PRINCIPLES OF TENDON REPAIR
flexor and extensor tendons is confirmed by review of the operative
logs of orthopaedic operating rooms and emergency facilities. The
techniques used to treat lacerated flexor and extensor tendons are
reviewed in Chapter 48 and Chapter 49,
while this chapter provides principles and basic concepts for
addressing tendon injuries. Some principles are well accepted, but
others are still in the development stages.
practices, clinical experience, and interpretation of experimental
animal studies. An understanding of the anatomic features of the
tendon, the nutrient pathways to the tenndon, and the physiology of the
healing process is essential to the formulation of a conceptual
approach to tendon repair. Flexor and extensor tendons have
distinguishing anatomic characteristics. Although there has been
considerable experimental interest in the flexor tendon, there have
been few investigations of the extensor tendon.
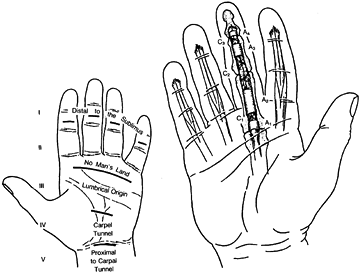
Nine flexor tendons pass through the synovium-lined carpal tunnel (zone
IV) and enter the palm (zone III), where they are covered by a filmy
paratenon. Two flexor tendons proceed to each finger (one to the thumb)
and have an intricate arrangement within the digit (zone II). The
superficialis tendon decussates into two tendon slips, which pass
around the profundus tendon near the metacarpal phalangeal joint, and
subsequently insert as a chiasma on the palmar surface of the middle
phalanx. The profundus tendon passes through the decussation and
inserts at the distal phalanx (zone I).
 |
|
Figure 47.1.
Diagram of the flexor tendon zones and the location of the annular and cruciate fibro-osseous pulleys. (From Kleinert HE, Kutz JE, Cohn M. Primary Repair of Zone 2 Flexor Tendon Lacerations. In: Hunter J, Schneider L, eds. American Academy of Orthopaedic Surgeons: Symposium on Tendon Surgery in the Hand. St. Louis: CV Mosby, 1975.) |
function to each finger, although the musculotendon unit to the small
finger is frequently hypoplastic. The four profundus tendons have a
common muscle belly. Consequently, the flexion of any individual distal
phalanx by the profundus tendon usually results in movement at the
distal joint of the other three. This has been referred to as the quadriga effect.
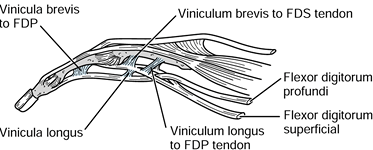
unique environment; they pass through a synovium-lined fibro-osseous
sheath. The tendons are attached to the periphery solely by two filmy,
vascularized mesenteric vincula (longum and breve), which are located
near the midportion and at the insertion of both tendons, respectively.
The vinculum breve is located at the insertion of the superficialis
tendon and is intricately associated with the vinculum longum of the
profundus tendon (Fig. 47.2); damage to the superficialis tendon insertion necessarily affects the blood supply to the midportion of the profundus tendon.
 |
|
Figure 47.2. The common origins of the vinculum breve to the superficialis tendon, and the vinculum longus to the profundus tendon.
|
pulleys that are regularly interrupted longitudinally by a filmy
synovium-lined membrane; together they form a continuous tunnel through
which the tendons pass. The pulleys hold the gliding tendons against
the phalanges and prevent tendon bow-stringing during finger motion.
The pulleys are also thought to have a passive role in pumping fluid
into the tendon during flexion and extension.
central core (the endotenon) that is composed primarily of acellular,
longitudinal, collagenous fibers that are sparsely interspersed with
fibroblasts. A thin epitenon layer of cells surrounds the central core.
The epitenon cells are fibroblasts that may have different functional
properties and characteristics from the internal fibroblasts of the
endotenon. The epitenon cells are arranged in a matrix that is presumed
to have a proteoglycan composition.
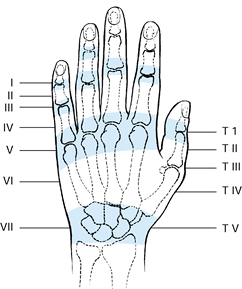
The extensor tendons enter the hand through a series of synovium-lined,
retinacular compartments on the dorsum of the wrist. Unlike the flexor
tendon, these tendons are attached to the retinacular compartments by
long, mesotendinous, synovial sheets rather than by narrow, filamentous
vincula. On the dorsum of the hand, the tendons emerge from the
retinacular compartments and proceed individually to each digit. The
extensor tendons are interconnected by tendinous bands known as
juncturae tendinum. Distal to the metacarpophalangeal joint, the
extensor mechanism forms an intricate arrangement of fibers that arise
from the extrinsic extensor tendons and from the intrinsic
musculotendinous units. This intricately arranged, digital, aponeurotic
mechanism
is essential to the synchronous movement of the three digit joints.
 |
|
Figure 47.3.
Extensor tendon zones. (Redrawn with permission of WB Saunders Co. From Kleinert HE, Schepel S, Gill T. Flexor tendon injuries. Surg Clin North Am 1981;61:267.) |
the tendon in zone II is an obvious nutrient pathway to the flexor
tendon (11), diffusion of nutrients into the tendon is a more significant pathway within the digital sheath (41). Synovial fluid produced by the lining cells of the digital sheath is an obvious source of diffusible nutrients (47).
Corporeal tissue fluid can also effectively diffuse into and maintain
the viability of tendons, as shown by experiments in which tendon
segments were placed in an abdominal wall diffusion chamber and in an in vitro tissue culture environment (27).
(zone VII) is surrounded by filmy paratenon. This vascularized
connective tissue is thought to be the source of nutrients to the
tendon. Within the carpal tunnel (zone IV), the tendons are surrounded
by a synovial mesotenon that arises from the floor of the carpal
tunnel. The nutrition of the flexor tendon within the carpal tunnel has
not been investigated, but it is presumed to be derived from the
synovium.
(zone III) receive nutrients from the mesotendinous synovial sheets
arising from the floor of the compartments, with diffusion and
perfusion serving as nutrient pathways (39). The filmy paratenon vascularizes the tendons on the dorsum of the hand and digit (zones I–VI).
The traditional concept is that peripheral fibroblasts from the
surrounding connective tissue invade the laceration site and serve as
the source of reparative cells; the tendon itself is thought to have no
intrinsic capacity for repair. However, in vitro
studies of various experimental animals have shown that the epitenon
cells migrate into and across the laceration site along a fibrin
lattice, and collagen fibers formed by the epitenon and endotenon
fibroblasts bridge the laceration sites (38,40,42,55). The repair site is vascularized by proliferation of vascular channels from within the proximal end of the tendon (18).
These studies suggest that the tendon is a viable structure that
possesses the intrinsic capacity for participating in the repair
process.
by the application of tension at the laceration site. Fibroblast
migration, protein synthesis, and collagen deposition are increased by
application of tensile forces to chicken tendons (5,61). Static tension applied to in vitro rabbit tendon segments showed increase in strength at the repair site (45), and intermittent cyclic tension enhanced the cellular response of lacerated chicken flexor tendon segments in vitro (65).
demonstrated that the anticipated loss of tensile strength within the
first 2 weeks following tendon repair can be avoided by actively
mobilizing the tendon at the repair site.
tendon, potentially restricting tendon excursion during flexion and
extension. Although the adhesions may contribute to the strength of the
healing tendon, it is likely that the adhesions are not essential to
the reparative process but only represent the response of the
surrounding tissue to trauma (19). The concept
of the tendon’s intrinsic capacity for healing is attractive to
surgeons, because it eliminates the need for fibrous adhesions at the
repair site and encourages rehabilitation protocols that prevent their
formation and attachment to the tendon. Nevertheless, restrictive
adhesions following tendon repair continue to be the bane of surgeons
who perform flexor tendon surgery.
fibroblasts from the periphery, but studies have not yet specifically
defined this process.
extensor tendon laceration by careful clinical examination. Begin the
examination with a visual inspection of the hand. A disruption of the
continuity of the tendon should be suspected by the location of the
wound and the resting position of the digit. For example, a skin
laceration immediately overlying a flexor tendon on the palm, in
association with a digit whose resting position is not as flexed as the
adjacent fingers, suggests a lacerated flexor tendon. However, remember
that sharp, stiletto-type, penetrating objects
can lacerate tendons through a small, inconspicuous skin wound at a distance from the tendon.
motion of the joint mobilized by the tendon. The flexor profundus
tendon to each digit can be examined by asking the patient to flex the
distal joint of the involved digit (Fig. 47.4);
inability to flex the distal interphalangeal joint indicates a
lacerated profundus tendon. A laceration of both profundus and
superficialis tendons is apparent when the patient is unable to
actively flex the proximal and distal interphalangeal joints. Testing
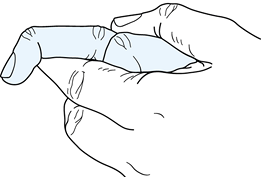
for a suspected superficialis tendon laceration in the presence of an
intact profundus tendon is more difficult. The surgeon must eliminate
the flexion force of the profundus tendon by making use of the quadriga
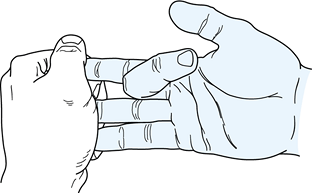
effect (Fig. 47.5). To do this, manually hold
the distal joints of the adjacent digits in full extension while the
patient attempts to flex the involved finger. An inability to flex the
involved digit actively at the proximal interphalangeal joint indicates
a lacerated superficialis tendon.
 |
|
Figure 47.4. Examination of an intact flexor profundus tendon.
|
 |
|
Figure 47.5.
Examination of an intact flexor superficialis tendon using the quadriga effect to eliminate the flexion force of the profundus tendon. |
patient to extend each digit. Failure to achieve complete extension
indicates a tendon laceration. The ability to obtain full extension may
be misleading, however, because this may be accomplished in the
presence of an extensor tendon laceration by the interconnecting
junctura tendinum from an adjacent tendon. The examiner must test the
comparative strength of digital extension against resistance; a digit
that is being extended solely by an interconnecting junctura tendinum
has a weaker extension force than the adjacent fingers with intact
extensor tendons.
wound site. In many instances, exposure is necessary to confirm the
diagnosis of tendon laceration if the clinical examination of the
tendon is not conclusive, or if the extent of injury to adjacent
structures is not apparent. This occurs in patients who are not able to
cooperate fully (children or incoherent patients, for example), as well
as in those with partial or incomplete tendon lacerations when function
of the digit is thought to be sustained by the adjacent intact
structures, such as a junctura tendinum or intact vincula. The burden
of proof about whether a flexor or extensor tendon laceration exists
rests with the surgeon; if this cannot be determined by clinical
examination, surgical exploration of the wound is indicated.
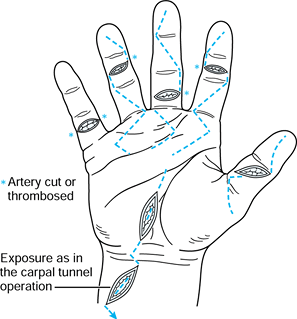
and expose the tendon ends. Generally speaking, proximal or distal
extension of the original wound is achieved nthrough a zigzag approach,
as described by Bruner (8), incorporating the original skin laceration (Fig. 47.6).
Lacerated flexor tendons frequently retract into the palm or forearm
unless prevented by an intact vinculum or lumbrical muscle. A second
proximal incision may be necessary
to
retrieve the retracted tendon and pass it into the original wound site.
Fortunately, proximal retraction of lacerated extensor tendons occurs
infrequently because the tendon is usually held in place by the
junctura tendinum or the synovial mesotendon beneath the retinaculum.
 |
|
Figure 47.6. Proximal and distal incisions extending from the laceration site to obtain exposure.
|
expose the flexor tendon ends, avoid cutting the fibro-osseous pulleys,
particularly A2 and A4, and limit the incisional
area to the intervening synovial portion of the sheath. This is best
accomplished by an L-shaped (or hockey stick) incision, which
facilitates closure of the digital sheath (34).
injuries is to identify any associated injuries to the adjacent bones,
nerves, or blood vessels. Before repairing the lacerated tendon, define
the injuries to these associated structures. If possible, repair all
the injured structures during the initial surgery. The sequence of
repair is usually bone, ligament, tendon, nerve, and important blood
vessels. Not all vessels require reanastomosis; only those that are
essential to the viability of the injured part. Additionally, the
surgeon may choose to perform a delayed repair of crushed or
explosion-injured nerves. In replantation surgery, this sequence of
repair may be altered in the interest of quickly restoring circulation
to the digit.
However, if appropriate facilities and skilled personnel are not
available and the wound is properly irrigated and debrided, repair may
be delayed up to 21 days with satisfactory clinical results (36,46,58).
manipulation is associated with increased adhesions and the formation
of restrictive scar tissue (9,48).
This principle cannot be overemphasized. If possible, handle only the
exposed, cut end of the tendon with the forceps. Do not apply crushing
clamps to the tendon, and keep puncture holes made by suture needles or
forceps inserted into the tendon substance to a minimum. Frequently,
the surgeon draws the proximal and distal ends of the lacerated tendon
into the wound site and holds them in position with straight taper
needles placed transversely through the tendon and the adjacent soft
tissue; this allows the repair to be accomplished without tension,
minimizing repeated manipulation of the tendon ends.
lacerated tendon ends to allow the healing process to reestablish the
continuity of the disrupted collagen bundles and the smooth gliding
surface of the tendon. Restore the tubular form to the tendon, avoiding
both gapping and bulging of tendon tissue at the repair site. This is
particularly important for flexor tendon injuries within the digital
sheath (zone II).
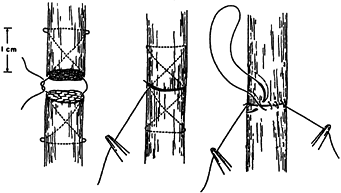
flexor tendon repair. Most of them include a “core” suture, which
passes through the endotenon substance of the tendon ends across the
laceration site to provide strength and stability to the repair until
healing takes place (Fig. 47.7). Additionally,
a peripheral suture is placed circumferentially around the laceration
site. Although this peripheral suture has been referred to as an epitenon suture, I prefer the term circumferential or peripheral suture
because the technique of placing it includes more than the single layer
of epitenon cells on the surface of the tendon. A properly placed
circumferential suture coapts and invaginates the tendon ends, helping
to prevent postoperative adhesions. This circumferential suture was
once considered to be primarily of cosmetic importance to “tidy-up” the
repair site; however, recent studies have noted the importance of this
suture to improve tensile strength and gap resistance (31,33,53,68).
 |
|
Figure 47.7.
A modified Bunnell core suture and circumferential running suture. (From Kleinert HE, Kutz JE, Cohen M. Primary Repair of Zone 2 Flexor Tendon Lacerations. In: Hunter J, Schneider L, eds. American Academy of Orthopaedic Surgeons: Symposium on Tendon Surgery in the Hand. St. Louis: CV Mosby, 1975.) |
II and for extensor tendons is not as critical, because the healed
tendons do not have to glide in a confined sheath. Nevertheless, the
surgeon is encouraged to use careful atraumatic techniques to minimize
postoperative adhensions.
important that the suture be strong and nonreactive. Monofilament
stainless steel is the strongest and least reactive of the available
suture materials, but it is difficult to use without kinking or
crimping the material, which potentially weakens the suture and leads
to breakage. Most surngeons
use
one of several nonabsorbable synthetic materials: 3-0, 4-0, or 5-0 for
core sutures, depending on the size of the tendon, and 5-0, 6-0, or 7-0
for the circumferential suture.
should be repaired at the time of tendon repair. Repair of the digital
sheath at the time of zone II flexor tendon injuries has become popular
on the premise that this enhances tendon nutrition by restoring the
synovial environment (34). This concept is contradicted by studies that show that tendons heal in an extra–synovial fluid environment (27),
as well as by studies that indicate that the integrity of the digital
sheath does not affect the uptake of nutrients into the tendon (51).
Experimental animal studies following tendon laceration and repair have
produced conflicting results about whether sheath closure or
reconstruction minimizes adhesions and improves tendon gliding (22,50,64).
Sheath closure in the presence of an edematous swollen tendon may
produce relative narrowing of the fibro-osseous tunnel and restrict
tendon gliding. Be meticulous if you are performing sheath closure at
the time of tendon repair, taking care not to compromise the
cross-sectional diameter of the fibro-osseous tunnel.
during passive digital motion, active digital motion, and active
digital motion against resistance have been measured clinically in
patients undergoing median nerve decompression at the carpal tunnel (59).
Passive motion generates up to 0.9 kilograms force (kgf) in the digital
flexor tendon. Active flexion against no resistance generates up to 3.9
kgf. Grasp against resistance generates up to 6.4 kgf, and tip pinch up
to 12.0 kgf. Tendon repair rehabilitation protocols usually employ
digital active or passive grasping activities. Consequently, 3.9–6.4
kgf is considered an appropriate approximation of the flexor tendon
forces required to flex an uninjured finger. It should be noted that
edema and increased tissue viscoelasticity associated with an injured
digit probably result in increased resistance to flexion; therefore,
the forces required to flex an injured digit following tendon
laceration and repair are likely to be increased (23).
techniques (e.g., Kessler, Bunnell) is approximately 2–3 kgf, as
measured in human cadaveric tendons (2,6,66). These tensile strength values have been shown (44,67)
to diminish more than 30% to 40% in experimental studies in immobilized
tendons during the early (5–10 days) postoperative period; these values
recover at approximately 2–3 weeks.
repair site can increase the tensile strength of the repair.
Traditionally, suture techniques with two strands across the repair
site were used (9,28). Increasing the suture strands to four nearly doubles the strength of two-strand repairs in human cadaver tendons (32,54). A six-strand suture technique described by Savage (57) has an ultimate tensile strength of 6.8 kgf.
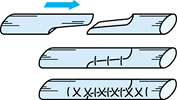
technique has been shown to increase the tensile strength. A
longitudinal continuous running suture along both margins of the
repaired tendon as described by Becker (Fig. 47.8) (4) increases the strength to 4.0 kgf, and a cross-stitch suture described by Silfverskiold and Anderson (Fig. 47.9) (60)
increases strengths to 6.4 kgf in sheep cadaver tendons. The latter
authors also reported tensile strength values of more than 10 kgf using
a nylon mesh sleeve sutured to the tendon across the laceration site;
again, the
testing was performed in sheep cadaver tendons, and the values may not be comparable to studies using human cadavers.
 |
|
Figure 47.8.
Diagram of the Becker suture. (Redrawn with permission. From Becker H. Primary Repair of Flexor Tendons in the Hand without Immobilization. Preliminary Report. Hand 1978;10:37.) |
 |
|
Figure 47.9.
Drawing of the criss-cross circumferential suture. (Redrawn with permission. From Silfverskiold KL, Anderson CH. Two New Methods of Tendon Repair. An in Vitro Evaluation of Tensile Strength and Gap Formation. J Hand Surg 1993;18A:58.) |
the repair site also influences the tensile strength of the repair.
Comparison of knot position in human cadavers showed that tendon
repairs with knots located within the repair site had 60% to 80% of the strength of repairs having the knots located outside the repair site (3). Similar in vivo studies in canines (52)
showed that four-strand suture repairs with the four knots located
within the laceration site had 65% the tensile strength at 1 week after
repair compared to tendon repaired with knots located away from the
repair site. This increased to 75% at 3 weeks, and there was no
significant difference in the tensile strength related to knot location
by 6 weeks. The explanation for this difference is that knots located
within the repair are subject to all the tensile forces applied at the
laceration site. When the knots are placed outside the laceration site,
the tensile forces are in part dissipated and absorbed by the friction
between the suture material and tendon fibers; therefore, more tensile
forces can be applied to the repair site before the suture fails. The
four suture strands crossing the laceration site occupied 7% of the
cross-sectional area of the tendon at the laceration site. Locating the
four knots within the laceration site increased the occupied
cross-sectional area to between 18% and 26%. The observation that there
was no difference in the tensile strength at 6 weeks indicates that the
knots within the repair site did not interfere with the healing process.
tensile strength related to the use of a locking or grasping suture
configuration, as well as over whether more than one locking or
grasping loop per suture strand increases the strength of the repair.
The controversy is in part related to poorly defined terms:
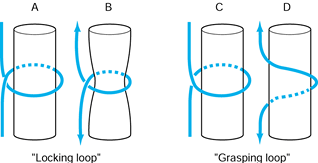
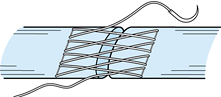
Unfortunately, the terms locking and grasping have often been used interchangeably. It is necessary to define a locking suture as originally defined by Pennington (49), who recognized the important relationship between the longitudinal and transverse components of the core suture (Fig. 47.10).
When the transverse component is passed superficial (palmar) to the
longitudi- nal component, it tightens around bundles of tendon fibers
as tensile forces are applied; this is a locking suture.
When the transverse component is passed deep (dorsal) to the
longitudinal component, the suture does not tighten around the tendon
bundles but rather pulls through the tendon fibers as tensile forces
are applied; this is a grasping suture.
 |
|
Figure 47.10. Definitions of locking loop and grasping loop. In the locking loop configurations (A), the suture (B) tightens around the fiber bundles when tension is applied to the suture ends. In the grasping loop configuration (C), the suture (D)
pulls through the fiber bundles without tightening around them when tension is applied to the suture ends. (Redrawn with permission of WB Saunders Co. From Hotokezaka S, Manske PR. Difference between Locking Loops and Grasping Loops: Effects on 2-Strand Core Suture. J Hand Surg 1997;22A:995.) |
the effect of the locking suture configuration as defined by Pennington
on tensile strength and gap formation using stainless steel suture
material. They found no significant differences using one, two, or
three locking loops per suture strand. In a subsequent study,
Hotokezaka and Manske (26) compared locking and
grasping loop suture configurations using polyester suture material.
They confirmed the previous observation that additional locking loops
do not contribute to increased strength or resistance to gap formation.
One locking loop per suture strand had greater tensile strength than
one grasping loop and was equal in tensile strength to two locking
loops and two grasping loops. With respect to gap strength, one locking
loop was equal to two locking loops and one grasping loop; however, gap
formation was greatest using two grasping loops per suture strand as
the added suture material pulled through the tendon allowing a larger
gap. These authors conclude that a single locking loop per suture
strand is preferred when using two-strand suture techniques.
showed that placement of core sutures in the dorsal half of the tendon
provided stronger repairs than core sutures placed in the volar half of
human cadaver tendons. This was particularly evident using curvilinear
testing techniques, whereby the tensile strength values were determined
with the tendon in its native environment within the flexor sheath of
the digit. It has not been conclusively established whether the
improved
tensile
strength is related principally to the potential mechanical advantage
of the dorsal placed suture during simulated digital flexion, or to a
real biological difference between the dorsal and volar aspects of the
tendon.
circumferentially around the periphery of the repair site. Formerly, it
was thought that this circumferential suture served only to tidy-up the
repair, but recent cadaver studies have shown that it also contributes
to the tensile strength and gap resistance of the repair site (30,33,53,68).
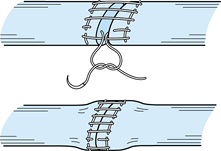
Tensile and gap strength values, in general, are increased according to
the number of suture strands. Additionally, tensile strength is related
to the suture configuration (30); simple
sutures add approximately 1 kgf, mattress sutures add approximately 2
kgf, and the locking mattress suture described by Lin (33) (Fig. 47.11) and the cross-stitch suture (Fig. 47.10) (60)
add approximately 3 kgf. Finally, increasing the depth of
circumferential sutures has also been shown to increase the tensile
strength and gap resistance (14).
 |
|
Figure 47.11.
Drawing of locking mattress circumferential suture. (Redrawn by permission. From Lin G-T, An K-N, Amadio PC, Cooney WP. Biomechanical Studies of Running Suture for Flexor Tendon Repair in Dogs. J Hand Surg 1988;13A:553.) |
following tendon repair have focused on minimizing tension at the
repair site to allow the tendon to heal. Complete immobilization has
been found to diminish the strength of the repair site, however, and to
encourage attachment of fibrous adhesions from the peripheral tissue to
the repaired tendon (32,67).
Ideally, these peripheral adhesions will become long and filmy strands
of scar tissue, which do not impede tendon excursion; however, they
often are tight, dense bands that restrict tendon gliding. In
experimental animals, a program of early passive motion effectively
restored the gliding surface and increased the strength at the repair
site of canine flexor tendons at 3 weeks after surgery (19,20).
Consequently, carefully supervised and controlled passive range of
motion of the digit has been advocated in the treatment of repaired
flexor tendons in the early postoperative period (15,35). Early controlled mobilization has also been advocated following extensor tendon repair (7).
Aoki et al. showed that the observed loss of tensile and gap strength
at the repair site during the initial 2 weeks could be obviated by
applying tension to the repaired tendon (1). Several authors have reported good results using active tendon mobilization protocols following flexor tendon repair (13,16,56,62). Active mobilization has also been advocated following extensor tendon repair (12,17).
the digital sheath follows the previously noted principles, but it also
takes into consideration the ease or difficulty of suture placement.
The core consists of two two-strand locking sutures (3-0 braided
polyester) as described by the Pennington (49) modification of the Kessler technique (28),
resulting in four strands across the repair site. (If surgical exposure
allows, a third locking suture can be placed, resulting in six
strands.) The knots for each of the two sutures are tied within the
repair site. The circumferential suture is a criss-cross suture (60) using 6-0 Prolene.
protective active mobilization; the specific protocol utilized depends
on the ability of the patient to understand and conform to the
guidelines. Treat reliable patients with a removable splint as
described by Cannon (10); this allows active
digital flexion with wrist extension and active digital extension with
wrist flexion. Between exercise sessions, hold the digit in full
extension at the proximal interphalangeal joint (with the wrist and
metacarpophalangeal joints flexed 30°) to prevent flexion contracture
of that digital joint.
(rubber band traction) similar to that originally described by Lister
et al. (35); modifications include passing the
rubber band through a pulley located at the distal palmar crease in the
hand, as well as including all four digits in the traction device.
Additionally, encourage patients to assist the passive flexion by
actively flexing the digit during exercise periods.
An additional figure-eight can be added at the surgeon’s discretion.
Postoperatively, early motion is obtained using dynamic splinting (7).
evolve. Perhaps it is best to regard these principles as dynamic and
ever-changing concepts that the surgeon must reconsider as they are
modified and redefined. Only in that way will the objective of
restoring the continuity of the collagenous fibers and reestablishing
the gliding surface of the tendon be realized.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
M, Kubota H, Pruitt DL, Manske PR. Biomechanical and Histologic
Characteristics of Canine Flexor Tendon Repair Using Early
Postoperative Mobilization. J Hand Surg 1997;22A:107.
JA, Dovell S, Thomas J, et al. A Comparison of Results of Extensor
Tendon Repair Followed by Early Controlled Mobilization Versus State
Immobilization. J Hand Surg 1989;14B:18.
D, Flemming AFS, Harris SB, Foster AJ. The Rupture Rate of Acute Flexor
Tendon Repairs Mobilized by the Controlled Active Motion Regimen. J Hand Surg 1994;19B:607.
RH, Khabie V, Cahill CJ. The Revascularization of Healing Flexor
Tendons in the Digital Sheath. A Vascular Injection Study in Dogs. J Bone Joint Surg Am 1991;73:868.
RH, Vande Berg JS, Manske PR, Akeson WH. The Early Stages of Flexor
Tendon Healing: A Morphologic Study of the First Fourteen Days. J Hand Surg 1986;10:776.
MN, Manske PR, Kubota H, Aoki M. The Effect of Immobilization,
Immediate Mobilization and Delayed Mobilization on the Resistance to
Digital Flexion Using a Tendon Injury Model. J Hand Surg 1997;22A:464.
TF, Light RR, Bunch WH, et al. The Effect of Immediate Constrained
Digital Motion on the Strength of Flexor Tendon Repairs in Chickens. J Hand Surg 1987;2A:590.
M, Tajima T. Experimental Investigation of Healing Process of Tendons
with or Without Synovial Coverage in or Outside of the Synovial Cavity.
J Niigata Med Asso 1981;95:532.
H. Double Loop Locking Suture: A Technique of Tendon Repair for Early
Active Mobilization. Part I. Evolution of Technique and Experimental
Study. J Hand Surg 1990;15A:945.
WW, Manske PR, Dunlap S, et al. Effect of Various Methods of Restoring
Flexor Sheath Integrity on the Formation of Adhesions after Tendon
Injury. J Hand Surg 1990;15A:48.
O, Diao E, Lotz JC, Hariharan JS. Comparative Mechanical Analysis of
Dorsal Versus Palmar Placement of Core Sutures for Flexor Tendon
Repairs. J Hand Surg 1995;20:801.
B, DeMoura W, Ferder M, et al. The Fate of Tendon Healing after
Restoration of the Integrity of the Tendon Sheath with Autogenous Vein
Grafts. J Hand Surg 1985;10A:790.
