TUMORS OF THE HAND
extremity will always be challenging; it is sometimes complex and may
also be frustrating. Considerable skill and careful thought are needed
to analyze diagnostic, treatment, and reconstructive problems, as well
as to treat the patient to ensure survival and function while treating
the tumor. The first issue is that an enlarging mass that represents an
invasive or life-threatening tumor in the upper limb is unusual;
circumspection and attention to detail should govern the approach to
all tumors.
(distal to the elbow, soft-tissue neoplasms predominate when serious
diagnoses are considered); the majority are reactive nodules and cysts,
connective tissue proliferations, foreign bodies, infections (that
mimic neoplasms), and posttraumatic sequelae. Table 74.1 and Table 74.2
list tumors of the soft-tissues and hard tissues, respectively. Lesions
that are small and appear innocent can be deadly, whereas large and
seemingly worrisome masses may be easy to treat (Fig. 74.1).
The surgeon who evaluates and treats tumors of the hand needs to be
familiar with limb anatomy, tumor biology, and the range of treatment
possibilities and their limitations. Ideally, the hand surgeon or
orthopedist is part of a team consisting of medical and surgical
oncologists, reconstructive surgeons, and therapists, who can offer
state of the art experience in integrating care of the tumor, balancing
patient survival against the desire to retain function. Lesions in the
hand more often present earlier in their course than those at other
sites, just because they are—by definition—more likely to be
superficial and easily noticed. However, hand tumors that require a
clean surgical margin will have a significant and often profound
functional impact after treatment.
 |
|
Table 74.1. Tumors of Soft Tissue
|
 |
|
Table 74.2. Tumors of Hard Tissue
|
 |
|
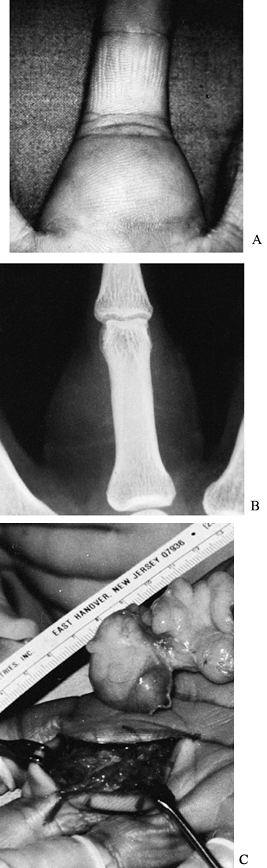
Figure 74.1. A: A 43-year-old man with an enlarging phalangeal mass of considerable size. B: Radiographs reveal the soft-tissue tumor as having a fluid density suggestive of a lipoma. C: The diagnosis was confirmed at surgery and the large lipoma removed.
|
Surgeons not practicing in a musculoskeletal or cancer treatment center
are not likely to see enough cases of aggressive and invasive but
oncologically benign
tumors or primary malignancies to be fully comfortable or entirely competent in their evaluation and management (58,102,131). Principles and definitions of tumor treatment are not different for the upper limb, although anatomic issues are.
-
Hyperplasia: An accumulation of cells resulting from accelerated proliferation.
-
Hamartoma: Congenital anomaly in which a tissue island is effectively excluded from regional maturation
P.2011
and
cellular differentiation. This lesion has a structure similar to its
tissue of origin; it is often multicentric and/or hemicorporal in
locations. Hyperplasias and hamartomas stop growing when skeletal
maturity is achieved. -
Neoplasia: A new growth of cells that enlarges in an atypical, autonomous, progressive course.
-
Latent (stage I) lesions are entirely
quiescent. Latent tumors in bone and soft tissue may require only
observation to ensure they have been correctly categorized. -
Actively growing lesions remaining in a
limited anatomic zone are considered a separate category (stage II).
Actively growing tumors may be controlled by local intralesional or
marginal excision. -
Aggressive (stage III) lesions are
characterized by regional invasion and spread (in a sense, they meet
the generic minimum standards for Campanacci’s neoplasia). Aggressive
tumors require surgical margins wide enough to effect en bloc
excision encompassing the entire lesion, and generally with a
contiguous margin (“cuff”) of normal surrounding tissue to minimize
recurrence.
Nonetheless, there are as yet no standards of appropriate or safe
surgery based on reproducible clinical data, because of the limited
number and varying histologic types of cases historically reported
using several methods and classifications (15,38,102,128,129,147).
Therefore, what exactly constitutes a safe, clean surgical margin, and
how much function need be sacrificed to ensure a patient’s survival,
are not irrefutably established (17,50,67).
Use and reporting of larger numbers of cases with the same staging
system will make treatment comparisons and definitions easier and more
accurate. However, the anatomic implications of
compartmentalization—based on the presence of a tissue barrier that
inhibits easy extension of tumor cells—has a more limited application
in the hand. That is to say, it is easy to understand a medial or
lateral compartment in the thigh, and a volar or dorsal compartment in
the forearm. However, the flexor sheath in the thumb extends from its
interphalangeal joint to the wrist, and it communicates with the entire
flexor compartment of the elbow. Yet, is the flexor surface of the
thumb truly different from the flexor surface of the index where the
synovial sheath is not continuous? The critical issue is that a
surgical margin is not compromised when it endangers the primary goal
of treatment—that is, permanently ridding the patient of the tumor.
Musculoskeletal tumor surgery requires local control; but surgery now
needs to be seen as part of a treatment paradigm that includes medical
and radiation therapies, delivered to the patient or the tumor region
before, during, and after the operation in appropriate cases (17,18,38,76,98,102,108,145,156).
 |
|
Table 74.3. Surgical Stages
|
careful and thorough history, physical examination, and addition of
laboratory and imaging studies to determine the local and systemic
status and stage of the tumor. These principles are discussed in more
detail in Chapter 126. We emphasize that plain
radiographs, technetium and/or gallium scintigraphy, computerized
tomography (CT; extremity and chest), and magnetic resonance imaging
(MRI) of the limb are required antecedents to surgical biopsy (34,41,62,79,103,140).
CT scans and MRI can be particularly helpful in delineating whether a
specific lesion is contained within a bone or a soft-tissue
compartment, or if it has already extended across a natural barrier (Fig. 74.2). Imaging data are essential to planning the biopsy incision location and the extent of subsequent treatments.
 |
|
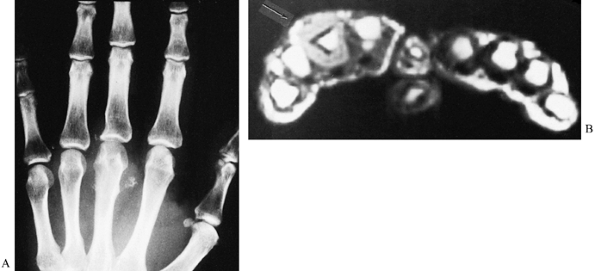
Figure 74.2. A:
Radiograph showing a soft-tissue mass with calcifications, plus erosion of the third metacarpal and proximal phalanx in a 27-year-old man. The findings suggest an aggressive tumor, quite possibly a (malignant) synovial sarcoma. B: MRI demonstrates absence of marrow involvement by this well-defined circumferential soft-tissue tumor (arrow). These findings clearly support the diagnosis of benign, noninvasive neoplasm, but one that would require dorsal and palmar incisions for complete excision. Synovial chondromatosis was confirmed at surgery. |
interpretation have improved notably in the past decade.
High-resolution CT with reconstructed images of various views, and MRI
sequences, sometimes enhanced with gadolinium, have improved
identification of anatomic structures, have refined preoperative
differential diagnosis, and have redefined and clarified tumor margins.
tissue sample. The biopsy of a potentially malignant lesion should be
planned as carefully as that of the definitive surgical resection. The
biopsy track will later have to be incorporated into the planned
resection to allow for its removal with the tumor. Open incisional
biopsy with intraoperative frozen section reviewed by an experienced
musculoskeletal pathologist to confirm the acquisition of an adequate
tissue sample remains the standard. Definitive diagnosis may require
permanent sections and special techniques. Improvements in
cytopathologic tissue processing have advanced the utilization of
fine-needle aspiration and core needle biopsies in the diagnosis
management of sarcoma. Cytopathology requires considerably greater
attention to detail by both the surgeon and the pathologist to ensure
that a complete and accurate diagnosis is made. Reliable
immunohistochemical and cytogenic evaluations could potentially be
obtained by needle aspirates, but presently fine-needle biopsy is
confined to academic musculoskeletal and cancer centers where a large
experience can be accumulated.
critical to the outcome of treatment. If a surgeon or the team
available at the institution is unprepared, ill equipped, or
inexperienced in completing all diagnostic studies and proceeding with
definitive, comprehensive management, the patient should be referred
before the biopsy is performed (86). The
patient and surgeon must be prepared for the unexpected. “Benign
growths” that otherwise appear “different” need to be biopsied; avoid
an invasive attempt at excision of an unknown lesion (Fig. 74.3) that may contaminate surgical margins with tumor cells.
 |
|
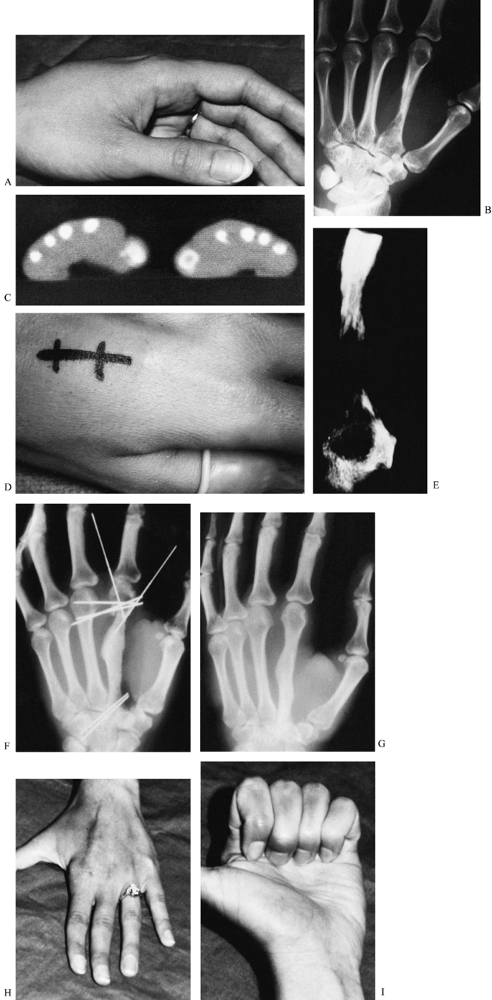
Figure 74.3. A:
Clinical appearance of the hand in a 24-year-old woman who reported a 10-week history of an enlarging, painful mass on the dorsum of the hand. B: Radiographs revealed endosteal invasion of the second metacarpal diaphysis and metaphysis without new bone formation, suggestive of a rapidly growing lesion. C: The CT scan revealed actual cortical destruction along the ulnar side of the metacarpal shaft. D: A small dorsal longitudinal incision (located so as to be entirely excisable) was used for the incisional biopsy under tourniquet control, without exsanguination. E: Radiograph of specimen. Permanent sections revealed an aneurysmal bone cyst, which was treated at a separate operation by en bloc second-metacarpal resection and iliac graft replacement. F: To maintain functional skeletal continuity, the second metacarpal head was transfixed to the third before bone resection. The Kirschner wires were left in place until union was solid. G: Therapy was started 2 weeks after surgery and motion recovered rapidly. H,I: Follow-up clinical photographs. |
bloodless field. Tourniquet control is safe for performing the biopsy
and when treating tumors of the hand. Do not exsanguinate the extremity
because this may cause dissemination
of
seed tumor cells—or bacteria—from the affected site. Elevate the arm
for 2–4 minutes before inflating the pneumatic tourniquet. Do not use
intravenous Bier block anesthesia (which requires exsanguination) when
performing a biopsy or when removing an undiagnosed or known serious
hand tumor. Very small tumors up to 1.0 cm in diameter can be
effectively biopsied and excised simultaneously if limited to one
tissue plane or compartment. Skin defects can be covered with synthetic
semiocclusive materials until biopsy results are known. Hemostasis is
required for deep biopsy wounds; therefore, release the tourniquet
before wound closure to ensure a dry field. Do not use drains. Biopsy
of bone tumors is best done by making a cortical window and harvesting
a limited sample. Postoperative care and splinting follow routine
principles.
utilizing intralesional or marginal surgical resection, often described
as shelling out or bony curettage. Tumors with special characteristics
and those that ought to have additional consideration because of their
anatomic location and biologic potential will be discussed in later
sections.
Hand anatomy and function make the treatment of these lesions special
compared to other musculoskeletal sites. Treatment of the hand tumor
may limit function and impair body image more severely than radical
surgery performed elsewhere in the body. The application of historic
“tumor margin minimums” to achieve a pure surgical cure may create a
potentially unnecessary and debilitating functional deficit,
particularly in larger tumors. Neoadjuvant therapy as discussed in Chapter 126
is important in treating aggressive tumors, as tumor shrinkage allows
more limited operative excision with clean margins; this permits
preservation of hand function while not risking survival of the patient.
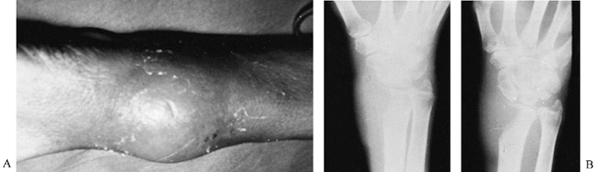
primary malignancies in the lung or kidney, the breast, or the
gastrointestinal and hematopoietic systems (Fig. 74.4).
These malignancies may require only limited-intervention (e.g.,
palliative) surgery in conjunction with systemic therapies for an
underlying but previously occult primary tumor.
 |
|
Figure 74.4. Clinical (A) and radiographic (B)
appearance of a painful, enlarging mass and osteolysis of the radius in a 70-year-old man. Incisional biopsy disclosed bronchogenic carcinoma; the arm was irradiated. |
distinguished from those that are only locally invasive and regionally
destructive. Nonmalignant tumors can be safely and adequately handled
by en bloc excision as long as the tumor
is contained within the specimen. Aggressive but nonlethal tumors do
not justify excision of important nerves and vessels, with rare
exceptions. Improvements in imaging techniques and nonsurgical care
allow the musculoskeletal oncologist to cut closer than the traditional
3–5 cm margin for bone and 1–2 cm margin for soft tissue without an
increased risk of local recurrence. Close, but safe, negative margins
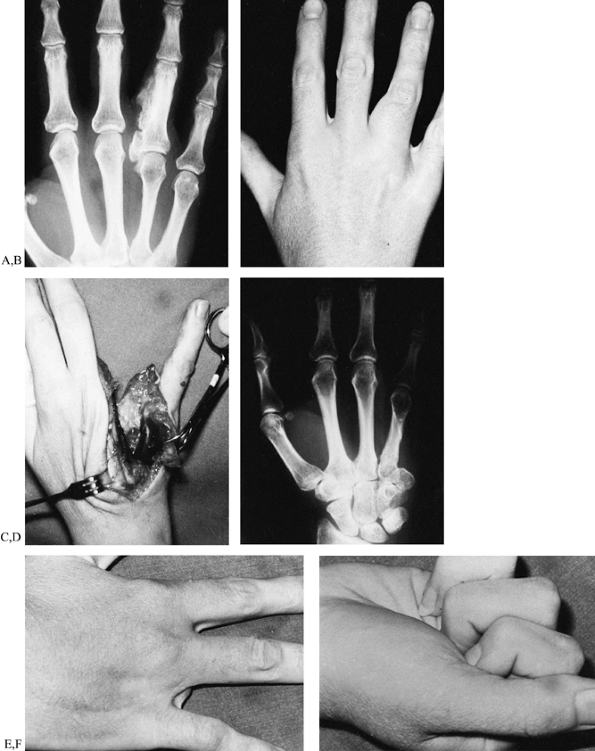
decrease the loss of function in the extremity (Fig. 74.5).
This approach requires intensive and extensive multidisciplinary
preoperative planning from the musculoskeletal radiologist, radiation
and medical oncologists, the oncologic surgeon, and the reconstructive
upper-extremity surgeon. At the time of excision, carefully examine the
margins of the specimen and sample histologically together with the
pathologist. If the tumor
has
not been cleanly resected, the margins have to be expanded; additional
regional adjuvants and systemic treatments may need to be added. If a
safe and adequate tumor resection will destroy hand function,
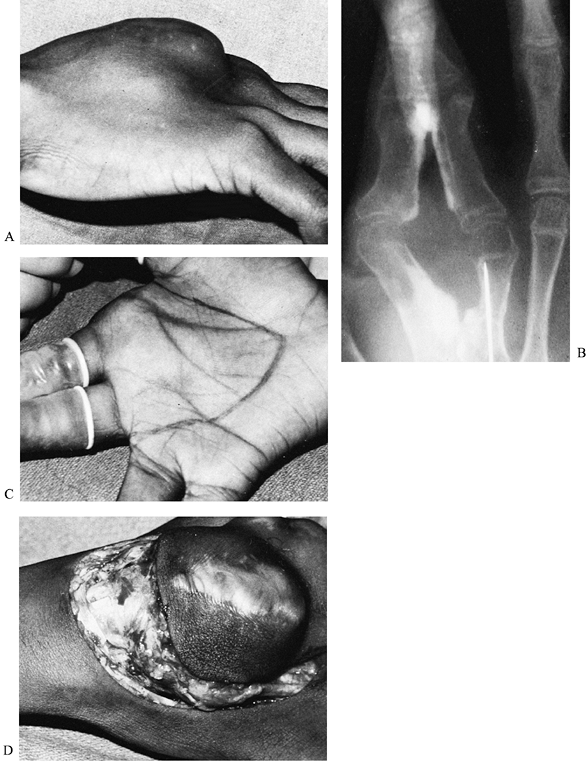
amputation is advised (Fig. 74.6).
 |
|
Figure 74.5. Radiographic (A) and clinical (B)
appearance of a slowly enlarging bony mass of the ring finger, which proved to be a parosteal osteogenic sarcoma. The patient was successfully treated by resection of the fourth ray and transposition of the fifth ray (C,D). She continues to be free of disease more than 7 years after surgery (E,F). |
 |
|
Figure 74.6. A,B:
After several local resections for a desmoplastic fibroma (extra-abdominal desmoid), a 14-year-old presented with an enlarging, painful, and radiographically destructive mass at the same site. The surgical plan was subtotal hand amputation with resection of the second and third rays. C,D: At surgery, the tumor was found to extend into the dorsal wrist; a below-elbow amputation was performed. |
local flaps, and skin grafts. Do not use distant pedicle flaps for
primary closure because of the risk of residual tumor cells in the
resection area contaminating an otherwise clean donor area. Finger
fillet flaps and local rotation flaps
from
portions of retained fingers, for example, are ideal for coverage in
such circumstances. Complex reconstructive procedures that include
vascularized soft tissue, bone, or composite flaps require integrated
preoperative planning; they require a team of specialists in surgical
tumor excision and hand reconstruction working simultaneously. Even
seemingly traditional reconstructive procedures, such as pollicization,
can be done primarily at the time of tumor resection if planning has
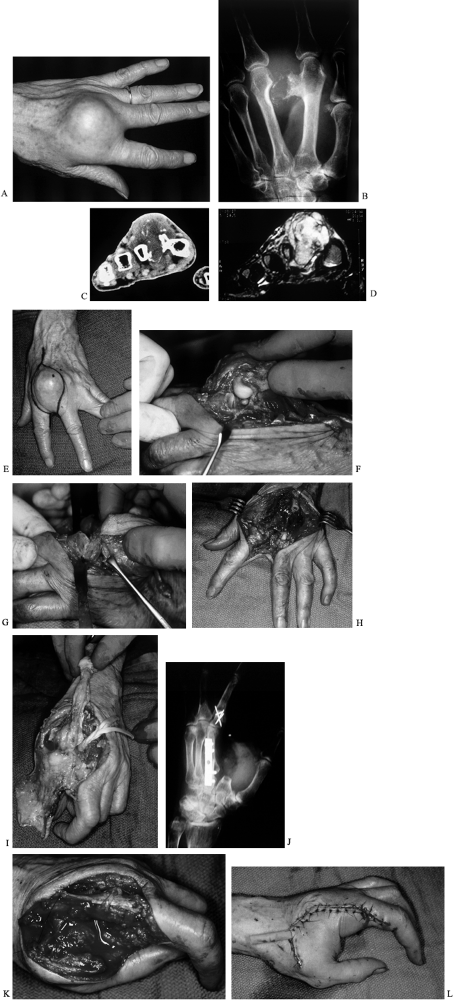
been adequate and the local conditions are appropriate (Fig. 74.7).
 |
|
Figure 74.7. This 68-year-old woman offers an example of these diagnostic and surgical principles. A,B:
Radiographs and clinical appearance of a hard, painless lesion present for at least 10 years but which expanded rapidly in the previous 8 months. C,D: CT and T2-weighted MRI images delineate the location and extent of this chondral and calcific tumor; it is seen to involve the second metacarpal and extensor tendons, but may not invade the middle finger MCP joint. Chest x-rays were clear; longitudinal biopsy of the dorsal surface proved chondrosarcoma—a “low-grade” malignancy. E. En bloc resection of the second metacarpal also including skin and extensor tendons of the index and middle fingers in the “reactive zone.” At surgery, tumor was found in the middle finger MCP joint (F), just volar to the metacarpal head. Resection included excision of the MCP joint en bloc with the surrounding tissues (G). H: Following en bloc marginal to wide excision, but preserving flexor tendons and neurovascular bundles (protected by the adductor pollicis muscle, as noted on preoperative MRI). I: Radial neurovascular bundle of the index finger was retained in the filet flap of skin, and its ulnar neurovascular bundle preserved to the proximal and middle phalanges of the index finger. J–K: Reconstruction of the middle finger was achieved by transposing the index proximal phalanx, PIP joint, and base of middle phalanx to form a (vascularized) “third metacarpal/middle finger MCP joint.” The index flexors were used as a jump graft to reconstruct the middle finger extensors (including pulley construction to keep the mechanism dorsal to the new bone); closure was achieved by transposing the filleted digital flap. (Case courtesy of CA Peimer and BR McGrath.) [From Peimer CA, ed. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill, 1996:226 (Fig. 99-4).] |
tissue and bone in the hand and forearm is surgery; however, the
judicious use of pre- and postoperative radiation and chemotherapy will
improve both local control and patient survival. Radiation therapy is
probably the most widely used adjuvant. It can improve local control of
malignant lesions when surgical margins are up to 1.0 cm in thickness.
Careful planning after diagnosis and before treatment by experienced
radiation and medical oncologists is required. Palliative external beam
radiation can be used for patients with painful lesions in the hand and
upper extremity. Preoperative radiation therapy and regional or
systemic medications are useful if a lesion is close to a vital and
potentially preservable structure. Brachytherapy can be similarly
useful but may be difficult to utilize and more typically requires
additional or special soft-tissue coverage methods. Chemotherapy
management of osteogenic sarcoma, Ewing’s sarcoma, and rhabdomyosarcoma
is now well documented as discussed in Chapter 126, Chapter 128, and Chapter 129.
However, present chemotherapy methods have not yet been proven to
augment survival of patients with soft-tissue sarcomas in the hand
other than rhabdomyosarcoma.
found more often in women. The most common sites are the dorsal carpus,
generally arising from the scapholunate ligament, or at the palmar
wrist, with origin from the scaphotrapezial and/or trapeziometacarpal
joints. They often occur spontaneously as well as after trauma and in
patients with osteoarthritis (7,88,96,162).
The ganglion cyst may also arise on the palmar surface of the
fibrosseous (flexor) sheath; these are termed retinacular cysts,
sesamoid ganglia, or seed or pea ganglia. These occur as very small but
firm, even hard, and often painful lesions over the palmar aspect of
the metacarpophalangeal joint.
and it is not a true neoplasm. The lesion is typically connected to an
underlying joint capsule or tendon sheath by a stalk that may be
tortuous. The lesion may be tender as it expands or when it is
impacted, and it hurts at the extremes of joint motion. Surgical
excision is recommended for these lesions when they are unsightly or
painful, interfere with function, and have not responded to
conservative care such as needle aspiration and steroid injection. Our
preferred technique for surgical excision is to aspirate and suction
the cyst empty to improve the visualization and retraction of
surrounding structures. This allows it to be excised through an
extensile but small incision. Excision is usually curative in 95% of
cases if the cyst and its stalk are removed with a very small cuff of
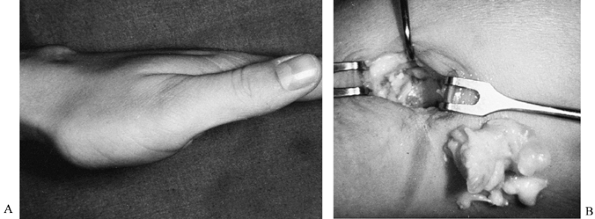
contiguous joint capsule (Fig. 74.8).
 |
|
Figure 74.8. A: A recurrent mass following two previous excisions of a dorsal radiocarpal ganglion cyst (performed elsewhere). B: At surgery, the cystic lesion, contiguous capsule, and satellite cysts were removed en bloc with the dorsal aspect of the scapholunate ligament. The lesion has not recurred.
|
arises dorsally from the distal interphalangeal joints of the fingers
and thumb. These cysts may involve the distal germinal nail matrix,
causing nail depression and grooving secondary to chronic local
pressure. Spontaneous rupture or patients’ attempts at aspiration can
lead to serious complications such as septic arthritis and
osteomyelitis.
mucus cyst must include its stalk, local small osteophytes, and joint
capsule. Protect the terminal (extensor) tendon
and
germinal nail matrix. Closure of the skin may require a small rotation
flap, skin advancement, or skin graft. If the underlying joint is
arthritic and painful, arthrodesis may be indicated (see Chapter 72).
-
Avoid overly vigorous retraction to
protect nearby sensory nerves, tendons, and vessels. It is not uncommon
for the volar wrist ganglion to “envelope” the radial artery; in such
cases, the lesion need not be peeled off the artery. Leave a small
piece of cyst wall on the artery. As long as the rest of the tumor and
its stalk are removed, recurrence is avoidable while the risk of injury
to the artery is lessened considerably.
The original injury may be entirely unrecognized or unremembered, given
the frequency with which small cuts occur in the hand. The mass is
attached to skin and contiguous subcutaneous tissue; it contains
keratin and may enlarge to a significant size. Carefully excise these
cysts, taking care to avoid spilling the contents or damaging adjacent
normal anatomy. Intraosseous components need to be curetted.
of soft-tissue (skin and skin organ) malignancies of the hand. It is
most commonly seen in older patients, and those with a history of
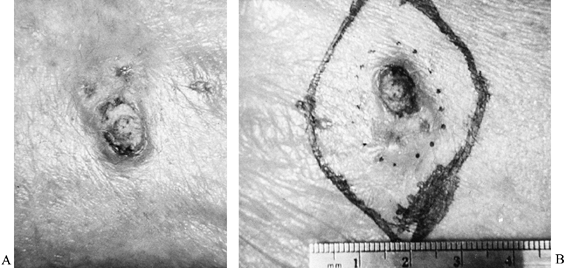
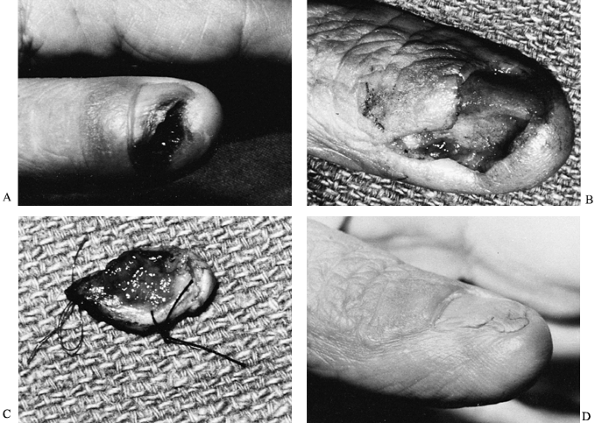
chronic exposure to chemicals, ionizing radiation, and the sun (21,28,29,33,52,64,94,116). Logically, this tumor is found most often on exposed, often dorsal, skin surfaces, and it may be accompanied by ulceration (Fig. 74.9). The nail bed is a less common site for this lesion (Fig. 74.10).
In contrast to keratoacanthoma (discussed next), these true
malignancies are actually less rapidly growing. Because primary
squamous cell carcinoma rarely metastasizes, it is amenable to cure by
local excision, including clean margins of greater than 1 cm of normal
skin. The depth of excision required depends on the size and extent of
the lesion. Removal of superficial tumors leaves a defect that is
coverable by direct local flap advancement or by skin graft. Treat
tumors that extend into adjacent tendon or bone by en bloc resection or local amputation.
 |
|
Figure 74.9. A:
This slowly growing skin ulcer on the hand of a 72-year-old retired construction worker was diagnosed by dermatologic punch biopsy as squamous cell carcinoma. B: Excision included a 1.5 cm cuff of normal skin. The tumor reactive zone is marked with a dotted circle. |
 |
|
Figure 74.10. A:
A chronic ulcer beneath the thumbnail of a retired dental hygienist was diagnosed as squamous cell carcinoma by dermatologic punch biopsy. B: Full-thickness excision of the radial half of the sterile and germinal nail matrix plus eponychium was performed. C: Suture tags were used for pathologic orientation. D: The frozen section confirmed tumor-free margins; a skin graft was applied to the resection zone. |
is clearly nodal enlargement or the primary tumor is quite large.
Sentinel node biopsy to evaluate the draining node basin is more common
with melanoma (see later) (3,100,130,146).
squamous cell carcinoma. It is found in the same sites and locations
and in the same older patient group as squamous
cell carcinoma, but it is usually a much more rapidly growing tumor (69). It is usually diagnosed at excisional biopsy. Recurrences are uncommon, and the lesion is rarely locally aggressive.
frequent skin cancer that too often is lethal. As the frequency of
chronic sun exposure has increased, the incidence of the melanoma
epidemic has increased as well (11,54,56,80,81,122,124,137,148,153,154).
These tumors may be dark, multicolored, and multivariate, or they may
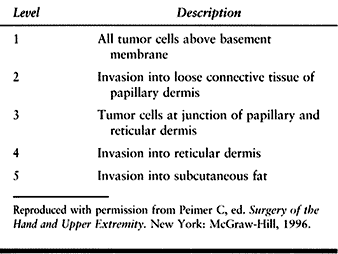
have no pigment at all. Lesions are graded according to the level of
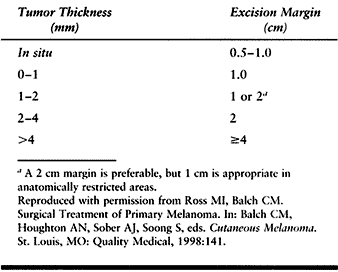
skin invasion and the size or thickness of the tumor (Table 74.4). Most tumors can be treated surgically with a clean margin of 1 cm for level I or II lesions, to 5 cm
for level III, IV, or V lesions and nodular lesions (Table 74.5).
 |
|
Table 74.4. Criteria for Levels of Tumor Invasion as It Relates to Skin Histology
|
 |
|
Table 74.5. Margins for Melanoma Excision
|
lymphatic mapping is being used in an increasing number of these cases,
as well as in certain circumstances of squamous cell carcinoma (70,95,106,158).
with lymphatic mapping requires participation of an experienced tumor
surgeon familiar with the precise methods required (117).
In overview, this is an inventive application of two vital-staining
techniques used to precisely identify the primary (sentinel) lymph node
in a node-basin draining an extremity. The idea behind the technique is
that if this sentinel node can be accurately and reproducibly located,
and it is found to be tumor free on biopsy, node dissection is not
warranted. On the other hand, if this node is positive for tumor, at
least additional node sampling, or a full node-basin dissection, will
be required for staging and treatment. The method utilizes a
combination of one visual and one nuclear stain. The affected extremity
is injected with a microquantity of fat-based, colored dye and
simultaneously with a microquantity of radioactive tracer. Both are
absorbed into the lymphatic system. After approximately 1–2 hours, a
Geiger counter is applied to the axilla to locate the first (sentinel)
node in the chain with significant uptake; this site is
marked
on the skin. Immediate surgical biopsy is aided by a sterile nuclear
counter probe and the fact that the node has also been stained from the
visible dye.
absolutely predictable for all tumors, but it has been highly
dependable for common tumors of squamous cell origin.
enlarging, multicolored, or irregularly bordered lesion leads to early
treatment. Early treatment—before the tumor has invaded and
metastasized—significantly increases chances for survival. The use of
chemotherapy and other adjuncts has not been uniformly helpful, however.
Dupuytren’s disease, and fibromatoses, which have variable
presentations and occur at different ages (2,49,68,82,109,115,117,135).
patients 10 to 25 years of age and are more common in blacks and others
with darker skin color, most often with a family history. A keloid may
continue to grow, albeit slowly, for months or years, or involute
spontaneously. Surgical excision and radiation usually provide only
temporary relief. Intradermal steroid injections and use of elastic
garments can be helpful.
subset includes subdermal fibromatosis in infancy, infantile dermal
fibromatosis/juvenile fibromatosis, calcifying aponeurotic fibroma, and
palmar fibromatosis (i.e., Dupuytren’s disease). These lesions
sometimes need to be evaluated by an experienced histopathologist to
distinguish them from aggressive processes and malignant fibrosarcomas
or malignant fibrous histiocytomas. The benign fibromatoses tend to
recur locally if incompletely excised.
sarcomas in the hand and forearm. However, it is the most common
soft-tissue tumor from childhood to young adulthood. The tumor tends to
differentiate “toward” typical striated muscle. Rhabdomyosarcomas have
been subdivided into embryonal, alveolar, and pleomorphic subtypes.
Often these tumors present as painful enlargements, but numbness or
paresthesias secondary to nerve compression may be the original symptom.
found in the hand; overall, it is reported as the second most common in
frequency for this tumor. When presenting in the hand, the neoplasm may
develop in an interosseous muscle with secondary bone changes, and with
enlargement evident on the palmar or dorsal regions of the hand.
surgical and medical oncology combined with radiation therapy. Control
the disease locally by wide surgical resection, radiation, or the
combination. Current survival rates are improving (17,18,36,62).
excision is indicated, especially for larger masses. Malignant fibrous
histiocytoma (MFH) seems to be the most common primary soft-tissue
sarcoma distal to the elbow in adults. The majority of MFH cases are in
patients 50 to 70 years of age. Although these sarcomas predominate in
the lower extremity, particularly the proximal thigh, the upper limb is
the next most common site. This tumor can manifest a wide range of
histologic and clinical features; it may invade along subcutaneous or
intermuscular planes. Most often, MFH arises in muscle and deep fascial
structures and enlarges within its compartment of origin. The tumor is
macroscopically solitary but often multilocular. Although it appears
well circumscribed, this malignancy has satellite lesions nearby,
within “reactive tissues” surrounding the mass. Micrometastases are
common when dissection is performed through the tumor pseudocapsule.
See Chapter 129 for a discussion of the treatment of MFH.
low rate of recurrence after local removal. Such tumors arise from the
tendon sheath (tenosynovial fibromas) or adjacent to the fingernails
(periungual).
nonmetastatic but are likely to recur. Recurrent lesions will
progressively invade and may destroy (in conjunction with repeated
attempts at surgical cure) function in the affected limb. Effective
local treatment requires a wide en bloc excision, or even an amputation for recurrent and destructive cases (Fig. 74.6).
required because of the tendency of these tumors to recur. Treat
recurrences promptly. These tumors are notable because they are more
aggressive clinically than their histologic appearance would suggest.
They behave like low grade sarcomas and may be quite difficult (89,110,121).
malignancy. It can be found in anatomic areas that have undergone
chronic traumatization or burns. Treatment varies with tumor location,
grade, and stage; en bloc excision or amputation is needed (2,32,48,60,65,71,72,122) (see Chapter 129).
or benign. Most lesions are benign and congenital; many may be
considered hamartomatous, and these are believed to be due to failure
of differentiation of embryonic vascular channels. Acquired lesions
include arteriovenous fistulae, false and true aneurysms, pyogenic
granulomas, and glomus tumors (30,60,97,118,136).
uncommon. Whether congenital or acquired, it is an arteriovenous shunt.
These tumors can be difficult to excise if they are not localized to a
limited anatomic region. They may involve adjacent bone, nerve, tendon,
muscle, and subcutaneous tissue as well as skin. Lesions not
effectively treatable by debulking may necessitate digital or ray
amputation. Some limited lesions can be effectively treated by ligating
the feeding vessels. In the upper extremity, when these malformations
occur, most are low-flow and their treatment relates to the aesthetics
and function of the extremity (118). The
high-flow lesions risk a neonate’s survival, necessitating emergency
treatment by a skilled pediatric cardiovascular team to reverse the
process.
abnormality in the hand. Following trauma, patients develop a painful,
pulsatile, locally tender mass (65,125).
The diagnosis is made by arteriography. Treat with excision. If
excision will compromise blood flow to the distal tissues, simultaneous
arterial/microvascular reconstruction will be needed. True aneurysms
are a rarity but not unknown.
will fade significantly, or disappear entirely, by the time the child
is about 2 years of age. Cavernous hemangiomas, which are more
extensive and deeper, may compress or compromise adjacent structures.
Treat surgically only those lesions that are enlarging and painful.
Preoperative arteriography helps to determine the probability of
distal-part survival following tumor resection, or the need for
vascular reconstruction, if possible (Fig. 74.11). At surgery, release the tourniquet before wound closure to ensure
hemostasis, as vigorous and persistent local bleeding may occur following extirpation (30,118,160).
 |
|
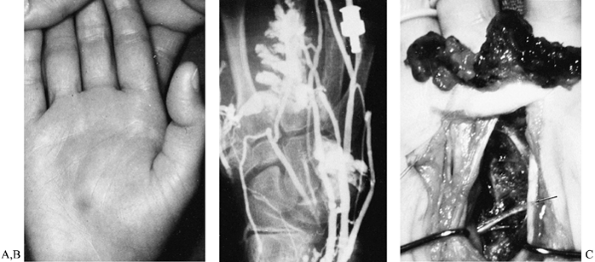
Figure 74.11. A: An enlarging, painful cavernous hemangioma in a 9-year-old. B: The venogram best demonstrated the lesion, but angiography revealed good flow to all digits. C: Dorsal and palmar incisions were required to remove the tumor. Note preservation of the motor branch of the ulnar nerve (arrow).
|
undiagnosed for long periods. They tend to occur in adults 20 to 60
years of age and are commonly subungual. They are very painful.
Patients may complain of relatively short-term or acute onset of pain,
marked local tenderness, and extreme sensitivity to cold at the site of
the lesion. Careful inspection of the tissues in the area of pain often
reveals a small, round nodule, or a bluish discoloration under the skin
or nail, which is also characterized by marked local tenderness.
Patients may note severe pain if an ice cube is applied to the site of
the suspected lesion. MRI is very useful in diagnosing glomus tumors,
especially when patients have localized obscure pain and no other
positive findings (141).
the nail, excision requires removal of the nail and longitudinal
incision of the nail matrix with removal of the lesion beneath
(including curettage of bone, where appropriate). Reapproximate
accurately the sterile and/or germinal matrix with fine, absorbable
sutures. Replace the nail or insert nonadherent gauze in the eponychial
fold. Neither need be sutured to the cuticle.
malignancy. Now, chronically immunocompromised patients such as those
with acquired immunodeficiency syndrome (AIDS) or those who have had an
organ transplant can suffer from these sarcomas (9,53,84,87).
KS appears as a reddish purple or reddish brown skin tumor or nodule on
the extremities or trunk and may be an early indication of human
immunodeficiency virus (HIV) infection or progressing AIDS. These
lesions should be treated palliatively, in conjunction with the
patient’s medical management. Where warranted, local treatment may be
requested by the primary physician, but this is not common.
benign lesion arising from the Schwann cell. It is the most common
tumor of the peripheral nerves, nerve trunks, and their branches. It is
more frequent (or more frequently noticed) on a flexor surface. These
tumors are generally less than 4.0 cm in diameter; masses 5.0 cm or
more in diameter may be malignant (43,78,133,144).
In general, symptoms are the result of nerve compression. Surgical
treatment requires extirpation. Use magnification during the surgical
dissection to minimize injury to adjacent and secondarily compressed,
atrophic, but otherwise normal fascicles. Because the neurilemoma is
well encapsulated, it can be dissected from the surrounding fasicles.
Recurrences are unusual following excision.
variant; rather, it arises as a complication of neurofibromatosis.
Malignant schwannoma requires wide excision and primary reconstruction;
amputation may be needed for the aggressive, poorly differentiated
tumors, which can have an extremely bad prognosis (see Chapter 129).
cells, axons, and fibrous tissue, which occurs as a solitary lesion or
may be part of multiple nerve and cutaneous tumors (von
Recklinghausen’s disease). Solitary neurofibroma is unlikely to recur
following excision; however, multiple plexiform neurofibromas cannot be
enucleated easily. Symptomatic plexiform neurofibromas that require
removal usually require complete resection. Nerve grafting is necessary
for reconstruction (107,138,144).
name implies; its cause is unknown. The lesion is rare. It usually
appears as a slow-growing painless mass in childhood. In the median
nerve, its presence is associated with symptoms like those of carpal
tunnel syndrome (4,85,119,123,138).
symptomatic patients operative exploration is warranted and should
include nerve decompression. At the carpal tunnel, release of the
transverse carpal ligament and a small incisional biopsy of the nerve
is done. Division of the antebrachial fascia may be necessary.
Occasionally—perhaps rarely—epineurotomy is appropriate. Intraneural
dissection often results in functional loss and is best avoided
primarily.
Children with this present with a grotesquely enlarged finger that may
need amputation. More than one finger may be affected. Early treatment
with physeal arrest is warranted. Some advocate removal of the branches
of the digital nerve with preservation of the nerve trunk.
Unfortunately, gradual, local neurologic deterioration can be
anticipated in many of these cases, necessitating nerve excision or
partial amputation.
upper limb. It is more often found in women, and a bit more commonly on
the flexor surface of the hand. Patients
complain
of a slowly enlarging, painless, and often asymptomatic mass that at
presentation may be of such impressive size that it interferes with
function. Symptoms of compression neuropathy (when the lesion is in the
carpal tunnel or a similar location) may be the presenting complaint.
Excision of the tumor and pseudocapsule is usually a straightforward,
curative procedure (83,101,113,135,157).
xanthoma) is a soft-tissue lesion that is not related to, and should
not be confused with, a giant cell tumor of bone. The two are of
different origin, presentation, frequency, and behavior. These lesions
are common on the flexor surface of the hand, where tenosynovial
tissues predominate. Most of these tumors are found in the fingers,
wrist, or palm. As they slowly enlarge, they have the potential to
envelope tendons, invade bones and joints, and to surround
neurovascular structures. Treatment is by thorough local excision while
avoiding injury to adjacent anatomy (Fig. 74.12).
The incidence of recurrence is reported to exceed 15%, but recurrence
quite probably occurs from incomplete, inadequate excision (49,99,113,115). Should a recurrent tumor present, reexcision is indicated.
 |
|
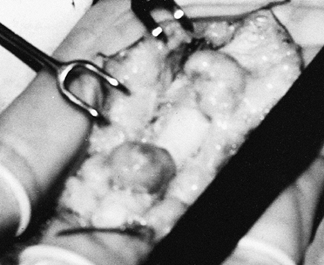
Figure 74.12.
Giant cell tumor of tendon sheath must be completely removed to prevent recurrence, while preserving the flexor pulleys and avoiding injury to the neurovascular bundles. |
among the more common primary soft-tissue malignancies in the distal
portion of the upper extremity, distal to the elbow (1,6,8,19,22,35,46,47,61,74,77,93,105,111,114,126,139,149,152,155).
similar aggressive behavior and their tendency to present in a
confusing and atypical fashion. Their spread is seemingly
unpredictable, and they can include skin, vessels, and lymphatics at
secondary regional and distant sites. Unfortunately, these neoplasms
have a grave prognosis. For that reason, an otherwise benign-appearing,
solid soft-tissue lesion in the hand or wrist must be approached with
caution. Incisional biopsy with appropriate technique (Chapter 129) is important, as the tumor may turn out to be one of these potentially lethal sarcomas.
multidisciplinary evaluation and staging, and surgical and medical
treatment, including axillary node dissection (increasingly preceded by
sentinel node biopsy) is advised. Radiation has been helpful in some
cases; the value of radiation and chemotherapy is not yet well defined
or entirely dependable, but it is increasingly part of the primary
treatment.
bone tumor in the hand. In the vast majority, the tumor presents as a
pathologic fracture. It is most common in young adults. This speckled
osteolytic lesion may be discovered incidentally on radiographs taken
for another reason (Fig. 74.13), or because of enlargement of a finger or hand. However, pain is unusual without fracture.
 |
|
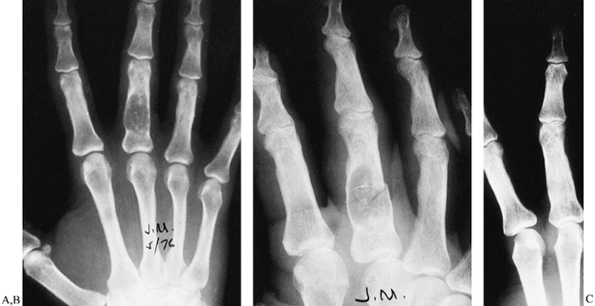
Figure 74.13. A:
Expansile lytic lesion of the proximal phalanx of the middle finger was found incidentally on radiographs done for a little finger injury. Speckled radiographic appearance is typical for an enchondroma. The patient declined treatment of this relatively large, albeit asymptomatic, lesion. B: Two years later, a radiograph following a minor trauma showed a pathologic fracture through the slightly further enlarged tumor. C: Curettage and bone grafting were performed to treat the tumor and stabilize the phalanx. Graft incorporation and remodeling occurred without incident. |
Plan to treat larger and enlarging tumors, as well as those associated
with fractures. The presence of a fracture through the lesion does not
ensure spontaneous tumor resolution during the course of bone healing.
These tumors are amenable to adequate curettage. Most commonly,
autogenous bone graft is added; but thorough curettage is the most
important surgical step.
fracture simultaneously, to minimize morbidity and avoid double
disability. In some patients, the addition of internal fixation,
commonly with Kirschner wires, plus bone graft will be needed to
stabilize the curetted infracted bone. Delay of surgical treatment
until the bone has healed is a more traditional approach, but this can
prolong a patient’s disability because the hand has to recover first
from
the
fracture and then from subsequent surgery. Furthermore, delay in
treating the lesion delays the diagnosis, should the osteolytic tumor
prove to be other than an enchondroma (12,73,112).
Start postoperative hand therapy as soon as practical, considering the
skeletal stability following trauma and treatment, in order to minimize
tendon adhesions and joint stiffness.
common than solitary lesions, but these multiple tumors have a tendency
to be larger and unilateral, and they are associated with skeletal
deformities (Fig. 74.14) (16,51).
Ollier’s disease is probably not hereditary. Maffucci’s syndrome is
Ollier’s disease with associated angiomas of skin or soft tissue. The
hand is most commonly involved, with tumors generally beginning in the
metaphysis but gradually extending distally into the diaphysis, or
proximally to the former epiphysis in adults. These lesions require
curettage and en bloc excision; often the bone deformity will need osteotomy for finger realignment.
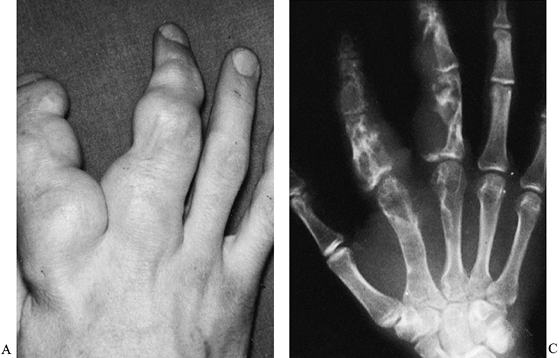 |
|
Figure 74.14. Multiple enchondromatosis caused extensive deformity and dysfunction in this patient with a positive family history.
|
higher with multiple growing lesions and should be suspected if tumors
become painful, begin to enlarge, or are associated with progressive
deformity. Do an incisional biopsy to rule out malignancy.
unknown. Findings of histologic atypia in a cartilaginous lesion should
not be regarded as a necessarily clinically worrisome issue. Consider
the clinical presentation and histologic situation together—especially
in the older or at-risk patient. Most malignancies are low grade. (See Chapter 128.)
as a hereditary problem that may cause significant and generalized
skeletal deformities. Although the tubular bones of
the
hands are less commonly affected than the radius and ulna, they may be
involved. Symptoms are generally caused by the presence of a bony mass,
and complaints may include deformity (in the growing skeleton) and
progressively diminished mobility because of bony block-to-joint
motion. Treatment should include removal of the space-occupying lesion
while preserving skeletal integrity and nearby soft tissues. The risk
of malignant transformation of lesions in the hand is extremely low.
The significant problem associated with these tumors is the need for
reconstructive surgery to restore functional alignment of the (small
and large) bones in the forearm, wrist, and hand (20,24,25,143).
osteotomy) require great care to avoid injury to the growing physis
when the osteochondroma is found, because they typically are close to
the physeal plate. Bowing of the radius or ulna, shortening of the
forearm, and limitation of forearm rotation can be a reconstructive
challenge necessitating tumor treatment and lengthening of any involved
bones. Removal of the mass will not, per se, correct the deformity or
necessarily restore lost joint motion, which may be accompanied by
secondary fibrosis (see Chapter 127).
in the hand and wrist. It is usually a tumor of younger adults and may
be associated with multicentric foci when found in the upper limb. The
behavior of multicentric GCTs is different from that of solitary
lesions. Those of the small tubular bones should always be considered
more aggressive than those presenting in the larger bones (10,26,42,55,90,104,120,132,134,142,151,161).
Small multicentric foci are not easily diagnosed on routine
radiographs; do a bone scan when a GCT of bone is suspected to avoid
missing the multicentric variant. Patients complain of pain and
enlargement of the part, and localized tenderness. Pain may be due to
an impending or pathologic fracture.
substitutes, bone cement, and other techniques have been used for GCT
for various locations. When in the distal portion of the upper
extremity, these tumors are likely to recur, however (Fig. 74.15). It is our opinion that GCTs of tubular bones in the hand and of the distal radius and ulna should be treated by en bloc excision or by ray resection with simultaneous replacement and reconstruction
with bone graft, vascularized graft, or osteoarticular allograft,
appropriate to the specific case. The precise surgical technique and
approach depend on the particular lesion and patient. Tailor
postoperative rehabilitation to the particular reconstructive
procedure. See Chapter 127 for a more extensive discussion.
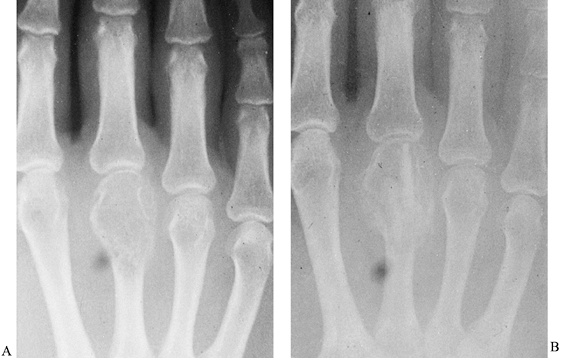 |
|
Figure 74.15. A:
Eccentric, lytic epiphyseal lesion of the third metacarpal proved to be a giant cell tumor of bone, which was treated by curettage and grafting. B: Tumor recurrence is seen as an enlarging zone of osteolysis, including graft destruction, nearly 4 months after curettage. Ray resection was required. |
It is relatively uncommon in the hand. Most often it is a solitary,
expansile lesion originating in the metaphysis of a long bone that has
a “blown-out” appearance on the radiograph. ABC tends to be more common
in the second and third decades, with a slight predominance for women. En bloc
excision is the most dependable treatment. Radiographically, this tumor
may be confused with GCT of bone, or with bone cyst, osteosarcoma, or
Ewing’s sarcoma. ABC, however, has a much lower recurrence rate than
GCT of bone.
bone-forming tumors that often present as painful but otherwise
radiographically obscure lesions in young patients. The propensity for
a locus in the hand is not high, but it has to be considered in
patients with localized pain and apparent enlargement of a small bone
in the hand with periosteal a endosteal new bone formation (Fig. 74.16).
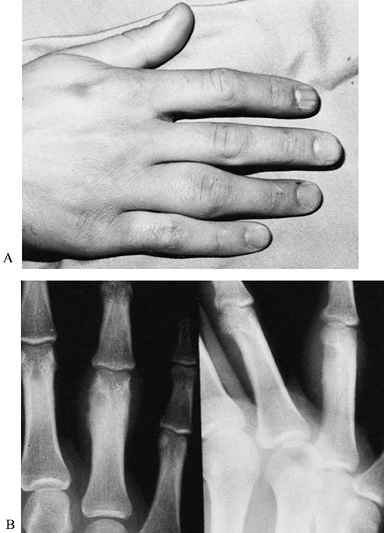 |
|
Figure 74.16. A:
Swelling and pain of 2 years’ duration in a 17-year-old boy whose radiographs show retrocondylar bone formation surrounding a central, lucent nidus. B: At surgery, the diagnosis of osteoid osteoma was confirmed and the lesion was successfully treated by curettage. |
diagnosis. If plain films are nonspecific, a CT scan of the lesion
using fine cuts is most likely to identify the nidus, which is
diagnostic. Excision without recurrence is typical if the tumor nidus
is excised (5,13,37,39,71,75,91,110).
Of the various subtypes, the parosteal, periosteal, and low-grade
central osteosarcomas have a lower incidence of metastasis and a higher
overall survival than osteoblastic, chondroblastic, fibroblastic, and
telangiectatic osteosarcomas.
multidisciplinary therapies have improved survival to nearly 70% in
some series. Frequently, preoperative (neoadjuvant) chemotherapy is
combined with other methods, including surgery. Pulmonary metastasis is
the primary cause of
death;
however, limb salvage following regional or systemic neoadjuvants,
combined with simultaneous (and most often complex microvascular)
reconstruction, can salvage a useful hand without increased morbidity
and mortality (see Chapter 126 and Chapter 128).
treatment for hand tumors rather than making an attempt to review every
tumor or related condition that may present in the hand. Certain
specific neoplasms have been described because they are common or could
lead to death.
hand must be part of an oncology team. This team must be experienced
and prepared to diagnose, biopsy, and excise these tumors, and
simultaneously reconstruct the hand. Such a team includes
musculoskeletal oncologists, medical oncologists, reconstructive
surgeons, and radiation therapists, as well as experienced
musculoskeletal pathologists. Treatment should achieve a cure, and
function should be preserved whenever possible. Balancing these
considerations may not be easy, but it is important to the patient’s
daily function, and ultimately to their survival.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JJ, Cruse CW, Rapaport D, et al. Intraoperative
Radio-lympho-scintigraphy Improves Sentinel Lymph Node Identification
for Patients with Melanoma. Ann Surg 1996;223:217.
AC, Wallace PF. The Dorsal Ganglion of the Wrist: Its Pathogenesis
Gross and Microscopic Anatomy and Surgical Treatment. J Hand Surg 1976;1:228.
CM, Urist MM, Karakousis CP, et al. Efficacy of 2-cm Surgical Margins
for Intermediate-thickness Melanomas (1-4 mm): Results of a
Multi-institutional Randomized Surgical Trial. Ann Surg 1993;218:262.
F, Bacchini P, Fabbri N, et al. Osteosarcoma, Low-grade Intraosseous
Type, Histologically Resembling Parosteal Osteosarcoma, Fibrous
Dysplasia, and Desmoplastic Fibroma. Cancer 1993;71:338.
PW, Bell RS, Bowen CV, et al. Limb Salvage Surgery and Adjuvant
Radiotherapy for Soft Tissue Sarcomas of the Forearm and Hand. J Hand Surg [Am] 1997;22:495.
JJ, Peimer CA, Mindell ER, et al. Primary Malignant Tumors of the Upper
Extremity: Retrospective Analysis of 126 Cases. J Hand Surg [Am] 1985;10:805.
GR, McElfresh EC, Peterson HA, Wicklund PT. Management of Deformities
of the Forearm in Multiple Hereditary Osteochondromas. J Bone Joint Surg Am 1984;66:670.
FJ, Sim FH, Frassica DA, et al. Survival and Management Considerations
in Post Irradiation Osteosarcoma and Paget’s Osteosarcoma. Clin Orthop 1991;270:120.
PA, Wexler LH, Venzon DJ, et al. Sarcomas of the Hand and Foot:
Analysis of Local Control and Functional Result with Combined Modality
Therapy in Extreme Preservation. Int J Radiat Oncol Biol Phys 1994;29:735.
FE, Soule EH, Coventry MB. Fibrous Xanthoma of Synovium (Giant-Cell
Tumor of Tendon Sheath, Pigmented Nodular Synovitis). A Study of 118
Cases. J Bone Joint Surg Am 1969;51:76.
DL, Wen DR, Cochran AJ. Management of Early-stage Melanoma by
Intraoperative Lymphatic Mapping and Selective Lymphadenectomy. Surg Oncol Clin North Am 1992;1:247.
WC, Truong NP, Totty WG, Gilula LA. Pictorial Review: Magnetic
Resonance Imaging of Benign Soft Tissue Masses of the Hand and Wrist. Clin Radiol 1995;50:519.
PW, Harrison LB, Leung DH, et al. Long-term Results of a Prospective
Randomized Trial of Adjuvant Brachytherapy in Soft Tissue Sarcoma. J Clin Oncol 1996;14:859.
DS, Rapaport DP, Tanabe KK, Ross MI. Lymphatic Mapping and Sentinel
Lymphadenectomy. In: Balch CM, Houghton AN, Sober AJ, Soong S, eds. Cutaneous Melanoma, 3rd ed. St. Louis, MO: Quality Medical, 1998;227.
DF, Harris MN, Ackerman AB. Diagnosis and Management of Cutaneous
Malignant Melanoma. In: Roses DF, Harris MN, Ackerman AB, eds. Major Problems in Clinical Surgery. Philadelphia: WB Saunders, 1983:346.
S, Corson JM, Demetri GD, et al. Prognostic Factors Predictive of
Survival for Truncal and Retroperitoneal Soft-tissue Sarcoma. Ann Surg 1995;221:185.
CL Jr, Stidham KR, Ricci WM, et al. Surgical Management of Regional
Lymph Nodes in Patients with Melanoma. Experience with 4682 Patients. Ann Surg 1994;219:120.
WK, Rapaport DP, Soong SJ, et al. Prognostic Clinical and Pathologic
Features. In: Balch CM, Houghton AN, Sober AJ, Soong S, eds. Cutaneous Melanoma, 3rd ed. St. Louis, MO: Quality Medical, 1998:11.
RM, Thorson EP, Raskind JR. Allograft Replacement with Distal
Radioulnar Joint Fusion and Ulnar Osteotomy for Treatment of Giant Cell
Tumors of the Distal Radius. J Hand Surg [Am] 1990;15:929.
CS, Bauer HC. Local Recurrence of Soft Tissue Sarcoma a Risk Factor for
Late Metastases. 379 Patients Followed for 0.5–20 Years. Acta Orthop Scand 1994;65:553.
U, Cascinelli N, Adamus J, et al. Thin Stage I Primary Cutaneous
Malignant Melanomas: Comparison of Excision with Margins of 1 or 3 cm. N Engl J Med 1988;318:1159.
BC, Keus RB, Nieweg OE, Kroon BB. Complications of Combined
Radiotherapy and Isolated Limb Perfusion with Tumor Necrosis Factor
Alpha + /– Interferon Gamma and Melphalan in Patients with Irresectable
Soft Tissue Tumors. J Surg Oncol 1997;65:88.
