FRACTURES AND DISLOCATIONS OF THE SHOULDER GIRDLE AND HUMERUS
II – FRACTURES, DISLOCATIONS, NONUNIONS, AND MALUNIONS > Upper
Extremity > CHAPTER 15 – FRACTURES AND DISLOCATIONS OF THE SHOULDER
GIRDLE AND HUMERUS
relatively long clavicles and the synovial joints at both ends of this
bone, exemplifies an appropriate compromise between the claims laid
upon the skeleto-muscular system concerning stability and mobility.
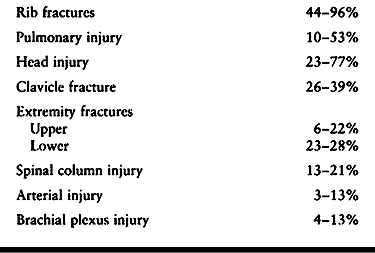
fractures in the body and 3% of all injuries involving the shoulder
girdle. Although scapular fractures are relatively uncommon injuries,
they are important not only because of the problems they create for
shoulder function but also because they may be the harbinger of other
injuries. Scapular fractures usually are diagnosed in patients who have
sustained violent blunt trauma, and up to 96% of patients with those
fractures have associated injuries (2). For that reason, scapular fractures have been referred to as “sentinel injuries” (Table 15.1) (51,138).
 |
|
Table 15.1. Common Injuries Associated with Scapula Fractures (2,86,103,138)
|
The muscles keep the scapula relatively protected from injury and help
explain why most fractures occur only after high-energy impact and why
fractures of the scapular body frequently can be treated
nonoperatively. The lateral aspect of the scapula has three bony
processes, each related to important soft tissue structures that can
influence surgical decision making:
 |
|
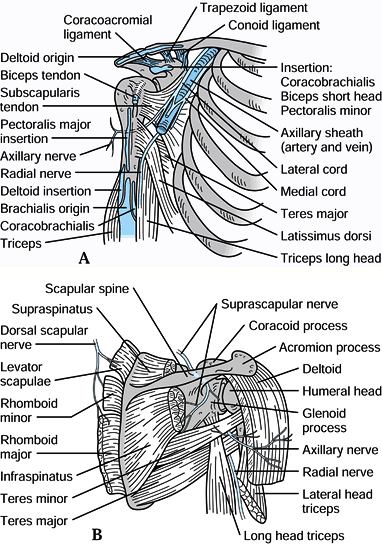
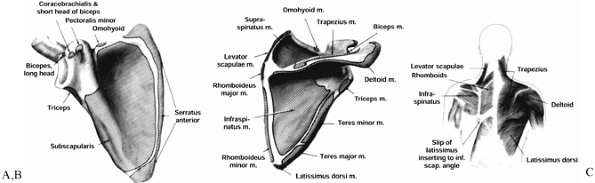
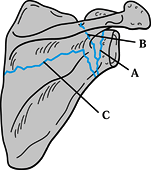
Figure 15.1. A: Muscle attachments on the anterior aspect of the scapula. B: Muscle attachments on the posterior aspect of the scapula. C:
Posterior muscles covering the scapula. (From Butters KP. Fractures and Dislocations of the Clavicle. In: Rockwood CA Jr, Green DP, Bucholz RW, Heckman JD, eds. Fractures in Adults. Philadelphia: JB Lippincott, 1996;1165.) |
-
The coracoid process
projects anteriorly, superiorly, and laterally from the
anterior-superior border of the scapula. The pectoralis minor,
coracobrachialis, and short head of biceps originate from it (Fig. 15.2),
and all three muscles may contribute to the deforming forces acting on
scapular fractures. The conoid and trapezoid ligaments, which make up
the coracoclavicular ligament complex, originate from the coracoid
process and insert on the inferior surface of the clavicle (Fig. 15.2).
The ligaments are key stabilizing structures for the distal clavicle,
and their integrity is an important factor in deciding whether or not
surgical treatment is indicated. The suprascapular nerve traverses the
superior border of the scapula through the scapular notch located just
medial to the base of the coracoid process, and the brachial plexus and
axillary artery and vein travel below the coracoid process adjacent to
its base (Fig. 15.3).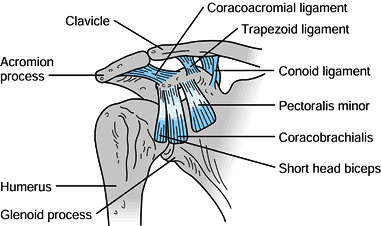 Figure 15.2. Important soft tissue attachments of the coracoid process.
Figure 15.2. Important soft tissue attachments of the coracoid process.![]() Figure 15.3. A: Relationships of the vital neuromuscular structures to the anterior shoulder girdle. B: Relationships of the important neurologic structures to the posterior shoulder girdle.
Figure 15.3. A: Relationships of the vital neuromuscular structures to the anterior shoulder girdle. B: Relationships of the important neurologic structures to the posterior shoulder girdle. -
The acromion is the lateral extension of the scapular spine. It curves anteriorly to form the acromioclavicular joint with the clavicle (Fig. 15.2 and Fig. 15.3). The supraspinatus and infraspinatus muscles travel below the acromion to insert into the humeral head (Fig. 15.1 and Fig. 15.3).
The potential for impingement on these muscles from a fractured and
tilted acromion is an important determinant of operative treatment. The
coracoacromial ligament is a stout ligament that runs between the
coracoid process and the anterior-inferior aspect of the acromial
process (Fig. 15.2). Occasionally this ligament
may be used to stabilize a distal clavicle rendered unstable by injury,
but it should be preserved whenever possible to maintain the integrity
of the coracoacromial arch (122). -
The glenoid process (neck)
is the lateral extension of the scapular body and supports the glenoid
fossa, which is the articular platform for the humeral head. Relative
to the scapular spine, the glenoid fossa is retroverted an average of
6° (129). Posteriorly, the suprascapular nerve
and artery run along the base of the glenoid process as they exit the
spinoglenoid notch before supplying the infraspinatus muscle (Fig. 15.3). These structures are at risk for injury during a posterior approach to the glenoid and from displaced fracture fragments.
relevance for surgical decision making. The thin bone that makes up a
large portion of the scapular body is not well suited for internal
fixation. There are, however, regions that have bone stock adequate to
accommodate screw or wire fixation, including the coracoid process, the
acromial process, the base of the scapular spine, the glenoid process,
and the lateral scapular border (44).
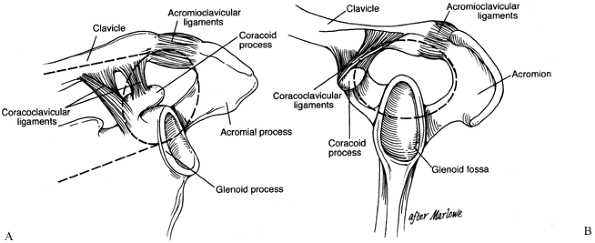
should be understood because it provides a useful framework for
considering shoulder injuries and their treatment. The SSSC is a bony
and soft tissue ring composed of the glenoid process, the coracoid
process, the acromial process, the
coracoclavicular
(CC) ligaments, the acromioclavicular (AC) joint, and the distal end of
the clavicle. This ring is supported by two bony struts: the clavicle
superiorly and the lateral border of the scapula inferiorly (Fig. 15.4).
 |
|
Figure 15.4. Superior shoulder suspensor complex. A: Anteroposterior view of the bone–soft-tissue ring and superior and inferior bone struts. B:
Lateral view of the bone–soft-tissue ring. (© 1995 American Academy of Orthopaedic Surgeons. Reprinted from Goss TP. Scapular Fractures and Dislocations: Diagnosis and Treatment. J Am Acad Orthop Surg 1995;3:22, with permission.) |
AC separations or isolated distal clavicle fractures are typically
stable injuries and respond well to nonoperative treatment. When the
SSSC is injured in two places, however, a so-called double disruption (Fig. 15.5),
one or both of the injuries may become significantly displaced because
the ring is left unstable. Double disruptions often lead to delayed
union, malunion, or nonunion and secondarily to decreased strength and
muscle fatigue and, possibly, neurovascular compromise. Osteoarthrosis
may result when one of the disruptions involves a joint surface.
Examples of double disruptions of the SSSC include a clavicle fracture
together with an AC joint disruption, a glenoid
neck
fracture with either an AC joint disruption or clavicle fracture, and
unusual combinations such as ipsilateral fractures of the acromion and
coracoid process (45).
The most common sequela is anterior, medial, and inferior displacement
of the scapula secondary to the weight of the arm and the combined pull
of the pectoralis major, pectoralis minor, and latissimus dorsi muscles.
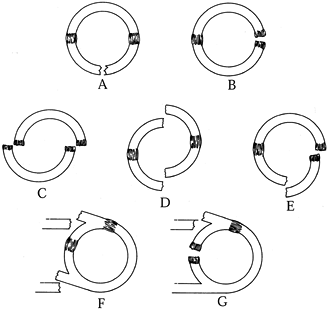 |
|
Figure 15.5. Types of traumatic ring/strut disruptions. Single disruptions of the bone–soft-tissue ring may be a break (A) or a ligament disruption (B). Double disruptions of the bone–soft-tissue ring may be a double-ligament disruption (C), a double break (D), or a combination of a bone break and a ligament disruption (E). Other double disruptions may be a break of both struts (F) or a break of one strut and a ring disruption (G).
(© 1995 American Academy of Orthopaedic Surgeons. Reprinted from Goss TP. Scapular Fractures and Dislocations: Diagnosis and Treatment. J Am Acad Orthop Surg 1995;3:22, with permission.) |
diagnosis of a scapular fracture in an acutely injured patient. In one
large series, one third of the scapular body fractures were not
diagnosed or recorded at the time of the patients’ admission to the
hospital (138). Scapular fractures may be
evident on the chest x-ray obtained as part of the initial evaluation
of a multiply injured patient. More often, special-view
radiographs—including scapular anteroposterior, scapular lateral, and
axillary views—are necessary to better define the fracture pattern.
Computed tomography (CT) scans are useful for detailed assessment and
preoperative planning of intraarticular and glenoid neck fractures.
Frequently they can be obtained when the patient goes to the CT scanner
for evaluation of other injuries.
regions because the criteria for operative and nonoperative treatment
and the expected outcomes vary accordingly. The regions and the
approximate incidence of scapular fractures that occur in each are
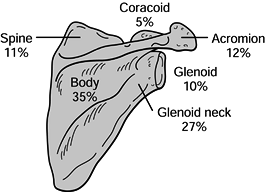
scapular body (35%), glenoid neck (27%), acromion (12%), scapular spine
(11%), glenoid fossa (10%), and coracoid process (5%) (Fig. 15.6) (2). Diagnosis and treatment of these fractures are discussed by anatomic region.
 |
|
Figure 15.6. Incidence of scapular fractures by anatomic location. (From Ada JR, Miller ME. Scapular Fractures: Analysis of 113 Cases. Clin Orthop 1991;269:174.)
|
therefore, extreme force usually is necessary to produce scapular body
fractures. Because of the rich blood supply to this region and the
stabilizing effect of the surrounding muscular envelope, healing
usually occurs uneventfully, and nonunion is rare. Focus treatment on
symptomatic relief. Prescribe ice to the local area and sling
immobilization of the arm for comfort. Within a week of injury, begin
pendulum exercises and advance to the use of overhead pulleys and
active-assisted range-of-motion exercises as soon as the patient’s
symptoms allow. Malunion with significant displacement may produce a
snapping or grating sensation with scapulothoracic motion, but patients
rarely complain of restricted motion or pain that limits function (2).
(a) fracture of the anatomic neck; (b) fracture of the surgical neck;
and (c) fracture of the inferior neck coursing inferior to the scapular
spine to exit along the medial scapula border, leaving the superior
portion of the glenoid intact (Fig. 15.8) (46).
These fracture patterns can be identified on the standard shoulder
trauma x-ray series, but CT scans are helpful in clearly defining the
fracture anatomy and assessing associated injuries to the shoulder
girdle.
 |
|
Figure 15.7. Three major fracture patterns of the glenoid process. A: Anatomic neck. B: Surgical neck. C:
Fracture of the inferior neck, which then courses along the inferior aspect of the scapular spine to exit at the medial border of the scapula. (Redrawn from Goss TP. Fractures of the Glenoid Neck. J Shoulder Elbow Surg 1994;3:42.) |
 |
|
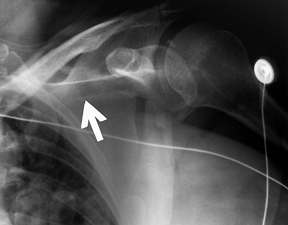
Figure 15.8.
Anteroposterior radiograph illustrating a type C glenoid neck fracture. This is not a complete fracture of the glenoid neck because the superior portion of the glenoid remains intact. |
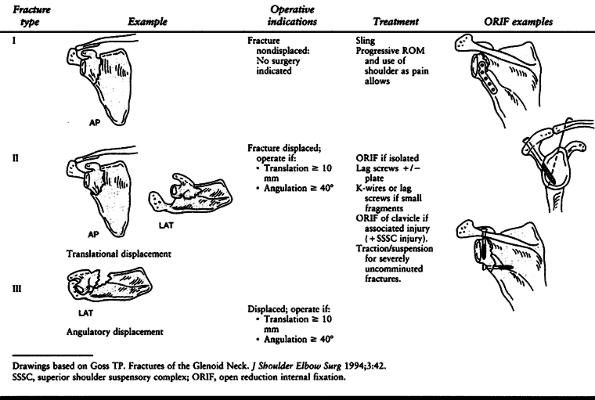
is important for deciding treatment. Excessive translation and
angulation can lead to shoulder dysfunction from altered rotator cuff
mechanics and pain. Therefore, we consider glenoid neck fractures as
one of two types, as suggested by Goss (46).
Type I fractures are undisplaced or insignificantly displaced; type II
fractures are displaced at least 1 cm or angulated at least 40° (Table 15.2).
Any fracture pattern (A, B, or C) may be either type I or type II. Type
I fractures are usually stable and heal satisfactorily because the
superior shoulder suspensory complex is disrupted in only one place.
Therefore, treatment is nonoperative and aimed at symptom relief.
Provide an arm sling for patient comfort and ice the injured region.
Instruct the patient to begin gentle pendulum exercises within 1 week
and progressively increase use of the arm and shoulder as pain allows.
The goal is to promote healing without developing debilitating shoulder
stiffness, which often follows prolonged immobilization.
 |
|
Table 15.2. Glenoid Neck Fractures
|
If the displaced glenoid neck fracture is an isolated injury without a
secondary disruption of the SSSC, then treat the fracture via open
reduction and internal fixation (ORIF) through a posterior approach.
Use interfragmentary fixation and/or a buttress plate as the fracture pattern dictates.
fracture, there is an associated disruption in the SSSC, rendering the
shoulder complex unstable. Most often the clavicle is fractured also;
less commonly, the acromioclavicular joint is disrupted. Several
authors have described this combination of injuries and the need for
ORIF of one or both of them. Herscovici (55), Rikli (117), and Goss (46)
suggest that ORIF of the associated injury—a clavicle fracture, for
example—would indirectly reduce the glenoid neck fracture and is
sufficient to restore stability to the shoulder complex. Leung et al. (81)
recommended ORIF of both injuries, suggesting that more rigid fixation
would allow more vigorous rehabilitation and better final results.
-
If the displaced glenoid neck fracture is
an isolated injury, then approach the fracture directly through a
posterior approach. Retract the deltoid with an appropriate retractor
rather than detaching it from its origin on the scapular spine whenever
possible. Retract the infraspinatus superiorly and the teres minor
inferiorly to reach the glenoid neck. -
Use either a 3.5-mm reconstruction plate
as a buttress plate or interfragmentary screw fixation to stabilize the
fracture depending on the fracture configuration and fragment size (Table 15.2). -
If the glenoid neck fracture is
associated with a second injury such as a clavicle fracture or
acromioclavicular joint disruption, then address the injury that is
more accessible first. Usually that means open reduction and plate
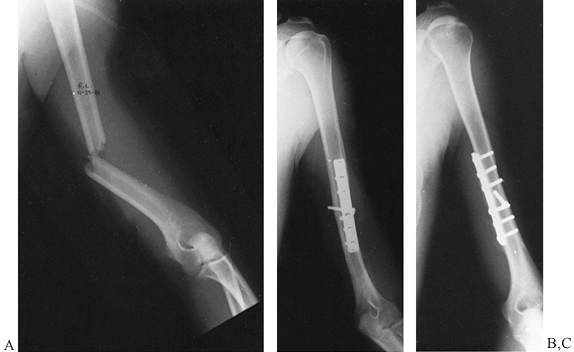
fixation of the clavicle (Fig. 15.9). If the
fixation is solid and reduces the associated glenoid neck fracture
within the criteria listed above, then no further internal fixation is
required. If the glenoid neck remains displaced beyond acceptable
criteria, however, then we openly reduce and fix that fracture also.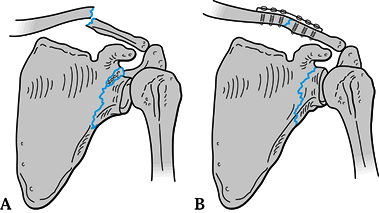 Figure 15.9. A: Displaced glenoid neck fracture associated with a clavicle fracture. B: Indirect reduction of glenoid neck fracture after open reduction and internal fixation of the clavicle.
Figure 15.9. A: Displaced glenoid neck fracture associated with a clavicle fracture. B: Indirect reduction of glenoid neck fracture after open reduction and internal fixation of the clavicle. -
Postoperatively, apply a sling and swathe or shoulder immobilizer and prescribe narcotics and local ice for
P.437
pain control. Instruct the patient to begin progressive passive
range-of-motion (ROM) exercises 48 to 72 hours after surgery. Allow
functional use of the shoulder within defined limitations depending on
the surgical approach and associated injuries. Patients should strive
to achieve full shoulder motion by 8 to 12 weeks after surgery. Begin
strengthening exercises at 12 weeks. The patient should avoid heavy
lifting with the injured arm until that time.
10% of all scapular fractures, and only 10% of these are significantly
displaced (2,62,86).
If a glenoid fracture is suspected from physical exam (pain, swelling,
ecchymosis, crepitus) or after review of a chest radiograph, obtain a
shoulder trauma series to confirm the diagnosis, rule out a dislocation
of the glenohumeral joint, and help determine if surgery is indicated.
A CT scan is helpful for delineating the articular fracture pattern and
is recommended before operative treatment.
fractures not associated with humeral head subluxation or dislocation
nonoperatively with early progressive ROM exercises as the patient’s
pain decreases. Progressive shoulder strengthening begins when shoulder
ROM has returned to normal. The majority of patients with glenoid
cavity fractures can be treated in this fashion. Functional outcome is
usually excellent, but it can take 6 to 12 months to reach maximum
recovery.
can lead to decreased range of motion, painful osteoarthrosis, and
chronic instability (2,29,76).
Although the exact amount of displacement or stepoff of the articular
surface necessary to cause significant morbidity is unknown, many
authors believe that residual displacement of 5 to 8 mm should be
reduced and stabilized in accordance with the principles of displaced
intraarticular fractures in other anatomic regions (51,67).
The indications, approaches, and techniques for operative treatment of
these injuries are dependent on the degree of displacement and the
fracture pattern.
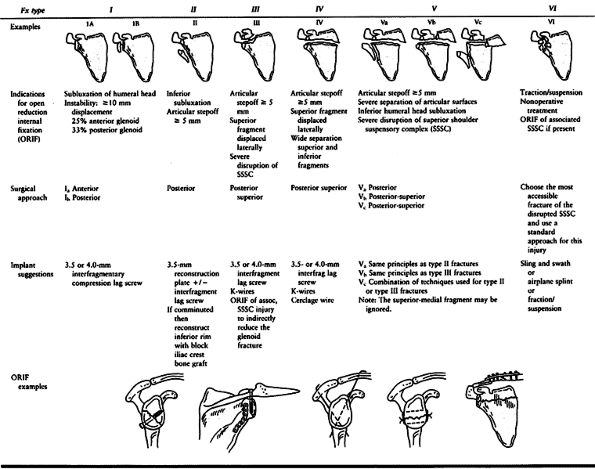
Type I fractures represent a substantial portion of the glenoid rim and
occur when a lateral force drives the humeral head into the glenoid
rim. These should be differentiated from small capsular avulsions of
bone associated with shoulder dislocations. They are considered
unstable if they are displaced at least 1 cm or involve at least 25% of
the anterior rim or 33% of the posterior rim (6,51,76,133).
 |
|
Table 15.3. Glenoid Rim and Glenoid Cavity Fractures (44,62)
|
several different fracture patterns depending on the position of the
humeral head at the time of injury and the direction of the applied
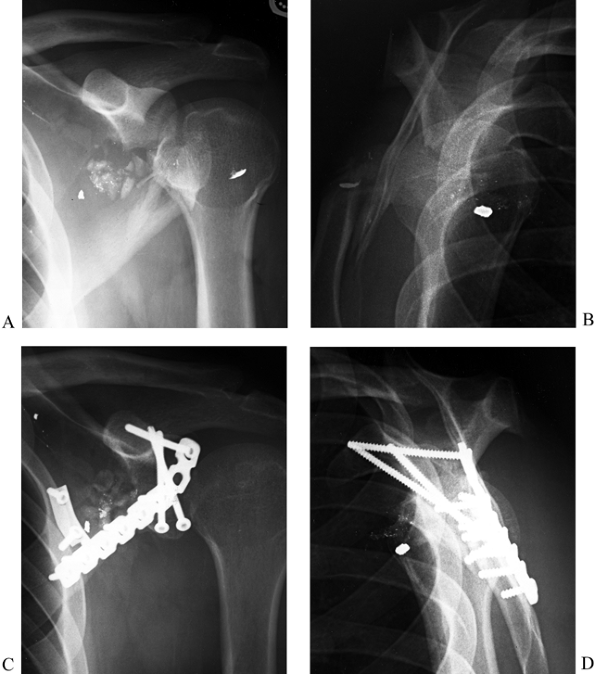
force. Type II fractures that are displaced 5 mm or more or associated
with inferior subluxation of the humeral head require open reduction
and internal fixation (47,51) (Fig. 15.10).
 |
|
Figure 15.10. A: Anteroposterior radiograph illustrating a comminuted type II glenoid fossa fracture. B: Lateral radiograph of fracture. C: Postoperative AP radiograph illustrating interfragmentary lag screws utilizing the coracoid process for good screw purchase. D: Postoperative lateral radiograph.
|
internal fixation if there is an articular stepoff of 5 mm or more,
lateral displacement of the superior fragment, or wide separation of
the inferior and superior fragments. A posterosuperior approach is
recommended for reduction of the fragments (44)
and placement of a 3.5-mm or 4.0-mm interfragmentary lag screw from the
superior fragment into the inferior fragment. When there is an
associated injury to the SSSC, reduction and fixation of the glenoid
surface may indirectly reduce the second injury of the double SSSC
disruption. However, open reduction and fixation of the associated
injury should be performed as well if fixing the glenoid surface does
not reduce the second injury adequately. Type V fractures require open
reduction and internal fixation if there is an articular stepoff of 5
mm or more, inferior displacement of the inferior glenoid fragment with
subluxation of the humeral head, wide separation of the joint surfaces,
or an associated SSSC injury leading to a separated superior glenoid
fragment (44).
amenable to open reduction and internal fixation. Several nonoperative
approaches to these difficult fractures have been advocated, including
(a) a sling and swathe followed by early ROM; (b) immobilization in a
shoulder abduction (“airplane”) splint and early ROM above the level of
the splint as the patient’s symptoms allow; and (c) traction-suspension
therapy with ROM within the ranges allowed by the traction setup (44,141).
glenoid rim and fossa is to achieve a smooth articular surface and
enough bony stability to allow full shoulder function. Frequently
that
can be achieved nonoperatively. In cases in which fracture displacement
is significant and anatomic alignment cannot be achieved by closed
manipulation, open reduction and internal fixation are indicated.
Surgical approach, fixation techniques, and postoperative
rehabilitation follow the principles outlined above for treatment of
glenoid neck fractures.
defined as the bony continuation of the scapular spine lateral to the
spinoglenoid notch. Acromion fractures account for 8% to 12% of all
scapula fractures (2,86)
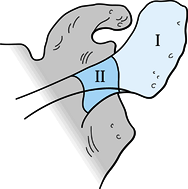
and can occur in two areas: zone 1 fractures occur in the anatomic
acromion, and zone II fractures occur more medially near the scapular
spine and descend toward the spinoglenoid notch (Fig. 15.11) (107). Zone 1 fractures have been subclassified by Kuhn et al. (75)
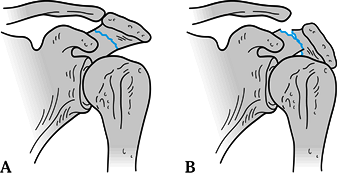
into three main types: Type IA fractures are avulsion injuries of the
tip; type 1B fractures are nondisplaced, true fractures of the acromion
(Fig. 15.12A); Type II fractures are displaced
but do not decrease the subacromial space; Type III fractures are
displaced such that the subacromial space is decreased (Fig. 15.12B).
 |
|
Figure 15.11. Zone I: Location of fractures occurring in the anatomic acromion. Zone II: Location of fractures occurring in the medial acromion between the spinoglenoid notch and the anatomic acromion.
|
 |
|
Figure 15.12. A: Nondisplaced acromion fracture; operative treatment not indicated. B: Displaced acromion fracture resulting in a decreased subacromial space; this fracture requires ORIF.
|
treated nonoperatively. Although nonunion or pseudarthrosis may result
from fractures treated nonoperatively, most of these are not painful
and do not lead to functional impairment (75).
The occasional nonunion that is painful can be treated with bone
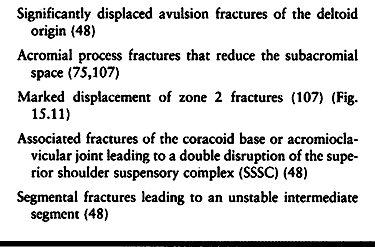
grafting and internal fixation. There are however several indications
for acute surgical treatment (Table 15.4).
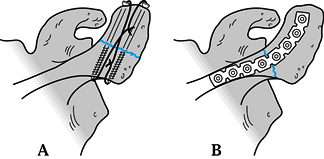
Avulsion fractures can be repaired with heavy suture through drill
holes in the acromion. Zone 1 fractures are best stabilized with a
tension band construct, either with cannulated 4.0 mm partially
threaded screws or Kirschner (K-) wires and either 18-gauge wire or
heavy suture (Fig. 15.13A). Zone 2 fractures are best stabilized with a 3.5 mm reconstruction plate (Fig. 15.13B and Fig. 15.14).
 |
|
Table 15.4. Indications for Operative Treatment of Acromion Fractures
|
 |
|
Figure 15.13. A:
Tension band construct of a zone I acromion fracture using cannulated 4.0 screws and 18 gauge wire. K-wires can be used as well. B: Zone II fracture fixed with a contoured, curved 3.5-mm reconstruction plate. |
 |
|
Figure 15.14. A:
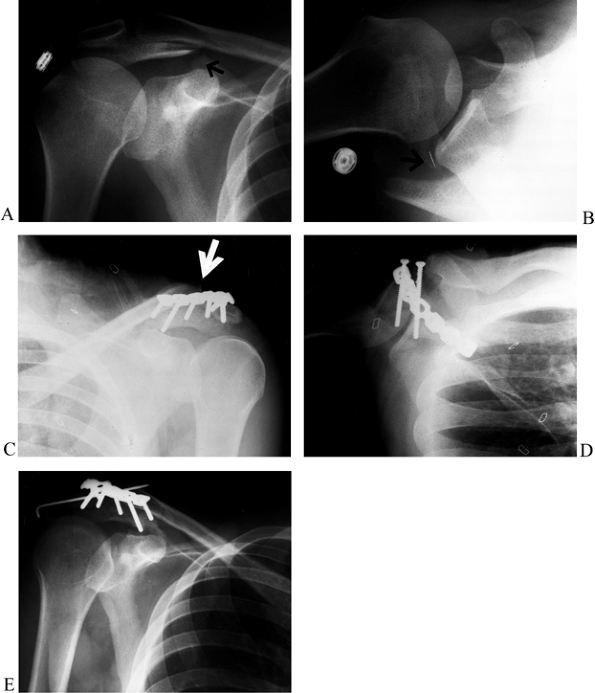
Anteroposterior radiograph showing a displaced fracture of the acromion at the junction of zone I and zone II with a type III pattern (i.e., tilted down toward the humeral head, causing impingement). B: Axillary view showing the widely displaced acromion fracture. C: After fixation of the acromion fracture with two parallel cannulated 4.0-mm screws and a neutralization 3.5-mm reconstruction plate, the AC joint was displaced, revealing a double disruption of the SSSC. The AC joint was reduced and pinned with a single 0.062 K-wire. D: Postoperative axillary view before placement of the K-wire, demonstrating the hardware placement. E: Anteroposterior radiograph 4 weeks postop showing the single 0.062 K-wire that was used to pin the AC joint. Note the bend in the end of the K-wire to prevent migration. The patient had full range of motion of his shoulder. |
with fixation of the acromial process fracture alone (47).
If the second injury involves the acromioclavicular joint, however,
then temporary fixation with an additional pin across the AC joint may
be necessary. If pins are used around the shoulder girdle complex, they
must be bent to avoid migration and should be removed once the injured
structures have healed.
nonoperatively with a sling to support the arm for comfort. Begin
pendulum and active-assisted ROM exercises within the first several
days. The patient may increase the use of the involved extremity as her
pain allows.
that require ORIF, we prefer a tension band technique with two
cannulated 4.0 screws and heavy number 5 suture or 18 gauge wire. Do
not use screws that project beyond the bony surface because they could
eventually cut the suture running over their edges. For more medial
acromion fractures, we recommend using a curved pelvic 3.5 mm
reconstruction plate which has oval holes and allows angled screw
insertion if necessary (Fig. 15.14). Use standard surgical approaches (see Chapter 1) to the shoulder girdle to expose, reduce and internally fix the fracture.
wound is sealed. If the deltoid required surgical repair due to
traumatic injury or take-down during surgery, avoid active abduction
and forward elevation for 4–6 weeks. In all other cases, begin
active-assisted exercises within the first 2 weeks to avoid shoulder
stiffness. Have the patient use a sling when sitting up or walking, and
a pillow under the ipsilateral elbow when in bed to relieve the
downward pressure on the acromion from the weight of the arm.
rotator cuff, deltoid and trapezius. Fractures in this region are
commonly associated with scapular body fractures and can lead to some
degree of pain and weakness. In one series, 70% of patients with
scapular spine fractures had abduction weakness and pain at rest from 3
to 7 years after their injury and many of these patients also had night
pain (2). Nevasier (100)
referred to this phenomenon as a “pseudorupture” of the rotator cuff
caused by hemorrhage and scarring of the supra- and infraspinatus
muscles related to the fractures which are frequently comminuted and
displaced. Because patients with these injuries may develop problems
either acutely or on a delayed basis, some authors advocate that
scapular spine fractures should be reduced and surgically stabilized (2,51). To date, there are no published reports that provide definite indications for surgery.
The typical mechanism of injury is an avulsion fracture caused by
traction on the conjoined tendon and/or the coracoclavicular ligaments.
Occasionally a direct blow by the humeral head at the time of
glenohumeral dislocation produces the fracture (48,108). In general, coracoid process fractures can be treated nonoperatively (48,50,84),
although associated injuries may warrant surgery. Prescribe a sling for
patient comfort for the first week or two, and then begin progressive
range of motion exercises. Avoid heavy lifting and weight training for
8 to 12 weeks since the short head of the biceps brachii and the
pectoralis minor (as well as the coracobrachialis) muscles originate
from the coracoid and forceful contraction of those muscles might
displace the fracture (Fig. 15.2).
exceptional cases. For example, a coracoid fracture in a high
performance athlete who relies on the precise functioning of his/her
upper extremity warrants ORIF. Another indication for surgery is an
associated injury such as a type III AC disruption (see below),
acromion fracture, glenoid neck fracture, or clavicle fracture that
results in wide displacement of the associated injury or both injuries.
In most cases, fixation of the associated injury is all that is needed,
and doing so indirectly reduces the coracoid process fracture and
allows it to heal in satisfactory position. Delayed treatment of an
avulsion fracture is occasionally necessary in patients who develop
soft tissue irritation from the displaced bony fragments. In those
cases, reduce and internally fix large fragments, or, if the bony
fragments are small, excise them and suture the conjoined tendon to the
remaining coracoid process.
CT scan will delineate the presence of these injuries and assist in
choosing a treatment plan and surgical strategy. Anterior and posterior
45° oblique views may be beneficial to show fracture displacement (45).
If AC or distal clavicle injuries are suspected, AP shoulder
radiographs obtained while 10-lb weights are suspended from the
patient’s wrists can be used to accentuate the disruptions and aid in
the diagnosis.
injuries is an indication for surgery. Only recently has attention in
the literature been focused on the complex nature
of these injuries (45),
and, therefore, precise criteria for operative intervention are
unknown. The surgeon must rely on his judgment as to the likelihood
that the displacement will lead to delayed or nonunion and/or adverse
biomechanical and functional outcomes. The more widely displaced the
injured structures are, the more likely surgical intervention will be
beneficial.
straightforward. Identify the injury that is easiest to approach
surgically, perform the site-appropriate reduction and stabilization
utilizing the techniques described above, and then evaluate the
secondary disruption for persistent displacement and instability. Once
the initial injury has been reduced and stabilized, it usually produces
an indirect reduction of the secondary injury and makes the whole
shoulder complex sufficiently stable so that ORIF at the second site is
not necessary. For example, ORIF of the clavicle will usually reduce
and stabilize an associated glenoid neck fracture, and K-wire fixation
or coracoclavicular ligament reconstruction in selected type III AC
separations and all type V AC separations (see below) will reestablish
the clavicle as the stable superior buttress of the shoulder girdle (Fig. 15.9).
the cases, except those with pins across the AC joint, prescribe sling
support for 1 to 3 weeks in conjunction with pendulum exercises. After
2 to 3 weeks, start active assisted ROM exercises and add gentle
strengthening exercises after 6 and 8 weeks. By 3 months, most patients
should have full shoulder motion and be using their shoulders for all
activities of daily living.
forequarter amputation caused by severe blunt trauma and traction to
the upper extremity. Beneath an intact layer of skin, there is
disruption of the scapular rotators, subclavian vessels and the
brachial plexus (22,109).
Bone and joint injuries such as AC separations, clavicle fractures, and
sternoclavicular (SC) disruptions complete the clinical picture.
severe injuries that necessarily divert the resuscitation efforts to
life-saving maneuvers. In addition, ipsilateral musculoskeletal
injuries may mistakenly be identified as the cause of neurovascular
deficits. However, careful attention to the mechanism of injury (thrown
motorcyclist or farming accident), critical assessment of the position
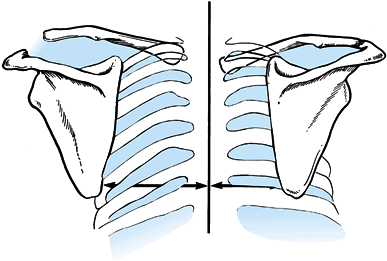
of the scapulae in relation to the spinous processes (the medial
borders should normally be equidistant from the spinous processes) (Fig. 15.15),
and a thorough physical exam (absent pulses, neurologic compromise,
severe swelling over the shoulder region) can lead the resuscitation
team to the correct diagnosis. Further workup includes upper extremity
angiography to identify the level of the arterial lesion and guide the
surgical strategy for vascular repair.
 |
|
Figure 15.15.
Diagnosis of scapulothoracic dissociation can be verified by observing significant lateral displacement of the scapula and shoulder girdle on the affected side. This can be determined by comparing the distance from the medial border of the scapula to the spinous processes between the affected and unaffected sides. (From Butters KP. Fractures and Dislocations of the Clavicle. In: Rockwood CA Jr, Green DP, Bucholz RW, Heckman JD, eds. Fractures in Adults. Philadelphia: JB Lippincott, 1996;1189.) |
restore perfusion to the affected limb. Frequently, that requires a
saphenous vein graft to repair the avulsed subclavian or axillary
arteries or a shunt. Next, explore the brachial plexus and
reapproximate any transected nerves with appropriate microsurgical
techniques. It is much easier to perform the nerve repairs acutely than
later, when they are encased in scar. Finally, establish bony stability
where needed such as by plating or pinning a clavicle fracture or
reconstructing the AC joint to restore the length and stability of the
shoulder girdle, protect the neurovascular repairs, and create a stable
environment for soft-tissue healing.
was recommended for patients who presented with complete neurologic
deficits in the affected limb (22,31).
Recent improvements in microsurgery, however, have allowed surgeons to
salvage limbs that might have been amputated previously. Partial
neurologic deficits generally have a good prognosis in terms of return
of function and sensation (31). The decision to
amputate a revascularized, viable limb because of neurologic or other
soft-tissue injuries should be delayed and made only after
multidisciplinary consultation and discussion with the patient and
family. More specific indications and a discussion of treatment options
regarding brachial plexus injuries are
beyond the scope of this chapter but are found elsewhere in this book (see Chapter 60).
to avoid when managing scapular fractures. The first is the failure to
recognize a double disruption of the SSSC. If one overlooks one or both
disruptions in the suspensory complex, further displacement and
instability of the shoulder complex may result when movement is
initiated. Conversely, a rehabilitation program that is not vigorous
enough can be equally problematic and result in excessive shoulder
stiffness. A well-designed and supervised exercise program is the key
to success.
nerve when operating on the scapula. The nerve may be injured at the
spinoglenoid notch by careless medial dissection during the posterior
approach to the glenoid neck or excessive traction on the infraspinatus
muscle. Finally, avoid placing K-wires across the AC joint or into the
scapula wherever possible. In rare cases when they are used temporarily
to fix small fragments, bend the ends of K-wires to prevent pin
migration. Remove them 4 to 6 weeks postoperatively before beginning
vigorous shoulder motion to avoid pin breakage and interference with
motion.
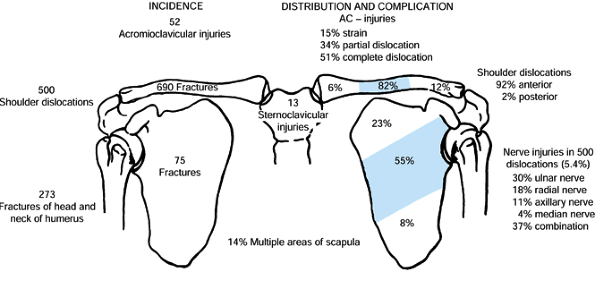
fractures and 35% to 43% of shoulder girdle injuries. Fractures occur
most commonly in the middle third of the bone (76%–82%) and less often
in the distal (12%–21%) and medial (3%–6%) thirds (103,127) (Fig. 15.16).
Proximal clavicle fractures tend to occur in elderly men; middle-third
fractures tend to occur in children (typically undisplaced),
adolescents (displaced), and young male adults (comminuted);
distal-third fractures are frequent in middle-aged patients (103).
 |
|
Figure 15.16.
An analysis of 1,603 shoulder girdle injuries among which were 690 fractures of the clavicle. (From Rowe CR. An Atlas of Anatomy and Treatment of Midclavicular Fractures. Clin Orthop 1968;58:29.) |
clavicles has been likened to a yoke and serves to keep the shoulder
girdle positioned laterally throughout a wide range of motion,
enhancing upper extremity function (82). The
clavicle also provides a base for muscular attachments of the shoulder
girdle, allows maximum upper extremity range of motion for better
positioning of the hand, protects vital neurovascular structures,
facilitates optimum respiration and circulation via the attached
secondary muscles of respiration, and contributes to the cosmetic
appearance of the neck region (82,90).
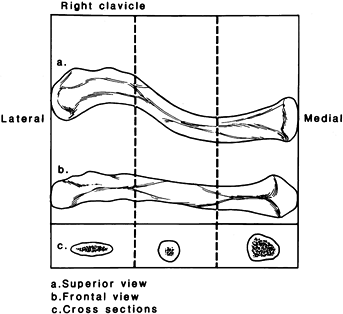
The clavicle is an S-shaped bone, concave arteriorly at its lateral end
and convex anteriorly at its medial end, and subcutaneous throughout
its entire length. The cross-sectional anatomy changes along its
lateral-to-medial course from flat to tubular to prismatic (Fig. 15.17).
The junction from the flat region to the tubular region is a stress
riser; that fact, coupled with the effect of the first rib acting as a
fulcrum near the same spot over which the clavicle rides as
superior-lateral load is applied to it, explains the high incidence of
fractures in that region. The medial end of the clavicle is firmly
attached to the sternum and first rib by stout ligaments, the only
articulating connection of the upper extremity to the axial skeleton.
Laterally, the clavicle is attached to the coracoid process by the
coracoclavicular ligaments (Fig. 15.2) and to the acromion by the acromioclavicular ligaments.
 |
|
Figure 15.17. The clavicle appears straight when viewed from the front (B) but as an S-shaped double curve when viewed from above (A). The lateral end of the clavicle is flat in cross section (C),
whereas the medial aspect is more tubular. (From Craig EV. Fractures and Dislocations of the Clavicle. In: Rockwood CA Jr, Green DP, Bucholz RW, Heckman JD, eds. Fractures in Adults. Philadelphia: JB Lippincott, 1996;1111.) |
neurovascular structures with the clavicle help explain some patterns
of injuries seen clinically and highlight potential complications to
avoid when treating them. For example, because of their insertions, the
sternocleidomastoid and trapezius muscles pull the medial aspect of the
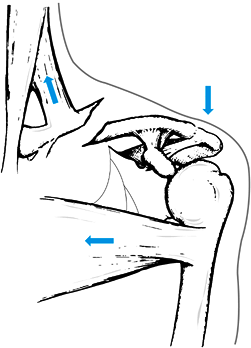
clavicle superiorly after clavicle fractures (Fig. 15.18).
The subclavian artery and vein as well as the brachial plexus pass
between the junction of the middle and medial third of the clavicle and
the first rib and need to be protected during plating of clavicle
fractures. The strong tubular portion of the clavicle, clothed on its
underside by the subclavius muscle and fascia, overlies these vital
structures, which may account for the low incidence of neurovascular
injury associated with clavicle fractures.
 |
|
Figure 15.18. Muscle forces acting on fractures of the middle third of the clavicle to render them potentially unstable.
|
clavicle: an AP view on a large film that includes the upper third of
the humerus, shoulder girdle, and upper lung field and a 45° cephalad
“oblique” view (126). Together these views are
sufficient for evaluating most clavicle fractures except those in the
medial third and will show associated musculoskeletal injures, screen
for a pneumothorax, and show both the superior–inferior and
anterior–posterior displacement of the clavicle. In fractures that have
been treated with internal fixation or in cases of questionable
nonunion, an abduction lordotic view is an alternate method of getting
a second view at 90° for a more complete representation of the clavicle
(116). Distal third fractures are more
difficult to assess because of overlapping bony structures.
Anterior–posterior oblique stress films with the patient standing and
while 10-lb weights are suspended from each wrist should be sufficient
to diagnose an unstable distal clavicle fracture or acromioclavicular
separation. Computed tomographic scans are useful for evaluating medial
clavicle fractures because plain films are
usually inadequate for assessing anterior–posterior displacement.
according to location in the bone because prognosis and treatment vary
according to type. Group I (middle-third) fractures are the most
common, accounting for approximately 80% of all clavicle fractures.
Approximately 97% of fractures in this group are mild to moderately
displaced and can be treated nonoperatively, with certain exceptions,
outlined below. However, 3% of middle-third clavicle fractures are
completely displaced and shortened. This small group of fractures
accounts for 90% of nonunions in middle-third fractures and therefore
may warrant early open reduction and internal fixation (139).
the bone and account for approximately 10% of clavicle fractures.
Critical to the behavior and treatment of these injuries is the
condition of the coracoclavicular ligaments. If they are both
disrupted, the shoulder girdle loses its superior strut, and the
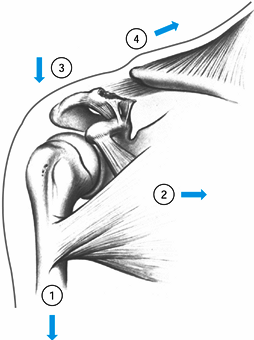
fracture displaces because of four main forces (95) (Fig. 15.19).
If the ligaments are not disrupted, the same displacement pattern will
still occur if the ligaments are attached to an inferior butterfly
fragment (111). The displacement caused by
these forces may account for the reported 30% nonunion and 45% delayed
union of these fractures when treated nonoperatively (32).
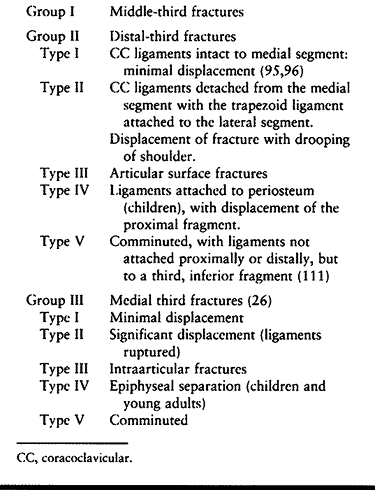
Group II fractures can be further subdivided into five subtypes based
on fracture location because the anatomic details of the fracture play
a role in treatment options (Table 15.5; Fig. 15.20).
 |
|
Figure 15.19. The four displacing forces that act on the clavicle and shoulder girdle in type II fractures: (1) the weight of the arm, (2) the latissimus dorsi, the pectoralis major and minor muscles, (3) scapular rotation, and (4)
the trapezius muscle. The first three forces act only the distal segment, while the trapezius muscle pulls the shaft upward and backward. (From Neer CS II. Fractures of the Distal Third of the Clavicle. Clin Orthop 1968;58:45.) |
 |
|
Table 15.5. Classification of Clavicle Fractures
|
 |
|
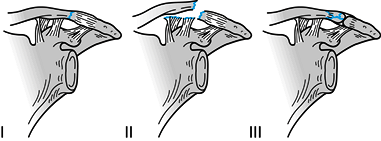
Figure 15.20.
Fractures of the distal clavicle. Type I fractures occur lateral to the coracoclavicular ligaments. Type II fractures functionally detach the coracoclavicular ligaments from the medial fragment of the clavicle. Type III fractures are intraarticular and involve the acromioclavicular joint. Types IV and V are not shown. |
includes a complete history, a careful neurovascular exam of the
shoulder and ipsilateral upper extremity, and appropriate imaging. The
mechanism and events surrounding the injury as well as the patient’s
preexisting medical problems provide useful information about the
amount of energy and forces which produced the fracture as well as
important clues about associated injuries. Most clavicle fractures are
closed, isolated injuries sustained in low energy falls (103);
however, they also occur in high energy accidents. Although
neurovascular injuries and open fractures are uncommon, subclavian
artery and vein injuries, severe chest trauma, and brachial plexus
injuries all have been reported. Unfortunately, associated injuries
often are missed initially (9,61,118,127); look carefully for them to avoid delays in diagnosis.
Group I, middle one-third, clavicle fractures can generally be treated
nonoperatively. A figure-of-eight bandage with or without plaster
reinforcement has long been recommended and is one very good treatment
option (Fig. 15.21) (3,26,119,127). See Chapter 10
for a description of the use of a plaster bolero. The goal of this
method is to reoppose the bone ends as much as possible by
simultaneously raising the lateral fragment upward and backward while
depressing the medial fragment. The advantage of a figure-of-eight
brace is that it leaves the ipsilateral hand free for use while
splinting the fracture helps keep the patient’s shoulders back and the
clavicle out to length, minimizing the chance of the bone healing in a
shortened position. The disadvantages of this method include the
difficulty many patients have keeping the brace adjusted properly and
the potential skin problems caused by the brace, as well as impairment
of patients’ agility, personal hygiene needs, and comfort while
sleeping (5).
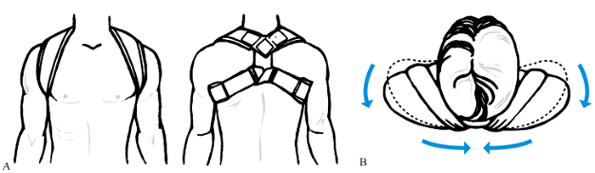 |
|
Figure 15.21. A: A commercial figure-of-eight support. B:
Superior view of the patient, showing how the figure-of-eight support pulls the shoulder up and backward. (From Dameron TB Jr, Rockwood CA Jr. Fractures and Dislocations of the Shoulder. In Rockwood CA Jr, Wilkins KE, King RE, eds. Fractures in Children, Philadelphia: JB Lippincott, 1984;618.) |
Although a sling does nothing to correct shortening or displacement at
the fracture site, it is often more comfortable and convenient for
patients than a figure-or-eight brace and yet leads to the same rate of
union and excellent function as can be achieved with more restrictive
treatment methods (5,105).
middle-third clavicle fractures heal uneventfully and with little or no
functional limitations. The nonunion rate for middle-third clavicle
fractures ranges from 0.1% in Neer’s series of 2,235 patients to 15% in
Hill’s series of 242 consecutive patients (57,94).
Higher rates of nonunion in middle-third clavicle fractures have been
associated with high-energy fractures, wide displacement (1–2 cm),
refracture, soft tissue interposition by the trapezius muscle, and
operative treatment (57,65,83,94,127,139,145).
The most commonly encountered indication is an open fracture, which
should be treated with thorough wound irrigation, debridement, and
stable internal fixation (49). The exception
may be an isolated clavicle fracture with a small (grade I) inside-out
puncture wound, which could be treated by irrigating and debriding the
wound and treating the fracture with sling immobilization. If the wound
requires surgical treatment anyway, however, then rigid internal
fixation of the fracture at the same time is indicated,
as the wounds heal much better when the clavicle is stabilized because the fractured ends tend to displace into the wound.
 |
|
Table 15.6. Indications for Operative Treatment of Middle-Third Clavicle Fractures
|
fractures are less common. Associated neurovascular injuries that do
not improve with attempted reduction warrant surgery to decompress and
prevent further damage to the underlying structures (9).
Fractures that are displaced upward to the point of tenting and
threatening the integrity of the skin may need reduction and fixation
if closed maneuvers fail to reduce the clavicle. As outlined above,
double disruptions of the SSSC that include a clavicle fracture require
reduction and stabilization of at least one of the injuries. Surgical
treatment of the clavicle fracture is logical in this setting because
it is usually the most accessible and simplest disruption to fix.
operative intervention. It has been shown that middle-third clavicle
fractures with more than 2 cm of displacement or 15 mm of shortening
are at increased risk for nonunion (34,57,83).
Thompson reviewed more than 100 middle-third clavicular nonunions
reported in the literature and found that 90% of the original fractures
had displacement greater than 100%, overriding more than 1 cm, or had
severe comminution (139). Although fractures
with this much displacement are uncommon, accounting for 3% of all
middle-third clavicle fractures, they warrant open reduction and
internal fixation.
need operative management. If a patient has associated ipsilateral
upper extremity fractures treated surgically and would benefit from
early mobilization of the affected limb, then ORIF of the clavicle
fracture is indicated. Patients with bilateral upper extremity injuries
including a clavicle fracture benefit from immediate clavicle fixation
because it frees up one upper extremity so the patient may perform
independent activities of daily living, and patients with lower
extremity fractures and a clavicle fracture benefit from fixation of
the latter because it facilitates the use of crutches or a walker for
early mobilization. Finally, stabilization of clavicle fractures is
indicated for patients with multiple ipsilateral rib fractures because
it helps stabilize the hemithorax, resulting in less pain and better
pulmonary hygiene (102). Other relative indications for the surgical treatment of clavicle fractures are listed in Table 15.6.
clavicle fractures: intramedullary devices, plates, and external
fixators. Intramedullary fixation can be accomplished with smooth or
threaded K- wires, Steinman pins, Knowles pins, Hagie pins, or
cannulated screws (26,77,101,102,150).
The advantages of using intramedullary devices are several: less
surgical dissection and soft tissue stripping is needed, and the
hardware is less prominent. Disadvantages include possible pin
migration and poor rotational control during elevation of the extremity
above shoulder level. Most techniques using intramedullary devices
utilize the S-shaped curve of the clavicle for hardware placement, a
small anterior incision, exposure of the bone ends, and retrograde
insertion of the chosen pin or device. Once the fracture is reduced,
the pins are advanced back across the fracture into the anterior cortex
of the medial fragment. Ngarmukos (102) has had
good success with two 2-mm smooth K-wires inserted retrograde into the
medial fragment and then antegrade into the lateral fragment. The ends
of the wires are bent down around the clavicle proximally. If these
pins back out, they are very prominent and easy to remove. Two wires
are used to prevent rotation. Removal of K-wires is recommended once
the fracture has healed (102). In contrast, Knowles pins and screws do not need to be removed unless hardware-related symptoms develop (101).
Biomechanically, plate fixation is superior to intramedullary fixation
because it better resists the bending and torsional forces that occur
during elevation of the upper extremity above shoulder level. Patients
treated with plate fixation can be allowed full range of motion once
their soft tissues have healed. Disadvantages of plate fixation include
the necessity for increased exposure and soft-tissue stripping;
potential damage to the supraclavicular nerves, which cross through the
surgical field; slightly higher infection rates (15); and the risk of refracture after plate removal (15). Despite these shortcomings,
plate fixation utilizing careful surgical technique and appropriate use
of autogenous bone grafting is an excellent method of treatment for
these injuries.
stripping are prerequisites for optimum outcome. Many investigators
have recommended the use of a 3.5-mm AO/ASIF dynamic compression plate
(DCP) or a low-contact dynamic compression plate with at least three
screws (six cortices) in both the medial and lateral fragment and an
interfragmentary lag screw whenever the fracture pattern allows it.
Bostman (15) found no difference between using
3.5-mm DCPs and 3.5-mm AO/ASIF reconstruction plates; both provided
acceptable fixation and rigidity. One-third of tubular plates have a
high rate of fatigue failure when used for clavicle fractures and
should be avoided (15,113).
The plates should be precontoured and placed superiorly (best) or
anteriorly (second best). Autogenous bone graft should be used in
comminuted fractures with bone loss.
reported a 100% union rate and normal shoulder function without pleural
injury and only two low-grade pin site infections utilizing a
Hoffman-type fixator for selected indications. It may be indicated for
severe open fractures with poor quality overlying skin. Ballmer and
Hertel (8) recommended eternal fixation with a
small AO external fixator for severely displaced fractures with extreme
angulation and tenting of the skin, open fractures, and fractures
associated with neurovascular injury. External fixation may also be
indicated for treatment of clavicle fractures in the face of infection
or infected nonunions following plate removal. Even in these cases,
plate fixation should be considered first and used whenever possible.
based on their classification. Type I fractures are stable because of
the intact surrounding ligaments and can be treated effectively with
sling immobilization and progressive use of the shoulder as pain allows
(96). Most type I fractures heal within 4 to 6 weeks with little to no residual shoulder dysfunction.
nonoperatively because of the deforming forces. The trapezius pulls
upward and posteriorly on the medial fragment, which is no longer
tethered by the coracoclavicular ligaments and thus becomes widely
displaced from the lateral fragment. Some authors have reported
satisfactory results after nonoperative treatment of these fractures,
but management is difficult (26,104,119). In contrast, Neer (95,96) and Edwards (32)
both reported a nonunion rate of approximately 30% in type II fractures
treated nonoperatively and a union rate of 100% when these fractures
were treated operatively with either coracoclavicular screws or
transacromial K-wires (32,95,96).
Based on the high rates of delayed union and nonunion, difficult
rehabilitation and residual shoulder pain associated with nonoperative
treatment in several studies, many surgeons recommend operative
treatment of type II fractures (26,32,43,56,95,96,118).
and coracoclavicular banding or taping with or without
acromioclavicular fixation utilizing dacron or other synthetic
materials (56). Each of the techniques has been
used successfully; the choice of one technique over another should be
based on the size of the distal fragment, patient and fracture anatomy,
and surgeon’s preference. If there is a noncomminuted, 2- to 3-cm
distal piece, then a small-fragment AO T-plate or two K-wires with a
tension band placed outside the acromioclavicular joint are good
choices for fixation. On the other hand, comminuted and/or small distal
fragments require transacromial wire fixation, coracoclavicular screw
fixation, or coracoclavicular ligament repair. Operative treatment can
be problematic. Kona et al. (72) reported 19
cases of type II distal clavicle fractures treated with a variety of
surgical techniques that had an overall 32% nonunion rate, 30%
infection rate, and 56% unsatisfactory results. Three nonunions and all
five patients with a poor result had transacromial K-wire fixation,
prompting the authors to recommend avoiding any transacromial wire
fixation for these fractures.
of the distal clavicle generally can be treated nonoperatively with a
sling for support (104) and gradual return to
normal use of the extremity as pain allows. If the fracture is
unstable, however, then treatment should be similar to that for type II
fractures. In severely comminuted fractures, primary excision can be
performed with repair or reconstruction of the coracoclavicular
ligaments using the Weaver-Dunn procedure (143)
as necessary to stabilize the clavicle. If painful posttraumatic
arthrosis or osteolysis develops, the distal clavicle can be resected (93).
and can mimic an AC joint dislocation. Most heal well with nonoperative
treatment unless severely displaced (26). If
operative treatment is necessary, then open reduction and suture repair
of the periosteal sleeve is recommended. Type V fractures can be
treated by following the same principles used to treat type II injuries.
compared the results of distal clavicle excision for idiopathic
arthritis in 27 patients and for posttraumatic pathology in 34 patients
and found no difference. On the other hand, Eskola et al. (33)
reported that a poor result was more common in patients who had had a
fracture of the distal clavicle compared to patients with primary
osteoarthritis and warned against
performing
the procedure in athletes or patients with physically strenuous
occupations. The authors also found that patients did better if less
than 10 mm was excised from the lateral clavicle.
Surgical treatment of medial clavicle fractures is rarely indicated and
limited to cases in which there is wide displacement of the fracture
fragments or impingement on vital neurovascular structures. When
treated operatively, the fracture may be fixed using heavy sutures
passed through drill holes in the bone or, alternatively, with a small
low-profile plate. Pin fixation should be avoided because of the
possibility for hardware migration into subjacent vital organs.
fractures nonoperatively. Our preference is to support the ipsilateral
extremity with a sling for comfort. As the fracture begins to heal and
becomes less painful, we instruct the patient to begin active-assisted
ROM exercises. Once the fracture is clinically stable, discontinue the
sling and begin overhead exercises and gentle strengthening. Healing
takes 4 to 8 weeks in most patients. Symptoms dictate the return to
desired activities. Avoid contact sports for at least 6 weeks, and then
the shoulder must be carefully padded when the athlete returns to play.
-
Use Langer’s lines for the skin incision and expose the superior surface of the clavicle.
-
Avoid undermining the skin flaps and
damaging the supraclavicular nerves. Limit the subperiosteal dissection
to the fracture site. -
Reduce and align the fracture with a
pointed tenaculum and place a lag screw across the fracture whenever
possible. If regaining the length of the clavicle is difficult, a small
AO external fixator can be used to hold the bone ends out to length;
this is rarely necessary. -
Precontour the chosen plate and place it
superiorly on the clavicle. It is important to achieve solid fixation
with three screws (six cortices) on each side of the fracture. -
Place a small drain in the subcutaneous layer and perform a plastic closure with a subcuticular suture.
clavicle fractures with a sling to support the injured extremity for 1
to 2 weeks. Patients are instructed to start pendulum exercises within
the first week and to begin using the involved extremity as their pain
allows.
of the lateral fragment and the stability of the fracture. If a type II
fracture is diagnosed using stress films, but the nonweighted x-rays
show little displacement, then the fracture is treated nonoperatively
with a sling for 6 to 8 weeks. Follow-up x-rays after 1 to 2 weeks are
essential to rule out further displacement. If, however, a type II
fracture is easily diagnosed without weighted views and is widely
separated (100% the diameter of the shaft), then we recommend open
reduction and internal fixation using wire fixation.
-
Make an incision in Langer’s lines and expose the fracture site with careful soft-tissue handling techniques.
-
If the lateral fragment is large enough
(2–3 cm), use a wire tension band (18 gauge) combined with two
extraarticular K-wires drilled across the fracture from lateral to
medial (Fig. 15.22). Bend the wires to avoid pin migration.![]() Figure 15.22. Anteroposterior (A) and superior (B)
Figure 15.22. Anteroposterior (A) and superior (B)
views of the shoulder showing placement of two K-wires and a tension
band used to stabilize a type II distal clavicle fracture. -
Anatomic fracture reduction usually
reapproximates the torn coracoclavicular ligaments sufficiently for
healing, but reinforce them with sutures whenever possible. -
If a Type III fracture is an extension of
a type II fracture or is comminuted and unstable, then we prefer to
treat this injury with a superiorly placed tension band of mersiline or
dacron tape with an additional coracoclavicular loop. Transacromial
K-wires may or may not be used depending on the amount of comminution
of the clavicle. We do not advocated acute distal clavicle resection
unless there is severe comminution and wide displacement of the
fragments. Assess the stability of the coracoclavicular relationship
ligaments so that repair or reconstruction of the coracoclavicular
ligaments can be addressed at the same time.
for comfort but remove it twice a day to perform pendulum exercises and
active-assisted ROM exercises below shoulder level. We encourage
patients to use the affected extremity for routine activities of daily
living below shoulder level as their pain allows. After 3 to 4 weeks,
when the wound is completely healed and the patient is comfortable,
institute active-assisted ROM exercises above shoulder level and full
return to activities of daily living. Most patients resume all normal
activities 8 to 12 weeks postoperatively. If the plate is prominent and
bothersome to the patient, it can be removed after the fracture heals,
but this is not routine.
handling were significant factors in the rates of nonunion and other
complications reported in the earlier literature. Therefore, whatever
approach is chosen, take great care to avoid excessive soft-tissue
stripping, work to achieve solid fixation, and plan on acute bone
grafting for fractures that are highly comminuted or where there is
bone loss. When exposing middle-third clavicle fractures, be cognizant
of the small supraclavicular nerves that cross through the operative
field and try to preserve them.
treatment of distal clavicle fractures include pin migration, coracoid
fracture (89), lysis of drill holes in the clavicle when dacron tape is used (89), wound-healing problems (56), K-wire failure (32), and infection (72).
To prevent pin migration, always bend the ends of the pins. Although
coracoid fracture and lysis of drill holes have been reported after use
of synthetic tapes to stabilize chronic AC separations, this may not be
a problem when they are used to help stabilize distal clavicle
fractures if bony union occurs and relieves stress on the implants (43).
Minimize wound-healing problems and infection by not undermining skin
flaps, by handling soft tissues carefully, and by waiting for acutely
damaged skin to improve before operating. Use stout K-wires, such as
two 2-mm pins as opposed to smaller pins, to decrease the chance for
hardware failure. Never use threaded wires. If distal clavicle
resection is done acutely, carefully assess the stability of the
remaining clavicle after the resection. If it is unstable, repair or
reconstruct the coracoclavicular ligaments.
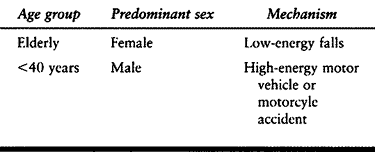
the elderly and are twice as common in women as in men. Typically they
result from falls from a standing height. Although much less common,
proximal humeral fractures do occur in younger male patients also, but
these usually are the result of high-energy motor vehicle or motorcycle
collisions (Table 15.7) (123).
Approximately 85% of proximal humeral fractures are nondisplaced by
Neer’s criteria and can be treated nonoperatively. The remaining 15% of
proximal humeral fractures require careful decision making based on an
understanding of the anatomy of the proximal humerus, the nature of the
injury, the quality of the patient’s bone, and the limitations of the
fixation devices currently available.
 |
|
Table 15.7. Causes of Fractures of the Proximal Humerus
|
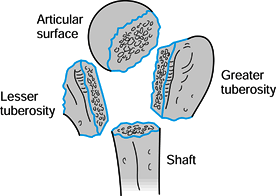
surgeons to recognize that fractures of the proximal humerus occur
along the lines of the physeal scar, potentially creating four separate
fragments (Fig. 15.23). Neer (97,98)
later incorporated this concept into his widely known classification
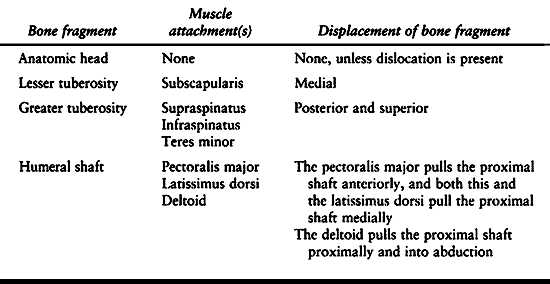
system for proximal humeral fractures. Muscular attachments to these
fragments create the various patterns of displacement seen on the
initial x-rays (Table 15.8). The vascular
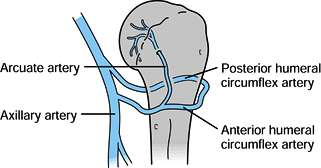
supply to the proximal humerus has been well studied and described by Gerber et al. (42) and Laing (78) (Fig. 15.24). Utilizing injection techniques, Gerber et al. (42)
found that the anterolateral branch of the anterior humeral circumflex
artery ascends within the intertubercular groove lateral to the biceps
tendon and enters the humeral head where the groove meets the greater
tuberosity. Once the anterolateral branch becomes intraosseous, it
becomes the arcuate artery, which supplies the entire epiphysis and all
but small portions of the proximal humerus. The posterior circumflex
artery supplies only a small portion of the posteroinferior head and
the posterior portion of the greater tuberosity. There are abundant
anastamoses between arteries, but they occur proximal to the point
where the anterolateral branch becomes intraosseous. If there is
arterial damage close to this point, the humeral head is at risk for
osteonecrosis. If the blood supply is disrupted more proximally, then
the humeral head will more likely remain viable and perfused through
anastamoses to the anterolateral branch.
 |
|
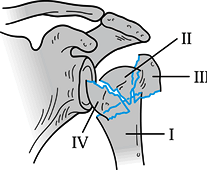
Figure 15.23. Four segments of the proximal humerus as described by Codman.
|
 |
|
Table 15.8. Displacement Patterns in Fractures of the Proximal Humerus
|
 |
|
Figure 15.24. Blood supply to the proximal humerus.
|
associated injuries. The mechanism of injury is important. A fracture
sustained in a high-energy accident in a young person is more likely to
be associated with other injuries than is a low-energy fall in an
elderly person. Follow Advanced Cardiac Life Support (ACLS) principles
when prioritizing the evaluation of a patient with a shoulder injury
(see Chapter 14). During the physical
examination, always note the appearance of the injured shoulder
compared to the opposite side. An anterior fracture-dislocation will
cause a fullness or bulging in the anterior aspect of the shoulder,
whereas a posterior fracture-dislocation may leave a hollow impression
in the anterior aspect of the shoulder. Assess the radial and ulnar
artery pulses and perform a distal sensory and motor neurologic exam.
Nerve injuries occur in association with proximal humeral fractures and dislocations in up to 45% of cases (28);
they are more common in elderly patients and in the presence of a
hematoma. Careful written documentation of the neurologic exam may seem
tedious at the time of the initial evaluation but is essential for
comparison later in the patient’s treatment course. Sensory loss does
not always correlate with motor dysfunction (28), and this should be noted. If pulses are absent, then a vascular consultation may be indicated.
classification and treatment of proximal humeral fractures. A standard
trauma series should include a true AP radiograph of the scapula, a
lateral scapular view, obtained with the patient in a 60° anterior
oblique position, and an axillary view. Computed tomography scans
provide the most reliable information and are helpful in several
circumstances including the evaluation of intraarticular fractures to
assess the degree and nature of damage to the joint surface and the
evaluation of fracture displacement, particularly the greater and/or
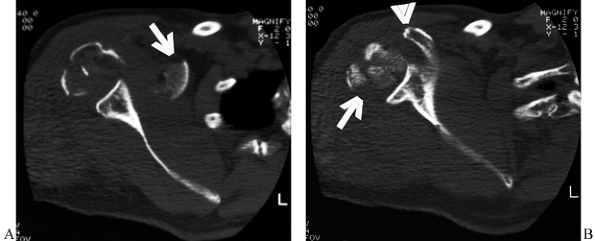
lesser tuberosities (Fig. 15.25).
 |
|
Figure 15.25.
Computed tomographic scans are helpful in evaluating fractures of the humeral head and the degree of articular surface involvement and in ruling out fractures of the glenoid surface. In this case, (A) the extensive comminution of the humeral head is evident (arrow) at the level of the coracoid process (arrowhead). Inferiorly (B), medial displacement of a segment of the humeral head is evident (arrow) at the level of the inferior glenoid, but there is no damage to the glenoid surface. |
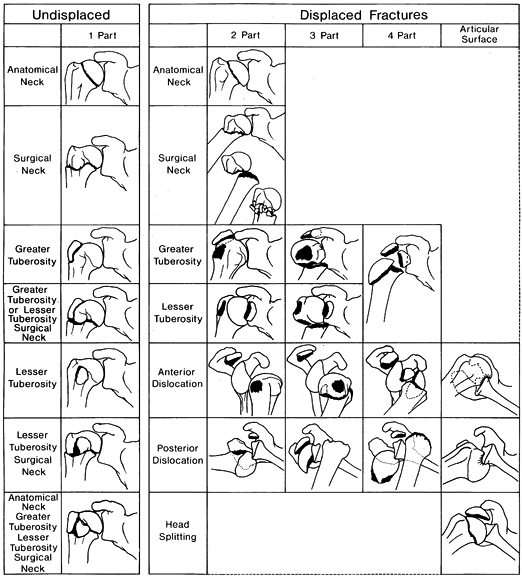
head, greater tuberosity, lesser tuberosity, humeral shaft—Neer, in
1970, devised his classic four-part classification of proximal humerus
fractures (Fig. 15.26) (97,98).
To be considered displaced, one or any combination of the four bone
fragments must be separated by 1 cm or more and/or angulated more than
45°. Fractures that do not fulfill these criteria are considered non-
or minimally displaced and referred to as a “one part fracture.” Neer
emphasized the relationship between displacement and the vascular
supply to the head of the humerus. The greater the number of displaced
fragments, the higher the risk of osteonecrosis. For example, a
two-part fracture of the greater tuberosity is much less likely to
develop osteonecrosis than a four-part fracture.
 |
|
Figure 15.26. Neer’s four-part fracture classification.
|
other fracture patterns that do not fit neatly into Neer’s scheme (AO
classification). One such addition is the so-called four-part valgus
impacted fracture (64), which seems to have a lower risk of osteonecrosis (26%) than the four-part fractures Neer described (90%) (Fig. 15.27) (97).
Head-splitting and impression fractures are special fractures that do
not fit the four-part scheme. Head impression fractures are graded by
the percent of head involvement: less than 20%; between 20% and 45%;
and greater than 45% (12). The AO
classification is more detailed but also more cumbersome and is used
mostly as a research tool. The Neer classification has been shown to
have poor intra- and interobserver correlation (11), but
it is still the most widely used and commonly accepted classification scheme for proximal humeral fractures in North America.
 |
|
Figure 15.27.
Four-part valgus impaction fracture. (Redrawn from Jakob RP, Miniaci A, Anson PS, et al. Four-Part Valgus Impacted Fractures of the Proximal Humerus. J Bone Joint Surg 1991;73-B:295.) |
nonoperatively. Factors important in the decision-making process
include the fracture pattern and classification as well as the
patient’s general health, age, occupation, and avocations. Consider
also whether additional benefits might be gained from surgical
stabilization of the fracture so a patient with multiple injuries could
begin moving the arm and shoulder sooner.
to “one-part fractures” amenable to nonoperative treatment and should
always be tried first. This may occur at the level of the surgical neck
of the humerus (106). Also, two-part
fracture-dislocations of the shoulder where the displaced part is the
greater tuberosity may be amenable to nonoperative treatment if the
shoulder can be reduced and the greater tuberosity fragment reduces.
These fractures require close follow-up to make sure they do not
displace later as a result of the muscle forces acting on them.
fractures nonoperatively are similar to those for managing other
shoulder girdle injuries nonoperatively. The goal is to provide comfort
and pain relief acutely for the patient and begin rehabilitation as
promptly as possible. Immobilize the arm in a sling or collar and cuff
initially (see Chapter 10), and begin gentle
pendulum exercises within a few days. Once the proximal fragments move
together as a unit with the humeral shaft as the shaft is rotated
passively, active ROM exercises can be started, usually within 14 days (73).
nonoperative management. If reduction maneuvers in the emergency
department are unsuccessful, attempt a closed reduction in the
operating room under general anesthesia. If the fracture is
irreducible, then an open reduction is indicated to look for possible
soft tissue interposition at the fracture site. If the fracture is
reducible but unstable, percutaneous pins or an interlocking
intramedullary nail can be used to stabilize and secure the reduction.
If an open reduction is performed, a small clover-leaf plate, modified
clover-leaf plate (35), or a blade plate (66) (Fig. 15.28)
can be used to fix the fracture. In osteopenic bone, however, plates
and screws should be avoided because of the risk of hardware pullout
and failure. Ender’s nails with a figure-of-eight tension band
neutralize rotational and translational forces and provide adequate
fixation to allow early ROM (Fig. 15.29).
Tension band suturing or wiring alone usually do not provide fixation
rigid enough to allow early ROM in patients with osteoporosis.
 |
|
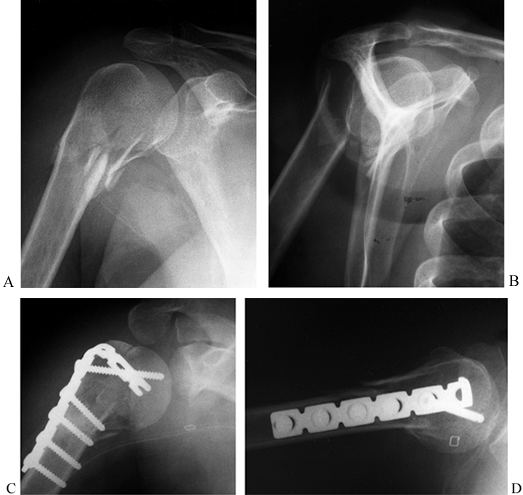
Figure 15.28. A:
Preoperative AP radiograph of a two-part surgical neck proximal humerus fracture. Note that although the greater tuberosity is fractured, it is not displaced and therefore not considered a displaced “part.” B: Scapular lateral view of fracture. C: Postoperative AP radiograph showing reduction and fixation with a 4.5 reconstruction plate fashioned into a “blade” plate. The patient also had a #5 nonabsorbable suture placed in a figure-of-eight tension band, which cannot be visualized. D: Postoperative axillary view of fracture fixation. |
 |
|
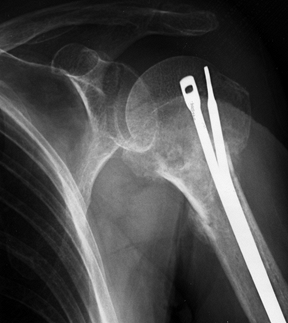
Figure 15.29.
Anteroposterior radiograph illustrating the use of two Ender’s rods and #5 nonabsorbable suture tension band to stabilize an unstable, comminuted, two-part proximal humerus fracture in an elderly patient with osteopenic bone. |
reduce closed and to stabilize with internal fixation, even when open
reduction is performed. Theoretically, the blood supply to the head is
totally disrupted, making osteonecrosis very likely. Therefore,
hemiarthroplasty may be indicated for these fractures, especially for
elderly patients (131). In young patients and
those with good bone stock, every attempt should be made to preserve
the humeral head and perform ORIF. Other salvage procedures or a
delayed hemiarthroplasty can always be done later if osteonecrosis
develops or the fixation fails.
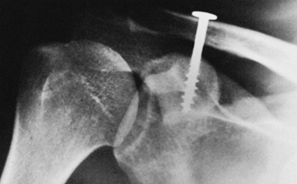
greater tuberosity can sometimes be treated nonoperatively (Fig. 15.30),
but they must be watched carefully, and management can be difficult. If
the greater tuberosity fragment displaces, then open reduction and
internal fixation may be indicated (Fig. 15.31). McLaughlin (88)
found that shoulder function correlated well with residual greater
tuberosity displacement. Fragments displaced more than 1.0 cm lead to
permanent disability, but fragments displaced less than 0.5 cm rarely
caused problems. Fragments displaced between 0.5 cm and 1.0 cm lead to
prolonged recovery time, and 20% of cases require late reconstruction
for persistent pain. In patients with good quality tissues, heavy
nonabsorbable suture or wire in a figure-of-eight tension band
construct placed into the tendonous insertion of the rotator cuff
provides satisfactory fixation to allow early motion. If the bone
quality is good, a lag screw with a washer is a good technique to
provide additional fixation as long as the screw head does not impinge
on the acromion (25). These fractures are
commonly associated with anterior glenohumeral dislocation and
longitudinal rotator cuff tears. Repair the rotator cuff as part of the
surgical procedure.
 |
|
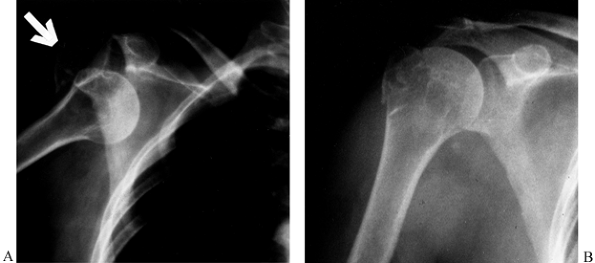
Figure 15.30. Two-part fracture-dislocation of the glenohumeral joint with avulsion of the greater tuberosity (arrow) (A). Reduction produced near-anatomic reapproximation of the tuberosity fragment (B).
This injury can be managed nonoperatively but requires careful follow-up and monitoring to make sure there is no subsequent displacement. |
 |
|
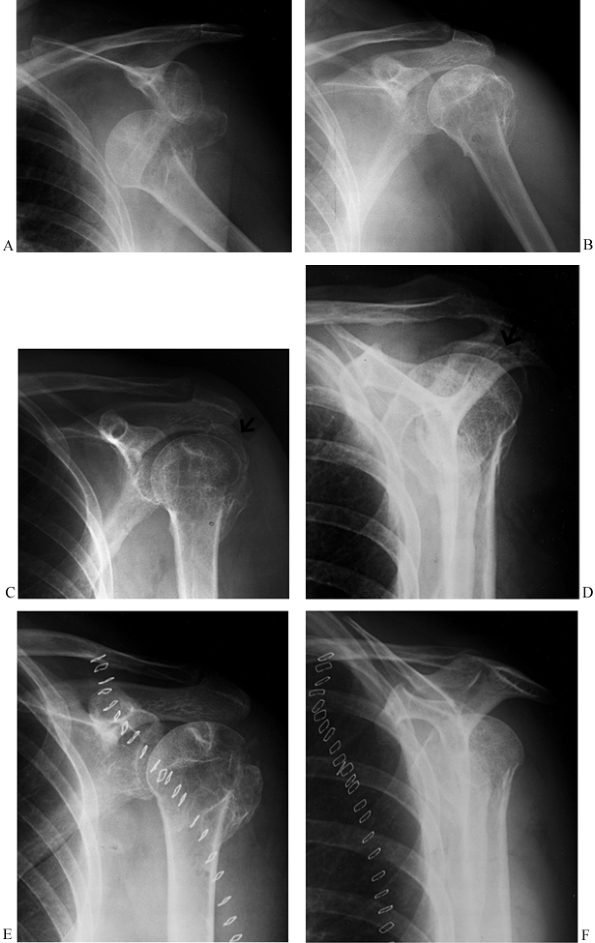
Figure 15.31. A: Anteroposterior radiograph showing a two-part greater tuberosity fracture-dislocation before reduction. B:
Anteroposterior radiograph showing the fracture-dislocation reduced with no apparent residual displacement of the greater tuberosity fragment. There is evidence of an old injury to the proximal humerus, however. C: Anteroposterior radiograph 10 days later showing displaced greater tuberosity fragment. D: Scapular lateral view showing the displaced greater tuberosity fragment. E: Postoperative AP radiograph showing reduction of the greater tuberosity (i.e., the fragment can no longer be seen above the humeral head). F: Postoperative scapular lateral view. |
less common, and when they do occur, they are frequently associated
with posterior glenohumeral dislocations. Following reduction of the
shoulder dislocation, the lesser tuberosity fragment may reduce to a
satisfactory position. If that is the case, then immobilize the arm and
shoulder in neutral or slight external rotation (12). If the lesser tuberosity remains significantly displaced (greater than 1 cm), then ORIF is indicated.
difficult to treat with closed reduction and short-term immobilization
because of the rotational forces exerted on the humeral head by the
rotator cuff muscles, which are attached to the tuberosities (Table 15.8). However, a recent prospective, randomized study by Zyto et al. (153)
comparing nonoperative versus tension-band osteosynthesis failed to
demonstrate any difference in function between the two groups at 1 and
2 years, although the surgical group had more anatomic position of the
tuberosities. Although this study may be reassuring for the patient and
surgeon faced with a fracture where surgery is contraindicated, the
general view expressed in the literature is that open reduction and
internal fixation form the treatment of choice for these injuries,
especially in younger, active patients with good bone quality, whereas
older patients with poor bone stock should be treated with an
arthroplasty.
approach is to use threaded K-wires or cannulated screws percutaneously
or through limited incisions (63,115). Some authors recommend open reduction and fragment fixation with wires or heavy nonabsorbable suture (24,97,131), but others advocate open reduction and internal fixation with plates and screws (Fig. 15.32) (35,136).
With all the techniques, the treatment objective is to provide stable
fixation for early ROM without disrupting any remaining blood supply to
the bone fragments and to avoid implants that will cause subacromial
impingement or other complications such as pin migration. Many studies
have reported poor results and unacceptable rates of osteonecrosis (74)
utilizing plate and screw fixation for three-part fractures. In elderly
patients with osteopenic bone, screws and K-wires may fail because of
poor fixation. Therefore, plates and screws should be reserved for
younger patients with good bone quality. On the other hand, patients
with severe osteopenia may not be good candidates for suturing or
wiring. For those patients, a hemiarthroplasty may be the best option (24).
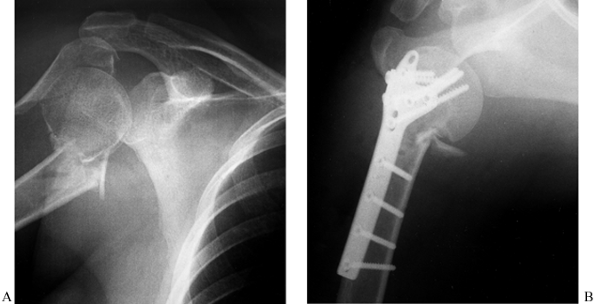 |
|
Figure 15.32. A: Preoperative AP radiograph of a comminuted three-part proximal humerus fracture. B: Postoperative AP radiograph illustrating the use of a clover-leaf plate and #5 nonabsorbable suture tension band as fixation.
|
Once again, younger, active patients with good bone stock are the
exception; for them, open reduction and internal fixation should be
attempted first, even though the risk of nonunion and osteonecrosis is
high (12,24,52,97,134). Stableforth (134)
reported the results of a prospective, randomized study comparing
nonoperative treatment versus hemiarthroplasty for four-part displaced
proximal humeral fractures and compared the results to cases of
impacted four-part fractures treated nonoperatively. He found that the
prosthesis group and
the
impacted group had similar results, which were dramatically better in
terms of function and range of motion than the group with displaced
fractures treated nonoperatively. All patients had some pain; in the
patients with displaced fractures treated nonoperatively, however,
functional disability and sleep difficulties because of pain were far
more common.
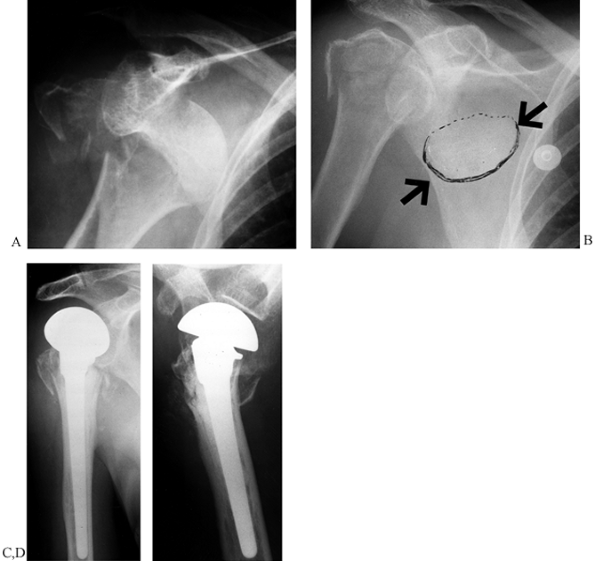 |
|
Figure 15.33. Comminuted fracture-dislocation (A)
of the proximal humerus in a 76-year-old woman with osteoporosis. Attempted reduction left a large section of the humeral head articular surface displaced medially and inferiorly (arrows) (B). This was treated with a cemented hemiarthroplasty (C,D). |
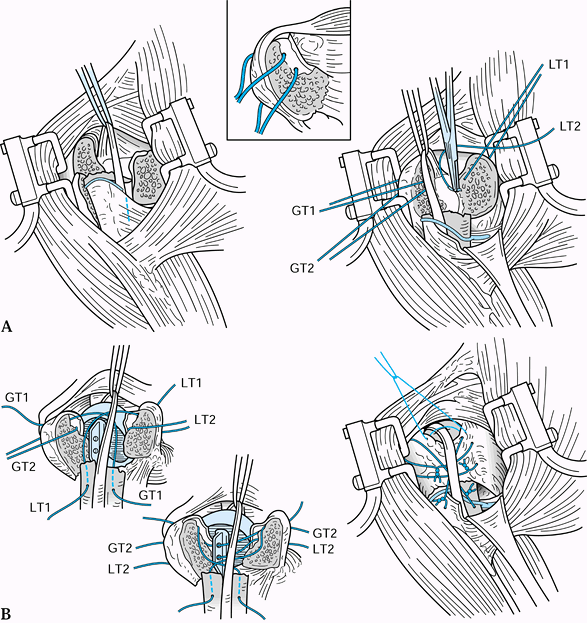 |
|
Figure 15.34. Hemiarthroplasty. A: Deltopectoral approach for a four-part fracture. B:
Prosthetic replacement with four-part fracture. GT1, greater tuberosity and suture #1; GT2, greater tuberosity and suture #2; LT1, lesser tuberosity and suture #1; LT2, lesser tuberosity and suture #2. |
-
Use an anterior deltopectoral surgical approach (see Chapter 1). Do not detach the deltoid muscle from the clavicle.
-
Identify all fracture fragments and clean them of hematoma. Excise the free floating head fragment.
-
Preserve the tuberosity fragments with
the attached rotator cuff muscles. The long head of the biceps is the
key to the rotator interval between the lesser and greater
tuberosities. Insert heavy, nonabsorbable sutures into the edges of
these fragments for traction and later repair of both the greater and
lesser tuberosities. -
Prepare the intramedullary canal of the
humeral shaft according to the instructions provided by the
manufacturer. Restoration of proper humeral length and retroversion
(30° to 40°) is essential for optimum glenohumeral mechanics. Select
the appropriately sized prosthesis and cement the prosthesis in place.
The humeral head replacement is cemented high enough for the
tuberosities to be returned to their normal position. The fin is just
posterior to the biceps groove. -
Reapproximate the tuberosities to each other with the two heavy sutures seen in Figure 15.34
and to the prosthesis to restore the rotator cuff. Harvest bone graft
from the excised humeral head and pack it around the secured
tuberosities to promote healing. Pass cross sutures for each tuberosity
through drill holes in the shaft, the holes in the fin of the
prosthesis, and around the opposite tuberosities. All sutures are tied
with both tuberosities reduced below the articular surface. Close any
remaining gaps in the rotator cuff with heavy, nonabsorbable sutures. -
Initiate supervised passive and active-assisted ROM exercises early to prevent stiffness.
anatomic head is impacted into the humeral shaft in valgus position, is
one subtype of comminuted proximal humeral fractures that warrants
special attention (Fig. 15.27). The incidence
of osteonecrosis after these fractures is approximately 26% versus up
to 90% in the classic Neer displaced four-part fracture, most likely
because the fracture fragments remain in close proximity to each other
and the blood supply to the humeral head is preserved. Jakob et al. (64)
recommended elevation and repositioning of the fractured head fragment
where necessary to make a smooth joint surface and improve glenohumeral
mechanics. Closed reduction can be attempted and open reduction
performed where necessary. Then stabilize the fracture using limited
internal fixation; K-wires usually suffice. In cases where the head
fragment is laterally or posteriorly displaced, the vascular supply is
probably disrupted, and hemiarthroplasty becomes the treatment of
choice.
humeral head are treated based partially on the degree of comminution.
If there are two large pieces amenable to fixation, perform open
reduction and internal fixation with two or more interfragmentary lag
screws. If the articular surface is comminuted, then hemiarthroplasty
is the technique of choice.
dislocations with anterior head impaction against the posterior rim of
the glenoid (Fig. 15.26). Acute impression
fractures that involve less than 20% of the joint surface can be
treated nonoperatively with the arm held in external rotation for 6
weeks (52). If the impression fracture is
between 20% and 45% of the joint surface and less than 6 months old,
transfer the lesser tuberosity into the defect and secure it with a
screw. Shoulder spica immobilization with the shoulder in external
rotation for 6 weeks is recommended postoperatively (130).
Impression fractures involving more than 45% of the joint surface or
associated with dislocations present for more than 6 months should be
treated with hemiarthroplasty. In these cases, the usual retroversion
of 30° to 40° is not recommended. Instead, cement the prosthesis in
neutral rotation to help restore stability to the glenohumeral joint.
their shoulders are immobilized for 24 to 72 hours in a shoulder
immobilizer or sling and swathe. Once the surgical incision is dry and
any drains are removed, however, patients should begin circumduction
pendulum exercises. If good fixation was achieved, patients start
active-assisted ROM early (a pulley system works well). If the deltoid
was released from the acromion and repaired, only passive exercises
should be performed for the first 4 to 6 weeks to allow for healing of
the deltoid. In general, the rehabilitation of a proximal humeral
fracture should follow a program very similar to that proposed for
other shoulder procedures.
nonoperatively, and two-part fractures as well if they can be reduced
and held in good position. We recommend an attempt at closed reduction
in the emergency room with conscious sedation. To reduce these
fractures, apply longitudinal traction while the arm is gently flexed.
Adduction of the distal shaft will help improve medial translation as
the shoulder is flexed. Counterpressure within the axilla may be
helpful to stabilize the humeral head during reduction. Position the
proximal shaft under the humeral head and impact it. We do not
recommend more than one or two attempts at closed reduction in the
emergency department. Further attempts at closed reduction should be
done under general anesthesia in the operating room.
easily reduced but unstable. Insert two pins from the proximal lateral
and proximal anterior shaft directed into the humeral head, and add a
third pin from the greater tuberosity into the proximal shaft. For
fractures that can not be reduced, we perform an open reduction and fix
the fracture internally. We use a blade plate for young patients with
good bone quality and Ender’s rods with tension band nonabsorbable
sutures in patients with osteopenic bone.
are displaced more than 5 mm. We use a deltoid-splitting approach,
being careful not to split the deltoid more than 5 cm from the lateral
edge of the acromion to avoid axillary nerve injury. If the fragment is
large enough, we use a 4.5-mm or 6.5-mm interfragmentary lag screw with
a washer to compress the fragment. We add a figure-of-eight tension
band suture through the rotator cuff insertion for additional fixation.
Avoid placing the screw high in the tuberosity fragment where it would
impinge on the acromion.
fractures in patients with sufficient bone stock to hold the fixation.
Patients with poor bone stock are treated with hemiarthroplasty.
Similarly, young patients (second to fourth decades) with four-part
fractures may be candidates for attempts at ORIF, but all others are
treated with hemiarthroplasty or total shoulder arthroplasty if the
glenoid has arthritic changes.
especially in patients who have been victims of electroconvulsive
therapy and grand mal seizures. Careful radiographic assessment of
patients with suspected shoulder injuries is critical. The axillary
view is frequently the most useful for diagnosing posterior
fracture-dislocations and should be obtained as part of all shoulder
trauma workups. A careful neurovascular examination of the involved
extremity should not be deferred or neglected because axillary artery
injuries and brachial plexus injuries are common in this setting, with
incidences of 5% and 6.2%, respectively (134),
especially in patients with high-energy mechanisms. A palpable pulse
may be present because of the collateral circulation around the
shoulder girdle, but look for signs of vascular injury including an
expanding hematoma, paresthesias, and pallor. More commonly, the
axillary nerve is in danger of injury with fractures of the proximal
humerus. In addition, iatrogenic axillary nerve injury is possible if
the deltoid is split too far distally (>5 cm from the lateral edge
of the acromion) to expose a greater tuberosity fracture.
medial location may cause impingement syndrome with as little as 5 mm
displacement. Osteotomy and replacement of the greater tuberosity to
its anatomic position for large displacements or exostectomy and
acromioplasty for small displacements may be required. Three-part
proximal humerus malunions are much more complex and typically require
prosthetic replacement to obtain satisfactory results. Nonunion may
occur even in two-part fractures at the surgical neck and is frequently
caused by overdistraction using hanging arm casts, soft-tissue
interposition, inadequate immobilization, overly aggressive physical
therapy, an uncooperative patient, or preexisting glenohumeral
stiffness (135).
head replacement is a difficult problem and can be avoided in most
cases by not removing too much bone from the fragments and by securely
fixing the fragments to each other, the prosthesis, and the humeral
shaft. Avascular necrosis may occur in up to 90% of four-part fractures
(97), although other authors have reported lower rates (136).
Humeral head replacement usually provides satisfactory pain relief.
Postoperative stiffness is a particularly troublesome problem for many
patients. Factors important in the development of a stiff shoulder
include prolonged immobilization, development of heterotopic
ossification, significant malunion, and nonunion. It is far better to
prevent stiffness by a well-organized physiotherapy program than to
treat stiffness after it has developed. Finally, inferior subluxation
of the humeral head after closed or operatively treated proximal
humeral fractures may appear worrisome, but in most cases it is
transient and resolves within 1 to 3 weeks as the deltoid and rotator
cuff muscles regain their normal tone. If the inferior subluxation
follows hemiarthroplasty and does not resolve, the prosthesis may be
positioned incorrectly too far down the shaft after excessive bone was
removed from the proximal humerus, and this may be an indication for
revision.
injuries. There is a wide array of good options for their treatment and
controversy over the best methods for many situations. Appropriate
decision making for operative and nonoperative treatment depends on a
thorough understanding of the regional anatomy, fracture pattern and
classification, and factors unique to the injured patient.
the upper border of the insertion of the pectoralis major proximally to
the supracondylar ridge distally (146). On
cross section the shaft is cylindrical proximally, and it narrows in
the AP diameter distally as it approaches the supracondylar ridges. The
deltoid muscle inserts on the deltoid tuberosity, which is on the
anterolateral surface of the proximal shaft and extends to a level
roughly halfway down the arm. The medial and lateral intermuscular
septae divide the arm into anterior and posterior compartments. The
posterior surface is covered by the triceps muscle and serves as the
origin for the lateral and medial heads of that muscle; those muscle
origins delineate the spiral groove. The radial nerve courses through
the spiral groove with the profunda brachii artery as they traverse
from the posterior arm compartment proximally to the anterior
compartment distally. The anterior compartment contains three muscles,
the coracobrachialis, biceps brachii and brachialis, and the brachial
artery and median nerve. The ulnar nerve passes from the anterior to
the posterior compartment as it travels from proximal to distal and
enters the cubital tunnel.
humeral shaft fracture are the muscle insertions and the effect of
gravity. Displacement of the proximal fragment will depend in large
part on whether the fracture occurs proximal or distal to the
insertions of the deltoid and pectoralis major muscles as outlined
above in the section on proximal humeral fractures (Fig. 15.35).
The distal fragment is always distracted to some degree by the weight
of the limb, and this phenomenon is the key to some methods of
nonoperative fracture management.
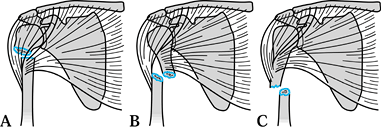 |
|
Figure 15.35.
The muscular attachments to the proximal humerus shaft will cause predictable deformity depending on the location of the fracture. A: The proximal fragment is abducted and externally rotated by the rotator cuff muscles when the fracture occurs above the level of the pectoralis insertion. B: The deltoid muscle displaces the distal fragment proximally and laterally when the fracture occurs between the deltoid and the pectoralis major. C: Fractures distal to the insertion of the deltoid muscle result in abduction of the proximal humerus. |
fractures that is accepted universally. In principle, however, humeral
shaft fractures can be classified by their location in the bone
(relative to the muscle insertions), fracture
pattern, and associated soft-tissue injuries (Table 15.9)
because these factors influence treatment decisions. Transverse
fractures of the midshaft usually occur from low-energy trauma, but
they unite slowly and have a higher rate of nonunion. Oblique and
spiral fractures have a larger fracture surface area and usually
progress to union without surgery. Oblique fractures in the distal
third of the shaft may be difficult to immobilize nonoperatively and
are more often associated with neurologic injuries (59).
Segmental fractures are difficult to reduce and maintain in
satisfactory alignment, and nonoperative management may lead to
nonunion at one or both fracture levels. Comminuted fractures are
especially challenging because they may be difficult to align and hold
by closed methods and also are difficult to fix surgically.
 |
|
Table 15.9. Factors Influencing Treatment of Humeral Shaft Fractures
|
for humeral shaft fractures. Open fractures need urgent surgical
management and have a lower rate of complications when treated with
adequate debridement and stable fixation (20).
Treatment of radial nerve injuries is controversial, as outlined below,
but may be an indication for surgery. Fractures associated with
vascular injuries require surgical stabilization to help protect the
vascular repair.
Several methods of treatment have been described and work well. Options
include a hanging arm cast, coaptation splints, Velpeau sling,
collar-and-cuff sling, abduction humeral splint or shoulder spica cast,
and functional brace. The goals of each treatment method are to keep
the patient comfortable, establish union with acceptable alignment, and
restore full function (see Chapter 10).
with a U-shaped plaster coaptation splint that extends well above the
fracture line laterally. Medially, the plaster should cup the elbow but
not extend very high. If the splint is brought up toward the axilla on
the medial side, it frequently ends at the level of the fracture and
levers it into varus. A sling is also provided for patient comfort.
After 7 to 10 days, when the acute swelling subsides, we switch to a
functional brace. The humeral functional brace was first described by
Sarmiento in 1977 (130). It works by
compressing the soft tissues circumferentially to produce fracture
alignment, and it has been shown to be very effective for treating
closed humeral shaft fractures (7,130,148,149).
 |
|
Table 15.10. Possible Indications for Operative Treatment of Humeral Shaft Fractures
|
requires a cooperative patient who is able to use gravity to help
maintain fracture alignment. This requires that the patient sleep
propped up in bed to avoid angulation at the fracture site, which may
occur in the supine position. This does not apply to the multiply
injured patient, who is often intubated, at bed rest, and not able to
use gravity in the reduction and alignment of the humeral fracture.
When there are other fractures of concern, it is advantageous to have
free use of the injured upper extremity to facilitate rehabilitation (10,16). This is particularly true in patients with bilateral humeral fractures.
the proximal radius and/or ulna, it leaves the elbow unstable or
“floating.” Nonoperative treatment of this lesion leads to high rates
of nonunion, malunion, and elbow stiffness (79,121). In this setting, both the humerus and forearm fractures should be internally fixed to achieve optimum results (79).
and they have been endorsed by several authors since then: a maximum of
3 cm of shortening, 20° anterior or posterior angulation and 30° of
varus. United fractures with up to this much deformity still allow full
functional use of the upper extremity. If attempts at closed reduction
can not produce or maintain the fracture within these parameters, then
surgery is indicated. Closed reduction may be lost in fractures that
are managed nonoperatively because of muscle forces, the inability of
the patient to comply with the physician’s instructions, or the
inability to immobilize the fracture adequately in obese patients.
The Holstein-Lewis fracture, an oblique fracture in the distal third,
is also well known for its association with radial nerve injury (59).
Management of the radial nerve injury is controversial. Most injuries
are neurapraxias or axonotmesis, and 90% will resolve in 3 to 4 months (41,69). The problem is in diagnosing the remaining 10% that will not recover and deciding when surgical exploration is indicated.
months following a closed injury if is there is no evidence of
neurologic recovery (4,114).
The advantages of waiting include the fact that enough time would have
passed for recovery from neurapraxia, the associated fracture will have
healed, and the results of secondary nerve repair may equal those of
primary repair. If the initial injury is open, the nerve is more
likely—64% in one series (38)—to be partially
or completely lacerated or incarcerated in the fracture site, which is
one reason to explore these injuries early.
associated with open fractures, including any penetrating injury, and
any time there is a nerve palsy that develops after fracture reduction
or surgical treatment. Also, at the time of surgery, particularly when
humeral shaft fractures are plated, the radial nerve is carefully
dissected out and identified along its entire course through the
operative field so that there is no question about its integrity
postoperatively. If there is a radial nerve palsy associated with a
closed fracture that is present from the time of initial presentation,
we treat it expectantly. If there is no recovery clinically by 6 weeks,
we obtain a baseline electromyelogram (EMG); an EMG before 3 weeks is
not useful. If there is no clinical improvement by 12 weeks, a second
EMG is obtained; if it confirms that there is no interval recovery of
nerve function, then we explore the nerve surgically with the intention
of performing neurolysis, repair, or sural nerve grafting as the
intraoperative findings dictate.
defined fractures ununited by 4 months as delayed unions and ununited
by 8 months as nonunions. In contrast, based on their retrospective
reviews, Mast et al. (85) and Foulk and Szabo (39)
determined that fractures of the humeral shaft that have not begun to
unite at 6 to 10 weeks probably will not unite unless the treatment is
changed. This is consistent with the literature, which states that the
average time to union of these fractures is 8 to 12 weeks (7,21,71). To optimize outcome and minimize the patient’s disability time, surgery should be performed
as soon as a delayed union or nonunion is suspected (see Chapter 27).
-
Use Henry’s anterolateral exposure or a
midline posterior approach for fractures within the distal third of the
humeral shaft (see Chapter 1). The plate may
be placed on the posterior or anterolateral aspects of the bone. Use
generous incisions to avoid excessive retraction on the soft tissues
with careful soft tissue dissection and bone-handling techniques. -
Identify and protect the radial nerve and others as necessary.
-
Reduce the fracture as accurately as
possible and use lag screws for interfragmental compression wherever
possible (oblique or spiral fractures). Then apply the chosen plate, in
compression mode whenever possible. At least six cortices of screw
purchase are needed in the major fragments, both proximal and distal to
the fracture (Fig. 15.36), and some authors recommend eight to 10 cortices on each side of the fracture (152).
In transverse and short oblique fracture patterns, a compression plate
works especially well. Prebending the plate over the fracture site
ensures closure of the fracture on the cortex opposite the plate.![]() Figure 15.36. A: Radiograph of an acute middiaphyseal humerus fracture associated with a vascular injury to the brachial artery. B:
Figure 15.36. A: Radiograph of an acute middiaphyseal humerus fracture associated with a vascular injury to the brachial artery. B:
Postoperative AP radiograph illustrating proper fixation principles
utilizing an interfragmentary lag screw and a compression plate. C: Postoperative lateral radiograph.
compression plate (DCP) for use on the humerus because the offset hole
alignment may allow better fracture fixation and because of
biomechanical test results on femur fractures. Testing results on the
femur were extrapolated to the humerus, but the need for such a large
plate has not been tested directly on humeral fractures. A 4.5-mm broad
DCP may be best for large patients, but a narrow 4.5-mm DCP should be
used in patients with smaller bones. Dabezies treated 44 consecutive
acute fractures of the humeral shaft with plate osteosynthesis (27).
Union was achieved in all at an average of 12 weeks. Nine fractures in
small patients were plated with the 3.5-mm DCPs, and all healed.
shaft fractures, particularly if the fracture is plated or openly
reduced. Cancellous bone grafts should be used in
fresh
fractures where there is bone loss, particularly for a cortical defect
or comminution. In an open fracture with significant soft-tissue
damage, bone grafting is useful but is withheld until there is adequate
soft-tissue coverage to cover the graft. This may be at the time of
delayed primary closure or skin grafting or when the fracture is plated
after initial temporary stabilization with an external fixator.
Cancellous bone grafting also should be used routinely in treating
nonunions.
et al. reported 100% union with a good ROM of the shoulder and elbow in
27 patients with multiple injuries treated acutely using plate
osteosynthesis for the humerus (37). Similar results have been reported by others (10,142). Plating also works well for humeral nonunions and is still considered the gold standard (92,124,125), although union rates for nonunions treated with intramedullary fixation and plates are nearly equivalent (147).
diaphyseal fractures including the humerus. They can function as
load-sharing devices and are subjected to smaller bending loads than
plates because plates sit further from the bone’s mechanical axis.
There is less stress shielding of the cortex with nails than plates,
and the risk of stress fracture after implant removal is less with
nails than plates. Less dissection is necessary to insert them, and the
fracture site need not be disturbed directly. They are more technically
demanding to insert properly, however, and they should not be used when
there is a radial nerve palsy that requires exploration anyway or to
treat nonunions when there is a true pseudarthrosis that requires open
debridement. Two types of intramedullary devices have been used:
flexible nails and rigid interlocking nails.
-
Place the patient in a semireclining
(beach-chair) position close to the edge of the table with a rolled
towel between the shoulder blades. -
Prep the entire extremity from the neck to the fingertips and drape the arm free.
-
Make a longitudinal incision starting at
the lateral acromion and extending distally approximately 4 cm in line
with the deltoid. Split the deltoid muscle in line with its fibers for
2 to 5 cm but not further to avoid injuring the axillary nerve. -
Next, incise the supraspinatus tendon
laterally in line with its fibers to expose the lateral edge of the
humeral head articular surface and the medial edge of the greater
tuberosity. -
Make a starting hole with an awl in the
sulcus between the articular surface and the greater tuberosity. This
entry point is directly in line with the medullary canal of the
proximal humerus and lessens the risk of creating an iatrogenic
fracture of the proximal humerus, particularly if the shaft fracture is
proximal or has fracture lines that extend proximally. -
Use a T-handled hand reamer to enter the intramedullary canal through the starting hole made by the awl.
-
Then, with fluoroscopic assistance,
reduce the fracture and pass a 3-mm bulb-tipped guide wire down the
shaft, across the fracture site, and into the cancellous bone of the
metaphysis of the distal humerus. -
If reaming is indicated, ream the
proximal medullary canal using a flexible reamer, beginning with the
end-cutting 8-mm reamer. Do not ream distally in the humerus where the
medullary canal thins and flattens. In general, open fractures should
not be reamed. -
Overream the canal 0.5 mm in young patients with hard bone to avoid incarcerating the nail.
-
Carefully determine nail length using a
second guide wire of identical length and/or a preoperative template of
the opposite humerus. This is important because if the nail is too
long, it may impinge in the distal humerus, causing potential
distraction of the fracture site, fracture of the distal humerus, or
nerve injury. -
Once the canal has been reamed to the
appropriate diameter (9 to 11 mm), exchange the reaming guide wire for
a smooth-tipped wire through a plastic tube in standard fashion and
drive the selected nail across the fracture over the guide wire. -
Reaming may be advantageous when treating
femur or tibia fractures because it allows placement of a larger nail,
which is mechanically stronger; these advantages are less important in
humeral shaft fractures (1), however, and reaming is detrimental to the endosteal blood supply of the humerus (115).
Reserve reaming for cases in which the intramedullary canal is too
narrow to accommodate a small interlocking nail. If the canal is
reamed, it is important to be aware of the possibility of injuring the
radial nerve during the reaming, especially in midshaft fractures. If
there is any doubt about soft-tissue interposition at the fracture
site, make a small incision laterally and palpate the fracture site
during the reaming to be sure the radial nerve is not damaged. It is
not likely that the small incision will negate the advantages of
“closed” intramedullary nailing. -
Whether or not reaming is done first,
once the rod is inserted the proximal end must be driven flush with the
cortical surface of the proximal humerus or below it to avoid nail
impingement in the subacromial space. -
Carefully check the reduction and length
of the humerus clinically and radiographically. Then irrigate and close
the wound in standard fashion. Apply a shoulder immobilizer for
postoperative comfort.
be inserted together to stabilize the fracture, and they do not control rotation (30,53). Rigid interlocked nails provide better stability and rotational control (54).
They may be inserted retrograde or antegrade, but if inserted antegrade
through the rotator cuff they frequently cause subacromial impingement
symptoms and must be removed after the fracture heals.
-
Place the patient in the supine or prone position on a fully radiolucent operating table, according to the surgeon’s preference.
-
The Ender nails can be inserted using
either closed or open technique, but radiographic control
intraoperatively with a C-arm fluoroscope is essential. -
Prepare and drape the entire upper extremity free, including the shoulder.
-
The Ender nails are inserted through the
midportion of the posterior cortex of the distal humerus, approximately
2 cm proximal to the olecranon fossa. For this reason, make a
longitudinal midline vertical incision on the posterior aspect of the
arm, approximately 3 to 5 cm in length, just proximal to the tip of the
olecranon. -
Incise directly down through the
subcutaneous fat and deep fascia in the midline and split the muscle
fibers of the triceps in the midline, down to the distal humerus, which
is exposed by subperiosteal dissection. -
Identify the superior edge of the
olecranon fossa and then use a 2- to 3.5-mm drill point to outline an
oval-shaped entry site on the posterior surface of the humerus with the
distal end approximately 15 to 20 mm proximal to the upper edge of the
olecranon fossa. The flat ends of the Ender nails will be left lying on
the external surface of the humerus and must be placed sufficiently
proximally that impingement from the olecranon is avoided. The oval
hole outlined with the drill points should be 10 to 15 mm in width and
20 to 25 mm in length along the longitudinal axis of the humerus,
depending on the size of the bone and the size of Ender nails to be
used. Usually, two Ender nails are placed. This hole must be large
enough to accommodate these two pins side by side and be long enough to
avoid accidental fracture of the proximal cortex from the pressure of
the pin. -
Use a small thin osteotome to connect the
drill holes and remove the window. Use a small-angled rongeur to smooth
out the edges of the hole and make it large enough to accommodate two
Ender nails of appropriate size. -
Ender nails are available in 3-, 4-, and
5-mm diameters. In the average humerus, 3-mm pins will be used. In some
very large individuals, a 3- and a 4-mm pin, or on occasion two 4-mm
pins, may be used. -
Determine length by pulling on the arm to
bring the fracture out to length, laying an Ender nail on the arm as
close to the bone as possible, and then visualizing it with the
fluoroscope. -
Next insert the first Ender nail with the
insertion tool. This T-wrench not only allows one to drive the Ender
nail but to control it in rotation which is essential for reducing the
fracture and for spreading the pins within the humeral head. Sometimes
it may be necessary to bend the tip of the pin gently in order to
obtain the position desired. -
Drive the pin under fluoroscopic control
to the fracture. Reduce the fracture with longitudinal traction and
then, under fluoroscopic control, insert the tip of the pin into the
medullary canal of the proximal fragment. Twist the pin to reduce the
fracture and drive it proximalward to within 1 cm of the subchondral
bone of the humeral head. In dealing with a fracture of the cervical
neck, the same technique can be used. -
With one pin in place, it is then fairly
simple to drive a second pin beside the first, placing the tip of the
pin into the opposite portion of the humeral head. This divergence of
the pins provides better fixation and superior control over rotation. -
Impact the fracture to improve stability when the configuration allows.
-
Close the wound in a routine fashion, dress sterilely, and support the arm in a shoulder immobilizer.
-
Postoperatively, Ender nails do not
provide absolute stability; therefore, some motion at the fracture site
can occur during the early phases of healing. Gentle pendulum exercises
can almost always be initiated as soon as the patient is reasonably
comfortable. Active use of the hand, wrist, and elbow are essential. As
soon as the first callus is visualized at the fracture site, which is
normally between 4 and 8 weeks, there is usually sufficient stability
that the patient can begin active motion and overhead rehabilitation
exercises.
fractures definitively with external fixation. This technique should be
reserved for open fractures with significant soft-tissue injuries (17,58,150)
and for the rare patient with multiple injuries where stabilization is
important and speed essential. It stabilizes the fracture without
adding further soft-tissue injury from the surgical trauma. Typically a
single frame is placed along the lateral aspect of the arm with two
pins above and two below the fracture site. If the Shanz pins are
placed laterally, then a limited open approach is recommended to avoid
injury to the radial nerve. The frame is usually used as a temporary
fixation device until the soft tissues heal and the fracture can be
treated with another device or replaced by a functional brace.
used for 2 to 3 days until the incisional and muscle pain decrease and
the swelling diminishes. Keep an axillary pad in place to prevent skin
maceration. Institute gentle ROM exercises within a few days, depending
on the stability of the fixation. It is important to stress the
importance of vigorous finger, wrist, and elbow motions to prevent
stiffness. Add shoulder pendulum exercises as the patient’s pain and
fracture stability allow and advance to active-assisted shoulder ROM
exercises within a few weeks. A sling is suggested until full muscle
function returns without pain. Strengthening exercises are avoided for
12 weeks or until the fracture heals.
-
The most troublesome complication is
fracture of the humerus through the entry hole, which results in a
supracondylar fracture. Avoid this by being certain that the entry hole
is smooth, without stress risers, and is large enough to avoid pressure
on the adjacent bone by the Ender nails. If there is any question,
always use the smaller, more flexible pins to avoid iatrogenic fracture
of the cortex. -
Do not use this technique for fractures
in the distal half of the humerus. Look for microcracks in the distal
fragment, as these can lead to comminution of the distal fragment if
this technique is used. -
Assure appropriate placement of the pins
in the proximal fragment by careful visualization with the fluoroscope
at multiple angles through as wide an arc of motion as can be obtained
with the fluoroscope and no less than 90°. -
Avoid retrograde migration of the Ender
nails by assuring that the fit in the canal is snug. If the pins are
somewhat loose, the risk of migration can be minimized by wiring the
tips of the pins together or by securing the pins to the distal
fragment with either a screw or malleable wire through the eye of the
Ender nail secured in the bone of the humerus. -
Avoid elbow pain from impingement by
assuring that the distal ends of the pins lie flat against the cortex
and do not impinge on the olecranon fossa.
not had the same rate of success as intramedullary nailing of femur and
tibia fractures. In addition, problems from subacromial impingement
have been reported in almost every series utilizing antegrade humeral
nailing. Therefore, for humeral shaft fractures treated operatively,
particularly because of failed nonoperative treatment, open fractures,
or fractures with neurovascular complications, we prefer open reduction
and internal fixation with plates and screws using an anterolateral
approach. Bone grafting is used following the principles outlined
above. We are careful to identify the radial nerve and retract it
gently out of the way to avoid injury to it, verifying that it is
intact at the conclusion of the case. We treat nonunions with plate
fixation (Fig. 15.37) also, but we prefer to
make a posterior approach to the humeral shaft and dissect out the
radial nerve to be sure it is not injured or incarcerated in the
nonunion site (see Chapter 27 for more detail on nonunions).
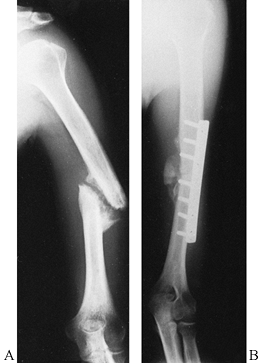 |
|
Figure 15.37. A: Anteroposterior radiograph of a middiaphyseal humeral nonunion. B: Postoperative AP radiograph illustrating proper surgical technique utilizing a compression plate and autogenous bone grafting.
|
patient who needs a load-sharing device to facilitate rehabilitation,
but only if those fractures can be reduced easily so that there is no
question about possible soft-tissue interposition at the fracture site.
Our list of indications for intramedullary nails also includes
pathologic fractures, fractures associated with osteopenic bone, and
fractures underlying burns.
is a major concern. Fortunately, the humerus is surrounded by a
well-vascularized muscular envelope, and when careful soft-tissue
techniques are used, infection rates of less than 1% should be
anticipated (14). Grade III open fractures have
higher rates of infection after plate fixation, which has led some
authors to recommend external fixation for these injuries. However,
deep infections in pin sites from external fixators can be equally
problematic.
but are seen even more commonly after plate fixation of the delayed
unions and nonunions where screw fixation to the bone may be suboptimal
because of disuse osteopenia. These problems may be minimized by
operating sooner when nonunion is suspected, using a longer plate with
at least six and preferably eight cortices above and below the
fracture, adding cancellous bone graft, and using a cast brace
postoperatively to augment the fracture fixation and decrease stress on
the implants.
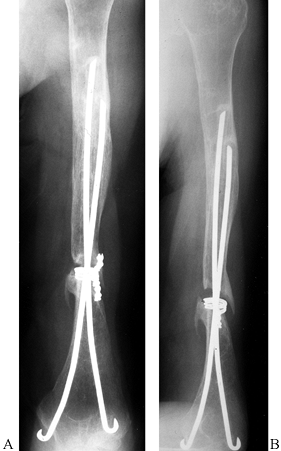 |
|
Figure 15.38.
Nonunion in a patient referred for treatment of a humeral shaft fracture treated with Rush rods and cerclage wiring. This was rotationally unstable, as seen by these attempted AP (A) and lateral (B) views, which demonstrate rotation at the nonunion site. Combining open reduction and wiring with intramedullary devices risks devascularizing the fracture site, a risk factor for nonunion, and is not recommended. |
antegrade intramedullary nailing of humeral shaft fractures is
subacromial bursitis and impingement, causing shoulder pain and
decreased ROM. Be sure to use the proper length nail to ensure that the
proximal end is flush with the greater tuberosity or buried beneath its
cortex. Also, distraction at the fracture site is a pitfall to avoid at
all costs, as it can cause a nonunion. If retrograde nailing is done
through the condyles, there is a risk of condylar fracture. If this
does occur, the condyles should be reattached with lag screws at the
time of nailing.
fixation of humeral fractures is elbow stiffness. We prefer not to use
external fixation for humeral fractures, but when it is necessary, we
do everything possible to start elbow motion early. Avoid using
fixators that span the elbow and prevent motion.
management technique after open fractures may increase the potential
for delayed or nonunion. This problem may be reduced by bone grafting (14).
An alternative is to use the external fixator temporarily until
soft-tissue coverage has been obtained and then convert to another
method of fixation such as plates. Unfortunately, pin tract infections
that develop in the interim may preclude internal fixation. In some
cases, the temporary fixator may be removed after the soft tissues heal
and the fracture is treated to union with a functional brace. (See Chapter 11 for the principles of external fixation.)
injuries. When they occur in association with fractures, fracture
management often dictates the treatment strategy and
follows
the principles outlined in the previous sections of this chapter. The
evaluation and management of glenohumeral, acromioclavicular, and
sternoclavicular joint injuries and instability are covered separately
in detail in other chapters of this textbook (see Chapter 78 and Chapter 80).
However, the principles of diagnosing and treating acute dislocations
of the shoulder joints are important to keep in mind when considering
fractures of the shoulder girdle and therefore they are reviewed here,
also.
usually as a result of a sports injury or a fall, but many other
etiologies have been reported. Effective treatment depends on making
the correct diagnosis promptly as well as recognizing and treating
associated injuries. Injury classification also helps direct treatment
appropriately.
direction, chronicity, and mechanism. The direction describes the
position of the humeral head relative to the glenoid and is most often
anterior, but may be posterior, inferior (“luxatio erecta”), or,
rarely, intrathoracic. The dislocation may be acute or chronic.
Unreduced dislocations presenting or diagnosed after 3 weeks are
considered chronic (see Chapter 80).
atraumatic. Traumatic instabilities are further characterized as a
subluxation or true dislocation of the joint. Atraumatic instabilities
can be voluntary or involuntary and may be related to congenital
general ligamentous laxity. Some patients with voluntary instability
are able to dislocate and relocate their glenohumeral joints almost
painlessly. Those patients are poor surgical candidates.
radiographically in the same way as patients with shoulder girdle
fractures (see above). Critical to evaluating the joint are two views,
one of which is a lateral view that shows the glenoid fossa well, to
rule out a posterior dislocation. The axillary or transaxillary (Y)
views are best for that purpose.
demonstrating the Hill-Sachs lesion, which is a defect in the
posterolateral aspect of the humeral head caused by impaction of the
humeral head against the anterior glenoid rim at the time of an
anterior dislocation. Impaction lesions of the glenoid rim, the
counterpart of the Hill-Sachs defect, are difficult to demonstrate with
plain radiographs and are seen better with CT scans. Magnetic resonance
imaging studies may be helpful for evaluating rotator cuff pathology
but are not indicated for acute trauma. As in all cases, postreduction
radiographs should always be obtained after the joint is reduced.
Usually the mechanism of injury is forced external rotation of the
shoulder while the arm is abducted, levering the humeral head against
the anterior capsule. As the restraining effect of the anterior glenoid
labrum and capsule are exceeded, the head of the humerus dislocates
anteriorly and usually comes to rest below the coracoid process. In
approximately 85% of patients, the glenoid labrum is detached from the
anterior glenoid rim (a Bankart lesion). The remaining cases occur with
interstitial stretching or frank rupture of the capsule without
significant detachment of the labrum (Fig. 15.39).
This has been proposed as a possible explanation for the decreased
incidence of recurrent dislocation in older patients (>40 years).
Anterior shoulder dislocation is usually an injury of adolescents and
young adults, with most patients being between the ages of 12 and 50
years.
 |
|
Figure 15.39. Some of the anatomic lesions around the glenohumeral joint that may result in or from instability.
|
anteriorly in the subcoracoid position, and there is loss of the normal
lateral and posterior contours because of the vacant glenoid.
Neurovascular examination is usually normal, but there may be an
axillary nerve palsy. This can be difficult to detect before reduction
because pain prevents the patient from contracting the deltoid or
abducting
the
shoulder. Decreased sensation to light touch laterally over the deltoid
may be the only detectable sign of an axillary nerve injury but is
frequently subtle and unreliable. Less commonly, other neurovascular
injuries occur (13).
the dislocation is the treatment of choice. The key to reduction of any
joint dislocation is muscle relaxation, as evidenced by the ease with
which most joints can be reduced under general anesthesia with complete
muscle paralysis. General anesthesia is rarely necessary to reduce
acute shoulder dislocations, however, unless there is interposed soft
tissue or an associated fracture with an incarcerated bony fragment.
back to antiquity. Most rely on the principles of traction, leverage,
or rotational forces, and there is considerable overlap between
methods. One of the safest and most effective means of reducing an
anterior shoulder dislocation relies on traction and countertraction
and is best accomplished with an assistant (Fig. 15.40), although it can be done with one person using her foot against the patient’s chest wall for countertraction.
 |
|
Figure 15.40.
Traction–countertraction maneuver for reducing an anterior glenohumeral dislocation. (Reprinted with permission from Rockwood CA Jr, Thomas SC, Matsen FA III. Subluxations and Dislocations about the Glenohumeral Joint. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults, 3rd ed. Philadelphia: JB Lippincott, 1991:1091.) |
well. It is relatively easy on the patient, can be performed by one
individual without force, and is usually successful. The key to this
method is avoidance of traction, which induces secondary muscle spasm,
increasing the patient’s pain and making reduction more difficult.
Elevate the patient’s elbow above the midcoronal plane and then gently
externally rotate it using a hand placed on the patient’s wrist. If
done slowly, it takes little effort to externally rotate the patient’s
shoulder. Slowly abduct the extremity during this process to about 90°.
It is important to continue external rotation beyond the midcoronal
plane. Reduction is usually accomplished by these maneuvers, but if it
is not, then the arm is adducted fully across the chest and internally
rotated as in the last two maneuvers of Kocher’s method.
reduced the patient’s pain is decreased dramatically and promptly. For
that reason, it is important not to administer an excessive dose of
sedation and narcotics to achieve the reduction, particularly in older
patients; this is because once the joint is reduced and the pain
stimulus has diminished, the residual effects of the medications may
cause dangerous respiratory depression. After the reduction, obtain
confirmatory radiographs. Immobilize the shoulder in a sling or
shoulder immobilizer. The duration of immobilization is controversial,
but most authors recommend 4 to 6 weeks total, during which time a
rehabilitation program is initiated. The goal is to avoid recurrent
dislocations without excessive stiffness. Patients older than 40 should
be immobilized for less time (2 weeks) because they are more likely to
have problems from stiffness and rotator cuff injuries than from
recurrent instability (70).
the initial diagnosis is missed, and that often leads to major shoulder
dysfunction. The mechanism of injury is a posteriorly directed force
acting on an adducted and internally rotated humerus. It can also
result from violent muscle contractions and therefore may be seen after
grand mal seizures, electroshock treatment, or electrocution injuries
and should be ruled out in patients complaining of shoulder pain after
such events.
internally rotated, and the patient is unable to abduct or externally
rotate the joint. It may be possible to feel the fullness of the
humeral head posteriorly unless the patient is examined late, when
chronic swelling masks this finding. If the diagnosis is made months
after the initial injury,
there may be atrophy of the shoulder musculature despite the return of limited abduction and external rotation.
be reduced by rotating the arm to the neutral position and gently
levering the humeral head anteriorly into the glenoid. Reduction of
posterior dislocations may be difficult because they are often
diagnosed late. Chronic dislocations are much more difficult to treat.
They may be irreducible by closed methods and can present with muscle
contracture, periarticular fibrosis, associated fractures, or
heterotopic bone formation. Commonly, open reduction is necessary
together with joint debridement and attempts at soft-tissue repair or
muscle advancement into the defect in the humeral head.
are stable may be treated with a sling. If postreduction examination
reveals significant instability, however, then the shoulder should be
immobilized in neutral or slight external rotation. A variety of
orthoses are available to hold the extremity in that position.
Rehabilitation of the injured structures is based on principles
outlined above for other shoulder injuries, with an emphasis on
strengthening the rotator cuff muscles.
irreducible in the absence of fractures, but it does happen, usually
because of soft-tissue interposition in the joint or because of severe
muscle spasm in large, muscular patients. In those cases, general
anesthesia may be necessary to obtain reduction. Occasionally an open
reduction is necessary when closed reduction fails in spite of general
anesthesia. An anterior deltopectoral approach is recommended for most
dislocations, although associated injuries or wounds may warrant an
alternate approach.
at the time of surgery and the stability achieved after the reduction
and repair of any injured soft tissues. Generally, the shoulder should
be immobilized in a shoulder immobilizer or sling and swathe
immediately postoperatively. Pendulum exercises can be started in 1 or
2 days, and a graduated rehabilitation program instituted within the
first 2 weeks. Less stable injuries require longer periods of
immobilization.
dislocation is recurrent dislocation. The rate of recurrence correlates
with patient age: 70% to 90% for patients younger than 20 years, 60%
for patients between 20 and 40 years of age, and only 10% for patients
older than 40 (60). The key to minimizing
recurrence is patient education about what joint positions are likely
to be unstable based on the original direction of dislocation and
careful adherence to a well-designed rehabilitation program that
strengthens the weakened shoulder muscles. Those joints that are
unstable despite these precautions warrant surgical treatment for
chronic instability (see Chapter 80).
following glenohumeral dislocations are usually transient. Brachial
plexus injuries are uncommon, but their presence suggests a more
violent shoulder injury. Most often they are neurapraxias and recover,
but more significant injuries also occur, such as in scapulothoracic
dissociation (see above). Isolated axillary nerve palsies usually
resolve within 3 months. In the interim, the shoulder should be
supported to prevent persistent inferior subluxation and stretching of
the soft tissues. Rotator cuff injuries are more likely in older
patients in association with glenohumeral dislocations and should be
suspected in patients over 40 with persistent weakness of shoulder
abduction (144).
subluxations (“shoulder separations”) account for approximately 15% of
all shoulder girdle dislocations (18,140).
The true incidence is probably much higher because patients with
low-grade injuries may not seek medical attention. The mechanism of
injury is typically a direct blow or fall on the shoulder, in which the
acromion is driven downward, tearing the AC joint capsule and
restraining ligaments. The most common mechanisms are sports related,
but they also occur in motor vehicle accidents. The same mechanism of
injury that causes acromioclavicular joint disruptions can also cause
sternoclavicular dislocations and fractures of the clavicle or acromion.
The classification hinges on a combination of physical examination and
radiographic findings, and as with most classifications in
orthopaedics, higher numbers indicate more severe and complex injuries.
Types I, II, and III are most common. Types IV through VI are uncommon
and usually are caused by higher-velocity injuries; they are
characterized by the unusual displacement of the clavicle. It is
important to thoroughly evaluate the shoulder girdle clinically and to
study the radiographs carefully to avoid overlooking other injuries
that may occur in combination with AC joint injuries.
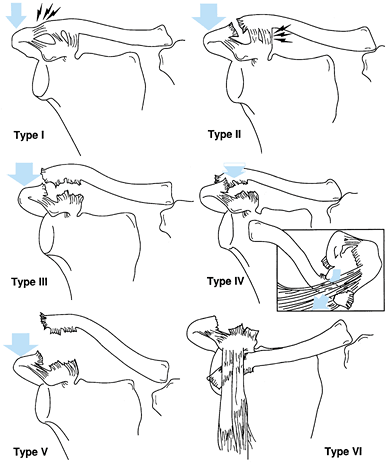 |
|
Figure 15.41.
Classification of acromioclavicular (AC) joint injuries. Type I, sprain of the AC ligaments; type II, AC joint disruption with sprained coracoclavicular (CC) ligaments; type III, complete disruption of the AC joint and CC ligaments; type IV, complete disruption of the AC joint and CC ligaments and posterior displacement of the distal clavicle into the trapezius muscle; type V, wide displacement of the distal end of the clavicle (one to three times normal distance) associated with detachment of the deltoid and trapezius muscles from the distal clavicle; type VI, inferior displacement of the distal clavicle below the coracoid process associated with detachment of the deltoid and trapezius muscles from the distal clavicle. |
disruptions, but both may be treated symptomatically with an arm sling
for patient comfort. Symptoms usually subside within 1 to 2 weeks, but
the patient should be cautioned against heavy use of the shoulder until
shoulder motion is painless and equal to the preinjury status.
Treatment for type III injuries is controversial. Many authors
recommend nonoperative management with a sling for comfort, pointing to
the higher complication rate from operative treatment (40,137).
A Kenny-Howard sling can be used to maintain joint reduction but
requires a motivated, compliant patient and diligent attention to
detail to be effective and avoid skin complications (137).
Others advocate early surgical treatment, and a wide variety of
surgical methods have been described for treating this lesion. The
occupational demands and goals of the patient are critical to decision
making. Type III injuries can be treated late if symptoms persist after
initial nonoperative treatment (see Chapter 78), and that is our preferred approach.
usually treated surgically because of the extreme displacement of the
clavicle and detachment of the deltoid and trapezius muscles. Many
different operations have been devised, including transarticular pins,
ligamentous repair, synthetic slings (mersilene), and screws securing
the clavicle to the coracoid to reduce the AC joint.
-
Position the patient in the beach-chair
position on the operating room table and pay particular attention to
supporting and securing the patient’s head and neck appropriately. -
Drape the shoulder so that free access is
possible from the middle of the patient’s neck to the elbow and from
the medial border of the scapula posteriorly to the midline of the
chest anteriorly. -
Make a 6- to 10-cm-long strap-like
incision in Langer’s lines beginning 2 cm posterior to the clavicle and
carried anteriorly to a point just medial to the coracoid process,
centered over the AC joint. -
Undermine the skin flaps to expose the AC joint, the distal 5 cm of the clavicle, and the anterior deltoid muscle fibers.
-
Detach the anterior deltoid from its
origin on the clavicle enough to visualize the base of the coracoid
process and the coracoclavicular ligaments as well as the remnants of
the AC joint capsule and ligaments. In some cases, the deltoid and
trapezius muscle fascia may have been stripped away as part of the
initial injury. -
Grasp the distal end of the clavicle with a towel clip or tenaculum, retract it, and debride the joint.
-
Tag the torn ends of the coracoclavicular
(CC) ligaments and any remnants of the AC joint capsule or ligaments
with nonabsorbable sutures but do not tie them yet. -
Reduce the clavicle to the acromion and drill a 3/16-in. hole through the clavicle, aiming for the base of the coracoid process. Use a smaller drill bit (9/64 in.) to make a hole through the base—not the waist—of the coracoid.
-
Measure the distance from the top of the
clavicle to the bottom of the coracoid process with a depth gauge and
then insert the proper size Bosworth screw. Be sure the screw enters
the hole in the coracoid process and does not skive off medially or
laterally. -
Obtain intraoperative radiographs to verify proper screw placement before beginning the final steps of the procedure. See Figure 15.42.
![]() Figure 15.42.
Figure 15.42.
Postoperative anteroposterior x-ray of the shoulder with Bosworth screw
in place. Note that the acromioclavicular joint has been reduced and
the coarse lag threads of the screw are well seated into the coracoid
process. -
Tie the previously placed sutures in the
CC ligaments and remnants of the AC joint capsule and ligaments. Then
turn the screw one half-turn tighter to draw the
P.475
clavicle
slightly closer to the coracoid and relieve any tension on the sutures.
Remember that the primary purpose of the screw is to temporarily hold
the clavicle reduced vertically and horizontally to the scapula and to
take tension off the coracoclavicular ligaments until they have healed. -
Repair the deltoid to the clavicle and anterior acromion with nonabsorbable sutures passed through small drill holes in bone.
-
In some cases it may be possible or
desirable to repair the AC joint disruption using local soft-tissue
remnants and reinforcing the repair with pins across the joint or other
material looped around the clavicle and coracoid. -
Position and prep the patient and expose the AC joint as described above.
-
For pin fixation of the AC joint, most
authors recommend the use of small, smooth or threaded Steinman pins.
The technique of exposure and hardware placement follows the principles
outlined for treating distal clavicle fractures (see above). -
If heavy sutures, tape (mersilene), or
other synthetic material is used to reinforce the repaired CC
ligaments, expose the base of the coracoid as described for the
Bosworth screw. -
Pass a curved vascular clamp under the
coracoid process, carefully hugging the bone with the clamp as it is
passed. It is usually easier to pass the clamp from medial to lateral. -
Grasp the selected material with the
clamp and pass it under the coracoid and then over the top of the
clavicle. Tie it securely to reapproximate the clavicle to the
coracoid, keeping the AC joint reduced, and then tie the sutures
previously placed in the CC ligaments. -
Anchor the reinforcing loop to the clavicle with additional nonabsorbable sutures.
sling for the first 2 weeks, but be allowed to perform activities of
daily living as their comfort allows. The sling can be discontinued
after 2 weeks, but heavy lifting should be avoided for 6 weeks. If a
Bosworth screw or pins are used, remove them 8 weeks after surgery;
normally that is done in the office with local anesthetic. Regardless
of the fixation or repair technique selected, patients should avoid
contact sports for 12 weeks.
recover excellent shoulder function. Heavy laborers and patients who
use their shoulders frequently for overhead lifting may develop chronic
symptoms, but they are rarely severe or disabling. For patients with
significant symptoms or complaints related to chronic AC dislocation,
several successful surgical options are available (see Chapter 78).
The simplest treatment is excision of the distal 1 to 1.5 cm of the
clavicle. Other options rely on reconstructing the CC ligaments,
usually in combination with distal clavicle excision as in the
Weaver-Dunn procedure (143). Acromioclavicular
arthritis may develop after AC joint injuries, for which resection of
the distal end of the clavicle (Mumford procedure) is an effective
treatment.
Because they are unusual and sometimes subtle, their diagnosis is often
delayed, especially in the multiply injured patient. Anterior
dislocations are significantly more common than posterior dislocations,
but because of their potential for associated mediastinal compression,
posterior injuries are more frequently reported. The reported ratio of
anterior to posterior injuries varies from approximately 3 to 1 to 20
to 1 (99).
ball-and-socket joint having approximately 30° of anterior and
posterior slide and upward elevation. It is also capable of 25° to 50°
of rotation around the long axis of the clavicle. The ligamentous
structures binding the medial clavicle to the sternum are very strong (Fig. 15.43),
and therefore traumatic dislocations are usually indicative of
significant force and often accompany rib and sternal fractures and
other chest injuries. Most dislocations result from direct trauma, but
indirect forces transferred from lateral
compression on the shoulder also may injure the sternoclavicular joint.
 |
|
Figure 15.43. The sternoclavicular (SC) joint area. A: Normal anatomy. B:
Dislocated SC joint. The articular disc ligament serves as a check-rein, preventing medial displacement of the clavicle. (Redrawn with permission from Rockwood CA, Green DP. Fractures in Adults. Philadelphia: Lippincott-Raven, 1996.) |
tender and swollen. The most striking finding is the asymmetry between
the injured and uninjured SC joints. If the joint is dislocated
anteriorly, there may be an appreciable prominence, and if the
dislocation is posterior, there may be a hollow depression. Posterior
dislocations may present with breathing or swallowing difficulties or
even cyanosis secondary to compression of the trachea, esophagus, or
underlying vascular structures (36,80).
Plain radiographs of the sternoclavicular joint are difficult to obtain
and interpret, as mentioned above with fractures of this region.
Computed tomography scans are best for evaluating suspected joint
injuries.
to reduce but often recur when pressure is removed from the medial end
of the clavicle. Whether or not the reduction can be maintained,
isolated SC joint injuries should be treated symptomatically. Provide a
figure-of-eight clavicle strap or a sling for comfort. The anterior
prominence and mild asymmetry persist in most cases but are not
functional problems. Operative reduction and internal fixation for
acute cases and resection of the medial end of the clavicle for chronic
cases have been reported but are rarely indicated (see Chapter 78).
because of their potential for compression of the underlying
structures. Numerous reduction methods have been advocated. Usually,
manual traction on the clavicle is sufficient. It is helpful to place a
rolled towel posteriorly between the patient’s scapulae and apply
posteriorly directed force on the lateral clavicle to help lever the
medial end of the clavicle anteriorly at the SC joint. Sometimes a
sterile towel clip is useful as a handle for grasping the clavicle and
guiding the reduction.
irreducible. Make an incision anteriorly directly over the joint and
reduce the clavicle under direct visualization. In the absence of
fractures, soft-tissue repair alone is recommended rather than internal
fixation so as to avoid complications from the latter.
necessary, and after reductions of SC joint dislocations, a
figure-of-eight clavicle strap is recommended because it keeps the
patient’s shoulders up and back, helping maintain the reduction. Unlike
anterior dislocations, posterior dislocations are usually stable after
reduction.
joint are uncommon and are related primarily to posterior dislocations.
These include compression or laceration of the carotid sheath or
subclavian vessels and injury to the trachea or esophagus. Neurologic
sequelae are rare, but compression of the recurrent laryngeal nerve and
thoracic outlet syndrome have been reported. Complications related to
chronic anterior subluxation or dislocation of the sternoclavicular
joint are usually cosmetic. The residual prominence over the
sternoclavicular joint may be tender but is best treated with benign
neglect (see Chapter 78).
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
S, Barrios RH, Martinez-Peric R, Losada JI. Surgical Treatment of the
Radial Nerve Lesions Associated with Fractures of the Humerus. J Orthop Trauma 1993;7:211.
MJ, Beauchamp CG, Kellam JK, McMurtry RY. The Results of Plating
Humeral Shaft Fractures in Patients with Multiple Injuries. J Bone Joint Surg 1985;67B:293.
J, Adler LM, Blank JE, et al. Evaluation of the Neer System of
Classification of Proximal Humeral Fractures with Computerized
Tomographic Scans and Plain Radiographs. J Bone Joint Surg 1996;78A:1371.
RJ, Bosse MJ, Poka, A, Burgess AR. Intramedullary Stabilization of
Humeral Shaft Fractures in Patients with Multiple Trauma. J Bone Joint Surg 1986;68A:960.
EJ, Banta CJ, Murphy CP, D’Ambrosia RD. Plate Fixation of the Humeral
Shaft for Acute Fractures, With and Without Radial Nerve Injuries. J Orthop Trauma 1992;6:10.
RJ, Dixon GL, Bach AW, et al. Internal Fixation of Fractures and
Nonunions of the Humeral Shaft. Indications and Results in a
Multicenter Study. J Bone Joint Surg 1985;67A:857.
RD, Hawkins RJ, Grainger RW. A Comparative Analysis of Operative vs.
Nonoperative Treatment of Grade III Acromioclavicular Separations. Clin Orthop 1985;193:150.
M, Kirchner R, Baumgaertel F, et al. Treatment of Unstable Distal
Clavicular Fractures with and without Lesions of the Acromioclavicular
Joint. Injury Int J Care Injured 1996;27:47.
BF, Bradway JK, Cofield RH. Open reduction and internal fixation of
displaced intra-articular fractures of the glenoid fossa. J Bone Joint Surg 1993;75A:479.
T, Rodriquez-Merchan C, Munuera-Martinez L. Fractures of the Coracoid
Process: Presentation of Seven Cases and Review of the Literature. J Trauma 1990;30:1597.
C, Parkpian V, Patradul A. Fixation of Fractures of the Midshaft of the
Clavicle with Kirschner Wires: Results in 108 Patients. J Bone Joint Surg 1998;80B:106.
A, Petersson C, Redlund-Johnell I. The Natural Course of Lateral
Clavicle Fracture: 15 (11–21) Year Follow-up of 110 Cases. Acta Orthop Scand 1993;64:87.
A, Redlund-Johnell I, von Scheele A, Petersson CJ. Shortening of
Clavicle after Fracture: Incidence and Clinical Significance, a 5-Year
Follow-up of 85 Patients. Acta Orthop Scand 1997;68:349.
BL, Butterfield SL, Daffner RH, O’Keefe RMJ. The Abduction Lordotic
View of the Clavicle: A New Technique for Radiographic Visualization. J Orthop Trauma 1991;5:392.
D, Regazzoni P, Renner N. The Unstable Shoulder Girdle: Early
Functional Treatment Utilizing Open Reduction and Internal Fixation. J Orthop Trauma 1995;9:93.
D, Jupiter JB, Miller ME, Ada JR. Injuries to the Shoulder Girdle: Part
II. Fractures of the Clavicle. In: Browner BD, Jupiter JB, Levine AM,
Trafton PG, eds. Skeletal Trauma, Vol 2. Philadelphia: WB Saunders, 1998;1670.
CA, Williams GR, Young DC. Injuries to the Acromioclavicular Joint. In:
Rockwood CA, Green DP, Bucholz RW, Heckman JD, eds. Rockwood and Green’s Fractures in Adults. Philadelphia: Lippincott-Raven, 1996;1354.