Legg-Calvé-Perthes Syndrome
controversial topics in all of pediatric orthopaedic surgery. The
debate about its etiology and pathogenesis continues, and there is no
unanimity regarding treatment. This chapter will review what is known
about the condition, point out where controversies exist, and highlight
the problems in decision making regarding treatment.
young children. The condition was described independently in 1910 by
Legg (1), Calvé (2), Perthes (3), and Waldenstrom (4,5). In the late 19th century, however, Hugh Owen Thomas (6), Baker (7), and Wright (8)
described patients with supposed hip joint infections that resolved
without surgery, whose histories were consistent with
Legg-Calvé-Perthes disease. Maydl (9), in 1897, reported this condition and thought it was related to congenital dislocation of the hip (10).
Recent findings, discussed in this chapter’s section on pathogenesis,
suggest that Legg-Calvé-Perthes “disease” may more appropriately be
called a syndrome.
who were limping after injury. This paper was published in 1910. He
called this condition an “obscure affectation of
the hip” and postulated that pressure secondary to injury caused flattening of the femoral head (1).
In that same year, Calvé reported ten cases of a noninflammatory
self-limiting condition that healed with flattening of the
weight-bearing surface. He postulated that the cause of this condition
was an abnormal or delayed osteogenesis. He reported coxa vara and
increased femoral head size in these patients; on physical examination,
all of the patients had decreased abduction (2).
Perthes simultaneously reported six cases of what he termed “arthritis
deformans juveniles.” He postulated that this was an inflammatory
condition (3). In his description of the condition, Waldenstrom postulated that the disease was a form of tuberculosis (4,5).
He reported on a 9-year-old boy who had experienced symptoms for 2
years. Examination of a portion of the excised head revealed numerous
cartilage islands throughout and “strings” connecting the cartilage of
the joint and the physeal plate. Perthes noted that the marrow spaces
were widened, with fatty infiltration; he saw no evidence of
inflammation. He believed that the cartilage islands were new, and that
this was an osteochondritis and not a tubercular process (11). Schwartz (12),
an associate of Perthes, described the pathologic changes in a
7-year-old boy with a 2-year history of symptoms and reported similar
findings. Waldenstrom (13) suggested the use of the term coxa plana to make the description of the disease consistent with that of other hip deformities, such as coxa vara and coxa valga. Sundt (14,15)
published the first monograph on Legg-Calvé-Perthes syndrome, reporting
on 66 cases and the pathology of the condition. The essential feature
in all of his cases was the cartilaginous islands in the epiphysis.
Sundt attributed the disease to an “osteodystrophy due to dysendocrinia
of a hereditary disposition.” He believed that individuals so
predisposed would get Legg-Perthes disease after they sustained an
injury (i.e., infection or trauma) to the hip. Sundt was the first to
introduce the modern concept of the “susceptible child.”
curettage findings in a 10-year-old child with an 8-month history of
symptoms, described areas of bone necrosis, granulation tissue, old
bone with new bone formation, and osteoclasts. He interpreted these
findings as an inflammatory and infectious process. In 1922, Riedel (17)
reported on two cases and presented the histology. He described the
thickening of the articular cartilage and noted that the junction
between the bone and the articular cartilage was filled with blood. He
also noted that the physeal plate was destroyed and that there were
many cartilage rests. Dead bone was surrounded by a rich granulation
tissue, and many giant cells were present. He also noted that farther
away from the main disease process, the marrow was fibrotic with
inflammatory infiltrates. Riedel was the first investigator to notice
that there were blastic and clastic changes working at the same time on
the same bone trabeculae. In his second specimen, he found regeneration
of the cartilage in the subchondral area, cell atrophy, and some
inflammatory cells. That same year, Waldenstrom (18)
proposed the first radiographic classification of the disease process
on the basis of the data from 22 patients who were followed up until
the completion of their growth. Since then, most of the orthopaedic
literature has centered on the etiologic, epidemiologic, and prognostic
factors in Legg-Calvé-Perthes disease and follow-up of various
treatment modalities (19,20,21,22,23).
but cases have been reported in children from 2 years of age to the
late teenage years. It is more common in boys than in girls by a ratio
of 4 or 5 to 1 (25). The incidence of bilaterality has been reported as 10% to 12% (24,26). Although the incidence of a positive family history in patients with Legg-Calvé-Perthes syndrome ranges from 1.6% to 20% (10,24,27,28,29,30,31,32,33), there is currently no evidence that this syndrome is an inherited condition (34).
reported on a series of 310 index patients with Legg-Calvé-Perthes
syndrome. They noted that, of the children of index patients with the
syndrome, only 2% had Legg-Calvé-Perthes syndrome. All twins in this
series were discordant, including one monozygotic pair. Eleven percent
had abnormal birth presentations, including breech and transverse,
compared with the 2% to 4% incidence that would be expected in the
general population. There is a higher incidence of Legg-Calvé-Perthes
syndrome in later-born children, particularly the third to the sixth
child, and a higher percentage in lower socioeconomic groups (35,36). Parents of the children with the syndrome also tend to be older than those in the general population (24,33,37).
geographic areas, particularly in urban rather than rural communities,
giving rise to the suspicion of a nutritional cause, possibly a trace
element deficiency (35,36,37,38,39,40,41,42,43).
There is also a recently reported strong association (33% of patients
with the syndrome) of Legg-Calvé-Perthes disease with the psychological
profile associated with attention deficit hyperactivity disorder (44). Malloy and Macmahon as well as Lappin et al. (45,46,47) noted that birth weight was lower in children with the syndrome. Harrison et al. (48)
reported that children with Legg-Calvé-Perthes syndrome lagged behind
their chronological age, and 89% of the involved individuals had
delayed bone age. Ralston (49) and others
demonstrated that this delay in skeletal maturation averages 21 months,
but that during the healing stages of the disease there was recovery of
height and weight through increased growth velocity (49,50,51). Race may also be a factor in the frequency of incidence of this condition. There is
a higher frequency of occurrence of Legg-Calvé-Perthes syndrome among
the Japanese, other Asians, Eskimos, and Central Europeans, and a lower
frequency of occurrence among native Australians, Americans, Indians,
Polynesians, and persons of African origin (10,40,52,53).
reported that boys with the syndrome were 1 inch shorter and girls with
the syndrome were 3 inches shorter compared with healthy children.
Burwell et al. (55,56,57) and others (35,58,59)
demonstrated that children with the syndrome are smaller in all
dimensions, except for head circumference, and shorter in the distal
portions of the extremities as opposed to the proximal portions. Loder
et al. (60), in a more recent study,
demonstrated that bone age of the pelvis in boys was less delayed than
that of the hand and wrist. In patients who have the disorder at a
young age, the shortness in stature tends to correct during
adolescence, whereas patients who have the disorder at an older age
tend to be small throughout life (33). Eckerwall et al. (61)
followed up 110 children with the disorder in a longitudinal study and
showed that these children were shorter at birth, remained short during
the entire growth period, and their growth velocity never changed.
Burwell et al. (57) demonstrated an abnormality
of growth hormone–dependent somatomedin in boys with Legg-Calvé-Perthes
syndrome, whereas Tanaka et al. (62), Fisher (27), and Kitsugi et al. (63) reported contrary results.
The effects of growth hormone on postnatal skeletal development are
mediated, in part, by the somatomedins (insulinlike growth factors) (64).
Somatomedin C insulinlike growth factor-1 (IGF1) is the principal
somatomedin responsible for postnatal skeletal bone maturation (64).
Plasma IGF1 levels have been reported to be significantly reduced in
children with the disorder during the first 2 years after the diagnosis
of Perthes disease. These alterations were accompanied by a tendency
toward growth arrest and impaired weight gain. An acceleration in
growth and weight gain is believed to accompany the healing stages of
the disease, although a recent report by Kealey et al. disputes this
growth acceleration following the active stages of the disease (65).
proteins. However, levels of the major binding protein, insulinlike
growth factor binding protein 3 (IGFBP3), are normal during the first 2
years after the diagnosis of Perthes disease (66,67).
Low levels of circulating IGF1 and failure of IGF1 to increase normally
during the prepubertal years in patients with Perthes disease, in
conjunction with reportedly normal growth hormone levels, raise the
possibility of decreased responsiveness of growth-plate chondrocytes
and hepatocytes (64). The combination of
moderately reduced IGF1 levels with normal IGFBP3 has been reported in
normal-variant short-statured children. The skeletal maturation delay
and retarded bone age reported in patients with Perthes disease, in
conjunction with the findings described in the preceding text, could be
considered to be a retention of the infantile hormone pattern (65).
and this could be related to the reportedly higher incidence of Perthes
disease in low-income families (42). The
disproportionate skeletal development affecting the distal portions of
the body reflects a tendency toward infantile body proportions. This
correlates with the reduced IGF1 levels in the presence of normal
levels of binding proteins (68). Controversy
still exists in that a recent study by another group of investigators
reported results opposite to those reported by Neidel et al. (67),
with serum levels of IGF1 being normal and those of IGFBP3 being lower
in children with Perthes disease, compared with controls (69).
Another recent study, although confirming the skeletal maturation delay
in children with Perthes disease, demonstrated no difference in IGF1
(measured with IGF2-blocked binding sites) and IGFBP3 serum
concentrations with respect to bone age (70).
This group disputed the claims of disturbance of the
hypothalamic-pituitary-somatomedin axis in patients with Perthes
disease. The reported differences in the various studies, in some
cases, may be partly attributable to the methods used for measuring
IGF1. There is an increased incidence of hernia in patients with
Legg-Calvé-Perthes syndrome and their first-degree relatives. There is
also an increased incidence of minor congenital abnormalities in
patients with the syndrome (28,71,72,73).
unknown. Many etiologic theories have been proposed. In the early part
of the 20th century, most investigators thought that it was a disease
of an inflammatory or infectious nature (3,4,5,74,75,76). Phemister (16,77) believed that the disease was an infectious process, although tissue cultures were negative. Axhausen (74)
believed that it was caused by bacillary embolism in which the
infection either was not manifested, or was too weak and healed
quickly. As late as 1975, Matsoukas (78) demonstrated an association between Legg-Calvé-Perthes syndrome and prenatal rubella.
investigators to be the cause, or a significant contributing factor, of
Perthes disease (1,79,80,81,82,83,84,85).
As with most childhood orthopaedic conditions, a significant number of
patients may relate an episode of trauma to the onset of symptoms.
Europe, thought that Legg-Calvé-Perthes syndrome was of congenital
origin, and that there was a relationship between this disease and
congenitally dislocated hips (83,84,85,86,87,88,89,90). Glimcher (91)
proposed that cytotoxic agents of external or endogenous origin may be
responsible for bone cell death. A recent report showed the association
of Perthes with delayed ossification of the proximal femoral epiphysis (92). At one time, Perthes disease was believed to be related to hypothyroidism (93,94,95); this has since been disproved (89,90). Recent reports demonstrate moderately
increased plasma concentrations of free thyroxin and free
triiodothyronine in patients with Perthes disease, compared with
controls (68).
It has yet to be conclusively determined whether the aforementioned
factors contribute to causing Perthes disease, and whether IGF1 is at
reduced levels in the early disease stages as reported. These findings
do, however, provide additional evidence that growth-related systemic
abnormalities exist in patients with Legg-Calvé-Perthes syndrome (68).
reported three cases of Legg-Calvé-Perthes disease among 25 patients
with acute transient synovitis. Although all hips with Perthes disease
have synovitis, especially early in the course of the disease, and many
have persistent synovitis for years (98,99,100,101,102),
a review of the literature reveals that an average of 1% to 3% of
patients with a history of transient synovitis later develop
Legg-Calvé-Perthes syndrome (103,104,105,106,107,108). Chuinard (109) and Craig et al. (81,82) have proposed that excessive femoral neck anteversion is a causative factor in the development of Legg-Calvé-Perthes syndrome.
embarrassment. An insufficient blood supply to the proximal femur has
been elucidated by many authors. The terminology used in the literature
varies. However, there are three main sources of blood to the proximal
femur: an extracapsular arterial ring, the ascending cervical
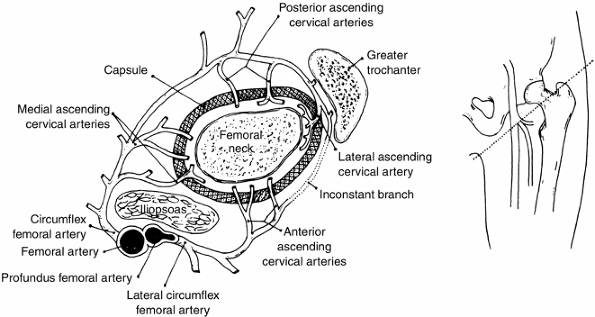
(retinacular branches) vessels, and the artery of the ligamentum teres (110) (Fig. 25.1).
The extracapsular ring is formed mostly by the medial and lateral
femoral circumflex vessels. This ring gives rise to the ascending
cervical branches, which are extracapsular, and these in turn give rise
to the metaphyseal and epiphyseal branches. The anterior portion of the
extracapsular ring is formed primarily by the lateral femoral
circumflex artery. The posterior, lateral, and medial aspects of the
ring are formed by the medial femoral circumflex artery. Chung (110)
found that the greatest volume of blood flow to the femoral head comes
through the lateral ascending cervical vessel (the termination of the
medial femoral circumflex artery), which crosses the capsule in the
posterior trochanteric fossa. Trueta and Pinto de Lima (111,112), and Chung (110) demonstrated that the anterior vascular anastomotic network (Fig. 25.1)
is much less extensive than the posterior anastomotic network,
particularly in specimens taken from patients 3 to 10 years of age,
which correlates with the age range of Legg-Calvé-Perthes syndrome.
Chung also demonstrated that the anterior anastomotic network was
incomplete more often in boys, which correlates with the male
predominance found in Legg-Calvé-Perthes syndrome. Ogden (113)
reported the presence of vessels crossing the physeal plate in some of
his specimens, but Chung disagreed, suggesting instead that the vessels
do not actually cross the plate, but pass through the peripheral
perichondral fibrocartilaginous complex.
used selective angiograms to demonstrate obstruction of the superior
retinacular artery in patients with Legg-Calvé-Perthes syndrome. In a
recent angiographic study, Atsumi et al. showed that 68% of subjects
with Perthes disease had interruption of the lateral epiphyseal
arteries at their origin (115). In 1973, Sanchis et al. (116) proposed the second infarction theory. They experimentally infarcted the femoral head of animals
labeled with tetracycline. They were unable to produce a typical
histologic picture of Legg-Calvé-Perthes syndrome with only a single
infarction. With a second infarction, however, they were able to show a
more characteristic histologic picture of Legg-Calvé-Perthes syndrome.
Inoue et al. (117)
later correlated this double-infarction theory with human histologic
material. Clinical correlation for this theory is provided by reports
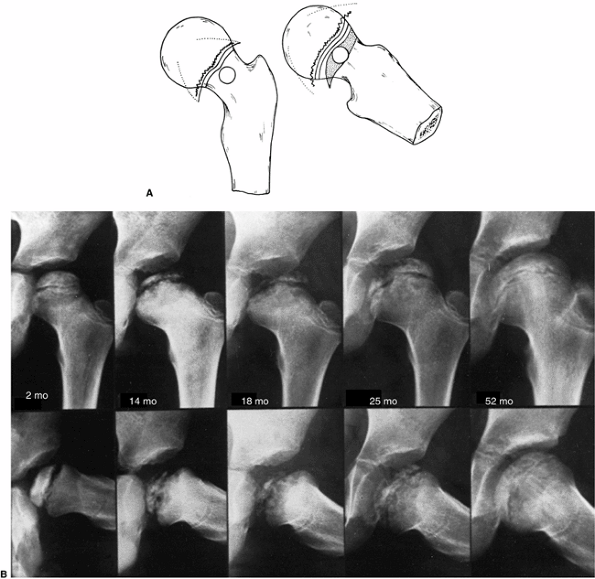
of recurrent Perthes disease (118,119) (Fig. 25.2). Salter and Thompson (120,121)
proposed that Legg-Calvé-Perthes syndrome is a complication of aseptic
necrosis, and that a fracture manifested radiographically by a
subchondral radiolucent zone initiates the resorptive phase. Kleinman
and Bleck (122) demonstrated increased blood
viscosity in a group of patients with Legg-Calvé-Perthes syndrome,
possibly leading to decreased blood flow to the femoral epiphysis.
Vascular embarrassment, caused by intraosseous venous hypertension and
venous obstruction, has been demonstrated by several authors (34,123,124).
 |
|
Figure 25.1
The blood supply to the normal proximal femur in a child. (From Chung SMK. The arterial supply of the developing proximal end of the human femur. J Bone Joint Surg Am 1976;58:961.) |
Thrombophilia induced by low levels of protein C or protein S, or by
resistance to activated protein C, has been associated with the
development of osteonecrosis and with arterial thrombosis (127,128,129,130).
These investigators have suggested routine screening of: the levels of
protein C, protein S, and lipoprotein(s); plasminogen activator
inhibitor activity; and stimulated tissue-plasminogen activator
activity in patients with Perthes syndrome (127).
They believe that routine coagulation screening of children with
Legg-Perthes disease has an additional advantage because of the
familial nature of the autosomal dominant coagulopathies. These
disorders are associated with thrombotic events in 60% of adult family
members. The authors believe that the diagnosis of a coagulation
disorder in a child with Legg-Perthes disease can and should lead to
studies in first-degree relatives, with the goal of preventing
thrombotic events in families. More recent literature has refuted the
role of thrombophilia in causing Perthes disease (131,132,133,134,135).
syndrome should be put in perspective. Few human specimens have been
studied, and each such specimen represents only one stage in the
disease process. Most specimens are from curettage or core biopsies,
which show only one portion of the involved head at a time.
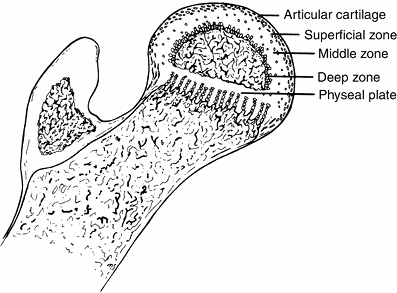
secondary center of ossification is covered by cartilage comprising
three zones (Fig. 25.3). The superficial zone
has the morphologic properties of adult articular cartilage. Beneath
this zone is the zone of epiphyseal cartilage, which is histochemically
different. The zone becomes thinner as the skeleton matures and the
epiphyseal bone enlarges in size. Underneath the epiphyseal cartilage
is a thin zone formed by small clusters of cartilage cells that
hypertrophy and
degenerate. Capillaries penetrate this zone from below, and bone forms at a much slower rate than in the metaphysis (136).
 |
|
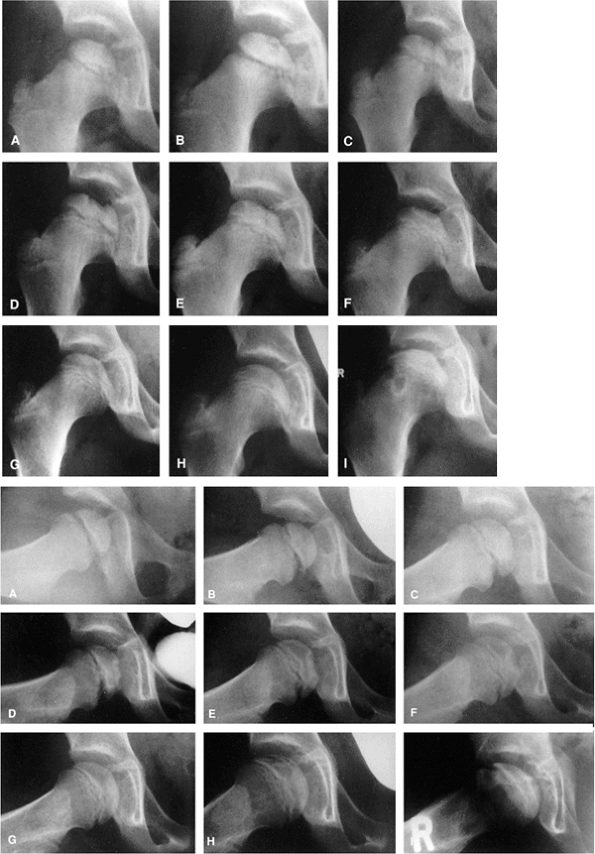
Figure 25.2
A girl, 4 years and 8 months of age, was treated for left hip Perthes disease (late fragmentation phase) beginning in January 1983. Anteroposterior (right, A–I) and Lauenstein (see pg. 1044, A–I) views of the right hip at different stages, January 1983 to December 1987. A: View of the right hip at the time of initial presentation with no signs of involvement (January 1983). B: Early involvement, patient still asymptomatic (September 1983). C–F: Progressive healing of the right femoral epiphysis at May 1984 (C), August 1984 (D), May 1985 (E), and November 1985 (F). G: Femoral head was completely healed by December 1986. H: Recurrent changes in the density of the femoral head and a subchondral fracture that involves less than 50% of the head (Catterall group 2) was seen in June 1987. I: Complete involvement of the ossific nucleus (Catterall group 4) with diffuse metaphyseal reaction and cysts in December 1987. (From Martinez AG, Weinstein SL. Recurrent Legg-Calvé-Perthes’ disease: case report and review of the literature. J Bone Joint Surg Am 1991;73:1081.) |
were described as early as 1913. These and current studies demonstrate
that the superficial zone of the epiphyseal cartilage covering the
affected femoral head is normal but thickened. In the middle layer of
the epiphyseal cartilage, however, two types of abnormalities are seen:
areas of extreme hypercellularity, with the cells varying in size and
shape and often arranged in clusters, and areas containing a loose
fibrocartilage-like matrix. These abnormal areas in the epiphyseal
cartilage have histochemical and ultrastructural properties that are
different from normal cartilage and fibrocartilage. Areas of small
secondary ossification centers are evident, with bony trabeculae of
uneven thickness forming directly on the abnormal cartilage matrix (136,137,138,139,140,141).
The superficial and middle layers of epiphyseal cartilage are nourished
by synovial fluid and continue to proliferate, whereas only the deepest
layer of the epiphyseal cartilage is dependent on the epiphyseal blood
supply and is affected by the ischemic process (136,139,142,143,144,145,146,147,148,149,150).
 |
|
Figure 25.3 Proximal femur in a child.
|
syndrome. It shows evidence of cleft formation with amorphous debris
and extravasation of blood. In the metaphyseal region, endochondral
ossification is normal in some areas, but in others the proliferating
cells are separated by a fibrillated cartilaginous matrix that does not
calcify (Fig. 25.5). The cells in these areas
do not degenerate but continue to proliferate without endochondral
ossification, leading to tongues of cartilage extending into the
metaphysis as bone growth proceeds in adjoining areas (136,137,141,151,152,153).
demonstrated thickening, abnormal staining, sporadic calcification, and
diminished evidence of ossification in the deep zone of the articular
cartilage of the unaffected hip. They also demonstrated that the
physeal plate in these unaffected hips is thinner than normal, with
irregular cell columns and cartilage masses remaining unossified in the
primary spongiosa.
acetabulum (154). In the human specimens described by Ponseti (137),
the physeal plate lesions were longstanding, as shown by the fact that
there was only necrotic bone in the femoral head and no evidence of
repair. Catterall et al. reported similar cartilaginous lesions in a
patient with Catterall group 1 disease, in which there is no sequestrum
formation (139,141) (Fig. 25.6).
The various reported physeal plate and epiphyseal plate lesions
resemble the lesions that Ponseti and Shepard produced in rats by
administering aminonitrils (155). These
epiphyseal and physeal plate changes, in conjunction with the unusual
and precarious blood supply to the proximal femur, make the femoral
head vulnerable to the effects of physeal plate disruption.
 |
|
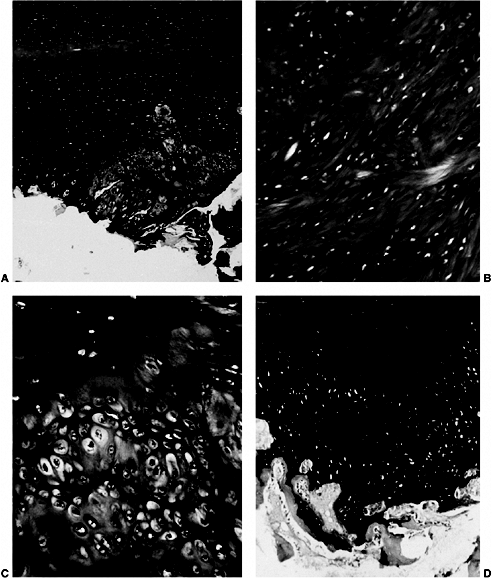
Figure 25.4 A:
Superficial zone cartilage and epiphyseal cartilage of the femoral head. The superficial zone cartilage is normal and is Alcian blue-positive. The epiphyseal cartilage stains with periodic acid-Schiff, but only the perilacunar rims stain with Alcian blue. In the epiphyseal cartilage, there is an area of disorganized abnormal Alcian blue-positive cartilage. (Alcian blue with 0.6 mol per L magnesium chloride; original magnification, × 5.) B: Abnormal area of epiphyseal cartilage. The matrix has a fibrillated appearance and is strongly Alcian blue-positive. (Alcian blue with 0.6 mol per L magnesium chloride; original magnification, × 100.) C: Junction between the normal and abnormal epiphyseal cartilage. Normal cartilage is periodic acid-Schiff positive, whereas the abnormal cartilage is very cellular and retains Alcian blue positivity at high concentrations of magnesium chloride. (Alcian blue with 0.7 mol per L magnesium chloride; original magnification, × 165.) D: Extensive area of abnormal epiphyseal cartilage in the femoral head. Bone seems to form directly on the abnormal cartilage. Abnormal cartilage retains intense Alcian blue positivity at a high concentration of magnesium chloride, but loses that positivity and becomes strongly positive to periodic acid-Schiff at the bone-cartilage junction. (Alcian blue with 0.7 mol per L magnesium chloride, periodic acid-Schiff, and Weigert hematoxylin stains; original magnification, × 40.) (From Hresko MT, McDougall PA, Gorlin JB, et al. Prospective reevaluation of the association between thrombotic diathesis and Legg-Perthes disease. J Bone Joint Surg Am 2002;84(9):1613–1618.) |
 |
|
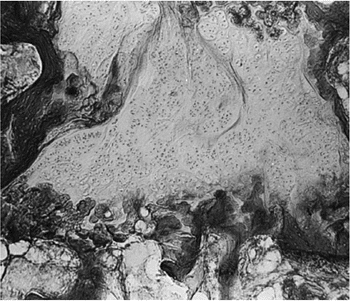
Figure 25.5
Photomicrograph showing a large area of cartilage between the bone trabeculae of the femoral neck. (Original magnification, × 80.) (From Kealey WD, Mayne EE, McDonald W, et al. The role of coagulation abnormalities in the development of Perthes’ disease. J Bone Joint Surg Br 2000;82(5):744–746.) |
confirm that the histologic abnormalities are accompanied by
irregularities of ossification in other epiphyses, especially Kohler
disease of the navicular (71,137,156). Harrison and Blakemore (157),
studying 153 consecutive patients with unilateral Legg-Calvé-Perthes
disease, found that 48% had contour irregularities in the contralateral
normal capital epiphysis compared with 10% of the matched controls.
Kandzierski et al. reported that 35% of patients with Perthes disease
showed changes in the unaffected proximal femur in the first radiograph
(145). Aire et al. (158)
demonstrated that the unaffected hip showed anterior and lateral
flattening at the time of diagnosis of the affected hip. These data
suggest that Legg-Calvé-Perthes disease is a generalized process
affecting other epiphyses, and therefore should not be referred to as a
disease but should be called Legg-Calvé-Perthes syndrome.
minimal trauma, may interrupt the continuity of retinacular vessels,
causing necrosis (136,137).
This finding, in conjunction with the aforementioned epidemiologic,
histologic, and radiologic data, supports the belief that
Legg-Calvé-Perthes syndrome may be a localized manifestation of a
generalized disorder of epiphyseal cartilage in the susceptible child (10,34,50,58,72,136,148,159,160).
classified into four stages: initial, fragmentation, reossification,
and healed. These stages are important in the formulation of treatment
decisions that will be discussed later in this chapter. In the initial
stage (18,161), one of
the first signs of this condition is failure of the femoral ossific
nucleus to increase in size because of a lack of blood supply (Fig. 25.7).
The affected femoral head appears smaller than the opposite, unaffected
ossific nucleus. Widening of the medial joint space, as initially
described by Waldenstrom (18,162) (Fig. 25.7),
is another early radiographic finding. Some researchers have theorized
that widening is caused by synovitis. Others have proposed that this
finding is secondary to decreased head volume caused by necrosis and
collapse and a secondary increase in blood flow to the soft tissue
parts, such as the ligamentum teres and pulvinar, causing the head to
displace laterally (161,163). Synovitis is indeed present in patients with Perthes disease to varying degrees (98,99,101,102,164,165),
but the medial joint space widening is probably most often an apparent
radiographic phenomenon secondary to epiphyseal cartilage hypertrophy (Fig. 25.8).
The bony epiphy-sis begins to fragment, and there are areas of
increased radiolucency and increased radiodensity. Increased
radiodensity at this stage may be caused by new bone forming on old
bone (169,170,171,172,173) and thickening of existing trabeculae (171). The subchondral radiolucent zone (i.e., crescent sign) first described by Waldenstrom (162,174), and later brought to wider attention by Caffey (175), is one of the very early signs of Legg-Calvé-Perthes syndrome in the fragmentation stage (Figs. 25.2 and 25.10). According to Salter and Thompson (121) and Salter and Bell (176),
this radiographic finding results from a subchondral stress fracture,
and the extent of this zone determines the extent of the necrotic
fragment.
Radiographically normal bone density returns, with radiodensities
appearing in areas that were formerly radiolucent. Alterations in the
shape of the femoral head and neck become apparent (Fig. 25.9).
 |
|
Figure 25.6 A:
Catterall group 1 disease shows anterior femoral head involvement with no evidence of sequestrum, subchondral fracture line, or metaphyseal abnormalities. B: Catterall group 1 disease 1 week to 5 years after onset of symptoms. |
proximal femur may have residual deformity from the disease and the
repair process (Fig. 25.9).
be compared with aseptic necrosis after fracture of the neck of the
femur or traumatic dislocations of the hip. In these situations, the
vascular insult to the femoral head usually heals rapidly without going
through the prolonged stages of fragmentation and repair that are seen
in children with Legg-Calvé-Perthes syndrome (136,177,178).
Legg-Calvé-Perthes syndrome come about in many ways. First, there is
growth disturbance in the epiphyseal and physeal
plates.
In the physeal plate, this may result in premature closure with
resultant deformity, such as central physeal arrest, causing shortening
of the neck of the femur and trochanteric overgrowth (179,180) (Fig. 25.11).
The repair process itself may cause physical compaction resulting from
structural failure and displacement of tissue elements (91).
During the healing process, the femoral head will deform according to
the asymmetric repair process and the applied stresses. The molding
action of the acetabulum during new bone formation may also play a role
in this process (181,182). With deformity of the femoral head, the acetabulum, particularly its lateral aspect, is deformed secondarily.
 |
|
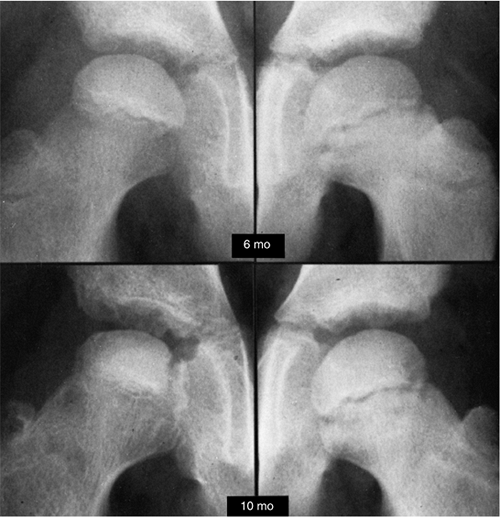
Figure 25.7
Anteroposterior radiogram of a hip in a patient who developed Legg-Calvé-Perthes disease. On the initial film, taken 6 months after the onset of symptoms, the right ossific nucleus is smaller than the left, and the medial joint space is widened. Note also the retained density of the ossific nucleus compared with the normal hip and the relative osteopenia of the viable bone of the proximal femur and pelvis. Ten months after the onset of symptoms, the evolution of the radiographic changes is seen. (From McKibbin B, ed. Recent advances in Perthes’ disease. Edinburgh: Churchill Livingstone, 1975.) |
 |
|
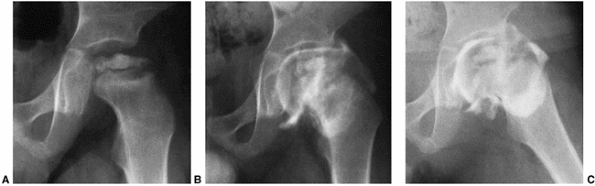
Figure 25.8 A boy, 4 years and 9 months of age, with Catterall group 4 disease and at-risk status. A: Plain radiograph. B:
Arthrogram in neutral abduction, adduction, and rotation. There is enlargement and flattening of the cartilaginous femoral head, and the lateral margin of the acetabulum is deformed by the femoral head. C: Arthrogram in abduction and slight external rotation. The femoral head hinges on the lateral edge of the acetabulum, further deforming the lateral acetabulum. Slight pooling of dye is seen medially. Note that the widened joint space is an apparent widening, not a real widening, and that it is secondary to continued growth of the superficial zone of cartilage in the absence of growth of the ossific nucleus. |
 |
|
Figure 25.9 A:
Catterall group 4 disease shows involvement of the whole head of the femur, with either diffuse or central metaphyseal lesions and with posterior remodeling of the epiphysis. B: Catterall group 4 disease, 2 months to 52 months after onset of symptoms. Note the stages: 14 months, fragmentation; 18 months, early reossification; 25 months, late reossification; 52 months, healed. Note also the growth-arrest line and evidence of reactivation of the growth plate along the femoral neck. |
changes in shape secondary to the disease process itself. The deepest
layer of the articular cartilage is nourished by the subchondral blood
supply. This layer is often devitalized in Legg-Calvé-Perthes syndrome (139,142,143,144,145,146,149). The superficial layers that are nourished by synovial fluid continue to proliferate, causing an increase in the thickness of
the articular cartilage. With trabecular collapse and fracture and
articular cartilage overgrowth, significant femoral head deformities
develop that are manifested clinically as loss of abduction and
rotation (Fig. 25.8).
 |
|
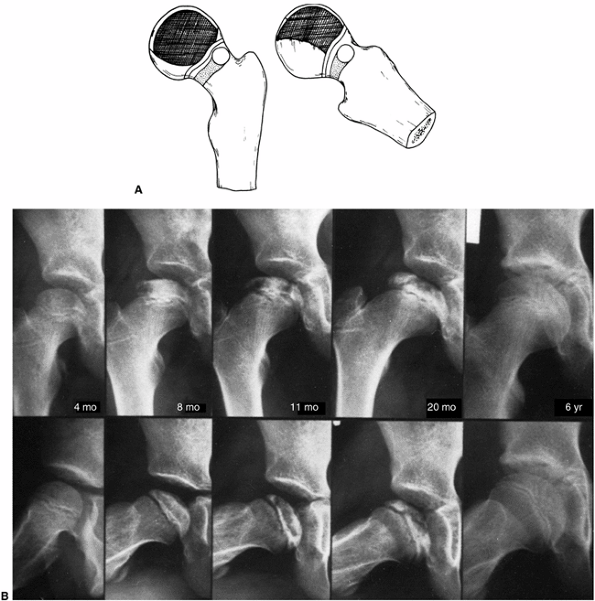
Figure 25.10 A:
Catterall group 3 disease shows large sequestrum involving three-fourths of the femoral head. The junction between the involved and the uninvolved portions is sclerotic. Metaphyseal lesions are diffuse, particularly anterolaterally, and the subchondral fracture line extends to the posterior half of the epiphysis. The lateral column is involved. B: Catterall group 3 disease, 4 months to 6 years after symptom onset. Note the involvement of the lateral pillar, as well as the subchondral radiolucent zone on the radiograph taken 8 months after onset of symptoms. |
have demonstrated that the new blood vessels arise from the metaphysis
and the metaphyseal periosteum, and penetrate between the epiphysis and
the joint cartilage into the epiphysis. Other investigators have shown
metaphyseal vessels penetrating the physeal plate into the epiphysis (113,184).
When the blood supply of the subchondral area is restored, it generally
comes from the periphery and moves to the center, first restoring
endochondral ossification at the periphery and causing asymmetric
growth (98,149) (Fig. 25.12).
In addition, there is abnormal ossification of the disorganized matrix
of the epiphyseal cartilage. Finally, there is periosteal bone growth
and reactivation of the physeal plate along the femoral neck, with
abnormally long cartilage columns leading to coxa magna and a widened
femoral neck (136,137).
 |
|
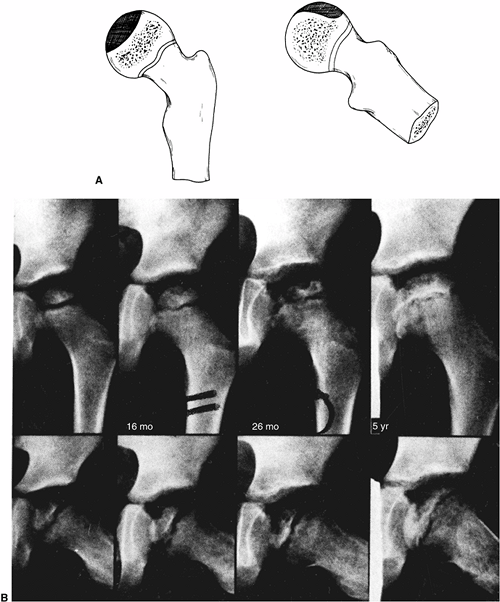
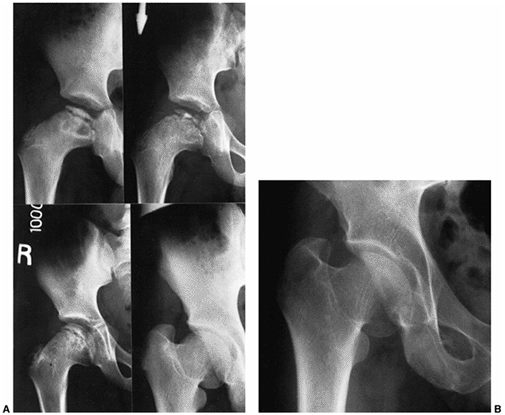
Figure 25.11 A: A 6-year-old boy with Catterall group 4 disease. At 6 years and 2 months of age, fragmentation stage (upper left). At 6 years and 9 months of age, early reossification stage (upper right). At 8 years and 9 months of age, healed (lower left). At 16 years and 2 months of age, skeletally mature (lower right). The patient’s hip healed with a central physeal arrest pattern. B:
A 51-year-old patient at 45-year follow-up. He was asymptomatic and had a full range of motion (Iowa Hip Rating, 95 of 100 points). At maximal fragmentation the hip is classified as showing Catterall group 4, Salter-Thompson type B, and lateral pillar type C disease. |
influenced by the duration of the disease. This, in turn, is
proportional to the extent of the epiphyseal involvement, the age of
the patient at the time of onset of the disease, the remodeling
potential of the patient, and the stage of disease when treatment is
initiated. An additional factor is the type of treatment chosen (185,186,187,188).
Legg-Calvé-Perthes syndrome: coxa magna, premature physeal arrest
patterns, irregular femoral head formation, and osteochondritis
dissecans (180,189). Coxa magna (Fig. 25.9)
develops with ossification of the hypertrophied articular cartilage,
and also from reactivation of the physeal plate along the femoral neck.
This also occurs in conjunction with periosteal new bone formation
along the femoral neck.
of two patterns of arrest: central or lateral. In the central arrest
pattern, the femoral neck is short and the epiphysis is relatively
round (Fig. 25.11). There is trochanteric
overgrowth and mild acetabular deformity. In the lateral arrest
pattern, the femoral head is tilted externally (Fig. 25.13). There is also trochanteric overgrowth. The epiphysis is oval, with a corresponding acetabular deformity (180,189).
certain patterns of physeal arrest, or it may be an iatrogenic
deformity from attempts at “containment” of a noncontainable head (Fig. 25.14).
After the femoral head becomes deformed and is no longer containable
within the acetabulum, the only motion that is allowed is in the
flexion and extension plane, with abduction leading to hinging on the
lateral edge of the acetabulum. This hinge abduction causes acetabular
deformity, leading to femoral head deformity (190,191,192).
 |
|
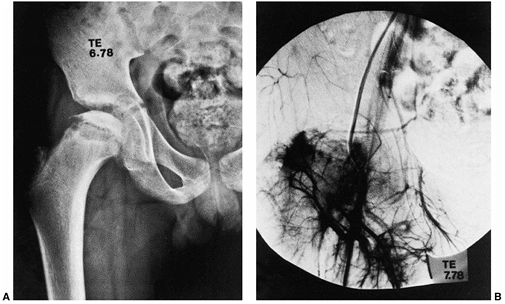
Figure 25.12 A: A 12-year-old boy with total femoral head involvement in the early fragmentation stage of the disease. B:
Subtraction arteriogram demonstrating the avascularity of the central portion of the femoral head, with increased vascularity at the periphery. (Courtesy of J. G. Pous, MD, Montpellier, France.) |
 |
|
Figure 25.13
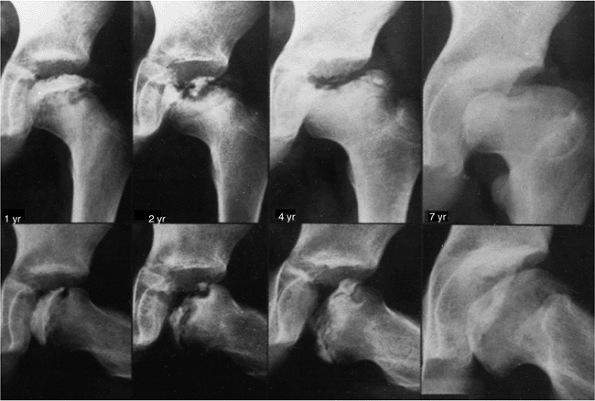
A 7-year follow-up from presentation in a patient with Catterall group 4 disease, who had a lateral growth-arrest pattern. At maximal fragmentation, the radiographic classification would be Salter-Thompson type B and lateral pillar type C disease. (From Weinstein SL. Perthes’ disease: an overview. Curr Orthop 1988;2:181.) |
 |
|
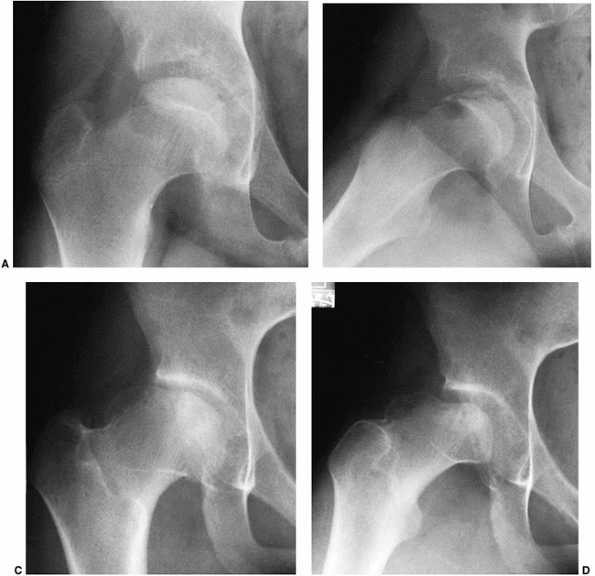
Figure 25.14 A girl, 11 years and 3 months of age, with Catterall group 3 disease had a noncontainable femoral head, yet was treated for a long time in an abduction brace. A, B: Anteroposterior radiograph in the early fragmentation stage (A) and Lauenstein radiograph in the early fragmentation stage (B). C, D: At age 14 years, the patient was skeletally mature, and had an irregular femoral head. Anteroposterior radiograph (C) and Lauenstein radiograph (D). (From Weinstein SL. Perthes’ disease: an overview. Curr Orthop 1988;2:181.)
|
deformity that occurs in Legg-Calvé-Perthes syndrome is osteochondritis
dissecans (Fig. 25.15). This usually occurs when there is late onset of disease, and with prolonged, ineffectual repair (180,189,193,194,195).
treating physician knows what would happen to the patient in the
absence of treatment (natural history), and what factors prognosticate
an adverse outcome. The treating physician must determine which of
these adverse prognostic factors can be affected by treatment. A
treatment plan is then initiated, and long-term follow-up determines
whether treatment favorably alters the course of the disease over the
long term. The fundamental problem in developing treatment plans for
patients with Legg-Calvé-Perthes syndrome is the paucity of natural
history data (72,196,197,198,199).
untreated hips of Murley and Lloyd-Roberts with a matched control group
of 51 hips treated with a weight-relieving caliper. The average age at
diagnosis was 4 years and 6 months, and the average
follow-up
was 10 years and 5 months, with a range of 4 to 18 years. The patients
were evaluated according to the grading system of Sundt, which requires
some subjective assessments (200).
The 10-year average follow-up in this series was too short to determine
the outcomes for patients and thus the natural history of the disease,
because most patients with childhood hip disease do well regardless of
the radiographic appearance in their early years (201,202,203,204). In addition, no data are presented on the interobserver or intraobserver reliability of the outcome criteria.
 |
|
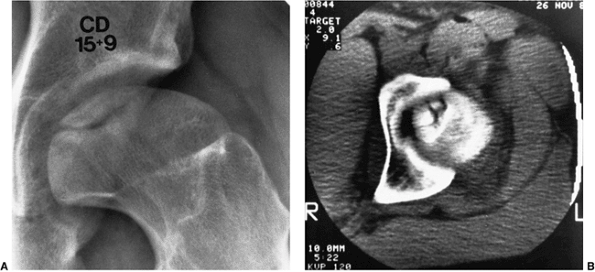
Figure 25.15 A:
A 15-year-old boy, whose disease started at 8 years and 6 months of age, returned to the physician with pain and synovitis. Anteroposterior radiograph demonstrates osteochondritis of the femoral head. B: Computed tomographic (CT) scan shows multiple fragments that appear as one on the radiograph. |
around the British Isles. The average follow-up in this series was only
6 years, and the results were graded according to the aforementioned
system of Sundt (200). The outcomes in this group of patients (Table 25.1)
are widely quoted in the literature as a comparison for outcomes of
various treatment modalities. Unfortunately, very few articles in the
literature use the same grading system for outcomes, and the follow-up
of this group is too short to be defined as natural history.
is not a natural history study but a study of patients from three
centers, treated by different methods. This study attempted both to
establish a relation between residual deformity and degenerative joint
disease and to identify clinical and radiographic factors in the active
phase of disease that would be predictive of hip deformity and
degenerative joint disease. Therefore, as will be further discussed
later in the chapter, decision making with reference to treatment is
difficult because of the lack of true long-term natural history data.
natural history, there are many long-term follow-up studies of patients
with Legg-Calvé-Perthes syndrome. The long-term studies that are
available suffer from the faults of retrospective long-term reviews in
that most series contain only small numbers of patients, with many of
the original patients not traced; original radiographs often are not
available. Many of the longer series contain patients diagnosed in the
years 1910 to 1940, when little was known about the disease, prognostic
factors, and radiographic classifications. In most series, patients are
combined regardless of what are now known to be prognostic factors: the
extent of epiphyseal involvement, age at onset of the disease, age at
the
beginning of treatment, and stage of the disease at treatment
initiation. Various treatment modalities are combined in many series,
and control groups are generally absent. Because of these inherent
problems, and the fact that different grading systems are used in
judging clinical and radiographic end results, all of which lack
interobserver and intraobserver reliability data, it is difficult to
compare and contrast the various reported series. Despite these
shortcomings, a great deal has been learned about the prognosis in
Legg-Calvé-Perthes syndrome.
|
TABLE 25.1 RESULTS FOR 97 UNTREATED HIPS
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
that results can improve with time, because remodeling potential
continues until the end of growth (72,205) (Figs. 25.9 and 25.10).
Mose wrote that, “for a precise prognosis, conclusions from any
measurements ought not be made before the patient reaches the age of
16, when growth stops” (204). Reviews of the outcomes of treatment modalities before skeletal maturity must be viewed as preliminary reports.
(70% to 90%) patients with Legg-Calvé-Perthes syndrome are active and
free of pain. Most patients maintain a good range of motion, despite
the fact that few have normal-appearing radiographs. Clinical
deterioration and symptoms of increasing pain, decreasing range of
motion, and loss of function are observed only in patients with
flattened irregular heads at the time of primary healing, and in
patients with premature physeal closure, as indicated by femoral neck
shortening, femoral head deformity, and trochanteric overgrowth (186) (Fig. 25.13).
reported a 33-year follow-up of 35 patients. Twenty-eight of the 35
patients were free of pain, with 34 of 35 functioning without
restrictions. In a 34-year follow-up, Hall (207) reported satisfactory results in 71% of 209 cases. Perpich et al. (208)
reported a 30-year follow-up of 37 patients. The average Iowa Hip
Rating was 93 of a possible 100 points. Eighty-five percent of the
patients had good clinical results, despite the fact that only 33% had
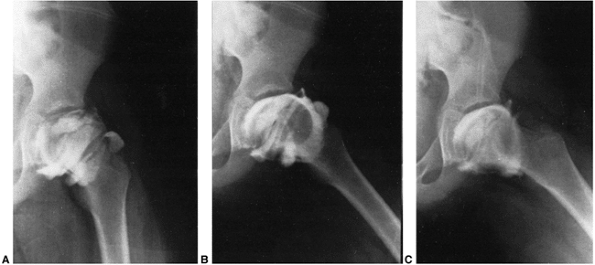
spherical femoral heads, as rated by the Mose Sphericity Scale (204) (Fig. 25.16). Forty-three percent of the patients had poor Mose ratings; however, of these patients, 76% had good clinical results.
patients for an average of 30 years and noted that 80% were fully
active and free of pain, whereas only 40% were radiographically normal.
He followed 16 of these patients for an additional 11 years (210)
and noted that, despite the fact that only one-third of them had good
anatomic results, “deterioration rarely occurred and many patients had
no pain and [maintained] normal activity.”
patients (106 hips), all of whom had noncontainment treatment, for an
average of 35 years. At maturity, 61% had poor results by the Mose
criteria. In a final follow-up, 48% had evidence of degenerative joint
disease. However, at an average 35-year follow-up, only 4% had
undergone total hip arthroplasty, with an additional 13% having
clinical symptoms significant enough to warrant arthroplasty. Ippolito
et al. (202) reported on 61 patients with an
average follow-up of 25 years. Only 19% of their patients had poor
results, as measured by the Iowa Hip Rating, at final follow-up. W.J.
Cumming (personal communication, 1997) reported on 82 patients with 95
involved hips treated by prolonged frame recumbency, with an average
follow-up of 38 years. Only 10% of the patients had required
arthroplasty at follow-up, with an additional 10% having symptoms
significant enough to warrant arthroplasty.
 |
|
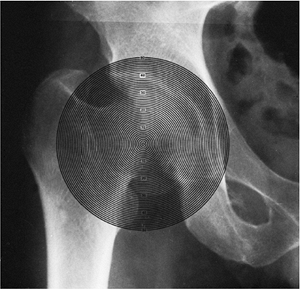
Figure 25.16 Mose Sphericity Scale.
|
reported on 30 nonoperated hips with an average 36-year follow-up. This
series is representative of other 20- to 40-year long-term series
reported in the literature. The average Iowa Hip Rating for these 30
patients was 91 points. The typical patient had minimal shortening,
absent or mild hip pain, and minimal or no functional impairment with
respect to their jobs and activities of daily living. Ninety-two
percent of the patients had Iowa Hip Ratings higher than 80 points, and
only 8% of them had undergone arthroplasty.
begins to deteriorate. In another study of the Iowa group of patients
at 48-year follow-up, McAndrew and Weinstein (203)
reported that only 40% of patients maintained an Iowa Hip Rating of
better than 80 points. Forty percent of the patients had undergone
arthroplasty, and an additional 10% had disabling osteoarthritis
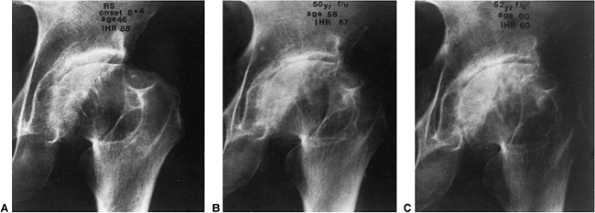
symptoms but had not yet undergone arthroplasty (Fig. 25.17).
Further, at 48-year follow-up, 50% of the patients had disabling
osteoarthritis and pain, and an additional 10% had Iowa Hip Ratings of
less than 80 points. The prevalence of osteoarthritis in this group of
patients was ten times that found in the general population in the same
age range (186). Mose followed a group of patients into the 7th decade of life. All of the
patients with irregular femoral heads had degenerative arthritis. Of
those patients with femoral heads that Mose classified as “normal, ball
shaped,” no patient had degenerative joint disease by the middle of the
4th decade, but 67% had severe degenerative arthritis by the middle of
the 7th decade (204).
Therefore, the follow-up studies beyond 40 years demonstrate marked
reduction of function, with most of the patients developing
degenerative joint disease by the 6th and 7th decades (180,199,202,203,204).
 |
|
Figure 25.17
This patient had disease onset at 8 years and 3 months of age. At 46 years of age (38-year follow-up), the Iowa Hip Rating was 88 points (A). At 58 years of age (50-year follow-up), there was a loss of 21 points on the Iowa Hip Rating, to 67 (B). At 60 years of age, just before arthroplasty, the Iowa Hip Rating was 60 points (C). (From Weinstein SL. Legg-Calvé-Perthes’ disease: results of long-term follow-up. In: Fitzgerald RH Jr, ed. The Hip: Proceedings of the Thirteenth Open Scientific Meeting of the Hip Society. St. Louis, MO: Mosby, 1985:28.) |
Legg-Calvé-Perthes syndrome, certain clinical and radiographic features
have been identified that have prognostic value (186,190,201,212,213,214,215,216) (Table 25.2).
The most important prognostic factor in determining the outcome is the
residual deformity of the femoral head, coupled with hip joint
incongruity (217,218,219).
Femoral head deformity and joint incongruity are multifactorial
problems. They are interrelated with all of the other prognostic
factors. It must be kept in mind that Legg-Calvé-Perthes syndrome
represents a growth disturbance of the proximal femur; the epiphyseal
and physeal cartilage is abnormal. Other key factors involved in the
development of deformity include the extent of epiphyseal involvement
and the varying degrees and patterns of premature physeal closure
associated with this condition (220).
|
TABLE 25.2 PROGNOSTIC FACTORS
|
|
|---|---|
|
established a relation between residual deformity and degenerative
joint disease. This was accomplished by retrospectively examining the
long-term outcomes of patients from three different centers treated by
various methods (e.g., bed rest, spica cast, ischial weight bearing,
brace, crutches, cork shoe lift on the normal side, combination of
methods). They attempted to identify clinical and radiographic factors
in the active phase of the disease that were predictive of the
development of hip deformity. They proposed a radiographic
classification of deformity relating to long-term outcome (Table 25.3).
The more deformity there was at maturity (i.e., the higher the Stulberg
classification), the worse the long-term outcome. However, as noted
from long-term follow-up studies, it is the class 5 hips that
deteriorate the earliest; they usually have significant symptoms by the
end of the 4th decade (201,202,203,204).
Patients with aspherical congruency (Stulberg class 3 and 4 disease)
may have satisfactory outcomes for many years, with most patients
undergoing significant functional deterioration in the 5th and 6th
decades of life (201,202,203,204).
This classification scheme, which attempts to classify a
three-dimensional deformity using two-dimensional parameters, has been
shown to have poor interobserver and intraobserver reliability (221). The general principles expressed by Stulberg et al. (199),
however, have been shown to have validity with reference to long-term
outcome studies. That is, the more out of round the femoral head is,
and the greater the discrepancy between the shape of the femoral head
and the shape of
the acetabulum, the greater the chance of development of early degenerative joint disease.
|
TABLE 25.3 STULBERG CLASSIFICATION
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||
have confirmed Waldenstrom’s original finding that partial or anterior
femoral head involvement leads to a more favorable prognosis than
whole-head involvement. Catterall (72,138,196)
demonstrated the importance of the extent of epiphyseal involvement
with regard to prognosis, and he proposed four groups on the basis of
the presence or absence of seven radiographic signs observed in 97
untreated hips (Figs. 25.6, 25.9, 25.10, and 25.18). He compared the final radiograph with the initial radiograph, using the clinical grading of Sundt (200);
90% of the patients who had good results were in group 1 or 2, whereas
90% of those who had poor results were in group 3 or 4. This commonly
used classification has been criticized as being difficult to use in
that there may be a great deal of interobserver error (213,225,226,227).
It also has been criticized as being insufficiently prospective,
because it may take up to 8 months for the hip to be far enough into
the fragmentation phase to show the extent of epiphyseal involvement (228,229).
Furthermore, it also has been noted that the classification may change
when radiographs taken during the initial phase are compared with those
taken at maximal fragmentation (229,230).
described a simplified two-group classification based on prognosis and
determined by the extent of the subchondral fracture line, which
appears early in the course of the disease: in group A less than half
of the head is involved (Catterall groups 1 and 2), and in group B more
than half of the head is involved (Catterall groups 3 and 4). The major
distinguishing factor between groups A and B is the presence or absence
of a viable lateral column of the epiphysis. This intact lateral column
(i.e., Catterall group 2, Salter-Thompson type A) may shield the
epiphysis from collapse and subsequent deformity (Fig. 25.18).
the height of the femoral head has been described as important by
several investigators (72,207,213,231,232). Hall (207)
reported on the long-term follow-up (34 years) of 209 hips. He
considered loss of femoral head height, as seen on the initial
radiograph, to be an important prognostic sign. All of his patients in
whom there had been a loss of 2 mm or more of height of the femoral
head in the affected hip, compared with the unaffected hip, had
unsatisfactory results in adult life. Patients in whom the height of
the femoral head was within 2 mm of that of the unaffected hip on the
initial radiograph had good results in all but six cases.
radiographic classification based on the radiolucency of the lateral
pillar of the femoral head on anteroposterior (AP) films during the
fragmentation phase of the disease (Table 25.4) (Figs. 25.11, 25.13, and 25.19).
The lateral pillar occupies the lateral 15% to 30% of the femoral head
width on an AP radiograph. The central pillar occupies approximately
50% of the head width, and the medial pillar occupies 20% to 35% of the
medial aspect of the head width on an AP radiograph.
|
TABLE 25.4 LATERAL PILLAR CLASSIFICATION
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
||||||||
 |
|
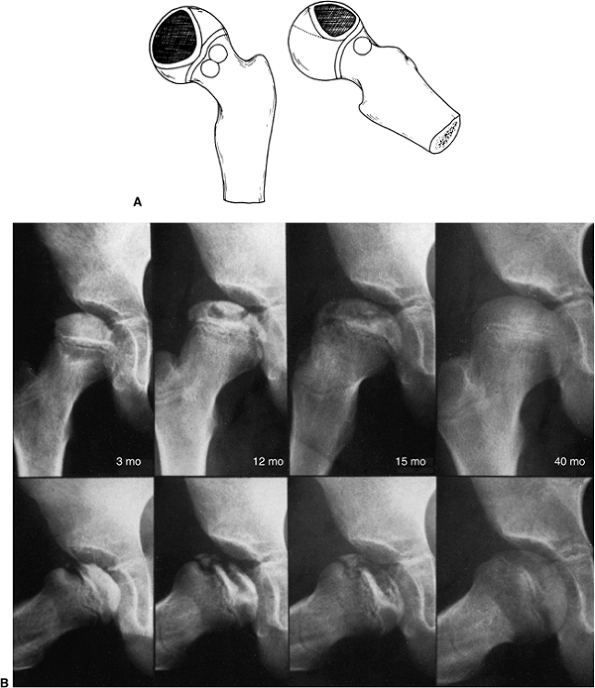
Figure 25.18 A:
Catterall group 2 disease showing anterolateral involvement, sequestrum formation, and a clear junction between the involved and uninvolved areas. There are anterolateral metaphyseal lesions, and the subchondral fracture line is in the anterior half of the head. The lateral column is intact. B: Catterall group 2 disease. Three to 40 months after onset of symptoms, the lateral pillar is still intact. |
Intraobserver reliability was reported to be 0.78, with a good
correlation of outcome, as measured by the classification of Stulberg
et al. (199). The importance of the integrity
of the lateral column is seen in other classifications, with patients
in Salter-Thompson type A and Catterall groups 1 and 2 having intact
lateral columns. The results of treatment in long-term outcome studies
show this to be an important prognostic factor (202,213,230,233,234) (Fig. 25.20). The reliability of this classification and its utility in Perthes disease will require further study (226,235).
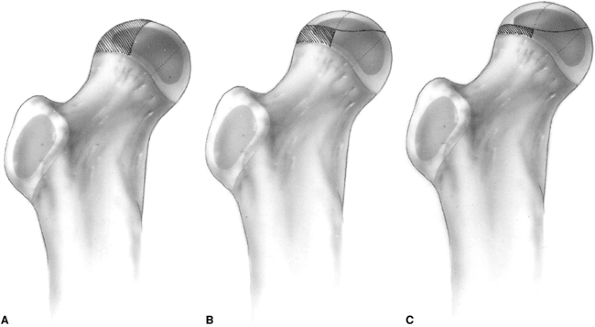 |
|
Figure 25.19 Lateral pillar classification (see Table 25.4 for a description of A, B, and C).
|
Results in untreated patients show that there were no poor results in
patients who did not have two or more of the radiographic at-risk signs
during the active stage of the disease. Radiographic at-risk signs
include the Gage sign (a radiolucency in the lateral epiphysis and
metaphysis) and calcification lateral to the epiphysis. These two signs
are indicative of early ossification in the enlarged epiphysis. They
are present only when the head is deformed. These signs are present
when the changes are reversible with treatment (72,238).
A third at-risk sign is metaphyseal lesions. These metaphyseal
radiolucencies may herald the potential for a growth disturbance of the
physeal plate (153,239,240). The final two at-risk signs are lateral subluxation and a horizontal growth plate (241).
Lateral subluxation is indicative of a widened head. A horizontal
growth plate (adducted hip) is indicative of a developing femoral head
deformity that, if left untreated, will lead to fixed deformity, hinge
abduction, and subsequent further deformity. These radiographic at-risk
signs are manifested clinically as loss of motion and adduction
contracture. Catterall reported no poor results in patients who did not
manifest at-risk signs. The validity of the Catterall classification
and the at-risk signs has been confirmed by several series (229,242,243,244,245,246,247,248,249), but questioned by others (202,213,230,250).
lateral and superior subluxation, which are indicative of significant
growth disturbance and flattening of the femoral head, as the key
factors associated with the development of class 3 and class 4 hips and
poor long-term outcome (i.e., after 40 years). Disease onset after the
age of 9 years and partial femoral head involvement, particularly
anterosuperior quadrant involvement, were associated with the
development of a class 5 hip and the early onset of degenerative joint
disease (i.e., 3rd to 5th decade of life).
epiphyseal involvement. In general, the greater the extent of
epiphyseal involvement, the longer the duration and course of the
disease. End results are worse with prolonged disease duration (205,213,251,252).
The extent of epiphyseal involvement is also related to the sex of the
patient in that girls affected by Legg-Calvé-Perthes syndrome have a
poorer prognosis than boys (253,254).
This may be explained by the fact that there are more girls (who tend
to be more skeletally mature than comparably aged boys, and hence have
less remodeling potential) with Catterall group 3 or group 4 disease,
which are the groups associated with a less favorable prognosis (159).
significant factor related to outcome; only deformity is more
significant. Eight years seems to be the watershed age in most
long-term series (186,203,255,256,257,258);
however, some authors believe that the prognosis is markedly worse for
long-term outcome in patients older than 6 years at the onset of the
disease (202). W.J. Cumming (personal
communication, 1997) estimated that 45% of patients with onset of
Perthes disease after the age of 6 years have undergone arthroplasty by
age 60 years. Patients older than 11 or 12 years, even with Catterall
group 2 or Salter-Thompson
type A disease, may have poor anatomic and clinical results, even with treatment (259). Age at healing, however, is probably a more important factor (Fig. 25.11). The overall skeletal maturation delay (49) in patients with Legg-Calvé-Perthes syndrome, and the usual compensation for this delay during the pubertal growth spurt (50),
contribute to the favorable prognosis in the young patient. The more
immature the patient at the time of entering the reossification stage,
the greater the potential for remodeling. At-risk signs are also less
likely to occur in younger patients, particularly those younger than 5
years.
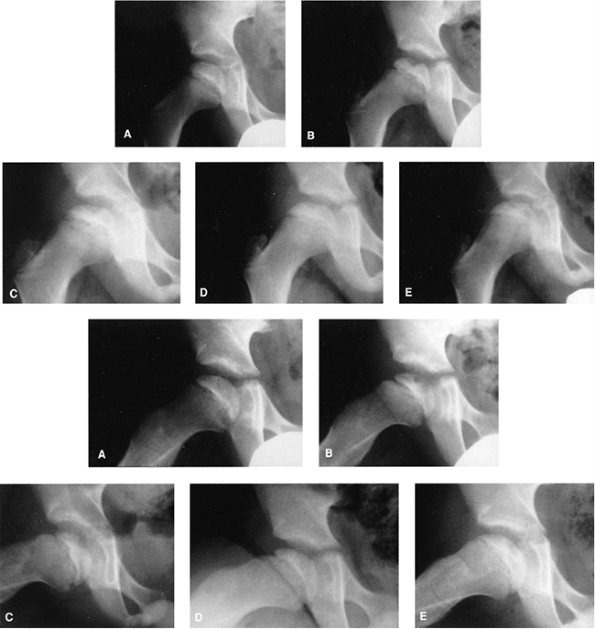 |
|
Figure 25.20 Anteroposterior (top, A–E) and lateral (bottom, A–E) views of a 7-year-old boy who presented with hip pain and a limp. A: At presentation, the patient was in the initial radiographic stage of the disease; his prognosis was indeterminate. B:
Six months after presentation, he had minimal loss of height of the lateral pillar and some radiolucency in that region, as well as significant bone resorption centrally. Note how the lateral pillar maintains its height throughout the course of the disease. C–E: One year (C), 18 months (D), and 3 years (E) after onset of disease. The patient had only mild symptoms on occasion, and maintained good range of motion throughout the course of the disease. Only symptomatic treatment was provided. |
the prognosis, is the shape of the femoral head and its relationships
to acetabular shape (congruency) and joint motion. The shape of the
acetabulum depends on the geometric pattern within it during growth (181,260,261). In addition, the acetabulum continues to have significant potential for development until the patient is 8 or 9 years
of age (85,87,262).
If a young patient develops a deformity of the proximal femur because
of Perthes disease, the immature acetabulum conforms to the altered
shape of the femoral head. This may lead to the development of an
aspherical congruency (Stulberg classes 3 and 4) that may be compatible
with normal function for many years. In older patients (whether “older”
means older than 6 years or older than 8 years is subject to debate),
the acetabulum cannot conform to the shape of a deformed femoral head;
there is thus a greater chance of the development of an incongruous
relation between the two, leading to early degenerative joint disease (199,201,203,204,261,263).
 |
|
Figure 25.21
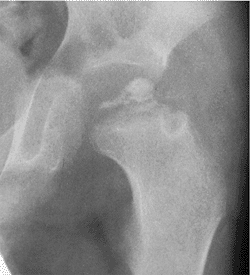
A boy, 6 years and 5 months of age, with Catterall group 4 disease demonstrates all of the at-risk signs: Gage sign, calcification lateral to the epiphysis, metaphyseal lesions, lateral subluxation, and horizontal growth plate. |
in a study of 80 patients with Legg-Calvé-Perthes syndrome, showed
interference with physeal growth in 90% of them, with 25% having
premature physeal closure. They demonstrated a direct correlation
between the severity of physeal involvement and deformity of the
femoral head. Clarke and Harrison (264)
reported that 47% of 31 patients who presented with painful hips after
Legg-Calvé-Perthes syndrome, at an average age of 27 years, showed
evidence of premature physeal closure.
used a transparent protractor with concentric circles drawn at 2 mm of
radial difference to evaluate the shape of the femoral head. Mose
further developed Goff’s method and applied it clinically. This is the
most commonly used method of measuring sphericity (104,266,267,268) (Fig. 25.16).
It is not clear from the criteria of Mose whether the measurement under
consideration is the difference between the outline of the femoral head
on the AP and lateral radiographs, or the deviation from a given
circle, measured in millimeters, on either the AP or the lateral
radiograph, or a combination of these two parameters. This variability
in the application of the method of Mose et al. is evident in the
literature on Legg-Calvé-Perthes syndrome (244,245,269,270,271,272,273,274).
superimposed on the AP and lateral radiograms. In the author’s
practice, if the outline of the femoral head is a perfect circle in
both projections, it is rated good; less than 2 mm of deviation is
rated fair; and more than 2 mm of deviation from a circle, in the AP or
lateral projection, is rated poor. Regardless of the measurements used,
it is important to realize that, with growth and remodeling of the
femoral head and acetabulum, the various parameters used for measuring
head deformity and congruency may change.
measured at skeletal maturity, are probably the most reliable
indicators of prognosis and the development of degenerative joint
disease. Catterall (72) showed, in a follow-up
of untreated patients, that 33% of the patients improved in anatomic
grade. Twenty percent of these patients improved two anatomic grades;
all of these patients were younger than 5 years at the time of onset of
the disease. However, it must also be remembered that the various
deformities of the femoral head and anomalies in acetabular congruency
are three-dimensional parameters that cannot be measured adequately on
two-dimensional radiographs. Thus far, the only existing radiographic
parameter that correlates with good clinical outcome is a perfectly
spherical femoral head. Loss of sphericity by itself, however, does not
necessarily lead to a poor long-term result (186,188,217).
which demonstrated that after the femoral head is in the reossification
stage of the disease, it will not deform further. If a treatment for
femoral head deformity is to be successful, it must be instituted early
in the course of the disease, that is, in the initial or fragmentation
stage, hence the importance of radiographic staging of patients.
present with a history of an insidious onset of a limp. Most patients
do not complain of much discomfort, unless specifically questioned
about this aspect. Pain, when present, is usually activity-related and
relieved by rest. Because of the mild nature of the symptoms, most
patients do not present for medical attention until weeks or months
after the clinical onset of disease. The pain is generally localized to
the groin, or referred to the anteromedial thigh or knee
region.
Failure to recognize that the thigh or knee pain in the child may be
secondary to hip pathology may cause further delay in the diagnosis.
Some children present with more acute onset of symptoms. Seventeen
percent of patients with Legg-Calvé-Perthes syndrome may have a history
of related trauma (24,27,276).
presents with limited hip motion, particularly abduction and medial
rotation. Early in the course of the disease, the limited abduction is
secondary to synovitis and muscle spasm in the adductor group; however,
with time and the subsequent emergence of deformities, the limitation
of abduction may become permanent. Longstanding adductor spasm
occasionally leads to adductor contracture. The Trendelenburg test in
patients with Legg-Calvé-Perthes syndrome is often positive. These
children most commonly have evidence of thigh, calf, and buttock
atrophy from disuse secondary to pain. This is additional evidence of
the longstanding nature of the condition before detection (1,2,3,4,5,185,265).
Limb length should be measured; inequality is indicative of significant
collapse of the femoral head and a poor prognosis. Evaluation of the
patient’s overall height, weight, and bone age may be helpful in ruling
out skeletal dysplasias or growth disorders in the differential
diagnosis, and may provide confirmatory evidence of the disorder.
Laboratory studies are generally not helpful in Legg-Calvé-Perthes
syndrome, although they may be necessary for ruling out other
conditions (see section on differential diagnosis).
in the AP and frog-leg lateral positions are used in making the initial
diagnosis, and also for assessing the subsequent clinical course. These
radiographs are generally sufficient for the assessment of the patient
and subsequent follow-up evaluations. From the plain radiographs, the
extent of epiphyseal involvement (e.g., Catterall groups 1 to 4;
Salter-Thompson type A or B; lateral pillar type A, B, or C) and the
stage of the disease (initial, fragmentation, or reossification) can be
determined. According to Salter and Thompson, if appropriate
radiographs are taken within 4 months of the clinical onset of the
disease, the subchondral radiolucent zone will be detectable (121).
Catterall, however, states that this sign is helpful in only 25% of the
cases, because it is present only transiently in the early phases of
the disease (72). It is most important, while
following the course of the disease, to view all radiographs
sequentially and compare them with previous radiographs, so as to
assess the stage of the reparative process and determine the constancy
of the extent of epiphyseal involvement. Additional radiographic or
imaging studies are rarely necessary but may be helpful in the initial
assessment and also in the follow-up of the condition (277,278,279).
may be helpful in the early stages of the disease, when the diagnosis
is in question, particularly if the differential diagnosis is between
transient synovitis and Perthes disease. Some investigators consider
scintigraphy to be helpful in determining the extent of epiphyseal
involvement and the prognosis (271,280,281,282,283,284,285,286,287,289).
medical centers. It appears to be sensitive in detecting infarction,
but cannot yet accurately portray the stages of healing. Its role in
the management of Perthes syndrome has yet to be defined. In the
future, magnetic resonance imaging not only may help the clinician in
the diagnosis, but may shed additional light on the underlying
pathology of the condition (98,287,290,291,292,293) (Fig. 25.23).
radiographs (Fig. 25.24). It can be used to demonstrate the hinge abduction (Fig. 25.8, 25.25, and 25.26) phenomenon with abduction of the leg (163,190,191,217,294).
Arthrography, in conjunction with plain radiography or computed
tomography, also may be useful in the diagnosis of osteochondritis
dissecans secondary to Perthes disease. Arthrography is most useful for
assessing the shape of the femoral head and its relation to the
acetabulum, both of which are necessary for treatment decisions (Figs. 25.25, 25.26, 25.27).
Where there is severe flattening of the femoral head, arthrography is
helpful in determining containability before any treatment is started,
whether it is Petrie casts or surgery. It is also useful in determining
the best position of containment (e.g., internal or external rotation,
and abduction or adduction) if surgical management is considered.
 |
|
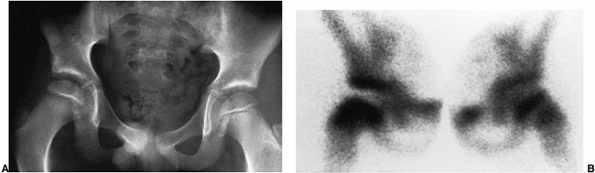
Figure 25.22 An 8-year-old boy with right hip pain. A:
Anteroposterior radiograph demonstrates a slight increase in width and medial joint space; the femoral ossific nucleus is slightly smaller than the one on the opposite side. B: Technetium 99 radionuclide scan demonstrates decreased uptake in the entire right femoral head, with increased vascularity in the neck. |
 |
|
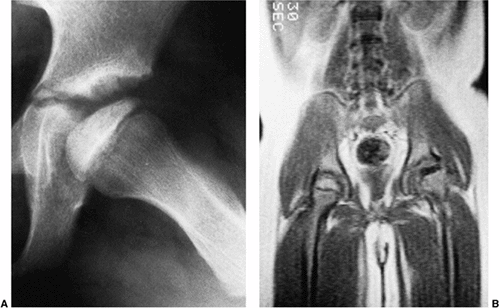
Figure 25.23 A 6-year-old boy with Catterall group 3 disease in the early fragmentation stage. A: Plain radiograph shows apparent sparing of the posterior head. B:
Magnetic resonance image demonstrates a complete absence of signal on the affected side. (Courtesy of Peter Scoles, MD, Case Western Reserve Medical School, Cleveland, Ohio.) |
plain radiographs are usually sufficient for making a diagnosis of
Legg-Calvé-Perthes syndrome (Table 25.5).
Diagnosis early in the initial phase of the disease requires that it be
differentiated from conditions such as septic arthritis, whether
primary or secondary to proximal femoral osteomyelitis, and toxic
synovitis (295,296,297).
A complete blood count including white cell differential, erythrocyte
sedimentation rate, C-reactive protein, and hip joint aspiration and
analysis of the fluid may be necessary in order to rule out infection.
In patients with Legg-Calvé-Perthes syndrome all laboratory results are
usually normal except the erythrocyte sedimentation rate, which may be
slightly elevated. In early cases, if all of the laboratory and plain
radiographic studies are normal, but doubt regarding the diagnosis
persists, radionuclide scanning or magnetic resonance imaging may be
helpful.
|
TABLE 25.5 DIFFERENTIAL DIAGNOSIS
|
|
|---|---|
|
 |
|
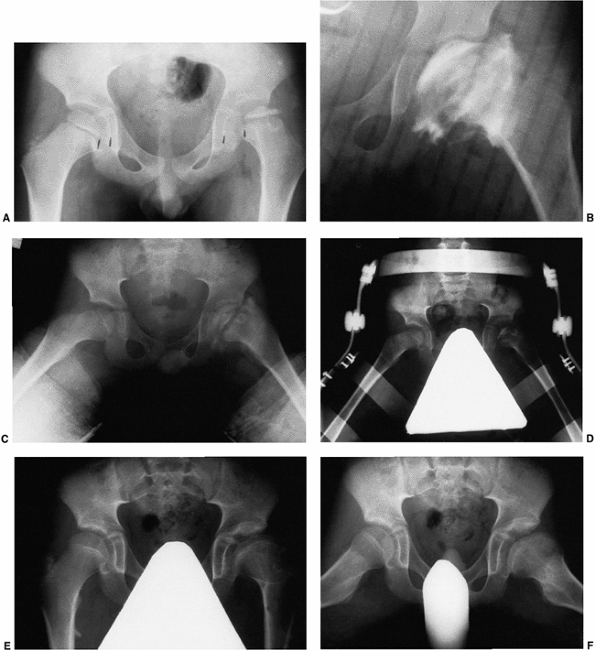
Figure 25.24 A 5-year-old boy with Catterall group 4 disease and at-risk status. A: Anteroposterior radiograph on presentation. B: Anteroposterior arthrogram, in the same position as in C,
after 10 days of traction. Note the relation between the lateral acetabular margin and the lateral margin of the cartilaginous femoral head, as well as the severe flattening of the femoral head. C: Anteroposterior radiogram in Petrie broomstick abduction plasters. The patient was maintained in casts for 6 weeks. D: Anteroposterior radiograph with pelvis abduction orthosis (weight bearing). E: Anteroposterior radiograph at age 13 years. Note residual deformity. F: Lauenstein radiograph at age 13 years. |
 |
|
Figure 25.25
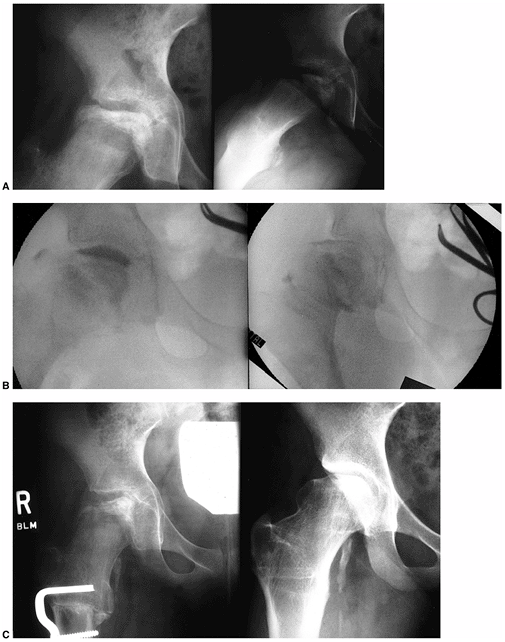
An 11-year-old girl with Catterall group 4 Perthes disease. The range of motion of the hip showed marked restriction of abduction (20 degrees) and rotation (10 degrees internal and external). All movement was painful. A: Preoperative anteroposterior (left) and Lauenstein (right) views. B: Intraoperative arthrograms demonstrating hinging on the lateral aspect of the acetabulum in abduction (left) with good congruity in adduction (right). C: Six-month (left) and 7-year (right) follow-ups after abduction osteotomy. At 7-year follow-up, the patient was free of pain, with 40 degrees of abduction, 20 degrees of adduction, flexion to 130 degrees, and 20 degrees of internal and external rotation. She has been pain-free since her surgery. |
 |
|
Figure 25.26
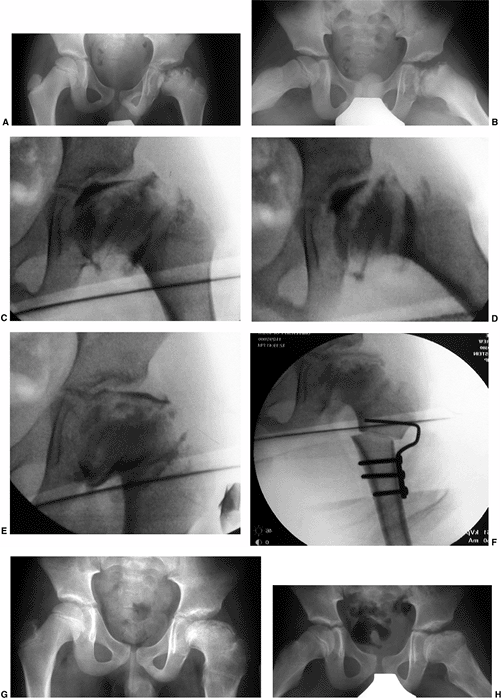
A 9-year-old boy presenting with hip pain, nonresponsive to nonsurgical measures. Clinical abduction is to 10 degrees; adduction is to 40 degrees with the hip in extension. A: Antero-posterior view of pelvis showing total femoral head involvement in reossification stage of disease. B: Lauenstein view. C: Arthrogram in neutral position showing considerable flattening of femoral head and slight impingement on lateral edge of the acetabulum. D: Arthrogram in abduction demonstrating hinge abduction. E: Arthrogram in adduction demonstrating reasonable congruity between femoral head and acetabulum; note normal contour of lateral acetabular edge. F: Abduction osteotomy allowing 45 degrees of abduction and 0 degrees of adduction. G: Three years postoperatively. Patient is pain-free with 45 degrees of abduction, 10 degrees of adduction, and good rotation. H: Lauenstein view 3 years postoperatively. |
disorders such as hypothyroidism and multiple epiphyseal dysplasia must
be considered (298,299,300).
In patients with bilateral involvement, particularly those with
atypical radiographic features, care should be taken to obtain a
detailed family history, measurements of height and weight should be
recorded, and a bone survey should be done in order to rule out a
metabolic or genetic condition (see Chapters 6 and 7).
The possibility of Meyer dysplasia, a benign self-resolving condition,
must be considered in children younger than 4 years of age (301).
believed that treatment could not prevent degenerative joint disease.
Because there is a paucity of long-term natural history data available,
the question must be raised whether the outcome of Legg-Calvé-Perthes
syndrome can be altered by any particular treatment. Although surgical
management has become very popular today, long-term series of patients
with uniform treatment, and matched for age, gender, stage, and extent
of epiphyseal involvement, are necessary in order to determine the most
effective treatment of Perthes syndrome.
Treatment must be considered only for those patients who have an
otherwise known poor prognosis based on prognostic factors gleaned from
long-term follow-up. It is difficult to formulate specific treatments
for patients because the natural history of the condition is not well
known.
Also,
most studies of current treatment methods lack interobserver and
intraobserver reliability as regards classifications of the extent of
epiphyseal involvement and outcome measures, and all the studies lack
control groups. These factors, and other variables that exist in most
series, make it difficult to support a “best” method of treatment.
 |
|
Figure 25.27 Arthrogram of a 6-year-old boy with Catterall group 4 disease. A: Neutral position. B:
Abduction, external rotation, and slight flexion (the position that would be maintained by an abduction Scottish Rite-type orthosis). C: Abduction and internal rotation (the position that would be maintained by a varus derotation osteotomy or innominate osteotomy). |
features of the various disease classification schemes. Under these
protocols, no treatment is warranted in patients with a good prognosis
(i.e., those with Catterall group 1, Salter-Thompson type A, or lateral
pillar type A disease) (Table 25.6). Patients
with a poor prognosis should be considered for treatment. These would
include patients with Catterall groups 3 and 4 disease, Salter-Thompson
type B disease, and lateral pillar type C disease. There is another
large group of patients whose prognosis is indeterminate; these
patients require careful follow-up, because they may need treatment at
a later date. This group includes patients with Catterall group 2
disease (good prognosis in 90% of cases) and patients with lateral
pillar type B disease. Because we have learned that the two major
prognostic factors in outcome are deformity of the femoral head and age
of the patient, these two factors must be taken into account in the
decision-making process. Patients with deformity (arthrographically
and/or clinically documented by persistent loss of motion, particularly
abduction) younger than 8 years should be considered for treatment.
Patients older than 8 years, especially girls, should be considered for
treatment even in the absence of deformity. In the absence of
treatment, such patients have a poor prognosis (257,258,304)
because their growth potential is insufficient for any deformity of the
proximal femur to be compensated for by a corresponding change in the
shape of the acetabulum.
|
TABLE 25.6 TREATMENT OF LEGG-CALVÉ-PERTHES DISEASE
|
|
|---|---|
|
at-risk signs (i.e., if they lose range of motion and have pain), or if
they demonstrate several of the radiographic at-risk signs regardless
of the extent of epiphyseal involvement. If the patient is already in
the reossification or healing stage of the disease, little further
deformity ensues, and no treatment is indicated unless the patient has
symptoms (see the section “Treatment Options in the Noncontainable Hip and the Late-presenting Patient with Deformity” later in this chapter).
the femoral head was reossified. These methods were based on the
premise that weight relief would prevent the mechanical deformation of
the head and early degenerative joint disease (89,305,306,307).
These modalities included prolonged, strict bed rest, often in the
hospital, and bed rest with or without various periods of traction on
special frames or in spica casts. These methods of treatment were
associated with disuse atrophy of muscles, osteopenia, shortening of
the involved extremity, loss of thoracic kyphosis, urinary calculi,
social and emotional problems, and high hospital costs (160,209,210,270,308,309,310,311,312,313,314,315).
Legg-Calvé-Perthes syndrome was challenged as early as 1927, when Legg
stated that, “while the process suggesting weakness of bone structure
is going on it is theoretically sound to allow no weight bearing but in
practice relief from weight bearing in no way affects the end results” (316). In addition, prolonged immobilization and bed rest do not influence the radiographic course of the disease (228,248,249,317,318,319). Harrison and Menon (312) pointed out, as had Pauwels and others (160,320,321), that even at rest or during minimal activity significant forces act on the femoral head.
The essence of containment is that, in order to prevent deformities of
the diseased epiphysis, the femoral head must be contained within the
depths of the acetabulum, thereby equalizing the pressure on the head
and subjecting it to the molding action of the acetabulum. Containment
is an attempt to reduce the forces through the hip joint by actual or
relative varus positioning (329). Containment may be achieved by nonoperative or operative methods (330).
The femoral head represents more than three-fourths of the sphere and
the acetabulum only half of the sphere. Therefore, no method of
containment can provide for a totally contained femoral head within the
acetabulum during all portions of the gait cycle (149,327,328,331).
and 5), stop growth disturbance, and thereby prevent degenerative joint
disease. Attainment of these goals requires that each patient be
assessed clinically and radiographically. Clinically, the patient is
evaluated for clinical at-risk signs such as loss of motion,
contracture of the joint, and pain. AP and frog-leg lateral radiographs
are evaluated so as to determine the radiographic stage of the disease,
the extent of epiphyseal involvement (Catterall group, Salter-Thompson
type, or lateral pillar classifications), and the presence of
radiographic at-risk signs. If treatment is to succeed in controlling
subsequent deformity, it should be initiated in the initial or
fragmentation phase of the disease (75,275).
none of the clinical or radiographic at-risk signs; if he or she has
Catterall group 1 or 2, Salter-Thompson type A, or lateral pillar type
A disease; or if the disease is already in the reossification stage. A
child who demonstrates clinical or radiographic at-risk signs
regardless of the extent of epiphyseal involvement should receive
treatment (185). Even patients with Catterall
group 2 disease (or lateral pillar type B disease) who are at risk may
have poor results without treatment (72) (W.J. Cumming, personal communication, 1997).
definitive method of treatment chosen is restoration of motion. Motion
of the joint enhances synovial nutrition and cartilage nutrition (332,333).
This tenet of treatment cannot be overemphasized. The series that
recorded the greatest success in treating patients for extensively
involved femoral heads is that of Brotherton and McKibbin (323).
These patients were treated with bed rest and containment. The end
results in these patients were superior to those in another long-term
study of patients treated with bed rest and containment on a frame (209,210).
The only difference between the two treatment regimens was that in the
former series motion was always maintained. Restoration of motion can
be accomplished by bed rest alone, or with skin traction and
progressive abduction to relieve the muscle spasms. The author
recommends bed rest at home with nonsteroidal anti-inflammatory drugs
(NSAIDs) on a round-the-clock basis and then reassessment in 1 week to
assure that range of motion has considerably improved (to at least 45
degrees of abduction). Occasionally, surgical release of the contracted
adductors may be necessary. Restoration of motion allows abduction of
the hip, which reduces the forces on the hip joint and allows
positioning of the uncovered anterolateral aspect of the femoral head
in the acetabulum. Mobilization of the hip joint can also be achieved
by rest followed by the use of progressive abduction plasters to
stretch the hip adductor muscles while allowing hip flexion and
extension. A full or almost full range of motion is usually obtainable
within 7 to 10 days of treatment. Because of early deformity, complete
abduction and internal rotation may not always be obtainable.
Persistence of an adduction contracture is always associated with a
serious femoral head deformity and will not respond to bed rest or to
bed rest with traction (334).
the femoral head actually can be contained and, if so, in what position
this is best accomplished (335) (Fig. 25.27).
Arthrography can reveal any flattening of the femoral head that may not
be seen on plain radiographs. More importantly, it can demonstrate the
hinge abduction phenomenon (190,191,217) (Fig. 25.8, 25.25, and 25.26).
Demonstration of the hinge abduction phenomenon, or the inability to
contain the hip, is a contraindication to any type of containment
treatment. Serious damage to the femoral head and acetabulum may result
from trying to contain a noncontainable head (Fig. 25.14).
Arthrography should be performed under general anesthesia. This also
provides an opportunity to examine whether muscle spasm, contracture,
or mechanical deformity is responsible for any apparent fixed
deformities.
controversial, and there is disagreement regarding whether operative or
nonoperative treatment is more beneficial. Although operative treatment
is becoming increasingly popular, there is considerable debate about
the benefits of each operative procedure. The shortage of natural
history studies for comparison of the results of different modalities
of treatment is another reason for the difficulty in resolving this
controversy. In addition, the variability of criteria for inclusion of
patients in studies, the use of different measurements to assess
outcomes of treatment, the lack of interobserver and intraobserver
reliability data, and the lack of untreated control groups make
comparisons difficult.
was devoted to describing the use of abduction bracing in the treatment
of Perthes disease. Over the years, this modality has been replaced by
more promising surgical interventions and is now rarely used by
pediatric orthopaedic surgeons. It is mentioned in this edition for the
historical perspective of the evolution of methods of treatment. Today
the most widely used methods to maintain containment are femoral
osteotomy, innominate osteotomy, and the use of a procedure earlier
regarded as a salvage procedure, namely, lateral shelf acetabuloplasty.
There is also growing interest in combined femoral and innominate
surgical procedures.
This series proved that weight bearing, with the femoral head
contained, was not harmful. This method of treatment allows for weight
bearing and maintenance of range of motion of the hip in the contained
position. Successful results of this technique have been reported (336).
Petrie casts are currently used by some surgeons after muscle-release
procedures and capsulotomies for reducing femoral heads that are
deformed and subluxated (337), or for maintaining containment after surgical treatment.
regaining knee and ankle motion, and to avoid the occasional flattening
of the femoral condyles seen in patients treated with broomstick
plasters, orthopaedists turned to the use of removal abduction
orthoses, as typified by the Newington abduction brace (270), the Roberts orthosis (338), the Houston A-frame brace (339), and the Toronto Legg-Calvé-Perthes orthosis (169,340). These devices provide containment in the abducted internal rotation position.
They allowed free motion of the knee and ankle. Containment was
provided by the abduction of the brace and the hip flexion required for
walking with the legs in abduction. These devices are less cumbersome
than other braces and are well tolerated by patients. On arthrography,
the position of containment that would be maintained by an abduction
orthosis of this variety would be demonstrated by abduction, slight
flexion, and external rotation (Fig. 25.27). Containment had to be demonstrated on a radiograph with the patient in the weight-bearing position and in the brace (Fig. 25.24).
The brace was worn on a full-time basis until the femoral head was in
the reossification stage, when there was no further risk of collapse.
Full-time bracing ranged from 6 to 18 months. The negative aspects of
bracing included prolonged treatment times and the need for compliance
on the part of the patient. Some patients did not tolerate the brace
for psychological reasons (343). This type of treatment was also difficult for girls and older patients to accept (344).
reported on 31 patients (34 hips) with severe Perthes disease
(Catterall groups 3 and 4) that had been treated with weight-bearing
abduction orthoses. The mean age of the patients when first seen was 6
years, and the mean duration of follow-up was 7 years. At follow-up,
applying the Mose criteria, no hip had a good result, 35% had fair
results, and 65% had poor results. On the basis of the classification
of Stulberg et al., there were 41% class 2 results, 53% class 3 and 4
results, and 6% class 5 results. With respect to the lateral column, of
the 20 hips in which collapse occurred, only 10% had Stulberg class 2
results, 35% had class 3 results, 45% had class 4 results, and 10% had
class 5 results. By comparison, in the 14 hips in which collapse of the
lateral column did not occur, 86% had Stulberg class 2 results and only
14% had class 3 results. Class 4 or 5 results were not found in hips in
which a collapse did not occur. The authors concluded that although
containment is the most widely accepted principle of treatment of
Legg-Calvé-Perthes disease, little clinical
information
supports the contention that bracing in abduction and external
rotation, as provided by the Atlanta Scottish Rite orthosis and its
modifications, is effective.
 |
|
Figure 25.28 An abduction orthosis.
|
34 patients with Catterall group 3 or 4 disease, with an average age at
diagnosis of 8 years. The average follow-up duration in this series was
6 years and 9 months. At follow-up, there were no Stulberg class 1
results, 3 class 2 results, 24 class 3 results, 6 class 4 results, and
1 class 5 result. The same investigators also arrived at a similar
conclusion concerning the use of this orthotic device in the treatment
of Perthes disease. In both of these studies the issue of compliance is
not documented, and as with all studies of Perthes disease in the
literature, control groups other than historic controls are absent.
Because the radiographic outcomes in both of these studies were poor,
it is questionable whether the orthosis itself adds anything to the
treatment other than maintenance of range of motion. As expected, most
patients with Perthes disease in both series were doing well
clinically, as do most patients over the short term regardless of the
extent of the deformity. The long-term prognosis for all but the
Stulberg class 1 and 2 hips is guarded.
today by pediatric orthopaedic surgeons in the treatment of Perthes
disease. Because of the nihilistic attitude that many physicians share
with regard to treatment for Perthes disease, they have begun treating
patients with only maintenance of range-of-motion programs, including
stretching exercises, nighttime abduction splinting, home traction, and
other combinations. Long-term follow-up studies of these nonoperative
range-of-motion regimens are needed in order to determine their
efficacy.
are advocated by many investigators. Surgical containment methods offer
the advantage of early mobilization and the avoidance of prolonged
bracing or cast treatment. In addition, no end point for discontinuing
treatment is required (337). Surgical
containment may be approached from the femoral side, the acetabular
side, or both sides of the hip joint. Procedures used for obtaining or
maintaining containment in Legg-Calvé-Perthes syndrome are those that
were originally used in the treatment of problems associated with
developmental hip dysplasia and dislocation. The discussion that
follows relates only to hips that are “containable” (relatively full
range of motion, congruency between the femoral head and the
acetabulum) and in the initial or fragmentation phase of the disease.
offers the theoretical advantage of deep seating of the femoral head
and positioning of the vulnerable anterolateral portion of the head
away from the deforming influences of the acetabular edge (238,334,347,348,349,350). The varus position reduces the forces exerted by the joint on the femoral head (329,347).
This procedure is thought to relieve the intraosseous venous
hypertension and improve the disturbed intraosseous venous drainage
reported in Legg-Calvé-Perthes syndrome, thereby speeding the healing
process (123,124,144,347,348,351). A number of investigators disagree with these perceived benefits (352,353,354).
full range of motion, congruency between the femoral head and the
acetabulum, and the ability to contain the femoral head in the
acetabulum in abduction and internal rotation (337,355) (Fig. 25.27).
This assessment usually requires arthrography if the femoral head is
well into the fragmentation phase. The procedure must be performed
early, in the initial or fragmentation stage of the disease, in order
to have any effect on later femoral head deformity (179,224,347).
considered. Varus osteotomy, with or without derotation, usually
requires the use of internal fixation and external mobilization in
plaster for 6 weeks. The patient must incur the inherent risks and
costs associated with at least one surgical procedure and most likely a
second surgical procedure for hardware removal. The limb is temporarily
shortened by the procedure. The varus angle must not exceed a
neck-shaft angle of less than 110 degrees. The varus angle generally
decreases with growth (190,356,357);
however, if there has been physeal plate damage secondary to the
disease, this remodeling potential may be lost, and the patient may
have permanent shortening and temporary or permanent weakness of the
hip abductors (144,357,358,359,360,361).
The proponents of varus osteotomy, with or without derotation, report
70% to 90% satisfactory anatomic results using this method (144,224,347,348,358,360,362,363,364,365,366,367,368,369,370,371,372).
redirection of the acetabulum, providing better coverage for the
anterolateral portion of the femoral head. The femoral head is placed
in relative flexion, abduction, and internal rotation with respect to
the acetabulum in the weight-bearing position. Any shortening caused by
the disease can be corrected (120,337,355,360,373,374,375,376,377).
Prerequisites for innominate osteotomy include restoration of a full
range of motion, a round or almost round femoral head, and congruency
of the joint, demonstrated arthrographically. Treatment must be
performed early in the course of the disease, and the head must be well
seated in flexion, abduction, and internal rotation.
of the iliopsoas muscle is always released at the musculotendinous
junction, and any residual contractures of the adductor muscles are
released by subcutaneous adductor tenotomy (120,337).
The osteotomy is fixed by two or three threaded pins for internal
fixation. Partial weight bearing may be resumed in a cooperative child
several days after surgery; however, in an uncooperative patient,
immobilization in a spica cast for 6 weeks is required.
associated risks and cost factors of the surgical procedure and the
procedure for pin removal. Additionally, the operation is performed on
the normal side of the joint. This procedure may increase the forces on
the femoral head by lateralizing the acetabulum and increasing the
lever arm of the abductors (347), although this
supposition has so far not been substantiated. Innominate osteotomy may
also cause a persistent acetabular configuration change where there was
a previously normal acetabulum, leading to loss of motion, particularly
flexion (378). Satisfactory anatomic results from this procedure range from 69% to 94% (337,360,373,374,375,379,380,381,382,383).
neither method of surgical containment, innominate or femoral
osteotomy, may effectively shield an extensively necrotic segment of
the femoral head from stress (327,328,384,385). Wenger (188)
reported a high incidence of complications in surgically treated
patients, even when the accepted methods were used and prerequisites
were met.
plus innominate osteotomy have been reported in patients with severely
involved Catterall group 3 or 4 disease. This combined procedure has
the theoretical advantage of maximizing femoral head containment while
avoiding the complications of either procedure alone. The femoral
osteotomy directs the femoral head into the acetabulum, while
theoretically reducing any increasing pressure or stiffness of the
joint that would result from the pelvic osteotomy. The coverage
provided by the innominate osteotomy reduces the degree of correction
needed from the femoral osteotomy, thereby minimizing the complications
of excessive neck-shaft varus, associated abductor weakness, and limb
shortening. Advocates of this procedure also believe that permanent
correction of the deformity, early weight bearing, and shortened
treatment time are obtained. The disadvantages of the procedure include
those mentioned for varus osteotomy and innominate osteotomy alone.
Surgical time is increased, potential blood loss is magnified, and the
combined procedures are technically more difficult. Satisfactory
anatomic results from this combined procedure are reported in up to 78%
of patients. As would be expected in short-term follow-up, the clinical
results are excellent (386,387,388,389,390).
The prerequisites for this operation include those for both varus and
innominate osteotomies, in a patient who probably would not achieve
satisfactory coverage from either procedure alone.
primary method of management in children older than 8 years with
Catterall group 2, 3, or 4 disease with or without at-risk signs,
lateral pillar type B or C disease, and Salter-Thompson type B disease;
if subluxation is present, it must be reducible on a dynamic arthrogram
(304,391) (Fig. 25.29).
Contraindications include hips that do not meet the aforementioned
criteria and the presence of hinge abduction. Only one
intermediate-term follow-up study exists, but proponents of this method
of treatment believe that containment of the femoral head by shelf
arthroplasty, before significant deformity can develop, improves
remodeling of the femoral head (304,392).
The shelf procedure may cover the anterolateral portion of the head,
preventing subluxation and lateral overgrowth of the epiphysis. Risk
factors for poor results with this technique are age older than 11
years, female gender, and Catterall group 4 disease.
This procedure, originally introduced for the treatment of
developmental hip dysplasia, is theoretically better able to cover the
deforming femoral head. Only longer follow-ups will let physicians know
if this will offer better long-term results compared with more commonly
applied osteotomies.
of hip distraction (arthrodiastasis) for periods of 4 to 5 months, with
or without soft tissue release, in older children with Perthes disease (395,396). A judgment about the usefulness of these procedures will have to await further follow-up.
episode indicative of loss of containment, such as recurrent pain or
loss of range of motion, must be treated aggressively with rest,
traction, and reassessment of containment clinically and possibly
radiographically.
(reossification) of the disease, those with noncontainable deformities,
and those who have lost containment after undergoing either surgical or
nonsurgical containment procedures present a management challenge.
These patients
usually demonstrate hinge abduction on arthrography and have an extremely poor prognosis without additional treatment (186,203,237,337,397,398).
They generally present with persistent pain, shortening of the involved
extremity, and a fixed deformity, generally 10 to 15 degrees of fixed
flexion and 15 to 20 degrees of fixed adduction (237,398). The salvage procedures to be considered at this point include abduction extension osteotomy (237,337,363,366,367,398,399,400,401),
lateral shelf arthroplasty, Chiari osteotomy, and cheilectomy. These
procedures must be viewed as salvage procedures, with each having
specific limited aims, which may include pain relief, correction of
limb-length inequality, increasing femoral head coverage, improvement
in movement of the joint, and strengthening of a weak abductor (398).
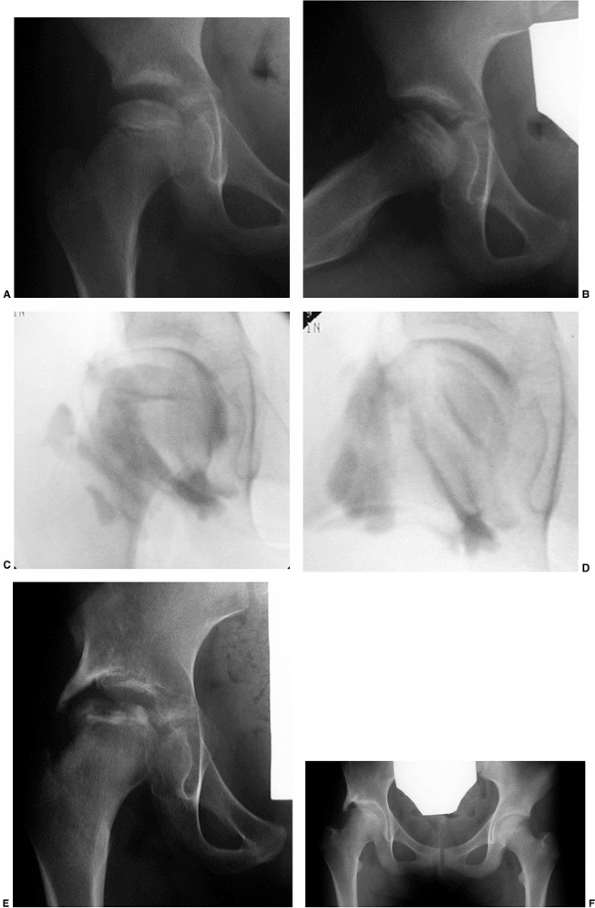 |
|
Figure 25.29 An 8-year-old girl with total femoral head involvement in the initial stage of disease. A: Anteroposterior view. B: Lateral view. C: Intraoperative arthrogram in neutral position showing very mild flattening of the femoral head. D: Arthrogram in abduction showing no evidence of impingement. E: One year after shelf arthroplasty. F: Five years after shelf arthroplasty.
|
regardless of their age with a noncontainable hip or patients with a
painful hip after healing who demonstrate hinge abduction, abduction
extension osteotomy should be considered. Abduction extension osteotomy
of the femur is indicated
when arthrography demonstrates that the congruency of the joint is improved by the extended adducted position (Figs. 25.27 and 25.28).
The preliminary results with this modality of treatment indicate
improvement in limb length, decrease in limp, and improvement in
function and range of motion (398). This
procedure may be applied in either the active or the late stage of the
disease, when arthrography demonstrates that the congruency of the
joint is improved by the extended adducted position. This modality of
treatment allows for realignment of the congruent position of the hip
in the neutral weight-bearing position. Short-term results are
promising (337,366,367,398).
“salvage” situations, including lateral subluxation of the femoral
head, inadequate coverage of the femoral head, and hinge abduction
associated with severe Legg-Calvé-Perthes disease (402,403,404,405,406,407).
In this scenario, the shelf would cover the enlarged femoral head in
hopes of improving the outcome, much as shelves are used as salvage
procedures in residual hip dysplasia cases (Fig. 25.30). One recent report does demonstrate resolution of hinge abduction (408).
Using this method in the presence of hinge abduction would not be the
author’s preference as it does not reduce the lateral impingement of
the head in abduction.
deformed femoral head, but does not reduce the lateral impingement in
abduction and may exacerbate any existing abductor weakness (237,398).
Chiari osteotomy may be useful in the enlarged, poorly covered femoral
head that is beginning to develop symptoms of early degenerative joint
disease. Although good preliminary results have been reported (400,401,402,403,404,405,406), the role of Chiari osteotomy in the treatment of Legg-Calvé-Perthes syndrome has yet to be defined.
It is indicated only in patients with functionally limiting and
restricted range of motion. The procedure must be performed only after
the physis is closed; otherwise, a slipped capital femoral epiphysis
may ensue (399,409).
Although cheilectomy may produce gratifying results with regard to
improved range of motion, in some cases increasing stiffness may occur
secondary to capsular adhesions at the osteotomy site (337).
In addition, shortening associated with the femoral head deformity is
not corrected. The entity of femoral acetabular impingement is
discussed more fully in Chapter 24. Little long-term information, other than for cheilectomy, exists for impingement syndromes in Perthes disease.
pain, several treatment options are available. Symptomatic treatment
with antiinflammatory agents and protective weight bearing may be used
to promote healing. Persistent pain may warrant attempts at
revascularization. This may include drilling of the fragment through
the femoral neck and internal fixation, either percutaneously with pins
or open with devices such as the Herbert screw. If the fragment becomes
detached and cannot be reattached and causes mechanical catching
symptoms, it may require removal (191,410). There is a paucity of information on the natural history of the condition and the results of treatment.
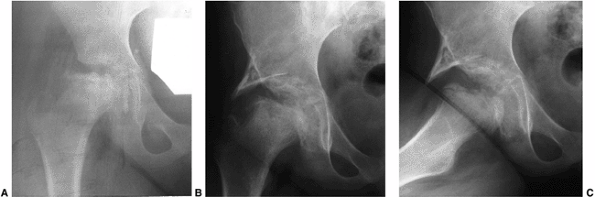 |
|
Figure 25.30 A 9-year-old girl with Catterall group 4 and lateral pillar type C disease treated with lateral shelf arthroplasty. A: Note the marked loss of epiphyseal height. B–C: Anteroposterior (B) and Lauenstein (C)
radiographs 1 year after operation. Note that the femoral head is in the reossification phase. The involved side is 1 cm shorter than the other. Abduction and internal rotation are to 15 degrees, external rotation is to 25 degrees, and flexion is to 100 degrees. (Courtesy of Fred Dietz, MD, University of Iowa, Iowa City, Iowa.) |
Such patients may develop a Trendelenburg gait and pain secondary to
muscle fatigue. This has rarely been a significant problem in long-term
reviews (186). However, distal and lateral advancement of the greater trochanter may be necessary (411,412).
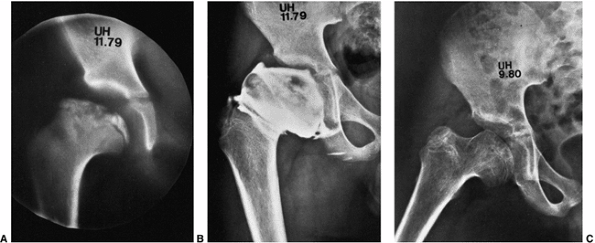 |
|
Figure 25.31
A boy, 8 years and 6 months of age, with Catterall group 4 disease. The range of motion of the hip included flexion of 140 degrees, extension of 0 degree, abduction of 20 degrees, adduction of 30 degrees, internal rotation of 10 degrees, and external rotation of 30 degrees. A: Polytome indicating superolateral growth arrest. B: Arthrogram demonstrating femoral head flattening and enlargement, and deformation of the peripheral acetabulum. C: Abduction radiograph 7 months after cheilectomy. Range of motion at this time was 130 degrees of flexion, 20 degrees of extension, 50 degrees of abduction, 50 degrees of adduction, 45 degrees of internal rotation, and 40 degrees of external rotation. (Courtesy of J.G. Pous, MD, Montpellier, France.) |
are matched for age, gender, degree of epiphyseal involvement, and
other diagnostic factors, compared with an untreated control group,
will no doubt be required in order to determine the most effective
treatment for Legg-Calvé-Perthes syndrome. As fundamental understanding
of Legg-Calvé-Perthes syndrome increases, so does understanding of how
various treatment modalities influence this complex growth disturbance.
J. Sur une forme particuliere de coxalgie greffe sur des deformations
caracteristiques de 1′extremite superieure de femur. Rev Chir 1910;42:54.
RM, Carrie AW, Walker NF, et al. Coxa plana: its genetic aspects and
results of treatment with long Taylor walking caliper. J Bone Joint Surg Am 1959;41:1959.
RG, Dangerfield PH, Hall DJ, et al. Perthes’ disease: an anthropometric
study revealing impaired and disproportionate growth. J Bone Joint Surg Br 1978;60:461.
B, Joseph B, Chacko V, et al. Altered skeletal growth in Perthes’
disease: an anthropomorphic study of children from rural India. J Pediatr Orthop B 1995;4:91.
R, Farley FA, Herring JA, et al. Bone age determination in children
with Legg-Calvé-Perthes disease: a comparison of two methods. J Pediatr Orthop 1995;15:90.
G, Wingstrand H, Haaglund G, et al. Growth in 110 children with
Legg-Calvé-Perthes’ disease: a longitudinal infancy childhood puberty
growth model study. J Pediatr Orthop B 1996;5:181.
J, Zander D, Hackenbroch MH. Low plasma levels of insulin-like growth
factor I in Perthes’ disease: a controlled study of 59 consecutive
children. Acta Orthop Scand 1992;63:393.
J, Zander D, Hackenbroch MH. No physiologic age-related increase of
circulating somatomedin-C during early stage of Perthes’ disease: a
longitudinal study in 21 boys. Arch Orthop Trauma Surg 1992;111:171.
JSE, Zander D, Rutt J, et al. Normal plasma levels of IGF binding
protein in Perthes’ disease: follow up of a previous report. Acta Orthop Scand 1993;64:540.
J, Boddenberg B, Zander D, et al. Thyroid function in
Legg-Calvé-Perthes’ disease: cross-sectional and longitudinal study. J Pediatr Orthop 1993;13:592.
T, Enomoto H, Takahashi K, et al. Decreased levels of IGF binding
protein 3 in serum from children with Legg Calvé Perthes’ disease. Acta Orthop Scand 1998;69:125.
H, Nicolai RD, Hauffa BP, et al. Skeletal immaturity, IGF-I and IGFBP-3
serum concentrations in Legg-Calvé-Perthes’ disease. Klin Padiatr 1996;208:339.
A, Lloyd-Roberts GC, Wynne-Davies R. Association of Perthes’ disease
with congenital anomalies of genitourinary tract and inguinal region. Lancet 1971;1:996.
DJ, Harrison MHM, Burwell RG. Congenital abnormalities and Perthes’
disease: clinical evidence that children with Perthes’ disease may have
a major congenital defect. J Bone Joint Surg Br 1979;61:18.
G, Monzali GL. Osteocondrite postridut Lattante iva nella L.C.A.:
l’osteochondrite apre reduction de la luxation congenitale de la
hanche. Lattante 1957;28:537.
H, Kitakoji T, Katoh M, et al. Delayed ossification of the proximal
capital femoral epiphysis in Legg-Calve-Perthes’ disease. J Bone Joint Surg Br 2003;85(1):121–124.
RW, Corrigan KF, Joistad AH Jr, et al. Thyroid function in
Legg-Calvé-Perthes’ disease: a new approach to an old problem. Clin Orthop 1954;4:160.
G, Lohmander LS, Wingstrand H. Increased levels of proteoglycan
fragments and stromelysin in hip joint fluid in Legg-Calvé-Perthes’
disease. J Pediatr Orthop 1997;17:266.
G, Hochbergs P, Wingstrand H, et al. Sonography and intracapsular
pressure in Perthes disease: 39 children examined 2–36 months after
onset. Acta Orthop Scand 1994;65:575.
H, Bauer G, Brimar J, et al. Transient ischemia of the proximal femoral
epiphysis in the child: interpretation of bone scintimetry for
diagnosis in hip pain. Acta Orthop Scand 1985;56:197.
RB, Thompson GH. Legg-Calvé-Perthes’ disease: the prognostic
significance of the subchondral fracture and a two group classification
of the femoral head involvement. J Bone Joint Surg Am 1984;66:479.
K, Posan E, Harsflavi J, et al. The most severe forms of Perthes’
disease associated with the homozygous Factor V Leiden mutation. J Bone Joint Surg Br 2004;86(3):426–429.
CJ, Crawford A, Roy D, et al. Association of antithrombotic factor
deficiencies and hypofibrinolysis with Legg-Perthes disease. J Bone Joint Surg Am 1996;78:3.
C, Glueck HI, Greenfield D, et al. Protein C and S deficiency,
thrombophilia, and hypofibrinolysis: pathophysiologic causes of
Legg-Perthes disease. Pediatr Res 1994;35:383.
MT, McDougall PA, Gorlin JB, et al. Prospective reevaluation of the
association between thrombotic diathesis and Legg-Perthes disease. J Bone Joint Surg Am 2002;84(9):1613–1618.
IV, Maynard JA, Weinstein SL, et al. Legg-Calvé-Perthes’ disease:
histochemical and ultrastructural observations of the epiphyseal
cartilage and the physis. J Bone Joint Surg Am 1983; 65:797.
H, Baadsgaard K, Sager P. The vascular supply to the femoral head
following dislocation of the hip joint: an experimental study in
newborn rabbits. Acta Orthop Scand 1965;35:264.
G, Karski T, Kozlowski K. Capital femoral epiphysis and growth plate of
the asymptomatic hip joint in unilateral Perthes disease. J Pediatr Orthop B 2003;12(6):380–386.
SA, Henderson RC, Renner JB, et al. Magnetic resonance evaluation of
“metaphyseal” changes in Legg-Calvé-Perthes’ disease. J Pediatr Orthop 1993;13:602.
EA, Bennett GA. Osteochondritis deformans of the hip (Legg-Perthes’
disease) and renal osteitis fibrosa cystica: report of a case with
anatomic studies. Arch Pathol 1942;33:866.
M, Moriya H, Tsuchiya K, et al. Arthrography of early Perthes’ disease:
swelling of the ligamentum teres as a cause of subluxation. J Bone Joint Surg Br 1989;71:413.
RD, LaViolette D, Buckley HD, et al. Studies of bone metabolism. I. A
comparison of the metabolism of strontium in living and dead bone. J Bone Joint Surg Am 1955;37:143.
RA, Brockenshire FA, Barber JR. Avascular necrosis of the femoral
capital epiphysis after traumatic dislocation of the hip in children. J Bone Joint Surg Br 1961;43:43.
F, Rebouillat J. Long-term development of primary osteochondritis of
the hip (Legg-Perthes-Calvé): apropos of 60 hips with a follow-up of
more than 30 years. Rev Chir Orthop 1987;73:561.
B, Kanat IO. Subchondral bone cysts, osteochondritis dissecans, and
Legg-Calvé-Perthes’ disease: a correlation and proposal of their
possible common etiology and pathogenesis. J Foot Surg 1988;27:75.
JA, Williams JJ, Neustadt JN, et al. Evolution of femoral head
deformity during the healing phase of Legg-Calvé-Perthes’ disease. J Pediatr Orthop 1993;13:41.
PF. The clinical observations after healing of Calvé-Perthes’ disease
compared with final deformities left by that disease and the bearing of
those final deformities on ultimate prognosis. Acta Radiol 1926;5:1.
J, Weinstein SL, Spratt K, et al. Stulberg classification system for
evaluation of Legg-Calvé-Perthes’ disease: intrarater and interrater
reliability. J Bone Joint Surg Am 1999;81:1209.
GC, Catterall A, Salamon PB. A controlled study of the indications for
the results of femoral osteotomy in Perthes’ disease. J Bone Joint Surg Br 1976;58:31.
H, Kalenderer O, Eryanilmaz G, et al. Intraobserver and interobserver
reliability of Catterall, Herring, Salter-Thompson and Stulberg
classification systems in Perthes disease. J Pediatr Orthop B 2004;13(3):166–169.
C, Frizziero P, Turra S. Prognostic value of Catterall and Herring
classification in Legg-Calve-Perthes disease: follow-up to skeletal
maturity of 32 patients. J Pediatr Orthop 2002;22(3): 345–349.
PH, Ross R, Hamalainen M, et al. Catterall grouping of Perthes’
disease: an assessment of observer error and prognosis using the
Catterall classification. J Bone Joint Surg Br 1980;62:428.
P, Tudisco C, Caterini R, et al. The Herring lateral pillar
classification for prognosis in Perthes’ disease: late results in 49
patients treated conservatively. J Bone Joint Surg Br 1995;77:739.
JF, Shantharam SS, Gelinas C. Comparison of lateral pillar
classification and Catterall classification of Legg-Calvé-Perthes’
disease. J Pediatr Orthop 1993;13:200.
K, Fabry G, Moens P. Legg-Calve-Perthes disease in patients under 5
years of age does not always result in a good outcome. Personal
experience and meta-analysis of the literature. J Pediatr Orthop B 2003;12(3):222–227.
F, De Rosa V, Zekri H, et al. Confirmation of the early prognostic
value of bone scanning and pinhole imaging of the hip in
Legg-Calvé-Perthes disease. J Nucl Med 2003;44(11):1761–1766.
JM, Dimon JH III, Meehan PL, et al. Preliminary experience with the
Scottish Rite Hospital abduction orthosis for Legg-Perthes’ disease. Clin Orthop 1980;150:49.
T, Kollmannsberger A, Fischer M, et al. Ultra- sonographic evaluation
of Legg-Calvé-Perthes’ disease based on sonoanatomic criteria and the
application of new measuring techniques. Eur J Radiol 1992;15:101.
RL, Roderique JW, Brown DC, et al. The relationship of isotopic bone
imaging findings to prognosis in Legg-Perthes’ disease. Clin Orthop 1980;150:23.
C, Sahlstedt B, Lonnerholm T, et al. Conventional radiography and bone
scintigraphy in the prognostic evaluation of Legg Calvé Perthes
disease. Acta Radiol 1996;37:561.
C, Lonnerholm T, Moberg A, et al. Legg-Calvé-Perthes’ disease:
comparison of conventional radiography, MR imaging, bone scintigraphy
and arthrography. Acta Radiol 1995;36:434.
M, Wook-Cheol K, Kubo T. Preliminary report on usefulness of magnetic
resonance imaging for outcome prediction in early stage Legg Calvé
Perthes disease. J Pediatr Orthop B 1999;8:161.
D, Kasser JR, Villegas-Medina OL, et al. Cartilaginous and growth
disturbances in Legg-Calvé-Perthes disease: evaluation with MR imaging.
Radiology 1995;197:767.
A, Hattori T, Noritake K, et al. Legg-Calvé-Perthes disease in the
evolutionary period: comparison of magnetic resonance imaging with bone
scintigraphy. J Pediatr Orthop 1995;15:362.
T, Lamminen A, Merikanto J, et al. The value of MRI in early Perthes’
disease: an MRI study with a 2 year follow-up. Pediatr Radiol 1997;27:517.
JM, Zink WP. Arthrographic classification of Legg-Perthes’ disease.
Presented at the Annual Meeting of the American Academy of Orthopaedic
Surgeons, Las Vegas, NV, 1981.
U, Bunger C, Krebs B, et al. Blood flow in the juvenile hip in relation
to changes of the intraarticular pressure: an experimental
investigation in dogs. Acta Orthop Scand 1983;54:182.
PE Jr, Schantz K, Bollerslev J, et al. Bilateral femoral head dysplasia
and osteochondritis: multiple epiphyseal dysplasia tarda,
spondyloepiphyseal dysplasia tarda, and bilateral Legg-Perthes’
disease. Acta Radiol 1988;29:705.
Al. Osteochondritis deformans coxae juvenilis, or Perthes’ disease: the
results of treatment by traction in recumbency. Br J Surg 1936;24:166.
MHM, Menon MPA. Legg-Calvé-Perthes’ disease: the value of x-ray
measurement in clinical practice with special reference to the
broomstick plaster method. J Bone Joint Surg Am 1966;48:1301.
BJ, McKibbin B. Perthes’ disease treated by prolonged recumbency and
femoral head containment: a long-term appraisal. J Bone Joint Surg Br 1977;59:8.
A, Bowen JR, Guille JT, et al. Treatment of the collapsed femoral head
by containment in Legg-Calve-Perthes disease. J Pediatr Orthop 2003;23(1):15–19.
JM, Meehan P, Counts G, et al. Ambulatory abduction brace for
Legg-Perthes’ disease. Presented at the Annual Meeting of the American
Academy of Orthopaedic Surgeons, Dallas, TX, 1974.
WW, Hopper WC, Purvis JM. The Scottish rite orthosis for Legg-Perthes’
disease. Presented at the Annual Meeting of the American Academy of
Orthopaedic Surgeons, Dallas, TX, 1978.
PL, Angel D, Nelson JM. The Scottish Rite abduction orthosis for the
treatment of Legg-Perthes’ disease: a radiographic analysis. J Bone Joint Surg Am 1992;74:2.
V, Poussa M, Yrjonen T, et al. Intertrochanteric varus osteotomy for
Perthes’ disease: radiographic changes after 2–16 year follow-up of 126
hips. Acta Orthop Scand 1991;62:549.
DY, Seong SC, Choi IH, et al. Changes of blood flow of the femoral head
after subtrochanteric osteotomy in Legg-Perthes’ disease: a serial
scintigraphic study. J Pediatr Orthop 1992;12:731.
T, Tillberg G. Coxa plana: a radiological comparison of the rate of
healing with conservative measures and after osteotomy. J Bone Joint Surg Br 1976;58:25.
IK, Deluca PA, Gage JR. A comparative study of ambulation-abduction
bracing and varus derotation osteotomy in the treatment of severe
Legg-Calvé-Perthes’ disease in children over 6 years of age. J Pediatr Orthop 1988;8:676.
PD, Desai SS, Millis MB. Comparison of femoral and innominate
osteotomies for the treatment of Legg-Calvé-Perthes’ disease. J Bone Joint Surg Am 1988;70:1131.
KJ, Price CT, Kupiszewski SJ, et al. Results of femoral varus osteotomy
in children older than 9 years of age with Perthes disease. J Pediatr Orthop 2001;21(2):198–204.
GA, Eijer H, Haverkamp D, et al. Intertrochanteric osteotomy in young
adults for sequelae of Legg-Calvé-Perthes’ disease—a long term
follow-up. Int Orthop 2004;28(1):44–47.
OA, Hemmer S, Wunsche P, et al. The adult hip after femoral varus
osteotomy in patients with unilateral Legg-Calvé-Perthes disease. J Pediatr Orthop B 2003;12(1):33–37.
P, Halmai V, Shaikh S, et al. Long-term results of derotational femoral
varus osteotomy in Legg-Calvé-Perthes disease: 26-year follow-up. Orthopedics 2003;26(5):487–491.
MJ, Catterall A, Hashemi-Nejad A. Valgus extension osteotomy for’hinge
abduction’ in Perthes’ disease. Results at maturity and factors
influencing the radiological outcome. J Bone Joint Surg Br 2000;82(4):548–554.
T, Marttinen EJ, Merikanto JE. Outcome of Perthes disease in unselected
patients after femoral varus osteotomy and splintage. J Pediatr Orthop B 1997;6:229.
G, Wallin J. External rotational positioning of the leg after
intertrochanteric combined varus derotational osteotomy in Perthes’
disease. Arch Orthop Traumatol Surg 1997;116:108.
B, Srinivas G, Thomas R. Management of Perthes disease of late onset in
southern India: the evaluation of a surgical method. J Bone Joint Surg Br 1996;78:625.
RB. The scientific basis for innominate osteotomy for
Legg-Calvé-Perthes’ disease and the results in children with a bad
prognosis. Orthop Trans 1985;9:203.
S, Kehl D. An evaluation of Perthes’ disease: comparison of nonsurgical
and surgical means. Presented at the Annual Meeting of the American
Academy of Orthopaedic Surgeons, Las Vegas, NV, 1981.
AM, Paterson DC, Sutherland AD. A comparison between innominate
osteotomy and hip spica in the treatment of Legg-Perthes’ disease. Clin Orthop 1982;163:141.
H, Wenger DR. Surgical correction of “functional retroversion” and
“functional coxa vara” in Legg-Calvé-Perthes disease and epiphyseal
dysplasia: correction of deformity defined by new imaging modalities. J Pediatr Orthop 1997;17:247.
H, Wenger DR. “Functional retroversion” of the femoral head in
Legg-Calvé-Perthes disease and epiphyseal dysplasia: analysis of
head-neck deformity and its effect on limb position using
three-dimensional computed tomography. J Pediatr Orthop 1997;17:240.
JK, Leonidou O, Pettas N. Acetabulum augmentation for
Legg-Calvé-Perthes disease: 12 children (14 hips) followed for 4 years.
Acta Orthop Scand Suppl 1997;275:103.
PA, Mullhall KJ, Kearns SR, et al. Triple pelvic osteotomy in
Legg-Calve-Perthes disease using a single anterolateral incision. J Pediatr Orthop B 2003;12(6):387–389.
E, Ezra E, Wientroub S, et al. Treatment of severe late onset Perthes’
disease with soft tissue release and articulated hip distraction: early
results. J Pediatr Orthop B 2004;13(3): 158–165.
RW, Guille JT, Bowen JR. Shelf arthroplasty in patients who have
Legg-Calvé-Perthes’ disease: a study of long-term results. J Bone Joint Surg Am 1991;73:1338.
GJ. Surgical treatment of coxa plana. Presented at the Joint Meeting of
the Orthopaedic Associations of the English Speaking World. J Bone Joint Surg Br 1964;46:779.
SM, Moon ES, Yoon TR, et al. Fate of the osteochondral fragments in
osteochondritis dissecans after Legg-Calvé-Perthes’ disease. J Bone Joint Surg Br 2002;84(7):1025–1029.
