Giant Cell Tumor of Bone
II – Specific Bone Neoplasms and Simulators > 5 – Benign Bone Tumors
> 5.6 – Giant Cell Tumor of Bone
aggressive tumor. It remains one of the most challenging of the benign
bone tumors to treat. It is also unique from all other benign bone
tumors except chondroblastoma in that it can metastasize. Metastases
from GCT of bone almost always arise in the lung and rarely are fatal
or cause significant morbidity at the site of metastasis.
-
Etiology is unclear.
-
5% to 10% of all bone tumors
-
Female:male 1.3 to 1.5:1
-
Age distribution
-
Rare prior to skeletal maturity
-
Most (~75%) occur between 18 and 40 years.
-
-
Skeletal distribution
-
Nearly always involve epiphysis, but epicenter is in metaphysis
-
Rare pediatric giant cell tumors are metaphyseal but may erode across physis into epiphysis.
-
-
Can occur in nearly any bone in the skeleton
-
Most common sites of occurrence (half of all lesions occur about the knee)
-
Distal femur
-
Proximal tibia
-
Distal radius
-
Sacrum (anterior body)
-
Proximal humerus
-
Proximal femur
-
Distal tibia
-
-
Rare in small bones of hands and feet
-
immunohistochemical studies have suggested that the cells are of a
histiocytic nature. More recently, the stromal cells have been
suggested to be of osteoblastic lineage based on their expression of
alkaline phosphatase, osteocalcin, and Cbfa1.
The
stromal cells are thought to produce RANKL, which leads to
osteoclastogenesis, which in turn leads to osteolytic destruction of
bone (Fig. 5.6-1).
GCT of bone was at one time described as osteoclastoma. The destructive
nature of GCT, in combination with its typical location in the
epiphysis, explains the clinical course. The articular cartilage is
initially spared. The lesion spreads across the epiphysis and
eventually destroys the cortex and can form a soft tissue mass. Without
the structural support of the underlying subchondral bone, pathologic
fractures through the articular surface are not uncommon. Unchecked,
this leads to severe secondary degenerative changes.
-
Dense cellular pattern of monocytes admixed with numerous giant cells
-
Nuclei of the monocytes appear identical to the nuclei in the giant cells.
-
Following Enneking’s classification of benign tumors, GCT of bone is most commonly active or aggressive.
-
Latent: rare in GCT
-
Active: any symptomatic or growing lesion
-
Aggressive (Fig. 5.6-2)
-
GCT is probably the most aggressive of the benign tumors.
-
Radiographically lesions can be extremely destructive of bone, resembling osteosarcoma.
-
Along with aneurysmal bone cyst and chondroblastoma, GCT is one of the three classically aggressive benign bone tumors.
-
-
-
GCT defies the classic definition of benign tumors in that it can metastasize, although it does so rarely.
-
One of only two benign bone tumors that metastasize: GCT and chondroblastoma
-
 |
|
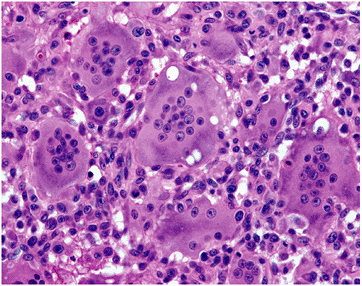
Figure 5.6-1
Photomicrograph of GCT. The nuclei of the giant cells and the monocytes appear similar, leading some to theorize that the giant cells occur from fusion of the monocytes. Currently this is not thought to be accurate. |
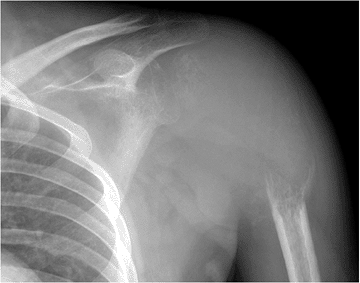 |
|
Figure 5.6-2 GCT in a 32-year-old man. The proximal humerus has been nearly completely destroyed by the lesion.
|
-
The radiographic findings associated with GCT are often strongly suggestive of the diagnosis.
-
Other benign lesions in the differential
would include chondroblastoma and aneurysmal bone cyst (ABC), although
chondroblastomas are more common in the skeletally immature patient and
are usually isolated to the epiphysis, and an epiphyseal location would
be unusual for the ABC. -
Aggressive GCT can be mistaken for any of
the primary or secondary bone malignancies that may extend into the
epiphysis (telangiectatic or fibroblastic osteosarcoma, Ewing sarcoma,
or metastatic disease). -
Clear cell chondrosarcoma is the one
malignancy that classically occurs in an epiphyseal location and should
be considered in the differential diagnosis.
-
Generally relate to the extent of the joint involvement
-
Patients initially present with dull achy pain of the involved joint.
-
Extension of the lesion into the soft
tissues and physical examination consistent with a soft tissue mass are
relatively late findings. -
Extension of the lesion into the joint is unusual and is associated with complaints similar to osteoarthritis.
-
Epiphyseal location: lesion is centered
in the metaphysis of long bones but in skeletally mature patients
uniformly involves the epiphysis and frequently extends to the
subchondral bone -
Radiolucent: GCT is uniformly destructive of bone without matrix mineralization
-
Eccentric: initially GCT is eccentric but spreads across the epiphysis with progression
-
Extension to articular cartilage: extends to the subchondral bone, but initially preserves the joint
-
Confined to bone: late spread breaks through the cortex
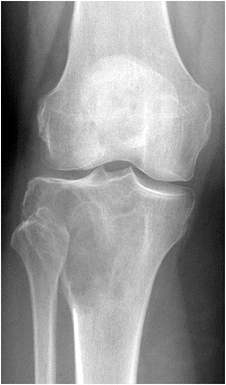 |
|
Figure 5.6-3
GCT of the proximal tibia. The lesion involves the epiphysis, is eccentric in location and destructive of bone, and extends to the articular cartilage. |
-
Orthogonal radiographs
-
Magnetic resonance imaging (MRI) to assess local extent
-
Chest radiograph and/or computed
tomography (CT) scan of the chest as baseline for subsequent comparison
in search of pulmonary metastases -
Bone scan to assess for multicentricity
-
Biopsy to confirm diagnosis
performed as a staging study in all patients to evaluate for
multicentricity. GCT has the potential to metastasize. Initial and
follow-up evaluation should include chest radiographs or a CT scan of
the chest.
aggressive. Because the differential diagnosis would include malignant
lesions, including osteosarcoma, biopsy is usually required for
diagnosis. Rarely does the lesion warrant nonoperative treatment. GCT
is typically progressive and juxta-articular in location in young
adults. Progression into the joint can cause irreversible damage to the
joint necessitating joint replacement in a young patient. Occasionally,
GCT of the axial skeleton is treated conservatively, either with
radiation or observation.
-
Central inoperable lesions of the axial skeleton
-
Lesions that would cause significant morbidity to operate on (i.e., sacrum or spine)
-
Rarely indicated, except perhaps in the rare patient with an asymptomatic (latent) lesion
-
Simple curettage: high recurrence rate
-
Extended intralesional curettage (with
power bur and other adjuncts such as phenol, laser coagulation, or
liquid nitrogen): lower, more acceptable recurrence rate -
Primary excision
-
Negligible recurrence rate
-
Overtreatment for most GCTs
-
May be indicated in expendable bones (e.g., fibula)
-
-
Consider surgical adjuvants to extend margin of curettage
-
Argon beam laser
-
Phenol
-
Liquid nitrogen
-
-
Need to fill defect from curettage
-
Cement (Fig. 5.6-4)
-
Advantages
-
Immediate stability to allow weight bearing
-
Exothermic reaction during curing theoretically may provide additional antitumor effect.
-
Provides discrete radiological margin with bone that improves ability to discern local recurrence
-
-
Disadvantages
-
Nonbiological reconstruction
-
Cement against subchondral bone or cartilage alters biomechanics and may lead to premature degenerative arthritis.
-
-
-
Bone graft
-
Advantages
-
Biological reconstruction
-
-
Disadvantages
-
More difficult to distinguish local recurrence from resorption of bone graft
-
Large defects more often require prophylactic fixation and prolonged restricted weight bearing during graft incorporation.
-
-
-
Possible prophylactic fixation, depending on the extent of the lesionP.162
-
Advisable for most large defects in long bones if bone graft used
-
Advisable for poorly contained defects in long bones if cement used
-
Threaded Steinman pins within intramedullary canal and cement
-
Large screws within intramedullary canal and cement
-
-
-
-
Joint-sacrificing procedures (en bloc excision) will require implants and instrumentation for reconstruction (Fig. 5.6-5).
 |
|
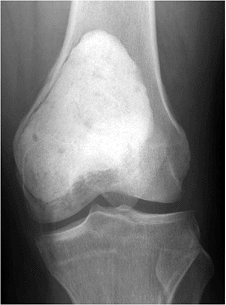
Figure 5.6-4
Postoperative radiograph of GCT in the distal femur. The lesion has been extensively curetted and the defect filled with cement. The subchondral space was packed with bone graft. |
 |
|
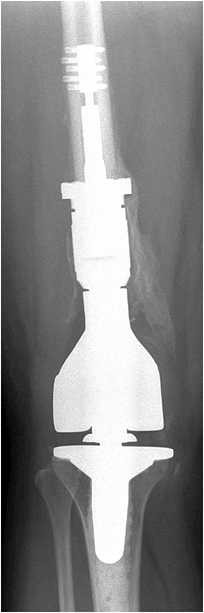
Figure 5.6-5
Postoperative radiograph of the patient with GCT of the distal femur that had progressed to destroy the joint surface. This patient underwent resection of the distal femur and reconstruction with a megaprosthesis. |
-
Elimination of pain
-
Preservation of joint (if possible)
-
Restoration of function
-
Low recurrence rate
-
Initially, GCT was treated with simple curettage.
-
Unacceptably high recurrence rates led to more aggressive treatment (i.e., wide excision).
-
Wide excision of periarticular lesions requires sacrificing the joint.
-
This returned the patient’s function to
acceptable levels, but long-term results suffered in young patients
undergoing reconstruction with megaprostheses.
-
-
More recently GCT has been treated by a technique known as extended curettage.
-
The lesion is approached through a longitudinal incision.
-
The overlying cortex (intact or not) is exposed.
-
A wide cortical window is made in the bone.
-
It is important that the window be
sufficiently large to allow adequate visualization of the entire
lesion; attempting to work around a corner or blindly curettage the
lesion results in an unacceptable recurrence rate.-
Windowing large enough to see all aspects of the defect is referred to as “exteriorization” of the defect.
-
-
The lesion is then curetted to normal cortical or
P.163
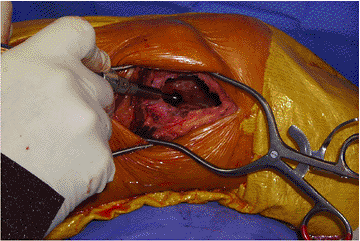
cancellous bone. A high-speed bur is then used to extend the margin circumferentially around the entire lesion (Fig. 5.6-6).![]() Figure 5.6-6
Figure 5.6-6
Intraoperative photograph of extended curettage of a GCT of bone. A bur
can be seen within the lesion through the cortical window in the distal
femur. -
A water-pick is then used to irrigate and again visualize the lesion. The previous steps are repeated as necessary.
-
An adjuvant, such as argon beam laser, phenol, or liquid nitrogen, is used to again extend the margin of the resection.
-
The bony defect is then bone-grafted or cemented according to surgeon preference.
-
Cementation is most commonly used as it allows extensive curettage without concern for structural weakness.
-
-
-
Oncologic complications
-
Recurrence: rate depends on technique used, as discussed in “Results and Outcome” section
-
Malignant degeneration: extremely rare, almost always related to treatment (secondary to radiation therapy)
-
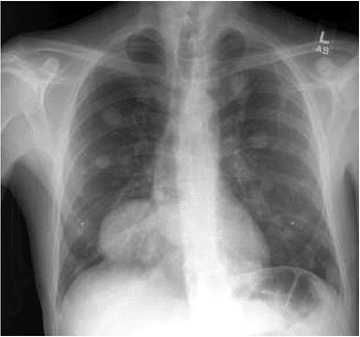
Metastasis: rare, perhaps 3% (Fig. 5.6-7)
-
Most behave in a benign fashion.
-
Treatment options
-
Nonprogressive: observation
-
Progressive and limited: surgical resection
-
Progressive and extensive: chemotherapy
-
-
-
-
Surgical complications
-
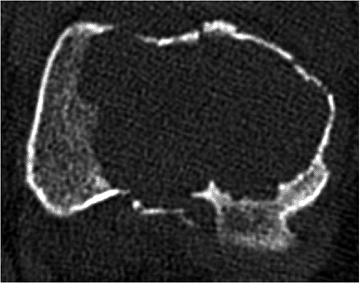
Pathologic fracture (Fig. 5.6-8)
-
Degenerative arthritis of adjacent joint
-
-
Simple curettage
-
Most studies have shown approximately 30% to 47% recurrence rate with simple curettage.
-
-
Extended curettage
-
Approximately 0% to 25% recurrence rate with curettage, bur, and an adjuvant
-
-
En bloc excision
-
Essentially no recurrence with primary excision
-
Significant morbidity from reconstructive procedures
-
 |
|
Figure 5.6-7 Chest radiograph of a patient with metastatic GCT of bone; note the multiple pulmonary lesions.
|
-
Feigenberg et al (2003) reported a
recurrence rate of 23% with various combinations of surgery and
radiation (many of which were in the pelvis, sacrum, or spine). One
patient developed a radiation-induced sarcoma 22 years later.
Patients undergoing joint-replacement surgery will require extensive
postoperative rehabilitation. Patients undergoing extensive curettage
may require protected weight bearing if bone graft was used to fill the
defect. Because of the low risk of metastases, these patients should be
followed with periodic chest x-rays or chest CT scans. GCT has also
been known to recur 20 years or more after the primary tumor, so these
patients should be followed or at least made aware of the risk of late
recurrence.
 |
|
Figure 5.6-8 Axial-cut CT scan of the distal femur in a patient with GCT. A pathologic fracture in the sagittal plane is apparent.
|
R, Fabbri N, Bettelli G. Curettage of giant cell tumor of bone. The
effect of surgical technique and adjuvants on local recurrence rate. Chir Organi Mov 1990;75(1 Suppl):206.
R, Sudanese A, Baldini N, et al. Phenol as an adjuvant in the control
of local recurrence of benign neoplasms of bone treated by curettage. Ital J Orthop Traumatol 1985;11(3):381–388.
SE, Lorentzon R, Boquist L. Giant-cell tumor of bone. A demographic,
clinical, and histopathological study of all cases recorded in the
Swedish Cancer Registry for the years 1958 through 1968. J Bone Joint Surg [Am] 1975;57(2):167–173.
RJ, Springfield DS, Motwani HK, et al. Recurrence of giant-cell tumors
of the long bones after curettage and packing with cement. J Bone Joint Surg [Am] 1994;76(12):1827–1833.