Cellular and Molecular Biology
multi-cellular organism, be it a mouse or a human, depends on the
complex and exquisitely regulated control pathways that govern the
proliferation and subsequent differentiation of cells. The critical
nature of the pathways that control cellular proliferation and growth
is perhaps best illustrated by the fact that most cancers are the
result of either inherited or induced defects in these control systems.
explaining the temporal pattern of cellular activity that is required
for one cell to divide (i.e., produce two identical daughter cells).
The cell cycle is divided into four active phases and one inactive
(quiescent) phase that may be more accurately considered outside of the
cell cycle (Fig. 13-1, Table 13-1),
since it is not involved in the production of a daughter cell. Although
cells from different tissues may show vastly different proliferation
rates, the length of the cell cycle is fairly constant, approximately
24 hours; the difference in proliferation rate is the result of
differences in the length of time that cells remain in the quiescent
phase.
proliferation, it should not be surprising to find that the cell cycle
contains a number of “checks and balances” through which the integrity
of DNA replication, spindle formation, and chromosome separation can be
ensured. The feedback pathways by which these checks are made are known
as checkpoints.
-
Passage is necessary before DNA synthesis can be initiated.
-
Failure to progress leads to accumulation of cells in G1.
-
Passage is necessary for the tetraploid cell to split into daughter cells via the process of mitosis.
-
Failure to progress leads to accumulation of cells in G2.
-
Radiation therapy and chemotherapy can cause blockade of the G2/M checkpoint.
-
Central effectors of cell cycle control at the molecular level
-
Disturbances in cyclin—Cdk interactions have been implicated in loss of cell cycle control in neoplastic conditions:
-
p53, a tumor suppressor gene, modulates the activation of cyclin D.P.310
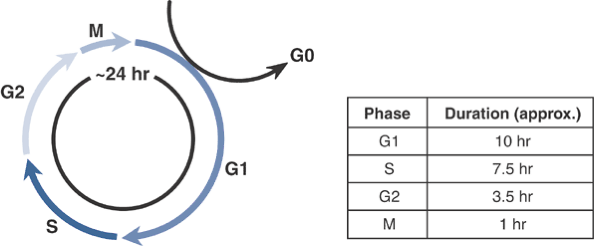 Figure 13-1
Figure 13-1
The cell cycle. Under culture conditions, growing cells divide
approximately every 24 hours. Cycle times in vivo are more variable
since fully differentiated cells may exit the cell cycle and remain in
G0 for protracted periods. -
Mutations in p53 are present in a very large proportion of cancers.
-
-
Synthetic analog of thymidine that is incorporated into newly formed DNA
-
Incorporation provides information on both magnitude and timing of DNA synthesis within a cell population.
-
Autoradiography
-
Location of radiolabeled BrdU is visualized by exposing the cells or tissues to x-ray film.
-
-
Immunohistochemistry
-
Detection within cells or histological specimens using antibodies.
-
-
Enzyme-linked immunoabsorbent assay (ELISA)
-
Detection and quantification using antibodies for in-vitro specimens
-
-
A DNA-binding dye (such as propidium iodide) is used to label the DNA.
-
Cells flow past a laser light source; scattering of beam provides measures of both cell number and size.
-
By combining information on cell size,
number, and intensity of propidium iodide, it is possible to quantify
the proportion of cells in the G1, S, and G2/M phases (Fig. 13-2).-
Flow cytometry is also a useful technique for documenting programmed cell death (see later).
-
Flow cytometry can also be used to quantify BrdU incorporation (see earlier).
-
|
Table 13-1 Phases of the Cell Cycle
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
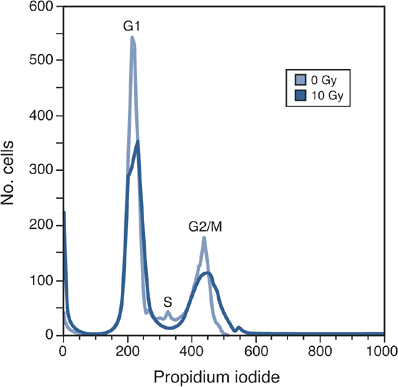
Figure 13-2
Analysis of the cell cycle using flow cytometry. Cells that have been stained with propidium iodide can be classified into three populations—G1, S, and G2/M—based on the amount of DNA that they contain. Treatment with X-irradiation (10 Gy in this example) decreases the G1 fraction and promotes the build-up of cells in G2/M. Note also the appearance of a large spike to the left of the G1 peak; this spike (known as sub-G1) represents cells with reduced DNA content, likely the result of either apoptosis or necrosis (see later). |
While the net effect of each mechanism is death of the cell, the
fundamental morphological and molecular features of apoptosis and
necrosis are vastly different.
-
Death from the inside out
-
Organized cascade of intracellular processes
-
Ultimately leads to digestion of the cellular DNA and fragmentation of the cell into apoptotic bodies
-
Critical role in the regulation of tissue structure and function
-
Important during growth and differentiation
-
Stimuli that promote or inhibit apoptosis
have the capacity to deregulate normal growth and differentiation,
leading to an increased risk of neoplasia.
-
Cell dies from the outside in.
-
Most often the result of direct injury to the plasma membrane
-
Once the membrane is damaged, the
stability of the cell’s internal milieu is lost and cellular components
may be released directly into the extracellular space.
-
Release of cytosolic and organellar components into surrounding tissues has an important secondary effect.
-
Typically induces a local inflammatory response in vivo
-
This effect is not seen in vitro, since a
cell culture environment cannot replicate the innate immune response
that is seen in vivo.
-
Nuclear condensation, blebbing, and fragmentation
-
Recognized best by labeling nuclei with fluorescent dyes (e.g., Hoechst 33258; Fig. 13-3)
-
-
Depending on the type of tissue, apoptotic cells may be removed by phagocytes.
-
Inflammatory changes are not a feature of apoptosis.
-
-
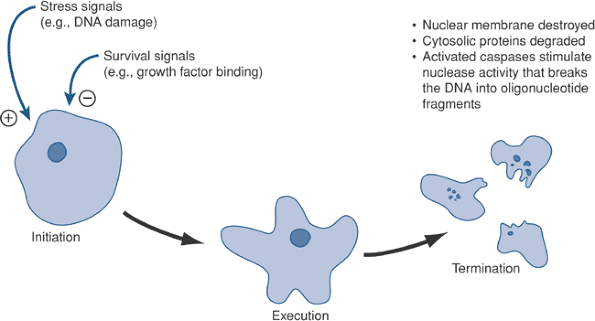
Initiation
-
Although the plasma membrane remains intact during
P.312
the early stages of apoptosis, there is redistribution of a glycolipid,
phosphatidyl serine (PS), from the inner layer to the outer layer. As a
result, PS becomes “visible” on the outside of the cell.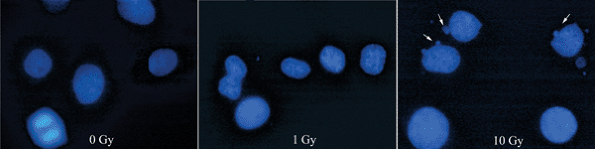 Figure 13-3 Morphological signs of apotosis. Radiation causes a dose-dependent increase in the incidence of cells with nuclear blebbing (arrows). Hoechst stain, 100 × original magnification.
Figure 13-3 Morphological signs of apotosis. Radiation causes a dose-dependent increase in the incidence of cells with nuclear blebbing (arrows). Hoechst stain, 100 × original magnification.![]() Figure 13-4
Figure 13-4
Key steps in apoptosis. The healthy cell needs to be stimulated by
growth factors to survive. Loss of these factors, or the addition of
stress signals, can push the cell toward programmed cell death.
-
-
Execution/activation
-
As a result of changes in mitochondrial
membrane permeability, cytochrome c migrates from the inside of the
mitochondria into the cytosol. -
Caspase activation then ensues, leading
to further changes in the permeability of intracellular organelles such
as the mitochondria.
-
-
Termination
-
The nuclear membrane is destroyed.
-
DNA damage is mediated via caspase-activated DNase (CAD).
-
CAD activity is usually controlled by an inhibitor, iCAD, but this is cleaved via the effector caspases (Fig. 13-5).
-
Cytosolic proteins are cleaved and organelles break up.
-
-
DNA damage from irradiation (UV or x-ray) or chemotherapy
-
DNA damage activates p53 pathways, leading to the transcription of p21.
-
p21 blocks the ability of cyclin D/Cdk to
phosphorylate the retinoblastoma (Rb) gene product; this blocks the
movement of cells through the G1/S transition. -
p53 activation also stimulates apoptosis via a direct effect on Bax (see later).
-
-
Loss of key trophic factors (e.g. serum withdrawal in cultured cells)
-
Growth factors are critical “survival signals” for cells; their loss will tip the balance towards programmed cell death.
-
-
Fas ligand
-
Signals via the Fas (or Apo2) receptor (CD 95)
-
Receptor activation enables FADD (Fas-associated death domain) binding, which in turn activates cas-pase-8.
-
-
Tumor necrosis factor-α
-
Signals via TNF receptor-1 (TNFR1)
-
Binding can have both pro- and anti-apoptotic effects:
-
Pro-apoptotic effects are mediated via FADD binding to the death domain on the activated receptor (see above).
-
Alternatively, the death domain can bind
a protein known as RIP (receptor interacting protein); in this case,
binding leads to activation of the nuclear factor kappa B (NF-κB) or
the Jun kinase pathway, both of which inhibit apoptosis.
-
-
-
“Cellular stress,” including free radicals
-
Cysteine-dependent aspartate-specific
proteases, or CASPases, are the central component of the normal cell’s
machinery for programmed cell death. -
Not all of the caspase family members are
involved in apoptosis; caspase 1, for example, is a key regulator of
IL-1 activity. However, caspases 3, 6, 7, 8, and 9 are
P.313
situated at pivotal control points in apoptotic signaling pathways.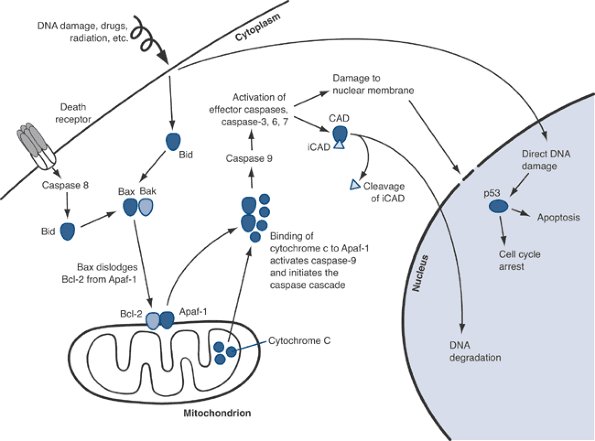 Figure 13-5
Figure 13-5
Apoptosis pathways. Activation of Bid (via death receptors or direct
DNA damage) and Bax/BAK leads to release of Apaf-1 from the
mitochondrial membrane. Apaf-1 is then free to bind to cytochrome c
that is released from the interior of the mitochondrion. The
interaction of Apaf-1 and cytochrome c activates the caspase cascade,
ultimately leading to membrane damage, DNA degradation, and the
dissolution of cellular proteins and organelles. -
Based on their structure and function, caspases can be considered either activators or effectors:
-
Activators such as caspases 8 and 9 amplify the initial signal and activate downstream effector caspases.
-
Effectors such as caspases 3, 6 and 7 mediate direct effects on cellular processes and structures.
-
-
Intracellular control of apoptosis is
determined by the balance between pro- and anti-apoptotic signaling
molecules in the Bcl-2 family. -
Anti-apoptotic molecules include Bcl-2 and Bcl-XL.
-
Bcl-2 tethered to the outer mitochondrial membrane binds to an adapter protein known as Apaf-1.
-
Binding of Bcl-2 anchors the Apaf-1, preventing its release into the cytoplasm.
-
Overexpression of Bcl-2 is associated with malignancies, particularly in B-cell leukemias (hence the name).
-
In this instance, neoplasia is the result
of decreased cell death from apoptosis (i.e., enhanced survival) rather
than increased cell proliferation.
-
-
-
Binding of pro-apoptotic molecules such as Bax, Bak, or Bid dislodges Apaf-1, which is now free to enter the cytoplasm (see Fig. 13-5).
-
Changes in the permeability of the mitochondrial membrane allow cytochrome c to diffuse out into the cytoplasm.
-
Apaf-1 and cytochrome c then bind, leading to the activation of caspase 9.
-
-
Microscopy: with or without special
stains (e.g., Hoechst stains will stain the DNA and facilitate the
identification of nuclear blebbing; see Fig. 13-3) -
TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling) stains on histological sections
-
Immunohistochemical staining technique in which the 3′ ends of fragmented DNA are labeled and then detected using an antibody-based technique.
-
TUNEL staining is not specific to apoptosis but can be useful if combined with morphological evidence of apoptosis.
-
-
Annexin V
-
Phosphatidyl serine (PS) is a phospholipid normally found on the inner surface of the plasma membrane.
-
-
In response to apoptotic stimuli, PS becomes exposed on the outer surface of the plasma membrane.
-
Exposed PS is free to bind to annexin V, a reaction that is readily confirmed by flow cytometry.
-
-
Caspase assays
-
Direct measurement of activated caspase family members provides a robust method for confirming and quantifying apoptosis.
-
Most commonly used markers are caspases 3 and 7.
-
Activated caspases may be detected by Western blotting, ELISA, or immunohistochemistry.
-
and survive in vivo is dependent on and regulated by a vast array of
local and systemic signals, including growth factors, cyto-kines, and
hormones. One of the most remarkable characteristics of cells is that
they have the capacity to integrate this multitude of signals, be they
stimulatory or inhibitory, into a coherent response in the form of
alterations in cellular activity. The stimulus ← response pathway can
be broken down into three stages.
-
Perception of the signal at either the cell surface or within the cell
-
This is usually made possible by the possession of specific receptors that can interact with the stimulus.
-
-
Intracellular signaling pathways
-
These convey signals from the activated receptor, through the cytoplasm to the nucleus.
-
Several key signaling pathways have been
implicated, including intracellular Ca ++, phosphoinositols, cyclic
AMP, and intracellular kinases (e.g., tyrosine kinase).
-
-
Effector pathways
-
Proto-oncogenes such as c-fos and c-jun
appear to be important targets; these proto-oncogenes encode
transcription factors that interact with the DNA molecule, facilitating
the expression of genes that control DNA replication, cellular
differentiation, and synthetic activity.
-
our ability to identify and characterize many of these intracellular
signaling pathways and their effects on the cell.
beyond the scope of this book. The goal of the following discussion is
to use some specific examples of signaling pathways that are important
in musculoskeletal tissues as a means of conveying general themes that
are broadly applicable across many tissue types.
-
Growth factors are biologically active
factors that control the growth, differentiation, and survival of
target cells via interactions with specific receptors. -
Growth factors play an important role in
the regulation of both normal physiological processes such as growth
and development and pathological conditions caused by disease or injury. -
Mutations in growth factors, their
receptors, or downstream signaling pathways have been implicated in the
development of a range of human cancers.-
Oncogenes: genes whose overexpression leads to cancer
-
Tumor suppressor genes: genes whose underexpression (or inactivation) is associated with cancer
-
-
Cytokines can be considered a specialized subset of growth factors.
-
Cytokines are small, usually locally
acting secreted proteins that are classically described as being the
products of immune cells. -
However, it is now recognized that cytokines are synthesized by (and act on) a wide range of cell types throughout the body.
-
Although cytokines usually function locally either on the cells that secrete them (autocrine pathway) or on adjacent cells (paracrine pathway), they can occasionally act on distant tissues via an endocrine mechanism.
cytokines is clearly outside the scope of this chapter. What follows is
intended as a general overview of those factors with greatest relevance
to the musculoskeletal system, with the aim of this discussion being to
describe the key biological actions of these molecules as well as to
highlight the signaling pathways by which these factors control the
activity of target cells.
-
Sources
-
Most IGF-I is synthesized in the liver in response to growth hormone.
-
Mineralized matrix contains abundant reserves of IGF that are released by bone resorption.
-
-
FunctionsP.315
-
Potent regulators of both osteoblast and chondro-cyte function
-
IGFs promote osteoblast survival and stimulate the proliferation and differentiation of chondrocytes.
-
-
Sources
-
Found in high concentrations in platelets and endothelial cells
-
Consists of homo- or heterodimers of two
isoforms, A and B (i.e., PDGF-AA, PDGF-AB, or PDGF-BB), although
biological effects appear to be similar for all variants
-
-
Functions
-
Primary physiological role appears to be to stimulate wound repair.
-
Stimulates cell proliferation and differentiation in a wide variety of cell types, including chondrocytes and osteoblasts
-
-
Sources
-
Two distinct forms exist, acidic FGF (aFGF) and basic FGF (bFGF).
-
FGFs are expressed in a variety of
tissues, including the central nervous system, vasculature,
musculo-skeletal system, and the developing mammary gland. -
Upregulation of FGF expression has been
associated with malignant transformation in solid tumors (breast,
prostate cancer) and hematological malignancies (including acute
myeloid leukemia).
-
-
Functions
-
FGFs play a critical role in embryonic development, homeostasis, and tissue regeneration.
-
FGFs also stimulate endothelial cell proliferation, increasing vascularization at sites of injury and healing.
-
Both forms are also mitogenic for both fibroblasts and osteoblasts, although bFGF is the more potent of the two forms.
-
-
Sources
-
Three isoforms of TGF-β are found in humans.
-
Synthesized in a latent form by osteoblasts and platelets
-
Latent TGF-β is activated by extracellular proteases.
-
Activation by osteoclast-derived proteases may provide a mechanism for coupling bone resorption with subsequent bone formation.
-
Bone matrix is the most abundant source of TGF-β1 and TGF-β2.
-
-
Functions
-
Multifunctional growth factor has been
implicated in the control of cellular growth and differentiation in
healthy and neoplastic tissues. -
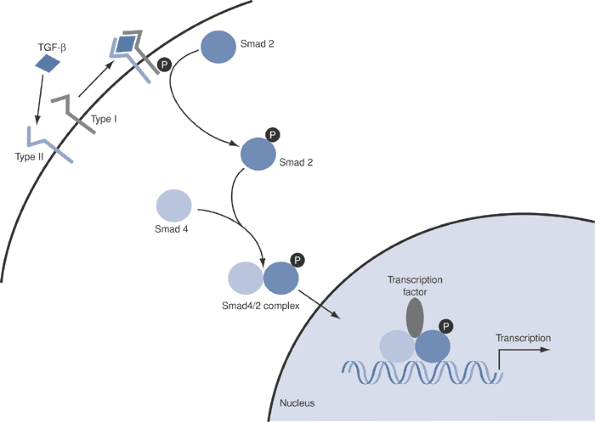
Signaling involves the Smad pathway (Fig. 13-6).
-
-
Sources
-
Originally identified through the
groundbreaking work of Marshall Urist and colleagues, who identified
the osteoinductive capacity of demineralized bone powder
-
-
Functions
-
BMPs are members of the TGF-β superfamily, sharing both structural features and signaling pathways (see Fig. 13-6).
-
BMPs have been shown to play critical roles in the regulation of bone and cartilage formation.
-
BMP-6 is an important paracrine regulator of growth plate function (see Chapter 14, Growth and Development of the Skeleton).
-
BMP-2 and BMP-7 (also known as osteogenic
protein-1 [OP-1]) stimulate new bone formation and show tremendous
promise in applications such as spine fusion, fracture repair, and
cartilage repair/regeneration.
-
-
-
Major sources include monocytes and macrophages.
-
Functions
-
Stimulates matrix metalloproteinase (MMP)
activity and the production of other pro-inflammatory cytokines,
including IL-6 (see below) -
Implicated in both osteoarthritis and rheumatoid arthritis
-
Stimulates the proliferation of synovial fibroblasts and T cells
-
A soluble IL-1 receptor antagonist
(anakinra) is currently approved for the treatment of rheumatoid
arthritis. The drug has a short half-life in vivo, however, and a
better approach may be to deliver the molecule via gene therapy.
-
-
-
Sources
-
Originally identified as a key factor in B-cell immunology
-
It is now recognized that IL-6 is a
pleiotropic growth factor that is expressed by multiple cell types,
including macrophages, fibroblasts, endothelial cells, glial cells,
keratinocytes, and synovial cells. -
IL-6 binds to a surface receptor and activates gp 130.
-
-
Functions: pro-inflammatory cytokine that
plays a key role in the pathophysiology of postmenopausal osteoporosis
and other conditions of pathological bone loss, including aseptic
implant loosening, osteomyelitis, and tumor osteolysis
-
Sources: synthesized by many cell types,
perhaps most importantly synovial macrophages, fibroblasts, bone marrow
monocyte-macrophages, and osteoblasts -
Functions
-
Pro-inflammatory cytokine that stimulates bone resorption and cartilage destruction in vitro and in vivo
-
Implicated in the pathogenesis of rheumatoid arthritis
-
Stimulates the release of pro-inflammatory IL-6 from synovial macrophages, fibroblasts, and chondrocytesP.316
![]() Figure 13-6
Figure 13-6
Smad pathways involved in BMP and TGF-β signaling. Binding of TGF-β
leads to phosphorylation of the receptor, which in turn phosphorylates
Smad 2. Phosphorylated Smad 2 binds to Smad 4 and the complex crosses
into the nucleus, where it binds transcription factors and activates
gene expression. -
Soluble TNF-α antagonists (etanercept, infliximab) are available for the treatment of rheumatoid arthritis.
-
-
-
Sources: products of the cyclooxygenase (COX) pathway of arachidonic acid metabolism
-
Functions
-
Potent mediators of inflammation in a range of diseases
-
Prostaglandins of the E series appear to be particularly important within the musculoskeletal system.
-
Locally applied prostaglandin E2 (PGE2) stimulates bone resorption.
-
Systemic administration of PGE2 is
anabolic for the skeleton; this effect is the result of enhanced
osteoblast recruitment and/or differentiation.
-
-
Selective inhibitors of the inducible COX
enzyme (COX-2) are highly effective in the management of joint
inflammation and bone loss in patients with arthritis.
-
presence of specialized connections between adjacent cells. In
musculoskeletal tissues, the most common junctions are gap junctions
and adherens junctions.
-
Function: to provide selective passage of
small-molecular-weight molecules, Ca+ + ions, and signaling molecules
such as cyclic AMP. -
Cell types: Gap junctions have been identified in osteo-blasts, osteocytes, and fibroblasts.
-
Components
-
Connexins are the major transmembrane components of gap junctions.
-
Connexin 43 is the predominant connexin in osteo-blasts.
-
Plays key roles in osteoblast differentiation, apoptosis, and response to mechanical strain
-
-
-
Function
-
Act as dimers
-
Cytoplasmic end of the cadherin binds via α- and β-catenins to the actin cytoskeleton.
-
Extracellular domain binds via a Ca + + -dependent mechanism to cadherins in adjacent cells.
-
Loss of adherens function impairs osteoblast differentiation, indicating a critical role in this process.
-
Cell types and components
-
Cadherins are transmembrane proteins (>40 variants).
-
E-cadherin found on epithelial cells
-
N-cadherin on muscle, neural tissues
-
-
-
cartilage are functional composite materials consisting of cells
embedded within an extracellular matrix (ECM). In both cases, the
matrix is composed of proteoglycans mixed with collagen fibers.
Interactions between cells and the surrounding matrix appear to play a
critical role in the following.
-
Mechanical forces
-
Potent regulators of the extracellular matrix in musculoskeletal tissues
-
Regulate the synthesis of extracellular matrix proteins by osteoblasts, chondrocytes, and fibroblasts
-
Capacity to modulate cell survival via effects on apoptosis
-
In osteocytes, mechanical stretch
activates extracellular signal regulated kinases (ERKs) via effects on
integrins and the cytoskeleton.
-
-
Primary sensor of the mechanical force: matrix itself
-
-
Osteoblast—matrix interaction
-
Degradation products of type I collagen modulate the activity of osteoblasts.
-
Osteoblasts control local bone resorption by regulating the differentiation of osteoclasts via the RANKL-OPG axis (see later).
-
-
Osteoclast—matrix interaction
-
Osteoclast interactions with the matrix (via the sealed zone) are mediated via integrin binding.
-
Removal of bone by osteoclastic activity
may also lead to changes in the mechanical properties of bone; these
changes then serve to signal for increased new bone formation.
-
-
Microtubules: polymeric complexes of tubulin that play a crucial role in organizing the mitotic spindle
-
Actin filaments: polymers of actin that
are essential for cell movement and, in combination with myosin, for
contractility in myocytes -
Intermediate filaments: vimentin and vimentin-related proteins are most significant in connective tissues. They are:
-
Critical to the cell’s ability to resist mechanical stress
-
Particularly abundant in cells that need to stretch (epithelial cells, myocytes, fibroblasts)
-
-
Interactions between the cytoskeleton and
the ECM are modulated via interactions between matrix proteins and
membrane-tethered integrins. -
Integrins
-
Transmembrane heterodimers consisting of one α and one β subunit
-
Interact with RGD
(arginine-glycine-aspartate) sequences that are present in a variety of
extracellular matrix components, including collagen, fibronectin, and
laminin -
Alterations in integrin expression can modulate cell—ECM interactions.
-
Particularly important in the context of
tumor cell metastasis, in which it is known that expression of certain
integrins (e.g., αVβ3) is associated with an increased risk of skeletal
metastasis
-
-
-
Source
-
Derived from the mesenchymal cell lineage
-
-
Morphology and molecular markers
-
Variable morphology, with active cells being cuboidal and more quiescent (lining) cells having a flattened appearance (Fig. 13-7)
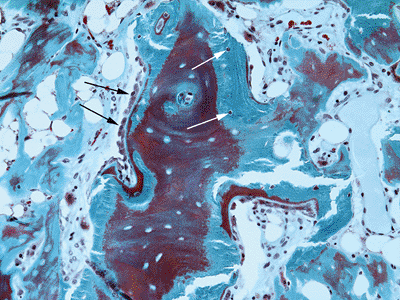 Figure 13-7 Osteoblast morphology. Active osteoblasts (black arrows) are seen as cuboidal cells that overlie the osteoid seam. Osteocytes (white arrows)
Figure 13-7 Osteoblast morphology. Active osteoblasts (black arrows) are seen as cuboidal cells that overlie the osteoid seam. Osteocytes (white arrows)
are flattened cells embedded in lacunae within the bone. Modified
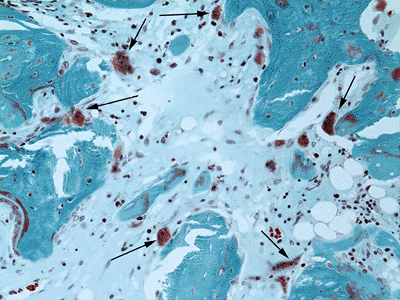
Masson-Goldner trichrome stain, 200 × original magnification.![]() Figure 13-8 Large, multinucleated osteoclasts (arrows) attached to and resorbing bone. Modified Masson-Goldner trichrome stain, 200 × original magnification.
Figure 13-8 Large, multinucleated osteoclasts (arrows) attached to and resorbing bone. Modified Masson-Goldner trichrome stain, 200 × original magnification.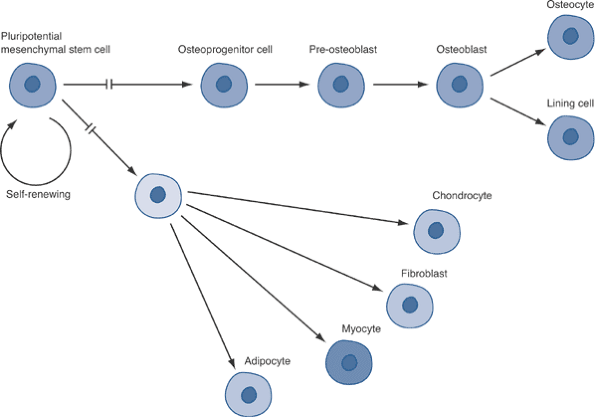 Figure 13-9
Figure 13-9
Differentiation pathways for mesenchymal cells. The potential for
proliferation decreases as cells move from left to right (i.e., with
advancing differentiation). -
No specific molecular markers, although osteocal-cin, osteonectin, and alkaline phosphatase are often used for this purpose
P.318 -
-
Function
-
Primary function: form mineralized bone matrix
-
Bone matrix (40% of the dry weight of bone) is first formed as nonmineralized osteoid containing:
-
Type I collagen (90% of the protein in bone)
-
Noncollagenous proteins such as proteoglycan, osteocalcin, osteopontin, osteonectin, bone sialoprotein, and thrombospondin
-
-
Bone mineral (60% of the dry weight of bone) is mainly hydroxyapatite, Ca10(PO4)6(OH)2.
-
-
Osteoblasts also control bone turnover via the RANKL-OPG axis (see later).
-
-
Source
-
Terminally differentiated form of the osteoblast
-
Formed as osteoblasts become encased in mineralized bone
-
-
Function
-
Osteocytes lie within lacunae but connect
with each other via long cellular extensions that run through channels
(canaliculae) in the bone.P.319Table 13-2 Key Factors in Regulation of Osteoclast Differentiation Via The Rankl-Opg AxisName Location Function Effect on Osteoclast Differentiation RANK Receptor activator of nuclear factor kappa-B (NF-κB) Surface receptor on osteoclast precursor cells Binding with RANKL activates upregulation of genes under transcriptional control of NF-κB Upregulation RANKL Receptor activator of nuclear factor kappa-B ligand Osteoblasts and stromal cells Binds to cognate receptor RANK Upregulation OPG Osteoprotegerin Soluble molecule Decoy receptor for RANKL; prevents binding of RANKL to RANK Downregulation -
Forms an extended network of interconnected cells, rather like a neuronal net
-
Osteocyte communication plays a critical role in the response of the skeleton to mechanical loading (or unloading).
-
-
Source
-
Derived from the hematopoietic cell lineage
-
Multinucleated cells formed by the fusion of mono-nuclear precursor cells
-
-
Morphology and molecular markers
-
Multinucleated cells (Fig. 13-8)
-
Osteoclast markers: tartrate-resistant acid phosphatase (TRAP) 5b, the vitronectin receptor (CD 51), and the calcitonin receptor
-
-
Function
-
Primary function: resorb mineralized bone
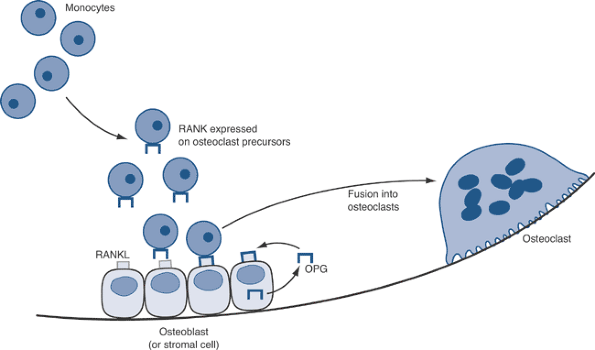
![]() Figure 13-10
Figure 13-10
The RANKL-OPG axis. Monocytes that are stimulated with M-CSF (via the
c-fms receptor) become osteoclast precursors. These cells bind RANKL on
the surface of local osteoblasts and/or stromal cells. Binding of RANKL
to its receptor, RANK, leads to NF-kB activation, gene transcription,
and the formation of functional multinucleated osteoclasts.
Osteoprotegerin (OPG), the decoy receptor for RANKL, antagonizes RANKL
binding to RANK, thereby blocking osteoclast formation. -
-
Cells attach to bone matrix via a complex of membrane invaginations, the so-called ruffled border.
-
Ruffled border has large surface area for secretion and absorption.
-
Sealed space contained within the ruffled border is acidified by the osteoclast’s proton pump.
-
Low pH favors the activity of secreted matrix metalloproteinases (MMPs) and lysosomal proteases such as cathepsin K.
-
-
-
Source: derived from mesenchymal cell precursors
-
Function: differentiation into one of two fates:
-
Growth plate chondrocytes (see Chapter 14)
-
Articular chondrocytes (see Chapter 15)
-
-
Morphology and molecular markers
-
Molecular markers
-
Articular chondrocytes: type II collagen
-
Proliferative growth plate chondrocytes: type I collagen
-
Hypertrophic growth plate chondrocytes: type X collagen
-
-
Chondrocytes are embedded within a water-rich matrix that contains both collagenous and noncollagenous proteins.
-
-
Source: derived from mesenchymal cell precursors
-
Function: Within the musculoskeletal system, fibroblasts are found in the bone marrow, synovial membrane, ligament, and tendon.
-
Morphology and molecular markers
-
Spindle-shaped morphology
-
Characterized by the expression of type I and type III collagen, as well as fibronectin
-
Tendon and ligament fibroblasts are
highly differentiated and express type I and type IX collagen, along
with biglycan and decorin.
-
-
Osteoblasts, osteocytes, chondrocytes, fibroblasts, myocytes, and adipocytes are all ultimately derived from a common precursor.
-
Exception: Osteoclasts are derived from hematopoietic cell line.
-
Commitment to one lineage rather than any other appears to depend on the expression of tissue-specific transcription factors:
-
Osteoblasts: Cbfa-1 and osterix
-
Chondrocytes: Sox proteins
-
-
Osteoclasts are derived from a circulating hematopoietic precursor.
-
Stimulation of monocytes precursors involves M-CSF, which binds to a specific surface receptor (c-fms).
-
Activated monocytes (now considered to be committed osteoclast precursor cells) fuse together to form the osteoclast.
-
The differentiation of osteoclast
precursors into mature, functional osteoclasts is controlled by several
regulatory pathways, of which the RANKL-OPG axis is best understood (Table 13-2; Fig. 13-10). -
Therapies that target the RANKL-OPG signaling pathway show promise in the treatment of osteoporosis and tumor osteolysis:
-
Options include monoclonal antibodies to RANKL or delivery of OPG as either recombinant protein or as a gene therapy product.
-
JA, Glimcher MJ, Cooper RR, et al. Bone biology. II: Formation, form,
modeling, remodeling, and regulation of cell function. AAOS Instr Course Lect 1996;45:387–399.