Management of Fractures
Properly instituted injury prevention programs can be effective in
reducing the number and severity of pediatric trauma events (3).
The fracture rate increases as children grow, with the incidence
peaking in early adolescence. New sports and recreational activities
such as rollerblading and skateboarding have created new injury
patterns, with an increase in the incidence of distal radius fractures (4,5).
Fortunately, most fractures are minor. Only 20% require reduction, and
greenstick and torus fractures constitute approximately 50% of
fractures in children (6,7). Therefore, the management of pediatric fractures is
often straightforward, but one must know when to intervene and when to
allow nature to take its course. The skeleton of a young child behaves
differently than the skeleton of an older child. There are also
musculoskeletal differences in biomechanics, anatomy, and physiology
between children and adults. It is the purpose of this chapter to
highlight the unique aspects of managing fractures in children, and to
delineate age-appropriate treatment guidelines.
vascular channels than the bones of adults. This results in a lower
modulus of elasticity (8). Therefore, a given
stress applied to a specific area of pediatric bone results in more
strain than the same stress on the adult bone. Bending strength is
lower than in adult bone, but the low modulus of elasticity allows for
greater energy absorption before failure. Pediatric bone also has the
capacity for plastic deformation in which the bone does not return to
its original shape. Microscopic mechanical failure, not evident on
routine radiographs, occurs through oblique slip lines or
microfractures on the compression side of the bone. This produces no
fracture hematoma and minimal periosteal reaction during healing.
Plastic deformation results from an applied load after the yield point
is reached, but before the breaking point. This partly explains why
greenstick fractures can occur when a bending load is applied. Much of
the strain energy is dissipated through plastic deformation of the
concave cortex, while tension failure occurs on the convex side. This
prevents larger, more catastrophic failure from occurring.
torus or “buckle” fractures from compression failure. This usually
occurs at the metaphyseal—diaphyseal junction, where the more rigid
diaphyseal cortex meets the thinner metaphyseal cortex. The less rigid
metaphyseal cortex buckles and deforms without complete failure,
creating a relatively stable injury. The rate at which the force is
applied also influences the fracture type. Higher load rates more often
result in complete fracture (8). Complete
fractures display transverse, oblique, or spiral configurations,
depending on how the injury force was applied. Comminuted fractures are
less common in children than in adults, because pediatric bone can
dissipate energy before failure, and its porosity inhibits fracture
line propagation. The smaller diameter of pediatric bone also affects
its strength compared with adult bone. The polar moment of inertia of a
structure, which measures resistance to torque, is proportional to the
fourth power of the radius of the structure. Therefore, a relatively
small increase in the cortical diameter results in a large increase in
a bone’s ability to resist torsional load.
bone. In young children, the epiphysis is largely cartilaginous and
transmits injury forces to the metaphysis. Increasing ossification in
the epiphysis occurs with age and imparts more rigidity to the
epiphysis. This increased rigidity partially explains why epiphyseal
fractures and separations are more commonly seen in older children.
major anatomic features that distinguish children’s bones from those of
adults. The physis, also called the growth plate or the epiphyseal plate,
is the area of growing cartilage that contributes length to the growing
bone. The epiphysis is the region at the end of the bone. The epiphysis
forms a secondary center of ossification and determines the shape and
size of the articular surface. An apophysis is a secondary growth
center at a site of tendon attachment. Apophyses are extraarticular and
do not contribute to longitudinal growth. Each of these structures
ossifies at predictable times during growth and thus provides
information regarding skeletal maturity. Lack of ossification in young
children and variable patterns of ossification during growth make the
diagnosis of injury an even greater challenge in children than in
adults.
thick, vascular, and highly osteogenic periosteum. The cambium or
osteogenic layer lies directly on the cortex of bone. The outer fibrous
layer provides attachment for muscles and ligaments. Between these two
layers lie elastic fibers that allow the fibrous layer to be stripped
from the bone, leaving the osteogenic layer intact (9).
Muscle and periosteum are firmly attached to the bone in only a few
places, such as the linea aspera. The periosteum is also firmly
attached to the growth plate at the perichondrial ring of LaCroix,
which helps stabilize the physis. The thick periosteum in children is
rarely torn circumferentially after a fracture and often remains intact
on the compression side of the injured bone. This intact periosteal
sleeve may then be used as a tension band for fracture reduction and
stabilization. Intact periosteum may also prevent soft tissue
interposition and facilitate reduction. Conversely, the periosteum
itself may become interposed between the fracture fragments, and the
longitudinally torn periosteum may interfere with reduction by allowing
the bone to “buttonhole” through the periosteal defect.
radius fracture in children with increased body mass and decreased bone
mineral density (10,11). However, this relationship has not been confirmed for fractures at other sites (12). Fracture patterns also vary with age due to changing skeletal maturation.
than fractures in adults, and nonunion is rare. Most pediatric
fractures unite by secondary fracture healing, which occurs without
rigid immobilization and involves a combination of intramembranous and
endochondral ossification. Fractures cause cellular injury and hematoma
formation. Blood, marrow, and necrotic cells release cytokines that
stimulate inflammation and the proliferation of stem cells (13).
The clot gives rise to platelet-derived growth factor and transforming
growth factor β leading to general cellular proliferation. Stem cells
produce bone morphogenetic proteins that cause cellular
differentiation. The exact relation between these factors and other
factors involved in the coordination of fracture healing is the subject
of ongoing research (14). During the second
stage of fracture healing, angiogenesis occurs. This stage of bone
healing is facilitated in children by the highly vascular periosteum
and preservation of a viable muscle envelope. The periosteum also
contributes to bone formation by membranous ossification within 10 to
14 days of injury. Angiogenesis is followed by the formation of soft
callus. Low oxygen tension and fracture motion promote cartilage
formation as the initial stage of endochondral ossification. This
cartilage is subsequently removed and replaced by hard callus with
woven bone (9,14). Ultimately, the ossification phase gives way to a more prolonged phase of fracture remodeling.
It is explained in part by the osteogenic potential of the periosteum
and the magnitude of the vascular response in children (9).
The periosteum is vital to fracture healing and should be preserved as
much as possible. Large segments of bone may regenerate and remodel, so
long as the periosteal tube is intact. Remodeling at the fracture site
occurs by bone resorption on the convexity and deposition on the
concavity of the fracture. This phenomenon of “bone drift” is well
recognized clinically and has been quantified in the rabbit model (15).
However, most of the remodeling occurs by reorientation of the growth
plates, with improvement in the overall alignment of the limb.
Asymmetric and longitudinal growth of the physis contributes to this
remodeling. Therefore, measurement of angular remodeling at the
fracture site gives an inadequate picture of the overall limb alignment
as remodeling occurs (15,16).
Remodeling capacity depends on the number of years of growth remaining,
the proximity of the fracture to a rapidly growing physis, the
magnitude of angular deformity, and the plane of angulation relative to
adjacent joints. Remodeling may continue for 5 to 6 years after
fracture, so long as growth occurs during the period of remodeling (16).
The rate of remodeling is minimally influenced by age, but the
completeness of remodeling may be limited by the number of years of
growth remaining (16,17).
Fractures in the plane of joint motion and near a rapidly growing
physis have the greatest capacity to remodel. Fractures with smaller
degrees of malunion are more likely to remodel completely.
Remodeling of rotational deformity has also been noted in children, but this is less predictable than angular remodeling (15,18). It has been postulated that torsional remodeling occurs by helical growth of the physis (15).
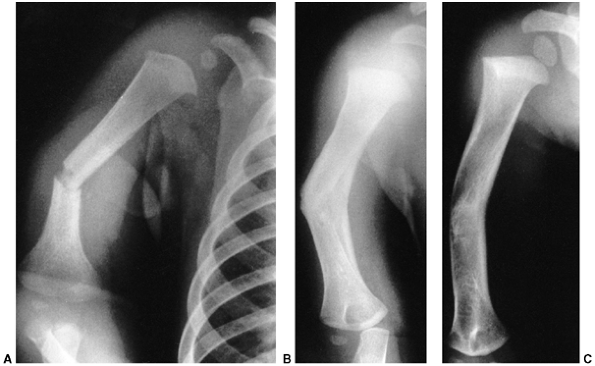 |
|
Figure 33.1 Periosteal bone formation. A:
Complete fracture of the humeral diaphysis in a 6-month-old child. The periosteum is presumed to be intact on the compression (concave) side. B: Four weeks later, the periosteum has formed a complete column of new bone. C: Six months after injury, there has been significant remodeling, with a 50% correction of the angular deformity. |
healing in children is the potential for growth stimulation after
fracture. The age of the patient and the amount of periosteal stripping
influence the amount of growth stimulation (9).
This phenomenon is most commonly reported after femoral fractures in
children between the ages of 3 and 9 years, but it also occurs after
other fractures (19,20,21).
The exact mechanism for growth acceleration is unknown, but increased
blood flow to the growth plate and release of periosteal tension after
fracture are potential causes. Hyperemia as a cause of overgrowth is
supported by the observation that growth acceleration may occur with
conditions that cause increased vascularity, such as congenital
vascular anomalies, inflammatory conditions, and tumoral disorders (19).
Transverse sectioning of the periosteum also produces overgrowth.
Periosteal stripping or division close to the growth plate increases
the effect of transverse periosteal release, but longitudinal incision
of the periosteum does not cause growth acceleration (9,22). Hemicircumferential release of the periosteum causes asymmetric growth and subsequent angular deformity (23).
This phenomenon is most often seen clinically after fracture of the
proximal tibial metaphysis in children, when the medial periosteum is
torn transversely while the lateral periosteum remains intact. These
findings support the observation that the periosteum acts as a
mechanical restraint on epiphyseal growth through its attachments to
the perichondrial ring (24). In older children, premature physeal closure has been noted after diaphyseal fracture (25,26).
Perhaps the variable growth responses after fracture can be partially
explained by differences in periosteal damage or asymmetric hyperemic
responses after fracture.
and contain the cells responsible for bone growth. Longitudinal growth
occurs as columns of cartilage form and undergo endochondral
ossification. Fracture of the growth plate generally heals within 3 to
4 weeks, because this is a well-vascularized region that is growing
rapidly and forming bone at the time of injury.
Several anatomic features are pertinent for understanding trauma to the
growth plate. The germinal zone of cartilage formation is located on
the articular side of the physis. This zone consists of resting cells,
which initiate the process of long-bone growth by dividing to form the
zone of proliferating cartilage. The hypertrophic zone is next. This is
a zone of maturation where the chondrocytes begin to enlarge. On the
metaphyseal side of this zone, vascular buds grow into the degenerating
columns of cartilage and initiate provisional calcification as the
chondrocytes degenerate. Remodeling into lamellar bone in the
metaphy-sis rapidly follows provisional calcification.
tension or shearing forces. Compressive forces tend to cause fracture
through bone. Bright et al. (27), using rapid-loading techniques to simulate in vivo
forces, demonstrated that the weakest zone is the zone of provisional
calcification. However, fracture failure was rarely limited to one
zone, and propagating cracks traversed, at least partially, the upper
germinal zones in 85% of the animals tested. In younger animals, the
fracture was more likely to traverse only the zone between the
hypertrophic cells and metaphyseal bone, avoiding the germinal zones.
This is clinically relevant because injury to the germinal zone may be
more likely in older children and can cause arrest of bone growth.
perichondrial ring of LaCroix surround each physis circumferentially at
the periphery. These structures constitute a separate growth center
that provides growth of the physis in width. The groove of Ranvier
consists of resting and proliferating cells, whereas the ring of
LaCroix consists of cartilage cells that extend toward the metaphysis
and become continuous with the metaphyseal periosteum. The groove and
ring provide support to the physis and resistance to physeal
separation. Additional stability is provided by large and small
undulations of the growth plate. The smaller projections are called mammillary processes.
Larger contours include the lappet formation, which is the overlapping
shape of the physis as it cups the metaphysis. The lappet formation is
readily appreciated where the anterior portion of the proximal tibial
growth plate extends distally as the tibial tuberosity. Muscular,
capsular, and ligamentous attachments to the epiphysis may provide
additional stability. However, these structures can also transmit force
to the growth plate, resulting in characteristic fracture patterns that
vary with the specific anatomy of a joint.
development. There is strict separation of the epiphyseal and
metaphyseal circulation, because blood vessels do not cross the growth
plate. There are two patterns of blood supply to epiphyses (28).
Some epiphyses are completely intracapsular (e.g., the proximal femur
and the proximal radius). Blood vessels for these epiphyses must enter
the epiphysis around the periphery of the growth plate and through a
narrow region between the articular cartilage and the physis. This type
of blood supply is very susceptible to damage during physeal separation
or occlusion of supporting vessels. The second pattern of epiphyseal
blood supply is more common, and is seen in epiphyses that are
extracapsular (e.g., the proximal tibia and the distal radius).
Vessels
that supply circulation to the epiphysis and the growth plate penetrate
directly through the side of the epiphysis where it is covered with
periosteum and capsular attachments. Extracapsular epiphyses are less
vulnerable to devascularization when physeal separation occurs.
The peak age for injury to the growth plate is early adolescence (11 to
12 years), and physeal fracture is uncommon in children younger than 5
years. Boys are affected twice as often as girls (29,30).
Several classification systems have been proposed since Foucher first
described different types of physeal fractures in 1863 (30). The most widely used classification of physeal fractures is that described by Salter and Harris (31). There are five types of fractures in this classification. Rang has added a sixth that is commonly recognized (Fig. 33.2) (32).
entire growth plate without evidence of a metaphyseal fragment. This
type of fracture is most commonly seen in infants and young children.
The epiphyseal fragment may be nondisplaced or minimally displaced,
making diagnosis difficult. Localized swelling and point tenderness may
confirm the diagnosis. The prognosis for resumption of growth is
excellent with a few notable exceptions, such as physeal separation of
the proximal or distal femur. Partial growth arrest may occur with more
severe trauma, or when periosteum is entrapped in the physis (33,34).
fractures. The fracture line passes through a portion of the growth
plate and exits through a triangular segment of the metaphysis that
remains attached to the intact portion of the growth plate. The
metaphyseal fragment (Thurston Holland fragment) is on the compression
side of the fracture. The prognosis for resumption of growth is
generally excellent, but the risk of growth disturbance varies with the
location of the fracture. Type II fractures of the distal radius rarely
lead to physeal closure (35), but type II fractures of the distal femur cause growth disturbance in approximately 50% of patients (36).
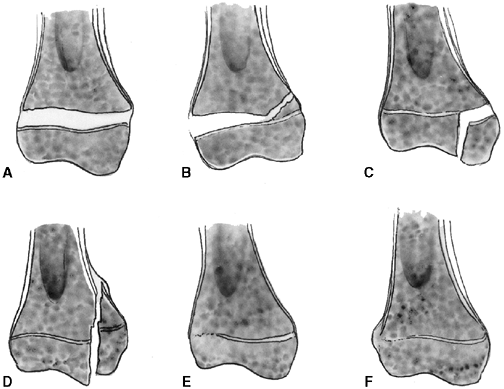 |
|
Figure 33.2 Salter-Harris classification. A: Type I is a transepiphyseal separation without evidence of a metaphyseal fragment. B:
In type II, the fracture line is through the physis, exiting into the metaphysis, leaving a small triangular portion attached to the physeal plate (i.e., Thurston Holland fragment). C: The type III fracture is an intraarticular fracture, with the fracture traversing the physis and exiting through the epiphysis. D: Type IV describes a vertical fracture line that is intraarticular. It passes through the epiphysis, physis, and metaphysis. E: Type V fracture describes a crush injury to the physis that usually is not apparent on initial injury films. F: Type VI fracture is a localized injury to a portion of the perichondrial ring. Subsequent healing produces bone formation across the perimeter of the physis, connecting the metaphysis to the epiphysis. |
a portion of the growth plate, then crosses the epiphysis and the
articular surface. The prognosis for resumption of growth is more
guarded with this injury, and depends on the vascularity of the physis
and damage to the germinal zone. These fractures are more common in
older children in whom growth arrest may not be problematic (29). Anatomic reduction is recommended to reduce the risk of growth arrest and to restore the congruity of the articular surface.
vertically. These fractures are intraarticular and traverse the
epiphysis, physis, and metaphysis. In type IV fractures, a relatively
small proportion of the physes is affected by injury, yet the risk of
growth arrest is high (31). Precise anatomic reduction is recommended to realign the physis and restore the articular surface.
the growth plate. This injury may not be apparent on radiographs
because it does not always involve fracture fragment displacement.
Growth arrest is common. Crush injury to the germinal physeal cells can
occur in combination with other Salter-Harris fracture patterns.
This may result from ligamentous avulsion, direct trauma, burn, or
other forces. Localized growth arrest may occur and lead to asymmetric
growth with angular deformity.
result from compromised vascularity of the physis, damage to the
germinal cells, and bone bridge formation between epiphyseal and
metaphyseal bone (37). Destruction of the
epiphyseal vasculature leads to central growth arrest followed by
complete epiphysiodesis. In contrast, destruction of the metaphyseal
vasculature may temporarily interfere with ossification but does not
result in growth arrest (38). Direct injury to
germinal cells of the growth plate can also lead to physeal arrest, but
small areas of physeal damage—less than 7% of the total area—do not
usually cause permanent growth disturbance (39,40).
Bone bridge formation can also develop after a Salter-Harris type IV
fracture when displacement allows the epiphyseal bone to remain in
contact with the metaphyseal bone. Peripheral defects result in greater
deformities than do central defects of the same size because of their
location. Also, small central defects may yield to the force of growth
in the remaining viable growth plate.
kind of deformity that eventually develops. Complete growth arrest may
produce limb-length discrepancy without angular deformity. The amount
of discrepancy depends on the growth rate of the affected physis and
the age of the child. Contralateral epiphysiodesis should be considered
as a treatment option as soon as complete growth arrest is diagnosed.
No treatment is required when there is minimal growth remaining or the
resultant lower-extremity discrepancy will be less than 2 cm at
maturity. Limb lengthening is a treatment option instead of
contralateral epiphysiodesis when the projected discrepancy will be
greater than 5 cm.
than complete arrest because partial arrest may result in length
discrepancy combined with angular deformity, joint incongruity, or
both. Early recognition is desirable to minimize complications. Partial
growth arrest can be recognized as early as a few months after
fracture, or may take up to 2 years to become evident. A sclerotic
bridge of bone or blurring and narrowing of the growth plate is often
visible on plain radiographs if the x-ray beam is tangential to the
physis. Another early sign of growth disturbance is the development of
an oblique growth arrest line (41) (Fig. 33.3). Magnetic resonance imaging (MRI) may also be useful to detect early physeal arrest (42,43).
The most common type is a peripheral bar, which produces an angular
deformity. The second type is a central bar, which acts as a central
tether and results in tenting of the physis with eventual articular
surface distortion. The third pattern of bar formation is referred to
as a linear bar and involves portions of
the central and peripheral physis. This last type is often the result
of a Salter-Harris type IV fracture that has healed in a displaced
position.
growth-plate damage, depending on the location of the bone bridge, the
size of the bar, and the amount of growth remaining. Once identified,
partial arrest can be surgically converted to complete arrest to
prevent further angulation. This method can also be combined with
osteotomy when angular deformity has already developed. Contralateral
epiphysiodesis may be performed when the discrepancy is less than 2 cm,
or lengthening through the osteotomy site can be performed to equalize
limb lengths (44). Bilateral epiphysiodesis
with or without osteotomy is particularly appropriate for growth
disturbances of slowly growing physes that do not contribute
significant length to the limb, such as the distal tibia. This approach
can also be utilized instead of bar excision in juvenile patients.
This has been performed for bars that involve as much as 40% of the
physis. Recurrence of the bar and growth arrest are common following
the procedure, so physeal distraction is recommended only for patients
who are near skeletal maturity (45). The
authors no longer perform physeal distraction because of limited
indications, complications, and pain associated with this technique.
50% of the physis is damaged and more than 2 years of growth remain in
the affected growth plate. This procedure
may
eliminate the need for osteotomy if the angular deformity is less than
20 degrees. Success rates are variable, and results are more successful
when the bar involves less than 25% of the growth plate (46,47,48).
Before surgical excision, it is necessary to clearly delineate the
extent and location of the bar. Plain radiography should be performed
with the beam centered on the growth plate and tilted in the same plane
as the growth plate. Helical computed tomographic scanning and MRI have
largely replaced traditional tomography (49). Three-dimensional reconstruction using these techniques allows accurate identification of the size and location of the bar (50).
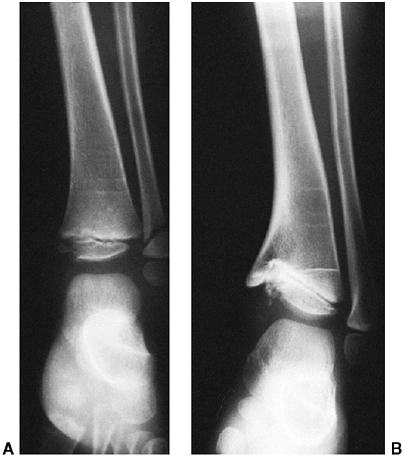 |
|
Figure 33.3 Distal tibial growth arrest. A: Distal tibial physeal Salter-Harris type IV injury treated with cast immobilization without reduction. B:
Two years later, there is varus angulation to the distal tibia from a medial physeal bar. The Harris growth arrest line is not parallel to the distal physis, and does not extend across the entire width of the metaphysis. |
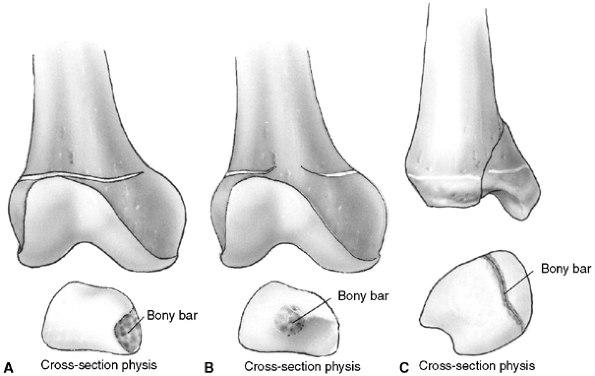 |
|
Figure 33.4 Growth arrest patterns. A: Type I is peripheral growth arrest with a peripheral bony bar. B: Type II is central growth arrest with central physeal tethering. The peripheral physis and perichondrial ring are intact. C:
Type III is combined growth arrest, demonstrating a linear bar involving the peripheral and central portions of the physeal plate. This type of growth arrest is more typical after a Salter-Harris type III or IV fracture. |
excision of the overlying periosteum and removal of the abnormal bone
until normal physeal cartilage is uncovered. Central bars are
approached through a metaphyseal window or through an osteotomy when
angular correction is necessary. An alternative approach is to remove a
wedge of metaphyseal bone and replace it after the bar resection has
been completed (51). The peripheral margins of
the physis are carefully preserved. Intraoperative fluoroscopy may help
guide the approach to the bone bridge. A linear bar is approached
directly where it contacts the periphery of the growth plate. The bone
bridge is then resected from one side to the other, creating a tunnel
that follows the original fracture line. A high-speed burr, small
curettes, and a dental pick facilitate the removal of unwanted bone
without damaging the normal physis. Loupe magnification, a headlight,
and a dental mirror can facilitate visualization of the normal growth
plate. Alternatively, an arthroscope can be used to verify complete
excision. Interposition material is inserted to prevent the bone bridge
from recurring. Fat and methylmethacrylate (Cranioplast) are the two
most commonly used materials for interposition, but cultured
chondrocytes or other biologic tissue may prove useful in the future (52).
The material must remain in contact with the physis during further
growth. This is accomplished by securing the interposed substance to
the epiphysis. Radio-dense markers are usually inserted in the bone to
facilitate the measurement of subsequent growth (Fig. 33.5).
difficult to evaluate because there is great variation in location and
extent of physeal bars. Also, long-term follow-up is difficult to
achieve. Some excellent results have been reported, and partial growth
has been restored in many patients (46). Williamson and Staheli (47)
noted excellent results in 50% of cases with an average follow-up of 2
years. However, others have reported resumption of growth in only 33%
of cases (48). Recurrences and additional
surgical procedures are common. This may be because of incomplete
resection, reformation of the bar, or migration of the interpositional
material (53). Parents should be advised that
the results of physeal bar excision are unpredictable. The resected
growth plate may grow more slowly than the opposite physis, or
premature closure may develop after a period of initial growth.
for physeal bars that have produced less than 20 degrees of angulation.
In addition, the bar should involve less than one-third of the growth
plate, and the affected physis should have more than 4 cm of growth
remaining. In patients who meet these indications, an osteotomy or
lengthening may be avoided or postponed.
 |
|
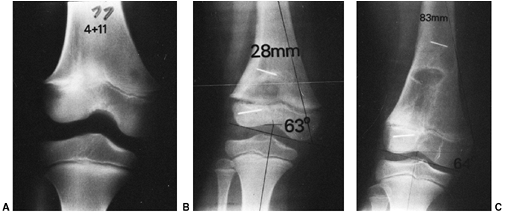
Figure 33.5 Physeal bar resection. A: A distal physeal bar is depicted in this anteroposterior hypocycloidal tomogram. B:
This condition was treated with bar excision and insertion of Cranioplast. Five months later, the physis remains open, and the two metal markers inserted at the time of surgery are 28 mm apart. There is residual femoral tibial valgus deformity. C: Four years later, there has been some improvement in the femoral tibial alignment, and growth of the distal femur has resumed. The markers are 83 mm apart. |
authors prefer bilateral complete epiphysiodesis prior to the
development of a discrepancy of more than 2 cm or a symptomatic angular
deformity. When deformity is already present without significant
discrepancy, as in the ankle region, then osteotomy with bilateral
epiphysiodesis is preferred. Growth contribution from the distal tibia
and fibula rarely warrants physeal bar excision after 10 years of age.
In cases of physeal bars involving more than one-third of the growth
plate, and where more than 4 cm of growth is remaining, the authors
prefer to perform complete arrest of the growth plate, osteotomy, and
lengthening. The involved extremity may be overlengthened to
accommodate growth of the opposite limb, or bilateral epiphysiodesis
can be performed to limit the amount of lengthening that is required.
from ages 1 to 18 years. Deaths from accidental injury in this age
group exceed the total number of deaths from the next nine causes
combined (3). Death from trauma is most often
attributable to head injury, but preventable deaths still occur. These
are most often caused by airway obstruction, pneumothorax, and
hemorrhage (54,55).
Children respond differently to trauma than adults. The child’s
vascular system can maintain systolic blood pressure for a prolonged
period in the presence of significant hypovolemia. Tachycardia may be
the only sign of impending hypovolemic shock, which can occur
precipitously. Hypothermia poses a greater problem for children because
they have a higher ratio of surface area to body weight than adults.
The child also has greater capacity to recover from neurologic injury
and hypoxia. For these reasons, all children should be treated
aggressively, with the expectation of survival and recovery of
function. The optimal timing of fracture management is uncertain,
although early fracture stabilization may facilitate patient care (56).
Several scoring systems have been developed to predict outcome and
assess the need for transport to major trauma centers. The Glasgow Coma
Scale is helpful in assessing cortical brain function (57) (Table 33.1).
Sequential assessment can help detect worsening or recovery from brain
injury. A child with a Glasgow Coma Scale score lower than 8 has a
significantly worse prognosis for survival. The score obtained at 72
hours after injury is more predictive of permanent impairment (58).
The Pediatric Trauma Score is an effective triage tool and a reliable
predictor of injury severity, although it gives an open metacarpal
fracture the same weight as an open femur fracture (59) (Table 33.2).
resuscitation, and blood replacement. Systematic, multidisciplinary
management of all organ systems is essential. Musculoskeletal injuries
are common in the severely traumatized child and may be initially
overlooked. Children with multiple injuries should be treated as though
they have a cervical spine injury until this can be ruled out
clinically
or radiographically. Regional examination of the spine, pelvis,
shoulders, and extremities should be complete, especially if the child
cannot communicate.
|
TABLE 33.1 GLASGOW COMA SCALE
|
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
conventional methods to meet the general needs of the patient. The
Mangled Extremity Severity Score (MESS) may be used in children to
assist the physician with immediate management decisions (60). Armstrong and Smith (61) have recommended the following principles for children with major trauma:
-
Make sure that any child with a major long-bone fracture does not have any other significant injuries.
-
Early treatment of the fractures should be compatible with the general care of the patient.
-
Fracture care should consider the need for early mobilization of the child.
-
Care of fractures should facilitate the management of associated soft tissue injuries.
-
The initial method of fracture management should be the definitive method, whenever possible.
-
Fracture care must be carefully individualized.
-
Treat all children as though they are going to survive.
|
TABLE 33.2 PEDIATRIC TRAUMA SCORE
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
penetrating wounds. The tibia is the most commonly involved site in
children and adults. Open tibial fractures are discussed in the section
on tibial fractures. The femur, forearm, humerus, and other bones may
also sustain open injuries.
This classification system is appropriate for children as well as
adults, and provides a method of assessment for purposes of management
and prognosis.
initiation of antibiotic and tetanus prophylaxis. Surgical irrigation
and debridement of open fractures should be performed to minimize the
subsequent risk of infection. Surgical intervention within 6 hours has
been the standard recommendation (63). However,
moderate delay of surgical management for lesser grades of open
fracture may not be associated with an increased infection rate when
antibiotics are administered early (64,65).
At the time of surgery all necrotic or devitalized material is removed.
Debridement of devitalized bone is not necessary in children if the
bone is clean and can be adequately covered with soft tissue (66).
Bone should be stabilized to create optimal conditions for soft tissue
recovery. Cultures can be obtained, but their value for subsequent
management is questionable (67). Partial wound closure over a drain is acceptable for clean Type I and Type II injuries (68,69).
Antibiotics are generally used for 72 hours. Cephalosporins are used
for Type I injury, and an aminoglycoside is added for Type II
or
Type III injury. Patients with open wounds are returned to the
operating room in 48 to 72 hours for repeat irrigation, debridement,
and possible delayed primary closure or flap coverage. Early soft
tissue coverage is advantageous and may require local or free-flap
reconstruction (69).
Vacuum-assisted closure (VAC) aids considerably in wound management and
can decrease the need for tissue transfers in pediatric patients (70).
|
TABLE 33.3 CLASSIFICATION OF OPEN FRACTURES
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
complications reported in the literature for open fractures in adults.
These fractures take longer to heal and have more complications than
closed injuries (71,72). However, the overall complication rates in children are lower than those in adults with similar injuries (72,73). This is especially true for children younger than 12 years (72,73).
of interstitial pressure in a closed osteofascial compartment,
resulting in inadequate circulation to the nerves and muscles of that
compartment. There is little documentation of the incidence of
compartment syndrome, but it has been reported in numerous anatomic
regions, including the abdomen. Increased intracompartmental pressure
may develop after athletic exertion, relatively minor injuries, and
major trauma (74).
obstruction of venous outflow from the compartment. This contributes to
further swelling and increased pressure. When the pressure increases
above the arteriolar circulatory pressure to muscle and nerve, ischemia
will occur, leading to irreversible damage to the contents of the
compartment. Muscle and nerve damage commences as soon as 4 to 6 hours
after the onset of abnormal pressures.
alert the clinician to the possibility of impending or established
compartment syndrome. Excessive pain requiring increased medication is
often the earliest symptom (75). This is
rapidly followed by clinical findings that include sensory changes
associated with nerve ischemia within the compartment, excessive pain
with passive movement of the muscles within the compartment, and loss
of active movement of those muscles. Distal pulses and peripheral
capillary refill are unreliable indicators of compartment syndrome.
Peripheral circulation may be normal because major arterial blood flow
through the compartment is preserved in the presence of increased
pressures, which eliminate microvascular perfusion to the muscles and
nerves within the compartment. The injured extremity should not be
elevated when compartment syndrome is suspected because this maneuver
reduces mean arterial pressure, causing a reduction in perfusion that
leads to further ischemia.
signs and symptoms which may be difficult to assess accurately in
children. For this reason children may have more advanced signs of
compartment syndrome at the time of definitive diagnosis. Tissue
pressure measurements should be obtained whenever the diagnosis is in
doubt (76) (Fig. 33.6). The pressure threshold for fasciotomy is
not clearly established. Mubarak and Owen (77)
recommend fasciotomy if compartment pressure exceeds 30 mm Hg. Others
recommend fasciotomy if the pressure is greater than 35 to 40 mm Hg or
within 30 mm Hg of the patient’s diastolic pressure (76).
 |
|
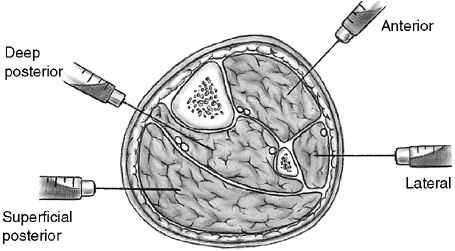
Figure 33.6
Orientation and entry points for measurement of compartment pressures. (From Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am 1994;25: 677, with permission.) |
 |
|
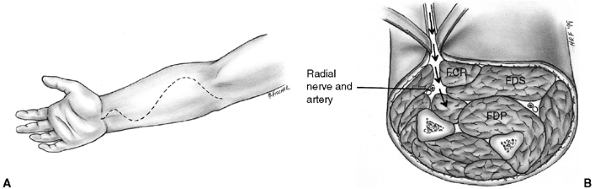
Figure 33.7 The Henry approach is used for decompression of the volar forearm compartments. A: Skin incision crosses the elbow crease and enters the palm along the thenar eminence. B: The superficial fascia is divided. The deep compartment is approached by retracting the flexor carpi radialis (FCR) to the ulnar side. The superficial radial nerve and brachioradialis are retracted radially. FDP, flexor digitorum profundus; FDS, flexor digitorum sublimus.
|
The two-incision technique facilitates decompression of the deep
posterior compartment. The fibula should be left intact. Devitalized
muscle is debrided when necessary, but extensive debridement is usually
performed 36 to 72 hours later, when muscle viability is more readily
determined. Approaches for foot compartment syndromes are discussed in
the section on metatarsal fractures.
 |
|
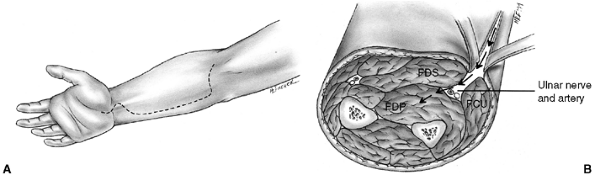
Figure 33.8 The volar ulnar approach (modified McConnell exposure) is more direct for decompressing the forearm compartments. A: Skin incision extends along the ulnar side of the forearm. B: The ulnar nerve and artery are retracted to expose the deep flexor compartment. FDP, flexor digitorum profundus; FDS, flexor digitorum sublimus; FCU, flexor carpi ulnaris.
|
When sedation or dissociative anesthesia is used, it is advisable to
follow the guidelines for monitoring and management that have been
established by the American Academy of Pediatrics (86).
blocks, and intravenous regional anesthesia. Hematoma blocks are
particularly useful for distal radius fractures. After appropriate skin
preparation, a needle is inserted into the fracture hematoma. The
hematoma is aspirated, and 3 to 10 mL of 1% or 2% lidocaine (maximum
dose is 3 to 5 mg per kilogram of body weight) is injected into the
fracture
site. Volume of injection should be less than 10 mL because hematoma
block can increase carpal tunnel pressure and increase the risk of
neurologic complications (87).
It is the author’s opinion that the technique of hematoma block is
often difficult when the hematoma is small, as in greenstick fractures,
or when the fracture hematoma has already coagulated.
 |
|
Figure 33.9 A:
Single-incision fasciotomy may be used to decompress all four compartments of the leg. Excision of the fibula is not necessary. B: Double-incision fasciotomy allows a more direct approach to the deep posterior compartment of the leg. (From Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am 1994;25:677, with permission.) |
The primary site of the nerve block is thought to be small, peripheral
nerve branches. For this reason, it is suggested that nerve blockade is
better achieved with a larger volume of dilute anesthetic (81).
Intravenous access is obtained in a vein of the dorsum of the hand of
the injured extremity. It is not necessary to have additional
intravenous access to the opposite extremity (82).
The arm is then exsanguinated by elevation, or by gentle application of
an elastic bandage. A single blood pressure cuff or tourniquet is then
rapidly inflated above the elbow to 100 mm Hg greater than systolic
blood pressure. The cuff may be taped to avoid Velcro failure, and the
inflation tube may be cross-clamped to prevent premature cuff
deflation. Two cuffs may be used to minimize tourniquet discomfort, but
this is not usually necessary for fracture reductions. Lidocaine,
diluted with normal saline to a 0.125% solution, is then administered
in a dose of 1 to 1.5 mg per kilogram of body weight (81).
The tourniquet should be kept inflated for at least 20 minutes to
permit the lidocaine to become fixed to the tissue, thereby minimizing
the risk of lidocaine toxicity after the cuff is deflated. This method
has proven most effective for forearm fractures and less effective for
supracondylar, finger, and hand fractures (88).
the wrist, or digital blocks can be useful for reduction of certain
hand and finger fractures and dislocations. Axillary block has proved
to be a safe technique for a variety of upper-extremity fractures in
children (89). In most children, the axillary
sheath is superficial and the pulse is more easily identified than in
adults, who generally have more subcutaneous fat. Intravenous access in
the uninjured extremity is recommended and can be used to administer
low-level sedation before axillary block. The arm is abducted and
externally rotated to a 90 degree–90 degree (“90–90”) position; 1%
plain lidocaine is used at a dose of 3 to 5 mg per kilogram of body
weight. Cramer et al. (89) recommend the
transarterial method of administration. A 23-gauge butterfly needle is
inserted through the axillary artery during continuous aspiration.
Approximately two-thirds of the lidocaine is slowly injected into the
sheath on the opposite side of the artery. Periodic aspiration is
performed to minimize the risk of intraarterial injection. The needle
is withdrawn just to the superficial side of the sheath, and the
remaining lidocaine is injected. This anesthetic technique provides
prolonged pain relief for complex manipulations but requires
considerable cooperation from the patient.
consciousness that maintains protective reflexes and retains the
patient’s ability to maintain an airway independently. During conscious
sedation, the patient can respond appropriately to verbal commands or
physical stimulation. Deep sedation is defined as a more profound state
of unconsciousness, accompanied by partial or complete loss of
protective reflexes and inability to respond purposefully to verbal or
physical stimuli. The American Academy of Pediatrics has developed
specific guidelines for monitoring and managing pediatric sedation (86).
These guidelines state that the risks of deep sedation may be
indistinguishable from those of general anesthesia. When the guidelines
are followed, the risk of adverse events can be reduced. Administration
of chloral hydrate was associated with higher risk, but the effects of
nothing by mouth (NPO) status are not statistically significant (90).
agents for intravenous sedation. Narcotics provide analgesia, whereas
benzodiazepines are primarily sedatives. These
drugs
act synergistically to induce controlled sedation and analgesia.
Intravenous access and continuous monitoring of pulse and oxygen
saturation are advised. Respiratory rate and blood pressure should be
monitored periodically. An emergency cart with resuscitation equipment
should be immediately available. Nasal oxygen and personnel skilled in
airway management increase the level of safety.
reported satisfactory pain relief in 98% of patients who were sedated
by titrating meperidine (Demerol) and midazolam (Versed). The target
doses were 2 and 0.1 mg per kilogram of body weight for meperidine and
midazolam, respectively. Meperidine is less potent than morphine, but
it has a slightly faster onset and some euphoric properties (82).
Fentanyl, a potent narcotic, is sometimes used as a substitute for
meperidine because it reaches peak analgesia within 2 to 3 minutes and
has a shorter duration of action than meperidine. Hypoxemia and apnea
are not uncommon after sedation with midazolam and fentanyl. Monitoring
of oxygen saturation is essential because hypoxemia can occur in the
absence of apnea. If respiratory depression occurs, reversal agents
should be administered. Naloxone is used to reverse the narcotic
effect, and the benzodiazepine is reversed with flumazenil (82).
It induces a trancelike state that combines sedation, analgesia, and
amnesia with little cardiovascular depression. It has been suggested
that protective orotracheal reflexes are preserved, but respiratory
depression is dose-related (91,92).
The intramuscular or intravenous route may be used to administer
ketamine. The intravenous route permits titration and more rapid onset
of action, with quicker recovery. The target intravenous dose is 1 to 2
mg per kilogram of body weight. The intramuscular dose is 4 mg/kg, and
a repeat dose may be given after 10 to 15 minutes if necessary.
Ketamine increases upper-airway secretions, so atropine or
glycopyrrolate are recommended before sedation. Emergence
hallucinations are more common in children older than 10 years.
Therefore, ketamine may be a less desirable choice in older children.
When ketamine is used in older children, low-dose midazolam (0.05
mg/kg) can reduce the risk of emergence reactions (84).
|
TABLE 33.4 MEDICATIONS MOST COMMONLY USED FOR SEDATION IN PEDIATRIC FRACTURE REDUCTION
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||
the shoulder. The clavicle is flat laterally, triangular medially, and
has a double curve that is convex anteriorly in the medial third and
convex posteriorly in the lateral third. The scapula is a large, flat,
triangular bone that is connected to the trunk by muscles only. The
spine arises from the dorsal surface of the scapula and forms the
acromion laterally. The coracoid process arises from the anterior
surface. The clavicle and scapula are attached at the acromioclavicular
joint and held in place by the coracoclavicular ligaments. The clavicle
connects the shoulder girdle to the axial skeleton at the
sternoclavicular joint. This joint is very mobile and allows the
clavicle to move through an arc of 60 degrees and accommodate a wide
range of scapular rotation. The shoulder girdle articulates with the
humerus through the glenohumeral joint. This joint is a ball-and-socket
joint that is supported primarily by the articular capsule and
surrounding muscle. Thus, the shoulder mechanism functions as a
universal joint, allowing freedom of motion in all planes.
treat and rarely require reduction or surgical stabilization. The wide
range of motion in this region contributes to rapid remodeling and
accommodates modest residual deformity.
The most common injuries are fracture of the clavicle and brachial
plexus palsy. The differential diagnosis should also include proximal
humeral physeal separation, septic arthritis of the shoulder,
osteomyelitis, and nonaccidental injuries. Lack of arm movement in the
neonatal period is the most common clinical finding for each of these
problems. Pain, swelling, and crepitus may be noted when fracture has
occurred. Often, fracture of the clavicle at birth is undetected until
swelling subsides and the firm mass of healing callus is noticed in the
midshaft of the clavicle. Parental reassurance and gentle handling are
all that are required for managing fracture of the clavicle at birth.
Clavicle fracture is occasionally confused with congenital
pseudarthrosis of the clavicle. Pseudarthrosis can be distinguished
from fracture of the clavicle at birth by the absence of pain and by
radiographic features of established pseudarthrosis.
physeal separation of the proximal humerus. This injury may be
difficult to diagnose radiographically because the proximal humeral
epiphysis does not ossify until 3 to 6 months of age. Ultrasonography,
MRI, or joint aspiration may facilitate diagnosis in questionable
cases. Closed reduction is not indicated because healing is rapid and
remodeling is certain. Immobilization for 2 to 3 weeks provides comfort
and allows union to occur.
the most common portion injured is the shaft. The mechanism of injury
is usually a fall on the shoulder or excessive lateral compression of
the shoulder girdle. The subclavian vessels, brachial plexus, and apex
of the lung lie beneath the clavicle but are rarely injured at the time
of fracture.
adolescents is supportive; reduction is not attempted, except for
fractures with extreme displacement. Both a figure-eight harness and a
sling may be used initially to provide comfort. After a few days the
sling and harness may be discontinued. Sports are avoided for
approximately 8 weeks. Uneventful, rapid healing is the rule, although
displaced fractures may heal with a visible subcutaneous prominence.
Parents should be advised that, regardless of alignment, healing will
produce a bump that will remodel over the course of several months.
Indications for surgery are rare but include open fractures, severe
displacement with the bone end impaled through the trapezius, and
irreducible tenting of the skin by the bone fragments. Even in these
severe cases internal fixation is rarely required. Nonunion after
clavicle fracture has been reported in adolescents, but it responds to
bone grafting and plating (94).
approximately 17 years of age, but the physis does not close until 20
to 25 years of age (95). Therefore,
displacements of the medial end of the clavicle are usually physeal
separations that mimic sternoclavicular dislocation (96).
These are Salter-Harris type I or II injuries, although the epiphyseal
fragment is not visualized well on radiographs. The direction of
displacement can be anterior or posterior. Posterior displacement by
fracture or dislocation can cause dysphagia or respiratory compromise,
especially when the child’s head and neck are extended. Apical lordotic
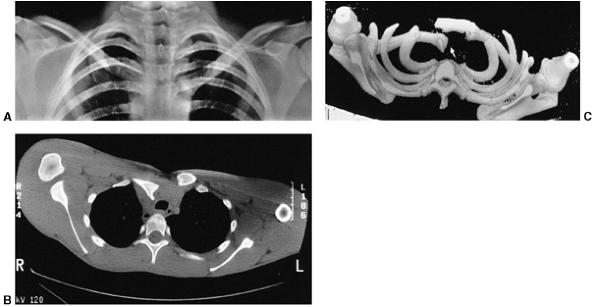
radiographs are helpful, but computed tomography (CT) scans best
visualize the deformity (Fig. 33.10).
Posterior displacements are reduced under general anesthesia for
complete relaxation. The reduction maneuver entails hyperextension of
the clavicle combined with longitudinal arm traction. It may also be
necessary to capture the medial clavicle with a percutaneous towel clip
and then pull in an anterolateral direction. Ligamentous repair and
stabilization with a figure-eight nonabsorbable suture is recommended
following reduction (97,98).
A shoulder immobilizer or figure-eight harness is used for
immobilization postoperatively. Anteriorly displaced epiphyseal
separations are less stable, and partial redisplacement may occur.
However, remanipulation or surgical treatment may be unnecessary
because fracture remodeling will occur.
similar to adult acromioclavicular separation. A fall onto the point of
the shoulder drives the acromion and scapula distally. This results in
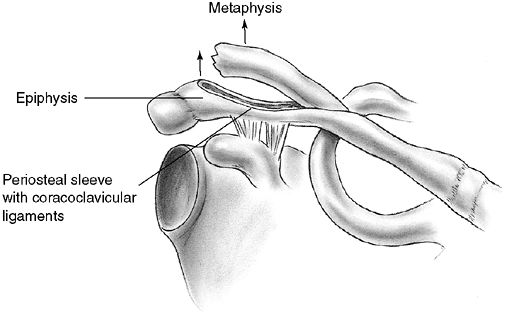
distal clavicular physeal separation. This is because the distal
epiphysis of the clavicle remains a cartilaginous cap until the age of
20 years or older (95), whereas the
acromioclavicular and coracoclavicular ligaments are firmly attached to
the thick periosteum of the clavicle. Typically, the lateral metaphysis
displaces through the injured dorsal periosteum, leaving the ligaments
intact and the epiphyseal end of the clavicle reduced in the
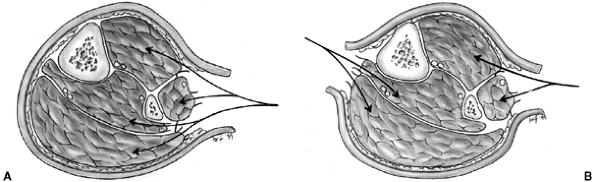
acromioclavicular joint (99) (Fig. 33.11).
Because these injuries represent physeal disruption with herniation of
bone from the periosteal tube, tremendous potential for healing and
remodeling exists.
 |
|
Figure 33.10
Sternoclavicular separation. This 14-year-old boy sustained an injury to the right clavicle during a wrestling match when his shoulder was compressed against his chest wall. He complained of shortness of breath, especially when he extended his neck. A: The anteroposterior radiograph demonstrates asymmetry of the sternal position of the clavicle. B: The computed tomographic scan demonstrates posterior displacement of the medial end of the right clavicle, which is near the trachea (arrow). C: A three-dimensional reconstruction, with a cephalic projection, demonstrates the posterior and midline displacement of the clavicle. |
support with a sling or shoulder immobilizer for 3 weeks. Reduction and
fixation are unnecessary, except for the rare instance in which the
clavicle is severely displaced in an older adolescent (100).
the age of 16. Fracture or physeal separation of the distal clavicle is
more common, and has been called pseudodislocation of the acromioclavicular joint (101).
Tenderness over the acromioclavicular joint and prominence of the
lateral end of the clavicle are present with fracture, physeal
separation, and joint separation. Radiographs demonstrate increased
distance between the coracoid process and the clavicle, compared with
the opposite side. The MRI can distinguish among these three similar
injuries, but it is rarely necessary because the treatments are
similar. When true joint separation occurs, the injury may be a sprain,
subluxation, or dislocation. These have been classified as grades I to
III, depending on the severity of injury to the acromioclavicular and
coracoclavicular ligaments (102).
 |
|
Figure 33.11
Lateral clavicle fracture-separation. The swelling and dorsal prominence of the clavicle may suggest an acromioclavicular separation. However, the distal epiphysis of the clavicle and acromioclavicular joint remain reduced. New bone forms from the periosteum, with subsequent remodeling of the prominence. (From Ogden J. Distal clavicular physeal injury. Clin Orthop 1984;188:68, with permission.) |
conservative, without attempting reduction. Therefore, it is
unnecessary to determine the degree of separation by stress radiography
with handheld weights. A sling or shoulder immobilizer is used for 3
weeks, followed by a graduated exercise program. Even in competitive
athletes, shoulder strength and range of motion are not impaired after
rehabilitation (103,104,105).
In complete separations (type III), the clavicle remains prominent but
is usually asymptomatic. The occasional patient who develops late
symptoms of pain and stiffness may be relieved by resection of the
distal clavicle (104).
should be suspected whenever there is shoulder tenderness or swelling
after trauma. These fractures are usually the result of a severe,
direct blow of high energy. Therefore, initial evaluation should
include a diligent search for more serious chest injuries, such as rib
fractures, pulmonary or cardiac contusion, and injury to the
mediastinum. Fractures of the scapula can involve the body, glenoid, or
acromion. Avulsion fractures of the scapula have also been reported,
and are a result of indirect trauma (106). The CT scan is quite helpful for evaluating scapular fractures and associated injuries.
immobilization with a sling and swathe, followed by early shoulder
motion after pain has subsided. The scapular body is encased in thick
muscles, so displacement is rare and well tolerated after healing (100).
Fractures of the acromion or coracoid require surgery only when
severely displaced. Glenoid fractures are the most likely to require
reduction and internal fixation. Intraarticular fractures with more
than 3 mm of displacement should be restored to anatomic positions.
Large glenoid rim fractures can be associated with traumatic
dislocations. An anterior approach is recommended for anterior glenoid
fractures, and a posterior approach is used for scapular neck and
glenoid fossa fractures (107).
Atraumatic shoulder dislocations and chronic shoulder instability are
discussed elsewhere in this book. Traumatic shoulder dislocation in the
adolescent age group is more common. Approximately 20% of all shoulder
dislocations occur in persons between the ages of 10 and 20 years. Most
displace anteriorly and produce a detachment of the anteroinferior
capsule from the glenoid neck (i.e., Bankart lesion).
and adolescents is nonsurgical, with gentle closed reduction. This is
accomplished by providing adequate pain relief, muscle relaxation, and
gravity-assisted arm traction in the prone position. An alternative
method is the modified Hippocratic method in which traction is applied
to the arm while countertraction is applied using a folded sheet around
the torso. After reduction, a shoulder immobilizer or sling is used for
2 to 3 weeks before initiating shoulder muscle strengthening. The most
frequent complication is recurrent dislocation, which has an incidence
between 60% and 85%, usually within 2 years of the primary dislocation (109,110). Posterior dislocations of the shoulder may also recur and require surgical stabilization in children (111). A more detailed discussion of this injury and its treatment is found elsewhere in Chapter 32.
of the humerus. The proximal humeral physis is an undulating structure
that forms a tentlike peak in the posteromedial humerus quadrant, near
the center of the humeral head. The glenohumeral joint capsule extends
to the metaphysis medially. Therefore, a portion of the metaphysis is
intracapsular. The proximal humeral physis remains open in girls until
14 to 17 years of age and in boys until 16 to 18 years of age.
dislocation in adults usually result in a proximal humeral fracture in
children and adolescents. These are usually Salter-Harris type II
epiphyseal separations or metaphyseal fractures. Metaphyseal fractures
are more common before the age of 10, and epiphyseal separations are
more common in adolescents. The distal fragment usually displaces in
the anterior direction, because the periosteum is thinner and weaker in
this region. Posteriorly, the periosteal sleeve is thicker and remains
intact. The proximal fragment is flexed and externally rotated because
of the pull of the rotator cuff, whereas the distal fragment is
displaced proximally because of the pull of the deltoid muscle.
Adduction of the distal fragment is caused by the pectoralis major
muscle. The long head of the biceps may be interposed between the
fracture fragments and may further impede reduction (112).
Remarkably, this is a relatively benign injury because of the rapid
rate of remodeling with growth and the wide range of shoulder motion (113,114).
minimally angulated. They are managed in a shoulder immobilizer for 3
to 4 weeks, followed by range-of-motion exercises and gradually
increased activity.
the treating physician. The alarming radiographic appearance invites
overtreatment. These fractures are difficult to reduce and almost
impossible to maintain in a reduced position by closed methods.
Traction and cast immobilization are not recommended because these
techniques are inconvenient, cumbersome, and have not been shown to
improve results. Current options for management include immobilization
without attempting reduction, and reduction under anesthesia with
percutaneous pinning. Authors who have studied these options have
concluded that most severely displaced fractures should be treated by
sling and swathe immobilization (113,114,115).
Complete displacement, 3 cm overriding, and 60 degrees of angulation
may be accepted in patients who are more than 2 years from skeletal
maturity. Up to 45 degrees of angulation can be accepted until physeal
closure (112) (Fig. 33.12).
Closed or open reduction under anesthesia with percutaneous pinning may
be indicated for fractures with a greater amount of deformity, open
fractures, vascular injuries, and severe displacement (100) (Fig. 33.13), or in situations in which tenting could lead to skin breakdown.
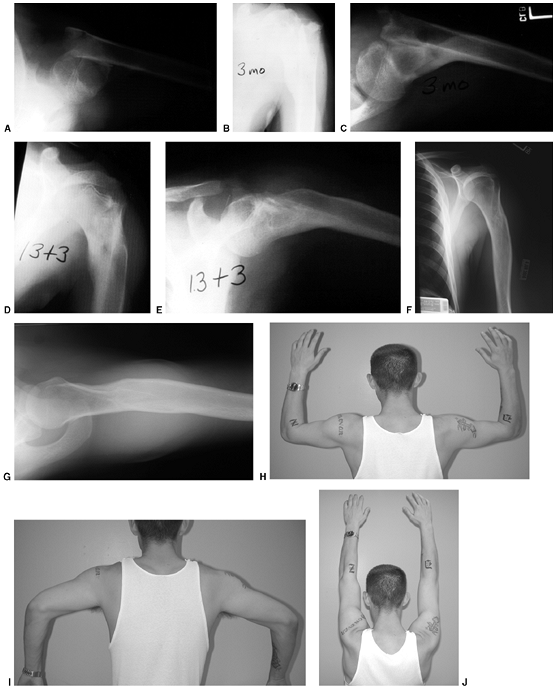 |
|
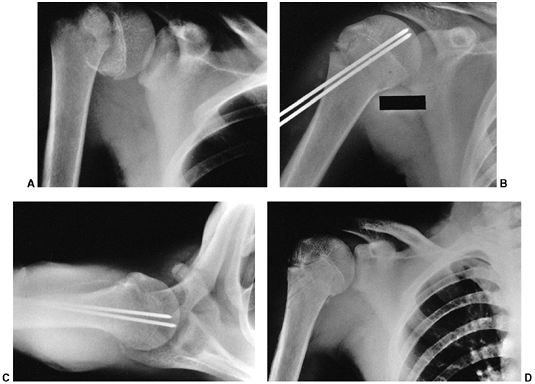
Figure 33.12 Proximal humeral fracture-separation in a boy aged 12 years, 3 months. A: The initial fracture displacement was treated with sling and swath. B, C: Three months after injury, healing and early remodeling are evident. D,E: One year after injury, remodeling continues. F, G: Four years after injury, remodeling is complete. H–J: The patient has recovered full range of motion, but has a 1 cm arm-length discrepancy.
|
 |
|
Figure 33.13 Salter-Harris type II fracture of the proximal humerus in a 15-year-old adolescent. A:
Displaced fracture with 70 degrees of angulation. The proximal fragment is abducted and externally rotated, because of the rotator cuff attachments. The shaft is displaced proximally by the pull of the deltoid muscle, and is generally adducted by the action of the pectoralis muscle. The distal fragment is also internally rotated if the arm is placed in a sling. B, C: Anteroposterior and lateral radiographs after closed reduction and percutaneous pinning. The arm is externally rotated and abducted with longitudinal traction to achieve this position. D: Final alignment after removal of the pins 4 weeks later. |
because of difficult delivery. Other humeral shaft fractures in
children younger than 3 years are often the result of nonaccidental
injury (116). In any instance of delay in
seeking medical attention, inconsistent history of injury, or evidence
of concurrent injuries, there is an increased likelihood of inflicted
trauma. However, there is no particular pattern of fracture that is
diagnostic of child abuse. Fractures seen in older children are usually
the result of blunt trauma. The radial nerve is susceptible to injury,
because it is fixed by the intramuscular septum as it passes lateral to
the humerus at the junction of the middle and distal thirds. The
prognosis for radial nerve recovery is excellent. Nerve injury with
closed fractures of the humerus should be observed for 3 months before
considering intervention.
fractures. Infants may be treated with gentle positioning and a small
coaptation splint, or the arm may be splinted in extension, using a
tongue blade and tape. Healing is prompt in infants and young children.
It is the authors’ opinion that up to 45 degrees of angulation can be
accepted in children younger than 3 years. Older children may be
treated with a coaptation splint and a sling to maintain alignment of
the arm. A hanging arm cast or collar and cuff may also be used, but a
U-slab coaptation splint allows better pain relief and control of the
fracture. Occasionally, an abduction splint or pillow is necessary to
control varus alignment. In older children and adolescents, complete
displacement and 2 cm of shortening are acceptable. In the proximal
shaft, one can accept 25 to 30 degrees of angulation. Fracture
deformity closer to the elbow is more visible. Up to 20 degrees of
angulation is acceptable in the middle third and 15 degrees in the
distal third of the humeral shaft (117).
Greater degrees of deformity are usually unacceptable cosmetically,
although they may remodel without causing functional problems.
Indications for surgery include open fractures, multiple injuries, and
ipsilateral forearm fractures in adolescents (i.e., “floating elbow”).
Fixation techniques include the use of flexible intramedullary nails,
antegrade insertion of a Rush rod, and compression plating. Open or
comminuted fractures can be stabilized with an external fixator until
union is complete or until fracture stability and wound healing permit
converting to splint immobilization.
with an elbow injury. Only full range of motion, complete absence of
swelling, and normal radiographs warrant the diagnosis of elbow sprain
or contusion. Any swelling or restriction of movement necessitates
thorough evaluation, sometimes with comparison radiographs of the
opposite elbow whenever there is doubt regarding normal anatomy. Small
fractures that appear to be avulsions should be accurately diagnosed,
because they may indicate a major injury. Arthrography,
ultrasonography, and MRI have been used to successfully diagnose occult
elbow trauma in children (118,119,120). These techniques should be considered whenever there is doubt regarding the diagnosis.
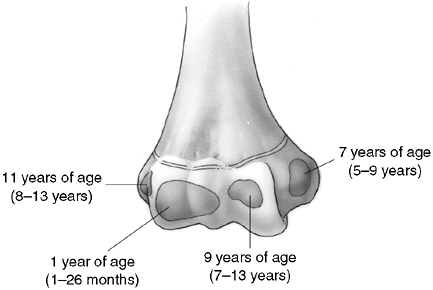
in young children because of the large cartilage composition of the
distal humerus. There are also multiple ossification centers that
appear at different ages (Fig. 33.14). The
capitellum is the first to appear, at 6 months of age, followed by the
radial head and the medial epicondyle at 5 years of age. The trochlea
ossifies at 7 years, and the lateral epicondyle and olecranon appear at
9 and 11 years of age, respectively. The lateral epicondyle, trochlea,
and capitellum coalesce to form a single epiphysis by 12 years of age.
Ossification centers are intraarticular except for the medial and
lateral epicondyles.
articulations: the radiohumeral, ulnohumeral, and radioulnar joints.
There are two fat pads: one in the olecranon fossa posteriorly, and the
other in the coronoid fossa anteriorly. Displacement of the posterior
fat pad may be visible on radiographs after elbow trauma. This is a
reliable indication of intraarticular effusion (121).
The anterior fat pad is sometimes seen under normal conditions, and
does not necessarily indicate joint effusion. Most of the distal
humerus has good collateral circulation, with most of the intraosseous
blood supply entering posteriorly. Caution should be exercised to avoid
disrupting this posterior blood supply during surgical exposure of
fractures (122). The trochlea and medial
condyle are particularly vulnerable to avascular necrosis because they
are perfused by sets of nonanastomotic nutrient vessels that enter the
bone posteriorly and medially (123).
 |
|
Figure 33.14
Ossification of the secondary centers of the distal humerus. The average ages are specified, and the age ranges are indicated. The ossification ranges are earlier for girls than for boys. The lateral epicondyle, capitellum, and trochlea coalesce between 10 and 12 years of age, subsequently fusing to the distal humerus between 13 and 16 years of age. This is about the time that the medial epicondyle fuses to the proximal humerus. |
 |
|
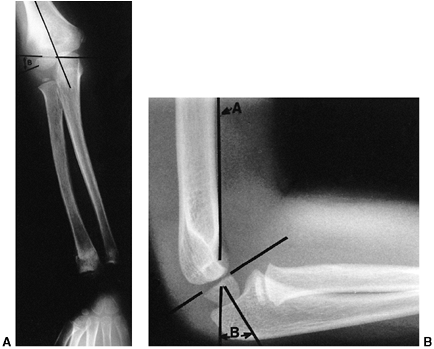
Figure 33.15 Radiographic lines of the distal humerus. A:
The Baumann angle is formed between the capitellar physeal line and a line perpendicular to the long axis of the humerus. As this angle becomes smaller, more elbow varus will occur. This angle should be compared with that of the contralateral, uninjured elbow with a similar anteroposterior view of the distal humerus. B: Line A is the anterior humeral line, which atypically passes through the middle of the capitellum. Angle B demonstrates the anterior angulation of the capitellum relative to the humeral shaft. This is approximately 30 degrees. As angle B becomes smaller, the fracture site is moved into extension. Fracture alignment with the capitellum behind the anterior humeral line produces a hyperextension deformity and a loss of elbow flexion. |
There are several helpful radiographic lines and angles that can be
measured to determine if there is adequate postinjury alignment; a
comparison view of the other elbow may be valuable as a reference (Fig. 33.15).
All measurements are subject to the inaccuracies caused by elbow
positioning, and this should be kept in mind when making clinical
decisions. The Baumann angle is used to assess the varus attitude of
the distal humerus, usually after a supracondylar elbow fracture. It is
the angle formed between the capitellar physeal line and a line
perpendicular to the long axis of the humerus. This angle normally
should be within 5 to 8 degrees of the same angle in the contralateral
elbow. An anteroposterior view of the distal humerus, positioned
parallel to the radiographic plate, is necessary to reduce the
variation of the Baumann angle that occurs when the arm is rotated. Ten
degrees of rotation produces a 6-degree change in the angle (124).
Another measure of coronal alignment is the medial epicondylar
epiphyseal angle. This angle is measured between the long axis of the
humerus and a line through the medial epicondylar physis (125).
It has the advantage of being reliably measured while the elbow is held
in flexion (i.e., Jones view), as during the reduction process. The
medial epicondylar epiphyseal line ranges from 25 to 46 degrees. This
angle is not reliable for children younger than 3 years or older than
10 (125). Sagittal alignment may be determined
by the lateral capitellar angle, which indicates the normal
forward-flexed position of the capitellum. This angle averages 30 to 40
degrees. The anterior humeral line offers a similar means to assess the
position of the capitellum and is measured on a true lateral
radiographic projection. A line along the anterior humeral cortex
should pass through the center of the capitellum.
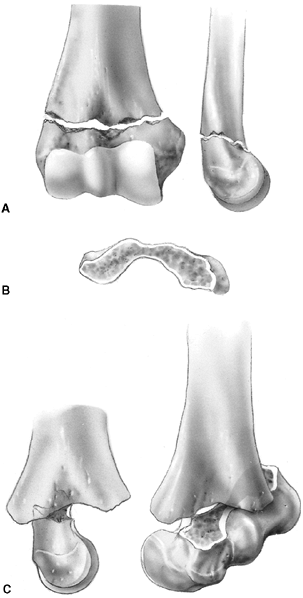
mechanism of injury is a hyperextension load on the elbow from falling
on the outstretched arm. The distal fragment displaces posteriorly
(i.e., extension) in more than 95% of fractures. The medial and lateral
columns of the distal humerus are connected by a very thin area of bone
between the olecranon fossa posteriorly and the coronoid fossa
anteriorly. The central thinning and the surrounding narrow columns
predispose this area to fracture. As the elbow is forced into
hyperextension, the olecranon impinges in the fossa, serving as the
fulcrum for the fracture. The collateral ligaments and the anterior
joint capsule also resist hyperextension, transmitting the stress to
the distal humerus and initiating the fracture (126) (Fig. 33.16). Flexion type supracondylar fractures result from a direct fall onto the flexed elbow.
type I, nondisplaced or minimally displaced; type II, angulated with
moderate disruption but with a portion of the cortex maintaining
end-to-end contact; and type III, completely displaced. Supracondylar
fractures with medial impaction may appear to be nondisplaced but may
result in cubitus varus attributable to unacceptable angulation (128).
After complete fracture, a small amount of rotational malalignment
allows tilting of the fragments because of the thin cross-sectional
area in the supracondylar region. This may also lead to malunion with
cubitus varus or, less commonly, cubitus valgus.
including the ipsilateral forearm. The incidence of nerve injury is
approximately 15%; most often, nerve injury is a neuropraxia that
resolves spontaneously within 4 months. The nerve that gets injured is
related to the position of the displaced fragment (129).
Median nerve injuries, including injury to the anterior interosseous
nerve, are more common with posterolateral displacement of the distal
fragment. Radial nerve injuries are seen more often with posteromedial
displacement.
 |
|
Figure 33.16 A:
The typical orientation of the fracture line in the supracondylar fracture. Sagittal rotation of the distal fragment generally results in posterior angulation, although, less commonly, it can be flexed. B: The cross-sectional area through the fracture demonstrates the thin cross-sectional area of the supracondylar region. C: Any horizontal rotation tilts the distal fragment. Typically, medial tilting occurs, producing cubitus varus. The lateral projection readily demonstrates this horizontal rotation, producing a fishtail deformity. In this instance, the distal portion of the proximal fragment is obliquely profiled, although there is a true lateral view of the distal humeral fragment. |
treated with an above-elbow cast for 3 weeks. Any medial buckling or
impaction of the medial metaphysis may indicate a fracture that
requires reduction. This fracture is a diagnostic trap, because the
collapse of the medial column may be very subtle (Fig. 33.17).
The Baumann angle, or the medial epicondylar epiphyseal angle, should
be carefully measured bilaterally; more than 10 degrees of varus
impaction warrants closed reduction and percutaneous pinning (CRPP). It
is difficult to maintain the reduction by cast immobilization alone,
and residual deformity will not remodel (128).
injuries, with an intact or nondisplaced posterior cortex. Type II
fractures, in which the capitellum is posterior to the anterior humeral
line, have an unacceptable amount of extension. Many of these are
stable after closed reduction and casting in 90 to 100 degrees of
flexion (130). When more than 100 degrees of
flexion is required for maintenance of reduction, percutaneous pinning
is recommended, with immobilization in less than 90 degrees of flexion (131). Weekly follow-up for 2 weeks is recommended following closed management to diagnose and treat any loss of reduction.
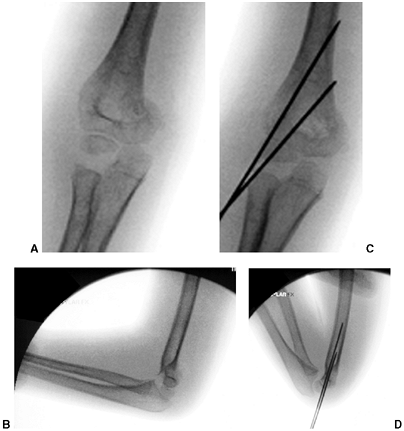 |
|
Figure 33.17 Type II supracondylar humerus fracture with medial impaction and varus alignment. A, B:
Anteroposterior and lateral views of a type II supracondylar humerus fracture with medial impaction. Note that although there is little displacement on the lateral view, the Baumann angle is 0 degrees on the anteroposterior. C, D: Anteroposterior and lateral intraoperative views of the distal humerus after the impacted fracture was reduced and fixed with divergent lateral pins. Note that on the anteroposterior, the Baumann angle is restored, and on the lateral, the anterior humeral line intersects the capitellum. The reduction was maintained during the postoperative period. |
displaced. Treatment begins with a complete assessment of perfusion and
nerve function. Neurovascular problems are frequent, and fracture
management may be altered if neurovascular compromise is present. In
the absence of neurovascular compromise, displaced fractures can be
splinted and managed safely in a delayed manner as long as the child is
closely monitored (132,133). Primary CRPP is the preferred treatment for type III injuries (134) (Fig. 33.18).
Displaced supracondylar fractures treated by closed reduction and
casting have a higher incidence of residual deformity than those
treated with reduction and pinning (134). Closed reduction and casting also has a higher risk of Volkmann ischemic contracture than treatment with early pinning (134).
experience has demonstrated that two or three laterally placed pins are sufficient for stabilization (137).
The authors prefer laterally placed pins without a medial pin except in
unusual circumstances. When a medial pin is used, one should be aware
that extreme elbow flexion could result in ulnar nerve subluxation from
its groove and increase the risk of damage during pinning. When
possible, it is advisable to place the lateral pin first to provide
provisional stability so that the medial pin can be inserted with the
elbow in less than full flexion, placing the ulnar nerve farther
posterior. It is also advisable to make a small incision over the
medial epicondyle and dissect with a hemostat, so that the medial pin
can be placed directly on the bone. Anatomic alignment is preferred,
but this may be difficult to achieve in some cases. When the quality of
reduction is in doubt, comparison radiographs of the opposite elbow can
be obtained intraoperatively. The Baumann angle should be within 5 to 8
degrees of the angle on the contralateral side. As long as fixation is
secure, it is the author’s opinion that one may accept up to one-third
translation of the distal fragment, 30 degrees of malrotation, and 20
degrees of extension after pinning (capitellum anterior to the anterior
humeral line). Initial immobilization should be in a nonconstrictive
splint or cast with the elbow in less than 90 degrees of flexion. The
authors prefer a cast that has been bivalved and spread with the elbow
in approximately 70 degrees of flexion. Oral analgesics are usually
sufficient for pain relief. The need for intravenous narcotics may
indicate ischemia. Immobilization is continued for 3 to 4 weeks, at
which time the pins are removed and active range of motion is initiated.
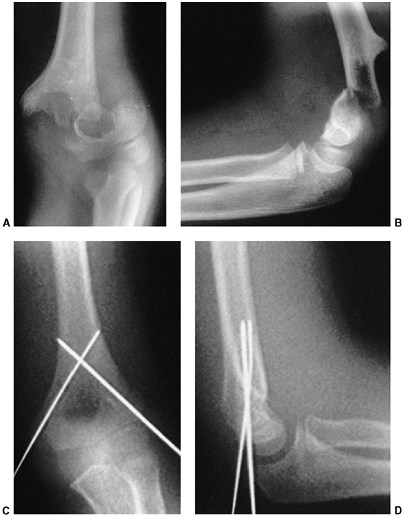 |
|
Figure 33.18 Type III supracondylar humerus fracture. A: This type III fracture demonstrates lateral displacement. B:
The lateral projection also shows flexion of the distal fragment. The treatment of this less common position is the same as that for extension fractures. The posterior periosteum is torn, and hyperflexion of the elbow will excessively forward-flex the distal fragment. The elbow is best pinned at slightly less than 90 degrees of flexion, because it is technically difficult to pin the elbow in extension. C and D: Anteroposterior and lateral postreduction and pinning films. |
reduction through a medial approach, adding a lateral incision, if
necessary. An anterior surgical interval can also be used, and is
recommended if the neurovascular structures need to be exposed. The
posterior approach should
be
used cautiously because it disrupts any remaining intact soft tissue
and may disrupt the primary vascular supply to the distal humeral
fragment (122,123).
Complete vascular disruption is uncommon, because the thick local
muscle envelope protects the artery. Vascular evaluation after
reduction requires differentiation of the pulseless extremity that is
pink and viable from one that is cold and pale with vascular
insufficiency. The child who has a well-perfused hand but an absent
radial pulse after satisfactory closed reduction does not necessarily
require routine exploration of the brachial artery (129,138,140,141).
The pulse usually returns within 48 hours. Likewise, the absence of a
Doppler-detected pulse at the wrist is not an absolute indication for
arterial exploration. The collateral circulation is vast and often
provides enough distal perfusion despite brachial artery occlusion.
When the hand is warm and normal in color with brisk capillary refill
and normal oxygen saturation, the authors recommend careful observation
with noncircumferential immobilization and less than 70 degrees elbow
flexion. There is no convincing evidence of a clinical problem with
cold intolerance or exercise-induced muscle fatigue for the hand
surviving on collateral vascularity, but long-term studies addressing
the problem are lacking (141).
avascular, cold, pale hand), especially if there is nerve palsy or
inadequate reduction, anterior open reduction is recommended.
Frequently, the neurovascular bundle is found kinked at the fracture
site, and liberation of the artery restores the pulse. There may also
be evidence of brachial artery injury. Vascular reconstruction should
be performed if the vessel does not respond to local measures (e.g.,
release of tether, adventitia stripping, lidocaine, papaverine) and the
hand remains avascular. The fracture should be stabilized before
vascular repair. After reconstruction, there is a significant rate of
asymptomatic reocclusion and residual stenosis, although the hand
remains well perfused (141).
Patients with preoperative nerve injury should undergo reduction and
fixation as described. Exploration of the nerve is not necessary when
the reduction is anatomic. However, failure to obtain anatomic
reduction may indicate that the nerve is interposed at the fracture
site, and exploration may be indicated.
preoperatively may represent a preexisting nerve deficit that was
undetected at the time of the initial examination or an iatrogenic
injury sustained during reduction. Median and radial nerve injuries are
more frequently seen because of the initial trauma and may be observed
when the reduction is anatomic. Postoperative ulnar nerve deficits are
more often iatrogenic and usually result from placement of the medial
pin (129,142,143).
Recommendations for management of this complication vary from
observation to exploration. When iatrogenic ulnar neuropathy is
suspected, it is the authors’ opinion that the medial pin should be
removed without exploration. Spontaneous recovery of most neural
injuries following elbow fracture is expected within 2 to 6 months. If
there has been no recovery of function by 4 to 6 months after injury,
then exploration is indicated. The results of late neurolysis or repair
are usually favorable in children (144).
supracondylar fracture. This deformity represents fracture malunion and
rarely results from partial growth arrest of the medial condylar growth
plate. Malunion may be avoided by careful attention to anatomic
reduction and secure fixation at the time of initial management.
Cubitus varus is generally considered a cosmetically acceptable
deformity, but increased risk of lateral condyle fracture, tardy ulnar
palsy, and posterior shoulder instability has also been reported (145,146,147). Osteotomy to correct deformity may be performed at any age, but complications are not uncommon (148).
Full-length radiographs of both arms are recommended preoperatively for
accurate planning. Simple uniplanar closing wedge osteotomies have the
lowest complication rates, but lateral condylar prominence may
compromise the cosmetic result in patients older than 12 years (148,149). When osteotomy is required in older patients, the authors translate the distal fragment medially to avoid lateral prominence.
result from high-energy trauma. Increased risks of compartment syndrome
and secondary displacement of the forearm fracture have been reported (150,151,152).
Percutaneous pinning of both the supracondylar and forearm fractures is
recommended if the forearm fracture requires reduction (150,152,153). This allows less constrictive immobilization and reduces the risk of redisplacement.
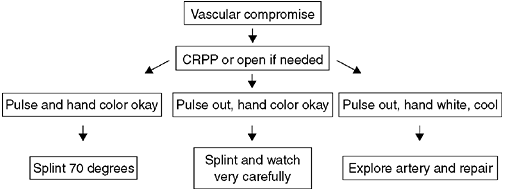 |
|
Figure 33.19
Management of a supracondylar humerus fracture with vascular compromise. This algorithm shows management of a supracondylar humerus fracture associated with vascular compromise. CRPP, closed reduction percutaneous pinning. |
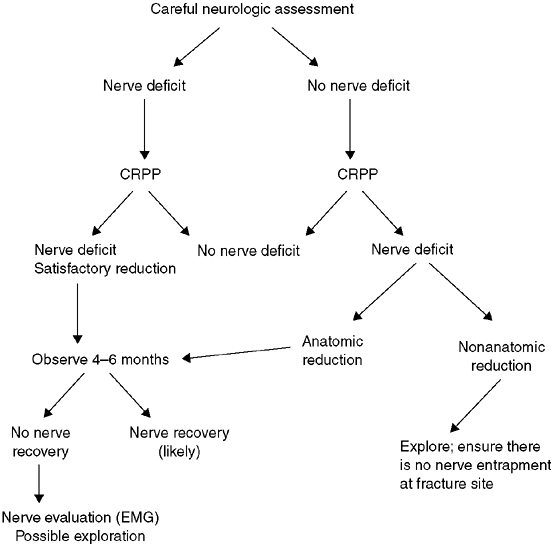 |
|
Figure 33.20 Management strategy with supracondylar humerus fracture and neurologic deficit. CRPP, closed reduction percutaneous pinning; EMG, electromyogram.
|
supracondylar fracture. The mechanism of injury is axial impaction with
resultant intraarticular fracture. This injury occurs predominantly in
adolescents around the time of physeal closure, but it can also occur
in younger children. The capitellum and trochlea usually are separated
from each other, and the two are separated from the proximal humerus.
restoring the anatomic alignment of the articular surface. Closed
reduction and percutaneous fixation has been reported, with
transcondylar fixation followed by pinning or flexible nailing of the
supracondylar component of the fracture (154,155).
When open reduction is necessary, a posterior approach is recommended.
This can be accomplished by splitting the triceps, reflecting a
distally based tongue of triceps, or by olecranon osteotomy (156,157).
Comminution of the articular surface is rare in children, so the
triceps-splitting approach is usually adequate without olecranon
osteotomy. Extensive dissection of the fragments should be avoided to
minimize the risk of avascular necrosis of the trochlea. Transverse
fixation of the trochlea to the capitellum is performed first, and this
unit is secured to the distal humerus with sufficiently strong crossed
pins or cancellous screws. Alternatively, 2.5-mm reconstruction plates
are applied to the medial and lateral columns of the distal humerus.
When open reduction is performed, internal fixation should be stable.
This allows motion during the early postoperative period (156,158).
The recommended period of immobilization should be 3 weeks or less. The
authors prefer rigid internal fixation with early motion in patients
who are near skeletal maturity.
primarily in infants and young children. The mechanism of injury
involves rotatory shear forces, resulting in a Salter-Harris type I or
type II fracture pattern. Abuse should be suspected (159).
This injury may present a diagnostic challenge because of the lack of
ossification of the distal humerus in young children. Diagnosis of this
fracture should be considered in any young child with significant soft
tissue swelling and crepitus on elbow motion. This fracture is most
often confused with elbow dislocation and lateral condyle fracture.
Elbow dislocation is rare in young children; the forearm is displaced
laterally and the long axis of the radius is lateral to the
capitellum.
A lateral condyle fracture in a 2- to 3-year-old child may be confused
with transphyseal separation, especially if there is joint subluxation.
However, the subluxation associated with lateral condyle fracture is in
the lateral direction, whereas distal humeral physeal separations
usually displace in a medial direction (Fig. 33.21).
Arthrography, MRI, or ultrasonography can help confirm the diagnosis of
separation of the entire distal humeral physis. Arthrography is a
helpful adjunct at the time of definitive treatment to confirm the
quality of fracture reduction (Fig. 33.22).
supracondylar fractures. Closed reduction and plaster immobilization
frequently lead to cubitus varus that does not resolve (146,159).
Therefore, fixation with two small-diameter, laterally placed pins is
recommended after reduction. Fractures diagnosed after 7 to 10 days
should not be manipulated because healing is rapid and growth arrest
may result from vigorous attempts at reduction. For fractures with late
presentation, it is better to wait and perform supracondylar osteotomy
for residual deformity.
common elbow fracture in children. This injury is usually the result of
a varus force on the supinated forearm, in which the extensor longus
and brevis muscles avulse the condylar fragment. The peak age range for
this injury is 5 to 10 years, but it is often seen in older or younger
children.
physis and the articular surface. It is a Salter-Harris type IV injury
in most cases, but a significant portion of the fragment is unossified,
especially in children younger than 5 years. Growth disturbance is more
common than is generally recognized (160).
Fortunately, growth disturbances are usually minor because the distal
humerus only contributes 2 to 3 mm of longitudinal growth per year in
children older than 7 years (161). The injury
is identified by a thin lateral metaphyseal rim of bone, but the
fracture line may continue through unossified cartilage, across the
physis, and into the elbow joint. An oblique radiograph of the
internally rotated distal humerus usually provides the best view of
this fracture.
unossified cartilage of the distal humerus. The Milch classification is
unreliable and has limited clinical usefulness (160,162). Postmortem studies by Jakob et al. identified three stages of lateral condylar displacement (163) (Fig. 33.23).
These stages provide a useful classification for lateral condyle
fractures. This classification, on the basis of the observations of
Jakob et al., is more useful than the Milch classification with regard
to treatment choices. Stage I has an intact cartilage hinge.
This
fracture pattern is inherently stable. Stage II is a complete but
minimally displaced fracture with disruption of the articular cartilage
hinge. This fracture may displace further and lead to nonunion. Stage
III has major rotational displacement with loss of soft tissue
stability. Severely displaced fractures lead to symptomatic malunion or
nonunion unless they are reduced and stabilized.
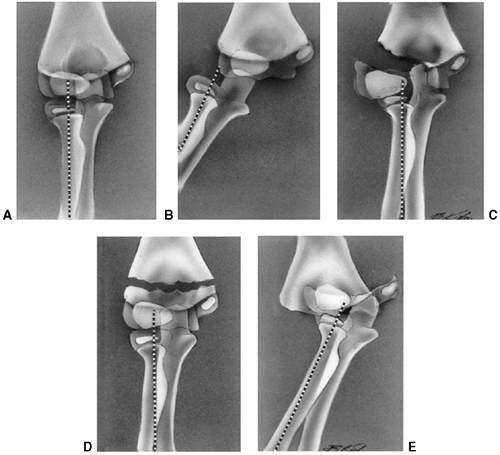 |
|
Figure 33.21 A: Normal elbow demonstrating the alignment of the radius with the capitellum. B: In a dislocation of the elbow, there is disruption of the radiocapitellar alignment. Most dislocations are posterolateral. C: In a displaced lateral condyle fracture, there is again disruption of the radial capitellar alignment. D:
Supracondylar elbow fracture, in which the radius and capitellum remain aligned, despite displacement of the distal humeral fragment. E: Fracture-separation of the distal humeral physis. The radiocapitellar relation is preserved, and typically the distal segment is posteromedially displaced. (Adapted from DeLee J, Wilkins K, Rogers L, et al. Fracture separation of the distal humeral epiphysis. J Bone Joint Surg Am 1980;62:46, with permission.) |
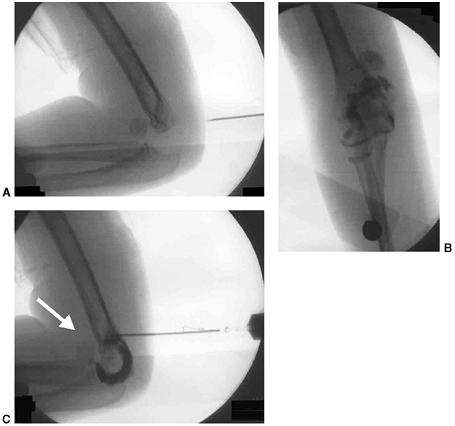 |
|
Figure 33.22
Arthrogram technique. Although many are trained to use the lateral radiocapitellar joint as the entry point for an elbow arthrogram, the olecranon fossa approach is easier and more reliable on the swollen elbow of a small child. This 6-year-old child presented with distal humeral fracture. The surgeon could not distinguish a distal humeral physeal fracture versus a lateral condyle fracture. In the operating room, 5 mL of radiographic dye was injected into the olecranon fossa. A: Lateral image, just prior to injection. B, C: Anteroposterior and lateral image after injection. The distal humerus was clearly delineated as intact, while a lateral condyle fracture fragment (arrow) can be seen anteriorly. |
fracture line may be difficult. When the fracture gap is equal medially
and laterally along the fracture line, there is a very high risk of
displacement due to absence of a cartilage hinge (164). Fractures with initial displacement of 3 mm or more also tend to displace further and have a higher incidence of nonunion (165). MRI has been recommended when there is doubt about the presence of an intact cartilage hinge (166).
However, the present authors prefer to assess fracture stability in
questionable cases by radiographic follow-up weekly for 2 weeks. It is
often necessary to remove the cast to obtain adequate anteroposterior,
internally rotated oblique, and lateral radiographs of the distal
humerus (Fig. 33.24). Gentle cast removal and radiography will not cause displacement if the fracture is inherently stable.
 |
|
Figure 33.23 Jakob classification of stages of displacement of a lateral condyle fracture. A: Stage I displacement with an intact articular surface. B: Stage II displacement. The articular surface is disrupted, but the fracture is minimally displaced. C: Stage III displacement with fragment rotated.
|
assessment of fragment stability. Fractures with initial displacement
of 3 mm or more tend to displace further and have a higher incidence of
nonunion (165); therefore, it is the authors’
preference to pin all fractures that are displaced 3 mm or more. Some
lateral condyle fractures that are displaced 3 mm may have an intact
cartilage hinge and heal
with cast immobilization (166,167) (Fig. 33.25).
Careful follow-up for minimally displaced fractures is essential, so
the surgeon should consider pinning minimally displaced fractures when
compliance with follow-up is doubtful (168).
When initial displacement is 3 mm or less and the patient is reliable,
weekly follow-up for 2 weeks with radiographs that are taken without
the cast is recommended to determine stability. Any further
displacement warrants surgical stabilization. Healing for minimally
displaced fractures is complete when bridging callus is identified
(usually seen posteriorly on the lateral radiograph). This usually
occurs by the fourth week of immobilization, but immobilization for up
to 12 weeks may be required in some cases (165).
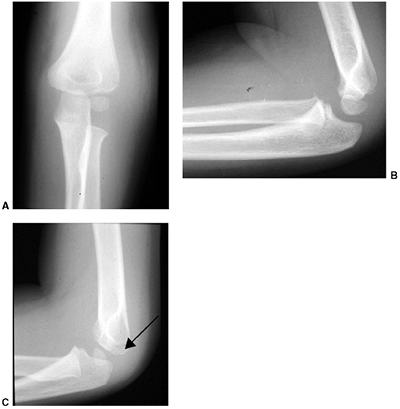 |
|
Figure 33.24 A minimally displaced lateral condyle fracture may be best visualized on the internal oblique radiograph. Anteroposterior (A), lateral (B), and internal rotation oblique (C) radiographs.
|
An arthrogram may provide confirmation of joint surface congruity when
closed pinning is performed. However, many of these fractures display
rotation of the condylar fragment, which is difficult to correct
accurately by closed reduction. Primary open reduction with restoration
of articular congruity is preferred in most cases requiring surgery. In
severe cases, the condylar fragment may be rotated 180 degrees. A
standard lateral approach is utilized between the triceps posteriorly
and the brachialis/extensor carpi radialis longus anteriorly. Incision
of the anterior joint capsule facilitates exposure. Soft tissue
stripping posteriorly should be avoided to reduce the risk of avascular
necrosis of the capitellum and trochlea. Stabilization can be achieved
with two smooth pins crossing the fracture site and exiting the
opposite cortex. The pins and cast are removed 3 to 6 weeks after
surgery, depending on the extent of healing seen on postoperative
radiographs.
mal-union is an area of controversy. Minor degrees of malunion are well
tolerated. Correction of malunion more than 6 weeks after fracture is
difficult (163,170).
Remodeling obscures fracture lines and interferes with restoration of
anatomic reduction following osteotomy. Excessive stripping of the
condyle to facilitate reduction may result in avascular necrosis and
greater joint stiffness. Two surgical approaches are described in the
literature for management of symptomatic patients with malunion.
Supracondylar osteotomy, combined with ulnar nerve transposition, has
been performed with satisfactory improvement in function (163). Intraarticular osteotomies, with partial reduction, have also achieved satisfactory results (171).
more. Delayed union and nonunion has been attributed to fracture
instability, exposure to synovial fluid, and decreased vascularity due
to the large articular surface of the fracture fragment. Long-term
sequelae of nonunion include ulnar neuritis, progressive valgus
deformity, and elbow instability with decreased strength. In
established nonunions, the lateral condyle fragment should be fixed in
a position that preserves the best functional range of motion,
realizing that anatomic restoration is not possible (Fig. 33.26) (165,171,172).
Bone grafting is recommended to achieve union. Any residual valgus
deformity can be corrected with a supracondylar osteotomy. Ulnar nerve
transposition may be needed, especially if there are preoperative
symptoms (173).
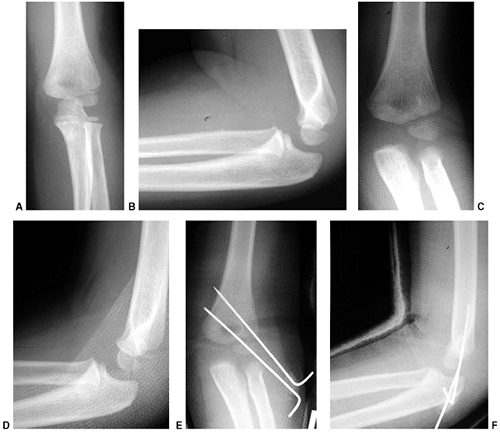 |
|
Figure 33.25 The drifting lateral condyle fracture. A, B:
Anteroposterior and lateral radiographs at presentation. This lateral condyle fracture has only approximately 2 mm of displacement on the anteroposterior view. No displacement is noted on the lateral view. The child was placed in a long-arm cast and a follow-up 1 week later was recommended. C, D: Anteroposterior and lateral radiographs taken 1 week after injury show further displacement of the lateral condyle fracture with 5 mm of separation of the lateral condyle from the distal humerus. Open reduction and pinning was performed. E, F: Radiographs taken in the cast 4 weeks after open reduction and pinning show anatomic alignment and early healing. |
Asymmetric growth of the lateral condyle or incomplete reduction can
produce mild cubitus varus. Fishtail deformity, or deepening of the
trochlear groove, may result from central growth arrest or from
avascular necrosis. This deformity rarely compromises function, but may
predispose to later condylar fracture (160).
Dissolution of the medial condyle or the lateral condyle has also been
reported following distal humerus fracture in children (175). Corrective osteotomies with nerve decompression or transposition can modify the deformity and symptoms.
does not involve the articular surface. This ossification center does
not appear until the second decade. This injury is
often
misdiagnosed as an avulsion fracture of the lateral condyle. Treatment
is usually immobilization followed by early motion, when comfortable.
Displacement greater than 5 mm may lead to joint stiffness. If this
occurs, early excision of the displaced epicondyle should be considered.
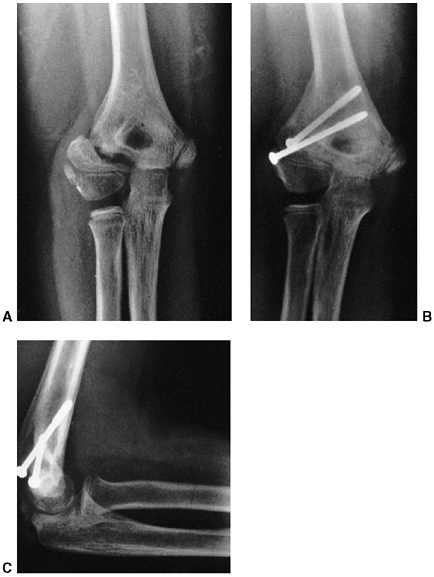 |
|
Figure 33.26 Nonunion of the lateral condyle. A:
Anteroposterior view of a child who sustained this fracture 4 months earlier. Symptoms consisted of pain and decreased range of motion. B: Anteroposterior projection after stabilization of the condylar fragment with bone screws and bone grafting of the nonunion. C: Lateral projection. |
valgus load is applied to the extended elbow. The displacement is
encouraged by the pull of the forearm flexor muscle group, which is
attached in this region. The medial collateral ligament, which also
originates from this apophy-sis, may play a role in the initial
fracture displacement, especially when the fracture is associated with
an elbow dislocation. This injury typically occurs in children between
the ages of 9 and 14 years, later than the peak age for most other
elbow fractures. In younger children, the entire unossified medial
condyle may be fractured, giving the appearance of medial epicondylar
fracture. Almost 50% of medial epicondyle fractures occur concomitantly
with posterolateral elbow dislocation. The medial epicondyle may be
trapped in the joint after reduction. When this occurs, the ulnar nerve
may also be in the joint, and vigorous attempts at closed manipulation
should be avoided.
indicated when the epicondyle is trapped in the joint. Otherwise,
management is controversial. There are advocates for the closed
treatment of this injury regardless of the magnitude of displacement (176,177).
Nonunion is a frequent result of closed management, but many patients
are asymptomatic. Valgus instability to stress testing has been
suggested as an indication for surgical stabilization (178).
However, most fresh medial epicondyle avulsion injuries will
demonstrate instability, so most patients will require surgery if this
test is used. Late instability after closed management has been
reported, but this complication is rare unless the epicondylar fragment
has been excised (177,178). Excellent results have also been reported after surgical stabilization of moderately displaced fractures (179).
Although nonsurgical management can produce good results, it is the
authors’ preference to perform open reduction and internal fixation for
medial epicondyle fractures when displacement is greater than 5 mm (Fig. 33.27).
Surgical intervention for lesser degrees of displacement is also
considered for highly competitive gymnasts or throwing athletes who
have injured their dominant elbow.
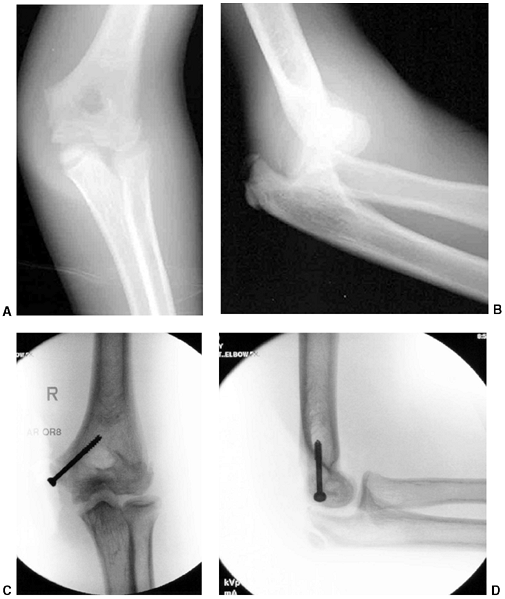 |
|
Figure 33.27 Medial epicondyle fracture with elbow dislocation. A, B: Anteroposterior and lateral views of the elbow showing an elbow dislocation with associated medial epicondyle fracture. C, D:
Intraoperative images after reduction of the elbow dislocation and open reduction of the medial epicondyle fracture with interfragmentary screw fixation. |
with a cannulated bone screw. When reducing this fracture, it is
important to understand that the fragment tends to rotate anteriorly
from its posteromedial origin. Stable fixation allows early
postoperative range of motion. Preadolescents may require fixation with
smooth Kirschner wires and cast immobilization for 3 weeks to minimize
the risk of growth arrest of the apophysis.
unusual injury. Medial condyle fracture may be misdiagnosed as medial
epicondyle avulsion in children between the ages of 5 and 7 years,
because the epicondylar ossification center is visible on radiographs
approximately 2 years before the trochlea ossifies. If a child with an
unossified trochlea presents with a swollen elbow, it is important to
examine the radiographs for a chip or flake of bone from the
metaphysis, which indicates medial condyle fracture. The mechanism of
injury is similar to that for medial epicondylar fracture, but medial
condyle fracture is a much more serious injury, because it involves the
articular surface. If the condyle is displaced more than 2 mm, open
reduction and internal fixation is recommended (180).
in the 7- to 12-year age group. Approximately 50% are isolated
injuries; associated fractures, most commonly of the proximal ulna, are
found in the other 50% (181). Associated
injuries should be treated independently as indicated for that
particular fracture. Radial neck fractures are predominantly
Salter-Harris type I or II injuries. The
radial
head is largely cartilaginous and is rarely injured in children. The
mechanism of injury is usually valgus stress, with compression of the
radial neck from a fall on the extended elbow. Fracture displacement
can result in angulation and translation, with or without complete
separation of the radial head from the shaft. Angulation after union
may remodel, especially in younger children. Union with translation may
limit motion because of a cam effect that prevents the radial head from
rotating in a circle. Approximately half of the children who sustain
fractures of the radial neck will have some permanent limitation of
forearm rotation. Factors leading to a poor prognosis are age greater
than 10 years, angulation greater than 30 degrees, displacement greater
than 3 mm, delayed treatment, associated injuries, and open reduction (182,183).
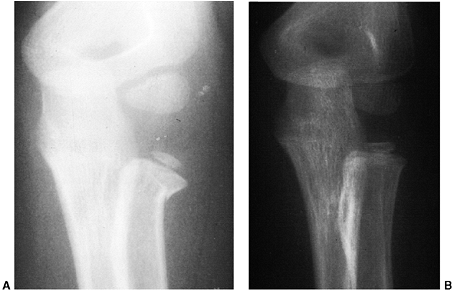 |
|
Figure 33.28 Impacted radial neck fracture in a boy who is 6 years and 5 months old. A: Angulation measures 30 degrees. B: Nine months later, alignment is normal without treatment. The child re-gained full range of motion.
|
of angulation and translation. The plane of maximum angulation can be
determined by multiple radiographic views or by fluoroscopy. When
angulation is greater than 30 degrees or translation is greater than 3
mm, closed reduction usually should be attempted because anatomic
alignment is associated with better outcome. However, closed reduction
may fail, and one must decide whether to accept suboptimal alignment or
to resort to open reduction, with increased risks in each case. Age is
important in this situation (183). For a child younger than 10 years (Fig. 33.28),
the authors will accept up to 45 degrees of angulation and 33%
translation before resorting to open reduction. In a child older than
10 years, up to 30 degrees of angulation and 3 mm of translation may be
accepted.
of traction and varus stress, combined with digital pressure over the
radial head. Alternatively, the radial shaft should be forced laterally
while the radial head is held in place (Fig. 33.29) (184),
or the forearm can be pronated as the elbow is maximally flexed.
Wrapping the arm firmly in an Esmarch bandage produces compression and
elongation forces, which may also reduce the fracture. Alternatively, a
percutaneous pin introduced proximally or a flexible intramedullary
wire introduced distally can be used to manipulate and stabilize the
proximal fragment (185,186,187).
Open reduction is performed when these methods fail to produce an
acceptable reduction. Another indication for open reduction is a
displaced Salter-Harris type IV fracture involving more than one-third
of the articular surface. Fixation may be achieved with intramedullary
fixation from the distal metaphysis (Figs. 33.30 and 33.31) or by
distally inserted Kirschner wires placed obliquely across the fracture.
A transarticular pin through the humerus should be avoided.
Percutaneous fixation is removed in 3 to 4 weeks to begin elbow range
of motion. If the metaphyseal fragment is large enough, a minifragment
screw can be used instead of Kirschner wires or flexible retrograde
nailing.
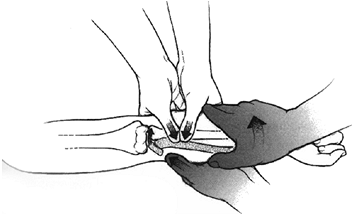 |
|
Figure 33.29
Displaced radial neck reduction technique using manipulation of the proximal radius with the elbow extended and hand supinated. (From Neher CG, Torch MA. New reduction technique for severely displaced pediatric radial neck fractures. J Pediatr Orthop 2003;23:626–628.) |
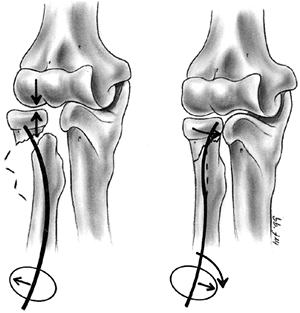 |
|
Figure 33.30
Radial neck fractures may be reduced by introduction of a percutaneous wire from the distal metaphysis. The wire is rotated after it engages the proximal fragment. [From Gonzalez-Herranz P, Alvarez-Romera A, Burgos J, et al. Displaced radial neck fractures in children treated by closed intramedullary pinning (Metaizeau technique). J Pediatr Orthop 1997;17 (3): 325–331.] |
loss of forearm rotation, radioulnar synostosis, injury to the
posterior interosseous nerve, nonunion, premature physeal arrest, and
avascular necrosis of the radial head (182,183). These complications are observed without treatment, unless they are progressive or disabling.
children, the spongy bone and thick surrounding cartilage often prevent
significant displacement. Older children and adolescents are more
likely to have displaced fractures and associated injuries. The most
common mechanism of olecranon injury is an avulsion or flexion injury
that disrupts the posterior periosteum and fractures the cortex (Fig. 33.32).
Extension injuries leave the posterior periosteum intact, but often
result in angulated fractures and associated fractures attributable to
varus or valgus forces acting on the extended elbow. Olecranon
fractures may also result from a direct blow that causes comminution
but minimal displacement because the periosteum remains intact.
Children with osteogenesis imperfecta can present with an olecranon
sleeve fracture (Fig. 33.33).
or cast immobilization for 2 to 3 weeks. When displacement is greater
than 4 mm in metaphyseal bone, or there is a greater than 2 mm joint
step-off in the anterior two-thirds of the coronoid fossa, closed
reduction is recommended. Open reduction with internal fixation is
performed when closed reduction fails, or when maintenance of reduction
is difficult because of fracture instability (188,189,190).
Internal fixation is achieved by standard osteosynthesis, using the AO
tension band technique or its modification, which involves placing the
distal wire hole anterior to the axis of the intramedullary Kirschner
wires (191). This provides additional compression forces across the articular surface of the olecranon (Fig. 33.34).
In younger children, heavy nonabsorbable suture may be used in place of
the figure-eight wire because healing is rapid. An alternative is to
place two divergent compression pins or screws through percutaneous
incisions (192).
young children; the peak incidence is in the second decade of life.
Often there is an associated fracture, most commonly of the medial
epicondyle, but occasionally of the coronoid process or the radial
neck. Elbow dislocation is predominantly a male injury (70%) involving
the nondominant arm (60%). The most common pattern is posterolateral
displacement of the proximal radius and ulna articulation from the
humerus, without disruption of the radioulnar articulation (193).
longitudinal traction, usually in the emergency department, after
establishing pain control and muscle relaxation. The surgeon should be
aware of possible interposed soft tissue or bony fragments, including
the ulnar nerve or medial epicondyle. A posterior splint is applied for
2 weeks; thereafter, elbow range of motion is initiated to minimize the
risk of fixed contracture.
be minimized for stable reductions by initiating early motion 2 weeks
after injury (193). Stiffness, in the absence
of fracture, usually resolves within 6 to 8 months. The authors
recommend avoiding the use of passive stretching devices for at least 4
months. The authors recommend excising associated avulsion fractures if
range of motion has not returned to a functional range (30 to 100
degrees) by 6 months after dislocation. When avulsion fractures are
absent, surgical release of contracture may be required if the range of
motion is less than functional 8 months or more after injury (194,195).
Neurapraxia involving the median or ulnar nerves occurs in
approximately 10% of dislocations and usually resolves within 3 months.
rare injury. The radial head dislocates anteriorly. Most of these
injuries actually represent occult Monteggia injuries, with
plastic deformation of the ulna and anterior dislocation of the radial head (Fig. 33.35). Treatment consists of closed reduction and immobilization, with the elbow in flexion and supination.
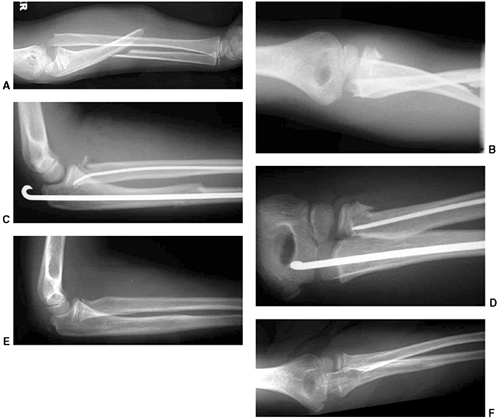 |
|
Figure 33.31 Monteggia-equivalent fracture treated with intramedullary (IM) reduction and fixation. A, B:
Anteroposterior and lateral view of a Monteggia-equivalent fracture with a displaced, oblique ulna fracture and completely displaced radial neck fracture, but intact radiocapitellar joint. C, D: The ulna was fixed with an IM nail, and the radial neck fracture was reduced and pinned using the Metazeau technique (shown in Fig. 33.30). E, F: Late follow-up after implant removal shows good alignment of the ulna and radiocapitellar joint. (Case courtesy of Ken Noonan, MD) |
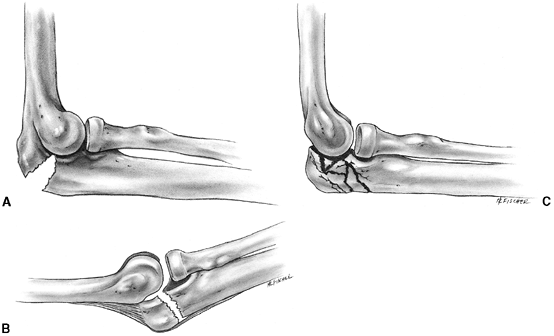 |
|
Figure 33.32 Olecranon fractures. A:
Avulsion or flexion injury is most common and requires reduction in extension. Internal fixation is frequently necessary to maintain reduction. B: Extension-type fracture leaves the posterior periosteum intact and may be reduced by flexing the elbow. C: Comminuted fractures result from a direct blow and may be minimally displaced. |
 |
|
Figure 33.33
Olecranon sleeve fracture. This lateral radiograph taken at the time of injury shows an olecranon sleeve fracture in a 10-year-old child with a mild osteogenesis imperfecta. |
congenital radial head dislocation falls and injures the elbow.
Congenital dislocation of the radial head is distinguished from
traumatic dislocation by the fact that congenital dislocation is
usually in a posterior direction, and the articular surface of the
radial head is convex or flat, rather than having the normal concave
contour.
injury in children between 1 and 4 years of age. Typically, the child
is being held by the hand and suddenly falls, or is pulled upward. Pain
is variable. The child will not move the arm and will hold it in a
slightly flexed and pronated position. Radiographs are not indicated
initially and are normal because the injury consists of subluxation of
the annular ligament, rather than true joint subluxation (196) (Fig. 33.36).
When longitudinal traction is applied to the child’s pronated arm, the
annular ligament slips off the radius and rides up onto the broader
radial head, like a rope that has jumped out of the groove in a pulley
wheel. The child is unwilling to move the elbow until the stretched
annular ligament is reduced.
degrees, then fully and firmly supinating the forearm. A click or snap
is often felt as the ligament snaps back into place. Occasionally, it
is necessary to fully pronate, and then supinate, the forearm to
achieve reduction. This is especially true for delayed cases. The child
should begin to move the arm within a few minutes after reduction. If
this is not the case, then radiographs should be obtained to rule out
occult fracture, and the arm may be immobilized in a splint for 2 to 3
days. Normal use of the arm should be expected within 1 day after
splint removal. Recurrences are not uncommon but are treated in the
same manner as the initial injury. Eventually, children outgrow this
condition, and long-term sequelae have not been reported.
There may be an increasing incidence of distal forearm fractures
related to increased body mass, decreased bone mineral content, and
changing patterns of activity such as rollerblading (5).
Most forearm fractures occur in children older than 5 years. The
location of the fracture advances distally with increasing age of the
child, probably because of the anatomic changes in the
metaphyseal-diaphyseal junction that occur with maturity (197).
The younger child’s radius is more elastic and has a gradual transition
of the diameter of the radius from shaft to metaphysis, whereas the
transition in diameter is more abrupt at the metaphyseal—diaphyseal
junction in the older child. The distal forearm is the site of 70% to
80% of fractures of the radius and ulna. Most of these are nonphyseal.
Physeal separations are more likely in early adolescence because of the
more adult shape of the radius, which concentrates stress closer to the
epiphysis. In 10% to 15% of patients, forearm fractures are associated
with elbow fractures. This highlights the importance of a thorough
clinical and radiographic examination of the injured extremity.
straight bone with a triangular cross section. The radius has a more
complex, curved shape with a cylindrical proximal portion, a triangular
middle portion, and a flattened distal third. The radius rotates around
the ulna during forearm supination and pronation. There are three areas
of soft-tissue interconnection between the radius and ulna. Proximally,
there is the radioulnar articulation, which is stabilized by the
annular ligament. Centrally, the shafts of the two bones are connected
by the interosseous membrane, which is wider distally. The fibers run
obliquely from the ulna distally to the radius proximally. This helps
transmit force to the ulna during load-bearing activities. Distally,
the triangular fibrocartilage complex stabilizes the radioulnar joint
by means of the ulnar collateral ligament and the volar and dorsal
radiocarpal ligaments. The proximal and distal radioulnar joints are
most stable in supination, and the interosseous membrane is widest with
the forearm in a position of 30 degrees of supination. Because of these
interconnections, both bones are usually injured at the time of
fracture. When only one bone is broken, there is frequently damage to
the proximal or distal radioulnar articulation.
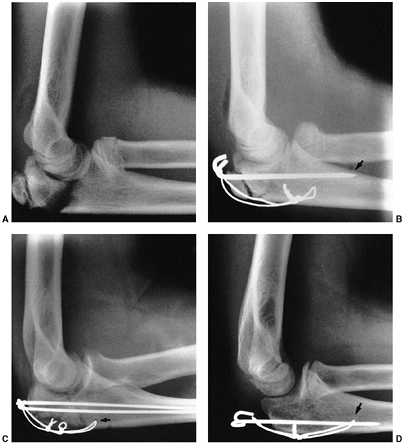 |
|
Figure 33.34 Olecranon fracture. A: Lateral projection showing displaced intraarticular olecranon fracture. There is also an impacted radial neck fracture. B:
Treatment with standard tension band technique. The slight variation from standard fixation is to capture the anterior cortex of the ulna with the Kirschner wires (arrow). C: Standard AO technique, used in a similar case, with parallel intramedullary Kirschner wires with the tension band transverse hole inferior (arrow). D: Modified AO technique performed by placing the transverse hole anterior to the Kirschner wires (arrow). The compressive force is anterior to the pin, which prevents the articular surface of the semilunar notch from gapping. Ideal joint compressive forces are obtained with placement of the Kirschner wires down the middle axis of the ulna and the transverse hole anterior to this axis. |
three major categories: fracture-dislocations, fractures of the
midshaft, and distal fractures. Fracture-dislocations include the
Monteggia and Galeazzi lesions. Midshaft fractures of the radius and
ulna tend to follow three injury patterns: plastic deformation,
greenstick fracture, and complete fracture. Distal fractures are either
metaphyseal fractures or physeal separations. Each type of injury
presents unique features with regard to mechanism of injury,
recognition, and management.
dislocation is less common than other types of forearm fractures.
Misdiagnosis may result if radiographs of forearm fractures fail to
clearly demonstrate the elbow and wrist joints. The peak incidence
occurs between 4 and 10 years of age. The Monteggia lesion is more
common and involves dislocation of the radial head. The usual mechanism
of injury is a fall on the hyperextended arm. The Galeazzi lesion
involves
fracture of the radius with dislocation of the distal radioulnar joint, or distal ulnar physeal fracture.
 |
|
Figure 33.35 Dislocated radial head with plastic deformation of the ulna. A:
A 10-year-old boy presented with elbow and forearm pain after a fall. Radiograph shows complete dislocation of the radiocapitellar joint with plastic deformation of the ulna (note the anterior bow of the ulna demonstrated by a line drawn along its subcutaneous border). B: Contralateral, uninjured arm. Note the straight subcutaneous border. |
pediatric forearm fracture, including plastic deformation and minimally
angulated greenstick fractures of the ulna. When the posterior border
of the ulna deviates from a straight line on a true lateral radiograph,
dislocation of the radial head should be suspected (198).
A true lateral radiograph of the elbow provides the best assessment of
the radiocapitellar joint. In the normal elbow, a line drawn down the
long axis of the radial shaft will bisect the capitellum regardless of
the position of flexion or extension of the elbow (199) (Fig. 33.37).
Congenital dislocation of the radial head is distinguished from
traumatic dislocation by the facts that congenital dislocation is
usually posterior and the articular surface of the radial head is
convex. In contrast, anterior dislocation of the radial head is common
with Monteggia fracture-dislocation (200,201).
Type I involves anterior radial head dislocation (i.e., ulnar deformity
apex anterior), which accounts for most childhood Monteggia injuries (Fig. 33.38).
Type II fracture is the least common and entails posterior or
posterolateral radial head dislocation. In type III lesions, the radial
head is dislocated laterally, and the ulna fracture is usually in the
proximal metaphyseal region. Type III injury accounts for 25% to 30% of
pediatric Monteggia injuries. Type IV fracture-dislocations involve
anterior dislocation of the radial head, in combination with fracture
of the radius and ulna. This may be considered a variant of the type I
lesion. There are numerous Monteggia equivalents that represent a
multitude of variations. For example, the ulna fracture may be combined
with a radial neck fracture rather than a simple radial head
dislocation (Fig. 33.31). Segmental fractures and plastic deformation of the ulna are other forms of Monteggia equivalent injury.
because stable, anatomic reduction of the ulna can maintain anatomic
reduction of the radial head. Most Monteggia injuries in children
younger than 12 years can be managed successfully by closed reduction
and above-elbow casting with the elbow in full supination and 90 to 110
degrees of flexion (200,202).
Weekly follow-up with good quality elbow radiographs is suggested for 2
to 3 weeks to detect any recurrent radial head subluxation. Transient
nerve palsies, most commonly of the posterior interosseous nerve, occur
in approximately 10% of patients.
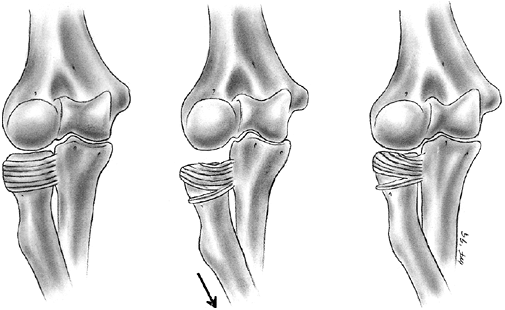 |
|
Figure 33.36
Pulled elbow, or “nursemaid’s elbow,” occurs as the radial head moves distally. The annular ligament is partially torn and displaced onto the radial head. (From Rang M. Children’s fractures, 2nd ed. Philadelphia: JB Lippincott, 1983, with permission.) |
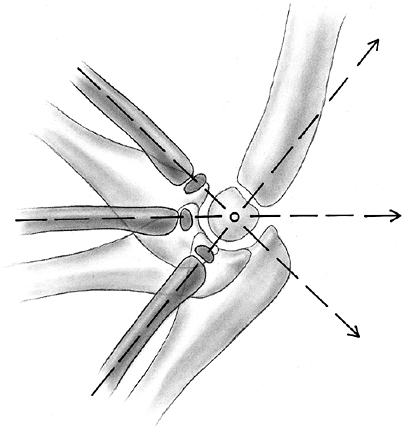 |
|
Figure 33.37
A true lateral radiograph of the elbow joint allows assessment of the integrity of the radiocapitellar joint. A line drawn down the long axis of the shaft of the radius will bisect the capitellum in all positions of flexion and extension. (From Smith F. Children’s elbow injuries: fractures and dislocations. Clin Orthop 1967;50:25, with permission.) |
unstable. Instability requiring surgical stabilization is more likely
when there is an oblique fracture, or very displaced fracture, of the
ulna (203). The percutaneous insertion of an
ulnar intramedullary pin is a simple and effective way to manage this
problem. Alternatively, the ulnar shaft can be plated. On rare
occasions, when the radial head is not
reduced
after correction of ulnar length and alignment, open examination of the
joint is indicated. Interposition of the annular ligament or an
intraarticular osteochondral fragment may be found. Transcapitellar
pinning of the reduced radial head should be avoided whenever possible.
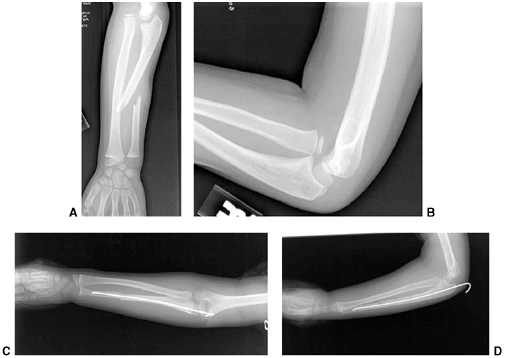 |
|
Figure 33.38 Type I Monteggia fracture reduced and stabilized with an intramedullary nail in the ulna. A, B:
Anteroposterior and lateral radiographs of a type I Monteggia fracture with an unstable, widely displaced, oblique fracture of the ulna and an anterior dislocation of the radial head. C, D: Anteroposterior and lateral views after closed reduction of the radiocapitellar joint and stabilization of the ulna fracture with a titanium elastic nail. |
associated with Monteggia fracture-dislocations. Patients presenting
less than 3 weeks after injury with persistent dislocation may be
treated by closed reduction. If the ulna can be reduced and stabilized,
the radial head will usually reduce and remain stable. Open reduction
is indicated if closed reduction fails, or when the injury is more than
4 weeks old. Chronic radial head dislocation has been surgically
treated by open reduction. Following excision of the interposed
fibrocartilaginous mass, the annular ligament can be reconstructed from
remnants of the ligament, from a strip of triceps fascia, or from other
tissues (204). Corrective osteotomy of the ulna
to restore length and alignment has been associated with improved
results, with or without annular ligament reconstruction (205,206).
with dislocation of the distal radioulnar joint. This is an uncommon
injury in children. The triangular fibrocartilage complex is disrupted,
and the distal ulna is dorsally displaced, as viewed on a lateral
radiograph. Injury to the distal radioulnar joint is frequently
overlooked, and persistent joint subluxation is responsible for poor
long-term results (207).
The Galeazzi equivalent injury is more common in children and consists
of a fracture of the radius with a physeal fracture of the distal ulna (208).
Treatment consists of closed reduction of the radius and ulnar physis
with above-elbow casting in full supination. Open reduction may be
necessary if the ulna is buttonholed through periosteum, blocking
reduction. Occasionally, it is necessary to place a smooth Kirschner
wire across the joint or the fracture to maintain reduction. Long-term
problems with this injury include premature physeal arrest with ulnar
shortening and loss of supination (209).
is possible because of the elastic properties of young children’s
bones. This injury represents a series of microfractures that are not
seen on radiographs. Bowing of both bones may occur, but plastic
deformation of one bone is often associated with complete or incomplete
fracture of the other forearm bone. Swelling and pain are usually less
severe with plastic deformation than with complete fractures. This
often facilitates examination. Reduction is recommended when deformity
is cosmetically unacceptable, or there is greater than 45 degrees loss
of forearm rotation. Remodeling capacity of this type of fracture is
limited after 6 years of age. In children younger than 6 years, 15 to
20 degrees of angulation can be accepted, but more than 10 degrees
angulation in older children may not remodel (210,211).
general anesthesia because a prolonged corrective force must be applied
to permanently straighten the bone. The position of immobilization
follows the principles outlined in the discussion of greenstick
fractures, which follows.
deformation of the opposite cortex characterize greenstick fractures.
Most greenstick fractures represent a rotational malalignment, in
addition to angular deformity (212). Apex-volar
greenstick fracture is the most common type, and results from excessive
supination forces applied to the distal segment, combined with axial
load. The child presents with the palm facing the apex of the fracture
deformity (Fig. 33.39). Apex-dorsal deformity
results from excessive pronation force applied to the distal segment;
the child presents with the palm facing down, relative to the apex of
the fracture deformity (Fig. 33.40).
Occasionally, greenstick fractures of the radius and ulna are caused by
a direct force producing angular deformity without much malrotation.
Reduction is indicated when shaft angulation is greater than 15 degrees
in a child younger than 10 years, or greater than 10 degrees in an
older child (213,214).
Judgment is required because the rotational component of greenstick
fracture of the forearm diaphysis does not always correlate with the
amount of angulation. Reduction is indicated if forearm deformity was
immediately obvious at the time of injury, or when there is rotational
deformity of 45 degrees or more in children younger than 10 years, or
30 degrees or more in older children.
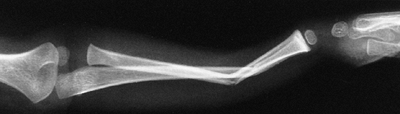 |
|
Figure 33.39
Coupled relation of rotation and angulation. This fracture demonstrates volar angulation. The mechanism of injury was falling on the outstretched hand. When the fractures of the radius and ulna are at different levels, angulation cannot occur without rotation. Note the anteroposterior appearance of the elbow and the lateral projection of the wrist. This deformity is corrected with pronation of the distal fragment. |
relief for one quick reduction attempt that is usually successful.
Reduction is accomplished by reversing the injury mechanism. Apex-volar
deformity (apex of fracture in the direction of the palm of the hand)
is reduced by pronating the wrist while applying pressure to the volar
surface of the forearm. Apex-dorsal deformity (apex of fracture in the
direction of the dorsum of the hand) is reduced by supinating the wrist
while applying pressure to the dorsal surface of the forearm.
Completing the fracture of the opposite cortex is unnecessary, although
this facilitates reduction and often occurs during the reduction
maneuver. After reduction, the arm is immobilized in a well-molded
sugar-tong splint or a bivalved long-arm cast. Three-point pressure is
essential for the maintenance of reduction. Weekly follow-up is
recommended for 2 to 3 weeks after reduction. It is generally necessary
to change the cast for remolding as swelling subsides during this
period. Six weeks of immobilization are usually adequate, but
refracture is a risk when immobilization is discontinued too soon.
This may be due to impaired healing of the fractured side because the
intact cortex reduces micromotion needed for periosteal new bone
formation. Lack of compression also leads to gap widening of the
fractured
cortex
rather than gap closure. One method to reduce the risk of refracture is
to complete the fracture of the intact cortex at the time of reduction.
Another option is to use a longer period of immobilization in a cast or
removable splint.
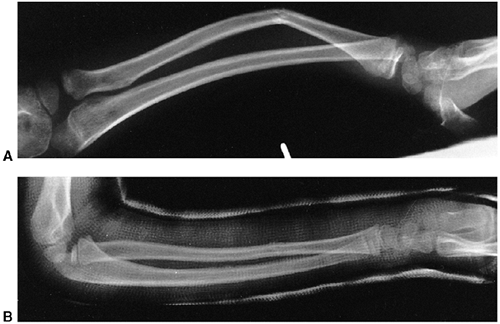 |
|
Figure 33.40 Coupled relation of rotation and angulation. A:
The radiograph shows deformity with pronation of the distal fragment and dorsal angulation of the radius. The ulna has undergone plastic deformation. When a single bone angulates, it must rotate around the other. B: Alignment is restored with supination of the distal fragment. The lateral projections of the elbow and the wrist are now matched. |
higher-energy trauma than do greenstick fractures. The proximal
segments usually assume a position dictated by muscle forces because
the muscle actions are unrestrained by an intact cortex. When the
fracture is in the proximal third, the proximal fragment is frequently
in supination due to the unrestrained actions of the biceps and
supinator muscles. When the fracture is more distal, the pronator teres
has a neutralizing effect on the proximal fragment, causing it to
assume a position of neutral rotation (212).
nonunions and serious complications are rare regardless of method of
management. The principal concerns are the possibilities of residual
deformity and loss of forearm rotation. Closed reduction is recommended
for low-energy minimally displaced fractures, but intramedullary
fixation is an increasingly popular solution for the management of
unstable fractures in children older than 8 years.
-
Younger children with more distal fractures have the best prognosis (213,216,217).
-
Bayonet apposition in the middle and distal third of the shaft does not compromise forearm rotation (218,219).
-
Rotational alignment of the radius is more critical than the rotational alignment of the ulna (212,220). Alignment with 45 degrees of malrotation in the radius can be accepted in younger children (217,218).
-
Midshaft angulation of 15 degrees or less is acceptable in children younger than 8 years (213,221).
-
Midshaft forearm fractures in children older than 8 years should be maintained with 10 degrees or less of angulation (218,222).
-
Residual loss of supination (fixed
pronation) is more difficult to accommodate than loss of pronation.
When in doubt for complete fractures, immobilize the forearm in neutral
or moderate supination (223). -
Immobilization with the elbow extended
may help maintain reduction for proximal-third fractures that are
unstable in flexion. Elbow extension also helps maintain reduction for
most children younger than 4 years. Incorporate the thumb to avoid cast
slippage (224). -
Gentle molding can make improvements in alignment, either in a new cast or by remanipulation for 1 to 3 weeks after injury (225,226).
relaxation are required because more than one attempt may be necessary.
Regional (i.e., Bier or axillary block) or general anesthesia is often
preferred. The reduction technique typically involves increasing the
angular deformity, applying longitudinal traction to lock in place and
straighten the fracture, and then correcting any malrotation by
supinating or pronating the forearm. If this is unsuccessful, it is
often helpful to apply traction by using finger traps for a period of
10 minutes before another attempt is made. Observing the bone widths at
the fracture site and matching their contours on radiographs allow
assessment of rotational alignment. Comparison radiographs of the
opposite extremity, in various degrees of rotation, may also be helpful
in determining rotational alignment (217).
Alternatively, the position of the bicipital tuberosity may serve as a
guide to rotation because the bicipital tuberosity is 180 degrees
opposite the radial styloid and thumb (212).
should maintain a straight lateral border along the ulnar side. A flat
interosseous mold along the volar forearm
should
create an oval shape to the cast. Fracture stability is improved with
at least 50% bone apposition. If one bone disengages, shortening may
occur, followed by increasing angulation. This may respond to
remanipulation or require surgical stabilization. Weekly reevaluation
is recommended for the first 3 weeks. Union is usually complete in 6 to
8 weeks. Nonunion is rare, and closed treatment can produce excellent
results in more than 95% of patients when the fractures can be
maintained within the guidelines stated previously (218,223,227) (Fig. 33.41).
and when closed management has failed. Internal fixation can also
facilitate management and can improve results for refractures with
displacement, most open fractures, and unstable floating elbow injuries
(152,153,215,228,229,230,231,232,233). Intramedullary fixation is usually preferred for children and adolescents (234,235,236). Fixation is achieved by insertion of a small-diameter, flexible pin; a 1.5 to 2.5 mm diameter is sufficient for most cases (234). The ulnar pin can be inserted
proximally, just lateral to the tip of the olecranon, or distally in
the flare of the ulna. The radial pin is inserted in the distal
metaphysis, avoiding penetration of the growth plate. Transphyseal
pinning through the distal radius has also been reported without
physeal arrest (235,237).
When the entry point is metaphyseal, an oblique drill hole is made in
the metaphyseal cortex, and the wire is introduced into the medullary
canal. Following insertion, the pin is tapped or gently rotated into
the medullary canal until it passes the fracture site (Fig. 33.43).
Occasionally, it is necessary to perform a limited open reduction at
the fracture site to facilitate passage of the wire. Tourniquet time
should be kept to a minimum, and multiple attempts at closed pinning
should be avoided in order to decrease the risk of compartment syndrome
(238). Both bones are usually stabilized, but one is sufficient in some cases (239,240).
It is usually best to bend the pins and leave them under the skin for 3
to 5 months because of the possibility of delayed union and also to
reduce the risk of refracture (235). Supplemental casting is recommended until union is satisfactory, approximately 6 weeks after fixation.
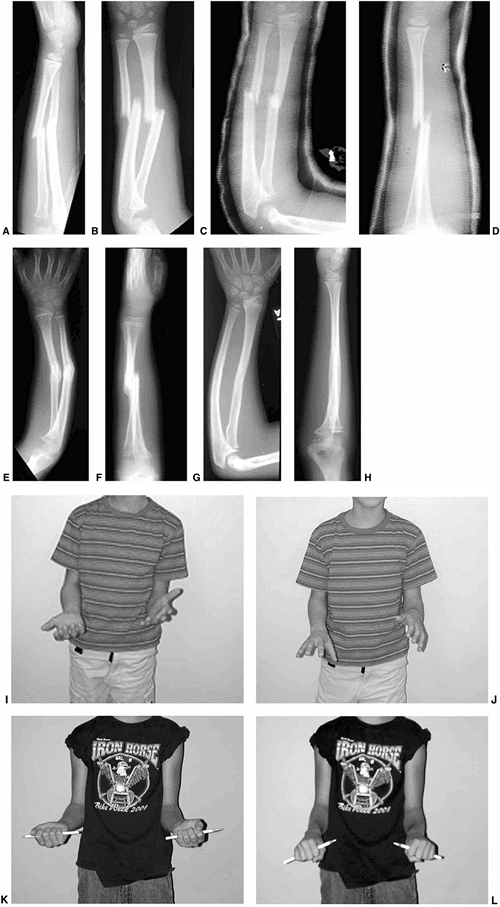 |
|
Figure 33.41 Malunion and remodeling of a diaphyseal radius and ulna fracture in a 7-year-old boy. A, B:
Initial anteroposterior and lateral views of the forearm; the completely displaced radius and ulnar fracture were treated with closed reduction and casting. C, D: These radiographs were taken 1 week after reduction. There is acceptable angulation of the ulna (approximately 15 degrees) and bayonet apposition of both bones, seen on the lateral view. Note that there is no molding of the cast—in particular, note that the cast lacks a straight ulnar border. E, F: These anteroposterior and lateral radiographs of the forearm at the time of cast removal 6 weeks after injury show considerable angulation on the anteroposterior and shortening with bayonet apposition on the lateral. G, H: Three years later, there is extensive remodeling, although the ulnar bow persists. I, J: Clinical pictures after cast removal show 45 degrees of pronation and supination. K, L: Six years after the injury, pronation and supination are symmetric with the uninjured side. |
 |
|
Figure 33.42 Treatment of a high-energy, unstable radius and ulna fracture with single bone intramedullary fixation of the radius. A:
Anteroposterior radiograph of the left forearm of a 12-year-old boy who fell 20 ft while skiing. Although this radiograph shows satisfactory bayonet alignment of both the radius and ulna, his fracture was so unstable and his forearm was so swollen that the surgeon opted to use single bone fixation of the radius and a splint rather than closed reduction and a well-molded cast. B, C: Anteroposterior and lateral view of the forearm taken 8 weeks after a titanium elastic nail was placed in the radius. Neither fracture site was opened. Both bones have healed in satisfactory alignment. |
this technique may be indicated for comminuted fractures, or for
adolescents who are within 1 year of skeletal maturity. Plating
requires more dissection and has the disadvantage that hardware removal
may become necessary later, with longer surgical time and the added
risk of neurovascular injury and refracture (236,241).
management, but remodeling may be surprising and function may return to
normal despite malunion (218,222,227,242).
A 3- to 6-month period of observation is recommended when deformity is
less than 20 degrees. Shaft deformities greater than 30 degrees should
be corrected as soon as some strength and motion have been regained,
usually 2 to 3 months after the initial injury. Deformities between 20
and 30 degrees may require early correction depending on the clinical
appearance, the age of the child, and the location of the malunion.
Distal deformity in children younger than 8 years is more likely to
remodel (213). Results are better when deformity is corrected within 1 year of the initial injury (243). Intramedullary fixation is usually sufficient for younger children (244). Older children benefit from plate fixation to begin early motion after osteotomy to correct malunion (Fig. 33.44).
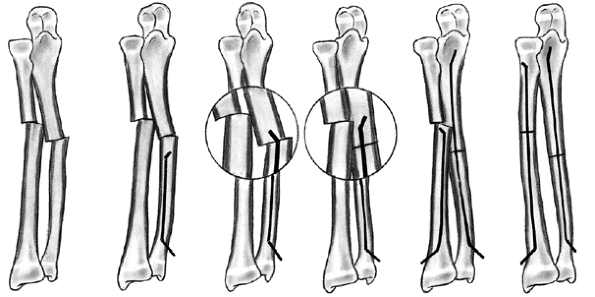 |
|
Figure 33.43
Surgical nailing procedure for flexible intramedullary nails. The ulnar wire can be introduced digitally, as shown, or through the olecranon. (From Verstreken L, Delronge G, Lamoureux J. Shaft forearm fractures in children: intramedullary nailing with immediate motion. A preliminary report. J Pediatr Orthop 1988;8:450, with permission.) |
refracture, nonunion, compartment syndrome, nerve injuries, and
synostosis. Refracture has been addressed as an indication for surgical
stabilization when alignment cannot be maintained by closed means.
Nonunion often requires surgical intervention, but long-term sequelae
are uncommon.
The risk of compartment syndrome may also be increased when closed
intramedullary fixation is difficult and tourniquet time is prolonged (238). Treatment of compartment syndrome is discussed elsewhere in this chapter.
fractures, but reports of this are uncommon. Nerve entrapment from
forearm fracture is rare (245). Management of
neurologic deficits following forearm fracture is similar to management
for deficits following supracondylar fractures.
children but may occur following closed or open management of forearm
fracture. Results of resection are better in adults than in children.
However, successful resections have been reported for nonarticular
midshaft cross-union (246,247).
common. Increased risk of distal radius fracture has been noted in
children with decreased bone mineral density and increased body mass (10,11).
Fractures of the distal radius and ulna are usually caused by a fall
onto the hand with the wrist in a pronated, extended position.
toward patient comfort and protection of the forearm from further
injury. Immobilization with a splint for 3 weeks without further
follow-up is sufficient for managing these injuries (248,249).
However, torus or unicortical fractures should be differentiated from
minimally displaced or angulated bicortical fractures because the
latter have a propensity for secondary angulation (250,251).
A well-molded cast is recommended for complete fracture. Follow-up
radiographs are recommended 1 week after complete bicortical fractures
when there is any displacement or angulation greater than 10 degrees (250,251).
 |
|
Figure 33.44 Malunion of the forearm in a 14-year-old child. A: Anteroposterior view of the alignment after closed reduction and casting. B: The lateral projection shows acceptable alignment. C: The anteroposterior projection 12 weeks after treatment shows malunion of the radius and ulna. D: A lateral projection shows dorsal angulation. Clinical evaluation demonstrated only 15 degrees of rotation of the forearm. E: An anteroposterior projection after osteotomy and internal fixation with 0.35-mm compression plates. F:
The lateral projection shows restoration of anatomic alignment. The range of motion was improved significantly, with full supination but a loss of the last 20 degrees of pronation. |
potential for remodeling because of their proximity to the distal
growth plate. Bayonet apposition with 15 degrees of angulation and 1 cm
shortening may be accepted until early adolescence (252). Friberg observed that a dorsal tilt up to 20 degrees will remodel so long as there are 2 years of growth remaining (17,253,254).
So long as the growth plate remains open, 50% of the remodeling occurs
in the first 6 months, and the remaining 50% in the next 18 months (17,253,254).
Deformity greater than 20 degrees may also remodel, but this is less
predictable, especially in older children. Remodeling capacity is
similar for dorsally angulated and palmarly angulated fractures (255).
The guidelines for acceptable residual angulation are age-dependent and
serve only as a general indicator of expected results. Immobilization
should be attempted to avoid angulation greater than 20 degrees. When
malunion occurs, dorsal tilt (apex-volar angulation) of up to 35
degrees can be accepted in children younger than 5 years. This
decreases to 25 degrees for children between 5 and 12 years of age. In
older children, the dorsal tilt should be controlled at less than 15
degrees to ensure a good outcome. Radial deviation remodels less than
angulation in the plane of flexion and extension. Therefore, radial
deviation should be kept to less than 15 degrees in children younger
than 12 years and to less than 10 degrees in older children (256,257,258).
of closed reduction and immobilization in a plaster cast. In complete
fractures, the distal fragment is usually dorsally displaced, but the
dorsal periosteum is intact. Reduction may be difficult to achieve but
should be attempted initially. The deformity is increased to relax the
intact periosteal hinge; longitudinal traction is then applied with
digital pressure at the fracture site until length is restored. The
angular deformity is then corrected. More reduction force is required
if the ulna is intact, and pronating the distal segment during the
reduction maneuver may assist in fragment realignment. After reduction,
the wrist should be
placed
in slight palmar flexion and ulnar deviation. The cast is molded with
three-point pressure dorsally over the distal fragment, centrally on
the volar surface of the forearm, and proximally on the dorsal surface
of the forearm. This cast molding technique is designed to counter the
tendency for later radial and dorsal fracture displacement. An
above-elbow splint or bivalved cast is recommended for immobilization,
but it has been demonstrated that a well-molded, below-elbow cast can
also effectively stabilize these fractures (259).
distal-third, metaphyseal fracture may be difficult to achieve by
closed manipulation. Displacement and angulation may be accepted so
long as alignment is within the described guidelines. However,
redisplacement is more common when reduction is incomplete (260).
Supination of the forearm has been recommended to reduce the pull of
the brachioradialis and thereby reduce the incidence of delayed dorsal
angulation (261). Fractures that do not
maintain their positions can be remanipulated. Percutaneous pinning has
been recommended to avoid repeated manipulation (260,262).
A randomized, controlled trial of above-elbow cast management versus
the additional insertion of a Kirschner wire determined no significant
difference in clinical outcome. However, 21% of patients in the group
managed only by cast required remanipulation, compared to none in the
group managed with pinning (263). Regardless of
method of immobilization, fracture alignment should be monitored
closely for the first few weeks after injury. It is the authors’
preference to use percutaneous pins when completely displaced distal
radius fractures cannot be satisfactorily reduced (guidelines listed
previously), or in the rare instance when an ipsilateral supracondylar
fracture makes pinning of both injuries the safest treatment (Fig. 33.45).
This can be performed by metaphyseal pinning, by transstyloid pinning
through the growth plate, or by utilizing the intrafocal leverage
technique of Kapandji (264,265,266) (Fig. 33.46).
A modified Kapandji technique for pinning Smith fractures in children
utilizes a dorsal entry site with the tip of the pin as a buttress to
prevent volar displacement (267).
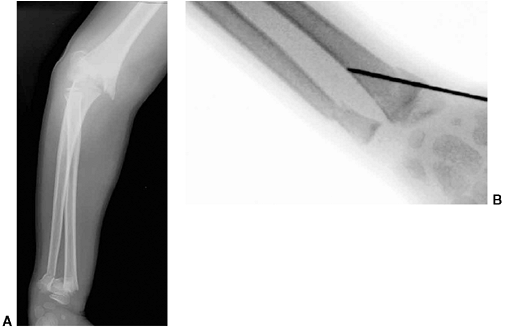 |
|
Figure 33.45 Fixation of a distal radius fracture with an ipsilateral supracondylar fracture. A:
This anteroposterior radiograph of the forearm and elbow of a 6-year-old boy shows a completely displaced distal radius fracture and a type III supracondylar humerus fracture. The arm was swollen and the hand was perfused, but the radial pulse could not be palpated. Because of the risk of forearm compartment syndrome, the surgeon elected to do closed pinning of both the supracondylar fracture and the distal radius fracture. B: Intraoperative image after K-wire fixation was used for the distal radius fracture. After pinning, the arm and elbow were splinted with the elbow in 30 degrees of flexion. |
can remodel. Cases with a dorsal tilt of 35 degrees and radial tilt of
15 degrees should be given a chance to remodel before considering
osteotomy. Galeazzi-type fracture may also be diagnosed. Galeazzi-type
fracture-dislocation should be suspected when an isolated radius
fracture is associated with fracture of the ulnar styloid (208). Distal physeal separation of the ulna is uncommon but often leads to premature physeal closure (209).
second most common physeal injury, with phalangeal fractures being the
most common (29). Distal radius physeal
fracture accounts for approximately 15% of all forearm fractures, with
70% of these injuries occurring in children older than 10 years (35,197).
Eighty percent are Salter-Harris type I or II injuries. More complex
injuries are uncommon but have higher rates of premature growth arrest.
Anatomic reduction may be required for complex physeal fractures, but
anatomic reduction is unnecessary for type I or II physeal injuries of
the distal radius because growth usually resumes and provides
remodeling. Before 10 years of age, 20 degrees of angulation and 40% of
displacement can be accepted without reduction for type II injuries (35).
and II are similar to those previously described for distal radius
metaphyseal fracture. Multiple reduction attempts may lead
to growth-plate damage. Two or more attempts have produced growth arrest in slightly more than 25% of these patients (268).
Repeat manipulation more than 10 days after injury may further damage
the growth plate because physeal healing has already commenced. Cast
immobilization can usually be discontinued 4 weeks after injury.
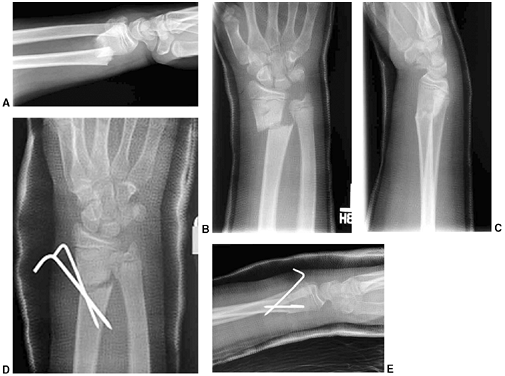 |
|
Figure 33.46 Kapandji pinning. A: Initial radiograph of a displaced distal radius and ulna fractures. B, C:
Anteroposterior and lateral view of the wrist after best attempt at reduction shows persistent angulation and displacement of the radius. The surgeon chose to do a repeat closed reduction and used the Kapandji pinning technique to reduce and fix the radius fracture. D, E: After Kapandji pinning and casting, a satisfactory reduction is maintained. |
displaced fracture is rare, and routine follow-up is not required.
However, the authors recommend follow-up 4 to 6 months after complex
distal radius and ulna physeal fracture to allow early detection of
growth arrest prior to the development of a deformity. If growth arrest
occurs, corrective lengthening osteotomy using iliac crest graft has
been successful in restoring alignment and function (269,270).
Thoracolumbar spine injuries are less common in children than in
adults, but the incidence is difficult to determine. The reported
incidence may be too low because some children with trauma severe
enough to cause spinal fracture may die from associated injuries (273).
Approximately two-thirds of thoracolumbar spine fractures in adults are
in the region of T12–L2, but the distribution of pediatric and
adolescent spine fractures is more uniform throughout the thoracic and
lumbar spine (274).
Complete examination is essential when evaluating a child with multiple
injuries because spine fractures are occasionally overlooked (275,276).
Examination may reveal tenderness, swelling, ecchymosis, or a palpable
defect posteriorly along the spinous processes. A seat belt mark across
the abdomen or injury of an abdominal organ should increase the index
of suspicion. Any loss of sensory or motor function should be
accurately documented.
more flexible than the adult spine and allows greater deformation
without fracture. This increased musculoskeletal elasticity is not
shared by the spinal cord and may lead to the occurrence of spinal cord
injury without radiographic abnormality (SCIWORA) (277).
The disproportionately large head size and other structural features in
children place the cervical and upper thoracic region at greatest risk
for spinal cord injury. Trauma to the lower thoracic or lumbar spine in
children is rarely associated with spinal cord injury. The prognosis
for recovery from incomplete neurologic injury is better in children
than in adults, but complete lesions rarely improve (271).
is suspected, but these may be difficult to interpret. A CT scan or an
MRI or both are indicated for evaluation of most patients when
thoracolumbar injuries are suspected or known to be present (276).
A CT scan is especially helpful to evaluate the bony structures.
Sagittal and coronal reconstruction can be used to evaluate alignment
and spinal canal encroachment. MRI is more useful than CT scan to
evaluate the spinal cord, intervertebral discs, and other soft tissue
structures (276). An MRI is indicated in all cases with neurologic deficit.
ossification centers, one each for the left and right sides of the
neural arch and one for the body. The junction of the arches with the
body occurs at the neurocentral synchondrosis. This junction is visible
radiographically until the age of 3 to 6 years. It lies just anterior
to the base of the pedicle and can be misinterpreted as a congenital
anomaly or fracture in younger children. Secondary centers of
ossification occur in flattened, disc-shaped epiphyses superior and
inferior to each vertebral body. These centers provide longitudinal
growth but do not cover the entire vertebral body (278).
Ossification of these growth plates at the age of 7 to 8 years creates
the radiographic impression of a groove at the corner of each vertebral
body. This groove is circumferential around the upper and lower end
plates of each vertebra. The ligaments and discs attach to this groove,
which is therefore an apophyseal ring. The ring apophysis develops its
own ossification center by the age of 12 to 15 years and fuses with the
remainder of the vertebra at skeletal maturity (279).
allows classification of adult fractures, and also has relevance for
the pediatric population. According to this theory, the thoracolumbar
spine consists of anterior, middle, and posterior columns. The anterior
column includes the anterior longitudinal ligament, the anterior half
of the vertebral body, and the anterior portion of the annular
ligament. Middle column structures are the posterior half of the
vertebral body, the posterior anulus, and the posterior longitudinal
ligament. The posterior column includes the neural arch, the ligamentum
flavum, the facet joint capsules, and the interspinous ligament. Spinal
stability is primarily dependent on the status of the middle column (281).
three-column theory to classify minor or major thoracolumbar fractures.
Minor injuries include isolated fractures of the posterior elements.
Major fractures are subdivided into compression fractures, burst
fractures, seatbelt-type injuries, and fracture-dislocations.
Compression of the anterior column is usually stable and results from
axial loading in flexion. Lateral compression fractures of the
vertebral body may also occur. Further compression results in a burst
fracture that is unstable because the middle column becomes involved.
Lap-belt injuries (Chance fractures) are unstable because they disrupt
the posterior and middle columns by flexion and distraction forces.
Fracture-dislocations usually involve all three columns and result from
various combinations of forces.
children; these include most cases of SCIWORA, posterior limbus or
apophyseal fractures, and fractures associated with child abuse.
Most thoracolumbar spine fractures in children and younger adolescents
are minor, stable, and without neurologic deficit. Simple bed rest and
gradual resumption of activities are generally sufficient for
management of these injuries. In the active athlete with an acute
fracture of the pars intraarticularis, a thoracolumbar-sacral orthosis
(TLSO) is recommended for 6 to 8 weeks in an attempt to obtain union.
Underlying causes of bone fragility, such as leukemia, should be
considered when trauma has been minimal. Multiple compression injuries
are not uncommon. Remodeling with restoration of anterior vertebral
height has been observed in children younger than 13 years (272,274).
When wedging of the thoracic or lumbar vertebra is less than 10
degrees, treatment consists of bed rest until the patient is
comfortable, then gradual resumption of activities. When wedging is
greater than 10 degrees and the Risser sign is less than 3,
immobilization in hyperextension is recommended for a period of 2
months, followed by bracing for 1 year or more (272).
Surgical stabilization is recommended when compression is greater than
15 degrees, or approximately 50% compression of the anterior vertebra,
compared to posterior vertebral height. Surgical stabilization is also
recommended when lateral compression is greater than 15 degrees (273,282). The
authors follow these guidelines, although prolonged bracing after initial treatment is usually avoided.
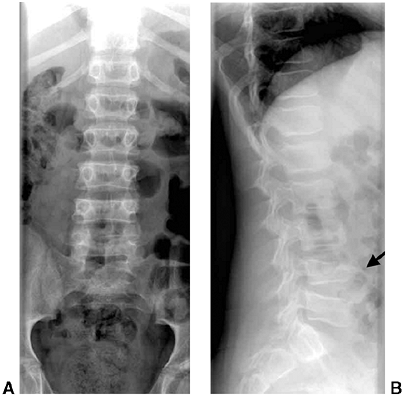 |
|
Figure 33.47 L4 compression fracture. A: These anteroposterior (A) and lateral (B)
radiographs were taken after a 13-year-old rear-seat passenger was involved in a motor vehicle accident. There is a 40% compression of L4 (indicated by black arrow). A computed tomography (CT) scan showed no evidence of a burst fracture. He was treated in a thoracolumbar-sacral orthosis (TLSO) for 8 weeks. |
those for compression fractures in children and adolescents. These
injuries may be managed nonoperatively when the posterior column is
intact, deformity is minimal, and there is no neurologic injury (272,283,284,285). However, progressive kyphosis has been noted in some patients treated nonoperatively (283).
Nonsurgical treatment usually consists of hyperextension casting for 2
to 3 months and bracing for an additional 6 to 12 months. Surgical
decompression and instrumentation are recommended for patients with
greater degrees of deformity or with neurologic compromise (284,285).
Posterior distraction and instrumentation may achieve decompression by
ligamentotaxis with reduction of the retropulsed fragments (286).
Anterior decompression has been recommended in the presence of multiple
nerve root paralysis, but the role of anterior decompression and
instrumentation remains controversial (287).
the use of lap-belt restraints, when the lap belt slides up the torso
and rests over the abdomen instead of the proximal thighs and hips (287).
The incidence of Chance fractures in children has increased since the
introduction of mandatory seat-belt laws. Fortunately, this injury has
a better prognosis in children than in adults (288). Neurologic deficits are infrequent, but intraabdominal injury is common and obscures the diagnosis of spine trauma.
consists of cast immobilization for 8 to 10 weeks when there is minimal
displacement and the fracture line goes through bone. Posterior
surgical stabilization is indicated in the presence of displacement,
neurologic deficits, or when there is a significant ligamentous
disruption. Instrumentation and fusion one level above and one level
below the fracture may be required (Fig. 33.48). In some patients one-level posterior fusion is sufficient (275,287).
This can be achieved by spinous process wiring and cast immobilization,
or by hook/rod and pedicle screw fixation in older children.
young adult and presents clinically like a herniated nucleus pulposus.
It often results from the patient’s lifting a heavy object, but may
result from falls or twisting injuries. The patient may describe a
“pop” at the time of injury, followed by radiculopathy. Delayed
diagnosis is common (289). Takata et al. (290)
described four types of growth-plate injuries to the spine.
Nonoperative management is rarely successful regardless of the type (284,289).
MRI, CT scan, or both should be used to determine the exact location
and configuration of the lesion. Surgical excision is then performed by
piecemeal excision of the limbus fragment. In order to completely
remove bony impingement, the authors recommend laminectomy with direct
exposure rather than relying on minimally invasive techniques.
uncommon unless there is an accompanying neurologic deficit. Inadequate
stabilization or late deformity may be problematic when there is an
associated neurologic deficit, or after wide laminectomy (291,292).
In the absence of spinal cord injury, remodeling is more likely in
younger children, particularly when the iliac apophysis is incompletely
ossified (Risser sign <3) (272,274).
However, remodeling can occur in older children. Spontaneous remodeling
and redevelopment of the spinal canal has been observed in adults after
burst fractures with canal encroachment (293).
End-plate injury has been correlated on MRI with disc degeneration, but
back pain is uncommon after spine fractures in children (294).
The immature pelvis is more malleable than that of an adult, largely
because of a greater component of cartilage and the greater flexibility
of adjacent joints. This allows greater energy absorption before
fracture. The flexibility of the pediatric pelvis also permits single
breaks in the
pelvic ring to occur. Avulsion fractures often result from athletic injuries (discussed in Chapter 32).
Other types of pelvic fractures in children are often the result of
high-energy trauma. Most unstable pelvic fractures are caused when a
motor vehicle strikes a pedestrian (296,297). Most pediatric pelvic fractures are stable and minimally displaced.
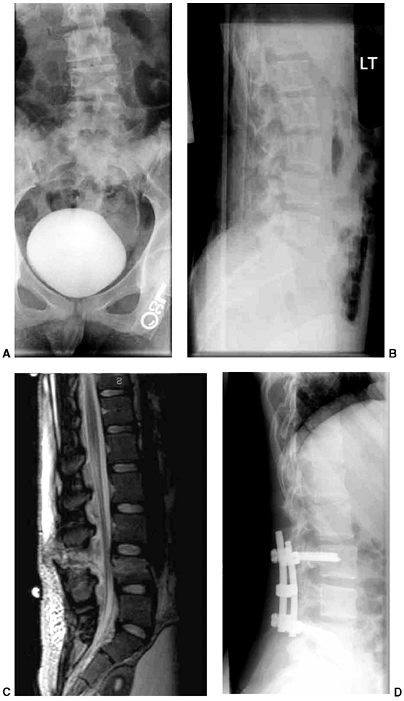 |
|
Figure 33.48 L4 Chance fracture with bony and ligamentous disruption. A, B:
These anteroposterior and lateral radiographs of the lumbosacral spine were taken upon presentation of a 14-year-old girl who was involved in a high-speed motor vehicle accident. She was a front-seat, restrained passenger. She had no neurologic deficits. An injury at the L4 level was suspected from the radiographs. C: This magnetic resonance imaging (MRI) (T2-weighted, sagittal image) shows a fracture of the L-4 vertebral body with complete posterior ligamentous disruption. All posterior tissues were disrupted except the skin and superficial subcutaneous tissue. On the basis of the extent of the posterior ligamentous injury, the surgeon elected to perform a posterior instrumented spinal fusion from L3 to L5. D: This lateral radiograph taken 6 months after surgery shows maintenance of alignment at the fracture site and signs of early fusion. |
These associated injuries include head injuries, intraabdominal trauma,
urologic disruptions, and fractures. Mortality rates are lower in
children with pelvic injury than in adults, but death occurs in 3% to
5% of children with juvenile pelvic trauma (295,296,297).
Death is most frequently related to head injury, but exsanguination
from fractures or visceral injuries can occur, and the risks of
hemorrhage and associated visceral injuries correlate with fracture
patterns. Patients with bilateral anterior and posterior
fractures
are at greatest risk, whereas isolated pubic ramus fractures have the
lowest risk of hemorrhage and intraabdominal injury (298,299).
associated injuries, including neurologic deficits. Any laceration
should be inspected to determine whether an open fracture has occurred.
Rectal examination is indicated to look for hemorrhage signifying bone
penetration into the rectum and to verify intact perineal sensation
(i.e., sacral plexus function). Pelvic stability should be tested with
anterior and lateral compression of the pelvis. Peripheral arterial
circulation should also be noted. Plain radiographs are useful for
screening but may be difficult to interpret. Pelvic inlet (40 degrees
caudal), outlet (40 degrees cephalad), and Judet (45 degrees oblique)
views can help define the fracture pattern and the potential
involvement of the acetabulum. However, these views have largely been
replaced with CT scan, with or without three-dimensional reconstruction.
the ischium, the pubis, and the ilium. These come together at the
acetabulum to form the triradiate cartilage. Secondary ossification
centers can be confused with fractures. These appear at the apophyses
in patients between 13 and 16 years of age. The apophyses that are
principally associated with avulsion injuries are located on the
ischial tuberosity, the anterior inferior iliac spine, and the anterior
iliac crest. Secondary centers of ossification can also develop along
the pubis and the ischial spine. Several other normal variants can also
be confused with fractures. An area of particular confusion is at the
junction of the inferior pubic ramus and the ischium. Before
ossification, this junction can have the appearance of a fracture,
especially when ossification is asymmetric. A swelling may also occur
in this area and can simply be observed when asymptomatic.
Plain radiographs allow reliable determination of fracture types,
although CT scanning may be helpful in questionable cases or when
surgical intervention is anticipated (301). The authors prefer the classification proposed by Watts (302):
-
Avulsions
-
Fractures of the pelvic ring (stable and unstable)
-
Fractures of the acetabulum
It should be noted that, because of the elasticity of the child’s
pelvis, diastasis of the pubic symphysis can occur in children without
instability of the sacroiliac joint posteriorly. In young children,
this fracture usually represents separation at the bone-cartilage
junction rather than joint disruption.
 |
|
Figure 33.49
This anteroposterior pelvis radiograph shows typical bilateral pubic rami fractures in a 6-year-old boy hit by a car. He was permitted weight-bearing activities as tolerated with crutches immediately after injury. |
vertical plane but unstable in the transverse plane. Mechanisms include
lateral compression causing, for instance, pubic and ischial ramus
fractures with contralateral sacral fracture.
Alternatively, anterior compression may cause an “open-book” type of injury with pubic diastasis.
 |
|
Figure 33.50
This anteroposterior pelvis radiograph of a 4-year-old child shows a left iliac wing fracture and pubic rami fractures. He was permitted weight-bearing activities as tolerated when comfortable. |
(straddle injuries), which rarely displace in children; vertical shear
fractures through the ipsilateral anterior and posterior pelvic rings;
and anterior ring fractures with acetabular disruption.
result with a minimum of treatment. Stable fractures are managed by bed
rest with gradual resumption of weight bearing as tolerated. Unstable
pelvic fractures with minimal displacement, which do not involve the
acetabulum, can be managed with prolonged bed rest (1 to 2 months). Up
to 3 cm displacement may be acceptable in children younger than 10
years, but the acceptable amount of displacement in all age-groups is
controversial (304). Careful observation with
frequent radiographs is recommended, so that any additional
displacement will be recognized and treated. Union and remodeling occur
reliably in younger children in spite of moderate displacement (305,306).
Unstable pelvic fractures with greater amounts of displacement,
especially in older children, should be reduced with traction or open
reduction. The authors recommend reduction of pubic diastasis
(open-book) fractures when the diastasis exceeds 3 cm, or when
stability is required for urologic reconstruction. Stabilization after
reduction can be achieved with external and/or internal fixation for
unstable open-book or other type B fractures (300,303) (Fig. 33.51).
Type C fractures may require internal fixation of posterior injury, in
addition to anterior stabilization with external fixation or other
means. Surgical stabilization is also appropriate to reduce
retroperitoneal blood loss and to facilitate mobilization of the child
who has multiple injuries.
Precise restoration of joint congruity is recommended except for stable
posterior fracture-dislocations with concentric reductions (Watts type
I fractures). Nondisplaced acetabular fractures are managed by closed
reduction
(Watts
type II fractures). Multiple fragments with instability (Watts type
III) are best managed by open reduction with stable internal fixation (303,307). Early motion is also recommended (303).
Comminution is often less severe in children than in adults, and the
results of surgical management are generally satisfactory. Fractures
with central dislocation of the hip have a poor prognosis regardless of
surgical or nonsurgical management. Traction may be utilized for these
fractures, or surgical management may be attempted when comminution is
minimal. Poor results after anatomic reduction may still occur with all
types of acetabular fractures due to the magnitude of the initial
trauma (308).
 |
|
Figure 33.51 A rare unstable pediatric pelvic fracture requiring internal fixation. A: This pelvic radiograph shows widening of the right sacroiliac joint. B: This computed tomography (CT) scan cut confirms disruption of the right sacroiliac joint. C: This unstable pelvis was treated with iliac bolt fixation.
|
Fractures that cause premature closure are usually nondisplaced and do
not require open reduction. Children younger than 10 years are at the
greatest risk for this complication. Disturbance in growth leads to the
development of a shallow acetabulum because the triradiate cartilage is
responsible for growth in the height and width of the acetabulum. Hip
subluxation may follow, necessitating redirectional pelvic osteotomy.
injuries can be challenging, but long-term complications are rarely
attributable to the bony structures. Untreated, severely displaced
fractures can result in a limp, rotational deformity, or limb-length
discrepancy. Acetabular injuries are at risk for developing traumatic
arthritis in spite of anatomic reduction. Premature closure of the
triradiate cartilage can be problematic in younger children. However,
residual morbidity is more often due to associated injuries, especially
traumatic brain injury.
 |
|
Figure 33.52 Traumatic hip dislocation in a 3-year-old boy. A:
This anteroposterior pelvis radiograph was taken upon presentation of a 3-year-old boy who twisted his leg while running down the stairs. B: This anteroposterior pelvis radiograph was taken immediately after closed reduction in the emergency room. On long-term follow-up, there was no avascular necrosis or any other sequelae. |
representing only 5% of all pediatric dislocations. Most hip
dislocations are posterior, but anterior and obturator dislocations can
occur (310,311). The
mechanism of injury depends somewhat on the age of the child. Hip
dislocations in children younger than 10 years are frequently the
result of mild trauma because joint laxity is common and the acetabulum
is largely cartilaginous (Fig. 33.52) (310).
Dislocations in children older than 10 years are more often the result
of moderate or severe trauma. Dislocation from moderate trauma may
result in spontaneous, incongruous reduction and capsular interposition
(312) (Fig. 33.53).
This is rare but easily misdiagnosed in children and adolescents. Any
suggestion of joint-space widening should be investigated with a CT
scan.
muscle relaxation and analgesia. Early closed reduction within 6 hours
of injury is recommended. Reduction is rarely difficult in children,
but gentle reduction is especially important in the adolescent age
group. Reduction with image guidance should be considered in the
adolescent age group because occult epiphyseal injury may be present,
and epiphyseal separation can occur during attempted reduction. The
reduction technique for posterior dislocation requires hip and knee
flexion, usually with adduction of the hip. Longitudinal traction is
then applied while an assistant stabilizes the pelvis. The limb is then
extended, internally rotated, and abducted. After the hip is reduced,
the stable arc of motion should be assessed. Postreduction
radiographs
should include the opposite hip to confirm a concentric reduction with
symmetrical joint spaces. A CT scan is recommended after hip reduction
in children older than 10 years or in younger children if instability
or joint-space widening is noted. A CT scan is not necessary in the
younger child with a stable concentric reduction and no evidence of
acetabular fracture on plain radiographs. The child younger than 8
years should be immobilized in a spica cast or abduction pillow with
strict bed rest for 4 to 6 weeks to reduce the risk of recurrent
dislocation. Stable, closed reductions in older children can be managed
with activity restriction and decreased weight bearing for 6 weeks to
allow capsular healing and reduction of posttraumatic inflammatory
response.
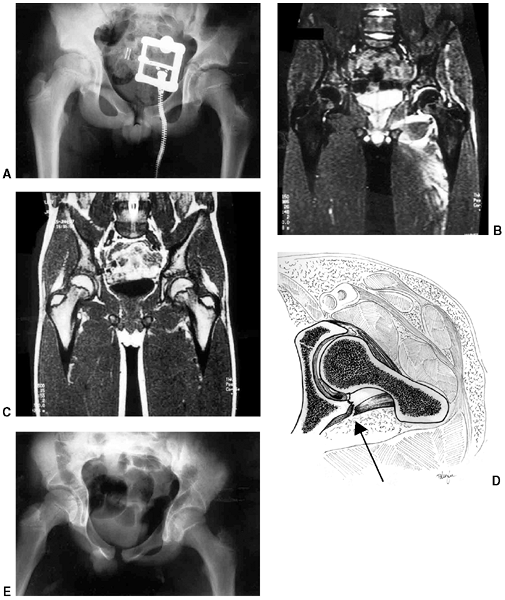 |
|
Figure 33.53 Hip dislocation with entrapped tissue. A:
Initial anteroposterior pelvis radiograph of a 15-year-old boy following an injury to the left hip in a soccer game. Joint asymmetry was attributed to a joint effusion. B, C: A magnetic resonance imaging (MRI) performed 4 days after the injury shows a joint effusion and mild incongruity. Because of persistent symptoms and concerns about interposed tissue, he was taken to the operating room and an arthrotomy was performed through a posterior approach. D: This drawing illustrates the intraoperative findings. There was an inverted labrum and capsule trapped in the hip joint (arrow). Once this was freed, complete hip joint reduction was achieved. E: Anteroposterior pelvis radiograph after surgery shows concentric reduction. The boy was followed up for more than 3 years, and there was no evidence of avascular necrosis or other late sequelae. (From Price CT, Pyevich MT, Knapp DR, et al. Traumatic hip dislocation with spontaneous incomplete reduction: a diagnostic trap. J Orthop Trauma 2002;16:730–735, with permission.) |
closed reduction, nonconcentric reduction, bone or soft tissue
fragments within the joint, or a large acetabular rim fragment with
instability. Open reduction is recommended from the direction of the
dislocation, such as the posterior interval (i.e., Kocher-Langenbeck
approach) for posterior dislocations. Postoperative management after
open reduction is the same as that after closed reduction.
is 3% to 10%, approximately half that in adults. The risk of avascular
necrosis is diminished when reduction is achieved within 6 hours of
injury (310,311). Nerve
injury has been reported in 5% of children with hip dislocation, but
recovery occurs spontaneously in 60% to 70% of these patients (313).
The long-term prognosis for arthritis is related to the severity of the
trauma, associated fractures, and treatment delays beyond 24 hours.
However, some satisfactory results have been reported when neglected
traumatic dislocations have been treated by traction and open reduction
(314). Coxa magna in the absence of avascular
necrosis has been observed in many children after traumatic hip
dislocation. The cause is probably reactive hyperemia
secondary to extensive soft tissue injury. Coxa magna does not seem to influence clinical outcome.
In contrast to adult hip fractures, pediatric and adolescent hip
fractures are usually the result of high-energy trauma, because
considerable force is required to produce a fracture in this age group.
The exceptions to this are infants who have been subjected to child
abuse, and fracture through a pathologic lesion of the femoral neck
(e.g., bone cyst). Complications are frequent and have been reported in
15% to 60% of patients (315,316). Prompt and appropriate management may reduce the risk of subsequent complications.
The medial portion becomes the epiphyseal center of the femoral head,
ossifies at around 4 months of age, and forms the proximal femoral
physis. The lateral portion of the proximal femur forms the greater
trochanter physis, with ossification of the epiphysis by 4 years of
age. Injury to the proximal femur can affect one or both of these
centers of growth. The proximal femoral physis is responsible for the
metaphyseal growth of the femoral neck and provides approximately 15%
of the total length of the femur. The greater trochanter helps shape
the proximal femur, and damage to this apophy-sis in children younger
than 8 to 10 years may produce an elongated, valgus femoral neck (318,319).
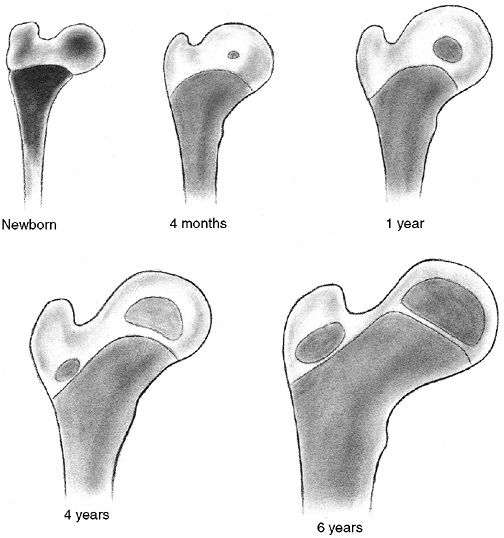 |
|
Figure 33.54
Development of the growth centers in the proximal femur. (From Edgren W. Coxa plana: a clinical and radiological investigation with particular reference to the importance of the metaphyseal changes for the final shape of the proximal part of the femur. Acta Orthop Scand 1965;84:24, with permission.) |
femur is jeopardized by these fractures, and the extent of damage
greatly affects the final outcome. The dominant arterial source for the
femoral head is the lateral epiphyseal vessels, which are the terminal
extension of the medial femoral circumflex artery. These
posterosuperior and posteroinferior vessels are found at the level of
the intertrochanteric groove, where they penetrate the capsule and
course along the femoral neck toward the head (320,321) (Fig. 33.55).
The lateral circumflex system can supply blood to a portion of the
anterior femoral head until 2 to 3 years of age, after which it
primarily supplies the metaphysis. In children older than 14 to 18
months, the proximal femoral physeal plate becomes an absolute barrier
to the metaphyseal blood supply and prevents direct vascular
penetration of the femoral head (320,321).
Thus, the epiphyseal and metaphyseal circulation remain separate until
complete physeal closure occurs. The vessels of the ligamentum teres do
not contribute a significant portion of the blood supply to the femoral
head, especially in children younger than 8 years.
the vascular leash intact but kinked and occluded until realignment is
established (322). This has been demonstrated by arteriography before and after reduction of an unstable slipped capital femoral epiphysis (323). Vascular
disruption as a cause of avascular necrosis is supported by the fact
that the magnitude of displacement is a prognostic factor for the
development of necrosis (324).
It has also been suggested that prompt decompression of the
intracapsular hematoma contributes to the restoration of normal
vascular flow and reduces the incidence of femoral head necrosis (325,326,327,328).
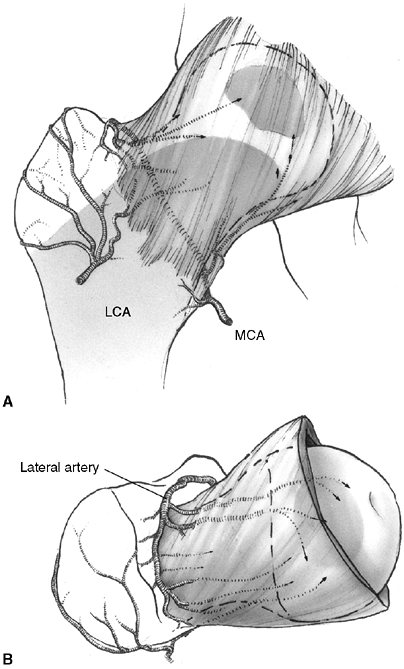 |
|
Figure 33.55 Arterial supply of the developing proximal femur. A: The anterior view demonstrates the lateral circumflex artery (LCA), which supplies the metaphysis and greater trochanter. The medial circumflex femoral artery (MCA) is the dominant vessel to the femoral head. B: The superior view shows the lateral ascending artery, which sends numerous epiphyseal and metaphyseal branches (arrows)
that supply the greatest volume to the femoral head and neck. These ascending cervical branches traverse the articular capsule as the retinacular arteries. The interval between the greater trochanter and the hip capsule is extremely narrow, and is the area where the lateral ascending cervical artery passes. This may be a site of vascular compression or injury. |
confirmed high intracapsular pressures with decreased blood flow on
bone scan. Following aspiration and fixation, repeat bone scans
demonstrated restoration of blood flow (329). Other studies have also reported high intracapsular pressures that are reduced by joint decompression (329,330,331).
Soto-Hall et al., in 1964, noted that intraarticular pressures
increased when intracapsular hip fractures were manipulated by placing
the leg in internal rotation and extension (332). The increased joint pressure during reduction of fractures in this position has been confirmed by other authors (329,331).
Therefore, it is the present authors’ opinion that reduction of
intracapsular fractures may improve vascularity by restoring normal
arterial position. However, reduction may also lead to increased
intracapsular pressure unless the hip is decompressed. Prompt
reduction, internal fixation, and decompression are recommended in
order to restore circulation in a timely manner. This approach to the
management of hip fractures and unstable slipped capital femoral
epiphyses in children has been associated with a decreased risk of
avascular necrosis (325,327,328,333).
Type I fractures are transphyseal separations. Physeal separation in
infants is occasionally seen as a birth fracture or as a result of
intentionally inflicted injury. Obstetric fracture-separations have
excellent clinical results, without avascular necrosis, although
diagnosis and treatment may be delayed (335). Children younger than 2 years with type I fracture also have a good prognosis without surgical management (336).
Transepiphyseal separations in older children result from more severe
trauma, but separation has been reported during reduction of hip
dislocation in the adolescent age group (337).
When the epiphyseal fragment is dislocated from the acetabulum, the
risk of avascular necrosis approaches 100%. However, the incidence of
avascular necrosis is variable when the femoral head remains within the
joint (334).
the epiphyseal plate and the base of the neck. They constitute
approximately 50% of all fractures of the proximal femur (334).
Complications are frequent with type II fractures. The incidence of
avascular necrosis approaches 50% to 60%, and the nonunion rate is 15%.
Premature physeal closure may also occur, but because growth of the
proximal femur is approximately 15% of the total limb (338), clinically important leg-length discrepancy is unlikely to occur in older children.
basal neck, region of the femoral neck. This is the second most common
type of hip fracture in children. Avascular necrosis occurs in 30% of
displaced fractures. Malunion has been reported in 20%, and nonunion
occurs in 10%, of these patients. These problems may be lessened by
precise fracture reduction, combined with compression across the
fracture site by means of cancellous bone screws (i.e., lag technique) (334,339).
and are associated with the least risk of damage to the femoral head
vascular supply. The incidence of avascular
necrosis
is between 0 and 10%. Varus deformity is the most likely complication,
but this may correct with growth in younger children (334,339,340).
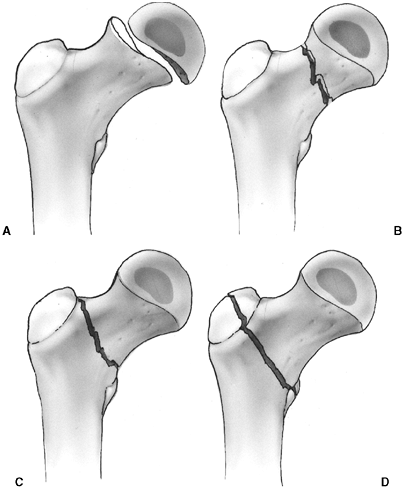 |
|
Figure 33.56 Delbet’s classification for proximal femur fractures. A: Type I is a transepiphyseal fracture. B: Type II is a transcervical fracture. C: Type III is a cervicotrochanteric fracture (basicervical). D: Type IV is an intertrochanteric fracture.
|
cases. The risk of avascular necrosis may be minimized with reduction,
joint decompression, and stable fixation within 24 hours of injury (316,326,328,341).
It is possible that treatment within 6 hours, as is recommended for hip
dislocations, would further decrease the incidence of avascular
necrosis, but there are no current reports of reduction and
decompression within 6 hours. Delay in treatment may be necessary
because of associated injuries or other considerations.
appropriate for minimally displaced fractures, and for children younger
than 2 years (342). In children aged 2 to 12
years, stabilization of the reduced fracture may be accomplished with
two smooth pins supplemented with spica casting. In older children,
fixation across the physis is recommended. Open reduction is often
necessary if the epiphysis is dislocated. This is performed through a
posterior approach for posterior fracture-dislocations. At the time of
open reduction, curettage of the physeal plate has been recommended in
an attempt to encourage revascularization of the femoral head (337).
and the patient is younger than 6 years, a spica cast alone can yield
good results (343). Displaced fractures can
usually be reduced by closed methods, but a small incision to open the
hip capsule is recommended because this could decrease the risk of
avascular necrosis (325,326,327,328). Ng and Cole (326)
studied the effect of early hip decompression on the frequency of
avascular necrosis. It had no apparent value in type I fractures. For
the type II and type III fractures, 41% of 54 patients treated without
hip decompression developed avascular necrosis whereas only 8% of 39
patients with hip compression developed avascular necrosis. As
previously discussed, prompt reduction, internal fixation, and
decompression are recommended by the present authors in an attempt to
restore circulation in a timely manner. Fixation is achieved by the
percutaneous insertion of two or three cannulated bone screws into the
metaphyseal portion of the proximal fragment (Fig. 33.57).
If the proximal metaphyseal fragment is too small for secure fixation,
smooth pins can be placed across the physis to allow subsequent growth.
Stable fixation of the fracture should be given priority over
preservation of the proximal femoral physis (334).
Spica cast immobilization is used to augment fixation in children,
especially when smooth pins have been used. In patients aged 12 years
or older, threaded screws may be placed across the physis for better
fixation and to avoid the use of a spica cast. Alternatively, a hip
screw with a supplemental pin to control rotation may be used in older
children. Caution is advised when using compression hip screws in
children, because dense bone may generate heat necrosis of the femoral
neck during reaming. An additional smooth pin is recommended to improve
rotational stability (Fig. 33.58). Open
reduction is occasionally required if suitable alignment is not
obtained by closed means. An anterolateral (Watson-Jones) approach is
recommended for type II fractures.
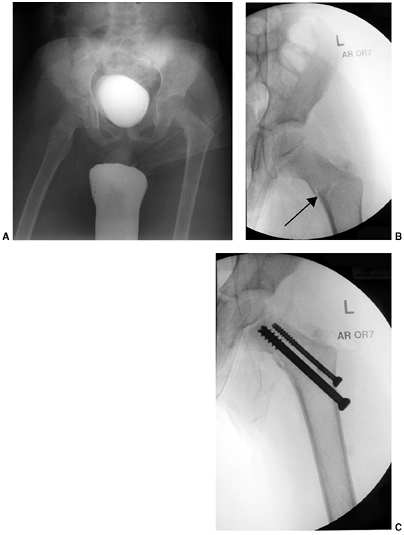 |
|
Figure 33.57 Hip fracture in an 8-year-old boy treated with internal fixation. A:
Initial radiograph of an 8-year-old boy who fell approximately 50 ft from a ski lift, sustaining a left femoral neck fracture and a pneumothorax. A Delbet type C femoral neck fracture was diagnosed. The surgeon also appreciated that the proximal femur was in varus. B: This anteroposterior intraoperative radiograph was taken immediately after closed reduction of the fracture (arrow). The neck shaft angle was corrected. C: Two screws (7.3 mm and 0.45 mm) were used for internal fixation. In this patient, there was enough room between the fracture and the growth plate, and the physis did not have to be crossed. After fixation, the child was protected in a spica cast for 6 weeks. |
except when surgical fixation improves general management. Nondisplaced
fractures in this region can be managed by spica cast immobilization
and close follow-up in younger children. Displaced fractures in infants
and toddlers may be treated with early closed reduction and casting so
long as the neck-shaft angle does not decrease to less than 115
degrees. Displaced fractures in older children
can
also be managed by skeletal traction followed by cast immobilization.
However, the authors recommend surgical stabilization in children older
than 6 years to reduce the risk of malunion and avoid prolonged
immobilization. Interfragmentary screws may provide sufficient
stability when combined with cast immobilization, but a pediatric-sized
hip screw with side plate, or an angled blade plate, is preferred. The
physis should be avoided. The femoral neck should be stabilized before
reaming, and reaming should be performed slowly to avoid heat necrosis
of the cortical bone. Adolescents are treated in the same manner as
adults, with stable fixation across the physis using a sliding hip
screw or angled blade plate. This avoids the need for a supplemental
spica cast for adolescent patients.
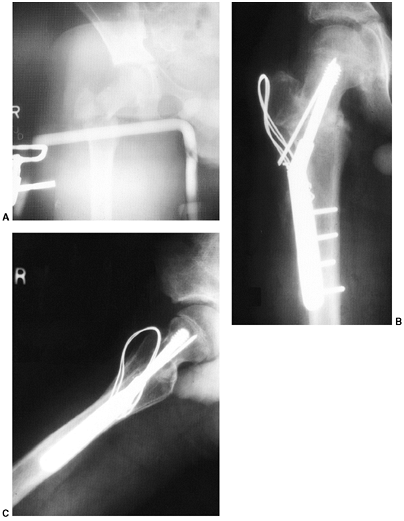 |
|
Figure 33.58 Comminuted type III femoral neck fracture. A: The anteroposterior projection shows a cervicotrochanteric fracture with an associated fracture of the greater trochanter. B, C:
Open reduction with internal fixation was necessary. Adolescent-sized lag screw fixation was supplemented with a smooth pin for rotational stability. The trochanter may be stabilized with Kirschner wires instead of a tension band. Spica cast immobilization is indicated when fixation does not cross the growth plate. |
proximal femur in children are osteonecrosis of the femoral head,
malunion, and nonunion. Other complications include infections,
premature closure of the proximal femoral growth plate, and
chondrolysis. Exact complication rates are difficult to determine
because of changing patterns of treatment. Prompt, accurate reduction,
joint decompression, and appropriate internal fixation and
immobilization reduce all of these complications (316,326).
the femoral head, just the portion of the femoral neck between the
fracture and the physis, or the entire femoral neck and head (344). Necrosis of the femoral head occurs
in approximately 30% of all hip fractures in children and often leads to poor results (334). Diagnosis of avascularity can be reliably determined by MRI as early as 2 weeks following the fracture (345),
or by isotope bone scan 4 months after the fracture. Children with
osteonecrosis who are younger than 12 years can be treated with
containment, with or without prolonged non—weight-bearing activities.
These patients have a possibility of recovering satisfactory function (346,347).
The outcome for older children with osteonecrosis of the femoral head
is poor. The authors recommend treating adolescents in a manner similar
to that for adults, with early detection and core decompression before
the occurrence of subchondral fracture (348).
Treatment is recommended as soon as the diagnosis is established.
Subtrochanteric valgus osteotomy is preferred, with bone grafting,
internal fixation, and application of a spica cast (334,349).
Supplemental vascularized bone grafting with a vascular-pedicle graft
from the iliac crest should be considered when there is a large defect
in the femoral head or neck (350).
reported patients, but this complication has a lower incidence when
internal fixation is used (334). Remodeling may occur in younger patients (340). Subtrochanteric osteotomy is recommended for persistent deformity.
Boys sustain this injury 0.25 times more often than girls. There is a
bimodal age distribution, with a peak incidence at 2 to 3 years of age
and another peak in adolescence. The cortical thickness of the femur
increases rapidly after 5 years of age, and this may explain the
decreasing incidence of femur fracture in late childhood. Intentional
injury should always be considered in young children, but there are no
distinguishing clinical parameters or fracture patterns to help
determine which injuries are inflicted and which are accidental (351).
In infants younger than 1 year, child abuse has been identified as a
cause in 65% of patients when obvious causes such as motor vehicle
accidents are eliminated (351). In children
aged 1 to 5 years, the incidence of abuse decreases. Children in this
toddler age group may sustain fractures with relatively minor trauma
from causes such as falling from a low height or tripping while
running. In the 4- to 7-year age group, approximately half of the
femoral shaft fractures are caused by bicycle accidents. In the
adolescent age group, motor vehicle accidents account for most of the
femur fractures.
“It does not require a physician to diagnose a fractured femur.”
However, the physician must carefully examine the patient completely.
Children and adolescents with femur fracture have a 35% to 40%
incidence of associated injuries. Some of these injuries are occult,
such as femoral neck fracture, hip dislocation, ligamentous instability
of the knee, and visceral injuries (352,353).
Hemodynamic instability or steadily declining hematocrit does not occur
because of an isolated, closed femur fracture. Other sources of blood
loss must be sought in these patients (353).
available for femur fractures in children and adolescents. Each of
these options can yield satisfactory results when used properly (354).
The surgeon managing femoral fractures in children and adolescents is
expected to select and perform the technique that is most appropriate
under a variety of circumstances.
the principal determinants of management. Younger children are less
likely to require surgical stabilization or prolonged traction.
High-energy trauma is more likely to require surgical intervention or
prolonged immobilization. At one end of the spectrum there are
low-energy injuries in young children that are managed with closed
reduction and immediate spica casting (Fig. 33.59).
At the other end of the spectrum, high-energy injuries in adolescents
are managed with early surgical stabilization. Occasionally, however,
infants with severe trauma require surgical stabilization of femoral
fractures to facilitate management.
to control, but residual deformity is better tolerated because of
multidirectional hip motion. Deformity is also less obvious with
proximal fractures because thick thigh muscles hide residual
angulation. Proximal fragments tend to flex, abduct, and externally
rotate because of the unopposed action of the iliopsoas, hip abductor,
and external rotator muscles. The proximal fragment of a midshaft
fracture also tends to flex, abduct, and externally rotate, but the
deformity is less extreme because of adductor and hamstring attachments
to the proximal fragment. More distal fragments are easier to control
and produce little proximal fragment angulation, except for
supracondylar fractures, which tend to hyperextend because of the
posterior pull of the gastrocnemius muscle on the short distal
fragment. Fractures of the distal femur require more precise alignment
because the deformity is more visible and remodeling in the coronal
plane is limited. Supracondylar fractures of the femur require
management that is similar to that for distal femoral physeal
separations (355).
approximately 10 years of age in girls and 12 years of age in boys.
Long-term studies have demonstrated that up to
25 degrees of midshaft angulation in any plane can be expected to correct satisfactorily in children younger than 13 years (16).
Remodeling continues for up to 5 years following fracture.
Approximately 25% of remodeling occurs at the fracture site, whereas
75% is attributed to physeal reorientation and longitudinal growth (15,19).
Fracture translation may also contribute to realignment of the
mechanical axis. Rotational remodeling may also occur, but the precise
amount is unpredictable (18,356). Clinically significant rotational deformity is uncommon, even when failure of remodeling has been documented (357,358).
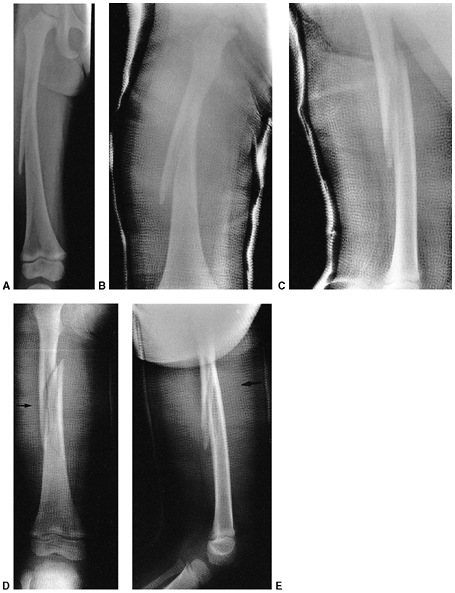 |
|
Figure 33.59 Spiral fracture of the femoral shaft in a 4-year-old child. A:
The anteroposterior projection shows some initial varus angulation. The child was placed in an early fit spica cast after closed reduction. B: One week later, the fracture has drifted into varus alignment. The cast is not molded correctly to prevent this tendency. C: The lateral projection shows adequate alignment and an acceptable amount of shortening. D: Anteroposterior projection after application of a new cast. The lateral aspect of the cast is molded to correct the varus alignment (arrow). E: The lateral projection shows the flat anterior mold. There is a tendency for these fractures to angulate anteriorly, and it is important to have a flat anterior mold proximal to the fracture (arrow). |
Unfortunately, most authors reporting the overgrowth phenomenon do not
distinguish between “catch-up growth,” which compensates for fracture
overriding, and true overgrowth, which results in the fractured femur
being longer than normal. Growth stimulation is greater when overriding
is greater and may continue for 5 years after fracture. Average total
growth stimulation is approximately 1 cm. In a prospective study of
children younger than 12 years, Hougaard observed that all fractures of
the femur healing with 3 cm or less discrepancy with the
contralateral leg spontaneously recovered to less than 2 cm discrepancy (20).
Therefore, any femoral fracture that heals in a shortened position
should be observed for several years to determine the final outcome (Fig. 33.60).
True overgrowth has been reported after reduction with internal
fixation, but the risk of overgrowth in this circumstance has not been
clearly defined (354). Parents are usually more
understanding when the injured femur grows longer than when the femur
is allowed to heal in a markedly shortened position.
choices. Age is used here with the understanding that the children are
of average weight and maturity.
fractures because of birth trauma or abuse. Osteogenesis imperfecta and
other metabolic disorders should also be suspected. Thick periosteum
and rapid hematoma consolidation usually prevent worrisome shortening
or angulation. Healing is rapid, and remodeling potential is great. One
can accept 3 cm of shortening and 30 degrees of angulation in
both
the coronal and sagittal planes. Infants may be treated with
application of a Pavlik harness (best if the infant is 6 months old or
younger) or a conventional spica cast (360).
Immobilization for 2 to 3 weeks is usually sufficient for infants less
than 4 months, and 4 to 6 weeks for infants aged 6 to 12 months.
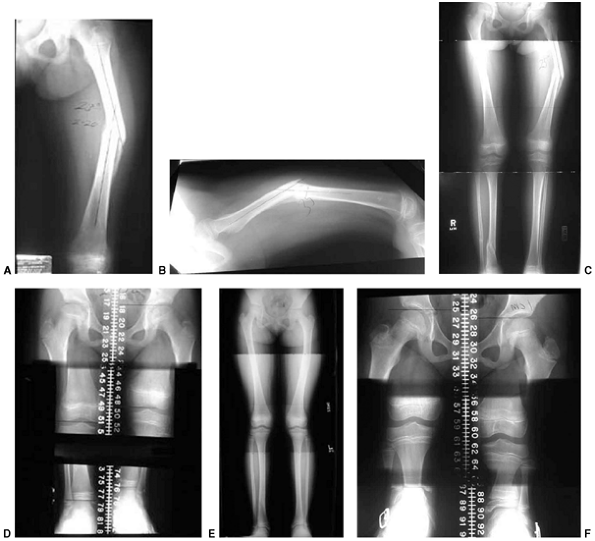 |
|
Figure 33.60 Femoral shaft fracture in a 7-year-old child with varus and flexion malunion. A, B:
Anteroposterior and lateral radiographs taken 10 weeks after femoral shaft fracture in a 7 + 4-old girl. There are 23 degrees of varus malalignment and 35 degrees of flexion. C, D: Full-length standing film and scanogram document lower-extremity alignment and a 2-cm leg-length inequality. E, F: Twenty months later, remodeling has nearly eliminated the deformity, and the scanogram shows 1 cm discrepancy. |
1 and 6 years are usually treated with early spica cast application.
All aspects of spica cast treatment are easier for preschool children
than for older children (361). Children in this
age group also heal rapidly, thus immobilization time is brief. The
spica cast may be applied in the emergency department under conscious
sedation. Alternatively, splinting is used for comfort until a spica
cast can be applied within 48 hours after injury. Alignment in the cast
should be as close to normal as possible, but full functional recovery
can be expected in this age group if shortening at the time of union is
no greater than 3 cm and angulation is less than 20 degrees in any
plane. Fractures in the distal third should be angulated no more than
15 degrees.
managed by a wide variety of methods, depending on the severity of the
fracture and social circumstances. Low-energy injuries without severe
displacement or shortening may be managed by early spica cast
immobilization (362,363).
However, children in the 6- to 10-year age group require a longer
period of immobilization than do younger children. This immobilization
and dependency may pose a problem if both parents are working or if the
child lives with a single working parent. Surgical stabilization may be
beneficial in these and similar social circumstances. High-energy
fractures with severe displacement, comminution, or shortening greater
than 3 cm require maintenance of length in addition to alignment. This
may be achieved by incorporating a traction pin in the spica cast or by
traction (Fig. 33.61) for 2 to 3 weeks before
cast immobilization to keep the fracture aligned and out to length
during the period of early callus formation (364,365,366).
Alternatively, surgical stabilization with internal or external
fixation achieves the same objectives and allows early mobilization.
Although it has been demonstrated that up to 25 degrees of angulation
in any plane will remodel in this age group (17),
recommended guidelines for alignment in this age group are up to 15
degrees varus or valgus, 15 degrees of anterior and/or posterior
angulation, and up to 20 mm of shortening.
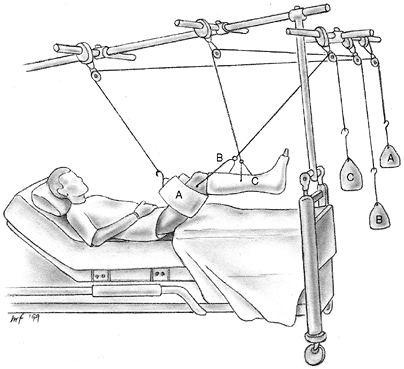 |
|
Figure 33.61
Balanced traction with a distal femoral pin, a thigh sling, and cast suspension of the leg. This is easily converted to 90-90 traction for more proximal fractures. Weight A supports the thigh, weight B provides traction, and weight C supports the leg in a cast. |
Accurate restoration of length and alignment is desirable in older
children because of limited remodeling potential. No more than 10
degrees of varus or valgus, 10 degrees of anterior or posterior
angulation, and 15 mm of shortening should be accepted in this age
group. Surgical stabilization permits early mobilization and return to
school and social activities. Various techniques for stabilization are
discussed in the following text. It is generally agreed that rigid,
reamed intramedullary nailing through a piriformis fossa should be
avoided
because of the risk of iatrogenic avascular necrosis of the femoral head (367). The risk of this complication is present as long as the proximal femoral physis remains open.
child younger than 6 years. Children up to 10 years of age may also be
treated by early spica casting for low-energy, closed femur fractures
that have less than 2 cm overriding at rest (362,363,368).
Under anesthesia, gentle longitudinal compression may be applied to
verify that the fracture does not shorten more than 3 cm (telescope
test) (362). If the fracture shortens more than
3 cm, then surgical stabilization, use of traction, or pins and plaster
may be more appropriate than early spica casting. After fracture
alignment is obtained, the popliteal area and all bony prominences
should be well padded prior to cast application. The authors recommend
the 90-90 position with gentle traction on the leg while the hip and
knee are held in slight abduction with 90 degrees of flexion at the hip
and knee (369,370).
Care must be taken to avoid excessive traction in order to avoid
peroneal nerve palsy and posterior compartment syndrome of the leg (371,372).
Applying the long leg portion of the cast as an initial step may
facilitate cast application and maintenance of alignment. After
casting, patients are usually discharged within 24 hours. Follow-up
visits to monitor alignment are recommended at 1 and 2 weeks after
reduction. It is often helpful to tape a paper clip or other small
metallic object outside the cast before radiographic examination. This
can be used to identify the fracture site if wedging of the cast is
required.
instituted form of management for almost any femur fracture in any age
group. However, skin traction is sufficient for children of average
weight (< 80lb) who are younger than 8 years (364).
Traction and casting are rarely the treatment of choice today, however,
because excellent results can be obtained from early spica casts in
children younger than 6 years, or internal and external fixation in the
older child.
The simplest is the 90-90 position for preadolescents and for managing
proximal femur fractures. Adolescents are usually placed in balanced
traction with a thigh sling and adjustable knee support to allow 45
degrees of knee flexion. Skeletal traction entails insertion of a pin
into the metaphysis of the distal femur, using a medial-to-lateral
direction to avoid potential injury to the femoral artery. Local
anesthesia and intravenous sedation provide sufficient analgesia for
pin insertion. Proximal tibial pins are avoided due to the risk of
growth arrest. However, proximal tibial growth arrest is more likely to
result from the mechanism of injury than from pin insertion (375).
Optimal femoral pin placement is approximately 2 cm proximal to the
distal femoral growth plate and is aligned parallel to the knee joint
to reduce the risk of malalignment (376). A
traction bow is attached to the pin, and traction is applied. Periodic
radiographs are obtained to guide the weight and direction of traction
vectors needed to restore alignment. After relative fracture stability
has been obtained (17 to 21 days) and callus formation is confirmed on
radiographs, a cast is applied for an additional 4 to 8 weeks.
length-unstable fracture patterns (i.e., significant comminution), some
very proximal or distal fractures, or soft tissue damage as in open
fractures or burns (Figs. 33.62 and 33.63) (377).
Other relative indications include polytrauma, in which the child’s
general medical condition favors rapid and bloodless stabilization,
which is possible with this technique. It may be considered as a form
of ambulatory traction enabling the child to be partially weight
bearing and independent several days after sustaining the injury. Some
authors report few complications with this method, whereas others
report many complications (354,378,389,380).
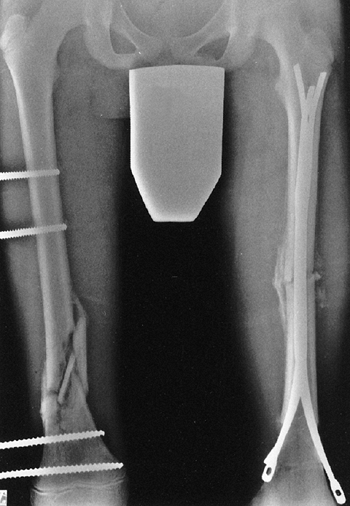 |
|
Figure 33.62
Bilateral femur fractures. The distal location, proximity to the growth plate, and marked comminution of the right femur fracture are best managed through the application of an external fixator. Although external fixation could have been applied to the contralateral fracture, intramedullary fixation was chosen because of the transverse pattern of the fracture. |
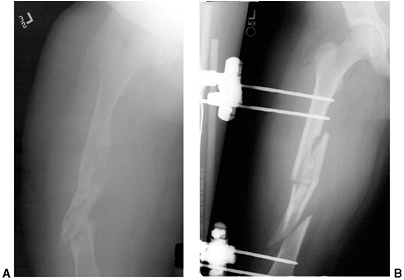 |
|
Figure 33.63 Femur fracture treated with external fixation. A:
This anteroposterior radiograph of a skeletally immature 13-year-old boy with a comminuted, length-unstable femur fracture. He had other multiple injuries, including an ipsilateral open tibia fracture. B: This anteroposterior radiograph was taken immediately after application of external fixator. (Courtesy of Theodore J. Ganley, MD) |
above and below the fracture site and attaching them to an external
frame. Preferred pin location should be far enough away from the
fracture site for the pins to avoid the fracture hematoma. However, the
pins should also avoid penetrating the cortex of the femoral neck or
the distal femoral physis. Under fluoroscopic guidance, final alignment
can be adjusted as the pins are secured to the body of the fixator.
external fixation are pin-track infections, delayed union, and
refracture after device removal. Pin-track inflammation and superficial
infection requiring oral antibiotics are common (379,380).
Rarely, deep infection may require intravenous antibiotics or pin
removal. In this instance, fixator removal before complete union can be
managed by application of a cast. Delayed union and refracture are more
frequent with fracture sites that have been opened, or with transverse
and short, oblique, midshaft fractures that are anatomically reduced (381,382).
Delayed healing has also been associated with excessively rigid
constructs, lack of dynamization, and premature removal of the fixation
device (380,383). With
careful attention to indications, surgical technique, and postoperative
management, external fixation may be successfully used to treat
pediatric femoral fractures.
Rigid nails are ideally suited for adults, because they can be locked
proximally and distally to control shortening and rotation. However,
the use of reamed rigid nails introduced through the piriformis fossa
has been associated with avascular necrosis of the femoral head in
children and adolescents (367). Rigid nails in
children younger than 13 years have also been associated with growth
disturbance with femoral neck deformity, including coxa valga and
thinning of the femoral neck (387,388).
For these reasons, it is recommended that rigid, reamed nails
introduced through the pyriformis fossa should be avoided unless the
proximal femoral physis is completely closed.
advantage of being applicable to the small, young child without risking
damage to the trochanter, the femoral neck, or the vascular supply to
the femoral head (389). A transverse or stable
fracture pattern is best for this method of internal fixation. Flexible
rods are commonly inserted retrograde from the distal femoral
metaphysis toward the proximal end of the femur. Two C-shaped nails,
one inserted medially and one laterally, usually provide sufficient
stability when three-point intramedullary contact is obtained (390,391) (Fig. 33.64).
The size of the titanium nail should be approximately 40% of the
diameter of the femoral canal at its most narrow point. The authors
recommend passing the second nail when the first nail has been inserted
just past the fracture site. Other options for insertion include a
unilateral approach distally, inserting one C-shaped nail and one
S-shaped nail. When the fracture is very distal, nails may be
introduced proximally in the region of the greater trochanter.
Additional nails may be added for stability. Flexible nails are less
stable in heavier, older children, and for comminuted fractures (392).
The addition of a cast can supplement unstable internal fixation, but
this partially defeats the advantages of surgical stabilization. New
types of flexible nails that allow proximal and distal locking are
currently being developed to help manage unstable fractures (393).
Submuscular bridge plating, and subcutaneous, minimally invasive
percutaneous osteosynthesis with locking compression plates, are newer
techniques that are being utilized for pediatric femur fractures (396,397)
(Fig. 33.65).
Plate fixation may be suitable for comminuted fractures and for
fractures that are located in sites difficult to secure with an
intramedullary nail or external fixator. The advantages of plate
fixation for femur fractures are anatomic reduction and early
mobilization. Disadvantages of conventional compression plating include
extensive dissection with additional blood loss, device failure, and
the risks associated with plate removal. Submuscular bridge plating
reduces the amount of dissection required, but subsequent removal
through limited incisions may be problematic. Minimally invasive
percutaneous osteosynthesis with locking compression plates may avoid
these difficulties, but this technique requires advanced technical
skill.
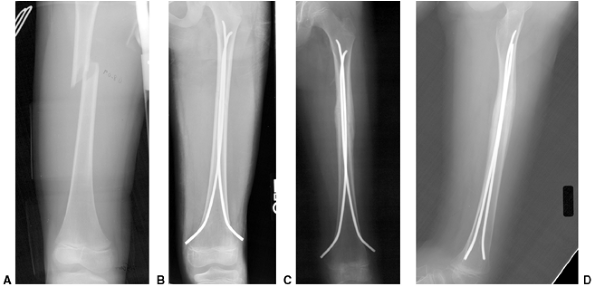 |
|
Figure 33.64 Titanium elastic nail fixation of a midshaft femur fracture in an 11-year-old girl. A: Initial radiograph of the short, oblique, midshaft femur fracture. B:
Anteroposterior radiograph taken immediately after internal fixation with titanium elastic nails. For this patient, the surgeon bent the distal nail away from bone, and this can cause soft tissue irritation. C, D: Anteroposterior and lateral radiographs of the femur taken 6 months after internal fixation. There is good healing and maintenance of an anatomic alignment. The nails were removed shortly thereafter. |
in complex circumstances as seen in children with head injuries,
multiple trauma, open fractures, floating knee, or high subtrochanteric
fractures. Children with head injuries or multiple trauma benefit from
more aggressive fracture stabilization so that they can be transported
and mobilized (55). The general management of
open fractures is discussed earlier in this chapter. External fixation
is recommended for open fractures with severe soft tissue injury, but
grade I and many grade II open femur fractures can be managed in
standard fashion after appropriate wound care.
The fracture usually is the result of high-energy trauma. Knee ligament
damage occurs in approximately 10% of these patients and is better
assessed after fracture stabilization. A juxtaarticular fracture
pattern and fractures in children older than 10 years have worse
prognoses for early and late problems (398). Surgical stabilization of at least one bone is recommended in most cases (398,399,400).
high-energy trauma. It may be difficult to maintain alignment by closed
treatment because the proximal fragment flexes, abducts, and externally
rotates. Union in the anatomic position is rarely achieved with closed
treatment, but remodeling potential is great in this anatomic region.
Fractures that are closer to the greater trochanter and fractures in
children older than 8 years more often result in malunion (401,402).
Early spica cast application in the “sitting” position may suffice in
very young children, but traction in the 90-90 position is frequently
necessary until callus formation is visible on radiographs.
Alternatively, the authors recommend that children older than 6 years,
or younger children with unstable fractures, should be considered for
surgical stabilization.
the most frequent complication of pediatric femur fractures.
Compartment syndrome, neurovascular injuries, nonunion,
and
infections may also occur. These latter complications are more frequent
after open injuries and are discussed elsewhere. Management principles
are similar to those in adults. Malunion and limb-length discrepancy
may resolve with remodeling so long as the deformities are within
management guidelines discussed previously. Osteotomy is performed to
correct persistent deformity in older children, or when remodeling is
incomplete in younger children after a period of observation.
Persistent limb-length discrepancy is rarely severe and is usually
managed by epiphysiodesis of the contralateral extremity at an
appropriate age.
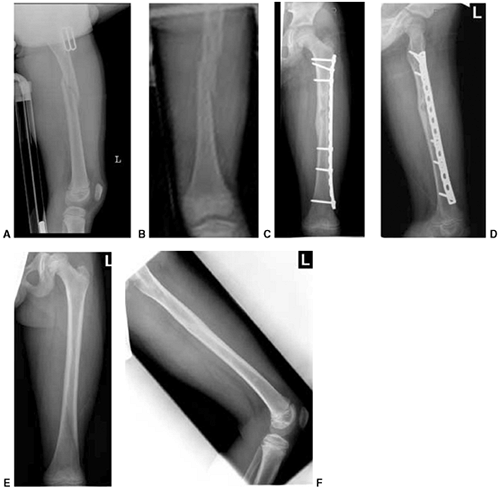 |
|
Figure 33.65 Submuscular plating of a left femur fracture in an 8-year-old boy injured while playing football. A, B: Anteroposterior and lateral radiographs of the length-unstable femoral shaft fracture. C, D: Anteroposterior and lateral radiographs taken 2 months postoperatively. There was clinical and radiographic union. E, F:
Late follow-up after plate removal. Anteroposterior and lateral radiographs taken 2 years after injury. (Courtesy of Ernest L. Sink, MD) |
patellar dislocations and soft tissue injuries of the juvenile knee are
discussed in Chapter 32. Significant trauma to
the knee in children usually results in a fracture instead of a
ligamentous injury. The attachments of the joint capsule and the
surrounding ligaments expose the pediatric knee to certain
characteristic avulsions and physeal injuries. The child presenting
with an acute hemarthrosis of the knee may have a soft tissue injury, a
fracture, or both. Stress views are sometimes useful if there is
concern for physeal fracture. MRI has been used to provide additional
information, but this modality may be less accurate in children than in
adults (403,404).
Radiographically silent osteochondral fractures have been noted in 7%
to 67% of juvenile patients undergoing arthroscopy for acute
hemarthrosis (405,406).
However, arthroscopy is rarely necessary for the initial management of
acute hemarthrosis in children younger than 13 years. Arthroscopy may
be useful in older children when it could lead to definitive management
(407,408).
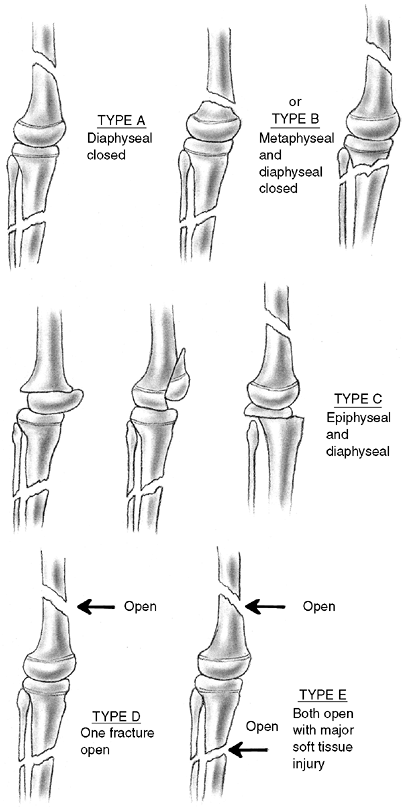 |
|
Figure 33.66 Classification of the “floating knee” in children. (From Letts M, Vincent N, Gouw G. The “floating knee” in children. J Bone Joint Surg Br 1986;68:442, with permission.)
|
configuration and is securely anchored to the metaphysis. Any fracture
at this location, whether displaced or not, confirms considerable
trauma. These fractures constitute approximately 5% of all long-bone
physeal fractures and 1% to 2% of all fractures in skeletally immature
patients (29,409). The
diagnosis is easily made except in cases of nondisplaced fractures.
Nondisplaced fractures may mimic ligamentous injuries on clinical
examination. However, careful inspection will reveal that the area of
tenderness and swelling is proximal to the joint line. Stress
radiographs with the patient sedated can be used to demonstrate the
fracture, but these are rarely needed. When the epiphysis is displaced,
the direction of displacement reflects the direction of the injuring
force. Hyperextension of the knee produces anterior epiphyseal
displacement, and valgus or varus stress produces medial or lateral
displacement, respectively. Direct impact in the knee-flexed position
causes posterior displacement of the femoral epiphysis.
femoral condyle usually results from valgus force on the knee. The
medial collateral ligament and joint capsule transmit this force to the
condyle, producing the fracture. Spontaneous reduction is frequent and
may obscure the diagnosis. The Salter-Harris type III injury to the
medial condyle may appear innocuous but is often associated with
cruciate ligament damage and intraarticular osteochondral fragments (410).
of the distal femoral epiphyseal separations, but these injuries are
not benign and have a 50% incidence of growth arrest (411,412,413). It has been observed that growth arrest is closely related to the severity of displacement (411,413).
caused by severe trauma and have the greatest likelihood of physeal
arrest (413). Fractures in the adolescent age
group are often the result of less severe trauma but still have a 50%
risk of growth disturbance. Close follow-up is recommended to detect
partial or complete growth arrest. In contrast to these fractures in
the juvenile and adolescent age groups, distal femoral separations in
infants and toddlers have excellent remodeling potential and rarely
lead to growth disturbance.
long-leg or cylinder cast for 3 to 4 weeks, until the fracture site is
nontender. Close follow-up is warranted to detect any tendency toward
displacement. Displaced distal femoral physeal separations should be
treated with closed reduction under general anesthesia (411).
After reduction, fixation is recommended using crossed percutaneous
pins to prevent redisplacement while the leg is in a cast. Pins may be
placed from distal to proximal (Fig. 33.67);
although this means that the pins are within the knee joint, the
procedure rarely leads to a secondary joint infection. Alternatively,
pins may be placed proximal to distal to avoid traversing the synovium
of the knee joint.
metaphyseal fragment is large enough, fixation can be accomplished with
percutaneous insertion of one or two cancellous bone screws through
this fragment (Fig. 33.68).
and IV injuries, open injuries, and fractures associated with
neurovascular disruption. Salter-Harris type III and IV injuries
require precise alignment to restore the articular surface and reduce
the risk of growth arrest. Because blocks to reduction are usually on
the tension side, the fracture is approached from that side. At least
two cannulated screws, 0.65 mm in diameter, are recommended to ensure
stability. Cast immobilization is also used for 3 to 4 weeks,
especially if there is only sufficient bone for a single screw.
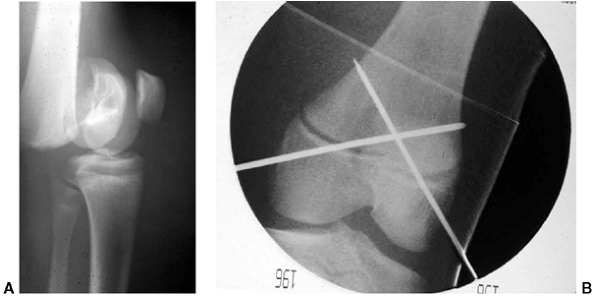 |
|
Figure 33.67 Salter-Harris type I distal femoral fracture. A:
This lateral radiograph shows a completely displaced Salter-Harris I distal femur fracture in a 12-year-old boy. He had a complete peroneal nerve palsy at presentation. B: Intraoperative anteroposterior radiograph after anatomic closed reduction and crossed-pin fixation. Pins were pulled at 4 weeks. He sustained a complete growth arrest of his distal femoral physis, detected approximately 6 months later. |
in a long-leg or cylinder cast for 3 to 5 weeks. Smooth pins can be
removed in the clinic. Gentle range of motion with progressive weight
bearing and strengthening is permitted following immobilization.
Follow-up consists of evaluation for ligamentous laxity and observation
for early signs of growth disturbance. MRI at 3 to 6 months after
injury may identify a growth arrest (42,43).
Complete closure of the opposite femoral physis should be performed in
adolescents when postinjury growth arrest will result in more than 1 cm
of limb-length inequality. Management of physeal closure in younger
children and management of partial physeal arrest have been discussed
in a previous section of this chapter.
fracture” have been used interchangeably to describe avulsion of the
tibial attachment of the anterior cruciate ligament. The tibial
eminence consists of two bony spines and is located between the medial
and lateral plateaus of the tibia. The anterior cruciate ligament
attaches to the medial spine, but nothing attaches to the apex of the
lateral spine. Between these spines are the attachments of the menisci.
Avulsion of the tibial eminence in children is usually caused by a fall
from a bicycle, sporting injuries, or some other indirect trauma to the
knee. The typical age range for this injury is 6 to 15 years.
-
Type I. Minimally displaced, with only slight elevation of the anterior margin.
-
Type II. Hinged posteriorly, producing a beak-like appearance on the lateral radiograph.
-
Type III. Completely displaced and elevated from its bed.
This would suggest that the fibers of the anterior cruciate ligament
are stretched before bone failure. Residual laxity has not led to
functional deficits or subjective feelings of knee instability. A more
troublesome problem is failure to regain full knee extension. Wiley and
Baxter (418) carefully evaluated knee range of
motion and determined that 60% of all patients lost more than 10
degrees of extension. This loss of motion was noted in all type III
injuries that were treated with closed reduction, and in approximately
one-half of the type III injuries treated with open reduction.
Long-term functional results are generally excellent except for
displaced type III fractures (417,418,419).
immobilized in a cylinder cast for 4 to 6 weeks. This is followed by
range-of-motion exercises, strengthening, and gradual resumption of
activities.
completely displaced fractures. Aspiration of the joint and injection
of local anesthetic facilitates reduction. Reduction is attempted by
fully extending the knee and applying an above-knee cast. If
radiographs after reduction are inconclusive or demonstrate inadequate
reduction, MRI or
arthroscopic
evaluation may be necessary to determine whether the posterior
attachment is disrupted, and whether there is meniscal entrapment. The
preferred position of immobilization after reduction is controversial.
Some authors recommend immobilization in extension (420,421),
whereas others recommend immobilization with 20 to 30 degrees of knee
flexion to relieve tension on the anterior cruciate ligament (421).
The present authors recommend immobilization in extension following
reduction because loss of extension after union is more problematic
than joint instability. Hyperextension should be avoided because this
position becomes uncomfortable. When reduction cannot be achieved for
type II fractures, open reduction is indicated in a manner similar to
that for type III injuries.
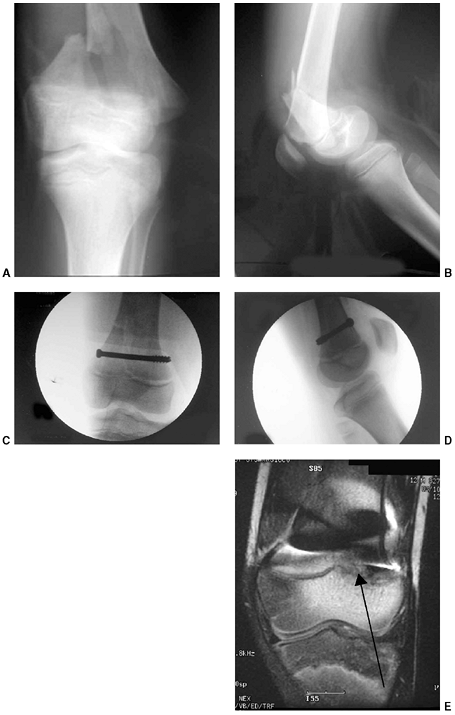 |
|
Figure 33.68 Salter-Harris type II distal femur fracture treated with screw fixation. A, B:
Anteroposterior and lateral radiographs of a 13-year-old boy tackled in a football game. Radiographs show a Salter-Harris type II distal femur fracture. C, D: Intraoperative anteroposterior and lateral radiographs after reduction and internal fixation with a 0.73-mm screw. E: Magnetic resonance imaging (MRI) obtained approximately 6 months after injury, showing growth arrest of the lateral aspect of the distal femoral physis. |
Hallam has performed anatomical and clinical studies that suggest that
arthroscopic removal of the entrapped meniscus can be followed by
reduction with immobilization in extension (420). However, other authors recommend internal fixation. This can be achieved by using a small intraepiphyseal cancellous screw (423) (Fig. 33.69).
Alternatively, sutures or wires can be passed that enter the
osteochondral fragment and exit through the periphery of the epiphysis (424,425). When fixation is secure, early mobilization can be initiated to avoid loss of motion.
common after tibial eminence fracture. These problems are usually mild
and rarely interfere with function. Arthrofibrosis can also occur
following arthroscopic surgical management. Manipulation under
anesthesia should be approached with caution due to the risk of distal
femoral physeal separation in skeletally immature patients.
Occasionally, patients will present late with malunion that limits knee
extension. This can be treated with an anterior closing wedge osteotomy
and internal fixation (421,426).
of the proximal tibial epiphysis. It develops a secondary ossification
center and serves as the insertion site of the patellar tendon.
Fracture of the tibial tubercle is an injury of the adolescent knee
joint, usually occurring in boys between 13 and 16 years of age (427).
During this period of growth, the proximal tibial physis is usually in
the process of physiologic closure. The tibial growth plate begins
closing centrally, proceeds centrifugally, and finally proceeds
distally to include the tubercle. The mechanism of injury for avulsion
of the tubercle is forceful quadriceps contraction against resistance
(e.g., jumping). There may be a preceding history of Osgood-Schlatter
disease (428,429).
The ability to perform a straight-leg raise should also be tested. The
diagnosis is confirmed on a lateral radiograph by identifying the
displaced fragment and a high-riding patella. A classification was
proposed by Watson-Jones and modified by Ogden et al. (429) (Fig. 33.70).
Type I fracture is through the distal ossification center; type II is
at the junction of the tubercle and the tibial ossification centers;
and type III involves the articular surface of the tibia. A type
IV fracture has been suggested, consisting of avulsion of the entire proximal tibial epiphysis.
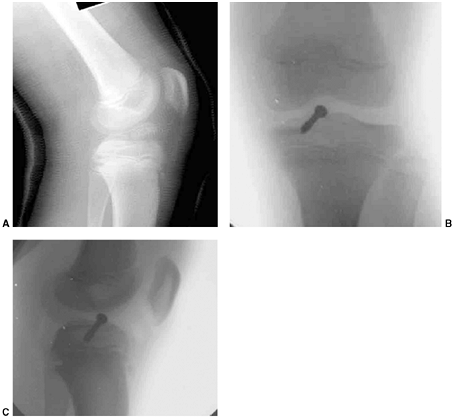 |
|
Figure 33.69 Type III tibial eminence fracture fixed with arthroscopic reduction and internal fixation. A:
Lateral radiograph of a type III tibial eminence fracture that presented after an attempt at closed reduction and casting at another center. The knee was more flexed in the cast than is recommended, and the fracture was still widely displaced. The decision was made to perform arthroscopic reduction and internal fixation. At the time of arthroscopy, the intermeniscal ligament was found trapped between the fragment and the tibia, blocking reduction. B, C: Anteroposterior and lateral views from the image intensifier following arthroscopic reduction and screw fixation of the fracture. |
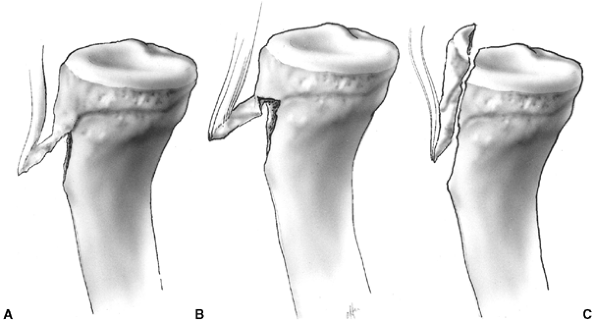 |
|
Figure 33.70 Classification of tibial tuberosity fractures. A: Type I fracture through the secondary ossification center. B:
Type II fracture located at the junction of the primary and secondary ossification centers. Sometimes this fragment is in two pieces. C: Type III is an intraarticular fracture (Salter-Harris type III). This can also be a two-part fracture. |
nonsurgical, but most of the other types require anatomic reduction and
internal fixation (427). This can be accomplished by open reduction and insertion of cancellous bone screws (Fig. 33.71).
There is usually a large retinacular injury that should be repaired. In
younger children with significant growth remaining (an unusual
scenario), smooth pins placed obliquely across the fracture can be
substituted for screws. This procedure can be supplemented with a
tension band suture or wire to the tibial metaphysis if pin fixation is
insecure. After knee immobilization for 3 to 4 weeks, active range of
motion is begun.
in tibial deformity because they occur toward the end of growth.
However, several authors have reported acute compartment syndrome of
the leg after this injury (430). This may be
because the tubercle is near the anterior compartment fascia, and
bleeding from the fracture enters this compartment. Pape et al. (430)
observed that compartment syndrome may be more frequent in patients who
are managed by closed reduction or by percutaneous methods. Bolesta and
Fitch (428) recognized this potential complication and performed prophylactic fasciotomy when anterior compartment swelling was present.
patients after tibial tubercle avulsion fracture. This is caused by
premature arrest of the anterior aspect of the growth plate. Patients
with more than 1 year of growth remaining should be observed for
development of this deformity. Bilateral proximal tibial epiphysiodesis
is generally the preferred procedure when deformity is mild. Greater
degrees of deformity may necessitate proximal tibial flexion osteotomy
to restore normal alignment (431).
in adults. This may be because the patella is largely cartilaginous
until adolescence. The child’s patella is also more mobile than the
adult’s and is subjected to less tensile force during quadriceps
contraction. In adolescents the patellar anatomy approaches that of the
adult, and the patella is more likely to be damaged by direct trauma.
Osteochondral fractures have also been reported in 15% to 70% of
children and adolescents sustaining acute patellar dislocations (408).
in children than in adults. Palpation may reveal a defect, but
palpation can be difficult to perform because of pain and tense
hemarthrosis. Lack of function and abnormal movement of the extensor
mechanism on physical examination are useful indicators of patellar
injury. Radiographs can be difficult to interpret. Bipartite patella
(i.e., secondary ossification center) may be painful and can be
confused with nondisplaced fracture. The characteristic location of a
bipartite patella—the superolateral portion of the patella—can be a
clue to diagnosis. A fractured patella may be difficult to diagnose
accurately due to incomplete
ossification.
The patellar sleeve fracture is a type of patellar fracture that is
unique to younger children. The age range for patellar sleeve fracture
is 8 to 12 years. This injury consists of an avulsion of the
cartilaginous portion of the distal patella from the ossification
center (432,433) (Fig. 33.72).
Lateral radiographs may demonstrate only patella alta and a very small
fragment of bone attached to the distal unossified cartilage. MRI can
be diagnostic when there is doubt regarding the nature of the injury.
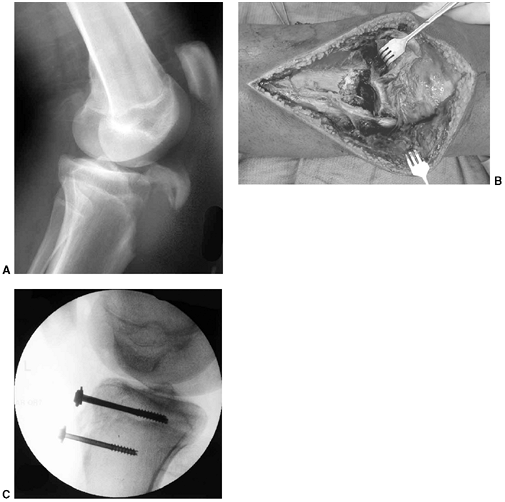 |
|
Figure 33.71 Type III tibial tubercle fracture. A:
The initial lateral radiograph of a 14-year-old boy who sustained a tibial tubercle fracture after landing from a jump in a basketball game. The radiograph shows complete displacement, with the fracture line extending into the articular surface of the tibial plateau. B: Intraoperative photograph showing exposure through a midline anterior knee incision. The typical massive soft tissue injury is illustrated, with tears of the medial and lateral retinacula and stripping of the anterior surface of the tibia. C: Lateral image intensifier view taken immediately after open reduction and internal fixation of the fracture. |
The patella is a sesamoid bone that grows by apposition, so growth
disturbance is uncommon. Nondisplaced fractures with intact extensor
mechanisms may be treated by immobilization in extension for 4 to 6
weeks. Open reduction with internal fixation is indicated for displaced
fractures. Stability may be achieved by means of the AO tension band
technique with either nonabsorbable suture or wire.
fracture in children, and account for 10% to 15% of all pediatric
fractures (6,409). Many of these are so-called toddler’s fractures,
or low-energy nondisplaced fractures occurring from minor falls or
twisting injuries. Motor vehicle accidents and high-energy injuries are
more common in older children.
This is probably because of the supporting ligamentous structures and
the shape of the physis. The fibula buttresses the tibia laterally and
the physis slopes downward anteriorly in the region of the tibial
tubercle. The medial collateral ligament inserts on the metaphysis in
addition to the epiphysis. Many of the musculotendinous units that span
the knee do not insert on the proximal tibial epiphysis.
Therefore, varus and valgus stresses are not transmitted to the tibial epiphysis.
 |
|
Figure 33.72
This lateral radiograph shows a patellar sleeve fracture in a 10-year-old child. Note the patella alta and the small osteochondral fragment 2 cm distal to the inferior pole of the patella. |
direct force to the knee, as in a fall from a height, lawn mower
injuries, or motor vehicle accidents. Hyperextension injuries from
sports may also injure this region. Lawn mower injuries are seen in
younger children, but most patients with tibial epiphyseal fractures
are older than 12 years at the time of injury (435,436).
Most proximal epiphyseal injuries are Salter-Harris type II fractures
demonstrating posterolateral or posteromedial displacement. These are
followed in frequency by type I separations. Hyperextension fractures
threaten the popliteal artery because the artery is tethered to the
posterior aspect of the tibia by its branches. The posterior branch
passes under the arch of the fibers of the soleus muscle, whereas the
anterior branch passes into the anterior compartment just distal to the
growth plate (Fig. 33.73).
or cannulated screw fixation is recommended when the fracture is
unstable, when vascular repair is necessary, or when early motion is
required. Precise reduction is recommended because future growth may be
impaired and residual deformity may not correct spontaneously.
Displaced type III and IV fractures are intraarticular injuries. Also,
precise reduction and internal fixation is advisable to preserve joint
function and reduce the risk of premature physeal closure. These
fractures should be followed closely for growth arrest, which occurs in
25% to 33% of patients regardless of the type of physeal separation (436).
fixation. If pulses are abnormal, or the foot is cool or discolored, a
vascular surgeon should be consulted and the integrity of the vessels
evaluated with an angiogram or magnetic resonance angiography (MRA). In
the absence of vascular findings, close observation after reduction is
imperative. When a period of ischemia has exceeded 4 hours or vascular
repair is required, prophylactic fasciotomy is recommended (74). This prevents reperfusion compartment syndrome from developing.
commonly in children between the ages of 2 and 8 years. The usual
mechanism of injury is a valgus force applied to the extended knee
producing an incomplete fracture of the tibia, with or without fracture
of the fibula. The lateral cortex of the tibia may be buckled,
impacted, or may undergo plastic deformation. Pronounced displacement
is uncommon except in instances of high-energy trauma. This
innocuous-appearing fracture is often in more valgus than one readily
appreciates from the initial radiographs. A comparison
radiograph of the opposite tibia is helpful to determine the true deformity of the injured leg.
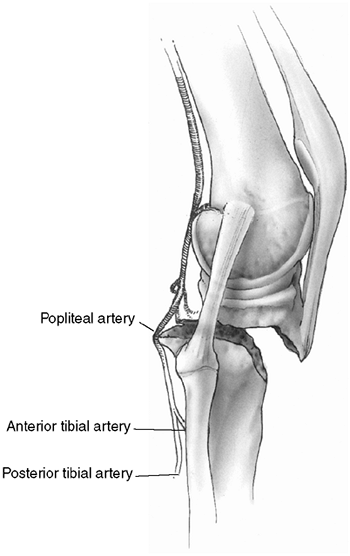 |
|
Figure 33.73
Proximal epiphyseal fracture. The distal tibial segment is displaced posteriorly, producing vascular occlusion of the popliteal artery. |
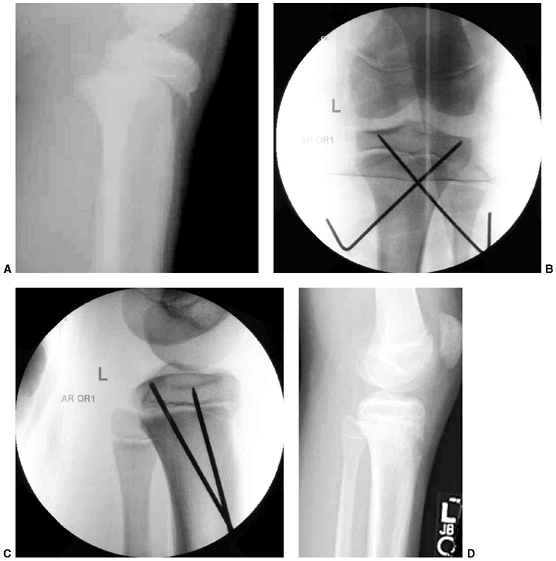 |
|
Figure 33.74 Proximal tibial physeal injury. A: This initial lateral radiograph shows a displaced proximal tibial physeal fracture. B, C: Intraoperative images after closed reduction and internal fixation with two Kirchner wires. D: Lateral radiographs 6 weeks after injury show maintenance of anatomic alignment.
|
immobilization. Occasionally, open reduction is necessary due to
interposed periosteum and pes anserinus. After reduction, the knee is
placed in extension. This position allows effective three-point cast
molding to generate a varus force. The extension position also
facilitates subsequent radiographic interpretation. The authors
recommend that reduction should be within 5 degrees of the opposite
intact tibia as judged by a line through the physis and a line down the
tibial shaft. The cast is maintained until healing is complete, usually
6 weeks. Families should be counseled about the possibility of
posttraumatic genu valgum. Displaced proximal tibial fractures in
adolescents sometimes require surgical stabilization to control
alignment and expedite mobilization. During the 48 hours after closed
reduction and casting, the extremity should be closely monitored for
signs of excessive compartment swelling.
Close review of the initial postreduction radiographs occasionally
reveals that an incomplete reduction was responsible for at least part
of the deformity. However, nondisplaced and anatomically aligned
fractures can also develop progressive valgus deformity (437).
Increasing angulation begins several weeks after fracture and usually
ceases by 12 months after fracture. The cause of this problem remains
somewhat obscure, but it probably results from selective overgrowth of
the medial tibial
physis (437).
Overgrowth of the tibia with tethering by the intact fibula has also
been postulated as a possible cause, but progressive valgus deformity
can develop even when the fibula is fractured. Treatment of this
deformity for children younger than 5 years consists of observation (438). Valgus deformity usually resolves spontaneously in this age group with the development of a slightly S-shaped tibia (438,439,440). Tibial osteotomy and hemiepiphyseal stapling have been reported for correction of more severe deformities (438,441). In the authors’ opinion, osteotomy should be avoided because of the high risk of recurrent deformity (439).
Although rarely indicated, the authors recommend temporary
hemiepiphyseal stapling to correct deformity in children older than 5
years who have a deformity greater than 15 degrees.
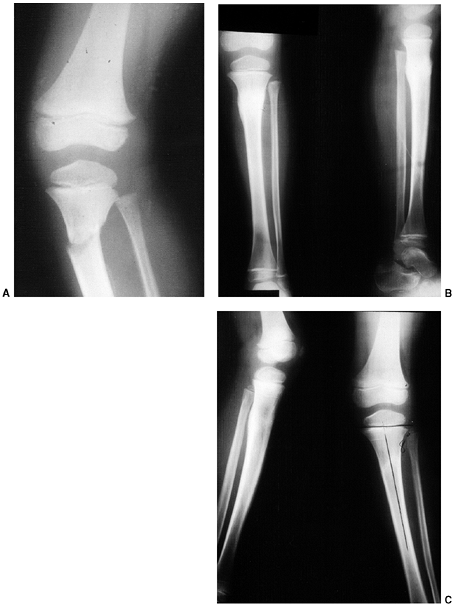 |
|
Figure 33.75 A: Radiograph of a proximal metaphyseal fracture of the tibia with valgus deformity. Closed reduction was performed. B: Radiographic appearance at the time of union demonstrates satisfactory alignment. C: Radiographs 8 months later demonstrate valgus alignment attributable to asymmetrical growth stimulation.
|
and comminuted displaced fractures (442,443).
In infants and young children, the tibial shaft is relatively porous
and is more likely to bend, buckle, or sustain a nondisplaced spiral
fracture than to fracture completely. The surrounding periosteum is
strong and imparts stability to the fracture site. This limits
displacement and shortening. In contrast, the adolescent tibial shaft
is composed of more dense cortical bone and a thinner, weaker
periosteum. Fractures in the adolescent age group are more often the
result of high-energy trauma and are associated with greater fracture
displacement, comminution, and slower healing rates than in younger
children.
Infants and toddlers can correct approximately 50% of residual
angulation with growth. In children older than 10 years, only 25% of
the axial malalignment improves. Hansen and Grieff (442) reported only 13.5% correction of angular deformity with subsequent growth, but Shannak (443)
demonstrated that one-third of children with more than 10 degrees of
angulation at healing had persistence of the angulation at final
follow-up assessment. In general, varus malalignment seems to remodel
more completely than valgus deformity. Although long-term studies show
that moderate angulation is well tolerated (444),
the authors recommend that attempts should be made to maintain
alignment within 10 degrees of angulation in any direction for children
older than 6 years and within 15 degrees of angulation for younger
children (442,443,445).
Rotational deformity may not remodel, although external rotation
deformity is better tolerated than internal rotation deformity (443).
the ability to compensate for shortening decreases with age. Children
younger than 5 years show the greatest capacity. However, growth
acceleration greater than 5 to 7 mm is unusual (446). In a review of 142 tibial fractures, Shannak (443)
reported an average of only 4.35 mm of growth acceleration. Comminuted
and long spiral fractures displayed the greatest amount of overgrowth,
including those that were treated with anatomic reduction and internal
or external fixation. Overgrowth is not routinely seen in girls older
than 8 years or boys older than 10 years.
children. Toddler’s fracture is seen in the 1- to 4-year age group. A
mildly traumatic event may have been observed, but often the child
presents with an acute limp of unknown cause. Approximately 20% of
these acutely limping toddlers have sustained occult fractures, and
half of these fractures are in the tibia (447).
Low-energy torsional forces, as when the child twists a leg, usually
cause these fractures. The child limps or refuses to walk on the
affected lower limb. Examination may reveal a point of tenderness or
subtle swelling in the distal third of the leg, but often the
examination is unremarkable. Radiographs may show a fracture, but
frequently the fracture line is not initially evident. Toddler’s
fracture is differentiated from pathology of the hip and femur by the
child’s ability to crawl, in addition to the finding of a normal range
of motion of the hip. Infectious processes need to be considered in the
differential diagnosis, but these can usually be diagnosed by the
presence of fever and laboratory studies demonstrating increased
sedimentation rate, C-reactive protein, and leukocyte count. A triphase
bone scan may help establish the diagnosis when pain and limp are
severe and the workup remains equivocal (448).
MRI is more specific but usually requires sedation in these young
patients. Treatment is initiated when fracture is suspected, and the
diagnosis is usually confirmed 10 to 14 days later, when periosteal new
bone has formed.
in all age groups consists of immobilization in a short-leg cast for
distal fractures or long-leg cast for fractures proximal to the
midshaft. Immobilization is continued until union has occurred, usually
3 to 4 weeks for toddlers and 6 to 10 weeks for older children.
A rotational or twisting force results in a spiral or oblique fracture
at the junction of the middle and distal thirds of the tibial shaft.
The most common mechanism of injury is indirect trauma such as sports
accidents or falls. The intact fibula imparts stability, but it may
have plastic deformation that interferes with reduction of displaced
tibial fractures. The intact fibula may also contribute to the
development of varus angulation.
above-knee cast, with the knee flexed to 30 degrees and the ankle in 15
degrees of plantar flexion to minimize varus muscle forces and prevent
recurvatum (445,449). Unstable, displaced fractures may require surgical stabilization with external fixation or flexible nails (Fig. 33.76).
Angulation greater than 10 degrees in any direction should be
corrected, except in children younger than 6 years, in whom 15 degrees
may be accepted (442,443,444,445).
common in older children. These fractures result from high-energy
trauma and are frequently unstable. Open fractures of the tibia are not
uncommon and account for 4% of all tibial fractures in children and
adolescents (450). Soft tissue damage and
periosteal stripping predispose to more severe complications such as
compartment syndrome, delayed union, and infection. Inherent fracture
stability after reduction is variable.
achieve when the fibula is fractured, but there is a tendency for the
fracture to drift into valgus and procurvatum because of the greater
muscle bulk posterolaterally in the leg. An above-knee cast is used for
4 to 8 weeks until initial stability has been achieved. Immobilization
may then be continued with a patella-tendon-bearing cast until healing
is complete. Final axial alignment should be within 10 degrees in any
direction.
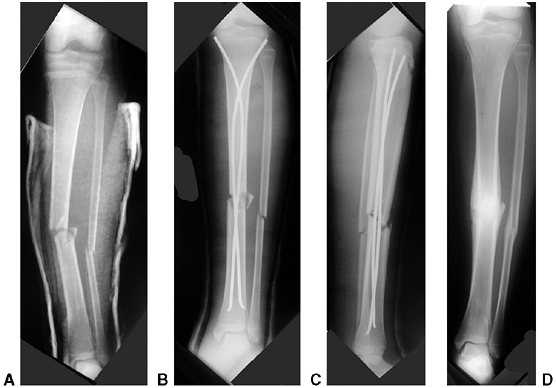 |
|
Figure 33.76 Titanium elastic nail fixation for an unstable tibia fracture in a skeletally immature girl. A:
This anteroposterior radiograph shows a transverse midshaft tibia and fibula fracture. This was a high-energy, closed injury with possible impending compartment syndrome. The surgeon elected to perform internal fixation. Because the proximal tibial physis was open, titanium elastic nailing was chosen over standard solid tibial nailing. B, C: Plain radiographs taken 1 week after internal fixation of the tibia fracture with antegrade titanium elastic nailing. D: Anteroposterior radiograph taken 6 months after injury. The fracture is healed in approximately 5 degrees of varus, within the range of a satisfactory result. |
maintain alignment or facilitate rehabilitation. The techniques for
surgical stabilization are similar to the techniques discussed with
regard to femur fractures. Fixation techniques include the use of
flexible intramedullary nails introduced through the metaphysis, plates
and screws, and external fixation. Additional indications for surgical
stabilization of tibial fractures include associated head injuries,
multiple trauma, floating knee, vascular injury, and open fractures
requiring wound access.
discussed earlier in this chapter. Open fractures of the tibia in
children are the result of high-energy trauma, with associated injuries
in 25% to 50% of these patients (63,450).
intravenous antibiotics and tetanus prophylaxis, followed by aggressive
wound irrigation and debridement (63,450,451,452). Surgical intervention within 6 hours has been the standard recommendation (63).
However, moderate delay of surgical management for lesser grades of
open fracture may not be associated with an increased infection rate
when antibiotics are administered early (64,65).
Clean, grade I open wounds may be loosely closed over a drain after
adequate debridement, but most wounds should be left open with repeated
debridement before soft tissue coverage (451).
Debridement of devitalized bone is not necessary in children if the
bone is clean and can be adequately covered with soft tissue (66).
Surgical stabilization facilitates wound management. External fixation
is generally preferred, but satisfactory results have been reported
with plates and screws and with flexible intramedullary fixation (63,450,451,452).
Children younger than 12 years require less aggressive surgical
management, heal faster, have lower infection rates, and have fewer
complications than older children. Children older than 12 years have
fracture patterns and complications that are similar to those in the
adult population. However, limb salvage and reconstruction has a higher
rate of success than in the adult population (454).
of all pediatric fractures and one out of every six physeal fractures
(17%) (3,29). The same
mechanisms that produce spiral fractures of the tibial shaft in younger
children may produce epiphyseal fractures of the ankle in older
children. Tillaux and triplane fractures are specific injuries that
occur as the distal tibial growth plate begins to close (455). Tillaux and triplane fractures are referred to as transitional fractures because they occur in adolescents during the transition to skeletal maturity.
fibular epiphyses appear between the ages of 6 months and 2 years. The
medial malleolar extension forms at around 7 to
8
years of age and is complete by the age of 10. Closure of the distal
tibial growth plate begins centrally, proceeds medially, and ends on
the lateral side. This sequence of closure is responsible for
transitional fracture patterns. The fibular physis lies at the level of
the talar dome, and closes 1 to 2 years later than the distal tibia.
dorsiflexion only, rendering this region susceptible to injury from
twisting or bending forces. Medial stability is provided by the deep
fibers of the deltoid ligament that attach the medial malleolus to the
body of the talus. The lateral ligament complex consists of anterior
and posterior talofibular ligaments and the calcaneofibular ligament.
Strong ligamentous structures also bind the distal tibia to the fibula
at the level of the joint. The anterior tibiofibular ligament is
important in the pathomechanics of transitional fractures. Ligaments
around the ankle principally attach to the epiphyses distal to the
level of the growth plate. This anatomic arrangement transmits injury
forces to bone and results in physeal fractures in older children and
adolescents.
or minimally displaced fractures. This is particularly true for distal
fibular physeal separations and Tillaux fractures. Swelling may be
minimal. Careful palpation usually reveals that the most tender area is
the growth plate rather than the joint or ligaments. Displaced
fractures are painful, with visible deformity due to the subcutaneous
nature of the ankle joint. The position of the foot relative to the
tibia provides evidence of the mechanism of injury and indicates the
direction of manipulation required for reduction. Motor, sensory, and
vascular assessments should be performed before reduction. Plain
radiographs usually confirm the diagnosis and define the fracture
pattern. CT scanning, with or without three-dimensional reconstruction,
is useful for evaluation and management of transitional fractures. An
MRI does not offer great advantage over plain radiography except to
evaluate complications such as growth arrest (456).
children, whereas a variety of epiphyseal injuries may be seen in older
children. Fractures with syndesmosis disruption are uncommon in
children until late adolescence. The most common avulsion injury is
avulsion of the tip of the lateral malleolus, followed by separation of
the distal fibular physis (457).
mechanism of injury, by type of growth-plate injury, and by
combinations of both systems (458,459).
Classifications based on mechanism of injury have been proposed to help
guide reduction, but these classifications have been formulated
independently of clinical examination. Also, children rarely have
comminution or syndesmosis disruption, which have poor prognoses in
adult classification schemes. In children, the steps necessary for
reduction are usually evident when the clinical examination of foot
position is combined with the radiographic appearance. Classifications
have also been proposed that combine mechanism of injury with the type
of growth-plate injury, but these classifications can be confusing and
difficult to remember. The authors agree with Vahvanen and Aalto (457),
who stated: “The simultaneous use of the classifications based on both
type of trauma and type of epiphyseal lesion for classifying ankle
fractures in children has led to unsatisfactory and unnecessarily
complex groupings. In children the mechanism of trauma can often not be
identified, and experimental work, such as what Lauge-Hansen did to
support the mechanism-of-trauma classification in adults, is lacking in
children.” Those authors subsequently proposed a simple classification
to guide management and predict outcome. According to their system,
ankle fractures in children can be classified into two categories (457):
-
Group I. Low-risk, including avulsion fractures and epiphyseal separations (Salter-Harris types I and II)
-
Group II. High-risk, including fractures
through the epiphyseal plate (Salter-Harris types III, IV, and V) and
transitional fractures -
In this classification scheme,
transitional fractures would be considered high-risk. The present
authors prefer to consider transitional fractures as a separate
category because of the distinct pathoanatomy of these injuries.
Spiegel et al. (460) used a slightly different
classification of high-risk and low-risk pediatric ankle fractures,
with a third category for transitional fractures.
involve the growth plate, and all Salter-Harris type I and II physeal
injuries. Nondisplaced distal fibular physeal fractures are treated
with a weight-bearing short-leg cast for 4 to 6 weeks. Children with
nondisplaced, low-risk ankle fractures involving the tibia are placed
in a short-leg cast and limited to non—weight-bearing activities for 2
to 3 weeks; they are allowed full weight-bearing activities thereafter.
Union occurs at approximately 6 weeks after injury. Follow-up
radiographs in the cast are recommended 7 to 10 days after initial
treatment to ensure maintenance of alignment.
This can be attempted in the emergency room, but complete muscle
relaxation may be required for successful manipulation. Following
reduction, a residual physeal gap of greater than 3 mm may indicate the
presence of entrapped soft tissue. This may lead to a greater risk of
physeal closure unless open reduction is performed to remove interposed
tissue (34). A flap of
periosteum is usually found, but tendons or neurovascular structures
can also become interposed. Internal fixation with smooth pins or
metaphyseal screws can be used after open reduction, but this is not
required if the fracture is stable clinically. Minor amounts of
displacement and angulation can be accepted, especially in children
younger than 8 years, because these injuries are usually extraarticular
and have good prognoses for resumption of growth. Immobilization in an
above-knee cast (non—weight-bearing) is recommended for the first 3
weeks after closed reduction. A below-knee, weight-bearing cast is then
applied until union is obtained. A total of 6 weeks of immobilization
is usually sufficient.
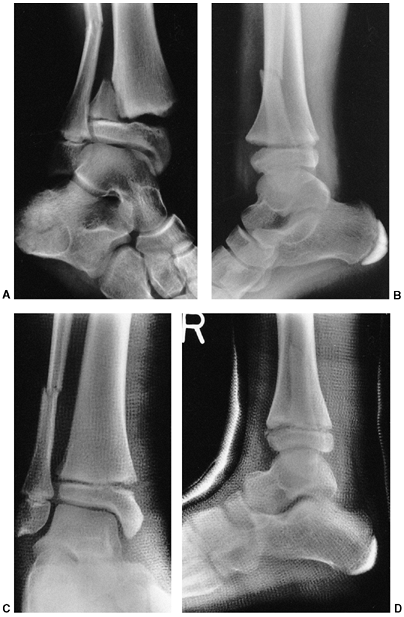 |
|
Figure 33.77 Pronation and external rotation ankle fracture. A:
This anteroposterior radiograph demonstrates a Salter-Harris type II fracture of the distal tibia. The Thurston Holland fragment is lateral. The fibular fracture is transverse and located well above the fibular physis. B: Lateral projection of the same injury. C: This fracture was treated with closed reduction and application of an above-knee cast. D: A lateral radiograph demonstrates acceptable alignment. This fracture was managed successfully with closed reduction. |
fractures. They are intraarticular, usually with joint instability.
Hairline fractures in which the fracture line is 1 mm or less on all
views can be managed by immobilization in a long-leg cast. Greater
degrees of displacement require accurate reduction. Salter and others (461,462)
have noted that reduction of these fractures must be “perfect” to
restore the articular surface and minimize the risk of growth arrest.
Closed reduction may be attempted for displaced fractures, but is
rarely successful. Open reduction is usually performed with fixation
using intraepiphyseal smooth Kirschner wires or small, cannulated
screws (Fig. 33.78).
Lintecum and Blasier (463)
described a technique of direct visualization and reduction through an
anterior arthrotomy incision. This was accompanied by percutaneous
fixation with cannulated screws inserted medially or laterally. Every
effort should be made to avoid crossing the growth plate with internal
fixation devices. However, restoration of articular integrity is more
important than preserving growth at the ankle. The distal tibial and
fibular epiphyses contribute only 5 to 7 mm of longitudinal growth per
year. When unstable fractures occur in children older than 12 years, it
is occasionally advisable to place internal fixation devices across the
physis and perform epiphysiodesis to avoid subsequent angular deformity
if sufficient growth remains.
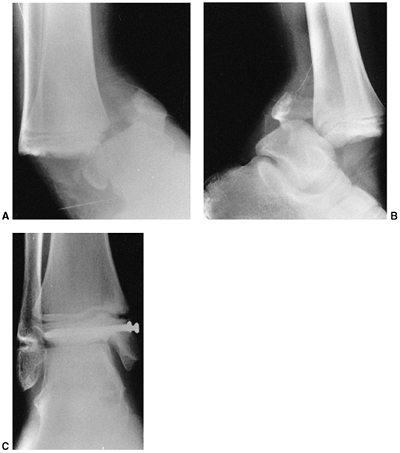 |
|
Figure 33.78 Supination-inversion ankle fracture. A:
The anteroposterior radiograph demonstrates a bimalleolar ankle fracture with ankle dislocation. There is a Salter-Harris type I fracture of the distal fibula and a Salter-Harris type III fracture of the medial tibial epiphysis. B: Lateral projection. C: Anteroposterior radiograph after open reduction and internal fixation. Transepiphyseal screws are used to avoid fixation crossing the growth plate. The joint surface is restored to anatomical alignment. No fixation was required for the fibula fracture. A smooth Kirschner wire can be placed across this physis, if needed, for ankle stability. |
Tillaux and triplane injuries. These fractures occur as the growth
plate is in the process of closing, so growth disturbance is not a
concern. Restoration of articular congruity is the objective of
treatment.
force, and consists of avulsion of the anterolateral portion of the
distal tibial epiphysis by the anterior tibiofibular ligament. This is
a biplane Salter-Harris type III injury that can be difficult to detect
on plain radiographs. Closed reduction is often successful and is
performed by internal rotation and immobilization in an above-knee
cast. The quality of reduction should be accurately documented. CT
scans are helpful if plain radiographs are inconclusive. Open reduction
with internal fixation is indicated when joint surface step-off after
closed reduction is greater than 2 mm (455,464) (Fig. 33.79). An anterolateral approach is used, and the fracture is fixed with cancellous screws crossing the physis.
the foot. On anteroposterior radiographs it appears as a Salter-Harris
type III fracture, but on the lateral projection it appears to be a
type II fracture (Fig. 33.80). The triplane
fracture may be a two-part or a three-part fracture, but greater
degrees of comminution can occur. The CT scans of two-part fractures
reveal a single fracture line on the horizontal section through the
epiphysis. Three-part fractures
demonstrate
three radiating fracture lines (“Mercedes sign”) on the transverse
section through the epiphysis. An extraarticular type of triplane
fracture can also occur when the fracture line exits through the medial
malleolus beyond the articular surface (455).
When seen early, initial management consists of attempted closed
reduction with internal rotation and application of a long-leg cast. CT
scan is recommended to confirm reduction (Fig. 33.81). Open reduction with internal fixation is indicated when joint surface incongruity exceeds 2 mm, when the patient
presents late, or after failed closed reduction (Fig. 33.82) (455,464,465).
The anterolateral approach is satisfactory for reduction and fixation
of two-part fractures. Three- and four-part fractures generally require
anterolateral and posteromedial exposures. In rare circumstances in
which articular congruity cannot be assured by direct visual inspection
or radiographic evaluation, arthroscope-assisted reduction can be
helpful.
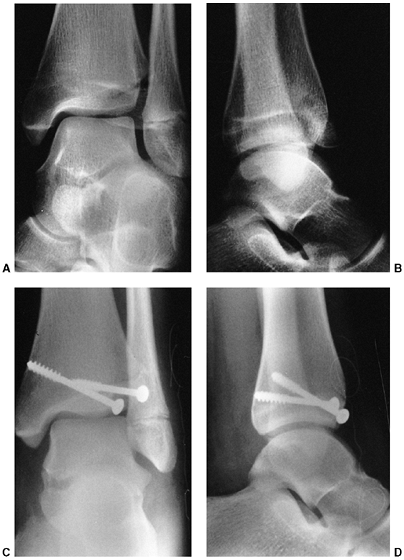 |
|
Figure 33.79 Transitional fracture. A: The anteroposterior radiograph shows displacement of the anterolateral distal epiphysis (Tillaux fracture). B: The lateral radiograph demonstrates anterior displacement and rotation of the fragment. C: Postoperative radiograph after open reduction and internal fixation with cancellous bone screws. D:
Lateral radiograph. In this instance, the screws can cross the physeal line because this injury occurred in an adolescent after the growth plate had begun physiologic closure. |
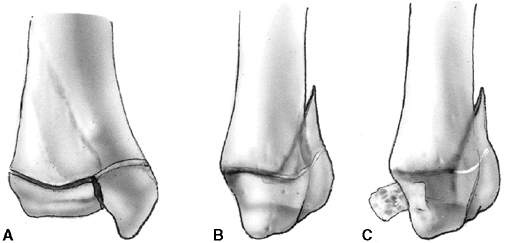 |
|
Figure 33.80 Triplane fracture. A: On the anteroposterior radiograph, the fracture appears as a Salter-Harris type III fracture of the distal tibial epiphysis. B: On the lateral view, the fracture appears as a Salter-Harris type II fracture of the distal tibia. C: In the three-part triplane fracture, the anterolateral epiphyseal fragment is displaced as a separate fragment.
|
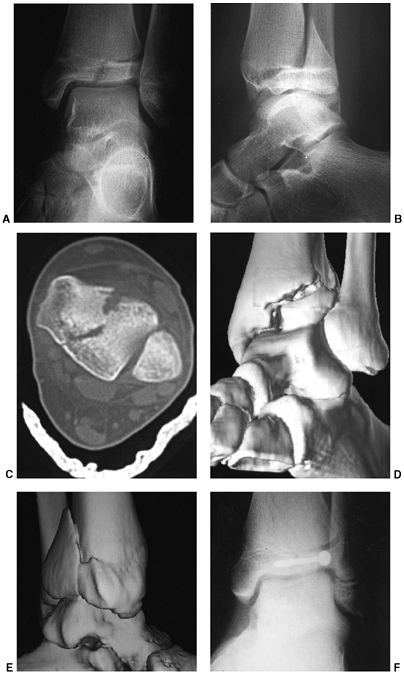 |
|
Figure 33.81 Triplane fracture of the distal tibia in a 12-year-old girl with computed tomography (CT) evaluation. A: The anteroposterior radiograph shows a Salter-Harris type III fracture. B: The lateral radiograph shows an apparent Salter-Harris type II fracture. This indicates a triplane injury. C: CT through the epiphysis confirms a two-part fracture. D, E: Three-dimensional reconstruction demonstrates the fracture from the anterolateral and posteromedial views. F:
Closed reduction was unsuccessful. Arthroscopically assisted open reduction was performed. The fracture was stabilized with a single anterolateral cannulated screw inserted percutaneously. |
to joint incongruity and growth disturbance. Both of these are
influenced by the adequacy of reduction. Kling et al. (462)
reported that 19 of 20 Salter-Harris type III and IV fractures that
were treated with accurate open reduction and internal fixation healed
without growth disturbance. In contrast,
five
of nine similar fractures that were treated by closed means developed
bone bridges. The distal tibia and fibula grow 5 to 7 mm per year.
Therefore, leg-length discrepancy is rarely a major problem, except in
younger children. The more common problem is angular deformity due to
asymmetric growth arrest. Complete epiphysiodesis, with or without
contralateral epiphysiodesis, should be considered as soon as growth
disturbance is recognized. Other alternatives for management of growth
arrest are discussed in the injury to the physis section of this
chapter. When ankle deformity has occurred, transphyseal osteotomy is a
successful method of correction (466).
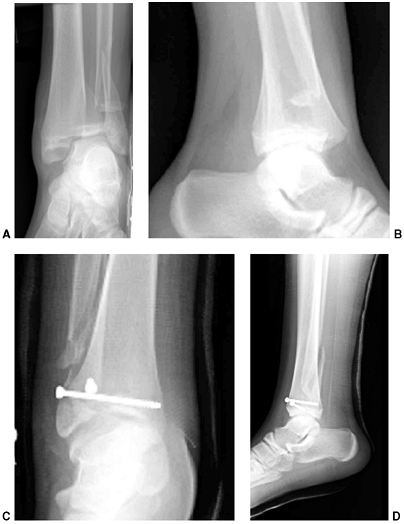 |
|
Figure 33.82 Triplane fracture. A, B: Anteroposterior and lateral triplane fracture in a 14-year-old. C, D:
Anteroposterior and lateral radiograph after closed reduction and internal fixation of the distal tibia. No fixation was chosen for the distal fibula. |
The amount of gap that can be accepted without step-off is not clearly
defined, but it may be slightly greater than 2 mm. Although every
attempt should be made to restore articular surfaces without placing
fixation across the physis, it is the authors’ opinion that maintenance
of articular integrity is a higher priority than preservation of growth
around the ankle.
However, fractures of the tarsal bones are uncommon in children and
account for less than 1% of all fractures in childhood. Most of these
are nondisplaced and may be an underreported cause of limping in
toddlers (468,469). The
rarity of fractures of the midfoot and hindfoot is attributed to the
fact that the juvenile foot is very flexible, with a large component of
cartilage until late adolescence. The ossification center of the medial
cuneiform does not appear until 4 years of age. Some of the secondary
ossification centers do not appear until 10 years of age or later.
Therefore, there are numerous ossification centers in various stages of
development. This can make radiographic
interpretation
difficult. Comparison radiographs of the opposite foot should be
obtained when the diagnosis of a fracture is in question. A CT scan or
MRI is often helpful to evaluate complex injuries.
first and fifth metatarsals to form a tripod for weight bearing and
shock absorption. Ligamentous structures, joint capsules, bone
geometry, and dynamic muscular forces maintain longitudinal and
transverse arches. The talus is a complex bone that links the tibia to
the foot and bears all the forces of body weight. It is composed of
three parts: head, neck, and body. The talus is supported by the
calcaneus, and the head of the talus articulates with the tarsal
navicular bone. The cuboid laterally and the three cuneiforms medially
constitute the distal row of tarsal bones.
through the ankle joint and accommodates pronation-supination motion
through the obliquely oriented subtalar joint. This articular function
requires the talus to be largely covered with articular cartilage,
leaving few avenues of entry for nutrient vessels. Therefore, the talus
is particularly susceptible to osteonecrosis after fracture. The
navicular is also susceptible to osteonecrosis and lies between the
head of the talus and the cuneiforms. Idiopathic or posttraumatic
necrosis of the navicular is a self-limited condition without long-term
sequelae. However, this condition, also known as Kohler disease, can cause pain and have the radiographic appearance of a fracture.
architecture that is much more cancellous and has a thin cortical wall
with less articular covering than the talus. The calcaneus has a direct
weight-bearing function through the complex subcutaneous septae and the
thick skin of the heel pad. Anteriorly, transmission of longitudinal
forces is achieved at the calcaneocuboid joint. The calcaneus is
susceptible to compression failure and collapse of the outer cortical
shell when subjected to direct impact, such as the force sustained in a
fall from a height. In addition, the wedge-like lateral process of the
talus may be driven into the superior region of the calcaneus between
the posterior and middle or anterior surfaces of the subtalar joint.
This intraarticular mechanism of injury is seen more often in adults
and older adolescents. It results in division of the calcaneus into
anterior and posterior fracture fragments.
forced dorsiflexion. However, there is a 25% to 30% incidence of
associated medial malleolar fractures, which suggests a supination
component to the deforming force (470). Most
talar neck fractures in children are nondisplaced. Most displaced
fractures are from high-energy trauma. Displacement jeopardizes the
tenuous blood supply of the talus because the neck region is the
primary site of vascular penetration into the talus. Fortunately, there
are numerous vascular anastomoses within the body of the talus. The
principal blood supply penetrates the neck from within the tarsal canal
that is formed by the sulcus of the calcaneus and the sulcus of the
talus at the base of the neck. The other major blood supply is a
deltoid branch from the posterior tibial artery that enters the medial
body of the talus along the deltoid ligament (471).
-
Type I. Minimally displaced fracture of the distal talar neck (the incidence of osteonecrosis is low)
-
Type II. Minimally displaced fracture of the proximal neck or body (the risk of osteonecrosis is low with this type also)
-
Type III. Displaced talar neck or body fracture (osteonecrosis is more likely)
-
Type IV. Talar neck fracture with dislocation of the body fragment (osteonecrosis is expected in these fracture-dislocations)
the anteroposterior view) and displaced less than 2 mm can be managed
closed (473). The foot is placed in slight
plantar flexion to reverse the mechanism of injury. Immobilization in a
non—weight-bearing below-knee cast is continued until union is evident,
usually at about 6 weeks. Then a full—weight-bearing cast is used for
an additional 4 to 6 weeks. Displaced fractures require open reduction
with internal fixation. The anteromedial approach is preferred for
fragment reduction. Kirschner wires or cannulated screws can be placed
anterior to posterior or retrograde, depending on the location of the
fracture (Fig. 33.83). Lag screws are recommended because they may eliminate displacement better than smooth wires (470). Cast immobilization with non—weight-bearing activities is maintained until union is achieved.
healing of the fracture, and the long-term outcome may be satisfactory.
Prolonged non—weight-bearing in a cast yields the best results when
osteonecrosis has developed (473). Subchondral
lucency may become visible in the body of the talus 6 to 8 weeks after
fracture (Hawkins sign). This sign results from disuse osteopenia, and
indicates that the body of the talus is vascularized.
may produce osteochondral fracture of the medial or lateral margin of
the talar body. This lesion should be suspected when a “sprained ankle”
does not improve as expected. Medial lesions tend to be posteromedial
and result from inversion,
plantar
flexion, and external rotation. Lateral lesions tend to be
anterolateral and result from inversion and dorsiflexion. Plain
radiographs of the ankle mortise in plantar flexion and dorsiflexion
may be necessary to visualize the fracture. A CT scan or MRI is useful
in problematic cases.
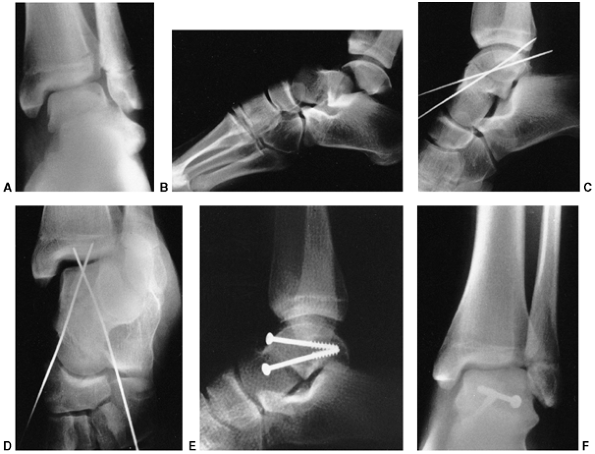 |
|
Figure 33.83 Talus fracture. A:
Anteroposterior radiograph of the ankle of a 13-year-old gymnast who injured her foot during a dismount. It appears that the head and neck of the talus are displaced laterally toward the fibula. B: The lateral projection shows a type III talus fracture with subluxation of the talonavicular joint. C: The intraoperative film shows provisional fixation with Kirschner wires. The fracture is reduced with plantar flexion of the foot. D: Another intraoperative anteroposterior view shows anatomical alignment of the talar neck with the body. The entry sites for the screws are in the nonarticular portion of the talar neck. E: The postoperative film shows cancellous screw placement. F: An anteroposterior radiograph shows restoration of the normal alignment of the ankle. Compared with the injury radiograph, there is no longer a prominence of the talar neck laterally. |
immobilization for 6 to 8 weeks. Many patients become asymptomatic in
spite of persistent defects (474). Drilling and
pinning, or removal of the loose fragment, is indicated if symptoms
persist after a period of immobilization. This frequently can be
accomplished arthroscopically.
dorsiflexion-inversion or twisting injury to the foot. These fractures
are easily missed because the initial symptoms are similar to those of
an ankle sprain (475). A high index of
suspicion and good quality anteroposterior radiographs of the talus are
required for diagnosis. More commonly, the patient presents with
persistent symptoms after an “ankle sprain” (476).
The diagnosis can be made with stress radiographs or CT scanning.
Displaced fractures are often associated with other fractures.
nondisplaced injuries. When the patient presents late or an acute
fracture is displaced, small fragments can be excised, but large
fragments should be treated with reduction and internal fixation.
sparing the subtalar joint, or intraarticular. Extraarticular fractures
are more frequent in younger children (75% of cases), whereas
intraarticular fractures account for most calcaneus fractures in
adolescents and adults. Fracture of the calcaneus may be minimally
displaced and can be easily overlooked in children. Delay in diagnosis
occurs in 30% to 50% of cases (477,478,479).
Swelling, pain, or localized tenderness after a fall should alert the
clinician to the possibility of calcaneus fracture. Multiple
radiographic views are recommended for diagnosis. However, CT scan has
evolved as the best method for imaging calcaneal fractures, both for
the assessment of displaced fractures and occasionally for the
diagnosis of occult fractures.
classified calcaneal fractures in children as extraarticular,
intraarticular, or those with loss of the insertion of the Achilles
tendon and significant soft tissue injury (e.g., lawn mower injury).
Intraarticular fractures in adults have been further classified by
Sanders et al. (480) to help plan surgical management and predict outcome.
prognoses. Closed injuries are usually treated with 4 to 6 weeks of
cast immobilization and progressive ambulation as tolerated. Displaced
avulsion fracture of the tuberosity of the calcaneus is an
extraarticular fracture that requires reduction (481).
This may be accomplished by closed reduction using direct pressure over
the Achilles insertion while the knee is flexed and the ankle is
plantar-flexed to relax the posterior calf muscles. Open reduction with
internal fixation is recommended if closed reduction is unsuccessful.
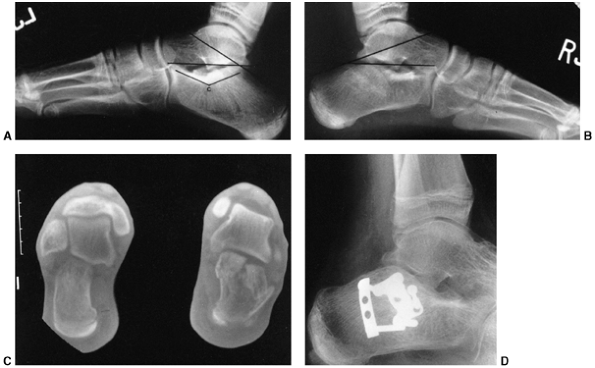 |
|
Figure 33.84 Calcaneus fracture. This 12-year-old boy injured both feet after jumping off a second-story deck. A:
The lateral radiograph of his left foot reveals a minimal fracture of the body of the calcaneus. The Bohler angle is subtended by a line connecting the anterior process of the calcaneus to the highest part of the posterior articular surface, intersecting a line along the most superior point of the calcaneal tuberosity. This angle normally is 25 to 40 degrees, and usually is compared with the contralateral side. The crucial angle (c) is directly related to the shape of the overlying lateral process of the talus. In axial compression fractures, the lateral process is driven into the calcaneus, and the crucial angle is distorted. B: The radiograph of the more significantly injured right calcaneus shows flattening of the calcaneus, reduction in the Bohler angle, and flattening of the crucial angle. C: The computed tomography scan demonstrates displacement of the posterior facet of the calcaneus, with impaction of the lateral fragment and widening of the body of the calcaneus. The lateral wall is fractured. This injury should be treated with open reduction and internal fixation. D: After reduction of the posterior facet, a cancellous bone screw is placed through the lateral joint fragment into the sustentacular fragment, securing the subtalar reduction. The lateral wall can be buttressed with a contoured Y-shaped plate or a small H-shaped plate. The Bohler angle and the height of the calcaneus are restored. |
younger children after nonsurgical management of intraarticular
calcaneal fractures (482,483). However, open reduction and internal fixation is recommended for most displaced intraarticular fractures (484).
The preferred approach is through a lateral, L-shaped incision, lifting
the peroneal tendons within their sheath and protecting the sural
nerve. The lateral wall of the calcaneus is folded down to reveal the
medial side and allow elevation of depressed central fragments.
Internal fixation of the posterior facet is achieved by placing
subchondral cancellous screws into the medial sustentaculum. The
lateral wall is buttressed with an H-shaped or Y-shaped plate (Fig. 33.84). Excessive
comminution of the articular surface precludes this type of surgery (480). Long-term results in children are usually good.
the cuboid or cuneiform bones are rare in children but can have
long-term sequelae (485,486).
Fracture of the base of the second metatarsal is usually an indication
of associated tarsometatarsal joint injury. These injuries are often
misdiagnosed and may occur more commonly than recognized (487) (Fig. 33.85).
The mechanism of injury may be direct impact but, more commonly, forced
plantar flexion of the forefoot combined with a rotational force
produces midfoot injuries (488). Heel-to-toe
compression of the foot can also produce these injuries. Dislocations
or displaced fractures require closed reduction. Percutaneous pinning
and cast immobilization may be necessary to maintain reduction.
The second, third, and fourth metatarsals are most commonly injured.
The mechanism of injury producing metatarsal fracture is usually direct
trauma or crush to the foot. Associated swelling can be significant and
may cause compartment syndrome.
usually avulsion injuries. These injuries can cause diagnostic
confusion. A transverse fracture at the junction of the metaphysis and
diaphysis is called a Jones fracture. This
fracture has a high incidence of nonunion. An oblique avulsion fracture
through the tuberosity of the fifth metatarsal may be confused with the
normal secondary ossification center of the apophysis or avulsion of
the apophysis. The apophysis does not extend into the joint. Stress
fractures may also occur at this location. Prolonged casting is
frequently required for fractures at the base of the fifth metatarsal,
and healing should be verified before resumption of activities.
 |
|
Figure 33.85 Tarsometatarsal joint injury. A file cabinet landed on the dorsum of this 4-year-old girl’s foot. A:
There is widening between the first and second metatarsals, and a small fragment of bone is seen in the space. This suggests a partial incongruity, with lateral subluxation of the meta-tarsals. B: The contralateral foot shows a normal relation of the tarsometatarsal joint. |
fractures can be immobilized in a below-knee cast for 4 to 6 weeks,
with weight bearing as tolerated. Surgical treatment is indicated for
open fractures, displaced fractures of the metatarsal heads, and
displaced intraarticular fractures (Fig. 33.86).
Kirschner wire fixation is usually adequate, but the pinning technique
requires securing the metatarsophalangeal joint in a reduced position.
If this is not done, extension contracture of the metatarsophalangeal
joint can result in development of a prominent and painful metatarsal
head. The wires are left in place for 3 to 4 weeks with
non—weight-bearing immobilization, followed by weight bearing in a cast
until union is complete.
when severe pain and swelling develop after injury. The inciting trauma
is often substantial, such as a foot run over by a car or crushed by a
heavy object. Fasciotomy is indicated if compartment syndrome is
confirmed. Compartment syndrome of the foot can involve any of the four
compartments: medial (i.e., abductor hallucis), central (i.e., flexor
brevis, lumbricals, quadratus), lateral, and interosseous (489).
Fasciotomy may be performed through a medial approach, with incision
from the medial malleolus to the first metatarsal head. The
neurovascular bundle
is
identified and released, including the tarsal tunnel. This releases the
medial compartment and allows retraction for exposure of the central,
lateral, and interosseous compartments from the plantar side.
Alternatively, two longitudinal dorsal incisions are centered over the
second and fourth metatarsals. Blunt and sharp dissection is performed
through each interspace to release all compartments. The dorsal
approach can also be combined with a medial incision to release the
medial compartment.
Fractures of the phalanges can be treated with closed management, such
as buddy taping and use of a hard-soled shoe. The exception to this is
an open fracture, which most often occurs to the proximal phalanx of
the great toe and may require open debridement and stabilization with
Kirschner wires. The physis of the great toe’s proximal phalanx
underlies the nail bed and may be injured in the same fashion as a nail
bed avulsion of the hand (490). Antibiotics
should be prescribed if there is concern about a communicating skin
breach. Obvious contamination requires debridement.
 |
|
Figure 33.86 Tarsometatarsal displacement. A:
Anteroposterior projection of the foot of a 14-year-old boy who sustained a plantar flexion injury in a motor vehicle crash. There is complete dislocation of the first metatarsal—cuneiform joint and medial displacement. There are fractures of the second, third, and fourth metatarsal shafts. The ipsilateral tibial fracture was treated with intramedullary fixation. The swelling in the foot was attributed to the tibial shaft injury, and diagnosis of the foot injury was delayed. B: The postoperative anteroposterior radiograph demonstrates reduction and pinning of the fracture-dislocation. |
IE, Williams SM, Dow N, et al. How many children remain fracture-free
during growth? A longitudinal study of children and adolescents
participating in the Dunedin Multidisciplinary Health and Development
Study. Osteoporos Int 2002;13(12):990–995.
S, Melton LJ III, Dekutoski MB, et al. Incidence of childhood distal
forearm fractures over 30 years: a population-based study. JAMA 2003;290(11):1479–1485.
DL, Loro ML, Pitukcheewanont P, et al. Increased body weight and
decreased radial cross-sectional dimensions in girls with forearm
fractures. J Bone Miner Res 2001;16:1337–1342.
A, Jones IE, Taylor RW, et al. Bone mineral density and body
composition in boys with distal forearm fractures: a dual-energy x-ray
absorptiometry study. J Pediatr 2001;139:509–515.
D, Jones G. The association between bone mineral density, metacarpal
morphometry, and upper limb fractures in children: a population-based
case-control study. J Clin Endocrinol Metab 2003;88:1486–1491.
K. Remodelling after distal forearm fractures in children II: the final
orientation of the distal and proximal epiphyseal plates of the radius.
Acta Orthop Scand 1979;50:731.
A, Gaynor T, Mubarak SJ. Premature physeal closure following distal
tibia physeal fractures: a new radiographic predictor. J Pediatr Orthop 2003;23:733–739.
JM, Gruber HE, Phieffer LS. Physeal fractures, part I: histologic
features of bone, cartilage, and bar formation in a small animal model.
J Pediatr Orthop 2002;22:703–709.
PM, Wikstrom B, Hirsch G. The influence of transphyseal drilling and
tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop 1998;18(2):149–154.
EA, Vainionpaa S, Vihtonen K, et al. The effect of trauma to the lower
femoral epiphyseal plate. An experimental study in rabbits. J Bone Joint Surg Br 1988;70(2):187–191.
KJ, Price CT, Sproul JT, et al. Acute correction and distraction
osteogenesis for the malaligned and shortened lower extremity. J Pediatr Orthop 1998;18(2):178–186.
LJ, Rivara FP, Grady MS, et al. Predictors of survival and severity of
disability after severe brain injury in children. Neurosurgery 1992;31(2):254–264.
BJ, Beaupre LA, Jones CA, et al. The effect of time to definitive
treatment on the rate of nonunion and infection in open fractures. J Orthop Trauma 2002;16:484–490.
MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting,
and intramedullary nailing in patients who have a fracture of the
tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am 1991;73(9):1316–1322.
JK, Buckley SL, Alexander AH, et al. Analgesia for the reduction of
fractures in children: a comparison of nitrous oxide with intramuscular
sedation. J Pediatr Orthop 1995;15(1):73–77.
EC, Mencio GA, Walker LA, et al. Ketamine sedation for the reduction of
children’s fractures in the emergency department. J Bone Joint Surg 2000;82A:912–918.
Academy of Pediatrics Committee on Drugs. Guidelines for monitoring and
management of pediatric patients during and after sedation for
diagnostic and therapeutic procedures. Pediatrics 1992;89(6 Pt 1):1110–1115.
J, Olerud C. Neurological complications of dynamic reduction of Colles’
fractures without anesthesia compared with traditional manipulation
after local infiltration anesthesia. J Orthop Trauma 1987;1(1):43–47.
RG, Stevens PM, Scott SM, et al. Mini-dose Bier block intravenous
regional anesthesia in the emergency department treatment of pediatric
upper-extremity injuries. J Pediatr Orthop 1994;14(4):534–537.
GM, Nowakowski R, Troshynski TJ, et al. Risk reduction in pediatric
procedural sedation by application of an American Academy of
Pediatrics/American Society of Anesthesiologists process model. Pediatrics 2002;109:236–243.
PM, Bae DS, Kadiyala RK. Short-term outcomes after surgical treatment
of traumatic posterior sternoclavicular fracture-dislocations in
children and adolescents. J Pediatr Orthop 2003; 23:464–469.
CA, Bassett GS, Sullivan S, et al. Retrosternal displacement after
physeal fracture of the medial clavicle in children. J Bone Joint Surg 2001;83B:1168–1172.
PB, Alexander MJ, Frejuk J, et al. Comprehensive functional analysis of
shoulders following complete acromioclavicular separation. Am J Sports Med 1988;16(5):475–480.
BA, Silberstein MJ, Rende RJ, et al. Arthrography in the diagnosis of
fractures of the distal end of the humerus in infants. J Bone Joint Surg Am 1986;68(4):599–602.
RS, Markowitz RI, Dormans J, et al. Ultrasonographic evaluation of the
elbow in infants and young children after suspected trauma. J Bone Joint Surg 1994;76A(12):1804–1813.
JM, Merrot T, Piclet B, et al. Anterior approach versus posterior
approach to surgical treatment of children’s supracondylar fractures:
comparative study of thirty cases in each series. J Pediatr Orthop B 1998;7:307.
J, Ishizue K, Gomez M, et al. Alteration of Baumann’s angle by humeral
position: implications for treatment of supracondylar humerus
fractures. J Pediatr Orthop 1993;13(4): 521–525.
A, Gupta SP, Sharma JC. Determination of medial epicondylar epiphyseal
angle for supracondylar humeral fractures in children. J Pediatr Orthop 1993;13(1):94–97.
TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment
pressures in children with supracondylar fractures. J Pediatr Orthop 2002;22:431–439.
SR, Hoffinger SA, Townsend DR. Early vs. delayed reduction and pinning
of type III displaced supracondylar fractures of the humerus in
children: a comparative study. J Orthop Trauma 1999;13:51–55.
LE, McKellop HA, Hathaway R. Torsional strength of pin configurations
used to fix supracondylar fractures of the humerus in children. J Bone Joint Surg Am 1994;76(2):253–256.
SS, Hahar AT, Miesen BS, et al. Displaced pediatric supracondylar
humerus fractures: biomechanical analysis of percutaneous pinning
techniques. J Pediatr Orthop 2002;22:440–443.
BA, Kasser JR, Emans JB, et al. Management of vascular injuries in
displaced supracondylar humerus fractures without arteriography. J Orthop Trauma 1990;4(1):25–29.
JC, Matthews JG, Benoit RL. Blind pinning of displaced supracondylar
fractures of the humerus in children. Sixteen years’ experience with
long-term follow-up. J Bone Joint Surg Am 1974;56(2):263–272.
S, Tredwell SJ, Beauchamp RD, et al. Management of pulseless pink hand
in pediatric supracondylar fractures of humerus. J Pediatr Orthop 1997;17(3):303–310.
RO, Dutkowsky JP, Kasser JR, et al. Neurologic complications after
K-wire fixation of supracondylar humerus fractures in children. J Pediatr Orthop 1991;11(2):191–194.
I, Bayrakci K, Tasbas B, et al. Posterior instability of the shoulder
after supracondylar fractures recovered with cubitus varus deformity. J Pediatr Orthop 2002;22:198–202.
FR, Kasser JR, Trepman E, et al. Uniplanar supracondylar humeral
osteotomy with preset Kirschner wires for posttraumatic cubitus varus. J Pediatr Orthop 1994;14(4):471–478.
HK, Lee EH, Balasubramaniam P. The lateral condylar prominence: a
complication of supracondylar osteotomy for cubitus varus. J Bone Joint Surg 1990;72B:859–861.
A, Reis M, Molina M, et al. Supracondylar fractures of the humerus
associated with ipsilateral forearm fractures in children: a report of
forty-seven cases. J Pediatr Orthop 2001;21: 307–312.
AY, Celebi L, Murath HH, et al. Closed reduction and percutaneous
fixation of supracondylar fracture of the humerus and ipsilateral
fracture of the forearm in children. J Bone Joint Surg Br 2003;85(8):1169–1172.
AD, Yiannakopoulous CK. Closed reduction and percutaneous stabilization
of pediatric T-condylar fractures of the humerus. J Pediatr Orthop 2004;24:13–16.
S, Mortensson W, Thomasson B. Prediction of the stability of minimally
displaced fractures of the lateral humeral condyle. Acta Radiol 1988;29(3):367–370.
JC, Richards JF Jr, Saltzman RI. Prevention and treatment of non-union
of slightly displaced fractures of the lateral humeral condyle in
children. An end-result study. J Bone Joint Surg Am 1975;57(8):1087–1092.
BD, Herman MJ, Crisci K, et al. Fractures of the lateral humeral
condyle: role of the cartilage hinge in fracture stability. J Pediatr Orthop 2002;22:8–11.
F, Leet AI, Jacopin S, et al. Lateral humeral condyle fractures in
children: a comparison of two approaches to treatment. J Pediatr Orthop 2004;24(4):385–391.
AC, Peterson HA, Shaughnessy WJ. Fishtail deformity following fracture
of the distal humerus in children: historical review, case
presentations, discussion of etiology, and thoughts on treatment. J Pediatr Orthop 2000;9B:309–318.
P, Ravn P, Hansen L, et al. Osteosynthesis of medial humeral epicondyle
fractures in children: 8-year follow-up of 33 cases. Acta Orthop Scand 1994;654:439.
S, Vaishya MS, Klenerman L. Management of radial neck fractures in
children: a retrospective analysis of one hundred patients. J Pediatr Orthop 1993;13:232–238.
AK, Von Laer L. Displaced fractures of the radial neck in children:
long-term results and prognosis of conservative treatment. J Pediatr Orthop 1998;7B:217–222.
P, Alvarez-Romera A, Burgos J, et al. Displaced radial neck fractures
in children treated by closed intramedullary pinning (Metaizeau
technique). J Pediatr Orthop 1997;17(3): 325–331.
MC, Graham HK. Olecranon fractures in children; Part 1: a clinical
review; Part II: a new classification and management algorithm. J Pediatr Orthop 1999;19:559–569.
P, Giacomelli M-C, Karger C, et al. Surgical technique and preliminary
results of a new fixation concept for olecranon fractures in children. J Pediatr Orthop 2003;23:398–401.
RB, Zaltz C. Anatomic investigations of the mechanism of injury and
pathologic anatomy of “pulled elbow” in young children. Clin Orthop 1971;77:134–143.
L, Delronge G, Lamoureux J. Shaft forearm fractures in children:
intramedullary nailing with immediate motion: a preliminary report. J Pediatr Orthop 1988;8(4):450–453.
SD, Comstock CP, Mubarak SJ, et al. Intramedullary Kirshner wire
fixation of open or unstable forearm fractures in children. J Pediatr Orthop 1999;19:329–337.
der Reis WL, Otsuka NY, Moroz P, et al. Intramedullary nailing versus
plate fixation for unstable forearm fractures in children. J Pediatr Orthop 1998;18:9–13.
PSH, Lam CY, Ng BKW, et al. Percutaneous transphyseal intramedullary
Kirschner wire pinning: a safe and effective procedure for treatment of
displaced diaphyseal forearm fracture in children. J Pediatr Orthop 2004;24:7–12.
JM, Beslikas T, Kapras EA, et al. Surgical treatment of unstable
diaphyseal both-bone forearm fractures in children with single fixation
of the radius. Injury 2000;31:591–596.
BJ, Olson S. Combined entrapment of the median and anterior
interosseous nerves in a pediatric both-bone forearm fracture. J Orthop Trauma 1990;4:197–199.
CE, Munshi NI, Currie L. Audit of patient satisfaction with
self-removable soft cast for greenstick fractures of the distal radius.
J Clin Eff 1997;2:14–15.
K. Remodelling after distal forearm fractures in children I: the effect
of residual angulation on the spatial orientation of the epiphyseal
plates. Acta Orthop Scand 1979;50:537.
K. Remodelling after distal forearm fractures in children III:
correction of residual angulation in fractures of the radius. Acta Orthop Scand 1979;50:741.
R, Gschwentner M, Pechlaner S, et al. Remodeling capacity and
functional outcome of palmarly versus dorsally displaced pediatric
radius fractures in the distal one-third. Arch Orthop Trauma Surg 2004;124:42–48.
RP, Danielsson LG. Dorsally angulated solitary metaphyseal greenstick
fractures in the distal radius: results after immobilization in
pronated, neutral, and supinated position. J Pediatr Orthop 1990;10(1):90–92.
KY, Chan WS, Lam TP, et al. Percutaneous Kirschner-wire pinning for
severely displaced distal radial fractures in children. J Bone Joint Surg 1995;77B:797–801.
GJ, Cowan B, Annan IH, et al. Management of completely displaced
metaphyseal fractures of the distal radius in children: a prospective
randomised control trial. J Bone Joint Surg 2002;84B:413–417.
PSH, Lam CY, Ng BKW, et al. Percutaneous transphyseal intramedullary
Kirschner wire pinning: a safe and effective procedure for treatment of
displaced diaphyseal forearm fractures in children. J Pediatr Orthop 2004;24:7–12.
BS, Esterhai JL Jr, Das M. Fracture of the distal radial epiphy-sis.
Characteristics and surgical treatment of premature, post-traumatic
epiphyseal closure. Clin Orthop 1984;185:90–96.
MK, Jakob RP, McGanity PL. Growth disturbance of the distal radial
epiphysis after trauma: operative treatment by corrective radial
osteotomy. J Pediatr Orthop 1990;10(3):411–415.
KK, Anders M, Ralph H, et al. A modeling capacity of vertebral
fractures exists during growth—an up to 47-year follow-up. Spine 2003;28(18):2087–2092.
PP, Voght MT, Ward WT. Pediatric spinal cord injury without
radiographic abnormality (SCIWORA): the absence of occult instability
and the lack of indication for bracing. Spine 2002; 27:2788–2800.
DA, Alander DH, Senica KM, et al. Burst fractures of the second through
fifth lumbar vertebrae. Clinical and radiographic results. J Bone Joint Surg Am 1996;78(8):1156–1166.
H, Mekhail A, Vogel LC, et al. Issues in surgical treatment of
thoraco-lumbar injuries associated with spinal cord injuries in
children and adolescents. Am J Orthop 2002;31:647–651.
L, Fontijne Q, Stijnen T, et al. Spontaneous remodeling of the spinal
canal after conservative management of thoracolumbar burst fractures. Spine 1998;23:1057.
LI, Serlo WS, Tervonen OA, et al. Post-traumatic findings of the spine
after earlier vertebral fracture in young patients. Spine 2000;25:1104–1108.
D, Karaiskakis M, Velmahos GC, et al. Pelvic fractures in pediatric and
adult trauma patients: are they different injuries? J Trauma 2003;54:1146–1151.
JS, Flynn JM, Koffler KM, et al. Analysis of the cause, classification,
and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop 2001;21:446–450.
JS, Flynn JS, Katz MA, et al. Role of computed tomography in
classification and management of pediatric pelvic fractures. J Pediatr Orthop 2001;21:148–151.
JM, Wong KL, Yeh GL, et al. Displaced fractures of the hip in children:
management by early operation and immobilisation in a hip spica cast. J Bone Joint Surg 2002;84B:108–112.
W. Coxa plana. A clinical and radiological investigation with
particular reference to the importance of the metaphyseal changes for
the final shape of the proximal part of the femur. Acta Orthop Scand 1965;(Suppl. 84):1–129.
JR, Cary JM. The effects of trochanteric epiphyseodesis on growth of
the proximal end of the femur following necrosis of the capital femoral
epiphysis. J Bone Joint Surg 1980;62A: 785–794.
GP, Cole WG. Effect of early hip decompression on the frequency of
avascular necrosis in children with fractures of the neck of the femur.
Injury 1996;27(6):419–421.
R, Johnson LH, Johnson RA. Variations in the intra-articular pressure
of the hip joint in injury and disease. A probable factor in avascular
necrosis. J Bone Joint Surg Am 1964;46: 509–516.
JE, Abrahams MS, Dobbs MB, et al. Early reduction, arthrotomy, and
cannulated screw fixation in unstable slipped capital femoral epiphysis
treatment. J Pediatr Orthop 2002;22(3): 352–358.
SL, Sturm PF, Tosi LL, et al. Ligamentous instability of the knee in
children sustaining fractures of the femur: a prospective study with
knee examination under anesthesia. J Pediatr Orthop 1996;16(2):206–209.
J, Molenaar C, Van Linge B. Rotational deformities after femoral shaft
fractures in childhood: a retrospective study 27–32 years after the
accident. Acta Orthop Scand 1981;302:27.
DA, Mooney JF III, Cramer KE, et al. Comparison of Pavlik harness
application and immediate spica casting for femur fractures in infants.
J Pediatr Orthop 2004;24(5):460–462.
KC, Thompson JD, Sponseller PD, et al. A prospective study of early
spica casting outcomes in the treatment of femoral shaft fractures in
children. J Pediatr Orthop 1995;15(1):30–35.
RN, Nicholson JT, Chung SM. Long-term results in the treatment of
femoral-shaft fractures in young children by immediate spica
immobilization. J Bone Joint Surg Am 1976;58(7): 945–951.
JF, Killian JT, Alonso JE. Improved treatment of femoral shaft
fractures in children utilizing the pontoon spica cast: a long-term
follow-up. J Pediatr Orthop 1995;15(1):36–40.
RA, Garvin KL, Huurman WW. Avascular necrosis of the femoral head after
closed intramedullary shortening in an adolescent. J Pediatr Orthop 1995;15(1):24–26.
AP, Schenck RC, Sponseller PD, et al. Peroneal nerve palsy after early
cast application for femoral fractures in children. J Pediatr Orthop 1992;12:25–28.
AP, Schenck RC, Sponseller PD, et al. Peroneal nerve palsy after early
cast application for femoral fractures in children. J Pediatr Orthop 1992;12:25–28.
JR, Mubarak SJ, Wenger DR. Tibial physeal closure and genu recurvatum
after femoral fracture: occurrence without a tibial traction pin. J Pediatr Orthop 1990;10(5):653–657.
E, Sagiv S, Porat S. External fixation or flexible intramedullary
nailing for femoral shaft fractures in children. A prospective,
randomised study. J Bone Joint Surg Br 1997;79(6): 975–978.
H, Hjorth K, Rehnberg L, et al. External fixation of displaced femoral
shaft fractures in children: a consecutive study of 98 fractures. J Orthop Trauma 2003;17:250–256.
JH, Austin SM, Warner WC, et al. Interlocking intramedullary nailing of
femoral-shaft fractures in adolescents: preliminary results and
complications. J Pediatr Orthop 1994; 14(2):178–183.
P, Burgos-Flores J, Rapariz JM, et al. Intra-medullary nailing of the
femur in children. Effects on its proximal end. J Bone Joint Surg Br 1995;77(2):262–266.
S, Overgaard S, Nielsen J, et al. Internal fixation of femoral shaft
fractures in children and adolescents: a ten- to twenty-one-year
follow-up of 52 fractures. J Pediatr Orthop B 1996;5:195.
JM, Luedtke LM, Ganley TJ, et al. Comparison of titanium elastic nails
with traction and a spica cast to treat femoral fractures in children. J Bone Joint Surg 2004;86A:770–777.
SD, Drvaric D, Darr K, et al. Stabilization of pediatric diaphyseal
femur fractures with flexible intramedullary nails (a technique paper).
J Orthop Trauma 1992;6(4):452–459.
WE, Roposch A. Elastic stable intramedullary nailing for unstable
femoral fractures in children: preliminary results with a new method. J Trauma 1999;47:372–378.
H, Kalenderer O, Eryanilmaz G, et al. Biological internal fixation of
comminuted femur shaft fractures by bridge plating in children. J Pediatr Orthop 2003;23:184–189.
MJ, Bathgate B, Gillingham BL, et al. Correlation of MRI and
arthroscopic diagnosis of knee pathology in children and adolescents. J Pediatr Orthop 1998;18(5):675–678.
C. Correlation of arthroscopic and clinical examinations with magnetic
resonance imaging findings of the injured knees in children and
adolescents. Am J Sports Med 1998;26:2.
LM, Scholz S, Rusch M. Characteristic patterns and management of
intra-articular knee lesions in different pediatric age groups. J Pediatr Orthop 2001;21:14–19.
JS, Pavlov H, Morris VB. Salter-Harris type-III fracture of the medial
femoral condyle occurring in the adolescent athlete. J Bone Joint Surg Am 1981;63(4):586–591.
SJ, Harvey JP Jr. Fractures of the distal femoral epiphyses. Factors
influencing prognosis: a review of thirty-four cases. J Bone Joint Surg Am 1977;59(6):742–751.
MS, Foreman ES, Micheli LJ. Laxity and functional outcome after
arthroscopic reduction and internal fixation of displaced tibial spine
fractures in children. Arthroscopy 2003;19: 1085–1090.
PJB, Fazal MA, Ashwood N, et al. An alternative to fixation of
displaced fractures of the anterior intercondylar eminence in children.
J Bone Joint Surg 2002;84B:579–582.
AS, Milano G, Tartarone M, et al. Arthroscopic treatment of malunited
and nonunited avulsion fractures of the anterior tibial spine. Arthroscopy 1998;14:233–240.
P, Panuel M, Faure F, et al. Acute fracture of the distal tibial
physis: role of gradient-echo MR imaging versus plain film examination.
Am J Roentgenol 1996;166:1203.
PG, Cooperman DR, Laros GS. Epiphyseal fractures of the distal ends of
the tibia and fibula. A retrospective study of two hundred and
thirty-seven cases in children. J Bone Joint Surg Am 1978;60(8):1046–1050.
JU, Jensen IE, Rasmussen F, et al. Osteochondral lesions of the talar
dome in children. A 24 (7–36) year follow-up of 13 cases. Acta Orthop Scand 1994;65(1):110–112.
R, Fortin P, DiPasquale T, et al. Operative treatment in 120 displaced
intraarticular calcaneal fractures. Results using a prognostic computed
tomography scan classification. Clin Orthop 1993;290:87–95.
RJ, Brown HP, Stein RE, et al. Avulsion fracture of the tuberosity of
the calcaneus in children. A report of four cases and review of the
literature. J Bone Joint Surg Am 1995;77(10): 1568–1571.
F, Faldini C, Piras F, et al. Surgical versus non-surgical treatment of
calcaneal fractures in children: a long-term results comparative study.
Foot Ankle 2000;21:825–832.
A, Benaroch TE, Guy P, et al. Clinical outcome of pediatric calcaneal
fractures treated with open reduction and internal fixation. J Pediatr Orthop 2004;24:178–180.
DR, Guille JT, Horn BD, et al. The stubbed great toe: importance of
early recognition and treatment of open fractures of the distal
phalanx. J Pediatr Orthop 2001;21:31–34.
