OSTEOTOMIES ABOUT THE HIP—ADULTS
– JOINT RECONSTRUCTION, ARTHRITIS, AND ARTHROPLASTY > Lower
Extremity > CHAPTER 104 – OSTEOTOMIES ABOUT THE HIP—ADULTS
ago, the number of osteotomies about the hip for the treatment of adult
hip disease has understandably decreased. However, despite the
advancements in joint replacement technology, the challenges remain
much as they were in the early 1970s. Even an optimal hip replacement
is not expected to survive for a lifetime in a healthy patient younger
than 50 years, especially given today’s longer life expectancy.
Survivorship of joint replacements in younger adults is possibly less
than 10 years (21,25,27,30,40,41,56,102,126,143,152).
host of other potential problems are encountered in young adult
patients [defined as being 20–50 years old (7)] who undergo total joint replacement. Complications such as these call for alternative treatments for the patient with a
life expectancy exceeding 20 to 25 years. What is to be done for these
patients? Many are told that they must modify their activities and
palliate their pain until they reach an appropriate age, while their
hip continues to deteriorate. A significant portion of these patients,
particularly if identified before they develop advanced arthritis, can
be successfully treated with an osteotomy of the pelvis or proximal
femur (or both).
hips do not last a lifetime. In addition, studies have suggested that
many hips—actually a majority—that develop osteoarthritis have
preexisting pathology (7,150,151,169,170 and 171).
Such secondary arthritis is generally the result of a biomechanical
abnormality affecting the joint. The major predisposing diseases
include developmental dysplasia of the hip (DDH), Legg-Calvé-Perthes
(or Perthes) disease, slipped capital femoral epiphysis (SCFE),
osteonecrosis, and trauma, of which the most common is DDH. Reports
vary, but taken together the prevalence of significant osteoarthritis
with these diseases by 50 years of age is between 20% and 50%. For
dysplasia, the risk is probably 25% to 50%, depending on the severity
of involvement, in spite of current treatment methods (33,109,176). For Perthes disease, the chance for painful arthritis by 50 years of age is around 50% (7,91,147,149,173). In the case of SCFE, it is only about 20%, but it certainly varies with the severity of the slip (22,53,118).
Often in these patients the childhood hip disease was subclinical and
went undiagnosed until the onset of adult hip pain. Nevertheless, many
of these patients required hip replacement surgery before 50 years of
age.
result in excessive shear and compressive stresses on the hyaline
cartilage and subchondral bone. When the biomechanical tolerance and
potential for repair of the joint are exceeded, progressive secondary
degeneration occurs. Additionally, as the life expectancy of our
population increases, so does the opportunity for an underlying hip
condition to express itself as symptomatic secondary degenerative
arthritis.
Prior to the development of this operation, diagnosing dysplasia in
painful adult hips did not seem as important. Most of the surgical
procedures were of a salvage nature for relatively more advanced
arthritis such as valgus–extension intertrochanteric or Chiari
osteotomies, and these did not correct the primary abnormality. Partly
because of this “salvage” mindset, physicians have not been taught a
sense of urgency about making the diagnosis or referring patients for
early surgical consultation. Indeed, the presence of moderately severe
acetabular dysplasia is frequently overlooked by nonpediatric
radiologists, primary care physicians, and even orthopaedists.
Increased education and discussion about dysplasia and the other causes
of secondary hip arthritis can facilitate earlier treatment in younger
adults with painful hips.
importance of early diagnosis for these at-risk hips. When an anatomic
abnormality exists that exposes the joint cartilage to high stresses,
and this situation is correctable by osteotomy, the potential long-term
benefits of reorienting or realigning the joint must be considered.
Salvage of a young patient’s joint cartilage holds more promise for a
good life-long result than does an early implant arthroplasty. In other
cases, an osteotomy may serve as a bridge to carry the patient safely
into the age range appropriate for total joint replacement. In this
chapter, we do not support the indiscriminate selection of osteotomy
for any adult hip disease, as the majority of patients with established
hip arthritis are best managed by total hip replacement (THR) surgery.
In this chapter, we define the indications for, and appropriate
selection of, osteotomies of the pelvis and femur for specific problems
in the adult hip.
treatment with an osteotomy do not give a history of a childhood hip
disorder. They may have vague anterior groin or greater trochanteric
pain, which has been treated for years as a “groin pull” or
“trochanteric bursitis.” They complain of difficulties with athletics
and endurance. Some may come with radiographs that have been
interpreted as normal despite the presence of significant dysplasia.
of fitness or presence of obesity. Observe gait and check for
Trendelenberg’s sign. Check the range of motion in all planes.
Carefully document the actual and apparent leg lengths as well as
strength and neurologic status of the lower extremities. Examine other
joints as they may relate to the affected hip, especially the back,
contralateral hip, and ipsilateral knee. Provocative tests are useful
in patients with suspected pathology in the labrum of the hip,
including any with dysplasia (30,42,73,88).
These include the apprehension test of hip extension and external
rotation, as well as the impingement test of flexion–adduction–internal
rotation (2,3,26,34,39,42,81,92).
When an angular proximal femoral osteotomy is being considered, examine
the hip for the position of comfort. Arthroscopy and magnetic resonance
imaging (MRI) are useful in assessing labral pathology and avascular
necrosis (AVN) (27,67,81,92). With the exception of MRI for AVN, these studies should not be considered mandatory prior to osteotomy.
This nearly lateral view is taken in the standing position with the
greater trochanter of the affected hip against the cassette and the
ipsilateral foot placed parallel to the cassette. Rotate the
contralateral leg, and with it the pelvis, backward about 20°, or just
enough so that the hip joints are not superimposed on one another. This
special oblique view provides the best radiographic documentation of
the anterior coverage of the femoral head, and it is a true lateral
view of the proximal femur.
 |
|
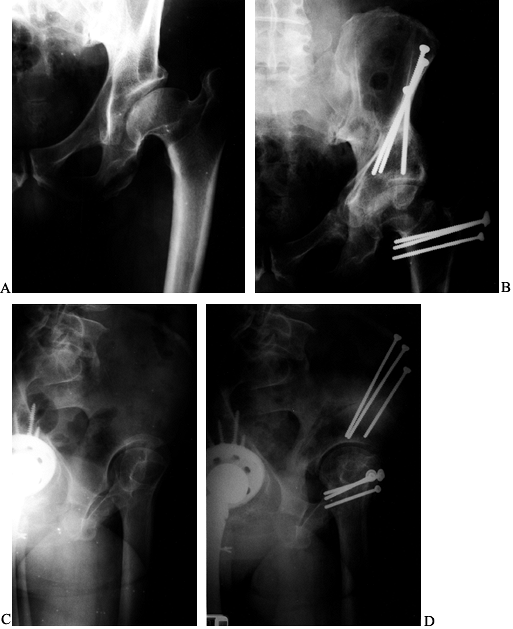
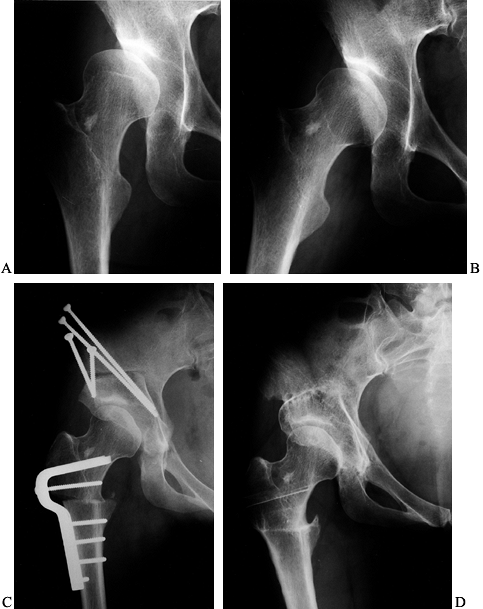
Figure 104.1.
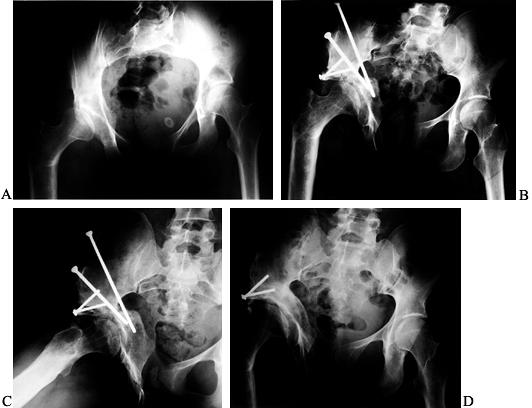
Preoperative radiographic evaluation of the hip. This patient has avascular necrosis of the hip and is being evaluated for an intertrochanteric osteotomy. A: AP view. B: Frog-lateral view. C: AP view in adduction. D: Postoperative AP radiograph after valgus osteotomy. E: Postoperative lateral view. F: AP view after hardware removal showing good preservation of the joint space. G: Lateral view after hardware removal. |
 |
|
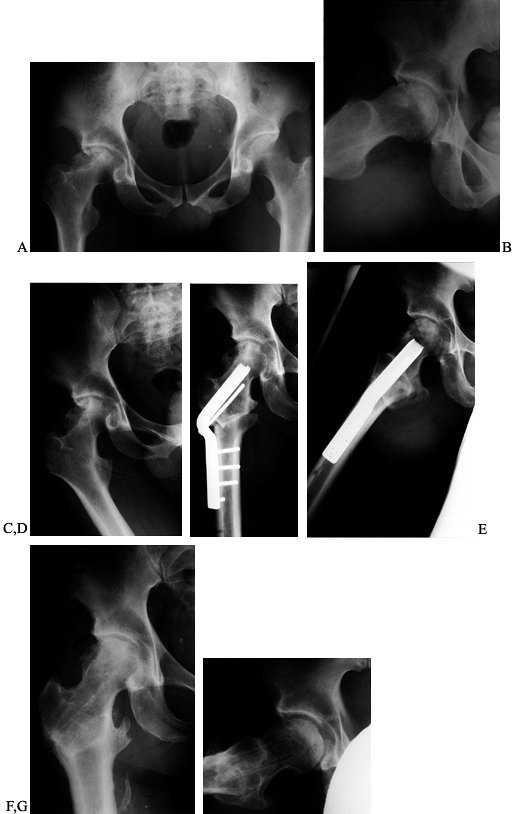
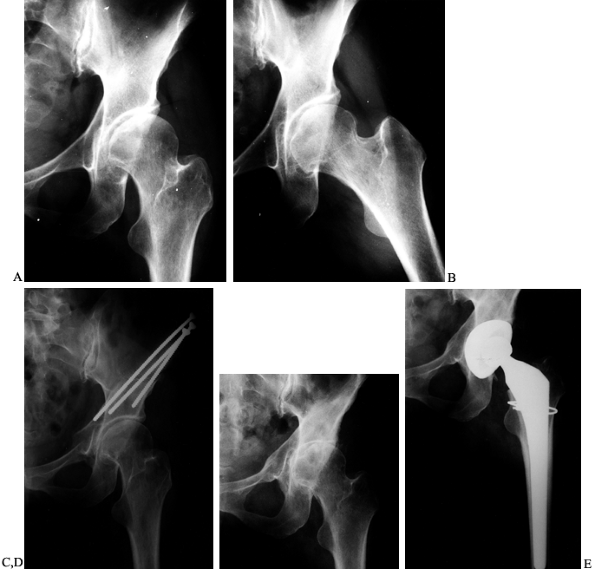
Figure 104.20. PAO for Perthes disease. A:
AP view of the left hip in a 52-year-old man with a history of bilateral Perthes disease. The contralateral hip had already been treated with a primary and revision total hip arthroplasties. B: AP view of the hip 3 months after PAO and trochanteric advancement. C: Preoperative false-profile view. D: False-profile view 6 months postoperatively. At 1 year he used a cane only rarely and was working without restrictions. His preoperative back and hip pain was relieved. |
rotation provides a preview of the potential enhancements of coverage afforded by a rotational acetabular osteotomy (Fig. 104.23A, Fig. 104.23B).
Functional views of the hip with the femur in abduction and adduction
permit characterization of the joint space and demonstration of the
adequacy of motion in the required plane prior to any angular
intertrochanteric osteotomy (ITO) (Fig. 104.1A, Fig. 104.1C).
Performing these views under fluoroscopy is another alternative; this
can provide simultaneous information about the positions of relative
comfort. An AP radiograph with the hip in adduction and flexion
approximates the position of the joint after a valgus–extension ITO (Fig. 104.1C).
If the overall alignment of the lower extremity will be changed by an
osteotomy, take a full-length standing radiograph for measuring the
biomechanical axis. This allows prediction of the effect of the surgery
on the weight-bearing axis, especially as it may affect the knee.
 |
|
Figure 104.23. Failure after an initially successful PAO. A: AP radiograph of the left hip in a 47-year-old woman with hip pain for less than a year. B: Abduction–internal rotation functional AP view of the left hip. C: AP view of the left hip early after PAO. D: By 1½ years postoperatively, arthritis had progressed and pain increased. E: A total hip arthroplasty was required 3½ years after the PAO when the patient was 50 years old.
|
can help with spatial localization of the necrotic segment of the
femoral head (137,138).
Anterior lesions can be visualized on an AP of the hip taken with the
hip in about 30° of flexion. The posterior femoral head can be better
seen with the hip in extension with the central beam of the x-ray
angled caudally about 40°. An MRI is usually performed in patients with
AVN. It helps to localize and quantify the head involvement as well as
assess the contralateral hip for a disease that is often bilateral.
The images and sometimes additional three-dimensional plastic models
can provide a more detailed understanding of coverage deficiencies that
may be present. These CT scans are not necessary, however, to determine
which patients are appropriate for surgery. In fact, most decisions
regarding reconstructive surgery of the hip can be made on the basis of
high-quality plain radiographs.
considered for an osteotomy around the hip joint. In patients older
than 60 years, there must be a compelling reason to choose a hip
osteotomy over implant arthroplasty. Being over 60, however, is not by
itself an absolute contraindication to a hip osteotomy. A patient’s
physiologic age and life expectancy are more relevant than chronologic
age. The actual status of the joint cartilage and lifestyle of the
patient are more important than any assumptions about arthritis or
activity based solely on a patient’s age.
chapter have the best results when performed for clearly correctable
biomechanical abnormalities in joints with at most mild arthrosis. Good
range of motion also correlates with a better long-term outcome for hip
osteotomies. Deviation from these principles may still yield good
results, but at an increased risk of failure. One of the most difficult
decisions frequently encountered is determining just how much arthrosis
is too much. The risks of increased stiffness,
pain,
or other modes of clinical failure increase with the degree of
preoperative degenerative changes. The amount of risk that the surgeon
and patient are willing to assume may be tempered by other factors such
as the patient’s age and surgical options. Figure 104.2
shows a patient with a dysplastic hip whose arthritis progressed
rapidly, leading to cancellation of a planned periacetabular osteotomy.
Figure 104.3 by comparison shows a patient with moderate preoperative arthrosis who has done well after a periacetabular osteotomy.
 |
|
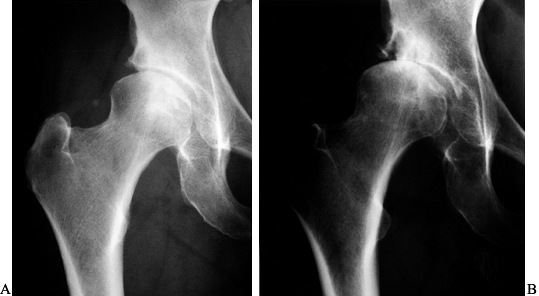
Figure 104.2. A:
AP radiograph of the left hip in a healthy and active 52-year-old woman with a 4-year history of hip pain. Periacetabular osteotomy was discussed as the probable treatment of choice. B: This AP radiograph taken 7 months later showed rapid progression of the arthritis, requiring cancellation of the osteotomy that was planned. |
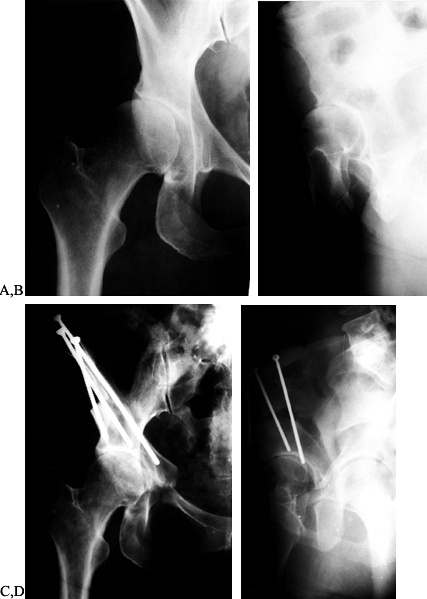 |
|
Figure 104.3. AP radiograph of the right hip (A) and a false profile view (B) of a 43-year-old woman with right hip pain and moderately advanced arthrosis. A periacetabular osteotomy is seen on the AP (C) and false-profile views (D).
The patient had a delayed union of the pubis, but by 8 months after surgery she was asymptomatic and back to full activity with no limp. |
risks and benefits of the procedure, including the possible need for an
extended period of rehabilitation. Moderate obesity should be
considered a relative contraindication and morbid obesity a nearly
absolute contraindication. Absolute contraindications to osteotomy
include neuropathic arthropathy, severe osteopenia, inflammatory
arthritis, and active infection. Relative contraindications include a
stiff joint, advanced age or arthrosis, and smoking.
complications and provide for reproducible results. An AP radiograph
with a magnification marker in the plane of the bone allows scaled
digitization of the image for computer planning (78,108,130) (see Chapter 26).
The computer facilitates the exploration of many possible surgical
plans with accuracy and speed. Sound planning, and successful surgery,
does not require computer-based planning. In fact, the majority of the
world’s leading osteotomy surgeons have never used, or relied on,
computer planning. The principle is that careful planning of the goals
of the surgery and the specific surgical techniques must be considered
prior to surgery. Issues that can be planned preoperatively include
osteotomy level, angulation, wedge resection, leg length, displacement,
effect on mechanical axis alignment, fixation choice(s), bone grafting,
and compatibility with future THR. Bring all the relevant preoperative
planning drawings or computer printouts to the operating room and post
these for easy reference during the procedure. Attention to detail and
technique during the surgery follows from attention to the procedural
details before the surgery.
procedure for patients with arthritis caused by acetabular dysplasia.
In general, young patients with fixed subluxations and/or femoral head
deformities are well suited for this procedure. It is a capsular
interposition arthroplasty and an extra-articular acetabular
augmentation utilizing a slightly inclined (distal–lateral to
proximal–medial) supra-acetabular iliac osteotomy. Chiari initially
described this procedure in 1955, and he later reported the results of
more than 600 cases in 1974 (31). His original
methods included a relatively minimal exposure through an anterolateral
approach. He made a slightly upsloping, inferiorly concave osteotomy
just above the joint capsule. He rarely used internal fixation and
patients were immobilized in a hip spica cast. Most of Chiari’s
patients were children, although adults were included in his study, and
104 had coexisting arthritis. Subsequent reports by Chiari and others
on the results in adults have better defined the indications and
outcomes (28,76,128,136,177). Many of Chiari’s large group of patients would now be treated with a rotational pelvic osteotomy.
displaced superior fragment forms a lateral shelf over the
pathologically lateralized and uncovered femoral head. The proximal
fragment can be manipulated anteriorly as well as laterally. Through
metaplastic transformation, this new buttress and the interposed
capsule produce a fibrocartilaginous articular surface. This broadens
the surface area available for weight bearing. The coverage also
prevents progression of the subluxation and is not dependent on joint
congruity.
usually around 1.5 cm, which reduces the resulting joint reaction
forces. The inferior fragment is hinged on the symphysis pubis, and
displacement actually slightly increases the already abnormal
inclination of the acetabulum. This is more than compensated for by the
medialization and improved coverage (162,179).
Additionally, the osteotomy increases hip abduction, an essential goal
for many patients. Although the Chiari procedure is conceptually one of
the easier pelvic osteotomies to visualize, its results are very
dependent on precise surgical technique (46).
have a limp and a Trendelenberg gait preoperatively. Usually only the
antalgic portion of a limp will be improved by the procedure. Some
authors (see later) have reported an improvement in the Trendelenberg
lurch in a majority of patients, attributing the improvement to the
medialized hip center and to advancement of the greater trochanter in
some patients. In general, advise patients that their Trendelenberg
lurch may not improve and can worsen postoperatively. This is, in part,
because the traditional surgical approach involves stripping of the
tensor and much of the abductor musculature from the lateral iliac wall.
indications and contraindications for Chiari osteotomy. We use Chiari
pelvic osteotomy in young adults and middle-aged patients with DDH,
refractory hip pain, and an uncovered femoral head, in whom acetabular
redirection is not indicated, usually because of incongruity or fixed
subluxation of the femoral head. This is most often secondary to hip
dysplasia, although Chiari also included patients with coxa magna after
Perthes disease (or childhood AVN). The procedure is contraindicated
when the proximal femur or acetabulum can be repositioned to provide
coverage for a congruous joint using an acetabular redirection with or
without femoral osteotomy. Another contraindication is lack of hip
mobility. At least 70° (to 90°) of flexion and 10° (to 20°) of
adduction are required. A relative contraindication is physiologic age
over 45 years, especially with significant arthritic changes. This is
because of the poorer results in this age group as well as the
competitive benefits of THR. Other relative contraindications include a
totally uncovered hip or severe proximal migration prohibiting access
to the appropriate iliac slope for the osteotomy and resulting in
insufficient breadth of the iliac wing and dangerous proximity to the
sciatic notch. This last problem may be overcome with intraoperative
traction in some patients.
 |
|
Table 104.1. Indications and Contraindications for Chiari Osteotomy
|
have demonstrated, where there is significant coxa vara or incongruity
(femoral head flattening), a valgus–extension ITO can be combined with
the Chiari procedure. This moves the broader surface of the head into
the weight-bearing zone, where superior–lateral eccentric loading of
the head would otherwise occur. As with most intertrochanteric
osteotomies, the decision is guided by preoperative adduction and
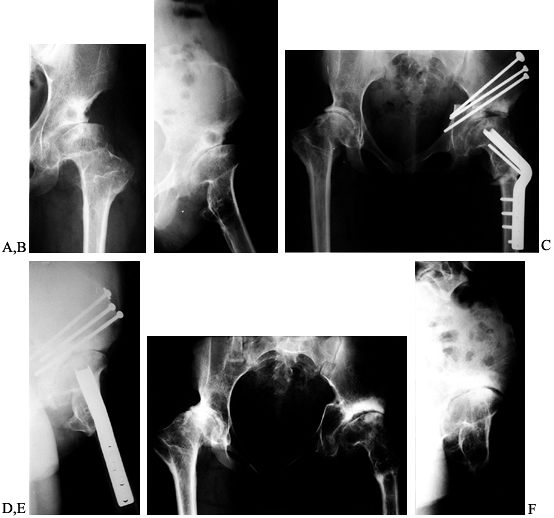
abduction radiographs (31,89,136,). Figure 104.4
shows a 17-year-old patient with bilateral dysplasia and arthritis who
underwent a combined Chiari and valgus–extension ITO on the left. At
over 3 years this hip has no pain or limp.
 |
|
Figure 104.4. Chiari osteotomy combined with a valgus ITO. Preoperative AP view of the left hip (A) and false-profile view (B)
in a 17-year-old girl with bilateral hip dysplasia, arthritis, and leg-length inequality. Postoperative AP view of the pelvis (C) and false-profile view of the left hip (D) after Chiari osteotomy with simultaneous extension ITO and femoral shortening of 3 cm. AP view of the pelvis (E) and false-profile view (F) 3 years after surgery. The patient had no left hip pain and no Trendelenberg sign, but she elected for total hip replacement on the contralateral hip within the next year. |
-
Position the patient supine on a
radiolucent table, with the pelvis and leg draped free, or on a
fracture/traction table with the leg slightly abducted and externally
rotated. Utilize routine antibiotic and thromboembolic prophylaxis
throughout the procedure. -
Make a 10-cm-long anterolateral
ilioinguinal incision beginning at the iliac crest and extending
medially. The less cosmetic iliofemoral (Smith-Peterson) approach
P.2730P.2731
may be preferred for more exposure or in larger patients, or for combined pelvic and femoral osteotomies through one incision. -
Develop the interval between the
sartorius and tensor facia lata muscles. Retract the tensor laterally
and preserve the lateral femoral cutaneous nerve medially. -
Expose the iliac wing by subperiosteal
dissection medially and laterally. Minimize lateral stripping to
minimize injury of the hip abductor muscles. -
Release the tensor, sartorius, and direct head of the rectus femoris from their origins off the ilium.
-
Expose the anterior and lateral hip joint capsule. Do not incise or damage the capsule, especially superiorly.
-
Separate the tendon of the rectus femoris
from the capsule and divide the reflected head. The curved insertion of
the rectus serves as a marker for the level of the osteotomy. -
Dissect subperiostially from the capsule
to the sciatic notch posteriorly and place a blunt retractor, such as a
relatively radiolucent flexible metal ribbon. Similarly, place a
retractor medially after careful dissection to the greater sciatic
notch. -
Radiographically confirm the starting
point and direction of the osteotomy with a pin drilled into the pelvis
just superior to the capsule. -
Use curved osteotomes to complete the
semicylindrical osteotomy, inclining 15° superiorly from inferolateral
to superomedial. A less steep inclination may be necessary in the case
of proximal head migration. In such a case, the osteotomy could exit
into the sacroiliac joint, which would block the desired displacement. -
A Gigli saw can be used to cut the most
posterior portion from the sciatic notch anteriorly about 1 cm. This
reduces the danger of injuring the neurovascular structures in the
sciatic notch from an osteotome or bone splinters or spikes.
Alternatively, a Gigli saw or power saw can be used for the entire cut.
The senior author (RS) prefers the Gigli technique for safety and
simplicity. -
After carefully completing the osteotomy
of the medial cortex, displace the osteotomy. Release any traction on
the leg and abduct the hip. Displace the inferior fragment medially
1–1.5 cm but at times up to 2.5 cm (76) as
needed to provide 80% to 100% lateral coverage of the femoral head.
Because the sacroiliac joint allows some lateral motion of the proximal
fragment, not all of the osteotomy displacement results in hip center
medialization. Confirm that the displacement has occurred both
posteriorly and anteriorly, because a posterior hinge may remain (13). -
Evaluate any remaining deficiency in
coverage, which is common anteriorly. Harvest a supplemental
corticocancellous shelf graft from the iliac wing as needed. Wedge this
into the osteotomy, or otherwise secure the graft. -
Place internal fixation from the iliac
wing across the osteotomy to eliminate the need for a spica cast in
adults. We use long 4.5 mm AO (Synthes, Paoli, PA) solid cortical
screws. Figure 104.5 shows a patient with a
Chiari osteotomy in which a supplemental graft was used. After more
than 4 years, she remains asymptomatic using no walking aids.![]() Figure 104.5. Chiari osteotomy with a bone graft. A: AP view of the pelvis in a 29-year-old woman with a history of a prior Sutherland pelvic osteotomy and increasing hip pain. B: AP view of the pelvis 1 month after Chiari osteotomy. C: Lateral view 2½ months after the Chiari osteotomy. D:
Figure 104.5. Chiari osteotomy with a bone graft. A: AP view of the pelvis in a 29-year-old woman with a history of a prior Sutherland pelvic osteotomy and increasing hip pain. B: AP view of the pelvis 1 month after Chiari osteotomy. C: Lateral view 2½ months after the Chiari osteotomy. D:
AP view of the pelvis 4 years after surgery. The patient is
asymptomatic, working full time, using no walking aids, and has a
moderate limp because of a short right leg. -
Where more than 75% displacement of the
osteotomy is required, consider placement of a bone graft medially to
assist with healing. -
Confirm the radiographic appearance of
the osteotomy and check hip range of motion, to be certain that there
is no block to flexion anteriorly. -
Irrigate the wound and close it in layers over a suction drain.
-
Take care to repair all muscles, suturing
the tendons back to bone through drill holes where initial release was
from bone. Avoid injury to the lateral femoral cutaneous nerve.
tolerated. Do not allow active flexion or abduction exercises
initially, to allow repaired muscles to heal. Weight bearing is partial
for 6 weeks. Most patients can bear full weight by 3 months if healed,
with longer healing times expected for osteotomies with greater
displacements.
the first few years and remains reduced in 50% to 60% at 15–20 years
follow-up. Because pain relief is a primary goal of this surgery in
adults, the reported rates of good and excellent results in the
literature are similar. Lack et al. (76)
reported 75% good results at 15 years in 100 hips in patients older
than 30 years at the time of operation. During these 15 years, 20% of
patients required THR at an average time of 11.5 years after osteotomy.
Windhager et al. (177) also summarized the
results of many patients who had undergone Chiari osteotomy at a mean
follow-up of 25 years, showing 51% continued good results out of 236
patients. In patients who had developed pain, the period of significant
pain relief was an average of 17 years. Zlatic et al. (179) reported 87% complete or nearly complete pain relief in adults at the 9-year follow-up. De Waal Malefijt et al. (36)
described 61% good results in their adult patients at 5 years, with the
poor results mostly attributable to technical errors. Betz et al. (14)
reported 88% good or excellent results in 24 patients with follow-up
ranging from 3 to 20 years. When performed as a time-buying salvage
operation for the hip, it seems reasonable to expect 10–15 years of
good service from the Chiari osteotomy.
and normalize in others. Graham et al. (55)
found no change in the Trendelenberg test after 58 osteotomies were
followed for 3.5 years. Other authors have reported that the
Trendelenberg gait was the same or slightly worse at short- and
long-term follow-up (28,36,179). By contrast, another group of authors reported improved Trendelen- berg gait without osteotomy of the greater trochanter (63,85,128).
Osteotomy of the greater trochanter with advancement shows more
significant improvement in the Trendelenberg test. For example, Matsuno
et al. (89) described a lateral approach with
greater trochanteric advancement. They reported only 2 of 66 patients
with a Trendelenberg gait at a minimum 6-year follow-up, and 80% good
results in 100 adult cases at 9 years.
does not. Severe limitation of motion has been reported, but its
incidence may be reduced by the use of internal fixation and early
physical therapy. In general, there is not a strong relationship
between preoperative radiographic findings and clinical results
following Chiari osteotomy (28,63).
the importance of an intact labrum, as determined by preoperative hip
arthrography, for good results following Chiari osteotomy in adults. In
their series, all 23 hips with normal labra had good or excellent
results at 4 years, whereas 1 of 21 with torn labra and 10 of 20 with
detached labra had fair or poor results. The authors felt that a
detached labrum was a
contraindication
to the procedure. Gadolinium-enhanced MRI is now our preferred method
for evaluating the labrum. If the capsule is opened at the time of
surgery, either for inspection or resection of a labral tear, secure
repair is essential, as an intact capsule is a prerequisite for a
successful Chiari result.
arthritis on the outcome after Chiari osteotomy. As may be expected, in
adults the results are best and most durable in patients who are
younger and have no signs of arthritis at the time of surgery. Reynolds
(128) found that without severe dysplasia or
arthritis, patients achieved significant and lasting improvement in 29
of 32 cases (90%) at a 5-year follow-up. However, those with more
severe dysplasia and/or arthritis failed in 9 of 12 cases. Calvert et
al. (28) found significant correlation between
either younger age at operation or absence of arthritic changes on
preoperative radiographs and Harris hip scores of 49 hips at a 14-year
follow-up. Windhager’s (177) review of patients
most of whom had undergone Chiari osteotomy, found that the oldest
third of the patients developed the worst arthritis at a 25-year
follow-up and that subjectively poor results were seen in patients with
preoperative signs of arthritis. In a report of subsequent Chiari
patients, Lack et al. (76) more clearly defined
the effects of age in an adult-only population. They showed 80% good
results for patients younger than 45 years of age at operation (age
range, 30–44 years) and only 50% good results for patients older than
44 years of age.
intuitive concept that a prior Chiari facilitates cup insertion
techniques is not valid.) Such an arthroplasty should be viewed as a
revision acetabulum with the presence of scar, bone defects, and
anatomic distortions anticipated. Especially important is the
recognition that the anteroposterior host bone diameter is much less
than the perceived superoinferior or mediolateral dimension. It is
necessary to avoid excessive reaming of the anterior and posterior
walls. Furthermore, the projecting shelf of bone anteriorly and
laterally may be a source of impingement that predisposes to
dislocation of the hip replacement if not dealt with at the time of
arthroplasty by resection of bone.
 |
|
Table 104.2. Complications of the Chiari Osteotomy
|
candidates for redirectional acetabular osteotomy, the Chiari procedure
offers a good choice when surgery is required (139).
The more ideal patients do not have advanced arthritic deterioration
and are much too young to be reasonable candidates for THR. In these
properly selected patients, 10–20 years of significant pain relief may
be expected, making the Chiari osteotomy a durable salvage option.
osteotomies can be classified as shelf procedures. These bone-grafting
surgeries are cousins of the Chiari type of osteotomy in that they are
capsular interposition arthroplasties. They rely on capsular metaplasia
into fibrocartilage as well as bony remodeling to provide coverage over
an otherwise deficiently contained femoral head. In these procedures,
an iliac bone graft is created or inserted immediately superior to the
hip capsule. A variety of techniques, shapes, and fixation methods have
been described. A common feature among these methods is the importance
of graft placement in direct apposition to the superior capsule and the
prevention of graft displacement. Because of these considerations, most
authors have recommended postoperative spica casting (129,145,146,153,167). The
use of casts and the dependence on bony remodeling are part of the
reason that this type of procedure, when done alone, is probably best
reserved for pediatric patients. In addition, the need for certain
pediatric procedures, such as open reduction of hip dislocations,
provides access to the hip joint and an opportunity for supplemental
shelf grafting. The use of simple shelf augmentations in adults has
diminished markedly since the development of redirectional acetabular
osteotomies.
adults is as an adjunct to another coverage osteotomy, particularly
that of Chiari. As discussed in the preceding section on the Chiari
osteotomy, a shelf graft is a useful supplement to extend the capsular
arthroplasty surface, usually anteriorly but also laterally (74).
Conceptually, the combination of these procedures is logical because
they both are designed to rely on metaplasia of the joint capsule to
fibrocartilage and can be applied to the same patients. The shelf
procedures have the benefit of being able to be tailored to adjust
coverage in an anterior or posterior direction more than is possible
with a Chiari osteotomy.
than with other pelvic osteotomies, even when using a slotted
technique. Hardware breakage, recurrent subluxation, or graft failure
due to displacement can occur. Many authors recommend spica casting;
however, complications from the cast itself can be significant (175),
especially in large adolescents or adults. When combined with another
primary procedure, almost any of the described shelf augmentations can
be performed with little increase in surgical risk or technical
difficulty. Our experience supports the use of a shelf procedure in
combination with a Chiari osteotomy, but we do not any longer perform
shelf grafting alone or in combination with periacetabular osteotomy.
In the award-winning, retrospective review of 130 hips with so-called
idiopathic or primary osteoarthritis, Stulberg and Harris (151)
discovered a 48% incidence of dysplasia of the hip as the etiologic
factor. Additionally, Cooperman et al., in a minimum 22-year follow-up
study of 32 hips in 20 patients with acetabular dysplasia (center–edge
angle < 20°), found a 100% incidence of roentgenographic evidence of
degenerative joint disease (33).
three variations of clinical presentation. In an early one, there may
be muscle-related trochanteric or anterolateral discomfort only. In
another subgroup, there is sudden onset of pain, giving way, and
clicking. These patients have the so-called acetabular rim syndrome,
often associated with a tear of the acetabular labrum. Finally, there
are those who present with already-established arthritis, secondary to
the underlying dysplasia.
15 years as the most effective procedures for adults with prearthritic
or early arthritic symptoms due to the adult sequelae of childhood
dysplasia of the hip (5,10,44,49,51,59,61,64,66,79,96,97,99,101,109,113,114,115 and 116,140,147,148,155,156,158,165,168,178) (Fig. 104.6).
Congruency of the hip is a near absolute requirement. Dislocation and
fixed subluxation are contraindications, as is the presence of
significant arthritis. The periacetabular (Bernese or Ganz) osteotomy
offers the most theoretical and practical advantages, and it has
largely supplanted the role formerly played by varus–extension ITO of
the femur for adult dysplasia.
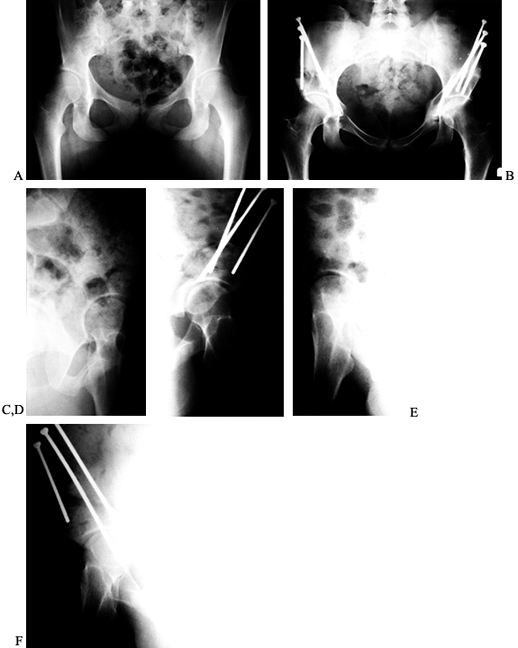 |
|
Figure 104.6. Classic periacetabular osteotomy (PAO). A: AP radiographic view of the pelvis in a 20-year-old woman with bilateral hip pain for over a year. B:
AP view of the pelvis 1 week after a right PAO and 7 months after a left PAO. By 3 months after the second PAO, the patient had no pain or limp bilaterally. C: False-profile view of the left hip preoperatively. D: False-profile view postoperatively. E: False-profile view of the right hip preoperatively. F: False-profile view postoperatively. |
stated that rotational osteotomies of the pelvis were the most
worthwhile operations he had performed in his illustrious career as
both a children’s and an adult reconstructive hip and knee surgeon. It
was not uncommon in his experience to have an enduring good result 30
years after the osteotomy (personal communication, 1989).
pelvic osteotomies introduced and used over the last 40 years,
including the spherical dome or dial (Wagner, Ninomiya and Tagawa,
Eppright), single innominate (Salter), double innominate (Sutherland),
triple innominate (LeCoeur, Hopf, and Steele), and the juxta-articular
triple (Tonnis), among others. All share the same main principle of
redirecting the position of the acetabulum to reduce sheer forces and
decrease compressive forces on the articular cartilage. Rotational
osteotomies do not result in an increase of the amount, size, or volume
of articular cartilage or supporting bone. The quantity of cartilage
that already exists is rotated into a more favorable position to
achieve one or more objectives. These objectives include achievement of
a more horizontal position of the acetabular sourcil in the frontal
plane as visualized on AP radiograph, enhanced appearance of femoral
head coverage on the frontal radiograph, and enhanced anterior coverage.
these procedures. One is the erroneous concept that rotational pelvic
osteotomies increase articular cartilage volume or amount. The apparent
increase seen on AP radiographs after successful redirections is
accomplished at the expense of loss of coverage or contact elsewhere
(usually medial and posterior). The losses elsewhere may have
implications for future THR as well as for instability of the hip in
cases of extreme correction. Another myth is the
concept
that the hip will be totally normal after the osteotomy. Many hips
manifest radiographic evidence of joint space narrowing, early
osteophytes, and subchondral sclerosis that reflect some element of
permanent and irreversible damage to articular cartilage and the joint
environment. Although regression of some of these elements (sclerosis
and cysts) may occur after favorable architectural changes, the joint
is never as normal as a joint unaffected by dysplasia.
changed, commensurate with the improvement in the reliability of pelvic
rotational osteotomies. Whenever a rotational osteotomy can be
performed, it is preferable to a Chiari. However, in cases of fixed
subluxation in young patients, impressive results lasting many years
are possible, frequently aided by concomitant valgus ITO, as previously
described.
in the mid 1980s, and the other rotational osteotomies, is the ability
of this technique to achieve three-dimensional freedom of the
acetabulum without transection of the posterior column of the ilium. In
Figure 104.7, note the superior appearance of
the acetabulum on the right side in this 33-year-old woman who had a
triple innominate osteotomy of the left hip followed by a PAO several
years later on
the
right. The right side has no residual dysplasia and is 100% free of all
symptoms. Three or four 4.5 mm screws achieve secure fixation of the
acetabular segment to the ilium above. Full-weight-bearing gait without
limp is sometimes possible within 8 weeks of surgery. Further, the
segment can be rotated anteriorly as well as laterally to customize the
coverage enhancement based on variations of pathology. Importantly,
these changes are accomplished without lateralization of the center of
rotation of the femoral head. In rare cases, deliberate medial or
posterior rotation can be done to correct less common types of
dysplasias.
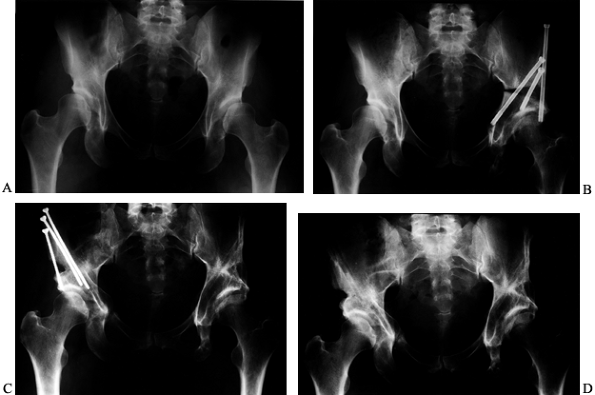 |
|
Figure 104.7. Triple (Steele) osteotomy on the left hip and PAO on the right hip in the same patient and by the same surgeon. A: AP view of the pelvis in a 33-year-old woman with bilateral hip dysplasia and pain in the left hip. B: AP view of the pelvis following left triple (Steele) pelvic osteotomy. C:
AP view of the pelvis 4 years later following the onset of right-sided hip pain and performance of a right PAO. Patient was working full time without restrictions before 6 months after PAO. Recovery from the triple osteotomy was more prolonged. D: AP view of the pelvis 1½ years after the PAO. The patient had occasional symptoms on the left secondary to some restriction of motion, but no pain. |
at the Kantonspital in Bern, Switzerland, and was based on their
experience in surgery for pelvic fractures. Understandably, the
surgical approach was borrowed from that used successfully for complex
double-column fractures with mobilization of the femoral neurovascular
bundle and the development of distinct surgical “windows” requiring
complete reflection of the tensor fascia and gluteal muscles off the
lateral wall of the ilium. Although this exposure afforded good access,
problems were encountered, including femoral vein thrombosis, a high
incidence of heterotopic ossification and long-lasting or permanent
limp due to abductor muscle insufficiency, among others.
modified their approach to expose the medial side of the ilium only,
avoid- ing stripping the lateral wall of the tensor fascia and gluteal
muscles. Matta (personal communication, 1992) devised a double oblique
fluoroscopic intraoperative view to permit radiographic visualization
of the difficult osteotomy of the ischium without direct surgical
exposure through a medial window. Santore and Stevens (personal
communication, 1993) perfected the use of grooved subperiosteal
retractors to cut the pubic bone with a Gigli saw from the direct
anterior approach without access through a medial window. An expanded
discussion of the technique for this osteotomy is warranted because of
its emergence as the procedure of choice for symptomatic DDH.
-
Position the patient supine on a fully
radiolucent operating table. Be certain that fluoroscopic visualization
of the operated hip is excellent on all views. Do a wide preparation
and drape the involved hip to well above the iliac crest. Drape the
extremity free (Fig. 104.8).![]() Figure 104.8. Drape the iliac crest free (right shown here). The planned incision along the anterior iliac crest is outlined.
Figure 104.8. Drape the iliac crest free (right shown here). The planned incision along the anterior iliac crest is outlined. -
Make a longitudinal Smith-Peterson type
of incision over the iliac crest and extending several inches distal
and lateral to the anterior superior iliac spine (ASIS). -
Incise the deep fascia lateral to the tensor muscle medial border to protect the lateral femoral cutaneous nerve.
-
Make an oblique osteotomy of the medial
half of the ASIS and reflect the sartorius and iliacus muscles medially
in continuity with this bone and each other (Fig. 104.9). Figure 104.9.
Figure 104.9.
Do a splitting osteotomy of the anterior superior iliac spine (ASIS)
with the attached origin of the sartorius muscle. The rake to the right
is retracting the sartorius medially. -
Incise and detach the indirect and the direct heads of the origin of the rectus femoris and reflect them medially.
-
Do not strip any of the muscles from the lateral aspect of the ilium, including the tensor.
-
Address the superior pubic ramus first.
Expose it with careful subperiosteal dissection, first superiorly, then
inferiorly. Protect the obturator nerve and vessels, which lie directly
beneath the pubic arch using subperiosteal retractors. Transect the
superior pubic ramus with a Gigli saw (Fig. 104.10).![]() Figure 104.10. Perform an osteotomy of the superior pubic ramus by exposing it through the incision (Fig. 104.8, Fig. 104.9)
Figure 104.10. Perform an osteotomy of the superior pubic ramus by exposing it through the incision (Fig. 104.8, Fig. 104.9)
and placing dual retractors around the pubic bone. A Gigli saw has been
passed around the pubic bone and will be used to cut it safely from
posterior to anterior. -
Next, address the ischium near its
attachment to the body of the ilium 1 cm below the joint. Approach the
ischium underneath the iliopsoas tendon and place the cutting edge of a
specially designed 40° angled osteotome (Synthes, Paoli, PA) in the
proper position under fluoroscopic control using the previously
described double oblique view to ensure that the ischial cut does not
violate the joint space and is oriented in the correct trajectory. This
trajectory is from inferolateral to superomedial. The ischium is not
transected.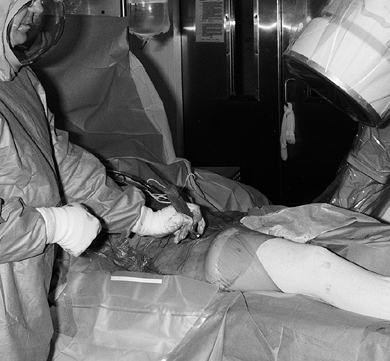 Figure 104.11.
Figure 104.11.
For the osteotomy of the ischium, an angled AO (Synthes) osteotome is
positioned under fluoroscopic control to cut it partially through from
inferolateral to superomedial. -
Cut the ilium using an oscillating saw
from the ASIS toward the sciatic notch, stopping 10–15 mm short of the
pelvic brim (iliopectinate line) (Fig. 104.12). Then use a curved standard ½-inch osteotome to cut across the pelvic brim as originally described by Ganz et al. (51).
Next, use the angled Synthes osteotome to cut parallel to the posterior
column intersecting with the previous cut made in the ischium.![]() Figure 104.12.
Figure 104.12.
Cut the ilium with a power saw from just inferior to the ASIS toward
the sciatic notch, stopping approximately 15 mm above the pelvic brim,
which is marked by the iliopectinate line (refer to Fig. 104.17). -
The final cut frees the acetabulum from
its continuity with the ilium. Insert the angled osteotome into the
medial osteotomy of the quadrilateral surface and transect the ilium
from medial to lateral, carefully avoiding the hip joint. -
Mobilize the free acetabular segment with
an osteotome inserted as a lever into the osteotomy and/or with the aid
of a Schanz screw inserted into the fragment (Fig. 104.13, Fig. 104.14). Ensure that the fragment is completely mobile and then reduce it back to its original position.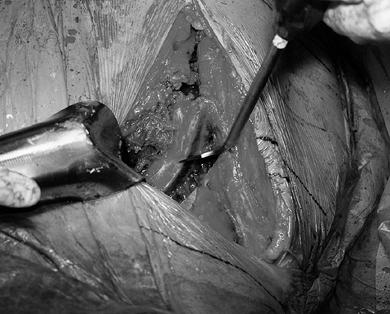 Figure 104.13.
Figure 104.13.
After cutting the lamina quadrilatera with an angled Synthes osteotome
(not shown), mobilize the acetabular fragment with the same osteotome.
The osteotome is shown in the iliac osteotomy with the ASIS just above
the osteotome.![]() Figure 104.14. More extensive anterior mobilization is shown here.
Figure 104.14. More extensive anterior mobilization is shown here. -
Arthrotomy of the hip joint may now be
performed with optimum timing since recovery of clear fluid suggests
that the hip joint has not been penetrated. Inspect the labrum of the
hip and resect any tears or detachments (Fig. 104.15).
The capsule may be left open to allow assessment of possible
impingement of the femoral neck caused by anterior reorientation of the
acetabulum. Figure 104.15. Now re-reduce the acetabulum and perform an anterior arthrotomy to inspect the labrum, which was normal in this hip.
Figure 104.15. Now re-reduce the acetabulum and perform an anterior arthrotomy to inspect the labrum, which was normal in this hip. -
Reorient the acetabulum fragment to
improve both lateral and anterior coverage of the femoral head. This
can be continuously monitored under fluoroscopic control using the AP
and double oblique views. In the frontal plane, achieve a horizontal
sourcil without under- or overcorrection. Anterior coverage enhancement
is just as important as the lateral coverage. Multiple attempts are
often necessary until optimal radiographic appearance is achieved.
Confirm the final position with an AP radiograph before placing
definitive fixation. -
Now fix the osteotomy by inserting three
or four AO 4.5 or 3.5 mm pelvic screws from the superior iliac crest in
an inferomedial direction into the acetabular segment (Fig. 104.16, Fig. 104.17).
Whenever possible, direct a screw approximately 145 mm in length into
the tear-drop area of the acetabulum at the far medial aspect of the
acetabular segment. Be certain that the osteotomy is stable.![]() Figure 104.16.
Figure 104.16.
Internally fix the osteotomy in the corrected position with four 4.5 mm
cortical AO (Synthes) screws. This provides very rigid fixation.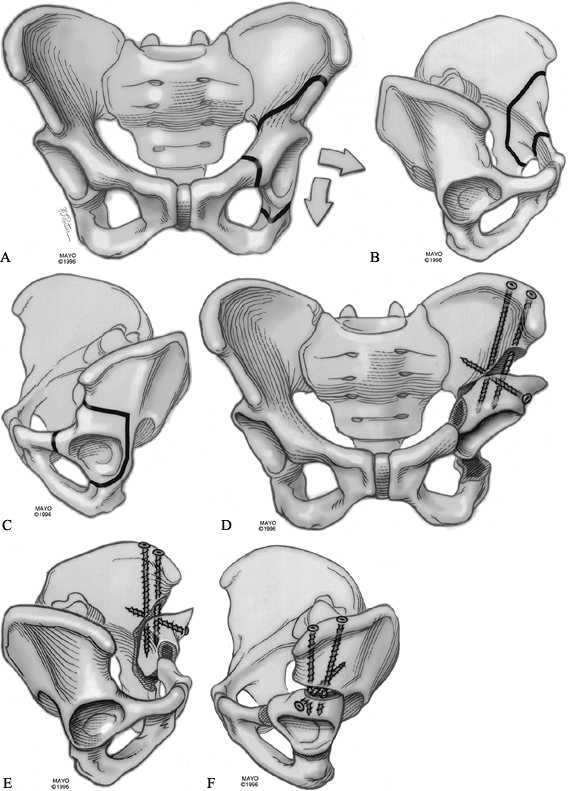 Figure 104.17. Periacetabular osteotomy. Cuts drawn on an AP view of the pelvis for the left hip (A). Cuts seen on the inner wall of the pelvis (B). Cuts seen on the outer wall of the pelvis (C). Osteotomy with screw fixation seen from the front (D), inside (E), and outside (F).
Figure 104.17. Periacetabular osteotomy. Cuts drawn on an AP view of the pelvis for the left hip (A). Cuts seen on the inner wall of the pelvis (B). Cuts seen on the outer wall of the pelvis (C). Osteotomy with screw fixation seen from the front (D), inside (E), and outside (F).
The method of screw fixation varies. The senior author (RS) prefers
three or four screws from the top of the iliac crest into the
acetabular fragment. (From Trousdale RT, Ganz RG. Periacetabular
Osteotomy. In: Callaghan JJ, Rosenberg AG, Rubash HE, eds. The Adult Hip, Vol. 1. New York: Lippincott-Raven, 1998:792, with permission.) -
Ensure hemostasis, place two drains deep
in the wound, and perform a layered closure. Reattach the ASIS
osteotomy, which contains the origins of the sartorius and rectus
femoris tendons with strong (#5) nonabsorbable suture (Fig. 104.18). Take care to avoid injury to the lateral femoral cutaneous nerve during the fascial layer closure (Fig. 104.19).![]() Figure 104.18. Appearance of the ilium after repair of the ASIS osteotomy.
Figure 104.18. Appearance of the ilium after repair of the ASIS osteotomy. Figure 104.19. Final appearance of the repair of the abdominal wall and fascia before skin closure.
Figure 104.19. Final appearance of the repair of the abdominal wall and fascia before skin closure.
with a history of bilateral Perthes who had already had a contralateral
joint replacement. He underwent a combined PAO and trochanteric
advancement and by 1 year was working without restrictions and had rare
need of a cane.
between a triple pelvic (Steele) osteotomy on the left and a PAO on the
right by the same surgeon. Note the better correction obtained after
the PAO. Recovery after the “triple” was more prolonged than after the
later PAO.
surgery if they can tolerate it. Start the patient walking with
physical therapy as soon as tolerable, utilizing a walker or two
crutches to bear the weight of the limb. Physical therapy is not
prescribed after discharge from the hospital. Weight bearing as
tolerated with crutches is permitted after 6 weeks. Full weight bearing
without support is usually achieved at 10–12 weeks after surgery.
fully healed, as they can be a source of discomfort. Very long screws
deep within the ilium can eventually fail due to micromotion within the
pelvis. Removing these prior to breakage avoids complications that
might lead to a later total hip arthroplasty.
bilateral osteotomies. We suggest spacing these 6–12 months apart so
that the initially operated hip is rehabilitated before operating on
the second hip.
with bilateral DDH and classic indications for PAO. She underwent
osteotomies 7 months apart and had no pain or limp by 3 months after
the procedure.
 |
|
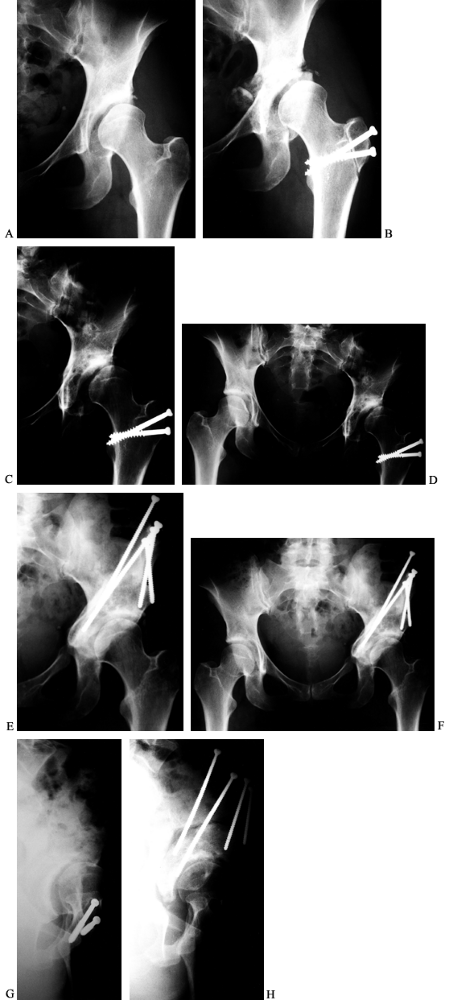
Figure 104.21. Revision of a previous, inadequate PAO. AP view of the left hip in a 31-year-old woman with dysplasia and pain (A). This AP view of the left hip shows an attempted periacetabular procedure done elsewhere (B). AP views of the hip (C) and pelvis (D)
over a year later show subluxation of the hip with poor coverage and the patient had increasing pain. A revision PAO was done. At 3 months after surgery, the patient was already walking without aids and limping only late in the day. At 8 months after surgery, it is healed (E,F). False profile view of the left hip preoperative before revision (G) and postoperative when healed (H). |
 |
|
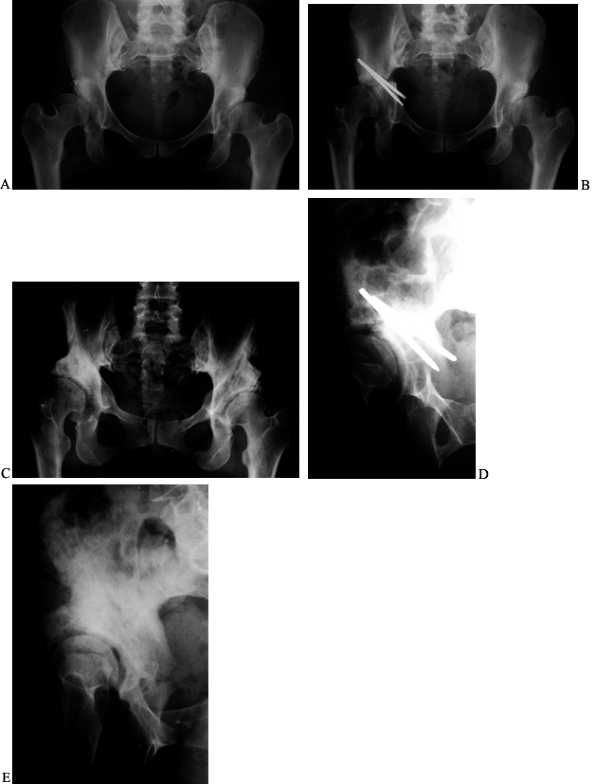
Figure 104.22. PAO salvage of a failed Chiari osteotomy. A: AP radiograph of the pelvis in a 37-year-old woman prior to Chiari osteotomy for the right hip done elsewhere. B: Postoperative radiograph after the Chiari procedure. C:
This radiograph of the pelvis is 4 years after a salvage PAO on the right. Patient had no pain and no limp on left but did have a Trendelenberg limp on right. D: This false-profile view of the right hip is just prior to salvage PAO. E: A postoperative radiograph. |
have shown that the incidence of significant complications decreases
impressively with increasing experience. A 2.9% incidence of serious
complications persists after gaining considerable experience.
Life-threatening hemorrhage; intra-articular penetration or fracture;
posterior-column fracture; malunion; nonunion; malposition of the
socket; hardware breakage; paralysis in the distribution of the
sciatic, obturator, or femoral nerves; thrombosis; phlebitis; pulmonary
embolization; heterotopic ossification; hardware-related bursitis;
numbness in the distribution of the lateral femoral cutaneous nerve;
herniation of bowel into the operative field; and vascular injury can
occur. Blood loss can be substantial and intraoperative use of the cell
saver and postoperative use of reinfusion drains are recommended.
complications deserve added emphasis, since the operation is ideally
suited to young adults with early, often minimal, symptoms. Figure 104.21 shows a 31-year-old woman
who was seen after a failed attempt at a dome-type osteotomy. A PAO was
done proximal to the previous cuts with an excellent radiographic and
clinical result.
established. The senior author (RS) has performed the operation on
patients up to the age of 60. Patients above the age of 50 must be
screened very carefully. They should be excellent candidates in terms
of all physiologic and radiographic criteria.
conversion of a failed PAO to a THR because of progression of arthritis
is virtually indistinguishable from a routine primary THR, especially
for surgeons who use the posterior approach for THR. One area for
particular attention is the anterior impingement in the case of
excessive anterior displacement of the osteotomy segment at the time of
the index osteotomy. Additionally, after complications such as gross
medial or proximal displacement or malposition, major intra-articular
fracture or heterotopic ossification, the conversion can be quite
difficult (Fig. 104.23).
significantly aided by adjunctive ITO. However, even in cases of
moderate concomitant valgus of the upper femur, PAO alone is usually
sufficient and is associated with a much-enhanced speed of recovery. In
cases of high valgus neck–shaft angle (greater than 145°), varus with
extension helps to restore more physiologic biomechanical parameters,
including offset, greater trochanter-tip-to-center-of-femoral-head
parallelism and to correct anterior extrusion of the femoral head by
virtue of the extension component in the sagittal plane (60).
The senior author (RS) prefers to perform a precisely planned ITO
first, through a separate straight lateral incision in the supine
position, followed by the PAO under the same anesthetic. Figure 104.28(A), Figure 104.28(B)
illustrates a 29-year-old woman from Japan who underwent
varus–extension ITO to normalize abductor mechanics, followed by PAO
under the same anesthetic. She is asymptomatic, now 6 years after
surgery.
 |
|
Figure 104.28. Combined varus ITO and PAO. A: AP view of the right hip of a 29-year-old woman with several years of right hip pain. B: Abduction–internal rotation AP view of the right hip prior to surgery. C:
AP view of the right hip following PAO and simultaneous varus–extension ITO. The patient required 4 months of rehabilitation to achieve a normal unassisted gait. D: AP view of her right hip 2 years later, when she had no limp and was playing tennis. |
incidence of combined ITO/PAO. Up to 6 months or more is required
before normal walking resumes, and limp may persist for up to a year
after the surgery. Circumstances that add to the appeal of the ITO
include a significant, ipsilateral leg-length inequality and/or limb
malrotation. The varus osteotomy can be planned to equalize the leg
lengths. A transverse, non-wedge-resecting technique allows full
correction of any malrotation. When the leg lengths are equal
preoperatively, an open hinge technique for the varus should be
utilized, which minimizes the shortening inherent in varus osteotomies.
An intraoperative adjunctive ITO is rarely, if ever, indicated. A
better strategy would be to readjust the position of the PAO to
maximize coverage.
high-riding greater trochanter is seen in adults as a sequela to
childhood Perthes disease (11). Options for
treatment include lateral–distal advancement of the greater trochanter
alone, ITO of the upper femur alone, PAO alone, or a combination of PAO
of the acetabulum and distal–lateral advancement of the greater
trochanter. The preference of the senior author (RS) has been the
latter option, which addresses the two principal features responsible
for disability: the acetabular dysplasia and the dysfunction of the
abductors related to the high-riding trochanter. A unique technical
consideration in this subset of patients is the close anatomic
relationship of the lesser trochanter to the ischium. This requires
that the ischial osteotomy be done on the medial surface of the
iliopsoas tendon. Great care must be taken to avoid injury to the
neurovascular structures in this region. The trochanteric osteotomy
must be done prior to the repositioning of the acetabular segment into
its new position. Otherwise, the position of the trochanter limits your
ability to laterally rotate the acetabular segment fully. Once the
periacetabular segment has been rotated and fixed, the trochanter is
advanced and fixed.
fragmentation, good mobility of the hip, and good joint congruence.
Blood loss is greater and postoperative rehabilitation more difficult
after this combined procedure than after isolated PAO alone. Although
trochanteric advancement alone would address the abductor insufficiency
and augment endurance for walking and standing, the opportunity to
combine definitive correction of the dysplasia with the biomechanical
enhancement of the abductors in one surgery and one rehabilitation
period is a significant advantage of this strategy.
52-year-old man with disabling pain and abductor weakness. PAO and
trochanteric lateralization were combined under one anesthetic. He
returned to full-time work 8 months after surgery.
minimal (Tonnis grade I) arthritis was present at the time of surgery (159).
In this subgroup, Harris hip score improved from 58 to 94. No patient
required conversion to a total hip arthroplasty. Conversely, in the
nine hips with Tonnis grade III changes preoperatively, the average
Harris score improved only modestly, from 59 to 67. Five (56%) were
converted to THR and 8 of 9 (89%) had a Harris score below 70 by the
time of final follow-up. Therefore, patients with evidence of grade III
(severe) arthritis are not suitable candidates, based on an 88% failure
rate within 5 years of the osteotomy.
the average Harris score went from 67 to 89 and one of the 18 was
converted to a THR. Overall, there were 31 secondary operations in this
series of 42 patients (42 hips), for a total of 73 operations in the
4-year (average) period including the index osteotomy, indicative of
the nontrivial nature of the decision to perform major pelvic osteotomy
in adults.
cohort of 75 periacetabular osteotomies by the group in Bern,
Switzerland, revealed a 73% incidence of good or excellent results (141).
The average age of the patients was 29 (range, 13–56) and 18% had been
converted to THR, with 49 subsequent operations in the 71 hips
available for follow-up. Unfavorable outcomes were associated with
older chronologic age at time of surgery, presence of significant
arthritis, and insufficient anterior coverage at the time of surgery.
The average age was 33.6 (range, 19–51) with 17% excellent, 59% good,
12% fair, and 12% poor at the follow-up (averaging 4 years after
surgery). There were 17 subsequent procedures (26% incidence) including
10 adjunctive intertrochanteric osteotomies, five THRs, and two
resections of heterotopic ossification. At the time of submission for
publication, there were three more pending conversions to THR, for a
total conversion rate of 8 of 66 (12%).
follow-up of 2 years (range, 2–7) demonstrated a rate of conversion to
THR of 4%. The average age of patients in the series was 29 (range,
10–55) (111).
reported on the minimum 2-year follow-up of 123 procedures in 115
patients (161). Overall, 83% of the hips were
rated as good or excellent at final follow-up. The average preoperative
Harris score of 65 increased to 89 at final follow-up. As in other
series, there were a high number of subsequent surgeries, that is, 70
in 44 patients, including seven THRs at an average of 41 months after
the PAO (5.6%). The frontal plane center–edge angle of Wiberg increased
from 6° (range, -20° to +20°) to 29° (range, -10° to +60°). The
anterior center–edge angle of Lequesne (80)
increased from an average of 3° to an average of 23°. In this series,
PAO alone or in combination with ITO or trochanteric advancement was
utilized successfully in multiple underlying conditions, including
Legg-Calvé-Perthes disease (10 hips), Charcot Marie Tooth disease (four
hips), epiphyseal dysplasia (three hips), SCFE (one hip), posttraumatic
dysplasias (one hip), and postinfectious dysplasia (one hip).
of 1,400 juxta-articular pelvic osteotomies, 138 in adults, at an
average 7.3 years after surgery (157). Although
pain relief was seen in 82%, Trendelenburg gait was common and 81% of
adults had no change or worsening of limp compared with preoperative
status. Best results were seen in patients with concentric,
nonarthritic hips preoperatively.
technically demanding, associated with a relatively high incidence of
perioperative complications and reoperations, and should be utilized
with caution in patients with advanced arthrosis on screening
radiographs.
Several authors have reported on the acute treatment of SCFE with
immediate corrective ITO accompanying the pinning of the slipped
epiphysis (8,134). This approach has been abandoned because of excessive occurrence of AVN and chondrolysis (47,50,163). Most patients with SCFE are now treated with pinning in situ (29), and any resulting deformity is addressed later, depending on its severity and the symptoms (144).
Because of the high risk of early-onset degenerative arthritis,
treatment of a symptomatic moderate to severe SCFE is better performed
early, once the child has matured and results of initial treatment are
evident. This allows for maximal remodeling and preempts years of
abnormal biomechanics that can cause significant cartilage damage (134).
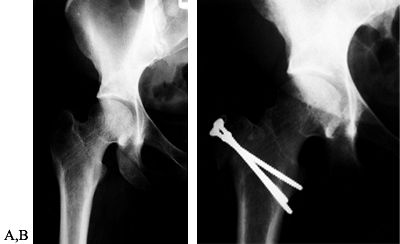
Occasionally, the deformity of relative greater trochanteric overgrowth
can occur because of proximal femoral physeal arrest following an SCFE (71). This may be amenable to a simpler trochanteric osteotomy, as in the patient in Figure 104.24, with advancement of the greater trochanter.
 |
|
Figure 104.24. Isolated advancement of the trochanter for SCFE. A:
AP view of the right hip in a 36-year-old woman with a history of a SCFE treated in adolescence. Patient had lateral hip pain and a Trendelenberg limp that was worse when she was fatigued. B: AP view of the right hip 1 month after isolated trochanteric advancement. By 9 months after surgery, the Trendelenberg limp was gone, although the patient had some end-of-day fatigue. |
posterior and varus (medial) displacement of the epiphysis relative to
the rest of the femur, with the posterior displacement usually the most
significant. Additionally, the femoral shaft is relatively externally
rotated as part of the deformity. The external rotation is directly
related to the
posterior
slip of the epiphysis. When the slip angle is 30° to 60°, correction of
all three components of the deformity is preferred, utilizing an ITO.
The angulation of the ITO is flexion and valgus with internal rotation
of the distal fragment as needed. The so-called triplane ITO was
proposed by Imhauser (68) and introduced to the English literature by Southwick (144). It is, in fact, a biplane ITO with additional rotational correction included when necessary.
documentation of the slip angle on biplane radiographs. On physical
examination, document the range-of-motion deficits, leg lengths, and
positions of comfort. On radiographs, confirm the mobility and
congruency of the hip in adduction and extension (the relative
direction that the femoral head will need to move after a
valgus-flexion ITO). These measurements determine the degree of
correction that will need to be made in each plane.
which is described in detail in the next section of this chapter. The
following details require attention:
-
The flexion component of the osteotomy
corrects for the posterior slip of the epiphysis. This requires
insertion of the blade plate so that the chisel is angled with the
distal aspect rotated anteriorly as it is inserted into the proximal
fragment. Correction of the posterior displacement with an anterior
repositioning of the proximal segment will result in the plate sitting
flush on the lateral surface of the femoral shaft. -
In this same osteotomy, orient the cuts
to produce a closing wedge laterally to correct the varus deformity. A
full lateral wedge resection, equal to the magnitude of the planned
valgus, is necessary to avoid lengthening of the limb. -
To measure the rotational correction,
insert parallel Kirschner wires (K-wires) above and below the osteotomy
for referencing. Even more important, though, is to monitor the patella
for anterior position prior to fixation. -
Check the range of motion of the hip
after fixation is in place to identify any bony blocks to motion,
especially in flexion or adduction. If present, this may require
excision of impinging bone or repositioning of the fragments. With
detailed preoperative planning and technique, surgical complications
such as limb-length inequality, rotational or angular malposition, and
nonunion can be minimized.
the results of 68 hips in 55 patients with 11- to 22-year follow-up. Of
these, only 27% showed early degenerative changes and one had severe
coxarthrosis and complained of pain. Five hips had significant
limitation of motion, with one patient dissatisfied by this. Imhauser
felt that these results represented a large improvement over historical
controls.
pinning of the epiphysis in the osteotomy group but used spica casting
postoperatively for all acute slips. There were no cases of AVN in the
55 patients. Three hips required a second operation, all for bony
impingement blocking either adduction or
flexion;
all of these had subsequent excellent results. All hips experienced ½–1
inch of shortening. Southwick stated that slips greater than 60° could
not be fully corrected because of excessive shortening of the femur. In
those patients with more than a 5-year follow-up, he reported 21
excellent, five good, and two fair results.
reported on 51 patients with unilateral chronic slips with angles of
30° to 60° who were treated by early corrective ITO. The average
patient age was 12.6 (girls) to 14.6 years (boys), and mean follow-up
was 24 years, with the surgery done an average of 6 months after the
first symptoms. At the latest exam, 55% of the patients had no symptoms
or radiographic deterioration, 28% had moderate osteoarthritis, and 17%
had severe osteoarthritis. The authors stated that this 17% represents
a significant improvement over what they believe to be a 28% to 40%
chance of developing early onset degenerative arthritis if these severe
slips are left uncorrected.
history of SCFE and serve as the control for most of the series in the
literature. Goodman et al. (53) in 1997 studied
more than 2,600 skeletons and found that the morphology of the hip
after the slip was a major risk factor for the development of
high-grade osteoarthrosis. In a Swedish study (118) of 30 untreated grade 3 slips (see Chapter 172)
with at least a 20-year follow-up, 57% had pain and 43% had limitations
in walking activity, despite an average age of less than 40 years. In a
distillation of these studies, Aronson et al. (8)
concluded that by the fourth decade, severe SCFE increases the risk of
hip osteoarthritis by a factor of 20 over the normal population.
Aronson et al. (8) reported on a series of
Bombelli’s patients, which included 24 grade 2 slips corrected by
primary triplane ITO. The group with more than a 2-year follow-up had a
39% gain in total arc of motion after having had a preoperative mean
slip angle of 58°. Less aggressive bony wedge removal than that
described by Southwick (144) resulted in fewer
problems with limb shortening. All were pain free at 2–10 years
follow-up, with no cases of AVN, chondrolysis, infection, or nonunion.
for osteoarthritis of the hip against which other surgical options must
be weighed. Even with modern advances, however, hip replacement cannot
yet be offered to younger adults routinely. Early failures, primarily
due to rapid wear and loosening, have been well documented in patients
younger than 50 years (30,40,41,155).
Proximal femoral osteotomy therefore remains an important and effective
treatment option for the painful osteoarthritic hip in adults.
ITO has a record of success in the treatment of osteoarthritis of the
hip, whether primary or secondary. Most authors have reported better
results from ITO in patients with secondary hip arthritis, such as from
dysplasia, SCFE, or Perthes disease (77,86,87,99,103,104,122,127).
These patients have a definable biomechanical predisposition to develop
degenerative arthritis. Because the predisposing deformity can often be
improved or even completely normalized by ITO, it is understandable
that results have been superior in this group. Surgical intervention
may both relieve pain and decelerate further deterioration of the hip.
have had better long-term results than those who had valgus ITO. The
selection criteria for those receiving a varus osteotomy, however,
often resulted in these patients being younger, with less radiographic
arthrosis and better joint congruity. Therefore, patient selection has
been biased, making comparisons between these procedures in the
literature difficult. In fact, with the advent of modern periacetabular
rotational osteotomies, the indications for isolated ITO in the adult
have narrowed considerably, particularly for varus ITO. Currently, the
most common osteotomy of the proximal femur indicated for significant
adult arthritis is a valgus–extension ITO. Valgus and extension
refer to the position of the distal fragment at the osteotomy site.
Therefore, a valgus–extension ITO moves the proximal fragment and the
hip joint itself toward adduction and flexion. For this reason,
therefore, passive positioning of leg in adduction–flexion
preoperatively anticipates to some degree the status of the hip after a
valgus–extension osteotomy.
attention when considering a varus or valgus ITO. In addition to
performing a routine evaluation, determine the position of comfort by
history and exam. Relief of pain in adduction suggests improvement from
a valgus osteotomy. Perform functional radiographs in abduction and
adduction, and/or examine the patient under fluoroscopy. With the hip
positioned to replicate the planned effect of an ITO, joint congruency
and the remaining joint space should be improved, or at least similar.
A false-profile view will
reveal any deficiency in anterior coverage, which may favor the addition of an extension component to the osteotomy.
inequality because there is a profound potential effect of ITO. Given
equal leg lengths preoperatively, routinely remove wedges from the
osteotomy site with valgus osteotomies, or preserve maximal length in
varus ITO, using no wedges at all. Preserving or improving limb length
almost always takes precedence over the goal of obtaining total
apposition of the raw cut surfaces at the osteotomy site.
document overall limb alignment preoperatively by displaying the
mechanical axis (see Chapter 26 and Chapter 32).
All things being equal, lateral shaft displacement will accompany a
valgus ITO, and relative medial displacement of the distal fragment
occurs with a varus ITO. This is necessary to accommodate future total
hip stem insertions and minimize any frontal plane mechanical axis
derangement, which may predispose to later knee (or ankle) problems.
Nevertheless, planning for the placement of a future total hip stem
generally takes priority over shaft displacement when considering final
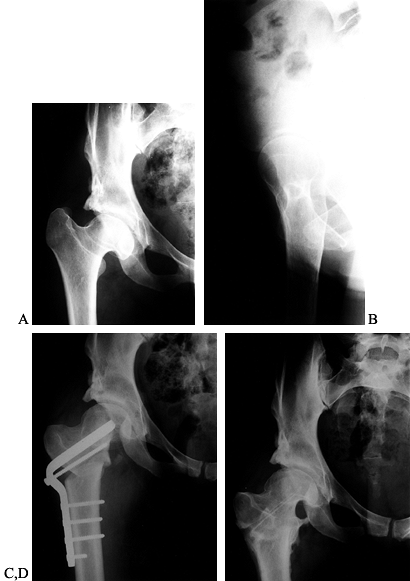
position of the fragments after osteotomy. The patient in Figure 104.25(A), Figure 104.25(C), Figure 104.25(E) demonstrates the importance of limb-length planning. Her leg was 1½ inches short before, but equal in length and pain
free after a valgus ITO with no wedge and lateral shaft displacement.
 |
|
Figure 104.25.
Leg-length discrepancy and arthritis treated with valgus ITO. This 32-year-old woman with a history of hip dysplasia, childhood hip surgeries, and treatment-related osteonecrosis. A: AP view of the right hip. B: False-profile view. The right lower extremity was 1½ inches (3.75 cm) short. C: AP view of the right 6 months after a valgus ITO. D: AP view of the right hip 15 months postoperative. After hardware removal, the patient had no pain, a 95° flexion arc, and equal leg lengths. |
has studied and categorized the morphologic features of osteoarthritis
of the dysplastic hip, including a nomenclature for the osteophytes
that develop. The vast majority of patients (95% of Bombelli’s
valgus–extension series) have superolateral rather than medial
degenerative changes. The superolateral group also had the best results
in Bombelli’s series as well as those of others (54,77).
utilize the noninnervated medial “capital drop” osteophytes as a new
fulcrum along with the “floor” osteophytes of the acetabulum. This more
medial fulcrum acts as a new center of rotation for the hip joint.
Lateral impingement is reduced, and weight-bearing forces are moved
away from the painful superolateral femoral head and toward the medial
osteophytes, and the lever arm of the body weight is reduced. “Roof”
osteophytes also play a role. The additional extension component to the
osteotomy allows improved anterior coverage of the femoral head and
elimination of any fixed flexion contracture. Extension, which refers
to the relative position of the distal fragment at the osteotomy site,
is therefore “apex-anterior.” Ideally, these features improve joint
congruity and the radiographic joint space, as well as increasing the
weight-bearing surface area. In patients with some flexion (with or
without adduction) contracture, a valgus–extension ITO moves the
restricted anatomic range of motion closer to the functional range of
motion needed for daily activities. This reduces a major source of pain
and impingement. The extension component also relaxes stresses of
hyperlordosis on the lumbar spine and often permits patients to rest
without pain in the supine position for the first time in years.
shows a patient who had a valgus ITO on the left with later recurrence
of some pain at 7 years. He had a mild valgus ITO on the right for
protrusio-pattern arthritis.
 |
|
Table 104.3. Indications and Contraindications for Valgus (and Varus) Extension Intertrochanteric Osteotomies
|
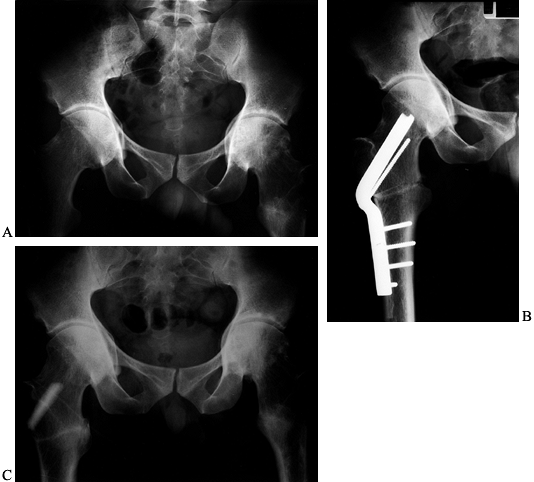 |
|
Figure 104.26. Arthritis with bilateral protrusio acetabulae treated with a valgus ITO. A: AP view of the pelvis in a 28-year-old with a history of a left ITO done elsewhere 4 years earlier with initial pain relief. B: AP view of the right hip early following a mild valgus–derotation right ITO. C:
AP view of the pelvis 1 year after surgery. By 2½ years, the patient rated the right hip as “excellent,” while symptoms had recurred on the left. |
-
Position the patient supine with the
operative hip at the edge of a radiolucent operating table. Place a
bump under the sacrum and prepare and drape the hip and leg free. -
Perform a lateral approach to the
proximal femur through a straight lateral incision, or one that curves
anteriorly in the proximal limb if access to the hip joint is needed. -
Reflect the vastus lateralis muscle
anteriorly from the posterolateral aspect of the femoral shaft, and
release the origin from the vastus tubercle. The femoral neck need not
be routinely exposed if good fluoroscopic images are obtained. -
Slide a K-wire along the anterior surface of the femoral neck to mark the anteversion of the femoral neck.
-
Mark the site of the chisel insertion by
drilling a transverse 7/64-inch K-wire at the level of the vastus
tubercle, (usually) parallel to the first K-wire and at an angle in the
frontal plane that corresponds to the preoperative plan. Confirm by
fluoroscopy. -
Generally, we use a 120° or 130° plate
for a valgus ITO. For a 30° valgus correction with a 130° plate, insert
the wire at 80° from the lateral cortex of the shaft and
P.2753
parallel to the femoral neck pin on the lateral view (Fig. 104.27).
Make fine adjustments to the start point and lateral position based on
AP and lateral imaging. The wire should be in the center or slightly
inferior portion of the neck on the AP view, and in the center of the
neck on the lateral view. The greater trochanter is posterior, so the
blade should enter anterior to the midtrochanteric line to avoid
exiting the posterior femoral neck.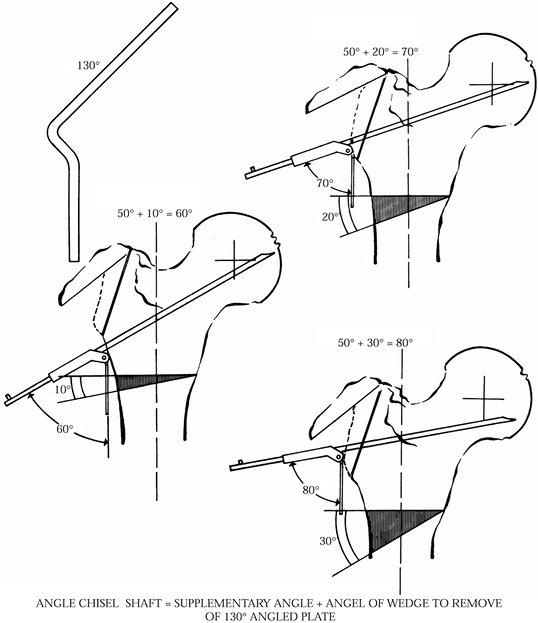 Figure 104.27.
Figure 104.27.
Classic Bombelli geometric preoperative planning strategy for 10°, 20°,
and 30° valgus osteotomies, using the 130° angled plate. (From Bombelli
R, Aronson J. Biomechanical Classification of Osteoarthritis of the
Hip. In: Schatzker J, ed.The Intertrochanteric Osteotomy. New York: Springer-Verlag, 1984:115, with permission.) -
Drill in a final reference K-wire
perfectly parallel to the wire marking the course of the blade, but 1–2
cm superior to it in the greater trochanter. This will help guide
placement of the seating chisel if the initial wire loosens or needs to
be bent or removed. Remove the initial extraosseous femoral neck wire. -
Clean the blade insertion site of any remaining soft tissue.
-
Prepare the lateral cortex for the
seating chisel using the AO (Synthes) 4.5 mm triple drill guide in the
site of the second K-wire and rotated posterior to the femoral shaft
for the desired amount of extension. Confirm that the passage for the
chisel through the neck will be safe by checking a lateral radiographic
view with drill bits in the outer holes of the triple guide. Use the
router as needed to further develop this opening in the outer cortex to
prevent bone splintering or incarceration of the seating chisel. -
Now slowly impact the seating chisel,
parallel in both planes to the reference K-wire and rotated posteriorly
for the desired extension component. Preset the attached adjustable
angle guide on the chisel to be parallel to the femoral shaft to
monitor the frontal plane insertion angle, that is, 80° to the shaft.
Check multiple AP and lateral images during insertion. -
Back the chisel out several times as it gets deeper, to prevent incarceration.
-
Prior to doing the femoral osteotomy,
score the anterior cortex longitudinally as a reference for rotation,
or insert reference K-wires above and below the osteotomy. -
Leaving the chisel in place, perform the
osteotomy 1.5 cm below the chisel, 90° to the shaft. The preoperative
plan should place the osteotomy at the upper border of the lesser
trochanter. Cut slowly with an oscillating saw using retractors to
protect the posterior soft tissues. Use copious irrigation to prevent
overheating. Adduct and externally rotate the leg vigorously after the
osteotomy. This permits access to the iliopsoas tendon insertion into
the lesser trochanter. This is released from the lesser trochanter with
the electrocautery device. -
Next, correct the rotation prior to removing any bone wedges.
-
Then use the oscillating saw to remove a
biplanar wedge of appropriate size from the proximal end of the distal
fragment. For example, if 30° of additional valgus and 10° of extension
of the proximal fragment were desired, a wedge that combined these two
amounts would be removed. The cuts can be made sequentially rather than
as a single cut; it is technically easier to do two separate cuts but
more bone is usually sacrificed. The location of the apex of the wedge
removed relative to the medial-lateral width of the femur determines
the degree of apposition of the surfaces of the osteotomy and the gain
in length. Placing the apex at the medial cortex maximizes the contact
area in the osteotomy and minimizes the gain in length. -
Remove the seating chisel and carefully
insert the blade plate by hand along the same path. Impact to final
depth while checking images in two planes. Lateralization of the shaft
is built into the selected blade plate according to preoperative
planning, but it can be fine-tuned at this stage by the final depth of
blade insertion. For instance, if the plan calls for 15 mm of lateral
shaft displacement,
P.2755
and
the seating chisel depth of insertion was 70 mm, use an 85 mm 130°
plate to achieve full insertion and 15 mm of lateral shaft displacement. -
Insert a 4.5 mm cortical screw into the
head fragment through the most proximal hole in the plate before use of
the compression device. -
Without using excessive force on the
implant, manipulate the fragments and dissect soft tissues as needed to
allow easy apposition of the bone ends. Before osteosynthesis, resect
any planned wedges from the proximal surface of the distal fragment. -
Apply a Verbrugge clamp (Synthes, Paoli,
PA) and approximate the plate to the lateral cortex of the shaft. With
the osteotomy reduced, tighten the clamp. Pay attention to the
displacement in the sagittal plane to avoid creating difficulties with
future femoral stem placement. -
The calibrated compression device is now
used. Avoid aggressive compression, which may place the lateral bone
bridge at risk. Make sure that the patella is in an anterior position
to control leg rotation. -
Gently flex the hip and knee to confirm normal rotational alignment.
-
Insert one or two screws in the side
plate. Check images in two planes for implant position and fragment
alignment. If satisfactory, then complete screw insertion. -
Use any removed bone as graft around the osteotomy after thorough irrigation.
-
Insert a drain and close the wound in layers.
toe-touch weight-bearing the day after surgery. Do not allow straight
leg raises or active abduction exercises during the initial 6 weeks.
Begin weight bearing with two crutches at 6 weeks after surgery and
keep the patient bearing only partial weight until bony healing and
strength allows transition to one crutch or cane, usually between 12
and 14 weeks. We remove the implants at 12–24 months postoperatively.
patients previously selected for varus osteotomy are usually candidates
for a PAO. Some patients with acetabular and femoral deformities may
require both procedures. Indications for an isolated varus ITO include
minimal femoral head deformity and little or no acetabular dysplasia, a
valgus neck–shaft angle, signs of early lateral joint arthritis, and
improved joint congruency on abduction radiographs (99). Medial displacement of the femoral shaft has been suggested in the past as the most important part of an ITO (37,95,127).
Experience over the past 20 years, however, has shown that good results
do not depend on medial displacement, and that problems with healing as
well as with future femoral stem placement increase with the degree of
displacement. In fact, deliberate medial displacement of the distal
fragment is strongly contraindicated for this reason.
of the leg is guaranteed if wedges are resected. An opening wedge
technique can preserve some length but is associated with a longer time
to complete bony union. A Trendelenberg gait is common and may persist
permanently. Simultaneous or staged osteotomy and distal transfer of
the greater trochanter may be required to combat abductor dysfunction.
A bulge from the prominence of the greater trochanter due to varus
positioning can be undesirable and can predispose to bursitis over the
plate and trochanter. A longer period of rehabilitation should be
anticipated following a varus ITO. Figure 104.28
shows a case of combined varus ITO with PAO. The patient had a 4-month
rehabilitation to a normal unassisted gait and was playing tennis with
no limp at 1 year.
similar to that for valgus–extension ITO, but there are some
significant differences.
-
Position the patient, expose the hip, and
insert guide wires in a fashion similar to that described previously
for the extension–valgus ITO. -
Generally, a 90° blade plate is utilized
for a varus ITO. For a 15° varus correction with this plate, insert the
guide wire at 75° from the lateral cortex of the shaft and parallel to
the femoral neck pin on the lateral view (Fig. 104.29).
Make fine adjustments based on AP and lateral imaging. The wire should
be in the center or slightly inferior portion of the neck on the AP,
and down the center of the neck on the lateral view. The greater
trochanter is a posterior structure, so the blade should enter anterior
to the midtrochanteric line to avoid exiting the posterior femoral neck.![]() Figure 104.29. A:
Figure 104.29. A:
Use of AO (Synthes) chisel device and geometry for 10°, 20°, and 30°
varus osteotomy. Rarely are varus osteotomies of greater than 15°
indicated. B: Varus ITO: (1) full wedge, (2) partial wedge, (3) no wedge. No wedge with medial displacement and impaction (4)
is the authors’ preferred method. (From Bombelli R, Aronson J.
Biomechanical Classification of Osteoarthritis of the Hip. In:
Schatzker J, ed. The Intertrochanteric Osteotomy. New York: Springer-Verlag, 1984:129,130, with permission.) -
Insert a final reference K-wire perfectly
parallel to the wire marking the course of the blade, but 1–2 cm
superior to it in the greater trochanter. This will help guide
placement of the seating chisel if the above wire loosens or needs to
be bent or removed. Remove the initial extraosseous femoral neck wire. -
Prepare the insertion site for the blade plate as described for the extension–valgus ITO and insert the chisel.
-
Leaving the chisel in place, perform the
transverse osteotomy 1.5 cm distal to the chisel using an oscillating
saw as described previously. -
Without wedge resection, impaction and
compression of the medial cortex of the proximal fragment into the
canal of the distal fragment can provide adequate bone contact without
further reduction in length from wedge resection. -
Carefully control rotation of the distal segment.
-
Any additional medial displacement of the shaft, beyond what occurs as a consequence of pure in situ varus, is avoided, to facilitate future THR stem insertion.
-
Insert the blade plate and secure it to the shaft of the femur as described previously. Then use the compression jig.
-
Gently flex the hip and knee to confirm that normal rotational alignment has been maintained.
-
Insert one or two screws. Check images in
two planes for implant position and fragment alignment. If
satisfactory, then complete screw insertion. -
Irrigate the wound, insert a drain, and close the wound in layers.
-
Postoperative procedure is similar to that of the extension–valgus osteotomy already described.
has helped to define the modern indications and technique for the
procedure. Good long-term results are particularly sensitive to proper
patient selection, preoperative planning, and intraoperative technical
details. Despite the success and ongoing advances in total hip
arthroplasty, there is growing evidence supporting the continued
viability of ITO as an important treatment option for arthritis of the
hip. In 1980, Morscher (103) reviewed the
results of 263 patients at his own institution and compared them with
the results of the Swiss multicentric study of more than 2,200 ITOs
from 1970. Sixty percent of his patients were more than 50 years old.
Sixty-eight percent of patients with arthritis secondary to dysplasia
had a varus ITO, with good to excellent results in 88%. They performed
varus osteotomies in patients with lateral joint space narrowing and
sclerosis, minor head deformities, and congruency in abduction. Valgus
ITOs were done for pronounced head deformities and congruency in
adduction. Summarizing their overall results, they had excellent
long-term outcomes in one-third of all patients and satisfactory
results in another third; one-third eventually needed total hip
arthroplasty. Additionally, they noted that the group of long-term
failures were considered failures early, although many went more than 5
years before conversion to an artificial joint.
the results of 150 valgus–extension ITOs in patients with severe
arthritis. Mean follow-up was 6 years, and 45% were older than 65
years. In patients under 50 years at the time of surgery, with
secondary arthritis, 83% were pain free at the 10-year follow-up, with
77% good or excellent results. Overall results were good in 68% at the
3-year follow-up and 62% at the latest follow-up. Results for patients
with concentric hips and primary osteoarthritis were only half to
two-thirds as good as for posttraumatic or other secondary arthritis.
Radiographic improvement of the joint space was seen in 60% of all
patients.
the results of 277 valgus–extension osteotomies in patients with a mean
age of 52 years. At 11–15 years of follow-up, they reported 67% good or
excellent results. Results were better in those under 40 years old (76%
good or excellent). Patients with unilateral disease had better results
(77% good or excellent) than those with bilateral disease (40% good or
excellent). Good results were obtained in 68% if preoperative flexion
was 60° or more compared to only 31% if flexion was less than 60°. As
with the preceding study, a mechanical etiology for arthritis predicted
results almost twice as good (71% good or excellent) as for patients
with primary arthritis (42% good or excellent). Maistrelli et al. (87), Bombelli et al. (18,19), and others (9,23) have recommended a minimum biplane correction of 20° to obtain their expected results. Santore and Bombelli (132)
reported the results of 45 patients with a mean age of 50 years. At the
11-year (average) follow-up they had 75% good to excellent results,
which included 35 valgus and 10 varus ITOs. The average Harris hip
score increased from 44 to 84. Unlike in previous reports, older
patients or those with preoperative hip stiffness did not have worse
outcomes.
varus ITO, in part because of the success of rotational pelvic
osteotomies. Most reports include a combination of pelvic and femoral
varus and valgus osteotomies, although when done for arthritis, an
extension component has usually been combined with both types.
Morscher’s (103) summary of the large Swiss
series reported the varus ITO results separately, with nearly 90% good
to excellent long-term outcomes. Detenbeck et al. (38)
reported a series of 59 ITOs according to the method of Pauwels in
patients with a mean age of 57 years. Results at 2½ years were
satisfactory in 80% (32 excellent, 15 good). The majority of patients
had a varus procedure, and these results (89% satisfactory) were
superior to the results in valgus osteotomy (65% satisfactory). These
results cannot be directly compared because of the different selection
criteria for each group. In a more recent report, Iwase et al. (70)
followed 110 hips with dysplasia more than 20 years after ITO, with
nearly equal numbers of varus and valgus osteotomies. Again, varus
osteotomy was selected for early coxarthrosis (mean age, 25 years) and
valgus for end-stage disease (mean, age 37 years). Using the endpoint
of a Harris hip score of less than 70 or a salvage surgery, the 10- and
15-year survival rates for varus osteotomy were 89% and 87% and for
valgus ITO were 66% and 38%, respectively. The younger age range in
this series was almost certainly a factor in the excellent long-term
outcomes, which also included 82% good to excellent 20-year results for their varus osteotomies.
reported on 31 valgus–extension ITOs in patients with a mean age of 43
years, including four that had a concomitant Chiari osteotomy. The good
results in these osteotomies had a survivorship of 85% at 5 years, 67%
at 10 years, and 51% at 15 years. Good radiographic results correlated
with the size of the preoperative osteophytes, particularly the width
of the capital drop and length of the roof osteophytes. D’Souza et al. (43)
published a series of 25 hips (6 varus–extension and 19
valgus–extension osteotomies) with a mean age of 38 years and average
follow-up of 7 years. Four hips (16%) were converted to total hip
arthroplasty at an average of 8 years after ITO, with a survival rate
at 12 years of 67%. Schneider (137), in a
report with 12–15 years of follow-up, also found that the median time
to conversion to total hip arthroplasty was 8 years. Despite the fact
that many of the studies in the literature have included inhomogeneous
groups and less than ideally selected patients, similar selection
criteria and results in properly selected patients have emerged over
the years. There is a growing consensus that at 10 years or more after
surgery, 70% to 80% of patients continue to have satisfactory results
following an appropriate angulation ITO (77,87,103,120,123,132,135,137).
pelvis and proximal femur for several reasons. Some patients have pain
related to the implants. Even patients who do not have tenderness to
palpation over the hardware can experience pain relief after removal.
With pelvic osteotomies, long cortical fixation screws are used, often
more than 130 mm in length. These are at some risk for fatigue failure
due to repetitive strain in the pelvis. Removal of these screws from
the iliac crest is a simple outpatient procedure if performed before
screw breakage, but is otherwise much more complicated. Patients who
have an osteotomy of the pelvis or proximal femur are at increased risk
for requiring a subsequent THR; therefore, we feel that it is prudent
to remove implants that might interfere with subsequent surgery.
proximal femur are not specific for osteotomy surgery and will not be
discussed in detail here. These include anesthetic complications, blood
loss and transfusion requirements, infection, deep venous thrombosis,
and perioperative medical problems such as urinary tract infection or
myocardial infarction. These complications are best avoided by treating
most major osteotomy patients like total hip arthroplasty patients.
Common total joint replacement protocols are generally applicable and
may include autologous blood donation, cell-saver for certain cases,
perioperative antibiotic and thromboembolic prophylaxis, and use of an
arthroplasty-style operating theater. As osteotomy patients are, by
selection, physiologically younger than total joint patients, surgery
is better tolerated, and nonspecific complications such as those listed
previously are unusual.
-
Trochanteric bursitis or painful bursae over implants (24,112)
-
Malunion, that is, undercorrection, overcorrection, malrotation, retroversion (PAO only)
-
Delayed union and nonunion
-
Implant breakage
-
Penetration of the hip joint
-
Loss of fixation
-
Changes in leg length
-
Abductor weakness and limp
-
Difficulty with subsequent THR (154)
-
Nerve injury, especially of the lateral femoral cutaneous nerve (PAO)
-
Intraoperative fracture of the femoral neck, acetabulum, or the lateral bone bridge below the blade of the blade plate
-
Clinical failure in spite of a
well-performed procedure, including increased hip joint stiffness, and
persistent or increased pain. Figure 104.23
shows a 47-year-old woman who, after a technically successful PAO,
maintained a pain-free status for 6 months, followed by failure
requiring THR. The degree of difficulty of the THR was no different
from that of a standard primary THR.
routinely care for fractures around the hip joint. The most common
complication seen with osteotomy of the pelvis or proximal femur is
bursitis. The nearly subcutaneous position of the greater trochanter
and iliac crest makes prominent hardware nearly unavoidable. This is
commonly seen over large-fragment screw heads at the iliac crest
following pelvic osteotomy, and laterally over a blade plate in the
proximal femur. Routine removal of the implants usually eliminates this
problem. When varus of the proximal femur is present or when the
greater trochanter has been lateralized, trochanteric bursitis can
persist despite hardware removal, because of the prominence of the
greater trochanter (2).
an ITO around the hip is overcorrection when performing a varus
proximal femoral osteotomy. This may lead to abductor weakness, an
unresolvable Trendelenberg limp, marked leg-length inequality, and
patient dissatisfaction. Overcorrection, undercorrection, and
rotational malalignment can all lead to a problematic malunion.
Malunion after osteotomy can result from several potential sources,
including malpositioning following a poor or inadequate preoperative
plan; error in technical execution of a good plan; or postoperative
loss of a good position obtained in the operating room secondary to
other problems such as delayed union, poor internal fixation, or
patient noncompliance.
adequate radiographic workup and skillful preoperative tracing or
computer templating. For many osteotomies, a complete plan includes
having multiple options or variations clearly spelled out. Other than
inappropriate patient selection, error in technical execution of a good
osteotomy plan is perhaps the most common complication described in the
literature, and it is also the complication most within the surgeon’s
control. Sufficient experience with these osteotomies is required to
avoid these errors.
surgical exposure, good radiographic control with both the image
intensifier and confirmatory plain films, and the skill and patience
for precision. With rigid internal fixation and good immediate
alignment and position, a loss of position is unlikely. However,
premature weight bearing or delayed union can predispose to such a
shift. Helping to prevent delayed union and fostering bony healing is
somewhat within the surgeon’s immediate control, as discussed next.
be minimized by following a few principles. Rigid internal fixation is
critically important. If there is any question of stability at the time
of surgery, ensure stability by reengineering the osteosynthesis. As
long as fixation and other factors are appropriate, however, even
point-contact apposition (as in a varus ITO) can be expected to heal,
although restricted weight bearing may be required for a longer period
of time.
gaps in the pelvis or upper femur. Exceptions include significantly
displaced Chiari osteotomies, markedly displaced periacetabular pubic,
ischial, or iliac cuts, and nonunions of the proximal femur or pelvis.
penetration, and cutout are all directly related to the problem of
achieving stability and union of the osteotomy and/or fracture site.
Documented recent breakage of fixation implants generally indicates
that the osteotomy has not healed. Whether implant breakage is a
significant clinical problem depends on the stability remaining in the
construct and the stage of healing. If the osteotomy or proximal
femoral fracture is unstable, then broken implants make revision
surgery more difficult and more urgent. Hardware penetration into the
hip joint following cutout of an implant from the femoral head also
calls for an expedited revision to prevent additional damage to the
articular cartilage of the hip.
joint that can be either an asset or a liability is the potential for
affecting leg length. This is most prominent with proximal femoral
osteotomy but can be seen with pelvic osteotomies as well. The Chiari
procedure tends to cause shortening, which can be significant depending
on the slope of the osteotomy and the degree of displacement. A PAO can
result in a slight lengthening, although this is typically minimal
(less than 1 cm). A valgus ITO has the potential for lengthening of
several centimeters. If the operative leg is short, this can be a
principal benefit and can contribute to the indications for the
surgery. This is commonly seen with malunions, femoral neck nonunions
with collapse, congenital varus deformity, and SCFE. However, if leg
lengths are equal or the operative leg is long, then full-wedge
resection combined with removal of an additional segment of bone from
the distal fragment may be necessary because of the inherent
lengthening effect of a valgus ITO.
around the hip accompanies varus ITO. For major angular corrections
(> 15° of varus), resection of any bone wedges essentially
guarantees a noticeable amount of limb shortening. The patient needs to
understand this aspect of the surgery before the fact. With minimal or
no wedge resection and rigid fixation, maximum length can be preserved
during a varus ITO. Accuracy in preoperative planning combined with
careful intraoperative verification can help to ensure that the
expected leg lengths are obtained following any hip osteotomy.
lasting and the source of patient dissatisfaction following surgery. A
chronic limp not caused by pain is usually secondary to abductor
dysfunction. Procedures that damage or shorten the abductors or place
them at a mechanical disadvantage are most likely to cause a limp or a
Trendelenberg lurch. These include pelvic osteotomies that involve
release of the abductor origin laterally for exposure, Chiari
osteotomies, and varus osteotomies of the proximal femur. This type of
limp can take a year or more to improve or can be permanent. The
patient should, of course, be educated about this before surgery. In
some cases, a preoperative Trendelenberg limp can be improved after
osteotomy. This is usually seen when greater trochanter advancement is
performed or when a valgus ITO improves suboptimal abductor mechanics.
biomechanical axis through the knee. This can lead to secondary knee
pain and arthritis after a hip procedure.
In
frontal plane angular osteotomies, a valgus ITO places a valgus stress
on the knee, whereas a varus ITO generates varus forces and increases
medial joint loading. Lateral displacement of the shaft (distal)
fragment at the time of a valgus ITO can correct the mechanical axis
and eliminate abnormal forces at the knee secondary to the hip surgery.
The converse is true for varus ITO. In the rare patient receiving a
valgus ITO who also has medial knee arthritis, some lateral mechanical
axis shift may be desirable, and therefore less lateral displacement of
the osteotomy is indicated. In addition to frontal plane displacement,
anterior shaft displacement generally accompanies a flexion ITO, and
posterior displacement accompanies an extension ITO. The significance
of this displacement that is not seen on the AP radiograph should not
be underestimated when planning for a subsequent THR. Despite the
importance of displacement, as discussed, if a choice must be made
between obtaining the full displacement desired and obtaining a
proximal femur compatible with future total hip stem placement, the
displacement that is compatible with the future THR is preferred.
Of the pelvic osteotomies discussed, the Chiari procedure creates the
most difficulty for acetabular component placement, which is more fully
discussed in the Chiari osteotomy section of this chapter. The
periacetabular rotational osteotomies generally do not alter the
difficulty of cup placement. While better superior and lateral coverage
is obtained in an otherwise dysplastic hip, this is at the expense of
posterior and inferior coverage, leaving a partially deficient bed of
host bone for acetabular fixation. The most significant postosteotomy
arthroplasty challenges come on the femoral side when a previous ITO
with excessive displacement or subtrochanteric osteotomy has been done.
Intraoperative perforation of the femoral shaft or fracture, component
malalignment, and compromised fixation, whether cemented or uncemented,
may potentially lead to early failure of the femoral component (12,1,42).
necessary prior to proceeding with a THR, although this is usually done
at the time of the hip replacement. The presence of an implant such as
a blade plate from a previous osteotomy can be a source of problems for
a subsequent THR. Simultaneous implant removal and THR surgery results
in slightly increased operative time and possibly an increased
infection rate for the THR. The presence of open screw holes can
diminish the effectiveness of cement pressurization when a cemented
femoral component is used. For these reasons, we prefer early hardware
removal of fixation devices years prior to any implant arthroplasty, as
advocated by Ferguson et al. (45) at the Mayo Clinic.
The femoral, obturator, and sciatic nerves are generally well
protected, and no major cutaneous nerves are at risk with the straight
lateral incision. Nerve injury is more common after pelvic osteotomy.
The lateral femoral cutaneous nerve is at risk with any anterior
exposure of the pelvis. Despite every attempt to preserve this nerve,
anterolateral thigh numbness and paresthesias are quite common. The
typical injury to the nerve is a traction neuropraxia. The majority of
these palsies completely resolve with time, but some patients do have a
permanent sensory deficit. The major nerves that pass the hip joint are
all at risk with a pelvic osteotomy, particularly in a rotational
periacetabular procedure. The femoral nerve is close to the anterior
and medial retractors. The obturator nerve is at risk during the pubic
osteotomy. Use of a Gigli saw for the pubic cut may reduce the chance
for damage to the obturator neurovascular bundle. The sciatic nerve is
in jeopardy at the sciatic notch, whether from laceration from an
osteotome or bone spike, or from traction. With complete pelvic
osteotomies into the sciatic notch area, such as a Chiari, use of a
Gigli saw for this cut, as well as specialized or malleable retractors,
may minimize the risk of sciatic injury. Avoid paralytic anesthetic
agents, as they preclude recognition of any inadvertent stimulation of
a motor nerve.
apply to the femoral head or to the acetabulum, depending on the
location of the osteotomy. Osteotomies closer to the joint surface are
more likely to disrupt the critical blood supply to the mobilized joint
fragment. Therefore, as a general rule, the smaller and more isolated
the fragment, the greater the risk of AVN. This is one of several
reasons that we (and others) prefer the Bernese PAO over the spherical
rotational or dial acetabular osteotomies. It also supports our
preference for intertrochanteric over femoral neck or cuneiform
proximal femoral osteotomies. Minimize the soft-tissue stripping during
surgery to reduce the chances of AVN. The remaining blood supply of
either a rotated acetabular fragment or femoral head is via the
ligamentum teres capitis and the capsule, especially its posterior
anastomoses. When an incision into the capsule is necessary, use a
minimum of dissection to respect the importance of this circulation. In
some cases, such as in a SCFE (with or without reduction) or a femoral
neck fracture nonunion, the risk of AVN is significant. In patients who
have surgery within a year of injury, it may be difficult to determine
the potential role that an osteotomy plays in increasing this risk.
related to creation of a thin or short fragment are fracture and
intra-articular hardware. The dial osteotomy is performed closer to the
articular surface than a triple pelvic or Bernese periacetabular
procedure. Difficulties in stabilizing the fragment or in avoiding
intra-articular penetration are more challenging with the dial
osteotomy or similar procedures. On the femoral side, cuneiform and
femoral
neck osteotomies and cases of femoral neck nonunion provide less
proximal bone for safe placement of fixation hardware. Quality
fluoroscopic images in multiple planes with some use of live
fluoroscopic visualization may be necessary to document safe screw
placement. Trauma surgeons have described the use of a sterile
esophageal stethoscope along the quadrilateral plate (or, in fact,
against any nearby bone) as a method of audibly detecting the
unmistakable scraping sound of an intra-articular screw in
radiographically uncertain cases (4).
We have not found this to be necessary. Radiographically uncertain
screws generally do not have to be tolerated in osteotomy surgery, for
which screw placement options are usually more varied than they are in
complex pelvic fracture osteosynthesis.
postoperative fracture is the lateral bone bridge of the proximal
fragment of an ITO. A 15–20 mm bridge of intact bone is needed between
the seating chisel entry site and the osteotomy for safety. The
integrity of this bone must be assessed during surgery, especially in
revision or femoral nonunion cases. Change from a higher-angle blade
plate to a lower-angle blade plate may be required to ensure stable
osteosynthesis. This should be ascertained in preoperative planning,
rarely during surgery itself.
or increased stiffness following osteotomy. Heterotopic ossification
can appear after an otherwise uncomplicated osteotomy, more commonly on
the pelvic side. It is usually an incidental finding but can cause
symptomatic stiffness. Increased stiffness independent of pain in the
hip is more common after surgery in patients with more advanced
arthritis. Persistent or increased pain is also more common in patients
with worse preoperative degenerative changes. Strict adherence to
described surgical indications will minimize the number of such
clinical failures. The guidelines to patient selection previously
discussed are not rigid but serve as guides by which the patient and
surgeon can come to understand the potential risks and benefits of a
given procedure. Once the surgeon understands the patient and the
patient is educated about the treatment options, together they can
decide whether an osteotomy will best serve the patient’s needs.
this field has been the adoption of the Bernese PAO as the procedure of
choice for the painful hip with prearthritic or early arthritic changes
secondary to underlying dysplasia in adults.
the hip as an interest area within the field of orthopaedic surgery
should include osteotomies of the pelvis and upper femur as well as
arthrodesis when indicated, in addition to primary and revision
arthroplasty.
careful preparation and detailed, virtually step-by-step planning prior
to any osteotomy surgery. Particularly with regard to the PAO, when the
best results are achieved prior to irreversible radiographic
deterioration, complications could leave a patient much worse off than
before the surgery, and at a young age. Careful study, multiple
cadaveric dissections, and visits to centers with extensive experience
are valuable building blocks. This surgery should not be undertaken
casually or infrequently.
organs, capable of extensive remodeling, filling-in of gaps and
defects, and healing of arthritis. The art of patient selection is as
important as the ability to perform the surgery. Obesity, lack of
motivation, and unrealistic expectations are as important
contraindications to surgery as an adverse radiographic variable. On
the other hand, the pleasure of observing periarticular joint space
remodeling, relief of pain, and restoration of function after a
successful osteotomy is one of the most rewarding aspects of
reconstructive surgery in adults.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
MM, Sugano N, Matsui M, et al. Dome Osteotomy of the Pelvis for
Osteoarthritis Secondary to Hip Dysplasia. An over Five-Year Follow-up
Study. J Bone Joint Surg Br 1993;75:222.
J, Bombelli R, Benedini A, Santore RF. Slipped Capital Femoral
Epiphysis: A Functional Comparison of in Situ Pinning and Primary
Osteotomy. Techniques in Orthopedics 1989;4:64.
MK, Evans DC. The Pelvic Osteotomy of Chiari: An Anatomical Study of
the Hazards and Misleading Radiographic Appearances. J Bone Joint Surg Br 1976;58:164.
R, Gerundini M, Aronson J. The Biomechanical Basis for Osteotomy in the
Treatment of Osteoarthritis of the Hip: Results in Younger Patients. Hip 1984:18.
DW, Mickelson MR, Ponseti IV. Slipped Capital Femoral Epiphysis.
Long-term Follow-up of One Hundred and Twenty-one Patients. J Bone Joint Surg Am 1981;63:85.
JA, Lohmander S. Current Concepts Review. Operative Treatment of
Osteoarthrosis. Current Practice and Future Development. J Bone Joint Surg Am 1994;76:1405.
HP, Reineck FT, Wixson RL, et al. Total Hip Relacement in Patients
Younger Than Thirty Years Old: A Five-year Follow-up Study. J Bone Joint Surg Am 1981;63:1426.
C, Hofmann S, Neuhold A, et al. Lesions of the Acetabular Labrum:
Accuracy of MR Imaging and MR Arthrography in Detection and Staging. Radiology 1996;200:225.
Waal Malefijt MC, Hoogland T, Nielsen HKL. Chiari Osteotomy in the
Treatment of Congenital Dislocation and Subluxation of the Hip. J Bone Joint Surg Am 1982;64:996.
SR, Sadiq S, New AMR, et al. Proximal Femoral Osteotomy as the Primary
Operation for Young Adults Who Have Osteoarthrosis of the Hip. J Bone Joint Surg Am 1998;80:1428.
R, ed. Alternatives to THA: Pelvic and Femoral Osteotomies in the
Treatment of Early Osteoarthrosis of the Hip in the Young Adult. Semin Arthroplasty 1998;8:1.
R, Klaue K, Vinh TS, et al. A New Periacetabular Osteotomy for the
Treatment of Hip Dysplasias, Technique and Preliminary Results. Clin Orthop 1988;232:26.
DA, Feighan JE, Smith AD, et al. Subclinical Slipped Capital Femoral
Epiphysis. Relationship to Osteoarthrosis of the Hip. J Bone Joint Surg Am 1997;79:1489.
E, Inao S, Okamoto T, Ando M. Valgus-extension Osteotomy for advanced
Osteoarthritis in Dysplastic Hips. Results at 12 to 18 Years. J Bone Joint Surg Br 1997;79:609.
DK, Wroblewski BM. Long-term Results of Low-friction Arthroplasty for
Degenerative Joint Disease in Patients 30 Years of Age or Younger Clin Orthop 1986;211:43.
O, Casillas M, Ganz R. Indications for Intertrochanteric Osteotomy
after Periacetabular Osteotomy for Adult Hip Dysplasia. Clin Orthop 1998;347:19.
A. Huftpfannenverlagerung jurch Doppelte Beckenotsteotomie zur
Behandlung der Huftgelenksdysplasie und Subluxation bei Jugendlichen
und Erwachsenen. Z Orthop 1966;101:559.
SC, Hwang YF, Liu HC, et al. Triple Innominate Osteotomy and Rotational
Acetabular Osteotomy in the Treatment of Congenital Hip Dysplasia. J Formos Med Assoc 1997;96:91.
T, Hasegawa Y, Kataoka Y, et al. Long-term Results of Intertrochanteric
Varus Osteotomy for Arthrosis of the Dysplastic Hip (Over 10 Years’
Follow-up). Arch Orthop Trauma Surg 1995;114:243.
T, Hasegawa Y, Kawamoto K, et al. Twenty Years’ Follow-up of
Intertrochanteric Osteotomy for Treatment of the Dysplastic Hip. Clin Orthop 1996;331:245.
W, Windhager R, Kutschera HP, Engel A. Chiari Pelvic Osteotomy for
Osteoarthritis Secondary to Hip Dysplasia. Indications and Long-term
Results. J Bone Joint Surg Br 1991;73:229.
M, DeSeze S. Le Faux Profil du Bassin. Nouvell Incidence Radiographique
pour l’Etude de la Hanche. Son Utilite dans les Dysplasies et les
Differentes Coxopathies. Rev Rhum Mal Osteoartic 1961;28:643.
G, Fusco U, Avai A, Bombelli R. Osteonecrosis of the Hip Treated by
Intertrochanteric Osteotomy. A Four- to 15-year Follow-up. J Bone Joint Surg Br 1988;70:761.
GL, Gerundini M, Fusco U, et al. Valgus-Extension Osteotomy for
Osteoarthrosis of the Hip: Indications and Long-term Results. J Bone Joint Surg Br 1990;72:653.
RE, Harris WH. Medial Displacement Intertrochanteric Osteotomy in the
Treatment of Osteoarthritis of the Hip. A Long-term Follow-up Study. J Bone Joint Surg Am 1984;66:878.
MB, Kaelin AJ, Schluntz K, et al. Spherical Acetabular Osteotomy for
Treatment of Acetabular Dysplasia in Adolescents and Young Adults. J Pediat Orthop B 1994;3:47.
MA, Maar DC, Krackow KA, et al. Total Hip Replacement without Cement
for Non-inflammatory Osteoarthritis in Patients Who Are Less Than
Forty-five Years Old. J Bone Joint Surg Am 1993;75:740.
E, Feinstein R. Results of Intertrochanteric Osteotomy in the Treatment
of Osteoarthritis of the Hip. In: Schatzker J, ed. The Intertrochanteric Osteotomy. New York: Springer-Verlag, 1984.
SB, Ganz R, Müller M. The Prognosis of Untreated Acetabular Dysplasia
of the Hip. A Study of Radiographic Factors That Predict the Outcome. J Bone Joint Surg Am 1995;77:985.
SB, Kijewski PK, Millis MB, et al. The Planning of Orthopaedic
Reconstructive Surgery Using Computer-aided Simulation and Design. Comput Radiol 1988;12:33.
M, Iwata H, Sugiura S, et al. The Evaluation of Intertrochanteric
Osteotomy in Relation to Osteotomized Angle and Leg-Length Discrepancy
for Osteoarthritis of the Hip. Clin Orthop 1980;152:277.
S, Ninomiya S, Takatori Y, et al. Long-term Outcome of Rotational
Acetabular Osteotomy: 145 Hips Followed for 10–23 Years. Acta Orthop Scand 1998;69:259.
T, Yamamura M, Nakamitu S, Suzuki K. The Displacement of the Femoral
Head by Rotational Acetabular Osteotomy: A Radiographic Study of 97
Subluxated Hips. Acta Orthop Scand 1992;63:33.
T, Saito S, Ohzono K, et al. Chiari Pelvic Osteotomy for
Osteoarthritis: The Influence of the Torn and Detached Acetabular
Labrum. J Bone Joint Surg Br 1990;72:765.
G, Hansson LI, Sandstrom S. Slipped Capital Femoral Epiphysis in
Southern Sweden. Long-term Result with No Treatment or Symptomatic
Primary Treatment. Clin Orthop 1984;191:95.
R, Wilson MG, Poss R. Isolated Proximal Femoral Osteotomy for Treatment
of Residua of Congenital Dysplasia or idiopathic Osteoarthritis of the
Hip. Five to Ten-year Results. J Bone Joint Surg Am 1996;78:1462.
CS, Atkinson RE, Salvati EA, et al. Conventional Hip Arthroplasty for
Degenerative Joint Disease in Patients between the Ages of Forty and
Sixty Years. J Bone Joint Surg Am 1984;66:745.
RF, Bombelli R. Long-term Follow-up of the Bombelli Experience with
Osteotomy for Osteoarthritis: Results at 11 Years. In: The Hip. Proceedings of 11th Meeting of the Hip Society. St. Louis, MO: Mosby, 1983:106.
H, Igarashi H, Taneda H, Azuma H. Rotational Acetabular Osteotomy for
Severe Dysplasia of the Hip with a False Acetabulum. J Bone Joint Surg Am 1996;78:871.
K, Boll KL, Kofod S, et al. Total Hip Replacement after
Medial-displacement Osteotomy of the Proximal Part of the Femur. J Bone Joint Surg Am 1989;71:692.
DH, Porter ML. The Long-term Results of Charnley Low-friction
Arthroplasty in Young Patients Who Have Congenital Dislocation,
Degenerative Osteoarthrosis or Rheumatoid Arthritis. J Bone Joint Surg Am 1997;79:1599.
HH. Triple Osteotomy of the Innominate Bone. A Procedure to Accomplish
Coverage of the Dislocated or Subluxated Femoral Head in the Older
Patient. Clin Orthop 1977;122:116.
SD, Cordell LD, Harris WH, et al. Unrecognized Childhood Disease: A
Major Cause of Idiopathic Osteoarthritis of the Hip. Hip 1975;212.
PM, MacKenzie JR, Callaghan JJ, Johnston RC. Total Hip Arthroplasty
with Cement in Patients Who Are Less Than Fifty Years Old: A Sixteen-
to Twenty-two-year Follow-up Study. J Bone Joint Surg Am 1994;76:863.
Y, Ninomiya S, Nakamura S, et al. Long-term Follow-up Results of
Rotational Acetabular Osteotomy in Painful Dysplastic Hips: Efficacy in
Delaying the Onset of Osteoarthritis. Am J Orthop 1996;25:222.
RT, Ekkernkamp A, Ganz R, et al. Periacetabular and Intertrochanteric
Osteotomy for the Treatment of Osteoarthrosis in Dysplastic Hips. J Bone Joint Surg Am 1995;77:73.
R, Schai PA, Exner GU. SCFE: A Long-term Follow-up Study after Open
Reduction of the Femoral Head Combined with Subcapital Wedge Resection.
J Pediatr Orthop 1998;7B:43.
H, Holder J. Treatment of Osteoarthritis of the Hip by Corrective
Osteotomy of the Greater Trochanter. In: Schatzker J, ed. The Intertrochanteric Osteotomy. New York: Springer-Verlag, 1984.
G. Studies on Dysplastic Acetabula and Congenital Subluxation of the
Hip Joint: With Special Reference to the Complication of
Osteoarthritis. Acta Chir Scand Suppl 1939;83:1.
R, Pongracz N, Schonecker W, Kotz R. Chiari Osteotomy for Congenital
Dislocation and Subluxation of the Hip: Results after 20 to 34 Years
Follow-up. J Bone Joint Surg Br 1991;73:890.
H, Sano S, Nagata Y, et al. Modified Rotational Acetabular Osteotomy
(RAO) for Advanced Osteoarthritis of the Hip Joint in the Middle-aged
Person. First Report. Arch Orthop Trauma Surg 1990;109:121.