Entrapment Neuropathies of the Lower Extremity
mapping and monitoring subacute and chronic denervation.13 One study correlating quantitative EMG and MR studies of the anterior tibial muscle depicted a high correlation between the two techniques in chronic denervation.15 In this same study MR imaging depicted denervation in additional muscles that were not, however, studied with EMG. In another large study of 90 patients with clinical evidence of peripheral neuropathy, MR examination demonstrated a sensitivity of 84% and a specificity of 100% compared to EMG studies.17 In a small subgroup of patients, however, abnormal EMG activity was noted in more muscles or in the absence of muscle involvement on STIR MR images.17 Comparison of MR studies with EMG in 40 patients with neurogenic foot drop revealed agreement in 92% of the patients.18 In a small percentage of these patients, MR imaging detected more widespread muscle disease than found on the EMG studies. In one patient a follow-up EMG study confirmed the MR finding.18
 |
|
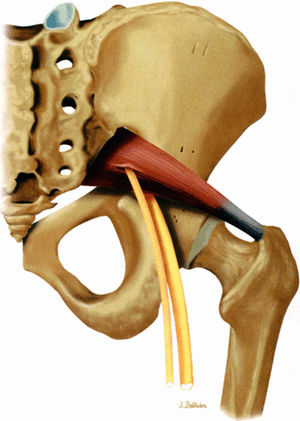
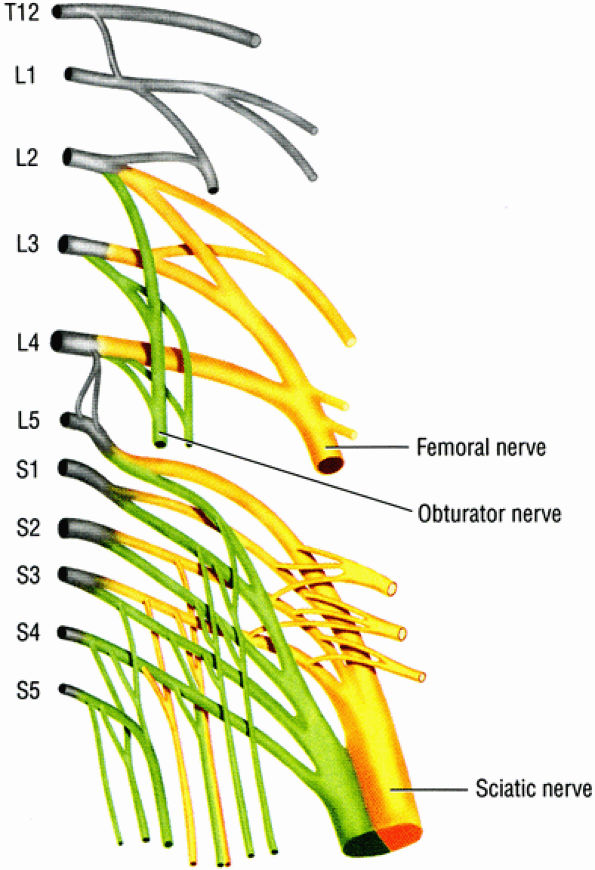
FIGURE 6.1 ● The origins of the three major nerves supplying the lower extremity: the sciatic nerve, the femoral nerve, and the obturator nerve.
|
 |
|
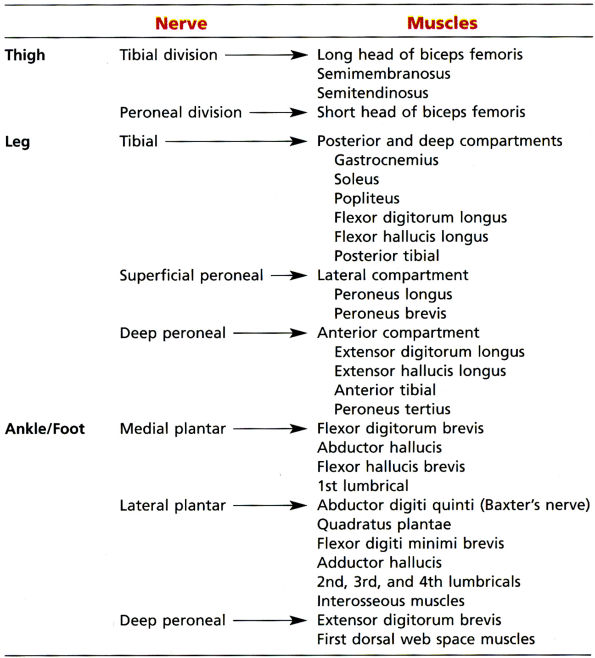
TABLE 6.1 ● Major Motor Innervations of the Lower Extremity
|
-
The etiology of neuropathy may be related to mechanical or dynamic causes.
-
Nerve entrapment may result in motor, sensory, and autonomic deficits.
-
Nerve injury is classified in order of severity from neurapraxia, to axonotmesis, to neurotmesis.
the symptoms manifest only after a superimposed external force, such as an ill-fitting shoe, aggravates the nerve compression. Trauma, surgery, and ankle inversion injuries are known to cause stretch injuries to many of the nerves of the lower extremity. Hormonal changes, preexisting polyneuropathy, and systemic diseases such as diabetes mellitus and rheumatoid arthritis can also predispose to nerve entrapment.
-
Motor function impairment, such as weakness, paralysis, and muscle atrophy
-
Sensory deficits, such as pain and paresthesias
-
Autonomic deficits, such as dry skin and ulceration
-
Neurapraxia, or first-degree nerve injury, is an early and reversible segmental conduction block without axonal disruption ranging from transient ischemia (such as occurs from crossing the legs) to myelin sheath injury. Neurapraxia usually resolves within hours, days, or months with no residual sequelae.
-
Axonotmesis, or second-degree nerve injury, represents more advanced nerve damage, in which a focal disruption of the axon produces Wallerian degeneration distal to the site of injury. However, the intact investing connective tissues around the nerve allow axonal regeneration at a rate of approximately 1 to 2 mm a day, and the prognosis is still relatively good. Depending on the distance between the site of nerve injury and the muscle affected, axonotmesis can resolve within weeks to months.
-
Neurotmesis, or third-degree nerve injury, is characterized by an irreversible and complete axonal and connective tissue disruption. In addition to significant functional impairment, neurotmesis can produce painful neuromas secondary to unsuccessful attempts at nerve regeneration.
-
Nerve visualization requires imaging in all three orthogonal planes using high-resolution T1-weighted images and fluid-sensitive fat-suppressed images (e.g., FS PD FSE or STIR).
-
MR intravenous contrast is used to assess mass lesions associated with nerve compression.
-
Normal nerves do not enhance with intravenous contrast administration.
-
MR T2 neurography selectively emphasizes fluid signal from nerves.
-
Chronic nerve damage is associated with fatty infiltration and decreased muscle bulk.
-
Partially reversible nerve damage displays increased signal on T1- and T2-weighted images.
-
In the double crush syndrome, a proximally compromised nerve is more susceptible to entrapment distally.
-
Distal entrapment affects the proximal nerve in the Valleix phenomenon.
-
A chemical shift-selective pulse (CHESS), which suppresses fat signal from around and within the nerves
-
High TE values (approximately 90 msec), which suppress signal from adjacent muscle
-
Radiofrequency saturation pulses, which suppress fluid signal from vessels
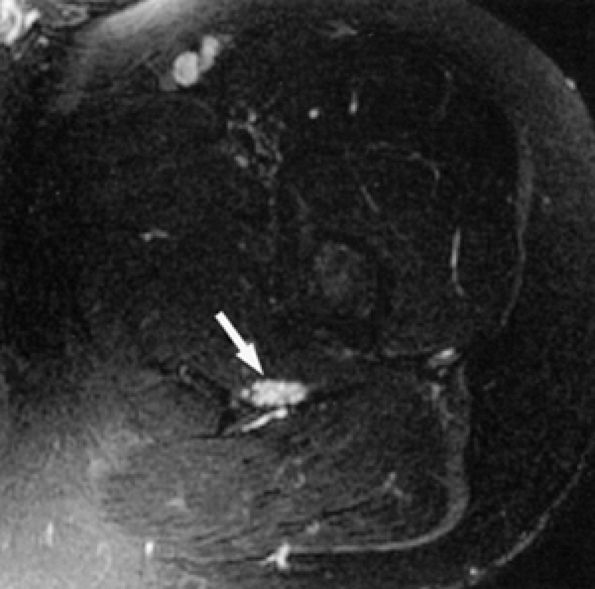 |
|
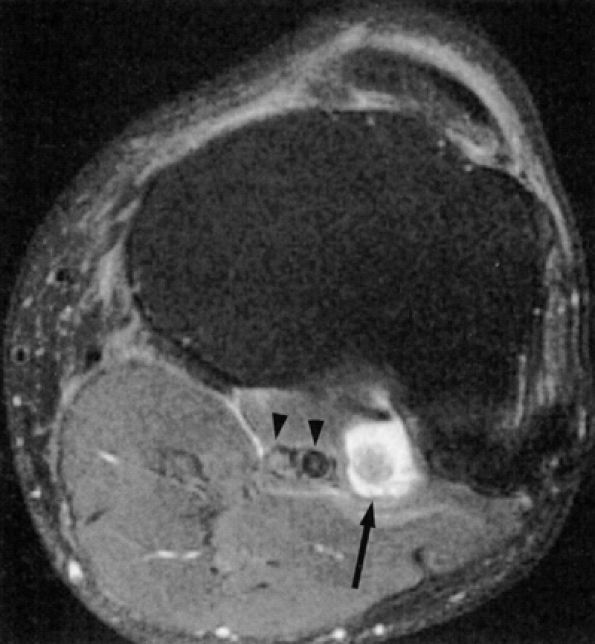
FIGURE 6.2 ● MR neurography of a young patient with sciatic neuritis. An axial T2-weighted fat-suppressed image (TE 90 msec, 3T) demonstrates increased signal in the sciatic nerve fascicles (arrow). Note suppression of signal from adjacent muscles and vessels.
|
-
Peripheral nerves display intermediate signal intensity on T1- and PD-weighted images with a mild increase on fluid-sensitive sequences (signal intensity usually within the intermediate range on FS PD FSE images).
-
The fascicular anatomy of larger nerves demonstrates honeycomb morphology on axial images.
-
Nerves are seen as ovoid to round structures on axial MR images.
-
Longer segments of a nerve can occasionally be appreciated on coronal and sagittal images.
-
The fascicular anatomy of larger nerves demonstrates a honeycomb appearance, best appreciated on high-resolution axial MR images. This pattern is difficult to visualize in the smaller nerves.
-
On axial MR images, it is not uncommon to detect major divisions of the larger nerves before they actually branch away from each other. The peroneal and tibial divisions of the sciatic nerve can be visualized as they descend together in the thigh. Similarly, in the distal knee, the common peroneal nerve is subdivided into its two major branches, the superficial and deep peroneal nerves, long before the two divisions physically separate from one another.
-
The saphenous nerve travels in close proximity to the sartorius muscle throughout most of its course in the thigh and knee.
-
The common peroneal nerve courses along the posteromedial aspect of the biceps femoris muscle.
-
Identifying the medial plantar nerve in the foot is made easy by following the flexor hallucis longus tendon.
-
Direct evidence of nerve pathology consistent with alterations in the course, morphology, and signal characteristics of the affected nerve
-
Indirect signs of entrapment evidenced by muscle signal and size alterations compatible with denervation
-
Nerve swelling and hyperintensity may represent an advanced process of nerve damage with fascicular and endoneurial edema.
-
Muscle denervation is associated with motor nerve entrapment.
-
On fluid-sensitive images hyperintense muscle signal represents subacute denervation (denervation edema).
-
Course deviations. Mechanical deviation of the nerve from its normal course may be related to static conditions associated with mass effect such as bone spurs, scar, soft tissue tumors, ganglia, and aberrant muscles. These processes are easily depicted with MR imaging. Course deviation and entrapment of a nerve, however, can be dynamic and may be appreciated only in certain predisposing limb positions, such as foot pronation in medial plantar nerve entrapment or ankle plantar flexion and dorsiflexion in superficial peroneal nerve entrapment secondary to a muscle hernia.2 These types of entrapments can be missed on routine MR studies and may require additional studies following changes in limb positioning.
-
Morphologic and signal changes. Size and signal alterations also represent direct MR evidence of nerve pathology. Nerve swelling is compatible with edema at the fascicular level and may be a late sign of disease. In the smaller nerves, however, size differences can be difficult to assess. When present, edema may accentuate the honeycomb fascicular appearance of the nerve. Conversely, focal effacement of the normal fascicular pattern may also be encountered.19 The increased nerve signal is believed to reflect endoneurial edema and may also represent a fairly advanced process. Since most normal nerves have mildly increased signal on fluid-sensitive images, assessment of signal alterations can be challenging. The increased signal in asymptomatic individuals is usually mild and also involves the whole course of the nerve, whereas pathologic increased signal is typically focal and is usually found in areas susceptible to entrapment.
-
Muscle denervation. Motor nerve entrapment can be inferred when there is MR evidence of muscle denervation. Muscle denervation is characterized by signal alterations seen on both T1- and T2-weighted images. These alterations serve to localize the site of entrapment and provide information on the extent of motor damage.18 Bright signal on T2-weighted images is consistent with a fluid shift, due to muscle cell atrophy, from intracellular to extracellular compartments without a significant increase in the total water content of the muscle.35 Increased blood perfusion to the muscle may also contribute to denervation signal alterations. Increased muscle signal on fluid-sensitive images has been labeled as acute denervation edema, although strictly speaking it may be more accurate to refer to it as subacute denervation. Reversal of the muscle signal to normal may be encountered once the entrapment has resolved.16,36
-
Fatty infiltration. In chronic nerve damage there is fatty infiltration and decreased muscle bulk indicative of relatively irreversible muscle atrophy. Chronic denervation is depicted as bright muscle signal on T1-weighted images. Often, ongoing nerve damage manifests as increased signal on T1- and T2-weighted images. This constellation of findings indicates an ongoing partially reversible process. Rarely, muscle pseudohypertrophy related to excessive fatty infiltration and true muscle hypertrophy have also been noted with chronic denervation.37
-
The double crush syndrome is a phenomenon in which a proximally compromised nerve has a decreased threshold for injury and as a result is more susceptible to entrapment distally.2,25,38,39,40,41 As a result, it is not uncommon to find two sites of entrapment present simultaneously. Spinal stenosis, for example, predisposes to nerve entrapment more distally. Simultaneous entrapment of the medial plantar nerves at both the tarsal tunnel and at the foot may also occur.
-
In the Valleix phenomenon, a distal entrapment affects the more proximal nerve.2,25,42 This process may, in fact, be the same as the double crush syndrome. Both entities can produce an odd distribution of muscle denervation signal, and the concomitant presence of two entrapment sites should be carefully looked for.
-
Selective muscle involvement is a common occurrence that manifests clinically, electrodiagnostically, and on MR examination as selective damage of only a few muscles along a specific nerve distribution. Sciatic nerve injury, for example, typically affects the peroneal division and spares the tibial division, partly because the former is more superficial, has larger fascicles and less connective tissue, and is fixed at two places. Similarly, entrapment of the common peroneal nerve usually affects the deep peroneal nerve more than the superficial peroneal nerve because the former is in closer proximity to the fibular head. This selective fascicular involvement can also occur within a single nerve branch,
P.1056
and its etiology is not always known.19 On MR images it is depicted as signal alterations in only a select group of muscles along a single nerve distribution. For example, it is not unusual for only the plantaris and popliteal muscles to depict muscle denervation changes in tibial nerve entrapment. Similarly, denervation signal isolated to the anterior tibial muscle with relative sparing of the extensor digitorum longus muscle is often depicted in common peroneal neuropathy. Neurography may illustrate greater involvement of isolated nerve fascicles.![]() FIGURE 6.3 ● Proximal tibial entrapment in a 49-year-old patient with neuropathic foot pain. Symptoms resolved following an intra-articular steroid injection of the knee. This axial T2-weighted fat-suppressed image depicts a loose body in the popliteal muscle bursa (arrow), abutting on the neurovascular structures (arrowheads).There are many variations in muscle innervation and many communicating nerve loops in the lower extremity, particularly in the foot region.43 For example, the deep peroneal nerve may, in rare instances, supply muscles such as the adductor hallucis and flexor hallucis brevis, muscles typically innervated by the lateral and medial plantar nerves. This variability can affect the distribution of signal alterations within denervated muscles and may produce puzzling MR patterns. Familiarity with variations in innervation aids in interpreting unexpected muscle denervation signal alterations.
FIGURE 6.3 ● Proximal tibial entrapment in a 49-year-old patient with neuropathic foot pain. Symptoms resolved following an intra-articular steroid injection of the knee. This axial T2-weighted fat-suppressed image depicts a loose body in the popliteal muscle bursa (arrow), abutting on the neurovascular structures (arrowheads).There are many variations in muscle innervation and many communicating nerve loops in the lower extremity, particularly in the foot region.43 For example, the deep peroneal nerve may, in rare instances, supply muscles such as the adductor hallucis and flexor hallucis brevis, muscles typically innervated by the lateral and medial plantar nerves. This variability can affect the distribution of signal alterations within denervated muscles and may produce puzzling MR patterns. Familiarity with variations in innervation aids in interpreting unexpected muscle denervation signal alterations. -
Finally, the distance from the site of entrapment to the innervated muscle should be considered when searching for muscle denervation abnormalities. Proximal damage to the peroneal division of the sciatic nerve may depict denervation signal in the leg or foot. This signal alteration may be missed if only the thigh is being imaged. Similarly, MR imaging of a painful foot may overlook a more proximal entrapment in the leg or thigh (Fig. 6.3). It is important to pay careful attention to signal changes on sagittal and coronal planes, where larger portions of the limb are illustrated, to help avoid this pitfall. If the clinical suspicion for entrapment is high and no abnormalities are noted on the initial study, imaging a more distal or proximal section of the limb can also be performed.
-
Sciatic neuropathy is associated with total hip replacements, especially revisions.
-
Sciatic neuropathy more commonly affects the peroneal nerve division.
-
The piriformis syndrome is related to spasticity, irritability, or inflammation of the piriformis muscle.
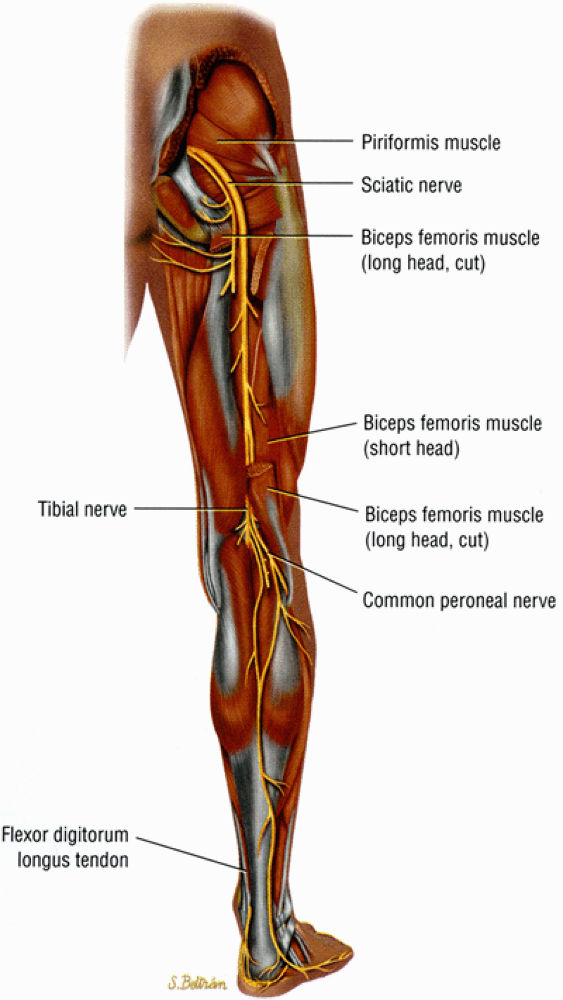 |
|
FIGURE 6.4 ● A posterior view of the right lower extremity demonstrating the sciatic nerve and its tibial and peroneal divisions.
|
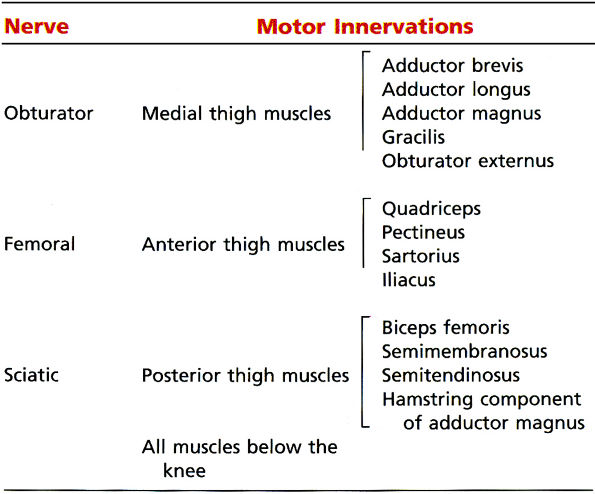 |
|
TABLE 6.2 ● Motor Distribution of the Sciatic Nerve
|
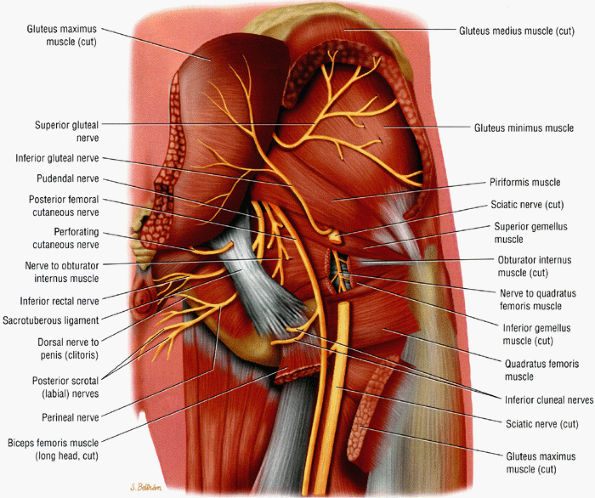 |
|
FIGURE 6.5 ● A posterior view of the sciatic nerve in the greater sciatic foramen. The nerve descends anterior to the piriformis muscle and successively posterior to the superior gemellus, obturator internus, inferior gemellus, and quadratus femoris muscles.
|
-
The sciatic nerve is highlighted by fat as it courses in the greater sciatic foramen anterior to the piriformis muscle and lateral to the internal iliac vessels.
-
The nerve follows the course of the piriformis muscle and can be traced as it curves around the ischial spine into a more posterior and lateral location in the upper thigh. There the nerve is seen within an abundant stripe of fat between the greater trochanter and the ischial tuberosity (Fig. 6.8).
-
In the upper thigh the nerve is anterior to the gluteus maximus muscle and posterior, successively, to the superior gemellus, the obturator internus, the inferior gemellus, and the quadratus femoris muscles. Further down the nerve is anterior to the hamstring muscles and posterior to the adductor magnus muscle.
-
There is a gradual decrease in the amount of fat surrounding the nerve as it descends down the thigh. Finally, the nerve is flattened between the biceps femoris and the adductor magnus muscles.
-
In the lower thigh and proximal knee, as the adductor magnus muscle mass progressively disappears, the nerve is again highlighted by a significant amount of fat.
-
Not infrequently, the tibial and peroneal divisions of the nerve can be detected as they descend side by side in a common sheath before they diverge from each other in the proximal knee (see Fig. 6.8B).
-
The posterior femoral cutaneous nerve, located posterior to the sciatic nerve, can sometimes be identified as it migrates into a more superficial location in the upper thigh.
 |
|
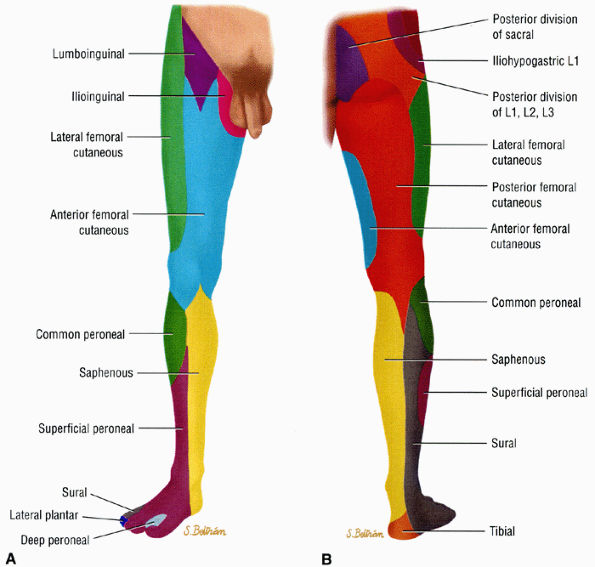
FIGURE 6.6 ● Anterior (A) and posterior (B) views of the sensory innervation of the lower extremity.
|
 |
|
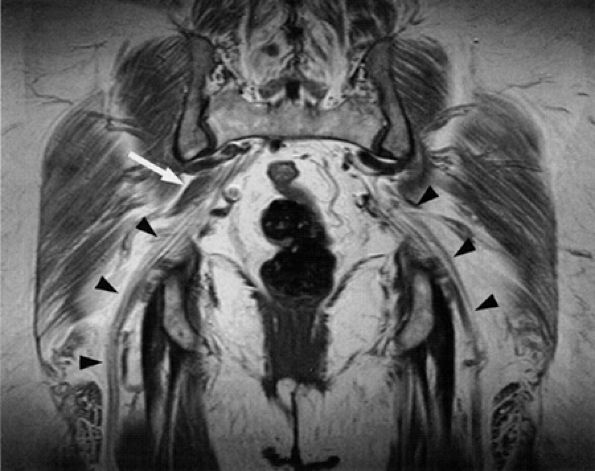
FIGURE 6.7 ● Coronal T1-weighted image of the sciatic nerve demonstrating the normal fascicular pattern of both sciatic nerves (arrowheads). A slip of the right piriformis muscle is noted (arrow).
|
 |
|
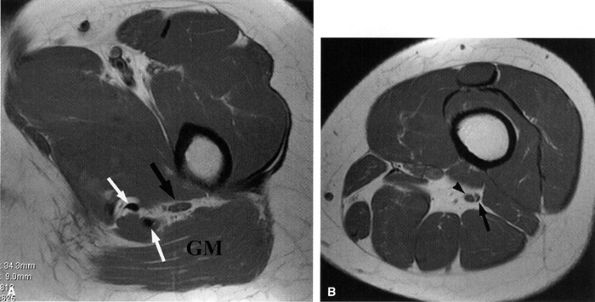
FIGURE 6.8 ● Normal sciatic nerve. (A) An axial PD-weighted image at the upper thigh demonstrates a normal ovoid-shaped sciatic nerve (black arrow) anterior to the gluteus maximus muscle (GM), highlighted by the fat. Note the proximity of the nerve to the hamstring tendons (white arrows). (B) An axial PD-weighted image at the middle thigh depicts the tibial (arrowhead) and smaller peroneal (arrow) divisions of the sciatic nerve in a common sheath before they diverge in the proximal knee.
|
-
The peroneal division is fixed at two points (at the sciatic foramen and at the fibular head), whereas the tibial division is fixed only at the sciatic foramen.
-
The peroneal division has larger fascicles with less protective surrounding connective tissue.
-
The peroneal division is more superficial and lateral than the tibial division.
and in the plantar surface of the foot (tibial division) are other manifestations of sciatic neuropathy.
-
The greater sciatic foramen is bordered superiorly and laterally by the sacrum and the iliac bone and by the sacroischial and sacrotuberous ligaments inferiorly and medially.
-
The piriformis muscle occupies a large part of the foramen as it extends from the sacrum to the greater trochanter (see Fig. 6.5).
-
A bursa is often found between the muscle and the bone.
-
The sciatic nerve traverses the greater sciatic foramen anterior to the piriformis muscle and is susceptible to compression due to loss of sciatic foramen volume, trauma, inflammation, local ischemia, and anatomic variations.
 |
|
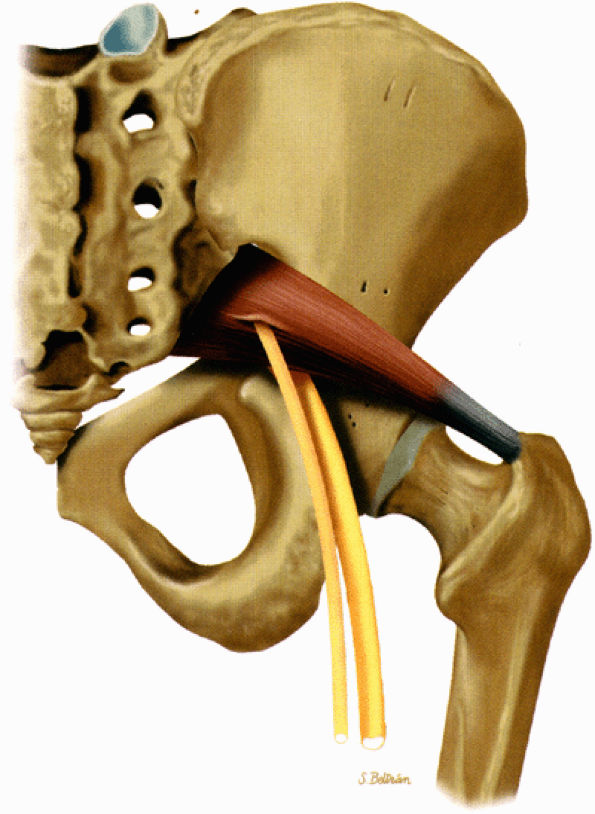
FIGURE 6.9 ● The piriformis muscle pierced by the peroneal division of the sciatic nerve (anatomic variation).
|
affecting the sciatic nerve such as amputation neuromas (Fig. 6.15), can also be evaluated on MR images.
 |
|
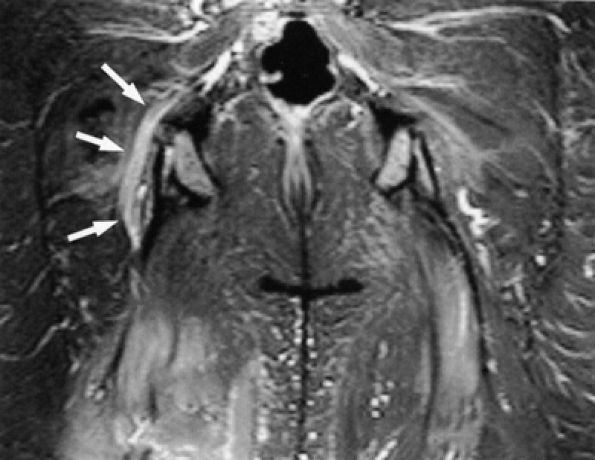
FIGURE 6.10 ● Sciatic neuropathy associated with rhabdomyolysis. Increased signal in the sciatic nerve (arrows) is shown on this coronal fluid-sensitive fat-suppressed image. Note the increased signal in multiple muscles.
|
 |
|
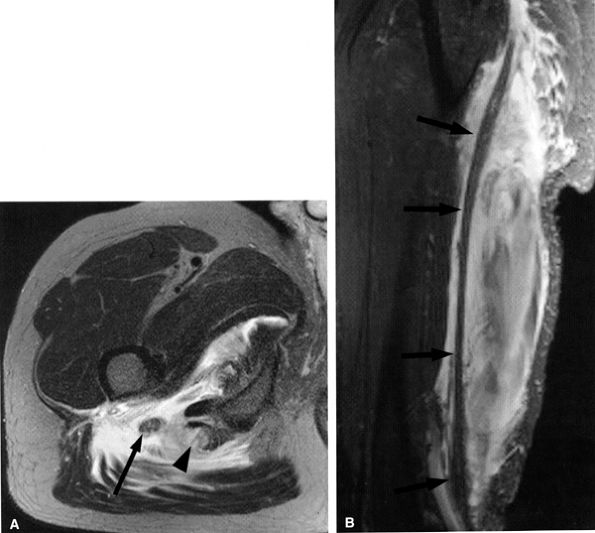
FIGURE 6.11 ● Hamstring tendon tear with secondary edema around the sciatic nerve. Axial T2-weighted (SA) and sagittal T2-weighted fat-suppressed (B) images show partial avulsion of the conjoined tendon of the biceps and semitendinosus muscles (arrowhead) with extensive edema around the sciatic nerve (arrows).
|
 |
|
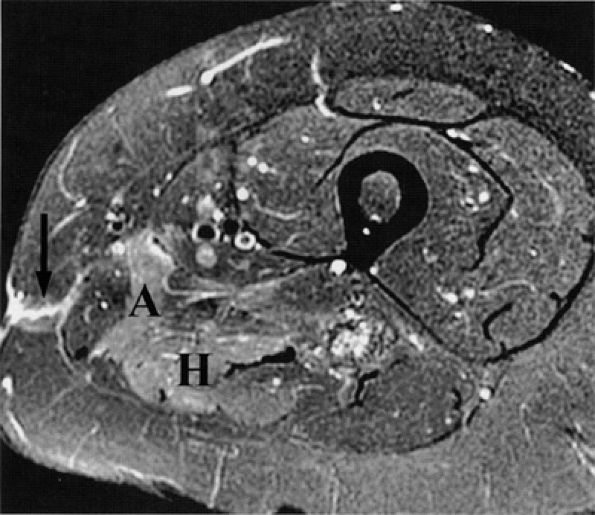
FIGURE 6.12 ● Denervation edema after resection of a sciatic nerve tumor. This axial T2-weighted image demonstrates denervation edema in the hamstring muscles (H) and adductor magnus muscle (A).
|
 |
|
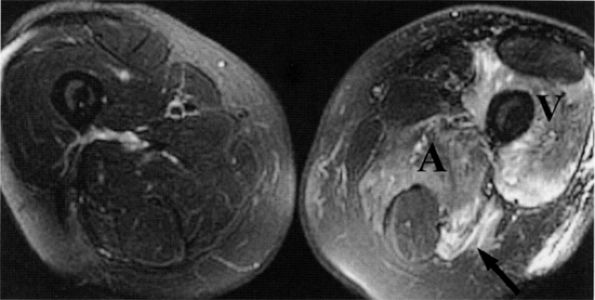
FIGURE 6.13 ● Lumbosacral plexopathy with denervation edema in the femoral, obturator, and sciatic nerve distribution. Increased signal in the vasti muscles (V) and adductor magnus (A) and hamstring muscles (arrow) is shown on this axial T2-weighted fat-suppressed image.
|
 |
|
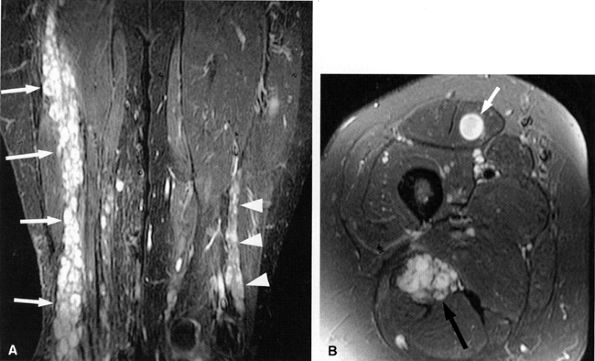
FIGURE 6.14 ● Plexiform neurofibromatosis. (A) Coronal T2-weighted fat-suppressed image depicting fusiform enlargement and increased signal of the right (arrows) and left (arrowheads) sciatic nerves. (B) Axial T2-weighted fat-suppressed image demonstrating the “bag of worms” appearance of the right sciatic nerve (black arrow). Note an intramuscular neurofibroma in the rectus femoris muscle (white arrow).
|
 |
|
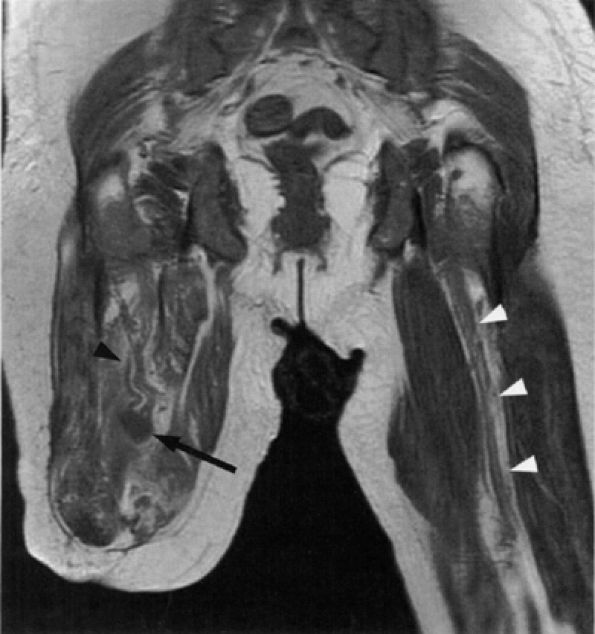
FIGURE 6.15 ● Amputation neuroma. Coronal T1-weighted image demonstrating an amputation neuroma in the right sciatic nerve (arrow). Compare the thickened fascicular pattern of the nerve proximal to the neuroma (black arrowhead) with the normal left sciatic nerve (white arrowheads). (Courtesy of Laercio Rosenberg, MD, Sao Paulo, Brazil)
|
 |
|
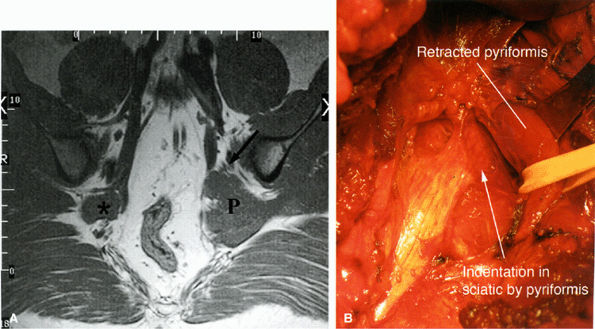
FIGURE 6.16 ● Surgically proven left piriformis muscle syndrome. (A) Axial T1-weighted image demonstrates a markedly enlarged left piriformis muscle (P) displacing the sciatic nerve (arrow). The normal right piriformis (asterisk) is much smaller. (B) A posterior view during surgery demonstrates indentation on the left sciatic nerve from the piriformis muscle. (Courtesy of Stephen Russel, MD, NY)
|
-
Significant asymmetry in the piriformis muscles is noted between the symptomatic and asymptomatic sides (Figs. 6.16 and 6.17). Mild asymmetry of the muscles, however, is often present in asymptomatic individuals.
-
Presence of selective atrophy49,50 or hypertrophy of the piriformis muscle and accessory muscle slips47,52
-
Detection of the sciatic nerve or one of its divisions within the substance of the piriformis muscle. An intramuscular location of the sciatic nerve, however, may be a normal variation in up to 10% of individuals.
-
Increased signal in the nerve and in the piriformis muscle may also be seen.49
 |
|
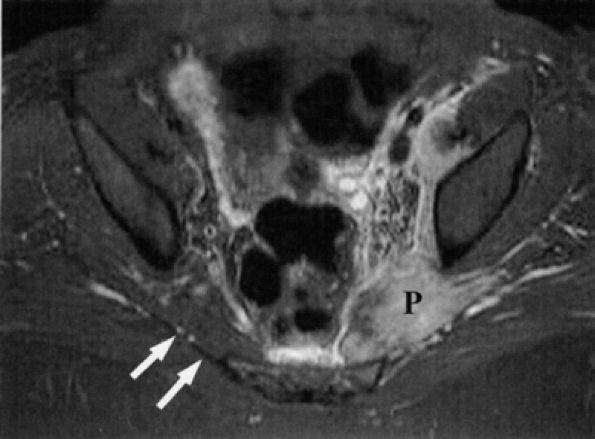
FIGURE 6.17 ● Piriformis muscle syndrome in a patient kicked by a horse in the left buttock. This axial T2-weighted fat-suppressed image shows engorgement and increased signal in the left piriformis muscle (P), with edema in the region of the sciatic nerve. Compare with a normal right piriformis muscle (white arrows). (Courtesy of Frank Crnkovich, MD, Colorado)
|
-
The saphenous nerve is the largest branch of the femoral nerve.
-
Femoral nerve compression frequently occurs underneath the inguinal ligament.
-
Anterior compartment (thigh) muscle atrophy is associated with femoral nerve entrapment.
-
Saphenous nerve entrapment occurs in the adductor canal.
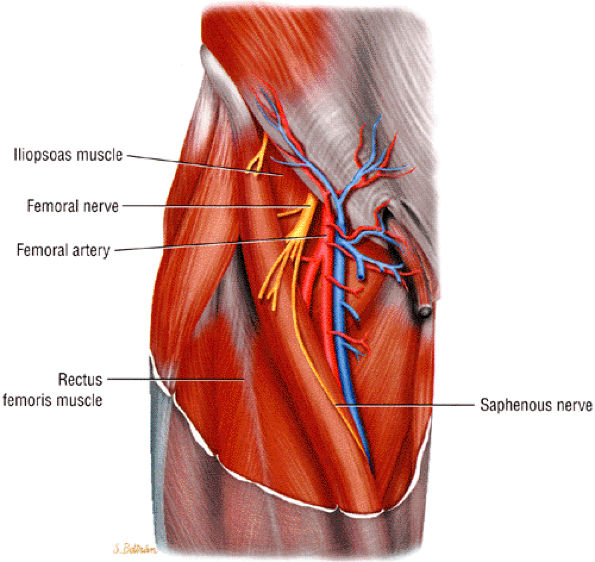 |
|
FIGURE 6.18 ● The femoral nerve and its branches.
|
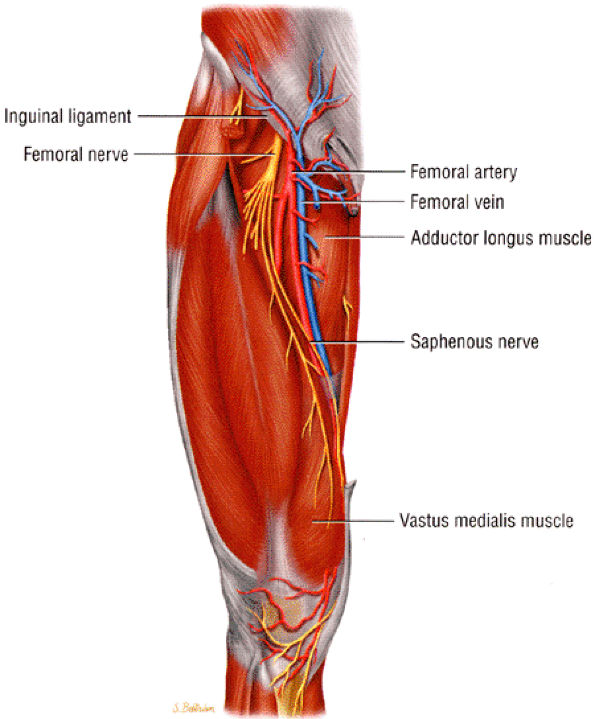 |
|
FIGURE 6.19 ● The saphenous nerve as it descends down the thigh to enter the adductor canal.
|
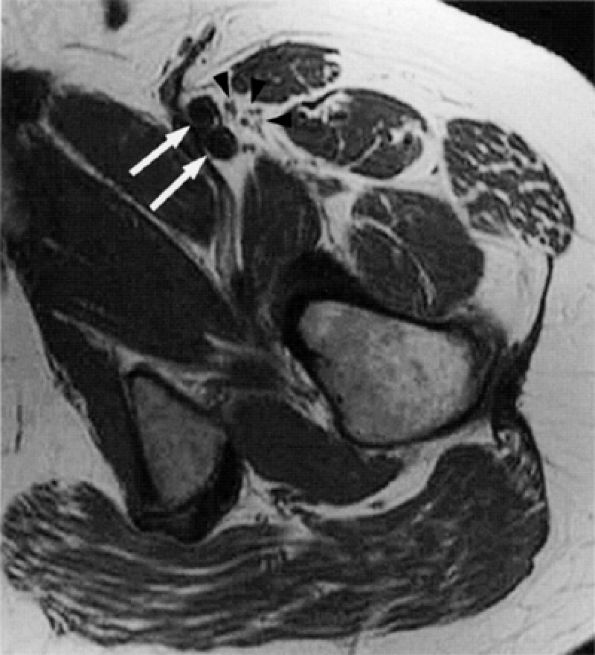 |
|
FIGURE 6.20 ● The normal femoral nerve in the upper thigh. Axial T1-weighted image demonstrating the normal bundles of the femoral nerve (arrowheads) lateral to the femoral vessels (arrows).
|
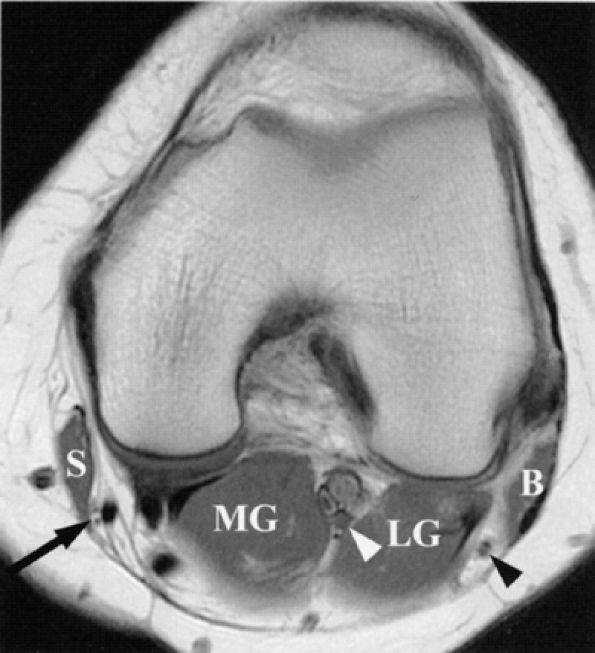 |
|
FIGURE 6.21 ● Normal saphenous, tibial, and common peroneal nerves in the knee. An axial PD-weighted image depicts the branches of the saphenous nerve (arrow) lateral to the sartorius muscle (S); the tibial nerve (white arrowhead) between the lateral (LG) and medial (MG) heads of the gastrocnemius muscle; and the common peroneal nerve (black arrowhead) between the gastrocnemius muscle and biceps femoris muscle (B). Note that the common peroneal nerve is already split into its deep and superficial branches.
|
-
Buckling and decreased knee extension (quadriceps weakness)
-
Decreased hip flexion
-
Difficulty arising from a sitting position and climbing up stairs (iliopsoas muscle weakness)
-
Atrophy of the muscles of the anterior compartment
-
Absent knee jerk
-
Sensory deficits in the anterior and medial lower thigh, and medial knee, leg, and foot
arthroscopy and occurs in 7% to 22% of patients undergoing arthroscopic meniscal repair.59,67,68,69,70,71 Nerve injury has also been noted in patients with posterolateral instability, possibly related to stretching of the nerve.72
 |
|
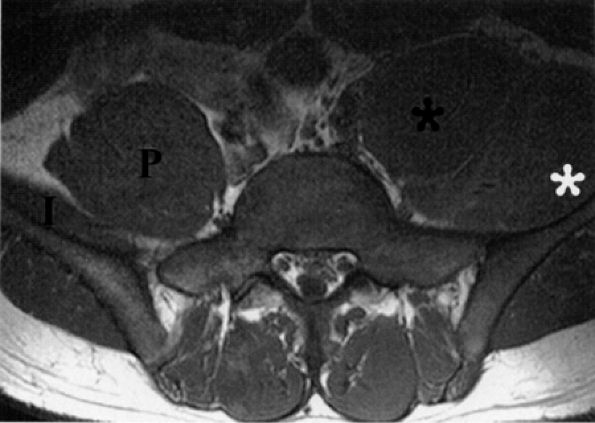
FIGURE 6.22 ● Femoral neuropathy secondary to sports-related iliacus muscle tear. Axial T1-weighted image demonstrating enlargement of the left iliac (white asterisk) and psoas muscles (black asterisk). The anteriorly displaced femoral nerve is not visualized. Compare with the right normal iliac (I) and psoas (P) muscles.
|
-
Entrapment neuropathy is usually associated with trauma and postsurgical injury to the inguinal area and pelvis.
-
Pelvic tumors, including metastatic extension, may cause obturator neuropathy.
-
Groin pain in athletes is associated with involvement of the anterior branch of the obturator nerve distal to the obturator tunnel.
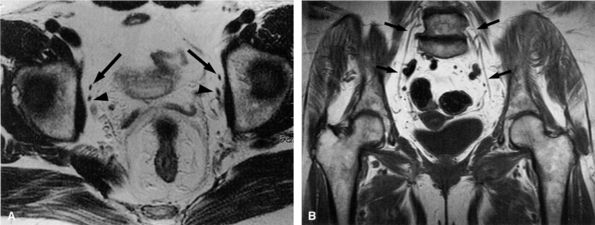
structure (Fig. 6.24). Occasionally, the nerve is seen on anterior coronal images (see Fig. 6.24B). The nerve can be traced as it originates from the lumbar spine and traverses inferiorly and anteriorly surrounded by a large amount of pelvic retroperitoneal fat. The nerve is located anterior to its accompanying vessels and can be followed as it dives under the superior pubic ramus to enter the obturator foramen. The anterior division of the nerve is depicted in the thin fat plane posterior to the pectineus and adductor longus muscles and anterior to the adductor brevis muscle. The posterior branch is identified in a fat stripe between the adductor brevis and adductor magnus muscles.
 |
|
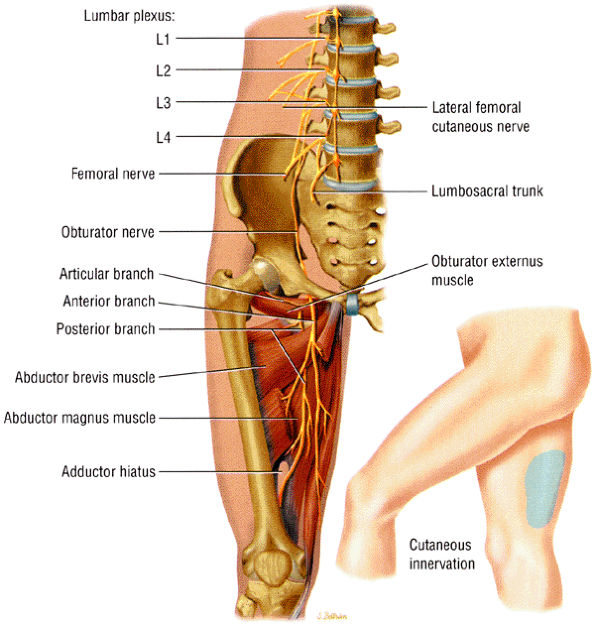
FIGURE 6.23 ● The normal obturator nerve. The insert shows the sensory distribution of the nerve along the medial thigh.
|
 |
|
FIGURE 6.24 ● Normal obturator nerves in the pelvis. (A) Axial T1-weighted image depicting the nerves (arrows) medial to the acetabula and anterior to the obturator vessels (arrowheads). (B) Coronal T1-weighted image demonstrating the nerves (arrows) surrounded by fat.
|
-
Pain in the groin or medial thigh. Radiation of pain to the medial knee can occur and is related to innervation of the knee joint by the posterior branch of the obturator nerve.
-
Complete sensory loss (uncommon due to overlapping innervation by other cutaneous nerves)
-
Adductor muscle weakness, which may be initially masked since the adductor magnus muscles are also innervated by the sciatic nerve
-
Weak thigh adduction and wide gait due to abducted hip in advanced cases
-
Atrophy of the medial thigh
 |
|
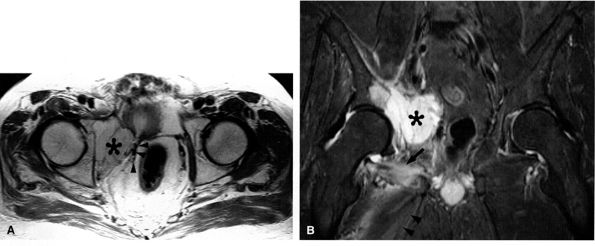
FIGURE 6.25 ● Acetabular metastasis with obturator neuropathy and denervation edema. (A) An axial PD-weighted image depicts a hyperintense destructive acetabular mass (asterisk) medially displacing the obturator neurovascular structures (arrowheads). (B) This coronal T2-weighted fat-suppressed image of the pelvis demonstrates the acetabular mass (asterisk) medially displacing the pelvic structures. Subacute denervation edema in the obturator externus (arrow) and adductor muscles (arrowheads) is noted.
|
-
Entrapment neuropathy occurs under the inguinal ligament or as the nerve perforates the fascia lata.
-
The term meralgia paresthetica is also used to refer to neuropathy of the lateral femoral cutaneous nerve.
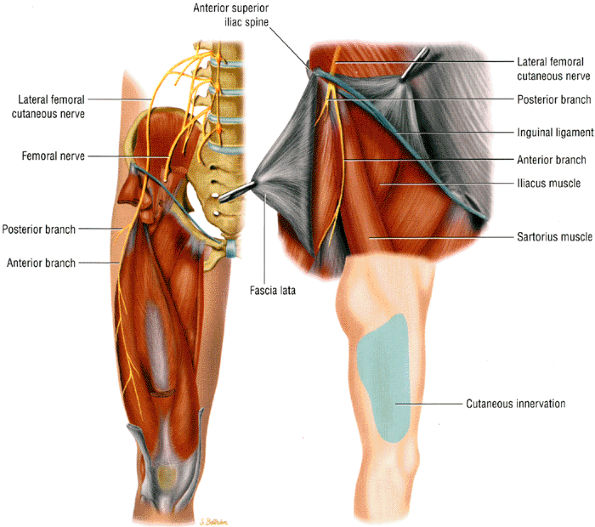 |
|
FIGURE 6.26 ● The lateral femoral cutaneous nerve as it dives under the inguinal ligament close to the anterior superior iliac spine. The insert shows the sensory distribution of the nerve along the lateral thigh.
|
-
Under the inguinal ligament, where the nerve makes a 70° to 90° turn as it bends around the anterior superior iliac spine
-
As the nerve perforates the fascia lata
-
Compression due to pelvic and retroperitoneal tumors
-
Trauma such as avulsion fracture of the ASIS
-
Surgical procedures, including spine surgery,92 acetabular fracture fixation, pelvic osteotomy, laparoscopic hernia repair, and iliac crest bone-graft harvesting
-
Posttraumatic neuroma following pelvic injury
-
Nerve transaction
-
Stretching of the nerve due to prolonged leg and trunk hyperextension
-
Leg length discrepancy
-
Scoliosis
-
Prolonged lithotomy position during surgery
-
Prolonged hip flexion to reduce incisional pain during the postoperative period93
-
Extended periods of standing, with secondary increased tension of the abdominal and fascia lata muscles
-
External compression of the nerve by seat belts, weight gain (obesity, pregnancy), and tight clothing (girdles and military belts94)
-
Anatomic variations predisposing to entrapment91,95,96,97
-
The common peroneal nerve trifurcates into the recurrent articular branch to the knee capsule and the superficial peroneal and deep peroneal nerves.
-
Common peroneal neuropathy usually occurs as the nerve crosses the fibular neck or as it pierces the peroneus longus muscle.
-
Extrinsic compression may be associated with intraneural and extraneural ganglia originating from the proximal tibiofibular joint. Tracking proximally along the articular branch of the common peroneal nerve occurs with intraneural ganglia.
-
Above the knee joint, the nerve can be easily traced as it migrates (highlighted by an abundant amount of fat) from a location medial to the short head of the biceps femoris muscle to a more lateral position posterior to the lateral head of the gastrocnemius muscle (see Fig. 6.21).
-
Occasionally the nerve travels within a narrower, fat-filled passage between the lateral head of the gastrocnemius muscle and a prominent or hypertrophied short head of the biceps femoris muscle.
-
The nerve then curves around the fibular neck to enter the anterior compartment of the leg.
-
The deep and superficial branches of the nerve usually diverge from each other at that level or just proximal to it. Typically, the deep peroneal nerve, which is anterior to the superficial peroneal nerve, hugs the fibula more closely.
-
It is sometimes difficult to appreciate the common peroneal nerve and its branches as they travel around the fibular neck. The two branches are better seen as they dive underneath the peroneus longus muscle. In older or heavy patients, the peroneus longus muscle is less bulky and a fat plane is identified between the muscle and adjacent fibula (Fig. 6.28).
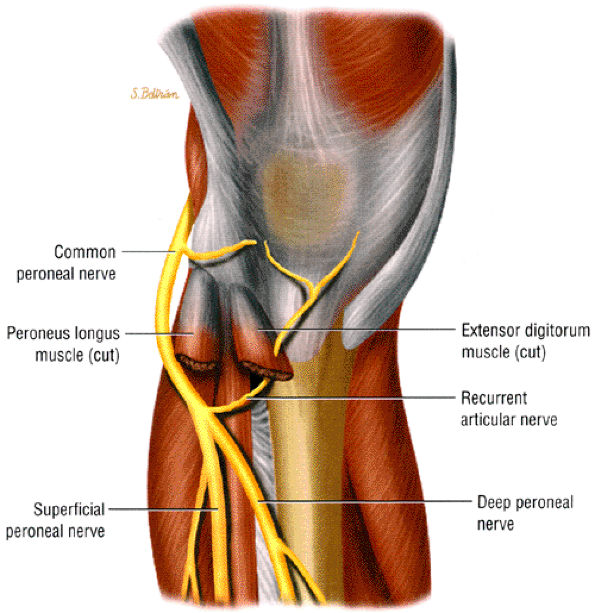 |
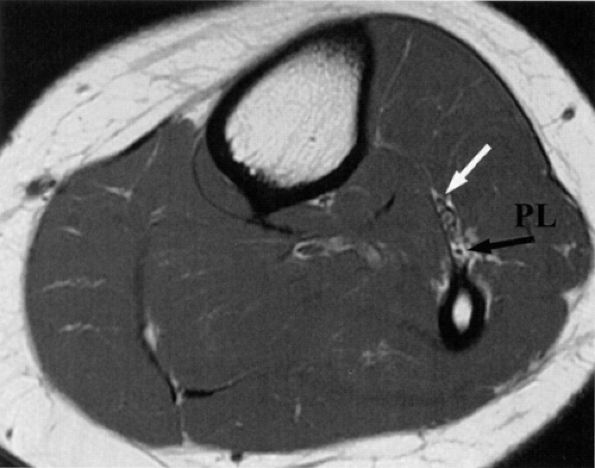 |
|
FIGURE 6.28 ● The normal peroneal nerves. Axial PD-weighted image of the proximal leg depicting the deep (white arrow) and superficial (black arrow) peroneal nerves, deep to the peroneus longus muscle (PL).
|
gastrocnemius muscle hernia,115 confluence of the origins of the soleus and peroneus muscles, and aberrant muscles can also produce entrapment of the nerve.116
-
Non-enhancing, homogenous masses with MR fluid signal features were depicted with a connection (tail) between the superior tibiofibular joint capsule and the ganglion cyst, best appreciated on either axial or sagittal images.
-
In many instances the ganglia and tail had a multilobulated appearance simulating varicosities.
-
An enlarged articular branch (arising from the deep peroneal nerve) may also be found.
-
Signal alterations in muscle were isolated to the anterior compartment, consistent with selective deep peroneal branch involvement.
-
Osteoarthritic changes of the knee and proximal tibiofibular joint may also be present.
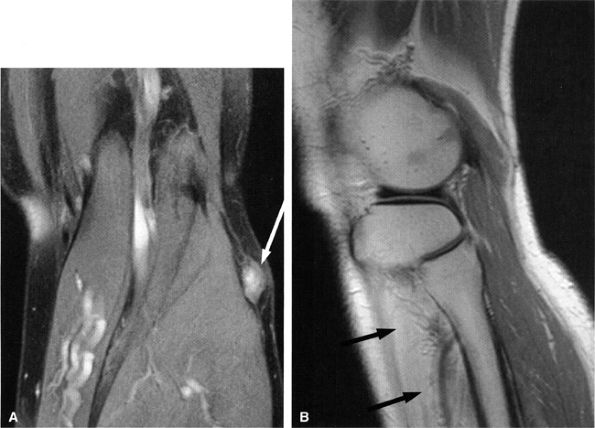 |
|
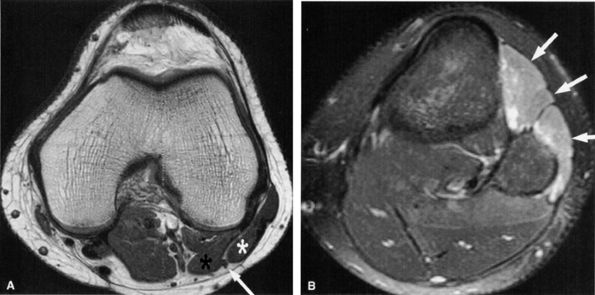
FIGURE 6.29 ● Common peroneal neuropathy secondary to a nerve sheath tumor. (A) Coronal T2-weighted fat-suppressed image demonstrating a nerve sheath tumor of the common peroneal nerve (arrow). (B) Sagittal T1-weighted image showing denervation atrophy of the anterior tibial muscle (arrows).
|
 |
|
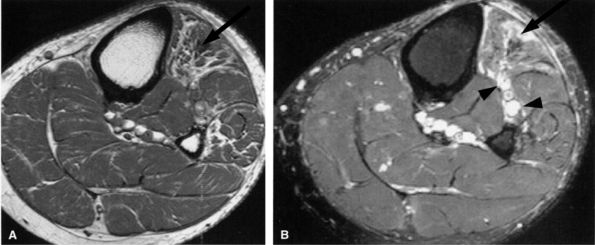
FIGURE 6.30 ● Common peroneal neuropathy with denervation atrophy and edema secondary to varicosities. (A) Axial T1-weighted image demonstrates denervation atrophy of the anterolateral muscles, predominantly the anterior tibial muscle (arrow). (B) Axial T2-weighted image depicts varicosities (arrowheads) next to the peroneal nerves and denervation edema of the anterior tibial muscle (arrow).
|
 |
|
FIGURE 6.31 ● Surgically proven common peroneal neuropathy and denervation secondary to a hypertrophied biceps femoris muscle. (A) Axial T1-weighted image showing the common peroneal nerve (arrow) entrapped between a hypertrophied short head of the biceps femoris muscle (white asterisk) and the lateral head of the gastrocnemius muscle (black asterisk). (B) Axial T2-weighted fat-suppressed image displaying denervation edema in the anterolateral compartment muscles (arrows).
|
-
The deep peroneal nerve provides motor supply to the extensor muscles of the foot and toes and carries sensation from the dorsal first web space.
-
Potential sites of entrapment include the superior extensor retinaculum and, in the anterior tarsal syndrome, the inferior extensor retinaculum or talonavicular joint.
rather a flat space between the medial and lateral malleoli defined superiorly by the inferior edge of the extensor retinaculum and inferiorly by the talonavicular joint. Just above the ankle joint the nerve divides into a lateral motor branch, which supplies the extensor digitorum brevis muscle. The medial branch, mostly sensory, continues dorsal to the talonavicular joint and middle cuneiform and between the bases of the first and second metatarsals to provide sensation and occasional motor supply to the first web space.
 |
|
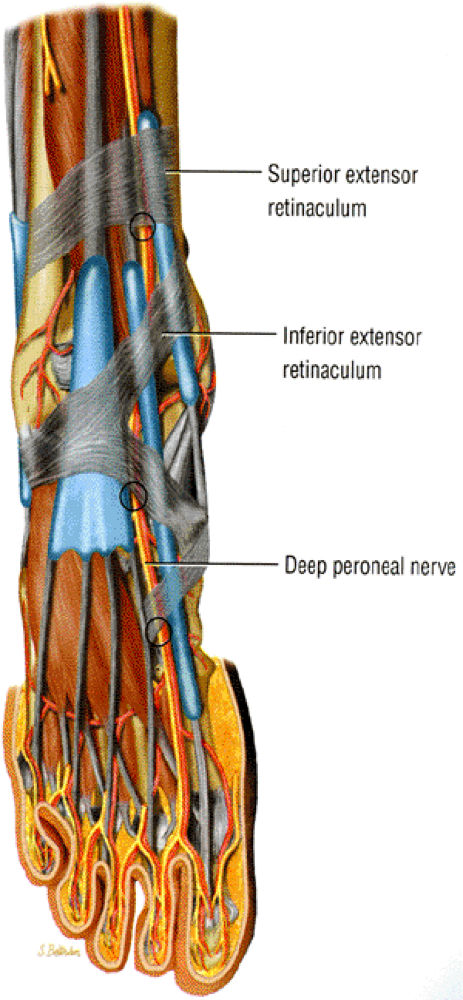
FIGURE 6.32 ● Anterior view of the foot demonstrating the three most common locations for deep peroneal nerve entrapment.
|
-
In the very proximal leg the deep peroneal nerve is noted within a small triangle of fat just anterior to the interosseous membrane, coursing first lateral and then anterior to the anterior tibial artery (see Fig. 6.28).
-
More distally, the nerve is again noted lateral to the anterior tibial artery.
-
In the proximal leg the nerve is deep to the anterior tibial and extensor digitorum longus muscles and, more distally, deep to the extensor digitorum and extensor hallucis longus muscles.
-
The deep peroneal nerve is easier to identify in older individuals and in patients with abundant fat; in younger athletic individuals, muscle bulk may obscure the nerve.
-
As the nerve descends down the leg into the ankle, it also migrates anteriorly and medially and becomes more superficial.
-
On axial images in the ankle region, the nerve can be followed within the dorsal fat between the extensor hallucis longus and extensor digitorum longus tendons. Approximately 5 cm above the ankle joint, the deep peroneal nerve is found deep to the superior extensor retinaculum. Approximately 1 cm above the ankle joint, it is seen deep to the superomedial band of the inferior extensor retinaculum. Proximal to the talonavicular joint, the nerve is deep to the inferomedial band of the inferior extensor retinaculum.
-
Distal to the ankle, oblique coronal (short axis) images of the midfoot are optimal for tracing the lateral and medial branches of the deep peroneal nerve (Fig. 6.33).
-
The anterior tarsal tunnel is seen as the space between the inferior edge of the extensor retinaculum and the talonavicular joint.
-
The lateral branch of the deep peroneal nerve can usually be identified as it courses laterally and terminates in the extensor digitorum brevis muscle.
-
The main medial branch is usually medial to the dorsalis pedis artery between the extensor hallucis longus and extensor hallucis brevis tendons. It is found within a small amount of dorsal fat between the first and second cuneiforms and between the first and second metatarsal bases.
-
At the forefoot the deep peroneal nerve can be difficult to distinguish from the adjacent vessels due to its small size, paucity of fat, and proximity to the underlying bones.
 |
|
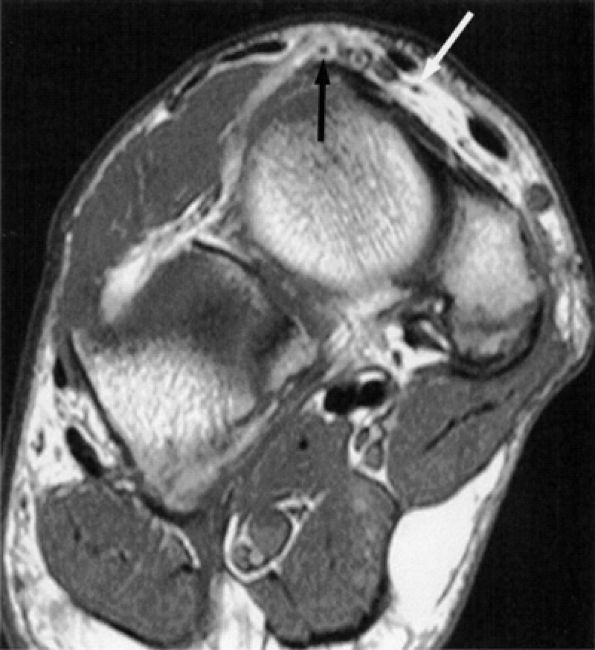
FIGURE 6.33 ● The normal deep peroneal nerve at the talonavicular joint. An oblique coronal PD-weighted image demonstrates the medial (white arrow) and lateral (black arrow) branches of the deep peroneal nerve separated by the dorsalis pedis vessels.
|
dull ache on the dorsum of the foot, pain, numbness, and a positive Tinel sign at the first web space may be seen. EMG studies are useful in distinguishing deep peroneal neuropathy from L5 and common peroneal neuropathies.
 |
|
FIGURE 6.34 ● Deep peroneal neuropathy secondary to a dorsal ganglion. (A) Oblique coronal T2-weighted fat-suppressed image depicts a ganglion (arrow) dorsal to the second cuneiform (asterisk). (B) A more distal oblique coronal T1-weighted image demonstrates denervation atrophy of the first dorsal interosseous muscle (arrow).
|
-
Due to the small size of the nerve, signal alterations and increased girth of the nerve may be difficult to appreciate.
-
Acute and subacute muscle denervation secondary to isolated entrapment of the nerve in the leg manifests as increased signal on fluid-sensitive images in the anterior compartment muscles, including the anterior tibial, extensor hallucis longus, extensor digitorum longus, and peroneus tertius muscle.
-
Increased T1 signal due to fatty infiltration and atrophy of the muscles is noted with more advanced entrapment.
-
Sparing of the lateral compartment muscles (the peroneus longus and peroneus brevis) is noted since the latter are supplied by the superficial peroneal nerve.
-
Tibial or fibular fractures with displaced fragments may be seen impinging on the nerve.
-
A mass effect due to tumors or ganglia, soft-tissue edema due to anterior compartment syndrome, muscle contusions, and infarcts may all be indirect MR features of deep peroneal entrapment neuropathy.
-
At the ankle and dorsal foot, injury to the deep peroneal nerve can be surmised when obliteration of the fat along the course of the nerve and its branches is identified. Mass effect by ganglia or tumors may also be depicted (Fig. 6.34).
-
Entrapment of the medial branch of the deep peroneal nerve produces bony and soft-tissue abnormalities such as talonavicular osteophytes, first metatarsal and first cuneiform osteoarthritis and spurs, a prominent os intermetatarseum, edema, and exuberant periostitis associated with stress fracture of the base of the second metatarsal, and first and second cuneiform fracture fragments.
-
Isolated muscle denervation of the extensor digitorum brevis is indicative of entrapment neuropathy of the lateral branch of the deep peroneal nerve. Because of the variability in innervation of the intrinsic muscles of the first web space, entrapment of the deep peroneal nerve may also demonstrate signal alterations in the first dorsal interosseous muscles (see Fig. 6.34B) and, less commonly, in the lateral head of the flexor hallucis brevis and oblique head of the adductor hallucis muscle.
 |
|
FIGURE 6.35 ● The superficial peroneal nerve as it pierces the deep fascia of the lateral compartment. Inversion injury can cause stretching of the nerve against the fascia.
|
-
The superficial peroneal nerve provides motor innervation to the peroneus longus and brevis muscles.
-
Entrapment occurs as the nerve pierces the deep fascia of the leg.
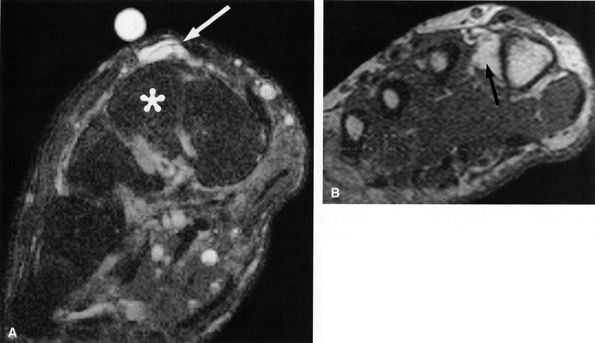
of the lower leg and dorsum of the foot with sparing of the first web space (innervated by the deep peroneal nerve). The pain is worsened with activity. Focal swelling, point tenderness, and exacerbation of symptoms with pressure occur approximately 10 to 12 cm above the ankle joint, where the nerve exits the deep fascia. When muscle herniation is the causative agent, a focal mass accentuated by resisted dorsiflexion may be seen.24 Prior trauma, particularly chronic ankle sprain, has been noted in approximately 25% of patients with superficial peroneal neuropathy. The neuropathy is most likely related to traction and stretching of the nerve.2 Impingement on the nerve following prior anterior compartment fasciotomy has also been described.
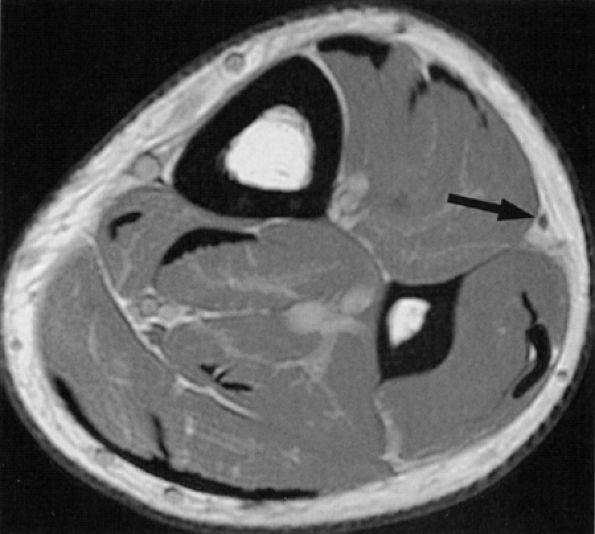 |
|
FIGURE 6.36 ● The normal superficial peroneal nerve. Axial PD-weighted image of the distal leg illustrating the nerve (arrow) in the subcutaneous fat, after it has pierced the fascia.
|
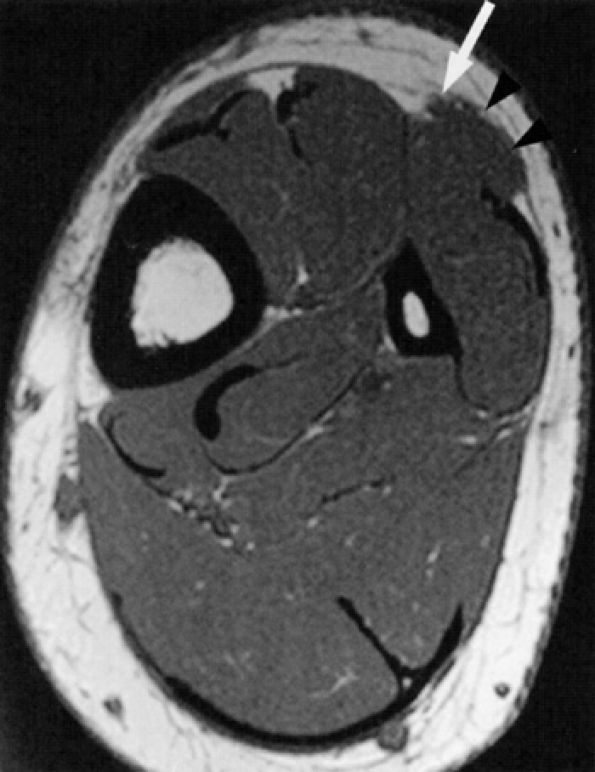 |
|
FIGURE 6.37 ● Superficial peroneal neuropathy secondary to muscle herniation. Axial T1-weighted image shows fascial herniation of the peroneus longus muscle (arrowheads) abutting on the superficial peroneal nerve (arrow).
|
-
The tibial nerve is the largest division of the sciatic nerve.
-
Tibial neuropathies include:
-
Proximal tibial neuropathy
-
Tarsal tunnel syndrome
-
Medial plantar neuropathy
-
Lateral plantar neuropathy
-
Morton's neuroma (interdigital neuropathy)
-
Joplin's neuroma (medial plantar proper digital neuropathy)
-
-
The tarsal tunnel syndrome is caused by trauma, space-occupying lesions, or foot deformities.
-
Distal foot entrapment neuropathies include:
-
Medial calcaneal neuropathy
-
Medial plantar neuropathy
-
Lateral plantar nerve entrapment and entrapment of the nerve to the abductor digiti minimi
-
-
Morton's neuroma is the result of damage to the interdigital nerve by entrapment or ischemia and represents a degenerative process.
-
Joplin's neuroma is an entrapment neuropathy of the plantar proper digital nerve.
-
Proximal tibial neuropathy (in the leg)
-
Tarsal tunnel syndrome
-
Medial plantar neuropathy
-
Lateral plantar neuropathy
-
Interdigital neuropathy (Morton's neuroma)
-
Medial plantar proper digital neuropathy (Joplin's neuroma)
of the leg between the two heads of the gastrocnemius muscle before proceeding anteriorly to the soleus muscle. The tibial nerve descends in an oblique fashion becoming more superficial approximately 15 cm above the ankle joint, at which point it travels medial to the Achilles tendon. It then proceeds deep to the flexor retinaculum (the lancinate ligament) within the tarsal tunnel. In the calf the tibial nerve provides motor innervation to the deep and superficial posterior compartment muscles, including the plantaris, popliteus, gastrocnemius, soleus, posterior tibial, flexor digitorum longus, and flexor hallucis longus muscles. Most of the sensory and motor innervation of the foot is supplied by the tibial nerve.
 |
|
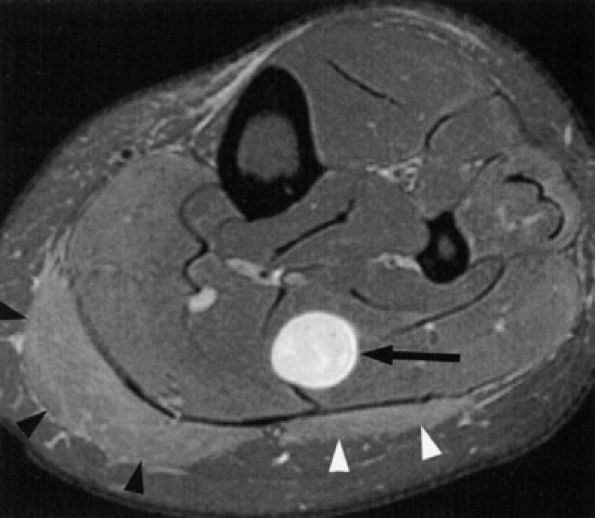
FIGURE 6.38 ● Proximal tibial neuropathy and muscle denervation in the leg secondary to a nerve sheath tumor. Axial T2-weighted fat-suppressed image demonstrates a hyperintense nerve sheath tumor (arrow) in the soleus muscle, in the region of a tibial nerve ramus, with secondary denervation edema in the medial (black arrowheads) and lateral (white arrowheads) heads of the gastrocnemius muscle.
|
 |
|
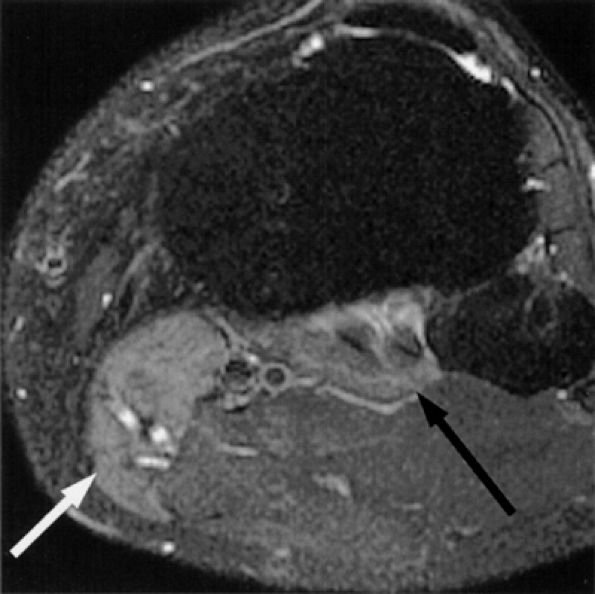
FIGURE 6.39 ● Proximal tibial neuropathy with denervation edema. Axial T2-weighted fat-suppressed image demonstrates denervation edema of the popliteus (black arrow) and medial head of the gastrocnemius muscles (white arrow).
|
in the knee (see Fig. 6.21) and finally anterior to the soleus muscle in the calf. A gradual and significant decrease in the surrounding fat in the calf may make it difficult to separate the nerve from the adjacent muscles and vascular bundle. The nerve continues its downward medial course until it becomes superficial in the distal calf, posterior to the posterior tibial muscle and medial to the flexor hallucis longus muscle. At this level it is again highlighted by abundant fat.
 |
|
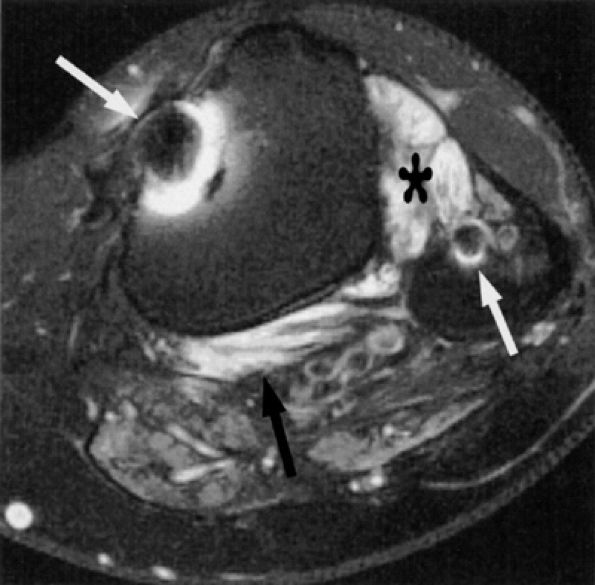
FIGURE 6.40 ● Tibial and common peroneal neuropathy secondary to a traumatic spinal root avulsion. Axial T2-weighted fat-suppressed image shows muscle denervation edema in the popliteus muscle (black arrow) (innervated by the tibial nerve) and in the anterolateral compartment muscles (asterisk) (innervated by the common peroneal nerve). Note postsurgical metallic susceptibility artifacts (white arrows) in the tibia and fibula.
|
-
The upper tibiotalar tunnel, at the ankle region, is bordered by the distal tibia and medial malleolus laterally and the deep aponeurosis of the leg medially.
-
The lower talocalcaneal tunnel, in the hindfoot (in strict anatomic terms the true tarsal tunnel), is bordered by the talus and calcaneus laterally and the flexor retinaculum medially (Fig. 6.41).
 |
|
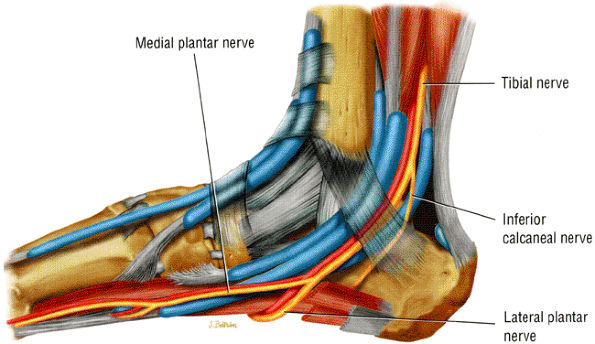
FIGURE 6.41 ● The tarsal tunnel. The tibial nerve and its branches descend, deep to the flexor retinaculum, along with the flexor tendons and vascular structures.
|
branches, one to the periosteum of the medial calcaneal tuberosity, one to the abductor digiti minimi, and one to the flexor digitorum brevis muscle. The long plantar ligament and the quadratus plantae may also be innervated by the inferior calcaneal nerve.11
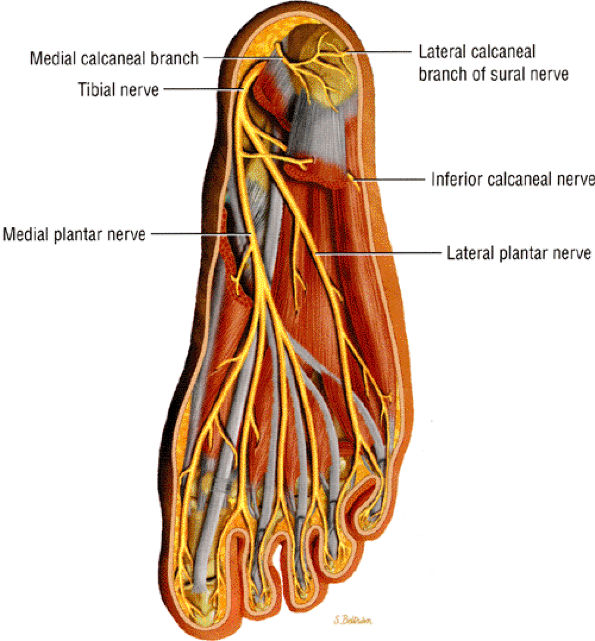 |
|
FIGURE 6.42 ● Posterior view of the foot depicts the tibial nerve and its medial and lateral plantar nerve branches.
|
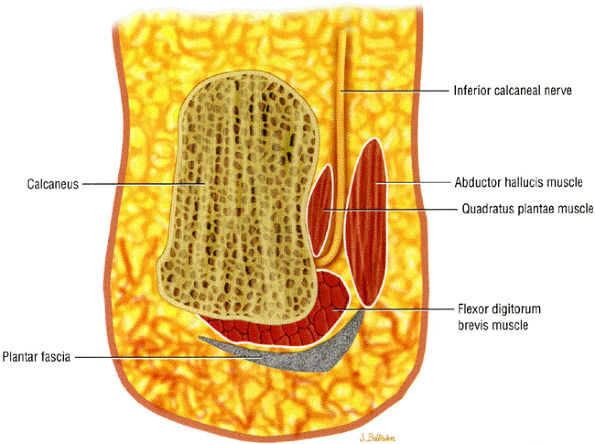 |
|
FIGURE 6.43 ● Posterior view of the foot demonstrates the inferior calcaneal nerve (Baxter's nerve) making a sharp, almost 90°, turn from a vertical to a horizontal position as it courses toward the calcaneus.
|
-
The thin septa separating the tunnel into multiple compartments are seen as delicate, linear low-signal structures extending from the flexor retinaculum toward the calcaneus. The most common of these is the transverse interfascicular septum, which subdivides the tunnel into lower and upper chambers and is seen on axial or coronal images of the ankle as a low-signal-intensity fine line extending from the abductor hallucis muscle toward the calcaneus and quadratus plantae muscle.
-
The tibial nerve and its branches can be traced on axial and coronal images of the ankle and then on oblique coronal images of the foot. Thin-section sagittal images can sometimes demonstrate long portions of the nerves in a single slice.
-
In the upper tunnel, the tibial nerve is depicted as an oval structure of intermediate signal intensity deep to the aponeurosis of the leg, in close proximity to the tibia and medial to the flexor hallucis longus tendon.139 The nerve can be distinguished from its accompanying posterior tibial artery and vein since it maintains its position lateral to the vessels (see Fig. 6.44A). The vessels, therefore, are found more superficially, closer to the retinaculum.
-
Occasionally, the medial calcaneal nerve is seen on either axial or sagittal images as it branches off the tibial nerve proximal to the flexor retinaculum.
-
The division of the tibial nerve into the medial and lateral plantar nerves is usually noted within the tunnel but may be seen proximal to it.
-
The lateral plantar nerve is found more posterior and lateral to the medial plantar nerve, in the lower chamber, between the muscle bellies of the abductor hallucis and quadratus plantae muscles (see Fig. 6.44B). Not infrequently, the first branch of the lateral plantar nerve, the inferior calcaneal or Baxter's nerve, can be followed as it takes off from the lateral plantar nerve and dives toward the abductor digiti minimi muscle (Fig. 6.45).
-
The nerve is usually seen in close proximity to the quadratus plantae muscle on axial or coronal images of the ankle.
-
Distal to the tunnel, the medial and lateral plantar nerves are easiest to identify on oblique coronal images of the foot (Fig. 6.46). Accompanied by the more medially located medial plantar artery, the medial plantar nerve and its branches are noted at the talonavicular joint within a fat plane between the abductor hallucis medially and the quadratus plantae muscle laterally. The nerve is plantar to the flexor hallucis longus and flexor digitorum longus tendons as they cross each other at Henry's knot (Fig. 6.47). At the bases of the
P.1086
metatarsals the nerve is bordered by the abductor hallucis muscle medially and the flexor digitorum brevis laterally. The lateral plantar nerve and its branches are seen more laterally within the fat planes between the abductor digiti minimi and the flexor digitorum brevis muscles. The nerve is accompanied by the more laterally located lateral plantar artery.
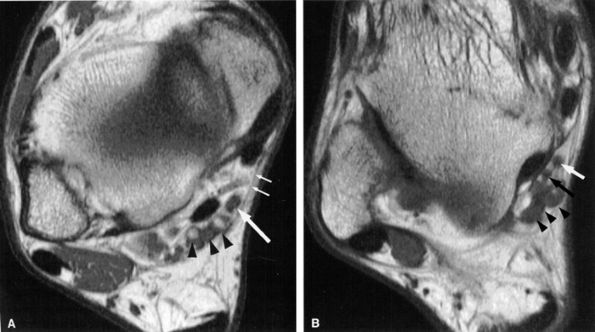 |
|
FIGURE 6.44 ● The normal tarsal tunnel. (A) Axial T1-weighted image of the upper tunnel demonstrates the tibial nerve (arrow) deep to the flexor retinaculum (small arrows) and anteromedial to the tibial vessels (arrowheads). (B) Axial T1-weighted image in the lower tunnel demonstrates the medial (white arrow) and lateral (black arrow) plantar nerves deep to the tibial vessels (arrowheads).
|
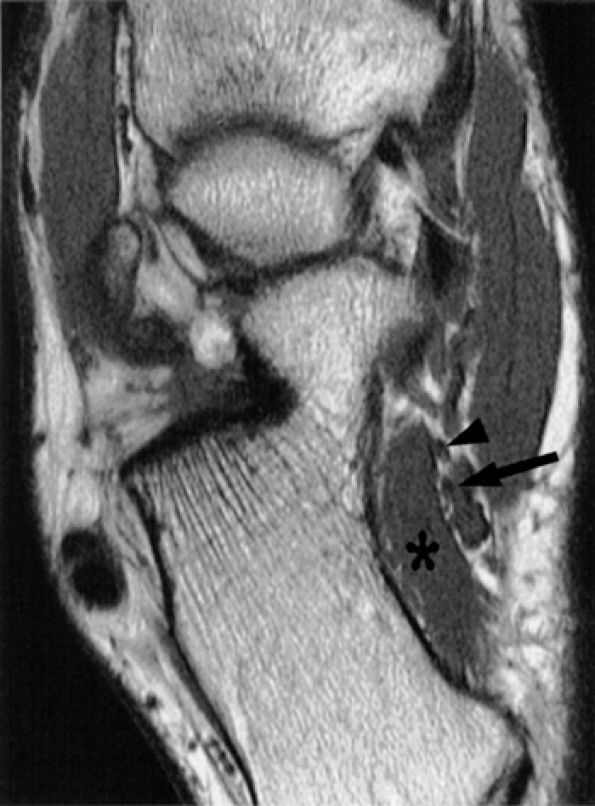 |
|
FIGURE 6.45 ● The inferior calcaneal nerve. The inferior calcaneal nerve (arrow) is shown in close proximity to the quadratus plantae muscle (asterisk) and posterior to the lateral plantar nerve (arrowhead) on this axial T1-weighted image.
|
 |
|
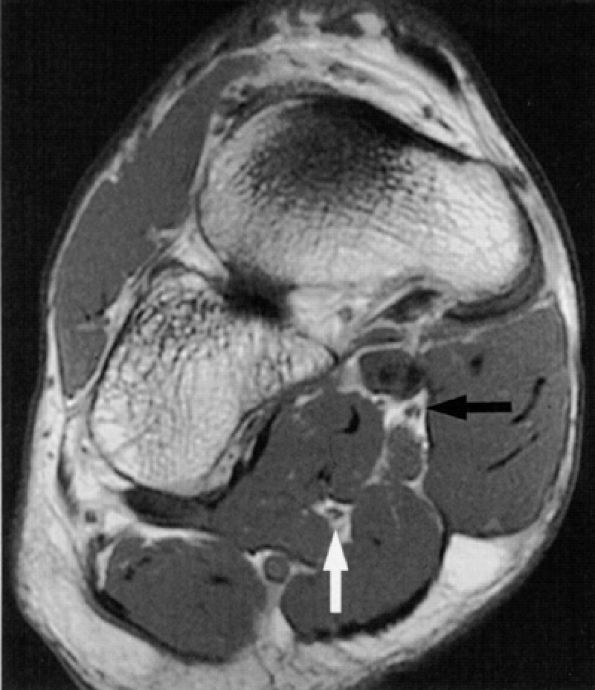
FIGURE 6.46 ● The normal medial (black arrow) and lateral (white arrow) plantar nerves in the midfoot as noted on an oblique coronal PD-weighted image.
|
 |
|
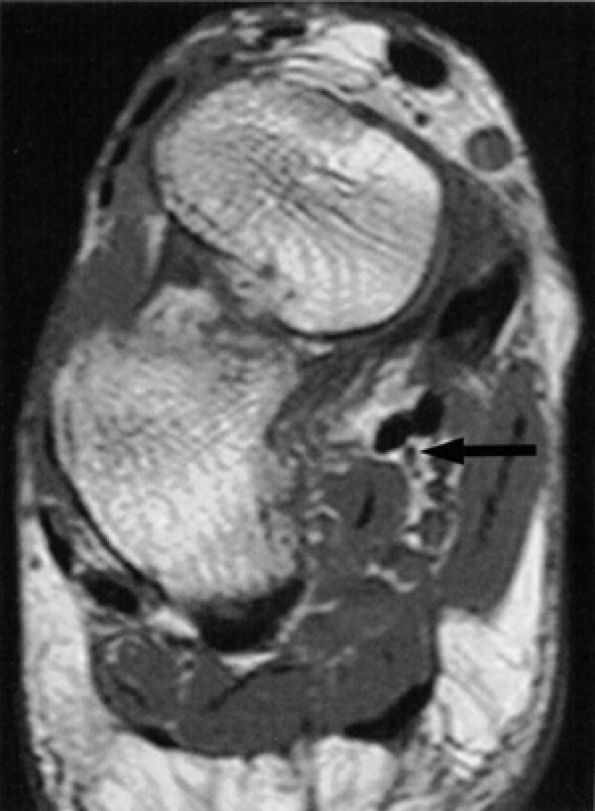
FIGURE 6.47 ● The medial plantar nerve at Henry's knot. The close proximity of the nerve (arrow) to the flexor hallucis longus and flexor digitorum longus tendons as they cross each other at Henry's knot is shown on an oblique coronal PD-weighted image.
|
-
Sharp, radiating or shooting pain, worsened with activity
-
Paresthesia, dysesthesia, and numbness along the distribution of the nerve and its branches
Thus, imaging can play a contributory role in the diagnosis of tarsal tunnel syndrome.
 |
|
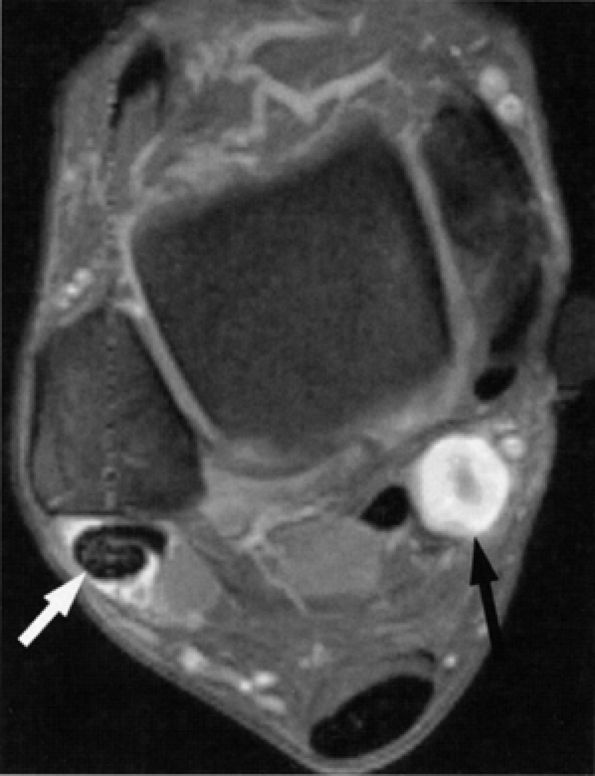
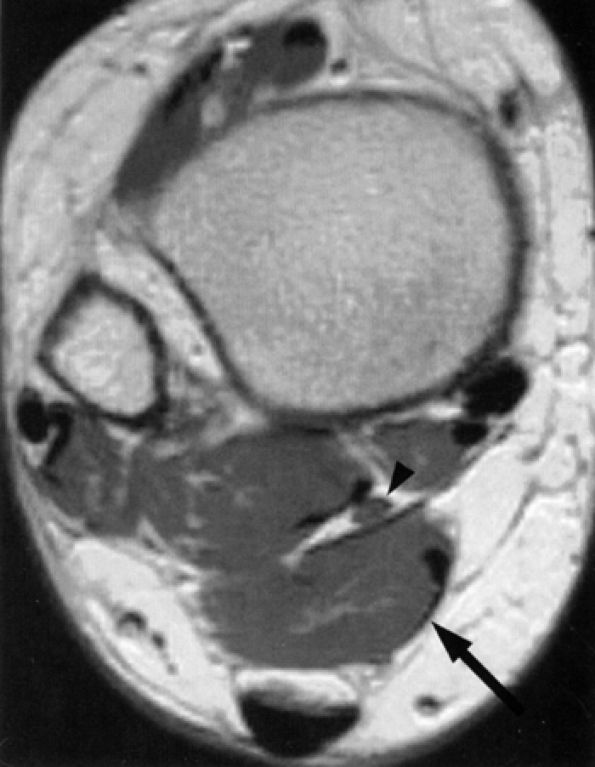
FIGURE 6.48 ● Tarsal tunnel nerve sheath tumor. Axial T1-weighted fat-suppressed post-gadolinium image demonstrating a hyperintense nodule (black arrow) with a hypo-intense center (“target sign”) in the tarsal tunnel. Note incidental finding of peroneal tendon tenosynovitis (white arrow).
|
 |
|
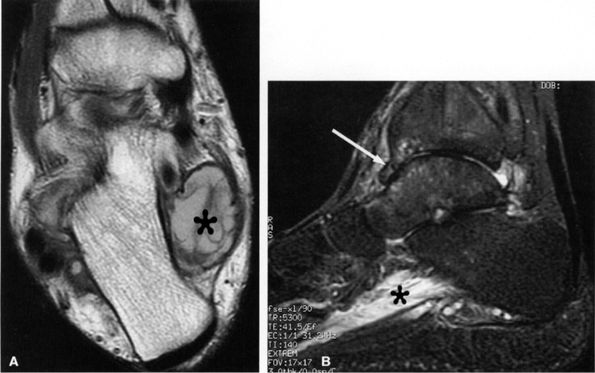
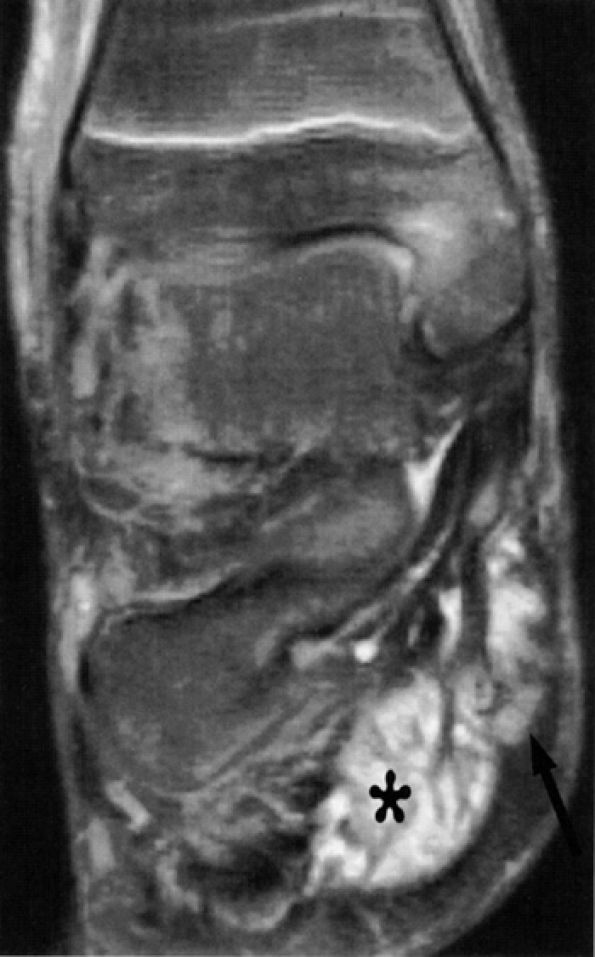
FIGURE 6.49 ● Tarsal tunnel syndrome and medial plantar nerve denervation edema due to proliferative synovitis. (A) Axial T2-weighted image demonstrates a synovial mass (asterisk) in the tarsal tunnel. (B) Sagittal T2-weighted fat-suppressed image illustrates denervation edema in the flexor digitorum brevis muscle (asterisk). Note associated osteoarthritic changes in the anterior tibiotalar joint (arrow).
|
fracture fragments, pigmented villonodular tenosynovitis, and low-signal scar tissue.11,153,162,165
 |
|
FIGURE 6.50 ● Accessory soleus muscle. Axial T1-weighted image illustrates an accessory soleus muscle (arrow) anteriorly displacing the tibial nerve (arrowhead) in the distal leg.
|
almost half of the cases are secondary to athletic activity, particularly distance running, with secondary hypertrophy of the abductor hallucis muscle. It is believed that up to 15% of athletes with chronic unresolving heel pain suffer from entrapment of the inferior calcaneal nerve.2 A hypermobile, pronated foot predisposes to stretching of the nerve.
 |
|
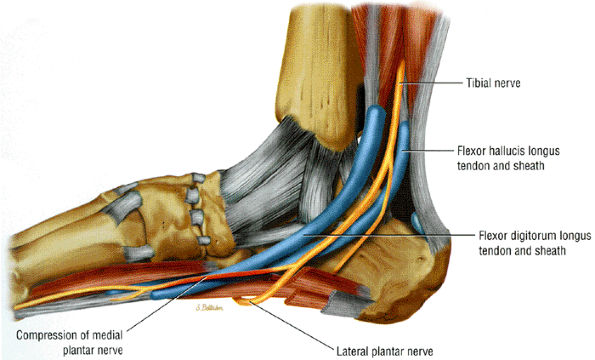
FIGURE 6.51 ● Compression of the medial plantar nerve at the knot of Henry.
|
-
Deep to or by the fascial edge of a hypertrophied abductor hallucis muscle
-
At the medial edge of the quadratus plantae muscle, where the nerve changes from a vertical to a horizontal course
-
Near the medial calcaneal tuberosity (see Fig. 6.43)
-
Tenosynovitis with tendon sheath distention at the intersection of the flexor digitorum longus and flexor hallucis longus tendons in the talonavicular region may occur in entrapment of the medial plantar nerve.
-
Muscle denervation edema or atrophy of the abductor hallucis and flexor digitorum brevis muscles, seen on MR images of the ankle, is compatible with medial plantar nerve entrapment (Fig. 6.52).
-
Denervation of the first lumbrical and of the flexor hallucis brevis muscle, also consistent with medial plantar nerve entrapment, is better seen on MR images of the foot (Figs. 6.53 and 6.54).
-
Abductor hallucis muscle hypertrophy and plantar fasciitis with medial calcaneal spur formation and adjacent soft-tissue edema are suggestive of entrapment of the first branch of the lateral plantar nerve.
-
In our experience, incidental MR detection of denervation edema and atrophy of the abductor digiti quinti muscle is not uncommon and most likely reflects a clinically missed entrapment of the first branch of the lateral plantar nerve (Figs. 6.55 and 6.56).
-
Decreased bulk, fatty atrophy, and increased signal on fluid-sensitive images of the intrinsic muscles of the foot in a diabetic patient are commonly secondary to peripheral neuropathy (Fig. 6.57).170,171,172
 |
|
FIGURE 6.52 ● Medial plantar neuropathy. Denervation edema of the flexor digitorum brevis (asterisk) and abductor hallucis muscles (arrow) is shown on this coronal T2-weighted fat-suppressed image of the ankle.
|
 |
|
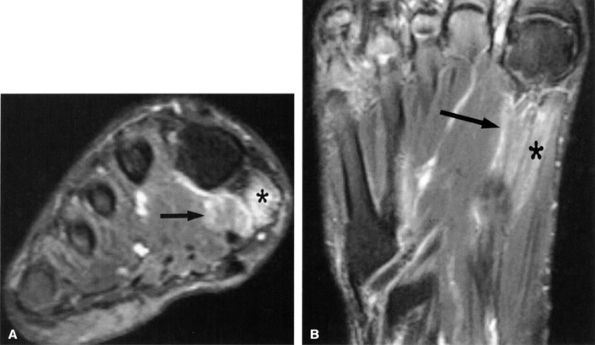
FIGURE 6.53 ● Medial plantar neuropathy. Oblique coronal (A) and axial (B) fluid-sensitive fat-suppressed images of the foot show denervation edema in the flexor digitorum brevis (arrow) and abductor hallucis (asterisk) muscles.
|
 |
|
FIGURE 6.54 ● Medial plantar neuropathy. Oblique coronal T1-weighted image of the foot shows denervation atrophy of the flexor digitorum brevis muscle (arrow).
|
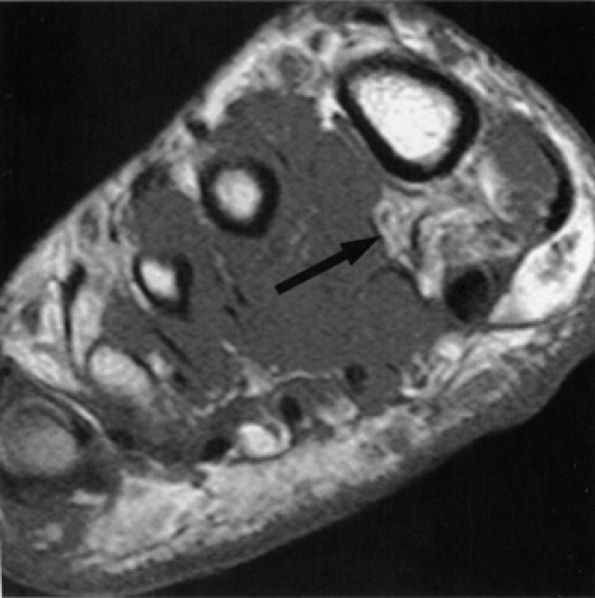
 |
|
FIGURE 6.55 ● Neuropathy of the inferior calcaneal nerve (Baxter's neuropathy). Coronal T1-weighted image of the ankle demonstrating denervation atrophy of the abductor digiti quinti (arrow). Note metallic susceptibility artifact secondary to a distal fibular fixation plate and screws.
|
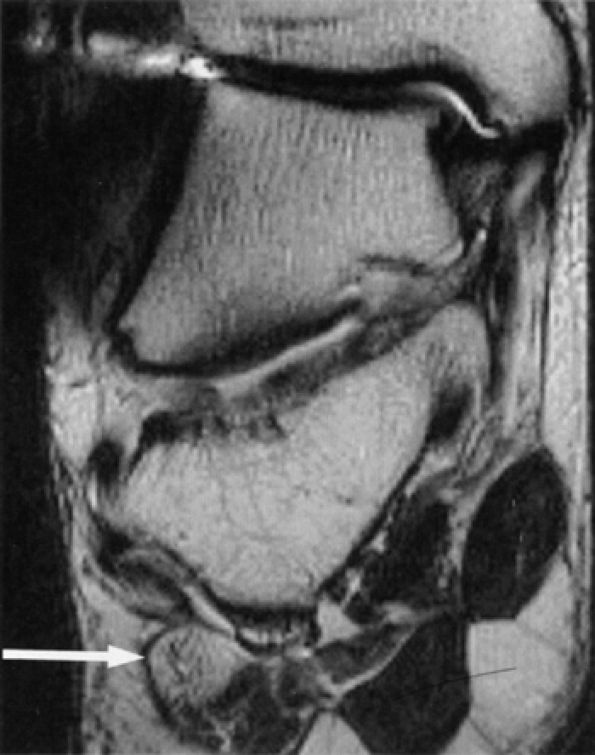
 |
|
FIGURE 6.56 ● Neuropathy of the inferior calcaneal nerve (Baxter's neuropathy) in a 66-year-old patient with tarsal tunnel varicosities. Sagittal (A) and axial (B) T1-weighted images of the ankle demonstrate denervation atrophy of the abductor digiti quinti muscle (asterisk). (C and D). Normal abductor digiti quinti muscle (asterisk) in an asymptomatic patient for comparison.
|
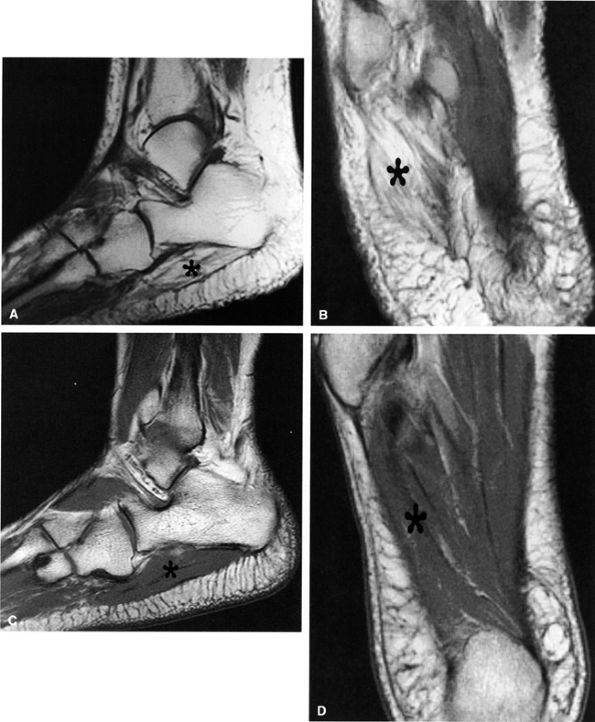
 |
|
FIGURE 6.57 ● Lateral plantar neuropathy. Oblique coronal fluid-sensitive fat-suppressed sequential images show denervation edema in the first, second, and third interosseous muscles (arrows).
|
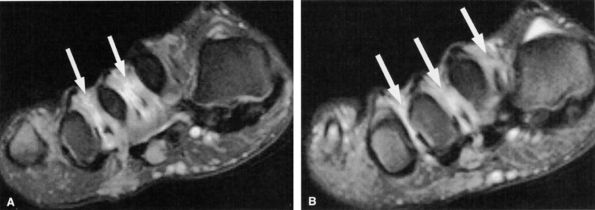
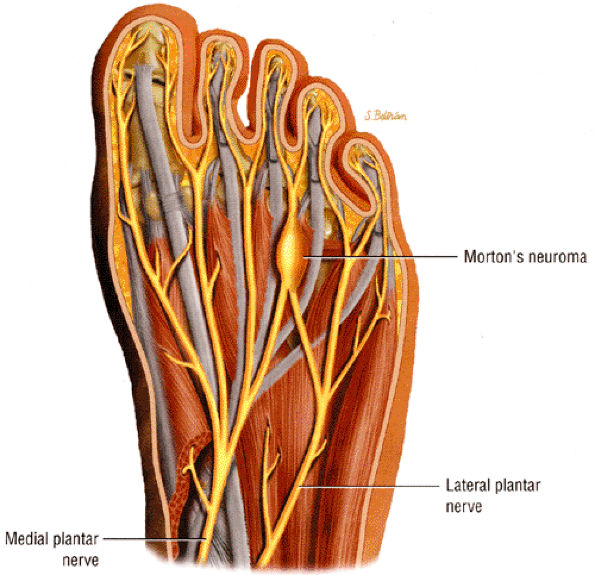 |
|
FIGURE 6.58 ● A third interdigital Morton's neuroma at the confluence of the terminal branches of the medial and lateral plantar nerves.
|
description of this condition by Thomas G. Morton in 1876.175,176 Initially the lesion was thought to affect the third intermetatarsal space exclusively. However, later studies revealed that although the second and third intermetatarsal spaces are the most common locations for Morton's neuroma, other intermetatarsal spaces can also be affected.177,178,179
-
An oval or dumbbell-shaped mass of intermediate to low signal on both T1- and T2-weighted MR images located in the intermetatarsal space. The mass frequently extends into the plantar subcutaneous fat and may be associated with intermetatarsal bursitis (Fig. 6.59).
-
The low signal of the lesion reflects its predominant histologic composition of dense fibrous tissue.
-
T1-weighted images are optimal for detecting the neuroma since they provide good contrast between the lesion and the adjacent fat.
-
T2-weighted images serve to confirm the diagnosis as well as to exclude other diagnostic possibilities, such as true neuromas, intermetatarsal bursitis, ganglion cysts, and synovial cysts.178,193,194,195
-
The usefulness of STIR and contrast-enhanced fat-saturated T1-weighted images is controversial due to variable signal intensity of the neuroma on these sequences.193
-
Intravenous gadolinium injection does not provide sufficient additional information to warrant its routine use.177,184
-
The lesion is centered in the neurovascular bundle, within the intermetatarsal space and on the plantar side of the transverse metatarsal ligament.
-
The lesion is well demarcated.
-
The signal intensity of the lesion is similar to that of skeletal muscle on TI-weighted images and less than that of fat on T2-weighted images (see Fig. 6.59).
in the shape of the neuroma and in its location relative to the metatarsal heads were also reported. Optimal visualization of Morton's neuroma is obtained in the prone position.
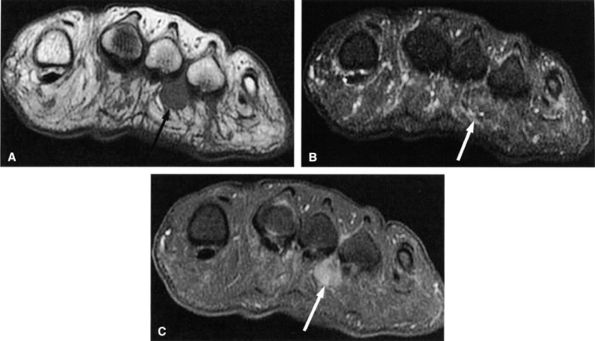 |
|
FIGURE 6.59 ● Third intermetatarsal Morton's neuroma. Dumbbell mass (arrows) of intermediate to low signal on both T1-weighted (A) and T2-weighted (B) images extends from the intermetatarsal space to the plantar aspect of the foot. (C) The mass enhances after gadolinium administration.
|
ganglia, entrapment secondary to gastrocnemius injury,206 and fibrous bands are additional causes of sural nerve entrapment.
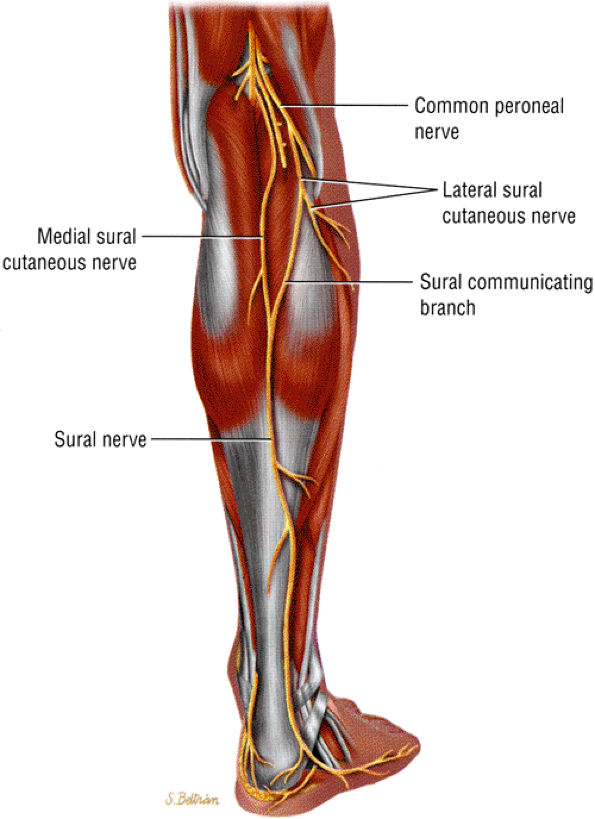 |
|
FIGURE 6.60 ● The posterior leg, showing the formation of the sural nerve from the tibial and peroneal contributions.
|
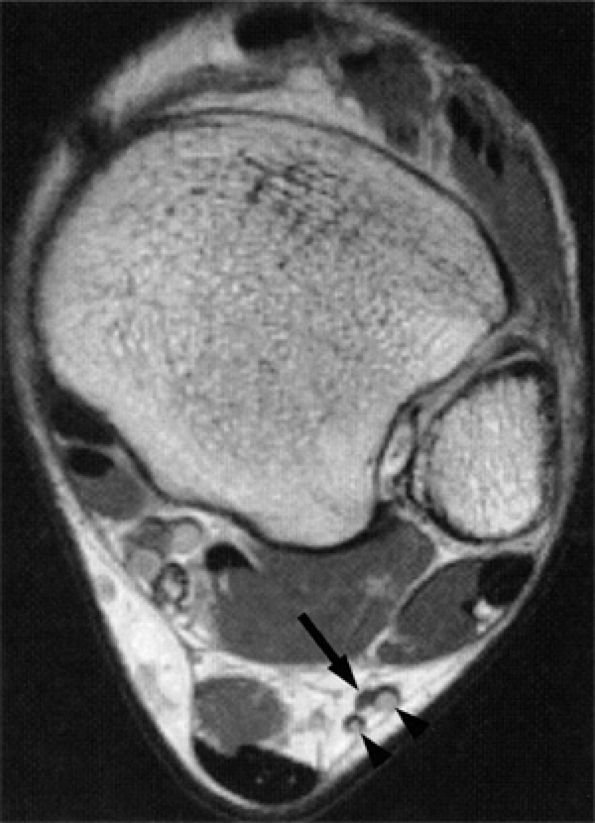 |
|
FIGURE 6.61 ● The normal sural nerve. Axial T1-weighted image demonstrates the sural nerve (arrow) deep to subcutaneous vessels (arrowheads).
|
 |
|
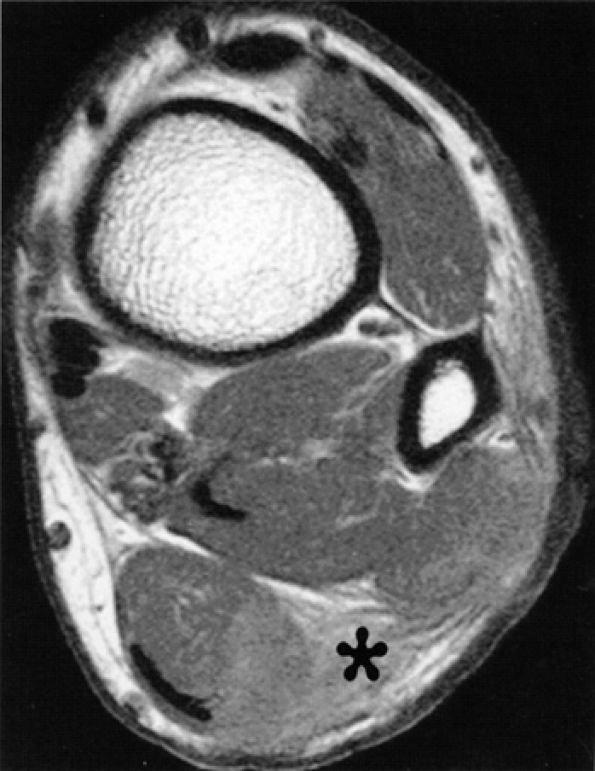
FIGURE 6.62 ● Sural nerve laceration in a 20-year-old male patient. Axial T1-weighted image demonstrates edema and obliteration of fat planes in the region of the sural nerve (asterisk). Note concomitant lacerations of the soleus and peroneal muscles.
|
 |
|
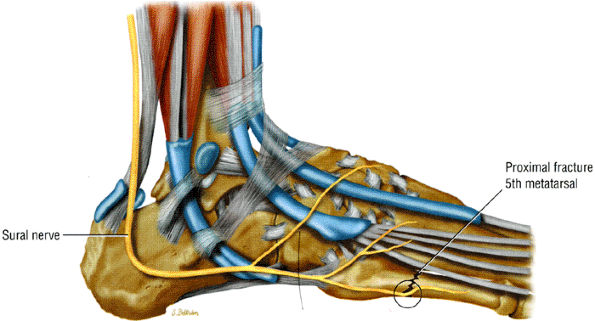
FIGURE 6.63 ● Sural nerve entrapment secondary to a fracture of the base of the fifth metatarsal.
|