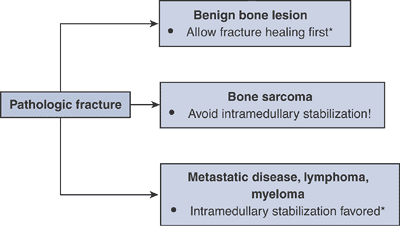
Pathologic Fractures
– Evaluation and Management of Musculoskeletal Oncology Problems > 4
– Treatment Principles > 4.5 – Pathologic Fractures
abnormal bone. The abnormality in the bone may be due to metabolic
diseases (such as osteoporosis, osteomalacia, or Paget’s disease),
benign lesions, sarcomas, lymphoma, metastatic disease, and myeloma.
This section focuses on management of pathologic fractures in bone
sarcomas and in disseminated malignancy (metastatic disease and
myeloma).
underlying diagnosis should be established prior to embarking on a
treatment plan (Algorithm 4.5-1). The most
serious mistake one can make is to assume that a pathologic fracture is
due to a disseminated malignancy only to find out after the surgical
procedure that the actual diagnosis is that of a bone sarcoma.
Other than for some benign bone lesions and very carefully selected
cases of widely disseminated metastatic disease, biopsy is often
necessary to establish the diagnosis.
-
Presence of a fracture should not preclude an adequate preoperative work-up.
-
Many benign bone lesions can be managed without biopsy initially, allowing healing of fracture if specific criteria are met:
-
Proximal femoral benign lesions with displaced pathologic fractures heal with malunion if not treated operatively.
-
Intramedullary reamings are not a good source of biopsy material.
-
Await final frozen section diagnosis before proceeding with operative intervention.
 |
|
Algorithm 4.5-1. General treatment of pathologic fractures. *General treatment principles only.
|
clinician with two difficult decisions. First, initial fracture
management must be carefully executed to minimize complications and, in
the case of high-grade sarcomas, to allow completion of neoadjuvant
chemotherapy. Second, since pathologic fracture has been associated
with a poorer overall prognosis, a decision must be made between
amputation and limb-sparing surgery, taking into account the associated
fracture and its initial treatment.
|
Table 4.5-1 Alternatives for Initial Management of Pathologic Fracture Through Bone Sarcoma
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Minimize tumor contamination of uninvolved areas
-
Avoid spreading tumor proximally and distally within intramedullary canal.
-
Avoid exposure of vital neurovascular structures to tumor.
-
Avoid spreading tumor to uninvolved muscle compartments.
-
Avoid loading pulmonary vasculature with tumor-laden reamings.
-
-
Allow completion of neoadjuvant chemotherapy.
-
Preferred: casting or traction
-
Less viable alternatives
-
Spanning external fixation
-
Limited internal fixation with plate/screws
-
-
Contraindicated: intramedullary stabilization
-
Contaminates uninvolved proximal and distal bone, soft tissues
-
Reamings may disseminate tumor into pulmonary vasculature.
-
-
Fracture hematoma increases local tumor dissemination.P.94
-
Difficulty predicting extent of tumor
-
Consequent increased risk for local recurrence
-
-
More difficult to manage resection intraoperatively, particularly with unstable fracture or deformity
-
Consequent increased risk of tumor spillage or positive margin
-
-
With adjuvant chemotherapy, osteosarcoma
and Ewing sarcoma oncologic survival outcome equivalent between
amputation and limb-sparing surgery
malignancies, including metastatic disease, myeloma, and lymphoma, are
to relieve pain, preserve function, and maintain independence. These
goals may be accomplished by means of surgery, bracing, radiotherapy,
chemotherapy, bisphosphonates, or a combination. Consideration for
nonorthopaedic care should be given in consultation with medical and/or
radiation oncologists. Medical oncologists should be consulted to
estimate expected survival.
-
X-rays of entire long bones and adjacent joints should be obtained to evaluate for other lesions.
-
Consider complete staging to assess for extent of disease.
-
Extent influences the prognosis, which should be considered prior to operative intervention.
-
Impending pathologic fractures may become apparent in other bones.
-
-
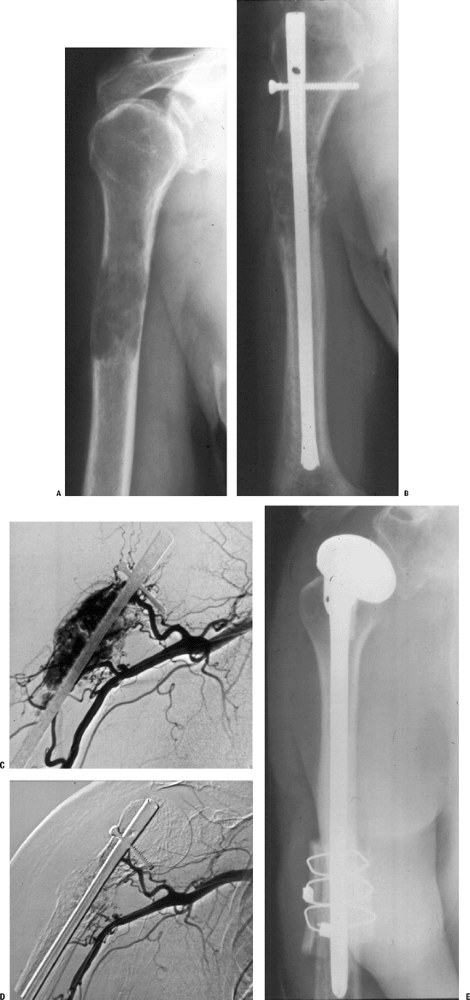
Consider preoperative embolization for renal cell metastases due to their high propensity for intraoperative hemorrhage (Fig. 4.5-2).
|
Table 4.5-2 Surgical Principles for Pathologic Fractures Due to Disseminated Malignancy
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
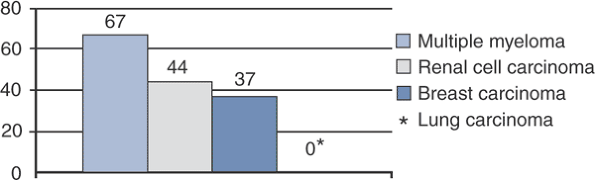
Figure 4.5-1
Graph of pathologic fracture healing rates according to underlying disease. (Data from Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res 1983;178:297–302.) |
-
Disease recurrence
-
Radiosensitive tumors are less likely to recur locally following postoperative irradiation (especially breast cancer, myeloma; Fig. 4.5-3).
-
Relatively radioresistant tumors must be watched closely (especially renal cell carcinoma; see Fig. 4.5-2).
-
-
Failure of fixation
-
Tumors with higher potential to heal fractures are less likely to incur fixation failure (e.g., myeloma; see Fig. 4.5-1).
-
Tumors with lower potential to heal
fractures are more likely to incur fixation failure (e.g., lung
carcinoma), but patients may not live long enough to have a problem.
-
 |
|
Figure 4.5-2 A lytic lesion secondary to metastatic renal cell carcinoma (A) has been treated with intramedullary stabilization and radiotherapy (B). Due to progression of the tumor, embolization and resection was elected. Pre-embolization (C) and post-embolization (D) angiography of the lesion shows the diminished tumor blush. (E) The proximal humerus has been resected and reconstructed with an allograft prosthetic composite reconstruction.
|
 |
|
Figure 4.5-3 Anteroposterior view of pelvis in woman with a lytic metastatic breast cancer lesion in the right intertrochanteric region (A) responded well to radiotherapy, with a sclerotic healing response (B).
|
-
Goal should be to allow full weight bearing immediately postoperatively.
-
Importance of postoperative radiotherapy
-
Lower chance of disease recurrence
-
Lower chance of hardware failure and need for second operative intervention
-
Better functional outcome
-
-
Proximal femur
-
Femoral neck
-
Long-stem cemented hemiarthroplasty is the gold standard for both impending fractures and after the fact.
-
Long stem is absolutely indicated when
there are distal shaft lesions, as these lesions serve as stress risers
and may progress. The long stem protects the remainder of the femur. -
Cementing provides immediate stability.
-
Hemiarthroplasty is inherently more stable than total hip arthroplasty.
-
Total hip arthroplasty is not needed
unless there is a significant acetabular lesion that requires operative
fixation (see acetabular section).
-
-
Potential complications
-
Embolization has been reported, sometimes
with catastrophic consequences, so adequate precementing hydration,
oxygenation, canal lavage, and consideration of venting should be done. -
Occult penetration of the femoral cortex
by guide rods, reamers, broaches, and/or femoral stems may occur
through unrecognized distal lesions.
-
-
-
Intertrochanteric region (Figs. 4.5-4 to 4.5-6)
-
Surgical options: Depend on extent of destruction, adequacy of proximal femoral bone to accept internal fixation (Table 4.5-3)
-
-
Subtrochanteric region
-
Gold standard is the proximally and distally locked intramedullary reconstruction nail.
-
Bone cement is absolutely indicated only
if screw fixation is inadequate or when segmental destruction creates
loss of femoral continuity, but bone cement can be used at the
surgeon’s discretion. -
When subtrochanteric lesions extend
proximally to the point that inadequate fixation will be achieved with
an intramedullary nail, a proximal femoral replacement long-stem
cemented endoprosthetic hemiarthroplasty should be considered.
-
-
-
Femoral diaphysis
-
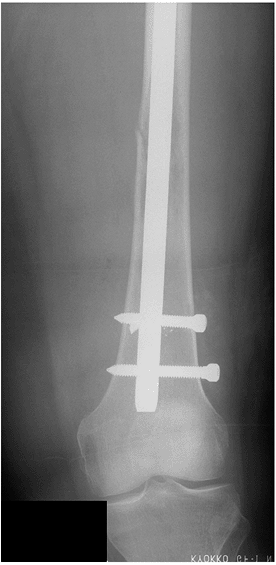
Gold standard is the proximally and distally locked intramedullary reconstruction nail. (Fig. 4.5-7).
-
Rationale: Proximal prophylactic fixation
of the neck region protects a common site of femoral metastases, where
a standard intramedullary nail would leave the femoral neck unprotected.
-
-
Distal femur
-
Surgical options: Depend on exact location of lesion, extent of destruction (Table 4.5-4)
-
Distal femoral plate and screw options are dictated by availability of distal bone.
-
Dynamic condylar screw requires approximately 5 cm of intact distal bone.
-
Distal femoral blade plate requires approximately 3 cm of intact distal bone.
-
Condylar buttress plates, particularly
newer locking condylar plates, are necessary for severely compromised
distal bone with less intact bone or partially compromised distal bone;
these plates are generally preferred for pathologic distal femur
fractures. -
Bone cement is generally indicated as an adjunct to these fixation options.
-
-
-
 |
|
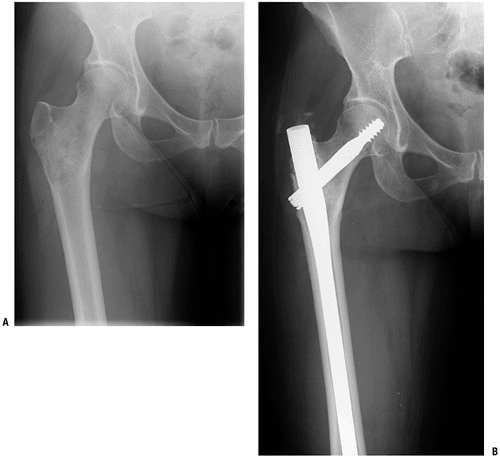
Figure 4.5-4 A woman with an impending pathologic right intertrochanteric femur fracture due to metastatic breast carcinoma (A) underwent prophylactic stabilization with a cephalomedullary locked nail (B).
|
-
Nonacetabular (ilium, pubic ramus)
-
Nonoperative care: observation, irradiation
-
Operative care: usually not indicated
-
-
Acetabulum (Table 4.5-5 and Fig. 4.5-8)
-
Proximal humerus
-
Nonoperative treatment: Sarmiento-type fracture brace with proximal Galveston extension, irradiation
-
Prophylactic stabilization and fracture fixation depend upon location and extent of bone destruction (Table 4.5-6 and Fig. 4.5-9).
-
-
Humeral diaphysis
-
Nonoperative care: Sarmiento clamshell fracture brace, irradiation
-
Operative care: depends on location and presence or absence of segmental destruction (Table 4.5-7 and Fig. 4.5-10)
-
-
First-line care should be nonoperative with local irradiation and/or systemic agents.
-
Surgical options, which consist of
subtotal or total scapulectomy, are reserved for failure of
nonoperative care or when a solitary metastasis from renal cell
carcinoma can be resected for potential cure.
 |
|
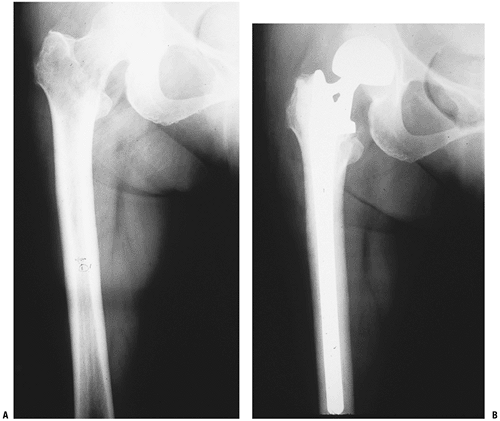
Figure 4.5-5 Another patient with intertrochanteric disease felt too extensive to be amenable to stabilization (A) was treated with a calcar replacement cemented long-stem hemiarthroplasty (B).
|
-
First-line care should be nonoperative
with local irradiation and/or systemic agents and a sling or
figure-of-eight immobilizer as needed. -
Resection should be reserved for failure of nonoperative care.
-
General considerations
-
Anterior spinal involvement far outweighs posterior element involvement.
-
Cervical spine metastases result in a lower incidence of neurologic deficit than thoracic or lumbar spinal metastases.
-
Plain films and bone scan may miss
lesions, so magnetic resonance imaging (MRI) is the test of choice when
spine metastases are suspected. -
Because of the overlap in radiographic
features between metastatic disease and benign degenerative conditions
and hemangiomas, a tissue diagnosis should almost always be obtained
prior to any treatment. -
Indications for surgical treatment
-
Failure of nonoperative treatment (bracing, radiation, chemotherapy, hormonal manipulation) to relieve pain
-
Need for diagnostic tissue
-
Spinal instability (pathologic fracture, progressive deformity, or neurologic deficit)
-
Clinically significant neural compression
-
-
Spiegel showed a significantly shorter
survival duration for vertebral metastases patients with more than one
site of visceral metastases, so surgical intervention was not
recommended in those patients.
P.100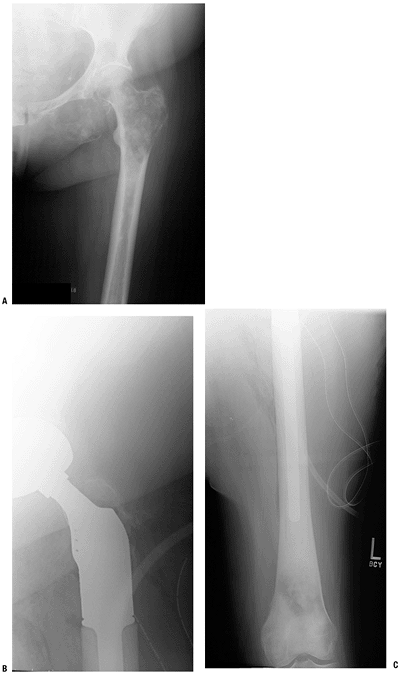 Figure 4.5-6 Even more extensive proximal femoral metastatic destruction here (A) was treated by resection of the proximal femur with a megaprosthesis reconstruction (B,C).P.101Table 4.5-3 Surgical Options for Pathologic Fracture of The Intertrochanteric Femur
Figure 4.5-6 Even more extensive proximal femoral metastatic destruction here (A) was treated by resection of the proximal femur with a megaprosthesis reconstruction (B,C).P.101Table 4.5-3 Surgical Options for Pathologic Fracture of The Intertrochanteric FemurSurgical Option Immediate Stability? Protects Entire Bone? Unique Potential Complications Indication Dynamic hip screw, side plate, and cement Technique-dependent; requires bone cement No Device failure if fracture nonunion Adequate proximal bone to achieve fixation Femoral reconstruction nailing Depends on bone quality, cement supplementation Yes Device failure if fracture nonunion Adequate proximal bone to achieve fixation Calcar replacement long-stem cemented hemiarthroplasty Yes If long stems are used Embolization, instability Inadequate proximal bone for fixation Proximal femoral replacement megaprosthesis hemiarthroplasty Yes If long stems used Embolization, instability Extensive destruction to below lesser trochanter ![]() Figure 4.5-7
Figure 4.5-7
A distal diaphyseal pathologic femur fracture secondary to metastatic
disease was stabilized with a proximally and distally locked
cephalomedullary nail. -
-
Cervical spine
-
Nonoperative treatment
-
Bracing, irradiation, chemotherapy, hormonal manipulation, bisphosphonates
-
-
“Patient at risk” for developing
progressive neurologic deficit will have three characteristics and
should be treated surgically:-
>50% vertebral body involvement
-
Exceeding White and Panjabi criteria for instability
-
>3.5 mm of subluxation
-
>11 degrees of adjacent angulation
-
-
Crescendo pain
-
-
Surgical options
-
Anterior corpectomy and stabilization is the procedure of choice for solitary or two-level C3–C7 anterior lesions.
-
Indications for posterior approach with laminectomy and stabilization
-
Occiput to C3 lesions
-
Cervicothoracic junction
-
Multilevel involvement with posterior epidural lesions (common in prostate carcinoma)
-
-
-
-
Thoracic spine
-
Nonoperative treatment
-
Bracing (Jewett brace for T7–L2), irradiation, or medical measures, as for cervical spine
-
-
Thoracic spinal instability criteria
-
Translational deformity
-
Collapse of >50%
-
Denis three-column involvement
-
Involvement of same column in adjacent levels
-
-
Surgical options
-
Anterior approaches for anterior lesions:
corpectomy and polymethylmethacrylate (PMMA), cage, or allograft
reconstruction, depending upon patient prognosis. -
Posterior approaches for posterior lesions: laminectomy and stabilization with or without fusionP.102
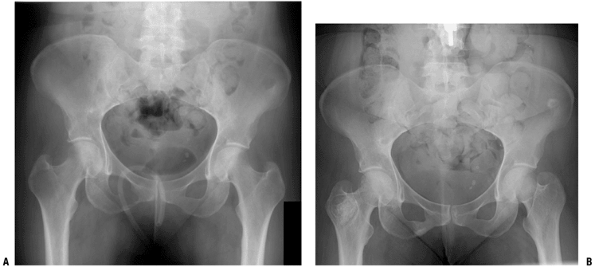
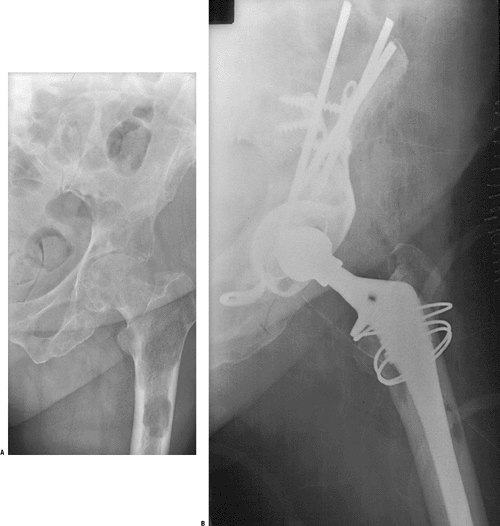 Figure 4.5-8 A patient with metastatic breast cancer involving the superior and medial acetabulum as well as the proximal femur (A) underwent reconstruction using a protrusio cage, reinforced cement, and a long-stem cemented femoral prosthesis (B).
Figure 4.5-8 A patient with metastatic breast cancer involving the superior and medial acetabulum as well as the proximal femur (A) underwent reconstruction using a protrusio cage, reinforced cement, and a long-stem cemented femoral prosthesis (B).-
Combined approaches for anterior
corpectomy and posterior stabilization are generally preferred if
multiple-level disease and instability exist.
-
-
-
-
Lumbar spine
-
Nonoperative treatment
-
Bracing (Jewett brace for T7–L2, lumbosacral corset for L3–S1), irradiation, or medical measures, as for other levels)
-
-
Surgical options
-
Upper lumbar lesions: anterior approach,
corpectomy and PMMA, cage, or allograft reconstruction with or without
plate stabilization -
Lower lumbar lesions: posterior
decompression and stabilization or combined anterior corpectomy and
reconstruction with posterior stabilization
-
-
-
Classification systems designed to provide guidance for intervention in spinal metastases are as follows:
-
Harrington classification (Table 4.5-8)
-
Kostuik two-column, six-segment concept
of stability, defined instability, and hence the need for surgical
intervention, as destruction of three or more segments -
Tokuhashi’s six variables (general
medical condition, number of extraspinal bone metastases, number of
vertebral metastases, visceral metastases, primary tumor type, presence
of neurologic deficit), with 0 to 2 points assigned per variable,
suggested that an excisional operation be done for cases with 9 or more
points and a palliative operation be done for those with 5 or fewer
points.-
Enkaoua’s prospective evaluation of the
Tokuhashi system revealed median survival of 24 months if the Tokuhashi
score is above 7 and 5 months for a score of 7 or less, but the series
was limited to patients with renal, thyroid, and unknown primary
carcinomas.
-
-
Tomita’s 2001 scoring system consists of
three prognostic factors (grade of malignancy 1/2/4 points, visceral
metastases 0/2/4 points, number of bone metastases 1 or 2 points),
totaling 2 to 10 points. Patients with 2 or 3 points are recommended
for wide or marginal excision, 4 or 5 points marginal or intralesional
excision, 6 or 7 points palliative surgery, 8 to 10 points nonoperative
approach.
-
-
Distal humerus
-
Nonoperative care: Sarmiento-type hinged elbow brace, irradiation
-
Operative care: depends on extent of destruction, presence of proximal lesions (Table 4.5-9 and Fig. 4.5-11)
-
-
Forearm
-
Nonoperative care: bracing, irradiation and/or medical management
-
Operative care: rarely indicated
-
Surgical options: intramedullary nailing, plate/screw, or combination
-
-
Wrist and hand
-
Nonoperative care: observation, irradiation and/or medical management
-
Operative care: rarely indicated
-
Surgical options: palliative amputation, resection
-
-
Tibia and fibula
-
General considerationsP.103P.104Table 4.5-4 Surgical Options for Pathologic Fracture of The Distal Femur
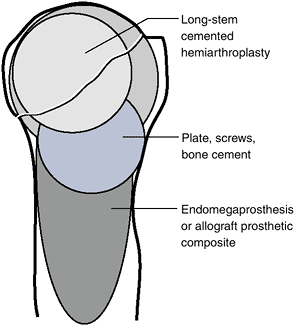
Surgical Option Immediate Stability? Protects Entire Bone? Unique Potential Complications Indication Curettage and cementing Only for impending pathologic fractures No Fracture if lesion progresses Small metaphyseal or epiphyseal eccentric defects Curettage, cementing, and plate/screw fixation Depends on bone quality, cement supplementation No Device failure if fracture nonunion Adequate distal bone to achieve fixation; no proximal lesions Retrograde intramedullary femoral nail Depends on bone quality No Fracture proximal to end of nail Distal diaphyseal lesion not amenable to antegrade nailing; no proximal lesions Distal femoral replacement megaprosthesis total knee arthroplasty Yes If long stems used Embolization Extensive destruction distal femur precluding other options Table 4.5-5 Modified Harrington Classification of Pelvic Insufficiency and Suggested ManagementClass Description Management 0 Supra-acetabular defect that does not penetrate acetabular subchondral plate Nonoperative care vs. curettage, cementation, and reinforcement pins/mesh I Contained defect involving acetabulum with intact rim and superior dome Curettage and cementing of defect with cemented cup total hip arthroplasty with or without protrusio ring II Medial wall acetabular defect Cemented protrusio cage total hip arthroplasty III Deficiency of rim or dome Option 1: Cemented protrusio cage total hip arthroplasty with curettage of supra-acetabular bone and reinforcement pins (Fig. 4.5-8) Option 2: Saddle prosthesis IV Resection of lesion required for cure Option 1: Saddle prosthesis Option 2: Allograft acetabular prosthetic total hip arthroplasty composite reconstruction P.105Table 4.5-6 Surgical Options for Pathologic Fracture of The Proximal HumerusSurgical Option Immediate Stability? Protects Entire Bone? Unique Potential Complications Indication Long-stem cemented hemiarthroplasty Yes Yes Shoulder instability Epiphyseal and limited metaphyseal involvement Plate, screws, and bone cement Dependent on bone quality, cement supplementation No Loss of fixation, fracture below device Metaphyseal lesions with enough intact proximal bone Endoprosthetic proximal humeral replacement (EPHR) Yes If long stems used Poor shoulder function Extensive epiphyseal/metaphyseal and proximal diaphyseal destruction Allograft prosthetic composite (Fig. 4.5-2) Yes, but must protect cuff reconstruction If long stems used Allograft infection and fracture risk Same as EPHR but patient with longer potential survival -
Tibial metastatic lesions far outweigh
pathologic fractures, likely because tibial lesions become symptomatic
earlier than femoral lesions. -
Protection of the entire tibia is of less
concern than in femur or humerus due to the less frequent occurrence of
distal lesions.
-
-
Proximal tibia
-
Nonoperative care: bracing (patellar
tendon–bearing orthosis or knee–ankle–foot orthosis, often with
drop-lock knee hinges), irradiation/chemotherapy/hormone
manipulation/bisphosphonates -
Surgical options: depend on location, extent of bone destruction (Table 4.5-10)
-
-
Tibial diaphysis
-
Nonoperative care: patellar tendon–bearing orthosis, irradiation, chemotherapy, hormone manipulation, bisphosphonates
-
Gold standard is antegrade proximally and distally locked tibial nail.
-
Need for cement depends on need to
re-establish continuity of the bone; cement is indicated for segmental
destruction of the bone.
-
-
Distal tibia
-
Nonoperative care: patellar
tendon–bearing orthosis or ankle–foot orthosis, irradiation,
chemotherapy, hormone manipulation, bisphosphonates -
Surgical options depend upon exact
location and extent of destruction. The techniques parallel those of
the proximal tibia in concept. Small lesions can be curetted and
cemented, and larger lesions require plate fixation to complement the
cemented defect.
-
-
-
Ankle and foot
-
General considerations
-
Rare site of metastatic disease
-
-
Gold standard is nonoperative care with
short-leg boots or pressure-relief shoes combined with irradiation,
chemotherapy, hormone manipulation, and bisphosphonates. -
Surgical options include amputation for palliation.
-
 |
|
Figure 4.5-9 Proximal humeral surgical options according to location.
|
destructive bone involvement by metastatic disease and myeloma is
important in order to educate the patient and to give him or her the
opportunity to potentially avoid an emergent hospitalization and the
added pain of a fracture. Furthermore,
because
of the lower likelihood of fracture healing in the setting of
malignancy, potential long-term complications of fracture fixation in
these patients may be avoided by prophylactic stabilization. Guidelines
for prediction of pathologic fracture risk continue to evolve.
|
Table 4.5-7 Surgical Options for Pathologic Fracture of The Humeral Diaphysis
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
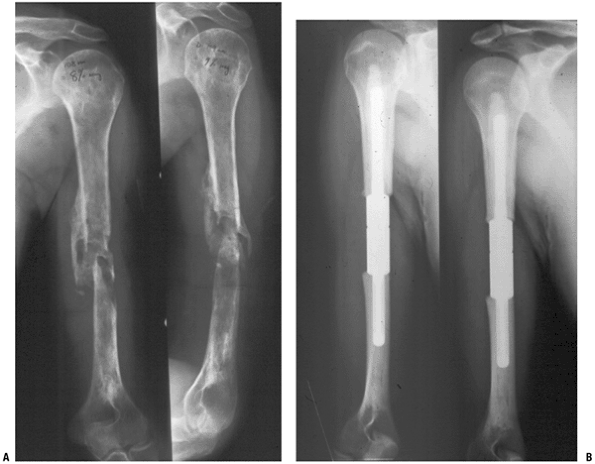 |
|
Figure 4.5-10 Failure of earlier plate fixation of this pathologic humeral shaft fracture (A) was salvaged with a cemented intercalary humeral spacer (B).
|
|
Table 4.5-8 Harrington Classification of Metastatic Spine Tumors and Suggested Management
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Table 4.5-9 Surgical Options for Pathologic Fractures of the Distal Humerus
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
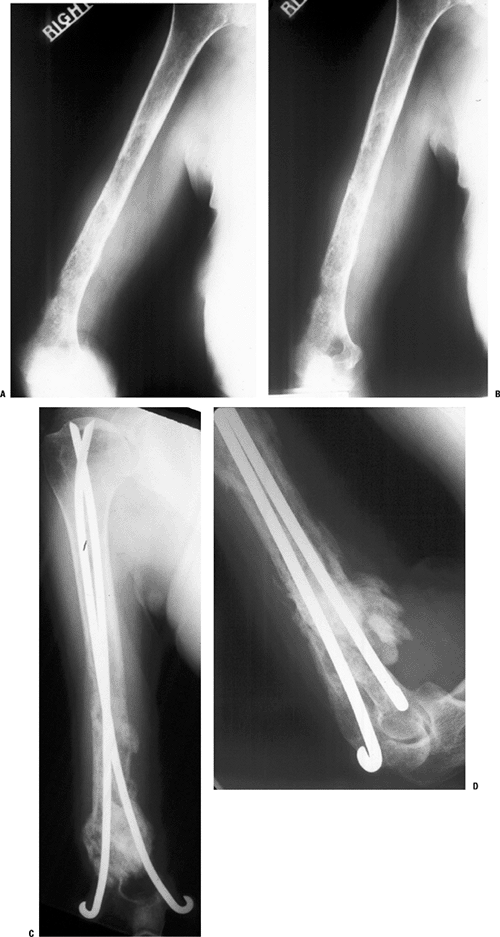 |
|
Figure 4.5-11 A patient with metastatic breast carcinoma involving much of the humerus with an impending distal third fracture (A,B) was treated with dual cemented Rush rod fixation, with good results (C,D).
|
-
More than half of width of bone destroyed
-
>2.5 cm of bone destruction
-
Avulsion of lesser trochanter
-
Pain unresponsive to radiotherapy
This classification scheme is sensitive and poorly specific for
prediction of pathologic fracture risk, but nonetheless it remains a
useful objective tool.
-
Osteoclasts are the cellular mediator of bone resorption in metastatic disease and myeloma.
-
Osteoclast inhibitor drug class: bisphosphonates
-
Etidronate (Didronel)
-
Pamidronate (Aredia)
-
Alendronate (Fosamax)
-
Risedronate (Actonel)
-
Zoledronate (Zometa)
-
Ibandronate (Boniva)
-
-
Types of efficacy demonstrated for bisphosphonates
-
Tumors with documented efficacy of bisphosphonates
-
Breast carcinoma
-
Multiple myeloma
-
Prostate carcinoma
-
Lung carcinoma
-
Renal carcinoma
-
-
Indications for bisphosphonates in metastatic disease
-
Hypercalcemia
-
Bone metastases in breast, prostate, lung, or renal carcinoma
-
Multiple myeloma
-
the disease has become refractory to hormonal treatment, chemotherapy
is usually employed. Monoclonal antibodies directed to the human
epithelial growth factor receptor 2 (HER2/neu), which contributes to
breast cancer tumorigenesis, may also be used for palliative care.
Breast cancer metastases to bone are responsive to radiotherapy.
|
Table 4.5-10 Surgical Options for Pathologic Fractures of the Proximal Tibia
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Table 4.5-11 Mirels Scoring System
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||
|
Table 4.5-12 Mirel’S Recommendations for Prophylactic Stabilization Based Upon Total Score
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
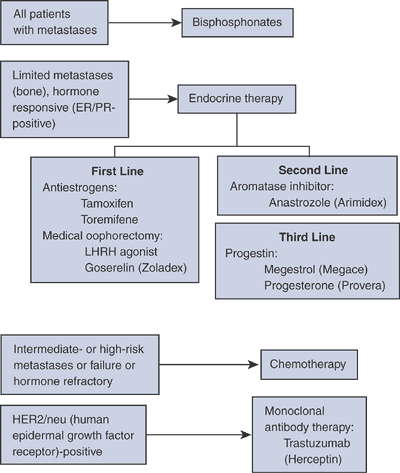
Algorithm 4.5-2. Systemic treatments for metastatic breast cancer.
|
cancer has consisted primarily of androgen suppression by hormonal
manipulation. Chemotherapy combinations have shown some promise against
hormone-refractory disease in recent years. Bisphosphonates are
successful for decreasing bone pain associated with prostate
metastases. Radiotherapy is very useful for prostate cancer. When there
is a single predominant symptomatic site producing pain, external beam
radiotherapy is useful. However, when patients have diffuse bone
metastases with multiple symptomatic sites, or when tissue tolerance
has been maximized by external beam therapy, systemic radionuclide
therapy may be useful. Strontium-89 is the most common radionuclide
used for systemic radiotherapy.
viewed as small cell lung cancer and non-small cell lung cancer. Small
cell lung cancer is typically treated with chemotherapy, whereas until
recently non-small cell has been viewed as poorly responsive to
chemotherapy. Recent trials suggest some efficacy of chemotherapy in
addition to radiotherapy for unresectable non-small cell lung carcinoma.
 |
|
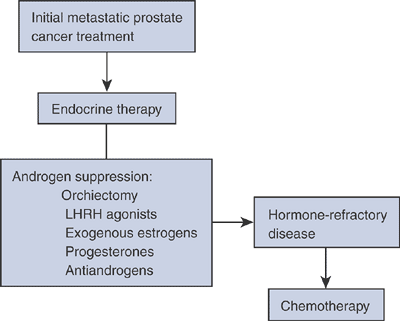
Algorithm 4.5-3. Systemic treatments for metastatic prostate cancer.
|
prognosis, and median survival is only 3 to 6 months. Systemic
treatments are of limited usefulness except as research protocols to
date. Radiotherapy provides effective palliation of bone metastases,
although response is not as good as for breast or prostate cancer
patients. Bisphosphonates play a similar role for lung cancer as for
other metastatic tumors, reducing both pain and the number of skeletal
events.
treatment of metastatic renal carcinoma. Immunotherapy is the typical
front-line treatment, although the efficacy of treatment has been
called into question by a randomized phase III placebo-controlled trial
that failed to show an advantage to treatment. Typical agents are
interleukin-2, interferon 2-α, or combinations. The efficacy of
chemotherapy has yet to be established. Pain relief from radiotherapy
for renal metastases is seen in 50% of patients, less than those of
breast, prostate, and lung cancers. Furthermore, radiation doses
greater than 60 Gy are often required, and duration of radiotherapy
response is frequently limited. Anti-angiogenics are beginning to play
an increasing role as well.
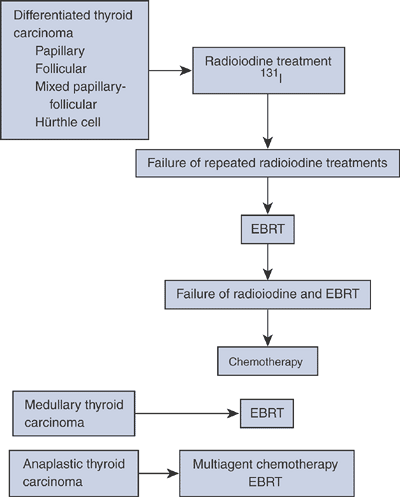
differentiation, which dictates treatment and prognosis. The majority
of these tumors are differentiated, and radioiodine treatment for
patients with increased uptake on radioiodide scans is the mainstay.
 |
|
Algorithm 4.5-4. Nonoperative treatments for metastatic thyroid cancer according to subtype. EBRT, external bean radiation therapy.
|
 |
|
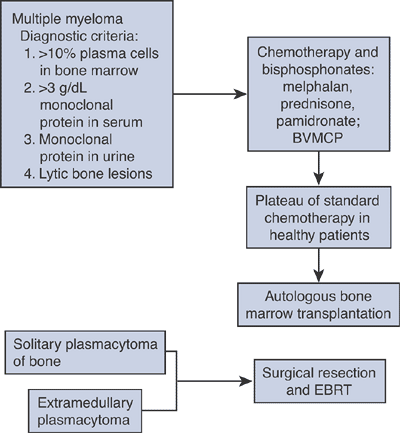
Algorithm 4.5-5.
Standard nonoperative treatment for multiple myeloma and related conditions. BVMCP, carmustine (BCNU), vincristine, melphalan, cyclophosphamide, prednisone; EBRT, external beam radiation therapy. |
with established multiple myeloma. Autologous stem cell transplantation
using peripheral blood stem cells offers a survival advantage over
standard chemotherapy maintenance. The opportunity for cure of multiple
myeloma may only be afforded by allogeneic bone marrow transplants, but
this comes with a price: the treatment-related mortality rate following
allogeneic transplant approaches 30% and is highest in patients >60
years old.
extramedullary plasmacytoma, however, are treated by surgical resection
(when feasible) and external beam radiotherapy. However, many of these
patients will progress to full-blown myeloma over 10 to 15 years.
G, Ferrari S, Longhi A, et al. Nonmetastatic osteosarcoma of the
extremity with pathologic fracture at presentation: local and systemic
control by amputation or limb salvage after preoperative chemotherapy. Acta Orthop Scand 2003;74(4):449–454.
E. A., L. Doursounian, et al. (1997). “Vertebral metastases: a critical
appreciation of the preoperative prognostic tokuhashi score in a series
of 71 cases.” Spine 22(19):2293–8.
Y., H. Matsuzaki, et al. (1990). “Scoring system for the preoperative
evaluation of metastatic spine tumor prognosis.” Spine 15(11):1110–3.
PW, Smalley SR, Cozad SC, et al. Role of postoperative radiation
therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys 1995;31:43–49.