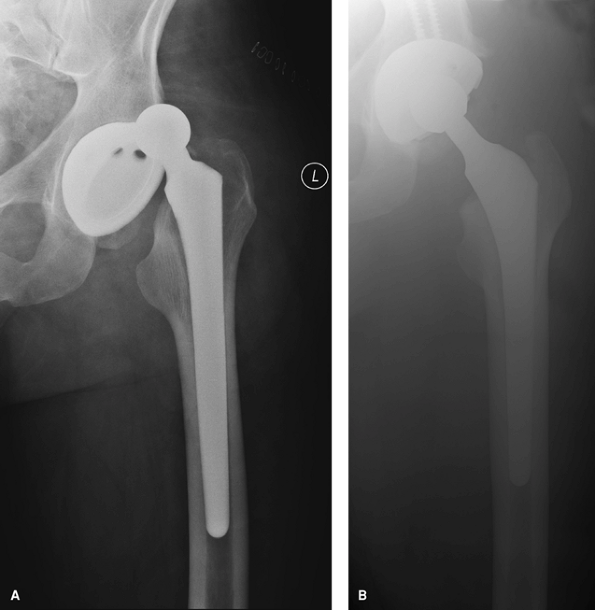 |
|
Figure 13-2 A:
Anteroposterior radiograph of a dislocated total hip arthroplasty demonstrating a vertically positioned acetabular component and resultant hip dislocation. B: Anteroposterior radiograph of the same patient after revision total hip arthroplasty in which the cup was revised and repositioned and the femoral head was upsized to a 32-mm-diameter head. |
evaluated the role of postoperative functional restrictions on the
prevalence of dislocation. This study found removal of commonly used
restrictions (extremes of range of motion, abduction pillows, elevated
toilet seats and chairs) did not increase the prevalence of
dislocation, but conversely promoted lower costs and a higher level of
patient satisfaction. The postoperative time frame of dislocation has
also been shown to be important in predicting future dislocations. The
greatest risk of dislocation occurs within the first 3 months after
surgery. Dislocations that occur beyond 5 weeks have been shown to have
a higher rate of recurrent dislocation than those in patients who had
their initial dislocation within the first 5 weeks.
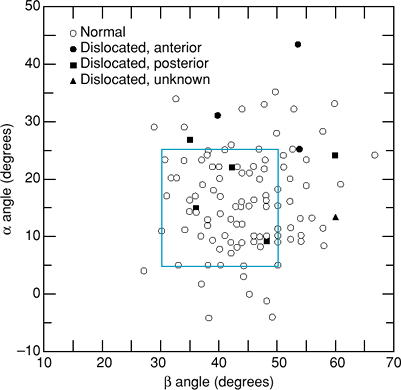 |
|
Figure 13-3
Schematic diagram illustrating the safe zone of 45 ± 10 degrees vertically and 15 ± 10 degrees of anteversion as the range of acetabular component position that will provide the highest stability. α angle represents cup anteversion; β angle represents cup abduction. (Reproduced with permission from Lewinnek GE, Lewis JL, Tarr R, et al. Dislocation after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60[2]:217–220.
) |
posterior, with anterior dislocation being much less frequent. Dorr and
associates have proposed a classification system based on increasing
severity of the cause: Type I dislocations can be attributed to
malposition of extremity, Type II dislocations are caused by
soft-tissue imbalance, and Type III by component malposition.
recognize all of the contributing factors that can lead to dislocation
and avoid them. When a dislocated THA is encountered, the immediate
treatment is closed reduction with either conscious sedation in the
emergency department or general anesthesia in the operating room. The
usual method of reduction is longitudinal traction with the hip in
slight flexion. Care must be taken not to dislodge or dissemble a
well-seated, modular component. Postreduction immobilization in the
form of a brace and reinforcement of motion precautions can be used to
avoid redislocation. For recurrent hip instability, the underlying
cause of dislocation should be addressed (Fig. 13-3).
Operative management should focus on correcting the cause of the
dislocation, and surgical planning should include all possible revision
options: modular liner and head exchange, trochanteric advancement, use
of a constrained acetabular liner, or revision of one or both
components. Patients should also be counseled regarding expectations as
the results of revision surgery for recurrent instability are mixed.
surrounding the hip joint is a frequent complication of THA, with a
reported incidence between 0.6% and 61.7%. The extent of HO may vary
from slight to complete bony ankylosis (Fig. 13-4).
The cause and pathogenesis of HO are not clear but are related to the
duration of the surgical procedure and to the amount of soft tissue
dissection. HO is associated with such conditions as ankylosing
spondylitis, Forestier disease, posttraumatic arthritis, and in some
males with considerable bilateral osteophytic osteoarthritis. Surgical
approach may also increase the risk of HO after THA, with anterior and
anterolateral approaches demonstrating an increase in the possibility
of HO compared with the transtrochanteric and posterior approaches.
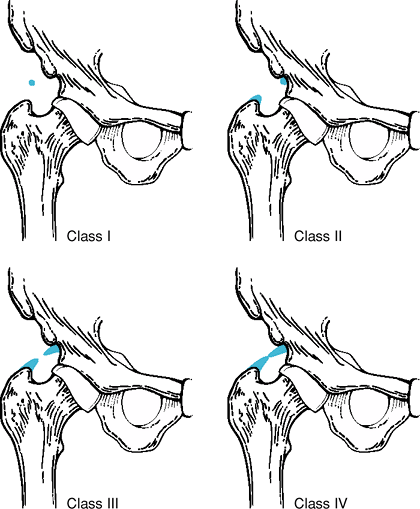
Grade 0 has no ossification, grade I represents one or two isolated
areas of ossification each <1 cm in diameter, grade II represents
more widespread isolated areas of ossification along the proximal femur
or acetabular rim, grade III ossification covers more than half of the
distance between the femur and pelvis but does not bridge the entire
distance, and grade IV ossification bridges the entire distance between
the femur and pelvis.
a priority. Various treatment modalities have been developed to reduce
the incidence of HO following THA. Low-dose radiation has been shown to
help in the prevention of HO. Various radiation protocols have been
described including a single preoperative or postoperative (800 cGy)
dose. If irradiation is chosen for prophylaxis postoperatively, it is
recommended that cementless porous implants should be adequately
shielded or a cemented implant be used. Nonsteroidal anti-inflammatory
drugs (NSAIDs) inhibit prostaglandin synthesis and may interfere with
the inflammatory response and subsequent development of heterotopic
bone. Indomethacin has been used successfully. Bisphosphonates have the
ability to prevent mineralization of osteoid but have no inhibitory
effect on the formation of osteoid matrix itself, and clinical trials
have not shown a significant benefit in the prevention of HO formation
from this treatment.
however, some patients may develop local signs of inflammation such as
erythema, effusion, tenderness, and loss of motion. Assessment of the
extent and severity of HO is made on radiographic analysis. HO may
become visible as early as 3 to 4 weeks postoperatively.
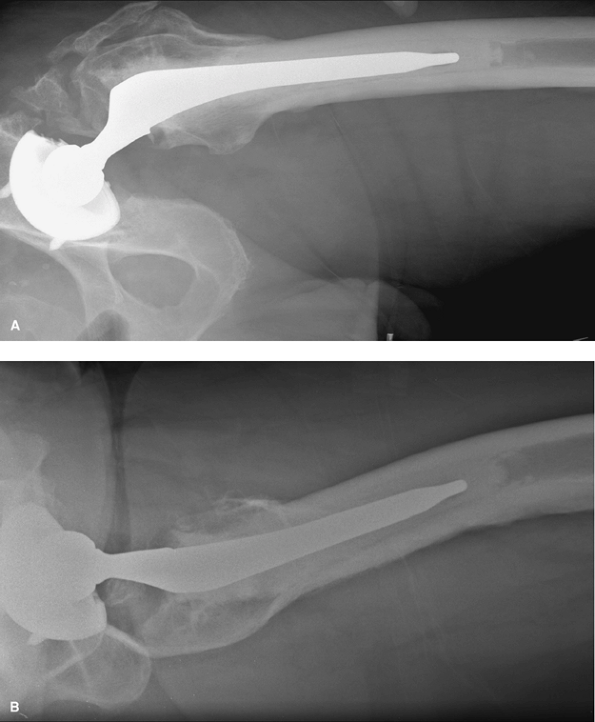 |
|
Figure 13-4 Anteroposterior (A) and lateral (B)
radiographs of a patient several years after left total hip arthroplasty demonstrating severe heterotopic bone formation in the soft tissues adjacent to the left total hip arthroplasty. |
excision will eradicate it. If surgical excision is warranted because
of limitation of motion, the procedure should be delayed until about 6
to 12 months after the index arthroplasty to permit maturation of the
HO and the development of a fibrous capsule (which allows for more
precise dissection of planes and reduces the amount of trauma to the
surrounding tissues).
complication following THA and can adversely affect an otherwise
excellent outcome. Patient dissatisfaction from this complication is
the most common cause of litigation against the orthopaedic community.
The true prevalence of postoperative LLD is difficult to quantify
because of marked variation in reporting methods and in the
interpretation of its clinical significance.
careful preoperative templating from standardized radiographs and by
taking intraoperative measurement of limb-length differences and offset
with various measurement methods.
postoperative LLD, it is important first to determine if the LLD is a
true discrepancy or an apparent discrepancy secondary to a flexion or
abduction contracture of the hip. In many cases, patients have a
postoperative abduction contracture of the hip, which gives them a
pelvic obliquity, resulting in an operated leg that seems too long.
When these patients stand with the feet close together, the
contralateral normal
hip
will be in an adducted position and will feel shorter than the leg on
the operated side. These patients should be asked to stand with their
feet widely separated so that both hips are equally abducted; in doing
so the pelvis becomes level and the legs then seem equal in length. For
these patients, an appropriate program of abductor muscle strengthening
often is helpful.
 |
|
Figure 13-5
Schematic diagram outlining the Brooker classification of heterotopic bone formation around a total hip arthroplasty (Reproduced with permission from Brooker
AF, Bowerman JW, Robinson RA, et al. Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1635. ) |
overlengthening, it is important to determine the amount of true
lengthening. This can be accomplished with the use of osseous landmarks
or blocks under the foot. It is also important to discuss with the
patient the reason for dissatisfaction and his or her expectations of
treatment. Revision to correct a substantial postoperative LLD is
seldom indicated because the procedure is fraught with the possibility
of postoperative hip instability. As a result, although patient
dissatisfaction may be great, surgery to correct true overlengthening
is not frequently performed. LLD that is not associated with back or
hip pain, sciatica, or recurrent dislocation should almost always be
treated nonoperatively by placing a shoe lift on the short side.
Revision arthroplasty is considered to be the last course of action in
patients with ongoing symptoms of instability, gait dysfunction, and
low back pain, and one recent study has reported success with revision
for true overlengthening (although this was performed for less than one
half of 1% of total hip surgery performed at their institution).
and an aging population, the rates of revision THA have been
increasing. Revision THA can be undertaken for any reason, but commonly
occurs as a result of periprosthetic fracture, wear, or osteolysis.
femoral components of a THA are complications that a reconstructive
surgeon must be able to manage. These fractures can occur
intraoperatively or postoperatively. The incidence of intraoperative
acetabular periprosthetic fracture has been reported to be <0.2%.
The acetabulum can fracture from impaction forces incurred while
employing a press-fit technique into an acetabulum that has been
underreamed by 1 or 2 mm in relation to the acetabular component. Other
contributing factors include osteopenia and Paget disease.
Postoperative periprosthetic acetabular fractures usually occur as a
result of bone loss from osteolysis but can also occur because of
traumatic fracture.
and can also occur intraoperatively or preoperatively. A review of the
Mayo clinic joint registry demonstrated an intraoperative femoral
periprosthetic fracture rate of 1% in primary THA and 7.8% in revision
THA. These fractures are more common when the femoral component is
inserted without cement at the time of primary THA. A similar increased
prevalence of fracture with cementless femoral stems is seen in the
revision setting. Revision THA with impaction grafting is associated
with a higher incidence of intraoperative and postoperative fractures.
Postoperative periprosthetic fractures of the femur occur in from 0.1%
to 2.1%.
intraoperative periprosthetic acetabular fractures that includes those
occurring around the anterior wall, transverse, inferior lip, and
posterior wall fractures. Peterson and Lewallen classified
postoperative periprosthetic acetabular fractures into two types: Type
I is a clinically and radiologically stable acetabular component, and
type II is an unstable acetabular component. The Vancouver
classification is the one most commonly used for periprosthetic femur
fractures and considers three important factors: the site of the
fracture, the stability of the implant, and the quality of the
surrounding bone stock. For those that occur intraoperatively,
type A fractures are proximal metaphyseal (not extending into the
diaphysis), type B fractures are diaphyseal (not extending into the
distal diaphysis and therefore not precluding diaphyseal long-stem
fixation), and type C fractures are distal fractures extending beyond
the longest extent of the longest revision stem and can include the
distal metaphysis (Table 13-4). Each type is
subclassified into subtype 1, representing a simple cortical
perforation, subtype 2, representing a displaced linear crack, and
subtype 3, representing a displaced, or unstable fracture.
|
TABLE 13-4 Vancouver Classification of Intraoperative and Postoperative Femur Fractures
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
fractures in which the implant is loose and there is a severe loss of
bone stock. Type C fractures occur distal to the stem and can be
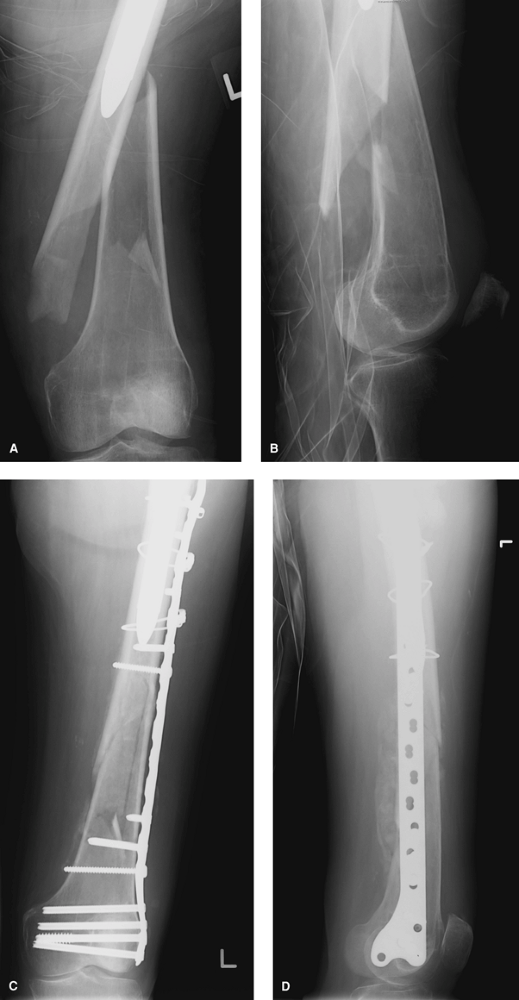
treated independently of the arthroplasty above (Fig. 13-6).
for stability. Stable fractures can be treated conservatively whereas
unstable fractures require fixation and postoperative weight-bearing
restrictions. Early postoperative acetabular fractures are treated
according to their pattern. Patients with stable, minimally displaced
acetabular fractures, in whom a cementless component has been augmented
with screw fixation, can be treated conservatively with union expected
in most cases. Late postoperative acetabular fractures are usually
associated with significant osteolysis and often warrant operative
intervention.
the time of surgery should be addressed at that time with simple bone
grafting (type A1), cerclage wiring (type A2), or with the use of wires, cables, cortical strut grafts, trochanteric claw plates, and a diaphyseal-fitting stem (types A3, B1, B2, and B3).
Diaphyseal fractures should be bypassed by at least two cortical
diameters with a diaphyseal-fitting stem. If the fracture occurs distal
to a well-impacted femoral stem (type C), the stem should be retained
and the fracture should be treated with extramedullary strut and cable
augmentation or formal open reduction and internal fixation.
Intraoperative fractures that are identified in the immediate
postoperative period should be evaluated fully with radiographs to
determine extent. Most of these fractures are stable, minimally
displaced, do not compromise the fixation of the prosthesis, and will
unite successfully without complication.
fractures are usually stable and can be treated with protected weight
bearing and avoidance of abduction for 6 to 12 weeks. Internal fixation
is considered if the greater trochanter has displaced >2.5 cm or if
the patient has pain, instability, and abductor weakness. Type AL
fractures are rare, but if they involve a large portion of the calcar
femorale, they may result in loss of implant stability and therefore
revision THA is necessary. Type B1 fractures should be treated with open reduction and internal fixation with or without cortical strut grafts. B2
fractures are treated with revision to a longer femoral stem and
fracture fixation with cerclage wires with or without cortical strut
grafts. Patients with B3 fractures often require structural
allograft replacement of the proximal femur with an
allograft-prosthetic-composite revision, tumor prosthesis, or a custom
implant. Patients with type C fractures are treated with standard open
reduction and internal fixation.
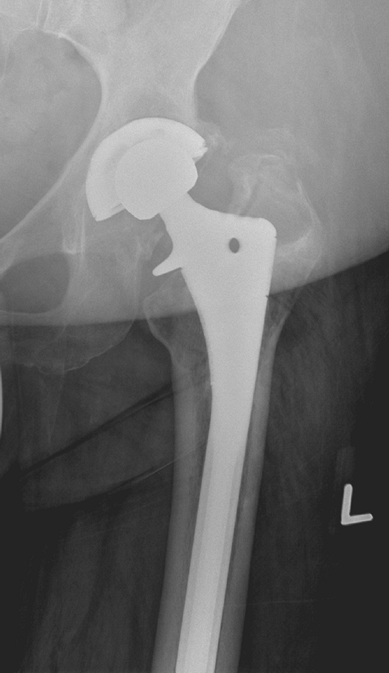
following total hip arthroplasty present a difficult problem because
patients are usually asymptomatic and satisfied with the function of
their existing hip replacement (Fig. 13-7). The
problem becomes more difficult because there is a reported morbidity
and risk of complications associated with surgical revision for
polyethylene wear and osteolysis, and the long-term outcomes of
surgical procedures done to address these problems are unknown.
 |
|
Figure 13-6 Anteroposterior (A) and lateral (B) radiographs of a patient with a Vancouver C periprosthetic fracture. Postoperative anteroposterior (C) and lateral (D) radiographs of the same patient following internal fixation with a locking condylar plate.
|
 |
|
Figure 13-7
Anteroposterior radiograph of a 46-year-old woman demonstrating eccentric wear of the acetabular polyethylene component and severe periprosthetic osteolysis. |
wear, it is not by itself a reason for revision. Revision is indicated
only when polyethylene wear is extensive and complete wear-through is
imminent. For stable acetabular cups, exchange of the polyethylene
liner is recommended. Even if the acetabular component and the locking
mechanism have a bad track record with significantly increased revision
rates, strong consideration should be given to cementing a polyethylene
liner into the retained well-fixed shell. Revision of the acetabular
shell is indicated if it is unstable or nonmodular, if hip stability
cannot be achieved, or if the thickness of the replaced polyethylene
liner is <6 mm thick. Modular femoral heads should be exchanged if
possible when the stem is retained in a revision procedure as the
degree of surface roughness of the femoral head may influence future
wear and subsequent osteolysis. The rationale behind the surgical
treatment of excessive wear is twofold: to prevent complete
wear-through that could damage the inside of the metal shell, and to
replace the debris-producing bearing surfaces with surfaces that wear
less.
osteolysis is based on the likelihood of the patient developing
complications related to the osteolysis (such as cup loosening) during
his or her lifetime. An operation usually is indicated if the lesion is
rapidly increasing in size or if the lesion is eroding away cortical
support of the cup. Treatment is determined on a case-by-case basis; in
general, revision is indicated in most but not all symptomatic patients
and some but not all asymptomatic patients. Although some morbidity is
associated with liner exchange (particularly instability), concern
revolves around the bone loss associated with a full cup revision and
uncertainty as to whether the revised cup will gain bone in-growth.
cardiac, gastrointestinal, renal, or postoperative mental status
changes. These are best prevented through careful preoperative
assessment (often in conjunction with an internist and
anesthesiologist) to identify any modifiable risk factors. Management
of these problems involves addressing the system involved. Rarely, THA
can result in mortality. Death from cardiac arrest during THA has been
described in association with insertion of a cemented long-stem femoral
component. When faced with this scenario, excessive pressurization of
the cement should be avoided, consideration should be given to
placement of a venting hole distal to the femoral isthmus, and invasive
hemodynamic monitoring should be used.
TK, Morrey BF, Ilstrup DM. The elevated-rim acetabular liner in total
hip arthroplasty: relationship to postoperative dislocation. J Bone Joint Surg. 1996;78A:80–86.
SA, Lachiewicz PF, Kelley SS. The influence of patient-related factors
and the position of the acetabular component on the rate of dislocation
after total hip replacement. J Bone Joint Surg. 1997;79A:1202–1210.
EL, Parvizi J, Ciminiello M, et al. The role of patient restriction in
reducing the prevalence of early dislocation following total hip
arthroplasty. A randomized, prospective study. J Bone Joint Surg. 2005;87A:247–253.
AF, Bowerman JW, Robinson RA, et al. Ectopic ossification following
total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973 55(8):1629–1635.
M, Neal B. Non-steroidal anti-inflammatory drugs for preventing
heterotopic bone formation after hip arthroplasty (review). Cochrane Database Syst Rev. 2005;3:1–29.
RC, Cooperstein LA, Kruger MP, et al. Acetabular anatomy and
transacetabular fixation of screws in total hip arthroplasty. J Bone Joint Surg. 1990;72A:502.
PA, Greidanus NV, Masri BA, et al. The prevention of periprosthetic
fractures of the femur during and after total hip arthroplasty. Instr Course Lect. 2003;52:301–308.
KF, Claus AM, Sychterz CJ, et al. Relationship between polyethylene
wear and osteolysis in hips with a second-generation porous-coated
cementless cup after seven years of follow-up. J Bone Jt Surg Am. 2003;85:1095–1099.
KM, Sychterz CJ, Orishimo KF, et al. Polyethylene liner exchange for
excessive wear and osteolysis: a report of 10 cases. J Arthroplasty. 2002;17:798–804.
