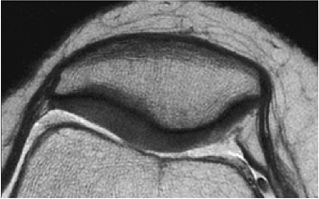
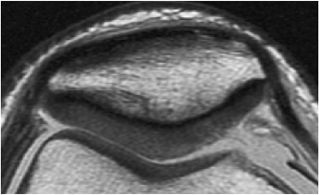
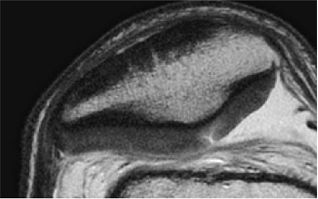
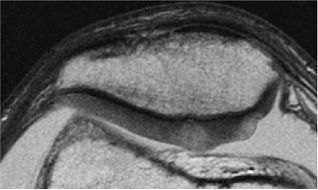
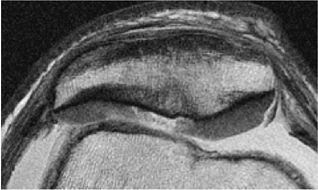
Articular Cartilage
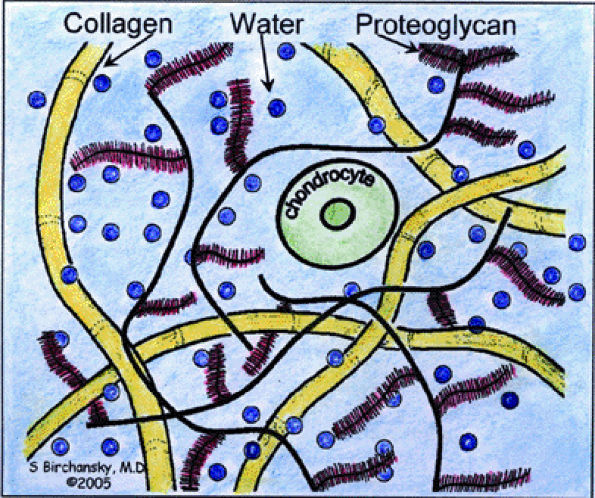
both compressive and tensile loads. The solid collagen fibers are of importance in maintaining tensile stiffness and strength.1 However, given their ratio of length to thickness in structure, collagen is unable to withstand high compressive loads, which is a property maintained by the integrity of the proteoglycan component.
 |
|
FIGURE 7.1 ● Schematic diagram of cartilage structure.
|
 |
|
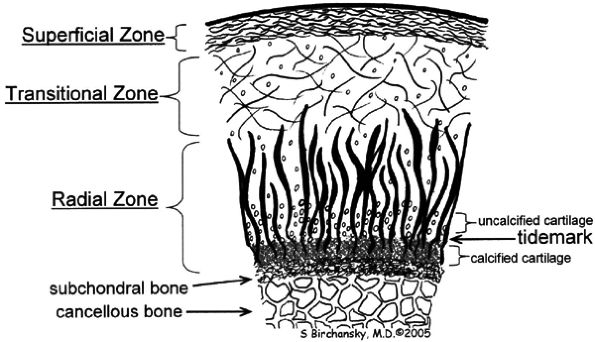
FIGURE 7.2 ● Schematic diagram of cartilage zonal histology.
|
-
The most superficial or tangential zone (lamina splendens) represents 10% to 20% of the total thickness of cartilage, reflecting collagen fibers that are oriented parallel to the articular surface. The superficial zone resists shear and has the highest collagen content of all zones.1 Chondrocytes in this layer differentially express proteins with lubricating and protective functions, yielding little proteoglycan content.7 Despite the importance of the superficial zone, it is typically not visualized as separate from the transitional zone on standardized cartilage imaging performed at clinically relevant field strengths.
-
In the transitional or middle zone, representing 40% to 60% of the cartilage thickness, collagen fibers are randomly oriented.1 The inhomogeneity of fiber orientation acts to distribute stress more uniformly across loaded tissue.8
-
The deep or radial zone represents 30% of the cartilage thickness and has highly ordered fibers that are perpendicular to the articular surface. The radial orientation of the collagen fibers coalesces as bundles; they then cross the tidemark, defined as the interface between the arular cartilage and the calcified cartilage beneath it, forming a system that anchors the cartilage to underlying bone.9
-
The calcified zone is separated from the radial zone by its boundary tidemark. The tidemark also represents a potential shear plane for articular cartilage defects in the adult skeleton. Deep to the calcified cartilage lies the subchondral bone plate.10
-
With a traditional T1-weighted sequence, fluid is of intermediate to lower signal intensity with poor contrast against the intermediate-signal-intensity articular cartilage.
-
Heavily T2-weighted sequences, with echo times in excess of 80 msec, depict the cartilage as being relatively hypointense compared to high-signal-intensity fluid. It is important to remember, however, that because of the normal stratification of the extracellular matrix in cartilage, T2 values are shorter in the radial zone (closer to the subchondral plate) than in the transitional zone, where the collagen is more randomly oriented and T2 values are prolonged. This normal stratification can be perceived on appropriate cartilage-sensitive scans and provides insight into the cartilage ultrastructure (Fig. 7.3).
-
With longer echo times there is poor delineation between the basilar components of the cartilage and the subchondral plate. This may account for factitious thickening of the subchondral plate and thinning of the cartilage (see Fig. 7.3).
arthroscopy in cartilage evaluation, and found that images acquired using the gradient-echo sequences provided excellent interobserver and intraobserver agreement (kappa = 0.72 – 1.0), with the exception of the lateral tibial plateau, which showed moderate to substantial agreement (kappa = 0.56 – 0.72). The more convex architecture of the lateral tibial plateau may make evaluation in this location subject to partial volume errors and reduced accuracy.18
 |
|
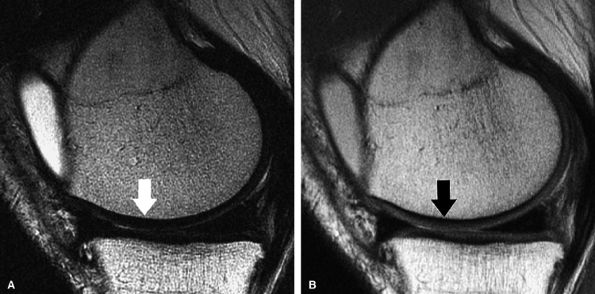
FIGURE 7.3 ● (A) Sagittal fast spin-echo MR image of the knee in a 39-year-old man performed on a high-field system (1.5 Tesla) demonstrates poor distinction between the subchondral plate and adjacent basilar components of cartilage at a TE of 110 msec (white arrow). (B) Corresponding image with all acquisition parameters held the same except for a moderate TE of 27.5 msec demonstrates improved cartilage–subchondral bone distinction (black arrow).
|
 |
|
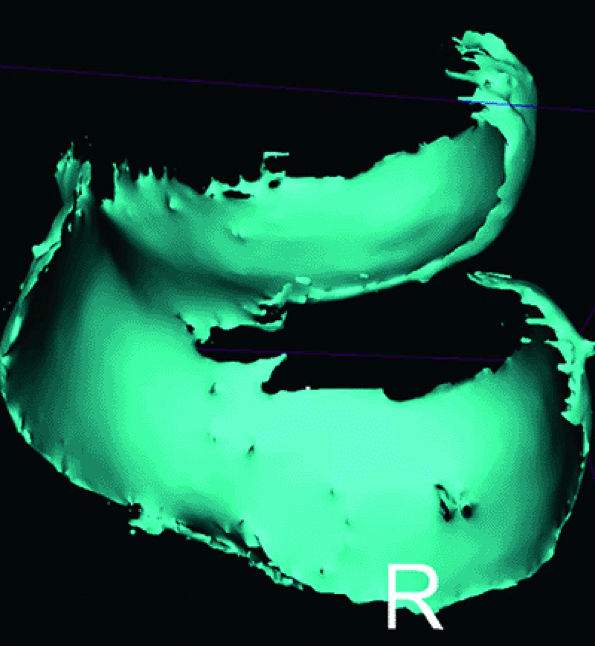
FIGURE 7.4 ● Three-dimensional cartilage model built from a semi-automated segmentation algorithm, with the cartilage subtracted from the subchondral bone. MR data were obtained from a 3D fat-suppressed, T1-weighted gradient-echo acquisition. These models may eventually prove helpful in surface replacement techniques.
|
Attention to echo time is also important. For fat-suppressed T1-weighted gradient-echo sequences, given the relatively short T2 relaxation times of cartilage, fairly short echo times (preferably 10 msec) are generally recommended. As Eckstein et al. reported, an echo time of 11 msec led to underestimation of tibial cartilage thickness (compared to CT arthrography as the standard).22
envelope, providing more efficient use of imaging time. Fast spin-echo images are cartilage-sensitive due to an inherent magnetization transfer contrast and exchange of off-resonance magnetization between slices, the net effect of which is to saturate the bound pool of hydrogen nuclei, resulting in a decrease in signal intensity from the free pool.36 This exchange results in relatively high signal intensity from fluid compared to the lower signal intensity of articular cartilage, providing an effective differential contrast among menisci, articular cartilage, and fluid in the meniscosynovial recesses (Fig. 7.5). With arthroscopy as a standard, Potter et al. found that articular surface evaluation using spin-echo sequences had a sensitivity of 87%, a specificity of 94%, and an accuracy of 92%, with minimal interobserver variability (kappa = 0.93).18 These findings of reproducibility support the use of MR imaging as a noninvasive and objective outcome measure of surgically manipulated cartilage and in the longitudinal evaluation of traumatic and degenerative cartilage lesions.
 |
|
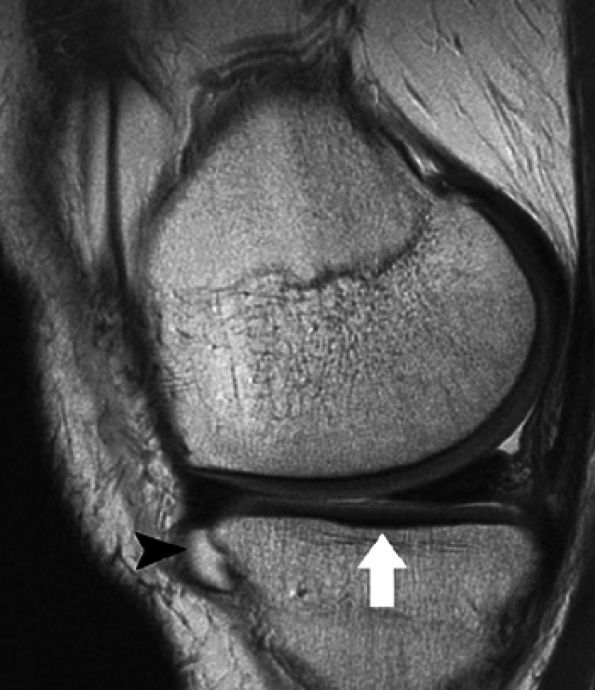
FIGURE 7.5 ● Sagittal fast spin-echo MR image of the knee in a 50-year-old patient demonstrates differential contrast for the high-signal-intensity joint fluid within the meniscosynovial recess (arrowhead), the intermediate signal intensity of hyaline cartilage, and the low signal intensity of meniscal fibrocartilage. Over the tibial plateau cartilage, note the gray-scale stratification (arrow) with lower signal intensity in the basilar components.
|
to minimize chemical shift misregistration as well as to reduce inter-echo spacing. Decreasing inter-echo spacing will minimize the edge blurring routinely encountered with fast spin-echo techniques performed at correspondingly narrower bandwidths with wider inter-echo spacing. The effect of chemical shift causes a misregistration at the interface between fat (subchondral bone) and water (cartilage), resulting in factitious loss of the subchondral plate, abnormal signal hyperintensity in the adjacent cartilage (due to the frequency shift summation), and inaccurate assessment of cartilage morphology (Fig. 7.7).
 |
|
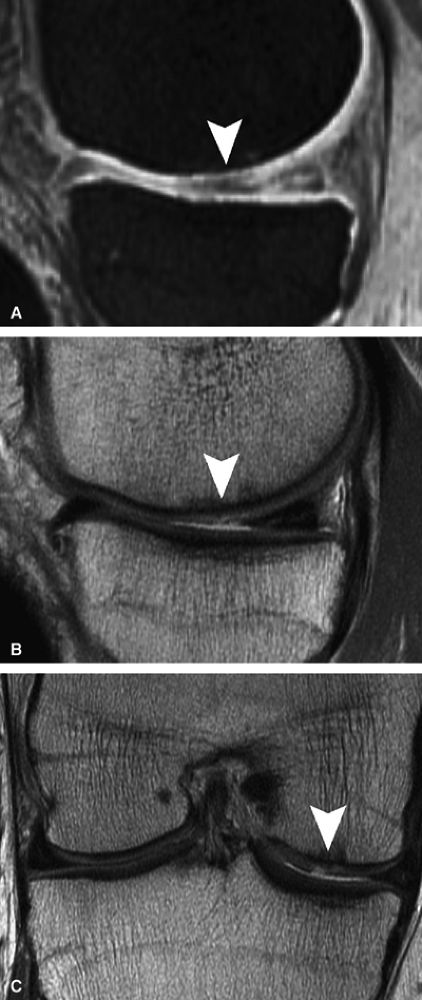
FIGURE 7.6 ● (A) Sagittal fat-suppressed T1-weighted gradient-echo MR image sequence of the knee demonstrates a focal high-grade partial-thickness cartilage defect overlying the medial femoral condyle (arrowheads). In the equivalent time required to acquire this single pulse sequence, cartilage-sensitive fast spin-echo MR sequences in two planes—sagittal (B) and coronal (C)—can be obtained, in which the depth of the lesion is much better delineated. (Reprinted by permission of SAGE Publications, Inc., from Am J Sports Med, in press.)
|
 |
|
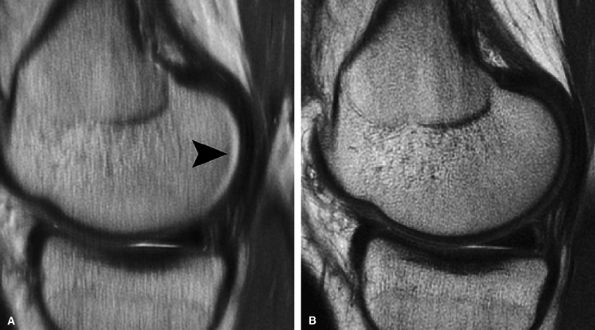
FIGURE 7.7 ● Sagittal fast spin-echo MR images of the knee in a 28-year-old man performed on a high-field system (3 Tesla). (A) There is chemical shift misregistration and image blurring at a receiver bandwidth of 10 kHz (arrowhead). (B) Corresponding image acquisition with all parameters held the same except for the use of a wider receiver bandwidth of 62.5 kHz. Attention to imaging technique is imperative to provide reproducible cartilage imaging.
|
-
Driven equilibrium Fourier transform (DEFT) imaging uses an additional 90° RF pulse to drive the recovery of longitudinal magnetization, resulting in high signal intensity from tissues with long T1 relaxation times, such as the synovial fluid adjacent to the articular cartilage. Gold et al. compared 3D DEFT imaging with proton density-weighted and T2-weighted fast spin-echo imaging and reported that sensitivity for full-thickness lesions was 50% for the fast spin-echo images compared to 67% for the 3D DEFT images; specificity was 80% for the FSE images compared to 100% for the 3D DEFT images.39 The authors also noted that artifacts were accentuated on the 3D DEFT sequence compared to the fast spin-echo images.39 The 3D DEFT images, however, subjectively provided superior cartilage-to-fluid contrast compared to more traditional cartilage-sensitive techniques.40
-
Several gradient-echo pulse sequences achieve cartilage-to-fluid contrast by the use of a steady state, including DESS, SSFP, and WS-bSSFP. Double echo steady state (DESS) acquires and combines two gradient echoes, providing both high T2 contrast and representative joint morphology.41 Refocused steady state free precession (SSFP)
P.1106P.1107
techniques (also known as True-FISP, FIESTA, and balanced FFE) require all imaging gradients to be fully re-wound between excitation pulses, resulting in high transverse coherence and relative T2/T1 weighting.42 Fat suppression may also be used in the steady state free precession pulse sequences, providing optimal cartilage-to-fluid contrast at the expense of signal-to-noise. Relative fat and water separation may be achieved with phase-sensitive techniques without the expense of the increased scan time needed when using frequency-selective fat suppression.43 Water selective balanced steady state free precession pulse sequences (WS-bSSFP) have also been explored. Kornaat et al. compared WS-bSSFP with moderately low in-plane resolution fast spin-echo techniques in 10 patients with arthritis and determined that the contrast-to-noise between cartilage and surrounding tissue was optimized with a 20° to 25° flip angle; the contrast-to-noise ratio was higher on the WS-bSSFP sequences than on fast spin-echo protocols or on T1-weighted gradient-echo techniques.44 In a comparison study of 3D T1-weighted gradient-echo and 3D SSFP imaging, Reeder et al. noted superior fat/water separation with good cartilage-to-fluid contrast and reduced acquisition times using 3D SSFP sequencing in a small cohort of 10 knees of five volunteers.45 -
Multiple echo techniques, in which a series of identically phase-encoded gradient echoes are sampled per line in K-space and unipolar readout gradients are used to avoid off-resonance effects, have also been developed.46 In a study evaluating the patellar joint using a slice thickness of 3 mm (1.5-mm gap) and arthroscopy as the standard, these techniques had a sensitivity of 79%, a specificity of 82%, and accuracy of 81% in detecting grade 2 or higher lesions.46 Interobserver agreement was also good (kappa = 0.68).46
-
Short echo time projection reconstruction imaging of cartilage is a technique to detect ultrashort species in cartilage, potentially improving visualization of cartilage structure.47 Gold et al. described a method to obtain information from ultrashort echo time species in cartilage using projection-reconstruction spectroscopic imaging, disclosing spectra from voxels across the zones of articular cartilage that are reconstructed at the water frequency (Fig. 7.8).48 The latter technique also uses fat suppression to minimize chemical shift misregistration.
|
TABLE 7.2 ● Arthroscopic and MRI Correlation
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
 |
|
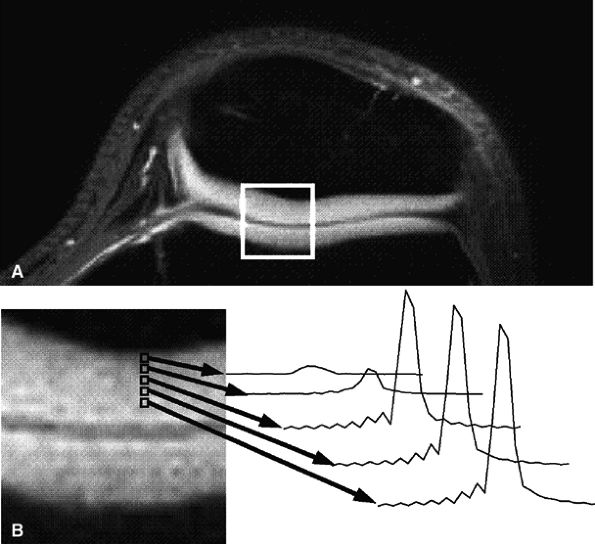
FIGURE 7.8 ● Axial MR images of the patellofemoral joint in a 25-year-old healthy volunteer using projection-reconstruction spectroscopic imaging sequence (TE = 200 μsec). (A) Water-frequency image. (B) Magnified image of articular cartilage from box in (A), along with spectra of the patellofemoral cartilage. Note the decreasing line width and increasing peak area as voxels progress from the cartilage–bone interface to the articular surface. (Reprinted by permission of
American Roentgen Ray Society, from Am J Roentgenol. 1998; 170:1223-1226.
) |
-
A relatively hypointense radial zone
-
A higher-signal-intensity transitional zone
-
A thinner, hypointense superficial zone
of truncation during the Fourier transform, resulting in a factitious laminar appearance that is accentuated on short TE gradient-echo sequences, particularly those with relatively low in-plane resolution.57 Truncation artifact occurs at high signal interfaces and is most typically seen in the spinal cord at the spinal fluid–cord interface, creating a “pseudo-syrinx.” It is important to remember that the laminar appearance of cartilage is markedly different on clinical MR imaging systems compared with high-field microscopy systems (or clinical systems fitted with special gradient inserts and/or optimized small sample coils). It is our experience that a trilaminar appearance of cartilage on microscopy systems indeed reflects the ultrastructure of the cartilage, and with an in-plane resolution of 300 to 350 μ, a consistent bilaminar appearance is seen on fast spin-echo techniques (with or without frequency-selective fat suppression). In most cases, however, the resolution of the superficial zone (representing approximately 10% of the overall cartilage thickness) is beyond that of standardized clinical protocols.
 |
|
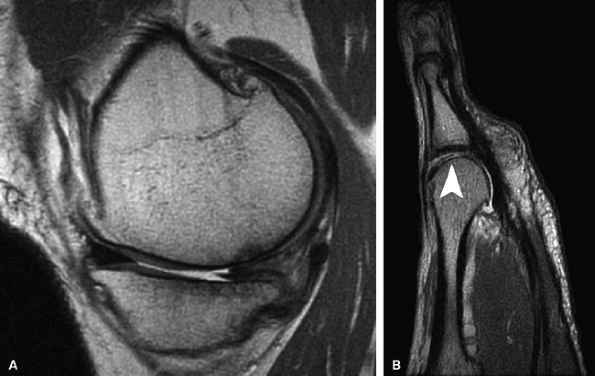
FIGURE 7.9 ● Fast spin-echo MR images obtained in a 0.7-Tesla open unit. (A) Sagittal image of the knee in a 40-year-old patient demonstrates a chronic tear of the posterior horn of the medial meniscus, full-thickness cartilage loss, and sclerotic changes in the subchondral bone over the medial compartment. (B) Sagittal image of the forefoot in a 48-year-old patient demonstrates active cartilage delamination (arrowhead) and flap formation over the head of the second metatarsal.
|
 |
|
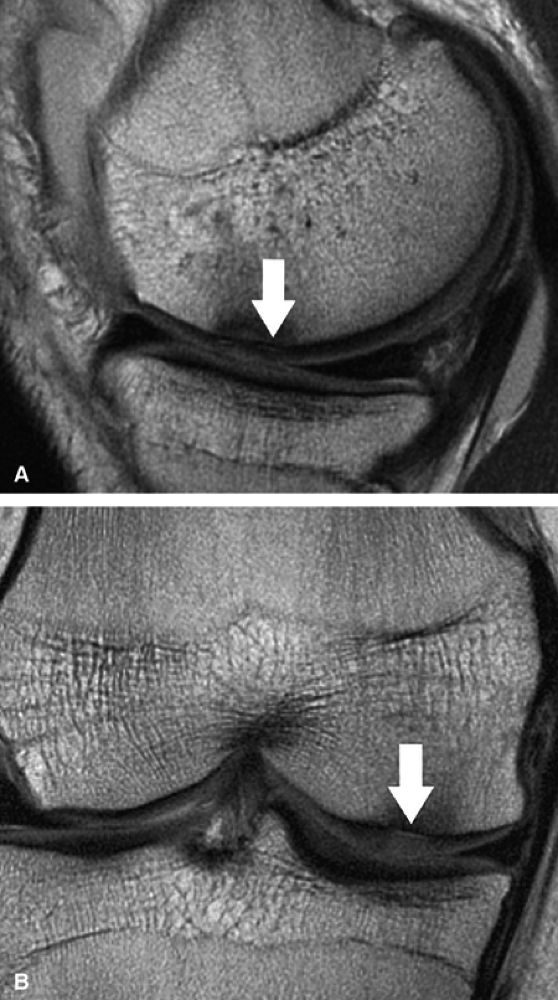
FIGURE 7.10 ● Sagittal (A) and coronal (B) fast spin-echo MR images of the knee in a 24-year-old professional football player. There is hyperintensity in the radial zone, indicating deep surface delamination (arrow), adjacent to the tidemark with no flap formation. There is also associated sclerosis of the subchondral plate. The patient underwent microfracture with a good clinical outcome.
|
-
Sodium MR imaging
-
No contrast required
-
Standard for newer FCD techniques
-
Requires multinuclear spectroscopy and special coils
-
Limited by longer scan times to obtain adequate signal-to-noise ratio
-
-
Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC)
-
Indirect T1 following Gd-DTPA penetration → index of [Gd]cartilage proportional to the [GAG]tissue
-
Requires intravenous injection of gadolinium, followed by exercise and delay before scanning
-
-
T1 rho (T1ρ) imaging
-
No contrast required
-
Spin-locking pulse requires more RF power (specific absorption rate may limit number of slices)
-
Less susceptible to regional field inhomogeneities
Collagen assessment: Assess collagen orientation throughout cartilage thickness-
T2 mapping
-
No contrast required
-
Subject to magic angle and chemical shift effects in vivo
-
-
the use of negatively charged gadolinium salt contrast agents (delayed gadolinium-enhanced MR imaging of cartilage [dGEMRIC]).
-
A 90° pulse to flip magnetization into the y axis
-
A spin-locking pulse along the y axis, “locking” the magnetization in the transverse plane
-
A second 90° pulse to drive the magnetization back to the z axis
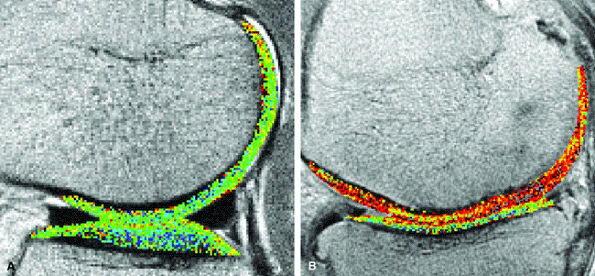 |
|
FIGURE 7.11 ● Sagittal T1 gadolinium-enhanced images of the knee demonstrate global and focal ranges of glycosaminoglycan distribution (T1Gd) index. (A) The lateral compartment in a 26-year-old female professional dancer shows high-range (blue-green) T1Gd values for the tibial plateau and the weight-bearing zones of the femoral condyle compartments. (B) The medial compartment in a 78-year-old woman with moderately severe OA demonstrates low-range T1Gd values (red). (Reprinted by permission of
American Roentgen Ray Society, from AJR Am J Roentgenol. 2004; 182:167-172.
) |
parallel to the long axis of the patient.80 When the angle between the external field and the hydrogen nuclei in collagen reaches approximately 55°, there is a corresponding prolongation of T2 relaxation time according to the relationship 1/T2 = k(3cos2&thetas; – 1). In the radial zone, where the collagen is highly ordered, expected prolongation of T2 has been noted in both high field strength microscopy systems at 7 Tesla as well as in images acquired on 1.5-Tesla units.80 Recognition of the magic angle effect in cartilage is important since it may produce apparent increased signal intensity in the radial zone at 55° (Fig. 7.12). Since the magic angle effect may hamper the measurement of cartilage regions of interest, caution should be used to avoid sampling T2 values at the magic angle.
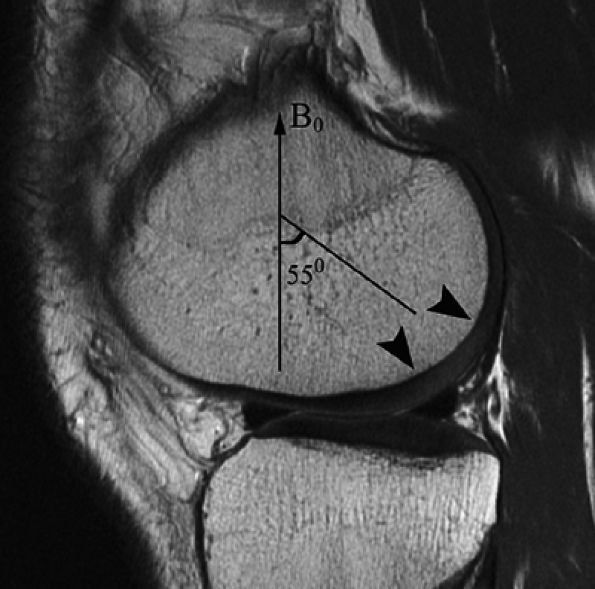 |
|
FIGURE 7.12 ● A sagittal fast spin-echo MR image of the knee in a 26-year-old man demonstrates the magic angle effect (arrowheads), causing prolongation of T2 with focal loss of gray-scale stratification at 55° relative to the external magnetic field (B0), indicating highly ordered structure components in the cartilage matrix. Note the normal gray-scale stratification of the remaining medial femoral condyle and tibial plateau.
|
a 7-Tesla magnet, several changes, including a decrease in superficial zone thickness, an increase in total cartilage thickness over the submeniscal regions, and a shift in the maximum T2 of the articular surface were found. These findings indicate that the collagen of the superficial zone is disrupted in early stages of OA.90 Further, the increasing T2 of the superficial zone was detected in OA specimens obtained from portions of the tibia covered by meniscus, suggesting that these regions may show earlier signs of cartilage degeneration, before it is evident in other (more central) areas of the joint. These regional differences are most likely due to alteration in structural properties related to weight-bearing areas of the joint.84,90
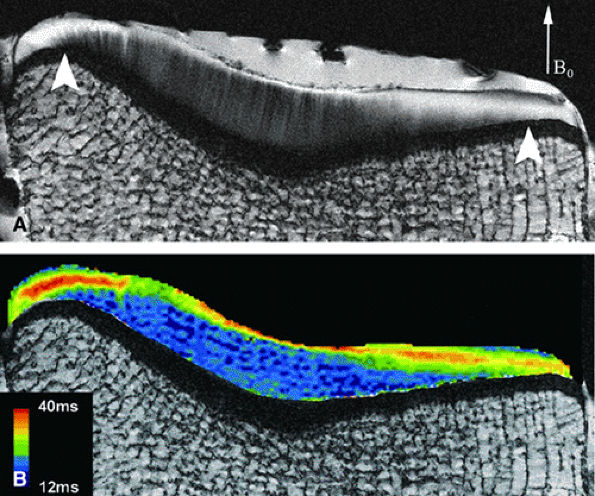 |
|
FIGURE 7.13 ● (A) Lateral tibial plateau of a 35-year-old woman. B0 = main magnetic field. In the central region of the plateau, prominent radial striations extend across a thick deep layer. Minor fibrillation is seen at the low-signal-intensity surface. In submeniscal and tibial eminence regions (arrowheads), the transitional layer is much thicker. (B) On a corresponding T2 map, changes in T2 parallel changes in signal intensity. Peak T2 values are located in the middle of transitional layer. (Reprinted by permission of
American Roentgen Ray Society, from AJR Am J Roentgenol. 2004; 182:311-318.
) |
between MRI and mechanical parameters, especially between T2 and Young—s modulus.91 Similarly, it has been shown that prolongation of T2 occurs early following meniscectomy, correlating with observable biomechanical changes using indentation probe testing as well as polarized light microscopy.92
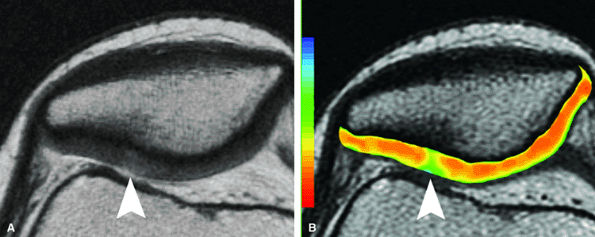 |
|
FIGURE 7.14 ● (A) Axial fast spin-echo MR image of the knee in a 25-year-old avid marathoner with anterior knee pain demonstrates focal increased signal (arrowhead) affecting normal-thickness cartilage of the lateral patella facet with subchondral sclerosis. (B) Corresponding quantitative T2 relaxation time map demonstrates geographic loss of stratification and prolongation in T2 values (arrowhead) throughout the thickness of the cartilage at this site.
|
 |
|
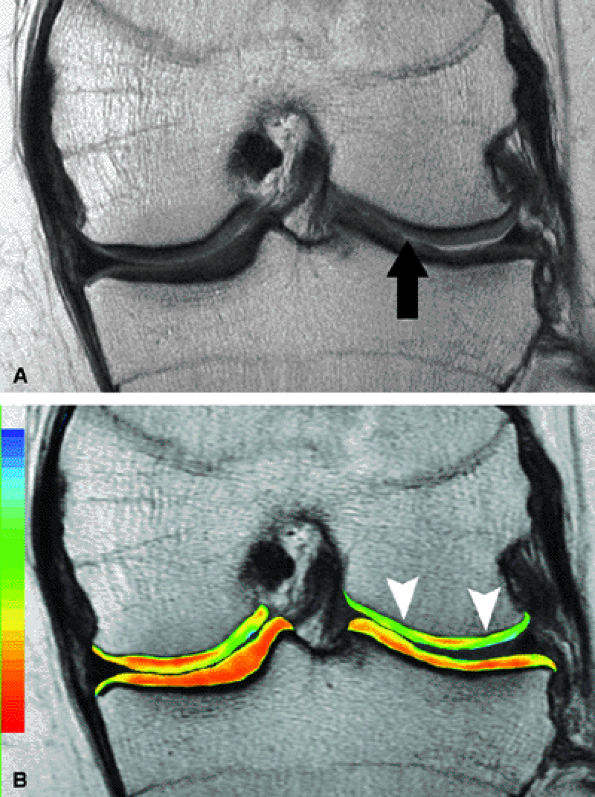
FIGURE 7.15 ● (A) Coronal fast spin-echo MR image of the knee in a 15-year-old girl with a previous partial meniscectomy demonstrates mild fibrillation of the cartilage overlying the lateral tibial plateau (arrow). (B) Corresponding quantitative T2 relaxation time map of the femorotibial articular cartilage coded to capture T2 values ranging from 0 to 100 msec demonstrates prolongation of T2 values over the plateau, but with unexpected prolongation over the lateral femoral condyle (arrowheads), which appeared normal on standard fast spin-echo cartilage-sensitive imaging. Green and blue reflect longer T2 values, yellow intermediate, and orange the shorter values.
|
articular cartilage homeostasis, with biopsy samples demonstrating degeneration of chondrocytes with loss of matrix as well as osteocyte necrosis and empty lacunae.95 Spindler et al., in a prospective evaluation of patients who underwent ligament reconstruction, found cartilage lesions, most commonly located over the lateral femoral condyle, in 46%.96 Although there does not seem to be a correlation between articular cartilage lesion size and postoperative clinical outcome, patients with a high-grade partial or complete cartilage defect seen at the time of reconstruction had significantly lower subjective scores compared to those without defects.97 MR follow-up studies demonstrate cartilage thinning adjacent to the site of the initial osteochondral lesion.98
 |
|
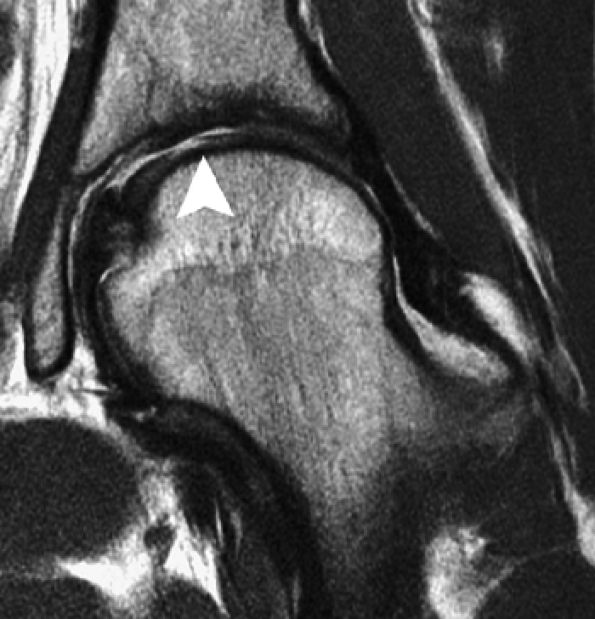
FIGURE 7.16 ● Coronal fast spin-echo MR image of the hip in a 28-year-old patient with active cartilage delamination and flap formation (arrowhead) over the weight-bearing aspect of the femoral head.
|
 |
|
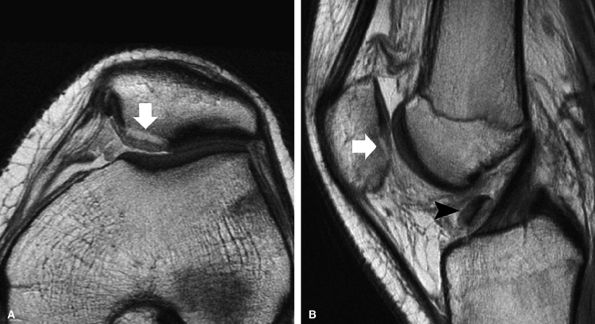
FIGURE 7.17 ● Axial (A) and sagittal (B) fast spin-echo MR images of the knee in a 15-year-old boy following a patellar dislocation demonstrate an osteochondral fracture of the medial facet (arrows). The displaced osteochondral fragment is seen against the synovial reflection of the ACL (arrowhead). Note the presence of high-signal-intensity bone marrow, low-signal-intensity subchondral plate, and intermediate-signal-intensity cartilage in the displaced fragment, distinguishing it from an isolated chondral shear.
|
 |
|
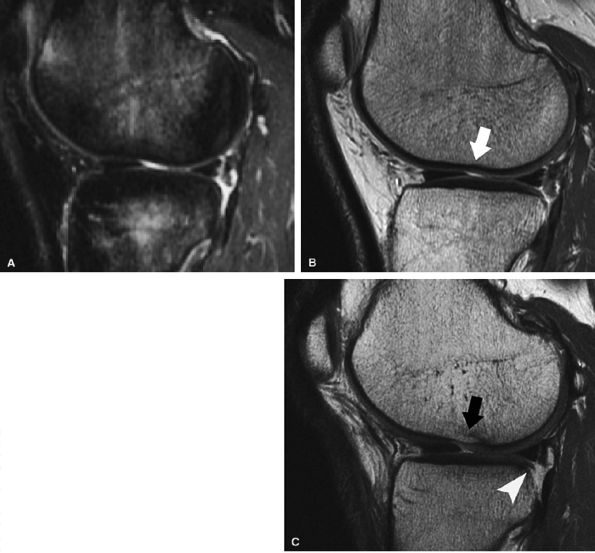
FIGURE 7.18 ● Prospective MR evaluation of a bone bruise in the knee of a 28-year-old man with a complete ACL tear. Sagittal fat-suppressed (A) and fast spin-echo (B) MR images obtained at the time of injury demonstrate characteristic bone marrow edema with compression of cartilage over the lateral femoral condyle (white arrow). A sagittal MR image obtained 19 months later (C) demonstrates proud subchondral bone with focal cartilage loss over the condyle (black arrow), as well as flap formation over the tibial plateau (arrowhead). (Reprinted by permission of SAGE Publications, Inc., from Am J Sports Med, in press.)
|
 |
|
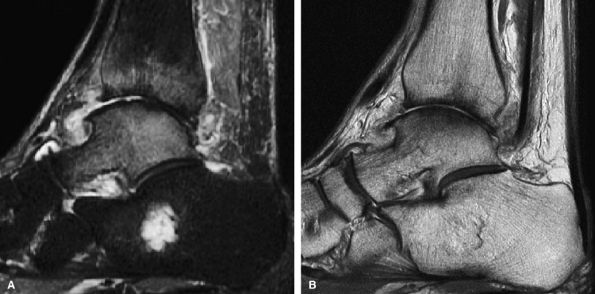
FIGURE 7.19 ● (A) Sagittal fat-suppressed MR image of the ankle in a 64-year-old man with OA of the tibiotalar joint demonstrates bone marrow edema pattern over both sides of the joint. (B) The corresponding cartilage-sensitive MR image demonstrates full-thickness cartilage loss, anterior osteophyte formation, and subchondral sclerosis. The presence of bone marrow edema over both sides of a joint in the setting of OA does not indicate superimposed fracture or osteonecrosis.
|
-
Signal intensity and cartilage morphology (thickness)
-
Subchondral bone marrow edema or cysts
-
Subchondral flattening or depression
-
Osteophytes
-
Synovial thickening/joint effusion
-
Loose bodies
-
Integrity of the cruciate ligaments, collateral ligaments, and menisci103
 |
|
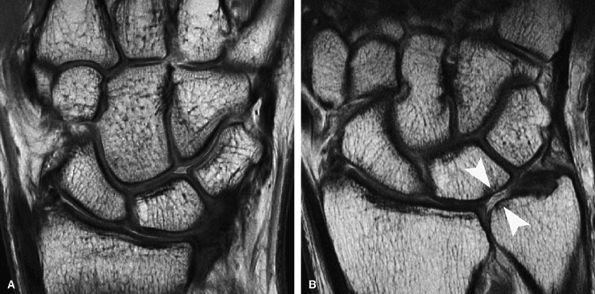
FIGURE 7.20 ● (A) Coronal fast spin-echo MR image of the wrist in a 28-year-old patient with normal articular cartilage. (B) A 61-year-old patient with ulnolunate impaction syndrome demonstrates full-thickness cartilage loss over the distal ulna and proximal pole of the lunate (arrowheads), with sclerotic change in the subchondral bone. Note also the full-thickness central defect in the articular disc.
|
 |
|
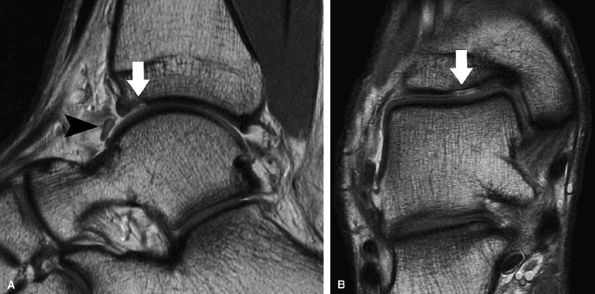
FIGURE 7.21 ● Sagittal (A) and coronal (B) fast spin-echo MR images of the ankle in a 16-year-old patient demonstrate an osteochondral shearing injury (arrows) over the anterior margin of the tibial plafond. A free cartilaginous fragment (arrowhead) in the anterior recess is present. Cartilage over the talar dome is preserved.
|
-
Formation of reparative cartilage by recruitment of multipotential stem cells released from subchondral bone.
-
Does not preclude other subsequent forms of cartilage repair.
-
Performed using arthroscopic approach.
-
Very small holes are created, to a depth of 4 mm, through the subchondral plate.
-
Early postoperative period
-
Reparative cartilage is hyperintense to native cartilage
-
Subchondral bone marrow edema pattern
-
-
Late postoperative period
-
Signal intensity of reparative cartilage decreases as it matures
-
Subchondral bone marrow edema pattern diminishes
-
Overgrowth of subchondral bone may be present
-
-
Degree of filling of defect by reparative tissue
-
Morphology of reparative tissue
-
Presence or absence of delamination
-
Peripheral integration (fissures at repair–native tissue interface)
-
Assessment of host cartilage
 |
|
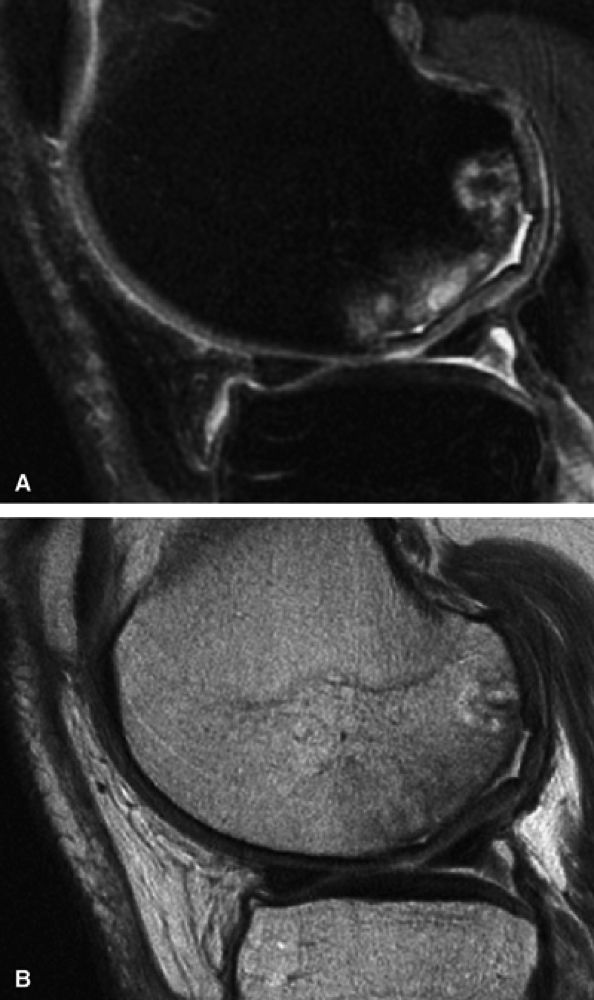
FIGURE 7.22 ● Sagittal fat-suppressed (A) and fast spin-echo (B) MR images of the knee in a 57-year-old man with extensive osteonecrosis and subchondral collapse of the posterior margin of the lateral femoral condyle. Note the secondary delamination of the overlying cartilage with fluid imbibition between subchondral bone and cartilage.
|
cartilage, Mithoefer et al. noted an underlying bone marrow edema pattern, followed by progressive filling and a decreasing bone marrow edema pattern.111 Overgrowth of subchondral bone has also been described following microfracture,111,112 resulting in corresponding thinning of the overlying repair cartilage as well as the potential for impaction of the opposing surface of the joint during active loading. Although osseous overgrowth is a not uncommon finding, it does not correlate with a statistically significant worsening in clinical outcome.111 The significance of subchondral bone overgrowth is not yet certain, but it might reflect excessive removal of the subchondral bone during debridement and removal of the calcified cartilage layer, thus providing a stimulus for enchondral ossification (Fig. 7.24).114 Postoperative peripheral integration may be incomplete, and discernible fissures can be seen in the majority of patients on postoperative MR imaging.111
 |
|
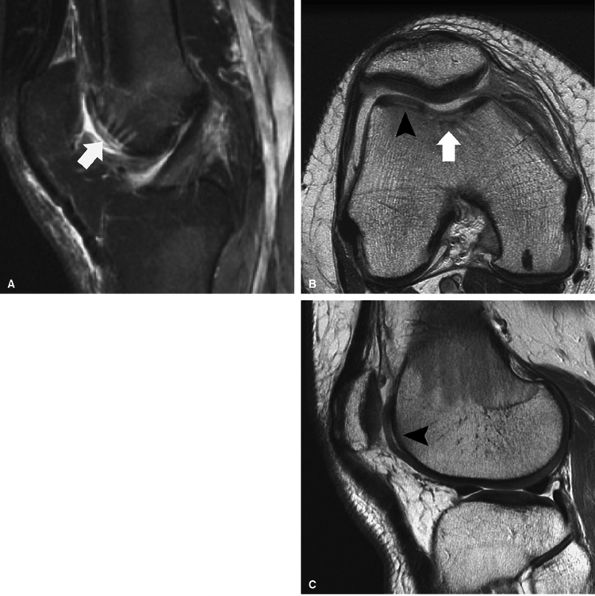
FIGURE 7.23 ● Sagittal inversion recovery (A) and axial fast spin-echo (B) MR images of the knee in a 31-year-old patient obtained 7 weeks following microfracture over the trochlea. Note the hyperintense reparative fibrocartilage over the defect (arrows). Sagittal fast spin-echo MR image (C) also demonstrates basilar delamination of cartilage without flap formation (arrowhead) adjacent to the area of microfracture, over the lateral margin of the trochlea.
|
 |
|
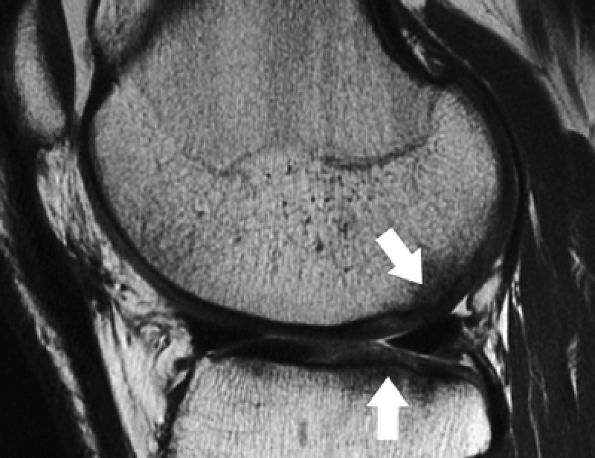
FIGURE 7.24 ● Sagittal fast spin-echo MR image of the knee in a 19-year-old patient, performed 15 months following microfracture of the posterior margin of the lateral femoral condyle and 12 months following microfracture of the lateral tibial plateau, demonstrates proud subchondral bone formation (arrows) and hyperintense reparative fibrocartilage over the sites of cartilage repair.
|
 |
|
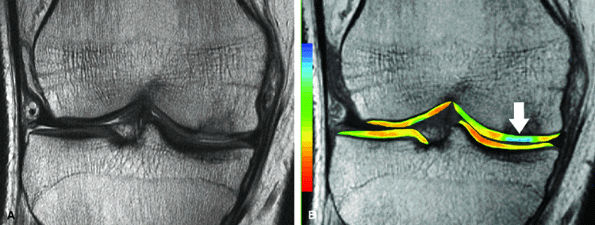
FIGURE 7.25 ● (A) Coronal fast spin-echo MR image of the knee in a 24-year-old professional basketball player obtained 3 months following microfracture of the medial femoral condyle demonstrates good fill by hyperintense reparative fibrocartilage. (B) Corresponding quantitative T2 relaxation time map coded to capture T2 values ranging from 0 to 100 msec demonstrates prolongation of T2 relaxation times (arrow), reflecting the reparative fibrocartilage and a less organized matrix. Green and blue reflect longer T2 values, yellow intermediate, and orange the shorter values.
|
-
Restoration of articular cartilage surface by osteochondral plugs harvested from a different region of the knee in the same individual.
-
Plugs are typically harvested from the far anterior margin of the condyle or the side of the intercondylar notch.
-
Technique is suitable for a large defect (several plugs are typically used).
-
One-stage procedure is performed using arthroscopic or open-joint approach.
-
Osteochondral plugs are harvested from relatively non–weight-bearing areas of the knee and transplanted to the site of articular defect in the same individual.
-
Multiple plugs can be transferred.
-
Early postoperative period
-
Subchondral bone marrow edema pattern
-
-
Late postoperative period
-
Subchondral bone marrow edema pattern diminishes
-
-
Degree of filling of defect by transplanted osteochondral plugs
-
Restoration of radius of curvature of joint surface
-
Presence or absence of displacement
-
Peripheral integration of repair cartilage and osseous components
-
Morphology of autologous bone
-
Assessment of host cartilage
-
Do not mistake low-signal condensation of trabeculae at the periphery of the plug using the “press fit” technique for lack of osseous integration.
-
Repair can be performed for a large defect.
-
Articular cartilage resurfacing with one or more osteochondral plugs harvested from a human cadaver.
-
Repair is suitable for large defects (several plugs may be used).
-
No donor-site morbidity.
-
Fresh or cryopreserved cadaveric plug(s) are harvested aseptically and transplanted.
-
Osteochondral allograft plugs may be pressed to fit in position or fixed by biodegradable or metallic pins if deemed mechanically unstable at the time of surgery.
-
Early postoperative period (0–3 months)
-
Graft bone marrow edema pattern
-
-
Late postoperative period (3–6 months)
-
Graft bone marrow edema pattern diminishes
-
-
Features of rejection
-
Graft bone marrow edema pattern persists after 6 to 12 months
-
Graft collapse
-
Fluid signal intensity at graft–host interface
-
-
Degree of filling of defect by transplanted osteochondral plugs
-
Restoration of radius of curvature of joint surface
-
Morphology of repair
-
Presence or absence of displacement
-
Peripheral integration of repair cartilage
-
Incorporation of allograft bone
-
Assessment of host cartilage
-
Relative signal intensity from the repair cartilage using a standardized ROI on an MR imaging workstation
-
Presence or absence of delamination
-
Nature of the interface with the native cartilage (presence or absence and/or size of fissures)
-
Percentage fill of the lesion using both coronal and sagittal images
-
Assessment of the integrity of the articular cartilage in the surrounding environment, including cartilage in the adjacent and opposite surfaces
with autologous osteochondral transplantation are closer to that of the remaining portion of the joint (Fig. 7.28).
 |
|
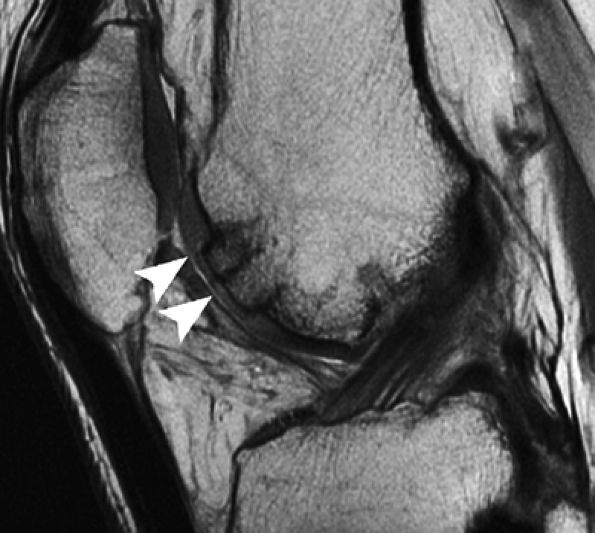
FIGURE 7.26 ● Sagittal MR image of the knee in a 37-year-old patient obtained 12 months following autologous osteochondral transfer to the trochlea demonstrates that although the osseous components of the plugs are proud relative to subchondral bone, the repair cartilage is flush with that of the native cartilage, with restoration of the radius of curvature of the joint surface. Note the high signal intensity at the repair–native cartilage interface (arrowheads).
|
 |
|
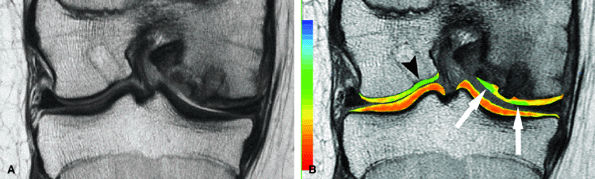
FIGURE 7.27 ● (A) Coronal fast spin-echo MR image of the knee in a 13-year-old girl obtained 3 months following autologous osteochondral transfer for osteochondritis dissecans of the medial femoral condyle. Despite the relatively thin cartilage over the proud osseous component of the osteochondral plug, there is good restoration of the radius of curvature of the articular surface. Note that the donor site in the lateral femoral condyle close to the notch is filled with autologous bone, covered by reparative material that is hyperintense to native cartilage. (B) Corresponding quantitative T2 relaxation time map coded to capture T2 values ranging from 5 to 100 msec demonstrates prolongation of T2 relaxation times at the margins of the osteochondral lesion (arrows). There is prolongation of T2 values at the margins of the plugs (arrows) and over the donor site (arrowhead), reflecting reparative fibrocartilage and a less organized matrix. Green and blue reflect longer T2 values, yellow intermediate, and orange the shorter values. (Reprinted by permission of SAGE Publications, Inc., from Am J Sports Med, in press.)
|
 |
|
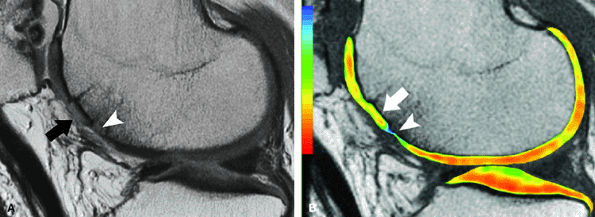
FIGURE 7.28 ● (A) Sagittal fast spin-echo MR image of the knee in a 53-year-old patient, obtained 2 years following autologous osteochondral transfer of the central trochlea. Note the hyperintensity of the cartilage of the osteochondral plug (black arrow) and adjacent inferior trochlear groove chondral loss (white arrowhead). (B) Corresponding quantitative T2 relaxation time map demonstrates mild prolongation of T2 values (white arrow) relative to the remaining normal cartilage within the joint, with preservation of T2 stratification. Note the full-thickness cartilage loss at the inferior margin of the plug, where there is marked prolongation of the T2 values (white arrowhead).
|
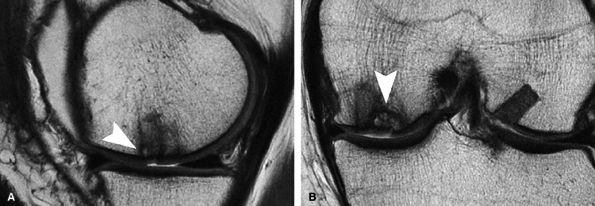
restoration of the normal fatty signal intensity of the plug, and lack of displacement. The use of both cartilage-sensitive and fat-suppressed imaging is therefore essential in assessing not only the integrity of the articular surface but also the degree of host subchondral bone turnover at the site of plug incorporation. The presence of low signal intensity on all pulse sequences strongly suggests loss of bone viability, which may lead to eventual implant failure (Fig. 7.30). Often multiple plugs are used, sometimes requiring instrumentation, and appropriate modification of pulse sequence parameters is necessary to reduce the associated susceptibility artifact in the case of metallic fixation. Alternatively, a “press fit” fixation
may be used, and in this situation care must be taken to avoid mistaking the low-signal-intensity compression of trabeculae at the side wall of the implant for failure of the plug to incorporate (Fig. 7.31).
 |
|
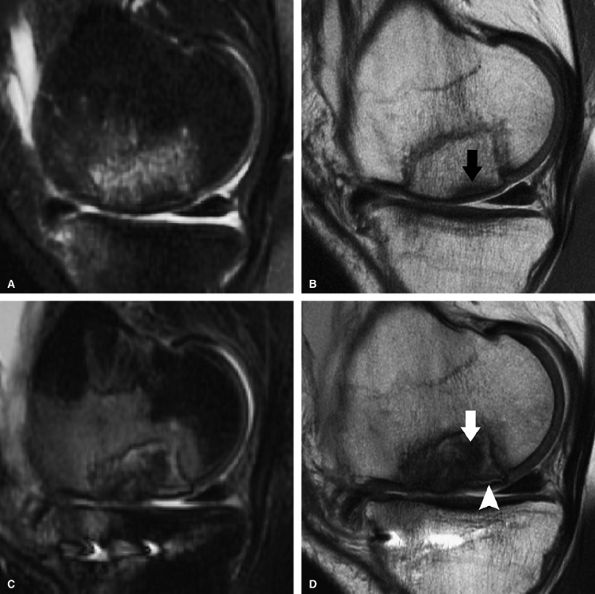
FIGURE 7.29 ● Sagittal (A) and coronal (B) fast spin-echo MR images of the knee in a 41-year-old patient obtained 3 months following autologous osteochondral transfer using multiple plugs. There is overall good bony incorporation of the osteochondral plugs, but with focal cystic change and collapse of subchondral bone centrally (arrowhead in A and B). Note that the donor site (B) over the lateral aspect of the intercondylar notch is filled with hyperintense material, likely representing reparative fibrocartilage.
|
 |
|
FIGURE 7.30 ● Sagittal inversion recovery (A) and fast spin-echo (B) MR images of the knee in a 36-year-old patient performed 1 year following fresh-frozen allograft transplantation in the medial femoral condyle. The allograft is slightly proud with persistent bone marrow edema pattern, both within the graft and at the graft-host interface, associated with mild subchondral collapse (black arrow in B). Corresponding inversion recovery (C) and fast spin-echo (D) MR images obtained a year and a half later, with interval tibial osteotomy, demonstrate further collapse of the allograft bone, with the areas suspicious for the presence of nonviable bone (white arrow), which is of low signal intensity on both pulse sequences. Note also the fluid imbibition (arrowhead in D) beneath the graft subchondral plate.
|
 |
|
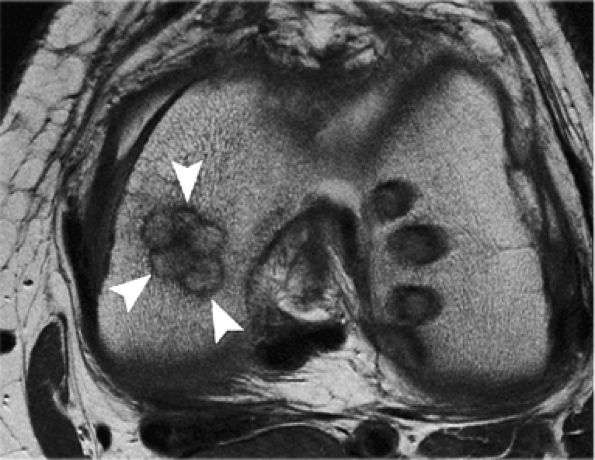
FIGURE 7.31 ● Axial fast spin-echo MR image of the knee performed 3 months following autologous osteochondral transfer using multiple plugs to restore a large osteochondral defect of the medial femoral condyle. Note consolidation of trabeculae (linear low signal intensity) around the plugs (arrowheads) as a result of the “press fit” fixation. Donor sites are seen in the lateral femoral condyle, close to the notch.
|
-
A matrix scaffold
-
Cells
-
Signaling molecules, including growth factors or genes105
 |
|
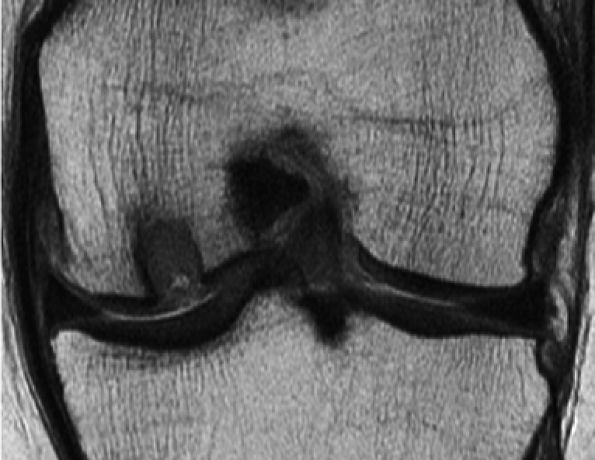
FIGURE 7.32 ● Coronal fast spin-echo MR image of the knee in a 61-year-old patient obtained 3 months following placement of a synthetic scaffold bone graft substitute. Note the intermediate signal intensity characteristics of the synthetic plug.
|
-
Articular cartilage resurfacing technique using tissue-cultured autologous articular cartilage cells.
-
Two-stage procedure:
-
Healthy articular cartilage is harvested using arthroscopy, and chondrocytes are extracted and grown in tissue culture for 3 to 5 weeks.
-
Periosteum is harvested via arthrotomy and sewn over the cartilage defect with the cambium layer facing toward the defect. The edges of periosteum are secured with fibrin glue or suture. The cultured chondrocyte suspension is then injected under the periosteum.
-
-
Early postoperative period
-
Reparative cartilage is hyperintense to native cartilage and periosteal cover
-
Subchondral bone marrow edema pattern
-
± Hypertrophy of periosteal cover
-
-
Late postoperative period
-
Signal intensity of reparative cartilage decreases as it matures, approaching that of adjacent cartilage
-
Subchondral bone marrow edema pattern diminishes
-
-
Degree of filling of defect
-
Morphology of repair
-
Presence or absence of displacement
-
Peripheral integration with adjacent native cartilage and underlying bone
-
Assessment of host cartilage
-
Porosity for cell migration
-
Biodegradability for physiologic remodeling
-
Bonding to allow for peripheral integration
-
Biocompatibility with the surrounding environment105
-
Hyperintense repair cartilage in the early period of 0 to 8 weeks
-
A transitional phase of 3 to 6 months with lower, more inhomogeneous signal intensity
-
A remodeling phase with the signal approaching that of the adjacent cartilage123
 |
|
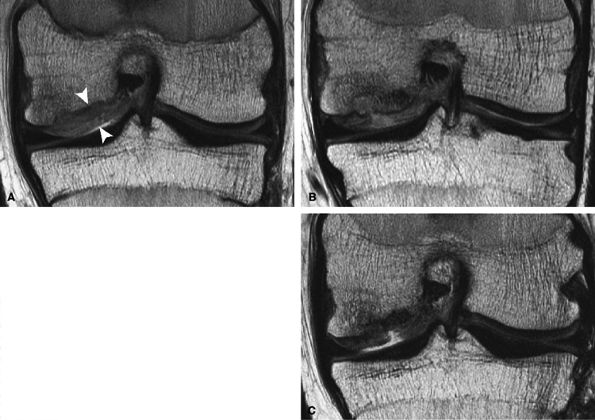
FIGURE 7.33 ● Coronal fast spin-echo MR images of the knee in a 15-year-old boy obtained following ACI for osteochondritis dissecans of the medial femoral condyle. (A) At the 3-month follow-up, the repair cartilage is proud and hyperintense relative to native cartilage, consistent with graft hypertrophy (arrowheads). (Reprinted by permission of
Lippincott Williams & Wilkins, from Clin Orthop Rel Res. 2004;422:214-23.
) (B) At the 8-month follow-up, there is further focal increased signal intensity of the graft, now associated with subchondral bony overgrowth. (C) At 27 months of follow-up, a focal full-thickness defect with partial delamination is demonstrated. |
glycosaminoglycan levels were more comparable to the adjacent and remote hyaline cartilage.125
 |
|
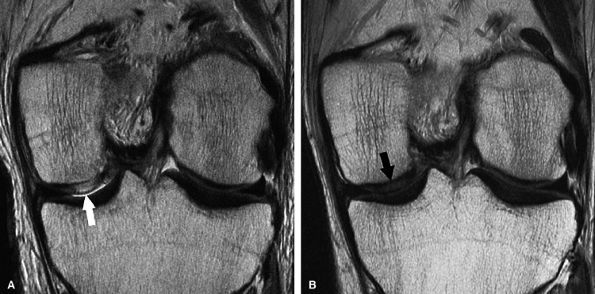
FIGURE 7.34 ● Coronal fast spin-echo MR images of the knee in a 31-year-old man obtained following ACI. (A) Six weeks following surgery the graft is hyperintense relative to the intact, hypointense overlying periosteal cover (arrow). (B) Twenty months following surgery there is incorporation of periosteum to what is now isointense reparative cartilage, such that periosteal cover is no longer distinct (arrow). (Reprinted by permission of
Lippincott Williams & Wilkins, from Clin Orthop Rel Res. 2004;422:214-23.
) |