PRINCIPLES OF TREATMENT OF INFECTION AND ANTIMICROBIAL THERAPY
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Infection and Hemophilia > CHAPTER 132 – PRINCIPLES OF
TREATMENT OF INFECTION AND ANTIMICROBIAL THERAPY
Associate Professor of Orthopaedic Surgery, Hospital of the University
of Pennsylvania, Department of Orthopaedics, Philadelphia,
Pennsylvania, 19104.
infection, diagnostic options, and antimicrobial treatment. No attempt
is made to discuss the specific management of individual infectious
problems such as acute or chronic osteomyelitis, infectious arthritis,
or infected total joint arthroplasty, because these issues are covered
in Chapter 73, Chapter 133, Chapter 134, Chapter 135, Chapter 150, and Chapter 176. Nor does this chapter include information on universal precautions and protection of the health care worker.
musculoskeletal infection include an understanding of normal human skin
flora, bacterial virulence factors, antibiotic resistance, nonspecific
and specific immunity factors, and immune deficiency states. Also
important are the impact of surgical technique, skin preparation, and
antibiotic prophylaxis.
including erythrocyte sedimentation rate (ESR), C-reactive protein
(CRP), and the newer polymerase chain reactions (PCR). Roentgenographic
imaging tools include plain x-ray studies, tomograms, computer-assisted
tomography (CT scans), magnetic resonance imaging (MRI), and nuclear
medicine studies (99m Tc bone scans, gallium- and indium-labeled white
blood cell scans, and IgG). Culture, antibiotic susceptibility testing,
and biopsy are also discussed.
complication of an orthopaedic surgical procedure. Because most
procedures are considered “clean,” the expected incidence of infection
is approximately 2%. The most common offending pathogens in these types
of infection are Staphylococcus aureus and Staphylococcus epidermidis.
Because of the morbidity associated with these infections, the benefits
of routinely using prophylactic antibiotics are believed to outweigh
the risks to the patient. These risks can be reduced by obtaining a
detailed history of adverse reactions to medication. Prophylaxis may be
further enhanced with a good understanding of the antibiotic
sensitivities of the organisms in a given institution (24).
tissues, and it is usually due to streptococcus, although a significant
number of cases are caused by S. aureus.
Often, the patient has experienced a minor trauma, or an insect sting
or bite. This event may have allowed bacteria to gain entry into the
subcutaneous tissues. The findings of tenderness, erythema, and
lymphangitis or lymphadenopathy will allow you to make the diagnosis.
Most cases of uncomplicated cellulitis can be simply treated with oral
antibiotics. Admit patients to the hospital whose cellulitis is
complicated by immunodeficiency, high fever, or systemic toxicity for
administration of intravenous (IV) antibiotics and observation.
involves the soft-tissue fold that surrounds the fingernail. It is
commonly caused by the introduction of S. aureus
into this paronychial tissue by a hangnail, manicure instrument, or
tooth. Early use of warm soaks and oral antibiotics, as well as resting
of the involved digit can halt the infection. As the condition
progresses, there may be a need for incision and drainage of the
abscess, followed by parenteral or oral antibiotics, depending on the
extent of the infection.
a finger or thumb. Numerous fibrous septae tether the skin to the bone
and compartmentalize the pulp space; thus, a felon is composed of a
series of small closed-space infections, which must be individually
incised and drained. Pain and swelling in the finger develop quickly as
the abscess enlarges. The expanding felon can break down the septae and
extend proximally up the finger, in some cases producing osteitis or
osteomyelitis. Treatment involves incision and drainage as well as
systemic antibiotic therapy (see Chapter 73).
emergency room. The most common infective organisms are normal flora of
the mouth such as Streptococcus viridans, Bacteroides, S. epidermidis, S. aureus, and Peptostreptococcus.
Irrigate and debride bite wounds adequately, and leave them open.
Direct antibiotic therapy at the most common infecting organisms.
Treatment of dog bites may be complicated by the threat of rabies. All
efforts should be made to discover the rabies status of the dog.
Prophylactic antirabies treatment must be considered.
the wounds are more difficult to drain adequately. It is estimated that
more than 80% of cat-bite wounds become infected compared with 5% of
dog bites. In cat bites, the common infecting organisms include P. multocida and S. aureus (Table 132.1).
 |
|
Table 132.1. Antimicrobial Therapy for Bite Wounds
|
but may also be the result of enteric gram-negative rod infections.
Clostridial spores germinate in the anaerobic environment of necrotic
tissue. C. perfringens ferments
carbohydrates and produces large amounts of carbon dioxide. The gas
causes high pressure locally that, in turn, limits blood flow to the
area and further enhances the anaerobic environment. The presentation
includes progressive pain and edema of the involved extremity with a
foul-smelling serosanguineous discharge. Plain-film radiographs or CT
scan may show gas in the soft tissues. Treatment includes widespread
irrigation and debridement, high-dose administration of antibiotics,
and hyperbaric oxygen.
with high mortality (40%) and morbidity rates. Most cases of
necrotizing fasciitis follow minor trauma or recent surgery, with the
highest incidence seen among patients with small-vessel diseases such
as diabetes mellitus. The clinical manifestations include extensive
dissection and necrosis of the superficial and often deep fascia. The
infection undermines adjacent tissue and leads to marked systemic
toxicity. Thrombosis of the subcutaneous blood vessels leads to
necrosis of the overlying skin. Numbness or analgesia replaces the
initial local pain as the infection involves the cutaneous nerves. It
is most commonly associated with Group A streptococcus but may also be
the result of infection by staphylococcus, or gram-negative enteric
bacteria. With careful use of bacteriologic techniques, anaerobes, such
as Peptostreptococcus, Bacteroides, and Fusobacterium
sp, can be isolated in 50% to 60% of cases. It is an emergency
situation, and treatment involves wide surgical debridement of the
infected area and administration of IV antibiotics.
neuroparalytic disease that is caused by the exotoxin of the
gram-positive anaerobe Clostridium tetani.
Treatment involves proper wound care and prophylactic treatment with
formalin-inactivated tetanus toxoid. Immunization for tetanus has a
well-established schedule, and indications for reimmunization can be
easily determined on the basis of the wound sustained and the patient’s
immunization history. Patients with no previous history of immunization
or very severe wounds may benefit from the administration of tetanus
immune globulin (Table 132.2).
 |
|
Table 132.2. Tetanus Immunization Recommendations
|
because of the prevalence of vascular insufficiency, neuropathy, and
immune deficiency. Cultures of these infections generally reveal them
to be polymicrobial in nature.
are commonly associated with Pseudomonas. Pseudomonas infection is
serious and requires aggressive debridement and antibiotic therapy.
its progression, and ultimate response to intervention in a given
patient. They can be separated into factors relating to the infecting
organism, the patient, the surgeon, and the choice of optimal therapy.
cause infection and disease. One of the most commonly neglected yet
important concepts to understand is the fact that a diverse flora is
associated with the normal skin, mucous membranes, and the respiratory,
urogenital, and gastrointestinal mucosa from birth until death. The
human
body harbors numerous bacteria, with the composition of this flora
remaining relatively stable within body regions during a person’s
lifetime. In some instances, the flora may aid the host by competing
for microenvironments with other pathogenic bacteria, but in other
circumstances, if given the opportunity, these bacteria may be the
actual cause of disease. The main influences determining the
composition of the natural flora of the human body are pH, temperature,
redox potential, and oxygen, water, and nutrient levels.
orthopaedic surgical infections are skin flora. The variety of
microenvironments provided by skin allows for the wide diversity of
dermal flora. Different bacteria characterize the following four
regions: (1) the axilla, perineum, and toe webs; (2) hand, face, and trunk; (3) upper arms and legs; and (4)
fingernails and toenails. Sites with more potential for occlusion, such
as the axilla or perineum, harbor more bacteria than open areas like
the arm or trunk. It is believed that the increased moisture,
temperature, and skin surface lipids allow for the higher numbers of
organisms. These more occluded regions may also have higher levels of
gram-negative bacteria.
is found most abundantly in the nasal cavity and perineum, with levels
ranging from 10% to 40%. Its prevalence rises to 67% on vulvar skin. In
those individuals with dermatologic conditions, such as psoriasis or
atopic dermatitis, the prevalence may be more than 80%.
Propionibacteria are common in areas rich in sebaceous glands and are
associated with trichomycosis axillaris and acne vulgaris. Propionibacterium acnes
is the most common offending species. Other bacteria seen less commonly
on the skin include streptococci, micrococci, and gram-negative rods.
in addition to bacteria, various species of fungi can also be isolated,
including Aspergillus, Penicillium, Cladosporium, and Mucor. The conjunctival and respiratory mucosal flora when cultured can show Corynebacteria, Neisseriae, Moraxellae, Streptococcus, and Staphylococcus. The primary organisms of the oral flora are Streptococcus viridans
and β-hemolytic streptococcal species that are often involved in
periodontal disease and dental caries. Studies have shown that a
transient bacteremia of these species occurs during dental procedures
and up to 25% of the time after brushing one’s teeth. This bacteremia
may be of concern in those patients with implanted prosthetic devices,
which may become seeded during these events. The bowel and bladder also
acts as reservoirs for a wide variety of disease-causing gram-negative
bacilli, enterococci, anaerobes, and the gram-positive rod C. perfringens, which may be involved in gas gangrene of the extremities (24).
microorganism, is affected by such factors as the number of infecting
organisms, route of entry into the body, and virulence factors that
bacteria have evolved to invade, cause disease, and evade the host’s
defenses. Virulence can be measured experimentally by determining the
dosage of bacteria required to cause death or disease in a given
subject. These calculations refer to the inoculum required to cause
death or symptoms in at least 50% of the population, the so-called
lethal dose (LD50) or effective dose (ED50) respectively.
species or may be found in a broad range of organisms. One such factor
is the presence of pili or fimbria that allow bacterial adherence to
host cells or aid the bacteria in escaping host defenses. The pili of Streptococcus pyogenes and Escherichia coli allow the organisms to attach to the mucosa, whereas the pili of Neisseria gonorrhoeae also function as an inhibitor of phagocytosis.
bacterial protective mechanism. These capsules act to interfere with
opsonization by immunoglobulins, phagocytosis, and intracellular
killing of the bacteria. There are numerous bacteria that produce such
capsules including Streptococcus pneumoniae, E. coli, Bacteroides, Salmonella, and Haemophilus influenzae. Protein A, expressed by S. aureus, is antiphagocytic and binds to the Fc portion of IgG, preventing complement binding and opsonization. Protein M of S. pyogenes inhibits phagocytosis.
organisms. It is a naturally occurring bacterial outer-cell membrane
component that has systemic effects on the host, causing a generalized
sepsis that can progress to shock. Exotoxins are specifically produced
for release by bacteria, generally exerting their effects over a
limited site of action or on particular cell types or receptors. There
are multiple classes of exotoxins, including neurotoxins, cytotoxins,
and enterotoxins.
that affects only the inhibitory neurons in the central nervous system
(CNS). The result is unopposed firing of motor neurons, leading to
violent muscle contractions and a spastic paralysis known as tetanus. S. aureus
produces a leukocidin that destroys polymorphonuclear cells as well as
an α-toxin that functions to cause lysis of monocytes and platelets,
resulting in the formation of pus and abscesses.
Pseudomonas aeruginosa secretes elastase, collagenase, and lecithinase, as well as an exoprotease that cleaves and inactivates immunoglobulins. S. pyogenes produces the clot-lysing enzyme streptokinase, as well as the enzymes streptolysin O and S. IgA protease, produced by S. pneumoniae, N. gonorrhoeae, and H. influenzae, destroys the secretory immunoglobulins.
of prosthetic joint infections involves adhesion of the organism to the
biomaterial. The implant elicits a host response that results in the
coating of the prosthetic material and potential pathogen in a layer of
proteins including fibronectin, vitronectin, collagen, and fibrinogen.
This “extracellular slime” acts to bind the organism and foreign body
together. The adhesion of S. aureus to
bioprosthetic materials is also mediated by adhesin molecules belonging
to the microbial surface components recognizing adhesive matrix
molecules (MSCRAMM) family of microbial cell surface proteins. The
organism further encases itself in glycocalyx that probably includes
glycerol techoic acid. This extracellular slime isolates bacteria from
the immune surveillance of the host. Furthermore, the glycocalyx
stimulates monocytes to produce prostaglandin E2, which acts
to inhibit T lymphocyte proliferation, B-lymphocyte blastogenesis, and
immunoglobulin production. It interferes with white cell chemotaxis and
degranulation, as well as immunoglobulin opsonization, and inhibits
antimicrobial therapy from locally reaching effective levels to exert
their actions.
infection is the presence of antibiotic resistance. The issue of
resistance is an ever-growing problem in medicine and is discussed in
detail as it relates to each specific antibiotic.
onslaught of invading organisms and keeping the body free from
infection. This process involves the two distinct arms of the immune
system, innate or nonspecific immunity and acquired or specific
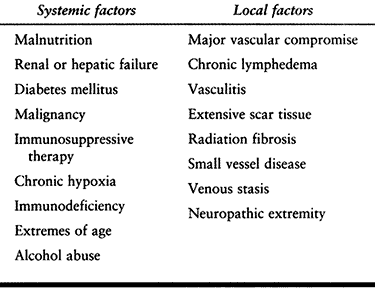
immunity (Table 132.3).
 |
|
Table 132.3. Systemic and Local Factors That Affect Host Response to Infection
|
physiologic barriers of the human body, as well as the inflammatory
reactions that begin once these barriers are compromised. This
nonspecific immune system protects from all invasion nondiscriminately.
physical barrier of the skin and mucous membranes. These surfaces
provide an effective barrier to the entry of most microorganisms. In
addition, the sebaceous glands, associated with the hair follicles,
produce sebum, lactic and fatty acids, maintaining the skin pH between
3 and 5, inhibiting the growth of most bacteria. Even small breaks in
the skin resulting from wounds or abrasions are potential routes for
infection. Biting insects harboring pathogenic organisms may also
introduce these pathogens subcutaneously or systemically as they feed.
This is the mechanism of spread for such diseases as malaria, bubonic
plague, and Lyme disease.
the complement system. Lysozyme enzymatically cleaves bacterial cell
wall peptidoglycan and is capable of killing invading bacteria.
Interferon exerts systemic effects that generally induce an antiviral
state throughout the entire body. Complement is a group of serum
proteins circulating in an inactive proenzyme state. Once activated,
the cascade amplifies a nonspecific immune reaction that ultimately
destroys the invading organism.
antigen-recognizing cells of the immune system that are on constant
surveillance. Once confronted by any foreign particle or organism,
these cells, best characterized by macrophages, internalize these
particles and degrade them through lysozymal enzymes into
macromolecular pieces. These pieces can then be presented to
lymphocytes to begin the process of developing acquired immunity.
is the inflammatory response to local tissue damage. The four cardinal
signs of inflammation—rubor,
tumor, calor, and dolor—have classically described this response. The events taking place during inflammation are (1) local tissue damage, (2) vasodilation, (3) increased capillary permeability, and (4)
an influx of phagocytic cells. These responses are mediated by the
release of local inflammatory mediators such as histamine, kinins,
prostaglandins, leukotrienes, and chemotactic factors.
cell-mediated responses. Unlike innate immunity, the acquired immune
system exhibits specificity, diversity, memory, and self and non-self
recognition. A discussion of the mechanism of this specific immunity is
beyond the scope of this chapter; it is important, however, to
understand the factors that may affect the ability of the body to mount
an adequate immune response.
functional reticuloendothelial and polymorphonuclear cells, as well as
competent B and T lymphocytes. Patients with deficiencies in any of
these cell lines are particularly vulnerable to infection. Deficiencies
may be present in patients with neoplasia involving the bone marrow,
those receiving certain medications (including patients taking
corticosteroids or immunosuppresives in the case of transplant or
rheumatoid arthritis), and patients with systemic illnesses such as
sickle cell disease. Tannenbaum et al. (50)
followed the clinical progress of 19 patients who had undergone both
transplant and arthroplastic procedures. Their findings suggested that
patients who had undergone orthopaedic procedures before transplant
surgery had the same rate of infection as the general population.
Patients who underwent orthopaedic procedures following
transplantation, however, were at a significantly higher risk of
developing infection in the prosthetic joint. Their study concluded
that this increased risk was most evident in those patients whose
immunosuppressive protocol included the use of cyclosporine A (50).
infection continues to spread in the United States and throughout the
world. The virus has a trophism for CD4, or so-called “helper” T
lymphocytes, infecting and ultimately destroying them. The CD4
lymphocytes play a crucial role in orchestrating the activation and
actions of both humoral and cell-mediated immunity. When patients have
lost significant activity of their CD4 cells, they are no longer
immunocompetent and are given the diagnosis of acquired
immunodeficiency syndrome (AIDS). These patients are at risk of
developing opportunistic infections as well as infections seen in
healthy individuals. Corticosteroid use, lymphomas, nutritional
deficiencies, obesity, uremia, and widespread radiation therapy, as
well as the other factors, may all be causes for immunocompromise in
patients. In the case of patients with longstanding diabetes, vascular
compromise along with neuropathy can allow small wounds in the distal
lower extremities, in the absence of proper care and adequate
inflammatory and immune responses, to become limb-threatening
infections.
cytotoxic medication, and those with hematologic malignancies may
develop neutropenia, with absolute neutrophil counts below 500
cells/ml. At this point, such patients are vulnerable to infections
from S. aureus and gram-negative
enterobacteria, as well as certain fungi. These patients may not have
high fever or appear toxic because of their lack of immune response.
The index of suspicion should be very high in such patients, and a
fever of 38°C (100.5°F) or higher should warrant a thorough evaluation.
humoral or complement systems are vulnerable to infection as well,
particularly with encapsulated organisms such as N. meningitidis, H. influenzae, and S. pneumoniae.
The same holds true for patients who have undergone splenectomy.
Nonfunctional complement also predisposes for infections from S. aureus and gram-negative enterobacteriae.
cell-mediated immunity, serum complement levels, and neutrophil
chemotaxis and bactericidal activity. The basal metabolic requirements
of patients who have undergone major mechanical trauma or severe burns
may rise to 200% of normal levels.
evaluation is the history and physical examination. Measure weight and
height, assess caloric intake, and evaluate medical conditions that may
affect nutritional status. On physical examination, look for evidence
of weight loss, loss of subcutaneous fat, muscle wasting, and the
presence of sacral or tibial edema or ascites. Anthropomorphic
measurements such as skin-fold thickness and muscle circumference are
often not useful. Clinical palpation of the triceps muscle, however,
can often provide an excellent estimate of nutrition, because extensors
tend to lose muscle mass faster than flexors.
nutritional evaluation. Measurement of visceral proteins, renal and
liver function, serum electrolytes and minerals, and hematologic
evaluation, as well as delayed cutaneous hypersensitivity testing, may
all play a role in developing the overall nutritional assessment of a
patient. Malnourishment has been defined in the past by laboratory
values of serum albumin levels below 3 to 4 mg/dl, serum transferrin
below 150 mg/dl, and total lymphocyte counts below 1500 cells/mm3. Additionally, the absence of significant immune reaction to skin testing may indicate a malnourished state.
the time of surgery has been associated with prosthetic joint
infections. Patients with such infections may continue to have episodes
of transient bacteremia, seeding the entire body with bacteria, and
increasing their risk for developing infection. Screen patients
thoroughly for the presence of pulmonary, genitourinary, skin, and
dental infections preoperatively. Treat and eliminate the infection
before proceeding with any orthopaedic procedures.
a patient play a tremendous role in the development and subsequent
progression of infection. When treating a patient with an open wound
after trauma or infection, be certain to irrigate the wound adequately,
removing the greatest load of bacteria and foreign debris possible. At
the same time, debride the wound well, and remove all nonviable tissue,
making certain that the remaining tissue has an adequate blood supply.
In the case of open fractures, the use of prophylactic antibiotics with
early irrigation and debridement can minimize the risk of an infection
that may jeopardize healing. The length of surgery may also influence
the incidence of infection. In addition, orthopaedic techniques, such
as reaming, can disrupt local blood supply and alter host–cell, humoral
factor, and antibiotic penetration to the site.
polymethylmethacrylate cement (PMMA) can contribute to the potential
for infection. PMMA significantly increases the likelihood for
infections with S. epidermidis and S. aureus. In vitro
studies have shown the toxicity of PMMA monomer on the bactericidal
serum factors, terminal complement components, phagocytosis, lymphocyte
function, and intracellular killing by polymorphonuclear cells. PMMA
monomers may reach toxic concentrations surrounding implanted
prostheses. When closing the wound, it is important to eliminate dead
space, have satisfactory drainage of hematomas, and provide adequate
soft-tissue coverage.
the incidence of infection, because infection may occur whenever the
protective barrier of the skin is broken. Proper preparation of the
skin before incision can decrease the risk of contamination
significantly (7).
remove the soil and transient flora found on skin, to reduce resident
microbial counts to subpathogenic levels in a short period of time with
the least amount of tissue irritation, and to inhibit the rapid rebound
growth of microorganisms. Although the normal flora of the skin and
hair can never be eradicated, the total number of organisms can be
markedly reduced through the use of agents such as iodine iodophors,
alcohol, hexachlorophene, and chlorhexidine. These preparation agents
have excellent activity against gram-positive bacteria and good
activity against gram-negative bacteria and fungi, reducing microbial
activity 100-fold within minutes. It must be kept in mind that the
sebaceous glands and hair follicles of normal skin where bacteria
reside and multiply can never be sterilized because of the poor
penetration of these agents.
resident flora that may be attached or absorbed into the epidermal
layers. Studies have measured that approximately 20% of resident skin
flora is not removed by standard preparation techniques. A recent study
comparing clean and sterile prep kits showed no difference between the
efficacy of either kit, a fact that has significant financial
implications for medical centers. The cost of prep kits assembled in
the hospital is significantly less than that of disposable kits
provided by vendors. When necessary, perform hair removal in the
operating room. Studies have shown that shaving of the operative site
the night before surgery can produce conditions optimal for the
reproduction of bacteria, increasing the risk of infection (43).
the operating room environment remain a source for contamination.
Bacteria may enter the wound directly or indirectly through gloves or
instruments that may become contaminated during skin preparation and
patient draping. These bacteria are believed to be mostly gram-positive
and shed by personnel in the room, particularly during periods of
increased activity. Conventional operating rooms may have as many as 10
to 15 bacteria per cubic foot. Lidwell reported the development of
infection following 1.5% of total hip arthroplasties performed in
conventional operating rooms and only 0.6% of procedures performed in
ultra-clean air operating rooms. Sir John Charnley advocated the use of
rapid air-change systems, along with multiple instrument trays in
orthopaedic operating rooms. Studies have found the number of airborne
bacteria can be reduced by approximately 50% in such ultra-clean air
rooms, and perhaps again by 50% with the use of body exhaust suits.
Other studies have shown no benefits to the use of ultra-clean air, and
one series examining the use of horizontal laminar flow actually
revealed an increase in the rate of infection in total knee
arthroplasty (6,41,45,51,56).
related to airborne bacterial levels. Standards have been recommended
for ultra-clean air operating rooms: fewer than 10 colony-forming units
per cubic meter (CFU/m3) within 30 cm of the wound and 20 CFU/m3 at the level of the operating table within the remaining clean-air enclosure (6,45).
bacterial counts were found to be 4.4 times higher during prepping and
draping of the extremity with an unscrubbed, ungowned leg holder, and
2.4 times higher with a scrubbed and gowned leg holder as compared with
intraoperative levels. Another study examined the levels of bacterial
contamination in two layers of latex gloves during operative
procedures. It was found that the outer glove used exclusively for
draping is the most significantly contaminated, and that changing this
outer glove at appropriate times during surgery greatly minimizes the
rates of contamination (34).
The use of ultraviolet light in operating rooms has also been shown to
decrease the incidence of wound infection by reducing airborne bacteria.
is another important decision for the treating surgeon. The incidence
of infection in clean surgery should be less than 2%. For clean surgery
that involves the implantation of foreign material, grafts,
polymethylmethacrylate cement, or prosthetic devices, prophylaxis is
well accepted and justified, because this practice provides benefits
that outweigh the expected risks. Prophylactic antibiotics should also
be used in cases of major devascularization, impaired host defenses, or
suspected wound contamination.
should be bactericidal, have low toxicity and good tissue penetration,
be low in cost, and effective against the most commonly suspected
infecting agents. S. aureus and S. epidermidis
are the most common pathogens involved in at least half of prosthetic
joint infections and 70% to 90% of wound infections in clean surgery.
Gram-negative bacilli are involved to a much lesser extent.
First-generation cephalosporins have been favored in this country for a
variety of reasons. They are nontoxic, inexpensive, and effective
against the potential infective organisms. The administration of
antimicrobial agents for a short duration (24 to 48 hours)
postoperatively is effective in preventing infection (44).
entering the operating room has been shown to be effective in this
capacity. Ideally, the infusion should be completed 30 minutes before
surgery to ensure adequate antibiotic levels at the time of skin
incision. An additional 1-g dose should be administered if the duration
of surgery is longer than 4 hours. Cefazolin 500 mg every 8 hours can
be continued for 24 hours postoperatively.
or coagulase-negative staphylococcus, vancomycin alone or in
combination with another agent, such as gentamicin, has been shown to
be effective. Patients with a history of severe allergic reaction
(urticaria, angioedema, anaphylaxis) to a penicillin or cephalosporin
should receive 1 g of vancomycin 2 hours before incision and every 12
hours thereafter for 24 hours. Recent studies investigating
prophylactic use of the glycopeptide antibiotic teicoplanin against
methicillin-resistant staphylococcus species in patients unable to
tolerate vancomycin have shown it to be as efficacious as any other
regimen (31,37,44,57,59).
cemented arthroplasty as well as antibiotic-impregnated polymethyl
methacrylate beads has been shown to be effective in reducing the risk
of infection. Josefsson et al. (22) followed a
prospective randomized group of 1688 consecutive total hip
arthroplasties comparing the use of gentamicin-impregnated bone cement
with systemic antibiotics. They found a decreased risk of deep
infection in those patients with antibiotic-impregnated cement, but
this effect was limited to the first year after operation.
retrospectively looked at 10,905 total hip arthroplasties performed in
Norway from 1987 to 1995. They compared patients who had received no
prophylactic antibiotics, systemic antibiotic prophylaxis only, cement
antibiotic prophylaxis only, and both systemic and cement antibiotic
prophylaxis. The lowest rate of revision arthroplasty was found among
those patients who had received both systemic and cement antibiotic
prophylaxis. Those receiving only systemic antibiotic prophylaxis had a
revision rate 4.3 times higher, those with only antibiotic bone cement
6.3 times higher, and those with no prophylaxis having a rate of
revision surgery 11.3 times higher (6,8,12,19,51,52).
These beads have been found to be cheaper than systemic antibiotics yet
provide the same efficacy. They are able to keep local antibiotic
concentration at levels that would be toxic systemically (29). Keating et al. (23) showed a decrease from 16% to 4% in infection rates among open tibia fractures. Cho et al. (10)
followed 54 patients with chronic osteomyelitis of the long bones
treated with local antibiotics and bone grafting an average of 4 years,
finding that 55% of these patients were completely free of infection.
patients who undergo dental procedures is controversial. A recent
survey has shown that a majority of orthopaedists (n = 44) and dentists
(n = 36) believe that patients with prosthetic joints should receive
prophylactic antibiotics before dental procedures (81% and 66%,
respectively) (47). Three approaches have been outlined in the literature.
antibiotic use based on the cost, the adverse effects from antibiotic
agents, the potential for the emergence of antibiotic resistance, and
the lack of a clear cause-and-effect relationship. Others suggest
routine prophylaxis before dental and other procedures known to cause
transient bacteremia for at least 2 years after arthroplasty. Yet
others
recommend the use of prophylaxis only in those patients with conditions that predispose for infection (27,53).
on 1,000 patients who had undergone 1,112 joint replacements and were
followed an average of 6 years. Their arthroplastic procedures were
performed between 1966 and 1980. The patients in this series were never
advised to take antibiotic prophylaxis before dental or surgical
procedures. Of these 1,000 patients, only three were found to have
developed prosthetic joint infections, and two of these patients
suffered from rheumatoid arthritis. Waldman et al. (54)
retrospectively looked at 3,490 patients who had had total knee
arthroplasty between 1982 and 1993. Of these patients, 62 developed
late infections of the prosthetic joint. Of these cases, seven were
strongly associated with prior dental procedures, representing 11% of
these late infections. The study concluded that the majority of
infections after dental procedures occurred in those patients with
systemic illnesses such as rheumatoid arthritis and diabetes mellitus.
first-generation cephalosporin is an appropriate choice because its
antimicrobial spectrum includes most organisms found in the oral
cavity. Cephalexin 1 to 2 g 1 hour before the procedure and 0.5 to 1 g
at 4 to 6 hours is recommended. Alternatively, 3 g of amoxicillin 1
hour before the procedure and 1.5 g 6 hours after the initial dose is
effective as well. Patients with allergy to penicillin should receive
600 mg of clindamycin 1 hour before the procedure and 6 hours later or
erythromycin 0.5 to 1 g 1 hour before the procedure and 0.5 g 4 to 6
hours after the first dose.
procedures, although its true value has never been established. In
vitro systems have shown that colony counts of S. aureus, S. epidermidis, E. coli, and Pseudomonas
sp can be reduced 12% to 56% with saline irrigation alone. Several
studies have shown a decrease in colony counts in wounds and a decrease
in infection rates for general surgical procedures with the use of
antibiotic devices. It has been shown, however, that the use of power
irrigation increases the removal of bacteria by a factor of at least
100 over bulb-syringe irrigation of the same volume, regardless of the
solution used (2,5).
wide spectrum of activity and have low toxicity. The addition of
polymyxin, bacitracin, neomycin, or a combination of these agents is
most effective for this purpose (19).
patient’s history plays a key role in guiding further diagnostic
procedures and what intervention is most suitable. A history of
systemic illness or medication use that may lead to immune depression
are important clues as to the environment in which an infection has
developed and what microorganisms may be responsible. A history of
prior surgery, infections at other sites, open fracture, or
instrumentation can also point to a likely etiology of infection.
infections, such as fever, chills, redness, nausea, swelling, and
tenderness, may not be present in the context of an orthopaedic-related
infection. The most reliable symptom in these situations is pain in the
affected region, but this finding may be very subtle and easily missed,
perhaps presenting as an innocuous chronic ache. When examining the
involved musculoskeletal component, note local signs of stasis,
hypoxia, drainage, edema, erythema, warmth, induration, tenderness,
painful range of motion, and evidence of previous trauma or surgery.
Evaluate joint range of motion above and below the involved region and
perform a full neurovascular examination (15).
consist of a complete blood count (CBC), ESR, and CRP. In evaluating
the CBC, the classic finding of a leukocytosis (white count greater
than 12,000) is associated with the presence of infection. This finding
may not be consistent, especially in infections with a more indolent
course. When leukocytosis is present, however, the differential cell
count will show an increase in the relative number of neutrophils, as
well as a shift to more immature polymorphonuclear cells in circulation.
It is not a specific test for infection and may be elevated in a number
of systemic conditions such as advanced age, pregnancy, morbid obesity,
and while a patient is using certain medications such as heparin and
oral contraceptives. It is also elevated in a variety of pathologic
conditions including infection, inflammation, collagen vascular
diseases, recent surgery or fracture, certain malignancies, myocardial
infarction, gastrointestinal, thyroid, and renal diseases (11). The ESR reflects an increase in the acute-phase reactants produced by the liver in response to inflammation or infection.
of the aggregation of erythrocytes, followed by other serum proteins.
These positively charged proteins coat red blood cells (RBC), which
normally repel each other because of their negative surface charges.
The coated RBCs assume rouleaux formations and settle in the patient’s
blood sample, with the rate measured as the ESR in millimeters per
hour. Normal values by the Westergren method are less than 16 mm/hour
for men and 25 mm/hour for women (11).
space infections, 84% to 100% of patients with proven infection about a
total hip replacement, and 71% to 97% of children with hematogenous
osteomyelitis have an elevated ESR (46). The
ESR has been found to rise within 2 days of infection in children with
infectious arthritis of the hip. The value of ESR is in following the
response of infection to therapy through serial observations. Return of
the ESR to normal may take many weeks, despite effective treatment of
the infection.
liver that is also a nonspecific marker of inflammation and infection.
Its temporal relationship to infection, however, makes it a much more
useful tool in the diagnosis of infection as well as its response to
intervention. Levels of CRP rise within 6 hours of infection, reaching
their peak within 48 hours, and fall to near-normal levels within 1
week of proper intervention (26).
include a cell count and Gram stain of joint fluid or localized
collection aspirates. These immediate procedures can determine the
presence of septic arthritis as well as quickly yielding a likely
pathogen. Cell counts greater than 100,000 with a differential greater
that 75% neutrophils increases the likelihood of infectious arthritis.
These images may show soft-tissue swelling, localized bone destruction,
periosteal thickening, or loosening of fracture fixation hardware or
total joint arthroplasty. The findings of bone destruction are rarely
present until 2 to 3 weeks into the course of an infection, after a
significant proportion of bone matrix has been lost. The value of such
plain films is limited but can be helpful in following the course of a
patient’s illness. If the suspicion of infection remains high even
after this initial evaluation, the use of other imaging modalities that
are able to detect disturbances of normal soft-tissue architecture,
joint inflammation, or the presence of fluid collections may be
necessary.
bone and soft-tissue architecture of an axial and appendicular
skeleton, one disadvantage is the scatter phenomenon that occurs when
metal is present in or near the area of bone visualized. This scatter
results in a significant loss of image resolution (38,49).
surgical practice, mainly because of its superior ability to evaluate
soft-tissue structures. Any infectious or inflammatory process produces
localized edema in the affected area, which will be readily detected by
T2 images. The fat of the marrow cavity that normally appears bright
white on T1 images loses its intensity in cases of intramedullary
osteomyelitis, whereas the marrow cavity signal is increased in T2
images. This finding reflects the replacement of the marrow fat with
edema and inflammatory cell infiltrates. MRI may also detect bony
destruction earlier than plain x-ray studies and CT scans because these
signal changes reflect the earlier processes of bone destruction that
may not be visible by conventional radiographic studies. As with CT
scans, metallic implants in the region of interest may produce focal
artifacts, thereby decreasing the usefulness of the image (38,49).
sequestra and subtle hardware loosening in cases of osteomyelitis or
involvement of subchondral bone in septic arthritis. Ultrasound can be
used to localize abscesses or fluid collection and may aid in its
proper drainage. Arthrography can be used in cases of infectious
arthritis after initial synovial fluid samples have been taken. The
contrast material once visualized allows determination of whether the
joint was properly aspirated.
diagnosis of infection. Contrary to the previously discussed imaging
modalities, which give structural or anatomic representations of the
patient, isotope scanning attempts to deliver functional or physiologic
information. Radionuclide scanning cannot directly show infection or
the structural changes that may be associated, but it can reveal
inflammatory processes throughout the body and the bone’s attempt to
heal. It is for this reason that radionuclide scanning has lower
specificities in differentiating infection, because any source of
inflammation or bone turnover can yield a false-positive result (26).
detection of infection include technetium 99m, gallium citrate, and
indium-labeled leukocyte scans. Technetium 99m is by far the most
commonly employed bone-scanning procedure and depends on uptake of the
technetium by active osteoblasts. Any physiologic process that causes
rapid turnover of bone appears as an area of increased uptake on the
technetium bone scan, including infection, inflammation, metastatic
tumor, degenerative joint disease, and postsurgical and posttraumatic
changes. Photopenia reflects areas of minimal uptake of isotope and may
be associated with decreased area blood flow, certain tumors,
vasospasm, and impingement of the local blood supply by soft-tissue
swelling.
three-phase bone scan that acts to improve the overall specificity of
the test. The three phases consist of the flow phase, the equilibrium
phase, and the delayed phase. The initial flow phase reflects blood
flow throughout a region and large vessel patency. The equilibrium
phase shows the passage of technetium into the smaller vessels of a
given area and its subsequent diffusion throughout the tissues,
revealing the relative blood supply to that area. The delayed phase of
bone scanning is measured at a time of 3 to
4 hours after injection, when most of the remaining isotope has been taken up by osteoblasts.
bone scanning, you can determine the true location of increase
physiologic activity. Osteomyelitis will show increased uptake of
technetium 99m at flow, equilibrium, and delayed phases of bone
scanning. Conversely, in the case of cellulitis, the findings at
initial flow and equilibrium phases show increased activity but low or
normal activity at the delayed phase. At the same time, osteoarthritis
shows decreased uptake of isotope at initial flow and equilibrium
phases but increased uptake at the delayed phase (32,33).
leukocytes in vitro. These cells are then reinjected into the patient,
with imaging taking place 24 to 48 hours later. When a focal area of
bone shows increased uptake relative to the surrounding bone, the scan
is considered positive. Indium scanning has found a significant role in
the diagnosis of acute osteomyelitis. However, the presence of chronic
infections, in which the predominant inflammatory cell type is
lymphocytic, indium has a much lower sensitivity.
be 100% sensitive in the diagnosis of acute osteomyelitis and 60%
sensitive in chronic osteomyelitis. A negative indium scan usually
means that osteomyelitis is not present. Combining the indium scan with
a technetium 99m scan can increase the specificity of the test. A
number of conditions including fractures, arthritis, osteosarcoma,
eosinophilic granuloma, pigmented villonodular synovitis, and
neuropathic arthropathy have been found to show false-positive results
on indium-111—labeled leukocyte scans (20,30,58).
leukocytes, thus reflecting the increased numbers of white blood cells
that migrate into infected areas. It was hoped that the use of gallium
citrate, when used in concert with technetium 99m scanning, could
distinguish infection from other processes. Despite the initial
promising results, sequential technetium-gallium scanning has
demonstrated only 50% sensitivity, 78% specificity, and an accuracy
rate of only 62% in the detection of low-grade musculoskeletal sepsis.
These values are far inferior to the 83% sensitivity, 94% specificity,
and 88% accuracy found with indium-111—labeled leukocyte scanning alone
(36).
indium-111—labeled monoclonal immunoglobulin directed toward
granulocyte cell surface antigens (Leukoscan). Becker et al. (4)
performed scintigraphy in 53 patients at 1 to 6 hours and at 24 hours
after injection of the labeled antibody. The overall sensitivity of
Leukoscan and indium-111—labeled leukocytes was found to be 90% and
83.9%, respectively, the specificity 84.6% and 76.5%, respectively, and
the accuracy 87.9% and 81.3%, respectively. Hakki et al. (18)
prospectively compared the efficacy of Leukoscan, indium-111—labeled
white blood cell scabs, and technetium 99m bone scans in diagnosing 74
patients with suspected musculoskeletal infections. Their findings
showed a sensitivity of 93%, 85%, and 92% for Leukoscan,
indium-111—labeled white cells, and technetium 99m bone scans,
respectively. Specificity was 89%, 75%, and 52%, and accuracy was 90%,
79%, and 74%, respectively.
physiologic and structural changes caused by infection. These
modalities, however, are not capable of giving an actual bacteriologic
diagnosis that can aid in developing a treatment plan and proper choice
of antibiotic chemotherapy. By properly using the laboratory
evaluations available, the surgeon can isolate the responsible pathogen
and determine its susceptibility to antibiotics (3).
useful because they are usually polymicrobial and generally reflect the
normal flora of the skin in the area. Mousa (40)
prospectively compared sinus tract cultures with those obtained
intraoperatively. The samples were found to be of value provided that
they were obtained by deep probing of the tract and aspiration with a
syringe. Swabbing of the sinus tract as well as the findings of S. epidermidis
were found to be unreliable in the diagnosis. Of the 115 operative
samples taken, 102 sinus tract isolates were identical to the operative
cultures, with a specificity of 95.7% and predictive value of 90.3%.
Lee (25) retrospectively reviewed the wound
cultures of 245 open fractures. Only 8% of organisms grown on
predebridement cultures eventually caused infections. Among
culture-negative patients, 7% went on to develop infection. Only 22% of
the organisms cultured ultimately caused infection. These results
suggest that culturing open fracture wounds is essentially of no
medical value.
Gram stains allow for quick determination of gram-positive or
gram-negative bacteria, as well as their morphology. Gram stains help
select early appropriate broad-spectrum antibiotic therapy. More
specific antibiotics can be used when sensitivity results have returned.
the predictive value of intraoperative frozen sections in detecting
infection in patients undergoing revision total joint arthroplasty.
Infection was defined as five polymorphonuclear cells per high-power
field in at least five distinct microscopic fields. Their investigation
showed a 100% sensitivity, with all patients found to have positive
intraoperative cultures also having positive frozen sections. In
addition, their investigation showed a specificity of 96%.
bacteria and fungi. Culture the initial specimens taken for aerobic,
facultative anaerobic, and obligate anaerobic bacteria. If these
specimens fail to yield any growth or there is little response from the
patient to antibiotic intervention, other cultures may be considered to
detect unexpected infectious agents such as mycobacteria and fungi.
methods, testing for the patients’ serum bactericidal activity while on
antibiotic therapy, and direct measurement of serum antibiotic levels. In vitro
methods include serial dilutions of antibiotic and antibiotic disc
diffusion. These tests attempt to determine the concentration of
antibiotic necessary to inhibit further growth of bacteria on an agar
gel medium or in broth.
of antibiotic incubated with bacteria attempting to determine the
concentration at which the growth of bacteria is halted. This
concentration is termed the minimum inhibitory concentration (MIC). If
bacterial growth is stopped by serum levels of an antibiotic when given
in normal dosages and routes, the bacteria are said to be sensitive.
After determination of the MIC, the minimal bactericidal concentration
(MBC) can be determined, reflecting the lowest concentration of
antibiotic at which 99.9% of all bacteria are killed. Ideally, an
antibiotic will have an MIC that is equal to the MBC—that is, the
antibiotic most effective in eradicating the pathogen will have the
lowest toxicity (28).
antibiotic-impregnated discs placed in the broth or agar gel medium to
determine the susceptibility of bacteria. By measuring the zone of
inhibited bacterial growth, the MIC may be determined when compared
with a standardized reference. The results of this test are reported as
susceptible, intermediate, or resistant, depending on the size of the
inhibited growth zones (28).
the effectiveness of the chemotherapeutic treatment. Samples of the
patient’s serum at peak and trough antibiotic levels are used. Peak
serum titers of greater than 1:8 have been associated with good
outcomes in the resolution of infection.
patient’s blood to assist in determining whether the levels are within
the reference range for effectiveness, as well as in below levels.
infection have their shortcomings and limitations. Culture
investigation remains the only definitive modality for the
identification of the pathogenic organism. However in 7% to 15% of
patients with periprosthetic infections, no pathogen can be cultured (16).
With the increasing availability of molecular biologic techniques, the
surgeon has gained a valuable tool in detecting and differentiating
pathogens in orthopaedic infections. The predominant molecular
technique available in this capacity is PCR. PCR depends on the
activity of the heat-stable Taq polymerase, which through repeated
sequences of DNA replication, can tremendously amplify even minute
samples of bacterial DNA.
sequences in bacteria, it is now possible to identify a pathogen based
solely on its DNA. In patients with suspected infection, this molecular
technique can be used to prove infection and provide the likely
organism when all other diagnostic modalities have failed. In addition
to PCR, other molecular techniques under investigation include ligase
chain reaction, reverse transcriptase PCR, branched-chain DNA reaction,
monoclonal antibodies directed against unique bacterial proteins,
direct detection of target RNA through Northern blotting, in situ hybridization of RNA with labeled complementary DNA sequences, Southern blotting of DNA, and Western blotting of proteins.
prescribed agents in the treatment of infections of bones, joints, and
soft tissue. Included in this class are the penicillins,
cephalosporins, carbapenems, and monobactams. Penicillins consist of a
thiazolidine ring coupled with a β-lactam ring, to which is attached a
side chain. The nucleus of this molecule is the chief structural
requirement for biologic activity, and any chemical or metabolic
alteration of this portion causes a loss of all significant
antibacterial activity. The side chain determines many of the
antibacterial and pharmacologic characteristics of the particular
penicillin.
These cell walls are composed of murein (peptidoglycan) and are
essential for the normal growth and development of bacteria.
Peptidoglycan is a heteropolymeric component of the cell wall that
provides rigid mechanical stability by virtue of its highly
cross-linked latticework structure. In gram-positive bacteria, the
thickness of this cell wall may be as much as 100 molecules, compared
with a thickness of only 1 or 2 molecules
in gram-negative organisms. Synthesis involves an N-acetylglucosamine-N-acetylmuramic
acid (GlcNAc-MurNAc) disaccharide unit that is attached to a
bactoprenyl lipid carrier molecule in the cytoplasmic membrane.
Autolytic enzymes open sites in the existing cell wall where the new
disaccharide units will be placed.
 |
|
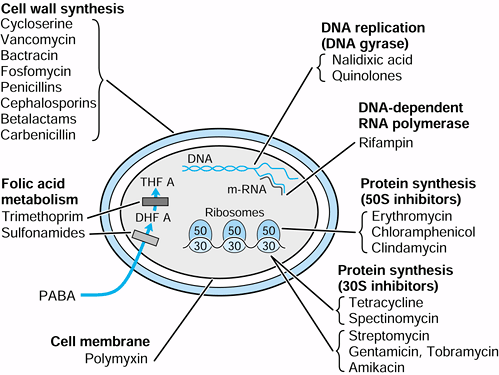
Figure 132.1. Location of antibiotic activity in bacterial cell.
|
and oriented, transpeptidase enzymes cross-link the peptide of the new
GlcNAc-MurNAc moiety with that of another. The key targets of the
penicillins in this process are the transpeptidases. By binding tightly
to the active site of these enzymes, they inhibit the cross-linking of
the cell wall components and reduce the overall tensile strength these
cells need to resist osmotic lysis.
referred to as penicillin-binding proteins (PBP), because their initial
identification was based on their ability to bind with more complex and
varied forms of penicillin. The PBP are a diverse group of
transpeptidases and carboxypeptidases that are involved in various
aspect of cell wall synthesis, and as such, each β-lactam antibiotic
may have different affinities for selected PBP. Once the formation of
the bacterial cell wall is inhibited, two normally expressed classes of
autolysins seem to be responsible for the cidal action of β-lactam
antibiotics.
and penicillin V (the phenoxymethyl derivative), are very similar in
their antimicrobial spectrum for aerobic gram-positive organisms (Table 132.4 and Table 132.5).
They remain the agents of choice in gonococcal and streptococcal
arthritis and soft-tissue infections. Penicillin G is five to 10 times
more active than penicillin V against gonococcus sensitive to
penicillin. The sole virtue of penicillin V in comparison to penicillin
G is its stability in an acid environment. Therefore, it is better
suited for absorption from the gastrointestinal tract. On an equivalent
oral dose, penicillin V may yield plasma concentrations two to five
times greater than those provided by penicillin G. Once absorbed, both
penicillin G and penicillin V are widely distributed in the body and
excreted by the kidneys.
 |
|
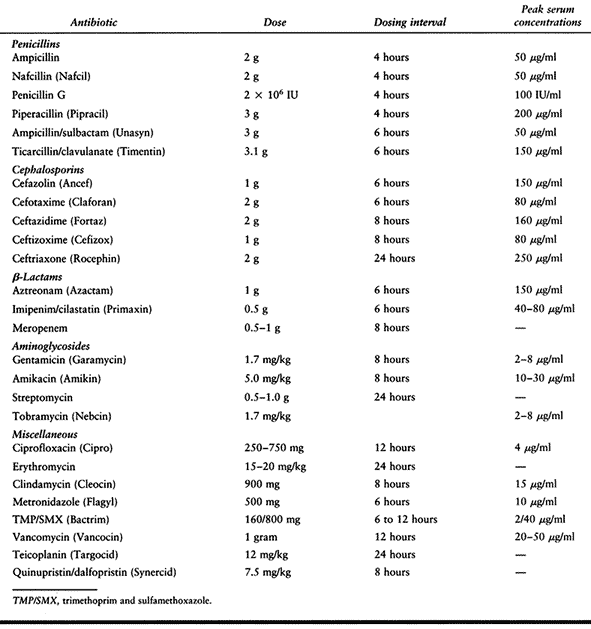
Table 132.4. Dosing of Parenteral Antibiotics Commonly Used in Orthopaedic Infections
|
 |
|
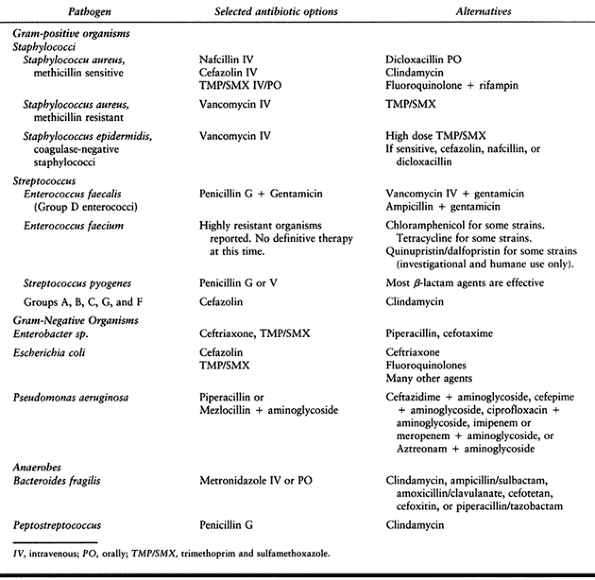
Table 132.5. Pathogenic Organism and Selected Treatment Options
|
plasma concentrations within 15 to 30 minutes, but this value declines
rapidly because of its short half-life (30 minutes). Many attempts have
been made to maintain the concentration of penicillin G doses given
parenterally. Probenicid has been found to block renal tubule secretion
of penicillin, but it is rarely used in this capacity. Instead,
repository preparations of penicillin may be used, the two favored
compounds being procaine penicillin G and benzathine penicillin G.
These preparations allow for slow release of antibiotic from the site
of injection, while maintaining bactericidal blood concentrations over
an extended period of time.
mold was grown, and the realization that changing the side chain moiety
could yield a wide variety of new properties. Investigators soon found
a highly reactive precursor of penicillin, 6-aminopenicillanic acid,
could be obtained by treating penicillin with an amidase. At that
point, an almost infinite number of synthetically generated organic
acids could then be fused with 6-aminopenicillanic acid to produce the
semisynthetic penicillins.
penicillin antibiotics. First, there was the need for a more
acid-stable penicillin that was orally available. Second, penicillin G
and V were effective against gram-positive aerobes but effective only
against a handful of gram-negative bacteria. Finally, the issue of
penicillin-resistant strains of bacteria had already begun to become a
matter of great concern.
amoxicillin, and bacampicillin) were developed to extend the spectrum.
These antibiotics are effective against many gram-negative bacteria but
are only about one-half as effective against gram-positive bacteria as
is penicillin G. Their amino group allows them to traverse the charged
outer membrane of gram-negative bacteria easily and reach bactericidal
concentrations. They are also acid stable and readily bioavailable
through oral dosing. Their main limitation is that they are readily
hydrolyzed by β-lactamase enzymes and are not effective against P. aeruginosa. The aminopenicillins are effective against non-β-lactamase—producing strains of E. coli, H. influenzae, Proteus, Salmonella, and Shigella.
The carboxypenicillins carbenicillin and ticarcillin were the first
antipseudomonal agents developed. They are ineffective against
gram-positive organisms but
have excellent activity against enteric rods and Pseudomonas.
Piperazine penicillins, such as piperacillin, and the
ureidopenicillins, such as mezlocillin and azlocillin, are even more
effective against enteric bacteria and Pseudomonas,
as a result of their high affinity for gram-negative PBP.
Unfortunately, all of the antipseudomonal penicillins are sensitive to
β-lactamase, are generally more toxic than their predecessors, and must
be provided parenterally.
through a variety of means: Chromosomal changes in the affinity of PBP
for penicillins, reduced antibiotic uptake, and decreased activities of
bacterial autolysins are all possible
mechanisms.
The most commonly encountered resistance to penicillins, however, is
based on the enzymatic destruction and inactivation of the β-lactam
ring. Different microorganisms elaborate a number of distinct
β-lactamases, although most bacteria are capable of producing only one
form of the enzyme. The substrate specificities of these enzymes are
relatively narrow, and they can often be described as either
penicillinases or cephalosporinases. Other “broad-spectrum” enzymes may
also be found that are capable of hydrolyzing a variety of β-lactam
antibiotics.
of β-lactamase, secreting it extracellularly. In staphylococcus, the
information for penicillinase is encoded on a plasmid that can be
readily transferred by bacteriophage to other bacteria. In
gram-negative bacteria, β-lactamases are found in much lower
concentrations; their
location
in the periplasmic space, however, between the inner and outer cell
membranes makes them ideally located for maximal protection of the
microbe. In gram-negative organisms, β-lactamases are encoded in either
a plasmid or the chromosome, allowing it to be transferred by
conjugation with another bacterium as well. Whereas the staphylococcal
enzyme is inducible by substrates, the gram-negative β-lactamases may
be inducible or constitutive (35).
methicillin, nafcillin, and the isoxazolyl penicillins (oxacillin,
cloxacillin, and dicloxacillin). These penicillins are more toxic and
have less activity than penicillin G against gram-positive cocci, but
it is their ability to resist hydrolysis by the β-lactamases produced
by staphylococci that allows them to remain the drug of choice in most
staphylococcal disease.
the term “methicillin-resistant Staphylococcus.” Most often, the
laboratory will test staphylococci with an oxacillin-impregnated disk,
reporting the organism as “resistant to oxacillin.” Oxacillin is the
commonly used class-disk for all penicillinase-resistant penicillins.
Although it is not specifically stated, this use indicates a resistance
of the organism to all other penicillinase-resistant penicillins
including methicillin, dicloxacillin, nafcillin, and cloxacillin. These
resistant organisms are also usually resistant to all available
cephalosporins (17).
penicillins has been the development of penicillin-like molecules that
carry little antibacterial activity but can act to inactivate
β-lactamases. The β-lactamase is able to cleave the β-lactam ring of
the penicillin analog but in the process forms a stable but inactive
complex with the cleaved ring, a process referred to as suicide
inactivation. The commercially available combinations of penicillins
and analogs include ampicillin/sulbactam, amoxicillin/clavulanic acid,
ticarcillin/clavulanic acid, and piperacillin/tazobactam. In the cases
of the ampicillin/sulbactam and amoxicillin/clavulanic acid, the
combination has extended the antibiotic spectrum to include
β-lactamase—producing strains of Bacteroides
fragilis, Enterobacter, E. coli, Haemophilus ducreyi, H. influenzae,
Klebsiella, Moraxella catarrhalis, N. gonorrhoeae, Proteus,
Providencia, S. epidermis, and methicillin-sensitive S. aureus.
the largest group of β-lactam antibiotics. The cephalosporins are
semisynthetic penicillin derivatives of 7-aminocephalosporanic acid,
consisting of a β-lactam ring coupled with a six-membered
dihydrothiazine ring (compared with the five-membered ring of
penicillins). In contrast to penicillins, cephalosporins can have up to
three side chains attached to the nucleus of the molecule. The R1 group
is attached to the same site as the R group of penicillin and
determines the antibacterial properties of the molecule. The R2 group
determines the metabolic and pharmacokinetic properties of the
molecule, and the R3 group acts to increase the resistance of
cephalosporins to the action of β-lactamase.
groups, referred to as generations, depending on their efficacy against
gram-negative bacteria. Some of the more recently developed
cephalosporins are referred to as “fourth generation,” but this
classification has yet to achieve widespread acceptance.
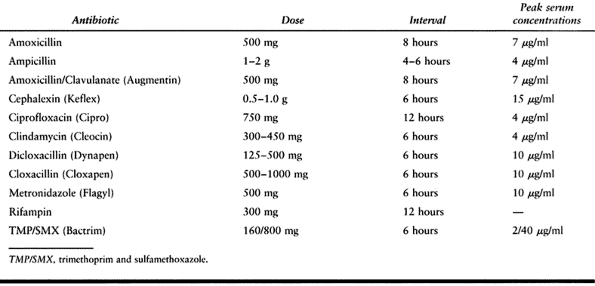 |
|
Table 132.6. Dosing of Oral Antibiotics Commonly Used in Orthopaedic Infections
|
against gram-negative bacteria, whereas they have comparable to
slightly less efficacy against gram-positive organisms compared with
first-generation cephalosporins. Second-generation cephalosporins are
not generally recommended for treatment of infections caused by
gram-positive organisms because they are more expensive, often need to
be given parenterally, and offer no real advantage over
first-generation cephalosporins. Cefuroxime is highly effective against
β-lactamase—producing strains of H. influenza and N. meningitidis,
two major causes of meningitis in children and young adults. Other
second-generation cephalosporins such as cefotetan and cefoxitin can
provide good coverage against N. gonorrhoeae, including β-lactamase—producing strains, as well as enteric rods such as E. coli, Klebsiella, and Proteus. Second-generation cephalosporins are not effective against P. aeruginosa.
the actions of β-lactamase because of their unusually large R groups.
Although these antibiotics have an excellent spectrum of coverage for
gram-negative bacteria, they have very poor efficacy against
gram-positive bacteria. Third-generation cephalosporins are highly
effective against β-lactamase—producing strains of N. gonorrhoeae, N. meningitidis, H. influenzae, M. catarrhalis, and most enteric bacteria including Citrobacter strains, E. coli, Klebsiella, Morganella, Proteus, Providencia, Salmonella, and Shigella. Their excellent coverage is believed to be
a result of the strong affinity for gram-negative PBP and resistance to
β-lactamase. Of the third-generation cephalosporins, ceftazidime has
the strongest activity against P. aeruginosa.
exhibit activity against gram-negative bacteria similar to that of the
third-generation cephalosporins while having the efficacy of
first-generation cephalosporins in their activity against certain
gram-positive bacteria. This trait has prompted some clinicians to
refer to these antibiotics as fourth-generation cephalosporins, whereas
others view them as only extended-coverage third-generation
cephalosporins.
from thienamycin. Although thienamycin is not suitable for human use,
its formimidoyl derivative imipenem has been shown to be the broadest
spectrum β-lactam antibiotic available. Imipenem is resistant to most
β-lactamases, can penetrate well into gram-negative bacteria, and is
also active against anaerobes. Thus, it is active against
β-lactamase—producing strains of Acinetobacter, Listeria, N. gonorrhoeae, N. meningitidis, P. aeruginosa, S. pneumoniae, gram-negative rods, and a number of strict anaerobes. Although it does not act as a cidal agent against Enterococcus faecalis, imipenem can act as a static agent against this organism.
located on the brush border of proximal renal tubules. Cilastatin, a
specific inhibitor of dihydropeptidase-1 is given in a 1:1 ratio with
imipenem to block inactivation and decrease the risk of renal tubular
necrosis. Do not administer imipenem-cilastatin to individuals with CNS
lesions (such as strokes or head injuries), a history of convulsions,
or renal insufficiency. Reports have indicated that as many as 12% to
32% of these patients may develop convulsions as a consequence of this
treatment. Because of this propensity to cause seizures and its high
cost, imipenem is generally reserved for gravely ill patients with
multiple-pathogen nosocomial infections. Meropenem is a newly approved
carbapenem with a similar spectrum of activity as imipenem. It does not
require dosing of cilastatin and carries a lower risk of seizures (17,21).
solely of a single ring. The first such antibiotic created was
aztreonam. In contrast to imipenem, aztreonam is a narrow-spectrum
antibiotic, having good activity against only N. gonorrhoeae, N. meningitidis, P. aeruginosa,
and most gram-negative enteric bacteria. Aztreonam is resistant to
gram-negative β-lactamase but is readily inactivated by plasmid-encoded
β-lactamase. The spectrum of aztreonam can be broadened with the
addition of a penicillin with gram-positive activity, such as nafcillin
or cloxicillin, or alternatively with an aminoglycoside. It also
appears that patients with allergy to penicillin show no such reaction
to aztreonam (17,21).
 |
|
Table 132.7. Selected Adverse Effects of Antibiotic
|
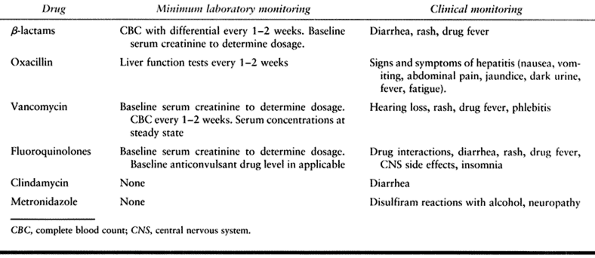 |
|
Table 132.8. Minimum Monitoring for Drug Toxicity
|
of the population, estimated to be between 1% and 5% of adults. The
commercially available preparations of penicillin act as excellent
allergens. What is actually the allergenic component in these
preparations is not the penicillin G itself but the incomplete
penicillin molecules present in the mixture. Some molecules are the
result of breakdown of penicillin while in storage, whereas others are
incomplete molecules secreted by the fungus on production. These
molecules act as haptens combining with the patient’s own serum
proteins to create an antigen capable of eliciting a vigorous response.
ranging from a wheal and flare at the site of injection to massive and
immediate anaphylaxis that may result in death if it is not properly
treated. Systemic anaphylaxis is life threatening. It is for this
reason that physicians regularly ask their patients about a history of
penicillin sensitivity, keep patients in the office after administering
the antibiotic, and have emergency equipment and medications available.
Such a reaction requires the attachment of IgE immunoglobulin to mast
cells in the subcutaneous tissue of patients, therefore necessitating a
previous exposure to penicillin. It must also be kept in mind that all
penicillins are cross-sensitizing and cross-reacting.
sickness—type reaction that may produce urticaria, pruritis, joint
swelling, and respiratory complications up to 2 weeks after the patient
has received penicillin. This particular reaction can occur on the
patient’s first exposure to penicillin. IgG-mediated hemolytic anemia
can also occur in some patients, caused by complement-mediated lysis of
red blood cells coated with penicillin.
about reactions to penicillin administration in the past. For those
patients with an unclear history who must receive penicillin,
penicillin breakdown products are commercially available that can be
injected intradermally. A wheal and flare will be seen in those
patients with an allergy to penicillin, necessitating the use of an
alternative antibiotic. In cases in which the choice is between
penicillin administration and death, patients can be desensitized to
penicillin by sequential oral or parenteral administration of small
doses over several hours.
allergic to cephalosporins. In those patients whose reactions are not
mediated by an IgE mechanism, cephalosporins can safely be used, but in
those patients with a clear history of IgE-mediated penicillin allergy,
cephalosporins are best avoided.
penicillins includes nausea, vomiting, and diarrhea. These effects are
more pronounced with the broad-spectrum penicillins, such as ampicillin
and amoxicillin. In cases in which massive doses of penicillin are
administered, there can be direct cation toxicity (Na+, K+). Methicillin, nafcillin, and other penicillins have been known to cause granulocytopenia occasionally, especially in children.
than does nafcillin. The antibiotic binds to the basement membrane of
the renal tubules and becomes a target of antibody binding that, in
turn, activates complement. Carbenicillin can cause hypokalemic
alkalosis and transaminase elevation in serum and can induce hemostatic
defects, leading to bleeding tendencies. Ampicillin frequently causes
skin rashes, some of which are not related to allergic reaction.
cefotetan, and cefoperazone frequently cause hypoprothrombinemia and
bleeding disorders. Administration of vitamin K, 10 mg twice weekly,
can prevent such an outcome. Moxalactam can also interfere with
platelet function and has induced severe bleeding. Its use has been
largely abandoned. Cephalosporins can cause severe disulfiram-like
reactions in patients who ingest alcohol or alcohol-containing products.
tobramycin, and kanamycin. They exert their action by binding to the
30s ribosomal subunit in bacteria, disrupting the conformation of mRNA,
and forcing erroneous tRNA binding to the codon. At high
concentrations, aminoglycosides can bind to both the ribosomes and
mRNA, disrupting protein translation beyond initiation as well as
permanently altering the ribosome, rendering it functionally useless.
limited by several factors. First, because they require active protein
production in the bacteria to exert their effect, they cannot be used
with agents that reversibly interfere with protein synthesis. It is for
this reason that aminoglycosides are not bactericidal in the presence
of chloramphenicol. Second, the rate of bacterial killing by an
aminoglycoside increases with the drug concentration, with the limiting
factor being the rate at which the antibiotic enters bacterial cells.
Third, aminoglycosides are effective only under aerobic conditions,
thus they are ineffective against obligate anaerobic bacteria. Fourth,
aminoglycosides are ineffective in areas of high acid or salt
concentrations. Fifth, because of their poor penetration into the host
cells, they offer little activity against intracellular bacteria.
Finally, aminoglycosides are relatively toxic with a limited
therapeutic window. High trough levels of aminoglycosides are
classically associated with ototoxicity and nephrotoxicity.
gram-negative rods. By administering aminoglycosides with a β-lactam,
the antibiotics work synergistically to reduce the dosage, increase the
therapeutic margin, and broaden the spectrum of the aminoglycosides.
Through this combination therapy, clinicians can avoid the toxicities
associated with this antibiotic.
mechanisms. In order to create sufficient concentrations in bacteria,
the aminoglycosides must be transported intracellularly by means of a
specific carrier protein. Bacteria that generate lower levels or weakly
binding transport protein are innately resistant to aminoglycosides.
Another mechanism that confers resistance to bacteria is the presence
of a number of enzymes that deactivate the antibiotic by means of
acetylation, phosphorylation, or adenylation. These chemical
modifications do not allow the aminoglycoside molecule to interact with
bacterial ribosomes to exert their bactericidal activity (35).
nephrotoxicity, and their dosage must be adjusted on the basis of the
patient’s baseline renal function. Ototoxicity is another adverse
effect of the antibiotic, manifesting mainly as vestibular dysfunction
owing to the destruction of hair cells by prolonged drug trough levels
in excess of 10 mg/ml. Loss of hearing can occur as well, and it is
occasionally extensive and irreversible (17,21).
fairly toxic when administered systemically. The bactericidal action of
bacitracin works by blocking the dephosphorylation of the bactoprenyl
carrier molecule after it has donated its GlcNAc-MurNAc disaccharide to
the growing peptidoglycan chain. As a result, no bactoprenyl phosphate
is left available to receive to new disaccharide monomeric subunits to
be incorporated into the existing murein, halting cell wall synthesis.
administered parenterally, and therefore, it is restricted to topical
and oral use. It can be found in topical creams for use in treating eye
and skin infections caused by staphylococci and streptococci, as well
as oral preparations for the treatment of pseudomembranous colitis
caused by Clostridium difficile. Its use in this capacity is possible because of its poor absorption from oral intake.
producing proteinuria, hematuria, and nitrogen retention. Its systemic
use has been virtually abandoned. Topical application rarely causes
hypersensitivity reactions or systemic toxicity.
interferes with the action of peptidyl transferase, inhibiting the
formation of a peptide bond and arresting peptide chain elongation.
Because of its bacteriostatic nature, it is generally not administered
in combination with a bactericidal antibiotic. Chloramphenicol’s
ability to penetrate host cells is at the same time a great advantage
and a drawback. Its accumulation in the host cell’s cytoplasm allows
the antibiotic to be used against intracellular organisms. At the same
time, these cytoplasmic concentrations interfere with ribosomal protein
synthesis, depressing bone marrow activity and causing a pancytopenia.
medication leads to toxic levels, and leads to the condition known as
gray-baby syndrome, with vomiting, flaccidity, hypothermia, gray color,
shock, and collapse. One idiosyncratic reaction to the medication is
the development of an aplastic anemia. It is not related to dosage or
length of use,
but
the odds of developing this condition are increased by extended use of
the medication. It is for these reasons that clinicians in the United
States have limited its use.
sp. Resistance to chloramphenicol is mostly limited to enteric
bacteria. Three modes of resistance have been described in these
organisms. The mechanism most commonly found is a gene coding for an
enzyme that is able to acetylate both chloramphenicol and tetracylines,
thereby inactivating them. The second method of resistance involves the
ability of certain bacteria to limit their permeability to the
antibiotic. The final resistance factor discovered is a mutation in the
50s subunit of the bacterial ribosome that results in poor recognition
of the antibiotic by its target (35).
upset with nausea, vomiting, and diarrhea. Chloramphenicol may also
prolong the half-lives of many drugs including phenytoin, tolbutamide,
chlorpropamide, and warfarin. This effect is attributed to the
inhibition of liver microsomal enzymes by the antibiotic.
developed with broad-spectrum effectiveness against most gram-negative
and some gram-positive bacteria. Their proposed mechanism of action is
through binding to the α-subunit of bacterial DNA gyrase (topoisomerase
II), inhibiting the process of DNA supercoiling, a necessary step in
the replication of DNA. The fluoroquinolones can be used to treat
infections in most parts of the body including the urinary tract and to
treat gonorrhea, bacterial diarrhea, and infections of the skin, bones,
and joints as well. They are generally effective against N. gonorrhoeae and S. aureus, and they are usually effective against P. aeruginosa.
fluoroquinolones are nausea, vomiting, and diarrhea. Occasionally,
headache, dizziness, insomnia, abnormal liver function tests, or skin
rash may develop. Concomitant administration of theophylline and
fluoroquinolones can lead to elevation of theophylline levels with
increased risk of toxic effects, especially seizures (Table 132.9).
Fluoroquinolones have been shown in animal models to damage immature
cartilage, but no study has documented this finding in the human
pediatric population.
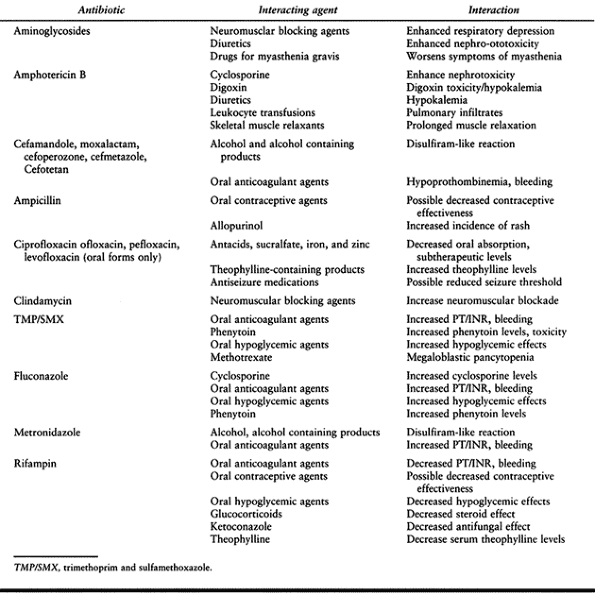 |
|
Table 132.9. Selected Important Drug Reactions
|
have become extremely important tools against methicillin-resistant
staphylococci. These antibiotics are macromolecules, consisting of
seven amino acids at their core, with all glycopeptides sharing a
homology of five of these amino acids. Because of their size, the
glycopeptide antibiotics cannot effectively penetrate the outer
membrane of the gram-negative bacteria and therefore can have no effect
on these organisms. Their inability to penetrate the cytoplasmic
membrane of gram-positive bacteria restricts their activities to the
metabolic processes of the microbe occurring outside this membrane.
configuration, with a central cleft binding tightly to its target. The
active sites of vancomycin and teicoplanin have been shown to recognize
tripeptides with a stereochemical configuration of L-D-D. This
configuration can be found only within the MurNAc pentapeptide, where
an L-amino acid can be found at position 3 with two terminal
D-alanines. When vancomycin is administered to susceptible
gram-positive bacteria, it first attaches to all available L-D-D
residues in the cell wall.
antibiotic attaches to the L-D-D of GlcNAc-MurNAc moeities that are
attached to the bactoprenyl transferring lipid. Once in this position,
the glycopeptide acts as a steric hindrance, blocking peptidoglycan
transglycosidase from transferring the disaccharide unit to the
peptidoglycan growth point. Moreover, the attachment of vancomycin to
the acyl-D-alanine-D-alanine of non-cross-linked dipeptides already
incorporated in the cell wall interferes with their ability to be
cross-linked.
A glycopeptide antibiotic can also be used in combination with an
aminoglycoside to treat infections caused by highly resistant E. faecalis.
the use of vancomycin has occasionally been associated with ototoxicity
and nephrotoxicity, the highly purified preparations of vancomycin that
are now commercially available are generally considered safe for most
patients and make these toxicities mild. Vancomycin is an irritant to
tissue, and patients may develop phlebitis at the site of injection as
well as a nonimmune-mediated histamine release referred to as the “red
man syndrome.” This reaction can largely be prevented by the
administration of antihistamine and slow infusion. Vancomycin is
excreted through the kidneys, and in patients with altered renal
function, peak and trough blood levels of the antibiotic should be
checked to avoid any of the previously mentioned toxicities.
sp primarily owing to the production of a membrane-bound protein known
as VanA. VanA is a D-alanine-D-alanine ligase that synthesizes other
mixed dipeptides, replacing the D-alanine-D-alanine within MurNAc.
These VanA-containing enterococci no longer express the L-D-D target
for the binding of glycopeptide antibiotics and are therefore rendered
resistant to
its actions. VanA has been found to be transferred to other bacteria via conjugation. Less commonly, Entercoccus
sp express VanB or VanC proteins whose modes of action are most likely
similar to that of VanA but have not been found to be transferred by
conjugation (17,21,35,37,57,59).
lincomycin. The difference between these two antibiotics is the
presence at position 7 of a chlorine group on clindamycin that is a
hydroxyl group on lincomycin. This difference allows clindamycin to be
more easily absorbed orally and more active against anaerobic bacteria.
The lincosamides have the same receptor on the 50s subunit of the
bacterial ribosome as chloramphenicol. Lincosamides, however, are able
to cause a more rapid dissociation of the ribosome into its constituent
50s and 30s subunits.
that of penicillin G and erythromycin, but it is used primarily in the treatment of infections caused by the strict anaerobe B. fragilis, especially in patients with known reactions to penicillin. It is sometimes used to treat abscesses or sepsis caused by other Bacteroides sp, Actinobacillus, Actinomyces, Capnocytophaga, Clostridium, Flavobacterium, Fusobacterium, or Peptostreptococcus. Most of these bacteria are anaerobic or microaerophilic.
infections, and in combination with gentamicin to treat complicated
pelvic inflammatory disease. Although the development of
pseudomembranous colitis is seen with prolonged use of any antibiotic,
it is most commonly associated with clindamycin. Clindamycin
effectively eradicates the normal flora of the colon, leaving it prone
for superinfection by C. difficile. The newly dominant C. difficile elaborates a potent exotoxin responsible for the colitis. The colitis can be treated with either metronidazole or vancomycin.
exhibit resistance to macrolide antibiotic. The resistance appears
linked to the same mechanisms that alter the bacterial 50s ribosomal
subunit and produce resistance to the macrolides (17,35).
contain a lactone ring. Until recently, erythromycin was the only
macrolide antibiotic available. The increasing incidence of resistant
strains has led to the development of the newer macrolides azithromycin
and clarithromycin. These antibiotics are bacteriostatic at low
concentration and bactericidal at high concentration. Macrolides bind
to ribosomes, allowing the initiation and formation of a short peptide
sequence, but inhibit the process of translocation and elongation soon
thereafter. This blocked complex becomes unstable, and eventually the
ribosomal fragments are released from the mRNA strand.
spectrum of action similar to that of penicillin G, resistance to
β-lactamase, oral availability, and absence of major toxicity. Most
adverse reactions are related to disturbances of the gastrointestinal
tract. Erythromycin is used in treating Legionnaires’ disease,
diptheria, pertussis, and atypical pneumonia cause by Mycoplasma or Chlamydia.
The newer macrolides have broadened the spectrum of this class of
antibiotics. Azithromycin has also been shown to be effective against Borrelia burgdorferi, the causative agent of Lyme disease; H. influenzae; and Toxoplasma gondii. Clarithromycin is unusual in its effectiveness against Mycobacterium avium-intracellulare and several of the atypical mycobacteria.
of the 50s ribosomal subunit in the bacteria resulting in an inability
of these subunits to recognize the antibiotic (35).
include anorexia, nausea, vomiting, and diarrhea. Erythromycin can also
produce acute cholestatic hepatitis (fever, jaundice, impaired liver
function), probably as a hypersensitivity reaction. Other allergic
reactions include fever, eosinophilia, and rashes. Erthyromycins can
also inhibit cytochrome P450 and increase the effects of
anticoagulants, digoxin, cyclosporine, and antihistamines.
that contain fatty acids, multiple positive charges, and a long alkyl
side chain. These properties allow polymyxins to act as cationic
detergents, binding avidly to lipopolysaccharide and
phosphotidylethanolamine in gram-negative outer membranes but poorly to
phosphatidylcholine, a constituent of human cell membranes. Therefore,
polymyxins are effective against gram-negative bacteria.
polymyxin B and polymyxin E. Because of their toxicity, polymyxins are
not the drug of choice in the treatment of any bacterial infection.
Instead they can be used as a second-line drug to treat severe or
life-threatening infections caused by P. aeruginosa or other gram-negative rods when such infections have not responded to standard therapies.
administration are rare. Systemic levels of polymyxins can cause
paresthesias, dizziness, and lack of coordination, which disappear when
the drug has been excreted. Very high blood levels (greater than 30
mg/ml) can cause respiratory paralysis. Polymyxins may also cause
proteinuria and hematuria.
the β-subunit of DNA-dependent RNA polymerase in bacteria. It allows
the creation of the first phosphodiesterase bond to form in the RNA but
then blocks any subsequent bond formation, and effectively terminates
chain initiation in RNA synthesis. Resistance to rifampin is based on a
mutation of the gene coding for the β-subunit of the bacterial
DNA-dependent RNA polymerase that will not allow for proper binding of
rifampin. Because of the high rate of spontaneous mutations that create
resistance to rifampin, its use has been limited to (1) long-term treatment of tuberculosis and leprosy, (2) prophylaxis for those exposed to patients with meningitis due to H. influenzae type b or N. meningitidis, and (3) combination therapy with vancomycin, nafcillin, and ciprofloxacin in the treatment of endocarditis or osteomyelitis caused by S. aureus or S. epidermidis.
sweat, tears, and contact lenses of which patients should be warned.
Occasional adverse reactions include rashes, thrombocytopenia,
nephritis, and impairment of liver
function.
Rifampin induces the cytochrome P450 system of the liver and increases
the elimination of anticoagulants and contraceptives. Likewise,
administration of rifampin with ketoconazole, cyclosporine, or
chloramphenicol results in significantly lower serum levels of these
medications.
that are capable of penetrating well into host cells. They are most
effective against rapidly multiplying bacteria. This group of
antibiotics include tetracycline, doxycycline, demeclocycline, and
minocycline. The tetracyclines exert their action by binding to the 30s
bacterial ribosomal subunit and inhibiting the correct positioning of
the incoming tRNA, thereby inhibiting peptide elongation. The earlier
tetracylines were not well absorbed from the intestinal tract and, as a
result killed much of the normal flora, resulting in colitis. However,
with the development of the more lipophilic semisynthetic tetracyclines
doxycycline and minocycline, intestinal absorption has increased and
reduced the incidence of colitis.
reasons for discontinuing tetracycline. These symptoms can usually be
controlled by administering the antibiotic with food. Use in children
under the age of 12 can impair bone development and stain developing
teeth. Tetracyclines can probably impair hepatic function and may also
result in liver necrosis, especially in pregnant patients. Renal
tubular acidosis and other renal injuries resulting in nitrogen
retention have been attributed to the administration of outdated
tetracycline preparations. Intravenous injection of tetracycline can
lead to venous thrombosis, whereas intramuscular injection produces
painful local irritation and should be avoided. Administration of
systemic tetracycline, especially demeclocycline, can induce
sensitivity to sunlight or ultraviolet light, particularly in
fair-skinned individuals. Dizziness, vertigo, nausea, and vomiting have
been particularly noted in patients receiving minocycline.
Resistance to tetracyclines is based on a plasmid-encoded active efflux
system. Although tetracycline entry into the cell is not altered, an
active system for pumping the antibiotic out of the bacteria is
established (35).
that also has striking bactericidal effects against most anaerobes,
including Bacteroides and clostridia. It
exerts its antibacterial effect through the formation of short-lived,
highly cytotoxic intermediates that develop in anaerobic conditions. It
is well absorbed when taken orally, metabolized by the liver, and may
accumulate in patients with hepatic insufficiency. Metronidazole is
considered for use in anaerobic and protozoal infections, and
antibiotic-associated enterocolitis. Adverse effects of the medication
include nausea, diarrhea, stomatitis, and peripheral neuropathy with
prolonged use. Advise patients to avoid alcohol ingestion because of
metronidazole’s disulfiram-like effect.
different points in the chemical pathway that produces dihydrofolic
acid for bacterial cells. Dihydrofolic acid acts as a reducing agent in
bacteria and is necessary for normal cellular processes. Because of
their actions on the same pathway, TMP/SMX act synergistically against
susceptible bacteria as well as some parasites. Resistance to TMP/SMX
develops when enzymes in the dihydrofolic acid pathway mutate and no
longer bind these medications as avidly. Because of the use of two
medications on the same chemical pathway, however, the development of
resistant strains is somewhat hampered (35).
effects owing partly to allergy and partly to direct effects. The most
common adverse effects include fever, skin rashes, photosensitivity,
urticaria, nausea, vomiting, and diarrhea. Other effects include
stomatitis, conjunctivitis, arthritis, hepatitis, exfoliative
dermatitis, polyarteritis nodosa, Stevens-Johnson syndrome, and
psychosis. Sulfonamides can produce anemia (hemolytic or aplastic),
granulocytopenia, thrombocytopenia, or leukemoid reactions. These are
rare except in certain high-risk patients. Sulfonamides cause hemolytic
anemia in patients with glucose-6-phosphate dehydrogenase deficiency,
and sulfonamides taken near the end of pregnancy increase the risk of
kernicterus in the newborn infant.
an antifolate drug, especially megaloblastic anemia, leukopenia, and
granulocytopenia. These effects can be prevented by the simultaneous
administration of folinic acid.
interferes with the synthesis of mycolic acid, an essential component
of the cell wall complex of Mycobacterium tuberculosis.
When used as prophylaxis for tuberculosis, it can be given as
monotherapy. With the rise of multidrug-resistant TB, however, INH must
be given in combination with other antimycobacterial drugs. Another
antimycobacterial drug ethionamide is closely related to INH and has
the same mode of action.
20% percent of patients, are on the peripheral nervous system and CNS.
These are related to a relative pyridoxine deficiency and include
peripheral neuritis, insomnia, restlessness, muscle twitching, urinary
retention, and even convulsions or psychotic episodes. Most of these
complications can be avoided by the administration of pyridoxine
(vitamin B6). Isoniazid has been associated with
hepatotoxicity with abnormal liver function tests, clinical jaundice,
and multilobular necrosis.
inhibits the growth of tubercle bacilli and other mycobacteria.
Pyrazinamide is well absorbed from the gastrointestinal tract and
widely distributed throughout the body. Tubercle bacilli develop
resistance to pyrazinamide fairly readily, but there is no
cross-resistance with INH or other antimycobacterial drugs. The major
adverse effects of pyrazinamide include hepatotoxicity (in 1% to 5% of
patients), nausea, vomiting, drug fever, and hyperuricemia.
discussed earlier. It is also used as a front-line chemotherapeutic
agent against mycobacterial infections.
the synthesis of cellular metabolites by an unknown mechanism. In
combination with other medication, it is used in the short-term
treatment of tuberculosis. Ethambutol may cause reduced visual acuity,
optic neuritis, and perhaps retinal damage in about 0.8% of patients
but rarely in patients with normal renal function.
and inhibits the formation of peptidoglycan cross-links by acting as a
competitive inhibitor of the transpeptidase enzymes. Cycloserine is
indicated primarily as a second-line agent in the treatment of
tuberculosis. Its use has been associated with convulsions, other
serious CNS dysfunction, and psychotic reactions.
aminoglycosides previously discussed and possesses the same adverse
effects. Most tubercle bacilli are inhibited by streptomycin, but the
drug can exert its effects only on extracellular bacteria because only
10% of the antibiotic can penetrate cells that harbor the intracellular
pathogen.
of action and adverse effects as the sulfonamides. For many years,
dapsone was the treatment of choice against Mycobacterium leprae.
With the rise of dapsone resistance among leprosy bacilli during the
past few years, standard treatment of leprosy now combines dapsone with
other antimycobacterial drugs.
of developing a selectively toxic antifungal agent has been difficult.
It has been solved by the use of agents that inhibit the synthesis of
unique fungal cell wall or membrane. Thus, most antifungal agents act
by binding or inhibiting the synthesis of ergosterol, a steroid found
only in fungal cell membranes. In high concentrations, however, these
antibiotics can have toxic effects on human cells as well, making the
treatment of systemic fungal disease difficult.
They are selectively toxic to fungi because of their ability to
preferentially bind ergosterol. This binding disorganizes the lipid
bilayer, and fungal metabolites leak out of the damaged cell. In
addition to their ability to bind ergosterol, the polyenes can bind to
cholesterol in the human cell membrane. It is for this reason that
amphotericin B is toxic when used IV, and high-dose therapy may damage
renal basement membranes. This is not a problem with nystatin, because
it is not given intravenously. Regardless of its toxic effects,
amphotericin remains the drug of choice in the treatment of systemic
aspergillosis, blastomycosis, candidiasis, coccidiomycosis,
cryptococcosis, histoplasmosis, mucormycosis, and sporotrichosis.
Nystatin is used in the treatment of cutaneous and mucocutaneous
Candida infections. Because nystatin is not absorbed well from the gut,
it is often used in the treatment of gastrointestinal candidiasis.
chills, fever, vomiting, and headache. The severity of these reactions
may be diminished by reducing the dosage temporarily; administering
aspirin, phenothiazines, antihistamines, or corticosteroids; or
stopping injections for several days. Therapeutically active amounts of
amphotericin B commonly impair renal and hepatocellular function, and
produce anemia.
antifungal effect by blocking the synthesis of ergosterol through the
inhibition of the cytochrome P-450 enzyme lanosterol-14-demethylase.
When the available ergesterol for cell growth is depleted and there is
no additional supply of ergesterol produced, fungal growth ceases. The
azole antifungals can be used topically in the treatment of
dermatophyte infections, chronic mucocutaneous candidiasis,
candidiasis in immunodepressed individuals, chromoblastomycosis, paracoccidiomycosis, and cutaneous or lymphatic sporotrichosis.
vomiting, nausea, diarrhea, rashes, and sometimes impairment of hepatic
function. Fluconazole and ketoconazole inhibit cytochrome P450 and may
increase serum concentrations of phenytoin, cyclosporine, oral
hypoglycemic drugs, and anticoagulants.
treatment of fungal infections. In fungal cells, flucytosine is
enzymatically converted to fluorouracil, and acts to terminate RNA
replication, and thus protein synthesis. Flucytosine can be used as a
single agent to treat Candida infections of the urinary tract, and is
used in combination with amphotericin B in the treatment of systemic
candidiasis or cryptococcosis. As much as 15% of fungal isolates,
however, rapidly develop resistance to flucytosine when it is used as a
single agent. In addition, flucytosine may be toxic in some patients,
with prolonged serum levels causing bone marrow depression with
leukopenia or thrombocytopenia, hair loss, and abnormal liver function.
and is used to treat dermatophyte infections that are not responsive to
topical antibiotics. It may also be administered orally to eradicate
infections that are localized in the stratum corneum. It is believed
that griseofulvin acts as a colchicine-like agent, interfering with
cellular microtubule assembly and inhibiting fungal mitosis. Although
griseofulcin is effective against dermatophytes, it has limited
effectiveness for the treatment of tinea versicolor or tinea nigra. It
is well tolerated, with headache being the most commonly reported
adverse reaction.
allylamine class of antifungal agents available. They are used mostly
to treat dermatophyte infections, such as athlete’s foot and ringworm.
These antibiotics are fungicidal and work through allosteric inhibition
of squalene epoxidase, leading to an accumulation of high levels of
squalene and inhibition of ergosterol synthesis (39).
macrolide-lincosamide-streptogramin family and function to inhibit
protein synthesis by interfering with bacterial ribosomal function.
Pristinamycin is a naturally occurring antibiotic whose existence has
been known for several years. The novel antibiotic Synercid is composed
of two synthetic derivatives of pristinamycin, quinupristin, and
dalfopristin mixed in a 30:70 ratio, respectively. Either component
used alone has a bacteriostatic effect, but when they are combined, the
antibiotic is a powerful bactericidal agent. Consequently,
quinupristin/dalfopristin has improved activity against pathogens
resistant to macrolides and lincosamides. In addition,
quinupristin/dalfopristin is also active against pathogens resistant to
β-lactams and glycopeptide antibiotics owing to its differences in
chemical structure and mode of action. This and other novel antibiotics
in development will be important against emerging strains of resistant
bacteria in the future (14,17,39).
severity and in many instances the relative lack of blood supply, may
require high doses of antibiotic over prolonged periods of time. This
antibiotic therapy may begin in the operating room but will need to
continue throughout the patient’s stay at the hospital and while at
home. With the introduction of the Hickman, Broviac, and PICC types of
indwelling catheters, one of the obstacles to home antibiotic therapy
has been conquered. In addition, the training of visiting home nurses
to educate patients and supervise the infusion of antibiotics, as well
as the efforts of pharmaceutical services to provide adequate
resources, has made this goal of long-term therapy far more achievable (14).
antibiotic remain, however. For those antibiotics with known
toxicities, such as the aminoglycosides or vancomycin, blood-level
monitoring is necessary to avoid toxic side effects. Audiology testing
is needed for a baseline before beginning therapy and may be needed at
some point during the course of treatment to avoid vestibular and
cochlear damage. Other forms of antibiotics may require serial complete
blood counts and liver function tests to avoid toxicity. As mentioned
earlier, serum bactericidal concentrations (SBC) can be measured to
ensure that the antibiotic concentrations remain effective. An SBC
greater than 1:16 at peak concentrations and greater than 1:2 at trough
concentrations correlate with successful treatment of deep-seated
infections (39).
scheme: *, classic article; #, review article; !, basic article; and +,
clinical results/outcome study.
JO, Apostoles S, Christensen G, et al. The Efficacy of Various
Irrigation Solutions in Removing Slime-Producing Staphylococcus. J Orthop Trauma 1994;8:390.
JB, Volz RG. Efficacy of a Topical Antibiotic Irrigant in Decreasing or
Eliminating Bacterial Contamination in Surgical Wounds. Clin Orthop 1984;184:114.
WW, Salvati EA, Klein R, et al. Antibiotic-impregnated Bone Cement in
Total Hip Arthroplasty. An In Vivo Comparison of the Elution Properties
of Tobramycin and Vancomycin. Clin Orthop 1993;296:242.
B, Engesaeter L, Vollset SE, et al. Antibiotic Prophylaxis in Total Hip
Arthroplasty: Review of 10,905 Primary Cemented Total Hip Replacements
Reported to the Norwegian Arthroplasty Register, 1987 to 1995. J Bone Joint Surg [Br] 1997;79-B:590.
SL, Galloway KP. Local Antibacterial Therapy for the Management of
Orthopaedic Infections: Pharmacokinetic Considerations. Clin Pharmacokin 1995;29:36.
G, Gudmundsson G, Kolmert L, et al. Prophylaxis with Systemic
Antibiotics Versus Gentamicin Bone Cement in Total Hip Arthroplasty: A
Five-year Survey of 1688 Hips. Clin Orthop 1990;253:173.
JF, Blachut PA, O’Brien PJ et al. Reamed Nailing of Open Tibial
Fractures: Does the Antibiotic Bead Pouch Reduce the Deep Infection
Rate? J Orthop Trauma 1996;10:298.
JT, Calhoun J, Cobos J. In Vitro Evaluation of Antibiotic Diffusion
from Antibiotic-impregneted Biodegradable Beads and
Polymethylmethacrylate Beads. Antimicrob Agents Chemother 1997;41:415.
JE, Brown ML, Hauser ME, et al. In-111-Labeled Leukocyte Scintigraphy
in Suspected Orthopaedic Prosthetic Infection: Comparison with Other
Imaging Modalities. Radiology 1988;168:235.
A, Debbia EA, Bacca D, et al. Multidrug-Resistant Gram-Positive
Pathogens. An Update on Current Microbiological Patterns. Drugs 1997;54:11.
SF, Berg EW, Saunders EA. Efficacy of Double-Gloving as a Barrier to
Microbial Contamination During Total Joint Arthroplasty. J Bone Joint Surg [Am] 1981;63-A:811.
KD, Brown ML, Dewanjee MK et al. Comparison of Indium-Labeled-Leukocyte
Imaging with Sequential Technitium-Gallium Scanning in the Diagnosis of
Low-Grade Musculoskeletal Sepsis. J Bone Joint Surg [Am] 1985;67A:465.
EA, Robinson RP, Zeno SM, et al. Infection Rates after 3175 Total Hip
and Total Knee Replacements Performed with and without a Horizontal
Unidirectional Filtered Air-flow System. J Bone Joint Surgery [Am] 1982;64:525.
MK, Scarbrough F, Powell BJ. Dental Care and the Prosthetic Joint
Patient: A Survey of Orthopaedic Surgeons and General Dentists. J Am Dent Assoc 1994;125:429.
RD, et al. Staphylococcal Exopolysaccharides Inhibit Lymphocyte
Proliferative Responses by Activation of Monocyte Prostaglandin
Production. Infect Immun 1992;60:922.
DA, Matthews LS, Grady-Benson JC. Infection around Joint Replacements
in Patients Who Have Renal or Liver Transplantation. J Bone Joint Surg [Am] 1997;79-A:36.
DK, Abreu SH, Callaghan JJ, et al. Diagnosis of Infection by
Preoperative Scintigraphy with Indium-labeled White Blood Cells. J Bone Joint Surg [Am] 1987;69-A:1353.
