FRACTURES AND DISLOCATIONS OF THE ELBOW AND FOREARM
II – FRACTURES, DISLOCATIONS, NONUNIONS, AND MALUNIONS > Upper
Extremity > CHAPTER 16 – FRACTURES AND DISLOCATIONS OF THE ELBOW AND
FOREARM
incorporates chapters from the second edition that were written by
Bernhard G. Weber, H. David Moehring, Jerald L. Cooper, Robert D.
D’Ambrosia, Bruce A. Mallin, and T. David Sisk.
serious joint injuries, constituting only about 4.3% of all fractures.
In contrast to shaft fractures, joint fractures are almost always
treated by open reduction and internal fixation; conservative treatment
is the exception.
treatment of fractures of the distal humerus have advanced
substantially over the past 20 years and now are quite sophisticated,
the rate of complications remains quite high (21,69,83,86,87,91,92 and 93,116,124,169).
Such complications include nonanatomic reduction of articular surfaces,
malunion, nonunion, residual stiffness of the elbow, heterotopic
ossification, posttraumatic osteoarthrosis, and generalized functional
disability. These injuries are being treated more often now with
coexisting multiple injuries or in an extremity with complex
ipsilateral open injuries, which previously may have led to amputation.
In the very elderly, osteopenia often compromises internal fixation,
and total elbow arthroplasty is a reasonable alternative (32).
problems to orthopaedic traumatologists because of the high occurrence
of comminution, the large and complex articular surfaces involved, the
close proximity of important nerves and vessels, and the poor
soft-tissue coverage. In spite of the best treatment, the result may be
poor because of the magnitude of the original injury. In the vast
majority of fractures, ideal treatment consists of early open reduction
with replacement of any lost bone, and rigid anatomic fixation of the
joint surfaces and adjoining metaphyseal bone, with early aggressive
active range-of-motion and strengthening exercises (69,94,169).
As with all intraarticular fractures, good technique requires careful
preoperative planning, adequate exposure of the joint and fracture,
biomechanically sound internal fixation, and protection of
neurovascular structures. Flexibility on the part of the surgeon and
the availability of a wide range of plates and screws are necessary (2,20,87,93,122,130,171,172).
osteopenic and the patient is over 70 years old can be nearly
impossible to fix. Primary total elbow arthroplasty is a reasonable
alternative in these patients, particularly if they have coexisting
arthrosis or rheumatoid arthritis. Cobb et al. (32)
performed arthroplasty in 21 elbows in 20 consecutive patients, whom
they followed for a mean duration of 3.3 years with a minimum follow-up
of 2 years. Fifteen elbows had an excellent result, five had a good
result, and one was not ratable. Of these, one required revision for a
fracture of the ulnar component sustained in a fall.
radial-capitellar, the trochlear-olecranon, and the proximal
radial-ulnar. The distal humerus consists of two divergent osseous
columns, one lateral and one medial, which flare and are separated by
the thin or absent bone of the olecranon fossa, and are connected
together distally by the trochlea. The end of the lateral column
becomes the capitellum. The lateral column is in approximately 20° of
valgus relative to the midline of the humeral shaft, whereas the medial
column is at a 40° to 45° angle. Viewed from a lateral perspective, the
capitellum is tilted 30° to 40° anteriorly, and the trochlea 10° to 20°
(Fig. 16.1). The articulation between the
trochlear notch of the olecranon and the trochlea is the most important
joint of the elbow because it provides the flexion–extension arc of
elbow motion as well as approximately half of the intrinsic stability
of the elbow (83). Reconstruction of the normal
shape of the trochlea is therefore critical to restoration of good
motion and stability in the elbow. Because the majority of
intraarticular fractures split through the narrow waist of the trochlea
with some comminution, there is a tendency during internal fixation to
narrow the trochlea. Avoid this, however, because it creates a
noncongruous elbow, which will interfere with motion and lead to
osteoarthrosis.
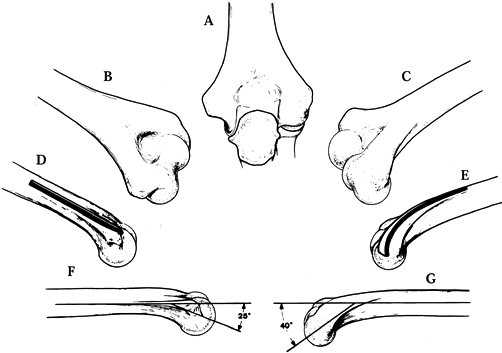 |
|
Figure 16.1. Surgical anatomy of the distal humerus. A: Aspect from the back. B: Posteroulnar aspect. C: Posteroradial aspect. D: Fixation plate along the ulnar pillar, flat profile. E: Fixation plate along the radial pillar, bent and twisted contour. F: Tilt of the trochlea humeri and its crest, 25°. G: Tilt of the capitellum and its crest of 40°.
|
notch of the olecranon surrounds almost 100° of the trochlea, making the elbow one of the most stable joints in the body (119,138).
The olecranon, in its articulation with the trochlea, contributes
significantly to anterior–posterior stability, as well as varus,
valgus, and rotatory stability. Resection of increasingly larger
amounts of the proximal end of the olecranon leads to a linear decrease
in the stability of the elbow. Resection of only 25% of the olecranon
process decreases the resistance of the elbow to valgus load by 50%.
The coronoid process plays an important role, in that it resists
posterior displacement of the elbow joint, particularly in flexion, and
the anterior band of the medial collateral ligament attaches near the
base of the coronoid process; therefore, large fractures involving the
coronoid process may result in incompetence of this ligament. In spite
of its small size, the radial head contributes to stability and the
transmission of longitudinal force across the elbow joint. The load
borne by the radial–capitellar joint is maximal when the forearm is
pronated and the elbow extended and loaded longitudinally. The
continuity of the radial head is most important when there is
associated ligament instability in the elbow and in particular when
there is incompetence in the intraosseous membrane and distal radial
ulnar joint of the forearm (an Essex-Lopresti lesion).
fracture of the distal humerus requires reconstruction of an
equilateral triangle consisting of the lateral column, the medial
column, and the trochlea. Fortunately, even in the elderly, the
cortical bone of the medial and lateral columns usually provides good
purchase for bone screws. In a biomechanical study of internal fixation
of the distal humerus, Helfet and Hotchkiss (61)
found that double-plate construction with the two plates at right
angles, a medial plate on the medial column and a posterior plate on
the lateral column, provided the strongest fixation regardless of
whether the plate was a one-third tubular or a 3.5 mm reconstruction
plate. Schemitsch et al. (154) looked at plates
of two designs placed in five different configurations. They found that
when cortical contact was present, dual plates placed medially and
laterally—whether at 90° to each other or in the same plane—provided
equivalent rigidity. When a cortical gap was present, however, they
found that the combination of an anatomically designed lateral buttress
J-plate and a medial reconstruction plate gave the greatest rigidity.
In practice, 3.5 mm reconstruction plates or their equivalent,
particularly in titanium, give the best opportunity to obtain a close
fit of the plates to the complex surfaces of the distal humerus, and
when placed posteriorly on the lateral condyle and medially on the
medial condyle, they provide the strongest construct. Interfragmentary
screw fixation from the capitellum through the trochlea usually ensures
good stability of the articular surface. Reestablishment of good bone
contact throughout the construct, using tricortical bone graft from the
iliac crest if necessary, substantially improves fixation and chances
for union.
source of complications if the fixation fails. The biomechanically
soundest technique appears to be a chevron
type
osteotomy through the nonarticular portion of the olecranon, predrilled
and fixed with interfragmentary screw fixation augmented by a tension
band wire.
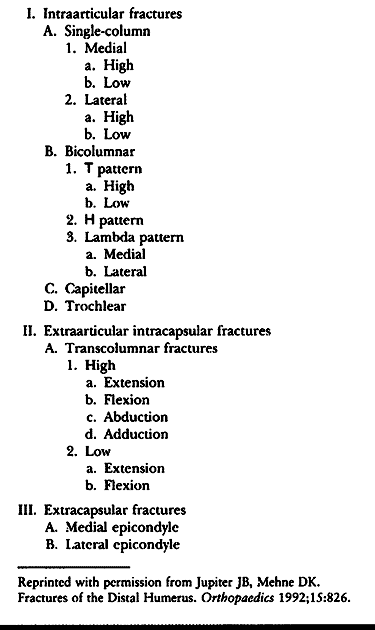
is the most commonly used scheme for determining treatment and clinical
research. The Orthopaedic Trauma Association and the International
Society for Fracture Repair recently expanded the AO classification (125)
to provide a more detailed breakdown to enhance the accuracy of
clinical reports. Their system, however, contains 38 separate fractures
of the distal humerus. Jupiter and Mehne (86)
divide distal humerus fractures into three major groups: extracapsular,
transcolumnar, and intraarticular, which includes single- and
bicolumnar fractures and articular fractures involving the capitellum
and the trochlea (21,86) (Table 16.1).
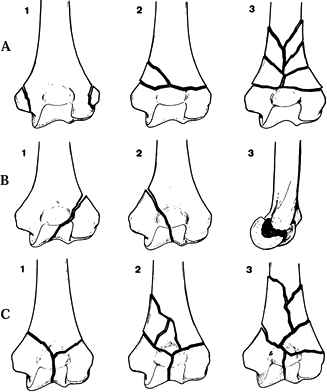 |
|
Figure 16.2.
A-O Classification of distal humeral fractures. Type A—Extraarticular fractures: A1 epicondylar avulsions; A2 supracondylar fractures; A3 supracondylar fractures with comminution. Type B—Unicondylar fractures: B1 fracture of the lateral condyle; B2 fracture of the medial condyle; B3 tangential fracture of the condyle. Type C—Bicondylar fractures: C1 T- or Y-shaped fractures; C2 T- or Y-shaped fractures with comminution of one or two pillars; C3 extensive comminution of the condyles and pillars. |
 |
|
Table 16.1. Classification of Fractures of the Distal Part of the Humerus
|
proximal to the olecranon fossa, whereas low T-fractures traverse the
olecranon fossa just proximal to the trochlea. The latter may be the
most common fracture pattern in the elderly, and they are difficult to
treat because of the small distal fragment. Y- or T-fractures begin in
the center of the trochlea, probably due to the olecranon trochlear
ridge being impacted into the trochlea, which causes a fracture line to
propagate vertically from the trochlear notch and then split to cross
each column obliquely. An H-fracture is a bicolumnar fracture with a
fracture of the medial column around the medial epicondyle and a split
of the lateral column into a T or Y pattern, producing a
free-floating
trochlear fragment. In a medial lambda fracture, the proximal fracture
line exits medially and the lateral fracture line extends distally to
the level of the lateral epicondyle. In a lateral lambda fracture, the
fracture is high on the lateral column and exits near the medial
epicondyle with the intraarticular component next to the capitellum. A
particularly difficult fracture is a shear fracture in the frontal
plane involving the anterior portion of the capitellum and lateral half
of the trochlea. This results in the characteristic double-arc sign
seen on the lateral radiograph of Figure 16.3.
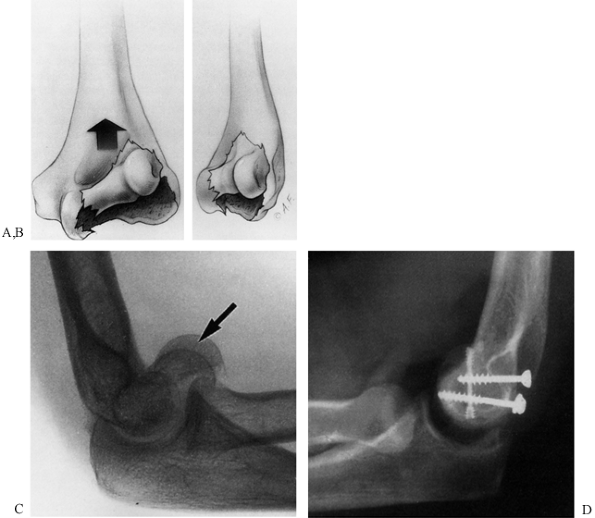 |
|
Figure 16.3. Coronal shear fracture of the distal end of the humerus. A:
This vertical shear fracture involves the capitellum and most of the trochlea, resulting in separation, proximal migration, and rotation of the distal articular surface of the humerus. B: This anterior lateral oblique view shows that the main fracture line is in the coronal plane. C: Lateral radiograph showing the double-arc sign (arrow): One arc represents the lateral ridge of the trochlea and the other, subchondral bone of the capitellum. D: Postoperative lateral radiograph showing anatomic reduction of the fracture and internal fixation with two 4.0 mm AO screws and a Herbert screw. (With permission from McKee MD, Jupiter JB, Bamberger HB. Coronal Shear Fractures of the Distal End of the Humerus. J Bone Joint Surg [Am] 1996;78:49.) |
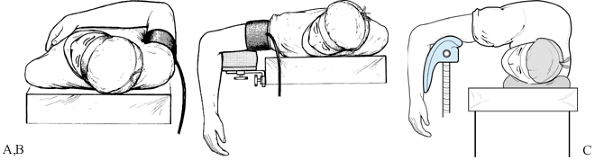
is good for lateral approaches to the elbow. Holding the elbow in
position is difficult, however, in complex fractures. If using the
supine position, place an arm board alongside the table, on which the
medial side of the elbow can rest when doing
lateral approaches. For medial approaches, a small hand table is useful. For the posterior approach, use the prone position (Fig. 16.4B),
with the upper arm supported on an arm rest and the elbow flexed to
90°. This position is useful for less complex fractures and when the
anterior iliac crest is not needed for structural bone graft. This
position is less convenient for the anesthesiologist. For complex
fractures, I prefer the lateral decubitus position (Fig. 16.4C),
supporting the upper arm on a well-padded obstetric knee support. The
advantage of this position is that it makes both the anterior and the
posterior iliac crests on the ipsilateral side available. In addition,
the elbow can be flexed to 120°, which improves exposure when using a
comprehensive posterior triceps-splitting approach, and the entire
upper extremity can be easily lifted off the holder if needed. In
general, these procedures tend to be long, and positioning can be
uncomfortable for the patient, so use a general anesthetic in most
cases. A tourniquet is useful for the initial dissection, particularly
when identifying and transferring the ulnar nerve. (Use a sterile
tourniquet.) Once the soft-tissue dissection has been completed, the
tourniquet can be released and removed, thus avoiding prolonged
tourniquet times.
 |
|
Figure 16.4. Various positions for surgical repair of fractures around the elbow. A: Supine position. B: Prone position with the upper arm on an arm rest. C: Lateral decubitus position with the arm over a well-padded obstetric knee support.
|
For fractures of the lateral column and capitellum, a lateral approach
is best. For medial column and epicondylar or trochlear fractures, a
straight medial approach works well. For nonarticular supracondylar
fractures, a triceps-splitting approach suffices if plates do not need
to extend beyond the upper edge of the olecranon fossa onto the medial
and lateral columns. For intraarticular T-type fractures, a
comprehensive posterior exposure is best, using either the
comprehensive triceps-splitting approach described in Chapter 1, or an olecranon osteotomy reflecting the olecranon superiorward by splitting the triceps both medially and laterally.
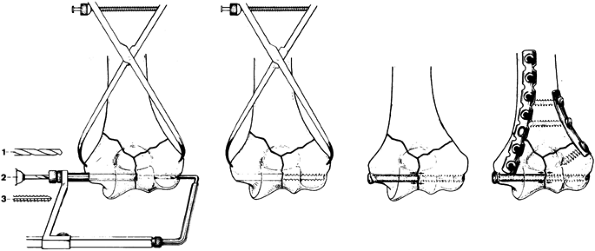
-
Prepare a midline screw hole for the
fixation screw using the lag technique. Drill a 4.5 mm hole to just
opposite the apex of the elbow joint. Place a guide, and drill with a
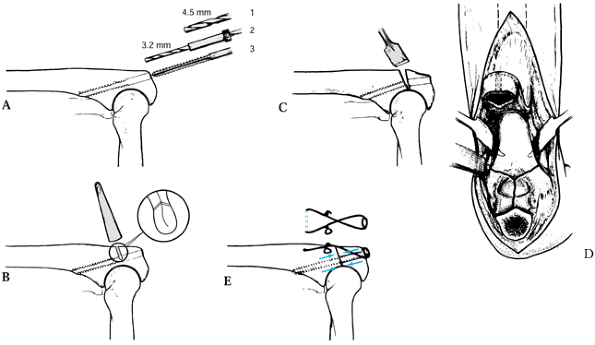
3.2 mm drill through the anterior cortex (Fig. 16.5A). Tap the hole.![]() Figure 16.5. Osteotomy of the olecranon. A: Screw hole preparation. B: Incomplete osteotomy. C: Final crack for osteotomy. D: Approach to the distal humerus. E: Lag screw and tension band fixation.
Figure 16.5. Osteotomy of the olecranon. A: Screw hole preparation. B: Incomplete osteotomy. C: Final crack for osteotomy. D: Approach to the distal humerus. E: Lag screw and tension band fixation. -
Using a water-cooled oscillating saw, make a chevron osteotomy with the apex distalward (Fig. 16.5B).
Cut down to but not through the subchondral bone of the olecranon.
Place this at the apex of the joint where there is almost no articular
cartilage. -
Complete the osteotomy with an osteotome, levering the proximal piece of the olecranon to crack through into the joint (Fig. 16.5C). Take care to not injure the articular cartilage of the humerus.
-
Split the triceps medially and laterally in line with the olecranon and reflect it proximally (Fig. 16.5D)
-
Repair the olecranon with a 4.5 mm
cortical screw using the lag technique, augmented by a tension band
wire placed around the head of the screw (Fig. 16.5E).
approaches, identify and protect the ulnar nerve. In most cases,
transfer of the nerve anteriorly is recommended to avoid impingement on
the nerve by plates or screws placed medially, and to avoid
incorporation of the ulnar nerve into periarticular scar during
healing. Techniques for transfer of the ulnar nerve are described in Chapter 51, Chapter 52, and Chapter 57. I prefer to transfer into a subcutaneous pocket, taking care to release the medial intermuscular
septum in the arm, and dissecting the ulnar nerve well down into the
flexor carpi ulnaris to avoid any tension or kinking of the nerve
proximally and distally. Try to maintain soft-tissue attachments to all
bone fragments to preserve their blood supply. This is most important
for the articular fragments and less important for smaller metaphyseal
fragments.
-
Identify all fracture fragments and clean
them of organizing hematoma. Often there are free fragments. It is
useful to make a drawing of the distal end of the humerus on a cloth or
a piece of paper on the back table and use this to assemble the
fragments in their correct orientation. -
Once all the fracture surfaces and
fragments are identified, develop a strategy for assembling the
fracture. A few minutes of careful thought spent working out a strategy
may save an hour or more of operating time because assembling the
fracture anatomically, maintaining it in position, and applying
fixation can be extremely demanding technically.
and the fragments on the other column can be assembled against the
intact column. If both columns are fractured, particularly if there is
bone loss, the difficulty increases. In looking at the fracture, think
about the sequence of assembling the fragments, because not
infrequently the fragments key into each other in such a way that if
the order of reduction is not appropriate it may be impossible to
assemble the fracture after it is partially fixed.
multifragmented fracture into a simple two-part fracture, assembling
the articular pieces as one unit and the metaphyseal fragments as
another unit, and then finally joining them together. Another approach
is to assemble one column and fix it in stable anatomic position and
then assemble the other column against it. Nonanatomic reduction of
fracture lines cannot be tolerated, particularly in the early phases of
reconstruction, because a small error in the assembly of one fragment
or fracture line will multiply itself as the reconstruction proceeds,
making achievement of an anatomic reduction impossible. Loose articular
fragments, particularly those from the groove of the trochlea, are
often too thin or too small to hold with internal fixation, even
resorbable pins. Often these will reduce into a stable position if held
in position as the trochlea and trochlear
capitellar fragments are internally fixed, keying these into place and locking them in position.
-
Anatomic reduction and preliminary fixation with Kirschner wires
-
Interfragmentary lag screw fixation of the articular condyles
-
Fixation of the lateral column with a well-molded posterior plate
-
Fixation of the medial column with a medial plate extending down to, and on occasion wrapping around, the medial epicondyle
-
Multiple interfragmentary screws, usually
through the plates but sometimes independent of the plates, to secure
the fracture construct together -
Cannulated screws are preferred by some
surgeons because these can be placed over the preliminary Kirschner
wires (K-wires), which greatly simplifies fixation. Keep in mind,
however, that these screws are much more expensive and weaker than
standard screws.
 |
|
Figure 16.6. Screw fixation of the two condyles in a Y-fracture. A: Screw hole preparation. B: Screw hole for a lag screw. C: Lag screw inserted. D: Plate fixation of the condyles to the shaft.
|
-
Reduce the fracture and hold it securely
with a sharp bone holding forceps. Under fluoroscopic control, insert a
K-wire from the medial epicondyle through the center of the lateral
aspect of the capitellum over which a cannulated screw will be
inserted, or use the anterior cruciate ligament (ACL) guide, as
depicted in Figure 16.6A. -
Insert a 4.5 or 4.0 mm cancellous lag
screw, depending on the size of the elbow, and compress the fracture.
In most cases, the fracture surfaces are sufficiently irregular that
compression makes them stable. If the fragments tend to rotate as the
screw is tightened, place a second K-wire more proximally to control
rotation. -
Next, reduce the proximal fragment to the
reassembled distal fragment and secure the position with a 2 mm K-wire
inserted from the medial epicondyle across the medial limb of the
fracture to exit the lateral cortex of the proximal fragment, and a
second wire from the lateral condyle across the T-component to exit the
medial cortex of the proximal fragment. -
Then apply plates to both columns (Fig. 16.6D).
This shows the use of a one-third tubular plate laterally along the
supracondylar ridge, and a 3.5 mm reconstruction plate along the
posterior aspect of the medial column. I prefer to place a small
reconstruction plate along the medial aspect of the medial column and
the posterior aspect of the posterior column (Fig. 16.7).
With the medial location, it is usually possible to get a minimum of
two and sometimes three screws into the distal fragment. In most
fractures, the plate can stop just proximal to the prominence of the
epicondyle. In more comminuted fractures, it may be necessary to bend
the plate around the tip of the epicondyle, represented by the dotted
lines on Fig. 16.7A, which adds still another screw. Transfer the ulnar nerve if this is
P.489
done. Note the three-dimensional contouring required for the plate on the lateral condyle (Fig. 16.7B, Fig. 16.7C and Fig. 16.7D).
These plates must be contoured exactly, so that the fracture does not
displace. Use templates and bend them to match the bone. I prefer
titanium plates because they are more forgiving and are less likely to
displace the fracture.![]() Figure 16.7. Plate fixation of a Y-type fracture of the humerus with dual plate fixation. A:
Figure 16.7. Plate fixation of a Y-type fracture of the humerus with dual plate fixation. A:
Use of 3.5 mm reconstruction plates: The lateral plate is molded to fit
the posterior aspect of the lateral column and a second plate is molded
to fit along the medial supracondylar ridge. B–D: Note that contouring the plate to fit the lateral column requires a twist in three dimensions. -
For simple single-condyle fractures, an
interfragmentary screw across the condyles and a one-third tubular
plate conformed to fit the supracondylar ridge act nicely as a buttress
plate (Fig. 16.8). Fix the articular portion of
the fracture with an interfragmentary lag screw. Then contour a
semitubular plate to fit snugly on the supracondylar ridge and secure
it with two screws proximally. This plate has a buttress function that
prevents proximal migration of the fractured condyle. Insert a third
screw distally.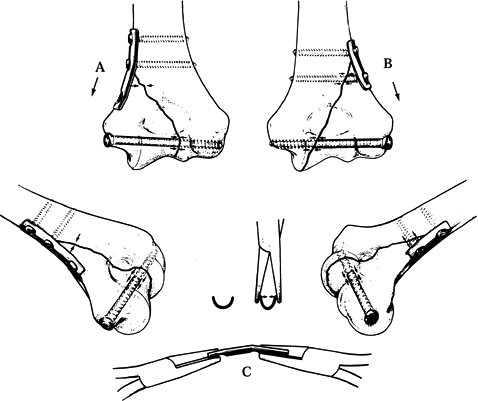 Figure 16.8. Methods for fixation of unicondylar fractures of the humerus. A: Technique for a fracture on the ulnar side. See text for details. B: A similar technique is illustrated for a fracture on the opposite side. C: Contouring of the plate with pliers.
Figure 16.8. Methods for fixation of unicondylar fractures of the humerus. A: Technique for a fracture on the ulnar side. See text for details. B: A similar technique is illustrated for a fracture on the opposite side. C: Contouring of the plate with pliers. -
Coronal shear fractures are difficult to
fix, and success depends on the size of the fragment. Alternatives for
fixation include multiple K-wires, resorbable pins, resorbable screws,
Herbert screws, and 4.0 or 2.7 mm cancellous screws. Because the
anterior fragment is small, fixation nearly always has to be through
the articular surface; therefore, all implants need to be countersunk
below the articular surface.
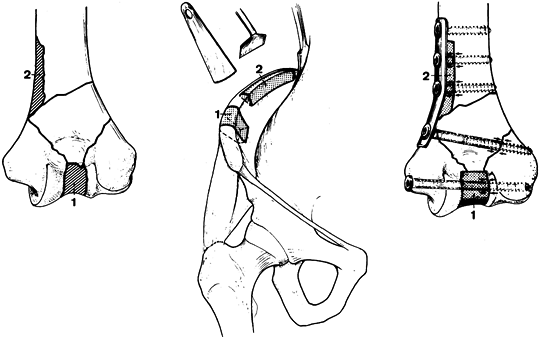
metaphyseal portion of the olecranon fossa (which usually does not
require replacement), fragmentation of the supracondylar ridge, or loss
of the midsubstance of the trochlea. The superior margin of the iliac
crest provides ideal graft for replacing these deficiencies (Fig. 16.9).
 |
|
Figure 16.9. Bone grafting for bone defects. A: Examples of bone defects. B: Harvesting corresponding bone grafts from the iliac crest. C: Incorporating the bone grafts into the corresponding defects.
|
-
After completion of the fixation, take
anteroposterior (AP) and lateral plain radiographs to confirm the
reduction and ensure that the internal fixation has been appropriately
placed. Sometimes, it is wise to leave the K-wires placed for
preliminary fixation, because they add to the strength of the
construct. If left, be certain that they are not protruding excessively
from the opposite cortex, and cut them flush with the near cortex if
you plan to leave them permanently. -
Next, carry the elbow through a full
range of motion to be certain that there is no micromotion or evidence
of weakness in the construct, particularly at the ends of range of
motion. If such is detected, add more fixation or plan to use
postoperative bracing, which prevents the elbow from reaching the range
of motion that causes micromotion in the fracture site. -
Place a small suction drain at the level
of bone and close the wound in layers. Apply a bulky sterile dressing
and splint the elbow in nearly full extension. This position
accommodates swelling better, and most patients rehabilitate better
from the extended position than from the flexed position.
 |
|
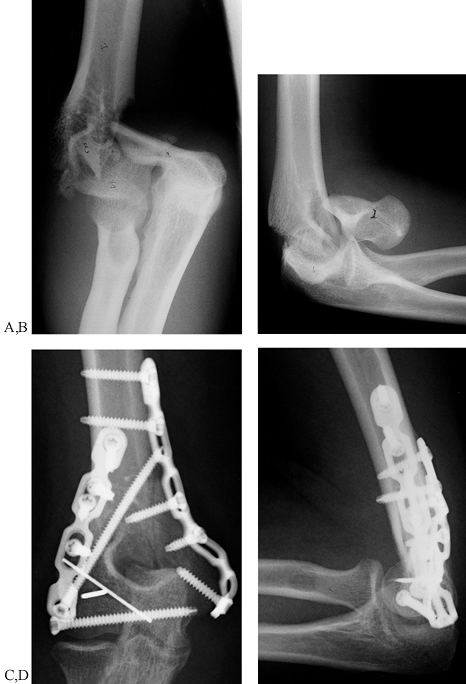
Figure 16.10. Comminuted intraarticular supracondylar fracture of the humerus with double plate fixation. A:
AP radiograph showing extreme comminution of the distal humerus in a 17-year-old girl who fell off a horse. The medial and lateral columns are fractured. There is comminution around the olecranon fossa. The junction between the trochlea and the capitellum is comminuted, with the majority of the trochlear notch consisting of two completely free fragments. B: Lateral radiograph showing the comminution with complete anterior displacement of the capitellum, which is dislocated free from the radial head. C: Postoperative AP radiograph showing reconstruction of the articular components with a lateral to medial lag screw. The two K-wires provide additional fixation for the free fragments. The integrity of the medial and lateral columns has been restored with 3.7 titanium Alta (Howmedica, Rutherford, NJ) reconstruction plates. The fracture is anatomic. D: Lateral radiograph of the fixation showing anatomic reduction. It was possible to start immediate motion in this elbow. At 6 months follow-up, the fracture had healed and the patient’s range of motion was from 10° to 135° of flexion, with full supination and pronation. Note that this was done with a comprehensive posterior triceps-splitting approach, and olecranon osteotomy was not necessary. |
entire extremity above the level of the heart. Observe carefully for
excessive swelling and compartment syndrome. Once swelling has abated
and the drain has been removed, remove the bulky compression dressing,
apply a supportive brace, and begin active range-of-motion exercises.
Set fixed limits on the brace if necessary, according to an examination
under anesthesia.
and work on the range of motion from that position. Discontinue the
braces as soon as feasible. If the patient has
difficulty
gaining range of motion, use dynamic flexion and extension splints on
alternating nights. Avoid resistive exercises and any heavy use of the
extremity until fracture healing has occurred, which is generally in
10–14 weeks.
surprisingly good results in the treatment of shear fractures of the
distal articular surfaces of the humerus in six patients. All united
without evidence of osteonecrosis. Functional outcomes were good, with
an average flexion contracture of 15° with further flexion to 141°. In
T-type bicondylar fractures, Helfet and Schmeling (62) reported an average of 75% excellent to good results.
Numerous treatment methods have been advocated, but none is clearly
superior. The fracture is usually caused by either direct trauma to the
olecranon or a fall on the outstretched hand. Restoration of stable
elbow function and rapid mobilization are the goals of treatment.
have an intact triceps aponeurosis are generally grouped separately.
With this injury, the patient can extend the injured elbow against
gravity without causing displacement of the fragments. If there is a
question about stability, examine under fluoroscopy to document it.
to the fracture line and degree of comminution. A modification of
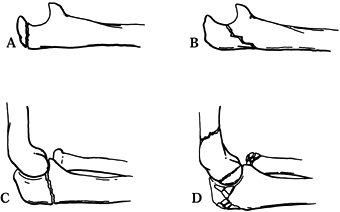
Colton’s classification (34) separates these into four types (Fig. 16.11):
 |
|
Figure 16.11. Colton classification of olecranon fractures. A: Type I, avulsion of the olecranon process. B: Type II, fracture from the deepest portion of the semilunar notch. C: Type III, fracture at the most distal portion of the olecranon.
|
-
Type I is an avulsion fracture, which may be either intra- or extra-articular (Fig. 16.11A). The fragment is often small (less than 50% of the olecranon process) and may have an oblique or a transverse fracture line.
-
Type II (Fig. 16.11B)
is a fracture that runs transversely or obliquely from the deepest
portion of the semilunar notch to the subcutaneous border. With
increased injury force, comminution of a centrally depressed wedge
fragment can be present. -
Type III (Fig. 16.11C)
involves a fracture through the more distal part of the olecranon and
may be associated with anterior subluxation of the radius and ulna.
Here the fracture line is near the tip of the coronoid process and may
produce a variant of the Monteggia lesion. -
Type IV (Fig. 16.11D)
is a severely comminuted olecranon fracture, usually caused by major
direct trauma, and is often accompanied by other elbow injuries.
Multiple fracture lines are seen with frequent depression of fragments.
Associated fractures of the radial head, distal humerus, and forearm
can be seen.
of the previous classifications to be useful to him for clinical
decision making; therefore, he proposed the Mayo classification:
-
Type I: Undisplaced fracture
-
Type II: Displaced stable fracture:
Fracture fragments are separated by more than 3 mm, but the collateral
ligaments of the elbow are intact and the forearm is stable in its
relationship to the humerus. This type can be subclassified as
noncomminuted type IIA or comminuted type IIB. -
Type III: Displaced unstable fracture:
The fracture is displaced and the forearm is unstable in relationship
to the humerus. This is a fracture dislocation, and it can be
subcategorized into noncomminuted type IIIA or comminuted type IIIB.
The latter is the most difficult to treat and has the poorest prognosis.
four different methods of tension band wiring. They compared a
monofilament wire in a traditional figure-eight loop with K-wires
inserted from the tip of the olecranon into the anterior cortex or
intramedullary, to similar constructs using cables in both
figure-eight
and circular loops. They found that insertion of the K-wires from the
tip of the olecranon into the anterior cortex and the use of cable
provided superior fixation to intramedullary K-wires and monofilament
wire. In a prospective randomized study, Hume and Wiss (74)
compared tension band wiring to plate fixation. Although plate fixation
required longer operative time, it did not have an increased
complication rate, and the clinical results were superior to tension
band wire. Postoperative loss of reduction occurred in 53% of patients
fixed with tension band wires and in only 5% after plating. With
plates, good clinical results were 26% more common, and good
radiographic results were 39% more common.
maintaining the elbow in a semiflexed position (to 90° of flexion, if
tolerated) with a cast or splint. Obtain repeat radiographs at 5–7 days
to ensure maintenance of reduction. Early, protected range of motion
may begin at about 1 week. Rarely, one encounters an undisplaced
fracture in a person in whom nonunion or displacement is not tolerable,
such as in a professional throwing athlete. A tension band wiring can
be applied with minimal exposure, which allows more aggressive early
rehabilitation with minimal risk of displacement.
II–IV and Mayo types II–III) has been the topic of a significant amount
of research and discussion through the years. Alternative methods
include open reduction and internal fixation or fragment excision, and
reconstruction of the triceps mechanism (49,70,77,176). I favor anatomic reduction with internal fixation when possible, with early mobilization.
wiring of the intraarticular fracture restores the continuity of the
extensor mechanism and allows early range of motion. For the
extraarticular fragment, treatment depends on fragment size and patient
age. In the young person with a significant bony fragment, open
reduction and internal fixation allows bone-to-bone healing. If the
patient is older or the fragment size is limited, excision and
reattachment of the triceps mechanism is preferred.
internal fixation options include tension band wiring with associated
K-wires or a screw (or screws) (79), and
intrafragmentary compression screws with or without neutralization
plating. I prefer a tension band wire with K-wires or a single screw of
appropriate size. This method transforms tension forces into
compressing forces across the fracture site (176).
Active elbow flexion increases compression and stability because of the
tension band construct. Plate fixation plus an interfragmentary
compression screw is more stable than a tension band wire but requires
more exposure (74).
those that propagate from the distal third of the olecranon are
potentially unstable. The biceps and brachial muscles may cause
anterior subluxation of the radial head and displacement of the distal
fragment of the ulna. This adds a compressive force to the posterior
surface of the olecranon, making tension band wiring less effective (71).
Therefore, I recommend rigid internal fixation with interfragmentary
compression screws augmented by a neutralization plate. In some cases,
a reverse obliquity of the fracture line may result in a proximal
fragment too small for plate fixation. In this case, use an
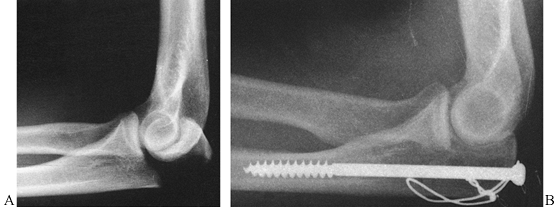
intramedullary compression screw to prevent anterior subluxation (Fig. 16.12). Failure to recognize
this specific fracture pattern may result in unsatisfactory fixation.
 |
|
Figure 16.12. A:
A type III olecranon fracture in which the proximal fragment is insufficient for plating. Gross instability with anterior subluxation of the radius and ulna is noted at the time of surgery. B: Fixation using an intramedullary cancellous screw and tension band wiring. |
(comminuted) injury, the fracture is frequently complex and is usually
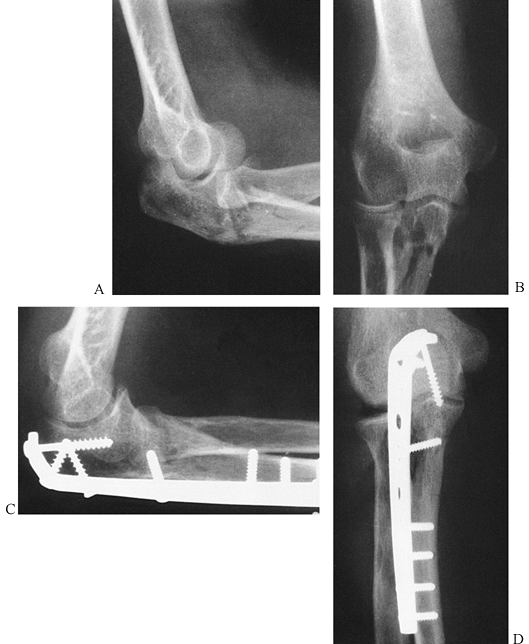
a challenge to reduce and fix (Fig. 16.13). If
the fracture extends beyond the coronoid process, length must be
restored. Fractures of the coronoid process must be adequately reduced
and fixed to preserve stability (117). Reconstruct the joint surface using interfragmentary screw compression wherever possible. Bone graft is
frequently needed. Then contour a 3.5 to 3.7 mm reconstruction plate to
fit along the posterior–lateral cortex to buttress the fragments and
help neutralize the deforming forces (Fig. 16.13C, Fig. 16.13D). A semitubular plate is usually too weak (152). Other possibilities for fixation include tension band wiring and the use of modified plates such as the hook plate (173).
Tension band wiring alone is often unstable, allowing telescoping of
the fragments with loss of articular congruity. Treatment of these
difficult fractures must be individualized, with fixation based on the
fracture pattern.
 |
|
Figure 16.13. Type IV olecranon fracture. A: Lateral view of a comminuted olecranon fracture with an associated radial head fracture and subluxation. B: AP view. C: Contoured plate fixation along the posterolateral cortex with interfragmentary compression where possible. D: AP view after fixation.
|
proximal fragment in an olecranon fracture did not significantly impair
power or stability (49). However, as discussed in the earlier section on biomechanics,
there is a nearly linear relationship between loss of any portion of
the olecranon and increasing instability in the elbow with loss of
power in extension (4,138,176).
Excision in young active patients is not recommended unless it is the
only alternative available. In the elderly or inactive patient for whom
comminution or poor bone quality limits the ability to internally fix
the olecranon, approximately 40% of the proximal olecranon can be
excised as long as the triceps is securely reattached so that early
motion is possible (104). This will generally result in acceptable range of motion and power in sedentary patients.
-
Position the patient prone with the
extremity free over the side of the operating table and with the
humerus supported on a movable padded arm board (122).
This provides direct visualization of the posterior aspect of the
elbow, allows free flexion and extension, and permits the entire
extremity to be supported if desired. Prepare and drape the extremity
free. A sterile tourniquet is useful. -
Make a dorsal longitudinal incision
beginning 2.5 cm proximal to the olecranon, and gently curve laterally
around the olecranon process to avoid crossing directly over its tip,
as this may lead to a painful scar. Carry dissection down sharply to
the fascia overlying the triceps tendon, and develop medial and lateral
thick fasciocutaneous flaps. Identify and protect the ulnar nerve; it
does not need to be mobilized in the vast majority of cases. Inspect
the fracture site and clean the fragments. -
Make a small drill hole in the distal
fragment to accept one tine of a reduction forceps. Insert the other
tine into the proximal fragment, reduce the fracture anatomically, and
compress it. Sometimes, two bone-holding forceps are necessary (Fig. 16.14A).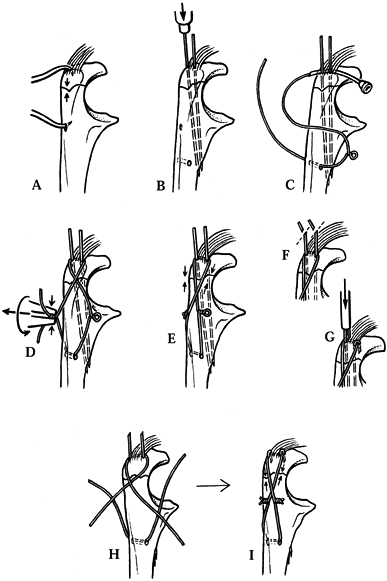 Figure 16.14. Tension band wire fixation of an olecranon fracture. See text for a full description. A: Reduction of the fracture with reduction tines. B: Initial fixation with two 1.6 mm K-wires and placement of the transverse drill hole in the distal fragment. C: Initial passage of the 18-gauge malleable wire with a loop bent in one side. D: Twisting the malleable wire on both sides to apply compression. E:. Compression across the fracture. F: Cutoff of excess length of the K-wires. G: Wires bent over and pounded into the olecranon, trapping the tension band wire. H,I: Accomplishment of the same fixation using double wires. (From Müller MS, Allgöwer M, Schneider R, Willenegger H. Manual of Internal Fixation, 2nd ed. New York: Springer-Verlag, 1979, with permission.)
Figure 16.14. Tension band wire fixation of an olecranon fracture. See text for a full description. A: Reduction of the fracture with reduction tines. B: Initial fixation with two 1.6 mm K-wires and placement of the transverse drill hole in the distal fragment. C: Initial passage of the 18-gauge malleable wire with a loop bent in one side. D: Twisting the malleable wire on both sides to apply compression. E:. Compression across the fracture. F: Cutoff of excess length of the K-wires. G: Wires bent over and pounded into the olecranon, trapping the tension band wire. H,I: Accomplishment of the same fixation using double wires. (From Müller MS, Allgöwer M, Schneider R, Willenegger H. Manual of Internal Fixation, 2nd ed. New York: Springer-Verlag, 1979, with permission.) -
About 1 cm volar to the dorsal border of the ulna, drill a 2.5 mm transverse hole in the distal fragment (Fig. 16.14B).
Next, drill two parallel 1.6-mm-diameter K-wires from the tip of the
olecranon at the insertion of the triceps across the fracture and
exiting the anterior cortex. These provide best fixation if they are
close to the articular surface. Visualization with a C-arm fluoroscope
on the lateral view during insertion is helpful. Use of a three-hole
pointed guide ensures that the wires are parallel. -
Take an 18-gauge malleable wire of
appropriate length, twist a loop into its midsection, and pass it volar
to the proximal end of the K-wires through the triceps mechanism. Be
certain that the wire is against the K-wires and the olecranon. Pass it
in a figure-eight fashion through the transverse hole in the distal
fragment (Fig. 16.14C). -
With the wires crossed, use a wire
twister to tighten the free ends of the wire together. Tighten the loop
alternately on opposite sides to produce even compression across the
fracture site. Bend the free ends of the wire over, making certain that
they are not prominent, and cut off the excess wire (Fig. 16.14D, Fig. 16.14E). -
Next, cut off excess K-wire, bend it into
a sharp loop, and pound it into the tip of the olecranon across the
tension band wire (Fig. 16.14F, Fig. 16.14G).
Carry the elbow through a full range of motion to test the stability of
the construct, and close the wounds in layers. Apply a bulky soft
dressing, and immobilize the arm in a splint in about 30° of flexion.
full range of motion is possible. Patients must avoid any lifting or
heavy use of the elbow until it is healed. This is a problem in
multiply injured patients who spend the majority of their time in bed,
because they tend to use their upper extremities to move themselves
about the bed. Warn patients to avoid this. Where the fracture is
comminuted or the bone osteoporotic and fixation marginal, determine
the safe range of motion intraoperatively and apply a postoperative
brace, setting the limits of the brace in the safe zone and allowing
the patient early active motion. Once the fracture is healed, which is
usually in 6–10 weeks, a full rehabilitation program is possible.
-
If there is segmental comminution in the
fracture, tension band wiring is contraindicated because it will narrow
the fossa of the olecranon and produce an incongruous joint. These
require an intercalary bone graft and plate fixation. (This procedure
will be described later.) -
Perfect position of the K-wires can be
obtained by inserting them retrograde from the fracture site out the
tip of the olecranon, and then inserting them antegrade after reduction
of the fracture. This usually eliminates the need for a fluoroscope. -
Because of the cartilage at the insertion
of the triceps into the olecranon, and because of the bulk of the
triceps insertion, getting the malleable wire against the K-wires and
directly against bone can be challenging. Sometimes, drilling a
transverse hole through the olecranon just beneath the K-wires is
easier. -
An 18-gauge wire may be too bulky in
petite patients. For small patients, use a 20-gauge wire. Avoid
unnecessary kinks or twists in the malleable wire, because this can
lead to premature failure. -
To avoid excessive penetration of the
K-wires from the anterior cortex, insert them through the cortex and
then withdraw them approximately 1 cm; when the wires are pounded home,
this will place them in optimal position. -
Bending the K-wires to fit over the
malleable wire can be difficult. Leave the K-wire protruding 2–3 cm.
Grasp it with thin-nosed pliers and place over the wire a small metal
suction tip; use it to bend the wire to a 90° angle. Cut off all but 5
mm of the bent end. Then twist the wire 90° to bring it over the
malleable wire. Make a small stab wound in the triceps to accept the
K-wire and pound it home with a small punch. Be certain (with a lateral
radiograph or fluoroscopic image) that the K-wire is fully buried,
trapping the malleable wire.
of the ulna with anterior dislocation of the radial head, the Monteggia
fracture–dislocation is a term now used to describe almost all ulna
fractures associated with radial–humeral joint disruption. Although the
ulna fracture is seldom undiagnosed, the associated dislocation of the
radial head may elude the examiner in as many as one quarter of the
cases (15,52).
Regardless of the radiographic view of the elbow, a line drawn down the
central axis of the radius and through the center of the radial head
should always pass through the middle of the capitellum. Any shift in
this alignment indicates subluxation or dislocation of the radial head.
Always look for this when fractures of the ulna are present.
The categories have in common a dislocation of the radial–humeral joint
associated with a fracture of the ulna or with lesions at the wrist.
Four types of fracture–dislocations are described, based on the
direction of the radial head dislocation. A series of equivalent
injuries is also described with respect to the mechanism of injury and
treatment:
 |
|
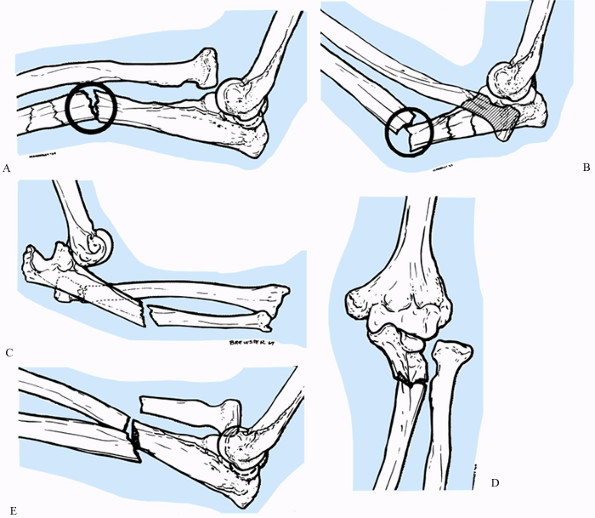
Figure 16.15. Bado classification of Monteggia fracture–dislocations. A: Type 1. Ulnar diaphysis fracture with anterior angulation and associated anterior dislocation of the radial head. B: Type 2. Posterior dislocation of the radial head and fracture of the ulnar diaphysis with posterior angulation. C:
Type 2–equivalent lesion with posterior dislocation of the elbow, fracture of the ulnar shaft and posterior angulation, and fracture of the head or neck of the radius. D: Type 3. Fracture of the ulnar shaft and associated lateral radial head dislocation. E: Type 4. Fracture through the proximal third of the radius and ulna at the same level, with associated anterior subluxation of the radial head. (From Reckling FW, Cordell LD. Unstable Fracture-Dislocations of the Forearm. The Monteggia and Galeazzi Lesions. Arch Surg 1968;96:999, with permission.) |
-
Type 1: Anterior dislocation of the
radial head associated with fracture of the ulnar diaphysis at any
level with anterior angulation (Fig. 16.15A): the most common Monteggia lesion, seen in about 60% to 80% of cases (10,134) -
Type 2: Posterior or posterolateral
dislocation of the radial head and fracture of the ulnar diaphysis with
posterior angulation (Fig. 16.15B, Fig. 16.15C) -
Type 3: Lateral or anterolateral dislocation of the radial head accompanied by a fracture of the ulnar metaphysis (Fig. 16.15D)
-
Type 4: Anterior dislocation of the
radial head associated with fractures of the proximal third of the
radius and ulna at the same level (Fig. 16.15E)
-
Anterior dislocation of the radial head alone
-
Fracture of the ulnar diaphysis with a fracture of the neck of the radius
-
Fracture of the neck of the radius
-
Fracture of the ulnar diaphysis with
fracture of the proximal third of the radius, where the radius fracture
is proximal to the fractured ulna -
Fracture of the ulnar diaphysis with anterior dislocation of the radial head and fracture of the olecranon
-
Posterior dislocation of the elbow and fracture of the ulnar diaphysis, with or without fracture of the proximal radius
are epiphyseal fractures of the dislocated radial head or fractures of
the neck of the radius. Bado described no equivalents for Monteggia
lesion types 3 and 4.
fracture dislocation is more likely to result in disability and
permanent stiffness of the elbow (15,19,134,140).
To the surgeon, this lesion is possibly the most challenging forearm
injury. To enhance the chances of obtaining a satisfactory result, the
following are needed:
-
Anatomic and rigid internal fixation of the ulna fracture, with bone grafting as needed
-
Anatomic reduction of the radial head dislocation
-
Sufficient period of adequate immobilization in unstable injuries, and early motion in stable injuries
skeletally mature patient, for whom it is generally accepted that
operative intervention is indicated. However, the Monteggia lesion in
children has been successfully treated by closed reduction and casting.
Open treatment in a child with this injury is the exception.
classification. In most series, type 1 injuries account for 60% to 80%
of cases. In the series of Ring et al. (138,140),
however, Bado type 2 was most common. The preferred treatment for type
1 is compression plate fixation of the ulnar fracture, with
supplemental autogenous bone grafting if comminution is significant.
Anatomic reduction and restoration of length is mandatory or the radial
head will remain subluxed. Usually, the radial head reduces
spontaneously after fixation of the ulna. If it does not, then either
reduction of the fracture is inadequate or there is soft-tissue
interposition. Inadequate reduction of the ulna
mandates
repeat reduction and fixation. Soft-tissue interposition, seen in fewer
than 10% of cases, requires open visualization of the radiohumeral
joint. A torn and interposed annular ligament is the usual offender;
this requires reduction of the radial head and repair of the ligament.
Interposition of the radial nerve is a rare cause of the unreduced
radial head.
dislocation of the radial head, a fracture of the radial head or neck
may be seen. Some surgeons perform early excision in these cases (15,19);
however, I prefer to fix the radial head fracture because of its
contribution to stability of the elbow. Radial head fractures may be
seen in any of the other Monteggia lesion types as well. Treatment of
the
ulna fracture is anatomic restoration and plating (Fig. 16.16).
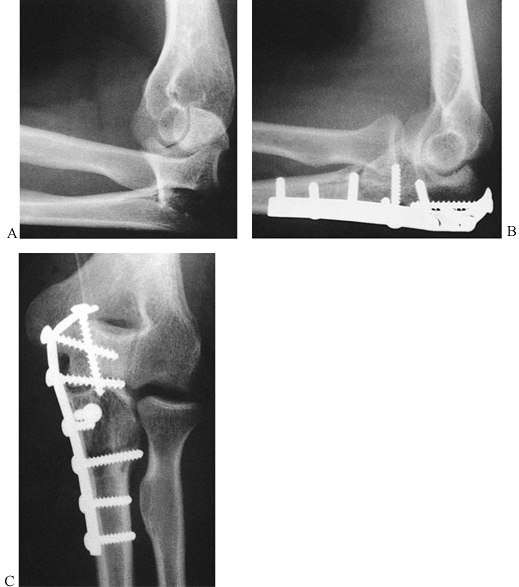 |
|
Figure 16.16. Type 2 Monteggia lesion. A: Posterior radial head dislocation in association with a proximal ulna fracture. B,C:
Anatomic restoration of the ulna fracture with plate fixation and interfragmentary compression, and reduction of the radial head. |
and is best treated closed. However, if seen in an adult, treat as
described for a type 2 lesion.
fixation of the radius and ulna fractures clearly constitutes the
treatment of choice. Radial head reduction usually follows anatomic
reduction of both bone fractures.
-
The operative approach to stabilize the
Monteggia lesion is initially the same as for the isolated ulnar
fracture. Position the patient supine with the involved arm on an arm
board or across the patient’s chest. Alternatively, place the patient
in the lateral decubitus position with the arm supported on a bolster
and the elbow flexed 90°. Prepare and drape the arm free. A sterile
tourniquet is useful. -
Make an incision just lateral to the
subcutaneous border of the ulna, centered over the fracture site,
carrying the dissection through the subcutaneous tissue. Identify the
fascia and periosteum between the extensor carpi ulnaris and the flexor
carpi ulnaris muscles, and split this layer directly over the
subcutaneous border. Take care to preserve soft-tissue attachments to
bone. Limit the placement of retractors and bone clamps to the
subperiosteal level; this will avoid damage to the ulnar nerve and
artery, which lie under the flexor carpi ulnaris in this dissection. -
Identify the bone fragments and clean
them of hematoma. Reduce the fracture anatomically, taking care to
fully restore length because this is necessary to reduce and stabilize
the radial head. Reduction can be difficult, especially in comminuted
fractures, and in particular when there is a large free coronoid
process. Sometimes, attaching the plate initially to the proximal
fragment with a single screw and then using it to reduce the fracture
is helpful. -
Try to use 3.5 mm regular or
reconstruction plates or their equivalent. Semitubular plates tend to
be too weak, but they do fit nicely along the subcutaneous border of
the ulna and contour around the proximal tip of the olecranon. In very
comminuted fractures, I often use a 3.5 mm plate as the primary plate
and then use a two- to four-hole one-third tubular plate as a second
plate to buttress comminution. Three plate locations are possible: the
subcutaneous border, the lateral surface, or the medial surface. In
most cases, the medial surface is best because it requires the least
contouring and provides better fixation in the proximal fragment. The
screws are directed from medial to lateral, so bicortical purchase can
be obtained, whereas a plate over the subcutaneous border precludes
bicortical fixation in the proximal fragment. Sometimes the plate works
best laterally; however, the proximity of the dissection to the radial
head increases the risk of a radial–ulnar synostosis. Plates along the
subcutaneous border may work best when there is substantial comminution
along this border that needs buttressing, but they have the
disadvantage noted previously and in addition are quite prominent
beneath the skin and therefore usually require removal (Fig. 16.16). -
Bone-graft comminuted fractures and those
with missing bone substance. Small deficiencies that are nonarticular
can be bone grafted with cancellous bone harvested from the iliac
crest. Segmental loss or deficiencies in the articular surface often
require a structural tricortical bone graft from the superior crest of
the ilium to maintain length. As long as the congruity of the humeral
ulnar joint is preserved, segmental deficiencies in the articular
surface are usually well tolerated. -
Should intervening soft tissue prevent
radial head reduction, open the radiohumeral joint; this is likely to
occur in fewer than 10% of cases. The joint may be visualized by
extending the incision proximally toward the lateral epicondyle of the
humerus. Detach the anconeus muscle and the ulnar extensor muscles
subperiosteally from the ulna and retract them anteriorly. Take care to
protect the radial nerve and its branches, as the posterior
interosseous nerve passes over the radial neck. Although uncommon, the
nerve itself may even be the soft tissue blocking reduction. Once the
joint is opened, reduce the radial head and repair the annular ligament
under direct visualization if necessary. -
Ensure hemostasis and close the wound in
layers. Apply a sterile dressing and then splint the forearm in a
position that offers the best stability for the radial head, which is
generally 90° of flexion and full supination. Occasionally, the reverse
is true.
-
Special consideration is necessary if the
Monteggia lesion is seen more than 4–6 weeks after injury as a result
of inadequate treatment or misdiagnosis. Significant ulnar malunion
requires corrective osteotomy, and an ulnar nonunion requires
reduction, plating, and bone grafting. It is best to preserve the
radial head, but persistent dislocation may require resection. The
minimally subluxed radial head is probably best left alone, especially
if adequate function is present. Minor degrees of ulnar angulation may
also be acceptable in these late-presenting cases. A less-than-optimal
result is often seen in these patients. -
Combinations of different types of
internal fixation are often necessary in these difficult fractures. If
the fracture has been plated but the proximal fragment is short or
osteoporotic, adding a tension band wire to the plate may significantly
increase the strength of the construct. It is important to fix large
coronoid process fragments. This can be difficult and is discussed in
more detail in the next section. -
In comminuted, unstable, displaced
fractures (Mayo type IIIB), internal fixation alone may be inadequate.
In these cases, Morrey (117) uses a
distraction-type external hinge fixator (DJG, Howmedica, Rutherford,
NJ). He begins with continuous passive motion with the distraction
device in place, and then he encourages active assisted motion. He
removes the fixator under anesthesia at approximately 4 weeks and then
relies heavily on splints to protect the collateral ligaments. To help
restore motion, he uses adjustable splints to encourage motion in one
direction at night and the opposite motion during the day.
supination for about 2–3 weeks. For type 1, 3, and 4 lesions, maintain
flexion at 90°. Position type 2 lesions at 70° of flexion. After
removal of the cast, begin active motion. Avoid extremes of motion to
prevent recurrent radial head subluxation. A motion restricting brace
may be helpful.
-
Type I is a small chip fracture that has no clinical importance but suggests the possibility of an elbow dislocation.
-
Type II involves 50% of the coronoid process and may or may not be associated with an unstable elbow.
-
Type III is a fracture of the entire coronoid process.
symptomatic, although careful evaluation of the elbow to look for major
ligamentous injury is essential.
If fixation is not possible, the elbow may be stabilized with the
distraction external fixator without fixation of the fragment.
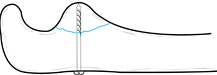 |
|
Figure 16.17. Fixation of a type 3 fracture of the coronoid process with a 4.0 mm lag screw.
|
fixation are indicated if the fracture is not too comminuted and
therefore unfixable. Loss of fixation or redisplacement of these is
common; therefore, Regan and Morrey (135)
neutralize the deforming forces across the elbow with a distraction
hinge external fixator. In comminuted fractures, they do not excise the
bone fragments, as this can lead to chronic elbow instability, but
stabilize them with #5 nonresorbable sutures to the shaft of the ulna
using the distraction device to provide stability. Early motion in all
of these fractures is important.
were able to obtain satisfactory results in 90% of patients with type I
fractures and 67% with type II, but only 25% for type III, showing the
difficulty of these fractures.
The vast majority of these injuries result from an axial load
transmitted across the radiocapitellar joint with the forearm in a
pronated position. The key factors in clinical decision making are
elbow motion and stability. Assessment of the distal radioulnar joint
is essential so that associated Essex-Lopresti–type injures (44,163,170) do not result in mismanagement and increased disability.
Often a posterior fat pad sign, with or without an anterior fat pad
sign, is all that is seen, indicating a hemarthrosis secondary to a
radial head fracture. The radiocapitellar view, a modified lateral of
the elbow joint with the tube angled 45° toward the radial head, has
been shown to be useful in detecting and evaluating fractures of the
head of the radius (56).
-
Type I: Undisplaced (Fig. 16.18A)
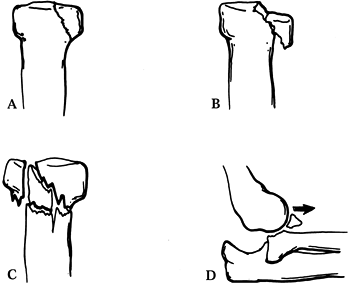 Figure 16.18. Mason’s classification of radial head fractures. A: Type I radial head fracture, nondisplaced. B: Type II injury with marginal fracture and displacement. C: Type III radial head fracture demonstrating comminution of the entire head. D:
Figure 16.18. Mason’s classification of radial head fractures. A: Type I radial head fracture, nondisplaced. B: Type II injury with marginal fracture and displacement. C: Type III radial head fracture demonstrating comminution of the entire head. D:
Type IV injury—ma radial head fracture in association with an elbow
dislocation. (From Mason ML. Some Observations on Fractures of the Head
of the Radius with a Review of 100 Cases. Br J Surg 1954;42:123, with permission.) -
Type II: Marginal fracture with displacement (Fig. 16.18B)
-
Type III: Comminution involving the entire head (Fig. 16.18C)
association with an elbow dislocation. This may result in altered
treatment and prognosis; thus others have designated this as a type IV
injury (Fig. 16.18D).
provides a much more detailed classification, which includes fractures
of both the head and neck, both as isolated injuries and in combination
with fractures of the ulna. Because all clinical studies published to
date use the Mason classification, I use it here.
and thus to retain adequate motion and joint stability. To evaluate
function acutely, aspirate the elbow and then instill local anesthetic
into the elbow joint. This affords two benefits. First, it decompresses
the joint and relieves pain (68). Second, once
the elbow becomes relatively painless, it is possible to determine
whether there is a bony block to motion or not. This procedure is
important in all radial head fractures in which closed treatment is
being considered.
disadvantages of radial head excision. Advocates feel that it allows
early motion and less morbidity for most type II and III fractures (77).
Others have disputed this mode of therapy, noting complications of
subluxation of the distal radioulnar joint, elbow pain, and cubitus
valgus deformity following excision (25,45,110,114,138).
Since the radial head has a role as a significant stabilizer of the
elbow, recommendations for early excision have probably been
overstated. Proponents of saving the head in all fracture types note
that late excision is still a viable option that has been shown to give
good results (18,117,118,126,179).
radial migration, prosthetic replacement with silicone rubber has been
proposed (60,120,121,161).
I no longer use silicone implants because they are unstable and lead to
silicone synovitis. This view is supported by others (112).
Certainly, the routine use of a prosthesis in the treatment of radial
head fractures is not favored. Situations in which its use may be
indicated are fractures associated with elbow or distal radioulnar
instability, for which a rigid stable replacement such as a metallic
head or allograft might be useful. We have used both at the University
of California Davis Medical Center, but our experience and that of
others is limited, and effectiveness is not yet proven (82,162).
consistent, reliably satisfactory results in Mason type II, III, and IV
fractures, I recommend reconstruction of the radial head and neck with
anatomic restoration of the radiohumeral joint, despite its technical
difficulty. Current indications for open reduction and internal
fixation are as follows:
-
The main fragment includes more than one quarter of the articular surface.
-
The fragment is displaced more than 2 mm.
-
There are additional lesions of the
capitellum or fracture of the proximal ulna, as well as a ruptured
collateral ligament or distal radioulnar joint injury (51). -
Clinical exam reveals less than 70° of forearm rotation in both directions, or less than 20° to 140° of flexion.
the radial head, and miniplates can be used on the neck. Try to place
fixation where it will not encounter the proximal radioulnar joint
(sigmoid notch). Countersinking the screw head is necessary if it is
placed within the articular circumference. An alternative method of
fixation is the Herbert differential pitch bone screw, which allows for
placement beneath the articular surface (98). Absorbable pins may be used as well (127).
Long-term studies evaluating operative reconstruction of these injuries
are needed before there will be widespread acceptance (117). Treatment is based on the fracture type.
aspiration and evaluation for any block to motion. If there is no bony
block to motion, then begin early active range of motion after pain has
subsided. Initial splinting provides comfort while allowing full
pronation and supination. Take frequent follow-up radiographs to check
for displacement.
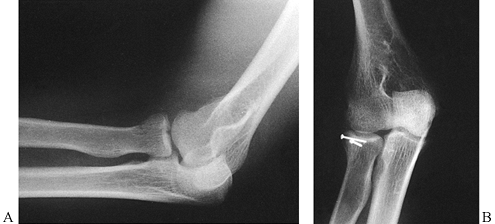
fracture pattern is amenable to internal fixation, I favor anatomic
restoration (Fig. 16.19). Otherwise, nonoperative treatment is advocated, again with aspiration and evaluation of motion (53). If problems persist, perform late excision.
 |
|
Figure 16.19. Mason type II radial head fracture. A: Marginal fracture of the radial head involving more than one third of the articular surface. B:
Open reduction and internal fixation with two minifragment screws, obtaining anatomic reduction and interfragmentary compression. |
treated with complete excision within 48 hours after fracture. The
elbow will be stable to valgus stress if the medial collateral ligament
is intact. There appears to be no advantage to removal of just the
displaced fragments. If examination under anesthesia in type III
injuries reveals satisfactory motion, pursue nonoperative treatment,
with late excision of the radial head if the result is not
satisfactory. However, late excision of the radial head after type III
fractures does not give nearly as good results as it does after type II
fractures (4,117). With
the increasing sophistication of mini-fragment fixation systems, more
surgeons are attempting to reconstruct and internally fix these
fractures (45,120,147). For stable elbows, Morrey (117)
advocates excision, and I agree. In complex fractures with instability
of the elbow or an Essex-Lopresti lesion, however, treatment is
exceedingly difficult. Fixation of the radial
head and neck with a miniplate and screw system may be essential to a stable elbow.
the elbow together with a radial head fracture, by initial reduction of
the dislocation and then treatment of the radial head according to the
principles just described. Preservation of the radial head in these
injuries is important to maintain stability and thus is highly
recommended (25,81,117,179).
When extirpation of the head is unavoidable, consider repair of the
torn ligaments or use of a distraction external fixation or a temporary
radial head prosthesis.
-
Position the patient supine with a pad
under the ipsilateral shoulder. Prepare and drape the arm free and rest
it on the patient’s chest or a small arm table. Use a sterile
tourniquet. -
Use a Kocher approach (see Chapter 1).
Incise the skin beginning at the lateral humeral epicondyle. Extend
obliquely across the radial head, ending at the posterior border of the
ulna. Identify and split the fascia between the anconeus muscle and the
extensor carpi ulnaris muscle, then spread these two muscles and their
associated fascia to bring the underlying capsule of the radiohumeral
joint into view. To aid exposure, detach part of the superior anconeus
from the lateral epicondyle of the humerus. Pronate the forearm to roll
the radial nerve and its posterior interosseous branch to a more
anterior position. Then incise the posterolateral aspect of the
capsule. Take care not to carry the incision past the annular ligament
because this risks injuring the posterior interosseous nerve. Also,
avoid applying too much tension with retractors anteriorly or distally
because this also risks nerve injury. -
Identify the fracture fragments and clean
them of hematoma. In Mason type II fractures, if the fragment has good
soft-tissue attachments, try to maintain them. Reduce the fracture and
internally fix it with a 2.7 mm screw using lag technique. Try to use
two screws if the size of the fragment will permit. Other screw sizes
may be indicated for smaller or larger fragments. Countersink the screw
heads beneath the articular surface. -
In comminuted fractures, the entire
radial head may be free fragments. Assemble the fragments on the back
table, internally fixing them in a similar manner. If a portion of the
radial head is intact, fix the preassembled radial head to the intact
fragment. If the entire head is comminuted, it can be entirely
assembled and internally fixed on the back table. At this point, you
are dealing with a neck fracture. -
To internally fix a neck fracture,
determine the portion of the radial head and neck that does not
encounter the sigmoid notch, and use it for the site of a minifragment
T-plate. Fashion this plate to fit the contour of the radial head and
neck. Securely fix it using compression techniques if possible. Examine
the construct before closure to determine its stability and the safe
range of motion. -
Thoroughly irrigate the joint and wound
and close the wound in layers. Apply a sterile dressing and splint the
elbow in the most stable position. -
For resection of comminuted fragments of the radial
P.504
head, remove all fragments and thoroughly irrigate the elbow joint.
Using a high-speed burr, smooth off the stump of the proximal radius
level with or slightly distal to the proximal edge of the annular
ligament. Purse-string the capsular remnants and annular ligament
around the stump of the proximal radius to increase its stability. Be
certain not to restrict motion.
-
Replacement of the radial head with an
allograft is no different from fixation of a radial neck fracture as
described above. Secure fixation is required if union is to occur. -
Prosthetic replacement with a metallic
prosthesis is rarely performed and is specific to the few prostheses
available. Please see the manufacturer’s instructions. The surgical
approach and soft-tissue techniques are roughly the same.
determined the safe and stable range of motion under anesthesia prior
to wound closure, apply a motion-limiting brace and begin active motion
as soon as the patient’s pain and soft-tissue swelling are controlled.
Dynamic splinting after 6 weeks may be helpful in gaining range of
motion, as previously described. Avoid resistive exercises or heavy use
of the elbow until fracture union has occurred.
-
Holding and reducing these small
fragments, which are in many cases covered entirely by articular
cartilage, can be very challenging. Insertion of a 1.6 mm K-wire into
the fragments to act as a “joystick” helps to grasp the fragments,
reduce them, and hold them in position while internal fixation is
applied. -
Prior to reduction of a free fragment, drill the screw hole. This guarantees good position of the screw and easier insertion.
-
When using the T-plate, if there is
insufficient space proximal to the annular ligament, the plate can be
slid under the ligament and a screw inserted through a small stab
wound. Take care to avoid injury to the branches of the posterior
interosseous nerve. -
Sometimes, after fixation, the radial
head remains unstable and tends to sublux. This is usually the result
of nonanatomic reduction of an associated ulnar fracture. First, be
certain that the ulnar fracture is out to length and anatomic. If
instability continues and cannot be controlled by positioning of the
elbow, insertion of a 2 mm K-wire from the radius into the ulna will
provide temporary stability. This increases the risk of synostosis of
the radius and ulna. Leave it sufficiently prominent that removal is
easy at 3 weeks, when there is usually sufficient stability to begin
motion.
It is usually sustained by active older children, adolescents, and
young adults. Elbow injuries occurring in younger or older patients are
more likely to involve fractures. Pure dislocations or those with small
periarticular fractures are the subjects of this section.
outstretched arm. Motor-vehicle accidents are also a common cause of
elbow dislocation, frequently with associated systemic injury. These
are often higher-energy injuries, and the mechanism may involve axial
compressive loading on a slightly flexed elbow. In most cases, a
posterolateral dislocation occurs with tearing of the radial collateral
ligament and lateral capsule (157). Associated
fractures are more likely to occur in higher-energy injuries. With
hyperextension of the elbow, the capsular constraints are torn and the
humerus is driven through the capsule anteriorly, tearing the
brachialis muscle. The anterior portion of the medial collateral
ligament appears to be the primary stabilizer in resisting valgus
stress and is a pivot point that allows the radius and ulna to
dislocate posteriorly when the lateral ligamentous constraints are torn
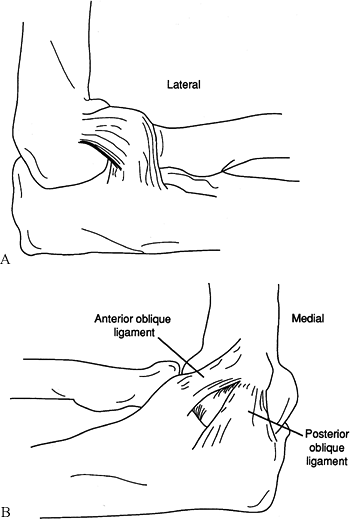
(Fig. 16.20) (72,157).
The final position is usually posterolateral, but depending on the
resultant force vector, straight posterior or posteromedial dislocation
may occur.
 |
|
Figure 16.20. The collateral ligaments of the elbow. A: Lateral. B: Medial. (Redrawn with permission from Rockwood CA, Green DP. Fractures in Adults. Philadelphia: JB Lippincott, 1974.)
|
uncommon. The rare anterior dislocation occurs with extreme
hyperextension and may be associated with extensive tearing of the
brachialis musculature, or neurovascular injury. Straight lateral
dislocations are also rare and are associated with extensive tearing of
the medial ligamentous restraints. The reverse is true for the uncommon
medial dislocation or subluxation. Divergent dislocations are rare
high-energy injuries associated with extensive soft-tissue injury to
the interosseus membrane, joint capsule, and collateral and annular
ligaments (Fig. 16.21). Usually, this strong
musculoligamentous complex binds the radius and ulna securely, so that
both bones dislocate together in a linear fashion. Therefore, isolated
dislocation of either bone is uncommon, although dislocation of the
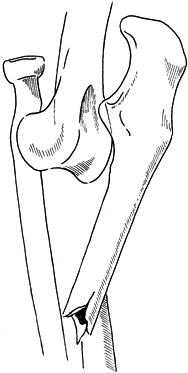
radial head is frequently associated with fractures of the proximal ulna (139) (i.e., Monteggia type 1 injury) (Fig. 16.22).
In children, the ulnar fracture can be subtle, consisting only of mild
ulnar bowing in association with a dislocation of the radial head,
which is usually anterior but uncommonly may be posterior or lateral.
Occasionally, the radial head remains dislocated after attempted
reduction of an elbow dislocation. Congenital or developmental
dislocation of the radial head with superimposed acute trauma may
present difficulty in diagnosis. These conditions are usually
bilateral, and radiographic evaluation of the opposite elbow is helpful
in securing the diagnosis.
 |
|
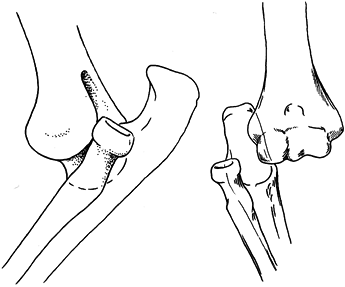
Figure 16.21. Divergent dislocation of the elbow.
|
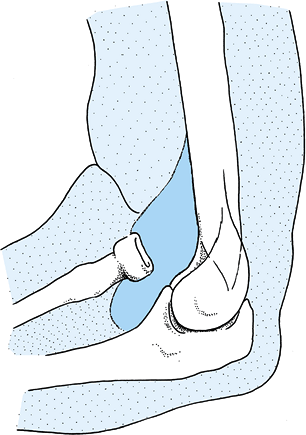 |
|
Figure 16.22. Anterior dislocation of the radial head. The radius and capitellum are not colinear.
|
associated with dislocation of the distal radioulnar joint
(Essex-Lopresti injury). This injury has been discussed previously with
radial head fractures.
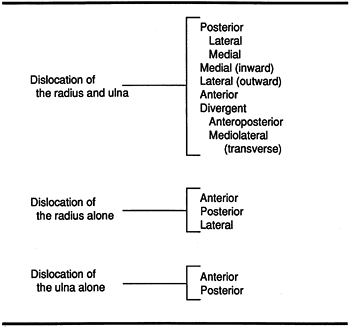
This system includes uncommon varieties of dislocation. However, from a
practical standpoint, approximately 90% of elbow dislocations
encountered are posterior or posterolateral (Fig. 16.24).
 |
|
Figure 16.23. Classification of adult elbow dislocations. (From Stimson LA. A Treatise on Fractures. Philadelphia: Henry C. Lea’s Son, 1890, with permission.)
|
 |
|
Figure 16.24. Posterolateral elbow dislocation.
|
swollen and usually held in slight flexion, often supported by the
uninjured hand. The forearm is foreshortened, and the olecranon and
radial head are prominent posteriorly in the typical dislocation.
Neurovascular function is usually intact but needs to be accurately
assessed. With rare anterior dislocation, neurovascular injury may be
present. Crepitus or extensive ecchymosis implies a fracture
dislocation. Soft-tissue abrasions about the elbow may exist, although
open dislocation is uncommon.
dislocation. They should be scrutinized carefully for associated
periarticular fracture, especially of the radial head, coronoid process
of the ulna, or medial epicondyle (Fig. 16.25).
The latter fracture is seen more commonly in children and is the most
likely culprit in irreducible elbow dislocation. When fracture of the
medial epicondyle accompanies elbow dislocation, it usually reduces
with elbow relocation. Occasionally, it is incarcerated in the joint,
preventing reduction and requiring surgical treatment. In the unlikely
event that physical examination suggests a vascular injury, obtain an
arteriogram promptly.
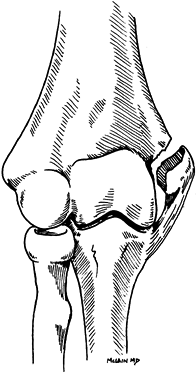 |
|
Figure 16.25. Fracture of the medial epicondyle.
|
manipulation. Closed reduction usually is not difficult. Most elbow
dislocations can be reduced in the emergency room under conscious
sedation. Various methods of reduction have been described. All
techniques involve correcting any lateral or medial displacement. Figure 16.26
depicts a method in which the arm hangs off the end of a well-padded
table and the patient is prone and relaxed. With the elbow flexed at
90°, apply gentle but firm traction on the forearm while grasping the
distal humerus with your opposite hand, and apply thumb pressure on the
posteriorly displaced olecranon. Some physicians advise mild
hyperextension to diminish impingement of the coronoid process, but
this is usually not necessary. After reduction, gently flex and extend
the elbow to ensure that reduction is complete and to ascertain
stability. Confirm reduction on radiographs.
 |
|
Figure 16.26. Closed reduction of a posterior dislocation of the elbow. (Redrawn with permission from Rockwood CA, Green DP. Fractures in Adults. Philadelphia: JB Lippincott, 1974.)
|
posterior displacement of the forearm, accompanied by gentle traction.
The rare divergent dislocation usually requires separate reductions of
the ulna and then of the radius. Gross instability or residual
subluxation may require open reduction and repair of the annular
ligament and capsuloligamentous complex.
periarticular fracture are not difficult to reduce. Inability to reduce
an elbow dislocation promptly requires careful reevaluation of
radiographs and physical findings to ascertain the impediment. The most
frequent indications for operative intervention are irreducible
dislocation, associated fractures, incongruous joint after reduction,
gross instability or neurovascular injury, and unreduced (chronic)
dislocations (44).
dislocation is an entrapped medial epicondyle. This is somewhat more
common in children. In most cases, the fracture of the medial
epicondyle reduces with reduction of the elbow dislocation. An attempt
may be made to reduce the incarcerated fragment by having the patient
activate the flexor–pronator muscle mass to which the fragment may be
attached. Otherwise, treatment is by open reduction and extraction of
the fragment from the elbow joint, followed by internal fixation or
excision of the fragment, depending on its size. Before skeletal
maturation, significant displacement should be treated by open
reduction and pinning of the medial epicondylar fragment.
when fractures and severe ligament injuries are associated, treatment
can be difficult. For the most part, treatment of these is focused on
treatment of the fractures, which usually results in stability of the
elbow. This is discussed in detail in the preceding section.
inability to reduce an acute dislocation, and it may involve entrapment
of the annular or collateral ligaments, or buttonholing of the radial
head through the posterolateral capsule. The joint may be asymmetric or
incongruous after reduction for the same reasons. Fragments remaining
in the joint or interposed soft tissue require surgical removal. Small
fragments that do not compromise joint congruency or interfere with
motion can be observed and, if necessary, removed at a later date.
reduction. Occasionally, the lateral capsuloligamentous complex and,
rarely, the medial ligamentous structures are extensively torn,
necessitating repair because of gross instability
(Fig. 16.27) (44,64,81).
Use strong absorbable sutures anchored through drill holes in bone to
ensure postoperative stability and allow early mobilization. With
medial side instability, repair the medial collateral ligament,
especially its anterior portion. Gross instability may require both
medial and lateral capsuloligamentous repair. Postoperatively, maintain
the elbow in the position of greatest stability until sufficient
healing has taken placed to allow protected graduated exercises,
usually in a dynamic splint (72,157).
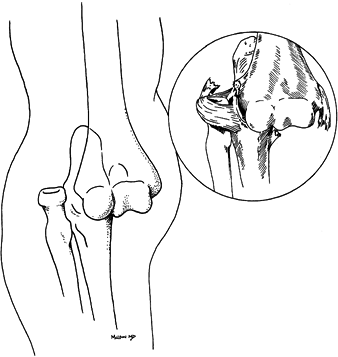 |
|
Figure 16.27. Ligamentous injury accompanying elbow dislocation.
|
anesthesia for reduction. After 2–3 weeks, open reduction is often
necessary, usually through a long extensile posterolateral approach.
Dislocations existing beyond this time present with progressively
increasing musculotendinous retraction, scarring, and articular
degeneration. Arthrodesis or arthroplasty may be indicated for the
significantly symptomatic patient.
described recurrent posterolateral rotary instability of the elbow as a
clinical entity that can be distinguished from dislocation of the elbow
and is unrelated to dislocation of the radial head. Their patients
complained of locking or snapping of the involved elbow. Diagnosis is
based on the lateral pivot-shift test of the elbow, which involves
supinating the forearm and applying a valgus moment and an axial force
to the elbow while flexing it from full extension. Flexion past about
30° produces a sudden palpable and visible reduction of the
radiohumeral joint. The essential lesion is felt to be laxity or
detachment of the lateral ulnar collateral ligament. Surgical
intervention involves restoring the functional integrity of this
ligamentous complex.
Therefore, start motion early in joints that are stable after
reduction. A brief period of immobilization (1–5 days) is all that is
necessary for stable injuries. Immobilize in a padded posterior splint
with the elbow flexed to 90°. Initiate gentle passive and progressive
active assistive and active range of motion as soon as practical.
radial head or coronoid process are unusual, but they should be
protected for longer periods in the position of maximal stability.
Usually, this is 90° or more of flexion at the elbow. Advise the
patient of the symptoms and signs of repeat dislocation. As with
fractures requiring reductions, a repeat examination at 24–48 hours is
recommended, with radiographs taken to confirm that reduction has been
maintained. Follow the patient closely during the first 10–14 days; if
dislocation recurs, it can be promptly recognized and treated (75).
Numerous studies have shown that patients who are mobilized early have
the best results and are most likely to regain normal or near-normal
elbow extension.
radiohumeral, proximal radioulnar, distal radioulnar, and radiocarpal)
constitute a delicately arranged mechanism that may be disrupted in any
or all of its parts by a shaft fracture of the radius or ulna.
Shortening, angulation, or malrotation of either of these bones will
cause functional problems at the wrist or elbow. If functional
disability is to be avoided following fracture, precise anatomic
reduction is necessary; even a very minor nonanatomic variation of
healing may constitute a malunion. Because the muscles of supination
and pronation apply rotational and angulatory forces to the radius and
ulna, maintenance of reduction after displaced or unstable fractures
requires rigid internal fixation (24).
Treatment of fractures of the radius or ulna depends on the amount of
displacement and the degree of stability of the fracture pattern.
straightforward. It is based on the level of the fracture(s), from
proximal to distal; the bone(s) that are involved; the degree of
displacement, angulation, and rotation; the presence or absence of
overriding, segmentation, or comminution; and whether the fracture is
open or closed. The Orthopaedic Trauma Association and the
International Society for Fracture Repair system (125) classifies shaft fractures into 36 types; these are not clinically useful, although they are used in clinical investigation.
level of the coronoid process, extending to about midshaft, and that
occur in conjunction with dislocation of the radial head are known as
Monteggia fractures and have been discussed previously in this chapter (1,9,10,15,19,40,133,134,151,158).
characterized by rotational problems; these are discussed later in this
chapter.
present with varying configurations, depending on the mechanism by
which the injury was inflicted and the degree of violence involved (37).
The fractures are typically transverse or short oblique if they are
simple. Alternatively, in high-energy injuries, they may exhibit
extensive comminution or segmentation, with significant soft-tissue
involvement.
“nightstick fractures,” an obvious reference to the mechanism of
injury. Fractures of the radius with disruption of the distal
radioulnar joint are termed Galeazzi or Piedmont fractures (90,108,109,113).
with careful attention to the condition of the soft tissues. Often the
deformity of the forearm with fractures of one or both bones will be
evident immediately. Refrain from excessive manipulation to avoid
further damage to soft tissues. Note any evidence of neurologic or
vascular compromise. Check the patient periodically to confirm the
continuing integrity of circulatory and neurologic function.
planes of the entire forearm, including both the elbow and the wrist
joints. Fractures of the radius and ulna can be complicated by subtle
injuries to these joints. Radial–ulnar disassociation with rupture of
the central band of the intraosseous ligament can be difficult to
detect and is often diagnosed late (166,170).
Especially when fractures of the proximal radius and/or radial head and
neck show shortening, look for evidence of proximal subluxation of the
radius at the distal radioulnar joint. Comparison films to the opposite
normal side may be necessary. On clinical examination, more pain than
would be expected and tenderness at the distal radioulnar joint should
suggest this injury.
articulation with the distal humerus at the elbow and runs virtually
subcutaneously distally to the ulnar styloid at the wrist. The radius
is bowed along its length from the widened distal end, which
articulates with the carpus, to just distal to the bicipital tuberosity
(at the insertion of the biceps tendon). There, it angles at about 13°
opposite to the bow to articulate with the capitellum. The radius and
ulna form a joint at the distal end, where the strutlike radius sweeps
and rotates around the relatively fixed distal ulna with motions of
pronation and supination.
proper interosseous space must be maintained for normal motion to be
achieved. Schemitsch and Richards (153), as
discussed previously, showed that restoration of the radial bow is
related in a directly linear fashion to the quality of the outcome.
Normal rotation of the forearm requires a normal bow in the radius. The
normal maximum radial bow, measured by the distance between the radius
in the ulna across the interosseous membrane, is 15 mm. To get 80% of
normal range of motion, this bow must be within 1.5 mm. The same
relationship is related to grip strength. Both the amount of bow and
the location are important.
the importance of the interosseous ligament to the axial stability of
the distal radioulnar joint in cadavers. They showed that osteotomy of
the radius allows axial movement of the radius of 3 mm and division of
the triangular fibrocartilage increases the movement to 6 mm, whereas
division of the central band of the interosseous membrane produced more
than 10 mm of proximal migration of the radius. In mechanical tests of
cadaver intraosseous membranes, Wallace et al. (170)
demonstrated elongation of about 10 mm of the central band before
failure. In a preliminary study, they demonstrated that ultrasound may
be of value in detecting tears of the interosseous membrane.
when the elbow is in valgus alignment with contact of the radial head
against the capitellum, the main pathway for load transmission from the
hand to the elbow is through the radius. When the hand was loaded, with
the forearm in neutral rotation, the mean force in the proximal
ulnar–humeral joint was only 12%, whereas when the elbow was in varus
alignment and the forearm in neutral rotation, 93% of the force was
transferred via the interosseous membrane through the ulna. It is
evident that transfer of a load from the wrist through the radius and
ulna to the elbow is complex and
dependent
on the anatomy of the wrist, the soft-tissue attachments between the
radius and the ulna, and the position of the forearm. Radial–ulnar
load-sharing changes significantly when the distal end of the radius is
shortened by as little as 2 mm, emphasizing the need for anatomic
reduction.
different levels of the radius and ulna are associated with various
deformities after fixation. For example, a fracture in the proximal
third of the radius, occurring between the supinator insertion and the
pronator teres insertion, results in supination of the proximal
fragment and pronation and angulation of the distal radius. In addition
to supination, the biceps brachii tends to flex the proximal radial
segment. If the fracture occurs distal to the insertion of the pronator
teres, both fragments are likely to be found in neutral rotation (Fig. 16.28).
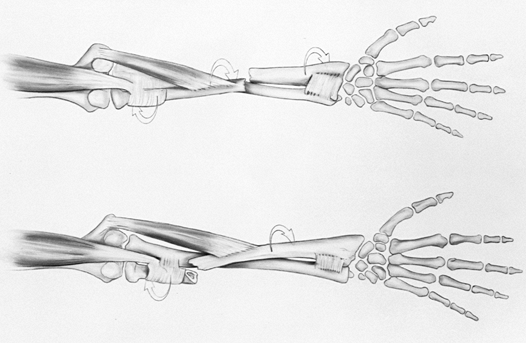 |
|
Figure 16.28. Rotational deformities vary according to the level of the radial fracture. (After Watson-Jones R. Fractures and Joint Injuries, vol. 2, 4th ed. Edinburgh: E & S Livingstone, 1955. In: Rockwood CA, Green DP, eds. Fractures, 2nd ed. Philadelphia: JB Lippincott, 1984:443, with permission.)
|
pronates the distal radial fragment. In the presence of an intact
radius, fractures of the ulnar shaft are comparatively less affected.
Muscles attaching to the proximal ulna are resisted by the stability of
the elbow joint. The radial head, of course, is not restricted by that
joint. Distally the ulna has little mobility and relatively weak muscle
attachments, so it is not often displaced.
essentially undisplaced and stable, may be successfully treated by
closed methods (14,33,35,36,149,150).
This requires meticulous attention to molding of the plaster to
maintain the necessary relationships among the radius, ulna, and
interosseous membrane. The fracture must be evaluated weekly to ensure
that no loss of position has occurred. The technique for closed
treatment is well outlined in an excellent book by Charnley (31). See Chapter 10
for additional information on application of upper extremity casts.
Isolated ulnar shaft fractures with less than 10° of angulation can be
treated with casts or braces (149,150).
287 isolated fractures of the ulnar shaft with prefabricated functional
braces resulting in a 99% union rate and good to excellent functional
results in 96% of the cases.
surgical treatment of displaced or unstable forearm fractures, assuming
that adequate alignment and proper rotation of the fragments can be
obtained and maintained by closed methods. Angulation in the plane of
joint motion is most likely to improve with growth and remodeling.
However, rotational deformities and loss of normal interosseous space
cannot be expected to improve with growth and remodeling, even in very
young patients. The cut-off ages are in the range of 10–12 years in
girls and 12–14 years in boys. At these ages, surgical treatment must
strongly be considered for displaced fractures of the forearm.
including isolated fractures of the ulna with angulation greater than
10°, Monteggia fracture–dislocations, and Galeazzi fractures.
debridement, administration of antibiotics, and stabilization of the
fracture by internal or external fixation (see Chapter 12).
Compartment syndrome, which must be treated by fasciotomy, also
warrants stabilization of fracture fragments by internal or external
fixation.
as practical, preferably before the onset of swelling. With delay,
fracture blisters secondary to swelling can develop. Ruptured fracture
blisters or abrasions that are more than 6–8 hours old may be
contraindications for surgery. At least 7–10 days may be required for
abraded skin and fracture blisters to heal and for swelling to subside.
that immediate fixation of open fractures of the forearm of all grades
can be done with minimal risk of infection if thorough irrigation and
debridement are done. Only 1 of 49 open fractures plated primarily
became infected, and that resolved with treatment.
forearm fractures after considering the level of the fracture
(especially important in radius injuries), the protection afforded any
neurovascular structures that may be encountered, and the best means of
providing efficient exposure with minimal soft-tissue damage. Open
wounds may require a modification of the “ideal” surgical approach.
-
Straight ulnar approach to the ulnar shaft
-
Volar antecubital approach to the proximal radius
-
Dorsolateral approach to the radial shaft, from the radial head to the distal quarter of the shaft
-
Palmar approach to the distal third of the radius
or partially turned toward the side contralateral to the fracture. In
this way, the arm can be pronated across the chest to facilitate access
by the surgeon and the assistant.
The dorsolateral approach provides an excellent extensile approach to
the radius from the elbow to the wrist. The key to the exposure is the
identification and meticulous reflection of the posterior interosseous
nerve along with the supinator muscle off the proximal third of the
radius. Exposure of the middle third presents no problems. The distal
third of the radius is usually approached volarly, but the dorsal
approach is useful as well.
established the principles used today in plate fixation of forearm
fractures. The most appropriate treatment for unstable fractures of the
bones of the forearm is open reduction and internal fixation using
plates and screws. Anderson et al. (5,6 and 7)
used the 4.5 mm, round-holed, narrow plate in their series and
established the efficiency of plate fixation of the forearm bones in
North America. These plates are rather large for the forearm and have a
22% incidence of refracture of the bone after plate removal. The 3.5 mm
AO-ASIF dynamic compression plate; its more recent version, the low
contact dynamic compression (LCDC) plate; and similar plates such as
the Alta reconstruction titanium plate are now the most commonly used
plates in adults and have made it possible to apply these techniques to
even small-boned patients such as adolescents and most women.
had a union rate of 98.5%, with 92% excellent or satisfactory
functional results, and no refractures after removal of the 3.5 mm
plates. Newer, equivalent-size titanium plates are now available that
are stronger yet more flexible than stainless-steel plates.
-
Place the patient supine on the operating
table. A hand table is useful. Prepare and drape the entire upper
extremity. Use a tourniquet. -
Expose the affected bone(s) using one of the surgical approaches described previously.
-
Expose the fracture site with as little
soft-tissue stripping as possible. Up to 2 mm of periosteal stripping,
limited to each side of the fracture site to ensure anatomic reduction,
should be sufficient. It is unnecessary to strip the periosteal sleeve
completely around the shaft of either the radius or ulna to apply a
plate. Expose the bone only directly under the plate (174).
Some surgeons advocate applying the plate extraperiosteally, but I see
no advantage in that. Be sure that no important structure, such as a
nerve, major vessel, or tendon, is trapped beneath the plate. -
If the fracture configuration results in
a stable reduction, reduce it and then fit the plate to the bone. If
the fracture is not stable due to a butterfly fragment, initial
interfragmentary fixation with a lag screw may be worthwhile if it can
be done easily without devascularizing the fragment. Otherwise, always
apply the plate to one fragment first with a snug (but not tight) screw
next to the fracture site. The second major fragment along with the
comminuted zone can be reduced to this plate–bone combination. This
greatly facilitates reduction and minimizes soft-tissue stripping. Use
only pointed tenaculum-type bone-holding forceps. These can be applied
without soft-tissue stripping and do not crush soft tissues. -
Contour the plate so that accurate bony
contact is made over its entire length. Use of the eccentric holes in
the dynamic compression plates has eliminated the need for an external
compressing device, but in some cases the external compressor is
helpful in reducing the fragments. After applying the plate to one of
the major fragments, if necessary use the articulated tension device to
distract the fragments by placing the hook end against the end of the
plate. This facilitates reduction. Then reverse the hook to apply
tension to the plate and to compress the fracture. Fix the plate to the
other major fragment using the appropriate screws (Fig. 16.29).![]() Figure 16.29.
Figure 16.29.
Use of the articulated tension device to assist in initial reduction
and for subsequent stabilization of comminuted fragments. (From Müeller
ME, Allgöwer M, Schneider R, Willenegger H. Manual of Internal Fixation, 2nd ed. New York: Springer-Verlag, 1979, with permission.) -
Comminution may make it impossible to
achieve perfect alignment and good screw fixation in the comminuted
area. Use “biologic” fixation techniques for reduction of the
comminuted fragments. Apply a minidistractor or external fixator with
pins inserted at sites remote from the comminuted or segmental portion.
Distract the fragments sufficiently to obtain good alignment. Then
bridge the comminuted or defect areas by attaching a well-contoured
plate over the intact extreme
P.513
ends
of the fracture. The plate acts as a strut over the comminuted area.
Graft the comminuted area with autologous cancellous bone for any bone
loss (Fig. 16.30).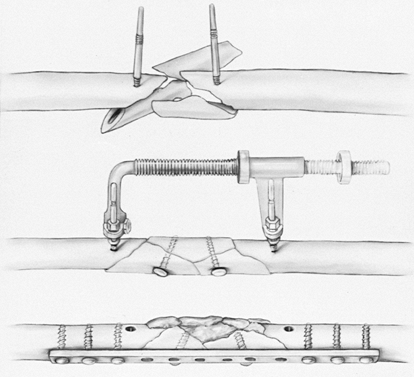 Figure 16.30.
Figure 16.30.
Use of the distractor to assist with reduction and preliminary
stabilization of comminuted fractures. (From Müeller ME, Allgöwer M,
Schneider R, Willenegger H. Manual of Internal Fixation, 2nd ed. New York: Springer-Verlag, 1979, with permission.) -
In general, use 3.5 mm dynamic compression plates with 3.5 mm cortical screws with 1.25-pitch threads (122).
This pitch allows the screws to grip the cortex of the bone with
additional threads, which improves the pullout strength. Also, these
screws are much stronger than the earlier designs of 3.5 mm cortical
screws. The 3.7 mm titanium reconstruction modular (Alta) plates as
well as the AO-LCDC and similar plates work well. The screws must
achieve good fixation in at least five cortices on each side of the
fracture. Increase this to six to eight cortices in unstable fractures
or in poor-quality bone. -
Lag screw fixation across the fracture
and any associated fragments increases the strength of the construct by
up to 40%. Usually these screws are applied before axial compression of
the fracture by the plate. The most accurate placement is achieved by
placing drill holes in the bone fragments before reduction, using
either a “threaded-hole first” or “gliding-hole first” technique. -
Ideally, place the plate on the tension
side of the bone, which is dorsolateral on the radius and dorsal on the
ulna. In the distal third of the radius, plates are best placed on the
volar aspect to avoid Lister’s tubercle and the extensor tendons.
Semitubular or one-third tubular plates are recommended only in unusual
circumstances, because they are quite weak. Examples of fixation are
shown in Figure 16.31, Figure 16.32, Figure 16.33 and Figure 16.34.![]() Figure 16.31. A closed Galeazzi fracture in a 25-year-old man. A: Preoperative AP and lateral radiographs. B:
Figure 16.31. A closed Galeazzi fracture in a 25-year-old man. A: Preoperative AP and lateral radiographs. B:
Postoperative radiographs show fixation with a six-hole, 3.5 mm dynamic
compression plate. Note the bicortical screws in the end holes and the
separate interfragmentary screw. (From Chapman MW, Gordon JE, Zissimos
AG. Compression Plate Fixation of Acute Fractures of the Diaphysis of
the Radius and Ulna. J Bone Joint Surg [Am] 1989;71:159, with permission.)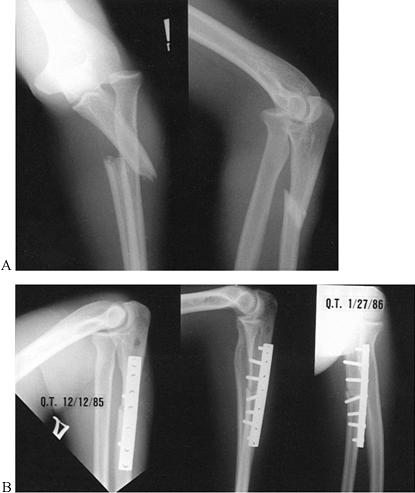 Figure 16.32. A closed Monteggia fracture in an 18-year-old man. A: Preoperative AP and lateral radiographs. B:
Figure 16.32. A closed Monteggia fracture in an 18-year-old man. A: Preoperative AP and lateral radiographs. B:
Postoperative radiographs show fixation with a seven-hole, 3.5 mm
dynamic compression plate, augmented by an olecranon bone graft. Note
the use of the center hole as the site for an interfragmentary screw.
(From Chapman MW, Gordon JE, Zissimos AG. Compression Plate Fixation of
Acute Fractures of the Diaphysis of the Radius and Ulna. J Bone Joint Surg [Am] 1989;71:159, with permission.)![]() Figure 16.33. A Gustilo type IIIA open fracture of the radius and ulna secondary to a high-velocity gunshot wound in a 32-year-old man. A: Preoperative AP and lateral radiographs. B:
Figure 16.33. A Gustilo type IIIA open fracture of the radius and ulna secondary to a high-velocity gunshot wound in a 32-year-old man. A: Preoperative AP and lateral radiographs. B:
Postoperative radiographs showing fixation with ten-hole, 3.5 mm
dynamic-compression plates on the radius and ulna. These were augmented
by a tricortical iliac-crest bone graft to the radius and cancellous
bone to the ulna at the time of delayed primary closure, 3 days after
injury. C: AP and lateral radiographs
showing union at 33 months. A normal range of motion was achieved.
(From Chapman MW, Gordon JE, Zissimos AG. Compression Plate Fixation of
Acute Fractures of the Diaphysis of the Radius and Ulna. J Bone Joint Surg [Am] 1989;71:159, with permission.)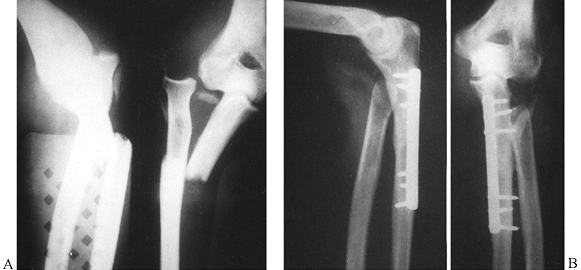 Figure 16.34. A: Monteggia type 1 fracture with associated fracture of the radial head. B:
Figure 16.34. A: Monteggia type 1 fracture with associated fracture of the radial head. B:
The ulna was treated with open reduction and internal fixation with a
compression plate; the radial head was excised. Today, some would make
more of an effort to fix the radial head fracture. -
Generally, bone grafting of fresh
fractures that have been accurately reduced and firmly fixed is
unnecessary. If unexpected comminution is encountered, or if you prefer
not to harvest a bone graft, collagen–calcium phosphate graft material
has been proven to be as efficacious as autologous cancellous bone (28).
It would appear that routine bone grafting of fractures that are well
reduced and have good bone contact is unnecessary, whereas fractures
with defects probably do best when the defects are filled with
autologous cancellous bone. Complete segmental defects require
structural
P.514P.515P.516
bone graft, such as tricortical graft from the iliac crest (Fig. 16.33), or, for larger defects, a vascularized fibular graft (55,84). -
After stabilization, close only the
subcutaneous layer and the skin. Use suction drains that are brought
out from the operative site through percutaneous stab incisions.
Drainage prevents the development of a postoperative hematoma. There is
no need to close the deep fascia; doing so may produce a compartment
syndrome.
performed autologous cancellous bone grafting at the time of delayed
primary closure of all open fractures of the forearm that were plated.
They also bone grafted those with comminution and bone deficiency. They
had no nonunions in 49 open fractures and an overall rate of union of
98.5% in 129 fractures. Wright et al. (177)
studied 198 forearm fractures treated with plate fixation with and
without bone grafting. Their union rate in those that were not grafted
was 98%. On that basis, they concluded that bone grafting of comminuted
forearm fractures was not necessary.
dressing with a palmar or dorsal splint for a few days postoperatively
while soft-tissue swelling decreases. Remove drains within 24 to 48
hours, depending on the amount of drainage. Keep the forearm elevated
10 cm above the heart. Begin active and passive range-of-motion
exercises of the fingers, wrist, and elbow shortly after surgery.
exercises of the hand, wrist, and elbow. Obtain radiographs just before
wound closure and then again 6 weeks and 3 months postoperatively, and
then every 4 to 6 weeks until union occurs. Allow protected use of the
limb throughout healing, but prohibit contact sports and the lifting of
heavy objects of more than 2 kg during this time. Union usually occurs
in 16–24 weeks, at which time a full rehabilitation program without
restrictions can begin.
laboratory study compared the stability of LCDC plates and fluted
intramedullary rods for the fixation of fractures of the radius and
ulna. They showed that the intact ulna is more important than the
radius to forearm stability in bending and torsion. Therefore, if the
radius is fractured but the ulna remains intact, medullary nailing will
produce constructs with greater stiffness, particularly in torsion,
than if the ulna is fractured and the radius is intact. In the case of
fractures of both bones, they found that nails resulted in
significantly less stiffness in torsion as well as in distraction and
compression as compared
to plates. Torsional stiffness with intramedullary nails was 2% of intact specimens, whereas with plates it was 83%.
popularized intramedullary nailing of forearm fractures in the late
1950s and the 1960s. Medullary nailing was attractive at the time
because closed treatment of displaced forearm fractures in adults was
not achieving acceptable levels of function and the principles of plate
fixation and the smaller stronger plates had not yet been popularized
by the AO group. In addition, the percutaneous techniques of
intramedullary nailing that avoided open incisions was appealing
because infection rates in open internal fixation at that time were
higher than they are now. As pointed out in the previous discussion on
the biomechanics of internal fixation, plates offer superior stability
to nail designs currently available. Nailing with smooth nonlocking
nails such as rush rods often requires additional immobilization in a
long-arm cast, which interferes with restoration of forearm motion.
Other problems of intramedullary nailing include the following:
-
Small intramedullary canals may preclude nailing or require the use of very small nails.
-
Medullary canals less than 3 mm in diameter contraindicate intramedullary nailing with most devices.
-
The double curve of the radius makes
nailing, especially with closed technique, difficult. Restoration of
the normal bow of the radius can be difficult. -
If open reduction is required to place
intramedullary nails, then plate fixation is preferable because
medullary nails offer less rigid fixation, and the combination of
occluding the medullary canal and stripping the periosteum
devascularizes the fracture to the point that the risk of nonunion is
higher. -
Distraction at the fracture site and loosening with nonlocking nails can lead to nonunion.
-
Combining intramedullary nailing, such as
for the ulna, with plate fixation of the radius results in a higher
incidence of nonunion in the ulna than when plate fixation is used for
both, because there is often persistent gapping at the fracture site of
the ulna.
and others offer improved fixation due to their proximal and distal
cross-locking capability. These designs (e.g., the Sage nail) often
have fluted cross sections, and this offers additional fixation. There
is little published on these newer nails, nor is a prospective
randomized study comparing plates to nails available; therefore, their
effectiveness remains unproven at present.
-
Poor skin conditions precluding open surgery at the fracture site
-
Diaphyseal fractures in patients with severe osteoporosis, where screws would not be expected to hold
-
An occasional segmental fracture not suitable for plate fixation
-
An occasional patient with multiple
injuries for whom intramedullary nailing can be done more quickly and
with less soft-tissue trauma, when access to the extremity is necessary
and cast mobilization is contraindicated
infection precluding surgery, an open growth plate, and a medullary
canal smaller than 3 mm in diameter.
His nail has a triangular cross section, which provides reasonable
rotational stability if good cortical contact is obtained. The ulnar
nail is relatively straight, but the nail for the radius is
specifically contoured to maintain the normal anatomic bow of the
radius, and it is designed to enter the radius on the dorsal lateral
aspect of the radial styloid. Newer locking nail systems have evolved
from the Sage nail. In the radius, the ForeSight nail (Smith and
Nephew, Memphis, TN) is similar to the Sage nail but is designed to
enter on the dorsum of the radius 5 mm proximal to the joint line, just
lateral to Lister’s tubercle. The True-Flex nail (Encore Orthopaedics,
Austin, TX) and the SST stainless steel nail (Biomet, Warsaw, IN) are
both designed to enter on the ulnar side of Lister’s tubercle on the
dorsum of the distal radius. The techniques for locking nails are
specific to each appliance. The reader is referred to the
manufacturer’s instructions.
nails is fairly generic to intramedullary nailing of the forearm.
Although nailing can be done using closed technique under fluoroscopic
control, Sage performed his nailings open and this is the technique
discussed here.
-
Be certain that a complete set of Sage
nails and insertion equipment is available. This consists of nails for
the radius and ulna, a combination driver and extractor, a 3 mm drill,
and two reamers that are slightly smaller than their respective nails.
Nails for the ulna are available in 4 and 5 mm diameters and three
lengths (22.9, 25.4, and 27.9 cm). Nails for the radius are available
in similar diameters and are available in 1.3 cm increments from 20.3
to 27.9 cm. -
Position the patient supine on the
operating table. For nailing of the ulna alone, it is convenient to
place the patient’s arm across his chest. For other nailings, place the
arm on a hand table. This description applies to nailing of both bones.
Nail the ulna first. -
Deliver the end of the proximal fragment
into the wound and insert an ulnar nail to test for fit. If too small,
enlarge it first with a 3.2 mm drill and then with a reamer. Drive the
reamer through the tip of the olecranon until the tip can be felt
beneath the skin. -
Select an ulnar nail of correct length and diameter.
-
With the elbow flexed to 90°, drive the
nail retrograde up the proximal fragment and through a small stab
incision, and bring it out the tip of the olecranon. -
Reduce the fracture anatomically and
drive the nail into the distal fragment. Do not seat the nail
completely until radiographs are taken and the radius is fixed. -
Expose the fracture of the radius through an appropriate incision.
-
Deliver the fractured ends into the wound and check for nail size in the medullary canal.
-
Reduce the fracture and lay a nail along
the radial border of the forearm to determine length. The nail should
extend from the styloid to within 1.3 cm of the radial head. -
Flex the wrist over a folded towel and deviate it ulnarward so that the radial styloid is accessible (Fig. 16.35).
Make a longitudinal incision 2.5 cm long over the radial styloid, and
carry it down to the bone at its proximal end but only through the skin
distally. Reflect the periosteum.![]() Figure 16.35. Medullary nailing of the radius. The nail enters the bone at the radial styloid. (Courtesy of Dr. F. P. Sage.)
Figure 16.35. Medullary nailing of the radius. The nail enters the bone at the radial styloid. (Courtesy of Dr. F. P. Sage.) -
Drill a hole with a 3.2 or 4.8 mm drill
through the exposed cortex, and then gradually angle the handle
distally until the drill is directed toward the lateral epicondyle of
the humerus. Advance the drill for 5 or 6 cm, thus producing an oval
hole at the point of insertion and a channel that nearly parallels the
medullary canal (Fig. 16.36). Figure 16.36.
Figure 16.36.
Sequence of insertion of the prebent Sage nail for the radius, steps 1
to 7. A hole is drilled in the distal radius, entering the medullary
canal from the radial styloid. The nail is bent as it advances in the
canal and finally springs back to its prebent shape, maintaining the
radial bow. (From Stewart MJ. Intramedullary Fixation of Forearm
Fractures. In: Larmon WA, ed. Instructional Course Lectures of the American Academy of Orthopaedic Surgeons, Vol. 15. Ann Arbor, MI: J. W. Edwards, 1958:50.) -
Insert the point of the nail with the
nail rotated so that its dorsal or long bow parallels the long arc of
the radius. With the wrist in flexion and ulnar deviation, insert the
nail by hand in a proximal direction as far as possible. If it cannot
be pushed in by hand for 6 cm, the angle of insertion is too acute.
Withdraw the nail, and drill the channel of insertion more obliquely.
Then thread the driver on the nail and, while the nondominant hand
exerts pressure to depress the nail toward the ulna, drive the nail in
with the dominant hand. If marked resistance is met, angle the nail
back and forth a few degrees, and then drive it in with moderate blows
until it reaches the fracture. If there is an undisplaced butterfly
fragment at the fracture, hold it with a clamp; reduce the fracture,
and drive the nail into the proximal fragment, leaving 1.3 cm exposed
at the radial styloid. -
Check the position of the fracture and
the position and length of the nail with AP and lateral radiographs of
the radius and ulna. If the nails are of correct length, finish seating
them (Fig. 16.37). Observe the fractures under
direct vision to be sure that no distraction occurs. As recommended by
Sage, place autogenous iliac bone grafts about all fractures of the
radius and ulna fixed by medullary nails (Fig. 16.38).![]() Figure 16.37. Displaced fracture of the radius treated with a prebent Sage nail. Note excellent maintenance of the radial bow.
Figure 16.37. Displaced fracture of the radius treated with a prebent Sage nail. Note excellent maintenance of the radial bow. Figure 16.38.
Figure 16.38.
Displaced both-bone fracture treated with Sage nails. Both fractures
are united at 2 years; nails have been removed. Note that there is some
loss of the normal radial bow, which is one of the problems that can
occur with nailing.
-
Avoid excessive stripping of bone
fragments, because nailing interferes with the revascularization of the
fracture through the endosteal canal, so preservation of the periosteum
and muscle attachments is important. To ensure union, bone graft all
fractures nailed with open technique. -
Do not drive nails into a tight medullary
canal, because comminution of the bone, penetration of the nail through
the bone, or incarceration may occur. -
Be certain that the nail at the styloid
is not near any of the wrist tendons, which could be abraded by running
over the end of the nail, leading to rupture.
and the forearm in neutral rotation. Continue the cast for 8–12 weeks,
at which time enough bridging callus is usually present. The use of
functional cast bracing may permit earlier mobilization of the wrist
and elbow if the nailing is stable. Locked nails do not usually require
cast mobilization. Gentle early active range of motion can be
encouraged in most cases.
modified titanium Street forearm nail, reported a 97.5% rate of union
and 92% good to excellent results in 50 patients with 75 fractures of
the forearm. DePedro et al. (42), using the
Lefèvre locking nail, experienced a 100% union rate in 20 patients
treated with isolated ulna or both-bone fractures.
Remember, if the radial fracture is displaced or shortened, the distal
radioulnar joint must be subluxed or dislocated. Failure to appreciate
the distal radioulnar joint injury may result in compromised functional
results and significant symptoms in the wrist, even though the fracture
has been internally fixed and has healed. Open reduction and internal
fixation of the radial fracture in anatomic position is required. The
distal radioulnar joint disruption can usually be treated by closed
means. After recognizing that the ligamentous structures and articular
disc have been ruptured, they are almost always adequately reduced if
the radial alignment and length are restored and rigidly internally
fixed, and if the forearm is kept in a fully supinated position for 6
weeks. Open reduction of the distal radioulnar joint is required only
if closed reduction in supination fails to achieve reduction. The
Galeazzi type of fracture–dislocation usually requires cast
immobilization for 6 weeks, with the forearm supinated for soft-tissue
healing and stabilization of the distal radioulnar joint.
the middle and distal thirds without distal radioulnar disruption has
been referred to as the “fracture of necessity,” or the Piedmont
fracture. The former term was coined to indicate that open reduction
and internal fixation are necessary to produce acceptable functional
results. The muscle forces acting on this fracture result in angulation
of the fracture toward the ulna, compromising the interosseous space
and resulting in loss of pronation and supination even if union is
complete.
difficult primarily because of the dangers to the deep branch of the
radial nerve when exposing and reducing the fracture (as discussed
previously).
involvement of the elbow or wrist joint are treatable with functional
braces. Sarmiento et al. (150) reported on the
outcomes in 287 patients. Union occurred in 99%, and overall good to
excellent functional outcomes occurred in 96% of their patients.
Fractures in the distal third did best, averaging a loss of 5° of
pronation, compared to an average loss of 12° of pronation in fractures
in the proximal third. Fractures of the ulna associated with disruption
of the proximal radial ulnar joint are Monteggia fractures (discussed
earlier). In adults, plate fixation of these fractures is nearly always
necessary (Fig. 16.34). Indications for plate fixation of fractures in the middle and distal third include the following:
-
Open fractures for which soft-tissue management requires stabilization of the bone in nearly anatomic position
-
Displaced both-bone fractures
-
Segmental fractures
-
Fractures for which satisfactory position cannot be obtained
-
An occasional patient, such as a dentist
or musician, who needs to return early to a highly skilled occupation
requiring fine use of the upper extremity; such a patient may require
immediate internal fixation.
injury resulting in fractures of the humerus and both bones of the
forearm. These high-energy injuries are often the result of direct
trauma with severe open fractures involving bone loss and accompanying
neurologic or vascular injury (84,178).
The principles of treatment do not differ much from those already
described for open fractures. In some cases, the magnitude of
soft-tissue injury and bone loss precludes effective internal fixation;
therefore, external fixation in two planes bridging the elbow joint
from the proximal humerus to the distal forearm is necessary. If the
level of wound contamination is not excessive and comminution is not
too severe, then primary plate fixation of the humerus and the forearm,
accompanied by local or free-flap coverage if necessary, usually gives
the best results, because this gives the best opportunity to restore
the anatomy of the forearm and permits early joint motion and
rehabilitation. Yokoyama et al. (178) reported on 15
floating elbow injuries, one of which led to immediate amputation. In
10 of these, immediate internal fixation was performed, and in three
the fractures were internally fixed on a delayed basis. In spite of
this severe injury, 10 of the 15 had a good or excellent results.
Complications were common, however, including one deep infection, two
nonunions of the humerus, two nonunions of the forearm, one malunion of
the humerus, and one forearm bone refracture.
discussion of open fractures. Except for fractures that are unsuitable
for plate fixation due to location or comminution, or those that are
extraordinarily contaminated, immediate plate fixation is usually
indicated (26,27,28,29 and 30,57,58,112). The next best alternative is external fixation (8,137). Fractures in the epiphyseal areas may be suitable for fixation (16,41). In 49 open fractures plated immediately, Chapman et al. (29) had only one infection (2%), and this resolved with treatment. Others (5,6 and 7,112) have shown similar results.
essential. Stable fixation with minimal soft-tissue dissection is also
required. Initially, leave the wounds open or use an antibiotic bead
pouch. Cover exposed bone and neurovascular structures by muscle.
Administer intravenous antibiotics. Delayed primary wound closure, flap
coverage if necessary, and bone grafting are usually indicated 5 days
or more after injury if the wound is free of evidence of infection.
excellent to good late results in 77 intraarticular fractures of the
distal humerus treated by open reduction and internal fixation. Nerve
injuries resulting from the fracture occurred in 26 patients, and 49%
had heterotopic bone formation; the results were less favorable in
patients with open fractures and those with multiple injuries. Helfet
and Schmeling (62) reported an incidence of
only 4% of heterotopic ossification, 4% infection, 7% ulnar nerve
palsy, 5% failure of fixation, and 2% nonunion. This rate of
complications is what would be expected with today’s modern techniques.
Neither of these authors mention postoperative stiffness, which in my
experience is the primary reason patients have poor outcomes (50,167).
extremity fractures is most commonly due to inadequate fixation, poor
patient cooperation, or poor-quality bone (17,67,73,106,136,159).
The nature and magnitude of the original injury, because of
comminution, may also lead to failure, but the devascularization
associated with comminution and severe soft-tissue injuries is more
likely to lead to infection or to failure of union. In the absence of
infection, the most common causes of failure in the reconstruction of
supracondylar fractures of the humerus are poor preoperative planning
and failure to follow the principles outlined in this chapter. This can
lead to the use of inadequate appliances, or to failure to achieve
sufficient stability in the fracture site that early rehabilitation can
be tolerated without failure. Fortunately, reconstruction of nonunions
and intraarticular malunions of the distal humerus is surprisingly
successful, with final functional arcs of motion averaging 100° (83,84,85,103) (see Chapter 27 for more details).
forearm are failure to choose a plate of adequate length; using plates
that are too small, such as one-third tubular plates; and failure to
eliminate micromotion in the fracture site. Interfragmentary
compression with screws should always be done, if feasible.
In fractures with bone loss or substantial comminution, or where there
has been significant soft-tissue stripping, I believe that bone
grafting is prudent. Collagraft (Zimmer, Warsaw, IN) (28)
has been shown in forearm fractures to be equal in efficacy to
autologous cancellous bone graft. This eliminates the morbidity of
taking an iliac crest graft. On the other hand, securing a small amount
of cancellous bone through a stab incision on top of the iliac crest
incurs minimal morbidity. Fortunately, with good technique, union rates
of 97% to 98% can be expected. Most nonunions respond well to repeat
plate fixation and bone grafting (see Chapter 27).
by the original injury, operative manipulation of the nerve, inadequate
release of the soft tissues around the nerve in a transfer, and
postoperative fibrosis around the nerve. The best approach is to avoid
any additional ulnar neuropathy by gentle neurolysis of the nerve and
well-executed anterior transfer. Ulnar nerve neurolysis and
transposition during reconstruction after failure of primary treatment
of elbow fractures has a reasonable chance of providing good pain
relief, improvement in sensation,
and even improvement in hand strength and dexterity (102).
elbow and may coexist with injury of the brachial artery. In fractures,
there may be direct contusion or stretching of the nerve over displaced
fragments, and in dislocations the nerve can slip around the medial
condyle and be stretched across the back of the trochlea. Trapping of
the nerve in the trochlear sulcus with compression is possible. In the
vast majority of cases, the injury is a neuropraxia, which responds to
reduction of the elbow and observation. The posterior interosseous
branch of the radial nerve is vulnerable in type I Monteggia fractures
because of anterior dislocation of the radial head (175).
The vast majority of these recover with nonoperative care. In closed
injuries, exploration of any of these nerves around the elbow and the
forearm is rarely indicated. If recovery is not seen by the time the
fracture heals, then nerve conduction and electromyographic and nerve
conduction studies are indicated to determine the nature of the lesion
and the need for surgical exploration.
distal humerus and with injuries around the elbow, any evidence of
impaired circulation, unless it returns to normal immediately after a
closed reduction, merits an arteriogram. With a disruption of the
brachial artery, the collateral supply about the elbow often prevents
loss of limb, but poor circulation can lead to long-term pain and
dysfunction. Obtain vascular surgery consultation if there is any
question of whether a brachial artery exploration and repair are
indicated.
dislocations but is more common after high-energy displaced fractures
of the distal humerus, elbow, and forearm. At the time of irrigation
and debridement of open fractures about the elbow and in the forearm,
it is usually fairly easy to do an open or semipercutaneous fasciotomy
of the deep fascia of the compartment in which you are operating. This
prophylactic procedure adds little morbidity and may prevent an
occasional compartment syndrome. After internal fixation of elbow and
forearm injuries, do not close the deep fascia, because closure can
precipitate a compartment syndrome. Postoperatively monitor patients
carefully for signs of compartment syndrome, measure intracompartmental
pressures when there is any question, and proceed with fasciotomy when
the diagnosis is made. In the forearm, fasciotomy usually requires
release from proximal to the lacertus fibrosis to the midpalm,
including release of the carpal tunnel (see Chapter 13).
actually unusual in the absence of associated predisposing causes such
as head injury, high-energy trauma, or thermal injury (88,99,138,165) (Fig. 16.39) (see Chapter 124).
Prophylactic treatment with diphosphonate, nonsteroidal
anti-inflammatory agents such as indomethacin, and low-dose radiation
therapy have been advocated and used with success in total hip
replacement and other disorders. I have no experience with using
prophylaxis for HO around the elbow. The treatment consists of
resection of the heterotopic bone after full maturation, usually in
combination with a soft-tissue release of the elbow to gain range of
motion (99) (see Chapter 27).
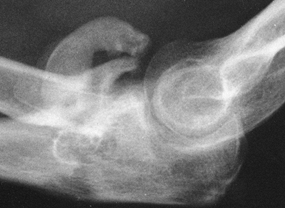 |
|
Figure 16.39.
Heterotopic ossification following prosthetic replacement of the radial head and tension band wire fixation of a fracture of the olecranon. |
fractures of both bones at the same level, particularly when plate
fixation of both bones is performed and the hematoma between the bones
communicates (88). Minimize the risk of
synostosis by limiting dissection so that the hematoma between the two
fractures does not communicate, by not placing bone graft in the
interosseous space, and by beginning early pronation and supination.
Maturation of a synostosis usually requires 12 or more months. Although
attempts to surgically remove a synostosis early may result in a
recurrence, Jupiter and Ring (88) showed
slightly improved results when resection was performed before 12 months
in 17 limbs treated by resection and interposition fat graft (see Chapter 27).
usually due to a fracture of the coronoid process with displacement,
severe comminution that precludes fixation, or failure of fixation
(discussed in more detail in Chapter 27). Use of a functional external fixator in unstable elbows may avoid this complication. Moritomo et al. (114)
have described successful reconstruction of the coronoid process for
chronic dislocation of the elbow using a graft from the olecranon in
two cases.
fractures, even in the presence of open fractures, is unusual, with
infection rates generally below 2% (29). When
acute infection occurs early after plate fixation and the plate is
stable, irrigation and debridement of the wound, combined with
administration of intravenous antibiotics, usually results in
resolution of infection. An antibiotic bead pouch may be helpful.
Low-grade persistent infection can often be managed by appropriate
antibiotic coverage until the fractures are healed. Plate removal at
that point usually results in resolution of the infection. Major
infections, particularly when there is loose fixation, usually require
removal of the implants and conversion to external fixation (see Chapter 135).
demonstrated that retention of diaphyseal plates, with a mean follow-up
of more than 8 years, results in no significant change in forearm bone
density and grip strength. Mih et al. (107)
compared 62 patients who had their forearm plates removed at an average
of 19 months after insertion, to 113 patients who retained their
forearm plates. The complication rate was much higher in those in whom
the plates were removed, including seven refractures through screw
holes or through the original fracture site. They recommended against
routine removal of forearm plates. Factors that predispose to
refracture are the degree of initial displacement and comminution, the
use of large screws (implants using screws larger than 3.5–3.7 mm in
diameter are rarely necessary), premature removal of the plate, and
lack of postremoval protection (29,67,142).
To avoid this complication, I use titanium plating systems with screws
3.7 mm in diameter or smaller, and I do not remove these plates unless
they are symptomatic or the patient desires removal. In some athletes,
such as football linemen, for whom there is substantial risk of trauma
to the forearm, removal may be indicated to avoid the stress riser at
the end of the plate, but avoidance of refracture may require skipping
a playing season.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
S, Fay GF, MacAusland WR Jr. Treatment of Olecranon Fractures;
Indications for Excision of the Olecranon Fragment and Repair of the
Triceps Tendon. J Trauma 1962;2:597.
O, Ekengren K, von Schreeb T. Fractures of the Head and Neck of the
Radius: A Clinical and Roentgenographic Study of 310 Cases. Acta Chir Scand 1957;112:115.
MW, Bucholz R, Cornell C. Treatment of Acute Fractures with a
Collagen-Calcium Phosphate Graft Material: A Randomized Clinical Trial.
J Bone Joint Surg [Am] 1997;79:495.
JM, Obletz BE. Comminuted Fractures of the Distal End of the Radius
Treated by Skeletal Transfixation in Plaster Cast: An End-Result Study
of 33 Cases. J Bone Joint Surg [Am] 1966;48:931.
HS, Cady GW. Treatment of Fractures of the Radius and Ulna with
Compression Plates: A Retrospective Study of 119 Fractures in 78
Patients. J Bone Joint Surg [Am] 1972;54:1167.
GS Sr, Edwards GS Jr, Jupiter JB. Radial Head Fractures with Acute
Distal Radioulnar Dislocation. Essex-Lopresti Revisited. Clin Orthop 1988;234:61.
HS, Sullivan FL, Urbaniak JR. Anterior Capsulotomy and Continuous
Passive Motion in Treatment of Post-Traumatic Contracture of the Elbow.
J Bone Joint Surg [Am] 1992;74:1229.
A, Norman A, Rosen H. Radial Head-Capitellum View in Elbow Trauma:
Clinical Application and Radiographic-Anatomic Correlation. Am J Radiol 1984;143:355.
RB, Mendoza RM, Williams DN. Problems in the Management of Type III
(Severe) Open Fractures: A New Classification of Type III Open
Fractures. J Bone Joint Surg 1984;24:742.
BJ, Clement DA, Rothwell PNR. Fractures of the Radial Head—The Benefit
of Aspiration: A Prospective Controlled Trial. Injury 1987;18:44.
DJ, Genley MB, Schemitsch EH, Tencer AF. A Biomechanical Comparison of
Two Methods of Fixation of Fractures of the Forearm. J Orthop Trauma 1995;9:198.
JB, Gerhard HJ, Guerrero J, et al. Treatment of Segmental Defects of
the Radius with Use of the Vascularized Osteoseptocutaneous Fibular
Autogenous Graft. J Bone Joint Surg [Am] 1997;79:542.
JB, Goodman LF. The Management of Complex Distal Nonunions in the
Elderly by Elbow Capsulotomy, Triple Plating, and Ulnar Nerve
Neurolysis. J Bone Joint Surg 1992;1:37.
R, Schmit-Neuerburg KP, Sturmer KM, Walz M. Intra-Articular Fractures
of the Distal Humerus. Surgical Treatment and Results. Clin Orthop 1989;241:238.
MD, Jupiter JB, Toh CL, et al. Reconstuction after Malunion and
Nonunion of Intraarticular Fractures of the Distal Humerus. J Bone Joint Surg Br 1994;76:614.
H, Tada K, Yoshida T, Kawatsu N. Reconstruction of the Coronoid for
Chronic Dislocation of the Elbow. Use of a Graft from the Olecranon in
Two Cases. J Bone Joint Surg Br 1998;80:490.
PT, Schein AJ, Siffert RS. Use of ASIF Compression Plates in Selected
Shaft Fractures of the Upper Extremity. A Preliminary Report. Clin Orthop 1970;71:208.
K, Boistman O, Tokkanen P. Treatment of Radial Head Fractures with
Absorbable Polyglycolide Pins: A Study on the Security of Fixation in
38 Cases. J Orthop Trauma 1994;8:94.
MJ, Williams JL, Marshall MP, et al. Biomechanical Comparison of
Fixation Methods in Transverse Olecranon Fractures: A Cadaveric Study. J Orthop Trauma 1997;11:565.
FP. Medullary Fixation of Fractures of the Forearm: A Study of the
Medullary Canal of the Radius and a Report of 50 Fractures of the
Radius Treated with a Prebent Triangular Nail. J Bone Joint Surg [Am] 1959;41:1489.
EH, Richards RR. The Effect of Malunions on Functional Outcome after
Plate Fixation of Fractures of Both Bones of the Forearm in Adults. J Bone Joint Surg [Am] 1992;74:1068.
RM, Hotchkiss RN, Slater RR Jr. The Use of Frozen-Allograft Radial Head
Replacement for Treatment of Established Symptomatic Proximal
Translation of the Radius: Preliminary Experience in Five Cases. J Hand Surg 1997;22A:269.
WA, Mast JW. Internal Fixation of Forearm Diaphyseal Fractures: Double
Plating versus Single Compression (Tension Band) Plating—A Comparative
Study. Orthop Clin North Am 1980;11:381.
JR, Hansen PE, Beissinger SF, Aitken MS. Correction of Post-Traumatic
Flexion Contracture of the Elbow by Anterior Capsulotomy. J Bone Joint Surg [Am] 1985;67:1160.
RR, Schmeling GJ, Schwab JP. The Necessity of Acute Bone Grafting in
Diaphyseal Forearm Fractures: A Retrospective Review. J Orthop Trauma 1997;11:288.