MANAGEMENT OF THE PATIENT WITH FAILED LOW-BACK SURGERY
VIII – THE SPINE > Disc Injury and Degeneration > CHAPTER 149 –
MANAGEMENT OF THE PATIENT WITH FAILED LOW-BACK SURGERY
Associate Professor, Department of Orthopaedic Surgery, Chief, Division
of Spine Surgery, Georgetown University Hospital, Washington, D.C.,
20007.
back pain following previous surgery on the lumbar spine is a complex
challenge. It is estimated that, in the United States alone, more than
300,000 lumbar laminectomies and 70,000 fusions are performed annually;
at least 15% of these patients fail to achieve long-lasting pain relief
(15,36).
represents a unique challenge and opportunity; many patients can be
made better with appropriate operative or nonoperative treatment, but
there is a great chance of succumbing to the assumption that another
operation, in the absence of objective indications, will be the
solution to the patient’s problem. The inherent complexity of these
cases necessitates an approach to evaluation that is precise and
unambiguous—one that, it is hoped, will lead to accurate identification
of the source of the patient’s pain and to appropriate treatment.
prevention. Although the technical aspects of performing surgery on the
lumbar spine are very important, proper patient selection is probably
the most important factor in avoiding postoperative failure (see Chapter 144). Long et al. (25) reviewed 78 patients with so-called failed back surgery
syndrome (FBSS) in a chronic pain program. They noted that, when
original records were reviewed from before the first operation, 68% of
these patients failed to fulfill any objective criteria available in
either the orthopaedic or neurosurgical literature for surgery.
Fifty-six percent were found to have an underlying psychiatric
abnormality.
most common factor associated with failure. Thus, it is clear that the
initial decision to operate is the most important one and should be
arrived at only when clear identification of the source of the
patient’s pain is made and objective criteria for the proposed surgery
are met. See the objective patient evaluation system in Chapter 144. Once low-back surgery has failed, the potential for a solution is limited.
distinguish between a mechanical source for the complaint from
nonmechanical causes. The types of mechanical conditions that respond,
in select cases, to revision surgery include recurrent disc herniation,
discogenic pain, segmental instability of the spine, and spinal
stenosis. Nonmechanical entities that can lead to recurrent symptoms
include local scar tissue formation (either arachnoiditis or epidural
fibrosis), abdominal or pelvic disorders, systemic medical diseases, or
psychosocial instability. These nonmechanical problems will not be
helped by additional spinal surgery.
accurate diagnosis. Although this factor is seemingly obvious, this
essential step is often neglected and the rehabilitation of the patient
is therefore inadequate.
with recurrent symptoms following previous lumbar spine surgery is
essential. Use a standardized form to detail the medical history and to
list any and all previous back operations, including dates and the type
of operation performed. Consider obtaining previous operative notes.
The symptom complex before the original operation can help to determine
the appropriateness of the procedure itself.
pain relief and the length of the pain-free interval to determine the
source of the patient’s current pain. No relief of preoperative
sciatica suggests failure to relieve root compression, which may be due
to a retained disc fragment, surgery at the wrong level, or an improper
original diagnosis. If the patient relates that the preoperative
sciatica was relieved, note the percentage of relief and duration of
that improvement. A pain-free interval of more than 6 months, and
certainly more than 12 months, suggests that the patient’s pain may be
caused by recurrent disc herniation. A pain-free interval lasting only
2 to 6 months, particularly with the gradual recurrence of symptoms,
suggests that epidural fibrosis may be the cause of the pain (13).
lumbar spine. It is well documented that each subsequent operation,
almost regardless of the diagnosis or procedure, carries a poorer
prognosis for an eventual good result. This fact alone does not mean
that a patient’s fifth or even sixth operation, in the presence of
clear-cut objective indications of nerve root compression or
instability, might not be beneficial. The surgeon and patient need to
be aware, however, of the diminishing statistical likelihood of success
with each successive surgery. It has been shown that second operations
have only, on average, a 50% chance of success, and in patients
undergoing a third or more operation, symptomatic worsening is as
likely as symptomatic improvement (37).
pain predominates, a herniated disc or spinal stenosis is most likely,
although scar tissue is also a possibility. Predominance of back pain
suggests segmental instability, discogenic back pain, or perineural
scar tissue. Mechanical symptoms—with clear-cut worsening of the pain
with sitting and standing, and relief at rest—point more toward
instability or discogenic back pain, whereas pain at rest suggests scar
tissue, or the remote possibility of tumor or infection. The presence
of back and leg pain may be due to spinal stenosis or scar tissue.
and the existence of any tension signs, such as a positive straight-leg
raise test or femoral nerve stretch test. Obtain the results of any
dependable previous examination and compare the patient’s preoperative
and postoperative status. If the neurologic picture is unchanged from
before the previous surgery, and tension signs are negative, mechanical
pressure is unlikely. If, however, a new neurologic deficit has
occurred since the last surgery, or if tension signs are present, nerve
root compression is often found. The presences of a tension sign is not
necessarily pathognomonic for mechanical nerve root compression because
epidural or perineural fibrosis can result in a tension sign.
when possible, those that led to the original decision for surgery.
These studies may give valuable information as to the appropriateness
of the original procedure, as well as the etiology of the current
problem.
which may reveal the extent of the laminectomy defect, the level of the
previous operation, changes consistent with spinal stenosis, and
evidence of instability on dynamic films. Perform plain films and
dynamic films with the patient standing (weight bearing). Assess any
evidence of abnormal motion, progressive deformity, or progressive
anterolisthesis (Fig. 149.1). Plain radiographs, including
dynamic views, also help assess the quality of any fusion mass that may
be present. On a lateral view, a successful interbody fusion is
indicated by the continuity of bone between the outer margins of the
adjacent vertebral bodies. A posterior fusion mass can be difficult to
evaluate, particularly at L5–S1; a Ferguson anteroposterior (AP) view
(with the x-ray beam tilted cephalad 30° to run parallel to the L5–S1
disc space) highlights the fusion mass between the L-5 transverse
processes and the sacral alae.
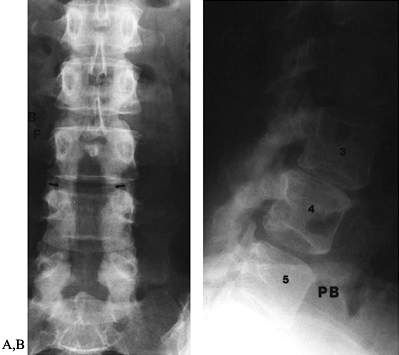 |
|
Figure 149.1.
A 49-year-old woman approximately 1 year after a decompressive laminectomy, with worsening low-back pain and recurrent leg pain. A: The AP view demonstrates the extent of the laminectomy defect (arrows). B: On the lateral view, subluxation of L-3 on L-4, not present before surgery, is seen, demonstrating postlaminectomy instability. |
such as screw loosening or breakage, rod breakage, progressive
deformity across the fused levels, and evidence of motion on lateral
flexion and extension views. Plain radiographs are relatively sensitive
(90% to 95%) but fairly nonspecific (37% to 60%) in detecting
pseudarthrosis following lumbar spine fusion (2,6).
value. Although extradural compression is well seen on myelography,
distinction between the presence of disc material and epidural scar
formation is limited (8). Myelography is most helpful in confirming the diagnosis of arachnoiditis when it is otherwise uncertain.
sensitivity for demonstrating changes of arachnoiditis. We still use it
quite frequently in assessing spinal stenosis in a patient who has
undergone previous surgery. The size of the spinal canal, the presence
of bony defects and the extent of posterior element resection, and
hypertrophic bony changes causing stenosis are all well visualized (Fig. 149.2) (34).
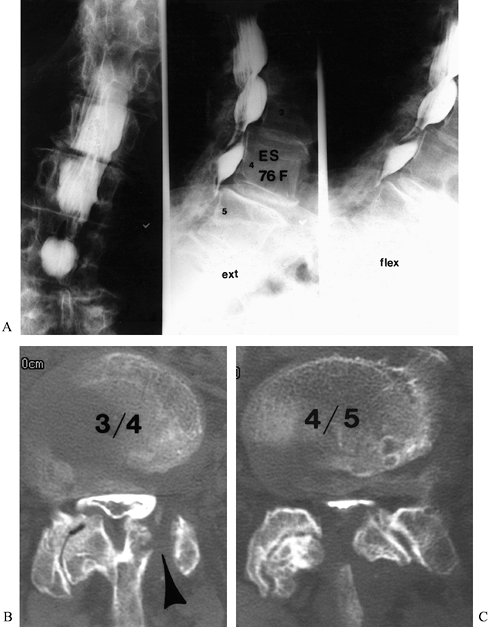 |
|
Figure 149.2.
A 76-year-old woman, 9 months after a left L3–L4 hemilaminectomy, with persistent back and leg pain. This shows AP and lateral flexion and extension views with myelography (A) Multi-level stenosis, most severe at L3–L4 and L4–L5; (B and C) axial postmyelogram CT images, which clearly define the pathologic anatomy. The failure of the previous decompression to address the pathology is appreciated (arrowhead). |
hardware placement. Although metallic scatter diminishes the quality of
the images, careful scrutiny of the bony windows following plain CT
scanning can usually establish whether or not a screw has broken out of
the pedicle (usually medial) and is causing nerve root compression.
exception, the most helpful diagnostic tool for imaging the lumbar
spine that has previously undergone surgery. The most noteworthy use of
MRI has been in the diagnosis of recurrent disc herniation, using
images obtained before and after the injection of intravenous
paramagnetic contrast material (Gadolinium-DTPA). MRI has 100%
sensitivity, 71% specificity, and 89% accuracy (19).
A nonenhancing soft-tissue mass causing nerve root compression is
strongly suggestive of recurrent disc herniation, whereas Gd-DTPA
enhancement suggests the presence of scar tissue (Fig. 149.3).
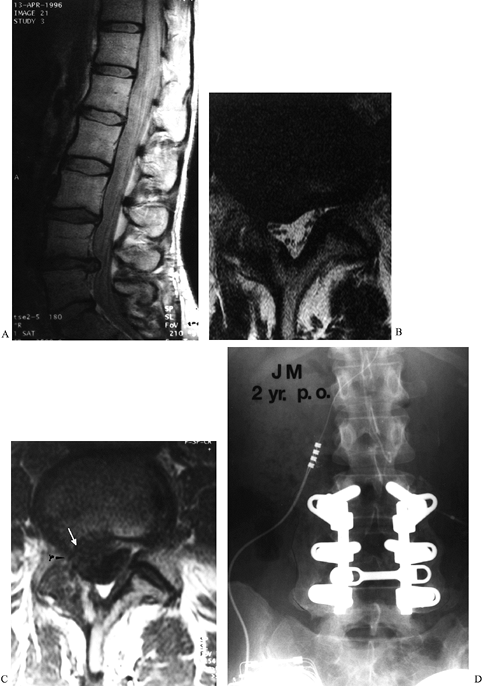 |
|
Figure 149.3.
A 37-year-old woman with a history of three previous discectomies who had recurrent, severe right leg pain, numbness, and a positive straight-leg raising sign. (A) The sagittal MRI demonstrates an apparent disc herniation at L4–L5; (B) the axial T2 image, without contrast, through the L4–L5 disc space demonstrates a soft-tissue mass consistent with disc herniation; (C) the T1 image, following contrast administration. The absence of contrast-enhancement of the mass (white arrow) is diagnostic of recurrent disc herniation, rather than epidural fibrosis; (D) a solid fusion 2 years following repeat discectomy and fusion; the patient continued to have significant back pain. |
surgery, gadolinium-enhanced MRI frequently demonstrates pathologic
changes and may suggest recurrent disc herniation, despite a good
clinical result. Take care not to overinterpret gadolinium MRI in the
early postoperative
period; overreliance on this study may lead to negative findings on repeat surgical exploration (4).
inflammatory processes such as discitis, and in fact, it is the test of
choice when a postoperative disc space infection is suspected.
Decreased signal intensity in the disc on the T1-weighted images and
increased signal on the T2-weighted images, particularly with
enhancement following Gd-DTPA injection, all suggest an inflammatory
process (28).
noninflammatory degenerative changes in the lumbar discs. Although the
significance of disc degeneration in the lumbar spine remains
controversial, MRI unquestionably gives the best picture of the discs
involved with degenerative changes, the extent of disc desiccation,
bulging, and reactive changes in the vertebral bodies. It may be
beneficial in the evaluation of a patient with persistent mechanical
back pain following a lumbar discectomy in whom discogenic back pain is
considered a potential diagnosis (18).
the patient whose previous back surgery has failed. The indication for
discography is to assess the reproduction of the patient’s
characteristic pain on disc injection and to compare it with the
injection of control levels above and below. It should be stressed
that, although discography gives a clear picture of abnormal disc
morphology, this information rarely contributes meaningfully to
surgical decision making and should not be used except in the context
of reproduction of the patient’s pain.
generator continues to be debated. Proponents believe that reproduction
of pain during disc injection, in a manner and distribution concordant
with the patient’s characteristic pain complaints, identifies that disc
as the source of the pain (Fig. 149.4).
Conflicting reports regarding the specificity of discography have
appeared, with Holt in 1968 reporting a false-positive rate of 37% (17), compared with Walsh et al., who recently noted no false-positive results in their study of normal subjects (38).
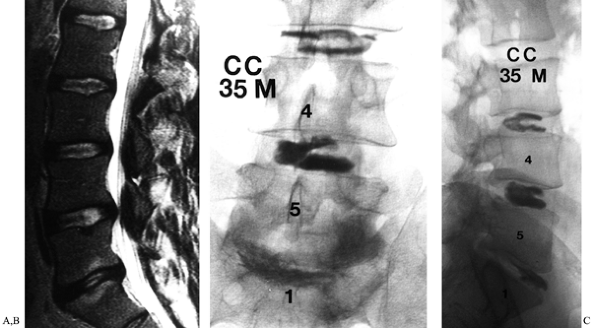 |
|
Figure 149.4. A 35-year-old man, 3 years following right L5–S1 discectomy, with persistent incapacitating back and right buttock pain. (A) This is a T2-weighted MRI demonstrating degeneration of the disc, with reactive changes in the adjacent vertebrae; (B and C)
AP and lateral discographic images. Injection of the L5–S1 disc reproduced the patients characteristic back and buttock symptoms, whereas injection at L4–L5 and L3–L4 discs was only minimally uncomfortable. |
controversial, with its ability to predict the pain generator as well
as to predict the results of surgical intervention still unproven. We
agree with the North American Spine Society position statement on
discography, which advocates discography only in the evaluation of a
patient with unremitting spinal pain of more than 4 months’ duration
and only when the
patient and physician have decided that surgical treatment is under consideration (29).
the conditions that contribute to FBSS, including recurrent or
persistent disc herniation (12% to 16%), lateral (58%) or central (7%
to 14%) stenosis, arachnoiditis (1% to 16%), epidural fibrosis (6% to
8%), and instability (5%). Superimposed on many of these conditions is
discogenic back pain, a relatively common cause of back or leg pain
following surgery.
pain such as pancreatitis, diabetes mellitus, or an abdominal aortic
aneurysm. Other systemic disorders to be considered include
fibromyalgia, ankylosing spondylitis, and osteoporosis or osteomalacia.
specific factors such as alcoholism, drug dependence, depression, and
the presence of compensation or litigation issues. Strongly weigh such
factors when calculating the risk–benefit ratio of surgery. People with
profound emotional disturbances and those involved in litigation rarely
derive significant benefit from additional surgery (37).
Even in the face of a specific orthopaedic diagnosis, make every
attempt to address psychosocial problems such as drug dependence and
depression before considering further surgery; in many cases, once a
patient’s underlying problem has been successfully treated, the somatic
back complaints and disability improve.
caused by a herniated disc. First, the disc that caused the original
symptoms may not have been completely removed, as can occur if the
surgery was performed at the wrong level, if inadequate decompression
was performed, or if a fragment of disc material was simply left
behind. The predominant complaint is leg pain, and the neurologic
findings, tension signs, and radiographic pattern remain unchanged from
presurgical findings. The distinguishing feature is that there is
typically no pain-free interval; this patient will have awakened from
surgery complaining of the same pain that he or she had preoperatively.
Patients in this group are helped by a correctly performed discectomy.
the previously decompressed level. In this case patients complain of
recurrence of sciatica and have similar neurologic findings and tension
signs. The distinguishing characteristic in this group is the presence
of a well-defined pain-free interval that is usually of 6 months’
duration or longer. The diagnosis is confirmed with gadolinium-enhanced
MRI; a recurrent disc herniation is avascular, with only a thin
enhancing rim at the periphery of the lesion (19). If nonoperative treatment fails, repeat discectomy is indicated in this group of patients.
different level or on the opposite side. In this case, patients will
also describe a pain-free interval of 6 months or longer following
their original surgery. Otherwise the development of their symptoms,
with leg pain predominating, is similar to that for a typical disc
herniation. A tension sign is usually present, as are appropriate
neurologic findings. A neurologic deficit should be different from that
associated with the original operation, because the source of the pain
is compression of a different nerve root. Repeat surgery in these
patients has the same prognosis as a primary discectomy.
previous back surgery can result in either back or leg pain but
typically causes both. The etiology may be progression of the patient’s
underlying degenerative spine disorder, failure to decompress the
patient’s stenosis adequately at the time of the original operation,
overgrowth of a previous posterior fusion mass, or transition syndrome.
degenerative changes and frequently instability at a level adjacent to
a previous lumbar fusion. The patient’s report of a pain-free interval
will vary when LSS is the cause of the symptoms; failure to recognize
and relieve stenosis at the time of the original procedure may result
in no pain-free interval whatsoever. Alternatively, a period of months
or even many years may pass before stenosis develops in a patient who
has undergone an otherwise successful operation.
patients with postoperative LSS do not differ significantly from those
of patients without prior surgery. Back and leg pain are typically
seen. Worsening of the leg symptoms with walking or standing is a
common finding, but not essential to the diagnosis, and many patients
with LSS do not report neurogenic claudication. A normal neurologic
examination is common, and neurologic findings, when present, are
usually subtle. Tension signs are usually negative (14,33).
may display facet degeneration, decreased interpedicular distance,
decreased sagittal canal diameter, and disc
degeneration.
Degenerative spondylolisthesis and degenerative scoliosis are commonly
seen in patients with stenosis of the spinal canal and lateral
recesses. Neuroradiographic imaging of the postoperative patient with
suspected LSS may be accomplished using plain CT, postmyelographic CT,
or MRI.
and parasagittal views of the thecal sac and foraminal narrowing, and
to identify disc degeneration, which may be helpful in planning for a
fusion. Its sensitivity in identifying other causes of back pain in
this population, including metastatic disease and occult infection, is
also an advantage. State-of-the-art technology in MRI has provided
sufficient bony detail to diagnose adequately facet overgrowth,
osteophyte formation, and other causes of LSS in most patients. This is
our routine test of choice (Fig. 149.5). In
some patients with previous surgery, however, it is helpful to use
postmyelographic CT scanning, which still provides better bony detail
and shows encroachment on the thecal sac and on the nerve roots in the
lateral recesses and foramina. Postmyelographic CT is not as specific
as MRI in identifying and differentiating postoperative scar tissue
from normal soft tissue, when differentiation is a consideration (5).
 |
|
Figure 149.5.
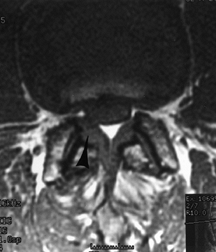
A 52-year-old man with recurrent back and right leg pain 8 years following a lumbar decompression and fusion from L4–S1. On T1-weighted axial MR images, right lateral recess stenosis at L2–L3 (arrows) is clearly demonstrated. Following repeat decompression and extension of his fusion to L-2 he had near-complete pain relief. |
having failed nonoperative treatment, has at least a 70% chance of
obtaining satisfactory results following surgery. If nonoperative
treatment is unsuccessful, thorough decompression of any bony or
soft-tissue compression is likely to relieve symptoms significantly.
If, however, a significant component of the compression is due to
epidural fibrotic scar, then the results of surgery are far less
predictable. Patients undergoing repeat decompression who have either
pre-existing instability or in whom instability may result from the
decompression should also undergo a posterolateral fusion at the
involved levels (20).
can cause mechanical back pain following previous surgery. Instability
results from the spinal motion segment’s inability to bear physiologic
loads; the result is abnormal motion between two vertebrae (42). Most commonly, it causes back pain, but leg pain or neurologic findings from dynamic stenosis may also be seen.
motion on flexion and extension radiographs or by the development or
worsening of spinal deformity (Fig. 149.6).
Instability following lumbar spine surgery may be the result of a
pre-existing condition, as in a patient with spondylolisthesis treated
with decompression alone, or it may be the result of an excessively
wide or aggressive decompression. It is not uncommon to see either
frontal or sagittal plane instability occur in a patient who has had
unilateral thinning of the inferior facet and pars, resulting in facet
fracture (16). Unilateral facet resection is
commonly believed to be benign, but this degree of resection in the
presence of an incompetent disc, particularly after an extensive
discectomy, may lead to instability. Another sign of instability would
be painful motion occurring at the site of a pseudarthrosis.
 |
|
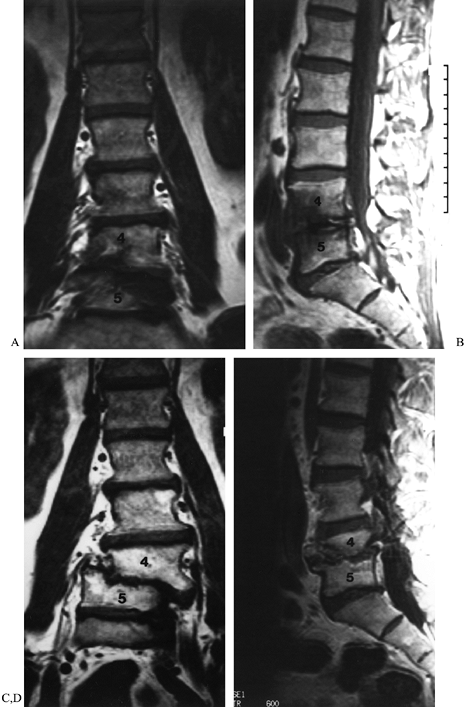
Figure 149.6. A 53-year-old man 18 months following a decompressive laminectomy at L4–L5, with discectomy, for degenerative stenosis. (A and B) Coronal and sagittal MRIs demonstrating the alignment of his lower lumbar spine before surgery; (C and D) similar images taken 16 months later, demonstrating progressive development of deformity, indicative of instability.
|
pain, although 20% to 25% report radiating leg symptoms with weight
bearing. The physical examination is frequently negative, although some
patients have a characteristic reversal of normal spinal rhythm on
return from forward bending (30). A key to
diagnosis in these patients is the plain radiograph. Weight-bearing
lateral flexion and extension views are diagnostic for instability when
they demonstrate
-
Sagittal plane translation greater than 12% of the AP diameter of the vertebral body,
-
Relative sagittal plane rotation greater than 11°,
-
Sagittal translation greater than 25% at L5–S1,
-
Relative rotation greater than 19° (4).
instability, indirect evidence may be seen in the patient who following
surgery has developed
-
Progressive deformity in either the sagittal or frontal planes;
-
Short-segment angular collapse at the level of the decompression.
postoperative instability and may result in dynamic stenosis, with leg
pain resulting from root compression in the concavity of the collapse (Fig. 149.7).
Scrutinize the plain AP radiograph for evidence of extensive or
excessive resection of the posterior elements, such as the pars
interarticularis and facet joints, which can lead to instability.
 |
|
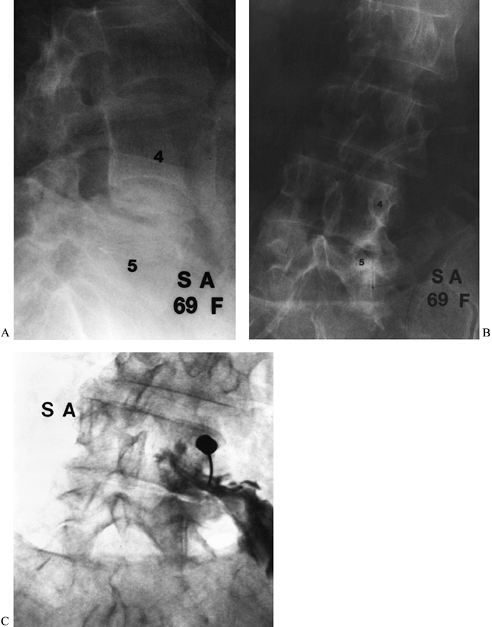
Figure 149.7. A 69-year-old woman who underwent two prior laminectomies at L4–L5 with no relief of her right buttock pain. (A and B) Lateral and PA views of the lumbar spine. The frontal view demonstrates asymmetric collapse on the right at L4–L5; (C)
a captured image during selective nerve root infiltration of the L-4 root, which completely relieved her pain, strongly suggesting that L-4 root compression was the cause of the symptoms. |
symptomatic patient, spinal fusion, facet injections, or discography
may help clarify the precise origin of the patient’s symptoms. Rule out
other possible causes of back pain before performing repeat surgery.
may vary, a certain subset of patients is believed to suffer from
primary disc-related or discogenic pain. The existence of this entity
continues to be debated, as does a reliable method of diagnosis. The
difficulty in arriving conclusively at the diagnosis of discogenic back
pain is magnified in the patient who has had prior back surgery because
of the potential contributions of instability, epidural fibrosis, and
generalized deconditioning.
back pain following previous surgery had a history of leg pain as well
as significant back pain before the initial operation. A period of
improvement in leg pain following the surgery is noted, but very often
the back pain continues unabated or even worsens. Gradual worsening of
the leg pain is frequently reported, although this symptom may be
related to epidural fibrosis. The pain is typically relieved by rest.
Generalized limitation of motion of the lumbar spine is seen on
examination, but otherwise the physical examination is usually
unremarkable.
including dynamic views to rule out instability. MRI, with or without
gadolinium, may demonstrate disc degeneration at the previous surgical
site, and possibly at other levels of the lumbar spine.
three types of signal changes in the vertebral bodies adjacent to a
degenerated disc degeneration. Type I changes show decreased T1
intensity and increased T2 intensity, which correlates histologically
with disruption and fissuring of the endplate and vascularized fibrous
tissue within the marrow of the vertebral body. These changes, which
can be suggestive of vertebral osteomyelitis, can be differentiated
from infection by the absence of increased signal intensity on
T2-weighted images.
and T2-weighted images. Histologically, these changes represent yellow
marrow replacement in the vertebral body. Finally, Type III changes
show decreased signal intensity on both T1- and T2-weighted images,
reflecting relative absence of marrow in the vertebral body; this
finding correlates with bony sclerosis seen on plain radiographs. The
significance of these discogenic changes in the vertebral bodies, as
seen on MRI, has not been clearly defined; such changes, when present,
would suggest that the intervening disc is the source of the pain.
the invasive nature of the procedure and the potential risks, in
particular discitis, perform discography only in patient’s in whom you
are considering fusion and they have agreed to proceed. The morphologic
picture seen with contrast injection typically correlates closely with
the MRI of disc degeneration, but it is the patient’s report of
reproduction of his or her characteristic pain that is essential in
attempting to determine that a given disc is the pain generator. Do not
use extensive sedation during the test because it renders the patient’s
feedback meaningless. It is also important to inject three or even four
levels to find at least one control level. If every level injected
reproduces the patient’s pain pattern, then the test result is
unreliable, and surgery based on this discogram is less likely to
result in adequate pain relief.
clearly reproducing the patient’s characteristic pain pattern, then the
patient may be a candidate for surgery. It should be noted there is no
conclusive evidence that a confirmatory discography can predict
surgical success. The patient and surgeon should be aware that no
spine-fusion technique for discogenic back pain has been conclusively
shown to have a high success rate.
rather than relying solely on posterolateral fusion. These techniques
are in evolution, but success depends on using abundant autologous
iliac bone graft with adequate graft–endplate contact, adequate
stabilization provided by the implant, and a minimum of destruction of
normal anatomy. Available techniques include
-
Transforaminal interbody fusion (TLIF) combined with transpedicular instrumentation,
-
Anterior lumbar interbody fusion (ALIF), or
-
Posterior lumbar interbody fusion (PLIF) with fusion cages packed with autologous bone, and
-
ALIF with structural allograft replacement.
causes of back or leg pain in patients who have had previous back
surgery. Scar tissue occurring beneath the dura is commonly referred to
as arachnoiditis. Scar tissue can also form extradurally, compressing
either the cauda equina or the nerve root, and is referred to as
epidural fibrosis.
The condition may be present in varying degrees of severity, from mild
thickening of the meninges to solid adhesions. The scarring may be
severe enough to obliterate the subarachnoid space and block the flow
of contrast agents. The etiology of this condition has been attributed
to many factors; prior surgery and particularly a history of
myelography with oil-based contrast are frequent precipitating factors.
A dural tear with blood mixing with cerebrospinal fluid (CSF) or a
postoperative infection may also play a role in its pathogenesis.
The
exact mechanism by which arachnoiditis develops from these events is
not clear. There is no uniform clinical presentation for arachnoiditis.
previous operation and a pain-free interval lasting from 1 to 6 months.
Often, the patient complains of back and leg pain. Physical examination
is inconclusive; alteration in neurologic status may be on the basis of
a previous operation. Myelography, CT, and MRI can all be helpful in
confirming the diagnosis (43).
Reconstructive or decompressive surgery has not proven effective in
eliminating the scar tissue or significantly reducing the pain. Salvage
procedures such as spinal cord stimulation or implantation of a
morphine pump have been advocated, with some promising results reported
(40).
steroids, transcutaneous nerve stimulation, operant conditioning,
bracing, and patient education have all been tried. None leads to a
cure, but all can provide symptomatic relief for varying periods of
time. Patients should be detoxified from narcotics and encouraged to
pursue physical activity as much as possible. Gabapentin (Neurontin)
and amitriptyline (Elavil) are pharmacologic adjuncts that may be
effective. Treating patients with arachnoiditis is a real challenge,
and the physician must be willing to devote time and patience to
achieve optimal results.
equina or directly on the nerve roots is a common occurrence. This
epidural scar tissue can act as a constrictive force around the neural
elements and may cause postoperative pain. Although most patients have
radiographic evidence of epidural scar tissue formation, only an
unpredictable few become symptomatic.
at almost any time, from several months to years after surgery. The
onset is typically gradual, with complaints of back pain, leg pain, or
both. Commonly the neurologic examination is normal, but the presence
of a tension sign may occur due to nerve root constriction from
fibrotic changes. The diagnosis is best differentiated from recurrent
disc herniation or LSS by gadolinium-enhanced MRI.
for epidural fibrosis. Prevention may be the best answer, and fat,
Gelfoam, and other interpositional membranes have been suggested to
minimize the formation of scar tissue following laminectomy (22).
Once scar has formed, decompressive surgery with the goal of resecting
scar tissue has not proven successful because of the almost inevitable
recurrence of even worse fibrosis. It is our experience, however, that
a fibrosed nerve root may be more susceptible to the deleterious
effects of instability or stenosis than a nerve that has not been
surgically treated.
lumbar disc surgery. Its pathogenesis is postulated to be direct
inoculation of the avascular disc space at the time of discectomy, but
it is not completely understood (1,9). The onset of symptoms usually occurs 2 to 4 weeks following surgery.
pain. Pain is unremitting, even at rest, and sometimes extends to the
buttocks. Pain does not usually follow a dermatomal pattern down the
leg. The patient may have a low-grade fever. Physical examination
usually reveals marked paraspinal spasm and rigidity, and pain is
present with any type of motion. Straight-leg raising may be limited,
but the presence of a true tension sign or new neurologic abnormality
is unusual. Occasionally, a superficial wound infection is seen, but in
most cases, wound healing has been uneventful.
blood cell count with a differential, erthrocyte sedimentation rate
(ESR), and a C-reactive protein level. Plain radiographs are usually
normal in the early stages; later, endplate erosion may be seen, but it
may not be present for several weeks. Contrast-enhanced MRI is the test
of choice in suspected disc space infection. Increased signal intensity
in the disc space on T2-weighted images suggests discitis, which can be
confirmed by enhancement of the disc space with use of gadolinium.
antibiotics, or combinations thereof. Initially, place the patient on
bed rest to immobilize the lumbar spine, with or without a brace or
corset. Begin empiric antibiotic treatment and continue it for 6 to 12
weeks. Cefepime, a third-generation cephalosporin with improved
staphylococcal coverage as well as pseudomonicidal properties, is
administered, giving 1 to 2 g every 12 hours. If the patient fails to
respond rapidly to antibiotics and immobilization or manifests
constitutional signs and symptoms, perform a needle biopsy of the
affected disc space. Open biopsy is reserved for patients who fail to
respond to treatment, as evidenced by improvement in pain and decline
of the ESR, or for patients with neurologic compromise (9).
Once the patient is comfortable at bed rest and is afebrile, institute
progressively increasing activity as symptoms allow. Most authors
report good long-term results with resolution of infection and adequate
pain control.
reported to occur in as many as 40% of cases. Risk factors include a
history of cigarette smoking, multiple-level fusion, and instability
that has not been adequately addressed with either internal or external
immobilization at
the time of fusion (32).
Nonunion may occur with or without instrumentation, although the
presentation may be somewhat delayed in cases in which rigid internal
fixation is initially used. Patients with persistent symptoms due to
pseudarthrosis complain primarily of back pain. Leg pain may be
present, but direct causes of nerve root compression should be sought,
and an assumption that the pseudarthrosis is the cause of the leg pain
is frequently unwarranted.
patients may say that their symptoms never improved following surgery,
or they may report many months or even years of relatively good pain
relief. It should be noted that unlike a simple discectomy, in which it
is not uncommon for the patient to describe truly complete relief of
pain, patients who have undergone spine-fusion surgery, even when it is
successful, rarely describe complete relief of their symptoms. Patients
who have undergone internal fixation, however, are more likely to
describe a clear-cut pain-free interval that begins to deteriorate when
the implant either loosens (the most common mode of failure) or breaks.
back pain. It has long been recognized, however, that the correlation
between radiographic failure of fusion and symptoms is uncertain. It is
very difficult to identify accurately the source of the patient’s pain
following lumbar fusion; solid fusion is no guarantee of pain relief,
and many patients with an obvious nonunion do remarkably well.
with a pseudarthrosis revision fusion. Undertaking repeat surgery for
pseudarthrosis repair in the absence of motion at the affected level
and without a thorough search for alternative causes for the patient’s
symptoms has limited chances for success. Most authors report
compromised results after such surgery, particularly when leg pain is
noted in the absence of a compressive etiology (24).
pseudarthrosis with plain radiographs, including Ferguson AP and
weight-bearing, dynamic lateral radiographs. Solid fusion, either
posteriorly or anteriorly, should eliminate virtually all motion on
flexion and extension views. Although the landmarks may be somewhat
difficult to identify, careful scrutiny of dynamic views can usually
identify whether or not motion is taking place (Fig. 149.8).
 |
|
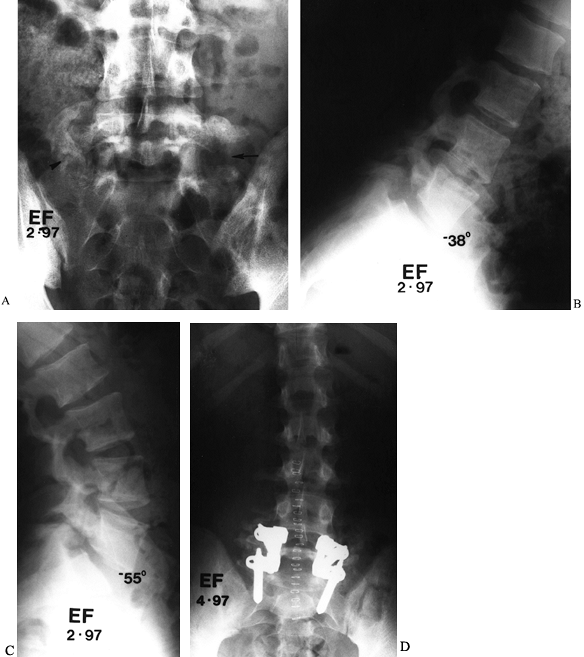
Figure 149.8. A 17-year-old man, 18 months following L5–S1 fusion for spondylolysis, who now reports worsening low-back pain. (A) A Ferguson AP view shows abundant fusion mass on the right, although a defect can be seen (arrowhead), whereas on the left, most of the graft has been resorbed (arrow). Lateral flexion and extension radiographs demonstrate 17° of angular motion; (B) clear-cut evidence of pseudarthrosis, which was confirmed at surgery; (C), clear-cut evidence of pseudarthrosis, which was confirmed at surgery; (D) revision fusion posteriorly, with transpedicular instrumentation, led to complete pain relief.
|
times, a serpiginous cleft in the fusion mass can be visualized.
Although a number of other radiographic modalities, including CT
scanning and single photon emission computed
tomography
(SPECT) scanning, have been suggested to diagnosis nonunion, we rely
almost exclusively on plain radiographic findings of motion or
progressive deformity to identify the patient who is likely to benefit
from repeat surgery.
pseudarthrosis clearly; additionally, the correlation between
pseudarthrosis and symptoms in a given patient is uncertain. For these
reasons, an aggressive attempt at nonoperative treatment is indicated.
When the nonsurgical approach is unsuccessful, revision surgery may be
undertaken. A failure rate as high as 50%, both clinically and
radiographically, has been reported, however. Lauerman et al. (24)
reported improved results in patients who had undergone only one prior
operation on the lumbar spine and in patients who had a clear-cut
original indication for fusion, such as spondylolisthesis.
following previous surgery on the lumbar spine, there is always another
operation that can be considered. Experience tells us, however, that
the results following revision surgery on the lumbar spine are
frequently unsatisfactory, and particularly when there is a history of
two or more previous operations, the patient has a significant chance
of being made worse rather than better with another surgery (37).
In light of this, treat nonoperatively most patients who have failed
prior surgery, even when it is possible to identify an etiology of
their pain that is potentially amenable to surgery.
well as some more specific interventions. Realistic goals for pain
relief are essential. Close questioning of the patient often reveals
that he or she is significantly better now than before the previous
operation; any consideration of further surgery simply to “get rid of
all of the pain” is likely to be unsuccessful and is unwarranted.
Furthermore, it is apparent on questioning some patients that there has
been almost no postoperative attempt at rehabilitation. These patients
respond quite readily to a generalized back exercise and aerobic
exercise program with judicious use of medication.
-
Weight reduction when appropriate;
-
A defined program of aerobic exercise, particularly involving walking, riding an exercise bicycle, or swimming;
-
A supervised program of active physical therapy consisting of specific back stretching and strengthening exercises; and
-
Use of nonsteroidal anti-inflammatory medications.
narcotic usage. Elavil is useful for the patient with chronic pain and
sleep disturbance, as are several other antidepressants. Neurontin, an
antiepileptic, appears to be beneficial in some patients with chronic
radicular pain.
in the patient with chronic pain and are becoming increasingly popular.
It is up to the individual physician to decide to what extent his or
her practice includes prescribing these medicines. The authors find it
more effective, in most cases, to refer such patients to a pain
management center for pharmacologic management. A final adjunct that is
occasionally useful is external immobilization, which may be provided
by something as simple as a lumbar corset or as elaborate as a
custom-made polypropylene lumbosacral orthosis. It is widely believed
that these devices decondition the lumbar musculature, although there
is little objective evidence to document this belief. Corsets and
orthotics do, however, provide significant pain relief for many
patients, and they are particularly effective in elderly patients.
fibrosis or recurrent mechanical compression from stenosis or disc, a
trial of lumbar epidural steroids is worthwhile. The long-term benefits
are quite variable, but a certain percentage of patients will obtain
lasting relief or will tolerate a more aggressive program of
rehabilitation once the inflammatory radicular symptoms are controlled.
Local trigger-point injections, facet joint blocks, and sacroiliac
injection may also be tried, although none of these methods has
consistently proven effective.
failed prior low-back surgery is persistent, unacceptable pain that has
failed to respond to aggressive and persistent nonoperative treatment.
In addition, it is explained by and correlates with either objective
evidence of instability, mechanical nerve root compression, or both.
The challenge in managing patients who have had prior back surgery, and
the primary reason for the increased rate of failure with further
surgeries, is the difficulty of clearly correlating the patient’s pain
with the radiographic findings. Adherence to guidelines that are as
strict as or stricter than those used for primary surgery is essential.
Our experience has been that attempts to extend these indications leads
to consistently unsatisfactory results. Further, viewing fusion as a
generically applicable salvage procedure for previously unsuccessful
back surgery rarely results in significant and long-lasting pain relief.
on patients who fit into one of three categories. These include
patients who have
-
Radicular leg pain and confirmatory
evidence of nerve root compression on high-quality neuroradiographic
imaging that demonstrates either recurrent disc herniation or LSS not
caused by epidural fibrosis; -
Back pain due to radiographically
documented instability, as confirmed either by progressive deformity
(scoliosis or spondylolisthesis), excessive motion on flexion and
extension lateral radiographs, or a failed fusion with motion
demonstrated on dynamic radiographs; -
Back pain believed to be emanating from one or two painful degenerated discs, confirmed on pain-provocation discography.
on this subset of patients, it should be stressed that only a
relatively small percentage of patients with FBSS fit into one of these
three categories.
include the presence of a clearly documented progressive neurologic
deficit. Although this condition is uncommon, it does occasionally
occur, more often in elderly patients with severe stenosis. A
progressive neurologic deficit is an indication for urgent surgery.
Cauda equina syndrome, a distinctly rare occurrence in patients who
have had unsuccessful back surgery, merits emergent imaging and
surgical treatment. Finally, one occasionally encounters the patient
with radiographic evidence of progressive spondylolisthesis or
progressive collapsing scoliosis, which itself suggests the need for
surgical stabilization. It rarely occurs in the absence of concurrent
incapacitating pain but might be a situation in which a more aggressive
approach is called for.
has gained increasing popularity in North America as an adjunct to
lumbar fusion. This trend has, in several ways, complicated the
approach to the treatment of patients whose back surgery has failed.
First, an increasing number of patients are undergoing lumbar spine
fusion, and unfortunately, in many cases, it has been carried out in
the absence of traditional, objective indications. The usual result is
failure. The presence of the implant itself raises several technical
considerations relating to the possible need for repeat surgery,
including the significance of screw breakage, implant loosening,
infection, and malposition of one or more screws. Finally, adverse
publicity related to these
devices
has led to a climate in which either medicolegal concerns or, at the
least, undue patient anxiety further clouds a complicated clinical
picture.
metal alloys that have a very low incidence of true allergy; therefore,
allergy is rarely, if ever, the cause of pain. Failure can occur in one
of several ways, but mechanical failure does not necessarily represent
an indication for removal of the implant or revision surgery. Screw
breakage is the most dramatic mode of failure, but with current
technology, it is quite rare. A broken screw does not preclude the
possibility of a successful fusion and, therefore, is not an absolute
indication of clinical failure (26,39).
relief of back pain following an instrumented lumbar fusion, now has
the sudden recurrence of pain and is noted to have new screw breakage
may well have had a nonunion that was adequately stabilized when the
implant was intact. Such a patient would benefit from revision fusion.
pedicle and vertebral body. This is much more common than screw
breakage. The loosening is seen as a small zone of radiolucency about
the screw on routine radiographs. There is no clear-cut relationship
between screw loosening and symptoms, and unless failure of fusion and
motion on flexion and extension views are demonstrated, continued
observation is indicated.
with the use of these bulky implants, and the rate of infection has
been reported to be from 2% to 5% (35). Acute
and subacute infection is readily diagnosed, but late infection may
represent the source of recurrent back pain after a relatively long
pain-free interval. Consider infection when evaluating the patient with
the late onset of pain after an otherwise successful fusion. On CT
scan, look for a fluid collection around the implant. Aspirate the
wound to look for purulent fluid. Send any fluid aspirated for Gram’s
stain and culture.
the fused levels, another possible source of nerve root compression in
patients who have implants in place is a misplaced screw. Although most
patients have symptoms early from a misplaced screw, it is not uncommon
for radicular pain to develop weeks, months, or even years later (Fig. 149.9).
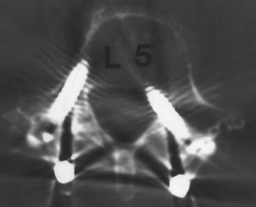 |
|
Figure 149.9.
A 59-year-old woman who, 1 month following revision fusion with transpedicular instrumentation, reported worsening left leg pain and weakness. A plain CT scan demonstrates, on the bone windows, medial placement of the L-5 screw, correlating with her symptoms. Prompt screw removal led to complete resolution of her leg pain, although she had mild residual weakness. |
bony windows, is a sensitive modality for identifying a screw placed
outside the pedicle. Because screw misplacement is asymptomatic in as
many as 20% of patients, close correlation between the patient’s signs
and symptoms and the root compromised by the screw in question is
essential before deciding on repeat surgery (41).
The most common location for screw impingement is medial to the
pedicle, particularly at L-5, but it is important to check for the
possibility of an S-1 screw placed through the sacral ala, lateral to
the sacral body, which brings it into proximity to the L-5 root,
passing over the brim of the sacrum. Once the diagnosis of screw
malposition, which is causing symptoms, has been made, screw removal is
indicated.
A rigidly fixed pedicle screw implant may temporarily provide stability
to an unstable motion segment, but if solid bony fusion is not
achieved, loosening commonly occurs. Therefore, mechanical back pain
may recur after a pain-free interval. No radiographic modality has
consistently proven accurate in diagnosing nonunion in the presence of
a transpedicular implant (23). We rely on the
presence or absence of motion on flexion and extension lateral views to
decide whether further surgery in indicated.
pedicle screw instrumentation is present is routine implant removal. It
is unusual for a patient to be so thin that the implants are palpable
or cause pressure problems with sitting in a hard-backed chair. The
role of routine implant removal is uncertain, and significant pain
relief, if a solid fusion is present, occurs in only about one patient
out of three (10). To the patient requesting
implant removal, we explain the uncertainty regarding the chances for
improvement of their pain as well as the fairly significant surgery
required to remove these devices. If the patient wishes to proceed
under these circumstances, we will remove the implants and explore the
fusion. We inform our patients that if a pseudarthrosis is found,
further bone grafting and revision instrumentation will be carried out.
surgeries can be a challenge. The technique of a repeat laminectomy or
a repeat fusion is somewhat different from first-time surgery. The risk
of complications is certainly greater, with the ever-present danger of
a dural tear or neurologic injury.
same as that for the initial procedure—to decompress the neural
elements without injury or excessive hemorrhage. Unfortunately, once
the spine has already undergone surgery, the anatomy is not as clear
and a great deal of scar tissue can be present. Thus, several technical
aspects of a repeat laminectomy are different from those of a primary
procedure.
is not possible to strip the paraspinal muscles away with impunity
because of absence of the spinous processes, lamina, or ligamentum
flavum at the sites of previous surgery.
-
Begin the approach at a new level with
normal anatomy and normal protection of the cauda equina. Find the
normal depth of the posterior elements and cauda equina, and carefully
extend the dissection into the area of the laminectomy defect. -
Working laterally, identify and expose the facet joints.
-
Proceeding distally, define the pars
interarticularis at the caudal base of the superior articular facet;
follow the pars further distally and medially onto the remaining lamina
and inferior articular facet of the next-lower facet joint. -
Carefully scrutinize the preoperative
plain radiographs, CT scan, and MRI scan to determine the extent of
previous resection. It is not uncommon to encounter a pars or facet
fracture unexpectedly. -
Beginning at each facet joint, use sharp
curets and a Penfield dissector to subperiosteally expose the remaining
normal posterior elements while minimizing risk of injury to the dura
and underlying cauda equina.
neural elements is determined, to remove the extradural scar tissue
directly over the dura. This is a technically difficult procedure with
the potential for a great deal of hemorrhage and a strong possibility
of dural injury. Even if the scar tissue can be successfully removed,
there is no reliable means available to prevent its regrowth. We
recommend, for the most part, that extradural scar tissue be left
intact; remove only the tissue covering the area of previously
documented nerve root compression.
nerve roots laterally and remove any mechanical (nonscar) tissue
pressure from them. Do so by extending the laminectomy from the new
level down the lateral gutters, leaving the central scar tissue as is:
-
Use sharp curets to follow the medial
border of the laminectomy defect ventrally, developing a plane between
the epidural scar tissue and the residual bone. -
Once this plane is developed, introduce a
Kerrison rongeur at a 45° angle, and undercut the bony encroachment,
usually arising from the medial overhang of the superior articular
facet. -
Carry this decompression out proximally,
distally, and laterally until all bony overgrowth has been removed back
to the medial wall of the pedicle. -
You may also use an osteotome to remove
the most medial portion of the facet, thereby gaining entry to the
spinal canal and nerve roots. -
If the goal of the repeat procedure is
decompression of recurrent stenosis, extend the laminectomy laterally
to the pedicle on either side at whatever levels are radiographically
involved. -
Leave the midline epidural fibrosis intact.
-
If a central laminectomy is required
either proximal or distal to the previous laminectomy, proceed in
standard fashion, first developing the interval between the caudad half
of the lamina and the underlying ligamentum flavum. Then resect the
lamina piecemeal. -
Once normal dura is encountered
proximally, reverse direction, working caudally to remove the
intervening ligamentum flavum from the underlying dura. -
If, at the junction of the previous
decompression and the ligamentum flavum, you find adherent scar tissue,
leave a small amount of ligamentum flavum over the thecal sac if it
cannot be safely dissected free. -
Address the nerve roots laterally, as previously described.
herniation is common. Many of the same caveats as described for repeat
decompressive laminectomy apply. Usually, a recurrent disc herniation
occurs in a patient who has had a previous relatively limited
hemilaminotomy, with the majority of the posterior elements being
preserved.
-
Expose the affected side only by
carefully dissecting along the involved laminae and following them
laterally as they join to become the facet joint. Place a retractor
lateral to the facet joint to visualize the previous laminotomy. -
Scar tissue that is encountered can be
thinned out, but as with a decompressive laminectomy, attempts to
remove epidural fibrosis from the dura completely increase the risk of
injury and are not indicated in most cases. -
Once the hemilaminotomy has been
completely defined, use either a Kerrison rongeur or osteotome to
remove a small portion of residual bone, first from the inferior facet
of the cephalad level and then from the medial aspect of the superior
facet of the caudad level. -
This new entry into the spinal canal and
lateral recess is slightly more lateral to the original hemilaminotomy
site. Extend the exposure until you are flush with the medial wall of
the pedicle. -
The traversing nerve root is usually
encased in a layer of scar tissue of variable thickness, but can be
palpated with a Penfield dissector. -
Carefully dissect along the lateral border of the root to mobilize it and retract it medially. Expose the underlying disc.
-
Although it can be fairly time consuming, careful mobilization of the nerve root is essential to avoid root injury.
-
Once the root is safely retracted medially, expose the underlying disc and resect it in standard fashion.
-
Whether or not to fuse in the face of a
recurrent disc herniation is a highly controversial decision; we favor
fusion when the patient has significant back pain or when there is any
suggestion of instability. We are more inclined to fuse at the L4–L5
level than at L5–S1. -
A second recurrence (third disc herniation) merits strong consideration of fusion.
the specific goal of repairing a nonunion unless other potential causes
of pain have been excluded. We rarely undertake nonunion repair in
cases in which evidence of motion on dynamic lateral x-ray studies or
progressive deformity has not been documented. Once the decision has
been made to proceed with revision fusion, several technical points
facilitate the procedure.
-
If there has been a prior laminectomy, expose carefully, starting at normal levels as described above.
-
Carry the exposure lateral to the facet joints and out all the way to the tips of the transverse processes on each side.
-
In cases in which there is a laminectomy
defect from previous surgery and no further decompression is planned,
use a paraspinal muscle-splitting approach, which affords excellent
visualization of the facet joints, pars interarticularis, and laterally
placed fusion mass. It also facilitates pedicle screw placement. -
After exposure, carefully explore the fusion mass.
-
In places where fixation devices are
still in place, it is very difficult to determine whether the fusion is
solid until the instrumentation is completely removed. Therefore, once
exposure has been obtained, disassemble the implant and remove it
piecemeal. -
Remove the fibrous scar tissue to visualize the fusion mass clearly.
-
Use Cobb elevators and sharp curets to
remove all soft tissue from the dorsal cortex of the fusion mass
extending from cephalad to caudad and from the most medial extent to
the lateral margins of the transverse processes and fusion mass. -
A well-consolidated fusion is easy to
strip, although it is not uncommon to find islands of fibrous tissue
surrounded by bridging trabecular bone. Soft tissues are attached
strongly to nonunions and are difficult to strip. -
Once the fusion has been completely
exposed, check not just for continuity but for adherence as well to the
proximal and distal spinal elements; occasionally, well-formed bone is
not adherent to the transverse process or, more commonly, the sacral
ala. -
In order to verify the adequacy of
fusion, take an osteotome and carefully remove the dorsal cortex of the
fusion mass to verify that there is underlying cancellous bone in
continuity with the transverse processes and alae. -
When a defect in the fusion mass is
found, it is frequently narrow and wanders in a serpiginous fashion
through the fusion. It can take fairly extensive exploration of a
defect to document that it does indeed track through the entire fusion
mass and allows motion. -
If the fusion is found to be solid, close
the wound in standard fashion. If a defect in the fusion mass is found,
use curets and a high-speed burr to remove all soft tissue from the
dorsal aspect of the defect. -
Complete excision of all soft tissue is
not necessary; remove the accessible dorsal soft tissue and decorticate
the area around the defect. Then apply abundant autologous bone graft
to the nonunion. -
Then stabilize the nonunions with a pedicle screw system and apply compression across the nonunion.
-
Identification of the normal landmarks
for pedicle screw placement can be difficult. Use interoperative
fluoroscopy for identification of the appropriate starting point and
path for the screws.
formed fusion mass, nonunion is seen in many patients in whom there has
been complete or near-complete resorption of the previously placed bone
graft. It is more common in patients in whom an allograft is used and
in smokers.
In these patients, it is usually readily apparent that the original fusion has failed.
-
Thoroughly expose out to the tips of the transverse processes.
-
Carefully decorticate the transverse processes and sacral ala.
-
Apply a massive bone graft to provide the best chance for successful repair.
-
In these cases, in which there is no stabilization from the original fusion, rigid internal fixation is essential.
previously failed fusion is interbody fusion. It can be performed
through a transforaminal or posterior approach or, more commonly,
through an anterior approach.
-
Use allograft, if desired, as a block
graft to provide stability, but supplement it with autologous bone from
the iliac crest or from the adjacent vertebral body. -
We consider performing a combined
posterior and anterior fusion in patients in whom a previously
well-done fusion with rigid fixation has led to nonunion, in smokers,
or in cases in which it is determined, at the time of posterior
exploration, that there has been sufficient attenuation of the bone
posteriorly to suggest that the chances of obtaining a solid fusion are
minimal (Fig. 149.10).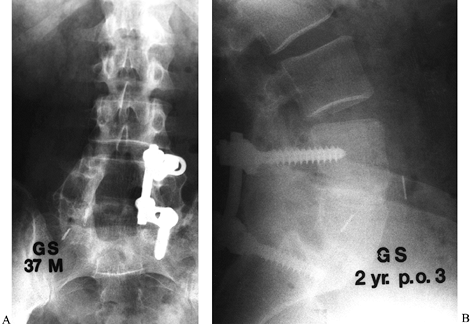 Figure 149.10. A 37-year-old man 2 years following his third attempt at L4–L5 and L5–S1 fusion for isthmic spondylolisthesis. A:
Figure 149.10. A 37-year-old man 2 years following his third attempt at L4–L5 and L5–S1 fusion for isthmic spondylolisthesis. A:
At the last posterior surgery, malpositioned left L-4 and L-5 screws
were removed and it was elected only to instrument the right side.
Because of the recurrent pseudarthrosis, and the ability to instrument
only one side, it was elected to proceed with anterior interbody
fusion, using a femoral ring allograft at both levels. B:
At 2 years follow-up, solid fusion is seen, and the patient, who had
not worked in 3 years, is minimally symptomatic and back to full-time
employment.
undergoing revision low-back surgery is similar to that for primary
surgery. Although the hospital stay is usually unchanged, the overall
length of recovery can be prolonged compared with first-time surgery.
The patient should be prepared for an extended time away from work or
other pressing duties. Fixation after revision fusion may be less than
ideal; therefore, we commonly supplement fixation with a brace. We use
a physical therapist to mobilize the patient after surgery by
facilitating transfers and ambulation; otherwise, we delay back
rehabilitation for 3 to 4 months following surgery.
The early postoperative period following a revision operation is not
the time to withdraw narcotic medication.
Work
with a pain management specialist to lessen narcotic usage gradually
with a goal of discontinuing narcotic medication altogether by 6 to 12
months postoperatively. Although this is an extended period of time, it
is impractical to assume that quicker withdrawal is possible.
greater in the patient who has undergone previous back surgery.
Although each dural tear is different, certain basic principles always
apply and certain steps should be followed.
visualization of, and entry into, the spinal canal. Although a large
majority of dural tears do not result in any long-term morbidity, the
repair of an intraoperative tear is time consuming and bears with it
the potential for persistent CSF leakage, wound problems, and nerve
root injury. The risk of dural tear is increased in repeat surgery
because previous resection of the posterior elements obliterates the
usual landmarks. Other risks include the difficulty of separating scar
tissue from the dura to develop a plane between the thecal sac and
nerve root, and the pathologic anatomy related to whatever is causing
recurrent neural compression.
-
Lessen the risk of dural injury by
beginning the deep exposure of the posterior elements, proximally and
distally, where there are retained normal spinous processes and laminae. -
Dissect proximally and distally along a
normal lamina to the facet joints and then work caudally from the
proximal end and distally from the cephalad end to expose the length of
the entire laminectomy defect safely. -
Expose the preserved facet joints and pars interarticularis susperiosteally, and leave a layer of scar tissue over the dura.
-
Use a curet to define the medial border of the retained posterior elements.
-
Rather than try to directly peel off or
resect scar tissue from the dura, resect a small amount of normal,
retained lamina or medial facet to expose an area of spinal canal,
thecal sac, or nerve root uninvolved with scar tissue. Entry to the
canal in such a way usually permits decompression without dural injury.
that is contributing to the nerve root or thecal sac compression, then
the technique described earlier is less likely to be adequate and the
risk of a dural tear is increased.
CSF, obscuring the extent of the damage. The surgeon’s first impulse is
to try to see the tear by using suction in the approximate area of the
problem. This is a mistake, because the individual nerve roots may be
sucked out of the thecal sac, causing significant neurologic damage.
Suction should be used only over a cottonoid so that no further damage
to the nerve roots is done. After visualizing the tear, place a piece
of Gelfoam over the injury site, cover it with a large cottonoid, and
complete the original procedure. The patient’s head may be tilted
downward into the Trendelenburg position to decrease the flow of CSF
into the wound.
repair of the dural tear. The goal is to achieve a watertight closure;
if not, a CSF fistula can form, raising the risk of meningitis or a
subarachnoid cyst. A dry operative field with hemostasis maintained
throughout the repair is essential. Similarly, achieve adequate
exposure in both the cephalocaudad and mediolateral directions in order
to define the extent of the tear adequately and to allow access for
repair. Failure to maintain hemostasis and to obtain adequate exposure
are the two most common causes of difficulty in repairing a dural tear.
Magnification loupes and adequate lighting also facilitate the repair.
-
For simple dural lacerations, we prefer
4-0 or 5-0 silk sutures on a tapered one-half circle needle. A running
locking suture or simple sutures incorporating a free fat graft provide
a watertight closure. -
If a tear is large or irreparable,
harvest a fascial graft from the lumbodorsal fascia and suture it
around the periphery of the defect with interrupted silk sutures. -
If the defect is in an inaccessible area,
introduce a small tissue plug of muscle or fat through a second midline
durotomy, pulling the tissue plug into the tear, thereby obliterating
the tear from inside the dura (12). -
Use Fibrin glue to reinforce the dural repair if there is any question about the adequacy of the repair.
-
Test the repair by placing the patient in
the reverse Trendelenburg position and performing a Valsalva maneuver
to increase intrathecal pressure. Close the fascia with a heavy,
nonabsorbable suture, which must be watertight.
possibility of the development of a draining fistula if there is
persistent CSF leakage. Keep the patient on bed rest for at least 3 or
4 days to reduce pressure on the repair while it heals.
can be difficult to make. If relatively clear drainage occurs, consider
the possibility of a dural leak. Similarly, a history of headaches when
the patient sits or stands suggests CSF leakage. No completely reliable
noninvasive diagnostic technique is available at present. The presence
of glucose
in
the fluid draining from an incision is not a reliable determinant,
because glucose is normally present in both noninflammatory and
inflammatory exudates. The best diagnostic test, a myelogram performed
with water-soluble contrast medium, is recommended if a dural leak is
suspected but the diagnosis is uncertain. Once a postoperative CSF leak
is diagnosed, pursue aggressive treatment. In the early postoperative
period, placement of a subarachnoid drain for 4 to 5 days has been
reported with good results (21).
If this procedure is unsuccessful or a leak is diagnosed late, timely
return to the operating room for dural repair is in order.
evaluation of the patient who has had previous back surgery is
essential. In many cases, the problem resulted from inadequate or
incorrect indications for the original surgical procedure. In such
patients, further exploratory surgery is not warranted and would lead
only to further disability. Another surgery is indicated only when
objective findings for a specific diagnosis are present.
operation, it must be appreciated that the surgery is usually more
extensive than the original operation with certain inherent risks. One
must approach the spine at a new level to identify the normal anatomy
of the neural elements and visualize the appropriate nerve root or
roots laterally, leaving the midline epidural scar tissue intact.
procedure, repair it in a watertight fashion. If nonunion of a prior
fusion is suspected, then carefully explore the fusion mass when a
nonunion is found, perform a thorough decortication, removal of scar
tissue, massive bone grafting, and rigid fixation.
repeat back surgery must realize that the chance of returning these
patients to a pain-free status is low. Depending on the type of
previous surgery and the patient’s symptoms, usually some form of
permanent impairment persists. These patients need counseling and must
be strongly encouraged to resume as functional a role as possible in
society.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
SD, Davis DO, Dina TS, et al. Contrast-enhanced MR Imaging Performed
After Successful Lumbar Disc Surgery: Prospective Study. Radiology 1992;182:59.
NF, Schonstrom NSR, Spengler DM. Role of Computed Tomography and
Myelography in the Diagnosis of Central Spinal Stenosis. J Bone Joint Surg [Am] 1985;67:240.
S, Bartleson JD, Onofrio BM, et al. Lumbar Spinal Stenosis: Clinical
Features, Diagnostic Procedures, and Results of Treatment in 68
Patients. Ann Int Med 1985;103:271.
SH, Eismont FJ, Green BA. Closed Subarachnoid Drainage for Management
of Cerebrospinal Fluid Leakage After an Operation on the Spine. J Bone Joint Surg 1989;71-A:984.
A, Kiviluoto O. Prevention of Epidural Scar Formation After Operations
on the Lumbar Spine by Means of Free Fat Transplants. Clin Orthop 1976;115:92.
JN, Spratt KF, Spengler D, et al: Spinal Pedicle Fixation: Reliability
and Validity or Roentgenogram-based Assessment and Surgical Factors on
Successful Screw Placement. Spine 1988;13:1012.
