Clavicular Fractures: Open Reduction Internal Fixation
The majority of clavicular fractures can be treated by nonoperative
methods, which have been traditionally thought to have fewer
complications than surgery. This reasoning probably has prevailed
because internal fixation methods are typically reserved for severe
fractures.
arm sling or a figure-of-eight harness. Until recently, nonoperative
treatment of clavicular fractures was thought to be associated with
high rates of union and a low probability of complications. The
deformity that often accompanies conservative care of these fractures
was thought to be of cosmetic concern only and that full function was
typically observed after healing (2). However,
many of the reports regarding clavicular fractures have included data
from children and adolescents who have the capability for rapid healing
and remodeling of residual deformities (2,3).
Complication rates following displaced clavicular fractures in adults
and elderly patients show that outcomes in these patients are much less
satisfactory than for the young. Nonunion or malunion of a clavicular
fracture can lead to a variety of undesirable outcomes including pain,
deformity, weakness, neurovascular symptoms, and decreased function (4).
The increasing recognition of adverse outcomes following fracture has
led to a renewed interest in internal fixation of displaced clavicular
fractures in adults.
biomechanics of the clavicle and shoulder is essential if rational
treatment is to be provided. The clavicle has an S-shaped
configuration, and when viewed from medial to lateral, it has an
anterior convex to concave curvature. The medial portion of the
clavicle is cylindrical, but the lateral portion is relatively flat.
The typical medullary canal is very small due to the thick cortical
bone that surrounds it. Medially, the clavicle is held in position by
three very strong ligaments: the sternoclavicular, costoclavicular, and
interclavicular ligaments. On the lateral end, the acromioclavicular
and the two coracoclavicular ligaments, the conoid and the trapezoid,
serve to anchor the clavicle to the scapula. The osseous surface of the
clavicle serves as the origin for many of the important muscles of the
upper limb including the platysma, sternocleidomastoid, pectoralis
major, subclavius, deltoid, and trapezius. Furthermore, the clavicle
shields important underlying neurovascular structures such as the
brachial plexus
and
the subclavian vessels. It also holds the distinction of being the only
bone that connects the upper limb to the axial skeleton.
Biomechanically, the clavicle acts as a strut that holds the shoulder
girdle away from the thorax. Surgical excision of part or the entire
clavicle results in decreased strength and stability of the shoulder
girdle, especially when the patient is reaching across his or her body (5).
describe the wide variety of clavicular fractures. The most commonly
used system was proposed by Allman (6) and is
used to divide fractures into three basic categories: group I, middle
third fractures; group II, lateral third fractures; group III, medial
third fractures. Neer (7,8)
subdivided group II fractures into separate subgroups based on the
extent of the associated ligamentous injury: In type I fractures, the
coracoclavicular ligaments remain intact; in type II fractures,
disruption of the coracoclavicular ligaments is associated with a
corresponding upward displacement of the medial fragment; type III
fractures involve the articular surface of the acromioclavicular joint.
However, most physicians base treatment on the direction and degree of
fragment displacement.
This correlates with biomechanical studies that have shown that the
weakest point of the clavicle lies at the transition region between the
curves where the bone is found to be thinnest and lacks any muscular or
ligamentous support (9). Of the remaining fractures, 15% occur in the distal third and less than 5% involve the medial third of the clavicle.
clavicular fractures with the peaks found in data obtained from people
in the second/third and sixth/seventh decades of life (1,10).
This specific pattern can be explained if the mechanism of injury is
taken into consideration. Most of the fractures occurring in the second
and third decades of life are found in males, and they usually result
from violent or high-energy injuries (e.g., bicycle and motor vehicle
accidents as well as sports injuries). In these cases, direct trauma to
the point of the shoulder causes the compressed clavicle to fail (9).
For patients over 60 years, the majority of clavicular fractures is in
osteoporotic bones and is the result of simple falls from a standing
height onto an outstretched hand.
for most clavicular fractures, surgery is indicated in certain
circumstances. In these particular situations, operative fixation is
thought to yield the best clinical results in terms of alignment,
union, and early mobilization. The main indication for internal
fixation of a clavicular fracture is displacement and/or shortening
greater than 15 to 20 mm in young, healthy, active individuals (11).
Although the clavicle has good healing and remodeling capabilities,
significantly displaced fractures have been shown to cause pain and
decreased patient satisfaction due to cosmetic deformity and functional
limitations (11). Relative indications for internal fixation of clavicular fractures include the following:
-
open fractures;
-
associated vascular injury;
-
progressive neurological deficits;
-
gross displacement with skin tenting that will likely lead to skin breakdown;
-
significant medialization of the shoulder girdle;
-
torn coracoclavicular ligaments with distal fracture;
-
ipsilateral fractures of the clavicle and scapula (floating shoulder);
-
multiply injured patients;
-
bilateral clavicular fractures; and
-
complex, ipsilateral, upper-extremity fracture.
and physical examination should be performed. Specific information
should be obtained regarding the mechanism of the injury, the degree or
magnitude of pain, paresthesia, or loss of function. A proper physical
examination should include a thorough inspection of the injured
shoulder for signs of swelling, ecchymosis, deformity, skin tenting, or
compromise (Fig. 1.1). Shoulder asymmetry may
or may not be detected when comparing the injured and contralateral
sides. Typically, a displaced clavicular fracture can be diagnosed by
observation and be based on clinical deformity. Palpation along the
entire length of the clavicle is very accurate as the fracture site is
very tender and a step-off deformity can often be appreciated.
acromioclavicular joints as well as the scapula and shoulder joint for
focal areas of tenderness that may reveal associated injuries. Due to
the close proximity of numerous other important structures, the
physical examination should include a careful assessment of
neurovascular or lung pathology. Brachial plexus injuries, while rare,
can occur in conjunction with clavicular fractures (13,14)
and can be detected through detailed neurological testing in the upper
limb. The upper extremity must also be assessed for evidence of
vascular compromise by comparing the temperature, color, peripheral
pulses, and blood pressure of the injured limb to those characteristics
of the contralateral extremity. If the comparison yields no
differences, then vascular damage did not occur. An angiogram should be
obtained if the physician suspects vascular injury associated with the
clavicular fracture. In patients with high-energy injuries, physical
examination of the lung fields and a chest x-ray should be performed
because pneumothorax occurs in 3% of patients (2).
confirmed with a radiograph of the injured shoulder. Usually, a
standard anteroposterior (AP) view of the clavicle is enough to
establish the definitive diagnosis (Fig. 1.2).
It is important that the AP radiograph includes the sternoclavicular
and acromioclavicular joints so that any disruption or fracture of
these joints can be ruled out. An apical oblique view, with the x-ray
beam angled 20 to 60 degrees cephalad, minimizes the interference of
the thoracic cage and improves visualization of the clavicle (Fig. 1.3) (15).
To better analyze the integrity of the coracoclavicular ligaments in
clavicular fractures of the lateral third, physicians should view the
effect of weight-bearing radiograph (4.5 kg) (3).
 |
|
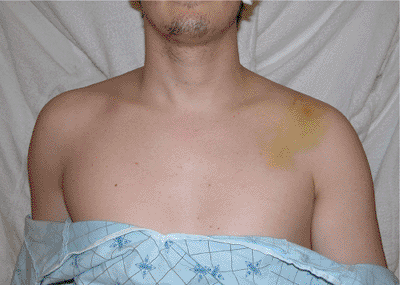
Figure 1.1. Clinical photograph showing swelling and ecchymosis over the left clavicle.
|
 |
|
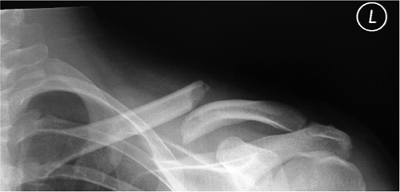
Figure 1.2. Preoperative AP radiograph demonstrating a midclavicular fracture with shortening and overriding of the fracture.
|
special attention if any posterior displacement or intra-articular
extension is found. Because it will provide the optimal visualization
of the fracture and sternoclavicular joint, a computed tomography (CT)
scan is often helpful when a complex, medial, clavicular fracture is
present.
 |
|
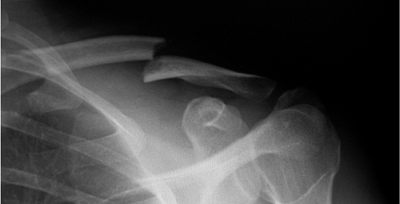
Figure 1.3. An apical oblique view helps minimize interference of the thoracic structures when viewing the fracture.
|
characteristics is essential to any orthopedic surgical procedure.
Although the emphasis of this chapter is on plate fixation of acute
clavicular fractures, other surgical procedures, with varying results,
have been described. Intramedullary pins or nails are a viable
treatment alternative because they allow for limited exposure and
soft-tissue disruption. However, numerous case reports have been cited
regarding pin migration out of the bone and into the lung (16), ascending aorta (17), pulmonary artery (18), abdominal aorta (19), and even the spinal canal (20).
Although rare, the potential for migration still exists, even when
precautionary measures such as using threaded pins or bending the pin
at the end, are used.
option for clavicular fractures than pin fixation for several reasons.
First and foremost, intramedullary fixation fails to provide rotational
control at the fracture site. Plate fixation controls both length and
rotation as well as providing compression in length stable fracture
patterns. The stable construct obtained after plate fixation allows for
early use of the upper extremity, unlike the postoperative
immobilization that is often required after intramedullary pin fixation.
anesthesia. Some surgeons advocate a regional nerve block in
combination with general anesthesia for postoperative pain control. The
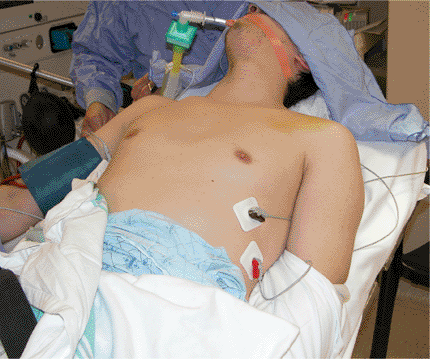
patient is placed in a beach-chair (semisitting) position with a small
pad behind the shoulder blade and the involved upper extremity tucked
into the side (Fig. 1.4) and square draped (Fig. 1.5).
We have found that in typical circumstances the arm need not be free
draped to obtain or maintain a fracture reduction. The opposite iliac
crest is square draped if autologous bone grafting will be required in
patients with severely comminuted fractures.
An oblique incision is made along the superior surface of the clavicle.
The skin and subcutaneous tissue are raised as a flap, protecting any
obvious cutaneous nerve branches, and reflected upward allowing the
underlying myofascia to be identified (Fig. 1.7).
This layer, including the deltopectoral muscle attachment, is raised as
contiguous flaps and is preserved so that a two-layered closure can be
achieved over the plate (21).
 |
|
Figure 1.4. The patient is placed in the beach-chair position with a small pad behind the involved shoulder. The arm is draped at the side.
|
 |
|
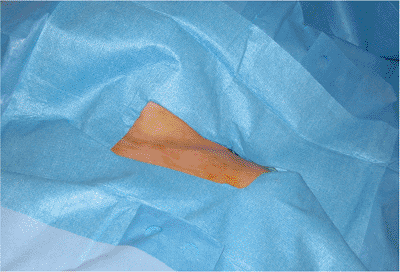
Figure 1.5. Placement of a sterile covering over the patient with an appropriate sized opening to address the clavicle fracture.
|
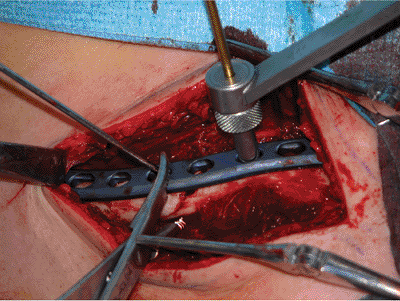
fixed with a 2.0-mm Kirschner wire aimed perpendicular to the fracture
line (Fig. 1.11). A 3.5-mm limited-contact
dynamic-compression (LCDC) plate with a minimum of 6 holes (10-hole
maximum) is applied superiorly and held in position with a reduction
clamp (Fig. 1.12). The first screw is inserted
in a hole on the side of the fracture site opposite where the K wire
and the reduction clamp are positioned (Fig. 1.13).
Extreme care should be taken in drilling and placing the screws to
avoid injury to the subclavian structures and the lung. It is generally
advisable to place a protective instrument along the inferior surface
of the clavicle to prevent the drill from inadvertently damaging any
vital structures. A minimum of three screws should be placed on either
side of the fracture such that purchase is achieved through all six
cortices of bone.
 |
|
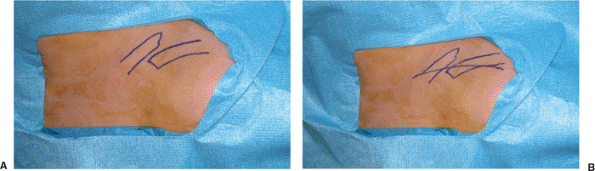
Figure 1.6. A. The proximal and distal ends of the fracture are marked on the skin. B. The skin incision is then performed between these two points.
|
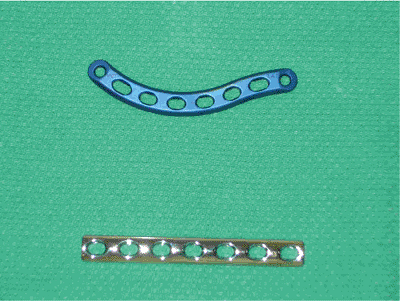
S-shaped clavicle than it is to contour a standard compression plate,
as has been previously described (22), and the
LCDC plate is stronger than a similarly sized pelvic-reconstruction
plate. More recently, we have used anatomic plates precontoured to fit
the clavicle. Use of precontoured plates saves time, as extensive
intraoperative contouring is not required, and their low-profile
anatomic shape minimizes prominence, especially medially where a
straight plate tends to project anteriorly (Fig. 1.14).
Because the most common fracture pattern for midshaft clavicular
fractures is transverse, the plate is applied in the compression mode
to maximize compression.
 |
|
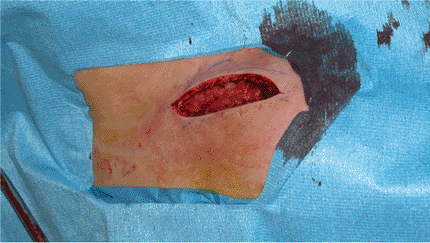
Figure 1.7.
The subcutaneous tissues are incised, revealing the myofascial layer underneath, which is dissected as a contiguous flap superiorly and inferiorly and preserved for later closure. |
 |
|
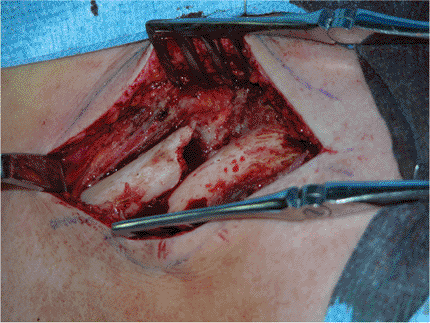
Figure 1.8. The fracture site is exposed.
|
 |
|
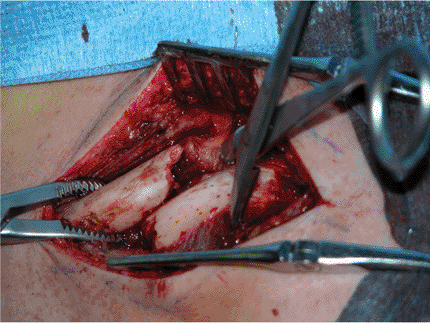
Figure 1.9. The proximal and distal fragments are mobilized with small-fragment reduction forceps.
|
 |
|
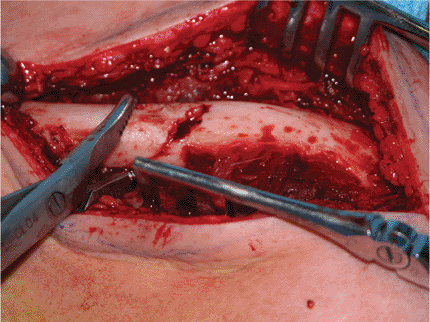
Figure 1.10.
The distal fragment is distracted, elevated, and derotated, and the fragments are reduced. A small defect is present in the center due to fracture comminution. |
 |
|
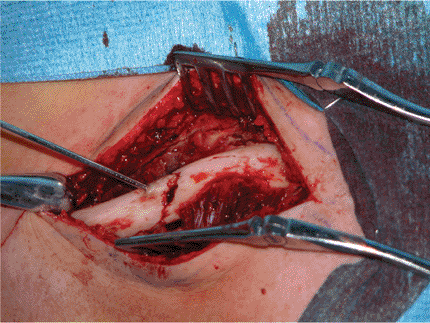
Figure 1.11. Following reduction, the fracture site is temporarily fixed with a 2.0-mm K wire aimed perpendicular to the fracture line.
|
 |
|
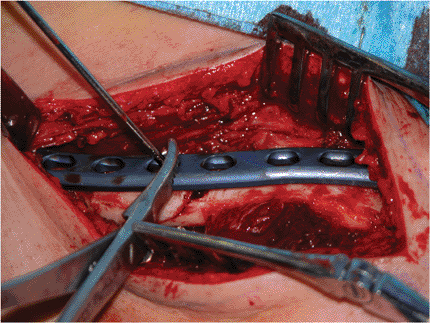
Figure 1.12.
After temporary transfixion with a 2.0-mm K wire, a precontoured plate is applied. The use of a precontoured plate saves operative time and reduces soft-tissue irritation at the proximal and distal ends. |
 |
|
Figure 1.13.
The first screw should be positioned in a hole on the opposite side of the fracture where the K wire and the reduction clamp are securing the plate. |
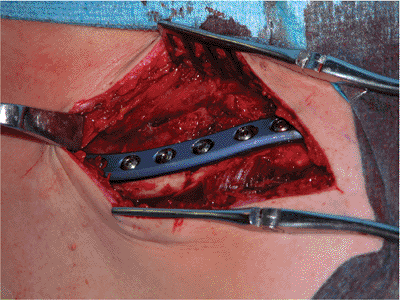
stability of the construct insured, the field is copiously irrigated
with normal saline (Fig. 1.15). A standard
closure is then performed in layers with use of no. 1 absorbable
sutures for the myofascia, no. 2–0 absorbable sutures for the
subcutaneous tissue, and clips or a subcuticular stitch for the skin
(Figs. 1.16 and 1.17).
Secure wound closure is essential to insure adequate soft-tissue
coverage of the bone as well as a decreased incidence of wound
hematoma. Drains are not used. Infiltration with long-acting local
anesthetic may be used at this time to help manage postoperative pain (Fig. 1.18). After the surgery, the arm is placed in a sling or shoulder immobilizer (Fig. 1.19).
 |
|
Figure 1.14.
Comparison between a standard 3.5-mm LCDC plate and an anatomic plate contoured to fit the clavicle. A precontoured plate saves operative time and minimizes the prominence of the hardware. |
 |
|
Figure 1.15.
The wound is irrigated and local bone graft can be packed around the fracture site both anteriorly and posteriorly (if needed). Care is taken to avoid placing excessive amounts of bone inferiorly, where it may interfere with the thoracic outlet. |
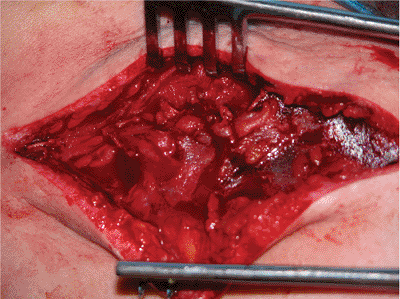 |
|
Figure 1.16. Both the myofascial and the subcutaneous layers are closed with interrupted absorbable sutures.
|
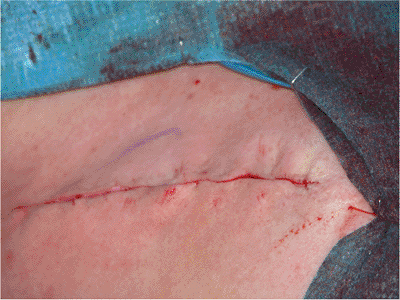 |
|
Figure 1.17. The skin layer is closed with clips or a subcuticular stitch.
|
-
malunion or nonunion with shortening (>15 mm, often 2 to 3 cm);
-
angulatory deformity (>30 degrees at the fracture site) or translation (>1 cm);
-
symptoms consistent with thoracic outlet syndrome;
-
chronic pain with repetitive, overhead, or resisted activity;
-
pain when using shoulder straps or backpacks;
-
dissatisfaction with the appearance or asymmetry of the shoulders; or
-
substantial disability detected on patient-oriented limb specific health measures.
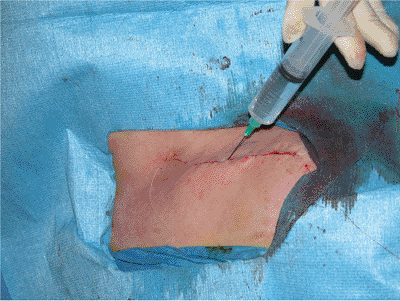 |
|
Figure 1.18. Long-acting local anesthetic may be injected to help manage postoperative pain.
|
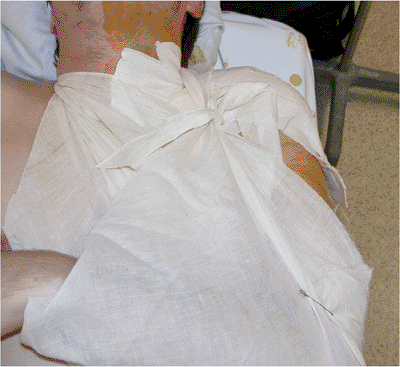 |
|
Figure 1.19. After the surgery, the arm is placed in a conventional sling.
|
based on clinical and radiographic data, is used to identify the amount
and degree of deformity as well as the correction that will be
required. Also, if the malunion or nonunion caused shortening, then
length should be restored. If the clinical and radiographic data show
that shortening exceeds 1 cm, an intercalary bone graft may be required
to compensate for bone loss. In patients with symptomatic malunions, an
osteotomy must often be performed through the extensively remodeled
fracture. In these patients, a combination of osteotomes and a
microsagittal saw (cooled continually with irrigation throughout the
cutting) is used to re-create the original fracture line. The medullary
canal is opened with a 3.5-mm drill bit in each fragment. An
intercalary bone graft can then be placed where necessary. A small
notch should be made with the saw in each fragment prior to the
corrective osteotomy as a means to measure the clavicular length. The
distal fragment is typically rotated anteriorly, such that its flat,
superior surface faces anteriorly rather than superiorly. Malrotation
is best corrected by redirecting superiorly the flat, superior surface
of the distal fragment, creating similar clavicular surfaces on both
sides of the osteotomy. Caution should be taken to protect the
underlying neural and vascular structures, and no attempt should be
made to explore or formally decompress the brachial plexus.
unite, plate fixation is an excellent treatment option. With atrophic
nonunions, the iliac crest is draped for supplemental autologous bone
graft. In hypertrophic nonunions, augmentation of the nonunion site
with iliac crest bone graft is unnecessary because abundant local bone,
which is resected from the bone ends, may be used for this purpose.
When packing the osteotomy or nonunion site with local callus or
autologous bone graft, care should be taken not to place an abundant
amount of bone graft inferiorly because the formation of a large callus
could impinge on the underlying neurovascular structures.
in a sling or shoulder immobilizer for comfort. Patients begin pendulum
exercises during the first postoperative week and active-assisted
motion at 2 weeks. Immediate motion of the elbow and shoulder are
encouraged to improve function and to restore patient independence. The
sutures or staples are generally removed in 10 to 14 postoperative days.
the first 8 weeks with standard radiographs to ensure that the
reduction is maintained and that the hardware has not loosened or
changed position. Often, the standard AP view of the clavicle may not
provide adequate visualization of the fracture because the overlying
hardware obscures the fracture site. Riemer et al (23)
recommended an abduction-lordotic view, with the shoulder abducted to
135 degrees and the x-ray beam angled 25 degrees cephalad. This results
in the superior rotation of the clavicle so the plate no longer
obstructs the view of the fracture site. By combining this view with a
standard AP view, one can visualize almost 90 degrees of the clavicle
for an accurate assessment of fracture healing.
full active and passive motion is initiated and the patient is weaned
from the sling. Resistance and strengthening activities are allowed
when radiographs reveal union, typically at 6 to 8 weeks postinjury.
Complete shoulder rehabilitation is recommended before the patient
resumes any throwing, racquet, contact, or collision sports.
following plate osteosynthesis of acute clavicular fractures. Early
studies suggested that open reduction and internal fixation of
clavicular fractures were associated with a high-nonunion rate (2,3). However, more recent
reports using modern small-fragment implants have shown vastly improved
outcomes. The first of these studies involved a small number of
patients and reported 100% union rates following primary plate fixation
of midclavicular fractures (2,24). In a larger study (122 patients), Poigenfurst et al (21)
achieved union in 96% of patients treated with plating for acute
clavicular fractures. The five nonunions healed after re-operation and
secondary plating. Bostman et al (26) studied
103 patients with midclavicular fractures treated with primary plate
fixation. They documented a union rate of 97.1% with only 3 nonunions
and 3 delayed unions. More recently, Shen et al (27)
conducted the largest research project involving plate fixation of
acute clavicular fractures in which 232 patients were followed for a
minimum of 3 postoperative years. They reported a union rate of 97%
with the mean time to union 10 weeks; no patients were shown to have
any impairment in their shoulder strength and range of motion. Overall,
the results of these studies demonstrate that internal fixation of
acute clavicular fractures leads to high rates of union and very good
functional results. To our knowledge, no randomized, controlled trials
have been published in which nonoperative treatments were compared to
internal fixation of acute clavicular fractures.
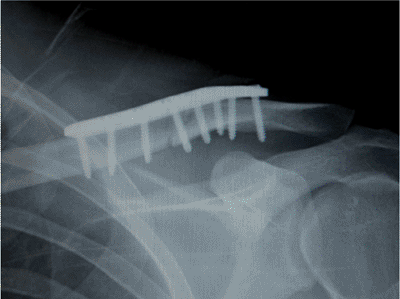 |
|
Figure 1.20.
Postoperative radiograph demonstrating correction of the deformity, apposition of the bone ends, and accurate placement of the plate. |
fracture are uncommon. Vascular injuries, ranging from acute
lacerations to trauma-inducing thrombosis or aneurysm, are rare but
limb-threatening injuries. Brachial plexus injuries tend to be mostly
traction neurapraxia from which the patient usually recovers within 3
to 4 months of injury. Excessive callus formation at the fracture site
has been associated with thoracic outlet syndrome (28,29).
Although rare, chronic impingement of the neurovascular structures
between the callus and the underlying first rib can lead to symptoms of
arm pain, paresthesia, and weakness.
include malunion, nonunion, and posttraumatic arthritis of the
sternoclavicular and acromioclavicular joints. Malunion of the clavicle
after plating is very rare and is the result of hardware failure or
technical error. If a clavicular fracture fails to unite by 4 to 6
months following the surgery, it can be classified as a nonunion (21,23,25,26,27).
Although the exact cause of a nonunion is unknown, several systemic and
local factors have been implicated in its development. Systemic factors
include smoking, alcoholism, poor nutrition, and the presence of a
chronic systemic disease. Poorly reduced or insecurely fixed fractures
can also contribute to the development of a nonunion. Schwarz and
Hocker (30) reported a nonunion rate of 12%
following surgical fixation of clavicular fractures with 2.7-mm dynamic
compression plates. They attributed their high
failure
rate to using plates of inadequate length and size and concluded that a
minimum of three screws or six cortices must be placed on either side
of the fracture site to achieve adequate stabilization. The development
of symptomatic arthritis of the sternoclavicular or acromioclavicular
joints following a clavicle fracture is dependent on the initial
fracture pattern. Even with accurate surgical fixation of the clavicle,
patients with intra-articular fractures are at risk for developing
posttraumatic degenerative arthritis
clavicular fracture carries a small risk of infection, blood loss, and
hardware-related prominence or failure. Deep infection can lead to
loosening of the hardware, soft-tissue ulceration, and the development
of a nonunion. However, the incidences of postoperative infection
following clavicular plating remain few. In their study of 103
patients, Bostman et al (26) reported that only
five patients developed deep infection requiring re-operation and
removal of the hardware, and only three patients developed superficial
infection, which subsided in each case with the use of antibiotic
therapy. With similar results to Bostman (26), Shen et al (27) reported only a single case of deep infection and four cases of superficial infection in their 232 patient population.
fixation requires early recognition and aggressive treatment. Although
a long-term antibiotic regimen and local debridement are the standard
treatments, hardware removal is also necessary to control the infection
in the majority of patients. Superficial infections can be managed with
local irrigation and debridement along with a short course of oral
antibiotic therapy.
complication remains shoulder stiffness after prolonged immobilization.
Close follow-up with a physician or physiotherapist is necessary to
insure that the patient is performing range-of-motion exercises. Most
patients regain full shoulder range of motion and strength.
hardware failure and soft-tissue irritation with possible wound
dehiscence. Hardware failure is a rare occurrence when appropriate
issues are addressed in the selection and insertion of the plate.
Loosening and breakage of the plate can often be predicted by
insufficient purchase of the screws during the procedure or selection
of an inadequately sized plate (30).
plate and screws, many patients require later hardware removal. Shen et
al (27) reported that 171 of their 232 patients
had their hardware removed, with two of these patients suffering a
refracture after hardware removal. Generally, refracture after plate
removal is rare as long as 12 months have passed since the fixation of
the fracture.
KM, Da Silva VF, Preston DN, et al. Brachial-plexus injury after
clavicular fracture: case report and literature review. Can J Surg 1991;34:264–266.
MD, Seiler JG, Jupiter JB. The application of the limited contact
dynamic compression plate in the upper extremity: an analysis of 114
consecutive cases. Injury 1995;26:661–666.
BL, Butterfield SL, Daffner RH, et al. The abduction lordotic view of
the clavicle: a new technique for radiographic visualization. J Orthop Trauma 1991;5:392–394.
K, Matsuda K, Sakai Y, et al. Late thoracic outlet syndrome secondary
to malunion of the fractured clavicle: case report and review of the
literature. J Trauma 2001;50:332–335.
