ARTHROSCOPIC SURGERY OF THE WRIST
III – THE HAND > Reconstructive Procedures > CHAPTER 75 –
ARTHROSCOPIC SURGERY OF THE WRIST
His primitive arthroscope had only limited utility, but over the next
several decades he continued to improve the quality of arthroscopic
equipment. In the late 1970s, an arthroscope suitable for use in small
joints was developed, leading to Chen’s description of wrist
arthroscopy in 1979 (9). Over the last 20
years, wrist arthroscopy has advanced from a diagnostic to a
therapeutic procedure as the principles of open surgical procedures
have been adapted to the arthroscope (1,14,15,23,30,33,36,38,48,49,50,63,66).
As in larger joints, this less invasive method may have decreased
morbidity and shortened recovery time. Only time and careful evaluation
of treatment outcomes will determine the ultimate benefit of each
specific procedure.
indications. It can be used to confirm findings suggested by other
diagnostic studies as well as for the diagnosis of
wrist pain of unknown etiology. Indications for wrist arthroscopy are listed in Table 75.1.
 |
|
Table 75.1. Indications for Wrist Arthroscopy
|
-
Position the patient supine on the
operating table with the upper extremity supported on a hand table.
Place a padded pneumatic tourniquet on the upper arm. In our
experience, inflation of the tourniquet is required in approximately
50% of cases. -
Because of the small joint volume and the
tightness of the wrist capsule, visualization requires distraction.
Obtain distraction through suspension of the extremity from overhead.
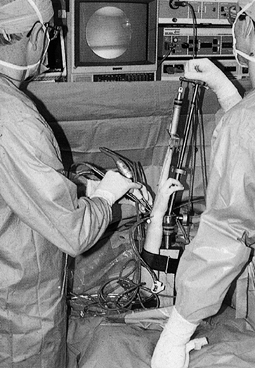
We prefer a distraction tower placed on the hand table (Fig. 75.1), as this allows conversion to open procedures without the need for redraping.![]() Figure 75.1.
Figure 75.1.
Arthroscopic setup. Strap the extremity to the base of a traction tower
placed on the hand table. Suspend all four fingers from the tower with
finger traps. Ten to 15 pounds of traction is usually sufficient.
categories: Scopes, probes, grasping instruments, and cutting
instruments. Wrist arthroscopes are generally 2.5–3.0 mm in diameter.
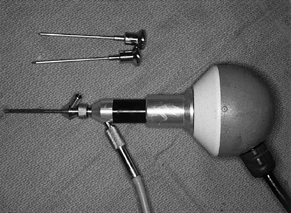
An oblique viewing angle of 30° provides excellent visualization (Fig. 75.2).
 |
|
Figure 75.2. Top to bottom: A 2.5 mm arthroscope, cannula, and camera. A 30° viewing angle provides the best perspective
|
Palpation with a blunt probe can help the arthroscopist define normal
anatomy as well as identify pathology such as a triangular
fibrocartilage complex (TFCC) injury.
 |
|
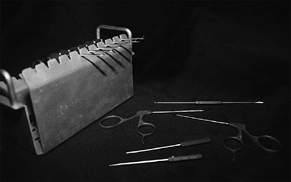
Figure 75.3. Arthroscopic instruments.
|
18-gauge needle is a versatile arthroscopic instrument. The needle is
commonly used to localize the joint prior to creation of a portal. It
can also be used to spear or manipulate loose bodies, and to resect the
edges of a central TFCC tear. More sophisticated manual instruments
include basket forceps and suction punches (Fig. 75.3).
full-radius resectors and arthroscopic burrs. Full-radius resectors are
used for soft-tissue excision such as synovectomy and debridement of
ligament tears. They are not well designed for work with firmer tissues
such as articular cartilage. These instruments function most
efficiently at lower speeds in the range of 400 rev/min (64).
At higher speeds, there is insufficient time for tissue to enter the
aperture of the tip. Arthroscopic burrs are useful for treatment of
osseous lesions as well as for salvage procedures. These instruments
work most efficiently at speeds greater than 1,200 r/min (64).
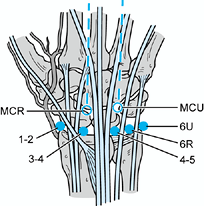
on the dorsal aspect of the wrist. The radiocarpal portals are numbered
according to their relation to the extensor compartments of the wrist.
For example, the 3-4 portal is situated between the third and fourth
extensor compartments (Fig. 75.4). There are
also two mid-carpal portals, as well as portals for the distal
radioulnar joint. Portals are established in the following manner.
 |
|
Figure 75.4. The mid-carpal ulnar (MCU) portal
is best located under arthroscopic view after the MCR portal is established. A gap between the proximal and distal carpal rows can be palpated along the midline of the fourth metacarpal. The 1-2 portal is located in
the dorsal aspect of the anatomic snuffbox, just radial to the extensor pollicis longus tendon. Care must be taken to avoid injury to the radial artery. This portal provides visualization of the radial styloid, scaphoid, and distal radius. The 3-4 portal is most
commonly used for radiocarpal arthroscopy. The portal is established directly distal to Lister’s tubercle (approximately 1 cm) in the radiocarpal joint. It lies between the third and fourth extensor compartments. The 4-5 portal provides
access to the ulnar wrist. It is located just ulnar to the fourth compartment and approximately 1 cm distal to Lister’s tubercle. This portal should be established with arthroscopic visualization. The 6R portal also provides
access to the ulnar aspect of the wrist. It is located distal to the ulnar head and radial to the extensor carpi radialis tendon. The portal is made just proximal to the triquetrum to avoid injury to the TFCC. Like the 4-5 portal, the 6R portal is established under arthroscopic visualization. The 6U portal can be used as
an inflow or outflow portal. It is located ulnar to the extensor carpi ulnaris. Under arthroscopic visualization, the needle is inserted just distal to the ulnar styloid. The mid-carpal radial (MCR) portal
is established 1 cm distal to the 3-4 portal, along a line bordering the radial edge of the third metacarpal. A soft depression between the proximal and distal carpal rows can be palpated. |
-
Determine the correct site by palpation of the surface anatomy.
-
Confirm the portal site by inserting an 18-gauge hypodermic needle into the joint.
-
Use a scalpel to make a 3 mm longitudinal incision through the skin only.
-
Use a hemostat to bluntly dissect to the level of the wrist capsule.
-
Introduce the arthroscopic sheath into the joint with a blunt trocar.
establish subsequent portals under arthroscopic visualization. The
portals are described in Figure 75.4.
for the diagnosis of soft-tissue wrist pathology. However, with the
emergence of magnetic resonance imaging (MRI) and arthroscopy, the
usefulness of this modality has been challenged (11,20,27,46,51).
Although it can be performed as a single radiocarpal injection, the
triple injection arthrogram as described by Zinberg et al. (70)
is considered a better test. Inject the radiocarpal joint first. If no
dye leakage is observed, inject the mid-carpal and distal radioulnar
joints after the dye from the first injection has been cleared.
Communication of dye between compartments indicates a tear.
incidence of positive findings in the asymptomatic, contralateral wrist
has led many investigators to question the significance of
arthrographic findings (8). Obstruction of
perforations by synovitis, or a flap acting as a one-way valve can lead
to false-negative tests. Positive tests give no indication of the size
of a tear or the degree of instability (62). A
number of studies have revealed arthroscopy to have higher specificity
and sensitivity than arthrography for the diagnosis of wrist
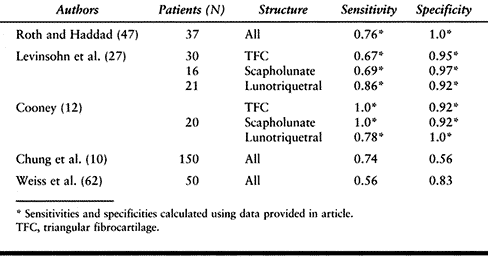
soft-tissue injuries (Table 75.2) (11,12,27,47,62).
 |
|
Table 75.2. Arthrography versus Diagnostic Arthroscopy
|
has become the most widely used imaging modality. An early study
suggested that MRI is inferior to arthrography for the diagnosis of
tears of the triangular fibrocartilage, but the MRI technology utilized
at that time has become obsolete (20). Many
older studies relied on the “arthrogram effect,” which occurs when
fluid (high signal intensity on a T2-weighted image) courses through
the perforated structure. Two recent studies comparing
higher-resolution MRI scans with arthroscopic evaluation demonstrated
sensitivity of 100% and specificity of 90% for MRI in the diagnoses of
TFC tears (46,58).
ability to localize the site of a TFCC tear with 100% sensitivity and
75% specificity. In the diagnosis of scapholunate injuries, MRI has
been reported to have a specificity of 40% to 90% and a sensitivity of
100% (24,71). MRI is
much less reliable for the diagnosis of lunotriquetral (LT) tears,
demonstrating a sensitivity of 0% to 50% and specificity of 100% (24,71).
modern technology, involves intracarpal injection of dye followed by
MRI of the wrist. This procedure theoretically combines the best
aspects of both arthrography and MRI. Using this technology, Scheck et
al. (51) diagnosed scapholunate ligament
injuries with a sensitivity and specificity of 90%. Further
investigation of this technique will be required before an assessment
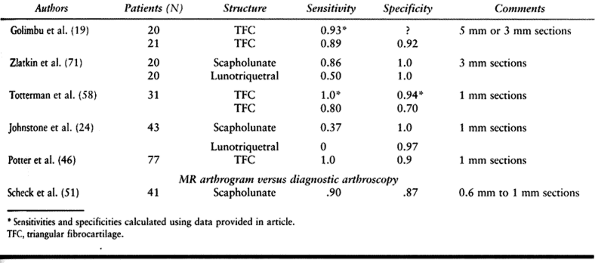
of its utility can be made. Table 75.3 reviews the results of studies comparing these modalities to diagnostic arthroscopy.
 |
|
Table 75.3. MRI versus Diagnostic Arthroscopy
|
diagnosis and classification of intercarpal wrist pathology. It enables
the surgeon to assess the size and stability of a tear and identify the
presence of associated synovitis or chondral defects.
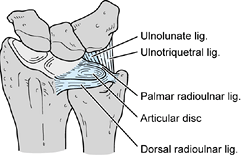
composed of dorsal and volar radioulnar ligaments, the ulnar collateral
ligament, the articular disc, the sheath of the extensor carpi ulnaris
(ECU) tendon, and the ulnolunate and LT ligaments (Fig. 75.5) (43).
The complex arises from the articular cartilage on the corner of the
sigmoid notch of the radius and inserts into the base of the ulnar
styloid as well as the ulnolunate and ulnotriquetral ligaments. The
dorsal and volar radioulnar ligaments are fibrous thickenings within
the dorsal and volar edges of the triangular fibrocartilage. The
central disc is the thinnest portion of the complex (10). The vascularity of the TFC has been well studied (5,56).
Like the knee’s meniscus, only the peripheral 25% of the TFC is
vascularized; therefore, only peripheral tears are considered reparable.
 |
|
Figure 75.5. The triangular fibrocartilage complex (TFCC)
is a ligamentous, cartilaginous structure composed of the following components: dorsal and volar radioulnar ligaments, the ulnar collateral ligament, the articular disc, the sheath of the extensor carpi ulnaris tendon, and the ulnolunate and lunotriquetral ligaments. |
ulnar-neutral individuals, the complex transmits 20% of axially applied
loads from the ulnar carpus to the distal ulna (43).
The percentage of the load transmitted is directly proportional to the
ulnar variance. The TFCC is also the major stabilizer of the distal
radioulnar joint (42,43 and 44).
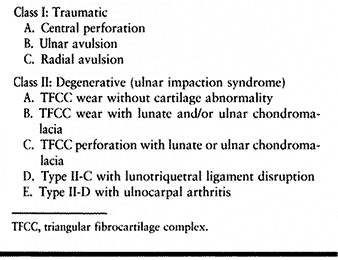 |
|
Table 75.4. Palmer’s Classification of TFCC Lesions
|
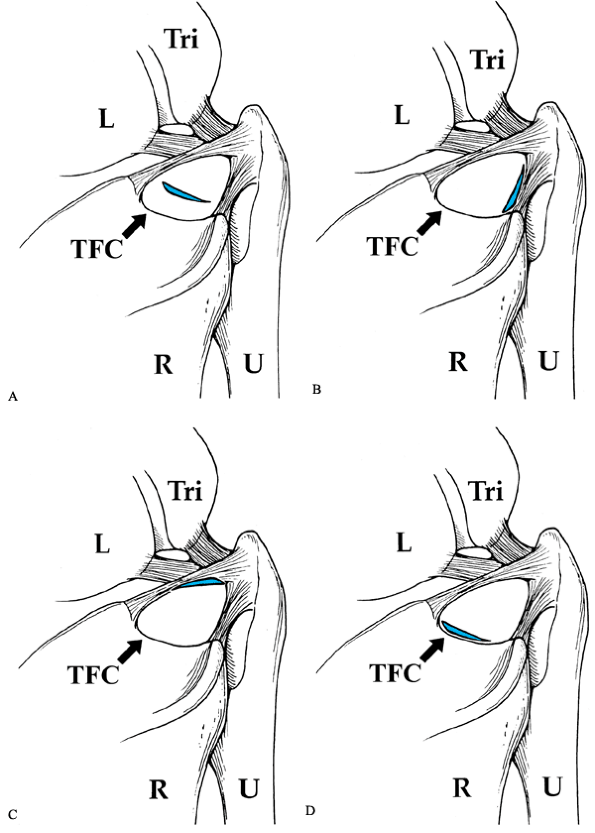 |
|
Figure 75.6. Palmer classification of traumatic triangular fibrocartilage (TFC) tears. R, radius; U, ulna; L, lunate; TRI, triquetrum. A: Class I-A. B: Class I-B. Can be purely soft tissue or with ulnar styloid base fracture. C: Class I-C. D: Class I-D.
|
extension–pronation force applied to an axially loaded wrist (a fall on
an outstretched hand) or a dorsal rotation injury (29). These tears can also occur as the sequelae of a distal radius fracture.
subluxation, LT injury, pisotriquetral arthritis, chondral lesions of
the ulnar carpus, ulnar artery thrombosis, and ulnar neuropathy at the
wrist. The patient may report having fallen on the pronated wrist, and
twisting or distraction injury to the wrist. The patient may also
report ulnar-sided, mechanical wrist pain, which is usually not
disabling and is frequently accompanied by clicking.
tenderness, a positive response to a TFCC compression test (axial
loading of the ulnarly deviated wrist), and varying degrees of
instability of the distal radioulnar joint and supination of the ulnar
carpus. Concomitant LT ligament injury may be present if there is
tenderness over the LT interval, and if an LT ballottement test and a
shuck test are positive (see Chapter 43).
posteroanterior (PA) and lateral views. Carpal alignment, ulnar styloid
morphology, and ulnar variance can be assessed through these views.
Clenched-fist radiographs in full ulnar deviation may show evidence of
dynamic variance changes. The role of more advanced imaging studies
such as arthrography, triple-phase bone scan, and MRI remains
controversial.
distal radioulnar joint, the majority of TFCC injuries can be treated
with 4 weeks of immobilization (Fig. 75.7) (37).
A peripheral tear would be expected to heal because of its excellent
vascular supply. In some patients, a local injection of corticosteroid
to relieve inflammation, as well as a short course of wrist therapy,
may be helpful. Many central tears may become asymptomatic despite
their inability to heal (37).
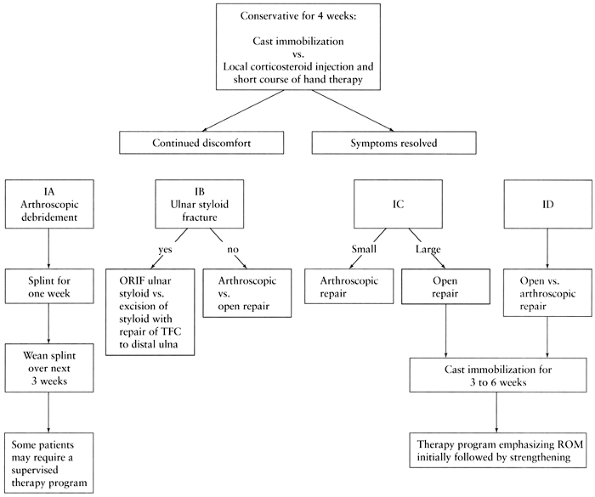 |
|
Figure 75.7. Treatment of traumatic triangular fibrocartilage complex (TFCC) tears.
|
significantly symptomatic TFCC injury after failure of an adequate
conservative treatment program. Central perforations can be treated
with either open or arthroscopic debridement. Peripheral tears should
be repaired using either arthroscopic or open methods. The technique of
arthroscopic repair varies with the location of the tear.
-
Evaluate the radiocarpal joint through the 3-4 portal (Fig. 75.8A).
![]() Figure 75.8. A:
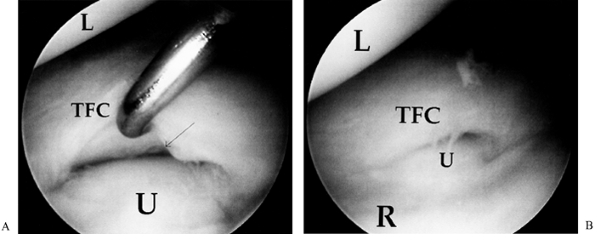
Figure 75.8. A:
Class I-A central traumatic tear of the TFCC. Note that the ulnar head
is deep to the radius, indicating negative ulnar variance. B:
Class I-A after debridement. Note the stable TFC rim and normal distal
ulnar articular surface. L, lunate; TFC, triangular fibrocartilage; U,
ulna; R, radius. -
Use the 4-5 or the 6R portal for instrumentation.
-
Perform a synovectomy with a full-radius resector. This important step allows proper visualization of pathology.
-
Debride the central two thirds of the TFCC with a suction punch or full-radius resector (Fig. 75.8B).
A banana blade can also be used to resect the unstable portion of the
TFCC. Do not use the distal ulna as a cutting board. Avoid injury to
the dorsal and volar radioulnar ligaments and the peripheral TFCC; such
injury will lead to destabilization of the distal radioulnar joint
(DRUJ). -
Postoperatively, splint the wrist
intermittently and initiate a therapy program stressing range-of-motion
(ROM) exercises for 3–4 weeks. Then initiate a graduated strengthening
program. Results from the literature are shown in Table 75.5.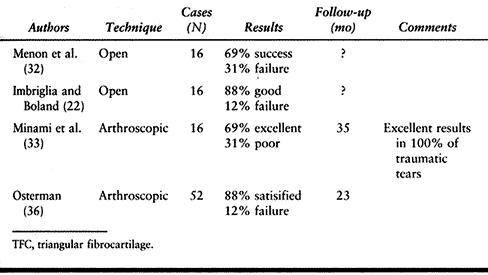 Table 75.5. Debridement of Central TFC Tears
Table 75.5. Debridement of Central TFC Tears
-
Evaluate the radiocarpal joint through
the 3-4 portal. A key to the diagnosis of a peripheral tear is the loss
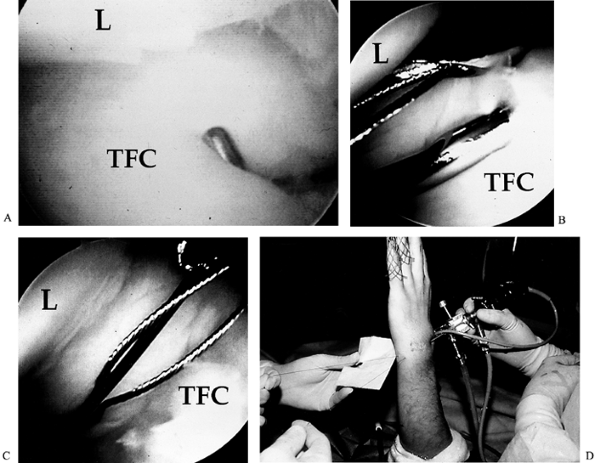
of normal tension in the TFCC. A probe will sink into the lax tissue (Fig. 75.9A).![]() Figure 75.9. A: Peripheral I-B tear. Note that the probe sinks into the lax TFC. L, lunate; TFC, triangular fibrocartilage. B: Out-to-in repair: Needles are passed through the capsule and the TFC; wire loop suture is passed through one needle. C: 2-0 PDS suture is passed through the other needle and lassoed by a wire loop. D:
Figure 75.9. A: Peripheral I-B tear. Note that the probe sinks into the lax TFC. L, lunate; TFC, triangular fibrocartilage. B: Out-to-in repair: Needles are passed through the capsule and the TFC; wire loop suture is passed through one needle. C: 2-0 PDS suture is passed through the other needle and lassoed by a wire loop. D:
Suture tied at the capsule level through a 1 cm incision radial to the
extensor carpi ulnaris. Generally, one to three sutures are used. -
Use the 4-5 or the 6R portal for instrumentation.
-
Perform a synovectomy using a full-radius resector.
-
Debride the edges of the tear with the full-radius resector.
-
Make a 1 cm longitudinal skin incision radial to the ECU tendon. Then sharply open the radial aspect of the ECU tendon sheath.
-
Retract the ECU and place two needles
from the meniscal repair kit through the floor of the sheath and across
the tear under arthroscopic visualization (Fig. 75.9B). -
Pass a 2-0 PDS (Meniscal MenderII, Instrument Maker, Inc., Okemos, Michigan) suture through one needle.
-
Retrieve the suture with a wire loop passed through the other needle (Fig. 75.9C).
-
Remove both needles, reapproximate the
tear, and tie the suture over the dorsal wrist capsule. Multiple
sutures may be necessary (Fig. 75.9D). -
Postoperatively, place the upper
extremity in a long-arm splint with the forearm in neutral rotation and
the wrist in neutral flexion and mild ulnar deviation. After
P.2044P.2045P.2046
suture
removal, change to a long-arm cast for immobilization for a total of 6
weeks. Then splint intermittently and begin a therapy program stressing
ROM initially, followed by a graduated strengthening program. Outcomes
are detailed in Table 75.6.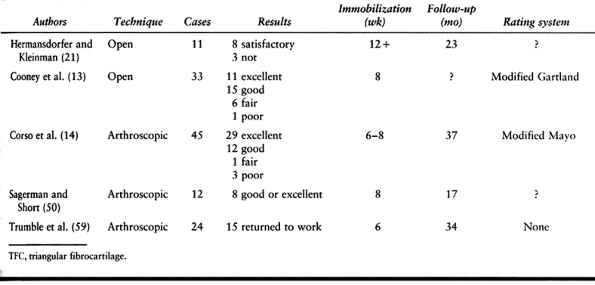 Table 75.6. Repair of Peripheral TFC Tears
Table 75.6. Repair of Peripheral TFC Tears
triangular fibrocartilage from the sigmoid notch of the distal radius.
Normally, the articular cartilage of the distal radius continues around
the medial corner and into the sigmoid notch. The triangular
fibrocartilage originates from this articular location. Given this
cartilage barrier, the healing potential for the TFCC at this site
should be poor. If the cartilage is disrupted manually or by fracture,
however, the triangular fibrocartilage can be reattached to
vascularized bone. To perform this repair arthroscopically, we use an
alignment guide similar to that described by Jantea et al. (23).
In this scenario, we use either 0.35 or 0.45 Kirschner wires (K-wires)
to pin the triangular fibrocartilage back to the radius. The pins are
left percutaneous and removed at 4 weeks.
-
Evaluate the radiocarpal joint through the 3-4 portal.
-
Use the 4-5 portal for instrumentation.
-
Use a burr to debride the TFC attachment site to allow healing to fresh cancellous bone.
-
Temporarily fix the TFC to the radial attachment site with a K-wire directed from the ulnar wrist.
-
Place an alignment guide over the K-wire.
-
Make a longitudinal incision between the first and second extensor compartments. Dissect bluntly to the wrist capsule.
-
Drill an 18-gauge spinal needle through the jig into the radius, exiting at the radial reattachment site and the TFC.
-
Place a second 18-gauge needle parallel to the first.
-
Place a 2-0 PDS suture through one needle.
-
Retrieve the suture with wire loop placed through the other needle.
-
Reapproximate the tear and tie the suture over the wrist capsule.
-
Postoperatively, place the upper
extremity in a long-arm splint with the forearm in neutral rotation and
the wrist in neutral flexion and mild ulnar deviation. After suture
removal, change to a long-arm cast to immobilize the wrist for a total
of 6 weeks. Then splint intermittently and begin a therapy program
stressing ROM initially, followed by a graduated strengthening program.
and no randomized controlled studies have been published. Because of
the lack of normalized data, it is difficult to compare the results of
open versus arthroscopic treatment of TFCC injuries.
TFCC tears include improved visualization of the tear as well as any
associated intracarpal pathology and, in the case of TFCC debridement,
earlier postoperative mobilization. Although the available data
indicate that arthroscopic treatment offers comparable or superior
results, further investigation with longer follow-up is warranted.
chronic increase in the load borne by the ulnar side of the wrist. This
problem tends to be progressive once it becomes symptomatic; therefore,
surgical correction is often advised. The etiology of this “ulnar
impaction syndrome” may be primary or secondary (Table 75.7).
 |
|
Table 75.7. Causes of Ulnar Impaction
|
who have ulnar-sided wrist pain and no history of recent trauma. Such
patients may have had a distal radius fracture in the past. On physical
exam there is distal ulnar tenderness. A TFCC compression test is
positive and there may be LT instability. Take PA and lateral plain
film radiographs to assess ulnar variance and DRUJ congruity. Cystic
lesions may be present in the ulnar lunate or distal
ulna.
A grip view shows the presence of dynamic ulnar impaction, and MRI
reveals focal signal intensity changes in the ulnar part of the lunate
and tears of the TFCC.
aspect of the wrist. Treatment options include ulnar shortening
osteotomy, partial ulnar-head resection, ulnar salvage procedures,
including the modified Darrach procedure and hemiresection arthroplasty
of the distal radial ulnar joint (Fig. 75.10).
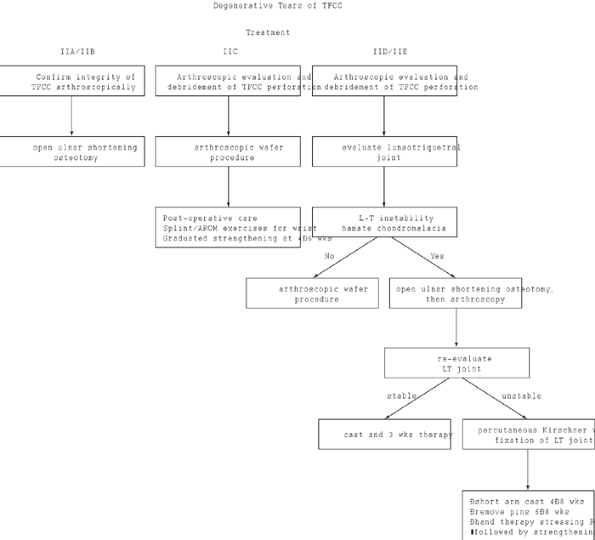 |
|
Figure 75.10. Treatment of degenerative tears of the TFCC.
|
-
Perform triangular fibrocartilage tear
debridement as described previously. Note that the triangular
fibrocartilage is usually fibrillated. The ulnar head has significant
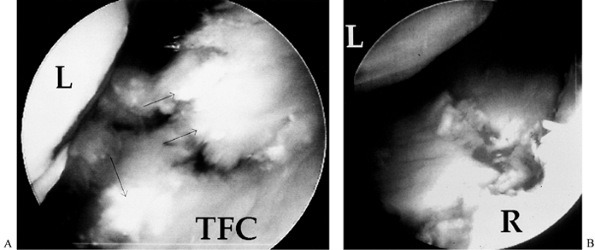
chondromalacia and is prominent (Fig. 75.11).![]() Figure 75.11. A: Degenerative TFC tear. Note the fibrillated appearance of the TFC. B: Degenerative changes of the ulnar head, which is prominent. Arthroscopic wafer resection of the ulna was performed.
Figure 75.11. A: Degenerative TFC tear. Note the fibrillated appearance of the TFC. B: Degenerative changes of the ulnar head, which is prominent. Arthroscopic wafer resection of the ulna was performed. -
Using a 4 mm burr introduced through the
6R portal, excise the distal ulna through the perforation in the TFCC.
Rotate the forearm as you do so to allow even leveling of the joint.
Knowing the size of the burr will help you to assess the depth of your
resection. -
Negative ulnar variance of 2 mm is acceptable.
No studies directly comparing the results of the open versus the
arthroscopic procedure have been published. Although the available data
indicate that arthroscopic treatment offers comparable or superior
results, further investigation with longer follow-up is warranted.
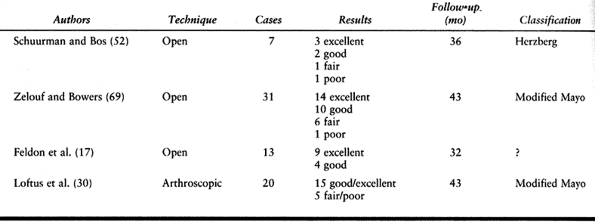 |
|
Table 75.8. Results of the Wafer Procedure
|
structure comprising dorsal, volar, and central components. The fibrous
dorsal and volar components are the major stabilizers, while the
membranous central component adds little to the ligament’s strength (6).
history, physical examination, and radiography and arthroscopy. Some
patients have experienced a fall on the outstretched hand with the
wrist in dorsiflexion, but often there is no history of trauma.
soft-tissue swelling. There is tenderness over the dorsal scapholunate
interval and over the anatomic snuff box and possibly the scaphoid
tuberosity. There is decreased wrist motion and a scaphoid shift test
is positive.
radiographs including neutral rotation PA, lateral, and clenched-fist
views. The key radiographic features of scapholunate dissociation are
an increased scapholunate gap (PA and clenched-fist PA views), palmar
flexion of the scaphoid (lateral view), and cortical ring sign, which
represents an axial projection of flexed scaphoid (PA view).
and characterizing scapholunate injuries. The intrinsic scapholunate
ligament can be easily seen from either the 3-4 or an ulnar radiocarpal
portal. An ulnar portal is generally better as it allows easy access
for a palpating probe through the 3-4 portal and provides excellent
visualization of the volar, central, and dorsal portions of the
ligament. Important information regarding ligament instability can also
be obtained through mid-carpal arthroscopy. The alignment of the
concave surface of
the scapholunate joint is more easily assessed because the overlying interosseous ligaments are absent.
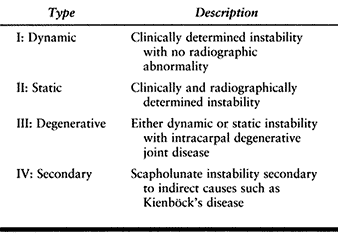
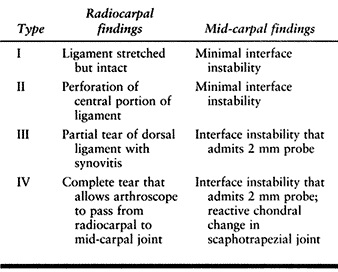
developed a classification system for scapholunate injuries. This
system is based on physical and plain radiographic findings (Table 75.9). Scapholunate ligament injuries can also be classified according to their arthroscopic appearance (Table 75.10).
 |
|
Table 75.9. Watson’s Classification of Scapholunate Instability
|
 |
|
Table 75.10. Arthroscopic Classification of Scapholunate Ligament Injuries
|
instability has been described. The open surgical treatment options for
acute and chronic cases of scapholunate ligament lesions are described
elsewhere (see Chapter 41). As mentioned
previously, arthroscopy has a definite role in diagnosis but its
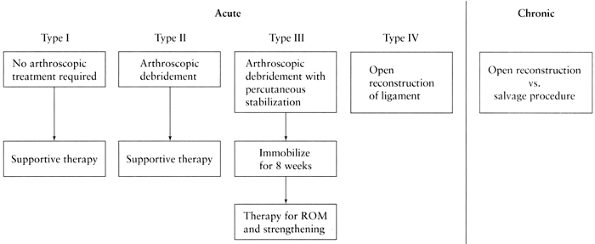
therapeutic role remains unproven. Arthroscopic options include
ligament debridement with or without reduction and percutaneous pinning
(Fig. 75.12).
 |
|
Figure 75.12. Treatment of scapholunate instability.
|
sufficient in cases of perforation of the central portion of the
ligament without evidence of scapholunate interface instability (class
II). In separate studies with short-term follow-up, Ruch and Poehling (49) and Weiss et al. (63) both demonstrated improvement in 85% of their cases.
unstable scapholunate intervals is based on the belief that a stable
ankylosis can result from immobilization and adhesion formation along
pin tracks across the joint. Whipple (66)
reported a series of 40 patients with scapholunate instability treated
in this manner with 2- to 7-year follow-up. He determined that 85% of
patients treated within 3 months of symptom initiation who had less
than a 3 mm difference in the scapholunate gap as compared with the
normal contralateral side maintained stability and comfort. He noted
only a 53% success rate if these selection criteria were not met.
-
Arthroscopically evaluate the radiocarpal and mid-carpal joints.
-
Insert the scope via the 6R portal.
-
Use the 3-4 portal as an instrument portal.
-
Debride the interosseous scapholunate ligament with a suction punch or full-radius resector.
-
Transfer the scope to the ulnar mid-carpal portal.
-
Debride the STT articulation through the radial mid-carpal portal.
-
Insert 0.045-inch K-wires from the dorsal
wrist into the scaphoid and lunate. These will be used as “joysticks”
to manually reduce the scapholunate joint. -
Insert 0.045-inch K-wires into the
proximal scaphoid via the anatomic snuff box (avoid the radial artery,
dorsal radial sensory nerve, and tendons). A 14-gauge angiocatheter can
be used as a soft-tissue protector. -
Reduce the scapholunate interval using
dorsally placed K-wires. Extend the scaphoid and flex the lunate.
Confirm the reduction arthroscopically. -
Drive the radially based K-wires across
the scapholunate interval. Add one more pin for a total of three. The
goal is to create enough fibrosis to provide stability.
C-shaped structure with three components. There are fibrous dorsal and
volar stabilizers and a membranous central portion.
exam, and radiologic evaluation. The patient’s history may include a
fall on the dorsiflexed wrist. The patient has ulnar-sided, mechanical
wrist pain, frequently with clicking. Physical exam reveals point
tenderness over the LT joint. An LT shock test is positive:
dorsal/volar translation of the triquetrum and pisiform while the
lunate is stabilized causes crepitus and pain.
variance on the PA view to help rule out ulnocarpal impaction. Bone
scans will reveal increased uptake over the LT interval.
degenerative tears secondary to ulnocarpal impaction. Treat the latter
with an ulnar carpal decompression procedure. Mild tears can be treated
conservatively with nonsteroidal anti-inflammatory medications, local
corticosteroid injections, and brief periods of
immobilization.
Treatment options for more severe injuries include ligament repair,
ligament reconstruction, and intercarpal arthrodesis. Arthroscopic
treatment, including ligament debridement and reduction with
percutaneous pinning, is also possible. Debridement is indicated in
cases of partial, stable ligament tears (49).
Complete tears with LT instability can be treated with arthroscopic
reduction and percutaneous stabilization if there is no associated
volar intercalated segmental instability or positive ulnar variance (39). Such cases require open procedures.
-
The LT ligament is best viewed through the 6R or the 6U portal.
-
Debride LT tears with a full-radius
resector introduced via the 3-4 portal. Evaluate LT stability via the
radial mid-carpal portal. A stepoff or gap at this articulation, and
proximal hamate chondromalacia indicate significant instability. -
Reduce the LT articulation under arthroscopic visualization from the radial mid-carpal portal.
-
Insert multiple 0.045-inch K-wires across the LT interval.
reported 80% good to excellent results in 20 patients treated in this
manner at 32-month follow-up. They concurred with Pin et al. (45),
noting the importance of differentiating isolated LT tears from cases
of ulnar impaction, as the latter group requires an additional
procedure—ulnar shortening osteotomy or arthroscopic wafer—to
decompress the ulnocarpal joint.
surface in cases of intraarticular distal radius fracture has become
the standard of care since Knirk and Jupiter reported their findings in
1986 (25). They showed that 91% of patients
with articular incongruency of 1–2 mm after reduction developed
radiocarpal arthritis within 6 years. These findings were confirmed in
additional studies (7,34).
Wrist arthroscopy has become a valuable tool in the treatment of a
small percentage of these fractures. Arthroscopy allows visual
assessment of the congruency of the articular surface as well as for
the diagnosis of concomitant ligamentous injury (18).
It can be used in conjunction with various fixation techniques such as
percutaneous pinning, external fixation, and open reduction with
internal fixation. The indications for arthroscopic assistance in the
treatment of distal radius fractures include the following:
-
Intraarticular fractures with more than 2 mm of intraarticular stepoff
-
Any distal radius fracture with a suspected associated carpal ligamentous injury
syndrome, severe soft-tissue injury, absent median nerve function, or
open joint injury.
If done more acutely, visualization might be obscured by bleeding, and
after 7 days, fractures become more difficult to reduce. Palpation of
extensor tendon intervals may be difficult on account of soft-tissue
swelling. Other landmarks, such as the radial border of the long finger
and the mid axis of the ring finger (longitudinal axes of the 3-4 and
4-5 portals, respectively) can usually be identified. Fluoroscopy may
aid in placing needles and establishing portals. Traction is essential
as ligamentotaxis helps with gross fracture reduction. Traction can be
applied through the standard wrist tower, through finger traps and
weight suspended off the end of a hand table, or, in cases where
external fixation is indicated, through the fixator itself.
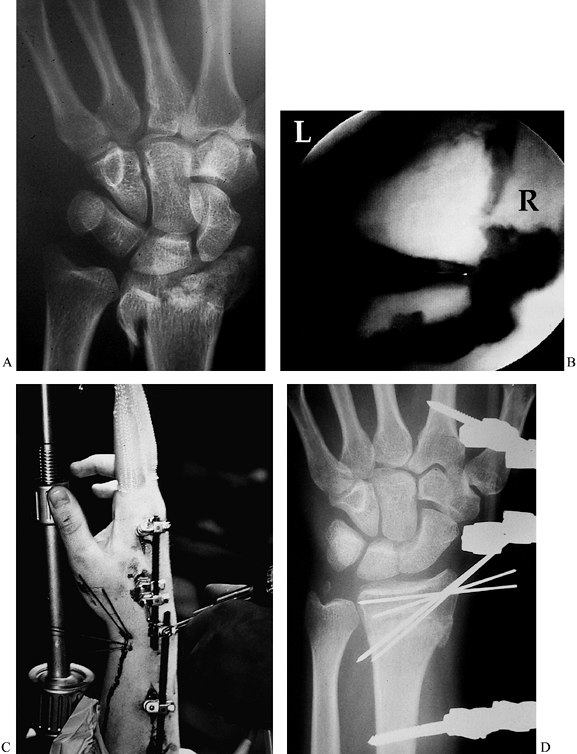 |
|
Figure 75.13. A: Comminuted intraarticular distal radius fracture. B: Arthroscopic view showing disruption of the articular surface. L, lunate; R, radius. C: Arthroscopic reduction with use of both external and internal fixation. D: Postoperative reduction with anatomic alignment of the articular surface.
|
-
Evaluate the radiocarpal joint via the 3-4 portal.
-
Establish an outflow cannula at the 6U
portal. Note: It is critical to maintain outflow to limit fluid
extravasation and prevent compartment syndrome. -
Thoroughly debride the joint of fibrin clot and debris; use a full-radius resector introduced via the 6R portal.
-
Reduce fractures with 0.062-inch K-wires placed percutaneously and used as joysticks.
-
Stabilize the fragments with
percutaneously placed 0.045- or 0.062-inch K-wires. Reduce the radial
styloid fragment first and stabilize it with two 0.045-inch K-wires or
a cannulated screw. -
If present, next reduce the lunate
“die-punch” fragment to the radial styloid by elevating the impacted
fragment with one or more K-wires. Stabilize the die-punch fragment
with two transverse 0.045-inch K-wires running from the radial styloid.
Note: Melone four-part fractures usually require
open reduction of the volar die-punch fragment. Perform this procedure
through a limited, volar incision placed between the finger flexors and
the ulnar neurovascular bundle. Elevate the fragment and stabilize it
with a small buttress plate. -
Bone-graft metaphyseal defects through limited incisions once the fragment fixation is completed.
-
Evaluate the wrist for associated soft-tissue injuries.
radius fractures treated by one of us (ALO), a significant number had
associated soft-tissue injuries of the wrist, including scapholunate
and LT ligament tears as well as injuries to the TFCC (15).
of patients with intraarticular distal radius fractures have described
excellent results with the use of arthroscopically assisted reduction.
At an average 27-month follow-up, Wolfe reported seven of seven
patients returning to work with range of motion and maximal grip
strengths averaging 92% and 98% of the uninjured
side, respectively. Over the same average follow-up period, Culp and Osterman (15) reported 22 good or excellent results out of 27 cases.
These fractures tend to occur in young, male laborers. Thumb spica-cast
immobilization is the standard treatment for the vast majority of
scaphoid fractures. Because the scaphoid has a high rate of delayed
union and nonunion, prolonged immobilization (a minimum of 3 months) is
required. Open reduction and internal fixation are indicated for
displaced fractures, as well as in cases of nondisplaced fractures in
patients for whom cast immobilization presents an undue hardship.
Arthroscopic reduction and internal fixation also has a role in the
treatment of patients with scaphoid fractures.
-
Scaphoid displacement is best assessed via the mid-carpal radial (MCR) portal.
-
Evacuate hematoma and debris with a full-radius resector via the mid-carpal ulnar (MCU) portal.
-
If necessary, reduce the fracture through
either direct manipulation of the scaphoid or the use of percutaneously
placed joysticks. -
Stabilize the fracture with an 0.045-inch K-wire.
-
Make a 2 cm incision radial to the flexor
carpi radialis and centered over the scaphotrapezial joint. Continue
dissection bluntly to the volar joint capsule. Elevate the capsule and
excise the volar tubercle of trapezium. -
Place the arthroscope in the 4-5 portal
and introduce the target hook of a Whipple compression jig through the
1-2 portal. Seat the hook on the dorsal aspect of the scaphoid 1–2 mm
radial to the scapholunate ligament. Slide the guide barrel down to
exposed articular surface of scaphoid and compress the jig. Use the
guide pin to confirm correct placement. -
Place an accessory pin to control rotation.
-
Place an appropriate-length Herbert–Whipple screw.
-
Immobilize the extremity in a thumb spica splint for 10 days.
-
Initiate protected wrist mobilization at 2 weeks.
series of 20 cases of scaphoid fracture treated with arthroscopically
assisted reduction and internal fixation. All fractures healed by the
12-month follow-up. The period of cast immobilization ranged from 3–4
weeks.
traumatic disorders, rheumatoid arthritis, pigmented villonodular
synovitis, infections, and crystal deposition disorders such as gout
and pseudogout. The differential diagnosis can often be narrowed by
determining the location of hypertrophied synovium as well as the
extent of its inflammation. The most common reason to perform
arthroscopic synovectomy is debridement of tissue that is obscuring
visualization. For example, symptomatic ligament tears are often
associated with a localized synovitis; to diagnose and treat the
injury, the synovial tissue must be removed. Removal of tissue is best
accomplished with either a full-radius resector or a more aggressive
side-cutting blade. Synovectomy is most efficiently performed with
shaver speeds less than 600 rpm and oscillation of the cutting blade (64).
rheumatoid or septic arthritis. In patients with rheumatoid arthritis,
whose symptomatic wrists are resistant to medical management but who
have preserved joint spaces, open or arthroscopic synovectomy may
provide symptomatic relief. The arthroscopic technique offers superior
visualization and easier access to the synovium of all the wrist
compartments than does open synovectomy. Usually, a complete
synovectomy can be performed by alternating the arthroscope and
full-radius resector between the 3-4 and 6R portals. Occasionally, the
1-2 or 6U portals may be required. Arthroscopy of the mid-carpal joint
and the DRUJ is also performed. To examine the DRUJ, distract the wrist
while it is in supination. Establish the viewing portal proximally and
place the working portal just proximal to the TFCC.
associated dorsal tenosynovitis. This tenosynovium can be removed only
through an open procedure. Additionally, extensor tendons weakened and
displaced by the disease process are at increased risk for injury
during establishment of arthroscopic portals.
described the treatment by arthroscopic carpal synovectomy of a series
of 18 patients with rheumatoid arthritis. All patients noted a decrease
in their pain and significant improvements in range of motion. Overall,
morbidity and complications for arthroscopic synovectomy, particularly
in terms of postoperative wrist stiffness and length of rehabilitation
time, have been shown to be less severe than those incurred by open
synovectomy (54).
decompression. Arthroscopic management of sepsis has been shown to be
effective in larger joints such as the knee and shoulder. Although no
studies have addressed the role of arthroscopy in the treatment of
wrist sepsis, its use is a logical consideration. In a series by one of
us (ALO), six patients with septic arthritis were treated with
arthroscopic drainage and appropriate antimicrobial therapy, and all
had a successful outcome. Until the efficacy of this approach is fully
established, it should be employed with caution.
associated with either joints or tendon sheaths. They represent the
majority of all soft-tissue tumors of the hand and wrist. The dorsal
wrist ganglion is the most common of these cysts. Patients generally
request treatment for their ganglia for cosmesis or because of
discomfort or functional disturbances. Current treatment options
include no treatment, aspiration with or without cortisone injection,
and surgical excision. No treatment is not an unreasonable course of
action in light of the spontaneous resolution rates of 28% to 58% that
have been reported (38). Aspiration, with or
without cortisone injection, has a reported success rate of 35% to 50%,
whereas recurrence rates after surgical excision have been as high as
40%.
the ganglion stalk and a small portion of the dorsal wrist capsule.
Since the majority of dorsal wrist ganglia arise from the scapholunate
interval, arthroscopic identification and excision of the stalk are
possible in many cases. The advantages of arthroscopic excision over
open excision are believed to be decreased wrist stiffness and an
earlier return to function. Additionally, the arthroscope allows the
identification of any additional carpal pathology that might be present.
-
Circle the ganglion with a marker (Fig. 75.14A).
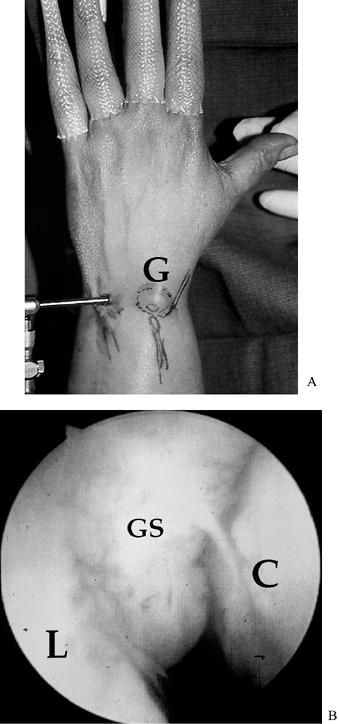
![]() Figure 75.14. A: Dorsal ganglion usually arises from the area of the 3-4 portal. Viewing approach should be from the 6R portal. G, ganglion. B: Arthroscopic view of ganglion stalk prior to resection with full radius shaver. GS, ganglion stalk; L, lunate; C, radius.
Figure 75.14. A: Dorsal ganglion usually arises from the area of the 3-4 portal. Viewing approach should be from the 6R portal. G, ganglion. B: Arthroscopic view of ganglion stalk prior to resection with full radius shaver. GS, ganglion stalk; L, lunate; C, radius. -
Approach the radiocarpal joint via the 6R portal to give best access to the stalk.
-
Look for the stalk of ganglion arising from the dorsum of the scapholunate interval (Fig. 75.14B).
-
If the stalk is not visualized (and it is
not visualized one third of the time) resect 1 cm of dorsal capsule at
the dorsal scapholunate angle with a suction punch or motorized shaver. -
If the stalk can be visualized, debride
it with a suction punch or motorized shaver via a portal placed through
the ganglion (usually the 3-4 portal). -
Resect 1 cm of the dorsal capsule with a suction punch or motorized shaver.
-
Complete your arthroscopic evaluation of the wrist (include the mid-carpal joint).
-
Ensure that the extraarticular sac has ruptured.
-
Immobilize the wrist in a volar splint for 1 week.
-
Use a Futuro splint as needed for comfort for up to 3 weeks with early active ROM exercises for the wrist.
-
Avoid strenuous use of the hand until 6 weeks after surgery.
The ganglion stalk was visualized in 61% of cases. At an average
follow-up of 16 months, there were no recurrences. Five patients
developed increased grip strength and range
of
motion postoperatively, whereas two experienced losses in both
categories. The remainder were unchanged. Additional intraarticular
pathology was identified in half the cases. The average return to work
time was 3.5 weeks.
There are a variety of causes of cartilage damage. Most often these
lesions occur secondarily because of an existing wrist problem such as
ligamentous instability, fracture, or inflammatory arthritis. Chondral
defects can occur primarily as well. For example, the presence of a
medial facet on the lunate is associated with an increased incidence of
chondromalacia at the proximal pole of the hamate.
majority of them are not identifiable radiographically. In cases where
the wrist pain is refractory to conservative treatment, arthroscopy can
have a diagnostic and therapeutic role. Chondral lesions can be
categorized with the system devised by Outerbridge (Table 75.11) (40).
 |
|
Table 75.11. Outerbridge Classification
|
continues to be debated. The majority of the data published on this
topic comes from studies performed on arthritic knees. While several
authors have reported a significant decrease in pain after arthroscopic
lavage and/or debridement, these studies have been limited by follow-up
periods of a year or less (4,16,28). The work of Mosely et al. (35) suggests that the benefit of arthroscopic treatment in these patients is no more efficacious than a placebo.
treatment of Outerbridge lesions grades I–III involves debridement of
the accompanying synovitis and fibrillation. Chondral lesions are
smoothed to reduce mechanical symptoms and to minimize the production
of intraarticular debris. Thick chondral flaps can be debrided with a
suction punch, whereas thin flaps are best debrided with a motorized
shaver.
controversial. While no treatment has been shown to cause repair or
regeneration of hyaline cartilage, chondral abrasion appears to
stimulate fibrocartilage repair. This fibrocartilage patch probably
cannot hold up under physiologic loads over time but it may bind to
adjacent hyaline cartilage protecting against extension of the injury.
degenerative arthritis. These bodies may produce mechanical symptoms
such as pain, locking, and catching. Whereas large loose bodies can
often be visualized on plain radiographs, small ones often cannot.
Arthroscopy provides a minimally invasive means of removing loose
bodies. When performing this surgery, it is best to obtain radiographs
on the day of the procedure to help confirm the location of radiopaque
bodies. Use the portal closest to the loose body for the arthroscope. A
separate inflow portal helps to force the body toward the arthroscope.
A percutaneously placed hypodermic needle can act as a skewer if
necessary. Small loose bodies can be retrieved with a basket forceps.
Larger ones may require morcellization with a motorized shaver.
may provide symptomatic relief to patients with early radiocarpal
arthritis. It is particularly useful in the treatment of patients in
the first stage of scapholunate or scaphoid nonunion advanced collapse.
It is often only a temporizing measure (31). The procedure can be performed either arthroscopically or in open fashion.
-
Perform a standard arthroscopic evaluation, synovectomy, and debridement as necessary.
-
Insert the arthroscope via the 3-4 portal and identify the radioscaphocapitate and long radiolunate ligaments. Note: These ligaments must be preserved. Introduce a 3 or 4 mm burr through the 1-2 portal and excise the styloid under arthroscopic visualization.
-
Fluoroscopically confirm the amount of styloid removed.
-
Arthroscopically confirm the decompression of radial impingement by radially deviating the wrist.
-
Splint the wrist intermittently for 3 weeks.
-
Initiate active ROM exercises at 1 week and begin a strengthening program at 4 weeks.
preserving motion in patients with later stages of scapholunate or
scaphoid nonunion advanced collapse (57,68).
Its success is based on the preservation of the articular surfaces of
the proximal capitate and the lunate fossa of the radius. Arthroscopy
can allow for evaluation of these surfaces as well as the performance
of the procedure itself.
-
Perform a standard arthroscopic evaluation, synovectomy, and debridement as necessary.
-
Confirm that the proximal pole of the capitate and lunate fossa have acceptable articular surfaces.
-
Establish a 4-5 viewing portal.
-
Introduce a 4 mm burr via the 3-4 portal.
-
Remove the proximal scaphoid.
-
Initiate excision of the lunate from its radial side. Note: Take care to avoid injuring the lunate fossa and proximal capitate.
-
Protect the proximal capitate with a Freer elevator introduced via a mid-carpal portal.
-
Small osteotomes help to morcelize the bones.
-
Remove large fragments with pituitary rongeurs.
-
Confirm decompression of radial
impingement arthroscopically by radially deviating the wrist. Perform
radial styloidectomy as necessary. -
Immobilize the wrist for 4 weeks, then
splint intermittently while initiating ROM exercises. Introduce
strengthening exercises at 8 weeks.
identified 17 complications in 214 cases of diagnostic wrist
arthroscopy. Reflex sympathetic dystrophy was most common (3.7%),
followed by dorsal radial sensory nerve neuropraxia (2.3%) and tendon
problems (0.9%). All complications resolved with nonoperative treatment.
than 3%. The majority of these complications were related more to
percutaneous pin fixation than to the arthroscopy. The morbidity was
directly related to the technical difficulty of the procedure.
complications into four categories: (a) complications related to
traction, (b) complications related to establishment of portals and
insertion of instruments, (c) procedure-specific complications, and (d)
miscellaneous complications. A list of these complications and the
means of avoiding them appears in Table 75.12.
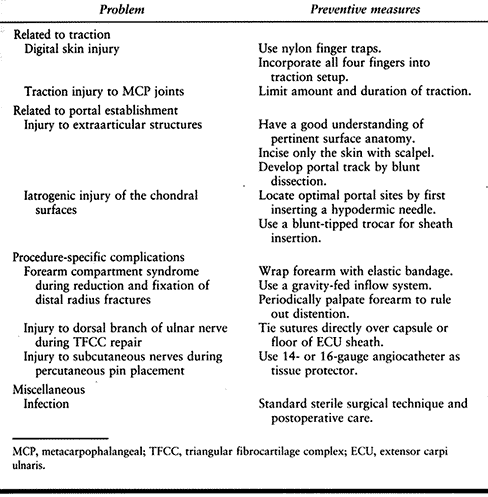 |
|
Table 75.12. Complications of Wrist Arthroscopy
|
arthroscopy is a relatively safe procedure that offers excellent
visualization of the wrist with minimal soft-tissue dissection. Its
diagnostic value is unquestionable. Its therapeutic usefulness
continues to expand.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
C, Richards RS, Roth JA, Patterson SD. Complications of Diagnostic
Wrist Arthroscopy. Presented at 51st annual meeting of American Society
for Surgery of the Hand, Nashville, TN, 1996.
JKL, Amadio PL, Cooney WP. Open Reduction and Internal Fixation of
Displaced, Comminuted Intra-articular Fractures of the Distal End of
the Radius. J Bone Joint Surg Am 1989:71:839.
RM, Stern PJ, Wyrick JD, Michaels SE. The Relevance of Ligament Tears
and Perforations in the Diagnosis of Wrist Pain: An Arthrographic
Study. J Hand Surg [Am] 1994;19:945.
SJ, Savoie FH, Geissler WB, et al. Arthroscopic Repair of Peripheral
Avulsions of the Triangular Fibrocartilage Complex of the Wrist: A
Multi-center Trial. Arthroscopy 1997;13:78.
P, Terrono AL, Belsby MR. Wafer Distal Ulna Resection for Triangular
Fibrocartilage Tears and/or Ulnar Impaction Syndrome. J Hand Surg [Am] 1992;17:731.
WB, Freeland AE, Savoie FH, et al. Intraarticular Soft Tissue Lesions
Associated with an Intraarticular Fracture of the Distal End of the
Radius. J Bone Joint Surg Am 1996;78:357.
CR, Kursunoglu-Brahme S, Schwaighofer B, et al. Is MR Imaging Better
than Arthrography for Evaluating the Ligaments of the Wrist? In Vitro
Study. AJR Am J Roentgenol 1990;154:337.
JE, Boland DS. Tears of the Articular Disc of the Triangular
Fibrocartilage Complex: Results of Excision of the Articular Disc. J Hand Surg 1983;8:620.
DJ, Thorogood S, Smith WH, Scott TD. A Comparison of Magnetic Resonance
Imaging and Arthroscopy in the Investigation of Chronic Wrist Pain. J Hand Surg [Br] 1997;22:714.
JB, Palmer AK. Disorders of the Distal Radioulnar Joint and Triangular
Fibrocartilage Complex: An Overview. In: Lichtman DM, Alexander AH,
eds. The Wrist and its Disorders. Philadelphia: Saunders, 1997:393.
JB, Palmer AK, Short WH. Arthroscopic Wafer for Ulnar Impaction
Syndrome. Presented at 52nd annual meeting of the American Society for
Surgery of the Hand, Denver, CO, 1997.
A, Ishikawa J, Svenago N, Kasashima T. Clinical Results of Treatment of
Triangular Fibrocartilage Complex Tears by Arthroscopic Debridement. J Hand Surg [Am] 1996;21:406.
JB, Wray NP, Kuykendall D, et al. Arthroscopic Treatment of
Osteoarthritis of the Knee: A Prospective, Randomized,
Placebo-Controlled Trial. Am J Sports Med 1996;24:28.
AL, Bednar JM, Gambino K, Mahony K. The Natural History of Untreated
Symptomatic Tears in the Triangular Fibrocartilage. Presented at the
51st annual meeting of the American Society for Surgery of the Hand,
Nashville, TN, 1996.
HG, Asnis-Ernberg L, Weiland AJ, et al. The Utility of High-Resolution
Magnetic Resonance Imaging in the Evaluation of the Triangular
Fibrocartilage Complex of the Wrist. J Bone Joint Surg Am 1997;79:1675.
RJ, Kubitzek C, Hierner R, et al. The Scapholunate Interosseous
Ligament in MR Arthrography of the Wrist: Correlation with Non-enhanced
MRI and Wrist Arthroscopy. Skeletal Radiol 1997;26:263.
RG, Ferlic DC, Clayton ML, McClure DC. Arterial Anatomy of the
Triangular Fibrocartilage of the Wrist and its Surgical Significance. J Hand Surg [Am] 1986;11:258.
SM, Miller RJ, McCance SE, Meyers SP. Lesions of the Triangular
Fibrocartilage Complex: MR Findings with a Three Dimensional
Gradient-Recalled-Echo Sequence. Radiology 1996;199:227.
APC, Akelman E, Lambiase R. Comparison of the Findings of Triple
Injection Cine-arthrography of the Wrist with Those of Arthroscopy. J Bone Joint Surg Am 1996;78:348.
JD, Stern PJ, Kiethaber TR. Motion-preserving Procedures in the
Treatment of Scapholunate Advanced Collapse Wrist: Proximal Row
Carpectomy Versus Four-Corner Arthrodesis. J Hand Surg [Am] 1995;20:965.
DS, Bowers WH. The Feldon Wafer Procedure—A Review of 35 Cases.
Presented at the 52nd Annual Meeting of the American Society for
Surgery of the Hand, Denver, CO, 1997.