Complications After Total Knee Arthroplasty
II – Knee > Part C – Operative Treatment Methods > 26 –
Complications After Total Knee Arthroplasty
arthroplasties are performed each year. Total knee arthroplasty (TKA)
is one of the most successful procedures in orthopaedic surgery, and
there are excellent reported long-term results with survivorship rates
of >90% at 15 years.1,2,3
In recent years surgical techniques have been changed and new
technologies have been introduced for TKA. Patients are now more
informed and are requesting newer technologies, better implants, less
pain, less blood loss, and quicker recovery from a joint replacement.
The introduction of minimally invasive techniques, preemptive
analgesia, progressive rehabilitation, computer-assisted surgery, and
new materials not only have changed the daily practice for orthopaedic
surgeons but also have added new challenges. Beyond the scope of these
advances, surgeons must keep in mind that TKA is a major surgery with
associated morbidity. This chapter will focus on the postoperative
complications including traumatic periprosthetic injuries, the pitfalls
of minimally invasive surgery, wound healing problems, nerve injury,
and postoperative infection. Also, issues associated with stiffness,
tissue balancing, and instability will be addressed.
arthroplasty during the first 90 days is 0.2% to 0.7%. Increased risk
is associated with advanced age, comorbidities, and revision
procedures. In a study of >3,000 consecutive TKAs performed by one
surgeon, the overall mortality rate was 0.46% during the first 90 days
in patients with an average age of 70 years.4 Gill et al.4
reported a risk of mortality 16 times higher in patients with cardiac
comorbidities such as previous myocardial infarction, ischemic heart
disease, and cardiac failure compared with those with no comorbidity.
Patients older than 85 years of age had a 14-times increase in the
chance of death when compared with patients younger than 85 years of
age, with a reported rate of 4.65%. The mortality rate in the Medicare
population undergoing primary TKA is reported as 0.6% to 0.7% during
the first 90 days in two studies.5,6
The overall morbidity rates in >80,000 patients during the first 90
days after primary TKA identified in a Medicare population were the
following: acute myocardial infarction, 0.8%; pulmonary embolism, 0.8%;
pneumonia requiring hospitalization, 1.4%; and infection requiring
irrigation and debridement, 0.4%.5,6
has become a topic of increasing interest. Two recent studies have
reported lower mortality and morbidity rates associated with surgeons
and hospitals performing a larger volume of TKAs. Katz et al.5
identified a 30% reduction in mortality rate for patients receiving a
TKA in hospitals that perform >25 of these procedures per year.
Surgeons performing >50 procedures per year had a 40% lower risk for
deep wound infection compared with surgeons performing <12 per year.
A steady decline of deep infection was independently related to
hospital volume as well, with a reported 40% reduction for hospitals
performing >200 cases per year versus those doing <25 per year.
The risk of pneumonia also diminished independently for surgeons and
hospitals performing >12 and 25 cases, respectively.
are rare after total knee replacement. The reported prevalence for
distal femoral fractures ranges from 0.3% to 2.8%7 and for tibial fractures from 0.4% to 1.7%.8 Patellar fractures occurred in 0.05% when unresurfaced9 and ≤21% with resurfacing.10
traumatic, with a higher incidence seen in patients with osteopenia.
Femoral notching may increase the incidence of periprosthetic fractures
of the distal femur when both medial and lateral cortices are notched.
Nondisplaced fractures with well-fixed implants can be treated by
nonoperative intervention with a high success rate. Surgical
intervention is required in the setting of displaced supracondylar
fractures, and the method of fixation is determined by implant
stability. Supracondylar fractures associated with well-fixed femoral
components can be treated by several techniques including retrograde
nails, blade plates, condylar screw plates, condylar buttress plates,
and locked condylar plates.
condylar locking plates have become standard treatment methods owing to
the preservation of fracture hematoma and minimal soft tissue
dissection. Whether the fracture is suitable for a retrograde nail is
determined by the length of the distal bone fragment from the fracture
to the intercondylar notch. Adequate bone length of the distal fragment
is needed for placement of the two distal locking screws. If the most
distal aspect of the nail protrudes into the notch, some surgeons have
successfully removed this portion after inserting the interlocking
screws.11 The design of the femoral
component must also be considered because posterior stabilized systems
may preclude insertion of the retrograde nails through a solid cam and
post mechanism. Locked plates inserted with minimal soft tissue
disruption offer many advantages over retrograde nailing, including
rigid fixation with locked screws, ability to combine with posterior
stabilized systems, and potentially better fixation in osteopenic
patients.12
supracondylar fractures require a different treatment approach. In
certain cases, the periprosthetic fracture can be addressed first and
allowed to heal prior to revision of the loose femoral implant.
Postponing component revision until fracture healing offers several
advantages including less bone loss, ease of revision TKA, reduced need
for cortical strut allograft, and less need for augments, wedges,
stems, and constrained or hinged prostheses.8
Combined fixation of the fracture with revision knee arthroplasty is a
technically demanding procedure that may require extensive allografts
and a hinged prosthesis or oncologic distal femoral replacement
prosthesis. Principles include restoration of the joint line,
preservation of fixed components, and proper femoral rotation based on
a rectangular flexion gap with the tibial component. Bulk allograft may
be necessary to restore condylar bone loss. The use of extensive bone
cement at the fracture site is discouraged because of risk of nonunion.
a stable anatomic position are amenable to nonoperative treatment.
Displaced and unstable fracture patterns associated with well-fixed
total knee components usually are treated with open reduction and
internal fixation with buttress plates or locking plates. Revision
total knee replacement is indicated when the fracture involves the
tibial component or when the implant is loose. Long-stemmed tibial
components should be used to bypass the fracture site and are often
secured with a hybrid cement technique. Additional plating or use of
bulk allograft may be required based on the fracture pattern and bone
loss.
risk for fracture in nonresurfaced patellae is minimal. Extensive
resection with a patella thickness of <15 mm can predispose to
fracture.13 A three-peg design has
reduced patellar strain and has a decreased likelihood of fracture
compared with a larger single peg. Several other risk factors for
patellar fracture have been identified and include overstuffing of the
femoropatellar joint, use of oversized femoral components, component
malrotation, and placement of the femoral component in too much flexion.14
important factor leading to avascular necrosis (AVN) and eventual
patellar fracture after total knee replacement. The patellar blood
supply may be compromised when a median parapatellar approach is
combined with a lateral release. Scuderi et al.15
demonstrated a 56.4% incidence of reduced blood flow to the patella
when a lateral release was performed following a parapatellar approach.
However, when a medial subvastus approach is used, there is less risk
for AVN when combined with a lateral release because the superior
geniculate artery is preserved. No data are available to show that the
decreased exposure and reduced soft tissue violation of minimally
invasive surgery has an effect on patellar blood supply and associated
fractures.
classified patellar fractures based on fixation of the patellar
component, integrity of the extensor mechanism, and quality of the
residual patellar bone stock. The fractures are classified as type I
with a stable implant and an intact extensor mechanism, type II with
disruption of the extensor mechanism, and type III with a loose
patellar component and reasonable bone stock (>10 mm thickness,
IIIA) or poor bone stock (<10 mm thickness or comminution
prohibiting fixation or resurfacing, IIIB). In this study comprising 78
patella fractures, about half were classified as type I and were
treated successfully with observation or immobilization.
treated with surgical intervention. However, type II fractures were
associated with a high complication rate of 50% and a reoperation rate
of 42%. Open reduction internal fixation was rarely successful owing to
a very thin and small piece of bone. Other surgical options included
partial or total patellectomy with repair and advancement of the
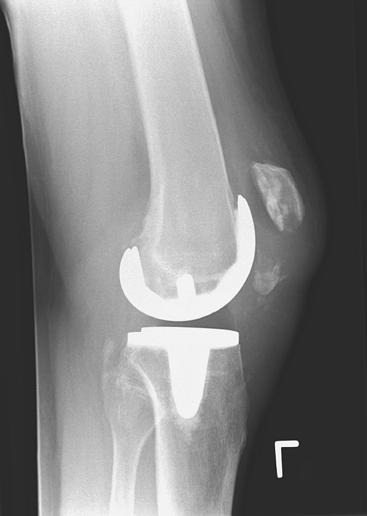
extensor mechanism. Figure 26-1 shows an open
reduction internal fixation of a type II fracture with complete rupture
of the extensor mechanism. Intraoperatively, it was felt that the
remaining distal pole of the patella was large enough for fixation; it
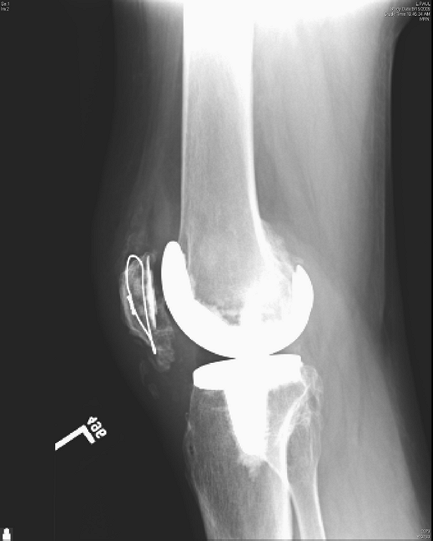
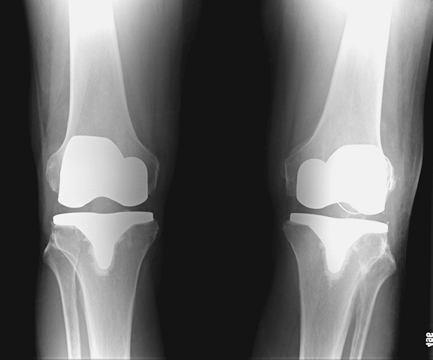
ultimately healed without an extension lag or quadriceps weakness (Figs. 26-2, 26-3, 26-4).
salvaged with an allograft reconstruction consisting of tibial
tubercle, patellar tendon, patella, and quadriceps tendon that was
first described by Emerson et al.16 Nazarian and Booth17 modified this technique by tightly tensioning the
repair in full extension and reported improved early results. Burnett et al.18
reported a series of 20 consecutive reconstructions with one group
having minimal tension in extension whereas the second group was
tightly tensioned intraoperatively. Loosely tensioned allografts
resulted in persistent extensor lag and clinical failure. The tightly
tensioned reconstructions were all clinically successful with an
average postoperative extensor lag of 4.3 degrees.
 |
|
Figure 26-1 Fracture of the inferior patella pole with complete extensor mechanism disruption.
|
 |
|
Figure 26-2 Lateral view of left knee with healed repair of patella fracture and extensor mechanism without functional deficit.
|
 |
|
Figure 26-3 Skyline view of repaired and healed patella pole fracture and ruptured extensor mechanism. Au: Is expansion of s/p correct?
|
 |
|
Figure 26-4 Anteroposterior view of bilateral total knee arthroplasties and healed left patella fracture.
|
arthroplasty occur infrequently but should be suspected in higher-risk
patients who are immunosuppressed, malnourished, taking steroids, or
have diabetes or rheumatoid arthritis, as well as those with a history
of multiple surgeries or prior infection in the operative knee.
surgery. The treatment of postoperative drainage is not clearly
presented in the orthopaedic literature but is based on sound clinical
judgment. Drainage should be more concerning to the surgeon when it
continues after 5 days and if it is associated with diffuse erythema,
purulence, or profuse volume. Persistent drainage, particularly of
serosanguineous character, usually is an indication for aspiration and
consideration of open irrigation and debridement.
risk for subcutaneous fat necrosis and potential wound drainage.
Application of an incisional vacuum sponge has been introduced and
promoted by orthopaedic trauma surgeons to potentially reduce early
drainage and wound breakdown in the morbidly obese. After skin closure,
an incisional vac with a ½-inch-wide strip of sponge is directly
applied to a nonadhesive dressing over the closed incision. After 2 to
3 days, the vac dressing and sponge are removed and replaced with a dry
dressing (MB Harris, personal communication, 2005).
often a granulomatous reaction to the suture material. The perplexing
diagnosis of suture granuloma is more commonly discussed in the general
surgery literature, with only a handful of orthopaedic cases being
reported. Three cases of culture-negative granulomatous reactions to
Vicryl suture were reported within 9 weeks after total hip arthroplasty
by Sayegh et al.19 All cases were
successfully treated with excision of the affected tissue, debridement
of the joint capsule, and extensive wound lavage. The implants were
left in place and the patients were treated with antibiotics pending
the negative culture reports, at which time the antibiotics were
discontinued. Regarding suture abscesses in total knee replacement, we
recommend removal of visible sutures associated with superficial
reactions and more formal debridement and antibiotic coverage for
deeper cases.
but should be more closely observed if profuse and continuous.
Temporary immobilization of the knee and avoidance of early motion can
often result in spontaneous resolution. However, significant
intra-articular hematoma with incisional leakage and excessive soft
tissue expansion with impending skin necrosis are indications for
prompt formal surgical evacuation with hemostasis. Evacuating the
hematoma by squeezing the wound or probing are strongly discouraged
because of the potential for retrograde contamination.20
recognition and is based on the size, depth, and location of the
defect. Superficial skin necrosis <4 cm2 with remaining
coverage of bone and tendon may be treated with wet to dry dressing
changes or a wound vac. Close observation is imperative to avoid deeper
penetration and possible contamination of the prosthesis. An early
plastic surgical consult is strongly recommended. For deeper and larger
defects of >4 cm2, a plastic surgeon should plan for local flap coverage. Ries23
described the use of a medial gastrocnemius flap or latissimus free
flap for defects over the patellar tendon and tibial tubercle. Five of
the six patients who underwent flap coverage required two-stage
revision total knee replacement. Additional adjunctive treatment
measures include immobilization as well as appropriate antibiotic
therapy with infectious disease consultation.
contamination, and the presence of biomaterials places patients
undergoing joint replacement at increased risk for the development of
deep infection. The incidence of deep infection has been reported to
range from 1% to 2.5% in primary TKA and approaches 5.6% in revision
TKA. Factors leading to deep infection must be considered with respect
to the microbiologic characteristics of the infecting organism, the
host, wound, and operative technique.24
bacterial contamination because of a self-perpetuating enlarging
immunoincompetent fibroinflammatory zone that develops around the
implants.25 Bacteria may adhere to
the implant based on the surface characteristics and the intrinsic
properties of the bacteria. Once adherent, bacteria can encase
themselves in a hydrated biofilm matrix of polysaccharide and protein.
Sessile, biofilm-encased bacteria are less susceptible to antibiotics
than free-floating bacteria.25 This quality of deep bacterial infection of TKA underlies its difficulty in eradication without complete hardware removal.
deep infection as well. Patients with decreased immunity, prior history
of deep infection, and higher contamination loads have incidence rates
of deep infection between 3% and 10%.25
Patients with decreased immunity include those with rheumatoid
arthritis, diabetes mellitus, organ transplantation, obesity, HIV, poor
nutritional status, and hemophilia. Patients with increased
contamination loads include those undergoing revision total joint
replacement and those with surgical duration >2.5 hours. There is
evidence that preoperative nasal screening, topical treatment, and
specific perioperative antibiotic prophylaxis in combination with
vancomycin reduces the incidence of MRSA infection in orthopaedic
operated patients to almost zero.27,28
early and accurate diagnosis. Classic clinical presentation of an
infected TKA is characterized by increasing persistent pain, warmth,
effusion, and less frequently, erythema. Patients with prolonged
postoperative pain should be suspected to be infected and should be
evaluated for infection. Aspiration of a suspected infected TKA should
be performed early and before the first administration of antibiotics.
specificity, as well as increase the chance of identification of the
infectious organism with susceptibilities.30
In a two-stage reconstruction of an infected TKA after hardware
removal, followed by a 6-week period of intravenous antibiotic therapy,
antibiotic therapy should be discontinued for a ≥10 ten days prior to
aspiration. Aspiration has been shown to have a 74% positive predictive
value and 94% negative
predictive value, although rates have been identified in studies of ≤100% sensitivity and specificity.24
Newer techniques of polymerase chain reaction (PCR) may increase the
utility of aspiration in sensitivity but may be associated with an
increased false-positive rate. One recent study evaluated the
differential gene expression by white blood cells, using a commercially
available gene chip. They identified expression of genes from
neutrophils present at the site of infection that was different than
that expressed at a site of aseptic inflammation. These findings may
lead to potential future simple lab tests that can distinguish the
causes of inflammation in total joint arthroplasty.29
rate (ESR) and C-reactive protein (CRP) level. However, the sensitivity
and specificity of ESR has been reported as low as 60% in one series.24
Therefore, blood tests may be useful for screening but should not be
used for the definitive diagnosis of deep infection. Radionucleotide
studies, such as indium-labeled leukocyte scans, have been used.
Sensitivity and specificity have been reported between 80% and 94%.
Increased scan activity can be present in ≤90% of tibial and 65% of
femoral components ≥1 year after implantation.24,30
classification system of deep infection and can help guide appropriate
management. Deep infections have been classified as those with positive
intraoperative cultures, early postoperative infection, acute
hematogenous infection, and late chronic infection.
setting of revision TKA for presumed aseptic loosening. Culture results
must be interpreted in conjunction with preoperative examination
findings and the overall clinical scenario. Multiple intraoperative
cultures can help resolve the dilemma of whether a positive
intraoperative culture represents contamination or infection. Greater
than two of five positive intraoperative cultures can indicate true
infection. If felt to be a true-positive, treatment with a 6-week
course of suppressive antibiotics can be curative in 90% of patients.30 This approach is similar to direct-exchange arthroplasty for low-grade infections.
after implantation. Acute hematogenous infection occurs with seeding of
the joint from another primary site of infection, such as urinary tract
infection or pneumonia. Invasive procedures leading to transient
bacteremia, such as colonoscopy and dental procedures, may contribute
to deep joint infection. Presentation is with local inflammation of
acute onset and systemic toxicity. Prompt surgical intervention is
mandatory, as delays of >2 weeks are associated with decreased rates
of implant salvage. A success rate of 60% to 80% has been found with
treatment with retention of the implants and multiple debridements. In
a study of 24 patients with infected TKA presenting within 30 days of
the index procedure or with <30 days of symptoms (acute hematogenous
group), Mont et al.31 reported that
the implants were successfully retained in 83% of patients after one to
three procedures. On the other hand, Deirmengian et al.32 reported on a series of 31 TKA patients with disappointing results of infections with Staphylococcus aureus. Only one was treated successfully with early debridement, liner exchange, and retention of the implants.
index TKA and involves extension of the infection through the capsule,
with or without sinus formation. Onset is more gradual, with slow
deterioration of function and increase in pain. The treatment of late
chronic infections has received much attention in the literature of the
past 30 years. Single-stage revision in the presence of low-grade
organisms has been reported. However, most reports favor a two-stage
approach with placement of a temporary antibiotic-impregnated cement
spacer after a thorough debridement of the knee joint.33
The current recommended dosage is ≥3.6 grams of antibiotics per 40 g of
acrylic cement for effective elution kinetics and sustained therapeutic
levels of antibiotics.34 We
currently use 2 g of vancomycin and 3.6 grams of tobramycin per 40 g of
acrylic cement. Premixed antibiotic cements with 1 gram of gentamicin
are not recommended for the treatment of deep infection, and an
inadequate dosage of antibiotics within bone cement has been described
as a cause of treatment failure.35
Intravenous antibiotics appropriate for the infecting organism are
administered for 6 weeks, followed by a second-stage implantation of a
permanent prosthesis using low dose antibiotic-impregnated bone cement.
Success rates have been identified for two-stage replantation of 80% to
93% when using an antibiotic cement spacer.36,37,38,39,40,41
the two-stage treatment algorithm lead to knee stiffness and may
compromise bone stock. Multiple studies have examined the use of an
articulated spacer technique. A comparison of static with articulating
spacers identified improved preservation of bone stock, increased ease
of exposure during replantation, and no apparent increase in
reinfection when using articulated spacers.38
One recent study showed the average range of motion with the
articulated spacer was 110 degrees, which was not significantly
different than the motion after replantation.33 Success rates for eradication of infection with the PROSTALAC spacer were found to be 91%.45
Multiple articulating designs have been described, including
all-antibiotic–laden cement, cement and metal composites, and
replacement of the original components after autoclave sterilization
and loose antibiotic cement technique.33,3637433940
Many variations of spacer design have been described as well, ranging
from ball-and-socket type molding, commercially available PROSTALAC
designs, and metal-polyethylene-cement composites.45,44
Most involve the intraoperative production of separate femoral and
tibial casted or sculpted cement spacers that mimic the design of the
metal implants and allow for motion at the cement/cement interface.38,433940 All designs show success rates ≥90% for infection eradication and improved patient function with the articulating spacer.
disappointing and disabling occurrence. Gait studies have suggested
increased difficulty of walking occurs with increasing flexion
contracture and that flexion of 67 degrees is required
for normal gait.46
Stair climbing requires 83 degrees of flexion, rising from a seated
position requires 93 degrees, and tying shoelaces requires 106 degrees.47
criteria used. Severe postoperative stiffness has been defined in the
literature as flexion <75 degrees and/or the presence of a knee
flexion contracture ≥15 degrees.46,48
However, others have also suggested that an arc of motion <70
degrees or a flexion contracture >20 degrees with a total range of
motion <45 degrees constitutes postoperative stiffness. Prior
studies have indicated an incidence of stiffness as high as 12%. Two
institutions recently found the incidence of stiffness after TKA to be
from 1.3% to 3.7%, based on large consecutive series of >1,000
primary TKAs in both studies.46,4
can all contribute to a stiff knee after TKA. The range of motion
before the index arthroplasty is the most common preoperative predictor
of decreased motion after TKA.46,47
This preoperative stiffness can occur from extensor mechanism or
capsular contractures. Although these structures may be released during
the index procedure, their elasticity may be restricted owing to
chronic fibrosis.49 Body habitus may
also decrease postoperative motion. Obese patients with short stature
have earlier impingement of posterior soft tissues, decreasing total
flexion.51 Other patient factors
such as posttraumatic arthritis, juvenile rheumatoid arthritis,
ankylosing spondylitis, and keloid formation may increase the risk for
stiffness.49,51 Patient noncompliance with postoperative rehabilitation protocols often results in suboptimal knee motion.51 Whether minimally invasive techniques and preemptive analgesia reduce the incidence of stiffness remains unclear at this time.
postoperative stiffness. These may include overstuffing of the
patellofemoral articulation by oversizing the femoral component or
increasing patellar thickness. A recent study found that on average,
passive knee flexion decreased 3 degrees for every 2-mm increment of
patellar thickness.50 Patella height should be restored and not altered.51,52
Appropriate balancing of the flexion and extension gaps is essential.
Gap balancing techniques with spacer blocks or tensiometers help to
assess and match intraoperatively composite implant thickness. Femoral
or tibial malrotation can be avoided by using the gap balancing
technique, in minimally invasive TKA, because anatomic landmarks are
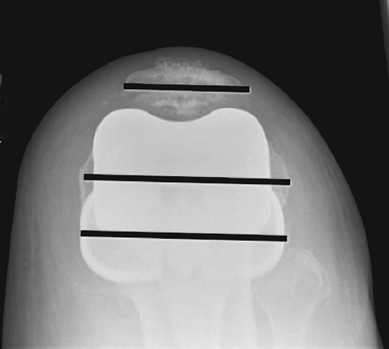
not very reliable (Fig. 26-5). Postoperatively,
the patellar axis should be parallel to the transepicondylar axis and
the tibial axis. Skyline views in 50 to 70 degrees can demonstrate
appropriate alignment (Fig. 26-6). An
excessively tight flexion and/or extension gap, a tight posterior
cruciate ligament (PCL), and femoral and/or tibial malrotation with
limited bearing excursion are associated with highly conforming
prosthetic designs.52
and can be avoided with the use of spacer blocks. If the posterior
femoral condylar bone resection is less than the thickness of the
posterior condyles of the femoral component, the flexion gap will be
decreased.52 In this scenario, the
extension gap will be larger than the flexion gap. Erroneous selection
of an increased tibial polyethylene thickness to balance the extension
gap will further limit flexion. Also, positioning the femoral component
too posteriorly, in excessive malrotation in the coronal plane, or
placing a component with a larger anteroposterior dimension than the
patient’s anatomy will result in a tight flexion gap.51
Failure to remove posterior osteophytes sufficiently can block the full
sagittal excursion of the tibial polyethylene and prevent full flexion.
These osteophytes can also tense the posterior capsule in extension,
causing a paradoxic block to full extension as well.46
Decreased extension may result if the distal femoral resection is too
distal, particularly in the setting of a pre-existing flexion
contracture. A recent study showed that an average value of 9 degrees
of femoral contracture is corrected for every 2 mm of distal femoral
resection.54
 |
|
Figure 26-5
Balancing gap technique: Medial and lateral soft tissues are tensioned equally and the femoral size determined (anterior referencing). This creates an equal flexion and extension gap without the use of anatomic landmarks. |
 |
|
Figure 26-6 Skyline view demonstrating patellar axis, transepicondylar axis, and tibial long axis being parallel.
|
The femoral component should optimally be at right angles to the
anatomic axis of the femur in the sagittal plane. A hyperflexed
component can lead to early cam-post impingement and loss of extension
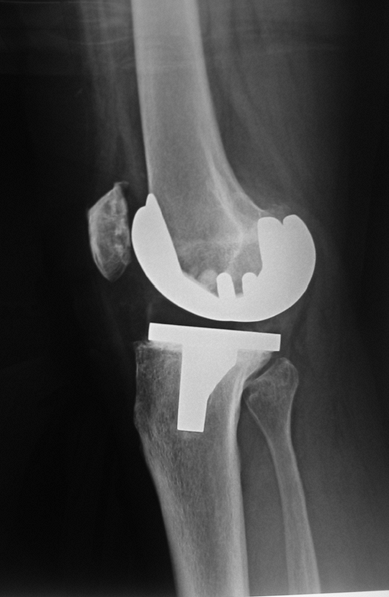
in implants. Figure 26-7 shows a lateral knee
radiograph of a PCL-retaining TKA, the femoral component in about 15
degrees of flexion and the tibial component with a slope of 15 degrees.
This can be tolerated with a PCL-retaining design, but a PS design
would lead to peg impingement.
 |
|
Figure 26-7
Lateral view of a total knee replacement showing a PCL-retaining TKA with about 15 degrees of femoral component flexion and a slope of approximately 15 degrees. In a posterior substituting (PS) design, peg impingement is model specific, but may occur as soon as the combined added angle totals 10 degrees. |
the risks associated with anterior femoral cortical notching. It has
been suggested that tibial slope in the sagittal plane should equal the
patient’s bony anatomy preoperatively. An up-sloped tibial cut (i.e.,
higher posterior than anterior) will lead to a decreased posterior
joint space and decreased flexion. Increased down-slope will increase
flexion but my lead to anterior tibial translation and early posterior
polyethylene wear. Sagittal plane tibial component balance is more
critical in PCL-retaining knees, whereas a flat tibial slope is more
appropriate in PCL-substituting knees that depend on the cam and post
mechanism for sagittal plane behavior of the prosthesis.
posterior cruciate ligament in PCL-retaining knee designs. The PCL may
result in overtightening in flexion, and imbalance of the flexion and
extension gaps will lead to stiffness. Paradoxically, a lax PCL leading
to flexion instability may also lead to stiffness, as anterior femoral
translation occurs with increasing knee flexion. This can induce
earlier posterior impingement and extensor mechanism tightening,
decreasing ultimate flexion.51
Excessive elevation of the joint line with a cruciate retaining implant
may lead to patella infera, which has been associated with patellar
pain and limited motion.47,49 Joint line elevation of 3 to 10 mm can substantially increase PCL tension and limit flexion.
Preoperative patient education, appropriate postoperative analgesia,
and aggressive postoperative rehabilitation help to maximize
postoperative motion and function. Continuous passive motion (CPM)
machines have been useful adjuncts in the immediate postoperative
period, but several studies indicate no significant benefit at 1 year
after TKA.51,49
stiff knee replacement remains controversial. Closed manipulation under
anesthesia has been shown to be effective when performed within 6 to 12
weeks after primary TKA. Surgical options after 3 months include
arthroscopic arthrolysis for focal adhesions, open arthrolysis for
general arthrofibrosis, and only if necessary, component revision.49
Reports of series with arthroscopic arthrolysis and PCL release with a
manipulation showed an improvement in only 43% of knees, whereas
another group showed an average increase of motion by 30.6 degrees.49,55
Open arthrolysis with radical scar excision and ligamentous rebalancing
has shown some promise. A “pie crust” quadricepsplasty followed by a
gradual manipulation has been recommended.51 Others suggest a quadriceps snip at the time of exposure with similar benefits.49
A recent study combining aggressive arthrolysis with a customized
rehabilitation protocol showed a mean increase in range of motion from
63 degrees to 94 degrees in 94% of knees. However, a flexion
contracture averaging 9 degrees remained in 39% of the patients.
Sixty-seven percent of the patients had Knee Society scores of good or
excellent, with improvement from 34 to 77 points.46
situations in which an identifiable, intrinsic problem is associated
with stiffness. These include situations as discussed above, such as
component malposition, incorrect sizing, joint line displacement,
inadequate bone resection, and improper soft tissue balancing. One
recent study evaluating patients with revision of femoral and tibial
components in the setting of stiffness showed Knee Society scores
improved from 38 to 87 and arc of motion improved from 55 degrees to 82
degrees in 93% of knees.48 Another report suggested less promising results, with only 10 of 15 patients satisfied
with outcomes, Knee Society scores from 28 to 65, and an increase of arc of motion from 40 degrees to 73 degrees.56
Successful revision of a stiff knee involves identification of
extrinsic sources of stiffness that are uncorrectable, such as
ipsilateral hip arthrodesis, neurologic disorders, longstanding
extrinsic muscle tightness, and systemic inflammatory conditions.
Identification of the cause of failure preoperatively or
intraoperatively and assessing correction of the problem after
placement of the new prosthesis is associated with the best results.52
most common causes of aseptic failure. Although reported incidence
rates range from 1% to 2% in all primary total knee replacements,
symptomatic instability may account for ≤10% to 20% of patients
undergoing revision surgery. Instability may present as medial-lateral
instability or flexion instability.
total knee replacement. Greater preoperative deformity requiring large
surgical correction and aggressive ligament releases may lead to
difficulties with obtaining stability.58
One study of patients with preoperative valgus deformity averaging
>10 degrees noted that 17% of patients had instability
postoperatively. In addition, the patients with postoperative knee
instability had significantly higher postoperative knee pain than those
with stable knees.2 Increased strain
on the ligaments of the knee may occur with conditions that alter the
mechanics of the knee during gait. Neuromuscular pathology such as
quadriceps weakness or hip abductor weakness may lead to increased
medial forces at the knee, leading to ligamentous laxity and
instability. Valgus forces at the knee may be increased by mechanical
instability at the ankle with posterior tibialis tendon rupture or at
the hip with a valgus alignment of an ipsilateral hip arthroplasty.58
collateral ligament damage owing to difficult surgical exposure. The
use of minimally invasive instrumentation may ease implantation in
these patients. Assessment of component position and appreciation of
ligament balance can be more difficult with the increased soft tissues
and weight of a large limb. Increased thigh circumference will cause a
wide-based gait, which increases stresses on the medial collateral
ligament. Any or all of these factors may contribute to postoperative
instability in obese patients undergoing TKA.
type of instability pattern. This type of instability may result from
collateral ligament imbalance or failure, incomplete correction of
preoperative deformity, and component malalignment and/or failure.
ligaments when balancing soft tissues for a fixed axial deformity
causes an asymmetric extension gap, leading to medial-lateral
instability. Inadvertent damage to the medial collateral ligament (MCL)
also leads to instability in extension. Care must always be taken to
protect the MCL when performing the medial proximal tibial cut as well
as the medial posterior femoral condylar cut. In this setting, medial
collateral ligament advancement or reconstruction alone with
postoperative bracing has been recommended. However, more predictable
stability can be attained by combined repair of the MCL and use of a
constrained implant.
lead to axial instability because of imbalance between medial and
lateral ligaments and soft tissues. For example, an uncorrected varus
deformity will produce a lax lateral sleeve and tight medial sleeve,
causing a varus thrust during ambulation. Patients with medial-lateral
laxity may compensate by walking with a stiff-legged gait to avoid the
pain associated with a thrust or sensation of buckling of the knee.59
Reconstruction in this situation should be directed toward
re-establishing the joint line and appropriate tension in the soft
tissue envelope. Asymmetric instability resulting from improper bone
cuts or bone loss often requires the use of modular augments or
structural bone grafts.
overresection of the distal femur, component loosening, and soft tissue
laxity of the medial and lateral collateral ligaments. An overresected
distal femur will lead to a larger extension gap than flexion gap.
Choosing a thin polyethylene component that fills only the flexion
space will lead to a loose extension gap and associated medial-lateral
instability as well as genu recurvatum during gait. Loosening of the
femoral or tibial component will present as apparent instability on
exam. The loose component will tilt with stress, giving the appearance
of an unstable opening joint.59
Global soft tissue laxity may occur in patients with connective tissue
disorders such as rheumatoid arthritis or Ehlers-Danlos syndrome. This
can result in persistent laxity and instability if not recognized at
the time of primary TKA.
flexion instability. This may occur with overresection of the posterior
femoral condyles, undersizing the femoral component, and excessive
tibial slope.59 All of these causes
lead to a flexion gap that is larger than the extension gap. If a thin
polyethylene insert is chosen that fills only the extension gap,
flexion instability will result. Patients present with recurrent
effusions and a sense of instability without buckling. They will often
mistrust the stability of their knee when descending stairs, and there
is often associated start-up pain. Posterior translation of the tibia
in flexion leads to areas of soft tissue tenderness anteriorly and can
be exacerbated by weakness of the extensor complex.59,60
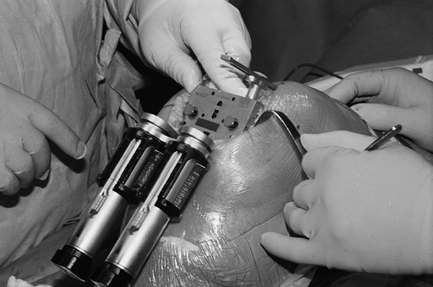
This could be prevented by using the gap balancing technique: First the
extension gap is balanced and the correct insert thickness selected
using a spacer block or a tensiometer. Second, femoral rotation is not
based on anatomic landmarks since it is sometimes impossible in
mini-invasive techniques. A tensiometer is inserted and medial and
lateral soft tissues are tensioned. Blocks are used to determine
femoral component size, and the anterior cut is completed. Figure 26-4 shows the positioning of a tensiometer in combination with
a cutting block to demonstrate how an equal flexion gap could be created using this technique.
is critical to prevent stiffness as well as instability in
PCL-retaining implants. As mentioned previously, a tight PCL will limit
flexion and may contribute to postoperative stiffness and increased
polyethylene wear. Typically, lift-off or rollback is observed. A PCL
release using a “pie crust” technique of tight fibers, a superior
release of the origin, or posterior release of the insertion easily
balances the flexion gap. An overreleased PCL may lead to an
incompetent ligament, with paradoxic roll-forward of the femur and
flexion instability. Flexion instability after cruciate-retaining TKA
has been reproducibly treated with revision to a posterior stabilized
TKA with careful balancing of the flexion and extension gaps.60
stabilized TKA has been classically identified as dislocation of the
cam and post mechanism. However, with contemporary implant designs, the
jump distance has been significantly increased and the prevalence of
frank dislocation is approximately only 0.15%.60
Excessive posterior slope or overresection of the posterior femoral
condyles in a PCL-sacrificing TKA can lead to flexion instability
because of the associated increased flexion space. This can allow the
tibia to subluxate and produce instability with or without dislocation.
There have been reports of tibial post fracture, requiring revision.
Post failure may be related to increased stresses on the tibial post in
the setting of a loose flexion space.61
Reconstitution of the flexion space in revision TKA for flexion
instability is accomplished with the use of posterior femoral
augmentation combined with a larger femoral component. Alternatively,
more distal femoral bone resection can be made if the bone stock
allows, creating symmetric flexion and extension gaps that can be
filled with a larger polyethylene insert.60
cited reasons for revision TKA are complications involving the extensor
mechanism and patellofemoral joint. Historically, patellofemoral
instability after TKA ranged from 10% to 35%. Recent improvements in
prosthetic design and surgical technique have lowered these rates to 1%
to 12%.67 Complications include patellar subluxation or dislocation, patellar component wear, and loosening.
of the most frequent causes of patellofemoral complications. Limb
alignment, preparation of the patella, prosthetic design, and soft
tissue balance all contribute to the stability of the patellofemoral
joint. Nonsurgical treatments such as bracing and physical therapy are
rarely effective in correcting structural abnormalities that lead to
patellofemoral maltracking.67
Treatment should be directed by the cause. Computed tomography (CT)
scan is the most accurate and reliable way to assess component
positioning and its impact on stability. One study using CT to analyze
component rotational alignment found that the combined amount of
internal rotation of femoral and tibial components correlated directly
with the severity of patellofemoral instability.64
If malposition is present, revision of one or both components may be
indicated. Lateral retinacular release, with or without vastus medialis
advancement may also help align the extensor mechanism axis.
potentially devastating complication. Multiple retrospective studies
examining large consecutive series (>1,000) identify an incidence of
0.3% to 1.3%.65,68 Subclinical palsy may occur in higher numbers but may be diagnosed only by electrodiagnostic testing.
no definitive causal relationships have been documented. Conditions
associated with peroneal nerve injury include severe flexion and valgus
deformity correction, preoperative neuropathy, postoperative epidural
analgesia, external leg compression, tourniquet time, and rheumatoid
arthritis (RA).
severe valgus and flexion contractures is associated with increased
postoperative peroneal nerve palsy. The average preoperative valgus in
the patients who developed peroneal palsy ranged from 18 degrees to
23.3 degrees, and average flexion contracture ranged from 15.5 degrees
to 22 degrees.65 The incidence
ranges from 3% to 10% for correction of knees with severe valgus and
flexion deformity. The mechanism of nerve injury has been suggested to
relate to narrowing of the extraneural and intraneural microvasculature
associated with stretching of the nerve within its surrounding soft
tissue.55 An anatomic study
examining the risk of direct injury to the nerve during releases to
allow for correction of valgus deformity measured a mean bone to nerve
distance of 1.49 cm at the level of the standard tibial resection.
Those authors concluded that the nerve is adequately protected at the
posterolateral corner of the knee, but that care should be taken when
performing a “pie crust” release.55
The sensory block may allow the patient to position the leg in a way
that directly compresses the nerve. Also, the patients may tolerate
excessive motion in extension or overly constrictive dressings leading
to nerve palsy. The epidural may mask a peroneal nerve palsy occurring
at the time of surgery and delay diagnosis and initiation of treatment.
associated with development of peroneal palsy. It is theorized that
nerves with prior compromise are more susceptible to a second insult,
often termed the “double-crush” phenomenon.65 No studies have associated diabetic neuropathy with increased risk for peroneal nerve palsy.
53% of patients who developed peroneal palsy had a diagnosis of RA,
which was significantly higher than the prevalence of RA in their
cohort of 1,476 patients. Their patients did not have higher amounts of
preoperative valgus or flexion contracture, suggesting that peroneal
palsy in RA patients may be via a mechanism unrelated to the deformity
of the knee.
electromyogram (EMG) changes in the peroneal and tibial nerves, but the
clinical significance of these changes is unclear. The mechanism is
thought to be related to both ischemia and mechanical deformation, but
most changes have been identified directly beneath the cuff. One study
identified tourniquet time of >120 minutes as an independent risk
factor for peroneal palsy.65
Tourniquet release, allowing a reperfusion interval of 10 to 30
minutes, followed by reinflation has been recommended to extend the
duration of tourniquet time if needed.69
One recent study reviewed a consecutive series of >1,000 patients
undergoing TKA with a tourniquet time of >120 minutes. The overall
incidence of neurologic complications was 7.7% in this population.
Complete neurologic recovery occurred in 89% of patients with peroneal
nerve palsy.69
given its superficial anatomic location as it winds around the fibular
head. Direct compression on the peroneal nerve by constrictive
dressings has been suggested to play a role in the development of
palsy. In addition, the development of postoperative hematoma has been
identified as a rare source of compression on the nerve leading to
palsy.
includes immediate removal of any constrictive dressings and flexion of
the hip and knee to approximately 20 degrees and 45 degrees,
respectively. This can be accomplished by elevation of the extremity on
several pillows. Initial short-term treatment should be observation and
an ankle/foot orthotic (AFO) device for foot drop. Surgical exploration
has been indicated if no functional recovery is noted after 3 months
from onset, particularly if the AFO is not tolerated. The routine use
of surgical decompression remains controversial, despite several
reports of full recovery following open exploration.
The less severe the initial palsy, the more likely it is to completely
resolve. Despite varying percentages of complete peroneal nerve
recovery, most patients have demonstrated good functional capacity
after TKA.
DR, Insall JN, Scott WN, et al. Total knee replacement in young, active
patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79:575–582.
JN, Barrett J, Mahomed NN, et al. Association between hospital and
surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86-A:1909–1916.
RN, Umlas ME, Rodriguez JA, et al. Supracondylar femoral fracture above
a PFC posterior cruciate-substituting total knee arthroplasty treated
with supracondylar nailing. A unique technical problem. J Arthroplasty. 1996;11:637–639.
PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above
total knee arthroplasty utilizing the Less Invasive Stabilization
System (L.I.S.S.). Injury. 2001;32(suppl 3):SC64–75.
RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture
after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop Relat Res. 1990;260: 154–161.
RS, Berger RA, Paprosky WG, et al. Extensor mechanism allograft
reconstruction after total knee arthroplasty. A comparison of two
techniques. J Bone Joint Surg Am. 2004;86-A:2694–2699.
S, Bernard L, Stern R, et al. Suture granuloma mimicking infection
following total hip arthroplasty. A report of three cases. J Bone Joint Surg Am. 2003;85-A:2006–2009.
M, Robinson D, Thornton M, et al. Therapeutic embolization of the
genicular arteries for recurrent hemarthrosis after total knee
arthroplasty. J Arthroplasty. 2001;16:935–937.
C, Lonner JH, Booth RE Jr. The Mark Coventry Award: white blood cell
gene expression: a new approach toward the study and diagnosis of
infection. Clin Orthop Relat Res. 2005;440:38–44.
MA, Waldman B, Banerjee C, et al. Multiple irrigation, debridement, and
retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12:426–433.
C, Greenbaum J, Lotke PA, et al. Limited success with open debridement
and retention of components in the treatment of acute Staphylococcus aureus infections after total knee arthroplasty. J Arthroplasty. 2003;18(suppl 1):22–26.
SM, Czajka J, Fuchs MD, et al. Antibiotic-loaded articulating cement
spacer in the 2-stage exchange of infected total knee arthroplasty. J Arthroplasty. 2004;19:768–774.
AA, Goldberg T, Tanner AM, et al. Treatment of infected total knee
arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125–131.
GV, Berend KR, Berend ME, et al. The effects of varus tibial alignment
on proximal tibial surface strain in total knee arthroplasty: the
posteromedial hot spot. J Arthroplasty. 2002;17:1033–1039.
BC, Scott RD. The effect of patellar thickness on intraoperative knee
flexion and patellar tracking in total knee arthroplasty. J Arthroplasty. 2006;21:650–655.
BC, Scott RD. The effect of distal femoral resection on passive knee
extension in posterior cruciate ligament-retaining total knee
arthroplasty. J Arthroplasty. 2006;21:161–166.
HD, Schwartz JB, Math KR, et al. Anatomic risk of peroneal nerve injury
with the “pie crust” technique for valgus release in total knee
arthroplasty. J Arthroplasty. 2004;19:40–44.
GJ, Jacofsky DJ, Pagnano MW, et al. Functional results after revision
of well-fixed components for stiffness after primary total knee
arthroplasty. J Arthroplasty. 2005;20:133–138.
MP, Jensen TT, Husted H. Secondary knee instability caused by fracture
of the stabilizing insert in a dual-articular total knee arthroplasty. J Arthroplasty. 2004;19:941–942.
TT, Hebl JR, Gali B, et al. Anesthetic, patient, and surgical risk
factors for neurologic complications after prolonged total tourniquet
time during total knee arthroplasty. Anesth Analg. 2006;102:950–955.
SK, Scott RG, Breidahl W, et al. Computer-assisted knee arthroplasty
versus a conventional jig-based technique. A randomised, prospective
trial. J Bone Joint Surg Br. 2004;86: 372–377.
RS, Beksac B, Phongjunakorn A, et al. Minimally invasive total knee
replacement through a mini-midvastus incision: an outcome study. Clin Orthop Relat Res. 2004;428:74–81.
M, Wolke B, Czupalla H, et al. Positioning of total knee arthroplasty
with and without navigation support. A prospective, randomised study. J Bone Joint Surg Br. 2003;85:830–835.
CM, Tetsworth KD, Calhoun JH, et al. An articulated antibiotic spacer
used for infected total knee arthroplasty: a comparative in vitro
elution study of simplex and palaces bone cements. J Orthop Res. 2005;23(1):27–33.
RC, Galat DD, Komistek RD. Correlation of compartment pressure data
from an intraoperative sensing device with postoperative fluoroscopic
kinematic results in TKA patients. J Biomech. 2005;38(2):333–339.
