BIOMECHANICS AND FUNCTIONAL ANATOMY OF THE SHOULDER
IV – SPORTS MEDICINE > Shoulder > CHAPTER 76 – BIOMECHANICS AND
FUNCTIONAL ANATOMY OF THE SHOULDER
muscles that links the arm to the trunk. Large arcs of motion in all
three planes allow the shoulder to perform a multitude of functions
that include reaching, lifting, carrying, pushing, propelling, and
placing the hand. Intricate synergy among the controlling muscles
provides the necessary functional precision. Shoulder function,
however, is determined not only by joint structure but also by the mode
of dynamic control.
shoulder complex, rims and discs that Codman grouped under the gross
classification of ligaments (9). These
structures limit motion by the lack of tissue elasticity in the line of
their collagen fibers (3% to 5% stretch). On the other hand, they allow
motion in all other directions according to the characteristics of the
ground substance. The bands, having a gellike proteoglycan ground
substance binding the linearly aligned collagen fibers, are the most
flexible. Stiffness of dense fibrous tissue or fibrocartilaginous
stroma of the discs and labral rim limits their mobility to mild
bending. Each joint has a unique system of ligaments.
joint. Three skeletal segments are used: the humerus, the scapula, and
the clavicle. The most prominent patterns of motion are arm elevation
and rotation. Lesser actions are depression, horizontal motion, and
support of the hanging arm. Each has specific biomechanical
requirements of the passive structures and selected patterns of muscle
action.
Of these, the glenohumeral joint is dominant both by its extensive
range of motion and by clinical concerns. The second most important
functional site is the scapulothoracic articulation. Mobility of the
scapula on the thorax depends on the quality of the sternoclavicular
and acromioclavicular joints. These provide the skeletal link between
the scapula and the trunk.
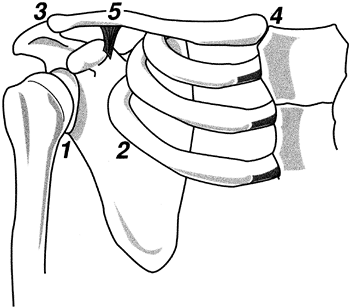 |
|
Figure 76.1. The four critical areas of motion within the shoulder complex: (1) glenohumeral, (2) scapulothoracic, (3) acromioclavicular, and (5) sternoclavicular. Clavicular rotation (4) is the result of motion at 3 and 5.
|
favors mobility over stability. The ball-shaped humeral head is
approximately one third of a sphere (6), with an average radius of 26.9 mm in men (women’s radii are 13% smaller) (64).
Opposing it is a shallow bony socket formed by the glenoid fossa of the
scapula. The bony surface of the glenoid fossa opposes only the central
35% of the humeral head, but a dense fibrous glenoid labrum enlarges
the socket contact area to 75% (4,60). The flexibility of this dense fibrous rim lessens the shock of impact during aggressive motion.
vertical plane and 5 mm along its anteroposterior axis. In the
superoinferior direction, the labrum increases the total socket depth
50%; horizontally it adds 60%.
Recent measurements of the cartilaginous surfaces, however, have found
that both surfaces accurately approximate a sphere, with the glenoid
contour not differing significantly from the humeral shape (64).
Further study has identified the peripheral cartilage of the glenoid to
be thicker than the center. Humeral articular cartilage thickness is
quite uniform.
three basic planes. Relative to the transverse axis of the elbow, the
humeral head is retroverted 20° to 35° (61). It
also makes a 45° vertical angle with the longitudinal axis of the
humerus. These angulations align the central axis of the humeral head
with the center of the glenoid fossa of the scapula as it rests on the
posterior, lateral aspect of the chest wall (55). Scapular alignment defined in the quiet standing position is approximately 30° anterior to the body’s transverse plane (30).
by the glenoid labrum, which deepens the socket, a capsule that
encloses the joint, and several critically aligned linear bands
(commonly called ligaments). The function of each structure is to
prevent displacement of the humeral head beyond the margins of the
glenoid socket.
Minor differences in labral depth have been found among the areas
(varying from 3.0 to 3.8 mm), with the anterior and inferior being
slightly greater than others (27). Average
socket depth is doubled by the labrum. No correlation has been found
between the size of the labrum and that of the glenoid (27). There is no consistency between right and left labra (44). The shape of the labrum also has been found to differ with the state of humeral head rotation (39). This latter fact supports the greater detail found by arthroscopy (11).
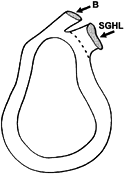 |
|
Figure 76.2.
The glenoid labrum. Its peripheral margin is attached to the rim of the glenoid fossa. While both the tendon of the long head of the biceps muscle (B) and the superior glenohumeral ligament (SGHL) appear to arise from the superior margin of the labrum, the biceps tendon also has a bony attachment. (Adapted from Harryman DT et al. The Role of the Rotator Interval in Passive Motion and Stability of the Shoulder. J Bone Joint Surg Am 1992;74:53, with permission.) |
chondrocytes. Fibrocartilage is present only at the base of the more inferior portion of the labrum.
Proximally, the labrum resembles a meniscus, with a roughly triangular
cross section, which is loosely attached to the glenoid rim by thin
connective tissue that stretches easily [mimicking a superior labral
tear from anterior to posterior (a SLAP lesion)]. The superior area (12
o’clock) of the labrum inserts directly into the biceps tendon, and
some of its collagen fibers extend over the rim to the tubercle. The
interval between the biceps tendon insertion site (the supraglenoid
tubercle) and the glenoid rim is a small (5 mm) recess.
8 o’clock) appears as a rounded, dense, fibrous tissue extension of the
articular cartilage, which is firmly attached to the glenoid rim. In
this area, there is a narrow fibrocartilagenous transitional zone
joining the labrum to the hyaline cartilage margin (11,39).
joint’s concave contour and the labrum. In the presence of a
compressive force, the joint resists passive translation (34).
Passive stability in the superior and inferior directions is
approximately twice that available against anterior and posterior
forces. This difference correlates with the relative depth of the
glenoid fossa. Excision of the labrum reduces the joint’s resistance to
translation by about 20% (36).
horizontal plane only at the extremes of external rotation and
extension (the cocking position) (28). Then
there is a 4 mm posterior displacement, corrected by either flexion or
derotation. During all other arcs of motion, the humerus remains
centered on the glenoid.
joint. Its attachment on the scapula most often is about 1 cm beyond
the labrum, but approximately 20% of the capsule is contiguous with the
base of the labrum. On the humerus, the capsule inserts primarily into
the anatomic neck. Inferiorly, the capsular attachment drops down to
the surgical neck of the humerus (7). Preservation of glenohumeral stability by an intraarticular vacuum appears to be an important function of the capsule (25,35).
glenohumeral contact. Venting the capsule allows easy distraction and
reduces the forces required for joint translation (8,23).
A cadaver study of the dependent arm with an intact capsule found no
subluxation even with sectioning of all the supporting muscles (35).
Puncture of the capsule, however, allowed the humeral head to drop half
the height of the glenoid, whether the muscles were intact or released.
provides structural reinforcement of the glenohumeral ligaments (GHL)
to preserve passive joint stability (Fig. 76.3) (7).
By their point of attachment on the humerus, these otherwise thin
tissues have been designated as the superior, middle, and inferior
glenohumeral ligaments (7). Initially, investigators considered these structures inconsistent (14), but recent investigations have found that they vary in size rather than presence or absence (7,18,39,45,66).
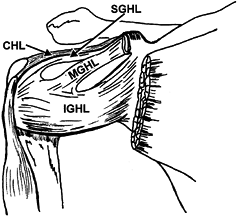 |
|
Figure 76.3. Ligaments of the shoulder joint, anterior view. CHL, coracohumeral ligament; SGHL, superior glenohumeral ligament; MGHL, middle glenohumeral ligament; IGHL, inferior glenohumeral ligament. (Adapted from Ferrari DA. Capsular Ligaments of the Shoulder. Am J Sports Med 1990;18:20, with permission.
|
labrum just anterior to the tendon of the long head of the biceps
brachii at the level of the coracoid base (Fig. 76.2, Fig. 76.3, and Fig. 76.4) (7,39,66).
It passes under the supraspinatus muscle and inserts on the anatomic
neck of the humerus, medial to the anterosuperior base of the lesser
tuberosity.
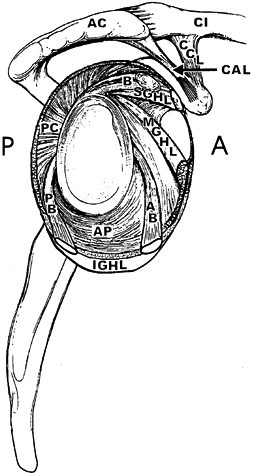 |
|
Figure 76.4. Ligaments of the shoulder as they encircle the glenoid fossa. B, tendon of the long head of the biceps; SGHL, superior glenohumeral ligament; MGHL, middle glenohumeral ligament; CAL, coracoacromial ligament; CCL, coracoclavicular ligaments. Components of the inferior glenohumeral ligament: AB, anterior band; AP, axillary pouch; PB, posterior band; PC,
posterior capsule. (Adapted from O’Brien et al. The Anatomy and Histology if the Inferior Glenohumeral Ligament Complex of the Shoulder. Am J Sports Med 1990;18:449, with permission.) |
the middle glenohumeral ligament (MGHL) arises just inferior to the
superior GHL and inserts along the middle area of the anatomic neck of
the humerus, opposite the lesser tuberosity (Fig. 76.3 and Fig. 76.4) (39,66).
glenohumeral ligament (IGHL). It is also very broad, arising from the
lower half of the labrum (anterior, inferior, and posterior). The
especially thick superior margin is designated the superior band, while
the rest of the ligament is called an axillary pouch (Fig. 76.4). Both the superior band and the anterior pouch insert on the anatomic neck of the humerus (Fig. 76.3). The posterior pouch dips down to insert on the surgical neck (39,66).
With the arm dependent (abduction zero), all three ligaments are
visibly slack. Strain measurements, however, register tension in the
superior and middle GHLs (46). The addition of
external rotation introduces tension (visible on radiographic
examination of tissue markers) in the middle GHL and the superior band
of the IGHL (66). Placing the arm in 45° of
abduction further tightens the superior band of the IGHL. External
rotation moves the superior band across the middle third of the joint
and the inferior margin tightly under the humeral head. Abduction to
90° increases IGHL tautness and its coverage of the lower half of the
joint (Fig. 76.5). The major strain is in the superior (anterior) band (46). There is little measurable tension in the MGHL and visibly it appears slack.
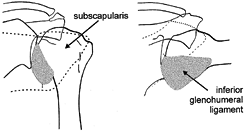 |
|
Figure 76.5. Change in the “ligamentous” anterior wall of the shoulder joint with arm position. Left: No elevation, no rotation; subscapularis tendon covers the anterior glenohumeral surface. Right:
90° abduction and 90° external rotation; the inferior glenohumeral ligament covers lower half of the glenohumeral joint, replacing upwardly displaced subscapularis tendon. (Adapted from Turkel SJ. Stabilizing Mechanism Preventing Anterior Dislocation of the Glenohumeral Joint. J Bone Joint Surg Am 1981;63(8):1208, with permission.) |
It forms a “bridge-like anterior leading edge” over the rotator
interval between the supraspinatus and subscapularis muscles (17).
Arising from the lateral base of the coracoid process, it joins the
capsule on its exterior surface and extends to both the greater and
lesser tuberosities, forming part of the roof of the bicipital tendon
sheath (Fig. 76.3) (8,17,18).
The coracohumeral ligament, in combination with the superior GHL, also
limits maximal forward flexion (by obligatory translation) and external
rotation (26).
Its greater base inserts on the underside of the acromion beyond the
tip, creating a mass that varies considerably in thickness (2.0–5.6
mm). The coracoid insertion is on the apophysis (22).
Studies of the skeletal arch have shown degenerative changes limited to
the anterior third of the acromion. The frequency of degenerative
findings is related to the slope and length of the acromion and the
height of the arch (17). Acromial thickness and
breadth are unrelated. The finding of an eburnated facet on the
underside of the acromion is consistent with a recent mechanical study
that showed the coracoacromial ligament passively restrains humeral
subluxation in the shoulder with a deficient rotator cuff (17,19).
of ribs is not a true joint. Instead, a thin layer of loose areolar
tissue separates the surfaces, allowing the scapula to glide freely in
all directions. Incongruity between the angle of the ribs and the
scapula is an occasional problem. The clavicle provides skeletal
contact between the scapula and the trunk.
junction between the scapula and the thorax. It is expendable, however,
if excised cautiously (29,58).
People without a clavicle (congenitally or by surgical resection) have
markedly increased scapular protraction and slightly reduced (10%)
strength of arm elevation but no significant instability (29).
Partial surgical excision must be designed to retain clavicular
stability by retaining the coracoclavicular ligaments or
sternoclavicular ligaments to a short medial segment. Otherwise, it is
necessary to remove the entire shaft (1).
Functional stability is retained by preserving the periosteal sheath
and muscular attachments. Replacement of the bone with a fibrin strip
also has been recommended (58).
joints at either end of the clavicle. Medially, the sternoclavicular
joint is the articulation between the shoulder girdle and the trunk. At
the lateral end, the acromioclavicular joint allows the scapula to
rotate on the clavicle.
This articulation consists of a large clavicular end; a small notch in
the superior, lateral corner of the sternal manubrium; and the
costocartilage of the first rib (3,69).
While the joint is grossly saddle shaped, the vertical convexity is
much greater than the anteroposterior concavity. Mobility is enhanced
by a complete articular disc attached superiorly to the clavicle and
inferiorly to the cartilage of the first rib.
end approximately 5 cm above the sternum is maintained by a dense
thickening of the superior and posterior portion of the capsule (3).
Simulation of arm weight (4.5 kg) suspended from the acromional end of
the clavicle has been shown to depress the clavicle 50%, but twice that
load caused little additional displacement. Depression of the clavicle
was accompanied by a forward movement. Conversely, the sternal end of
the clavicle moved upward and backward against the dense
posterosuperior part of the capsule. Avulsion of the ligament from the
sternum occurred with an 18 kg weight. Gray’s Anatomy emphasizes the mass of the anterior sternoclavicular ligament without a reference to Bearn (3,69).
the costoclavicular ligaments, reinforced by a dense anterior
sternoclavicular ligament and a thin posterior ligament. On the
inferior surface, there is a dense, complex costoclavicular ligament
spanning the first rib and clavicle. This arrangement provides good
stability during the significant range of motion that occurs in all
three planes (29).
is unique because its major stabilizing ligaments are remote
(coracoclavicular) despite there being a discrete local ligament lying
on the superior surface of the joint (acromioclavicular ligament) (21).
Restraint of posterior displacement of the clavicle and posterior axial
rotation have been identified as the primary function of the
acromioclavicular ligaments.
demonstrated that joint stability is gained mainly from the bipartite
coracoclavicular ligament, which is about 2 cm medial to the
acromioclavicular joint. The conoid component of the coracoclavicular
ligament complex is the primary restraint to anterior and superior
rotation, and to anterior and superior displacement of the clavicle.
The trapezoid ligament (vertical or horizontal) provides a much lower
level of clavicular restraint. The relative contributions of these
ligaments change with the direction and magnitude of loading and
displacement.
 |
|
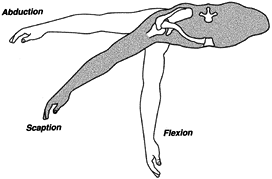
Figure 76.6.
The basic planes of arm elevation: flexion (sagittal plane), scaption (plane of the scapula on the thorax), and abduction (coronal plane). |
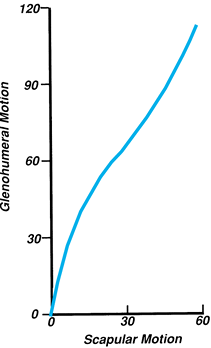
maximal ranges of the types of arm elevation. Theoretically, the
maximum is 180°, but few people have such mobility: Men average 168°,
women 175° (16). Within the full range of arm elevation, humeral displacement contributes about 120° and the scapula 60°.
 |
|
Figure 76.7.
Glenohumeral rhythm during scaption. The gross ratio of humeral to scapular motion is 2:1. During the first and last 30° of arm elevation, however, the humerus moves with minimal scapular participation. |
With the arm adjacent to the trunk, the range is greatest (180°), with
external rotation being the larger arc (108° versus 71°) (5).
Raising the arm to 90° reduces the total range to 120° and limits
external rotation to 90° (forearm vertical). With full flexion or
abduction, only a jog of rotation is possible. The relative
contributions of humeral and scapular motion to arm rotation have not
been determined.
paths, with the direction being defined by the motion of the acromion.
Upward rotation is the largest arc (60°). An undefined degree of
downward rotation accompanies humeral extension behind the body line.
Planar displacement of the scapula may be vertical or horizontal.
and depression. Horizontal scapular displacement is described by two
terms. Moving away from the vertebral spine is either scapular abduction or protraction; conversely, movement toward the spine is scapular adduction or retraction. The second term for each motion is preferred to avoid confusion with the descriptions of total arm motion.
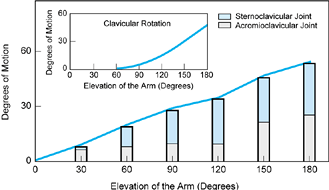
motion combined with clavicular rotation provide the scapular mobility
used in arm elevation (Fig. 76.8). The same motion patterns occur in flexion and abduction.
 |
|
Figure 76.8. The components of scapular elevation (heavy line). The contributions of the sternoclavicular joint (darker shading) and the acromioclavicular joint (lighter shading) motion differ during the range of arm elevation. Clavicular rotation (inset) is an essential component of terminal arm elevation.
|
rise of the clavicle, reaching 30° during the first 90° of arm
elevation, with little gain thereafter. Clavicular protraction and
rotation also take place at the sternoclavicular joint (29). By the end of full-arm elevation (180°), the clavicle has rotated 50°.
motion (15°) during the first and last 40° of arm elevation with no significant action in between (Fig. 76.8). Clavicular rotation is essential for the terminal arc of acromioclavicular mobility (29).
The sigmoid contour of the clavicle relatively lengthens the
coracoclavicular ligaments as the bone rotates; this lengthening
permits the scapular rotation needed to complete the last 60° of arm
elevation.
functions: movement of the arm and dynamic stabilization of the
glenohumeral joint (49). The 14 muscles contributing to these functions fall into four functional groups:
-
Three heads of the deltoid (anterior, middle, posterior)
-
Four rotator cuff muscles (supraspinatus, subscapularis, infraspinatus, teres minor) plus the biceps as an optional supplement
-
Two axiohumeral muscles (pectoralis major and latissimus dorsi) plus the teres major
-
Scapular muscle group (serratus anterior, trapezius, rhomboid, and levator scapulae)
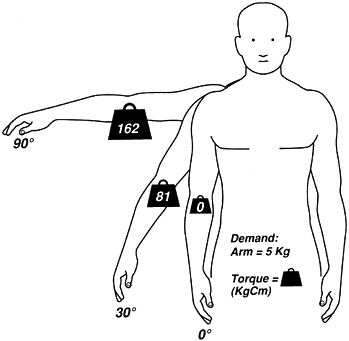
increasing moment resulting from the arm’s weight must be overcome by
increasing muscle force. Consequently, the torque that muscles must
provide at 90° is twice that needed at 30° (Fig. 76.9).
Arm elevation combined with preservation of glenohumeral stability
involves three muscle groups: the deltoids, the rotator cuff, and the
scapular rotators.
 |
|
Figure 76.9.
The influence of arm position on the demands (torque) imposed on the shoulder muscles. Arm weight is 5 kg; arm’s center of gravity is represented by the weight sign. At 0° (arm vertical), there is no torque. At 30° abduction, lateral displacement of the arm weight (functional lever) creates a torque of 81 kg-cm. Arm elevation of 90° further displaces the arm’s center of gravity, presenting the greatest passive torque (162 kg-cm). |
the primary source of arm elevation. Their relative participation
varies with the plane of motion (50). Middle
deltoid action is strong in all three planes of arm elevation. Anterior
deltoid involvement is greatest in flexion and scaption. The posterior
deltoid has a minor role except during abduction (Fig. 76.10).
 |
|
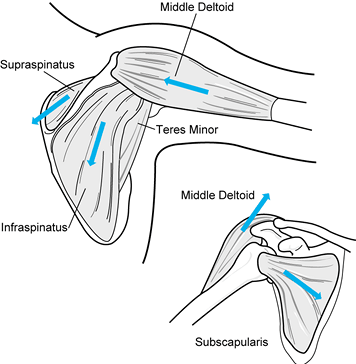
Figure 76.10. The line of pull (arrows)
of the deltoid and rotator cuff muscles. Note that the deltoid force direction has changed from being relatively vertical to being horizontal with increased arm elevation. The more downward alignment of the subscapularis muscle reflects the greater mass of vertical fibers. |
the humerus. While each provides an arc of motion, they also function
in synergy to stabilize the glenohumeral joint.
superior surfaces, is primarily an abductor. It also stabilizes the
glenohumeral joint by compression (Fig. 76.10) (30).
joint anteriorly, has a distribution of its fibers that fans out from
horizontal to vertical. The fibers’ common
function is internal rotation. The larger vertical mass provides a downward (humeral depression) force (Fig. 76.10) (30).
The subscapularis is an essential component of the dynamic anterior
wall that preserves joint stability during arm abduction and external
rotation. With the arm dependent, the subscapularis tendon lies across
the anterior surface of the glenohumeral joint. As the arm is elevated
or externally rotated, the tendon moves upward, leaving the lower
portion of the humeral head uncovered (66).
the shoulder joint, also has a fan-shaped dispersion of its fibers. Its
actions are external rotation and humeral depression. The smaller,
adjacent teres minor is a second external rotator and is most active
with the arm above horizontal (54).
has a potential for influencing shoulder as well as elbow motion. The
long head is more intimately involved than the short head, however, as
the tendon closely contacts the humeral head. During normal function,
the biceps brachii long head primarily serves the elbow independently
of shoulder action (41).
latissimus dorsi—extend from the chest wall to the proximal end of the
humerus. They contribute to shoulder joint stability by two very
different roles. With the arm dependent, these two large muscles
provide a depressor force, which protects the glenohumeral joint from
upward shear when the hand is being used to support the body weight.
Conversely, during arm elevation and external rotation, these muscles
oppose anterior shear. As it crosses the front of the joint, the
pectoralis major contributes directly to the dynamic anterior wall in
synergy with the subscapularis, while the latissimus acts indirectly by
retracting the humerus toward the muscle’s origin. The teres major
supplements the latissimus.
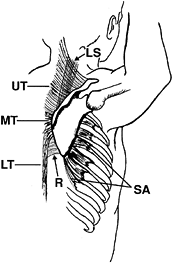
accomplished by synergistic action of the two largest scapular muscles
(the serratus anterior and trapezius) (Fig. 76.11).
In addition, these muscles also displace the scapula across the chest
wall. Two other muscles, the rhomboids and levator scapulae, act as
supplementary stabilizers.
 |
|
Figure 76.11. Scapular muscles: lateral view with arm abducted and externally rotated (cocked). SA, serratus anterior; UT, upper trapezius, MT, middle trapezius, LT, lower trapezius; R, rhomboids; LS, levator scapulae. (Adapted from Pink MM, Perry J. Biomechanics. In: Operative Techniques in Upper Extremity Sports Injuries. St. Louis: Mosby, 1996:113, with permission.)
|
the vertebral border of the scapula toward the lateral rib cage,
creating scapular protraction. The lower four segments, by their
insertion on the inferior angle of the scapula, induce upward rotation.
from the vertebral spinous processes to the upper border of the
scapular spine, provides scapular retraction. Upward rotation is
accomplished by synergistic action of the upper and lower portions of
the trapezius.
the scapula toward the vertebral spines. The levator scapulae, lying
between the neck and its insertion on the superior medial corner of the
scapula, serves as a stabilizing pivot by resisting depression.
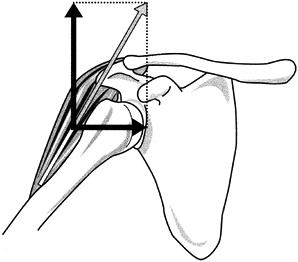
within the joint. Their impact on stability relates to the angle that
the muscle’s line of pull makes with the plane of the glenohumeral
joint. Also involved is the displacement of the action line from the
center of rotation of the joint. A force perpendicular to the face of
the glenoid creates compression and augments joint stability. In
dynamic cadaver studies, muscle compression has proved to significantly
increase the joint’s resistance to translatory displacement (8). Conversely, forces parallel with the glenoid induce shear and lead to joint instability (Fig. 76.12) (56).
 |
|
Figure 76.12. Joint forces (dark arrows) resulting from the muscle’s line of pull (light arrow). Perpendicular line to glenoid (horizontal line) represents compression. Parallel line (vertical) indicates shear. Note that at this arm position, the shear force of the deltoid is greater than its compression force.
|
clavicle, and scapular spine and extend distally to the mid-humeral
shaft, have a vertical alignment when the arm is down. As the arm is
raised, the muscles become increasingly horizontal, which markedly
changes their alignment with respect to the plane of the glenohumeral
joint (48,49,56).
results in a shear force 1.73 times greater than the compression force.
Consequently, there is a tendency for upward displacement of the
humerus on the glenoid that threatens joint stability and impingement
of the rotator cuff against the acromion. Inspection of 200 scapulae
showed a 22% incidence of an eburnated facet on the underside of the
acromion (17).
The subscapularis, infraspinatus, and teres minor muscles’ activity
provides downward force to counteract the deltoid’s upward glenohumeral
shear and reduce the necessary abduction force by 41% (63).
the deltoid becomes more horizontal and the muscle’s
shear-to-compression force ratio is reversed (S/C = 0.70) (56). Joint stability is correspondingly increased.
fast arm elevation in all three planes confirm this synergy between the
rotator cuff and the deltoid (30,50,51).
In all positions of arm elevation (flexion, scaption, and abduction),
the supraspinatus is a strong synergist of the deltoids (Fig. 76.13).
During flexion, the stronger action of the anterior deltoid induces a
posteriorly directed horizontal shear, which is opposed by greater
infraspinatus muscle action (Fig. 76.13A).
Similarly, in abduction the posterior head of the deltoid is more
active. This creates a forward-directed shear that is resisted by the
subscapularis (Fig. 76.13C). When these muscle synergies fail to occur, dynamic instability results (37).
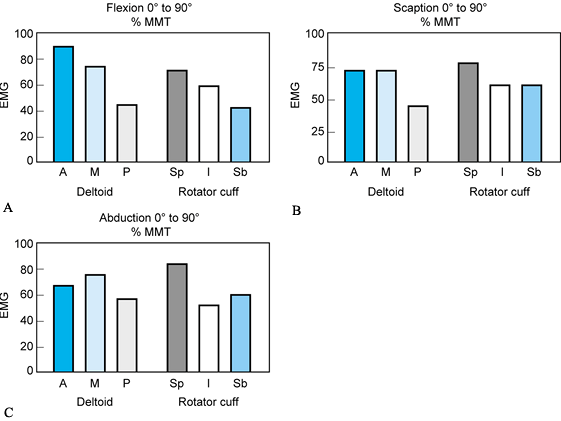 |
|
Figure 76.13. Relative intensity of the glenohumeral muscles during free arm elevation indicated by EMG. A: Flexion. B: Scaption. C: Abduction. Deltoid: A, anterior; M, middle; P, posterior. Rotator cuff muscles: Sp, supraspinatus; I, infraspinatus; Sb, subscapularis. Height of bar indicates muscle effort compared to EMG of maximum manual muscle test (MMT).
|
contribute to superior glenohumeral stability. During abduction,
patients with isolated proximal biceps detachment were found to have
increased superior displacement of the humeral head (68). Experimentally, the presence of a biceps force added torsional rigidity (57). Hence, the biceps brachii long head can be considered an accessory joint stabilizer, to be used when needed.
of an obligatory deltoid/rotator cuff force couple is contradicted by
the finding that the arm can be raised overhead with complete paralysis
of either the deltoid or the supraspinatus (65).
Selected anesthetic blocks of the axillary nerve innervating the
deltoid, or of the suprascapular nerve innervating the supraspinatus
and infraspinatus, have confirmed this clinical observation (10,67). Each nerve block reduced arm elevation strength approximately 50% but did not prevent a full range of motion.
provide partial substitution. For the deltoid, there is the long head
of the biceps, the clavicular head of the pectoralis major, and the
coracobrachialis. Supplements to the supraspinatus are the
subscapularis and teres minor. Thus, inability to elevate the arm fully
is not an essential sign of either a rotator cuff tear or deltoid
paralysis.
elevation by upwardly rotating the glenoid fossa. This action also
preserves muscle length of the glenohumeral muscles. In addition,
upward displacement of the acromion reduces the threat of humeral
impingement. The relative intensity
of contraction of the serratus anterior and the different divisions of the trapezius varies with arm position (13,38). The basic synergy was found by EMG to be between the lower serratus anterior and upper trapezius (Fig. 76.14) (13,38). During flexion, the need for scapular protraction as well as upward rotation made the serratus anterior the dominant muscle.
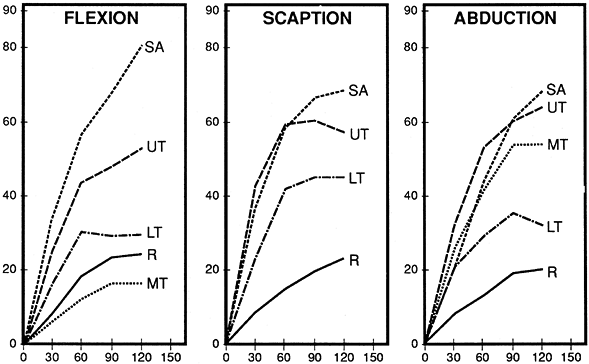 |
|
Figure 76.14.
Relative intensity of scapular muscle activity during arm elevation. Vertical axis: EMG as a percentage of maximum muscle test. Horizontal axis: degrees of arm elevation. SA, serratus anterior; UT, upper trapezius; MT, middle trapezius; LT, lower trapezius; R, rhomboid. |
Conversely, abducting the arm in the body’s coronal plane includes
scapular retraction. The serratus anterior was less intense, while both
the upper and middle heads of the trapezius showed strong EMG (30,38).
Scaption, as a neutral position between scapular abduction and
adduction, displays a balanced synergy between the two rotator systems
(serratus anterior and trapezius) (51). Lower trapezius activity was slightly increased in scaption but otherwise was of just moderate intensity.
trapezius is highly dependent on the plane of arm elevation used.
Clinical experience with cases of isolated paralysis of either the
serratus or the trapezius has shown that the patient may still raise
the arm to approximately 90° but that full overhead reach cannot be
accomplished (unpublished data).
Inman et al. postulated participation of the levator scapulae as a
component of the serratus force couple, but this finding was not
supported by EMG (30). A recent study of the
levator scapulae during arm elevation, however, showed a moderate level
of activity, which increased as the arm moved higher (Pink M,
unpublished data, 1998). The findings of incomplete scapular mobility
in a recent case of levator scapulae tendon strain support the concept
of this muscle’s being a central stabilizer.
This rotation changes the coordination between the deltoid and rotator
cuff from a synergy to a sequence. The mechanics of a baseball pitch
provides one example: Cocking of the shoulder begins with elevation and
horizontal extension, which are quickly augmented by aggressive
external rotation to tense the propulsive muscles maximally. The rapid
reversal of shoulder motion into accelerated internal rotation enables
the propelling forces to throw the ball at high speed (Fig. 76.15).
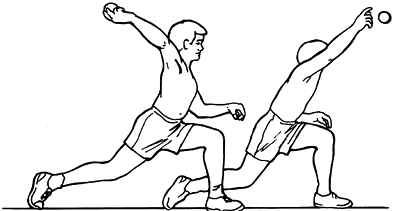 |
|
Figure 76.15.
The humeral rotation used in pitching. This is also representative of throwing and the tennis serve. Maximum external rotation of cocking and the internal rotation of acceleration are displayed. (Adapted from Glousman R et al. Dynamic Electromyographic Analysis of the Throwing Shoulder with Glenohumeral Instability. J Bone Joint Surg Am 1988;70:220, with permission.) |
relies on moderately intense deltoid (anterior, middle, and posterior) and supraspinatus activity to raise and retract the arm (15,31).
Further cocking of the arm by maximal external rotation shifts the
dominant muscle control to the rotator muscles (infraspinatus, teres
minor, subscapularis), while the deltoids’ role is reduced. Rapid
reversal of dominant muscle control to the shoulder internal rotators
(upper subscapularis, latissimus dorsi, teres major, and pectoralis
major) first protects the anterior joint from strain at the end of
cocking. Then, as the external rotators relax, these anterior muscles
whip the arm forward with a strong propelling force, which throws the
ball at high speed (Fig. 76.15) (15).
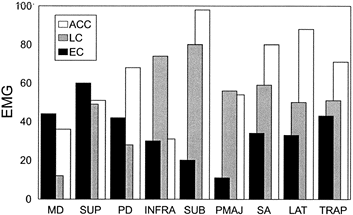 |
|
Figure 76.16. EMG representation of relative intensity of muscular action during the dynamic rotational phases of pitching. Muscles: MD, middle deltoid; SUP, supraspinatus; PD, posterior deltoid; INFRA, infraspinatus; SUB, subscapularis; PMAJ, pectoralis major; SA, serratus anterior; LAT, latissimus dorsi; TRAP, trapezius. Phases: EC, early cocking; LC, late cocking; ACC, acceleration.
|
cocking adds upward rotation of the scapula to increase the range of
arm motion (53). There also is vigorous levator scapulae activity (15). The fact that trapezius participation in early cocking and follow-through is much less than that of the serratus (24)
suggests that the scapular retraction function of the trapezius is
useful as a stabilizer but is not a dominant source of motion.
rotation as a primary driving force, but they also rely on timely input
of all the other shoulder muscles at their appropriate intensities. The
threats to anterior and posterior joint stability are enhanced by the
momentum imparted to the arm by the forward drive of the trunk and legs
and the forearm inertia created by the flexed elbow, which creates a
significant torque against the tissues of the glenohumeral joint.
of the arm, such as hammering, swimming, and using the hand to raise
the body from a sitting position or to propel a wheelchair.
The simultaneous action of the teres minor counterbalances the internal
rotation action of the propelling muscles. Serratus anterior synergy
adds the scapula to the length of the pulling extremity. Peak effort
occurs as the arm is perpendicular in the water (approximately 90° of
shoulder flexion).
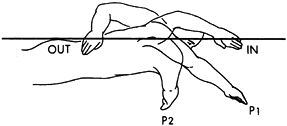 |
|
Figure 76.17.
The propulsion phase of a freestyle swim stroke. This begins shortly after hand entry into the water and continues until the vertical line has been passed. (Adapted from Scovazzo et al. The Painful Shoulder during Freestyle Swimming: An EMG Cinematographic Analysis of 12 Muscles. Am J Sports Med 1991;19:577, with permission.) |
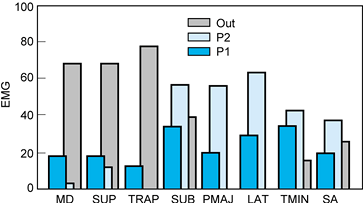 |
|
Figure 76.18. EMG representation of the relative intensity of muscle action during a freestyle swim stroke. Muscles: MD, middle deltoid; SUP, supraspinatus; TRAP, trapezius; SUB, subscapularis; PMAJ, pectoralis major; LAT, latissimus dorsi; TMIN, teres minor; SA, serratus anterior. Phases: P1, pull, early; P2,3, pull, late; OUT, hand exit.
|
to reach overhead (arm elevation mechanics) for a brief period. Arm
depression then is initiated to propel the body. Soon after the arm has
passed its peak propulsive alignment, the rhomboid, trapezius, and
posterior deltoid muscles lift the arm from the water, and the
mechanics of reach begin. Now the middle and anterior deltoids,
supraspinatus, and subscapularis advance the arm in this recovery phase.
critical function for patients with spinal cord injury who rely on
manual transfer (Fig. 76.19) (42,52).
An EMG study of this maneuver found that the latissimus dorsi, lower
trapezius, and sternal pectoralis major are the major lifting forces.
The serratus anterior, while not significant in the vertical rise,
shows strong activity when the body weight is shifted from one arm to
the other in the depression transfer (52). A study of the prone push-up exercise shows the serratus anterior to be a major force when the
trunk is elevated on the arms (40).
Seldom does any head of the deltoid participate during the raise, but
it is active in body positioning. Propelling a wheelchair relies on a
downward forward force against the wheel rim. The muscles most active
during the propulsion phase are the pectoralis major and the anterior
deltoid (43).
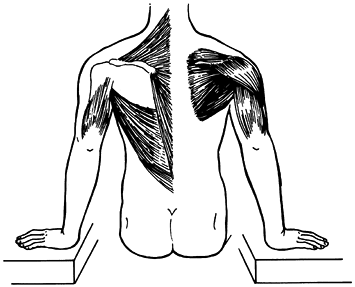 |
|
Figure 76.19. Body raise; manual transfer of the body from a sitting position. Left side: raise muscles [latissimus dorsi, trapezius, pectoralis major (not shown), and triceps]. Right side: positioning muscles (deltoid, rotator cuff).
|
use the same mechanics. The upper trapezius and levator scapulae
muscles stabilize the scapula. Glenohumeral stabilization is more
indirect. Experimentally, a strong downward tug on the arm fails to
elicit any response by the longitudinal muscles (triceps, biceps, and
anterior and middle deltoids) (2). Instead, the
supraspinatus and to a lesser degree the posterior deltoid respond. The
fact that both of these latter muscles have a horizontal alignment
identifies compression, rather than lift, as the primary force needed
to hold the humeral head into the glenoid fossa. This finding has been
confirmed by studies of passive translation (36).
The former term is preferred, as there is less chance for confusion
with scapular motion. The pectoralis major, pectoralis minor, and
serratus anterior bring about horizontal flexion. Often, the levator
scapulae also participates (13).
horizontal flexion are the clavicular head of the pectoralis major and
the subscapularis (47). During the
arm-retraction phases of pitching (late cocking) and swimming (early
recovery), there is prominent action by the rhomboid, trapezius,
posterior deltoid, and infraspinatus (24,47,62).
A study of horizontal circumduction confirmed the posterior deltoid and
added the infraspinatus as the dominant horizontal extensor muscles of
the glenohumeral joint (47).
contact between the humeral head and the glenoid fossa. Mobility is
enhanced by the flexibility of the glenoid labrum, limited glenohumeral
ligamentous restraint, and the small contact areas of the clavicular
articulations. The selective exchange among the various muscle groups
must simultaneously provide dynamic stability as well as the wide
versatility of motion that characterizes arm function. To meet these
objectives, a continual balancing is necessary among the external
glenohumeral muscles, the rotator cuff musculature, and the muscles
controlling the scapula.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JV, Bazant FJ. Factors Preventing Downward Dislocation of the Adducted
Shoulder Joint: An Electromyographic and Morphological Study. J Bone Joint Surg Am 1959;41:1182.
VP, Gagliardi JA, Murnane TG, et al. Glenohumeral Ligaments and
Shoulder Capsular Mechanism: Evaluation with MR Arthrography. Radiology 1995;196:27.
Freitas V, Vitti M, Furlani J. Electromyographic Study of Levator
Scapulae and Rhomboideus Major Muscles in Movements of the Shouder and
Arm. Electromyogr Clin Neurophysiol 1980;20:205.
EL, Wang VM, Kelkar R, et al. The Coracoacromial Ligament Passively
Restrains Anteriorsuperior Humeral Subluxation in the Rotator Cuff
Deficient Shoulder. Ortho Trans 1996;20:633.
P, Schuller U, Wiedermann E. The Intra-articular Pressure of the
Shoulder: An Experimental Study on the Role of the Glenoid Labrum in
Stabilizing the Joint. Arthroscopy 1992;8:166.
DT II, Sidles JA, Harris SL, Matsen FA III. The Role of the Rotator
Interval Capsule in Passive Motion and Stability of the Shoulder. J Bone Joint Surg Am 1992;74:53.
TB. The Movements of the Shoulder Joint. A Plea of the Use of the
‘Plane of the Scapula’ as the Plane of Reference for the Movements
Occurring at the Humero-scapular Joint. Br J Surg 1937;25:252.
AR, Williams GR, Williame JL, Lannotti JP. Kinematics of the
Glenohumeral Joint: Influences of Muscle Forces, Ligamentous
Constraints, and Articular Geometry. J Orthop Res 1996;14:986.
EG, Jobe FW, Perry J, et al. Electromyographic Analysis of Recurrent
Posterior Instability of the Shoulder. In: Post M, Morrey BF, Hawkins
RJ, eds. Surgery of the Shoulder. St. Louis: Mosby Year Book, 1990:112.
PJ, Jobe FW, Pink MM, et al. Comparative Electromyographic Analysis of
Shoulder Muscles during Planar Motions: Anterior Glenohumeral
Instability versus Normal. J Shoulder Elbow Surg 1996;5:118.
S, Newsam C, Gronley JK, Perry J. Stroke Characteristics and Upper
Extremity Motion of Spinal Cord Injured Patients during Wheelchair
Propulsion. Arch Phys Med Rehabil 1992;73:954 (abst.)
SJ, Gronley JK, Newsam CJ, Perry J. Electromyographic Activity of
Shoulder Muscles during Wheelchair Propulsion by Paraplegic Persons. Arch Phys Med Rehabil 1996;77:187.
SJ, Neves MC, Arnoczky SP, et al. The Anatomy and Histology of the
Inferior Glenohumeral Ligament Complex of the Shoulder. Am J Sports Med 1990;18:449.
PW, Nuber GW, Mileski RA, Lautenschlager E. The Contribution of the
Glenohumeral Ligaments to Anterior Stability of the Shoulder Joint. Am J Sports Med 1990;18:579.
J, Gronley JK, Newsam CJ, et al. Electromyographic Analysis of the
Shoulder Muscles during Depression Transfers in Subjects with Low-Level
Paraplegia. Arch Phys Med Rehabil 1996;77:350.
J, Hoffer MM, Antonelli D, et al. Electromyography Before and After
Surgery for Hip Deformity in Children with Cerebral Palsy. A Comparison
of Clinical and Electromyographic Findings. J Bone Joint Surg Am 1976;58:201.
M, Perry J, Browne A, et al. The Normal Shoulder during Freestyle
Swimming: An Electromyographic and Cinematographic Analysis of Twelve
Muscles. Am J Sports Med 1991;19:569.
MW, Harner CD, Fu FH. The Role of the Long Head of the Biceps Muscle
and Superior Glenoid Labrum in Anterior Stability of the Shoulder. Am J Sports Med 1994;22:121.
AK. Mechanics of Elevation of Glenohumeral Joint. Its Application in
Rehabilitation of Flail Shoulder in Upper Brachial Plexus Injuries and
Poliomyelitis and in Replacemant of the Upper Humerus by Prosthesis. Acta Orthop Scand 1973;44:668.
L, Browne A, Pink M, et al. The Painful Shoulder during Freestyle
Swimming: An Electromyographic and Cinematographic Analysis of Twelve
Muscles. Am J Sports Med 1991;19:577.
