Meniscal Structure, Function, Repair, and Replacement
located on the medial and lateral aspects of the knee joint. They
function to absorb shock and dissipate load between the distal femur
and the proximal tibia. The knee may encounter forces two to four times
body weight while walking and almost double that while running. Due to
the high shear stresses that arise across the knee joint during
activity, injury to the menisci is quite common. Epidemiologic studies
indicate an annual incidence of meniscal tears of 60 to 70 per 100,000
people. In addition, these lesions are highly associated with both
chronic and acute anterior cruciate ligament (ACL) tears as well as
tibial plateau fractures. Initial treatment of these lesions is usually
conservative. However, the natural history of meniscal tears depends on
the specific location and characteristics of the tear within the
meniscus. Due to the relatively avascular nature of the tissue, central
injuries will often not heal. Repair and regeneration of normal
cartilage is an area of increased interest in the orthopaedic community
and a popular area for research. The focus of this chapter will be the
anatomy, structure, and function of the menisci and how this pertains
to potential treatments, including regeneration, repair, and
replacement.
to be “functionless remnants of intra-articular leg muscles.” We now
know that these wedges of fibrocartilage play a critical role in normal
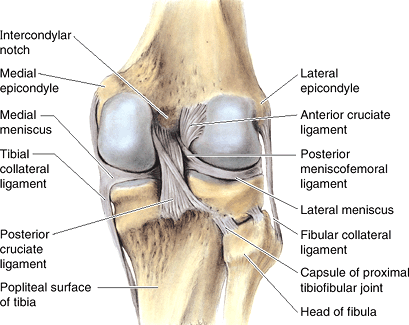
knee kinematics. There are medial and lateral menisci, which are each
divided into thirds: anterior horn, middle, and posterior horn. The
anterior and posterior horns of each meniscus attach to intercondylar
fossa (Figs. 22-1 and 22-2).
-
C-shaped
-
~3.5 cm in length
-
Greater diameter than the lateral meniscus
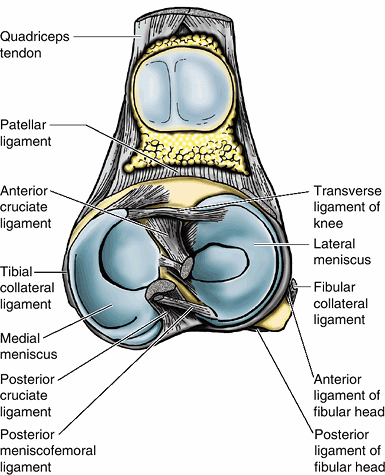 Figure 22-1
Figure 22-1
Superior view of the tibial plateau cruciate ligaments and menisci of
the knee joint. (Courtesy of Dr. W. Kucharczyk, Chair of Medical
Imaging, University of Toronto, and Clinical Director of Tri-Hospital
Magnetic Resonance Centre, Toronto, Ontario, Canada.)![]() Figure 22-2
Figure 22-2
Posterior view of the knee demonstrating meniscofemoral ligaments.
(Courtesy of Dr. W. Kucharczyk, Chair of Medical Imaging, University of
Toronto, and Clinical Director of Tri-Hospital Magnetic Resonance
Centre, Toronto, Ontario, Canada.) -
Posterior horn wider than anterior horn
-
Coronary ligaments attach the meniscus to capsule.
-
Deep bundle of the medial collateral ligament (MCL) is condensation of capsule that attaches to the meniscus at its midportion.
-
Transverse meniscal ligament connects the
anterior horns of the medial and lateral meniscus, but the attachment
to anterior horn of the medial meniscus can be variable. -
Multiple attachments to medial meniscus
are postulated to result in less mobility, to which the higher
incidence of medial meniscal tears (three times as common as lateral
meniscus) has been attributed.
-
Semicircular
-
Covers larger tibial surface area than medial meniscus
-
Anterior and posterior horns are the same width.
-
More mobile, weaker coronary ligament than medial meniscus
-
No attachment to capsule in area of popliteal hiatus
-
Meniscofemoral ligaments attach posterior
horn of lateral meniscus to medial femoral condyle and posterior
cruciate ligament (PCL).-
Ligament of Humphrey is anterior to PCL.
-
Ligament of Wrisberg is posterior to PCL.
-
One or the other may be present, but not both, in 70% to 90%.
-
proteins, chondrocytes, water, and other matrix components, each of
which plays a role in the biomechanics of the meniscus.
-
Predominant protein is collagen, which contributes 60% to 70% of the dry weight.
-
Specialized orientation to respond to large stresses (Fig. 22-3)
-
Peripheral fibers: arranged circumferentially following the contour of the meniscus
-
Woven in a mesh fashion superficially
-
Thicker and oriented more parallel at deeper layers
-
-
Radially oriented fibers: interposed
fibers that act as “ties” to prevent longitudinal tears and provide
structural stability. In addition, this arrangement helps the meniscus
to function as a “wet sponge” (see below).
-
-
Primarily composed of type I collagen (90% of total collagen)
-
While mature cartilage has no progenitor
cells, immature cartilage has a stem cell population, and it has been
hypothesized that the stem cells
P.445
differentiate into chondrocytes, which secrete type I collagen due to the tensile loads on the menisci.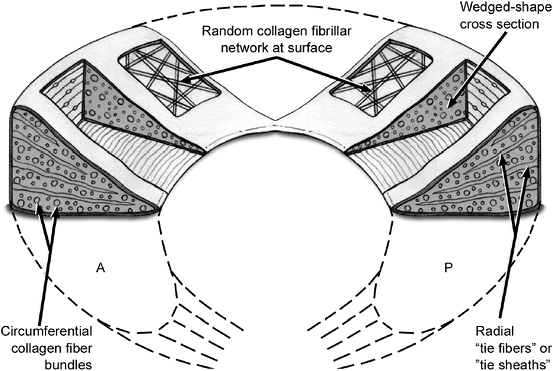 Figure 22-3 Collagen fiber ultrastructure and orientation within the meniscus.
Figure 22-3 Collagen fiber ultrastructure and orientation within the meniscus.
-
-
Less common collagen components: types II, III, V, VI
-
Larger proportion of type II collagen in the central, inner regions
-
-
-
Remaining dry weight of the meniscus comes from other proteins, including proteoglycans and elastin.
-
Interaction of collagen and proteoglycans
(“wet sponge” function): Aggregan, a common proteoglycan, combines with
glycosaminoglycans to form a macromolecule that binds positively
charged sodium ions. These ions then associate with the negative charge
on the water molecules by hydrogen bonding. When a force that spreads
the collagen fibrils apart is applied, these bonds are broken and water
exudes from the tissue. When the force is relaxed, the water is drawn
back into the cartilage by the electrostatic charge of the
glycosaminoglycans. Thus, force is dissipated through the cyclic
disruption and reformation of the hydrogen bonds with the
glycosaminoglycans.
-
-
Meniscal water content: 65% to 75%
-
Cellular component: fibrochondrocytes
-
Terminology based on microscopic appearance as well as the fibrocartilaginous matrix that they synthesize
-
Secrete collagen, proteoglycans, and enzymes for cartilage metabolism
-
Differing morphologies based upon depth
-
Superficial zone: fusiform cells
-
Remainder: more rounded
-
-
Predominately anaerobic metabolism
-
Few mitochondria
-
Prevalent Golgi complexes and endoplasmic reticulum
-
-
after birth to assume its adult, relatively avascular composition by 10
years of age (Table 22-1). In the adult, only
the peripheral third carries meaningful blood supply to either
meniscus. The menisci derive their blood supply from the superior and
inferior branches of the medial and lateral geniculate arteries as well
as the middle geniculate artery. These vessels form a perimeniscal
capillary plexus.
-
A reflection of vascular synovial tissue gives a limited vascular supply to the peripheral 1 to 3 mm of the menisci.
-
In the adult form, Arnoczky and Warren demonstrated that the peripheral 10% to 25% of the lateral meniscus is vascularized.
-
The peripheral 10% to 30% of the medial meniscus is vascularized.
-
-
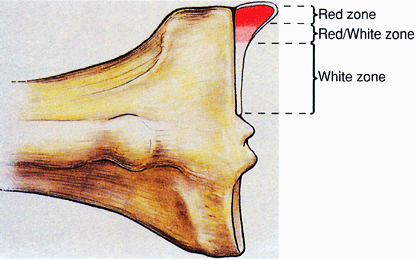
Red-and-white classification system
-
Divides the meniscus into an inner, middle, and peripheral third (Figs. 22-4 and 22-5)
-
Inner third = white/white zone (poor healing potential for tears)
-
Middle third = red/white zone (intermediate healing potential)Table 22-1 Vascularity of the Menisci
Age Vascularity Birth Entire meniscus 9 months old Peripheral two thirds 10 years old to adult Peripheral third ![]() Figure 22-4
Figure 22-4
Distribution of healing zones within the meniscus. (From Miller MD,
Warner JJP, Harner CD. Meniscal repair. In Fu FH, Harner CD, Vince KG. Knee Surgery. Baltimore: Williams & Wilkins, 1994.) -
Peripheral third = red/red zone (good healing potential)
P.446 -
-
Exception: Region of the lateral meniscus
at the popliteal hiatus, which is devoid of capsular attachments, is
avascular and effectively a watershed area, resulting in poor healing
potential.
-
-
Less vascular central two thirds depends upon synovial fluid and diffusion.
-
Joint fluid: ultrafiltrate of blood plasma combined with protein products
-
Protein products provide joint lubrication: hyaluronic acid, lubricin, collagenase, prostaglandins, and other enzymes.
-
Ultrafiltration removes red blood cells
and clotting factors, prevents formation of intra-articular fibrin clot
in response to injury.-
Hemarthrosis associated with ACL reconstructions actually promotes healing of associated meniscal repairs.
-
-
-
Nutritional molecules transported by diffusion to intra-articular structures: proteins, glucose, and other metabolic molecules
-
-
Joint forces help to distribute these nutrients into the deep layers of the meniscus.
within the menisci are distributed along the periphery. This includes
both myelinated and unmyelinated fibers. Dye and colleagues mapped the
internal structures of the knee and demonstrated that centrally located
meniscal tissue gave minimal pain awareness. In contrast, the more
peripheral tissues produced more pain awareness. Moreover, there are
mechanoreceptors in the anterior and posterior horns of the menisci
that may contribute to proprioceptive feedback at the extremes of
flexion and extension.
coefficient of friction, on the order of 0.002. For comparison, the
frictional coefficient of the knee is lower than that of ice on ice.
Synovial fluid, fluid extrusion from the meniscal cartilage, elastic
deformation of the articular cartilage, and fluid film formation all
contribute to the normal kinematics of the knee. The menisci have
several functions. They act as shock absorbers, minimize friction,
share load, reduce contact stresses, limit extremes of motion, and have
proprioceptive feedback mechanisms.
-
Menisci transfer 50% to 70% of the load with the knee in extension.
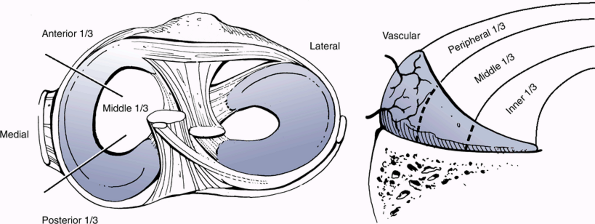 Figure 22-5
Figure 22-5
Superior view of healing zones within the meniscus. (After Siliski JM,
Leffers D. Dislocations and soft tissue injuries about the knee. In
Browner BD, Jupiter JB, Levine AM, et al, eds. Skeletal Trauma. Philadelphia: WB Saunders, 2003.) -
Menisci transfer 85% to 90% of the load with the knee in flexion.
-
Shock absorption capacity is reduced by 20% after men-iscectomy.
-
After medial meniscectomy, there is a 50% to 70% decrease in femoral condyle contact area.
-
100% increase in contact stresses
-
Relationship of shape of medial femoral
condyle and medial tibial plateau (i.e., round on round) buffers the
increased contact stress after meniscectomy.
-
-
After lateral meniscectomy, there is a 40% to 50% decrease in femoral contact area.
-
200% to 300% increase in contact stresses
-
Relationship of shape of lateral femoral
condyle and lateral tibial plateau (i.e., flat on flat) results in a
greater impact following meniscectomy than on the medial side.
-
-
Forces on medial meniscus are increased in ACL-deficient knee.
-
Increase in anterior tibial translation by 58% with medial meniscectomy in ACL-deficient knee at 90 degrees of flexion
-
The excursion of the lateral and medial meniscus is determined by their attachments (anterior to posterior).
-
Medial meniscus: average excursion 5.1 mm
-
Lateral meniscus: average excursion 11.2 mm (fewer capsular attachments)
-
-
Maximum excursion occurs at the extremes
of flexion and extension, which provides proprioceptive information to
the central nervous system through mechanore-ceptors with type I and II
nerve endings in anterior and posterior horns.
-
Increased conformity from menisci
enhances viscous hydrodynamics for fluid-film lubrication and helps
maintain low coefficient of friction.
-
Implied by results after meniscectomy
-
After meniscectomy: increased joint space narrowing, osteophyte formation, squaring of condyles, cyst formation
that lead to a decrease in the elasticity of the cartilage. The
permeability of cartilage increases, which undermines its ability to
absorb loads. The cartilage framework loses structure and total
collagen content is decreased. Proteolytic enzymes such as
metalloproteinase and cytokines such as IL-1 that have been implicated
in the catabolism of cartilage are more prevalent.
-
Increased calcium pyrophosphate dehydrate crystals
-
Decreased proteoglycan content
(chondroitin and keratin sulfate; increased ratio of chondroitin 6
sulfate to chondroitin 4 sulfate) -
Decreased elasticity
-
Most common meniscal pathology (Table 22-2)
-
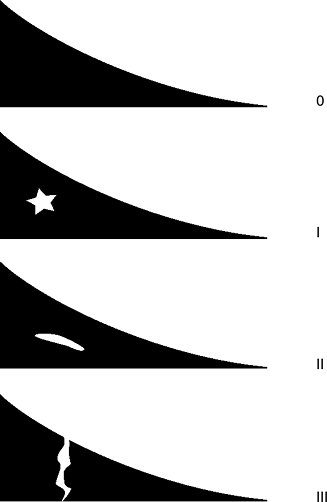
Several classification systems (Figs. 22-6 and 22-7)
-
Mechanisms of injury
-
Excessive shear force exceeding yield stress of cartilage
-
Normal forces acting on degenerative tissue
-
-
Common tears and associated injuries
-
Most common: 81% oblique or vertical longitudinal
-
Prevalence of degenerative tears (especially posterior horns) increases with advancing age.
![]() Figure 22-6
Figure 22-6
Magnetic resonance imaging classification of meniscal tears. (Thaete
FL, Britton CA. Magnetic resonance imaging. In Fu FH, Harner CD, Vince
KG, et al, eds. Knee Surgery. Philadelphia: Williams & Wilkins, 1994.)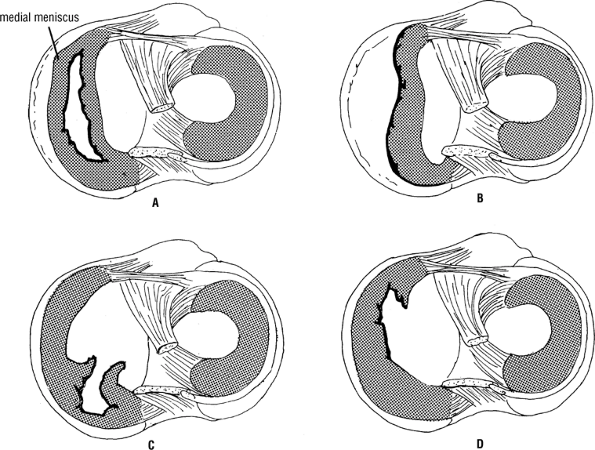 Figure 22-7
Figure 22-7
Tears of the medial meniscus of the knee joint. (A) Complete
bucket-handle tear. (B) The meniscus is torn from its peripheral
attachment. (C) Tear of the posterior portion of the meniscus. (D) Tear
of the anterior portion of the meniscus. (From Snell RS. Clinical Anatomy, 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2003.) -
Acute ACL tears associated with bucket-handle lateral meniscus tears
-
Chronic ACL tears associated with medial meniscus tears
P.448 -
-
Parameniscal cysts
-
Lateral meniscal tears more common
-
Horizontal tears more commonTable 22-2 Common Sites of Meniscus Tears
Injury Location Most common Medial meniscus Associated with ACL Lateral meniscus: bucket-handle tear Degenerative change Tear of midbody and posterior horns Meniscal cyst Lateral meniscus, horizontal tear
-
management is an appropriate first-line treatment. The focus of therapy
is to control symptoms and delay surgical intervention. Patient
response to these modalities is often idiosyncratic and unpredictable.
Given the relative lack of vascular-ity and neural supply, it is not
surprising that not all meniscal tears cause symptoms. Some may heal
spontaneously or remain asymptomatic.
-
Short, stable vertical longitudinal tears (< 1 cm)
-
Partial-thickness tears (<50%)
-
Small radial tears (<3 mm)
minimizing pain and inflammation, increasing flexibility and strength,
and optimizing function for activities of daily living. Nonoperative
modalities include physical therapy, activity
modification,
heat/cold therapy, bracing, patient education, and topical, systemic,
and intra-articular medications. However, many of these lesions persist
and surgical intervention may be indicated, including meniscectomy,
meniscal repair, and meniscal replacement (Table 22-3).
|
Table 22-3 Surgical Intervention for Meniscal Lesions
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
meniscal tear to heal it must be located peripherally within the
vascular zones of the meniscus. Furthermore, this blood supply must
allow for all the inflammatory mediators essential to the healing
response. This includes formation of a fibrin clot, resorption, and
eventual fibrous scar tissue. Weiss reported on complete healing in 65%
of stable vertical longitudinal tears at a follow-up arthroscopic
examination. Moreover, radial tears that extend to the synovial fringe
are healed with a fibrovascular scar tissue in 10 weeks (see Fig. 22-7).
Similar to articular cartilage, this scar tissue does not retain all of
the same biomechanical properties as the original meniscus. Some
remodeling does occur, although the meniscus may never assume its
original strength.
joint line pain, and effusions often persist and require surgery to
remove the offending agent.
-
All mobile fragments that can be pulled past the inner margin of the meniscus into the center of the joint
-
Remaining meniscal rim should be smoothed to prevent further tearing and stress risers.
-
A perfectly smooth rim is not necessary.
-
A probe should be used repeatedly to gain information about the mobility and texture of the remaining rim.
-
Meniscocapsular junction and peripheral meniscal rim should be protected.
-
Both manual and motorized resection instruments should be used.
-
In uncertain situations, more rather than less meniscal rim should be left.
cost of progression of degenerative disease. This dilemma has
encouraged many physicians and scientists to search for interventions
that can aid in healing, repair, regeneration, and in some cases
replacement.
characteristics of a given meniscal tear. Tears within the red/red zone
have the vascular supply to form a fibrin clot and subsequent scar.
Lesions within the red/white zone have this capacity as well, albeit to
a lesser extent. Lesions within the white/white zone do not have
reparative capacity and will not heal even with excellent surgical
technique. These lesions are a common indication for partial
meniscectomy. Operative techniques for repair include open,
arthroscopic, inside-out, outside-in, and all inside.
-
Complete vertical longitudinal tear >10 mm long
-
Meniscal tear within the peripheral 10% to 30% of the meniscocapsular junction
-
Meniscal tear that can be displaced by probing, thus demonstrating instability
-
Meniscal tear without secondary degeneration or deformity
-
Meniscal tear in an active patient
-
Meniscal tear associated with concurrent ligament stabilization or in a ligamentously stable knee
-
Accessory vascular channels from the periphery can be made to promote a healing response to central lesions.
-
Meniscal trephination is a procedure
through which horizontally oriented holes are made to provide a
vascular channel while minimizing the detrimental biome-chanical
effects on the circumferentially oriented collagen fibers. -
Synovial abrasion can be used to provoke a vascular response.
-
Exogenous fibrin clots have been shown to
release che-motactic mediators that may stimulate dormant
fibro-chondrocytes to synthesize the matrix components necessary for
meniscal repair.
meniscectomy are closely tied to the vascular supply. Furthermore, the
scar tissue that forms does not retain all the same biome-chanical
properties as the original meniscal tissue. Animal experiments have
shown regeneration of “meniscal-like” tissue after total meniscectomy.
This tissue remodels and by 7 months has the microscopic appearance of
fibrocartilage. However, the radius of this tissue is only a fraction
of the original meniscus. In addition, this was after total
meniscectomy with excision into the peripheral vasculature. In
specimens with subtotal meniscectomies or those with concomitant
synovectomies, meniscal regrowth was limited. The long-term function of
the tissue that does regenerate is still a subject of controversy and
has limited clinical applications at the current time. Many
investigators have advocated collagen scaffolds on which to produce
tissues. These products have yet to prove their efficacy in the
orthopaedic community.
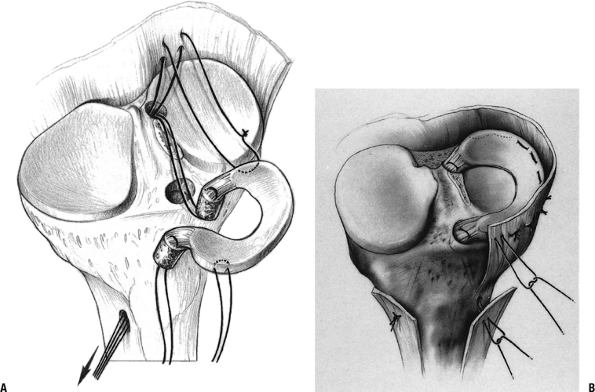
regenerate, many physicians have turned to meniscal transplantation as
a potential solution. Several techniques have been described, but
meniscal transplants with appropriately sized bone plugs have had good
results (Fig. 22-8). Cryopreserva-tion has been
used to transplant allografts with viable fibro-chondrocytes, which
have a theoretically higher healing potential. However, studies
comparing fresh, cryopreserved allografts and deep-frozen allografts
showed similar incorporation features. Analysis 6 months
postoperatively demonstrated a normal histologic pattern, normal
tensile properties, increased water content, and decreased proteoglycan
content. The long-term results are still a topic of debate. As with
other transplant procedures, meniscal transplant
does carry the risk of viral transmission: Nemzek and colleagues demonstrated the transmission of a virus in a feline model.
 |
|
Figure 22-8
Diagram of a meniscal allograft transplant. (From Goble EM, Kane SM, Wilcox TR, et al. Meniscal allografts. In McGinty JB, Caspari RB, Jackson RW, et al, eds. Operative Arthroscopy. Philadelphia: Lippincott-Raven, 1996.) |
-
Previous total or near-total meniscectomy
-
Joint line pain
-
Early osteoarthritic changes
-
Normal anatomic alignment
-
Ligamentously stable knee or amendable to ligament reconstruction
to change the way meniscal injuries are treated. Meniscal scaffolds
continue to improve. Mesenchymal stem cells with regenerative capacity
and their application to the meniscus are being studied. Gene therapy
using recombinant retroviruses to deliver growth factors, cytokines,
and other matrix components may also aid the regenerative process.
Cultured fibro-chondrocytes may also allow meniscal growth.