FAILED SHOULDER ARTHROPLASTY: REVISION AND ARTHRODESIS
– JOINT RECONSTRUCTION, ARTHRITIS, AND ARTHROPLASTY > Upper
Extremity > CHAPTER 103 – FAILED SHOULDER ARTHROPLASTY: REVISION AND
ARTHRODESIS
However, when complications occur after shoulder arthroplasty, the
resulting functional deficits can be profound, significantly limiting
activities of daily living. Proper indications, surgical technique, and
good postoperative rehabilitation of primary shoulder arthroplasty can
avoid most complications; however, some are unavoidable. It is
important to be aware of potential complications and to be prepared to
address them as they arise.
factors that may be contributing to the failure, keeping in mind that
the rotator cuff, capsule, and ligaments, although invisible on
radiographs, are often more important than the position of the
components. When contemplating revision surgery, you must have a
reasonable idea of what you expect to accomplish at reoperation;
exploratory procedures are less likely to succeed than is the
correction of an identifiable problem.
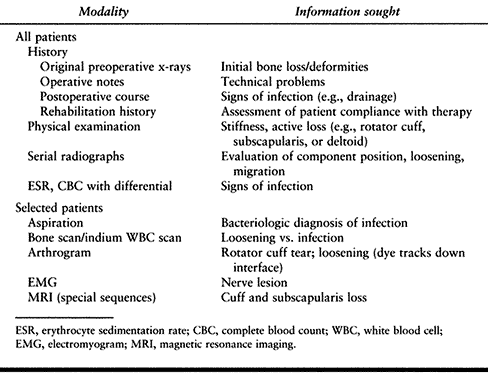
careful and methodical evaluation is essential to planning appropriate
treatment (Table 103.1). Review all previous
records, including the original preoperative radiographs, prior
operative reports (including information about the make and size of the
previous implants), and follow-up treatment and therapy records. Ask
the patient specifically about recent dental work as well as about any
episodes of fever, chills, redness, or drainage that might indicate
infection. The therapy history is important both to assess whether the
exercise regimen was appropriate and to gain a sense of the patient’s
reliability and compliance with aftercare: a revision to release scar
will be of little help if the patient will not perform the
postoperative range-of-motion exercises. Review all available
postoperative radiographs, not just the most recent ones. Although
different beam angles and technique can make comparison difficult, it
is often possible to see increasing periprosthetic lucent lines or
frank component migration, indicative of loosening.
 |
|
Table 103.1. Evaluation of Failed Shoulder Arthroplasty
|
range of motion. Loss of passive motion may be an indication either
that contractures were not adequately released at the first operation
or that the postoperative exercise regimen failed to maintain motion.
Increased external rotation combined with an active internal rotation
lag (loss of terminal active internal rotation) suggests a loss of the
subscapularis insertion. Loss of terminal active internal rotation is
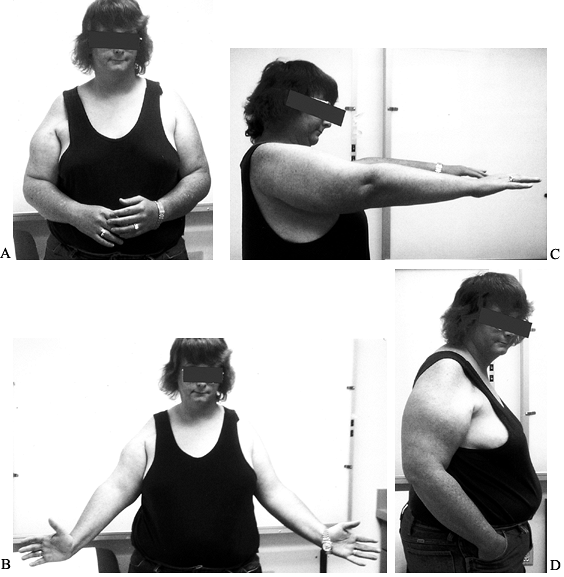
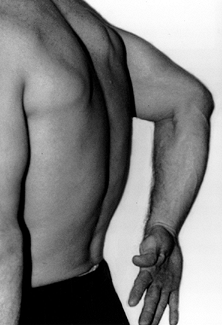
revealed by the lift-off test or the stomach press sign. In the
lift-off test (Fig. 103.1), the patient puts
the hand of her affected arm on her lower lumbar spine and is asked to
lift the dorsum of her hand off her spine. The test is positive if she
is unable to lift her hand off her back, indicating compromise of the
subscapularis tendon. Weakness of elevation and external rotation may
suggest loss of the rotator cuff, but always consider a nerve or
brachial plexus injury. Assessing elbow and hand motor function can be
helpful in this differential. Investigate other sources of pain that
may be referred to the shoulder, such as a cervical radiculopathy.
 |
|
Figure 103.1.
The lift-off test can be used to evaluate the integrity of the subscapularis. From Gerber C, Farron A, Hersche O. Isolated Ruptures of the Subscapularis. J Bone Joint Surg Am 1997;78:1015, with permission. |
both the glenoid and the humeral components should be shown in
profile), an outlet lateral view, and an axillary view.
Fluoroscopically positioned films can be used to achieve precise
orientation (39).
The humerus is seen in profile when the arm is externally rotated by an
amount equal to the retroversion in which the humeral component was
originally placed. The glenoid is generally best seen when a true AP of
the scapula is taken: This gives the best imaging of the cement mantle
and of lucent lines.
and a complete blood count with differential on all cases of failed
arthroplasties. If these are normal, and there is no other indication
of infection, then we do no further evaluation. If there is some
suspicion, then joint aspiration followed by a bone and indium-labeled
white blood count scans are performed. In some situations, a biopsy for
culture and pathologic examination may be performed.
computed tomographic (CT) scans were unable to provide adequate images
in the presence of a large metal implant, current techniques allow some
information to be obtained. MRI scans with special sequences can show
subscapularis avulsions and can demonstrate massive cuff deficiency and
cuff muscle atrophy. Small tears at the supraspinatus insertion,
however, are often obscured by implant artifact. CT arthrography with
the use of digital subtraction software can provide useful information
in cases of suspected glenoid loosening, and it allows an accurate
evaluation of glenoid bone stock, especially in patients who have had a
hemiarthroplasty.
the original replacement (improper indications for surgery or poor
patient education), or they may have occurred during the surgery
(technical errors), immediately after (e.g., improper aftercare), or
years after (e.g., trauma or late loosening). Nevertheless, it is most
useful to classify prosthetic failure by the mode of failure at the
time the patient presents for treatment. The most common types of
failure after shoulder arthroplasty are listed in Table 103.2
 |
|
Table 103.2. Causes of Failure in Total Shoulder Arthroplasty
|
joint requires accurate alignment of the prosthetic joint surfaces and
careful balancing of the surrounding soft tissues. Instability
following arthroplasty can result from failure to achieve one or both
of these objectives and is best considered according to the direction
of instability (Table 103.3).
 |
|
Table 103.3. Instability Patterns and Related Factors
|
following total shoulder replacement have been reported to be as high
as 18.2% (18). There are some conflicting reports
about the most common instability pattern seen after arthroplasty (5,18,72,73).
A collective review of clinical series published before 1988 found that
anterior instability occurred most frequently, followed by posterior
and inferior instability (18). In contrast, Boyd et al. (5)
reported a series of 127 shoulders and found that 23% of shoulders
demonstrated superior migration. When approaching prosthetic
instability, categorization according to the direction of subluxation
is useful for developing a rational approach to treatment.
Determine whether the instability is acute, recurrent, or chronic. A
dislocation in the early postoperative period may be caused by
unprotected movement or trauma, not by a structural problem; closed
reduction and motion within a protected range, avoiding extension and
external rotation, may lead to a stable arthroplasty. Once chronic or
recurrent instability is present, look for etiologies such as
subscapularis rupture and component malposition.
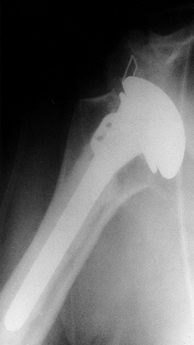 |
|
Figure 103.2.
Postoperative radiograph of a total shoulder replacement performed for osteoarthritis, demonstrating anterior dislocation. Closed reduction with protected range of motion provided a satisfactory outcome with no recurrence of instability. |
replacement is more common than was previously recognized. Etiologies
include inadequate surgical repair, use of a sharp-edged or an
oversized humeral head, and an overly aggressive physical therapy
program. Frank dislocations do not always result, but there is usually
some element of static anterior subluxation, best appreciated on the
axillary view.
Careful evaluation of passive range of motion can reveal increased
external rotation compared to that of the contralateral extremity. Take
scapular AP and axillary radiographic views to assess prosthetic
stability and version. In the case of chronic subscapularis rupture,
static anterior subluxation may be observed on the axillary view. An
MRI with digital subtraction software, or an arthrogram, is useful for
assessing the integrity of the subscapularis. A CT arthrogram with
digital subtraction can determine component version and stability.
seven cases of subscapularis rupture following arthroplasty. All
patients underwent repair, with rerupture occurring in three cases.
These patients underwent reoperation using an Achilles tendon allograft
as a static anterior restraint. Wirth and Rockwood (73)
described pectoralis major transfer for subscapularis deficiency. This
technique transfers the pectoralis major tendon over the conjoint
tendon to the greater tuberosity. Recently, transfer of the pectoralis
under the conjoint tendon has been described as a salvage procedure for
chronic loss of the subscapularis (H. Resch, personal communication,
1998).
may lead to increased anterior humeral head translation but rarely
causes frank dislocation unless the mal- alignment is severe. However,
if inadequate retroversion
in
combination with soft-tissue deficiencies is found, revision of the
humeral component, and reconstruction of the anterior soft-tissue
deficiencies must be considered (18,72,73). Wirth and Rockwood reported on 18 anterior instabilities in 1994 (72).
Four shoulders were treated with closed reduction and immobilization
and 14 underwent operative treatment. Clinical results were guarded
regardless of treatment (72).
arthroplasty is often seen in association with an attenuated or
dysfunctional rotator cuff. When the dynamic stabilizing effect of the
rotator cuff is lost, the humeral head migrates superiorly because of
the unopposed pull of the deltoid. Component malposition, including
humeral lengthening and an inferiorly placed glenoid component, is a
less common cause of superior instability. Subtle superior subluxation
of the humeral component is not always painful (5,73). However, eccentric superior loading of the glenoid component has been linked to glenoid loosening (25).
greater concern. This can be especially severe if both cuff deficiency
and loss of the passive stabilizing effect of the coracoacromial arch
(resulting from surgical decompression) are present. Options for this
difficult problem include attempts to reconstruct the cuff with direct
repair or tendon transfers; revision of the humeral component to place
it in increased retroversion, and/or to replace it with a wider head
that is less able to escape between the remaining acromion and the
coracoid; and coracoacro- mial arch reconstruction with fascia or bone
grafts. However, little information exists to support the efficacy of
any of these approaches, and early results have been disappointing (21).
arthroplasty is an infrequent complication and is most commonly seen in
patients undergoing arthroplasty for acute fracture of the proximal
humerus. In fracture cases, normal bony landmarks have been
obliterated, making intraoperative determination of appropriate humeral
length extremely difficult. Neer (53)
emphasized the importance of maintaining proper tension on the
myofascial sleeve of the shoulder. Preoperative templating using the
contralateral extremity can be a useful method to determine prosthetic
height. Intraoperatively, prosthetic height should allow for 50%
inferior translation. If greater translation occurs, more humeral
length is required. This may require the addition of bone graft to
augment humeral shaft length. Inferior subluxation may also result from
nerve injury with deltoid paralysis.
sling support to prevent permanent capsular stretching. When an
axillary nerve injury is present, electrical stimulation may help to
prevent atrophy of the deltoid. Inferior subluxation resulting from
insufficient component height is difficult to correct with revision
surgery, but it may be improved if the myofascial sleeve can be
properly retensioned.
replacement usually presents as intermittent subluxation rather than as
recurrent or chronic dislocation, and it is often related to technical
problems at the initial procedure. Component malposition most commonly
occurs on the glenoid side because erosion of the posterior glenoid is
a common occurrence in osteoarthritis of the shoulder. When not
recognized and corrected at the time of surgery, increased glenoid
retroversion can result. Furthermore, this can be compounded by
posterior capsular redundancy secondary to longstanding posterior
humeral subluxation and inadequate release of anterior contractures,
which then force the head posteriorly.
soft-tissue healing is complete may allow nonoperative treatment to be
effective, especially when component malposition is not severe.
Exercises can be modified to avoid flexion and internal rotation, and
in severe cases a brace may be worn temporarily to keep the arm in
slight extension and slight external rotation between exercise
sessions. The patient may be told to perform activities with the palm
up.
subluxation is longstanding, you may need to consider revision surgery.
A malpositioned glenoid may require revision into a more appropriate
angle. Revision of a modular humeral stem is not usually required
unless it is also malpositioned. When there is no apparent glenoid
retroversion, or if the soft tissues are also imbalanced, posterior
capsulorrhaphy can be performed to eliminate redundancy. This may be
performed from an anterior approach if component revision is also being
performed. Place imbricating sutures in the posterior capsule. The
imbrication may be side to side, or, if glenoid revision is being
performed, suture anchors may be placed in the posterior glenoid and
the sutures placed through the posterior capsule before implanting the
new component. If no component malposition is present, a posterior
approach may be used for the capsulorraphy.
repair, two had no pain but were unable to raise their arms, and one
patient had intermittent pain but refused further surgical
intervention. Cofield (14)
reported five rotator cuff ruptures in his review of 77 unconstrained
Neer prostheses. One patient suffered severe pain, had limited range of
motion, and was considering further surgery. The remaining four
patients had no pain but had functional weakness.
replacement for osteoarthritis with an initially intact cuff may
present with pain and increasing weakness. Base the decision to repair
the cuff on the degree of symptoms and functional compromise. If the
humeral component was placed too proud, revise the humerus at the time
of cuff repair, lest the compression against the acromion and the
increased stress in the cuff tendon as it winds around the overly
superior head lead to a retear. In such a situation, the cuff is most
easily repaired while the prosthetic head is out.
-
In the absence of humeral malposition,
you may approach tears involving the supraspinatus and/or infraspinatus
tendons from a standard superior deltoid-splitting approach. -
Preserve the coracoacromial arch for stability, but shell out and remove any large acromial spurs.
-
If the coracoacromial ligament must be detached to remove a spur, then reattach it with transosseous sutures to the acromion.
-
Identify and mobilize the torn rotator
cuff. Fixation to the tuberosity presents a unique challenge. Bone
tunnels can sometimes be created for fixation despite the presence of a
cemented humeral component. When severe osteopenia is present, you may
achieve bone augmentation with commercially available plastic
reinforcement devices (Cuff Link, Innovasive, Marlborough, MA) or with
the use of small plates. Suture anchors can also facilitate repair. -
Repair the rotator cuff tissue with modified Mason-Allen stitches (28).
-
Meticulous repair of the deltoid incision is essential.
exercises postoperatively for 6 weeks, staying within the limits
determined at surgery. Follow with a graduated stretching program.
Delay resistive exercises until 3 months postoperatively (53).
This rate is lower than those seen in replacement of the hip and knee,
which range from 0% to 3%. This may be in part a result of the
excellent soft-tissue coverage and abundant vascularity present in the
shoulder. The hip and knee literature have identified rheumatoid
arthritis, male sex, skin ulcers, diabetes, postoperative urinary tract
infections, trauma, and revision arthroplasty as potential risk factors
for the development of sepsis (23,69).
Prosthetic replacement for shoulder trauma has higher rates of
infection than that for arthritis, with rates ranging from 3.1% to 4.8%
(13,18,72). This increase is likely because of the additional soft-tissue trauma, bone damage, and hematoma.
found no preoperative investigation to be a reliable indicator for
active sepsis in an arthroplasty. They found a 20% false-negative rate
for joint aspiration, a 40% false-negative rate for triple-phase bone
scans, and a 13% false-negative rate for intraoperative cultures.
Analysis of preoperative blood work showed that white blood cell counts
were usually within normal limits, and in 13% the sedimentation rate
was normal. (These problems occur in the hip and knee as well.) When
infection is suspected in a patient with shoulder arthroplasty, a
thorough evaluation is required. When in doubt, an open biopsy sent for
pathologic evaluation and culture is the most reliable assessment tool.
In contrast, treatment options for infected total hips and knees in-
clude irrigation and debridement, immediate exchange arthroplasty or
staged reimplantation, and resection arthroplasty. The application of
these principles to infected shoulder arthroplasties has been reported
recently (13).
treatment algorithm has yet to be defined. Given the poor functional
results for activities performed at or above shoulder level with
resection arthroplasty (15,52), reimplantation is the preferred treatment (Fig. 103.3).
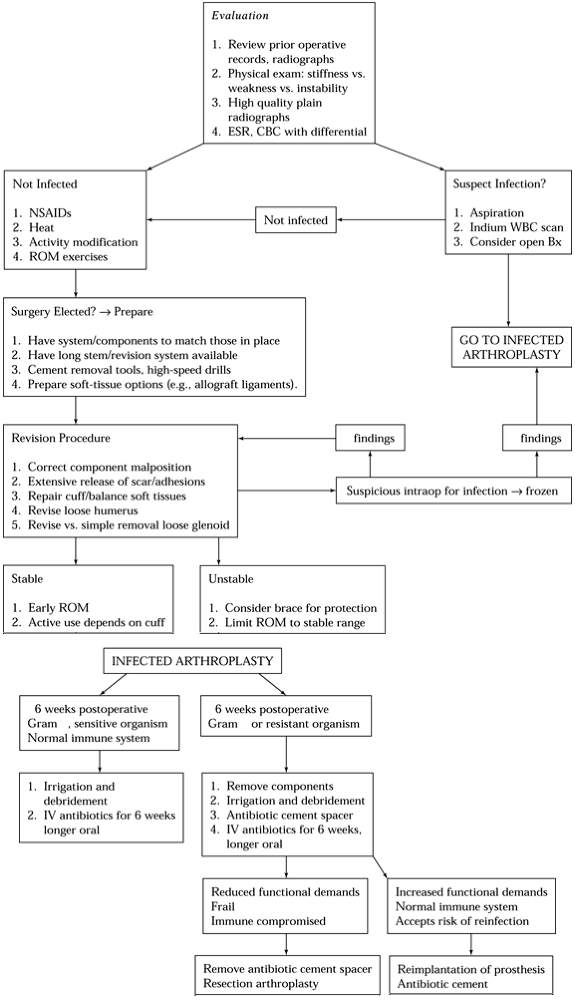 |
|
Figure 103.3.
Treatment algorithm for infection following shoulder arthroplasty. ESR, erythrocyte sedimentation rate; CBC, complete blood count; NSAIDs, nonsteroidal anti-inflammatory drugs; WBC, white blood cell count; ROM, range of motion. |
16 patients with septic shoulder arthroplasties, showed that
reimplantation of another prosthesis after treatment of the deep
infection is a reasonable option. These patients had no recurrence of
infection at an average follow-up of 47 months. Base decisions about
timing and whether to do another arthroplasty on the relative virulence
of the organism, the chronicity of the infection, and the overall
health of the patient. In rare cases of early infection (less than 6
weeks after surgery) by antibiotic-susceptible organisms, irrigation
and debridement without component removal may be considered by
inference from the hip literature; however, data for the shoulder are
limited.
for prosthetic extraction available, as well as flexible osteotomes,
ultrasonic cement removal systems, and specialized cement removal
instrumentation.
-
Use an extended deltopectoral incision. Excise sinus tracts as part of the skin incision.
-
Tissue planes are often obliterated by
the infection. Employ caution throughout the procedure to protect the
neurovascular structures. -
Release the upper 1 cm of the pectoralis major tendon if necessary to facilitate exposure.
-
Identify the subscapularis, and localize the axillary nerve along the inferior margin of the muscle.
-
Gently free the anterior surface of the
subscapularis tendon from the posterior surface of the conjoined
tendon. Take care to protect the musculocutaneous nerve. It lies an
average of 66 mm from the tip of the coracoid, with a range of 31 to 82
mm (24). -
Release the subscapularis tendon from the
proximal humerus. Identify and remove sutures used previously for
subscapularis closure, because they may act as a nidus of infection.
prosthesis and cement mantle need to be removed entirely. Take great
care in these cases, as the risk for humeral fracture is high. The
loose, infected humeral stem and cement mantle can often be removed as
one piece.
-
If the cement mantle is well fixed, break it with thin, flexible osteotomes and extract the prosthesis.
-
An ultrasonic cement removal system may
be helpful to free the humeral stem and assist in clearing the canal of
remaining cement. The use of intraoperative fluoroscopy and x-rays is
of great assistance. -
Take multiple cultures from the prosthesis and canal, and send the interface tissue for pathologic analysis.
-
Remove glenoid components: Cut the face
of the glenoid components from the underlying pegs or keel with an
osteotome. Then remove the peg or keel from the glenoid with curets and
osteotomes. In the presence of an osteolytic glenoid, removal of the
implant is best performed with the use of a high-speed burr to minimize
the risk of fracture. -
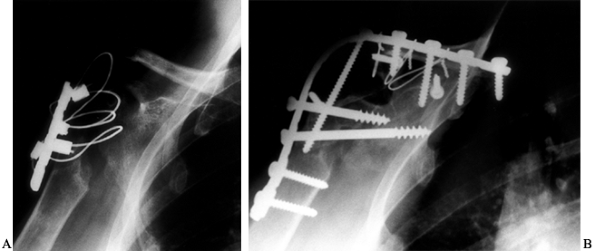
Place an antibiotic-impregnated polymethylmethacrylate cement hemiarthroplasty spacer in the humeral canal (Fig. 103.4). Palacos (Smith & Nephew Richards, Memphis, TN) is used because of its superior elution characteristics (20,34).
The spacer provides local antibiotic delivery, eliminates dead space,
and prevents contracture of the soft-tissue envelope about the shoulder.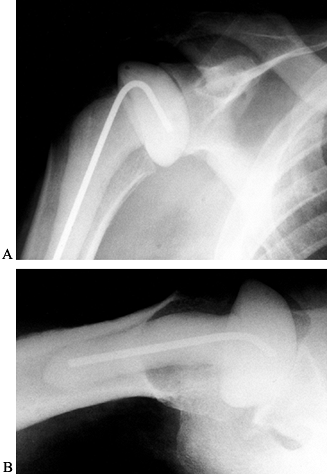 Figure 103.4. Anteroposterior (A) and lateral (B) radiographs of an antibiotic spacer placed after removal of an infected prosthesis.
Figure 103.4. Anteroposterior (A) and lateral (B) radiographs of an antibiotic spacer placed after removal of an infected prosthesis. -
Repair the subscapularis with
nonabsorbable monofilament sutures, and close the deltopectoral
interval over the suction drains. Leave drains in place for 48 hours.
the humeral stem inserter to the fixed prosthesis. The use of a
slap-hammer attachment allows the extraction force to be transmitted
along the long axis of the humeral stem. This substantially decreases
the force required to remove the prosthesis.
-
Place the patient on appropriate intravenous antibiotics.
course of antibiotics, usually at least 6 weeks. When the patient’s
functional demands are modest, when the organism is virulent or
resistant to antibiotics, or when immunologic compromise is present,
consider simple removal of the cement spacer and a resection
arthroplasty.
of arthritic shoulder conditions, and the increased functional demands
of patients with shoulder implants have led to concern over implant
loosening. Aseptic loosening
in
hip and knee prostheses is well documented and is thought to be induced
by wear particles that stimulate giant cell–mediated bone resorption (31,35,36,38,57,67).
A recent study characterized the particulate debris generated in the
total shoulder arthroplasty when radiographic evidence of osteolysis
was present. The particles were larger and more varied in shape than
those in total hip arthroplasties that required revision (70). No information is currently available regarding the immune response generated by particles of this size.
few revisions for symptomatic component loosening, particularly on the
humeral side. Indeed, aseptic loosening of cemented humeral components
is extremely rare. In their 1982 series, Neer et al. (56)
reported only two patients with such loosening among 94 total shoulders
with radiographic evidence of humeral loosening. Although both were
symptomatic, neither underwent revision arthroplasty. Information
regarding press-fit humeral stems is more varied, but the rate of
loosening would appear to be, if anything, higher than that of cemented
stems. Cofield (16) reported that less than 1%
of cemented humeral stems demonstrated radiographic evidence of
loosening, compared to 12% of uncemented stems. Several stems with
textured surfaces have been introduced to facilitate bone adherence to
the prosthesis, but long-term follow-up is lacking (16,22,47).
A new problem introduced by these designs is the difficulty of removing
a malaligned or infected ingrowth stem that is not loose.
the glenoid component. Lucent lines about the glenoid component have
been reported in 30% to 83% of cases (18,53),
with progression occurring in 12% to 16% of cases. The clinical
significance of these radiolucent zones is unclear. Several authors
have attributed glenoid lucencies to technical errors occurring at the
time of glenoid placement (7,12,63,64). Neer (53)
believed that radiolucencies about a glenoid component could be caused
by a number of factors, including inconsistent radiographic technique,
variable density and strength of glenoid bone stock, stress shielding
caused by the glenoid component, and disuse osteoporosis. Both Neer (53) and Brems (7)
reported higher incidences of radiolucent lines in cases performed
earlier in their series, and that it might be related to inexperience
with the technique in earlier cases. Clear radiographic evidence of
humeral loosening is pres- ent when a completely circumferential
radiolucent line of greater than 1 mm width is seen to progress at the
bone–cement/prosthesis interface, or when the prosthesis changes
position on serial plain radiographs.
In a series of 70 total shoulder replacements followed for 5–11 years,
complete lucent lines of 1.5 mm or more were found in one third of the
glenoid components, but only three components required revision (32).
However, unlike the situation with a lower extremity, the use of one
arm may be decreased by compensating with the other arm; thus, the rate
of revision may underestimate the true rate of component loosening.
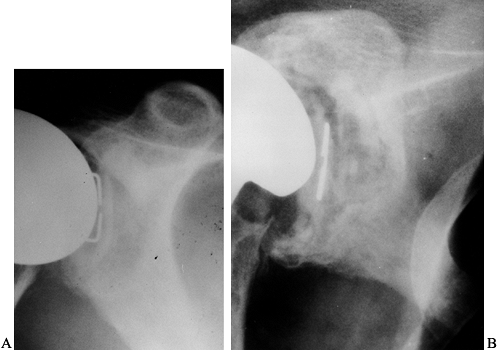 |
|
Figure 103.5. A: One-year follow-up radiograph of a total shoulder replacement performed for osteoarthritis. B: At the 15-year follow-up, progressive lucency is seen around the glenoid.
|
preoperative planning. Special instrumentation is often required,
including high-speed motorized burrs, thin flexible osteotomes, and
ultrasonic cement removal systems. Have a full complement of prostheses
available, including long and short stems. Use prior operative notes to
identify the size and make of the failed implant. Have full
instrumentation for both insertion and removal available for the
system, as well as multiple humeral head sizes. Even if you prefer a
different system (which may be employed if all prior components are
removed), situations may arise in
which
the prior system is useful (e.g., if the stem is well fixed but the
modular head needs to be resized). Careful preparation will give you
greater flexibility during the operation. Hold preoperative antibiotics
until intraoperative cultures have been obtained.
-
In making a long deltopectoral approach,
incorporate the prior incision when possible. The coracoid serves as an
important landmark. -
Open the deltopectoral interval, and release the upper 1 cm of the pectoralis tendon to facilitate exposure.
-
Identify the underlying subscapularis tendon, and localize the axillary nerve along its inferior margin.
-
Release the subscapularis from the proximal humerus, preserving as much tendon length as possible.
-
Examine the humeral component for malposition.
-
When the stem is loose, it can be removed
from the shaft with little damage to the humerus. Removal of a
porous-coated stem can be extremely difficult, especially if the
textured coating extends to the distal stem. Begin removal with bone
and/or cement removal at the stem. This can be done with small curved
curets and thin osteotomes. -
Place an extraction device, and pull it
in line with the stem. Use instruments specifically designed for this,
to minimize the force required for extraction. -
If attempts at removal fail, it may be
necessary to cut a longitudinal slot anteriorly in the humeral shaft,
one third of its circumference in width and three quarters of the
stem’s length long, giving it a laterally based soft-tissue hinge, to
extract the stem (Fig. 103.6). In a standard-length prosthesis, this lies well above the spiral groove, minimizing risk to the radial nerve.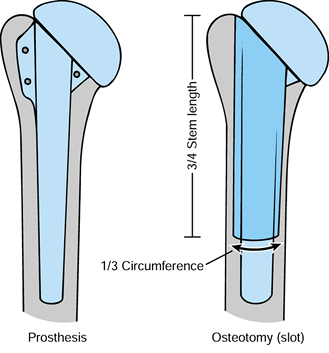 Figure 103.6. Osteotomy used to extract a well-fixed prosthesis.
Figure 103.6. Osteotomy used to extract a well-fixed prosthesis. -
With the stem removed, take cultures and
frozen sections from the bone–implant interface. If more than 10
polymorphonuclear cells are seen per high-power field, place an
antibiotic spacer until final cultures and pathology are obtained. If
there is no evidence of infection, remove the antibiotic spacer and
implant final components in a second operation. If a modular humeral
component does not need to be revised but glenoid work is indicated,
remove the head and leave the stem in place. Protect it from damage. -
Retract the humerus posteriorly to visualize the glenoid.
-
If a humeral head component is being
converted to a total shoulder replacement because of glenoid wear and
pain, perform a glenoid resurfacing at this time. -
If revision of a glenoid component is
required, clear the soft tissue from the rim of the component. If the
component is loose, it can be removed easily. If the face of the
glenoid component is well fixed but worn or malpositioned, it can be
cut from the underlying pegs or keel with a sharp, curved osteotome.
The keel or
P.2709
pegs can be extracted from the glenoid with a high-speed burr or curved osteotomes. -
Remove the cement, and obtain cultures and frozen section.
glenoid bone stock is sufficient for implant support and the cuff
muscles are intact or repairable (53,54 and 55).
Otherwise, removal of the glenoid component with conversion to a
hemiarthroplasty is preferred. Both approaches may yield good results.
Although glenoid bone deficiency can be bone grafted (55),
this is rarely performed. Central defects can be treated with impacted
particulate graft with cement fixation of the glenoid component, or as
a staged grafting procedure. In staged grafting, the defect is packed
with cancellous allograft and a hemiarthroplasty is performed. When
graft consolidation occurs, a glenoid is implanted at a later date,
usually 6 weeks to 3 months. Rim defects can be treated with
corticocancellous bone graft fixed with screws. When screw fixation is
not feasible, secure the graft with heavy absorbable sutures.
-
Place a humeral trial component to
determine implant stability and motion. Take care to properly size the
components; this is critical for implant stability and postoperative
motion. -
Place heavy nonabsorbable sutures around
the lesser tuberosity and humeral neck for repair of the subscapularis,
then implant the humeral stem with antibiotic-impregnated cement. -
In cases where humeral slotting was
necessary to remove the implant, close the osteotomy and secure it with
cerclage wires or cables. Maintain complete visualization of the
osteotomy during cementation, as extravasation of cement can occur,
which may lead to thermal injury of the radial nerve.
the preoperative condition of the surrounding soft tissues. Neer and
Kirby (54) found that, although pain relief is
often possible when specific causes are found, functional recovery is
limited. Results of published series are listed in Table 103.4.
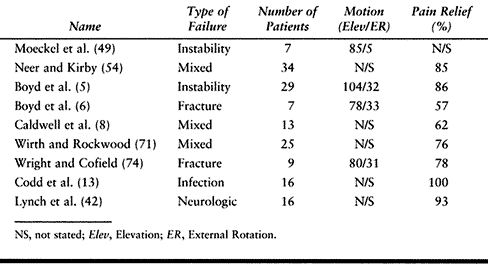 |
|
Table 103.4. Results of Revision of Failed Shoulder Arthroplasty
|
fortunately, a rare occurrence. The rates of injury are much lower than
the 0% to 3% reported for total hips and knees. The cause of nerve
injury is most often traction or compression, but accidental
transection is possible. Injuries to the axillary, musculocutaneous,
ulnar, and radial nerves have been reported (18,42,72,73). Most injuries are neuropraxias that resolved with time. Lynch et al. (42)
reviewed 417 patients undergoing total shoulder ar- throplasty and
found that 18% of shoulders had a neu- rologic deficit after surgery.
Most of those deficits involved the upper and middle trunks of the
brachial plexus. Of the 18 shoulders affected, four had lesions that
interfered with their rehabilitation. This series identified the long
deltopectoral approach, use of methylmethacrylate, and shorter
operative times as risk factors for injury.
have a careful neurologic evaluation postoperatively. If a deficit is
identified, institute physical and occupational therapy to maintain
joint mobility. When indicated, provide support with splints. If there
is no clinical evidence of neurologic recovery in 4–6 weeks, obtain
electromyography and nerve conduction studies to document the extent
and type of injury and to provide a baseline for monitoring improvement
in nerve function. If no improvement is seen in 3 months, consider
exploration of the nerve for neurolysis or nerve grafting.
Boyd et al. reported seven patients with humeral shaft fracture
following shoulder arthroplasty. Two were treated nonoperatively: One
developed a painful nonunion but refused surgical intervention, and the
other attained union but required revision for a loose glenoid
component. Five patients underwent operative intervention when
conservative treatment failed after an average of 8 weeks of treatment.
Two of those patients developed radial nerve palsies following the
fracture, which prompted early surgical exploration. All fractures
united.
-
Type A: The fracture is centered at the tip of the prosthesis and extends proximally for one third the length of the stem.
-
Type B: The fracture is centered at the tip of the prosthesis with slight proximal extension.
-
Type C: The fracture is distal to the tip of the stem.
humerus fractures, and those in categories A and B had a greater risk
of nonunion. The authors recommended surgical treatment for type A
fractures, operative treatment for type B fractures when satisfactory
alignment could not be achieved with closed means or if no evidence of
union was present by 3 months, and nonoperative management for type C
fractures. Groh et al. (30) reported on four
postoperative fractures that occurred an average of 14 months after
arthroplasty. They were treated with a fracture brace and early
range-of-motion exercises. At an average of 9 weeks, all fractures had
united.
reliable rules for the treatment of these fractures. We prefer
conservative treatment with immobilization in a functional brace if the
fracture is well aligned and has a long oblique pattern with a stable
stem. However, if the fracture is unstable or the humeral stem loose,
we recommend open reduction and internal fixation.
-
Use Henry’s extensile anterolateral exposure (see Chapter 1). Make a longitudinal incision along the lateral aspect of the arm to expose the biceps tendon.
-
Retract the biceps medially to reveal the
brachialis muscle. Avoid injury to the musculocutaneous nerve. Identify
and protect the radial nerve if it is in the region of the fracture. -
Split the brachialis fibers longitudinally to expose the humeral shaft. Visualize the entire fracture.
-
You must evaluate the humeral stem
intraoperatively, either with imaging or by direct examination. If the
stem is stable and well fixed, reduce the fracture and stabilize it
with a large plate, using screws and cables to fix it to the bone
depending on its location and configuration. -
Autologous bone graft is recommended to increase the chances for union (4).
-
When the humeral stem is loose, perform revision shoulder arthroplasty with a long-stem humeral component.
-
Reduce the fracture, and fix it with cerclage cables or wires before the final implantation of the prosthesis stem.
for shoulder arthrodesis have become limited. However, glenohumeral
arthrodesis remains an important and valuable treatment option for
restoring useful function in situations where arthroplasty either has
failed or is not indicated. Whereas the optimal results seen with
glenohumeral arthrodesis do not compare with those seen with
conventional arthroplasty, it should be noted that when arthrodesis is
indicated, significant and meaningful improvement in quality of life
can be obtained. Although arthrodesis is performed uncommonly, numerous
reports show that a functional fusion can be obtained reliably with an
acceptable complication rate (2,17,33,40,51,59,60,61 and 62,65).
obtain a solid bony fusion between the proximal humerus and the scapula
that alleviates pain and allows function through scapulothoracic motion
(Fig. 103.7).
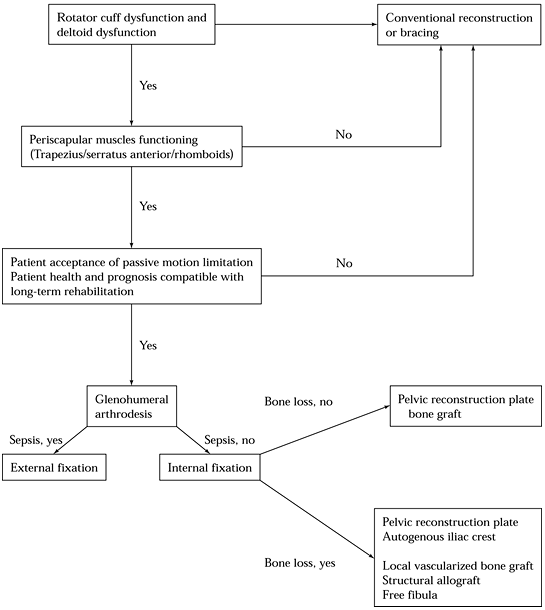 |
|
Figure 103.7. Algorithm for indications and type of shoulder arthrodesis for failed shoulder arthroplasty.
|
Glenohumeral arthrodesis is a reliable means of obtaining shoulder
stability and function sufficient for most activities of daily living
in patients in whom this would not otherwise be attainable (33,59).
Shoulder stability may come with significant loss of motion because all
glenohumeral rotation is lost. In some patients who do not have much
glenohumeral motion, the additional loss of motion is negligible.
Shoulder arthroplasty is preferred when the final outcome provides
stability and good pain control.
 |
|
Table 103.5. Indications for Shoulder Arthrodesis
|
arthrodesis because of scapulothoracic motion. The procedure reliably
controls pain originating in the glenohumeral joint. Counsel patients
carefully about the advantages and disadvantages of glenohumeral
arthrodesis. Either arthrodesis or arthroplasty is generally hard to
reverse. Glenohumeral fusion after a failed arthroplasty, and
arthroplasty after takedown of an established glenohumeral fusion are
both technically challenging surgical procedures with compromised
results.
glenohumeral arthrodesis is sufficient strength of the axial and
scapular musculature to maintain stability of the scapula. This
includes adequate trapezial function. The patient should be in
sufficiently good health to withstand major surgery. Additionally,
because postoperative recovery (including strengthening of periscapular
musculature) can be prolonged, glenohumeral arthrodesis requires a
mature patient with long-term goals. This is particularly relevant in
patients needing reconstruction after tumor resection.
flail shoulder. Paralytic shoulder secondary to anterior poliomyelitis,
upper-trunk brachial plexus lesions, and axillary nerve palsy in the
presence of a nonfunctional rotator cuff are indications for
arthrodesis. The flail shoulder is a particularly strong indication for
an arthrodesis when good elbow and hand function and strong
periscapular musculature are present (44,45,59,61,65).
In addition to limited function, patients with flail shoulders may have
significant pain associated with inferior luxation of the humeral head.
not necessarily a contraindication to glenohumeral arthrodesis. A
concurrent procedure to restore elbow flexion can be performed, either
staged or simultaneously. Indeed, an arthrodesis can significantly
improve the results of an elbow flexorplasty by stabilizing the arm in
reference to the axial skeleton and by placing the arm in a more flexed
position. For the completely flail upper extremity, a shoulder
arthrodesis can be performed with an elbow flexorplasty, to improve
quality of life, even with a dysfunctional hand (59,61,75) (see Chapter 59, Chapter 60 and Chapter 178).
arthrodesis is controversial. Most agree that deltoid dysfunction alone
is not a sufficient indication—rotator cuff dysfunction is generally
required in addition. The restoration of rotator cuff and/or deltoid
function with muscle transfers is difficult and generally requires
multiple surgeries.
upper extremity surgeon; for example, in the case of an axillary nerve
laceration, cable grafting can give surprisingly reliable functional
return. Other nerve grafting and muscle transfer procedures are
available that may make shoulder fusion unnecessary (see Chapter 59, Chapter 60 and Chapter 178).
While it remains an important indication, arthroplasty has become more
accepted. The surrounding musculature is often preserved and functional
despite prior sepsis, making it amenable to prosthetic reconstruction (13). Thus, if the septic arthritis can be eradicated, an arthroplasty is preferred.
of both deltoid and rotator cuff functions. When function of the
deltoid and/or rotator cuff muscles is preserved, a revision
arthroplasty is preferred. Anterior superior instability, however, may
best be treated with arthrodesis. In these people, the rotator cuff as
well as the anterior deltoid is usually dysfunctional. Incompetence of
the coracoacromial arch can result in migration of the prosthesis
anteriorly and superiorly into a subdeltoid, almost subcutaneous,
location. In addition to significant dysfunction, these patients often
have severe pain. Because periscapular musculature is still intact, a
successful arthrodesis could alleviate the problem and provide
significant functional gains. However, arthrodesis after failed
shoulder arthroplasty is difficult because of the loss of proximal
humeral bone, in particular the head of the humerus.
been an indication for glenohumeral fusion. More recently, arthrodesis
does not appear to be a good alternative for failed shoulder
instability reconstructions. Outcome data suggest that many people who
obtain arthrodesis in this situation continue to be unhappy,
persistently perceiving instability in the glenohumeral joint despite a
radiographic fusion (59). Additionally, pain
from the glenohumeral joint is often persistent despite any obvious
objective reasons. Before exploring arthrodesis as a surgical
alternative, exhaust every other treatment option. Assess the patient’s
psychological makeup carefully.
cuff tear, osteoarthritis, or rheumatoid arthritis, advances in
conventional reconstructive procedures have made glenohumeral
arthrodesis relatively contraindicated. The deciding factor in each of
these three clinical scenarios is the function of the rotator cuff and
deltoid. Loss of these two important muscle groups makes arthrodesis
the best choice in most patients.
choice following removal of periarticular shoulder tumors when the
proximal humerus can be preserved. Because the rotator cuff and the
deltoid are often removed as part of the resection, stability is
difficult to achieve without arthrodesis. After aggressive bone
resection, vascularized bone graft or allograft reconstruction may be
necessary to achieve an arthrodesis (11,41,43).
to be performed, do not use the trapezius for a muscle transfer,
because this is an extremely important muscle for function and pain
relief following arthrodesis.
method of shoulder reconstruction is available. In general, if the
rotator cuff or deltoid still functions, then a total shoulder or
hemiarthroplasty can be performed. Additionally, do not do a
glenohumeral arthrodesis in the presence of a flail scapula. In
patients who have partial periscapular function, lost trapezius
function is a contraindication to arthrodesis.
recovery to achieve bone healing as well as periscapular strengthening,
it is relatively contraindicated in people who cannot tolerate
prolonged rehabilitation, those who have limited life spans, and those
in whom bone healing would be a problem. For this reason, shoulder
arthrodesis is generally contraindicated in patients who are
physiologically over the age of 70 years.
extra-articularly or intra-articularly, or with both methods, using
external or internal fixation. Extra-articular arthrodesis is not used
often now and was primarily indicated in patients with tuberculous
arthritis in whom there were concerns about disseminating an
intra-articular infection (29,58,68).
external and/or internal fixation. External fixation is now rarely
indicated because of the superiority of internal fixation. It is
indicated in patients with an active infection of the shoulder.
Fixation can be achieved by using half-pins in the clavicle, acromion,
scapular spine, and glenoid neck (37,40,51).
glenohumeral arthrodesis. Its advantages are rigid fixation, better
maintenance of arthrodesis position, and decreased length of time in
plaster or orthotic immobilization. The postoperative care is easier.
-
Place the patient in an upright
beach-chair position with the entire arm, including the scapula, draped
free. Place a bump along the medial border of the scapula to position
the scapula laterally. -
Make an extensile deltopectoral incision
that extends vertically from the spine of the scapula 2–3 cm medial to
the acromioclavicular joint and then down a line from the coracoid
process toward the deltoid insertion (Fig. 103.8A) (see Chapter 1).
This avoids placing the incision directly over the plate. In patients
with paralytic shoulders and complete atrophy of the deltoid,
soft-tissue coverage of the plate is a concern.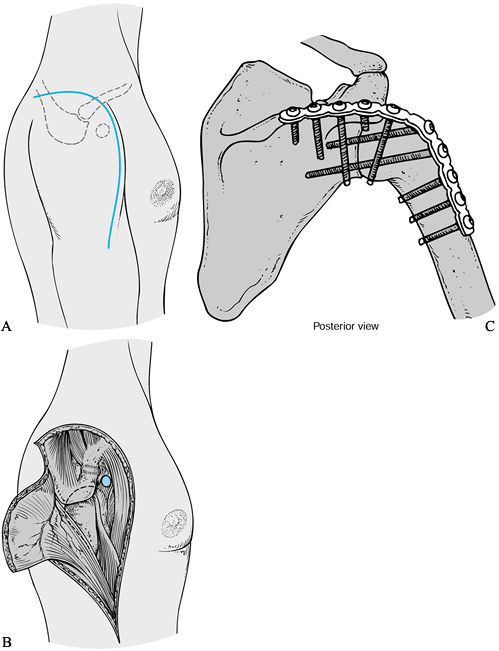 Figure 103.8. A: Incision for an extended deltopectoral approach for the shoulder arthrodesis. B:
Figure 103.8. A: Incision for an extended deltopectoral approach for the shoulder arthrodesis. B:
Reflect the deltoid off the lateral clavicle and acromion as needed for
exposure. Release of a portion of the insertion into the humerus is
helpful. C: Ten-hole 4.5 mm AO
reconstruction plate contoured along the spine of the scapula over the
acromion and onto the humerus, and fixed with multiple lag screws. -
Develop a subcutaneous lateral flap at the level of the deep fascia.
-
Identify the deltopectoral interval, and
remove the deltoid from the anterolateral clavicle as well as the
anterior and lateral acromion. If distal extension is necessary, also
release the anterior two thirds of the deltoid insertion. In comparison
to a muscle-splitting approach, releasing the deltoid from the anterior
and posterior maintains it for coverage of the plate and spares the
axillary nerve, avoiding a possible neuroma (Fig. 103.8B). -
Release the anterior and superior rotator
cuff directly off its humeral attachment, and retract it medially. Do
not excise it. Maintaining the rotator cuff musculature for later
repair can help contain bone graft. -
Dislocate the humeral head anterolaterally to expose the glenoid.
-
Place a posterior glenoid retractor, and
decorticate the glenoid. A standard glenoid reamer from a total
shoulder set is helpful for concentric decortication of the glenoid. -
Decorticate the humeral head using large
curets and a rasp. Maintain the semicircular contour of the proximal
humerus to provide a congruent surface area for fixation against the
glenoid. -
For fixation, we do not generally
recommend superior translation of the humeral head against the
undersurface of the acromion. Rather, center the humeral head on the
glenoid. This provides a large concentric area of contact, and the
glenoid vault is an important area for obtaining screw purchase for
fixation and compression of the humeral head against the scapula. -
Apply iliac crest bone graft to bridge the gap between the humeral head and the acromion.
-
Alternatively, Richards et al. (61)
reported a technique in which the humeral head is translated superiorly
for bony abutment against both the undersurface of the acromion and the
glenoid. Using this method, bone grafting was not necessary to achieve
fusion in most cases. This technique is preferred by many. -
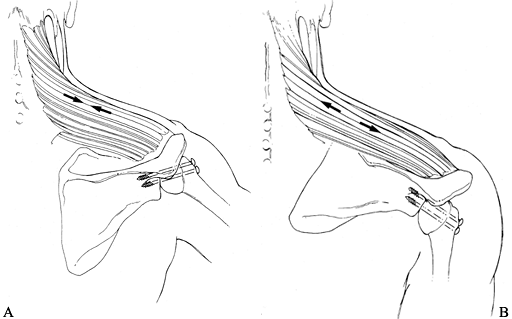
Position the arm for fusion, using an arm
holder. Our preferred position is at 20° of abduction, 20° to 30° of
forward flexion, and 40° of internal rotation (Fig. 103.9).![]() Figure 103.9. A–D:
Figure 103.9. A–D:
Clinical photographs of a patient’s arm fused in 20° of abduction, 30°
of forward elevation, and 40° of internal rotation. The patient is able
to achieve sufficient motion for activities of daily living.
Early recommendations from the American Orthopaedic Association
established criteria of 45° to 50° of abduction, 15° to 25° of forward
flexion, and 25° to 30° of internal rotation (1).
Additionally, the method for measuring the position of fixation has
varied. Early recommendations used the medial border of the scapula in
reference to the long axis of the humerus. Now most surgeons use the
side of the body as the reference point and place the arm at 20° to 30°
abduction from the side of the patient.
observed that the previous recommendation of 45° to 50° for abduction
was excessive. This position could result in uncomfortable scapular
winging when the arm was resting at the side. He noted that shoulder
flexion and internal rotation were important for activities of daily
living, and some abduction was required to provide clearance for
axillary hygiene. Richards et al. (59) noted
that the optimum position of glenohumeral arthrodesis is one that
allows the hand to be raised in the midline with elbow flexion so that
the mouth can be reached. Abduction should not exceed that which allows
the arm to rest comfortably at the side. To make it easy, Richards
recommends placing the hand on the face and setting the shoulder at 30°
of abduction, 30° of forward flexion, and 30° of internal rotation.
-
It is important to note that regardless
of the position of fusion, the arm will rest at the side a significant
amount of the time. Thus, a significant amount of scapular winging
would occur if the arm were fused in too much abduction, causing
chronic pain due to excessive tension on the trapezius (Fig. 103.10).
 |
|
Figure 103.10. The ideal position for arthrodesis is in less than 30° of abduction. A: The shoulder is fused in excessive abduction. B: When the arm rests at the side, excessive tension occurs in the upper trapezius which can cause chronic pain.
|
-
Once the position of fusion is
established, fix the proximal humerus to the glenoid with two 6.5 mm,
partially threaded cancellous screws with washers, either independently
or through a precontoured plate. -
Next, contour a 10- to 14-hole, 4.5 mm
pelvic reconstruction plate to fit over the spine of the scapula across
the acromion and down the lateral side of the proximal humeral shaft.
The length of the plate is dictated by what is necessary to obtain
three bicortical screws distal to the surgical neck of the humerus as
well as three cortical screws into the spine of the scapula. Often, a
screw can be placed through the acromion into the proximal humerus in a
superior-to-inferior direction (Fig. 103.8C).
Use either 4.5 mm cortical or 6.5 mm cancellous screws depending on the
quality of the bone. Securely compress the head of the humerus to the
glenoid using lag technique. -
Test the entire construct for stability.
If there are concerns about the strength of fixation, a spica cast or a
rigid orthosis may be necessary. -
In many circumstances in which an arthrodesis is necessary, significant scapular and proximal humeral osteopenia has occurred (Fig. 103.11A).
Screw purchase in the scapular spine can be poor. The strength of the
fixation can be improved by placing cerclage wires around the pelvic
reconstruction plate and the base of the acromion (Fig. 103.11B).![]() Figure 103.11. A:
Figure 103.11. A:
Radiographs of a patient who had a previously failed hemiarthroplasty
and hypoplastic glenoid. Because there was bone loss on the humerus and
a hypoplastic glenoid, she went on to nonunion after an unsuccessful
attempt at arthrodesis. B: Revision
glenohumeral arthrodesis was performed using plate fixation and local
vascularized bone graft. Note that plate fixation to the spine of the
scapula was augmented with three cerclage wires.
inadequate internal fixation, use a soft, removable abduction pillow.
It will unload the weight of the arm and is easily removed for hygiene;
thus it is better tolerated than the orthosis for the 8- to 12-week
period that is often necessary to obtain a solid fusion. Once clinical
or radiographic fusion has been obtained, place the patient on a
rehabilitation program for periscapular strengthening. Give particular
attention to strengthening the trapezius, rhomboids, and serratus
anterior. Throughout the postoperative period, employ elbow
range-of-motion and hand exercises in conjunction with shoulder
rehabilitation.
proximal humeral or glenoid bone loss such as after failed total
shoulder arthroplasty is challenging. Autogenous bone grafting is
always indicated in these situations. Additionally, vascularized local
bone graft transfers can be effective. The axillary border of the
scapula provides a convenient and excellent source for local
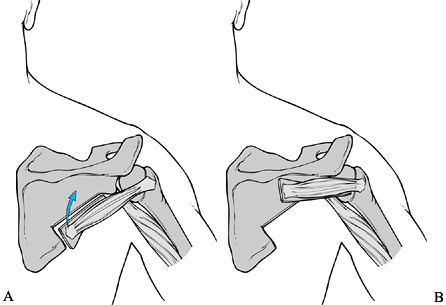
vascularized bone graft (Fig. 103.12). The
axillary border of the scapula is well vascularized by the circumflex
scapular artery and muscular attachments of both the teres minor and
teres major. To utilize this transfer, extend the vertical saber
incision posteriorly approximately 7 cm.
 |
|
Figure 103.12. Schematic of the technique for local vascularized bone grafting. A: Muscle pedicle bone graft developed from the lateral border of the scapula. B: Graft transferred to bridge the glenohumeral joint posteriorly.
|
-
Take the subcutaneous dissection to the
superficial deltoid fascia, and release the deltoid off the posterior
spine, exposing the infraspinatus and teres minor. -
Split the infraspinatus–teres minor
interval up to the glenoid neck, and place multiple drill holes in the
scapula along the line between the infraspinatus and teres minor. -
Sharply free the axillary border away
from the scapular body by using an osteotome to connect the drill
holes. Leave the glenoid intact. Superiorly, the transverse cut is
inferior to the glenoid neck and inferiorly the cut is proximal to the
inferior angle of the scapula, which leaves the important attachment
site of the serratus anterior. Protect the origin of the teres minor
from the scapula, as it maintains the vascularity. -
Rotate the axillary border bone graft
horizontally, and fix it to the scapular body just inferior to the
scapular spine, as well as the proximal humerus laterally (Fig. 103.12). -
Intercalary allograft or a free microvascularized fibular graft might be helpful in very difficult fusions with loss of bone
internal fixation and supplemental external support is generally over
90%. Earlier series, which relied primarily on external support,
achieved union but required immobilization for at least 6 months. Pain
relief after shoulder arthrodesis is less predictable, with many
patients reporting some postoperative discomfort (33,59).
Overall patient satisfaction after arthrodesis has not been universally
established. A recently published functional outcome analysis of 33
patients found that the single best predictor of the ability to perform
activities of daily living was preoperative diagnosis. Satisfaction
rates were highest in osteoarthritis, brachial plexus injuries, and
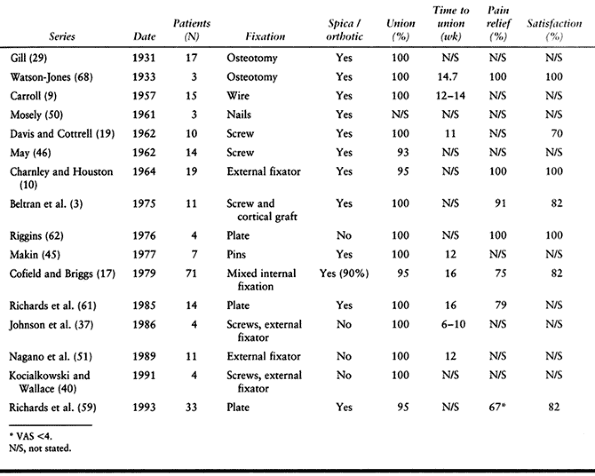
failed total shoulders (59). Published results of shoulder arthrodesis are listed in Table 103.6.
 |
|
Table 103.6. Results of Shoulder Arthrodesis
|
bone, where a stress riser distal to the plate is dangerous. It is not
surprising that a high fracture rate can occur in this area. Cofield
and Briggs (17) reported a fracture rate
of 15%. Because this complication often occurs with only minor trauma,
closed treatment is often successful. If a glenohumeral arthrodesis is
established and internal fixation of a distal humerus fracture becomes
necessary, then removal of the proximal plate is recommended for the
future to prevent a stress riser.
In general, avoid removal of in- ternal fixation devices until you
achieve an arthrodesis. Following this, remove all internal fixation
and perform repeat irrigation and debridements and treat with
intravenous antibiotics as necessary to eradicate the infection. If the
internal fixation has loosened in the presence of infection, remove it
and convert to external fixation augmented by an external orthosis.
reliable measure of outcome for glenohumeral arthrodesis. Nonunion is
rare. In Cofield’s (17) series of 71 shoulders, only 4% went to nonunion. Richards et al. (59) reported similarly high fusion rates.
large range of flexion and rotation positions. Glenohumeral abduction,
in contrast, is poorly tolerated in adults at angles greater than 40°.
This is because of the significant scapular winging and trapezial
tension that occur when the arm is resting at the side. When
malposition causes significant pain or dysfunction, an osteotomy may be
required. It is generally performed in the humerus distal to the
glenohumeral fusion.
can improve functional activities and relieve pain. Careful
preoperative evaluation is critical to the planning and success of
these procedures. Preoperative counseling is important to provide the
patient with realistic postoperative expectations. With realistic
expectations and a clear preoperative plan, good clinical results can
be expected in these challenging clinical situations.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
J, Freiberg JA, Colonna PC, Pemberton PA. A Survey of End Results on
Stabilization of the Paralytic Shoulder. Report of the Research
Committee of the American Orthopaedic Association. J Bone Joint Surg [Am] 1942;24:699.
PM, Hawkins RJ. Fracture of the Humeral Shaft Associated with Total
Replacement Arthroplasty of the Shoulder. A Case Report. J Bone Joint Surg Am 1992;74:617.
GL, Dines DM, Warren RF. Revision Shoulder Arthroplasty. Presented at
the Annual Meeting of the American Shoulder and Elbow Surgeons, San
Francisco, CA, Feb., 1993.
TP, Yamaguchi K, Pollock RP, et al. Infected Shoulder Arthroplasties:
Treatment with Staged Reimplantation versus Resection Arthroplasty. Orthop Trans 1996;20:59.
XA, Connor PM, D’Alessandro DF. Anterosuperior Instability of the
Shoulder. Presented at the 15th Open Meeting of the American Shoulder
and Elbow Surgeons, Anaheim, CA, February 7, 1999.
VP, Satku SK, Mitra AK, Pho RW. Function following Limb Salvage for
Primary Tumors of the Shoulder. Ten Patients Followed 4 (1–11) Years. Acta Orthop Scand 1994;65:55.
W, Thrum CB, Hamilton SGL. Designing an Implant by CT Scanning and
Solid Modelling: Arthrodesis after Excision of the Upper Humerus. J Bone Joint Surg Br 1986;68:208.
RR, Sherman RMP, Hudson AR, Waddell JP. Shoulder Arthrodesis Using a
Modified Pelvic Reconstruction Plate: A Review of Eleven Cases. J Bone Joint Surg Am 1988;70:416.
TP, Jasty MJ, Harris WH. Periprosthetic Loosening in Total Hip
Arthroplasty. Polyethylene Wear Debris and the Concept of the Effective
Joint Space. J Bone Joint Surg Am 1992;74:849.
MA, Agrawal CM, Mabrey JD, et al. Isolation and Characterization of
Polyethylene Wear Debris Associated with Osteolysis following Total
Shoulder Arthroplasty. J Bone Joint Surg Am 1999;81:29.