Developmental Hip Dysplasia and Dislocation
the Pediatric Orthopaedic Society of North America, and the American
Academy of Pediatrics have endorsed the name change of this entity from
CDH to DDH, because the latter is more representative of the wide range
of abnormalities seen in this condition (2).
more encompassing and is taken in the literal sense of organ growth and
differentiation, including the embryonic, fetal, and infantile periods.
This terminology includes all cases that are clearly congenital and
those that are developmental, incorporating subluxation, dislocation,
and dysplasia of the hip. Because this change in terminology has not
yet been incorporated into the International Classification of Diseases, the term “CDH,” which has existed in the literature for years, will continue to be used in many publications.
terminology used in discussing the condition. What different
investigators mean by “instability,” “dysplasia,” “subluxation,” and
“dislocation” varies considerably. In this chapter, the term “DDH”
denotes developmental dysplasia of the hip and encompasses all the
variations of the condition described. Within this spectrum are two
entities: dysplasia and dislocation. In the newborn, the term dysplasia refers to any hip with a positive Ortolani
sign. This sign is the sensation the examiner feels upon provoking the
femoral head into subluxation (i.e., partial contact between the
femoral head and acetabulum), dislocation (i.e., no contact between the
femoral head and the acetabulum), or reduction from either of these
positions. It is often difficult to make the distinction between these
two entities, especially given the subtleties of arthrographic and
ultrasonographic classifications. Because further subclassification in
the newborn has no influence on treatment, the author prefers to use
the term dysplasia to encompass these entities and other variations. The term dislocation refers here only to complete, irreducible dislocations.
there must be a genetically determined balance of growth of the
acetabular and triradiate cartilages and a well-located and centered
femoral head. Embryologically, the components of the hip joint, the
acetabulum, and the femoral head develop from the same primitive
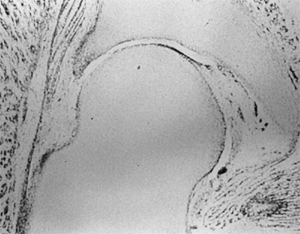
mesenchymal cells (3,4,5,6) (Fig. 24.1).
A cleft develops in the precartilaginous cells at about the 7th week of
gestation. This cleft defines the acetabulum and the femoral head. By
the 11th week of intrauterine life, the hip joint is fully formed (5,6,7). Theoretically, the 11th week is the earliest time at which a dislocation could develop, although this rarely happens (7).
Acetabular development continues throughout intrauterine life,
particularly by means of growth and development of the labrum (3,6).
In the normal hip at birth, the femoral head is deeply seated in the
acetabulum and held within the confines of the acetabulum by the
surface tension of the synovial fluid. It is extremely difficult to
dislocate a normal infant’s hip, even after incising the hip joint
capsule (8,9). The
retaining force is similar to that of a suction cup. Hips in newborns
with DDH are not merely normal hips with capsular laxity; they are
pathologic entities.
 |
|
Figure 24.1
Embryonic hip. The components of the hip joint, the acetabulum, and the femoral head develop from the same primitive mesenchymal cells. A cleft develops in the precartilaginous cells at about the 7th week of gestation, defining the acetabulum and the femoral head. |
the acetabular cartilage complex is extremely important to the
continuing development of the hip joint (3,7,10,11,12). The growth of these two members of the hip joint is interdependent.
is a three-dimensional structure that is triradiate medially and
cup-shaped laterally. The acetabular cartilage complex is interposed
between the ilium above, the ischium below, and the pubis anteriorly.
Acetabular cartilage forms the outer two-thirds of the acetabular
cavity, and the nonarticular medial wall of the acetabulum is formed by
a portion of the ilium above, the ischium below, and portions of the
triradiate cartilage.
The fibrocartilaginous labrum is at the margin of the acetabular
cartilage, and the joint capsule inserts just above its rim (13) (Fig. 24.3).
Each phalangis is composed of very cellular hyaline cartilage. This
cartilage contains many canals. Each side of each limb of the
triradiate cartilage has a growth plate. One phalangis
is
oriented horizontally between the ilium and the ischium. One phalangis
is oriented vertically and interposed between the pubis and the
ischium. The third phalangis is located anteriorly and slanted
superiorly between the ilium and the pubis (Fig. 24.4).
The triradiate cartilage is the common physis of these three pelvic
bones. Interstitial growth within the triradiate cartilage causes the
hip joint to expand in diameter during growth (14).
 |
|
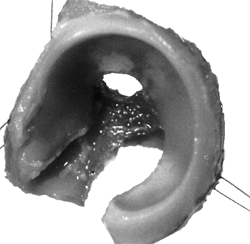
Figure 24.2
Normal acetabular cartilage complex of a 1-day-old infant. The ilium, ischium, and pubis have been removed with a curet. The lateral view shows the cup-shaped acetabulum. (From Ponseti IV. Growth and development of the acetabulum in the normal child: anatomical, histological and roentgenographic studies. J Bone Joint Surg Am 1978;60:575.) |
This is important in understanding normal growth and development and
the shape of the acetabulum in skeletal dysplasias and injury. The
labrum, or fibrocartilaginous edge of the acetabulum, is at the margin
of the acetabular cartilage. The hip joint capsule inserts just above
the labrum. The capsule insertion is continuous with the labrum below,
and with the periosteum of the pelvic bones above.
 |
|
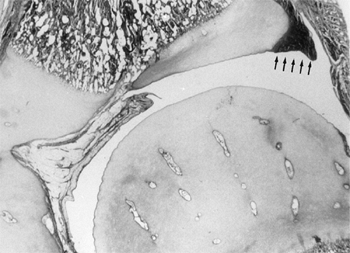
Figure 24.3
Coronal section through the center of the acetabulum in a full-term infant. Note the fibrocartilaginous edge of the acetabulum, the labrum (arrows), at the peripheral edge of the acetabular cartilage. The hip capsule inserts just above the labrum. |
 |
|
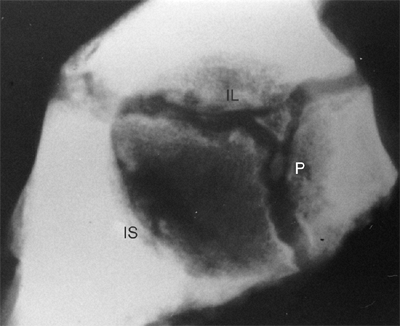
Figure 24.4
Lateral radiograph of the acetabulum of a 9-year-old girl. Two centers of ossification are seen within the cartilage adjoining the pubis (P) and appear to be developing within the vertical phalange of the triradiate cartilage. The positions of the ischium (IS) and the ilium (IL) are indicated. (From Ponseti IV. Growth and development of the acetabulum in the normal child: anatomical, histological and roentgenographic studies. J Bone Joint Surg Am 1978;60:575.) |
the side that articulates with the femoral head. On the opposite side
is a growth plate, with its degenerating cells facing toward the pelvic
bone that it opposes. New bone formation occurs in the metaphysis
adjacent to the degenerating cartilage cells. Growth of the acetabular
cartilage occurs by means of interstitial growth within the cartilage
and appositional growth under the perichondrium. This fact is most
important when considering various innominate bone osteotomies, because
surgical injury (by aggressive periosteal stripping or osteotome
placement) to this important area may jeopardize further acetabular
growth.
including the greater trochanter, the intertrochanteric zone, and the
proximal femur, is composed of cartilage. Between the 4th and 7th
months of life, the proximal femoral ossification center appears. This
bony centrum and its cartilaginous anlage continue to enlarge (although
at a slowly decreasing rate) until adult life, at which stage only a
thin layer of articular cartilage remains over it. The proximal femur
and the trochanter enlarge by appositional cartilage cell proliferation
(16).
and the femoral neck isthmus (16) (Fig. 24.5).
A balance among the growth rates of these centers accounts for the
normal configuration of the proximal femur, the relation between the
proximal femur and the greater trochanter, and the overall width of the
femoral neck. The growth of the proximal femur is affected by muscle
pull, the forces transmitted across the hip joint by weightbearing,
normal joint nutrition, circulation, and muscle tone (16,17,18). Any alterations in these factors may cause profound changes in the development of the proximal femur (19,20).
 |
|
Figure 24.5
The proximal femur in an infant has three physeal plates: the growth plate of the greater trochanter, the growth plate of the proximal femoral physeal plate, and the growth plate of the femoral neck isthmus connecting the other two plates. |
the trochanteric and femoral growth plates along the lateral border of
the femoral neck and is a reflection of their previous common origin.
This growth cartilage contributes to the lateral width of the femoral
neck and remains active until maturity.
determines the femoral neck configuration in the adult. Disturbances in
growth in any of these three growth plates, by whatever mechanism,
alter the shape of the proximal femur. Hyperemia secondary to surgery
or inflammatory conditions may stimulate growth in any or all of these
growth plates (16).
approximately 30% to the overall growth in length of the femur, and 13%
to the growth of the limb. Any damage to or disruption of the blood
supply to this plate disrupts the growth at this plate and results in a
varus deformity because the trochanter and the growth plate along the
femoral neck continue to grow (16,21).
Partial physeal arrest patterns may be caused by damage to portions of
the proximal femoral physeal plate. The relation between the growth of
the trochanter and the physis of the proximal femur should remain
constant; it is measured by the articular trochanteric distance, which
is the distance between the tip of the greater trochanter and the
superior articular surface of the femoral head. The greater trochanter
is usually classified as a traction epiphysis, depending on normal
abductor pull for growth stimulation. The trochanter, like the proximal
femur, grows appositionally.
with unreduced dislocations suggest that the main stimulus for the
concave shape of the acetabulum is the presence of a spherical femoral
head (10,15,22,23,24). Harrison determined that the acetabulum failed to develop in area and depth after femoral head excision in rats (15).
He also demonstrated atrophy and degeneration of the acetabular
cartilage, although the growth plates of the triradiate cartilage
remained histologically normal, as did the length of the innominate
bones. These experimental findings are characteristic of humans who
have had untreated hip dislocations (Fig. 24.6).
during development, several factors must act in concert. There must be
a reduced spherical femoral head. There must also be normal
interstitial and appositional growth within the acetabular cartilage,
and periosteal new bone formation must occur in the adjacent pelvic
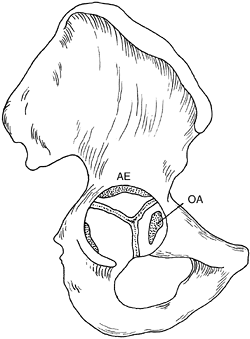
bones (10,11). The depth of the acetabulum is further enhanced at puberty by the development of three secondary centers of ossification (Fig. 24.7). These three centers are homologous with other epiphyses in the skeleton (11,15). The os acetabulum
develops in the thick cartilage that separates the acetabular cavity
from the pubis. The os acetabulum is the epiphysis of the pubis and
forms the anterior wall of the acetabulum. The epiphysis of the ilium,
the acetabular epiphysis, forms a major
portion of the superior edge of the acetabulum. A third, small
epiphysis also forms in the ischial region and contributes to its
normal growth (11,15,25).
balanced growth of the proximal femur, the acetabular and triradiate
cartilages, and the adjacent bones. This balance, which is probably
genetically determined, may be faulty in DDH. There is ample evidence
to suggest that an adverse intrauterine environment also plays an
important role in the pathogenesis of hip dysplasia (11,26,27,28,29,30).
 |
|
Figure 24.6 Untreated dislocation of the hip. Note the lack of the concave shape and the shallowness of the acetabulum.
|
femoral head and the acetabulum is lost. The femoral head can be made
to glide in and out of the acetabulum, with a palpable sensation known
clinically as the Ortolani sign (11,18,31,32).
DDH in the newborn refers to a spectrum of anatomic abnormalities, from
mild dysplastic changes to the severe pathoanatomic changes that are
found in the rare idiopathic teratologic dislocation, and more commonly
in teratologic dislocations associated with conditions such as
myelomeningocele and arthrogryposis.
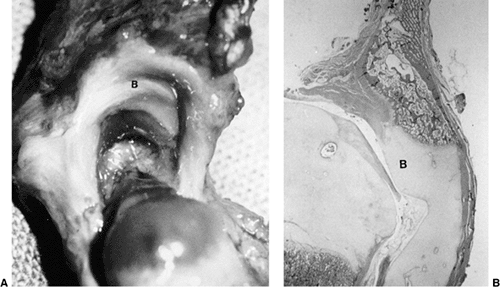
DDH is a hypertrophied ridge of acetabular cartilage in the superior,
posterior, and inferior aspects of the acetabulum. This ridge was
referred to by Ortolani as the neolimbus (18,32). The neolimbus is composed of hypertrophied acetabular cartilage (9,31) (Fig. 24.8).
There often is a trough or groove in the acetabular cartilage caused by
secondary pressure of the femoral head or neck. It is over this ridge
of acetabular cartilage that the femoral head glides in and out of the
acetabulum, with the palpable sensation referred to as the Ortolani
sign (9,18,32).
 |
|
Figure 24.7 Diagram of the right innominate bone of an adolescent. The os acetabulum (OA) is shown within the acetabular cartilage adjoining the pubic bone. The acetabular epiphysis (AE)
is within the acetabular cartilage adjoining the iliac bone, and another small epiphysis is within the acetabular cartilage adjoining the ischium (left). (Adapted from Ponseti IV. Growth and development of the acetabulum in the normal child: anatomical, histological and roentgenographic studies. J Bone Joint Surg Am 1978;60:575.) |
are reversible in the typical newborn with DDH, because there is a 95%
success rate of treatment using simple devices such as the Pavlik
harness and the von Rosen splint (33). These
pathologic changes are typical of 98% of DDH cases that occur at or
around birth. However, approximately 2% of newborns have teratologic
(antenatal) dislocations not associated with a syndrome or
neuromuscular condition (25,28).
In these rare cases, the pathologic and clinical findings are similar
to those seen in late-diagnosed DDH, which is described later in this
chapter.
different from that described for the normal hip. This is particularly
true for late-diagnosed cases. The primary stimulus for normal growth
and development comes from the femoral head within the acetabulum (10,23,24).
When there is a delay in diagnosis and treatment, some aspects of
normal growth and development are lost. The femoral head must be
reduced as soon as possible, and the reduction must be maintained to
provide the stimulus for acetabular development. If concentric
reduction is maintained, the acetabulum has the potential for recovery
and resumption of normal growth and development for many years (34,35,36).
 |
|
Figure 24.8 A:
Right acetabular cavity and femoral head of a newborn baby with bilateral congenital hip dysplasia. There is an acetabular bulge (B) or neolimbus along the upper acetabular cartilage, and the acetabular cavity is small. B: Frontal section of the same hip. The femoral head is very large in relation to the acetabular cavity. Note how the labrum is everted and adheres to the joint capsule above. The neolimbus (B) is composed of hypertrophied acetabular cartilage. (From Ishii Y, Weinstein SL, Ponseti IV. Correlation between arthrograms and operative findings in congenital dislocation of the hip. Clin Orthop 1980;153:138.) |
The resumption and adequacy of acetabular development is a
multifactorial problem that depends on the age at which the reduction
is obtained and on whether the growth potential of the acetabular
cartilage and the proximal femur is normal. The capacity of the
acetabular cartilage to resume normal growth depends on its intrinsic
growth potential, and whether it has been damaged by the subluxated or
dislocated femoral head or by various attempts at reduction. In the
patient with DDH who has been treated, especially in late-diagnosed
cases, accessory centers of ossification contribute to acetabular development (Fig. 24.9). Accessory centers of ossification
in the acetabulum are seen in only 2% to 3% of normal hips, and they
rarely appear before 11 years of age. However, among patients treated
for DDH, the centers may be present in as many as 60% of hips, usually
appearing 6 months to 10 years after reduction (34,35,36,37,44) (Fig. 24.9). These accessory centers
form in the peripheral acetabular cartilage, and may be a primary
abnormality of dysplasia or, more likely, they are a secondary
abnormality caused by pressure damage from the femoral head and/or neck
in the subluxated or dislocated position or by damage secondary to
closed or open treatment (see later discussion on obstacles to
reduction). In treated DDH cases, these accessory centers of
ossification should be sought on every sequential radiograph so as to
determine whether acetabular development is progressing, as they may
coalesce to form a normal acetabulum (Fig. 24.9).
This is an important factor to consider when deciding if surgical
intervention is necessary to correct residual acetabular dysplasia.
Although the presence of these centers indicates continued growth in
the acetabular cartilage, they may be indicative of injury to the
cartilage in this area. Their presence does not assure normal
acetabular development.
factors play a key role, with the incidence of DDH as high as 25 to 50
in 1000 live births among Lapps and Native Americans and a very low
rate among the southern Chinese population and persons of African
descent (29,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59).
One study reported a tenfold increase in the incidence of DDH among the
parents of index patients and a sevenfold increase among siblings
compared with the incidence among the general population (45). There is some suggestion that anteversion of the femoral neck or acetabulum may be an etiologic factor (29,31,37,60,61,62).
degrees of joint laxity, or a combination of both. Intrauterine
mechanical factors, such as breech position or oligohydramnios, and
neuromuscular mechanisms such as myelomeningocele, can profoundly
influence genetically determined intrauterine growth (5,6,63,64).
The first-born child is more likely to be affected than subsequent
children. Any of the factors contributing to an “adverse” intrauterine
environment may influence the development of the hip joint, and
postnatal influences may also contribute to the development of DDH (5,30,65,66,67).
 |
|
Figure 24.9 A:
An 18-month-old girl with bilateral high dislocations. Note the poorly developed acetabula with well-developed secondary acetabula. B: At 33 months of age, the irregular ossification centers in the left and right hip have coalesced, with a slight improvement in the acetabular index. C: When the girl is 7 years of age, an anteroposterior view shows the appearance of the accessory centers of ossification in the periphery of the acetabulum. D: The accessory centers of ossification are somewhat better appreciated in the abduction view at 7 years of age. E: An anteroposterior view at 8 years of age shows the coalescence of the accessory centers of ossification, increasing the depth of the acetabulum. Note the excellent sourcil formation. F: The accessory centers of ossification are well demonstrated in an abduction view at 8 years of age. |
The unstretched abdominal muscles and the primigravida uterus may
subject the fetus to prolonged periods of abnormal positioning, forcing
the fetus against the mother’s spine. This restraint limits fetal
mobility, especially hip abduction. The high rate of association of DDH
with other intrauterine molding abnormalities, such as torticollis and
metatarsus adductus, lends some support to the theory that the
“crowding phenomenon” plays a role in the pathogenesis (8,71,72,73). Oligohydramnios, which is associated with limited fetal mobility, also is associated with DDH (8,71).
The left hip is the most commonly affected hip; in the most common
fetal position, this is the hip that is usually forced into adduction
against the mother’s sacrum (8,47,71).
children delivered in the breech presentation. In the general
population, breech presentations occur in approximately 2% to 4% of
vaginal deliveries. Carter and Wilkinson (26,27) reported that 17% of children with DDH had a breech presentation; Salter reported the incidence as 23% (74). Twice as many girls as boys are born breech (75). Fifty-nine percent of breech presentations are first-born children (26,27,75).
Ramsey and MacEwen demonstrated that 1 of 15 girls born breech has
evidence of hip instability. In animal studies, the prolonged
maintenance of an abnormal position, such as the breech position, is
associated with the production of DDH (23,24).
the development of DDH. In societies that use swaddling (i.e., hips
forced into adduction and extension) in the immediate postnatal period,
the incidence of DDH is high, possibly as a result of the forceful
positioning of the legs in extension and adduction, counter to normal
newborn hip flexion and hamstring contractures (30,47,57,62,67,76,77,78,79).
of DDH has been addressed by many investigators. Newborns with DDH may
have capsular laxity. Hip capsular laxity has been implicated in the
pathogenesis of DDH, because the diagnostic test for DDH, the Ortolani
sign, depends on the head gliding in and out of the dysplastic
acetabulum over a ridge of abnormal acetabular cartilage. Proponents
argue that because reversible dysplasia can be produced in animals by
producing ligamentous laxity, the acetabular dysplasia seen in DDH is a
secondary phenomenon (23,24,30,31,74,80). LeDamany demonstrated that the acetabulum is shallowest at birth (61). Ralis and McKibbin confirmed LeDamany’s anatomic work in a small number of patients (80).
They too demonstrated that the acetabulum was shallowest at birth and
that this, combined with the normal laxity in the joint of the infant,
makes the time around delivery a high-risk period for dislocation (80,81). These anatomic experiments were repeated by Skirving and Scadden in African neonates (29);
in the African neonate, the acetabulum was deeper more frequently and
in a narrower range, possibly explaining why DDH is almost nonexistent
among persons of African descent. This finding also provides indirect
evidence that acetabular dysplasia is a primary cause of DDH.
The laxity may allow some instability without a positive Ortolani sign.
In postmortem examination of seven stillborn infants, the hips
demonstrated instability with a negative Ortolani sign; arthrograms
demonstrated slight pooling of the contrast media medially. On gross
examination, the hip capsules were stretched, and the femoral heads
could be pulled slightly away from the acetabula. However, the hips
were anatomically and histologically normal, unlike the postmortem
findings reported for all infants with positive Ortolani signs (9,11,32,55,83,84).
In addition to the normal physiologic capsular laxity expected in the
newborn, DDH is not a feature of conditions characterized by
hyperlaxity, such as Down, Ehlers-Danlos, and Marfan syndromes (32).
factors, a high-risk group of patients can be identified. This group
includes any patient who has more than one of the factors listed in Table 24.1. If an infant manifests any combination of these factors, the physician should be alert to the possibility of DDH.
|
TABLE 24.1 HIGH-RISK FACTORS FOR DEVELOPMENTAL DYSPLASIA OR DISLOCATION OF THE HIP
|
|
|---|---|
|
ethnic factors and by the diagnostic criteria used by the examining
physician. Another important factor is the diagnostic acumen of the
examiner. The age of the patient at the time of diagnosis must be taken
into account, because the physical findings and manifestations of the
condition change with increasing delay in diagnosis (8,9,11,30,32,55,71,82,83,84,85,86,87,88).
Despite screening programs for newborns, some cases are not detected,
and some evidence suggests that a few cases may arise after birth (29,85,90,91,92,93,94,95,96,97,98,99).
Moreover, whether acetabular dysplasia is primary or secondary to an
unrecognized dislocation or subluxation that has reduced spontaneously
remains uncertain.
Fellander et al. likened this diagnostic sign to the femoral head
gliding in and out of the acetabulum over a ridge and referred to this
palpable sensation as the ridge phenomenon (100).
This ridge, over which the femoral head glides in and out of the
acetabulum, is composed of hypertrophied acetabular cartilage (9,31,32,84) (Fig. 24.8). Ortolani named the ridge the neolimbus.
to describe this diagnostic sign. High-pitched soft tissue clicks are
often elicited in the hip examination of newborns. These clicks are
usually transmitted from the trochanteric region or the knee and have
no diagnostic significance (100). This poor
understanding of the pathoanatomy of the primary diagnostic sign of DDH
in the newborn has no doubt led to overdiagnosis and overtreatment of
infants (101,102,103,104).
referred to as the “click of exit.” The Barlow maneuver is a
provocative maneuver in which the hip is flexed and adducted and the
femoral head is palpated to exit the acetabulum partially or completely
over a ridge of the acetabulum (85). Many physicians refer to the Ortolani sign as the click of entry,
which is caused when the hip is abducted, the trochanter is elevated,
and the femoral head glides back into the acetabulum. Some physicians
make treatment decisions on the basis of whether they feel that the hip
is Ortolani-positive rather than Barlow-positive, the general opinion
being that the Barlow-positive hip is more stable and hence may
stabilize spontaneously. Because Ortolani and LeDamany described the
palpable sensation as the femoral head exits or enters the acetabulum,
the author prefers to use the Ortolani sign to refer to both the
palpable sensation of subluxating or dislocating the hip and to
reducing a subluxated or dislocated hip. The author also makes no
distinction in treatment between patients exhibiting the Ortolani sign
and those with the Barlow sign.
every 100 newborns examined has evidence of some hip instability (i.e.,
positive Ortolani or Barlow sign), although the incidence of true
dislocation is reported to be between 1 and 1.5 cases per 1000 live
births (9,43,85,93,94,95,96,101,105,106,107,108,109,110).
newborns and are usually associated with other generalized conditions,
such as arthrogryposis, myelodysplasia, and other syndromes. These
perinatal teratologic dislocations are at the extreme end of the DDH
pathologic spectrum and account for only 2% of cases in newborn
examination series (9,47,48,111,112). They are usually manifested because of the secondary adaptive changes more characteristic of the late-diagnosed case.
Limited abduction is a clinical manifestation of the various degrees of
shortening of the adductor longus that are associated with hip
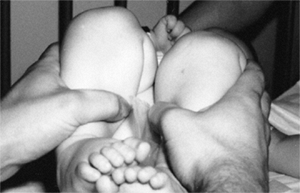
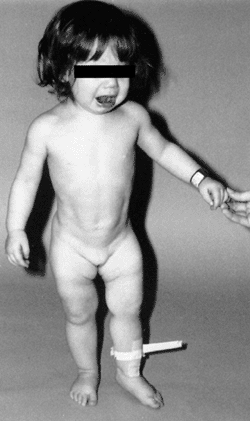
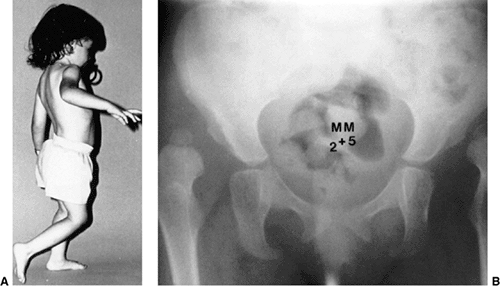
subluxation or dislocation (48). Other manifestations of late-diagnosed DDH may include apparent femoral shortening, also called the Galeazzi sign (Fig. 24.11); asymmetry of the gluteal (113), thigh, or labial folds (114); and limb-length inequality (Fig. 24.12). In patients with bilateral dislocations, clinical findings include a waddling gait and hyperlordosis of the lumbar spine (Fig. 24.13).
development are impaired. With increasing age at detection and
reduction, and particularly in children older than 6 months, the
obstacles (intraarticular and extraarticular)
to
concentric reduction become increasingly difficult to overcome by
simple treatment methods such as use of the Pavlik harness, and closed
or open reduction usually must be performed under general anesthesia.
Restoration of normal acetabular development is less likely as age at
detection increases (25,31,43,109,113,114,115,116,117).
 |
|
Figure 24.10 A 15-month-old child with left hip dislocation. Note the limited abduction of the hip.
|
 |
|
Figure 24.11 A 15-month-old girl with developmental dislocation of the left hip. Note the apparent femoral shortening.
|
to reduction include the contracted adductor longus and the iliopsoas.
These muscles are shortened because of the hip being in the subluxated
or dislocated position, allowing secondary muscle-shortening.
 |
|
Figure 24.12
A 1-year-old girl with developmental dislocation of the left hip was referred for toe walking. Note the apparent limb-length inequality and the asymmetry of the thigh and labial folds. |
late-diagnosed DDH include the ligamentum teres, the transverse
acetabular ligament, the constricted anteromedial joint capsule, and,
rarely, an inverted and hypertrophied labrum (31,118).
The most significant intraarticular obstacle to reduction, however, is
some degree of anteromedial hip capsular constriction (31,119,120,121,122,123).
The ligamentum teres may be thickened, and it may become the primary
obstacle to reduction in some cases. In children of walking or crawling
age, the ligamentum teres may be significantly elongated and enlarged.
Its sheer bulk precludes concentric reduction without excision of the
ligament. The transverse acetabular ligamentum may hypertrophy
secondary to the constant pull of the ligamentum teres on its
attachment at the base of the acetabulum (31,123). This effect decreases the diameter of the acetabulum.
The acetabular labrum may be iatrogenically inverted, and may be an
obstacle to reduction in patients previously treated with unsuccessful
closed reductions. Arthrograms are often misinterpreted as showing an
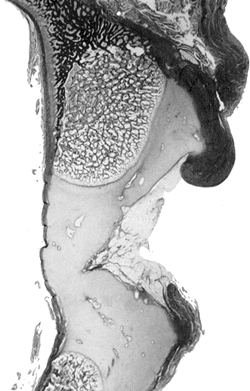
inverted labrum (124); the shadow thought to be the inverted labrum or limbus is instead the neolimbus (originally described by Ortolani) (9,31,125,126) (Fig. 24.15).
This neolimbus is epiphyseal cartilage and is almost never an obstacle
to reduction. It must not be removed, because removal impairs
acetabular development (9,127).
If the surgeon feels that this tissue is somehow impairing reduction,
it should be radially incised but never excised. The cartilage of the
neolimbus may be primarily abnormal or may be damaged by a traumatic
open or closed reduction. A response to this damage may be responsible
for the appearance of the previously discussed accessory centers of ossification seen in treated cases of DDH (9) (Fig. 24.9).
ultrasonography has gained popularity worldwide as a screening tool.
Its cost-effectiveness has yet to be documented for screening for DDH
on a wide scale.
Many proponents strongly recommend that ultrasonography be used as a
routine screening tool in the newborn nursery and that it be used
extensively in the management of all DDH problems (140,141,142). The use of ultrasonography in orthopaedic practice was pioneered by Graf in Austria in the 1970s (143,144). Harcke et al. in the United States (145,146,147,148), Terjesen et al. in Norway (149,150,151,152), and Clarke in Great Britain (153,154,155) have been the prime evaluators of this tool for the diagnosis of DDH and other hip disorders.
 |
|
Figure 24.13 A girl, 2 years and 5 months of age, with bilateral hip dislocations. A: Note the waddling gait and hyperlordosis. B:
Radiograph shows high bilateral dislocations and poorly developed acetabula with well-developed secondary acetabula where the femoral heads articulate with the ilia. |
 |
|
Figure 24.14
A coronal section of the acetabulum demonstrates the interned hypertrophic labrum (limbus) extending over the margin of a slightly thickened acetabular cartilage. The thick capsule extends upward above the inverted labrum, from which it is separated by a shallow groove. In this section through the ilium, the growth plate is slanted upward laterally, but endochondral ossification is normal. At the margin of the roof, periosteal bone growth is retarded. (From Ponseti IV. Morphology of the acetabulum in congenital dislocation of the hip: gross, histological and roentgenographic studies. J Bone Joint Surg Am 1978;60:586.) |
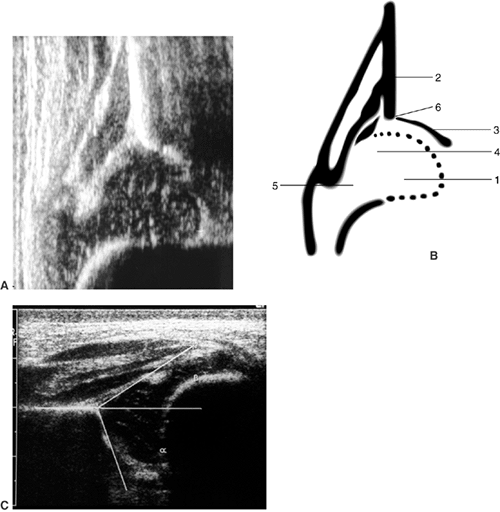
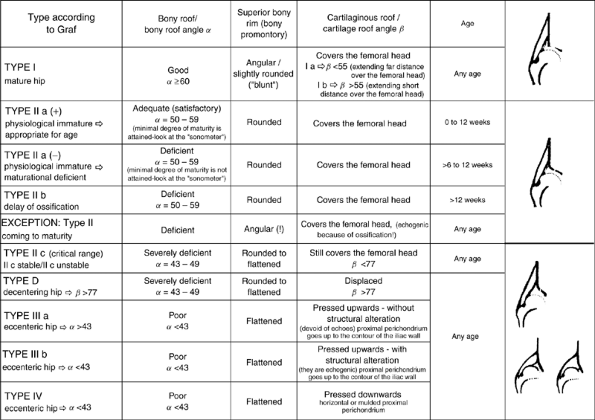
The morphologic assessment, as pioneered by Graf, focuses primarily on
critical evaluation of the anatomic characteristics of the hip joint (Fig. 24.16).
This is accomplished by measuring two angles on the ultrasound image:
the α angle, which is a measurement of the slope of the superior aspect
of the bony acetabulum, and the β angle, which evaluates the
cartilaginous component of the acetabulum. The hip is classified into
four types and several subtypes according to various factors (143,144) (Fig. 24.17).
In the evaluation of Terjesen et al., the percentage of acetabular
coverage of the femoral head (i.e., percent coverage) is a key
measurement (149,150,151,152).
practiced in Europe, but it has been criticized because of substantial
interobserver and intraobserver variations in the measurement of
angles, particularly the β angle (146).
observed in real time and in multiple planes provides a means of seeing
what occurs during the Ortolani or Barlow maneuver. The use of dynamic
ultrasonography, as popularized by Harcke et al. (145,146,147), has been criticized for being excessively operator-dependent and requiring a subjective assessment of the findings.
treatment of DDH are not universally established. Because there are
many controversies yet to be resolved, ultrasonography cannot be
advocated as a routine screening tool, even though (146)
it is used as such in Europe. Prospective longitudinal studies
documenting the outcomes of minor anatomic abnormalities found in
ultrasonographic
examinations need to be completed (162,163).
Its routine use in newborn nurseries has resulted in overdiagnosis
(above the expected incidence) of DDH and cannot be considered
cost-effective (164,165,166,167). Its use in only high-risk infants may eventually prove cost-effective (167,168).
However, Clarke et al. showed that screening all high-risk infants and
all infants who had any abnormality on physical examination did not
reduce the prevalence of late-diagnosed cases (154,155,169).
 |
|
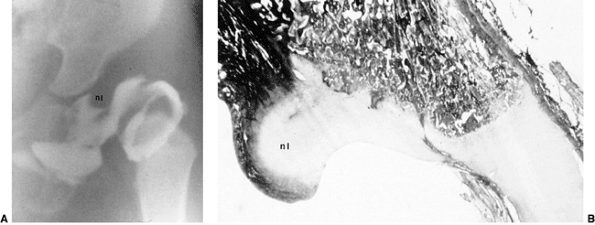
Figure 24.15 A: Arthrogram of the left hip in a 15-month-old child with complete dislocation. Note the shadow of the neolimbus (nl). B: A histologic specimen demonstrates hypertrophied acetabular cartilage of the neolimbus (nl), consistent with the arthrographic appearance in (A).
|
 |
|
Figure 24.16 A: Ultrasonography of a normal newborn. B: Anatomic drawing of hip landmarks (after Graf): 1, femoral head; 2, iliac limb; 3, bony acetabular roof; 4, acetabular labrum; 5, joint capsule; 6, osseous rim. C: The α and β angles are identified on this ultrasonograph of a newborn.
|
 |
|
Figure 24.17 Hip types based on ultrasonographic results. (Courtesy of Prof. R. Graf, Stolzalpe, Austria.)
|
Ortolani-positive infants to assess stability at the completion of
treatment (146). An ideal use for ultrasonography is for “guided reduction” of a dislocated hip in an infant (170),
in other words, for monitoring the progress of reduction of a
subluxated or dislocated hip being treated in a Pavlik harness.
Ultrasonography is used at 7- to 10-day intervals to check the progress
of reduction of the hip and its stability during Pavlik harness
treatment. This may temporarily obviate the need for radiographic
evaluation. Other uses for ultrasonography in the treatment of DDH
include monitoring of the hip position while the patient is in traction
before attempting reduction and evaluating closed reductions in the
operating room. The distinct advantage of ultrasonography is that it
provides some anatomic evaluation of the hip joint without exposing the
infant to radiation.
evaluation and whether an orthopaedic surgeon, the treating physician,
or a radiologist with expertise in ultrasonography should perform the
evaluation. In the newborn, DDH is not a radiographic diagnosis; the
diagnosis should be made by clinical evaluation, which may be enhanced
by ultrasonography if the examination results are questionable. Some
investigators have used vibration arthrometry (171,172,173).
diagnosis of DDH should be confirmed by radiography. Many radiographic
measurements can be made, but there are wide interobserver and
intraobserver variations in these measurements (174,175). Because it is difficult to standardize the radiographic positioning of infants, many centers use positioning frames (176).
is essential to notice changes in the radiographic measurements over
time, and not to make significant decisions based on a single
radiograph. The classic radiographic
features of late-diagnosed DDH include: an increased acetabular index (28,177,178,179,180,181,182); disruption of the Shenton line; a widened pelvic floor (183); an absent teardrop figure (184,185,186,187,188,189,190);
delayed appearance of the femoral ossific nucleus on the involved side
or dissimilar sizes of the ossific nuclei; abnormality in Smith
centering ratios (23,24,36);
decreased femoral head coverage; and failure of the medial metaphyseal
beak of the proximal femur, and, subsequently, the secondary
ossification center, to be located in the lower inner quadrant defined
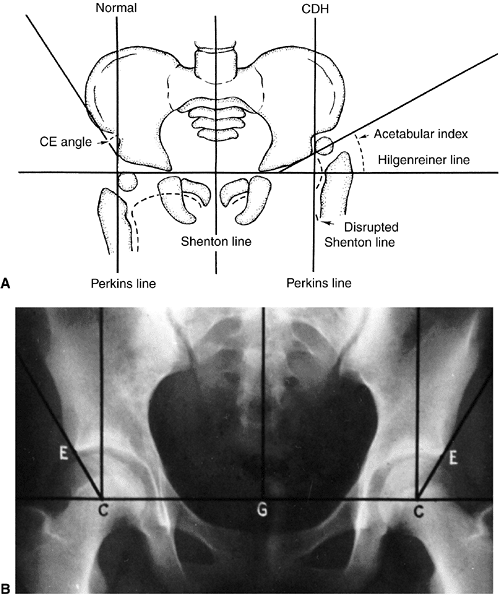
by the Hilgenreiner and Perkins lines (87,191,192) (Fig. 24.18A).
When the triradiate cartilage is closed, the acetabular angle of Sharp
(i.e., from the inferior edge of the teardrop figure to the edge of the
acetabulum) is a useful measurement of acetabular dysplasia (193).
Measurement of the center-edge (CE) angle becomes useful only in the
patient who is more than 5 years of age, and is most useful in the
adult patient (44) (Fig. 24.18B).
Radiographs show only the ossified portion of the pelvic bones and the
proximal femur. Excellent acetabular coverage of the femoral head may
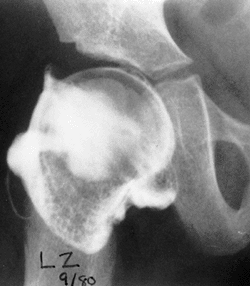
be found, albeit by unossified cartilage (Fig. 24.19). If this cartilage does not ossify, the residual dysplasia may eventually lead to subluxation and degenerative joint disease.
dysplasia during the first few years of life. After 3 or 4 years of
age, the Shenton line should be intact on all views of the hip;
thereafter, any disruption of the Shenton line indicates an abnormality
in the relation between the proximal femur and the acetabulum. As will
be emphasized in the section on methods of treatment, this relation
must be restored in order to prevent degenerative joint disease in
later life (25,44,57,62,116).
diagnosis and evaluation, as well as for documentation of femoral
head–acetabular relationships after closed or open reduction (194,195).
With advances in software, this modality will no doubt provide useful
information in the future, but the need for anesthesia in infants and
children limits its utility (196,197,198,199).
 |
|
Figure 24.18 A: Radiographic parameters. CDH, congenital dysplasia of the hip; CE Angle, center-edge angle. B: Center-edge angle of Wiberg. C, Center of the femoral head; E, bony edge of the acetabulum; G, gravity line.
|
 |
|
Figure 24.19
Arthrogram of a 5-year-old girl 3 years after open reduction. Note the excellent coverage of the femoral head by unossified acetabular cartilage. |
quite variable. Yamamuro and Doi followed up 52 patients whose hips had
positive Ortolani signs for a 2-year period without treatment for the
first 5 months. Of the 12 they called “dislocated” hips, 3 (25%) were
radiographically normal at 5 months of age. Of the 42 which they called
“subluxable” hips, 24 (57%) were normal at 5 months (79).
More than 60% of these stabilize during the first week of life, and 88%
stabilize during the first 2 months, without treatment. The remaining
12% become true congenital dislocations and persist in the absence of
treatment. Pratt et al. did a follow-up, for an average of 11.2 years,
of 18 “dysplastic” hips in patients who had been diagnosed on the basis
of clinical and radiographic parameters at an age younger than 3
months., They found that 15 of these hips were radiographically normal (78).
diagnosed as having DDH on the basis of clinical and radiographic
criteria at younger than 3 months. He found that 26% of the femoral
heads became completely dislocated, 13% had partial contact of the
femoral head with the acetabulum, 39% remained located but retained
dysplastic features, and 22% were normal (47).
birth, some may go on to subluxation (partial contact of femoral head
with the acetabulum) or dislocation, and some may remain located
(intact Shenton line) but retain anatomic dysplastic features. Because
it is not possible to predict the outcome of unstable hips in newborns,
all newborns with clinical hip instability, as manifested by a positive
Ortolani or Barlow sign, should be treated.
the presence or absence of two factors: a well-developed false
acetabulum and bilaterality (26,57,66,115,200,201).
Wedge and Wasylenko demonstrated only a 24% chance of a good clinical
outcome with a well-developed false acetabulum, but with a moderately
developed or absent false acetabulum, the patients had a 52% chance of
a good clinical outcome (57,66).
Of 42 patients with complete dislocations, 13 had radiographically
confirmed degenerative joint disease, such as loss of joint space, cyst
formation, sclerosis, osteophyte formation, and flattening of the
femoral head. Of these 13 patients, 10 (76%) had poor clinical outcomes.
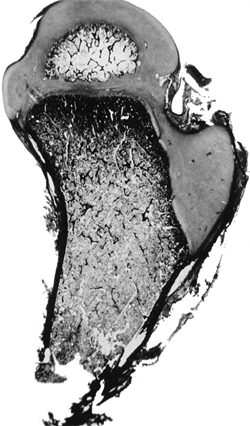
This 74-year-old man had no hip or thigh pain and only mild backache
for 5 years before his death. The femoral head had no articulation with
any portion of the ilium, and was covered with a thickened, markedly
elongated hip joint capsule. The only degenerative changes were where
the lesser trochanter abutted the overhanging superior acetabular rim.
In the absence of a false acetabulum, most patients with complete
dislocations do well, maintaining good range of motion with little
functional disability (Fig. 24.20). Completely dislocated hips with
well-developed false acetabula are more likely to develop
radiographically visible degenerative joint disease changes and poor
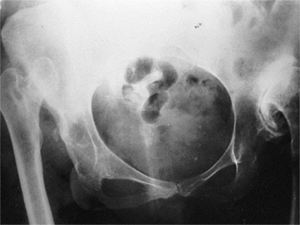
clinical outcomes (Fig. 24.21). Factors that lead to the formation or lack of formation of a false acetabulum remain unknown (25).
 |
|
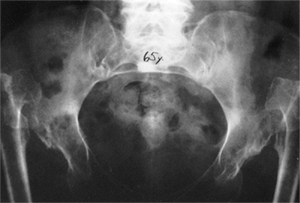
Figure 24.20
A 65-year-old woman with bilateral, untreated developmental dislocations of the hips complained of some low back pain, but had no hip pain. She had a waddling gait and hyperlordosis. |
 |
|
Figure 24.21
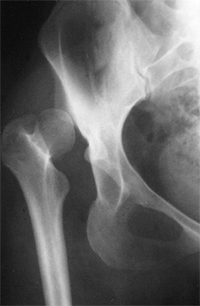
Radiograph of a 43-year-old woman with complete dislocation of both hips. She is asymptomatic on the right side but has disabling symptoms from the left hip. She has no false acetabulum on the right, but has a well-developed false acetabulum on the left with secondary degenerative changes. [From Weinstein SL. Natural history of congenital hip dislocation (CDH) and hip dysplasia. Clin Orthop 1987;225:62, with permission.] |
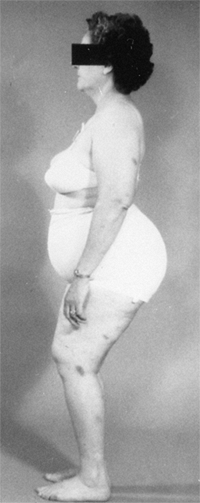
dislocations. It is thought that this pain is secondary to the
hyperlordosis of the lumbar spine that is associated with bilateral
dislocations (25,57,66,202,203,204) (P. Melvin, R. Johnston, I.V. Ponseti, personal communication) (Fig. 24.22).
of limb-length inequality, ipsilateral knee deformity and pain,
scoliosis, and gait disturbance are common. Limb-length inequalities of
as much as 10 cm have been reported in patients with unilateral
dislocations. These patients develop flexion–adduction deformities of
the hip, which may lead to valgus deformities of the knee. The valgus
knee deformity is often associated with attenuation of the medial
collateral ligament and degenerative joint disease of the lateral
compartment, although some medial compartment disease has also been
described (25,57,66,116,201), (P. Melvin, R. Johnston, I.V. Ponseti, personal communication).
The same factors that are involved in the development of secondary
degenerative disease in the false acetabulum and in the associated
clinical disability in bilateral cases affect unilateral dislocations
also.
untreated patients is important because of the likelihood that these
findings can be extrapolated to residual dysplasia and subluxation
after treatment (116,200,205,206,207,208,209,210).
 |
|
Figure 24.22
A 45-year-old woman with bilateral complete dislocations, hip flexion deformity, and marked hyperlordosis. The patient’s only reported symptoms concerned her back. |
All subluxated hips (i.e., those in which there is some contact between
the femoral head and the acetabulum) are by definition anatomically
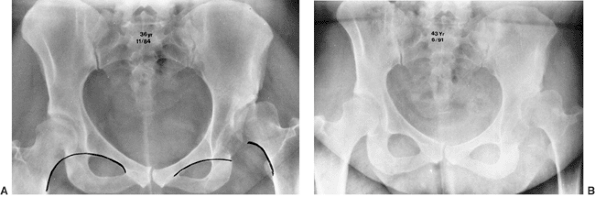
dysplastic. On film, the major difference between radiographic dysplasia and radiographic subluxation is determined by the integrity of the Shenton line. In radiographic subluxation,
the Shenton line is disrupted and the femoral head is superiorly,
laterally, or superolaterally displaced from the medial wall of the
acetabulum. In radiographic dysplasia, the normal Shenton line relation is intact (57,66,211,212) (Fig. 24.23).
In the literature describing the natural history of DDH, these two
radiographic and clinical entities are often not separated. Moreover,
secondary degenerative changes may convert a radiographically dysplastic hip into a radiographically subluxated hip (202,204,208,209,212,213,214) (Figs. 24.24 and 24.25).
subluxation and dysplasia, but the natural histories of these two
radiographic entities are different. Residual radiographic subluxation
after treatment of DDH invariably leads to degenerative joint disease
and clinical disability (25,65,67,116,202,207,209,212). The rate of deterioration is directly related to the severity of the subluxation and the age of the patient (209,212).
 |
|
Figure 24.23 Radiographic subluxation and dysplasia. A:
A 36-year-old woman with bilateral anatomically abnormal (dysplastic) hips. The left hip is radiographically subluxated, with the Shenton line disrupted, and the right hip is radiographically dysplastic, with the Shenton line intact. B: Seven years later, note the marked loss of joint space in the secondary acetabulum of the left hip and very early disruption of the Shenton line on the right. The right hip is asymptomatic, and the left hip is about to undergo total hip arthroplasty. |
radiographic acetabular dysplasia leads to secondary degenerative joint
disease, especially in women, although there are no predictive
radiographic parameters (202,208,209,212,214,215).
The reasons for degenerative changes in radiographically dysplastic
hips are probably mechanical in nature and related to increased contact
stress over time. A certain threshold of “overpressure” (product of
time and pressure, involving years of exposure to pressure above a
2-megapascal (MPa) level, leading to damage) (216,217)
may correlate with poor long-term outcome. Aspherical femoral heads
(e.g., secondary to aseptic necrosis) tend to experience even more
severe degrees of overpressure. It appears that radiographic
degenerative joint disease correlates with the magnitude of the
overpressure and the time of exposure (216). This overpressure may cause problems at a much younger age, with symptoms of “acetabular rim syndrome” (see Page 1021) (218).
visible. Cases of radiographic hip dysplasia are either diagnosed only
incidentally on the basis of radiographs taken for other reasons, or
after the patient develops symptoms (26,27,44,75,214).
Stulberg and Harris found that 50% of their patients with radiographic
dysplasia and degenerative joint disease had radiographic evidence of
dysplasia in the other hip (214). Melvin et
al., in their unpublished 30- to 50-year follow-up of DDH, demonstrated
that 40% of the patients with DDH showed radiographic evidence of
dysplasia in the opposite hip (P. Melvin, R. Johnston, I.V. Ponseti, personal communication).
It has been estimated that 20% to 50% of degenerative joint disease of
the hip is secondary to subluxation or residual radiographic acetabular
dysplasia (44,57,212,214,215,219,220,221).
Wiberg suggested that there was a direct correlation between the onset
of radiographically determined degenerative joint disease and the
amount of dysplasia as measured by the decrease in the CE angle (44) (Fig. 24.16).
degenerative joint disease and its relation to the severity of
radiographic acetabular dysplasia, reviewed the 17 cases on which
Wiberg based his conclusions (212). They
concluded that 7 of 17 hips were actually subluxated. These subluxated
hips were the most anatomically dysplastic; their CE angles averaged 2
degrees, and all 7 had radiographically seen degenerative changes by
the time the patients were 42 years of age. The other 10 hips in
Wiberg’s series were radiographically dysplastic. They had intact
Shenton lines and an average CE angle of 10 degrees. None of these
patients developed radiographic degenerative joint disease before 39
years of age; however, degenerative changes became apparent
radiographically by 57 years of age. In this review of Wiberg’s series,
the decrease in CE angle was associated with an increase in anatomic
acetabular dysplasia and an increased likelihood that the hip would be
subluxated. Subluxation was the primary factor in the development of
degenerative joint disease in this group. Subluxation predictably leads
to degenerative joint disease and clinical disability over time.
radiographic evidence of acetabular dysplasia (i.e., CE angle less than
20 degrees, but without subluxation and with the Shenton line intact),
at an average follow-up of 22 years (212). All
the patients eventually developed radiographic evidence of degenerative
joint disease. However, there was no linear correlation between the CE
angle and the rate of development of degenerative joint disease, as had
been suggested previously by Wiberg. A decreased CE angle was
associated solely with increasing radiographic evidence of acetabular
dysplasia and not with subluxation, because patients with subluxation
had been excluded in this series. Cooperman et al. demonstrated that
radiographic evidence of acetabular dysplasia leads to radiographically
detectable degenerative joint disease, but the
process
may take decades. This study also demonstrated that the conventional
radiographic parameters used for describing dysplasia (e.g., CE angle,
acetabular index of Sharp, percent coverage, depth, inclination) could
not predict the rate at which a radiographically confirmed dysplastic
hip joint would develop radiographic evidence of degenerative joint
disease.
 |
|
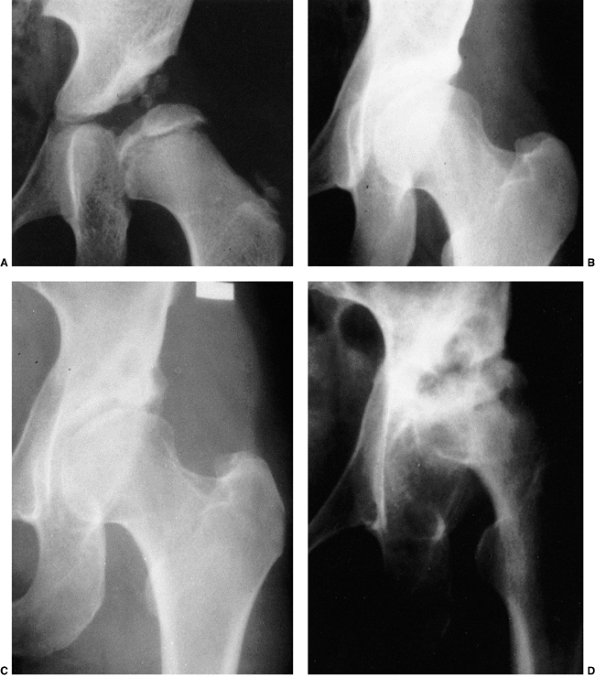
Figure 24.24
Anteroposterior radiographs made after closed reduction of developmental dislocation of the hip that had been performed when the patient was 2 years and 4 months of age. A: Thirty-nine months after reduction, when the patient was 5 years and 7 months of age, the accessory centers of ossification are visible in the acetabular cartilage. B: Fifteen years after reduction, when the patient was 17 years of age, the Shenton line is intact and there is mild, acetabular dysplasia. C: Forty-two years after reduction, when the patient was 44 years of age, degenerative changes are present. D: Fifty-one years after reduction, when the patient was 53 years of age, the hip is subluxed and shows severe degenerative changes (Iowa Hip Rating, 48 of 100 points). The patient subsequently underwent total hip replacement. (From Malvitz TA, Weinstein SL. Closed reduction for congenital dysplasia of the hip: functional and radiographic results after an average of thirty years. J Bone Joint Surg Am 1994;76:1777.) |
 |
|
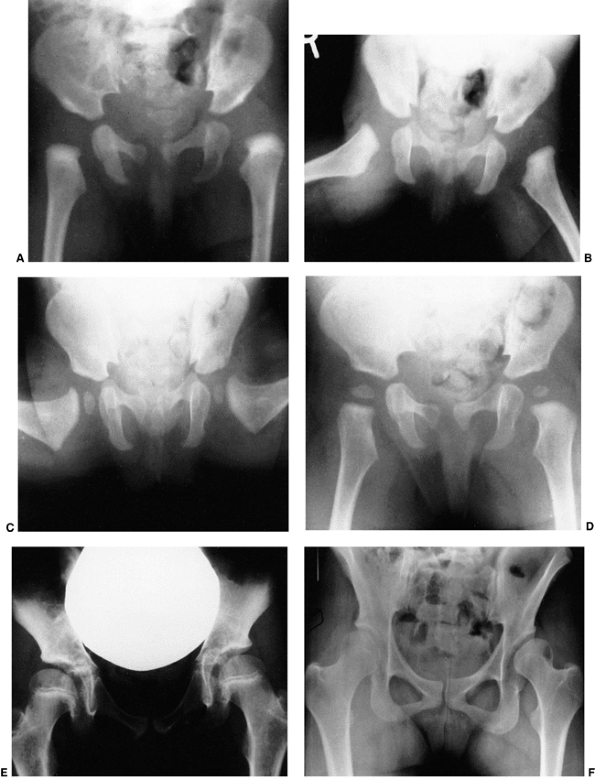
Figure 24.25 A: Anteroposterior view of a 4-month-old girl with left hip dislocation and right hip subluxation. B: Abduction view. C: Abduction view at 7 months of age, 3 months after closed treatment. D: Anteroposterior view at 7 months of age, 3 months after closed treatment. E: Anteroposterior view at 7 years of age. Note the mild anatomic dysplasia of both hips. F:
Anteroposterior view at 15 years of age. Note the bilateral anatomic dysplasia. The right hip is radiographically dysplastic, and the left hip is radiographically subluxated. |
radiographic picture of degenerative joint disease that is uniquely
associated with preexisting acetabular dysplasia (214).
In 80% of patients with dysplasia, the CE angle is usually less than 20
degrees, but acetabular shallowness, as measured by acetabular depth,
affects all of these patients. The investigators also demonstrated that
the CE angle, the criterion most commonly used to quantitate dysplasia,
could be affected by many factors, including positioning for the
radiographs and the changes accompanying the normal development of
degenerative joint disease. The secondary degenerative changes in a
dysplastic acetabulum may give the hip a normal-appearing CE angle. In
their series of 130 patients with primary or idiopathic degenerative
joint disease, Stulberg and Harris were able to demonstrate that 48%
showed evidence of primary acetabular dysplasia, and that acetabular
dysplasia frequently occurred in women with degenerative joint disease.
radiographic evidence of acetabular dysplasia and degenerative joint
disease comes from the southern Chinese population. In an epidemiologic
study from Hong Kong, where the incidence of childhood hip disease is
low, the incidence of adult osteoarthritis (nontraumatic) was also
shown to be low (53,54).
Patients with the most severe subluxation usually had the onset of
symptoms during the 2nd decade of life. Those with moderate subluxation
presented during their 3rd and 4th decades, and those with minimal
subluxation usually experienced the onset of symptoms around the 5th
decade.
rarely have the classic signs of degenerative joint disease such as
decreased joint space, cyst formation, double acetabular floor, and
inferomedial femoral head osteophytes. The only radiographic feature
present at the onset of symptoms may be increased sclerosis in the
weight-bearing area. This increased sclerosis is secondary to
increasing osteoblastic stimulation in response to the decreased
weight-bearing surface area; the increase of the normal per- unit load
strains the bone. The mechanism of pain in these instances is open to
speculation.
symptoms is 36.6 years in women and 54 years in men. Severe
degenerative changes become evident radiographically approximately 10
years later, by 46.4 years of age in women and 69.6 years of age in men.
 |
|
Figure 24.26
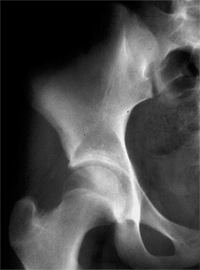
The “gold standard” normal hip at maturity: note intact Shenton line, well-developed and appropriately shaped teardrop, down-sloping sourcil, and normal gothic arch above the sourcil. |
of symptoms at a younger age than patients with complete dislocations
do. After pain and radiographically evident degenerative disease start,
the disease progresses rapidly. Harris reported that symptoms of
degenerative joint disease associated with radiographic evidence of
acetabular dysplasia occurred early in life and that almost 50% of the
patients in his series with acetabular dysplasia had their first
reconstructive procedure before 60 years of age, with fewer than 5%
having their first reconstruction after 60 years of age (215).
well-developed teardrop; a normal femoral neck-shaft angle; an intact
Shenton line; a down-sloping sourcil and a well-developed gothic arch (Fig. 24.26). Any deviation from this radiographic appearance may lead to degenerative joint disease over the long term (57,116,184,208,209,212,213,214).
development of the hip, the fundamental treatment goals in DDH are the
same, regardless of the age of the patient. The first goal is to obtain
reduction and maintain that reduction to provide an optimal environment
for the development of the femoral head and acetabulum (115). As has been demonstrated by many follow-up studies of
treated DDH, the acetabulum has the potential for development for many
years after reduction as long as the reduction is maintained (34,36,37). The femoral head and femoral anteversion can remodel if the reduction is maintained (209,222).
Further intervention is necessary only to alter an otherwise adverse
natural history, as in the treatment of residual dysplasia and the
prevention or treatment of subluxation. The later the diagnosis of DDH
is made, the more difficult it is to achieve these goals, the less
potential there is for acetabular and proximal femoral remodeling, and
the more complex are the required treatments. With increasing age and
complexity of treatment the risk of complications is greater, and the
patient is more likely to develop degenerative joint disease.
Triple diapers or abduction diapers have no place in the treatment of
DDH in the newborn. They give the family a false sense of security and
are generally ineffective. Any success with the use of triple diapers
or abduction diapers could be attributed to the natural resolution of
the disorder.
Although other devices are available (e.g., von Rosen splint, Frejka
pillow), the Pavlik harness remains the most commonly used device
worldwide (35,81,225,226,227,228,229,230,231,232,233,234,235,236).
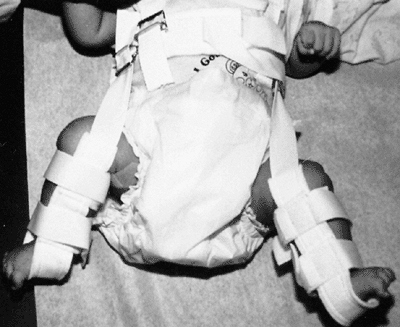
When appropriately applied, the Pavlik harness prevents the hip
extension and adduction that can lead to redislocation, but it allows
further flexion and abduction, which lead to reduction and
stabilization. By maintaining the Ortolani-positive hip in a Pavlik
harness on a full-time basis for 6 weeks, hip instability resolves in
95% of cases (236).
 |
|
Figure 24.27
Newborn with bilateral hip dislocations in a Pavlik harness. Appropriately applied, the harness prevents hip extension and adduction which can lead to redislocation, but allows further flexion and abduction, which lead to reduction and stabilization. |
months of age for any child with residual dysplasia, subluxation, or
complete dislocation. After 6 months of age the failure rate for the
Pavlik harness is greater than 50%, because it is difficult to maintain
the increasingly active and crawling child in the harness.
They pointed out that failures of treatment most often result from
problems related to the physician, the device, or the patient.
inappropriate application and persistence of inadequate treatment. The
Pavlik harness is contraindicated in patients with significant muscle
imbalance, such as those with myelodysplasia or cerebral palsy. It is
also contraindicated in patients who have significant stiffness of the
joints, such as children with arthrogryposis. The harness will fail if
it is applied in a child with excessive ligamentous laxity, as seen in
Ehlers-Danlos syndrome (33).
many factors. If treatment with the harness is to be successful,
physicians should be well versed in its appropriate application and the
adjustments that are necessary throughout the course of treatment. It
is important that the physician recognize when a treatment failure has
occurred, so as not to prolong treatment with the harness and cause
secondary pathologic changes, called Pavlik harness disease (238).
Persistence of treatment may damage the femoral head, injure the
acetabular cartilage, and impair future bone growth. An inappropriately
applied harness is a failure of the physician, not a failure of the
orthotic (237,239).
specific orthotic device. Not all Pavlik harnesses are the same; the
strap attachment sites vary. However, since the article by Mubarak et
al. in 1981, most harnesses on the market meet the requisite standards
that those authors outlined (33,237).
family, social, and educational situations make compliance impossible.
In these situations, the Pavlik harness would be inappropriate, and
closed reduction and casting may be the more judicious approach. The
family must be educated about the importance of the harness, its care
and maintenance, how the child should be bathed while wearing the
harness, and the consequences of failure. Family noncompliance can lead
to failure, and the use of a visiting nurse may be helpful in these
situations.
should be demonstrated to the family members. The chest halter strap
should be positioned at the nipple line, and the shoulder straps are to
be set to hold the cross strap at this level. The leg and foot stirrups
must have their anterior and posterior straps oriented anteriorly and
posteriorly to the child’s knees. Hip flexion should be set at 100 to
110 degrees. These straps should be in the anterior
axillary
line. The posterior abduction strap should be at the level of the
child’s scapula and adjusted to allow comfortable abduction within the safe zone (240),
which is defined as the arc of abduction and adduction, that is,
between redislocation and comfortable, unforced abduction. The
posterior strap acts as a checkrein to prevent the hip from adducting
to the point of redislocation. Ultrasonography is a useful means of
documenting relocation of the Ortolani-positive hip.
the Pavlik harness. If the Pavlik harness is used for stabilizing an
unstable hip (i.e., an Ortolani- or Barlow-positive hip), the harness
is used full time for 6 to 12 weeks after clinical stability is
achieved. The author’s preference is to use the harness full time for 6
weeks beyond the point when stability is reached. Most hips stabilize
in days to weeks. The harness is checked at 7- to 10-day intervals to
assess hip stability and to adjust the flexion and abduction straps to
allow for growth of the infant. In the author’s opinion, clinical
examination is usually sufficient to check on the progress at each
visit; but if uncertainty is present, ultrasonography may be used;
radiographs are unnecessary.
dislocation, the Pavlik harness may be used in a trial of
ultrasound-monitored reduction. In this case, the harness must be
applied with enough hyperflexion and abduction to point the femoral
head toward the triradiate cartilage. This situation is the ideal
indication for the use of ultrasonography to follow the reduction. When
the harness is used in this situation, the infant should be checked at
7 to 10 days to determine whether the reduction is being accomplished.
Clinical examination alone may be adequate, but initial radiographs or
ultrasound should be obtained in order to document adequate flexion and
redirection of the femoral neck toward the triradiate cartilage in the
harness. After clinical stability is achieved, radiography is not
indicated until approximately 3 months of age to assess acetabular
development (Fig. 24.28). Ultrasonography is an excellent means of documenting progress toward and completion of successful reduction (241).
success rate for the treatment of the Ortolani-positive hip, the
success rate for using the harness to guide the reduction of a
subluxated or dislocated hip in a child younger than 6 months of age is
85% (33,228,236,242).
complications; most of these complications are iatrogenic and can be
avoided. Inferior dislocations may occur with prolonged excessive hip
flexion (243,244,245).
Hyperflexion may also induce femoral nerve compression neuropathy; this
condition generally resolves after the harness is removed. It is
important during each examination to make certain that the patient has
active quadriceps function. Brachial plexus palsy may occur from
compression by the shoulder straps, and knee subluxations may occur
from improperly positioned straps.
popliteal fossa if great care is not taken in keeping these areas clean
and dry. Instruction with regard to bathing and skin care is essential.
treatment is damage to the cartilaginous femoral head and the proximal
femoral physeal plate (246,247).
This is usually secondary to forced abduction in the harness or to
persistent use of the harness, despite the failure of reduction, in a
complete dislocation.
of age in a Pavlik harness because of the child’s activity levels. In
this age group subluxated or dislocated hips should be treated by
closed or open means as necessary, because success rates using the
Pavlik harness are less than 50%.
treatment with the Pavlik harness, the obstacles to reduction are
different, treatment has greater risks, and the results are far less
predictable. The principal goals in the treatment of the late-diagnosed
patient are similar to those for the newborn. The goal is to obtain
reduction, to maintain that reduction so as to provide an adequate
environment for femoral head and acetabular development, and to avoid
proximal femoral growth disturbance (Fig. 24.29).
those who have failed a trial of Pavlik harness reduction, closed
reduction is indicated. In some centers, treatment by closed reduction
and spica cast immobilization is preceded by a period of skin or
skeletal traction (248,249,250,251,252,253,254,255,256,257,258) (Fig. 24.30).
Traction theoretically stretches contracted muscles, allows reduction
without excessive force, decreases the need for open reduction, and
reduces the incidence of proximal femoral growth disturbance resulting
from avascular necrosis (AVN). The use of prereduction traction, and
its effectiveness at accomplishing what it is touted to do, are
controversial topics (259,260). In 1991, Fish et al. sought the opinions of the members of the Pediatric Orthopaedic Society of North America on this topic (261).
Most pediatric orthopaedic surgeons thought that traction did reduce
the incidence of necrosis in the treatment of DDH. Only 5% of
responders did not use traction in their practice. In the author’s
opinion, this practice pattern has changed considerably over the last
13 years, with far fewer pediatric orthopaedic surgeons employing
prereduction traction.
of secondarily contracted muscles, such as the iliopsoas and adductor
longus; theoretically, this allows reduction without creating excessive
joint forces, thereby decreasing the incidence of AVN and reducing the
need for open reduction. These ideas are lacking the support of
scientifically valid studies (260).
 |
|
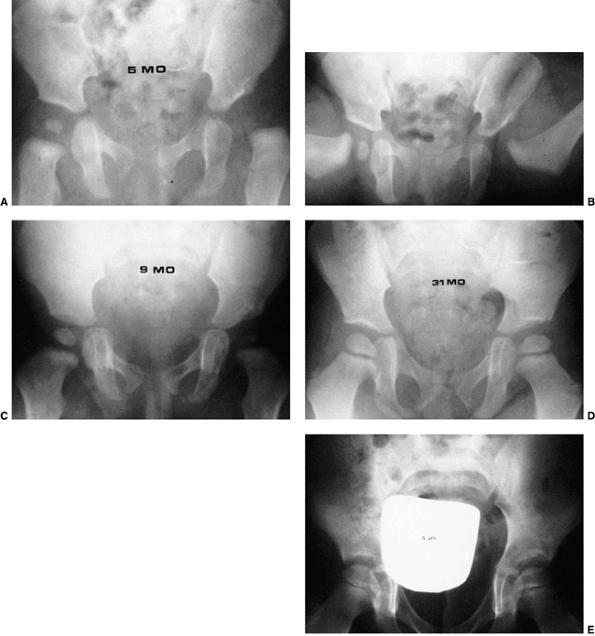
Figure 24.28 A 5-month-old child with left developmental dislocation of the hip. A:
Anteroposterior view of the pelvis at diagnosis. The acetabular index is increased, the medial floor of the acetabulum is widened, and the acetabular teardrop figure is absent. There is a well-developed secondary acetabulum, the Shenton line is disrupted, and the femoral ossific nucleus is decreased in size. The femoral head is located in the upper outer quadrant, as defined by Hilgenreiner and Perkins lines. B: Anteroposterior view of the pelvis with a hip Pavlik harness in place to demonstrate an excellent reduction. Note the hyperflexed position. C: Anteroposterior view of the pelvis at 9 months of age shows reduction, early appearance of the teardrop figure, and improvement in the acetabular index. D: Anteroposterior view of the pelvis at 31 months of age. There is marked improvement in the acetabular teardrop figure and acetabular development. E: Anteroposterior view of the pelvis at 5 years of age. There has been continued improvement in acetabular and femoral head development. |
quantify prereduction hip positions, and concluded that there was a
direct correlation between inadequate traction and the incidence of
growth disturbance (251). Weiner et al. found
that in patients younger than 1 year traction for longer than 21 days
substantially reduced the rate of growth disturbance (256).
Buchanan et al. recommended a minimum of 2 weeks of traction until
achievement of a traction station using the Gage and Winter scale (248).
Skeletal traction was gradually increased over several weeks, and an
average of 39% of body weight was usually required for achieving this
position. In contrast, Cooperman et al. studied 30 DDH hips with
aseptic necrosis and 30 hips without necrosis and found, at an average
39-year follow-up, that the degree of initial displacement that had to
be overcome in order to obtain reduction was comparable in both groups
and that it was not a factor in the development of proximal femoral
growth disturbance (262). Some of the worst
outcomes were seen in patients with minimal superior dislocation.
Schoenecker and Strecker demonstrated that the results of traction were
not as good as the results of femoral shortening in older patients with
DDH (263).
Gibson and Benson (210)
thought that although preliminary traction protects against growth
disturbance, there was no relation between the original degree of
displacement of the proximal femur and the final outcome (264). A recent study by Kutlu et al. (265),
looking at traction as a single variable in a series of patients
treated by closed reduction, showed that traction did not affect the
rate of necrosis.
 |
|
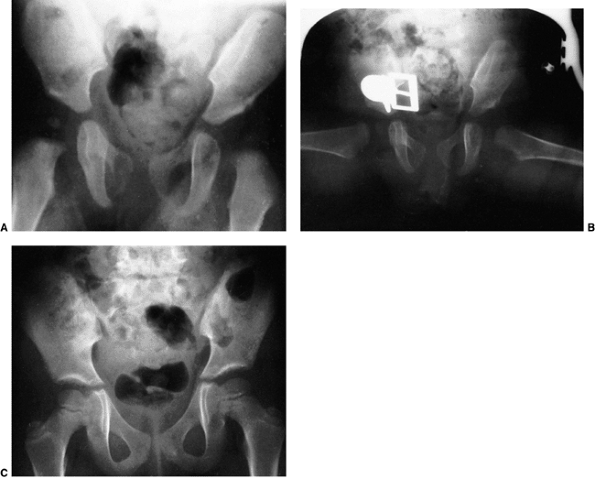
Figure 24.29 A 6-month-old girl with apparent left hip subluxation and acetabular dysplasia secondary to excessive anteversion. A:
Diagnostic anteroposterior view of the pelvis. Note the increased acetabular index, the poorly developed teardrop figure, and the small ossific nucleus. B: Anteroposterior view of the pelvis in the fixed abduction brace. Excellent reduction of the hip has been achieved. C: Anteroposterior view of the same patient at 5 years of age. The left hip appears normal. |
 |
|
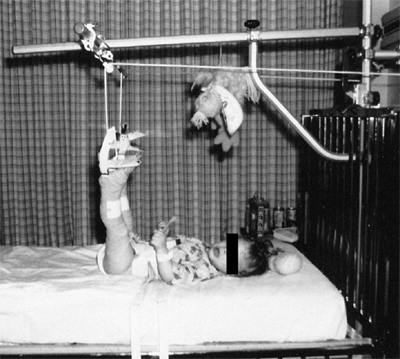
Figure 24.30 A 1-year-old child with bilateral hip dislocations in traction.
|
assessment of the adequacy of closed reduction and the need for open
reduction varies and is subjective. Several articles on open and closed
reduction without the use of preliminary traction report incidences of
proximal femoral damage comparable to those found in series in which
prereduction traction was used (43,266,267,268).
These researchers think that the main obstacles to reduction are
intraarticular and therefore would not be affected by the use of
traction. Controversy also exists about the amount of weight applied,
the direction of application of the force, and the duration of applied
traction. There are no clinical or experimental studies of the direct
effects of traction, and
there are no well-controlled studies that analyze the effect of traction as a single variable (260).
generally consider that 1 to 2 weeks of skin or skeletal traction are
sufficient. However, a recent report on the successful use of traction
to attain reduction in patients more than 6 months of age, reported a
mean time in traction of 8 weeks (269). Skin traction is the most commonly used method, although some physicians recommend skeletal traction (239). Skin tapes should be applied above the knee to distribute the traction over a large area (Fig. 24.30).
Elastoplast tape is applied loosely over tincture of benzoin from the
ankle to the upper thigh. It is important not to stretch the
Elastoplast tape at all; it should merely lie on the skin in a
circumferential manner, with each edge directly opposing the preceding
edge. Buck traction tapes are then applied from above the ankle to the
thigh and to the foot plate; weights may be added to both legs, so that
the buttocks “lightly” touch the bed. The author has used this method
without adverse consequences. The direction of application of the
traction forces (e.g., overhead, longitudinal, divaricated) and the
duration of traction (days to months) vary worldwide.
ischemia of the lower extremities; these are attributable to
inappropriate application. Neurocirculatory checks must be performed
frequently, and traction must be applied in a carefully supervised
manner.
This markedly decreases the costs associated with hospitalization.
Patients are usually hospitalized for 24 hours to allow their parents
to become familiar with the traction apparatus, to learn how to monitor
neurocirculatory status, and to become totally familiar with the
potential risks and danger signs. The patient and family must be
cooperative; a visiting nurse is often helpful in instituting this
program.
Gentle reduction must be done under general anesthesia. The hip is
gently manipulated into the acetabulum by flexion, traction, and
abduction. An open or percutaneous adductor tenotomy is usually
necessary in these cases because of secondary adduction contracture,
and for increasing the “safe zone” (arc of adduction–abduction in which
the hip remains located), thereby lessening the incidence of proximal
femoral growth disturbance.
Because large portions of the femoral head and acetabulum are
cartilaginous, arthrography is a useful tool in assessing the obstacles
to and the adequacy of reduction (271,273,274,275).
Dynamic arthrography using fluoroscopy helps to achieve both of these
goals. Intraoperative ultrasonography may also be used. The use of the
femoral head as a “dilating sound” to overcome the intraarticular
obstacles to reduction may cause damage to the femoral head and make
open reduction more difficult (238,276,277).
cast. Reduction, after casting, should be documented by radiography,
computed tomography (CT) scan, MRI, or ultrasonography (31,164,278,279,280,281,282,283) (Fig. 24.32).
The author prefers to use plaster of paris because of its
“moldability,” but some surgeons prefer to use synthetic materials. The
plaster must be well-molded dorsal to the greater trochanters so as to
prevent redislocation (Fig. 24.32). In the
author’s experience, most successful reductions are lost in the
postoperative period by poorly applied and molded casts. The “human
position” of hyperflexion and limited abduction is the preferred
position (284,285) (Fig. 24.33).
The amount of apparent hip flexion during cast application is often
greater than the flexion seen on radiographs. Wide, forced abduction or
forced abduction with internal rotation should be avoided because these
approaches are associated with an increased incidence of proximal
femoral growth disturbance (Fig. 24.34).
reduction in the plaster cast varies considerably. The author prefers
to maintain the plaster below the knee on the involved side and above
the knee on the uninvolved side for approximately 6 weeks, regardless
of the patient’s age. After that time, the plaster on the involved side
(or sides) is cut to above the knee to allow some hip rotation and
range of motion for the knee for an additional 6 weeks.
period after cast removal. Some use them on a full-time basis (except
for bathing) for several months, then part-time, usually during the
night and napping hours, until acetabular development has caught up
with that of the opposite, normal side (generally 18 to 24 months) or
surgical intervention is planned. The use of abduction orthotic devices
after reduction of DDH varies widely. The theory behind the use of the
fixed abduction brace is that there may still remain mild instability
that can be corrected by the fixed abduction brace that maintains the
stable relation between the femoral head and the acetabulum, yet allows
some hip mobility. Empirical evidence exists for its efficacy in cases
of mild hip instability (Fig. 24.29). The
complications associated with the use of a fixed abduction device
include skin breakdown and proximal femoral growth disturbance. It is
important to ensure, while positioning the device, that the hip is not
placed in extreme positions of abduction that could interrupt the
vascular supply to the proximal femur.
is indicated if there is failure of closed treatment, persistent
subluxation, soft tissue interposition, or reducible but unstable
reductions other than in extreme positions of abduction. There had been
a theory in recent years that the
incidence
of damage to the proximal femur is decreased if open reduction is
delayed until the femoral ossific nucleus is present (286,287). This notion has recently been dispelled (288,289,290).
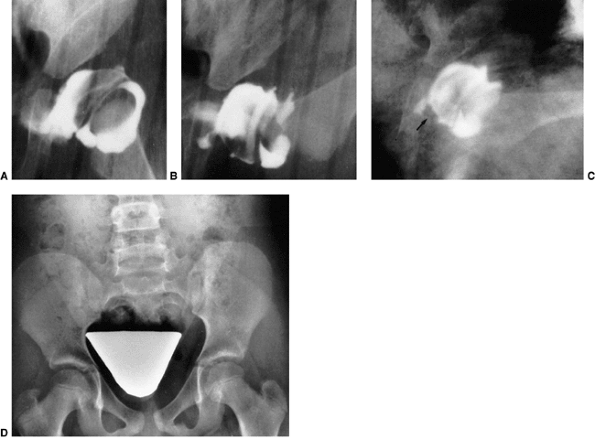 |
|
Figure 24.31 Arthrograms demonstrate closed reduction of developmental dysplasia of the left hip in an 8-month-old infant. A: Untreated. B: Reduced. There is no pooling of dye medially. The acetabular cartilage completely covers the femoral head. C: Reduction in a plaster cast. Note the arthrographic shadow of the transverse acetabular ligament (arrow). D: Nine years after reduction. Note the symmetric acetabular and proximal femoral development.
|
 |
|
Figure 24.32
This computed tomography (CT) scan documents a successful closed reduction of a right hip dislocation. The plaster cast is molded dorsal to the greater trochanters in order to help prevent redislocation. |
maintain the reduction, avoid damage to the femoral head, and provide
an optimal environment for acetabular and proximal femoral development.
Open reduction of a DDH may be accomplished through a variety of
surgical approaches (40,43,119,121,122,123,182,240,291,292,293,294,295,296,297).
reduction is the anterolateral Smith-Petersen approach with a modified
“bikini” incision, as described by Salter and Dubos (283).
This is a standard approach to the hip joint and is familiar to most
surgeons. In the late-diagnosed patient with DDH, any associated
capsular laxity can be plicated through this approach. If the surgeon
thinks that a secondary procedure, such as pelvic osteotomy, is
necessary, it also can be accomplished through the same surgical
approach (40,295,296,297).
approach is that the hip is immobilized in a functional position, with
minimal hip flexion and some degree of abduction. If this approach is
used in conjunction with a
capsular plication, the postoperative immobilization period is usually 6 to 8 weeks.
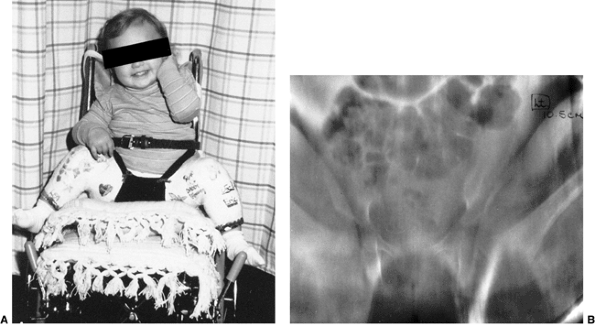 |
|
Figure 24.33 A:
The “human position” of hyperflexion and limited abduction is the preferred position after closed reduction. This patient underwent bilateral reductions. B: Single-cut tomogram documents the hyperflexed, minimal abduction position. |
with the various medial and anteromedial approaches, possible damage to
the iliac crest apophysis and the hip abductors, and postoperative
stiffness. If this approach is used in bilateral cases, the procedures
are usually staged at 2- to 6-week intervals.
approaching the hip joint directly over the site of the obstacles to
reduction (43,121,122,123,293,298,299,300,301,302,303). The medial approach described by Ferguson is in the plane between the adductor brevis and the adductor magnus (119,304).
Advocates of this approach claim that its advantages include minimal
soft tissue dissection, direct access to the medial joint capsule and
the iliopsoas tendon, avoidance of damage to the iliac apophysis and
abductor muscles, minimal blood loss, and excellent cosmesis. However,
it is a less familiar approach to most surgeons, and visualization is
somewhat impaired. Capsular repair cannot be accomplished through this
approach. The stability of the reduction is maintained only by the
postoperative cast. This approach is somewhat difficult to use in older
patients, and no concomitant surgical procedures can be performed
through the same incision. Concern has also been expressed about a
possible higher incidence of proximal femoral growth disturbance after
use of this approach (305).
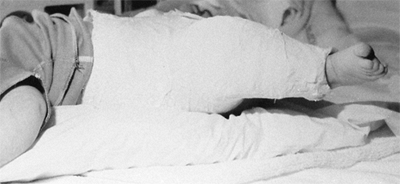 |
|
Figure 24.34
Wide abduction should be avoided. This position is associated with a high incidence of damage to the proximal femoral epiphysis and the physeal plate. |
the anteromedial approach originally described by Ludloff and modified
by Weinstein and Ponseti (43,109,115,121,264,306,307,308,309).
The approach is made in the interval between the femoral neurovascular
bundle and the pectineus muscle. The advocates of this approach cite
minimal blood loss (transfusion is never necessary) and the fact that
it is the most direct approach to the obstacles to reduction. There is
minimal muscle dissection in this approach; only the iliopsoas and the
adductor longus are sectioned. Both hips can be reduced during the same
operative procedure, the scar is extremely cosmetic, and there is no
damage to the hip abductor muscles or the iliac apophysis.
Postoperative stiffness is not a problem.
surgeons. Only open reduction can be accomplished; no secondary
procedures can be performed through this incision. It is difficult to
use in older patients because of the depth of the hip joint and the
difficulty with visualization. The surgeon who decides that capsular
plication should be performed cannot do it through this approach. The
medial femoral circumflex vessels (the primary blood supply to the
proximal femur) are in the operative field. Moreover, visualization is
claimed by some to be poor, and the approach is associated with a
higher incidence of aseptic necrosis (305,310). In the author’s experience of more than 200 cases, the visualization is excellent (Fig. 24.35)
and the incidence of aseptic necrosis during a minimum 4-year follow-up
is approximately 14%, which is well in line with the results of other
series of open reductions (123,311).
Capsular plication appears to be unnecessary in this age group, because
in a successful closed reduction the capsule tightens and the scar
induced by the surgical procedure helps to provide capsular stability.
This approach, however, depends on the placement of a well-molded cast.
The approach to casting after reduction is the same as that described
earlier for closed reduction. A certain degree of capsular stability is
gained through the prolonged postoperative immobilization that is
necessary. No residual stiffness has been reported.
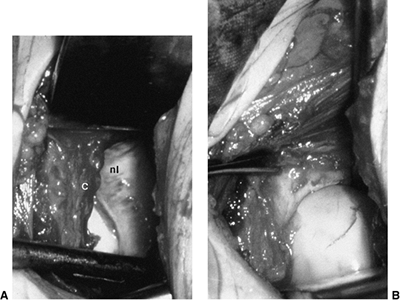 |
|
Figure 24.35 A: Open reduction through an anteromedial approach in a 14-month-old child. Note the anterior joint capsular edge (c) and the neolimbus (nl). B:
The hip is reduced under direct view. The femoral head is well seated in the acetabulum, and the anterior edge of the hip capsule is everted by a hemostat. |
abduction orthosis for varying periods of time, as discussed in the
preceding text. When using any of the approaches other than the
anterior approach with capsular plication, the author uses a fixed
abduction orthosis for 2 months on a full-time basis and then at night
and nap time until acetabular development has normalized (generally 18
to 24 months) or further surgical intervention is planned for
correcting residual dysplasia.
These give the treating physician an idea of whether the cartilage in
the region of the neolimbus in the periphery of the acetabulum has the
potential for ossification and normal acetabular development, or
whether secondary acetabular procedures will be necessary.
or femoral procedure is rarely required. The potential for acetabular
development after closed or open reduction is excellent and continues
for 4 to 8 years after the reduction (36,37,43,312,313,314).
The most rapid improvement in acetabular development—as measured by
parameters such as the acetabular index, development of the teardrop
figure, and thinning of the medial floor—occurs in the first 18 months
after surgery (34,36,37,43,312,315,316,317).
Femoral anteversion and any coxa valga associated with the untreated
condition have an excellent chance to resolve during this time.
However, some surgeons think that every child with DDH older than 18
months should undergo pelvic osteotomy accompanying open reduction,
because of the poor acetabular development potential (40,295,296,318,319).
of DDH, open reduction is usually necessary. In this age group, the
treating surgeon must also consider whether to perform concomitant
femoral shortening in conjunction with the open reduction. In children
older than 3 years, femoral shortening to avoid excessive pressure on
the proximal femur leads to far lower rates of proximal femoral growth
disturbance than does preliminary traction followed by open reduction (263,320,321,322,323) (Fig. 24.36).
Schoenecker and Strecker reported a 54% incidence of aseptic necrosis
with a 32% incidence of redislocation, when skeletal traction was used
in patients older than 3 years (263). A
theoretical advantage of open reduction accompanied by femoral
shortening is that it can be used for correcting any anatomic
abnormality, such as excessive femoral anteversion. The disadvantages
of femoral shortening include the need for a second incision and
internal fixation for the osteotomy, and a further operation for
hardware removal.
zone;” some surgeons advocate preliminary traction before open
reduction, but an increasing number of surgeons prefer to perform
concomitant femoral shortening (324,325,326).
In this age range, because the potential for acetabular development is
markedly diminished, many surgeons recommend a concomitant acetabular
procedure, either in conjunction with the open reduction or 6 to 8
weeks after it (327). The decision about
whether to perform a secondary acetabular procedure is subjective. The
author prefers to judge stability at the time of open reduction (328).
If good stability is evident, the author prefers to observe acetabular
development for the next few years, and if acetabular development is
not improving by radiographic criteria (e.g., decreasing acetabular
index, improvement in teardrop appearance and shape) (183), the author considers secondary acetabular procedures (329,330).
Most surgeons have been adopting earlier rather than later intervention
for residual dysplasia, as the results are more predictable with fewer
complications (317).
performed in this age group in conjunction with open reduction is
innominate osteotomy as described by Salter (40,331) and by Pemberton (39,332,334,335,336).
Anatomic deficiency of the acetabulum in this age group is usually
anterior, and the Salter innominate osteotomy gives anterior coverage,
although at the expense of posterior coverage. The Pemberton osteotomy
provides anterior coverage, and also various degrees of lateral
coverage, depending on the direction of the osteotomy cuts.
described by Smith-Petersen with the Salter modification is the ideal
approach, because it enables capsular plication, immobilization of the
hip joint in a more functional position, and innominate osteotomy, all
at the same time and through the same incision.
be accompanied by femoral shortening, and probably by a concomitant
acetabular procedure, depending on hip stability at the time of surgery
(317,323,326,337,338). The acetabular procedure may be performed at the time of
open reduction, 6 weeks later, or at a later date, depending upon acetabular development and the surgeon’s judgment.
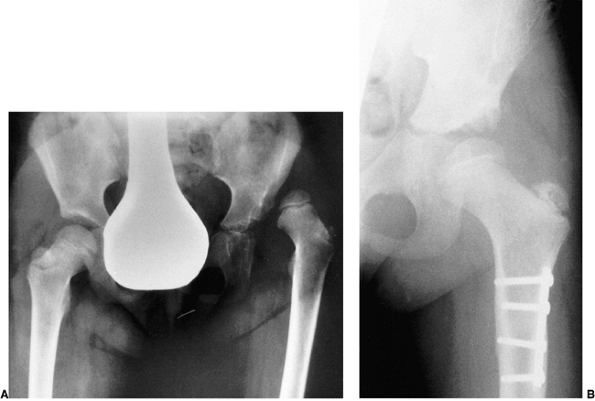 |
|
Figure 24.36 A: Preoperative anteroposterior radiograph of a 4-year-old girl with developmental dislocation of the left hip. B:
Eighteen months after reduction and femoral shortening, accessory centers of ossification are appearing in the lateral portion of the acetabular cartilage. |
it is the goal of treatment to have a radiographically confirmed normal
hip at maturity in order to prevent degenerative joint disease in the
future (Fig. 24.26). Hip subluxations must be
corrected. The evidence demonstrates that residual acetabular
dysplasia, even in the absence of subluxation, eventually leads to
degenerative joint disease, so this also should be corrected (339,340). The goal of treatment of DDH is to have a hip as anatomically normal as possible by the end of skeletal growth.
the relation between the acetabulum and the femur should be assessed.
Anatomic dysplasia can involve the acetabulum, the proximal femur, or
both. In patients with DDH, the deficiency is most commonly on the
acetabular side, or the dysplasia is significantly greater on the
acetabular side. If there has been a disturbance of proximal femoral
growth secondary to previous treatment, the femoral side may be more
dysplastic.
radiographs taken with the patient in the standing position, when
possible, and with the standard evaluations of acetabular development:
the acetabular index, the acetabular angle of Sharp, and the CE angle.
In most young children with DDH the acetabular deficiency is anterior,
and in adolescents and adults with DDH, the acetabular deficiencies can
be anterior, posterior, or global, (40,74,295,341,342,343,344,345) and there also may be evidence of acetabular retroversion (346). Excessive femoral anteversion can be ascertained clinically, but is most accurately measured by CT scan (347). There has been an increased interest in evaluating dysplasia by means of three-dimensional CT scan (210,345,348,349).
However, CT scan cannot show the cartilaginous component of the
proximal femur or the acetabulum; therefore, it is most useful for
mature patients or those close to maturity.
if they lead to subluxation of the joint: lateral subluxation with
extreme coxa valga or anterior subluxation with
excessive anteversion (182).
In general, patients with DDH have normal neck-shaft angles. They may
have persistent anteversion that gives the radiologic appearance of
subluxation (disrupted Shenton Line).
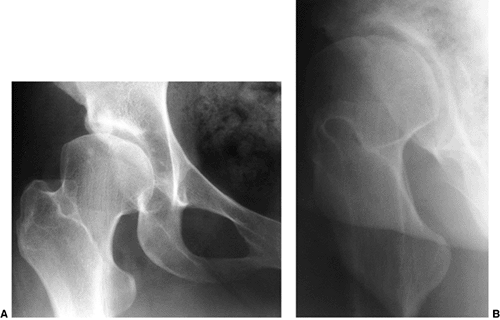 |
|
Figure 24.37
A 34-year-old woman with residual dysplasia, who had undergone closed reduction for right developmental dysplasia of the hip at 16 months of age. She now has mild pain. A: Anteroposterior view of the hip. B: False profile lateral view demonstrating anterior deficiency of the acetabulum. |
If acetabular dysplasia persists for 2 to 3 years after closed or open
reduction and the patient has residual anteversion, proximal femoral
rotational osteotomy may be considered. When the Shenton line is
disrupted, the proper relation of the proximal femur can usually be
restored by derotation osteotomy, with or without various degrees of
varus. The varus derotation osteotomy is used alone in such cases by
surgeons who think that redirection of the femoral head toward the
center of the acetabulum stimulates normal acetabular development (154,181,204,358,359,360,361,362,363,364,365,366,367).
If the proximal femoral varus derotation osteotomy is to be used for
“stimulating” more normal acetabular development in patients with
persistent femoral anteversion, it must be performed in children
younger than 4 years (358). After 8 years of age, no improvement in acetabular dysplasia can result from this procedure alone.
surgeon must ensure that the femoral head can be concentrically reduced
as seen on an anteroposterior pelvic radiograph with the patient’s legs
abducted 30 degrees and maximally internally rotated (Fig. 24.38).
This position allows visualization of the actual angle of the femoral
neck. If concentric reduction is not documented, this procedure should
be accompanied by open reduction.
from varus osteotomy should resolve by growth stimulation and
restoration of the normal neck-shaft angle (368).
In teenagers, however, more than a 15-degree lessening of the
neck-shaft angle may result in limb shortening. If the varus osteotomy
is excessive, it can cause lateralization of the shaft, shifting the
mechanical axis medial to the knee joint and leading to mechanical
abnormalities at the knee (369). Proximal
femoral osteotomy may, in some cases, be indicated in order to correct
residual deformity from a partial physeal arrest resulting from aseptic
necrosis; however, minimal literature exists on this procedure.
can be removed after 6 to 8 weeks. Internal fixation devices are
usually removed 12 to 18 months postoperatively; if they are not
removed in young children, they become encased within the proximal
femur, and this could pose problems if future operations become
necessary.
acetabular dysplasia and a disrupted Shenton line in whom there is no
potential for acetabular growth and remodeling, changing the
orientation of the proximal femur does not increase the weight bearing
area, but only shifts the weight-bearing area to another portion of the
femoral head (370,371,372).
Proximal femoral osteotomies in the adolescent or adult group are
indicated only as adjuncts to pelvic operations and in extreme cases of
coxa valga and subluxation (182,371) (Fig. 24.39).
acetabular dysplasia after closed or open reduction (or discovered
incidentally) depend on the age of the child and whether the patient
has symptoms (373). The goal of treatment is to
restore the anatomy to as near normal as possible by the time skeletal
maturity is attained. As discussed previously, after concentric
reduction is obtained and maintained, the potential for acetabular
development continues for many years (34,36,37,43,209). However, after 4 years of age, this potential for restoration of normal anatomy is markedly decreased.
theoretically provides for a better weight-bearing surface for the
femoral head, restores the normal biomechanics of the hip and reduces
contact pressures (345), and may increase the
longevity of the hip by preventing degenerative joint disease. However,
only prospective, long-term follow-up studies of these procedures can
provide unambiguous answers.
generally assessed by arthrography and by inspection at the time of
open reduction. In reduced hips with residual dysplasia, the author has
found that the problem is not one of deficiency of the acetabulum, but
a failure of the peripheral acetabular cartilage to ossify (Fig. 24.40).
In most cases, an arthrogram at the time of surgery shows excellent
coverage of the femoral head by the unossified acetabular cartilage.
This cartilage fails to undergo normal development because it is
intrinsically abnormal or because it was damaged by the femoral head in
the unreduced position, causing pressure necrosis. Given enough time,
some of this acetabular cartilage may resume normal ossification and
correct a large amount of the dysplasia. However, in the author’s
experience of a large number of patients treated with open and closed
reduction, this does not happen in many cases, and intervention should
be undertaken after the acetabulum has had a reasonable chance to
develop on its own (123,209,374).
Any osteotomy of the iliac bone and the neovascularity stimulated by it
in healing may increase the ossification of the otherwise unossified
acetabular cartilage. In any case, the redirection of the acetabulum
restores more normal bony anatomy and normal biomechanics that may also
be factors in stimulating ossification (239).
used widely until the mid-1950s, when Chiari described his medial
displacement osteotomy (375). Later, Salter,
Pemberton, and others described various pelvic osteotomies for
redirecting the acetabulum and covering the femoral head with articular
cartilage. Although intuitively it seems better to cover the femoral
head with articular cartilage than to rely on fibrous metaplasia, it is
impossible to be certain whether the long-term results of shelf
arthroplasty will be
less successful than those osteotomies that provide articular cartilage coverage (376,377).
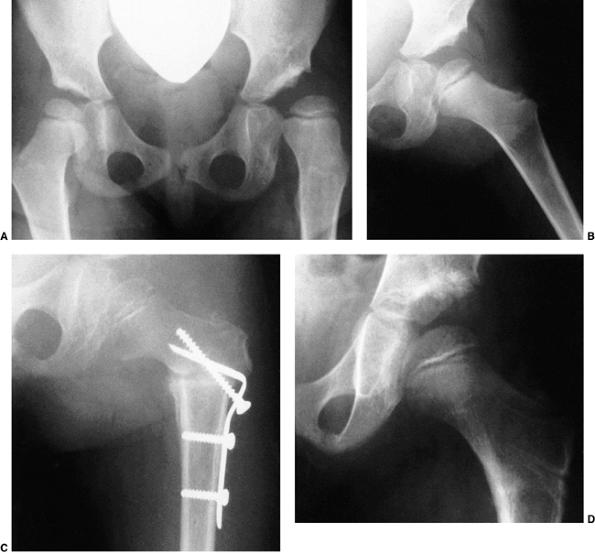 |
|
Figure 24.38 A 3-year-old girl 2 years after closed reduction. A: Anteroposterior view of the pelvis. Note the persistent acetabular dysplasia and apparent coxa valga. B:
The radiograph shows the leg abducted approximately 30 degrees and maximally internally rotated. The femoral head is seated well within the acetabulum, and the Shenton line is restored. C: Anteroposterior view of the left hip 6 weeks after varus derotation osteotomy. D: Anteroposterior view of the left hip 18 months after varus derotation osteotomy, with hardware removed. The Shenton line has been restored; persistent acetabular dysplasia remains, but development of the teardrop figure improved, and accessory centers of ossification have appeared in the periphery of the acetabular cartilage. |
acetabular dysplasia are divided into four groups. The first group
consists of osteotomies of the pelvis that redirect the entire
acetabulum. This redirection provides coverage of the femoral head by
acetabular articular cartilage. These osteotomies include the Salter
innominate osteotomy (40,74,295,296,297,327,378,379,380) (Fig. 24.40), the Sutherland double-innominate osteotomy (381), the triple-innominate osteotomies of Tonnis (182,382,383,384,385,386), Steel (387,388,389,390,391,392), and Ganz (369,393,394,395,396), the spherical osteotomies of Wagner (397,398,399) and Eppright (372,400), and others (401,402,403,404,405,406,407,408,409). These procedures involve complete cuts through various pelvic bones and rotation of the acetabulum.
include complete concentric reduction and release of muscle
contractures, including the iliopsoas and hip adductors;
a
congruous joint; and good range of motion. Rotational pelvic
osteotomies in the face of subluxation may lead to severe damage to the
femoral head. These procedures are best performed before 6 years of
age, but the age limits vary considerably, depending on the surgeon.
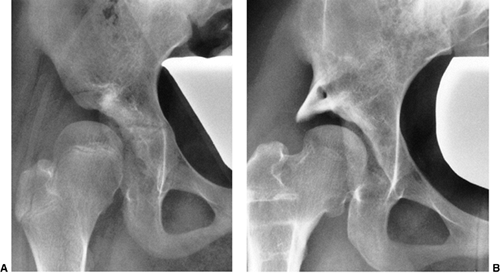 |
|
Figure 24.39
A 10-year-old girl whose developmental dysplasia of the hip was diagnosed at 5 years of age. She had previously undergone open reduction, but had residual proximal femoral and acetabular deformities. A: Preoperative anteroposterior radiograph of the pelvis. B: Three years after varus derotation osteotomy and Staheli slotted acetabular augmentation. |
symphysis pubis, is better performed in the infant, child, or
adolescent, because of the flexibility of the symphysis pubis in young
patients. However, this osteotomy can be performed in adults as well (297). The procedure is more likely to succeed when the CE angle is greater than 10 degrees.
although rarely performed today, aims to allow greater rotation of the
pelvic fragment by cutting through the pubis, instead of merely hinging
on it (381). Complications of this procedure
can involve injury to the spermatic cords, bladder, and urethra. The
triple-innominate osteotomy allows even greater coverage by means of
cuts of all three hip bones.
correction of acetabular dysplasia by one of the rotational procedures
is the amount of dysplasia that needs to be corrected. The amount of
coverage obtained by osteotomies such as the Salter procedure is
limited, whereas osteotomies that cut all three pelvic bones provide
the ability to obtain greater coverage (367,410,411,412).
The closer the cuts are placed to the acetabulum, the greater the
femoral head coverage. The triple-innominate osteotomies described by
Tonnis and Ganz provide greater rotational possibilities than the one
described by Steel (Fig. 24.41). The
osteotomies that are closest to the acetabulum (e.g., Eppright, Wagner,
and Naito procedures) provide the greatest potential for redirection,
but these require significant technical expertise and have higher rates
of complications.
acetabuloplasties that involve incomplete cuts and hinge on different
aspects of the triradiate cartilage, such as the acetabular procedures
described by Pemberton (39,314,316,332,334,335,336,413,414,415) and Dega (416,417,418,419,420).
These procedures can theoretically decrease the volume of the
acetabulum because they depend on the triradiate cartilage as the
fulcrum.
that osteotomies that depend on the triradiate cartilage as the fulcrum
must be done in patients with open triradiate cartilage. Procedures
which involve hinging on the triradiate cartilage, such as the
Pemberton osteotomy, have the potential to injure the triradiate
cartilage and cause premature closure, but these complications are not
common (414,416).
Procedures that must cross the triradiate cartilage and that would
definitely induce closure, such as the Ganz osteotomy, cannot be done
in patients with open triradiate cartilage.
involves placing bone over the hip joint capsule on the uncovered
portion of the femoral head. These procedures provide coverage of the
femoral head by capsular fibrous metaplasia (421,422). They include the various shelf procedures (423,424,425,426,427,428,429) and the medial displacement osteotomy described by Chiari (375,376,377,422,430,431,432,433,435,436). In 1981, Staheli and Chew introduced a modification of a previously
described shelf arthroplasty, and this gained widespread acceptance for
use on its own in significant anatomic dysplasia, and also in
conjunction with various rotational procedures as an augmentation to
provide increased femoral head coverage (429) (Figs. 24.39, 24.42).
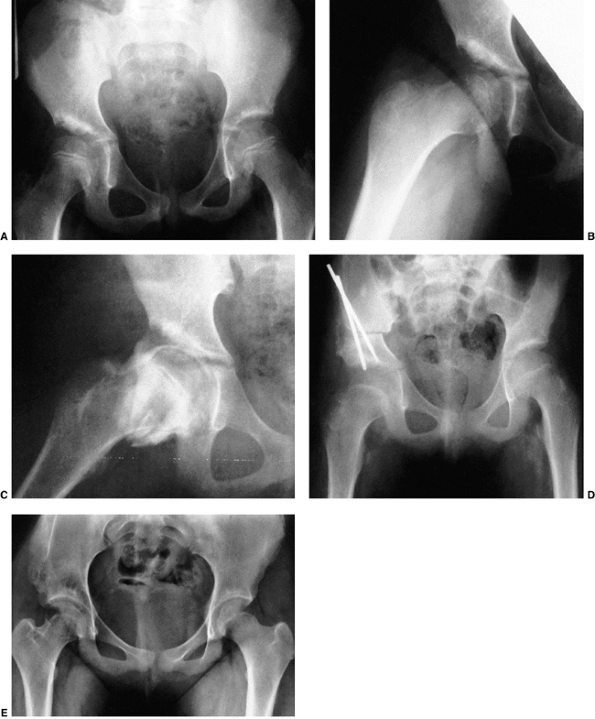 |
|
Figure 24.40 An 8-year-old girl with residual right acetabular dysplasia. A: Anteroposterior radiograph of the pelvis. B:
Antero-posterior view of the pelvis with abduction, flexion, and slight internal rotation; the femoral head appears slightly uncovered. C: Similar view with the addition of an arthrographic dye. Note the excellent coverage of the proximal femur by unossified acetabular cartilage. D: Immediately after the innominate osteotomy. E: Four years after the innominate osteotomy. |
may be performed in well-reduced hips, but they are usually reserved
for hips that lack significant femoral head coverage because of
inability to acquire such coverage with articular cartilage by
means of one of the other procedures mentioned in the preceding text (413,414).
Many of these procedures can be performed in patients with early
degenerative changes in the hope of delaying the necessity for
arthroplasty or fusion. Further discussion of these issues is beyond
the scope of this chapter.
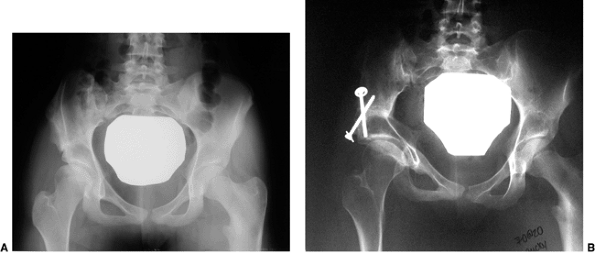 |
|
Figure 24.41 A 16-year-old patient with residual dysplasia after treatment for developmental dislocation of the hip (DDH) and pain. A: Preoperative anteroposterior radiograph. B: Anteroposterior radiograph 2 years after a triple innominate osteotomy.
|
symphysis pubica, with the distal fragment displacing medially and
upward (Figs. 24.43 and 24.44).
This medialization results in reduction of the lever arm in order to
reduce joint loading. Abductor muscle function is theoretically
improved. Patients may limp for as long as 1 year. There is some
concern that bilateral Chiari osteotomies may interfere with a woman’s
ability to deliver children. This is one of the few procedures for
which long-term results are available, and these show that, in the
absence of subluxation and degenerative joint disease, good long-term
outcomes may persist for many years (128,432,437,438,439,440,441,442,443,444,445,446,447).
above groups, such as addition of a shelf to a Salter or Pemberton
innominate osteotomy when the surgeon feels that inadequate coverage
has been obtained by the primary procedure.
in the case of the asymptomatic mature patient with hip dysplasia. For
the asymptomatic adolescent with minimal radiographic dysplasia
(because degenerative arthritis is a probability but not a certainty),
the author prefers to inform the family about the potential for an
adverse natural history and recommend surgery only at the onset of
symptoms. There is usually a long interval between the onset of
symptoms and degenerative joint disease as evidenced on radiographic
images (66). The patient can be reassured that
if symptoms develop surgical treatment can help in avoiding long-term
degenerative joint disease. However, faced with an adolescent with
radiographic evidence of subluxation, regardless of the symptoms, the
author recommends surgical correction, because without treatment an
adverse natural history is certain.
in young adults as a primary disease, or as a residual of childhood hip
disease. These patients may present with sudden onset of sharp groin
pain. They may describe symptoms such as sudden “locking” or a clicking
sensation (412). These sensations are
precipitated by movements that combine hip flexion, adduction, and
internal rotation. Patients may also experience sudden “giving way”
sensations. As the site of acetabular rim overload is usually anterior,
symptoms are provoked on physical examination by the so-called
impingement test. This test brings the anterior aspect of the femoral
neck in contact with the anterior acetabulum by internally rotating the
hip as it is gradually flexed to 90 degrees and adducted. In acetabular
rim syndrome, the patient’s original symptom of pain (usually in the
groin) will be reproduced. Films taken in a weight-bearing situation
and false profile lateral views will show evidence of dysplasia, as
previously discussed. One may also see evidence of an acetabular rim
fracture suggestive of the rim overload (218,412). A gadolinium-enhanced MRI arthrogram of the hip is the
best test for assessing labral disease (448,449), and a three-dimensional CT scan is the best diagnostic study for ascertaining the acetabular deficiencies.
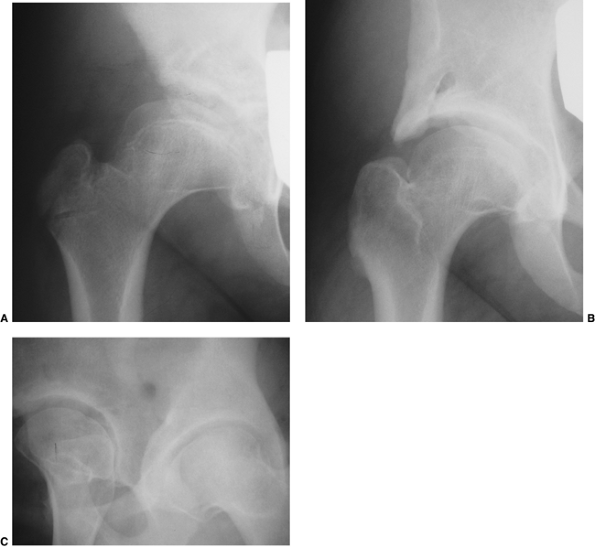 |
|
Figure 24.42 A:
Preoperative anteroposterior radiograph of an 8-year-old patient with residual dysplasia and proximal femoral growth disturbance. B: Anteroposterior radiograph 2 years after shelf arthroplasty. C: False profile lateral radiograph 2 years after operative procedure. |
very minimal evidence of arthritis, one of the joint- preserving
operations (Ganz, Salter, Tonnis, Naito, Steel, etc., as described in
the preceding text) is indicated in order to try to improve an
otherwise poor long-term prognosis. A radiograph with the leg in
maximal abduction must demonstrate that the femoral head is reduced,
covered, and congruent and that good joint space is maintained. These
are absolute prerequisites for considering a periacetabular osteotomy
(PAO) (Fig 24.41). Labral pathology may have to be dealt with in conjunction with the PAO procedure.
late teens or early adult years, hip fusion or total joint arthroplasty
may be the only treatment alternatives available. These circumstances
are rare among patients younger than 30 years and are beyond the scope
of this text.
treatment of DDH involves various degrees of growth disturbance of the
proximal femur, including the epiphysis and the physeal plate. This is
commonly referred to by the term aseptic necrosis. Because there has never been a study of a pathologic specimen from a patient with what is called aseptic necrosis, the author prefers to use the term proximal femoral growth disturbance (209).
These growth disturbances can be precipitated experimentally by
creating vascular injuries in animals; the results resemble the growth
disturbances seen in humans with treated DDH. The disturbance to growth
may be caused by vascular insults to the epiphysis or the physeal
plate, or by pressure injury to the epiphyseal cartilage or the physeal
plate (262,276,277,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464).
The blood supply to the proximal femur is described in Chapter 25.
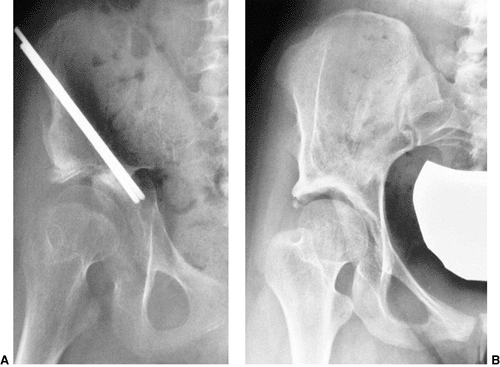 |
|
Figure 24.43 An 11-year-old girl with pain and residual right hip subluxation with severe acetabular dysplasia. A: Immediately after the right Chiari osteotomy. Note the additional graft placed anteriorly. B: Eight years after the operation, there is excellent remodeling of the acetabulum with sourcil development.
|
only in patients who have been treated. This may also occur in the
other normal hip in a patient who has been treated for the involved hip
(465,466). The reported incidence of proximal femoral growth disturbance varies from 0% to 73% (209,211,410,411,467,468,469). Different opinions exist about the reasons for this variation (470,471,472). The use of prereduction traction (201,209,211,248,251,265,455,467,473), adductor tenotomy (279,457,474), open or closed reduction (43,119,124,305,455,475,476,477), the force applied during reduction (467,474,478,479), the position in which the patient is immobilized postoperatively (242,248,251,285,276,444,458,460,461,462,480), and the age at reduction (248,251,256,458) have all been implicated
as etiologic factors. Others think that the incidence may be much less variable than the means by which it is assessed (209,471).
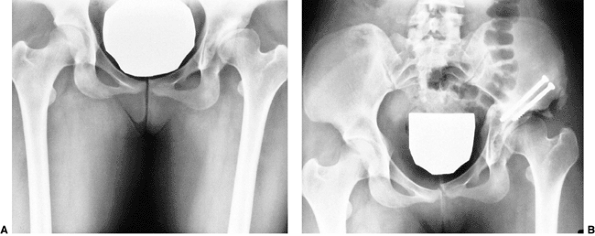 |
|
Figure 24.44
This girl underwent open reduction of the left dysplastic hip at 18 months of age. She presented at 17 years of age with subluxation, as seen on the radiograph. A: Preoperative anteroposterior view of the pelvis. B: Eight weeks after Chiari osteotomy. Note the additional graft placed anteriorly. |
necrosis published by the German Society for Orthopaedics and
Traumatology (3,318),
conservatively treated hips and operatively treated hips were evaluated
in order to determine the factors associated with the development of
ischemic necrosis (182). The factors associated
with necrosis included: high dislocations and dislocations with
inversion of the labrum; narrowing of the introitus between the
superior labrum and the transverse ligament in the position of
reduction; inadequate depth of reduction of the femoral head (greater
than 3 mm from the acetabular floor); the age of the patient (older
than 12 months); immobilization in 60 or more degrees of abduction for
joint instability; and adductor tenotomy.
Thomas et al. concluded that there was some association between the
reported incidence in a given series and the rigor with which the
diagnosis had been sought (471). Buchanan et
al. concluded that if signs of growth disturbance were not present
within 12 months of reduction they were highly unlikely to appear (248).
stressed that it may be difficult to identify this group early, and
studies reporting growth disturbances with follow-up periods of less
than 12 years must be regarded as preliminary and may not reflect the
actual incidence of proximal growth disturbance (455).
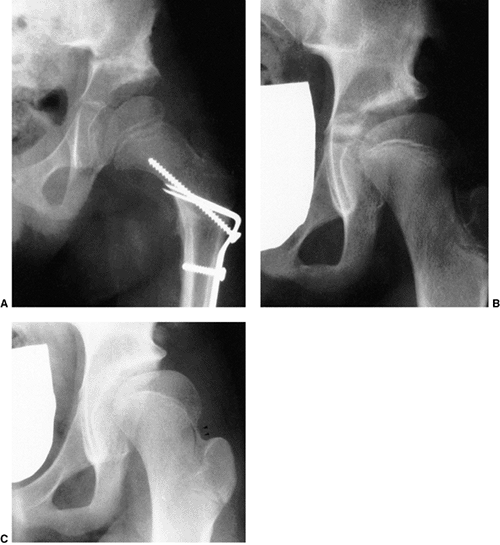 |
|
Figure 24.45 A:
Anteroposterior view of a 5-year-old girl, 3 months after varus derotation osteotomy, an innominate osteotomy for residual dysplasia. She had undergone an open reduction procedure 3 years previously. B: Anteroposterior view at 9 years of age. Note the valgus tilt to the proximal femur. C: Anteroposterior view at 11 years of age. Note the lateral tether, a typical type II physeal plate tether, and how the tether produces hip subluxation. A physeal growth arrest pattern may not be evident until a patient is 9 years of age or older. |
Younger patients have a lower rate of growth disturbance. Kalamchi and
MacEwen, however, documented an increase in the incidence of the severe
form (type IV) in younger patients (455). Salter et al. (285) and Ogden (460,461)
proposed that the femoral head in DDH is most vulnerable to ischemic
changes during the first 12 to 18 months of life, when it is composed
mostly of cartilage. According to some orthopaedic specialists the risk
of total head involvement becomes somewhat less after the appearance of
the femoral ossific nucleus, although, as mentioned in the preceding
text, this concept has recently been challenged (256,288).
of proximal femoral growth disturbance have been documented in the
clinical setting and in experimental studies. These include extremes in
positioning of the proximal femur in abduction and abduction with
extreme medial rotation. Extremes in position can cause compression of
the medial femoral circumflex vessel as it passes the iliopsoas tendon
and compression of the terminal branch between the lateral femoral neck
and the acetabular margin (285,460,461).
Anatomic and experimental investigations have persistently shown that
strong medial rotation with concomitant abduction, and extreme
abduction alone (i.e., the Lorenz position), can compromise the blood
flow to the capital femoral epiphysis. If the hip is maximally abducted
against firm resistance, the blood flow can be completely or almost
completely arrested. The same is true in forced medial rotation. The
blood vessels, and consequently the blood supply to the proximal femur,
can be occluded by compression, either outside the femoral head or as
the vessels cross through the epiphyseal cartilage (285,277,464).
Schoenecker et al. showed a diminution of epiphyseal profusion with
increasing pressure, which was relieved after the external fixation
device was removed (276,463).
used in cases of unrelieved adduction contracture, as seen in
dislocations, uniformly result in severe growth disturbances of the
epiphysis (284,285,463).
the vulnerable epiphyseal cartilage and the physeal plate. This has
been experimentally shown by Law et al. (457) and by Schoenecker et al. (463). These studies and others demonstrated the severe effects of cartilage necrosis (182,285,277).
These effects can also be precipitated by circumscribed pressure, such
as using the vulnerable femoral head as a “dilating sound” to overcome
the intraarticular obstacles to reduction.
apposition to the acetabulum in order to induce regression of the
obstacles to reduction (124). The idea is that
sustained pressure from the femoral head causes the labrum to adapt
itself to the spherical contour of the head. This maneuver can be used
for obtaining reduction, but the price may be an increased incidence of
necrosis (182,474); and
hence the author’s unwillingness to use the femoral head as a “dilating
sound” to obtain closed reduction. Although the use of prereduction
traction has been implicated as a factor in reducing the incidence of
necrosis, the German Orthopaedic Study Group did not find this to be
the case (182).
make the femoral head overcome the intraarticular obstacles to
reduction can lead to severe necrosis (182). If
closed reduction is attempted, in the author’s opinion the only
acceptable result is an anatomically perfect reduction; otherwise, the
hip must be reduced openly so as to prevent damage to the vulnerable
femoral head (182,481,482).
The author disagrees with the inclusion of coxa magna, because coxa
magna is often seen after open reduction as a result of the stimulation
of blood flow to the proximal femur (483,484,485).
It is also often difficult to ascertain whether some of the residual
deformities seen after treatment for DDH are alterations in the
proximal femur secondary to disturbances that occurred before the
reduction, or whether they are the result of complications associated
with the reduction. One of the most common deformities seen is the
flattening of the medial aspect of the proximal femur, which occurs
because of pressure of the femoral head lying against the ilium before
reduction.
represents damage to the epiphyseal cartilage or merely multiple
ossification centers that eventually coalesce. These areas may be
analogous to the accessory centers of ossification seen in the
periphery of the acetabulum. This pattern usually does not result in
growth disturbance of the proximal femur. Only long-term follow-up
studies of this entity can resolve this issue.
|
TABLE 24.2 SALTER’S CLASSIFICATION OF GROWTH DISTURBANCE OF THE FEMORAL HEAD
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
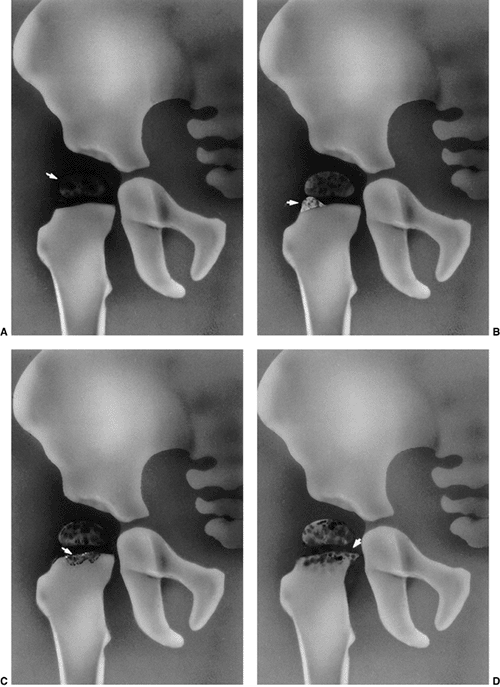 |
|
Figure 24.46 Classification of proximal femoral growth disturbances. A: Group I. B: Group II. C: Group III. D: Group IV. (Adapted from Kalamchi A, MacEwen GD. Avascular necrosis following treatment of congenital dislocation of the hip. J Bone Joint Surg Am 1980;62:876.)
|
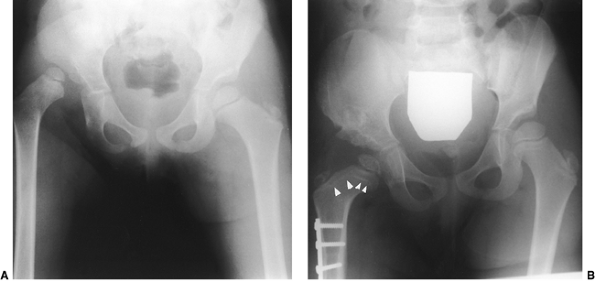 |
|
Figure 24.47 A 3-year-old girl with right developmental dysplasia of the hip. A: Preoperative radiograph. B:
Ten months after operative open reduction, femoral shortening, and Pemberton osteotomy. Note the presence of growth arrest line at the proximal femur (O’Brien lines; arrows); also note similar lines on the opposite, normal hip. |
necrosis, emphasizing the growth disturbances associated with various
degrees of physeal arrest (455). This classification (Fig. 24.46)
puts all the growth disturbances seen in the ossific nucleus into one
category if they are not associated with physeal involvement. Bucholz
and Ogden provided an additional classification based on patterns of
vascular supply resulting in partial or total ischemia (451).
There are few studies documenting the interobserver or intraobserver
reliability of these classifications of growth disturbance. As many as
25% of hips may not fit into any particular classification.
identification of growth disturbance lines in predicting future
deformity of the proximal femur (486,487) (Fig. 24.47).
These growth arrest lines may provide the physician with early evidence
of a future problem. However, the utility of this approach must await
long-term follow-up studies.
The results indicate that any alteration or disturbance of proximal
femoral growth decreases the longevity of the hip. As previously
discussed, recent attention has focused on femoral acetabular
impingement syndrome in patients with residual deformities of the
proximal end of the femur, and their effect on the induction of labral
tears and early osteoarthritis. (489,490,491)
reduction must be maintained by corrective femoral and/or acetabular
procedures (492,493). With arrest of the proximal femoral physeal plate, trochanteric overgrowth ensues, producing an abductor lurch (Fig. 24.48). If the problem is identified, greater trochanteric physeal plate arrest may be carried out, and this may maintain articular
trochanteric distance if performed in children younger than 8 years (21,454,455,494,495); otherwise, distal transfer of the greater trochanter may be necessary (417,456,457,458,496,497,498).
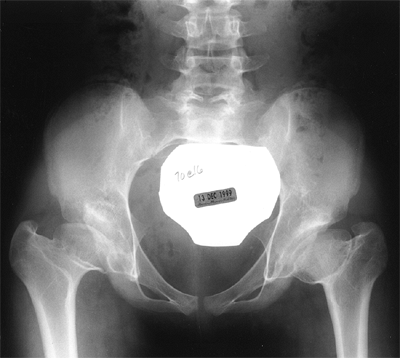 |
|
Figure 24.48
A 14-year-old girl with residual dysplasia, type III growth arrest, and trochanteric overgrowth. The patient had bilateral open reductions at 14 months of age. She had a type III growth arrest of the proximal femur with resultant disturbance of growth and corresponding acetabular deformity and trochanteric overgrowth. Her Trendelenburg test was negative. Some patients with this deformity have an abductor lurch, necessitating distal transfer of the greater trochanter. |
detection. This results in a 95% success rate of treatment with a low
risk of complications. It is the initial treating physician who has the
greatest chance of successfully achieving a normal hip. Orthopaedic
surgeons must educate primary care colleagues in making the diagnosis
early and initiating prompt referral.
HM, Dunn PM. Controlled trial of immediate splinting versus
ultrasonographic surveillance in congenitally dislocatable hips. Lancet 1990;336:1553.
J, Jarvis J, Uhthoff HK, et al. The fetal acetabulum: a
histomorphometric study of acetabular anteversion and femoral head
coverage. Clin Orthop 1992;281:48.
IV. Morphology of the acetabulum in congenital dislocation of the hip:
gross, histological and roentgenographic studies. J Bone Joint Surg Am 1978;60:586.
IV. Growth and development of the acetabulum in the normal child:
anatomical, histological and roentgenographic studies. J Bone Joint Surg Am 1978;60:575.
JM. Histological study of the fetal development of the human acetabulum
and labrum: significance in congenital hip disease. Yale J Biol Med 1981;54:255.
JR, Canny JM. The effects of trochanteric epiphysiodesis on growth of
the proximal end of the femur following necrosis of the capital femoral
epiphysis. J Bone Joint Surg Am 1980;62:785.
D, Effmann E, Broda K, et al. The development of the upper end of the
femur with special reference to its internal architecture. Radiology 1980;137:71.
WS, Ireton RJ, Coleman CR. Sequelae of experimental dislocation of a
weight-bearing ball-and-socket joint in a young growing animal. J Bone Joint Surg Am 1958;40:1121.
S, Garfin S, Vance R, et al. Pitfalls in the use of the pavlik harness
for treatment of congenital dysplasia, subluxation and dislocation of
the hip. J Bone Joint Surg Am 1981;63:1239.
NH, Lloyd-Roberts GC, Gallien R. Acetabular development in congenital
dislocation of the hip with special reference to the indications for
acetabuloplasty and pelvic or femoral realignment osteotomy. J Bone Joint Surg Br 1975;57:46.
FT, Healey JH. Osteoarthrosis and congenital dysplasia of the hip in
family members of children who have congenital dysplasia of the hip. J Bone Joint Surg Am 1990;72:1510; erratum 1991;73:293.
DL, Barnett CR, Arnold WD, et al. Untreated congenital hip disease: a
study of the epidemiology, natural history and social aspects of the
disease in a Navajo population. Am J Public Health 1965;55:1.
JM, Morcuende JA, Delgado E, et al. Radiologic pelvic asymmetry in
unilateral late diagnosed developmental dysplasia of the hip. J Pediatr Orthop 1995;15:753.
JF, Wright JG, Hedden DM. Is there a difference between the
epidemiologic characteristics of hip dislocation diagnosed early and
late? Can J Surg 1995;38:437.
JM, Goldsmith CH. Morphometric study of the fetal development of the
human hip joint: significance for congenital hip disease. Yale J Biol Med 1981;54:411.
K. Prevention of congenital dislocation of the hip: the Swedish
experience of neonatal treatment of hip joint instability. Acta Orthop Scand Suppl 1984;208:1.
T, Ishida K. Recent advances in the prevention, early diagnosis, and
treatment of congenital dislocation of the hip in Japan. Clin Orthop 1984;184:34.
MA. Screening for congenital dislocation of the hip, scoliosis, and
other abnormalities affecting the musculoskeletal system. Pediatr Clin North Am 1986;33:1335.
M. Mehad: the Saudi tradition of infant wrapping as a possible
aetiological factor in congenital dislocation of the hip. J R Coll Surg Edinb 1989;34:85.
K. Preluxation of the hip joint: diagnosis and treatment in the newborn
and the diagnosis of congenital dislocation of the hip joint in Sweden
during the years 1948-1960. Acta Paediatr Scand Suppl 1961;129:1.
G, Nachemson A, Palmen K. Screening of children with congenital
dislocation of the hip joint on the maternity wards in Sweden. J Pediatr Orthop 1983;3:271.
AC, Cundy P, Foster BK, et al. Late diagnosis of congenital dislocation
of the hip and presence of a screening programme: South Australia
population-based study. Lancet 1999;54:1514.
H, Danielsson LG. Screening of neonatal instability and of
developmental dislocation of the hip. A survey of 132,601 living
newborn infants between 1956 and 1999. J Bone Joint Surg Br 2002;84(6):878–885.
M, Gotoh E. Significance of inguinal folds for diagnosis of congenital
dislocation of the hip in infants aged three to four months. J Pediatr Orthop 1990;10:331.
T, Ono Y, Kitakoji T, et al. Soft tissue interposition after closed
reduction in developmental dysplasia of the hip: the long term effect
on acetabular development and aseptic necrosis. J Bone Joint Surg Br 1999;81:385.
JA, Weinstein SL, Dolan L, et al. DDH: results of treatment after
reduction by an anteromedial surgical approach at a minimum four year
follow-up. J Bone Joint Surg 1997;79A:810.
JN. Congenital dislocation of the hip: acetabular deficiency in
adolescence (absence of the lateral acetabular epiphysis) after
limbectomy in infancy. J Pediatr Orthop 1989;9:640.
MKD, Evans JDC. The pelvic osteotomy of chiari: an anatomical study of
the hazards and misleading radiographic appearance. J Bone Joint Surg Br 1976;58:163.
V, Reuveni A, Pery M, et al. Ultrasonography in developmental
displacement of the hip: a critical analysis of our results. J Pediatr Orthop 1989;9:154.
J, Kotwicki T, Ponitek T, et al. Ultrasound measurements of the newborn
hip. Comparison of two methods in 657 newborns [comment]. Acta Orthop Scand 1998;69(1):21–24.
H, Bicimoglu A, Koparal S, et al. Assessment of variations in the
measurement of hip ultrasonography by the Graf method in developmental
dysplasia of the hip. J Pediatr Orthop B 2001;10(2):89–95.
DS, Clegg J, Al-Chalabi AN. Routine ultrasound screening for neonatal
hip instability: can it abolish late-presenting congenital dislocation
of the hip? J Bone Joint Surg Br 1994;76:534.
PA, Harcke HT, Bowen JR. Effective use of ultrasound in the management
of congenital dislocation and/or dysplasia of the hip. Clin Orthop 1990;252:176.
MH, Clarke NM, Campbell MJ, et al. Comparison of audible sound
transmission with ultrasound in screening for congenital dislocation of
the hip. Lancet 1990;336:421.
NM, Harcke HT, McHugh P, et al. Real-time ultrasound in the diagnosis
of congenital dislocation and dysplasia of the hip. J Bone Joint Surg Br 1985;67:406.
NMP, Clegg J, Al-Chalabi AN. Ultrasound screening of hips at risk for
CDH: failure to reduce the incidence of late cases. J Bone Joint Surg Br 1989;71:9.
K, Markestad T, Lie RT. Congenital dislocation of the hip: a
prospective study comparing ultrasound and clinical examination. Acta Paediatr 1992;81:177.
KM, Markestad T, Lie RT, et al. Cost effectiveness of alternative
screening strategies for developmental dysplasia of the hip. Arch Pediatr Adolesc Med 1995;149:643.
K, Markestad T, Lie RT. Developmental dysplasia of the hip: a
population based comparison of ultrasound and clinical findings. Acta Pediatr 1996;85:64.
KJ, Tegnander A, Bredland T, et al. Universal or selective screening of
the neonatal hip using ultrasound? A prospective, randomized trial of
15, 529 newborn infants. J Bone Joint Surg Br 2002;84(6):886–890.
G, Clarke NMP. Monitoring treatment of developmental dysplasia of the
hip with the pavlik harness: the role of ultrasound. J Bone Joint Surg Br 1997;79:719.
S, Terjesen T, Kvistad KA, et al. Acetabular angles and femoral
anteversion in dysplastic hips in adults: CT investigation. J Comput Assist Tomogr 1991;15:115.
S, Terjesen T, Kvistad KA. Computed tomography measurements of the
acetabulum in adult dysplastic hips: which level is appropriate? Skeletal Radiol 1991;20:267.
M, Donoghue VB, Gorman WA, et al. Radiographic screening at four months
of infants at risk for congenital hip dislocation. J Bone Joint Surg Br 1992;74:704.
VA, Piggott H. Acetabular floor thickening and femoral head enlargement
in congenital dislocation of the hip: lateral displacement of femoral
head. J Pediatr Orthop 1983;3:22.
J, Morcuende JA, Weinstein SL. Predictive value of the teardrop figure
in congenital dislocation of the hip: a quantitative study, Annual
Meeting 1994.
JT, Matan S, Coleman SS, et al. The predictive value of the development
of the acetabular teardrop figure in developmental dysplasia of the
hip. Presented at the American Academy of Orthopaedic Surgeons Annual
Meeting, San Francisco, CA, 1993.
JM, Morevende JA, Weinstein SL. The teardrop in congenital dislocation
of the hip diagnosed late: a quantitative study. J Bone Joint Surg Am 1996;78:1048.
JM, Matan A, Coleman SS, et al. The predictive value of the development
of acetabular teardrop figure in developmental dysplasia of the hip. J Pediatr Orthop 1997;17:165.
NS, Suzuki S, Kasahara Y, et al. Prediction of reduction in
developmental dysplasia of the hip by magnetic resonance imaging. J Pediatr Orthop 1996;16:254.
BJ, Hugosson C, Jacobsson B, et al. Magnetic resonance imaging study of
acetabular morphology in developmental dysplasia of the hip. J Pediatr Orthop 1993;13:314.
JC, Howell P, Nockler I, et al. Relationship of pain to the
radiological anatomy of the hip joint in adults treated for congenital
dislocation of the hip as infants: a long-term follow-up of patients
treated by three methods. J Pediatr Orthop 1986;6:539.
TA, Weinstein SL. Closed reduction for congenital dysplasia of the hip:
functional and radiographic results after an average of thirty years. J Bone Joint Surg Am 1994;76:1777.
ME, Dickson JH, Cain TE, et al. Surgical therapy for congenital
dislocation of the hip in patients who are twelve to thirty-six months
old. J Bone Joint Surg Am 1984;66:412.
NA, Brown TD, Weinstein SL. The effect of contact pressure elevations
and aseptic necrosis on the long-term outcome of congenital hip
dislocation. J Orthop Res 1990;8:504.
JK, Cavadias AX. One-stage surgical procedure for congenital
dislocation of the hip in older children: long-term results. Clin Orthop 1989;246:30.
PH, Lynskey TG, Catterall A. Congenital hip dysplasia: problems in the
diagnosis and management in the first year of life. J Pediatr Orthop 1987;7:568.
D, Lehman WB, Tenenbaum Y, et al. Pavlik harness versus Frejka splint
in treatment of developmental dysplasia of the hip: bicenter study. J Pediatr Orthop 1993;13:311.
BJ, Burger JD, Bos CF, et al. Frejka pillow and Becker device for
congenital dislocation of the hip: prospective 6-year study of 104
late-diagnosed cases. Acta Orthop Scand 1993;64:305.
F, Bensahel H, Canadell J, et al. The Pavlik harness in the treatment
of congenital dislocating hip: report on a multicenter study of the
European Paediatric Orthopaedic Society. J Pediatr Orthop 1988;8:1.
A, Wierusz-Kozlowska M, Kruczynski J. Abduction treatment in late
diagnosed congenital dislocation of the hip: follow-up of 1,010 hips
treated with the Frejka pillow 1967-76. Acta Orthop Scand Suppl 1990;236:1.
A. The functional method of treatment using a harness with stirrups as
the primary method of conservative therapy for infants with congenital
dislocation of the hip, 1957 [classical article]. Clin Orthop 1992;281:4.
JP, Round J, Taylor G, et al. The natural history of developmental
dysplasia of the hip after early supervised treatment in the Pavlik
harness. A prospective, longitudinal follow up. J Bone Joint Surg Br 2002;84(3):418–425.
RG, Birch JG, Herring JA, et al. Use of the Pavlik harness in
congenital dislocation of the hip: an analysis of failures of
treatment. J Bone Joint Surg Am 1990;72:238.
PS, Lasser S, MacEwen GD. Congenital dislocation of the hip: use of the
Pavlik harness in the child during the first 6 months of life. J Bone Joint Surg Am 1976;58:1000.
JJ, Kaelin A. Inferior (obturator) dislocation of the hip in neonates:
a complication of treatment by the Pavlik harness. J Bone Joint Surg Br 1992;74:708.
Gonzalez J, Albinana J. Obturator dislocation in developmental
dislocation of the hip: a complication during treatment. J Pediatr Orthop B 1996;5:129.
S, Kashiwagi N, Kasahara Y, et al. Avascular necrosis and the Pavlik
harness: the incidence of avascular necrosis in three types of
congenital dislocation of the hip as classified by ultrasound. J Bone Joint Surg Br 1996;78:631.
JR, Greer RBIII, Cotler JM. Management strategy for prevention of
avascular necrosis during treatment of congenital dislocation of the
hip. J Bone Joint Surg Am 1981;63:140.
JR, Winter RB. Avascular necrosis of the capital femoral epiphysis as a
complication of closed reduction of congenital dislocation of the hip. J Bone Joint Surg Am 1972;54:373.
J, Schleberger R, Steffen R. Closed reduction by two-phase skin
traction and functional splinting in mitigated abduction for treatment
of congenital dislocation of the hip. Clin Orthop 1990;258:27.
A, Paavilainen T. The effect of traction treatment on the results of
closed or open reduction for congenital dislocation of the hip: a
preliminary report. In: Tachdjian MO, ed. Congenital dislocation of the hip, New York: Churchill Livingstone, 1982:365.
WB, Grant AD, Nelson J, et al. Hospital for Joint Diseases’ traction
system for preliminary treatment of congenital dislocation of the hip. J Pediatr Orthop 1983;3:104.
DS, Hoyt WA, Odell HW Jr. Congenital dislocation of the hip: the
relationship of premanipulation traction and age to avascular necrosis
of the femoral head. J Bone Joint Surg Am 1977;59:306.
DL, Lee CS, Kolman B. Derotational femoral shortening for developmental
dislocation of the hip: special indications and results in the child
younger than 2 years. J Pediatr Orthop 1995;15:768.
PL, Strecker WB. Congenital dislocation of the hip in children:
comparison of the effects of femoral shortening and of skeletal
traction in treatment. J Bone Joint Surg Am 1984;66:21.
A, Ayata C, Cevat Ogun T, et al. Preliminary traction as a single
determinant of avascular necrosis in developmental dislocation of the
hip. J Pediatr Orthop 2000;20:579–584.
WK, Anderson MB, Alpert J, et al. The value of preliminary traction in
the treatment of congenital dislocation of the hip. J Bone Joint Surg Am 1990;72:1043.
E, Choi IH, Guille JT, et al. Prognostic factors in congenital
dislocation of the hip treated with closed reduction: the importance of
arthrographic evaluation. J Bone Joint Surg Am 1992;74:1140.
PL, Lesker PA, Ogata K. A dynamic canine model of experimental hip
dysplasia: gross and histological pathology, and the effect of position
of immobilization on capital femoral epiphyseal blood flow. J Bone Joint Surg Am 1984;66:1281.
BG, Kasser JR, Hey LA, et al. Post reduction computed tomography in
developmental dislocation of the hip. Part I. Analysis of measurement
reliability. J Pediatr Orthop 1997;7:626.
EE, Accardo NY. Incidence of avascular necrosis of the femoral head in
congenital hip dislocation related of the degree of abduction during
preliminary traction. J Pediatr Orthop 1981;1:307.
RB, Kostiuk J, Dallas S. Avascular necrosis of the femoral head as a
complication of treatment for congenital dislocation of the hip in
young children: a clinical and experimental investigation. Can J Surg 1969;12:44.
LS, Schneider DJ, Berlin JM, et al. The contribution of the ossific
nucleus to the structural stiffness of the capital femoral epiphysis: a
porcine model of DDH. J Pediatr Orthop 1999; 19(4):433–437.
LS, Berlin JM, Schneider DJ, et al. Chrondronecrosis of the hip. The
protective role of the ossific nucleus in an animal model. Clin Orthop 2000;377:265–271.
SS, Schoenecker PL, Anderson AM, et al. The prognostic importance of
the ossific nucleus in the treatment of congenital dysplasia of the
hip. J Bone Joint Surg Am 1998;80:1719.
SJ, Schoenecker PL, Anderson AM, et al. The prognostic importance of
the ossific nucleus in the treatment of congenital dysplasia of the
hip. J Bone Joint Surg Am 1998;80(12):1719–1727.
SJ, Bassett GS, Gordon JE, et al. Reduction of a dislocation of the hip
due to developmental dysplasia. Implications for the need for further
surgery. J Bone Joint Surg Am 2003;85-A(2):239–243.
H, Tanabe G, Miyake Y. A new open reduction treatment for congenital
hip dislocation: long-term follow-up of the extensive anterolateral
approach. Acta Med Okayama 1990;44:223.
MG, Arntz CT, Staheli LT. Open reduction through a medial approach for
congenital dislocation of the hip: a critical review of the Ludloff
approach in sixty-six hips. J Bone Joint Surg Am 1993;75:1334.
RB. Role of innominate osteotomy in the treatment of congenital
dislocation and subluxation of the hip in the older child. J Bone Joint Surg Am 1966;48:1413.
RB, Dubos J-P. The first fifteen years’ personal experience with
innominate osteotomy in the treatment of congenital dislocation and
subluxation of the hip. Clin Orthop 1974;98:72.
RB, Hansson G, Thompson GA. Innominate osteotomy in the management of
residual congenital subluxation of the hip in young adults. Clin Orthop 1984;182:53.
E, Adyaman S, Omeroglu H, et al. Medial approach open reduction for
congenital dislocation of the hip using the Ferguson procedure: a
review of 31 hips. Arch Orthop Trauma Surg 1991;110:169.
G, Milella PP. Indications for treatment of congenital dislocation of
the hip by the surgical medial approach. In: Tachdjian MO, ed. Congenital dislocation of the hip, New York: Churchill Livingstone, 1982:385.
JN, Bernard AA, Dwyer NS. Early results of medial approach open
reduction in congenital dislocation of the hip: use before walking age.
J Pediatr Orthop 1988;8:288.
PE, Chingren GL, Klaaren HE, et al. Open reduction for congenital
dislocation of the hip using the Ferguson procedure: a review of
twenty-six cases. J Bone Joint Surg Am 1979;61:915.
DE, Karol LA, Colby S, et al. Results of medial open reduction of the
hip in infants with developmental dislocation of the hip. J Pediatr Orthop 2003;23(1):1–9.
RC, Capelli AM, Schoenecker PL. Pemberton osteotomy for the treatment
of developmental dysplasia of the hip in older children. J Pediatr Orthop 1998;18:254.
O, Sosna A, Vavra J. Indications and surgical technique for open
reposition of congenital dislocation of the hip after Ludloff [author’s
translation]. Acta Chir Orthop Traumatol Cech 1976;43:233.
R, Ortolani M Jr. Open reduction (Ludloff approach) of congenital
dislocation of the hip before the age of two years. Isr J Med Sci 1980;16:276.
JM, Dolan LA, Weinstein SL, et al. Long term outcome after open
reduction through an anteromedial approach for congenital dislocation
of the hip. J Bone Joint Surg Am 1997;79:810.
ST, Tanabe T, Ogawa R. Acetabular development after open reduction for
developmental dislocation of the hip: 15 year follow up of 22 hips
without additional surgery. Acta Orthop Scand 1998;69:17.
DI, Broughton NS, Cole WG, et al. The predictability of acetabular
development after closed reduction for congenital dislocation of the
hip. J Bone Joint Surg Br 1988;70:733.
RK, Jones RS, Vergroessen DA, et al. Simultaneous open reduction and
salter innominate osteotomy for developmental dysplasia of the hip. J Bone Joint Surg Br 1996;78:471.
P, Jankovic L. Combined procedure of open reduction and shortening of
the femur in treatment of congenital dislocation of the hips in older
children. Clin Orthop 1976;119:60.
MJ, Johnson LO, Quanbeck DS, et al. One stage treatment of congenital
dislocation of the hip in children three to ten years old: functional
and radiographic results. J Bone Joint Surg Am 1998;80:336.
RD, Roach JW, Wenger DR, et al. One-stage treatment of congenital
dislocation of the hip in older children, including femoral shortening.
J Bone Joint Surg Am 1989;71:734.
P, Brzuske A. Salter innominate osteotomy for the treatment of
developmental dysplasia of the hip in children: results of
seventy-three consecutive osteotomies after twenty-six to thirty-five
years of follow up. J Bone Joint Surg Am 2002;84-A(2):178–186.
HG, Catterall A, Hashemi-Nejad A, et al. Test of stability as an aid to
decide the need for osteotomy in association with open reduction in
developmental dysplasia of the hip. A long-term review. J Bone Joint Surg Br 2000;82-B(1):17–27.
S, Tanabe T, Ogawa R. Acetabular development after open reduction for
developmental dislocation of the hip. 15 year follow up of 22 hips
without additional surgery. Acta Orthop Scand 1998;69(1):17–20.
J, Dolan L, Spratt K, et al. Acetabular dysplasia after treatment for
developmental dysplasia of the hip. Implications for secondary
procedures. J Bone Joint Surg 2004;86B:876–886.
T, Kiefer GN, Coleman SS. Pemberton osteotomy for residual acetabular
dysplasia in children who have congenital dislocation of the hip. J Bone Joint Surg Am 1993;75:643.
WV, Vogt M, Grudziak J, et al. Severin classification system for
evaluation of the results of operative treatment of congenital
dislocation of the hip: a study of intraobserver and interobserver
reliability. J Bone Joint Surg Am 1997;79:656.
RS. The management of late diagnosed congenital dislocation and
subluxation of the hip: with special reference to femoral shortening. J Bone Joint Surg Br 1979;61:7.
WP, Staheli LT, Chew DE. The effectiveness of the Salter innominate
osteotomy in the treatment of congenital dislocation of the hip. J Bone Joint Surg Am 1986;68:79.
GC, Harris NH, Chrispin AR. Anteversion of the acetabulum in congenital
dislocation of the hip: a preliminary report. Orthop Clin North Am 1978;9:89.
HW, Wenger DR. The morphology of residual acetabular deficiency in
childhood hip dysplasia: a three dimensional computed tomographic
analysis. J Pediatr Orthop 1997; 17:637.
D, Lehman WB, Grant AD. 2-D and 3-D computed tomography and magnetic
resonance imaging in developmental dysplasia of the hip. Orthop Rev 1992;21:1189.
P, Genant H, Steiger P, et al. Three-dimensional digital displays in
congenital dislocation of the hip: preliminary experience. J Pediatr Orthop 1989;9:532.
CM, Sartoris DJ, Tyson R, et al. Acetabular alterations in untreated
congenital dysplasia of the hip: computed tomography with multiplanar
reformation and three-dimensional analysis. J Comput Assist Tomogr 1986;10:84.
N, Nagoya MD, Mieno T. Determination of acetabular coverage of the
femoral head with use of a single anteroposterior radiograph: a new
computerized technique. J Bone Joint Surg Am 1993;75:1318.
JR, Bowen JR, MacEwen GD. Varus derotation osteotomy in the treatment
of persistent dysplasia in congenital dislocation of the hip. J Bone Joint Surg Am 1985;67:195.
G. Intertrochanteric femoral osteotomy with concentric reduction of the
femoral head in treatment of residual congenital acetabular dysplasia. Clin Orthop 1976;119:48.
DM, Glover SD, Benson MK. Congenital dislocation of the hip presenting
after the age of three years: a long-term review. J Bone Joint Surg Br 1989;71:745.
M, Stryhal F. Behavior of the proximal femur during the treatment of
congenital dysplasia of the hip: a clinical long-term study. J Pediatr Orthop 1991;11:506.
JE, Holloway GM, Waugh W. Growth of the proximal femur after
varus-derotation osteotomy in the treatment of congenital dislocation
of the hip. Clin Orthop 1982;162:61.
SS, Szoke G, Murray G, et al. Femoral remodeling after subtrochanteric
osteotomy for developmental dysplasia of the hip. J Bone Joint Surg Br 1996;78:917.
RW, Wilson MG, Poss R. Isolated proximal femoral osteotomy for
treatment of residua of congenital dysplasia or idiopathic
osteoarthritis of the hip. J Bone Joint Surg Am 1996; 78:1462.
J, Weinstein SL, Morcuende JA, et al. DDH acetabular remodeling after
open or closed reduction: timing for secondary procedures. Orthop Trans 1996;20:298.
MB, Hall JE. Transiliac lengthening of the lower extremity: a modified
innominate osteotomy for the treatment of postural imbalance. J Bone Joint Surg Am 1979;61:1182.
DH, Moore M. Clinical and radiographic outcome of patients treated with
double innominate osteotomy for congenital hip dysplasia. J Pediatr Orthop 1991;11:143.
D. A new technique of triple osteotomy for acetabular dysplasia in
older children and adults. Abstracts of the 14th World Congress of the
Society of International Chirurgiae Orthopaedicae et Traumatologiae,
Kyoto, 1978.
JT, Forlin E, Kumar SJ, et al. Triple osteotomy of the innominate bone
in treatment of developmental dysplasia of the hip. J Pediatr Orthop 1992;12:718.
JS, Saluja R, Eilert RE, et al. Evaluation of the biomechanics of the
hip following triple osteotomy of the innominate bone. J Bone Joint Surg Am 1996;78:855.
R, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment
of hip dysplasias: technique and preliminary results. Clin Orthop Relat Res 1988;232:26.
MB, Kaelin AJ, Schluntz K, et al. Spherical acetabular osteotomy for
treatment of acetabular dysplasia in adolescents and young adults. J Pediatr Orthop B 1994;3:47.
T, Yamaura M, Nakamitu S, et al. The displacement of the femoral head
by rotational acetabular osteotomy: a radiographic study of 97
subluxated hips. Acta Orthop Scand 1992;63:33.
MM, Masuhara K, Nishii T, et al. Early deterioration after modified
rotational acetabular osteotomy for the dysplastic hip. J Bone Joint Surg Br 1997;79:220.
Kleuver M, Huiskes R, Kauer JM, et al. Three dimensional displacement
of the hip joint after triple pelvic osteotomy. A postmortem
radiostereometric study. Acta Orthop Scand 1998; 69(6):585–589.
Kleuver M, Kapitein PJ, Kooijman MA, et al. Acetabular coverage of the
femoral head after triple pelvic osteotomy: no relation to outcome in
51 hips followed for 8-15 years. Acta Orthop Scand 1999;70(6):583–588.
L, Grudziak JS, Kruczynski J, et al. Combined one stage open reduction
femoral osteotomy and Dega pelvic osteotomy for DDH. Presented at the
American Academy of Orthopaedic Surgeons Annual Meeting, San Francisco,
CA, 1993.
M, Zwierzchowski H. One-stage hip reconstruction with Dega’s
transiliacal osteotomy in the treatment of congenital hip dislocation
in children. Beitr Orthop Traumatol 1990;37:571.
S, Higuchi F, Inoue A, et al. Changes in the interposed capsule after
Chiari osteotomy: an experimental study on rabbits with acetabular
dysplasia. J Bone Joint Surg Br 1992; 74:463.
C, Tanaka S, Yamamuro T. The Spitzy shelf operation for the dysplastic
hip: retrospective 10 (5-25) year study of 124 cases. Acta Orthop Scand 1992;63:273.
R, Pongracz N, Schoenecker W, et al. Chiari osteotomy for congenital
dislocation and subluxation of the hip: results after 20 to 34 years
follow-up. J Bone Joint Surg Br 1991;73:890.
HM, Matussek J, Baum C. Long term results of Salter and Chiari hip
osteotomies in developmental hip dysplasia: a survey of over 10 years
follow up with a new hip evaluation score. Arch Orthop Trauma Surg 1998;117:222.
Malefijt MC, Hodgland T, Nielsen HKL. Chiari osteotomy in the treatment
of the congenital dislocation and subluxation of the hip. J Bone Joint Surg Am 1982;64:996.
WH, Bourne RB, Oh I. Intra-articular acetabular labrum: a possible
etiological factor in certain cases of osteoarthritis of the hip. J Bone Joint Surg Am 1979;61:510.
T, Saito S, Ohzono K, et al. Chiari pelvic osteotomy for
osteoarthritis: the influence of the torn and detached acetabular
labrum. J Bone Joint Surg Br 1990;72:765.
DI, Broughton NS, Cole WG, et al. Avascular necrosis following closed
reduction of congenital dislocation of the hip: review of influencing
factors and long-term follow-up. J Bone Joint Surg Br 1990;72:557.
E, Ando M. The pathogenesis of femoral head deformity in congenital
dislocation of the hip: experimental study of the effects of articular
interpositions in pigs. Clin Orthop 1993;288:303.
D, MacEwen GD. Growth disturbance of the proximal part of the femur
after treatment for congenital dislocation of the hip. J Bone Joint Surg Am 1991;73:410.
JA. Anatomic and histologic study of factors affecting development and
evolution of avascular necrosis in congenital hip dislocation. In: The hip: proceedings of the second annual meeting of the hip society. St. Louis, MO: Mosby, 1974:125.
PL, Bitz DM, Whiteside LA. The acute effect of position of
immobilization on capital femoral epiphyseal blood flow: a quantitative
study using the hydrogen washout technique. J Bone Joint Surg Am 1978;60:899.
J. Avascular necrosis of the proximal femur in developmental
dislocation of the hip: incidence, risk factors, sequelae, and MR
imaging for diagnosis and prognosis. Acta Orthop Scand Suppl 1996;268:1.
IH, Dunin AJ, Cole WG, et al. Avascular necrosis after open reduction
for congenital dislocation of the hip: analysis of causative factors
and natural history. J Pediatr Orthop 1989;9:525.
J. Avascular necrosis after non operative treatment of developmental
hip dislocation: prognosis in 36 patients followed 17-26 years. Acta Orthop Scand 1995;66:239.
WK. Vascular epiphyseal changes in congenital dislocation of the hip:
results in adults compared with results in coxa plana and in congenital
dislocation without vascular changes. J Bone Joint Surg Am 1951;33:284.
EN, Gerratana FJ, Gage JR. Open reduction for congenital hip
dislocation: the risk of avascular necrosis with three different
approaches. J Bone Joint Surg Am 1986;58:1000.
R. Congenital dislocation of the hip: a review and assessment of
results of treatment with special reference to frame reduction as
compared with manipulative reduction. J Bone Joint Surg Br 1960;42:253.
T, Millis MB, Griffin PP. The early identification and classification
of growth disturbances of the proximal end of the femur. J Bone Joint Sung Am 1986;68:970.
HW, Morcuende JA, Dolan LA, et al. Acetabular development in
developmental dysplasia of the hip complicated by lateral growth
disturbance of the capital femoral epiphysis. J Bone Joint Surg Am 2000;82-A(12):1692–1700.
EH, Huo MH, DeLuca PA. Early innominate osteotomy as a treatment for
avascular necrosis complicating developmental hip dysplasia. J Pediatr Orthop B 1997;6:138.
