ARTHROPLASTY AND ARTHRODESIS OF THE ELBOW
– JOINT RECONSTRUCTION, ARTHRITIS, AND ARTHROPLASTY > Upper
Extremity > CHAPTER 102 – ARTHROPLASTY AND ARTHRODESIS OF THE ELBOW
Associate Professor, Orthopaedic Surgery, Hand and Upper Limb Centre,
University of Western Ontario, St. Joseph’s Health Centre, London,
Ontario, N6A 4L6, Canada.
infrequently performed with the advent of successful replacement
arthroplasty. Resection arthroplasty should be avoided due to the gross
instability that results from removal of the distal humerus and
proximal ulna. The most common indication for resection arthroplasty is
a failed infected total elbow arthroplasty (88).
Interposition arthroplasty continues to be used in selected patients
with elbow arthritis. The procedure consists of interposing fascia or
dermis between the humerus and ulna while maintaining the overall shape
of these bones to preserve stability.
arthroplasty of the elbow is incapacitating pain and stiffness due to
old sepsis, prior trauma, hemophilia, or inflammatory arthropathy. With
improved prosthetic arthroplasties of the elbow, interposition
arthroplasty is now most commonly used for young patients with
posttraumatic arthritis for which the results of total elbow
arthroplasty are less reliable. Patients with more than 50% loss of
articular cartilage and joint incongruity should be considered for an
interposition arthroplasty rather than a surgical debridement.
Interposition arthroplasty can be considered in young patients whose
elbows are ankylosed but whose functional demands require motion.
Patients must be able to cooperate in the postoperative rehabilitation
program, which is an essential part of the success of interposition
arthroplasty.
debridement before interposition arthroplasty. Delay arthroplasty until
all epiphyses are closed. Patients with a poor soft-tissue envelope
should have flap coverage before any type of elbow arthroplasty.
Consider elbow arthrodesis or tendon transfers before interposition
arthroplasty for
patients
with inadequate muscle power for flexion and extension. Avoid
interposition arthroplasty in heavy laborers because pain relief is
often incomplete. Patients who require ambulatory aids for walking may
develop increased elbow instability after surgery, which may impair
their ability to ambulate. Accept an ankylosed elbow in a functional
position because the results of interposition arthroplasty in such
circumstances are less reliable than for other indications.
interposition arthroplasty. Of these, the most popular have been the
use of fascia lata or skin (5,6,33,41,50,55,78).
Some surgeons have advocated the use of an articulated external fixator
to distract the elbow in the postoperative period to improve the
outcome (55). There are no comparative series
evaluating the efficacy of adding a distraction device to an
interposition arthroplasty. In spite of this, distraction interposition
arthroplasty has become increasingly popular because it allows
immediate postoperative elbow motion while maintaining stability.
Distraction of the joint in the postoperative period may have an
additional advantage by allowing the interposition material to become
biologically attached to the underlying bone before being subjected to
compression and shear loading.
-
With the patient supine, prep and drape the arm and inflate a sterile tourniquet.
-
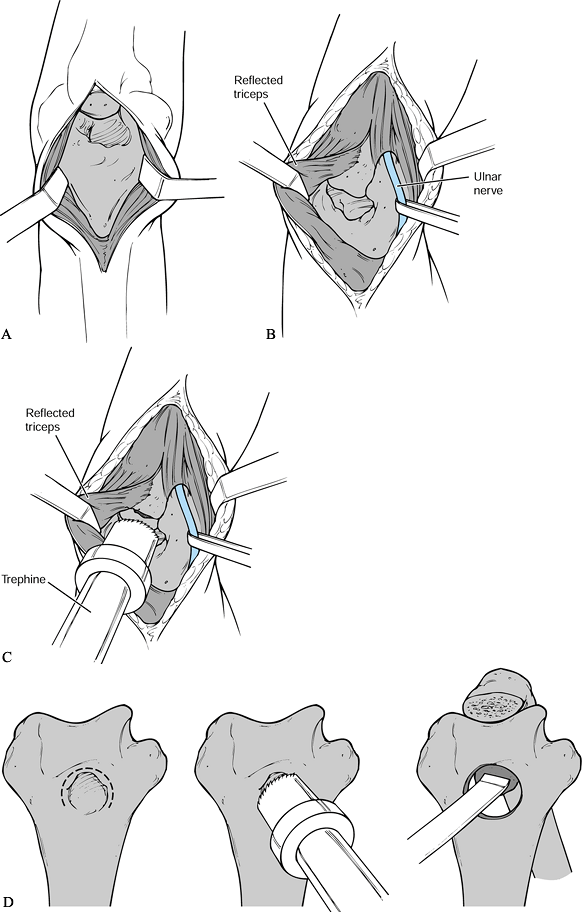
Make a midline posterior elbow incision, staying just lateral to the tip of the olecranon (15). Elevate a full-thickness lateral flap and identify the Kocher interval between the anconeus and extensor carpi ulnaris (Fig. 102.1).
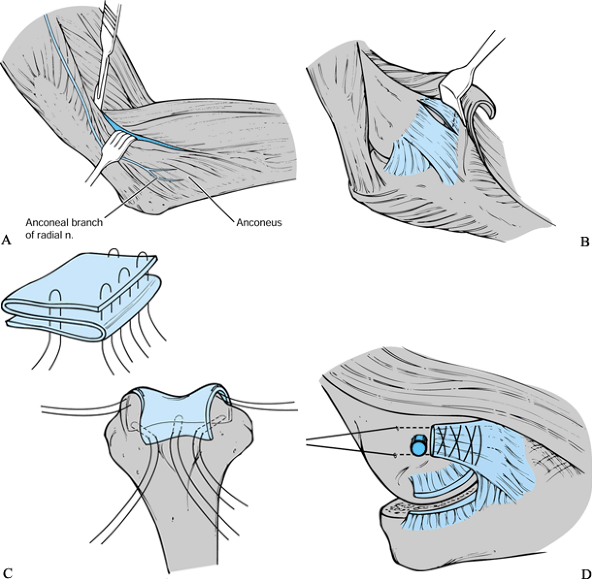 Figure 102.1. Interposition arthroplasty technique: A: The Kocher interval is identified between the anconeus and the extensor carpi ulnaris. B:
Figure 102.1. Interposition arthroplasty technique: A: The Kocher interval is identified between the anconeus and the extensor carpi ulnaris. B:
The lateral collateral ligament complex is detached from its humeral
origin, and the elbow is subluxated to achieve wide access to the
joint. C: The fascia lata is folded onto
itself and wrapped over the articular condyles of the distal humerus
and secured with nonabsorbable sutures. D:
The elbow is reduced, and the lateral ligaments are repaired through
drill holes in the lateral epicondyle using nonabsorbable sutures. -
Elevate the common extensor origin off
the lateral epicondyle and supracondylar ridge anteriorly. Reflect the
anconeus posteriorly. -
Detach the lateral collateral ligament
complex from its humeral origin to achieve wide access to the elbow.
The triceps may need to be elevated off the olecranon in continuity
with the anconeus, and the medial collateral ligament may have to be
partially released in some patients to achieve adequate exposure,
particularly in those with very stiff elbows. -
Excise the anterior and posterior capsule and remove any osteophytes to improve motion.
-
Using a burr, smooth and contour the
greater sigmoid notch of the olecranon and the trochlea of the distal
humerus. Take care to minimize removal of subchondral bone because this
often results in excessive bone resorption in the postoperative period. -
If possible, preserve the radial head to
maximize the stability of the elbow. It can be debrided if it is mildly
arthritic; however, it should be excised if it is involved with
advanced disease. -
Make a 25 cm incision over the proximal
lateral thigh. Remove a 10 cm wide by 25 cm long portion of fascia
lata. Do not close the fascial defect. -
Fold the fascia lata onto itself to
create a three-ply graft. Wrap the fascia over the articular condyles
of the distal humerus, and secure it with #1 braided nonabsorbable
sutures. -
Reduce the elbow and repair the lateral
ligaments through drill holes in the lateral epicondyle. Close the
fascia between the anconeus and extensor carpi ulnaris to augment
lateral elbow stability.
motion while protecting the interposed graft tissue during healing.
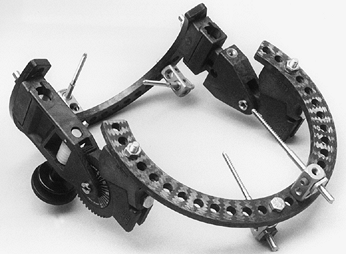
Options include the Dynamic Joint Distractor (Howmedica, Rutherford,
NJ) developed by Morrey (Fig. 102.2) or the Compass Universal Hinge (Smith & Nephew Richards, Memphis, TN) developed by Hotchkiss (Fig. 102.3).
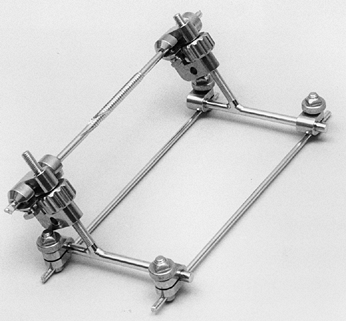 |
|
Figure 102.2. Dynamic Joint Distractor.
|
 |
|
Figure 102.3. Compass Universal Elbow Hinge.
|
which is placed through the axis of rotation of the distal humerus as
well as two pins for fixation in the ulna. Adjustable distraction of
the joint is permitted. This device maintains stability of the elbow
while allowing early active and passive mobilization. It is easy to
apply; however, because the intraarticular axis pin is left in the
distal humerus, it may have a higher incidence of postoperative joint
sepsis.
pins in the humerus and two pins in the ulna. All pins are placed
remote from the axis of the joint, which reduces the possibility of
postoperative infection. Distraction of the joint is permitted, and a
worm gear mechanism allows the patient controlled passive postoperative
mobilization of the elbow. This hinge is bulkier and somewhat more
difficult than the Dynamic Joint Distractor to apply.
-
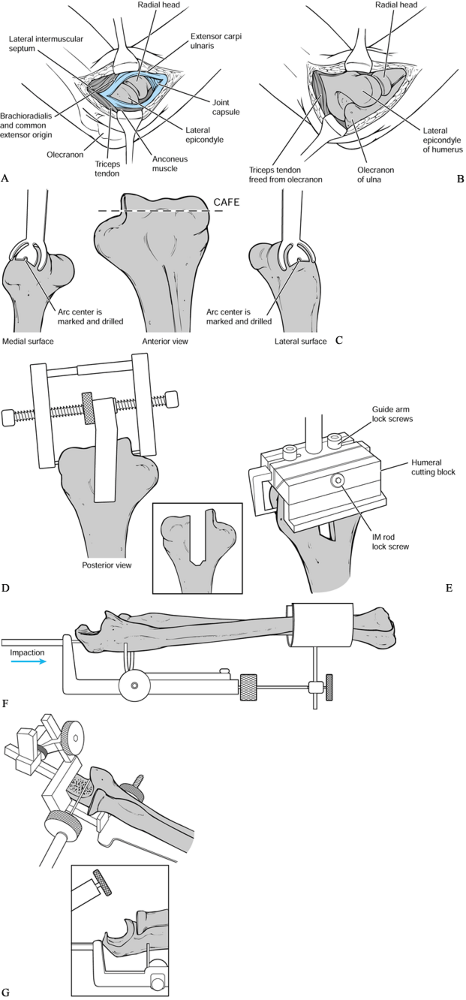
Identify the axis of motion of the elbow,
passing from the center of the radius of curvature of the capitellum
laterally to just anterior and distal to the medial epicondyle, and
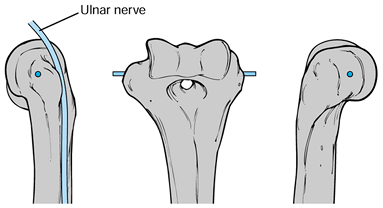
place a guide pin. Take care to identify and protect the ulnar nerve (Fig. 102.4).![]() Figure 102.4. Elbow flexion axis.
Figure 102.4. Elbow flexion axis. -
Reduce the elbow joint and repair the soft tissues as previously described.
-
Mount the fixator on the central axis pin
and apply the remainder of the pins for the articulated external
fixator. Distract the joint 2 to 3 mm to prevent dislodgement of the
graft during early active and passive mobilization.
With an axillary catheter in place, the patient and therapist can
initiate immediate postoperative passive range of motion.
Alternatively, employ a continuous passive motion machine. Use ice
packs to control edema. Commence active assisted motion after removal
of the axillary block. Throughout the postoperative period, maintain
elbow and forearm motion, along with meticulous pin site care. Remove
the elbow distraction device 6 weeks after surgery. Begin passive
stretching at 6 weeks and strengthening at 12 weeks. Provide resting
extension and static progressive flexion splints after fixator removal
to help regain motion under the supervision of a physical therapist.
good results with distraction interposition arthroplasty. Pain was
reduced, although at least half the patients had some residual
discomfort. Motion increased from an average arc of 27° preoperatively
to 107° postoperatively (Fig. 102.5). Unlike
earlier reports of interposition arthroplasty without distraction, the
stability achieved with this approach has been good. Others (5,6,33,41,50,78) have reported good results from interposition arthroplasty without distraction (Table 102.1).
 |
|
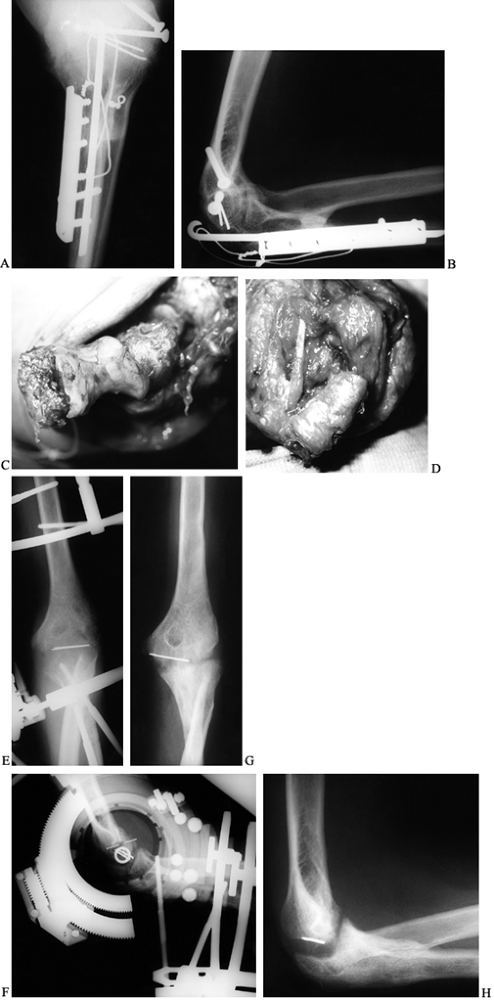
Figure 102.5.
Interposition arthroplasty: A 22-year-old woman with posttraumatic ankylosis of the elbow following a fracture of her distal humerus and forearm. A: Preoperative anteroposterior (AP) radiograph demonstrating a loss of joint space and heterotopic ossification. B: Preoperative lateral radiograph. C: Intraoperative appearance of the articular surface of the distal humerus with extensive loss of the articular cartilage of trochlea and absence of capitellum. D: Interposition graft secured in place on the distal humerus. E: Postoperative AP radiograph in Compass Elbow Hinge demonstrating joint distraction. F: Postoperative lateral radiograph in Compass Elbow Hinge showing correct articular alignment. G: One-year postoperative anterior posterior radiograph showing maintenance of joint space. H: One-year postoperative lateral radiograph demonstrating some postoperative resorption of the distal humerus. The patient achieved adequate functional outcome with motion from 30° to 130°, minimal pain, and no instability. |
 |
|
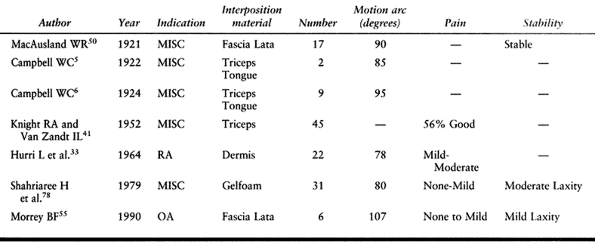
Table 102.1. Interposition Arthroplasty Results
|
formation, infection, bone resorption, and incomplete pain relief have
been reported following interposition arthroplasty. Minimize the
incidence of these complications by careful patient selection and
meticulous operative technique. Avoid bone resorption by preservation
of the subchondral cortex when performing the intraoperative joint
debridement. Minimize infection by careful pin site care and early
removal of any distraction device. Patients who are left with residual
pain and instability following interposition arthroplasty may be aided
by an elbow arthrodesis or a prosthetic arthroplasty.
patients with osteoarthritis. Even though there is increasing
experience with arthroscopic debridement, there are limited clinical
reports available documenting the medium- and long-term results of this
procedure (see Chapter 82). As a consequence, open debridement is preferred for selected patients with primary or secondary osteoarthritis.
the prevalence of osteoarthritis was reported at 2% of 1,000 patients
attending a fracture clinic. This disorder is most commonly found in
men, particularly those involved in heavy manual labor. Osteoarthritis
occurs secondary to trauma and other disorders such as Panner’s
disease, osteochondritis dessicans, and synovial osteochondromatosis.
Cubital tunnel syndrome is commonly associated with elbow
osteoarthritis, up to 40% in one series (66).
At present, the primary indication for debridement arthroplasty is the
younger patient with evidence of loose bodies or impinging osteophytes
and a preserved joint space. Older patients with advanced arthritis may
be better candidates for a total elbow arthroplasty. Patients who have
impingement pain—that is, pain at the extremes of motion with minimal
pain in the central arc—are considered ideal. Patients with pain
throughout motion generally have more articular destruction and may
require an alternative procedure such as an interposition or prosthetic
arthroplasty. Patients with a well-preserved ulnohumeral joint but
advanced involvement of the radial capitellar joint can be considered
for a radial head excision in combination with a debridement
arthroplasty. Patients must be able to understand and cooperate in the
postoperative rehabilitation program if a successful result is to be
obtained from a debridement arthroplasty.
alternate procedures such as interposition arthroplasty as described
above or total elbow replacement. Consider patients with a poor
soft-tissue envelope for flap coverage, either concomitant with
debridement or before it.
arthroplasty of the elbow. All involve resection of impinging
osteophytes and removal of loose bodies. Excise the radial head only if
it is involved by advanced disease.
including posterior, medial, and lateral approaches. The use of a
posterior midline incision provides the most flexibility while
performing the surgical procedure. Regardless of the surgical approach,
remove all osteophytes, particularly on the olecranon and coronoid
process, which typically impinge as the elbow is extended and flexed,
respectively.
routinely perform an anterior ulnar nerve transposition as part of the
operative procedure. Particularly in patients who lack elbow flexion
preoperatively, increased motion of the elbow after surgery may stretch
the ulnar nerve and exacerbate these symptoms.
-
Make a midline posterior elbow incision
centered just medial to the tip of the olecranon. Develop a
full-thickness medial skin flap and perform an anterior ulnar nerve
transposition. -
Expose the anterior aspect of the elbow
by developing the interval between the pronator teres and flexor
carpiradialis, as described by Hotchkiss (Fig. 102.6) (32). Excise the tip of the coronoid process if it is redundant and remove any loose bodies. Excise the anterior capsule.
P.2672
Clear the coronoid and radial fossi of any impinging osteophytes so
that near-full elbow range of motion is achieved at the completion of
the debridement.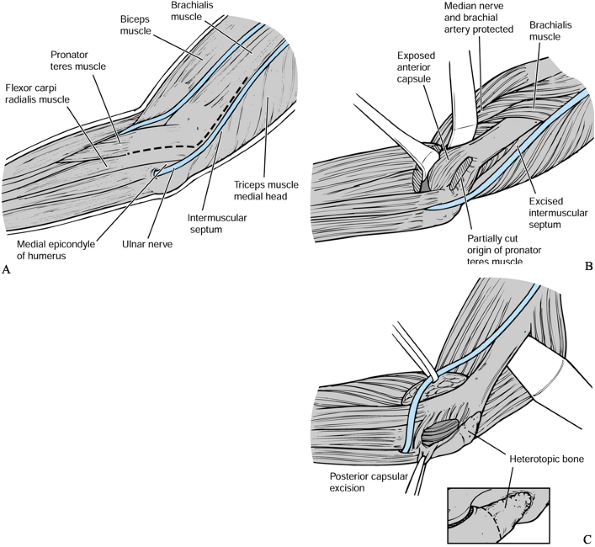 Figure 102.6. Debridement arthroplasty—medial approach: A:
Figure 102.6. Debridement arthroplasty—medial approach: A:
After isolation and transposition of the ulnar nerve, the interval
between the pronator teres and flexor carpi radialis is developed to
expose the anterior aspect of the elbow. B: The anterior capsule is excised, as well as any loose bodies or impinging osteophytes. C: The triceps is elevated posteriorly to expose the posterior capsule, which is excised. -
Elevate the triceps off the posterior
aspect of the humerus. Excise the tip of the olecranon and remove any
osteophytes or loose bodies from the olecranon fossa. Resect the
posterior capsule to optimize the postoperative range of motion.
approached using lateral muscular intervals. Preserve the lateral ulnar
collateral ligament. Visualization of the coranoid process is more
difficult than with a medial approach.
-
Make a midline posterior elbow incision
centered just lateral to the tip of the olecranon. Develop a
full-thickness lateral skin flap and identify the interval between the
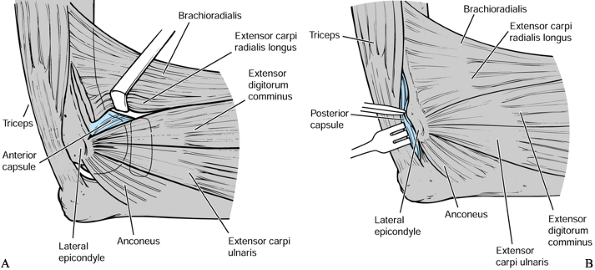
extensor carpi radialis longus and extensor digitorum comminus (Fig. 102.7) (8).
An approach through this interval provides adequate access to the
anterior aspect of the elbow while avoiding damage to the lateral ulnar
collateral ligament.![]() Figure 102.7. Debridement arthroplasty—lateral approach: A:
Figure 102.7. Debridement arthroplasty—lateral approach: A:
The anterior capsule can be approached from the lateral side of the
elbow through the interval between the extensor carpi radialis longus
and extensor digitorum communis. B: The olecranon and olecranon fossa are exposed by elevating the triceps off the posterior aspect of the humerus. -
Excise the anterior capsule and any osteophytes, as described for the medial approach.
Humeral fenestration is particularly useful when the olecranon and
coronoid fossae are obliterated by hypertrophic osteophytes.
 |
|
Figure 102.8. Outerbridge-Kashiwagi arthroplasty: A: The triceps tendon is split in the midline, and the olecranon fossa is exposed. The tip of the olecranon is excised. B:
Alternatively, expose the ulnar nerve and partially elevate the triceps off the tip of the olecranon to expose the olecranon fossa. C: The distal humerus is fenestrated using a Cloward drill. D: By working through the fenestration of the distal humerus, osteophytes can be removed from the coronoid and a partial excision of the anterior capsule achieved. |
-
Make a midline posterior elbow incision
centered just medial to the tip of the olecranon. Perform a midline
split in the triceps tendon to visualize the posterior aspect of the
distal humerus. If there is a pre-existent ulnar neuropathy, transpose
the ulnar nerve anteriorly during the same procedure. Alternative
exposure can be obtained by elevating the triceps from medial to
lateral off the tip of the olecranon. -
Fenestrate the distal humerus using a
Cloward drill. Ensure central placement of the drill on the olecranon
fossa such that the medial and lateral columns of the distal humerus
are not violated. Direct the drill 40° cephalad to correlate with the
shaft-capitellar angle of the distal humerus. The drill should exit the
anterior aspect of the elbow in the region of the coronoid fossa. -
Working through the fenestration in the
distal humerus, remove any coronoid osteophytes with an osteotome and
excise the anterior capsule (Fig. 102.9).![]() Figure 102.9. Debridement arthroplasty: A:
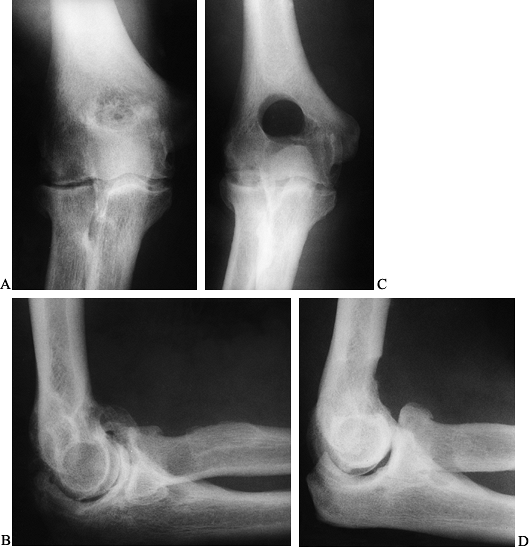
Figure 102.9. Debridement arthroplasty: A:
Anteroposterior radiograph of a 52-year-old laborer with persistent
stiffness and pain at the extremes of motion. Note preservation of the
ulnohumeral and radiocapitellar joint spaces with obliteration of the
normal radiolucency seen in the region of the olecranon fossa. B: Lateral radiograph showing osteophytes on the coronoid as well as the olecranon. C: Postoperative anteroposterior radiograph showing fenestration of the central portion of the distal humerus. D: Lateral radiograph showing adequate excision of the olecranon and coronoid osteophytes.
preoperatively or in the recovery room to facilitate postoperative pain
control. Begin elbow motion immediately after surgery with a continuous
passive motion machine for 48 hours. Following the discontinuation of
the axillary block, commence active motion. Use extension splinting at
night to minimize postoperative flexion contractures. A static
progressive flexion splint may be used intermittently during the day to
optimize postoperative elbow flexion.
Although preoperative pain is generally markedly reduced, many patients
have mild residual pain, particularly with heavier activities. Gains in
motion have been modest, on the order of 20° to 25°. The procedure
provides durable pain relief in spite of the tendency to redevelop
osteophytes at long-term follow-up (66).
 |
|
Table 102.2. Debridement Arthroplasty Results
|
formation, infection, ulnar neuropathy, and incomplete pain relief have
been reported following debridement arthroplasty. The most common cause
of persistent pain is incomplete osteophyte excision and advanced
articular degenerative changes. With careful patient selection and
meticulous operative
technique, patient satisfaction has been high with this procedure.
resurfacing are all terms that have been used to describe categories of
elbow arthroplasties. These terms are somewhat misleading because even
“unconstrained” implants have some intrinsic constraint by virtue of
the interlocking shape of their articular surfaces (39). A more simplified classification designates implants as linked or unlinked.
tissues as well as the intrinsic constraint of the implant to maintain
joint stability (40). The decision to use an
unlinked or a linked arthroplasty depends on the amount of bone
destruction, the status of the capsuloligamentous tissues of the elbow
joint, and the surgeon’s experience with these devices. Numerous
unlinked implants have been developed. The most popular designs in
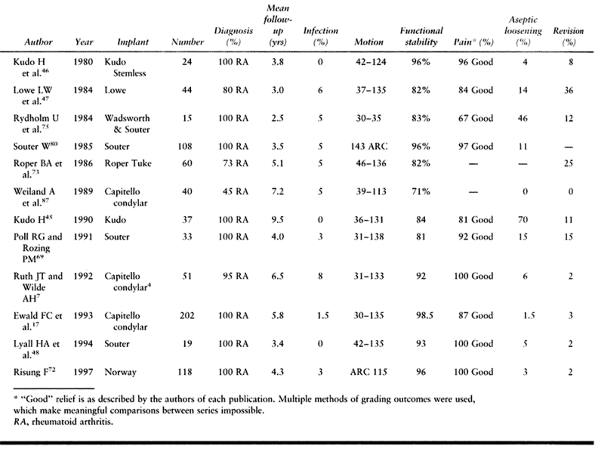
current use are the Capitellocondylar (Johnson & Johnson) (10,16,17,74,76,87), Kudo (44,45 and 46), Pritchard ERS (Depuy), Sorbie (Wright Medical) (79), and Souter (Howmedica) (48,54,69,75,80).
designed without stems on either the humeral or ulnar components. A
high incidence of mechanical loosening was reported with these early
designs (44,46,47,75);
however, improved results have been reported with newer stemmed
devices. At present, there is no consensus as to whether a radial head
replacement should be used in conjunction with the humeral and ulnar
components. Although there is a theoretical basis for a radial head
component from the perspective of load transfer, this has not been
confirmed by clinical experience (85).
elbow to optimize ligamentous balance and maintain joint stability.
Differences between implants include the type and length of
intramedullary fixation and the shape of the articular surfaces. For
example, the depth of the trochlear groove is much greater in the
Souter implant than the capitellocondylar design (Fig. 102.10).
The intrinsic constraint of these two devices is clearly different,
which may have implications with regard to the incidence of dislocation
and loosening. The more constrained the implant, the greater the
transfer of loads to the bone–cement interface and, hence, the greater
potential for loosening. Conversely, less constrained devices may have
a higher tendency to postoperative instability or dislocation.
 |
|
Figure 102.10. A: The humeral component of the Souter total elbow arthroplasty has a deep trochlear groove. B: The capitellocondylar design has a shallower trochlear groove with much less intrinsic constraint.
|
rheumatoid arthritis comprise the most common indication for total
elbow replacement. Joint space loss, with relatively well-preserved
bone stock and adequate capsuloligamentous tissues, is the main
indication for an unlinked design. Patients with advanced bone
destruction and joint instability are best treated with a linked
device, as is discussed later in this chapter. Patients with rheumatoid
arthritis who have less joint destruction may be candidates for
synovectomy (either open or arthroscopically), with or without radial
head excision as a means to defer arthroplasty. Other forms of
inflammatory arthritis, such as hemophilia, are also indications for
total elbow arthroplasty. Posttraumatic arthritis is an increasingly
common indication for replacement arthroplasty; however, careful
consideration should be given to patient age and functional demands
because failure rates have been higher in these patients. Patients with
periarticular nonunions and acute fractures generally require a linked
implant due to bone loss.
ligaments are the most frequent contraindications to unlinked
arthroplasty. Previous infection has been considered an absolute
contraindication to elbow arthroplasty. However, in selected patients,
replacement arthroplasty can be considered despite remote sepsis. A
staged debridement
of
the elbow that yields negative cultures should be performed before
proceeding with an elbow arthroplasty in the face of previous
infection. Elbow arthrodesis is considered a relative contraindication
for replacement arthroplasty. The conversion of an elbow fusion to an
arthroplasty is challenging, and the outcome is unpredictable. A linked
arthroplasty will usually be required due to bone loss and ligamentous
insufficiency. Paralysis of muscles about the joint or inadequate soft
tissues would be considered relative contraindications if they can not
be improved preoperatively.
One of the principles of treatment for most unlinked implants is to
preserve the medial collateral ligament. Accordingly, most surgical
approaches are through an extended Kocher approach (Fig. 102.12).
Sublux the elbow on the intact medial collateral ligament to achieve
adequate exposure for placement of the components. Management of the
ulnar nerve is controversial, but most surgeons recommend
identification and protection of the nerve with or without formal
transposition.
 |
|
Figure 102.11. Humeral, ulnar, and radial components of the Sorbie-Questor total elbow arthroplasty.
|
 |
|
Figure 102.12. Sorbie total elbow arthroplasty: A:
Through an extended Kocher approach, the lateral collateral ligament complex is identified and detached from the lateral epicondyle. B: The triceps is elevated off the olecranon from lateral to medial and the elbow is subluxated on the intact medial collateral ligament to achieve adequate exposure for placement of the components. C, D, E: After identification of the axis of rotation of the distal humerus appropriate bone cuts are made and the distal humerus is prepared. CAFE, central axis of rotation. F, G: By using a specialized ulnar cutting jig, the greater sigmoid notch of the ulna is prepared. (Redrawn from “The Sorbie-Questor Total Elbow System” brochure, with permission from Wright Medical Technology.) |
-
Make a midline posterior elbow incision just medial to the tip of the olecranon to avoid damage to cutaneous nerves (15). Identify and transpose the ulnar nerve into a subcutaneous pocket through a medial flap.
-
Make bone cuts and open medullary canals by using instruments specific for the implant to be done.
-
Before cementing the definitive
components, perform a trial reduction and ensure congruent tracking of
the arthroplasty. Flex and extend the elbow with the forearm held in
pronation to tension the medial collateral ligament and close the
lateral side of the elbow that has been destabilized by the surgical
exposure. If there is maltracking, reposition one or both of the
components. -
Perform a linked arthroplasty if there is
instability due to an incompetent medial collateral ligament or
persistent incongruity in spite of component repositioning. An unlinked
elbow arthroplasty that is unstable or maltracks intraoperatively
usually continues to function poorly postoperatively. -
Prepare the medullary canals with pulse
lavage, dry thoroughly, and insert antibiotic-laden cement retrograde
by using a cement gun. Pressurize the cement if the medullary canal is
adequately occluded with cement restrictors (18). -
Insert the components and reduce the
elbow. Hold the elbow at 90° flexion and full forearm pronation while
the cement sets. Elbow reduction allows the components to align with
one another and enhances implant tracking. -
Place drill holes through the lateral
epicondyle to accommodate sutures that reattach the lateral collateral
ligament complex. Close the fascia of the Kocher interval to augment
lateral elbow stability, as has been described by Cohen et al. (8). Reattach the triceps tendon to the olecranon using nonabsorbable sutures. Close the skin over suction drains. -
Evaluate elbow stability throughout
motion. Splint the elbow in a position of stability, generally 60° of
flexion, taking care to avoid any pressure over the posterior incision (Fig. 102.13).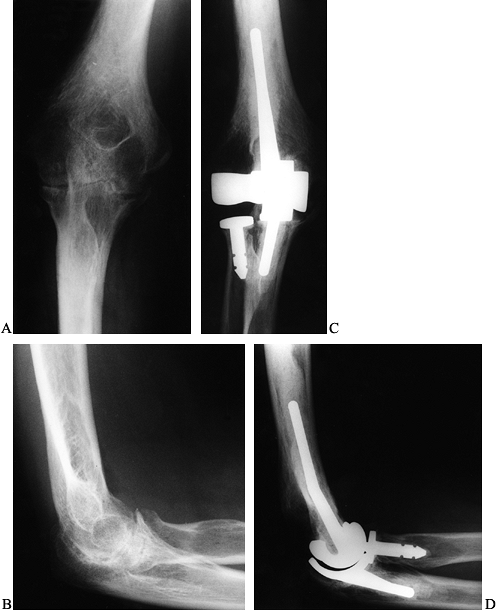 Figure 102.13. A:
Figure 102.13. A:
AP radiograph of a 44-year-old man with polyarticular rheumatoid
arthritis of 20 years’ duration. Note complete loss of joint space with
relative preservation of bone architecture. B: Lateral radiograph. C:
Postoperative anteroposterior radiograph following Sorbie total elbow
arthroplasty. The patient had complete relief of pain and restoration
of a functional arc of elbow motion. D: Lateral postoperative radiograph.
splint at 90°. Have the patient perform elbow flexion and extension
with the forearm fully pronated for 6 weeks to protect the lateral
ligament repair. Active assisted flexion and passive gravity-assisted
extension protect the triceps tendon repair. Have the patient avoid
extension past 30° for the first 3 to 4 weeks to guard against
posterior subluxation or, worse, dislocation of the elbow. Permit
forearm pronation and supination only with the elbow at 90° of flexion.
Apply a resting extension splint 4 to 6 weeks after surgery if the
patient is having difficulty achieving extension. Continue this
treatment for 12 weeks. Counsel patients not to lift more than 5 kg and
not to participate in any upper extremity impact sports (e.g., golf,
tennis) after total elbow arthroplasty.
The inventor of this implant has had excellent results, with only a
1.5% incidence of postoperative dislocation. However, other surgeons
have reported instability in up to 20% of patients (87). The mechanical loosening rate of this implant has been low in patients with rheumatoid arthritis.
 |
|
Table 102.3. Unlinked Arthroplasty Results
|
reported good functional results with the Souter total elbow
arthroplasty as well as a low incidence of loosening and instability.
elbow arthroplasty are infection and instability. Infection is
difficult to eliminate. Immediate surgical debridement is essential if
the implant is to be retained. Intravenous antibiotics followed by
suppressive antibiotics may allow retention of the implant. If the
infection is acute, either in the postoperative period or from a
hematogenous source, then consider debridement. Manage late, indolent
infections, or persistent sepsis in spite of debridement, by removal of
the implant and all cement. Reimplantation of an infected elbow
arthroplasty remains controversial; however, a staged revision
arthroplasty may be successful.
arthroplasty often requires revision to a linked design. Carefully
evaluate the radiographic position of the components before revision.
If one or both components are malpositioned, then consider
repositioning. If the component position is adequate, perform closed
reduction and casting to allow capsule and ligament healing. If the
elbow remains unstable after a period of immobilization and the
component position is acceptable, repair or reconstruction of one or
both of the collateral ligaments may stabilize the arthroplasty. If
stability cannot be achieved by component repositioning or ligament
reconstruction, consider revision to a linked arthroplasty.
surgery. If the ulnar nerve is not protected and transposed, it may be
compressed while the elbow is subluxed to place the components. The
ulnar nerve may be damaged by power instruments or cement leakage due
to its proximity to the olecranon.
may preclude the successful placement of an unlinked design. Consider
intraoperative conversion to a linked arthroplasty. Open reduction and
internal fixation of the columns can be performed in patients with good
bone quality and reconstructible fractures. Cortical perforation or
fracture of the humerus or ulna can occur due to the small diameter of
the medullary canals. Extravasation of cement with damage to neural
structures may occur. Careful repair of the triceps tendon to the
olecranon is required to prevent triceps disruption and postoperative
extension weakness.
release during surgery or a delay in postoperative rehabilitation.
Heterotopic ossification can occur, particularly in patients with
hypertrophic osteoarthritis. Wound healing problems are uncommon with
postoperative extension splinting and suction drains. Delayed wound
healing is occasionally seen in patients with rheumatoid arthritis,
particularly those on steroids. Treat minor areas of superficial dermal
necrosis by delayed mobilization and extension splinting. Consider
surgical debridement, with or without flap coverage, in patients with
full-thickness skin loss to avoid secondary infection.
rate (13,26,59,64).
The advent of improved loose-hinge linked designs has improved
survivorship equaling that of unlinked elbow arthroplasties at
medium-term follow-up. Biomechanical data suggest that muscle
activation about the elbow may allow semiconstrained linked
arthroplasties to function within the laxity of their loose hinge (65).
This limits loading of the bone–cement interface and may explain the
lower mechanical loosening rates relative to a fixed hinge. Linked
devices have a significant advantage over unlinked designs because
instability is uncommon. Commonly used linked devices include the
Morrey-Coonrad (Zimmer) (7,9,27,58,79), GSB (Gschwend-Scheier-Bahler) (Allopro) (28,29,30), and the Osteonics (43) prostheses.
arthroplasty from rheumatoid arthritis to posttraumatic arthritis, bone
loss due to tumors, nonunions, and fractures. Be careful when
considering total elbow arthroplasty in patients who do not have
rheumatoid arthritis. Those with high functional demands and single
joint disease will have a higher mechanical failure rate due to the
excessive loading they place on their implants. In addition, consider
age, vocation, and avocations of the patient. Some linked designs can
be employed with loss of the distal humerus up to the level of the
proximal aspect of the olecranon fossa and loss of the proximal ulna to
the level of the coronoid (58). Most linked
designs do not incorporate a radial head component, and therefore, the
presence of the proximal radius is not required.
have a higher risk of postoperative infection for any total elbow
arthroplasty, including linked designs. Do not consider
total
elbow arthroplasty for patients who cannot be expected to comply with
the postoperative restrictions. Such surgery requires a satisfactory
soft-tissue envelope, adequate muscle power, and a functional hand.
implant design. The author’s current preferred linked implant is the
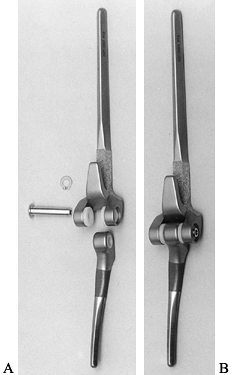
Morrey-Coonrad design (Coonrad III) (Zimmer, Warsaw IN) (Fig. 102.14).
 |
|
Figure 102.14. Morrey-Coonrad linked total elbow arthroplasty: A: Humeral component with beaded flange, ulnar component with polymethylmethacrylate (PMMA) precoat, axis pin, and locking clip. B: Appearance after assembly of the arthroplasty.
|
-
Position the patient supine with a
sterile tourniquet in place. Make a midline posterior elbow incision
centered just medial to the tip of the olecranon. Elevate a
full-thickness fasciocutaneous flap and transpose the ulnar nerve into
an anterior subcutaneous pocket. -
Use a Bryan-Morrey approach (57) for patients without significant distal humeral bone loss (Fig. 102.15).
Elevate the triceps off the olecranon from medial to lateral in
continuity with the anconeus to expose the distal humerus. This creates
a continuous sling of the extensor mechanism.![]() Figure 102.15. Triceps-reflecting Bryan-Morrey approach: A: A midline posterior elbow incision is used. B:
Figure 102.15. Triceps-reflecting Bryan-Morrey approach: A: A midline posterior elbow incision is used. B:
After identification and anterior transposition of the ulnar nerve, the
triceps is elevated off the olecranon from medial to lateral in
continuity with the anconeus to expose the distal humerus. -
Use a triceps-sparing approach (58,68) in elbows with deficient distal humeral columns (Fig. 102.16).
Work through either side of the triceps while preserving its attachment
to the olecranon. Take care to identify and protect the ulnar nerve.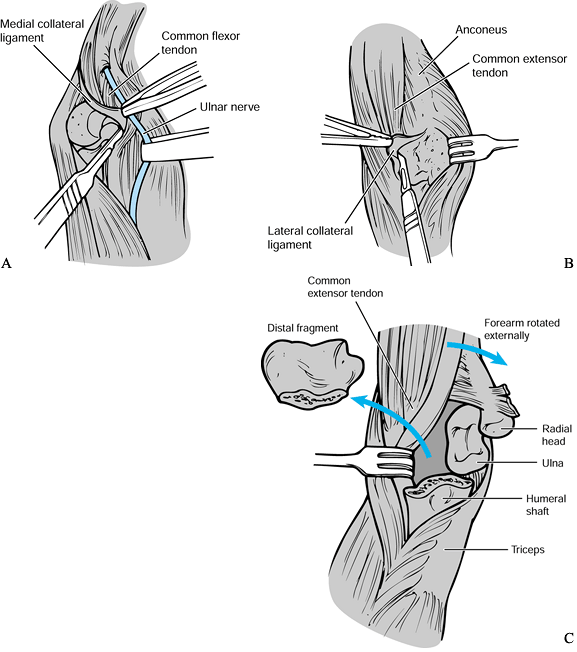 Figure 102.16. Triceps-sparing approach: A: The medial collateral ligament is divided. B: The lateral collateral ligament and lateral common extensor origins are released from the lateral epicondyle. C: The distal humerus and ulna are delivered through the lateral muscular interval.
Figure 102.16. Triceps-sparing approach: A: The medial collateral ligament is divided. B: The lateral collateral ligament and lateral common extensor origins are released from the lateral epicondyle. C: The distal humerus and ulna are delivered through the lateral muscular interval. -
Divide the humeral origins of the
collateral ligaments to dislocate the elbow and facilitate exposure of
the proximal ulna and distal humerus (Fig. 102.17).
Excise the tip of the olecranon and coronoid to avoid restricting
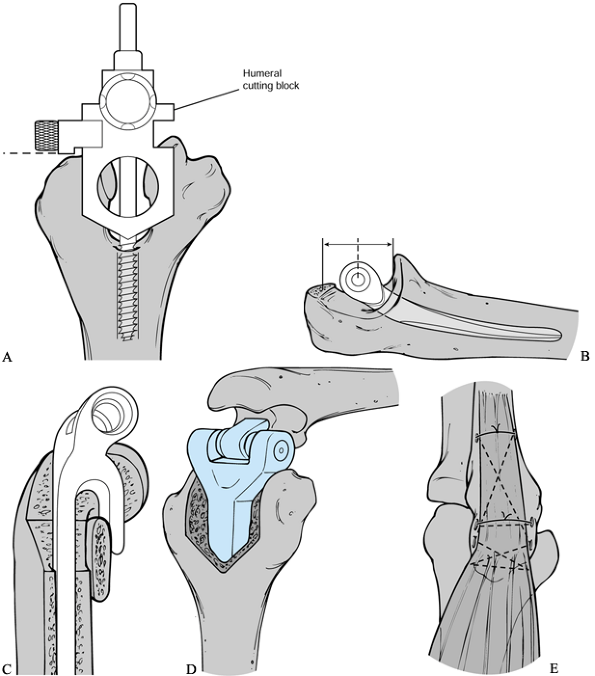
postoperative elbow motion by abutting the humeral component.![]() Figure 102.17. Morrey-Coonrad total elbow arthroplasty surgical technique: A:
Figure 102.17. Morrey-Coonrad total elbow arthroplasty surgical technique: A:
After obtaining exposure of the distal humerus, the intercondylar area
is opened and an alignment stem is placed into the humeral canal. A
humeral cutting block sits on the capitellum, and appropriate saw cuts
are made. B: The ulnar canal is opened
with a burr, and the tip of the olecranon is excised. Ulnar rasps are
used, and a trial placement of the components and reduction of the
joint is performed. C: Cement is injected, and the components are inserted. A bone graft is placed behind the humeral flange. D: The components are connected with an axis pin and locking clip. E:
The triceps is reattached to the olecranon with nonabsorbable sutures
through drill holes. (Modified from “Coonrad/Morrey Total Elbow:
Surgical Technique” brochure, with permission from Zimmer Corp.) -
Open the humeral canal in the midline at
the level of the olecranon fossa and place the humeral cutting block
over the intramedullary guide. Excise the intercondylar portion of the
distal humerus using a microsagittal saw. Prepare the humeral canal
with appropriate-sized rasps and place a trial humeral component. Use a
humeral component with a 100 cm stem for most patients; however, a 150
cm component can be used for those with deficient distal humeral bone
stock or severe osteopenia. Use a 100 cm stem if there is shoulder
disease that may necessitate the need for a shoulder arthroplasty in
the future. -
Excise the radial head if it is diseased.
Open the medullary canal of the ulna with a power burr and widen with
ulnar rasps. Insert trial components and articulate the implant. Ensure
that an adequate range of motion is achieved without impingement of the
olecranon, coronoid, or radial head on the implant. -
Plug the humeral and ulnar canals with cement restrictors. Lavage and dry the canals and inject antibiotic-laden
P.2685P.2686
cement retrograde using a cement gun. Cement the ulnar component first
and allow the cement to cure. Inject the humeral canal and insert the
component. Place an autogenous local cancellous bone graft beneath the
anterior flange of the humeral component. Articulate the components
using an axis pin before fully seating the humeral component. -
Hold the elbow in extension until the
cement sets. Reattach the triceps to the olecranon with sutures through
drill holes. Ensure that the ulnar nerve is secured anteriorly in a
subcutaneous pocket. Close the wound over suction drains and splint the
elbow in extension with anterior plaster slabs (Fig. 102.18).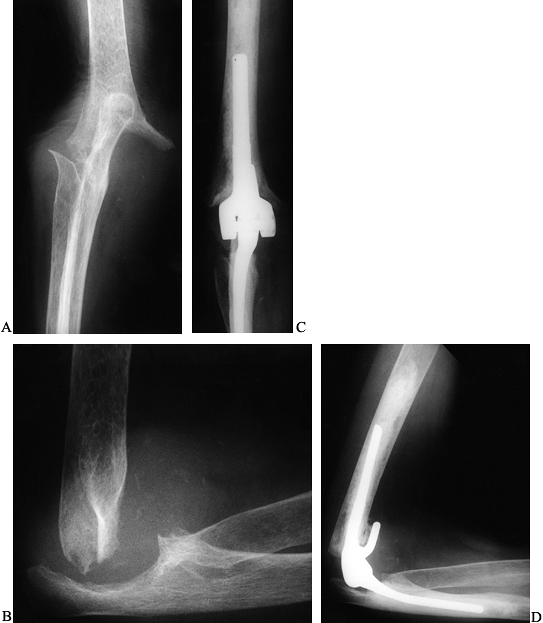 Figure 102.18. A:
Figure 102.18. A:
AP radiograph of a 68-year-old woman with a 30-year history of
polyarticular rheumatoid arthritis. Note the advanced bone loss and
articular destruction. The patient presented with a painful unstable
elbow. B: Lateral radiograph. C: Postoperative AP radiograph following a Morrey-Coonrad arthroplasty through a triceps-sparing approach. D: Lateral radiograph.
48 hours postoperatively. Have the patient perform gravity-assisted
extension exercises to protect the triceps repair for a 6-week period.
If a triceps-sparing approach was used, active assisted extension
exercises can be initiated immediately. Have the patient use a night
extension splint for 12 weeks to optimize elbow extension and a 90°
daytime resting splint for 6 weeks. Long-term restrictions are
identical to those recommended for an unlinked arthroplasty.
 |
|
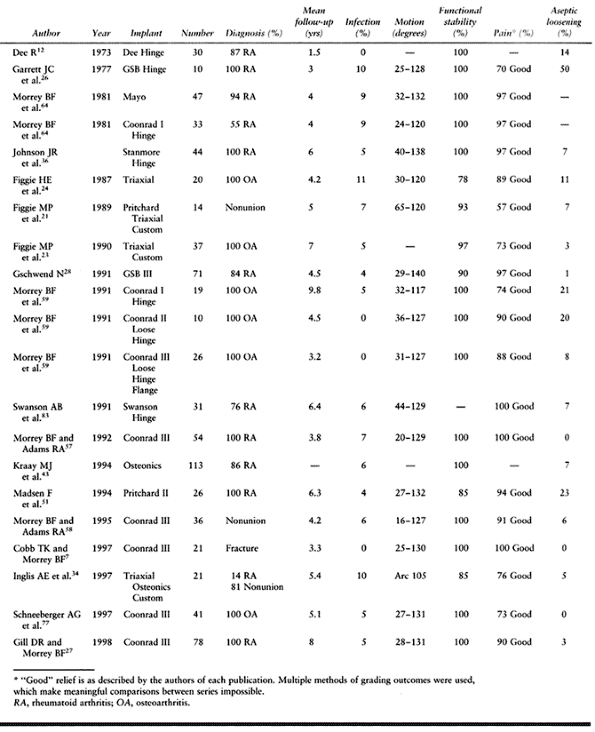
Table 102.4. Linked Arthroplasty Results
|
A semiconstrained metallic polyethylene bushing was added, and
excellent clinical results have been reported with this modified
implant (30). Although the loosening rate of this implant has been low, disassembly of the linkage mechanism has occasionally occurred.
The major difference has been that elbow instability is uncommon, given
appropriate intraoperative coupling of the implant. Failure of the
linkage mechanism requiring revision has been reported with a number of
available linked devices. This has been a particular problem for the
Triaxial device (52), but failure of the linkage bearings has also been reported in patients with Morrey-Coonrad prostheses (77).
The mechanical loosening rate of these devices in younger patients with
posttraumatic arthritis is of concern at short- to medium-term
follow-up (77). Osteolysis has been seen in
some patients. Fracture of the ulnar component has occurred with the
Morrey-Coonrad device in patients after falls or heavy lifting (27).
design and implantation, the increasing numbers of implants being
performed, as well as the tendency to extend the indications to
younger, more active patients have resulted in failures requiring
revision.
arthroplasty is mechanical loosening. This may be asymptomatic or
present with an insidious onset of pain. Occasionally, loosening
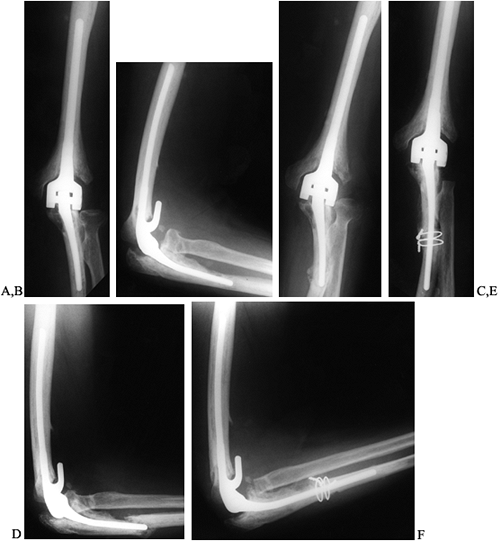
presents as an acute fracture due to cortical thinning from osteolysis (Fig. 102.19).
Patients with progressive osteolysis, even in the absence of
significant symptoms, should be considered for revision before the
development of extensive bone loss (Fig. 102.20).
 |
|
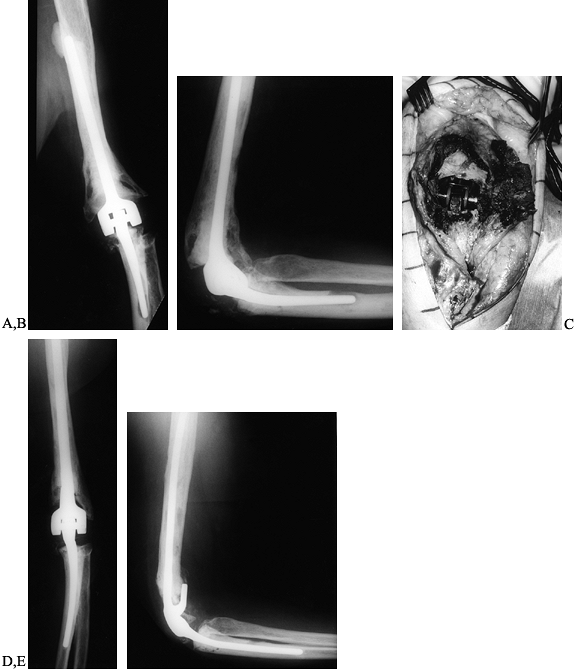
Figure 102.19. A:
AP radiograph of a well-functioning Morrey-Coonrad total elbow arthroplasty 4 years following the index procedure for posttraumatic arthritis. B: Note the osteolysis evident at the tip of the ulnar component. C: AP radiograph following a pathologic fracture of the ulna at the site of the aforementioned osteolysis 1 year later. D: Lateral radiograph. E: AP radiograph following a revision of the ulnar component. Note the cement leakage evident at the tip of the ulnar component through a cortical perforation. F: Lateral radiograph. |
 |
|
Figure 102.20. A:
AP radiograph of a 67-year-old woman 10 years after revision total elbow arthroplasty for posttraumatic arthritis. The patient has mild aching pain in the elbow that is worse following activities. Cement leakage is evident at the tip of the humeral component. B: Lateral radiograph. Note the radiolucency around the ulnar component and the osteolysis evident at the tip. C: Intraoperative photograph of titanium synovitis from a loose total elbow arthroplasty. D: AP radiograph following revision to a long-stem cemented Morrey-Coonrad total elbow arthroplasty. This gave the patient complete pain relief and return of function. E: Lateral radiograph demonstrating autogenous bone graft behind the flange of the humeral component. |
Removal of well fixed, cemented implants often results in bone loss,
which complicates the revision. Component failures from polyethylene
wear of the coupling mechanisms may occur, particularly in younger,
more active patients. Component fracture is occasionally seen and
requires revision of the fractured component (Fig. 102.22).
An infected total elbow arthroplasty that has not responded to
debridement requires removal and conversion to an excisional
arthroplasty (59,62). Delayed reimplantation has been reported and may be an option for selected patients (89).
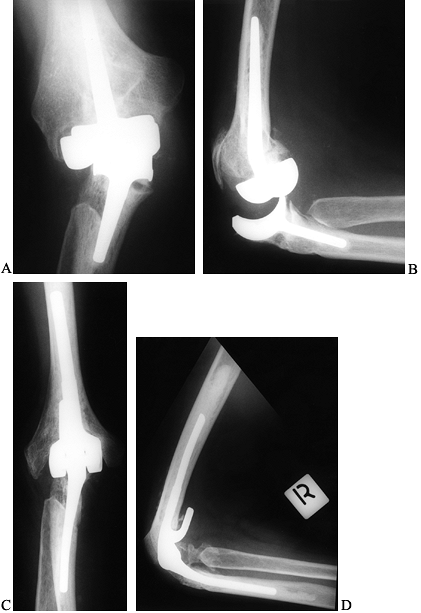 |
|
Figure 102.21. A: AP radiograph of a 51-year-old woman with longstanding rheumatoid arthritis 18 months after capitellocondylar arthroplasty. B: Lateral radiograph showing persistent joint subluxation. C: Postoperative AP radiograph following revision to a linked arthroplasty. D: Lateral radiograph with pain-free elbow at 4-year follow-up.
|
 |
|
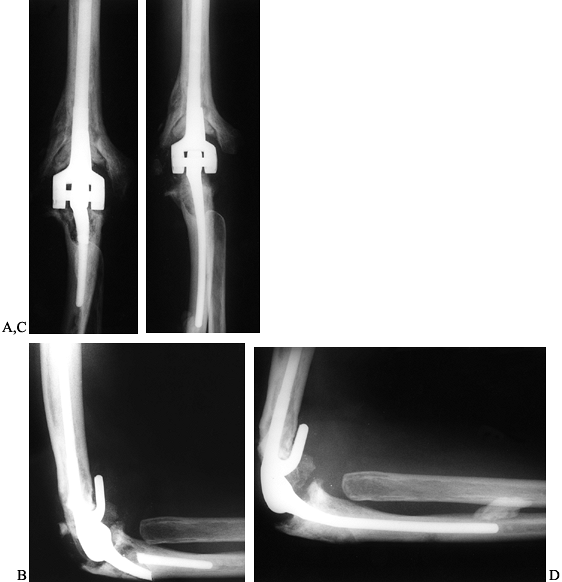
Figure 102.22. A:
AP radiograph of a 32-year-old man 3 years after total elbow arthroplasty for posttraumatic arthritis. The patient developed pain and a sense of instability of the elbow following lifting a heavy object. B: Note the fracture that has occurred through the beaded portion of the ulnar component. In newer versions of this implant, these beads have been removed to avoid the stress riser effect of their application. C: AP radiograph following revision of the ulnar component with impaction grafting of the proximal ulna using autogenous iliac crest bone graft. D: Lateral radiograph demonstrating some cement leakage from a cortical perforation in the ulna. |
with recent or remote history of infection, as outlined earlier.
Consider coverage procedures before proceeding with revision
arthroplasty for patients with a poor soft-tissue envelope. Consider
excisional arthroplasty (13) or allograft or total elbow arthroplasty (TEA) composite reconstruction (11,25) for patients with inadequate bone stock.
procedures due to bone loss, which occurs frequently with implant
loosening and extraction (38). Exceptions to
this would be a stable, unlinked design with aseptic loosening of one
or both components, which can be successfully revised while preserving
the soft tissues and bone (27).
stemmed implants. Standard linked designs can typically be used with or
without composite allograft reconstruction, even in the most difficult
revision cases (38). Other surgeons have reported using custom designed revision implants with good results at short-term follow-up (20,21,24).
Ultrasonic cement removal devices have simplified the removal of cement
from the small medullary canals. Use a sterile tourniquet to facilitate
extended surgical exposure as needed.
-
Use previous skin incisions, if possible,
to avoid flap necrosis. Identify and protect the ulnar nerve, even if
it has previously been transposed. -
Use a triceps-sparing approach, if
possible, to avoid detachment of the triceps from the olecranon, which
is often atrophic from previous surgery. Use a Bryan-Morrey or extended
Kocher approach with elevation of the triceps from the olecranon if the
patient has preserved distal humeral bone. -
Extract loose implants and remove all
loose cement from the canals by using standard instruments or an
ultrasound device, as indicated. Consider using a cortical window for
retrograde removal of well-fixed implants. Leave well-fixed cement that
does not interfere with component placement rather than risk cortical
perforations and fractures. Remove all cement in cases of infection by
using a cortical window in the humerus or ulna, or both. -
Expose the radial nerve in patients
undergoing cement removal from the humerus because damage has been
reported from power instruments and cement leakage through a cortical
perforation at the time of component reinsertion (38). -
Consider the ulnar bow toward the radius,
which predisposes the ulna to cortical perforation at the time of
cement removal with straight-stemmed revision instruments. Expose the
subcutaneous border of the ulna to guide cement removal. -
Place the trial implants and assess the
need for cancellous or structural autograft or allograft bone. Use
antibiotic-impregnated cement, taking care to avoid cement leakage from
cortical perforations and consequent damage to nerve structures.
Perform a meticulous soft-tissue closure over suction drains and splint
the elbow in full extension.
motion may be indicated. Immobilize patients with tenuous soft coverage
in an anterior splint with the arm in the extended position for as long
a period as necessary to ensure adequate soft-tissue healing.
Immobilization of up to 3 weeks allows restoration of a functional arc
of motion while avoiding problems from wound slough. The therapy
program generally follows that of a primary elbow arthroplasty but may
need to be adjusted in patients with significant bone loss who have had
structural allografts. If the triceps tendon is left in continuity,
both active assisted flexion and extension can begin in the early
postoperative period.
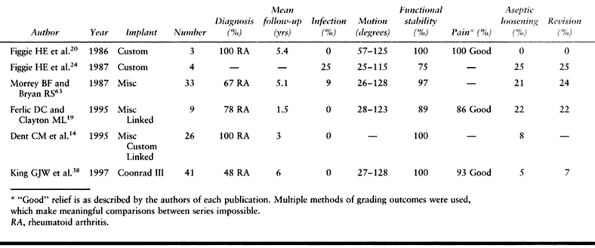
demonstrate functional results and survivorship rates approaching those
of primary elbow replacement at short- to medium-term follow-up (Table 102.5) (14,19,21,24,38,63).
Patients with posttraumatic arthritis have had a higher failure rate
than those with rheumatoid arthritis, similar to those undergoing
primary elbow replacement (38).
The complication rate has been higher with revision total elbow
arthroplasty, even in experienced hands, indicating that revision total
elbow arthroplasty should be performed only by those with extensive
experience with primary elbow arthroplasty.
 |
|
Table 102.5. Revision Arthroplasty Results
|
due to the prolonged operative times and the extent of surgical
exposures. Intraoperative fractures frequently occur with component or
cement removal. Cortical perforation of the humerus has resulted in
injury to the radial nerve from cement removal devices and from cement
leakage at the time of arthroplasty replacement. Nonunion of allografts
has been a problem, particularly when they are placed in a subcutaneous
location. Further experience with these grafts is required. Aseptic
loosening and failure of the articulating bearings have been reported
at longer term follow-up.
indicated to relieve pain and restore stability in situations for which
other reconstructive techniques are contraindicated. A successful
fusion generally provides good pain relief; however, the loss of motion
significantly impairs the patient’s functional abilities (60,67) and therefore even a successful arthrodesis renders only a fair outcome.
fused at 90° of flexion; however, because there is no truly good
position for a stiff elbow, a trial of immobilization in different
positions is recommended to allow the patient to participate in the
decision. Generally, an arthrodesis at approximately 45° of flexion
provides the best appearance and function for activities such as
writing and bimanual tasks. This however, compromises the patient’s
potential for self-care. A 90° or 110° position allows the use of the
arm for eating, but prevents many extrapersonal activities and care of
the perineum.
posttraumatic unilateral arthritis of the elbow in a young, healthy,
active patient. These are patients who are not candidates for or who
will not tolerate the limitations imposed by an interposition or
replacement arthroplasty. Patients with postinfectious arthritis,
tuberculous arthritis, or chronic osteomyelitis may also be candidates
for elbow arthrodesis. Arthrodesis is occasionally used as an option
for patients with juvenile rheumatoid arthritis; however, interposition
arthroplasty or total joint replacements are preferred because of the
already impaired function of their adjacent joints. Elbow arthrodeses
have been attempted following failed total elbow arthroplasty, but
fusions in these circumstances are very difficult to achieve and
fraught with complications.
the spine, shoulder, wrist, or hand, are poor candidates for elbow
arthrodesis because they cannot compensate for the loss of elbow
motion. Bilateral elbow disease is a relative contraindication.
and combined internal and external fixation. Plate fixation is
preferred in patients undergoing arthrodesis without active sepsis.
-
Use a triceps-sparing posterior approach. Transfer of the ulnar nerve is usually required
-
Apply a 10- to 12-hole dynamic
compression plate with eight cortices of fixation above and below the
elbow that is contoured to the posterior aspect of the distal humerus
and proximal ulna. A tensioning device facilitates compression of the
arthrodesis. -
Fashion the bone ends to maximize bone
apposition and to fit the needs of the position selected for fusion.
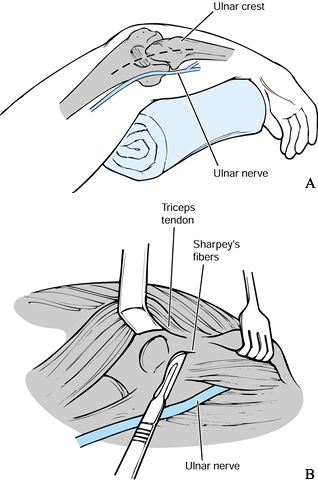
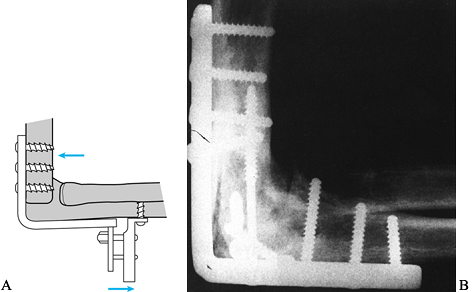
Use an autogenous iliac crest bone graft to promote fusion (Fig. 102.23).![]() Figure 102.23. Elbow arthrodesis–plate fixation: A: Dynamic compression plate and tensioning device. B:
Figure 102.23. Elbow arthrodesis–plate fixation: A: Dynamic compression plate and tensioning device. B:
Radiographic appearance of a successful arthrodesis. (From McAuliffe
JA, Burkhalter WE, Ouellette EA, Carneiro RS. Compression Plate
Arthrodesis of the Elbow. J Bone Joint Surg 1992;74:300, with permission.) -
When there is evidence of ongoing or
recent infection, perform a staged radical debridement with the removal
of all internal fixation from the elbow. Treat patients with active
sepsis initially with an external fixator using a triangulated lateral
half-frame to achieve stabilization of the elbow while allowing
adequate debridement and soft-tissue coverage (Fig. 102.24).
Following debridement, perform delayed cancellous bone grafting to
assist in achieving union. External fixation can be used as a
definitive technique to achieve arthrodesis or subsequently
P.2695
changed to internal fixation by means of a plate.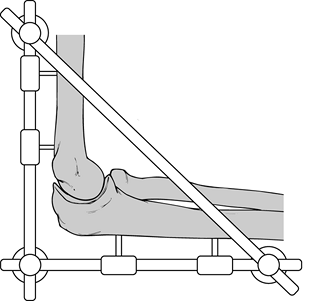 Figure 102.24. Elbow arthrodesis–external fixation.
Figure 102.24. Elbow arthrodesis–external fixation.
occurs. Take care to ensure hand, wrist, and shoulder motion are
preserved. It is important to maintain rotation of the forearm because
the radius is not usually involved in the fusion except under
exceptional circumstances.
Functional outcome studies have yet to be reported on patients with
elbow arthrodesis due to the current infrequency of this procedure. In
the series by McAuliff et al. (49), successful fusion was achieved in all but one patient despite a lack of postoperative immobilization.
 |
|
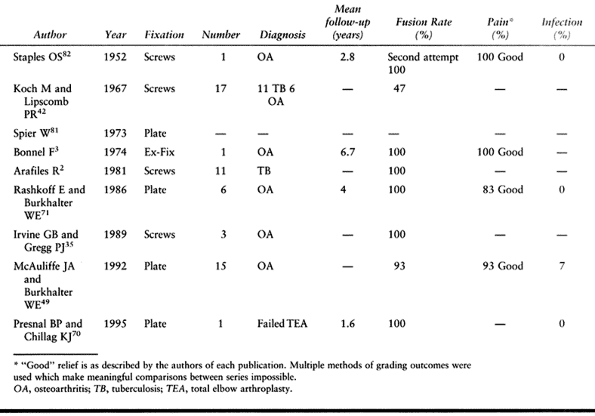
Table 102.6. Arthrodesis Results
|
patients undergoing elbow arthrodesis. The current use of more reliable
methods of internal fixation and autogenous cancellous bone has
improved the frequency of fusion. Nonunions still occur. Careful
debridement of dead and devitalized tissue, as well as control of
infection, are critical to achieve union of an elbow fusion.
Soft-tissue coverage is a concern with placement of posterior bulky
hardware. Late fractures have been reported through a solid arthrodesis
as well as adjacent to internal fixation from a stress riser effect.
Avoid removal of internal fixation from an elbow fusion. However, if
symptoms from hardware persist, waiting at least 18 months
postoperatively
will
allow cortical remodeling and may decrease the likelihood of a
fracture. Give appropriate protection to support the arm following
hardware removal in these patients.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study. (Note: For the
references below, page number precedes volume number.).
HE, Inglis AE, Ranawat CS, Rosenberg GM. Results of Total Elbow
Arthroplasty as a Salvage Procedure for Failed Elbow Reconstructive
Operations. Clin Orthop 1987;185:219.
MP, Inglis AE, Mow CS, Figgie III HE. Salvage of Non-Union of
Supracondylar Fracture of the Humerus by Total Elbow Arthroplasty. J Bone Joint Surg 1989;1058:74A.
JC, Ewald FC, Thomas WH, Sledge CB. Loosening Associated with G.S.B.
Hinge Total Elbow Replacement in Patients with Rheumatoid Arthritis. Clin Orthop 1977;170:127.
DRJ, Morrey BF. The Coonrad-Morrey Total Elbow Arthroplasty in Patients
Who Have Rheumatoid Arthritis. A Ten to Fifteen-Year Follow-Up Study. J Bone Joint Surg 1998;1327:80-A.
N, Loehr J, Ivosevic-Radovanovic D, et al. Semiconstrained Elbow
Prostheses with Special Reference to the GSB II Prosthesis. Clin Orthop 1988;104:232.
R, Pisan M, Lambert S, Ballmer F. Operative Management of the Stiff
Elbow: Sequential Arthrolysis Based on a Transhumeral Approach. J Shoulder Elbow Surg 1997;82:6.
H, Iwano K. Total Elbow Arthroplasty with a Non-Constrained
Surface-replacement Prosthesis in Patients Who Have Rheumatoid
Arthritis: A Long-Term Follow-Up Study. J Bone Joint Surg 1990;355:72A.
HA, Cohen B, Clatworthy M, Constant CR. Results of the
Souter-Strathclyde Total Elbow Arthroplasty in Patients with Rheumatoid
Arthritis. J Arthroplasty 1994;279:9.
EH, Ewald FC, Thornhill TS. Results of Total Elbow Arthroplasty after
Excision of the Radial Head and Synovectomy in Patients Who Had
Rheumatoid Arthritis. J Bone Joint Surg 1996;1541:78A.
AG, Adams R, Morrey BF. Semicon- strained Total Elbow Replacement for
the Treatment of Post-Traumatic Osteoarthritis. J Bone Joint Surg 1997;1211:79A.
E, Vella M, Ewald FC. Radial Head Replacement in Capitellocondylar
Total Elbow Arthroplasty: 2 to 6 year Follow-up Evaluation in
Rheumatoid Arthritis. J Arthroplasty 1991;67:6.