REVISION AND SALVAGE AFTER SURGERY FOR SPINAL DEFORMITY
VIII – THE SPINE > Spinal Deformity > CHAPTER 163 – REVISION AND
SALVAGE AFTER SURGERY FOR SPINAL DEFORMITY
fusions and fusion with casting, to fusion using Harrington and Luque
instrumentation, to the current generation of segmental systems that
attempt to correct spinal deformity in three planes. Regardless of the
treatment method, there have always been some patients who lose
correction—either because of a pseudarthrosis or by progression of the
curve—or who have poor results because of pain. Although acute failures
can be treated by immediately reinstrumenting and augmenting the
fusion, patients who have lost correction over time typically require a
more aggressive approach to reconstruction. To successfully manage
these patients, the surgeon must understand why the loss of correction
occurred, develop a sound preoperative plan, and choose a surgical
approach designed to provide a painless arthrodesis, good correction of
deformity, and a well-balanced trunk.
in the way fusions are performed, the way fusion levels are selected,
and the types of instrumentation used to stabilize the spine (2,7,9,21). Despite technical advances, the following remain the most common causes of failure:
-
Pseudarthrosis
-
Fixation failure
-
Inadequate fusion length
-
Progression of deformity due to the “crankshaft” phenomenon
-
Addition of new segments to the old curve
-
Progression of an untreated, secondary curve
reported the results of 196 patients treated by cast correction and
posterior fusion. They noted that 46 patients (23%) required
reoperation for pseudarthrosis repair or a combination of
pseudarthrosis repair and osteotomy. McMaster and James (20)
reviewed the experience of a number of authors and concluded that
pseudarthrosis rates ranged from 3.3% to 68.3% (average, 22.5%) in
patients treated without internal fixation. When internal fixation was
used, this rate was significantly lower (2% to 17%; average, 6.4%).
With current instrumentation techniques, a pseudarthrosis rate of
between 2% and 5% is typical in adolescent idiopathic scoliosis (1,5,7,10).
However, other deformity groups remain at much greater risk. Adults
treated for idiopathic and paralytic scoliosis have a 10% to 15%
incidence of pseudarthrosis (12,23,25,26,28). Patients with myelodysplasia and paralytic scoliosis have a 20% to 45% incidence of pseudarthrosis (18,22).
First, the fusion technique itself has improved over the years.
Decortication of transverse processes, removal of facet joints, and
meticulous exposure of the lumbar transverse processes have all
resulted in improvements in fusion rates. Second, the use of autograft
bone to augment the fusion mass has greatly improved success rates.
Finally, the use of spinal instrumentation and the subsequent
improvement in instrumentation constructs have further reduced
pseudarthrosis rates. Still, some failures are inevitable. The surgeon
has no control over the patient’s age at the time of surgery, the
nature of the deformity (paralytic, congenital, or those associated
with neurofibromatosis or Marfan’s syndrome), or the location and
severity of the curve at presentation. All of these factors have an
impact on fusion rates. Regardless of technique or instrumentation,
pseudarthroses will continue to occur in patients with severe and
recalcitrant curves.
the addition of pedicle screws, instrumentation failures are
inevitable. Excessive distraction and rotational forces applied to
large, rigid curves can result in hardware displacement if the hook
fractures through the lamina. Poor purchase of the hook over a deformed
or rotated lamina may result in hook displacement at either end of the
curve. Transverse process fractures may compromise the
transversopedicular claw even in a well-designed scoliosis construct.
Patients with poor bone quality or osteomalacia are also at increased
risk for hardware displacement. Rod breakage, seen in 7% to 10% of
Harrington constructs, is less common with current segmental systems
but can still occur as either an acute or a late complication (7).
Whether fixation is lost because of failure through the bone or failure
of the hardware itself, the patient is exposed to a great risk of
pseudarthrosis, pain, and loss of any correction gained at the initial
surgery.
reported that, among adult patients requiring revision surgery, the
initial spinal fusion had been too short in a significant number.
Cummine et al. (4) found that 40 of 59 patients
requiring reconstruction for failed scoliosis fusion had curve
progression due to incorrect selection of fusion levels at the initial
operation. Inadequate fusion length may result in the following
situations:
-
The initial surgery stopped short of the appropriate end vertebra, leaving part of the primary curve unfused.
-
The surgeon inadvertently selected a
fusion level that did not address all the involved segments,
particularly when there were a number of parallel end-vertebrae in the
primary curve. -
Sagittal malalignment was not corrected, leaving an unfused kyphosis that tended to increase over time (17).
-
In children fused at a young age,
progression can be seen even when the initial fusion correctly
addressed the entire primary curve. Over time, additional vertebrae not
part of the original scoliotic curve may become involved.
at an early age. These patients, through growth and remodeling,
experience an increase in curve magnitude without an increase in curve
length. One of the earliest descriptions of what is now considered the
“crankshaft” phenomenon was provided in Ponseti and Friedman’s 1950
paper on progressive deformity after fusion (24). Letts and Bobechko (16)
reviewed the outcome of children undergoing spine fusion before the age
of 8 years and found that 26% had significant curve progression despite
successful arthrodesis. They found that progression was an even bigger
problem in children fused at less than 4 years of age. Curve
progression was greatest during the rapid growth phase, and the
patients who had the greatest problem with this type of curve
progression were those with a congenital scoliosis requiring fusion at
a very early age. The progressive bending of an apparently solid fusion
mass has been explained as the continued growth of the
anterior vertebral elements within a spinal segment that has been fused (tethered) posteriorly (6,14).
As the anterior elements elongate, the vertebral bodies are forced
farther out from the midline, increasing both the sagittal and
scoliotic deformities. Anterior fusion at the time of the initial
posterior surgery prevents this phenomenon.
imbalance and deformity despite appropriate initial treatment and a
successful primary fusion. In these patients, the progression occurs in
the secondary curve either above or below the primary curve, resulting
in significant trunk imbalance and an increase in apparent deformity.
Whether this happens because of relentless progression of the secondary
curve or the patient’s progressive inability to compensate for the
fused primary curve, the problem generally begins with a mild to
moderate compensatory curve that, with age, becomes more severe and
structural.
the adult population. Curve progression in the lumbar segments results
in low back pain, degenerative disc disease, translational deformities,
and, in some cases, nerve root compression in the concavity of the
curve. Once these curves become structural, correction may not be
possible without simultaneously addressing the previously fused primary
curve.
Generalized pain may involve either the convexity or the concavity of
the curve. In the thoracic region, pain over the convexity of the curve
usually involves paraspinous muscles overlying the ribs and bony
prominence. Patients may also complain of subscapular or interscapular
pain in the region of the rhomboid and levator muscle attachments. Pain
on the concavity may be localized to the paraspinous muscles or may be
radicular in nature. In thoracolumbar and lumbar curves, pain in the
concavity is frequently associated with degenerative disease and facet
arthrosis. Translational shifts, commonly seen in degenerative curves
of the lumbar spine, may cause severe and debilitating pain related to
both segmental instability and muscle spasm, as well as nerve root
impingement and stenosis.
patients as for any patient with chronic low back pain. Patients
complaining primarily of pain without evidence of curve progression
warrant a full course of nonoperative treatment, including physical
therapy, anti-inflammatory medications, pain behavior modification, and
a trial of bracing, before entertaining surgical options. All
reasonable conservative measures should be explored prior to scheduling
surgery.
previous fusion, suspect a pseudarthrosis. Pain from pseudarthrosis
usually increases with activity and improves with rest. The area may be
tender; however, if spinal instrumentation is in place, palpation may
not produce symptoms. The patient may complain of a popping or grating
sensation with movement, fatigue at the end of the day, or a sense that
the deformity is progressing. Fixation failure, broken hardware, and
progressive deformity strongly suggest the presence of a
pseudarthrosis. Standard tomography is the most reliable method of
demonstrating the defect.
suffer at the levels immediately caudal to the fusion mass. This may be
related either to discogenic pain or to facet arthrosis. Cochran et al.
(3) demonstrated a proportionally greater
incidence of degenerative disc disease as fusions are carried more
distally. Their study showed that patients with fusions to L-2 had a
20% incidence of symptomatic disc degeneration; those with fusions to
L-3, a 40% incidence; those with fusions to L-4, a 60% incidence; and
those with fusions to L-5, an 80% incidence. Changes in spinal
mechanics result in degeneration of the intervertebral disc and facet
joints at the junction of the fused and mobile segments. Disc prolapse,
facet hypertrophy, and translational deformities may produce spinal or
foraminal stenosis. Patients with these disorders typically have
radicular symptoms as well as back pain. Discography, done correctly,
is frequently helpful in confirming the diagnosis of a painful disc
below a previous fusion (2,11).
common symptom among patients presenting for revision surgery. Patients
frequently sense that the curve has changed in magnitude or contour,
and they complain of changes in shoulder alignment, height, or comfort
when sitting or standing. They may also complain of alteration in
waistline contour or discomfort in the flank caused by impingement of
their ribs on the iliac crest. Patients may develop progressive
deformity in either the sagittal or coronal plane, or both.
to progression of the deformity, it is a more particular concern in
patients with a previous fusion; increasing curvature
below
a solid fusion, which allows no compensation, can generate a
disproportionate imbalance relative to the change in curvature. With
age, a previously compensatory curve may become structural,
compromising the patient’s ability to compensate for the original,
static curve. As trunk imbalance increases, the patient’s function is
progressively impaired, and the pain may increase dramatically.
complain of dyspnea, pulmonary dysfunction is much more common in
neuromuscular and postpoliomyelitic patients (4,17). Winter et al. (31) and Weinstein et al. (29)
noted that patients with idiopathic scoliosis develop pulmonary
insufficiency only when their curves become severe. However, advanced
age may significantly aggravate this problem in patients undergoing
revision surgery. Scoliosis surgery is more often done to prevent pulmonary complications in high-risk patients than to treat existing or progressive dysfunction.
scoliosis radiographs to carefully document the magnitude of deformity
and establish the correct end-vertebrae. The lateral view is
particularly important because sagittal imbalance is common and
debilitating in these patients. Loss of normal lordosis, or marked
lateral decompensation, may cause the patient to stand with one or both
knees flexed; it is important that upright radiographs be taken with
the knees extended to adequately represent the deformity. Other studies
include supine, oblique radiographs to evaluate the fusion mass, and
tomograms to confirm the presence of pseudarthrosis. Assess spinal
rigidity through side-bending views in scoliotic patients, and use
hyperextension views, supine over a bolster, for kyphotic deformities.
Side-bending films should visualize the full extent of both upper and
lower curves, assessing the cervicothoracic junction in thoracic curves
and the lumbosacral junction in thoracolumbar or lumbar curves. Use
magnetic resonance imaging (MRI) or myelography to evaluate any
neurologic abnormality in a patient with progressive scoliosis.
Diastematomyelia, tethered cord, syrinx, and cord or root compression
may be ruled out by using these studies.
important in adults, where pulmonary complications are common and
potentially life-threatening. Pulmonary function testing is indicated
in any patient complaining of dyspnea and in all those with
neuromuscular or chronic pulmonary disease. Patients with a severe
thoracic lordosis, with a reduced AP chest diameter, may also need
pulmonary function testing even if asymptomatic. A medicine
consultation is often appropriate for middle-aged patients preparing to
undergo a major spine reconstruction, and it is particularly important
in those with underlying cardiovascular, pulmonary, or renal disease.
initiated 3–4 weeks prior to the operation. Prescribe supplemental iron
for any patient donating her own blood.
early identification of the problem. If a pseudarthrosis or failure of
fixation is identified in the early postoperative phase,
reinstrumentation can be carried out easily without osteotomies or
major reconstruction. Likewise, if progression of an adjacent
compensatory curve is identified early, it may be corrected by simply
extending the fusion while the curve is still supple. If these problems
are neglected for long, however, compensatory curves may become
structural and pseudarthroses may lead to progressive deformity and
decompensation. In these patients, reconstruction and hardware revision
can be challenging.
progressive deformity. In cases with no loss of correction, the surgeon
may address the pseudarthrosis directly, retaining or replacing
hardware as necessary.
-
After exposing the fusion mass over its
full length, strip the thickened periosteum laterally with Cobb
elevators. Carefully expose the cortical bone of the fusion mass
throughout its length and from transverse process to transverse process. -
At the pseudarthrosis, the periosteum
will be bound down to the fibrous tissue insinuated into the bony
defect. At this point, it becomes difficult to strip the periosteum
away, and the persistent fibrous tissue left behind reveals the defect. -
The fusion defect is usually located at
the level of unfused facets. Using curets and rongeurs, remove this
fibrous tissue completely from the pseudarthrosis.
deformity, correct the deformity at the time of the pseudarthrosis
repair. Better curve correction at the time of revision surgery
correlates with a significantly better fusion rate (15).
Increased age, lower lumbar pseudarthrosis, and nonsegmental
instrumentation are associated with a significantly higher rate of
salvage failure.
-
If segmental instrumentation is in place
and in good alignment, do not remove it to treat the pseudarthrosis.
Revise hardware that has failed or is loose, however. -
Remove and replace nonsegmental
instrumentation with segmental fixation: Pseudarthrosis repair using
Harrington rods is successful in fewer than 60% of patients, while
repair using segmental instrumentation has provided 100% success in
some series (15,30). -
Substitute a shorter compression
construct at the site of the pseudarthrosis, if possible, to avoid
reinstrumenting the entire spine. -
In a mature fusion, insert compression hooks through the dorsal cortex of the fusion mass without entering the neural canal.
-
Place pedicle screws in the thoracolumbar
region to restore fixation where hooks have failed. Position pedicle
screws under fluoroscopic or stereotactic control. -
Once the pseudarthrosis has been debrided
of all soft tissue, decorticate the facets and pack the defect with
cancellous bone. Then reapply the instrumentation, decorticate the
fusion mass extensively with a gouge, and reapply local and autograft
bone to obtain fusion.
adult patients with risk factors for delayed healing or recurrent
pseudarthrosis, perform anterior interbody fusion in conjunction with
the posterior repair. Particularly in pseudarthroses of the lower
lumbar spine or where fusion crosses the lumbosacral junction, anterior
interbody fusion increases the chances of a successful arthrodesis.
Likewise, in patients with pseudarthrosis and progressive deformity,
anterior release and fusion may be necessary to obtain sagittal
correction and maximize the chance of a solid fusion. The debilitating
sagittal imbalance associated with the lumbar flatback syndrome is most
successfully corrected through a combined approach, coupling anterior
osteotomy and interbody fusion with posterior osteotomy or
pseudarthrosis repair and instrumentation (13).
usually heralded by the sudden onset of severe pain. Pain is usually
well localized to the region of failure, and the displaced hook or rod
is sometimes palpable through the skin. There is sometimes an
associated episode of trauma, and symptoms may begin several days
before the instrumentation actually displaces. If the patient’s curve
was particularly rigid, or if poor bone quality or severe rotation made
fixation particularly difficult, have a higher index of suspicion for
hardware displacement.
-
First, if correction has not been lost
and the patient’s pain is tolerable, postpone surgical revision and
treat the patient in a rigid orthosis until fusion occurs. Solid fusion
of the operated segments will eliminate the problem of the displaced
hardware, and any prominent hardware can be removed at a later date if
necessary. -
A second option is to reoperate, and
either reinsert the dislodged hook or remove it, shortening the
construct. This may reduce the patient’s pain and eliminate the
prominent hardware, but it could lead to a loss of correction.-
If the dislodged hook is surgically replaced, take care to see that other fixation hooks are not displaced during the revision.
-
Carefully inspect the lamina under which
the hook was seated to ensure that it is not fractured and that the
bone is sound enough to allow rigid fixation; reinstrumenting a damaged
or disrupted lamina is unwise. -
If you choose to remove the displaced
hook and shorten the instrumentation construct, you may significantly
compromise construct stiffness. Only when fusion is established and
curve progression is unlikely does removal of an end-hook make good
sense.
-
-
The third option is to revise the
hardware with a modified construct. This is most often necessary in
patients with end-hook displacement, particularly those with rigid
curves. In these cases, revising the terminal segment fixation may
salvage the overall construct.-
Where a single hook has pulled out or dislodged, a claw configuration may provide rigid fixation and salvage the construct.
-
Salvage a transverse process fracture by applying a sublaminar hook in place of the transverse process hook.
-
Use one or two pedicle screws on the convex side to salvage hook failure in lower lumbar curves (Fig. 163.1).
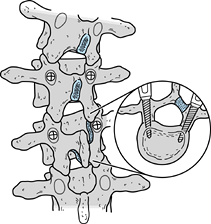 Figure 163.1.
Figure 163.1.
Pedicle screws can be used to salvage sites where hooks have loosened
or pulled through the lamina. Orientation of the pedicles is determined
on preoperative computed tomography. Pedicle screw fixation allows the
surgeon to reinstrument the spine without extending the fusion to
adjacent segments.
-
pullout that it is unsalvageable, you must make a critical judgment. If
shortening the construct to the next intact lamina will compromise
fixation of the appropriate segments of the curve, then the
instrumentation must be extended over a longer segment. This may
necessitate removal of the previous instrumentation.
-
In some cases it may be possible to
salvage and lengthen an instrumentation construct by using “domino” rod
connectors, extending the construct to the next stable level. This
provides a bulky construct, however, which is more acceptable in the
lumbar region than in the upper thoracic segments. This technique is
particularly useful if fusion must be extended to the sacrum. Short
segmental rods may be fixed to the sacrum either directly, using
pedicle screws, or using a Galveston technique to instrument the iliac
wings. These rods are then joined to the original construct using the
“domino” connectors.
must be replaced. All hook and screw attachments should be checked in
the process. Reactive bone often reinforces the hook or screw insertion
site, and these implants may be left in place. If the hook or screw
site has been eroded by excessive motion or has fractured, a new
fixation point must be chosen, or a different implant used—exchanging a
pedicle screw for a hook, for example.
multiple pseudarthroses, adding unfused segments or bending the fusion
mass may require multiple osteotomies, reinstrumentation, and extensive
fusion before the spine is adequately corrected, stable, and balanced
over the sacrum.
-
When a progressive curve develops below a
previous thoracic fusion, correct the upper curve when you correct the
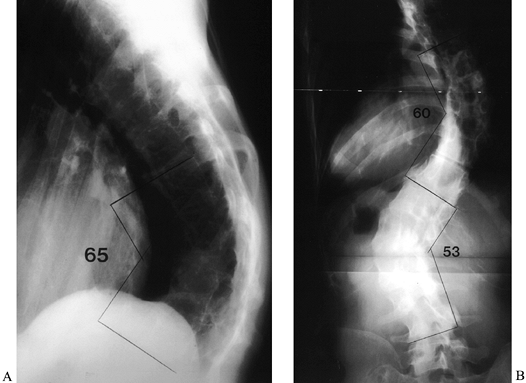
lumbar curve to avoid causing significant coronal imbalance (19) (Fig. 163.2).![]() Figure 163.2. AP (A) and lateral (B) views of a 36-year-old woman with Marfan syndrome who presented 20 years after an in situ
Figure 163.2. AP (A) and lateral (B) views of a 36-year-old woman with Marfan syndrome who presented 20 years after an in situ
thoracic fusion for scoliosis (T-6 to T-12). She had low-back pain,
progressive shoulder asymmetry, and progressive thoracic and lumbar
deformities. The lateral view shows a significant thoracolumbar
kyphosis, centered at T-9, which resulted in sagittal imbalance. The
thoracic fusion was substantial and solid. (From McLain RF. Revision
and Salvage in Deformity Surgery. Semin Spine Surg 1993;5:214, with permission.) -
Use preoperative radiographic studies to
determine the stiffness of the previously fused curve, as well as the
potential to correct the progressive compensatory curve. -
If pseudarthrosis is contributing to curve progression, obtain tomograms to document the extent and location of the defect.
-
Obtain a bone scan to confirm the level
of pseudarthrosis in difficult cases. Oblique radiographs can often
identify the suspicious level.
necessary to perform anterior release and interbody fusion prior to
posterior reconstruction. Both procedures may be performed under one
anesthetic, but the two procedures in combination are time-consuming
and demanding on both surgeon and patient. If excessive blood loss
occurs or the patient experiences problems, perform a two-staged
procedure.
-
For the anterior procedure,
place the patient in a lateral decubitus position. Establish
appropriate monitoring lines prior to positioning the patient: Arterial
and central venous lines are usually indicated, and some patients may
require Swan-Ganz catheterization to more carefully monitor pulmonary
pressures and cardiac output. -
Position the patient with the convexity of the most rigid and severe curve upward.
-
Use a transthoracic or thoracolumbar
surgical approach, taking one rib, or in some cases two, to expose the
anterior spinal column. -
Expose as many interspaces as can be reached and excise the discs.
-
Isolate and ligate segmental vessels on one side only, and spare particularly prominent vessels.
-
If the patient has a severe kyphosis,
peripheral vascular disease, or any other risk factor for cord
ischemia, place a “bull-dog” vascular clip across the segmental vessels
to temporarily occlude segmental flow. Spinal cord monitoring may then
determine whether the vessel is crucial to cord perfusion. -
Once the interspaces are exposed, remove
the discs with a scalpel and rongeurs, and remove the endplates with a
sharp osteotome directed away from the spinal canal. In very rigid
deformities, it may be necessary to release the entire posterior
longitudinal ligament and lateral annulus before correction can be
obtained. This is not necessary in more supple curves. -
Correct kyphotic segments by distracting
the disc space anteriorly with a large vertebral body spreader and
inserting a tricortical graft or titanium cage to restore sagittal
lordosis. -
At the end of the anterior procedure,
pull the pleura, or the paraspinous muscles in the thoracolumbar
region, gently over the spinal column to prevent graft extrusion. -
Place a chest tube and close the wound.
-
For the posterior part of the procedure,
turn the patient or dress the wound and return the patient to the
intensive care unit in anticipation of a staged posterior fusion. -
Perform the posterior reconstruction
through the old surgical wound. Expose the old fusion mass and the
spinous processes and lamina of the secondary curve. -
Obtain a radiograph after the rigid and compensatory curves are fully exposed, to verify levels.
-
Perform thoracic osteotomies at levels previously chosen in the preoperative plan (Fig. 163.3).
Center the initial osteotomy over the apex of the curve, and subsequent
osteotomies at every second level above and below the apex for the
length of the previous fusion (8).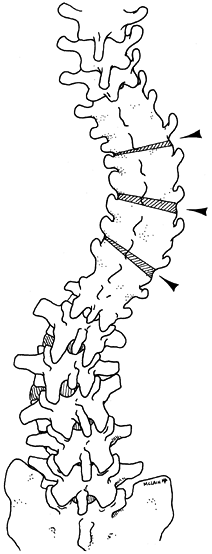 Figure 163.3. The preoperative plan for the patient in Figure 163.2.
Figure 163.3. The preoperative plan for the patient in Figure 163.2.
To correct the progressive lumbar curvature without precipitating a
marked thoracic imbalance, multiple osteotomies were planned as
indicated. The initial osteotomy was centered over the curve apex;
subsequent ones were located at every second level above and below the
apex. (From McLain RF. Revision and Salvage in Deformity Surgery. Semin Spine Surg 1993;5:214, with permission.) -
Take care to complete the osteotomy
across the full width of the fusion, and to take a wide enough wedge to
allow correction of the scoliosis. -
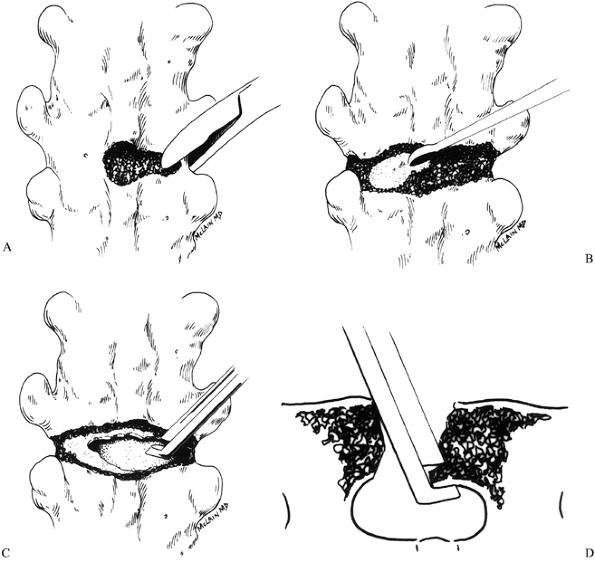
Remove the outer cortex with either a
burr or rongeurs, creating a defect that spans the fusion mass just
below the transverse processes (Fig. 163.4A).![]() Figure 163.4. Osteotomy technique. See text for details. (From McLain RF. Revision and Salvage in Deformity Surgery. Semin Spine Surg 1993;5:214, with permission.)
Figure 163.4. Osteotomy technique. See text for details. (From McLain RF. Revision and Salvage in Deformity Surgery. Semin Spine Surg 1993;5:214, with permission.) -
After exposing the anterior cortex,
penetrate it on the convexity with a burr or curet, creating a window
large enough to admit the Kerrison rongeur. Use the Kerrison punch to
complete the osteotomy across the full width of the fusion mass (Fig. 163.4C). -
After completing the osteotomy, use the
Kerrison punch to undercut the margins of the osteotomy defect. This
will reduce the chances that the cut edge of the fusion mass might
impinge on the thecal sac when the osteotomy is closed, a particular
risk when a kyphotic deformity is being corrected. Cut the osteotomies
so that they are wider posteriorly than anteriorly (again, particularly
if a kyphotic deformity is being corrected) (Fig. 163.4D). -
Once the osteotomy is complete, the
spinal segments above and below the cut should be independently mobile.
Position hooks or pedicle screws proximally and distally for correction
of both scoliotic curves.
-
If the fusion mass is sufficient, place
sublaminar wires or hooks through the dorsal cortex of the fusion
without entering the canal. -
Carefully prepare and decorticate the
area of the spine that was not previously fused, and pack the facet
joints with cancellous bone graft prior to instrumentation. -
Once the segmental wires or hooks have
been placed, apply the rods and correct the deformity either by
tightening the segmental wires to bring the spine to the contoured rod
or by gently rotating the rod, and sequentially distracting and
compressing the hook combinations (Fig. 163.5).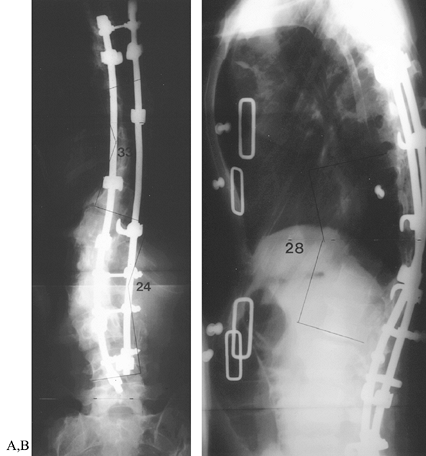 Figure 163.5. Postoperative radiographs of patient in Figure 163.2.
Figure 163.5. Postoperative radiographs of patient in Figure 163.2.
After multiple osteotomies from T-6 to T-10, segmental instrumentation
was used to correct the double curve to less than 50% of the
preoperative deformity. Both sagittal and coronal deformities were
improved, and shoulder asymmetry was corrected. (From McLain RF.
Revision and Salvage in Deformity Surgery. Semin Spine Surg 1993;5:214, with permission.) -
Pack local bone and iliac crest autograft around the osteotomy sites and over the previously unfused segment of spine.
pneumothorax. If he has not undergone an anterior procedure and a chest
tube was not previously placed, it is important to obtain a radiograph
in the operating or recovery room to look for a pneumothorax and to
place a chest tube if necessary.
-
Encourage the patient to sit up in bed or on a bedside chair on the first postoperative day.
-
Independent ambulation is not possible until a molded thoracolumbosacral orthosis (TLSO) is available.
-
The TLSO is usually molded by postoperative day 3 or 4, when the chest tube is removed.
-
Begin transfers and ambulation when the brace arrives, and discharge the patient when he is independent.
not be needed at night after the third month. Wean the patient from the
TLSO by 5–6 months, and begin physical rehabilitation for lifting and
trunk strengthening at 6–8 months. Take oblique radiographs to evaluate
the maturing fusion and thus ensure that the timing of brace removal
and rehabilitation is appropriate.
surgery, and even more frequent when it is revision surgery.
Complications in revision scoliosis surgery include the following:
-
Pneumothorax, pneumonia, and/or respiratory insufficiency
-
Infection
-
Persistent or adjacent-level pseudarthrosis
-
Instrumentation failure
-
Neurologic injury
-
Sagittal or coronal imbalance
-
Thromboembolic disease and pulmonary embolism
-
Death (a 1% to 2% mortality rate)
that the risks associated with scoliosis surgery appear to go up with
each decade of life. The overall complication rate for primary surgery
ranges from 53% to 62%, with a mortality rate of roughly 1.5% (23,25,26). The complication rates in patients undergoing revision surgery are even more daunting. Floman et al. (8)
reported that 52% of revision patients had a serious complication; 10%
developed pseudarthrosis, 14.5% had a significant neurologic
complication, 11% had significant pulmonary complications, and 1.6%
died (other complications made up the remaining 12.9%). Considering the
young age of this group of patients (average, approximately 21 years),
this is a high rate of complications. Kostuik (11)
discussed outcomes in 31 patients undergoing revision surgery for
scoliosis. The overall incidence of pseudarthrosis in this group was
23%, as opposed to a 6.5% incidence in adults undergoing primary
fusion. He noted a 10% incidence of pulmonary complications, including
nine pneumothoraces.
patients undergoing reconstructive surgery for failed scoliosis fusion.
The overall complication rate was 71%, with two postoperative deaths
(3.4%) in the group. They noted a 17% incidence of pseudarthrosis, a 5%
incidence of pulmonary complications,
and an 8% incidence of deep wound infections. There was one fatal and one nonfatal pulmonary embolism in this study group.
-
Monitor patients postoperatively to
maintain an adequate blood pressure. Postoperative hypotension can lead
to cord ischemia, particularly after extensive anterior dissection or
multiple procedures that may have compromised collateral blood flow.
Case reports have documented transient and permanent neurologic
injuries associated with episodes of postoperative hypotension (27,30).
and the routine use of prophylactic antibiotics have significantly
reduced the incidence of pulmonary embolism and deep wound infections
over those seen in previous studies. Likewise, the use of autograft
bone and improved techniques in instrumentation and surgical fusion
should reduce pseudarthrosis rates. Nonetheless, complications in
revision scoliosis surgery are likely to remain high, particularly in
older patients and those with neuromuscular or paralytic disorders.
management, surgical technique, and instrumentation technology will not
eliminate the common complications associated with reconstructive
surgery of the scoliotic spine. Early recognition of curve progression
or pseudarthrosis remains the most reliable way to limit the complexity
of these challenging reconstructions. In those patients who do develop
rigid curvatures with marked loss of correction, careful preoperative
planning is the key to a good surgical result.
T, Irstram L, Nachemson A. Long-term Anatomic and Functional Changes in
Patients with Adolescent Scoliosis Treated by Harrington Rod Fusions. Spine 1983;8:576.
AS, Richards BS. Preventing the Crankshaft Phenomenon by Combining
Anterior Fusion with Posterior Instrumentation. Does It Work? Spine 1995;20:1392.
WC, Bradford DS, Transfeldt EE, Ogilvie JW. Management of
Pseudarthrosis after Arthrodesis of the Spine for Idiopathic Scoliosis.
J Bone Joint Surg 1991;73:222.
C, Dickson J, Harrington P, et al. Results of Harrington
Instrumentation and Fusion in the Adult Idiopathic Scoliosis Patient. J Bone Joint Surg Am 1975;57:797.
Dam BE, Bradford DS, Lonstein JE, et al. Adult Idiopathic Scoliosis
Treated by Posterior Spinal Fusion and Harrington Instrumentation. Spine 1987;12:32.
RB, Denis F, Lonstein JE, Dezen E. Salvage and Reconstructive Surgery
for Spinal Deformity using Cotrel-Dubousset Instrumentation. Spine 1991;16:S412.