Principles of Mangled Extremity Management
One – General Principles: Basics > Principles of Treatment > 12 –
Principles of Mangled Extremity Management
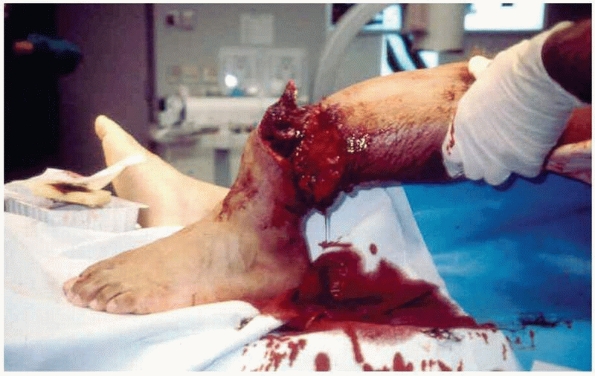
extremity so severe that salvage is often questionable and amputation
is a possible outcome. This injury is always a result of high-energy
trauma caused by some combination of crush, shear, blast, and bending
forces. Associated fractures usually verify the high-energy forces of
the mechanism of injury by exhibiting extensive comminution patterns.
The skin is often degloved with large areas of loss secondary to
avulsion or ischemia and the fascial compartments are typically
incompletely opened by explosion or tear. Muscle tissues are typically
damaged at both local and regional levels by direct as well as indirect
injury. Furthermore, soft tissue planes are usually extensively
disrupted and, when present, contaminants generally infiltrate all of
these planes (Fig. 12-1). Not only are the
injury patterns themselves complex, but the medical, psychological, and
socioeconomic impacts that these injuries have on the patient make
their management a difficult task, even in the most experienced of
hands.
the management of the mangled extremity have occurred during times of
war, the majority of limb-threatening injuries seen in practices today
are the result of high-speed motor vehicle collisions. Modification of
passenger restraints, vehicle safety engineering, and the legislation
of seatbelt and air-bag protection appear to be decreasing the
mortality rate associated with motor
vehicle
crashes. As a result, the incidence of severe lower extremity trauma
may be increasing. In the United States, injuries to the lower
extremity account for over 250,000 hospital admissions annually for
patients 18 to 54 years of age. It is estimated that over half of these
admissions result from a high-energy mechanism.31
Orthopaedic surgeons providing emergency department trauma coverage
need to understand the historical concepts surrounding the care for
these injuries as well as recent modifications of these concepts based
on numerous advances in technology combined with a better understanding
of the long-term clinical outcomes of these injury patterns.
 |
|
FIGURE 12-1 A typical example of a limb-threatening injury at presentation.
|
limb-threatening lower extremity injury has plagued patients and
surgeons alike. Until the implementation of amputation, most severe
open fractures resulted in sepsis and these injuries were often fatal.2
In its infancy, amputation itself usually entailed a very high
mortality rate, often from hemorrhage or sepsis. Amputations performed
during the Franco-Prussian War and American Civil War carried mortality
rates ranging from 26% to as high as 90%.22,87
As amputation techniques improved, so did our understanding of the
concepts of bacterial contamination and infection. By the mid-1880s,
through the pioneering works of Pasteur, Koch, and Lister on bacterial
contamination and infection, there was a rapid increase in the use of
antiseptic agents, soon followed by the introduction of aseptic methods
and then mortality rates rapidly declined.115
Subsequently, topical sulfa agents were introduced just before World
War I and systemic antibiotics became available during World War II and
the Korean War.87,89
Through advances in surgical technique, as well as through a better
understanding of microbial prophylaxis and treatment, extremity
injuries that were once considered to be life threatening have now been
rendered, at the very least, survivable.
reducing mortality in the treatment of patients with a life- or
limb-threatening injury of the extremity, many patients and physicians
have historically perceived amputation as a failure of therapy and have
fought aggressively to salvage the mangled limb. Although a pioneer in
the field of amputation, Ambrose Paré knowingly risked his own life
over limb when he insisted on conservative management of his own open
tibia fracture rather than amputation. Not only did he survive the
injury, but his documentation of the conservative treatment of a
potentially limb- and life-threatening injury serves as one of the
first known documented cases of “limb salvage.” Nevertheless, for
centuries to come, most complex extremity injuries were routinely
treated with amputation. After World War II, medical and surgical
training became more specialized and numerous developments in the
civilian medical arena led to a revolution in the management of
limb-threatening battlefield injuries, which dictates our treatment
today. Arterial repair and bypass were attempted on a wide scale during
the Korean and Vietnam Wars, subsequently reducing the amputation rates
in extremities with vascular injuries from 50% to 13%.51,52,53,87,96
Over time, similar advances in all aspects of wound and fracture
management have improved our ability to reconstruct the severely
injured extremity. Limbs that would have required an amputation 20
years ago are now routinely entered into complex reconstruction
protocols. The development of second- and third-generation antibiotics
and microsurgical tissue transfers14,62,102,111 and the use of temporary intraluminal vascular shunts,56
wound irrigation strategies, and tissue-friendly fracture fixation
methods have combined to make initial limb salvage, at the very least,
feasible in most cases. Furthermore, by using massive autogenous grafts
and/ or osteoinductive materials,20,32,40,60,64 as well as through the technique of bone transport,26,80,92,97
delayed large-segment bone defect reconstruction has become routine.
Although limb salvage has become technically feasible, the initial
assessment and management of the patient and the injury are paramount
in determining whether salvage is advisable.
presentation and can often be distracting to the treating surgeon and
medical team. Because these injuries are usually the result of a
high-energy mechanism, routine trauma protocols should be followed that
first address the patient as a whole and not just the injured
extremity, because 10% to 17% of these patients will have an associated
life-threatening injury.16,67
Evaluation should begin by following the principles of Advanced Trauma
Life Support (ATLS). Once the patient has been stabilized and the
primary and secondary trauma surveys have been completed, a thorough
orthopaedic evaluation is mandatory. This should include a
determination of the time of injury, mechanism of injury, the age of
the patient, and the presence of any other social or medical
comorbidities. Prophylactic antibiotics should be administered as soon
as possible and tetanus prophylaxis should be administered as
indicated. The injured extremity should first be evaluated for adequate
perfusion and, if a vascular injury is suspected, vascular surgery
consultation should be obtained. The soft tissue wound should be
inspected and the pattern of soft tissue injury and contamination
should be noted. If possible, a cursory removal of any gross
contamination via irrigation should be performed before dressing the
wounds and immobilizing the extremity, especially if a fracture
reduction or joint reduction is thought to be necessary before
transport to the operating room for initial wound débridement. A
detailed motor examination and sensory examination should be performed
and documented, both before and after any manipulation of the
extremity. The presence of an associated compartment syndrome should be
entertained and ruled out. Radiographic evaluation should include two
orthogonal views of any involved joints or long bones, as well as the
joint above and below any confirmed fractures. Photographs of the
extremity should be obtained whenever possible. These can provide
invaluable documentation of the extent of the initial injury and,
during the course of treatment, serve as a visual record of progress to
or away from a functional salvaged extremity.29
extremity in question, but a comprehensive musculoskeletal examination
should be performed to rule out any concomitant musculoskeletal
injuries. In the case of a polytrauma patient with a mangled extremity,
the initial diagnostic workup and treatment of any life-threatening
injuries can often be time consuming and precede the management of the
injured limb; therefore, a sterile dressing should be applied to all
wounds and the limb immobilized as soon as possible to prevent any
ongoing
soft
tissue damage until proper débridement and stabilization procedures can
be performed in the controlled setting of the operating room.
vascular insult. Arterial injuries usually present with either hard or
soft signs suggestive of injury. Examples of hard signs that should be
documented and investigated include pulsatile bleeding, the presence of
a rapidly expanding hematoma, a palpable thrill, or audible bruit, as
well as the presence of any of the classic signs of obvious arterial
occlusion (pulselessness, pallor, paresthesia, pain, paralysis,
poikilothermia). Soft signs of arterial injury include a history of
arterial bleeding, a nonexpanding hematoma, a pulse deficit without
ischemia, a neurological deficit originating in a nerve adjacent to a
named artery, and the proximity of a penetrating wound, fracture, or
dislocation near to a named artery.84
In addition to observing for these hard and soft signs of vascular
injury, a formal vascular examination should be conducted. The skin
color and time required for capillary refilling of the skin of the
distal extremity should be compared with and documented against that of
the uninjured contralateral side. The distal extremity should be
evaluated for the presence of palpable peripheral pulses and/or Doppler
signal. The limb with gross deformity secondary to fracture or
dislocation with questionably palpable pulses or reduced Doppler
audible flow should undergo immediate gentle reduction of the deformity
and immobilization of the reduced limb in an effort to relieve possible
kinking or compression of the vascular structures. Subsequently, pulse
assessment of the distal extremity should again be performed and
documented after any reduction maneuvers. Arterial pressure indices
(APIs) should also be obtained in the presence of a history of
pulselessness in the extremity or if the vascular status of the distal
extremity remains unclear even after reduction attempts have been made
to restore reasonable alignment to the extremity. APIs are obtained by
first identifying the dorsalis pedis and posterior tibial arteries of
the injured extremity using a Doppler probe. Next, a blood pressure
cuff is placed proximal to the level of injury and then inflated to a
suprasystolic level causing cessation of the normal Doppler signal. The
cuff is then slowly deflated and the pressure at which the Doppler
signal returns identifies the ankle systolic pressure to the injured
limb. This procedure should then be repeated on the contralateral
extremity as well as in the arm (brachial pressures). The pressure in
the injured extremity is then compared with the pressure in the arm or
the unaffected extremity and reported as a ratio of the normal systolic
pressure (e.g., if the brachial systolic pressure is equal to 120 mm Hg
and the systolic pressure in the injured limb is equal to 90, then API
is reported as 0.75). If the API is lower than 0.90 or distal pulses
remain absent despite reduction, angiography and/or vascular surgery
consultation is indicated.
identified, treatment should first address restoration of arterial
inflow and skeletal stabilization. In the patient with a pulseless but
perfused limb, the priority and sequence of vascular and orthopaedic
repair depend primarily on the experience and availability of both the
orthopaedic and vascular teams. At times, if the fracture is relatively
stable and will require little manipulation, immediate arterial repair
should precede bony stabilization. However, if the fracture is
excessively comminuted, displaced, or shortened, rapid bony
stabilization should be performed before any attempts at vascular
repair. Not only will this aid in the exposure of the vascular injury,
but doing so brings the limb out to its proper resting length, ensuring
the vascular repair is of sufficient length to allow for further
manipulation and reduction of the extremity with less risk of vascular
complications after the repair has been completed.55
ischemia, the restoration of arterial inflow should be the highest
priority and consideration should be given to temporary intraluminal
vascular shunting of the extremity.56,57,86
The insertion of an intraluminal shunt can rapidly restore arterial
inflow and allow for a more detailed examination to better determine
the extent of the injury and whether the limb is indeed salvageable.
Because the shunt will hold up to fairly vigorous manipulation, it will
also allow for a more thorough débridement and appropriate
stabilization of the bone and soft tissues. Once the débridement has
been completed and the bony injury temporarily or definitively
stabilized, formal vascular repair can then either proceed immediately
or in a delayed fashion if the patient remains in extremis.
of arterial inflow to an ischemic and traumatized limb. The diminished
arterial inflow during the ischemic period combined with the
“reperfusion injury” that occurs after arterial repair can result in
interstitial fluid leakage and elevated compartment pressures.
Fasciotomies should be performed after any revascularization procedure
in the mangled extremity.67,68,78
While most vascular and general surgeons are adequately trained to
perform decompressive fasciotomies, ideally, these should be performed
by or under the supervision of the orthopaedic surgeon to ensure
adequate compartment decompression as well as appropriate fasciotomy
incision placement that will not compromise later bony and soft tissue
reconstructive procedures.
department and photographs have been taken for the medical record, any
open wounds should be gently rinsed with a copious amount of normal
saline and dressed with sterile gauze.23 The dressings should be left in place until the patient reaches the operating room for definitive débridement.
prevent the possibility of exsanguination, but it should not be
inflated unless absolutely necessary to prevent further ischemic injury
to the extremity. Once the tourniquet is in place, the splint and
dressings can be removed and the extremity again examined for
perfusion. Although typically referred to as “irrigation and
débridement,” the first and most important step is a thorough
débridement of the wound. This should be done in a methodical manner to
ensure adequate removal of any contaminating material and devitalized
tissues. The skin and subcutaneous tissue should be addressed first.
While the initial open skin wounds are obvious, the energy imparted at
the time of injury typically produces a shock wave that causes
stripping of the soft tissues. Acute traumatic injuries to the
extremity typically result in so-called zones of injury. A gradient of
energy extends peripherally from the site of impact, variably damaging
tissues along its path. A central zone of necrotic tissue exists at
and
around the point of impact and greatest injury. These tissues are
typically nonviable regardless of the intervention. Surrounding this
area lies a zone of marginal stasis. This ischemic penumbra consists of
tissue that is variably injured and may or may not survive despite
appropriate intervention. Finally, at the periphery of the injury
exists a zone of noninjured or minimally injured tissue that, while not
subject to the primary injury, could be at risk from the delayed
physiological responses to the primary area of injury.41,68
To address these zones of injury, the open wounds should be extended or
separate extensile incisions should be performed to adequately assess
and debride the wound. These incisions should be axially aligned and
thoughtfully placed so as not to create “at-risk” flaps or preclude any
later reconstructive efforts.
muscle, fat, fascia, skin, and other nonviable tissue within the
central zone of injury should be removed. Muscle should be tested for
viability based on its contractility, consistency, color, and capillary
bleeding (the four Cs), and if it is found to be nonviable, it should
be debrided, regardless of the expected functional loss. While the
amount of tissue damage seen on the initial débridement can be quite
extensive, the quantity of tissue necrosis from the delayed response to
the injury within the zone of marginal stasis can far exceed the loss
and destruction caused by the initial traumatic injury. Because the
exact degree of expected tissue loss and necrosis cannot be determined
easily at the time of initial débridement, serial débridements will be
required until the identification and removal of all nonviable tissue
has been achieved and wound homeostasis obtained.
in the initial management of the limb at risk. Stabilization of the
bony skeleton prevents ongoing soft tissue damage, promotes wound
healing, and is thought to protect against infection. In an animal
study, Worlock et al.124 examined
the rate of infection and osteomyelitis associated with stable and
unstable skeletal fixation. They reported that the infection rate in
the unstable group was nearly double that in the skeletal stabilization
group.
location of the bony injury, the degree of soft tissue injury, and the
overall condition of the patient at the time of initial operative
management. Stabilization options range from splint immobilization or
skeletal traction to internal fixation. While no one technique has
proved to be superior to all others in all clinical situations, in
general, the more severe the injury, the greater is the need for direct
skeletal fixation to provide improved access to the traumatic wound.
Immediate intramedullary stabilization or plate fixation of type I, II,
and IIIA open fractures remains an accepted treatment strategy.
However, most limb-threatening injuries present as type IIIB or type
IIIC open fractures. These injuries are perhaps most judiciously
managed with temporizing external fixation. External fixation in this
setting offers many advantages. It can be applied relatively quickly
and without the use of fluoroscopy while still providing excellent
stability and alignment of the limb until definitive fixation can be
performed. External fixation also allows for redisplacement of the
fracture fragments for a more thorough evaluation and débridement of
the soft tissues during any repeat procedures. Once wound homeostasis
has been obtained, conversion to definitive internal fixation can be
performed on a delayed basis with good results.3,103,104
External fixation can also be chosen as the form of definitive fixation
for diaphyseal fractures, but multiple studies have found this approach
to have slightly higher complication rates and poorer outcomes when
directly compared with intramedullary fixation. Henley et al.46
prospectively compared unreamed intramedullary nailing with external
fixation in patients with type II, IIIA, and IIIB open fractures of the
tibial shaft. Both groups underwent identical soft tissue management
before and after skeletal fixation. Their study showed that those
patients in the intramedullary nail fixation group had significantly
fewer incidences of malalignment and underwent fewer subsequent
procedures than did those in the external fixation group. Tornetta et
al.116 also reported on the early
results of a randomized, prospective study comparing external fixation
with the use of nonreamed locked nails in type IIIB open tibial
fractures. Again, both groups had the same initial management, soft
tissue procedures, and early bone grafting. They found that the
intramedullary nail treatment group had slightly better knee and ankle
motion and less final angulation at the fracture site. They also
concluded that that the nailed fractures were consistently easier to
manage, especially in terms of soft tissue procedures and bone
grafting. Furthermore, they thought the intramedullary nailing was
preferred by their patients and that it did not require the same high
level of patient compliance as external fixation. Using data obtained
through the Lower Extremity Assessment Project (LEAP), Webb et al.121
reviewed 156 patients with the combination of a fractured tibia in
association with a mangled lower extremity. One hundred five patients
with 17 type IIIA, 84 type IIIB, and 4 type IIIC tibial fractures had
follow-up to 2 years. The authors found that definitive treatment with
a nail yielded better outcomes than definitive treatment with external
fixation. In their series, the external fixation patients had a
significantly increased likelihood of both infection and nonunion.
oxygen in a chamber under increased barometric pressure. This results
in a supraphysiological arterial oxygen saturation level, creating an
expanded radius of diffusion for oxygen into the tissues that results
in increased oxygen delivery at the periphery of certain wounds. As a
result, HBO is thought to enhance oxygen delivery to injured tissues
affected by vascular disruption, thrombosis, cytogenic and vasogenic
edema, and cellular hypoxia as a result of trauma to the extremity.
beneficial in the peripheral zone of injury where tissue that is
variably injured may or may not survive despite other appropriate
interventions. Injured but viable cells in this area have increased
oxygen needs at the very time when oxygen delivery is decreased by
disruption of the microvascular supply.54,91
As such, HBO can be applied in an effort to mitigate this process of
secondary injury in extremity trauma and minimize the resultant tissue
loss at different points in both the pathological and recovery
processes.41
extremity trauma are observational with fairly anecdotal reports on its
efficacy. However, in 1996 Bouachour et al.11 performed a randomized placebo-controlled human trial of HBO as an
adjunct to the management of crush injuries to the extremity.
Thirty-six patients with crush injuries were assigned in a randomized
fashion, within 24 hours after surgery, to treatment with HBO (session
of 100% O2 at 2.5 atmospheres [atm] for 90 minutes, twice daily, over 6 days) or placebo (session of 21% O2
at 1.1 atm for 90 minutes, twice daily, over 6 days). Both treatment
groups (HBO group, n = 18; placebo group, n = 18) were similar in terms
of age; risk factors; number, type or location of vascular injuries,
neurological injuries, or fractures; and type, location, or timing of
surgical procedures. The authors found complete wound healing without
tissue necrosis in 17 of the 18 HBO patients and in 10 of the 18
control patients. While two patients in the control group eventually
required amputation, no patients in the HBO group went on to
amputation. Furthermore, a decreased number of surgical procedures such
as skin flaps and grafts, vascular surgery, or eventual amputation were
required for patients in the HBO group compared with the placebo group.
A subgroup analysis of patients matched for age and severity of injury
showed that HBO was especially effective in patients older than 40 with
severe soft tissue injury. They concluded that HBO improved wound
healing and reduced the number of additional surgical procedures
required for treatment of the injury, and that it could be considered a
useful adjunct in the management of severe crush injuries of the limbs,
especially in patients over 40 years old.
case series and a small number of randomized studies seem to suggest a
potential benefit of HBO therapy as an adjunct to the management of the
severely traumatized limb. However, if efficacious, HBO use in the
mangled extremity patient will be selective as many patients are
critically ill and are often unable to travel to receive and to
tolerate HBO therapy. At this time, more data and stringent clinical
investigations are needed to determine the exact indications for,
optimal timing of, and appropriate duration and dosage of HBO therapy
before it can be recommended in the routine management of complex
injuries of the limb.
However, a few principles are worth discussing here. The first
addresses the type of soft tissue coverage selected in the
reconstruction pathway. While multiple options for coverage exist, such
as skin grafts, local flaps, or free flaps, complications will occur
with each. Pollak et al.94 found
that 27% of high-energy tibia injuries requiring soft tissue
reconstruction had at least one wound complication within the first 6
months after injury. They also found that the rate of complication
differed based on the type of flap coverage. For limbs with the most
severe osseous injury (OTA type C fractures), treatment with a
rotational flap was 4.3 times more likely to lead to an operative wound
complication than was treatment with a free flap. The rate of
complications for the limbs with less severe osseous injury did not
differ significantly based on soft tissue coverage selection. Based on
this information, one should be very cautious when selecting a local
flap in the setting of high-energy trauma as the flap, although
originally healthy in appearance, may have indeed been included in the
initial zone of injury.
timing of the soft tissue reconstructive procedure. The primary
argument for early soft tissue reconstruction is to reduce the risk of
nosocomial contamination because of repeated exposures of the
vulnerable wound to the hospital environment. Some recent data have
brought into question the efficacy of early soft tissue reconstruction.
When analyzing a subset of patients with open tibial fractures in
association with a mangled extremity, Webb et al.121
failed to observe any advantages related to the performance of early
muscle flap wound coverage within the first 72 hours after the injury.
In contrast, multiple authors have indeed shown that early
reconstruction (within 72 hours) reduces postoperative infection, flap
failure, and nonunion rates as well as the risk for the development of
osteomyelitis.33,37,39,47 Others have recommended muscle flap coverage on a more delayed basis (7 to 14 days).125
Recently, with the advent of negative pressure wound therapy (NPWT) and
the decreasing availability of surgeons trained in rotational flaps and
free tissue transfer, there seems to be a trend toward increased delays
until definitive soft tissue reconstruction procedures are performed.
While NPWT can be a very effective tool in the initial soft tissue
management of high-energy open fractures, its routine use in open tibia
fractures has not been found to reduce the overall infection rates
compared with historical controls nor has it been shown to reduce the
need for free tissue transfer or rotational muscle flap coverage in
these injuries.28 Bhattacharyya et al.4
recently evaluated whether the use of NPWT could allow for a delay of
flap coverage for open tibia fractures without a subsequent increase in
the infection rate. The authors concluded that despite the routine use
of NPWT before definitive soft tissue reconstruction in patients with
Gustilo type IIIB fractures, patients who underwent definitive soft
tissue coverage within 7 days had significantly decreased infection
rates compared with those who underwent soft tissue coverage at 7 days
or more after injury (12.5% versus 57%).
reconstruction are often inevitable; however, based on a preponderance
of evidence, it still appears that soft tissue coverage should be
performed as early as possible once both the patient and the wound bed
appear stable enough for such a procedure.
the field of amputation during World War I and World War II, wrote,
“Injury, disability, or deformity incompatible with life and function
indicates amputation. The surgeon must use his judgment as to whether
the amputation is indicated and at what level it can safely be done.”63
Since that time, numerous physicians caring for the patient with a
mangled extremity have delineated a multitude of clinical factors to
help better guide in the decision-making process in the setting of a
potentially salvageable versus an unsalvageable limb injury66 (Table 12-1).
In 2002, factors that influenced the mangled extremity treatment
decision process were studied by Swiontkowski and the LEAP Study Group.112
Orthopaedic and general trauma surgeons caring for the mangled limbs
were surveyed to determine the factors they typically used to make a
reconstruction or amputation treatment decision. More than 33% of 52
orthopaedic surgeons
indicated
that plantar sensation was the most important determinant for limb
salvage. The severity of the soft tissue injury (17%) and limb ischemia
(15%) followed in importance. No orthopaedic surgeon ranked the
patient’s Injury Severity Score (ISS) as a critical factor. In
contrast, 33 general trauma surgeons from the same centers ranked the
ISS as the most critical determinant (31%), followed by limb ischemia
(27%) and plantar sensation (21%). An analysis of the patient, injury,
and surgeon characteristics determined that the soft tissue injury
(i.e., the extent of muscle injury, deep vein injury, skin defects, and
contamination) and the absence of plantar sensation were the factors
considered to be most important at the time to predict amputation.
Patient characteristics and the experience level of the surgeon did not
appear to influence the decision process. Of important note, the
orthopaedic surgeon was responsible for the initial treatment decision
in all cases. General trauma surgeons participated in the
decision-making process 58% of the time and plastic surgeons
contributed to the process 26% of the time. While all of these
variables play a key role in decision making by the orthopaedic surgeon
and the trauma team, a few of these warrant further discussion, as new
evidence suggests that we should reconsider the importance of some of
these factors.
|
TABLE 12-1 Limb Salvage Decision-Making Variables
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
can often be a long, drawn-out, and difficult decision for both the
patient and the treating surgeon. However, on rare occasions, the
decision for amputation can be quite simple. Amputation is generally
the only treatment option in cases of a severely injured extremity with
an irreparable vascular injury or in the setting of prolonged warm
ischemia (longer than 6 hours).67 In
some instances, when the patient’s life would be threatened by attempts
to save the limb, the dictum of “life over limb” supersedes the
feasibility issue of limb salvage, and amputation should be the only
option despite the presence of a potentially salvageable limb.
Immediate amputation should also be considered in patients critically
injured with significant hemodynamic instability, coagulopathy, or an
injury constellation that would preclude the multiple surgeries
required for limb salvage.66,67
In these cases, an immediate guillotine amputation is performed to
minimize the soft tissue wound area. This amputation is then revised to
a formal closure once the patient’s condition is improved.
is critical to the salvage of an extremity is difficult to trace.
Although the LEAP Study Group’s112
decision-making analysis supported the inclusion and perceived
importance of plantar sensibility, the fact that this was an
established treatment axiom at the time of this study may have driven a
self-fulfilling prophesy phenomenon. Because surgeons believed that
absent plantar sensation was a reason to amputate a limb, they acted
accordingly. Indeed, the literature before 1980 warns of neuropathic
ulcers and chronic complications associated with absent plantar
sensation. Johansen,58 Howe,50 and Russell99 and their colleagues, however, describe a confirmed
avulsion or complete transection of the tibial nerve as their
definition of absent plantar sensation in their limb salvage
algorithms. Lange et al.67 considered complete tibial nerve disruption in adults to be an absolute indication for amputation.
the limb is performed in the emergency department. Once in the
operating room, additional dissection of the deep posterior compartment
to assess the tibial nerve is usually considered unwise, as surgical
exploration of the nerve within the zone of injury is contraindicated
because doing so causes additional soft tissue injury. Therefore, in
many centers, the absence of initial plantar sensation has been
considered the same as a physiological disruption of the nerve.
Ischemia, compression, contusion, and stretch can temporarily affect
the function of the tibial nerve. Once these factors resolve, nerve
function typically returns. In the face of no sensory return,
orthopaedic surgeons have successfully demonstrated the ability to care
for the insensate foot in other conditions (diabetes or incomplete
spine lesions) through education and shoe modifications. Furthermore,
the orthopaedic oncology literature has documented cases of limb
salvage in the face of tumor with acceptable results after sciatic,
peroneal, or tibial nerve resection.5,12
used the variations in physician practice patterns to explore the
outcomes of patients admitted to the LEAP Study with absent plantar
sensation. They examined the outcomes of a subset of 55 subjects
without plantar foot sensation at the time of initial presentation. The
55 patients were divided into two groups depending on their hospital
treatment (i.e., insensate amputation group [n = 26] and insensate
salvage group, the study group of primary interest [n = 29]). In
addition, a control group was constructed from the parent cohort so
that a comparison could also be made to a group of patients in whom
plantar sensation was present and whose limbs were reconstructed. The
sensate control group consisted of 29 subjects who were matched to the
29 insensate salvage subjects on four limb injury severity
characteristics (i.e., severity of muscle, venous, and bony injury as
well as the presence of an associated foot injury). Patient and injury
characteristics and functional and health-related quality of life
outcomes at 12 and 24 months after injury were compared
between subjects in the insensate salvage versus the other study groups and considered significant if p ≤ .005.
significantly worse outcomes at 12 or 24 months after injury compared
with subjects in the insensate amputation or the sensate control
cohort. Among those with a salvaged limb (insensate salvage and sensate
control groups), equal proportions (55%) had normal foot sensation at 2
years after injury regardless of whether plantar sensation was reported
as intact (sensate control group) or absent (insensate salvage group)
on admission. Pain, weight-bearing status, and percentage of patients
who had returned to work were similar for subjects in the insensate
salvage group compared with subjects in the insensate amputation and
the sensate control groups. Furthermore, there were no significant
differences noted in the overall, physical, or psychosocial Sickness
Impact Profile (SIP) scores between subjects without plantar sensation
whose limbs were salvaged (insensate salvage group) and subjects who
had undergone amputation (insensate amputation group) or subjects with
intact sensation whose limbs were salvaged (sensate control group).
More than one half of the patients initially presenting with an
insensate foot and treated with limb reconstruction had regained normal
sensation at 2 years. At 2 years, only two patients in the insensate
salvage group and one patient in the sensate control group had absent
plantar sensation. In this cohort, initial plantar sensation was not
found to be prognostic of long-term plantar sensory status or
functional outcomes. Based on these data, the authors concluded that
plantar sensation should not be included as a factor in the decision
making for limb salvage in lower extremity trauma.
injured lower extremity is difficult, several researchers have
attempted to enumerate certain indications for amputation or quantify
the severity of the trauma to establish numerical guidelines for the
decision to amputate or salvage a limb. These lower extremity injury
scoring systems all vary in terms of the factors considered relevant to
limb salvage and the relative weights assigned to each element. These
scoring systems were validated by the developers and demonstrated a
high sensitivity and specificity in predicting limb salvage at the time
of their design.
proposed a decision-making protocol for primary amputation in type IIIC
open tibial fractures. They suggested that the occurrence of one of two
absolute indications (complete tibial nerve disruption in an adult or a
crush injury with warm ischemia time longer than 6 hours) or at least
two of three relative indications (serious associated polytrauma,
severe ipsilateral foot trauma, or a projected long course to full
recovery) warranted amputation. This protocol, however, presented
several limitations in that only a minority of cases can be resolved
based on the absolute indications and that the relative indications
were quite subjective. Furthermore, this protocol did not address
individual patient variables such as age, medical comorbidities,
occupational, and other psychosocial factors that can have a
significant effect on the overall outcome and no subsequent clinical
studies were performed to validate this protocol.
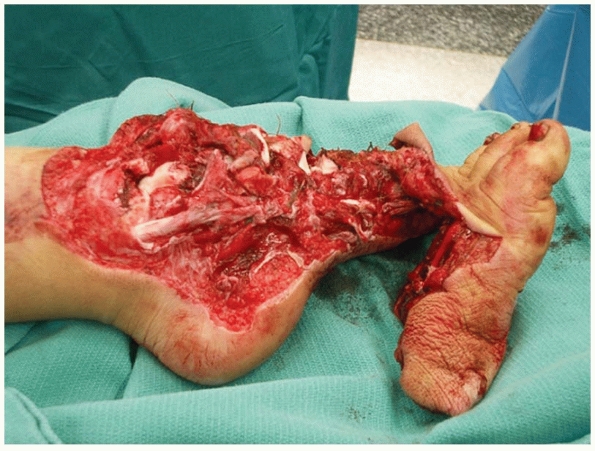 |
|
FIGURE 12-2 Another example of a “mangled extremity.” Note the extensive degree of both bony and soft tissue injury.
|
Over a 10-year period, six scoring systems were published that valued
different injury components as critical to the treatment decision42,45,50,58,81,99,110 (Table 12-2).
These components were assigned arbitrary weights and the summation
scores were used to establish “cutoffs” for limb salvage or amputation.
In this study, the authors included 17 patients over a 3-year period
who met their criteria of a mangled extremity syndrome (defined by
three of four organ/tissue systems —integument, nerve, vessel,
bone—injured in the same extremity). These patients’ charts were
retrospectively reviewed and their injuries classified according to a
point system based on the degree of integumentary, nervous, vascular,
and osseous injury. Additional scoring schemes were also included to
address patient age, the time lag to treatment, preexisting medical
comorbidities, and the presence or absence of shock. In their series,
they found that 100% of patients with an MESI score of greater than 20
underwent either primary or secondary amputation. From their data, they
suggested that if applied prospectively, the MESI could have been used
to identify those patients in their series who ultimately underwent
amputation and guide
their
treatment at the time of initial evaluation. They suggested that their
scoring system could help better identify the salvageable versus the
unsalvageable extremity. Unfortunately, the MESI had numerous faults.
Five of the 17 cases studied were injuries to the upper extremity. The
MESI scoring system can also be both cumbersome and somewhat subjective
in nature, making it prone to interobserver variability and difficult
to apply during the initial evaluation of the patient. These factors
prevented its widespread acceptance and application in orthopaedic
practice.
|
TABLE 12-2 Index Domains
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||
|
TABLE 12-3 Mangled Extremity Syndrome Index (MESI)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
was introduced in 1987 as another scoring system to help predict
amputation versus salvage in patients with combined musculoskeletal and
vascular injuries of the lower extremity. The PSI ascribes points based
on information from four key categories (level of arterial injury,
degree of bone injury, degree of muscle injury, and interval from
injury to treatment) (Table 12-4). In the
initial retrospective analysis, all 12 patients in the salvage group
had PSI scores of less than 8, while 7 of 9 in the amputation group had
scores of 8 or higher. The authors concluded that the PSI determined
the likelihood of amputation with a sensitivity of 78% and a
specificity of 100%. Although less complex than the MESI, it still had
similar faults in that many of the scores attributed were subjective in
nature and thus prone to interobserver variability. And as with the
MESI, the information necessary to complete the scoring can be
difficult to ascertain readily during the patient’s initial evaluation.
|
TABLE 12-4 Predictive Salvage Index System (PSI)
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||
Like the PSI, the MESS system is also based on four clinical criteria
(skeletal/soft tissue injury, shock, ischemia, and patient age), and it
was developed through the retrospective review of 26 severe lower
extremity open fractures with vascular compromise. It was then
validated in a prospective trial involving 26 patients at two separate
trauma centers. In both the prospective and retrospective studies, all
salvaged limbs had had scores of 6 or lower and an MESS score of 7 or
greater had a 100% positive predictive value for amputation.
Russel et al.99 proposed the Limb Salvage Index (LSI) (Table 12-6).
In this study, the authors performed a 5-year retrospective review of
70 limbs in 67 patients. Their proposed index was slightly more complex
in that it quantified the likelihood of salvage according to the
presence and severity of arterial injury, nerve injury, bone injury,
skin injury, muscle injury, and venous injury as well as the presence
and duration of warm ischemia. They reported that all 59 limbs with an
LSI score of less than 6 were able to undergo successful limb salvage,
while all 19 patients with an LSI score of 6 or greater had
amputations. Criticisms of the LSI are that it is very detailed and
requires a thorough operative evaluation to complete the initial
scoring. Furthermore, because accurate scoring of the skin category
requires a prior knowledge of the treatment and final outcome, the LSI
is essentially ineffective during the initial phases of treatment.
|
TABLE 12-5 Mangled Extremity Severity Scoring System (MESS)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TABLE 12-6 Limb Salvage Index (LSI)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
modified the MESS by including nerve injury in the scoring system and
by separating soft tissue and skeletal injury. Their modification was
named the NISSSA (Nerve Injury, Ischemia, Soft tissue Injury, Skeletal
Injury, Shock, and Age of patient) scoring system (Table 12-7).
Subsequently, the authors applied the MESS and the NISSSA to
retrospective data of 24 patients previously treated for
limb-threatening injuries. The authors found both the MESS and the
NISSSA to be highly accurate in predicting amputation. The NISSSA was
also found to be more sensitive (81.8% versus 63.6%) and more specific
(92.3 versus 69.2%) than the MESS in their patient population. Despite
the improved statistical outcomes when comparing the NISSSA to the
MESS, it inherently retains all the faults of the MESS scoring system
while increasing its complexity. The NISSSA has also not been validated
in prospective clinical trials.
|
TABLE 12-7 NISSSA Scoring System
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
helped highlight certain key factors considered relevant to limb
salvage, each system, in and of itself, is not without its own
limitations. First, while these scoring systems were validated by the
developers and demonstrated a high sensitivity and specificity in
predicting limb salvage in their respective studies, the development of
the lower-extremity Injury Severity Scores has been flawed by
retrospective designs and small sample sizes. In each study, with the
exception of the small prospective series in which the MESS system was
validated, each proposed classification system was applied
retrospectively to patients with known outcomes, rather than
prospectively to patients with unknown outcomes. Another important flaw
in the development of the scoring systems lies in the fact that
component selection and weighting in all of the indices were affected
by the clinical bias of the index developers. The NISSSA and LSI
include the result of the initial plantar neurological examination.
Age, the presence of shock, severity of contamination, and time to
treatment are included in some of the other scoring strategies. While
each of
these
factors plays a key role in decision making, strict reliance on certain
criteria with disregard to others via strict adherence to a scoring
system might lead to premature amputation in an otherwise salvageable
situation. As an example, the commonly cited MESS assigns an additional
point if the patient is above the age of 29, a point for normal
perfusion with a diminished pulse, and points for transient or
persistent hypotension without qualifying cause or response to
treatment. The suggested MESS threshold score for amputation is 7.
Using the MESS, for example, a 30-year-old patient (1 point) with a
high-energy open tibia fracture (3 points), with normal perfusion but a
diminished pulse secondary to spasm or compression (1 point), who has
persistent hypotension before laparotomy related to a spleen injury (2
points) would undergo amputation at the conclusion of the laparotomy
despite the fact that the limb perfusion will likely return to normal
and splenectomy and appropriate resuscitation will resolve the
patient’s hypotension.
other authors have attempted to validate several of the proposed
scoring systems. Although it was originally devised to assess injuries
to the lower limb, Slauterbaeck et al.107
applied the MESS to the high-energy injuries of the upper extremity. In
their series, they retrospectively reviewed the data of 37 patients
with 43 mangled upper extremities and found that all 9 upper extremity
injuries with an MESS of greater than or equal to 7 were amputated and
34 of 34 with an MESS of less than 7 were successfully salvaged. Based
on their findings, they concluded that the MESS system was an accurate
predictor of amputation versus salvage when applied to the upper
extremity. Conversely, Togawa et al.114
also retrospectively applied the MESS to patients with severe injuries
of the upper extremity with associated arterial involvement. In their
series, they successfully salvaged two of three upper extremity
injuries with an MESS score of 7 or higher with good functional
outcomes. They concluded that because of the decreased muscle mass in
the upper extremity compared with the lower extremity and the increased
collateral circulation and tolerance to ischemia seen in the upper
extremity, the MESS score was inappropriate for application to the
upper limb.
|
TABLE 12-8 Clinical Usefulness of Limb Salvage Scores
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
both attempted to apply the MESI retrospectively to each of their
patient populations. Both authors determined that the MESI inaccurately
predicted amputation versus salvage. Furthermore, they found that MESI
scores were often only approximate at best because many of the
variables required surgical intervention for accurate determination of
the scores, which negated its usefulness as a prediction tool in the
acute phase of assessment and treatment.
evaluated the MESS, LSI, and PSI limb salvage score strategies. They
retrospectively applied each limb salvage scoring system to 58 lower
limb salvage attempts over a 10-year period. Failure of the
reconstruction effort was defined as an amputation or functional
failure at 2 years. A limb was considered to be a functional failure
based on the ability to walk 150 feet without assistance, climb 12
stairs, or independently transfer. Based on their data, they were not
able to support use of any of the three scores to determine limb
treatment.
any of the limb salvage scores, the LEAP Study prospectively captured
all of the elements of the MESS, LSI, PSI, NISSSA, and the Hanover
Fracture Scale110 at the time of each patient’s initial assessment and critical decision making.8
The elements were collected in a fashion so as to not provide the
evaluator with a “score” or impact on the decision-making process. The
analysis did not validate the clinical utility of any of the lower
extremity injury severity scores. The high specificity of the scores
did, however, confirm that low scores could be used to predict limb
salvage potential. The converse was not true, though, and the low
sensitivity of the indices failed to support the validity of the scores
as predictors of amputation (Table 12-8). The authors
concluded that lower extremity injury severity scores at or above the
amputation threshold should be used cautiously by surgeons deciding the
fate of a mangled lower extremity.
sensitive (all amputated limbs will have scores at or above the
threshold) and 100% specific (all salvaged limbs will have scores below
the threshold). In the decision to amputate, high specificity is
important to ensure that only a small number (ideally, none) of
salvageable limbs are incorrectly assigned a score above the amputation
decision threshold. A high sensitivity is also important to guard
against inappropriate delays in amputation when the limb is ultimately
not salvageable. Unfortunately, few clinical scoring systems perform
ideally and the limb salvage scoring systems have proved to be no
exception.
extremity trauma, the prototypical injury is the severe open tibial
fracture. However, in reality these injuries often occur in conjunction
with severe crushing-type injuries to the ankle, hindfoot, and forefoot
and this factor should also be carefully considered when opting for
salvage versus amputation. Myerson et al.85 and others120,122
have shown that despite successful salvage and treatment of crush
injuries to the foot, a substantial proportion of these patients will
continue to have pain, often neuropathic in nature, and poor functional
outcomes.
also assessed the effect of foot injuries on functional outcomes in the
multiply injured patient. They matched 28 multiply injured patients
with foot injuries against 28 multiply injured patients without foot
injuries and compared their outcomes using the Short Form-36 (SF-36),
the Western Ontario and McMaster Universities and Osteoarthritis Index
(WOMAC), and the modified Boston Children’s Hospital Grading System.
They found that the outcome of the multiply injured patients with foot
injuries was significantly worse than that of the patients without foot
injuries when using any of the three outcome measures. Postinjury
evaluation also showed that not only were the physical scores affected
in the patients with associated foot injuries, but also the pain and
social and emotional health perceptions were dramatically reduced
compared with a control population of trauma patients without foot
injuries. When using the SF-36, the patients in their study were
similar to patients with well-recognized chronic debilitating
conditions such as congestive heart failure, ischemic heart disease, or
chronic obstructive pulmonary disease. In a similar study, Tran and
Thordarson,117 using validated
outcome instruments such as the SF-36, the American Academy of
Orthopaedic Surgeons (AAOS) lower limb core questionnaire, and the AAOS
foot and ankle questionnaire,59,90
found that the multiply injured patients with associated foot injuries
in their study had had dramatically lower Physical Function (38.9
versus 80.7), Role Physical (a perception of their physical function,
41.1 versus 87.5), Bodily Pain (50.6 versus 81.8), and Social Function
(67.9 versus 96.6) compared with the control group of multiply injured
patients without associated foot injuries. By use of the AAOS
questionnaire, their study also addressed specific lower extremity
musculoskeletal endpoints. All five of these scales also showed
significantly lower scores for factors such as pain, treatment
expectations, satisfaction with symptoms, and shoe comfort in those
patients with associated foot injuries.
severity of injury to the ipsilateral foot, one should proceed
cautiously when recommending salvage in the face of severe crush
injuries to the foot. In this situation, a given tibial injury or
“mangled” lower limb with concomitant severe injuries to the foot might
preclude achieving reasonable limb function despite the feasibility of
salvage and amputation may indeed be a better long-term option.
medical comorbidities such as coronary heart disease and chronic
obstructive pulmonary disease in a patient with potentially limb
threatening injury, but it also can be used early as a prognostic
variable to help inform the patient of potential long-term treatment
complications and perhaps better guide treatment recommendations. Both
basic science and clinical studies have consistently documented
suspected links between cigarette smoking and complications of the
fracture healing process. Several studies have provided preliminary
evidence of a link between smoking and delayed bone healing and
nonunion,* infection,34,77,113 and osteomyelitis.34,105
Laboratory studies have also shown that nicotine reduces
vascularization at bone healing sites, and this is associated with
delayed healing in animal models.25,49,119 Smoking has also been associated with decreased immune function.61,69,106
been the presence of many potential confounding variables that may have
also affected the outcomes, thus refuting the overall impact of smoking
on such negative outcomes as delayed union, nonunion, and infection.
Patient age, education, and socioeconomic status have all been shown to
have deleterious effects on overall health status, access to treatment,
treatment compliance, and other health behaviors, which may have
affected the higher complication rates seen in some of the smoking
cohorts. In an effort to address these issues, Castillo et al.15
used data from the LEAP project to determine if cigarette smoking
increased the risk of complications in patients with a limb-threatening
open tibial fracture, while adjusting for the previously mentioned
confounders. They were able to demonstrate that current smoking and
even a previous smoking history independently placed the patient at an
increased risk for nonunion and infectious complications. Current
smokers and previous smokers were 37% and 32%, respectively, less
likely to achieve union than nonsmokers. Current smokers were also more
than twice as likely to develop an infection and 3.7 times more likely
to develop osteomyelitis than were nonsmokers. Furthermore, previous
smokers were also 2.8 times more likely to develop osteomyelitis than
were patients without a prior history of tobacco use.
with increased bone healing complications in the patient with a
limb-threatening injury, but also smoking can significantly threaten
the likelihood of success of the soft tissue portion of the
reconstructive effort. Smoking is associated with a significant
reduction in peripheral blood flow. Sarin et al.100
have shown that blood flow to the hand is reduced as much as 42% after
smoking just one cigarette. Cigarette use has also been shown to
negatively
affect peripheral blood flow in free transverse rectus abdominus flaps.7 Microsurgeons have reported poor outcomes after digital replantation in smokers. Chang et al.18
noted that approximately 80% to 90% of cigarette smokers will lose
their replanted digits if tobacco use occurred within 2 months before
their surgery. Cigarette use has been shown to lead to increased local
flap and full-thickness graft necrosis compared with nonsmoking status.38
Smoking has also been shown to adversely affect the success and
complication rates associated with microvascular free tissue transfer.
Reus et al.95 studied the incidence
of free tissue transfer survival and complications in nonsmokers,
active smokers, and patients who had discontinued smoking before
surgical intervention. In their series, they found that complications
occurred more often in active smokers, with these complications often
occurring at the interface between the flap and its bed or an overlying
skin graft. They also found that smokers required more secondary
surgical procedures at the recipient site to accomplish ultimate wound
closure. Lovich and Arnold70
examined the effect of smoking on various muscle transposition
procedures. They performed a retrospective review of 300 pedicled
muscle flap procedures and determined that active smokers had a
significantly higher complication rate than nonsmokers and smokers who
had previously quit. In the smoking group, they noticed a higher
incidence of both partial muscle flap necrosis and partial skin graft
loss with most of these complications occurring in the immediate
postoperative period. Not only is smoking associated with an increased
complication rate at the recipient site, but smokers have also been
shown to have an increased rate of complications at the donor site.17
current cigarette smoking places the patient with a limb-threatening
injury at increased risk for both osseous and soft tissue complications
These factors must be discussed at length and weighed very carefully
with the patient before embarking on a prolonged course to salvage a
mangled limb.
return of the patient to as close to a preinjury level of performance
and social interaction as possible are dependent on the interaction of
the patient, the patient’s environment, the injury, and the treatment
course (Fig. 12-3). Understanding the potential
impact of elements outside of the surgeon’s control—the patient and the
patient’s environment—is critical to the development of an effective
care plan. Through data obtained by the LEAP Study group, Mackenzie et
al.71 were able to characterize and
help provide the medical community with a better understanding of the
type of patients who face the challenge of amputation versus salvage in
the face of a limb-threatening injury. In that study, most of the
patients were male (77%), white (72%), and between the ages of 20 and
45 years (71%). These patients were often less educated, as only 70%
were high school graduates versus a national rate of 86%. These
patients were often impoverished. Significantly more of the patients
(25%) lived in households with incomes below the federal poverty line
compared with the national rate (16%). This patient cohort also had
significantly higher rates of uninsured individuals (38%) and had
double the national average of heavy drinkers. Not only do these
patients typically present with socioeconomic challenges, but many will
have psychological and psychosocial issues, which can make the
treatment plan and recovery even more of a challenge. Patients in this
study were also found to be slightly more neurotic and extroverted and
less open to new experiences compared with the general population. No
significant differences were detected between the characteristics of
patients entered into the reconstruction or amputation groups.
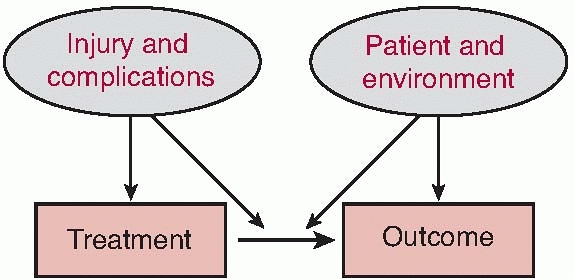 |
|
FIGURE 12-3 Factors influencing treatment decision and outcome.
|
care for patients with mangled lower extremities. Compared with the
general population, patients with limb-threatening injuries have fewer
resources, which can potentially limit their access to rehabilitation
services and affect their ability to accommodate to residual
disability. These patients are typically employed in more physically
demanding jobs, which may impede efforts to return to work, and they
have poorer health habits, which may complicate recovery. The
personality traits identified in this population could also predispose
these patients to a more difficult recovery.
as early as possible, the correct treatment pathway for the patient.
The surgeon must weigh the fact that, in most cases, limb
reconstruction is possible given the appropriate application of current
techniques and counterbalance the expected result of salvage against
that which is possible with amputation. Prosthetic bioengineering
innovations have significantly improved the function and comfort of
lower extremity amputees. Most series reporting on the results of limb
salvage or amputation are single center, small, and retrospective.
Their conclusions provide a glimpse into the complexity of the clinical
decision-making process, but these studies alone should not be used to
guide clinical decisions.
optimal treatment option in the setting of the mangled extremity.
Georgiadis et al.36 retrospectively
compared the functional outcomes of 26 patients with successfully
reconstructed Grade IIIB open tibia fractures with the outcomes of 18
patients managed with early below-knee amputation. Five patients in the
reconstruction group required a late amputation to treat infection
complications. The reconstruction patients had more operations, more
complications, and longer hospital stays than did patients treated by
early amputation. The functional outcomes of the 16 successful
reconstructions were compared with the outcomes of the early amputation
patients. They found that the reconstruction patients took more time to
achieve full weight
bearing
and were less willing or able to return to work. Validated outcomes
instruments were used to assess the quality of life for a subset of the
patients. Significantly more limb salvage patients considered
themselves to be severely disabled and impaired for both occupational
and recreational activities. The authors concluded that early
below-knee amputation resulted in a quicker recovery with less
long-term disability.
a retrospective review of 72 acute Grade IIIB open tibia fractures
requiring soft tissue reconstruction from 1983 to 1988, also showed
that while limb salvage can be successful, over 50% of the patients in
the salvage group had severe limitations in the salvaged limb by
objective motion measurements, and 48% of the patients in the salvage
group at least intermittently required the use of an assistive device
for ambulation after complete healing. They also found that in the
salvage group, the long-term employment rate was 28% and no patient
returned to work after 2 years of unemployment. In contrast, 68% of
trauma-related lower extremity amputees from their institution over the
same time period returned to work within 2 years.
claim that patients undergoing amputation often have shorter initial
hospital stays, decreased initial hospital costs, and a higher
likelihood of resuming gainful employment, thus decreasing the
financial burden of this life-altering injury.
retrospectively compared below-knee amputees with patients receiving
complex reconstructions after a Grade IIIB or IIIC open tibial
fracture. They also concluded that for the first 4 years after injury,
amputation resulted in lower mean annual hospital costs than
reconstruction and amputation patients required 3.5 interventions and
12 months of rehabilitation compared with an average of 8 interventions
and 30 months of rehabilitation for the reconstruction patients.
However, amputation patients were reported as having a higher dollar
cost to society, a figure that was inflated by adding the amounts of
permanent disability assigned to an amputee compared with a
reconstruction patient. Despite this fact, the authors eventually
concluded that functional outcome based on pain, range of motion,
quadriceps wasting, and walking ability was better in the
reconstruction group than in the amputation group and therefore limb
reconstruction was advisable (although the data to support this
conclusion was soft and no patient-directed outcome measures were used.)
touted reconstruction as the preferred option in the management of the
mangled extremity. They retrospectively evaluated 55 Grade IIIB and
IIIC tibia fractures cared for over a 12-year period. The SF-36 was
used as the primary outcomes measure. Although both groups had SF-36
(Physical Component) outcomes scores as low as or lower than those of
many serious medical illnesses, successful salvage patients had
significantly better physical subscale scores than did amputees. Both
groups had psychological subscores similar to a healthy population.
Furthermore, 92% of their patients preferred their salvaged leg to an
amputation at any stage of their injury, and none would have preferred
a primary amputation. Based on their findings, the authors suggested
that a below-knee amputation was an inferior option to a successfully
reconstructed leg.
less costly in the short term, reconstruction may be more cost
effective compared with amputation when lifelong prosthetic costs are
taken into account. Smith et al.108
reviewed hospital and prosthetist records for 15 of 20 patients who
survived initial trauma and eventually underwent isolated below-knee
amputation from 1980 through 1987. Using the medical record and the
billing records of the prosthetist, they calculated the number of
prostheses fabricated and the overall prosthetic charges since the
initial amputation. They found that during the first 3 years, the mean
number of prostheses acquired per patient was 3.4 (range, 1 to 5), with
an average total prosthetic charge of $10,829 (range, $2,558 to
$15,700). Over the first 5 years, the mean number of prostheses
acquired per patient increased to 4.4 (range, 2 to 8), with average
total prosthetic charges of $13,945 (range, $6,203 to $20,070). Williams123
also compared hospital costs and professional fees of 10 patients with
Ilizarov limb reconstruction to the hospital costs, professional fees,
and prosthetic costs of 3 patients with acute and 3 patients with
delayed lower extremity amputation. The average treatment time was
higher in the Ilizarov reconstruction group. The hospital costs and
professional fees for the amputation group averaged $30,148 without
prosthetic costs, while the total cost of the Ilizarov limb
reconstruction averaged $59,213. However, with projected lifetime
prosthetic costs included, the average long-term cost for the amputee
was estimated to be $403,199. Thus, he concluded that Ilizarov limb
reconstruction is a more cost-effective treatment option than
amputation when long-term prosthetic costs are considered.
limb reconstruction has best been analyzed through information
collected via the LEAP Study. MacKenzie et al.75
compared the 2-year direct health care costs and projected lifetime
health care costs associated with both treatment pathways. The
calculated patient costs included the initial hospitalization, all
rehospitalizations for acute care related to the limb injury, any
inpatient rehabilitation, outpatient physician visits, outpatient
physical and occupational therapy, and the purchase and maintenance of
any prosthetic devices. When the costs associated with
rehospitalizations and postacute care were added to the cost of the
initial hospitalization, the 2-year costs for reconstruction and
amputation were similar. However, when prosthesis-related costs were
added, there was a substantial difference between the two groups
($81,316 for patients treated with reconstruction and $91,106 for
patients treated with amputation). Furthermore, the projected lifetime
health care cost for the patients who had undergone amputation was
three times higher than that for those treated with reconstruction
($509,275 and $163,282, respectively). Based on these estimates, they
concluded that efforts to improve the rate of successful
reconstructions have merit and that not only is reconstruction a
reasonable goal, but it may result in lower lifetime costs to the
patient.
studies offer important insight into the various arguments for
amputation or salvage of the mangled extremity, they are also somewhat
contradictory, which is likely a result of the retrospective design and
small sample sizes in many of the series. The research teams could not
adequately assess or control for the injury, treatment, patient, and
patient environment variables that could influence the outcome.
outcomes of a large cohort of patients from eight Level I trauma
centers who underwent reconstruction or amputation following an open
tibial shaft fracture. The hypothesis was that after controlling for
the severity of the limb injury, the presence and
severity
of other injuries, and patient characteristics, amputation would prove
to have a better functional outcome than reconstruction. Detailed
patient, patient environment, injury, and treatment (hospital and
outpatient) data were collected for each patient.73
The SIP was used as the primary outcome measurement. The SIP is a
multidimensional measure of self-reported health status (scores range
from 0 to 100; scores for the general population average 2 to 3, and
scores of greater than 10 represent severe disability). Secondary
outcomes included the limb status and the presence or absence of a
major complication that required rehospitalization. Five hundred
sixty-nine patients were followed over 2 years. No significant
difference was detected at 2 years in the SIP scores between the
amputation and the reconstruction patients. After adjustment for the
characteristics of the patients and their injuries, patients who
underwent amputation had outcomes that were similar to those who
underwent limb reconstruction.9
environmental variables in the LEAP Study also identified a number of
predictors of poorer SIP scores. Negative factors included the
rehospitalization of a patient for a major complication, a low
education level, nonwhite race, poverty, lack of private health
insurance, a poor social support network, a low self-efficacy (the
patient’s confidence in being able to resume life activities), smoking,
and involvement with disability-compensation litigation (Table 12-9).
To underscore the combined influence of these multiple factors on
outcome, adjusted SIP scores were estimated for two subgroups of
patients. A patient with a high school education or less, poor social
support, and rehospitalization for a major complication had a mean
adjusted SIP score of 15.8. A comparable score for a patient with some
college education, strong social support, and an uncomplicated recovery
was 8.3 (Table 12-10). Although patients with
substantial economic and social resources and no complications could
not function at the level of a healthy adult of similar age and gender
(SIP typically less than 4), they were still significantly better off
than those without such resources.
reconstruction were more likely to be rehospitalized than were those
who underwent amputation (47.6% versus 33.9%). At 2 years, nonunion was
present in 10.9% of the reconstruction patients and 9.4% had developed
osteomyelitis. Additional operations were anticipated for 5% of the
amputation patients and for 19% of the reconstruction patients. The
levels of disability, as measured by the SIP, were high in both groups.
More than 40% of the patients had an SIP score of greater than 10,
reflecting severe disability. Except for scores on the psychosocial
subscale, there was significant improvement in the scores over time in
both treatment groups. Return to work success was disappointing. At 24
months, only 53.0% of the patients who underwent amputation and 49.4%
of those who underwent reconstruction had returned to work.
|
TABLE 12-9 Predictors of Poor Outcome Found in the LEAP Study after Adjusting for Extent of Injury
|
|
|---|---|
|
|
TABLE 12-10 Adjusted Sickness Impact Profile (SIP) Scores Estimated for Two Patient Subgroups
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
||||||||
reexamined the outcomes of patients originally enrolled in the study to
determine whether their outcomes improved beyond 2 years and whether
differences according to the type of treatment emerged. Three hundred
ninety-seven of the 569 patients who had originally undergone
amputation or reconstruction of the lower extremity were interviewed by
telephone at an average of 84 months after the injury. Functional
outcomes were assessed with use of the physical and psychosocial
subscores of the SIP and were compared with the scores obtained at 24
months. On the average, physical and psychosocial functioning
deteriorated between 24 and 84 months after the injury. At 84 months,
half of the patients had a physical SIP subscore of 10 or more points,
which is indicative of substantial disability, and only 34.5% had a
score typical of the general population of similar age and sex. There
were few significant differences in the outcomes according to the type
of treatment, with two exceptions. Compared with patients treated with
reconstruction for a tibial shaft fracture, those with only a severe
soft tissue injury of the leg were 3.1 times more likely to have a
physical SIP subscore of 5 points and those treated with a
through-the-knee amputation were 11.5 times more likely to have a
physical subscore of 5 points. There were no significant differences in
the psychosocial outcomes according to treatment group. At 7-year
follow-up, patient characteristics that were significantly associated
with poorer outcomes included older age, female sex, nonwhite race,
lower education level, living in a poor household, current or previous
smoking, low self-efficacy, poor self-reported health status before the
injury, and involvement with the legal system in an effort to obtain
disability payments. Except for age, predictors of poor outcome were
similar at 24 and 84 months after the injury. These results confirmed
the previous conclusion of the LEAP Study that limb reconstruction
results in functional outcomes equivalent to those of amputation.
The results also showed that regardless of the treatment option, long-term functional outcomes are likely to be poor.
Level I trauma centers must be cautioned against. In the Level 1 trauma
center, surgeons should advise their patients with mangled lower limbs
that the functional results of reconstruction are equivalent to
amputation. The reconstruction process requires more operations and
more hospitalizations and is associated with a higher complication
rate. At 2 years, both patient groups are significantly disabled, and
only 48% are returned to work. Both patient groups show evidence of
lingering psychosocial disability. Given the “no outcome difference” at
2 years, patients and surgeons can be comfortable recommending or
selecting limb-preservation surgery. Efforts to minimize complications
and hastened fracture union might improve the outcome of the
reconstruction patients.
improvements in outcome might require greater emphasis on nonclinical
interventions, such as early evaluation by vocational rehabilitation
counselors. The study also confirms previous research that found both
self-efficacy and social support to be important determinants of
outcome.30,76
Interventions aimed at improving support networks and self-efficacy may
benefit patients facing a challenging recovery. Surgeons also need to
acknowledge the long-term psychosocial disability associated with the
mangled extremity, regardless of the treatment. Posttraumatic stress
disorder screening and appropriate referral of patients for therapy may
need to become a proactive part of the postoperative treatment plan.79,82,83,109
also identified a number of clinical issues that can be used by the
surgeon in planning amputation level and stump coverage. There were no
significant differences between above-knee amputations and below-knee
amputations in return to work rates, pain, or SIP scores. Patients with
through-knee amputations had SIP scores that were 40% higher than those
patients who received either a below-knee amputation or an above-knee
amputation. Patients with through-knee amputations also demonstrated
significantly lower walking speeds. Physicians were less satisfied with
the clinical, cosmetic, and functional recovery of through-knee
amputations compared with above-knee and below-knee amputation. Thus,
as a generality, in the adult trauma population, a through-knee
amputation should be avoided whenever possible.
adversely affect the outcome in this study, suggesting that efforts to
preserve the knee are worthwhile.72
Furthermore, patient outcomes were not affected by the technical
sophistication of the prosthesis, although patients with
higher-technology prostheses were more satisfied. These findings will
challenge the physician who currently fits a patient with a
sophisticated (and expensive) prosthesis and the results underscore the
need for controlled studies that examine the relationships between the
type of prosthetic device, the fit of the device, and its functional
outcomes.24,72
lower extremity is a difficult one, which relies not only on the
expertise of the orthopaedic surgeon but also on the input of his
subspecialty colleagues (general trauma surgeons, vascular surgeons,
and plastic surgeons) as well as the patient. The decision to
reconstruct or amputate an extremity cannot depend on limb salvage
scores, as all have proved to have little clinical utility. Using
current technology and Level I trauma center orthopaedic clinical
experience, combined with multispecialty support, current data appear
to suggest that the results of limb reconstruction are equal to those
of amputation following severe lower extremity trauma, and this
observation should encourage the continued efforts to reconstruct
severely injured limbs. Ideally, the patient with a mangled extremity
should be directed to an experienced limb injury center, where
strategies to minimize complications, address related posttraumatic
stress disorder, improve the patient’s self-efficacy, and target early
vocational retraining may improve the long-term outcomes in patients
with these life-altering injuries.
P, Marti-Garin D, Murias-Alvarez J, et al. External fixation and
secondary intramedullary nailing of open tibial fractures. A
randomized, prospective trial. J Bone Joint Surg Br 1997;79:433-437.
T, Mehta P, Smith M, et al. Routine use of wound vacuum-assisted
closure does not allow coverage delay for open tibia fractures. Plast
Reconstr Surg 2008;121:1263-1266.
J, Wittig JC, Kollender Y, et al. Sciatic nerve resection: is that
truly an indication for amputation? Clin Orthop 2002;399:201-204.
DI, Debats IB, Boeckx WD, et al. Risk factors and blood flow in the
free transverse rectus abdominis (TRAM) flap: smoking and high flap
weight impair the free TRAM flap microcirculation. Ann Plast Surg
2007;59:364-371.
MJ, MacKenzie EJ; the LEAP Study Group. A Prospective Evaluation of the
Clinical Utility of the Lower-Extremity Injury Severity Scores. J Bone
Joint Surg 2001;83: 3-14.
MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of
reconstruction or amputation after leg-threatening injuries. N Engl J
Med 2002;347:1924-1931.
MJ, McCarthy ML, Jones AL, et al. The insensate foot following severe
lower extremity trauma: an indication for amputation? J Bone Joint Surg
Am 2005;87A: 2601-2608.
G, Cronier P, Gouello JP, et al. Hyperbaric oxygen therapy in the
management of crush injuries: a randomized double-blind
placebo-controlled clinical trial. J Trauma 1996;41:333-339.
AD, Gold JS, Graham D, et al. Resection of the sciatic, peroneal, or
tibial nerves: assessment of functional status. Ann Surg Oncol
2002;9:41-47.
CW, Orme TJ, Richardson HD. The rate of pseudarthrosis (surgical
nonunion) in patients who are smokers and patients who are nonsmokers:
a comparison study. Spine 1986;11:942-943.
RC, Bosse MJ, MacKenzie EJ, et al. Impact of smoking on fracture
healing and risk of complications in limb-threatening open tibia
fractures. J Orthop Trauma 2005; 19:151-157.
DW, Reece GP, Wang B, et al. Effect of smoking on complications in
patients undergoing free TRAM flap breast reconstruction. Plast
Reconstr Surg 2000;105: 2374-2380.
EP, Bosse MJ, Robb G. Reconstruction of large diaphyseal defects,
without free fibular transfer, in Grade-IIIB tibial fractures. J Bone
Joint Surg Am 1989;71A: 994-1004.
TK, Gabrielsen TA, Campbell DC, et al. Cigarette smoking and nonunion
after ankle arthrodesis. Foot Ankle Int 1994;15:64-67.
C. The history of fracture treatment. In Browner BD, Jupiter JB, Levine
AM, Trafton PG, eds. Skeletal trauma. Philadelphia: Saunders;2003:3-28.
JK, MacKenzie EJ, Smith DG, et al. Prosthetic device satisfaction among
patients with lower extremity amputation due to trauma. Toronto,
Ontario, Canada:Orthopaedic Trauma Association 18th Meeting Abstracts
2002.
TK, Whitesides TE Jr, Heller JG, et al. Nicotine on the
revascularization of bone graft. An experimental study in rabbits.
Spine 1994;19:904-911.
F, Roukoz S. Compound tibial fractures with bone loss treated by the
Ilizarov technique. J Bone Joint Surg Br 1991;73B:316-321.
AB, Best AK, Schemitsch EH, et al. Salvage after severe lower-extremity
trauma: are the outcomes worth the means? Plast Reconstr Surg
1999;103:1212-1220.
BT, Kortesis B, Punger K, et al. The use of negative-pressure wound
therapy (NPWT) in the temporary treatment of soft-tissue injuries
associated with high-energy open tibial shaft fractures. J Orthop
Trauma 2007;21:11-17.
CK, Stewart KJ, Gillilan RE, et al. Self-efficacy mediates strength
gains during circuit weight training in men with coronary artery
disease. Med Sci Sports Exerc 1986; 18:531-540.
EA, Corso PS, Miller TR, Associates. Incidence and Economic Burden of
Injuries in the United States. New York: Oxford University Press; 2006.
MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting,
and intramedullary nailing in patients who have a fracture of the
tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am
1991;73A:1316-1322.
JW, Starr AJ, Early JS. Early wound complications of operative
treatment of calcaneus fractures: analysis of 190 fractures. J Orthop
Trauma 1999;13:369-372.
TJ, Vander Kolk CA, Hoopes JE, et al. Microvascular soft-tissue
transplantation for reconstruction of acute open tibial fractures:
timing of coverage and long-term functional results. Plast Reconstr
Surg 1992;89:478-487.
GM, Behrens FF, Joyce MJ, et al. Open tibial fractures with severe
soft-tissue loss. Limb salvage compared with below-the-knee amputation.
J Bone Joint Surg Am 1993;75A:1431-1441.
S, Majumder S, Batchelor AG, et al. Fix and flap: the radical
orthopaedic and plastic treatment of severe open fractures of the
tibia. J Bone Joint Surg Br 2000;82B: 959-966.
S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic
protein-2 for treatment of open tibial fractures. J Bone Joint Surg
2002;84:2123-2134.
RT, Gould RJ, Peclet M, et al. The mangled extremity syndrome (M.E.S.):
a severity grading system for multisystem injury of the extremity. J
Trauma 1985;25: 1147-1150.
DJ, Lee SS, Goulet JA. Success of exchange reamed intramedullary
nailing for femoral shaft nonunion or delayed union. J Orthop Trauma
2000;14:178-182.
EJ, Agel J, Selznick HS, et al. Deleterious effect of smoking on
healing of open tibia-shaft fractures. Am J Orthop 2002;31:518-521.
DL, Howey T, Sanders R, et al. Limb salvage versus amputation.
Preliminary results of the Mangled Extremity Severity Score. Clin
Orthop Relat Res 1990;80-86.
MB, Chapman JR, Agel J, et al. Treatment of type II, IIIA, and IIIB
open fractures of the tibial shaft: a prospective comparison of
unreamed interlocking intramedullary nails and half-pin external
fixators. J Orthop Trauma 1998;12:1-7.
R, Lambert SM, Muller S, et al. On the timing of soft-tissue
reconstruction for open fractures of the lower leg. Arch Orthop Trauma
Surg 1999;119:7-12.
R, Strebel N, Ganz R. Amputation versus reconstruction in traumatic
defects of the leg: outcome and costs. J Orthop Trauma 1996;10:223-229.
HR Jr, Poole GV Jr, Hansen KJ, et al. Salvage of lower extremities
following combined orthopedic and vascular trauma. A predictive salvage
index. Am Surg 1987; 53:205-208.
CW. The primary repair of wounds of major arteries; an analysis of
experience in Korea in 1953. Ann Surg 1955;141:297-303.
TK, Pai MP. The effect of varying ambient oxygen tensions on wound
metabolism and collagen synthesis. Surg Gynecol Obstet 1972;135:561-567.
WM, Taffet R, DeLong WG Jr, et al. Early exchange intramedullary
nailing of distal femoral fractures with vascular injury initially
stabilized with external fixation. J Trauma 1994;37:446-451.
K, Bandyk D, Thiele B, et al. Temporary intraluminal shunts: resolution
of a management dilemma in complex vascular injuries. J Trauma
1982;22:395-402.
K, Daines M, Howey T, et al. Objective criteria accurately predict
amputation following lower extremity trauma. J Trauma 1990;30:568-572.
NA, Liang MH, Daltroy L, et al. American Academy of Orthopaedic
Surgeons lower limb outcomes assessment instruments. Reliability,
validity, and sensitivity to change. J Bone Joint Surg Am
2004;86A:902-909.
AL, Bucholz RW, Bosse MJ, et al. Recombinant human BMP-2 and allograft
compared with autogenous bone graft for reconstruction of diaphyseal
tibial fractures with cortical defects. A randomized, controlled trial.
J Bone Joint Surg Am 2006;88A: 1431-1441.
R, Singh SP, Savage SM, et al. Effects of cigarette smoke on immune
response: chronic exposure to cigarette smoke impairs antigen-mediated
signaling in T cells and depletes IP3-sensitive Ca(2+) stores. J
Pharmacol Exp Ther 2000;293:166-171.
RK, Shaw WW. Reconstruction of the lower extremity with microvascular
free flaps: a 10-year experience with 304 consecutive cases. J Trauma
1989;29:1086-1094.
P, Tarkin IS, Frink M, et al. [Voluminous bone graft harvesting of the
femoral marrow cavity for autologous transplantation: an indication for
the “Reamer-Irrigator-Aspirator” (RIA-) technique.] Unfallchirurg
2008;111:469-472.
A, Usenius JP, Aarnio M, et al. Are smokers a risk group for delayed
healing of tibial shaft fractures? Ann Chir Gynaecol 1993;82:254-262.
RH. Limb reconstruction versus amputation decision making in massive
lower extremity trauma. Clin Orthop Relat Res 1989;92-99.
RH, Bach AW, Hansen ST Jr, et al. Open tibial fractures with associated
vascular injuries: prognosis for limb salvage. J Trauma 1985;25:203-208.
MJ, Smith JM, Gould M. Treatment of the mangled lower extremity after a
terrorist blast injury. Clin Orthop Relat Res 2004;88-96.
CG, Caggiula AR, Knopf S, et al. The effects of nicotine on the immune
system. Psychoneuroendocrinology 1998;23:175-187.
EJ, Bosse MJ, Kellam JF; LEAP Study Group. Characterization of the
patients undergoing amputation versus limb salvage for severe lower
extremity trauma. J Orthop Trauma 2000;14:455-466.
EJ, Bosse MJ, Castillo RC, et al. Functional outcomes following
trauma-related lower-extremity amputation. J Bone Joint Surg Am
2004;86A:1636-1645.
EJ, Bosse MJ, Kellam JF, et al. Characterization of patients with
high-energy lower extremity trauma. J Orthop Trauma 2000;14:455-466.
EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability
following severe lower-limb trauma. Results of a 7-year follow-up. J
Bone Joint Surg Am 2005; 87A:1801-1809.
EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with
amputation or reconstruction of a limb-threatening injury. J Bone Joint
Surg Am 2007;89A: 1685-1692.
EJ, Morris JA Jr, Jurkovich GJ, et al. Return to work following injury:
the role of economic, social, and job-related factors. Am J Public
Health 1998;88: 1630-1637.
DR, Shah S, Elliott J, et al. The Ilizarov method in nonunion,
malunion, and infection of fractures. J Bone Joint Surg Br
1997;79B:273-279.
ML, MacKenzie EJ, Edwin D, et al. Psychological distress associated
with severe lower-limb injury. J Bone Joint Surg Am 2003;85A:1689-1697.
MD, Yoo DJ, Zdero R, et al. Combined single-stage osseous and soft
tissue reconstruction of the tibia with the Ilizarov method and tissue
transfer. J Orthop Trauma 2008;22:183-189.
MG, Heckman JD, Corley FG. Severe open fractures of the lower
extremity: a retrospective evaluation of the Mangled Extremity Severity
Score (MESS). J Orthop Trauma 1994;8:81-87.
AJ, Michaels CE, Moon CH, et al. Posttraumatic stress disorder after
injury: impact on general health outcome and early risk assessment. J
Trauma 1999;47: 460-466.
JG, Svoboda JA, Parks SN. Use of temporary intraluminal shunts in
selected peripheral arterial injuries. J Trauma 1986;26:1094-1096.
SA, Willis MD. Initial management of open fractures. In: Rockwood and
Green’s Fractures in adults (pp. 390-391), 6th ed. Philadelphia:
Lippincott Williams & Wilkins, 2006.
instruments and information: lower extremity instruments. American
Association of Orthopaedic Surgeons web site. Retrieved February 24,
2009, from http:// www.aaos.org/research/outcomes/outcomeslower.asp.
A. Dix Livres de la Chirurgie avec la Magasin des instruments
Necessaires a Icelle. 7, Chapter 13. Paris: Jean le Royer, 1564.
AN, McCarthy ML, Burgess AR. Short-term wound complications after
application of flaps for coverage of traumatic soft-tissue defects
about the tibia. The Lower Extremity Assessment Project (LEAP) Study
Group. J Bone Joint Surg Am 2000;82A: 1681-1691.
WF III, Colen LB, Straker DJ. Tobacco smoking and complications in
elective microsurgery. Plast Reconstr Surg 1992;89:490-494.
RS, Weitzman AM, Tracey WJ, et al. Simultaneous treatment of tibial
bone and soft-tissue defects with the Ilizarov method. J Orthop Trauma
2006;20:197-205.
WL, Sailors DM, Whittle TB, et al. Limb salvage versus traumatic
amputation. A decision based on a seven-part predictive index. Ann Surg
1991;213:473-480.
MA, Finnegan M, Natarajan R, et al. Effect of smoking on tibial shaft
fracture healing. Clin Orthop Relat Res 1999;184-200.
AE, Lower R. Late results of free-muscle flaps and delayed bone
grafting in the secondary treatment of open distal tibial fractures.
Plast Reconstr Surg 1989;83:77-84.
KA, Gerich T, Jakob RP. Sequential intramedullary nailing of open
tibial shaft fractures after external fixation. Arch Orthop Trauma Surg
1997;116:32-36.
KA, Schillig B, Jakob RP. Treatment of complex tibial shaft fractures.
Arguments for early secondary intramedullary nailing. Clin Orthop Relat
Res 1993; 269-274.
HJ, Patzakis MJ, Holtom PD, et al. Limb salvage for chronic tibial
osteomyelitis: an outcomes study. J Trauma 2000;48:484-489.
SP, Kalra R, Puttfarcken P, et al. Acute and chronic nicotine exposures
modulate the immune system through different pathways. Toxicol Appl
Pharmacol 2000;164: 65-72.
JR, Britton C, Moneim MS, et al. Mangled extremity severity score: an
accurate guide to treatment of the severely injured upper extremity. J
Orthop Trauma 1994;8:282-285.
DG, Horn P, Malchow D, et al. Prosthetic history, prosthetic charges,
and functional outcome of the isolated, traumatic below-knee amputee. J
Trauma 1995;38: 44-47.
AJ, Smith WR, Frawley WH, et al. Symptoms of posttraumatic stress
disorder after orthopaedic trauma. J Bone Joint Surg Am
2004;86A:1115-1121.
NP, Barbey N, Veuskens A, et al. The incidence of osteitis in open
fractures: an analysis of 948 open fractures (a review of the Hannover
experience). J Orthop Trauma 1993;7:473-482.
MF, MacKenzie EJ, Bosse MJ, et al. Factors influencing the decision to
amputate or reconstruct after high-energy lower extremity trauma. J
Trauma 2002;52: 641-649.
JS, Cotler HB, Sasso RC, et al. Postoperative infections in spinal
implants. Classification and analysis—a multicenter study. Spine
1991;16:981-984.
S, Yamami N, Nakayama H, et al. The validity of the mangled extremity
severity score in the assessment of upper limb injuries. J Bone Joint
Surg Br 2005;87B: 1516-1519.
P III, Bergman M, Watnik N, et al. Treatment of grade-IIIb open tibial
fractures. A prospective randomised comparison of external fixation and
nonreamed locked nailing. J Bone Joint Surg Br 1994;76B:13-19.
T, Thordarson D. Functional outcome of multiply injured patients with
associated foot injury. Foot Ankle Int 2002;23:340-343.
DC, Schemitsch EH, McKee MD, et al. Do foot injuries significantly
affect the functional outcome of multiply injured patients? J Orthop
Trauma 1999;13:1-4.
SW, Lee SS, Lin SS, et al. Hyperbaric oxygen therapy mitigates the
adverse effect of cigarette smoking on the bone healing of tibial
lengthening: an experimental study on rabbits. J Trauma 1999;47:752-759.
LX, Bosse MJ, Castillo RC, et al. Analysis of surgeon-controlled
variables in the treatment of limb-threatening type-III open tibial
diaphyseal fractures. J Bone Joint Surg Am 2007;89A:923-928.
MO. Long-term cost comparison of major limb salvage using the Ilizarov
method versus amputation. Clin Orthop Relat Res 1994;156-158.
P, Slack R, Harvey L, et al. The prevention of infection in open
fractures: an experimental study of the effect of fracture stability.
Injury 1994;25:31-38.
MJ, Brumback RJ, Manson PN, et al. Acute and definitive management of
traumatic osteocutaneous defects of the lower extremity. Plast Reconstr
Surg 1987;80: 1-14.
