The Child with a Limb Deficiency
congenital limb deficiency in the population, and what is reported
varies widely, from 1 per 4264 in Canada (1) to 5 per 10,000 in Australia (2) to 310 per 10,000 in Tayside, Scotland (3). This fact illustrates that this information should be interpreted with caution because the methods of gathering it vary.
health planning agencies, the fact is that to the orthopaedic surgeon,
a child with a limb deficiency is not a common problem. Most limb
deficiencies seen in childhood are congenital in origin. This is
followed at a distant second by those caused by trauma and lastly by
those caused by tumors. This infrequency creates a lack of experience
for the practioner in a complex problem, creating a need for these
patients to be seen in an organized program in which knowledge and
experience are available.
The prevalence of tibial deficiencies is far less, and is reported to
be approximately one per million live births. The incidence of proximal
femoral focal deficiency (PFFD) ranges from 1 in 50,000 to 1 in 200,000
live births. Although this is a more common anomaly than fibular
deficiency, the explanation lies in the difficulty in separating
congenital short femur from true PFFD.
precisely known. However, two general facts are easily accepted.
Although upper-extremity amputations of all types are unusual, in
children congenital amputations are far more common than acquired
amputations. In one multicenter review, 85% of bilateral deficiencies
were congenital (6). In addition, one
congenital upper-extremity deficiency, below-elbow transverse
deficiency, is more common than all others, combined. Few physicians,
other than those working in a limb-deficiency program, will have much
experience with these amputations.
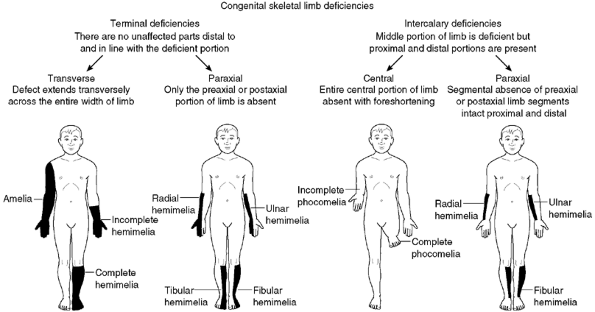 |
|
Figure 31.1
Diagrammatic representation of the Frantz and O’Rahilly classification of congenital limb deficiencies. (From Frantz C, O’Rahilly R. Congenital skeletal limb deficiencies. J Bone Joint Surg Am 1961;43:1202, with permission) |
many paths, and most clinics choose to use some combination of these
classification systems to best categorize the child’s deficiency. These
different classifications are an attempt to convey the particular
anomaly more precisely.
This system was widely adopted in the United States, and is still
widely used by clinicians today to describe longitudinal deficiencies.
However, it was not acceptable to European physicians because of
problems created in translating terms such as “hemimelia.” This led to
the classification system devised by the International Standards
Organization (ISO) and the International Society for Prosthetics and
Orthotics (ISPO) (8).
seven descriptive terms derived from Greek root words. Three of the
terms are from the root word melos, meaning limb:
amelia, absence of a limb; hemimelia, absence of a large part or half of a limb; and phocomelia, or a flipperlike limb (phoke;
seal). The hemimelias can be divided into complete, partial, and
paraxial hemimelias. The remaining four terms refer to the hands (cheir), feet (pous, podos), metacarpals (daktylos), and phalanges (phalanx) (Table 31.1).
complete, when the entire distal half of the limb is missing; partial,
when only a portion of the distal half is missing; and paraxial, when
either the preaxial or postaxial side of the distal portion of the limb
is missing. The hemimelia is named after the missing portion of the
limb. Therefore, a complete absence of the fibula is a fibular
hemimelia.
of the parts of the limb distal to and in line with the defect are
affected; or intercalary, in which the parts of the limb proximal and
distal to the defect are present. Finally, the terminal and intercalary
deficiencies are further divided into transverse deficiencies, in which
the defect extends across the entire limb, and longitudinal
deficiencies, in which only the pre- or postaxial portion is affected.
present at birth (ISO 8548-1 Method of Describing Limb Deficiencies
Present at Birth) was adopted by the ISO and the ISPO (8,9).
In this system, the deficiency is first described as transverse, in
which the entire limb is missing; or as longitudinal, in which all or
part of one or more bones in a limb are missing. In a longitudinal
deficiency (“paraxial” in the Franz and O’Rahilly classification),
there may be parts of the limb present distal to the deficiency. In
the
transverse deficiencies, the part of the segment in which the limb ends
is named, and extent may be stated. A complete loss of the limb distal
to the tibial tubercle would be “transverse leg upper third.” In a
longitudinal deficiency, the bone or bones missing are named and
described as “partial” or “total.” Therefore, a fibular deficiency with
part of the distal fibula present would be described as “longitudinal
fibula partial.” Because this system does not characterize deficiencies
(e.g., PFFD, phocomelia, and amelia), these terms may still be used.
surgical) amputations (ISO 8549-2.1) uses three adjectives: trans,
disarticulation, and partial. The term trans is used to describe any
amputation across the axis of a long bone. A child who has an
amputation performed through the upper third of the tibia is not a case
of below-knee (B-K) amputation in this classification but one of
“transtibial, upper third.” “Disarticulation” refers to any amputation
through a joint. Therefore, a Syme amputation is an ankle
disarticulation. “Partial” refers to any amputation distal to the wrist
or ankle joint. Therefore, a Boyd amputation is a partial foot
amputation, with qualifiers added to distinguish it from a Chopart
amputation.
can be caused: errors in the genetic control of limb development;
disruption of the developing arterial supply, such as “the subclavian
artery supply disruption sequence;” (10) and intrauterine amputation from amniotic bands.
congenital amputation in the past was the mechanical amputation of
limbs by amniotic bands. Streeter, whose name is associated with this
concept of limb deficiency, actually felt that the bands and
constrictions were due to an intrinsic defect in the growth of the
fetal limb (11). There is, however, evidence
that amniotic bands can form a constriction around the developing limb
that interferes with the growth of the limb. The resulting constriction
can lead to any degree of damage, from a constriction band around a
limb that is otherwise normal to a complete transverse amputation. The
previously developed limb has actually been recovered at the time of
birth, indicating the mechanism (12). Most children with amniotic band syndrome have either craniofacial abnormalities or other evidence of band formation.
limb is a complex phenomenon that requires the precise interaction of a
large number of genes and their effects, which are described in Chapter 1 and other review articles (13,14).
The opportunity for errors in this system is great, and animal
experimentation has identified the probable mechanism in several limb
anomalies.
supply to the tissues explains the overlap of many of the common
orthopaedic conditions seen, for example, Poland syndrome, Klippel-Feil
syndrome, Mobius syndrome, Sprengle deformity, and transverse limb
deficiencies. There are several possible mechanisms by which this
disruption may occur. For more details, the reader is referred to an
excellent review article (15).
importance to the clinician. With genetic control and vascular
disruptions playing a large role in the development of the limb and
having consequences in other organs and systems, the association of
limb deficiencies with other abnormalities creating syndromes is
likely. There are two implications when children present with limb
deficiencies; a thorough examination for other abnormalities is
necessary and any heritable genetic defect should be identified. The
possibility of a teratogen always arises in the parent’s mind. Although
there may be many suspected agents, to date, thalidomide remains the
only drug proven to have caused many congenital limb deficiencies, and
other teratogens do not appear to play a role in the congenital limb
deficiencies of today. The role of retinoic acid and low cholesterol on
gene expression is discussed in Chapter 1.
and not transmissible (transverse below-elbow), a few are (tibial
deficiency and cleft hand and foot). This is often something parents
desire to know. Understanding the cause of the deficiency is important
to the resolving of the guilt that parents will initially feel. The
possibility of a transmissible defect is certainly something their
affected offspring will also need to know. A recent study from the
Medical Birth Registry of Norway showed that children born to a mother
with a limb deficiency had a relative risk of 5.6% of having the same
defect as the mother (16). This is similar to
the relative risk of clubfoot. For the physician, knowing the existence
of other problems and the natural history of the syndrome is necessary
for the care of the child.
different—something that no child wants to be. However, almost all
children are different in various ways. Some differ in physical
appearance, some in physical ways that are not immediately visible, and
some in personality and intellectual development. The more children
perceive themselves to be different from peers, the more they
understand their disability. Children’s understanding of their
disability is general and incomplete at 6 years of age, but within a
few years, around the age of 8 or 9 years, they come to a much more
complete understanding of their handicap (17).
Therefore, if parents have not discussed this with the child, they can
expect the more difficult questions from their child to begin at around
this age.
isolation. This, in turn, can have negative effects on the development
of self-esteem, body image, and the child’s identity, which are
developed through the interaction with parents, teachers, friends,
classmates, and others. As children develop these interactions, the
issue of “first appearance” becomes important because it serves as a
clue to perceived personal characteristics, and can be an obstacle to
further healthy interaction. Children in peer groups tend to devalue
those with handicaps, a factor that may greatly interfere with these
relationships (18). Parents especially understand this and fear for their child in this regard.
nonhandicapped child’s reaction to various handicaps, showing that
children prefer other children without handicaps, and that they dislike
some handicaps more than they dislike others (19,20,21).
In addition, it is known that young adults show signs of anxiety when
face-to-face with a handicapped person. However, there is some evidence
that young children do not share their parents’ values toward various
handicaps when young, but between the ages of 6 and 18 years, they
gradually develop values almost identical to those of their parents (20).
This would suggest that these values may be subjected to modification
among young children and emphasizes that organized discussions with
classmates in school about the child’s handicap may be of great value.
differences hold for children, there is evidence demonstrating that the
age of the patient, the gender, the degree of limb loss, or
socioeconomic status are not predictors of low self-esteem or of
depressive symptoms. Rather, social factors, for example, stress and
hassle, parental discord, and social support from classmates, parents,
and teachers, along with the child’s own perceptions of competency and
adequacy, gained through peer acceptance, scholastic achievement, and
athletic accomplishments, play the largest role in the development of
self-esteem (22,23,24).
physicians, therapists, prosthetists, and teachers is that although
limb deficiency is the visible problem and is subject to little
modification, the important factors in the development of self-esteem
are independent of the deficiency and can be positively affected.
adult amputee are as different as are the child and the adult. Acquired
amputation in the adult is usually of the lower extremity and involves
only one limb. In the child, it is most often congenital and more
frequently has upper- and multiple-limb involvement.
that children are born dependent and are naturally in the process of
becoming independent, whereas adults and older children are independent
and far less changeable. The child with a congenital amputation or
congenital deformity requiring an amputation will adapt far better to a
missing limb than will an older child or adult who suddenly loses a
limb. In addition to their adaptability in the physical arena, they
will have far more adaptability in the psychosocial arena.
juvenile amputees. The congenital amputee has a difference; the
acquired amputee must adjust to a loss. The difference is dependent on
the age and the deficiency. A child born with bilateral amelia will
learn to use the feet for all activities of daily living, as will a
child with traumatic loss of both upper limbs at an early age. However,
bilateral upper-extremity loss in the older child who has functioned
with both hands for years will not be so well compensated for by use of
the feet.
amputation first presents, one of the first concerns has to be the
parents. The child does not need a doctor yet, but the parents do. The
issues around the child’s deficiency, especially if amputation or
surgery is required, are not urgent. However, the mental turmoil in the
parents’ lives is urgent. They did not expect to give birth to anything
but a healthy, normal child, unless forewarned by ultrasonography. They
are now there to see a doctor, with the certainty that their child is
anything but healthy and with the unrealistic desire for an expert to
make the deficient limb normal.
guilt. What did I take or do during my pregnancy to cause this? Even
worse, they may be wondering from which side of the family did this
affliction come. It is a time of anticipated joy that has turned into a
period of great stress for both the individuals and, often, for the
marriage. Taking time at the first visit and again looking for the
opportunity later to explain what is known about the cause of these
problems is essential. Obtaining a genetic consultation, even when the
deficiency is not known to be hereditary or caused by teratogens, can
be therapeutic in this regard. It is usually much more thorough than
what the orthopaedic surgeon can do and is done by an expert in that
particular area, giving the parents additional reassurance.
about the child’s future. Will he walk? Can he play sports? Will she
have children of her own? The parents probably have never known a child
or an adult with an amputation and have no frame of reference to answer
these questions. Although one may try to anticipate and answer many of
their questions, it is not possible to alleviate their fears through
conversation. The best one can do during the first visit of the parents
is to gain their confidence and give them realistic hope. Fortunately,
there are usually no emergent
decisions to be made, and there is time to help the parents answer these questions for themselves.
are a good time for the parents to meet children with similar
deficiencies and to see what their child might actually be like in the
future. This makes known the previously unknown, and the problems
become easier to deal with. In addition, the parents can see the
various surgical options that might be recommended for their child.
Because there is no need for intervention in the congenital deficiency
for a few months to a few years, the parents have time to learn about
their child’s problem. Introducing the family to other families who
have children with similar limitations is one of the most important
interventions. A recent study has demonstrated that although parents
benefit from the support of friends and health professionals, they do
not receive the level of support they need (25). This support is found by contact with other parents whose children have similar disabilities.
encouraged by well-meaning friends, many parents will ask about a
miracle cure—whether the doctor can sew on a new arm, for example. The
Internet can be both helpful and harmful and is used by virtually all
parents with a child with a limb deficiency. What the parents often
find may be more advertisement than information, and they usually have
difficulty placing their child in the correct context of the
information they read. Carefully listening to and explaining what they
have read or heard will usually suffice for most. Seeing other patients
and talking to their parents is also of great help because they are
parents who went through the same thing, and they likely asked the same
questions.
careful about what they tell the parents. In an effort to help the
parents feel better, although not knowing how to deal with this
difficult role, physicians may offer false hope and mention treatments
that are totally unrealistic. Physicians who do not know, or who do not
wish to take on this role, should assure the parents that they are
referring them to the best possible care rather than tell them not to
worry and that medical science has amazing cures today.
(as well as all members of the team) is important. In this regard, the
first thing the parents must be made to understand is that all the
decisions will be theirs. No one will make them do anything they do not
wish to do. In this regard, it becomes the role of the physician to
educate the parents through repetitive explanation and answering
parents’ questions. It is helpful if this process occurs not only with
the physician but also with the therapist and prosthetist, all of whom
should be working together. In fact, the parents will usually hear and
retain more information from a knowledgeable therapist or prosthetist
than from the physician. Again, nothing helps like seeing other
patients and parents.
It is important for the treating physician to recognize the factors
that affect their decision and to do his or her best to educate the
parents. Frequently, the child will have a near-normal appearing foot,
and all the parents see is a small amount of shortening. The child may
be able to walk in the first few years of life, and the parents may not
understand the progressive shortening that will develop. Along with
these observations, they share the popular public belief that modern
science can cure everything—next year, if not now. They have all heard
of miraculous lengthening of limbs in the popular press, and more
recently, the “successful” transfer of limbs. It can be difficult to
align the parents’ expectations with reality.
the child with a limb deficiency is more normal than abnormal. During
this first year, the parents need to resolve their disappointment and
loss, accept the child, and see the potential for the future. They need
to bond to the child and begin to think of the child as an independent
person. Most parents will begin to see their children this way as they
grow and develop. Again, this process can be accelerated by seeing
other children with similar problems. One recommendation that often
needs repeating to the parents is that children tend to acquire the
fears of their parents and that supporting any activity the child
expresses interest in will allow the child to develop his or her
natural abilities to the fullest.
considered a specialized area of practice. There are several reasons
for this. First, these anomalies are rare to any one practitioner
outside a limb-deficiency center. The experience of an orthopaedic
surgeon, therapist, or prosthetist who has treated several of these
different anomalies can be important. Second, all patients and parents
benefit by knowing they are not alone. In particular new parents, and
later their children, will benefit immensely by seeing other parents
and children like themselves. No amount of explanation, pictures, or
movies can educate parents so well as talking to other parents like
themselves and seeing children like their own.
prosthetist, a physical and occupational therapist, and a social worker
or child psychologist, all of whom are knowledgeable in normal
childhood development and who can anticipate the deviations that will
occur in development. The adult acquired amputees know what they had
and what they want. The child and parents of a congenital amputee know
little, and need education and guidance that usually cannot be provided
by an orthopaedic surgeon referring the patient to a prosthetist. The
parents will be making decisions for their child that the child will
live with for the remainder of his or her life, and they are
acutely
aware of this responsibility. The professionals caring for the family
must provide the necessary education and framework in which the parents
can make these decisions.
The child has a condition he or she will adapt to. This is not a
disease that will be cured. Hence, the condition should not be
“medicalized,” but rather treated within the context of family, home,
school, and play, not through clinic visits.
in a congenital limb deficiency remains relatively constant. This
principle has been established for congenital short femur (28,29,30), and fibular hemimelia (31,32).
Clinical experience indicates this to be true for the tibial hemimelias
also. It is important for the clinician and the parents to be aware
that this percentage difference will translate into significant
differences as the limb grows. Therefore, in discussing centimeters of
shortening and planning treatment, it is important to calculate what
the discrepancy will be at maturity.
constant, it is possible to calculate the ultimate discrepancy in
centimeters at an early age. Knowing the percentile height of the
child, the length of the femoral and tibial segments of the normal limb
can be estimated from the Green and Anderson growth charts (33) (Tables 31.2 and 31.3).
Then, knowing the length of the normal segments and the percentage by
which the affected segments are short, the length of the affected
segments at maturity can be estimated.
eventual discrepancy at maturity is clinically valid, the clinician
should be aware of the effect that surgical procedures could have on
the growth of the limb. Following amputation, the epiphysis of the bone
may not grow at the normal rate. Christi et al. showed that in 20 B-K
amputations in children, only three tibias grew at the expected rate (34).
The congenital group of tibias grew to 36% of what would have been
expected, and the acquired group grew to 53% of the expected level.
Various reasons for this may be the lack of stress across the growth
plate, the decreased blood flow to the bone, or the result of the
congenital insult that produced the limb deficiency.
limb-deficient child is best understood in the context of the purpose
of the amputation in an already limb-deficient child and the
development of the child. The amputation is done for two reasons: to
correct a severe discrepancy in length or to enhance prosthetic fitting.
designed to aid prosthetic fitting of congenital deficiencies, are
performed at the time the children are ready to walk, as indicated by
their pulling to stand. For children with tibial and fibular
deficiencies who will be treated with amputation, pulling to stand and
cruising are the signals that the child is ready to begin walking. This
would be the time for an amputation and prosthetic fitting, so that the
child can maintain a normal developmental sequence. In some unusual
cases in which the deformed extremity is interfering with crawling and
other prewalking activities, amputation may be performed earlier.
However, prosthetic fitting should wait until it will be of some value.
It is very difficult to keep a prosthesis on a crawling child.
usually be done when the child is ready to walk, but definitive
surgical treatment to permit a more functional prosthesis will be done
later for technical reasons.
the treatment of choice, but the difference in length must be
compensated for before lengthening at a later age. Occasionally, it
will be necessary to fit such patients with nonconventional prostheses,
when shoe lifts are not sufficient.
impression among both parents and surgeons that with early amputation
the child does not experience the loss in body image that accompanies
amputation at a later stage. Also important is that as a general rule,
the earlier the amputation, the better the adaptation of the child’s
neurologic plasticity to the alteration.
most common problem in juvenile amputees. Its occurrence is reported to
be between 4% and 35%, and depends on the cause of the amputation, the
age of the patient at the time of amputation, and the bone involved (35,36,37).
It occurs most commonly following traumatic amputation or elective
amputation through a bone. It is seen in congenital amputations because
of amniotic band syndrome but not in those due to failure of limb
development. It is not seen in amputations through joints (38).
Overgrowth occurs most often in below-knee amputations, with the
problem being present in the fibula more often than in the tibia, and
in transhumeral amputations. Recurrence is common and is felt by some
to be more common during periods of rapid growth when bone turnover is
high (e.g., adolescence).
responsible for bone overgrowth. Aitken disproved these theories when
he demonstrated by implanting metallic markers that the overgrowth took
place distal to the end of the bone (35,39).
The new bone is formed by periosteal and endosteal bone. Overgrowth
results from the typical process of wound contracture as has been
demonstrated by Speer (40). Following
amputation through a bone, the periosteum continues to grow. As it
grows over the end of the bone, it grows over the open medullary canal,
where it contracts and is drawn into the canal from which it can
continue to grow, producing the overgrowth at the end of the bone.
|
TABLE 31.2 GIRLS: LENGTHS OF THE LONG BONES INCLUDING EPIPHYSESA
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
prosthetic use. An antalgic gait with decreased stance time may be
noticed. Decreased range of motion, to limit pulling
of
the skin at the end of the limb, is an additional symptom. Clinically,
the patient presents with tenderness and pain at the end of the
residual limb. There may be inflammation, bursal formation, or the bone
end may be protruding through the skin. Commonly, the bony spike can be
palpated within a small, tender bursa. Careful medical supervision can
often anticipate the problem, which in turn allows the patient to plan
for surgical correction. Prosthetic adjustments may help delay surgical
revision, but will seldom be sufficient with significant overgrowth.
|
TABLE 31.3 BOYS: LENGTHS OF THE LONG BONES INCLUDING EPIPHYSESA
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
overgrowth, various plastic and metallic devices have been used to cap
or plug the end of the bone. However, these techniques have been
abandoned because the results were not as good as with the use of
biologic material (41,42). Marquardt reported in the mid-1970s, in the German literature, on the
capping of the bone end with a cartilage-bone graft. More recently,
Tenholder et al. reported comparable results with the use of a
polytetrafluoroethylene felt pad (43).
Various reports for both acquired and congenital amputees indicate
generally favorable results, with most revisions being for technical
reasons (41,42,44,45).
prosthetic modification and other conservative measures should be used
first unless a bony spike is felt. If revision surgery is necessary, a
capping procedure should be considered. In the case of a primary
amputation, it is advisable to use available parts from the amputated
portion of the limb to cap the end of the bone, if conditions permit.
The most common procedure is the use of the proximal fibula to cap the
tibia. As in any revision, adequate resection of the bone is essential
to provide a healthy soft-tissue envelope. (Fig. 31.2).
educate the child and parents in edema control to hasten return to
prosthetic use. In addition, range of motion and strengthening
exercises hasten, and may even be necessary to regain, full function of
the prosthesis. Users of myoelectric prostheses may need readjustment
of their electrodes and, for a while, may have difficulty activating
the prosthesis. This is because of swelling and reshaping of the limb,
which may alter the optimal sites for electrode placement.
amputation, the residual limb will be too short for satisfactory or
comfortable prosthetic fitting. In such cases it may be possible to
lengthen the residual limb. Watts has written an excellent review of
this subject and reports his experience with 32 limbs in 27 patients (46). Alekberov et al. report on six patients who had successful lengthening of 3.4 to 8.4 cm in congenital below-elbow segments (47). The remainder of the literature to date is largely case reports.
complications, and careful consideration needs to be given to the
potential benefits versus the possible complications. Skin problems are
frequent and often limit the length to be gained. Tissue expanders
generally do not provide the solution. Free tissue flaps may be used
when skin coverage is
inadequate.
Free flaps often remove sensation from the end of the residual limb,
and especially in the upper limb this can affect the function of the
limb both with and without the prosthesis. The child will be without
the use of the prosthesis for a prolonged period, an especially
difficult problem when the lower extremity is involved.
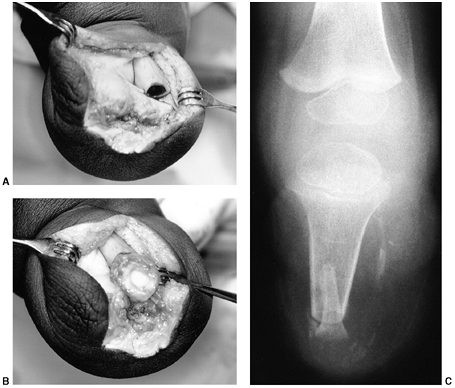 |
|
Figure 31.2 A, B:
The end of the tibia with the bony overgrowth removed and the head of the fibula inserted into the medullary canal of the tibia (Marquardt procedure). C: The anteroposterior view of the tibia 6 weeks after the procedure. |
Silas Weir Mitchell in the middle of the 19th century. He described
these sensations as replicas of the lost limb, some being painful and
some not. Phantom sensation of the limb is often described by patients
as the feeling that they can move the part, tell how the part is
positioned, or feel it itching or tingling. Phantom pain, however, is
perceived by the patient as just that—painful. It often is the same as
the pain before an amputation or may be cramping, shooting, burning, or
of any other characterization.
limbs do not have sensations of them; nor do these children experience
the phantom pain or phantom sensation seen in the acquired amputee (48). Recent reports call this commonly accepted truism into question (49,50).
Whatever this pain is, those who care for children with limb
deficiencies will recognize that these children do not have the same
problem as the true chronic phantom pain seen in adult amputees.
at least 20% of children with congenital limb deficiency and in 50% of
those who underwent amputation before age 6 (50).
In addition, 20% of the congenitally deficient group described the
sensations as painful, whereas 42% of the acquired amputees described
them as painful. To explain the phenomenon of phantom limb in a child
who has never had a limb, Melzack et al. have proposed that there is a
genetically or innately determined neural network that is distributed
in the cortex (not focal), which is responsible for the representation
of the limb, even though the limb bud development was not normal.
associated, and in general seen to be associated with, other pains;
such as, headache, bone, or joint pain (49,51).
The phantom sensations may occur frequently or rarely. They are often
triggered by a wide variety of stimuli. Feeling nervous or happy, not
wearing a prosthesis, being cold, or being ill are frequent triggers.
Fortunately, these sensations do not interfere with the child’s usual
activity, and most say they just try to ignore the sensations (52).
phantom limb pain, most likely because there is usually not one single
cause. Because many of these problems resolve with prosthetic
alterations or physical therapy modalities, a multidisciplinary
approach has proven to be the best intervention in evaluating and
properly treating the phantom limb phenomenon when it becomes a problem.
A heavy, tight shrinker, either worn inside the prosthesis or when the
prosthesis is off, may provide relief. Physical therapy interventions,
including weight bearing and graduating pressures such as tapping,
rubbing, and massage to the residual limb, have been reported to give
temporary or permanent relief. Rubbing and massaging the uninvolved
limb at similar points to those in which they are experiencing the
phantom limb sensation may provide relief. Various physical modalities
have been utilized in the treatment of phantom sensations in children,
including transcutaneous electrical nerve stimulation (TENS),
biofeedback, ultrasound, and the physical agents of heat and cold (54).
with phantom pain following an amputation, gabapentin (Neurontin,
Park-Davis) has proven a useful medication for some patients.
longitudinal deficiency that is either partial or complete. But this
does little to accurately portray the spectrum of deficiency that is
seen.
To be useful, a classification should guide treatment or aid in
prognosis. As treatment changes, it may be reasonable to expect that
classifications change.
radiographic appearance. Maffulli and Fixsen describe total aplasia of
the fibula and a forme fruste of the same condition in which the fibula and tibia are short to varying degrees (58,59). A more specific classification, which is probably the most widely used today, was proposed by Achterman and Kalamchi (31) (Figs. 31.3, Figs. 31.4, 31.5).
They correlated the classification with the discrepancy in length, and
recommended treatment on the basis of the classification.
classification on the basis of the functionality of the foot and the
limb-length discrepancy as a percentage of the opposite side (60). As they point out, however, the problem is picking the correct treatment for the individual patient.
fibular deficiency, including the parents’ desire, it remains unlikely
that classifications will provide anything more than a rough guide and
a method of comparing patients in different reports. In addition, the
protean effects on the femur, tibia, knee, ankle, and foot demonstrate
the difficulty of classifying this deficiency accurately by the
radiographic appearance of the fibula.
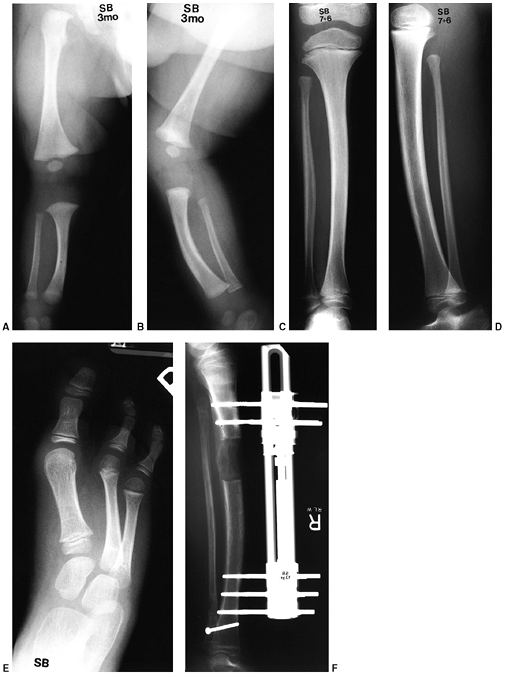 |
|
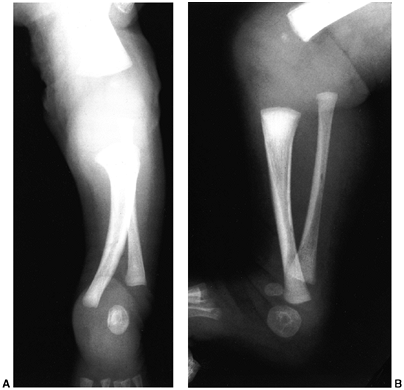
Figure 31.3 A, B:
The radiographs of a 3-month-old boy with type IA fibular deficiency of the Achterman and Kalamchi classification. Although the bones may seem normal to the casual observer, the proximal fibula is short. C, D: Anteroposterior and lateral radiographs of the same patient at the age of 7 years, 6 months. The shortening of the fibula is more apparent and the ball-and-socket ankle joint is easily seen. E: The foot at the same age, with the lateral two rays missing. F: At 13 years of age, the leg was lengthened by 7 cm. |
indicative of the widespread abnormality that may be present, along
with hypoplasia or absence of the fibula, which gives the deficiency
its name.
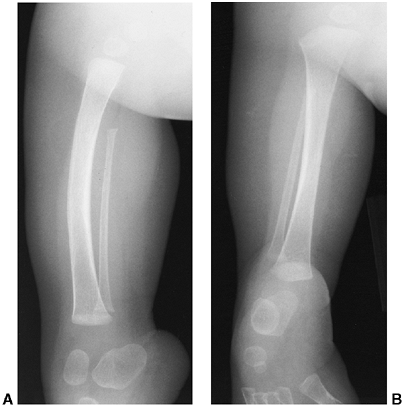 |
|
Figure 31.4 A, B:
Type IB fibular deficiency (Achterman and Kalamchi), in which the proximal fibula is missing. This type is often associated with proximal focal deficiency, as in this child. |
especially in the first year of life, can vary from practically normal
to severely deformed. However, the typical limb is characterized by a
rigid valgus foot, often with one or two lateral (postaxial) rays
missing, a shortened leg and (often) thigh, a valgus knee, and variable
anterior bowing of the tibia with a dimple over the apex (Fig. 31.5 E, F). Further examination will demonstrate an anteroposterior instability of the knee along with a small patella.
shortened in relation to the tibia. This may occur either proximally or
distally or both (Fig. 31.3). Often a portion of the fibula is absent in part (Fig. 31.4) or in its entirety (Fig. 31.5).
In those cases in which the fibula is of normal or near-normal length,
the diagnosis can be difficult during the first year of life.
Radiographically, the condylar notch of the femur is shallow and the
tibial spines are small.
 |
|
Figure 31.5 A, B:
Anteroposterior and lateral radiographs of a type II fibular deficiency (Achterman and Kalamchi), in which the entire fibula is missing. Note the missing lateral rays of the foot and the severe angulation of the tibia. C, D: The limb, 6 weeks after Syme amputation and an anterior closing-wedge osteotomy of the tibia. Placing the pin through the anterior cortex of the proximal fragment provides rigid fixation, which is not obtained if the pin is simply passed up the medullary canal. The pin was removed in the office at the time of cast removal. E, F: The clinical appearance of the same deficiency at the time of surgery in another patient. Note the short tibial segment, the valgus knee and foot, and the dimple over the tibia. |
reported femoral deficiencies in almost two-thirds of their patients.
Kalamchi noted that 70% of type I and 50% of type II deficiencies were
associated with shortening or deformity of the femur (62).
In some cases, the degree of valgus is more severe than can be
explained by the smaller lateral femoral condyle alone, and its
recurrence after correction speaks of a more dynamic cause.
deficiencies, but by skeletal maturity there is usually a valgus
deformity. The classic appearance of the ball-and-socket ankle joint in
these deficiencies is seen in Figs. 31.3 C, D. There is disagreement about the origin of this abnormality. Some authors feel that it is congenital (67), whereas others feel that it develops secondary to the tarsal coalitions (68).
If it is caused by the tarsal coalition, it is difficult to explain its
absence in tarsal coalitions without fibular deficiency. In slightly
more severe cases, if the fibula is present it is short and may not
reach the level of the ankle joint. The distal tibial epiphysis shows a
triangular appearance.
coalitions are present in most of the feet associated with fibular
deficiency. Grogan et al. noted such coalitions in 54% of anatomic
specimens, although the abnormality could be seen radiographically on
only 15% of the specimens because of the cartilaginous nature of the
coalition (69). The missing lateral rays are easily seen without radiographs.
are the limb-length discrepancy and the deformity and instability of
the foot and ankle. It is very important to realize that the
discrepancy will become worse with growth, and it is the ultimate
discrepancy at maturity that is important.
In reaction to the results of these early attempts to save the limbs,
several reports emphasized the advantages of amputation for severe
cases (32,71,72,73).
The indications are based primarily on the difference in length and the
functionality of the foot. Wood et al. recommended amputation for: a
discrepancy of 3 inches or more at the time of decision, or if
predicted at maturity; for a nonfunctional foot; for a limb that would
have severe cosmetic or functional problems; or for children who cannot
endure the psychologic trauma of repeated hospitalizations and surgery (73).
These recommendations were reaffirmed in a later publication from the
same institution, following up many of the same patients (32).
to which length can be restored, reflecting improvements in limb
lengthening. Westin et al. suggested amputation for any discrepancy
that would be greater than 7.5 cm at maturity (32). For Letts and Vincent, the number was greater than 10 cm (26), and for Hootnick et al. it was between 8.7 cm and 15 cm (74).
somewhat more acceptable alternative, the improved ability to lengthen
limbs has also made limb salvage a more feasible option. The
recommendations of Birch et al. are an effort to account for these
changes (60). They would recommend amputation
for those with a nonfunctional foot, regardless of leg length, unless
the upper extremities were nonfunctional. For those with a functional
foot, but a leg-length discrepancy of 30% or more, amputation would be
recommended. For those with a functional foot and a discrepancy of less
than 10%, epiphysiodesis or lengthening is reasonable. There is little
disagreement about these indications today.
regarding treatment lies, and the greater the discrepancy in length,
the greater the controversy. According to Birch et al., those patients
with a functional foot and a discrepancy between 10% and 30% are
candidates for either amputation or lengthening (60).
The parents, who are the decision-makers, are weighing the hope for
their child to retain the limb against what that will entail. They most
likely have never seen a child or adult with an amputation; they
visualize something horrible. At the same time they cannot really know
what a lengthened limb will be like at the end of treatment; they
imagine the limb will be normal. Although they may understand that they
will need two or three lengthening procedures, they cannot know what
the impact will be on their child or their family, what complications
they will encounter on the way, or how their child will look or
function at the end of the treatment.
lengthening in fibular deficiencies with discrepancies greater than 10
cm. These preliminary reports, using the Ilizarov methods, deal mainly
with the extent of length achieved, often before maturity, but with
little information on cosmetic and functional result (75,76,77,78,79).
what amount of length is required. The combined femoral and tibial
length for a girl of average height at maturity will be approximately
80 cm (33) (Table 31.2).
A 10% discrepancy would be approximately 8 cm, a 20% discrepancy would
be 16 cm, and a 30% discrepancy would be 24 cm. To achieve greater than
10 cm of length in a congenital limb deficiency with anteroposterior
knee instability, ankle instability, foot deformity, and congenitally
short soft tissues is a significant undertaking (79,80,81).
few and incomplete, but begin to give an appreciation of the problems
associated with lengthening severe deficiencies (55,82,83,84).
These reports conclude that lengthening should be reserved for those
with more normal feet and less discrepancy in length, although early
Syme amputation is the best treatment for the more severe problems.
Herring gives a philosophical perspective on the dilemma (85).
Birch et al. reviewed a series of adults who were treated with Syme
amputation in childhood. These authors conducted physical examination,
prosthetic assessment, psychologic testing, and physical performance
testing, and commented that the results of multistaged lengthenings for
this condition would have to match these results to be justified (86). They currently offer lengthening to patients whose limb-length discrepancy is 20% or less.
problems are the foot deformity, the discrepancy in length between the
two limbs, and the overall shortening in height because of two short
limbs. Without extenuating circumstances (e.g., nonfunctional upper
extremities), disarticulation of the foot and prosthetic fitting is the
best solution. For those children with nonfunctional upper extremities
who will use their feet for many of the activities of daily living,
amputation of the feet is not an option.
usually little discrepancy between the two limbs, but rather a
discrepancy between their height and what their normal height should
be. As they enter into their peer group this becomes an increasing
problem. This problem is most easily solved by the prosthetist. If
there is a significant difference between the length of the two limbs
that cannot be solved by prosthetic adjustment, lengthening of the
short limb becomes an attractive option.
the deficiency on one side is mild. The focus on the severe deficiency
distracts the examiner. The lesser deficiency will become apparent with
time, however, causing disappointment and loss of confidence.
seems to have been accepted for adults before it was accepted for
children, and its use in boys was advocated before its use in girls
because it was said that the Syme amputation produced an unsightly
bulkiness around the ankle. This resulted in many children receiving a
transtibial amputation rather than a Syme amputation. It was
subsequently learned, however, that the ankle does not enlarge
following amputation in a young child, and the cosmetic appearance is
excellent as the child grows.
amputation, rather than transtibial amputation, although only as a last
resort (70). Subsequent reports by Kruger and Talbott (72) and Westin et al. (32)
not only confirmed the advantages of the Syme amputation in both boys
and girls but also advocated its early use for severe deficiencies.
Several studies now confirm the value of Syme amputation (66,72,85,88,89,92).
the ability to bear weight on the end of the residual limb. One of the
major complications of the Syme amputation is migration of the heel pad
off the end of the residual limb. This is particularly true in
congenital limb deficiencies, in
which
the heel may be on the back of the tibia, making repositioning of the
heel pad on the end of the limb difficult or impossible. Although
suggestions to remove a piece of the Achilles tendon, suture the
extensor tendons into the anterior portion of the heel pad, or fix the
heel pad with a Kirschner wire may each have their place, most authors
agree that migration of the heel pad does not produce any
insurmountable problem for the patient or the prosthetist (65,88). Most other problems in patients with Syme amputation are caused by other effects of the underlying disorder (65,66,88,90).
Syme amputation in children, Herring et al. examined the functional and
psychologic status of 21 patients with a Syme amputation (65).
They noted that family stress was the factor that had the greatest
influence on the patients’ psychologic functioning, and that children
who had the amputation after several failed attempts at salvage were at
considerable risk for emotional disturbance. Green and Cary found that
patients were able to function at the average levels for their age
group, and the authors did not find that adolescents were less likely
to participate in athletics (93). In summary,
these studies indicate that Syme amputation may be compatible with the
athletic and psychologic function of a nonhandicapped child.
In the Boyd amputation, the talus is excised and the retained calcaneus
with the heel pad is arthrodesed to the tibia. The surgery was
initially devised to avoid the complication of posterior migration of
the heel pad seen in some children with Syme amputation. However, the
main complication of the Boyd amputation is the migration of the
calcaneus if arthrodesis is not achieved. This requires an additional
surgery, which is often conversion to a Syme amputation. An advantage
of the Boyd amputation is that with the retained calcaneus, the heel
pad tends to grow with the child, rather than remaining small as in the
Syme amputation. The Boyd amputation also adds length. This can be a
problem when children who do not have significant shortening of the
limb are fitted for various prosthetic feet and may require a shoe lift
on the normal side.
compared the two surgeries, and found the migration of the heel pad to
be the only complication in the Syme amputation, whereas the Boyd
amputation had more perioperative wound problems and migration or
improper alignment of the calcaneus. In the authors’ clinic, all of the
children, even bilateral amputees, can and do walk on the ends of their
residual limbs, either with or without the heel pad in place. If the
heel pad migrates posteriorly, it may alter the weight-bearing aspects
of prosthetic fitting, especially as the child gets older.
advantages to having the deficient limb shorter. The first is the
ability to make a narrower and more cosmetic ankle in the prosthesis.
The second is that some shortening, rather than additional lengthening,
makes it easier to fit more durable, energy-storing feet. In fibular
deficiencies, length is never a problem, because these limbs are always
short.
from nonexistent to severe. Severe bowing is usually seen in the more
severe deficiencies with complete absence of the fibula. Westin et al.
reported this to be of little consequence (32).
However, observations in the authors’ center have shown this to be a
frequent prosthetic problem, requiring osteotomy during the first
decade.
the weight-bearing axis that passes through the knee. If the foot is
placed at the distal end of the tibia (which the parents want for
cosmetic reasons), the ground reaction force places a large moment
through the toe-break area, leading to premature failure of the foot
component and skin problems caused by abnormal pressure. The problem is
then blamed on the foot component or the prosthetist.
significant bow at the time of Syme amputation. A small anterior
incision, removal of an anterior-based wedge of the tibia, and fixation
with a temporary Steinmann pin placed up through the heel pad solves
the problem and does not result in any delay in prosthetic fitting (Fig. 31.5).
When correction of the bow is necessary in older children who are
already in their prosthesis, the authors have preferred the Williams
rod technique, because this speeds resumption of prosthetic wearing.
known as a complication of fibular deficiency. It is one of the major
problems seen in the gait of children with this problem. At first, it
is merely cosmetic and can be accommodated with prosthetic alterations.
However, if it becomes more severe, it will increase the forces on the
lateral compartment and make good alignment impossible.
Initially, it was thought to result from tethering by the fibular
anlage; however, release of this lateral band does not prevent or
lessen the deformity (29).
anterior flexion along with the valgus, and attributed the problem to
an abnormality in growth in the lateral and posterior portions of the
proximal tibial physis (32). This problem is different from anterior bow in the diaphysis of the tibia.
the height of the lateral femoral condyle that was not present prior to
walking (64). There was a suggested relation
between the extent of anteromedial bowing of the tibia and subsequent
decrease in height of the lateral femoral condyle. They suggested that
tibial osteotomy might prevent the changes in the lateral femoral
condyle and correct the anteromedial bowing. If the deformity was
present in the lateral femoral condyle, they suggested temporary
stapling of the medial femoral condyle, since osteotomy has a very high
recurrence rate unless performed near the end of growth. The authors’
experience indicates that it is not as simple as this and that the
recurring nature of the valgus following good correction of alignment
suggests other causes of this problem.
fibular deficiency will require efforts to realign and stabilize the
ankle. There is renewed interest in this subject with attempts to
lengthen the leg.
stability to the foot in the absence of the fibula. Serafin gives the
first report of the technique in the English literature, and recounts
the various attempts at bone grafting and other procedures that were
described before Gruca developed his technique (95).
longitudinally. The medial segment is displaced proximally with the
talus, leaving the lateral fragment as a lateral buttress. Thomas and
Williams describe the early results in nine patients treated with this
procedure. The follow-up is short and the evaluation of function
incomplete (96). The surgery has not been widely used and would seem to have little to recommend it.
logical plan in conjunction with leg lengthening, but there are no
reports on its outcome. It is likely that this would also require
release of all of the tendons crossing the ankle joint to prevent foot
deformity. Drift of the foot through the physis or the fusion itself
with lengthening and over time seems a possibility.
type IA deficiencies, usually require no treatment. The authors have,
however, seen several children with increasing valgus during
adolescence or following leg lengthening who become symptomatic with
normal athletic activity. They have been successfully treated by a
Wiltse osteotomy of the distal tibia.
treated differently than management of a comparable Syme
disarticulation in an adult after trauma. In the child, the prosthesis
is designed to accommodate growth and to help stabilize knee laxity and
hyperextension through socket design and alignment. Emphasis is placed
on socket alignment and minimizing rotational forces acting on the knee.
to take all of the weight on the end of the residual limb, as intended,
all of the weight on the patellar tendon and flare of the proximal
tibial condyles, as in a transtibial amputation, or both. As the child
grows, it is a good idea to begin to shift some of the weight bearing
to the proximal structures to prepare the child for the time when full
weight bearing on the end may not be possible. Failure to shift weight
bearing proximally with age usually leads to problems with fittings
resulting from tolerance issues. This discomfort probably arises from
the small distal weight-bearing surface seen in many of the
congenitally deformed limbs. This is an important consideration in the
bilateral amputee, in whom disproportionate weight shifting to the
sound side is not possible for comfort.
condyles will be small at the time of amputation, will not grow to
normal size, and therefore, do not need to be trimmed as in the adult.
The brim of the socket is designed with supracondylar medial and
lateral trim lines, in an effort to control any knee valgus instability
and/or patellar instability. The type of suspension will depend on the
size of the distal end of the residual limb. If it is very large, an
obturator or window may be necessary. With further growth, the distal
end may not be sufficient for suspension, and a different design will
be necessary. These are discussed later.
are older and large enough to take advantage of them, it is necessary
that at least 4 cm of space be available at the distal end. If the
prosthetist is to offer the latest in technologic advances in
components, greater residual limb-length differences will be required.
This need can be anticipated, and an arrest of the distal or proximal
tibial and fibular physes performed at the appropriate time. This
length differential is usually not a problem in children with
congenital limb deficiency, because the deficient limb will usually be
shorter than the other limb. It is relevant in children with acquired
deficiency treated by Syme or, more often, Boyd amputations. Although
the longer lever arm of the Syme amputee tends to compensate for the
lack of more elaborate prosthesis components, when fit is possible,
they can be an advantage.
deficiencies, both based on the radiographic findings. Anatomic studies
of available specimens have not proven helpful in classification (97,98).
Type II is the absence of the distal tibia, with a proximal portion
that forms a relatively normal articulation with the femur (Fig. 31.7). Type III is distal tibiofibular diastasis (Fig. 31.8).
tibial deficiency of Kalamchi and Dawe into 1a and 1b. In neither is
the proximal tibia visible radiographically at birth. In type 1a it is
actually absent and without an extensor mechanism, whereas in type 1b
it is present as a cartilaginous remnant that will later ossify,
suggesting that the extensor mechanism is intact.
unusual variant with a diaphyseal and distal remnant of tibia but no
proximal tibia, as type 3. The diastasis of the distal tibiofibular
joint is type 4 in this classification.
the presence of active knee extension, which implies an adequate active
quadriceps muscle and insertion on the tibia. This usually depends on
the presence of a proximal tibial segment. Because this proximal
portion of the tibia may be present, but not be visible, in the Jones
type 1b
deficiencies, some authors have recommended direct surgical exploration, sonography (100),
or magnetic resonance imaging to detect its presence. This should
seldom be necessary because it is the extension power to the tibial
segment that is important, and not the presence of a tibial segment. A
good radiographic clue is that in patients who have a proximal portion
of the tibia, the distal femoral condyle is wider and the ossification
of the epiphysis is better than if it is not present.
 |
|
Figure 31.6 A, B: Radiographs of an infant with a type I tibial deficiency of Kalamchi and Dawe (99) (type 1a of Jones et al.) (120),
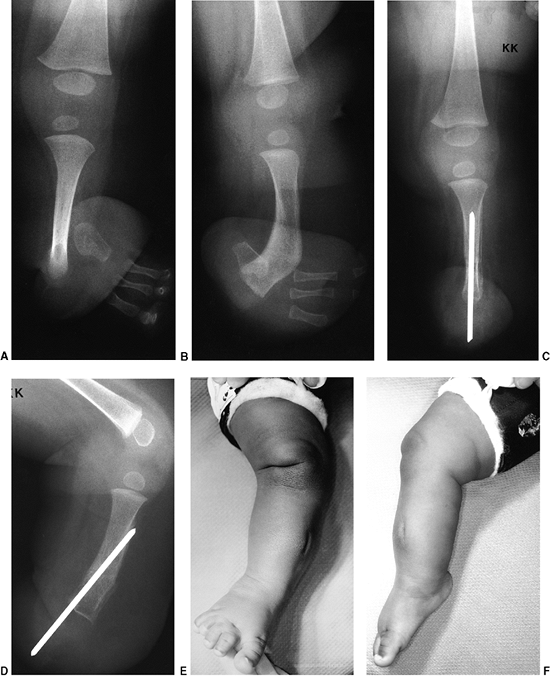
in which the entire tibia is absent. There was no extensor mechanism and no proximal tibia. If there were a proximal remnant of tibia that would later ossify, this would be a type 1b deficiency of Jones et al. C: The clinical appearance, with the medial deviation and severe equinus of the foot and the absence of any tibial structure below the distal femur. |
 |
|
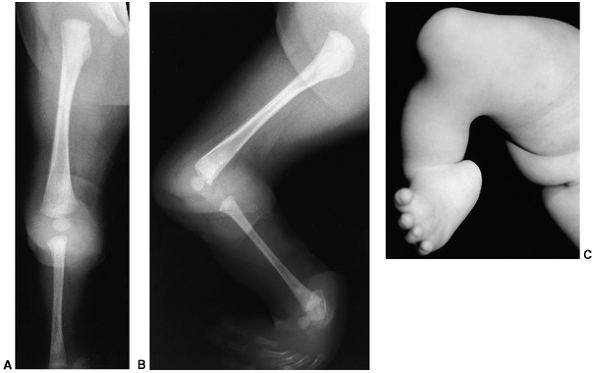
Figure 31.7 A:
Anteroposterior radiographs of a 4-month-old child with type I tibial deficiency of the right leg and type II deficiency of the left leg. B: On the right leg, he underwent a Brown procedure. Despite the favorable radiographic appearance 4 years after surgery, he developed a severe valgus/flexion deformity, and subsequently had a knee disarticulation on this side. On the left leg, he underwent a synostosis of the fibula to the tibia and a Syme amputation. It is best to excise the proximal remnant of the fibula when performing this procedure, because the continued growth of the proximal fibula produces a large prominence that will interfere with prosthetic fitting. This was resected at the time of his right knee disarticulation. |
 |
|
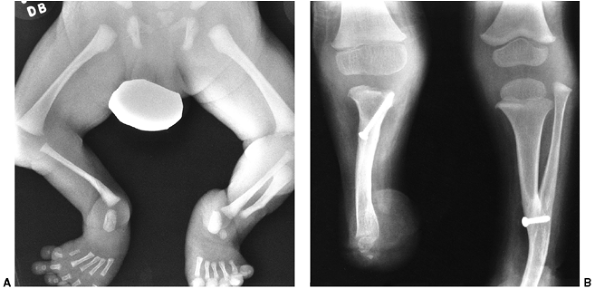
Figure 31.8 A, B:
Anteroposterior and lateral views of tibial deficiency of type III of Kalamchi and Dawe, or type 4 of Jones et al. This is sometimes referred to as a diastasis of the ankle mortise. Notice the shortened tibia and the disruption of the normal relation between the tibia and the fibula. |
association with fibular dimelia. Kumar and Kruger summarized the
sporadic reports until 1993 and presented the findings, associated
anomalies, and treatments in six patients (101).
In this deficiency, the tibia is absent and there is a duplication of
the fibula. There is a high incidence of other anomalies, including
visceral anomalies, in these patients. These authors recommended knee
disarticulation if the femur is of normal length, and fusion of the
fibula to create a sufficient lever arm if there is associated PFFD.
deficiency is a markedly shortened tibia with a rigid
equinovarus-supinated foot pointing toward the perineum (Fig. 31.6C).
Preaxial polydactyly is characteristic of tibial deficiencies, although
absence of the preaxial rays can also be seen. The fibula is relatively
long. Other congenital limb anomalies will frequently be seen in
association with tibial deficiency.
of type, frequently have other associated abnormalities, frequently
musculoskeletal (102,103). The incidence of associated anomalies is reported to be between 60% and 75% (99,104,105).
Although most of these anomalies are in the musculoskeletal system,
there may occasionally be problems in other organ systems (106).
Although it is not necessary for the orthopaedic surgeon to know all of
these syndromes, he or she must be aware of the need for a thorough
examination of the affected children, and of the high potential for
genetic transmission of the disorder (108,109). These patients should probably have formal genetic counseling.
1a of Jones is knee disarticulation. Without the presence of active
knee extension, there is no possibility for reconstruction of the leg.
Notwithstanding this common orthopaedic principle, there have been
attempts to centralize the fibula under the femoral condyle.
is commonly known in the United States, is the centralization of the
fibula under the femur. It was Brown’s recommendation that fibular
centralization be done only with active extension. Apparently, there
are occasional deficiencies in which some part of the extensor
mechanism inserts into the fibula. On reviewing the literature on the
results of Brown’s procedure, it is not apparent that this is always
present before surgery. The surgery has now been evaluated in several
clinical trials (102,103,113,114,115,116,117).
This procedure is distinct from synostosis of the fibula to a tibial
remnant, a point that may not always be clear in reports on the subject.
against it, preferring the early function obtained with knee
disarticulation (102,103,114). Loder (118)
examined 87 cases from the literature using the minimal requirements
for a good result, as suggested by Jayakumar and Eilert, of acceptable
gait, active knee motion of 10 degrees to 80 degrees of flexion,
varus/valgus instability less than 5 degrees, and no flexion
contracture (119). He found that 53 of the 55
cases of Jones type 1a deficiency treated by Brown’s procedure had a
poor result because of flexion contracture. This echoes the reported
experience of most others, and emphasizes the need for strong, active
knee extension, which is usually not present without a remnant of the
proximal tibia (102,113,114,116,117). Simmons et al. were satisfied with the results from their evaluation of Brown’s procedure (115). Their satisfaction was based more on the patients’ feelings than objective assessment.
However, it might be a wise clinical decision to consider quadriceps
function to be at least inadequate, if not absent, if it cannot be
observed during the first year of the patient’s life by an experienced
physician and therapist.
radiographically invisible cartilaginous remnant of the tibia is
present, it is important to assess it over time for ossification and
development, as well as to verify good active extension. It is possible
that this remnant will be present, but good active extension will be
absent or the remnant will not ossify. If there is active extension in
a child, but the remnant is not sufficiently ossified by 1 year of age,
the surgeon may choose to attempt to transfer the fibula to the
unossified segment or perform a Syme amputation, fit with a prosthesis,
and wait for ossification before performing the transfer.
deficiency with a very short limb, the best option may be to arthrodese
the fibula to the distal end of the femur. The goal of this procedure
is to increase the lever arm of the femoral segment, for the same
reasons that a knee fusion is performed in children with PFFD.
tibial remnant will ossify and form a satisfactory joint. In these
cases, it is usually best to create a synostosis between the existing
fibula and the tibial remnant to increase the length of the lever arm.
A Syme amputation is performed at the same time, and the patient is fit
with a below-knee prosthesis. When performing the synostosis, it is
important to achieve good alignment of the fibula in relation to the
knee joint. The residual proximal fibula should be removed to avoid
problems later with prosthetic fit.
and Dawe) are very unusual, and there is not much published experience.
Jones et al. reported one case that was bilateral (120).
They described a cartilaginous portion of the tibia, proximal to the
ossified portion, which was “under voluntary muscle control.” Their
patient was treated with excision of the proximal fibula and Syme
amputation. Fernandez-Palazzi et al. had two cases in their report.
Both were treated with Syme amputation, implying that there was an
active quadriceps mechanism (121).
classification) presents a unique problem. At birth, the foot is
deformed, often appearing like a clubfoot to the inexperienced. In
addition, the amount of tibial shortening that will result is not
apparent. All of this makes it difficult for the parents to accept
amputation. The difficulty for the surgeon is that this deformity is a
spectrum of deformity. Garbarino et al. have emphasized the distinction
between a short tibia with a varus foot and a true congenital diastasis
of the ankle joint (122). The former is usually amenable to reconstruction according to Schoenecker (105), whereas the true type 4 deficiency with diastasis of the ankle joint usually is treated with amputation (120,123).
deficiencies, but in general the follow-up is short and the problems of
a plantigrade foot and limb-length discrepancy are just beginning in
these patients (122,124,125,126). One patient followed up to the age of 15 years is described as having satisfactory ankle function and 6.5 cm of shortening (127),
while another followed up to the age of 10 years (6 years after
reconstructive surgery) is reported as having a stable ankle and
plantigrade foot, but projected limb-length discrepancy is not
mentioned (128).
al. reported on ten patients with Jones type 4 deficiencies, of which
nine had initial reconstruction of the foot. A Syme amputation was
subsequently done in six of them, usually at the parents’ request. Of
the four who retained the foot, two had contralateral deficiencies in
which the prosthesis accommodated the length discrepancy. One had a
lengthening of 4.6 cm and one remained 4.8 cm short.
to attempt to retain the foot, if the deformity is at the less severe
end of the spectrum, or if there is a significant contralateral
deficiency. In most other cases, Syme amputation seems most reasonable.
several prosthetic approaches to management. In children with type I
tibial deficiency who have been treated with knee disarticulation and
have a flare at the condyles, the prosthetic socket consists of a
nonischial weight-bearing design with rotational control achieved
through the intimate fit of the distal end of the socket over the
femoral condyles and a well-formed gluteal impression. Suspension is
usually achieved with the use of a segmented liner or bladder design
that allows the wider condyles to pass through, while maintaining
pressure over the femur just proximal to the condyles.
the need to fit with a transfemoral socket, rotational control is
achieved through proper contouring of the socket relative to the
femur—the musculature surrounding the femur has a slight triangular
shape in a cross-sectional view, with a flatter contour on the lateral
surface, especially proximally. This allows a locking of the
musculature which, with proper socket fit, decreases rotation. In
addition, a silicone sleeve suspension may be used in conjunction with
a pull-through strap to secure the liner. If all other procedures fail,
a standard Silesian belt (around the pelvis) may be utilized. The total
elastic suspension (TES) belt offers excellent suspension and
flexibility of form, and it aids in control of the prosthesis. However,
the Silesian belt and TES will interfere with grooming and toilet
training.
for children, there are differences of opinion as to when young
children are able to handle an articulated knee. Traditional
established practice is to first fit the child with a locked knee, and
allow an articulating knee at approximately 3 to 5 years of age. In
contrast, Wilk et al. (129) advocate the use of
articulating knees in children as young as 17 months. Children as young
as 11 months can be appropriate candidates for articulated knees (128).
The children learn how to handle the knee very quickly, and there is
very little need for any type of device to temporarily stabilize the
knee. The use of a knee joint at this stage permits more normal
development, allowing bent-knee sitting, side sitting, crawling and
kneeling on hands and knees, and easier pull to a stand. With a
pediatric knee, children can reduce or eliminate a circumducted gait
pattern.
preserved or the fibula has been joined to the tibial remnant, a
modified transtibial prosthesis or a Syme prosthesis is utilized.
Unlike the standard transtibial design, the socket will incorporate
supracondylar and suprapatellar proximal brim lines that will aid in
the control and stability of the knee and prevention of a
hyperextension moment, respectively. In some instances in which knee
stability is less than optimal, outside joints and a thigh cuff or
lacer may be required. These are used as a last resort and often
contribute to increased weakening of the musculature as a trade-off for
increased control and alignment.
These classifications range from attempts to unify all radiographic
defects of femoral development to a simple two-part classification
based on limb-length inequality. Some classifications are radiologic,
some functional, and some are designed to suit the authors’ preferred
treatment. In addition, no classification is able to account for the
length, radiographic, and muscle abnormalities, all of which are
important in the treatment and outcome.
It divides the true PFFD cases into four categories on the basis of the
radiographic findings. It is important to keep in mind that in PFFD, as
in other congenital deficiencies, the bone may be late in ossifying and
therefore may be present but unseen on radiographs. Also, these
different groups are not distinct, but rather form a continuous
spectrum.
absent, but will later ossify, and its presence is indicated by a
well-developed acetabulum. The subtrochanteric defect will eventually
ossify, establishing bony continuity, although usually with
considerable varus deformity. The location of this varus deformity in
the subtrochanteric region, rather than the femoral neck, is what
distinguishes PFFD from congenital coxa vara.
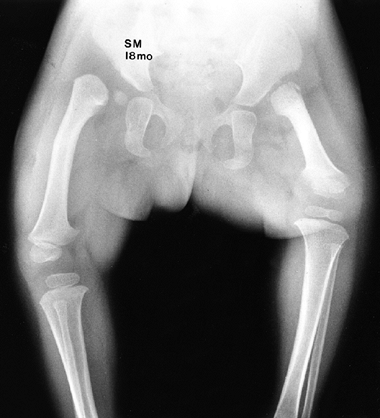 |
|
Figure 31.9
Anteroposterior pelvis of an 18-month-old child with bilateral proximal femoral focal deficiency (PFFD). The right hip is an Aitken class A and demonstrates the presence of the ossific nucleus and a good acetabulum. The femoral metaphysis lies above the level of the ossific nucleus. There is a cartilaginous connection between the metaphysis and the femoral head, which will usually ossify by skeletal maturity, but often with a significant varus deformity. The opposite hip is an Aitken class C PFFD. This patient demonstrates the difficulty with limb-length difference in some patients with bilateral PFFD. |
The femoral head is present, although its ossification may be delayed.
There is usually a bony tuft on the proximal end of the femoral shaft.
The defect will not heal spontaneously, and the proximal end of the
femur will be above the acetabulum.
The femoral shaft is shorter than in class B, and the entire proximal
portion of the femur, including the trochanters, will not appear.
often with only a tuft of irregularly ossified bone proximal to the
distal femoral epiphysis (Fig. 31.12). The lateral pelvic wall is flat, without hint of an acetabulum.
classifying femoral deficiencies into three groups for purposes of
treatment. Group A are those with congenital short femur indicated by
clinical hip stability, lack of significant knee flexion contracture,
and the foot of the affected extremity lying at or below the midpoint
of the opposite tibia. These patients may be candidates for limb
lengthening. His group B patients include those classified as Aitken
classes A, B, and C, whereas his group C represents the Aitken class D
patients. He recommends lengthening for his group A patients, and
prosthetic treatment for his group B and C patients.
bifurcated (not duplicated) femur. In this condition, the femur has two
distal ends, which form a “Y” (135). There is
always an associated Kalamchi and Dawe type I tibial deficiency without
active extension, and treatment is by knee disarticulation and removal
of the segment of femur in poorest alignment.
recognized. It will be bilateral in 15% of the cases. The femoral
segment is short, flexed, abducted, and externally rotated. The hip and
knee joints exhibit flexion contractures. The proximal thigh is bulbous
and rapidly tapers to the knee joint. Fibular deficiencies are so
common in association with PFFD that the valgus foot and other
characteristics of fibular deficiency are almost a part of PFFD (Fig. 31.13). PFFD is associated with fibular deficiency in 70% to 80% of cases (136). In addition, approximately 50% of the patients will have anomalies involving other limbs (130,136).
bulbous thigh and short femoral segment. Pistoning may be apparent
because of associated hip instability. The knee is always unstable in
the anteroposterior direction.
problems the patient experiences. The most obvious of these is the
shortening of the limb. Less obvious is the problem with hip function
and its relation to alignment of the limb. Because of the flexed and
externally rotated femoral segment, the knee remains flexed, and the
leg and foot are anterior to the axis of the body (Figs. 31.13 and 31.14D).
With or without skeletal hip stability, there is a deficiency of the
muscles around the hip, resulting in a significant lurch to shift the
center of gravity in single-leg stance. The knee will have varying
degrees of instability. The function of the foot will depend on the
severity of any associated deficiencies of the leg, for example,
fibular deficiency.
of PFFD than in that of almost any other congenital limb deficiency.
Fortunately, most of these decisions can be postponed until 2½ to 3
years of age, because this is the best age to perform these surgical
options. Before this age, several important decisions need to be made.
The first is whether the child is a suitable candidate for limb
lengthening.
may be judged to be suitable for lengthening if the predicted
discrepancy at maturity is not greater than 20 cm, the hip is, or can
be, made stable, and there is a good knee, ankle, and foot. In such
cases, multiple staged lengthenings can be planned. The timing and
staging of these procedures depends on the choice of the physician, but
will usually not start before the age of 3 years. Although it is
possible to obtain 20 cm length, there are as yet no good reports on
the functional outcome of such lengthenings in patients followed up to
maturity and into adulthood.
at maturity, or for any other reason lengthening is not chosen as a
treatment, a decision should be reached about the best approach to
prosthetic fitting.
unilateral PFFD is, as in other congenital deficiencies, to parallel
normal development. Therefore, when the child is ready to stand,
regardless of the treatment planned for the future, he or she is fitted
with a prosthesis to equalize the leg lengths and permit standing and
walking. The prosthesis is often called a nonconventional or extension prosthesis. It is designed to fit the extremity without any surgical modification to it (Fig. 31.14).
The flexion, abduction, and external rotation of the proximal segment
(the femur) are accommodated in the alignment. Although it is customary
to omit a knee joint, all efforts should be made to incorporate an
articulated prosthetic knee or outside knee joints. The level of the
prosthetic knee as compared to the contralateral side is of no
consequence. The benefit is to allow flexion and ground clearance and
thereby reduce compensatory patterns (128).
developmental functions: one-half kneel, squat, pull-to-stand, and
climb on toys and furniture. The authors have found that
infants
who are fitted with knees learn very rapidly to extend their hips to
control knee extension throughout all their movements. Less vaulting
and fewer deviations on the nonprosthetic side are also noted if a knee
joint is placed within the prosthesis. There is no knee joint in the
prosthesis if the length of the limb is too long to fit a prosthetic
foot that includes a knee.
 |
|
Figure 31.10 A:
Anteroposterior radiograph of the pelvis and limbs of a newborn girl with Aitken class B proximal femoral focal deficiency. Note the short femoral segment and the well-developed acetabulum, although the femoral head is not visible. B: By 5 years of age, the femoral head is ossified and the cartilaginous connection between the femoral head and the subtrochanteric region of the femur has undergone considerable ossification. However, a pseudarthrosis persists and a significant varus deformity has developed. C: The femur after correction of the varus with a spike type of osteotomy. D: The result 1 year later. Now faced with a projected discrepancy of 20 cm, the parents elect a van Nes rotationplasty. This was done with part of the rotation through the knee arthrodesis, and the remainder through the tibia. E: The radiographic result. The patient had one additional derotation performed through the midtibia at the age of 10 years. |
 |
|
Figure 31.11 The anteroposterior pelvis and limbs of a newborn boy (A) and at 3 years of age, just before surgery (B),
with Aitken class C proximal femoral focal deficiency. Note the very short femoral segment and the lack of acetabular development. The same patient is seen in (C) at the age of 12, following a Syme amputation and knee arthrodesis with preservation of the proximal tibial physis. There is still no appearance of a proximal femoral ossific nucleus. |
for the young child. However, as the child grows older, the limitations
of this prosthesis soon become apparent (Fig. 31.14D).
The continued flexion, abduction, and external rotation of the femoral
segment result in limb alignment anterior to the body’s axis, along
with the hip instability and the flexed knee, which is difficult to
contain within the prosthesis; all these are factors that make a very
poor lever arm to move the prosthesis. The foot frequently lies at the
level of the midcalf of the opposite limb, making the placement of the
knee joint less than ideal. The primary goal of surgical correction of
these problems is to make the limb a more efficient lever arm for the
prosthesis. The secondary goal is to provide a more cosmetic
prosthesis. Among the usual surgical choices are knee arthrodesis,
amputation of the foot, rotationplasty of the limb, and reconstruction
of the hip. Additional possibilities are iliofemoral arthrodesis, with
or without rotation, and prosthetic fitting without modification to the
limb. Thigh reduction by surgical resection and liposuction are of
great value in prosthetic fitting as the patient grows older.
different. In these children, the feet should be preserved and knee
fusion is not indicated. This is because these
children
will spend most of their lives walking without prostheses. The two
biggest problems in these children are foot deformities and unequal
limb lengths. Surgical release of the foot and long-term orthotic use
can usually provide for a useful foot. Limb-length discrepancy, when
significant, is more difficult because of the problem of shortening the
child more, on the one hand, and the difficulty of lengthening these
limbs, on the other. No firm recommendation can be made regarding this,
and each case should be decided on its own merits.
 |
|
Figure 31.12
Anteroposterior radiograph of the pelvis and femur of a boy aged 1 year and 5 months with Aitken class D proximal focal deficiency. There is little femur present, and no sign of acetabular development. He underwent knee arthrodesis and Syme amputation at 2½ years of age. |
children with PFFD. It creates a single, longer, and more efficient
lever arm, which is easier to contain within the prosthesis. This will
greatly enhance prosthetic function and reduce energy consumption.
Within 6 months of knee fusion and prosthetic fitting, the abduction
and flexion deformity at the hip joint will correct, thereby aligning
the limb under the axis of the body.
limb as a whole, it is usually desirable to remove at least one of the
growth plates at the knee at the time of fusion. This is usually the
case in Aitken class A, B, and C deformities. If this is not done, the
limb will remain too long for fitting of a suitable knee joint at
maturity. In some cases, depending on the length, it may be advisable
to remove both or neither growth plates (137). Calculation of the anticipated length of both limbs at maturity by means of the Green-Anderson growth charts (Tables 31.2 and 31.3), as described earlier, will help with the answer.
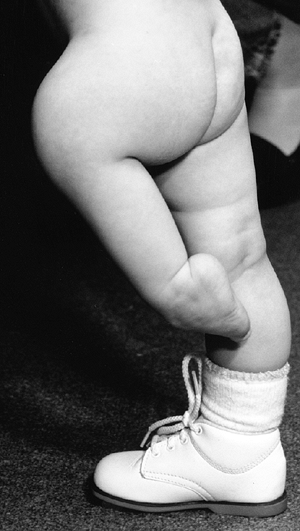 |
|
Figure 31.13
This photo of a 12-month-old girl who is pulling to the standing position demonstrates the clinical features of proximal femoral focal deficiency: a very short and bulbous femoral segment, which is flexed, abducted, and externally rotated. |
and 3 years of age. After excision of the epiphysis and physis from one
side, usually the femur, and the joint surface from the other, the
femur and tibia are fixed with a rigid rod, for example, a Rush rod
placed from proximal to distal. This is supplemented with a spica cast.
The patient is usually ready for prosthetic fitting in 6 weeks and for
ambulation as soon as the prosthesis is ready.
in most situations. One reason is length—the new lever arm, consisting
of the femur and the tibia, needs to be short enough to accommodate an
internal knee joint when the child is older. The foot adds unnecessary
length. In addition, as the foot grows, it becomes increasingly
difficult to accommodate in a cosmetically acceptable socket. The
advantages and disadvantages of the Syme and Boyd amputations have been
discussed. Amputation is best performed at the time of knee fusion.
the tibia, or a combination of both. The goal is to have the ankle of
the short limb at the level of the knee on the long limb at maturity.
The foot now functions like the residual tibia in a below-knee
amputation, thereby allowing the patient to function more like a B-K
amputee than one with a knee disarticulation (Fig. 31.15).
An obvious prerequisite is reasonable function of the foot and ankle.
Because this procedure is also used in the treatment of both malignant
skeletal tumors around the knee and traumatic loss of the knee with its
corresponding growth plates, it is important to keep in mind that these
patients are very different from those with PFFD. Although some lessons
from one group are applicable to the other, care must be taken.
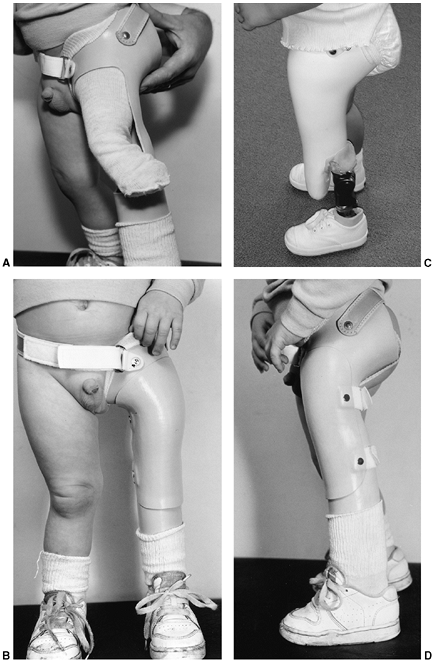 |
|
Figure 31.14
The nonconventional or extension prosthesis allows the child to “stand” on the prosthesis, extending his limb to the floor and accommodating the deformity. A, B: The nonconventional or extension prosthesis without a knee joint, which is usual. It is possible to add a knee joint to the prosthesis. C: The children rapidly learn to control the knee joint. D: Lateral view of the patient with the prosthesis, demonstrating why this prosthesis is not a good long-term solution. The knee is at the brim of the socket, is poorly contained, and provides a very poor lever arm. The weight-bearing line of the leg remains anterior to the axis of the body; the flexion and external rotation of the hip persist. |
for three cases of congenital deficiency of the femur. Initial reports
of rotationplasty for treatment of PFFD by Kostuik et al. (140) and Torode and
Gillespie (141) have been followed by more recent reports by Friscia et al. (142) and Alman et al. (143).
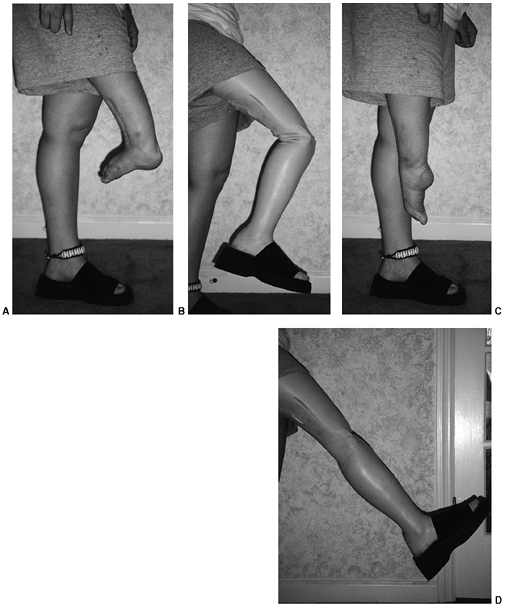 |
|
Figure 31.15
The results of a van Nes rotationplasty are seen in this 17-year-old girl with proximal femoral focal deficiency (PFFD) (same patient as in Fig. 31.10). With the ankle rotated 180 degrees, dorsiflexion of the ankle (A) results in flexion of the prosthetic knee (B), and plantar flexion (C) results in extension of the prosthetic knee (D). |
to achieve sufficient rotation at surgery or subsequent derotation with
growth. Kostuik et al. (140), in one of the
earliest reports on this procedure, recommended waiting to perform the
surgery until the child was older. However, this delays the procedure
many years beyond when the patient can learn to use the prosthesis and
benefit from the procedure. Because the complication is easily treated,
there seems to be little reason to wait. Subsequent reports have not
found this to be so great a problem.
of concern to patients, parents, physicians, and prosthetists is the
cosmetic appearance. It appears, however, that this problem is
overrated by medical staff, compared to the patients themselves. The
procedure has been more widely used and accepted in Canada than in the
United States,
except
in large limb-deficiency centers. Alman et al. found no difference in
the perceived physical appearance of children treated with
rotationplasty, compared to knee arthrodesis and Syme amputation (143). In the report of Friscia et al. (142),
one patient subsequently had a Syme amputation at the parents’ request.
Two recent studies evaluating the quality of life in patients who had
rotationplasty for sarcoma treatment demonstrated that although
physical function was less than that in healthy peers, psychosocial
adaptation and life contentment were about the same (144,145).
This emphasizes the importance of proper preparation of the parents and
of the patient, if she or he is old enough. This is best accomplished
by seeing other patients with a rotationplasty, along with the use of
videos of patients, teaching dolls, and so on.
sufficiently normal to serve as a knee. This is particularly important,
because up to 70% of children with PFFD will also have a fibular
deficiency on the same side. Although some valgus alignment of the foot
and ankle can be compensated for in the prosthetic alignment, the
deformity may tend to become greater with age. Severe valgus and
equinus deformities, with a deficient foot, are contraindications to
the procedure.
ankle strengthening, in particular, because these are the structures
that will power the new knee joint. Equinus position should be
emphasized, because this will place the foot in the best mechanical
position. Children who have mild equinus contractures of 30 degrees or
less will usually stretch these out with prosthetic use, and do not
need special attention preoperatively. Crutch training should be done
preoperatively, as in all elective surgery that will require crutch use
postoperatively.
compared to other procedures, has been documented both for patients
with tumor (146,147,148) and for those with PFFD (143,149).
These studies demonstrate that those with rotationplasty function
better than those with knee arthrodesis and Syme amputation, not quite
so well as those with a below-knee amputation, and not as well as those
who have rotationplasty for noncongenital conditions, for example,
tumor. Those with rotationplasty for noncongenital conditions probably
do better because of the normal hip function that remains one of the
major problems in those with PFFD.
prosthetic fitting will have hip instability. This is not only because
of the deficient bony anatomy, but also the deficient musculature. This
has resulted in some controversy about the value of surgical procedures
to stabilize the hip. Some feel that nothing of functional value is
gained and surgical intervention is not warranted (136,137,150), whereas others feel that surgical correction can be of value (28,151,152,153).
It is the authors’ opinion that in Aitken class A and B patients who
have a femoral head within the acetabulum, surgical correction of the
pseudarthrosis with correction of the varus deformity is beneficial (Fig. 31.10 B,C,D).
and consequent malalignment, and the bony stability of the
femoral-pelvic articulation. In those patients for whom lengthening is
planned, it is necessary to obtain good containment of the femoral
head, which may require an acetabular procedure. In these patients,
retroversion and varus are usually present, and should be corrected
prior to lengthening.
pseudarthrosis of the femoral neck. This can be repaired while, at the
same time, restoring more normal alignment. It may not be necessary to
wait until complete ossification of the femoral neck to perform this
procedure (154). Ossification may accelerate after realignment.
described. These procedures are an attempt to address the problem of
hip instability.
arthrodesis of the distal femoral segment to the pelvis in the region
of the acetabulum in four patients. The femur was flexed 90 degrees so
that it was perpendicular to the axis of the body. This results in knee
extension being equivalent to hip flexion, and knee flexion being
equivalent to hip extension.
described a rotationplasty in conjunction with iliofemoral arthrodesis.
In this procedure, the distal end of the femur is rotated 180 degrees
before it is joined to the ilium with its axis in line with that of the
body. The knee now functions as the hip joint, and the ankle now
functions as the knee joint, as in a van Nes rotationplasty.
significant problems in achieving an arthrodesis, and the distal
femoral segment cannot be allowed to grow too long. Additional surgical
procedures are to be expected. As yet, there are only very limited
reports on the functional advantages (155,156).
prosthetic fitting difficult in some patients and results in discomfort
for others. This problem usually manifests itself during adolescence
and can be resolved by thigh reduction with a combination of surgical
excision and liposuction.
with PFFD begins with the extension or nonstandard prosthesis, with or
without an activated knee joint (Fig. 31.14).
With the foot positioned in plantar flexion, the limb is cast proximal
to the hip joint, and the prosthesis fabricated with a prosthetic foot
positioned under the shortened limb. The socket, with ischial
containment,
has been called a “ship’s funnel” design because of the resemblance to
the engine air intake funnels of ocean vessels. This drastic socket
design is necessary because of the flexed hip and knee that must be
contained within the socket while attempting to gain ischial support.
equalize the length between the prosthetic and the sound limb, in
preparation for early ambulation, while affording time for surgical
decisions. There are four indications that have been identified
relating to the fitting of nonstandard prostheses (157):
-
When the patient is still too young for surgical conversion.
-
When the patient or parent refuses surgical intervention, and a prosthesis is necessary for ambulation.
-
In bilateral cases, when extra height or better balance is the goal.
-
When there is lower-extremity
involvement, combined with bilateral upper-extremity absence, requiring
the feet for activities for daily living (ADL).
chosen, the prosthesis resembles a knee disarticulation prosthesis,
except for the need for ischial weight bearing and high lateral brim
containment to aid in hip stability. Weight bearing is divided between
the ischium and the distal heel pad. Full distal weight bearing would
severely compromise hip function over a period of time, because of the
inherent instability of the hip with possible proximal migration of the
femur. Prosthetically, fusion of the knee with correction of the
angular deformities results in improved gait and ease of fitting
because of the single skeletal lever arm (158).
During growth, the child should be evaluated periodically for the
relative length of the two limbs so that, if needed, distal femoral
epiphysiodesis can be performed. This will allow fitting of an optimal
knee joint when the patient is fully grown, while maintaining the knees
at the same level.
than the opposite femoral segment, external knee joints may be used. As
the child grows, an internal four-bar knee can be used. More about the
indications and selection of knee joints is discussed later in this
chapter.
difficulty with prosthetic management. Movement within the prosthesis,
at the level of the anatomic knee, and the increased need for an
intimately fitted socket, foster a decreased stride length and
increased pelvic movement. However, in the child with an Aitken class D
PFFD and only a remnant of distal femoral epiphysis in which knee
fusion will have little to offer, this may be a suitable choice.
prosthesis with the ankle functioning as the new knee. This is a very
difficult prosthesis to align and fit, although it gives excellent
function (142,159). The
prosthesis has a lower padded foot socket that contains the rotated
foot in full plantar flexion. Lateral and medial external joints are
attached to the upper thigh section to increase stability and to
prevent hyperextension of the lower shank (159).
The original design incorporated a laminated thigh section with ischial
weight bearing. For patients with good hip stability, for example, in
those who had a tumor and trauma, the laminated section is often
replaced with a leather thigh lacer and no ischial weight bearing. It
is imperative for proper function that the external joints be aligned
with the axis of rotation of the ankle/subtalar complex, while
maintaining the line of progression. Failure to ensure this alignment,
regardless of the anatomic joint, will result in a poor gait pattern
and skin breakdown. The prosthetist should incorporate mechanical joint
placement with slight external rotation on a new prosthesis, in
anticipation of the mild internal derotation inevitable during growth.
lower-extremity amputations have been discussed previously. One
additional difference requires emphasis here: Upper-extremity
amputations are very visible. The hand is one of the most noticed parts
of the human body. Therefore, unlike the child with a below-knee
amputation who walks without a limp and can often match his or her
peers in physical activity, the child with an upper-extremity
amputation is more easily seen as different.
the upper extremity that are transverse in nature. The longitudinal
deficiencies, for example, radial club hand and the other classic
anomalies that are confined to the hand, are discussed in Chapter 23.
be treated with prosthetic fitting, if they are treated at all. An
upper-extremity prosthesis is a way to improve the ability of the
deficient limb to assist the intact limb in its activities; it does not
replace the function of the missing part as adequately as it does in
the lower limb.
substitute, lies in the function of the hand, with the rest of the limb
used to position the hand. In addition, the upper limb, or a
prosthesis, also plays a role in support, balance, and trunk stability.
In these latter functions, the prosthesis can do well, but in the main
function of prehension, it functions poorly when compared to the normal
hand. A prosthesis has no sensory feedback, which requires that the
child watch the prosthetic hand in use. This, plus the thought and
mental practice that goes into making a mechanical device work, makes
the upper-extremity prosthesis a far less efficient tool than the
lower-extremity prosthesis.
prescription. If the prosthesis does not afford the child a functional
gain or cosmetic benefit, he or she will be quick to reject it. The
prosthesis must aid the child in some function he or she wishes to do,
that is, an age-appropriate function that requires the use of two
hands. The reasons for some children becoming good users of a
prosthesis, whereas others with the same characteristics reject it, are
not well understood. Although the parents’ acceptance and compliance
are important, this is not the whole answer. The inability of the
prosthesis to substitute for a normal limb, and the hand in particular,
is also a partial explanation. These facts, coupled with the incredible
ability of the young child to learn to use one hand assisted by the
residual limb with minimal concession to activities that are assumed to
require two hands, must also be a significant factor.
very specific to the particular patient, and difficult to quantify. The
hours of use alone is not a good criterion. Many children will use the
prosthesis for specific tasks (riding a bike), and prefer to remove it
for others (swimming). Some children will wear it very little during
the summer while playing, but will wear and use it every day in school,
when different bimanual motor tasks are a significant part of the
activity.
what he or she actually does with it in normal activities can be very
different. Although some children develop an amazing facility with the
prosthesis in their everyday activities, many will demonstrate their
skill with the prosthesis only in the medical setting while they
continue to use the prosthesis much as they would their residual limb,
and not as a prehensile tool, during daily activities. Standardized
tests have been developed to measure spontaneous use versus voluntary
control as it relates to age-appropriate activities. The University of
New Brunswick test of myoelectric control is used by therapists to
assess the child’s ability to use the prosthesis in a controlled
situation. The Prosthetic Upper Extremity Functional Index is a
self-reported measure of the child’s functional abilities during daily
activities.
functional purposes, the importance of the hand in appearance must be
remembered. Besides the face, the hand (or its absence) is the most
readily noted feature of the body. Good cosmetic hands can be of great
help to children, especially adolescents, who desire them, and their
benefit to the child should not be minimized because of their
nonfunctional nature.
harder it is to replace the function with a prosthesis, and the less
will be the patient’s acceptance of it. Lack of heat dissipation,
weight, energy expenditure, concentration necessary to work it, and
lack of functionality are all reasons for children with more proximal
deficiencies to be less likely to use a prosthesis.
and manipulation of objects. Early in infancy, the upper extremity
reaches and touches objects within the visual fields and provides a
rich sensory feedback to the child. This sensory feedback is an
essential element of upper-extremity function. For the child with a
limb deficiency or high-level amputation, especially if bilateral,
sensation seems to be the single most desirable attribute of the
extremities. Therefore, if the upper extremities meet in the midline,
the child will usually reject a prosthesis. If the extremities will not
meet, or sometimes, when they meet where they cannot be seen, the
patient may prefer a prosthesis for the function it affords.
more individualized than a lower-extremity prosthesis. For those with a
unilateral below-elbow amputation, fitting with a passive hand at
approximately 4 to 6 months of age is an easy decision because it is
relatively inexpensive, well tolerated by the patient, and helpful in
deciding on fitting with a more complex prosthesis later. However, in
cases of high-level amputations, especially if they are bilateral, such
a program of routine fitting will frequently result in failure (6).
of the child. By 4 months of age, the child brings the hands to the
midline while supine, and props on the elbows while prone. Eye—hand
coordination develops as the hands are brought into the visual fields.
By 6 months, the child is beginning to prop on the extended arms when
sitting, and is rolling in all directions. Early prosthetic fitting
between 4 and 6 months allows the infant to incorporate the prosthesis
in all gross and fine motor functions that are developing.
primarily use adaptive performance techniques with their mouth, chin,
neck, shoulder, and feet. Therapeutic interventions should first focus
on promoting these techniques, and secondarily on adapting the
environment to assist the child in age-appropriate activities. Attempts
should not be made at this point to modify the child with prosthetics.
Children with high levels or complete absence of the upper limbs will
use their feet to accomplish everyday two-handed activities. The use of
the foot in play, and in ADL appropriate for the child’s stage of
development should be incorporated in all therapy home programs. It may
take considerable persuasion to win the parents to this view.
amputations should probably be given an opportunity for prosthetic use.
In addition to possible functional gains, the families experience a
significant benefit in knowing that all has been tried, and the
patients gain a valuable experience in having tried prostheses. A
multicenter review of bilateral upper-limb deficiencies showed that 50%
of patients were still wearing a prosthesis at age 17 years or more (6).
Fitting in such children should rarely be attempted before 1 year of
age, despite the parents’ anxieties. Fitting should be done to help the
child perform appropriate tasks for his or her stage of development, or
to aid in certain specific
activities.
It is usually best to fit a child with only one prosthesis at a time
because the problems with two may lead to early rejection.
less likely to fully accept a functional prosthesis than those with
lower levels of amputation. If body-powered components are used, the
patient has difficulty in controlling the devices because there is no
lever arm. Externally powered prostheses are heavy. The weight and
increased body heat due to the necessary suspension make this a
difficult prosthesis to wear. When coupled with the problems of
function in using an artificial shoulder, elbow joint, and hand, the
child will usually choose to function without the prosthesis.
affected bilaterally. The choices for these children are to help them
develop their lower extremities to substitute for the upper
extremities, to fit them with prostheses, or to attempt a combination
of both. There is universal agreement that there is no place for
attempting to limit the child’s use of the feet or attempting to
provide all of the child’s upper-extremity function with prostheses.
The feet are the best substitute for the hands. Children with bilateral
amelia and relatively normal lower extremities can usually master all
ADL, while leading full lives with family, children, and employment.
Until the physician becomes acquainted with a child or, preferably an
adult, with bilateral amelia, she or he cannot understand the extent to
which the legs and feet can substitute for the arms and hands. Most of
these children will reject prostheses.
the child with bilateral amelia and significant lower-extremity
anomalies that limit their substitution for upper-extremity function.
In such cases, unilateral fitting may be indicated, but is likely to
gain limited acceptance, and then only after many years of struggle.
They will need help in pushing to stand. In addition, the fear of
falling, because they cannot protect themselves, comes early. Helmets
or some protective headgear are needed until the child is independent
in gait. Therapy is directed at trunk control and strength, along with
training in the use of their feet for all activities. These children
are also prone to develop a progressive scoliosis, often before
adolescence. This presents a difficult problem. Bracing restricts the
use of their feet in activities of daily living. Surgical fusion does
the same. However, often these curves will require fusion, and in such
cases, as short a fusion as possible should be done.
appear to attach directly to the body. There are wide variations in
this deficiency. In some, the hands may be close to normal, with some
remnants of the arm bones, whereas in others the hand may be no more
than a single functionless digit with little residue of the arm.
Patients with phocomelia usually have some mobility in their residual
limbs, and therefore differ in one significant way from the child with
bilateral amelia—they have a sensate limb often capable of some
grasping function. The function of these limbs depends on the function
of the hand, the length and function of the arm itself, and the ability
to bring the hands together at the midline or to the face.
They should be encouraged from an early age to develop their foot
skills and the body strength that is necessary to use the feet. In
those children with better function and length, little else than
adaptive equipment to aid in dressing and so on may be necessary (Fig. 31.16E).
and limbs and to strengthen any muscle power in the residual digits may
prove of benefit. Adaptive equipment to aid in the use of the residual
limbs can be very useful for some activities such as feeding, dressing,
and so on. The residual limbs can manipulate switches for powered
prostheses, and therefore, the temptation to find for a prosthetic
solution to their problem arises. However, like the child with
bilateral amelia, these children will function in most activities by
substituting foot function for what they cannot do with their upper
extremities. Fitting the older child with a unilateral prosthesis for a
specific function may be indicated, but routine wear is not common.
Although some children with unilateral above-elbow amputation will
develop surprising facility with a prosthesis, they will often wear it
only for specific activities, such as sports, or for cosmetic reasons.
If there is a humeral segment of any reasonable length, the
humeral-thoracic pinch provides useful assistance for the normal
opposite extremity. Prosthetic use will be in accord with the patient’s
functional needs, and will generally relate to specific tasks. These
may be as limited as riding a bicycle or full-time use at school. These
patients should be offered the opportunity for prosthetic fitting.
deficiencies are more inclined to use their own body, rather than a
prosthesis. These children may benefit more from assistive devices than
from prostheses. Such patients will often prefer prosthetic fitting on
one side, where they use the humeral-thoracic pinch, with intact
sensory feedback on the other side and in their feet. Again, it is
important to make the distinction between the congenital and acquired
amputee. The congenital amputee will be less inclined to
use prostheses, and the traumatic amputee will be more inclined.
 |
|
Figure 31.16
A child with bilateral amelia or bilateral phocomelia, when the hands cannot meet in the midline, will use the feet for most activities. In such children, the use of the feet should be encouraged and developed from an early age (A). When older, these children will need the use of their feet to accomplish the activities of daily living (B, C). If there is any motor power in the extremities, they may be capable of useful function and assist the feet (D). When the hands can meet in the midline and have good motor power, excellent function is possible (E). |
deficiencies, Marquardt has recommended an angulation osteotomy of the
humerus (160). The osteotomy angles the distal
3 to 5 cm of the humerus anteriorly by 70 to 90 degrees. This allows
for suspension of the prosthesis without the usual shoulder cap, makes
it easier to put on, permits better shoulder motion, and gives better
control of rotation. This procedure should only be performed if the
humeral length is sufficient and there is a need for a unilateral
prosthesis.
of all of the upper-extremity deficiencies. It is more often the left
arm (Fig. 31.17). It is sporadic and without
known cause. Children with this deficiency present the most ideal
upper-extremity deficiencies for prosthetic fitting. Children with this
deficiency are unlike those with a transverse amputation through the
carpal bones, which usually have partial grasping function with
sensation. They are also unlike the above-elbow deficiencies in that
they have a normal shoulder and elbow that allow accurate placement of
a relatively light prosthetic terminal device.
are ideal candidates for prosthetic use, not all children will remain
good prosthetic users during their childhood. Scotland and Galway
reviewed the experience at the Ontario Crippled Children’s Center, and
found that 32% had stopped using their prosthesis, upon follow-up of 7
to 17 years (161). How many of these may resume use of the prosthesis in the work environment is unknown. They, like Brooks and Shaperman (162), noted greater acceptance of the prosthesis if fitting was done before the age of 2 years.
 |
|
Figure 31.17 A:
A typical patient with a congenital below-elbow amputation. There is usually enough length to fit a prosthetic arm and still permit good active elbow motion. B, C: Two different children with transverse incomplete forearm deficiency fitted with a myoelectric-powered hand performing common functions of childhood. Many of the children who use the prosthesis develop amazing skills in its use. |
22% of those fitted before 2 years of age, and 58% of those fitted
after, had stopped using their prosthesis. However, of those who
continued to use their prosthesis, there was no difference in the
extent of use between the two groups. The most common age at which
patients discontinued use of their prosthesis was at 13 years, and the
most common reasons they gave were that the prosthesis was cosmetically
unacceptable and that they could do everything without it. Sorbye (163)
reported that, of the patients in their clinic who were younger than 24
years, 87% were using their myoelectric prosthesis, and 65% of these
used it all day and for all activities. Hubbard et al. (164),
reporting on the Toronto experience, found that 70% of the below-elbow
amputees were using their prosthesis, whereas 30% had rejected it.
practice to recommend fitting around the age of 4 to 6 months with a
passive hand to aid in normal development. It is in this age range that
the child brings his or her hands to midline
while
supine, props on the elbows while prone, and then props on extended
arms while sitting and rolling over. This lightweight prosthesis is
used to have the child become comfortable with a prosthesis, and to
acquaint the child with the two-handed activities that a normal child
would perform, in an effort to develop the central cortical pathways
for bimanual dexterity.
passive prosthesis and the choice of a terminal device, a more
functional terminal device is fit between 15 and 18 months (27,165). Today, there are a number of terminal devices available (166).
There are two choices to power the device: battery (myoelectric) and
body (cables). Although there will be many factors to consider in the
selection (cost and funding, clinic philosophy, and parent choice),
virtually all centers today in North America are fitting most children
with myoelectric-powered terminal devices (Fig. 31.17). Table 31.4
compares the advantages and disadvantages of myoelectric and
body-powered terminal devices for the child with a congenital
below-elbow amputation.
common deficiency of the upper limb and occurs in a characteristic
pattern, with some or all of the proximal row of carpal bones
preserved. The existing flexion of the carpals on the radius allows for
limited grasping function, which along with normal sensation, makes
this an assistive hand for which no prosthesis can substitute (Fig. 31.18).
Occasionally, children will benefit from a volar opposition post for
certain activities. They will usually wear it only for certain tasks,
for example, as a guitar pick adapter or to grasp the handle bars on a
bicycle.
|
TABLE 31.4 COMPARISON OF MYOELECTRIC WITH BODY-POWERED PROSTHESES
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
have much more difficulty with the cosmetic aspect of their deficiency
than do those children with transverse amputations at higher levels. In
the older child and adolescent, it may be wise to explore the desire
for a cosmetic hand that would be used in certain circumstances or
would provide a psychosocial benefit. Some cosmetic hands have a
passive spring grasp to provide limited function.
The Ballif arm (circa 1400) was the first body-powered prosthesis to
introduce the use of prosthetic hand operation by transferring
shoulder movement to activate the terminal device (167).
A harness over the contralateral shoulder is connected with a thin
cable and housing to a terminal device. Through the use of scapular
abduction, the fixed cable is stretched over a greater distance and
causes the prosthetic hook or hand to open or close, depending on the
configuration of the terminal device. A good analogy is the braking
system on most bicycles. Most parents prefer the cable-operated
prosthetic hand over the cantered hook for cosmetic reasons.
Unfortunately, the hook is far superior in function, but has fallen
from favor because of the desire to have the prosthesis look as natural
as possible, even at the sacrifice of function. Most hooks are canted
in design, and this allows a full field of view of the device while
using it, compared to the mechanical hand that obstructs the view and
results in awkward arm positions to grasp objects.
 |
|
Figure 31.18
This young boy with a transverse transcarpal deficiency demonstrates the partial grasp at the flexor crease that, when combined with sensation, usually proves superior in function to a prosthesis. |
In both systems, a battery, relay switch, electric hand, and electronic
control system are present. It should be noted that the myoelectric
hand is the only terminal device available for children using the
externally powered prosthesis. In the myoelectric prosthesis (Fig. 31.19),
an electrode placed on the surface of the skin acts to pick up the
electromyographic (EMG) signal, which in turn is amplified with the
help of an electronic relay switch, and this, in turn, operates the
electric hand (168). The entire system is
generally referred to as a one-site or two-site system. This denotes
the number of electrodes that are used for signal recognition.
 |
|
Figure 31.19
This example of a myoelectric prosthesis, called the Otto Bock Electrohand, was made with a clear socket for teaching purposes. The proximal portion of the socket, which fits on the residual limb, contains the electrodes that pick up the signals from the muscles. This fits into the prosthesis, which contains the electrical and mechanical working parts of the hand. |
voluntary opening—automatic closing, rate-sensitive, and
level-sensitive. The first fitting of a myoelectric arm occurs at
younger than 2 years and utilizes a voluntary opening— automatic
closing (cookie-cruncher) configuration. The muscle signal opens the
electric hand, and relaxing of the signal causes the hand to close
automatically. This system is used for children under 4 years of age.
The rate-sensitive and level-sensitive control systems use one muscle
to control two functions, and are generally fitted to children over 4
years of age, when two sites are not available. The choice of a system
depends on the muscle signal strength, muscle control, and prosthetic
design factors (27).
system by 3 to 3½ years of age. The EMG signal of the flexors is used
to close the hand, whereas the EMG signal of the extensors is used to
open the hand. This system is used when children have established good
control and good use of their myoelectric prosthesis and have
demonstrated the ability to control both the flexors and extensors
independently of each other.
amputation are generally good candidates for switch-controlled
externally powered prostheses. The electrode is replaced with a
miniature switch that can be of a push-pull configuration, a
force-sensing resistor, or of a simple on-off design. The incorporation
of these switches into the prosthesis depends primarily on the level of
amputation and the design of the prosthetic socket or frame.
deficiencies, involving both the upper and lower limbs, is a challenge
that requires a team with experience to achieve the maximum function
for the patient. The problem of bilateral upper-extremity amputation
has been covered earlier. Children with one upper and one lower
extremity pose no problems beyond the management of each individual
limb. Children with bilateral knee disarticulation or transtibial
amputations will walk without support, and therefore a unilateral
upper-extremity amputation in association poses no special problem,
other than donning and doffing the prostheses. With bilateral
amputations above the knee disarticulation level, however, walking
without support is problematic; upper-extremity function is needed, and
a wheelchair may be required for long distances and to conserve energy.
children with bilateral upper-extremity amputation and lower-extremity
anomalies that might ordinarily be treated with amputation and
prosthetic fitting. No amputations of the lower extremity should be
performed until it is certain that the child will not require the use
of the feet for grasping activities.
pediatric age-group with this problem is the quadrimembral amputee
resulting from meningococcemia. In these patients, it is often
necessary to cover the residual extremities with split-thickness skin
grafts to maintain length. These grafts, if not adherent to bone, do
very well in the prosthesis, and are not a hindrance to fitting.
Treatment must be individualized for each patient with certain general
guidelines. The first is to help the patient maximize function with his
or her residual limbs. This is especially true with the upper
extremities, in which sensation is so important to function. Although
these children will become proficient in the use of bilateral
upper-extremity prostheses, if their residual limbs are long enough,
they will usually perform many of their daily activities, especially at
home, without their prostheses.
extremities of these children with quadrimembral loss at the same time.
Although the pressure to do so is enormous, it may result in actual
delay in functional recovery and rejection of the prostheses. In most
situations, it is best to first fit the lower extremities and achieve
ambulation. After this is accomplished, the upper extremity is fit.
there will usually come a time when the parents, and perhaps the child,
desire prosthetic fitting. Again, it is best to avoid fitting all four
extremities at once, but rather focus on meeting specific needs.
Although experience shows that most of these children will use their
prosthetic devices in a limited fashion, if at all, they and their
parents need and are entitled to this experience at least once (Fig. 31.20).
reasons. Although there are no good statistics, trauma is the major
cause of amputation in childhood (169). In this
group, power lawnmowers lead the list of causes, and most commonly, it
is a child riding with a family member. Motor vehicle accidents, farm
injuries, and gunshot wounds follow in that order (170).
In war-torn countries, land mines may be the leading cause of
amputation. Because amputation of the digits is most often caused by
machinery, upper-extremity amputations are more common than those of
the lower extremity. Boys are affected about twice as often as girls.
vascular catheterization, and burns are additional causes, and each has
its own unique set of circumstances. Indeed, the differences in
acquired amputation defy a detailed analysis. Some are semielective and
allow for some preparation of the patient and the parents, whereas
traumatic amputations do not. It is possible, however, to discuss the
common principles that are applicable to acquired amputations in
children.
extremity, it can be difficult to decide on amputation versus limb
salvage. The usual criteria and classifications that are applied to
adults are not easily transferred to children. It is often necessary to
gain the input of a vascular surgeon and others to make the best
assessment, while always remembering the tremendous healing and
adaptive capacity of the child, compared with the adult.
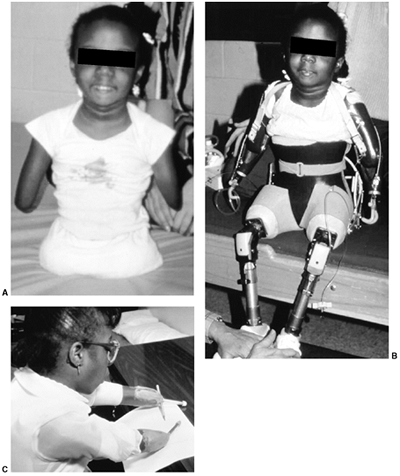 |
|
Figure 31.20 This child with congenital bilateral hip disarticulation and transhumeral amputations (A) was fitted with four prostheses (B), which she quickly abandoned in favor of her power chair and simple assistive devices (C).
|
is often wise to attempt salvage at the first surgery. However, in the
case of a mangled leg that will require vascular and nerve repair,
along with bone reconstruction and free tissue transfer, the decision
needs to be made more realistically. The more energy expended in saving
the limb, the higher the parents’ expectations of the result.
of amputation in trauma. The lack of preparation, coupled with the
parents’ expectations, usually dictates saving as much of the limb as
possible. This often results in a less functional level of amputation
than one that is more proximal. The most obvious example is
preservation of the talus and calcaneus, which the parents and child
may see as saving at least part of the foot, whereas the result may be
a worse gait, worse cosmesis, and more prosthetic problems than with a
Syme amputation.
length as possible in amputations of the long bones. This is especially
true in the femur, in which 70% of the growth
of
the bone occurs from the distal physis. A 5-year-old child with a
midthigh amputation will have less than ideal length as an adult. In
the leg, however, little is lost, so long as an adequate portion of
proximal tibia, in which most of the growth occurs, is preserved, and
in fact, shortening the bone to achieve good soft-tissue coverage may
be the best course.
suitable coverage for a residual limb that will bear weight in a
prosthesis, is not applicable to children, especially very young ones.
In children, skin grafts do make good coverage so long as they are not
adherent to the bone. Split-thickness skin grafts are frequently needed
to preserve length in meningococcemia, burns, and some cases of trauma.
In older children with traumatic amputations, free vascularized flaps
can provide excellent coverage.
problem of bony overgrowth. It is not necessary, or perhaps even
advisable, to remove the cartilage from the bone end. Tapering of the
bone ends, at the distal tibia, for example, is not necessary unless
the child is approaching adulthood. The bony prominences will not
develop to adult proportions, and therefore do not present a prosthetic
fitting problem. If a young child has a through-bone amputation, it may
be possible to salvage a portion of bone and cartilage from the
amputated part for capping the bone. This is similar to performing a
Marquardt procedure, and has the potential to substantially reduce
problems of overgrowth.
phase of the amputation. Often the surgeon has enough problems dealing
with the parents’ emotions, and can easily forget that the child also
needs emotional as well as physical attention.
Such is the case with children who have posttraumatic injuries, and are
electing surgical modification for better function and prosthetic
fitting. Children with gigantism, Klippel-Trenauney-Weber syndrome, and
malignant tumors not suitable for limb salvage also are in this
category. In many cases the need is obvious, and the child and parents
have come to their decision after careful consideration.
easy, and generally there is not complete acceptance of what is in fact
a life-saving procedure. In all cases, the more preparation by the
professionals and opportunities for the parents and patient to talk
with other patients, the better. It needs to be emphasized that the
challenge is to live, and that the surgery is necessary for that. The
options revolve around the functional and cosmetic aspects of the
different procedures.
is worthwhile to fit the acquired juvenile amputee in the immediate
postoperative period. In the young child with a congenital deficiency,
there seems little to be gained. However, in the older child,
especially when the amputation is caused by trauma, there can be large
psychologic benefits from placing the child immediately in a
postoperative prosthesis. This also aids in the control of edema and
phantom pain.
single most important guiding principle is that function should never
be sacrificed for cosmesis. When dealing with the adult population,
overall biomechanical forces resulting from prosthetic alignment can do
relatively little damage to skeletal integrity. This is not the case
for the pediatric patient, in whom skeletal development is ongoing.
Incorrect alignment can have far-reaching and often pronounced negative
results.
highest level of functional need of the patient is met through
prosthetic intervention, or through no intervention at all if
appropriate. The skilled prosthetist is able to assess anatomic and
functional deficiencies and recommend socket design and component
selection. In recent years, there has been a tremendous increase in the
prosthetic innovations and components available for the pediatric
amputee. Current knowledge of these components and their appropriate
use will generally be the responsibility of a prosthetist with special
interest and experience with children. In addition, he or she must
possess the clinical skills, medical knowledge, and the ability to
communicate that will enable timely and appropriate flow of knowledge
to the other team members and parents, so that realistic expectations
can be identified and achieved. Routine servicing of the prosthesis is
extremely important so that extensive repairs will be minimized and the
need for a new prosthesis recognized. Children are generally not happy
to be without their prosthesis.
of the prosthetist, team approach, integration of ever-changing
technology, and the severity of the deficiency. Physiologically, the
child is in a constant state of growth and the prosthetic device must
be designed to permit weight bearing and allow for the greatest amount
of growth without compromising fit and function. Most congenital
lower-extremity amputees are able to bear some weight on the distal end
of the residual limb, and the prosthetist is able to allow for a
slightly less intimate fit of the prosthetic socket than might be the
case for the acquired amputee. Unlike the adult, the child’s skin has
greater tolerance to skin breakdown. The increased activity levels of
the child amputee place tremendous expectations on the prosthetic
devices and the components. All of these factors
are
constant challenges to the prosthetic prescription, and emphasize the
need for a nonstructured approach to fitting. What may be suitable for
one child may be unsuitable for a different child with the same
anomaly. Rigid time schedules are discouraged, and developmental levels
should be used only as a rough guideline to aid the practitioner.
normally seen in the adult population. Disarticulations lead to long
residual limbs with knee centers lower than on the sound side. Ischial
containment sockets are not normally recommended (unless needed because
of hip instability or nondistal weight bearing) because of problems
with soft-tissue containment and diapers in infants. Location of bony
landmarks is obscured by fatty baby tissue, and casting is difficult
and nonexact.
prosthesis are generally standard within the profession. Upon referral
to a clinic, the child is assessed by the team, and a treatment
protocol is established. The stages involved in prosthetic fitting
include:
-
CASTING of the residual limb.
-
TEST FITTING of the modified interfacing socket.
-
DYNAMIC ALIGNMENT AND GAIT TRAINING.
-
DELIVERY of the completed prosthesis.
It is only after a well-fitting and comfortable socket-skin interface
is achieved that the additional components can be added and expected to
function as designed. Casting usually involves the placing of a casting
sock on the residual limb, marking all landmarks and wrapping
circumferentially with plaster or synthetic bandage. This becomes the
negative impression. It is then filled with molding plaster and
stripped, forming the positive cast ready for modification. This
positive cast is then modified to distribute forces and relieve
pressure in the socket for proper hydrostatic control of the residual
limb.
fabricated over the positive mold, the practitioner is able to
ascertain the areas of high and low pressure and to ensure that they
are directed over the appropriate areas. Common fitting problems can be
flagged and corrected before final socket design.
(CAD/CAM) has been used as an alternative tool to plaster casting and
modification of the prosthetic socket. In its most simplified form, a
residual limb is scanned with an optical laser. The information is
relayed to a computer, with which modifications can be made to the
scanned shape to allow for increased or decreased weight-bearing areas.
The finished design is transferred to a computerized milling machine to
form a positive model. This, in turn, is used to fabricate the finished
device. Slowly, CAD/CAM is becoming more widely used within the
profession, because of advantages of design reproducibility, record
keeping, and flexibility in remote locations (172).
Its advantages in the pediatric setting have yet to be proven. All
current CAD/CAM systems rely on surface topography of the residual
limb, and therefore disregard crucial data such as tissue density,
tissue mobility, and underlying skeletal structures (173).
prosthetist initially focuses on creating a prosthesis that will aid
the infant in preparation for ambulation, transitioning from crawling
to standing, with little consideration of gait at this point.
Sutherland concluded that mature gait patterns were established by 3
years of age (174), whereas others place the
time frame closer to 6 years of age. Early infant gait patterns are, in
fact, the processes of suppression of primitive reflexes and the
acquisition of postural responses (175).
Dynamic alignment is the manipulation of relative position of the
socket to the foot and knee while the prosthesis is moving through the
various stages of gait. Through the use of alignment mechanisms in the
components, the prosthetist is able to shift, tilt, and rotate the knee
and foot in relation to the socket. Once independent gait is
established in the infant, refinement to gait can be achieved through
further prosthetic alignment.
encompasses numerous biomechanical principles and their application to
the residual limb-socket interface, and it is the successful
manipulation of these forces that ensures a patient’s comfort and
function. The accommodation for differences in tissue compressibility,
pressure tolerance, underlying bony structures, and vascular integrity
are factors taken into account prior to socket design. Dynamic forces
exerted through ground reaction forces and resulting moments, including
torque and shear forces, increase the vulnerability of the skin-socket
interface.
extensive prosthetic intervention. The socket encompasses the amputated
pelvic remnant, and encloses the contralateral side for suspension. The
traditional socket design rises proximally to the waist, and fits
similarly to a Boston spinal orthosis. The diagonal socket is a
modified version of the standard design, and it affords a more
comfortable fit and increased flexibility. Prosthetic hips arc on a
single axis, and are mounted on the outside anterior distal aspect of
the affected side. Hip and knee flexion are easy to activate through
pelvic tilt, if the prosthesis is perfectly aligned. The hip is
anterior to the weight line and the knee is posterior. This allows for
a stable stance and a smooth gait. The endoskeletal system is used
exclusively for this level of
amputation
because of an increased level of cosmesis and decreased weight.
Children with amputations at this level can achieve remarkable gains
when fitting begins while the child is pulling to stand and when
therapeutic intervention and parental training are incorporated. It is
recommended that the knee be locked, initially, so that hip control can
first be learned. Once the child is walking independently, the knee can
be activated.
over the last 10 years. The quadrilateral socket was the socket design
of choice until 1987, when the ISPO formulated recommendations on the
narrow medial lateral (ML) socket design (176).
Variants of this design (ischial containment socket) continue,
including the contoured adducted trochanteric—controlled alignment
method (CAT-CAM), the normal shape, normal alignment (NSNA), and the
modified quad designs, to name a few (Fig. 31.21).
The underlying principle is to adduct the femur while locking the
ischial tuberosity within the socket, thereby providing a more
anatomically correct alignment during all phases of gait (177). The controversy over these designs has been increasingly dispelled, with further clinical experience.
utilized for the secure attachment of the socket to the residual limb.
These devices may provide auxiliary suspension which is attached to the
socket to suspend or enhance suspension. The suspension may be
incorporated in the socket itself, as in suction sockets, supracondylar
sockets, and so on.
until adequate development of the residual limb allows for silicone
suspension, at approximately 2 to 3 years of age. The Silesian belt
attaches to the anterior/medial aspect and the lateral aspect of the
transfemoral socket, and lies across the pelvis at the waist.
Tightening the Silesian belt prevents the socket from slipping
distally. The TES belt may be used instead of the Silesian belt. The
TES belt is a neoprene suspension system that is applied over the
proximal portion of the transfemoral socket and is then secured around
the waist, and it has become the suspension of choice for the
first-time prosthetic user.
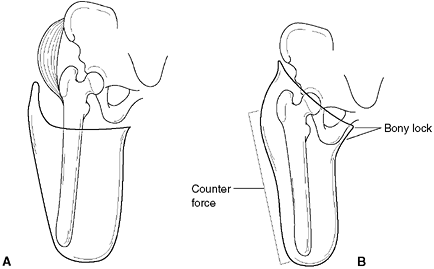 |
|
Figure 31.21 A:
The quadrilateral socket is useful for the young child, especially if end bearing is possible. However, it fails to stabilize the femoral segment in a transfemoral amputation. B: This has led to the popularity of the narrow medial lateral socket design, such as the ischial containment socket shown here. This design can prove impossible in small infants, because of the fatty thigh and buttocks as well as the diapers. |
population because of tight tolerances in fitting that cannot be
maintained by a growing child. With the suction socket, the residual
limb is pulled into a socket that incorporates a one-way valve in its
design (Fig. 31.22B). Once the valve is in
place, the amputee expels air every time the prosthesis is in contact
with the ground. During swing phase, the negative pressure within the
socket holds the prosthesis in place. Air that leaks into the socket is
quickly expelled through the one-way valve, and a constant negative
pressure is maintained. Total contact suction sockets are generally
used for the transfemoral amputee with a mature residual limb, and at
the completion of skeletal growth. Short limbs, volumetric changes, and
severe scarring are contraindications for the suction-suspended socket.
pediatric population. Although most amputations in children are
disarticulations, growth changes in the fibular deficiency often result
in a transtibial-level residual limb that is a distal-end
weight-bearing limb. The true transtibial socket is most often required
for the traumatic amputee. Total contact design allows for increased
pressure bearing over the patellar tendon, medial flare of the tibia,
medial shaft of the tibia, and lateral shaft of the fibula, and the
anterior and posterior compartments. Similarly, the weight-sensitive
areas most affected include the tibial crest, fibular head, distal
tibia and fibula, peroneal nerve, and the patella. The socket design is
composed of an outer shell, inner soft liner, and a cosmetic cover.
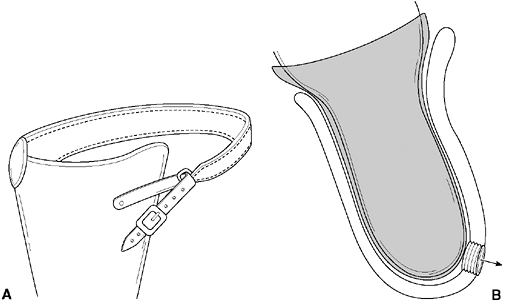 |
|
Figure 31.22 Methods of suspension for transfemoral prostheses. A:
The Silesian belt is almost universally used in the young pediatric patient to suspend a transfemoral prosthesis or occasionally a knee disarticulation or transtibial prosthesis (Fig. 31.15B). B: The suction socket is a tight-fitting socket design with a one-way valve that allows air to be expelled with weight bearing to maintain a suction fit on the residual limb. It is best suited for the older child or adolescent, who has a mature limb that is not changing in size. |
It provides the least suspension, and for that reason, is not often
suitable for young children. The supracondylar/suprapatellar (SCSP)
transtibial socket design (Fig. 31.23B) allows
for suspension without the need for belts or cuffs. The medial,
lateral, and anterior walls extend proximally, to fully enclose the
patella and femoral condyles. The supracondylar (SC) transtibial socket
(Fig. 31.23C) is almost identical to the SCSP
design, except the anterior proximal brim does not enclose the patella,
and therefore allows greater freedom and range of motion.
Contraindications for both the SCSP and SC design include obese or
muscular limbs and patients with heavy scarring around the knee.
The cuff is fabricated from leather and encompasses the femoral
condyles and patella. It is attached to the medial and lateral aspects
of the socket. The neoprene sleeve suspension is another useful
suspension in the pediatric prosthesis (Fig. 31.23E).
For the very active child, it provides a great level of security in
that the prosthesis will not come off. Recent advances in silicone and
urethane technology have increased comfort, flexibility, and cosmesis
of the sleeve suspension systems.
The liner is rolled onto the residual limb. At the distal end of the
liner is a serrated pin. Inside the distal end of the socket is a
shuttle or receptacle mechanism. Once the liner is donned, the amputee
places the limb in the socket, and the pin and shuttle engage and lock
into place. Pressing of a button hidden on the medial distal aspect of
the prosthesis releases the pin, and the residual limb can be removed
from the socket. Because of the physical characteristics of the liner,
the greater the distracting forces placed on the prosthesis, the
tighter the liner grips the residual limb. This system is used
extensively in young children. Where space is at a premium, a cushioned
silicone liner used in conjunction with a socket expulsion valve, and a
silicone sleeve allows the amputee to achieve a remarkable level of
suspension using a modified suction technique.
when the distal bulbous end is large and the medial malleolus is
prominent (Fig. 31.25A). The removable or
segmented liner socket incorporates a full foam liner that has been
built up to the same circumference as the distal bulbous end. A
laminated shell is then formed over this insert. The patient dons the
liner first, then slips this into the laminated receptacle (Fig. 31.25B).
An atrophied residual limb with a small heel pad is best suited for
this design, and the degree of cosmetic restoration will be very good.
The silicone or bladder prosthesis utilizes an inner elastic area that
stretches to permit passage of the bulbous end of the residuum through
the narrower circumference of the tibia and fibula, then constricts
once the distal end has passed through (Fig. 31.25C).
All of the above designs maintain total contact, and the proximal brim
is at the level of the patellar tendon. This ensures that the
biomechanical forces are adequately spread up to a load-bearing
landmark to increase comfort and function.
Although most are for adults, recently there are a number of new knees
available for children. The prosthetic knee is composed of the knee
mechanism or frame, and may contain a control unit. The control unit
consists of a pneumatic, hydraulic, or mechanical system, or some
combination of these three. The control unit responds to changes in
cadence and dampens sudden, abrupt changes.
The
faster a hydraulic or pneumatic unit is compressed, the faster the
energy is released, and this helps to regulate the lower shank of the
prosthesis. The prosthetic knee unit can be further subdivided into
single axis and polycentric types.
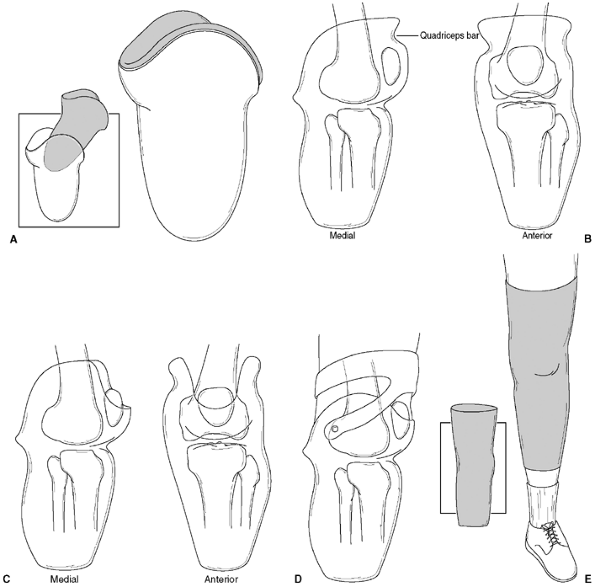 |
|
Figure 31.23 Common sockets and suspensions for transtibial pediatric amputees. A:
Patellar-tendon-bearing (PTB) socket is most useful for the mature patient. It gives the most freedom of motion, but the least secure suspension. B: The supracondylar suprapatellar (PTB-SCSP) design gives the most secure suspension and best knee control of any of the sockets that incorporate the suspension in the socket. C: The supracondylar (PTB-SC) socket eliminates the suprapatellar portion of the socket anteriorly, providing better range of motion but less control of hyperextension. D: The supracondylar cuff suspension is a common suspension used in the pediatric age range. E: The neoprene sleeve suspension provides very secure suspension for the very active amputee. This can also be used for the transfemoral amputee. |
it the need for sound practice in selecting the appropriate knee, on
the basis of amputation level, functional level, and body size. In
general, the single-axis internal knee without any control unit is the
first knee to be used on the child, because of its light weight, short
lever arm, and simplicity. In the single-axis knee joint, the lower
shank rotates around a single point in relation to the socket.
Internal polycentric knees move around a center of rotation that varies with the flexion angle of the lower shank (168). The four-bar linkage knee is the most common polycentric knee and the most widely used by prosthetists (179) (Fig. 31.26).
The inherent stability during stance, the fluid knee-flexion movement,
and the mechanical design to give more ground clearance during flexion
increase patient and practitioner confidence in the unit (180).
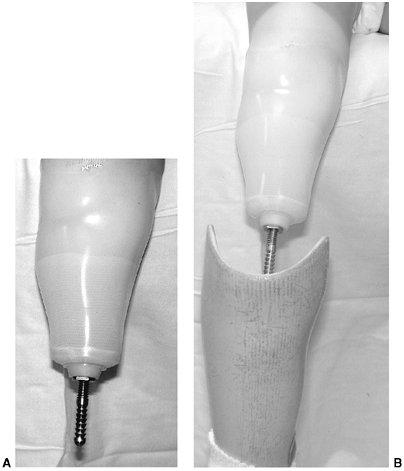 |
|
Figure 31.24
Silicone suspension liners (Triple S socket) have become very popular. The soft silicone liner has a serrated pin incorporated into the bottom of the liner. The patient rolls the liner on the residual limb (A), then inserts the limb into the prosthesis (B). At the bottom of the prosthesis is a socket in which the pin locks. It is released by pushing the button on the medial side of the prosthesis. |
boundaries and age distinctions for the prescribing of specific knees.
Traditionally, an articulating knee would be introduced in a congenital
amputee at approximately 3 to 4 years of age. This age was determined,
in part, by the limitations in the size and function of the components.
In the experience at the authors’ center, as well as others,
introduction of a prosthetic knee without a locking feature can be used
as the first prosthesis when the child first pulls to stand. A recent
report demonstrated the benefits of early fitting with articulated
knees in children as young as 17 months. All children learned to walk
with an articulated knee, despite their age differences (129).
control systems (e.g., hydraulic knees) can be incorporated into the
prosthesis. Most components carry specific weight guidelines, and many
children reach these ranges well before adulthood. For example, an
adult hydraulic polycentric knee is routinely used on 8-year-old boys
whose weight has surpassed 100 lb. This does not mean that every child
of a certain age and weight should have a particular knee. Placing a
sophisticated knee and control system on an individual who has neither
the hip range, muscle strength, nor residual limb length to activate
the knee often results in contralateral hip and lower back pain as well
as patient frustration.
the foot can have profound effects on the performance of the
prosthesis. Functionally, prosthetic feet can be categorized into five
main groups (181):
-
Solid ankle cushion heel
-
Single axis
-
Multiaxis
-
Elastic keel
-
Dynamic response
Recreation Systems, Inc. (TRS)] contains no articulating parts, and
foot motion depends on the various compressive properties of the
materials used between heel-strike and
toe-off (Fig. 31.27A).
It is generally considered when amputees require maximum late-stance
stability because of weak knee extensors, knee-flexion contractures, or
poor mid- to late-stance balance (182). The
SACH foot is used in pediatrics when the foot size is below 12 cm. The
Little Feet is a bolt on type SACH foot designed with unique energy
dynamics (Fig. 31.27B). The toes are very
flexible because of the use of an elastomer that more closely mimics
the child’s foot. A special removable heel core allows the foot to be
used “barefoot.”
 |
|
Figure 31.25 Common socket designs for Syme amputation prosthesis. A:
Obturator design is often needed if the distal end of the residual limb is large and bulbous or the medial malleolus is prominent. It is the least cosmetic. B: The removable or segmented liner consists of a complete separate foam liner, which has a split in the side to allow the distal end of the limb to pass. Once the patient applies the liner, he or she slides the limb covered by the liner into the prosthesis. The patient must have the manual dexterity and strength to use this suspension, which will eliminate some patients with hand anomalies from using it. Limbs with a small heel do best with this system. C: The bladder design has a built-up silicone sleeve, which the patient slides the limb past. This socket fits more loosely, and does not stabilize the heel pad as well as the other designs. |
that allow passive dorsi- and plantar flexion. By changing the hardness
of the bumper, the prosthetist is able to effectively change the
properties of the foot. The single-axis foot does not come in a size
suitable for the child amputee. This foot is best suited for the
transfemoral amputee, in whom full-foot contact with the ground is
necessary to increase stability. The multiaxis foot allows passive
dorsi- and plantar flexion, inversion, and eversion. The multiaxis foot
was once thought best suited for the amputee who because of uneven
terrain or a lifestyle that includes golfing or various sports requires
flexibility and some rotational control: It has now found its way into
the pediatric population. The multiaxial foot (College Park Truper
foot) (Fig. 31.27C) allows controlled
resistance in all planes—inversion/eversion, dorsiflexion and plantar
flexion, and transverse rotation about the ankle joint. This class of
foot has gained wide acceptance within the pediatric arena, in part
because of its ability to absorb forces at the ankle and reduce
transmission of these forces to the socket. This is particularly useful
when fitting a very short residual limb.
higher-functioning foot, the prosthetist can move the child into a
dynamic-response foot. This group of feet is distinguished by a spring
mechanism in the keel that deflects during gait (Fig. 31.27D).
The dynamic-response foot has found its way into competitive-level
sports as well as day-to-day activities. Although the variety of
componentry for children is still much less than for adults, there is a
wide variety of feet with different performance characteristics
available. It is important to use
components that will maximize performance, and at the same time be appropriate for the patient (183).
 |
|
Figure 31.26
Four-bar linkage is an internal polycentric knee that provides many advantages to the patient, including increased stability and better ground clearance during swing phase. As indicated in this illustration, the point of rotation varies with the degree of flexion. With the knee flexed, the leg folds under the thigh segment, and therefore is very useful for longer residual limbs. There is also a hydraulic version of the four-bar-linkage for children. |
Generally, children are not fitted with the highest-performance
dynamic-response feet, because of constant growth and weight changes
and the high costs associated with foot replacement. The involvement in
competitive sports is usually a good benchmark to initiate fitting
adolescents with the highest-performance dynamic-response feet.
choose a foot that will enhance gait, but also to choose the
complementary knee that will aid in controlling the foot during all
aspects of the gait cycle. A common mistake is to prescribe a
dynamic-response foot with a simple, friction-controlled knee that is
incapable of preventing uncontrolled heel rise. The same is true of the
transtibial amputee, who lacks the muscle strength to control the foot,
often resulting in premature muscle fatigue. In the selection of
multiple components, the prosthetist must marry the characteristics of
all components, so that maximum benefit can be available to the amputee.
partial-foot amputee is to ensure that adequate load-bearing is
designed into the prosthesis of choice. As a general rule, the more
proximal the level of amputation, the higher the prosthesis must fit
over the ankle complex and the more proximally it must fit on the tibia
and fibula. Tissue condition, function of the remaining foot complex,
and activity of the child all play a role in determining the
prescription and design of the prosthesis.
little more than a shoe filler. A carbon fiber insert to better control
forces from heel to toe-off may be incorporated in the shoe filler. In
the case of the very young child, no intervention may be required until
a need has been demonstrated, for example, the inability to keep the
shoe on, especially when the child becomes more active in sports.
This incorporates a cosmetic foot shell, silicone-laminated socket with
modified foot sole, and a posterior zipper for ease of donning and
doffing. The prosthesis is fabricated over a modified model of the
patient’s partial foot. The socket trim line is proximal to the
malleoli, and is fitted intimately to ensure adequate control. The
design of a partial-foot prosthesis may also include a removable
insert, to accommodate the need for corrective alignment of the
residual foot. The prosthesis is then cosmetically finished to resemble
the contralateral limb. Overall, this type of prosthesis is perfectly
suited for the child amputee, and resists premature wear and tear. If
needed, a partial-foot prosthesis should be prescribed once the child
is pulling to furniture, so that foot control will begin at an early
age. It should be noted that a low-profile insert (distal to the
malleoli), used in conjunction with a high-top boot, will offer
adequate function and cosmesis until a lower-cut shoe is requested by
the parent.
In the Chopart partial-foot amputation level, the prosthesis is
modified to encompass the calcaneus and talus, and this results in a
prosthesis that is often longer than the contralateral limb. The
prosthesis must encompass the ankle joint, and it often rises
proximally to the patellar tendon in an effort to reduce forces on the
tibial crest-socket interface. Selection of prosthetic feet is
compromised because of the lack of space distally, and commercially
available carbon foot plates require permanent attachment with
vulcanizing rubber cement. This negates any changes caused by growth,
and realignment to compensate for gait changes is virtually impossible.
and, at times, controversial. Where some clinics maintain rigid
protocols for terminal device selection, other clinics rely more on
patient and parent input, combined with historic
success
rates for device types. Clinics that maintain very high caseloads for
myoelectric devices, for example, will most likely have far more
experience in fitting externally powered prostheses, compared to a
clinic that may only see a handful of potential myoelectric candidates.
 |
|
Figure 31.27 A:
In the conventional style solid ankle cushion heel (SACH) foot, the length of the keel controls the toe lever arm, and therefore the hyperextension moment at the knee, while the compression of the material at the heel absorbs the forces at heel strike. B: The Little Feet type design incorporates unique energy dynamics with flexible toes all in sizes beginning at 10 cm length. The length of the keel controls the toe lever arm and thus the hyperextension moment at the knee, and the compression of the elastomer heel absorbs and deflects the forces at heel strike. C: The dynamic multiaxial TruPer foot allows rotation, inversion and eversion, flexion, and extension movements. The outer cosmetic shell can be exchanged for larger shells as the child grows. D: The Flex-foot is a dynamic-response foot with much different performance characteristics than the SACH foot or multiaxial foot. It is used for the older, stronger, and physically active child who has the physical ability to use such a foot. |
into hands and hooks, and they can be body-powered (cable and harness)
or externally powered (electric). Hands and hooks can be either
voluntary opening or voluntary closing. Patton lists the functional and
prescription criteria for the various terminal devices (166).
deficiency begins at 4 months of age in a passive prosthesis with a
stylized passive hand. There are several hands manufactured for this
age range (Fig. 31.29A). This allows for equal
arm lengths for the development of propping up on the amputated side
and greater acceptance by the parents. Following initial sitting
balance, the clenched-fist terminal device is exchanged for a small
infant passive hand. When the infant begins to reach out (at
approximately 15 to 18 months of age), the clinic team begins to assess
the need for either body-powered or externally powered prostheses. If
body-powered prosthesis is recommended, a cable-operated Hosmer 12P plastic-covered hook (Fig. 31.29B) or an ADEPT infant hand (Fig. 31.29C)
will be prescribed. The canted design of the 12P hook allows for
greater visual feedback to the wearer. In the event that a myoelectric
device is warranted, the Variety Village 0-3 (VV 0-3) electric hand (Fig. 31.29D) or the Otto Bock Electrohand (Fig. 31.19)
is used. In the pediatric VV 0-3 hand, the thumb and opposing two
fingers operate to form a three-point chuck grip. In the Otto Bock 2000
hand, the same principle is applied, except that from the open to
closed position, the thumb sweeps from a lateral position to meet the
two opposing fingers upon close.
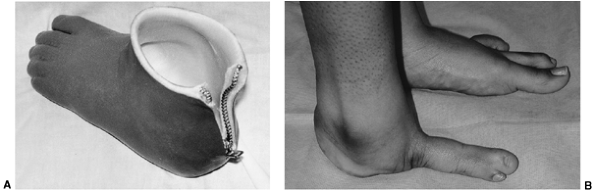 |
|
Figure 31.28 A:
The Lange silicone partial-foot prosthesis is a custom-made prosthesis that can incorporate a keel to aid in foot stability and push-off in gait. B: It is useful for children with partial amputations of the foot or congenital longitudinal deficiencies of the foot, shown here. It is not useful in feet with insufficient length, for example, those with the Chopart amputation. |
 |
|
Figure 31.29 A:
The TRS ALPHA Infant Hand is an option for the first prosthetic hand offering age-appropriate fine motor activities. This would commonly be fitted between 4 and 6 months of age. The hand incorporates a flexible thumb that allows objects to be placed for simple grasp and release functions. B: The ADEPT is a voluntary closing body-powered hook that is fitted at approximately 15 months of age, if the child is ready and a body-powered device is desired. C: The Lite-Touch is a voluntary closing hand that would find the same indications as the ADEPT hook. It looks a bit more like a hand, which often makes this option popular with parents. D: The Variety Village hand is one of the most commonly used myoelectric hands in the pediatric age group. The Otto Bock Electrohand is shown in Figure 31.19. Both of these electric hands are covered with a cosmetic glove. |
component sizes and versions allows for a relatively smooth transition
into adulthood. Prescription criteria are reviewed during each clinic
visit, and changes are made on the basis of the child’s changing needs.
In today’s environment of active children and sports activities, the
use of sports or other adaptive terminal devices is essential for the
amputee. TRS has developed numerous devices for use in sports and
recreational activities. These can be interchanged on the prosthesis,
so that only one socket is required.
referred to as an endoskeletal (internal structure) or exoskeletal
(external structure) prosthesis. Generally, transtibial, partial foot,
and transradial prostheses are constructed exoskeletally, and
transfemoral, knee disarticulation, hip disarticulation, transhumeral,
and shoulder disarticulation levels of prostheses are constructed
endoskeletally.
better suited to the growing child. There are various techniques and
materials used in the construction of the exoskeletal prosthesis.
Generally, following the completion of dynamic alignment, the
ready-to-be-finished prosthesis is placed within a transfer jig that
allows the socket to be separated from the foot, while maintaining
alignment. A rigid polyurethane foam is added, and the prosthesis is
cosmetically shaped to equal the sound limb. The structure is then
laminated with acrylic resin forming the outer “shell.” The advantages
of this construction are its increased durability, and that it is easy
to clean and structurally strong. The major disadvantages are the lack
of further alignment capabilities, and that they are less cosmetically
acceptable than some other types.
postoperative period as a temporary method to initiate ambulation,
while maintaining the ability to alter the alignment. This quickly
became the norm for fitting in the adult population, and has been used
primarily for knee disarticulation or transfemoral prosthesis. The
prosthesis is modular and composed of a pylon (tube) and connecting
hardware, and it allows for quick changing of damaged components. In
the event that realignment is necessary, the endoskeletal design
incorporates alignment jigs within the attachment couplings, and only
the cosmetic soft cover needs to be removed for adjustment. For these
reasons, advanced components for use by children tend to be engineered
for use in this system. The disadvantages of the endoskeletal system
are lack of durability of the cosmetic cover, increased maintenance,
and increased costs. This is outweighed by the increase in function and
ease of adjusting length.
care of the limb-deficient child is mostly nontraditional. In addition
to the traditional role, the physical/occupational therapist fills the
roles of teacher, advocate, friend, and liaison (186).
In some situations, all but the traditional role of therapist may be
filled by a nurse. In some cases, the role may be shared. What is
important is to recognize the need for all of these activities.
the educator. In this role, it is important that the therapist,
physician, and prosthetist all be of one mind regarding the patient’s
treatment. First is the education of the parents. Like
nurse-practitioners, the therapists will usually have more time and be
better heard than the doctor when relaying information to the parents
about their child. The therapist will be able to reinforce to the
parents the options that have been discussed at the initial meeting
with the physician. Most importantly, they can arrange for the family
and child to meet others with similar deficiencies during routine
sessions. Throughout the child’s life, the experienced therapist can be
of immense value to the child and young adult in anticipating problems
and helping with solutions.
the first meeting with the parents. The importance in this role is to
bring the parents to see the normal, as well as the abnormal, and to
ensure that the initial bonding to the parents occurs. The therapist
will frequently need to advocate for the patient to insurance companies
and other agencies to help provide for the patient’s needs. When the
child starts into day care and then school, the therapist will assist
in the child’s transition into a new world by educating the teachers
and the child’s peers about the child’s differences. This can be
extremely important for the child’s acceptance and socialization.
Later, the same role may be necessary with physical education teachers
and coaches to ensure that the child can participate in all the
activities he or she is able to.
liaison among the team members. This close teamwork can spare the child
and parents countless visits to clinics and
delays
in treatment: a very important goal in avoiding the medicalization of a
condition for which there is no cure, but only good management.
home/community based than hospital/office based, for many reasons.
First, the child’s condition will be permanent. This means adapting to
the environment in which the child exists. None of the child’s
activities (toileting, eating, dressing, play, sports) will take place
in the hospital or an office. Therefore, they should be learned in the
normal environment. Second, the parents will be with the child, and are
responsible for the child’s development and learning. They will have
unlimited access to the child for this “therapy.” Finally, the child
should not come to think of him- or herself as a medical problem, but
rather as a child with a difference that can be successfully adapted
to. Unnecessary hospital, clinic, or office visits are not a good way
in which to communicate this goal of independence.
situations, as it does in many diseases or postsurgical situations.
These are times when specific therapeutic exercises or the use of new
prostheses must be performed, supervised, and taught. During the first
months of life of a congenital amputee, the parents are seen by the
therapist every 6 to 8 weeks. The child’s development is monitored, and
the parents are taught activities appropriate for the stage of
development, for example, rolling over and coming to stand from
sitting. These visits are used to monitor the parents’ coping, to
listen and answer questions, review treatment options, and arrange for
the meeting with other parents and children.
the medical model is more appropriate, and the frequency of the visits
increases briefly, while the child and parent are taught the use of the
prosthesis. In addition to the usual goals of increasing or maintaining
motion and strength following surgery, the therapist plays a critical
role in edema control. This is very important if the patient is to
resume prosthetic wear quickly. Teaching and supervising elastic
wrapping and obtaining shrinkers are important postsurgical issues.
independent in donning and doffing the prosthesis, toileting, and other
activities with the prosthesis. Fitting of a new prosthetic component,
for example, a hydraulic knee joint, will usually require specific
training to maximize the benefits of the new components.
helpful in designing and modifying age-appropriate play activities,
With the approach of school age, emphasis switches to independence in
the activities of daily living, and later, to fine motor skills that
may be needed for classroom activities. Adaptations for sports, for
example, special terminal devices, if desired, or a swimming leg, are
important, as is the advocacy role to allow the children to participate
in all possible activities.
interventions is usually minimal. The child has now become fully aware
of his or her differences and their significance. The child will
usually dictate the needs. Appearance being important, more cosmetic
prostheses and improved gait become important issues.
defining independence. The therapist can play a critical role in
directing the child and parents to the appropriate agency for the rules
of the state, and to a source for evaluation and modifications to the
vehicle. As with any adolescent, the amputee should attend driver
education training, using modifications if needed. Modifications can
range from simple to complex. Switching the brake and gas pedals to
accommodate unilateral lower-limb loss is one of the most common
examples. Many amputees, even with bilateral lower-extremity loss,
drive without adaptations. This must be closely monitored and evaluated
by the state’s examiners. Hand controls are used most commonly in
bilateral lower-extremity loss. A ring adaptation can be used to modify
the steering wheel for upper-extremity amputees. A handicap license is
appropriate for individuals whose mobility is limited. The higher-level
amputee, and those with multilevel limb loss, may benefit from a
handicap parking license. All modifications are listed on the amputee’s
driver’s license.
independence, and the needs may be greater than what the child
anticipates. The therapist can be of great value in assessing the
situation, counseling the patient, and helping with the transition. The
Internet can prove to be a great resource in assisting college-bound
students and their families.
amputations should be encouraged to participate in sports and
recreational activities with their peers. The psychologic impact of
sports cannot be underestimated. Improving self-esteem and confidence,
gaining independence, learning to win and lose, developing
decision-making and problem-solving skills, and cooperating as a team
member are a few of the benefits that a child carries throughout his or
her life. Improving physical fitness, developing balance, strength,
coordination and motor skills, increasing endurance, and weight control
are benefits of physical activity.
adapted sports and recreation for individuals with physical and mental
impairments. The Paralympic and Special Olympics initiatives have been
the most obvious and have sparked an increase in availability of
programs for special-needs children. Laws also have been passed for
children to receive education in the least restrictive environments. PL
94-142 provides free and appropriate education for all
children with disabilities (186).
Physical therapy and recreation are related services included in this
legislation. This allows for adapted physical education to be included
in a child’s everyday school activities. Physical therapists need to be
aware of the resources and adaptations that provide accessibility of a
sport to a child within limits of his/her physical abilities in the
school and community settings. Gross motor achievements are a measure
of a child’s development. Learning to toss a ball, jump, run, hop, and
ride a bicycle are activities included on standardized developmental
screenings and tests. It is important that these activities be included
in the child’s plan of treatment, so that a child can be offered the
same age-appropriate physical challenges as his or her peers.
prostheses are available, depending on the degree and level of the
disability. These adaptations are too numerous to mention. Recreational
and sports-related terminal devices are available for the
upper-extremity amputee (187,188).
Adaptations can be as simple as raising the handlebars on a bicycle and
adding a toe strap to highly sophisticated prosthetic components
specific to each sport (189). Information and
resources for sports and adaptive recreation for the amputee can be
obtained through the Amputee Coalition of America (http://www.amputee-coalition.org;
telephone 1-888-267-5669). The Association of Children’s Prosthetic and
Orthotics Clinics is an excellent resource for services in geographical
regions (http://www.acpoc.org; telephone 847-698-1637).
M, Atkins D, Hubbard S, et al. Prosthetic devices for children with
bilateral upper limb deficiencies: when and if, pros and cons. In:
Herring JA, Birch JG, eds. The child with a limb deficiency, Rosemont, IL: American Academy of Orthopaedic Surgeons, 1998:397.
JN, Weaver DD. Subclavian artery supply disruption sequence: hypothesis
of a vascular etiology for Poland, Klippel-Feil, and Mobius anomalies. Am J Med Genet 1986;23:903.
R, Wilcos AJ, Lie TR. A population-based study of survival and
childbearing among female subjects with birth defects and the risk of
recurrence in their children. N Engl J Med 1999;340:1057.
J, Setoguchi Y, Rappaport L, et al. Effects of stress, social support,
and self-esteem on depression in children with limb deficiencies. Arch Phys Med Rehabil 1991;72:1053.
JW, Setoguchi Y. Effects of parental adjustment on the adaptation of
children with congenital or acquired limb deficiencies. J Dev Behav Pediatr 1993;14:13.
C. Congenital short femur: clinical, genetic and epidemiological
comparison of the naturally occurring condition with that caused by
thalidomide. J Bone Joint Surg Br 1980;62:307.
M, Messner MB, Green WT. Distribution of lengths of the normal femur
and tibia in children from one to eighteen years of age. J Bone Joint Surg Am 1964;46:1197.
JR, Meyer LC, Blackhurst DW. Operative treatment of bone overgrowth in
children who have an acquired or congenital amputation [see comments]. J Bone Joint Surg Am 1995;77:1490.
R, Israel R, Lacroix R, et al. Phantom limbs in people with congenital
limb deficiency or amputation in early childhood. Brain 1997;120:1603.
JA, Barnhill B, Gaffney C. Symes amputation; an evaluation of the
physical and psychological function in young patients. J Bone Joint Surg Am 1986;68:573.
DP, Holt GR, Ogden JA. Talocalcaneal coalition in patients who have
fibular hemimelia or proximal femoral focal deficiency. A comparison of
the radiographic and pathological findings. J Bone Joint Surg Am 1994;76:1363.
D, Boyd NA, Fixsen JA, et al. The natural history and management of
congenital short tibia with dysplasia or absence of the fibula. J Bone Joint Surg Br 1977;59:267.
HG, Wright JG, Cole WG, et al. The Ilizarov method for correction of
complex deformities: psychological and functional outcomes. J Bone Joint Surg Am 1996;78:1480.
CH Jr, Tooms RE, Edholm CD, et al. Failure of centralization of the
fibula for congenital longitudinal deficiency of the tibia. J Bone Joint Surg Am 1991;73:858.
D, Barnes J, Lloyd-Roberts GC. Congenital aplasia and dysplasia of the
tibia with intact fibula. Classification and management. J Bone Joint Surg Br 1978;60:31.
JL, Clancy M, Harcke HT, et al. Congenital diastasis of the inferior
tibiofibular joint: a review of the literature and report of two cases.
J Pediatr Orthop 1985;5:225.
GL. Complex congenital anomalies of the lower extremities: femoral
bifurcation, tibial hemimelia, and diastasis of the ankle. Case report
and review of the literature. J Bone Joint Surg Am 1984;66:453.
Nes CP. Rotation-plasty for congenital defects of the femur: making use
of the ankle of the shortened limb to control the knee joint of a
prosthesis. J Bone Joint Surg Br 1950;32:12.
JP, Gillespie R, Hall JE, et al. Van Nes rotational osteotomy for
treatment of proximal femoral focal deficiency and congenital short
femur. J Bone Joint Surg Am 1975;57:1039.
KM, Sprangers MAG, van der Eyen JW, et al. Quality of life in survivors
with a van Ness-Borggreve rotationplasty after bone tumour resection. J Surg Oncol 2000;73:192.
A, Hoffmann C, Gosheger G, et al. Malignant tumor of the distal part of
the femur of the proximal part of the tibia: endoprosthetic replacement
of rotationplasry. J Bone Joint Surg Am 1999;81:462.
E, Zernicke R, Setoguchi Y, et at. Energy expenditure during walking by
children who have proximal femoral focal deficiency. J Bone Joint Surg Am 1996;78:1857.
NJ, Hashemi-Nejad A, Fixsen JA. Natural history and treatment of
instability of the hip in proximal femoral focal deficiency. J Pediatr Orthop B 1995;4:145.
D, Stanitski DF. Early conservative and operative treatment to gain
early normal growth in proximal femoral focal deficiency. J Pediatr Orthop B 1997;6:59.
KL. Resection, rotationplasty, and femoropelvic arthrodesis in severe
congenital femoral deficiency. A report of the surgical technique and
three cases. J Bone Joint Surg Am 2001;83:78; Steel HH. Iliofemoral fusion for proximal femoral focal deficiency. In: Herring JA, Birch JG, eds. The child with a limb deficiency. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1998:99.
F, Stills S, McClellan B, et al. Does socket conuration influence the
position of the femur in above-knee amputation? J Prosthet Orthot 1990;2:94.
DH, Shurr DG, Golden JC, et al. Comparison of energy cost and gait
efficiency during ambulation in below-knee amputees using different
prosthetic feet—a preliminary report. J Prosthet Orthot 1988;1:24.
C. Physical therapy management in children with lower extremity limb
deficiencies. In: Herring JA, Birch JG, eds. The child with a limb deficiency. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1998:319.
