MANAGEMENT OF VASCULAR DISORDERS IN THE UPPER EXTREMITY
III – THE HAND > Microvascular Surgery > CHAPTER 33 – MANAGEMENT
OF VASCULAR DISORDERS IN THE UPPER EXTREMITY
Department of Orthopaedic Surgery, Johns Hopkins University School of
Medicine, Johns Hopkins Bayview Medical Center, Baltimore, Maryland
21224.
Department of Orthopaedic Surgery, Division of Surgical Sciences, Wake
Forest University School of Medicine, Winston-Salem, North Carolina,
27157.
flow significant enough to compromise cellular perfusion and function,
and ultimately to cause cellular injury or death. Clinical symptoms of
abnormal perfusion include pain, cold intolerance, numbness,
ulceration, and gangrene. Symptomatic vascular disorders of the upper
extremity interfere with health-related quality of life and diminish
function. More than 10% of the general population and 20% to 30% of
postmenopausal women suffer from abnormal microvascular flow secondary
to trauma, congenital deformity, systemic processes, or genetic
influences. This chapter presents an approach to the diagnosis and
management of vascular disorders.
trauma may accompany high-energy fractures or dislocations, and these injuries require careful evaluation (51).
The management of arterial injuries has improved dramatically since the
late 1940s. During World War II, only 3 of 2471 known reported cases of
acute arterial repairs by end-to-end anastomosis were successful (15).
operating microscope, microvascular instruments, and microsuture can
achieve excellent vascular patency and replantation of complete upper
extremity amputations. Thrombosis following the repair of noncritical
arterial injuries, however, is common, even with the sophisticated
microvascular techniques performed by experienced vascular surgeons.
Successful management of acute vascular disorders requires (a) an
understanding of the physiology of symptoms associated with vascular
trauma; (b) a thorough knowledge of the pertinent vascular anatomy (Fig. 33.1) (2,12);
(c) an assessment of associated injury to soft tissue, bone, and nerve;
and (d) an appreciation of the natural history of the treated and
untreated injury.
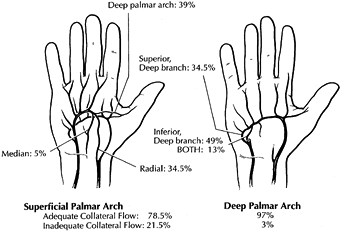 |
|
Figure 33.1.
The superficial palmar arch is completed by branches from the deep palmar arch, radial artery, or median artery in 78.5% of patients; the remaining 21.5% are incomplete. The deep palmar arch is completed by the superior branch of the ulnar artery, the inferior branch of the ulnar artery, or both in 98.5% of patients. (Modified from Koman LA, Urbaniak JR. Ulnar Artery Thrombosis. In: Brunelli, ed. Textbook of Surgery. Milano: Masson 1988:75; with permission.) |
complete transection (Type 1), partial transection (Type 2), and
nontransection (Type 3). These injuries can be further defined as
either critical, wherein the absence of arterial reconstruction will produce tissue necrosis requiring amputation, or noncritical, wherein tissue survival is independent of arterial reconstruction (Table 33.1 and Table 33.2). Chronic arterial injury and vasospastic problems may also be classified (Table 33.3 and Table 33.4).
 |
|
Table 33.1. Acute Vascular Injuries: Wake Forest University Classification
|
 |
|
Table 33.2. Noncritical Arterial Injury: Relative Indications for Reconstruction
|
 |
|
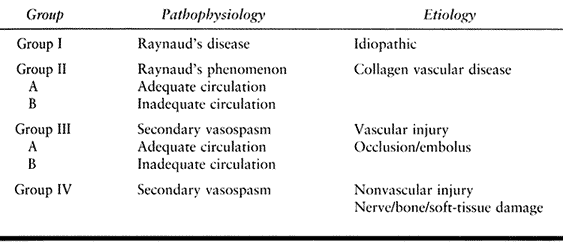
Table 33.3. Classification of Chronic Arterial Injuries for Treatment
|
 |
|
Table 33.4. Chronic Arterial Dysfunction: Wake Forest Classification of Occlusive, Vasospastic, and Vasoocclusive Disease
|
the site of injury. Vasoconstriction, intraluminal thrombosis, and
compression from surrounding soft-tissue hemorrhage result in a
cessation of arterial bleeding, depending in part on the diameter of
the vessel. The thrombosis usually propagates proximally and distally
to a patent branch not more than several centimeters from the zone of
injury. The amount of retraction and zone of injury determine whether
end-to-end repair or interposition grafting is required to restore
perfusion. Occasionally, arteries that are lacerated and transected
completely by sharp bone fragments may be tethered by the fragments;
retraction is prevented, and the result is uncontrolled hemorrhage.
secondary to lacerations from sharp glass or knives or bone fragments.
Iatrogenic injuries may follow diagnostic arterial cannulation for
hemodynamic monitoring, arterial blood sampling, or angiography, or
they may result from inadvertent puncture with drill bits or screws
(see later). The injured portions of the vessel wall retract and
constrict, but because of the segment of vessel that remains intact,
this retraction serves to enlarge the defect and increase the bleeding.
Distal ischemia may not occur because of persistent flow or collateral
circulation, but hemorrhage is typically much greater than with
complete transection and may not stop with directly applied pressure (42).
Although the normal coagulation mechanism may control bleeding, the
thrombosis may develop a communication with the arterial lumen and
stabilize (heal), expand (false aneurysm), or develop a communication
with an adjacent injured vein [arteriovenous fistula (AVF)] (6).
blunt direct trauma or indirect shock waves from a high-velocity
missile and may produce complete or partial flap tears of the intima or
intramural hemorrhage; both diminish lumen diameter and compromise flow
(Fig. 33.2). Potential sequelae may include thrombosis, periadventitial
constriction, and aneurysm; all may occur with or without ischemia and
may not be immediately symptomatic. After complete occlusion by
thrombosis, recanalization can occur and may restore circulation
without symptoms or may rethrombose and produce distant emboli.
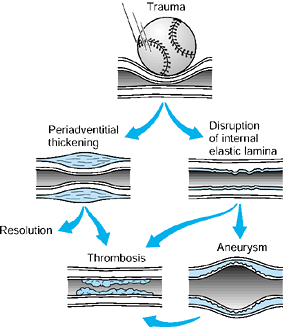 |
|
Figure 33.2.
A schematic depiction of occlusive mechanisms is shown. Acute or repeated trauma may cause direct intimal damage, leading to stasis and thrombosis (in most cases) or to aneurysm formation. Chronic low-grade trauma initially may produce periadventitial thickening; this results in constricted flow that may resolve spontaneously, may be relieved by surgical stripping of the adventitia, or may lead to subsequent intimal damage and secondary aneurysm formation, which may progress to thrombosis. (From Koman LA, Urbaniak JR: Ulnar Artery Thrombosis. In: Brunelli G, ed. Textbook of Microsurgery. Milan: Masson, 1988:75, with permission.) |
following acute arterial injury are based on the adequacy of the
collateral circulation, posttraumatic sympathetic tone, and vasomotor
control mechanisms (Fig. 33.3) (1,42).
In the presence of adequate collateral circulation and normal vasomotor
control, patients with arterial injuries infrequently require vascular
reconstruction (noncritical injury). An underlying neurologic,
soft-tissue, or osseous injury, however, may alter sympathetic control
in the remaining intact vessel or vessels, resulting in ischemic
symptoms from an otherwise insignificant injury. Therefore, adequate
anatomic vasculature may be compromised by inappropriate functional
control that produces vasospasm, arteriovenous shunting, and resultant
hypoperfusion. In the presence of inadequate collateral circulation,
arterial injury results in distal ischemia that increases sympathetic
tone, produces additional arterial spasm, decreases tissue perfusion,
induces ischemia, and escalates symptoms. The effects of an arterial
injury may be magnified in the presence of a concomitant nerve injury
or underlying vasomotor control abnormality.
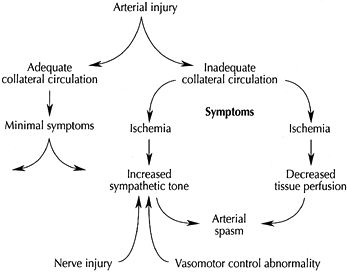 |
|
Figure 33.3.
Vascular injury precipitates a vicious circle of symptoms if insults occur at a crucial location, are associated with concomitant or pre-existing abnormalities of vasomotor control, or accompany nerve injury. (Reproduced from Koman LA, ed. Bowman Gray School of Medicine Orthopaedic Manual. Wake Forest University Orthopaedic Press: Winston-Salem, North Carolina, 1997, with permission.) |
collateral and subdermal plexus flow, there is uniform agreement that
the surgeon should revascularize one or both forearm vessels if both
the radial and ulnar arteries are disrupted, if the vessels in the
distal extremity are suitable, and if the patient’s overall condition
permits. During World War II, amputation (critical injury) after
arterial injury occurred in 26% of brachial artery injuries requiring
ligation below the origin of the profunda brachia and in 55% of
injuries above this level. The amputation rate after ligation of both
radial and ulnar arteries was 39%, whereas for single-vessel injuries
the amputation rate dropped to 5%—an indication that the majority of
single-vessel injuries are noncritical (25).
single vessels with adequate collateral circulation do not produce
significant symptoms, do not impair function, and do not initiate
significant cold sensitivity. Blood pressure decreases in the digits
served by the damaged vessel in spite of a compensatory increase in the
flow of the parallel artery (22). Symptoms depend on associated injuries and the ability of the remaining vasculature to respond appropriately to stress (39).
The effect of a nonrepaired noncritical vessel on the completeness of
nerve recovery following neurorrhaphy and the effect of the nerve
injury on the function of remaining parallel microvasculature is
unclear; data are conflicting.
suspicion based on the history of the injury. Vascular injury may occur
after penetrating wounds (80%) or fractures and needs to be considered
when patients have injuries to adjacent neural structures. Initial
physical examination may include profuse external bleeding (62%),
hypotension (18%), expanding or pulsatile hematomas, thrills, bruits,
and decreased peripheral pulses (43). A distal
pulse is not a reliable sign of vascular integrity, however, and it is
palpable in approximately one quarter of patients with a brachial
artery disruption and in 50% of patients with an isolated radial or
ulnar artery injury (24). Distal to arterial
disruption, a palpable pulse may be secondary to retrograde flow
through collateral circulation or wave transmission through the injured
segment. The Allen test, which may be obtained using Doppler
ultrasound, is the most accurate and reliable indicator of arterial
patency in the hand and can document the direction of flow and the
quality of the collateral circulation (2).
Doppler ultrasound, pulse echo real-time ultrasonography, color duplex
Doppler imaging, and radial or digital blood pressures (Table 33.5).
As noted, when performed with and without parallel vessel compression,
Doppler evaluation can detect the presence and direction of flow,
whereas digital pressures can quantify the amount of flow when
referenced to the brachial artery in the form of a digital brachial
index (DBI) or radial brachial index (RBI).
A
DBI or RBI of ≤0.7 or less indicates inadequate flow and supports
medical and surgical intervention; heavily calcified vessels, as seen
in patients with diabetes, may produce higher pressures, which must be
considered (8).
 |
|
Table 33.5. Direct Vascular Evaluation
|
For most critical penetrating arterial injuries, the preceding
assessments dictate obvious and appropriate surgical intervention.
Arteriography is sought preoperatively if (a) surgery may compromise
the patient or the extremity, (b) multilevel arterial damage is
possible or probable (e.g., shotgun wound), and (c) extensive
thrombosis or embolism is of clinical concern (e.g., injection or
cannulation injuries). Relative indications for arteriography after
blunt trauma include (a) suspicion of a proximal traction injury or
distal occlusion (thrombosis or embolism), (b) detection of a partial
arterial injury (e.g., intimal flap), and (c) suspicion of a false
aneurysm.
injuries but may also be required in cases in which the extent of
damage and quality of collateral circulation are in question.
Collateral flow can be assessed by the backflow from the injured
vessel, qualitatively by the pulsatile flow or by quantitative
measurement of the retrograde blood pressure (23,25).
Although excellent retrograde flow (DBI >0.7) confirms adequate
collateral circulation, poor flow may be transient secondary to
vasospasm or hypotension and not predictive of collateral flow
postoperatively. Alternative intraoperative measures available are
qualitative assessment of capillary refill (22) or pulp turgor, and quantitative digital plethysmography or laser Doppler flowmetry.
arterial injuries, assuming the absence of additional injuries or
problems. Absolute indications for noncritical arterial injuries are
not well defined, but relative indications include injuries in which
additional flow would help to maintain pulsatile digital flow capable
of responding to stress. Another relative indication is the presence of
injury to adjacent neural structures (i.e., ulnar artery and nerve).
use the proper instruments, appropriately sized needles, suture
materials of appropriate diameter, adequate magnification, and
meticulous microsurgical technique.
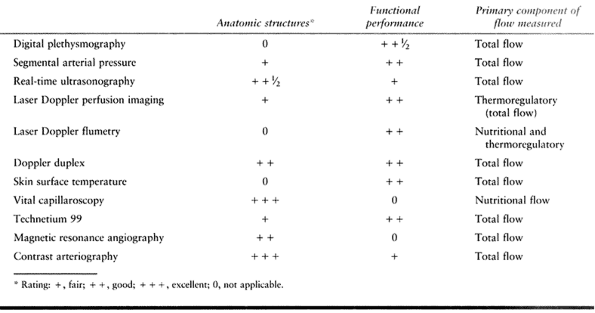
at the surgeon’s discretion. Instruments should be simple, corrosion
resistant, and fabricated with nonglare material, and they should
approximate accurately
(Fig. 33.4).
Scissors and needle holders should have nonlocking spring mechanisms
and should be long enough to rest comfortably in the thumb-index finger
web space. Sufficient length is important to minimize intrinsic muscle
fatigue, decrease hand tremors, and help minimize technical errors.
 |
|
Figure 33.4. Basic microsurgical instruments. A: Tying forceps may be used for tissue manipulation and suture handling and tying. B: Specialized forceps may be used to dilate vessels as well as to manipulate tissue and to handle sutures. C: Straight or adventitial scissors and curved or dissecting scissors (inset) are used for dissection, tissue preparation, and suture transection. D: Needles may be manipulated with curved or straight forceps or specialized needle holders.
|
tearing are essential, with tip sizes ranging from 0.2 to 0.6 mm.
Jeweler’s forceps (straight #5 and #3) traditionally have been used as
microsurgical forceps; however, their small tips are easily distorted,
rendering them ineffective. Additionally, they are unavailable in
lengths greater than 10 cm. Forceps made specifically for microsurgery
are longer, are constructed with forged or case-hardened metals, have
improved durability, and are not sharp. A variety of specialized
forceps are available for vessel dilatation, stretching, and tissue
manipulation.
should be 15 to 18 cm long, and their tips should be of forged or
case-hardened metal. Adventitial scissors are straight; dissecting
scissors have a gentle curve. Tips may be pointed or slightly blunted;
the latter are preferable for less experienced surgeons, because they
decrease the possibility of damage to vessels. Handles may be straight,
rounded, or flared. Many of the newer microscissors are counterbalanced
to improve their feel and maneuverability.
with finely forged tips that have smooth action, such as jewelers
forceps. Longer needle holders (15 to 18 cm), which are easier to
handle and control than short ones, help minimize false needle passes,
inadvertent vessel wall penetration, and trauma. Needle holders come
with straight or curved jaws. A gently curved jaw facilitates handling,
allowing the surgeon to roll the handle between the thumb and
forefinger to rotate the needle through the vessel wall. Locking needle
holders are not used with needles smaller than 150 to 200 µm. Locking
and unlocking may bend the needle and tear vessel walls.
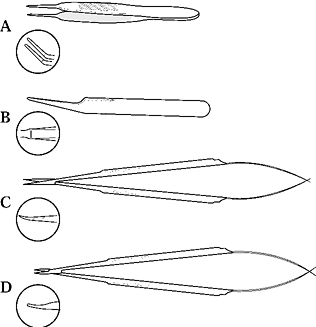
Vessel clamps are designed to hold the vessel, to prevent bleeding, and
to allow rotatory approximation. Disposable single and double clamps of
varying sizes and pressures are now available and are suitable for 0.4
to 2.0 mm vessels. Specialized clamps include stay suture–holding
frames, adjustable tension devices, and clamps with variably angled
blades. The ideal clamp has enough tension to hold the vessel without
damage and jaws 1.5 to 2.0 times wider than the diameter of the vessel.
A clamp that exerts excessive pressure will damage the intima and
media, and
produce
or increase the likelihood of thrombosis. Avoid using double-bar clamps
to overcome tension during vessel approximation. Clamps are designed as
anastomotic aids to facilitate vessel positioning, hemostasis, and
suture management; they are not designed to overcome tension.
 |
|
Figure 33.5. Microclamps. A large variety of microclamps capable of holding 0.3 to 2.0 mm vessels is available.
|
duct probes) are often helpful, but they may damage the intima if their
surfaces are rough or if they are mishandled. Instruments to provide
counterpressure are useful to facilitate needle passes in difficult
situations or to avoid inadvertent penetration and tethering of the
vessel.
pass through the vessel wall without bending and the smallest suture
strong enough to approximate the vessel. Standard needle sizes range in
diameter from 50 to 135 µm and in cord length from 2 to 5 mm. Suture
material in sizes 10-0 to 11-0 (35 to 14 µm) is available on most
needles.
Large vessels in the proximal forearm or arm may be repaired reliably
using loupe magnification (2.5× to 6.0×). Because magnification
requires concomitant increases in light to be effective, it is often
simpler to use an operating microscope than higher power loupes and a
headlamp. Loupe magnification of 3.5× to 4.5× may facilitate gross
dissection; vessel repair is then accomplished with the operating
microscope at 6.0× to 30.0×. Inspecting the vessel carefully under high
power identifies wall or intimal damage that would compromise patency.
Resect abnormal vessels before repair.
-
In general, perform rigid skeletal
fixation before vascular repair in cases involving skeletal trauma. In
critical injuries, arterial or venous shunting may be used to prevent
excessive warm ischemia. -
Explore wounds under loupe magnification using a tourniquet to provide a bloodless field.
-
Use extensile incisions that incorporate
traumatic wounds. If it is clinically indicated, perform fasciotomies.
Identify transected and injured structures proximally and distally in
normal tissue. Mobilize and tag them with vascular tapes or loops. -
Excise dead and necrotic tissue, and trim wound margins.
-
Identify the periadventitial plane of the
injured vessel proximally and distally to the transection site to allow
rapid and safe dissection. Dissect vessels from surrounding tissues and
place atraumatic vascular clamps on either side of the repair site. -
Ligate branches from the damaged artery
in the proximity of the transection or cauterize with a bipolar
cautery, leaving a 0.5 mm stump (Fig. 33.6). Manipulation of the media or intima is thereby minimized.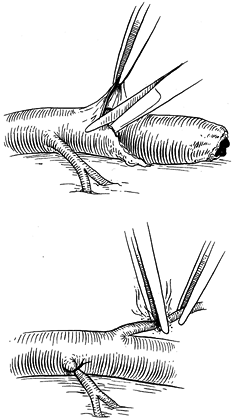 Figure 33.6.
Figure 33.6.
Periadventitial tissue may be dissected safely after identification of
the vessel lumen in the area of dissection. Avoidance of direct
manipulation of the lumen minimizes trauma. Branches may be ligated
with 8-0 nylon sutures or cauterized using a bipolar cautery. -
Remove the periadventitial tissue to
reveal the vessel wall and lumen to prevent debris from interfering
with passing or tying of sutures. -
Place but do not tie sutures if lacerated
flexor tendons are adjacent to or would interfere with vascular
anastomoses; after vascular repair, tendon approximation is possible
without additional dissection. The possibility of damaging the vessel
repair is thereby minimized. Performing tendon repair before vessel
anastomosis may make exposure of the anastomotic site more difficult
because of finger, hand, or wrist position, or because of the location
of the tendon. -
Inspect both portions of the vessel to
determine if the intima and media are suitable for anastomosis.
Hemorrhage within the media, intimal disruption and multiple stellate
tears, or telescoping of the intima are ominous findings and require
additional resection back to the level of normal vessel. Intimal damage
itself is an important
P.1118
but
not definitive factor in long-term patency. Intimal damage, however,
the most easily recognizable external sign of local trauma, may
indicate significant additional damage and is associated with a higher
rate of thrombosis. It is good practice to resect the vessel to see
relatively normal intima. -
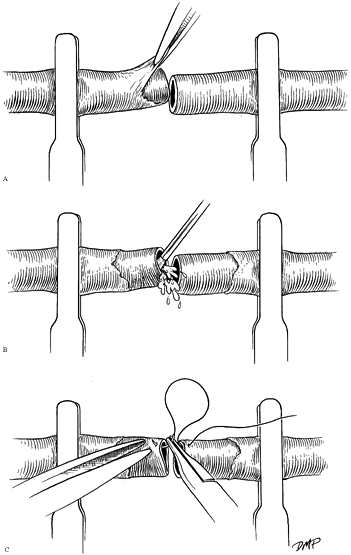
Place the vessel in an approximating clamp and repair it end to end (Fig. 33.7). Manage undue vessel tension by the use of reversed interposition vein grafting, in situ
vein grafting with valvulotomy, antegrade vein grafting with
valvulotomy, proximal and distal arterial mobilization, acute arterial
lengthening, or bone shortening.![]() Figure 33.7. Vessels may be placed in a bar clamp to facilitate positioning, control bleeding, and maximize ease of repair. A:
Figure 33.7. Vessels may be placed in a bar clamp to facilitate positioning, control bleeding, and maximize ease of repair. A:
Periadventitial tissue, which is highly thrombogenic if left within the
lumen, is debrided to prevent clot formation and to facilitate
visualization of the lumen. The vessel interior should then be
irrigated with heparinized saline (B) and inspected under high-power magnification before the repair (C).
-
Perform a primary end-to-end repair,
without placing excessive tension on the anastomotic site if possible.
In sharp, nonsegmental injuries or when minimal artery has been
resected, it is possible to achieve a tension-free anastomosis after
resection of damaged vessel ends by moderately mobilizing the artery
proximally and distally. -
Colored background material improves visualization and handling of suture, decreases glare, and improves contrast.
-
Perform the anastomosis with or without
vessel approximating clamps, which are used to hold the vessel in
position, not to overcome excessive tension. It is easier to gain
access to the anastomotic site by orienting the bar clamps with the
open end away from the surgeon (Fig. 33.7). -
Under the operating microscope, adjust
tension, expose the vessel, irrigate the lumens with heparinized
saline, and remove debris or loose adventitial tissue. Make a final
inspection of the intima, and, if the vessel is suitable, begin the
anastomosis. -
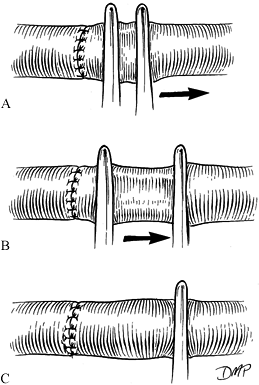
After ensuring that the vessel is not
twisted and that tension is not excessive, place stay sutures at 120°.
Approximate the front wall by halving the distance with each suture,
using a triangulation technique (Fig. 33.8).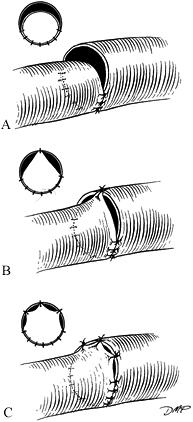 Figure 33.8. A:
Figure 33.8. A:
Minimal size discrepancies (10% to 20%) may be managed by careful
triangulation. The first suture is placed, halving the distance between
stay sutures. B: As the knot is tied, tension on the stay suture will ensure intimal apposition without buckling. C: Additional sutures are placed similarly. -
Rotate the bar vessel–approximating clamp 180° (open end toward surgeon) and approximate the back wall in a similar fashion.
-
Minimize size or spacing discrepancies by
applying tension to the adjacent sutures as you tie and triangulate the
first throw of the knot (Fig. 33.9). Gently
dilate the end of the smaller vessel, and cut it obliquely (not to
exceed 30°). Perform end-to-side anastomosis, V-section, and closure of
the end of the larger vessel, longitudinal slit opening of the end of
the smaller vessel, or interposition of appropriate graft material (Fig. 33.9).![]() Figure 33.9. Compensatory technique for size discrepancy. (A) End-to-side, (B) V-excision and closure, (C) V-opening, and (D) vein graft.
Figure 33.9. Compensatory technique for size discrepancy. (A) End-to-side, (B) V-excision and closure, (C) V-opening, and (D) vein graft. -
Repair forearm level injuries with
nonabsorbable 7-0, 8-0, or 9-0 sutures, and repair distal vessels with
9-0, 10-0, or 11-0 sutures. -
When the anastomosis is complete, inspect
it and remove the clamp. Additional sutures may be necessary, but early
leaks often stop spontaneously or you can stop them by placing fat over
the anastomosis. Spaces of 0.3 mm or less between sutures generally do
not require additional sutures. Technical errors in performing
anastomoses can also cause turbulence and thrombosis (Fig. 33.10). Confirm patency by direct observation or by patency testing (Fig. 33.11).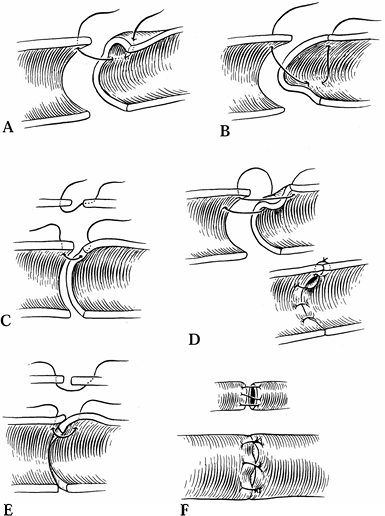 Figure 33.10. Technical errors in anastomosis are shown. Side wall (A), back wall (B), failure to penetrate full vessel (C), uneven lateral placement (D), and uneven approximation of intima (E, F).
Figure 33.10. Technical errors in anastomosis are shown. Side wall (A), back wall (B), failure to penetrate full vessel (C), uneven lateral placement (D), and uneven approximation of intima (E, F).![]() Figure 33.11. The technique for patency testing is illustrated. A: Using minimal pressure, the vessel is occluded distal to the anastomosis. B: Blood is milked from the vessel, which flattens between the forceps. C: The proximal forceps is released, and blood fills the flattened area.
Figure 33.11. The technique for patency testing is illustrated. A: Using minimal pressure, the vessel is occluded distal to the anastomosis. B: Blood is milked from the vessel, which flattens between the forceps. C: The proximal forceps is released, and blood fills the flattened area.
after appropriate debridement, you cannot mobilize the damaged vessel
to achieve end-to-end reapproximation (27).
Although it requires two anastomoses, vein grafting does not
significantly lower patency rates, even when this procedure is used in
more severe injuries. Several types of bypass grafts are reversed
interposition veins, nonreversed in situ valvulotomized vein segments, noncritical arterial grafts, and synthetic or allograft material.
-
For injuries to the radial artery, ulnar
artery, or proximal superficial arch injuries, use the cephalic vein
from the forearm, the distal saphenous or lesser saphenous vein, or a
dorsal vein from the foot; they are equally effective (27,37,40). The volar forearm is an excellent source of veins for the digital vessels. -
To harvest veins, use longitudinal
incisions, multiple transverse incisions, or endoscopic techniques. In
general, longitudinal incisions allow meticulous vascular dissection,
identification, and ligational branches, thus minimizing accidental
injury to superficial peripheral nerves and facilitating the procedure.
Harvest a segment of vein 15% to 20% longer than the measured defect.
Ligate all branches with 4-0 or 5-0 suture, and coagulate them with
bipolar cautery or microclip branches to prevent leakage (Fig. 33.6). -
Dilate the vein to inspect for injury, to
compress valves, and to detect wall defects or unligated branches; if
damage and problems persist after repair, harvest a new vein. -
At the time of surgery, dissect and
mobilize the ends of the severed artery, and position vascular clips
for later rapid identification. Typically, the vein graft is reversed
and placed in position, but nonreversed valvulotomized veins may be
used. -
Perform the more difficult anastomosis
first because the extra degree of freedom allowed by the mobility of
the vein graft makes repair easier and prevents technical errors.
Repair the anastomosis as described above. Discrepancies in size are
common with vein grafting; they can be overcome by using the techniques
previously discussed or by using nonreversed valvulotomized grafts (Fig. 33.3 and Fig. 33.9).
and nonreversed vein grafting are (a) the need for a large (greater
than 7 cm) graft segment, (b) multiple distal anastomoses, and (c)
significant size discrepancies between the proximal and distal ends.
This type of grafting prevents the large discrepancy in lumen size at
both ends found with reversed vein grafting and provides natural
branching for multiple distal grafts. Incise all valves before
revascularization. The risk of accidental vein damage is the limiting
factor with the use of this type of grafting. Historically, many
microvascular surgeons preferred reversed interposition vein grafting;
new valvulotomes, endoscopic techniques, and documented efficacy
support the judicious use of nonreversed valvulotomized vein grafts,
however.
freedom of orientation, longer theoretical patency, and ease of
dissection, few adequately sized donor arterial grafts are available
and the use of arterial grafts is limited. If reconstruction of one
artery is contraindicated and sufficient undamaged vessel is available,
use it as an interposition graft.
warm ischemia. Use commercially available shunts or heparinized
Silastic catheters. A variety of carotid endarterectomy shunts are
available and are sized (e.g., 3 mm distal, 3 mm proximal) to fit 2.5
to 5.0 mm vessels. Smaller vessels can be shunted using neurosurgical
ventriculoperitoneal shunts; however, these shunts require the surgeon
to stabilize them with either suture ligatures or vascular keepers or
clamps. Perform minimal dissection to avoid iatrogenic damage, place
the shunts, and perfuse the distal extremity. Do a fasciotomy if overt
or impending compartment syndrome exists.
assessment of the adequacy of the collateral circulation and is based
primarily on clinical judgement. Preoperative and intraoperative
assessment of color, capillary refill (22), turgor, and back bleeding combined with quantitative measures of perfusion provide an estimate of the
adequacy of the collateral circulation. Consider arterial
reconstruction as part of a patient-oriented plan. Assuming that limb
salvage is appropriate, circulation is restored in critical injuries or
in the presence of poor collateral circulation (37).
A DIB of 0.7 is a relative indication for reconstruction (refer to the
discussion on diagnosis of acute arterial injuries in the earlier
section on the physiology of arterial injuries). The final decision
regarding arterial reconstruction is often made in the operating room,
after exploration. If collateral flow is good, reconstruction is
optional and ligation is an appropriate option at the discretion of the
surgeon. If symptoms secondary to decreased arterial perfusion occur in
the perioperative period, elective arterial reconstruction may be
achieved without compromising long-term symptoms or function.
arterial repair of isolated and noncritical vessels. Postoperative
transfer to a facility for revascularization is appropriate if
subsequent symptoms warrant it, assuming adequate preoperative,
intraoperative, and postoperative evaluation and assessment have been
performed. The reconstruction of brachial artery lacerations,
particularly those injuries occurring above the origin of the profunda
brachia, is indicated, if possible, in most situations. For
single-vessel repairs of noncritical injuries, patency rates have
improved from a maximum (100%) postoperative thrombosis rate in 1967 to
a more acceptable rate, between 47% and 82% in 1982 (25). In combined radial and ulnar artery injury, it is preferable to reconstruct both vessels (25).
Although the ulnar artery is larger anatomically, the radial artery
often provides greater or equal flow in 88% of patients. If only one
vessel can be repaired without significant additional intervention,
assess distal perfusion after repair and base the decision regarding
the second vessel on clinical judgement. The need for repair of a
noncritical lacerated ulnar artery when an associated ulnar neurorhaphy
is performed is controversial. Combined ulnar nerve and ulnar artery
injury, however, is a relative indication for arterial reconstruction (43).
and cardiac catheterization most frequently involve the brachial and
radial arteries (4,31,47,53).
Repeated injuries to the arterial lumen following attempts to obtain
blood from the artery causes vessel lacerations and intimal flaps and
an increased likelihood of (a) pseudoaneurysm formation, (b) creation
of AVFs, and (c) acute thrombosis with possible distal embolization.
Despite a 23% incidence of occlusion of the radial artery following
cannulation, few symptoms arise because collateral circulation is
adequate, and the occluded vessel recanalizes spontaneously. In rare
instances, acute ischemia and distal embolization (more frequent in the
brachial artery) ensue (4).
capillary refill, petechiae, and, later, pain. Bedside assessments can
be performed using the Allen test or evaluation with a Doppler device.
Arteriography is rarely indicated and may be contraindicated following
cannulation events due to coexisting illnesses in this patient
population.
ischemia following cannulation injuries. In critically injured
patients, evaluation may require exploration of the involved vessel in
the intensive care unit or operating room under local anesthesia.
Management options include one or more of the following: thrombolytic
therapy, thrombectomy, embolectomy, and resection of the thrombosed
segment and surgical reconstruction.
assess the extent of injury. Results following exploration, primary
repair, patch grafting, and vein grafting are excellent, and wound
complications are infrequent and manageable. Consider thrombolytic
therapy for patients without complicating medical conditions but not in
the immediate postoperative period following extensive nonvascular
surgery.
distally is not an indication for emergency surgery. In an awake and
alert patient, monitor the extremity clinically. In an anesthetized or
unconscious (e.g., head injury) patient, objective monitoring through
use of the Allen test, placement of temperature probes, Doppler imaging
with hand-held instruments, color duplex instruments, and laser
perfusion instruments, or by measuring distal pressure (DBI) is
recommended. Arteriography is indicated to answer specific clinical
questions or to explain contradictory findings (20). Suspicion of proximal pathology unrelated to the cannulation injury is an indication for arteriography.
heparin unless it is contraindicated. The use of thrombolytic agents is
contraindicated in the presence of pseudoaneurysm, vascular laceration,
and in most postoperative conditions (e.g., following heart surgery).
In the presence of persistent distal ischemia (45),
active bleeding, or aneurysm, surgical exploration is indicated to
determine the extent of arterial damage. Surgery can include resection
and ligation, arterial reconstruction, and thrombectomy or embolectomy.
are secondary to medical procedures. They may also be self-inflicted
during drug abuse. Distal ischemia can occur following injection of a
variety of pharmaceutical products (e.g., barbiturates, propoxyphene,
and nonparenteral narcotics). Severe vascular events occur following
workplace injury involving solvents, paint products, and lubricants
injected through high-pressure devices, but fortunately intraarterial
injection is infrequent. Injection injuries can result in acute, severe
extremity ischemia on the basis of secondary vasospasm, chemical
endarteritis, arterial blockage by acid crystals, activation of the
clotting cascade, and localized compartment syndromes.
tool, a high-risk medical procedure, or drug abuse. Swelling, numbness,
and discoloration often accompany symptoms of acute arterial
insufficiency. Injection wounds often appear innocuous, but the
combination of the mass of injected material, chemically induced
inflammation, and increased interstitial pressure (compartment
syndrome) may produce secondary vasospasm, thrombosis, and skin and
soft-tissue necrosis. Diagnostic tests include Doppler evaluation,
color duplex imaging, arteriography, and interstitial (compartment)
pressure measurements. See Chapter 45 for more details.
beds is common, and systemic anticoagulation is the only option.
Treatment options include vasodilators, thrombolytic agents (e.g.,
urokinase) (55), steroids, heparin, and
low-molecular-weight dextran. Surgical management includes
revascularization of injured and repairable segments, fasciotomy, and,
in some cases, amputation. Revascularization is difficult because of
frequent involvement of small and distal vessels and diffuse distal
vascular injury. If a discrete and reconstructable obstruction or
obstructions exist, however, early exploration and embolectomy or
thrombectomy or arterial repair (or both) is indicated. Associated
compartment syndrome of the digits, hands, or forearms is common and an
indication to perform immediate fasciotomy.
evaluation, which may provide definitive information. Perform contrast
arteriography to determine whether or not reconstructable segmental
occlusion is present and to evaluate the extent of distal microvascular
compromise. Management depends on the etiologic agent, the location of
the insult or insults, and the extent of damage. Treatment may involve
one or more techniques, which may be performed sequentially or
simultaneously. Manage diffuse thrombosis and embolism involving
vessels of any size with thrombolytic therapy (e.g., urokinase), oral
vasodilators, and close observation. Discrete, distal small vessel
occlusion may respond to heparinization. It is important to monitor the
patient for signs or symptoms of compartment syndrome. Perform a
fasciotomy for elevated compartment pressures, as needed. Perform
revascularization and thrombectomy if it is technically possible and
clinically indicated. Repeat arteriography is often necessary to
document the extent of large vessel versus small vessel involvement and
to evaluate the effects of medical thrombolytic management.
trauma, atherosclerosis, proximal embolic events, systemic disease, and
hypercoaguable states. Significant arterial occlusion produces
ischemia, vasospasm, and stress-induced symptoms; venous involvement
may produce cyanosis and edema. Symptoms and signs depend on the
existence of collateral vessels and normal functioning vasomotor and
autonomic mechanisms (Fig. 33.2). Symptomatic
chronic vascular events produce pain, cold sensitivity, and numbness
and may cause ulceration, fibrosis from segmental cell death, and
gangrene. The relationship of trauma to occlusive disease is well
documented in laborers who use their palms as a hammer (40) or in individuals who participate in activities that expose them to repetitive palmar stress (e.g., baseball catchers).
clinical consequences of atherosclerosis and chronic trauma are
frequently similar, the etiologic mechanisms and prognosis differ. For
example, posttraumatic ulnar artery thrombosis has a much better
prognosis than does Buerger’s disease. Repetitive trauma may produce
localized thrombosis on the basis of periadventitial scarring and
compression (10). Alternatively, trauma may
cause intimal damage and expose the media, disrupt the internal elastic
lamina, and expose endothelial collagen. The result can include
aneurysmal dilatation, mural thrombosis, complete occlusion, and distal
embolic events. This process is worsened in the presence of a
hypercoaguable environment and stasis. In the presence of
atherosclerosis or systemic disease, vessels appear to be more
susceptible to trauma, and occlusion or aneurysm is more frequent.
Regardless of the etiology, level, or extent of occlusion, the adequacy
of collateral flow and sympathetic tone (Table 33.2) will affect clinical outcome and must be factored
into a clinically useful classification (Table 33.3 and Table 33.4).
palmar arch is the most common type of upper-extremity occlusion and
has been described as ulnar artery thrombosis or hypothenar hammer
syndrome (37,40).
Symptoms of pain, cold sensitivity, numbness, and weakness occur
following thrombosis, occlusion, or distal embolization of the ulnar
artery within the confines of Guyon’s canal (Fig. 33.12).
Signs include the presence of a pulsatile or pulseless mass, absent
flow through the ulnar artery by Allen’s test, decreased ulnar
sensibility, nailbed changes, decreased refill, diminished turgor,
ulceration, and gangrene.
Fortunately,
ulceration and gangrene rarely occur. Ulnar artery thrombosis occurs
most frequently in male laborers in the fifth decade of life. Patients
often have a history of using the palm of the hand as a hammer and of
using tobacco products.
 |
|
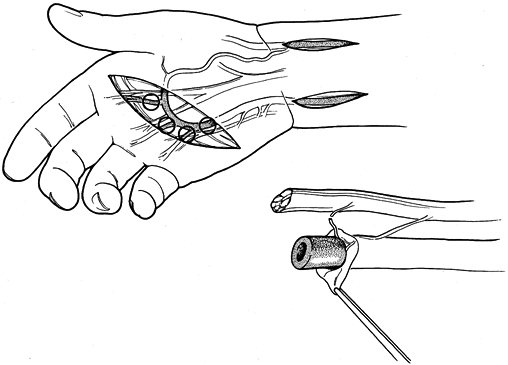
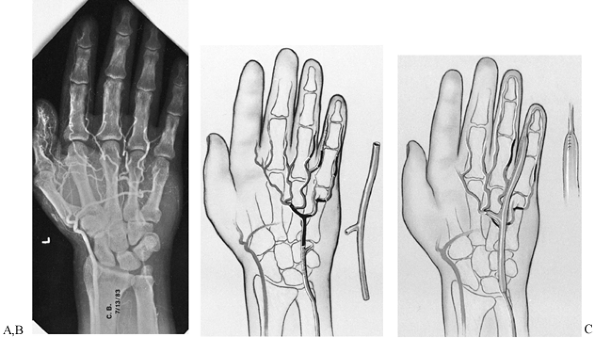
Figure 33.12. A:
A 52-year-old man sustained repeated trauma to the hypothenar area of his left hand. He presented with pregangrenous changes in his little finger. The arteriogram shows complete occlusion of the ulnar artery and superficial palmar arch with no flow to the proper digital artery to the little finger. The deep arch provides filling of the common digital artery to the ring and little fingers as well as the long and ring fingers, but there is no flow past the middle of the proximal phalanx in the proper digital artery on the radial side of the little finger. At surgical exploration, the ulnar artery, superficial arch, radial proper digital artery to the little finger, and proper digital artery to the ulnar side of the little finger were found to be thrombosed. B: Schematic depiction of the surgical findings in the same hand. The black areas represent areas of thrombosis; the cross-hatched areas represent areas of intimal damage. The reversed interposition 18 cm vein graft from the contralateral foot is shown next to the hand. C: One end of the graft was sewn end to end to the ulnar artery proximal to the area of intimal damage, and the other end was anastomosed end to end to the proper digital artery on the radial side of the little finger; one limb was placed end to end into the superficial palmar arch. Follow-up at 1 year (including Allen testing, high-resolution real-time ultrasonography, and Doppler evaluation) showed that the vein graft was patent. Symptoms were decreased, the finger ulceration had healed, and the patient was working. (From Koman LA, Urbaniak JR: Ulnar Artery Thrombosis. Hand Clin 1985:1:311, with permission from W.B. Saunders Co.) |
digital vessels may also occur. Radial artery thrombosis often involves
the deep branch of the radial artery within the anatomic snuffbox and
is seen in women with collagen vascular disease.
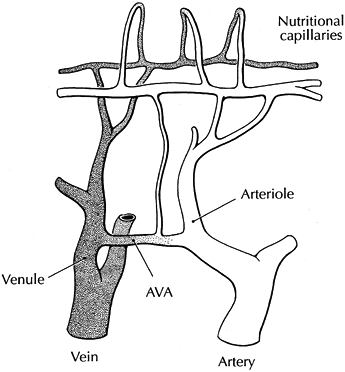
papillary capillary beds and nonnutritional thermoregulatory vessels.
Distribution of flow between and within these beds varies by anatomic
region (Fig. 33.13). Under normal conditions in
the digits, 80% to 90% of total flow passes through thermoregulatory
beds. In pathologic states, however, cellular hypoperfusion may result
secondary to decreased total flow, abnormal distribution of nutritional
and thermoregulatory components of flow, or both. Symptoms secondary to
anatomic damage (e.g., arterial thrombosis) are managed by either
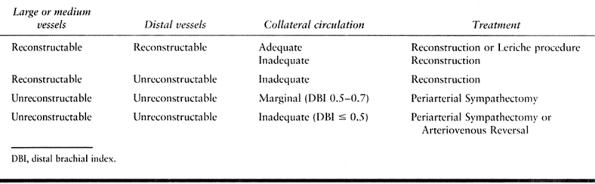
increasing collateral flow or restoring arterial flow (Table 33.6 and Table 33.7).
Symptoms secondary to abnormal functional (physiologic) control require
alterations in vasoconstrictive and vasodilatory tone that, in turn,
have a direct effect on collateral flow, total flow, and the
distribution of flow between the nutritional and thermoregulatory beds.
 |
|
Figure 33.13.
A schematic diagram of a microvascular bed from the skin surface is shown. Nutritional perfusion occurs in papillary capillaries and is dependent on appropriate arteriovenous shunting. Excessive shunting through arteriovenous anastomoses (AVA) prevents or limits (shunts) nutritional flow. Nutritional flow can also be reduced by arteriole vasoconstriction and a decrease in total flow. (Reproduced from Koman LA, ed. Bowman Gray School of Medicine Orthopaedic Manual. Winston-Salem, North Carolina: Wake Forest University Orthopaedic Press, 1985, with permission.) |
 |
|
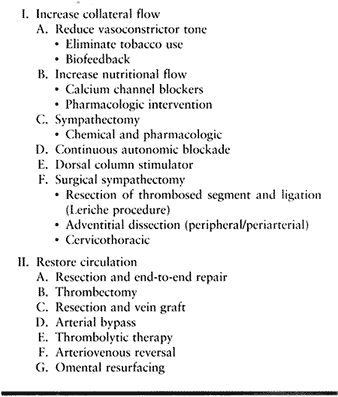
Table 33.6. Treatment Options of Symptomatic Thrombosis
|
 |
|
Table 33.7. Treatment Options of Symptomatic Thrombosis Based on Pathology
|
10% of cases. Although an Allen’s test demonstrating absence of flow
through the suspected artery confirms the diagnosis, recanalized
thrombosed vessels and aneurysms may demonstrate flow but will create
abnormal hemodynamics, pressure gradients, or the presence of distal
emboli. Doppler ultrasound, duplex scanning, plethysmography, digital
brachial indices, stress testing, and arteriography help delineate
structural and functional flow characteristics (35,36).
on sympathetic tone, the adequacy of the collateral circulation, and
functional capability. Collateral flow can be increased by altering
vasoconstrictor tone. Establish a regimen of biofeedback and oral
pharmacologic interventions combined with environmental modifications.
The patient should give up use of tobacco and caffeine, and should wear
gloves, hats, and scarves to reduce convective heat loss. Nicotine
patches used for smoking cessation do not adversely affect nutritional
microcirculatory perfusion (21).
Total flow may be maximized by the use of vasodilators such as
tolazoline and thorazine; calcium channel blockers or adrenergic
compounds may enhance nutritional flow. Partial sympathectomy may be
effected by intraarterial medications (e.g., guanethidine) or epidural
or brachial plexus sustained blocks, implanted dorsal column
stimulators (30), or surgical options.
physiologic pathology aids in the selection of appropriate treatment
options (Table 33.3 and Table 33.7).
Most patients with secondary vasospasm and occlusive disease have
adequate collateral circulation (Group IIIA) and require minimal
intervention, such as calcium channel blockers or mild sympatholytics
(Algorithm 33.1). Surgical options decrease vasoconstrictor tone,
restore blood flow, or both. Surgery to decrease abnormal sympathetic
tone includes resection and ligation of the thrombosed segment (Leriche
sympathectomy), adventitial dissection (peripheral or periarterial
sympathectomy), and cervicothoracic sympathectomy. Periarterial or
peripheral sympathectomy is considered a salvage procedure and is
reserved for otherwise unreconstructible disease with or without
adequate collateral flow.
risk–benefit ratio, arterial reconstruction is appropriate in selected
patients with adequate circulation (Group IIA and IIIA) and is the
treatment of choice for patients with inadequate circulation (Group IIB
and IIIB). Physical findings following surgical exploration that
support the use of arterial reconstruction include (a) absence of a
parallel arterial limb; (b) two-level or greater occlusion that
compromises potential collateral flow; thrombosis extending beyond the
origin or origins of common digital vessels; and (c) incomplete deep
and superficial arches (40). Group IIIB
patients, with secondary vasospasm and inadequate circulation, require
the restoration of arterial flow for maximal recovery (39).
Methods to restore arterial flow include use of thrombolytic agents and
a variety of reconstructive arterial surgery techniques.
nonembolic thrombosis of the subclavian and ulnar artery, and
intraarticular injection injury with medium vessel occlusion. Perform
thrombolysis by introducing a percutaneous catheter proximal to the
thrombus and introducing a thrombolytic agent, typically urokinase, a
tissue plasminogen activator. Monitor therapy clinically and with
serial arteriograms. Twelve to 72 hours of treatment may be necessary,
followed by 3 to 6 months of anticoagulation therapy. Thrombolytic
therapy is performed using interventional radiology or cardiology
consultants, selective catheterization into or just proximal to the
thrombosis is optimal, and a more acute occlusion may respond more
dramatically than a chronic condition. The current agent of choice is
urokinase, with the dose based on the patient’s body weight, response
to infusion, and the position of the catheter.
in conjunction with thrombolytic agents (i.e., urokinase) or following
proximal resection of thrombosed areas and arterial reconstruction. The
use of embolectomy catheters within the hand and digits is difficult
and may produce vascular damage. The choice of a Fogarty catheter is
based on the size of the arteries involved. In general, catheters 1 to
1.5 mm in diameter are used within the distal palm, whereas 2 to 3 mm
catheters are appropriate
at
the wrist and forearm level. It is difficult to introduce embolectomy
and thrombectomy catheters distal to the midpalm or distal palm without
causing injury; carefully assess the risk–benefit ratio for the
patient. After successful embolectomy, administer systemic
anticoagulation therapy for 5 to 7 days. Administer intraoperative
heparin by bolus (2,000 to 3,000 units), followed by a continuous
infusion of heparin based on body weight. A typical 70 kg patient
receives 600 to 1,000 units/hour. Obtain daily clotting studies and
adjust dosage accordingly. Follow this systemic therapy with an oral
anticoagulant (Coumadin) if it is clinically indicated.
procedures have the potential to improve symptoms, to aid in healing,
and to prevent premature amputation. End-to-end repair is rarely
indicated or possible because of excessive tension at the repair site
after removal of damaged artery. In instances in which vessels are
tortuous, end-to-end repair is possible after resection of the most
diseased segment. A critical evaluation of anastomotic sites is
crucial; if a compromised vessel is included in the reconstruction, the
likelihood of rethrombosis is high. For this reason, reversed
interposition autologous vein grafting is most frequently used, as
described above (Fig. 33.12 and Fig. 33.13).
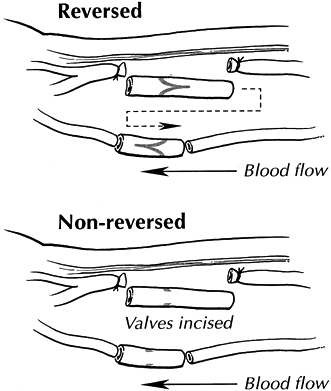
nonreversed interposition vein grafts requires incising the valves. The
exposure and technique are similar to those for reversed interposition
grafting, with the exception of vein placement and valvulotomy (Fig. 33.13).
The use of expendable donor arterial grafts is not described for upper
extremity reconstruction. Current data in the thoracic literature,
however, support the use of arteries in preference to veins for
coronary artery bypass and supports the expendability of internal
mammary arteries and the radial artery. Therefore, the use of a
contralateral radial artery graft for a critical upper extremity
vascular problem provides an alternative to vein grafting.
valvulotomized, or nonreversed valvulotomized vein grafts may be used
from the parallel forearm vessel or from the brachial artery to a
distal artery (Fig. 33.14).
 |
|
Figure 33.14.
Interposition vein grafting may be used to repair damaged arterial segments that would be under tension with an end-to-end repair. Reversed interposition grafts may be obtained from local veins (e.g., cephalic) after reversal. Alternatively, nonreversed veins may be used if the values are incised. (Reproduced from Koman LA, ed. Bowman Gray School of Medicine Orthopaedic Manual. Winston-Salem, North Carolina: Wake Forest University Orthopaedic Press, 1997; with permission.) |
or poor outflow secondary to arteriosclerosis, or end-stage
vasoocclusive or embolic disease may be treated with arterialization of
venous flow (5,26,34).
This procedure attempts to bypass the valves in the venous system by
reversing flow through arteriovenous anastomoses to nutritional
vascular beds (26,34). The proximal artery is identified, branches of the in situ
venous system are ligated, valves are incised, and arterial flow is
established through the venous system to the metacarpal phalangeal
level. Theoretically, the delivery of systemic arterial flow to vessels
of 150 µ or smaller renders distal valves relatively incompetent,
allows flow reversal within the microcirculation, and perfuses
nutritional capillaries retrograde through arteriovenous anastomoses.
Salvage procedures are occasionally appropriate and involve transfer of
omental free tissue to resurface and revascularize ischemic distal
areas, nourished by proximal arterial and venous anastomoses.
-
Explore the involved arterial tree
through an extensile incision. Excise damaged and compromised arterial
segments, and reconstruct using end-to-end repair or reversed
interposition grafting. -
End-to-end repair is difficult to achieve
unless the segment involved is short and tortuous. Do not perform
repair with the vessel under tension. -
Expose vessels, evaluate the vasculature,
and initiate resection and ligation or repair. It is important to
identify and excise damaged areas of artery. Often, areas of the vessel
that remain patent have damaged internal elastic lamina and are at risk
for future thrombosis. -
Resecting a central vessel segment that
includes patent branches proximally and distally increase the
probability that undamaged vessels are repaired. -
Harvest the appropriate-sized graft from
either the upper or lower extremity. Although the cephalic and basilic
veins are suitable, the distal saphenous vein at the ankle to midcalf
level for ulnar artery and brachial lesion is optimal for ulnar artery
lesions. The cephalic vein—if adequate—is used in radial artery
occlusion. In selected patients who lack appropriate donor veins, the
contralateral radial artery may be an option. -
Grafts, either in situ with the valves incised, or reversed, are appropriate. In situ
grafts overcome the size discrepancies created by reversed grafts, with
the larger proximal end needing to be anastomosed to the smaller distal
artery. Multiple terminal branches are common in antegrade grafts with
incised valves but are more difficult to find in reversed vein grafts. -
To accomplish in situ
and bypass grafts using a valvulotomized vein, perform end-to-side
repairs with an operating microscope. The grafts must be tensioned
appropriately to prevent kinking. -
In short uncomplicated grafts involving
the ulnar artery and superficial arch, it is appropriate to complete
both end-to-end repairs before deflating the tourniquet. -
When reconstructing the radial artery
within the anatomic snuffbox, perform the distal anastomosis (to the
deep arch and princeps pollicis) before performing the proximal repair
in order to facilitate the anastomosis. -
In larger or more complex grafts, deflate the tourniquet briefly in order to confirm flow through the vein graft.
-
Use an operating microscope unless there is a clinical contradiction.
-
After completing the more difficult
anastomosis, if possible, trim the graft to size (10% longer than the
defect), distend it with heparinized physiological saline (10 to 50
units/ml), and place it in its new bed in the wrist or palm. Take care
not to twist the graft. Use 8-0 or 9-0 nylon suture on 100 to 135 µm
needles for distal repair. -
Manage vessel size discrepancies using the most appropriate technique.
-
If two or more distal anastomoses are
necessary, accomplish end-to-end and end-to-side repairs in a series of
steps. Complex reconstructions may be achieved by end-to-side
anastomoses proximally and distally, Y grafts, and bypass grafting (Fig. 33.15).![]() Figure 33.15.
Figure 33.15.
Bypass grafting in the upper extremity is often advisable. For example,
in this patient with severe occlusive disease associated with diabetic
vasculitis and nephropathy and chronic dialysis, the brachial artery
and the origins of both the radial and ulnar artery are both thrombosed
(solid lines). Rather than dissecting the
entire brachium and forearm, bypass grafting was performed using
end-to-side repair into the brachial artery proximally and an
end-to-side repair into the radial artery distally. A reversed
interposition graft of 34 cm was used. (Reproduced from Koman LA, ed. Bowman Gray School of Medicine Orthopaedic Manual. Winston-Salem, North Carolina, Wake Forest University Orthopaedic Press, 1997; with permission.) -
Give systemic intraoperative heparin as
one or two 2,000 to 5,000 unit boluses immediately before releasing the
tourniquet. Irrigate all vessels with heparinized balanced solution. -
After all the anastomoses are complete,
deflate the tourniquet, remove the clamps, and check the repair or
repairs for patency and leaks. After obtaining hemostasis, close the
wound in a single layer from wrist to digits. Place a single Silastic
7-French drain adjacent to but not touching the vein graft, if
necessary.
-
Apply a large, bulky hand and forearm
dressing with dorsal plaster from fingertips to elbow with the hand in
a functional position. Leave the drain in 12 to 36 hours. -
Assess the circulation in the hand based
on clinical need. Commonly, evaluation every hour for 6 hours, then
every other hour for 24 hours, is appropriate. -
Advise patients to stop using tobacco and
caffeine. Place the patient in a warm, quiet environment. Start
administering systemic intravenous dextran 40 infusion at 20 ml/hour in
the operating room. In general, heparin is not necessary
postoperatively; but consider systemic anticoagulation if abnormal
distal vessels or marginal run-off compromise the anastomosis (37). Continue the infusion at 20 ml/hour for 2 to 5 days. -
Administer salicylates (125 mg/day). If
sedation is necessary, use chlorpromazine (25 mg p.o. tid). Narcotic
analgesia may be used as necessary.
pharmacotherapy are helpful in most patients with occlusive vascular
disease and may ameliorate signs and symptoms secondary
to
pathologic vasospasm in patients with adequate primary and collateral
circulation (Algorithm 33.1). When required, arterial reconstruction
can reduce symptoms of pain, improve function, increase distal arterial
perfusion, promote healing of ulcerations, and prevent gangrene in
extremities with inadequate collateral circulation (Algorithm 33.2) (39).
Successful reconstruction of occlusive lesions proximal to
unreconstructible lesions increases total digital flow and nutritional
flow distal to inoperable digital occlusions while simultaneously
decreasing symptoms, increasing function, and positively affecting
health-related quality of life (39). In contradistinction, symptomatic improvement from Leriche (44)
or peripheral sympathectomy procedures in patients with diffuse
vasoocclusive digital disease occurs via an increase in the ratio of
nutritional to total flow without increasing total microvascular
perfusion.
heart, subclavian artery, and superficial palmar arch. Upper extremity
embolic events account for less than 20% of arterial emboli, and 70%
are of cardiac origin. Cardiac emboli are created by mural thrombi that
form after myocardial infarction or in association with atrial
fibrillation. In general, cardiac emboli are large and affect the
brachial artery, whereas emboli of arterial origin (noncardiac) are
smaller, affecting wrist-level and digital-level vessels. Most emboli
originate in the subclavian artery; they are commonly produced by mural
thrombi secondary to thoracic outlet compression and poststenotic
dilatation. Emboli from aneurysms or thrombotic events in the wrist and
palm occur in and affect primarily the common and proper digital
arteries.
insidious in onset, embolism often produces acute pain, pallor, and
pulselessness. Paralysis occurs rarely and only after lesions proximal
to muscle units; thus, it is not present in distal events. Secondary
vasospasm may compromise undamaged collateral flow, and necrosis is
common in untreated digits. The classic blue finger progresses to white
and then black. The degree of damage depends on the amount of embolism,
the extent of undamaged collateral vessels, the presence or absence of
continued embolic events, and the source the of emboli.
embolism is to restore pulsatile flow and to normalize the distribution
of the components of the flow.
terminal vessels suggest embolic events. Symptoms of pain, pallor,
pulselessness, and paralysis are not specific, but an acute onset
suggests an arterial origin. Petechiae and varying degrees of cyanosis
are common. Take a complete history and do a physical examination;
obtain consultations for evaluation of embolic events and
echocardiogram. The extent and location of the occlusion can be
determined rapidly by segmental pressure measurements, and
arteriography and color duplex imaging can pinpoint the embolic source.
Arteriography using conventional techniques or magnetic resonance
imaging is important to determine the extent of the damage (16).
sulfate is the treatment of choice for large emboli. After embolectomy,
initiate anticoagulation with heparin followed by Coumadin for
approximately 3 months. Reperfusion injury following revascularization
may result in a compartment syndrome requiring release. Embolism of
arteries in the hand and the excision of embolic sources may require
surgical explorations. For acute situations that do not respond to
heparinization, use urokinase or streptokinase (17,55).
we perform arteriography immediately. If lesions are found, we initiate
thrombolytic therapy. If appropriate, we perform embolectomy, surgical
reconstruction, or both.
of the vessel wall, with subsequent hemorrhage and extravasation. The
resultant hematoma in the soft tissues becomes organized, fibrosed, and
recanalized. The lumen of this false vessel is in continuity with a
true vessel and is distinguished from it by the absence of endothelial
lining.
causing gradual dilation into a more uniform shape, compared with the
saclike appearance of a pseudoaneurysm. Whereas false aneurysms
commonly result from penetrating traumatic and iatrogenic incidents,
true aneurysms occur most frequently in areas of arterial circulation
that are exposed to repetitive trauma (e.g., the distal ulnar artery
and superficial arch).
trauma is unknown. False aneurysms of the upper extremity accounted for
27% of all of the false aneurysms recorded in the Vietnam vascular
registry (52). Aneurysms of the ulnar and
digital vessels are evenly distributed between the true and false type,
and ulnar artery aneurysms comprise the largest group of both true and
false aneurysms (52). False aneurysms are
frequently encountered in the axillary, brachial, anterior
interosseous, and radial arteries but may also involve the radial
artery within the anatomic snuffbox, the palmar arch, and the digits.
evaluation of an aneurysm may vary considerably between 2 weeks and 12
years. The natural history of both true and false aneurysm is
characterized by a slow progression leading to thrombosis, the
production of emboli, or both. The effect of thrombolytic agents on the
natural history is unknown. However, the degree of turbulence in the
re-created aneurysm and any abnormal coagulation properties are similar
to the pretreatment state and rethrombosis is possible. In fact,
because the components of Virchow’s triad for thrombosis (stasis,
turbulence, and hypercoagulability) is present, reocclusion is likely
to occur.
Symptoms of localized pain from soft-tissue and neural compression can
occur secondary to a mass effect; distal signs and symptoms occur most
frequently from embolization.
ultrasonography, color duplex scanning, and B mode imaging.
Arteriography, the gold standard, can define the extent of damage;
delineate distal embolic events; differentiate the aneurysmal mass from
an arteriovenous malformation, neural tumor, or malignancy; and, most
important, aid in preoperative planning by assessing collateral flow
and determining the possibility of reconstructing distal vessels (18).
(a) resection and ligation, (b) excision of the damaged wall and patch
graft, (c) resection with end-to-end repair, and (d) resection with
interposition graft. The choice of treatment is determined by
evaluating collateral flow and vasomotor tone. Resection of the
aneurysm has been found effective, along with proximal and distal
ligation of a noncritical radial or ulnar artery aneurysm; brachial
vessels or critical vessels, in general, however, require arterial
reconstruction. The long-term benefit of thrombolytic agents is unknown.
recanalization, and embolism. Because of the risk of thrombosis and
distal embolization, surgical treatment of both true and false
aneurysms is recommended. Aneurysms may occur in the presence of
adequate or inadequate circulation and may be accompanied by distal
occlusive and embolic disease. If collateral flow is adequate,
providing uniform digital pulp microcirculatory perfusion, either
resection plus ligation of the aneurysm or arterial reconstruction is
appropriate. When collateral flow is inadequate or occlusive, or
embolic events occur distal to the aneurysm, arterial reconstruction
using end-to-end repair or reversed interposition vein grafting or
other techniques is advised. (See previous discussion of preoperative
and intraoperative assessment and treatment techniques.) Until further
data are available, we feel that thrombolytic therapy is relatively
contraindicated in the treatment of upper extremity aneurysms.
trauma from catheterization or hemodialysis. Distal ischemia and
neurologic symptoms are created by shunting, which causes a so-called
steal phenomenon. Although most patients tolerate AVFs for dialysis,
complications are common (11,46).
Following end-to-end and end-to-side anastomosis of the radial artery
to the cephalic vein (radiocephalic AVF), digital blood flow to the
thumb may be reduced by up to 40% secondary to proximal shunting (steal
phenomenon) (11). Arterial insufficiency is
produced if distal perfusion decreases below a critical volume (DBI =
0.5). Dialysis-associated steal syndrome (DASS) occurs in 1% of
side-to-side radiocephalic fistulas and in 6.4% of patients who have
undergone forearm loop grafts (50a). The incidence increases
significantly in the presence of pre-existing occlusive disease distal
to the shunt.
on exertion and may produce a higher than expected incidence of median
and ulnar neuropathy. The incidence of median mononeuropathy following
radiocephalic AVF approaches 70%—significantly higher than the 2%
incidence in the contralateral hand. Neurologic symptoms are produced
by neural ischemia, elevated interstitial pressure, diminished systolic
pressure in the presence of pre-existing elevated carpal canal
pressure, and venous distention compressing neural structures. Shunt
reversal or banding may alleviate symptoms of arterial insufficiency
secondary to inappropriate shunting, but neurolysis of involved nerves
may be required.
A combination of neural or ischemic symptoms (whether due to a steal
phenomenon or emboli) and the presence of an AVF support the diagnosis,
which may be confirmed by an assessment of digital flow. A DBI of 0.5
to 0.7 is a harbinger of impending cell death and necrosis. Stress
testing may be helpful to delineate the pathophysiology. An assessment
of nutritional flow may be diagnostic. Arteriography is required to
differentiate between aneurysm and thrombosis, to document emboli, and
for preoperative planning.
sympatholytic agents, banding, and ligation. Surgical ligation of the
venous limb of the shunt, leaving the artery intact, may be required if
banding (decreasing shunt diameter) is inappropriate. The persistence
of excessive arteriovenous shunting may produce severe neurologic and
ischemic sequelae, which may require (a) shunt revision, (b) banding or
ligation of the shunt, or (c) neurolysis of compressed nerves, or a
combination thereof.
resistance through the shunt, increases blood flow distally in the
digits, and may reverse symptoms if the shunt is 4 mm or more in
diameter. Shunts smaller than 3 to 4 mm, however, may not produce
sufficient flow for effective dialysis. Banding is indicated if the
ulnar artery is patent and occlusion of the radial artery distal to the
fistula causes a documented increase in distal perfusion. It is also
indicated if occlusion of the venous limb increases digital flow and
banding allows adequate flow for dialysis. Occlusion of the radial
artery distal to side-to-side fistulas eliminates retrograde steal and
increases thumb blood pressure significantly (11).
Documentation of digital blood pressure before and after occlusion of
the radial artery below the fistula provides valuable information in
symptomatic patients with a DBI of less than 0.64 (Algorithm 33.3)
account the importance of vascular access in the dialysis-dependent
patient. Decisions require the combined input of the patient,
nephrologist, vascular access surgeon, and the hand surgeon. In
general, severe symptoms following an AVF require surgical
intervention. In the presence of pre-existing or acquired occlusive
disease distal to the AVF, severe symptoms are frequent and are
alleviated most effectively by elimination of the vascular steal, by
minimizing abnormal sympathetic tone, and by arterial reconstruction of
occlusive disease.
difficult and requires the involvement of the patient, the
nephrologist, and the vascular surgeon. Symptoms often include (a)
necrotic digital tips requiring amputation, (b) cyanotic digits, and
(c) neurologic impairment. Digital blood pressures and peripheral nerve
conduction velocity (PNCV) measurements guide the decision-making
process. If temporary occlusion of the radial artery distal to the
shunt reduces symptoms and improves capillary refill (22)
and the DBI is less than 0.5, we consider banding or ligating of the
shunt. If PNCVs are abnormal and the DBI is greater than 0.7,
associated neurologic complaints may be reduced by neurolysis with
excision of any venous compressive structure. If PNCVs are normal,
distal perfusion is poor, the DBI is £ 0.5, and graft reversal
represents an unacceptable burden, consider bypass grafting from the
brachial artery to a vessel distal to the fistula (Algorithm 33.3).
thromboangiitis obliterans (TAO), giant cell arteritis, Wegener’s
granulomatosis, Takayasu’s arteritis, polyarteritis nodosa, and
collagen vascular disease, and it is seen in association with
neoplastic disease. TAO (Buerger’s disease) is an inflammatory
occlusive disease of small and medium-sized vessels (9). Classic clinical characteristics include lower extremity involvement in young, predominantly male smokers (33).
The male-to-female ratio of patients with TAO, however, is declining,
with older patients more frequently seen and upper extremity
involvement more common (41). Although
arteriographic differentiation from atherosclerosis is relatively
obvious, TAO is difficult to differentiate from scleroderma and
collagen vascular disease. Histologically, arterial or venous
thrombosis with lymphocytic and polymorphonuclear leukocyte
infiltration into the thrombus and vessel wall is observed. If the
patient quits smoking, disease progression decreases and the need for
amputation is less likely.
-
Improve nutritional flow to improve the response to stress.
-
Correct inappropriate arterial-venous shunting.
-
Increase proximal and distal perfusion.
nutritional perfusion falls under the rubric of preoperative
management. Use calcium channel blockers and other pharmacologic or
mechanical means, including biofeedback, to decrease sympathetic tone,
decrease abnormal shunting, and increase nutritional flow.
requires (a) an understanding of the vascular anatomy and (b) an
assessment of physiologic control. Therefore, studies to evaluate the
form and function of the vascular tree are important and should include
an estimate of
-
Total flow—before and after stress [temperature and laser Doppler flow (LDF)],
-
Nutritional flow (LDF, vital capillaroscopy), and
-
Vascular anatomy (arteriography or magnetic resonance angiography) (16,20). Surgery should then be planned to
-
Decrease sympathetic tone and increase nutritional flow by periarterial sympathectomy, and
-
Increase total and nutritional flow by arterial reconstruction and periarterial sympathectomy (Table 33.7).
restoration of anatomic channels by using vascular reconstruction, if
necessary. The techniques used here are identical to those in acute and
chronic reconstruction.
maintains blood pressure by the peripheral modulation of vascular
resistance, directing blood to nutritional capillary beds, providing
appropriate thermoregulatory control, and preventing excessive blood
loss after trauma. However, inappropriate vasoconstriction may produce
symptoms of pain and cold intolerance, interfere with health-related
quality of life, and result in ulceration, gangrene, or both.
Pathologic vasospasm produces inappropriate arterial and venous tone
that persists in the presence of physiologic demands for increased flow
or contrary to specific cellular metabolic needs. Inappropriate cold
sensitivity, the most common symptomatic manifestation of vasospastic
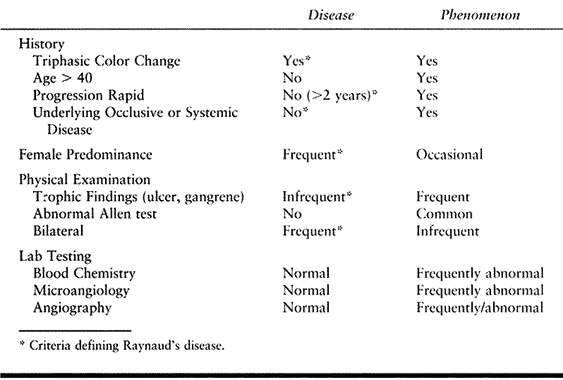
states, is frequent and affects 5% to 10% of the general population and
20% to 30% of premenopausal women. Vasospastic states are defined as
primary (Raynaud’s disease) in the absence of an identifiable etiology
or secondary (Raynaud’s phenomenon) in the presence of a causal
condition (Table 33.8).
 |
|
Table 33.8. Raynaud’s Disease versus Raynaud’s Phenomenon
|
inadequate vascular structure, inappropriate vascular control
mechanisms, or both. Adequate tissue perfusion requires delivery of
blood to nutritional capillary beds in sufficient quantity to fulfill
metabolic needs, appropriate oxygen-carrying capacity, and diffusion of
oxygen through intravascular spaces to functioning cellular structures.
In the majority of patients, oxygen and metabolic-carrying capacity
within the vascular system is adequate. In the absence of intravascular
fibrosis and cell death, symptoms are secondary to inadequate delivery
of blood products at a cellular level (ischemia). Vasospasm decreases
total flow, shunts blood through nonnutritional thermoregulatory
pathways, or, in some cases, does both.
normal vascular architecture, no identifiable underlying etiology, and
the presence of vasospasm. Group II patients exhibit Raynaud’s
phenomenon secondary to collagen vascular disease and may be classified
as either (a) those with normal circulation or (b) those with
compromised circulation. Group III patients have secondary vasospasm
due to vascular injury and may be classified as either (a) those with
uncompromised collateral flow or (b) those with abnormal collateral
flow. Group IV patients without pre-existing or acquired structural or
vascular abnormality have secondary vasospasm after sustaining an
injury to nerves, soft tissue, bone, or any combination thereof. These
categories provide criteria that allow treatment to be based on
anatomic and physiologic considerations.
from inadequate nutritional perfusion, insufficient delivery of oxygen
and metabolites to cellular structures, and the development of an
anaerobic and acidotic tissue environment. Raynaud’s phenomenon
classically has three stages that progress from normal to blanched
(white) to cyanotic (blue) to rubrous (red). Sympathetic hyperactivity
stimulated by exposure to cold or other stresses initiates intense
vasospasm with cessation of arterial inflow and the production of a
white, blanched, and cool digit (stage 1). Stasis within the digit
produces deoxygenated, pooled blood that appears cyanotic or bluish and
produces the characteristic blue hue seen in stage 2 of the phenomenon.
The rubor (redness) in stage 3 is secondary to reactive hyperemia from
a rebound vasodilatation initiated by the ischemia; reperfusion is
associated with burning pain, dysesthesias, or both. Therapeutic
approaches are designed to increase microvascular flow and maximize
capillary perfusion.
Pursue a diagnostic strategy to delineate physiologic events (i.e.,
vessel structure, vascular function and control, and intravascular
blood components) and to determine precipitating local or systemic
processes or disease (e.g., collagen vascular disease, arterial
occlusion, neurovascular compression, hematologic abnormality, trauma,
smoking, drugs or toxins, central nervous system disease, or primary
vasospasm). Specifically, diagnostic goals should
-
Differentiate vasospastic from vasoocclusive states.
-
Separate Raynaud’s disease from secondary Raynaud’s phenomenon.
-
Evaluate the effects of structural compromise versus functional impairment.
gangrene, which are associated with unilateral Raynaud’s symptoms, is
presumptive evidence of thrombosis or embolism.
ulcerations, and infections. Wrist-level and digital Allen testing is a
valuable screening test for distal occlusive disease and may be
enhanced by digital plethysmography and measurement of segmental
arterial pressures. Check capillary refill, turgor, skin integrity, and
sensibility. Perform appropriate nerve conduction studies if there is a
suspicion of neurologic dysfunction and neurologic symptoms. Use
laboratory tests to look for hematologic or collagen vascular
abnormalities.
may be employed in order to delineate the structural and functional
influences on perfusion of the upper extremity. Digital plethysmography
and temperature measurements with and without stress are most commonly
used. Pressure gradients of greater than 20 mm of mercury between two
levels in the same extremity or between the affected and contralateral
extremity, or a DBI or an RBI of 0.7 or less, suggest stenosis or
occlusion (8,48).
capillaroscopy to document pathognomonic capillary morphologic changes
including telangiectasias, abnormal capillary budding, and blunting,
dilation, or deformation of papillary capillaries. At present, vital
capillaroscopy is the only clinical method to assess directly the
nutritional components of digital blood flow. A quantitative and
qualitative assessment of digital perfusion is necessary to make an
appropriate diagnosis and to plan medical care. Detailed assessments of
digital temperature, laser Doppler flowmetry measurements of cutaneous
perfusion, and vital capillaroscopy before and after stress allow a
determination of the extent of arteriovenous shunting, the adequacy of
nutritional flow, and an estimate of total flow available (35,36,50).
and provides data regarding thermoregulatory flow components. Combined
temperature and plethysmographic data correlate with total perfusion (38).
Allen testing and pulse volume recordings provide data regarding
vascular structure, which may be delineated maximally by contrast
arteriography or—if available—magnetic resonance angiography (16,20).
Structural data, complemented by stress testing, provide a functional
assessment of extremity flow. A combination of both structural data and
functional information is mandatory for optimal evaluation (36).
assessment of vascular pathology and structural integrity. Indications
for arteriography include potential surgical intervention, unilateral
Raynaud’s phenomenon, progressive gangrene or ulceration, suspected
occlusive or embolic disease, and clinical concerns regarding vascular
structural integrity. For patients with decreased perfusion or
vasospasm, use isosmotic contrast agents to enhance diagnostic
capability
and vasodilation through intraarterial agents (e.g., tolazoline or
nitroglycerine) or regional anesthetic block (e.g., axillary block).
Vasospasm prevents visualization of vasculature that is otherwise
adequate structurally, and the incorrect diagnosis that could result
may affect treatment plans.
The treatment of vasospastic disorders must be individualized.
Management options include medical therapy, biofeedback, digital
sympathectomy, and microvascular reconstruction. Patients with
vasospastic symptoms, abnormal autonomic control, and absence of
occlusive disease (Group I, Raynaud’s disease), frequently respond to
nonsurgical modalities and improve spontaneously over time. For
patients in Group II (Raynaud’s phenomenon) and Group III (occlusive
disease), structural and functional information guides treatment
decisions. With inadequate collateral circulation (Groups IIB and
IIIB), vascular reconstruction is indicated, if possible. Although
nonsurgical methods or peripheral sympathectomy may reduce patient
symptoms, the prognosis is guarded. Patients in Group IV (secondary
vasospasm and normal vasculature) improve with correction of the
initiating traumatic event or with appropriate pharmacologic
intervention.
-
Decrease inappropriate vasospasm by nonsurgical management, if possible.
-
Improve nutritional blood flow in the
absence of occlusive disease using, in order (a) calcium channel
blocker, (b) biofeedback, (c) oral pharmacologic agents, and (d)
peripheral sympathectomy. -
In the presence of combined occlusive and
vasospastic problems, correct repairable occlusive disease with or
without periarterial sympathectomy (PAS). -
In the absence of repairable occlusive
disease consider, in order (a) periarterial (peripheral) sympathectomy,
(b) phenol or alcohol percutaneous proximal sympathectomy, (c) spinal
cord stimulators, and (d) arteriovenous reversal. (Note that the
up-regulation of peripheral receptors 6–12 weeks following traditional
cervicothoracic sympathectomy may reexacerbate clinical complaints.)
to stop smoking, avoid cold environments, alter activities to eliminate
those that precipitate symptoms, and use protective garments. Cessation
of smoking is the most important of these modifications. Patients who
live in cold climates have more significant symptoms, which may be
ameliorated by the use of mittens or hand-held heating devices. A
distinct relationship exists between cold exposure of the head and neck
and the reflex vasospasm of hands and feet. Advise patients to use
scarves and hats.
the conscious regulation of autonomic body processes. External monitors
are used initially, allowing patients to observe how they can elevate
their digital temperatures by changing their rate of breathing or
thought processes. Biofeedback significantly alleviates symptoms in
patients with groups I, IIA, IIIA, and nonneural Group IV etiology, but
it is less effective in patients with Group IIB and Group IIIB symptoms
(21).
or to mitigate sympathetic hyperactivity associated with vasospastic
and vasoocclusive disease. Pharmacologic intervention works best in
patients with adequate collateral circulation and redistributes flow to
nutritional capillary beds by decreasing inappropriate shunting (Fig. 33.13).
At present, calcium channel blockers are the drugs of choice for
vasospastic symptoms, provide the most reliable palliation of symptoms,
have an acceptable level of side effects, and present an excellent
risk–benefit ratio (14). Calcium channel
blockers prevent calcium influx in vascular smooth muscle and diminish
sympathetically driven vasoconstriction. Nifedipine, 10 to 30 ml tid,
or in long-acting form (30 to 60 ml qd), and amlodipine besylate
(Norvasc), 5 to 10 mg qd, are the drugs employed most frequently. Other
drugs include
-
Tricyclic antidepressants (amitriptyline, 25-75 mg QHS)
-
Serotonin reuptake inhibitors [fluoxetine
hydrochloride (Prozac), 20 mg qd; sertraline (Zoloft), 25 to 50 mg qd,
and paroxetine (Paxil), 20-50 mg qd] -
Sympatholytic drugs (prazosin
hydrochloride, 1 to 2 mg bid to tid; terazosin hydrochloride, 1 to 2 mg
qd; clonidine, 0.1 mg bid or by transdermal patch).
function or provide short-term to intermediate-term palliation. Group
IV patients with nonvascular injury seldom require surgery. Successful
surgical intervention depends on the underlying etiology, the extent
and location of vascular insults, and the extent of irreversible
end-organ damage. Surgical options include reconstruction of occluded
vessels and modification of sympathetic tone (Table 33.7).
cervicothoracic sympathectomy, Leriche sympathectomy (resection of
thrombosed or occluded vessels and ligation) (44), or peripheral periarterial sympathectomy (19,38,54).
sympathetic tone and improves peripheral blood flow by permanent
ablation of the cervical sympathetic trunk and may be performed through
an axillary or cervical incision, or by endoscopic technique. Chronic
sympathectomy secondary to cervicothoracic interruption of sympathetic
fibers decreases total peripheral flow and produces upregulation of
distal alpha-adrenergic receptors. It also decreases the efficacy of
subsequent peripheral sympathetic modifiers. This procedure, which is
often immediately effective, lasts only 6 to 12 weeks in many patients
and, after that time, may increase sympathetic tone. Temporary
posterior cervicothoracic sympathectomy by phenol injection under
computer tomography control is reversible and may be of benefit during
collateral vessel maturation.
Classically, Leriche observed that resection of a thrombosed segment of
occluded artery with ligation proximal and distal stumps decreases
abnormal sympathetic tone and improves distal perfusion through
collateral circulation. Therefore, peripheral sympathectomy is
performed by excision of thrombosed arteries with or without arterial
reconstruction. In the presence of Group IIIA occlusive disease and
secondary vosospasm, the structurally adequate but abnormally
functioning circulation will respond and deliver adequate distal flow
to fulfill metabolic requirements. However, in Group IIIB occlusive
disease, there is inadequate collateral flow and sympathectomy will be
inadequate to restore microvascular perfusion. In primary vasospastic
conditions with or without occlusive disease (Groups IIA and IIB),
peripheral sympathectomy of digital vessels or wrist-level vessels may
be necessary to decrease optimally abnormal tone. The Leriche
sympathectomy may be combined with multiple-level peripheral
sympathectomy and arterial reconstruction.
-
A Leriche sympathectomy involves the
excision and ligation of a thrombosed segment of artery, which
interrupts the sympathetic connections from the peripheral nerves to
the peripheral arteries as well as the sympathetic fibers that travel
on and within the adventitia of peripheral arteries (49).
It is preferable to begin in an area of near-normal tissue and to
continue the dissection into the area of occlusion. In the case of the
ulnar artery, transect the nerve of Henle as well as other connections
with the ulnar nerve while protecting the ulnar nerve. -
Periarterial sympathectomy decreases
spasm in the collateral circulation and decreases inappropriate
arteriovenous shunting, thereby increasing nutritional flow. If
collateral circulation is inadequate or if there are multiple levels of
occlusion, a Leriche-type sympathectomy may not provide sufficient
palliation. -
With a digital sympathectomy technique (Flatt and Wilgis) (19,54),
digital arteries and nerves are identified through Bruner zigzag
incisions from the distal palmar crease to the midportion of the
proximal phalanx. All connections between the digital nerve and artery
are transected, and the adventitia of the digital artery is stripped
from the artery under the operating microscope (19). -
A palmar or hand sympathectomy technique (Koman) (38) is used to achieve a sympathectomy of all four digits and the thumb. A description of the procedure follows:
-
Expose the radial and ulnar artery, the
superficial arch, and the three common volar digital arteries by means
of three palmar incisions: two parallel 3 cm longitudinal incisions at
the wrist and an oblique incision in the palm (Fig. 33.16).![]() Figure 33.16.
Figure 33.16.
Schematic representation of periarterial sympathectomy of the hand
using longitudinal incisions to expose the radial and ulnar artery and
an oblique incision in the palm to expose the superficial arch and the
origins of the common volar digital arteries (Koman technique) is
shown. A separate incision (not shown) may be used over the anatomic
snuffbox to expose the radial artery. After initial dissection under
loupe magnification, the operating microscope is used to strip the
adventitial fibers from the vessels and to sever connections with
parallel peripheral nerves. (Reproduced from Koman LA, ed. Bowman Gray School of Medicine Orthopaedic Manual. Winston-Salem, North Carolina, Wake Forest University Press, 1997; with permission.) -
Transect the connections between the
radial and ulnar arteries and their respective nerves over a 3 cm
segment. Dissect the adventitia of the radial and ulnar artery under
the operating microscope over a length of 2 cm. -
Similarly, after exposure of the proximal
portion of the superficial arch and the origins of the three common
volar digital arteries, all connections from the peripheral nerves to
the arteries are severed, and all periarterial adventitia containing
neural tissues is dissected free. -
If there are significant symptoms in the
thumb, make a fourth dorsal incision in the anatomic snuffbox, and
expose and mobilize the deep branch of the radial artery and the origin
of the deep arch. Remove the adventitia (Fig. 33.16) (38).
-
refractory symptoms and inadequate collateral circulation (Group IIB
and IIIB) (32,38).
Vascular reconstruction in Group IIA and IIIA patients should be
individualized as described previously. The goal of surgical
reconstruction is to increase digital perfusion pressure and
nutritional flow in ischemic digits (28,48)
by (a) end-to-end repair, (b) interposition grafting, or (c) bypass
grafting of the occluded vascular areas. Also important is functional
modification of sympathetic tone resulting from the peripheral
sympathectomy associated with the vascular resection. Successful
arterial reconstruction in patients with underlying collagen-vascular
disease increases total digital flow and nutritional flow, diminishes
symptoms, and promotes healing of ulcers (38,39).
unreconstructible distal occlusion, successful reconstruction of a
concurrent proximal occlusion increases total digital flow and
nutritional flow, decreases symptoms, and allows ulcers to heal (39).
In contradistinction, Leriche resection of the proximal occluded
segment combined with peripheral digital and palmar sympathectomy
improves nutritional flow by the elimination of inappropriate
arteriovenous shunting but does not increase total flow (38). The technique for reconstruction is similar to that used in occlusive disease.
pathophysiology, and the treatment goal should be directed at
appropriate distribution of digital flow to provide adequate
nutritional capillary flow sufficient to meet metabolic demands under
stressed and nonstressed conditions. Treatment decisions are aided by a
knowledge of structural and functional characteristics and the
classification of the vasospastic state (Table 33.4).
For example, Raynaud’s phenomenon with inadequate circulation (Group
IIB) is the condition most refractory to treatment and Group IIB
patients have the highest incidence of ulceration. Conversely, patients
in Group I (Raynaud’s disease) and Group IV (secondary vasospasm from a
nonvascular injury) have the best prognosis, rarely have ulcerations,
progress infrequently to gangrene, and often respond to oral
medications or biofeedback and improve spontaneously.
biofeedback are often effective in Group I (Raynaud’s disease), Group
IIA (Raynaud’s phenomenon with adequate circulation), and Group IV
(secondary vasospasm without arterial compromise). These patients have
normal vasculature, but abnormal vascular reactivity secondary to
abnormal sympathetic control. In patients with inadequate collateral
circulation or inadequate circulation (Groups IIB and IIIB), the
prognosis is guarded unless arterial reconstruction can be successfully
achieved. The use of periarterial sympathectomy in patients with
inadequate circulation is a salvage procedure.
is controversial. An increased digital temperature or pulse volume
recording and decreased symptoms after wrist or digital block with
lidocaine or bupivacaine confirm abnormal sympathetic tone and that
sympathectomy will provide palliation. Of note, in response to
sympathetic blockade, patients with vasoocclusive disease and
inadequate collateral circulation are unlikely to be able to increase
digital temperatures—a reflection of total flow. In a percentage of
these extremities, however, nutritional flow is maximized,
inappropriate arteriovenous shunting is decreased, ulcers heal, and
symptoms decrease. This group of patients benefits the most from
periarterial sympathectomy. The surgeon must have a thorough knowledge
of the diagnostic tests and their interpretation to identify them (38).
Although surgical statistics would be improved by eliminating this
group of patients, some of whom do not have sufficient flow to respond
successfully, it is this group of patients that needs a palliative
procedure the most. The role of arteriovenous reversal in vasoocclusive
disease represents an additional modality and deserves investigation.
Although short-term relief is dramatic, long-term efficacy has not yet
been demonstrated in the peer-reviewed literature.
scheme: *, classic article; #, review article; !, basic article; and +,
clinical results/outcome study.
EV. Thromboangiitis Obliterans: Methods of Diagnosis of Chronic
Arterial Lesions Distal to the Wrist with Illustrative Cases. Am J Med Sci 1929;178:237.
RJ, Hobson RW, Lee BC, et al. Reduced Dependency on Arteriography for
Penetrating Extremity Trauma: Influence of Wound Location and
Noninvasive Vascular Studies. J Trauma 1990;30:1059.
SC, Piccorelli GO, Shah PM, et al. Incidence and Results of Arterial
Complications among 16,350 Patients Undergoing Cardiac Catheterization.
J Vasc Surg 1989;10:113.
R, Danylewick R, Burdon T, et al. An Angiographic Study of Ischemia as
a Determinant of Neovascularization in Arteriovenous Reversal. Surg Gynecol Obstet 1988;166:28.
JJ, Chung KC, Gellad FE, et al. Efficacy of Magnetic Resonance
Angiography in the Evaluation of Vascular Malformations of the Hand. Plast Reconstr Surg 1995;99:136.
ER, Crump JM, Vines FS, et al. A Reassessment of the Role of
Arteriography in Penetrating Proximity Extremity Trauma: A Prospective
Study. J Trauma 1989;29:1041.
SM, Smith TL, Holden MB, Koman LA. Does Transdermal Nicotine Adversely
Affect Digital Microvascular Flow During Acute Cigarette Withdrawal? American Society for Reconstrucive Microsurgery 1997;64(Abstract).
AM, Baffour R, Burdon T, et al. A Demonstration of Vascular
Proliferation in Response to Arteriovenous Reversal in the Ischemic
Canine Hind Limb. J of Surgical Research 1989;47:341.
BM, Cuadros L, Jupiter JB: Interpositional Vein Grafts to Restore the
Superficial Palmar Arch in Severe Devascularizing Injuries of the Hand.
J Hand Surg 1988;13A:753.
CA, Yakubu MA, Howie CA, et al. Desensitization and Down-regulation of
Brain Alpha2-adrenoceptors by Centrally Acting Antihypertensive Drugs. Br J Clin Pharmacol 1990;30:131S.
MJHM, Jorning PJG, Joshi SR, et al. Epidural Spinal Cord Electrical
Stimulation Improves Microvascular Blood Flow in Severe Limb Ischemia. Ann Surg 1988;207:179.
TA, Marks J, Berrettoni BA, Seitz WH. Arteriovenous Reversal for Limb
Salvage in Unreconstructible Upper Extremity Arterial Occlusive
Disease. J Vasc Surg 1993;17:924.
LA, Smith TL, Smith BP, et al. Arterial Reconstruction in the Ischemic
Hand and Wrist: Effects on Microvascular Physiology and Health-related
Quality of Life. J Hand Surgery 1998;23A:773.
DA. Selective Heparinization of Venous Anastomosis in Latissimus Dorsi
Free Flaps to Cover Lower-extremity Soft-tissue Defects. Microsurgery 1991;12:301.
PA, Gilula LA, Anderson CB, et al. Complications Associated with
Arteriovenous Fistulas in Patients Undergoing Chronic Hemodialysis. Arch Intern Med 1978;138:1117.
JO, Corson JD, Bush HL Jr, LoGerfo FW: Management of the Upper
Extremity with Absent Pulses After Cardiac Catheterization. Am J Surg 1978;135:484.
H, Negita S, Teranishi Y, Sasa M. Release of Sympathetic
Neurotransmitter Evoked by Electrical Stimulation is Increased in the
Chronically Decentralized Artery. Jpn J Pharmacol 1993;63:285.