Embryology and Development of the Musculoskeletal System
is an unparalleled example of integrated cell behavior. The single cell
divides many times to produce the trillions of cells of the organism,
which form structures as complex and as varied as the eyes, limbs,
heart, and brain. This amazing achievement raises a multitude of
questions. How are the body’s specialized tissues and organs formed?
How do the different patterns form in the embryo and tell the different
parts what they should become? How do individual cells become committed
to particular developmental fates? Increased knowledge about
developmental biology comes from understanding how genes direct these
developmental processes. In fact, developmental biology has
become essential for understanding many other areas of biology and medicine.
microscopic anatomy, is fairly well described. Studies with
experimental animals, mainly the chicken and the mouse, have provided
insights into the relation between the tissues involved in normal
growth and differentiation. The molecular mechanisms underlying
developmental events are being discovered. An integration of the
approaches of genetics, molecular biology, developmental biology, and
evolutionary biology is taking place, resulting in an explosion in our
understanding of the importance of individual genes and of interactions
of cells and tissues in specifying the development of complex organisms
from single cells. One of the major reasons for the creation of and the
complementary relationship among these varying disciplines is the
existence of homology, both within organisms and among species. In
fruit flies, chickens, mice, and men, genes and their products are
often very similar in structure and function. For example, HOX
genes that convey body plan positional information are conserved among
species and are similar in structure and function, that is, they are
homologous. In complex organisms, the same gene is often used at
different stages of development and in different areas of the body to
perform similar functions.
genes. Humans have only 5 to 10 times as many genes as most bacteria.
The number of human genes presently identified at the completion of the
Human Genome Project appears to be only about 30,000, whereas the worm Caenorhabditis elegans, a simple metazoan, has 19,000 genes. What does increase dramatically as the complexity of an organism increases is the size of the genome. The human genome is 1,000 times larger than the typical bacterial genome. The density of genes decreases
with the increasing complexity of organisms, and, conversely, the
amount of nongene deoxyribonucleic acid (DNA) (both intra- and
intergene DNA) increases dramatically.
DNA has important regulatory roles. Biologic complexity increases more
by increasing the complexity of interactions of genes and gene products
than by increasing the number of novel genes (1). For example, major regulatory genes, such as the hedgehog genes, Wnt genes, and tgf-β
genes, undergo posttranslational modification that affects their
potency and diffusibility. Furthermore, these signaling proteins
undergo complex interactions with receptors and extracellular molecules
that both facilitate and inhibit their activity in complex feedback
loops. A large part of the complexity of signaling in multicellular
organisms occurs at the posttranscriptional level.
involve the evolution of new genes, arises from the creation of complex
proteins with multiple domains. Many proteins in complex organisms have
several similar domains and combinations of different domains. For
example, the fibroblast growth factor receptor 3 (FGFR 3)
gene (which causes achondroplasia and hypochondroplasia) encodes a
transmembrane protein with three immunoglobulinlike domains, two
tryosine kinase domains, and a transmembrane domain. Therefore, the
novel assembly of conserved and common gene products into proteins with
complex structures can be a complex process. These complex proteins
increase the number of possible protein–protein and protein–DNA
interactions, resulting in greater complexity of the signaling pathways
and information transfer. It is probable that there is a relation
between the large amount of unexpressed DNA and the development of
complex proteins with repeated domains. The unexpressed DNA regions
(introns) appear to facilitate the recombination of expressed DNA
regions (exons), thereby allowing the modular assembly of novel
proteins from existing “parts” (1).
identification of all human genes will mark, in many respects, the
beginning of our understanding of the process of complex interactions
that determine the development rather than the end of the story (2).
This is the major “theme” of this chapter—complex pathways of genes and
gene products recur in the development of the complex human organism,
and understanding these pathways forms the foundation of understanding
human development and the occurrence of disease.
development, followed by descriptive anatomy of limb development and
the formation of the vertebral column. It examines bone formation and
growth, and emphasizes the progress in the understanding of the
cellular and molecular mechanisms involved in these aspects of
development. Each section concludes with some observations that relate
developmental anatomy to the clinical problems faced by orthopaedic
surgeons.
embryonic period and the fetal period. During the embryonic period, the
body plan is completed and all major organs are established. The stages
of the embryonic period include fecundation, cleavage, gastrulation,
neurulation, and organogenesis. By the twelfth week of gestation, the
organism’s shape is fully formed and the remaining period of gestation
will involve growth and maturation of the organ functions.
divides in an ordered pattern to produce a ball of much smaller cells called blastula.
The cleavage of the zygote in mammals has several specific
characteristics. First, it is a relatively slow process. The divisions
occur approximately 12 to 24 hours apart. Second, there is a unique
orientation of the cells with relation to one another. The first
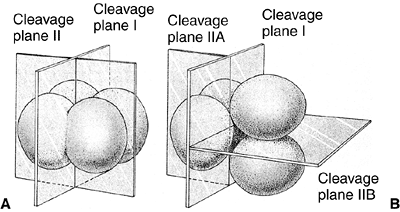
cleavage is a meridional division, but, in the second division, one
pair of cells divides meridionally and the other divides equatorially (Fig. 1.1). This type of cleavage is called rotational cleavage (3).
Third, there is an asynchrony in the early divisions. All cells do not
divide at the same time. Therefore, embryos do not increase evenly from
two- to four- to eight-cell stages but frequently contain an odd number
of cells. Fourth, the zygotic genome is activated early during cleavage
divisions to produce the proteins needed for the process to occur (4).
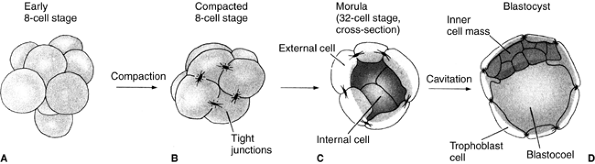
Finally, the most crucial difference between mammals and other species
is the phenomenon of compaction. At the eight-cell stage, blastomeres
form a loose arrangement of cells, but, after the third division, the
cells cluster together and form a compact ball, with the outside cells
being stabilized by tight junctions and the inner cells developing gap
junctions that allow the passage of small molecules and ions (Fig. 1.2).
 |
|
Figure 1.1 Comparison of early cleavage divisions in the sea urchin and in mammals. A: The plane of cell division in the sea urchin is perpendicular to the cells, whereas in mammals (B),
in the second division, one of the two blastomeres divides meridionally and the other divides equatorially. Early cell division in mammals is asynchronous—not all cells divide at the same time. (From Gilbert SF. Developmental biology, 3rd ed. Sunderland: Sinauer Associates, 1991, with permission.) |
 |
|
Figure 1.2 The cleavage of a mouse embryo up to the blastocyst stage. A: Early eight-cell stage with loose cell arrangement. B:
Compacted eight-cell stage. During the process of compaction, cells suddenly huddle together, maximizing their contacts. Tight junctions, which seal off the inside of the sphere, stabilize the outer cells. The inner cells develop gap junctions, thereby enabling the passage of small molecules and ions. C: Morula with differentiation between the external cells and the inner cell mass. D: Blastocyst before implantation. (From Gilbert SF. Developmental biology, 3rd ed. Sunderland: Sinauer Associates, 1991, with permission.) |
stage, and each of its cells can form any part of the later embryo or
adult. One example is seen in the development of a pair of identical
twins from a single fertilized egg. Similarly, this potential of the
embryonic cell can be demonstrated experimentally by using chimeras.
Chimeras are animals made by combining individual cells from early
embryos of genetically different strains of animals. The reaggregated
cells are then implanted in foster mothers. An analysis of the genetic
composition of the tissues of the developed animal shows that the
single cells form the four-cell stage, which can participate in forming
many different parts of the animal. These cells are said to be
totipotent (5,6).
generation of the cells that will form the placenta and the membranes
that surround the developing embryo. The cells of the compacted embryo
divide to produce a 16-cell morula. This morula consists of a small
group of internal cells (one or two) surrounded by a larger group of
external cells (7) (Fig. 1.2C).
The position of a cell at this stage determines whether it will form
extraembryonic structures or contribute to the embryo proper. The inner
cells will form the embryo and most of the external cells will form the
trophoblast. This structure will enable the embryo to receive oxygen
and nourishment from the mother, and will secrete hormones and
regulators of the immune response so that the mother will not reject
the embryo (8). By the 64-cell stage, the inner
cell mass and the trophoblast have become separate cell layers, neither
of which contributes cells to the other group. The establishment of a
distinction between these two cell types, therefore, represents the
first differentiation event in mammalian development.
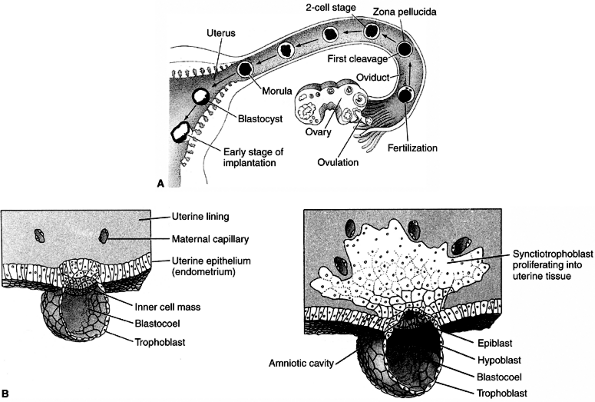
At the time of implantation, the exposed surface of the uterine lining,
the endometrium, is a single-layered epithelial sheet that forms
numerous tubular glands. Having adhered to the epithelium, the
trophoblastic cells penetrate it and erode it (Fig. 1.3B). The endometrium responds with a dramatic increase in vascularity and capillary permeability, the so-called decidual reaction (Fig. 1.4).
These processes are apparently mediated by estrogens produced by the
blastocyst and by the estrogen receptors in the wall of the uterus.
remarkable process in which the ball of cells of the blastula turns
into a multilayered structure and rearranges itself to form three
embryonic tissue layers known as endoderm, ectoderm, and mesoderm.
In addition, during gastrulation, the body plan of the organism is
established. Gastrulation, therefore, involves dramatic changes in the
overall structure of the embryo, converting it into a complex
three-dimensional structure.
 |
|
Figure 1.3 A: Development of human embryo from fecundation to implantation. B:
Tissue formation of human embryo between days 7 and 8. The inner cell mass will give rise to the embryo proper and the trophoblast to the placenta. The distinction between those two groups of cells represents the first differentiation event in embryonic development. (From Gilbert SF. Developmental biology, 3rd ed. Sunderland: Sinauer Associates, 1991, with permission.) |
understood. In sea urchins and insects, the phenomenon of gastrulation
is similar to what happens if a ball is punctured and then kicked: the
ball collapses and the inner surface on one side makes contact with the
other side, making a large dimple. In the embryo, by a complicated
invagination, a large area of cells on the outside of the embryo is
brought to lie inside it. Subsequent development of the embryo depends
on the interactions of the outer ectoderm, middle mesoderm, and inner
endoderm layers of cells. The ectoderm will give rise to the epidermis
of the skin and the nervous system; the mesoderm will give rise to
connective tissues including the bones, muscles, and blood; and the
endoderm will give rise to the lung and the lining of the gut and
associated organs.
positioned according to the body plan that is appropriate to the
species, and there is a process of differentiation of the functional
characteristics required of each part of the body plan. The
specification of the axes in mammals does not involve any maternal
component. The dorsoventral (DV)
axis
is established by the interaction between the inner cell mass and the
trophectoderm, whereas the anteroposterior (AP) axis may be set only at
implantation. The generation of the left-right asymmetry is under
genetic control. This vertebrate body plan will be maintained
thereafter as the embryo grows.
 |
|
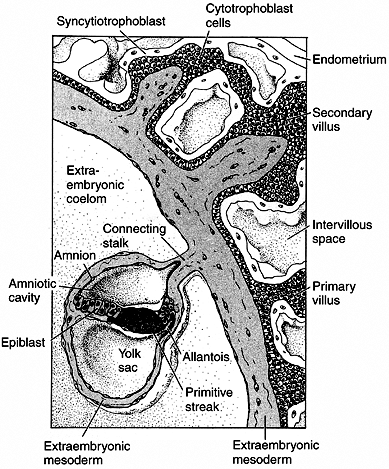
Figure 1.4
Placenta development in human embryo at the end of the third week of gestation. The trophoblast cells forming the placenta are coming into contact with the blood vessels of the uterus. The endometrium responds with a dramatic increase in vascularity and capillary permeability, the so-called decidual reaction. The trophoblast divides into the cytotrophoblast, which will form the villi, and the syncytiotrophoblast, which will ingress into the uterine cavity. The actual embryo forms from the cells of the epiblast. (From Gilbert SF. Developmental biology, 3rd ed. Sunderland: Sinauer Associates, 1991, with permission.) |
entire embryo. Cell migration in one part of the embryo must be
intimately coordinated with other cell movements occurring
simultaneously elsewhere. However, gastrulation depends on a relatively
simple repetition of basic cell activities: cells can change their
shapes by extending or contracting; they can group together or separate
by forming or breaking their adhesions with neighboring cells or with
the extracellular matrix; and they can secrete extracellular matrix
that constrains or guides their location or movement. These activities,
together with cell proliferation, underlie almost all morphogenetic
activities during gastrulation.
suggested that the maternal and paternal genomes (imprinting) have
different roles during mammalian gastrulation. Mouse zygotes can be
created that have only sperm-derived or only oocyte-derived
chromosomes. The sperm-derived embryos die without structures of the
embryo proper and with well-formed chorionic structures. Conversely,
the oocyte-derived embryos develop normally but without chorionic
structures (9,10). This observation has also been confirmed by using mouse chimeras (11). Therefore, the maternal and paternal genomic information may serve distinct functions during early development.
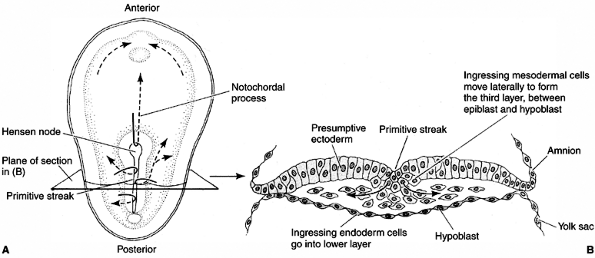
and endoderm—move to the positions where they develop into the
structures of the adult organism. The AP body axis of the vertebrate
embryo emerges, with the head at one end and the future tail at the
other. During the next stage of development, the main organs of the
body begin to emerge gradually (12). A major
set of interactions takes place between the mesodermal cells and the
ectoderm in the dorsal midline (Hensen node), and the ectoderm cell
layer will form the nervous system (Fig. 1.5).
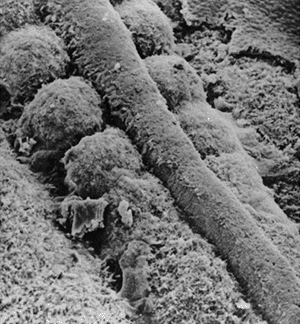
At the same time, the mesoderm on either side of the middle breaks up
into blocks of cells to form the somites, a series of repeated segments
along the axis of the embryo (Fig. 1.6) (13).
The interaction between the dorsal mesoderm and its overlying ectoderm
is one of the most important interactions in the entire process of
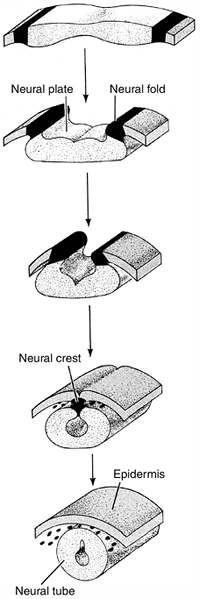
development. The process by which the flat layer of ectodermal cells is
transformed into a hollow tube is called neurulation (Fig. 1.7).
The first indication of neurulation is a change in cell shape in the
ectoderm. Midline ectodermal cells become elongated, whereas cells
destined to form the epidermis become flattened. The elongation of the
cells causes this region to rise above the surrounding ectoderm,
thereby creating the neural plate. Shortly thereafter, the edges of the
neural plate thicken and move upward to form the neural folds that will
subsequently fuse to form the neural tube beneath the overlying
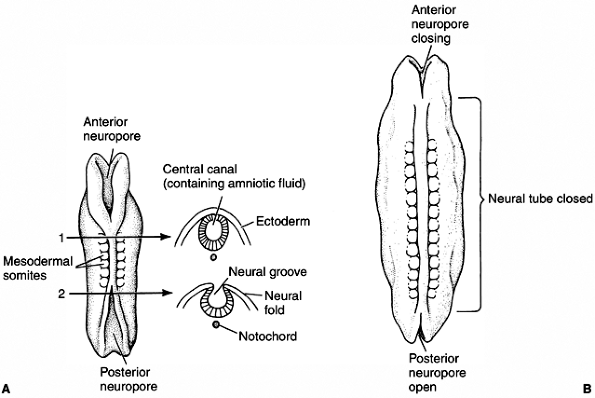
ectoderm. The formation of the neural tube starts near the anterior end
of the embryo and proceeds anteriorly and posteriorly. The open ends
are called anterior and posterior neuropores (Fig. 1.8).
In mammals, the failure of the anterior neuropore to close results in
anencephalia, and the failure of the posterior neuropore to close leads
to spina bifida. Nowadays, neural tube defects (NTDs) can be detected
during pregnancy by ultrasonography and chemical analysis of the
amniotic fluid.
changes in the shape of cells that are generated by the cytoskeleton
(microtubules and microfilaments). Differential cell divisions seen in
different regions of the neural plate also contribute to the size and
shape of this region. Cells directly adjacent to the notochord and
those cells at the hinges of the neural groove also help mold the
neural tube. Separation
of
the neural tube from the ectoderm (that will form the skin) requires
changes in cell adhesiveness. Although molecules that can induce the
formation of neural tissue, such as noggin protein, have been
identified, the induction of neurulation is caused by the inhibition of
bone morphogenetic protein (BMP) activity. The positional identity of
cells along the AP axis is encoded by the combinatorial expression of
genes of the four HOX complexes.
 |
|
Figure 1.5 Cell movements during the gastrulation stage. A:
Cells migrating through the Hensen node travel anteriorly to form the notochord. Cells traveling through the primitive streak will become the precursors of mesoderm and endoderm. B: Transverse section of the embryo. (From Gilbert SF. Developmental biology, 3rd ed. Sunderland: Sinauer Associates, 1991, with permission.) |
 |
|
Figure 1.6
Scanning electron microscopy showing the neural tube and the well-formed somites with paraxial mesoderm that has not yet separated into distinct somites. (Courtesy of K.W. Tosney.) |
will form the neural crest. These cells will migrate throughout the
embryo and will give rise to several cell populations—the neurons and
supporting glial cells of the sensory, sympathetic, and parasympathetic
nervous systems; the melanocytes of the epidermis; and the cartilage
and connective tissue components of the head. The mechanisms of neural
crest migration are not random but follow precise pathways specified by
the extracellular matrix. Differences in adhesiveness between the
anterior and posterior halves of the somites inhibit the neural crest
from migrating over the posterior halves. Presumptive dorsal ganglia
cells collect adjacent to anterior halves, giving them a segmental
arrangement.
simultaneously with neural tube development. Five regions of mesoderm
can be identified at the neurula-stage embryo—chordamesoderm, dorsal
(somitic) mesoderm, intermediate mesoderm, lateral plate mesoderm, and
head mesoderm (Fig. 1.9). The chordamesoderm
will generate the notochord, a transient organ whose functions include
inducing neural tube formation and establishing the body axis. The
dorsal (somitic) mesoderm will produce many of the connective tissues
of the body. The intermediate mesoderm will form the urinary system and
genital ducts. The lateral plate mesoderm will give rise to the heart,
blood vessels, and blood cells, and the body lining cavities. The head
mesoderm will contribute to the connective tissues and muscles of the
face.
and other organs are determined. Although the positions of the organs
are fixed, overt signs of differentiation are not yet visible. Each
region has, however, considerable capacity for regulation, so that, if
a part of the region is removed, a normal structure can still form.
 |
|
Figure 1.7
Diagrammatic representation of neural tube formation. The ectoderm folds in at the most dorsal point, forming a neural tube that is connected by neural crest cells, and an outer epidermis. (From Gilbert SF. Developmental biology, 3rd ed. Sunderland: Sinauer Associates, 1991, with permission.) |
specialized structures from an initially simple group of cells. The
cells of the body are genetically alike (i.e., they all have the same
DNA content) but are phenotypically different—some are specialized as
muscles, others as neurons, and so on. During development, differences
are generated between cells in the embryo, and these differences lead
to spatial organization, changes in form, and the generation of
different cell types. All these features are ultimately determined by
the DNA sequence of the genome. Each cell must act according to the
same genetic instructions, but it must interpret the instructions with
regard to time and space.
generated by a limited repertoire of cell activities. Similar to an
artist who moves from one part of a sculpture to another to achieve
first the overall shape of the figure and then the specific anatomic
features, using a selected number of instruments over and over again,
Nature also displays a comparable economy in choosing the processes and
molecular tools. The key to understanding development lies in cell
biology, in the processes of signal transduction, and in the control of
gene expression that results in changes of cell state, movement, and
growth. The most important fact in development is based on the
surprising finding that the developmental control genes are maintained
throughout the process of evolution. Therefore, for many genes
discovered in invertebrates, homolog genes have been identified in
vertebrates, and they have similar developmental roles in species
ranging from the fruit fly to fish to mouse to human.
processes, although they overlap with, and influence, one another
considerably. These are the emergence of pattern, cell differentiation,
and change in form or morphogenesis.
temporal arrangements of cell activities are organized within the
embryo so that a well-defined structure develops. Pattern formation is
crucial for the proper development of every part of the organism. In
the developing limb, for example, pattern formation enables the cells
to know whether to make the upper arm or the fingers, and where the
muscles should form.
mechanism by which the cells first acquire a positional identity, which
determines their future behavior. The ability of cells to sense their
relative positions within a limited population of cells and to
differentiate according to this position has been the subject of
intense research. Interestingly, pattern formation in many systems has
similar principles and, more strikingly, similar genes. Many of the
so-called homeotic genes that determine segment identity in Drosophila
(fruit flies) are present in vertebrates and appear to play similar
roles in the segmentation of structures such as the brain or the
vertebral column.
switches of railroad yards that direct trains onto one set of tracks
rather than another. Homeotic genes are involved in specifying regional
identity along the AP axis. The name “homeotic” comes from the fact
that mutations
in some of these genes result in what is called a homeotic transformation, in which one body structure replaces another. For example, in mice in which Hoxd-11
is mutated, the anterior sacral vertebrae are transformed into lumbar
vertebrae. Homeotic genes in all systems work similarly: they code for
proteins called transcription factors that control gene expression. In vertebrates and Drosophila,
the order of homeotic genes on the chromosome corresponds to their
temporal and spatial expression on the AP axis of the embryo.
 |
|
Figure 1.8 Neural tube formation in human embryos does not occur simultaneously throughout the ectoderm. A: At the initial stages, both anterior and posterior neuropores are open. B:
Closing of the neural tube proceeds both cranially and caudally. Failure to close the posterior neuropore at day 27 results in spina bifida, the severity of which depends upon how much of the spinal cord remains open. Failure to close the anterior neuropore results in lethal anencephaly. (From Gilbert SF. Developmental biology, 3rd ed. Sunderland: Sinauer Associates, 1991, with permission.) |
 |
|
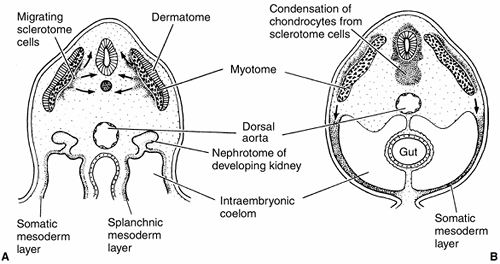
Figure 1.9 Mesoderm formation in human embryo. Diagram of a transverse section through the trunk of an early 4-week embryo (A) and a late 4-week embryo (B).
Sclerotome cells migrate from the somite, and these cells ultimately become chondrocytes. The remaining dermatome cells will form the dermis. The myotome will give rise to the striated muscles of the back and limbs. (From Gilbert SF. Developmental biology, 3rd ed. Sunderland: Sinauer Associates, 1991, with permission.) |
become structurally and functionally different from one another, ending
up as distinct types, such as muscle, bone, or cartilage. Because each
cell of the organism has the same genetic material, the achievement and
persistence of the differentiated state depends on a series of signals
that ultimately control the transcription of specific genes. In humans,
the zygote gives rise to about 250 clearly distinguishable types of
cells.
number of discrete kinds of cells, each with its particular repertoire
of biochemical activities and possible morphologic configurations. When
cells achieve a distinctive state of differentiation, they do not
transform into cells of another type. Differentiation leads to a
stable, irreversible set of cellular activities. At the organ level,
once an embryonic part is capable of realizing its prospective fate in
the absence of the conditions that established that capability, the
part is said to be determined. Pattern formation and cell
differentiation are very closely interrelated, as can be seen by
considering the difference between the upper and lower extremities.
Both contain the same tissues—muscle, cartilage, bone, and so on—yet
the patterns in which they are arranged are different. Essentially, it
is pattern formation that makes human beings different from rabbits or
chimpanzees.
the earliest stages of embryogenesis—the setting up of such basic
elements of body pattern as the head-to-tail and DV axis—also regulates
morphogenesis. These molecules and pathways have been conserved over
the course of evolution. Morphogenesis relies on a rather restricted
number of cellular activities and encompasses the formation of all
tissues and organs from the first embryonic tissue layers to the
completed limb, spine, or brain. However, before any tissue or organ
can form, some earlier steps must occur, steps that tell cells what
they are and what tissues they should form. These early steps take
place in the “control room” for development; morphogenesis is then what
happens on the “factory floor”—the actual assembly of the tissues and
organs that make up the organism. In addition, spatial patterns of cell
proliferation, folding of cell groups, rearrangement of cells, and cell
migration make important contributions to morphogenesis, the process
that shapes the embryo. Finally, as the embryo develops, cells begin to
differentiate, and this process culminates in the specialization of
cells for particular functions. Therefore, during development,
morphogenesis gives rise to structures appropriate to their position
within the embryo, and, within these structures, the differentiation of
individual cells and their interactions are spatially ordered.
abnormalities occur in 6% of all live births. Twenty percent of infant
deaths are caused by congenital anomalies. Approximately 3% of newborns
have significant structural abnormalities. At present, the causes of
approximately 50% to 60% of birth defects are unknown; chromosomal
abnormalities account for 6% to 7%; specific gene mutations cause 7% to
8%; environmental teratogens are responsible for 7% to 10% of defects;
and combined genetic predisposition with environmental factors causes
the remaining 20% to 25% of congenital abnormalities.
the new cells and tissues needed to provide an organism with its
correct complement of organs. Many of the molecules and pathways known
to control cell differentiation and growth during organ formation in
the embryo do not become obsolete in the adult. They help maintain and
repair tissues and regulate their response to signals from the external
environment. Some of these proteins are, or soon will be, in clinical
use, such as erythropoietin, which triggers red blood cell production;
platelet-derived growth factor (PDGF), for management of diabetic skin
ulcers; and BMPs for bone and cartilage regeneration. In malignant
disease, the control of cell activities, such as proliferation,
differentiation, and migration, appears to break down. An understanding
of the way in which cell behavior is coordinated in embryos could
therefore give insights into bone and cartilage regeneration and cancer
biology.
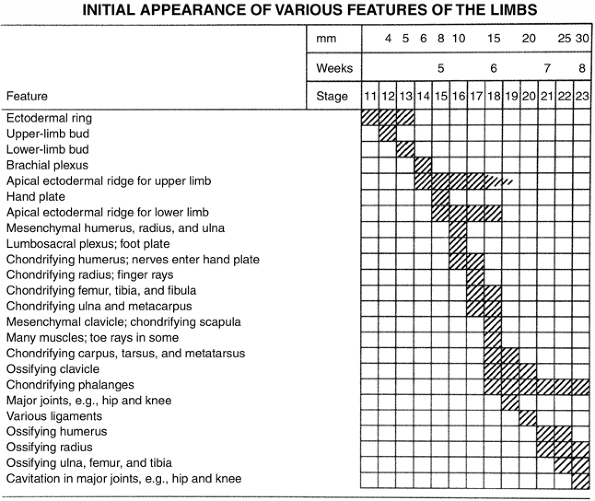
evident as a slight elevation on the ventrolateral body wall at the
level of the pericardial swelling. The lower limb elevation appears 2
days later just caudal to the level of the umbilical cord and develops
similarly to, but slightly later than, the upper limb (Fig. 1.10).
By this time, the neural tube is closed, all somites are present, and
the anlagen of the vertebrae and intervertebral discs are present. The
limb bud initially consists of loose mesenchymal tissue enclosed in an
epithelial ectodermal sheath. The skeletal elements and tendons develop
from the lateral plate mesenchyme, whereas the limb muscle arises from
somitic mesenchymal cells that migrate into the limb bud.
(PZ). Proliferation of these cells causes limb outgrowth. Cells begin
to differentiate only after leaving the PZ. The interaction between the
AER and the PZ is crucial for limb development. Experimental procedures
on chicken embryos reveal the following about the limb bud mesenchyme:
(a) if removed, the limb does not develop, (b) when grafted under the
ectoderm at a location other than the normal limb area, an AER is
induced and a limb will develop, and (c) lower limb mesoderm will
induce leg formation when placed under an upper limb AER. Grafting
experiments with the AER reveal that AER removal aborts further limb
development. The later the stage of limb development when the AER is
removed, the less severe is the resulting limb truncation (limb
elements develop from proximal to distal).
In
addition, AER grafting experiments show that an extra AER will induce a
limb bud to form supernumerary limb structures; and nonlimb mesenchymal
cells placed beneath the AER will not result in limb development, and
the AER withers (14).
 |
|
Figure 1.10 Timing of the appearance of limb features. (From Thorogood P. Embryos, genes and birth defects. New York: John Wiley & Sons, 1997:350, with permission.)
|
is necessary for the growth and development of the limb, and the limb
bud mesenchyme induces, sustains, and instructs the AER. In addition to
exerting a biochemical influence on the PZ, the tightly packed columnar
cells of the AER perform a mechanical function, directing limb shape by
containing these undifferentiated cells in a dorsoventrally flattened
shape. The length of the AER controls the width of the limb as well.
When all limb elements have differentiated, the AER disappears.
small finger), dorsoventral (DV, back of hand to palm), and
proximal-distal (PD)—are specified very early in limb development. The
AP and DV axes are fixed before morphologic differentiation of limb
components occurs. The PD axis is determined as the limb grows out. The
AP axis is set first, followed by the DV axis and then by the PD axis.
This progression has been shown by the rotation of transplanted limb
buds from their normal position and by the finding that, at different
stages, the limb bud retains the axis orientation of its original limb
position or develops the orientation of the host bud (11,15).
If this tissue is grafted onto the anterior aspect of a limb bud, a
duplication of digits that are a mirror image to the normally present
digits occurs (16). The cells for the new
digits are recruited from the underlying mesoderm, and the distal part
of the limb widens, as does the AER. If less tissue from the polarizing
region is grafted, fewer new digits develop (17).
This and other experiments suggest that a morphogenetic gradient of a
diffusible signal originating from the ZPA determines the AP axis. This
will be discussed in the section on the molecular biology of limb
development.
and the ectoderm of the limb bud at different stages of development.
The mesoderm specifies the axis initially, but, soon after limb bud
formation, the ectodermal orientation becomes preeminent. If the
ectoderm of a right limb bud is transplanted onto the mesenchyme of a
left limb bud, the distal limb that develops will be that of a right
limb with respect to muscle pattern and joint orientation (15).
 |
|
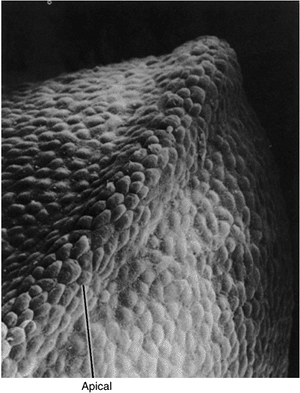
Figure 1.11
Scanning electron photomicrograph of an early chick forelimb bud with the apical ectodermal ridge at the tip of the limb bud. (Courtesy of K. W. Tosney.) |
a mesodermal cell remains at the tip of the limb bud in the PZ under
the influence of the AER. Once a cell leaves the tip, its position in
the limb is fixed. Young tips grafted on older limb buds will duplicate
existing limb elements, whereas older tips grafted on young buds will
form only distal elements. The best hypothesis to explain how this
information is passed is that the number of rounds of cell division
that occurs while under the influence of the AER determines the PD fate
of a cell. The support for this hypothesis comes from experiments in
which the limb bud is irradiated. The surviving cells of the irradiated
tip have to undergo several extra rounds of mitosis before they can
escape the influence of the AER and, thereby, gain positional
determination. In these experiments, intermediate limb elements are not
formed—just the preexisting proximal elements and the newly formed
distal elements (18).
undifferentiated mesenchymal cells in the limb bud results from
different signals than those conveying the axis and positional
information as described in the preceding text. The center of the limb
bud undergoes a condensation of cells that prefigures the skeletal
elements. This is the chondrogenic core, which begins at the body wall
and progresses distally with limb elongation. A rich vascular bed
surrounds the chondrogenic core. Immediately adjacent to the vascular
bed is a thick avascular zone that extends to the ectodermal sheath of
the limb bud. Although the signaling mechanism has not yet been
discovered, the ectoderm appears to control initial mesodermal
differentiation by maintaining the adjacent mesenchymal cells in a
flattened configuration, thereby preventing their differentiation into
chondrogenic cells. The central mesenchymal cells assume a rounded
shape and form the chondrogenic core (17,19).
This process of differentiation occurs proximally to distally. Early in
the seventh week, the cartilage anlage of the entire upper limb
skeletal elements, except the distal phalanges, is present.
Paddle-shaped hand plates are formed by the end of the sixth week and
condensations of cells form identifiable digital rays in the hand. The
same is true of the foot; the process occurs 1 week later. The cells
between the digital rays represent a loose mesenchyme that undergoes
programmed cell death (apoptosis) to create the separated fingers and
toes.
future skeletal structures and the vascular bed, the ingrowth of nerves
occurs, which is immediately followed by the development of muscle
tissue. All bones are prefigured in mesenchyme, followed by cartilage,
and then bone. The actual bone appears toward the end of the embryonic
period, first in the clavicle, mandible, and maxilla, between the sixth
and seventh weeks. Ossific centers appear soon after in the humerus,
then in the radius, the femur, the tibia, and finally in the ulna. Just
before birth, ossific centers appear in the calcaneus, talus, cuboid,
and distal femoral epiphysis, and proximal tibial epiphysis.
patterning of the vasculature are not clearly defined. Vascular cells
are believed to have an intrinsic capacity to form vessels and
branches, which is controlled by inhibitory signals extrinsic to the
angiotropic tissues. Well-developed veins appear on the postaxial
border of the limb buds and persist as the fibular and saphenous veins,
thereby permitting identification of the embryonic postaxial border
even in mature organisms. The early preaxial veins, such as the
cephalic and great saphenous veins, develop secondarily. The initial
arterial supply to the limb bud organizes into a single axial artery.
In the arm, this artery becomes the subclavian, axillary, brachial, and
anterior interosseous arteries. In the leg, the axial artery comes from
the umbilical artery and becomes the inferior gluteal, sciatic,
proximal popliteal, and distal peroneal arteries. The femoral and
tibial arteries develop secondarily.
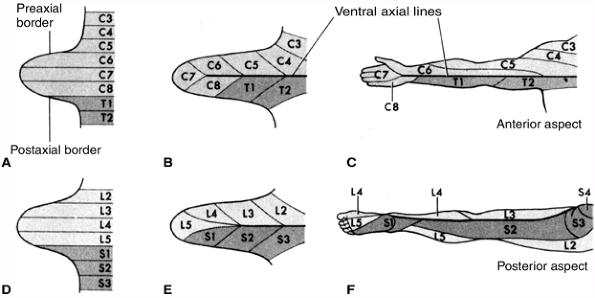
peripheral nerves are present by the fifth week. These plexuses
progressively invade their target tissues, and by the seventh week,
innervate the muscles and cutaneous tissues in the adult pattern. Each
dermatome represents a single dorsal root’s sensory fibers. From
cranial to caudal ends, the dermatomes of the limbs descend along the
preaxial border and ascend along the postaxial border of
the
limb. Overlapping and variability among individuals make the assessment
of dermatomal sensation nonspecific for single nerves (Fig. 1.12).
 |
|
Figure 1.12 Development of the dermatome pattern in the limb. A and D: Diagram of the segmental arrangement of dermatomes in the fifth embryonic week. B and E: The pattern is shown 1 week later as the limb bud grows. C and F:
The mature dermatome pattern is shown. The original ventral surface becomes posterior in the mature leg and anterior in the mature arm due to the normal rotation of the limbs. (From Moore KI, Persaus TVN. Before we are born. Essentials of embryology and birth defects, 4th ed. Philadelphia: WB Saunders, 1993:266, with permission.) |
migrate from the somatic layer of the lateral mesoderm during the fifth
week and surround the chondrogenic core of the limb bud. They develop
into dorsal and ventral groups from an undifferentiated mass, and
individual muscles gradually become distinct, again in a PD sequence.
Most anatomically distinct adult muscles are identifiable in the eighth
week. Mesenchymal cells develop into myoblasts (activation of a single
gene, MyoD1, is sufficient to turn a
fibroblast into a myoblast phenotype in tissue culture), which then
elongate, form parallel bundles, and fuse into myotubes.
Muscle-specific contractile proteins, actin and myosin, are
synthesized, and the myotubes form sarcomeres. By the eighth week, both
myotube development and innervation are sufficiently advanced for
movement to begin. By 12 weeks, the cross-striations of the myofibrils
are apparent in the cytoplasm of the myotube. Most muscle cells are
formed before birth, with the remaining cells developing in the first
year of life. The enlargement of muscles is caused by an increase in
cell diameter, with the creation of more myofilaments and elongation
along with the growth of the skeleton. Ultimate muscle size results
from genetic programming, exercise, and the hormonal milieu.
sixth week of development. A condensation of cells where the joint
develops is called the interzone. The
interzone cells differentiate into chondrogenic cells, synovial cells,
and central cells. The chondrogenic cells are adjacent to the
mesenchymal cells and form the articular cartilage. The central cells
form the intraarticular structures. The synovial cells differentiate
into both the tough fibrous capsule and the loose, vascular synovium.
Programmed cell death (apoptosis) leads to the cavitation that produces
the joint per se. Motion is necessary for
normal joint development, as is demonstrated by the host of conditions
that cause arthrogryposis, as well as by animal experiments that show
that joint anomalies can be created by paralyzing the developing fetus.
with parallel axes. The preaxial borders are cephalad and the postaxial
borders are caudad. The thumb and hallux are preaxial; the radius and
tibia, and ulna and fibula are homologous bones occupying the same
positions in the limb bud. The longitudinal axis at this stage passes
through the long finger and the second toe. During the fetal period,
the upper limb rotates 90 degrees externally (laterally) and the lower
limb rotates 90 degrees internally (medially). The forearm flexors come
to lie medially, and the forearm extensors, laterally. The leg
extensors lie ventrally, and the leg flexors, dorsally (Fig. 1.13).
biology and molecular genetic techniques has revealed much about how
the activation of individual genes at specific moments in development
causes the events that create complex organisms from single cells. The
mechanisms for differentiation and patterning are remarkably conserved
from
fruit flies to chickens to mice to humans. The limb is one of the best
studied body structures, and much information is available from the
study of animals, especially chickens, amphibians, mice, and fruit
flies. Much knowledge can be inferred from the observations that
certain genes and gene products are present at crucial moments in
development.
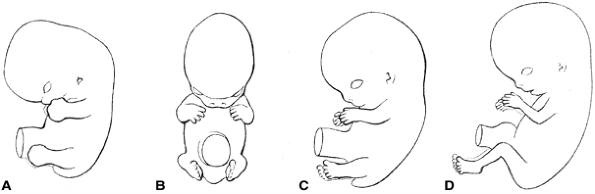 |
|
Figure 1.13 Normal limb rotation is depicted. A: At 48 days, the hand and foot plates face each other. B: At 51 days, elbows are bent laterally. C: At 54 days, the soles of the feet face each other. D:
The lateral rotation of the arms and medial rotation of the legs result in caudally facing elbows and cranially facing knees. (From Moore KI, Persaus TVN. Before we are born. Essentials of embryology and birth defects, 4th ed. Philadelphia: WB Saunders, 1993:265, with permission.) |
capacity to form limbs prior to any visible appearance of limb buds.
This has been demonstrated by transplantation experiments in chicken
embryos. The molecular signals specifying these cells are not known.
Early after specification, however, four genes of the tbx family of transcription regulators are expressed. Two of these genes specify hindlimb versus forelimb identity. Tbx4 is expressed only in the pre-hindlimb cells and tbx5 is found only in the pre-forelimb tissue (20). Pitx1, a homeobox-containing gene, acts prior to tbx4 and is also involved in hindlimb specification (20).
FGF-8) are the critical FGFs expressed during the initiation of limb
bud outgrowth. FGFs are a group of similar proteins that affect cell
proliferation, differentiation, and motility. During development, they
each play a role in mediating mesenchymal-epithelial tissue
interaction. FGF-10 is expressed first in
the lateral plate mesoderm at the site of the future limb bud. FGF-10
induces FGF-8 expression in ectodermal cells that will become the AER.
Some experiments suggest that FGF-8 and FGF-10 act in a positive
feedback loop; that is, the expression of each supports and promotes
the expression of the other. Mice in which FGF-10 function is
eliminated develop normally except for the complete absence of limbs
and failure of normal pulmonary development (21). Knockout of the gene encoding FGFR 2b, to which FGF-10 binds, similarly results in limblessness or severe limb truncation (22).
FGFR 2b is expressed only in the AER in the early limb, suggesting that
a major function of FGF-10 is limb ectoderm growth stimulation and
regulation. In addition to the FGFs and tbx genes, Wnt3a
plays a crucial role in early limb induction, maintenance of the AER,
and PD outgrowth, through interactions that have not yet been fully
elucidated.
gland and was named for its ability to stimulate the growth of
fibroblasts in a culture medium. So far, 22 different proteins have
been identified as belonging to the FGF
gene superfamily. FGFs participate in a wide variety of biologic
actions. These proteins play a crucial role in early embryonic
development and patterning of the lung, limb, and brain (in concert
with many different growth factors), and also in tumorigenesis,
angiogenesis, and wound healing. Four FGFR
genes have been identified, with 7 isoforms because of varying
ribonucleic acid (RNA) splicing. Several FGFs may bind to any
particular FGFR, but the strength of the binding is specific for a
given FGF and a given isoform of FGFR. The activation of FGFRs sends
signals to various downstream genes including src kinase, phospholipase C, PI3 kinase, and Stat1 (23).
Subsequently, retinoic acid was identified in the limb bud, in a high
concentration posteriorly and a low concentration anteriorly. Retinoic
acid on filter paper, placed anteriorly, induces mirror image digits to
those formed from the natural posterior gradient (25).
Nevertheless, retinoic acid is not a simple morphogen acting through a
gradient. Bathing an entire limb bud in retinoic acid should eliminate
the gradient, but instead, mirror image reduplication occurs. Also,
when retinoic acid concentration is at a minimum, the polarizing
activity of the ZPA is at a maximum. The action of retinoic acid is
complex and includes several classes of nuclear retinoic acid receptors
and retinoic-acid–binding proteins in the cytoplasm. These different
receptors and binding proteins are present in different amounts in
various parts of the developing limb, thereby suggesting a very complex
role for retinoic acid. If retinoic acid synthesis is
blocked,
limb bud outgrowth either does not occur or is severely stunted. At
present, it appears that retinoic acid acts by regulating the cells
that can express a protein called sonic hedgehog (26). It is likely that an HOX gene is an intermediate that is stimulated by retinoic acid, and the HOX
gene expression specifies the sonic hedgehog-expressing cells. If
retinoic acid receptors are blocked after sonic hedgehog expression has
been established, limb malformations still occur, suggesting some
additional role for retinoic acid in limb patterning perhaps through
its effect on HOX genes (26).
It also appears that the AER ectoderm modulates the formation of
cytoplasmic retinoic acid-binding proteins (CRABPs). CRABPs may act to
limit the amount of retinoic acid available to bind to nuclear
receptors, thereby limiting the differentiation-promoting effects of
retinoic acid on the undifferentiated mesenchymal cells of the limb
bud. This effect may be part of an interaction between AER and
mesenchymal cells that is necessary for both AER maintenance and
continued limb outgrowth, without premature specification of limb
elements such as cartilage (27).
normal development but also because it is a powerful teratogen. Various
retinoids, which are derivatives of vitamin A, have been used in
pharmacologic doses mainly to treat dermatologic conditions such as
acne, psoriasis, exfoliative dermatitis, and disorders of
keratinization. The retinoids have been associated, in animals and
humans, with multiple different birth defects and with the systemic
usage of retinoids. In addition, pregnancy is not recommended for up to
2 years after discontinuing their systemic use because of the prolonged
elimination half-life of these compounds (28).
as anemia and hyperlipidemia, may occur with systemic retinoid use.
These problems usually resolve with discontinuation of retinoid
therapy. Rheumatologic complications of retinoid use include
hyperostosis, arthritis, myopathy, vasculitis, and a condition
mimicking seronegative spondyloarthropathy (29).
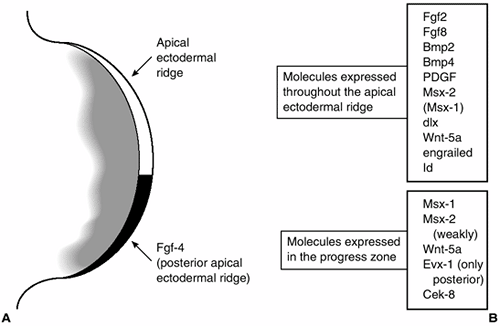 |
|
Figure 1.14
A diagram of the tip of the limb bud showing the apical ectodermal ridge and progress zone and some of the molecules that are expressed in these tissues. (From Thorogood P. Embryos, genes and birth defects. New York: John Wiley & Sons, 1997:109, with permission.) |
band of cells at the limb bud tip, lying between the dorsal and ventral
limb ectoderm. Engrailed-1 (En-1) is a homeobox-containing a
transcription factor whose expression is limited to the ventral limb
ectoderm (30). Radical fringe (R-fng) is a secreted factor that modulates the signaling that is expressed only in the dorsal ectoderm (31). Radical fringe is a homolog of the Drosophila gene fringe, which helps specify DV boundaries in the fruit fly (Fig. 1.14) (32,33).
The earlier the excision is carried out, the more proximal is the
truncation. Limb bud outgrowth can be sustained after excision of the
AER by the insertion of beads carrying FGFs. To obtain the most normal
limb development, two FGF-soaked beads must be placed so that the
polarizing region as well as the AER are mimicked (34).
The absence of the mechanical flattening of the limb bud that the AER
would have produced results in a bulbous limb bud and in the bunching
of the digits. Nevertheless, fully differentiated skeletal structures
of the limbs can be produced.
are expressed in the AER and each is able by itself to sustain limb bud
outgrowth (probably because of the ability of different FGFs to
activate the same receptors) (35,36,37). In vivo FGF-8 is found in the entire AER, whereas FGF-4 expression is limited to the posterior portion of the AER.
beneath the ectoderm. Shp2 maintains the AER, and influences PZ cell
shape or adhesion in order to regulate the recruitment of cells for
distal limb segments (38).
cells in the PZ on the basis of the length of time (number of mitoses)
the cell spends in the PZ, as discussed in the section “Developmental
Anatomy of the Limb” (39,40).
Some experimental work suggests that transforming growth factor βs
(TGFβs) act like a gradient from the AER to increase cell adhesion by
activating integrins—mediators of cell
adhesion. Perhaps, the longer a cell is in the PZ, the more TGFβ it
encounters, and the more integrins that are activated, leading to
greater cell adhesion, and, ultimately, more distal limb positional
information programmed into the cell.
posterior and proximal limb bud to induce duplication of digits when
grafted to an anterior position on another limb bud suggests that this
region of polarizing activity synthesizes a morphogen that acts like a
gradient to specify AP limb elements (16). If
it is acting through a morphogenetic gradient, the ZPA should give
different digit patterns when transplanted to different areas of the
limb bud—which is what it does (41).
Furthermore, if a physical barrier is placed between the anterior and
posterior parts of the limb bud, a normal number and order of digits is
formed in the posterior portion of the limb bud, and no digits are
formed anteriorly (Fig. 1.15) (42).
expressed in the ZPA. Activation of this gene, through cell
transfection, in anomalous locations in the limb bud will cause digit
duplication in the same manner as transplantation of the ZPA tissue (43).
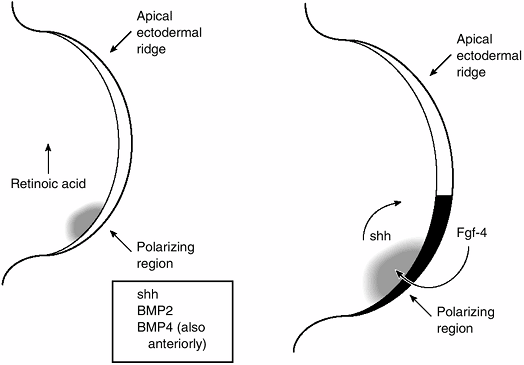 |
|
Figure 1.15
These two diagrams of the tip of the limb bud illustrate some of the molecules involved in the interaction between the polarizing region and mesenchyme of the progress zone that specify the anteroposterior (AP) axis of the limb. On the left, the arrow shows the direction of the decreasing concentration of retinoic acid. Retinoic acid acts by regulating cell production of sonic hedgehog (shh). Sonic hedgehog, in turn, stimulates bone morphogenetic protein-2 (BMP2), which is a homolog of a Drosophila segment polarity-specifying gene. BMP-4 expression overlaps that of BMP2 and is, therefore, probably involved in AP axis determination as well. The diagram on the right shows the feedback loop between shh and fibroblast growth factor-4 (FGF-4), an ectodermally expressed protein that appears to maintain the apical ectodermal ridge. (From Thorogood P. Embryos, genes and birth defects. New York: John Wiley & Sons, 1997:111, with permission.) |
is an evolutionarily conserved pathway that has been adapted to many
functions that are crucial in development. It plays a role in embryonic
development of the notochord, neural tube, brain, gut, and limb
(including the crucial ZPA, which specifies AP axis in the limb bud).
modification after translation, with cleavage of the protein and
addition of a cholesterol moiety. The cholesterol addition is necessary
for the attachment of hedgehog to the cell membrane and affects its
concentration and diffusion characteristics. Sonic hedgehog functions by causing the activation of a transmembrane protein named Smoothened. Smoothened causes phosphorylation of the GLI family of transcription factors. Patched, a transmembrane receptor for sonic hedgehog, acts to repress Smoothened. Sonic hedgehog
binds to Patched and stops its repression of Smoothened. To complete
this complex multiple feedback system, the phosphorylated transcription
factors effect the expression of Patched (44). At present it appears that sonic hedgehog, Smoothened, fusion (a serine-threonine kinase), and GLI 1 act as positive activators of the pathway, whereas Patched, suppressor of fusion, GLI 2, GLI 3, costal 2 (a kinesin-related protein), and Hip (hedgehog-interacting protein) are negative regulators of the hedgehog pathway (45,46).
is expressed mainly in Schwann cells and developing germline cells.
Invertebrates have only one hedgehog gene, with functions similar to sonic hedgehog in vertebrates (47).
are members of the TGFβ superfamily, and BMP2
is, specifically, a homolog of a fruit fly gene that specifies segment
polarity, thereby making it a good candidate for an axis-determining
gene. BMP2 is secreted from cells, and, therefore, its action extends over a larger area than just the cells in which it is produced. BMP4 expression overlaps that of BMP2, and it is probably involved in AP axis specification as well. Sonic hedgehog
in the mesenchyme also appears to participate in a positive feedback
loop with FGF-4 in the ectoderm, which may be important in maintaining
the AER and in supporting continued limb outgrowth and patterning (49). BMP2 and BMP4
also play roles in regulating the size and shape of long bones.
Overexpression of these genes appears to cause an increase in the
quantity of mesenchymal cells that differentiate into the chondrogenic
precursors of the skeleton.
protein) is expressed specifically in the prospective joint region. If
noggin is not present, GDF-5 is not expressed and BMP2 and BMP4
are not inhibited, resulting in a continuous, jointless skeleton. A
balance of activating and inhibiting signals seems to be necessary for
normal joint cavitation (51,52). Wnt14
is present in chicken interzone cartilage cells before joint
segmentation and may be an important initiator of synovial joint
formation. Other Wnt genes are expressed
in the presumptive joint region, and this common pathway may be vital
in the regulation of joint formation (53).
matrix interactions are involved in the mechanism of joint cavitation.
Specifically, CD44s, an isoform of a cell surface receptor that
interacts with hyaluronan, is found in a single layer of cells outlying
the presumptive joint cavity in rats (54). One
hypothesis is that the creation of a hyaluronan-rich, but
proteoglycan-poor and collagen II-poor extracellular matrix results in
a loss of cell adhesion and allows joint cavity formation (54).
pathway is involved in embryonic mesodermal induction, vertebrate
neural tube patterning, somite specification, hematopoiesis, AER
growth, AP axis specification, initiation of chondrogenesis, and
regulation of apoptosis of the interdigital mesenchyme of vertebrates (55).
activate a Type I and a Type II receptor simultaneously, causing the
Type II receptor to phosphorylate the Type I receptor. This activates
the Smad cascade. Restricted Smads are released from the Type I
receptor and combine with common mediator Smads. This complex moves
into the nucleus and activates the transcription of specific genes.
Smad activation is regulated by inhibitory Smads, which prevent the
activation, or by restricted Smads and common mediator Smads. The BMP/Smad signaling pathway is utilized for the cell differentiation function. BMPs
act through a different pathway that leads to p38 pathway stimulation
for control of apoptosis. P38 is a protein kinase that controls
cellular responses to cytokines and stress. BMP concentration, and
therefore its activity, is partly controlled by various antagonists.
Typically, the stimulatory activity of BMP is opposed by chordin,
noggin, and gremlin, which bind and/or inhibit BMP. In Xenopus mesodermal induction, follistatin acts to inhibit a BMP activator named Furine (57).
ectoderm and mesoderm in chickens have given conflicting results with
respect to DV patterning. Some experiments have suggested that the
ectoderm dictates the DV axis, whereas others claim that the mesoderm
dictates the DV polarity of the overlying ectoderm before limb bud
outgrowth (58). Recent evidence indicates that
DV patterning of the limb is more complex. Evidence suggests that
somites, and possibly the lateral somatopleuric mesoderm, provide DV
inductive signals in the prelimb bud (59,60). Once the limb bud forms, however, DV patterning control is transferred to the ectoderm (58).
Surgical rotation of the ectoderm gives results suggesting that the
ectoderm has a patterning effect only on the cells in the PZ. Several
genes involved in dorsal–ventral specification have been identified.
Evidence suggests that Wnt-7A, a secreted glycoprotein, confers the dorsal character to the ectoderm, and it stimulates Lmx–1b (30). Lmx-1b
is a homeobox gene that encodes a transcription factor that dorsalizes
the mesoderm. The ventral limb ectoderm expresses the
homeobox-containing transcription factor En-1. En-1 appears to suppress
Wnt-7A expression, thereby limiting the activity
of Lmx-1b to the dorsal mesenchyme (61). Evidence suggests that this interaction is operating mainly in the distal limb bud. Wnt-7A and En-1 do not modulate Lmx-1b activity in the proximal limb bud. Nonetheless, Lmx-1b is crucial in DV patterning in both the proximal and distal limb bud (62). In the absence of the Wnt-7A and Lmx-1b
dorsalizing signals, the entire limb bud is ventral. This suggests that
a ventral morphology is the “default” state in DV patterning that is
modified by the dorsalizing signals (63). Gene
knockout experiments of these major genes show that nail and hair
follicle density are mesodermally determined and exocrine gland
positioning is controlled by the ectoderm (63). The actions of several genes are important in determining more than a single axis. For example, Wnt-7A expression is necessary for normal AP digit formation and sonic hedgehog
is necessary for PD limb outgrowth, in addition to AP patterning. The
coordinated development of all three axes is regulated by the
interactions of several signaling genes. Wnt-7A, sonic hedgehog, and FGF-4 are promising candidates for this role (Fig. 1.16) (62,64).
several pathways. The originally described pathway or canonical pathway
is an evolutionarily conserved pathway by which Wnt genes control cell proliferation and differentiation through induction of specific gene expression. In the canonical pathway, Wnt binds to the receptors frizzled
and low-density lipoprotein (LDL) receptor-related proteins 5 and 6,
thereby activating the protein dishevelled and suppressing glycogen
synthase kinase-4β (GSK-3β). The result of these events is the
inhibition of β-catenin phosphorylation. If β-catenin becomes
phosphorylated, it is targeted for degradation. β-catenin changes
lymphoid enhancer factor/T-cell factor (LEF/TCF) from a transcription
repressor to a transcription enhancer. Simply stated, Wnts regulate the amount of β-catenin that stimulates LEF/TCF-mediated transcription (32).
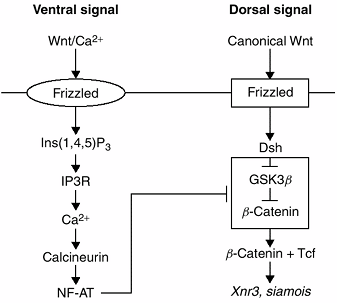 |
|
Figure 1.16 This figure presents a model of dorsoventral (DV) axis formation through the interaction of the Wnt/Ca2+ and the canonical Wnt pathways in a Xenopus (frog) embryo. The T-shaped bar indicates inhibition of the “dorsalizing” pathway by the “ventralizing” pathway.
|
pathways is that β-catenin is not involved. These pathways require
frizzled as the Wnt receptor, and activate Ca2+
flux, G proteins, and c-Jun N-terminal kinase intracellularly. Both
pathways are involved in multiple embryonic events including
gastrulation and limb formation.
the entire embryo. Parts of this pathway were probably coopted during
evolution for use in DV limb patterning. A recently proposed model for
embryonic DV patterning, developed from investigations of Xenopus, suggests that two Wnt pathways interact—the canonical Wnt and the Wnt/Ca2+ pathways. Wnt/Ca2+ mediates ventral signaling by suppressing the dorsalizing effects of the canonical Wnt pathway. Wnt/Ca2+ activates frizzled, which, through a series of steps, activates intracellular Ca2+
The protein calcineurin is thereby activated, ultimately resulting in
target gene activation. This is the ventralizing pathway. The dorsal
signal arises from activation of canonical Wnt
or from the accumulation of dishevelled. Dishevelled inhibits the
phosphorylation of β-catenin resulting in an accumulation of β-catenin
in the cytosol. β-catenin then moves into the nucleus to activate the
target genes Xnr3 and siamois. It appears that the target genes of Wnt2+ inhibit the canonical Wnt pathway somewhere between dishevelled and β-catenin (65).
genes are not expressed strictly along AP or DV axes, and the positions
where they are expressed differ in various areas of the limb. Human
limb malformations caused by mutations in HOX genes have been identified and will be discussed in the section on clinical significance. The activity of the HOX genes is overlapping and is sufficiently redundant such that a mutation of a single HOX gene generally results only in a minor limb anomaly.
ligament, and tendon development have not been identified. Exploration
of these mechanisms may ultimately be of great clinical utility.
 |
|
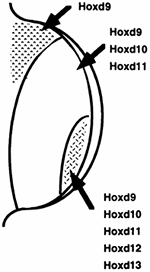
Figure 1.17 The pattern of expression of the HOX-D genes is shown. The overall HOX-D expression is sequential from the anterior to the posterior aspect of the limb bud; however, several individual HOX-D genes have clustered expression patterns. The pattern of HOX-D gene expression can be altered by polarizing signals, thereby implicating the HOX-D genes in limb pattern determination. (From Thorogood P. Embryos, genes and birth defects. New York: John Wiley & Sons, 1997:111, with permission.)
|
because of their complex developmental biology and their exposed
position outside of the body wall. Nearly all teratogens and
chromosomal anomalies have ill effects on limb development. Many single
gene mutations disturb the normal development of limbs as well. Rapid
advances in molecular genetics are leading to the identification of the
gene(s) responsible for many Mendelian disorders. Developmental
molecular biologists are identifying genes that create limb deformities
in animals whose homologous human genes may be responsible for other
specific limb defects.
Fifty percent of limb deficiencies occur as isolated defects and the
other 50% of deficiencies occur with associated malformations (69).
These associated malformations may be life threatening. The most common
associated malformations are musculoskeletal, head and neck,
cardiovascular, gastrointestinal, and genitourinary. As discussed
previously, destruction of the AER or of the PZ, or failure of
expression of critical signaling molecules, can result in truncation or
absence of limb development.
family of proteins involved in regulating cell proliferation,
apoptosis, and differentiation by sensing multiple inputs to cells. P63
has been shown to play a critical role in the formation and
differentiation of the AER, which is required for normal limb outgrowth
and patterning. Five syndromes caused by specific mutations of p63 have been identified (70). Approximately 10% of nonsyndromic split hand–split foot malformations (also called ectrodactyly or lobster claw deformity) are caused by p63 mutation (71).
EEC syndrome is characterized by ectrodactyly, ectrodermal dysplasia,
and cleft lip with or without cleft palate. Almost all cases of EEC are
caused by a mutation that causes a small number of amino acid
substitutions in the DNA-binding region of p63.
Ankyloblepharon-ectodermal defects–cleft lip/palate (AEC) syndrome
involves ectodermal dysplasia (more severe than EEC) and cleft lip
and/or palate, with generally minimal or absent limb deformity.
Limb-mammary syndrome is characterized by ectrodactyly and hypoplastic
or absent mammary glands and nipples. Adult syndrome has many features
in common with EEC and is characterized by ectrodactyly, syndactyly,
nail dysplasia, hypoplastic mammary glands, underdeveloped teeth, and
intense freckling.
genes are DNA-binding transcription factors and are crucial for the
development and patterning of the axial skeleton, limbs, CNS,
urogenital tract, genitalia, and gut. Three human disorders resulting
from HOX gene mutations have been identified (72).
It is surprising that mutations in genes such as these, which are
fundamental to development, result in relatively mild phenotypes. It
seems likely that redundancy of functions might exist in these very
important genes and also that many mutations are not compatible with
embryonic development. Synpolydactyly is an autosomal dominant disorder
characterized by syndactyly between the middle and ring fingers and
between the fourth and fifth toes, with variable presence of
hypoplastic digit duplications in the webs. Almost all cases result
from a polyalanine tract expansion in HOX-D13 (72).
Hand-foot-genital syndrome is characterized by short thumbs and short
great toes, urinary tract malformations, and hypospadias in men and by
Mullerian duct fusion defects in women. This autosomal dominant
disorder is commonly caused by a HOX-A13 nonsense mutation, although polyalanine repeat expansions have also been reported (73).
Guttmacher syndrome has features similar to those of hand-foot-genital
syndrome but differs in the occurrence of postaxial polydactyly of the
hands and uniphalangeal 2nd toe. Brachydactyly types D and E result
from HOX-D13 mutations (74).
primarily or exclusively, and 39 causative genes have been identified
for these disorders.
gene: postaxial polydactyly a/b, postaxial polydactyly a, preaxial
polydactyly iv, Grieg cephalopolysyndactyly syndrome (postaxial
polysyndactyly of hands, preaxial polysyndactyly of feet, and
dysmorphic facies), Pallister-Hall syndrome (congenital hypothalamic
hypopituitarism, imperforate anus, polydactyly, and various visceral
anomalies), and Acrocallosal syndrome (postaxial polydactyly,
hallux duplication, macrocephaly, and absence of the corpus callosum) (50,75,76,77,78).
GLI 3 is a DNA-binding transcription factor that is expressed in the
interdigital mesenchyme and joint-forming regions of the digits and is
also part of the sonic hedgehog–Patched–GLI pathway (see the section Anterior-Posterior Axis Determination/Zone of Polarizing Activity).
Sonic hedgehog utilizes cholesterol as a carrier molecule, and
Smith-Lemli-Opitz syndrome, which involves defective cholesterol
synthesis, is also characterized by polydactyly and brachydactyly (79).
The small patella and nail anomalies reflect dorsal pattern defects.
The iliac horns may represent duplications of spinous processes that
are normally only ventral structures.
deficiencies by prohibiting limb outgrowth or by causing necrosis of
already differentiated limbs (81,82). Amniotic bands result in amputation by interfering with vascular supply (69).
structures, may result from a temporary injury to the PZ as discussed
in the section “Developmental Anatomy of the Limb.”
synostoses in humans. Retinoic acid creates synostoses in animals when
applied during chondrogenesis of developing limbs (83).
factor in causing some disorders with synostosis as a feature. A
failure of aphotic cell death in the interdigital mesenchyme is
presumed to be the cause of some syndactyly.
an inherited condition. Triphalangeal thumb is the most common of these
disorders. In several affected families, this abnormality has been
linked to a region on chromosome 7, but the gene has not been
identified (84). Other families do not link to this locus, which implies that more than one gene mutation causes triphalangeal thumb.
anomalies, is usually an inherited condition. Given the complex
interactions that occur in limb pattern specification, it is not
surprising that a number of causes for this condition are being found.
disorder characterized by postaxial polysyndactyly of the hands and
preaxial polysyndactyly of the feet, as well as dysmorphic facies. GLI 3, a DNA-binding transcription factor, is the cause of this disorder (85). GLI 3
expression is restricted to the interdigital mesenchyme and
joint-forming regions of the digits. A mouse mutant with a defect in
the homologous gene has ectopic expression of both sonic hedgehog and FGF-4 in the anterior limb bud (86). Another mutation causing human polydactyly is in the HOX-D cluster of homeobox genes that has been implicated in digit specification. Synpolydactyly is caused by a mutation of HOXD-13 (87).
Smith-Lemli-Opitz syndrome is characterized by a variety of birth
defects including postaxial polydactyly and brachydactyly. Cholesterol
synthesis is defective in children with this syndrome, and sonic
hedgehog utilizes cholesterol as a transport molecule. It is possible
that the limb anomalies in this syndrome result from a distortion of
the normal sonic hedgehog gradient caused by cholesterol insufficiency.
disorders whose genetic causes are rapidly being discovered, giving
insight into the mechanisms of normal and disordered skeletal
development (see Chapter 8).
either singly or in groups, are believed to result from vascular
disturbances in the embryo or fetus. The best developed of the
hypotheses on vascular etiology is the subclavian artery supply
disruption sequence, which seeks to explain Klippel-Feil syndrome,
Poland anomaly, Mobius syndrome, absence of the pectoralis major,
terminal transverse limb deficiencies, and Sprengel deformity. A
disruption occurs when a normal embryo suffers a destructive process
with cascading consequences. Because all the tissues affected in these
various disorders receive their blood supply mainly from the subclavian
artery, it is hypothesized that a defect of arterial formation or an
injury to existing arteries causes these defects. The location and
extent of tissue abnormality is determined by the extent, location, and
timing of the interruption of normal blood supply. The observation
underlying this hypothesis is that the disorders listed above often
occur together in various combinations.
include vessel occlusion from edema, thrombus, or embolus; extrinsic
vessel compression caused by surrounding tissue edema, hemorrhage,
cervical ribs, aberrant muscles, amniotic bands, or uterine
compression; abnormal embryologic events, including delayed or abnormal
vessel formation and disruption of newly formed vessels; and
environmental factors such as infection, hyperthermia, hypoxia,
vasculitis, and drug effects. It is possible that some fetuses suffer
ischemia because of normal embryologic events that are
idiosyncratically not well tolerated, such as the rapid descent of the
heart and great vessels.
explaining combinations of congenital anomalies and their usually
sporadic occurrence. However, there are often combinations of anomalies
that are difficult to relate to a single vascular event, and it is not
easy to determine whether the anomalies caused the vascular
abnormalities or vice versa.
proportion of nonhereditary congenital limb anomalies. Constriction
rings are diagnosed by the occurrence of soft tissue depression
encircling a limb or injuries from amputations or disruptions.
Constriction rings are commonly multiple and may be broad or narrow.
The depth of the
ring
determines whether the limb distal to the ring is normal, hypoplastic,
engorged (from venous or lymphatic obstruction), or amputated (from
vascular insufficiency). Syndactyly, clubfoot, and clubhand have also
been associated with constriction rings.
Nonetheless, the syndrome is common, affecting 1 in 5000 to 15,000
births. The risk of recurrence of this syndrome in subsequent
pregnancies is low (69).
the species of the subphylum “Vertebrata.” The evolution of a vertebral
column to replace the notochord provided strength, flexibility, and
protection to the neural tube that conferred many advantages to
vertebrate species. Minor and even major anomalies of the vertebral
column are compatible with life and good function. Vertebral column
development depends on the appropriate prior development of the
notochord and somites.
mass of ectodermal cells proliferates and forms the archenteron—a tube
that migrates cranially in the midline between the ectoderm and the
endoderm. The floor of the archenteron forms the notochordal plate. For
a short time there is a direct connection between the primitive gut and
the amniotic cavity because the endodermal floor is not continuous and
the blastopore (the opening of the archenteron) communicates with the
amniotic cavity. This connection is obliterated by the end of the 3rd
week of gestation, and remnants of this connection are presumed to be
responsible for diastematomyelia.
that come from the ingress of cells from the epiblast during
gastrulation and, later, from the caudal eminence. This ingress of
cells forms the endoderm as well as the notochord and the paraxial
mesoderm (segmental plate). The notochord develops from cranial to
caudal end by adding cells as it develops. It is initially a solid rod
in which a small central canal develops. The notochord induces the
formation of the neural groove, which gradually closes to form a tube
with a central canal. By the 23rd day, the neural groove is closed
except at its most cranial and caudal ends. These openings are termed
the neuropores, which close by the end of
the 4th week. Closure of the neural tube progresses from cranial to
caudal end. It is hypothesized that failure of the neural tube to close
properly is the cause of NTDs such as myelomeningocele.
internalized through the primitive streak. These cells form the
paraxial mesoderm, which will become the somites and will ultimately
become the vertebrae. Presomitic cells cluster by increased adhesion
into distinct balls of epithelial cells surrounding the mesenchymal
cells, giving the embryo its first segmental organization. Positional
information is programmed into the somites by the time they are
morphologically distinct. For example, a thoracic somite will still
form a rib when transplanted into the cervical region. The positional
information is imparted during gastrulation (93,94).
Somites do not depend on interaction with the neural tube or notochord
for development, and fated cells will develop into somites in vitro.
sacral, and 4 or 5 coccygeal pairs of somites will develop. The first
somites are evident at 3.5 weeks, and 30 pairs are present at 4.5
weeks. Not all of the somites are visible simultaneously. Somites
develop a complex internal organization. The somite begins as a ball of
pseudostratified epithelium surrounding a central cavity, the
somitocoele. The central cavity becomes filled with mesenchymal cells.
Some of these cells, along with cells in the medioventral portion of
the somite, become the sclerotome. Cells from the sclerotome will form
the vertebral bodies and vertebral arches and emerge without the
epithelial portion of the sclerotome to surround the neural tube. In
addition to contributing to the sclerotome, cells from the central
cavity migrate to become the intervertebral discs and contribute to rib
formation. The dorsolateral wall of the somite is called the dermomyotome.
It separates into the dermatome laterally and to the myotome lying
between the dermatome and the sclerotome. The dermatome gives rise to
the dermis of the skin and the myotome supplies cells for muscles,
tendons, and fascia (Fig. 1.18).
medially, meeting around the notochord and separating it from the
dorsal neural tube and the ventral gut. The continuous perichordal
sheath is distinct from the more lateral, segmented sclerotomes.
Between the adjacent somites lie the transverse intersegmental
arteries. The segmental spinal nerves originally exit at the midportion
of the somitic sclerotomal mass. Resegmentation of the sclerotomic
tissues occurs at 4.5 weeks (Fig. 1.19). This
process of resegmentation occurs by variable rate mitosis and causes
each somitic sclerotome to thin cranially and condense caudally. The
transverse intersegmental arteries and spinal nerves traverse the
cellularly loose cranial portion of the sclerotome. The dense caudal
portion of each sclerotome unites with the cranial, less-condensed part
of the next sclerotome to form the primordium of the vertebra.
Therefore, the skeletal
portions
of the somites no longer correspond to the original segmentation. The
segmental spinal nerves that originally were in midsomite now lie at
the level of the disc. The intersegmental arteries located between
somites come to lie at the midportion of the vertebral bodies, and the
myotomes bridge the vertebrae. The densely cellular, caudal portion of
the sclerotome gives rise to the vertebral arch. The initially
continuous notochordal sheath segments into loosely cellular cranial
and densely cellular caudal portions. The cranial portion becomes the
vertebral centrum and the dense, caudal portion becomes the
intervertebral disc. The vertebral centrum surrounds the notochord and
forms the vertebral body. Notochordal cells in the vertebral centra
degenerate, although some remnants of the notochord may remain. The
notochordal cells persisting in the sacral or cervical areas may give
rise to chordomas in later life. The neural arches develop from ventral
to dorsal end, enclosing the neural tube, and unite during the fetal
period. The spinal nerves and the dorsal root ganglia arise at the
level of the somite and enter the myotome at the beginning of the 6th
week. The presence of ganglia is necessary for normal neural arch
segmentation. Although somites form from cranial to caudal end,
resegmentation progresses from midspine, both cranially and caudally.
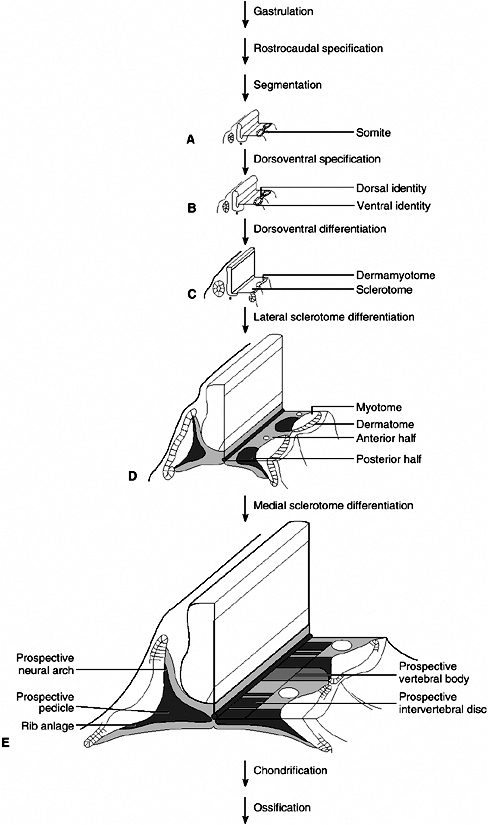 |
|
Figure 1.18 The progressive differentiation of the vertebral column is illustrated in (A) to (E). The dark areas in (D) and (E)
demonstrate the portion of the sclerotome that develops into the neural arch, the vertebral body, rib anlage, and the intervertebral disc. (From Thorogood P. Embryos, genes and birth defects. New York: John Wiley & Sons, 1997:282, with permission.) |
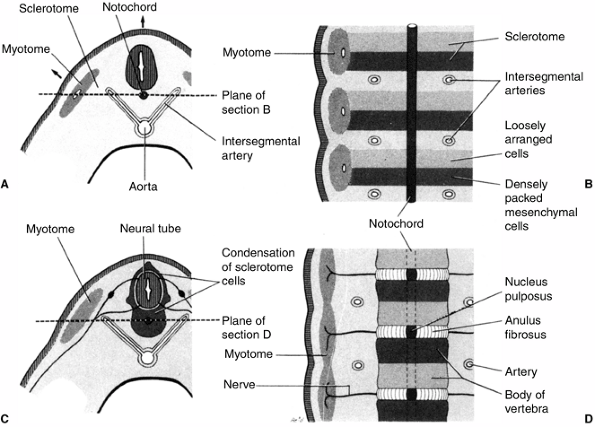 |
|
Figure 1.19 A: Transverse section through a 4-week embryo. The top arrow shows the direction of the growth of the neural tube and the side arrow shows the dorsolateral growth of the somite remnant. B:
Coronal section of the same stage embryo showing the condensation of sclerotomal cells around the notochord with loosely packed cells cranially and densely packed cells caudally. C: A transverse section through a 5-week embryo depicting the condensation of sclerotome cells around the notochord and neural tube. D: Coronal section illustrating the formation of the vertebral body from cranial and caudal halves of adjacent sclerotomes resulting in the segmental arteries crossing the bodies of the vertebrae and the spinal nerves lying between the vertebrae. (From Moore KI, Persaus TVN. Before we are born. Essentials of embryology and birth defects, 4th ed. Philadelphia: WB Saunders, 1993:257, with permission.) |
neuroectoderm) accumulate just before cranial closure of the neural
tube and are situated between the neural tube and the somites. These
cells become the peripheral nervous system sensory cells and nerve
fibers as well as the Schwann cells and melanocytes. Peripheral nerve
afferents and preganglionic fibers of the autonomic nervous system
develop from the neural tube, as do the brain and spinal cord. At each
cervical, thoracic, and lumbar somite, a corresponding ganglion
develops.
an overlapping fashion in the developing spinal column, and evidence
suggests that they specify the morphology of individual vertebra (95). It is possible to create transgenic mice through out-of-sequence
activation of certain HOX
genes that transform the atlas into a cervical vertebra with a body and
the lumbar vertebrae into thoracic-like vertebrae, with rib formation (96,97). Conversely, HOX gene inactivation can transform the axis into an atlas-like vertebra (98). Overlapping, redundant expression of HOX genes occurs, and inactivation of more than one adjacent HOX gene has been found to be necessary in order to alter vertebral morphology in some regions of the vertebral column (99). Retinoic acid application can alter the normal expression patterns of HOX
genes, and can create varying morphologic abnormalities depending on
the timing and location of its application. A particular retinoic acid
receptor (the γ receptor) is expressed only in prebone tissue. Its
inactivation causes transformation of the axis vertebra to an atlas and
C7 to C6 (100).
genes that contain a DNA-binding domain and are expressed in the
sclerotome in high levels during sclerotome condensation. Some evidence
suggests that a defect in specific PAX genes, or in genes they modify, may result in failure to form vertebral elements (101). The homeobox gene MSX2 is necessary for spinous process development in mice (102). Clearly, the interactions of genes and tissues involved in vertebral column formation are complex.
characteristic skeletal structure of vertebrates and it is remarkable
how even severe vertebral abnormalities are so well tolerated by the
organisms. Because vertebral column development has been highly
conserved during evolution, most, if not all, vertebral abnormalities
seen in other vertebrates can also be found in humans. In fact, mouse
genetics has led to the identification of probably all types of
vertebral abnormalities (103,104).
result in different vertebral malformations. In general, the earlier
the disruption in the developmental process, the more severe the
phenotype will be. The developmental processes that can be affected
include mesoderm formation during gastrulation, axial patterning,
notochordal mesoderm induction, somite formation, sclerotome
condensation, neural tube closure, and axial identity specification.
Whereas disorders in pattern formation result in specific prevertebral
phenotypes, general disorders of mesenchyme condensation or of
cartilage or bone formation affect the composition and, therefore, the
morphology of the skeleton. Misspecifications of vertebral identity,
called homeotic transformations, are
characterized by the presence of all the vertebral components but
usually with shapes characteristic of the adjacent vertebra.
during gastrulation leads to a block in the formation of the whole
vertebral column formation. Because gastrulation also generates the
other two germ layers, the embryo will have multiple congenital
abnormalities and will not survive (105).
Defects during sclerotome formation are compatible with life and
typically result in segmental vertebral agenesis. Because sclerotome
formation depends on the inductive activity of the notochord, it is
mainly notochord mutants that are found within this category. In the
affected region, somite ventralization is hindered, and the
corresponding vertebrae appear to be deleted.
formation lead to absence, truncation, or interruption of vertebral
column formation, disorders of somatogenesis are compatible with
vertebral development. However, multiple vertebral components can be
lacking or fused. Variations in number, shape, and position of
vertebrae are common developmental anomalies. Most columns have 24
segments including 7 cervical, 12 thoracic and 5 lumbar vertebrae.
However, columns with 23 or 25 elements are commonly observed, and they
are most likely related to differences in the number of elements of the
lumbar spine. This number difference may be a result of the last lumbar
vertebra being incorporated into the sacrum (sacralization) or the
first sacral vertebra being freed (lumbarization).
varied. The most common conditions are spina bifida, hemivertebra and
wedge vertebrae, and vertebral bars. Spina bifida occulta is a failure
to complete the neural arch, but without neurologic compromise. Failure
of the neural arch to fuse in the cervical spine, and sometimes in the
upper thoracic spine, is seen shortly after birth, but spina bifida is
most commonly seen at the level of the lumbosacral spine. This is a
normal finding in children as old as 2 years, in 50% of children as old
as 10 years, and in approximately 20% of adults. NTDs can be subdivided
into four subgroups:
-
A meningocele is a cyst that involves only the meninges but not the neural elements.
-
A myelomeningocele includes the abnormal elements as part of the sac.
-
A lipomeningocele is a deformity in which there is a sac containing a lipoma that is closely involved with the sacral nerves.
-
Rachischisis is a complete absence of skin and sac, with exposure of the muscle and dysplastic spinal cord.
caudal end. Failure to close properly is widely believed to be the
cause of most cases of myelomeningocele (106).
This hypothesis is supported by observations of early fetuses with
myelomeningocele and is consistent with animal models of NTDs (107).
The competing hypothesis, championed by Gardner, suggests that
overdistension and rupture of a closed neural tube causes NTDs (108,109).
Myelomeningocele has multiple causes resulting in a common phenotype.
An inherited predisposition to NTDs appears to be present in some cases
and is based on an increased incidence of NTDs in some families and a
variation in prevalence among different ethnic groups (110).
Furthermore, in a mouse model, NTD has been shown to result from a mutation in the gene PAX-3. PAX-3
is a homeobox gene that has been shown to play a role in the fusion of
the dorsal neural tube as well as in neural crest cell migration and
dermomyotome development (107). Environmental
factors are responsible for some proportion of NTDs, and multiple
teratogens have been identified that interfere with neurulation.
Examples include vinblastine, which disrupts actin microfilaments, and
calcium channel blockers, which interfere with microfilament
contraction (111,112).
Retinoic acid, hydroxyurea, and mitomycin C interfere with the timing
of neuroepithelial development and cause NTDs in animal models (113).
Folate supplements during pregnancy decrease the risk of NTDs in
subsequent children when a prior child has been affected; they also
decrease the incidence of NTDs in pregnancies of women without a prior
history of NTDs (106). The mechanism by which
folate protects against NTDs is unknown. The interaction of genes and
the environment that leads to myelomeningoceles and the molecular
pathology involved are under investigation (107).
hemivertebra and vertebral bars. Hemivertebra appears as a wedge,
usually situated laterally between two other vertebrae. As a
consequence, a lateral curvature of the spine develops. Vertebral bars
are caused by localized defects in segmentation and are observed most
frequently in the posterolateral side of the column, resulting in
absence of growth in that side. The outcome is a progressive lordosis
and scoliosis. When located anteriorly, vertebral bars lead to
progressive kyphosis. Klippel-Feil sequence is considered a defect in
cervical segmentation. Clinically, there is a short, broad neck; low
hairline; limited range of motion of the head and neck; and multiple
vertebral abnormalities.
Diastematomyelia is a longitudinal splitting of the spinal cord
associated with a bony or fibrocartilaginous spicule or septum arising
from the vertebral body. This is believed to result from remnants of
the early connection to the primitive gut or the amniotic cavity. It is
commonly associated with skin changes and abnormalities of the lower
extremities. A chordoma is a neoplasm that arises from notochordal
rests and is found especially in the sacrococcygeal region.
Sacrococcygeal teratoma is a neoplasm composed of multiple embryonic
tissues that can undergo malignant transformation.
cord) and the neural crest develops into most of the peripheral nervous
system. The spinal cord develops from the portion of the neural tube
that is caudal to the four occipital somites. The neural tube forms
from the folding of the neural groove and begins at the brain and
spinal cord junction. As the neural groove fuses, so does the neural
fold. Neural crest cells begin their migration from neural fold tissue
just after neural tube closure occurs in the spinal regions. Neural
crest cells migrate either beneath the surface ectoderm or between the
neural tube and the somite. Migration occurs through the extracellular
matrix along relatively cell-free paths. Neural crest cells form the
pia mater, the spinal ganglia, and the sympathetic trunks and ganglia.
is separated from the ventral basal laminae by a shallow groove—the
sulcus limitans. A thin bridge of tissue remains, connecting the two
halves of the alar and basal laminae named the roof plate and the floor plate.
The roof plate develops into the sensory pathways (dorsal columns) and
the floor plate develops into the motor pathways (ventral horns). The
notochord is necessary for floor plate induction, and the floor plate
appears to specify the DV organization of cell types in the developing
spinal cord.
ventral roots. The dorsal root ganglia develop from neural crest cells.
The axons of the ganglion cells form central processes that become the
dorsal roots and peripheral processes that end in sensory organelles.
direction, and motor neurons develop neural capabilities before sensory
nerves do. Autonomic nerve function is established last (54).
Movement is visible by ultrasonography 5.5 weeks postfertilization. The
spinal cord extends the entire length of the vertebral column during
the embryonic period. During fetal development, the vertebral column
grows more rapidly than the spinal cord. With some loss of caudal
spinal cord tissue, the caudal tip of the spinal cord ends at the
second or third lumbar vertebra in newborns. In the adult, the spinal
cord terminates at the inferior portion of the first lumbar vertebra.
Therefore, the lumbar and sacral nerve roots have an oblique course
below the conus medullaris before exiting from their intervertebral
foraminae, resulting in the formation of the cauda equina (Fig. 1.20).
fetal period and continues during the first year after birth. Schwann
cells myelinate the peripheral nerves, whereas ligodendrocytes
myelinate the axons within the spinal cord.
system is not well understood, but it probably will be interpreted in
the next decade. There is an intimate relation between the notochord
and the neural plate and tub, both of which are necessary for
differentiation of the floor plate or the
spinal
cord and for specification of ventral structures in the developing
spinal cord. Hepatocyte nuclear factor-3 appears to regulate sonic hedgehog (an important axis-specifying gene in the limb as well), which can induce ventral structures (114). PAX
genes have a dorsoventrally restricted expression in the developing
spinal cord. Dorsal structures can develop without the notochord, but
specific molecules may be necessary for complete dorsal specification.
For example, dorsalin-1, a member of the TGFβ family, can induce neural
crest cell differentiation (115). The transcription factor genes PAX 3 and GLI 3 are necessary for neural tube closure (116).
The large number of NTDs that occurs suggests that neural tube closure
does not have a large redundancy in its developmental regulation. Lmx-1b
is part of the control mechanism that results in different motor and
sensory nerve innervation patterns in the dorsal versus the ventral
limbs.
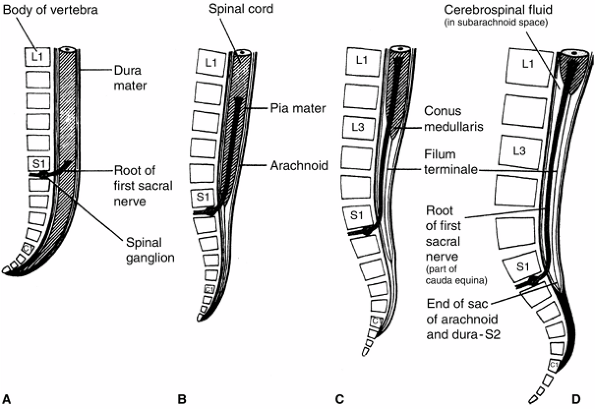 |
|
Figure 1.20 Illustration of the position of the spinal cord and meninges in relation to the vertebral column at 8 weeks (A), 24 weeks (B), birth (C), and adulthood (D). (From Moore KI, Persaud TVN. Before we are born: Essentials of embryology and birth defects, 4th ed. Philadelphia: WB Saunders, 1993:283, with permission.)
|
orthopaedic implications because of disordered control of limbs, but
these are not discussed here. Failure of closure of the caudal
neuropore may result in myelomeningocele. Other evidences suggests that
increased cerebrospinal fluid (CSF) pressure causes rupture of an
already closed neural tube at its weakest point—where it closed last.
In mutant animals, at least 10 genes have been identified that lead to
NTDs. Several teratogens such as valproic acid and vitamin A, or
exposure to hypothermia, can cause NTDs. Although the mechanism is
unknown, perifertilization supplementation with folic acid decreases
the incidence of NTDs in humans.
groove fails to form a neural tube. Myelomeningocele is associated with
uncovered neural tissue that has herniated into the dysraphic area of
the spine. Meningocele, in which the neural elements remain in their
normal locations, is probably a primary defect of the vertebral column
development rather than a primary neural defect. Ten percent of people
have spina bifida occulta, in which the vertebral neural arch fails to
develop fully and fuse, usually at L5 or S1.
numerous sizes and shapes of bones, each of which has precise functions
such as locomotion, protection of vital organs, provision of major
sites for hematopoiesis, and participation in calcium hemostasis and
storage of phosphate, magnesium, potassium, and bicarbonate.
Interestingly, the molecular composition of bone is remarkably
constant. Regardless of the animal species or the particular bone
considered, bone is always a two-phase composite substance made up of
two very different materials. The two components of bone are the
organic matrix, or osteoid, and the inorganic matrix. Various calcium
salts, primarily hydroxyapatite,
are
deposited in crystal form within and between the matrices. These
inorganic crystals give bone its rigidity, hardness, and resistance to
compression.
Mesenchyme arises primarily from the primitive streak and secondarily
from mesodermal segments and the lateral somatic and splanchnic
mesodermal layers (Fig. 1.9). In early embryos,
the mesenchyme acts as an unspecialized “packing material” but soon
differentiates into various tissues and organs.
(osteogenesis), and both involve the transformation of a preexisting
connective tissue into bone tissue. The transformation of fibrous
primitive connective tissue into bone is called intramembranous ossification. The replacement of cartilage by bone is called endochondral ossification.
Except the clavicle and the flat bones of the skull, all bones of the
appendicular and axial skeleton form by endochondral ossification.
cells derived from the neural crest interact with the extracellular
matrix secreted by the epithelial cells arising from the head. If the
mesenchymal cells do not contact this matrix, bone will not develop (117,118). The mechanism responsible for the conversion of mesenchymal cells into bone is still unknown. However, BMPs may play a significant role in this process.
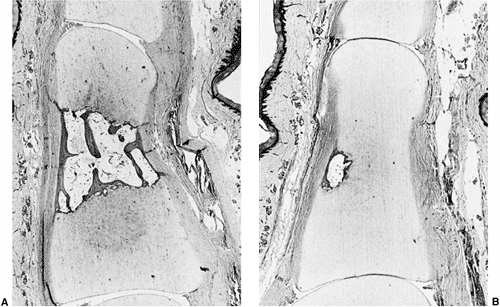 |
|
Figure 1.21 A: Formation of the primary ossification center of a phalanx. Note the central location of bone and bone marrow formation. B: Delayed ossification center formation in a case of digital duplication. (From Ogden JA. Pediatric orthopaedics, 3rd ed. Philadelphia: JB Lippincott, 1990:2, with permission.)
|
cells proliferate and condense into packed nodules. Some of these cells
differentiate into capillaries and others change their shape to become
osteoblasts. These cells are capable of secreting osteoid, the organic
extracellular matrix that will subsequently become mineralized. High
levels of alkaline phosphatase and the appearance of matrix vesicles
mark the commencement of ossification. The cells will eventually be
surrounded by calcified matrix and become osteocytes.
cartilaginous (or endochondral) ossification. The process begins with
the formation of a cartilage precursor or template. Mesenchyme cells
condense and proliferate, but instead of turning into osteoblasts, as
is the case in intramembranous ossification, they become chondroblasts.
These cells will then secrete the cartilage extracellular matrix. Soon
after the cartilaginous model is formed, the cells in the center become
hypertrophic and secrete a matrix that will subsequently be invaded by
capillaries. As this matrix is degraded and the chondrocytes die,
osteoblasts carried by the blood vessels begin to secrete the bone
matrix. Eventually, all cartilage is replaced by bone (Fig. 1.21).
This process appears to be dependent on the mineralization of the
extracellular matrix. Interestingly, a special, condensed mesenchymal
tissue, the perichondrium, surrounds the cartilage model. This tissue
is essentially the
same
as that surrounding the intramembranous centers of ossification, but in
the perichondrium the osteoprogenitor cells remain dormant for a time,
while the cartilage model is enlarged by the chondrocytes.
shaft, and proceeds outward from the medullary cavity and inward from
the periosteum in a repetitive sequence. As the cartilage model is
replaced by bone, extensive remodeling occurs. First, the medullary
cavity is created and enlarged by resorption of the bony struts and
spicules. Second, the developing bone continues to enlarge through both
interstitial and appositional growth. The same repetitive sequence of
events occurs in the epiphyseal centers of ossification. Once the shaft
and epiphyses are ossified, leaving the cartilage physeal plates
between them, each skeletal segment increases in size until maturity.
The initiation of the endochondral ossification process, as well as the
highly ordered progression of the chondrocytes through the growth
plate, must be under strict spatial and temporal control. In view of
the complexity of the process, it is remarkable that the human limb
bones can grow for some 15 years independently of each other, and yet
eventually match to an accuracy of 99.8%.
uniform, and different bones grow at different rates. Patterning of the
embryo occurs while the organs are still very small. For example, the
limb has its basic structure established when its size is about 1 cm
long. Yet, it could grow to be a hundred times longer. How is this
growth controlled? Most of the evidence suggests that the cartilaginous
elements in the limb have their own individual growth programs. These
growth programs are specified when the elements are initially
patterned, and involve both cell proliferation and extracellular matrix
secretion. An understanding of the processes of bone formation, growth,
and remodeling is fundamental in pediatric orthopaedics.
in all growth plates, is unique to the immature skeleton. Once the
growth plates have been formed, longitudinal growth of the bones occurs
by appositional growth of cells and extracellular matrix from within
the growth plate and by new bone formation on the metaphyseal side. The
rate of increase in the length of a long bone is equal to the rate of
new cell production per column multiplied by the mean height of the
enlarged cell. The rate of proliferation depends on the time the cells
take to complete a cell cycle in the proliferative zone and on the size
of this zone. Generally, the greater the number of chondrocytes and the
higher the plate, the faster the growth rate of the bone (Fig. 1.22).
In addition, total longitudinal growth for the life span of the growth
plate depends on the total number of progenitor cell divisions and the
number of divisions of each daughter cell. The number of cell divisions
is genetically determined, but the rate is influenced by hormonal and
metabolic factors.
structure as an organ, which depends on the integrated function of
three distinct components. The first component is the physeal
cartilage, which is divided into three histologically recognizable
zones: resting, proliferative, and hypertrophic. The second component
is the metaphysis, which is the region where calcified cartilage is
replaced by bone. The third component comprises the circumferential
structures known as the perichondrial ring of LaCroix and the groove of Ranvier.
Each of these components has its unique cellular architecture and
extracellular matrix biochemistry, and their integrated functioning
results in longitudinal and latitudinal bone growth. Interestingly,
although cartilage lacks blood vessels, to a large extent, the
metabolic activity of each zone depends on the blood supply system
around the physis.
center of ossification, contains chondrocytes that are widely dispersed
in an abundant matrix. The cells contain abundant endoplasmic reticulum
characteristic of protein synthesis but low intracellular and ionized
calcium content. The function of these cells is not well understood,
but data indicate that the resting zone is relatively inactive in cell
or matrix turnover, although it may be a source for the continuous
supply of chondrocytes to the proliferative zone.
by longitudinal columns of flattened cells parallel to the long axis of
the bone. These cells contain glycogen stores and significant amounts
of endoplasmic reticulum. The total calcium content is similar to that
in the resting zone, but the ionized calcium content is considerably
greater. The oxygen pressure is high in this zone (57 mm Hg), and this,
together with the presence of glycogen, suggests an aerobic metabolism.
Of the three zones, it has the highest rate of extracellular matrix
synthesis and turnover.
the cells to five to seven times their original sizes in the
proliferative zone. Electron microscopy studies suggest that these
cells maintain cellular morphology compatible with active metabolic
activity. Biochemical studies have demonstrated that the mitochondria
of the hypertrophic chondrocyte is used primarily to accumulate and
release calcium rather than for adenosine 5′-triphosphate (ATP)
production. In addition, these cells have the highest concentration of
glycolytic enzymes and synthesize alkaline phosphatase, neutral
proteases, and type X collagen, thereby participating in
mineralization. Because the growth
plate
is radially constrained by the ring of LaCroix, its volume changes are
expressed primarily in the longitudinal direction. In the last part of
this zone, there is a provisional calcification of the cartilage.
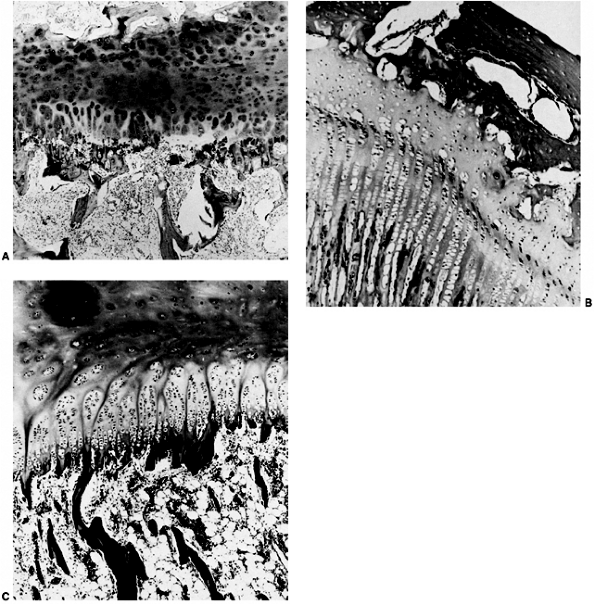 |
|
Figure 1.22 Variations on growth-plate morphology. A: Limited column formation in a slow-growing physis. B: Elongated cell columns in a fast-growing physis (distal femur). C: Some physis form clusterlike groups divided by longitudinal cartilaginous columns. (From Ogden JA. Pediatric orthopaedics, 3rd ed. Philadelphia: JB Lippincott, 1990:17, with permission.)
|
cartilage of the hypertrophic zone and the formation of the primary
spongiosa. Bone formation begins with the invasion of the hypertrophic
lacunae by vascular loops, bringing with them osteoblasts that initiate
the synthesis of bone. The osteoblasts progressively lay down bone on
the cartilage template. Subsequently, the initial woven bone and
cartilage of the primary trabeculae are resorbed by osteoblasts and
replaced by lamellar bone to produce the secondary spongiosa.
structures—a wedge-shaped structure, the groove of Ranvier, and a ring
of fibrous tissue, the ring of LaCroix. The groove of Ranvier consists
of active proliferative cells that contribute to the increase in
diameter, or latitudinal increase, of the growth plate. The ring of
LaCroix contains a thin extension of the metaphyseal cortex and the
fibrous portion of the groove of Ranvier and periosteum; this provides
it with a peripheral supporting girdle around the growth plate (Fig. 1.23).
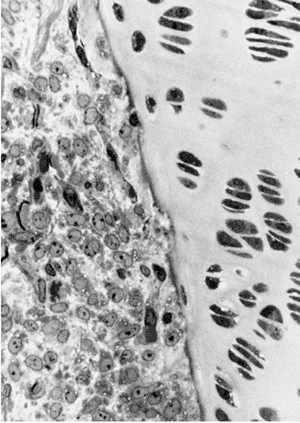 |
|
Figure 1.23
Photomicrograph of the zone of Ranvier demonstrating the demarcation between the cells of the growth plate and the mesenchymal cells of the zone of Ranvier. (From Ogden JA. Pediatric orthopaedics, 3rd ed. Philadelphia: JB Lippincott, 1990:19, with permission.) |
-
The epiphyseal arteries enter the
secondary ossification center and the terminal branches pass through
the resting zone and terminate at the uppermost of the proliferative
cells. -
The nutrient artery of the diaphysis
supplies the extensive capillary loop network at the junction of the
metaphysis and growth plate. -
The perichondrial arteries supply the
ring of LaCroix and the groove of Ranvier. Capillaries from this system
communicate with the epiphyseal and metaphyseal systems in addition to
the vessels of the joint capsule.
length. Because the metaphysis is larger than the diaphysis, some of it
must be trimmed during the process of remodeling. This process is
called funnelization. In the area called the cut-back zone,
osteoclasts resorb the peripheral bone of the metaphysis. In this way,
the metaphysis gradually narrows to the width of the diaphysis. The
epiphysis also grows in circumference by a process called hemispheration,
which is a process similar to that of the growth plate. Therefore, the
bone acquires its final shape by a combination of intramembranous
ossification at the diaphyseal level and endochondral ossification at
the epiphysis and growth plate, along with a process of elongation,
funnelization, hemispherization, and cylinderization (Fig. 1.24).
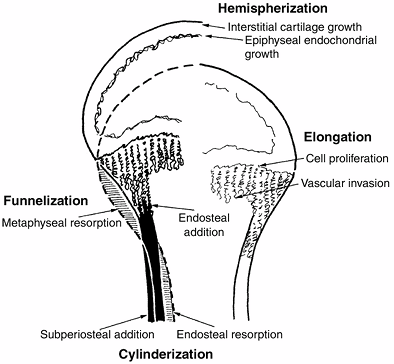 |
|
Figure 1.24
Diagrammatic representation of the remodeling process of bone during growth. Bone resorption and deposition result in longitudinal growth and shape changes of the epiphysis, metaphysis, and diaphysis. (Adapted from Ham AW. Some histophysiologic problems peculiar to calcified tissue. J Bone Joint Surg Am 1952;34:701, with permission.) |
is inherited from each parent in diploid organisms. Therefore, two
alleles of each gene are present, which may or may not be the same.
at the tip of the developing limb bud in chickens and mammals. This
structure interacts with the underlying mesoderm of the PZ in the
development of the limb bud.
cell death is unlike necrotic cell death in that no damage occurs to
surrounding tissue. It is characterized by fragmentation of the DNA and
shrinkage of the cell. It occurs widely during development.
corresponds to the blastula stage of other animal embryos. It is the
stage at which the embryo implants in the uterine wall.
organism. In most animals it is organized around two main axes, the AP
axis and the DV axis, with a plane of bilateral symmetry where it
exists.
structurally and functionally different from one another and become
distinct cell types, for example, muscle, bone, and cartilage cells.
after fertilization. This stage consists of a series of rapid cell
divisions without growth, dividing up the embryo cells into a ball of
much smaller cells called a blastula. Cleavage marks the beginning of a multicellular organism.
undergo a change in behavior and maximize their contact to form a
compact ball of cells. This is perhaps the most crucial difference
between mammalian cleavage and cleavages in all other types of animals.
of a cell such that its eventual cell type (or group of cell types) is
fixed. A determined cell will not alter its eventual cell type even
when grafted into other regions of the embryo.
(mouse) or blastoderm (chicken) that gives rise to the embryo proper.
In the mouse, it develops from cells of the inner cell mass.
into a multilayered structure and rearranges to form the three
embryonic germ layers. In addition, during gastrulation the body plan
of the organism is established.
will give rise to distinct types of tissue. Most animals have three
germ layers—ectoderm, mesoderm, and endoderm.
chromosomes (half the diploid number of chromosomes), and therefore
containing only one copy of each gene. In most animals the only haploid
cells are the gametes—the sperm or egg.
end of the primitive streak that will give rise to the notochord. It
corresponds to the Spemann organizer in amphibians.
The homeodomain is present in a large number of transcription factors
that are important in development, and are conserved—present with
relatively minor variations—in a large number of species of animals
from fruit flies to mice to humans.
(fruit fly). It plays an important part in specifying body segment
identity during development, for example, in specifying thorax versus
abdomen.
significant similarity in their nucleotide sequence and are derived
from a common ancestral gene, often, but not always, showing similarity
in function.
embryo derived from the inner cells of the morula, which form a
discrete mass of cells in the blastocyst. Some of the cells of the
inner cell mass give rise to the embryo proper.
cells that lies lateral and ventral to the somites and gives rise to
the tissues of the heart, kidney, gonads, and blood.
tissue, usually of mesodermal origin, whose cells are capable of
migration. Some epithelia of ectodermal origin, such as the neural
crest, undergo an epithelial-to-mesenchymal transition.
skeletomuscular system, connective tissues, and blood, and to internal
organs such as the kidney and heart.
formation based on its local concentration—often associated with a
gradient or threshold effect.
neural plate that migrate to different regions of the body and give
rise to the autonomic nervous system, the sensory nervous system,
melanocytes, and some cartilage of the head.
development of part of the embryo or the whole embryo. In amphibians,
the organizer usually refers to the Spemann organizer.
developing embryo acquire identities that lead to a well-ordered
spatial pattern of cell activities.
DNA transcription and that contain both a homeodomain and another
protein motif—the “paired” motif. PAX genes are related to the
pair-rule genes in Drosophila, which are expressed in transverse stripes in the blastoderm, each pair-rule gene being expressed in alternate parasegments.
cell or organism, as distinguished from the genotype that includes
alleles that are not expressed in the phenotype of the cell or organism.
the tip of the bud that result in limb outgrowth and that are under the
regulatory control of the AER.
both sides of the notochord. They give rise to muscles, the vertebral
column, and the dermis.
limb bud that has been identified in mice and chickens, and that is
critical in specifying the AP axis in the developing limb.
J, Belmonte JC. Extracellular modulation of the hedgehog: Wmt and TGF-β
signalling pathways during embryonic development. Curr Opin Genet Dev 1999;9:427–433.
BJ. A reexamination of the cleavage pattern in eutherian mammalian
eggs: rotation of the blastomere pairs during second cleavage in the
rabbit. J Exp Zool 1975;193:235–248.
MAH, Barton SC, Norris ML. Development of reconstituted mouse eggs
suggests imprinting of the genome during gametogenesis. Nature 1984;308:548–550.
JF, Errick J. Inductive activity and enduring cellular constitution of
a supernumerary apical ectodermal ridge grafted to the limb bud of the
chick embryo. Dev Biol 1976;50:16–25.
K. The effect of the ectoderm on the dorsoventral pattern of epidermis,
muscles and joints in the developing chick leg: a new model. Anat Embryol 1996;193:377–386.
JW, Gasseling MT. Ectoderm-mesenchymal interactions in the origin of
the wing symmetry. In: Fleischmajer R, Billingham RE, eds. Epithelial-mesenchymal interactions. Baltimore, MD: Williams and Wilkins, 1968:78–97.
M, Singley CT, Reiter RS. The influence of epithelia on cartilage and
loose connective tissue formation by limb mesenchyme cultures. Dev Biol 1981a;86:471–482.
CA, Holmyard DP, Millen KJ, et al. Examining pattern formation in
mouse, chicken and frog embryos with an En-specific antiserum. Development 1991;111:287–298.
SH, Rauskolb C, Wilson R, et al. A family of mammalian fringe genes
implicated in boundary determination and the notch pathway. Development 1997;124:2245–2254.
B, Bashirullah A, Dagnino L, et al. Fringe boundaries coincide with
notch-dependent patterning centers in mammals and alter notch-dependent
development in Drosophila. Nat Genet 1997;16:283–288.
L, Tickle C, Vogel A, et al. FGF-4 replaces the apical ectodermal ridge
and directs outgrowth and patterning of the limb. Cell 1993;75:579–587.
H, Nakagawa T, Yamamoto A, et al. The mesenchymal factor, FGF10,
initiates and maintains the outgrowth of the chick limb bud through
interaction with FGF8, an apical ectodermal factor. Development 1997;124:2235–2244.
MJ, Izpisua-Belmonte JC, Abud H, et al. Fibroblast growth factors
induce additional limb development from the flank of chick embryos. Cell 1995;80:739–746.
P, Richardson M, Brickell P, et al. Bone morphogenetic proteins and a
signalling pathway that controls patterning in the chick limb. Development 1994;120:209–218.
KJ, Reiter RS, Kurriger GL, et al. Spatial and temporal expression of
CD44 isoforms in the developing and growing joints of the rat limb. J Orthop Res 1998;16:100–103.
D, Schwarz EN, Rosier RN, et al. ALK2 functions as a BMP type I
receptor and induces Indian hedgehog in chondrocytes during skeletal
development. J Bone Miner Res 2003; 18(9):1593–1604.
M. Les phases d’activite morphogene du mesoderme somatopleural pendant
le developpement precoce du membre chez l’embryon de poulet. Ann Embryol Morphol 1971;4: 281–298.
JL, Lapointe F, LeDouarin NM. The dorsoventral polarity of the
presumptive limb is determined by signals produced by the somites and
by the lateral somatopleure. Development 1997;124:1453–1463.
RD, Ensini M, Nelson C, et al. Induction of the LIM homeobox gene Lmxl
by Wnt7a establishes dorsoventral pattern in the vertebrate limb. Cell 1995;83:631–640.
Y, Niswander L. Interaction between the signaling molecules Wnt7a and
SHH during vertebrate limb development: dorsal signals regulate
anteroposterior patterning. Cell 1995;80: 939–947.
T, Kume S, Amasaki Y, et al. The Wnt/calcium pathway activates NA-AT
and promotes ventral cell fate in Xenopus embryos. Nature 2002;417(6886):295–299.
DJ, Tabin CJ. Analysis of HoxD-13 and HoxD-11 misexpression in chick
limb buds reveals that Hox genes affect both bone condensation and
growth. Development 1997;124(3):627–636.
P, Kilpatrick MW, Toudjarska I, et al. Split-Hand/ split-foot
malformation is caused by mutations in the p63 gene in 3q27. Am J Hum Genet 2000;67:59–66.
D, Kan S, Oldridge M, et al. Missense mutations in the homeodomain of
HOXD13 are associated with brachydactyly types D and E. Am J Hum Genet 2003;72:984–997.
E, Perveen R, Donnai D, et al. De novo GL13 mutation in acrocallosal
syndrome: broadening the phenotypic spectrum of GL13 defects and
overlap with murine models. J Med Genet 2002;39:804–806.
U, Bornholdt D, Scott HS, et al. The phenotypic spectrum of GLI3
morphopathies includes autosomal dominant preaxial polydactyly type-IV
and postaxial polydactyly type-A/B; No phenotype prediction from the
position of GLI3 mutations. Am J Hum Genet 1999;65(3):645–655.
F, Hars C, Illien F. Molecular mechanism underlying limb anomalies
associated with cholesterol deficiency during gestation: implications
of hedgehog signaling. Hum Mol Genet 2003;12(10):1187–1198.
U, Blouin J-L, Solanki JV, et al. An autosomal dominant triphalangeal
thumb: polysyndactyly syndrome with variable expression in a large
Indian family maps to 7q36. Am J Med Genet 1996;66:209–215.
A, Kieny M, Mauger A. Developmental fate of the somitic mesoderm in the
chick embryo. In: Ede DA, Hinchliffe JR, Balls M, eds. Vertebrate limb and somite morphogenesis. Cambridge: Cambridge University Press, 1977:421–432.
M, Gruss P. Homeotic transformations of murine vertebrae and
concomitant alteration of Hox codes induced by retinoic acid. Cell 1991;67:89–104.
SR. Hoxb-4 (Hox-2.6) Mutant mice show homeotic transformation of a
cervical vertebra and defects in the closure of the sternal rudiments. Cell 1993;73:279–294.
O, Capecchi MR. Regionally restricted developmental defects resulting
from targeted disruption of the mouse homeobox gene hox-1.5. Nature 1991;350:473–479.
WJ. Diastematomyelia and the Klippel-Feil syndrome. Relationship to
hydrocephalus, syringomyelia, meningocele, meningomyelocele, and
iniencephalus. Cleve Clin Q 1964;31: 19–44.
Y, Epstein DJ, St JB, et al. Sonic hedgehog, a member of a family of
putative signaling molecules, is implicated in the regulation of CNS
polarity. Cell 1993;75:1417–1430.
