PRIMARY TOTAL HIP ARTHROPLASTY
– JOINT RECONSTRUCTION, ARTHRITIS, AND ARTHROPLASTY > Lower
Extremity > CHAPTER 105 – PRIMARY TOTAL HIP ARTHROPLASTY
and predictable procedure that provides pain relief, functional
improvement, and improved quality of life for several hundred thousand
patients each year worldwide (58,71,83,96).
The success of hip arthroplasty is predicated on proper patient
selection, use of well-designed implants, and skilled technical
execution of the procedure. Long-term results demonstrate that THA is
durable in many patient populations (1,10,17,62,78,93,94,120,134), although they also demonstrate durability problems in specific patient groups (19,21,35,37,67).
Durability also depends on implant type, method of fixation, and
technique of insertion. The excellent results of hip replacement have
driven patients and surgeons to expand the indications for the
procedure to patients with higher functional demands and more difficult
anatomic problems, with a consequent increase in the mechanical demands
on implant fixation and prosthesis-bearing surfaces (4,6,23,68,89,122).
this time, operative techniques and implant technology have matured.
Many principles that have contributed to the success of the procedure
are now widely accepted. At the same time, implants and surgical
techniques continue to evolve. Today, surgeons are confronted with many
choices of implant design, fixation type, and bearing surface. This
chapter reviews the indications, results, and techniques of primary
THA. Where possible, the focus is on broadly applicable principles.
Unresolved issues and unsolved problems are also addressed.
diagnosis, symptom severity, the degree of hip joint damage, and the
patient’s functional demands.
milder hip symptoms and less damage to the hip joint, and for those who
by virtue of age, activity level, or medical problems are not
candidates for operative treatment. The mainstays of nonoperative
treatment include nonsteroidal anti-inflammatory medications, activity
modification, use of a cane, and, when appropriate, weight loss.
Arthroscopy has a limited role: it is particularly useful to manage
symptomatic labral tears and intra-articular loose bodies. Pelvic and
femoral osteotomies have a limited but important role, particularly in
the treatment of younger patients with mild to moderate joint damage
and anatomy that is amenable to correction with joint surface
redirection. Many young patients with developmental hip dysplasia and
minimal arthritis are candidates for redirectional pelvic osteotomies.
Some younger adults with proximal femoral abnormalities due to
childhood hip problems such as Perthe’s disease or slipped capital
femoral epiphysis are candidates for intertrochanteric femoral
osteotomy. Selected patients with osteonecrosis of the femoral head may
benefit from femoral osteotomy or other procedures designed to preserve
the femoral head. Finally, arthrodesis is an option for some young
patients with a single destroyed hip joint and high activity demands,
but the operation is less well accepted by patients than it once was
(see Chapter 106).
more difficult to perform or less predictable with respect to outcome
than THA. With the exception of arthrodesis, alternatives to THA are
most successful when advanced hip arthritis is not present. For most
patients of middle age or older with severely damaged hip joints and
marked hip pain, THA offers the most reliable pain relief and
restoration of function.
patients considered for THA is essential. It ensures proper patient
selection and exclusion for the procedure, collection of critical
physical findings, and proper radiographic evaluation. Because most
candidates are older, many have significant comorbidities involving
cardiac, pulmonary, renal, hepatic, neurologic, or hematologic systems.
The patients should be evaluated, when appropriate, by an internist.
-
Determine the source of symptoms.
-
Determine whether the symptoms are severe enough to warrant consideration of THA.
-
Determine whether the patient has contraindications to THA.
caused by intra-articular hip pathology is felt in the groin, buttock,
lateral hip, “deep hip area,” thigh, knee, or a combination of several
of these anatomic areas. Pain is typically increased with weight
bearing and often is worse after the patient has been on his or her
feet for a period of time. Exclude spinal, neurologic, vascular, and
tumor problems as sources of the pain. Establish the symptom severity
by learning the pain intensity and frequency, the degree to which pain
limits ambulation, and the amount the pain limits use of stairs and
other activities, such as putting on shoes and socks, getting in and
out of vehicles, and sexual function. Ask what limitations the hip
problem places on the patient’s life, and consider whether the
limitations that bother the patient can realistically be improved with
arthroplasty. Search for contraindications for which THA should be
considered only cautiously (Table 105.1). Perform a preoperative medical evaluation.
 |
|
Table 105.1. Contraindications to Surgery
|
-
Establish the source of pain felt in the hip area and confirm the functional impact on the patient.
-
Make objective measurements of range of
motion and leg lengths that bear on the feasibility of performing a hip
arthroplasty and that impact on how the hip arthroplasty is performed. -
Establish whether the patient has adequate neurovascular function and muscle strength.
-
Identify any distant sites of infection that might elevate the risk of implant infection.
which the patient leans toward the affected hip in the stance phase of
gait, thereby transferring the center of gravity of the body closer to
the affected hip, and reducing
the
resulting joint reactive force. Pain is often present at the extremes
of hip motion, especially flexion combined with internal rotation. The
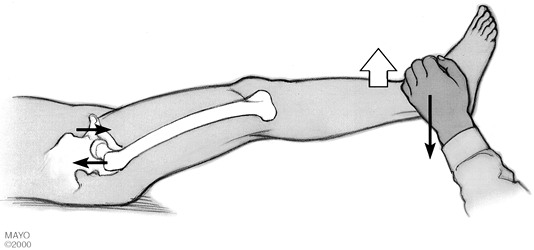
Stinchfield test, in which the patient performs a resisted straight-leg
raise in a supine position (Fig. 105.1),
selectively loads the hip and can distinguish hip joint pain from other
nearby musculoskeletal sources. Soft-tissue tenderness around the hip
can result from trochanteric bursitis or local soft-tissue problems
such as tendinitis. Record hip motion. Measure apparent and true leg
lengths. Check and record neurovascular status. Evaluate the muscle
strength, especially the hip abductors and quadriceps. Look for
evidence of local or distant infection including dental disease or
lower extremity ulcers.
 |
|
Figure 105.1.
The Stinchfield test: With the patient in a supine position, a resisted straight leg raise loads the hip joint selectively. Pain in the hip area (particularly the groin or proximal or anterior thigh) with this test suggests hip joint pathology. |
and the diagnosis leading to the hip pain, establish the severity of
hip disease, and provide information needed for preoperative planning
and templating. The standard radiographs needed for these purposes
include an anteroposterior (AP) film of the pelvis, an AP film of the
affected hip and upper part of the femur, and a lateral view of the
hip. Frog lateral or Lowenstein lateral films provide the best lateral
view of the proximal femur, but true lateral films of the hip are
necessary to provide a lateral view of the acetabulum. Use
magnification markers to aid templating.
magnetic resonance imaging (MRI) scan can demonstrate early
osteonecrosis of the femoral head. Radionucleotide bone scans identify
stress fractures and tumors. Some patients with radiographic evidence
of hip arthritis have atypical or complex symptoms that leave the
source of discomfort in doubt. Intra-articular injection of the hip
with a local anesthetic under fluoroscopic guidance is valuable to
confirm or exclude the hip as the source of pain.
hip replacement include significant hip pain or dysfunction, or both,
caused by intra-articular hip problems. This finding must be confirmed
by radiographic evidence that the hip joint is damaged sufficiently to
explain the symptoms. The symptom severity necessary to justify hip
arthroplasty requires judgment about the potential operative risks and
benefits in a given patient. The more severe a patient’s pain and the
more profound the disability, the stronger the indication for hip
arthroplasty. In distinction, the milder the symptoms and the fewer the
limitations, the more cautiously surgery should be recommended. Most
patients with pain that markedly limits ambulation, interferes with
sleep, or requires narcotic pain medication are strong candidates for
surgery. Patients with moderate to severe pain that occurs with
moderate activity such as walking several blocks also deserve strong
consideration for surgery. Although severe hip pain is the strongest
indication for surgery, marked functional loss associated with less
pain is a relative indication in some patients. Severe loss of function
due to hip stiffness, particularly if it is associated with a marked
flexion contracture, is a relative indication in a few patients. For
less debilitating symptoms, the surgeon and patient must assess the
degree to which the patient’s symptoms restrict activities that the
patient expects to resume after THA, and then they must balance the
risks with the potential benefits of the surgery.
arthroplasty is osteoarthritis. Other diagnoses include inflammatory
arthritis, osteonecrosis of the femoral head, developmental dysplasia
of the hip, other developmental or congenital hip abnormalities,
traumatic conditions leading to secondary degenerative arthritis of the
hip, some proximal femoral fracture nonunions, occasional acute
displaced proximal femur fractures that are not ideal for internal
fixation or hemiarthroplasty, and some tumors around the hip.
replacement. In most circumstances anatomically distant bacterial
infection should be treated before THA. Hip area pain in the absence of
demonstrable hip pathology is a contraindication to surgery. Age,
medical illness, or orthopaedic
comorbidities
that make it unlikely that the patient would obtain substantial
functional benefit from THA are relative contraindications to surgery.
Cognitive, behavioral, and substance abuse problems that may preclude
compliance with critical portions of the postoperative regimen, such as
hip dislocation precautions, are also relative contraindications.
long-term risk for complications should be considered more cautiously.
The threshold for surgery should be higher in patients with medical
comorbidities that put them at markedly elevated operative risk (34).
Young and very active patients who are at higher risk for mechanical
arthroplasty failure and subsequent multiple revision procedures should
consider arthroplasty cautiously. Finally, unrealistic expectations
should prompt a review of the goals of the procedure with the patient
and re-evaluation of the indications for elective surgery.
available. A surgeon must choose between cemented and uncemented
acetabular and femoral implants. If uncemented acetabular implants are
chosen, the surgeon must decide whether to augment the press-fit of the
socket with further fixation such as screws. If cemented femoral
implants are chosen, the surgeon must decide whether to use an implant
with a collar as well as whether to use an implant with a polished or a
rougher surface finish. If an uncemented femoral implant is chosen,
wedge-shaped metaphyseal filling proximally porous-coated implants,
tapered implants, and extensively porous-coated implants are all
available. Implant materials vary, the most common being
cobalt-chromium and titanium. Different surface finishes, including
porous-coated surfaces, plasma-sprayed surfaces, roughened titanium
surfaces, and hydroxyapatite-coated surfaces, are also all available.
The wide array of choices can make the best implant choice for a
specific patient difficult. See Chapter 100 for details on these various prostheses and their biomechanics.
the features associated with success or failure of cemented implants
and uncemented implants.
strong and durable fixation of THA implants to bone. Cement is stronger
in compression than tension. Voids in cement weaken it; technologies
such as centrifugation and vacuum mixing have been introduced to reduce
cement voids. Whether these technologies will reduce cemented implant
loosening rates is unproven. Cement must provide stability at two
interfaces: the interface between the cement and bone, and the
interface between the implant and cement. The bone-cement interface
gains strength from interdigitation of the cement with the
microstructure of cancellous bone and from the internal macrogeometry
of the bone into which the cement is implanted. Cement interdigitation
into bone is achieved by pressurizing the cement into the cancellous
bone microstructure. The best means of providing stability between the
implant and the cement is controversial. Some implants have roughened,
textured, or treated surfaces designed to enhance the strength of the
implant-cement bond (7,24,31,97).
Others have polished surfaces that may remain mechanically stable by
virtue of macrogeometry even if the stem is not bonded to the cement (13,27).
Stability in these cases may be maintained by slight subsidence of the
stem and engagement of the tapered stem in the tapered cement mantle.
Most cemented femoral implants appear to function best when the
component is surrounded by a continuous cement mantle (5).
Defects in the full-thickness cement mantle are associated with
premature loosening of some implant types and localized osteolysis (2,5,40).
as grit-blasted titanium or a ceramic such as hydroxyapatite (32,61).
Smooth press-fit implants that lack coatings or texturing to provide
for bone ongrowth or ingrowth have not enjoyed reliable long-term
fixation in patients with THA (39,76). For porous ingrowth to occur reliably, the optimal pore size appears to be 100 to 400 µm (20).
The most popular materials for biologic fixation have been
cobalt-chromium and titanium. Biologically active osteoconductive
ceramics have gained popularity, although it is unproven whether they
have advantages over porous metal surfaces. Regardless of the surface
material, a key prerequisite for bony fixation of uncemented implants
is stable initial fixation of the implant against bone. Micromotion of
less than 50 µm favors bone ingrowth, whereas larger amounts of
micromotion tend to allow only fibrous fixation (20).
For stable long-term biologic fixation to occur, the implantation must
be securely fit against bone of sufficient strength to resist forces
that could lead to micromotion before bone ingrowth occurs.
The indications for an uncemented socket are especially strong in young
patients, for whom cemented sockets have a higher loosening rate. I
reserve cemented sockets for patients with previous therapeutic pelvic
radiation and for elderly patients with very poor bone quality. Good
cases can be made for both cemented and uncemented femoral stems.
Regardless of the fixation method, femoral results depend on technique,
so choose a method or methods that you can perform well. I tend to use
uncemented femoral fixation for young active patients with good bone
quality and cemented fixation for older patients or those with poor
bone quality. Femoral canal morphology influences decisions about
femoral fixation (Fig. 105.5). Patients with a very small canal diameter and thick cortices (Dorr type A bone) (36)
are good candidates for uncemented implants. Patients with a typical
canal geometry (Dorr type B bone) are good candidates for either a
cemented or uncemented implant. Patients with a very large canal
diameter and thin cortices (Dorr type C bone) are often good candidates
for cemented fixation. Patients with large diameter femoral canals
appear to be at greater risk for thigh pain and stress shielding if
uncemented implants are used. I tend to use uncemented, fully
porous-coated femoral implants for patients with Dorr type A bone (Fig. 105.2), uncemented proximally coated metaphyseal filling implants for young patients with Dorr type B bone (Fig. 105-3), and cemented femoral
implants for older patients with Dorr type B bone and for patients with Dorr type C bone (Fig. 105.4).
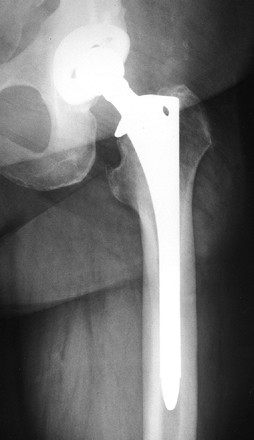 |
|
Figure 105.2. AP radiograph of an uncemented total hip arthroplasty using an extensively porous-coated femoral component.
|
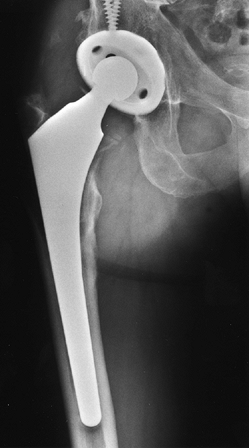 |
|
Figure 105.3. AP radiograph of an uncemented total hip arthroplasty using a proximally hydroxyapatite-coated uncemented stem.
|
 |
|
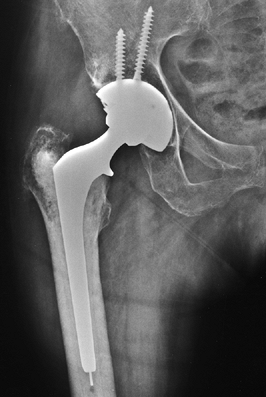
Figure 105.4. AP radiograph of a hybrid total hip arthroplasty using an uncemented socket and a cemented stem.
|
 |
|
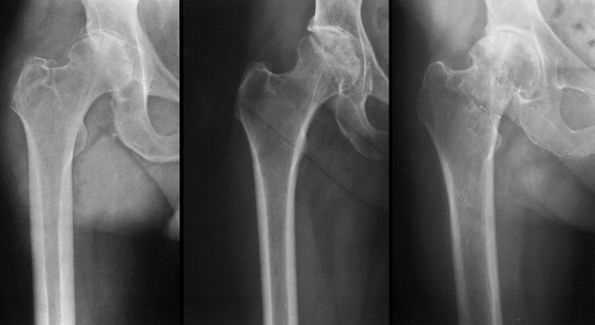
Figure 105.5. The Dorr classification of femoral bone morphology (36).
The proximal femoral morphology is helpful in determining the favored femoral implant geometry and type of fixation. From left to right: Type A, Type B and Type C bone. |
results, but loosening increases with time and is much more common
after the first decade compared with other components. Loosening (90,116,119) is more common in younger patients (4,21).
Acetabular loosening often causes fewer symptoms in the elderly.
Loosening of cemented sockets correlates with the quality of the
bone–cement interface achieved at surgery: better interfacescorrespond
to better implant survivorship. Cemented acetabular loosening is due in
part to linear osteolysis along the bone–cement interface caused by
particulate debris from the joint implant surface (114).
Factors such as younger age, and higher activity and large femoral head
size, which increase polyethylene wear, correlate with higher
acetabular component loosening rates.
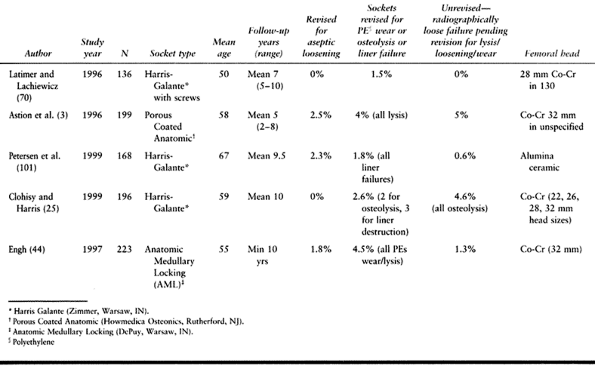
increase durability over cemented sockets. Midterm results demonstrate
that porous-coated uncemented sockets can become osteointegrated and
perform well clinically (25,50,52,70,84,102,112,132) (Table 105.2).
Uncemented porous-coated sockets now have been implanted for more than
a decade, so relevant comparison to cemented sockets is becoming
possible. Results demonstrate that some uncemented sockets can perform
at least as well as cemented sockets 10 years after surgery, even in
demanding patient populations (6). However,
some first-generation uncemented sockets experienced a high failure
rate related to a 32-mm head size, thin polyethylene, poor
polyethylene-socket congruity, and poor liner–shell locking mechanisms (3,136). Many of these problems have been eliminated or reduced with newer designs.
 |
|
Table 105.2. Uncemented Sockets
|
a low loosening rate, and implant fixation to bone appears to be more
favorable for uncemented sockets than for cemented sockets at a similar
time interval. Exceptions to these good results have been threaded,
nonporous-coated sockets and sockets with hydroxyapatite over a smooth
substrate surface, both of which have had higher loosening rates.
two main modes of failure have been osteolysis, and catastrophic
failure of the polyethylene liner. Catastrophic failures are typically
due to wearing through of very thin polyethylene, polyethylene
fracture, or failure of the
liner—shell locking mechanism (12).
Catastrophic failures have diminished markedly with improved locking
mechanisms and recognition that very thin polyethylene shells are
undesirable. Osteolysis remains the single biggest problem associated
with uncemented cups (Table 105.2). The three main strategies used to reduce osteolysis are
-
Reduce wear debris from the bearing surface.
-
Reduce wear debris from nonbearing surfaces.
-
Reduce access of the wear debris to bone.
-
Using smaller femoral head sizes
-
Avoiding titanium femoral heads
-
Avoiding oxidized polyethylene sterilized in air by gamma irradiation (see Chapter 100)
-
Improved liner–shell locking mechanisms
-
Avoiding impingement between the prosthetic neck and polyethylene liner
restricted absolutely from reaching the pelvic bone. Cups without holes
are used with the rationale that eliminating holes from the cup may
reduce debris access to bone. However, some cups without screw holes
have demonstrated high rates of pelvic osteolysis (the particulate
debris gains access to bone peripherally around the cup), so it is
unclear that this strategy reduces lysis rates (111).
The majority of uncemented sockets with midterm results demonstrating
good fixation had screws, pegs, or spikes to augment initial socket
fixation, and it is yet to be proven that uncemented cups placed
without fixation augmentation will perform as well.
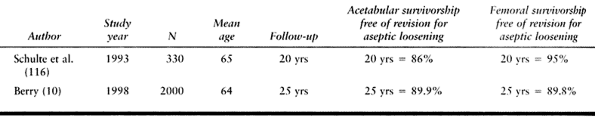
after THA (Table 105.3).
However, some early and some more modern designs have demonstrated high
failure rates. Modern cementing techniques demonstrate good long-term
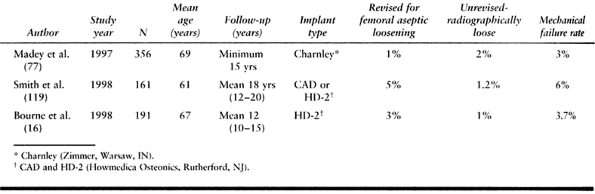
results when coupled with successful prosthesis design (16,56,77,91,119) (Table 105.4).
These techniques include use of a medullary canal plug and retrograde
filling of the canal with cement. Other innovations, such as pulsatile
lavage of the femoral canal, cement pressurization, cement porosity
reduction, and centralization of the implant in the cement mantle, may
further enhance durability of cemented implant fixation, but this
remains unproven.
 |
|
Table 105.3. Cemented Stems—First Generation, Charnley Total Hip Arthroplasty
|
 |
|
Table 105.4. Cemented Stems—Second Generation
|
critically on implant design. Implants with a broad medial border that
reduces medial cement stresses perform better than implants with a
sharp medial border (123). The implant needs to
remain mechanically stable in the cement mantle. There appear to be
several strategies that can successfully achieve this goal. The first
is use of smooth tapered stems that remain stable within the cement
even if the stem is not bonded to the cement (i.e., a taper within
taper arrangement). The second is use of rougher stems that remain
bonded to the cement by virtue of geometry and surface finish. The
surface finish of cemented implants has recently attracted much
attention, based on the finding that if rough surface implants debond
from the cement, osteolysis ensues (121). The
process is caused by the debonded rough stem abrading the surrounding
cement and creating a large amount of particulate debris. This problem
increases with adverse combinations of surface finish and stem
geometry. At present, there is no consensus that a single ideal surface
finish exists for all stems. Rather, it appears that there are certain
combinations of stem geometries and surface finish that function well
together and others that do not.
The problem of thigh pain often resulted from loose implants but also
occurred in some patients with well-fixed stems, probably secondary to
micromotion betweenthe distal tip and femoral endosteum or stress
concen-tration at the stem tip (22,127).
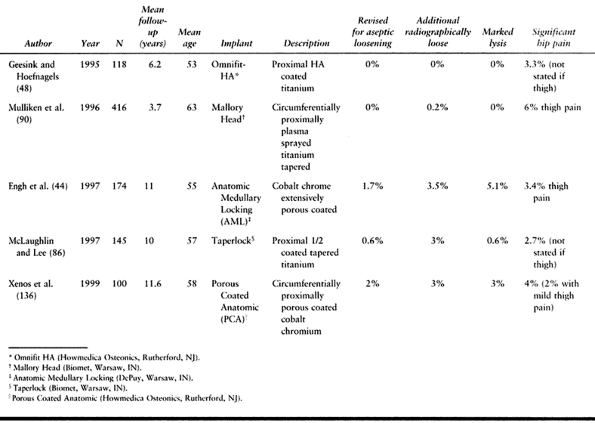
Uncemented femoral de-signs have improved, and now the best clinical
resultsreported with uncemented implants match those of ce-mented
implants (18,33,43,85,100,103,106,107) (Table 105.5).
 |
|
Table 105.5. Uncemented Stems
|
achieve axial and rotational stability in the femur. Implants fixed in
the metaphysis, at the metaphyseal–diaphyseal junction, or in the
diaphysis appear to work in some design iterations. A surface coating
that provides for long-term biologic fixation is necessary. Porous
coating with cobalt-chromium (43,55,100) or titanium beads, titanium fiber metal (84) or plasma spray (86,90), roughened titanium surfaces (106), and hydroxyapatite coatings (48,108)
have been be successful. The main goal of uncemented implants is to
improve long-term durability over cemented implants. Studies of
selected uncemented implants at 10 and more years are encouraging, but
longer follow-up is needed before definitive comparison to cement
fixation can be made.
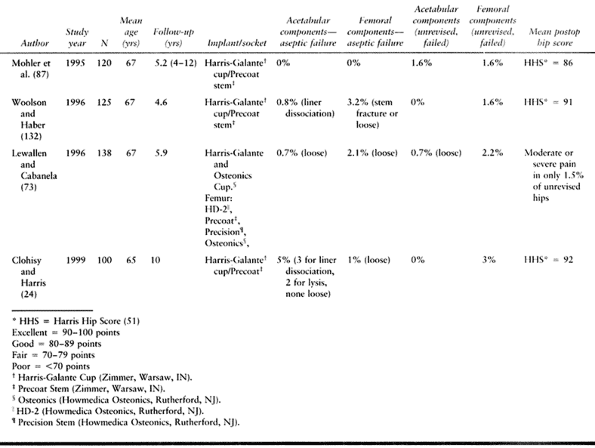
stem is commonly called hybrid hip replacement. The goal of this
combination of implants is to take advantage of the clinical
reliability, durability, and ease of use of uncemented sockets and
cemented femurs. The method has produced excellent midterm results and
is presently popular in North America (Table 105.6).
 |
|
Table 105.6. Hybrid Total Hip Arthroplasty
|
surgery reduces operative time and complications. In planning, also
address leg length and hip stability. Preoperative planning requires
information obtained from the patient’s history, physical examination,
and radiographs.
Both the AP and true lateral films of the hip and pelvis help determine
implant size. The goal is to optimize socket orientation and contact
with native bone and to restore the normal hip center of rotation when
possible. The optimal socket position is 40° to 45° of abduction on the
AP radiograph and 10° to 25° of anteversion on the true lateral
radiograph. Most surgeons prefer to target the lower range of
anteversion when an anterolateral approach to the hip is used, and the
higher range when a posterolateral approach is used. Anticipate areas
where bone removal or graft reconstruction will be required. In most
cases of degenerative disease, plan to remove a small amount of medial
bone to place the acetabular prosthesis in an appropriate position,
that is, touching the lateral aspect of the foveal bone
(radiographically, the teardrop). On the AP radiograph, note the amount
of socket left uncovered laterally. On the true lateral radiograph,
note the position of the socket relative to the ischium and anterior
wall of the acetabulum (Fig. 105.6B). Knowing
the expected relationship of the socket to the pelvic landmarks
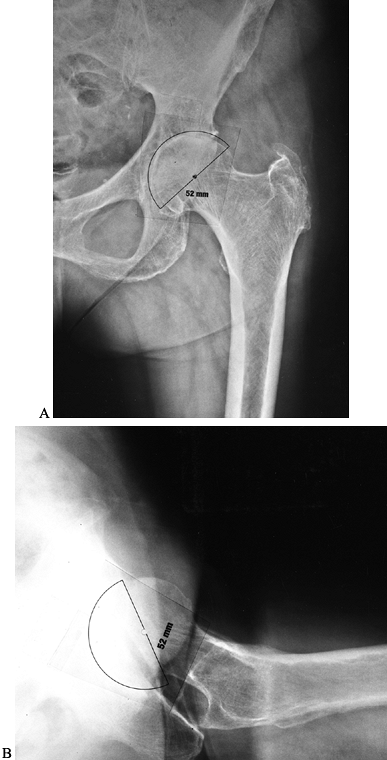
improves socket positioning during surgery. Finally, mark the socket
center of rotation on preoperative radiographs (Fig. 105.6A).
 |
|
Figure 105.6. A:
AP hip radiograph with acetabular component template in place. The cup is oriented in approximately 40° of abduction. The cup is positioned properly, with the medial socket against the lateral aspect of the radiographic teardrop. From the templating, the surgeon can anticipate the need to deepen the socket slightly by reaming away osteophytes medially so as to position the socket properly. The planned center of hip rotation is marked with a dot. B: True lateral hip radiograph with acetabular component template in place. The cup is oriented in about 20° of anteversion. From templating, the surgeon should expect that the rim of the acetabular component will be inset slightly with respect to the anatomic acetabular rim. |
factors and surgeon preference guide the choice of implant design and
fixation. Preoperative templating also plays a role in choosing the
implant design: certain femoral canal geometries are more favorable for
cemented implants, whereas others are better for uncemented implants.
Very poor bone quality, very large canal diameters, and marked canal
deformities are indications to consider cement fixation. In contrast,
excellent bone quality with thick cortices and a small canal diameter
are indications to consider uncemented implants more strongly. The Dorr
classification of canal morphologies (36) is helpful in organizing this decision-making process (Fig. 105.5).
radiograph. Properly sized cemented implants leave room for a cement
mantle of appropriate size and also leave adequate cancellous bone
after broaching for cement interdigitation (Fig. 105.7). Most templates show the outline
of the broach corresponding to each implant size, making it possible to
visualize the amount of intramedullary cancellous bone left after
broaching. Choose uncemented implants to fit and fill the part of the
femur in which that particular implant obtains fixation (Fig. 105.8A and Fig. 105.8B).
Different implants variously obtain fixation in the metaphysis, at the
metaphyseal–diaphyseal junction, and in the diaphysis. Identify the
femoral neck osteotomy level necessary to provide restoration of leg
length. When possible, choose a neck resection level that avoids long
modular femoral necks with a “skirt” because such devices allow less
motion before impingement between the prosthetic femoral neck and the
acetabular polyethylene (126). Choose a prosthesis that restores femoral offset (Fig. 105.9).
If an implant of proper size cannot restore offset, then consider a
stem design with a higher femoral offset. Many implant companies now
provide implants with high and standard offset options. High offset
stems commonly provide better restoration of femoral offset without
excessive leg lengthening in patients with
varus
femoral necks. Restoring femoral offset allows the abductors to
function more efficiently (thereby reducing limp) and also helps
restore soft-tissue tension (thus improving hip stability).
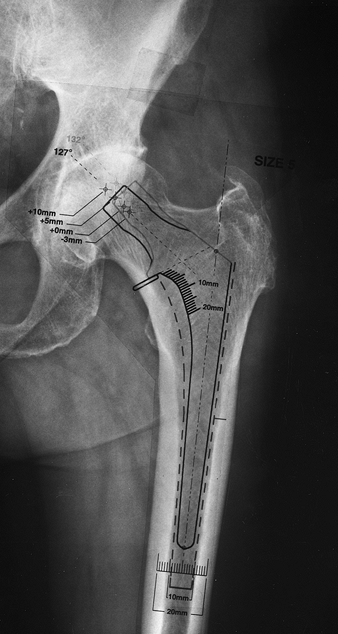 |
|
Figure 105.7. AP radiograph of the hip with template for a cemented femoral component. The template shows the broach envelope (a dotted outline, which simulates the minimum cement mantle), as well as the femoral component (solid line).
Note that the implant size chosen should leave some cancellous bone behind after broaching to provide for cement interdigitation. |
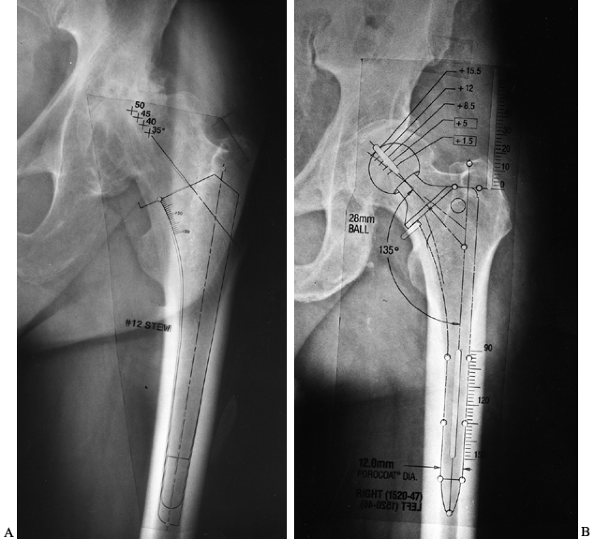 |
|
Figure 105.8. A:
AP hip radiograph template for a proximally coated uncemented stem. Note that with this type of implant, priority is given to obtaining a good fit in the metaphyseal and metaphyseal-diaphyseal junction areas. B: AP hip radiograph with a template for an extensively porous-coated uncemented stem. Note that with this type of implant, priority is given to getting a good fit in the diaphysis. |
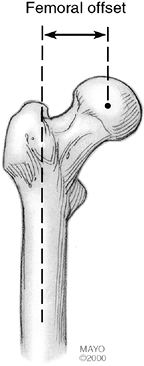 |
|
Figure 105.9.
The femoral offset is the horizontal distance from a line drawn through the center of the femoral canal along its long axis to the center of the femoral head. AP hip radiographs taken with the hip externally rotated tend to underestimate the true femoral offset. |
design, and size on leg length and femoral offset. Once the center of
hip rotation is determined by acetabular templating, the difference
between the femoral head center and the acetabular center on templating
predicts change in leg length: if the templated center of the femoral
head is superior to the templated acetabular center, the leg will be
lengthened by that amount, and if the templated center of the femoral
head is inferior to the templated acetabular center, the leg will be
shortened by that amount (Fig. 105.10). The
position of the templated femoral head center to the templated
acetabular center from medial to lateral corresponds roughly to femoral
offset restoration (Fig. 105.10).
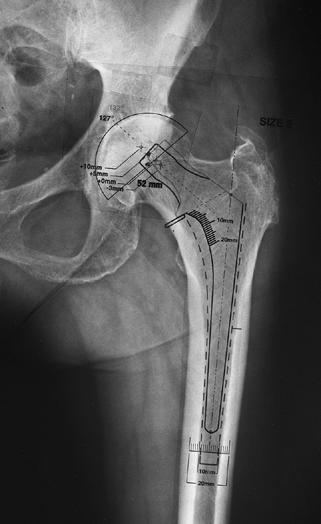 |
|
Figure 105.10.
AP hip radiograph with acetabular and femoral templates demonstrating the position of possible modular femoral neck lengths relative to the planned center of hip rotation (The inferior dot marks the center of the acetabular component.) Femoral head centers inferior to the planned center of the acetabulum shorten the leg relative to the preoperative condition, whereas femoral head centers superior to the planned center of the acetabulum lengthen the leg relative to the preoperative condition. Likewise, femoral head centers lateral to the planned center of the acetabulum reduce femoral offset relative to the preoperative status, whereas femoral head centers medial to the planned center of the acetabulum increase femoral offset relative to the preoperative status. The term femoral offset in this context is used as an approximation because true femoral offset refers only to the femur and is not dependent on the center of the acetabulum. The patient’s symptomatic leg was 4 mm shorter than the opposite side preoperatively so the femoral component position is chosen to provide proper leg length reconstitution with a +5 mm modular neck length. To obtain proper femoral offset, and thus to help restore soft tissue tension and abductor lever arm, a high-offset femoral component is chosen. |
center of rotation for each available neck length, and (for collared
implants) the relationship of the collar to the medial femoral neck.
-
Position the patient in either the
lateral or supine position. Advantages of the lateral position are that
soft tissues fall away from the wound, the assistant has better
visualization of the procedure, and both anterior or posterior
approaches to the hip joint are possible. The main disadvantage is the
possibility the pelvis may tilt during surgery, making acetabular
orientation difficult (Fig. 105.11). This
problem can be reduced by making sure the pelvis is held securely with
a positioning device. Advantages of the supine position include more
certain pelvis position and easier direct comparison of leg lengths
intraoperatively. The supine position restricts the surgical approach,
however: Only anterior hip dislocation is possible. Pad the opposite
leg and arms for all positions to prevent skin and nerve problems. Figure 105.11.
Figure 105.11.
The pelvis can roll when the patient is in the lateral decubitus
position. This is particularly common with the posterior approach, in
which femoral retraction tends to cause the pelvis to roll forward.
When the pelvis is rolled forward, the acetabular component anteversion
falsely appears to be greater than it is in reality. Failure to
recognize this problem can lead to insufficient acetabular component
anteversion and posterior hip instability. -
Give the first dose of antibiotics within
an hour before the skin incision is made. Perioperative antibiotics are
one of the most effective means of preventing infection. For most
patients, use a broad-spectrum, first-generation cephalosporin. For
patients with an allergy that precludes using cephalosporin, use
another intravenous antibiotic with a gram-positive bacterial spectrum.
Continue the prophylactic antibiotic for 24 to 48 hours after surgery. -
A variety of exposures are available for
THA. Most can be categorized as variations of anterolateral,
posterolateral, or transtrochanteric approaches (see Chapter 3).
The main advantage of anterolateral approaches is the low dislocation
rate; the main disadvantage is the potential for abductor muscle
dysfunction to cause a limp after surgery (owing to poor abductor
healing or abductor denervation). The posterior approach spares the
abductors, and postoperative limp is uncommon; the main disadvantage of
the posterior approach is a higher reported rate of hip dislocation.
Both anterior and posterior approaches have strong followings, and the
choice of approach is best left to the surgeon. Consider patients at
very high risk for posterior dislocation good candidates for an
anterolateral approach. I use both approaches selectively; I use the
anterior approach for patients at higher dislocation risk and the
posterior approach for patients judged at lower dislocation risk. The
transtrochanteric approach provides excellent hip exposure but is
associated with more blood loss and a risk of trochanteric nonunion or
migration. Reserve the transtrochanteric approach for unusual cases
with special anatomic considerations, such as marked proximal femoral
deformities or revision surgery. -
In the anterolateral exposure,
make an incision directly over the proximal lateral femur centered on
the greater trochanter. Curve the incision slightly posteriorly at the
proximal third of the incision. Divide the iliotibial band in the
direction of its fibers, and split the interval between the tensor
fascia lata and gluteus maximus. Reflect the anterior third of the
gluteus medius and the gluteus minimus in continuity with the anterior
third of the vastus lateralis from the proximal femur. To avoid injury
to the superior gluteal nerve, take care not to extend the incision in
the gluteus medius more than 5 cm from the trochanter (60,104).
Note that the gluteus minimus fibers run at 90° to those of the gluteus
medius. The anterior, superior, and inferior hip capsule can be excised
to gain exposure or can be preserved if exposure is satisfactory. Place
a retractor in the ilium to hold the
P.2783
soft tissues out of the field. See Chapter 3 for more details. -
In the posterior exposure,
make a Kocher-style incision centered over the greater trochanter. The
distal limb is parallel to the femur and the proximal limb is parallel
to the gluteus maximus muscle fibers. Split the iliotibial band in the
direction of its fibers and then divide the fascia of the gluteus
maximus in the direction of its fibers in line with the skin incision.
Split the gluteus maximus using a muscle-splitting technique. Identify
the sciatic nerve by visualization or palpation. Protect it throughout
the procedure. Identify the piriformis tendon and divide the fascia
along its superior border. Pass a periosteal elevator through the
interval between the gluteus minimus and the hip capsule. Detach the
short external rotators from the posterior femur to expose the
posterior hip capsule. Keep the short external rotator tendons as long
as possible and tag them with a suture. Control branches of the
circumflex femoral vessels on the deep border of the quadratus femoris
muscle. Perform a capsular incision inferiorly and superiorly that
extends from inferior to superior, then take the capsule down from the
femur, leaving it attached to the posterior wall of the acetabulum.
This creates a large posteriorly based capsular flap that can be
repaired later (101). Make the capsular flap the maximum size possible. See Chapter 3 for more details. -
Place a pin in the pelvis if you intend
to make direct leg length measurements intraoperatively, and mark a
point on the femur against which to compare measurements. -
Dislocate the hip and identify the lesser
trochanter. Use cutting guides or implant trials to mark the level and
orientation of the femoral neck osteotomy. Base the level on
preoperative planning relative to the greater trochanter, center of the
femoral head, and lesser trochanter. Cut the femoral neck with an
oscillating saw. If anterolateral exposure is used, retract the femur
posteriorly with a bone hook. If posterior exposure is used, retract
the femur anteriorly with a cobra retractor placed against the anterior
wall of the acetabulum. Make sure no soft tissues are interposed
between the retractor and the pelvis. In the posterior approach,
release the gluteus maximus tendon to facilitate anterior retraction of
the femur if needed. Anterior capsulotomy also helps translate the
femur anteriorly. Remove the labrum and visualize the entire rim of the
acetabulum. Proper acetabular preparation is not possible without
satisfactory acetabular exposure. Poor exposure increases the
likelihood of acetabular implant malposition and hip instability. Place
retractors carefully around the acetabulum to avoid injury to
neurovascular structures. If a posterior retractor is placed, make sure
it is against bone and that the sciatic nerve is protected. -
For a cemented acetabulum,
remove remaining soft tissues from the fovea. Good visualization of the
floor of the fovea provides a guide to determine the depth of reaming.
Remove large osteophytes around the inferior, posterior, and anterior
rim of the acetabulum with an osteotome or rongeur. Superior
osteophytes can usually be left in place. Preserve the transverse
ligament because this will help restrict inferior cement extrusion.
Remove remaining acetabular cartilage with a large curet. Use small
reamers to remove bone to, but not beyond, the base of the lateral
aspect of the fovea. The fovea corresponds to the teardrop
radiographically, and reaming usually should not be deeper than the
lateral fovea, except when a shallow socket is present. Use
sequentially larger reamers to create a hemisphere of bleeding
subchondral bone, keeping the reamer centered in the acetabulum. Strike
a compromise between excessive reaming, which removes all the
subchondral bone and leaves behind only very weak bone, and
insufficient reaming, which leaves a bed of only sclerotic bone. If
only weak bone is left, loosening can result from implant migration
through soft bone; conversely, if only sclerotic bone is exposed, a
poor bone–cement interface may result, leading to early loosening. -
Make holes for cement fixation into the
ilium, the pubis, and the ischium with a curet, large burr, or drill.
Some surgeons prefer a few large holes into each area, whereas others
prefer multiple smaller holes 1/8 to ¼ inch in
diameter. Larger holes remove more bone but expose more cancellous bone
for cement interdigitization. I prefer multiple moderate-sized drill
holes. Place a trial implant into the socket and position it
appropriately. Note its position relative to pelvic landmarks. Mix the
cement and wait until it reaches a doughy stage. Introduce the cement
into the socket and pressurize it into the bone. The cement can be
finger pressurized, or pressurized with a cement gun or special
acetabular pressurizers. A sponge inside a rubber glove works well for
this purpose but tends to extrude cement around the rim of the
acetabulum. I prefer to pressurize the cement into each part of the
acetabular surface with my thumb. -
Insert the socket into the acetabulum
with a positioning device and hold it securely in proper position until
all cement is hardened. The ideal socket position is 40° to 45° of
abduction and 10° to 25° degrees of anteversion. Many surgeons err on
the lower side of anteversion for the anterior approach and the higher
side for a posterior approach. Remove any cement around the periphery
of the socket before it hardens. Ensure that no large pieces of cement
remain after all the cement is hard. -
For an uncemented socket,
remove soft tissue from the fovea and large inferior, anterior and
posterior osteophytes. Excise the transverse ligament. Use a small
reamer to reach the lateral aspect of the fovea and then ream with
sequentially larger reamers to expose a bleeding
P.2784
hemisphere
of subchondral bone. Keep the reamers centered in the acetabulum by
judging the reamer position relative to the peripheral rim of the
socket. There is a tendency for power reamers to migrate superiorly and
posteriorly. Maintain pressure on the reamer to keep it centered. Use a
curet to remove cysts. Feel for a residual prominent rim of bone around
the periphery of the socket. This rim of bone may be left behind
inadvertently during the reaming process but should be removed with a
burr or reamer before inserting an implant. Place particulate
cancellous bone graft obtained from the reaming into any cysts and into
any defects left in the area of the fovea. Test the size of the
acetabular hemisphere with trial implants. Impact an uncemented implant
into the acetabulum. In most cases, choose an implant 1 or 2 mm larger
than the size of the last reamer to obtain a good press-fit. Position
the socket as recommended for cemented implants. Make sure the socket
is “bottomed out” (i.e., that it contacts bone not only peripherally
but also in the dome). I always use screws, fins, or pegs to augment
fixation, but some surgeons do so only selectively. If none are used,
it is imperative to make certain that the press-fit of the socket is
very rigid. Avoid placing screws anterior to a line drawn from the
anterosuperior iliac spine to the ischium to avoid injury to
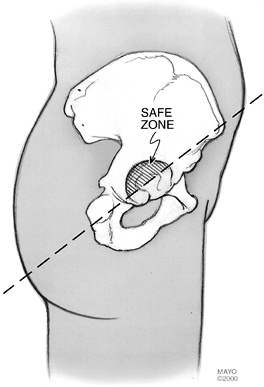
intrapelvic vascular structures (63,129) (Fig. 105.12). The best area is the superior dome of the acetabulum into the ilium. Place a trial liner in the socket.![]() Figure 105.12.
Figure 105.12.
The “safe zone” for acetabular screw placement (Redrawn from Wasliewski
RC, Cooperstein LA, Kruger MP, Rubash HE. Acetabular anatomy and the
transacetabular fixation of screws in total hip arthroplasty. J Bone Joint Surg
1990;72A:501, with permission.) In the anterior two quadrants, the risk
of neurovascular injury if a drill or screw extends beyond the far
cortex is high. In the posterior two quadrants, there is less risk but
the sciatic nerve still needs to be respected. -
Before preparing the femur, remove soft
tissues just medial to the greater trochanter to allow access to the
piriformis fossa. If these soft tissues are not removed, instruments
and the implant tend to be pushed into a varus position. Open the
femoral canal with a T-handled broach or awl. The proper starting point
is usually the piriformis fossa, which is located directly over the
medullary canal. Avoid an excessively medial starting point, which can
lead to varus alignment. There is a tendency with a high neck cut to
introduce instruments into the canal in flexion due to femoral neck
anteversion; avoid this problem by starting more posteriorly in the
femoral neck. -
For a cemented femur,
prepare the femur with broaches alone or reamers and broaches. Use
reamers only to start the canal preparation, and avoid excessive
reaming. Good remaining cancellous bone is required for cement
interdigitation. As the canal is prepared, avoid varus or valgus
orientation of the instruments. Broach the canal in proper anteversion,
usually about 15°. After the final broach is in place, use a calcar
planer to smooth the femoral neck. Perform a trial reduction and check
anterior and posterior hip stability, leg length, and soft-tissue
tension. Remove any loose, unsupportive, cancellous bone from the canal
with a uterine curet or canal brush. Place a cement restrictor at an
appropriate depth in the femoral canal to provide about 2 cm of cement
distal to the tip of the stem. Resorbable or permanent plugs
(polyethylene) are convenient. Plugs can also be created by the surgeon
from bone or methacrylate. Clean the intramedullary canal with
pulsatile lavage and dry with sponges or a vaginal packing gauze. Some
surgeons use sponges with thrombin or dilute epinephrine to reduce
canal bleeding. Keep the canal packed until you are ready to inject the
cement. The best means of preparing cement is controversial: Some favor
mixing under a vacuum, some favor centrifugation, and others use
neither. Both vacuum mixing and centrifugation were developed to reduce
cement porosity, which may improve cement strength. Introduce the
cement into the canal retrograde using a cement gun. Pressurize the
cement column to allow the cement to interdigitate into the cancellous
bone. Introduce the femoral component
P.2785
into the canal and hold it securely in proper position until all cement has hardened. -
The methods of implanting an uncemented femoral component
are specific to the implant design. Techniques associated with each
implant differ because of different instrumentation and because
different parts of the femur are used to gain fixation. Nevertheless,
several important points are common to insertion of most uncemented
femoral components. Obtain rigid fixation of the implant against
strong, hard bone. Recent understanding of uncemented implant fixation
has emphasized the importance of gaining rigid rotational stability of
uncemented implants. Proper and precise sizing of the uncemented
implant is essential to obtain initial rigid fixation and subsequent
biologic fixation. Prepare the femur with reamers or broaches until
contact is made in the appropriate area of the canal with hard
endosteal or cortical bone. A press-fit of the real implant is
predicated on having a cavity slightly smaller than the implant. Too
large a cavity from imprecise preparation or poor instruments provides
unsatisfactory initial implant stability. Too small a cavity can lead
to femoral fracture when the implant is inserted. Be sure to prepare
the femoral canal in the proper orientation. Avoid varus malalignment,
which gives the tactile impression that canal preparation is complete
prematurely and leads to implant undersizing. -
Perform a trial reduction before the real
femoral component is inserted and again after the real implant is
securely in place. Many surgeons perform this step after the real
socket has been placed, but some perform a trial reduction with both
trial acetabular and femoral implants. The goals of trial reduction are
to test hip stability and leg length, and to exclude prosthesis
impingement. Reduce the hip and test posterior hip stability with the
hip flexed, adducted, and internally rotated. Test the hip in the mid
zone of flexion with maximum adduction (the so-called “position of
sleep”). Test anterior stability of the hip in extension and external
rotation. Some surgeons take an AP radiograph of the pelvis with the
trial implants in place to check position and radiographically verify
restoration of leg length and center of rotation of the hip. -
If posterior stability is unsatisfactory,
determine the cause. Is there insufficient acetabular or femoral
anteversion? Is there soft-tissue or bony impingement between the femur
and pelvis? Is soft-tissue tension insufficient? If implants are not
ideally positioned, reposition the appropriate component. Small
adjustments in acetabular component position can be made using elevated
lip liners if modular uncemented sockets are used. Elevated lip liners
increase hip stability in one direction at the expense of hip stability
or range of motion in the opposite direction (69).
If impingement is present, try to remove the source. Commonly, some
remaining an-terosuperior hip capsule, part of the head of the rectus
femoris tendon, or the anterior aspect of the greater trochanter are
sources of impingement with the hip flexed and internally rotated.
Redundant posterior inferior capsule can cause impingement between the
femur and ischium with the hip in extension and external rotation. -
Test the soft-tissue tension. If it is
lax, a longer neck length may be needed. Soft-tissue tension is often
less in small women or in patients with profound muscle relaxation.
Relying solely on soft-tissue tension to choose neck length can lead to
inadvertent overlengthening of the limb. Sometimes soft-tissue tension
can be improved without increasing leg length by increasing the femoral
offset. Increase offset by using a high-offset femoral implant or by
placing the same implant deeper in the femur and using a longer neck
length. Make sure the prosthetic femoral neck does not impinge on the
acetabular polyethylene. If it does, consider repositioning the
acetabular component. Prosthetic impingement can lead to hip
instability or polyethylene wear. -
Judge whether leg length has been restored according to the preoperative plan (133).
If a pin was placed in the pelvis intraoperatively, compare the
distance from the pin to the fixed point marked on the femur (compared
with the same measurement performed before femoral neck osteotomy). Use
bony landmarks, including the position of the femoral head relative to
the greater trochanter and position of the femoral collar relative to
the lesser trochanter, to help estimate the leg length based on
preoperative templating. Compare the position of the patient’s knees
and heels to one another compared with their relative position before
femoral neck osteotomy. The legs must be in the same relative positions
for this to be a valuable test. An intraoperative radiograph of the
pelvis and both hips can provide information on leg lengths. None of
these tests of leg length are perfect, but all provide some information
concerning relative leg length in surgery. -
Irrigate the wound and make sure fragments of cement or bone are removed. Obtain good hemostasis.
-
To close the anterolateral approach,
reattach the gluteus minimus, medius, and vastus lateralis muscles to
their beds using strong absorbable or nonabsorbable sutures passed
through bone. Meticulous repair is necessary because failure of the
abductors to heal can cause limp and hip instability. -
To close the posterolateral approach,
reapproximate the capsule and short external rotators to the posterior
aspect of the abductors and the greater trochanter. If possible,
reattach the soft tissues with sutures passed through bone. This may
not be possible if the leg has been lengthened or if the femoral offset
is markedly increased. Avoid overtensioning the piriformis tendon,
which passes over the sciatic nerve. A good repair of
P.2786
the capsule and short external rotators may reduce the risk of early hip dislocation after a posterior approach (Fig. 105.13) (101).
Close the remaining tissues in layers. I prefer, as do others, to use
closed suction drains; some surgeons do not. Treat the patient with
intravenous antibiotics for 24 or 48 hours after surgery. There is no
evidence that longer treatment has any advantage.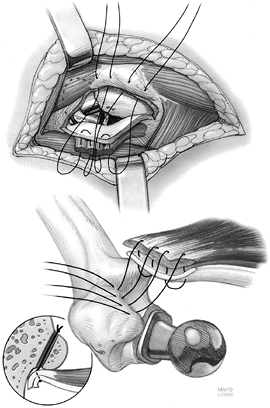 Figure 105.13.
Figure 105.13.
Diagram showing formal soft tissue repair of the posterior capsule and
short external rotators after posterior approach to the hip.
thromboembolic disease. Most North American surgeons use some
prophylaxis for this potentially life-threatening complication (75,92). Available measures include warfarin (28,74), low-molecular-weight heparin (29,98,105), unfractionated heparin, aspirin, dextran, and mechanical foot or leg compression devices (128,131). Warfarin or low-molecular weight heparin is widely used in North America (18,29,46).
If warfarin is used, the target international normalized ratio (INR) is
usually 1.5 to 2.2. The ideal treatment duration is not defined and
varies from 1 to 6 weeks. I prefer 2 to 3 weeks of treatment under
routine conditions, and 6 weeks for patients with a history of venous
thromboembolic disease or strong risk factors for thromboembolic
disease. Low-molecular-weight heparin should be used according to the
manufacturer’s recommendations concerning timing of administering the
first dose. If the drug is given too early, bleeding complications are
more common. See Chapter 5 for additional details.
surgery. Individualize the weight-bearing regimen according to the type
of implant fixation. Most surgeons allow cemented and hybrid hip
replacements to begin partial weight bearing immediately. The duration
of arm support varies, but by 6 weeks most patients can begin to make
the transition to a cane. The postoperative regimen after uncemented
THA is more controversial. Some allow immediate weight bearing, whereas
others restrict weight bearing for 8 to 12 weeks. Those who advocate
immediate weight bearing believe that it stimulates bone healing; those
who advocate protected weight bearing believe it provides time for
tissue ingrowth before maximally loading the femoral implant.
Show the patient hip positions that can lead to dislocation and
demonstrate how to avoid them. Show the patient how to rise from a
chair and get in and out of bed safely.
provide excellent pain relief and a very good level of function for
moderate activities. Studies have demonstrated a high level of patient
satisfaction after THA (58,71,83).
For patients with well-fixed implants, most decrements in function in
large series are due to increasing disability with age (62). When implants fail for mechanical reasons, many patients develop pain and poorer function (95)
and require revision surgery. Thus function free of mechanical failure
is considered a key parameter of arthroplasty success. Mechanical
failure rates depend on fixation method, implant design, and
demographic factors (Table 105.2, Table 105.3, Table 105.4, Table 105.5 and Table 105.6).
of perioperative antibiotics (45).
Careful surgical technique, low traffic in the operating theater, body
exhaust suits, and laminar flow may also reduce the risk of early
infection (47). Late prosthetic infection can
occur from bacteremia. Recent guidelines developed by the American
Academy of Orthopaedic Surgeons suggest that for 2 years after hip
arthroplasty, prophylactic antibiotics be given to all patients for
dental and other procedures that might cause bacteremia. The
recommendations for prophylaxis for dental or oral procedures for
patients with no penicillin allergy are for amoxicillin 2.0 g orally 1
hour before a dental procedure and for amoxicillin 2.0 g orally before
a gastrointestinal or genitourinary procedure. Alternative antibiotics
for dental or oral procedures are cephalexin 2.0 g orally or
clindamycin 600 mg orally 1 hour before the procedure, and for
genitourinary or gastrointestinal procedures, ciprofloxacin 750 mg
orally 1 hour before the procedure. For immunocompromised patients,
other patients at high risk for infection, and for certain high-risk
procedures, lifetime prophylaxis for dental or other invasive
procedures is recommended.
that requires prompt treatment. If the problem is identified early,
infection may be treated successfully with prompt surgical treatment
and proper antibiotics. More typically, however, treatment of the
infection requires implant removal. In North America, most surgeons
prefer a two-stage approach to treat most patients with infected
implants, but under specific circumstances, one-stage reimplantation
may also be considered (47) (see Chapter 135).
different series, from 0.3% to greater than 10%. In most large series,
the range is around 2% to 3%. The period of highest risk for
dislocation is the first 3 months after surgery, but there is always
some risk of dislocation, and first-time dislocations can occur even
years after a THA. Certain patient populations are at risk for
dislocation: females have a higher risk than males (130), and patients treated with a posterolateral approach are at higher risk than those treated with an anterolateral approach (130).
The risk of dislocation can be minimized by preoperative,
intraoperative, and postoperative measures. Preoperative measures
include appropriate patient selection, careful templating, and
consideration of an anterolateral approach for patients at high risk
for posterior dislocation. Intraoperative attention to component
position (especially the acetabulum), as well as soft-tissue tension,
care to seek and remedy impingement sources, and meticulous repair of
the tissues during closure help reduce the risk of dislocation.
Postoperative patient education and prophylactic abduction bracing for
selected patients are also helpful.
Before reduction, make a true lateral radiograph of the hip (in
addition to a standard AP radiograph) to confirm the direction of
dislocation. Most first and second dislocations are treated
nonoperatively with patient education and with an abduction bracing
program; however, re-current dislocations usually require reoperation.
Whenreoperation is considered, determine the etiology ofdislocation.
Component malposition, impingement, or inadequate soft-tissue tension
are the most frequent categories. Once the specific problem has been
identified, institute specific measures to address that problem.
Conversion to constrained sockets (49) or
bipolar implants are also effective means of managing dislocation, but
these measures are usually reserved for problem patients who have
failed previous operative attempts to gain hip stability or who have
markedly deficient abductor mechanisms. See Chapter 106 for additional details.
Injury to the sciatic nerve, particularly the peroneal division of that
nerve, is probably the most common injury. Women, patients with
peripheral neuropathy, and patients with lumbar disc disease are at
increased risk (115). Marked lengthening of the
leg is associated with a higher risk of palsy, but a specific cut-off
for the maximum amount of lengthening that can be performed safely has
not been identified. The etiology of nerve injury is unknown in many
cases (109,115).
Reoperation is indicated if hematoma is thought to contribute to the
palsy. Many patients recover significant function over time; some early
recovery of function and initial incomplete dysfunction correlate with
better likelihood of eventual complete recovery. Treatment during the
recovery period involves bracing with an ankle-foot orthosis, skin
care, and pain management for dysesthetic pain.
The main vessels at risk are the femoral artery anteriorly, the
obturator artery inferiorly, and the iliac vessels medial to the
pelvis. Avoid socket screw placement anterior to a line between the
anterior superior iliac spine and ischium to minimize the risk of iliac
vessel injury (63,129).
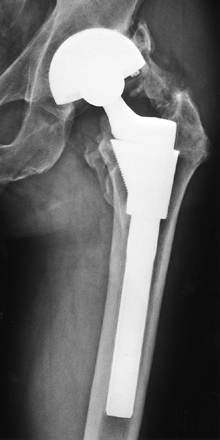
hip range of motion is uncommon (Fig. 105.14).
Patients at risk include those who have previously formed heterotopic
bone after another hip operation, patients with diffuse idiopathic
skeletal hyperostosis (DISH), patients with pelvic Paget’s disease, and
probably patients with active ankylosing spondylitis. Men are at higher
risk than women. Heterotopic bone formation can cause pain while the
process is active early after THA. The process may not be visible on
radiographs for 6 to 8 weeks after the operation. Mature ectopic bone
typically does not cause pain.
 |
|
Figure 105.14. AP hip radiograph demonstrating heterotopic bone formation. The patient had marked limitation of motion.
|
formation are available but must be instituted early (preferably within
3 days postoperatively) to be effective (30,66).
Nonsteroidal anti-inflammatory medicines given for a duration of 2 to 6
weeks after surgery reduce the risk of heterotopic ossification (65).
Low-dose radiation therapy (600 to 800 cGy) given in a single or
divided dose to the field at risk either immediately before or within
the first 3 days after surgery has also proven effective (54,99).
Radiation shielding is useful to avoid irradiation of bone around
uncemented implants and to avoid irradiation of trochanteric
osteotomies or abductor muscles. Patients that develop heterotopic bone
with marked limitation of range of motion can be treated effectively
with surgical excision of heterotopic bone. Most surgeons prefer to
wait 1 year after THA to excise the bone. If heterotopic bone is
excised, treat the patient with an effective method to prevent
recurrence. See Chapter 124 for additional information.
femur can occur during hip arthroplasty and are much more common during
insertion of press-fit uncemented implants (8).
Acetabular fractures are less common than femur fractures. Minimize
acetabular fractures by avoiding an excessive discrepancy between the
diameter of the final reamer and the outside diameter of the socket and
by testing with trial components before impaction of the final implant (64).
Most intraoperative acetabular fractures are minor cracks of the
posterior wall of the acetabulum that do not compromise acetabular
implant stability or pelvic integrity. More extensive fractures occur
rarely and may require stabilization of the pelvis with plates. When an
acetabular fracture is recognized intraoperatively, consider augmenting
fixation of the socket with screws (118).
Intraoperative femur fractures can be minimized by careful attention to
preoperative planning, proper implant sizing, and surgical technique.
Fractures occur most often during broaching and during implant
insertion. Patients with unusual femoral geometries and poorer bone
quality are at higher risk. A prophylacticcerclage wire around the
proximal femur may preventfracture during canal preparation or implant
seatingin patients at high risk for fracture. The pattern of
intraoperative fractures varies according to implant design.
Metaphyseal fractures of the femoral neck are most common with
proximally coated metaphyseal filling implants, whereas extensively
coated diaphyseal filling implants more often cause diaphyseal cracks (117).
Most small cracks that do not compromise implant fixation or femoral
integrity can be treated with cerclage fixation without changing
femoral component design. If the fracture compromises implant fixation,
a different implant is required; the type chosen depends on fracture
location and severity.
techniques have reduced the problem of implant loosening, but it still
is a major long-term problem. Choosing well-designed implants and using
meticulous surgical techniques are the most effective means to reduce
loosening.
For
cemented components, make sure implant alignment is optimum, obtain
good cement interdigitation with bone, and obtain an adequately thick,
circumferential cement mantle. For uncemented implants, size the
implant optimally and obtain secure fixation to good-quality bone. Most
patients with implant loosening develop pain: acetabular loosening is
most commonly felt in the buttock or groin, femoral loosening most
commonly in the thigh. Weight-bearing pain associated with loose
implants is often triphasic: patients experience pain with initiation
of weight bearing, feel better after a short distance of ambulation,
then develop more pain as they walk farther. The treatment of most
symptomatic loose implants for medically fit patients is revision
surgery (see Chapter 106).
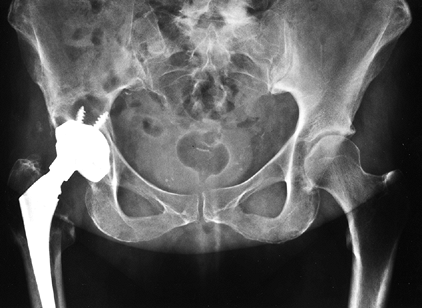
of the polyethylene bearing surface and associated osteolysis have
become limiting factors in the durability of many arthroplasties,
particularly in young active patients (Fig. 105.15).
Some wear of the polyethylene bearing surface of THA occurs in all
patients. Marked polyethylene wear leads to billions of submicron-sized
polyethylene debris particles in the periprosthetic space (57,110). This debris causes osteolysis (81)
by a pathway, the details of which are still being worked out, that
involves mediators of inflammation and a cellular response. Factors
known to correlate with increased volumetric polyethylene wear (and
hence more polyethylene debris) include large femoral head size (32 mm
or greater), thin polyethylene (less than 6 mm), titanium femoral
heads, and polyethylene oxidized by gamma irradiation in air (11,124).
There is some evidence that ceramic femoral heads against
polyethylene-bearing surfaces may produce less polyethylene wear.
However, to produce a dramatic reduction in the problem of polyethylene
wear and associated osteolysis, major efforts are being directed toward
the development of new bearing surfaces. These include
ceramic-on-ceramic surfaces, metal-on-metal bearing surfaces, and a new
class of highly cross-linked polyethylenes. Further clinical experience
is needed to determine whether these new bearing surfaces will
dramatically reduce the problem of bearing surface wear and osteolysis
without causing new unanticipated problems (135).
 |
|
Figure 105.15.
Marked polyethylene wear (note femoral head eccentricity in the socket) and associated extensive periprosthetic acetabular and femoral osteolysis. |
patterns based on access of the debris to the periprosthetic pelvic and
femoral bone (9,14,80,113,125).
When marked particulate debris–induced periprosthetic osteolysis
occurs, intervention should be considered. The specific type of
treatment is contingent on the status of implant fixation, the pattern
of osteolysis, and specific features of the acetabular and femoral
implants (see Chapter 106) (9,79).
changes the stress distribution in the proximal femur and leads to bone
remodeling (26,59).
Bone loss around femoral implants by this mechanism is called stress
shielding. The amount of bone loss that occurs, regardless of implant
design fixation type, correlates with the quality of bone prior to hip
implantation (82). Bone that is more osteopenic
preoperatively leads to a higher percent of postoperative bone loss.
Larger, stiffer, uncemented implants, and particularly extensively
coated implants in which distal bone ingrowth is possible, appear to be
at higher risk for marked stress shielding (43,44). If stress shielding occurs,
in most cases, only observation is recommended. To date, the primary
problem caused by stress shielding has been poor bone quality if a
further operation is required. As long as the stem continues to
function well, stress shielding appears to remain a clinically silent
problem.
according to their location and the fixation status of the femoral
component. An excellent classification system that helps guide
treatment is available (38). Treat fractures
involving the trochanters operatively if the displacement is
unacceptable or if the etiology is progressive osteolysis. Fractures
around the femoral stem or stem tip almost always need to be treated
surgically. Nonoperative treatment is associated with a high rate of
nonunions and malunions, and requires prolonged immobilization. Most
fractures around well-fixed stems can be treated with open reduction
and internal fixation using strut allografts and plates that allow
fixation with combinations of cerclage, wires or cables, and screws.
See Chapter 20 for more details. Fractures
around loose implants are treated with revision, usually to longer
stemmed implants in association with fracture stabilization with
cerclage fixation, strut allografts, or plates (72).
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
PP, Gie GA, Howie CR, Ling RSM. Localized Endosteal Bone Lysis in
Relation to the Femoral Components of Cemented Total Hip
Arthroplasties. J Bone Joint Surg 1990;72B:971.
DJ, Saluan P, Stulberg BN, et al. The Porous-coated Anatomic Total Hip
Prosthesis: Failure of the Metal-backed Acetabular Component. J Bone Joint Surg 1996;78A:755.
WT, Callaghan JJ, Sullivan PM, Johnston RC. The Results of Improved
Cementing Techniques for Total Hip Arthroplasty in Patients Less than
Fifty Years Old. A Ten-year Follow-up Study. J Bone Joint Surg 1994;76A:959.
RL, Mulroy RD, Harris WH. Improved Cementing Techniques and Femoral
Component Loosening in Young Patients with Hip Arthroplasty. A 12 Year
Radiographic Review. J Bone Joint Surg 1992;74B:385.
RA, Jacobs JJ, Quigley LR, et al. Primary Cementless Acetabular
Reconstruction in Patients Younger than 50 Years Old. 7- to 11-year
Results. Clin Orthop 1997;344:216.
DJ, Harmsen WS, Ilstrup JM: The Natural History of Debonding of the
Femoral Component from the Cement and Its Effect on Long-term Survival
of Charnley Total Hip Replacements. J Bone Joint Surg 1998;80A:715.
JD, Jacobs JJ, Tanzer M, et al. The Susceptibility of Smooth Implant
Surfaces to Peri-implant Fibrosis and Migration of Polyethylene Wear
Debris. Clin Orthop 1995;311:21.
RB, Rorabeck CH, Ghazal ME, Lee MH. Pain in the Thigh Following Total
Hip Replacement with a Porous-coated Anatomic Prosthesis for
Osteoarthrosis. A Five-year Follow-up Study. J Bone Joint Surg 1994;76A:1464.
RB, Rorabeck CH, Skutek M, et al. The Harris Design-2 Total Hip
Replacement Fixed with so-called Second-generation Cementing
Techniques. A Ten to Fifteen-year Follow-up. J Bone Joint Surg 1998;80A:1775.
CF, Garvin KL, Otterberg ET, Jardon OM. A Femoral Component Inserted
without Cement in Total Hip Arthroplasty. A Study of the Tri-lock
Component with an Average Ten-year Duration of Follow-up. J Bone Joint Surg 1998;80A:952.
JJ, Forest EE, Olejniczak JP, et al. Charnley Total Hip Arthroplasty in
Patients Less than Fifty Years Old. A Twenty to Twenty-five Year
Follow-up Note. J Bone Joint Surg 1998;80A:705.
WN, D’Antonio JA, Feinberg JR, Manley MT. Hydroxyapatite-coated Total
Hip Femoral Components in Patients Less than Fifty Years Old. Clinical
and Radiographic Results after Five to Eight Years of Follow-up. J Bone Joint Surg 1997;79A:1023.
JC, Harris WH. Primary Hybrid Total Hip Replacement, Performed with
Insertion of the Acetabular Component without Cement and a Precoat
Femoral Component with Cement. An Average Ten-year Follow-up Study. J Bone Joint Surg 1999;81A:247.
JC, Harris WH. The Harris-Galante Porous-coated Acetabular Component
with Screw Fixation. An Average Ten-year Follow-up Study. J Bone Joint Surg 1999;81A:66.
CW, Collis DK, Paulson R, et al. Comparison of Enoxaparin and Warfarin
for the Prevention of Venous Thromboembolic Disease after Total Hip
Arthroplasty. Evaluation During Hospitalization and Three Months after
Discharge. J Bone Joint Surg 1999;81A:932.
CW, Spiro TE, Trowbridge AA, et al. Use of Enoxaparin, a
Low-molecular-weight Heparin, and Unfractionated Heparin for the
Prevention of Deep Venous Thrombosis after Elective Hip Replacement. A
Clinical Trial Comparing Efficacy and Safety. J Bone Joint Surg 1994;76A:3.
JE, Cook SD, Thomas KA, Kay JF. The Effect of Operative Fit and
Hydroxyapatite Coating on the Mechanical and Biological Response to
Porous Implants. J Bone Joint Surg 1995;77A:97.
JA, Capello WN, Jaffe WL. Hydroxyapatite-coated Hip Implants:
Multicenter Three-year Clinical and Roentgenographic Results. Clin Orthop 1992;85:102.
JT, Harris WH. Postoperative Mortality after Total Hip Arthroplasty. An
Analysis of Deaths after Two Thousand Seven Hundred and Thirty-six
Procedures. J Bone Joint Surg 1998;80A:1291.
A, O’Sullivan T, Quinlan W. 16- to 25-year Follow-up Study of Cemented
Arthroplasty of the Hip in Patients Aged 50 Years or Younger. J Arthroplasty 1997;12:479.
E, Sarmiento A, McKellop HA, et al. The Cement Mantle in Total Hip
Arthroplasty. Analysis of Long-term Radiographic Results. J Bone Joint Surg 1994;76A:77.
CA, Hooten JP, Zettl-Schaffer KF, et al. Evaluation of Bone Ingrowth in
Proximally and Extensively Porous-coated Anatomic Medullary Locking
Prostheses Retrieved at Autopsy. J Bone Joint Surg 1995;77A:903.
CA, Bobyn DJ, Glassman AH. Porous-coated Hip Replacement: The Factors
Governing Bone Ingrowth, Stress Shielding and Clinical Results. J Bone Joint Surg 1987;69B:45.
CA Jr, Culpepper WJ III, Engh CA. Long-term Results of Use of the
Anatomic Medullary Locking Prosthesis in Total Hip Arthroplasty. J Bone Joint Surg 1997;79A:177.
B, Engesaeter LB, Vollset SE, et al. Antibiotic Prophylaxis in Total
Hip Arthroplasty. Review of 10,905 Primary Cemented Total Hip
Replacements Reported to the Norwegian Arthroplasty Register, 1987 to
1995. J Bone Joint Surg 1997;79B:590.
CW, Pellegrini VD, Totterman S, et al. Prevention of Deep-vein
Thrombosis after Total Hip Arthroplasty. Comparison of Warfarin and
Dalteparin. J Bone Joint Surg 1997;79A:1365.
DD, Capello WN, Callaghan JJ, et al. Salvage of a Recurrently
Dislocating Total Hip Prosthesis with Use of a Constrained Acetabular
Component: A Retrospective Analysis of Fifty-six Cases. J Bone Joint Surg 1998;80:502.
WH. Traumatic Arthritis of the Hip after Dislocation and Acetabular
Fracture: Treatment by Mold Arthroplasty. An End-result Study Using a
New Method of Result Evaluation. J Bone Joint Surg 1969;51A:737.
LI, Espehaug B, Vollset SE, Engesaeter LB. Early Aseptic Loosening of
Uncemented Femoral Components in Primary Total Hip Replacement. A
Review Based on the Norwegian Arthroplasty Register. J Bone Joint Surg 1995;77B:11.
WL, Lo TCM, DeSimone AA, et al. Single-dose Irradiation for the
Prevention of Heterotopic Ossification after Total Hip Arthroplasty. A
Comparison of Doses of Five Hundred and Fifty and Seven Hundred
Centigray. J Bone Joint Surg 1995;77A:590.
RD, Callaghan JJ, Hopkinson WJ, et al. The Porous-coated Anatomic Total
Hip Prosthesis, Inserted without Cement. Results after Five to Seven
Years in a Prospective Study. J Bone Joint Surg 1993;75A:77.
K, Bauer TW, Stulberg BN, et al. Characterization and Comparison of
Wear Debris from Failed Total Hip Implants of Different Types. J Bone Joint Surg 1996;78A:1235.
SS, Furia JP, Smith P, Pellegrini VD. Atrophy of the Proximal Part of
the Femur after Total Hip Arthroplasty without Cement. A Quantitative
Comparison of Cobalt-chromium and Titanium Femoral Stems with Use of
Dual X-ray Absorpiometry. J Bone Joint Surg 1995;77A:231.
LGH, Buxton RA. The Anatomy of the Superior Gluteal Nerve: Its
Relevance to the Direct Lateral Approach for Hip Arthroplasty. J Bone Joint Surg 1989;71A:1239.
P, Schmidt SA. Total Hip Arthroplasty: The Role of Anti-inflammatory
Medications in the Prevention of Heterotopic Ossification. Clin Orthop 1991;263:78.
S, Takaoka K, Saito N, Hisa K. Factors Affecting Aseptic Failure of
Fixation after Primary Charnley Total Hip Arthroplasty. Multivariate
Survival Analysis. J Bone Joint Surg 1997;79A:1618.
RJ, Burke DW, Harris WH. Elevated-rim Acetabular Components: Effect on
Range of Motion and Stability in Total Hip Arthroplasty. J Arthroplasty 1991;6:S53.
JR, Wollaeger J, Dorey F, et al. The Efficacy of Prophylaxis with
Low-dose Warfarin for Prevention of Pulmonary Embolism following Total
Hip Arthroplasty. J Bone Joint Surg 1997;79A:319.
AV, Mallory TH, Eberle RW, et al. Failure of Intraoperatively
Customized Non-porous Femoral Components Inserted without Cement in
Total Hip Arthroplasty. J Bone Joint Surg 1995;77A:1836.
SM, Callaghan JJ, Olejniczak JP, et al. Charnley Total Hip Arthroplasty
with Use of Improved Techniques of Cementing. The Results after a
Minimum of Fifteen Years of Follow-up. J Bone Joint Surg 1997;79A:53.
H, Herberts P, Wang YX, et al. Long-term Clinical and Radiological
Results of the Lord Total Hip Prosthesis. A Prospective Study. J Bone Joint Surg 1996;78B:884.
WJ, Herzwurm P, Paprosky W, et al. Treatment of Pelvis Osteolysis
Associated with a Stable Acetabular Component Inserted without Cement
as Part of a Total Hip Replacement. J Bone Joint Surg 1997;79A:1628.
JM, Pierson RH, Jacobs JJ, et al. Primary Total Hip Reconstruction with
a Titanium Fiber-coated Prosthesis Inserted without Cement. J Bone Joint Surg 1993;75A:554.
JP, Moore KD, Culpepper WJ, Engh CA: Total Hip Arthroplasty with
Porous-coated Prostheses Fixed without Cement in Patients who are
Sixty-five Years of Age or Older. J Bone Joint Surg 1998;80A:1648.
CG, Kull LR, Martell JM, et al. Total Hip Replacement with Insertion of
an Acetabular Component without Cement and a Femoral Component with
Cement. Four to Seven-year Results. J Bone Joint Surg 1995;77A:86.
MA, Maar DC, Krackow KA, et al. Total Hip Replacement without Cement
for Non-inflammatory Osteoarthrosis in Patients Who Are Less than
Forty-five Years Old. J Bone Joint Surg 1993;75A:740.
BD, Bourne RB, Rorabeck CH, Nayak N. A Tapered Titanium Femoral Stem
Inserted without Cement in a Total Hip Arthroplasty. Radiographic
Evaluation and Stability. J Bone Joint Surg 1996;78A:1214.
WF, Estok DM, Harris WH. Total Hip Arthroplasty with Use of So-called
Second-generation Cementing Techniques. A Fifteen-year-average
Follow-up Study. J Bone Joint Surg 1995;77A:1845.
L, Freund KG, Sørenson KH. Total Hip Arthroplasty with the Charnley
Prosthesis in Patients Fifty-five Years Old and Less. Fifteen to
Twenty-one Year Results. J Bone Joint Surg 1996;78A:73.
L, Freund KG, Sørenson KH. Long-term Results of Charnley Total Hip
Replacement. Review of 92 Patients at 15 to 20 Years. J Bone Joint Surg 1994;76B:245.
LT, Franzén H, Carlsson ÅS, Önnerfält R. Early Radiographic Loosening
Impairs the Function of a Total Hip Replacement. The Nottingham Health
Profile of 49 Patients at Five Years. J Bone Joint Surg 1994;76B:235.
CS, Walker RH, Colwell CW. The Femoral Component in Total Hip
Arthroplasty. Six to Eight-year Follow-up of One Hundred Consecutive
Patients after Use of a Third-generation Cementing Technique. J Bone Joint Surg 1994;76A:1130.
GD, Wessinger SJ, Hughes R, Harris WH. Routine Use of Adjusted Low-dose
Warfarin to Prevent Venous Thromboembolism after Total Hip Replacement.
J Bone Joint Surg 1993;75A:893.
VD, Gregoritch SJ. Postoperative Irradiation for Prevention of
Heterotopic Ossification following Total Hip Arthroplasty. J Bone Joint Surg 1996;78A:870.
MB, Poulsen IH, Thomsen J, Solgaard S. The Hemispherical Harris-Galante
Acetabular Cup, Inserted without Cement. The Results of an Eight to
Eleven-year Follow-up of One Hundred and Sixty-eight Hips. J Bone Joint Surg 1999;81A:219.
AA, Kraay MJ, Goldberg VM. Clinical and Radiographic Outcomes of Total
Hip Arthroplasty with Insertion of an Anatomically Designed Femoral
Component without Cement for the Treatment of Primary Osteoarthritis. J Bone Joint Surg 1999;81A:210.
Heparin Arthroplasty Group (The). RD Heparin Compared with Warfarin for
Prevention of Venous Thromboembolic Disease following Total Hip or Knee
Arthroplasty. J Bone Joint Surg 1994;76A:1174.
RP, Deysine GR, Green TM. Uncemented Total Hip Arthroplasty Using the
CLS Stem: A Titanium Alloy Implant with a Corundum Blast Finish.
Results at a Mean 6 Years in a Prospective Study. J Arthroplasty 1996;11:286.
CH, Bourne RB, Laupacis A, et al. A Double-blind Study of 250 Cases
Comparing Cemented with Cementless Total Hip Arthroplasty.
Cost-effectiveness and Its Impact on Health-related Quality of Life. Clin Orthop 1994;298:156.
TP, Guttmann D, Grecula M, Amstutz HC. The Relationship Between the
Design, Position, and Articular Wear of Acetabular Components Inserted
without Cement and the Development of Pelvic Osteolysis. J Bone Joint Surg 1994;76A:677.
TP, Harris WH. The Harris-Galante Porous-coated Acetabular Component
with Screw Fixation: Radiographic Analysis of 83 Primary Hip
Replacements at a Minimum of Five Years. J Bone Joint Surg 1992;74A:1130.
TP, Jasty M, Harris WH. Periprosthetic Bone Loss in Total Hip
Arthroplasty: Polyethylene Wear Debris and the Concept of the Effective
Joint Space. J Bone Joint Surg 1992;74A:849.
TP, Kwong LM, Jasty M, et al. The Mechanism of Loosening of Cemented
Acetabular Components: Analysis of Specimens Retrieved at Autopsy. Clin Orthop 1992;274:60.
KR, Callaghan JJ, Kelley SS, Johnston RC. The Outcome of Charnley Total
Hip Arthroplasty with Cement after a Minimum Twenty-year Follow-up. J Bone Joint Surg 1993;75A:961.
PF, Hozack WJ, Callaghan JJ, et al. Acetabular Fracture Associated with
Cementless Acetabular Component Insertion: A Report of Thirteen Cases. J Arthroplasty 1999;14:426.
SW, Estok DM, Harris WH. Total Hip Arthroplasty with Use of
Second-generation Cementing Techniques. An Eighteen-year-average
Follow-up Study. J Bone Joint Surg 1998;80A:1632.
DH, Porter ML. The Long-term Results of Charnley Low-friction
Arthroplasty in Young Patients Who Have Congenital Dislocation,
Degenerative Osteoarthritis, or Rheumatoid Arthritis. J Bone Joint Surg 1997;79A:1599.
SM, Callaghan JJ, Olejniczak JP, et al. The Effects of Surface
Roughness and Polymethylmethacrylate Precoating on the Radiographic and
Clinical Results of the Iowa Hip Prosthesis. J Bone Joint Surg 1999;81A:481.
PM, MacKenzie JR, Callaghan JJ, Johnston RC: Total Hip Arthroplasty
with Cement in Patients Who Are Less than Fifty Years Old. J Bone Joint Surg 1994;76A:863.
CJ, Wilde Ah, Borden LS, Marks KE: A Ten-year Follow-up of 100
Consecutive Muller Curved-stem Total Hip Replacement Arthroplasties. J Bone Joint Surg 1982;64A:970.
LC, Collier JP, Saum KA, et al. Impact of Gamma Sterilization on
Clinical Performance of Polyethylene in the Hip. The Otto Aufranc
Award. Clin Orthop 1995;319:28.
RM, Jacobs JJ, Sumner DR, et al. The Bone-implant Interface of Femoral
Stems with Non-circumferential Porous Coating. A Study of Specimens
Retrieved at Autopsy. J Bone Joint Surg 1996;78A:1068.
AG, D’Lima D, Venn-Watson E, et al. Polyethylene Wear after Total Hip
Arthroplasty: The Effect of a Modular Femoral Head with an Extended
Flange-reinforced Neck. J Bone Joint Surg 1998;80A:1641.
EJ, Hozack WJ, Rothman RH. Incidence of Thigh Pain after Uncemented
Total Hip Arthroplasty as a Function of Femoral Stem Size. J Arthroplasty 1996;11:304.
D, Harrison J, Glew D, et al. Comparison of the Use of a Foot Pump with
the Use of Low-molecular-weight Heparin for the Prevention of Deep-vein
Thrombosis after Total Hip Replacement. A Prospective, Randomized
Trial. J Bone Joint Surg 1998;80A:1158.
RC, Cooperstein LA, Kruger MP, Rubash HE. Acetabular Anatomy and the
Transacetabular Fixation of Screws in Total Hip Arthroplasty. J Bone Joint Surg 1990;72A:501.
ST, Haber DF. Primary Total Hip Replacement with Insertion of an
Acetabular Component without Cement and a Femoral Component with
Cement. Follow-up Study at an Average of Six Years. J Bone Joint Surg 1996;78A:698.
JS, Callaghan JJ, Heekin RD, et al. The Porous-coated Anatomic Total
Hip Prosthesis, Inserted without Cement. A Prospective Study with a
Minimum of Ten Years of Follow-up. J Bone Joint Surg 1999;81A:74.