REPLANTATION
limbs has been possible for nearly 40 years. The first successful
reattachment of a completely severed human limb was reported by Malt et
al. in Boston in 1962 (55). Komatsu and Tamai (50) have been credited with successful replantation of a completely severed digit in 1965. Zhong-Wei et al. (102)
reported the successful replantation of a severed digit the same year.
Over the past three decades, several microsurgery centers throughout
the world have achieved greater than 80% success rates in replanting
severed digits, hands, and major limbs (6,7,10,14,32,33,34,37,45,47,48,52,57,63,64,68,70,77,82,87,96,99,100 and 101,103,104).
Additional experience, subspecialization in microsurgery with the
increased knowledge and surgical skills in this field, improvements in
microscopes, ultrafine needles, microsuture materials, and other new
and improved instruments have made replantation the procedure of choice
for the management of many amputations (1,4,11,82,88,89).
anastomosis and survival of the replanted or revascularized part is the
technical adequacy of the microvascular repair (82).
Successful microsurgery requires familiarity with the operating
microscope and microsurgical instruments and the careful handling of
small vessels and surrounding tissues. These skills must be learned in
the animal laboratory, not in the clinical operating room. After the
surgeon has demonstrated proficiency in the repair of microvessels and
nerves, in the animal laboratory, then these skills may be transferred
to the operating room. In addition, replantation of amputated parts
should be done by surgeons who are thoroughly trained in surgery of the
upper extremity and hand and who have had sufficient clinical
experience to be able to predict the outcome following replantation of
a severed part.
replant an amputated extremity are the predicted morbidity to the
patient; survival of the replanted part; functional outcome of the
reconstructed limb; and the overall financial burden to the patient,
insurance carrier, or society (83). The
predicted function after replantation should be better than that of a
prosthesis or amputation revision. Because the ultimate functional
result may be unpredictable, the decision of whether to replant an
amputated part is often difficult, even for the experienced
reconstructive surgeon.
process. Based on the experience of more than 1,800 attempted
replantations over a 25-year period by my colleagues and me, we have
developed criteria for proper patient selection. The surgeon must
understand that not only viability but also recovery of useful function
determine success in replantation.
-
Level of amputation
-
Severity of injury (guillotine versus crush or avulsion)
-
Warm and cold ischemia times
-
Age of patient
-
Segmental injuries
-
General health of patient
-
Vocation
-
Predicted rehabilitation
make the decision regarding replantation because they will usually
request replantation even in cases where there is little chance of
survival or function of the replanted part. The final decision must
rest with the surgeon in collaboration with the patient. Social,
ethnic, and religious beliefs about the significance of loss of the
amputated part do influence the surgeon’s decision regarding
replantation, however.
replantation is rigid or absolute, and the criteria, although generally
similar for most replantation surgeons, vary with individual experience
(83). Future experience with replantation and
technological advances in externally powered prostheses are likely to
further alter these criteria. On the basis of this standard, good
candidates for replantation are those with amputations of the following
types:
-
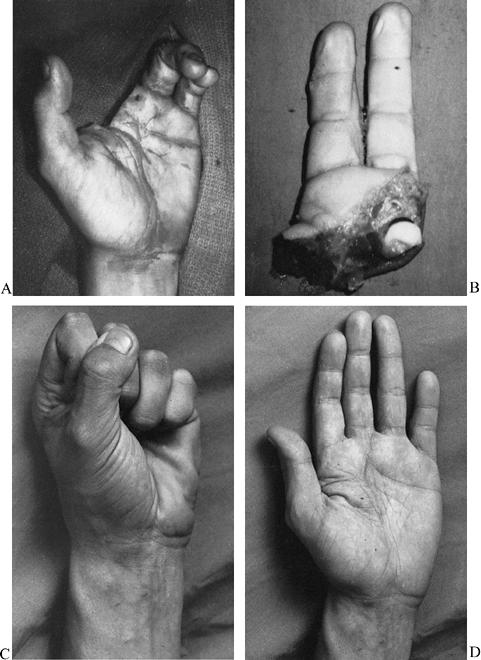
Wrist (Fig. 34.1)
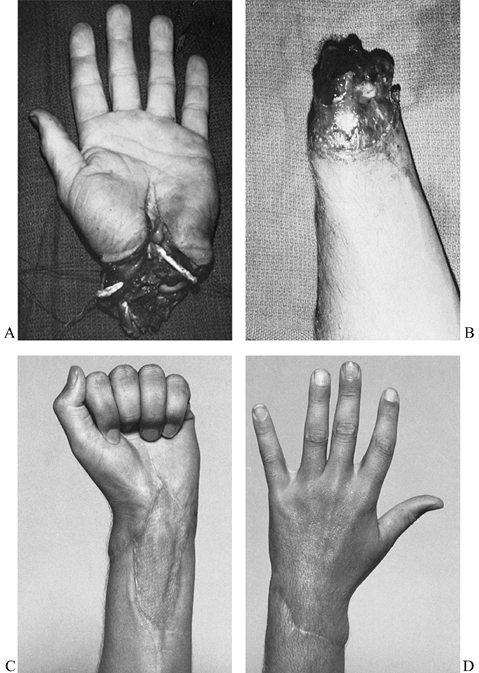 Figure 34.1. Good candidates for replantation include amputations at the level of the wrist. A,B: A complete amputation at the wrist level in a 21-year-old man. C,D: The amount of digital flexion (C) and extension (D)
Figure 34.1. Good candidates for replantation include amputations at the level of the wrist. A,B: A complete amputation at the wrist level in a 21-year-old man. C,D: The amount of digital flexion (C) and extension (D)
seen in this patient 3 years following successful replantation. (From
Porubsky GL, Urbaniak JR. Limb and Digital Replantation. In Flye MW,
ed. Principles of Organ Transplantation. Philadelphia: WB Saunders, 1989.) -
Partial hand (through metacarpal level of palm) (Fig. 34.2)
![]() Figure 34.2. A,B:
Figure 34.2. A,B:
Complete amputation of the left index and long finger through the
metacarpals in a 20-year-old man. The amputated part was reattached as
a composite. C,D: Flexion (C) and extension (D)
1 year later. The patient lacks 5 mm of touching the distal palmar
crease with the index finger and 4 mm with the long finger. He returned
to work as a meatcutter 3.25 months after the injury. -
Thumb (Fig. 34.3)
 Figure 34.3. A: Dominant thumb of a 16-year-old boy was avulsed by a nylon rope. B,C:
Figure 34.3. A: Dominant thumb of a 16-year-old boy was avulsed by a nylon rope. B,C:
Replantation resulted in less than 5 mm of two-point sensory
discrimination and individual interphalangeal and metacarpophalangeal
flexion and extension. (From Urbaniak JR. Replantation of Amputated Hands and Digits. American Academy of Orthopaedic Surgeons Instructional Course Lectures, Vol. 27. St. Louis: CV Mosby, 1978.) -
Multiple digits
-
Forearm
-
Almost any body part of a child
-
Elbow and above elbow (only sharply severed or moderately avulsed)
-
Individual digit distal to the flexor superficialis insertion
for replantation, if all other factors are favorable one should attempt
replantation of these amputated parts.
Usually, amputations proximal to the midforearm level are of a crushing
or avulsing nature, which produces considerable muscle trauma.
Myonecrosis and subsequent infection are common problems with this type
of replantation, particularly at the elbow or brachial level;
therefore, be extremely selective in choosing replantation at these
levels.
patients of all ages. A replanted thumb is far better than any type of
reconstructed thumb to replace one that has been amputated (5,31,58,68,73,95) (Fig. 34.3).
Attempt replantation even as far distal as the nail base if vessels for
revascularization can be located. If the patient is healthy, there is
no upper age limit (33,75).
For example, if the thumb and index finger have been completely
amputated in a crushing injury and the distal thumb has irrepairable
distal vessels, transpose the amputated index finger to the thumb
stump. This alteration
should result in excellent thumb function and cosmetic acceptability.
for replantation. Amputations at or distal to the distal
interphalangeal joint of the fingers or the interphalangeal joint of
the thumb can be successfully replanted if dorsal veins can be located
on the amputated part (21,25,26,36,41,43,54,59,75,81,91,98).
As a general rule, at least 4 mm of dorsal skin proximal to the base of
the nail plate must be present for locating veins suitable for repair.
insertion; an excellent functional result can be achieved, and the
operating time is not long (usually less than 4 h) (33,87).
Replantations at this level are usually successful (greater than 90%
viability rate), provide a good appearance, are not painful, allow good
function, eliminate the potential for painful neuromas, and permit an
early return to work (usually less than 3 months) (16,33,87).
body part because, if the reattached part survives, useful function can
be predicted (20,84).
neither are contraindications. Injuries generally not considered ideal
for replantation include:
-
Amputations in patients with other serious injuries or diseases
-
Amputations at multiple or segmental levels
-
Severely crushed or avulsed parts
-
Amputations in which the vessels demonstrate arteriosclerosis
-
Amputations in mentally unstable patients
-
Amputations with prolonged warm ischemia time (more than 6 h)
-
Individual finger amputations in the
adult at a level proximal to the superficialis insertion (proximal
interphalangeal joint or proximal)
however, there are no methods for replacing the most distal vessels in
the amputated part. Because arteriosclerotic plaques on the intima
often preclude functional patency following the reanastomosis of small
vessels, the arteries of older patients must be thoroughly evaluated
under the microscope before reattachment. In addition, patients with
diabetes mellitus may have diseased blood vessels, which would lessen
the likelihood that repaired vessels will remain patent. Again, the
experienced microsurgeon can determine the potential success of an
anastomosis by studying the vessel structure under a high-powered
microscope.
phalanges and midpalm or at the elbow and above the elbow, in the same
patient), replantation is usually not recommended because the time
commitment is large and the predicted outcome is uncertain (8).
decision regarding replantation. Mental instability is not uncommon in
patients who sustain completely amputated upper extremities, but it is
often difficult to determine the patient’s mental stability during the
limited preoperative assessment stage.
recommend not replanting the isolated finger amputation proximal to the
flexor superficialis tendon insertion. Although the cosmetic result is
excellent, the overall function of the hand is usually not improved; in
fact, this digit sometimes gets in the way. Special considerations,
such as for musicians, young women, and children, do influence
selection, however (72,87).
The patient with a replanted index finger that was amputated at the
base will usually bypass the replanted digit to oppose the thumb to the
long finger.
chances of viability, length of surgery and hospitalization,
anticipated functional outcome, predicted loss of time from work, and
cost compared with an amputation revision. Equipped with all of this
information, most patients and families will insist on replantation;
therefore, the ultimate decision rests with the surgeon. On many
occasions the final decision cannot be made until the vessels of the
amputated part are carefully evaluated under the operating microscope.
essential to achieve success in replantation. Generally, if the warm
ischemia time of the amputated part exceeds 6 h, do not attempt
replantation. If the amputated part contains muscle, the ischemia time
is much more critical. Muscle is extremely sensitive to ischemia,
compared with tissues such as skin, bone, and tendon (22,23).
and bacterial growth. All amputated parts should be cooled during the
transportation to a replantation center. If the part is cooled,
successful replantation is possible with cold ischemia times up to 12
h, or even longer if it is a digit; we have successfully replanted
amputated digits with cold ischemia times up to 30 h. However,
transportation of the amputated part and the patient should be as rapid
as possible.
-
Wrap the part with gauze moistened with
Ringer’s lactate or saline solution and place it in a plastic bag,
which is then placed on ice. -
Alternatively, immerse the part in Ringer’s lactate or saline solution in a plastic bag and place this bag on ice.
-
The part is less likely to become frozen (frostbitten).
-
The part is less likely to be strangled by the wrappings.
-
Instructions are easier to explain to the primary-care physician.
-
Maceration secondary to immersion is not a problem.
so only the equipment essential for replantation surgery is mentioned
here. A great variety of expensive microsurgery instruments are
unnecessary; the appropriate instruments must be available, however,
and the working ends must be well maintained for precision handling of
the small microstructures, fine needles, and suture material (Fig. 34.4) (11,83,88). Basic replantation surgery instruments include the following:
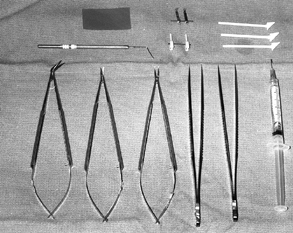 |
|
Figure 34.4. Basic microsurgery instruments for replantation. Left to right, bottom row:
Micro-Pots (angled) scissors, spring-loaded curved tipped microscissors, spring-loaded needle holder, two jeweler’s forceps, 30-gauge blunt needle syringe for irrigation. Top row: Lacrimal duct dilator (below), latex background material (above), microclip approximator (below), Van Beek nerve approximator (above), and small cotton absorbent sponge sticks. |
-
Surgical loupes
of 3.5× to 4.5× are necessary for the initial debridement, exploration,
and dissection of the amputated part and injured extremity. -
An operating microscope
with magnification to at least 20× is essential. Preferably, the
operating microscope should have a beam splitter and a double head that
allows the surgeon and the first assistant to visualize the same
microfield. Foot controls for focusing, zoom magnification, and
horizontal xy movement are ideal. We use the microscope for the repair of any vessels distal to the axilla. -
Microinstruments
should be at least 10 cm long to allow the handles to rest comfortably
in the thumb–index webspace. Instrument handles at least 15 cm long are
recommended for all microsurgery instruments to increase the
versatility of the instruments when operating in deep fields.
-
A spring-loaded needle holder and spring-loaded microscissors
-
Two sets of jeweler’s forceps
-
A microtipped dilator (lacrimal duct dilator)
-
A microirrigator (30-gauge smooth-tipped needle)
-
Various-sizes of (approximator) microclips mounted on some type of bar
disposable type with two plastic tips mounted on a metal sliding bar.
The use of a new clip or microapproximator for each procedure better
assures a constant pressure of less than 30 g/cm. Other helpful
equipment includes:
-
Small hemoclips to tag the vessels and nerves and to use when isolating or harvesting vessels
-
A small-tipped bipolar cautery, essential in isolating and mobilizing the vascular structures to be repaired.
is useful in highlighting the vessels and suture material. I prefer a
yellow background material, particularly if the lighting is low.
suggests the appropriate needle and suture size for amputations at the
wrist level or distal. An 8-0 or even 7-0 suture may be used proximal
to the elbow.
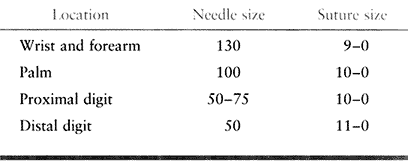 |
|
Table 34.1. Needle and Suture Sizes for Wrist Amputation
|
with bupivacaine, a long-acting local anesthetic. Regional anesthesia
is favored because of the peripheral autonomic block, which increases
vasodilation and peripheral blood flow (25).
Some inhalation anesthetics may result in vasospasm and diminished
peripheral blood flow. General anesthesia is necessary in the
uncooperative patient or for children 12 years old or younger. Keep the
operating room warm at all times to diminish peripheral vasospasm
caused by cooling of the body temperature.
into two subteams (here designated teams A and B) when the patient and
the amputated part arrive in the emergency room. This practice is more
critical with major limb replantations. Surgeons without microsurgical
experience may help prepare the amputated part and patient; however,
they must be experienced in dissecting the small vessels lest
irreparable damage may occur. They may, however, be quite helpful in
the bone trimming, bone fixation, and tendon repair and subsequent
wound coverage.
-
Immediately transport the amputated part
to the operating room for initial cleansing with Hibiclens and sterile
Ringer’s lactate solution. -
Place the prepared amputated part on a
bed of ice covered with a sterile plastic drape. If multiple digits are
involved, these may be cooled by storage in a refrigerator or under ice
packets until the appropriate time for replantation. Care must be taken
not to freeze them. -
Under the operating microscope or with operating loupes (depending on the size of the amputated part),
P.1152
carefully debride the part or parts and identify and tag the nerves and
vessels with small hemoclips. This identification and tagging of the
neurovascular structures will save time when microsurgeons have to work
in a bloody field at a later time. The ease in locating the tagged
structures, particularly in multiple amputations, is definitely
beneficial in the later stages of replantation, particularly if the
procedure is lengthy and fatiguing. -
Make bilateral longitudinal midlateral
incisions, which provide the most rapid and best exposure of the
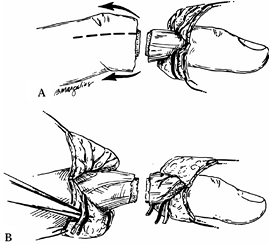
neurovascular structures on the digits (Fig. 34.5).
Place the incisions slightly toward the dorsum so that both the dorsal
and palmar skin flaps can be reflected to locate the veins, nerves, and
arteries with ease.![]() Figure 34.5. A: Bilateral longitudinal midlateral incisions. B:
Figure 34.5. A: Bilateral longitudinal midlateral incisions. B:
Neurovascular bundles exposed palmarly and veins exposed dorsally.
(From Urbaniak JR. Replantation in Children. In Serafin D, Georgiade
NG, eds. Pediatric Plastic Surgery. St. Louis: CV Mosby, 1984:1168.) -
Using magnification, isolate the digital nerves and vessels for a distance of 1.5 to 2 cm and tag them.
-
Identify the dorsal veins on the
amputated part by reflecting the entire dorsal flap of skin and
dissecting the subdermal tissue. In the amputated digit, locating and
labeling the veins may be delayed until after arterial anastomosis,
which makes identification of veins easier because of the backbleeding,
particularly if the patient and amputation stump are ready to receive
the amputated part. If the patient is not yet prepared to receive the
amputated part, however, then search for the veins at this time to
reduce the overall operating time. -
Continue additional debridement after isolation and labeling of the neurovascular bundles.
-
Shorten and trim the bone appropriately on the amputated digit.
-
Insert an intramedullary Kirschner wire
retrograde in the amputated part so the part is prepared for immediate
reattachment. If the bone is closely surrounded by soft-tissue
structures, a 16-gauge hypodermic needle serves as an excellent
protector guide for the 0.45 Kirschner wire.
-
Thoroughly evaluate the patient in the
emergency room with a physical examination, radiographs of the chest
and injured extremity, cardiogram, complete blood count, blood
chemistries, urinalysis, blood type and cross-match, and activated
partial thromboplastin time. -
Begin intravenous fluids and administer
intravenous antibiotics and tetanus prophylaxis if indicated. Insert an
indwelling urethral catheter if a long procedure is anticipated. -
Using magnification and tourniquet
ischemia, debride the stump and locate and label the neurovascular
structures in a manner similar to the preparation used by Team A on the
amputated part. -
It may be difficult to locate the
subcutaneous veins on the stump, particularly for the inexperienced
surgeon. Because successful replantation depends on the patency of an
adequate number of veins, this is a critical point
of the operation. Isolating veins in the digit may be tedious and
requires meticulous dissection and careful handling of these small
structures. After one good vein is located in the subcutaneous layer,
this vein may serve as a guide to direct the surgeon to similar veins
in the same subdermal plane.
replantation will vary slightly depending on the level of amputation
(digit versus levels proximal to the wrist) and type of injury (sharp
versus avulsion). The technique of replanting amputated digits is
described first because these amputations are much more common than the
more proximal amputations. The variations in technique used for major
limb replantations are described subsequently.
 |
|
Table 34.2. Order of Repair for Digit and Hand Replantation
|
not, in haste to reestablish blood flow, compromise on the irrigation
and debridement. A thorough wound debridement is essential. A pulsating jet lavage is useful in severely contaminated major limb replantations.
Excise all necrotic and potentially necrotic tissue, particularly muscle.
It is much easier to make repairs at the time of the initial
reconstruction than to reoperate secondarily through the repaired
structures of the replanted part.
Sufficient bone must be resected to ensure the ease of approximation of
normal intima in the vascular anastomosis. Additionally, bone
shortening allows easier skin coverage of the repaired veins,
particularly on the dorsum of the digits. The amount of resected bone
depends on the type of injury. In an avulsion or crushing type of
injury, more bone must be resected until normal intimal coaptation is
possible without tension. For the digit, it is usually necessary to
resect 5 to 10 mm of bone; in amputations proximal to the hand, 2 to 3
cm of bone resection may be indicated. In the avulsion type of injury,
even more bone resection is done. If it appears that a great deal of
bone must be resected in the digit or in partial hand amputations, use vein grafts to make up the deficit in the arteries and veins rather than doing excessive bone shortening.
shortening is seldom necessary, and instead they recommend vein
grafting when there is considerable intimal damage (52).
Bone shortening should usually be chosen over vein grafting, however,
because there is often concomitant damage to the nerves and other soft
tissues. These injured structures can be more easily managed after bone
shortening. However, do not hesitate to perform vein grafts when they
are indicated (for example, when the loss of a potentially functional
joint may result from bone shortening). This is particularly true for a
child, for whom excessive bone resection should be avoided because it
results in the excision or potential damage to the epiphysis and joint.
Do not hesitate to perform interposition vein grafts when they are
necessary; it is quicker, easier, and less
frustrating to perform an interposition graft than to redo a difficult
anastomosis several times, or even one time.
shortening is done on the amputated part to preserve maximal length on
the stump in case replantation fails. This is not always possible, as,
for example, when attempting to preserve joint function. Several
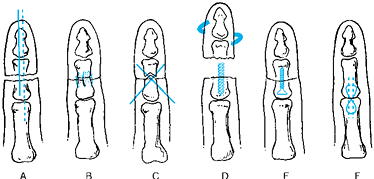
methods of bone fixation are available (Fig. 34.6) (41,79,86,97):
 |
|
Figure 34.6. Methods of bone fixation in replantation. A: Longitudinal intramedullary Kirschner wire or wires (an oblique Kirschner wire may be added to prevent rotation). B: Interosseous wiring. C: Crossed Kirschner wires. D: Intramedullary screw or bone peg. E: Miniplates and screws. F: Tension band technique.
|
-
Longitudinal intramedullary Kirschner wire or wires
-
Longitudinal intramedullary Kirschner wire plus oblique Kirschner wire to prevent rotation
-
Crossed Kirschner wires
-
Interosseous wiring
-
Intramedullary screw or bone peg
-
Miniplates and screws
-
Tension band technique.
replantations; however, certain methods may be preferred in specific
areas, such as near the joint or epiphyseal plates. When possible, it
is preferable to perform bone stabilization in a digit with double
axial Kirschner wires because the lowest nonunion and complication
rates occur with this method (97). This is the
easiest and quickest method when a motorized drill is used for careful
pin placement. A large-bore hypodermic needle (16-gauge) is often used
as a guide to prevent the surrounding soft tissue from
wrapping up near the bone ends. In
more than 1,800 replantations, we have experimented with all methods of
bone stabilization and prefer single or double axial Kirschner wire
fixation for the following reasons:
-
Speed and simplicity of the technique
-
Minimal bone exposure required
-
Minimal skeletal mass needed for fixation
-
Ease of reshortening or readjusting the bone approximation if deemed necessary
-
In children, the likelihood of physeal
damage is minimal with a single well-centered intramedullary
longitudinal pin. A second axial intramedullary pin is frequently used
for better stability (29).
the repaired neurovascular bundles, either by contact or by tethering
of the vessels or the protective retaining ligaments. I prefer crossed
Kirschner wires for arthrodesis of joints. A chevron bone cut aids
stabilization when crossed Kirschner wire fixation or the tension
wiring is used.
metacarpal stabilization in replanting a complete amputation at this
level. This method is easy and rapid and provides immediate stability.
Its use is limited in arthrodesis of other joints because some flexion
is usually desired. Immediate fixation allows early motion of the
digits; however, the screw is difficult to remove if the replanted
digit becomes infected. Bone pegs or other intramedullary devices can
be substituted for the screw. Intramedullary devices, however, are not
favored in highly contaminated wounds.
3, 6, and 9 o’clock in each end of the bone. Then place two strands of
24-gauge wire perpendicular to each other. This provides good stability
(86). It is particularly useful for fractures
in the metaphyseal areas or even for primary joint fusions. Immediate
or early motion is usually possible. It does take slightly more time
than intramedullary pins, but the amputated part can be prepared by
having the wires inserted in one end by one of the teams.
nonunion is rarely a significant problem in replantation of digits.
This method requires extensive bone exposure, with the possibility of
further soft-tissue damage, and it also requires more time. In major
limb replantation, however, it is the preferred method because of the
precision and rigid fixation obtained (10,32,61).
experienced hand surgeon, is obtaining proper digital alignment when
replanting multiple digits. This may present a problem even when
replanting a single digit. Check the relationships of the digits
frequently in extension and flexion. Observe the cascade and rotation
of fingertips; in general, when flexed, the fingertip should point to
the scaphoid bone. Most replantations do survive, so take great care to
achieve anatomic alignment and rotation of the replanted parts. In
adults there are occasional indications for primary silicone implant
arthroplasty with replantations at the joint level, such as an effort
to save proximal interphalangeal joint function in a musician. This is
particularly the case if one of the central digits (long or ring
finger) is amputated at the proximal interphalangeal joint level. It is
actually quicker and easier to perform a silicone implant arthroplasty
than a primary fusion; however, the indications are not common. In
addition to bone stabilization, whenever possible try to repair the
periosteum, capsule, and ligamentous structures to provide better
gliding of joints and tendons and to promote joint stability.
tendons primarily, just after bone stabilization. Use surgical loupes
in extensor tendon repair, and keep in mind the following procedures:
-
Using two horizontal mattress sutures of 4-0 polyester or absorbable suture is sufficient.
-
It is important to repair the entire extensor mechanism, which may include repair of the extensor hood or lateral band.
-
In some severe avulsion injuries, if no
extensor tendons are available for repair, I recommend extensor tendon
grafting as a secondary procedure. -
In the digits it is usually advisable to
perform interphalangeal joint arthrodesis because of the difficulty in
reconstructing the extensor mechanism as well as dealing with other
injuries. -
In children, primary extensor tendon grafting is favored over interphalangeal joint fusion (67,78,84,90).
replantations. Secondary repair is rather difficult because of the
excessive scarring around the repaired nerves, vessels, and joints.
Secondary flexor tendon repair in replanted digits usually requires a
two-stage silicone rod procedure in digits other than the thumb.
Because this procedure amounts to two additional surgical procedures,
it is wise to attempt primary repair as follows.
-
Take care not to provoke spasm or damage to the proximal arteries.
-
Try to retrieve the proximal stumps by flexing the wrist, massaging the palm, or extending the other digit.
-
If these methods are unsuccessful, insert
wall suction tubing in the proximal portion of the flexor sheath, turn
on the suction, and withdraw the proximal stump. -
If all of these methods fail, make proximal incisions to locate the tendons (57).
-
Blind grasping with some type of tendon retriever must not be performed because it may produce irreversible damage to the vital proximal vessels.
repair is to relieve any undue compression or tethering of the proximal
vascular tree that could be caused by proximal retraction of the
severed flexor tendons.
-
The type of material best suited for suture repair is 4-0 polyester on a double-armed curved needle.
-
The Tajima suture method is excellent for primary flexor tendon repair in replantation surgery (Fig. 34.7).
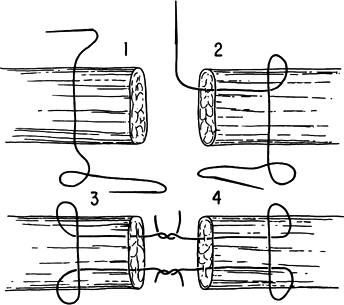 Figure 34.7. The Tajima suture method. (From Urbaniak JR. Replantation in Children. In Serafin D, Georgiade NG, eds. Pediatric Plastic Surgery. St. Louis: CV Mosby, 1984:1168.)
Figure 34.7. The Tajima suture method. (From Urbaniak JR. Replantation in Children. In Serafin D, Georgiade NG, eds. Pediatric Plastic Surgery. St. Louis: CV Mosby, 1984:1168.) -
With the Tajima method, sutures can be
placed in each end of the tendon initially, and the approximation
secured any time during the procedure. -
This delayed coaptation has the advantage
of allowing the digit to be held in full extension for better vascular
and nerve repair, particularly in the area of the proximal phalanx.
digits may require subsequent tenolysis; however, do not do a tenolysis
for at least 3 to 6 months after the primary repair.
be safely performed as long as 3 months after the initial replantation
or revascularization. Usually the two-stage silicone rod method is
indicated.
Anastomose the arteries after bone fixation and extensor and flexor
tendon repair. The recommended number of arteries to repair is as
follows:
-
With the digit, it is advisable to repair both arteries when possible.
-
At the palm or at the wrist level, anastomose all available arteries to increase the chances of survival (56).
Some microsurgeons recommend the repair of only one digital artery to
diminish the operating time; however, we have demonstrated that both
vessels, even in multiple digital amputations, should be anastomosed to
increase the survival rate (52).
-
Free the arteries from their surrounding
connective tissue for a distance of 1.5 to 2 cm. Perform this
dissection under the microscope with microsurgical instruments. Small
branches from the arteries may be safely coagulated with bipolar
cautery. -
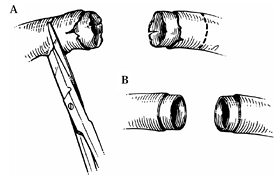
Dissect a severed artery until normal intima is visualized under high-power magnification. Only normal intima must be reconnected (Fig. 34.8).
![]() Figure 34.8. Preparation of the vessel. A: The damaged portion must be resected until normal intima is visualized under high-power magnification. B: Minimal adventitia is stripped to make the lumen clearly visible.
Figure 34.8. Preparation of the vessel. A: The damaged portion must be resected until normal intima is visualized under high-power magnification. B: Minimal adventitia is stripped to make the lumen clearly visible. -
The two most
critical factors in achieving successful microvascular anastomosis are
the surgeon’s skill and expertise and the ease of coaptation of normal
intima to normal intima with minimal tension. -
Assess the most distal and proximal
portions of the exposed vessels to ensure there is no damage to the
media or surrounding adventitia. Such an injury indicates the need for
further arterial resection and vein grafting.
-
After sharply trimming the opened end of
the proximal stump and enlarging it with jeweler’s forceps or a
dilator, an excellent pulsating blood flow must occur. -
Active spurting blood in a digital vessel should produce a persistent stream of at least 5 cm for about 20 to 30 seconds.
-
Do not use this vessel for repair.
-
Take steps to relieve the proximal vasospasm that is present.
-
Be certain that the patient is not
hypotensive, hypovolemic, cold, acidotic, or in pain, because all these
factors introduce unwanted vasospasm. -
Perform thorough irrigation of the proximal lumen with warm heparinized Ringer’s lactate solution to relieve vasospasm.
-
If it is certain that no proximal
mechanical block exists, place papaverine in a 1:20 solution with
normal saline directly on the proximal vessel or gently insert it into
the lumen with a blunt needle. This maneuver usually provides an
excellent pulsatile flow that lasts for at least 30 min. -
Exercise caution when using papaverine or
similar dilating agents because the flow induced may be temporary and
flow may be induced in vessels with damaged intima.
-
Insert the two vessel stumps into the approximating microclip. Coapt the ends without tension.
-
If undue tension is present, use an interpositional vein graft obtained from the palmar forearm.
-
Trim the vessel ends with sharp straight scissors so that no adventitia covers the ends of the lumen.
-
Carefully dilate the lumen of each end with jeweler’s forceps or a lacrimal duct dilator.
-
Irrigate the lumen with heparinized Ringer’s lactate solution and reinspect the intima for complete normalcy.
-
Just before beginning the anastomosis,
give an intravenous bolus of 3,000 to 5,000 U of heparin (decrease this
dose in young children).
-
Apply two stay sutures of 10-0
monofilament nylon 180° apart. Do not tie the sutures too tightly
because this will produce necrosis of the vessel wall. -
The distance from the needle insertion to
the cut end of the vessel should be about one or two times the
thickness of the arterial wall. -
Do not pinch or touch the intima with the jeweler’s forceps.
-
After the application of each knot,
carefully irrigate the anastomosis site with heparinized Ringer’s
lactate and examine it to ensure the suture does not pierce the back
wall. -
After the anterior wall is repaired, turn
over the clip approximator and again examine the lumen under
high-powered magnification to ensure that all sutures are properly
placed. -
Place additional sutures in the back wall in a similar manner.
-
Usually six to nine sutures are required to repair an artery 1 mm in diameter.
-
In the small distal vessels, particularly in children, sometimes only four or five sutures are necessary for the anastomosis.
arterial anastomosis, the fingertip will begin to turn pink if there is
adequate perfusion. Capillary refill should be excellent, and pulp
turgor good. If the amputated part has been without blood flow for
several hours and has been cooled for a prolonged period, a return of
adequate perfusion to the distal part will be delayed. Under these
circumstances, proceed with repair of the digital nerve and the
opposite digital artery. In addition to warming the replanted part,
this waiting period is often all that is necessary to achieve adequate
distal perfusion.
safely for each vascular anastomosis. The tourniquet is helpful in
diminishing blood loss as well as decreasing operating time. If the
microsurgery is skillfully and rapidly performed, the patency rate will
not be affected by the ischemia.
anastomosis to ensure that the anastomosis is patent and blood flow is
strong. The tourniquet may be inflated or deflated many times during
the procedure for nerve, artery, and vein repair.
-
Because all available microclips produce some degree of vessel wall damage, do not apply them for more than 30 min (72).
-
In a crushing or avulsion injury, and if
the procedure is lengthy, consider giving serial doses of heparin (500
to 1,000 U every hour) intravenously. Adjust this dosage for children. -
After completion of the anastomosis, release the microclips; the blood flow across the anastomosis site should be immediate.
may be shifted. For example, in a ring-finger avulsion, the proximal
ulnar digital artery may be shifted to the distal radial digital artery
if these ends are less traumatized. In most instances, however, a vein
graft is used if the distal artery is salvageable (85).
-
For replantation of thumbs that have been
avulsed or amputated, I prefer to use an interpositional vein graft
from the ulnar digital artery of the amputated thumb to the first
dorsal metacarpal artery, or to the dorsum of the hand, for an
end-to-side anastomosis into the radial artery at the wrist. For
expediency, the reversed vein graft is often anastomosed to the ulnar
digital artery of the detached thumb before bone fixation (13). -
The final stage is the connection of the
proximal end of the vein graft into the artery. We use interpositional
vein grafts in about 20% of our replantations to obtain reapproximation
of healthy arteries and veins. The palmar aspect of the wrist contains
veins 1 to 2 mm in diameter that are appropriate for replantation of
digits. -
Hint: If the use of vein grafts is
anticipated, harvest the vein grafts from the forearm in the initial
steps of replantation, using loupe magnification before bringing in the
operating microscope, in an effort to save time.
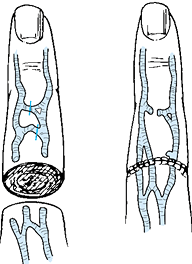
It may be necessary to mobilize or harvest veins to achieve this ratio (Fig. 34.9). Perhaps the greatest error in venous anastomosis is trying to repair veins under tension. If vein harvesting does not allow coaptation without undue tension, then vein grafts must be used. Following this principle will elevate the patency rate and diminish the surgeon’s frustration as well.
 |
|
Figure 34.9.
The mobilization or harvesting of veins is a useful method of achieving ease of approximation for vein reconstruction in a completely amputated digit. An attempt is made to repair two veins for each artery. |
-
The technique of vein repair is similar to that for arterial repair with only a few exceptions.
-
Generally fewer sutures are necessary at
the anastomosis site because the blood flow through the venous system
is not as strong as through the arterial system. -
Because the walls of the veins are more fragile than those of the arteries, minimize the application time of microclips.
-
The distance from the needle insertion to the cut in the vessel should be two to three times the thickness of the wall.
-
Use constant irrigation of the suture line with warm heparinized lactate solution to float the thin walls apart.
-
Do not waste time repairing the very
small veins; instead, connect only the largest veins. One large
repaired vein is more reliable than two smaller repaired veins.
impossible to locate a suitable dorsal vein. Various available options
are as follows:
-
Arteriovenous shunt.
If successful flow through a repaired digital artery is established,
retrograde flow from the unrepaired distal contralateral artery may be
seen. In the absence of a suitable vein in the distal part, connect
this digital artery to a proximal vein (arteriovenous shunt). This
procedure is successful in about half of the attempted cases in
providing sufficient venous drainage (76,93). -
Removal of the nail plate.
Removing the nail plate allows venous oozing from the nail bed. Every 1
or 2 h, have a physician, nurse, or family member stroke the nail bed
with a cotton-tipped applicator to stimulate bleeding from the nail
bed. In the interim, apply heparin-soaked pledgets to the exposed nail
matrix. -
Surgical leeches.
Surgical leeches have been successfully used when venous congestion is
apparent. Place the leeches, which are readily available from various
biological
P.1158
companies,
on the failing part until they become engorged with blood; remove them
and replace with fresh leeches every few hours (12,51). -
Venocutaneous fistula. The venocutaneous fistula technique involves the construction of a temporary venous return bypass using a venous graft (46).
Anastomose the proximal side of the venous graft to a vein at the
dorsum of the stump. Then suture the distal end of the graft to the
skin around a punch wound on the volar side of the replanted fingertip (Fig. 34.10).![]() Figure 34.10.
Figure 34.10.
The venous cutaneous fistula, a technique for reducing venous
congestion in replanted fingertips when distal dorsal veins are not
present. A cut is made in the volar pulp, and the vein graft extends
from this bleeding fistula to an easily located dorsal vein on the
proximal stump. (Modified from Kamai K, Sinokawa Y, Kishibe M. The
Venocutaneous Fistula: A New Technique for Reducing Venous Congestion
in Replantation Fingertips. Plast Reconstruct Surg 99:171;1997.) -
Periodic massage.
Periodic massaging of the distal segment to enhance venous drainage
improves the venous outflow temporarily but is usually not successful
in achieving viability of the part if it must be continued more than a
day or two. -
Skin grafts.
When there is an absence of skin on the dorsum of the replanted digit,
cover the anastomosed veins with a split-thickness or full-thickness
graft. -
Venous flaps. If there is a deficit of veins in addition to subcutaneous tissue and skin, cover the defect by a venous flap (27,42,60,80).
This flap may be based on a proximal pedicle from an adjacent digit or
freely harvested from the dorsum of the hand or foot or volar forearm.
A vein graft with its covering of subcutaneous tissue and skin serves
as a conduit for venous return as well as skin coverage.
arteries to decrease blood loss and maintain a bloodless field for
better vision (57,58).
By judicious use of the tourniquet, however, the artery may be repaired
first and a dry field maintained. This sequence provides the advantage
of earlier revascularization and allows easier location of the most
functional veins, detected by their spurting backflow. Additionally, if
the veins are repaired first, and a subsequent arterial anastomosis
fails to show adequate arterial inflow, the surgeon has wasted valuable
time on a nonsalvageable part.
interphalangeal joint or deep in the thumb–index webspace, the back
wall inside-out technique is favored over the conventional two-stage
microanastomosis (1). Use this method in cases
where limited exposure of the vessel ends may preclude the possibility
of inverting the vessel for back wall repair.
outside in, withdraw it, and pass it from inside out on the other
vessel. Begin the repair with the back wall and, by alternating
sutures, work the knots toward the front wall.
is sutured to the digital artery of the amputated fingertip before bone
fixation. This sequence allows for better exposure and an easier
anastomosis (40).
cements have been developed for small vessel repair, but none has
proven as successful as interrupted suture methods (44,53,72).
because the bone has been shortened, and no tension should be present
at the suture line.
-
Attempt primary repair in all replantations, when feasible.
-
If direct end-to-end repair is impossible, then use primary nerve grafts.
-
The median antebrachial cutaneous nerve of the ipsilateral extremity is ideal for primary nerve grafting in the digital nerve (62).
-
The nerves from a discarded segment such as a severely damaged digit may be used to bridge the gap.
-
The Van Beek nerve approximator (Fig. 34.4) is extremely helpful in repair of nerves in difficult areas.
-
Cut the nerve ends sharply until pouting fascicles are seen on each stump.
-
Align the fascicles on the freshly cut
nerve ends and approximate the ends without undue tension, using 8-0 to
10-0 monofilament nylon or polypropylene (prolene). -
Epineural repair after obtaining geographic fascicular alignment is the best method for the digital nerves.
-
Only two or three epineural sutures are necessary; more sutures are used in proximal injuries, where the nerve is larger.
-
Fibrin glue may be helpful but really does not save time in replantation (44).
-
Loosely approximate the skin with a few
interrupted nylon sutures. Usually the midlateral incisions are not
closed to allow for decompression of the digital vessels. Excise all
potentially necrotic skin, and place no tension on the skin during the
closure. Cover the vessels without constriction from the overlying skin
or sutures. A local flap or split-thickness graft may be necessary,
even for digital vessel coverage. Large defects may be covered by
“flow-through” arterialized venous flaps or pedicled venous flaps (27,42,60,80). Fasciotomies are indicated if the slightest pressure or constriction occurs. -
Cover the wounds with small strips of gauze impregnated with petrolatum or antibacterial grease. Never place these strips in a continuous or circumferential manner around the replanted part.
Although it is preferable not to cover the replanted part with any
dressing or splint, to allow free drainage and early active motion,
this is usually not feasible because some type of protective dressing
is necessary. The ideal dressing is bulky and uniformly compressive but
not constrictive. -
Extend the plaster splints above the elbow to prevent proximal slippage while the hand is being elevated (Fig. 34.11).
Apply plaster splints on one side of the hand (usually on the palmar
aspect) so that the dorsum of the hand can be inspected if there is a
postoperative problem. If flexor tendons have been repaired, however,
place the splints dorsally to prevent unwanted pull of the flexors
against the rigid plaster.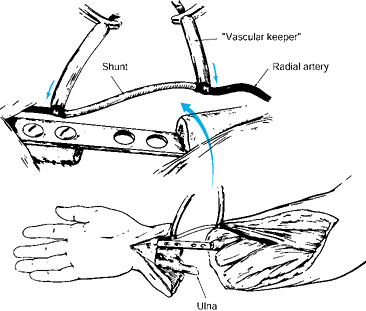 Figure 34.11.
Figure 34.11.
Digital temperature is monitored with a telethermometer and small
surface probes. These quantitative skin temperature measurements have
proven to be the most reliable indicators of replantation status. -
Then elevate the extremity in the bulky
compressive dressing by a soft (Styrofoam) cradle boot. Keep the
fingertips exposed for application of temperature probes and frequent
clinical observation. The environment of the bulky compressive dressing
seems to be well suited to the replanted part: often, blood flow
increases to the fingertips at the conclusion of the dressing
application. -
Do not change the dressing for about 10
days (particularly in children) because anxiety and discomfort produced
by the dressing change often incite potentially irreversible vasospasm
in the replanted part (22). If there is
excessive bleeding into the dressing, change the dressing immediately
to prevent constriction from the blood-soaked dressings.
lower extremity) is less common than amputation of digits or parts of
the hand. In replantation of limbs amputated proximal to the wrist
level or of amputations of a lower extremity below or above the knee,
principles similar to
those
for digit replantation are used with minor modifications. The major
difference is related to the increased amount of muscle tissue
involved. Because more muscle mass is present in a major limb
amputation, the duration of ischemia of the detached part is more
critical. Whereas amputated digits that have been detached for 30 to 36
h may be replanted with a high degree of success, an amputated arm at
the elbow area is in jeopardy if it has been avascular for 10 to 12 h,
even with appropriate cooling. Rarely are major limbs cleanly severed;
therefore, the muscle damage is usually quite severe. Extensive muscle
debridement of both the detached part and the proximal stump is
essential to prevent myonecrosis and subsequent infection. This
problem, associated with major limb replantation, seldom occurs in
digital reattachment (46).
-
If the amputated part and the patient
arrive in the operating room more than 4 h after injury, initiate
immediate blood flow into the detached part. This is best accomplished
by using some form of shunt, such as a Sundt (Huger Shulte Corporation,
Galeta, CA) or ventriculoperitoneal shunt, to obtain rapid arterial
inflow from the proximal vessel to the detached part (61) (Fig. 34.12).![]() Figure 34.12.
Figure 34.12.
A Sundt or ventriculoperitoneal shunt is used to obtain rapid arterial
inflow from the proximal vessel to the amputated part. This is
particularly useful for major limb amputations that arrive in the
operating room more than 4 h after injury. (From Urbaniak JR.
Replantation in Children. In Serafin D, Georgiade NG, eds. Pediatric Plastic Surgery. St. Louis: CV Mosby, 1984:1168.) -
Perform shunting before bone fixation
unless the bone can be rapidly stabilized and early blood flow
obtained. After establishing temporary blood flow, complete the
debridement, stabilize the bone, remove the shunts, and perform a
direct arterial repair or interposition vein grafting of the vessels.
-
For major limb replantation, the best
bone fixation is obtained with plates and screws. Cross-pin fixation of
the joint is usually the best method for stabilizing the amputations at
the joint level. -
Carefully plan adequate bone shortening
relative to the type of injury, tissue damage, proximity of the
epiphysis, and the particular limb involved. Usually the plate may be
secured to the amputated part after the initial debridement and then
fixed to the stump after blood flow has been established through the
shunts (if the ischemia time has been longer than 10 to 12 h).
injuries with extensive muscle and other soft-tissue damage, a great
deal of soft tissue, including skin, muscle, tendon, and nerve, must be
excised if the replanted part is to survive without infection. It is
therefore prudent to shorten the bone considerably when possible.
This sequence permits a physiologic washout of noxious agents, such as
lactic acid, in the distal part. If this order is reversed, with venous
repair first, the return of toxic metabolites to the systemic
circulation can cause serious consequences, even death. Giving
intravenous sodium bicarbonate before venous anastomosis is beneficial (23).
replantation. A common error is failure to decompress the replanted
limb adequately, leading to myonecrosis with subsequent infection.
Meshed split-thickness skin grafts provide some coverage of exposed
vessels. Other areas may be closed primarily or grafted several days
later.
-
In replantations of major limbs, return
the patient to the operating room within 48 to 72 h to evaluate the
state of the muscle tissue. -
Change the dressing with the patient under general or regional block.
hospitalized for 7 to 8 days. Major limb replantations, of course,
require longer periods, depending on the severity of the injury.
Intelligent, careful postoperative management is essential to achieve a
high success rate in replantation surgery (30).
and keep it warm at all times. The patient should stay in a warm,
comfortable room during the first week. If the recovery room is cooler
than normal room temperature, place a patient heater or a warmer over
the patient. Keep the patient’s room at a minimum of 72°F (22°C) and
prevent cool air drafts.
controversial. A few surgeons use none or perhaps only aspirin and di-
pyridamole; some use all or various combinations of anticoagulants (11,52,57,104). We prefer some type of anticoagulation for all patients.
amputations that are replanted or if the anastomosis was technically
easy and the blood flow was immediately brisk. In these patients, we
use aspirin, 300 mg, and dipyridamole, 50 mg, twice daily as well as
500 mg of intravenous low-molecular-weight dextran for 1 week. In
addition, we give chlorpromazine, 25 mg three or four times daily, for
a tranquilizing effect as well as to provide peripheral dilation. This
drug is valuable in relieving vasospasms secondary to anxiety, which
occurs particularly in children. In children, the dose is reduced to 10
mg three to four times daily by mouth.
intravenous heparin, 1,000 units every hour, in adults for 7 days.
Adjust this dosage in children, giving about 100 U/kg for 4 h. Adjust
this according to the activated partial thromboplastin time, which is
maintained at 1.5 times normal. If bleeding occurs into the dressing,
adjust the dosage and change the dressing immediately to prevent
constriction. Do not use heparin prophylaxis in amputations proximal to
the wrist level. Give appropriate antibiotics for 1 week.
The normal skin temperature of the digits is 31° to 35°C. Remember that
ambient temperature may influence the interpretation of the recordings.
Probes are usually placed on the replanted digits, on a normal digit
for control, and on the dressing for measurement of the ambient
temperature. An abrupt or even gradual change in temperature indicates
that the replant may be failing, and steps must be taken to improve the
blood flow. If the temperature drops below 30°C or shows more than a 2°
drop when compared with the normal digit, appropriate methods of
management must be undertaken immediately.
level or desire of the patient. If the patient is anxious and chooses
to remain in bed or appears to be having difficulty with blood flow to
the replanted part, then bed rest is advised for 3 to 5 days. If the
patient is vigorous or energetic, however, allow activity a day or two
after the replantation. Permit no smoking by the patient or in the
patient’s room, and all efforts should be made to keep the patient
tranquil (15).
because the dressing is not changed for at least 10 days, however,
immediate early motion is limited. In the patient who has had a
replantation distal to the superficialis insertion, encourage movement
of the digit the following day because there is really no stress on the
flexor or extensor tendons. Otherwise, begin protective active motion
against rubber bands and flexor and extensor outriggers, supervised by
a hand therapist, 1 to 2 weeks after the replantation. Especially for
replantation patients who live far from the treatment center, if more
than one digit has been replanted or a major limb has been replanted,
keep the patient in the hospital or in a nearby motel so that daily
therapy can be supervised by hand therapists.
-
Replantation in children under 10 years of age
-
Crush and avulsion injuries
-
Ring avulsion injuries
-
Poor proximal flow observed before the arterial anastomosis
-
Intermittent or inconsistent distal flow despite a technically good anastomosis
postoperative efforts are necessary to enhance the chances of survival.
Intravenous heparin is particularly beneficial in these difficult
replantations.
a regional sympathetic block is particularly helpful. Insert a #5
silicone urethral stent beside the median or ulnar nerve (depending on
the digits revascularized or replanted) to permit regional block to be
given in the postoperative course. The catheter exits from the dressing
and has a stopcock attached so that a nurse can give 5 mL of 0.25%
bupivacaine hydrochloride every 6 to 8 h. This drug provides regional
block anesthesia to diminish vasospasm and alleviate pain. This
procedure is particularly rewarding in children because it obviates the
use of axillary or brachial block if postoperative spasm develops. In
the postoperative period, we have rarely given an axillary or brachial
block since the early 1980s.
immediate postoperative period, as indicated by decreased skin
temperature, loss of capillary refill, diminished pulp turgor, or
abnormal color, immediately take the following steps:
-
Inspect the dressing to rule out any type of constriction. Administer intramuscular narcotics prior to inspection.
-
Depress or elevate the hand to improve the vascular status, depending on whether the problem is arterial or venous.
-
Give an intravenous bolus of heparin (3,000 to 5,000 U), which often incites recovery.
-
Give chlorpromazine to diminish anxiety and decrease vasospasm, if necessary.
-
Ensure that the patient is adequately hydrated and that the hematocrit is normal or near normal.
-
Alter the environment of the patient’s room (for example, increase the temperature and remove smokers or other agitating factors), if necessary.
-
Make all efforts to calm the patient, especially a child, as pain, fear, and anxiety may initiate peripheral vasospasm.
management measures, return the patient to the operating room. Once
this decision is made, do so within the first 4 to 6 h after the loss
of adequate perfusion. Seldom have we found reexploration to benefit
the patient if it occurs more than 1 to 2 days after replantation. If
the patient is returned to the operating room within the first 12 to 48
h, however, many potential failures can be salvaged by redoing the vein
graft, removing the thrombus, and usually grafting a previously
unrecognized damaged vessel segment.
replantations, loss of a replanted digit is rare secondary to
infection. The most common infections are pin tract infections, which
usually occur more than 4 weeks after the replantation and are easily
managed by removing the pins and putting the patient on antibiotics. We
have seldom found it necessary to admit a patient to the hospital for
pin tract infections.
replantations. The presence of large amounts of muscle that have been
rendered ischemic increases the likelihood of infection. To ensure
adequate muscle debridement, return all major limb replantations to the
operating room in 48 h for a “second look” procedure. At this time,
inspect the wounds thoroughly and debride any muscle necrosis that has
progressed since the first procedure. Infections in major limb
replantations may not become apparent until the second week (most
commonly in 8 to 10 days); if the infection involves the vascular
anastomosis, failure is almost inevitable.
patients who have undergone digital replantations. However, cold
intolerance is not unique to digital replantation and occurs with
almost equal incidence in other severe hand injuries, including
amputations that are not replanted (29). Cold
intolerance is related to the adequacy of digital reperfusion; for this
reason, try to maximize the number of arterial repairs. Cold
intolerance diminishes with time and usually resolves by 2 years (91). Pain is rare in successfully replanted limbs.
repaired in replantations, tendon adhesions are common, with diminished
motion. If this is severe, tenolysis may be performed as early as 3
months after replantation.
replantation. These complex injuries require multiple tendon, vessel,
and nerve repairs, and if care is not taken during bone fixation,
rotational malunion may occur.
Every
effort should be made to properly align digits before replantation.
Another form of malunion can occur in multiple digit replantations for
which it is difficult to identify the various digits at the time of
surgery. It may become apparent later that one digit has been exchanged
for another. This is primarily a cosmetic problem and does not result
in any diminished function.
80% viability in replantation of completely severed parts. Our team has
achieved 77% viability in replantations and 92% in revascularizations
of more than 1,800 amputations.
the experienced and proficient microsurgeon should be able to achieve
at least an 80% overall viability rate in replantations. The results,
based on our own long-term follow-up as well as reports from major
replantation centers, should be as follows:
-
The active range of joint motion should be about half of normal, depending on the level of injury.
-
Cold intolerance is a definite problem that usually subsides within 2 years.
-
Nerve recovery is comparable to the repair of an isolated, severed peripheral nerve (19).
-
Cosmesis is usually better than any amputation revision or prosthesis (29).
-
Near-normal growth may be anticipated in
amputations through the diaphyseal region of children. If the injury
involves the epiphyseal plate, growth will almost always be retarded,
although excessive growth has been reported (24,35,67). -
The best results are obtained in
replantations of the thumb, a finger distal to the insertion of the
superficialis tendon, and the hand at the wrist level.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
H, Shieh S, Hsu H. Multivariate Analysis of Factors Influencing the
Functional Recovery after Finger Replantation for Revascularization. Microsurgery 1995;16:713.
NK, Gerostathopoulos N, Efstathopoulos D, et al. Major Amputation of
the Upper Extremity. Functional Results after
Replantation/Revascularization in 47 Cases. Acta Orthop Scand [Suppl] 1995;264:7.
RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital Replantation at the
Level of the Distal Interphalangeal Joint and the Distal Phalanx. J Hand Surg 1989;14:214.
RB, O’Brien BM, Morrison A, MacLeod AM. Survival Factors in
Replantation and Revascularization of the Amputated Thumb—10 Years’
Experience. Scand J Plast Reconstruct Surg 1984;18:163.
SER, Adrichem LNA, Mulder HD, et al. Comparison of Laster Doppler
Flowmetry and Thermometry in the Postoperative Monitoring of
Replantations. J Hand Surg 1997;38:151.
N, Cooley BG, Kamiishi J. Clinical Outcome of Digital Replantation
using the Fibrin Glue-Assisted Microvascular Anastomosis Technique. J Hand Surg 1996;21B:573.
TF, Arnex ZM, Solinc M, Zaletel-Kragelj L. One Hundred Sixty-Seven
Thumb Replantations and Revascularisations: Early Microvascular
Results. Microsurgery 1996;17:259.
K, Sinokawa Y, Kishibe M. The Venocutaneous Fistula: A New Technique
for Reducing Venous Congestion in Replantation Fingertips. Plast Reconstruct Surg 1997;99:171.
JD, Kleinert HE, Tsai T-M. Methods and Results of Replantation
Following Traumatic Amputation of the Thumb in 64 Patients. J Hand Surg 1980;5:63.
People’s Hospital, Shanghai. Replantation of Severed Fingers: Clinical
Experience in 162 Cases Involving 270 Severed Fingers. Pamphlet July
1963.
Y, Ishikawa K, Isshiki N, Takami S. Fingertip Replantation with an
Efferent A-V Anastomosis for Venous Drainage: Clinical Reports. Br J Plast Surg 1993;46:187.
N, Sharzer LA, Starker I. Replantation and Revascularization at the
Transmetacarpal Level: Long-Term Functional Results. J Hand Surg 1990;15A: 328.
S, Nomura S, Yoshimura M, et al. A Clinical Study of the Order and
Speed of Sensory Recovery after Digital Replantation. J Hand Surg 1983;8:45.
T, Katsumi M, Tajima T. Replantation of Untidy Amputated Finger, Hand
and Arm: Experience of 99 Replantations in 66 Cases. J Trauma 1978;18:194.
C, Meyer VE, Kleinert HE, Beasley RW. Present Indications and
Contraindications for Replantations as Reflected by Long-term
Functional Results. Orthop Clin North Am 1981;12:849.