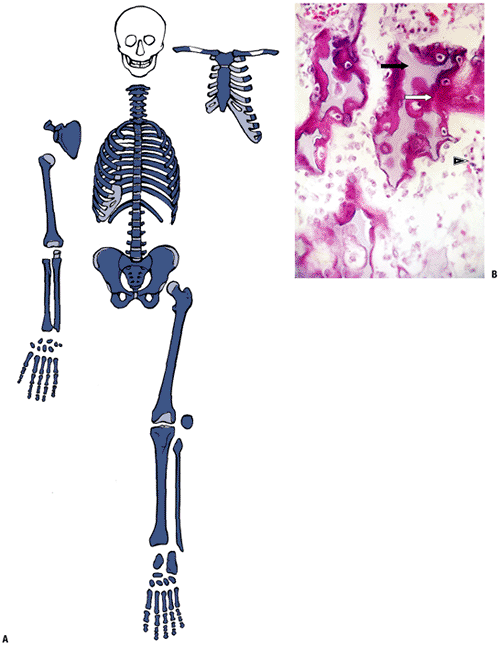
Growth and Development of the Musculoskeletal System
August 18, 2004. The Chairman of the Department of Orthopaedic
Pathology, Armed Forces Institute of Pathology, Washington, DC, and an
educator par excellence, he was in the
process of preparing this chapter with me (T.V.S.), and the chapter is
dedicated to his memory. Humble, brilliant, and a contributor in many
ways to the world of orthopaedics for 35 years, he will be missed.
ordered sequence of steps that begins in the embryo. Growth follows a
logical order of sequential appearance, maturation, and removal of
progressively more differentiated structures and tissues. The
morphology of this sequence will be demonstrated in this chapter. The
genetic mechanisms underlying this process are not fully known;
reference will be made, where appropriate, to areas of ongoing
investigation.
-
Central core gives rise to bones and joints.
-
Around central core: looser tissue from which muscles, tendons, ligaments, and vessels will develop
-
Ectodermal covering with a central condensation of mesoderm (Fig. 14-1)
|
Table 14-1 Origin of Skeletal Elements According to Mesodermal Site
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
-
Governed by relationship between the thickened distal ectoderm (apical ectodermal ridge [AER]) and the underlying mesoderm
-
Extremely rapid reproduction of large
numbers of cells in the central core as well as in the ectoderm results
in a rapid elongation of the limb bud. -
Several growth factors regulate activities at the AER and adjacent zones:
-
Fibroblast growth factors (FGFs;
especially FGF4 and FGF8) control cell differentiation in the
immediately adjacent limb bud along its longitudinal axis. -
Zone of polarizing activity (ZPA) at
posterior distal part of the limb bud controls anterior posterior
patterning through the protein sonic hedgehog (Shh).
-
-
Nerves extend outward from the central nervous system and attach themselves to developing muscles.
-
Innervation of bone and periosteum is by
sympathetic and sensory fibers that contribute to regulation of
vascular control and perception of nociceptive stimuli. -
Nerves accompany vessels into bone both into the marrow space and into the Haversian and Volkmann canal systems.
-
Unfolds from proximal to distal in a sequential manner (hip, then femur, then knee, then calf, and so forth; see Fig. 14-1)
-
Cartilage formation from the mesenchymal condensations begins during the sixth week.
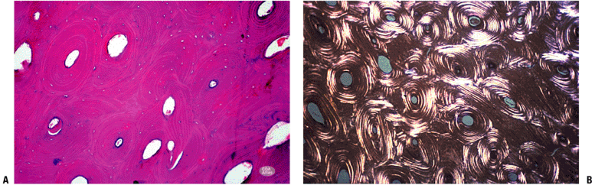
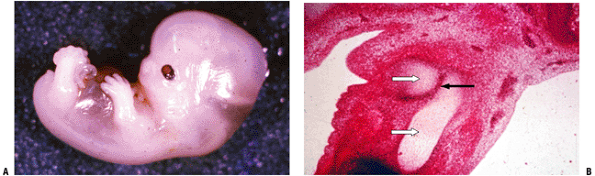 |
|
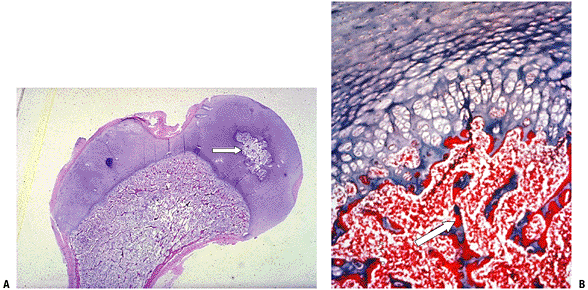
Figure 14-1
(A) Seven-week embryo with well-developed limb buds. Lower limb development lags behind upper limb throughout life. Rapidly developing cells are susceptible to insult. (B) Limb bud cross-section showing central condensation of immature cartilage cells (appearing as dark dots at this power) forming two early cartilage models with immature matrix production (white arrows), as well as the dark zone between the two cartilage models that will become a joint (black arrow). (Courtesy of the Armed Forces Institute of Pathology, Washington, DC.) |
-
To understand the further development of the limb bud, one must consider several fundamental aspects of bone development.
-
Bone is not created de novo but rather as an applique on or within a pre-existing mesenchymal framework. In skeletal embryogenesis, then, bone forms:
-
On a collagen framework called woven bone in the craniofacial bones and clavicle, or
-
On a calcified cartilage framework in the remainder of the skeleton.
-
Once bone has formed, bone modeling or remodeling involves bone deposition on previously made bone.
-
osteoclasts. Once formed, the skeleton attempts to maintain homeostasis
in the long run by balancing formation and deletion. A surgeon, Julius
Wolff, proposed in 1891 that mechanical stress determined the
architecture of bone in that bone is made in areas where there is
mechanical stress and is deleted where there is little stress.
Homeostasis in the skeleton involves a balance among mechanical,
metabolic, and circulatory influences acting on the effector cells,
osteoblasts and osteoclasts. The discipline of cell biology is now
demonstrating that the final pathways directing these cells in these
fields of influence involve polypeptide signaling substances, hormones,
and their receptor systems.
-
Osteoblast origin: subset of pluripotent stromal stem cell line
-
Preosteoblast (flat, spindle cell):
inducible osteoprogenitor cells (IOPCs) proliferate and make bone
morphogenic protein (BMP) and osteopontin-
Molecular regulator of osteoblast
differentiation is transcription factor Cbfa1; bone morphogenic
proteins 2 and 7 (BMP2, BMP7) regulate Cbfa1 gene expression (Box 14-1).
-
-
Proliferating osteoblast (cell is plumper, increasing rough endoplasmic reticulum)
-
Differentiating osteoblast
-
Mature osteoblast (cell is cuboidal, looks like a plasma cell) expresses alkaline phosphatase
-
Osteoblast, matrix mineralizing: most
osteoblasts undergo apoptosis, some become osteocytes, some remain on
the endosteal surface as bone lining cells-
Osteocalcin is expressed by the osteoblast in this stage.
-
-
Osteocyte, resident in bone
-
One in ten osteoblasts does not
participate in the extreme activity creating the osteoid seam but
becomes a resident osteocyte, responsible for maintaining the bone that
has been produced around it. -
Cells communicate through dendritic processes and gap junctions, forming a functional syncytium with the bone lining cells.
-
Life span of an individual osteocyte may be months (fetal bone) to years (adult bone).
-
-
Bone surface lining cell (BSLC)
-
Can either be resting or in an activated state making bone
-
Resting cells provide cytokine signal to activate bone remodeling.
-
May adopt fat cell morphology in the aging
-
and is either skeletal or extraskeletal; dysregulation of these
precursor cells may manifest clinically in diseases such as
fibrodyplasia ossificans progressiva, a genetic disease characterized
by the ossification of skeletal muscle and other connective tissues, or
in the periarticular heterotopic ossification that can complicate
skeletal trauma.
-
Calcified cartilage acts as a nidus on
which bone is deposited by osteoblasts derived from perivascular stem
cells arising from vessels in the adjacent marrow. -
Figure 14-4 is a
high-power cut showing a primary trabecula in a growth plate; primary
trabeculae anchor the growth plate to the rest of the bone and act as
an initial lattice for transmission of mechanical force. -
This region undergoes rapid remodeling in the fetus and young and remains the site of most remodeling of bone throughout life.
-
Primary trabeculae thus remodel rapidly,
with removal by osteoclasts of bone and calcified cartilage and
replacement by bone, thus forming a secondary trabecula.
-
Lamellar bone consists of layers of
collagen, each layer arranged at a different angle from its neighbor,
like plywood; this gives mechanical strength to the composite
structure. It can be applied to pre-existing woven bone or to lamellar
bone.-
In woven bone the lamellar bone is
applied in sheets as an inlay apposed to the inner surface of the
randomly oriented trabecula; the effect is to strengthen the construct. -
In lamellar bone the application of new bone is part of the modeling or remodeling process.
-
-
Woven bone formation is rapid, and the
bone that results is a mechanically weak, open mesh that must be
reinforced by a lamellar bone fill-in before significant weight
transmission.
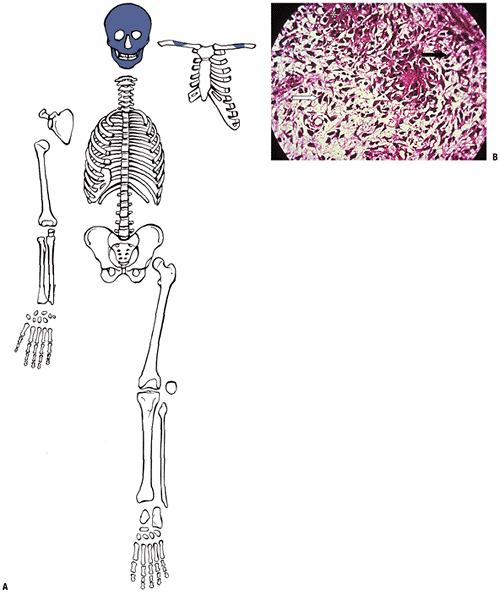 |
|
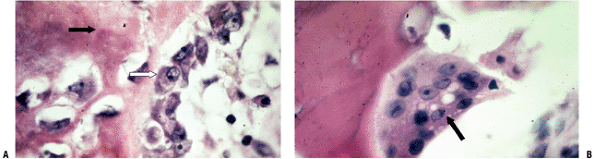
Figure 14-2
Woven bone formation. (A) Facial bones and central clavicle start from a collagenous framework. (B) Spectrum of cells making woven bone. Spindle-shaped mesenchymal cells (black arrow) change into plumper pre-osteoblasts. Pre-osteoblasts (white arrow) are larger cells not yet elaborating collagen. Osteoblast deposits collagen subunits into extracellular space, where they aggregate into the triple helix of collagen (dark-staining extracellular material; asterisks). (Courtesy of the Armed Forces Institute of Pathology, Washington, DC.) |
 |
|
Figure 14-3 Woven bone formation. (A) High-power view of osteoblasts depositing osteoid, forming woven bone. White arrow
points to an osteoblast that looks like a plasma cell. Cytoplasm is packed with rough endoplasmic reticulum for collagen production, packaged as subunits in the juxta-articular Golgi apparatus for excretion. Osteoid (collagen) is the deposited amorphous dark-staining extracellular material (black arrow) that then undergoes a complex biochemical preparation for mineralization for 10 days after deposition. (B) An osteoclast, a motile multinucleated giant cell packed with mitochondria for energy production, is shown actively deleting bone (arrow), creating a Howship’s lacuna under the ruffled border of its attachment site through dissolution of hydroxyapatite in a highly acidic local environment, and through enzymatic destruction of the collagen, marking the surface path of osteoclast. (Courtesy of the Armed Forces Institute of Pathology, Washington, DC.) |
-
Process whereby bone, formed initially in the embryo, is shaped into the adult form as growth occurs
-
Responsible for the large changes in bone structure during growth
-
Changes in bone structure occur on existing bone structure.
-
Bone structure alterations occur by independent action of osteoblasts and osteoclasts.
-
Bone resorption and formation may occur on different surfaces.
-
Process whereby current bone structure is maintained
-
Cannot cause large changes in bone structure at a given site
-
Changes in bone structure occur on or within existing bone structure (same as for modeling).
-
Osteoblasts and osteoclasts do not act
independently but are coupled through cytokine activity and mechanical
forces (different than for modeling). Table 14-2 outlines the essential features of osteoblasts and osteoclasts.-
Coupled bone deletion and formation at
the same site: Intracortical remodeling is affected by the bone
remodeling unit (BRU)—teams of osteoclasts excavating a hole in the
cortex (10 longitudinal surges of clastic activity over 2 months)
followed by osteoblasts filling in the hole (months) -
Cancellous bone remodeling occurs at many
sites in the honeycomb trabecular array, with activation of bone
surface lining cells, recruitment of marrow osteoclasts, creation of a
Howship’s lacuna (see Fig. 14-3), and activation of stem cells to become osteoblasts, which fill the defect.
-
preformed, the craniofacial bones as woven bone and the rest as a
multiplicity of cartilage models (Fig. 14-6). Subsequent growth of the models progresses in stages.
-
Before development of ossification centers:
-
Cartilage models undergo considerable growth in size:
-
By appositional induction at their surface (“perichondrium”) and
-
By internal cartilage cell proliferation and maturation
-
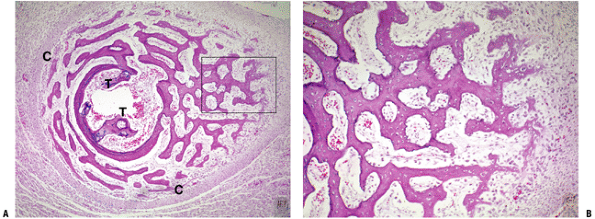
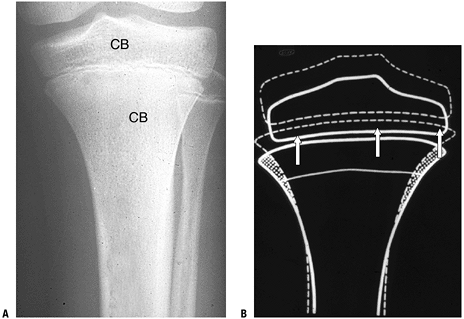
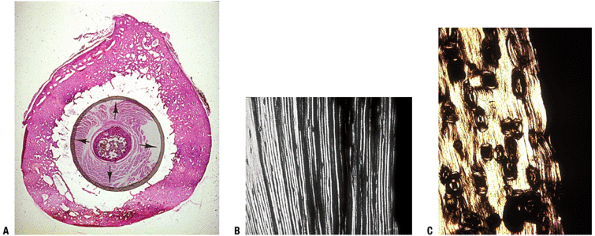
P.326![]() Figure 14-4
Figure 14-4
(A) Enchondral bone formation. Much of the skeleton arises from
cartilage model bones; these grow in length at growth plates. (B)
Enchondral bone formation: primary trabeculae formation. “Primary
trabeculae” form a latticework (like a honeycomb) of intersecting bone
structures, each formed by a process in which the calcified cartilage
core (dark blue-staining material, black arrow), a remnant of the growth plate cartilage, acts as a nidus on which woven bone (dark pink—staining material; white arrow) is deposited. In the surrounding marrow space, a fibrovascular stroma (arrowhead)
supplies stem cells and blood supply to support the enchondral bone
process. (Courtesy of the Armed Forces Institute of Pathology,
Washington, DC.)P.327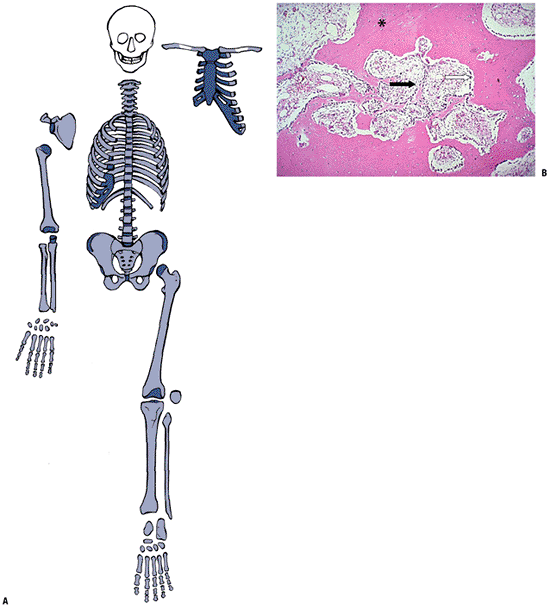 Figure 14-5
Figure 14-5
Bone modeled bone (lamellar). (A) Once created, bone throughout the
entire skeleton is altered through modeling and remodeling. (B)
Photomicrograph shows dense cortical bone (solid pink—staining
material; asterisk), which has been
tunneled through aggressive osteoclast action after a stress fracture.
Bone is being formed rapidly to refill the spaces, as generation after
generation of osteoblasts line up and deposit lamellar bone on the
margins of the space. Mature, adult cortical bone is a solid structure (asterisk)
and consists of osteons that have formed over time through repeated
stress-directed cutting cone activity. The marrow space is a
fibrovascular stroma (black arrow) that supports the lamellar bone formation (white arrow).
The process effectively fills in holes created by osteoclast activity
in an overuse situation. (Courtesy of the Armed Forces Institute of
Pathology, Washington, DC.)P.328Table 14-2 Essential Features of Osteoblasts and OsteoclastsOsteoblast (OB) Osteoclast (OC) Function Only bone-forming cell Only bone-deleting cell Cell cycle Stem cell ← pre-OB ← proliferating OB ← mature OB ← mineralizing OB ← apoptotic OB/osteocyte/ bone lining cell Marrow and circulating OC precursors ← fusion of OC precursors ← multinucleated OC ← apoptotic OC Cell function Expression of type I collagen,
ALP, osteonectin, osteopontin, bone sialoprotein, biglycan, decorin,
matrix Gla-protein, and bone acidic glycoproteinAttachment to the bone surface
← polarization of the cell surface into three distinct membrane
compartments ← formation of a sealing zone ← resorption ← detachment ←
cell death (apoptosis)Cytoplasm Cytoplasm packed with rough endoplasmic reticulum, stains blue with hematoxylin Cytoplasm packed with
mitochondria, for energy production associated with bone deletion.
Clear areas contain material for export from cell.Nucleus Eccentric nucleus with adjacent Golgi apparatus Multinucleated giant cell with
ruffled border isolating highly acidic environment for mineral
dissolution, enzymatic collagen removal, and abundant acid phosphataseSurvival As blast, up to 10 days
As resident osteocyte, months to years24 to 48 hours Efficiency Up to 100 osteoblasts required to fill bone deleted by one osteoclast; requires 3 months Digests 3 times its cell volume in 24 hours Clast 100 times more efficient than blast in terms of volume and creates its cavity in 1 day, while refill takes months Clast efficiency is responsible for symptoms related to acute overuse (stress reactions leading to stress fractures) ![]() Figure 14-6
Figure 14-6
Cartilage cell life cycle. Sequential cartilage maturation from early
cartilage model formation to emerging primary and secondary centers of
ossification. (A) Mesenchymal condensation early at sites of bone
model, adjacent perichondrium, and interzone (future site of joint).
(B) Differentiation and cellular function have produced cartilage
matrix of the bone model and collagen at the perichondrium. (C) Primary
center of ossification has formed with a sleeve of diaphyseal bone
around the marrow cavity, a trabecular array of woven bone, growth
plates, epiphyseal cartilage, and early joint space. (D) Fetal joint
now has fully formed with secondary center of ossification, articular
cartilage, joint capsule, and other elements as already listed. (E)
Emergence of ossification centers allows radiographic visualization.
Gross specimen correlates with radiograph, demonstrating secondary
center of ossification in the tibia but none in the fibula. (A-D,
Courtesy of Proceedings of Annual Canadian Orthopaedic Association Basic Science Course,
DE Sweet, lecture notes, Skeletal Radiologic/Pathologic Correlation,
1987–2003. E, Courtesy of the Armed Forces Institute of Pathology,
Washington, DC.) -
-
After development of ossification centers:
-
Emergence of sequential primary and then secondary ossification centers subdivides long bone cartilage models (see Fig. 14-6).
-
Epiphysis (secondary ossification center)
-
Epiphyseal growth plates (physeal growth center/ physis)
-
Metaphysis
-
Diaphysis
-
-
Emergence of secondary ossification
centers allows radiographic identification, providing a basis for
evaluating chronological versus biologic bone age.
-
condensation of mesenchymal cells, which then produce cartilage matrix
from the center of the bone, spreading outward toward the ends. This
step, differentiation of cells to become chondrocytes, is regulated by
a DNA transcription factor, Sox9, which controls the expression of
genes coding for collagen (col), proteoglycan (PG), or non-collagenous
proteins. Sox9, in conjunction with L-Sox5 and Sox6, binds to specific
enhancer regions in the promoters of these target genes to activate
gene transcription. Individual cartilage cells undergo sequential
stages of development, which lead to development of the primary center
of ossification.
-
Differentiation from uncommitted stromal cell
-
Rest
-
Reproduction
-
Maturation/matrix production
-
Hypertrophy
-
Matrix mineralization: first identifies primary center of ossification on x-ray
-
Senescence and death (apoptosis)
-
Initiation of chondrification
-
Begins near the center
-
Cartilage cells there reach the final stages of mineralization and terminal differentiation first (Fig. 14-7).
-
-
Woven bone formation
-
Change in cartilage maturation induces
the formation of a sleeve of coarsely woven bone at the level of
maximum cartilage cell hypertrophy (see Fig. 14-7). -
Stimulus for this induction is unknown.
-
Perichondrium changes to periosteum.
-
-
Vascular invasion
-
Death of cartilage cells may provide the signal for vascular invasion; it is likely that cytokines direct the process.
-
Invading vascular granulation tissue brings in endothelial cells and precursor cells that differentiate into blasts and clasts.
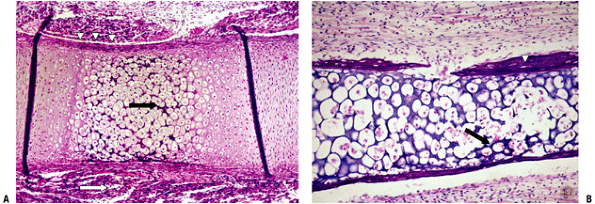 Figure 14-7
Figure 14-7
Primary center of ossification. (A) Central portion of cartilage model
where cartilage cell death (absence of cells centrally) has left behind
dark-staining calcified cartilage (black arrow),
which will act as a nidus for apposition of woven bone in the formation
of primary trabeculae; this defines enchondral bone formation. On
either end, the cartilage cells undergo growth through their cell
cycle, showing growth plate morphology. White arrow
indicates surrounding mesenchymal anlage forming early skeletal muscle.
(B) Later, vascular invasion occurs through a gap in the woven bone
that has formed from the inner layer of the periosteum. The cortex at
this time is a coarsely woven sleeve of bone, incomplete where the
vessel enters. Cartilage cells are dead; the calcified cartilage black arrow) is removed by osteoclasts emerging as precursors from the vessels; no bone is deposited yet on the cartilage cores. Arrowheads
indicate primitive cortex, arising from the cambium layer of the
periosteum, which is woven bone in the embryo, lamellar bone in the
child. (Courtesy of the Armed Forces Institute of Pathology,
Washington, DC.) -
-
Removal of chondrocytes and calcified cartilage
-
Monocytes and specialized giant cells
(chon-droclasts, analogous to osteoclasts) remove most of the dead and
dying chondrocytes and calcified cartilage (see Fig. 14-7).
-
-
Primary trabecula formation
-
Osteoblasts differentiate out of the cellular blastema and reinforce the remaining calcified cartilage remnants (Fig. 14-8).
-
Primary trabecula
-
Definition: the combination of calcified cartilage core with apposed woven bone
-
Array of interconnected primary
trabeculae consists of plates of bone that act to transmit force from
the cortex to the epiphysis and articular surface
-
-
-
Secondary trabecula formation
-
Secondary trabecula (Fig. 14-9)
-
Lacks calcified cartilage cores
-
Makes up the metaphyseal trabecular array in long bones and the core of cuboidal bones
-
Undergoes repeated remodeling throughout life
-
-
Remodeling of these occurs with removal
of those that are not needed mechanically, and reinforcement of those
that become the selected transmitters of mechanical force.
-
-
Growth plate formation
-
Growth plates (see Fig. 14-8)
become clearly visible on either end with identifiable zones of
function correlating with the stages of development of cartilage cells;
the entire bone model (diaphysis, metaphyses, growth plates) is
contained within a periosteal sleeve that merges with the perichondrium
on either end. This confluence of periosteum and perichondrium is
termed the perichondrial ossification groove of Ranvier, containing the
circumferential bony ring of Lacroix. -
Bony ring of Lacroix
-
This is a woven sleeve of bone maintaining its position at the level of the hypertrophic cartilage cells (see Fig. 14-8). It acts as a rigid cup supporting the cartilaginous epiphysis.
-
-
Ossification groove of Ranvier
-
An outer fibrous perichondrial layer formed by fibroblasts and collagen, continuous below with the periosteum
-
An inner layer of undifferentiated
loosely packed cells that become chondrocytes, providing lateral growth
adjacent to the germinal and proliferating layers -
Below this, the inner layer differentiates into osteoblasts that form the ring of Lacroix.
-
-
structure of the growth plate, where reserve cartilage cells in the
physeal growth center give rise to multiple successive generations of
proliferating clones of cartilage cells. The growth plate can be
divided into zones, as in Figure 14-9. There are a variety of descriptions of the cell layers, with confusion arising because some refer to morphology and others to cell function.
The cartilage cells follow the cycle that applies to cartilage at most
sites in the body: rest, proliferation, hypertrophy, and programmed
cell death or apoptosis. Matrix synthesis occurs throughout with
variation of content according to cell layer. As the chondrocyte
is the only cell present in the growth plate, it is responsible for all activities:
 |
|
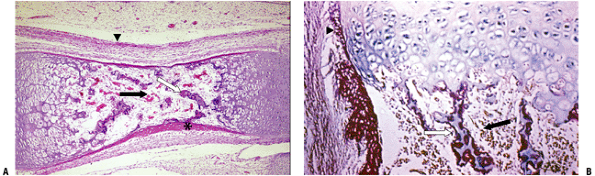
Figure 14-8 Primary center of ossification. (A) The initial cortex (asterisk)
is a sleeve of woven bone that ends on either end of the cartilage model at the hypertrophic zone of the growth plate. This is the site of the circumferential ring of Lacroix. Most of the calcified cartilage is gone centrally and replaced by a fibrovascular stroma (black arrow); there are a few primary trabeculae centrally (white arrow). The arrowhead indicates the several layers of periosteum. (B) Growth plate with woven bone reinforcement of primary trabeculae. The arrowhead indicates the termination of cortex at the hypertrophic zone. (Courtesy of the Armed Forces Institute of Pathology, Washington, DC.) |
 |
|
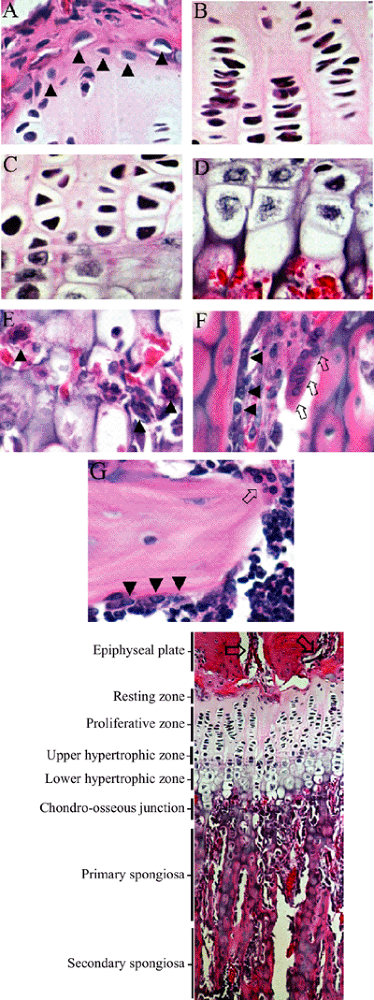
Figure 14-9 Growth plate morphology. (A) Resting zone: One cell per lacuna, large amount of PG; high ratio of extracellular matrix to cells. Arrowheads identify resting zone stem cells. 40 × magnification, H&E. (B) Proliferative zone:
Flattened disc morphology with occasional mitotic profile, may have many cells within a single large lacuna, highest rate of PG synthesis and turnover. 40 × magnification, H&E. (C) Upper hypertrophic (transition) zone: Characterized by loss of capacity to divide and increase in size, cartilage cells termed prehypertrophic, calcium storage begins, PG synthesis continues. 40 × magnification, H&E. (D) Lower hypertrophic zone: Matrix calcification, breakdown of nuclear envelope, and apoptosis of cells. 40 × magnification, H&E. (E) Chondro-osseous junction: Vascular invasion and multinucleate osteo-/chondroclastic matrix removal. Arrowheads show chondroclasts at transverse septa of evacuated lacunae. 40 × magnification, H&E. (F) Primary spongiosa: Endochondral bone formation over cartilaginous trabeculae by osteoblastic osteoid (pink) deposition over cartilage scaffold (blue-purple) and continuation of remodeling via osteo-/chondroclastic matrix resorption, removing cartilage scaffold core. Osteoblasts identified by black arrowheads, osteo-/chondroclasts by arrows. 40 × magnification, H&E. (G) Secondary spongiosa: Remodeling of secondary, osseous trabeculae by osteoblastic bone deposition over osteoid scaffold and continuation of osteoclastic matrix resorption. Osteoblasts identified by black arrowheads, osteoclasts by arrow. 40 × magnification, H&E. Bottom: Micrograph of 10-week-old male rat proximal tibial growth plate and metaphyseal primary and secondary spongiosa. Arrows identify epiphyseal vascular structures. 10 × magnification, H&E. |
-
Synthesis, maintenance, and breakdown of the collagen and extracellular matrix
-
Elaboration of the cytokines that participate in the direction of these processes
extracellular matrix acts as a reservoir in which there is interaction
among cells, the surrounding structure, integrins, circulating systemic
hormones, and local polypeptide signaling substances. The vessels also
seem to be intimately involved. Actual growth is a function of
increased cell mass (proliferation plus hypertrophy) and matrix
elaboration. All of this occurs in place, with the cells finally
undergoing senescence and programmed cell dropout (apoptosis) as
calcification of the surrounding cartilage occurs. This combined
activity essentially lifts or pushes the overlying epi-physis away from
the underlying metaphysis. The dynamics of chondrocyte differentiation
are such that a cell moves through resting, proliferating, and
hypertrophic stages, ending with calcification over 24 hours, with
apoptosis involving 18% of this time (see Fig. 14-9, Table 14-3).
-
Epiphyseal vessels supply the secondary center of ossification and the resting, proliferative, and hypertrophic zones.
-
Resting zone
-
Vessels perforate but do not supply cells.
-
Chondrocytes at this level have a low pO2 and their metabolism is anaerobic.
-
-
Proliferative zone
-
Vessels ramify at this level, providing a high-pO2 environment for the aerobic activities of cellular proliferation and matrix production.
-
-
Hypertrophic zone
-
Progressive drop-off of oxygen tension as
the now-hypertrophying cells pass through altered cell function,
terminal differentiation, and apoptosis. -
Adjacent cartilage becomes more and more calcified.
-
Metabolism becomes more and more anaerobic up to cell death.
-
-
-
Metaphyseal vessels supply the periphery
of the zone of vascular invasion; end loops from the nutrient artery
system supply the center.-
Vascular invasion
-
Space is created by deletion of most of the calcified cartilage by clasts emanating from the invading vessels.
-
The remaining calcified cartilage cores
act as templates for the application of woven bone by perivascular
cells that become osteoblasts; this is the primary trabecular array. -
Anatomy of terminal vessels
-
Arteriole loops back on itself beneath the last intact transverse septum of calcified cartilage.
-
Capillary ends in a venous sinusoid where flow is turgid and pO2 is very low, supporting bone formation.
-
-
Adjacent marrow becomes more and more
vascular to support the active process of primary trabecular bone
formation and subsequent internal remodeling.
-
-
cells, which then produce structure formed from proteins,
proteoglycans, and non-protein substances. Disorders of growth thus
represent problems in cell multiplication and differentiation, or in
formation of the elements of structure. Growth plate function is a
linked series of steps affecting the chondrocyte, stem cells, vascular
endothelial cells, osteoblasts, and osteoclasts, influenced by a
combination of systemic (endocrine) and local (paracrine or autocrine)
factors (Table 14-4). Each step in the growth
cascade has specific factors that influence and direct it; effects are
different between the developing embryonic and postnatal growth plate.
-
Mechanism of growth plate closure
-
Hormones driving growth diminish in activity.
-
Reduced proliferation of cartilage and
reduced hypertrophic chondrocyte size and column density produce a
radiographic narrowing of the growth plate. -
Cartilage becomes progressively more mineralized as the remaining cells go through a slower sequence, again seen on radiographs.
-
Clast activity works only on calcified cartilage, so that the final step is a surge of clast activity (see Fig. 14-11).
-
Estrogen is a key factor leading to growth plate closure.
-
Genetic disorders in estrogen handling,
either in the gene encoding aromatase enzyme that converts androgen to
estrogen, or in gene encoding estrogen receptor-[a]; in both the physes
fail to close, leading to increased height. -
Mechanism of estrogen-driven growth plate closure is unknown.
-
physiologically similar to, but slower than, physeal growth. It
primarily emanates from the subarticular (subchondral) cartilage rather
than the growth plate (physis). The timing of appearance of the
secondary centers of ossification (Fig. 14-12) is
unique for each bone. The morphology is identical to that of the
primary center as detailed earlier in the section “Stages of
Development in Primary Center of Ossification.“
|
TABLE 14-3 Growth Plate Structure and Function
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|
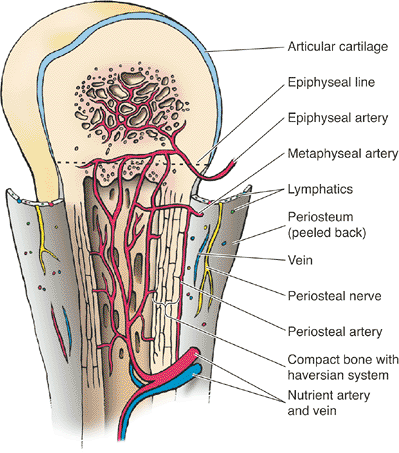
Figure 14-10 Blood supply to a typical growth plate. (From Moore KL, Dalley AF. Clinically Oriented Anatomy, 4th ed. Baltimore: Lippincott Williams & Wilkins, 1999.)
|
ossification grows circumferentially with normal physeal morphology
until impaction on the epiphyseal side of the growth plate occurs. Then
growth in that part of the epiphysis ceases, and a bony plate is laid
down on the epiphyseal side of the physis. From that moment on, growth
in the epiphysis is from subarticular cartilage (Fig. 14-13).
and subarticular growth plates as primary metaphyseal and epiphyseal
trabeculae, respectively (see Fig. 14-13). Itis subsequently modeled and remodeled into a lattice of interconnected bony plates compartmentalizing the marrow space.
-
Small round and square bones (e.g., talus, calcaneus)
-
Represents the central core of these bones
-
-
Long tubular bones
-
Confined to the epiphysis and metaphysis only
-
Minimal cancellous bone is present in the diaphysis along the inner “endosteal” surface of the cortex.
-
-
Mechanical
-
To support the subarticular bony plate
-
To transmit mechanical force from the articular surface to the cortex
-
-
Acid—base homeostasis
-
Enormous surface area of the body’s
trabecular and canalicular array provides the ionic resources for
mineral and acid-base homeostasis. -
Estimated surface area: 1,500 to 5000 m2
-
-
Reservoir for hematopoiesis
-
Initial extent: both axial and appendicular skeleton in the fetus and child
-
With skeletal maturation and cessation of growth, hematopoiesis recedes from limbs and remains within the axial skeleton.
-
-
Architectural orientation is largely determined by the changing patterns of force on each bone.
-
Quantity of cancellous bone depends on the total force applied.
-
The presence of disease or the
requirements of remodeling can induce a regional marrow transformation
from fatty marrow to fibrovascular tissue that may lead to emergence of
spindle cells, osteoblasts, and osteoclasts.
-
The cortex first appears as a sleeve of woven bone during the development of primary center of ossification (see Figs. 14-7 to 14-8).
-
Change in cartilage maturation induces
the formation of a central sleeve of coarsely woven bone that ends at
the level of maximum cartilage cell hypertrophy near the periphery of
the center of ossification. -
Stimulus for this induction is unknown but presumably is cytokine-driven.
-
Perichondrium merges with the growth
plate at the level of the reserve cells, contributing cells at the
lateral margin in a groove-like structure termed the ossification
groove of Ranvier (see Fig. 14-8). -
Inferior to this, the perichondrium
changes to periosteum, making bone instead of cartilage. The bone at
this level is termed the circumferential bony ring of Lacroix around
the hypertrophic zone (see Figs. 14-8A and 14-8B). -
Periosteum consists of an outer fibrous
layer and an inner cambium layer that produces an initial cortex of
woven bone extending from one growth plate to the other, ending at the
level of the hypertrophic zone.-
Starting as a solid fibrous structure, as
the periosteum migrates outward; its inner layer undergoes fibroclasia,
with most of the collagen being removed through clastic activity.
Table 14-4 Hormone/Vitamin Effects on the Growth PlateHORMONE/FACTOR SOURCE BIOLOGIC EFFECT ZONE AFFECTED Growth hormone Pituitary After birth, growth hormone
promotes cellular proliferation acting directly, and indirectly through
mediators, on the somatomedins, produced in liver and in CCs; direct
effect creates target CCs in which IGF-I induces replication of CCs.Throughout growth plate Somatomedins: Insulin-like growth factor (IGF) I and II Liver, plus local from CCs IGF receptors are present
throughout GP CCs; receptors for IGF-I have highest concentration in
PZ; response to IGF-I lessens with increasing maturity and HT of the
CCs; IFG-II is essential for normal fetal growth; IGF-I most active
during postnatal life.Throughout growth plate Thyroid hormone: T3 (3,5,3-triiodothyronine)3 T4 (thyroxine) Thyroid gland T4 and T3 together with IGF/IGF-II allow normal growth and maturation of CCs; excess T4 produces protein catabolism; deficient T4 produces growth retardation, cretinism, and abnormal degradation of mucopolysaccharides. Proliferative zone and upper hypertrophic transition zone Parathyroid hormone (PTH) Parathyroid Direct mitogenic effect on
CCs; stimulates prostaglandin synthesis through increase in
intracellular Ca and stimulation of protein kinase; is synergistic with
other growth factorsProliferative zone and upper hypertrophic transition zone Glucocorticoids (GC) Adrenal cortex Familial GC deficiency is
associated with tall stature; GC excess is associated with osteopenia
and growth retardation; direct effect is reduction in CC proliferation
and extracellular matrix synthesis; impaired growth is also an indirect
effect by interfering with other growth-modulating pathways.Proliferative zone Calcitonin Parafollicular cells of thyroid Accelerates GP calcification and CC maturation Lower hypertrophic and transition zone Estrogen (Eg) Ovary Growth spurt is probably
caused by elevated growth hormone plus low Eg levels. GP fusion is
probably caused by higher level of Eg.Probably proliferative zone Androgen (Ag) Testicles Ag stimulates longitudinal
growth, independent of Eg action, by direct local effect on CCs; until
skeletal maturity, Ags stimulate mineralization, increased glycogen,
and lipids in CCs, prostaglandin synthesis; supraphysiologic doses of
Ag depress growth and accelerate GP closure.Hypertrophic transition zone, primary spongiosa Vitamin A Diet Normal levels maintain CC
metabolism; deficient vitamin A impairs CC maturation, suppresses
growth, and alters bone shape; excess vitamin A produces fragile bones;
mechanism is increased lysosomal body membrane fragility.Hypertrophic transition zone Vitamin C Diet Cofactor in enzymatic
synthesis of collagen; stimulates mineralization in cultures of CCs;
mechanism is stimulation of matrix vesicle formation, and of synthesis
of ALP, collagen II, and collagen XProliferative zone and hypertrophic transition zone Vitamin D Diet; secondary from the sun; locally produced by CCs and osteoblasts Allows mineralization of newly
synthesized osteoid; 1,25-hydroxyvitamin D3 and 24,25-dihydroxyvitamin
D3 control autocrine regulators of matrix events: activity of enzymes
in matrix vesicles, of matrix proteins during growth, calcification,
and activation of growth factors; increases activity of alkaline
phosphatase and MMPsAll growth plate zones except hypertrophic transition zone; highest levels in proliferative zone Prostaglandins Ubiquitous Family of hormone-like
substances derived from arachidonic acid; mechanism of action is
variable depending on the prostaglandin; the precise role of
prostaglandin in the GP is unknown.All growth plate zones CC, cartilage cells; HT, hypertrophy. 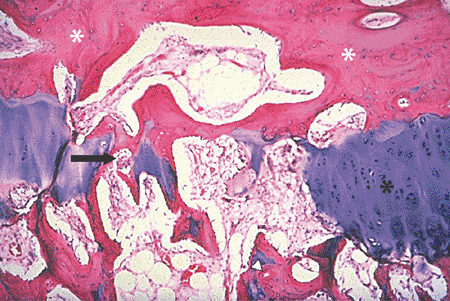 Figure 14-11
Figure 14-11
Growth plate closure occurs by a wave of osteoclast activity, driven by
a complex interplay of systemic hormones and local factors. The slide
shows the central region of a growth plate with the epiphyseal bone
plate above (white asterisks) and growth plate (black asterisk) below, with a gap in the middle. The gap has been created by repetitive osteoclast activity. An osteoclast (arrow) is deleting calcified cartilage on the left side of the gap. Secondary trabeculae (arrowhead) are seen below the gap. (Courtesy of the Armed Forces Institute of Pathology, Washington, DC.) -
-
-
Nature of the woven bone in the embryo
-
Explosive process with radiating
streamers of woven bone laid down in bursts of activity (also called
osteophytic or streamer bone) -
High cell-to-bone ratio; irregular and random collagen deposition; appearance of an open mesh (Fig. 14-14)
-
Newly formed woven bone is like chicken wire and is mechanically weak.
-
-
Woven bone of this immature nature is
seen throughout life in any situation requiring the formation of bone
very rapidly (fracture, periosteal response to acute bacterial
infection or aggressive tumor).
-
Once the woven bone structure is in
place, Wolff’s law demands a strengthening process: this occurs through
a filling-in of the interstices of the woven bone by a lamellar bone
fill-in, producing a composite structure that is flexible and strong. -
Process of primary osteon formation
-
Osteoblasts line up on the margins of the
woven bone trabeculae and slowly deposit bone in successive layers of
inlay lamellar configuration into the open spaces between the
trabeculae; this passive filling-in produces “primary” or passive
osteons. -
Completes the process of formation of a mechanically solid cortex in the fetus (Fig. 14-15)
-
-
In the embryo and the young child, the cortex is a composite structure:
![]() Figure 14-12 (A) Secondary center of ossification (arrow)
Figure 14-12 (A) Secondary center of ossification (arrow)
with central growth plate morphology. (B) At higher power the growth
plate morphology is seen above. The cancellous bone is formed initially
as primary trabeculae (arrow) with woven bone deposited on the calcified cartilage core. Primary trabeculae are also seen in Figure 14-8. (Courtesy of the Armed Forces Institute of Pathology, Washington, DC.)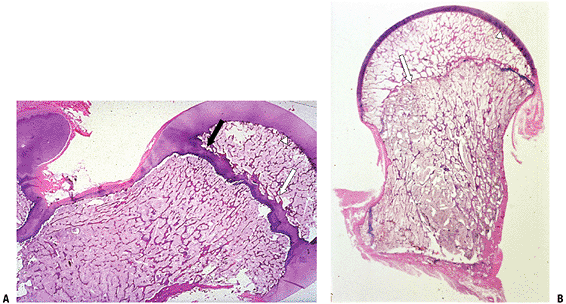 Figure 14-13 Epiphyseal development of the proximal femur. (A) Initial circumferential growth ceases as epiphyseal growth impacts physis (black arrow). (B) Bony plate (white arrow)
Figure 14-13 Epiphyseal development of the proximal femur. (A) Initial circumferential growth ceases as epiphyseal growth impacts physis (black arrow). (B) Bony plate (white arrow)
develops under mechanical forces on the epiphyseal side of the physis.
Remaining growth occurs only from subarticular cartilage (arrowhead).
In both A and B, the medullary space of the epiphysis and metaphysis
consists of an array of cancellous bone, a latticework of
interconnected secondary trabeculae. These secondary trabeculae
(lamellar bone only, no calcified cartilage core) replace the primary
trabeculae that first form on the metaphyseal end of the growth plate.
(Courtesy of the Armed Forces Institute of Pathology, Washington, DC.)![]() Figure 14-14
Figure 14-14
(A) Cross-section of fetal cortex through primary center of
ossification. The cambium layer (C) of the periosteum has deposited
coarse woven bone. Note primary trabeculae (T), fibrovascular marrow
space centrally. (B) Higher-power view of boxed area in A emphasizes
open mesh appearance of this woven bone. Note increasing maturity of
bone on the left versus new bone formation from osteoblasts on the
right (cambium layer of periosteum). This is also the appearance of
fracture callus at 2 to 3 weeks, at the onset of mineralization prior
to lamellar bone fill-in. This is the prototype image of woven bone:
random orientation of trabeculae, high cell-to-bone ratio,
fibrovascular marrow space. (Courtesy of the Armed Forces Institute of
Pathology, Washington, DC.)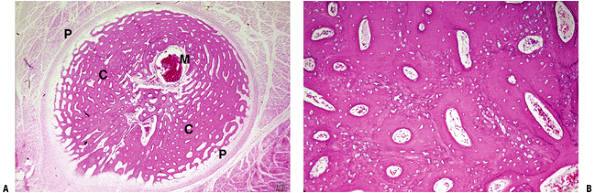 Figure 14-15
Figure 14-15
(A) Fetal cortex solid appearance after lamellar fill-in. (B) Higher
power shows high cell-to-bone ratio of woven bone ghost, with much
denser lamellar bone fill-in forming primary osteons. This mimics the
appearance of fracture callus at 5 to 6 weeks. M, marrow space; P,
periosteum; C, cortex (passive osteons). (Courtesy of the Armed Forces
Institute of Pathology, Washington, DC.)-
Outer primary structural element of the cortex
-
Peripheral successive rings of bone deposited by osteoblasts arising from the cambium layer of the periosteum
-
This is woven bone in the embryo and becomes lamellar as growth slows.
-
-
On either end of a fetal long bone are epiphysis and metaphysis with growth plate (see Figs. 14-8, 14-12, and 14-13),
and primary and secondary trabecular arrays forming the cancellous
network filling the medullary space. The primary trabeculae (primary
spongiosa), forming first, are replaced promptly by mechanically more
solid bone because of the high rate of turnover of this area. -
The processes of modeling and remodeling
continue to unfold, changing the shape and size of the growing bone,
respectively, and making the composite structure mechanically efficient.
-
Modeling is the mechanism by which bone alters its shape during growth.
-
Involves unequal removal and addition of bone on opposing surfaces (Figs. 14-16 and 14-17)
-
Bone removal and replacement occurs in bursts of activity.
-
Teams of clasts completely remove portions of cortex in the cutback zone.
-
Simultaneously, the inner surface of the adjacent bone (either cortex or trabecula) becomes the site of active bone formation.
-
-
Funnelization: Unbalanced removal and replacement allows the metaphysis to “funnel” down to diaphyseal diameter (see Fig. 14-17); removal is highly efficient and occurs in bursts, while replacement takes months (see Table 14-2).
-
Cortex, once it funnels to diaphyseal diameter, remains in a state of imbalance throughout life.
-
Cortical diameter enlarges over time through subp-eriosteal apposition of rings of lamellar bone.
-
Once growth ceases, the cortex gradually
thins as clastic removal on the medullary side of the cortex outstrips
the periosteal addition of bone.
-
and joints depend on a normal balanced relationship among metabolic,
mechanical, and circulatory factors. Balance of these factors explains
any shift in density in bone through clast and/or blast activation.
Each side of this triangle is ultimately driven by a combination of
local and systemic environmental and humoral influences (cytokines)
acting on blasts and clasts.
-
Osteonization: The mechanical
stress-driven formation of a resorption cavity and its refill with
concentric lamellar bone; this is the process by which cortical bone
increases enormously its mechanical properties, providing a durable
skeleton for a lifetime (Figs. 14-18, 14-19, and 14-20).
Once formed, the osteon’s lifetime is, in part, a function of the
activity of the individual. Turnover is rapid in the fetus and growing
child, and the osteon may be remodeled in a matter of months. -
Cutting cone activation (see Fig. 14-18):
This process, which osteonization relies upon, begins in the infant
femur at 1 year of age and the rest of the skeleton over the next
several years and continues throughout life. In 1964 Lent Johnson
provided this description of the formation of a cutting cone:P.342![]() Figure 14-16
Figure 14-16
Modeling. (A) Cancellous bone (CB) fills the medullary space of the
epiphyses and metaphyses of long bones, transmitting force from the
cortex to the articular surfaces. To maintain the shape of the bone end
as growth occurs, modeling must occur. Modeling is a complex process
with coordinated addition and deletion of bone to achieve an adult
configuration. (B) Diagram shows where bone must be deleted and added
as growth unfolds. At a given time the proximal tibia has the
appearance diagrammed by the solid line. Growth plate action, through
increased cell mass and volume and the secretion of matrix, pushes the
epiphysis away from the metaphysis. The epiphysis grows in width and in
height. The metaphysis widens superiorly and undergoes cutback
inferiorly, narrowing its width to that of the diaphysis. The diaphysis
undergoes lamellar bone apposition to increase its width, while there
is endosteal deletion of bone to maintain cortical width. All deletion
is performed by osteoclasts; addition of bone is by osteoblasts. The
subsequent bone has the contour outline by the dashed line. (Courtesy
of the Armed Forces Institute of Pathology, Washington, DC.)The cavity is formed by a broad, shallow, cone-shaped
osteo-clastic wave front, the cutting cone, containing an average of 6
osteoclasts in the plane of section. This is followed by a zone of
scattered clasts planing down the edges of Howship’s lacunae and
producing a slight further increase in diameter. The cutting cone is…
followed by a mesenchymal cell proliferation so that the spindle-shaped
cavity is at all times filled with connective tissue and blood vessels.
Finally, there is a very long, slowly closing cone of osteoblastic
lamellar refill. Osteoclasts are limited to the cutting cone and the
planing zone. Osteoblasts are present all along the face of the closing
cone. -
Osteon: The osteon represents a complex
cable, laid down in response to mechanical signals, glued to its
neighbors. The composite structure of each bone represents a
biomechanical structure of force transmission that is flexible, strong,
and capable of ongoing self-maintenance and repair by virtue of its
contained living elements. -
Wolff’s law is described by Robert Salter
as “remodeling of bone, occurring in response to physical stresses—or
to the lack of them—in that bone is deposited in sites subjected to
stress and is resorbed from sites where there is little stress.” The
precise mechanism explaining Wolff’s law is not yet determined.
-
The cancellous network is a honeycomb-like lattice of lamellar bone in which remodeling occurs on the trabecular surface.
-
Remodeling occurs in the following series of linked steps, termed coupling:
-
Quiescence: Bone surface lining cells at rest act as a barrier covering a thin layer of unmineralized osteoid.
-
Activation: Bone surface lining cells’
surface receptors for PTH, vitamin D, and IL-6 initiate the remodeling
process in response to:-
Hormone signals: changes in acid—base and calcium—phosphate homeostasis activate receptors for PTH and vitamin D
-
Mechanical signals: mediated via IL-6 signaling
-
-
Bone surface lining cells initiate a response to a hormonal or stress-induced signal:
-
Secretion of enzymes collagenase and stromelysin for the removal of the osteoid covering the bone surface
-
Secretion of cytokine IL-6, which acts on the marrow to initiate the formation and activation of osteoclasts
P.343P.344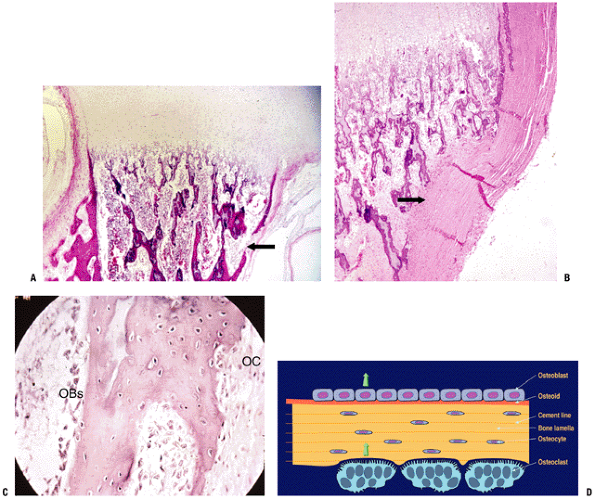 Figure 14-17 Modeling. (A) Proximal long bone whole mount specimen. Arrows show site of active metaphyseal cutback; osteoclast activity has temporarily deleted a portion of the cortex (arrow). (B) Another specimen shows a higher-power view of zone of complete cortical deletion adjacent to primary trabeculae (arrow).
Figure 14-17 Modeling. (A) Proximal long bone whole mount specimen. Arrows show site of active metaphyseal cutback; osteoclast activity has temporarily deleted a portion of the cortex (arrow). (B) Another specimen shows a higher-power view of zone of complete cortical deletion adjacent to primary trabeculae (arrow).
(C) Cortex at higher power in a different specimen shows bone modeling
with osteoclasts on one surface and blasts on the opposing surface. (D)
Diagram of modeling, with osteoblasts on one side of the cortex and a
site of cortical deletion with a Howship’s lacuna and osteoclast on the
other side. (A-C, Courtesy of the Armed Forces Institute of Pathology,
Washington, DC.)![]() Figure 14-18
Figure 14-18
Cortex: bone remodeling unit (cutting cone). (A) Photomicrograph shows
cortical bone (CB) being deleted by a team of osteoclasts. The trailing
vessels (V) are seen with activation of perivascular cells to
osteoblast lineage; osteoblasts lay down a cement line and then
sequential layers of lamellae. (B) Diagrammatic representation. (A,
Courtesy of the Armed Forces Institute of Pathology, Washington DC; B,
courtesy of Dr. P. Roughley.) -
-
Downregulation of osteoprotegerin, the decoy receptor for receptor activator of NF-Kβ-ligand (RANKL)
-
-
Resorption
-
With RANKL available, newly activated
osteoclasts link to RANKL and the bone surface lining cells through the
receptor activator of NF-kappa beta (RANK). -
This coupling made, the bone surface lining cells drop off and the osteoclast settles onto the newly exposed raw bone surface.
-
Osteoclast seals the site and elaborates
acid for solubilizing the mineral and the enzyme cathepsin K, which
eliminates the osteoid. -
Bone deletion liberates local stores of cytokines, including BMP and TGF-beta.
-
BMPs initiate osteoblast differentiation in the adjacent marrow; these cells then migrate in and fill the hole.
-
TGF-beta stimulates the osteoblasts to make osteoid and fill the hole.
-
-
Reversal: process by which osteoclasts stop removing
P.345P.346
bone and osteoblasts fill the defect. Initiation of this step involves
the deposition of a cement line by the first wave of osteoblasts.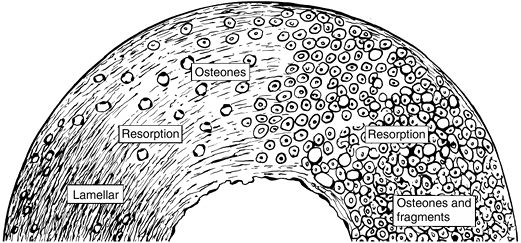 Figure 14-19
Figure 14-19
Cortical remodeling (osteonization). Over the course of a person’s
lifetime, cortex begins as a mechanically linked array of “primary” or
passive osteons, created by lamellar bone fill-in of the spaces between
the initial woven bone trabeculae of the fetal cortex. Mechanical
activity induces the activation of cutting cones that create
“secondary” or active osteons whose orientation and structure is
directed by Wolff’s law. On the left the cortex is primarily passive
osteons in the fetus; the subsequent bone structure changes with the
activity of a lifetime to fully osteonized bone with innumerable
interstitial fragments, as on the right.![]() Figure 14-20
Figure 14-20
(A) Adult cortical bone. Note mature haversian systems (secondary
osteons). (B) Polarized light view of adult cortex showing secondary
osteons and osteonal fragments. (Courtesy of the Armed Forces Institute
of Pathology, Washington, DC.)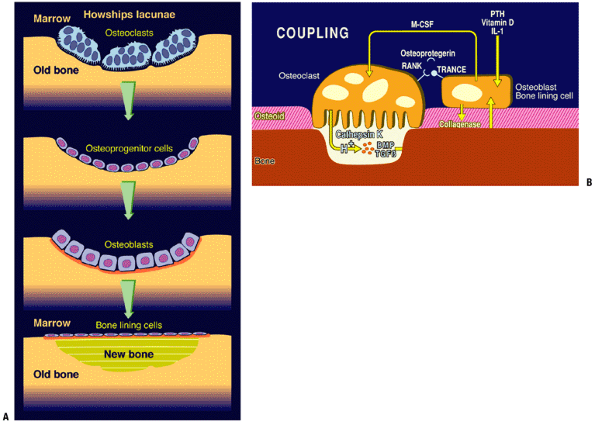 Figure 14-21
Figure 14-21
(A) Trabecular remodeling. Activation of bone surface lining cells
recruits marrow osteoclasts, which dig a cavity (Howship’s lacuna),
subsequently filled in by locally recruited osteoblasts. (B) The
process of activation, resorption, and formation explained
diagrammatically. (Courtesy of Dr. Peter Roughley.)![]() Figure 14-22
Figure 14-22
(A) Fetal femur plus thigh muscle compared to cross-section of adult
femur (woman in late 60s). Mechanism for cortical drift is periosteal
addition of lamellar rings with concomitant endosteal osteoclastic
removal. The process is not balanced so that as one ages, the cortex
becomes thinner as the bone contour expands. Note early cancellization
(porosity) of adult femur. (B) Polarized image of outer concentric
lamellar, not yet osteonized. These lamellae are laid down by the
cambium layer of the periosteum. (C) Polarized image of similar
lamellae undergoing osteonization. (Courtesy of the Armed Forces
Institute of Pathology, Washington, DC.) -
Formation: laying down of bone by osteoblasts
-
Once the cycle is complete, a subset of
the osteoblasts become bone surface lining cells and the surface again
becomes quiescent. -
Rates of remodeling
-
Remodeling of cortical bone: 2% to 3% per year
-
Remodeling of trabecular skeleton: 2 5 % per year
-
-
Changes over lifetime
-
Initially coarse and more cellular in the fetus
-
Lamellae become finer and finer as the individual ages.
-
The slow, more orderly pattern of
periosteal bone formation in late childhood, adolescence, and adulthood
results in successive layers of lamellar bone encompassing the cortical
circumference (circumferential lamellar bone), like the rings of a tree. -
Subsequent generation of “secondary” (stress-directed haversian systems; see Figs. 14-19, 14-20, and 14-21) will eventually convert the normal periosteal ap-positional bone formation into adult remodeled cortex (Fig. 14-22).
-