Tendon and Ligament Anatomy, Biology, and Biomechanics
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section IV – Basic Science > 24 – Tendon and Ligament Anatomy, Biology, and Biomechanics
24
Tendon and Ligament Anatomy, Biology, and Biomechanics
Brian J. Harley
Joseph W. Bergman
Tendons and ligaments act as the bonds that tie the body
together. Ligaments connect one bone to another at a joint, and tendons
connect bone to muscle. While the specific natures of their tasks
differ, tendons and ligaments share a great many features in their
construction and function.
together. Ligaments connect one bone to another at a joint, and tendons
connect bone to muscle. While the specific natures of their tasks
differ, tendons and ligaments share a great many features in their
construction and function.
Tendon
Tendon Anatomy, Structure, and Composition
Gross Anatomy
Macrostructure
-
Variable sizes and shapes: wide and flat to round and narrow
-
The larger the muscle unit, and therefore the potential force, the larger diameter the corresponding tendon
-
Unique features in regions of compression
-
Sheaths and bursa
-
Shield the tendon from abrasion and friction
-
-
Tendon assumes more of a cartilage-like appearance in these areas.
-
Synovial sheath
-
Encloses the path of the tendon
-
Provides a reservoir of fluid to hydrate and lubricate the tendon
-
-
-
Ultrastructure
-
While variety is seen in tendon macrostructure, all tendons are organized with a similar ultrastructure (Fig. 24-1).
-
Musculotendinous junction
-
Collagenous structure of the tendon blends with the muscle.
-
As the tendon fans out into the muscle,
the collagen fibrils connect to the myocytes, allowing for the
transmission of force from muscle to tendon.
-
-
EpitenonP.464
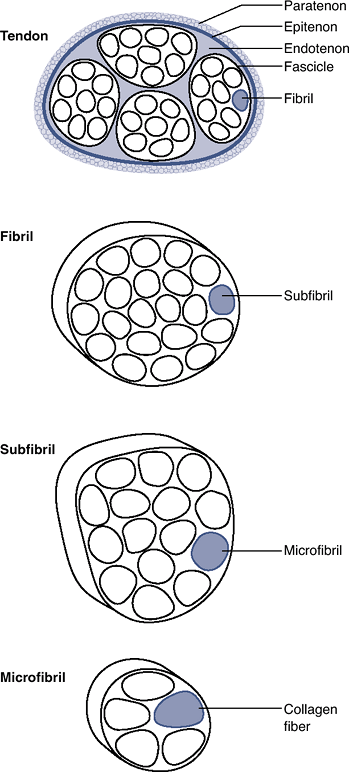 Figure 24-1 Tendon ultrastructure.
Figure 24-1 Tendon ultrastructure.-
Layer of organized tissue that tightly encircles the entire surface of the tendon
-
-
Endotenon
-
Layer of loose connective tissue between fascicles within a tendon
-
-
Paratenon
-
Layer of loose areolar tissue that substitutes for a tendon sheath; serves a nutritional role
-
Present in tendons not enclosed within a fibrous sheath (e.g., Achilles tendon)
-
-
Fibrils
-
Collagen molecules organized into microfibrils
-
Microfibrils assembled into subfibrils, fibrils, and fascicles
-
One or more fascicles compose a tendon.
-
Biochemistry
-
Collagen
-
Primary component of tendon
-
Type I collagen predominates, typically representing up to 85% of the dry weight.
-
Other collagens in tendon: III, IV, V, VI
-
Collagen provides a strong molecule capable of transmitting tensile loads.
-
Constructed from tropocollagen, a triple-helical polypeptide molecule
-
Ends of tropocollagen overlap at regular intervals, giving the collagen fiber a banded appearance.
-
Primarily oriented in the direction of expected tensile force
-
Variety of fibers that travel in an oblique or perpendicular direction and tie the longitudinally oriented fascicles together
-
-
-
Noncollagenous substances
-
Proteoglycans, proteins, and various supporting molecules, including elastin
-
Small variations in the concentrations of all these components are seen in tendons in differing locations and functions.
-
-
Matrix
-
Proteoglycans and glycosaminoglycans
-
Ionic charge on the side chains of these molecules causes them to spread out and occupy as large a volume as possible.
-
Water molecules are strongly attracted by this structure and are strongly restrained within the tendon.
-
Presence of water bound to the proteoglycan molecules allows the tendon to resist compressive loads.
-
Compressive load passes through the water
in much the way that the fluid inside a can of soda supports the thin
walls surrounding it. -
Resistance to fluid flow through the tendon also contributes greatly to its viscoelastic biomechanics.
-
-
Cellular Population
-
As a consequence of the mechanical
demands placed on the tendon, much of the structure of the tendon is
composed of mechanical elements.-
As a result, the tendon has a high structure to cellularity ratio.
-
-
Tenocytes and tenoblasts
-
Spindle-shaped cells
-
Subjected to significant mechanical loads
-
Poorly supplied with blood and nutrients
-
Stimuli triggering cellular responses
-
Mechanical load
-
Electric potential
-
Various chemical and cytokine messengers
-
Transforming growth factor (TGF) and interleukin (IL) groups, as well as a variety of extracellular molecular fragments
-
-
-
Function: responsible for maintaining the collagen and proteoglycan matrix
-
Normal tendon
-
Constant turnover of extracellular matrix
in response to chemical and mechanical damage that occurs from normal
day-to-day function
-
-
Acute tendon injury
-
Inflammatory response (including lysis
and removal of damaged molecules), followed by a regenerative,
depositional phase (by tenocytes and tenoblasts) -
Due to the paucicellular nature of tendon
combined with areas of poor blood supply, it is possible for the
cellular repair mechanism (tenocytes and tenoblasts) to become
overwhelmed.
-
-
Chronic tendon conditions
-
Inflammatory and lysis responses predominate.
-
Reparative response by tenocytes muted
-
Chronic tendinitis, tendinosis, and even tendon failure are manifestations of this state.
-
P.465 -
Blood Supply
-
Vascular anatomy of tendons
-
Blood vessels exist within the epitenon and endotenon.
-
Fine areolar structures that surround tendon bundles and supply individual fibrils and fibers
-
-
Variable blood supply depending upon location within tendon
-
Variable amount of blood supply at origin and insertion
-
Myotendinous junction allows an increased
microperforating blood supply to nourish a portion of the tendon
extending away from the junction.
-
-
-
Factors decreasing vascular incursion
-
Intense mechanical environment
-
Confined geometry of the tendon
-
Increased tensile and compressive forces within the extracellular matrix
-
-
Secondary path for nutrition in poorly vascularized regions
-
Diffusion of nutrients and oxygen from the adjacent synovial layers
-
Innervation
-
Neural elements within tendons
-
Two predominant mechanoreceptors, both of which sense pressure and tension within tendon
-
Rapidly adapting receptors: Pacinian corpuscles
-
Slow-adapting receptors: Ruffini endings
-
-
Free nerve endings less common
-
Sympathetic and parasympathetic innervation
-
-
Importance of tendon innervation
-
Important for normal function
-
Proprioceptive receptors communicate with gamma-muscle-spindle system to modulate joint position.
-
-
Recovery of mechanical function after an injury is not always accompanied by return of nervous function.
-
Unknown what effect this defect has on rehabilitation and musculotendinous action
-
-
Tendon Biomechanics
Tendon acts as a relatively rigid connector between the
motor unit of the muscle and the bone. Its first role is to transmit
tensile forces created within the muscle to initiate and modulate
motion. To accomplish this, tendon has one of the highest tensile
strengths of any material in the body. Collagen has a high tensile
strength (and high ultimate stress) along its longitudinal axis, and
the parallel orientation of the collagen fibrils within tendon takes
advantage of this strength. The clinical importance of these properties
is evident when the biomechanical properties of the healing tissue in a
tendon or ligament are compared to the intact state. The scar tissue is
weaker, therefore reducing the material properties of a tendon.
Increasing the total cross-sectional area of the tendon with this scar
tissue may allow for the same structural properties, however.
motor unit of the muscle and the bone. Its first role is to transmit
tensile forces created within the muscle to initiate and modulate
motion. To accomplish this, tendon has one of the highest tensile
strengths of any material in the body. Collagen has a high tensile
strength (and high ultimate stress) along its longitudinal axis, and
the parallel orientation of the collagen fibrils within tendon takes
advantage of this strength. The clinical importance of these properties
is evident when the biomechanical properties of the healing tissue in a
tendon or ligament are compared to the intact state. The scar tissue is
weaker, therefore reducing the material properties of a tendon.
Increasing the total cross-sectional area of the tendon with this scar
tissue may allow for the same structural properties, however.
Structural Properties of Tendons
-
Describes the properties of the whole
tissue complex, as in the entire tendon–bone insertion unit, in terms
of force and displacement (Fig. 24-2)-
Strength: overall force transmitted
-
Stiffness: ability of the structure to resist deformation when force (or load) is passed through it
-
Material Properties of Tendons
-
Describes properties according to cross-sectional area, in terms of stress and strain (Fig. 24-3)
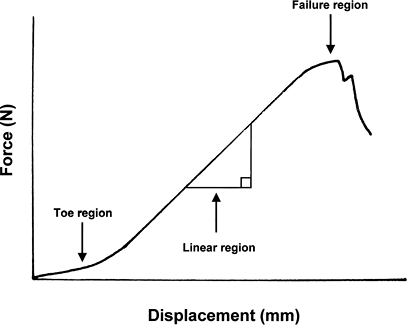
![]() Figure 24-2
Figure 24-2
Typical force–displacement curve during low-load tensile testing of a
tendon. A toe region exists representing the changes from crimp. The
linear region represents the high stiffness obtained from full
recruitment of collagen fibers. The failure region occurs with
sequential loss of continuity of fibers.P.466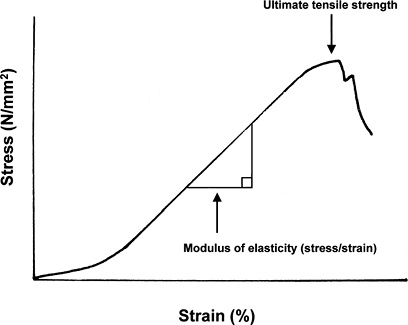 Figure 24-3
Figure 24-3
Typical stress–strain curve during low-load tensile testing of a
tendon. The slope of the linear region represents the modulus of
elasticity of the tendon. The ultimate tensile strength represents the
stress at failure of continuity of the tendon.-
Stress: force divided by cross-sectional area
-
Strain: amount of deformation of the structure divided by length over which the deformation takes place
-
-
Useful for comparing tendons at various
anatomic locations or comparing a healing tendon to an intact tendon,
or when relating tendons to ligaments
Low-Load Tendon Mechanics
-
The behavior of tendon at low loads
reveals a degree of laxity that allows some movement through the tendon
before load is passed to muscle. -
Crimp in tendons
-
At low loads, this “crimp” present in the collagen fibers straightens out before the collagen begins to conduct load (see Fig. 24-2).
-
-
Steepening of force–deformation curve
-
Not all of the collagen is crimped
equally, so there is a steepening of the force-deformation curve as
more and more fibrils are recruited.
-
-
Effect of loading rate
-
Most of the work performed on the
mechanics of tendon has loaded the tissue at a very slow rate of
loading, and in this quasi-static state the curve slowly steepens until
the elastic modulus of the tendon is reached (see Fig. 24-3). -
At higher loading rates, which more
accurately represent the condition in vivo, the structure displays a
viscoelastic behavior and acts as a stiffer structure.
-
Elastic Biomechanics
Structural and Material Properties
-
An elastic substance is one in which a force and displacement and stress and strain are linearly related.
-
Doubling a certain force will double the deformation.
-
Doesn’t matter if the force (load) is arrived at during loading or unloading
-
Elastic Properties
-
Tendon demonstrates very important elastic behavior.
-
Any force generated by a muscle is transferred to the intended bone with virtually no energy wasted.
-
-
Cycling between loaded and unloaded states does not waste energy.
-
Whatever energy is stored during loading of the material is released during its unloading.
-
Viscous Properties
-
A purely viscous substance is one that will deform infinitely given a particular force.
-
This is seen with fluids where rate of motion through the fluid is dependent on the force.
Viscoelastic Behavior
-
A time-dependent property of material
behavior whereby the tendon behaves partly as a viscous substance and
partly as an elastic substance -
Interaction of the viscous and elastic properties during loading
-
The collagen fibers take up most of the force and the loaded fibers tend to squeeze together.
-
The water in the extracellular matrix resists this inward movement of the collagen fibers.
-
Typically the water would quickly be squeezed out of the tissue by the tension on the tendon.
-
The proteoglycans and glycosaminoglycans within the matrix act as a colloid attraction force to keep water within the tendon.
-
-
The rate at which a tendon is loaded is very important.
-
The more quickly a tensile load is placed on the tendon, the more effective is the resistance to outward flow of the water.
-
-
-
Advantages of viscoelastic behavior in tendons
-
Load “sharing” between the elastic and viscous portions of the matrix
-
During very large loads, the viscous
nature of the tendon absorbs and dissipates energy. This helps to
protect the collagen from irreparable damage. -
At lower, cyclic loads, the tendon behaves more as an energy-conserving, elastic material.
-
-
Energy conservation
-
One of the important functions of tendon
is efficient transmission of energy. The most efficient way to transmit
force is with a very stiff structure. -
Within short time frames, the
viscoelastic behavior of tendon causes it to act like a much stiffer
and energy-conserving structure. -
Loss of energy
-
Due to the properties of creep,
force-relaxation, and mechanical hysteresis, upwards of 10% of total
work is lost during normal cycles of loading and unloading.
-
-
-
P.467
Tendon Injury, Healing, and Repair
Tendon dysfunction adversely affects overall orthopaedic
health. Degenerative and/or traumatic injuries to tendons throughout
the musculoskeletal system are a common cause of presentation to an
orthopaedic surgeon.
health. Degenerative and/or traumatic injuries to tendons throughout
the musculoskeletal system are a common cause of presentation to an
orthopaedic surgeon.
Injury Mechanisms
Direct Trauma or Laceration
-
Common example: laceration of the finger flexor tendon
Acute Application of Tensile Loads Exceeding the Strength of the Tendon
-
Results in partial or complete ruptures of the tendon
-
Distinct locations common
-
Avulsion of the tendon from its bone insertion
-
Healthy tendons can easily withstand
tensile forces larger than the maximum force generated by the muscles
or tolerated by the bones, so failures tend to occur at bony insertions. -
Common example: distal biceps tendon avulsion
-
-
Midsubstance rupture
-
Generally pre-existing pathology at the site of a midsubstance rupture
-
Common example: Achilles tendon rupture
-
-
Disruption at the musculotendinous junction
-
Typically caused by a very forceful eccentric muscle contraction
-
Partial loss of continuity of the muscle fibers close to the junction (complete loss of continuity of tendon is uncommon)
-
Muscles that cross two joints appear to be more susceptible.
-
Spontaneous healing the norm; surgical repair unusual
-
Common example: hamstring tear (forceful hip flexion with the knee extended)
-
-
Fatigue/Overuse Conditions
-
Repetitive loading of a tendon at loads
well beneath its ultimate failure load can damage the tendon beyond its
ability to repair the damage. -
Failure typically occurs at the site of tendon weakness.
-
Multifactorial etiology
-
Intrinsic and extrinsic causes
-
-
Clinical examples of tendinous fatigue failure
-
Tendon coursing around a sharp turn
-
Damaged by constant friction by a harder material
-
Common example: posterior tibialis tendon rupture
-
-
Tendon compressed between two hard surfaces
-
Damage from abrasive wear
-
Common example: rotator cuff tendon tear
-
-
Chemical or enzymatic damage
-
Slow attenuation of tendon macrostructure
-
Generally associated with signs and symptoms of inflammation
-
Common example: extensor tendon rupture in a patient with rheumatoid arthritis
-
-
Classification
Classification of intrinsic degenerative tendon disorders is covered in Table 24-1.
Clinical Presentation
Acute Injury
-
Due to a single traumatic event
-
No preceding symptoms
-
May be subclinical pre-existing pathology
in affected tendon, particularly if there is no externally applied
direct impact to the tendon
Chronic Injury
-
Etiology
-
Often thought of as “normal wear and tear” that exceeds the body’s ability to repair it
-
Root cause of problem is twofold:
-
Pathologic changes within “at-risk” portions of the tendon
-
Limited metabolic potential of the host cells in these locations
-
-
-
Factors that promote chronic injury
-
Increasing age
-
Conditions that impair normal vascularity (e.g., smoking)
-
-
Clinical presentation
-
Insidious onset of clinical symptoms
-
Subacute worsening with extension of the tear
-
Swelling at the site of the affected tendon(s) is appreciated by a clinician only in superficial locations.
-
When chronic injury is caused by
chemical/enzymatic damage from an inflammatory or autoimmune condition,
patients generally complain of pain and crepitus before rupture.-
If the inflammatory process is not
abated, the tendon can be damaged to the point where rupture occurs
with submaximal loading conditions. -
In many patients with rheumatoid disease,
the resultant tendon rupture is painless and manifests only as
deformity and decreased function on physical examination.
-
-
-
Histology of chronic tendon injury
-
Pre-existent tendon pathology typical
-
Abnormal tissue is usually seen in the
portions of the tendon with the lowest strains, not the tissue under
the highest tensile loads. -
Suggests a “stress-shielding”-related atrophy
-
Internal and/or external shear forces can propagate into complete tears.
-
-
Tendon Healing
Tendon healing is a complex process with significant
implications for clinical treatment. While the typical stages have been
defined histologically, the exact molecular signaling
implications for clinical treatment. While the typical stages have been
defined histologically, the exact molecular signaling
P.468
and
cellular control of the process remains poorly understood. Furthermore,
the different anatomic locations can affect the success of the process.
|
Table 24-1 Classification of Degenerative Tendon Disorders
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stages
Inflammation
-
Begins with hemorrhage at the time of tendon injury
-
Lasts for about 5 days
-
Inflammatory cytokines released and inflammatory cells recruited
-
Resultant granulation tissue has no intrinsic strength.
Proliferation
-
Begins within 3 to 5 days of injury
-
Continues until about 6 weeks after injury
-
Copious deposition of immature collagen fibrils that do not resemble the anatomy of normal tendon
-
Greater levels of types II, V, and VI collagen than normal
-
-
Fibroblasts migrate into wound.
-
Collagen is laid down in a haphazard fashion in first 2 weeks.
-
Fibers aligned perpendicular to the axis of tensile force
-
-
Extrinsic healing represents deposition from fibroblasts originating in the paratenon proliferating into the injury site.
-
Intrinsic healing represents deposition from fibroblasts within the epitenon and endotenon.
-
-
Fibrous bridge connects the injured ends by 2 weeks.
-
Mechanical strength remains well below that of the native tendon.
-
-
Gradual reorientation of the fibrils results in more normal-appearing microstructure over the last 4 weeks.
-
Repetitive, low tensile loading of the tendon has been shown to improve the healing response during this stage.
The proliferative stage is of major importance. If the
repair proceeds too slowly, the immature repair tissue will accumulate
damage, leading to a chronic tendinitis state, gapping of the tendon
ends, and possible failure. If this stage proceeds too vigorously, the
tendon will scar to its sheath or other surrounding structures.
Excessive scarring to the tendon will limit or deny its normal
excursion. Scarring is a particular problem in areas where the healing
tendon is in close association with a tendon sheath or retinacular
restraint.
repair proceeds too slowly, the immature repair tissue will accumulate
damage, leading to a chronic tendinitis state, gapping of the tendon
ends, and possible failure. If this stage proceeds too vigorously, the
tendon will scar to its sheath or other surrounding structures.
Excessive scarring to the tendon will limit or deny its normal
excursion. Scarring is a particular problem in areas where the healing
tendon is in close association with a tendon sheath or retinacular
restraint.
Remodeling
-
Begins at 6 weeks after injury and continues to beyond 1 year
-
Encompasses two phases: consolidation and maturation phases
-
Previously disorganized collagen slowly becomes more organized.
-
Increased intermolecular bonding increases strength.
-
-
While this remodeling never results in
the material properties of the original tendon, a structural strength
approaching that of the original tendon can be realized.
Interventions and Repairs
Surgical Repair
-
Surgical repair of a ruptured tendon accomplishes two ends:
-
Restoration of the musculotendinous unit function
-
Reduction of the gap between the healing
ends: With close apposition throughout the healing stages, a more
effective and stronger repair ensues.
-
-
Timing
-
Repair is best performed within 2 weeks of injury.
-
Retraction and secondary fibrosis of the muscle seen after 2 weeks can prevent coaptation of the lacerated ends.
-
-
Techniques
-
Key features of best techniques
-
Multiple suture strands
-
Some component of each strand placed perpendicular to the fascicle orientation to minimize cut-out
-
-
Specific techniques differ between the various sites of tendon injury.
-
Experts disagree on the “best technique” at any given site.
-
-
Goals of the repair
-
Gain sufficient mechanical strength to minimize gapping of tendon ends
-
Allow some early motion
-
Postsurgical Care/Physical Therapy
-
Benefits of early loads and motion on tendon healing
-
Monomeric collagen production, fibril orientation, and cross-linking are all enhanced by applied loads in the healing tendon.
-
Small amounts of motion applied early
have been shown to reduce the amount of paratendinous adhesions, which
can be detrimental to tendon gliding in the wound bed.
-
-
Repairs of tendons with sheaths (e.g., flexor tendons in finger)
-
Early passive motion protocols and light active loading in the immediate postoperative period are encouraged.
-
Closely supervised by a trained therapist
-
Patient education and compliance is of utmost importance.
-
Risk of detrimental paratendinous adhesions far outweighs the risk of rupture with contemporary techniques.
-
-
Repairs of tendons without sheaths (e.g., Achilles tendon)
-
Period of immobilization is often instituted.
-
Paratendinous adhesions are not as problematic to eventual outcome.
-
In the setting of an Achilles tendon
rupture with ragged tendon ends after repair, a larger quantity of
extrinsic paratendinous collagen production may be imperative to help
offset the limited intrinsic healing that will occur early on. Later
institution of active and passive motion protocols will generally
provide the mechanical stimulation needed to stimulate adequate
adhesive scar resorption.
-
-
Modalities
-
Ultrasound, electrical field stimulation, and suction cupping are common techniques used by therapists
-
Used for resolution of adhesive scar formation in the postoperative period
-
-
Only anecdotal evidence demonstrating any benefit over more traditional stretching and passive motion protocols
-
No high-quality studies
-
-
Ligament
Ligament Anatomy, Structure, and Composition
Ligaments are responsible for the passive transmission
of force between bones and are vital to joint stability. They connect
one location on a bone across a joint to another bone and are generally
short, flexible structures composed of fibrous tissue. As the force
applied to the ligament increases, more fibers are recruited to resist
the applied load. Ligaments are very similar to tendons with regard to
their structure, but their differing role leads to a variety of unique
features.
of force between bones and are vital to joint stability. They connect
one location on a bone across a joint to another bone and are generally
short, flexible structures composed of fibrous tissue. As the force
applied to the ligament increases, more fibers are recruited to resist
the applied load. Ligaments are very similar to tendons with regard to
their structure, but their differing role leads to a variety of unique
features.
Macrostructure
-
Variety of sizes and shapes
-
Correspond closely with the size and shape of the joint that they stabilize
-
-
Most are located on the external surfaces of the joints and tend to be wide and thin.
-
Example: collateral ligaments of knee
-
-
Some are located in protected positions within the articulation and shaped more like a cable.
-
Example: cruciate ligaments of knee
-
-
All ligaments are composed of bands, or bundles.
-
Bundles are not distinct on gross inspection.
-
During physiologic motion, different bundles are recruited to maintain joint stability.
-
Depending upon the position of the joint,
some portions of the ligament are minimally loaded, while other bundles
are under high tension.
-
Ultrastructure
-
Epiligament
-
Layer of organized tissue that tightly
envelops the surface of the ligament and through which a neurovascular
network enters the ligament.
-
-
Endoligament
-
Layer of loose connective tissue between the fascicles
-
-
FasciclesP.470
-
Ligaments, like tendons, are composed of a similar arrangement of microfibrils, subfibrils, and fascicles.
-
One or more fascicles make up a ligament.
-
-
Ligament–bone junction
-
Represents the critical region of load transfer from the ligament to the bone
-
It is a transitional zone with a complex arrangement.
-
There are two types of insertions:
-
Direct: insertion of ligament into bone only
-
Four morphologically distinct zones of transition: ligament → fibrocartilage → mineralized fibrocartilage → bone
-
-
-
Indirect: ligament fans out with connections into both bone and periosteum
-
Most common insertion type
-
Broader insertion zone
-
Superficial fibers insert into periosteum.
-
Deep fibers connect to bone obliquely via Shapey’s fibers.
-
-
Biochemistry
Collagen
-
Collagen represents about 70% of the dry weight of ligament.
-
This is less than tendon and one of the most significant differences between tendons and ligaments (Table 24-2).
-
Type I collagen predominates, with smaller proportions of types III, IV, V, and VI.
-
Slight variability in overall collagen composition between various ligaments is seen.
-
-
On a microscopic level, the collagen is constructed somewhat different than tendon.
-
Ligaments experience a less uniform loading pattern.
-
There is a more varied arrangement in fiber direction.
-
There is variety in density and orientation of fibrils even among different ligaments.
-
Noncollagenous Substances
-
Water is the primary component of ligament (70%).
-
Remainder of the dry weight is composed of proteoglycans, fibronectin, and elastin.
Cellular Population
-
There is a high structure to cellularity ratio.
-
As with tendon, the fibroblasts within
the extracellular matrix maintain the collagen and proteoglycan matrix.
They are subject to significant mechanical loads and operate in a field
of relative microvascularity. -
There is constant turnover of the
extracellular matrix in response to chemical and mechanical damage that
occurs from normal day-to-day function. -
The cellular reaction is similar to tendon.
|
Table 24-2 Differences of Tendons and Ligament Properties
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Blood Supply
Vascular Anatomy of Ligaments
-
Host cells are supplied via a network of blood vessels located in the endoligamentous and epiligamentous tissue.
-
Ligaments also receive a significant amount of blood supply at their insertions at bone.
-
A uniform microvascular network travels
along the epiligament, penetrating the ligament substance in a regular
longitudinal pattern. -
Ligaments are shorter and subject to minimal excursion compared to tendons, so a more consistent microvascular supply exists.
-
A more uniform process of matrix synthesis and repair results.
-
This represents a difference when compared with tendon (see Table 24-2).
-
Innervation
-
Neural elements within ligament
-
Generous nervous innervation within ligaments
-
Most are mechanoreceptors designed for detecting changes in pressure or tension.
-
Autonomic and nociceptive supply has also been documented.
-
-
Importance of ligament innervation
-
Proprioceptive receptors form part of an important ligament–muscular feedback loop that helps modulate joint function.
-
The recovery of proprioceptive function
after injury to a ligament is unpredictable and may contribute to
altered rehabilitation and long-term ligament function.
-
-
Regulation of blood flow in the normal and injured ligament
-
Mechanism for control of inflammation or repair in periarticular tissue
-
-
P.471
Ligament Biomechanics
Ligaments act as relatively rigid connectors of joints
that serve to stabilize and guide the bones through a range of motion.
The first role of the ligament is to transmit tensile forces without
failure, allowing for absorption of shock and dissipation of energy.
Ligaments are only part of the equilibrium of compressive and tensile
elements that ensure normal joint function. Furthermore, all the
ligaments in a specific joint work together, and at any given time only
distinct bundles of a given ligament are truly loaded. Early
biomechanical testing of ligaments tended to isolate individual
ligaments in vitro and involved nonphysiologic loading patterns; as
such, much early biomechanical work is not truly indicative of ligament
function in vivo.
that serve to stabilize and guide the bones through a range of motion.
The first role of the ligament is to transmit tensile forces without
failure, allowing for absorption of shock and dissipation of energy.
Ligaments are only part of the equilibrium of compressive and tensile
elements that ensure normal joint function. Furthermore, all the
ligaments in a specific joint work together, and at any given time only
distinct bundles of a given ligament are truly loaded. Early
biomechanical testing of ligaments tended to isolate individual
ligaments in vitro and involved nonphysiologic loading patterns; as
such, much early biomechanical work is not truly indicative of ligament
function in vivo.
Crimp
-
Ligament accomplishes these tasks by having the same sort of microscopic “crimp” seen in tendons, only to a greater degree.
-
At low loads, this crimp straightens out
and allows the ligament to accommodate a degree of lengthening or
shortening of the ligament without generating large loads. -
This is seen as a “toe” region on a force-displacement graph.
-
-
There is a gradual steepening of the force-deformation curve as more and more fibrils are recruited.
-
As the amount of load in the ligament begins to rise sharply, the ligament provides more stability to the joint.
-
Variability
-
The mechanical properties of ligaments closely correspond with anatomical location and in vivo loading conditions.
Ultimate Strength and Failure
-
As the loaded collagen fibrils in ligament reach their point of maximal load, there is ultimate failure.
-
Ligaments have a lower collagen content and a less uniform fibril and cross-linking arrangement than tendons.
-
More nonlinear load–deformation curve
-
Lower mechanical properties (ultimate tensile strength and elastic modulus) than tendons
-
|
Table 24-3 Grading of Ligament Injuries
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
Elastic Biomechanics
-
Ligaments demonstrate increased elastic behavior compared to tendons.
Viscoelastic Behavior
-
Allows for laxity that allows gradual assumption of force as the joint moves through a normal range of motion
-
Rapidly stiffens should an undesirable joint position occur
-
-
Provides load sharing and energy conservation to protect ligaments
-
The rate at which the ligament is loaded is important.
-
The more quickly a tensile load is placed, the more effective is the resistance to outward flow of the water.
-
Ligament Injury, Healing, Repair, and Reconstruction
In contrast to tendon injuries, ligament injury tends to
occur by a single mechanism. The application of a load to a joint that
exceeds the ultimate strength of part or all of the ligament results in
loss of competence of the ligament. While the mechanism of injury is
similar, the degree of injury to the ligament varies and has resulted
in the following classification.
occur by a single mechanism. The application of a load to a joint that
exceeds the ultimate strength of part or all of the ligament results in
loss of competence of the ligament. While the mechanism of injury is
similar, the degree of injury to the ligament varies and has resulted
in the following classification.
Clinical Presentation
-
Relative degree of injury is given in Table 24-3.
Location of Disruption
-
Midsubstance rupture
-
Bony avulsions
-
Second most common location
-
Most common in skeletally immature patients
-
Growth plate represents weak link.
-
Common example: avulsion of medial epicondyle of elbow
-
-
Diagnosed by radiographs
-
A variable degree of subclinical failure in the ligament also evident
-
Good prognosis from surgical repair
-
Bone attachments generally easily reattached
-
Common example: avulsion of medial malleolus of ankle
-
-
-
Chronic attenuation of ligament
-
Vast majority of ligament injuries occur after application of excessive traumatic force.
-
Chronic attenuation of ligament integrity occurs in conjunction with inflammatory disease.
-
Leads to slow loss of joint alignment and stability
-
Becomes progressively debilitating
-
Common example: rheumatoid degeneration of the metacarpophalangeal joints of the fingers
-
-
Ligament Healing
Ligament healing is similar to healing in most tissues
in the body and nearly identical to the process in tendons (see section
on tendon healing for description of stages).
in the body and nearly identical to the process in tendons (see section
on tendon healing for description of stages).
Healing Environment
Vascular Supply
-
More consistent vascular supply within torn ligaments than tendons
-
Speculation that certain ligaments may
fail to heal because of decreased vascularity and/or an environment
inhospitable to the vascular response-
All torn ligaments can generate an adequate hemorrhagic response.
-
Clinical example: anterior cruciate ligament of the knee, intra-articular location bathed in synovial fluid
-
-
How far the healing progresses along the
second stage (proliferation) seems to be the bigger variable, most
dependent on mechanical environment.
Mechanical Environment
-
Determines the quality of ligament healing during the proliferative stage
-
Secondary stabilizing ligaments must minimize loading on the healing ligament for the progression of the healing process.
-
Minimization of gapping at the torn ends
-
-
Institution of early and controlled joint motion further stimulates an ideal environment for ligament healing.
-
Clinical example: isolated injury to the
knee medial collateral ligament. Adequate secondary stabilizers
(cruciate ligaments and capsule); generally heals with an early motion
protocol.
Poor Healing Environment
-
Secondary constraints cannot compensate for the function of the injured ligament.
-
Excessive loading stretches repair tissue.
-
Excessive gapping prevents tissue reorganization and vascular networks.
-
-
Results in an incompetent repair and compromised joint stability long term
-
Surgical intervention is most indicated in these instances.
-
Clinical example: anterior cruciate ligament of the knee–no adequate secondary stabilizer for the function of the ligament
-
-
Joints that have suffered injury to more
than one ligament will predictably have poorer restoration of stability
and clinical function without surgical stabilization.-
Clinical example: combined medial collateral and anterior cruciate injury
-
Outcomes
-
Even when healing occurs in an ideal environment, normal ligamentous anatomy is not created.
-
Healed ligament demonstrates subtle but significant differences when compared to normal ligament (Box 24-1).
Box 24-1 Differences in Ligament Structure after Healing
|
Interventions and Repairs
Nonsurgical Treatment and Bracing
-
A brief period of immobilization is often
instituted as a first-line treatment for a ligamentous injury when the
joint is anticipated to be stable.-
Clinical example: elbow dislocation
-
-
A program of active motion protocols in
conjunction with a brace that minimizes detrimental loading of the
healing ligament will generally provide the mechanical stimulation
needed to maximize healing properties and stimulate adhesive scar
resorption.-
Clinical example: medial collateral ligament of knee injury
-
Surgical Repair
-
Surgical repair of a ruptured ligament accomplishes two tasks:
-
Restores the ligament structural properties
-
A portion of the joint-stabilizing function of the ligament is restored.
-
Reduces loading-induced deformation of the early granulation tissue
-
May also reduce loads on partially
injured secondary stabilizing ligaments within the same joint,
increasing the likelihood of improved healing in these structures as
well
-
-
Promotes apposition of the torn ligament ends for healing
-
Stronger repair with close apposition when compared with wide gaps.
-
No advantage to surgical repair when the torn ligament ends lay closely apposed naturally
-
If the joint retains adequate stability after ligament tear, surgical intervention may be detrimental to clinical outcomes.
-
-
Preferred technique for ligament repair differs between various sites.
-
In general, this is best accomplished via multiple suture strands.
-
Suture anchor fixation or sutures through bone tunnels are most effective for repair of ligament back to bony origins.
-
Even experts disagree on the “best technique” at any given site.
-
Goals
-
Gain sufficient mechanical strength to initiate early motion protocols
-
Minimize gapping
-
Ligament Reconstruction
-
In some instances, it is technically
impossible to generate adequate stability or create sufficient
apposition of torn ligament ends. -
In the setting of a joint with chronic
ligament deficiency, imbrication of the poorly organized local scar
tissue is ill advised.-
Clinical example: chronic anterior cruciate ligament tear of the knee
-
-
In these situations, ligament reconstruction with autograft or allograft substitutes has been extensively described.
-
Key features of these techniques
-
Substitution of grafts with structural properties meeting or exceeding native ligament
-
Secure graft fixation though bone tunnels
-
Anatomic reapproximation of ligament-bone insertions
-
“Isometric” placement: With increasing
knowledge of fiber bundles, truly isometric graft placement is not
possible for all knee positions. -
Ligament preconditioning: stress relaxation applied to graft before insertion and final tensioning
-
Postsurgical Care and Physical Therapy
-
When compared with joint immobilization,
motion applied to healing ligaments improves the biomechanical
properties of the ligament in the following manner:-
Increased size of scar
-
Increased size of collagen fibrils (little improvement in collagen fiber realignment, however)
-
Improved parallel sliding between fibers
-
Reduced synovial adhesions and adjacent connective tissue proliferation
-
Suggested Reading
Amiel D, Frank C, Harwood F, et al. Tendons and ligaments: A morphological and biochemical comparison. J Orthop Res 1984; 1: 257–265.
Buckwalter JA, Einhorn TA, Sheldon SR, eds. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. Rosemont, IL: AAOS Press, 2000.
Dienst M, Burks RT, Greis PE. Anatomy and biomechanics of the anterior cruciate ligament. Orthop Clin North Am 2002;33: 605–620.
Frank CB. Ligament healing: Current knowledge and clinical applications. J Am Acad Orthop 1996;4:74–83.
Gelberman RH, Woo SL, Lothringer K, et al. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg [Am] 1982;7:170–175.
Kirkendall DT, Garrett WE Jr. Clinical perspectives regarding eccentric muscle injury. Clin Orthop 2002;403S:S81–89.
Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 2003;84:649–698.
Lin, TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomechanics 2004;37:865–877.
Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med 2003;22:675–692.
Maganaris
CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology
of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med 2004;34:1005–1017.
CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology
of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med 2004;34:1005–1017.
Malcarney HL, Murrell GA. The rotator cuff: biological adaptations to its environment. Sports Med 2003;33:993–1002.