Spondylolysis and Spondylolisthesis
first to discuss the clinical significance of spondylolisthesis in a
report detailing a difficult delivery in a woman with slippage of the
5th lumbar vertebra on the sacrum (1). He described spondylolisthesis as a lumbosacral dislocation. The actual term spondylolisthesis was not coined until 1854 by Kilian (2). The word spondylolisthesis arises from the Greek roots spondylos, which means “vertebra,” and listhesis, meaning “slippage” or “movement,” referring to the forward slipping of one vertebra on the adjacent caudal vertebra. Spondylolysis
is a term used to refer to an isolated defect in the posterior elements
of the vertebra, specifically the pars interarticularis. The term
originates from the Greek roots spondylos, which means “vertebra,” and lysis,
meaning “break” or “defect.” The spondylotic defect is most common at
the L-5 vertebra in the pediatric and adolescent patient, and it can
secondarily result in spondylolisthesis, with L-5 slipping anteriorly
on S1.
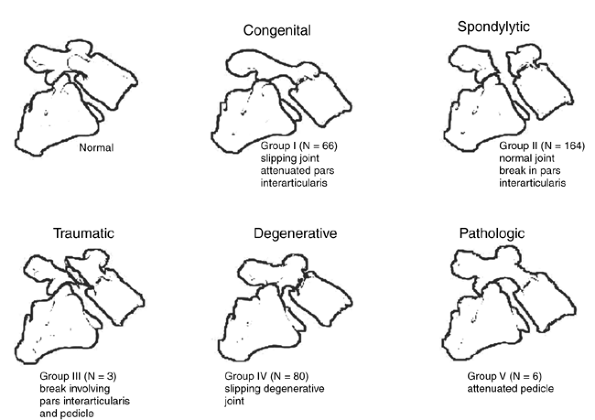
appearance and potential causality, they created a five-part
classification scheme. The slippage in group I, termed the “congenital”
type, was attributed to the attenuation of the pars interarticularis
with more transverse orientation of the facets than is normal, allowing
slippage of one vertebra on the next caudal level with an intact neural
arch. Patients in group II, termed “spondylytic,” were believed to have
morphologically normal L-5–S1 articulations, but a lytic lesion at the
pars interarticularis, permitting L-5 to slip forward on S1. Patients
in group III, the “traumatic” type, were also believed to have
morphologically normal L-5–S1 facet joints, with a discontinuity of the
pars interarticularis caused by an acute fracture creating the
instability. The condition of patients in group IV was classified as
“degenerative.” The authors hypothesized that arthritic changes in the
facet joints allowed slippage of the degenerative facet joint,
resulting in a spondylolisthesis. The condition of patients in group V
was labeled as “pathologic,” and they were believed to have attenuated
pedicles caused by a generalized or localized bone disease resulting in
the development of spondylolisthesis. Wiltse et al. later refined and
reorganized Newman and Stone’s classification, and at present this is
the best-known classification system (Table 21.1) (4,5,6).
Both of these classification systems rely on the radiographic
appearance of the anatomy to classify the spondylolisthesis, making no
attempt to be predictive of the likelihood of progression of the
condition. Only two of these types, specifically Wiltse Types I and II,
occur in children and adolescents and are the focus of this chapter.
 |
|
Figure 21.1 Newman and Stone classification of spondylolisthesis in 319 patients.
|
classification that relied primarily on identification of developmental
characteristics rather than the observed radiographic pathology (Table 21.2) (7).
The initial version of their classification divided spondylolisthesis
into “developmental” and “acquired” pathology. Developmental pathology
included lytic lesions, spondylolisthesis caused by elongation of the
pars interarticularis, and spondylolisthesis secondary to traumatic
events such as acute fractures or stress fractures. The acquired
pathology group consisted of iatrogenic, pathologic, and degenerative
conditions. In 1994, Marchetti and Bartolozzi restructured and refined
their classification system (8). In the updated
scheme, the developmental category was further subclassified into high
and low dysplastic groups, and within each of these dysplastic groups
the par interarticularis could be further described as osteolytic or
elongated. The acquired pathology group was further expanded to include
postsurgical (previously named iatrogenic),
pathologic, and degenerative conditions; traumatic lesions were moved
from the developmental to the acquired pathology category. The
classification scheme of Marchetti and Bartolozzi stresses the
importance of the developmental aspect of the pathology, highlighting
dysplasia of the posterior elements as a significant factor in the
development and progression of spondylolisthesis.
children and adolescents, the congenital or dysplastic group is the
less common. This type of spondylolisthesis occurs in a 2:1 ratio of
girls to boys (9,10) and accounts for between 14% and 21% of the overall cases, according to several published reports (9,11). Most congenital/dysplastic spondylolistheses do not progress beyond 50% slippage, mainly on account of
the severe symptoms created by the intact posterior bony neural arch
compressing the neural elements against the posterior margin of the
next caudal vertebral body. The children with congenital/dysplastic
spondylolistheses are at higher risk for neurologic injury (e.g., cauda
equina syndrome) than are those with isthmic spondylolisthesis.
|
TABLE 21.1 CLASSIFICATION OF SPONDYLOLISTHESIS BY WILTSE ET AL.
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
Although most published series combine both types of spondylolisthesis
(isthmic and congenital/dysplastic) with spondylolysis, most data in
the literature refer to the isthmic type of spondylolisthesis. Some
have suggested that the isthmic spondylytic defect may be caused by
congenital factors. With one exception (12), a defect in the pars interarticularis has never been found at birth (13,14,15,16,17,18).
In fact, the pathology seems to be rare in patients younger than 5
years, with only a few cases reported in children younger than 2 years (15,16,18,19).
Fredrickson et al. reported on the natural history of spondylolysis and
spondylolisthesis in a review of 500 children in the first grade. The
prevalence of spondylolysis was 4.4% at 6 years of age, increasing to
the adult rate of 6% at 14 years of age (13).
In addition, they documented and associated spondylolisthesis in 68% of
the 5-year-old children, which increased to 74% in adulthood; the
authors also implied that the development of spondylolisthesis after
the age of 6 years in children with spondylolysis is infrequent. Only 7
patients in
this
series developed further slippage; all slippages were minimal, and none
of the patients complained of pain. Virta et al. identified a 2:1 ratio
of occurrence in boys and girls (20).
In their review of 1,100 individuals in Finland ranging in age from 45
to 64 years, Virta et al. reported a 7% incidence of spondylolisthesis
in a population of individuals who had radiographic evaluation for back
pain.
|
TABLE 21.2 CLASSIFICATION OF SPONDYLOLISTHESIS BY MARCHETTI AND BARTOLOZZI
|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||
influenced by the racial or genetic background of the population
studied. African Americans have the lowest rate of spondylolisthesis,
1.8%, whereas Inuit Eskimos have a prevalence of 50%. South Africans
and whites fall in an intermediate range, 3.5% and 5.6%, respectively (21,22,23).
Rowe and Roche report a difference in the incidence of
spondylolisthesis depending on sex and race: for the male sex, the
incidence is 6.4% in whites and 2.8% in African Americans, and in the
female sex, it is 2.3% in whites and 1.1% in African Americans (24).
The role that gender plays in the natural history of spondylolisthesis
is illustrated by the fact that, despite the twofold higher number of
men the high-grade slips are four times more common in women. (For
grading of spondylolisthesis, see the Meyerding measurements presented
in Fig. 21.9 and the section in this chapter
entitled “Slip Percentage.”) Osterman et al. noted in their report that
the lower grades of spondylolisthesis are far more common at the time
of presentation: grade I, 79%; grade II, 20%; grade III, 1% (22). A slightly higher incidence of more severe slips has been reported by other authors (25).
There is also an increasing prevalence of spondylolisthesis in
individuals who participate in active sports, especially in physical
activities that accentuate lumbar lordosis. Gymnasts have long been
identified as an at-risk group for development of spondylolisthesis.
Jackson et al. noted an 11% incidence of bilateral pars
interarticularis defects in 100 female gymnasts (28).
An even higher rate of spondylolysis or spondylolisthesis has been
identified in a similar population of Asian female gymnasts. Other
sports involving chronic repetitive hyperextension, including college
football (specifically, linemen), weight lifting, and rugby also have
been implicated in the development of spondylolysis (29,30,31). Other sports implicated in the development of spondylolisthesis include pole vaulting and volleyball (5,32,33,34). Generally, the course of this pathology is relatively benign (35).
Saraste in a 20-year follow-up study of 255 patients with spondylolysis
and spondylolisthesis (35). In this study, 40%
of adults showed no progression of the slip, and 40% showed an
additional 1- to 5-mm slip. Spondylolisthesis was much more common than
spondylolysis, with approximately 22% of patients initially presenting
with only spondylolysis. Significant progression of the slip occurs in
a low percentage of cases, occurring in 4% of patients in the series
studied by Frennered et al., in 5% of the cases studied by Saraste, and
in 3% of the 311 patients in the series studied by Danielson et al. (35,36,37). In the series of Fredrickson et al., progression was shown to be unlikely (1.4%) after adolescence (13), whereas other authors have reported progression, attributed to disc degeneration, during adolescence (23,38,39).
Beutler reported a long-term follow-up of patients with
spondylolisthesis and documented that the progression of the slippage
declined with each decade (40). Various studies
in the literature have reported that women are more likely to present
at a younger age, and are at greater risk of slip progression in
higher-grade spondylolisthesis, and of having posterior element
dysplasia and lumbosacral kyphosis of more than 45 degrees (11,13,41,42,43,44).
In patients with pre-existing lumbar spondylolisthesis, traumatic
injuries usually do not aggravate the condition. Floman et al. reported
on 200 patients with thoracolumbar trauma and documented that major
axial skeletal trauma had little or no effect on pre-existing lumbar
spondylolisthesis (45).
the likelihood of progression of the spondylolisthesis. Some
researchers have associated the degree of slip at presentation with a
greater chance of slip progression (46,47,48), but others have not (35,36).
In the growing child, the amount of spondylolisthetic kyphosis or of
the slip angle, especially when severe, is associated with progression.
Other morphologic changes found with high-grade slips, for example,
dome-shaped sacrum and trapezoidal L-5, are secondary or adaptive
changes to the slip and have not been prognostic for slip progression (36).
remains unclear. A truly congenital etiology seems unlikely because,
with one exception (12), no evidence exists for the presence of the lytic pars interarticularis defect in the newborn (13,14,15,16,17).
Studies by Vaz et al., Legaye et al., and Labelle et al. suggest that
the intrinsic architecture of the pelvis may be an important parameter,
modulating the mechanical stresses experienced by the lumbosacral
junction (49,50,51).
This is confirmed by the higher incidence of spondylolysis in certain
sports, previously mentioned, and in Scheuermann disease (52). In addition, spondylolysis has not been reported in adults (average age of 27 years) who have never walked (53), suggesting that mechanical factors associated with upright posture may play a role.
along with the increased prevalence of spondylolysis and
spondylolisthesis among athletes who participate in sports involving
hyperextension, strongly suggest a mechanical etiology to the
development of spondylolisthesis (3,33,34,54,55,56).
Several authors have postulated that a fracture is the underlying
pathomechanical event in the development of a lytic spondylolisthesis (34,57,58,59,60,61). This may be either an acute traumatic event or secondary to an insidious fatigue failure during repetitive stress (62).
Wiltse et al. theorized that spondylolysis is a stress fracture in the
pars interarticularis, specifically due to repetitive microtrauma or
microstresses, with inadequate healing (63).
Although an acute fracture is the obvious cause of the acute traumatic
type of spondylolysis, more commonly patients present after a traumatic
episode. The impact of a traumatic event can be difficult to assess in
some patients.
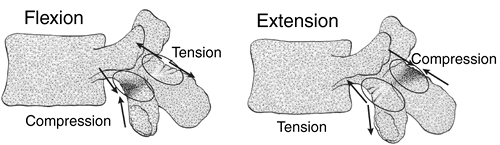
During flexion and extension, the pars interarticularis is cycled
through alternating compressive and tensile loads. During extension,
the pars interarticularis experiences posterior compressive forces and
anterior tensile forces (Fig. 21.2) (64).
The ability of the pars interarticularis to resist the compressive and
tensile forces during flexion and extension depends on the thickness of
the cortical bone (65). The overall resilience
of the pars interarticularis is undoubtedly high, as evidenced by the
generally low prevalence of spondylolisthesis in the population.
However, Hutton et al. have shown that fatigue fractures can be
precipitated during biomechanical testing (60,66).
Using 507 N of force at 100 cycles per minute, pars interarticularis
fractures could be created with as few as 1,500 cycles. Other cadaveric
specimens were able to tolerate 54,000 cycles before developing a
fracture.
ability to resist shear stress has been well-documented; in contrast,
the role of the intervertebral disc is less well understood. In the
intact, morphologically normal spinal motion segment, the
intervertebral disc contributes 60% of the total shear resistance (67).
A skeletally immature animal model of shear load forces demonstrated
that, in spines with pars interarticularis defects, the end-plate
(apophyseal ring) most likely was responsible for the anterior
listhesis (66,67).
Kajiura et al. confirmed these findings and demonstrated that the
increasing strength of the growth plate during skeletal maturity is the
likely reason for the infrequent occurrence of further slippage after
the completion of growth (68). In addition, the
slippage through the ring apophysis, causing a growth disturbance, can
lead to the development of a trapezoidal olisthetic vertebral body or
sacral rounding.
 |
|
Figure 21.2 Compressive and tensile forces experienced in the region of the pars interarticularis during flexion and extension.
|
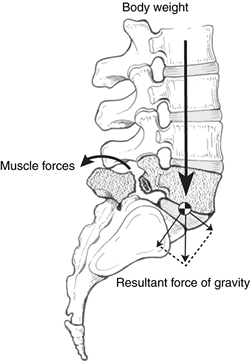
anatomic and biomechanical forces conspire to prevent spontaneous
healing of the fracture (Fig. 21.3). The shear
forces created by the body’s center of gravity tend to cause anterior
displacement of L-5 on the sacrum because of the effects of gravity,
muscular activity, and body movement. The posterior muscular forces
tend to extend the posterior elements, thereby tending to open the
spondylolytic defect and create the spondylolisthesis. These initial
events tend to precipitate a cascade of worsening biomechanics as the
center of gravity moves progressively anterior, causing a vector that
increases the shear forces at the lumbosacral junction. This situation
may be exacerbated by a low intercrestal line and small transverse
processes of L-5, resulting in muscular and ligamentous connections
between the pelvis and the spine that are not robust enough to resist
the forward slippage of the rostral vertebrae on the caudal vertebrae.
Loder has demonstrated in children with higher grades of lumbosacral
spondylolisthesis that the sacrum becomes more vertical as the slip
worsens (69). When the sacrum becomes more
vertical there is an increase in the thoracic lordosis; this is likely
an adaptive mechanism to maintain the normal upright posture.
significant factors in the development of lytic spondylolisthesis,
genetic considerations have been discussed by some researchers (70).
Familial studies have documented a high incidence (19% to 69%) of
spondylolysis and spondylolisthesis in first-degree relatives of
children with spondylolysis and dysplastic or isthmic spondylolisthesis
(16,71,72,73,74).
Wynne-Davies and Scott noted an increased incidence of dysplastic lesions in affected relatives (74).
First-degree relatives of patients with the dysplastic form of
spondylolisthesis had a prevalence of 33%, compared to 15% for isthmic
spondylolisthesis. These authors have suggested an autosomal dominant
genetic predisposition, multifactorial and with reduced penetrance.
Wiltse, on the other hand, suggested that a cartilaginous defect in the
vertebral analogue may be an autosomal recessive characteristic with
varying expressivity (56).
 |
|
Figure 21.3 Forces that affect distraction of spondylolytic defect at L-5.
|
 |
|
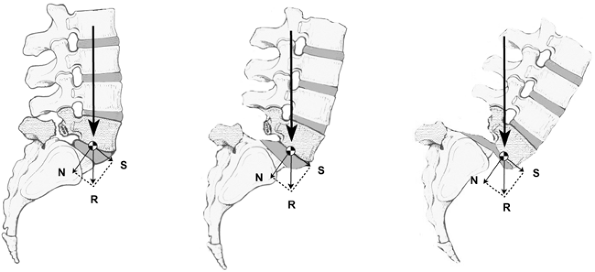
Figure 21.4 The alteration in pathomechanics as a spondylolysis proceeds from a low-grade spondylolisthesis to a high-grade slip.
|
predisposition to develop spondylolysis or spondylolisthesis may be
exacerbated by functional anatomic factors. The human bipedal gait
causes L-5 to be precariously balanced on the sacrum. In the “best
case” scenario, the anterior and posterior opposing forces are
neutralized so that L-5 remains solidly atop the sacrum in spite of its
inclined position; however, anything that unbalances these opposing
forces may precipitate a spondylolisthesis (Fig. 21.4).
Posterior elements already compromised by spina bifida or dysplastic
lumbosacral facet joints may not withstand even normal daily
activities. A low intercrestal line in a patient with short L-5
transverse processes may provide less robust muscular and ligamentous
attachments between the spine and the pelvis, predisposing the patient
to a spondylolisthesis. Undoubtedly, the etiology of spondylolisthesis
is multifactorial. However, mechanical forces are highly implicated
both in the development of lytic pars interarticularis defects and in
the development and progression of spondylolisthesis.
these must be distinguished from pain secondary to a spondylolisthesis.
Although back pain is often a presenting symptom in spondylolisthesis,
many asymptomatic spondylolytic defects are identified incidentally on
spine or pelvic radiographs. Spondylolisthesis incidentally discovered
during screening for low back pain after trauma is typically a stable,
chronic entity, probably not a result of the trauma and presenting
little, if any, risk of a catastrophic structural instability that
would result in neurologic sequela (45). Mild-to-moderate spondylolisthesis does not necessarily predispose to low back pain (75).
In spite of this, spondylolisthesis is found to be two to five times
more frequent in patients with low back pain. Patients with symptomatic
low back pain have a spondylolisthesis rate of 5.3% to 11%, whereas in
asymptomatic patients occult spondylolisthesis may occur in 2.2% (76).
Libson et al. have documented a twofold increase in the incidence of
spondylolisthesis in patients with symptomatic low back pain, compared
to asymptomatic patients (77). Wiltse and
Rothman identified 11% of 1,124 patients undergoing lumbosacral
radiographic examination for back pain as having either unilateral or
bilateral pars interarticularis defects (6).
Saraste described radiographic features that correlated with low back
symptoms: slip of greater than 25%, L-4 spondylolysis or
spondylolisthesis, and early disc degeneration at the level of the slip
(35). The most common period for the
spondylolysis and spondylolisthesis to become symptomatic is during the
adolescent growth spurt, between the ages of 10 and 15 years. However,
the degree of the deformity does not always match the degree of pain (35).
diagnostic and therapeutic process; although radiographic
investigations are important in defining the pathoanatomy, treatment is
typically based on the patient’s symptoms, history, and physical
examination. The presence of a spondylolisthesis should not be presumed
to be the cause of the patient’s back and/or leg symptoms. Muscular
strain induced by poor sagittal alignment and poor muscular tone could
also be the cause (78). Symptomatic
spondylolisthesis typically presents with back pain and/or neurologic
symptoms. Knowledge of the location and duration of the symptoms and
their association with various activities can be useful in developing a
causal relation between the radiographic pathology and the patient’s
symptoms. It is important to determine whether the pain is acute,
chronic, or an acute exacerbation superimposed on a chronic condition.
Progression of low-grade non-dysplastic slips in older adolescents is
uncommon, and it can be difficult to decide whether the symptoms are a
direct result of the spondylolysis or spondylolisthesis. The patient’s
exercise and activity history, as well as the quality and severity of
pain provoked by that activity, should be defined. The pain is usually
a dull, aching, low back discomfort and is localized to the low back
with occasional radiation into the gluteal region and posterior thighs.
This pain is most likely due to the instability caused by the pars
interarticularis defect, and is generally exacerbated by participation
in athletic or other physical activity, and relieved by rest or
restriction of activities. In a few cases, the pain may also follow an
acute
traumatic episode, usually involving hyperextension during athletic participation.
child’s posture or gait, usually noted by his or her parents, with or
without accompanying pain. This can be present in mild degrees of
spondylolisthesis, but is much more common in more marked degrees of
slip. Additionally, these patients may present with scoliosis. As the
degree of slip increases, the corresponding pain may cause a
muscle-spasm-induced atypical scoliosis. Concomitant rotatory
displacement of the spondylolisthetic segment can also create an
olisthetic curve. Conversely, the presenting symptoms may be adolescent
idiopathic scoliosis, with the spondylolysis or spondylolisthesis
detected incidentally on the radiographic evaluation of the scoliosis.
from radiculopathy. Radicular pain is atypical in the pediatric
patient, being more common in the adolescent and adult (44,79).
If present, aggressive treatment of the radiculopathy should be
undertaken along with management of the low back pain. The neurologic
symptoms that accompany spondylolisthesis may be either unilateral or
bilateral radiculopathy, and may be either intermittent or chronic. In
patients with spondylolisthesis and significant degenerative disease,
the resulting neuroforaminal compression may cause chronic
radiculopathy or neurogenic claudication. In addition, even in patients
with low-grade slips that are hypermobile, radiculopathy may be a
presenting complaint. Posterior to the nerve roots, the
fibrocartilaginous scar or hypertrophic callus that forms around the
lytic defect may be responsible for much of the neuroforaminal
compression. Anterior to the nerve roots, annular bulging of the disc
that results from the vertical collapse of the disc space and the
anterior translation of the rostral vertebral body may cause a
significant compression of the nerve root against the caudal surface of
the pedicle. Although the radiculopathy is usually caused by either
central or neuroforaminal stenosis at the level of the lytic defect and
impingement of the nerve roots exiting at the level of the defect,
compression of traversing nerve roots may cause radiculopathy in more
distal roots/dermatomes. Because spondylolysis and spondylolisthesis
are most common at L-5, the nerve roots most typically involved are at
L-5 and S1. In spite of this, the presence of radiculopathy in a
patient with radiographically documented spondylolysis or
spondylolisthesis should not cause the clinician to exclude other
possible etiologies for the radiculopathy, such as a disc herniation,
especially far lateral disc herniations. In patients who have central
stenosis with or without neuroforaminal narrowing, neurogenic
claudication may be the presenting neurologic symptom. This is also
common in patients with larger slips or slips that are hypermobile and
worsen with extended periods of standing. Although uncommon, high-grade
slips may be accompanied by acute or chronic cauda equina syndromes.
Hypermobile, high-grade slips may present as intermittent neurologic
symptoms.
determining the etiology of the back pain or radiculopathy. The mere
presence of a spondylolisthesis does implicate it in the patient’s
symptoms. Important physical examination parameters include body
habitus, coronal and sagittal alignment, and spinal mobility. The
physical examination findings depend on whether pain is present, as
well as on the degree of spondylolisthesis. In patients with
spondylolysis and mild spondylolisthesis, the back and gait
examinations may be completely normal, with no hamstring tightness.
With increasing degrees of spondylolisthesis there is usually some
degree of hamstring tightness. This may significantly restrict
straight-leg raising and forward bending, and may create postural and
gait changes. The compensatory increased lumbar lordosis caused by the
spondylolisthetic kyphosis creates a flattening of the buttocks
(“heart-shaped”), shortening of the waistline, a protuberant abdomen,
and a waddling-type gait pattern or Phalen-Dickson sign (11,47,80).
Some have attributed the hamstring tightness to signs of nerve root
irritation; in any case, the exact mechanism remains unclear but
typically resolves after solid bony fusion (80,81).
with a prominent L-5 spinous process. Palpation of the lumbosacral
region may also elicit a localized area of tenderness. In addition, the
child with a severe slip tends to stand with the hips and knees flexed
because of the anterior rotation of the pelvis, with the gait
examination demonstrating a shortened stride length caused by the
patient’s inability to extend the hips. Both static and dynamic
examinations are important for eliciting pertinent symptoms. Pain on
flexion and extension, with limitation of these motions, may suggest
hypermobility as the cause of the pain.
but on occasion may reveal a diminished or absent ankle deep-tendon
reflex or weakness of the extensor hallucis longus. Sphincter
dysfunction is very rare (82). Provocation of
neurologic symptoms during dynamic assessment, particularly
radiculopathy in a particular position, may also imply the presence of
hypermobility. Neurologic symptoms that correlate dermatome and myotome
levels with the level of stenosis or lytic instability are also
corroborative of the contribution of the spondylolisthesis to the
development of symptoms. Scoliosis, which may be seen at the time of
the presentation, is of the typical idiopathic type or, where there are
more advanced grades of decompensation, may be caused by reflexive pain
or spasm (“olisthetic scoliosis”). A thorough evaluation is essential
to rule out other causations of the individual’s pain and/or neurologic
findings, such as tumors of bone, spinal cord, conus or cauda equina,
disc herniation, and disc-space infection.
completely document the three-dimensional pathoanatomy of spondylolysis
and spondylolisthesis (83). Each modality
contributes a unique view of the various aspects of the pathology.
Typically, plain radiographs are obtained as the initial imaging
modality. These films should be obtained with the patient in an
upright, preferably standing position. Films of the patient supine may
not show subtle instability (84). A complete
plain radiographic investigation of a potential pars interarticularis
defect or spondylolisthesis includes the following views: routine
posteroanterior, spot lateral, right and left oblique, Ferguson
anteroposterior, and flexion-extension lateral. In addition,
long-cassette (14″ × 36″) anteroposterior and lateral thoracolumbar
scoliosis radiographs may be useful for documenting overall coronal and
sagittal alignments (Fig. 21.5).
identifying certain aspects of the pathology. The routine
posteroanterior and Ferguson anteroposterior projections may show spina
bifida occulta, pars interarticularis defects, lumbar scoliosis, or
dysplastic posterior elements (85). The lateral
views often allow identification of a pars interarticularis defect even
when a spondylolisthesis is not present. Oblique views will often
better define the pars interarticularis defect, also known as the collar on the well-known “Scotty dog” (Fig. 21.6). The diagnosis of spondylolysis may be missed in 30% of symptomatic young patients if a lateral radiograph alone is obtained (86). The Ferguson anteroposterior provides an en face
view of L-5 that may improve the visualization of the transverse
process and the sacrum and may more clearly identify a high-riding L-5
vertebral body. Flexion-extension views may uncover subtle
instabilities that are not apparent on static standing views.
Instability will almost certainly be underappreciated if only supine
views are obtained. Other important anatomic features that can be
identified on plain radiographs are rounding off of the anterior corner
of the sacrum, wedging or erosion of L-5 in higher-grade
spondylolisthesis, flexion at the S1-S2 disc, and bending of the sacrum
(87) (Fig. 21.14A). In
cases of a unilateral defect, the only finding may be sclerosis of the
facet, lamina, or pars interarticularis on the intact side opposite the
defect, secondary to increased bony stresses.
 |
|
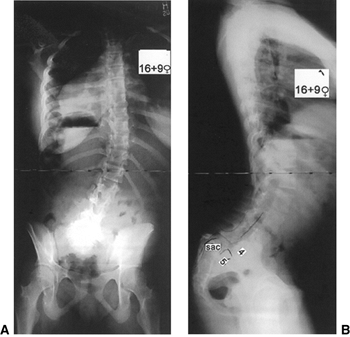
Figure 21.5 Long-cassette upright posteroanterior (A) and lateral (B) radiographs show olisthetic scoliosis and also marked forward sagittal vertical axis (SVA).
|
a distinct history of trauma, a bone scan may be useful for detecting
an acute fracture of the pars interarticularis or for excluding a bony
tumor. Bone scans may also provide information about the metabolic
activity, enabling the evaluation of whether the lytic defect will
heal. The most sensitive technique is a single-photon emission computed
tomography (SPECT) scan because of the improved detail that it provides
(88). An intensely “hot” SPECT scan suggests
that the defect is metabolically active and could benefit from a period
of immobilization or, failing this, direct osteosynthesis. A “cold”
SPECT scan, on the other hand, implies that the lytic defect is chronic
and metabolically inactive. These defects, when symptomatic, are not
amenable to nonsurgical treatment such as immobilization. Symptomatic
lytic lesions of the pars interarticularis that respond to local
anesthetic injections may be amenable to fusion or repair (89).
situations in which, although a pars interarticularis defect is
strongly suspected on clinical evaluation, it is not identifiable on
the lateral or oblique radiographs. The imaging of the pars
interarticularis defect will be optimal if the cuts are no greater than
1.5 mm apart; otherwise the defect may be missed. CT scans are useful
in order to clearly define the bony architecture of the posterior
elements (90). They can delineate the pars interarticularis defects in the axial plane even when no spondylolisthesis is present (Fig. 21.11 C,D).
On the axial images, the spondylolytic defect is identified as a linear
lesion of varying width with sclerotic osseous margins and hypertrophic
osteophytes. The lytic defect is usually identified in the axial image
either at, or immediately inferior to, the axial image containing the
pedicles of the involved vertebrae. CT scans can also provide excellent
visualization of complex anatomy in the
coronal
and sagittal planes when reformatted images are obtained. When combined
with myelography, CT scans provide excellent definition of even subtle
neurologic compression and are particularly useful for visualizing
high-grade slips and for patients with complex deformities. If a CT
myelogram is anticipated, then plain myelogram films should be obtained
at the time the CT scan is done in order to maximize the information
garnered. Plain myelography is useful in identifying the longitudinal
effect of either the spondylolisthesis or the intercanal compromise,
which may be secondary to hypertrophic bone and the fibrous cicatrix
formed as a result of the instability at the level of the pars
interarticularis defect.
 |
|
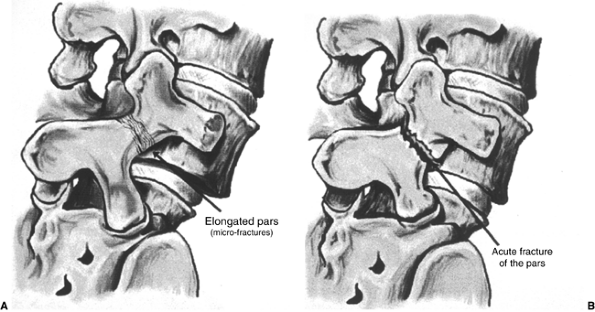
Figure 21.6 Sketches of an elongated pars interarticularis (A) and an acute fracture of the pars interarticularis (B) across the neck of the “Scotty dog.”
|
modality for the evaluation of the soft-tissue component of the
spondylolisthesis and can also help define the degree of associated
degenerative disc disease (39,91).
Degenerative disc disease at, above, or below the slip level may be the
cause of the patient’s pain because of nuclear degeneration or annular
injury. The MRI excels at visualizing the neural elements and the
surrounding soft tissue, and it is the optimal modality when there is a
neurologic deficit or the symptoms suggest a diagnosis other than
spondylolysis or spondylolisthesis. MRI may not be as precise as CT
myelography in distinguishing these soft tissues from the osseous
elements of the pathology in high-grade slips; however, this small
drawback is offset by the fact that MRI studies do not involve ionizing
radiation, myelographic dye that could precipitate an anaphylactic
reaction, or invasive techniques. MRI studies can identify both central
and foraminal stenosis (on parasagittal views) and provide a good
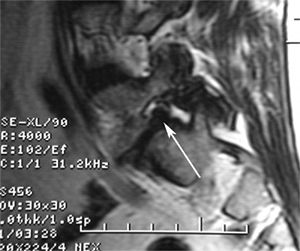
indication of the degree of neural compression (Fig. 21.7).
A consistent finding on MRI, especially in moderate- to high-grade
slips, is a large, bulging disc at the level of the spondylolisthesis,
trapping the exiting nerve root between the bulging anulus fibrosus and
the pedicle above. In addition, the MRI can document the degree of
encroachment on the neural elements by often
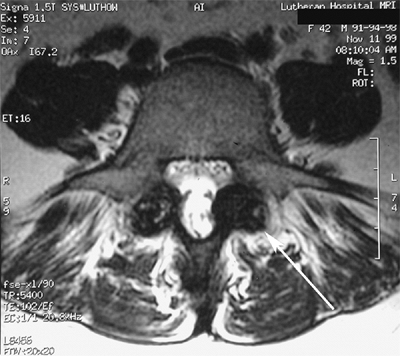
exuberant hypertrophic scar tissue which forms at the spondylolytic defect (Fig. 21.8).
The degeneration of adjacent discs can also be discerned by reviewing
the MRI. Often, in spite of the relatively normal appearance on plain
radiographs or CT scans, the MRI may document premature degeneration of
adjacent discs. The significance and the etiology of the degeneration
of these adjacent discs are unclear. It is likely that abnormal
biomechanics at the spondylolytic level probably result in increased
biomechanical stress at adjacent levels, which may, in turn,
precipitate degenerative disc disease at an accelerated rate. The MRI
and CT scans are also useful in identifying facet joint hypertrophy and
degeneration at the level of the slip and adjacent levels, as these
factors may also contribute to the patient’s low back pain or
discomfort.
 |
|
Figure 21.7 Parasagittal reconstructed magnetic resonance imaging (MRI) view of lytic spondylolysis at L-5.
|
 |
|
Figure 21.8 Axial magnetic resonance imaging (MRI) of bilateral pars interarticularis overgrowth (arrow) with foraminal narrowing.
|
intervention. When a pars interarticularis repair is contemplated and
the health of the involved disc is not certain, discography may provide
useful information about its functional quality. If a segmental fusion
is required because of severe disc degeneration at the level of a pars
interarticularis defect, and MRI shows degenerative changes at the
adjacent level, discography may be helpful in deciding whether the
fusion should include the adjacent degenerative level. Practically,
however, we rarely carry out discography in pediatric patients.
lumbosacral junction, consists of anterior translation of L-5 on S1,
with obligatory forward rotation of L-5 on S1 into lumbosacral
kyphosis. The degree of slip can be quantified using the Meyerding
classification, the percentage of slip described by Boxall et al. (Fig. 21.9), or the Newman classification which also describes angular slippage (Fig. 21.10A) (3,11,89,92,93).
Sagittal rotation, slip angle, and sacral inclination are all direct
measurements of the amount of lumbosacral kyphosis, and are assessed on
spot lateral radiographs of the lumbosacral area taken with the patient
in standing position (94).
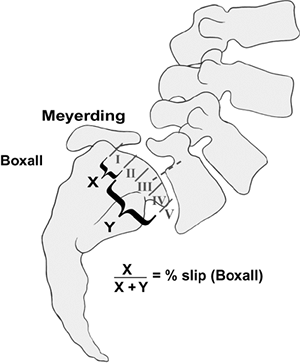 |
|
Figure 21.9 Meyerding and Boxall measurement techniques for grading spondylolisthesis.
|
grade 0 (spondylolysis) through grades I–IV (spondylolisthesis) and V
(spondyloptosis), is probably the most functional and widely used
technique (92) (Fig. 21.9).
The amount of anterior translation of the olisthetic vertebra on the
caudal level is measured at the posterior vertebral body line. This
classifies the spondylolisthesis into five grades: grade I (slip of 1%
to 25%), grade II (slip of 26% to 50%), grade III (slip of 51% to 75%),
grade IV (slip of 76% to 100%), and grade V (spondyloptosis).
Higher-grade spondylolistheses have been shown to be predictive of
spondylolisthetic progression (95). Boxall et al. describes a slip percentage which is more precise but requires exact measurements (96).
On the radiograph, a line is drawn along the posterior border of the
sacrum, and a perpendicular line is drawn at the upper end of the
sacrum. The anterior displacement of the posteroinferior corner of L-5
from the line along the posterior border of the sacrum is quantified as
the numerator. The width of S1 forms the
denominator,
and the slip is expressed as a percentage. In the situation of a
rounded superior end plate of S1, the anteroposterior width of L-5 is
used instead.
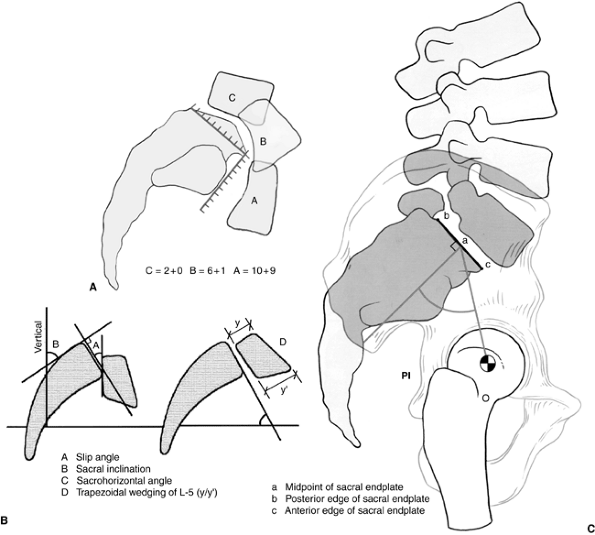 |
|
Figure 21.10 A:
Modified Newman grading system, combining horizontal measurements as the first number with vertical measurements as the second number. B: Radiographic parameters for angular measurements in the description of spondylolisthesis. C: Radiographic parameters and pelvic incidence (posterior instrumentation). |
inability to describe the important rotational component in the
sagittal plane of the subluxing rostral vertebrae. The modified Newman
classification takes this into account (Fig. 21.10A).
In this classification system, measurements are taken of both the
anterior displacement (first number) and the vertical/downward
displacement of the vertebral body in relation to the sacrum (second
number). The superior end plate and the anterior face of the sacrum are
divided into 10 equal segments. The first number is the position of the
posteroinferior corner of the L-5 vertebra with respect to the superior
end plate of S1, and the second is the position of the anteroinferior
corner of L-5 relative to the anterior surface of S1. A score by this
method utilizes both numbers, for example; 7 + 5, with the “7”
indicating the amount of sagittal slip and the “5” indicating the
amount of angular roll of L-5 over the sacrum.
continuous scale of 0 to 20 to be applied to each spondylolisthesis,
uniquely describing anterolisthesis and the degree of caudal migration
of the rostral vertebrae (3,89,93).
On the radiograph, a perpendicular to the line drawn at the posterior
cortex of the sacrum forms the sacral measuring line. A second line is
drawn along the inferior end plate of L-5, and the angle formed by
these two lines is the slip angle which, in the normal condition, is in
lordosis and is expressed by a negative number (94). Boxall et al. (11)
have used the line along the inferior edge of L-5 for their
measurement, but this edge is often difficult to visualize accurately,
and when slippage is considerable, the vertebral body is often
trapezoidal in shape. In such a situation, use of the inferior end
plate as reference may increase the measured slip angle by erroneously
adding the measurement of the kyphosis and the wedging of olisthetic
vertebra. Slip angles of greater than +45 degrees (kyphosis) correlate
with an increased risk of slip progression (11,36,48,95).
and is the angle between the posterior cortex of the sacrum and the
posterior cortex of the L-5 vertebral body. This sagittal rotation
angle (SRA) should approximately equal the slip angle measured as
described previously. In higher degrees of slip (translation or
angulation), L-4 may show retrolisthesis on L-5. In severe slips of
greater than 50%, the slip angle of L-4 in relation to the sacrum
should also be measured, as this will be the new lumbosacral slip angle
if surgical management is to be an L-4 to S1 fusion.
line along the posterior cortex of the sacrum and measuring the angle
between that line and a vertical line from the floor (a line drawn
parallel to the edge of the x-ray film) (Fig. 21.10B).
Normal sacral inclination is greater than 30 degrees; however, with
higher-degree slips, the sacrum usually becomes more vertical and
sacral inclination decreases.
sacropelvic and hip joints. Pelvic incidence is the angle between a
perpendicular-to-superior end plate of S1 and a line from the center of
the superior end plate of S1 to the center of the femoral head (Fig. 21.10C).
In normally aligned individuals, the gravity line should pass through
the hip joints. Increased pelvic incidence has been shown to correlate
with the degree of slippage (51,96,97).
level and are more common in high-grade slips. In L-5-S1
spondylolisthesis, the superior end plate of S1 undergoes bony
remodeling with resorption of the anterior lip of S1, creating a
rounded, dome-shaped surface. The cephalic level, usually the fifth
lumbar vertebra, becomes trapezoidal, specifically more narrow
posteriorly and wider anteriorly. The amount of L-5 wedging can be
measured in terms of the lumbar index, with references to the height of
the anterior aspect of the L-5 vertebra expressed as a percentage of
the height of the posterior aspect (Fig. 21.10B). Greater slips tend to have lower lumbar indices, and slip progression is more common with lower indices (13,35).
spondylolysis or spondylolisthesis fall into two main groups:
nonsurgical and surgical. The nonsurgical options include observation,
activity modification, physiotherapy, bracing or casting, and oral
medications. Surgical options include repair of a pars interarticularis
defect, fusion, decompression, reduction of the slip, or a combination
of these. The challenge lies in selecting the appropriate treatment for
each patient. In making this choice, one must consider the patient’s
symptoms, age, slip angle and grade, causation, and physical findings
(especially neurologic signs). The recommendations of Wiltse are still
generally accepted for treatment of children with spondylolysis or
spondylolisthesis (98) (Table 21.3).
-
Asymptomatic grades I and II: If the
child is less than 10 years old, follow up with radiographs every 6
months through 15 years, then annually until end of growth. No
limitations on activity are necessary in grade I. Patients with grade
II slips should avoid contact sports and activities requiring
repetitive lumbar hyperextension. -
Symptomatic grades I and II: Nonoperative
therapies should be tried. Contact sports and those calling for
hyperextension should be avoided. Fusion is indicated for patients who
are unresponsive to all nonoperative interventions. -
Grades III/IV: Surgical intervention is indicated regardless of symptoms.
|
TABLE 21.3 TRADITIONAL RECOMMENDATIONS OF TREATMENT
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
spondylolysis are asymptomatic. In the patient with minimal to no
symptoms, the follow-up will depend upon the child’s age and growth
potential. In the adolescent who has completed growth, no follow-up is
necessary. In the growing child, however, lumbar radiographs should be
obtained at the end of growth to monitor any potential for slip
formation. The child with an asymptomatic spondylolysis can be allowed
to participate in all sporting activities without restriction. In the
long term, most young patients with spondylolysis, nonoperatively
managed, maintain good functional outcome up to 11 years after
diagnosis (99). In fact, the unilateral defects
can undergo bony healing which may take up to 12 weeks; however, the
bilateral defects may undergo degeneration, with mild slip over time (100).
Beutler et al. reported on a 45-year follow-up of patients with
spondylosis and spondylolisthesis. Patients with unilateral pars
interarticularis defects never experienced slippage over the 45-year
period (40).
spondylolysis there is typically a long history of low back pain
associated with activity. Restriction of activity is recommended along
with physiotherapy emphasizing abdominal muscle and spinal extensor
muscle strengthening, with short-term bedrest reserved for only the
most exceptionally symptomatic patient. When the patient is
asymptomatic, physical activities are gradually resumed.
determining the duration of the child’s symptoms. Patients with
increased radiotracer uptake at the pars interarticularis during a
SPECT bone scan are typically classified as having undergone an acute
injury or fracture. In cases in which the scan is positive at the pars
interarticularis in a patient with more acute onset of symptoms,
immobilization in a cast or brace can be used for symptom abatement,
and to hasten healing at the pars interarticularis defect. The ideal
method is to immobilize this area with a body cast including one
thigh/leg in order to appropriately control the pelvis. Another less
onerous option is to use a thoracolumbar sacral orthosis (TLSO) with
thigh cuff extension. The use of a removable brace is better accepted
by the patient and family and will allow the individual to perform
strengthening exercises out of the brace. The downside to a removable
orthosis is the uncertainty of the patient’s compliance with wearing
the brace. Regarding healing of the pars interarticularis defect,
varying levels of success have been described, with “healing” occurring
in 3 to 4 months and typically being documented with oblique plain
radiographic views or repeat bone scans (100,101,102,103).
In the patient with a cold SPECT scan the use of a TLSO can be an
option if the back pain is bothersome; however, the goal in this
situation is not healing of the pars interarticularis defect, but
rather, the elimination or diminution of symptoms. Bracing for
approximately 3 months along with marked activity modification usually
results in complete resolution of symptoms. Once the patient is
asymptomatic, regardless of the status of the pars interarticularis
defect, gradual resumption of athletic activities can be initiated
without restriction. In cases that do not respond to nonoperative
treatment, surgery can be offered as a possible alternative.
young adults and adolescents. The ideal candidate has a spondylolysis
of less than a full grade I slip and no degenerative disc disease at
the olisthetic level, and has failed a full course of nonoperative
treatment of the symptoms, including immobilization. Given such
restrictive criteria of selection, this technique should be used
cautiously in patients beyond the adolescent years. The use of pars
interarticularis injections preoperatively can assist verifying that
the defect is the sole cause of the back pain. An MRI is necessary to
assess the involved intervertebral disc and vertebral end-plate in
order to identify any degree of disc degeneration or end-plate
destruction which would preclude a successful outcome (23,104).
He reported on 16 patients, out of whom 15 underwent fusion with this
technique. One patient required salvage with a posterolateral fusion
following failure of the pars interarticularis repair. Several other
surgical techniques have also been described (107,108,109,110). Pedersen and Hagen reviewed 18 patients treated with Buck’s technique and reported 83% satisfactory results (111).
Like Buck, they recommend pars interarticularis repair only in young
patients with no degenerative disc disease. Bradford and Iza presented
a technique of transverse process wiring bilaterally to fix the loose
posterior element and to facilitate pars interarticularis
osteosynthesis (112,113).
This technique has been modified with placement of pedicle screws as
anchor points for the wiring, rather than the transverse processes. Of
the 21 (of 22) cases available for follow-up, 90% obtained solid fusion
of the pars interarticularis defect, and 80% had a good or excellent
result. Bradford and Iza were also of the opinion that the technique is
best suited for patients younger than 30 years without degenerative
disc disease (113). This construct facilitates
a compressive osteosynthesis across the laminar defect. A sublaminar
hook/pedicle screw technique has been demonstrated to achieve improved
control over the fracture fragments, compared to the less predictable
laminar or spinous process wiring technique (Fig. 21.11).
This improved technique includes replacing the posterior wire with
bilateral, sublaminar hooks connected to the pedicle screws by a short
rod. This facilitates direct compression across the lytic defect and
provides improved control of the loose
posterior
element. In two small series of patients treated with sublaminar
hook/pedicle screw constructs, 70% to 100% demonstrated clinical pain
relief (107,108,114).
The direct pars interarticularis repair is ideal for spondylolysis at
the L-4 level and above, because it preserves lumbar motion segments.
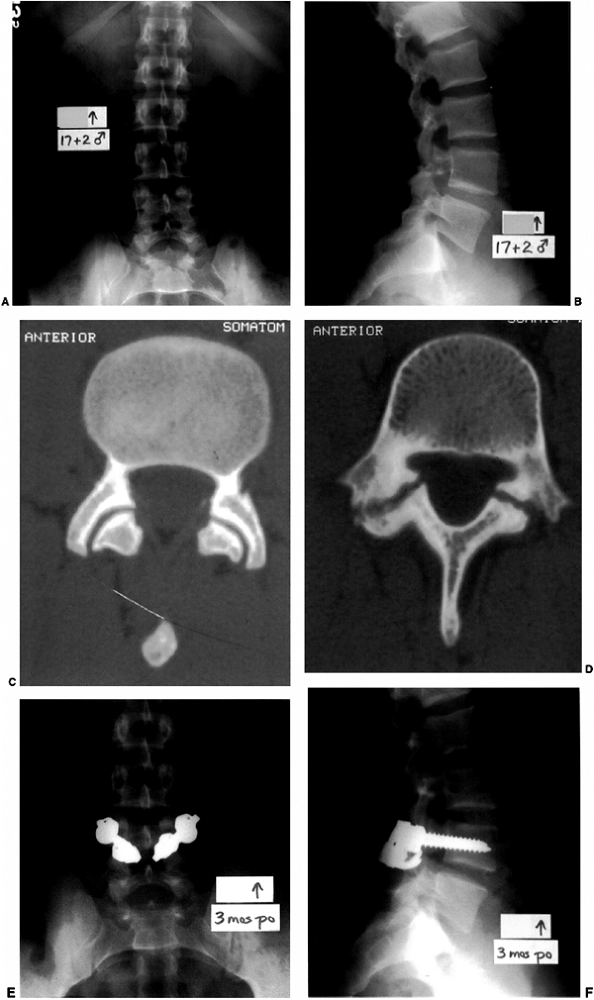 |
|
Figure 21.11 A and B:
A boy 17 years and 2 months old with a history of lumbosacral back pain and an associated L-4 bilateral spondylolysis. Normal facet joints at L-3–L-4 (C) contrast with the pars interarticularis defects of L-4 situated immediately caudal to the pedicles (D). E, F: Following the failure of conservative treatment, and because the magnetic resonance imaging (MRI) showed absence of disc degeneration at L-4–L-5, he underwent an instrumented pars interarticularis repair at L-4. This was performed with bilateral L-4 pedicle screws connected to infralaminar hooks at L-4, with compression forces applied across the pars interarticularis defects that were grafted with autogenous iliac crest bone. He experienced marked relief of lumbosacral pain postoperatively. |
in patients who are unresponsive to nonoperative treatment and who are
not candidates for direct pars interarticularis repair. Traditionally,
this has been performed through a midline skin incision with an
intertransverse process to sacrum fusion, utilizing autologous iliac
crest bone graft as described by Wiltse and Jackson (10).
Postoperative immobilization is dependent upon the surgeon’s preference
and various parameters relating to the patient. Spica casting for 3
months has been advocated on the basis of reports documenting high
levels of good and excellent outcomes (11,115,116,117,118,119,120). However, others report good results with no immobilization (103), or immobilization in a corset (121) or Boston brace (122).
The use of posterior spinal instrumentation (i.e., pedicle screw) is
gaining acceptance, because it usually obviates the need for external
immobilization and can rigidly maintain intraoperative correction.
Overall, though, it is extremely rare for patients to require a fusion
for a spondylolysis that fails conservative treatment.
fall into two categories: nonsurgical and surgical. The nonsurgical
options consist primarily of observation, activity modification,
bracing, and physiotherapy, and intervention in the form of medications
or injections (123).
Nonoperative management regimens have generally included observation,
activity modification, bracing, and physiotherapy, and several reports
have documented good short-term and long-term results (13,16,36,44,124,125,126,127).
Optimal candidates for successful nonoperative management appear to be
those with spondylolysis and low-grade spondylolistheses (128).
skeletally immature child is kept under observation for progression
with lateral lumbosacral radiographs taken with the patient in the
standing position. A slip of less than 50% in an asymptomatic mature
adolescent is also placed under observation. Observation is continued
if there is no change in the slip angle or the amount of the slip;
however, if there is progression of the spondylolisthesis or persistent
symptoms, surgical stabilization is indicated.
proper bending and lifting activities and development of a sustained
aerobic activities program. This program should aim at decreasing
recumbent and sitting activities in favor of aerobic activities in
order to facilitate achieving ideal body weight. A growing child who
presents with low back pain and a spondylolisthesis grade I or II is
advised to limit all physical activities which exacerbate the low back
pain. Resumption of almost all activities is possible after symptoms
have resolved.
This usually requires a period of 6 to 12 weeks in the brace. Once the
symptoms are relieved, activities can gradually be resumed.
Although passive modalities may be useful initially, when there is
acute pain, active physical therapy techniques are probably more
important in the long term. Examples of passive techniques are thermal
therapy, massage, phonophoresis, ultrasound, immobilization,
acupuncture, traction, and transcutaneous electrical nerve stimulation.
These may facilitate the patient’s acceptance of an active physical
therapy program by ameliorating acute symptoms. Active physical therapy
includes spinal flexibility exercises and muscle strengthening,
especially abdominal and posterior lumbar muscles. Pelvic stabilization
techniques are also important; these may involve isometric and
isokinetic exercises as well as aerobic conditioning.
be important. Nonsteroidal antiinflammatory drugs should be instituted
early and are the mainstay of drug therapy. Because the use of muscle
relaxants and narcotics remains controversial, we tend to avoid
prescription medication in children and teens, if at all possible. If
medications are to be used, the patient must be informed that narcotics
will not be prescribed beyond a week or two, and their purpose is to
facilitate the transition to physical therapy for managing the low back
pain. Muscle relaxants may likewise be useful in the early, acute
period to deal with muscle spasms secondary to injury.
persistently symptomatic spondylolisthesis which does not respond to
nonoperative management, and which causes pain that prevents normal
participation in daily and desirable physical activities. Additionally,
the skeletally immature patient with slippage greater than grade II or
the mature adolescent with a slip of more than 75% should be treated
surgically even in the absence of symptoms (42,47,124,129).
Surgical treatment options for symptomatic spondylolisthesis include
decompression, fusion, or a combination of these techniques. In grade I
and early grade II isthmic spondylolisthesis, the use of in situ
posterolateral fusion is well established. However, for higher grades
of spondylolisthesis, the decision-making process becomes more complex,
involving decisions about the number of levels that should be fused,
whether to aim for partial or complete slip reduction, whether to
include anterior fusion, and whether to use instrumentation and
postoperative immobilization.
adolescents and perhaps also in young adults, pars interarticularis
repair is a reasonable option if the caveats previously mentioned are
followed. In particular, the disc at the slip level should be lordotic
and without degenerative changes. Any degree of spondylolisthesis
indicates the likelihood of disc degeneration or end-plate destruction,
making MRI a necessity if a direct pars interarticularis repair is
being considered.
motor weakness, and a sensory deficit, and is confirmed with further
imaging studies. No correlation has been found between tight hamstrings
and the objective neurologic findings of weakness, sensory deficit, or
changes in reflexes (11). In an L-5-S1
spondylolisthesis, the L-5 root is usually implicated; the compression
occurs at the foraminal level, between the proximal part of the pars
interarticularis as it slips forward with the vertebral body, or is
caused by the fibrocartilaginous tissue at the pars interarticularis
defect. True nerve root compression is an indication for formal nerve
decompression. However, the presence of hamstring tightness is not by
itself necessarily a sign of nerve root compression.
be necessary if radicular or neurogenic claudication symptoms are
present. Decompression alone may be a useful technique in patients with
spondylolysis or a low-grade (grade II or less) spondylolisthesis when
the symptoms are primarily neurologic and there is little evidence of
instability. This situation is more common in adults than in children
or adolescents. However, even patients with presumed stability and
little back pain must be informed that decompression in the presence of
a lytic defect or a low-grade spondylolisthesis may increase
instability, causing low back pain. Intuitively, one would consider
foraminotomies either unilaterally or bilaterally rather than a
significant midline decompression in such a case. In 1955, Gill et al.
reviewed 18 patients treated with complete removal of the loose
posterior element (Gill laminectomy), and reported good results (130,131).
A long-term follow-up study of 43 patients, published in 1965, revealed
an increased slip in 14% of patients, but a 90% satisfactory result in
the group overall (132). These results,
however, have not been universally observed. Osterman et al. reported
on 75 patients with long-term follow-up averaging 12 years, and
although the initial results at the end of 1 year showed fair, good, or
excellent results in 83% of the patients, these results did not hold up
over time (133). When these same patients were
evaluated 5 years postoperatively, satisfaction ratings had dropped to
75%, and the spondylolisthesis had progressed in 27% of patients.
Marmor and Bechtol described a patient who progressed from a grade II
slip to a spondyloptosis after a Gill laminectomy (134).
In a more dismal review of 33 patients with a 7-year follow-up, Amuso
et al. reported 36% poor results with Gill laminectomy (135).
These authors did not observe any significant progression of the
spondylolisthesis and do not believe there is any correlation between
the progression of spondylolisthesis and poor results.
the Gill laminectomy as a stand-alone intervention is not a reasonable
procedure, especially in a growing child, and should always be
accompanied by a spinal fusion (130,131).
However, decompression is often an important part of the surgical
treatment of spondylolisthesis, and in patients with lytic defects a
Gill laminectomy is an efficient start for achieving a wide
decompression. It also often results in sufficient autologous bone for
fusion of that level. It
should
be noted that the Gill laminectomy alone does not decompress the
involved foraminal nerve root; to achieve this, an additional
dissection and formal nerve root decompression is necessary. Wiltse and
Jackson proposed that root decompression is rarely necessary and that
the tight hamstrings, abnormal reflexes, and motor weakness will
recover after posterior fusion alone (10).
Formal decompression of the nerve is assumed to give the affected nerve
root the optimal chance of recovery; however, this must be weighed
against the chance of increased slip, if one is considering a
decompression and fusion without instrumentation. In such a situation,
nerve root decompression with an instrumented fusion will permit an
adequate decompression of the affected nerve roots while stabilizing
the fusion segment in the desired position.
of symptomatic spondylolisthesis and is necessary when instability
(documented on lateral flexion and extension radiographs) and low back
pain exist. Fusion is probably also reasonable when performing
primarily decompressive surgery on patients whose main symptom is lower
extremity radiculopathy, but whose spondylolisthesis is grade III or
greater, especially in the presence of degenerative disc disease.
Available techniques include anterior and posterior procedures, either
alone or in combination. Posterior techniques include posterior lumbar
interbody fusions (PLIF) and posterolateral fusions with or without
instrumentation.
posterolateral uninstrumented fusions. In these studies, fusion rates
have ranged from 67% to 96%, with 60% to 100% of the patients showing
good results (25,41,122,136,137,138,139).
Although the outcomes of these multiple studies have been excellent,
the actual pseudarthrosis rate may be much higher than reported. Lenke
et al. critically evaluated 56 patients with isthmic spondylolisthesis
treated with in situ posterolateral fusions (140).
When strict grading criteria were used, only 50% of the patients had
bilateral solid fusions, 18% had unilateral solid fusions, and 21% had
pseudarthrosis (Fig. 21.12). Despite the high
rate of pseudarthrosis, overall clinical improvement was noted in more
than 80% of patients who had presented with preoperative symptoms of
back or leg pain or hamstring tightness.
of instrumentation in achieving improved fusion rates and improved
outcomes (141). Other groups have reported high
fusion rates (90% to 95%) and 90% excellent or good outcomes with
instrumented fusions for spondylolisthesis (142,143,144).
External immobilization postoperatively is typically not necessary,
because the spinal fixation of transpedicular instrumentation provides
sufficient rigidity; however, in cases with poor bone quality, an
adjunctive postoperative cast or brace may be helpful. In situ
posterolateral fusion continues to offer satisfactory results for
patients with grade I and some grade II spondylolistheses; the risks
are within reasonable limits, and this technique remains a good
approach for this category of patients. However, if the surgeon is
comfortable placing pedicle screws in children, the procedure can
provide definitive stabilization with less reliance on postoperative
immobilization in most circumstances (Fig. 21.13).
the customary procedure is a posterolateral one-level L-5-S1 fusion.
Extension of the uninstrumented fusion to L-4 may be indicated for
greater degrees of slip (i.e., more than 50%) for two main reasons (Fig. 21.14):
(a) in high-grade slips the transverse process of L-5 is displaced
anterior to the sacral ala, making it difficult to expose the
transverse process of L-5 without exposing the L-4–L-5 facet and L-4
transverse process; and (b) the fusion mass placed from L-5 to the ala
will be horizontal and under shear forces, whereas graft from the ala
to the L-4 transverse process will lie in a more biomechanically sound,
vertical direction. A two-level uninstrumented arthrodesis may also be
necessary in a slip of less than 50% if the transverse process of L-5
is very small and provides an insufficient posterior bed for the
fusion. With the advent of posterior instrumentation (i.e., pedicle
screws) and anterior structural support (i.e., cages), the need for
two-level fusion is decreasing, because of the high probability of
creating a stable, solid bony union of the one-level fusion, even for
high-grade slips. In addition, even a two-level L-4–sacrum
uninstrumented fusion is not guaranteed to heal in a grade III or IV
spondylolisthesis (Fig. 21.15). When pseudarthrosis and slip progression are noted postoperatively, revision fusion with instrumentation is indicated.
have a pathologic slip angle; therefore, surgeons prefer to treat such
patients with fusion in situ, unless there
is demonstrable instability on flexion-extension lateral radiographs.
However, with a more marked deformity in higher-grade
spondylolisthesis, especially in the presence of increased lumbosacral
kyphosis and extreme spondyloptosis, some degree of reduction is
necessary in order to realign the lumbar spine over the sacrum in a
position that will permit a solid fusion with acceptable sagittal
alignment (Fig. 21.16). Studies on in situ
fusions for higher-grade slips have reported pseudarthrosis rates from
0% to 60% and slip progression rates of as much as 25%, gait
disturbances, and persistent cosmetic deformity. These data have led
many to advocate reduction of high-grade slips, not only to address
these issues but also to save motion segments (14,25,118,121,143,145,146,147,148,149,150,151,152,153). Although there is some concern regarding the neurologic risk at the time of reduction, in situ fusions for high-grade slips have also been associated with adverse neurologic outcomes (125). Schoenecker et al. reported on 12 patients who developed
cauda equina syndrome after in situ
arthrodesis for high-grade spondylolisthesis. Seven of the 12 patients
had permanent neurologic injuries, which were attributed to the prone
positioning during surgery and the postural reduction of the deformity
during surgery (126).
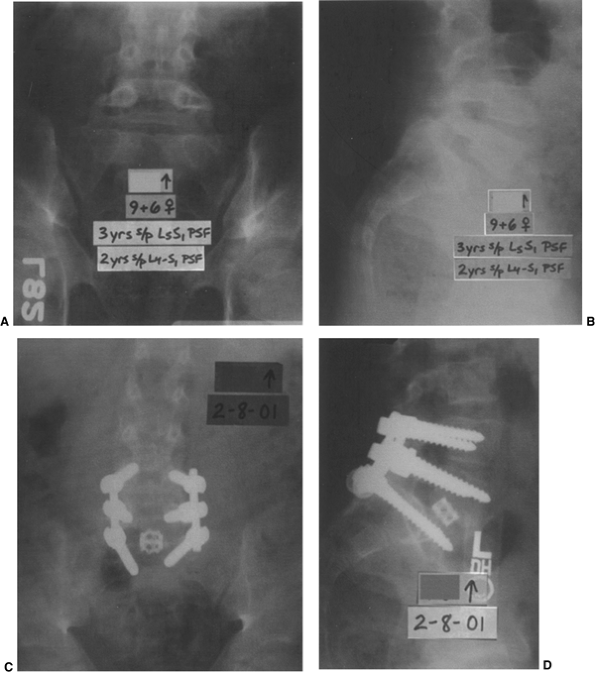 |
|
Figure 21.12 A and B: The patient is a girl 9 years and 6 months old who has undergone two prior in situ
fusions for a low-grade isthmic dysplastic spondylolisthesis at L-5–S1. She ultimately developed a solid fusion at L-4–L-5, and pseudarthrosis at L-5–S1, with continued lumbosacral pain. C and D: She then underwent a posterior instrumented revision fusion from L-4 to the sacrum as well as an anterior interbody fusion with a structural cage and bone graft at L-5–S1 in an attempt to alleviate the pain caused by her pseudarthrosis. |
reduction procedures, a sound surgical technique and adherence to
simple mechanical principles are important, including wide laminectomy
and complete bilateral nerve root decompression. Often nerve root
decompression must be performed beyond the vertebral column. This is
because of soft tissue constriction of the nerve roots within the
paraspinal muscles and iliolumbar ligaments, which may result in
neuropraxia caused by nerve root stretch during reduction of high-grade
slips. Complete discectomy is necessary in order to release the
olisthetic segment. Further
release
and mobilization of the olisthetic segment can be achieved by sacral
dome osteotomy, which facilitates reduction without necessitating
excessive vertebral distraction. Although excessive distraction may be
dangerous, judicious distraction is a useful maneuver to help achieve
reduction by lifting the L-5 body out and away from the pelvis. Various
techniques for achieving distraction have been reported since its
initial description by Jenkins in 1936 (154). External traction, that is, halo-femoral (83,137,155), passive positional reduction (151), temporary casting (152),
temporary intraoperative hook and rod constructs, and distraction using
pedicle screw instrumentation as a staged part of the surgical
procedure can all be useful (31,126,143,150,156,157,158).
If distraction is used across segments that are not to be included in
the final levels to be instrumented and fused, careful attention must
be given to the intervening soft tissue to make sure that the
uninstrumented facet joints are not injured. Overdistraction may result
in iatrogenic instability of those uninstrumented levels. If excessive
distraction is placed across the final instrumentation, the pedicle
screws may loosen, resulting in the loss of fixation postoperatively.
 |
|
Figure 21.13 A and B:
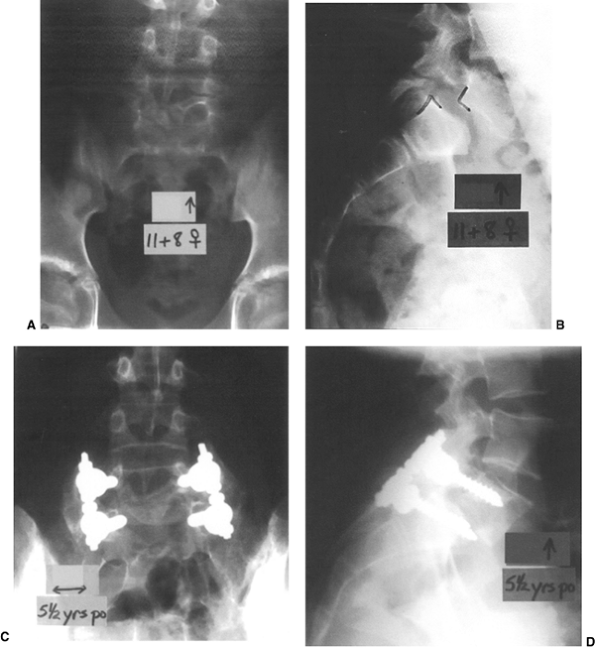
This patient, a girl 11 years and 8 months old, had 50% back pain and 50% leg pain from a low-grade L-5 spondylolisthesis. Following failure of conservative treatment, she underwent a posterior decompression and instrumented posterolateral fusion from L-5 to the sacrum with autogenous iliac crest graft laterally. C: At 5 years 6 months postoperatively, she had an excellent solid arthrodesis seen on this Ferguson posteroanterior view. D: The lateral view, showing a stable L-5–S1. |
 |
|
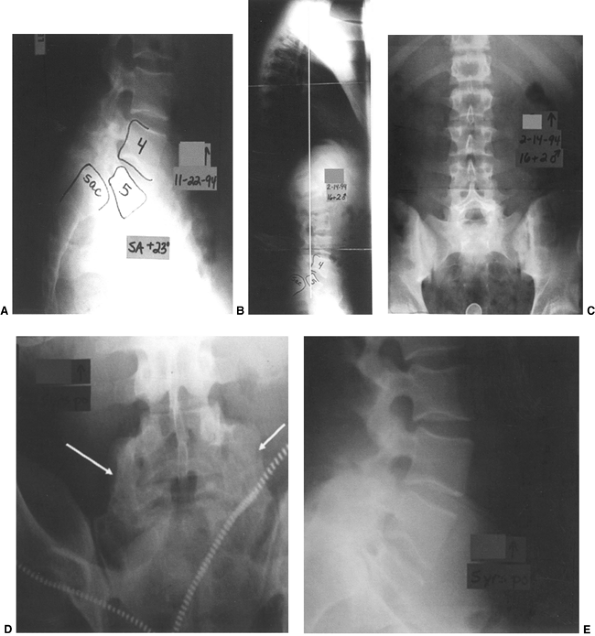
Figure 21.14
This 16-year-old boy had a grade III isthmic spondylolisthesis at L-5–S1. He had chronic lumbosacral back pain without radicular pain. He was treated with a bilateral Wiltse paraspinal approach, with autogenous iliac crest bone graft placement following decortication from L-4 to the sacral ala. Postoperatively, he was maintained in a bilateral pantaloon cast for 3 months, and then in a lumbar-sacral orthosis for an additional 3 months. A: Preoperative lateral x-rays of a Grade III slip with a +25° angle. B: Upright long cassette lateral x-ray showing good overall sagittal balance. C: Preoperative frontal x-ray showing large lumbar transverse processess. D and E: At 5 years postoperatively, he had a rock-solid fusion from L-4 to the sacrum, with stable alignment in his lateral view radiograph. His pain was totally relieved postoperatively. |
a newly available technique. This instrumentation, used in conjunction
with appropriate distraction, can constitute a powerful reduction
method. However, it must be stressed that without first achieving
appropriate soft tissue release through a combination of sacral dome
osteotomy, discectomy, and distraction to provide room to allow
posterior translation of the olisthetic segment, any attempt at
translation with reduction pedicle screws is likely to result only in
the fracture of the pedicles and dislodgement of the pedicle screws.
The possible complications associated with the reduction procedure,
both intraoperatively and postoperatively (159,160),
raises questions about whether reduction is necessary or even
desirable. Petraco et al. have suggested that complete reduction causes
excessive stretch of the L-5 nerve root and should therefore not be
performed (161).
However, this study did not consider the effect that an adequate
discectomy and sacral dome osteotomy have on shortening the spine,
thereby making full reduction safer. Proponents for full but judicious
and safe reduction point to the improved weight bearing, decreased
shear stress, and the improved bed for fusion provided by a full
reduction (143,162,163,164).
The improved biomechanical stability provided by full reduction with
anterior column support may allow us to perform a shorter
instrumentation and fusion because of the inherent stability found in
the fully
reduced
construct. Once acceptable alignment is obtained after reduction,
posterior instrumentation and anterior reconstruction with an intradisc
graft or cage are typically necessary for maintaining the rigidity of
the new lumbosacral alignment (143,157,162,163,164,165).
As one would expect with these complex spinal realignment or reduction
techniques, there is a risk of iatrogenic radiculopathy, and this must
be borne in mind during the decision-making process (79,160,164,165).
Perhaps a good compromise is a partial reduction of the translation and
kyphotic angle component of the slip to an acceptable level, which
usually entails less neurologic risk. As long as the L-5–S1 disc space
has
been repositioned adequately to allow placement of an intradiscal
support device, such as an Allograft wedge or cage, then the new
lumbosacral alignment should be biomechanically stable for fusion.
 |
|
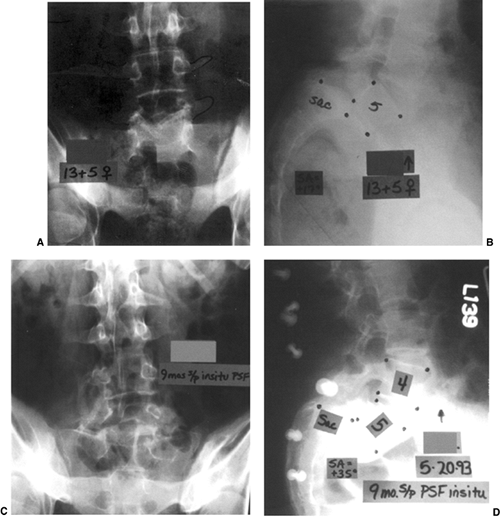
Figure 21.15
This girl, aged 13 years and 5 months, presented with a grade IV isthmic spondylolisthesis at L-5–S1. She had pain only in the back, and no symptoms in the legs. She underwent an in situ L-4–to–sacrum posterolateral fusion followed by 3 months of postoperative casting and 3 months of bracing. A and B: Preoperative AP and lateral radiographs of a Grade IV slip. C and D: Nine months postoperative AP and lateral radiographs showing increased slipage and a pseudarthrosis. She had, at that point, not only lumbosacral pain but also radicular leg pain caused by L-5 symptomatology. She then underwent a revision posterior decompression, partial reduction, instrumentation and fusion from L-4 to the sacrum and ilium. This was followed, at an early postoperative stage, by an anterior fibular strut graft placed between L-5 and the sacrum. At 1½ years after her revision surgery, she has a solid fusion with elimination of her preoperative symptoms. |
 |
|
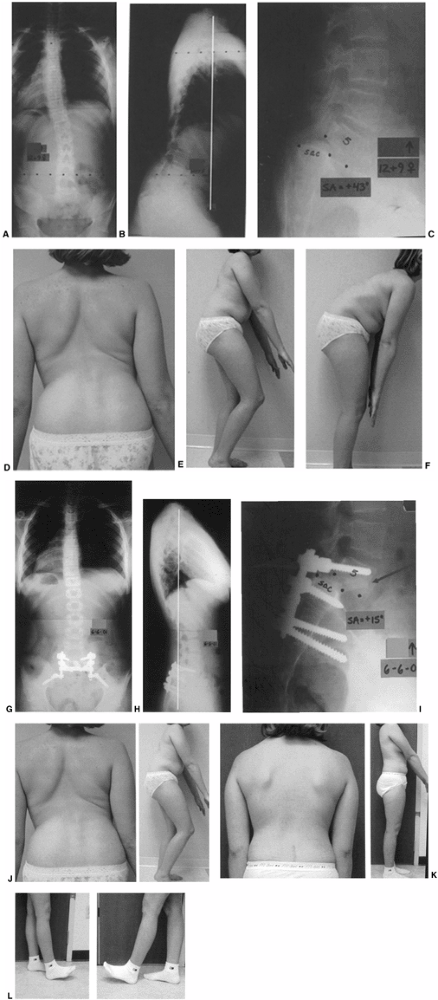
Figure 21.16
The patient, a girl 12 years and 9 months of age, had a grade IV dysplastic isthmic spondylolisthesis with a 43-degree kyphotic slip angle. Her anteroposterior radiograph shows mild olisthetic scoliosis (A), and her lateral radiographs show marked sagittal imbalance with a vertical sacrum (B, C). Preoperative clinical photos show her clinical deformity (D), with thoracic hyperextension, flattened buttocks, and flexed knees and hips, because of her significantly tight right and left L-5 nerve roots (E), and marked limitation on forward flexion (F). She was taken to surgery where she was positioned prone with her hips and knees flexed initially prior to her decompression. She had a dysplastic L-5 arch, and a spina bifida occulta just caudal to this. Following thorough decompression of the L-5 nerve roots, the right L-5 root was stimulated directly to obtain an electromyographic (EMG) distal recording corresponding to the innervation in the anterior tibialis of the L-5 root. On the left side, L-5–S1 disc excision and sacral dome osteotomy were performed to allow access into the disc and reduction of the spondylolisthesis. Spondylolisthesis was reduced in part by flexing the S1–iliac screw construct to meet the L-5 screw on the right side. Postoperative radiographs show the L-5–S1 instrumented posterolateral fusion (G, H) with partial reduction followed by a reamed anterior fibular graft (arrow) from L-5 to the sacrum (I). Note the improved coronal alignment as well as the excellent sagittal realignment following the reconstruction (G, H). Postoperatively, the clinical posture is normalized, with excellent ankle and toe dorsiflexion function noted bilaterally (J–L). |
of spondylolisthesis is reserved for cases in which it is necessary to
reestablish segmental lordosis, to increase the size of the available
fusion bed, or to increase the stability of the posterior
instrumentation. Multiple studies on anterior fusion alone have
reported overall good results when compared to posterior and
circumferential fusions (166,167,168,169).
Issues such as increased pseudarthrosis rate, and lesser correction of
slip angle, slip grade, sacral inclination, and sacral rotation raise
questions about the use of anterior fusion as a stand-alone procedure (166,167,168,169).
Some authors have advocated combining anterior structural grafts with
posterior pedicle screw constructs and posterolateral fusion; this
approach would theoretically improve fusion rates while reducing and
maintaining the spondylolisthesis in a more anatomic position (43,144,164,166,170,171,172).
La Rosa reported on 35 patients who underwent an instrumented
posterolateral fusion, out of whom 17 had an additional PLIF. At 2-year
follow-up the PLIF group had better correction of subluxation, disc
height, and maintenance of foraminal area; however, the clinical
outcomes for patients in the two groups were statistically equivalent (173).
technique for interbody fusion and posterior decompression for
high-grade spondylolisthesis. Along with posterior decompression and
fusion, this procedure additionally stabilizes the spondylolisthesis in situ by placing a fibular strut graft through the body of S1 and into the displaced body of L-5 through a posterior approach (174,175).
This technique has been shown to be effective in primary and revision
surgeries, and is associated with a low incidence of transient
neurologic deficits (142,164,176).
Hanson et al. reported on 17 patients who underwent combined partial
slip reduction with dowel fibular strut grafts for high-grade
dysplastic isthmic spondylolisthesis. Solid fusion was achieved in 16
of the 17 patients, and there were no cases of neurologic deficits (97).
treatment of high-grade spondylolisthesis. The wide exposure of the
spinal canal at the time of decompression provides ideal access for
performing a unilateral or bilateral PLIF, thereby permitting a
biomechanically sound fusion of all three columns of the spine (164).
The intervertebral structural grafts increase the surface area
available for fusion, and permit compressive load-sharing through
normal spinal biomechanics while opening up the narrowed neuroforamen.
Several series have reported excellent results on combining PLIF with
an instrumented posterior reduction and fusion (144,164,173).
with the patient ambulatory or in bed rest. The decision to use
immobilization will depend upon several factors such as the patient’s
body habitus, likelihood of compliance, preoperative slip grade and
angle, whether the surgery was primary or a revision, and the adequacy
of reduction and instrumentation purchase. If a TLSO is used, the thigh
must be included into the brace without a hinge joint, in order to
adequately control the pelvis. Brace use is continued for 3 to 4 months
postoperatively until solid fusion is noted radiographically.
patients with spondylolysis or minimal spondylolisthesis, with L-5 in a
lordotic position, and no degenerative changes at the olisthetic level.
A “hot” SPECT scan at the pars interarticularis implies a biologically
active defect, making osteosynthesis a viable option. A “cold” SPECT
scan is a relative contraindication to a direct pars interarticularis
repair, because the lack of biologic activity indicates little inherent
ability to create a bony union. In addition, the absence of neurologic
findings is essential, because the technique of direct pars
interarticularis repair is not amenable to a nerve root decompression.
pedicle screw and rod constructs provide ample support, particularly
when anterior interbody support is used. In the case of a mild or
moderately degenerated disc without significant collapse, a
posterolateral instrumented fusion alone may not be sufficient, even if
a wide decompression is not necessary and a large posterolateral
surface area exists for fusion. We prefer to always stabilize even
grade I slips so as to enhance our fusion rates. Posterior instrumented
fusions without abdominal subcutaneous fat (ASF) may be sufficient in
patients with significant disc-space collapse at the olisthetic level.
The inherent stability provided by the collapsed disc will decrease the
stress on the construct and the interface between the screws and the
vertebrae. However, in patients with a large or hypermobile disc,
anterior column support may be necessary for an effective
reconstruction.
instrumentation and fusion along with decompression will likely be
required. Anterior column support in the form of intradiscal cages or
Allograft wedges should be provided in order to ensure long-term
stability of the construct and to allow for maximum correction of
segmental sagittal alignment. The anterior column support can be
achieved by either an anterior lumbar interbody fusion (ALIF) or a
posterior lumbar interbody fusion/transforaminal lumbar interbody
fusion (PLIF/TLIF) technique. Another attractive option is to perform a
transperitoneal approach, which permits
quick
and easy access to the disc space, allowing complete disc removal and
placement of a large footprint interbody spacer while reestablishing
lordosis. Reestablishing or maintaining segmental alignment of the
olisthetic level will theoretically protect adjacent levels.
technique can be extremely demanding. Because each high-grade slip
presents with a unique combination of pathologic anatomy, biomechanics,
and clinical symptoms, all surgical techniques should be considered and
may be useful during any reduction and subsequent stabilization of the
vertebral column (164,170,174).
Slip angle reduction, anterior interbody support, and reconstruction of
the anterior column are important components of the reconstruction for
high-grade slips. With appropriate release, a complete discectomy, and
possible sacral dome osteotomy, anterior column reconstruction may
provide enough segmental stability to allow monosegmental fixation with
a high degree of success. This has been advocated by Harms et al. for
many years (159). Wide nerve root decompression
is an essential component in minimizing the development of iatrogenic
nerve root injury at the time of reduction of the slip, as has been
mentioned earlier in the Reduction of Spondylothesis.
III, in whom significant effort is required in order to achieve
reduction of the olisthetic segment, fixation distal to S1 (i.e., iliac
screws) should be provided for stability of the reconstruction. For
these higher-grade slips, a posterior approach for achieving an
anterior column reconstruction (i.e., PLIF/TLIF technique) is more
practical than an ALIF procedure, for several reasons. Because of the
forward flexion of the rostral vertebra, access into the disc space
anteriorly may be difficult or impossible. Because these higher-grade
slips often have significant posterior element dysplasia and
neuroforaminal narrowing, the nerve roots should be identified and
decompressed before vertebral reduction maneuvers are attempted.
Failure to achieve an adequate decompression before reduction may
result in severe irreversible nerve root injury. Usually by the time
the wide laminectomy and foraminal decompression are carried out for
bilateral nerve root decompression, the posterior aspect of the disc is
clearly exposed. This facilitates the PLIF/TLIF technique. Sacral dome
osteotomy, which is another useful technique for achieving release of
the deformity and facilitating reduction of high-grade slips, can be
performed through the posterior approach. The posterior approach is
also an advantage because of the expansive posterior decompression that
is accomplished with the Gill laminectomy.
augmented with fibular dowel grafts; posterior reduction as previously
discussed; or an L-5 spondylectomy. The posterior approach with sacral
dome osteotomy can be used effectively in many of these cases (Fig. 21.17). However, we occasionally prefer to treat this pathology with a Gaines procedure, which is a complete L-5 vertebrectomy (Fig. 21.18) (178,179).
The first stage of this procedure is an anterior L-5 corpectomy,
followed by a posterior approach to resect the posterior elements. This
technique is followed by the reduction of L-4 to the sacrum and
stabilization with pedicle screw instrumentation. Fusion is performed
anteriorly and posteriorly. The L-5 nerve roots are in extreme jeopardy
during anterior vertebral resection because they are usually trapped
and tethered under the pedicles of L-5, the posterior aspect of the
vertebral body of L-5, and the anterior cortex of the sacrum.
with most of the sources reporting less than 15%. Higher pseudarthritic
complication rates have been associated with higher-grade
spondylolisthesis and fusion in situ of these deformities (11,171).
displacement or kyphosis, can increase even in the presence of a solid
fusion (10,11,23,47,119,180).
However, it should be noted that the fusions reported on were
uninstrumented and were assessed with plain radiographs. CT scans may
have been able to demonstrate that some of the instances of increased
slippage were actually caused by the lack of adequate fusion. Further
slippage following an uninstrumented surgery is more common because the
removal of midline-stabilizing structures at the time of decompression
increases lumbosacral instability. This is also more common in higher
grades of spondylolisthesis, in patients with a greater degree of
anterior displacement, and in slip kyphosis.
reduction of spondylolisthesis; it is usually an L-5 nerve root lesion
and has varying recovery rates (79,160,164,165).
One series on partial slip reduction with the use of a dowel fibular
strut graft for high-grade slips reported no permanent neurologic
complications (97). In contrast, acute
postoperative cauda equina syndrome has been reported after a simple
posterolateral fusion without decompression or reduction (125,126).
This complication can occur through a midline or lateral
muscle-splitting incision when the patient is prone or laterally
positioned (115,126). The cause of this significant complication is not definitely known. However, in high-grade slips, the MRI scan can
demonstrate a cleaved intervertebral disc at the spondylolisthetic
segment, with the posterior half indenting the dural sac. The
development of cauda equina syndrome is likely secondary to acute
neural compression caused by this posterior disc fragment in a patient
with a marked slip, which partially reduces at the time of surgery,
causing further compression of the already at-risk neural elements.
Patients with high-grade slips and the congenital types of
deformities
are at an increased risk for neural compression at the time of surgery.
MRI can be helpful in these situations by permitting visualization of
the intervertebral disc pathology and neural compression, since such
neurologic deficits can occur in the absence of any preoperative
neurologic signs or symptoms (125,126).
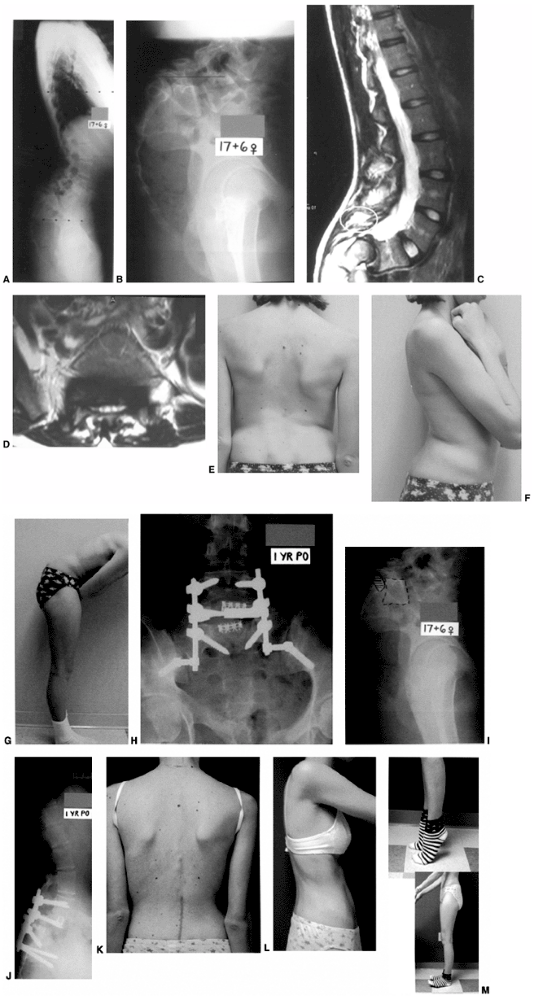 |
|
Figure 21.17 A, B: The patient, a girl 17 years and 6 months old, presented with an L-5–S1 spondyloptosis as well as L-4 bilateral spondylolysis. C, D:
Her preoperative sagittal and axial magnetic resonance imaging scans at L-5–S1 demonstrate a tight spinal canal with a pincer effect behind the posterior edge of the sacrum and the lumbosacral disc. E–G: Her preoperative clinical photos demonstrate prominent L-5 arch visible on the coronal as well as lateral clinical views. In forward flexion, she had limited flexion in her waist. H–J: She underwent a wide decompression and an instrumention of posterolateral fusion of L-4 to the sacrum and ilium which, aided by a proximal sacroplasty, resulted in marked reduction of her deformity. She also had transforaminal lumbar interbody fusions (TLIFs) at L-4–L-5 and L-5–S1 for anterior structural support and grafting. One year postoperatively, radiographs show excellent maintenance of alignment. K–M: Her postoperative clinical photos demonstrate her clinical alignment as well as excellent plantar and dorsal flexion of her feet bilaterally. |
optimal, because permanent neurologic deficits can occur
postoperatively, even after immediate decompression of iatrogenic
neurologic deficits. Patients with spondylolisthesis who experience
postoperative neurologic deficits should undergo MRI or CT myelogram
imaging of the neural elements in order to elucidate the causes of the
deficits. Any preoperative nerve root compression, irrespective of the
grade of the spondylolisthesis, should be decompressed. Adequate
decompression typically destabilizes the spine, however, increasing the
risk of slip progression, neurologic deficit, and pseudarthrosis and
making posterior stabilization with pedicle screw constructs an ideal
option. Intraoperative neuromonitoring can be useful at the time of
decompression and, if necessary, reduction, so as to minimize
iatrogenic neurologic deficits. However, somatosensory and
elecromyographic monitoring may not predict L-5 nerve root deficits,
and therefore intraoperative wake-up test(s) are strongly recommended.
Direct L-5 and S1 nerve stimulation is another technique for monitoring
nerve integrity more closely during various stages of the surgery.
Careful neurologic follow-up postoperatively is essential in all cases.
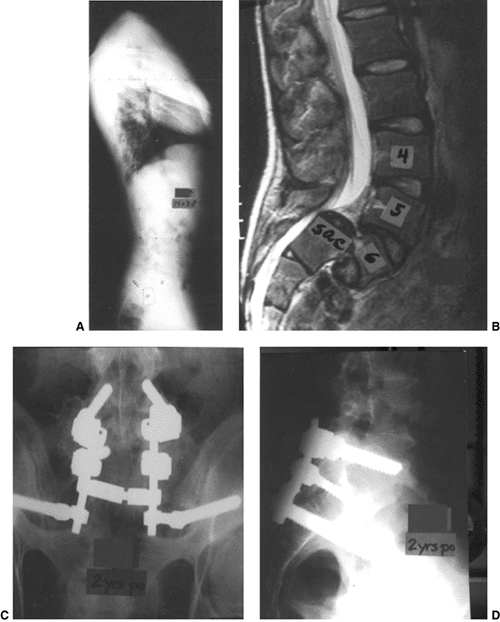 |
|
Figure 21.18 A, B:
The patient is a 15-year-old boy who presented with a severe L-6 spondyloptosis. The magnetic resonance imaging shows the complete anterior and distal position of the L-6 vertebral body below the sacrum. He had lumbosacral back pain and bilateral L-5 radicular pain. C, D: He underwent an L-6 spondylectomy with anterior and posterior fusion, and posterior instrumentation of L-5 to the sacrum. Two years postoperatively, he has an excellent circumferential fusion, with chronic dorsiflexion weakness to his feet and ankles bilaterally, but there is no need for any ankle-foot orthotics (AFO) or ambulatory aids. Although he had an excellent result as seen radiographically, his case demonstrates the high risk to the L-5 roots associated with the spondylectomy procedure in the treatment of severe spondyloptosis. |
and types of spondylolisthesis is a challenge for the pediatric spinal
specialist. Most patients with the more common low-grade slips will be
managed successfully with nonoperative treatment. Complete preoperative
work-up, detailed planning for intraoperative contingencies, technical
execution, and common-sense postoperative adjunctive immobilization and
activity restriction are all requirements for success. Severe cases of
spondylolisthesis are some of the most challenging pediatric spinal
problems encountered, and should be operatively treated only by those
with expertise in the pathoanatomy of the condition and with the
technical skills necessary for surgical success.
PC, Bartolozzi P. Classification of spondylolisthesis as a guideline
for treatment. In: Bridwell KH, DeWald RL, Hammerberg KW et al., eds. The textbook of spinal surgery, 2nd ed., Vol. 2. Philadelphia, PA: Lippincott–Raven Publishers, 1997:1211–1254.
L, Ronnemaa T, Osterman K, et al. Prevalence of isthmic lumbar
spondylolisthesis in middle-aged subjects from eastern and western
Finland. J Clin Epidemiol 1992;45:917–922.
K, Schlenzka D, Poussa M, et al. Isthmic spondylolisthesis in
symptomatic and asymptomatic subjects, epidemiology, and natural
history with special reference to disk abnormality and mode of
treatment. Clin Orthop Relat Res 1993;297:65–70.
AK, Danielson BI, Nachemson AL. Natural history of symptomatic isthmic
low-grade spondylolisthesis in children and adolescents: a seven-year
follow-up study. J Pediatr Orthop 1991;11:209–213.
EP, Twining P, Mulholland RC, et al. The prevalence of disc
degeneration associated with neural arch defects of the lumbar spine
assessed by magnetic resonance imaging. Spine 1989;14:977–981.
WJ, Fredrickson BE, Murtland A, et al. The natural history of
spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine 2003;28:1027–1035.
IE, Weinstein SL. Long-term follow-up of patients with grade-III and IV
spondylolisthesis: treatment with and without posterior fusion. J Bone Joint Surg Am 1987;69:960–969.
S, Osterman K, Hyvarinen H, et al. Progression of spondylolisthesis in
children and adolescents. A long-term follow-up of 272 patients. Spine 1991;16:417–421.
J, Duval-Beaupere C, Hecquet J, et al. Pelvic incidence: a fundamental
pelvic parameter for three-dimensional regulation of spinal sagittal
curves. Eur Spine J 1998;7:99–103.
HH, Roussouly P, Berthonnaud E, et al. Spondylolisthesis, pelvic
incidence, and spinopelvic balance: a correlation study. Spine 2004;29:2049–2054.
M, Kurowski P. The importance of mechanical factors in the etiology of
spondylolysis: a model analysis of loads and stresses in human lumbar
spine. Spine 1985;10:532–542.
FA. Lesions of the isthmus (pars interarticularis) of the laminae of
the lower lumbar vertebrae and their relation to spondylolisthesis. Surg Gynecol Obstet 1931;53:273–306.
RG, Maurer AH, Bonakdarpour A. Stress injuries of the pars
interarticularis: radiologic classification and indications for
scintigraphy. Am J Roentgenol 1985;145:763–766.
BM, Hutton WC. Variations in the amount and distribution of cortical
bone across the partes interarticularis of L-5: A predisposing factor
in spondylolysis? Spine 1979;4:163–167.
J, Troup JD. The structure of the pars interarticularis of the lower
lumbar vertebrae and its relation to the etiology of spondylolysis:
with a report of a healing fracture in the neural arch of a fourth
lumbar vertebra. J Bone Joint Surg Br 1973;55: 735–741.
K, Katoh S, Sakamaki T, et al. Vertebral forward slippage in immature
lumbar spine occurs following epiphyseal separation and its occurrence
is unrelated to disc degeneration: is the pediatric spondylolisthesis a
physis stress fracture of vertebral body? Spine 2004;29:524–527.
K, Katoh S, Sairyo K, et al. Slippage mechanism of pediatric
spondylolysis: biomechanical study using immature calf spines. Spine 2001;26:2208–2212.
RT. Profiles of the cervical, thoracic, and lumbosacral spine in
children and adolescents with lumbosacral spondylolisthesis. J Spinal Disord 2001;14:465–471.
T, Tamura T, Senzoku F, et al. Congenital laminar defect of the upper
lumbar spine associated with pars interarticularis defect: a report of
eleven cases. Spine 1991;16:353–355.
J, Abe H, Tsukimura Y, et al. Relationships between radiographic
abnormalities of the lumbar spine and incidence of low back pain in
high school and college football players: a prospective study. Am J Sports Med 2004;32:781–786.
L, Ronnemaa T. The association of mild-moderate isthmic lumbar
spondylolisthesis and low back pain in middle-aged patients is weak and
it only occurs in women. Spine 1993; 18: 1496–1503.
RD, Summerville DA, Treves ST, et al. Low back pain in adolescent
athletes: detection of stress injury to the pars interarticularis with
SPECT. Radiology 1991;180:509–512.
PB, Esses SI, Kostuik JP. Repair of pars interarticularis defect: the
prognostic value of pars interarticularis infiltration. Spine
1991;16(Suppl. 8):S445–S448.
MJ, Buckley J, Mawhinney R, et al. Magnetic resonance imaging and
discography in the diagnosis of disc degeneration: a comparative study
of 50 discs. J Bone Joint Surg Br 1986; 68: 369–373.
P, Templier A, Skalli W, et al. The association of sagittal spinal and
pelvic parameters with isthmic spondylolisthesis. J Spinal Disord 2002;15:24–30.
SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up
of early detected spondylolysis in young athletes. Am J Sports Med 2004;32:928–933.
P, Petit M. Direct repair of spondylolysis without spondylolisthesis,
using a rod-screw construct and bone grafting of the pars
interarticularis defect. Spine 1999;24:1252–1256.
E, Gerber B, Fasel J. Surgical treatment of spondylolisthesis by bone
grafting and direct stabilization of spondylolysis by means of a hook
screw. Arch Orthop Trauma Surg 1984; 103:175–178.
DS, Iza J. Repair of the defect in spondylolysis or minimal degrees of
spondylolisthesis by segmental wire fixation and bone grafting. Spine 1985;10:673–679.
PO, Johnston CE II. Analysis and treatment of poor outcomes following
in situ arthrodesis in adolescent spondylolisthesis. J Pediatr Orthop 1997;17:754–761.
JK, Lonstein JE, Winter RB, et al. Long-term evaluation of adolescents
treated operatively for spondylolisthesis. A comparison of in situ
arthrodesis only with in situ arthrodesis and reduction followed by
immobilization in a cast. J Bone Joint Surg Am 1992;74:693–704.
BL III, Donati NL. Spinal arthrodesis for severe spondylolisthesis in
children and adolescents. A long-term follow-up study. J Bone Joint Surg Am 1989;71:594–598.
D, Fielding J, Demarest L, et al. Spondylolisthesis: a critical review
of a consecutive series of cases treated by arthrodesis. J Bone Joint Surg Am 1955;37:767.
JN, Polly DW Jr, Van Dam BE. A study of the efficacy of nonoperative
treatment of presumed traumatic spondylolysis in a young patient
population. Mil Med 1995;160:553–555.
HD, Morley TR. Cauda equina lesions following fusion in situ and
decompressive laminectomy for severe spondylolisthesis. Four case
reports. Spine 1989;14:214–216.
PL, Cole HO, Herring JA, et al. Cauda equina syndrome after in situ
arthrodesis for severe spondylolisthesis at the lumbosacral junction. J Bone Joint Surg Am 1990;72:369–377.
GG. Long-term follow-up evaluation of a few patients with
spondylolisthesis treated by excision of the loose lamina with
decompression of the nerve roots without spinal fusion. Clin Orthop Relat Res 1984;182:215–219.
GG, Manning JG, White HL. Surgical treatment of spondylolisthesis
without spine fusion: excision of the loose lamina with decompression
of the nerve roots. J Bone Joint Surg Am 1955;37:493–520.
K, Lindholm TS, Laurent LE. Late results of removal of the loose
posterior element (Gill’s operation) in the treatment of lytic lumbar
spondylolisthesis. Clin Orthop Relat Res 1976;117:121–128.
C. Treatment of spondylolisthesis by posterolateral fusion, resection
of the pars interarticularis, and prompt mobilization of the patient.
An end-result study of seventy-three patients. J Bone Joint Surg Am 1966;48:1282–1300.
KH, Sedgewick TA, O’Brien MF, et al. The role of fusion and
instrumentation in the treatment of degenerative spondylolisthesis with
spinal stenosis. J Spinal Disord 1993;6: 461–472.
RL, Faut MM, Taddonio RF, et al. Severe lumbosacral spondylolisthesis
in adolescents and children. Reduction and staged circumferential
fusion. J Bone Joint Surg Am 1981; 63: 619–626.
JB, Waters PM, Hall JE. Technique for maintenance of reduction of
severe spondylolisthesis using L-4-S4 posterior segmental
hyperextension fixation. Orthop Trans 1987;11:113.
O, Frontino G, Bartolozzi P. Technique of anatomical reduction of
lumbar spondylolisthesis and its surgical stabilization. Clin Orthop Relat Res 1976;117:165–175.
I, Inoue S, Murata T, et al. Reduction and fusion of severe
spondylolisthesis using halo-pelvic traction with a wire reduction
device. Int Orthop 1980;4:107–113.
J, Jeszenszky D, Stoltze D, et al. True spondylolisthesis reduction and
monosegmental fusion in spondylolisthesis. In: Bridwell KH, DeWald RL,
Hammerberg KW et al., eds. The textbook of spinal surgery, 2nd ed., Vol. 2. Philadelphia, PA: Lippincott–Raven Publishers, 1997:1337–1347.
RA, Bradford DS. Technique for achievement and maintenance of reduction
for severe spondylolisthesis using spinous process traction wiring and
external fixation of the pelvis. Spine 1985;10:376–372.
DS, Boachie-Adjei O. Treatment of severe spondylolisthesis by anterior
and posterior reduction and stabilization. A long-term follow-up study.
J Bone Joint Surg Am 1990;72: 1060–1066.
JA, Spangfort E, Aaro S. Operative treatment of spondylolisthesis in
children and adolescents with tight hamstrings syndrome. Clin Orthop Relat Res 1980;147:192–199.
M, Zippel H, Perka C. Surgical management of severe spondylolisthesis
in children and adolescents. Anterior fusion in-situ versus anterior
spondylodesis with posterior transpedicular instrumentation and
reduction. Spine 1997;22:2036–2042.
TS, Ragni P, Ylikoski M, et al. Lumbar isthmic spondylolisthesis in
children and adolescents. Radiologic evaluation and results of
operative treatment. Spine 1990;15:1350–1355.
RW, Bridwell KH, Lenke LG, et al. Complications in the surgical
treatment of pediatric high-grade, isthmic dysplastic
spondylolisthesis: a comparison of three surgical approaches. Spine 1999;24:1701–1711.
H. The treatment of lumbar spondyloptosis or impending lumbar
spondyloptosis accompanied by neurologic deficit and/or neurogenic
intermittent claudication. Spine 1979;4:68–77.
Rosa G, Conti A, Cacciola F, et al. Pedicle screw fixation for isthmic
spondylolisthesis: does posterior lumbar interbody fusion improve the
outcome over posterolateral fusion? J Neurosurg Spine 2003;99:143–150.
HH, Cook SS. One-stage decompression and posterolateral and interbody
fusion for lumbosacral spondyloptosis through a posterior approach.
Report of two cases. J Bone Joint Surg Am 1982;64:415–418.
MD, Bohlman HH. Spondylolisthesis treated by a single-stage operation
combining decompression with in situ posterolateral and anterior
fusion. An analysis of eleven patients who had long-term follow-up. J Bone Joint Surg Am 1990;72:415–421.
