Skeletal Muscle Anatomy, Physiology, and Mechanics
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section IV – Basic Science > 26 – Skeletal Muscle Anatomy, Physiology, and Mechanics
26
Skeletal Muscle Anatomy, Physiology, and Mechanics
Bryan T. Kelly
William J. Robertson
Muscle Structure and Function
Myofibrils and Connective Tissue
-
Myofibrils represent the basic structural element of skeletal muscle (Fig. 26-1).
-
Along with their innervating nerves, myofibrils are responsible for the contractile function of skeletal muscle.
-
Myofibrils fuse together to form ribbon-like multinucleated cells called muscle fibers or myofibers.
-
Muscle fibers varying in length from a few millimeters to 50 cm.
-
Muscle is structurally organized by three connective tissue layers: the endomysium, perimysium, and epimysium (Table 26-1).
-
Endomysium: a delicate connective tissue layer (basement membrane) that surrounds each muscle fiber
-
Perimysium: an enveloping connective tissue layer that bundles multiple muscle fibers to form larger fascicles
-
Epimysium: a stronger, thick connective
tissue sheath that combines and surrounds muscle fascicles to form the
entire muscle belly
-
-
-
This connective tissue framework allows
individual muscle cells, nerves, and capillaries to work together
during muscle contraction.
Intracellular Organelles and Architecture
-
Below the layer of endomysium that covers each muscle fiber sits a plasma membrane called the sarcolemma (Fig. 26-2 and Table 26-2).
-
The ends of each fiber are attached to
the extracellular matrix (ECM) through the sarcolemma by way of protein
molecules called integrins.P.485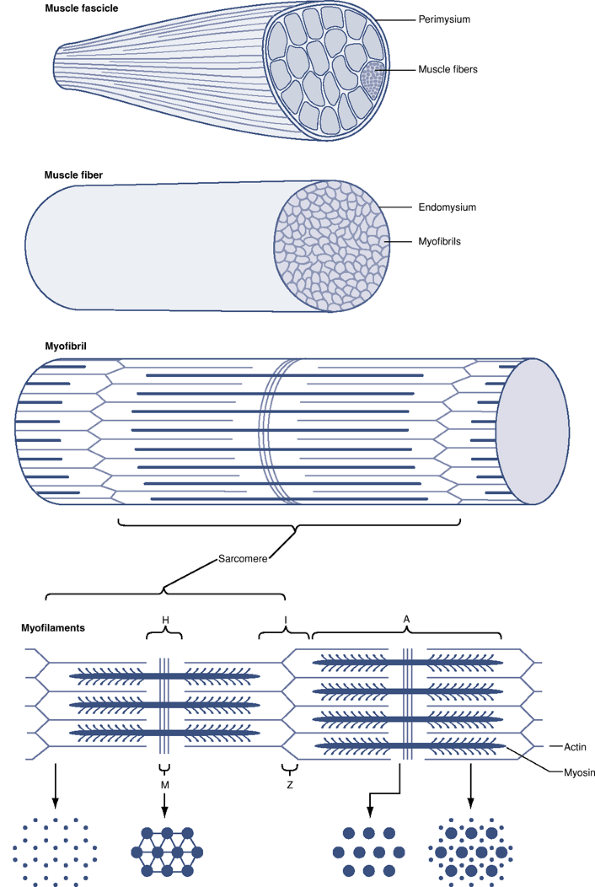 Figure 26-1
Figure 26-1
Schematic diagram of a skeletal muscle, showing a stepwise arrangement
of its components. Myofibrils are composed of thin and thick
myofilaments that provide the framework for muscle contraction. A
muscle fiber (myofiber) is made up of numerous myofibrils covered in a
delicate connective tissue layer (endomysium). Multiple muscle fibers
are grouped into a muscle fascicle. The connective tissue surrounding
each fascicle is called the perimysium. The fascicles, as a group, are
surrounded by epimysium and form a skeletal muscle.P.486Table 26-1 Muscle Structural OrganizationStructural Units of Skeletal Muscle Organized from Smallest to Largest Separating Structures Between Structural Elements of Skeletal Muscle Myofibrils Muscle fibers Endomysium Muscle fascicles Perimysium Muscle belly Epimysium Table 26-2 “Sarco” TerminologyTerm Definition Sarcolemma Limiting plasma membrane surrounding individual muscle fibers (myofibers) Sarcoplasm Fluid material within the sarcolemma surrounding the myofibrils
Contains Golgi apparatus, mitochondria, ribosomes, sarcoplasmic reticulum, glycogen, and lipid dropletsSarcoplasmic reticulum Network of closed sacs, rich in calcium ions, coursing around myofibrils in a longitudinal orientation
Communicate with cell membrane by way of transverse tubulesSarcomere Repeating structural units of the myofibril that span between Z lines ![]() Figure 26-2
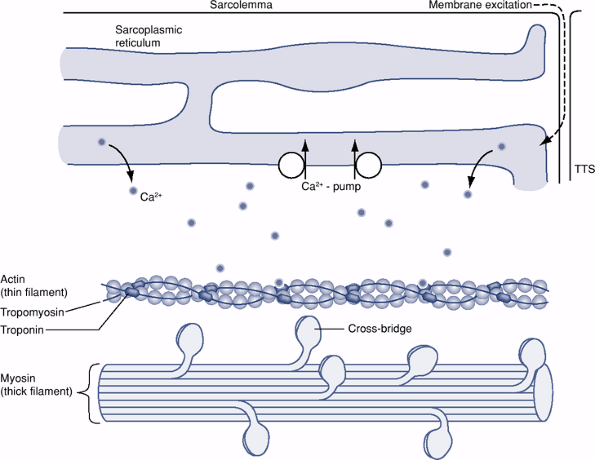
Figure 26-2
Schematic diagram showing some of the intracellular structures of an
individual muscle fiber. Depolarization originating at the motor
endplate travels down the sarcolemma membrane and continues via the
transverse tubules (TTS) to the sarcoplasmic reticulum. A resultant
depolarization of the calcium-rich sarcoplasmic reticulum releases
calcium into the sarcoplasm, where at high concentrations it binds to
troponin. A conformational change in troponin frees actin of
tropomyosin and allows for actin-to-myosin cross-bridging.P.487-
Integrins are a family of adhesions
receptors that provide muscle fibers with the strength to withstand
tensile forces at the myotendinous junction (MTJ), while also playing
vital roles such as cell-to-cell signaling, cell-to-ECM interactions,
and signal transduction.
-
-
Within the sarcolemma there exists a
fluid material, or sarcoplasm, that encompasses large numbers of
longitudinally oriented myofibrils and numerous nuclei that compose
each muscle fiber. -
The sarcoplasm contains a Golgi apparatus, many mitochondria, ribosomes, sarcoplasmic reticulum, glycogen, and lipid droplets.
-
The sarcoplasmic reticulum is a network of closed sacs that course around the myofibrils in a primarily longitudinal direction.
-
These sacs are rich in calcium ions.
-
They communicate with the cell membrane by way of transverse tubules.
-
Myofibril Structural Composition (see Fig. 26-1)
-
Each myofibril exhibits periodic cross-striations.
-
These striations are alternating light
and dark bands of isotropic and anisotropic materials, respectively,
composed of repeating units called sarcomeres that spans from one Z
line to the next Z line. -
The light and dark bands of a sarcomere are created by overlapping thin and thick filaments, also termed myofilaments.
-
These thin and thick filaments are made
up of specific proteins that, in the presence of calcium, are
responsible for force production within the muscle.
Thin and Thick Filaments
Thin Filaments
-
Thin filaments consist of three proteins (actin, tropomyosin, and troponin), which form the I band of a myofibril (see Fig. 26-2).
-
Major component of the thin filament is actin, consisting of two polymers:
-
G-actin, or globular actin
-
On binding with ATP, it polymerizes to the F form, or fibrous actin.
-
-
F-actin binds calcium tightly.
-
-
Actin has long, thin fibers attached to
its surface that inhibit contraction by blocking actin and myosin
cross-bridges. These fibers are called tropomyosin fibers and are
attached to actin by troponin molecules. -
Troponin is a three-subunit molecule
(troponin-I, troponin-T, and troponin-C) that can bind actin,
tropomyosin, and calcium, respectively. In the absence of adequate
calcium levels, troponin binds tropomyosin to actin and inhibits
contraction. -
A high enough concentration of calcium
within the sarcoplasm will allow calcium to bind to the troponin-C
subunit. A conformational change then results in the other troponin
subunits. These changes release the inhibitory effect of tropomyosin
and allow actin and myosin interaction. -
As the calcium concentration falls, the
conformation of troponin reverts back to its inhibitory form, shifting
tropomyosin to again stearicly inhibit cross-bridges.
Thick Filaments
-
Formed by the muscle protein myosin
-
Thick filaments span the midportion of the sarcomere forming the A band of a myofibril.
-
I bands contain only thin filaments,
whereas A bands contain both thick and thin filaments, except in the
central H zone, where only thick filaments are present (see Fig. 26-1). -
Myosin is the largest of the myofibril proteins, making up >50% of muscle mass.
-
Myosin molecule resembles a thin rod with two small, globular heads.
-
This globular end has significant ATPase
activity in the presence of ionic calcium and serves as a cross-bridge,
binding myosin with actin during muscle contraction.
Changes During Contraction
-
When a muscle fiber contracts, each fiber and each sarcomere shortens.
-
Thick and thin filaments are arranged so that they can slide past each during contraction.
-
With maximal contraction a sarcomere shortens 20% to 50% of its normal resting length.
-
The I band becomes shorter and the H zone usually disappears.
-
The A band does not change in length during contraction or relaxation.
-
-
A sarcomere can extend to 120% of its length during passive stretching, and the I band becomes longer.
Neuromuscular Interaction
-
Each nerve cell axon branches many times, providing each muscle fiber with a point of contact called the motor endplate.
-
This single nerve axon and all of the muscle fibers it innervates constitute a motor unit.
Motor Unit
-
Both the size of the motor unit and the number of motor units within a given muscle are variable.
-
Muscles that require fine motor control and coordinated movements, such as the extraocular muscles, have small motor units.
-
Larger, more powerful muscles, such as the gastrocnemius muscle, have large motor units.
Stimulus for Muscle Contraction
-
The arrival of an electrical impulse at the terminal axon leads to an inflow of calcium ions.
-
Acetylcholine-containing vesicles fuse with the axon membrane and release acetylcholine into the synaptic cleft.
-
Acetylcholine binds to receptors on the muscle cell membrane, causing an inflow of current and depolarizing the motor endplate.
-
Depolarization of the sarcoplasmic reticulum causes a release of calcium ions into the sarcoplasm.
-
This in turn causes all filaments within a muscle fiber to contract together.
Muscle Contraction
-
Muscle contraction is initiated by the release of ionic calcium into the sarcoplasm.
-
In absence of calcium, troponin and tropomyosin interfere with the formation of active complexes between actin and myosin.
-
With the binding of free calcium,
troponin undergoes a conformational change, releasing tropomyosin from
actin and allowing the formation of cross-bridges between actin and
myosin (see Fig. 26-2). -
Intracellular calcium also activates the myosin—ATP complex, which motors the sliding action between actin and myosin.
-
Energy provided by ATP drives the pulling of the actin molecule past myosin, shortening the fibril.
-
As long as the calcium concentration in
the cell is maintained at a high enough level, the myosin ATPase
remains active, permitting the fibril to stay in its contracted form. -
When the energizing impulse terminates, calcium is rapidly pumped out of the sarcoplasm.
-
As the calcium ion concentration drops, the calcium—troponin interaction is uncoupled.
-
Tropomyosin can again bind to actin, and
the troponin—actin—tropomyosin B complex impedes cross-bridging between
actin and myosin. -
Calcium reuptake by the sarcoplasmic
reticulum will not occur if there is insufficient ATP available, which
may lead to muscle contraction without electrical stimulation, as is
seen in rigor mortis.
Fiber Types and Adaptability
-
In lower mammals and other animals, muscles are generally composed entirely of either type I or type II fibers.
-
Human muscle is made up of a mixture of type I and type II fibers.
-
Muscle fiber type depends not on any intrinsic feature of the fiber itself, but on the motor neuron supplying that fiber.
-
All muscle fibers supplied by one particular axon will be of either type I or type II.
-
There are significant differences in the fiber types.
Type I Fibers
-
Type I muscle fibers are rich in the enzymes necessary for oxidative metabolism and are darker in appearance.
-
Contain a higher concentration of mitochondria and more capillaries per fiber than type II muscle fibers
-
When stimulated, they have a slow contraction or “twitch” time.
-
These fibers have increased resistance to
fatigue. * Type I fibers are well suited for activities related to
physical effort requiring strength and endurance that depend on the
metabolism of oxidative processes for energy.
Type II Fibers
-
Type II fibers obtain their energy through a much faster glycolytic process.
-
As glycogen stores are more rapidly
depleted than oxygen supplies, type II fibers are less suited to
continuous types of activity and are more suited to rapid alternating
effort. -
Those muscles most accustomed to slow, continuous work have a lower percentage of type II muscle fibers.
-
Type II fibers may be more prone to anatomic changes following altered energy demands than are type I.
-
Type II fibers tend to be smaller than
type I in children and in adults who do not carry out strenuous
physical exercise, although they increase in size with repeated
physical demands on the muscle. -
Type II fibers are subdivided into types IIa and IIb.
-
Type IIa fibers have an admixture of glycolytic and oxidative enzymes and show an intermediate twitch time.
-
Type IIb fibers (fast glycolytic fibers)
have the largest motor unit size, have the fastest rate of contraction,
and are most susceptible to fatigue.
-
Fiber Type Interconversion
-
It is generally accepted that the
relative percentage of type I and type II fibers in humans is
established genetically without a great deal of capacity for change. -
Evidence does exist for a training-dependent interconversion of type IIa and IIb fibers.
-
Endurance training can result in an increased percentage of type IIa fibers at the expense of IIb fibers.
-
Likewise, strength training and burst
exercises without an emphasis on endurance can lead to a percentage
increase in type IIb fibers and corresponding decrease in type IIa
fibers.
Muscle Injury
-
Type II muscle fibers are more susceptible to injury than type I fibers.
-
Other risk factors for injury include muscles that cross two joints and eccentric loading.
Growth and Development
Embryology of Skeletal Muscle
Myoblasts
-
Fusiform-shaped muscle progenitor cells that arise from the mesoderm cell population
-
Undergo mitosis in response to molecular signaling
-
When an adequate population of myoblasts exists, they fuse to form long multinucleated cells called myotubes.
P.489
Myotubes
-
Precursor of eventual skeletal muscle fibers
-
Continue to differentiate into large multinucleated cells called muscle fibers
-
Skeletal muscle can be identified by the seventh week of gestation.
Longitudinal Growth of Skeletal Muscle
-
Able to increase in length to accommodate for skeletal growth in the following ways:
-
In skeletally immature animals
-
Increasing muscle and tendon length: occurs at the musculotendinous junction rather than in the skeletal muscle midsubstance
-
Increasing the number of sarcomeres during longitudinal growth: individual sarcomere length remains relatively stable
-
-
In skele tally mature animals
-
The primary mechanism of muscle lengthening is elongation of the muscle belly.
-
-
Immobilization of Skeletal Muscle Under Stretch
-
Initially muscle fibers lengthen and the
sarcomeres stretch, resulting in an increased separation of the A bands
and I bands compared to their normal state. -
Over several weeks, additional sarcomeres
are added in series at the musculotendinous junction, allowing all
sarcomeres to return to their normal length. -
This addition of sarcomeres results in
muscles with increased protein and increased weight due to longitudinal
growth rather than an increase in cross-sectional area. -
As little as 2 weeks of this type of
immobilization shifts the length—tension curve to the right, resulting
in less passive force generated in response to a given stretch.
Energetics of Muscle
Energy Sources for Skeletal Muscle
Adenosine Triphosphate (ATP)
-
ATP is the immediate energy source for muscle contraction.
-
Has two terminal high-energy bonds that,
upon hydrolysis, provide a significant amount of chemical energy to
drive biologic reactions -
The stepwise hydrolysis of these phosphate bonds produces: ATP → ADP + inorganic phosphate → AMP + inorganic phosphate
Creatine Phosphate (CP)
-
Another source of high-energy phosphate bonds
-
Cannot be used as direct energy source; used instead to produce ATP from ADP
-
This reaction of ADP + CP → ATP + creatine is catalyzed by the muscle enzyme creatine kinase.
-
The total energy available from ATP hydrolysis is approximately enough for a person to sprint for less than 50 yards.
-
The total energy available from all the stored high-energy phosphate compounds is enough to sprint 200 yards.
-
The ability to replenish ATP is the limiting factor in many aspects of athletic performance.
-
Two metabolic pathways that maintain this ATP energy reservoir are the aerobic system and the anaerobic system.
Aerobic Metabolism
-
The primary source for ATP when oxygen is available
-
Uses glucose or fatty acids to produce ATP in large quantities
-
Glucose is broken into two pyruvate molecules, which enter the Krebs cycle.
-
Hydrogen molecules are removed from pyruvate and couple with oxygen to make water and release energy.
-
This energy results in the generation of high-energy phosphates that are coupled to ADP to form ATP.
-
The Krebs cycle results in 38 ATP from the oxidation of one glucose molecule.
-
Glucose is stored in the muscle cell in the form of glycogen.
Anaerobic Metabolism
-
The rapid hydrolysis of a glucose molecule into two molecules of lactic acid
-
Releases enough energy to convert two ADP molecules into two ATP molecules
-
The lactic acid produced causes acidosis and muscle fatigue.
-
This is the system relied upon when a large amount of energy is needed for a relatively short period.
Fat
-
Fat stores are abundant in the body and provide the largest potential source of energy.
-
Usually stored as triglycerides, which consist of three separate fatty acids
-
Variable-length fatty acid chains are cleaved by beta-oxidation into two carbon molecules called acetyl CoA.
-
Acetyl CoA enters the Krebs cycle.
-
-
The amount of ADP converted to ATP is dependent on the size of the fatty acid chain.
Protein
-
Under normal conditions, protein is not the primary source of fuel for muscle metabolism.
-
Deamination of amino acids produces ketoacids, which enter the Krebs cycle.
Use of Energy Sources
-
Anaerobic and aerobic systems function simultaneously and are not exclusive to any particular intensity level.
-
Both carbohydrate and fat are used as energy sources for any activity level.
-
Prolongation of aerobic exercise leads to
a shift from the metabolism of stored glucose and glycogen to the
oxidation of fatty acids.
Muscle Mechanics
Length vs. Tension
-
Passively stretched unstimulated muscle undergoes a period of lengthening before any tension develops.
-
Tension in the passively stretched muscle then rises in a nonlinear manner and approaches significant levels with high strains.
-
The length—tension relationship for
active skeletal muscle is similar to that of cardiac muscle and can be
explained by the myofibrillar ultrastructure. -
Actin and myosin overlap is required for force to be generated.
-
In a lengthened position this overlap is reduced, as is the maximum force generated.
-
As the sarcomere length decreases so does the cross-bridge overlap and the ability to generate force.
-
However, with continued sarcomere shortening, the filaments will collide and again the ability to generate force is decreased.
Isometric Contraction
-
With isometric (static) contraction, external muscle length does not change.
-
Internally, however, within the fibril the distance between the Z lines does shorten.
Isotonic Contraction
-
A constant internal force is produced and the muscle shortens.
-
Also defined as a dynamic exercise with a constant load or resistance
Eccentric Contraction
-
Contraction in which the external force
applied is greater than the internal force of the muscle, which causes
the muscle to lengthen while continuing to maintain tension -
Movement is controlled but not initiated.
-
During an eccentric contraction, muscle
can sustain greater tension than it can develop in isometric
contraction at any given muscle length. -
Because greater tension is generated, muscles are more vulnerable to rupture during eccentric contraction.
Isokinetic Contraction
-
Term means “constant force” and typically
is used to describe dynamic exercise performed through the range of
motion of a joint at constant velocity. -
Equipment used in isokinetic exercises
accommodates the exerted force to maintain the specified velocity
throughout the arc of motion. -
Because velocity does not change, the kinetic energy remains constant.
-
Isokinetic contractions are not part of normal physiologic muscle function.
Training Effects on Muscle
-
Skeletal muscle is a highly adaptable tissue.
-
Training can be aimed at increasing strength, endurance, and anaerobic or aerobic fitness.
Strength Training
-
High-resistance, low-repetition (1 to 15) exercise leads to increased strength and increased muscle cross-sectional area.
-
The majority of this size increase is likely due to muscle hypertrophy, but hyperplasia may also play a role.
-
Results in increased contractile protein content
-
Type II muscle fibers (higher in anaerobic metabolism) show a more pronounced hypertrophy than type I fibers.
-
Neurologic component, resulting in improved motor unit recruitment and better synchronized muscle activation
-
In poorly conditioned muscles, as few as 60% of the fibers fire simultaneously.
-
Well-conditioned muscles may fire over 90% of the fibers simultaneously.
-
Results in increased stores of energy-rich phosphagens
Endurance Training
-
Aerobic training
-
The challenge for the muscle is not to overcome high forces but rather to perform faster without fatigue.
-
The goal is not to increase muscle size but instead to adapt to use energy more efficiently.
-
Improvements in endurance result from better central and peripheral circulation and muscle metabolism.
-
Cardiovascular gains
-
Larger stroke volume, resulting in a lower resting heart rate (fewer beats are need to deliver the same amount of blood)
-
Improved blood flow through arterioles to the muscles
-
Increased muscle capillaries, especially in type I muscles
-
These advances allow the muscle to use oxygen more efficiently.
-
-
Muscle fiber changes
Aerobic (Sprint or Power) Training
-
High-intensity exercise for a few seconds to 2 minutes
-
Primarily driven by anaerobic metabolism
-
Relies on available muscle stores of ATP and the ability to perform anaerobic glycolysis
-
This training results in an increased level of stored phosphagens and an elevation in some enzymes involved in glycolysis.
-
Almost exclusively seen in fast-twitch type II muscle fibers
Muscle Injury and Repair
Mechanisms of Skeletal Muscle Injury
-
Can be caused by laceration, contusion, or strain
-
The most common injuries occurring during sports, 90% of which are contusions or strains.
-
Contusions result from a heavy compressive force, such as a direct blow to the muscle, and typically occur in contact sports.
-
Strains result from an excessive tensile force on the muscle, resulting in complete or, more commonly, partial tears.
-
These tears usually occur near the myotendinous junction, where the terminal sarcomeres are stiffer.
-
Muscle strains are typically seen in
muscles crossing two joints (gastrocnemius, rectus femoris,
semitendinosus), where potentially higher length-tension values can
occur.
-
-
Pathobiology of Muscle Injury
-
Skeletal muscle heals via a repair process.
-
Regardless of the underlying cause, the healing process is quite constant.
-
Three phases of muscle injury have been identified:
-
Destruction phase
-
Repair phase
-
Remodeling phase
-
Destruction Phase
-
Rupture and the ensuing necrosis of myofibers
-
Contused muscle tears at the point of impact, whereas muscle strains usually result in tears at the myotendinous junction.
-
The contusion injury is more superficial
in a contracted muscle vs. deeper in an uncontracted muscle, where it
is compressed against the underlying bone. -
The characteristic length of myofibers predisposes them to widespread necrosis starting at the rupture site.
-
The propagation of necrosis is halted
within hours by myofiber structures called contraction bands that act
like a system of fire doors, preventing further cell death.
-
-
Hematoma formation between the ruptured muscle ends
-
Muscle fiber-associated blood vessels are torn, allowing inflammatory cells to gain access to the injury site.
-
-
Inflammatory cell reaction
-
Macrophages and fibroblasts release chemotactic and growth factors.
-
In the acute phase, polymorphonuclear leukocytes (PMNs) are the most abundant cells at the injury site.
-
By the first day, PMNs are being replaced
by macrophages that aid in the proteolysis and phagocytosis of the
necrotic muscle tissue.
-
Repair and Remodeling Phase
-
As the phagocytosis subsides, two simultaneous processes (regeneration and scar formation) begin.
-
Regeneration of disrupted myofibers
-
Although myofibers are considered
postmitotic cells, undifferentiated reserve cells called satellite
cells allow for restoration of the contractile unit following injury.
During fetal development, a pool of these satellite cells is set aside
underneath the basal lamina of each muscle fiber.-
In responses to injury, these satellite cells proliferate and then differentiate into myoblasts.
-
Similar to their embryological development, myoblasts join together to form multinucleated myotubes.
-
Myotubes join with the injured ends of
the myofibers, allowing them to regain their normal cross-striated
appearance and structure over time -
Both myofiber ends join to a connective
tissue scar that has filled the injury gap by forming mini-myotendinous
junctions with the scar periphery.
-
-
Mature skeletal muscle contains two major populations of satellite cells: committed and stem.
-
Committed satellite cells are ready to begin differentiation to myoblasts immediately upon muscle injury.
-
Stem satellite cells first undergo
division before differentiation, providing another pool of satellite
cells should subsequent injury occur to the same muscle.
-
-
-
-
Formation of the connective tissue scar
-
Immediately after injury the gap between the ruptured muscle fibers is filled with hematoma.
-
Early fibrin and fibronectin
cross-linking forms granulation tissue within the gap that provides a
foundation for arriving fibroblasts and most importantly the initial
strength to withstand contractile forces. -
Type III collagen is soon synthesized, and type I collagen synthesis is initiated a couple of days later.
-
This initially large connective tissue scar condenses efficiently into a small scar composed mainly of type I collagen.
-
Early on, this scar represents the
weakest point of the injured skeletal muscle, but its tensile strength
increases considerably with the production of type I collagen. -
Approximately 10 days after injury, due to type I collagen cross-links, the scar is no longer the weakest link.
-
Rupture at this time most often occurs at the mini-myotendinous junction between the myofibers and scar.
-
The muscle still needs a relatively long time before its tensile strength is restored completely.
-
In reruptures or major trauma a dense scar tissue may result that acts as a barrier restricting myofiber regeneration.
-
With time, the connective tissue scar shrinks, bringing the myofiber ends closer to each other.
-
Whether these ends will ever fuse is still unknown.
-
Regenerating myofibers increase their tensile strength by reinforcing their adhesions to the extracellular matrix peripherally.
P.492 -
Classification
Table 26-3 covers the classification of muscle injuries.
Myositis Ossificans
-
A non-neoplastic formation of bone within skeletal muscle at a site of previous injury or hematoma
-
Relatively rare complication of muscle injury
-
Higher incidence in contact sports
-
Should be suspected if pain and swelling do not subside by 10 to 14 days after an injury
-
Radiographic detection of ectopic bone
may be seen as early as 18 days after injury, but often the formation
of ectopic bone lags behind symptoms for weeks.
|
Table 26-3 Clinical Classification of Muscle Injuries (Strain/Contusion)
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
Delayed Muscle Soreness
-
Muscle pain typically occurring 48 to 72
hours after intense exercise (can begin several hours after exercise
and peaks after 1 to 3 days) -
Primarily associated with eccentric exercise and varies depending on intensity and duration
-
Strength loss and muscle swelling are common signs.
-
Structural changes typically occur.
-
Z-band streaming, A-band disruption, and myofibril misalignment
-
Most severe at 2 to 3 days after exercise and tend to occur primarily in type IIb fibers
-
Ultrastructural damage is thought to lead to edema formation within the muscle, which in turn results in soreness.
-
Immobilization and Disuse
-
Muscle atrophy is among the initial changes seen with immobilization.
-
Atrophy results in a loss in muscle mass with associated reductions in muscle strength and endurance.
-
Decreased endurance, or higher
fatigability, is associated with the diminished ability to utilize fat
in aerobic pathways, decreased energy stores, and an increase in
intramuscular lactic acid levels.
-
-
Muscle changes depend on the amount of time that the muscle is immobilized and the position of immobilization.
-
Muscles immobilized under no tension undergo more detrimental changes than those immobilized while stretched.
-
Muscles immobilized in a stretched
position (i.e., hamstrings in a knee immobilizer) exhibit a decreased
strength and cross-sectional area, but due to the addition of new
contractile proteins and sarcomeres, the change in muscle mass is less
pronounced. -
Muscles immobilized in a shortened position can exhibit higher tensions for a given passive stretch, or stiffen.
-
Acutely Injured Skeletal Muscle
-
Advantages of early mobilization of an
acutely injured muscle: shown to induce more rapid capillary ingrowth,
results in better myofiber regeneration, produces more parallel
myofiber orientation, and regains muscle strength more quickly -
Disadvantages of early mobilization: associated with excessive scar formation at the injury site and increased rerupture rates
-
Advantage of immobilization for an acutely injured muscle: allows time for stronger, more efficient scar formation
-
Disadvantages of immobilization: can lead
to muscle fiber atrophy, excessive deposition of connective tissue
within muscle tissue, and a substantial delay in the recovery of muscle
strength
P.493
Treatment Recommendations
-
A short (3- to 5-day) period of relative
immobilization, allowing the scar tissue and myofiber ends to gain the
strength needed to withstand contractile forces and prevent rerupture -
After a few days, gradually, within the
limits of pain, begin active mobilization that will enhance the
penetration of muscle fibers through the connective tissue scar, limit
the size of the permanent scar, facilitate the proper muscle fiber
alignment, and restore the tensile strength of the muscle more rapidly.
Hormonal Effects on Skeletal Muscle
Insulin
-
Insulin increases glucose and amino acid
uptake by the muscle, increases glycogen synthesis and ribosomal
protein synthesis, and decreases protein catabolism and the release of
gluconeogenic amino acids.-
Results in an anabolic effect, increasing both protein and carbohydrate stores within the muscle
-
-
Insulin effects antagonized by glucocorticoids
-
Glucocorticoids accelerate protein degradation, decrease amino acid transport, and hinder protein synthesis.
-
Growth Hormone
-
Produced in the pituitary gland
-
By increasing muscle uptake of amino acids and protein synthesis, positively influences skeletal muscle synthesis
-
Growth hormone leads to a metabolic shift
that favors fatty acids metabolism, reducing amino acid and glucose
breakdown within the skeletal muscle. -
Evidence exists for the direct
interaction between growth hormone and skeletal muscle, as well as an
indirect influence through somatomedins and insulin-like growth factors
(IGF-I, IGF-II). -
Patients with excessively high growth
hormone levels exhibit selective hypertrophy of type I muscle fibers
and atrophy of type II fibers, often leading to weakness and fatigue.
Testosterone
-
Anabolic; increases protein synthesis and decreases the rate of protein catabolism within the muscle fiber
-
Testosterone and other androgens exert a growth-promoting influence on bone and skeletal muscle.
-
Results in increased muscle size
-
Androgens promote physeal closure of the long bones.
Muscle Stretching and Viscoelasticity
-
The viscoelastic properties of skeletal
muscle help explain the improved range of motion and decreased
stiffness seen after stretching and warm-up exercises.
Stress vs. Strain
-
Muscle is viscoelastic (i.e., it exhibits a time-dependent stress-vs.-strain relationship).
-
A certain muscle tension develops when stretched to a given length.
-
With time this tension decreases.
-
The phenomenon of diminished muscle stress with time is called stress relaxation.
-
Muscle stretched quickly is stiffer than muscle stretched more slowly.
-
Cold muscle is stiffer and therefore develops more tension than a warm muscle when stretched to a given length.
Creep
-
Skeletal muscle also undergoes creep.
-
Under a given load, muscle reaches an initial length and then slowly stretches with time.
Electromyography (EMG)
-
Means of detection and measurement of electrical currents passing across muscle cells
-
An electrical representation of neuromuscular activation during muscle contraction
-
Types of detecting electrodes
-
Skin electrodes
-
Applied to skin for use in detecting underlying muscle currents
-
Good for use in large muscle testing
-
-
Needle electrodes
-
Inserted directly into the muscle
-
Allow for the study of individual muscles and muscles not on the surface
-
-
-
EMG provides an excellent representation of muscle activation but a less accurate picture of the force being exerted.
-
EMG provides added insight into the muscles recruited during activities ranging from simple gait to complex movements.
-
EMG can help in the diagnosis of neuromuscular pathology.
-
In cases of neuropraxia
-
The nerve distal to the site of injury does not undergo necrosis.
-
Results in muscle silence and absent signal on EMG
-
-
In cases of axonotmesis or neurotmesis
-
The axons distal to the injury undergo necrosis.
-
Leads to degeneration of the motor endplate over several weeks
-
This causes depolarization of the individual muscle fibers.
-
Needle electrodes can detect these smaller action potential or fibrillations, indicating muscle denervation.
-
With regrowth of axons across the site of injury, the muscle may become reinnervated.
-
Reinnervation leads to large atypical motor units that produce abnormally large muscle potentials.
-
EMG detection of these giant polyphasic action potentials provides the examiner with objective evidence of muscle reinnervation.
P.494 -
Suggested Reading
Appell HJ. Muscular atrophy following immobilisation. A review. Sports Med 1990;10(1):42–58.
Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Orthop Surg 2001;9(4):227–237.
Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 1997;29(2): 197–206.
Caiozzo VJ, Utkan A, Chou R, et al. Effects of distraction on muscle length: mechanisms involved in sarcomerogenesis. Clin Orthop Relat Res 2002(403 Suppl):S133–145.
Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med 2004;34(12):809–824.
Fitts RH, Widrick JJ. Muscle mechanics: adaptations with exercise-training. Exerc Sport Sci Rev 1996;24:427–473.
Garrett WE Jr. Muscle strain injuries. Am J Sports Med 1996;24(6 Suppl):S2–8.
Gollnick PD, Matoba H. The muscle fiber composition of skeletal muscle as a predictor of athletic success. An overview. Am J Sports Med 1984;12(3):212–217.
Green HJ, Jones S, Ball-Burnett ME, et al. Early muscular and metabolic adaptations to prolonged exercise training in humans. J Appl Physiol 1991;70(5):2032–2038.
Greiwe JS, Hickner RC, Hansen PA, et al. Effects of endurance exercise training on muscle glycogen accumulation in humans. J Appl Physiol 1999;87(1):222–226.
Hughes CT, Hasselman CT, Best TM, et al. Incomplete, intrasub stance strain injuries of the rectus femoris muscle. Am J Sports Med 1995;23(4):500–506.
Ingalls CP. Nature vs. nurture: can exercise really alter fiber type composition in human skeletal muscle? J Appl Physiol 2004;97(5): 1591–1592.
Jarvinen TAH, Jarvinen TLN, Kaariainen M, et al. Muscle injuries: biology and treatment. Am J Sports Med 2005;33(5):745–764.
Kelso
TB, Hodgson DR, Visscher AR, et al. Some properties of different
skeletal muscle fiber types: comparison of reference bases. J Appl Physiol 1987;62(4):1436–1441.
TB, Hodgson DR, Visscher AR, et al. Some properties of different
skeletal muscle fiber types: comparison of reference bases. J Appl Physiol 1987;62(4):1436–1441.
Matoba H, Gollnick PD. Response of skeletal muscle to training. Sports Med 1984;1(3):240–251.
Nikolaou
PK, Macdonald BL, Glisson RR, et al. Biomechanical and histological
evaluation of muscle after controlled strain injury. Am J Sports Med 1987;15(1):9–14.
PK, Macdonald BL, Glisson RR, et al. Biomechanical and histological
evaluation of muscle after controlled strain injury. Am J Sports Med 1987;15(1):9–14.
Noonan TJ, Garrett WE Jr. Muscle strain injury: diagnosis and treatment. J Am Acad Orthop Surg 1999;7(4):262–269.
Ryschon
TW, Fowler MD, Wysong RE, et al. Efficiency of human skeletal muscle in
vivo: comparison of isometric, concentric, and eccentric muscle action.
J Appl Physiol 1997;83(3):867–874.
TW, Fowler MD, Wysong RE, et al. Efficiency of human skeletal muscle in
vivo: comparison of isometric, concentric, and eccentric muscle action.
J Appl Physiol 1997;83(3):867–874.
Seger JY, Thorstensson A. Effects of eccentric versus concentric training on thigh muscle strength and EMG. Int J Sports Med 2005; 26(1):45–52.
Sheffield-Moore M, Urban RJ. An overview of the endocrinology of skeletal muscle. Trends Endocrinol Metab 2004; 15(3): 110–115.
Taylor
DC, Dalton JD Jr, Seaber AV, et al. Experimental muscle strain injury.
Early functional and structural deficits and the increased risk for
reinjury. Am J Sports Med 1993;21(2):190–194.
DC, Dalton JD Jr, Seaber AV, et al. Experimental muscle strain injury.
Early functional and structural deficits and the increased risk for
reinjury. Am J Sports Med 1993;21(2):190–194.
Taylor
DC, Dalton JD Jr, Seaber AV, et al. Viscoelastic properties of
muscle-tendon units. The biomechanical effects of stretching. Am J Sports Med 1990;8(3):300–309.
DC, Dalton JD Jr, Seaber AV, et al. Viscoelastic properties of
muscle-tendon units. The biomechanical effects of stretching. Am J Sports Med 1990;8(3):300–309.
Thompson LV. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther 2002;32(2):44–57.
Yarasheski KE, Campbell JA, Smith K, et al. Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Physiol 1992;262(3 Pt 1):E261–267.