CHONDRAL INJURIES
difficult challenge. Because joint surfaces have a limited intrinsic
capacity for repair after they are damaged, they remain abnormal and
may deteriorate over time. The natural history of chondral injuries has
not been well defined, and there is a wide range of injuries from small
partial-thickness lesions to large full-thickness articular defects.
When considering treatment, you must include factors such as lesion
location, size, and depth, associated instability, malalignment, age,
and activity level (17). Although a broad range
of treatment methods has been developed and short-term successes have
been reported by many, no procedure has ever been shown to consistently
restore a damaged articular surface to normal. Controlled studies
comparing several current treatment methods for articular cartilage
injuries are underway; however, long-term follow-up data will be needed
before conclusions can be drawn.
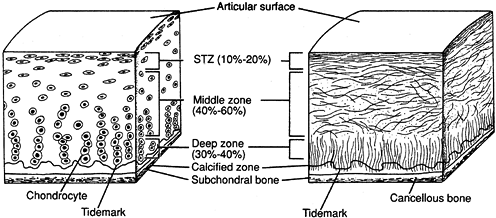
cartilage are defined by its composition and structure. Normal
articular cartilage has a highly organized structure composed of a
small chondrocyte population surrounded by a large extracellular matrix
(4,5). The matrix elements include collagen (primarily type II), proteoglycan aggrecan, glycoproteins, and water (4,18,20). Up to 80% of the weight of normal articular cartilage is water (4,22).
Within this matrix, chondrocytes synthesize developing articular
cartilage and play a role in both cartilage maintenance and degradation
(5).
and liquid) that provides its remarkable mechanical qualities. The
solid phase consists of collagen and proteoglycans, and the liquid
phase contains primarily water and electrolytes (4). When a compressive load is applied to a normal articular surface, the fluid phase takes the majority of the initial stress (5,22).
Fluid flows out of the matrix to absorb the force. After a period of
continuous loading, fluid has diffused out of the matrix, and the solid
phase bears the load. When the load is removed, the fluid flows back
into normal cartilage. In abnormal cartilage, the balance of
fluid is poorly controlled, leading to abnormal force transmission and abnormal water concentration (19). A cycle of progressive damage is thus initiated (Fig. 86.1).
 |
|
Figure 86.1.
Articular cartilage structure. (From Buckwalter JA, Mow VC, Ratcliffe A. Restoration of Injured or Degenerated Articular Cartilage. J Am Acad Orthop Surg 1994;2:193, with permission.) |
described a grading system for articular damage that was based on his
findings with open operative procedures for patellar chondromalacia.
This system was based on the size and gross appearance of surface
lesions and did not include factors such as lesion depth or location or
associated pathology on other joint surfaces. Although it was developed
in the prearthroscopic era and is highly subjective, it remains the
most widely used grading system for articular cartilage injury. Noyes
and Stabler (23) refined the Outerbridge
articular injury classification in 1989, basing their changes on
arthroscopic findings. The articular lesion is defined on the basis of
depth, diameter, and location. Lesions less than 10 mm are not
considered clinically significant (Table 86.1).
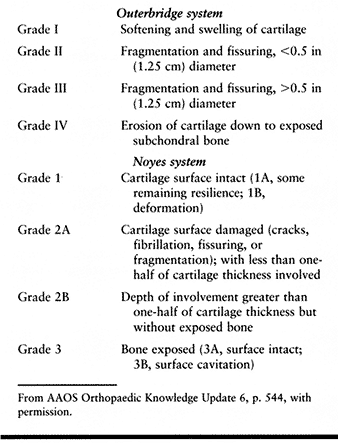 |
|
Table 86.1. Classification of Articular Injuries
|
Necrosis of damaged cells begins immediately and depends on injury
severity, blood supply, and oxygen content. Next, an inflammatory phase
is mediated by the vascular system, with an influx of undifferentiated
cells. A dense fibrin network forms the cellular “glue” to hold the
tissue together (20). Finally, tissue repair occurs with neovascularization of the fibrin network into granulation tissue and scar.
When a partial-thickness injury occurs, a chondral fissure or flap is
often produced. The injured chondrocytes undergo necrosis with some
matrix disruption, depending on degree of injury. But because the
articular
surface is avascular, the normal cycle of tissue repair cannot proceed.
Without a blood supply, no hematoma is formed, fibrin is not produced,
and the inflammatory phase cannot occur (5,18).
In addition, without a fibrin network, the repair phase is very
limited. The small amount of repair is generated solely by the
remaining viable chondrocytes, as no supply of undifferentiated cells
is available. These chondrocytes proliferate, but their response is
incomplete and short-lived.
Larger lesions with articular flaps and loose articular debris,
however, cause joint effusion with mechanical symptoms and have the
potential to progress to significant degenerative arthritis. Blunt
trauma to articular cartilage is a particularly insidious injury.
Initially, the gross appearance of the articular surface may be normal;
however, microscopic examination will reveal disorganized collagen,
increased water concentration, and decreased proteoglycan concentration
(5). This type of blunt articular injury can later progress and degenerate.
case of articular injury. Although the exact etiology is unknown, both
the juvenile and adult forms likely develop from exercise-related
repetitive subthreshold trauma that leads to subchondral stress
fractures (6). The articular injury results
when an osteochondral fragment becomes loose or separates. Ideally, one
can initiate conservative or minimally invasive treatment before an OCD
lesion separates. In the juvenile form, the open physes provide the
potential for spontaneous healing, and the prognosis is good. The adult
form, however, has little potential for healing and the prognosis is
poor, with frequent early degenerative arthritis.
physical examination because their clinical presentation may be
indistinguishable from or may coexist with a torn meniscus. Joint
effusion and mechanical symptoms may be absent, and persistent pain
after an injury may be the only complaint. Although a nonspecific
finding, point tenderness over an area of articular damage can
occasionally be elicited. The mechanism of articular injury is usually
a twisting or shearing force, but articular damage may result also from
direct trauma. The frequent association of chondral injury with chronic
anterior cruciate instability has been well documented (7,13,36).
presence of fat globules suggests a full-thickness chondral injury or
osteochondral fracture. A high index of suspicion must be maintained to
recognize a probable articular injury.
with conventional magnetic resonance imaging (MRI); however, the
majority of articular lesions are found at the time of arthroscopic
surgery. Specialized MRI using fast-spin echo sequences has been shown
to have enhanced accuracy for partial- and full-thickness articular
injuries and may allow preoperative diagnosis (29).
arthroscopy, and it is important to distinguish symptomatic lesions
from those that are incidental findings. Correlate preoperative
symptoms and physical findings, and look for associated pathology.
Because partial-thickness injuries tend to remain stable over time,
treat only those areas that have large articular flaps and impending
loose bodies. To avoid making grooves or channels in the remaining
cartilage surface, when debriding a lesion, rotate the cutting surface
of the arthroscopic debrider blade 90°, and use only the blade edge in
a tangential fashion to resect articular flaps (Fig. 86.2).
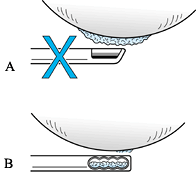 |
|
Figure 86.2.
Technique for arthroscopic debridement of loose chondral flaps. A: Do not debride with the mouth of the debrider directed toward the articular surface. B: Keep the mouth of the debrider at right angle (tangential) to the articular surface. |
healing and may cause degeneration of the underlying cartilage. Despite
the improved appearance of the articular surface after chondral
shaving, Kim et al. (16) showed that further
surface degeneration is common. Do not convert a partial-thickness
injury to a full-thickness defect. Although there is enhanced healing
potential when the subchondral bone is involved, there is little
assurance that the fibrocartilage repair response will be any better
than
a carefully debrided partial-thickness injury, and it may lead to further deterioration of the damaged joint surface.
appearance of partial-thickness articular damage, but it has not been
shown to stimulate healing. In addition, with a laser, there is the
potential for thermal damage to the cartilage deep to the point of
laser application that may later lead to development of a
full-thickness defect or osteonecrosis. Recently, radiofrequency energy
has been suggested as a means of stimulating articular healing (14), but further clinical studies are needed.
OCD are very different, the treatment principles are similar. When a
fragment separates from a weight-bearing surface, replace all
potentially viable fragments, as removal of these fragments has a
predictably poor outcome (6,33).
Only those occasional fragments from non-weight-bearing surfaces may be
removed with minimal adverse sequelae. Despite the frequent progression
of adult OCD to degenerative arthritis, aggressive treatment with
reattachment of the articular fragment may slow the progression or
lessen the severity of the arthritis. After a full-thickness defect
develops from an OCD lesion, one of the many treatments for focal
defects can be used. Lesion size and location will help determine which
treatment method is best. With osteochondritis dissecans, there may be
an associated large bone defect that may require supplemental bone
grafting.
have been developed for focal full-thickness chondral defects. These
techniques began with open debridement, spongialization, and osteotomy;
evolved into arthroscopic debridement, abrasion arthroplasty, and
microfracture; and now include autogenous osteoarticular mosaicplasty,
autogenous chondrocyte implantation, and allograft replacement. As is
often the case when many treatment options are used, no single method
has been shown to be superior on a consistent basis. Because some good
results have been reported with each technique, decision making for the
treatment of full-thickness defects is challenging.
basic techniques: (a) stimulation of intrinsic healing potential, (b)
alteration of loads, (c) transfer of autogenous tissue and cells, and
(d) transfer of allograft tissue. Exact guidelines and indications for
each technique are controversial. Many factors, including lesion
location, size, and depth, patient age, activity level, alignment, and
ligamentous instability, must be considered; and deciding which lesions
to treat is difficult, as the natural history of chondral injuries is
poorly understood (22). Equally important is the experience of the surgeon, as many of these techniques are technically demanding.
degenerative articular surfaces in 1959, many surgeons have developed
techniques to stimulate healing of worn or damaged articular surfaces (11).
Debridement with drilling, abrasion arthroplasty, and microfracture all
share the basic principles of removing loose debris and degenerative
cartilage, and penetrating the subchondral bone to produce bleeding.
Using hand awls, instead of power drills, to create subchondral holes
avoids thermal injury and creates microfractures of the trabecular bone
(24,34). This in turn stimulates a tissue healing response (Fig. 86.3; see also COLOR FIG. 86.3).
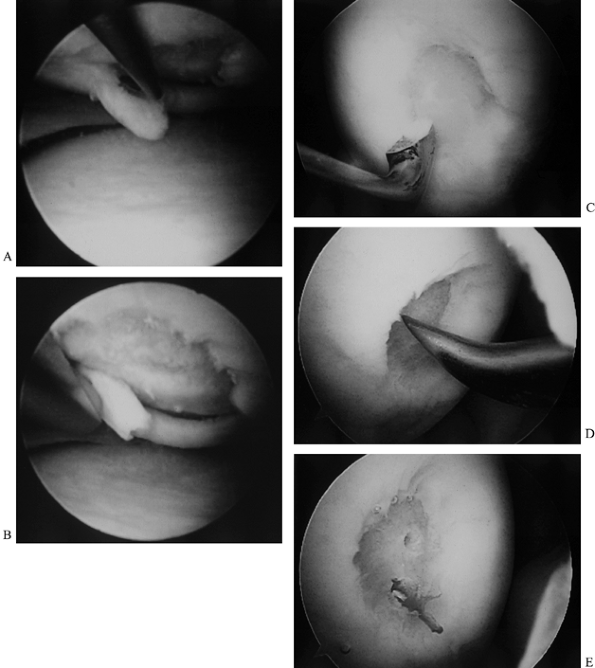 |
|
Figure 86.3. (See COLOR FIG. 86.3.) Technique for debridement, abrasion arthroplasty, and microfracture. A: Arthroscopic view shows a chondral flap tear on the medial femoral condyle. B:
The unstable flap and unstable edges of the cartilage are debrided. Note that this is a full-thickness defect exposing underlying subchondral bone. C: The debridement is completed by smoothing the edges with a curet. D: An awl is used to punch through (microfracture) the subchondral bone to bring cells into the defect. E: Two microfracture holes, with bleeding from one. |
cell line is released and, in the proper environment, can differentiate
into a chondrogenic cell line (20,24).
Although the perfect environment for chondrogenesis has not been
defined, factors such as protected postoperative weight bearing and
continuous passive motion (CPM) have been associated with enhanced
healing (34,35). Other
factors, such as transforming growth factor-β1, insulin-like growth
factor (ILG-1), and bone morphogenic protein (BMP), appear to play an
important role in the cartilage-healing environment and are being
studied (4,5).
penetration does not produce normal articular cartilage. Unlike hyaline
cartilage, which contains primarily type II collagen, the regenerated
cartilage from these techniques is fibrocartilage with a high
concentration of type I collagen (18). It is not as smooth or durable as normal hyaline cartilage and tends to deteriorate over time (4,17).
has a much better prognosis than a defect in an arthritic joint with
diffuse chondral degeneration. Several studies have shown that drilling
and abrasion of arthritic knees do not provide significant benefit over
arthroscopic debridement alone and may actually accelerate the
degenerative process (1,30).
Smaller defects also have a better prognosis than larger ones, and
unipolar defects have a better prognosis than bipolar “kissing
lesions.” Overall, the short-term outcome from abrasion, drilling, and
microfracture is fairly good, with variable degrees of symptomatic
improvement for several years. The fibrocartilaginous repair tissue
does deteriorate over time.
attempt to provide symptomatic relief for patients with damaged or
degenerative joints. Its underlying principle is to shift a force
concentration overload away from a damaged joint surface. Initially,
symptomatic improvement can be achieved for patients with isolated
unicompartmental disease, but results deteriorate with time. Insall et
al. (12) reported good and excellent results from
proximal tibial osteotomy in 85% of patients at 5 years but noted only
37% excellent results after 9 years. Some healing of articular lesions
has been documented at second-look arthroscopic surgery after proximal
tibial osteotomy (8), but preoperative arthroscopic evaluation has not been found to be a positive predictor of outcome (15).
consider osteotomy to correct significant associated limb malalignment.
The role of osteotomy in a normally aligned limb is limited because the
potential benefits are small, the potential complications are
significant, and the potential compromise of future prosthetic
reconstruction is well known (38).
attractive option, as it has the potential to transfer normal articular
cartilage into a damaged area. There are no risk of disease
transmission, no problem with tissue rejection, and a high rate of
union. In addition, chondrocyte viability is maintained with fresh
autogenous grafts (22). Unfortunately, the
supply of expendable autogenous osteoarticular grafts is limited, and
donor site morbidity is a major concern.
In animal studies, the articular cartilage in the transplanted
osteoarticular plugs has been shown to survive, with fibrocartilage
filling the area between the plugs. The donor sites were also found to
fill with fibrocartilage. Early clinical outcomes are promising, but
the long-term outcome with this procedure is not known.
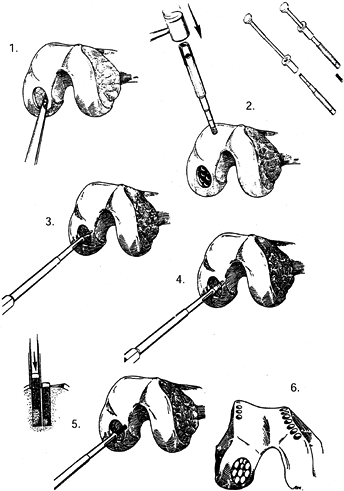 |
|
Figure 86.4.
Technique for osteoarticular mosaicplasty. 1: Preparation of recipient site. 2: Harvest of the grafts. 3,4: Preparation for the plug grafts. 5: Insertion of the plugs. 6: Completed mosaicplasty. (From Mandelbaum BR, Browne JE, Fu F, et al. Articular Cartilage Lesions of the Knee. Am J Sports Med 1998;26:855, with permission.) |
usually not sufficient expendable articular cartilage to fill the
defect by mosaicplasty. For femoral lesions, Outerbridge et al. (27)
reported the use of a lateral patellar autograft. Graft incorporation
was not a problem, and subsequent biopsy of transplanted articular
cartilage demonstrated that the hyaline cartilage survived. However,
donor site morbidity remains a major concern. Most of these cases
necessitated removal of the lateral third of the patella, and at an
average follow-up of 6.5 years, four patients had mild anterior knee
pain, two had flexion contractures, and five had nonpainful patellar
crepitation. Once again, short-term results are encouraging, but the
long-term outcome is unknown.
of articular defects has been reported both in animal models and in
humans. The principle behind these procedures is to provide a new
source of chondrogenic cells to repair the defect. Several series have
been published, and some successful regeneration of hyaline cartilage
has been achieved (21,25,26,31),
but there are no long-term data or controlled studies. The limited
supply of expendable perichondrium limits its use and makes periosteum
a more attractive option. Meticulous technique is required in both
graft harvest (to maintain cell viability) and graft fixation (to avoid
unstable or redundant repair tissue). There is evidence that this
technique works best in younger patients (5), and some problems with graft calcification have also been reported (2,24).
published their initial clinical series of 23 patients with
full-thickness chondral defects treated with autologous chondrocyte
implantation (ACI). Their technique, which was developed initially in
an animal model, involves a two-stage surgical procedure (9).
At arthroscopy, cartilage slices are harvested from the
non-weight-bearing trochlear margins or intercondylar notch, placed in
sterile medium, and sent to the laboratory. The chondrocyte
cells
are isolated and cultured over 14–21 days to increase the number of
cells 10- to 20-fold. A second open surgical procedure is performed in
which a periosteal patch is sutured over the chondral defect and the
cultured chondrocytes are injected under the patch. Fibrin glue is used
to achieve a watertight seal (Fig. 86.5).
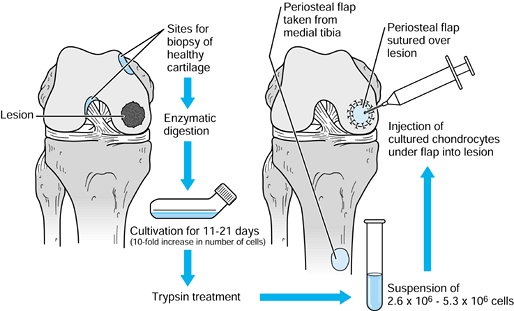 |
|
Figure 86.5.
Technique for autologous chondrocyte implantation. (Redrawn from Mandelbaum BR, Browne JE, Fu F, et al. Articular Cartilage Lesions of the Knee. Am J Sports Med 1998;26:856, with permission.) |
reported 87% good and excellent results with femoral condyle lesions
and noted repair of cartilage with a hyaline-like appearance at
second-look arthroscopy in 73% of these lesions. Defects involving the
patellofemoral joint did poorly, with only 28% good and excellent
results. This was felt in part to be due to underlying patellar
malalignment that was not corrected at the time of ACI.
demonstrated an abundance of type II collagen. Animal studies have
demonstrated that the repair is initiated by the injected chondrocytes,
which repopulate the defect, and not by the periosteal patch (9).
In addition, it is of particular interest that repeat arthroscopy on
the successfully treated femoral condyle lesions demonstrated
improvement of the hyaline-like repair tissue over time. Since the
initial clinical report, tremendous interest in ACI has been generated,
and the technique has been used in 1,896 cases recorded in the Genzyme
Tissue Repair Registry (Genzyme, Inc., Cambridge, MA). The early data
suggest moderate success with femoral defects, but few surgeons have
duplicated the success rate of Peterson and the early Swedish
experience (3). This underscores the importance
of meticulous surgical technique, careful patient selection, and
surgeon experience. Long-term controlled studies are in progress.
articular surfaces has been the subject of studies for many years. The
advantages of allografts include potential availability of young donor
cartilage, graft material from identical anatomic location
(orthotopic), and avoidance of donor-site morbidity. Disadvantages,
however, include possible disease transmission and immunologic
reactions (22). Freezing the allograft tissue
decreases the immunogenicity of the subchondral bone but damages the
viability of the transplanted chondrocytes (37).
been followed long enough to determine the long-term outcome, but
several authors have reported promising early results (32). The best results appear to be in unipolar traumatic lesions treated with young orthotopic allografts (22).
Osteoarticular allograft replacement should be reserved for patients
with massive osteoarticular defects that cannot be treated with other
methods. It can also be considered as a salvage procedure for patients
who have large defects for which conventional treatments failed.
injuries are controversial, it is clear that any underlying ligamentous
instability or malalignment must be corrected if the chondral injury
treatment is to succeed. Although we recognize that abrasion
arthroplasty and microfracture produce fibrocartilage repair tissue and
not hyaline cartilage, our bias is to use this technique as the initial
treatment for small and medium-sized focal chondral defects. It is an
easy, inexpensive, low-morbidity procedure that can be performed at the
time of initial arthroscopy. Should
this
fail, we use a secondary technique with ACI or osteoarticular plug
autografts (mosaicplasty). Previous penetration of the subchondral bone
does not appear to compromise outcome after ACI, as 7 of 23 patients in
Brittberg et al.’s (3) original series had undergone drilling initially.
lesions on weight-bearing femoral condyles in young active athletes, we
favor ACI or mosaicplasty as the initial procedure because abrasion
arthroplasty with microfracture is less likely to succeed (Fig. 86.6) (17).
In addition, for massive articular defects, particularly those
associated with significant bone loss, osteoarticular allograft is the
salvage option of choice. Until long-term data from controlled studies
are available, use the above recommendations as general guidelines.
Individualize the treatment of each patient, and perform only those
procedures in which you have mastered technical proficiency.
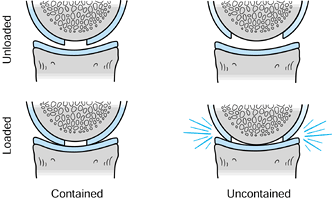 |
|
Figure 86.6.
Loading of contained and uncontained articular defects. (Redrawn from Mandelbaum BR, Browne JE, Fu F, et al. Articular Cartilage Lesions of the Knee. Am J Sports Med 1998;26:858, with permission.) |
ligament tears and meniscus injuries cannot be overemphasized. All too
often, a patient is referred for orthopaedic consultation with an MRI
scan showing a meniscus tear. The patient arrives, anticipating that a
“work order” for arthroscopic meniscectomy is all that is required.
After the simple procedure is completed, the patient assumes that he or
she will be “normal” again. The possibility of a chondral injury or
defect may never have been considered. If the findings at surgery are
primarily chondral damage and the outcome is not “normal,” the patient
becomes confused and upset. To avoid this, we recommend a brief
preoperative discussion of possible chondral damage with all patients
undergoing arthroscopic knee surgery. If MRI has been performed, we
review the images with the patient, explain the limitations of the
study, and discuss the possibility of additional findings at surgery
that may require additional treatment.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JM, Maschka K. The Arthroscopic Treatment of Unicompartmental
Gonarthrosis: A Five-year Follow-up Study of Abrasion Arthroplasty plus
Arthroscopic Debridement or Arthroscopic Debridement Alone. Arthroscopy 1989;5:25.
SJ, Beckers JM, Kuijer R, et al. Long-term Results of Rib Perichondrial
Grafts for Repair of Cartilage Defects in the Human Knee. Int Orthop 1997;21:313.
M, Lindahl A, Nilsson A, et al. Treatment of Deep Cartilage Defects in
the Knee with Autologous Chondrocyte Transplantation. N Engl J Med 1994;331:889.
Y, Masuhara K, Shiomi S. The Effect of High Tibial Osteotomy on
Osteoarthritis of the Knee: An Arthroscopic Study of 54 Knee Joints. Orthop Clin North Am 1979;10:585.
DA, Pitman MI, Peterson L, et al. The Repair of Experimentally Produced
Defects in Rabbit Articular Cartilage by Autologous Chondrocyte
Transplantation. J Orthop Res 1989;7:208.
DL, Urban WP, Caborn DNM, et al. Articular Cartilage Changes Seen with
Magnetic Resonance Imaging-detected Bone Bruises Associated with Acute
Anterior Cruciate Ligament Rupture. Am J Sports Med 1998;26:409.
L, Uribe JW, Saskin H, Markarian G. The Viability of Articular
Cartilage following Radiofrequency-generated Energy Treatment. Arthroscopy 1999;15:569(abst).
HK, Moran ME, Salter RB. The Potential for Regeneration of Articular
Cartilage in Defects Created by Chondral Shaving and Subchondral
Abrasion. J Bone Joint Surg Am 1991;73:1301.
ME, Kim HK, Salter RB. Biological Resurfacing of Full-thickness Defects
in Patellar Articular Cartilage of the Rabbit: Investigation of
Autogenous Periosteal Grafts Subjected to Continuous Passive Motion. J Bone Joint Surg Br 1992;74:659.
SW, Keeley FW, Salter RB. The Chondrogenic Potential of Free Autogenous
Periosteal Grafts for Biological Resurfacing of Major Full-thickness
Defects in Joint Surfaces under the Influence of Continuous Passive
Motion: An Experimental Investigation in the Rabbit. J Bone Joint Surg Am 1986;68:1017.
SW, Salter RB. The Repair of Major Osteochondral Defects in Joint
Surfaces by Neochondrogenesis with Autogenous Osteoperiosteal Grafts
Stimulated by Continuous Passive Motion: An Experimental Investigation
in the Rabbit. Clin Orthop 1986;208:131.
HK, Outerbridge AR, Outerbridge RE. The Use of a Lateral Patellar
Autologous Graft for the Repair of a Large Osteochondral Defect in the
Knee. J Bone Joint Surg Am 1995;77:65.
HG, Linklater JM, Allen AA, et al. Magnetic Resonance Imaging of
Articular Cartilage in the Knee: An Evaluation with Use of
Fast-spin-echo Imaging. J Bone Joint Surg Am 1998;80:1276.
JJ, Steadman JR, Silliman JF, Fulstone HA. Improvement of
Full-thickness Chondral Defect Healing in the Human Knee after
Debridement and Microfracture Using Continuous Passive Motion. Am J Knee Surg 1994;7:109.
RB, Simmonds DF, Malcolm BW, et al. The Biological Effect of Continuous
Passive Motion on the Healing of Full-thickness Defects in Articular
Cartilage: An Experimental Investigation in the Rabbit. J Bone Joint Surg Am 1980;62:1232.
MF, Warren RF, Marshall JL, Savatsky GJ. A Clinical and Radiographical
Analysis of 127 Anterior Cruciate Insufficient Knees. Clin Orthop 1988;227:229.
S, Dannucci GA, Sharkey NA, Pool RR. The Fate of Articular Cartilage
after Transplantation of Fresh and Cryopreserved Tissue-antigen-matched
and Mismatched Osteochondral Allografts in Dogs. J Bone Joint Surg Am 1989;71:1297.
