DISORDERS OF THE ROTATOR CUFF
disorders of the rotator cuff have become universally recognized as the
predominant cause of shoulder pain and impairment in athletes and the
general population as well. Pathologic conditions include tendinitis,
partial- and full-thickness tears of the supraspinatus and other
rotator cuff muscles, calcific tendinitis, and degeneration of the long
head of the biceps tendon. Recent advances in the management of these
disorders include increased recognition of the role of dynamic causes
of rotator cuff tendinopathy, an expanded role of arthroscopic
evaluation and treatment, and improved fixation methods of tendon
repair facilitating rehabilitation. Other conditions of the shoulder
discussed in this chapter include rupture of the pectoralis major and
adhesive capsulitis.
to etiology. In the younger patient, especially the athlete, repetitive
overuse, muscle imbalance and weakness, capsular contractures, and
coexistent glenohumeral instability are potential causes (24,27,30).
Abnormality of scapular rotation, because of the functional
interdependency of the shoulder complex, can cause secondary rotator
cuff dysfunction and pain (30,31).
In middle-aged and older patients, primary tendon degeneration as well
as morphologic alteration of the coracoacromial arch including
degeneration of the acromioclavicular joint are the principal causes of
symptomatic rotator cuff tendinopathy (8,12,38,39,40).
the rotator cuff tendons, especially the supraspinatus, against the
undersurface of the anterior acromion and the coracoacromial ligament
is a major mechanism for the production of rotator cuff pain (40).
Progressive, unchecked mechanical impingement against the
anterior-inferior acromion and coracoacromial (CA) ligament initiates a
cycle of tendon degeneration, for which Neer (40)
identified three stages, starting with edema and hemorrhage in the
tendon, progressing to fibrosis and tendinitis, and culminating in
spurs and eventual tendon rupture.
postulated that primary alteration of the coracoacromial arch reduces
the space for excursion of the rotator cuff tendons during humeral
elevation, precipitating tendon degeneration. An increased incidence of
tendinopathy has been noted in patients with a type II or III acromial
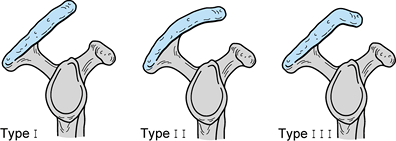
configuration (Fig. 79.1), as well as in those with ununited acromial ossification centers (38,39). It appears, however, that in a number of cases, hypertrophic changes in the coracoacromial arch develop after primary degeneration of the rotator cuff has occurred (44,47).
Further attrition can then occur as the degenerated rotator cuff
tendons are compressed against a thickened acromion and CA ligament.
 |
|
Figure 79.1. Acromial morphology: I, flat; II, curved; and III, hooked.
|
to recognize that tendon degeneration could occur on the undersurface
(articular), superior surface (bursal), or within the substance of the
supraspinatus tendon (mid-substance), creating partial-thickness
lesions in these locations. Some lesions produce symptoms, and an
unknown percentage progress to full-thickness tears. Most complete
tears become manifest as the result of minor trauma superimposed on
longstanding degeneration. Due to its intraarticular course, the long
head of the biceps tendon is subjected to the same degenerative
processes as the adjacent supraspinatus tendon.
humeral head during overhead motion leads to compression of the
supraspinatus against an unyielding but normal-shaped acromion and
coracoacromial ligament. Weakness and imbalance of the rotator cuff
muscles or the scapular muscles, glenohumeral subluxation, and
tightness of the posterior shoulder capsule all produce rotator cuff
pain as a result of dynamic compression (24,30,31).
shoulder pain. Particularly in the throwing athlete, tensile failure
from repetitive, eccentric overload occurs because the supraspinatus
and posterior cuff muscles resist the forces of internal rotation,
adduction, and distraction during the deceleration phase of throwing.
Typically, undersurface partial tears occur in the posterior part of
the supraspinatus as well as the infraspinatus (1).
Acute overload of a healthy tendon contracting against excessive
resistance occurs infrequently, resulting in massive rotator cuff
avulsion, or in isolated rupture of the pectoralis major or long head
of the biceps.
for symptoms is important because treatment regimens differ. Whereas
rotator cuff compression or impingement due to static abnormalities
(abnormal subacromial outlet) may not resolve without surgical
intervention, dynamic causes of rotator cuff compression most often
respond to appropriate nonsurgical measures or to surgical correction
of coexistent instability. Furthermore, rotator cuff tendinopathy that
is not associated with an abnormal subacromial outlet may not respond
to subacromial decompression (8).
treatment, considering the various etiologies and the spectrum of
rotator cuff injuries (Table 79.1). Using a
scheme prevents the tendency to generalize all cases of rotator cuff
pain as impingement syndrome. That sort of generalization leads to
automatic subacromial decompression for refractory cases.
 |
|
Table 79.1. Differential Diagnosis of Rotator Cuff Tendinopathy
|
the shoulder, it is by no means the cause of all cases of rotator cuff
tendinopathy. The tendency to refer to all instances of rotator cuff
tendinopathy as impingement can lead to the inappropriate use of
acromioplasty
for every refractory case, which is analogous to the misuse of lateral release for patellofemoral pain in the lower extremity.
with rotator cuff tendinitis usually describe an insidious onset of
shoulder pain located over the anterior superior aspect of the
shoulder. Pain occurs during or following overhead activity and is
relieved by rest. Like patients with acromioclavicular (AC) joint
pathology, some individuals with rotator cuff injury may experience
pain with cross-arm adduction. With progression, pain may occur at
rest, although usually it is less severe than that associated with
activity, and it may awaken the patient at night. Rotator cuff pain can
radiate laterally to the area of the deltoid attachment but not more
distal. Pain radiating below the elbow suggests radicular pain from
cervical or thoracic outlet disease.
stiffness of the shoulder may be elicited. A history of catching or
popping can occur in partial rotator cuff tears but more likely
indicates an internal derangement such as a labral tear, a superior
labral tear from anterior to posterior (a SLAP lesion) (60),
or loose body. Acute onset of pain in the absence of significant
trauma, coupled with marked pain on attempted motion, raises the
possibility of acute calcific tendinitis, infection, or acute brachial
neuritis.
older patients, to rule out degenerative disc disease with radicular
symptoms. In longstanding rotator cuff disease, inspection may reveal
atrophy of the supraspinatus, infraspinatus, and deltoid muscles, as
well as limited or asymmetric scapular rotation. Active and passive
forward flexion and abduction, as well as internal and external
rotation, may be reduced, compared with the unaffected side. Loss of
internal rotation identifies posterior capsular tightness. Throwers
often have increased external and decreased internal rotation of the
shoulder. Look for pain or weakness with manual muscle testing of
external rotation, abduction, and isolated supraspinatus. Elicit the
impingement sign by passive elevation of the arm against the
supraspinatus outlet, which is fixed by your hand to limit rotation.
Perform the maneuver in overhead elevation (40) as well as in abduction with forced internal rotation of the humerus (26).
instability, especially when there is pain with maximum abduction and
external rotation but no measurable laxity. Testing in the supine
position can relax the patient sufficiently to allow detection of
laxity. The relocation test may be of some benefit in distinguishing
anterior laxity from rotator cuff disease (61):
Apply a posterior force along the anterior aspect of the humeral head,
which has been subluxated by abduction and external rotation of the
arm. Accurate diagnosis may not be possible until examination is
performed under anesthesia and arthroscopy shows a Hill Sachs lesion or
a positive “drive-through” sign (66). Inferior laxity reproducing symptoms should alert you to the possibility of multidirectional instability.
A smaller tear confined to the supraspinatus may cause weakness only
with isolation of the supraspinatus (elevation of the arm, internally
rotated in the scapular plane). Marked weakness in external rotation
suggests a tear with posterior extension to include the infraspinatus.
Perform isolated testing of the external rotators of the rotator cuff
(infraspinatus
and
teres minor) in the lateral decubitus position with the elbow aligned
with the thorax. To test subscapularis function, have the patient
extend her arm and rotate it internally as much as possible, which
positions the hand on the lumbar spine region. Inability to lift the
hand posteriorly from the lumbar spine indicates weakness of the
subscapularis and has been termed the lift-off test (21).
 |
|
Figure 79.2. Inability to maintain humeral abduction.
|
joint pathology. Although cross-arm adduction usually reproduces the
pain of AC joint degeneration, it may be positive in patients with
impingement syndrome as well. Selective injections and specific imaging
as described below may be necessary to differentiate disorders of the
AC joint from those of the rotator cuff.
Use of a posterior or lateral subacromial approach is less painful than
anterior injection; because the entry angle of the needle is directed
upward toward the acromion, inadvertent laceration of the rotator cuff
is less likely. After injection of approximately 10 ml of 1% lidocaine,
repeat the examination. If rotator cuff tendinitis or a partial tear of
the bursal surface is present, the injection will provide a minimum of
50% and often nearly 100% relief of symptoms. If no relief is obtained,
the diagnosis is incorrect or the injection has not been delivered into
the bursa. If there is a full-thickness rotator cuff tear, pain will be
significantly relieved, but some weakness usually persists despite
injection. Continued pain and tenderness at the AC joint may warrant an
injection of 1–2 ml of lidocaine into this joint. If indicated in
selected cases, a steroid may be combined with the local anesthetic.
diagnosis of rotator cuff pathology. Obtain at least two perpendicular
views for every patient, consisting of an anteroposterior (AP) view of
the glenohumeral joint and a supraspinatus outlet view. The
supraspinatus outlet view is a lateral radiograph made in the plane of
the scapula with the x-ray beam directed 10° inferiorly. On the AP
view, nonspecific changes such as sclerosis and cysts in the area of
the greater tuberosity and anatomic neck can be seen, as well as a
decreased interval (normal, 7–15 mm) between the humeral head and the
acromion, which suggests a full-thickness tear of the cuff.
but an AP view in external rotation may better define the lesion.
Although advanced degeneration of the glenohumeral joint is usually
characterized by joint narrowing, effacement of the humeral head, and
spurring, especially inferiorly on the humeral head, early degenerative
joint disease (DJD) may not be recognized even using a true AP
radiograph of the glenohumeral joint. The outlet view defines any
anterior acromial spurs, CA ligament ossification, or abnormal
morphology of the acromion.
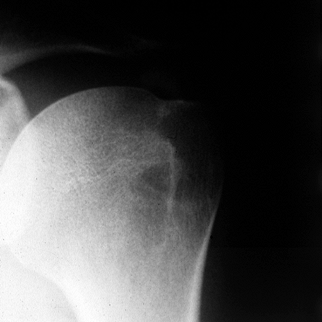 |
|
Figure 79.3. Anteroposterior radiograph of calcium deposits in a supraspinatus tendon located well medial to its insertion.
|
30° inferior tilt to identify any anterior prominence (spurring) of the
acromion (52). If AC joint pathology is
suspected, obtain a 10° inferiorly directed AP radiograph of that joint
with reduced voltage. An axillary view is often useful to identify an
os acromiale or a bony Bankart lesion. A computed tomography (CT) scan,
however, may be necessary for optimal definition of an os acromiale.
arthrography on a case-by-case basis. Ultrasound has been shown to be
helpful in identifying full-thickness tears, but it is less accurate
than arthrography or MRI and remains operator dependent (49).
MRI has largely replaced arthrography for the diagnosis of rotator cuff
disorders as it is noninvasive. It is very accurate in the diagnosis of
full-thickness tears (Fig. 79.4) but less helpful for tendinitis and partial-thickness tears (28). In as many as 50% of asymptomatic
individuals, MRI can demonstrate signal changes within the
supraspinatus tendon consistent with partial-thickness and complete
tears (58). Because incidental MRI-documented
changes increase with patient age, clinical symptoms and signs are more
important than MRI findings in determining treatment in older patients.
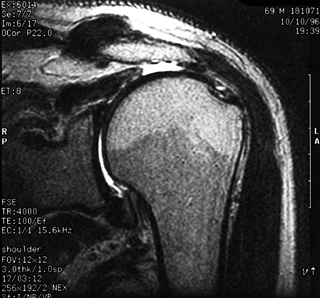 |
|
Figure 79.4. MRI demonstrating full-thickness tear of the supraspinatus with wide retraction.
|
In older patients particularly, DJD of the glenohumeral joint and
cervical radiculopathy must be considered. When mild, DJD may be not be
evident radiographically but can cause tendinitis-like symptoms (17,55).
Often, these patients have slight but detectable limitation of shoulder
motion. In younger patients, glenohumeral instability and degenerative
changes of the AC joint can be associated with symptoms and signs
suggestive of rotator cuff tendinopathy. Although preoperative laxity
testing may not reveal instability, it should be apparent with
examination under anesthesia and confirmed by arthroscopic findings. AC
joint tenderness, pain with cross-arm adduction, radiographic changes,
and a positive technetium-99 bone scan will confirm AC joint
degeneration. These conditions should be strongly suspected whenever
nonsurgical or previous surgical treatment for rotator cuff tendinitis
has been unsuccessful.
 |
|
Table 79.2. Pathologic Conditions of the Rotator Cuff
|
is unpredictable, establishing treatment guidelines remains
challenging. Tendinitis, the most common manifestation of rotator cuff
disease, usually responds to modification of activity or nonoperative
treatment and typically does not progress to frank tendon rupture. Even
in cases of a complete tear of the rotator cuff, some patients
experience clinical resolution (7,29) while others remain symptomatic. A few may progress to cuff-tear arthropathy, characterized
by superior migration of the humeral head, which initiates severe
impingement leading to relentless joint degeneration with collapse of
the head and erosion of the glenoid. In a retrospective review, Itoi
and Tabata (29)
noted satisfactory results in more than 80% of patients treated
nonsurgically for a torn rotator cuff. Patients whose symptoms were
present for more than a year and who had significant loss of motion and
strength were unlikely to respond to nonoperative treatment.
patients compensate for the functional loss of the supraspinatus? And
how do you identify the patient at risk for progressive, disabling
tendinopathy?
synergistic effects of the other rotator cuff muscles (subscapularis,
teres minor, and infraspinatus) together with the supraspinatus and
deltoid in effecting humeral elevation (46,57).
Because the other cuff muscles together can contribute to the power of
elevation in magnitude similar to the supraspinatus, individuals who
have adequate strength in these muscles may be able to function without
significant problems following a tear of the supraspinatus.
Patients with a significant anterior acromial hook (type III acromion)
are known to have a higher incidence of full-thickness tears (38).
It is also apparent that repair of smaller tears produces better
functional results than repair of larger tears. Assuming that an
increase in tear size can occur over time due to further tearing as
well as retraction, it would seem prudent to repair known tears earlier
rather than later, especially in the active patient, regardless of age.
for tendinitis. Surgery is indicated for those patients who fail to
respond to nonoperative measures. Although some have attempted to
define a specific period of time to try treatment without surgery, it
seems more reasonable to rely on the patient’s response to nonoperative
management and the functional demands of the shoulder as the basis for
determining future treatment. In this era of managed care, many
patients have had several months of nonsurgical care before they are
seen by an orthopaedist. In such cases, especially if there has been no
improvement, an additional lengthy period of nonoperative treatment is
unnecessary before surgery.
nonsurgical treatment program and reexamine the patient within 2
months. If there is minimal or no improvement, offer a subacromial
steroid injection. If there continues to be minimal improvement over
the next 2 months, then recommend subacromial decompression in cases
associated with a type II or III acromion.
nonoperative treatment for a minimum of 3 months. Failure to improve
with this regimen warrants arthroscopy. Many such patients will have
readily apparent articular or bursal surface, partial-thickness tears.
Others will have areas of thinning consistent with mid-substance tears
discernible only by probing. In these cases, articular and bursal
partial-thickness tears are debrided, or, if thickness is greater than
50%, repaired by open methods; mid-substance tears usually are excised
and repaired. The question of whether to perform acromioplasty to treat
tendinitis-like symptoms in the patient with a normal-shaped acromion
is unsettled at present because of the unpredictable response.
instability, posterior capsular contacture, and muscle imbalance,
direct nonoperative treatment toward correction of the primary
diagnosis. With the exception of glenohumeral instability, nonoperative
measures are nearly uniformly successful.
tears of the supraspinatus or infraspinatus tendons may not respond to
nonoperative measures directed at eliminating capsular contactures,
muscle strengthening, and alteration of throwing mechanics. Except in
the high-performance athlete, where nonoperative treatment is often
abandoned earlier because of nonmedical considerations, nonoperative
measures should be tried before arthroscopic debridement.
diagnosed, surgical repair is recommended. In less-active patients who
do not rely on the shoulder for work or sports, employ a trial of
nonoperative measures similar to the program used for impingement. If
there is no significant improvement in pain and function within 2 to 3
months, I recommend surgical repair. If the patient shows initial
improvement and maintains a satisfactory level of function, continue
additional nonoperative therapy or self-exercise and follow the patient
at regular intervals.
decreasing pain, stretching and range-of-motion (ROM) exercises, and a
rotator-cuff-specific strengthening program consisting of isometric and
isotonic exercises. Especially in cases of acute onset associated with
overuse, rest may be the single most beneficial modality. Determine any
specific ROM or strength deficits and then prescribe physical therapy
using ice, heat, ultrasound, electrical stimulation, and transcutaneous
nerve stimulation (based on patient response) to reduce pain in
conjunction with posterior capsule stretching, scapulothoracic
mobilization, and scapular strengthening exercises. Keeping within the
pain-free ROM, start a light resistive exercise program
emphasizing external rotator as well as supraspinatus strengthening.
antiinflammatory and a supervised treatment program is reason to offer
the patient a subacromial steroid injection. Give 40 mg of
methylprednisolone and 7–8 cc of 1% lidocaine, followed by rest for a
1-week period before the resumption of resistance exercises. As
symptoms subside and motion recovers, reintroduce the patient to
functional activity. For a swimmer, gymnast, or tennis player, for
instance, have a coach, therapist, or trainer monitor performance of
activity to ensure that proper mechanics are being used and the patient
remains pain free.
demonstrate any improvement after nonoperative treatment, or if you
suspect a rotator cuff tear. If the patient demonstrates improvement
with the program but pain returns with activity, try activity
modification. If unsuccessful, surgery is indicated. With a
nonoperative program, as many as two thirds of patients can expect
relief of symptoms, allowing continuation of activity.
ligament is the standard treatment for patients with rotator cuff
tendinitis due to mechanical impingement. Neer initially described
removal of a 1 cm anteriorly based wedge tapering approximately 2 cm
posteriorly to the undersurface of the acromion (40).
Others have since emphasized the importance of removing any acromial
overhang anterior to the anterior edge of the distal clavicle (15,57) as well as along the medial border of the AC joint.
-
It enables the surgeon to evaluate the
glenohumeral joint (for undersurface tears, for example, and SLAP
lesions, DJD, and loose bodies). -
It can be done on an outpatient basis.
-
It entails decreased perioperative morbidity and faster relief of pain.
that the procedure requires technical proficiency and can be difficult
to learn.
-
Place the patient in the beach-chair
position. Use a folded sheet to stabilize the shoulder along the medial
border of the scapula. Drape the arm free for manipulation, as manual
traction and rotation can facilitate exposure of the rotator cuff. -
Inject the subcutaneous tissues with 10
ml of 1% lidocaine with 1:200,000 epinephrine to decrease bleeding.
Make a 3 to 4 cm straplike incision parallel to and centered over the
anterolateral edge of the acromion. This cut allows posterior as well
as anterior extension, if necessary, to treat an associated rotator
cuff tear. Incise the skin and subcutaneous layer to the level of the
deltoid fascia. Obtain hemostasis with electrocautery. Insert a
self-retaining retractor to increase exposure of the underlying deltoid. -
Identify the deltoid attachment to the
acromion, the AC joint, and the lateral and anterior edge of the
acromion. Split the deltoid at the level of the AC joint, limiting its
distal incision to 3 cm to avoid injury to the axillary nerve
innervating the anterior head of the deltoid. It is usually necessary
to detach a short segment of the deltoid beginning at the AC joint and
extending laterally for about 1 cm. Use electrocautery to incise the
deltoid subperiosteally, and elevate its fibers from the anterior edge
of the acromion. This technique preserves a satisfactory cuff of tissue
for deltoid reattachment. -
Identify the coracoacromial ligament
running obliquely from the medial border of the anterior acromion to
the coracoid. Do not detach the ligament yet. Carefully open the bursa,
which may be adherent to the undersurface of the acromion; introduce a
fingertip to palpate the contour of the undersurface of the acromion.
If necessary, apply gentle longitudinal traction to the humerus to
increase exposure of the subacromial space. If placing a retractor
under the anterior acromion, use care, as excessive force can cause
fracture. -
Protect the rotator cuff with a malleable
retractor while performing the acromioplasty. Excise any portion of the
acromion extending anterior to the distal clavicle. Next, starting at
the superior cortex of the anterior acromion direct a 3/4-inch
osteotome or small oscillating saw to remove the anteroinferior
prominence of the acromion, tapering to exit about 1.5 cm posteriorly
on the undersurface of the acromion. A flat acromion is the goal. The
lateral deltoid will retain some connections to the osteotomized piece.
Incise along the deltoid margin with electrocautery to mobilize the
fragment anteriorly in one piece, pulling on it with a rongeur or
Kocher clamp. -
Next, detach a 1 cm segment of coracoacromial ligament and remove the osteotomized fragment and ligament stump.
-
Palpate the undersurface of the acromion.
File rough areas with a rasp and remove any spurs along the inferior AC
joint. Excise any residual bursal tissue to allow complete inspection
of the rotator cuff while rotating the humerus. If necessary, repair
any full- or partial-thickness tears of the rotator cuff. -
Repair the detached deltoid to its origin
using horizontal mattress stitches with #1 nonabsorbable suture, or
place small drill holes in the acromion for direct deltoid-to-bone
reattachment if the soft-tissue repair is tenuous. Repair the
longitudinal deltoid split with #0 absorbable sutures. Irrigate and
check for bleeding. Close the subcutaneous tissue loosely with inverted
3-0 absorbable suture and the skin with a subcuticular suture. Apply a
sterile dressing and place the arm in an immobilizer or commercial
pillow splint, either of which provides better support than a simple
sling.
-
Place the patient in either the
beach-chair or lateral decubitus position. Mark the outlines of the
acromion, AC joint, posterior and lateral portals, and, if needed,
anterior portal. Using gravity inflow or a mechanical pump, perform
diagnostic arthroscopy of the glenohumeral joint. If gravity inflow is
selected, it is helpful to add one ampule of 1:1,000 epinephrine per
3-liter container of irrigating solution to reduce bleeding. The
supraspinatus outlet should be readily visible during the procedure in
order to assess the amount of acromion to be removed. -
Replace the arthroscope, which is in the
posterior portal, with a dull trocar, and then withdraw the arthroscope
from the capsule and posterior deltoid. The trocar should move easily
in the subcutaneous tissue. Palpate the lateral border of the acromion
and advance the arthroscope sheath along this path toward the anterior
limit of the acromion. Reinsert the arthroscope. If the bursal space is
well visualized, proceed; if not, reinsert the trocar and advance the
arthroscope sheath in a slightly more medial direction until the bursal
space is entered. Taking the time to get adequate visualization at this
point is the key to a well-performed, quick arthroscopic procedure. -
Insert an 18-gauge spinal needle through
the lateral portal (2–3 cm distal to the lateral acromion and parallel
with the posterior border of the clavicle). Palpate the CA ligament,
the undersurface of the AC joint, and the acromion. If acceptable, make
the portal incision, and if you see vessels on the soft tissue around
the margins and undersurface of the acromion, electrocoagulate them
prior to debridement. Place a nonconducting canula through the lateral
portal (Fig. 79.5). Next, insert a 4.5 mm
aggressive motorized shaver to begin stripping the periosteum and soft
tissue covering the anterior acromion. Using the shaver or
electrocautery, remove the periosteum and fibrous tissue from the
lateral margin of the acromion as well, up to the point at which the
deltoid fibers attach. Medially, the AC joint may not be apparent until
externally balloted. The medial
P.2127
aspect
of the acromion must be identified, as it can be a cause of recurrent
symptoms if not adequately resected as well. Identify the
coracoacromial ligament and divide it from the anterior and medial
acromion, taking care not to injure the deltoid fibers immediately
underneath (Fig. 79.6; see also COLOR FIG. 79.6).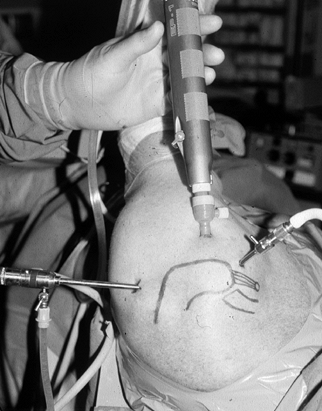 Figure 79.5.
Figure 79.5.
Arthroscopic set-up for subacromial decompression. Shaver is inserted
in the lateral portal created in line with the posterior axis of the
distal clavicle 2–3 cm distal to the lateral edge of acromion.![]() Figure 79.6. (See COLOR FIGURE 79.6) Coracoacromial ligament prior to division with electrocautery (undersurface of acromion is exposed for better visualization).
Figure 79.6. (See COLOR FIGURE 79.6) Coracoacromial ligament prior to division with electrocautery (undersurface of acromion is exposed for better visualization). -
Now that the anterior tip of the
acromion, both medial and lateral borders, are well seen, introduce a
round or elliptical burr from the lateral portal to begin the
acromioplasty. Resect the leading edge of the acromion flush with the
anterior edge of the distal clavicle (Fig. 79.7).
Next, by referencing the posterior margin of the distal clavicle, mark
the posterior extent of the acromioplasty by creating a shallow trough,
no deeper than 5 mm, medial to lateral. Now, switch the arthroscope to
the lateral portal and insert the burr posteriorly.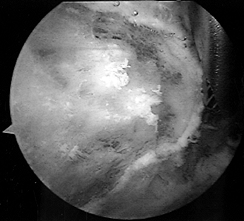 Figure 79.7. Arthroscopic burr resecting the anterior edge of the acromion flush with the distal clavicle.
Figure 79.7. Arthroscopic burr resecting the anterior edge of the acromion flush with the distal clavicle. -
Beginning at the level of the trough,
continue the acromioplasty by sweeping the burr from medial to lateral
and advancing it from posterior to anterior. Keeping the shaft of the
burr in contact with the undersurface of the acromion posteriorly will
result in an upward-sloping acromioplasty, protecting against either
inadequate or excessive bone resection (54). -
Reinsert the arthroscope into the
posterior portal to inspect the acromioplasty. The acromion should be
flat when viewed from both posterior and lateral (Fig. 79.8; see also COLOR FIG. 79.8).
Often, resecting from only one portal will result in a cup-shaped
configuration of the acromion, with prominent spurs anterolaterally and
anteromedially. Using these two portals both for viewing and for
planing the acromion will eliminate this possibility (Fig. 79.9).
At this point, if there are no spurs on the undersurface of distal
clavicle, debride the remainder of the coracoacromial ligament, using
the shaver. If possible, remove 1 cm to eliminate any possibility of
reattachment. Using the shaver in this manner will protect from
accidental deltoid detachment. Remove inferior spurs from the distal
clavicle without disrupting the AC joint itself.![]() Figure 79.8. (See COLOR FIGURE 79.8) Acromion planed flat when viewed from the posterior portal (A) and from the lateral portal (B).
Figure 79.8. (See COLOR FIGURE 79.8) Acromion planed flat when viewed from the posterior portal (A) and from the lateral portal (B).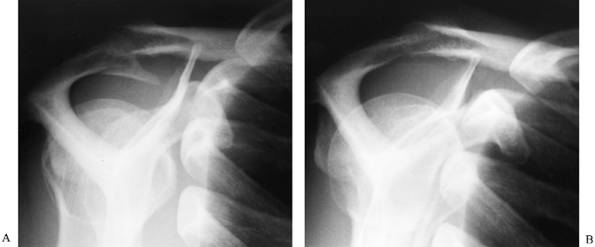 Figure 79.9. Radiographs showing an acromial spur before (A) and after (B) acromioplasty.
Figure 79.9. Radiographs showing an acromial spur before (A) and after (B) acromioplasty. -
Close the skin portals and apply a
nonbulky dressing incorporating a cryotherapy pad, if desired. Use a
sling or immobilizer for support.
performed, start immediate active and passive ROM exercises. Pendulum
exercises and motion performed supine are
best tolerated in the immediate postoperative period. Advise the
patient to avoid active abduction beyond 60° while standing or sitting
for the first 3–4 weeks; such
activity
may result in painful impingement due to postsurgical dysfunction of
the cuff. Initiate isometrics as early as 1–2 weeks.
Once ROM has been restored (in 4–6 weeks), start resistive exercises,
but advise the patient to avoid heavy resistance and contact for a
minimum of 3–4 months to prevent acromial fracture or reexacerbation of
symptoms. Usually, high-demand activities (tennis, swimming, throwing)
can be resumed within 4–6 months’ time.
the deltoid has been elevated or detached, then rehabilitation will
proceed more slowly. Do not allow the patient to perform significant
active flexion or abduction for approximately 4 weeks. Rehabilitation
then proceeds as above with return to activity requiring 1–2 additional
months on average.
tendinitis, intraoperative assessment of motion of the fragment directs
treatment. If the fibrous union between the fragments is secure,
perform routine acromioplasty. If the nonunion is mobile, perform
excision for small fragments (os acromiale), and open reduction and
internal fixation for larger fragments (meta- or mesoacromion). Center
a 4 cm incision over the anterior acromion and parallel to the acromial
border to expose the nonunion, which is debrided of fibrous tissue,
bone-grafted, and internally fixed with one or more small fragment
screws. Rehabilitation is similar to that for open acromioplasty with
active abduction delayed for 6 weeks.
in the recognition and treatment of partial-thickness tears to the
rotator cuff. The major issue in treating a partial-thickness tear is
deciding whether to perform debridement alone, debridement with
subacromial decompression, or excision and repair. This can prove to be
a considerable dilemma, particularly in the young, active patient.
and the articular side of the rotator cuff, it may prove difficult to
determine the exact depth of the lesion. Moreover, midsubstance lesions
may be extremely difficult to detect and measure because of the
relatively normal appearance of surfaces in such lesions. Careful
probing, use of a marking suture passing through both surfaces of the
lesion, and injection of methylene blue dye to observe penetration,
facilitate inspection and enable determination of the extent of a
partial-thickness lesion.
whereas those deeper than 50% are excised and repaired. Treat
bursal-sided lesions with an accompanying type II or III acromion with
decompression as well. If symptoms recur after debridement of a lesion,
perform excision and repair. For postoperative management, follow the
procedure outlined for subacromial decompression or repair, as relevant.
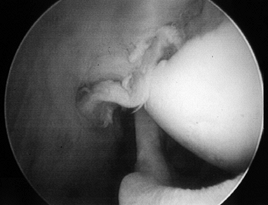 |
|
Figure 79.10. Arthroscopic debridement of a partial-thickness undersurface tear.
|
rotator cuff tears include direct repair of tendon to tendon,
advancement of tendon to bone, local tissue releases and advancement to
effect tendon-to-bone repair, and slight medialization of the repair
site. Exposure through a deltoid-splitting (mini-open) incision rather
than by detachment of the deltoid is ideal to minimize perioperative
morbidity and reduce the risk of deltoid avulsion postoperatively, but
the ultimate goal is a well-done repair. Deltoid detachment to expose
and repair a tear of the rotator cuff is preferable to a
deltoid-splitting incision with a compromised repair of the cuff.
Depending on the surgeon’s preference, open repair without arthroscopy
remains an acceptable option (for incision and initial steps, see the
section on Open Acromioplasty).
achieve direct tendon-to-bone healing. Suture anchors have been shown
to have superior mechanical properties over transosseous suture
fixation when loaded cyclically (10). Suture
anchors perform best when inserted at 45° angles to the bony surface
(in the manner of a tent stake). Transosseous fixation is improved by
using 10-mm-long bony bridges, exiting the tunnels 10 mm distal to the
tuberosity, and use of a plastic button to tie over in osteopenic bone (11).
Using both suture anchor and transosseous fixation with suture provides
optimum fixation, allowing early motion of the shoulder after rotator
cuff repair.
-
Place the patient in the lateral
decubitus or beach-chair position and perform arthroscopy in the
routine fashion. Arthroscopy is often helpful to identify the extent
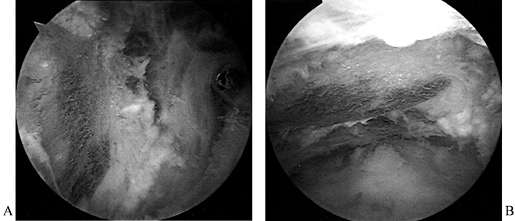
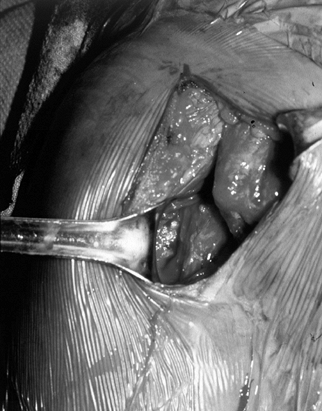
and reparability of a tear (Fig. 79.11 and Fig. 79.12). If the tear appears reparable with or without mobilization of the cuff, proceed with arthroscopic repair. (See below for the management of irreparable tears.) After decompression, perform arthroscopic mobilization of the cuff if desired, or proceed with open surgery.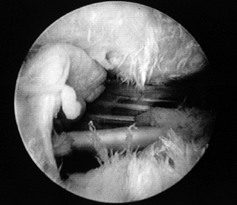 Figure 79.11.
Figure 79.11.
Arthroscopic view of a full-thickness tear with an obvious acromial
spur overhead and an intact biceps tendon beneath a slightly retracted
tear of supraspinatus.![]() Figure 79.12. Incision parallel to the acromion to expose a rotator cuff tear. Arm is still suspended from overhead traction.
Figure 79.12. Incision parallel to the acromion to expose a rotator cuff tear. Arm is still suspended from overhead traction. -
With the patient in the same position, make a strap incision paralleling the lateral edge of the acromion (Fig. 79.12).
Such an incision is preferable to a straight lateral incision, which
can result in scarring to the underlying deltoid raphe with painful
dimpling of the skin. -
Split the deltoid in line with its fibers at the anterolateral corner of the acromion (junction of the anterior and
P.2130
middle one third of the deltoid) (Fig. 79.13). Insert a smooth, self-retaining retractor to inspect the rotator cuff (Fig. 79.14). Gentle traction on the humerus often improves visualization.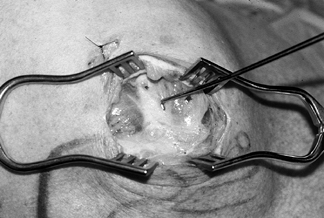 Figure 79.13.
Figure 79.13.
Raphe between the anterior and middle heads of a deltoid. After
splitting raphe, a self-retaining retractor allows more than adequate
exposure of the torn supraspinatus.![]() Figure 79.14. View of the torn rotator cuff using mini–open incision.
Figure 79.14. View of the torn rotator cuff using mini–open incision. -
Excise adherent bursal tissue to see the
actual rotator cuff. Inspect the articular surface for any delamination
in a horizontal plane. -
Retracted tears require mobilization.
These tears are usually triangular or ovoid in shape, principally
involving the supraspinatus tendon near its insertion. If mobilization
is needed, apply stay sutures (#2 nonabsorbable) to the edges of the
tear for traction. Start mobilizing with a blunt elevator or a finger
along the superior surface, working medially. Gain additional excursion
on the articular side by sharply incising the junction of the upper one
half of the capsule and the cuff, staying peripheral to the labrum. Do
not dissect more than 1 cm medial to the glenoid along the undersurface
of the supraspinatus in order to avoid injury to the suprascapular
nerve (65). Additional excursion can be gained
by releasing the coracohumeral ligament, which can retract the anterior
portion of the supraspinatus. -
Advance the mobilized cuff toward the
tuberosity. Observe whether the leading edge can be directly advanced
to bone (crescentic tear pattern) (Fig. 79.15).
If the tear is V-shaped, partial side-to-side repair near the apex may
be necessary, with the edge of the tear then advanced to the bony
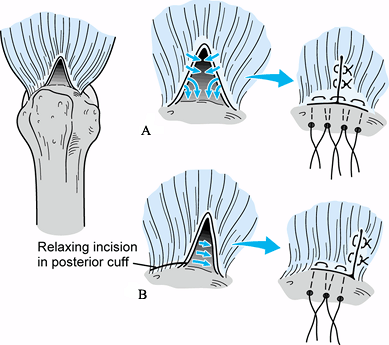
trough (Fig. 79.16). In some cases, the defect can be closed and secured to bone only by local transposition of tissue (Fig. 79.16).
Alternatively, use a partial side-to-side closure and medial repair
within 10 mm of the normal insertion (McLaughlin type V-Y repair).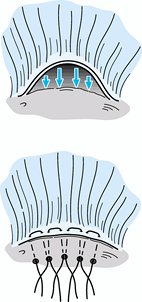 Figure 79.15. Direct advancement to bone of a crescentic-type tear using repair by transosseous sutures.
Figure 79.15. Direct advancement to bone of a crescentic-type tear using repair by transosseous sutures.![]() Figure 79.16. Triangular tear. A: Repaired by side-to-side repair and advancement to trough by transosseous sutures. B: Repaired by local transposition using a relaxing incision in the posterior portion of the cuff.
Figure 79.16. Triangular tear. A: Repaired by side-to-side repair and advancement to trough by transosseous sutures. B: Repaired by local transposition using a relaxing incision in the posterior portion of the cuff. -
Prepare the area for bony attachment by
lightly burring the cortical margin, leaving a shallow trough (5–7 mm)
with intact lateral cortical bone to minimize the risk of suture
pull-out. Insert interrupted horizontal mattress sutures with the
sutures exiting the bursal surface to help force the tendon against the
cancellous surface after repair. Using a small drill or awl of a
diameter slightly larger than the suture, place multiple holes exiting
lateral to the trough. For additional support, insert one or two suture
anchors (#2 nonabsorbable) just medial to the trough, inclined at a 45°
angle, as one would use to drive in a tent stake (Fig. 79.17).
Suture anchors provide a “belt-and-suspenders” repair. Pass the sutures
in the cuff edges using a suture passer or needle through the drill
holes laterally. With the lateral sutures tensioned to achieve
reduction, pass the sutures from the anchors at appropriate spots
through the cuff.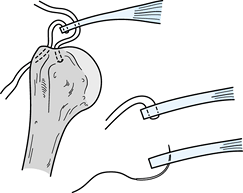 Figure 79.17.
Figure 79.17.
Combined repair with suture anchor and transosseous suture. Note medial
placement in the tendon for suture strands emanating from the anchor,
and horizontal mattress suture exiting the superior surface of the
tendon to facilitate compression against the cancellous surface of the
trough when tensioned and tied over the transosseous bridge. -
Perform repair with the arm as close to
neutral rotation as possible. Repair a longitudinal (side-to-side) tear
with interrupted #0 absorbable sutures. For tendon tears from bone,
apply traction through the sutures, passing laterally through the
tuberosity, and tie the anchor sutures. Tie the lateral sutures
individually, leaving only a small tag to minimize formation of bursal
adhesions. -
Check the integrity of the repair with
passive motion. Irrigate and remove any additional bursal tissue. Close
the deltoid split with interrupted #0 absorbable sutures. Close the
subcutaneous layer with deep sutures, and perform a subcuticular skin
closure. Apply a nonbulky dressing incorporating a cryotherapy pad. Use
an immobilizer for support or a commercial pillow splint for added
comfort.
consider the size of the tear, the type of repair (side-to-side versus
tendon-to-bone), how easy it was to achieve repair (degree of
retraction), intraoperative motion limits, and the age of the patient
(quality of bone and tendon). Other factors include use of an abduction
pillow versus a sling, the timing and extent of ROM exercises, and the
rate of progression to resistive exercises and eventual return to
activity. Patients typically do not progress in a straight-line fashion
but rather can be expected to experience minor setbacks or temporary
plateaus with regard to both postoperative pain and ROM. Patients do
not experience maximal recovery for up to 1 year after surgery in many
cases. Preoperative counseling is important to educate the patient
about individual variability and length of recovery.
without compromising the repair. If a longitudinal tendon split has
been repaired without detachment of the deltoid, immediate passive ROM
is acceptable, followed by active ROM excercise at 2–3 weeks. If the
patient is elderly with osteopenic bone and a large, retracted tear,
use of an abduction pillow for 4–6 weeks with only limited passive
motion is appropriate. If the tear involves a significant portion of
the infraspinatus, internal rotation may be tight and may pose a danger
to the repair. Use of an abduction splint in such cases may avoid the
extreme internal rotation imposed by a standard sling.
using pendulum exercises and supine ROM for 4–6 weeks within an arc of
motion of up to 75% of the limit that was observed at surgery. These
restrictions are necessary before allowing active assisted motion in
order not to threaten the repair. Overhead pulley exercises involve
active muscle contraction and should be delayed until 4–6
weeks
in most cases. Part-time use of the sling may be needed for up to 6–8
weeks. Once active control of the arm is achieved at 6–8 weeks, allow
isometric exercises. At 10–12 weeks, initiate light resistive
exercises. If pain develops, reduce exercises while maintaining motion.
Modalities such as ice and interferential stimulation are useful.
performed at 12 weeks without risk of tearing the tendon from its
repair site. In most cases, functional and progressive resistive
exercises can be initiated by 4 months. Most activities can be resumed
between 6 and 12 months.
despite extensive mobilization. The surgical options are then to
medialize the repair as much as 10 mm along the lateral articular
cartilage; perform a partial repair of the cuff; transpose the
subscapularis tendon superiorly; fill the defect with allograft tissue,
autograft tissue, or synthetic material; or transfer the latissimus
dorsi.
less maintains its mechanical advantage. Biomechanical and clinical
evidence of the force-couple relationship of the intact subscapularis
and infraspinatus to functionally substitute for the torn supraspinatus
lends support to the concept of partial repair. It eliminates the
unstable edges of a torn cuff, which may be the real cause of pain.
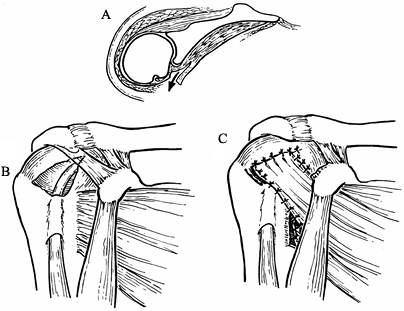
head, Cofield has reported success with transposition of the upper one
half of the subscapularis tendon to fill the defect in the
supraspinatus (13) (Fig. 79.18).
This procedure disrupts the important force-couple between the
subscapularis and infraspinatus–teres minor, which has been observed
clinically and experimentally to effectively substitute for a torn
supraspinatus. Because a transferred muscle loses approximately one
grade of power, both subscapularis and latissimus dorsi transfers may
be weakened.
 |
|
Figure 79.18. Subscapularis transposition to cover a supraspinatus defect. A.Cross section of the shoulder showing release of the subscapularis tendon. B.Defect in the supraspinatus tendon. C.Transposition
of the subscapularis tendon into the defect. (From Poppen NK. Soft-tissue Lesions of the Shoulder. In: MW Chapman, ed. Operative Orthopaedics, 2nd ed. Philadelphia: JB Lippincott, 1993:1651, with permission.) |
which can be successful in relieving pain when followed by a program to
strengthen the other cuff muscles, deltoid, and scapular rotators. If a
tear is found to be irreparable, then traditional acromioplasty should
be modified to prevent superior migration of the humeral head
postoperatively. Removal of the CA ligament in the presence of a
full-thickness tear can destabilize the glenohumeral joint leading to
superior dislocation (68). In these instances,
perform flattening, and even slight concave hollowing, of the acromion
to achieve decompression, while preserving the CA ligament attachment
to its anterior edge.
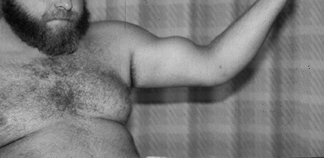
biceps can be managed by extraarticular tenodesis. In the case of
rupture, the deformity is obvious with distal migration of the muscle (Fig. 79.19).
Weakness is usually confined to supination activities (e.g., use of a
screwdriver), unlike distal biceps rupture, which causes elbow flexion
weakness. Isolated rupture is seen in young athletes and laborers, but
coexistent supraspinatus tendinopathy should be suspected in middle-
and older-aged patients.
 |
|
Figure 79.19. Biceps rupture with distal migration of the muscle belly.
|
detect. Pain similar to rotator cuff tendinopathy is present, and
tenderness anteriorly about the shoulder is common.
Speed’s test (resisted elevation of the supinated arm with the elbow extended) (22) and Yeargerson’s test (resisted supination with elbow flexed) (70) may be positive. Tenodesis for bicipital tendinitis, however, is unsuccessful in as many as 50% of patients (4), and nonsurgical measures like those used in rotator cuff tendinitis are used to manage this condition.
combined arthroscopic and open approach. Do an arthroscopic inspection
of the joint and rotator cuff, and resect the intraarticular portion of
the torn long head of the biceps. Two short incisions may be needed for
tenodesis: a 3 cm incision over the deltobicipital groove at the
mid-arm to locate the often distally retracted and entrapped long head
tendon, and a second 3–4 cm incision just anterior and distal to the
acromion to expose the intertubercular groove. This proximal incision
corresponds to the anterior portion of the saber-type incision used to
expose the rotator cuff. A suture passer is helpful to deliver a
retracted tendon into the proximal incision. Tenodesis is necessary
because direct repair is usually not feasible, except in the rare
instance of a tear at the musculotendinous junction (mid-arm level).
method is to place two suture anchors in the middle of the groove,
which has been lightly decorticated with a burr, and secure the tendon
with horizontal #2 nonabsorbable mattress sutures. Have the patient
wear a sling for 10–14 days, then allow active shoulder motion. Avoid
active use of the biceps for 4 weeks, and hold off on resistive
exercises until 10 weeks.
decompression are essentially equivalent, with approximately 75% to 90%
of patients having a good or excellent result. Advocates of the
arthroscopic approach note diminished perioperative pain, faster return
of motion, and earlier return to activity. The arthroscopic procedure
is technically demanding and, other than error in diagnosis, an
inadequate or poorly performed acromioplasty is the major reason for
failure of the procedure. Assuming a correct initial diagnosis, factors
associated with a poor result include the following:
-
Worker’s compensation cases (25)
-
Inadequate or excessive bone resection from the acromion
-
Failure to remove inferior distal clavicle spurs compromising the supraspinatus outlet (50)
-
Surgery performed in throwing athletes, some of whom possibly had coexistent glenohumeral instability (53,62,63)
be expected in 75% to 90% of patients. Relief of pain rates highest,
followed by lower scores for ROM and return of function. The following
are useful guidelines to predict success when treating patients with
rotator cuff tear:
-
In general, small and moderate tears have
better overall results (pain relief, motion, and function) than large
and massive tears (16,27). -
Duration of symptoms and preoperative weakness correlate with the size of the cuff tear (2,16).
-
Cuff integrity at follow-up correlates
with improved strength, motion, and function but does not affect pain
scores; large tears with involvement of the supraspinatus and
infraspinatus have a retear rate greater than 50% (23). -
Return to previous activity level for the high-level competitive athlete is approximately 50% (62), whereas more than 80% of recreational athletes can return to activity (6).
-
Rehabilitation time is prolonged by 25%
when distal clavicle excision for AC joint DJD is performed
concomitantly with rotator cuff repair (14). -
Persistent mechanical impingement and large or massive tears of the cuff are the two major reasons for surgical failure (5).
-
Although a good outcome for pain relief,
motion, and strength can be obtained in full-thickness tears treated by
decompression only (9), improvement in pain only is most likely (15,19), and repair offers the best results (37).
rotator cuff disorders include retear or failure of cuff repair,
infection, stiffness, acromial fracture, deltoid injury, and reflex
sympathetic dystrophy. While technical factors may be involved to some
degree, many of these complications are no different from those
associated with other procedures. The patient’s final outcome is
frequently dependent on how early a complication is recognized and
treated.
most important factors associated with a failed repair are continued
subacromial impingement and the presence of a large or massive rotator
cuff tear at initial surgery (5). Additional
treatment (revision of repair) is indicated if the patient experiences
return of pain. Revision cuff repair can relieve pain in nearly 80% of
such patients, although function is not likely to be improved
significantly.
abundant vascularity, but recognition may be difficult. The traditional
signs of heat, swelling, and redness are often not
present
initially. Diagnosis depends on suspicion of continuing or increasing
pain at rest and with movement of the shoulder. Infection is more
likely to develop following open surgery. If diagnosed early, infection
can respond to arthroscopic debridement and irrigation, followed by
appropriate antibiotic therapy. Established infection with necrotic
tissue and copious granulation tissue usually requires open
debridement. Preserve if possible any repair of the rotator cuff, even
if repeat debridement may be needed.
even after arthroscopic debridement. Early motion is the key to
prevention. If motion is slow to return during the first few weeks,
increase formal therapy sessions to ensure compliance with motion
exercises. Posterior capsular tightness can be a contributing factor in
regaining motion by causing impingement pain with exercises. If the
patient has achieved less than 60% of expected ROM by 6–8 weeks, then
repeat arthroscopy with lysis of subacromial adhesions is indicated.
After cuff repair, 12 weeks is required before the tendon is adequately
healed for manipulation to be performed.
Rapid advancement of resistive exercises in the postoperative period
may be a contributing factor. Fracture usually is not evident for
several months after the index procedure. Radiograph patients with new
onset of pain in the postoperative period. Treat large fragments by
fixation; small fragments can be arthroscopically excised.
the acromion can occur. Denervation results from distal extension of an
incision transecting the axillary nerve as it courses posterior to
anterior along the undersurface of the deltoid approximately 5 cm below
the acromion. Weakness results in the denervated portion of the
deltoid. Rarely, detachment of the deltoid occurs from inadvertent
arthroscopic release during decompression of the subacromial bursa and
by avulsion from its repair site following reflection during open
surgery. Excessive retraction can produce a defect inferior to the
acromion and a nonfunctioning segment of muscle. Early treatment by
reattachment through drill holes in the acromion can be successful if
performed within 6 weeks. After repair, maintain the shoulder in
abduction for 3–4 weeks.
middle-aged individuals predominantly. Calcification of a portion of
the supraspinatus tendon is usually followed by resorption of the
deposit and by reconstitution of the tendon. The clinical expression of
this degenerative process is highly variable. Incidental asymptomatic
calcification is common.
similar to tendinitis. Acute calcific tendinitis is quite dramatic,
with sudden onset of severe pain, exquisite tenderness, and pain that
increases with attempted shoulder motion. Differential diagnosis
includes trauma, acute infection, and acute brachial neuropathy.
The condition is not caused by mechanical impingement, but tendon
degeneration may be important (12). It appears that calcific tendinitis is preceded by fibrocartilaginous metaplasia of the involved portion of the tendon (64).
dense, well-defined opacities or are fluffy with irregular outlines.
The calcific deposit usually appears within the substance of the
supraspinatus tendon distinct from its bony insertion (Fig. 79.3).
Initially dense and well bordered, the deposit becomes irregular and
fluffy as the resorptive phase begins with rupture of calcium particles
into the subacromial bursa, which is usually associated with acute pain.
pain is likely to respond to temporary immobilization, nonsteroidal
anti-inflammatory drugs (NSAIDs), or subacromial injection of a steroid
such as dexamethasone directly into the deposit. Lavage using two
separate needles, one to inject lidocaine and sterile saline and the
other for aspiration, may be helpful. Most patients typically improve
regardless of treatment during the resorptive phase. In the
asymptomatic patient, treatment is not necessary. Some patients with
dense deposits develop symptoms of tendinitis, which are treated with a
nonsurgical program similar to that for noncalcific tendinitis. Failure
to improve with this regimen is an indication for surgery.
-
Use the same set-up as previously
described for shoulder arthroscopy. After glenohumeral visualization,
move the arthroscope into the bursa using the posterior portal
initially. -
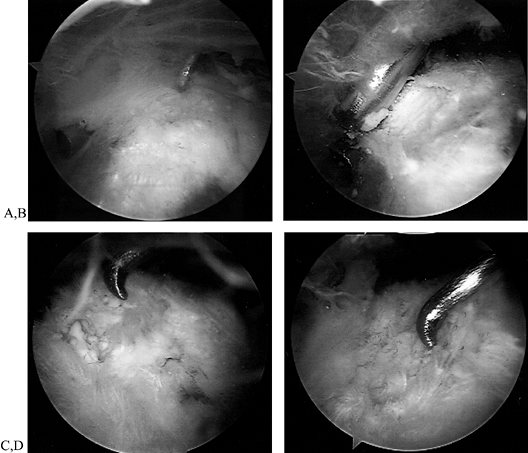
Visualize an area of increased vascularity on the bursal surface of the tendon (Fig. 79.20A; see also COLOR FIG. 79.20). Insert a probe or needle from the lateral portal to confirm the deposit by palpation. Uncover the deposit using a shaver (Fig. 79.20B). Note the chalklike flakes and nuggets (Fig. 79.20C). Continue debridement until the base is clean of calcium particles (Fig. 79.20D). A curet can be helpful in scraping out all remaining particles.
![]() Figure 79.20. (See COLOR FIGURE 79.20) Calcific tendinitis. A: Note inflamed area on bursal surface of the supraspinatus, which corresponds to an underlying calcific deposit. B: Unroofing of the calcific deposit by gentle shaving. C: Full extent of the deposit. D: After complete debridement, the tendon remains intact.
Figure 79.20. (See COLOR FIGURE 79.20) Calcific tendinitis. A: Note inflamed area on bursal surface of the supraspinatus, which corresponds to an underlying calcific deposit. B: Unroofing of the calcific deposit by gentle shaving. C: Full extent of the deposit. D: After complete debridement, the tendon remains intact. -
If the defect is more than 50% the
thickness of the tendon, do a repair; if it is less than 50% of that
thickness, nothing further is necessary. Do not perform acromioplasty
in either instance. -
Close the arthroscopic portals and place the arm in a supportive sling.
repair if appropriate. Otherwise, start early ROM allowing resistive
exercises by 3 weeks and return to activity at 4–6 weeks.
loss of motion unassociated with trauma, followed by partial if not
complete resolution over a prolonged time course, is commonly referred
to as adhesive capsulitis or frozen shoulder (12,41).
Adhesive capsulitis has been further classified as (a) primary or
idiopathic and (b) secondary, on the basis of a precipitating condition
or injury (35). Risk factors for the
development of primary adhesive capsulitis include female sex, middle
and older age, and diabetes. In fact, the first presentation of
diabetes can be adhesive capsulitis. Secondary frozen shoulder can
develop after trauma or surgery to the shoulder, cervical and
intrathoracic disease including malignancy, and as a consequence of
distant upper-extremity trauma (shoulder–hand syndrome).
the literature, ranging from no synovial changes, to vascular synovitis
and presence of intraarticular adhesions and capsular fibrosis, to no
such changes (67). More recently, it has been suggested that contracture of the rotator cuff interval is the responsible mechanism (48). Regardless, reduction in volume of the capsule is the result of the pathologic process.
three phases: freezing, frozen, and thawing, each representing
approximately a 6-month period. The initial process can be painful,
although in some patients loss of motion is the major complaint. Once
established, pain usually tapers off and restricted motion remains the
concern. Unfortunately, the condition can occur bilaterally, either
simultaneously or sequentially.
of secondary causes. Plain radiographs are unremarkable unless a
secondary cause is identified. A saline-contrast MRI or traditional
arthrogram will demonstrate decreased capsular volume with obliteration
of the inferior
capsular fold, and it will exclude abnormality of the rotator cuff. Perform these studies on a case-by-case basis.
for pain and may be indicated early in the disease because of the
inflammatory changes described. Similarly, an intraarticular steroid
injection (40 mg methylprednisolone) may be helpful. ROM exercises are
the centerpiece of treatment. Passive exercises, especially external
rotation and flexion stretching, use of a pulley, and distraction
techniques employed by therapists, are especially beneficial.
Ultrasound may be beneficial to increase capsular compliancy.
passage of time do not improve ROM. Although arthroscopy has been used
with limited success, the risk of incidental scuffing of the joint
surfaces does not warrant it, in my opinion. The preferred treatment is
manipulation.
-
Have the anesthetist administer propofol
or a similar short-acting agent while monitoring the patient. Position
the patient supine and have an assistant stand at the head of the table
to apply downward force on the scapula while you perform manipulation. -
Grasp the arm near the shoulder joint, not
at the elbow, to reduce the moment arm. Slowly but firmly begin flexing
the humerus, overcoming adhesions. Usually, at least 90° or more will
be easily obtained without excessive force. -
Grasp the arm at the elbow and begin to
rotate externally with less force than used for flexion. As resistance
increases, switch to flexion of the humerus until more motion is
gained, then return to external rotation. Alternate this pattern until
at least 80% of the normal ROM is gained. -
To reduce pain postoperatively and to
encourage active ROM by the patient, inject 20 ml of 0.5% Marcaine into
the glenohumeral joint. If possible, have the therapist begin working
with the patient in the recovery area.
usually the result of weightlifting or other sporting activity.
Complete and partial tears are recognized, with the shorter sternal
fibers most often involved. Rupture is the result of eccentric loads
applied to the muscle while the humerus is extended (18,69). Risk factors include male sex and steroid use.
pain and weakness of the shoulder. Examination shows ecchymosis along
the arm and upper chest wall, associated with pain and weakness with
attempted resisted adduction of the arm (Fig. 79.21). Because of swelling, no defect is usually palpable in the anterior axillary fold.
 |
|
Figure 79.21. Ecchymosis associated with pectoralis major rupture.
|
acute setting, differential diagnosis includes fracture, dislocation,
rotator cuff tear, and ruptures of the subscapularis and long head of
the biceps tendon. Chronic tears
are associated with pain and weakness with activity. On examination, there is a palpable defect in the anterior axillary fold.
used to identify complete tears, but MRI is more accurate,
differentiating complete from partial tears.
active patient. Successful repair of chronic tears has been reported as
well, however (32).
-
Under general anesthesia or interscalene block, place the patient in the beach-chair position with the arm draped free.
-
Make an incision along the deltopectoral
groove extending distal to the anterior axillary fold. Retract the
cephalic vein laterally with the deltoid, and in the lower incision
identify the avulsed pectoralis tendon (Fig. 79.22).![]() Figure 79.22. Avulsed pectoralis tendon retracted medially.
Figure 79.22. Avulsed pectoralis tendon retracted medially. -
Clear off the attachment area along the
lateral lip of the bicipital groove, protecting the biceps tendon.
Create a trough with a burr to maximize tendon-to-bone healing. -
Insert three #2 nonabsorbable suture
anchors in this area and pass the ends through the tendon in a
horizontal configuration. Alternatively, use drill holes exiting the
trough laterally. With the arm in adduction and slight internal
rotation, tie the sutures (Fig. 79.23).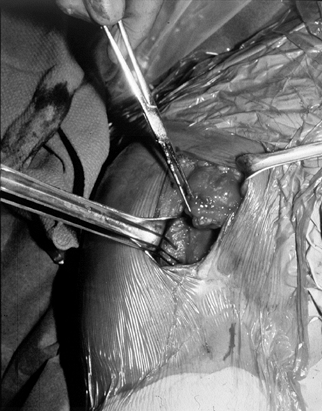 Figure 79.23. Repair of the pectoralis tendon to its bony bed.
Figure 79.23. Repair of the pectoralis tendon to its bony bed. -
Close the subcutaneous layer and skin in a routine manner. Apply an immobilizer for postoperative support.
4 weeks. Then allow forward flexion with the arm internally rotated. At
6 weeks, allow external rotation to neutral. Between 8 and 10 weeks,
start additional external rotation. Do not start resistive exercises
for internal rotation and adduction until 12 weeks. Return to activity
usually takes 4–6 months.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JR, Broussard TS, Carson WG. Arthroscopy of the Shoulder in the
Management of Partial Tears of the Rotator Cuff: A Preliminary Report. Arthroscopy 1985;1:117.
JE, Nirschi RP, Guidi EJ. Current Concepts Review: Debridement of
Partial-thickness Tears of the Rotator Cuff without Acromioplasty. J Bone Joint Surg [Am] 1998;80:733.
SS, Pagan JL, Wirth, MA, et al. Cyclic Loading of Anchor-based Rotator
Cuff Repairs. Confirmation of the Tension Overload Phenomenon and
Comparison of Suture Anchor Fixation with Transosseous Fixation. Arthroscopy 1997;13:720.
DJ, Dobozi W. The Influence of Distal Clavicle Resection and Rotator
Cuff Repair on the Effectiveness of Anterior Acromioplasty. Clin Orthop 1989;247:117.
WE Jr, Safran MR, Seaber AV, et al. Biomechanical Comparison of
Stimulated and Nonstimulated Skeletal Muscle Pulled to Failure. Am J Sports Med 1987;15(5):448.
DT II, Mack LA, Wang KY, et al. Repairs of the Rotator Cuff:
Correlation of Functional Results with Integrity of the Cuff. J Bone Joint Surg [Am] 1991;73:982.
JP, Zlatkin MB, Esterhai JL, et al. Magnetic Resonance Imaging of the
Shoulder: Sensitivity, Specificity, and Predictive Value. J Bone Joint Surg [Am] 1991;73:17.
FW, Kvitne RS, Giangarra CE. Shoulder Pain in the Throwing Athlete. The
Relationship of Anterior Instability and Rotator Cuff Impingement. Orthop Rev 1989;18:963.
TJ, Yerger B, Savoie FH III. Management of Rotator Cuff Tears: A
Comparison of Arthroscopic Debridement and Surgical Repair. J Shoulder Elbow Surg 1994;3:70.
S, Uhthoff HK. Acromial Enthesopathy and Rotator Cuff Tear: A
Radiologic and Histologic Post-mortem Investigation of the
Coracoacromial Arch. Clin Orthop 1990;254:39.
JC, Jiang CC, Wickewicz TL, et al. Changes in the Moment Arms of the
Rotator Cuff and Deltoid Muscles with Abduction and Rotation. J Bone Joint Surg [Am] 1994;76:667.
J, Fujimoto S, Nakagawa Y. Tears of the Rotator Cuff of the Shoulder
Associated with Pathological Changes in the Acromion: A Study in
Cadavera. J Bone Joint Surg [Am] 1988;70:1224.
CJ, Gentz CF. Ruptures of the Supraspinatus Tendon: The Significance of
Distally Pointing Acromioclavicular Osteophytes. Clin Orthop 1983;174:143.
CA, Lyons FR. Shoulder Impingement Syndrome: Diagnosis, Radiographic
Evaluation, and Treatment with a Modified Neer Acromioplasty. J Bone Joint Surg [Am] 1993;74:409.
JP, Krushell RJ, Masquelet A, et al. Anatomy and Relationships of the
Suprascapular Nerve: Anatomical Constraints to Mobilization of the
Supraspinatus and Infraspinatus Muscles in the Management of Massive
Rotator Cuff Tears. J Bone Joint Surg [Am] 1992;74:36.