Sports Medicine in the Growing Child
and young adults between the ages of 6 and 21 years engage in sports
programs that are held outside of school, and 3.5 million boys and 2
million girls participate in school sports programs (1).
activities warrant the risks involved, so an understanding of
sport-specific risks is crucial to provide a comprehensive approach to
address this concern.
specific risks and patterns of injury associated with different sports
can be determined and compared. From this data, it may be possible to
develop specific interventions designed to reduce the frequency of
injuries.
These studies, one from the National Athletic Trainers Association and
one from the Athletic Health Care System (Seattle) studied injury
patterns among children participating in high-school sports.
girls’ cross-country has the highest frequency of injury, followed by
football, wrestling, girls’ soccer, and boys’ cross-country (Table 32.1).
Of all the injuries reported, approximately 70% to 80% are minor (time
loss of 5 to 7 days from participating in the involved sport), whereas
4% to 8% result in a time loss of greater than 3 weeks from
participating in the involved sport (4) (Table 32.2).
younger children, due to falls, whereas lower-extremity injuries occur
more frequently in older children and adolescents (1,4).
recreational injuries are available from the United States Consumer
Product Safety Commission (CPSC). The CPSC operates the National
Electronic Injury Surveillance System (NEISS) whereby data are gathered
from the emergency departments of 100 hospitals throughout the United
States. These data are then used in conjunction with other models
involving the relation between emergency room visits and the number of
injuries treated outside hospital emergency rooms to arrive at an
estimate of the number of injuries treated for each specific age-group
in hospital emergency rooms, doctors offices, clinics, and ambulatory
centers. In the year 2000, eight of the most common recreational
activities accounted for 2.24 million medically treated injuries of the
musculoskeletal system in children from 5 years to 14 years of age (6,7). The cost to society of these injuries, estimated using an “Injury Cost Model,” was $33 billion (6,7).
specific sports and may lead to injury prevention by mandatory changes
in equipment. Face masks and helmets used in hockey, shin guards used
in soccer, and helmets used in baseball are examples of equipment
modification put into place after injury surveillance rates indicated
the need for change.
|
TABLE 32.2 ALL INJURY DATA CLASSIFIED ACCORDING TO AFFECTED BODY PART
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
the benefits, both physical and psychosocial, of participation in any
particular sport outweigh the risks of significant injury, and parents
should be advised of both the recognized benefits as well as the
sport-specific risks, in order to make an informed decision regarding
their child’s participation.
children and adults generally lag behind injury management strategies.
As participation in recreational and scholastic sports increases, there
is a desire to examine strategies of injury prevention to lower the
risk of injury.
preparticipation physical evaluation to identify medical problems such
as asthma or diabetes mellitus that affect training or participation
and previous significant injuries such as fractures and sprains that
should be assessed before clearing the athlete to participate (8).
of general health, physical fitness, strength, flexibility, and joint
stability and alignment should be performed (9).
preseason practice begins, and is the responsibility of the coach,
parent, and athlete. As well as general aerobic fitness, sport-specific
conditioning is recommended to prevent sport-specific injuries.
Athletes involved in throwing sports should work on strengthening and
stretching exercises for the shoulder girdle and upper extremity (10).
Controversy exists as to the benefit of stretching programs in the
prevention of muscle-tendon strains or apophysitis. There are no
studies that have proven the efficacy of stretching in reducing the
incidence of injury, but most coaches, trainers, and sports medicine
personnel continue to advocate their use (8).
injury prevention is the coach. The coach should be qualified in
sport-specific methods of training, injury prevention, injury
recognition, and proper rehabilitation of the injured athlete before
return to participation. An understanding and knowledgeable coach can
make a lasting impression on the athlete, especially at the youth level.
without any concern for overuse injury or effect on growth, as long as
the program is supervised and submaximal weights are employed.
structured activities enables an athlete to return to normal activity
or function.
assess the functional limitations of the injury, physical therapy is
actively involved in the rehabilitation process to enable the athletes
to resume their previous level of activity.
process and determine when joint functions, muscle strength, and
sport-specific functions are restored (11).
sprains, the need for supervised rehabilitation is questionable.
However, for major joint injuries such as significant ligament sprains,
fractures, and significant resistant overuse syndromes, physical
therapy will usually aid the athlete to a speedier return to activity
and may also prevent further or repetitive injuries (12).
of acute care when the limb is put at relative rest, and pain and
inflammation are controlled by ice, elevation, and compression (11,12,13).
The next phase, or intermediate phase, is aimed at the resolution of
pain and restoration of joint motion, flexibility, and strength.
pain and swelling, blood flow, and muscle spasm. It is the agent of
choice for nearly all acute injuries and even overuse injuries.
spasm and increases blood flow and soft-tissue relaxation. It has a
limited role in acute injuries or overuse syndromes when swelling and
inflammation are present.
exercise is initiated to improve joint range of motion and to stretch
and strengthen muscles.
mobilization in which the athlete moves the injured joint. Passive
mobilization utilizes another individual, usually a therapist, to move
the patient’s contracted joint. This technique is often complicated by
exacerbation of the injury, tearing or stretching of soft tissues, and
hemorrhage, and should only be done by an experienced therapist when
active mobilization has failed (11,12,13).
flexibility after an injury. Static stretching employs techniques in
which the involved or target muscle is stretched or maintained for
approximately 20 seconds. It is safer than ballistic stretching in
which sudden bounces or joint motions are permitted. Ballistic
stretching can cause activation of the stretch reflex and cause
muscle-tendinous strain and is not recommended after acute injuries.
program and includes isometric, isotonic, isokinetic, concentric and
eccentric, closed kinetic chain, and functional exercises.
exercises are most important in the early phase of rehabilitation after
injury because the injured joint or muscle is not moved. The exercises
are simple to perform and do not require specialized equipment. To the
patient’s relief, isometric exercises are relatively painless.
against fixed resistance while the joint moves through its arc of
motion. Examples are free weights and weight machines. Isotonic
exercises are initiated after pain and swelling subside and joint
motion is restored. Motor performance is superior following isotonic
exercise compared to isometric exercise.
contraction during specific activities and are usually provided by
specific and expensive therapeutic machines. The exercises are
performed at a constant velocity.
during exercise (e.g., biceps curl). Eccentric exercises involve
lengthening of the muscle while opposing gravity (e.g., elbow extension
with free weights after biceps curl). Significant increase of muscle
strength occurs with eccentric exercise (11,12). Eccentric conditioning is introduced during the latter stages of rehabilitation.
performing work (for example, foot on floor while performing squats)
whereas open chain kinetic exercises do not fix the body part (e.g.,
leg lift). Closed chain exercise improves agonist and antagonist muscle
contraction (13). These exercises are performed in the later stages of rehabilitation because they may cause pain.
involved in a specific sport and involve the integration of several
muscle groups working together. This is the final step in the
rehabilitation process before the athlete returns to the sporting
activity.
electrical stimulation to block pain impulses from the site of injury
or site of surgery (11,12,13).
Most sports injuries in the skeletally immature are amenable to
nonoperative management. Not all injuries require supervised
rehabilitation, but it has been shown to lessen recovery time and
decrease reinjury rates.
and the perception of societal reward for exceptional athletic success
are the major reasons why young athletes consider the use of these
substances (14).
scrutiny by international athletic organizations as well as the press
in an attempt to publicize their role in the performance of elite
athletes.
and proper diet, anabolic steroids have been shown to increase muscle
size and strength; but there is little, if any, evidence that their use
resulted in improved performance or increased aerobic capacity (14,15,16).
indicate patterns of use in up to 10% to 15% of boys and up to 2% to 4%
of girls among high-school students (17).
Anabolic steroid use is determined by a complex set of factors that
includes potential beneficial effects of anabolic steroids,
dissatisfaction with current body size and strength, a peer group
involved in their use, and a tendency toward risk-taking behavior (14,15,16,17).
forms. The oral form is metabolized in the liver and converted to
testosterone. The injectable form is directly absorbed into the
circulation and is therefore less hepatoxic than the oral form (16).
epiphyseal closure has been documented with intake of a single cycle of
anabolic steroids (14,15,16). In addition, strains and ruptures of the tendons have been noted in young individuals without any predisposing tendonitis (15,16).
elevation of liver enzymes, blood-filled cysts in the liver which may
rupture and cause fatal hemorrhage, and benign and malignant neoplasms (15).
(reversible) and an increase in total cholesterol with a reduction in
high-density lipoproteins (16,17). Prolonged use may lead to arteriosclerotic heart disease and cardiomyopathy (16,17).
testicular atrophy (14,15,16,17). Women may develop masculinization including hirsutism, deepening of the voice, and baldness (14,15,16).
pressure to avoid cheating are probably necessary. Imparting proper and
current medical knowledge without the use of scare tactics may help.
Encouragement and availability of proper programs in strength training,
conditioning, proper nutrition, and acquisition of sporting skills is
probably the best deterrent.
growing adolescents is a controversial topic. The controversy exists
because of the belief that weight training causes damage to the physes
or joints and the perceived association of weight training with
performance-enhancing drugs. Both are enough to cause parents to
question the potential benefits of weight training against its
perceived risks to their child.
In girls and prepubescent boys, it is postulated that a gain in
strength occurs due to enhanced recruitment of motor units rather than
muscular hypertrophy (18,19,20).
weight lifting in children, including distal radius and ulnar
fractures, distal radial epiphyseal fractures, patellofemoral pain,
clavicular osteolysis, pelvic apophyseal fractures, and meniscal tears (19,21,22,23). In adolescents, lumbosacral injuries such as disc herniation, spondylolysis, and spondylolisthesis are common injuries (24). Power lifting and Olympic-style weight lifting are not recommended for the skeletally immature (18).
However, there are no deleterious consequences to a well-supervised
program of weight training in the growing youth, provided the movements
are done in a slow, controlled fashion with submaximal weights (25,26).
recurrent, or habitual. Acute dislocations tend to occur in adolescent,
high-level athletes, whereas recurrent instability occurs in
individuals with well-known anatomic variants such as ligamentous
laxity, patella alta, and genu valgum (27).
Even with acute patellar dislocations there is commonly an underlying
anatomical abnormality that predisposes to the dislocation. The common
age for acute patellar dislocation is from 14 to 20 years (27,28,29).
requires an understanding of the anatomy of the extensor mechanism of
the knee. There are three distinct layers around the patellofemoral
joint as part of the extensor mechanism. The superficial layer involves
the fascia overlying the sartorius muscle. The second layer comprises
the patellar retinaculum and the medial patellofemoral ligament. The
final layer comprises the medial collateral ligament and the joint
capsule (30,31).
is the medial patellofemoral ligament. It arises from the adductor
tubercle and inserts along the medial patellar border on its superior
two thirds. The medial patellofemoral ligament varies widely in size,
shape, and strength, and provides from 50% to 80% of the restraining
force to lateral displacement (32,33,34,35,36,37,38).
to the knee. The foot is planted, knee flexed and in valgus, and an
internal rotation moment applied to the femur (39).
Patellar dislocation is commonly associated with this mechanism of
injury in basketball, football, baseball, gymnastics, and also in falls
(29).
side of the patella or to the lateral side of the knee with a valgus
force may cause patellar dislocation (40).
mechanism not unlike that for an anterior cruciate ligament (ACL) tear.
The patient may describe something moving out of position in the knee
and then popping back into place. The knee quickly becomes swollen and
the child is reluctant to move it.
reduced or relocated. In the rare instance where it has not reduced,
the child’s knee is usually still flexed. The patella may be palpated
on the lateral aspect of the lateral femoral condyle. If the knee is
passively extended and a gentle medial force applied to the patella,
the patella should reduce very easily. A large, tense hemarthrosis
accompanies an acute patellar dislocation. Plain radiographs,
specifically anteroposterior, lateral, and Merchant view (also known as
skyline views) should be carefully
evaluated for patellar reduction, lateral tilt, and osteochondral
fracture. The Merchant view is an axial view of the patellofemoral
joint with the knee flexed to a consistent 35 to 45 degrees (41) (Fig. 32.1).
dislocation is similar to an ACL tear, and careful examination to rule
out the latter must always be performed.
if required. If the patella cannot be relocated with the patient
supine, reduction can be facilitated by placing the patient prone. This
allows the hamstrings to relax and with gentle extension of the knee,
the patella will reduce.
 |
|
Figure 32.1
Tangential x-ray view for evaluating the patello-femoral joint. Merchant view allows the quadriceps mechanism to relax. The patella is not artificially held reduced in the distal femoral groove. |
depends on the presence or absence of an osteochondral fracture. The
incidence of ostoechondral fracture following patellar dislocation
ranges from 5% to 50% (42,43,44,45).
If an osteochondral fracture is detected, a knee arthroscopy is
recommended to visualize the fragment and to determine if the fragment
should be replaced or excised. If the fragment is greater than 2 cm and
has a significant bony component, fixation should be performed with any
number of fixation techniques: low-profile cannulated screws
countersunk to avoid abrasion, Herbert screws countersunk in the
articular cartilage, or bioabsorbable pins or screws (39).
In most cases, the fragment is smaller than 2 cm in diameter and should
be excised. If significant anatomic abnormalities also exist,
consideration of surgical correction at the time of treatment of the
osteochondral fracture should be considered (42,43).
osteochondral fracture involves brief immobilization, then vigorous
rehabilitation. The principles of rehabilitation have been elucidated
previously and are aimed at resolving the hemarthrosis, reducing the
pain, improving the range of motion, and increasing the strength of
both the quadriceps and hamstrings (29,38,40).
sports is permitted. There should be no effusion, full range of motion,
and approximately 80% of the strength of the uninjured knee prior to
resumption of athletic activities. The use of a patellar stabilization
brace is recommended during sports.
is imperative to obtain appropriate radiographs to rule out an
osteochondral fracture. If symptoms persist, magnetic resonance imaging
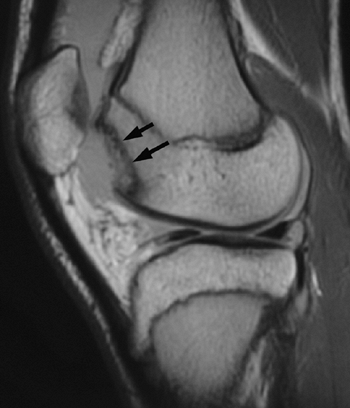
(MRI) may be helpful in visualizing a chondral or osteochondral defect (Fig. 32.2).
If no fracture is detected, rehabilitation is begun. If an
osteochondral fracture is detected, a knee arthroscopy determines
whether it should be excised (if the fragment is less than 2 cm with
very little subchondral bone) or replaced (if the fragment greater than
2 cm with significant subchondral bone). Replacement is accomplished by
an arthrotomy with the use of small cannulated screws countersunk to
the level of the subchondral component. Acute repair of medial
structures including medial patellofemoral ligament and patellar
retinaculum is carried out, and usually a lateral retinacular release
is done at the same time. The knee is immobilized for approximately 10
to 14 days in a soft dressing and knee immobilizer, followed by
vigorous rehabilitation.
 |
|
Figure 32.2
Lateral image of knee [magnetic resonance imaging (MRI)] with large osteochondral defect of lateral femoral condyle after acute patellar dislocation. Arrows (solid black) point to defect in lateral femoral condyle. |
several features which predispose to the recurrence. Anatomic factors
include an increased Q angle, increased femoral tibial valgus,
excessive external tibial torsion, femoral condylar dysplasia, patella
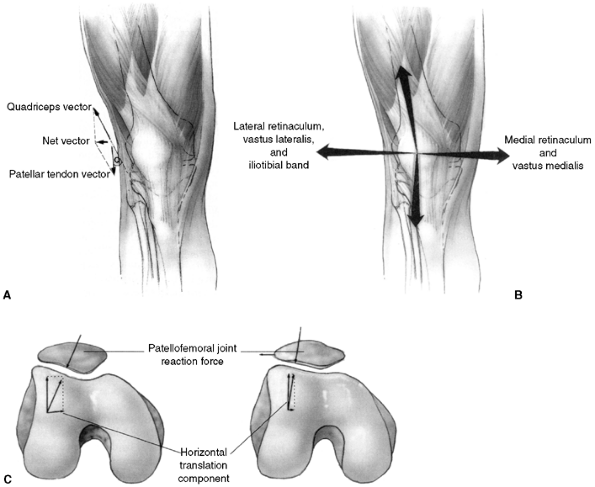
alta, and generalized ligamentous laxity (27,28,29,39,47,48,49). The Q angle is the angle formed by a long axis drawn along the quadriceps
mechanism from the anterior superior iliac spine (ASIS) to the midaxis
of the knee joint subtended by a long axis drawn along the patellar
tendon. A normal Q angle is 10 degrees or less (Fig. 32.3 A,B,C).
Children with recurrent patellar instability exhibit a positive
apprehension test. Apprehension is produced when an attempt is made to
displace the patella laterally with the knee flexed approximately 30
degrees.
four recurrences of patellar dislocation and the instability affects
his or her lifestyle. Correction of the anatomic variants is crucial
for the long-term outcome.
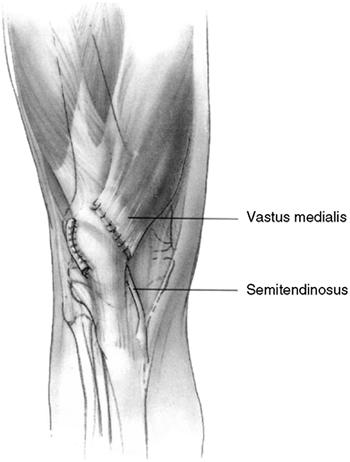
patella, including lateral retinacular release, reconstruction of the
medial patellofemoral ligament (MPFL), vastus medialis advancement with
medial reefing, or semitendinosis tenodesis.
 |
|
Figure 32.3 Patellofemoral biomechanics. A:
The Q angle relates the direction of pull of the quadriceps mechanism to that of the patellar tendon. These are the two most powerful forces exerted on the patella. Their vector sum is directed laterally. B: There are additional soft tissue forces applied to the patella. C: The laterally directed net vector is opposed by the patellofemoral articulation. If the groove is shallow, there is less potential resistance to horizontal translation than in knees with a deeper femoral groove. The dysplastic patellofemoral articulation results in less resistance to lateral translation, and therefore greater sheer forces on the articular surface. |
instability is rarely indicated. There are isolated reports of its
success in the treatment of recurrent patellar dislocation, but its
exact role in this condition remains to be determined (50,51).
Excessive lateral retinacular release combined with aggressive medial
reefing may result in iatrogenic medial subluxation, and must be
avoided (33,52,53). Lateral retinacular release usually must be combined with reconstruction of the MPFL or by vastus medialis advancement.
If the natural tissue is found to be deficient, the MPFL should be
reconstructed using a free hamstring graft, usually the semitendinosus (33). The graft is anchored by suture anchors at the adductor tubercle and to the medial superior border of the patella.
a vastus medialis obliquus (VMO) advancement distally and laterally
with medial reefing will correct the medial deficiency (54,55,56). In a child 12 years or younger, the medial reefing may be augmented by the semitendinosus tenodesis described by Galeazzi (57,58).
This graft reproduces the vector of the patellotibial ligament. It is
employed when there is persistent instability after already performing
a lateral release and MPFL reconstruction or VMO advancement (Fig. 32.4).
advocated as well. If the individual is a skeletally mature youth, the
tibial tubercle is osteotomized and shifted medially without distal
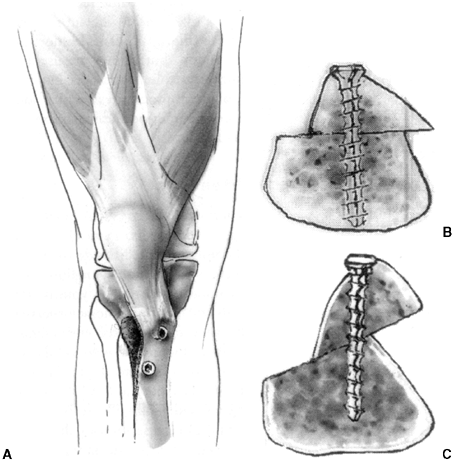
transfer (Elmslie-Trillat procedure) (59) (Fig. 32.5 A,B). A modification of the Elmslie-Trillat procedure is the Fulkerson procedure (60),
in which a more generous osteotomy of the anterior tibial tubercle is
performed and the tubercle transferred anteriorly and medially (Fig. 32.5C).
This procedure is primarily reserved for patellofemoral pain in adults
and is not recommended for instability in adolescents or young adults.
tibial tubercle apophysis, osteotomy is contraindicated because of the
potential for growth arrest and genu recurvatum. In these cases, the
patellar tendon may be split, and the lateral half delivered beneath
the medial portion of the patellar tendon and sutured to the periosteum
of the proximal tibia medially by direct suture or by suture anchors (61) (Fig. 32.6). Acceptable results can
be expected in up to 90% of cases employing the Roux-Goldthwait procedure (61).
 |
|
Figure 32.4
The Galeazzi procedure transfers the semitendinosus to the inferior pole of the patella. From there, it courses through a drill hole placed obliquely through the patella, exiting the superior lateral aspect. The tendon is then sutured to the soft tissues. This provides a medial tether and effectively alters the net vector of the patellar tendon toward the medial side. Typically, the vastus medialis is advanced approximately one-third the width of the patella. |
 |
|
Figure 32.5 A, B: The Elmslie-Trillat technique shifts the tibial tubercle medially. The tubercle stays in the same plane. C:
The Fulkerson modification involves an oblique cut that results in anterior translation as the tubercle is moved medially. This reduces the patellofemoral contact forces while shifting the pull of the patella medially. |
 |
|
Figure 32.6
The Roux-Goldthwait procedure splits the patellar tendon. The lateral half is transferred beneath the medial side and sutured to the periosteum along the metaphysis. This redirects the patellar tendon vector more medially. |
procedure is very important. It is crucial to move the knee early, and
immobilization in a removable knee immobilizer for 3 to 4 weeks is
sufficient for healing. Active range of motion and strengthening are
essential parts of the rehabilitation program and a resumption of
sports activity can be anticipated in 4 to 6 months.
instability include a thorough analysis of anatomic factors
preoperatively, and intraoperative evaluation of the reconstruction. I
employ a stepwise surgical protocol that almost always involves a
lateral retinacular release and medial VMO advancement. If the Q angle
is within normal limits and the patella is stable, there is no need for
further surgery. It is imperative that the knee is put through flexion
and extension to ensure normal patellar tracking and to ensure the VMO
advancement is not too ambitious, in which case it will limit flexion.
Reconstruction of the medial patellofemoral ligament by means of an
autogenous hamstring graft is an option in the adolescent who is
nearing or has attained skeletal maturity (33).
The graft is double stranded and attached by means of suture anchors to
the medial superior portion of the patella and to the adductor tubercle
area of the femur.
performed; the Roux-Goldthwait procedure is used for the skeletally
immature patients up to 14 years of age, and the Elmslie-Trillat
procedure for patients older than 14 years.
article of 1948, the deleterious late effects of partial or total
meniscectomy, namely, degenerative joint disease, in children and
adolescents were amplified. Other authors have provided similar
evidence with long-term results after meniscectomy in children and
adolescents (63,64,65,66,67,68,69).
Therefore, preservation of the meniscus, either partially or in its
entirety, is a principle of treatment of a torn meniscus in a young
patient.
The menisci have a blood supply which arises from the periphery, but
extends throughout the meniscus during intrauterine development. By
approximately 1 year of age the central third is avascular, and by the
age of 10 years the adult vascular pattern prevails (71).
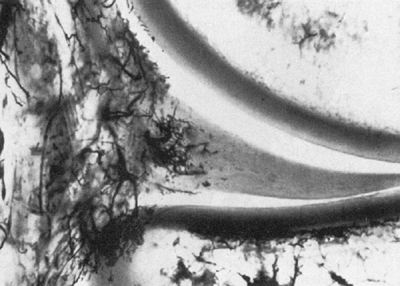
Arnoczky’s classical vascular injection studies revealed that the
peripheral 10% to 30% of the medial meniscus and the outer 10% to 25%
of the lateral meniscus have a vascular supply (72) (Fig. 32.7). Branches of the superior, inferior, medial, and lateral geniculate
arteries form a peripheral vascular plexus. Therefore tears in the
peripheral zone (so-called red-red zone) are capable of healing and
form the basis for the meniscal repair or suturing techniques that
should always be considered in children.
 |
|
Figure 32.7
Vascularity of the human meniscus, as seen in a frontal section of the medial compartment of the knee. Branching vessels from the perimeniscal capillary plexus penetrate the capsular third of the adult meniscus. (From Maffuli N, Testa V, Vapasso G. Mediopatellar synovial plica of the knee in athletes: results of arthroscopic treatment. Med Sci Sports Exerc 1993;25(9):985.) |
the fibers are oriented in a circumferential pattern. There are
additional radial, oblique, and vertically oriented fibers, which
resist hoop stresses (73,74).
there is normally approximately 2 to 3 mm of posterior translation as
the knee flexes. Contrast this with the more unstable lateral meniscus,
with up to 10 to 11 mm of posterior translation, and the reasons for
fewer lateral meniscal tears become more apparent (73,74).
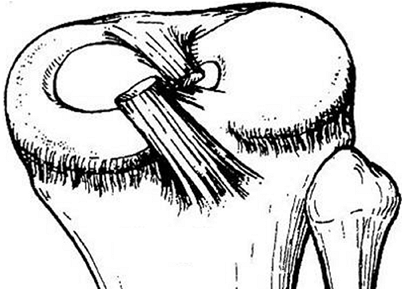
the child is the discoid meniscus. Almost always involving the lateral
meniscus, this abnormality can cause a loud “snapping” sensation. The
incidence of this condition has been reported to be as high as 3% to
5%, higher in the Asian population, and it occurs bilaterally in up to
20% of cases (75,76,77,78).
but there is no accepted explanation for its development. The lateral
meniscus does not acquire a discoid shape in its embryologic
development, and this condition appears to be an anatomic variant (70,71).
Discoid menisci are more prone to tears because of increased mechanical
stress transmitted to their larger surface area, and because of
hypermobility (75,79,80,81).
 |
|
Figure 32.8 Type I discoid meniscus covering entire lateral tibial plateau and with intact meniscotibial ligaments.
|
 |
|
Figure 32.9 Type II discoid meniscus covering 80% of lateral tibial plateau with intact peripheral attachments.
|
depends on the type of discoid meniscus. The discoid meniscus with
deficient peripheral attachments (Type III) presents in a young child
of 2 to 3 years of age as a “snapping knee syndrome.” As the knee is
brought from flexion into full extension, a painless, palpable, and
audible snap occurs. The child may also have painless giving way
resulting in unexplained falls. Type I and Type II discoid menisci do
not usually present until the child or adolescent actually tears the
discoid meniscus, which is prone to happen due to its large surface
area. These patients have joint-line pain and tenderness, and have an
effusion. Catching, locking, and giving way are also suggestive of
tears in a discoid meniscus if the location is lateral. This typically
occurs in the middle of the child’s 2nd decade of life as the child
approaches skeletal maturity, or in early adulthood. In other respects
Type I and Type II discoid menisci are asymptomatic.
diagnosis. Criteria to define a discoid meniscus include a transverse
meniscal diameter of more than 20% of the tibial width on transverse
images, or a transverse meniscal diameter of more than 15 mm and
continuity between anterior and posterior horns on three or more
successive sagittal images (83). MRI can detect tears within a discoid
meniscus, but is not sensitive enough to detect subtle peripheral
detachments. MRI has a high positive predictive value for discoid
menisci, but a low sensitivity (83,84).
In other words, a discoid meniscus will be present in almost all cases
when picked up by MRI, but MRI will miss many cases of discoid meniscus.
 |
|
Figure 32.10 Type III discoid meniscus covering 75% to 80% of lateral tibial plateau with deficient peripheral attachments.
|
patient is symptomatic or not. For stable, asymptomatic discoid menisci
detected by MRI or at arthroscopy, no treatment is indicated or
recommended. For the patient with symptoms strongly suggestive of a
torn discoid meniscus but not detected by MRI, arthroscopic examination
is warranted.
discoid meniscus is stable or unstable. If the discoid meniscus is
stable, “saucerization” of the central portion by arthroscopic or open
technique is indicated (75,85,86,87,88,89,90).
In a young child with the complete variety, it is often difficult to
visualize the portion of the meniscus to be resected arthroscopically.
It may be safer for the inexperienced arthroscopist to do a mini
arthrotomy to visualize the central portion of meniscus to be resected
as well as the stable peripheral rim. A peripheral rim of 6 to 8 mm of
meniscus should be left.
punches, scissor punches, and biters are employed with the knee in
flexion. Small arthroscopic shavers complete the saucerization
technique with the knee flexed and in the “figure 4” position.
peripheral stability. This is best accomplished by arthroscopic
technique. If peripheral detachment or instability is encountered,
meniscal suturing is indicated. The suture technique is best
accomplished by an inside-out technique and open incision
posterolaterally to identify and protect the common peroneal nerve when
the sutures are tied (85).
restricted range of motion for approximately 4 weeks. This may best be
accomplished by a bent-knee cast in very young children who cannot
cooperate with crutches or other measures.
discoid menisci are limited, but good results have been reported in up
to 75% of cases in a few selected series (80,81,82).
In addition, there is very little information as to the long-term
results of saucerization technique, especially as it relates to the
incidence or prevention of degenerative changes in the knee (75,87). Long-term studies will be needed to delineate and provide evidence of the efficacy of the treatment.
adolescents are rare. They are more commonly seen in conjunction with
an ACL tear or if the meniscus is abnormal in shape (i.e., discoid).
They are extremely rare in children younger than 10 years if the
meniscus is normal in size and shape (91).
Most meniscal injuries are longitudinal in children and adolescents,
with medial meniscal tears being more prevalent than lateral meniscal
tears. Approximately 30% of meniscal tears in patients younger than 20
years are repairable, and two-thirds of repairable tears of the
meniscus are seen in conjunction with ACL tears (92).
sports that involve twisting or pivoting motion, such as basketball,
soccer, and football (91,93).
Symptoms include pain along the joint line, swelling, giving way, and
locking. On physical examination, joint line tenderness and an
associated effusion are the most common findings. Differential
diagnosis should include: osteochondritis dissecans (OCD),
osteochondral fracture, patellofemoral pain and instability, tibial
eminence fracture, and even popliteal or patellar tendonitis or
synovial plica.
other conditions such as tibial eminence fracture, osteochondral
fracture, or OCD. The gold standard for delineation of meniscal
pathology by imaging is MRI, although normal signal changes in the
posterior horn of both the medial and lateral meniscus often result in
false-positive reports (83,84,94,95).
These signal changes probably represent persistent vascular
developmental changes and are normal variants in children and
adolescents. MRI is not a fail-safe tool with absolute accuracy and
should not be ordered as a screening test for intraarticular pathology.
treatment because they are usually large and often associated with ACL
pathology (73,75,96).
Meniscal suturing is the procedure of choice for most tears in the
red-red zone or red-white zone, but must be accompanied by ligament
reconstruction or protection of the knee by bracing if the ACL is torn (73,96,97).
Meniscal stabilization alone in an ACL-deficient knee will most likely
fail. ACL reconstruction techniques are discussed separately.
partial or complete meniscectomy, meniscal stabilization is
recommended. For middle-third (red-white zone) and outer-third (red-red
zone) tears, there is sufficient healing potential to warrant repair.
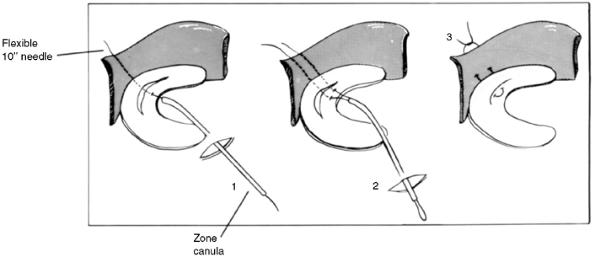
The most common technique of meniscal repair involves an inside-out
technique with sutures passed through cannulae (Fig. 32.11).
Care must be taken to avoid neurovascular structures, specifically, the
saphenous vein and nerve medially and the peroneal nerve laterally. It
is recommended that small skin incisions be made to identify the
sutures and ensure that the sutures are tied at the capsule, avoiding
any neurovascular structure.
potential problems of sutures. These devices are inserted as an
all-inside device and are most often employed for small posterior horn
tears. There are few, if any, reports of their use in children or
adolescents, and they are not recommended for widespread use.
 |
|
Figure 32.11 Diagram illustrating inside-out technique of meniscal suturing. (1) Suture passed through tear and outside knee. (2) Second suture passed. (3) Suture tied to approximate edges of meniscal tear.
|
knee motion are an important adjunct to the surgical repair of the
meniscal tear. Range of motion is restricted from 0 to 45 degrees for
the first 2 weeks, and flexion is restricted to less than 90 degrees
for the first 6 weeks. The patient is permitted only toe-touch weight
bearing for the first 6 weeks. Because most skeletally immature
patients undergoing meniscal repair also undergo an ACL reconstruction,
this protocol has become part of the rehabilitation following ACL
reconstruction.
immature patients are excellent, regardless of the necessity for
concomitant ACL reconstruction (98). Noyes and
Barber-Westin reported a success rate of 75% in patients younger than
20 years who underwent repairs extending into the avascular zone (99,100). In a group of patients 20 years or younger who underwent isolated meniscal repair, Johnson et al. (101) reported healing in 76% of the patients at 10-year follow-up.
after repair include concomitant ACL reconstruction, younger age of
patients, peripheral tears in the vascular zone, small-sized tears
<2.5 cm and time lapse from injury to surgery of less than 8 weeks (63,64,73,92,96,100,102,103,104).
described by Poncet in 1875, and controversy still exists as to the
mechanism of injury and appropriate treatment plan (105).
but with increasing participation in competitive sports, these injuries
are now often associated with various sporting activities. The
mechanism involved is usually a hyperflexion or hyperextension force,
with or without the combination of varus or valgus, and a rotational
moment about the knee (107,108,109).
before the fracture of the eminence. The residual sagittal laxity
observed in many of these patients is no doubt due to the in-substance
elongation of the ACL (107,111,112,113).
Type I fractures are minimally displaced, Type II are elevated
anteriorly but remain intact posteriorly through a hinge of bone and/or
cartilage (trap-door configuration), and Type III are completely
displaced and separated. Type IV fractures are displaced and comminuted.
and the ability of the surgeon to reduce it by knee extension. The
position of immobilization varies from surgeon to surgeon and is based
on the tension placed on the ACL in varying degrees of flexion. The
ligament is under increased tension at 0 degrees (posterolateral
bundle) and at 45 degrees flexion or greater (anteromedial bundle) (115).
As a consequence, a position from 10 to 30 degrees flexion has been
recommended, but the most important aspect is to reduce the fragment
into its bed. In a recent study, Kocher et al. (116) found a 26% incidence of medial meniscus entrapment in Type II injuries and a 65% incidence in Type
III injuries. Therefore, in order to remove the incarcerated meniscus
and allow for anatomic reduction, arthroscopic or open reduction should
be considered for Type III fractures and for Type II fractures that do
not reduce in extension. Numerous fixation methods have proved
effective, including various suture techniques, cannulated screws, and
more recently, bioabsorbable fixation pins (117).
The exact technique depends on the particular fracture pattern and the
surgeon’s experience with a particular technique. If the surgeon is
unable to fix the fragment by the arthroscopic technique, a small
arthrotomy is performed with reduction of the fracture and fixation by
one of several available methods.
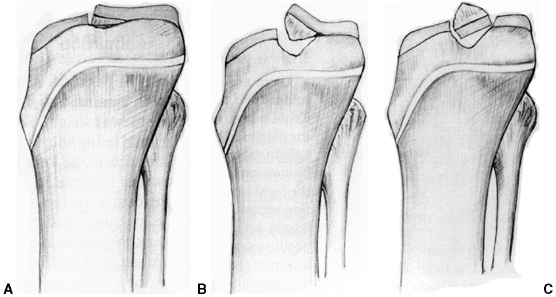 |
|
Figure 32.12 Meyers and McKeever classification of tibial eminence fractures. A: Type I, no or very minimal displacement. B: Type II, displaced but with intact posterior hinge. C: Type III, totally displaced eminence fracture.
|
cylinder cast for approximately 4 to 6 weeks. In Type II and Type III
injuries, an attempt is made to reduce the fragment closed by fully
extending the knee. If the patient resists the extension maneuvre,
aspiration of the hemarthrosis and injection with local anesthetic (5
to 10 mL of 0.25% bupivicaine hydrochloride, Abbott Laboratories) may
facilitate the reduction.
brought into full extension, I perform an arthroscopy with thorough
irrigation and joint lavage. Blocks to reduction of the eminence
fragment (meniscus or intermeniscal ligament) are removed and fixation,
usually with a small, l 4.0-mm cannulated screw, is performed (Fig. 32.13 A,B,C)
closed methods are employed. Active range-of-motion exercises and
strengthening should begin immediately after cast removal.
stable, early range of motion and strengthening is begun, but
protective weight bearing is enforced for approximately 6 weeks.
is regained and most of the strength restored, which often takes 3 to 4
months.
are generally excellent if the fragment is reduced. Some sagittal plane
instability is a common feature after treatment, but this objective
finding does not correlate with subjective symptoms in most patients (75,105,107,111,112,113,117). The core of treatment remains reduction of the fragment by closed means, open reduction, or arthroscopic technique.
been considered a relatively rare occurrence. Increased participation
in organized sports, especially by girls, and recent improvements in
diagnostic ability, including specialized imaging techniques such as
MRI, are responsible for the reported increase in incidence of this
injury (118,119,120,121,122,123,124,125).
Expectations from family and coaches about full return to athletic
activities by the injured child are based on well-publicized reports of
similar injuries in elite athletes. The decision about treatment of an
ACL disruption in the skeletally immature child should only be made
after all the risks and benefits of each treatment option are analyzed,
including nonoperative management with modification of the athlete’s
activities.
the Cleveland Clinic, five patients were younger than 12 years (125).
A recent study of insurance claims by players in youth soccer leagues
in the United States revealed approximately 550 claims for ACL injury
(both sexes) out of a total of 8215 claims. The total number insured
during the period was approximately 6 million, giving an incidence of
approximately 0.01% (126). Girls in this age group are two to nine times more likely to disrupt their ACL than boys (127).
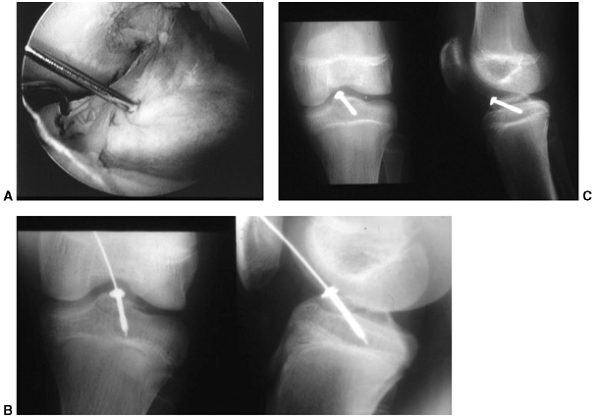 |
|
Figure 32.13 A:
Arthroscopic image of displaced tibial eminence fracture. Guide wire is transfixing eminence in place prior to insertion of cannulated screw. B: Screw and washer have been introduced over guide wire to hold the reduced eminence fracture. Note screw stops short of proximal tibial physis. C: Final intraoperative x-rays: Screw provides enough stability to allow early range of motion (2 to 3 weeks after surgery). |
intercondylar notch of the femur posteriorly and inserts on the tibia
at the anterior aspect of the tibial eminence. It comprises of two
distinct bundles, an anteromedial and a posterolateral, whose function
is to provide anteroposterior stability throughout the range of knee
flexion and extension. In extension, the posterolateral bundle
lengthens and the anteromedial bundle shortens. In flexion the
posterolateral bundle shortens and the anteromedial bundle lengthens.
The primary function of the ACL is to prevent excessive anterior
translation of the tibia, but it also aids in preventing
hyperextension, excessive varus and valgus, and internal rotation of
the tibia (115,128,129,130,131,132,133,134).
supply is from branches of the inferior medial and inferior lateral
genicular arteries, through the infrapatellar fat pad, and posteriorly
near its origin from branches of the middle genicular artery (135).
but other factors have been implicated, including differences in
strength and conditioning, especially proprioceptive training (136,137), increased joint laxity (137), differences in limb alignment (136,137), and hormonal factors (138). The reasons for the disparity in incidence are probably multifactorial (135).
documented in adults. Chronic ACL deficiency leads to intraarticular
damage including meniscal tear and chondral damage, especially if the
individual continues to participate in physically demanding sports
involving change of direction or rapid deceleration (139,140,141).
untreated ACL injury in the skeletally immature. It is assumed that
similar deleterious intraarticular pathology
ensues if knee stability is not obtained and there is no modification of the patient’s activities. Graf et al. (142), McCarroll et al. (122), and Angel and Hall (118)
have all reported on the high incidence of continuing symptoms,
meniscal tears, and inability to resume preinjury activity levels.
Recent studies have revealed a significant incidence of meniscal tears,
osteochondral lesions, and degenerative changes in skeletally immature
patients not undergoing ACL reconstruction (143,144).
in the skeletally immature patient—direct and indirect. In the younger
child, direct trauma to the anterior aspect of the knee is not uncommon
and may result in an ACL tear. In older children, as in adults,
indirect injury by a twisting motion is the most common mechanism. The
injury is commonly seen in participants of sports such as basketball,
soccer, football, gymnastics, hockey, and volleyball.
of injury. Almost always, the foot is planted or fixed and the
deforming force (often rotatory) gets applied to the knee. An effusion
usually develops quickly, but may not appear for several hours and is a
strong indicator of significant intraarticular pathology. Stanitski et
al. (123) found that 47% of patients aged 7 to
12 years with a traumatic effusion had an ACL disruption, and the
percentage increased to 65% if the patients were aged 13 to 18 years.
evaluating for an ACL tear. The presence or absence of an effusion or
any loss of motion is an important finding. In almost all acute ACL
tears, an effusion will be present due to a hemarthrosis. Loss of
extension may be due to impingement at the torn tibial stump of the
ACL. The Lachman test is performed with the knee in 20 to 30 degrees of
flexion and an anteriorly directed force applied to the tibia while the
femur is stabilized. Side to side difference and the presence or
absence of a firm end point are evaluated. The same test is performed
with the knee flexed to 90 degrees (anterior drawer test) if it is not
too painful for the patient. In addition, the pivot shift test is
performed, although the patient in the acute situation will often not
permit it. As the knee is brought from flexion of 45 to 60 degrees to
full flexion, a slight valgus and anterior force is applied to the
tibia. As the knee comes into full extension, a positive test occurs
when the tibia subluxates anteriorly on the femur, indicating a torn
ACL. As the knee is flexed, the tibia reduces. This physical finding is
easier to elicit in the patient with a chronic ACL-deficient knee.
injuries to the medial collateral, lateral collateral, and posterior
cruciate ligaments, because there may be associated ligamentous
disruptions. In the case of a very unstable knee (multiplanar
instability) in extension, careful evaluation of the vascularity and
neurologic status of the limb is mandatory. Multiplanar instability is
often indicative of a knee dislocation or distal femoral physeal injury
in which acute or delayed vascular impairment may occur.
injury is often difficult due to pain, swelling, and muscle spasm.
After 2 to 3 weeks of rest, ice, and gentle range of motion, the
patient should be reexamined, as the physical examination is often
easier to perform then.
patients with suspected knee ligament injury. Evaluation of the
radiographs should be made for bony avulsions, osteochondral fractures,
physeal fractures, and patellar dislocation or subluxation as well as
for degree of physeal closure.
controversial. It should not be done as a routine screening tool
because of its high rate of false-positive readings for meniscal tear
as well as its excessive cost. It is indicated in the patient who fails
to improve range of motion and has a persistent effusion after
conventional therapy, or in whom the physical examination is difficult
to interpret.
tears and osteochondral lesions, which may affect the decision
regarding surgery (Fig. 32.14 A,B). If the patient has clinical evidence of a torn ACL alone, an MRI is not indicated.
patients differ from their adult counterparts because of concern
regarding future skeletal growth. The treating orthopaedist must be
aware of the unique features of the growing child and the potential for
growth arrest associated with conventional surgical techniques. As a
consequence, each patient must be assessed individually to arrive at
the decision for surgical reconstruction based on the child’s activity
level, the parents expectations, and the child’s potential for further
growth. There is no need for urgent surgical reconstruction as the
incidence of arthrofibrosis is markedly increased in the acute
situation.
designed to restore the patient’s range of motion and muscle strength.
The rehabilitation program may take from 6 weeks to 3 months (135).
brace the child’s knee with an appropriate ACL brace should be
recommended. This facet of treatment should be undertaken by a
certified orthotist, and the brace will have to be custom-made for the
child. Cost for an ACL brace varies from $500 to $1500. The child is
allowed to resume limited participation in sports using the brace,
which is designed to
prevent anterior subluxation of the tibia with change of direction or stopping.
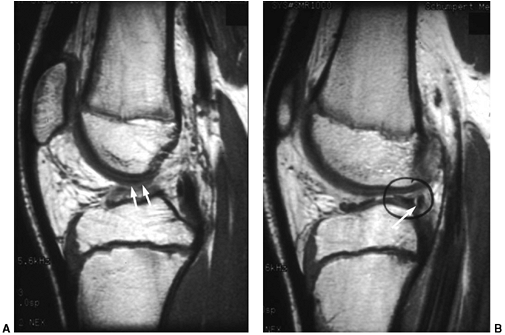 |
|
Figure 32.14 Magnetic resonance imaging (MRI) of a skeletally immature individual with disruption of anterior cruciate ligament (white arrows) (A), and peripheral separation of lateral meniscus (white arrow and black circle) (B)
|
of giving way while using the brace, full sports participation is
permitted. A frank discussion of the potential risks of continued
instability, namely, meniscal tears and chondral damage, should be
undertaken with the parents, and recommendations may be made for
modification of sports activity to decrease the exposure of the child
athlete to potential instability episodes.
with an ACL tear. If the child continues to experience episodes of
giving way or instability when using the brace, the risk of meniscal
tear and chrondral damage increases. If instability in the brace
persists or there are signs of meniscal tear, surgery is recommended to
stabilize the knee joint.
However, the inability of these procedures to control instability,
because of their nonanatomic location, results in certain failure.
of the potential for growth arrest, the isometry of the graft with
various techniques, and the specific properties of different grafts.
Stadelmaier et al. (147) and Guzzanti et al. (148)
have shown that continuing growth occurs in experimental animals if a
tendinous graft is employed through a small, centrally placed drill
hole traversing the physis. Edwards et al. (149)
have reported growth changes in experimental animals with a small,
centrally placed tendon graft to an “over-the-top” position on the
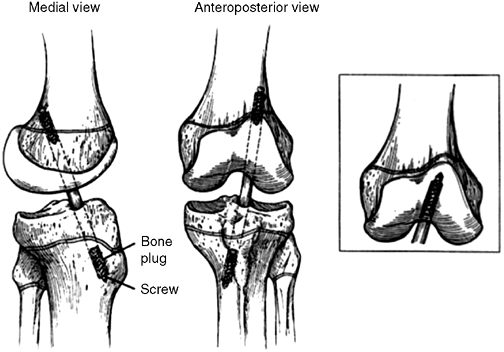
femur when excessive tension was applied to the graft.
through the intercondylar notch of the femur, through the posterior
capsule, and “over the top” of the lateral femoral condyle where it is
attached to bone by staples, a screw with a special spiked washer, or
sutures.
ACL-deficient knees in children, employing hamstring tendon grafts
through a small central drill hole in the proximal tibial physis,
various techniques for the femoral fixation including attachment via an
over-the-top position (150,151),
and drill holes exiting distal to the distal femoral physis or even
through small drill holes through the distal femoral physis (152).
have employed a unique classification system for ACL reconstruction in
the skeletally immature patient. Physis-sparing techniques that provide
a ligamentous reconstruction while avoiding both the proximal tibial
physis and distal femoral physis are used in young children (Tanner
stage 0-1) (153) (Fig. 32.15).
Critics of this technique remark on the lack of anatomically correct
orientation of this graft and therefore the inability to restore
isometry and normal knee kinematics.
tibial physis, and placement of the soft tissue hamstring graft
posterolaterally through the intercondylar notch to the lateral femoral
metaphysis (over-the-top position) (150,151). This technique is employed in children who are Tanner stage 2 (Fig. 32.16).
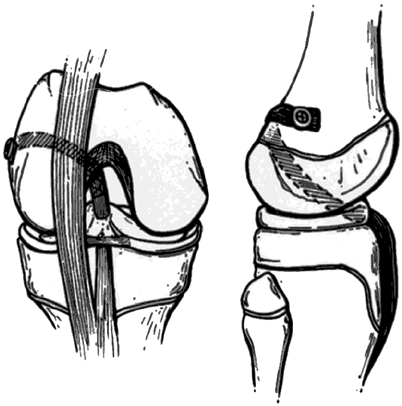 |
|
Figure 32.15
Physis-sparing technique of anterior cruciate ligament (ACL) reconstruction. ACL reconstruction employing patellar tendon graft. The graft is passed behind the intermeniscal ligament to an “over-the-top” position on the distal femur. No violation of either the proximal tibial or distal femoral physis occurs. |
reconstructions in the case of adults, but may employ smaller drill
holes and avoid any hardware crossing the physis (Fig. 32.17). Advantages of this technique are improved graft fixation and graft isometry (152). Excellent results are reported in adolescents close to maturity (152) (Fig. 32.18 A,B). Variations of this technique include the use of Achilles tendon or patellar tendon allograft (154).
children with 1 year or less of growth remaining as determined by a
wrist x-rays and the Greulich and Pyle atlas (Fig. 32.19 A,B).
patient’s compliance with a strict rehabilitation protocol. There are
standard ACL reconstruction protocols that restore range of motion and
strength and allow the graft to incorporate and mature. Return to
preinjury sports usually takes 6 months following surgery and should
not occur sooner.
from the distal femoral physis and proximal tibial physis may be
disturbed with conventional ACL reconstruction techniques. A trial of
bracing is warranted in this age group in an attempt to control their
instability and to wait until the child is near skeletal maturity. Full
sports participation is permitted if the brace successfully controls
episodes of instability and giving way. If the brace is unsuccessful, a
frank discussion regarding discontinuing sports or modifying activities
should be undertaken with the child and the parents.
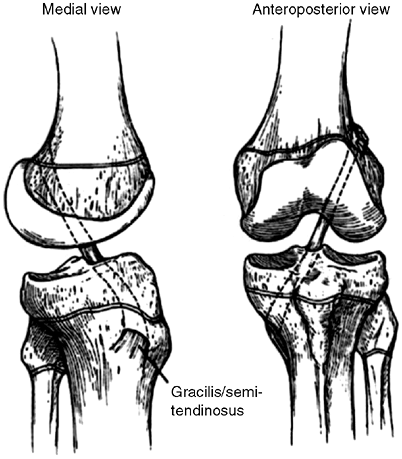 |
|
Figure 32.16
Anterior cruciate ligament (ACL) reconstruction using autogenous hamstring graft through tibial drill hole crossing proximal tibial physis to an over-the-top position on the distal femur. Hamstring graft is passed through the posterior knee capsule over the top of the lateral femoral condyle to a position on the distal femoral metaphysis where it is anchored with staples and/or sutures. |
participation and understand the potential risks of growth disturbance,
surgery should be a consideration.
predicted to have at least 2 years of growth remaining (11 to 12 years
of age in girls, 13 to 14 years of age in boys), I favor a partial
transphyseal reconstruction employing several strands of hamstring
tendon. The tibial drill hole should be 6 to 8 mm in diameter (larger
hole for older children) and oriented more vertically than that in a
standard ACL reconstruction in an adult. Rather than employing a
femoral tunnel, I favor placement of the graft to an over-the-top
position. The graft should not be tensioned as much as it is in the
adult.
maturity as demonstrated by bone age (13 years of age in girls and 15
in boys), I favor a complete transphyseal reconstruction (Fig. 32.17, 32.18A,B).
 |
|
Figure 32.17
Diagram of transphyseal anterior cruciate ligament (ACL) reconstruction. Note vertical orientation of drill holes; fixation should not encroach on either physis. |
the articular cartilage and underlying bone are involved. Originally
described by Paget (155) as “quiet necrosis,”
the condition remains an enigma as to etiology, pathogenesis,
prognosis, and treatment. Juvenile osteochondritis dissecans (JOCD) has
a much better prognosis than its adult counterpart and will be the
focus of this discussion. It is a lesion of the articular cartilage and
subchondral bone before closure of the growth plate and was originally
described by Roberts (156).
that removal and replacement of a portion of articular cartilage of the
distal femur in rabbits produced a lesion similar to OCD in humans.
Biomechanical studies using finite element analysis have demonstrated
that stresses are greatest in the subchondral bone of the medial
femoral condyle and are maximal at 60 degrees of flexion (168).
 |
|
Figure 32.18 A:
Anteroposterior radiograph of skeletally immature child who underwent transphyseal anterior cruciate ligament reconstruction utilizing autogenous hamstring tendon. Note vertical orientation of drill holes in proximal tibia and distal femur. B: Lateral radiograph of same patient. |
 |
|
Figure 32.19 A: Magnetic resonance imaging (MRI) (axial) showing small, centrally placed drill hole in proximal tibia (white arrow). B:
Lateral MRI image of same patient showing graft filling tibial tunnel. Note growth arrest lines on distal femur proximal to distal femoral physis (white arrows) indicating continued growth. |
fractures are similar to forces responsible for causing JOCD and
include impaction of a tibial spine, direct impaction forces resulting
in a “bone bruise,” joint compression forces, and rotational injury
patterns (169,170,171,172,173). In addition, Smillie (174)
has suggested that the juvenile form may be caused by a disturbance in
ossification of the epiphysis itself, resulting in the separation of
small islands of bone from the main bony epiphysis.
 |
|
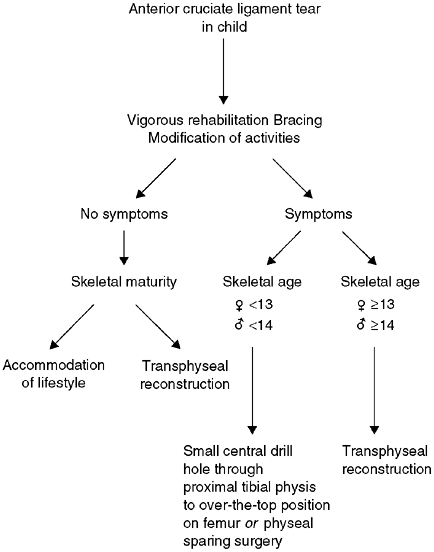
Figure 32.20 Algorithm for treatment of anterior cruciate ligament (ACL) tear in children.
|
classic article, noted that the basic process in OCD was aseptic
necrosis involving the subchondral bone. However, most other histologic
studies of OCD do not reveal ischemic changes, but a reparative process
at the interface between the fibrocartilage and the bone (175,176,177). With the advent of MRI to evaluate OCD lesions of the knee, it is apparent that ischemic changes of bone are rare.
discuss the familial nature of the lesion indicating an autosomal
dominant inheritance pattern. However, most other large series refute
the hereditary nature of the lesion (179).
including repetitive mechanical trauma or stress, in highly active
children and adolescents (180).
children. The pain is activity-related, and its location is
nonspecific. The patient may have complaints of giving way, locking, or
swelling. In a large multicenter study carried out by Hefti et al. (181), 32% of patients had no or minor pain at presentation.
involved femoral condyle. A positive Wilson test occurs with the knee
flexed 90 degrees and internally rotated. As the knee is extended, the
patient complains of pain that is relieved by external rotation. The
knee examination of
patients with JOCD is notoriously poor for detecting the presence of this disorder (182).
radiographs including a “tunnel view,” which is the best view for
seeing the lesion in the classic location on the lateral aspect and
posterior two-thirds of the medial femoral condyle.
A high-intensity signal on T2-weighted images between the lesion and
the surrounding subchondral bone means that synovial fluid from the
joint is present between the lesion and underlying subchondral bone,
implying instability of the lesion (188). In
children, the signal may represent a line of healing vascular
granulation tissue, but it is pathognomonic of instability in the adult
variety of OCD (189). MRI with gadolinium
enhancement improves the ability to assess lesion stability, and MRI
arthrography with gadolinium allows for virtually 100% ability to
assess stability (190).
In this study, patients with JOCD (patients with open physes) did
better than adults; 22% of these patients with JOCD had abnormal knees
at follow-up, whereas 42% of adults with OCD had abnormal knees.
that are stable at the time of presentation and in the classic location
as opposed to any other location. Patients who were less active had a
better result at follow-up than did active athletes. Patients with
stable lesions at diagnosis did better with conservative (nonoperative)
treatment than did those with surgery, regardless of the type of
nonoperative treatment. Conversely, patients with unstable lesions did
better with surgery than did those with nonoperative treatment. There
was no superior method of fixation or resurfacing, as the numbers in
these groups were too small for statistical analysis.
 |
|
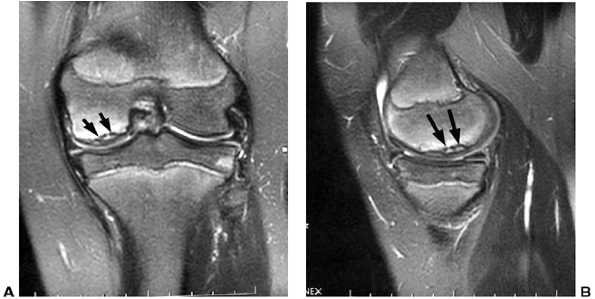
Figure 32.21 A: Magnetic resonance image (MRI) of a knee demonstrating osteochondritis dissecans lesion (black arrows)
in the classical location (lateral aspect of medial femoral condyle). The lesion appears to be stable, with an intact articular surface. B: Lateral image of same knee (black arrows outline lesion). |
prognosis in its adult counterpart. The goals of treatment in JOCD
include preservation of articular cartilage and stability of the lesion.
lesion, a period of nonoperative treatment is indicated. This usually
involves immobilization in a non-weight-bearing cylinder cast for 6 to
10 weeks. It is of interest that in the European multicenter study, the
results of all conservative treatment methods, including cast
immobilization, bracing, physiotherapy, and non—weight-bearing were the
same (181). Refraining from sports for 3 to 6 months is probably efficacious in children and adolescents with OCD lesions (156,183,185).
bone across the lesion; JOCD lesions heal in an average of 4 to 5
months (160,191,192).
period of nonoperative treatment including non—weight bearing for 6 to
10 weeks. Uncooperative patients should be managed in a cylinder cast.
Refraining from sports for up to 6 months is advisable to allow lesions
to heal.
of 6 months, or if the lesion is unstable, arthroscopic evaluation and
treatment are indicated. Guhl classified lesions arthroscopically as
(a) intact lesion, (b) early separated lesion, (c) partially detached
lesion, (d) salvageable craters and loose bodies and (f) unsalvageable
craters and loose bodies (193).
or retrograde manner to promote healing. The theory is that vascular
ingrowth occurs in the small channels created by the K-wires or drill.
Excellent results have been reported by several authors using the
transarticular drilling technique (160,190,191).
Some authors prefer not to violate the articular surface and use an
extraarticular drilling method with or without bone graft to stimulate
healing (185,193,194). Hefti’s multicenter study was more discouraging than these studies (181). In a subgroup of 58 patients demonstrating marked sclerosis, little benefit or healing was noted.
This can be accomplished by two or more divergent K-wires, by the use
of Herbert or other small fragment screws in which the heads of the
screws are countersunk below the articular surface, by bioabsorbable
pins or screws, or even autogenous bone pegs (195,196,197,198).
for 6 to 8 weeks after fixation. It is imperative to schedule a
second-look arthroscopy to see if the fixation is raised before the
resumption of weight bearing. If the screw heads are too prominent,
they are turned in further to prevent articular cartilage erosion.
freshened down to bleeding bone. The bed may require a small amount of
bone grafting before the OCD lesion is replaced and fixed. It is
important to achieve articular congruity at the completion of the
fixation procedure (189). The patient is kept
non—weight bearing for 6 to 8 weeks and on occasion rearthroscopy of
the knee is performed to evaluate if the screw heads are raised and
need to be countersunk further or eventually removed when the lesion is
definitely healed.
body or bodies are removed, and the edges of the articular cartilage
trimmed. Fragment excision alone appears to have poor long-term
results, although in the short-term knee function may be excellent. In
the European Pediatric Orthopaedic Society study, Hefti et al. (181)
reported 48% poor results after fragment excision alone. Because of the
poor results, they recommended some technique to restore the articular
surface. Anderson and Pagnani (199) also reported a preponderance of poor results in young patients at follow-up an average of 5 years after fragment excision.
These techniques need further refinement and development before they
can be widely recommended for use in children and adolescents.
injury that occurs when playing football, hockey, and even non-contact
sports such as soccer or basketball. If the force is severe enough, it
will result in muscle hemorrhage, followed by the formation of
granulation tissue over the course of the next few weeks, which can
mature into a dense collagenous scar and lead to significant disability
(210). It is therefore important to recognize this injury early to prevent long-term problems.
may occur. It is accompanied by thigh swelling, pain, and loss of knee
flexion.
be taken to rule out fracture and epiphyseal separation. The
differential diagnosis should also include osteomyelitis and tumor
(osteosarcoma or Ewing sarcoma), which can be ruled out with a careful
history and normal laboratory workup.
and elevation (RICE). The knee and thigh may be further protected by
employing a knee immobilizer and crutches. When the pain and muscle
spasm subside, gentle active range of motion is begun. Passive
stretching to increase knee flexion is not permitted and will
exacerbate bleeding and formation of scar tissue. Progressive
strengthening and exercise are permitted after 90 degrees of knee
flexion is obtained. Moderate—to—severe contusions take from 4 to 6
weeks, on an average, to heal before return to sports participation (211,212,213). Minor contusions take considerably less time.
allowing full participation in sports. Knee motion of at least 120
degrees, at least 80% strength of the opposite leg, and functional
agility are required (211,212,213). Special thigh guards can be employed to protect from further injury.
rare situation of compartment syndrome of the thigh and myositis
ossificans.
manifested by severe thigh swelling and pain after a significant
contusion, and has also been described after relatively minor trauma in
a patient with a bleeding disorder. Like its counterpart in the arm or
leg, it demands fasciotomy to prevent muscle necrosis (214).
severe quadriceps muscle contusion or after reinjury and occurs in up
to 20% of quadriceps contusions (211).
new bone may also be seen. By 3 to 6 months, the bony changes stabilize (214,215).
 |
|
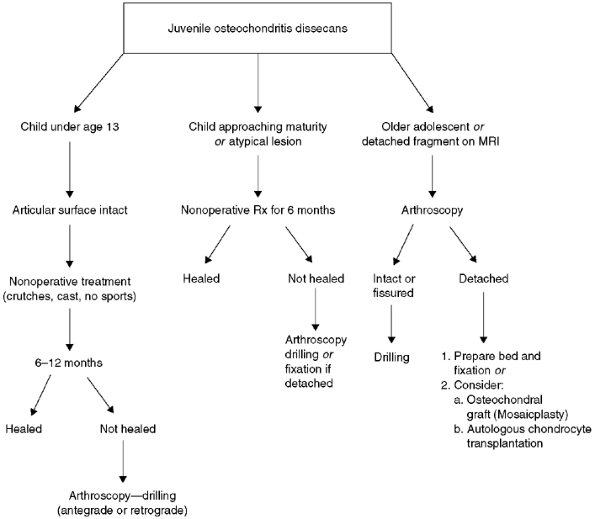
Figure 32.22 Algorithm for treatment of juvenile osteochondritis dissecans of the knee.
|
exhibits no functional deficit. No treatment is required if the patient
is functioning well, and full participation in sports is permitted.
case surgical excision should be undertaken, but only after the
myositis has matured, which usually takes 6 months. Plain radiographs
on a sequential basis will provide evidence that the lesion is mature
and not continuing to ossify. A bone scan may be helpful in showing the
lesion to be relatively quiescent in its uptake of radionucleotide,
which is suggestive of maturity of the lesion. Nonsteroid
antiinflammatory drugs (NSAIDs) may be used before and after excision
of the myositis ossificans to prevent recurrence, but there is no
evidence that NSAIDs are beneficial in this clinical situation.
Younger children are more likely to suffer an injury to the distal
fibular physis, whereas ankle sprains are more common in adolescents.
and inversion injury. The lateral ligaments; namely, the anterior
talofibular ligament, calcaneofibular ligament, and posterior
talofibular ligament are injured in that sequence.
effect of bony stability is minimized and the lateral ligaments become
the primary lateral stabilizers, with the anterior talofibular becoming
the most important (216).
and ligamentous injury is made primarily on the basis of the anatomical
location of the pain and tenderness. If the maximal tenderness is
directly over the distal fibula, a fracture or physeal injury is
suspected and x-rays are taken. If the maximal tenderness is directly
over the anterior talofibular ligament or calcaneofibular ligament,
there is little need for x-rays (217). If there
is excessive swelling and it is difficult to determine the exact area
of maximal tenderness, one should err on the side of caution and obtain
three views of the ankle—anteroposterior, lateral, and mortise.
Grade I sprains (mild) have no appreciable disruption of tissue and
there is minimal loss of function. Grade I sprains involve the anterior
talofibular ligament only. Grade II sprains (moderate) have some
disruption of tissue and partial loss of function with involvement of
the anterior talofibular and calcaneofibular ligaments. Grade III
sprains (severe) have significant or complete disruption of tissue with
involvement of all the lateral ligaments and even the deltoid medially.
There is marked loss of function. The type of sprain is best determined
by the anatomic location of the pain and swelling and the degree of
disability of the patient.
more prone to develop osteochondral fractures and chronic instability.
The diagnosis of interosseous ligament injury is based largely on the
mechanism of the injury, the physical findings, and in rare instances
radiographic findings.
conjunction with a deltoid ligament injury. They are seen when the
mechanism of injury is pronation—abduction, pronation —external
rotation, and supination—external rotation of the foot. If the
syndesmosis is significantly disrupted, squeezing the fibula and tibia
together proximally will cause pain distally at the site of the
syndesmosis in the ankle. In addition, increased side-to-side mobility
of the talus in the ankle mortise when the distal leg is grasped is
indicative of a syndesmosis injury. Plain radiographs that show
widening of the syndesmosis width greater than 5 mm are indicative of a
syndesmosis rupture.
into three phases. Phase I consists of rest and protection (brace,
cast, splint, crutches, and ice wrap); control of swelling (ice,
compression, and elevation), and early weight bearing. Phase II is
aimed at reducing residual swelling and restoring range of motion of
the ankle as well as strength, followed by low-impact aerobic training.
The final phase (Phase III) includes proprioceptive exercises and
sports-specific skills such as running, cutting, and jumping, with a
gradual return to sports. With the return to sports, the athlete may
benefit from an ankle stabilization brace or taping, although there is
no evidence that they prevent further injury (220).
athletes is distinctly uncommon. A careful clinical and radiographic
examination of the ankle and hindfoot is mandatory in patients with
continuing symptoms. It is important to differentiate between
functional instability and mechanical instability in the patient who
complains of giving way after an ankle sprain. Functional instability
is a subjective feeling of giving way during physical activity,
occurring in up to 50% of patients following an ankle sprain. Its exact
mechanism is unclear, but it is thought to be due to a disorder of
proprioception, muscle control, and ligamentous stability. Functional
instability is best managed with proprioceptive training (ankle tilt
board), muscular strengthening, and the use of ankle taping or bracing
for athletic activities.
stabilizing ligaments of the ankle and is demonstrated clinically by
the ankle drawer test and talar tilt stress radiographs. A side-to-side
difference of 10 mm or more of anterior talar translation and a talar
tilt of 9 degrees or more on stress radiographs is highly suggestive of
mechanical instability (221).
differential diagnosis includes tarsal coalition and osteochondral
fracture or OCD of the talar dome.
young athlete, ligamentous reconstruction may be necessary. A variety
of options exist to reconstruct the anterior talofibular ligament and
calcaneofibular ligament, among them the Evans procedure (222), Watson-Jones technique (223), and the Chrisman-Snook modification of the Elmslie procedure (224).
The most widely used reconstruction method is the Bröstrom repair, a
direct repair and imbrication of the anterior talofibular and
calcaneofibular ligaments (225). Biomechanical and clinical data support this anatomic reconstruction method (226,227).
injuries seen in adolescents and young adults. Avulsion fractures occur
primarily between the ages of 14 and 25 years and account for
approximately 15% of pelvic fractures in children (228,229,230).
or eccentric muscle contraction, which occurs with rapid acceleration
or deceleration.
activities such as sprinting and jumping sports, as well as soccer and
football (228). The same mechanism that would cause a muscle or tendon strain in an adult may cause an apophyseal avulsion in an adolescent.
contraction of the sartorius as seen in jumping or running, from the
anterior inferior iliac spine (AIIS) due to overpull of the straight
head of the rectus femoris, and from the ischial tuberosity due to
forceful contraction of the hamstrings (Fig. 32.23).
Avulsions of the AIIS are often seen in participants of sports
involving kicking action, and avulsions of the ischial tuberosity are
seen in gymnasts and hurdlers.
may not unite until 25 years of age, making ischial avulsions possible
even in early adulthood (230).
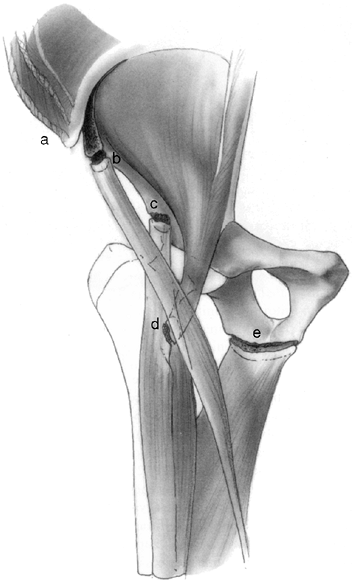 |
|
Figure 32.23
Avulsion fractures of the growing pelvis result from traction injuries where major muscle groups insert into or originate from apophyses about the pelvis. The abdominal and trunk muscles insert into the iliac apophysis (a). The sartorius originates from the anterior superior iliac apophysis (b). The direct head of the rectus femoris originates from the anterior inferior iliac apophysis (c). The iliopsoas inserts into the lesser trochanteric apophysis (d). The hamstrings originate from the ischial apophysis (e). |
antecedent prodromal pain signifying apophysitis before the avulsion.
Athletes with avulsions present with local pain, swelling, and
tenderness confined to the avulsed area. Pain is reproduced by active
or passive stretch of the involved muscle.
area of avulsion. Special views to place the ASIS or AIIS in profile
may help in delineating the avulsion. If the lesion is acute, the
diagnosis is usually straightforward.
inciting event, the radiographs may be misenterpreted as showing a
neoplasm or infection. A careful history and normal laboratory values
aid in making the correct diagnosis (Fig. 32.24).
avulsion fractures has generally been rest, followed by a specific
rehabilitation program. Metzmaker and Pappas (231)
outlined a five-stage rehabilitation program that consists of rest to
relax the involved muscle groups as well as ice wrap and analgesics,
initiation of gentle active and passive motion, resistance exercises
after 75% of motion is regained, stretching and strengthening exercises
with an emphasis on sports-specific exercises, and finally return to
competitive sports.
 |
|
Figure 32.24
Anteroposterior radiograph of right hip demonstrating how avulsion fracture of ischium may be mistaken for neoplasm or infection. |
and internal fixation have been recommended for isolated incidents, but
there appears to be no superiority of operative intervention over
conservative management (231).
competitive athletics may be prolonged. The earliest that such a return
can be expected is 6 weeks, but it is not uncommon for complaints to
persist for up to 4 to 6 months (228,232).
athlete are extremely rare. Overall, the incidence in children younger
than 12 years represents less than 5% of all glenohumeral dislocations (233,234,235,236,237,238).
relatively large humeral head articulating with a small, shallow,
glenoid fossa. The average vertical dimension of the adult humeral head
is 48 mm with an average transverse diameter of 45 mm. The glenoid
fossa, on the other hand, measures 35 mm in its vertical dimension and
25 mm in its transverse dimension (239). As a consequence, the range of motion in
each direction is accomplished at the expense of stability of the
joint. Although still considered a ball and socket joint, the shoulder
joint is similar to a golf ball on a tee (239).
|
TABLE 32.3 CLASSIFICATION OF DISLOCATION OF THE GLENOHUMERAL JOINT IN CHILDREN
|
||
|---|---|---|
|
mechanisms as those seen in adults, including significant falls on to
an outstretched hand and forced abduction and external rotation
injuries during contact sports.
with the arm held in slight abduction and external rotation. With
traumatic posterior dislocations, the arm is held adducted and in
marked internal rotation. With either dislocation, the normal rounded
contour of the shoulder is lost, and any attempt to move the shoulder
either actively or passively is extremely painful. The humeral head may
be palpated anteriorly in the subcoracoid position with anterior
dislocation or posteriorly with posterior dislocations.
 |
|
Figure 32.25
The drawer test for anteroposterior instability of the glenohumeral joint. The examiner, seated next to the patient, uses one hand to grasp the humeral head to translate it anteriorly and posteriorly, while stabilizing the scapula with the opposite hand and forearm. (Modified from Curtis RJ Jr, Rockwood CA. Fractures and dislocations of the shoulder in children. In: Rockwood CA Jr, Matsen FA III, eds. The shoulder. WB Saunders, Philadelphia, PA. 1990:991, with permission.) |
the diagnosis of recurrent shoulder instability, especially in the
young athlete. Assessments of active and passive range of motion,
strength of the shoulder girdle and upper arm musculature, as well as
examination of the cervical spine should be performed.
degrees of passive forward flexion are noted. Most important for the
assessment of instability are the evaluation of translation of the
humeral head on the glenoid and apprehension and relocation testing.
in the sitting or supine position. The sitting position requires a
relaxed cooperative patient, but the supine position is usually
preferred, especially with provocative tests for dislocation.
the shoulder in the neutral position, in external rotation for anterior
inferior testing, and in flexion and internal rotation for posterior
inferior translation. The amount of translation in each direction is
quantified and compared to the healthy shoulder (Fig. 32.25). A grading system has been employed as shown in Table 32.4 (241).
and externally rotating the shoulder 90 degrees in each direction. As
more force is gently applied, the athlete will
become
apprehensive of an impending dislocation and either adduct and
internally rotate the shoulder or demonstrate their concern by changing
facial expression or by making a sound.
|
TABLE 32.4 GRADING OF GLENOHUMERAL STABILITY BASED ON TRANSLATION OF THE HUMERAL HEAD
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||
flexed to 90 degrees and internally rotated with a posterior force
applied to the shoulder joint through the upper extremity.
examiner’s suspicions of shoulder instability, and are best performed
with the patient in the supine position. After the anterior
apprehension test has been performed, a hand is placed anteriorly over
the upper humerus and a posteriorly directed force is applied while
again performing the apprehension test. If positive, the relocation
test should relieve the patient’s apprehension and can be verified by
removing the posterior force to see if the apprehension returns.
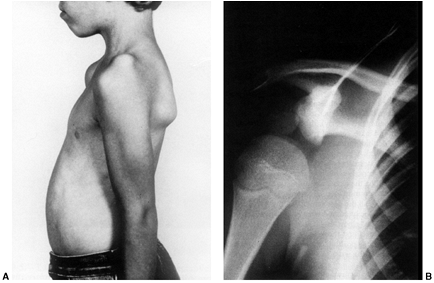 |
|
Figure 32.26 A: A patient with voluntary, atraumatic instability of left shoulder demonstrating “sulcus” sign. B: Anteroposterior view of the shoulder, demonstrating inferior subluxation of humeral head.
|
opposite manner, with a hand held over the posterior aspect of the
upper humerus (applying an anteriorly directed force) while the
posterior apprehension test is performed.
With the patient sitting, the humerus is grasped distally just above
the elbow, and an inferiorly directed force is applied while
stabilizing the scapula. A dimple or gap will appear over the lateral
shoulder as the humeral head is translated inferiorly. The sulcus sign
is pathognomonic of multidirectional instability (240).
often seen in multidirectional and voluntary dislocations, a number of
clinical tests should be quickly assessed, including thumb-to-forearm
abduction, hyperextension of metacarpal phalangeal joints (5th finger
greater than 90 degrees), elbow hyperextension greater than 10 degrees,
knee hyperextension greater than 10 degrees, and palms to floor with
knees extended (242). If the athlete has two or
more of these signs, the diagnosis and implications of generalized
ligamentous laxity should be considered. Surgery is generally
contraindicated in this group of patients.
confirm the direction of dislocation and to rule out fracture. However,
the diagnosis of anterior dislocation is readily apparent with the arm
held in slight abduction and external rotation with the humeral head
palpable anteriorly. If the treating physician is experienced in
diagnosis and management, reduction of the dislocation without prior
x-rays is permitted. On the playing field this is accomplished by
gentle traction on the arm in slight abduction, forward flexion, and
internal rotation prior to the onset of muscle spasm.
appropriate sedation and placing the patient prone with the arm hanging
free and 5 to 10 lb (2–5 Kg) of weight attached to the upper extremity.
then a specific strengthening program. The athlete should work
vigorously on the anterior rotator cuff (supraspinatus and
subscapularis) as well as the periscapular muscles following an
anterior dislocation. In the rare case of a posterior dislocation, the
posterior rotator cuff muscles or external rotators (infraspinatus and
teres minor) should be isolated and strengthened. Four specific
exercises have been shown to strengthen glenohumeral muscles (243):
-
“Press-up” exercise. In seated position,
the patient lifts his or her body from a chair by placing the hands on
the chair and extending the upper extremities. -
Elevation of the arm in the sagittal plane.
-
Elevation of the arm in the scapular plane with the arm internally rotated and thumbs pointing down.
-
Horizontal adduction from the prone position with the arm externally rotated.
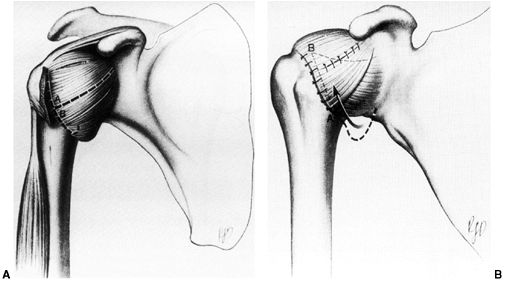 |
|
Figure 32.27 A: T-shaped incision in capsule in preparation for inferior capsular shift. B:
Diagram of capsule after inferior capsular shift. After preparing the posterior, inferior, and anterior humeral neck, the lower flap is brought upward, reducing the joint volume and eliminating redundant capsule. The upper flap is sutured over the lower one for further reinforcement. (From Neer CS. Shoulder Reconstruction. Philadelphia: WB Saunders, 1990, with permission.) |
The high rate of redislocation has prompted some surgeons to recommend
surgical repair of the Bankart lesion by arthroscopic technique
immediately after the initial dislocation (238,239,240,241,242,243,244,245,246).
This approach should be reserved for high-demand athletes until such
time as the evidence of the benefits of early surgical stabilization is
verified.
instability has been made, recommendations regarding surgical
stabilization are strongly advocated in younger patients. The decision
regarding surgical intervention is usually made after two or three
dislocations and the patient’s and family’s expressed desire to resume
an active lifestyle without the feeling of shoulder instability.
the instability is due to the so-called Bankart lesion, or avulsion of
the capsule and/or labrum from the anterior glenoid. Surgery, be it
arthroscopic or open technique, is aimed at restoration or repair of
this anterior capsulolabral complex to eliminate the Bankart lesion (245,246,247).
Careful preoperative evaluation of these patients is necessary to
differentiate them from the atraumatic group. In the latter group,
surgery is usually doomed to failure (240,241).
dislocation, in which the patient learns to voluntarily subluxate or
dislocate the glenohumeral joint and involuntary dislocation, in which
the dislocation occurs with a specific event such as carrying heavy
weight. Involuntary atraumatic dislocation is often seen in association
with generalized ligamentous laxity in connective tissue disorders such
as Ehlers-Danlos or Marfan syndromes (240).
glenohumeral joint is encountered in patients with a high incidence of
psychological or even psychiatric disorder (248,249).
Surgery should never be contemplated in this specific subset of
patients, but psychological support and rehabilitation will often help (240,248,249).
be contemplated after failure of a vigorous muscle-strengthening
program involving all the muscle groups of the shoulder for at least 6
to 12 months. The rare patient who fails this program may be a
candidate for an inferior capsular shift procedure, but the failure
rate will still be high due to the association with ligamentous laxity (240,241).
high demand for throwing will complain of pain and decreased ability to
throw. On examination, the athlete demonstrates signs of rotator cuff
impingement and inflammation, with pain and weakness on resisted
supination testing. There are subtle signs of glenohumeral instability
upon translation testing and provocative maneuvers. These patients
respond well to rest, cessation of throwing, and NSAIDs for 2 to 4
weeks, followed by a vigorous rehabilitation program once the pain has
resolved. Specific strengthening of the rotator cuff and scapular
stabilizers is employed and also an examination of the throwing
mechanics of these athletes. Results with these nonoperative regimes
are encouraging (238,250,251,252,253,254).
traumatic or anterior dislocation, and musculocutaneous nerve injury
has also been reported (239,240). In both cases, these injuries are almost all traction neuropraxias and will resolve spontaneously.
the anatomic location, course, and direction of these nerves is
essential to prevent iatrogenic injury (254,255,256,257).
adolescent, both the competitive athlete and nonathlete. Anterior knee
pain can occur as the result of a number of musculoskeletal conditions
and can prove challenging for the orthopaedist to sort out. These
conditions may include “chondromalacia patellae” or idiopathic anterior
knee pain, Osgood-Schlatter syndrome, Sinding-Larsen-Johansson
syndrome, synovial plica, or patellar or quadriceps tendinitis.
have a careful hip examination to rule out referred pain from hip
pathology such as slipped capital femoral epiphysis or
Legg-Calvé-Perthes disease. In particular, any loss of hip motion or an
abnormal gait demands careful clinical and radiographic evaluation of
the hip.
apophysitis of the tibial tubercle. This is an entity that is common in
athletes between 10 and 15 years of age, particularly in those involved
in jumping sports. Osgood-Schlatter syndrome is historically more
prevalent in boys, but occurs with some frequency in girls as well.
ossification center of the tibial tuberosity. It probably represents a
true avulsion or stress fracture of the tibial tuberosity ossification
center. Some authors have postulated that OSD occurs more frequently in
patients with patella alta (258), while others have postulated the exact opposite as a contributing factor, namely, patella infera or baja (259).
In the acute phase, pain and tenderness directly over the tibial
tubercle are noted. After the acute phase heals, the pain and
tenderness subside, and the only positive physical finding may be an
anterior mass. Pain in this phase occurs usually after physical
activity.
x-rays merely confirm what is already known from history and physical
examination. If the condition is bilateral, plain radiographs are not
necessary. However, in unilateral cases, x-rays should be ordered to
rule out other pathology such as tumor or infection.
internally rotated 10 to 20 degrees to place the tibial tubercle in
profile) usually show fragmentation of the tubercle or a loose ossicle
separate from the underlying tuberosity. Further investigations such as
MRI, CAT scans, or ultrasound are not necessary (Fig. 32.28).
had no symptoms or any limitation of function, 60% had pain when
kneeling, and most noted the presence of a prominent tibial tubercle (259). Osgood-Schlatter disease has been noted to be a predisposing factor
in cases of tibial tubercle fracture, but no direct cause-and-effect relation has been demonstrated (260,261,262).
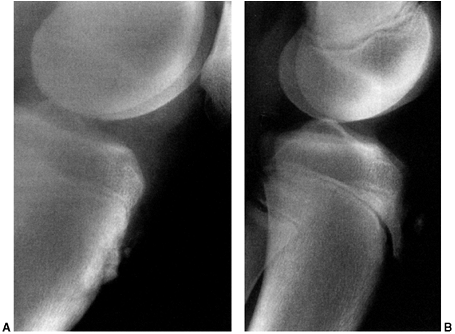 |
|
Figure 32.28 Osgood-Schlatter disease. A:
Typical radiographic findings include a prominence of the tibial tubercle with irregularity of the bone at the insertion of the patellar tendon. B: In some cases, a separate ossicle may form and not unite. If persistently symptomatic, this ossicle may require excision. |
nonoperative. Time, rest, and occasional immobilization usually result
in marked improvement of symptoms (263,264,265,266,267).
Activity should be limited until the pain resolves and the athlete
demonstrates a full painless range of knee motion. Use of ice wraps can
help the acute situation and NSAIDs may relieve some of the pain. After
the acute phase, a maintenance program of stretching, especially of the
quadriceps, and strengthening of the quadriceps and hamstrings may help
the athlete.
similar to Osgood-Schlatter disease, but affecting only the proximal
attachment of the patellar tendon to the inferior pole of the patella.
It typically affects children at a slightly younger age than is the
case with OSD; namely, 10 to 12 years.
pole of the patella. Radiographs may show calcification at the inferior
pole (Fig. 32.29). The condition is thought to be due to repetitive tensile stress at the junction of the tendon and bone (270,271).
the natural history of SLJ syndrome. Most patients respond to rest and
NSAIDs (272).
NSAIDs for the osteochondroses. In almost all cases where they are
administered, cessation of physical activity may be just as important
in relieving the symptoms. However, their use seems justified, given
the inflammatory nature of these conditions.
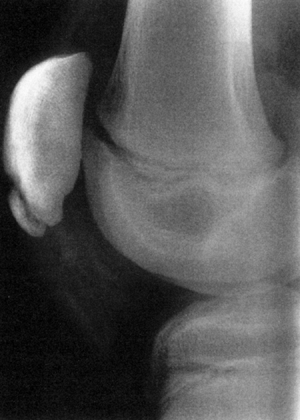 |
|
Figure 32.29
Sinding-Larsen-Johannson disease. The lateral radiograph best demonstrates the irregularity at the inferior pole of the patella. |
joint that can cause knee pain. With trauma or repetitive motion, the
plicae may hypertrophy, causing pain and signs of intraarticular
pathology (273,274,275,276,277).
patellar plica, which anatomically runs from the superior medial pole
of the patella or midpatella to the medial patellar fat pad (276). Other plicae which have been described are the suprapatellar, lateral, and infrapatellar plicae (277) (Fig. 32.30).
condition is a true symptomatic entity. The patient complains of
anterior or anteromedial knee pain, often after repetitive activities
such as running, jumping, or squatting. The athlete may complain of a
popping or snapping sensation with their knee in midflexion. Giving way
may also be a symptom.
over the plica as it comes over the medial femoral condyle to the
infrapatellar fat pad, and possibly a palpable snapping sensation as
the knee is flexed from 30 to 60 degrees of flexion while the patient
is weight bearing or standing. In some cases, synovial plicae are
associated with signs of lateral patellar instability.
have not proven to be of value. This syndrome is a clinical diagnosis
of exclusion after other causes of knee pain and popping have been
ruled out.
inflammation which lead to thickening and eventual fibrosis. With
significant fibrosis, changes in the articular surface and even
subchondral bone may occur (278).
including rest, ice wrap, NSAIDs, and a gradual strengthening program,
especially of the quadriceps and hamstrings, avoiding terminal
extension if pain is reproduced (279).
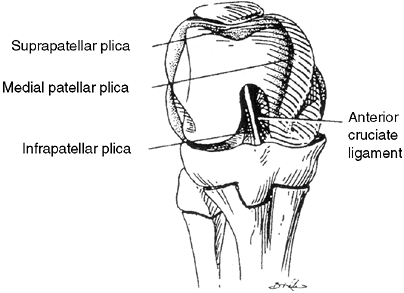 |
|
Figure 32.30
Plicae of the knee. The suprapatellar (superior) plica, the medial plica, which is the one most commonly symptomatic, and the inferior plica (ligamentum mucosum), which overlies the anterior cruciate ligament. |
nonoperative treatment. Arthroscopic resection of the plica is
indicated in those rare cases, with excellent results expected in 90%
of cases (275,280,281,282,283,284,285,286,287,288).
Simple division of the plica has been associated with recurrence and is
not recommended. At the time of surgical resection, the joint is
thoroughly examined for other causes of internal derangement such as
meniscal tear or patellar maltracking.
very little is known about this condition in children or adolescents.
Anatomic studies of the fat pad have revealed a densely innervated
tissue, and because of its anatomic location, its role as a possible
cause of anterior knee pain is debated (290,291,292,293,294).
It has been mentioned as a source of pain or pathology in conjunction
with other entities such as patellar tendinitis, meniscal tear, ACL
disruption and reconstruction, impingement after intramedullary nail
placement, and tibial osteotomy (278,294).
tendinitis or SLJ syndrome. In the latter condition, maximal tenderness
is at the inferior pole of the patella. In Hoffa syndrome, the maximal
area of tenderness is at the anterior joint line on either side and
deep to the patellar tendon.
NSAIDs. A diagnostic intraarticular local anesthetic injection directly
into the fat pad may be of help in those patients whose symptoms fail
to resolve. There is little information as to the efficacy of surgical
management for this condition (295). Because
such doubt exists as to the validity of the diagnosis, surgical
recommendations as a treatment option cannot be made (296).
that a patient’s complaints defy a plausible explanation. However,
anterior knee pain in children and adolescents can exist in the absence
of positive physical findings for any of the pathologic conditions
causing knee pain. In that case, the term “idiopathic anterior knee
pain” is used (296,297).
with insidious onset. The pain is often bilateral, more commonly worse
in one knee. The pain is made worse with physical activity such as
running, jumping, squatting, going up and down stairs, or after
prolonged sitting with the knee in flexed position.
malalignment” including excessive internal femoral torsion, external
tibial torsion, mild genu valgum with medial deviation of the patella,
and a tendency to pes planus or
foot
pronation. Patients typically point to the entire anterior aspect of
the knee as the location of the pain. The patients usually do not have
an effusion and have a full range of motion. The patella may be
hypermobile and have some evidence of maltracking, but without signs of
patellar subluxation or dislocation. There may be atrophy of the
quadriceps and patellar crepitus with flexion and extension of the knee
or a patellar compression test.
In most cases, the plain radiographs are normal. Unless the clinical
diagnosis is suggestive of another pathology, there is no indication to
proceed to other imaging studies such as MRI.
“Chondromalacia patellae” implies changes in the articulation between
the patella and femoral sulcus, including softening, fibrillation, or
erosion of the articular surface. In children and adolescents with
idiopathic anterior knee pain, however, the articular surface is often
normal. Articular cartilage has no sensory nerve endings, and with the
lack of articular cartilage changes in these patients, the source of
the pain is not definite. Therefore, one should avoid the use of the
term “chondromalacia patellae” in the case of patients with idiopathic
anterior knee pain.
Goodfellow reported on the natural history of untreated anterior knee
pain in adolescent girls followed up for 2 to 8 years (299). The symptoms of most of these patients resolved over time or were significantly improved.
consist of activity modification, flexibility exercises of the
quadriceps and hamstrings, strengthening exercises of the same muscle
groups, and the use of other modalities such as ice, heat, ultrasound,
and transcutaneous electrical muscle stimulation (299,300,301,302,303,304,305,306).
Some patients will benefit from foot orthotics, especially if pes
planus is a component of the problem. Knee orthotics such as a patellar
stabilization brace or patellar sleeve or strap may also be beneficial.
The orthopaedist must assume the role of the “knee psychiatrist” when
treating these patients, with a careful and complete clinical history
and physical examination, and almost evangelical enthusiasm for
nonoperative treatment. It is essential that the patient and his or her
family understand that the course of symptoms may be prolonged, but
with growth and maturation, activity modification, and an organized
rehabilitation program, there is excellent prognosis for return to
physical activities and improvement in symptoms (296).
young children. The common presentation is the discovery of an
asymptomatic mass by the mother of the affected child on the
posteromedial aspect of the knee at the popliteal crease. The age at
presentation is usually 4 to 8 years. Pain is an uncommon feature.
There is usually no associated intraarticular pathology in children
with this symptom (75).
Transillumination of the cyst or ultrasound can document the cystic
nature of the lesion and rule out solid soft-tissue lesions such as
rhabdomyosarcoma. MRI is only indicated when a cystic lesion is not
identified on ultrasound (83).
of the knee joint itself, between the medial head of the gastrocnemius
and the semimembranosus. Although it may be firmly attached to the
fascia of the medial gastrocnemius, it almost always communicates with
the knee joint. Spontaneous resolution of popliteal cysts tends to
occur, but this often takes up to 12 to 24 months (75).
probably be considered only in cases where rapid enlargement has
occurred or pain is a major feature. Recurrence rates are significant
after surgery, and the treatment of choice should be watchful waiting
and parental reassurance.
virtually every type of lower leg pain as a result of overuse. A more
appropriate term is shin pain.
The condition is limited to musculotendinous inflammations and
diagnosis should exclude stress fractures and ischemic disorders.
Nevertheless stress fracture and chronic exertional compartment
syndrome are part of the differential diagnosis of leg pain in the
running athlete.
in the literature. The pain is usually appreciated on the posteromedial
border of the tibia from an area approximately 4 cm above the ankle to
a more proximal level approximately 10 to 12 cm proximal. It was felt
that symptoms were due to an inflammation and overload of the posterior
tibial tendon (308,309). Drez (310)
has used the term “medial tibial stress syndrome” to describe this
pain. Postmortem studies have demonstrated that the site of pain along
the posteromedial border of the tibia corresponds to the medial origin
of the soleus muscle (311).
tenderness along the posteromedial border of the tibia, centered at the
junction of the proximal two-thirds and distal one-third. There is no
pain along the subcutaneous border of the tibia, and active and passive
motion of the foot and ankle are usually negative. Active resistant
plantar flexion and toe raises may elicit the pain.
there may be hypertrophy of the posterior tibial cortex with
subperiosteal lucency or scalloping. Faint periosteal new bone may be
present.
scan. In medial tibial stress syndrome, there is increased uptake in a
longitudinal pattern along the posteromedial tibial cortex at the exact
site of pain and tenderness (312,313). Tibial stress fractures demonstrate a transverse pattern of increased uptake (312,313).
multitude of causes and should be evaluated for a specific diagnosis.
Possible causes of shin pain include medial tibial stress syndrome,
stress fracture, exertional compartment syndrome, benign or malignant
tumor, infection, and other rare causes.
A study at the U.S. Naval Academy demonstrated that inactive recruits
were twice as likely to develop symptoms as were recruits who had been
actively training (315). In the same study, rest was shown to be the most important element of treatment.
nonsurgical treatment. Rest or restriction of causative factors is the
most important modality and is usually combined with ice, NSAIDs,
stretching of lower leg muscle groups, and the use of foot orthotics.
because tight heel cords have been shown to be more prevalent in
patients with shin splints (310,315).
days after cessation of activity, but recurrence is a common problem,
especially if the athlete returns to the preinjury activity level too
quickly. A gradual return to full exercise over 6 weeks is recommended.
after 6 to 12 months of nonsurgical management, surgery in the form of
release of the investing fascia overlying the medial soleus (the soleus
bridge) and division of the medial soleus origin and periosteum may be
indicated (308,315). Surgery in adults has been performed with success, but is rarely indicated in the growing child.
it is characterized by increased intracompartmental pressure that is
sufficient to cause pain in the leg and is usually precipitated by
running (316).
Muscle weight and size increase up to 20% during exercise and, because
of the unyielding compartment space, lead to increased pressure that
eventually exceeds the capillary filling pressure, causing ischemia and
pain (318). The ischemia is never severe enough
to cause muscle necrosis in exertional compartment syndrome. Indeed,
other studies have cast doubt on the ischemic theory of causation (319).
leg pain in one of the muscle groups of the lower leg after training,
usually running. The pain is initially dull and may persist after
training has ended. Paraesthesia on the plantar aspect of the foot or
the dorsum of the foot indicate involvement of the deep posterior
compartment or anterior compartment respectively. These are the two
most frequent compartments involved (316,317,320,321).
at rest. Examination of the foot and entire limb for mechanical axis
deviations and rotational abnormalities should be performed.
compartment syndrome, pressure measurements using either a slit
catheter or wick catheter should be performed to evaluate compartment
pressure both at rest and after exercise (317,318,322,323,324,325).
-
Pre-exercise pressure greater than 15 mm Hg.
-
A 1-minute postexercise pressure of greater than 30 mm Hg.
-
A 5-minute postexercise pressure in excess of 20 mm Hg.
pressure testing, including MRI, near infrared spectroscopy, and
thallium-201 single photon emission computed tomography scans, have
been inconclusive (326,327).
exercise-induced compartment syndrome, including the use of orthotics,
NSAIDs, activity modification, and physical therapy modalities have
been shown to be unsuccessful in cases where pressure measurements have
been obtained (315,317,320,321). These studies were performed only in adults.
Pressure measurements of all four compartments should be conducted
prior to surgery, as more than one compartment may be involved. The
tibialis posterior may reside in a separate, deep posterior
compartment, and careful evaluation should be undertaken after
decompression (319,329).
obvious cosmetic reasons. The fascia between the skin bridges must be
divided to ensure that the compartment is adequately released. In
release of the anterior and lateral compartments, care must be taken to
protect the superficial peroneal nerve. Visualization and protection of
the saphenous vein and nerve must be done with release of the
superficial and deep posterior compartments. The skin is closed
primarily, unlike an acute compartment syndrome.
to 4 days, but allowed ambulation and early range of motion of adjacent
joints. Strengthening is commenced when the wounds are healed, and the
patient can be expected to return gradually to running after 3 to 4
weeks.
bursitis is invariably associated with iliotibial band tendonitis or
“snapping hip syndrome.” Clinically, there is tenderness directly over
and distal to the greater trochanter. Pain is accentuated with
adduction and external rotation of the hip. The patient can often
voluntarily reproduce the snapping with a trick maneuver. Occasionally
the pain may also be experienced just above and at the knee on the
lateral epicondyle. Associated factors which may contribute to this
condition include a broad pelvis, leg-length discrepancy, and excessive
pronation of the foot (330).
Treatment is aimed at stretching the iliotibial band and decreasing the
inflammation with NSAIDs. In extreme cases or cases which fail to
resolve, local corticosteroid injection into the iliotibial band and
greater trochanteric bursa may be indicated.
symptoms, operative release or lengthening of the iliotibial band may
be required (330).
adolescents has increased in concert with the increase in their
participation in organized sports, especially sports involving running (331). Stress fracture should be included in the differential diagnosis of the child with overuse injury and pain.
that disrupts the normal bone-remodeling mechanism. This stress is
below the threshold needed to cause an acute fracture, but is
sufficient to interrupt the balance between bone formation and
resorption. Physical training, especially after prolonged inactivity,
results in stimulation of osteoblastic activity. At the same time there
is a concurrent stimulation of bone resorption (osteoclastic activity),
and it is believed that stress fractures occur early in this remodeling
period when the bone is weakened by osteoclastic resorption (332).
the proximal tibia, femoral neck, femoral diaphysis or distal femoral
metaphysis, medial malleolus, and metatarsals.
fractures are fractures due to an abnormal stress applied to a normal
bone. Insufficiency fractures occur when normal stress is applied to
abnormal or pathologic bone such as in children with osteogenesis
imperfecta, spina bifida, cerebral palsy, or rickets, or in some
patients on long-term steroids. Micheli has noted the infrequency of
fatigue or stress fractures in
children involved in free play (333).
Stress fractures almost always occur in children and adolescents
involved in strenuous repetitive activities such as running, jumping,
or dance (333,334).
adolescents is not known, but it is generally accepted that the
incidence is increasing due to their increased participation in
organized sports. A study of Israeli military recruits found that the
incidence of stress fracture in recruits younger than twenty years was
ten times greater than recruits who were 21 to 26 years of age (335).
In studies undertaken of military recruits, it was seen that those
recruits with foot deformity such as pes planus or pes cavus had a
higher incidence of stress fracture (332,334,339,340,341). Those recruits who were not physically fit and had smaller thigh muscle mass were more prone to develop stress fractures (336). In addition, smaller bone diameter, especially of the femur and tibia, was associated with a higher incidence (340).
Women with a lower bone mineral density (BMD), with low body fat, or
amenorrhea were more likely to develop stress fractures (337,340).
with stress injury, namely, the combination of muscle weakness, muscle
tightness, and ligamentous laxity (340). These
effects were magnified if the adolescents also had increased body
weight and height, any malalignment of the lower extremities, and were
involved in sports that demanded bursts of explosive strength (340).
stress fracture from a malignant bone tumor, especially with some
periosteal new bone formation. In addition, the physician must rule out
other conditions such as benign tumors (osteoid osteoma), infection,
inflammatory arthritis, or soft-tissue injury.
differentiating stress fracture from these other conditions. A history
of repetitive physical activity such as running, jumping, or dancing,
causing pain during the activity and relief with rest, should arouse a
high index of suspicion (333,334).
Night pain is not common in stress fracture, but is common in osteoid
osteoma or malignant bone tumors (osteogenic and Ewing sarcoma).
the foot and ankle, lower leg, and entire lower extremity alignment.
Stress fractures occur more commonly in athletes with foot
abnormalities such as pes cavus and pes planus (334,339,340,341).
tightness or flexibility, and ligamentous laxity should be part of the
physical examination (339).
radiographs with emphasis on bony detail, radionucleotide bone scans,
or, more recently, MRI.
following the onset of symptoms. Until resorption and the proliferative
healing response occur in trabecular bone, the x-ray films will appear
to be normal. In metaphyseal bone this may take from 10 to 21 days (342) (Fig. 32.31).
stress fractures, especially in the metaphyseal region, whereas
malignant bone tumors have a more widespread periosteal reaction with
lamination and permeative patchy destruction of the metaphyseal bone.
However, distinguishing a stress fracture from a malignant bone tumor
may be very difficult.
 |
|
Figure 32.31 A:
Anteroposterior radiograph of adolescent cross-country runner with pain in proximal tibia for 3 months. Reactive new bone medially (black arrow) is suggestive of stress fracture. B. Lateral radiograph of same patient. Arrow points to area of new bone formation posteriorly. |
especially in the patient in whom the history and plain radiographs are
inconclusive (344,345). MRI can distinguish stress fracture from both osteomyelitis and malignant bone neoplasm in most cases (Fig. 32.32).
signal intensity in the intramedullary area, continuous with the
cortical bone. On T1 images, there is decreased signal intensity in the marrow (345).
depends on the site of the injury, the severity of symptoms, and the
patient’s age (333,346,347).
is to modify or eliminate the activities that caused the stress
fracture. This may require cast immobilization or off-the-counter
orthotics for 4 to 6 weeks to allow sufficient bone deposition to occur.
prolonged symptoms or even nonunion. These include stress fractures of
the femoral neck, tibial diaphysis, medial malleolus, and tarsal
navicular (334).
problematic. If the fracture goes on to displacement, risk or the
complication of avascular necrosis (AVN) is high and has prompted the
recommendation for internal fixation (348).
Micheli recommends the use of a hip spica cast in noncompliant young
children, but internal fixation may be the conservative treatment of
choice in an active adolescent (347).
 |
|
Figure 32.32 Magnetic resonance imaging (MRI) of the same patient as in Figure 32.31. Arrow points to fracture line.
|
In the case of delayed union, a patellar—tendon—bearing (PTB) cast or
commercial orthosis should be employed for 4 to 6 months before surgery
is considered. If nonunion exists in the skeletally mature adolescent,
options to treat the nonunion include the use of an intramedullary nail
with or without bone grafting and fibular osteotomy (350).
In the skeletally immature individual with a dipahyseal tibial stress
fracture that fails to heal after 6 months of nonoperative treatment,
operative treatment is indicated. Excision of the fibrous nonunion and
autogenous bone grafting from the iliac crest, with possible fibular
osteotomy, has been employed successfully in this rare situation (350).
open reduction and internal fixation is recommended to facilitate early
healing and recovery (351).
fractures, excision of the nonunion site and autogenous bone grafting
has been recommended (352,353).
when they can return to sports. This is a complex decision which
involves healing of the stress fracture clinically, as evidenced by
loss of pain, healing of the stress fracture as indicated on
radiographs, and modification or elimination of inciting causes and
training methods.
The condition is bilateral in most cases and presents as diffuse pain
and tenderness over the prominence of the heel rather than exclusively
on the plantar aspect as is seen in plantar fasciitis (355).
in 1912, the condition is presumed to be an inflammatory condition of
the calcaneal apophysis. Radiographs are normal, and the condition in
all cases improves over time.
stretching exercise of the Achilles tendon and plantar fascia, and heel
pads or inserts may offer some relief from the discomfort (354,355,356). Cast immobilization may rarely be necessary for the patient with severe, incapacitating symptoms (355). Reassurance and time are the basis of treatment of this common condition.
specific stress reaction in the physis. This reaction is not uncommonly
seen in the distal radial physis of skeletally immature gymnasts who
load their wrists with significant compressive forces while
participating in vault and floor exercises (Fig. 32.33).
Radiographically the stress reaction is noted as a radiolucency and
irregularity on the metaphyseal side of the physis, similar to the
radiolucency seen in the medial aspect of the proximal tibia in
adolescent tibia vara and in the femoral neck in patients with slipped
capital femoral epiphysis.
radiographic abnormalities in the distal radius in up to 85% of
competitive gymnasts who were skeletally immature. The same study found
an increased incidence of positive ulnar variance in the wrists of
nonelite gymnasts (357). The hypothesis of the
study was that repetitive compressive forces to the distal radius led
to premature growth arrest resulting in a positive ulnar variance.
Positive ulnar variance has been associated with secondary wrist
problems including tears of the triangular fibrocartilage complex
(TFCC) and ulnar impingement syndrome.
same symptoms and radiographic abnormalities in the proximal humerus.
This condition is commonly seen in Little League pitchers (358) (Fig. 32.34).
 |
|
Figure 32.33
Anteroposterior radiograph of wrist of gymnast with 3-month history of wrist pain. Note radiolucency on metaphyseal side of growth plate (physis) indicative of stress physeal reaction. |
 |
|
Figure 32.34
Radiographs of the proximal humerus in a 14-year-old, right-handed baseball pitcher with shoulder pain that progressed over the final few weeks of the season. A: Widening and irregularity of the physeal plate are present in the right shoulder. B: Radiograph of the left shoulder is provided for comparison. |
on the discontinuation of the offending force or modification of
activities until the symptoms of pain subside. Baseball pitchers may
need to refrain from pitching for 2 to 3 months until the pain that
accompanies the throwing motion totally subsides.
should refrain from loading their wrists for up to 2 to 3 months to
allow the reactive changes to heal.
wrist flexors should be undertaken in gymnasts, and taping or bracing
to limit wrist dorsiflexion may help prevent recurrence.
exercise, the radiographic changes seen at the physis and metaphysis
will resolve.
injury when the athlete throws too frequently or uses a style that puts
too much stress on the elbow joint.
This valgus moment results in excessive tensile or distraction forces
on the medial aspect of the joint (medial epicondyle and ulnar
collateral ligaments) and excessive compressive forces on the lateral
aspect of the joint (radiocapitellar joint) (360,361) (Fig. 32.35).
Studies have also shown that side-arm throwing results in markedly
increased elbow pathology in the throwing athlete and should be
forbidden for the child athlete involved in throwing sports (365).
the facets of overuse injury in the young throwing athlete. The term
was originally proposed in 1960 and encompasses a spectrum of
conditions including medial epicondyle apophysitis, capitellar OCD,
radial head deformation, olecranon apophysitis, and flexion contracture
(363).
 |
|
Figure 32.35 Pitching produces a valgus moment (large curved arrow) at the elbow. There are compressive forces (straight arrows) across the radiocapitellar joint, and tension (curved arrows) across the medial epicondyle and medial collateral ligament.
|
athlete involved in throwing sports have been studied extensively in
recent years. Factors responsible include: age of the pitcher, skeletal
maturity, tissue strength, muscle strength, level of conditioning,
throwing speed and style, types of pitches thrown, number of pitches
per outing, and number of outings per season (362,363,364).
the young throwing athlete, 47% of pitchers complained of shoulder or
elbow pain, and 68% of the elbow pains were medial (362,365).
Twenty-eight percent of the pitchers complained of elbow pain
specifically, and throwing split finger pitches or sliders increased
the incidence of elbow complaints (365).
resist traction forces better than the apophyseal cartilage. These
repetitive stresses cause microfractures in the apophyseal cartilage
not unlike those seen in Osgood-Schlatter syndrome. The apophysis is
seen to widen on x-ray films and may eventually fail catastrophically,
resulting in a medial epicondyle avulsion, especially in a throwing
athlete.
epicondyle is elicited. The pain may also be increased if a valgus
stress is applied to the elbow or if resisted wrist flexion or
pronation is tested.
skeletally immature athlete is rest and avoidance of the offending
activity, and the elbow is no exception. Rest, ice, and the use of
acetaminophen or NSAIDs should result in improvement of symptoms. If
the athlete develops a true avulsion of the medial epicondyle,
treatment should consist of cast immobilization if the apophysis is
displaced 5 mm or less, or surgical fixation of the medial epicondyle
if displaced more than 5 mm in a throwing athlete or gymnast. Fixation
should be accomplished with a small cannulated screw, which allows
early range of motion and prevents elbow stiffness.
cortex. A fully threaded screw with washer is employed to provide
enough stability to allow early range of motion. The epicondyle is
positioned without removal of physeal cartilage. The screw is not
routinely removed unless it becomes prominent and symptomatic to the
patient.
are younger than 10 years; it is atraumatic and probably due to
variation in ossification rather than typical OCD. It is self-limited
and resolves spontaneously (366).
 |
|
Figure 32.36 Osteochondral lesion of the capitellum in a 15-year-old baseball player with a painful elbow. A: The subchondral plate of the capitellum appears intact. Subchondral cysts are present. B:
The computed tomography scan with three-dimensional reconstruction demonstrates the bony defect of the capitellum, including loss of the subchondral plate in that area. |
forces on the lateral side of the joint (radiocapitellar joint) during
the throwing motion, resulting in microfractures, edema, AVN, and
potential loose body formation (368,369,370).
and tenderness of the radiocapitellar joint to palpation lead to the
diagnosis. With progression of the disease, pain on forced flexion, a
flexion contracture, and snapping and locking from joint incongruity
and loose bodies may occur (Fig. 32.36).
lesions shown on magnetic resonance imaging (MRI) to be stable respond
favorably to prolonged rest and cessation of throwing. The lesions may
take up to 12 months to heal. MRI is helpful for visualizing any
disruption in the integrity of the articular cartilage (fluid behind
lesion) and helps direct surgical management. If the lesion is stable,
drilling of the involved fragment may promote vascular ingrowth and
healing of the lesion. If the lesion is unstable, the base should be
freshened and fixed with pins, screws, or bioabsorbable nails. Small,
unstable lesions or loose bodies are removed.
motion and strength. The decision to resume throwing sports is one that
should not be made until the lesion is healed and the elbow fully
rehabilitated.
When the elbow becomes painful, coaches, trainers, and medical
personnel should advise cessation of throwing. Fortunately,
nonoperative treatment is successful in alleviating most of the
conditions associated with “Little League elbow,” but the athlete,
parents, and coaches must be educated as to the reasons for the problem
and ways to prevent it from recurring.
overuse injuries that can occur to the skeletally immature athlete.
Despite these risks, the beneficial aspects of recreational and
organized sports far outweigh the hazards. Most important of all the
benefits is the development of an active and healthy lifestyle, which
offers countless rewards not only during childhood and adolescence but
also in adult life.
In: Sullivan JA, Anderson SJ, eds. Care of the Young Athlete. Rosemont,
IL: AAOS, American Academy of Pediatrics, 2000:121–130.
In Sullivan JA, Anderson SJ, eds. Care of the young Athlete. Rosemont,
IL: American Academy of Pediatrics. AAOS, 2000: 267–280.
In Sullivan JA, Anderson SJ, eds. Care of the young Athlete. Rosemont,
IL: AAOS and American Academy of Pediatrics, 2000:95–104.
Academy of Pediatrics. Committee on sports medicine. Weight training
and weight lifting: information for the pediatrician. Phys Sportsmed 1983;11:157–161.
DM, Fithian DC, Marangi KS, et al. Characteristics of patients with
primary acute lateral patellar dislocation and their recovery within
the first 6 months of injury. Am J Sports Med 2000;28(4):472–479.
D Jr, Edwards TB, Williams CS. Results of medial patellofemoral
ligament reconstruction in the treatment of patellar dislocation. Arthroscopy 2001;17(3):298–306.
CS, Stein BE, Matuz D, et al. Immediate surgical repair of the medial
patellar stabilizers for acute patellar dislocation. A review of eight
cases. Am J Sports Med 2000;28(6):804–810.
R, Nietosvaara Y, Kallio PE, et al. Operative versus closed treatment
of primary dislocation of the patella. Similar 2 year results in 125
randomized patients. Acta Orthop Scand 1997; 68(5):419–423.
JC, Pynsent PB, van Poortvliet JA, et al. Mechanical factors in the
incidence of knee pain in adolescents and young adults. J Bone Joint Surg 1984;66B(5):685–693.
R, Wissinger HA, Donaldson WF. Preliminary experience with a method of
quadricepsplasty in recurrent subluxation of the patella. J Bone Joint Surg Am 1975;57:600.
OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser
and Roux Goldthwait procedures for recurrent patellar dislocation. Clin Orthop 1979;144:27.
CR, Ogden JA. Development of the meniscus of the human knee joint:
morphological changes and their potential role in childhood meniscal
injury. J Bone Joint Surg Am 1983; 65:538–547.
PM, Patel DV, Marx CI. Congenital discoid lateral meniscus in children:
a follow-up study and evaluation of management. J Bone Joint Surg Br 1991;73:932–939.
TD, Paulos LE, Parker RD, et al. Discoid lateral meniscus: case report
of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy 1987;3:277–282.
MS, DiCanzio J, Zurakowski D, et al. Diagnostic performance of clinical
examination and selective magnetic resonance imaging in the evaluation
of intra-articular knee disorders in children and adolescents. Am J Sports Med 2001;29: 292–296.
CL. Correlation of arthroscopic and clinical examination with magnetic
resonance imaging findings of injured knees in children and
adolescents. Am J Sports Med 1998;26:2–6.
WD, Vittori JM. The incidence of healing in arthroscopic meniscal
repairs in the anterior cruciate ligament-reconstructed knee versus
stable knees. Am J Sports Med 1992;20: 176–181.
FR, Barber-Westin SD. Arthroscopic repair of meniscal tears extending
into the avascular zone in patients younger than twenty years of age. Am J Sports Med 2002;30:589–600.
MH, Noye FR, Barber-Westin SD. Arthroscopic repair of meniscal tears
that extend into the avascular zone: a review of 198 single and complex
tears. Am J Sports Med 1998;26: 87–95.
MS, Noyes FR. Arthroscopic evaluation of meniscal repairs after
anterior cruciate ligament reconstruction and immediate motion. Am J Sports Med 1991;19:489–494.
FR, DeLucas JL, Torvik PJ. Biomechanics of anterior cruciate ligament
failure: an analysis of strain-rate sensitivity and mechanisms of
failure in primates. J Bone Joint Surg Am 1974; 56(2):236–253.
JC, Weinberg HW, Wilson AS. The anatomy and function of the anterior
cruciate ligament as determined by clinical and morphological studies. J Bone Joint Surg Am 1974;56: 223–225.
BK, Lange RH, Fujisaki CK, et al. Anterior cruciate ligament tears in
skeletally immature patients: meniscal pathology at presentation and
after attempted conservative treatment. Arthroscopy 1992;8:229–233.
K, Pfeiffer R, Wang J, et al. ACL injury in pediatric and adolescent
athletes: Differences between males and females. Presented at: Annual Meeting of Pediatric Orthopaedic Society of North America. Amelia Island, FL: May 2003.
FG, Marhall JL, Monajem ARSA. The cruciate ligaments of the knee joint.
Anatomical, functional and experimental analysis. Clin Orthop 1975;106:216–231.
AA, Dawkins GPC. Functional anatomy of the anterior cruciate ligament.
Fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg 1991;73B:260–267.
E, Dick R. Knee injury patterns among men and women in collegiate
basketball and soccer. NCAA data and review of literature. Am J Sports Med 1995;23(6):694–701.
MJ, Bosco J, Sherman OH. Gender disparity of anterior cruciate ligament
injury: etiological theories in the female athlete. Bull Hosp Jt Dis 2000;59(4):217–226.
BK, Lange RH, Fujisaki CK, et al. Anterior cruciate ligament tears in
skeletally immature patients: meniscal pathology at presentation and
after attempted conservative treatment. Arthroscopy 1992;8(2):229–233.
PM, Datel DV, Zorilla P. The natural history and treatment of rupture
of the anterior cruciate ligament in children and adolescents. A
prospective review. J Bone Joint Surg Br 2002;84(1):38–41.
DM, Arnoczky SP, Dodds J, et al. The effect of drilling and soft tissue
grafting across open growth plates: a histological study. Am J Sports Med 1995;23:431–435.
TB, Greene CC, Baratta RV, et al. The effect of placing a tensioned
graft across open growth plates. A gross and histologic analysis. J Bone Joint Surg Am 2001;83(5):725–734.
MS, Micheli LJ, Yaniv M, et al. Functional and radiographic outcome of
juvenile osteochondritis dissecans of the knee treated with
transarticular arthroscopic drilling. Am J Sports Med 2001;29(5):562–566.
S. The hereditary multiple epiphyseal disturbance and its consequences
for the aetiologies of local malacias-particularly the osteochondrosis
dissecans. Acta Radiol 1954;25:296–299.
F, Beguiristain J, Krauspe R, et al. Osteochondritis dissecans: a
multicenter study of the European Pediatric Orthopaedic Society. J Pediatr Orthop B 1999;8(4):231–245.
Smet AA, Ilahi OA, Graf BK. Untreated osteochondritis dissecans of the
femoral condyle: prediction of patient outcome using radiographic and
MR findings. Skeletal Radiol 1997;26(8): 463–467.
E, Nosir H, Smothers C, et al. Juvenile osteochondritis dissecans of
the knee: healing prognosis based on x-ray and gadolinium enhanced MRI.
American Society for Sports Medicine-specialty day at the American
Academy of Orthopaedic Surgeons Annual Meeting. San Francisco,
CA:2001;44.
Smet AA, Ilahi OA, Graf BK. Reassessment of the MR criteria for
stability of osteochondritis dissecans in the knee and ankle. Skeletal Radiol 1996;25(2):159–163.
M, Palaniappan M, Khan N, et al. Osteochondritis dissecans of the knee
in children. A comparison of MRI and arthroscopic findings. J Bone Joint Surg Br 2002;84(2): 258–262.
P, Buzzi R, Bassi PB, et al. Arthroscopic drilling in juvenile
osteochondritis dissecans of the medial femoral condyle. Arthroscopy 1994;10(3):286–291.
J, Mubarak SJ, Chambers HG. Extraarticular drilling on osteochondritis
dissecans lesions of the knee in children and adolescents. Annual Meeting of Pediatric Orthopaedic Society of North America, Vancouver, 2000:75.
MN, Beaver RJ, Gross AE. The long-term success of fresh, small fragment
osteochondral allografts used for intraarticular post-traumatic defects
in the knee joint. Orthopedics 1992;15(10):1191–1199.
L, Minas T, Brittberg M, et al. Treatment of osteochondritis dissecans
of the knee with autologous chondrocyte transplantation: results at two
to ten years. J Bone Joint Surg Am 2003; 85:17–24.
M, Faxen E, Peterson L. Carbon fiber scaffolds in the treatment of
early knee osteoarthritis: a prospective 4 year follow-up of 37
patients. Clin Orthop 1994;307:155–164.
IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of
radiography in acute ankle injuries. Refinement and prospective
validation. JAMA 1993;269:1127–1132.
C, Fowles JV, Fallaha M, et al. Long-term results of surgical
reconstruction for chronic lateral instability of the ankle: comparison
of Watson-Jones and Evans techniques. J Trauma 1988;28:1330–1334.
OD, Snook GA. Reconstruction of lateral ligament tears of the ankle: an
experimental study and clinical evaluation of seven patients treated by
a new modification of the Elmslie procedure. J Bone Joint Surg Am 1969;51:904–912.
GB, Pinerola JJ, Caturano RW. Invertor versus evertor peak torque and
power deficiencies associated with lateral ligament injury. J Orthop Sports Phys Ther 1997;26:78–86.
In Sullivan JA, Anderson SJ, eds. Care of the young Athlete. Rosemont,
IL: American Academy of Orthopaedic Surgeons, American Academy of
Pediatrics, 2000:323–347.
H, Jobe F, Pink M, et al. Electromyographic analysis of the
glenohumeral muscles during a baseball rehabilitation program. Am J Sports Med 1991;19:264–272.
S, Richmond JC, Woodward JS. Two to five year follow-up of arthroscopic
bankart reconstruction using a suture anchor technique. Am J Sports Med 1997;25:809–812.
CR, Pierce DS, Clark JG. Voluntary dislocation of the shoulder: a
preliminary report on a clinical, electromyographic and psychiatric
study of twenty-six patients. J Bone Joint Surg 1973;55A:445–460.
RR, Hudson AR, Bertoia JT, et al. Injury to the brachial plexus during
putti platt and Bristow procedures: a report of eight cases. Am J Sports Med 1987;15:374.
JE, Cristini JA. Patella alta and patella infera. Their etiological
role in patellar dislocation, chondromalacia and apophysitis of the
tibial tubercle. J Bone Joint Surg Am 1975; 57(8):1112–1115.
JP, Trakru S, Meyer M, et al. Arthroscopic excision of symptomatic
medial plica. A study of 118 knees with 1-4 years follow-up. Acta Orthop Scand 1994;65(4):408.
U, Ruckstuhl J, Scherrer H, et al. Internal derangement of the knee
joint due to pathologic synovial folds: the mediopatellar plica
syndrome. Clin Orthop 1981;155:59.
RM, Lobenhoffer P, Latterman C, et al. Free nerve endings in the medial
and posteromedial capsuloligamentous complexes: occurrence and
distribution. Knee Surg Sports Traumatol Arthrosc 2000;8(2):68–72.
RM, Stauffer E, Friederich NF. Occurrence of free nerve endings in the
soft tissue of the knee joint. A histologic investigation. Am J Sports Med 1992;20(4):430–433.
B, Sienkiewicz P, Mankin H. Chronic exercise-induced compartment
pressure elevation measured with a miniaturized fluid pressure monitor.
Am J Sports Med 1988;16(6): 610–615.
D, Hargens A, Mubarek S, et al. Modified criteria for the objective
diagnosis of chronic compartment syndrome of the leg. Am J Sports Med 1990;18(1):35–40.
L, van Every B, Bennell K, et al. A prospective blinded evaluation of
exersice thallium–201 SPECT in patients with suspected chronic
exertional compartment syndrome of the leg. Eur J Nucl Med 2001;28:688–695.
D, Abramovitz A, Zelikovsky L, et al. Stress fractures in female
soldiers: an epidemiological investigation of an outbreak. Mil Med 1988;153:448.
TJ, Gudger TD, Obermeyer L. Stress fractures in 295 trainees: a
one-year study of the incidence as related to age, sex, and race. Mil Med 1983;148:666.
C, Ekenman I, Tornkvist H, et al. Stress fractures of the femoral neck
in athletes: the consequence of a delay in diagnosis. Am J Sports Med 1990;18:524.
AC, Shelbourne DK, McCarroll JR, et al. The natural history and
treatment of delayed union stress fractures of the anterior cortex of
the tibia. Am J Sports Med 1988;16:250.
S, Fleisig G, Andrews J, et al. Effect of pitch type, pitch count and
pitching mechanics on risk of elbow and shoulder pain in youth baseball
pitchers. Am J Sports Med 2002;30(4): 463–468.
