Peripheral Nerve Physiology, Anatomy, and Pathology
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section IV – Basic Science > 25 – Peripheral Nerve Physiology, Anatomy, and Pathology
25
Peripheral Nerve Physiology, Anatomy, and Pathology
Shikha Sethi
Brian J. Harley
Christian Custodio
Michael Stubblefield
A comprehensive understanding of peripheral nerve
anatomy and physiology is essential for understanding peripheral nerve
pathophysiology and mechanisms of peripheral nerve injury and
regeneration. Understanding peripheral nerve injury and cellular repair
is critical to clinical management of operative nerve injury,
microsurgical nerve repair, and emerging applications that target
intrinsic nerve cell functions to assist in nerve regeneration.
anatomy and physiology is essential for understanding peripheral nerve
pathophysiology and mechanisms of peripheral nerve injury and
regeneration. Understanding peripheral nerve injury and cellular repair
is critical to clinical management of operative nerve injury,
microsurgical nerve repair, and emerging applications that target
intrinsic nerve cell functions to assist in nerve regeneration.
Peripheral Nerve Anatomy
Gross Anatomy
General Organization
-
31 mixed spinal nerves emerge from the spinal cord:
-
8 cervical
-
12 thoracic
-
5 lumbar
-
5 sacral
-
1 coccygeal
-
-
Nerves emerge from the foramen of the vertebral bodies after the union of ventral and dorsal roots.
-
Autonomic, sensory, and motor fibers travel together in peripheral nerves to their destinations.
-
Nerves branch into dorsal and ventral rami upon exiting the foramen.
-
Dorsal rami
-
Small-caliber branches
-
Provide segmental innervation to dorsal paraspinal area
-
-
Ventral rami
-
Large-caliber branches
-
Cervical, lumbar, and sacral roots join together to form nerve plexuses to innervate the extremities.
-
Thoracic spinal nerves (except T1) do not
form plexuses but instead provide segmental innervation to large areas
of the ventral trunk.
-
-
P.475
Nerve Plexus
-
Coalescence of multiple spinal nerve ventral rami
-
Fairly consistent anatomic connections and exchanges within plexuses
-
Each root level still innervates specific dermatomal and myotomal segments.
-
-
At the distal aspect of the plexus, peripheral nerves form with representations from multiple spinal levels.
-
Four consistent locations
-
Cervical plexus
-
First four cervical roots
-
-
Brachial plexus
-
Lower four cervical and first thoracic ventral rami
-
-
Lumbar plexus
-
First three and a part of the fourth lumbar ventral rami
-
-
Sacral plexus
-
All sacral rami along with the fifth and a part of the fourth lumbar ventral rami
-
-
Peripheral Nerves
-
Each nerve may contain any combination of three possible nerve types:
-
Motor efferent fibers
-
Cell bodies in the spinal cord
-
Transmit motor information to muscles about when and how to act
-
Motor unit: individual motor neuron and the specific group of muscle fibers it innervates
-
-
Sensory afferent fibers
-
Cell bodies in dorsal root ganglia
-
Convey modality or quality, intensity, duration, and location of a stimulus from the periphery
-
Arise from specialized pain, thermal, tactile, and stretch (proprioceptive) receptors in the periphery
-
Terminal axons and presynaptic terminals
for sensory fibers may be at the spinal level of the corresponding
dorsal root ganglion or deeper in the central nervous system.
-
-
Sympathetic fibers
-
Originate in the intermediolateral cell column in the thoracic and upper lumbar spinal cord
-
Synapse at variable levels of the
paravertebral sympathetic ganglion and then travel as fibers within
mixed spinal nerves to end organs such as sweat glands, blood vessels,
and erector pili -
Fibers join the spinal nerve and can then branch into ventral and dorsal primary rami.
-
-
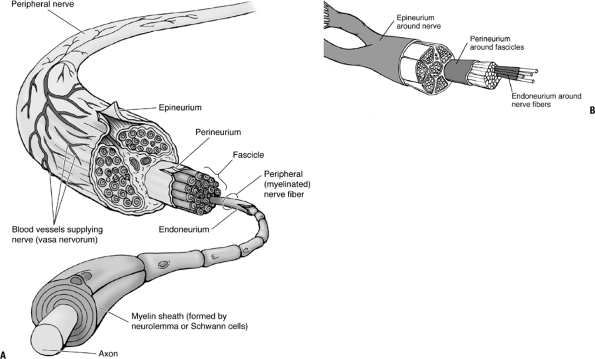 |
|
Figure 25-1
(A) Arrangement and ensheathment of peripheral, myelinated nerve fibers. All but the smallest peripheral nerves are arranged in bundles (fascicles), and the entire nerve is surrounded by the epineurium, a connective tissue sheath. Each small bundle of nerve fibers is also enclosed by a sheath, the perineurium. Individual nerve fibers have a delicate connective tissue covering, the endoneurium. The myelin sheath is formed by neurolemma (Schwann) cells. (B) Peripheral nerves are structured similarly to tendons, ligaments, and muscles, with long parallel fibers contained in bundles surrounded by connective tissue. (A from Moore KL, Dalley AF. Clinically Oriented Anatomy, 5th ed. Baltimore: Lippincott Williams & Wilkins, 2006. B from Hendrickson T. Massage for Orthopedic Conditions. Baltimore: Lippincott Williams & Wilkins, 2002.) |
Microanatomy
Nerves (Fig. 25-1)
-
The normal peripheral nerve is composed
of blood vessels, nerve fibers, and three levels of connective tissue
within which the fibers and vessels lie.P.476-
Epineurium
-
Outermost connective tissue layer
-
Represents up to 50% of the cross-sectional area of the nerve trunk
-
Loose meshwork of collagen and elastin fibers and is generally thicker where a nerve crosses a joint
-
Well-developed vascular plexus runs within the epineurium.
-
Functions to protect the nerve fiber bundles, called fascicles, within the nerve
-
Tough external epineurium surrounds periphery of nerve.
-
Loose internal epineurium occupies space between fascicles.
-
-
-
Perineurium
-
Thin, dense, multilayered connective tissue sheath that surrounds each fascicle
-
Tight basement membranes within the perineurium protect the endoneurial space by serving as a diffusion barrier.
-
Tensile strength of the perineurium helps maintain intrafascicular pressures.
-
Vascular structures traverse the perineurium obliquely to enter the endoneurial space.
-
-
Endoneurium
-
Delicate collagenous matrix with fibroblasts, mast cells, and a capillary network
-
Surrounds individual myelinated nerve fibers or groups of unmyelinated nerve fibers within a fascicle
-
-
Fascicles
-
All neurons within a peripheral nerve are bundled together into structures termed fascicles.
-
Fascicles are located within the internal epineurium.
-
Bounded by the perineurium
-
-
Fascicles are often grouped together into a larger unit.
-
Inner interfascicular epineurium bounds grouped fascicles.
-
Grouped fascicles can be easily divided along internal epineurial planes.
-
-
Major peripheral nerves will contain many grouped fascicles.
-
There is constant redistribution of fascicular organization along a peripheral nerve.
-
Interfascicular plexuses allow for interconnections.
-
-
Fascicles are more numerous and smaller where a nerve crosses a joint.
-
Smaller fascicles and more internal
epineurium between them allows for increased protection of nerve fibers
from external trauma and deformation.
-
-
-
As the nerve gives off branches along its course, the fascicles divide (see Fig. 25-1).
-
Small terminal nerves contain only one or two fascicles.
-
Example: digital nerve
-
-
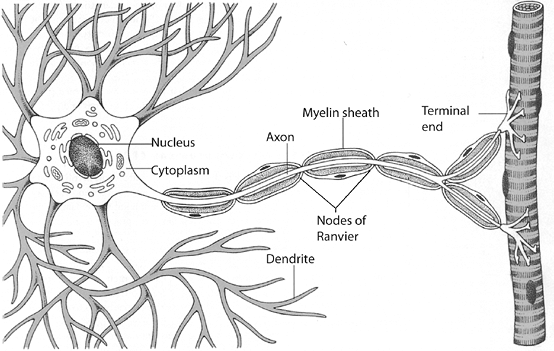 |
|
Figure 25-2 Neuron showing cell body, axon, dendrites, Schwann cells, myelin sheath, and nodes of Ranvier. (From Werner R, Benjamin BE. A Massage Therapist’s Guide to Pathology, 2nd ed. Baltimore: Lippincott Williams & Wilkins.)
|
Cellular Anatomy and Physiology
Neurons (Fig. 25-2)
-
Individual nerve fibers within the endoneurium of a peripheral nerve are termed neurons.
-
Neurons are extensions of a single nerve cell body.
-
-
Neurons are broken down into four distinct regions:
-
Cell body
-
Contains the nucleus of the nerve cell
-
Metabolic center of the nerve cell
-
Dorsal root ganglion contains the cell body for sensory nerve fibers.
-
Motor nerve cell bodies are found in the anterior horn cells of the spinal cord.
-
-
Dendrites
-
Thin processes that branch off the cell body
-
Receive inhibitory or stimulatory synaptic input from other cells
-
Synapse with both central and peripheral nervous systems
-
This input allows modulation of peripheral nerve function.
-
-
-
Axons
-
Each cell body gives rise to a single axon at its axon hillock.
-
Propagate electrical signals known as action potentials
-
Convey information over distances from cell bodies to nerve terminus
-
-
Axons may act at great distances from their cell bodies.
-
Example: Signal to extend great toe is
generated in the motor cell bodies of the anterior horn cells of spinal
nerve roots L5 and S1 and runs to the distal portion of the lower leg
to innervate the extensor hallucis.
-
-
-
Presynaptic nerve terminal
-
Located at the distal end of the axon
-
Action potential causes changes in ion exchange at terminus.
-
Release of neurotransmitter at the synaptic cleft or neuromuscular junction results.
-
-
P.477 -
Schwann Cells and Myelin Sheaths
-
Specialized macroglial cells are called Schwann cells.
-
Surround peripheral nerve axons and produce myelin
-
70% lipid
-
30% protein
-
High concentration of cholesterol and phospholipids
-
-
Myelin provides electrical insulation for the electrical impulse.
-
Allows propagation of electrical impulses at faster speeds and at higher frequencies
-
Myelinated Axons
-
Wrapped throughout their length by concentric, tight spirals of layers of the Schwann cell membrane
-
Schwann cells line up end to end along the course of a single axon.
-
Entire length of the axon is surrounded.
-
Small spaces (up to 1.0 mm) between adjacent Schwann cells called nodes of Ranvier
-
Up to 500 Schwann cells may myelinate a single axon.
-
Unmyelinated Axons
-
Surrounded as a group by processes of a single Schwann cell
-
Conduction through these axons is comparatively slower.
Peripheral Nerves
-
Contain both myelinated and unmyelinated fibers in an average ratio of 4:1 traveling within each fascicle
-
Pathologic processes that disrupt the myelin sheath can slow conduction or cause focal conduction block.
-
Axoplasmic Transport
-
Specialized transport processes within a nerve cell
-
All cellular proteins and neurotransmitters are produced in cell body.
-
Cell body may be at a significant distance from the terminal axon.
-
Multiple transport mechanisms
-
Fast and slow anterograde transport
-
Move cellular proteins from the cell body to the axon
-
-
Fast retrograde transport
-
Removes debris and breakdown products from the distal axon back to the cell body
-
-
-
Mechanisms proposed consist of carrier proteins binding to microtubules within the nerve cell.
-
Electrophysiology of Peripheral Nerves
Nerve cells communicate via electrical and chemical
impulses. Ion exchanges between the microenvironment inside and around
a nerve fiber create electrical potential differences in the nerve
cell. When certain threshold levels are reached, events such as release
of neurotransmitter vesicles, or initiation of an action potential,
occur.
impulses. Ion exchanges between the microenvironment inside and around
a nerve fiber create electrical potential differences in the nerve
cell. When certain threshold levels are reached, events such as release
of neurotransmitter vesicles, or initiation of an action potential,
occur.
Resting Cell Membrane and Electrical State
-
Neurons at rest have a negative potential within the cell between -50 and -80 mV.
-
Na+ and Cl– are concentrated on the outside of the neuron.
-
K+ and organic anions are concentrated on the inside.
-
-
Cell membrane is essentially impermeable to charged ions, except where specialized ion channels allow transit of charged ions.
-
Net ion flux across the membrane at rest is zero.
-
Actively maintained by the Na +/K+ pump
-
-
Excitatory and inhibitory neurons induce graded potentials in the nerve cell.
-
Act at cell body and dendrites
-
Membrane potential can become more or less negative as a result.
-
If sum of electrical activity received and processed reaches threshold, nerve depolarizes.
-
Depolarization
-
Depolarizing “all-or-nothing” initiation of an action potential
-
Brief change in the properties of the neuron cell membrane occurs.
-
Na+/K+pump then begins to pump Na+ out of the cell and restore resting negative potential to allow additional action potentials.
-
-
Depolarization begins at the axon hillock.
-
Na+ flows into this region of the axon first.
-
Adjacent areas of the axon become flooded with Na + and depolarize.
-
Depolarization propagates down the axon until the terminus is reached.
-
Saltatory Conduction
-
Process of signal conduction in a myelinated axon
-
Myelin insulates the nerve axon, allowing passive current flow to spread quickly through an area of myelination.
Nodes of Ranvier
-
Unmyelinated spaces between adjacent Schwann cells on myelinated nerves
-
Essential to fast impulse propagation
-
Large density of voltage-gated Na+ channels at nodes
-
When depolarization reaches the node of Ranvier, these Na+ channels open.
-
Na+ flows into the axon.
-
Depolarizes the nodal region
-
Action potential quickly spreads through the next length of myelinated territory to the next node.
-
-
Focal areas of demyelination can slow or stop saltatory conduction.
-
Example: Pressure on the peroneal nerve
as it traverses around the fibular head can cause demyelination and a
resultant mononeuropathy.
-
End-Organ Neurotransmission
-
Depolarization in a nerve cell extends to the presynaptic terminal at the end of the axon.
-
Depolarization in the presynaptic area allows nerve signal “communication” to the end organ.
-
Example: Calcium ion entry through
voltage-gated calcium channels facilitates neurotransmitter release at
the neuromuscular junction.
-
Classification of Nerve Fibers (Table 25-1)
-
Nerve fibers characterized by Erlanger-Gasser or Lloyd-Hunt classification
-
Based on nerve fiber diameter and conduction velocity or function
-
Larger-diameter fibers typically have higher conduction velocities.
-
|
Table 25-1 Nerve Fiber Types
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nerve Injury
Pathophysiology
-
Efficient nerve transmission requires intact axons and myelin.
-
Injury to nerves can cause conduction slowing or failure of transmission.
-
Nerve transmission may fail because of four possible mechanisms:
-
Axonal dysfunction
-
Example: nerve ischemia from tourniquet
-
Blocks oxidative metabolism needed to produce energy for nerve cell transport
-
Axonal transmission may recover soon after ischemia.
-
Axonal transport may take hours or up to a day longer to recover.
-
-
Axonal degradation (axonal neuropathy)
-
Can predominantly affect sensory or motor axons or both
-
Example: amyotrophic lateral sclerosis
-
Anterior horn cell degeneration producing preferentially motor dysfunction
-
-
Demyelination (demyelinating neuropathy)
-
Focal demyelination
-
Usually due to stretch or compression of nerve tissue
-
Usually at cutaneous sites where specific peripheral nerves run close to the skin surface and are vulnerable to injury
-
-
Generalized demyelination
-
Widespread slowing of conduction velocities
-
Example: Guillain-Barré syndrome
-
Acute inflammatory demyelinating poly-neuropathy
-
Muscle weakness, pain, and in severe cases respiratory paralysis
-
-
-
-
Transport system disruption
-
Example: chemotherapeutic agents
-
Some agents interfere with microtubule assembly.
-
Axoplasmic transport affected
-
-
-
Any combination of these disorders is possible.
-
Example: amyloidosis
-
Deposition of extracellular material between nerve fibers causes demyelination and loss of axonal continuity.
-
P.479
Anatomic Classifications of Nerve Injury
Radiculopathy
-
Neuropathy of the nerve roots before they join to form spinal nerves
-
Most radiculopathies are compressive.
-
Examples: herniated nucleus pulposus, spinal stenosis
-
-
Noncompressive radiculopathies may be seen.
-
Examples: diabetes, sarcoidosis, amyloidosis, vasculitis, infections
-
Plexopathy
-
Refers to nerve dysfunction at the nerve plexus
-
Most result from blunt trauma.
-
Examples: brachial plexopathy
-
Sudden traction on arm from motorcycle accident
-
Invasive breast cancer or lung cancer (Pancoast tumor)
-
Radiation fibrosis from cancer treatment
-
Idiopathic (Parsonage-Turner syndrome)
-
-
Peripheral Neuropathy
-
Generic term used to describe a large and heterogeneous group of disorders affecting fibers distal to the plexus
-
Large- and small-diameter motor and sensory fibers can be affected.
-
Lesion can occur anywhere distal to the plexus.
-
To the neuromuscular junction for motor nerves
-
To the small intradermal fibers for sensory nerves
-
-
Mixed motor and sensory axonal neuropathies are more common than pure motor or sensory neuropathies.
-
Muscle weakness is usually more prominent
distally, and sensory abnormalities are usually in a distal
stocking-and-glove distribution.-
Length-dependent dysfunction
-
Distal axon is affected more than the proximal axon.
-
Abnormality may be symmetric or asymmetric.
-
-
Etiology of neuropathy includes multiple disorders that can be grouped broadly into the following categories:
-
Inherited
-
Traumatic
-
Compressive
-
Autoimmune
-
Toxic
-
Metabolic
-
Infectious
-
Idiopathic
-
Neuromuscular Junction Disorders
-
A functioning neuromuscular junction is
required for efficient and effective transfer of impulses from motor
nerve to the muscle itself. -
Diseases affect synaptic transmission
between motor nerve and muscle and can affect either the presynaptic or
postsynaptic locations.-
Presynaptic
-
Example: Botulinum toxin compromises release of acetylcholine from the nerve terminal of the motor axon.
-
-
Postsynaptic
-
Example: myasthenia gravis–antibodies to acetylcholine receptors on the postsynaptic muscle membrane
-
-
Complex Lesions: Multiple Anatomic Locations
-
Neuropathies can coexist with radiculopathy and plexopathy.
-
Example: A patient with diabetes can have
a combination of radiculopathy, plexopathy, and neuropathy (diabetic
amyotrophy), median mononeuropathy at the wrist (carpal tunnel
syndrome), mononeuropathy multiplex from vasculitis, distal symmetric
poly-neuropathy, or an isolated small-fiber neuropathy.
-
Electrodiagnostic Testing
-
Frequently used in the assessment of peripheral nerve disorders
-
Helps characterize and localize disorders
-
Differentiate mononeuropathies from mononeuropathy multiplex and distal symmetric polyneuropathies
-
Differentiate acute vs. chronic, axonal vs. demyelinating, and motor vs. sensory involvement
-
-
Severity of a nerve lesion can often be determined.
-
Prognosis can sometimes be elucidated.
-
-
Other disorders known to cause similar signs and symptoms can be excluded.
P.480
Traumatic Nerve Injury
-
Direct trauma to the nerve may injure or sever the axons as well as the connective tissues surrounding the nerve fibers.
-
Motor and sensory function of the nerve will be adversely affected.
-
Resultant dysfunction of muscles and loss of sensation
-
-
Sympathetic fiber damage can induce dysfunction or hyperactivity of local sympathetic fibers.
-
Atrophy of skin and adnexal structures, changes in vascular and lymphatic flow
-
-
Classification of traumatic nerve injury
is based on the damage sustained by the nerve components and the
ability for spontaneous recovery (Table 25-2).-
Two schema widely used
-
Seddon’s classification the first to characterize injury types
-
Sunderland’s classification more precise
-
Grade I Injury (Neuropraxia)
-
Mildest grade of traumatic nerve injury
-
Dysfunction of conduction across a segment of a nerve
-
Conduction is evident in axon above and below site of injury.
-
-
Axonal continuity preserved
-
Wallerian degeneration does not occur.
-
-
Recovery is generally quick.
-
Pain and temperature return first, motor and fine touch last.
-
Recovery is complete within 3 to 4 months.
-
-
Clinical example: median nerve stretched at the time of a distal radius fracture causing hand dysesthesias
|
Table 25-2 Sunderland and Seddon Classifications of Traumatic Nerve Injury
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Grade II Injury (Axonotmesis)
-
Axon damage is seen but endoneurium is in continuity.
-
Wallerian degeneration occurs in distal axons.
-
Fibrillations and denervations evident in muscles
-
Neural connective tissue sheath, Schwann cells, and other supporting structures intact
-
-
Recovery of motor and sensory is prolonged but generally complete.
-
Preservation of the connective tissue architecture guides axonal regeneration to reinnervate distal target organs.
-
Grade III Injury
-
Axonal and endoneurial continuity is disrupted.
-
Perineurium is preserved.
-
Wallerian degeneration occurs in distal axons.
-
Fibrillations and denervations evident in muscles
-
-
Recovery is prolonged and often incomplete.
-
When axons regenerate, they may enter an incorrect nerve sheath.
-
Intraneural scarring at site of injury obstructs axonal regrowth.
-
Grade IV Injury
-
Axons and perineurial tissue are damaged but the epineurial nerve sheath is preserved.
-
Neural scarring is common and adversely affects axonal regrowth and repair.
-
Axonal regeneration is still possible without surgical repair.
-
Grade V Injury (Neurotmesis)
-
Endoneurium, perineurium, and epineurium are all disrupted or transected.
-
Substantial perineural hemorrhage and scarring develop.
-
Recovery through axonal regeneration cannot occur.
-
Surgical repair is only effective way to allow for axonal regeneration.
-
Clinical example: median nerve laceration at wrist with a knife
Grade VI Injury
-
A sixth grade of injury has been proposed that more closely describes injury to nerve in vivo.
-
Complex injury that involves combinations of milder and more severe grades of nerve injury within a single nerve
-
Recovery from grade VI injury is not uniform.
-
Varies across nerve segments and fascicles depending on the pattern of nerve injury
-
-
Clinical example: radial nerve injury at level of humeral shaft fracture
P.481
Mechanisms of Traumatic Nerve Injury
-
Eight mechanisms of traumatic injury to
peripheral nerves are known. Each causes specific damage to the neuron,
the Schwann cell, or the myelin sheath: -
Mechanical injury
-
Acute compression injury that can cause a focal demyelination
-
Typically results in a focal conduction block
-
Recovery occurs after remyelination of the axon.
-
Example: Prolonged use of a tourniquet may cause a mechanical injury.
-
-
If injury is severe enough or prolonged, axonal damage may also occur and recovery will be delayed.
-
Example: carpal tunnel syndrome
-
-
-
Crush and percussion injury
-
Focal compression injury to the nerve induced by hematomas and/or compartment syndrome following a fracture
-
High pressure induced in the surrounding tissue compromises the vascular supply.
-
-
Neuronal ischemia leads to subsequent
segmental demyelination, periaxonal and intramyelin edema, and axonal
interruption of nerves.-
Example: Delays in assessment and treatment of compartment syndrome can lead to nerve injury.
-
-
Double crush syndrome
-
A situation in which proximal compression injury renders the distal nerve more susceptible to compression injury
-
Likely a consequence of effects on axoplasmic transport
-
Example: carpal tunnel syndrome with a cervical radiculopathy
-
-
-
-
Stretch injury
-
Nerve stretches approximately 10% to 20% before structural damage occurs.
-
Axonal injury results from acute lengthening greater than the nerve can tolerate.
-
Axons are disrupted over long segments of the nerve.
-
No disruption of epineurium is observed, so there is no clear role for surgical repair.
-
Example: Traction on peroneal nerve during knee dislocation causes a resultant foot drop.
-
-
-
Laceration
-
Caused by blunt or penetrating trauma or combination thereof
-
Represents a progression of the stretch injury (type 3 above)
-
Stretch to the point where the nerve is grossly disrupted
-
Loss of continuity of both perineurium and epineurium
-
-
Nerves are not cleanly sectioned but are damaged in an irregular pattern over a large distance.
-
Nerve ends must be cut back to undamaged fascicles for successful repair.
-
Example: radial nerve laceration caused by open humerus fracture
-
-
-
Transection
-
Associated with penetrating trauma, typically sharp edge
-
Nerves are partially or completely severed, with resultant epineurial disruption.
-
Direct surgical repair usually favorable with minimal resection of nerve ends
-
Examples: stab wound, glass lacerations, surgical incisions
-
-
-
High-velocity trauma
-
Damage secondary to rapid tissue expansion in the track of a missile wound
-
Nerve injury a result of large amount of absorbed energy in surrounding tissue
-
Most frequently the nerve is not lacerated; rather, a mixed injury of axonotmesis and perineurial disruption.
-
Surgical repair is generally not required.
-
Example: gunshot wounds
-
-
-
Cold injury
-
Several hours of exposure to temperatures between -2.5°C and 10°C will slow or stop axoplasmic transport.
-
Prolonged exposure may cause permanent damage to peripheral nerves.
-
-
Frostbite will likely lead to necrosis of peripheral nerves.
-
-
Healing injury
-
Nerves can be damaged from the physiologic healing processes.
-
Adherence of nerves to scar tissue,
fracture callus, or heterotopic bone may limit nerve gliding and a
resultant decrease in function can be observed.
-
Pathophysiology of Nerve Injury
-
After damage to a peripheral nerve, complex changes occur throughout the neuron.
-
Cell body
-
Cell body and nucleolar swelling as well as nuclear eccentricity
-
The neuron shifts from nerve conduction mode to repair mode.
-
Increased production of cytoskeletal proteins and growth-associated proteins (GAPs)
-
Decreases in neurotransmitters and neurofilament proteins
-
-
-
Site of injury
-
Changes in local tissue oxygenation and energy delivery negatively affect axonal transport and nerve conduction.
-
The proximal segment of a transected nerve degenerates one or more internodal segments.
-
If the cell body survives, axonal sprouting begins.
-
-
Wallerian degeneration
-
Structured process of breakdown in the distal segment that occurs with axonal disruption
-
Axonal degeneration initiated within hours of injury
-
Myelin breakdown and phagocytosis progress distally to the target organ.
-
-
Macrophages
-
Play important roles in phagocytosis of cellular and myelin debris and stimulation of Schwann cells
-
-
Schwann cells
-
Proliferate and create endoneurial tubes (bands of Bunger) for regeneration of axons
-
-
-
Nerve conduction
-
Immediately after injury, stimulation
proximal to the injured site will demonstrate a reduced compound muscle
action potential (CMAP). -
Distal CMAP amplitude will be normal immediately after injury.
-
Reduced amplitude on distal stimulation is observed by 7 days.
-
Spontaneous denervation potentials may be observed on electromyographic examination of affected muscles within 2 to 5 weeks.
-
Nerve Regeneration and Repair
Nerve recovery involves orderly degeneration followed by
an orchestrated sequence of regeneration. The success of nerve
regeneration is variable and highly dependent upon the age of the
patient as well as the type of nerve injury. Little functional recovery
will occur if the end organ has irreversibly atrophied by the time it
is reinnervated, so the timing and location of nerve injury are also
critical to outcomes.
an orchestrated sequence of regeneration. The success of nerve
regeneration is variable and highly dependent upon the age of the
patient as well as the type of nerve injury. Little functional recovery
will occur if the end organ has irreversibly atrophied by the time it
is reinnervated, so the timing and location of nerve injury are also
critical to outcomes.
Regeneration
-
Axon regeneration occurs at rate of approximately 1 to 2 mm/day or 1 inch/month.
-
Process begins with formation of multiple nerve “sprouts” at the proximal axon stump.
-
Sprouts form a regenerating unit that contains multiple nonmyelinated axons surrounded by a single Schwann cell.
-
Regenerating units must first elongate through the zone of injury.
-
Scar tissue density, increasing gap length, and axonal misdirection all negatively affect the process.
-
Several cytokines and nerve substances
secreted by the distal end of the nerve and possibly by Schwann cells
mediate the process.
-
-
Axons that bridge the gap can then enter the distal endoneurial tubes.
-
The regenerating axon signals Schwann cells to begin myelination.
-
Reinnervation
-
Functional recovery of nerve function is
related to completeness of axonal regeneration and directed growth into
the correct endoneurial tubes.-
Lack of functional recovery is most likely a result of axon misdirection.
-
During neonatal development, selective reinnervation occurs as neurotropic factors guide axons to their target.
-
In adult models, this effect is greatly reduced and dependent upon nerve gap and axon orientation.
-
-
-
Progress of the healing nerve can sometimes be monitored with an advancing Tinel’s sign.
-
Recovery of motor function is possible only when the distance from the site of trauma to the endplate in the muscle is short.
-
Irreversible motor endplate atrophy and permanent loss of functional muscle fibers occur 12 to 18 months after initial injury.
-
-
Sensory function return after injury
tends to follow a pattern of pain recovery first, followed by slow
vibration sense, and then sensation to moving touch.-
Smaller-diameter, unmyelinated fibers
without specialized endplate receptors likely recover before the
larger, myelinated fibers that carry sensation from specialized
receptors. -
Deep tendon reflexes may or may not recover, even after only partial nerve injury.
-
Operative Nerve Repair
-
Nerve repair aims to reconstitute fascicular bundle organization after a transection or laceration.
-
Epineurial and group fascicular repair techniques have been described.
-
-
Reconnecting the proximal and distal ends of severed nerve fibers is thought to speed recovery and improve reinnervation.
-
Repair reduces the gap and the distance axonal sprouts must navigate before connecting with distal endoneurial tubes.
-
Magnification in the form of a microscope is typically used to improve the accuracy of nerve repair.
-
Healthy proximal axons must be identified before coaptation begins.
-
-
Nerve ends reconnected under excessive tension on the repair have been shown to have poorer functional outcomes.
-
Even the most accurate surgical repair does not ensure that regenerating axons find their appropriate distal endoneurial tubes.
-
Repair of pure sensory nerves demonstrates reliable return of function.
-
Example: digital nerve of finger
-
-
Variable outcomes after repair of mixed nerves
-
Regenerating axons frequently reinnervate incorrect target organ.
-
Example: ulnar nerve in forearm
-
-
Poor outcomes observed from proximal nerve repair
-
Combination of axonal misdirection as
well as end-organ atrophy during the lengthy regeneration process
results in predictably poor distal functional return. -
Example: brachial plexus
-
-
-
Neurotropic factors are being studied as
adjuncts to operative repair to assist regenerating axons to find their
end-organ targets.
Suggested Reading
Chaudhry V, Glass JD, Griffin JW. Wallerian degeneration in peripheral nerve disease. Neurol Clin 1992;10(3):613–627.
Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nature Rev Neurosci 2005;6(11):889–898.
Corfas G, Velardez MO, Ko CP, et al. Mechanisms and roles of axon-Schwann cell interactions. J Neurosci 2004;24(42):9250–9260.
Green DP, Hotchkiss RN, PedersonWC, eds. Operative Hand Surgery, 4th edition. Philadelphia: Churchill Livingstone, 1998.
Guzik
BW, Goldstein LS. Microtubule-dependent transport in neurons: steps
towards an understanding of regulation, function and dysfunction. Curr Opin Cell Biol 2004;16(4):443–450.
BW, Goldstein LS. Microtubule-dependent transport in neurons: steps
towards an understanding of regulation, function and dysfunction. Curr Opin Cell Biol 2004;16(4):443–450.
Johnson EO, Zoubos AB, Soucacos PN. Regeneration and repair of peripheral nerves. Injury 2005;36(Suppl 4):S24–29.
Lundborg G. Nerve regeneration and repair. A review. Acta Orthop Scand 1987;58:145–169.
Myers RR. Anatomy and microanatomy of peripheral nerve. Neurosurg Clin North Am 1991;2(1): 1–20.
Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve 1990;13:771–784.
Sunderland S. The connective tissue of peripheral nerves. Brain 1965; 88:841–854.
Whitwam JG. Classification of peripheral nerve fibres. An historical perspective. Anaesthesia 1976;31(4):494–503.
