The Upper Limb
the more common upper-limb and hand congenital anomalies, traumatic and
posttraumatic conditions, neuropathic problems, and growth deformities.
The sections of this chapter examine major diagnostic categories and
anatomic regions. The reader will find more information regarding
upper-extremity development (Chapter 2), fractures (Chapter 32), and limb deficiency (Chapter 30) in other chapters of this text.
should address the issues of function, growth, cosmetic deformity, and
the emotional concerns of the child and family. All are important
factors in determining a successful outcome. The orthopaedist’s goals
are to enhance the ability to place the hand in space; to improve
deficiencies in grasp, release, or pinch function; and to improve skin
mobility and sensibility (1). The treatment of
physeal abnormalities improves growth-related loss of motion and
function, and reduces pain and musculoskeletal deformity (2).
Extensive time and counseling are important so as to address the
concerns of the child and parents regarding the alteration in
self-image that can occur with any hand deformity.
appears 26 days after fertilization and 24 hours before the appearance
of the leg bud. Growth is in a proximal-to-distal manner. Development
is guided by the apical ectodermal ridge inducing the mesoderm to
condense and differentiate (3). The upper limb
anlage is initially continuous and extends to a hand paddle by day 31.
The digital rays develop by day 36 with fissuring of the hand paddle,
initially in the central rays, followed by the border digits.
Mesenchymal differentiation also begins in a proximal-to-distal manner
with chondrification, enchondral ossification, joint formation, and
muscle and vascular development. Joint formation and digital separation
require apoptosis, or programmed cell death. The entire process is
complete by 8 weeks after fertilization (4).
Other major organ system development is occurring simultaneously, which
explains the associated cardiac, craniofacial, musculoskeletal, and
renal anomalies that can occur with upper limb malformations.
Their genetic expression controls the timing and extent of growth by
regulating mesenchymal cells. At present, the understanding of the
genetic basis of limb development, and therefore of the occurrence of
congenital anomalies, is expanding rapidly (6,7,8,9,10,11,12). For example, a mutation at the HOXD13 site has been identified as a cause of polysyndactyly (13).
A further understanding of the role of genetics in limb development may
revolutionize the treatment of congenital deficiencies.
live births, with 1% being multiple anomalies. It has been estimated
that between 1 in 531 and 1 in 626 live births involve upper-extremity
anomalies (14,15). Only
1% to 2% of these congenital differences are the result of chromosomal
abnormalities. However, 75% of 233 missed abortions studied were noted
to have an abnormal karyotype, with 18% having a morphologic defect and
normal karyotype (16). At present, only a small
percentage of these are known to be caused by defined genetic events.
In most cases, the cause of the congenital malformation is still
unknown, but expanding genetic information provides optimism for
increased knowledge in the near future.
differences of the hand and upper limb. The present accepted
classification system for congenital differences was proposed by
Swanson (17) and revised by the Congenital Anomalies Committee of the International Federation of Societies for Surgery of the Hand (18).
This classification is based on embryologic failure, and defines
deficiencies as terminal or intercalary, with a subclassification into
longitudinal and transverse deficiencies. The subcategories are as
follows: (i) failure of formation of parts; (ii) failure of
differentiation of parts; (iii) duplication; (iv) overgrowth; (v)
undergrowth; (vi) constriction band syndrome; and (vii) generalized
skeletal abnormalities. However, there have been reports of
inconsistencies in classifying congenital anomalies of the upper limb
by this system. A more descriptive method has been shown to be valid (19,20).
classification group, but presents them by anatomic region. Caring for
the child with congenital differences involves more than surgical
skill. From the moment of birth, these children may potentially be
viewed by their parents, family, and society as being impaired;
eventually, they will begin to view themselves the same way (21,22).
It is critical that the treating surgeon helps provide the emotional
support and caring that allow the parents and child to appropriately
grieve the loss of a normal hand (23). It is helpful to provide them with in-depth knowledge of the cause and treatment options (24).
This process starts with the initial newborn visit, and continues
throughout the growth and development of the child into an independent,
self-reliant adult (25). Support groups are useful for many of these children and their families.
will not be impaired. They will merely develop their skills in a
“different” way from their peers. They may need the help of skilled and
caring parents, siblings, therapists, teachers, coaches, prosthetists,
and surgeons in order to achieve their goals and dreams. Being part of
helping these children grow into unique and independent adults is
exciting and rewarding for the surgeon.
central nervous system. It occurs in 5 in 1000 live births, and may be
caused by perinatal anoxia, intraventricular hemorrhage, or congenital
cerebral vascular accidents. It occurs most
commonly in premature infants weighing less than 1500 g (26,27).
The resultant hemiplegia or quadriplegia can lead to significant
upper-extremity deformities and functional deficits. In hemiplegia,
these individuals predominantly use the affected extremity as an assist
for the unaffected extremity. In the quadriparetic, both upper limbs
will have significant deformities and deficits. The quality of use of
an affected extremity is dependent on many factors, including the
presence of contractures, voluntary motor control, discriminatory
sensibility, learning disabilities, and visual deficits (28,29,30,31,32).
This section focuses on the deformities and deficits relating to elbow
flexion, forearm pronation, wrist palmar flexion and ulnar deviation,
finger flexion, and thumb-in-palm deformity in these patients.
Individuals with contractures greater than 60 degrees will either
perform activities with one hand or use the dorsum of the affected hand
or forearm to assist the unaffected hand. These individuals may benefit
from surgical correction of their pronation deformity in order to
improve the assistive function of that extremity. This can often be
performed with simultaneous procedures to improve thumb-in-palm, wrist
palmar flexion, or digital flexion deformities (35).
Although approximately 50% of these patients will have a flexion
contracture, most of these contractures are less than 30 degrees and do
not limit function (37,38).
There may be an associated radial head dislocation in a small number of
patients, and this should be assessed radiographically before operative
intervention (39). Patients with quadriparesis
have greater degrees of elbow flexion contracture. However, these
contractures rarely affect their ability to use their motorized
wheelchairs, computers, or communication boards. In the care-dependent,
nonfunctional quadriparetic, contractures may become severe enough to
affect hygiene and care. If skin breakdown develops or is imminent,
then surgery may be necessary.
Limited motor function occurs with: (i) poor release because of wrist
and finger flexor spasticity and weak digital extension; (ii)
inadequate grasp because of wrist palmar flexion spasticity and weak
wrist extension; and (iii) minimal pinch because of thumb-in-palm
deformity. Discriminatory sensibility is deficient in more than 50% of
these children. Poor voluntary control of the upper extremity limits
functional placement of the hand in space (31,33).
In addition, many of these children have visual and cognitive
abnormalities that further impair hand function. At best, most patients
with spasticity have assistive hand function.
forearm pronation, wrist and palmar flexion, thumb-in-palm, and
interphalangeal swan-neck deformities. These deformities may be a
combination of spasticity and contractures. Pronation deformity and
thumb-in-palm contracture seem to affect function the most (33). The combination of neurologic impairment and disuse affects growth in length and girth of the affected arm and hand (33).
In this useful scheme, there are four subgroups of patient function: 0
(no use), 1 to 3 (passive assist), 4 to 6 (active assist), and 7 and 8
(spontaneous use). Because spasticity changes with stress, growth, and
central nervous system changes, it may be difficult on any one visit to
accurately define a patient’s level of function. This system is used
with the input of the patient, family, and physical therapist in order
to best define a patient’s overall status. It is used for assessing the
outcome of treatments (35). Both the Melbourne
Assessment of Unilateral Upper Limb Function and the Pediatric
Evaluation of Disablility Inventory (PEDI) have been validated for
upper-limb function assessment in children with cerebral palsy. The
Melbourne Assessment of Unilateral Upper Limb Function has very high
internal consistency and high inter- and intraobserver reliability,
making it a reliable tool in assessing function and outcome of
interventions in patients with cerebral palsy (41,42).
include: observation of the patient’s growth and development; the use
of therapy, including splints; injections, such as phenol or Botox; and
performing surgical reconstruction.
treatment for children with cerebral palsy. The rationale is that,
although the central nervous system deficit is static, the peripheral
manifestations of spasticity and muscle imbalance are progressive with
growth. By maintaining range of motion with passive therapy, it is
hoped that contractures will be prevented (33,43).
In addition, it is hoped that the affected child is capable of learned
motor behavior leading to functional improvement over time,
developmentally, and through formal therapy (44,45).
At present, formal therapy is used during the period of infancy. This
is most intense in the first year of life, and progresses to a home
program with less formal supervision. In many states, early
intervention programs end at 3 years of age. Monitoring of function and
range of motion are performed less regularly thereafter, sometimes
through the school system. During growth spurts that increase
spasticity and lessen range of motion, or with specific activities that
the patient finds difficult to do, brief periods of formal therapy are
often reinitiated, but the therapeutic benefit has not been
statistically established (33).
nighttime splints. As Manske has observed, it is unclear whether they are cost-effective and alter long-term outcome (43).
No objective study has been performed. However, most caretakers use
splints in children with developing contractures. Daytime splints are
recommended only if they improve function in patients with dynamic
contractures. Recently, constraint therapy with casting of the
unaffected limb has been advocated in order to improve the function of
the affected limb in children with hemiplegia (46).
A single randomized study has shown this to be effective. It has been
shown that patients with hemiplegia do not maximally utilize their
motor capabilities in the affected limb in functional tasks (47).
Constraint therapy may better enable these patients to maximize their
motor function in the affected limb, but there are emotional issues
that make this treatment difficult for some families and caregivers. In
a small cohort of patients with cerebral palsy (48),
it has been shown that functional electrical stimulation (FES) is
effective in the short term (up to 3 months) in improving hand
function, when applied to the extensor muscles of the wrist and hand.
Its long-term effectiveness and applicability to all types, degrees,
and ages of patients with cerebral palsy is still unclear.
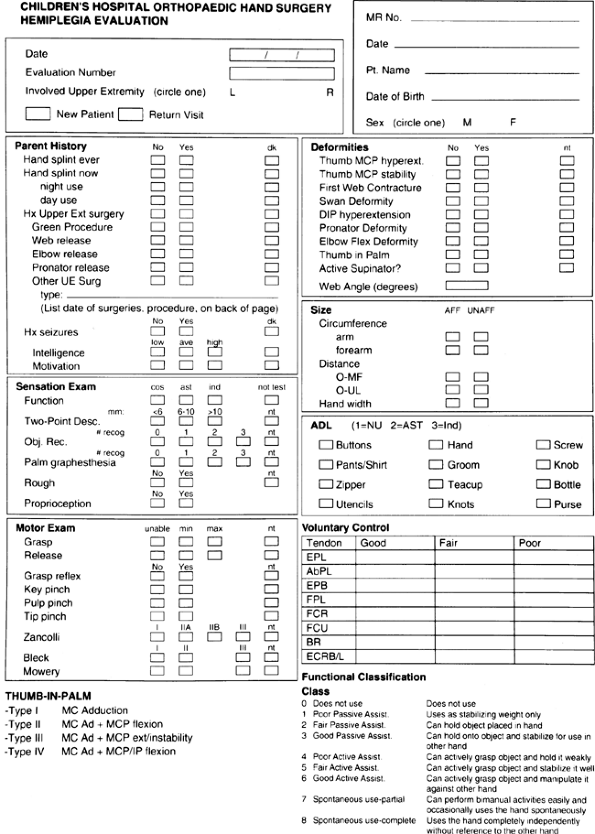 |
|
Figure 23.1 Data sheet for prospective analysis of hemiplegic function used at Children’s Hospital, Boston.
|
most common form of neuromuscular injection (35,49), replacing xylocaine (50,51) and phenol (52,53).
It is used at an initial dose of 1 to 2 U per kg of body weight, and
should not exceed 6 U/kg/month. Injection into pronator, flexor carpi
ulnaris (FCU), and thumb adductor are most often performed. Therapy
should be performed aggressively to stretch agonistic musculotendinous
units and strengthen antagonists. To date, Botox has been most
effective in patients with high motivation to train, good motor
learning capacity, and no fixed contracture or limiting spasticity (54).
Its role in patients with contractures is limited and less effective,
although these patients may show the greatest involvement. Its
effectiveness in young children has not yet been studied critically.
There are several ongoing prospective studies of Botox injections in
the upper extremity and hand, so more definitive information should
soon be available on the indications and effectiveness of its use in
all age groups and at all levels of involvement. At this stage, in our
institution, we use Botox injections in the upper limbs in (i) younger
patients with marked spasticity or developing contractures; and (ii)
older patients with limitations, for whom surgery is not indicated.
Complications involve the formation of antibodies to Botox that limit
further effective injections and leading to deterioration of upper limb
function for the first 1 to 3 weeks postinjection in some patients.
|
TABLE 23.1 HOUSE CLASSIFICATION OF UPPER EXTREMITY AND HAND FUNCTION FOR PATIENTS WITH CEREBRAL PALSY
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
cerebral palsy include: (i) contractures that cause hygiene and care
problems not solved by therapy, splints, or casts; (ii) muscle
imbalance or contractures that cause functional deficits that may be
improved by tendon transfers, musculotendinous lengthening, and/or
joint stabilization procedures; and (iii) cosmetic concerns (28,29,30,31,32,55).
It may be difficult to identify the individual who will have improved
function through surgical reconstruction. As Smith so aptly pointed
out, careful preoperative assessment is necessary in order to select
the appropriate patients and operations (27).
Video recordings of activities of daily living and validated
multiple-task assessment scales, such as the Jebsen scale, can be
helpful in defining functional limitations. Preoperative video
assessments and House classifications are reliable and useful for
surgical planning (56).
The best candidates are patients with hemiplegia and good voluntary
control, sensibility, and motivation. The principle of surgery is to
restore muscle imbalance by lengthening or releasing tight, spastic
muscles and by augmenting weak, stretched muscles by tendon transfers
and tenodesis procedures. Unstable joints need to be stabilized by soft
tissue or arthrodesis procedures in order to maximize the outcome of
tendon reconstruction. Multiple upper-extremity rebalancing procedures
performed under one anesthesia seem to be best. This can also be
performed in conjunction with simultaneous lower-extremity procedures
if the patient and surgeons can tolerate it. It cannot be stressed
enough to the patient and family that surgery will not achieve a normal
hand. Even the best outcome will result in deficiencies of function,
cosmesis, and sensibility. Patients, families, and surgeons need to be
realistic about the expected results. However, in properly selected
patients, surgery will clearly improve function and patient
satisfaction (35,57,58).
This is evident in individuals using the dorsum of the hand or forearm
for bimanual tasks. Thumb-in-palm deformity is usually markedly
improved with surgery (57). The goal must be
well defined and specific to the peripheral manifestation of the
incurable, central disorder of cerebral palsy.
He recommended release of the lacertus fibrosus, Z-lengthening of the
biceps tendon, and musculotendinous lengthening of the brachialis
fascia. In mild contractures, release of the lacertus fibrosus and
musculotendinous lengthening of the brachialis alone may be sufficient.
quadriparetics. The Z-lengthening of the biceps tendon and release of
the brachialis fascia advocated by Mital (36)
are not sufficient to obtain adequate release in these patients. In
patients with contractures greater than 90 degrees and skin breakdown,
extensive release of the muscle origins from the medial and lateral
epicondyles, biceps and brachialis tendons, and anterior elbow capsule
is necessary so as to solve the hygiene and care-related problems that
accompany these conditions.
in patients with hemiplegia. Patients are forced to use the dorsum of
the forearm for two-handed tasks. Wrist and finger flexion deformities
are commonplace in patients with hemiplegia. The FCU is usually the
major deforming force at the wrist. Transfer of the FCU to the wrist
extensors alleviates the deformity and improves the strength of the
antagonist. On occasion, the extensor carpi ulnaris (ECU) is the
primary deforming force, as noted by more ulnar deviation than palmar
flexion at the wrist. In these situations, the ECU is transferred to
the extensor carpi radialis brevis (35).
Simultaneous musculotendinous lengthenings of the finger flexors are
necessary if the extrinsic finger flexors are tight in the neutral
wrist position (32). Otherwise, the patient
will develop a disabling clenched fist postoperatively. Z-lengthenings,
superficialis-to-profundus flexor tendon transfers, and bony procedures
are reserved for patients with severe contractures and limited
function. In the unusual patient with passive digital extension but no
active digital extension, the FCU, ECU, or pronator teres (PT) can be
transferred into the extensor digitorum communis. This will improve
both wrist and digital extension.
grasp function, and make hygiene difficult to maintain in severe
contractures. Static contractures in the web space are corrected with
web-space Z-plasties and adductor releases. Hoffer et al. (59)
have shown by dynamic electromyography that release of the transverse
adductor alone may lead to better pinch postoperatively in selected
patients. At times, the static contractures include the flexor pollicis
longus and brevis, and these muscles need to be appropriately
lengthened or released. Dynamic rebalancing is performed with tendon
transfers to the weak abductors and extensors of the thumb. The donor
muscles used are numerous, and include the palmaris longus, flexor
carpi radialis, and brachioradialis, among others. The recipient
tendons include the extensor pollices brevis and longus and the
abductor pollicis longus. The treatment for each patient should be
individualized in order to correct his or her deformity and imbalance.
Finally, the metacarpophalangeal (MCP) joint should be stable
postoperatively. In most patients, this is achieved by muscle
rebalancing. On occasion, a capsulodesis or arthrodesis procedure
should be performed. Selected patients with thumb-in-palm deformity
respond very favorably to surgical intervention (57).
disabling swan-neck deformities of the interphalangeal joints. If the
fingers extend at the proximal interphalangeal (PIP) joint beyond 40
degrees and lock, the position can limit grasp and cause pain. Multiple
operations have been advised, including flexor digitorum superficialis
tenodesis (32), intrinsic muscle slide (32), lateral band rerouting (60),
spiral oblique ligament reconstruction, and resection of the motor
branch of the ulnar nerve. The lateral band rerouting procedure
provides both intrinsic and extrinsic rebalancing and is effective in
correcting the problem.
disabling dynamic spasticity and fixed contractures of the wrist and
hand benefit from surgical reconstruction. Over a 25-year experience,
House reported that, for 718 procedures in 134 patients with cerebral
palsy, the functional improvement was 2.6 functional levels on the
House scale of 0 to 9 (34). Patients with fair
and good voluntary control had significantly better improvement in
functional use scores than those with poor voluntary control. Often,
the more severely involved patients (House levels 0 to 2) respond best
to musculotendinous lengthenings, tenodesis, and joint stabilization
procedures. More functional patients (House levels 3 to 6) improve with
dynamic tendon transfers and releases. Both groups of patients tolerate
multiple simultaneous procedures (Fig. 23.2).
However, surgery will not create a normal hand. The goals of surgery
need to be realistic and attainable. In properly selected patients,
surgery will improve assistive function and cosmesis. For many of these
children, especially adolescents, and their families, the functional
and cosmetic improvements are quite marked and satisfying.
Proper preoperative selection so as to assess functional deficits and
the patient’s level of cooperation may lessen the risk of these
problems postoperatively (28). Hematoma formation, wound breakdown, and infection can occur after extensive elbow releases (61).
The institutionalized patients with quadriparesis may be most at risk.
If wound dehiscence occurs and the joint is exposed, coverage with a
rotation flap is the treatment of choice.
Fortunately, many infants with minor birth palsies recover fully. These
are the infants who initiate recovery of all muscle groups in the first
1 to 2 months of life. However, in other infants,
permanent
impairment does occur. It is most likely in infants who do not initiate
antigravity motor recovery before 5 to 6 months of life (64,65,66,67,68). Horner syndrome is clearly a major risk factor for a poor outcome (67).
Most infants have involvement of the upper trunk (C-5 to C-6, causing
Erb palsy and, often, additional involvement of C-6 to C-7). Less
often, the entire plexus (C-5 to T1) is affected. In rare instances,
the lower trunk (C-7 to T1, causing Klumpke palsy) is most affected.
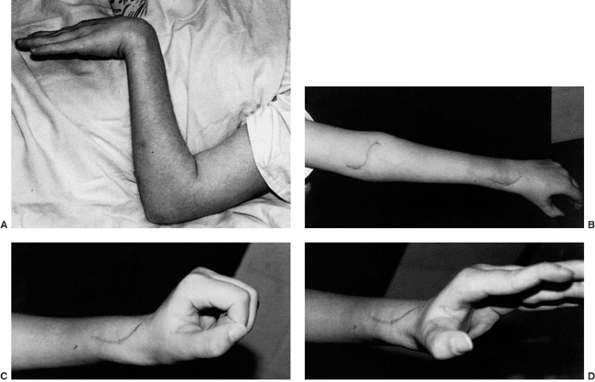 |
|
Figure 23.2 Clinical photographs of hemiplegic tendon transfers for improving hand and upper limb function. A: Preoperative view of dynamic elbow flexion, forearm pronation, wrist flexion, and ulnar deviation with poor assist function. B: Postoperative active elbow extension with maintenance of active elbow flexion. C: Postoperative active wrist extension with the thumb out of the palm for active pinch. D: Postoperative active grasp function with the thumb abducted and extended actively. (Courtesy of Ann Van Heest, MD)
|
for gestational age; prolonged labor; previous births with brachial
plexopathy; difficult delivery, including extraction techniques; and
fetal distress. Shoulder dystocia is the mechanical factor that leads
to an upper-trunk lesion in the difficult vertex delivery. Difficult
arm extraction in a breech delivery can result in an avulsion injury of
the upper trunk (69). The degree of impairment
is related to the level and magnitude of injury to the plexus. Neural
injury is defined by the type (stretch, rupture, avulsion) and severity
(Sunderland grades I to V). Prognosis by natural history has been best
defined by the spontaneous rate of recovery of muscle strength in the
first 3 to 6 months of infancy. Gilbert and Tassin (68)
described the recovery of antigravity biceps function in infancy as a
predictor of the outcome of spontaneous recovery. This finding was
confirmed in a similar study by Waters (67).
Their results showed that infants who did not recover biceps function
by 3 months of life were not normal after 2 years of age. Gilbert et
al. recommend microsurgical reconstruction of the plexus in the first 3
to 6 months of life for infants who fail to recover biceps function (68,70,71). Michelow et al. (72)
noted that return of biceps function alone had a 12% error rate in
predicting outcome, as defined by long-term antigravity muscle
strength. By using elbow flexion, elbow extension, wrist extension,
finger extension, and thumb extension (the Toronto scale), their error
rate for predicting outcome decreased to 5%. In this system, each
muscle group is scored as 0 (no motion), 1 (motion present but
limited), or 2 (normal motion), for a maximum score of 12. A score of
less than 3.5 predicted a poor long-term outcome without microsurgery.
In all studies, the presence of Horner syndrome, total plexus
involvement, and failure of
return of function by 3 to 6 months of life indicate a poor long-term outcome.
function can be limited. It is important to distinguish true paralysis
from the pseudoparalysis that comes with a neonatal clavicle fracture,
humeral fracture, or septic shoulder. There can be clinical overlap
because fractures also occur in infants with shoulder dystocia and
infantile brachial plexopathy. Plain radiographs will identify the
infant with clavicle and humeral fractures. In the neonate, these
fractures heal within 10 to 21 days. If restriction in range of motion
persists after 1 month, there will most likely be a concomitant
brachial plexopathy. In the rare infant with a septic shoulder there
will be evidence of systemic illness (altered vital signs, change in
appetite, toxicity), marked irritability with glenohumeral range of
motion, and abnormal white blood cell count. If there is doubt,
ultrasonography will reveal the effusion, and arthrocentesis will be
confirmatory.
examination is limited to observation of spontaneous activity and
stimulated movement by primitive reflexes in the infant. The Moro
startle reflex and the asymmetric tonic neck reflex can elicit
upper-trunk movement in infants in the first 6 months of life.
Classification of nerve injury in the ambulatory child has included
physical assessment according to the Mallet system. The modified Mallet
system classifies upper-trunk function by grading hand-to-mouth,
hand-to-neck, and hand-on-spine activity, global abduction, and global
external rotation from 0 (no function) to V (normal function). Grades
II, III, and IV are illustrated (Fig. 23.3).
The Toronto active motion scale is also utilized to define the degree
of motor recovery, grades I through IV being gravity-assisted and
grades V through VII being against gravity. These classification
systems have been shown to be reliable in intra- and interobserver
analysis (73).
the clavicle or proximal humerus. Radiographic assessment of the
severity of brachial plexus injury has used myelography, combined
computed tomography (CT) scan and myelography, and magnetic resonance
imaging (MRI). Kawai et al. (74) compared the
results of all three techniques with operative findings. MRI and
combined myelography and CT scan were more reliable than myelography
alone. The presence of large diverticulae and meningoceles was
indicative of root avulsion. Small diverticulae were diagnostic only
60% of the time. Electrodiagnostic studies, with electromyography and
nerve conduction studies, are diagnostic of avulsion if there is no
reinnervation after 3 months of age. However, the presence of
reinnervation does not indicate the long-term quality of muscle
recovery.
is critical to assessing and caring for an infant or child with
brachial plexus palsy (Fig. 23.4). The brachial plexus supplies every muscle of the upper extremity except the trapezius. It is made up of spinal cord nerve
root contributions from C-5 to T1. Prefixed cords (22% of the
specimens) receive a contribution from C-4. Postfixed cords are rare
(1%) and receive a contribution from T2. The C-5 and C-6 roots join to
form the upper trunk. The C-7 root alone becomes the middle trunk. The
C-8 and T1 roots become the lower trunk. Each trunk has an anterior and
a posterior division. The anterior divisions of the upper and middle
parts of the trunk form the lateral cord. The posterior divisions of
all three parts of the trunk form the posterior cord. The anterior
division of the lower trunk continues as the medial cord. The terminal
branches of the cords form the major nerves of the upper extremity. The
upper and lower subscapular and thoracodorsal nerves branch off from
the posterior cord before it bifurcates into the radial and axillary
nerves. The medial cord branches are the medial pectoral, medial
brachial cutaneous, and medial antebrachial cutaneous nerves,
terminating in the medial contribution to the median nerve and the
ulnar nerve. The lateral cord supplies the lateral pectoral nerve and
the lateral branch of the median nerve, and terminates as the
musculocutaneous nerve. In infantile brachial plexopathy, any of these
nerves can be affected. However, the most severe injuries are avulsions
of the nerve roots. The most common injuries are postganglionic
ruptures of the upper trunk.
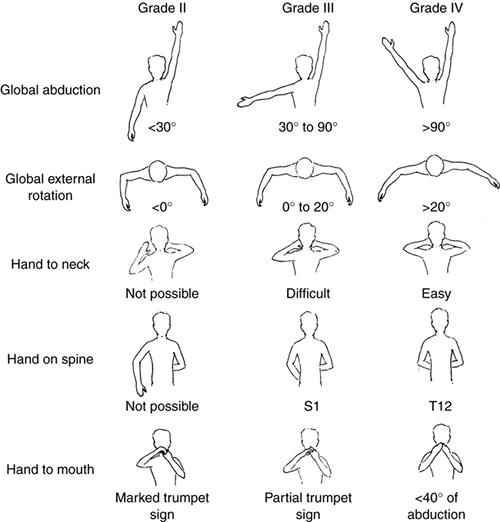 |
|
Figure 23.3
Mallet classification for function about the shoulder in patients with brachial plexus birth palsy. Grade 0 is no function; grade V is normal function; and grades II through IV are depicted for hand-to-mouth, hand-to-neck, external rotation, and hand-to-sacrum activity. |
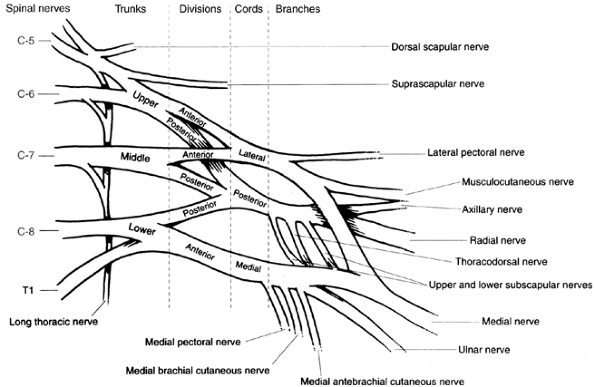 |
|
Figure 23.4 Brachialplexus anatomy.
|
brachial plexus birth palsies should be monitored for spontaneous
recovery in the first 3 to 6 months of life. During this time it is
important to maintain glenohumeral range of motion, especially passive
external rotation (75). This will lessen the risk of progressive glenohumeral dysplasia and dislocation (65,66,67,75,76,77,78)
Many infants will initiate recovery in the first 6 to 8 weeks of life,
and progress to a normal result. Infants who do not initiate recovery
until after 3 months of life may be candidates for microsurgery or
reconstructive surgery.
still debated. The range used clinically is from 1 month to after 6
months of life (63,67,68,69,70,71).
The indications include absence of biceps recovery, Toronto score less
than 3.5, and total plexopathy with Horner syndrome. At present, most
centers throughout the world agree that an infant with a flail
extremity and Horner syndrome should have microsurgical reconstruction
between 1 and 3 months of life. A child with complete absence of
upper-trunk function (shoulder abduction, elbow flexion) should have
surgery between 3 and 6 months of life. However, the controversy
regarding the best timing for microsurgery, whether it should be done
at 3, 4, 5, 6 or even 9 months, is still unresolved. This creates
difficulties for parents who are trying to do what is best for their
infants. There is an ongoing prospective study sponsored by the
Pediatric Orthopedic Society of North America (POSNA) which hopes to
resolve this issue. Microsurgery involves resection of the neuroma
and
bypass nerve grafting or nerve transfer procedures. On the basis of the
information published in peer-reviewed journals, there seems to be no
role for neurolysis alone in a patient at any age, especially in the
infant older than 6 months of age. The recommended surgical technique
involves exploration of the brachial plexus and reconstruction of
avulsion and nonconducting rupture injuries. If the proximal trunk or
nerve roots are intact, sural nerve grafting across the neuroma is
preferred. In the presence of an avulsion, intercostal and spinal
accessory nerve transfers are performed. The surgery will not restore
normal function, but there is improvement when compared to natural
history outcome alone (67,68).
The controversy regarding the exact timing of intervention (e.g.,
whether to intervene at 3 months of age or at 6 months of age in an
infant with no upper-trunk function) may not be resolved without a
prospective, randomized study.
external rotation weakness and internal rotation contractures about the
shoulder. This muscle imbalance will progressively alter the
glenohumeral joint (65,66) (Table 23.2). Function, especially with the arm in above-horizontal activities, will be impaired (64,66). These children clearly benefit from surgical intervention (66,72). In the situation of dislocation in an infant (65,76,78,79), open reduction and capsulorrhaphy or arthroscopic release and reduction are indicated (Fig. 23.5). Such children have limited external rotation that affects function.
(normal or mild increase in glenoid retroversion, grades I and II) or
slight posterior subluxation (mild, grade III), anterior
musculotendinous lengthening of the pectoralis major and/or
subscapularis muscles and posterior latissimus dorsi and teres major
transfer to the rotator cuff (77,80) will improve function (67).
In addition, dynamic rebalancing of the muscle forces about the
shoulder at a young age has the potential advantages of restoring more
normal anatomy and preventing progressive glenohumeral joint deformity.
Glenohumeral joint remodeling appears to have limited utility with
extraarticular musculotendinous rebalancing procedures alone. The
benefit of arthroscopic release and reduction, as opposed to open
reduction and stabilization, is still unclear in terms of long-term
remodeling of a dysplastic joint, but both have been shown to be
effective in reducing the humeral head, as verified by postoperative
MRI. In the older child with more established and progressive deformity
of the glenohumeral joint (more severe posterior glenoid flattening,
advanced grade III), development of a false glenoid (grade IV) (Fig. 23.6),
or humeral head dislocation and deformity (grade V), the deterioration
of the joint is usually too advanced to tolerate a soft-tissue
procedure. In these situations, humeral derotation osteotomy is
indicated and will also improve function (67).
|
TABLE
23.2 COMPUTED TOMOGRAPHY/MAGNETIC RESONANCE IMAGING (CT/MRI) CLASSIFICATION OF GLENOHUMERAL DEFORMITY IN CHRONIC BRACHIAL PLEXUS BIRTH PALSIES |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
osteotomy and tendon transfer. These patients are in the middle range
of deformity (grade III). To date, it has been difficult to identify
this small subset of patients preoperatively. Therefore, the transfer
alone is performed initially, and only if the result is suboptimal more
than 1 year later is the secondary-stage osteotomy performed. The role
of glenoid osteotomy, its risks, and its benefits are still being
defined as it relates to grade III and mild grade IV patients.
occur with a permanent Klumpke (C-8 to T1) or mixed brachial plexus
lesion. Contractures, bony deformity, and joint instability are the
result of muscle imbalance in a growing child. In the rare case of a
patient with residual C-8 to T1 neuropathy with recovery of C-5 to C-6
function, the elbow and forearm deformities are secondary to an intact
biceps muscle in the presence of weak or absent triceps, pronator
teres, and pronator quadratus muscles. Progressively, the biceps
creates an elbow flexion and supination deformity from unopposed
muscular activity. Soft-tissue contractures develop, followed by
rotation deformities of the radius and ulna (81). Radial head dislocation may occur (82).
The wrist and hand are often in extreme dorsiflexion because of
unopposed wrist dorsiflexors. In the position of forearm supination,
gravity further exacerbates the dorsiflexion deformity. The patient is
left without use of the hand, and performs bimanual activities using
the volar and ulnar forearm as an assist. Often, shoulder abduction and
internal rotation are required in order to improve assistive function.
Activities that require simultaneous elbow flexion and forearm
pronation, such as dressing, eating, and writing (83),
are significantly limited. In addition, the forearm and hand posture is
a major cosmetic concern to both the patient and the family (84).
supinator to a pronator. This will improve elbow extension and forearm
pronation. Surgically, the biceps tendon is identified as it inserts
into the radial tuberosity. By dissecting lateral to the tendon, the
brachial artery and median nerve are protected. A long Z-plasty of the
tendon is performed from the musculotendinous junction to the insertion
site. The distal attachment of the tendon is rerouted posteriorly
around the radial neck, from medial to lateral. Care must be taken to
stay adjacent to the radial neck so as to avoid injury or compression
of the radial nerve. The distal tendon is reattached to its proximal
counterpart in a lengthened position. This converts the biceps into a
forearm pronator (83,84,85).
 |
|
Figure 23.5
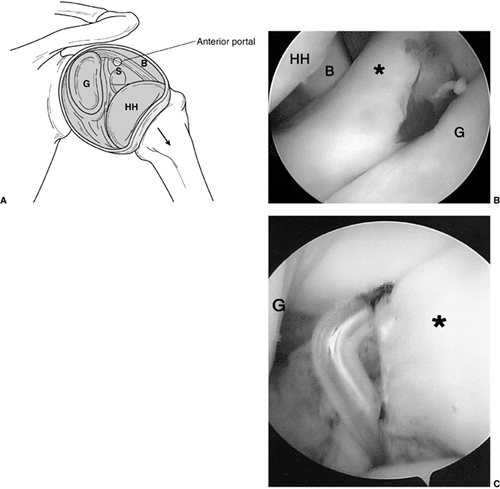
Intraoperative shoulder arthroscopy carried out on a patient with brachial plexus type III deformity for reduction of the glenohumeral joint by release of anterior middle and inferior glenohumeral ligaments, subscapularis. A: Illustration of normal arthroscopic anatomy of the shoulder from the posterior portal. (S, subscapularis, anterior capsule and ligaments; B, biceps; HH, humeral head; G, glenoid.) B: View from the posterior portal showing the thickened anterior ligaments and anterior aspect of the deformed glenoid. (* thickened anterior ligaments capsule and subscapularis; HH, humeral head; B, biceps; G, glenoid.) C: Electrocautery release of the anterior middle and inferior glenohumeral ligaments. A concomitant latissimus dorsi and teres major tendon transfer was perfomed along with posterior capsulorraphy. |
rerouting procedure alone is carried out, it will fail because of
recurrence of the deformity. Zancolli (83)
suggested performing simultaneous interosseous membrane release.
However, active pronation was maintained in only 50% of patients who
underwent this procedure. Bony correction of the forearm deformity can
be performed more predictably. Manske et al. (85) proposed staged procedures of tendon rerouting and forearm osteoclasis. Waters and Simmons (84)
described simultaneous tendon rerouting and osteotomy, using internal
fixation to avoid multiple operations and loss of alignment. In both
techniques, the forearm is positioned in approximately 20 to 30 degrees
of pronation.
their functional capabilities. Bimanual tasks, such as lifting,
carrying, and transferring, are easier. The affected extremity
becomes
a better assistive extremity to the unaffected side. The wrist and hand
now have greater assisted palmar flexion and resolution of their
dorsiflexion deformity. In addition, the patients are usually pleased
with the cosmetic results.
 |
|
Figure 23.6 A:
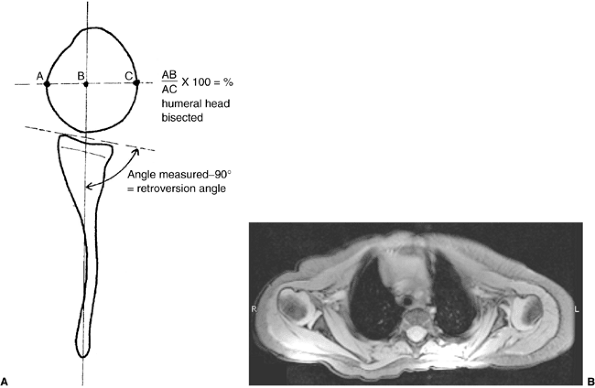
Schematic showing the method of measuring the glenoscapular angle (glenoid vision) and the percentage of posterior subluxation of the humeral head. To measure the glenoscapular angle, a line is drawn parallel to the scapula and a second line is drawn tangential to the joint. The second line connects the anterior and posterior margins of the glenoid.The cartilaginous margins are used on magnetic resonance images. The osseous margins are used on computed tomographic scans. The intersecting line connects the center point of the first line (approximately the middle of the glenoid fossa) and the medial aspect of the scapula. The angle in the posterior medial quadrant is measured with a goniometer (arrow), and 90 degrees is then subtracted from this measurement to determine the glenoid version. The percentage of posterior subluxation is measured by defining the percentage of the humeral head that is anterior to the same scapular line. The greatest circumference of the head is measured as the distance from the scapular line to the anterior portion of the head. This ratio [the distance to the anterior aspect of the humeral head (AB) divided by the circumference of the humeral head (AC), multiplied by 100] is the percentage of subluxation. B: Magnetic resonance imaging of a type IV deformity with posterior humeral head subluxation and the development of a false glenoid. The glenoid is markedly retroverted. The contralateral glenohumeral joint is normal for age. |
unknown cause that presents at birth with contractures of the joints
and muscle weakness (see Chapter 9). The incidence is approximately 1 in 3,000 live births (86).
The clinical syndrome is variable and includes classic arthrogryposis
(amyoplasia), distal arthrogryposis, and syndromic involvement (87).
The classification of arthrogryposis makes the distinction between
myopathic and neuogenic types; however, muscle biopsies and
electromyography have not been shown to be helpful in determining the
mode of therapy for these children (88).
Intelligence is usually average or above average. Sensibility is
normal. Upper-extremity involvement is frequent, with 72% of the 114
patients in the Gibson and Urs study being affected (89).
The wrist was most commonly involved, followed by the hand, elbow, and
shoulder. In the classic presentation, the elbow is usually contracted
in extension at birth. The shoulder is internally rotated with the
forearm pronated. Often, there is wrist palmar flexion and ulnar
deviation, and the fingers have flexion deformities. The thumb is
usually adducted and flexed in the palm (89,90,91).
These children often have incomplete syndactylies of all web spaces.
The first web-space contracture is usually the most functionally
significant. There is usually marked intrinsic muscle weakness. There
may be camptodactyly or symphalangism of the PIP joints. All of this
will limit hand function in these children (92).
passive and active elbow flexion is a significant functional liability
in these children. The goal of orthopaedic management
of
the arthrogrypotic elbow is to improve self-feeding and independent
hygiene skills by achieving both passive and active elbow flexion. The
goal of treatment of the hand is to improve pinch, grasp, and release
functions.
range of motion. Repetitive, gentle, passive manipulation of the
involved joints may progressively lessen the contracture. This process
is tedious and requires meticulous, gentle care by both the therapist
and the family. Corrective splints and serial casts have been used with
varying success. Caution is necessary because of the risk of fractures
or dislocations that can occur as a complication of aggressive
treatment of resistant contractures. At the elbow, the goal of therapy
is to achieve at least 90 degrees of passive elbow flexion by 2 years
of age. Most patients can achieve the desired passive elbow flexion
through therapy (93). However, if this is not obtained, operative posterior elbow release and triceps V-Y lengthening are recommended (93,94,95,96,97).
When passive elbow flexion is obtained, therapy should then emphasize
the use of adaptive trunk sway, head tilt, and table-assisted passive
elbow flexion to improve feeding and hygiene tasks. Finally, if
subsequent active elbow flexion does not develop, active elbow flexion
tendon transfer should be considered at approximately 5 years of age (93). Most of these children will have deficient biceps and brachialis musculature, and will fail to develop active elbow flexion.
passive range of motion and nighttime splinting. The goal of therapy in
the hand is to improve motion of the joints and digital strength.
Fortunately, in many children the condition improves with growth and
therapy during the first several years of life. Serial casting or
splinting is often used for the wrist deformity, but this procedure is
associated with a high rate of recurrence. If passive motion cannot be
improved, surgical releases and tendon transfers may be necessary (98).
achieve a functional arc of flexion at the elbow with manipulative
therapy, splints, and casts, are candidates for operative elbow
posterior capsulotomy and triceps lengthening. Surgery can be performed
when the child is 2 years of age. If it is delayed well beyond this
age, progressive bony deformity can occur. The goal of operative
intervention is to achieve at least 90 degrees of passive elbow
flexion. Initially, the dominant extremity should have surgery. The
presence of passive elbow flexion will improve independence in feeding,
hygiene, play, and school activities (84,93).
the elbow. The triceps tendon is incised in an inverted V. The angle of
the V should be acute enough to allow for appropriate triceps
lengthening. The ulnar nerve is protected during the medial incision of
the tendon. The distal flap of the triceps is elevated from the elbow
capsule, but often the triceps and the capsule are confluent distally.
The triceps lengthening alone usually does not improve passive elbow
flexion. A transverse incision in the elbow capsule is then made. Full
passive elbow flexion is gained. The triceps tendon is lengthened in a
V-Y manner at 90 degrees of flexion.
flexion of greater than 90 degrees and no active elbow flexion are
candidates for tendon transfer. The transfers to be considered include:
(i) triceps (93,94);
(ii) pectoralis major using the sternocostal origin; (iii) pectoralis
major using the entire musculature on a neurovascular pedicle (95); (iv) latissimus dorsi (93,96);
(v) lateral and proximal reinsertion of the flexor-pronator origin;
(vi) sternocleidomastoid with a free tendon graft; and (vii) pectoralis
minor with a free tendon graft. Each of these transfers has been
described in limited series in the arthrogrypotic elbow. Until
recently, no objective criteria had been proposed for comparing the
results of these various transfers (84,93).
The muscle considered for transfer must be expendable and of sufficient
strength to function actively against gravity after transfer. Each
transfer has its inherent negative attributes: triceps transfer may
weaken assistive ambulation in patients with lower-extremity
involvement, and may result in a flexion contracture; pectoralis major
transfer may create asymmetric breast appearance in women; Steindler
flexorplasty may worsen wrist and finger flexion contractures.
Information gained to date indicates that the triceps transfer is most
effective in improving strength, active range of motion, and function (84,93).
arthrogryposis. With transfer, it is usually successful in providing
active elbow flexion in a functional arc. However, the triceps is
important for crutch ambulation, rising from a sitting position, and
wheelchair transfers in patients with lower-extremity involvement and
should be used cautiously for tendon transfer in these children. This
operation involves the transfer of the antagonist to elbow flexion and
leaves the patient without an active elbow extensor postoperatively.
This can lead to progressive elbow flexion deformity with growth (93).
major muscle for elbow flexion. The first choice is transfer of the
sternocostal origin, as originally described by Clark (99).
This transfer can be problematic because the partial transfer may be
too weak to provide antigravity strength for feeding and facial
hygiene. In addition, the pectoralis major muscle crosses the shoulder
and may lose strength in trying to move both the shoulder and the elbow.
by Carroll and Kleinman (95) and Doyle et al. (100).
This operation has had favorable results in limited series of
arthrogrypotic elbows. It involves transferring the insertion of the
pectoralis major to the acromion. The origins of the clavicular and
sternocostal heads, with attached anterior rectus abdominis fascia, are
inserted distally into the proximal radius. The medial and lateral
pectoral nerves and the lateral thoracic vessels are preserved. This
transfer has the mechanical advantage of a linear contraction for elbow
flexion and does not involve the loss of any strength in stabilizing or
moving the shoulder. The proximal advancement of the insertion to the
acromion or clavicle improves the lever arm and mechanical advantage of
the transfer. In addition, the pectoralis minor can be transferred with
the pectoralis major for further strength of transfer. However, it may
create an asymmetric appearance of the breasts in women, and this has
been raised as an argument against transfer (84).
involvement, with weak triceps or pectorals, or with failed pectoralis
major or triceps transfers, a bipolar latissimus dorsi transfer, as
described by Zancolli and Mitre (96), may be
the optimal choice. Preoperative assessment of the strength of the
latissimus is important before transfer, but at times this is difficult
to assess. An experienced pediatric physical therapist with extensive
muscle evaluation experience may be helpful. Biopsy of the muscle has
been tried, but is not predictive of outcome with transfer.
infancy to obtain and maintain passive range of motion of the elbow.
This will frequently result in passive elbow flexion of greater than 90
degrees. If by 24 months of age nearly full passive elbow flexion has
not been achieved, elbow capsulotomy and triceps lengthening are
recommended. After 4 years of age, tendon transfer for elbow flexion in
the dominant arm is recommended, with consideration given to
intelligence, ipsilateral and contralateral upper-limb function,
lower-extremity involvement, and available motors for transfer. All
transfers have had success, but partial or complete triceps-to-biceps
transfer gives the most predictably good results (84,93).
FCU lengthening or transfer to the wrist extensors. Unfortunately, in
many of these children the transfer is more of a tenodesis procedure
than a dynamic transfer. In addition, there is often bony deformity,
even in the very young. Smith (27) had
recommended a proximal row carpectomy to correct the wrist flexion
contracture. However, there are frequently carpal coalitions present
that preclude that procedure. A dorsal, carpal, closing-wedge osteotomy
can correct the deformity in the presence of a carpal coalition. This
is an excellent procedure to correct the bone and wrist joint deformity
that does not respond to therapy (91).
Simultaneous FCU transfer can be performed to rebalance the wrist. An
alternative to carpal osteotomy is a dorsal closing osteotomy of the
radius and ulna dorsal osteotomies (87).
However, these create an “S” deformity to the distal forearm, and
physeal remodeling with growth tends to lead to recurrent wrist flexion
deformity.
Z-plasty syndactyly release. Care must be taken not to overrelease the
adductor, because it may be providing the bulk of the pinch strength.
Dynamic transfers for thumb abduction and extension are predominantly
tenodesis procedures because of the limited strength of the donor
muscles. Many of these children will have permanent limited motion and
strength in their hands. Fortunately, their high level of intelligence
allows them to be very adaptive in their functioning.
condition that may not be diagnosed until school age. It is usually an
isolated condition, but it may be present in association with other
congenital malformations and syndromes, including arthrogryposis and
Cornelia de Lange, Larsen, and nail-patella syndromes (101,102,103,104). It may be associated with radioulnar synostosis (105,106)
or other musculoskeletal anomalies, such as congenital hip dislocation,
club feet, brachydactyly, clinodactyly, tibial fibular synostosis,
congenital below-elbow amputation, and radial or ulnar club hand.
Dislocations associated with Madelung deformity or familial
osteochondromatosis (106) may be acquired, and will be considered elsewhere in this chapter.
It is defined by the direction of subluxation or dislocation. Most
congenital dislocations are posterior or posterolateral. It is
important to distinguish the congenital dislocation from the
posttraumatic dislocation. Because the condition frequently presents
late, this distinction can be confusing (103,107). This is especially true for unilateral anterior dislocations in otherwise healthy children (108,109,110).
Radiographic criteria have been established to distinguish this lesion
from a chronic, traumatic dislocation. These include: a small,
dome-shaped radial head; a hypoplastic capitellum; ulnar bowing with
volar convexity in the anterior dislocation and dorsal convexity in the
posterior dislocation; and a longitudinal axis of the radius that does
not bisect the capitellum. The presence of these characteristics in the
absence of any history of trauma to the affected elbow has been seen as
evidence of a congenital radial head dislocation (84,103,111,112,113,114,115,116).
In addition, bilateral involvement, the presence of other
musculoskeletal or systemic malformations, and a positive family
history make a congenital cause more likely.
after infancy. The most common reasons for presentation are (i) limited
elbow extension; (ii) posterolateral elbow mass/prominence; and (iii)
pain with activities, especially athletics (84,117).
The elbow extension loss is frequently less than 30 degrees, and not of
functional significance. This loss of motion is usually not noted early
in life. The mass may be noted in infancy. Radiocapitellar incongruity
can be a cause of pain and disability later in life (107,117).
Unfortunately, many children present late with pain resulting from
radiocapitellar articular changes. There is often chronic discomfort
with school and sports activities. On occasion, these children may
present with an acute loss of motion attributable to a loose
osteochondral fragment. Some individuals remain asymptomatic, and the
cosmesis of the deformity is their major concern.
valgus. A flexion contracture of up to 30 degrees often occurs with a
posterior subluxation/dislocation. Hyperextension and/or loss of
flexion may occur with an anterior dislocation. The radial head is
palpable in its dislocated position. A congenital dislocation is not
reducible by forceful manipulation, and should not be misinterpreted as
a nursemaid’s pulled elbow or a Monteggia lesion. There is usually
limited forearm rotation, with supination being affected more than
pronation. Clicking and crepitus may be present when there is
intraarticular pathology (84).
The longitudinal axis of the radius does not bisect the capitellum,
regardless of the angle of the radiograph. The radius and ulna are of
different lengths. The ulna is bowed, with volar convexity in an
anterior dislocation and dorsal convexity in the more common posterior
dislocation. The capitellum is hypoplastic. The radial head will be
dome-shaped, with a long, narrow radial neck.
 |
|
Figure 23.7
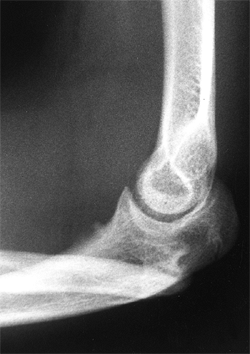
Lateral radiograph of congenital posterolateral dislocation of the radial head. There is evidence of tapering of the radial head and neck posteriorly, bowing of the ulna posteriorly, and a small dome-shaped radial head. These patients often have limited elbow extension and develop intraarticular pain at the abnormal radiocapitellar articulation in adolescence. |
not an indication for operative intervention. Many patients with this
disorder have no functional limitation and no pain. Their mild
limitation of motion may not restrict them in any significant way. The
degree of cubitus valgus is usually mild, and does not seem to put them
at risk for ulnar neuropathy. Therefore, in most cases a definitive
diagnosis followed by observation is most appropriate. If the patient
develops pain, functional or progressive limitation of motion, or
restriction of elbow-related activities, then surgery needs to be
considered (84).
would involve open reduction and restoration of normal anatomy. This
has led many surgeons to consider open reduction of a congenital
dislocation if the child presents in infancy (101,105,111,118).
The logic is that if the radial head can be reduced early in infancy,
the deformity of the capitellum and the forearm may not occur or
remodel with growth. This may prevent the long-term complications of
pain, loss of motion, and osteochondral loose bodies. However, there
have been only a small number of published cases of open reduction of
congenital radial head dislocations (105,118,119).
Techniques have included ulnar osteotomy and lengthening, radial
shortening and osteotomy, annular ligamentous reconstruction, and the
use of limb-lengthening devices to reduce the radial head (115,118,119,120).
Sachar and Mih’s report of open reduction through an anconeus approach,
followed by annular ligament reconstruction, is the most promising
series to date. They described seven cases of open reduction of a
congenitally dislocated radial head with good success (118).
Their operative findings included an abnormality of the annular
ligament that was surgically correctable. The indications for this
procedure, and the age limit, are still being defined in this
relatively rare condition. It is reasonable in specialized centers to
consider open reduction of the congenitally dislocated radial head in
the infant younger than 1 to 2 years of age, provided the family is
well informed of the limited nature of the
information regarding this procedure. Hopefully, clinical surgical
research in this area will define the indications and techniques for
open reduction and annular ligament reconstruction in congenital radial
head dislocations.
present later than infancy. Therefore, the most common procedures for
this problem are excision of loose bodies and excision of the radial
head. The indications for excision of a loose osteochondral fragment
are the presence of
pain,
clicking or locking, and loss of motion. Usually, degenerative changes
are too advanced for repair of the osteochondral fragment. There is
some controversy regarding the indications and timing for excision of
the radial head. In the skeletally immature patient, the concern is the
potential development of postoperative complications (see “Complications,”
below). These concerns have not been supported in the published
literature on excision of the congenitally dislocated radial head. Most
of these children do not present until adolescence with pain or
progressive restriction of motion. In our series, the youngest patient
with excision of a symptomatic congenital radial head without
complication was 8 years of age (107). However,
the presence of an asymptomatic dislocated radial head alone, without
painful, progressive restricted range of motion, is not an indication
for radial head excision. Indications for radial head excision must
include progressive pain, progressive loss of motion, and progressive
restriction of activities (84), regardless of age.
journal articles have denounced the concept of radial head excision in
the skeletally immature individual. Postoperative complications of
progressive cubitus valgus and potential associated ulnar neuropathy,
proximal migration of the radius with recurrent radiocapitellar
impingement, radioulnar synostosis, and reformation of the radial head
have been cited (111,112,121,122,123).
However, most of these problems occurred after radial head excisions to
treat trauma. The admonishment never to excise a radial head in a
skeletally immature individual still holds true in the posttraumatic
situation. These complications are rare after excision for congenital
radial head dislocations (107).
If it leads to recurrent radiocapitellar impingement, limitation of
motion, and/or pain, then repeat radial head excision should be
performed. Wrist pain does occur in the long term but seems to be mild
and nonrestrictive (107). Fortunately, iatrogenic radial nerve injury is rare.
rare. Mead and Martin described a family with aplasia of the trochlea
and humeroulnar dislocations (126).
Ulnotrochlear dislocations have also been seen in hyperelasticity
syndromes. These situations are rarer than the unusual posttraumatic
persistent or recurrent dislocation.
elbow motion that can affect function. The dislocation is usually
palpable on examination. There may be axial malalignment, such as
cubitus valgus. If severe, the valgus deformity can result in ulnar
neuropathy. In recurrent dislocations secondary to hyperelasticity or
associated with syndromes such as Rubinstein-Taybi syndrome (127),
the elbow instability is palpable and even audible on examination. On
occasion, the recurrent instability can lead to osteochondral injury
that will cause pain, clicking, or even locking on examination.
The dysplastic ulnotrochlear joint in ulnar club hand can lead to elbow
problems that limit motion and function. Ulnar dimelia, or mirror hand,
is exceedingly rare. The forearm and elbow in this condition consist of
two ulnae and no radius. This means that there are two olecranon
processes articulating with the distal humerus. There are usually two
poorly defined trochleae and no capitellum present. The olecranon
processes may face one another. There is significant limitation of
elbow and forearm rotation (84,132,133).
secondary centers, it may be difficult to define the dislocation
anatomically by plain radiography. MRI will be diagnostic, but will
require sedation or general anesthesia in infants. Ultrasonography may
be diagnostic in skilled hands (84).
elbow and forearm range of motion and strength, and this will affect
function. They must compensate with shoulder, wrist, or trunk range of
motion to perform recreational activities and activities of daily
living. If left unreduced, chronic arthritic pain could develop.
However, this is not well documented.
secondary to osteochondral injury. This can lead to osteochondral loose
bodies and arthrosis-like pain.
These cases and operations are rare enough that generalized comment is
difficult. The more abnormal the anatomy, the less likely that
operative intervention will be successful.
transposition of the biceps tendon insertion to the coronoid process,
and an anterior bone-block procedure have all been advocated (127,128). The choice or combination of procedures depends on the pathologic anatomy and the degree of instability.
with ulnar dimelia and ulnar club hand that may warrant surgical
reconstruction. Although ulnar dysplasia will be described in more
detail in the section dealing with the wrist, it is worthwhile to
discuss elbow reconstruction in this section. In type II ulnar club
hand there is partial absence of the ulna distally (128,130).
The proximal ulna articulates with the humerus but is usually unstable.
With growth, the radius migrates proximally, leading to progressive
loss of elbow flexion and extension. A supination deformity of the
forearm may develop that limits forearm rotation (130).
In these circumstances, creation of a single-bone forearm may improve
cosmesis, stabilize the forearm, and improve elbow motion (132,128). As described by
Bayne (128),
with this procedure the ulnar anlage is completely excised and the
adjacent ulnar artery and nerve are protected. Radial osteotomy is then
performed proximally. The radius is placed distal to the ulna in an
end-to-end manner. Intramedullary fixation is performed to connect the
proximal ulna to the distal radius. If there is significant bowing of
the radius distal to the osteotomy site, a second osteotomy is
performed with passage of the intramedullary wire. If it is difficult
to attain end-to-end fixation, then side-to-side fusion is acceptable.
Resection of the dislocated proximal radius can be performed
simultaneously or up to 6 months later. If there is any question of
neurovascular compromise, it is advisable to delay the proximal radius
excision (128). At the time of proximal radius excision, the posterior interosseus radial nerve should be exposed and protected.
deformity associated with ulnar dimelia should begin at the elbow with
excision of the lateral olecranon process (132).
Reconstruction of ligamentous structures may be necessary after
excision in order to provide elbow stability. Excision of the lateral
olecranon will reportedly provide improved passive elbow flexion and
extension, but limitation in active elbow flexion may continue because
of deficiencies in the biceps and brachialis musculature. Tendon
transfers for active elbow flexion have reportedly had limited success (132). This condition (and reconstruction) is so rare that in-depth analysis of treatment options is not possible.
differentiation of parts with skeletal involvement. In this section,
congenital radioulnar and elbow synostoses will be discussed.
a rare malformation of the upper limb. It is caused by a failure of the
radius and ulna to separate, usually proximally.
humerus, radius, and ulna are conjoined. Longitudinal segmentation
begins distally. For a time, the proximal ends are united and share a
common perichondrium. Genetic or teratogenic factors that are as yet
unknown may disrupt proximal radioulnar joint development, leading to a
bony synostosis. This represents a type I deformity. If rudimentary
joint development occurs before developmental arrest, a rudimentary
radial head will develop with a less severe degree of coalition. This
is a type II deformity (134).
Failure of formation of the proximal radioulnar joint at this stage of
differentiation will leave the forearm in its fetal position of
pronation. With rare exceptions (136), the forearm is fixed in pronation with congenital radioulnar synostosis (135).
event. There is a 3:2 ratio of boys to girls. Positive family histories
have been reported (102,137,138). It is a bilateral occurrence 80% of the time (139).
The condition is also seen in disorders such as acropolysyndactyly
(Carpenter syndrome), acrocephalosyndactyly (Apert syndrome),
arthrogryposis, acrofacial dysostoses of Najjar and mandibulofacial
dystosis, and Klinefelter syndrome and its variants (140,141).
event, there may be associated anomalies of the musculoskeletal,
cardiovascular, thoracic, gastrointestinal, renal, and central nervous
systems. Cardiac anomalies include tetralogy of Fallot and ventricular
septal defects. Thoracic anomalies include hypoplasia of the first and
second ribs and the pectoral musculature. Renal anomalies involve
anatomic malformations that can be screened by ultrasonography. In the
central nervous system, associated problems include microcephaly,
hydrocephalus, encephalocele, mental retardation, delay in attaining
developmental milestones, and hemiplegia. Musculoskeletal problems
include clubfeet, dislocated hips, polydactyly, syndactyly, and
Madelung deformity (84,105,139,142).
functional deficit. Generally, the degree of fixed forearm pronation
determines the disability and the age of presentation. The presence of
bilateral synostosis in marked pronation significantly limits function,
and leads to an earlier presentation. Most children will present for
evaluation by school age. Radioulnar synostosis is often first noted by
a teacher or daycare worker when comparing the affected child with
peers performing the same tasks (84).
difficulty in holding or using small objects such as spoons or pencils;
(ii) inability to dress owing to poor manipulation of belt buckles or
buttons; (iii) backhanded positioning when holding objects such as
bottles or toys; and (iv) difficulty competing in sports requiring
upper-extremity dexterity. Feeding and accepting objects with an open
palm are often difficult (84,139).
normal carrying angle and has a flexion deformity. The flexion
contracture is usually minimal. Shortening of the forearm is more
apparent in unilateral cases. Rotational hypermobility of the wrist
compensates for the lack of forearm rotation (136,138). Despite this ligamentous laxity, patients do not appear to develop symptoms of carpal instability.
approximately 40% of patients presented with pronation of less than 30
degrees, 20% had pronation fixed between 30 and 60 degrees, and 40% had
more than 60 degrees of pronation. Pronation of greater than 60 degrees
is most limiting.
synostosis show anatomic variations from minor radial head deformities
in patients with limited forearm rotation to full synostosis and
absence of the radial head in patients with no rotation (105) (Fig. 23.8).
The more extensive synostoses are usually fixed in more pronounced
pronation. Plain radiographic classifications have distinguished
partial and complete synostoses. In the partial synostosis there is
often a rudimentary radial head present, but it is posteriorly or
posterolaterally subluxated. In the complete synostosis the radial head
is absent, and the proximal radius and ulna are a single bony mass.
There is always an increased anterior bow of the radius. On occasion,
the synostosis can extend into the middiaphysis of the forearm.
forearm rotation and normal radiographs. MRI of the proximal radius and
ulna may reveal a cartilaginous synostosis that has yet to ossify or a
fibrous tether that limits motion (84).
radioulnar synostosis should be observed. Children can often compensate
for lack of forearm rotation if they have (i) synostosis in
neutral-to-mild pronation (less than 60 degrees), (ii) significantly
compensatory radiocarpal and intercarpal wrist rotation, and (iii)
unilateral disease (84). These children present
because they, their parents, and/or their teachers notice them
performing home, school, or recreational tasks differently from their
peers. However, when questioned extensively, it becomes apparent that
they are without pain or functional disability (138).
These children and their families are best served by counseling
regarding the diagnosis and functional issues of their problem, and
reassurance that operative intervention would be unlikely to improve
their condition.
rotation. Many surgical attempts to do so have been tried. Reported
procedures have included division of the bony bridge (135); resection of the synostotic proximal radius to save the bicipital tuberosity, with (143,144,145) and without (146)
muscle interposition; division of the interosseous membrane; and
muscle, fat, fascia, or silastic interposition after synostosis
excision (137,147). All
had limited success at restoring motion. Artificial joint replacement,
with a metallic swivel in the intramedullary canal of the radius
between the supinator and pronator teres, also failed (145). Tagima et al. (147)
reported improved forearm rotation with synostosis takedown, radial
osteotomy, and interposition of either a silastic or a free fascial
lateral arm flap. Intraoperatively, synostosis takedown procedures can
dramatically improve motion, but there is a high incidence of loss of
motion in 6 to 12 months after surgery. At present, the functional gain
does not seem to warrant this surgical intervention.
osteotomy. The goal is to place the hyperpronated hand in a more
functional position. The dominant extremity is given priority in
bilateral cases. It is easiest to perform the osteotomy through the
synostosis distal to the coronoid process. Before the procedure, an
intramedullary ulnar Kirschner wire is placed to maintain control of
the osteotomy. After completion of the osteotomy, the forearm can be
rotated into the desired position of correction and can be held in this
position by either percutaneous pins or external fixation (148).
Generally, patients undergoing derotation osteotomy have a fixed
preoperative position of 60 to 100 degrees of pronation. The final
corrected position is often 0 to 20 degrees of pronation (84). Ogino and Hikino advocated measuring the preoperative
compensatory wrist supination to define the desired operative osteotomy correction (136).
Once this position is achieved a second percutaneous Kirschner wire
transfixes the osteotomy site obliquely, from the proximal ulna to the
distal radius, across the derotated synostosis (Fig. 23.8). Because there is a high risk of compartment syndrome postoperatively (139,149),
it is important to avoid internal fixation that would require a second
operation for removal if neurovascular compromise occurs. Resection of
bone at the synostosis site (136), or dorsal and volar fasciotomies through the operative incision, lessen the risk of compartment syndrome postoperatively (84) and should be performed routinely.
 |
|
Figure 23.8 A:
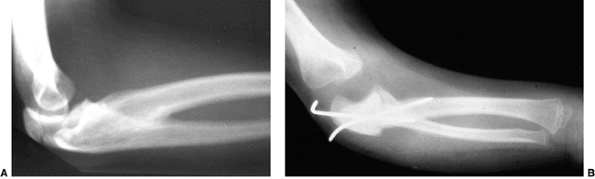
Preoperative radiograph of a congenital radioulnar synostosis. There is complete fusion of the proximal radius and ulna, and posterior dislocation of the radial head. The entire ulna is mildly hypoplastic. B: Postoperative radiograph of a derotation corrective osteotomy for this patient. A longitudinal wire is passed down the medullary canal of the ulna across the synostosis site. This Kirschner wire starts from the proximal ulnar apophysis. The osteotomy cut is performed through the synostosis. The transfixing wire is obliquely placed to secure the corrective derotation to a position of 0 to 20 degrees of pronation. |
noted to show significant improvement in function and cosmesis.
Bimanual tasks are easier. Single-handed tasks, such as holding a fork,
no longer require backhanding in extreme hyperpronation. Activities of
daily living, such as dressing and feeding, are performed more
independently and with less adaptive shoulder and trunk motion.
compartment syndrome. It has been reported in one third of patients
undergoing derotation osteotomy. This is attributable to changes in the
vascularity and volume of the forearm compartments with derotation
osteotomies in the range of 60 to 90 degrees. Compartment syndrome is
more common in osteotomies with greater than 85 degrees of rotational
correction. Prophylactic forearm fasciotomy, or resection of a segment
of synostotic bone, reduces the incidence of this complication. If
compartment syndrome is developing, the compressive dressings should be
removed promptly, and the limb should be placed horizontally at the
level of the heart. Compartment pressure measurements are routinely
performed in the presence of tense compartments in a child with the
clinical appearance of compartment syndrome. In pediatric patients an
increasing analgesia requirement and a high level of anxiety are the
most diagnostic clinical signs of compartment syndrome (150).
Removal of the oblique transfixing Kirschner wire is performed if
removal of dressings and proper elevation fail to improve the
situation. Removal of the oblique Kirschner wire allows the forearm to
rotate to its preoperative position, lessens the tension on the
interosseous vessels, and may reduce the pressure of the forearm
compartments. Finally, if these maneuvers do not resolve the problem,
emergent skin and fascia decompression is mandatory (84,139).
syndrome there is a risk of loss of operative correction. The
longitudinal ulnar Kirschner wire helps maintain control of the
osteotomy site and allows for controlled, repeat derotation 5 to 10
days later. Although more rigid internal fixation may seem more
desirable, it unnecessarily complicates the procedure, especially if
compartment syndrome develops.
in association with syndromic conditions. Humeroradial synostosis is
more common than ulnotrochlear synostosis (151152153).
The elbow flexion may range from 60 to 90 degrees. Often, there is also
limited or no forearm motion. On examination, there will be no elbow
motion.
It has been described in siblings with humeroradial synostosis,
indicating a potential genetic inheritance pattern. It frequently
occurs with phocomelia variants (151). The
limitation of elbow motion limits function, particularly if the
affected extremity is dominant. The placement of a functional hand in
space is limited by the lack of flexion-extension at the elbow.
Compensatory trunk, head, and shoulder motion is difficult to adapt.
Associated hand anomalies can further limit function (84).
motion have had minimal success. Techniques have included excision with
muscle, fat, silastic interposition, or distraction arthroplasties.
Although intraoperatively the motion can be improved, recurrence of the
synostosis usually develops postoperatively. The use of continuous
passive motion devices or distraction elbow hinge devices has not
improved results (84). If the ankylosis leads
to dysfunctional positioning of the hand in space, such as in the
presence of an ulnar clubhand, corrective osteotomy is indicated. Most
often, this is a derotation osteotomy at the level of the synostosis (151).
Correction of a marked flexion deformity acutely increases the risk of
neurovascular compromise. There is no role for total elbow
arthroplasty, because of the possibility of early mechanical failure (84).
hereditary osteochondromatosis, with between 30% and 60% of patients
affected in various series (154,155,156).
The most frequent problem seems to be distal ulnar osteochondroma,
which selectively slows the growth of the ulna in the presence of
continued radial growth. The resultant relative shortening of the ulna
can lead to progressive bowing of the radius and/or possible radial
head dislocation. At the wrist there is increased radial angulation of
the distal epiphysis, with ulnar deviation of the hand and ulnar
translocation of the carpus (156,157,158,159).
These deformities can lead to progressive loss of forearm rotation. If
radial head dislocation occurs, loss of elbow motion can occur and pain
may develop. This section focuses on the treatment of ulnar shortening,
progressive radial bowing, and radial head subluxation. The principles
outlined here for osteochondromatosis have also been used in congenital
syndromes with
forearm growth discrepancies, such as Conradi and Morquio syndromes (84).
with deformity secondary to osteochondromatosis of the upper extremity.
There is ample information on the indications for, and the results of,
surgical excision of osteochondromas and forearm reconstruction for
these patients with deformities (160,161,162). The Shriners group in St. Louis (163)
attempted to obtain natural history data by surveying their patients by
telephone. Their data suggest that adults with forearm, wrist, and hand
deformities from osteochondromatosis do reasonably well with activities
of daily living and occupational tasks. Unfortunately, their data were
limited because they could not reach many of their patients, and no
patients were examined.
indication for surgical excision. Excision of an osteochondroma will
not predictably improve growth or prevent recurrence with growth.
However, if the osteochondroma is a source of pain, limitation of
motion, or neurovascular or muscular impingement, then excision is
indicated. In addition, children with forearm osteochondromatosis may
present with progressive deformity, loss of pronation and supination,
and wrist or elbow pain related to joint subluxation. The limitations
of forearm rotation may be caused by impingement of osteochondromas
distally or proximally. When the loss of motion is secondary to
impingement alone, rotation will improve with osteochondroma excision (156,157).
In the presence of progressive forearm deformity, loss of rotation may
also be related to bony malalignment, proximal radial head subluxation,
or distal radioulnar joint dislocation. In these situations, rotation
and radiocapitellar alignment can be improved by corrective radial
osteotomy and ulnar lengthening (160). In the
presence of radial head dislocation, reconstruction is very difficult.
Attempts at reduction of the radial head by osteotomy or distraction
lengthening techniques have had limited long-term success. Radial head
excision has been advocated after skeletal maturity (161,162).
The creation of a single-bone forearm may be the necessary salvage
procedure in this complex deformity, and this can be performed at a
very young age with successful results (156,162,164,165).
either progressive deformity that limits motion or radiocapitellar
joint instability. The indications, specifically in terms of deformity,
are ulnar shortening by more than 1.5 cm, increasing distal radial
articular angle greater than 30 degrees, ulnar carpal translocation
greater than 60%, progressive radial bowing, and radial head
subluxation (160). The key to managing
radiocapitellar instability is to treat it before frank dislocation
occurs. Once the radial head is dislocated, obtaining and maintaining
reduction is difficult.
osteochondromatosis can be treated with a single-stage operative
correction. The ulnar shortening is addressed by simultaneous excision
of the osteochondroma and Z-lengthening of the ulna. After the
Z-osteotomy, distraction lengthening is carried out intraoperatively
with an external fixator. When the desired lengthening is achieved, a
plate is applied to maintain the length until bony healing is complete (84) (Fig. 23.9).
This lengthening technique is, in essence, a rebalancing of the forearm
skeleton. It realigns the proximal and distal radioulnar joints. In one
series, lengthenings of 1 to 2.3 cm, leading to a neutral ulnar
variance at the wrist, were obtained in a single stage (160). In most patients forearm rotation was improved by an average of 40 degrees (84,160). Results indicate improved range of motion and function with minimal risk of complications.
The option then is to perform serial lengthenings or gradual
distraction osteoclasis. Up to 13 cm of length has been obtained by
distraction techniques (166,167).
However, the rate of complications with distraction osteoclasis in the
forearm has been cited as between 60% and 100%. Therefore, forearm
lengthenings by distraction techniques should be performed cautiously
by those skilled in the technique. The techniques available for
distraction lengthening include unilateral external fixation frames (169,170,171), classic Ilizarov technique (170), and hybrid fixation using transverse Ilizarov wires fixed at 90 degrees to half pins (168,172).
Most surgeons performing distraction lengthening now use a hybrid
technique so as to lessen the risk of neurovascular and muscle
entrapment complications (166,167,168,172). The fixator
is preassembled as part of preoperative planning, with a half ring
proximally and a full ring distally. In situations requiring angular
correction, appropriate hinges need to be applied in order to obtain
correction. Because each case is unique, the specifics of application
are difficult to address in a review such as this. However, certain
principles need to be adhered to. The pins need to be placed in the
safe zone so as to lessen the risk of complications. Passive digital
flexion and extension need to be at the full range intraoperatively
after pin placement to ensure postoperative maintenance of motion. The
preferred site for corticotomy is the proximal ulna metaphysis to
enhance regeneration of bone (168).
Lengthening begins 3 to 5 days after surgery and progresses at a rate
of 1 mm per day, usually with an advance of 0.25 mm four times per day.
Maintenance of passive and active range of motion of the shoulder,
elbow, and digits is critical. Clearly, the loss of hand function is
not worth the advantage of increased forearm length. Prevention and
treatment of expected pin-track infection require meticulous pin care
and liberal use of oral antibiotics. After the desired lengthening is
achieved, the external fixator is left in place until there is
sufficient regenerate bone to allow removal without the risk of
fracture. In general, the fixator is left in place for at least twice
the time necessary to obtain lengthening (84).
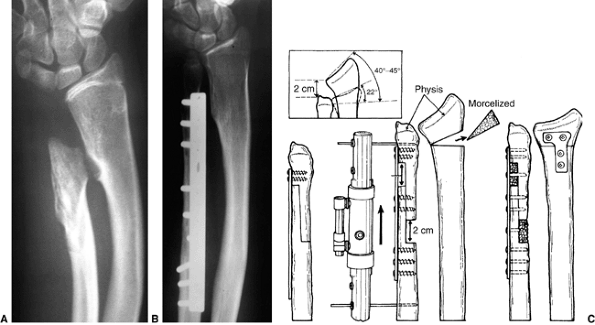 |
|
Figure 23.9 A:
Preoperative radiograph of a patient with osteochondromatosis, ulnar shortening, and mild radial deformity, with recent progressive loss of forearm rotation. B: Postoperative radiograph of single-stage lengthening of the ulna and radial osteotomy. C: Illustration of the lengthening technique. |
distraction technique has been used in an attempt to reduce the radial
head before correcting the forearm deformity (120,168,172).
A separate ring and olive wire are placed in the proximal radius.
Progressive distal migration of the radial head has been used for
radiocapitellar reduction. Once the radial head is reduced, the forearm
correction is performed as described in the preceding text. However,
recurrent subluxation, stiffness of the joint, and pain have occurred
after radial head reduction (84). The limited success of this procedure in this situation does not seem to warrant its use.
either painful radial head dislocation or radius and ulna instability
that is not salvageable by other means. In these circumstances, it can
result in a stable, pain-free extremity (161).
Radial head excision is performed to decompress the radiocapitellar
joint and improve the range of motion of the elbow. Correction of the
deformities of the radius and ulna is performed at the same time as the
radioulnar synostosis with internal fixation and bone grafting. Some
increased length can be achieved with the single-stage procedure. The
distal ulna and proximal radial resected bone are utilized as bone
graft. Neutral rotation to 20 degrees of pronation is desired. Although
this procedure is rarely indicated, these patients have excellent
long-term results (161).
summarized the cases of forearm pseudarthrosis in the medical
literature, and noted that, according to the published papers, 5% of
patients with neurofibromatosis have pseudarthrosis of the upper or
lower limb, whereas more than 50% of patients with congenital
pseudarthrosis of the forearm have definitive neurofibromatosis,
multiple café-au-lait spots, or a positive family history of
neurofibromatosis. Congenital pseudarthrosis is most often seen in the
tibia, but it has been described in all the long bones.
the radius alone in 15 cases, and both ulna and radius were involved in
11 cases. Twenty-three of these patients had either neurofibromatosis
(18 patients) or a positive family history of neurofibromatosis (5
patients). Reports of this disorder range from a single case to up to 6
patients (174).
treatment options for this problem outline the difficulty of obtaining
union with conventional cast immobilization or corticocancellous
autografting or allografting, with and without internal fixation
techniques. The role of distraction lengthening techniques for
congenital pseudarthrosis of the forearm is unclear. There are several
reports of the use of vascularized fibular grafts (174,175) to heal the
pseudarthrosis. These reports indicate a high rate of union when
vascularized fibular transfer is carried out. This is the preferred
treatment for this disorder at present. In the forearm, the fibula is
internally fixed to the proximal ulna. Distally, the fibula is secured
either to the soft tissues of the distal radioulnar joint and the
triangular fibrocartilage complex (TFCC) or to the residual distal
ulna, provided it is not too dysplastic and dysvascular. The vascular
anastomosis is end-to-side in relation to the ulnar artery. At the
donor site of a skeletally immature patient, the distal fibula is fixed
to the tibia so as to prevent valgus ankle instability after harvesting
a vascularized fibular graft (173,176).
The proximal fibular epiphysis can be transferred in the young patient
to allow for growth. In this situation, the separate vascular supplies
to the fibular diaphysis and epiphysis need to be preserved and
maintained with the transfer and vascular anastomosis. The soft-tissue
support of the lateral knee must be reconstructed.
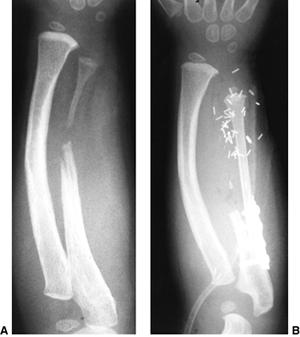 |
|
Figure 23.10 A: Preoperative radiograph of congenital pseudarthrosis of the ulna. Note the hypoplasia and tapering of the distal ulna. B:
Postoperative radiograph of the vascularized fibular transfer, with proximal epiphyseal and physeal transfer, to establish distal ulnar growth. If the patient is very young, the microvascular transfer must include revascularization of both the diaphysis and epiphysis of the fibula so as to obtain physeal growth. The most distal metallic clip indicates the top of the fibular epiphysis in the reconstructed distal radioulnar joint. |
The underdevelopment, or aplasia, of the radius is universally
associated with hypoplasia or absence of the thumb and of the radial
aspect of the carpus (179). The radial or preaxial deficiency has been classified by Bayne and Klug (177)
into four types, ranging from a present but defective distal radial
epiphysis (type I), to complete absence of the radius (type IV). James
et al. modified this classification system to include type N, which has
normal radial and carpal length thumb hypoplasia, and type O, which has
normal radial length but carpal abnormalities (180).
The severity of the radial deficiency determines the extent of the
associated deficiencies of the thumb, digits, ulna, and elbow (Fig. 23.11).
Therefore, the wide spectrum of anatomic deficiency includes mild
radial deviation of the wrist and minimal thumb hypoplasia; complete
absence of the thumb and radius; camptodactyly of the index, long, and
ring fingers; foreshortening of the ulna; and a stiff elbow.
radius is unknown. It has been postulated that injury to the apical
ectodermal ridge during upper limb development is the cause (7,148).
Factors such as intrauterine compression, an inflammatory process,
vascular insult, maternal drug exposure (thalidomide, insulin), and
irradiation have all been raised as possible etiologic causes (1).
There is no known genetic cause except when radial deficiency is
associated with other congenital abnormalities. The pattern of
inheritance is autosomal dominant or autosomal recessive, depending on
the syndrome. There are many associated congenital syndromes, including
cardiac, craniofacial, hematopoietic, musculoskeletal,
gastrointestinal, and renal organ syndromes. There are associated
chromosomal abnormalities, including trisomy 13, 18, and 21. The
occurrence is most often sporadic.
isolation, it is commonly associated with other congenital
malformations. Forty percent of patients with unilateral radial club
hand and 27% of patients with bilateral radial club hand have
associated malformations (181). It is
imperative that these problems be assessed by clinical, radiographic,
and laboratory evaluations as appropriate. These organ system
malformations may present in a nonsyndromic pattern. Congenital
cardiac, genitourinary, respiratory, skeletal, and neurologic problems
occur in children with radial dysplasia. Similarly, many syndromes have
been described in association with longitudinal deficiency of the
radius. The most common
are
Holt-Oram syndrome, Fanconi anemia, thrombocytopenia with absent
radius, and the “VACTERLs” syndromes of abnormal vertebrae, anus,
cardiovascular tree, trachea, renal system, and limb buds. (The classic
“VATER” syndrome comprised vertebral anomalies, anal atresia,
tracheoesophageal fistula, and renal anomalies.) (182,183,184). Holt-Oram syndrome is an autosomal dominant disease characterized by upper-limb malformations and major
cardiac malformations (185).
The gene for Holt-Oram syndrome has been identified. Fanconi anemia
also has an autosomal dominant inheritance pattern. In infancy, there
are usually characteristic facial features (microphthalmos, strabismus,
hearing deficits) (186). Pancytopenia often
does not present until later in childhood. Fanconi anemia can be
identified by a mitomycin C test, but it is now advocated that all
infants with radial-sided defects be assessed early for Fanconi anemia
with the diepoxybutane (DEB) test, since a delay in diagnosis can have
serious consequences (187). Thrombocytopenia
with absent radius is also from autosomal recessive inheritance. The
thrombocytopenia is present at birth. The platelet count usually
improves with growth, and hand surgery should be delayed until it is
safe (186,188).
 |
|
Figure 23.11 Classification of radial dysplasia types I through IV as represented by radiographs. A: In type I, the ulnar variance is positive as a result of the foreshortened distal radius. B: In type II, both the proximal and distal radial physes have deficient growth, with more radial shortening and ulnar bowing. C: In type III, the radius is partially absent. D: In type IV, the radius is completely absent.
|
have tried to clarify the spectrum of clinical deformity by classifying
radial club hands from type I to type IV. Type IV deficiency is the
most common. Type I deformity involves a defective distal radial
physis. This leads to a minor foreshortening of the radius and a
prominent distal ulna. Although there is mild radial deviation of the
wrist throughout life, problems with radioulnar incongruity, such as
triangular fibrocartilage tears, ulnocarpal impaction syndrome, and
distal radioulnar joint pain or loss of motion, usually do not occur.
The major clinical issue is the associated thumb hypoplasia with
opposition weakness. Type II deficiency involves limited proximal and
distal radial physeal growth. As a consequence, the wrist is more
radially deviated, and the ulna bows. The thumb hypoplasia is usually
more significant, with more deficiency of the radial carpus. Type III
deficiency is the absence of the distal two thirds of the radius. The
wrist is more severely deviated, and the hand has limited mechanical
support. The ulna is thickened and bowed. The associated thumb and
finger abnormalities of hypoplasia and camptodactyly are more common
and severe. Type IV deficiency involves complete absence of the radius.
The ulnar bowing is marked. The thumb is usually absent. The index,
long, and even ring fingers are often involved. The elbow may have
limited range of motion. There is marked limitation of hand, wrist, and
forearm function.
soft-tissue deficiencies on the preaxial or radial side of the hand,
wrist, and forearm. The severity of the soft-tissue loss parallels the
skeletal deficiency. The preaxial muscles arise from the lateral
epicondyle, and are normally innervated by the radial nerve. Therefore,
the radial wrist extensors and brachioradialis are either absent or
deficient. The pronator-flexor muscle mass is affected when its
skeletal insertion sites are absent or hypoplastic. These structures
may consist of only fibrous tissue (radial anlage) that maintains or
worsens the deformity of the wrist and hand with growth. Similarly, the
neurovascular structures will be affected. The posterior interosseous
and sensory branches of the radial nerve will be absent in a severe
deformity. The radial artery is usually absent. The ulnar nerve and
artery are usually present and unaffected. The blood supply to the hand
comes through the ulnar artery, and at times the interosseous vessels
or a persistent median artery. The median nerve is usually present and
serves as a neural supply to the hand along with the ulnar nerve.
However, the more severe the deformity of the hand and wrist, the more
limited the neural and vascular supply will be to the hand.
radius have an unaffected central nervous system. As with any
congenital upper limb malformation, children’s creative minds allow
them to perform all activities of life. However, they may need to use
adaptive mechanisms. These generally include a spherical grip and
lateral pinch to compensate for the absence of opposition (190).
Fifty to sixty-two percent of patients with radial dysplasia have
unilateral involvement. Even with severe unilateral radial dysplasia,
these children will adapt their skills by increasing their use of the
contralateral, normal hand and upper limb. Lamb (191)
noted no functional impairment in patients with unilateral involvement.
Individuals with bilateral involvement have more difficulty. Activities
of daily living, such as hygiene, eating, and dressing, are affected.
Adaptive techniques and alteration of clothes are often necessary.
However, as Bora et al. (192,193)
reported, despite these adaptive modifications, patients without
surgical correction were more limited than surgically treated patients.
Finally, the issue of the cosmetic and psychological impact of radial
dysplasia is difficult to quantify. The social setting is constantly
changing, and peer perception plays a major role in an involved
individual’s self-perception. Parental and family perception and coping
will surely have a profound effect on a developing child’s self-image.
There is limited objective psychologic information at present.
radial dysplasia: (i) unstable wrist with lack of support for the hand;
(ii) digital weakness secondary to radially deviated wrist; (iii)
intrinsic digital weakness and deformity; (iv) thumb hypoplasia or
aplasia that results in lack of opposition; and (v) foreshortened ulna.
All of these deformities affect function in the patient with radial
dysplasia. In addition, there are significant cosmetic deformities in
these children that can be improved by intervention.
children are corrective casting, bracing, and physical therapy. In
infancy, the first goal is to achieve passive correction of the radial
deviation deformity. In
mild
cases, this involves merely a home exercise program of wrist ulnar
deviation, extension, and distraction stretching. In more severe cases,
care involves corrective casting or splinting to gradually stretch the
contracted soft tissues, and then maintain the correction. The splints
are used in conjunction with a passive range-of-motion program. If
attempts to correct the static radial deviation contracture are not
successful within 6 to 12 weeks of vigorous therapy and skilled bracing
or casting, the use of distraction external fixation to obtain
soft-tissue and musculoskeletal alignment should be considered (194).
to maintain the correction. Again, in mild forms of the condition, this
can be done nonsurgically. A nighttime corrective splinting program
during infancy and times of rapid growth is useful. This treatment is
adequate for most type I and type II malformations. In severe cases,
the lack of a stable wrist out of the splint impairs hand and limb
function. These children are candidates for operative correction at 6
to 12 months of age.
and functionally limiting thumb deficiency. The surgical options for
the wrist contracture and lack of support for the hand have included
bone graft procedures to the ulna, centralization, radialization, and
wrist fusion (195). Surgical options for thumb
aplasia are pollicization and microvascular toe-to-thumb transfer.
Thumb hypoplasia can be surgically corrected with first web-space
deepening, MCP joint stabilization, and opponensplasty tendon transfer.
Less clear indications exist for the surgical treatment of digital
camptodactyly, ulnar foreshortening, and radial hypoplasia in type II
deformities.
 |
|
Figure 23.12
A patient with complete absence of the radius and thumb aplasia. Note the foreshortening of the forearm, 90-degree radial deviation at the wrist, and dimpling of the skin over the distal ulna, with the proximal and radially subluxated carpus and hand. |
lack of elbow flexion, such that the wrist deviation enables the
patient to perform hand-to-mouth and hand-to-neck activities; (ii)
severe index digital deformity and weakness that will result in failed
pollicization; and (iii) severe medical problems that pose a risk to
the patient’s well-being.
dysplasia involved improving the radial deviation deformity and lack of
wrist support for the hand by grafting bone to the ulna. Albee (196), Starr (197), Entin (198), and Riordan (199)
described the use of nonvascularized bone grafts from the proximal
fibula to the ulna in Y-configuration to support the carpus and hand.
These procedures resulted in significant short-term improvement.
However, the transplant usually failed to grow, leading to recurrent
deformity. Vascularized bone grafting has recently been advocated by
Vilkki (200), in rare circumstances. Centralization of the carpus over the third metacarpal has been a standard treatment (191,192,193).
Soft-tissue release of the radial contracture, contouring of the ulna
to match the carpus, and capsular reefing are performed. Stabilization
is performed with pin fixation until healing is achieved. The problem
with centralization is a high incidence of recurrence. Lamb (191)
advocated modifying the technique by notching the carpus to inset the
distal ulna. This lessened recurrence, but also decreased wrist motion
and increased early ulnar physeal closure postoperatively. Function is
clearly impaired when there is less than 30 degrees of wrist motion
postoperatively. Buck-Gramcko (201) introduced
radialization during the thalidomide crisis. The centralization
procedure is modified by aligning the ulna with the second metacarpal.
Tendon transfers from the radial aspect of the wrist (extensor carpi
radialis and flexor carpi radialis, if present) to the dorsal, ulnar
wrist are performed in order to rebalance the wrist and hand. The
quality of the radial muscles clearly affects the success of the
radialization procedure. With both centralization and radialization,
correction is performed at the wrist. If there is a concomitant ulnar
bow of greater than 30 degrees, ulnar osteotomy should also be
performed. This usually involves a multiple-level open osteotomy and
intramedullary fixation.
the wrist is not possible by splinting, casting, or therapy,
distraction and correction with an external fixator is performed. As
described by Kessler (194), this can be performed in infancy (Fig. 23.13).
Often, after 3 to 6 weeks of external fixation an open centralization
or radialization procedure is performed. Wrist fusion is not performed
in young patients, because this leads to loss of wrist motion and
potential loss of ulnar physeal growth. However,
Catagni et al. (202)
performed wrist fusion in conjunction with distraction lengthening in
adolescent and young adult patients with recurrent deformity.
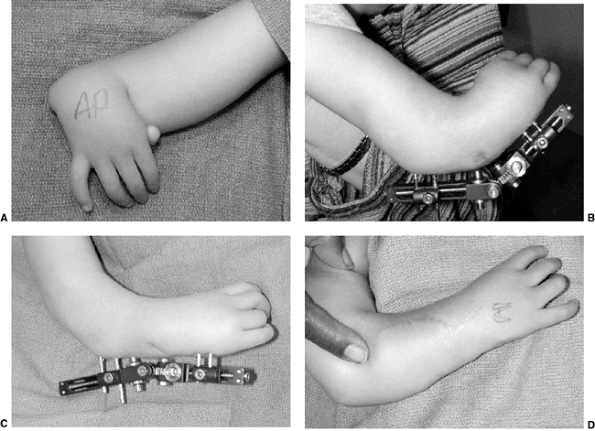 |
|
Figure 23.13
Clinical photographs of a patient undergoing progressive distraction lengthening to stretch the soft tissues, bring the hand out to length, and reduce the wrist over the ulna. This patient has a markedly foreshortened forearm. Preoperatively, there was volar, radial, and proximal subluxation of the carpus and hand that was not correctable with exercises and splinting. A–D: Preoperative (A), early postoperative after fixator application (B), after correction just before fixator removal (C), and final result after centralization (D). (Case and illustrations courtesy of Dr Allan Peljovich.) |
thumb reconstruction. Ideally, thumb reconstruction should be performed
before the child is 18 months of age, because the learning ability for
the pinch movement becomes limited once the central nervous system
matures to this stage. In mild forms of radial dysplasia, the thumb
hypoplasia causes functional problems involving decreased first web
space, MCP joint instability, and weak thenar muscles. The first web
space can be deepened with Z-plasties or rotation flaps (203,204).
Release of adductor and first dorsal interosseous fascia is often
necessary. The MCP joint can be stabilized with local fascia or use of
extra flexor digitorum superficialis tendon length for ligament
reconstruction. On occasion, MCP joint chondrodesis (fusion of the
proximal phalanx epiphysis to the metacarpal head) or arthrodesis is
appropriate. Opponensplasty is performed simultaneously with use of the
abductor digiti quinti (205), ring-finger flexor digitorum superficialis (94),
or accessory digital extensors. All have had reported success in
providing opposition strength. Thumb aplasia is best addressed with
pollicization (204,206).
Toe-to-thumb microvascular transfers have been reported, but to date
the results are less successful than those of index-finger
pollicization. Overall, the quality of the index-finger donor
determines the quality of the subsequent thumb. If there is significant
camptodactyly, the thumb will be stiffer, weaker, and less often used
in pinch activities than if the index has full passive mobility and
intrinsic and extrinsic strength. In a well-performed pollicization,
the results are functionally and cosmetically pleasing to the patient,
family, and surgeon.
ulnar physis are the two major complications of wrist reconstruction.
The occurrence of these problems
depends
on the procedure performed (centralization versus radialization) and
the quality of the preoperative musculoskeletal and soft-tissue
anatomy. With radialization, the goal is to dynamically rebalance the
wrist and maintain motion. If this fails to occur, radial deviation and
flexion deformity will recur with growth. In addition, if there is
limited elbow flexion, excessive flexion and radial deviation of the
wrist will be used by the patient to compensate while carrying out
activities of daily living such as oral hygiene and feeding. This
contributes to the recurrence rate.
procedures. The forearm is already foreshortened, and this is further
exacerbated by loss of distal growth. Because 70% to 80% of forearm
growth comes from the distal physis, postoperative growth arrest is a
major cosmetic and functional problem.
pollicization procedures can have poorer results in terms of opposition
strength and active range of motion (207). The opposition weakness may be improved by opponensplasty transfer (208,209),
but there should be a strong donor if the procedure is to succeed.
Otherwise, the patient will continue to compensate with lateral digital
pinch on the ulnar side of the hand.
common than either radial or central longitudinal deficiency. It is
classified as a failure of formation of parts. The incidence was found
by Birch-Jensen to be 1 in 100,000 live births (178). Ogden et al. cited a boy-to-girl ratio of 3:2, with only 25% of the patients showing bilateral involvement (210).
It also occurs as a part of rare, identified, inheritable syndromes,
such as ulnar mammary (Schnitzel) syndrome, Klippel-Feil syndrome (215,216),
and some nongenetic syndromes such as Cornelia de Lange syndrome. It is
associated with musculoskeletal system malformations in up to 50% of
cases. Contralateral upper-extremity deficiencies of phocomelia,
transverse arrest, radial deficiency, and aphalangia occur commonly.
Similarly, lower-extremity deficiencies, such as proximal femoral focal
and fibular deficiencies, occur in almost one half of the cases. Unlike
those with radial dysplasia, it is uncommon for patients with ulnar
deficiency to have associated major organ system malformations. Ogino
and Kato’s experimental data may explain this finding (217).
They produced major deficiencies in rat fetuses by injecting the
mothers with the antimetabolite Myleran. The timing of injection during
the gestational period determined the limb malformation produced. For
example, ulnar deficiencies were produced by earlier injections than
were radial deficiencies. Fetuses that had ulnar deficiencies had more
lethal cardiac malformations. This may explain why there are fewer
major organ system malformations and a lower incidence of ulnar
deficiency among live births.
Most clinicians use this system to define and establish treatment plans
for these patients. Type I deficiency is hypoplasia of the ulna. Both
distal and proximal physes of the ulna are present, but decreased in
growth. There is minimal, nonprogressive bowing of the radius, and a
variable presentation of hand malformations. Type II deficiency is the
most common type and involves partial absence of the radius. There is a
fibrous anlage extending from the distal ulna to the carpus. The hand
is ulnarly deviated, with bowing of the radius, and these deformities
may be progressive with growth. The elbow is stable if there is
sufficient proximal ulna present. Again, digital malformations or
absences are variable. Type III deficiency involves complete absence of
the ulna. There is no ulnar anlage. The radius, wrist, and hand are
usually straight. The elbow is unstable as a result of the lack of an
olecranon. Hand malformations and absences are common. Type IV
deficiency involves synostosis of the distal humerus to the proximal
radius. There is an ulnar anlage present from the distal humerus to the
carpus, with marked bowing of the radius and ulnar deviation of the
hand. Hand anomalies are common also in type IV deformities (Fig. 23.14).
classified ulnar deficiency by the presenting hand malformations. They
divided the disorder into four types according to the deficiency of the
first web space and thumb. Type A is a normal thumb and first web
space. Type B involves a mild web contracture and thumb hypoplasia.
Type C involves marked hypoplasia of the thumb with rotation of the
thumb into the plane of motion of the other digits. Type D is absence
of the thumb. In their series, 73% of the 55 patients had thumb
deficiencies. This was similar to Broudy and Smith’s findings of 16
hypoplastic thumbs and 5 absent thumbs in 26 patients with ulnar club
hand (220).
and usually internally rotated. The glenoid may be dysplastic. The
radial head is often dislocated, and range of motion of the elbow is
limited in up to 40% of cases (221). These
abnormalities make placement of the hand in space difficult. The hand
malformations limit pinch, grasp, and release functions. Reconstructive
surgery is indicated for improving hand and wrist orientation, thumb
opposition, and digital motion and strength.
history of untreated ulnar dysplasia. In 1927, Southwood stated, “From
the functional viewpoint, therefore, the deformed limb is much more
useful than its anatomical condition would lead one to expect” (222).
This malformation is not associated with central nervous system
deficiencies. As with all congenital malformations in individuals with
normal brains, the patients will perform activities well, but
differently. Treatment has to improve function and cosmesis, if it is
to be warranted.
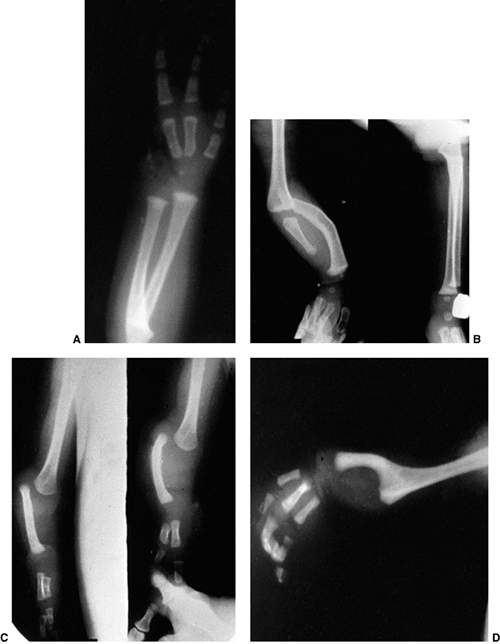 |
|
Figure 23.14 Classification of ulnar dysplasia types I through IV as represented by radiographs. A:
Type I dysplasia represents deficiency of both the proximal and distal ulnar physes, with foreshortening of the ulna and mild bowing of the radius. B: Type II is the most common type and represents partial absence of the radius. C: Type III involves complete absence of the radius with an unstable elbow joint. D: Type IV includes complete absence of the radius and humeroulnar synostosis. |
physical therapy and corrective casting or splinting. In types I and
III deficiencies, the mild ulnar deviation of the wrist and hand may be
improved with serial casting, splinting, and passive exercises starting
in infancy. In types II and IV deficiencies, the ulnar anlage may make
nonsurgical correction of the severe ulnar deviation of the hand and
wrist impossible.
the hand that is severely ulnarly deviated and that does not respond to
casting/splinting. There is limited information to allow for critical
evaluation of the options of (i) leaving
the
patient alone, (ii) performing excision of the ulnar anlage, and (iii)
corrective radial osteotomy. Some of the confusion exists because not
all of these deformities are progressive (223). As Flatt (1)
makes clear, it is difficult to critically evaluate the literature
because of limited objective measurements in previous studies. He
correctly points to the low incidence of this disorder as hampering
objective assessment of the therapeutic options. As with many rare
conditions, only multicenter, prospective studies can definitively
answer the questions.
interpretation of the treatment options, leading to reluctance to
pursue aggressive surgical intervention. Within these limits, an
attempt is made to outline treatment options and recommendations for
wrist deformity, elbow instability, and digital and thumb deficiencies.
progressive ulnar deviation of the wrist and hand of greater than 30
degrees. This can occur in types II and IV deficiencies (1,224).
Through an ulna-based incision, the anlage is identified as it inserts
into the carpus. The ulnar artery and nerve should be protected.
Resection should be performed until neutral positioning of the wrist
can occur intraoperatively. If there is associated marked ulnar
deviation of the radius, concomitant radial osteotomy can be performed.
However, it is imperative to assess the location of the radial head and
the status of forearm rotation before proceeding with anlage excision
and consideration of radial osteotomy.
rotation preoperatively in the type II deformity, anlage excision,
resection of the radial head, and creation of a single-bone forearm
should be carried out simultaneously. If there is acceptable forearm
rotation preoperatively, it is best to correct only the wrist deformity
and to monitor the status of the forearm and elbow with growth.
Resection of the radial head for cosmetic reasons should be performed
cautiously, because even the dislocated head may be providing some
elbow stability in these patients.
in improved cosmesis, but the loss of forearm rotation may cause an
unacceptable loss of function. In patients with type IV deficiency,
there may be associated internal rotation posture to the arm that
limits placement of the hand in space. If this is present, simultaneous
external rotation osteotomy of the limb and ulnar anlage excision
should be performed. This is clearly the case with patients with
bilateral deformity who are unable to reach their mouths preoperatively.
Syndactyly is common and should be corrected in infancy. Thumb
hypoplasia or absence should also be repaired in infancy. Broudy and
Smith (220) described a modified pollicization
procedure for the malpositioned thumb in the plane of motion of the
other fingers. Tendon transfers for intrinsic and extrinsic muscle
deficiencies of the thumb and fingers are indicated if there are
adequate donors available.
described a growth deformity of the distal radius. For reasons that are
still unknown, the volar, ulnar aspect of the distal radial physis
slows or stops growing prematurely. The continued normal growth of both
the ulnar physis and the remaining dorsal, radial aspect of the radial
physis results in ulnar overgrowth, carpal subluxation, and radial
articular deformity (Fig. 23.15). Madelung deformity usually occurs in girls, and is most often bilateral (226).
It may not become clinically apparent until the adolescent growth
spurt, which is when most patients present. It is generally sporadic in
presentation. It is also associated with Leri-Weill syndrome, a
dyschondrosteosis form of mesomelic dwarfism that is inherited in an
autosomal dominant manner (224,227).
In addition, Madelung deformity has been associated with Hurler
mucopolysaccharidosis, Turner syndrome, osteochondromatosis,
achondroplasia, and Ollier disease (228). True Madelung deformity should be distinguished from a posttraumatic or postinfectious deformity.
the age at presentation and the severity of the growth arrest.
Generally, by the time the affected children are brought for treatment,
there is marked deformity, limitation of motion, and activity-related
pain. Because the condition is usually bilateral, the subtle growth
deformity that occurs before the adolescent growth spurt is often
ignored. However, with early presentation there is a slight positive
ulnar variance and loss of the volar, ulnar aspect of the radial lunate
fossa (Fig. 23.15). The carpus subluxates
volarly and into the gap between the radius and the ulna. These
patients may have mild symptoms of ulnocarpal impaction with power grip
activities, and distal radioulnar joint incongruity with forearm
rotation. More often, individuals with Madelung deformity present late
with marked deformity. There is an increased tilt of the radial
articular surface from the dorsal radial corner of the styloid to the
volar, ulnar aspect of the depleted lunate fossa. The ulnar variance is
more positive, with carpal overlap and dorsal subluxation. The carpus
migrates more proximal into the increasing diastasis between the radius
and the ulna on anteroposterior radiographs. These patients have more
pain and limitation of motion, especially forearm rotation and wrist
extension.
previously, the arrest of the volar, radial aspect of the distal radial
physis causes subsequent deformity of the radiocarpal, radioulnar, and
ulnocarpal joints. Vickers and Nielsen (229), Linscheid (230), and Ezaki (226)
have described abnormal tethering of soft tissues from the distal
radius to the carpus and ulna. These have included aberrant ligaments (228,229) and pronator quadratus muscle insertions (230). It is unclear whether these structures are responsible for the growth deformity of the radius. Vickers
and Nielsen’s successful treatment of Madelung deformity by excision of
the volar tethering soft tissues and prophylactic physiolysis of the
volar radial physis indicates that there may be a causal relationship.
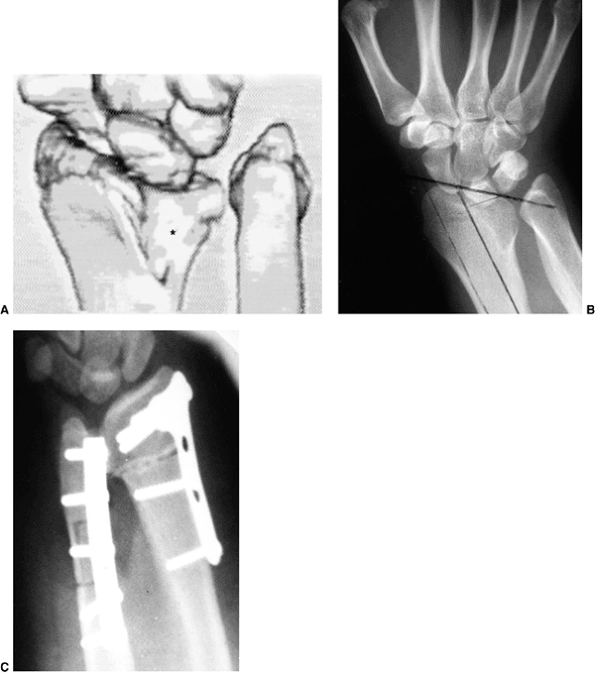 |
|
Figure 23.15
Magnetic resonance imaging (MRI) of severe Madelung deformity. Note that the lunate fossa (*) is markedly deficient and oriented ulnarly. B: Preoperative three-dimensional MRI of a patient with Madelung deformity and debilitating ulnocarpal and radioulnar pain. There is nearly complete deficiency of the lunate fossa and subluxation of the carpus ulnarly, volarly, and proximally. The ulna has a positive variance. C: Postoperative radiograph of a radiodorsal closing-wedge osteotomy of the radius and ulnar Z-shortening osteotomy in this patient. This procedure restored radial articular alignment, corrected the ulnocarpal impaction, and reduced the distal radioulnar joint. |
deformity advocated treatment only for symptomatic patients at skeletal
maturity (231). Originally, the mere presence
of the deformity was not an indication for operative intervention in
the asymptomatic patient, regardless of age. However, the growth
discrepancy is easier to treat if it is dealt with early. Young
patients become symptomatic and the range of function of the limbs
becomes restricted with increasing growth deformity. Vickers and
Nielsen advocated early intervention with physiolysis (229).
The treatment principle is similar to that for Blount disease, with
resection of the abnormal volar, ulnar physeal region of the radius and
fat interposition. At the same time, any aberrant, tethering anatomic
structures are excised. Their case series indicates restoration of
radial growth and prevention of progressive deformity. Some patients
with Madelung deformity can present at a very young age with marked
deformity and complete lack of a lunate fossa for carpal support. In
these patients it is difficult
to
carry out successful reconstruction surgery, and there is a high rate
of recurrent deformity with growth. Combined radial and ulnar
osteotomies are necessary for initial correction. Repeat surgery is not
uncommon. An alternative treatment for the patient presenting early is
to perform ulnar and radial epiphysiodesis in order to prevent
progressive or recurrent deformity and symptoms. In the patient with
bilateral disease, this treatment leads to foreshortened upper limbs
without side-to-side discrepancy.
deformity and symptoms is more problematic. The radial deformity can be
addressed by an osteotomy. Techniques described include a dome
osteotomy, dorsal radial closing-wedge osteotomy, or volar
opening-wedge radial osteotomy and bone grafting. The ulnar overgrowth
is treated with an ulnar-shortening procedure (Fig. 23.16).
Alternative methods of ulnar shortening include resection of the distal
ulna and a Sauve-Kapandji procedure. However, there may already be
significant deterioration of the articular cartilage, wrist ligaments,
or triangular fibrocartilage, resulting in continued pain and
limitation of motion postoperatively.
past as typical or atypical. Since 1992, the International Federation
of Societies of Surgery of the Hand has classified typical cleft hands
as cleft hands and atypical cleft hands as part of symbrachydactyly.
Cleft hands represent a partial or complete longitudinal deficiency in
the central portion of the hand. The elbow, forearm, and wrist are
usually normal. There are often ulnar and radial-sided syndactylies and
digital hypoplasia. Cleft hands often occur in conjunction with cleft
feet. In that situation, there is an autosomal dominant inheritance
pattern. However, the penetrance is variable, with approximately one
third of the known carriers of the gene having no malformations (232,233). Genetic identification has been localized to the p63 gene on chromosome 3q27 (234,235,236).
In addition, the phenotypic expression in affected individuals is
variable. Cleft hands are also associated with other syndromes and
malformations such as cleft lip/palate (ectrodactyly, ectodermal
dysplasia, cleft syndrome), other craniofacial syndromes, Cornelia de
Lange syndrome, congenital heart disease, ocular malformations, and
imperforate anus (233,237). The incidence is estimated at between 1 in 90,000 and 1 in 100,000 live births (178,238,239). Various classification schemes have been used. Most have focused on the nature of the deformities (240,241). Manske and Halikis proposed a classification system of cleft hands based on the thumb and first web space (242). This scheme aids the surgeon in surgical reconstruction decisions and therefore may be the most useful classification.
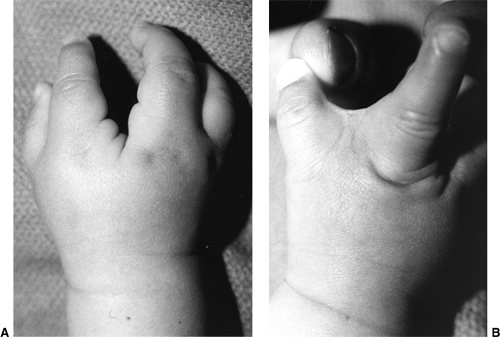 |
|
Figure 23.16 A: Cleft hand with absent middle ray. B:
Incomplete syndactyly of the first web space in the same patient. Closure of the cleft must include deepening of the first web space to maintain maximum hand function. |
digital deficiencies and simple syndactylies. It is a sporadic event
without genetic inheritance. There are no associated anomalies. The
feet are normal. It is a unilateral process, often with multiple absent
digital rays. There are often finger nubbins present, which is a
situation not seen in cleft hands. Symbrachydactyly is a transverse
deficiency that may or may not be a separate entity from transverse
absence of digits (233). These entities are distinct from the amputations associated with constriction band syndrome.
Symbrachydactyly should be viewed as a clinical entity distinct from cleft hand, with a very different treatment plan.
“The cleft hand is a functional triumph and a social disaster.” The
wide central cleft allows for outstanding grasp, release, and pinch
functions. Sensibility is normal. The cleft hand, therefore, functions
usually without limits.
cleft. However, surgical closure of the cleft must be accompanied by
appropriate treatment of the first web space and thumb to avoid
limiting the patient. The skin flaps designed for cleft closure,
however, must take into account the status of the first web space (Fig. 23.16).
If the first web space is normal or mildly narrowed (Manske types I and
IIA), simple cleft closure, such as with a Barsky flap (243),
can be performed. If necessary, a simultaneous but separate Z-plasty
widening of the first web space can be performed (Manske types IIA and
IIB). If the first web space has a marked syndactyly (Manske type III),
the flap designs use the redundant skin of the cleft closure to create
a first web space. The adduction contracture of the thumb is released,
and the index ray is transposed ulnarly at the same time. The
Snow-Littler, Ueba, and Miura flaps (244,245,246)
all involve transposition of the cleft skin to the first web space,
with simultaneous transposition of the index ray ulnarly. If there is a
transverse bone across the cleft, this must be removed in order to
prevent progressive deformity. Often there is a conjoined flexor and
extensor across the base of the cleft that has to be released.
Sometimes carpal closing-wedge osteotomy is necessary to close the
cleft. In addition, the stability of the index- and ring-finger MCP
joints should be maintained or restored. Associated fourth web space
syndactylies are separated with Z-plasties and skin grafts. There may
be associated camptodactyly or clinodactyly of the adjacent digits
requiring corrective splinting or surgery.
is probably the most individualized of that of any of the congenital
malformations. The range of options include (i) leaving the child
alone; (ii) nonvascularized transfers to the soft-tissue nubbins of the
phalanges (247,248); (iii) microvascular toe transfer(s) (249,250,251,252,253); (iv) web-space deepening; (v) digital distraction lengthening or bone grafting (254);
and (vi) use of a prosthetic. In addition, families and patients are
very interested in the possibilities of transplantation and laboratory
cellular growth of digits. There is no definitive answer at present.
The choice is greatly influenced by the family’s desires and the
surgeon’s experience and biases. There are few peer-reviewed published
studies regarding functional and cosmetic outcomes that would guide the
decision more objectively. However, there are clear principles to help
guide all parties as to the best choice for them.
a normal thumb and web space, all of the choices for treatment will
work. In this situation, treatment options focus on the quality of the
other digital rays. If the soft-tissue pockets of the digits are
adequate, nonvascularized transfer of the proximal phalanx of the toes
is a very good choice. Although it will not provide normal digital
length, it will provide stability for lateral pinch. This must be
performed before 18 months of age and include the periosteum and
collateral ligaments (247,255).
The proximal phalanx is harvested through an extensor-tendon-splitting
dorsal approach. The proximal phalanx is harvested extraperiosteally,
while protecting the neighboring tendons and neurovascular structures.
At the metatarsophalangeal joint, the collateral ligaments, dorsal
capsule, and volar plate are detached proximally from the metatarsal,
while leaving intact their distal attachments to the phalanx. At the
PIP joint, those soft tissues are left attached to the middle phalanx.
With transfer to the hand, the proximal soft tissues of the toe phalanx
are sutured to the corresponding soft tissues of the recipient site.
The best results for phalangeal survival and growth are realized when
this procedure is performed before 1 year of age. The quality of the
soft-tissue pocket clearly affects the outcome. Multiple phalangeal
transfers can be performed simultaneously. In the presence of a normal
thumb and first web, digital lengthening can be performed. In addition,
digital lengthening has been performed successfully after
nonvascularized toe phalangeal transfer (254).
Finally, prosthetic use has been tried. The major problems with
prostheses are that children function as well or better without them
because the prostheses are insensate. In the adolescent and adult, a
cosmetic prosthesis may be used for social reasons (240). It should be noted that the finest cosmetic prostheses are very expensive.
the web with release of the adduction contracture is appropriate. At
times, this may require resection or transfer of the index metacarpal (Fig. 23.16)
in order to achieve a useful web for pinch and grasp functions. If
there is absence of the thumb, then digital transposition or
microvascular transfer is indicated.
the patient is a child older than 2 years; the family is well-informed
about all aspects of the surgery and possible outcomes; there are
proximal nerves, vessels, tendons, and muscles available for creating a
viable and functional transfer; there is carpal or metacarpal support
for the transfer; and there is an experienced surgical team (217).
Unfortunately, although this procedure is being performed more commonly
nowadays, objective data regarding functional, cosmetic, and
psychologic outcomes are still minimal in relation to children.
disruption of the inner placental wall, the amnion. This early amnionic
rupture often results in oligohydramnios and amnionic bands. The
fibrous bands from the amnionic wall wrap around the digits, causing
constricting digital bands, amputations, and syndactylies (256,257,258). Streeter (259) was the first to propose that this syndrome is a mechanical deformation rather than a malformation.
It is associated with other musculoskeletal deformations in 50% of
cases, the most common being clubfeet. There may be devastating cleft
lip and facial deformations as a result of deforming amnionic bands.
There are no associated major organ system malformations.
affected. This may be because in a clenched fist, the ring finger is
the longest. The band may merely cause an indentation. However, if it
is circumferential, the constriction ring may lead to distal edema or
cyanosis (Fig. 23.17). Intrauterine amputations
are the result of vascular insufficiency caused by the tourniquetlike
bands. At times, this can be noted at birth with a necrotic or severely
compromised phalanx distal to a constricting band. Syndactyly occurs
when the bands attach adjacent digits (Fig. 23.18).
There are often skin clefts proximal to the syndactyly, indicating the
embryonic formation of a web space before the amnionic rupture and
subsequent deformation. The extremity proximal to the band is normal.
The development of the underlying tendons, nerves, vessels, and muscles
is also normal.
removal of the band to relieve vascular compromise. This is a rare
situation, usually seen only in the neonatal period. Removal of
neonatal constricting bands that are causing vascular compromise can
generally be performed outside the operating room. The band will
literally unravel or debride like an eschar. Improved venous drainage
is almost immediate.
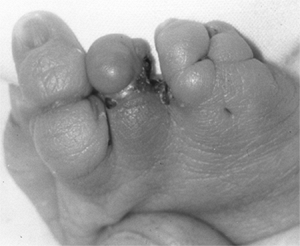 |
|
Figure 23.17
An amnionic band causing digital ischemia in a neonate in the newborn nursery. This condition is rare, and requires immediate removal of the band in order to prevent further soft-tissue digital loss. |
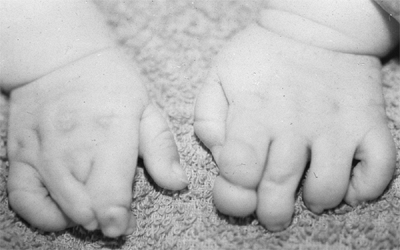 |
|
Figure 23.18
Bilateral amnionic band syndrome with deep constriction rings on the left hand and partial acrosyndactyly and amputations on the right hand. |
compromise or functional or cosmetic problems do not require treatment.
Constricting rings that cause distal deformity are treated with
excision of the constriction ring and staged Z-plasty reconstruction.
Complete excision of the ring is necessary to recontour the digit or
limb. The depth of excision can extend to the periosteum. Such digits
usually have chronic impaired venous outflow with marked distal
swelling. In these situations, it is imperative to preserve distal
venous drainage and the deep neurovascular structures. Careful
dissection of the veins, arteries, and nerves is performed on both
sides of the deep constricting band. These structures are then
delicately freed from the band to preserve their longitudinal
integrity. It is recommended that complete circumferential excision not
be performed in one procedure. Rather, excision up to 270 degrees at
one time may be safest for preservation of vascular inflow and outflow.
Z-plasties are performed after ring excision, so as to prevent
recurrence.
follows the basic principles outlined in the section on syndactyly. The
unique features of amnionic band syndrome are acrosyndactyly secondary
to constricting bands and the presence of epithelialized incomplete
web-space proximal to the syndactyly.
progressive deformity in digits of unequal length, early digital
separation is necessary. More often, the acrosyndactyly separation can
be performed after 6 months of life. There is usually limited skin for
coverage, and creative flap design is needed to cover all the web
spaces. Abundant skin graft is necessary. Distal release of complex
syndactylies may require excision of osseous or cartilaginous
synostoses. The embryologic remnant of the web is usually too distal
and small to
serve
as an acceptable web reconstruction. Excision of that epithelial tract
is usually performed. If that primitive web is used, it often must be
deepened secondarily.
the thumb. The reconstruction can include metacarpal or phalangeal
transposition from the index finger (261,262),
nonvascularized toe phalangeal transfer, or vascularized toe transfer.
It is imperative to reconstruct the thumb for pinch and proper grasp
and release functions in these patients.
central nervous system, these patients have outstanding hand function
after reconstruction. There are clear cosmetic differences, but minimal
functional differences between them and their peers.
Normal differentiation of digits occurs during the fifth to eighth
weeks of gestation. Failure of normal programmed cell death results in syn(together) dactylos(digits). The incidence is between 1 in 2000 and 1 in 2500 live births (1).
It can occur in isolation or as part of a syndromic condition. It is
often an inheritable condition, whether in isolation or as part of a
syndrome. It is bilateral and symmetric in up to 50% of patients. It is
more common in boys than in girls.
starts distally and proceeds proximally. Normally, the third web space
is the most distal web, followed by the second, fourth, and first web
spaces. The normal commissure of the web extends over 30% to 35% of the
length of the proximal phalanx (263). The
bones, joints, tendons, and neurovascular structures separate before
the skin does. If separation fails to occur or ends prematurely,
syndactyly results. If it extends over the entire length of the
phalanges, then it is complete. Complete syndactyly is when the web is
more distal than is normal (Fig. 23.19);
complex syndactyly is when there is osseous connection between the
digits; and simple syndactyly is when the digits are joined by skin
only (263). Acrosyndactyly involves webbing of
the tips of all the digits. Syndactyly can also be a part of other
major developmental problems in the hand that affect hand function,
such as brachydactyly, camptodactyly, clinodactyly, symphalangism, and
polydactyly. These are the most complicated syndactylies in terms of
surgical decisions and care. As noted in the preceding section,
syndactyly secondary to amnionic band syndrome is not a malformation,
but an in utero disruption, and will be considered separately.
hand. It is sequentially less common in the fourth, second, and,
finally, first web space (1). It may be
associated with syndactyly of the toes. In isolated syndactyly, often
one of the parents will have an incomplete syndactyly of the fingers
and/or toes. As mentioned in the preceding text, there are many
chromosomal, craniofacial syndromic, and generalized syndromic
conditions associated with syndactyly. These all need to be evaluated
before treating the syndactyly. The most important aspect of the hand
evaluation for syndactyly is determination of the quality of the
affected digits. In simple syndactyly, these digits are usually normal
except for their skin union. In more complex situations, the digits may
have malalignment, limited motion, and limited strength after surgical
separation. Plain radiographs will reveal osseous union and marked
joint and bony malalignment. However, in infancy, the areas of chondral
abnormalities in the joints, physes, and between digits exhibiting
syndactyly will not be visible on plain radiographs. MRI and
arteriography are
used only in very complex situations to define digital anatomy preoperatively.
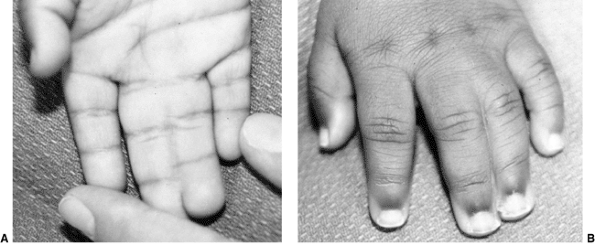 |
|
Figure 23.19 A, B:
A 1-year-old child with complete simple third web-space syndactyly. In this patient, the distal eponychial folds and nail plates are already separate. The underlying joints, tendons, nerves, and blood vessels should be separate and normal. |
undergo surgical separation. If the syndactyly does not extend beyond
the PIP joint, this will not limit function. However, it may affect
wedding ring wear in the third web space, and for this reason, some
patients request separation.
desire separation of the digits for functional and cosmetic reasons.
There are rare situations in which a family declines surgery for
complete syndactyly. Because of the discrepancy in the lengths of the
adjacent digits, this usually results in some degree of bony
malalignment and joint contracture. This is most marked in the border
digit syndactylies (first and fourth web spaces), and least marked in
the third web space. Leaving the digits joined also precludes
independent function of the affected digits. There are also syndromic
and chromosomal situations in which the overall health or mental
capacity of the patient does not warrant the risks of surgical
separation. Finally, there are situations of complex syndactyly in
which the affected digits are too hypoplastic, malaligned, or stiff to
warrant separation. Otherwise, the standard treatment is surgical
separation of the affected digits.
wish, that is, the simple division of the conjoined skin. The uncovered
soft tissues result in linear scars with long-term joint contractures,
digital malalignment, and loss of motion and function (223,264).
Standard treatment now consists of: (i) local rotation flap coverage
for the commissure; (ii) zig-zag incisions, avoiding the interdigital
creases to prevent scar contractures; and (iii) full-thickness skin
grafts to cover all areas of the digits not covered by local flaps (Fig. 23.20). In addition, special attention is given to the eponychial reconstruction with either local flaps or composite grafting (265).
anesthesia and surgical handling of the tissues are safe. There is some
controversy regarding the best age for surgery (1,266) but in most institutions it is performed at approximately 12 months of age (267).
After 6 months of age, the anesthesia risk is equivalent throughout
childhood. With magnification, surgery can be performed safely and
skillfully during infancy. The only controversy concerns surgical
healing and scarring. Neonatal releases result in more scarring. There
is some evidence that surgical release performed at approximately 18
months of age may result in less scarring and recurrent web
contractures than release during infancy (268). However, this is a very difficult developmental age for elective surgical intervention.
has had a higher rate of complications. Border digits of unequal length
need to be separated earlier to lessen angular and rotatory deformity
in the longer digit (Fig. 23.21).
joint, surgery usually involves the use of local flaps such as
double-opposing Z-plasties and “stickman” or “dancing girl” flaps.
Separation may not be to normal depth, but patients often prefer to
avoid skin grafting (269). If the incomplete syndactyly extends to the middle phalangeal region, full-thickness skin grafting is necessary.
of a dorsal rotation flap into the web, Z-plasty flaps the length of
the digits, and full-thickness skin grafts to
cover
the defects. It is important to have a vascularized flap for web skin
reconstruction. This is usually done with a dorsal rectangular flap but
may also involve a dorsal metacarpal island flap (270,271,272).
The fascial connections between the digits extending from Grayson and
Cleland ligaments need to be separated. Any synostosis or synchondrosis
union of the distal phalanges should be divided. Conjoined nails are
divided, and the exposed eponychial and paronychial regions are
reconstructed with local flaps or composite grafts (273,274).
If the common digital nerve extends beyond the desired web deepening,
epineural separation is performed proximally. If the common digital
artery bifurcates distally, ligation of one of the proper digital
arteries may be necessary for obtaining the desired separation. This is
one of the major reasons why surgery is not performed on both sides of
a single digit in multiple syndactylies.
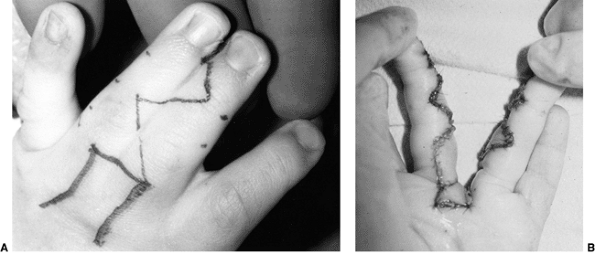 |
|
Figure 23.20 A:
Intraoperative photograph of a 1-year-old child with complete syndactyly treated with dorsal rotation flap coverage and Z-plasties, as outlined. Note the skin marks on the lateral borders of the ring and long fingers to outline the apex and base of each Z-plasty. This allows for precision placement of corresponding volar and dorsal Z-plasties. B: Intraoperative photograph after dorsal-to-volar rotation flap coverage for web space, Z-plasties, and full-thickness skin grafting. |
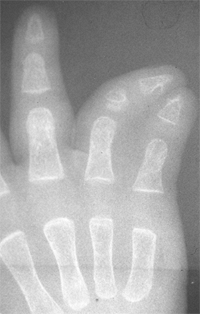 |
|
Figure 23.21
Radiograph of complex syndactyly of the fourth web space with progressive deformity of the ring finger. This should be released early in infancy to prevent progressive deformity. The abnormal middle phalanx of the ring finger may still require corrective osteotomy. |
underlying joints, bones, neurovascular structures, muscles, or
tendons. The separation of skin follows the same principles as in
complete syndactyly. If there is significant digital malalignment, skin
incisions may need to be modified so as to maximize coverage. After
separation of the skin, all abnormal connections of fascia, tendons,
bones, joints, nerves, and arteries need to be addressed individually.
Phalangeal deformity may require osteotomy. Instability of the joints
may require ligamentous reconstruction. Stiffness of the joints,
camptodactyly, or symphalangism may need to be dealt with subsequently.
Neural, vascular, and nail problems are managed in a manner similar to
those described for complete syndactyly. Tendon reconstruction is
performed primarily if possible. Brachydactyly is usually addressed
subsequently, if at all.
especially if it is bilateral. Adjacent webs are not separated
simultaneously. Generally, the first and third webs are separated
together, as are the second and fourth webs. Abundant full-thickness
skin graft is usually required. Sufficient time between procedures (3
to 6 months) lessens worries about flap necrosis and scar contracture.
In syndromic conditions, such as Apert syndrome, the acrosyndactyly is
more complex, and normalcy may never be achieved (275,276).
avascular digit, is almost never encountered and should be avoidable.
Adhering to the axiom of never operating on both sides of a digit
during the same procedure prevents the occurrence of this devastating
complication. Careful dissection of the digital vessels in complex
situations lessens the risk of avascularity at the initial or
subsequent operations. Preoperative vascular studies in complicated
situations prepare the surgeon and allow him or her to avoid
intraoperative surprises and dangers. Flap necrosis and scar
contracture are more common. The flaps should be secured without
tension, and their vascularity should be checked with deflation of the
tourniquet at the completion of the procedure. If in doubt, lessen the
tension and use skin graft. Skin graft failure is usually caused by
inadequate immobilization and excessive shear forces applied to the
grafts. Secure immobilization with the compressive dressing, long-arm
cast, and sling and swathe bandages is necessary for protecting the
grafts. Infection is rare but will result in marked scar contractures
that require reoperation. Long-term issues that have been reported
regarding skin graft sites include contracture formation, graft
breakdown (both incidences are seen more often with split thickness
grafts), hyperpigmentation, and hair growth (both incidences are seen
more often with full thickness grafts) (277).
Nail deformity and poor eponychial coverage are common when there is an
initial conjoined nail. The use of composite grafts seems to lessen the
incidence of these complications. Web-space creep is common with
growth, but often does not require reoperation unless digital
contractures develop. The use of local rotation flaps and skin grafts
will resolve this problem. Keloid formation is rare but has been shown
to be associated with primary digital enlargement before syndactyly
separation (278). Finally, angular deformity may require osteotomy or joint reconstruction.
occur on the radial (preaxial), central, or ulnar (postaxial) portion
of the hand. Preaxial, or thumb, polydactyly usually
occurs
in isolation and will be addressed in the section dealing with the
thumb. Central polydactyly is very rare and is usually associated with
syndactyly; the underlying digits are rarely normal. It can be
inherited in an autosomal dominant manner. It affects girls more than
boys, and is often bilateral. Postaxial or small-finger polydactyly has
a variable racial incidence, with the occurrence in African Americans
estimated to be as high as 1 in 300 live births, and that in whites
estimated to be 1 in 3000 live births (279,280,281). It is often bilateral. Polydactyly has been classified by Stelling (282) and Turek (283)
into three types. Type I involves soft tissue alone and is very common
in the African American population. Type II involves phalangeal
duplication articulating with a single or bifid metacarpal head. Type
III involves a complete ulnar ray duplication, including the
metacarpal. There is also a universal classification of polydactyly
proposed by Buck-Gramcko and Behrens (260) that
denotes the digit involved by a Roman numeral (I to V) and the extent
of bifurcation by abbreviation [DIST (distal), DIP (distal
interphalangeal), MID (middle), PIP (proximal interphalangeal), PROX
(proximal), MCP (metacarpophalangeal), MET (metacarpal), CMC
(carpometacarpal), C (carpal), IC (intercarpal), and RUD (rudimentary
polydactyly)]. The major issue for the surgeon is not the
classification of the polydactyly, but whether excision with
reconstruction, or reconstruction alone, is warranted. Only postaxial
soft- tissue polydactyly (type I, or rudimentary) can be treated by
excision alone. All other forms require reconstruction. Central
polydactyly is usually an isolated malformation, and postaxial
polydactyly in African Americans is almost always an isolated
malformation. Postaxial polydactyly in whites without a positive family
history may be associated with chromosomal abnormalities, other
syndromes, or other malformations.
Unfortunately, too often this has been performed with a suture ligature
by inexperienced hands. The result is a persistent nubbin caused by
incomplete excision of the base of the digit. If a suture ligature is
used, it must bring about necrosis of the entire digit. Otherwise, it
may be best to perform an elliptical excision under local anesthesia.
Care is taken to ligate the digital vessels with this excision. Other
than failure to completely excise the digit, complications are rare,
and the hand is normal afterward. There have been reports of traumatic
neuromas along with hypertrophic scars that have led to late problems
and the need for repeat surgery (284,285). The parents should be aware of the future genetic implications for them and their children.
In addition to excising the redundant parts, transfer of the hypothenar
muscles (abductor digiti quinti, flexor digiti quinti) from the sixth
to the fifth digit is necessary. In type II polydactyly, the MCP joint
collateral ligaments are also transferred to the reconstructed fifth
digit. If the metacarpal head is enlarged or bifid, intraarticular
osteotomy is appropriate. In this procedure, the origins of the
collateral ligament and the metacarpal physis should be preserved. In
type III polydactyly, the entire ray is resected, and the basilar joint
is stabilized. The outcome of surgery in both type II and type III
malformations is usually cosmetic and functional normalcy.
The major decision is whether it is feasible to achieve independently
functioning digits. The choices are to leave the digits conjoined, to
attempt reconstruction to a five-digit hand, or to perform ray
resections of part or all of the synpolydactyly. Often the involved
digits have bone and joint malalignment, hypoplasia, and poor motor,
nerve, and vascular supplies. The reconstructed digit is usually
smaller, stiffer, weaker, and malaligned. The family needs to be well
aware of this, so that their expectations are realistic as far as
surgical reconstruction and digital function are concerned. Treatment
decisions in this condition are often personal, based on the family’s
desires and the surgeon’s preferences.
It involves a flexion deformity of the PIP joint, most commonly in the
small finger. It may present in infancy or in adolescence. It may
involve a single digit or multiple digits (Fig. 23.23).
malformations. Its incidence is unknown but has been cited at less than
1% of the general population (288,289). Most cases appear in infancy, and there is equal gender distribution (290). Less commonly, it may first appear in adolescence, usually in girls (291).
Some cases are familial, with an autosomal dominant inheritance
etiology and variable penetrance patterns. Most cases are isolated,
without a positive family history. Up to two thirds of the patients
have bilateral small-finger involvement. It is important to distinguish
camptodactyly from neurologic causes of clawing or from posttraumatic
butonniere deformities (292).
The volar skin is usually tight. With growth, these anatomic
abnormalities cause PIP joint contracture and phalangeal bony
abnormalities. The distal proximal phalanx becomes narrowed volarly and
flattened dorsally, with loss of the normal contour of the head. The
articular surface can become incongruous, with notching of the base of
the middle phalanx (299). Initially, the patient can compensate for the PIP
joint flexion contracture by MCP and distal interphalangeal (DIP) joint hyperextension (Fig. 23.24).
This keeps the digital pulp of the affected fingers in line with the
other digital rays. Therefore, the mild contracture in the older child
may not need treatment. However, as the contracture continues to
progress beyond 30 degrees and toward 90 degrees, it becomes more
difficult for the patient to compensate. Camptodactyly is usually
progressive with growth.
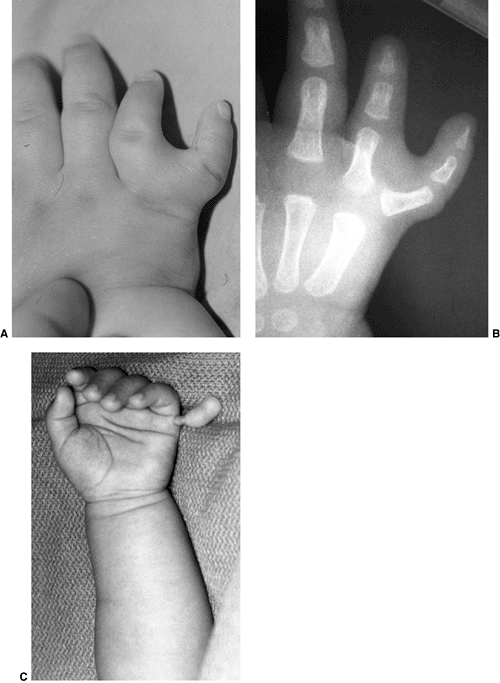 |
|
Figure 23.22 A: Complete postaxial polydactyly with phalangeal duplication with a conjoined metacarpal. B:
Radiograph of the same patient. Reconstruction will consist of excision of the duplicate phalanges, contouring of the bifid metacarpal head, and transfer of the metacarpophalangeal joint ulnar collateral ligament and the hypothenar musculature to the reconstructed fifth digit. C: Simple polydactyly with only soft-tissue attachment. This can be excised in the newborn nursery under local anesthesia. Care must be exercised while performing suture ligature; the entire stalk should be excised so as to avoid leaving a residual nubbin. |
flexor-extensor balance. The options for treatment are splinting and
surgical reconstruction. Most clinicians recommend an initial treatment
program of progressive passive extension and splinting with dynamic or
progressive static splints. Parents are instructed to perform frequent
home exercises for their infants; affected adolescents are similarly
instructed to perform a home program for themselves. The
goal is to achieve full passive extension. It is hoped that active extension will follow. Many clinicians (291,300,301,302)
report success with a splinting program in most cases. The best results
are in mild cases in young patients. The patients are followed until
the end of growth in order to treat recurrence if it occurs.
 |
|
Figure 23.23
An adolescent patient with marked camptodactyly of the small, ring, and long fingers. There are proximal interphalangeal joint flexion contractures in each digit, and the patient is actively hyperextending the metacarpophalangeal joints to compensate for those contractures. |
for surgery. McFarlane et al. strongly recommend surgical intervention
to reconstruct the aberrant insertion of the lumbrical (297,298). However, the published surgical results are disappointing in terms of outcome (297,298,301,303).
Surgery is reserved for severe contractures that are not amenable to
splinting treatment. Specifically, surgery shows best results in
patients in whom the finger is flexed into the palm and obstructs use
of the hand. The principle of surgical intervention is to correct the
abnormal anatomy. This involves release of the aberrant lumbrical (288,297,298) or flexor digitorum superficialis (294) insertion in conjunction with volar Z-plasties and PIP joint release (303).
Local flaps or full-thickness skin grafts are often necessary for volar
skin coverage. Tendon transfer to the extensor mechanism is performed
in patients with full passive extension of the PIP joint, but no active
extension (304). The results of soft-tissue
reconstruction often merely change the arc of motion rather than
normalize it. Preoperatively, patients have significant flexion
contracture. Postoperatively, they generally have difficulty achieving
full active and passive flexion.
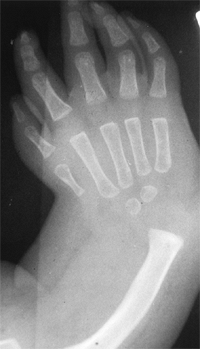 |
|
Figure 23.24
Radiograph of a thumb with type III B hypoplasia without a carpometacarpal joint and proximal thumb metacarpal. This is usually treated with pollicization. Less often, treatment is by reconstruction, potentially including a microvascular toe metatarsophalangeal transfer to form a thumb carpometacarpal joint. Note the radial dysplasia in this patient. |
joint, and these preclude restoration of normal motion or alignment.
Oldfield (305) and Flatt (1)
have stated that, in the presence of marked radiographic evidence of
bone and joint changes, corrective extension osteotomy may be most
effective at improving alignment and function. The published data about
this salvage operation are too limited to enable objective evaluation (288).
unusual to achieve a perfectly aligned and mobile digit. Parents and
patients should be aware of this from the outset. In addition,
deformity can recur with growth and persistent muscle imbalance.
the digit in the radioulnar plane. It is usually caused by a misshapen
middle phalanx. The middle phalanx is trapezoidal, with less height on
the radial side. This results in deviation at the DIP joint.
Clinodactyly is most often seen in the small finger and is usually
bilateral. This form of
clinodactyly has an autosomal dominant inheritance (306,307,308,309).
Clinodactyly is also frequently associated with syndromes (Holt-Oram,
Turner, Silver, and Cornelia de Lange) and chromosomal abnormalities
(trisomy 18 and 21), and should alert the primary neonatal examiner to
look for associated malformations or problems. For example,
clinodactyly of the thumb is seen in Rubinstein-Taybi syndrome (310,311) and diastrophic dwarfism (312,313).
In addition, it is common with other congenital abnormalities of the
hand. Mild clinodactyly, however, is commonplace in otherwise healthy
individuals. In these situations the major issue is cosmesis. Function
is affected only when the deformity is severe enough to impinge on the
adjacent digit during flexion.
cases are mild and nonprogressive, and therefore do not warrant
surgical intervention. Progressive, severe clinodactyly may interfere
with flexion and grip. In these rare situations, the progressive
deformity is secondary to altered physeal growth. The middle phalangeal
physis may be a bracket delta phalanx. Treatment options are bracket
resection and surgical realignment. Physeal bar resection and fat graft
interposition have been reported by Vickers (314,315)
to restore longitudinal growth and provide correction. Surgical
realignment can be in the form of opening-wedge, closing-wedge, and
reverse-wedge osteotomies. Osteotomy should be delayed until there is
sufficient ossification of the middle phalanx to allow for precise
cuts. Generally, this is around school age. The complications of
osteotomy are persistent deformity and loss of interphalangeal motion.
Loss of motion in a patient whose indication for surgery was purely
cosmesis is unacceptable to most patients, their families, and surgeons.
categories of congenital hand anomalies described by the American
Society and the International Federation of Societies for Surgery of
the Hand. Failure of formation occurs with aplasia of the thumb, and
this is often associated with other radial-sided longitudinal
deficiencies. Failure of separation occurs with thumb-index
syndactylies, which are common with other syndactyly syndromes.
Duplication is seen in the form of thumb polydactyly, undergrowth as
thumb hypoplasia, and overgrowth as macrodactyly and triphalangeal
thumbs. Also, thumb abnormalities are common as part of constriction
band syndrome or generalized musculoskeletal disorders. The list is
exhaustive and this section will cover the more common congenital thumb
malformations.
pollicis longus and its tendon sheath at the A1 pulley, where there is
a palpable mass (Notta nodule), representing the flexor pollicis longus
constriction at the A1 pulley. In the past, trigger thumbs were defined
as congenital. However, this condition is almost always
acquired in the first 2 years of life, as indicated by a prospective
screening of neonates, which failed to record any trigger thumbs (316,317).
The cause appears to be a size mismatch between the flexor pollicis
longus and the A1 pulley, leading to progressive constriction. Unlike
adult trigger digits, there does not appear to be an inflammatory
component (318); 30% of the cases are
bilateral. Isolated trigger thumbs have no associated syndromes.
However, trigger digits are seen with neurologic syndromes (trisomy 18)
and mucopolysaccharidoses (319). There is no
familial inheritance pattern. Patients with trigger thumb present at
ages ranging from infancy to school age. Often, the diagnosis is missed
until local trauma brings attention to the thumb. In the emergency
setting, the flexed interphalangeal joint can be mistaken for an
interphalangeal joint dislocation. Radiographs are misleading because
of limited phalangeal ossification. A palpable nodule at the A1 pulley
is diagnostic. If the trigger is longstanding, compensatory
hyperextension of the MCP joint develops to effectively bring the thumb
out of the palm. In addition, mild radial deviation of the
interphalangeal joint may develop, secondary to eccentric flexor pull.
found that 30% of trigger thumbs may resolve spontaneously. In infants
older than 1 year of age, less than 10% of trigger thumbs resolved
spontaneously. Ger et al. (321) found lack of
resolution in their patients after observation for 3 years. There is
limited evidence that splinting is of benefit (322),
and often it is not well tolerated. Surgical release of the
constricting A1 pulley and flexor tendon sheath is the treatment of
choice indicated in infants who show no spontaneous resolution by 1
year of age, and in any toddler or older child presenting with a locked
trigger thumb. Incision is made transversely in the digital crease to
lessen scarring. Care must be taken to avoid injury to the superficial
digital neurovascular bundles. The oblique pulley has to be preserved
so as to prevent flexor tendon bowstringing. Recurrence is extremely
rare.
associated with central nervous system disorders and syndromes (trisomy
18, mucopolysaccharidoses). The pathology appears to predominate at the
decussation of the flexor tendons under the A2 pulley, and not at the
A1 pulley alone. The triggering appears to occur as the flexor
digitorum profundus passes through the chiasm of the flexor digitorum
superficialis. Surgical recurrence is high in pediatric trigger digits.
This may be because A1 pulley release alone is not sufficient to solve
the problem (323). Further opening of the
chiasm or resection of a slip of the flexor digitorum superficialis is
often necessary to prevent recurrence (319).
deficient prehension and grasp. Thumb hypoplasia or aplasia can occur
in isolation, or be associated with other radial-sided deficiency
syndromes (Holt-Oram and Fanconi syndromes). It is seen universally in
radial dysplasia. It is also common in other congenital malformations (206,324),
including those of the cardiac, craniofacial, musculoskeletal, renal,
gastrointestinal, and hematopoietic systems. It may involve hypoplasia
of the metacarpals (Cornelia de Lange syndrome and diastrophic
dwarfism) or phalanges (Rubinstein-Taybi and Apert syndromes). The
finding of a thumb deficiency in a neonate should prompt a thorough
multiple-system examination for other malformations. In addition, a DEB
screening for Fanconi anemia is recommended for all infants with
radial-sided defects including thumb hypoplasia.
first web space, unstable MCP joint, thenar weakness, and
interphalangeal joint stiffness or instability. Buck-Gramcko (325) and Manske et al. (326) have modified the Blauth (327)
classification for thumb hypoplasia. This classification system is
useful from the point of view of treatment considerations. There are
five types of thumb hypoplasia in the Buck-Gramcko modification of the
Blauth classification (Table 23.3). A type I
thumb is essentially a normal thumb, except for diminished size. A type
II thumb is even smaller and narrower, with a contracted first web
space and thenar atrophy. Type III thumbs have marked atrophy or
absence of both the intrinsic and extrinsic musculature. The thumb is
globally unstable and underdeveloped. Manske et al. (326)
further subdivided type III thumbs into A and B categories. Type IIIA
thumbs have a stable carpometacarpal (CMC) joint, whereas type IIIB
thumbs have absence of the proximal metacarpal and trapezium. Type III
B thumbs have no basilar joint stability (Fig. 23.23). Type I thumbs are the classic pouce flottant or “floating thumbs.” A type V demonstrates complete aplasia of the thumb.
|
TABLE 23.3 MODIFIED BLAUTH CLASSIFICATION OF CONGENITAL THUMB HYPOPLASIA
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
thumb hypoplasia. Universally, the thumb ray bones are smaller and
narrower. The interphalangeal joint is usually underdeveloped and
stiff. The first web space is contracted in all except the rare type I
thumb. The MCP joint usually has ulnar collateral ligament
insufficiency, but may be globally unstable. The thenar intrinsics are
deficient, and they may be completely absent in the more severe forms
of hypoplasia. The thumb’s extrinsic musculature is progressively
deficient in the classification scheme. The major anatomic determining
factor for reconstruction is the status of the CMC joint. Type IIIB and
type IV thumbs have no basilar joints. Plain radiographs are helpful in
distinguishing the skeletal development, including the carpus and
distal radius.
hypoplasia is whether to do surgery or not. If left alone, these
children will adapt. They will use lateral pinch between the long and
index fingers. However, the deficiencies in pinch, grasp, and fine
motor activities will be significant. Surgical reconstruction can
improve function and cosmesis, and should be advised (328).
includes first web space deepening; opponensplasty, with either the
abductor digiti quinti or the ring-finger flexor digitorum
superficialis tendon; and MCP joint stabilization, with either
ligamentous reconstruction or arthrodesis. Blauth types I to IIIA
should be reconstructed according to these principles (Fig. 23.25).
The choice of first web-space deepening procedure includes two-part and
four-part Z-plasties or the use of dorsal rotation flaps from the index
finger, thumb, or hand. The degree of contracture determines the amount
of skin necessary to provide a normal depth and breadth to the web for
pinch and grasp activities. The abductor digiti quinti transfer for
opposition can be used in the infant with a relatively stable MCP
joint. Care must be taken to protect the proximal neurovascular pedicle
to the abductor muscle, with dissection near the pisiform (193).
In older children, or in patients with marked instability of the MCP
joint, the ring-finger flexor digitorum superficialis is used for
opposition. The additional tendon length can be used for ligamentous
reconstruction. In addition to the flexor digitorum superficialis
tendon, local fascia from the adductor can be mobilized for ligamentous
augmentation at the MCP joint. If soft tissue procedures fail, or the
instability at the MCP joint is too severe, fusion can be performed.
The physis of the proximal phalanx should be preserved so as to
maximize growth of the thumb ray. Chondrodesis of the metacarpal head
to the epiphysis of the proximal phalanx is desirable.
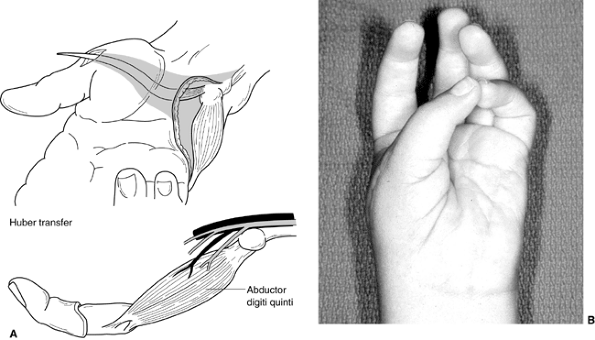 |
|
Figure 23.25 A:
Illustration of Huber opponensplasty transfer of abductor digiti quinti to the abductor pollicus brevis for a hypoplastic thumb reconstruction. It is imperative that the neurovascular bundle be preserved proximally during the transfer. B: Postoperative clinical photograph of opposition pinch in a patient with a Huber opponensplasty reconstruction. |
(type IV), or aplasia of the thumb (type V) are candidates for
pollicization of the index finger. The major area of controversy is
still the type IIIB thumb. In the absence of a CMC joint, the results
of reconstruction have been disappointing (329).
It is a difficult decision for the parents to accept pollicization in
these children, because of the appearance of the thumb. However,
reconstruction without a CMC joint leads to continued lateral pinch of
the index and long fingers, rather than use of the reconstructed thumb.
CMC joint reconstruction, with microvascular transfer of a toe
metatarsophalangeal joint, has rarely been performed in children with
type IIIB thumbs (329). This is an alternative
to pollicization in these children. Surgery is performed later and is
quite extensive. Pollicization involves the conversion of the
triphalangeal index finger, without a basilar joint and a narrow web
space, to a biphalangeal thumb with a CMC joint and a deep first web
space. The removal of the index metacarpal and the use of the index
metacarpal epiphysis as the trapezium enable the surgeon to properly
position and cover the thumb. The technique described by Buck-Gramcko
is used most commonly (203,204) (Fig. 23.26).
In the congenital absence of the thumb, this is better than
microvascular toe transfer. Thumb reconstruction or pollicization is
generally performed between 6 and 18 months of age. The quality of the
pollicized digit is dependent on the quality of the original index
finger, in terms of tendon function and joint motion. Patients with
thumb aplasia and radial dysplasia generally do more poorly, because
the involved index finger has deficient musculature and camptodactyly.
Manske and McCarroll performed secondary opposition transfers in
children with poor pollicization (208). The best results are seen in children who have aplasia alone, and normal index fingers (Fig. 23.27).
that there are two normal thumbs whereas, in fact, both thumbs are
hypoplastic. In isolation, thumb duplication is usually a sporadic
occurrence. It may be associated with genetic syndromes, such as
acrocephalopolysyndactyly (Nocack and Carpenter types) and Holt-Oram or
Robinow syndromes. If it is associated with a triphalangeal thumb or
with duplication of the great toe, it may be autosomal dominant, with
variable penetrance (330). The locus for preaxial polydactyly has been mapped to chromosome 7q36 (331)
inheritance. Preaxial polydactyly is a rare occurrence, with an
incidence estimated at 0.08 in 100,000 live births. There are many
different classification systems, including the universal (332), Marks and Bayne (333), and Wassel (334) systems. Classification by the Wassel system
is dependent on the number of bifid or duplicated phalanges or metacarpals, starting distally and progressing proximally (Fig. 23.28). A bifid distal phalanx is Wassel type I. A duplicated distal phalanx is type II (Fig. 23.28),
constituting approximately 20% of all thumb duplications. A duplicated
distal phalanx with a bifid proximal phalanx is type III. A thumb with
duplicated proximal and distal phalanges is type IV, which is the most
common type (approximately 40%) (Fig. 23.29).
Duplicated proximal and distal phalanges with a bifid MTP is type V.
Duplication of all phalanges and metacarpals is type VI. Any
duplication with a triphalangeal thumb is type VII, which accounts for
approximately 20% of thumb duplications.
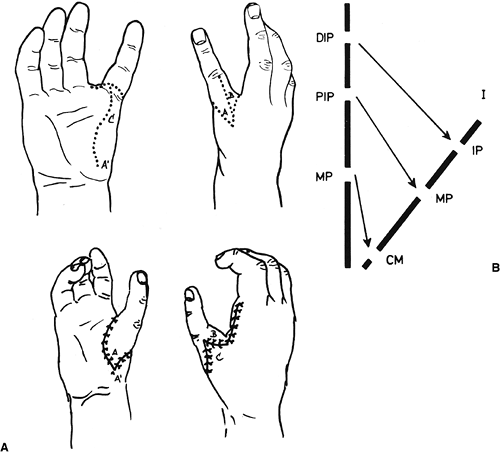 |
|
Figure 23.26 Illustrations of the pollicization procedure as popularized by Buck-Gramcko. A:
Outlines show the skin incisions that provide first web-space flap coverage as the pollex is positioned for opposition. The triphalangeal index finger is converted into a biphalangeal thumb. The index metacarpal is excised, except for the distal epiphysis. B: The changes in joints from the index finger to the thumb and the tendon transfers to provide opposition and pinch function are as follows: extensor indicis proprius → extensor pollicis longus; extensor digitorum II → abductor pollicis longus; interosseus palmaris I → adductor pollicis; interosseus dorsalis I → abductor pollicis brevis. DIP, distal interphalangeal; PIP, proximal interphalangeal; MP, metacarpophalangeal; IP, interphalangeal; CM, carpometacarpal; I number of finger. |
polydactyly. The nails, bones, joints, ligaments, muscles, tendons,
nerves, and blood vessels are split between the two digits. In
addition, there can be hypoplasia or aplasia of any of the normal
anatomic elements of a thumb. Plain radiographs will generally provide
definitive information regarding skeletal pathoanatomy. Careful
surgical exploration will define the soft-tissue anatomy.
improve function and cosmesis. Unlike postaxial polydactyly, ablation
in the nursery is not recommended, even for the simplest preaxial
polydactyly. Too often, the thenar musculature and collateral ligaments
insert on the radius-based digit and can be lost with simple excision (335,336,337).
thumb and reconstruction of the more developed thumb. Generally, the
radius-based digit is excised. The soft-tissue elements usually
bifurcate at the level of the skeletal split. Transfers of the shared
or aberrant tendons, nerves, and ligaments to the reconstructed thumb
are necessary in order to maximize outcome.
 |
|
Figure 23.27 A:
A 1-year-old child with thumb aplasia. The surgical incisions for index pollicization are outlined. These flaps provide for deep first web space. B: Long-term follow-up clinical photograph of a pollicization. |
The flexor pollicis longus usually inserts on the ulnar thumb, although
it can be bifid or insert on the radial thumb. The thenar musculature
and the radial collateral ligament to the MCP joint usually insert
radially, especially in the common type IV polydactyly (Fig. 23.29).
These should be transposed. The radial digital nerve may be present
only in the radial thumb, and should be transposed to the radial aspect
of the reconstructed thumb. The bifid proximal phalanx or metacarpal
should be excised in types II and IV, respectively. Primary phalangeal
or metacarpal osteotomy is indicated if there is axial malalignment.
The more developed thumb often has a larger nail and distal phalanx.
However, some Wassel type II malformations have almost equally sized
distal phalanges and nails (Fig. 23.30).
Surgical recombination of the distal phalanx and nail beds
(Bilhaut-Cloquet procedure) in types I and II malformations has been
disappointing because of poor nails, loss of joint motion, and physeal
closure. It still appears best to accept the more hypoplastic distal
phalanx than to consider recombination of the nail and phalanx (338).
Adduction contracture of the first web space should be treated with
Z-plasty to deepen the web space. The need for additional surgery with
growth may be as high as 40% of cases.
Reconstruction with osteotomy, tendon transfers, ligament
reconstruction, or arthrodesis may be necessary to improve both
function and cosmesis.
autosomal dominant manner. This is true whether they are associated
with thumb polydactyly (343) or are seen in
isolation. In inherited isolated triphalangeal thumbs, the genetic
marker has been localized to chromosomal region 7q36 (344).
The extra phalanx is the middle phalanx. It may be wedge-shaped or
rectangular. The thumb may be in a position of opposition or in the
plane of motion of the other fingers. The latter situation may indicate
an index finger duplication with an absent thumb. This concept is
supported not only by the clinical and radiographic appearance of the
most radial digit, but also by the dermatoglyphics (345). The triphalangeal thumbs that were studied contained the radial loops normally seen in the index finger and not in the thumb.
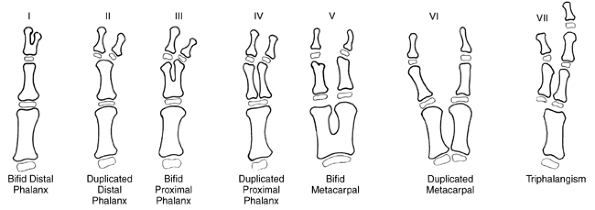 |
|
Figure 23.28
Wassel classification of thumb duplications. Type IV is the most common, with an incidence of 40%. Types II and VII have an incidence of approximately 20% each. |
type of triphalangism. Type I involves a delta middle phalanx with
radial deviation deformity (Fig. 23.31). Type
II involves a normal middle phalanx, but an opposable thumb. Type III
is an index-finger duplication with all digits in the same plane. In
types I and II triphalangism, the first web space is normal. In the
five-fingered hand (type III), there is a contracted first web space
that limits prehension. Similarly, usually the thenar musculature is
normal in type I and type II triphalangeal thumbs, whereas it is absent
in type III triphalangism. In addition, the triphalangeal thumb may be
hypoplastic and have associated extrinsic musculature weakness.
undergo surgical reconstruction. Patients with significant cosmetic
abnormalities and limitation of pinch prefer
reconstruction
for both functional and cosmetic reasons. A malaligned and elongated
triphalangeal thumb in the same plane as the other digits is
cosmetically anomalous and functionally limited for prehension
activities.
 |
|
Figure 23.29 A: An adolescent with the more common Wassel type IV thumb duplication. B:
Results at the conclusion of the surgical reconstruction. Surgery included excision of the radial proximal and distal duplicated digits, transfer of the thenars and metacarpophalangeal joint radial collateral ligament to the reconstructed thumb, and contouring of the skin by excision of redundancy and Z-plasties. |
In older children, or in children with abnormal phalanges and
interphalangeal joints, a combination of shortening osteotomy and
arthrodesis is preferred. In these situations, physeal growth of a
biphalangeal digit should be preserved. In the five-fingered hand, a
modified pollicization procedure is necessary so as to provide a deep
first web space and an opposable thumb.
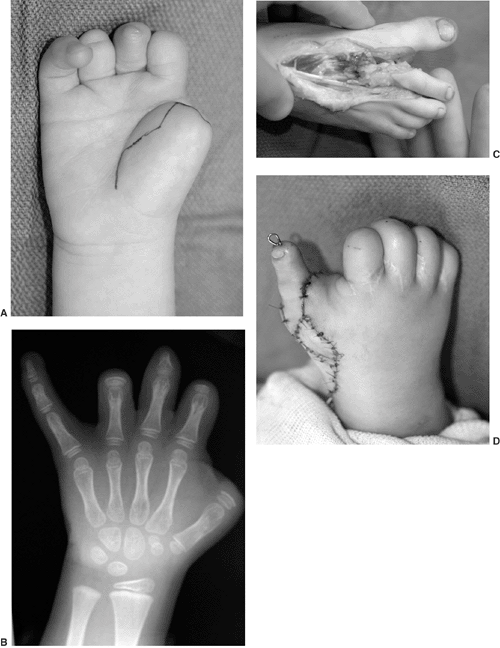 |
|
Figure 23.30 A:
Preoperative clinical photograph of a toddler with constriction band syndrome and congenital amputation of the thumb at the metacarpal–phalangeal joint level. B: Preoperative radiograph revealing the level of the congenital amputation through the proximal phalanx just beyond the epiphysis. C: Intraoperative anatomy of second toe donor harvest for microscopic transfer and thumb reconstruction. D: Immediate postoperative clinical photograph of toe transfer. This process led to normal thumb functioning and to a remarkable cosmetic result for the patient. |
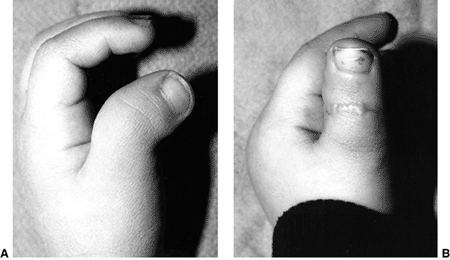 |
|
Figure 23.31 A: Triphalangeal thumb with a delta phalanx and a marked radial deviation deformity. B: Postoperative photograph after excision of the delta phalanx and rotation flap reconstruction of the soft tissues.
|
The two peak ages for these fractures are adolescence (from
sport-related activities) and infancy (from crush injuries). Most
fractures are nondisplaced, nonphyseal injuries. Physeal injuries,
however, can account for up to 40% of finger fractures (357,358),
with a Salter-Harris II fracture of the small-finger proximal phalanx
being the most common. Most pediatric hand fractures do well regardless
of treatment (359). Malunion and growth disturbance are rare (357,360).
However, there is a subset of pediatric hand injuries that will do
poorly if not recognized and treated appropriately. The purpose of this
section is to review fractures that are problematic.
of ossification. These appear between birth and 3 to 4 years of age.
The epiphyses of the phalanges are proximal. The epiphyses of the
metacarpals are distal, except for the thumb, in which the physis is
proximal. Distally in the thumb, there can be a second epiphysis or
pseudoepiphysis. Ossification of the phalangeal condyles is progressive
with growth, but in preschool children the condyles may be
predominantly cartilaginous. Radiographic evaluation of injuries in
young children may be difficult because of the chondral nature of the
epiphysis and the intraarticular portions of the condyles. It is
important to obtain true anteroposterior and lateral radiographs of the
injured digits. In a diagnostically confusing situation, MRI scans of
the fingers and hand should be performed.
in a young child, maximal protection is necessary. It is often
necessary to protect the preschool-age child with a long-arm mitten
cast. The older child often needs a short-arm mitten cast.
Single-finger splinting is difficult to maintain, even in an
adolescent. If the fracture is painful, or if it requires
immobilization to maintain reduction, casting of the entire hand is
appropriate. Fortunately, most pediatric fractures are nondisplaced and
stable (361). The outcome will be successful
regardless of immobilization technique. In these situations, the change
is made to simple buddy taping as soon as it is comfortable. However,
it is imperative not to mistake a problematic injury for a simple one
and treat it with benign neglect. Such a mistake will lead to long-term
loss of alignment, motion, and function, that may not be salvageable by
secondary surgical reconstruction.
secondary to crush injury. These injuries are most common in the
toddler and preschool age groups. The mechanism of injury is usually a
digit caught in a door. Adults, often parents, are frequently involved
in the accident, which makes the situation emotionally charged. The
injuries can include partial or complete amputation, nail-bed
laceration, and distal phalangeal fracture. All of these sites of
trauma need to be addressed in the care of the child. In addition, time
and energy need to be spent in helping the family cope with the
emotional trauma.
includes a partial amputation with a nail-bed laceration and a distal
phalangeal tuft fracture (Fig. 23.32). The
fractures are generally minor avulsions that heal without problems. The
partial amputation often extends dorsally through the nail bed, leaving
some volar pulp intact. The volar soft-tissue attachments maintain the
vascularity to the distal tip. Meticulous repair under conscious
sedation and/or local anesthesia usually leads to normal long-term
outcome.
The technique involves initial repair of the eponychial folds to
properly align and stabilize the digit. It is imperative to then
meticulously repair the nail-bed laceration with fine, absorbable
suture (i.e., 6-0 chromic suture) and loupe magnification to prevent
long-term nail deformity. The dorsal roof of the eponychium is
preserved by placement of a spacer for several days after repair. Even
when there is nearly complete amputation, with apparently nonviable
distal tissue, soft-tissue repair almost always leads to survival of
the tip. Uncomplicated repair of the partial amputation and nail-bed
laceration will generally heal without permanent damage to the nail or
phalanx. However, neglected nail-bed injuries are associated with a
high rate of permanent deformity (354,362).
 |
|
Figure 23.32
Typical distal phalangeal crush injury with nail and pulp laceration. Repair requires nail-plate removal, absorbable suture repair of the lacerated nail bed, and anatomic closure of the eponychial skin. |
nail, or distally, is more controversial. First, this is a much more
difficult situation emotionally for the child and family. The injury
often occurs while the child is under adult supervision. There is
usually significant stress and guilt associated with the amputation.
The parents universally want the tissue replaced. However, this injury
is beyond the trifurcation of the digital arteries near the level of
the DIP joint, and so the tissue is not replantable. The debate
concerns whether to suture the amputated part back on without vascular
anastomoses (a composite graft), or to allow healing by secondary
intention (363). Replacement of the amputated
soft tissue initially makes the family feel better, but usually is not
necessary. The amount of soft tissue amputated is small. The physis is
proximal and not affected. Subsequent growth will be normal. The
overall digital length, therefore, will be nearly normal. The cosmetics
of composite grafting and healing by secondary intention are generally
equal in the long term. Long-term sensibility and function seem to be
equivalent. In addition, in the short term, replacement of the
amputated part may be more stressful for the family and child, with
multiple dressing changes, superficial necrosis of the distal tip, and
slow healing. Therefore, if the soft-tissue loss is minor, reassurance
to the family of the long-term result, and treatment with serial
dressing changes are best. This is true as well for the situation in
which a minimal portion of exposed bone is debrided. However, if the
piece includes the eponychium or the entire sterile and germinal matrix
of the nail, composite grafting of the amputated part is preferred.
There is a chance that this will heal and preclude secondary
reconstructive surgery.
flexion posture of the distal phalanx, or mallet appearance.
Radiographs will reveal a displaced physeal fracture with dorsal
widening. Too often, these children are diagnosed as having mallet
finger and treated with splinting. This is not an extensor tendon
disruption. The extensor mechanism is intact because the terminal
tendon inserts into the more proximal epiphysis. The deformity is
caused by entrapment of the proximal nail bed (germinal matrix) in the
physeal fracture site (364). If not recognized
early, the open injury can become secondarily infected. The clinical
appearance of the finger and the radiographic physeal changes may be
interpreted as distal phalangeal osteomyelitis. However, antibiotic
treatment alone or surgical debridement of the distal phalanx is not
the proper treatment for this late-presenting fracture. Surgical repair
of the nail bed is necessary in order to prevent long-term nail and
distal phalanx growth problems. Under local, regional, or general
anesthesia, the nail plate should be removed. By flexing the distal
phalanx, the surgeon can gently extract the entrapped nail bed. The
germinal matrix should be meticulously repaired so as to avoid
long-term nail-plate problems. At times, this requires more proximal
exposure by raising an eponychial flap. After repair of the nail bed,
the nail plate can be replaced to preserve the dorsal roof of the
eponychium and to provide an internal splint for repair of the fracture
(364,365). Placement of a cautery hole in the nail plate will lessen the risk of subsequent hematoma and paronychial infection.
In this age group, physeal fracture is more common. In the adolescent,
mallet injuries with disruption of the extensor mechanism are more
common and adultlike. As long as there is no entrapment of the germinal
matrix, as noted in the preceding text, these injuries can be treated
with immobilization. Rarely, the entire epiphysis can be displaced with
the extensor mechanism (366,367).
If recognized early, this injury should be treated with open reduction
of the dislocated epiphysis. The rare, chronic mallet finger in the
young child has been treated successfully with tenodermodesis (368).
usually caused by crush injury. As the child attempts to extract the
affected digit, the condyles become entrapped and a fracture occurs in
the subcondylar region (347,356,357,361,369,370).
The condylar fragment displaces into extension and often malrotates.
The fragment is tethered by the collateral ligaments as it rotates
dorsally up to 90 degrees. The condylar fragment is small, and has a
precarious blood supply through the collateral ligaments. The
subcondylar fossa is obliterated, blocking interphalangeal flexion (Fig. 23.33).
If not properly recognized and treated, complications of malunion, loss
of motion, and avascular necrosis can occur. Too often, the severity of
the fracture is underappreciated in the urgent care setting. The
patient presents late, with inappropriate immobilization and a
significantly healed fracture. In addition, the fracture is unstable
and will often displace after closed reduction (371).
The treatment of choice is closed reduction and percutaneous pinning.
In a young child, this can be accomplished with a single oblique pin.
In an older child, crossed pins prevent malrotation. Placement of the
pins in the distal fragment requires careful localization of the
fragment and avoidance of the extensor mechanism. If open reduction is
necessary, the collateral ligaments should not be dissected from the
distal fragment. Careful dissection lessens the risk of avascular
necrosis.
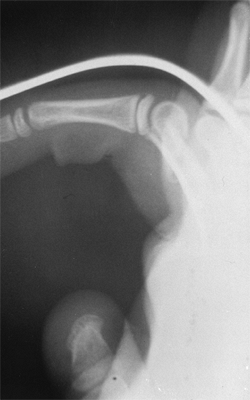 |
|
Figure 23.33
Displaced phalangeal neck fracture with loss of the subchondral fossa. This leads to loss of digital flexion. This fracture requires prompt attention, anatomic reduction, and pin stabilization. If the fracture is left in the position shown here in the splint, there will be a problematic malunion. |
avascular necrosis. The fracture has generally been healing too long
for successful closed reduction and percutaneous pinning to be carried
out. If there is still lucency along the fracture line, percutaneous
osteoclasis can be performed (372). A pin is
placed dorsally in the fracture site under fluoroscopic control, and
used for reducing the fracture. The subcondylar fossa can be
reconstituted, and the fracture can be pinned percutaneously.
subchondral fossa reconstruction can be performed if there is a bony
block to flexion (370). An average of 90 degrees of PIP joint flexion has been obtained with subchondral fossa reconstruction (370).
Remodeling of the fracture is rare because of the significant distance
from the physis, but it has been described in case reports of both
proximal and middle phalanx phalangeal neck fractures (373).
Observation may be appropriate, but only if the patient is young, there
is only malangulation and not malrotation, and the family is willing to
wait for 1 or 2 years.
small osteochondral fractures. These carry a high risk of nonunion,
malunion, and avascular necrosis (354,374).
This is particularly true in the middle phalanx if the injury is a
crush injury that alters the local blood supply. The fracture is
intraarticular, generally displaced, and requires anatomic reduction
for a successful outcome. Most often, the fracture has to be treated
aggressively with open reduction. The collateral ligament attachments
to the fragment are preserved so as to lessen the risk of avascular
necrosis. On occasion, bone grafting is necessary for maintaining
articular congruity and to prevent collapse. Even with well-performed
open reduction, complications of this fracture can occur in the young
patient (374). Avascular necrosis usually resolves by revascularization, but often not before collapse. Articular malunion can occur (Fig. 23.34). Loss of motion may not limit function.
is similar to that in adults. Anatomic reduction and pin fixation are
necessary to restore the joint surface and to prevent loss of reduction
that can occur with this unstable fracture. Generally, the procedure
can be performed closed, using distraction and a percutaneous towel
clip to obtain reduction (375). Open reduction
can be performed with a volar, midaxial, or dorsal approach. This is
appropriate for fractures that cannot be reduced closed or for
comminuted fractures. Restoration of an anatomic joint will lessen the
risk of loss of motion, malalignment, or long-term arthritis (Fig. 23.34).
young child, and more common in the teenager. The major issue in these
fractures is malrotation (357) (Fig. 23.35).
Frequently, children will not actively move the finger in the acute
setting to allow for accurate assessment of digital alignment. However,
close inspection of the nail plates will reveal the digital
malalignment. In addition, the examiner can test digital alignment by
tenodesis of the wrist. With passive wrist extension, the fingers flex
and point toward the volar scaphoid tubercle. Digital alignment is
generally symmetric. Children will tolerate this test, even when they
are in too much pain or are too frightened to actively move their
digits. Tenodesis assessment should be performed on all phalangeal and
metacarpal fractures, regardless of radiographic appearance. If closed
treatment is chosen, a finger should never be immobilized by itself,
but should be secured to the adjacent digits to prevent subsequent loss
of reduction and malrotation.
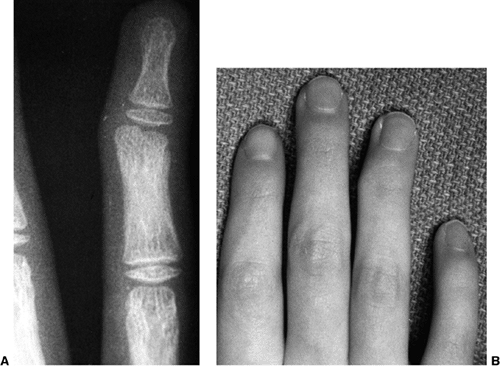 |
|
Figure 23.34 A: Radiograph of a displaced intercondylar fracture with articular malunion. B:
Clinical photograph of a similar patient. This injury requires acute anatomic reduction and pin stabilization to prevent long-term loss of motion, malalignment, and potential pain and arthritis. |
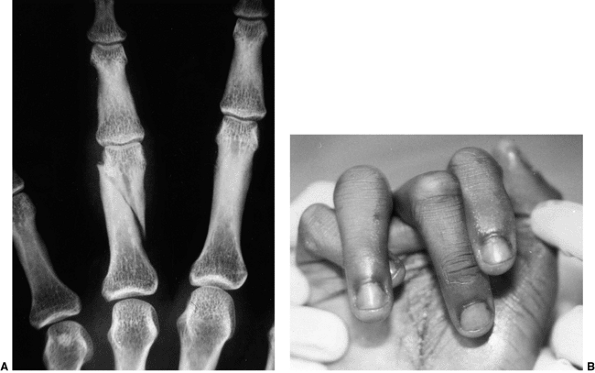 |
|
Figure 23.35 A: Displaced proximal phalanx fracture with malrotation. B:
Tenodesis testing reveals malrotation. By passively extending and flexing the wrist, the digits will passively flex and extend, respectively. Malalignment is evident. |
Although malrotation is uncommon, it is a major problem if missed until
after healing. The malrotated digit impairs the function of the
adjacent digits because the digits will overlap in flexion. At that
stage, malrotation can be corrected only with osteotomy.
A Salter-Harris II fracture of the small finger is the most common of
these fractures. Closed reduction of the abducted fracture is performed
in MCP flexion so as to lessen the restraint of the more distal web
space. The surgeon’s thumb or a cylindrical object such as a pencil can
be used. Postreduction stability is maintained by taping the digit
loosely to the adjacent digit and applying a short-arm mitten cast. The
less common type III physeal fracture requires open reduction if there
is more than 2 mm of diastasis or articular step-off.
common in adolescence. The mechanism is a closed fist injury, most
often to the small-finger metacarpal. These fractures are usually
juxtaphyseal and malaligned, with apex dorsal angulation. In the acute
setting, closed reduction and cast immobilization with three-point
fixation for 3 weeks is preferred for displaced fractures. This
includes volar-to-dorsal pressure on the metacarpal head and
dorsal-to-volar pressure on the more proximal shaft. Often, these
patients will not seek medical attention until there is significant
healing. Fortunately, the fracture is adjacent to the distal metacarpal
physis, and the flexion malunion can remodel if there is sufficient
growth remaining. This fact has led some clinicians to approach these
fractures with neglect to allow for remodeling. Indeed, depending on
the age of the patient, remodeling of the flexion deformity can occur.
However, malrotation will not remodel. In addition, if remodeling is
slow or fails to occur, the prominence of the metacarpal head in the
palm can be limiting.
They should be treated with closed reduction and percutaneous pinning.
Two smooth pins are placed, under fluoroscopic control, from ulnar to
radial, distal to the fracture site(s), from the 5th to the 4th or 3rd
metacarpal, as necessary. Late open reduction of the juxtaphyseal
fracture carries the risk of physeal injury and should be avoided.
Diaphyseal fractures of the fifth metacarpal carry a higher risk of a
malunion that will not remodel. Closed reduction and transverse
percutaneous pinning to the adjacent metacarpals, or intramedullary pin
fixation, is the treatment of choice. Corrective osteotomy may be
necessary in the severe, malunited diaphyseal fracture that fails to
remodel the flexion deformity with growth.
fractures, especially if there are multiple metacarpal fractures, is
malrotation. Active digital motion or passive tenodesis of the wrist
will reveal malrotation. Anatomic reduction and pin, screw, or plate
fixation will correct the malrotation.
in Salter-Harris III fractures of the proximal phalanx, and at the base
of metacarpal fractures. The type III physeal fracture of the thumb
proximal phalanx is the skeletally immature equivalent of an adult
ulnar collateral ligament disruption (378) (Fig. 23.36).
These fractures require open reduction and internal fixation in order
to restore stability of the joint and anatomic alignment of the joint
and physis. During surgical exposure, the surgeon should remember that
the ligament is intact. Therefore, after adductor takedown, the MCP
joint should be exposed through the fracture site rather than through
inadvertent incision of the ligament. The long-term results of anatomic
open reduction are excellent. Metaphyseal fractures at the base of the
thumb metacarpal often displace (379).
Immobilization and observation, even in displaced fractures, are
appropriate as long as there are at least 2 years of growth remaining,
because the malunited dorsoradial prominence will remodel during the
ensuing 6 to 12 months (362) (Fig. 23.37).
Most parents would prefer to wait for remodeling rather than have
operative reduction and pinning. In the markedly displaced and unstable
fracture, closed reduction and percutaneous pinning are appropriate.
The presence of a Bennett fracture with articular malalignment requires
anatomic alignment of the intraarticular component and pin fixation of
the thumb metacarpal to the adjacent second metacarpal and carpus.
wrist. The pain is often mild, rather than the severe pain a child or
parent expects from an acute fracture. This leads some patients and
families to ignore the acute injury
and
not present until late. When the child presents at the acute stage of
the injury, there will be tenderness to palpation over the anatomic
snuff-box (the region dorsoradially at the wrist between the extensor
pollicis longus and the extensor pollicis brevis tendons), over the
volar scaphoid tubercle, and upon axial compression of the thumb CMC
joint. Radiographs may reveal the fracture. The best view is an
anteroposterior view in 30 degrees of ulnar deviation (scaphoid view).
If the radiographs are diagnostic for the fracture, long-arm
immobilization in a thumb spica cast is best. If the radiographs are
negative, but the child has tenderness at the site of the injury, as
noted in the preceding text, protected immobilization for 2 weeks is
advised. Repeat clinical and radiographic examination should be
performed out of cast 2 weeks later if tenderness persists. If there is
still doubt about the diagnosis, MRI or CT scan is advised. The CT scan
shows the bony alignment better, but the MRI gives added information
about the vascularity to the proximal pole, and better information
about the cartilage surfaces in a young child. CT scans are used for
assessing acute fractures in adolescents. MRI scans are used for
assessing the acute injury in the child younger than 10 years of age.
In the acute setting, MRI scans have been diagnostic for the exact site
of injury, and can lead to appropriate treatment early (380,381).
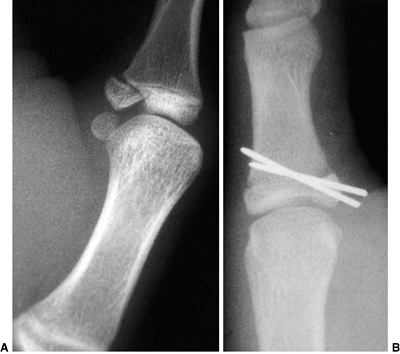 |
|
Figure 23.36 A:
Salter-Harris III displaced proximal phalanx fracture of the thumb. This fracture requires open reduction and internal fixation in order to restore articular congruity and ligamentous stability. B: Postoperative radiograph of a similar patient with pins in place. |
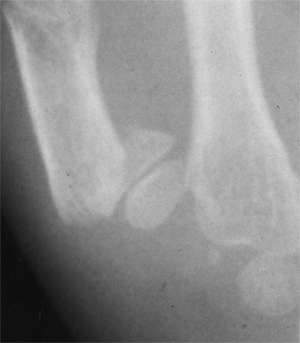 |
|
Figure 23.37
Displaced base in a metacarpal thumb fracture. Because this fracture is juxtaphyseal, adjacent to the carpometacarpal joint with universal motion, its remodeling potential is almost unlimited if the patient is younger than 10 years. Treatment choices are closed reduction and pinning, or immobilization in a cast to allow for biologic remodeling for the ensuing 6 to 12 months of growth. This patient was treated in a cast. |
in the skeletally immature patient were distal pole or avulsion
fractures (374). Distal pole fractures heal
readily with cast immobilization without risk of nonunion or avascular
necrosis. However, scaphoid wrist fractures are becoming more common in
the adolescent age group. These fractures can displace and carry the
same risks of nonunion and avascular necrosis in the child as they do
in the adult. Therefore, nonunions are becoming more commonplace (382,383). Treatment of an established nonunion in a child should be with open reduction, bone grafting, and,
potentially, internal fixation. Internal fixation screws have been used
in children with both acute displaced wrist fractures and established
nonunions (373,382,383) (Fig. 23.38).
It is difficult to determine whether a bipartite scaphoid, even if
bilateral, is congenital or posttraumatic. However, if it is
symptomatic, it should be treated in the same way as a traumatic
nonunion. The success of open reduction, bone grafting, and internal
fixation for a scaphoid nonunion is high in children (382,383). Proximal pole fractures and nonunions have now been described in children and adolescents (384). These fracture nonunions have been treated successfully with vascularized bone graft from the distal radius to the scaphoid.
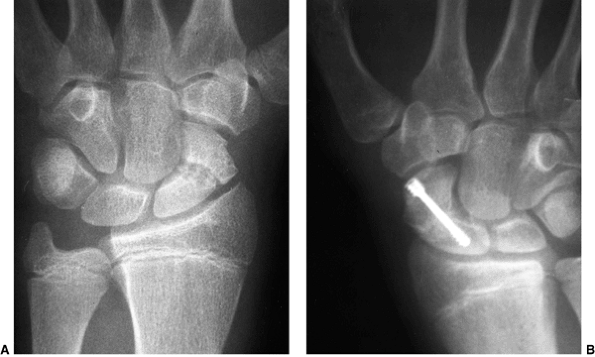 |
|
Figure 23.38 A: Scaphoid nonunion in an adolescent. B: Postoperative iliac crest bone grafting and internal fixation for a similar scaphoid fracture nonunion.
|
injuries. These patients often have generalized ligamentous laxity or a
hyperelasticity syndrome. The wrist pain is similar to the
patellofemoral knee pain and multidirectional shoulder instability pain
seen in this age group. Overuse, growth, and resultant muscle weakness
all contribute to instability of the joints. On physical examination,
there is often systemic evidence of generalized laxity (elbow cubitus
valgus, pes planus, knee hyperextension, passive hyperextension of the
index finger parallel to the dorsal forearm, and thumb abduction to the
volar forearm). At the wrist, there will be increased midcarpal
translation to volar and dorsal applied stress. This passive midcarpal
instability will be equivalent on both the affected and the unaffected
wrist. However, the affected side will often have painful clicking.
Plain radiographs may reveal a volar tilt to the lunate on the lateral
view. MRI scans and arthrograms appear normal. These children respond
to alteration of activities and strengthening, as in other
growth-related overuse injuries in the teenager. It is the author’s
experience that resistive strengthening with therapeutic putty is best.
rarely occur in children. Most descriptions of these injuries have been
case reports (385). However, it is the author’s
experience that these traumatic ligamentous injuries are occurring more
frequently with today’s higher level of athletic competition in this
age group. On physical examination, the tenderness is usually more
focal than in atraumatic ligamentous laxity wrist pain. Asymmetric
clicking with applied ligamentous stress testing is common. Static
injuries are more common in adults, and will show an increased
scapholunate distance and a flexed scaphoid on plain radiographs. Most
scapholunate injuries in children are dynamic injuries, with normal
plain radiographs. In addition, the scapholunate distance in children
has been difficult to interpret because of eccentric ossification and
the chondral nature of the carpus in the young. A recent study of
pediatric wrist radiographs developed an age standard for scapholunate
distances in both sexes (386). This will help
in the evaluation of pediatric carpal injuries. MRI scans may reveal
the ligamentous injury. Arthroscopic examination of the wrist is
diagnostic
and often therapeutic. Many of these injuries are partial tears of the
ligament, with an associated chondral lesion of the radius. This leads
to mechanical impingement that often responds favorably to arthroscopic
debridement. It is rare that there will be a complete ligamentous
disruption in this age group. However, symptomatic complete
scapholunate disruptions need to be treated with ligamentous
reconstruction.
to prolonged rest and therapy should be evaluated for intraarticular
pathology. As in scaphoid fractures and intercarpal ligamentous
injuries, the epidemiology of triangular fibrocartilage complex (TFCC)
tears has changed to include the adolescent. Physical examination for
TFCC tears includes ulnocarpal compressive testing, lunate-triquetral
stress testing, and stress testing of the distal radioulnar joint.
Painful clicking with these maneuvers, especially if asymmetric, may be
indicative of a tear. However, many children with nondissociative
laxity show similar findings on physical examination. Plain radiographs
are normal. The MRI may be diagnostic, but there has been a high
incidence of false-negative readings for adolescent TFCC tears. In
skilled hands arthroscopy is definitive for diagnosis, and often for
treatment (Fig. 23.39).
Most tears are associated with nonunion of ulnar styloid fractures,
radial growth, ulnar overgrowth, and/or ulnocarpal impaction syndrome.
Isolated tears also occur. In adolescents and children, these tears are
usually peripheral tears that respond well to surgical repair (387).
Isolated tears can be repaired arthroscopically. Tears associated with
radial and ulnar bony deformities are repaired at the time of
corrective osteotomy (Fig. 23.40). Treatment
should also include appropriate excision of the ulnar styloid nonunion,
shortening of the impacting ulna, osteotomy of the deformed radius, and
stabilization of the distal radioulnar joint, if necessary (387).
joints in children result in a tear of the volar plate. There may be
associated minimal Salter-Harris III physeal avulsions of the adjacent
phalanx (Fig. 23.41). These injuries are
stable. Treatment should be brief immobilization for comfort, followed
by buddy taping until the patient is asymptomatic.
Prolonged
treatment with splints can lead to PIP joint stiffness. True
interphalangeal dislocations occur less often. The dislocation is
usually dorsal, and it occurs more commonly at the PIP joint than at
the DIP joint. Closed reduction with distraction and dorsal-to-volar
manipulation are generally successful.
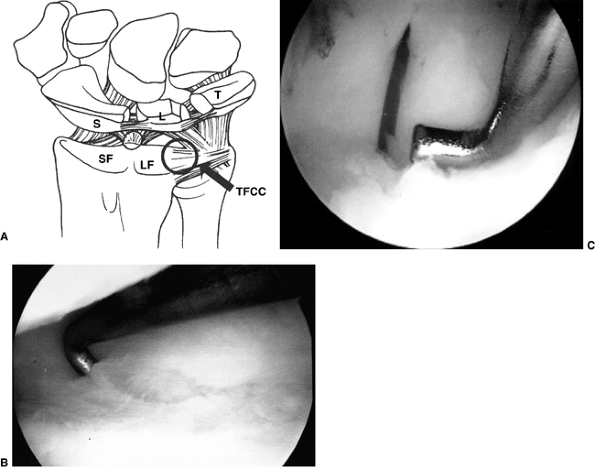 |
|
Figure 23.39 A: Illustration of intraarticular wrist anatomy, as seen from the dorsal 3/4 portal. (S, scaphoid; L, lunate; SF, scaphoid fossa; LF, lunate fossa; T, triquetrum; TFCC, triangular fibrocartilage complex). B:
Arthroscopic photograph of a triangular fibrocartilage complex (TFCC) peripheral tear. The tear is along the peripheral edge of the TFCC, where the blood supply enters and aids in healing. In this situation, repair is by arthroscopic suture techniques. C: Intraoperative photograph of arthroscopic suture repair of peripheral TFCC tear. |
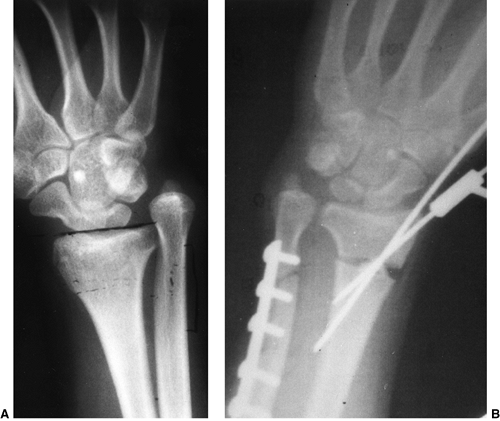 |
|
Figure 23.40 A:
Radiograph of ulnocarpal impaction associated with radial growth arrest, ulnar overgrowth, and a triangular fibrocartilage complex (TFCC) tear. B: Repair of the TFCC, ulnar shortening, and radial osteotomy with bone grafting were performed. |
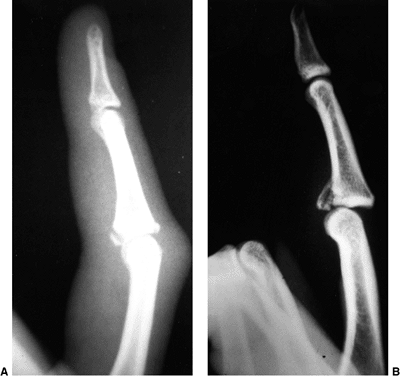 |
|
Figure 23.41 Volar plate injuries. A:
Nondisplaced minor volar and dorsal avulsion injuries. This injury requires minimal immobilization and prompt initiation of range-of-motion exercises to minimize the risk of permanent flexion contracture. B: This injury has a more significant fracture fragment and joint subluxation. It requires anatomic reduction of the proximal interphalangeal joint and an extension block splint to allow for motion within the range of joint stability. |
interposed volar plate can block reduction, demanding an open reduction
procedure. MCP joint dislocation of the thumb or index finger can be
simple or complex (371). Simple dislocations
are reducible in the emergency setting. Complex dislocations are
irreducible, and have an interposed volar plate blocking closed
reduction. Plain radiographic evidence of widening and lateralization
of the joint, as well as bayonet apposition of the proximal phalanx and
metacarpal, indicate an irreducible situation.
approach. In the volar approach, the radial neurovascular bundle is
tented just beneath the skin by the metacarpal head. It is imperative
to be cautious with the skin incision so as not to cause laceration. In
either approach, the volar plate should be incised so as to allow
reduction of the joint and anatomic realignment of the flexor tendons,
sesamoids, and collateral ligaments. Postoperative treatment is by
early protected motion with buddy taping and extension block splinting.
Chronic instability is rare, but limitation of MCP motion is not. The
digital neurapraxia secondary to the dislocation will resolve in this
age group.
for flexor tendon lacerations, is the same for the child as it is for
the adult. The diagnosis of flexor tendon injury, operative care, and
postoperative rehabilitation may be more difficult in the child. This
is especially true in the toddler and preschool-aged child, whose
ability for cooperation is limited. Often, the presenting digital
cascade and digital excursion with wrist tenodesis serve as the basis
for the diagnosis of flexor tendon laceration (Fig. 23.42).
If in doubt, the clinician should explore the wound under anesthesia.
Repair of the tendon lacerations in zones I and II requires meticulous
technique with fine sutures. Repair can be performed electively in the
first 1 to 2 weeks with equivalent results. However, if there is any
concern regarding the vascular status of the digit, repair should be
emergent, with exploration of the digital neurovascular bundles. In the
infant, the core suture may be as fine as 6-0 and the epitenon suture
may be 8-0. Postoperative immobilization in a cast for 4 weeks is
effective protection. Subsequent rehabilitation is necessary in order
to regain maximal passive and active motion.
(TAM) between early mobilization protocols and cast immobilization for
4 weeks in children younger than 15 years (392).
The results of isolated profundus tendon lacerations in zone I averaged
90% to 94% of normal TAM. Isolated profundus lacerations in zone I
averaged 71% to 78% TAM. Combined superficialis and profundus
lacerations in zone II averaged 72% TAM. However, if cast
immobilization continued beyond 4 weeks there was a significant
decrease in TAM, to 40% by 6 weeks. There was no difference in the
results according to age groups from birth to 15 years.
 |
|
Figure 23.42
Photograph of an altered digital flexion cascade with passive wrist extension tenodesis. This is diagnostic of a flexor tendon laceration in the long finger. |
results slightly. Postoperative tendon rupture is rare. Two-stage
reconstruction of unrecognized zone II lacerations in children younger
than 6 years has had poorer results than in adults, with a higher rate
of complications and a mean TAM of approximately 60% of normal. Results
were better with supervised rehabilitation (393).
lacerations in the adult apply to the child as well. Direct repair in
the emergency room under sedation, or in the operating room under
general anesthesia, is preferred. Cast immobilization in a protected
position of wrist dorsiflexion and digital extension is continued for 4
weeks after repair. Results are excellent with primary repair.
Associated fractures, dislocations, and flexor tendon injuries impair
the results.
are very common in children, and have been discussed in detail in the
section on distal phalangeal injuries. Treatment of more proximal,
complete digital amputations with replantation in children as young as
1 year of age is now standard. In children, the indications for
replantation are more liberal than in adults, and include
multiple-digit, thumb, midpalm, hand, and distal forearm amputations as
well as single-digit amputations in zones I and II. Crush amputations
from doors, heavy objects, or bicycle chains have a peak incidence at 5
years, whereas sharp amputations occur more commonly in adolescence.
Digital survival rates from replantation range from 69% to 89% in
pediatric series. More favorable digital survival was seen with sharp
amputations, body weight greater than 11 kg, more than one vein
repaired, bone shortening, interosseous wire fixation, and vein
grafting of arteries and veins. Vessel size generally exceeds 0.8 mm in
digital replants in children, and is not a technical problem for the
skilled microvascular surgeon. Index- and long-finger replants have
better survival than small-finger replants in children. A finger
survival rate of 95% was seen in children if prompt reperfusion
occurred after arterial repair with at least one successful venous
anastomosis, compared with zero survival if one or both of these
factors was absent (394). Neural recovery rates
far exceed those cited in adults, with return of two-point
discrimination of less than 5 mm often present (378).
Tenolysis may rarely be necessary after tendon repair. Two-stage flexor
tendon reconstruction in children has a higher rate of complications
than in adults. Growth arrest or deformity is more common if there is a
crush component to the amputation. These digits are rarely normal after
replanting, although the results in children are better than in adults,
in terms of sensibility and recovery of range of motion. Microvascular
toe-to-thumb transfer is a very successful alternative to pollicization
in the case of a failed thumb replant in a young child (395) (Fig. 23.43).
often present with a decreased neck line, limited motion about the
shoulder, or both. This is secondary to the embryonic failure of one,
or sometimes both, scapulae to descend in utero. The abnormal elevation
is in conjunction with hypoplasia and abnormal alignment of the
scapula. Most often the scapula is small and shaped like an equilateral
triangle rather than having a long medial border. The scapula in
Sprengel deformity usually has abnormal anterior bending
of
the superior pole into the convexity of the upper thoracic region.
There is often limited forward flexion and abduction of the shoulder
because of lack of normal scapulothoracic motion and malpositioning of
the glenoid. In up to 50% of cases there may be an associated
omovertebral bar, which consists of a fibrous, cartilaginous, or bony
connection between the superior medial angle of the scapula and the
cervical spine (397).
Frequently, there is associated abnormal regional anatomy including
scoliosis, spina bifida, clavicular abnormalities, rib anomalies, and
Klippel-Feil syndrome, among others (398,399). Systemic abnormalities include renal and pulmonary disorders. In 10% to 30% of the cases, the condition is bilateral.
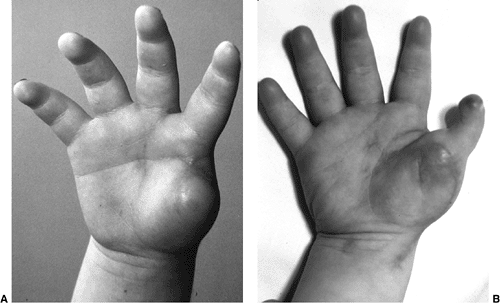 |
|
Figure 23.43 A: Traumatic amputation of the thumb in a 2-year-old child. B: Postoperative photograph of the same patient after microvascular toe-to-thumb transfer for thumb reconstruction.
|
grades the severity of deformity in a rudimentary way: grade I is mild,
with level glenohumeral joints and no deformity visible when the
patient is dressed; grade 2 has level glenohumeral joints, with a lump
in the neck region with the patient dressed; grade 3 is a moderate
deformity with 2 to 5 cm of shoulder elevation; and grade 4 is severe,
with elevation of the scapula to the vicinity of the occiput. The more
severe the deformity, the more likely there are to be limitations of
motion and function and associated regional anatomic anomalies. Surgery
is indicated in children with severe cosmetic and functional
limitations. Surgery does not correct the scapular hypoplasia, but is
indicated for improving shoulder motion by restoring more normal
positioning of the scapula and glenoid. This often consists of excising
any omovertebral connections and surgically derotating and caudally
relocating the scapula. Most of the procedures that are described
include extraperiosteal resection of the superior pole of the scapula (398,400). Subperiosteal resection is associated with a high rate of recurrence (401,402).
In addition to functional indications for surgery, most patients and
families welcome the cosmetic improvement in the appearance of the neck
line.
superior pole of the scapula and any omovertebral connections alone may
be satisfactory treatment. In the moderate and severe deformities, the
scapula is also derotated and moved more distally in order to bring the
glenoid into a more vertical orientation. The purpose of surgery is to
improve the neck contour along with shoulder motion and function.
Indications for functional improvement have been cited for preoperative
abduction less than 110 to 120 degrees (397,403). Surgery is recommended most often in patients between 3 and 8 years of age (404,405,406).
Surgery after 8 years of age is associated with the highest risk of
nerve impairment; clavicular osteotomy is recommended in the older
child in order to lessen the risk of brachial plexus impingement with
scapular descent.
The Woodward procedure moves the scapula distally by detachment and
reattachment of the parascapular muscles at their origins on the spinal
process. The Green procedure involves extraperiosteal detachment of the
scapular insertion of the paraspinal muscles, and reattachment after
the scapula has been moved distally with traction cables. Wilkinson and
Campbell described a vertical scapular osteotomy in conjunction with a
clavicular oseotomy in the older child for improving anterior release
and scapular relocation and for lessening the risk of neurologic
injury. The modified Woodward procedure includes resection of the
superior pole of the scapula in conjunction with surgical scapular
descent and realignment. The results have been reported to have
improved abduction in the range of 40 to 50 degrees (397,411) and achieved a satisfactory cosmetic result. Hypertrophic scar formation, however, has been cited often as a complication.
JWJ, Cairns JM, Gasseling MT. The role of the apical ridge of ectoderm
in the differentiation of the morphological structure and inductive
specificity of limb parts in the chick. J Morphol 1957;101:57.
T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of
233 missed abortions: factors involved in the pathogenesis of
developmental defects of early failed pregnancies. Hum Reprod 2003;18:1724.
JW, Setoguchi Y. Effects of parental adjustment on the adaptation of
children with congenital or acquired limb deficiencies. Journal of Dev Behav Pediatr 1993;14(1):13–20.
EA, Goldner LJ, Swanson AB. Surgery of the spastic hand in cerebral
palsy: report of the Committee on Spastic Hand Evaluation
(International Federation of Societies for Surgery of the Hand). J Hand Surg [Am] 1983;8(5 Pt 2):766–772.
HT, Mital MA, Lenzi WD. Treatment of pronation contractures of the
forearm in cerebral palsy by changing the insertion of the pronator
radii teres. J Bone Joint Surg Am 1981;63(4):645–652.
CA, Gelberman RH, Rhoades CE. Upper extremity tendon transfers in
cerebral palsy: electromyographic and functional analysis. J Pediatr Orthop 1985;5(1):69–72.
H. Melbourne Assessment of Unilateral Upper Limb Function: construct
validity and correlation with the Pediatric Evaluation of Disability
Inventory. Dev Med Child Neurol 2003;45(2):92–96.
A, Freivogel S, Voss A. Exploring a repetitive training regime for
upper limb hemiparesis in an in-patient setting: a report on three case
studies. Brain Inj 2002;16(12):1093–1107.
A, Freivogel S, Schmalohr D. Neurobehavioral aspects of recovery:
assessment of the learned nonuse phenomenon in hemiparetic adolescents.
Arch Phys Med Rehabil 2002;83(12): 1726–1731.
PA, Granat MH. Therapeutic effects of functional electrical stimulation
of the upper limb of eight children with cerebral palsy. Dev Med Child Neurol 2000;42(11):724–727.
JL, Koman LA, Gelberman R, et al. Arthrodesis of the
metacarpophalangeal joint of the thumb in children and adults.
Adjunctive treatment of thumb-in-palm deformity in cerebral palsy. Clin Orthop Relat Res 1990;253:75–89.
MA, Tomas ES, Stone L, et al. Percutaneous phenol block of the
musculocutaneous nerve to control elbow flexor spasticity. J Hand Surg [Am] 1990;15(2):340–346.
I, Larsen A, Taimo A, et al. Management of the upper limb with
botulinum toxin type A in children with spastic type cerebral palsy and
acquired brain injury: clinical implications. Eur J Neurol 2001;8(Suppl. 5):136–144.
PM, Zurakowski D, Patterson P, et al. Interobserver and intraobserver
reliability of therapist-assisted videotaped evaluations of upper-limb
hemiplegia. J Hand Surg [Am] 2004;29(2): 328–334.
LB, Komoto-Tufvesson Y, Salgeback S. Surgery of the spastic hand in
cerebral palsy. Improvement in stereognosis and hand function after
surgery. J Hand Surg [Br] 1998;23(3):334–339.
AC, Ekholm C, Carlstedt T. Hand function in children with cerebral
palsy after upper-limb tendon transfer and muscle release.[see
comment]. Dev Med Child Neurol 1998;40(9): 612–621.
MM, Perry J, Garcia M, et al. Adduction contracture of the thumb in
cerebral palsy. A preoperative electromyographic study. J Bone Joint Surg Am 1983;65(6):755–759.
PR, McCarroll HRJ, Hale R. Biceps tendon rerouting and percutaneous
osteoclasis in the treatment of supination deformity in obstetrical
palsy. J Hand Surg [Am] 1980;5:153.
PM. Comparison of the natural history, the outcome of microsurgical
repair, and the outcome of operative reconstruction in brachial plexus
birth palsy. J Bone Joint Surg 1999;81(5): 649–659.
G, Gilbert A, Helsen K. Obstetric brachial plexus palsy associated with
breech delivery. A different pattern of injury. J Bone Joint Surg Br 1996;78(2):303–306.
DS, Waters PM, Zurakowski D. Reliability of three classification
systems measuring active motion in brachial plexus birth palsy. J Bone Joint Surg Am 2003;85-A(9):1733–1738.
H, Tsuyuguchi Y, Masada K. Identification of the lesion in brachial
plexus injuries with root avulsion: a comprehensive assessment by means
of preoperative findings, myelography, surgical exploration and
intraoperative electrodiagnosis. Neuro-orthopaedics 1989;7:15–23.
ML, Edgerton BW, Kon DS, et al. Comparison of arthroscopic findings
with magnetic resonance imaging and arthrography in children with
glenohumeral deformities secondary to brachial plexus birth palsy. J Bone Joint Surg 2003;85(5): 890–898.
S, Floyd WE, Waters PM. Posterior dislocation of the humeral head in
infancy associated with obstetrical paralysis. A case report. J Bone Joint Surg 1993;75:1370–1375.
PR, McCarroll HRJ, Hale R. Biceps tendon rerouting and percutaneous
osteoclasis in the treatment of supination deformity in obstetrical
palsy. J Hand Surg [Am] 1980;5:233.
PB, Lidov HG, David WS, et al. Diagnostic value of electromyography and
muscle biopsy in arthrogryposis multiplex congenita. Ann Neurol 2003;54:790.
T, Takemitsu Y, Yagihara K, et al. Operation for chronic dislocation of
the radial head in children. Reduction by osteotomy of the ulna. J Bone Joint Surg Br 1987;69(4):639–642.
JO, Ovesen J, Gundorf CE. The stability of the elbow following excision
of the radial head and transection of the annular ligament. An
experimental study. Arch Orthop Trauma Surg 1987;106(4):248–250.
T, Hikino K. Congenital radio-ulnar synostosis: compensatory rotation
around the wrist and rotation osteotomy. [erratum appears in J Hand
Surg [Br] 1987 Oct;12(3):402]. J Hand Surg [Br] 1987;12(2):173–178.
T, Ogisho N, Kanaya F. Follow-up study of joint mobilization of
proximal radio-ulnar synostosis. Paper presented at: American Society
of Surgery of the Hand meeting, Cincinnati, OH, 1994.
P, Laville JM. Rotational osteotomy technique for congenital
radio-ulnar synostosis with central medullary nailing and external
fixation. Rev Chir Orthop Reparatrice Appar Mot 2002;88:812.
WB, Hall JE. One-bone forearm as a salvage procedure for recalcitrant
forearm deformity in hereditary multiple exostoses. J Pediatr Orthop 1993;13(5):587–591.
D. Lengthening and deformity of the upper arm. Paper presented at:
American Society of Surgery of the Hand Congenital Differences
Workshop, Salt Lake City, UT, 1994.
Y, Gomis R, Yoshimura M, et al. Congenital pseudarthrosis of the
forearm-two cases treated by free vascularized fibular graft. J Hand Surg Am 1981;6(5):475–481.
AJ, Weiss AP, Moore JR, et al. Vascularized fibular grafts in the
treatment of congenital pseudarthrosis of the tibia. 1990;72(5):654–662.
SK. Distraction lengthening and microvascular bone transplantation in
the treatment of radial club hand. Paper presented at: American Society
for Surgery of the Hand Congenital Differences Workshop, Salt Lake
City, UT, 1994.
A. Pollicizations in radial club hand. Paper presented at: Second
International Workshop on Congenital Differences of the Upper Limb,
Salt Lake City, UT, 1994.
PD, Dean JC, Duffty P, et al. Weyers’ ulnar ray/oligodactyly syndrome
and the association of midline malformations with ulnar ray defects. J Med Genet 1992;29(9):659–662.
P, Vardeu MP, Dalforno L, et al. Possible relationship between
ulnar-mammary syndrome and split hand with aplasia of the ulna
syndrome. Am J Med Genet 1992;44(6):807–812.
SW, Poorkaj P, Allen T, et al. Fine mapping of the autosomal dominant
split hand/split foot locus on chromosome 7, band q21.3-q22.1. [see
comment]. Am J Hum Genet 1994;55(1):12–20.
P, Kilpatrick MW, Toudjarska I, et al. Split-hand/split-foot
malformation is caused by mutations in the p63 gene on 3q27. Am J Hum Genet 2000;67:59.
LL, van Bokhoven H, Daack-Hirsch S, et al. Analysis of the p63 gene in
classical EEC syndrome, related syndromes, and non-syndromic orofacial
clefts. J Med Genet 2002;39:559.
E, Manservigi D, Garani GP, et al. Limb reduction defects in Emilia
Romagna, Italy: epidemiological and genetic study in 173,109
consecutive births. J Med Genet 1990;27(6):353–357.
D, Pereira JA. Proximal toe phalanx transplantation for bony
stabilization and lengthening of partially aplastic digits. Ann Chir Main Memb Super 1990;9(2):107–118.
S, Hosokawa K, Haramoto U, et al. A new technique for the treament of
syndactyly with osseous fusion of the distal phalanges. Ann Plastic Surg 2000;44:660.
AR, Rafols F, Masson J, et al. Keloid formation after syndactyly
reconstruction: associated conditions, prevalence, and preliminary
report of a treatment method. J Hand Surg [Am] 2004;29:201.
RJ, Kaplan EB. Camptodactyly in similar atraumatic flexion deformities
of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Am 1968;50:1187.
LA, Toby EB, Poehling GG. Congenital flexion deformities of the
proximal phalangeal joint in children: a subgroup of camptodactyly. J Hand Surg [Am] 1990;15:582.
T, Nakamura R, Tamura Y. Long-standing extended dynamic splintage and
release of an abnormal restraining structure in camptodactyly. J Hand Surg [Br] 1992;17(6):665–672.
DW. Langenskiold’s operation (physolysis) for congenital malformations
of bone producing Madelung’s deformity and clinodactyly. J Bone Joint Surg Br 1984;66:778.
Heest AE, House J, Krivit W, et al. Surgical treatment of carpal tunnel
syndrome and trigger digits in children with mucopolysaccharide storage
disorders. J Hand Surg [Am] 1998; 23(2):236–243.
JM, Meggitt BF. Trigger thumbs in children. A review of the natural
history and indications for treatment in 105 patients. J Bone Joint Surg Br 1974;56(1):153–155.
H, Amro S. Characterisitics of patients with hypoplastic thumb: a
prospective study of 51 patients with the results of surgical
treatment. J Pediatr Orthop B 2004;13:127.
M, Yoshizu T, Seki T. Reconstruction of a congenital hypoplastic thumb
with use of a free vascularized metatarsophalangeal joint. J Bone Joint Surg Am 1998;80:1469.
T, Sekiguchi J, Komuro Y, et al. “Face to face”: a new method for the
treatment of polydactyly of the thumb that maximises the use of
available soft tissue. Scand J Plast Reconstr Surg Hand Surg 2000;34:79.
H, Masatomi T, Shimada K, et al. Treatment of residual instability and
extensor lag in polydactyly of the thumb. [see comment]. J Hand Surg [Br] 1993;18(1):5–8.
MB, Dietz FR, Gurnett CA, et al. Localization of dominantly inherited
isolated triphalangeal thumb to chromosomal region 7q36. J Orthop Res 2000;18:340.
E, Nakamura R, Makino H. Triphalangeal thumb without associated
abnormalities: clinical characteristics and surgical outcomes. Plast Reconstr Surg 2001;108:902.
LA. Fracture patterns in children. Analysis of 8,682 fractures with
special reference to incidence, etiology and secular changes in a
Swedish urban population 1950-1979. Acta Orthop Scand 1983;202:1–109.
P, Iselin F, Frajman JM, et al. Fractures of the base of the first
metacarpal in children. Role of K-wire stabilisation. Chir Main 1999;18(3):184–190.
CM, Waters PM, Simmons BP. Nonunion of the scaphoid in children treated
by Herbert screw fixation and bone grafting. A report of five cases. J Bone Joint Surg Br 1995;77(1):98–100.
JL, Christian JD Jr, Goodwin HN, et al. A simplified technique for
treating the complex dislocation of the index metacarpophalangeal
joint. J Bone Joint Surg Am 1975;57(5): 698–700.
B, Rondhuis JJ, Karbowski A. Treatment of congenital elevation of the
scapula. 10 (2–18) year follow-up of 37 cases of Sprengel’s deformity. Acta Orthop Scand 1993;64:365.
JW. Congenital elevation of the scapula. Correction by release and
transplantation of the muscle origins. A preliminary report. J Bone Joint Surg Am 1961;43A:219.
