NEUROMAS
the injury site seek to grow and reestablish contact with their
respective end organs. Continuity or repair of the Schwann cell
endoneurial tube aids this process. Regenerating axons that do not grow
through the zone of injury and into the distal segment of endoneurial
tube become encased in scar and constitute a forme frustre of peripheral nerve regeneration.
an intact crushed or stretched nerve, or in the terminal end of the
proximal segment of a lacerated nerve, is called a neuroma.
The neuroma is usually well circumscribed, white, and rubbery or firm
in consistency. It may adhere to adjacent scar, skin, muscle, fascia,
tendon, periosteum, or bone.
injury, but only neuromas containing sensory nerve fibers can be
symptomatic. The digital nerves of the hand, the palmar and ulnar
cutaneous nerves, and the dorsal sensory branches of the radial and
ulnar nerves are pure sensory nerves and are especially prone to form
painful neuromas. As many as 30% of neuromas formed by these nerves
become symptomatic (7). Common digital nerves
in ray amputations appear more inclined to be painful than those of
digital amputations, possibly because the former contain more sensory
fibers.
consisting of randomly directed axons in a matrix of proliferative
mesothelial elements, primarily epineurium and endoneurium. There is a
disorganized mixture of axons with a predominance of small-diameter
unmyelinated fibers, frequently in whorl-type patterns surrounded by
Schwann cells, fibroblasts, collagen, macrophages, and capillaries.
Myofibroblasts have been consistently identified by electron microscopy
in painful neuromas. These cells may serve as histologic markers of a
symptomatic neuroma, but their pathophysiologic role remains
undetermined (1,5).
completely severed, unrepaired nerves; and amputation stump neuromas (28).
Neuromas-in-continuity include those with intact perineurium, those
with partial nerve division, and those that form after nerve repair or
grafting.
 |
|
Table 53.1. Classification (Sunderland)
|
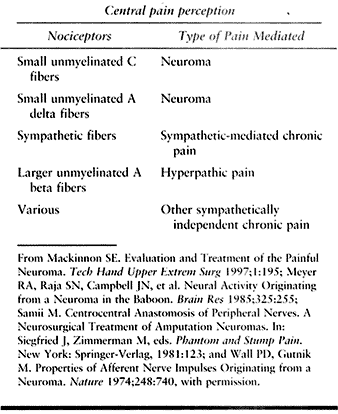
transmitted by afferent small unmyelinated C and A delta fibers. This
picture is sometimes confounded by the involvement of additional
adjacent afferent axons contained within the same sensory nerve (Table 53.2). Afferent impulses are biochemically mediated and may occur spontaneously or be triggered physically (Table 53.3).
In the gate theory of pain, nociceptors do not appear to override other
nociceptors, whereas touch or pressure may do so. Proximity of a
neuroma to skin, to bony prominences, and to the working surface (palm)
of the hand renders it more vulnerable to mechanical impulses (19,26,31).
 |
|
Table 53.2. Pathophysiology of Neuroma Pain
|
 |
|
Table 53.3. Biochemically Mediated Afferent Impulses
|
predisposition to heightened pain sensitivity and perception.
Psychosocial factors may also play a variable role in the perception
and expression of pain. Such factors include individual personality,
pain tolerance, and secondary gain. These elements may be difficult to
sort out and may confound diagnosis, treatment, and outcome.
diathesis similar to that seen in patients who develop chronic regional
complex pain occurs more frequently than expected by chance alone.
Symptomatic neuromas of the hand may be complicated by sympathetically
or independently mediated pain. There may be combinations of pain
involvement (see Table 53.2, Table 53.6).
Pain patterns are often refractory to treatment until the noxious
stimulation of the nociceptors is eliminated. Chronicity may lead to
dystrophic tissue changes. The impairment
and
consequent outcome of a symptomatic neuroma are worsened by the
complexity of such additional afferent nerve fiber involvement.
 |
|
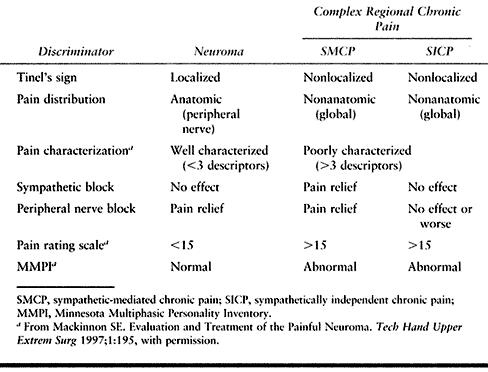
Table 53.6. Relating Diagnostic Discriminators to Pain Type, Etiology, and Pathophysiology
|
The pain may radiate peripherally in the sensory distribution of the
injured nerve, or proximally in the distribution of the nerve trunk. A
dysesthetic “trigger area” found in the same site may radiate pain
peripherally in the distribution of the injured nerve on percussion.
This pain is equivalent to a nonadvancing Tinel’s sign and may be
called a neuroma sign.
 |
|
Table 53.4. Diagnosis of Neuroma
|
anesthetic helps to confirm the diagnosis. Often there is corresponding
sensory deficiency or loss. The history, physical examination,
peripheral and sympathetic nerve blocks, and standardized pain and
psychosocial analysis in various combinations aid in establishing the
diagnosis, sorting out confounding factors, and predicting outcome.
Knowledge of sensory nerve anatomy in the hand and digits helps to
minimize such injuries. In both traumatic and iatrogenic lacerations,
careful identification of transected nerve ends and their repair at the
time of primary wound treatment improves the likelihood of functional
nerve recovery and decreases the risk of symptomatic neuroma formation.
In cases of segmental nerve injury with an identifiable and reparable
distal end, and in a clean and adequately vascularized bed, nerve
grafting accomplishes the same goals. In instances where nerve repair
or reconstruction is not possible and in some amputations, painful
neuromas may develop. The signs are identifiable early, and prompt
treatment is usually more effective than later measures.
painful neuroma is mild and is treated early. Medications, therapy,
physical modalities, and consultation with a pain management specialist
may be helpful (Table 53.5). If injections of
the neuroma are used, adding triamcinolone acetate to the injection
solution may produce a collagenase effect and soften adjacent scar
sufficiently to be curative or palliative in some cases (23).
Use as much as 10 mg of triamcinolone acetate (i.e., a 10 mg/ml
concentration diluted 1:1 or more with 1% lidocaine) and repeat three
times at 1- to 3-week intervals as needed. Advise patients that
depigmentation of the skin and atrophy of the subcutaneous fat may
accompany triamcinolone injection (15).
 |
|
Table 53.5. Nonoperative Treatment
|
demonstrated histologically after application of triamcinolone on a
freshly severed nerve, there is no histologic response to the
application of this steroid in the chronic neuroma. The reason for
improvement or resolution of neuroma pain after steroid injection in
the chronic neuroma remains unclear. The use of collagenase inhibitors
and gangliocidal agents on freshly cut nerve ends is an area of ongoing
investigation that may have future clinical impact (3).
indicated after confirmation of the diagnosis by injection of local
anesthetic and when physical measures such as desensitization and
injection with triamcinolone acetate fail to provide relief. The more
clearly the diagnostic discriminators demonstrate a pure neuroma pain
picture, the better are the outcome expectations following surgery (Table 53.6).
heal them. Take particular care when operating in the vicinity of the
major sensory nerves of the hand, in excision of ganglions. Other
tricky surgeries include decompression of de Quervain’s stenosing
tenosynovitis of the first dorsal compartment, carpal tunnel
decompression, palmar fasciotomy or fasciectomy for Dupuytren’s
disease, A1 pulley release for trigger digits, and tendon repair and reconstruction.
transections of the nerves should be identified and repaired early
whenever possible. Secondarily identified lacerations may merit nerve
grafting if direct suture cannot be performed (6).
In either instance, the preferred treatment for prevention of painful
neuromas is to restore the continuity of the nerve, allowing successful
axonal regeneration across the injury site.
electric current, a variety of artificial (e.g., polyglycolic acid,
collagen-filled) and autogenous (e.g., vein and muscle) conduits, and
the use of nerve allografts with immunosuppressors may soon play a
larger, more definitive role in secondary neuroma treatment by means of
nerve reconstruction (19,20).
Neurovascular island flaps and toe–hand transfers have been successful
for management of refractory digital neuromas when the distal portion
of the involved nerve is not available, when sensory restoration is
important, and in the reconstruction of an amputated digit (17,27).
neuroma-in-continuity forms with the intact perineurium as a result of
repetitive or cumulative trauma (e.g., bowler’s, jeweler’s, or
surgeon’s thumb) and does not respond to nonsurgical measures. A
neurolysis may be performed and the perineural sleeve of scar removed.
In some instances, the nerve may be translocated intact, deep to muscle
such as the thumb abductor or a lumbrical muscle in the palm (Fig. 53.1) (14).
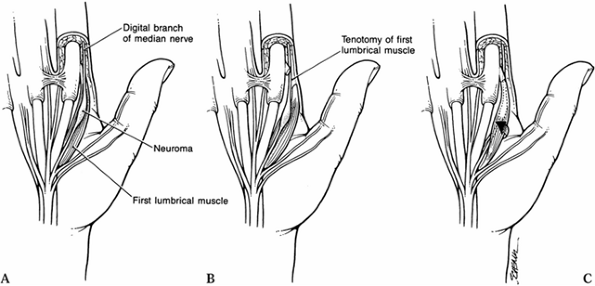 |
|
Figure 53.1. Submuscular transposition. A: Exposing the radial digital neuroma. B: Division of the lumbrical muscle. C: Mobilization of the lumbrical muscle and digital nerve.
|
-
Expose the radial digital neuroma at the base of the index fingers adjacent to the lumbrical muscle.
-
Divide the lumbrical muscle near its insertion.
-
Mobilize the lumbrical muscle and digital nerve.
-
Translocate the digital nerve beneath the muscle.
-
Reapproximate the muscle insertion by suture.
-
The lumbrical muscle now overlies and protects the neuroma-in-continuity.
are that each digital nerve end be identified, mobilized, and placed
under gentle tension, and that the sharply divided end be allowed to
retract 6–10 mm into healthy tissue proximal to the amputation stump (Fig. 53.2) (30).
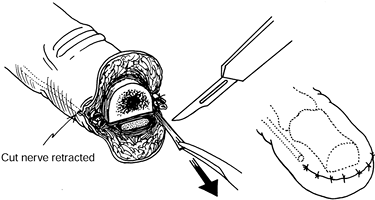 |
|
Figure 53.2. Simple immediate or early primary excisional neurectomy.
|
-
Identify and ligate the digital arteries.
-
Place the digital nerve under gentle tension.
-
Cut the digital nerve as far proximal as possible.
-
Allow the cut nerve end to retract 6–10 mm proximal to the amputated bone end.
nerve ligation, and capping of the nerve end have been tried with
varying success and are not recommended.
and does not respond satisfactorily to triamcinolone acetate injection,
desensitization, transcutaneous electrical nerve stimulation, or other
nonoperative measures. For excisional neurectomy to be successful in
the treatment of amputation neuromas, a stump revision must be
performed if there is a bony prominence representing a potential source
of trauma to the nerve end. Simple neuroma resection has become a
benchmark by which other measures may be gauged (Fig. 53.3).
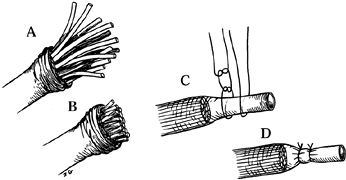 |
|
Figure 53.3. Funicular excision with epineural ligation. A: Mobilization of the epineurium. B: Cutting the funicular ends. C,D: Restoration and suturing of the epineurium.
|
-
Dissect the digital nerve and its neuroma away from the digital artery.
-
Place the digital nerve and its neuroma under gentle tension.
-
Resect the neuroma by cutting the digital nerve as far proximal as possible.
-
Allow the cut nerve end to retract into unscarred soft tissue 6–10 mm from the amputated bone end.
simple excisional neurectomy is one of initial improvement followed by
recurrent symptoms as the anatomic neuroma reforms in its new position.
A second excisional neurectomy may occasionally be helpful after a
failed initial procedure, but subsequent procedures offer diminishing
returns (30).
influences of sensory end organs, particularly those of the skin.
Transposition may remove the nerve end from local neurohormonal
influences at the site of injury. Transposition of a severed nerve end
to a protected environment away from skin, bony prominences, and the
working surface of the hand and avoidance of tension on the nerve may
provide the best physical protection of the severed proximal nerve end.
It may prevent, improve, or eliminate symptoms.
poor full-thickness skin coverage may often be protected by submuscular
translocation or by local or distal flap coverage, it may be easier and
more practical to transpose the nerve to a protected environment. An
area with better blood supply, less scar, and less tension seems to
have a salutary effect on a painful neuroma. Transposition has the
additional benefits of physically removing the nerve end from areas of
direct trauma, such as the working surface of the hand, bony
prominences (especially amputated bone ends), severely scarred areas,
and local neurohormonal influences. There are three types of
translocation procedures for cut nerve ends: subcutaneous,
intramuscular, and intraosseous.
-
To protect the nerve end or the neuroma
from direct trauma in the area to which it is transposed, place a
resorbable suture through the epineurium without violating the nerve,
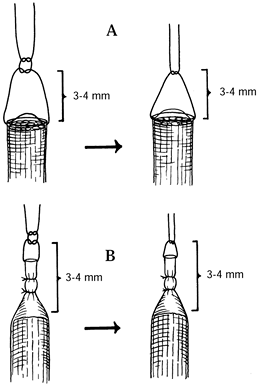
or through the capsule without violating the neuroma (Fig. 53.4, Fig. 53.5) (4).![]() Figure 53.4. Suture techniques for nerve transposition. A: Unligated nerve end. B: Ligated nerve end.
Figure 53.4. Suture techniques for nerve transposition. A: Unligated nerve end. B: Ligated nerve end.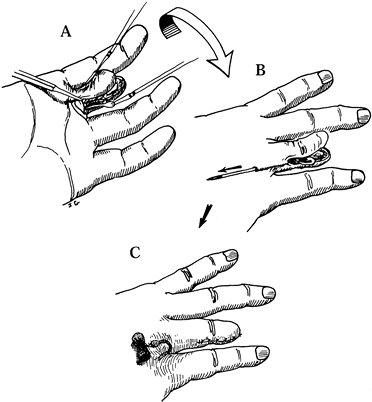 Figure 53.5. Subcutaneous dorsal web space transposition. A: Dissection and mobilization of the digital nerve and its neuroma. B: Suturing the perineurium. C: Tying the suture over a dental roll.
Figure 53.5. Subcutaneous dorsal web space transposition. A: Dissection and mobilization of the digital nerve and its neuroma. B: Suturing the perineurium. C: Tying the suture over a dental roll. -
Tie a knot 3–4 mm distal to the freshly
cut nerve end or neuroma to prevent its direct contact with the
structures to which it is transposed. -
After transposition of any type, inspect the nerve trunk to be certain it is neither under tension nor kinked.
-
In diffusely dysesthetic digital
amputation stumps with one palpable sensitive digital neuroma and one
nonpalpable insensitive neuroma, consider transposing both nerves. If
transposition is not done, the remaining digital neuroma sometimes
becomes significantly more painful, even though the transposed neuroma
becomes asymptomatic.
-
Mobilize the epineurium proximally exposing the fu-nicular ends.
-
Cut the funicular ends proximally.
-
Restore and suture the epineurium over the cut funicular ends without injuring them.
-
Dissect and mobilize the digital nerve
and its neuroma. Dissect the neuroma and its fibrous capsule in
continuity with its nerve proximally and mobilize it so that it can be
translocated without tension. -
Protect the digital artery.
-
Ensure hemostasis.
-
Resect the neuroma. Select a dorsal,
scar-free site away from bony prominences and local pressure or trauma.
The area chosen should place the neuroma dorsal to muscle, positioning
the muscle between the neuroma and the surface of the hand (13,18).
The web space is preferred for neuromas of the digital stumps and the
area between or adjacent to the metacarpals for those involving the
common digital nerves of the palm (Fig. 53.4, Fig. 53.5 and Fig. 53.6).![]() Figure 53.6. Intraosseous transposition. A: Drilling of two small holes in the far cortex opposite the initial drill hole. B: Drill two small holes in the near cortex distal to the initial drill hole.
Figure 53.6. Intraosseous transposition. A: Drilling of two small holes in the far cortex opposite the initial drill hole. B: Drill two small holes in the near cortex distal to the initial drill hole. -
Attach two fine sutures to the perineurium only, on opposite sides of the resected distal tip of the nerve.
-
Tunnel under or into the subcutaneous fat in the adjacent dorsal web space.
-
Tie a knot 3–4 mm distal to the nerve
fascicles to prevent them from directly abutting soft tissues, muscle,
or bone after transposition. -
Bring the needles out through the skin in the proximal portion of the dorsal web space.
-
Transpose the nerve into the depths of the subcutaneous tunnel in the dorsal web space.
-
Be sure the nerve is not kinked.
-
Be sure the nerve is not under excessive tension.
-
Tie the suture over a dental roll.
advocated, there is now evidence from both laboratory and clinical
studies that neuroma formation is suppressed by placing the transected
proximal nerve end directly into muscle substance (19).
The operative technique is similar to that of dorsal subcutaneous
transposition. Although the procedure of neuroma excision and
implantation of the transected nerve end into the brachioradialis has
been a successful method of treating symptomatic neuromas of the
superficial radial nerve, similar procedures placing the digital nerves
into the intrinsic muscles of the hand have been disappointing. Perhaps
muscle contracture, a relatively large muscle excursion in relation to
muscle size, pressure, traction on the nerve during use, or some
combination of these is at fault. Therefore, this procedure is not
currently recommended.
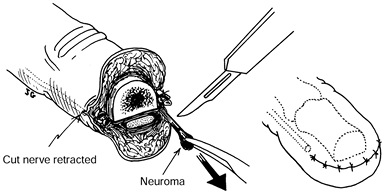
sensory nerve or of a neuroma with sensory neuroma resection produces
results at least comparable to those of subcutaneous transposition (2,9,12,21,22). Either of two techniques for intraosseous transposition may be used (Fig. 53.7).
 |
|
Figure 53.7. Simple late excisional neurectomy.
|
-
Dissect and mobilize the digital nerve and its neuroma.
-
Protect the digital artery.
-
Ensure hemostasis.
-
Resect the neuroma.
-
Attach two fine sutures to the perineurium only, on opposite sides of the resected tip of the nerve.
-
Drill a hole in the adjacent phalanx
large enough to accommodate the nerve without constriction and proximal
enough to avoid excessive nerve tension. -
Drill two small holes in the far cortex
opposite the initial drill hole using a small Kirschner wire or drill.
Pass the needles and suture ends through these holes. Tie the suture
over a dental roll or make a small skin incision prior to passing the
suture ends and tie them directly on the bone. Two alternative methods
follow: -
Alternative A: Drill two small holes in
the near cortex distal to the initial drill hole using a small
Kirschner wire or drill. Pass the needles and suture ends through these
holes. Tie the sutures directly on the bone. -
Alternative B: Suture the epineurium to
the periosteum adjacent to the initial hole and leave the nerve end
free within the bone. -
Be sure the nerve is not kinked.
-
Be sure the nerve is not under excessive tension.
nerve end against intramedullary bone. The epineurium may also be
sutured to the periosteum or to the bone at the site of its entry into
the intramedullary canal. In either technique, there should be no
tension on the nerve at any point. The angle that the transposed nerve
makes as it enters and courses through the bone should not be too
acute. In transposing a transected nerve into bone, it is important to
avoid tension on the proximal stump in any position in the arc of
motion of adjacent joints.
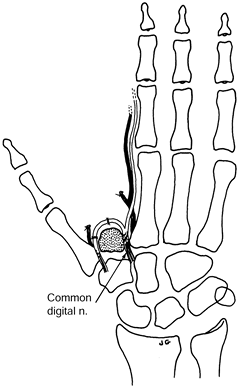
fascicles of the same diameter to either end of an autologous nerve or
fascicle transplant, artificially created by sharply dividing and then
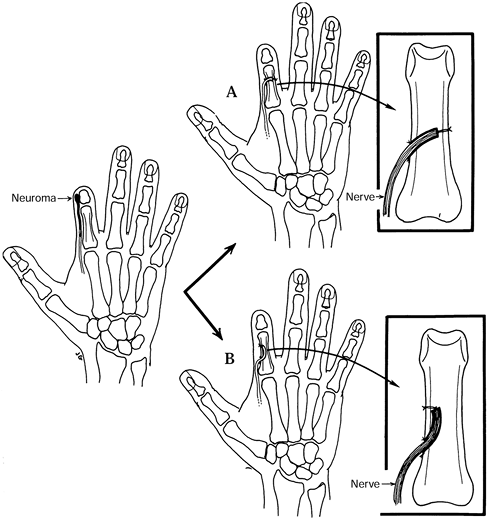
resuturing one of the nerves or fascicles 5–10 mm from its cut end (Fig. 53.8).
 |
|
Figure 53.8. Centrocentral coaptation for index finger ray amputation.
|
-
Ligate the digital arteries.
-
When done as a secondary procedure, resect the neuromas.
-
Intercalate a section of digital nerve
graft (from the amputated finger or other donor site) between the
digital nerves of the index finger. -
The nerve graft should be at least 5–10 mm in length and of similar diameter to the digital nerves.
-
Allow no tension at the suture lines.
-
Protect with adequate cover.
-
Keep away from skin suture lines.
-
Primary centrocentral coaptation is preferred, although secondary centrocentral coaptation can be done.
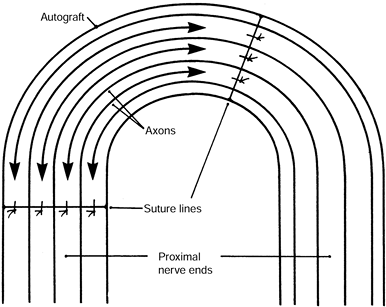
nerve or fascicle and the transplant, so that the physiologic
regeneration of the central axons course past the suture lines and
bypass each other at the midportion of the transplant (Fig. 53.9).
 |
|
Figure 53.9. Close-up of the centrocentral coaptation. The axons (arrows) course past the suture lines and interdigitate at the midportion of the intercalated nerve graft.
|
-
The axons recognize each other as “nontarget structures.” Therefore, the neuroma is small and nonsensitive.
-
Increased intraneural pressure reduces
axoplasmic flow, centrally inhibiting neural protein synthesis and
stopping axonal growth after 3–5 mm of axonal overlap. -
The nerve fascicles’ insulation from neurotrophic growth factors also inhibits neuroma formation.
performed at the same area, the suture lines may be offset stepwise to
minimize the chances of axonal compression by interfascicular
connective tissue proliferation. Protect the centrocentral junction by
adequate full-thickness cover and place it as far from the skin suture
lines as possible. This procedure is excellent for cases of digital or
ray amputation. It can be performed as a primary or secondary procedure
(8,10,11,16,26,27,29).
2–5 mm within the midportion of the transplant, as the increased
intraneural pressure created by this juncture mechanically reduces its
axoplasmic flow; this centrally inhibits neural protein synthesis. This
mechanism also minimizes the size of the intraneural neuroma within the
transplant.
neuroma formation by centrocentral union is that macromolecular
proteins in the distal nerve stump and sensory receptors (i.e.,
target-derived neurotrophic factors) stimulate axonal regeneration
locally at the site of injury and centrally at the nerve cell body by
retrograde axoplasmic transport. They may also guide the regenerating
axons to their target end organs after nerve repair or grafting is
performed. In the case of an unsatisfied proximal nerve end, these
neurotrophic factors may contribute to neuroma formation and its
symptoms. Centrocentral coaptation may insulate the central nerve
segment or the fascicle stumps from neurotrophic influences and confine
the regenerating axons to a nontarget environment, allowing the
regenerative process to cease.
dressing until pain at the operative site is minimal. In the case of a
successful operation, it takes 3 to 6 weeks for the pain to subside.
Thereafter, have the patient remove the protective dressing or splint 3
to 4 times each day for therapy. Therapy includes joint motion and
tendon excursion exercises, as well as scar softening, mobilization,
and desensitizing measures. Use warm water soaks, massage, and active
range-of-motion exercises.
are capable of gliding a limited distance in the tissue that surrounds
them. To the extent that this gliding is impaired by scar adhesion,
traction on the nerve may occur and may produce symptoms. We attempt to
restore gliding by early motion, scar massage, softening, mobilization,
and desensitization after neuroma surgery. Aerobic conditioning has
proven helpful to some patients (Table 53.7).
 |
|
Table 53.7. Postoperative Care and Rehabilitation
|
used to diminish swelling. The Isotoner glove is worn at night.
Vibration may be soothing and softens, mobilizes, and desensitizes the
scar area. Other physical measures to soften and desensitize the scar
include Silastic elastomer (Smith & Nephew, Menomonee Falls, WI),
maintained in place with Coban (Medical Products Division of 3M, St.
Paul, MN), which may be worn at night. Paraffin wax bath and
phonophoresis deliver deep heat and may be soothing. Carefully combined
with massage, they may help to stretch and soften scar. Continue these
methods until the patient’s pain is well within tolerance, resolved, or
has reached a point of maximum medical improvement.
physician and a program directed at functional recovery,
desensitization of the stump and scar, and early return to manual
activities (including work and recreation) play a very important role
in patient recovery.
single measure to prevent a symptomatic neuroma. Nerve grafting and
neurotized tissue transfer are a close second. These methods may also
restore sensory loss. Decompression and translocation of an intact
neuroma in continuity is also a highly reliable procedure. Each of
these procedures reestablishes nerve continuity. They are at least 80%
to 90% successful in avoiding or eliminating symptoms or making them
tolerable.
Repetition of this procedure for initial failure is also about 65%
effective. After two unsuccessful attempts at simple excisional
neurectomy, there is little yield from repeating the procedure again.
usually used as salvage procedures for symptomatic neuromas in which
the distal nerve segment is not available and in which the
reestablishment of sensation is not critical. These procedures are 80%
to 90% successful.
with complex pain. Patients who do not have adverse psychosocial
factors fare better than those who have them (25).
division can be achieved by repair using direct suture in freshly
lacerated lesions and by nerve grafting, primarily or secondarily, in
cases with nerve loss and those in which direct suture cannot be
accomplished without tension. In elective digital amputations or ray
resection, centrocentral coaptation or transposition may be selected
for digital nerve management. For traumatic amputations, the condition
of the wound at the time of surgery may dictate whether the transected
nerves are managed by simple excision or by a nerve-manipulating
procedure. If the wound is contaminated or if additional dissection
would jeopardize tissue viability, the transected nerve ends should be
managed by simple excision. Centrocentral coaptation or transposition
of the neuroma may be deferred and performed as a delayed primary
procedure when wound conditions permit or secondarily if a symptomatic
neuroma develops. Naturally, prevention of a symptomatic neuroma is
preferable to treatment of one.
include nerve grafting if a distal nerve end is available.
Decompression and submuscular translocation are quite reliable in
managing neuromas caused by repetitive or cumulative trauma that form
in continuity with the perineurium intact. Dorsal subcutaneous or
intraosseous transposition is effective in treating symptomatic
neuromas when no suitable distal nerve exists. Centrocentral coaptation
is an excellent method of managing transected digital nerves. Although
setting the standard for comparison, simple excision does not provide
as good or as reliable results for established neuromas as do the other
procedures described in this chapter.
symptomatic neuromas include removing the free nerve end from areas of
scarring, bony prominences, and the working surface of the hand.
Translocation or transposition can also remove a freshly cut nerve end
from local
neurohormonal
influences arising from denervated cutaneous sensory end organs. The
free nerve end must be under no tension and should lie in or adjacent
to well-vascularized tissues. There must be no kinking of the
translocated nerves.
may be excessive nerve tension, pullout, or kinking. Consider
performing a second surgical procedure to investigate the causes and
redo the transposition or relocate the nerve. The same is true in
failure of centrocentral coaptation.
suppressed by intramuscular transposition of the proximally transected
nerve, this method has proved ineffective for controlling neuroma pain
in the hand. Intrinsic muscle contracture causes a relatively large
excursion in relation to muscle size; pressure and traction have been
implicated.
correlates with the time from its formation. Chronic pain syndromes and
the establishment of central pain are time related and often involve
psychosocial and economic factors. The longer a painful neuroma goes
untreated, the less likely it is that any modality can be effective.
Treatment should be completed expeditiously, and the patient should
return to work, even if performing only light duty, as soon as possible.
treatment of a painful neuroma may be successful and is indicated. If a
second operation does not solve the problem, additional surgery
produces sharply diminished returns. Although another operation is
sometimes indicated, the physician should also consider alternatives.
The patient can, for example, be referred to a multidisciplinary pain
clinic as an alternative to additional surgery. These clinics provide
diagnostic and therapeutic nerve blocks. They are particularly helpful
with complex regional independent and sympathetically mediated pain.
moderated symptoms in some patients with complex pain components. There
has also been some success with both peripheral nerve and central
spinal cord stimulators. Although these methods for managing chronic
pain are not a panacea, some otherwise refractory problems have
responded favorably.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
MA, Hurst LC, Ellstein J, McDevitt CA. The Pathobiology of Human
Neuromas. An Electron Microscope and Biomechanical Study. J Hand Surg 1985;10B:49.
MD, Bunke JH, Campagna-Pinto D. Experimental Treatment of Neuromas in
the Rat by Retrograde Axioplasmic Transport of Ricin with Selective
Destruction of Ganglion Cells. J Hand Surg 1989;14A:710.
J, Barbera J, Abellan MJ, et al. Centro-central Anastomosis in the
Prevention and Treatment of Painful Terminal Neuroma. J Neurosurg 1985;63:754.
M. Centrocentral Anastomosis of Peripheral Nerves. A Neurosurgical
Treatment of Amputation Neuromas. In: Siegfried J, Zimmerman M, eds. Phantom and Stump Pain. New York: Springer-Verlag, 1981:123.
JW, Booth DM. Treatment of Painful Neuromas of Sensory Nerves in the
Hand: A Comparison of Traditional and Newer Methods. J Hand Surg 1976;1:144.