ANESTHESIA AND PAIN MANAGEMENT
management involve preventing or controlling the response to acute
(surgical) pain and chronic (pathologic) pain. The response to acute
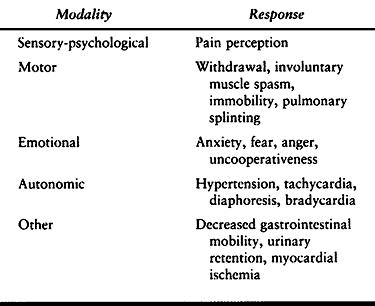
pain is motor (withdrawal), psychological (pain), emotional (fear), and
autonomic (hypertension, tachycardia) (Table 7.1).
The response to chronic pain is more complex because of the adaptations
that occur. A chronic pain patient’s response is somewhere between
stoicism and amplified emotion. The International Association for the
Study of Pain (IASP) defines pain as “An unpleasant sensory and
emotional experience associated with actual or potential tissue damage
or described in terms of such damage” (36a).
This definition does not include a description of how a patient in pain
looks. Only the patient’s description of his subjective experience
matters.
 |
|
Table 7.1. The Response to Noxious Stimulation
|
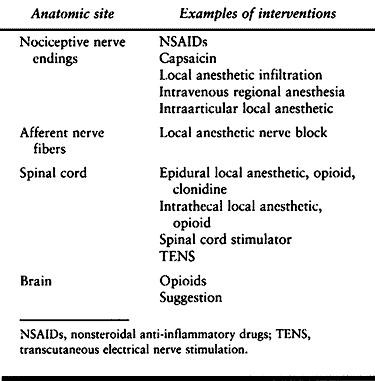
nociceptors, primary afferent fibers, ascending nociceptive tracts,
higher centers in the brain, descending pain modulating tracts, and
centers in the spinal cord. The detailed anatomy and physiology of pain
continues to be an area of active research. The sites where
interventions are currently available are listed in Table 7.2.
Despite the scientific knowledge about anesthesia and analgesia, the
practices of anesthesia and pain management have a decidedly empirical
nature. Drugs that block the motor and autonomic
response
to acute pain are well understood, as are the drugs that block nerve
conduction. Promising areas of research currently focus on the role of
the spinal cord, N-methyl-D-aspartate
(NMDA) receptors, and gamma-aminobutyric acid (GABA) receptors in the
mechanism of anesthesia and chronic pain.
 |
|
Table 7.2. Anatomic Sites for Pain Intervention
|
surgery without complications and usually with a quick return of
physiologic function. This should be accomplished while minimizing the
unpleasantness of the perioperative experience for the patient. There
are numerous options for controlling the response to surgery, but they
all have limitations and risks. Anesthetic complications arise from
invasive procedures, drugs, devices, and decisions. Complications are
avoided by assessing the patient, assessing the anesthetic implications
of the operation, selecting an anesthetic that will accomplish the
goals, modifying the anesthetic according to the patient’s response and
the operative requirements, and managing the risks. Risk management
consists of anticipating adverse effects, preventing them if possible,
looking for them, and controlling them. Even with careful planning,
unanticipated problems arise. Early recognition can often be gained by
vigilant routine monitoring. This is important because the evolution of
some problems is extremely rapid. Problem-solving skills and experience
are needed. The source of a problem is the operation, the patient, the
anesthetic, or sometimes a combination of these factors. Sometimes
multiple problems arise and need to be prioritized so that the most
rapidly fatal problems are dealt with first.
anesthetic risk management. Cardiovascular and pulmonary disease,
obesity, pregnancy, and many other conditions increase the possibility
for anesthetic-related complications. The subsets of orthopaedic
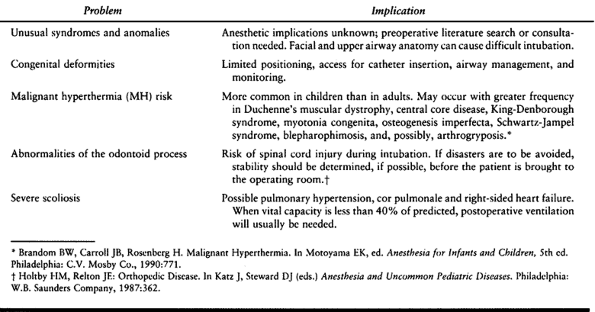
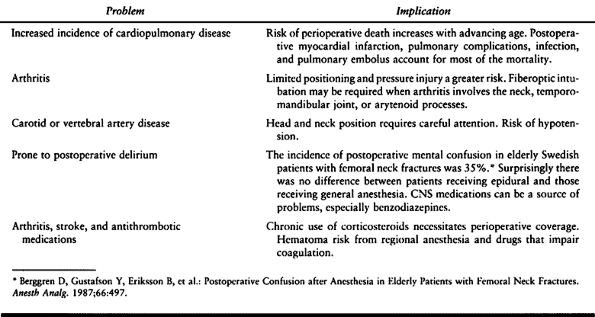
patients who require careful assessment are the pediatric, the
geriatric, and the trauma patients. Table 7.3, Table 7.4 and Table 7.5, respectively, summarize some of the specific considerations for these patients.
 |
|
Table 7.3. Pediatric Special Risks
|
 |
|
Table 7.4. Geriatric Special Risks
|
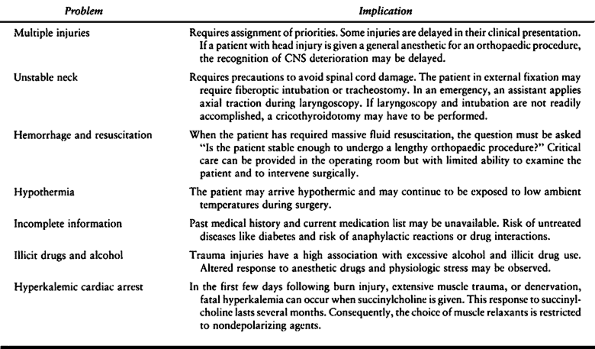 |
|
Table 7.5. Trauma Special Risks
|
Anesthesiologists’ Physical Status (ASA-PS) classification has been
used to grade the preoperative physical condition of surgical patients (38). The current, revised classification appears in Table 7.6 (34).
This classification has proven useful in assessing the effect of a
patient’s preoperative condition on the subsequent response to
anesthetic techniques and drugs. The ASA-PS communicates succinctly to
other anesthesiologists and surgeons the assessor’s evaluation of the
patient. It serves statistically as an ordinal covariate for an
individual patient. The distribution of
ASA-PS
in a patient population is useful in comparing one population with
another. A consistent correlation between ASA-PS and perioperative
mortality has been reported in two large series (27,51).
This consistency is evident despite the fact that ASA-PS does not
include a priori information about anesthetic or surgical risk, such as
known difficult airway or expected major blood loss. To be useful as a
covariate, the ASA-PS should be consistent between different rating
anesthesiologists. Consistency has been tested in a small series. When
presented with 10 different patient descriptions, 4 patient
characteristics that cause anesthesiologists to differ in their
assignment of ASA-PS are age, anemia, a history of previous myocardial
infarction, and obesity (36).
 |
|
Table 7.6. ASA Physical Status
|
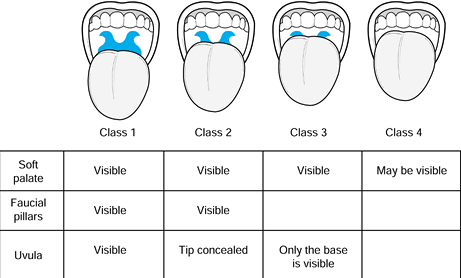
anatomy has been used to predict the likelihood of difficult
endotracheal intubation (25). This classification is described in Figure 7.1.
It basically estimates the relative sizes of the mouth and the tongue.
The mobility of the neck is an additional factor. This classification
has become a routine part of the preoperative assessment; although it
is not infallible, it indicates the possible need to prepare for a
difficult intubation. Patients with class 3 or 4 airways are
increasingly likely to require nonroutine methods for intubation.
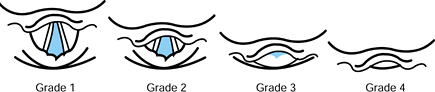
Laryngoscopy grades, described in Figure 7.2,
provide a succinct indication of the view during direct laryngoscopy.
Patients in grades 3 or 4 are likely to be difficult to intubate
because the glottic opening is not visible.
 |
|
Figure 7.1.
The Mallampati airway classification is based on assessing the relative size of the oropharyngeal cavity and the tongue. Classes 3 and 4 are predictive of difficult laryngoscopy. Limited neck mobility and a short neck are also predictive of difficulty. |
 |
|
Figure 7.2.
The airway grade classification is based on the view that is obtained by direct laryngoscopy. It is partially dependent on laryngoscopy technique and skill. |
“deep.” At one time, the stages of ether anesthesia were used to
describe the depth of anesthesia. With newer agents that are used in
combinations, the stages are difficult to identify by simple
observation and are no longer clinically relevant. It is common to rely
on the end-tidal anesthetic concentration (MAC, or minimum alveolar
concentration) as an indication of depth, although it is really a
measure of dose. Neurophysiologic correlates of anesthetic depth have
been proposed, but none has as yet been widely accepted. The bispectral
index, which is based on the computer-processed electroencephalogram
(EEG), is currently of interest.
regional, or local anesthesia with or without sedation. Anesthetic
agents are also broadly classified by route of administration,
pharmacologic class, and clinical application.
somatic. These classifications correspond loosely to pain arising from
nerves or pain from nonneural tissues. A third category comprising
psychogenic pain, arising from thought disorder, is considered to
represent few patients,
although many chronic pain patients fear that they will be placed in this category.
elimination of routine laboratory testing and x-rays, and the
elimination of routine preoperative admission the night before surgery
have been adopted to reduce the costs of surgical procedures. As with
other attempts to reduce costs, care must be exercised to ensure
adequate preparation for the operation and the anesthetic. Some
patients benefit from preoperative medical consultation and additional
testing and therapy. Others benefit from postponement of surgery until
they have recovered from acute illness or exacerbation of a chronic
illness. To avoid the cost of canceling a scheduled case on the day of
surgery, we have a system of preoperative screening and evaluation.
Patients are screened by means of a questionnaire that is completed
during the preoperative surgical clinic visit. Patients who score above
a threshold number of points are referred to the anesthesia presurgical
unit (PSU), where they are evaluated by either a nurse practitioner or
an anesthesiologist. In addition, all hospital admission requests (HAR)
for surgery are screened for patients who might benefit from early
evaluation. Timely medical consultations can thus be obtained. Patients
who have medical problems but do not need to be seen in the anesthesia
presurgical clinic are evaluated by chart review or telephone
interview, or both. The evaluation results are available by computer in
the operating room.
cancellations for anesthetic reasons. Issues that are addressed in the
PSU include identification of medical problems that should be evaluated
or controlled, recommendations for perioperative management of
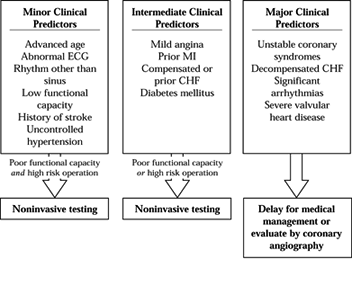
medications, and recommendations for preoperative testing. Algorithms
have been proposed for preoperative evaluation of patients with
symptomatic cardiovascular disease (2,14). See Figure 7.3
for an example. The American College of Cardiology/American Heart
Association and also the American College of Physicians have published
recommendations for evaluating patients who have coronary artery
disease (18a).
Factors that correlate with increased risk of perioperative cardiac
morbidity are the presence of acute congestive heart failure, recent
(within 2 months) myocardial infarction, unstable angina, diabetes,
limited exercise tolerance, and the type of operation. Guidelines for
preoperative testing have resulted in reductions in routine laboratory
tests, chest films, and electrocardiograms (ECGs), except in specific
populations. See Table 7.7 for the guidelines
that we use. Lastly, misunderstandings about the management of
medications can cause delays or cancellations. Our current
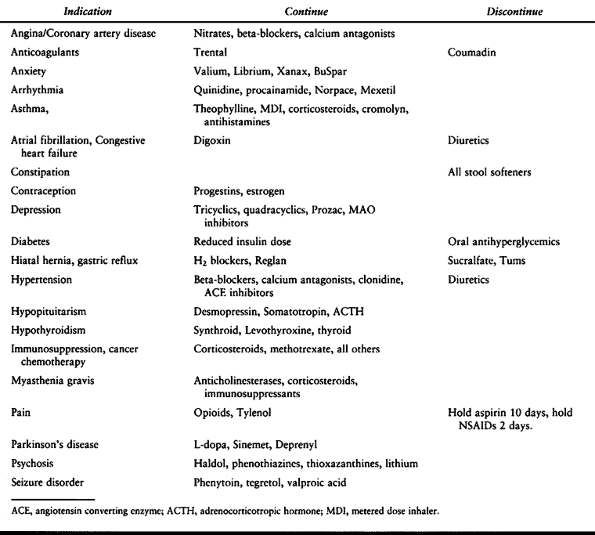
recommendations for medications to be continued or discontinued are
listed in Table 7.8. The type of anesthetic is
not selected in the PSU because that is decided by the anesthesiologist
who eventually cares for the patient in the operating room. Information
about the various types of anesthesia is provided, however, and one
hopes that this preliminary discussion will save time on the day of
surgery.
 |
|
Figure 7.3.
The initial steps in the guidelines. The clinical predictors are grouped according to significance for severe coronary artery disease. The patient’s functional capacity and the risk of the operation determine whether further testing or medical management are indicated. |
 |
|
Table 7.7. Guidelines for Preoperative Laboratory Testing*
|
 |
|
Table 7.8. Medications that Should be Continued or Discontinued Preoperatively
|
consideration of the operative requirements. Other factors are patient
illness and medications, expected duration of the procedure, position
on the table, blood loss, and preferences of surgeon, patient, and
anesthesiologist. It might appear that the risk of complications should
be least with local anesthesia and greatest with general anesthesia,
but patients under inadequate local anesthesia have experienced acute
myocardial infarction and patients under spinal anesthesia have
experienced respiratory arrest and anoxia. Reports that correlate
morbidity or mortality with type of anesthesia are difficult to
interpret when the type of anesthetic has not been randomly assigned.
Furthermore, factors such as the quality of postoperative respiratory
and nursing care influence the outcome from general anesthesia.
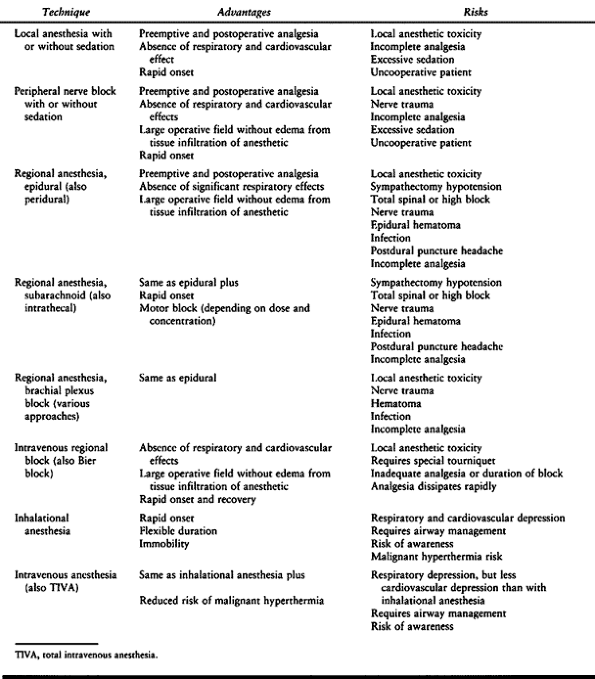 |
|
Table 7.9. Anesthetic Techniques. A Brief Summary
|
anesthetic are sufficient for limited procedures in cooperative
patients. The primary risks are local anesthetic toxicity and
inadequate analgesia. Allergy to local anesthetics is rare. Most
reactions are due to epinephrine absorption or high blood
concentrations of local anesthetic. Allergic reactions occur to the
antioxidant (bisulfite) (41) or the preservative (methylparaben) in the commercial preparations (32). Although they are extremely rare, allergies to both lidocaine and bupivacaine have been reported (7). Skin testing with a preservative-free agent will identify true allergy to local anesthetic (39).
Toxicity results from inadvertent intravascular injection or from
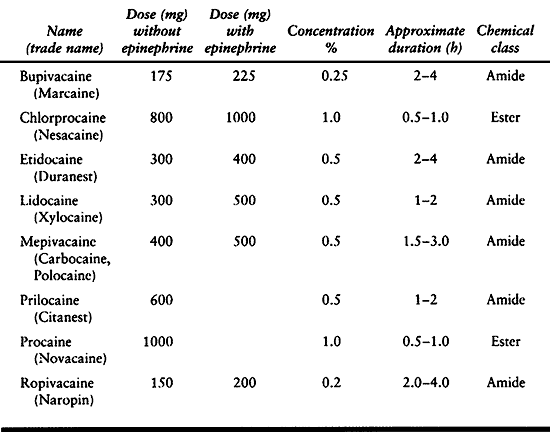
excessive dose. The maximum recommended dose depends on the size of the
patient, the site of injection, the particular agent, and the use of
epinephrine (Table 7.10). Frequently used
supplements are midazolam for sedation and amnesia, and an opiate such
as fentanyl for analgesia. Oxygen, pulse oximetry, periodic verbal
contact, and observation reduce the risk of hypoxia and respiratory
depression.
 |
|
Table 7.10. Recommended Maximum Local Anesthetic Doses for Infiltration
|
are the most serious complications resulting from high blood
concentrations of local anesthetic. A peak blood concentration occurs
immediately following intravascular injection or premature release of
the tourniquet during intravenous regional block. A delayed peak (20 to
30 min) occurs after regional blocks. The priorities in managing toxic
reactions are oxygenation, cardiovascular resuscitation, if necessary,
and suppression of seizures. Intractable ventricular dysrhythmias have
been associated with bupivacaine and etidocaine cardiotoxicity (1). Possible mechanisms are inhibition of calcium release from sarcoplasmic reticulum (24) or use-dependent block of sodium channels on myocardial cells (10). There may also be a central nervous system (CNS) contribution to the cardiotoxicity (6). The best treatment of this complication has not been determined, but recommendations include bretylium or amiodarone (19).
brachial plexus (axillary and interscalene) blocks are the most
commonly used techniques for regional anesthesia. Combination nerve
blocks, such as ankle block, sciatic-femoral block, elbow block, and
wrist block, are less commonly used but should be considered for
high-risk patients. Epidural and sometimes spinal anesthesia is
difficult to achieve in the foot or ankle (18). This may be due to the large size of the L-5 and S-1 nerve roots.
increase the time the surgeon waits before surgery can start. Clear-cut
landmarks for needle insertion and easily identified endpoints for
needle position [cerebrospinal fluid (CSF), arterial blood,
paresthesia] make a block easier to perform; obesity and spinal
deformity are sources of difficulty. A block that has been easy to
perform can nevertheless take up to 20 minutes to produce adequate
anesthesia. Neutralization of local anesthetics with sodium bicarbonate
may hasten the onset. Agents are usually selected for the duration of
block they produce. An epidural block with lidocaine lasts 1 to 2 hours
or 2 to 4 hours with bupivacaine. In the less vascular vicinity of
peripheral nerves, lidocaine lasts 2 to 4 hours and bupivacaine lasts 6
to 12 hours.
nitrous oxide analgesia, or general anesthesia can supplement regional
blocks that are anatomically incomplete or inadequate in duration. As
in the selection of anesthetic technique, the best choice depends on
the patient (pain tolerance), the surgical problem (need for
immobility), and the operation (time remaining to completion, intensity
of noxious stimulation). One particular danger is that intravenous
sedation and analgesia with or without nitrous oxide can progress
insidiously to general anesthesia without protection against aspiration
or without adequate ventilation and oxygenation. On the other hand, a
general anesthetic superimposed on an incomplete epidural or spinal
anesthetic can be complicated by the presence of a sympathetic block.
block. It starts to be a problem approximately 1 hour after tourniquet
inflation. Under general anesthesia, progressive hypertension is
noticed. Overtreatment can result in hypotension when the tourniquet is
released. Tourniquet
release
can also cause transient hypercapnia and elevation of intracranial
pressure in head-injured patients if ventilation is not temporarily
increased. There is a slight temperature elevation with tourniquet
inflation and a decrease after tourniquet release.
either systolic or diastolic blood pressure (BP)] is more common under
general anesthesia than under regional anesthesia. It is not prevented
by a sympathetic block. Technical details have been shown to have some
influence over the efficacy of a spinal (subarachnoid) block in the
prevention of tourniquet hypertension. The amount of anesthetic; the
particular anesthetic agent; the addition of epinephrine, clonidine, or
morphine; and even the concentration of added glucose have been shown
to have some effect. Differential blockade of some fiber types but not
of others may explain the occurrence of tourniquet hypertension despite
adequate sensory level as determined by pinprick. Tourniquet pain is
probably mediated by unmyelinated, slow-conducting C fibers.
Explanations include a gate theory mechanism of large fiber block and
small fiber activity. The superiority of intrathecal bupivacaine
compared with tetracaine may be due to the longer residence of
bupivacaine at the nerve fiber.
hypertension are to use subarachnoid block rather than general
anesthesia or epidural anesthesia, obtain an adequate pinprick level,
and add either epinephrine or clonidine but not glucose. Alkalinization
of the agent may improve the success with mepivacaine epidural
anesthesia so that it is comparable to an intrathecal block with
bupivacaine (47). Alkalinization is thought to
enhance the onset and intensity of a block by increasing the nonionized
form and by increasing neuronal pH.
A high incidence of venous thrombus embolism has been discovered after
tourniquet deflation. Metabolic products and cooled blood may be part
of the problem. When hypotension persists or is catastrophic, pulmonary
embolism becomes a serious concern.
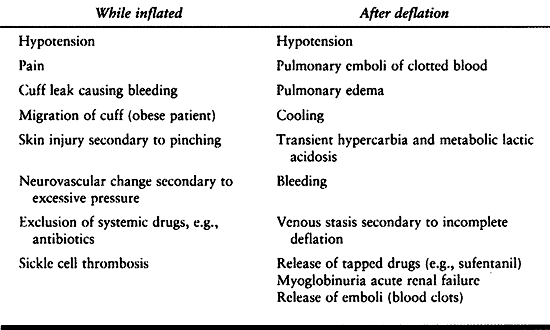 |
|
Table 7.11. Tourniquet Problems
|
under general anesthesia is between 60% and 70%. Regional anesthesia
[both spinal (11,49) and epidural (30)]
reduces the incidence of postoperative deep vein thrombosis to 20% to
40%. Proposed mechanisms include increased lower limb blood flow,
reduced activation of factor VIII, reduced viscosity, and enhanced
release of plasminogen activators. Additional
advantages
that have been claimed for regional anesthesia include a lower risk of
aspiration and pulmonary complications, reduced blood loss, prophylaxis
against phantom limb pain, reduction of the stress response, and the
ability to continue the analgesia postoperatively.
(spinal) block is hypotension from sympathectomy. Hypotension is more
likely to occur if a patient is hypovolemic or if the block is
extensive. Consequences of uncontrolled hypotension include nausea,
vomiting, apnea, stroke, and myocardial infarction. The side effect
that patients fear is permanent neurologic injury. Although this
complication is rare, it can occur from traumatic needle insertion,
infection, epidural hematoma, spinal cord ischemia, or cerebral
ischemia. See also the discussion in the section on Pitfalls and Complications.
spinal block, and this factor is sometimes used to advantage in
patients with potentially unstable cardiovascular responses.
Disadvantages of an epidural block are that it is usually less intense
than a spinal block and a larger dose of local anesthetic is required.
Thus, there is greater risk of an inadequate block, and there is risk
of either a total spinal block or local anesthetic toxic reaction from
accidental intrathecal or intravenous injection. It is usually assumed
that a failed spinal anesthetic is due to faulty technique or a
defective drug, but cases have been reported in which both
possibilities were excluded with the implication that certain patients
are truly resistant to spinal anesthesia. In some instances, these
patients were resistant to lidocaine but not to bupivacaine (40).
and with the use of larger gauge needles. A spinal headache may be
distinguished from meningitis by postural relief and by the absence of
obtundation, leukocytosis, and fever. Fluid, caffeine, and abdominal
binders offer some symptomatic relief. The headache is completely
relieved in 90% of patients by epidural injection of 10 to 20 ml of
freshly drawn, autologous blood. Untreated headaches can progress to
abducens nerve palsy or auditory deficits.
fascial sheath, the various approaches—axillary, subclavian, or
interscalene—all produce a similar result, provided that an appropriate
volume of local anesthetic is deposited. The distribution of anesthesia
is more extensive with the more proximal injection sites. An axillary
approach is used when the forearm or hand is the operative site.
Interscalene block is used when the elbow, upper arm, or shoulder is to
be operated on or when the axilla is unavailable. The technique of
eliciting paresthesias is associated with persistent postoperative
paresthesia, possibly due to nerve trauma (42). Location of nerves can be achieved less traumatically by using a nerve stimulator.
is rapid in onset and recovery. Tourniquet pain becomes a problem after
an hour. Risk of local anesthetic toxicity is significant, especially
during injection (leak under the cuff) and after release of the
tourniquet, because a toxic dose is deliberately placed
intravascularly. The tourniquet is usually deflated briefly and quickly
reinflated to release only part of the dose over 10 to 15 minutes.
applicable, there are sometimes reasons for considering alternatives.
Examples include a full stomach, obesity, reactive airway disease,
malignant hyperthermia (MH), generalized muscle disease, a need to
monitor neurologic status, and pregnancy. There can be advantages to
combining regional and general anesthesia. For long procedures and when
general anesthesia might cause excessive cardiac depression, regional
anesthesia reduces the amount of general anesthetic needed. When
postoperative analgesia with epidural opiates is indicated, a combined
anesthetic can be given for the procedure and the regional anesthetic
can be continued postoperatively using opiates with or without local
anesthetic. Whether the combination of general and regional anesthesia
reduces the risk of deep vein thrombosis has not been tested.
anesthesiologist with airway management challenges. The pediatric
patient who has skeletal deformity may also have facial and airway
malformation. Elderly patients with deformity of rheumatoid arthritis
may have cervical spine and arytenoid process arthritis. The trauma
victim may have facial or cervical spine injury and multiple
orthopaedic injuries. Key questions that should be answered before
undertaking airway management are
-
Is difficult intubation likely?
-
Is difficult ventilation likely?
-
Will the patient consent to or cooperate with airway management procedures?
-
Is a surgical airway needed?
-
Is awake intubation indicated?
-
Is it necessary to maintain spontaneous ventilation?
deformed or unstable cervical spine may require fiberoptic intubation.
Practice guidelines from the ASA (46) provide
recommendations regarding airway evaluation, preparations, and
management. A portion of the guideline algorithm listing airway
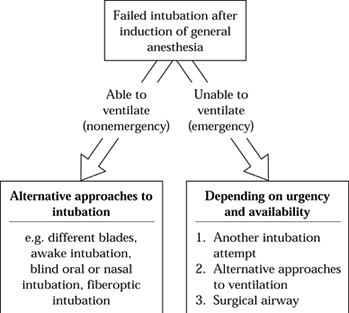
management options is shown in Figure 7.4. The
risk of hypoxic injury is probably greatest once anesthesia has been
induced and intubation has been unsuccessfully attempted. As long as
mask ventilation can be adequately provided and as long as there is no
risk of gastric content aspiration, fiberoptic intubation can be
performed under general anesthesia. Alternatively, the patient can be
allowed to recover from anesthesia for awake intubation. In pediatric
patients, awake fiberoptic intubation may not be an option. Finally,
some orthopaedic procedures present specific threats to a secure
airway. Operations that require repositioning of the patient to a
lateral or a prone position require special attention to avoid
accidental extubation or mainstem intubation.
 |
|
Figure 7.4.
A portion of the airway algorithm that proceeds from failure to intubate after inducing anesthesia. Other portions not shown involve the preoperative assessment and planning of airway management. |
problem of fluid management. During major procedures lasting more than
3 hours and with anticipated blood loss of more than 20% of blood
volume, an arterial catheter, central venous pressure (CVP) or
pulmonary artery (PA) catheter, and Foley catheter are commonly used.
Invasive monitoring is likely to be required in elderly patients
undergoing lengthy hip fracture surgery because the risk of myocardial
infarction correlates with the duration of surgery. Conservation of
blood by intraoperative hemodilution or induced hypotension also
warrants closer, more invasive monitoring.
cord function. Both methods in common use—somatosensory evoked
potentials and the wake-up test—mimpose restrictions on the anesthetic
agents. In the case of somatosensory-evoked potential monitoring
(posterior spinal cord function), nitrous oxide with narcotic and low
concentrations
of
volatile anesthetics can be used. A stable anesthetic depth is required
during the monitoring period, and nondepolarizing muscle relaxants are
given to suppress electromyographic (EMG) interference. When the
wake-up test (anterior spinal cord function) is used, low solubility
volatile anesthetics, such as desflurane or sevoflurane, will prevent
prolonged emergence. Recovery from nitrous oxide usually occurs in 5 or
10 minutes. A nerve stimulator can be used to maintain a partial
neuromuscular block, thus avoiding excessive movement during
reawakening.
venous air embolism is a possibility. Precordial Doppler ultrasound is
a sensitive monitor for air in the right side of the heart, and a
central venous catheter is one method for removing some of the air.
Hypotension during methylmethacrylate cementing of hip prostheses was
initially attributed to monomer escape into the circulation, causing
vasodilation or myocardial depression. Recent evidence implicates air
embolism as the cause of hypotension in up to 57% of arthroplasty
patients. This hypotension is usually inconsequential but may cause
impaired pulmonary blood flow and hypoxemia or paradoxical embolism.
Cardiac arrest has been reported to result from paradoxical embolism to
the coronary arteries (8). In recent years,
transesophageal echocardiography (TEE) has been used increasingly as a
less invasive alternative to pulmonary artery catheterization. Air and
thrombotic emboli can be visualized, as can ventricular dimensions and
wall motion. The primary limitations to more general use are the cost
of equipment and the substantial training and experience required for
interpretation.
applying any monitoring device is wasted if the person who should make
use of the information is inattentive, distracted, or inexperienced.
(pressure or stretch) or indirectly (decreased perfusion pressure or
impaired gas exchange). In addition, the transition to a new position
can cause loss of monitoring, loss of intravenous catheters, or loss of
the airway. Traction injuries to joints and nerves can also result from
position change. The major risks of prone position are injury to the
face, impaired inferior vena cava flow, and injury to nerves.
Cardiopulmonary function is not significantly different from that in
the supine position if the abdomen is not compressed. The lateral
position is more disturbing to cardiopulmonary function. Because of the
cephalad shift of the diaphragm during general anesthesia,
ventilation-perfusion matching is altered, resulting in arterial blood
gas changes. Thus, a patient undergoing hip surgery in the lateral
decubitus position will have better gas exchange with regional
anesthesia and spontaneous ventilation than with general anesthesia.
The lateral position places patients at risk for nerve injury, vascular
compression, and pressure injury to the dependent side of the face and
head. Of particular importance is the “axillary roll” to prevent
compression of the subclavian artery between the clavicle and humerus.
In the lateral position, the pulse oximeter placed on the dependent arm
can be used to detect arterial compression. Complications can occur
from intraoperative movement out of position, so the patient’s position
should be monitored. Finally, nonsupine positioning and draping limits
access to the patient for monitoring or other procedures.
preoperative deficits, replacement of ongoing insensible losses
(maintenance), compensation for third-space loss, and compensation for
blood loss. Anesthesiologists use additional fluid as a carrier for
drugs and to compensate for the vasodilation of anesthesia. The amount
of third-space loss is clinically unmeasured and depends on the degree
of tissue trauma. For orthopaedic procedures, 2 ml/kg/hour above
maintenance is commonly used. Errors in fluid management include
excessive reliance on BP or urine output as endpoints, because both are
indirect measures of fluid volume status. When BP or urine output are
not normal after giving the most liberal estimate that can be
justified, additional information is usually sought by measuring
cardiac filling pressures.
blood products. Transfusion risks are of continuing concern, but
improvements in donor screening and blood unit testing have improved to
the point where some are beginning to question the need to avoid
allogenic transfusion in favor of autologous donation (15a) (see Chapter 5).
Measures to avoid autologous transfusion of blood products include cell
saving, hemodilution, and induced hypotension. Blood loss during total
hip replacement can be reduced 30% by either induced hypotension or
regional anesthesia (48,52).
Tourniquet hypertension may lead to myocardial ischemia in patients
with coronary artery disease or ventricular hypertrophy. Many of the
serious complications occur with release of the tourniquet. To the
already established use of extremity tourniquet and induced hypotension
have been added measures to recover the patient’s blood loss and avoid
transfusion by using autologous blood (Table 7.13). A tourniquet is the traditional method to control blood loss during extremity surgery and to improve visibility.
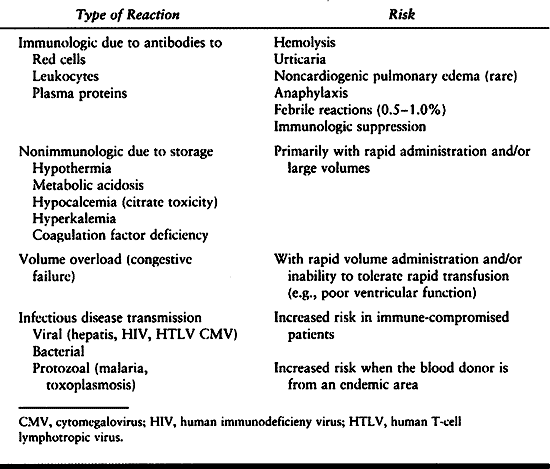 |
|
Table 7.12. Transfusion Risk
|
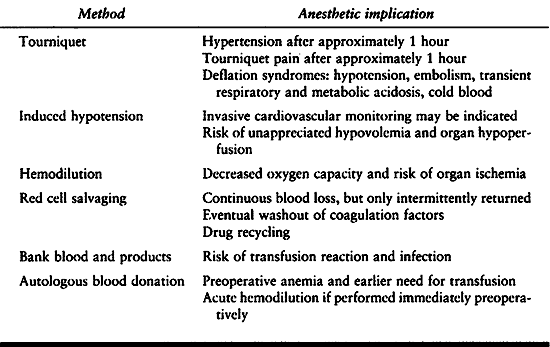 |
|
Table 7.13. Blood Conservation
|
should be reduced if systemic BP is reduced. Systemic BP can be reduced
by anesthetics, regional anesthesia, and vasodilating drugs. Inhalation
anesthetics produce both vasodilation and myocardial depression. The
possibility of organ ischemia and infarction has to be considered and
discussed with patients. Induced hypotension is contraindicated in
patients who have coronary artery disease, cerebrovascular disease,
chronic obstructive pulmonary disease, and anemia. Normovolemic
hemodilution is contraindicated if induced hypotension is used. How far
can systemic BP be safely reduced? Clinical studies suggest that a
systolic BP less than 65 mm Hg is the lower limit. Approximately 700 ml
of whole blood is conserved, and operative time is shorted (3,48).
The normal brain tolerates reduction to 55 to 60 mm Hg mean arterial
pressure (MAP). MAP is used rather than systolic BP because the
arterial catheter system can distort the arterial pressure waveform;
systolic pressure will be affected, but MAP will not. Because patients
with chronic hypertension are unable to autoregulate cerebral blood
flow (CBF) at 50 mm Hg MAP, their pressures should be reduced only to
50 mm Hg below their usual MAP. Contraindications to induced
hypotension include fever, anemia, and occlusive cerebrovascular
disease. Because hypocapnia will reduce CBF, a normal PaCO2
should be maintained. Isoflurane at high concentrations lowers MAP by
vasodilation but maintains cardiac output. When evoked responses are
monitored, nonanesthetic drugs such as sodium nitroprusside (SNP),
nitroglycerine (NTG), and trimethaphan are used to lower MAP. SNP has a
rapid onset, short duration, and consistent effect. It vasodilates with
little effect on cardiac output. The side effects of SNP include
cyanide toxicity, rebound hypertension from plasma renin activity,
coagulopathy, pulmonary shunting, reduced tissue oxygenation, and
hypothyroidism. Severe reductions in BP may impair spinal cord
perfusion enough to alter evoked responses. The risk of pressure injury
is increased by hypotension. Rebound hypertension can be prevented by
gradual return to normal MAP, propranolol pretreatment, or captopril.
Coagulopathy is due to platelet disintegration and inhibition of
platelet aggregation (22,28). Pulmonary shunting is due to the vasodilation of pulmonary vasculature (9). Tissue oxygenation may be preserved better with NTG, because SNP but not NTG diverts blood flow to arteriovenous shunts (20).
preparation and is limited by the time that it takes for red cell
production. Erythropoiesis is not stimulated until the hematocrit is
less than 30%. Exogenous erythropoietin can be used but increases the
cost of the procedure. There has not been a rigorous study of risk
versus benefits of preoperative donation. Mathematical modeling
predicts that the benefit is small. On the other hand, the patient
generally becomes anemic and the threshold for transfusion is reached
earlier. The patient who donates for herself is then exposed to risks
of transfusion, possibly unnecessarily. The administrative cost of
preoperative donation is more than for homologous transfusion.
donation is phlebotomy with isovolemic hemodilution. This is done with
the patient in the operating room but before surgical blood loss. The
patient’s blood is kept in the operating room. Diluted blood is lost,
and the patient’s own blood is returned to maintain hematocrit at the
transfusion threshold. As with preoperative donation, mathematical
modeling predicts little benefit unless large volumes of blood are
withdrawn (4 to 5 units) and surgical blood loss is large (2000 ml) (4).
In addition, either large volumes of crystalloid or colloid replacement
must be given to achieve hemodilution without hypovolemia.
blood, with little apparent risk to the uninfected patient. Blood is
collected by suction and then heparinized and washed. The concentrated
red blood cells, minus clotting factors and platelets, are then
available for reinfusion into the patient. The effect of
autotransfusion on coagulation has been studied using the Sonoclot
device (15). A brief period of
hypocoagulability, followed by a tendency to hypercoagulability, was
observed. The conventional cell-washing devices require collection of
1000 ml before the blood can be efficiently processed. This is a
significant disadvantage in smaller patients who may need to be
transfused with bank blood before their own washed cells are available.
posthemorrhage reinfusion of the patient’s own blood are well tolerated
under anesthesia because oxygen requirements are reduced. If anemia
persists postoperatively, increased oxygen consumption from shivering
should be prevented with small doses (12.5 mg) of intravenous
meperidine.
donor screening and blood testing. Of continuing concern in
orthopaedics are the studies that show increased rates of postoperative
infection in total joint replacement and spine surgery when allogenic
blood has been used (16,31,,50). Transfusion has also been implicated in tumor recurrence after cancer surgery.
Blood Component Therapy (37).
One effect of these guidelines is to encourage use of laboratory
testing to establish that indications for transfusion of a component
have been met. In this way, it is hoped that unnecessary component
therapy and attendant risks can be avoided.
perfluorochemicals, are under development. At present, limited clinical
trials are in progress. These substitutes are expensive and less
efficient than blood, but they may eventually be useful in normovolemic
hemodilution.
Risks of inadequate analgesia include myocardial infarction, impaired
ambulation and ventilation, and reduced cooperation. Risks of analgesia
include impaired consciousness, respiratory depression, and delayed
recognition of postoperative complications, such as myocardial
infarction or compartment syndrome.
is a rapid return to function. An increased interest in postoperative
pain management by anesthesiologists and hospital pharmacists is
reflected in the increasing availability of patient-controlled
analgesia (PCA) and regional opioid analgesia.
The neural basis for this clinical observation may reside in the
posterior horn of the spinal cord. Noxious stimulation produces an
activation of spinal neurons that persists and enhances the response to
repeated noxious stimulation. Numerous methods to reduce the need for
postoperative analgesia have been tried. The effective preemptive
techniques include opioid analgesia, local anesthetic infiltration of
the incision site, nonsteroidal antiinflammatory drugs (NSAIDs), and
regional anesthesia.
pharmacodynamic differences between patients, PCA is particularly
useful for severe postoperative pain. When given limited control for
self-administration of intravenous opioids to control postoperative
pain, the majority of patients do not become narcotic addicts. The
constant infusion (basal rate) provides consistent analgesia for the
patient who has constant pain at rest, and supplemental analgesia
(bolus doses) will be available for dressing change, position change,
or ambulation. Contraindications to PCA have included children, drug
addicts, and patients unable to understand or unable to operate the
device. In pediatric patients, there is risk that a parent will operate
the device or that the child will fail to use it. However, PCA has been
used successfully in children as young as 5 years old. Patients with
alcoholism or other drug addiction require larger doses. Whether these
patients are more difficult to wean from PCA remains controversial.
Risk of respiratory depression is greatest during sleep, following
abrupt reduction of painful stimulation, or following coadministration
of sedatives.
anesthesia can be continued postoperatively in infusion of local
anesthetic (5,33). Good
clinical demonstrations of benefit are currently lacking, but
theoretical advantages are improved tissue perfusion, prevention of
phantom pain, reduction in pulmonary complications, and prevention of
venous thrombosis. On the other hand, an insensitive extremity places
the patient at risk for unrecognized ischemic or pressure injury.
Sympathectomy may predispose the patient to hypotension. Accumulation
of the drug may result in local anesthetic toxicity.
intravenous infusion is based on the premise that an effective
concentration of spinal opioid can be achieved without producing
systemic analgesic concentrations that can be produced by intravenous
infusion. In addition, the side effects of spinal opioid should be
comparable or less than the side effects of intravenously administered
opioid. Morphine and fentanyl are the opioids that are commonly infused
epidurally. Because of its relatively higher lipid solubility, fentanyl
is more rapidly distributed to the epidural vascular structures and
removed to the systemic circulation. Consequently, its distribution in
the neuraxis is more restricted. Morphine migrates rostrally over
several hours, eventually reaching the brain stem. Both drugs can
produce respiratory depression.
fentanyl, produces analgesia without sympathectomy, anesthesia, or
impaired motor function. Pruritus, urinary retention, and sedation
occur commonly. A less frequent but significant hazard is delayed
respiratory depression from cephalic spread of the opiate, especially
morphine. Sedation from the upward spread of morphine usually precedes
respiratory depression. With daily monitoring, epidural catheters are
used for up to a week. Systemic infection and systemic anticoagulation
are contraindications.
epidural anesthesia. Epidural analgesia has only rarely been a cause of
permanent neurologic injury. An alarming
incidence
of this complication has recently been reported in patients receiving
low-molecular-weight heparin for thromboembolism prophylaxis. The issue
of anticoagulation and neuraxial anesthesia (both epidural and
subarachnoid) is the subject of a recent collection of expert reviews (21). Risks are summarized in Table 7.14.
Epidural hematoma frequently presents as persistent back pain with
patchy analgesia or motor weakness. Rapid diagnosis by magnetic
resonance imaging (MRI) and neurosurgical decompression is the only
hope for reversal of neurologic deficits. The diagnosis may be delayed
by the use of high concentrations of local anesthetic, intravenous
sedation or analgesia, or the inability to communicate with the patient.
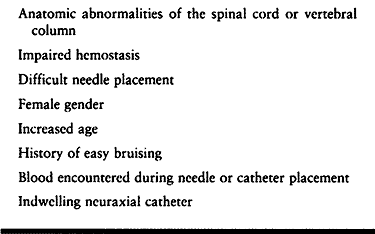 |
|
Table 7.14. Risk Factors for Epidural Hematoma
|
less dramatic in presentation than epidural hematoma. This can cause
delayed diagnosis. Signs of infection may not occur until later in the
course of the hospital stay or might not be evident until several days
after the catheter is removed. Thirteen cases of epidural abscess have
been reported in the literature. The estimated incidence is estimated
to be 1:13,000 patients. Daily examination and monitoring of the
catheter site for inflammation and suppuration is considered to be
essential, as are noting unexpected motor or sensory deficits. Clinical
factors that may interfere with the diagnosis include immobilization
that inhibits access to the patient’s back for examination of the
epidural site, use of local anesthetic concentrations that are high
enough to produce sensory and motor block, and the presence of wound
infection. Clinical signs that should raise suspicion of epidural
abscess include prolonged use of the epidural catheter, incomplete
analgesia, and new neurodeficits. If neural compression is not present,
then antibiotic therapy with or without percutaneous catheter drainage
may be successful. Otherwise, surgical drainage is necessary. The
risk–benefit ratio of epidural analgesia must be carefully considered
in patients who may be difficult to monitor postoperatively.
postoperative analgesia or for treatment of reflex sympathetic
dystrophy. Brachial plexus block by axillary or supraclavicular
approaches can be used for prolonged infusion, but it is difficult to
maintain a catheter in position. The interscalene approach is
associated with a high incidence of phrenic nerve paralysis. For
long-term infusions, the subclavian approach to the brachial plexus
provides a site that is easier to maintain in terms of catheter
stability and dressings. The long-acting local anesthetic bupivacaine,
in 0.125% to 0.25% solution, can be infused up to 5 ml/hour. A maximum
of 400 mg/24 hours is recommended. The primary hazard is accumulation
of drug to toxicity levels. Because bupivacaine is eliminated by the
liver, dose reduction or alternatives should be considered when hepatic
function is impaired.
diagnosis frequently consists of syndrome classification, and therapy
is largely empiric. Many problems can be handled in so-called block
clinics, but intractable problems may need the combined expertise and
resources of a multidisciplinary pain clinic. Psychological issues
assume a larger role for the patient who has to contend with chronic
pain.
multidisciplinary. Anesthesiology training provides skill in the safe
application of drugs by intravenous and perineural injection. The
following sections discuss the techniques that are used in our pain
management clinic.
avoidance of further injury and provides information about the injury.
When the source of pain has been identified, the benefits of pain may
no longer outweigh the cost in terms of suffering, reduced function,
and stress. Research into pain mechanisms has led to advances in the
management of postoperative and posttraumatic pain. Spinal cord opiate
receptors demonstrate the efficacy of intrathecal and epidural opiate
injections. When occupied by an agonist drug, opiate receptors on
primary afferent neurons inhibit the release of substance P. The spinal
gate control hypothesis explains the efficacy of transcutaneous
electrical nerve stimulation (TENS) and dorsal column stimulation (29).
Research has given objective support to the well-established clinical
observation that pain tolerance and experience are highly variable
between individuals as well as for the same individual. This
variability is
the rationale behind PCA devices, which allow the patient to self-administer analgesics within prescribed limits (17).
At present, clinical measurement of pain is based on patient responses,
such as the visual analog pain scale or the amount of drug delivered by
a PCA device. An objective measure is needed.
sympathetic-maintained pain (reflex sympathetic dystrophy and
causalgia) and phantom limb pain. The classic picture of
sympathetic-maintained pain in the acute phase is that of a closely
guarded, hyperesthetic, edematous, painful limb that is relieved by
sympathetic block. Pain and joint stiffness are consistent features of
the subacute and chronic stages, but the appearance of the affected
limb becomes one of disuse atrophy. When associated with a partial
nerve injury, this syndrome is referred to as causalgia. A series of
sympathetic ganglion blocks (cervicothoracic or stellate for the arm,
lumbar sympathetic at L-2 for the leg) frequently results in resolution
of the pain over time. Alternatively, intravenous regional bretylium
and TENS also provide relief. Physical therapy is an important
component of treatment.
encountered. The easiest to manage is stump pain due to local causes
such as scar, ischemia, infection, bony irregularities, and neuroma.
Treatment is focused on the cause. Phantom limb pain is notoriously
refractory to treatment. Unlike the nondebilitating phantom sensations
that amputees experience, phantom pain is severe and occurs in 5% to
10% of amputees. The pain is typically described as burning, cramping,
or crushing. Numerous therapies are available, including antidepressant
and anticonvulsant drugs, TENS, nerve blocks, and surgery, but no one
therapy is uniformly successful (43). When
amputation is preceded by pain, preoperative regional anesthesia that
is continued into the postoperative period may reduce the likelihood of
phantom pain.
relief of muscle pain and spasm by trigger point injections is
empirically based. The mechanism of pain and its relief are still
unsettled. Diagrams of trigger point locations and the pattern of
radiating pain to assist in the diagnosis and treatment can be found in
Travell and Simons’ text (44). Local anesthetic
injection is frequently used, but steroids, saline, and NSAIDs have
also been injected with reported relief. Local therapy directed at the
muscle is recommended after relieving the trigger point pain. The risks
are local bleeding, infection, trauma to nerves, and in the chest wall,
pneumothorax.
(RSD) and causalgia as complex regional pain syndrome (CRPS) type I and
type II, respectively. CRPS I is an infrequent sequela of skeletal and
soft-tissue injury, usually to an extremity. CRPS II is a complication
of partial injury to a mixed peripheral nerve. They are both under the
broader classification of neuropathic pain. Sympathetic blocks are
usually performed for the diagnosis or treatment of complex regional
pain syndrome. Effectiveness of the block is most easily documented by
the use of skin temperature liquid crystal strips. Skin temperature can
rise to 33° to 34°C. The duration of the block depends on the
pharmacokinetics of the particular local anesthetic, but the duration
of analgesia may last longer. Occasionally, patients experience
progressively longer periods of relief after repeated block. When
relief occurs, physical therapy should immediately follow the onset of
block. Other therapies for CRPS I include medications, such as
gabapentin, clonidine and terazocin, biofeedback, and spinal cord
stimulation.
anesthetic block of the stellate ganglion (inferior cervical ganglion
plus T-1 sympathetic ganglion). An anterior approach is commonly used.
Take care to avoid injection into the carotid artery, the vertebral
artery, or the dural sleeve. Other nerves that can be blocked are the
recurrent laryngeal, the phrenic, and branches of the brachial plexus.
anesthetic block of the lumbar sympathetic chain (L-1 through L-3). A
posterior approach is commonly used and is efficiently executed using
fluoroscopic guidance. A large volume of anesthetic is injected to
achieve cephalad and caudad spread of the solution along the
sympathetic chain. Take care to avoid posterior spread with resultant
nerve root block and intravascular injection into the inferior vena
cava (IVC) or aorta.
also be produced by placement of a brachial plexus catheter. The
subclavian approach is used because the catheter is less likely to be
dislodged. Perform this procedure with sterile conditions and
dressings, followed by frequent examination for possible infection of
the catheter skin site. The catheter can be used for continuous or
repeated local anesthetic injections in conjunction with physical
therapy.
drugs can provide long-lasting sympathectomy and relief of pain from
CRPS I. The procedure is similar to surgical anesthesia with the Bier
block technique. An intravenous catheter is placed in the distal part
of the extremity. This procedure may be difficult when venoconstriction
is part of the clinical presentation. Mechanical venous distention
can
be produced by inflating the double pneumatic tourniquet above venous
pressure range and then wrapping an Esmarch bandage in reverse
direction from proximal to distal to squeeze blood into the distal
portion of the venous system. If this procedure fails to produce
satisfactory venous dilation, a regional anesthetic block may have to
be performed, for example, a brachial plexus block or epidural block. A
regional block may also be used when a patient is unable to tolerate
the discomfort of tourniquet ischemia. Various sympatholytic drugs have
been injected (guanethidine, reserpine, and bretylium). At present,
only bretylium is available in the United States. Clinical trials with
guanethidine have been conducted to assess the efficacy of intravenous
regional sympathectomy in CRPS I. The results suggest that, despite the
clinical improvement that is observed in some patients under controlled
conditions, the efficacy is small.
block is too brief, prolonged sympathectomy is usually attempted using
radiofrequency or surgical ablation. Although the initial response may
be gratifying relief, frequently the pain returns. This is attributed
to incomplete ablation, but it may actually be a deafferentation
phenomenon. A more promising technique for lasting analgesia may be
achieved with spinal cord stimulation. The results of this relatively
recent therapy are still being evaluated in clinical trials. Individual
patients have experienced significant long-term relief.
relieving pain in 60% of patients at three months followup who have
acute lumbar radiculopathy (11a). With chronic
lumbar radiculopathy, there is less likelihood of benefit from epidural
steroids. In the lumbar region, 80 mg of methylprednisolone diluted in
normal saline are injected epidurally (8a,11a,41a). Watts and Silagy (52a)
in a meta-analysis of the literature concluded that epidural steroids,
up to 60 days followup, increased the odds ratio of pain relief (75%
improvement) to 2.61 (95% confidence interval: 1.9–3.8) when compared
with placebo. Although risks are minimal, they include adrenal
suppression, infection, and epidural abscess.
management of chronic cervical and lumbar spine pain and
radiculopathies. Peridural (epidural) injection, selective nerve
injection, sacroiliac joint injection, and facet injection of steroid
are offered empirically as pain reduction therapies. The specific
corticosteroid agents and dose, the number and interval between
injections, and the adjunctive therapies vary widely. Therapeutic
efficacy has not been established in a way that can be easily applied
to individual patients. Methylprednisolone (Depo-Medrol) and
triamcinolone (Aristocort) are commonly used. Our clinic uses
triamcinolone. The amount that causes significant side effects is
unknown and possibly varies from one patient to the next.
steroid technique for lumbar pain. The indications vary from very
conservative recommendations to an almost universal trial in patients
with back pain lasting over a few weeks. The recent onset of symptoms
or a recent exacerbation seem to be favorable prognostic signs for
relief by epidural steroids. The procedure is similar to that used for
epidural anesthesia. A sitting position is used to simplify the
location of midline, particularly in large patients. The sitting
position, however, tends to decrease the interspinous space, making
needle insertion more difficult. This can be overcome by using the
paramedian approach. There is concern that what is perceived as an
epidural injection is actually not in the epidural space. One often
quoted result is that there is a 30% failure rate without resorting to
fluoroscopy. Loss of resistance to air or saline injection can be
misleading if the needle leaves the interspinous ligament and enters
the paraspinous area. Quantification of the pressure required to infuse
saline through the needle and injection of local anesthetic to produce
a transient block have been recommended. Epidural needle placement
under fluoroscopy with contrast injection is considered by many to be
the gold standard and has been recommended when the patient’s size or
spine presents an anatomic challenge or when an injection without
fluoroscopy guidance has not resulted in relief. Some advocate
performing the initial steroid injection with fluoroscopy guidance in
all patients.
suspected of causing radicular pain, selective nerve root injections
are performed for both therapeutic and diagnostic reasons. This
procedure is done with fluoroscopy. For lumbar nerve root injections,
the patient is placed in a prone position with a pillow under the hips.
The image of the lumbar pedicles is a landmark for directing the needle
to the foramen beneath it. Outline the nerve root by injection of a
small amount of contrast. To avoid epidural block, inject only small
volumes of local anesthetic with steroid.
lumbar pain. Patrick’s test, pain on crossed-leg external rotation at
the hip joint, is one of the tests used to identify sacroiliac joint
pain. Injection can be performed with or without fluoroscopy.
joints is difficult to localize by examination. Imaging studies are not
reliable in identifying pain-producing pathology of the facet joints.
Frequently, a therapeutic trial injection of local anesthetic and
corticosteroid of the joint
capsule
or the medial branch nerve at various levels is undertaken, in search
of a pain-relieving procedure. When reproducible relief is achieved by
either intracapsular or medial branch nerve block, the patient becomes
a candidate for radiofrequency coagulation of the medial branch nerve.
Fluoroscopy is essential for both the medial branch and the
intracapsular injections. With the patient in prone position and with
the fluoroscopy tube positioned to identify the joint space, direct the
needle either toward the joint space or toward the medial, upper edge
of the transverse process. Facet block is a relatively low-risk
procedure. Excessive volume injection into the capsule can cause
capsular rupture.
entrapment, are also treated as neuropathetic pain syndromes. Before
spinal cord stimulation, neurolytic block with phenol, freezing
(cryoanalgesa), and radiofrequency coagulation neurolysis are usually
attempted. Neurolytic blocks are usually temporary, lasting from a few
weeks to months. Dysesthesias and pain are sometimes produced by
neuritis or possibly by the additional deafferentation. Because
precision of needle placement is essential, nerve stimulation and
fluoroscopy with contrast are usually employed.
administration. Long-term exposure to opioids can result in significant
tolerance and corresponding large doses. Drugs can be administered
continuously for spinal analgesia by means of a chronically implanted
catheter and pump. This method for opioid administration has been used
with success in the treatment of postlaminectomy pain syndrome. Not
every chronic pain patient is a suitable candidate for an intrathecal
pump. The patient’s pain must be suppressed by opioids, and the patient
must demonstrate psychological stability.
of deafferentation pain, a subset of neuropathetic pain syndromes that
do not respond to sympathetic or additional neuroablative procedures.
The primary therapies are medications, such as anticonvulsants,
antidepressants, opioids, and neurostimulation.
thromboembolism. When pulmonary embolism occurs intraoperatively, the
combined effects of embolism, anesthesia, and blood loss may be
catastrophic. Impaired cardiac output can lead to severe, refractory
hypotension and tissue ischemia. Occlusion of pulmonary blood flow can
lead to carbon dioxide retention and tissue acidosis. Embolism has
recently been detected using echocardiography following the release of
a thigh tourniquet during total knee arthroplasty. Sudden hypotension
at any time during a procedure presents a diagnostic problem, and one
of the considerations is the possibility of major pulmonary embolus.
The presence of a large increase in arterial-to-end tidal carbon
dioxide difference, elevated venous pressure, or evidence of decreased
peripheral perfusion, such as failing pulse oximeter, point to the
possibility of pulmonary embolism.
Orthopaedic patient populations who have frequent exposure to latex
products include patients with spina bifida, congenital urologic
anomalies, and spinal cord injuries. Approximately 40% of children with
myelomeningocele test positive for latex allergies. Because of this
high prevalence, it has become routine to treat patients in these
categories with latex allergy precautions. Latex-containing items used
in anesthesia include tourniquets for starting intravenous injections,
gloves, breathing circuit reservoir bags, intravenous infusion
injection ports, and stoppers on medication vials.
reported. Successful management depends on prompt recognition and
treatment. Anaphylactic and anaphylactoid reactions occur unexpectedly
and to a variety of foreign substances besides latex. The combination
of sudden, profound hypotension, tachycardia, cutaneous flush, and
bronchoconstriction is easily recognized. A delayed diagnosis may
result if significant hemorrhage is occurring and the cutaneous
flushing is not apparent. Treatment for anaphylaxis consists of 1 mg of
intravenous epinephrine, rapid infusion of crystalloid solution,
oxygen, and a bronchodilator if bronchoconstriction is evident (23a). For hypotension, 5 to 10 mcg of intravenous epinephrine is recommended (23a).
Further exposure to the antigen should be avoided. Corticosteroids and
antihistamine may be helpful in controlling symptoms. Repeat doses of
epinephrine may be needed after the initial resuscitation. (See Table 7.15.)
 |
|
Table 7.15. Treatment for Anaphylaxis
|
to potent inhalation anesthetics and succinylcholine. The incidence is
approximately 1:15,000 (pediatric) to 1:50,000 (adults). A higher
incidence of MH has been associated with some of the myopathies, such
as King-Denborough syndrome and central core disease. The abnormal
response of susceptible patients to inhalation anesthetics can be
demonstrated in muscle biopsy specimens in the presence of caffeine
stimulation. When a patient has been identified as MH susceptible by
history or muscle biopsy, the anesthetic agents must be restricted to
nontriggering drugs. An episode of MH is potentially fatal. Dantrolene
is an effective treatment. At least 36 vials of dantrolene should be
available in any location where general anesthesia is given.
fever, are easily recognized. Dilemmas arise because none of the
clinical signs is specific. The intraoperative diagnosis of MH is also
difficult when the onset is gradual. The possibility of MH is easier to
recognize when muscle rigidity, tachycardia, hypercapnia, and rapidly
increasing temperature follow shortly after exposure to the anesthetic.
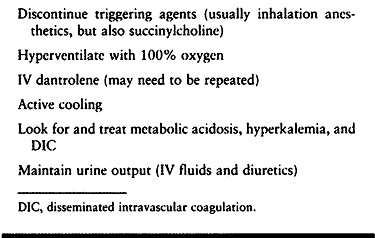
Management of MH is summarized in Table 7.16.
After a fulminant MH episode is controlled, the patient should be
observed in an intensive care unit for at least 24 hours because
recurrence is possible. Controversy exists among anesthesiologists as
to whether to proceed with elective surgery when succinylcholine causes
masseter spasm, an event that is sometimes followed by an MH episode.
Consultation regarding the preoperative preparation or crisis
management of MH is available through the Malignant Hyperthermia
Association of the United States (MHAUS) hotline number,
1-800-MH-HYPER. Additional information is available on the MHAUS
website, <www.mhaus.org>.
 |
|
Table 7.16. Management of Malignant Hyperthermia
|
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
College of Physicians. Guidelines for Assessing and Managing the
Perioperative Risk from Coronary Artery Disease Associated with Major
Noncardiac Surgery. Ann Intern Med 1997;127:313.
G, Desmonts JM, Couderc E, et al. Comparative Effects of Induced
Hypotension and Normovolaemic Haemodilution on Blood Loss in Total Hip
Arthroplasty. Br J Anaesth 1980;52:1039.
CM, Artru AA: Hexamethonium and Midazolam Terminate Dysrhythmias and
Hypertension Caused by Intracerebroventricular Bupivacaine in Rabbits. Anesthesiology 1991;74:89.
WR, Sacks GM, Schools AG, et al. Nearly Fatal Cardiovascular Collapse
During Total Hip Replacement: Probably Coronary Arterial Embolism. Anesth Analg 1991;72:245.
CW, Hondeghem LM. Mechanism for Bupivacaine Depression of Cardiac
Conduction, Fast Block of Sodium Channels during the Action Potential
with Slow Recovery from Block during Diastole. Anesthesiology 1985;62:396.
FM, Quince M, Laurenson VG. Deep Vein Thrombosis and Anaesthetic
Technique in Emergency Hip Surgery. Br Med J 1980;281:1528.
KA, Brundage BH, Chaitman BR, et al. Guidelines for Perioperative
Cardiovascular Evaluation for Noncardiac Surgery. Report of the
American College of Cardiology/American Heart Association Task Force on
Practice Guidelines. Committee on Perioperative Cardiovascular
Evaluation for Noncardiac Surgery. Circulation 1996;93:1278.
G, Schott U, Axelsson K, Carlberg M. Perioperative Autotransfusion and
Functional Coagulation Analysis in Total Hip Replacement. Acta Anaesthesiol Scand 1995;39:390.
A, Hernandez J, Benavides O, et al. Quality of Spinal Extradural
Anesthesia: The Influence of Spinal Nerve Root Diameter. Br J Anaesth 1975;47:41.
for Assessing and Managing the Perioperative Risk from Coronary Artery
Disease Associated with Major Noncardiac Surgery. American College of
Physicians. Ann Internal Med 1997;127:309.
J, Pitkanen MT, Kytta J, et al. Treatment of Bupivacaine-induced
Cardiac Arrhythmias in Hypoxic and Hypercarbic Pigs with Amiodarone or
Bretylium. Reg Anesth 1990;15:174.
J, Schonleben K, Spiegel H, et al. Nitroprusside- and
Nitroglycerin-induced Hypotension: Effects on Hemodynamics and on the
Microcirculation. World J Surg 1982;6:241.
P, Heal JM, Blumberg N. Infection of Suspected Infection after Hip
Replacement Surgery with Autologous or Homologous Blood Transfusions. Transfusion 1991;31:212.
Guidelines for Blood Component Therapy. A Report by the American
Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 1996;84:732.
RA, Sherman CJ. Prevalence and Characteristics of Chronic Phantom Limb
Pain among American Veterans—Results of Trial Survey. Am J Phys Med 1983;62:227.
GE, Miller RD, Stevens WC, Murray WR. Hypotensive Anesthesia for Total
Hip Arthroplasty: A Study of Blood Loss and Organ Function (Brain,
Heart, Liver, and Kidney). Anesthesiology 1978;48:91.
J, Louden JR, Vallance R. Spinal and General Anesthesia in Total Hip
Replacement: Frequency of Deep Vein Thrombosis. Br J Anaesth
1980;52:1117.
DJ, Vanek K, Ryan DH, et al: A Clinical and Immunologic Study of Blood
Transfusion and Postoperative Bacterial Infection in Spinal Surgery. Transfusion 1992;32:517.
CJ, Van Houten RJ, Hill RC. A Statistical Analysis of the Relationship
of Physical Status to Postoperative Mortality in 68,388 Cases. Anesth Analg 1970;49:564.
