OPERATIVE TREATMENT OF CHILDREN’S FRACTURES AND INJURIES OF THE PHYSES
IX – PEDIATRIC DISORDERS > CHAPTER 164 – OPERATIVE TREATMENT OF
CHILDREN’S FRACTURES AND INJURIES OF THE PHYSES
adults because of the rapid healing and remodeling of bone that occurs
in children. A perceptive surgeon realizes that children differ a great
deal from adults and care of their fractures can be affected by a
child’s preinjury status, the specific fracture mechanics of childhood
injuries, the response to injury, and the unique treatment problems and
complications that occur in the pediatric age group.
nonoperative, there are certain instances when surgical management is
required, desirable, or optional. Open surgical treatment is indicated
in certain physeal fractures where there is joint incongruence and
closed reduction has not led to satisfactory position, and where exact
reduction improves the chances of normal physeal growth. Open
reduction
should be performed when anatomic reduction is required for normal
function, as in a displaced, both-bone forearm fracture in an older
adolescent. Surgical treatment should be considered in children with
multiple trauma if stabilization of major long-bone fractures will
enhance nursing care and pulmonary management. It should also be
considered in children with major long-bone fractures (especially
femoral shaft) in the presence of a severe head injury.
internal fixation is to alleviate the psychological stress of either
children or parents associated with prolonged hospitalization. An
example is a 13-year-old boy with a closed midshaft femoral fracture
that would eventually heal with skeletal traction and spica-cast
treatment—a treatment that might take 8 weeks or more. A closed
intramedullary nail would allow rapid mobilization, discharge from the
hospital in a few days, and return to home and school within 1 week.
 |
|
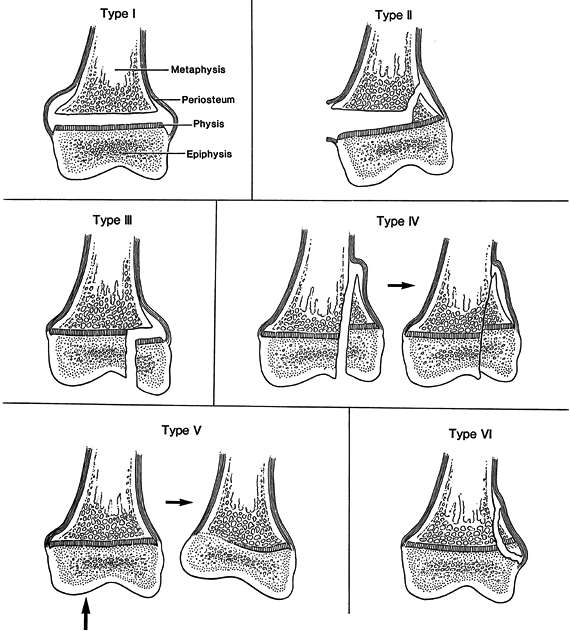
Figure 164.1.
Salter–Harris classification of physeal fractures. See text for description of types. (Redrawn from Salter RB, Harris WR. Injuries Involving the Epiphyseal Plate. J Bone Joint Surg Am 1963;45:587, with permission.) |
|
but some general principles will be reviewed here. Nondisplaced
fractures through the growth plate tend to be stable and require
immobilization without internal fixation. Minimally displaced
growth-plate injuries do not require reduction, and the chance of
growth arrest is not increased by leaving them displaced. Displaced
fractures requiring reduction should be treated early (within 48 hours)
because growth arrest is common after attempts at late reduction.
Atraumatic fracture reduction and suitable fixation (casting or
surgical) are mandatory.
exists and greater degrees of displacement are acceptable. However,
younger patients in whom physeal arrest develops have a greater
potential for deformity. Likewise, a growth plate that requires higher
energy to cause failure because of its geometry tends to have a higher
rate of problems with growth arrest. For instance, the distal femoral
and proximal tibial growth plates are only rarely injured but are
responsible for the majority of longitudinal and angular growth
abnormalities following growth-plate fracture. Salter–Harris types III
and IV fractures require anatomic reduction of both the growth plate
and the articular surface; thus they frequently require open reduction
with internal fixation.
of children’s fractures is beyond the scope of this chapter. Following
are descriptions of techniques that we have found useful for the
surgical treatment of common pediatric injuries, as well as uncommon
injuries requiring surgery. This chapter includes some generalizations
and personal preferences in both indications and treatment options;
readers should consult fracture textbooks and the scientific literature
for more extensive descriptions.
are most frequently seen in neonates and adolescents. Neonatal
fractures are typically Salter–Harris type I injuries caused by an
abduction–external rotation force imparted during the process of
delivery. Orthopaedic consultation is obtained in these cases because a
neonate will not actively move the involved extremity. Fracture of the
clavicle, Erb’s palsy, and infection are the main differential
diagnoses. Radiographs may not be helpful, although ultrasonography
yields a clear representation of this cartilaginous injury. Simple
immobilization of the arm to the trunk with a loose elastic bandage for
1–2 weeks allows complete healing.
metaphyseal fractures of the humerus. Most of these can be managed by
splinting because remodeling is rapid in this region and anatomic
reduction is not required for excellent function. Fortunately, physeal
growth arrest is rare and neurovascular injury uncommon. Closed
reduction is generally necessary only in patients near skeletal
maturity whose fracture has greater than 50° to 70° of angulation in
either the sagittal or the coronal plane. After initial muscle spasms
abate after treatment in a sling for 5–7 days, however, fracture
alignment frequently improves enough to eliminate the need for closed
reduction. If closed reduction does not yield an acceptable position,
reduction under anesthesia with shoulder spica-cast immobilization
usually suffices. On occasions when a spica cast may not be appropriate
(e.g., when there is a chest injury), surgical fixation may be
accomplished by introducing a large, smooth Steinmann pin into the
reduced humeral head through a 1 cm incision over the deltoid tubercle.
Bend the pin end to decrease the chance of proximal migration, and
immobilize the arm with a sling and swath. Image intensification is
necessary, and it is surprisingly difficult to place the pin in the
head with enough purchase to fix the fracture. Remove the pin at 3–4
weeks.
have the highest rates of complications of any pediatric fracture.
Volkmann’s ischemic contracture due to compartment syndrome, neurologic
or vascular compromise, and cubitus varus have historically complicated
the treatment of these fractures. Supracondylar fracture of the humerus
is
often a surgical emergency, and prompt reduction and stabilization will
reduce the incidence of complications. Although closed methods of
immobilization may be used, percutaneous pin fixation has emerged in
the last decade as the preferred method for unstable, displaced
fractures. Pin fixation, properly done, is a low-risk procedure that
provides excellent control of fracture fragments, nearly eliminating
the risk of cubitus varus that accompanies cast immobilization. In
addition, percutaneous pinning allows partial extension of the elbow
without loss of reduction, which is much safer when there is swelling
and vascular compromise.
extremity for neurovascular compromise or compartment syndrome (usually
in the flexor compartment of the forearm), and document the findings in
the chart. An absent radial pulse is an indication for prompt reduction
but is not in itself an indication for surgical exploration if the
capillary refill is intact and the hand well perfused after reduction
is accomplished. Neurologic deficits are common in supracondylar
fractures but generally disappear spontaneously within 3 months after
treatment. Exploration of the nerve is probably indicated only when
closed reduction is impossible in the face of a preexisting nerve
deficit (implying interposed nerve tissue in the fracture), or when
nerve deficit occurs coincident with reduction.
anteriorly displaced as a result of a flexion force applied to the
elbow. The remaining supracondylar fractures are caused by
hyperextension injuries of the elbow. They have been classified by
Wilkins (74) as follows:
|
posterolateral displacement. Regardless of the direction of
displacement of the distal fragment in an extension-type supracondylar
fracture, the posterior periosteum is generally intact and may be used
to assist reduction. Most supracondylar fractures occur with the
forearm in pronation; therefore, the distal fragment is internally
rotated relative to the proximal fragment. Thus, most are more unstable
after reduction with the arm internally rotated, a fact that has
implications when radiographs are obtained (see later discussion).
-
Place the patient in the supine position,
and administer a general anesthetic. Use an image intensifier in a
vertical position next to the table. The receiver can be used as a
minitable to set the arm on. -
Perform closed reduction by manually
distracting the fracture with the elbow slightly hyperextended and the
forearm in supination. Correct the medial or lateral displacement, and
then align the varus–valgus position of the arm to match the opposite
normal elbow. While still distracting, flex the supinated arm while
pushing posteriorly on the distal portion of the humeral shaft
(proximal fragment). Flex the elbow acutely, and temporarily hold it
flexed by wrapping a gauze or tape between the wrist and shoulder (Fig. 164.2A);
pronation of the forearm to “lock” the fracture is unnecessary if
percutaneous fixation is to be used. The pulse may not be palpable at
this time.![]() Figure 164.2. Surgical technique for percutaneous pinning of supracondylar fracture of the humerus. A: The hand and wrist are secured to the upper arm. B: AP and lateral image intensifier views are obtained by rotating the arm. C: A 14-ga needle is useful as a pin guide. See text for explanation.
Figure 164.2. Surgical technique for percutaneous pinning of supracondylar fracture of the humerus. A: The hand and wrist are secured to the upper arm. B: AP and lateral image intensifier views are obtained by rotating the arm. C: A 14-ga needle is useful as a pin guide. See text for explanation. -
Check an anteroposterior (AP) image,
using the image intensifier. Obtain a lateral view by externally
rotating the flexed arm on the image intensifier (Fig. 164.2B);
internal rotation can destabilize the fracture at this point and cause
loss of reduction. Exact anatomic reduction is unnecessary, but the
carrying angle should be restored. Some translation or angulation on
the lateral x-ray film is acceptable because it should correct with
remodeling. -
Percutaneous pinning requires two pins,
usually 0.045 Kirschner wires (K-wires), both of which may be inserted
from lateral and parallel or from medial, lateral, and crossed. If
crossed, they should not cross at the fracture site. Although crossed
pins have been shown to be biomechanically advantageous, two parallel
lateral pins are safer. An ulnar nerve palsy may result from injury to
the nerve at the time of insertion of a medial pin or from chronic
contact with the pin throughout the course of treatment. These
neurotmeses usually resolve in 3–4 months. When a medial pin is used,
massage the medial epicondyle for a few minutes to “milk out” edema to
be sure that the ulnar nerve is avoided, or insert the pin through a 1
cm incision under direct visualization. If the medial epicondyle cannot
be palpated, two lateral pins must be used. In either event, the pins
must pass through the distal fragment and engage the opposite cortex of
the proximal (shaft) fragment by passing just through the entire
cortex. We always use two lateral pins, if possible. -
To make pinning easier, insert a 14-gauge
needle into the periosteum of the distal fragment laterally at the
desired angle of the pin (Fig. 164.2C). Obtain
AP and lateral views (by external rotation), using the image
intensifier. After adjustment of the direction of the needle, insert an
0.045 K-wire through the needle, and drill it through the opposite
cortex of the proximal fragment. Place a second pin in a similar
manner. Withdraw the needles, bend the pins outside the skin to avoid
migration, and apply pin caps (Fig. 164.3).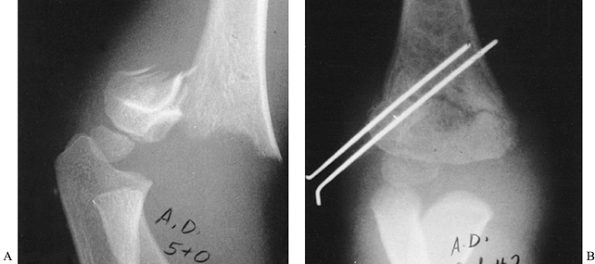 Figure 164.3.
Figure 164.3.
Displaced supracondylar fracture of the humerus fixed by closed
reduction and percutaneous pinning with two parallel lateral pins. Note
the engagement of the cortex of the medial proximal fragment. -
Extend the arm fully, and check the
carrying angle of the elbow; if it is not correct, repeat the preceding
procedure after a second reduction. Anatomic reduction on x-ray images
is not necessary, but cubitus varus will not remodel and must be
avoided. Flex the elbow to 90° (or less if the pulse disappears with
flexion), and immobilize with a posterior splint, sling, and swath.
position. Remove the pins and splint at 4 weeks after the fracture, and
begin motion as tolerated. Immobilization beyond 4 weeks is
unnecessary, and physical therapy is not appropriate.
fracture is compartment syndrome (Volkman’s ischemic contracture). It
is more common when there has been vascular compromise or massive
swelling has occurred, but it can appear in less dramatic clinical
situations. The hallmark feature of compartment syndrome is pain with
passive finger extension, but it is easy to misinterpret the
examination in a frightened or sedated child. Measure compartment
pressures if necessary; this is facilitated by the stability achieved
with percutaneous pinning.
and both fragments are engaged by the pins. If loss of position is
detected at 1 week, it may be possible to salvage a satisfactory
carrying angle by extending the arm fully, adjusting the carrying angle
back into valgus, and applying a long-arm cast with the forearm in
supination and the elbow extended. Do not remove the pins. Continue
immobilization for a total of 4 weeks.
pinning in the operating room. This is not advisable or possible after
approximately 10 days, as healing is too far advanced.
fractures may be associated with injury to the brachial artery. The
brachial artery and the median nerve are juxtaposed to the fracture
site and thus are subject to direct and stretch injury at the time of
fracture and reduction. A well-documented neurovascular examination
before closed reduction is mandatory to avoid unnecessary exploration
afterward. Brachial artery compromise may be due to acute thrombi,
intimal tears, laceration, transection, or entrapment within the
fracture site. Absence of a radial pulse or the presence of a mottled
arm and hand is an indication to proceed urgently to surgery for closed
reduction. Do not delay treatment because circulation returns with
fracture reduction. Because the site of vascular compromise is known,
angiography is usually not necessary.
extension, make a decision based on clinical examination of the hand.
If the fingers are pink and well perfused, it is safe to observe, even
if pulses are present. If the fingers are dusky, exploration of the
artery is indicated.
afterward, obtain a vascular surgery consultation. The vascular surgeon
may choose to obtain an angiogram with the image intensifier on the
operating-room table or proceed directly to exploration. If
revascularization is needed, closely monitor the patient for
compartment syndrome, and give serious consideration to performing
prophylactic forearm compartment releases.
consider an open reduction. Remember that anterior-to-posterior
translation and angulation are generally acceptable, and even the AP
radiograph does not need to be anatomic as long as the carrying angle
is satisfactory with the elbow extended. Open reduction usually proves
to be more difficult than anticipated. Use a surgical approach on the
side of the largest fracture gap. Periosteum and the brachial muscle,
nerve, and artery can all block reduction and should be looked for and
extricated. Open reduction has not been associated with increased
stiffness in children with this complication.
usually occur as the result of a fall on an outstretched hand and
consequently are Salter–Harris type IV intra-articular fractures, with
the initial failure beginning at the capitellar or trochlear surface.
Errors in interpretation of the radiograph can lead to a missed
diagnosis. Such fractures may be mistaken for type II fractures because
of their metaphyseal component (Fig. 164.4A),
but they are usually highly unstable injuries that require surgical
treatment. Lateral condylar fractures that are truly nondisplaced may
be treated nonoperatively, but they must be radiographed weekly because
they have a propensity to displace late.
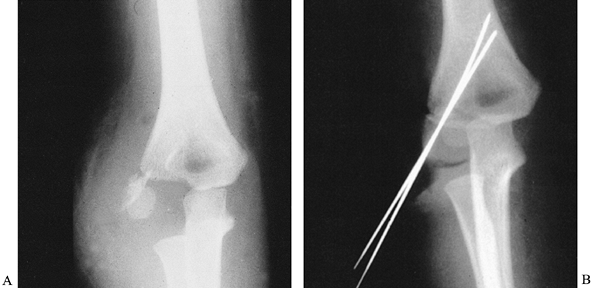 |
|
Figure 164.4. Lateral condylar fracture of the humerus treated by open reduction and pinning. A: AP radiograph of acute fracture. B: Fracture is anatomical after fixation with two K-wires.
|
seen in children approximately 2 years of age. They are usually
hyperextension injuries and are analogous to supracondylar fractures.
They can be distinguished from lateral condylar fractures by their
longer, more posterior metaphyseal fragment. Closed reduction
(occasionally together with percutaneous pinning) is appropriate for
these injuries.
condylar fractures is displacement, either acute or progressive, of the
visible fragments by more than 2 mm. Some have advocated closed
reduction and pinning for selected minimally displaced lateral condylar
injuries (47) as determined by intraoperative
arthrography. We generally perform open reduction because the joint
surface is often remarkably displaced.
-
Under tourniquet control, make a curved
longitudinal incision over the lateral humeral condyle. There is
usually a longitudinal rent in the brachioradialis muscle; develop this
interval and carefully expose the lateral margin of the condyle. Take
great care at this point to keep all subsequent dissection anterior to
the condyle because the blood supply of the capitellum lies posteriorly
and a major complication of this procedure is osteonecrosis of this
fragment. -
Open the elbow joint, and retract the
synovium anteriorly, using the long end of an army–navy retractor. The
distal fragment is frequently rotated up to 90° and may be much larger
than expected, including a sizable portion of the cartilaginous
trochlea. -
Gently clean the fracture ends of
hematoma and fibrous tissue, and reduce the fracture. Reduction may be
unstable. Sometimes, stability is facilitated by inserting a K-wire in
the fragment and using it as a “joystick” to control the fragment;
however, be careful to plan its insertion point so that it may be used
later to fix the fracture. -
Fix the fracture with two K-wires (Fig. 164.4B).
If the metaphyseal fracture is large, they may pass through it, but
often they must begin in the distal cartilaginous portion of the
condylar fragment. Leave a space of at least 3 mm between the wires,
and pass them just through the medial cortex of the proximal shaft to
ensure stability. Bring the wires out through small stab wounds in the
skin in the appropriate site. Bend the ends to prevent migration and
place pin caps. -
Close the wound with fine, absorbable suture. Apply a splint at 90° of elbow flexion.
and begin motion as tolerated. Immobilization beyond 4 weeks is
unnecessary.
fractures in children are missed diagnosis, nonunion, malunion, lateral
growth arrest, and cubitus valgus. Tardy ulnar nerve palsy is possible
late but fortunately is rare. Fractures treated by cast immobilization
only that do not heal by 8 weeks after the injury should be treated
with pin fixation and in situ bone
grafting of the metaphyseal portion. If an arthrogram shows contrast
agent between the capitellum and the trochlea, then do an open
reduction with pin fixation. This can be done as late as 8 weeks after
the injury. If contrast does not penetrate the fracture, then we pin
the fracture percutaneously to add stability and facilitate healing.
Leave wires in for 6 weeks, and then remove them.
treat. If the fracture is pain-free and there is no joint instability,
treatment is not required. This avoids the possibility of stiffness
secondary to bone grafting.
generally occur as a consequence of a fall on an outstretched hand that
cause buckling and impaction of the radial neck. They are uncommon
injuries. Their treatment is highly controversial because they often
have good potential for remodeling and the results of open reduction
are often poor. Despite the relatively minor appearance of some of
these fractures, they are significant injuries, and compartment
syndrome may occur.
children younger than 10 years. With greater angulation, closed
manipulative reduction or percutaneous reduction techniques are
indicated (Fig. 164.5).
Remember that open reduction can be complicated by elbow stiffness,
heterotopic ossification, growth arrest, and synostosis. A percutaneous
technique described by Metaizeau uses a curved K-wire inserted
retrograde into the canal from the distal radius (27). We have no experience with this technique, but it has been reported to be simple and effective.
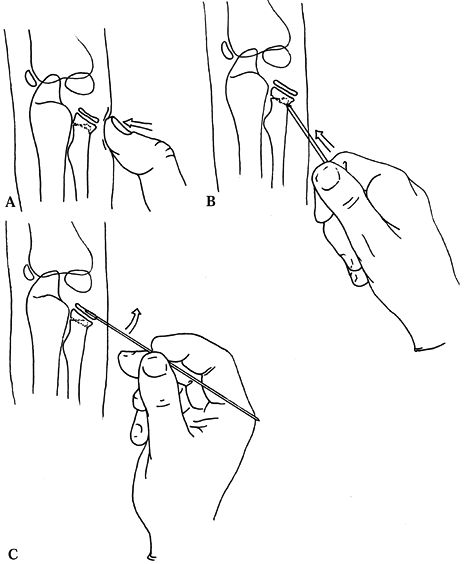 |
|
Figure 164.5.
Three options for management of a severely angulated radial neck fracture that may allow avoidance of open reduction. See text for description. |
-
Under general anesthesia, attempt a
closed reduction first; an assistant is helpful. Supinate the forearm,
apply traction and varus stress to the elbow, and place the thumb over
the radial head. By pronation and supination of the forearm, the
deformity may be palpable. When the radial head feels most prominent,
reduce the fracture by forceful pressure with the thumb (Fig. 164.5A).
An image intensifier may be helpful for localizing the deformity. If
reduction to 45° or less is obtained, accept the reduction, and
immobilize the elbow in a splint for 3 weeks. -
If reduction fails, rotate the forearm so
that the tilt of the radial head is maximal, and pass a Steinmann pin
percutaneously just below (distal to) the physis of the radial head,
using the image intensifier. Use the Steinmann pin to push the head
fragment back to an acceptable position (Fig. 164.5B). If this succeeds, immobilize the elbow for 3 weeks. -
If this fails in an older child with an
ossified radial head, a third option is to carefully pass an 0.035
K-wire transversely into the ossified radial head (be sure not to
damage the physis). Use this wire to manipulate the head fragment into
an improved position (Fig. 164.5C). -
In both of these percutaneous techniques, if the fracture is unstable, it may be held for 2–3 weeks with a small
P.4183
K-wire inserted percutaneously obliquely, usually from proximal to
distal, from the radial head to the shaft fragment. Be careful when
using pins for manipulation or fixation near the radial head not to
pass through the radius into the ulna; even one pass may cause
synostosis.
with particular concern about how much displacement of the fragment is
acceptable. The true significance of this fracture, however, is that
avulsion of the epicondyle is due to a subluxation or dislocation of
the elbow joint. Consequently, the fracture should be thought of as
similar to a medial collateral ligament injury, and the proper
treatment is dictated by the instability of the elbow and not by some
arbitrary degree of fracture displacement seen on radiographs.
guarded. Periarticular injury may accompany an elbow dislocation,
whether recognized or not, and can lead to permanent loss of elbow
motion of a magnitude unexpected in a child. Warn parents about this
early in the course of treatment. The medial epicondyle has a tendency
to enlarge because of hyperemia after surgery; thus, the cosmetic
result may be compromised after treatment.
and each child must be individually evaluated. The surgical technique
is straightforward, but take care to avoid injury to the ulnar nerve
during the dissection. Fixation may involve either K-wires or
small-fragment screws because the amount of growth remaining in the
usual patient is so small that cubitus varus will not develop.
is usually successful. Growing children exhibit excellent remodeling
potential, and angular and rotational deformities up to 15° are well
tolerated. In older children, treatment can be closed, as long as
reduction achieves satisfactory alignment, because union is rapid and
stiffness unlikely. Adolescents with both-bone forearm fractures,
however, represent a transitional situation between that of young
children (who tolerate imperfect reduction) and that of adults (who
generally require open reduction). We treat both-bone forearm fractures
in older adolescents with open distal radial or ulnar physes,
regardless of age, by performing closed reduction first; if the
reduction is anatomic or nearly so, we accept it and follow the child
with weekly radiographs until union occurs. If the reduction is not
acceptable, we proceed with open reduction, using either one-third
tubular plates or 3.5 mm compression plates and the same technique
employed in adults (see Chapter 16). In most
cases, it is wise to use the larger, 3.5 mm plate because nonunion is
not unusual in this age group. After open reduction, immobilize the
forearm in a long-arm cast until union occurs.
forearm fractures either cannot be reduced by closed means or, once
reduced, are too unstable to maintain the reduction in a cast (usually
when the fractures are at the same level in the bone). Unreducible
fractures may require a small incision to remove soft tissues blocking
the reduction. An intramedullary K-wire or flexible nail can then be
introduced either proximally through the olecranon in the ulna or
distally in the radial metaphysis. Flynn (22)
showed that intramedullary fixation of a single bone in both-bone
forearm fractures in conjunction with long-arm casting results in
excellent fracture fixation. The pins are left outside the skin and the
ends bent over. They can be removed in the office 4–6 weeks after
insertion.
the acceptable limits of reduction for pediatric forearm fractures. In
children younger than 9 years, 15° of angulation, 45° of malrotation,
and complete displacement can be accepted. In children age 9 years or
older, bayonet apposition, 30° of angulation, and 10° of malrotation
are acceptable. The closer the fracture is to the growth plate and the
younger the child, the greater is the remodeling potential in all
planes, except for rotational malalignment.
younger children (approximately 10 years) with distal both-bone
fractures in which the ulnar fracture is a greenstick fracture and the
radial fracture is displaced and translated dorsally with shortening of
approximately 1 cm. The radial fragment is often buttonholed through a
rent in the periosteum and cannot be reduced back to length. In such
cases, make a small dorsal incision, and pry the fragment back with an
elevator. Usually, no internal fixation is required after reduction is
achieved.
with massive pelvis injuries do not exhibit gross instability of the
fragments. Pubic symphysis widening is well tolerated and tends to
decrease after the child begins to walk. Proximal displacement of the
iliac bone is rare, but patients with fracture patterns susceptible to
displacement must be followed with serial radiographs. In rare
instances, they require external or internal fixation, as in adults.
For most children, bed rest followed by mobilization to a chair and
progression to weight bearing as tolerated, along with pain control, is
all that is required.
can usually be treated nonoperatively. When fragment displacement is
wide, assessment with computed tomography (CT) or, especially, magnetic
resonance imaging (MRI) will determine whether there is involvement of
the triradiate cartilage. The surgical principles for the management of
acetabular fractures in adults are outlined in Chapter 18.
They must be applied sparingly in children because triradiate cartilage
closure can be a serious complication in younger children, and
nonoperative treatment may be safer. Sometimes, cartilage joint
surfaces may remain intact, even though the underlying bone is
displaced, and these fractures need not be surgically treated (Fig. 164.6).
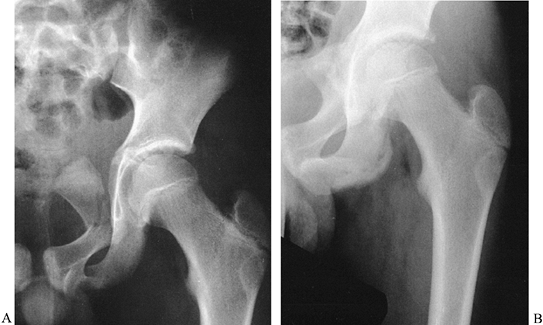 |
|
Figure 164.6.
An 11-year-old boy with a fracture of the pubic ramus apparently involving the acetabulum. In reality, the triradiate cartilage and acetabular articular cartilage were intact, and the acetabulum was normal 1 year later without reduction. |
seen in adolescent athletes, are known as transitional fractures
because they occur when the muscle forces approximate those in adults
but the bone is still immature. Surgical reattachment of the avulsed
fragment usually results in redisplacement; therefore, symptomatic
treatment is best for these injuries.
are dangerous injuries and do not behave similarly to their adult
counterparts. Because they are so rare, few orthopaedic surgeons have
extensive experience with them, and there is a natural tendency to
treat them as one would in adults, which can lead to significant
complications. Most proximal femoral fractures in children require
operative management (Fig. 164.7). General principles and guidelines for surgical management include the following:
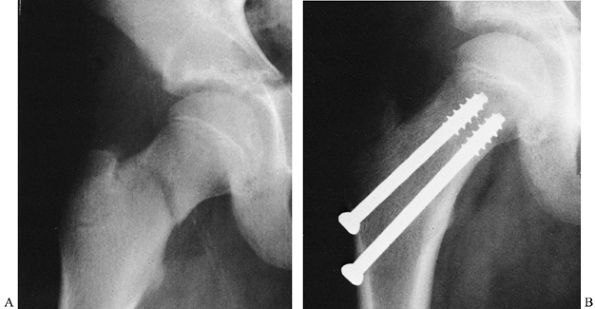 |
|
Figure 164.7. Operative fixation used for pediatric femoral neck fracture. The patient was immobilized in a spica cast.
|
-
Do not cross the proximal femoral physis
with internal fixation devices. The exception to this occurs in older
children with a very proximal femoral neck fracture (e.g.,
Salter–Harris type I fracture of the hip) in whom fixation into the
head is necessary and leg-length discrepancy may be addressed later.
Depending on the patient’s age and the fracture configuration, devices
may
P.4185
include pins, cancellous screws, cannulated screws, or specialized pediatric blade-plate or screw-plate devices. -
Use a spica cast as supplemental fixation
for all proximal femoral fractures, whether or not they are surgically
stabilized. For most children, we prefer a full double-spica cast
because it provides more effective mobilization. -
Treat most nondisplaced fractures of the
femoral neck in a spica cast. Internal fixation with a Steinmann pin or
cancellous bone screw, combined with a cast, is used by some surgeons
for additional protection against displacement. -
Gently reduce and internally fix displaced fractures of the femoral neck, and supplement this with a spica cast until union.
-
Intertrochanteric fractures of the femur
in children have a tendency to drift late into varus. If they are
nondisplaced, treat in a double-spica cast. Follow with serial
radiographs, and continue cast immobilization for 8–10 weeks. If they
are displaced, treat with closed reduction and fixation, using a
pediatric hip screw-plate device supplemented by spica-cast
immobilization. -
Most subtrochanteric fractures of the
femur are treated in 90°/90° traction with the use of a distal femoral
traction pin. A below-knee cast with a suspension loop to support the
leg makes this form of traction easy to adjust and comfortable for
children. Once callus is present, bring the leg into extension, and
apply a spica cast. An alternative to traction is operative reduction
and fixation with a screw and plate device, but this must be
supplemented with a spica cast during healing.
The involvement may be epiphyseal (partial or complete), physeal
(limiting growth potential or causing angulation of the femoral neck
with growth), or metaphyseal. Long-term follow-up of pediatric hip
fractures is therefore essential to allow prompt detection of
complications and timely intervention if required.
differs for young children and older children. Simple skin traction and
early spica-cast application generally work well for younger children
with fractures of the femoral shaft. Although such treatment leads to
shortening, the predictable overgrowth of 1–2 cm that occurs in
children 2–10 years of age allows excellent functional results.
Angulation of up to 15° in the frontal plane and up to 30° in the
sagittal plane will quickly remodel.
occur, prolonged traction (up to 4 weeks) may be required to ensure
maintenance of length before cast application. The callus that forms in
such patients may be flexible, and early angulation is common after
casting; often, the angulated femur then heals rapidly with resulting
malunion. The expense (both emotional and financial) of prolonged
traction may be considerable, and school education can be severely
disrupted. For these reasons, we often favor operative treatment of
femoral-shaft fractures in children older than 10 years.
(generally with a cast for additional protection) or external fixation;
however, we usually favor intramedullary fixation. There are two
general approaches to intramedullary fixation in children.
adolescents, standard intramedullary fixation with interlocking may be
used. Use nails as small as 9 mm in diameter, and take great care to
avoid penetrating the distal femoral physis with either the guidewire
or the nail. Keep the proximal entry site as lateral as possible; using
an entry guide pin is safer than using an awl to avoid inadvertently
slipping posteriorly. Standard interlocking techniques, when required,
can be safely applied to children (Fig. 164.8).
It is wise to leave the proximal rod a little “proud” to facilitate
later removal. Heterotopic bone often forms at the insertion site and
may be symptomatic, but the pain resolves when the rod and heterotopic
bone are removed 1 year postinjury. There have been reports of
avascular necrosis of the proximal femoral epiphysis with
intramedullary nailing, especially with larger nails or posterior and
medial insertion sites. For this reason, we prefer flexible nails, such
as the Ender nail (See Chapter 19 and Chapter 20).
In stable fractures, flexible intramedullary nails can be inserted
antegrade or retrograde without risk to the blood supply to the femoral
head.
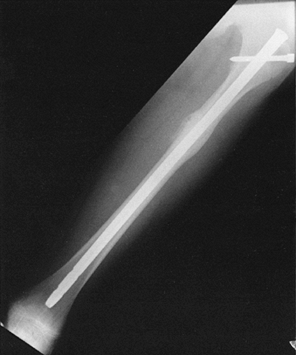 |
|
Figure 164.8. Interlocked intramedullary nail used to fix femoral-shaft fractures in children with open physes.
|
pediatric femoral fractures. Use a stable unilateral fixator along the
lateral aspect. Once callus appears, apply compression across the
fracture because early callus is soft and flexible and reducing
distraction may help the callus mature. Do not remove the fixator too
early, as malunion will occur. A main disadvantage of external fixation
is the high rate of refracture; it is difficult to tell when the
fracture is healed enough to discontinue the fixator. Dynamize the
fixator, if possible, to minimize the risk.
fractures include open femoral fractures and fractures in patients with
multiple injuries or a serious head injury. Grade I open femoral
fractures can be treated as outlined above after thorough irrigation
and debridement. Fractures with more extensive wounds may require
external fixation, although skeletal traction is often a viable option.
If a head injury is likely to lead to spasticity and posturing,
fixation of femoral fractures by one of the methods outlined previously
is helpful. Even in younger head-injured children, intramedullary
nailing with antegrade Ender nails, Rush rods inserted antegrade distal
to the greater trochanter, flexible Nancy nails, or external fixation
is usually necessary. It has been our experience that children with
head injuries recover neurologic function more completely than adults;
therefore, pay careful attention to the management of long-bone
fractures to avoid malunion (see Chapter 14 and Chapter 20).
-
Place the child supine on a fracture
table. Skin or foot traction usually suffices for children younger than
11–12 years with recent fractures, but the fracture should be reducible
under fluoroscopy before the skin incision is made. If necessary, use
skeletal traction while avoiding injury to the physes (see Chapter 20). -
For antegrade nailing, size the Ender
nail by holding it over the leg, with the eye at the greater
trochanter. The nail should end short of the distal femoral physis.
Make a longitudinal incision from the lateral prominence of the
trochanter proximally for about 5–7 cm. Incise the fascia lata to
expose the trochanter. The entry point is the flat lateral surface of
the trochanter (Fig. 164.9A).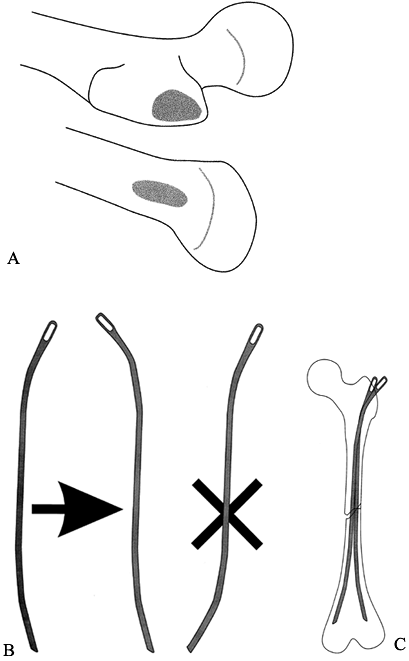 Figure 164.9. Approach for flexible (Ender) nailing of the femur. A: The entry point is generally at the lateral trochanter (for antegrade nail) or medial distal metaphysis (for retrograde nail). B: If an S-shaped nail is needed, perform the bending at the eyelet end of the nail to maintain proper entry shape. C:
Figure 164.9. Approach for flexible (Ender) nailing of the femur. A: The entry point is generally at the lateral trochanter (for antegrade nail) or medial distal metaphysis (for retrograde nail). B: If an S-shaped nail is needed, perform the bending at the eyelet end of the nail to maintain proper entry shape. C:
Multiple pins are used to fill the canal; a transverse, minimally
comminuted fracture pattern is best for flexible-nail techniques. -
Make an entry hole with a 6.5 mm drill,
and introduce a curved Ender nail. Keep it aligned with the
longitudinal axis of the femur. Gently tap it down the shaft, allowing
the oblique blunt end to “bounce” off the medial cortex and pass down
the canal. Verify its position on the image intensifier in two planes.
At the fracture site, use the curve of the nail to hook the distal
fragment, or make a small incision to openly reduce the fracture so
that the nail can be passed. Once it is past the fracture, trap the
nail distally so that it ends up in the lateral condyle, short of the
physis. Leave the eye of the nail outside the cortex proximally for
later removal. -
If the canal is large enough, insert a
second nail. Many surgeons bend the nail into an S shape so that it
will anchor in the medial condyle distally. Place the S curve at the
proximal end of the nail to maintain the proper orientation of the
oblique blunt entry tip (Fig. 164.9B). For most
pediatric patients, two nails across the fracture give sufficient
longitudinal stability. Adding a third (or occasionally a fourth) nail
is optional. These extra nails may be shorter, just long enough to pass
the fracture site and fill the canal. This helps align transverse
fractures (Fig. 164.9C). -
For retrograde nailing (usually for
subtrochanteric or intertrochanteric fractures), place the patient on
the fracture table with the legs abducted. Approach the distal medial
femur from the medial side, using a longitudinal incision just proximal
to the physis. Elevate the vastus medialis, and cauterize the leash of
geniculate vessels that sits against the bone. -
Make a 6.5 mm drill hole proximal to this
leash, and carefully insert the first Ender nail from below. There is
slightly more risk of penetrating the weaker cortex as the rod passes
up the canal, and adding a slight curve to the end of the nail helps
pass into the canal, as well as into the femoral neck. Reduce the
fracture as for antegrade nailing, and carefully rotate the nail as it
is inserted up into the neck. Stop short of the proximal physis.
Two-plane fluoroscopy is essential to accomplish this maneuver. Use two
or more nails as described for antegrade nailing.
Salter–Harris type I or II fractures. Unlike similar fractures in other
anatomic locations, these have a likelihood of growth arrest as high as
a 50%. This is both because high energy is required to fracture the
distal femur and because the physeal mamilary processes are frequently
sheared off during injury. Leg-length inequality can ensue from altered
growth of this rapidly growing physis.
accompanied by neurovascular injuries but not as frequently as are knee
dislocations in adults. They are often unstable and may require
internal fixation.
-
Use general anesthesia and muscle
relaxation for the reduction. Closed reduction may require surprising
force. Occasionally, the bone end will buttonhole through the
periosteum, making closed reduction impossible and necessitating open
reduction. Once the fracture is reduced, test the stability of the
reduction, as internal fixation is usually required. If there is a
large metaphyseal component, it may be possible to stabilize the
fracture by inserting a screw percutaneously across the metaphyseal
fracture parallel to the physis. Otherwise, stabilize the fracture with
two medium-sized, smooth Steinmann pins inserted from the medial and
lateral femoral condyles at a 45° angle. Be sure that the pins pass
into and just through the opposite cortex of the proximal fragment;
otherwise, the fracture may remain unstable. Drive the pins slowly so
as not to cause thermal damage. -
Bury the pin ends because they are
intra-articular and the risk of infection is great if they are left
protruding. After fixation, check the stability of the fixation. If it
is stable, apply an above-knee cast; if there is any question, use a
one-half hip spica cast with the knee in extension. -
Postoperatively, remove the pins under
general anesthesia after 3–4 weeks. Immobilization for 4–6 weeks is
sufficient for healing. Obtain follow-up radiographs every 3 months to
look for evidence of growth arrest; if it occurs, it can be managed by
methods outlined in Chapter 170 on leg-length
discrepancy. In older children, however, an early epiphysiodesis of the
contralateral distal femur may be a simple solution.
is characteristically a jumper’s injury and occurs most commonly in
boys 14 years of age. It usually happens when the patient lands, and
the quadriceps muscles contract to support the falling weight. The
avulsion may involve only the tubercle or may extend through the
condyles and the tibial articular surface of the knee. Use CT to
delineate the exact fracture pattern.
of the tibial tubercle. Because the fracture usually occurs in a physis
that is in the process of closing, it is unnecessary to avoid crossing
the growth plate because growth arrest that can cause hyperextension
will not be significant. For this reason, use fixation that provides
the optimal strength and stability.
easier in children than in adults because children possess excellent
healing potential (10,33,61,63,70).
Initially, administer antibiotics, and irrigate and debride all open
tibial-shaft fractures under a general anesthetic as described in Chapter 12.
In younger children with Gustilo type I injuries and little periosteal
injury, it is usually possible to treat the fracture with a long-leg
cast. Some children may exhibit overgrowth, but this is unpredictable.
In older children or
children with severe soft-tissue wounds, external fixation is usually required to manage the soft-tissue injury.
fixation for the vast majority of open tibial-shaft fractures with a
Gustilo type II or III wound. This allows excellent fracture control
for repeated wound debridements as required. Usually, a single
unilateral half-pin anterior frame is sufficient if supplemented by a
posterior splint or cast. In most cases, we have achieved excellent
immobilization and pain control, using a supplementary below-knee cast.
This can be placed directly over the fixator if fluff gauze is packed
in the recesses of the device, and the whole construct is then covered
with cast padding. It can be removed and replaced by splitting the cast
and opening it like a clamshell. Leave the fixator in place until
callus is present, which usually requires 8 weeks or more, or until pin
loosening occurs. Remove the fixator under a general anesthetic, and
apply a long-leg cast with the knee straight until the fracture has
united.
and the susceptibility of the distal tibial physis to fracture produce
a group of fractures that may require operative management (20,21,39,45,66).
The physis begins to close centrally, and then over 18 months to 2
years closure progresses medially, posteriorly, and laterally, sweeping
like the hand of a clock. The last portion of the physis to close is
the anterolateral corner (Fig. 164.10).
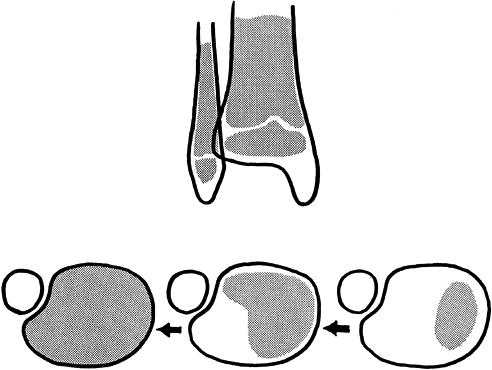 |
|
Figure 164.10.
The sweep of closure of the distal tibial physis is a process that takes 18 months to 2 years to complete. Fracture patterns often parallel this pattern. |
stresses may be directed to open physeal regions, leading to a specific
group of fracture patterns (Fig. 164.11).
Before physeal closure (age 11 years and younger), Salter–Harris type
II fractures are common and can usually be managed by nonoperative
means. When inversion is included in the mechanism, Salter–Harris type
III or IV injuries can be seen, and joint incongruence and physeal
alignment may necessitate open reduction if closed reduction is not
anatomic. It has been our experience that Salter–Harris type III
fractures are quite rare; if radiographs are taken in various degrees
of rotation, a metaphyseal fragment is usually detectable (type IV).
Plan surgical fixation to avoid the physis, if at all possible, because
growth remains in the distal tibia and a varus deformity may be a
complication of treatment.
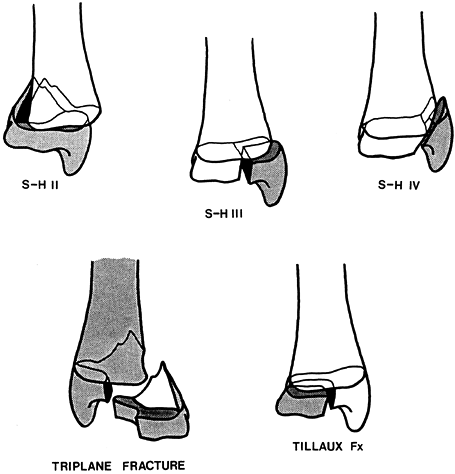 |
|
Figure 164.11. Common fracture patterns seen in the region of the distal tibial physis (S-H, Salter–Harris; Fx, fracture).
|
becomes common. This complex fracture may consist of two, three, or
occasionally more parts, and the fibula may be fractured (Fig. 164.11).
Open reduction may be required for joint incongruence. Interpret
standard radiographs cautiously because the fragments can be spread
posteriorly while reduced anteriorly, giving a false sense of security
on the AP view, or there may be out-of-plane fractures. A transverse
plane CT cut is often most helpful for exact delineation of the
fracture pattern and displacement. We generally recommend open
reduction if displacement after closed reduction is greater than 2 mm
or if there is an articular surface step-off visible on the AP view,
which is rare. Because the physis is in the process
of
closing, angular deformity does not occur if fixation devices cross it;
fixation may therefore be planned for maximum fixation effectiveness.
close, which occurs age 15 years or older, the remaining anterolateral
component may be avulsed by the ligaments of the anterior syndesmosis
during forced external rotation. This is known as a juvenile Tillaux
fracture, and surgical treatment is indicated if closed reduction does
not close the gap to 2–3 mm or less. Like the triplane fracture, this
fracture does not lead to late angular deformity because the physis is
nearly closed.
-
Make a medial or anteromedial incision
over the malleolus. Take care not to strip more periosteum than is
required, and do not further injure the physis. Carefully reduce the
fracture; a fluoroscopic image intensifier may be helpful. If a
Salter–Harris type IV fracture has a small metaphyseal component,
carefully remove it with a rongeur to allow better visualization and
alignment of the physeal plate (Fig. 164.12).![]() Figure 164.12.
Figure 164.12.
A metaphyseal fragment may be removed for better physeal visualization
when open reduction of a Salter–Harris type IV fracture of the distal
tibial epiphysis is performed.
-
Two incisions may be required. Reduce the
medial epiphyseal fragment first through a medial or anteromedial
incision, and fix it by stabilizing the posterior metaphyseal fragment
with small-fragment screws or K-wires. Reduce the lateral
(Salter–Harris type III) component through a lateral incision, and fix
it with a cancellous small-fragment screw. It is unnecessary to avoid
the physis because so little growth remains in the distal tibia. -
A Tillaux fracture is treated surgically in the same fashion as the Salter–Harris type III component of a triplane fracture.
and proceed with no alteration in growth of the extremity.
Occasionally, complete growth arrest will result in limb-length
inequality, or partial growth arrest through formation of a bony bar
will result in longitudinal and angular bone deformity. These
complications are less significant the closer a patient is to skeletal
maturity. In the lower extremity, if the limb-length difference is
projected to be greater than 2–3 cm at skeletal maturity, consider
treatment.
arrest on a patient’s (and parent’s) height, projected degree of
longitudinal or angular deformity, extent of physeal injury (size of
the bony bar), and the patient’s tolerance for the proposed treatment.
Partial arrests of more than 30% to 50% of the cross-sectional area of
the growth plate are not amenable to treatment designed to restore
growth; they can be treated by early contralateral epiphysiodesis (see Chapter 170) or bone lengthening (Chapter 171).
Children who are projected to be tall may be more easily treated by
epiphysiodesis of the noninjured side than very short children. Partial
arrest of less than 30% of the area of the growth plate in a patient
with at least 2 years of growth remaining may be considered for
excision of the bony bar if it is surgically accessible. The most
common sites requiring surgery are the distal femur, proximal tibia, or
distal radius, where significant loss of length will have functional
consequences.
some instances to treat by acute opening-wedge osteotomy. This gains
length and avoids complex, prolonged treatment. Such osteotomies,
utilizing a tricortical wedge of iliac bone and appropriate internal
fixation, heal rapidly in adolescents. If osteotomy is carried out
before skeletal maturity, remember to complete the growth arrest by
total epiphysiodesis to avoid recurrent deformity, with epiphysiodesis
of the opposite extremity if indicated.
location within the physis. Plain x-ray films, scanogram, and bone-age
determinations are important initially in determining which patients
should be considered for bar excision (7,8,11,14,19,31,43,75).
Standard tomography, trispiral tomography, CT, and MRI have each been
advocated for physeal mapping. Plain tomography has long been utilized
for characterizing physeal bars, but resolution is frequently
inadequate. Images must be taken in two projections, and radiation
exposure is quite high. Spiral and hypocycloidal tomography improve the
resolution, but radiation exposure and scanning time remain high.
the extremity within the scanner and multiple thin cuts. The transverse
section of these studies is inadequate, so sagittal and coronal
reconstructions must be used and detail is poor. Direct and specific
communication with the radiologist is frequently required to obtain
clinically useful images. Helical CT has been reported to offer many
advantages over other methods of growth-plate mapping. These include
excellent bony detail, diminished radiation exposure, ability to
manipulate the images into multiple perspectives, and significantly
decreased scanning times that obviate sedation or anesthesia (Fig. 164.13).
Advocates of MRI mapping cite the lack of ionizing-radiation exposure
and excellent detail afforded. Scanning times are prolonged, and
children frequently require sedation or general anesthesia. MRI data
can be processed by either three-dimensional (3D) rendering or 3D
projection to provide excellent detail to assist preoperative planning.
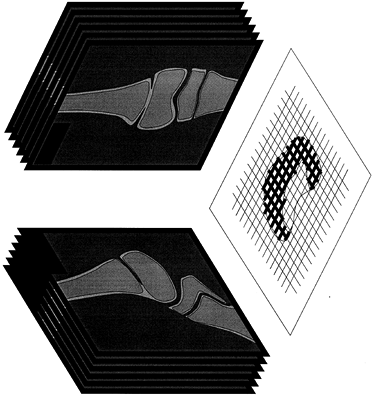 |
|
Figure 164.13.
Reconstruction of the position of a physeal bar by AP and lateral tomograms. Scaled graph paper is used to plot the presence of physeal bar on all radiographs in two planes; the resulting graph gives a good indication of the extent and location of the area of growth arrest. |
physeal arrest, the surgeon must decide whether to correct the
angulation and complete the epiphysiodesis, correct the angulation and
resect the physeal bar, or resect the physeal bar alone and allow
remodeling with growth. Even though there are no simple answers to this
dilemma, the basic guidelines used for management of postfracture
angular deformities may be applied. For example, a 25° flexion
deformity of the distal femur in an 8-year-old child might be expected
to remodel after resection of a peripheral posterior bar, but a similar
degree of varus deformity with a medial bar would not remodel,
necessitating concurrent osteotomy. Central bars that are readily
approached from a metaphyseal osteotomy site may lead to
a
decision to perform full early correction of an angular deformity. In
the upper extremity, completion of epiphysiodesis and closure of the
physis of the other forearm bone (usually the ulna) may be technically
easier and appropriate, given the functional unimportance of equal
upper-limb length.
with tomography or CT or MRI reconstructions. If a bar is 30% or less
of the total physeal area, resection has a fairly high likelihood of
success; with bars greater than 50% of the physeal area, failure is
almost certain.
If the bar is peripheral, it can be directly approached from the
surface. Approach central bars through a large metaphyseal window
proximal to the physis. If osteotomy is required, it is usually easiest
to perform a transverse osteotomy and position the limb to avoid
neurovascular damage; the bar is then approached from above through the
distal face of the osteotomy.
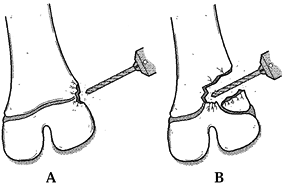 |
|
Figure 164.14. Approach to a physeal bar depends on its location. A: Peripheral bars are approached directly. B: Central bars may be approached through a metaphyseal window or osteotomy. Use a burr for this procedure.
|
-
Complete exsanguination and tourniquet control are essential for a dry field.
-
When resecting a peripheral bar, directly
expose the region of the bar, using an image intensifier as necessary
to confirm the location. With a #15 blade, sharply incise the
perichondrial ring and a small cuff of proximal periosteum at the
resection site, and completely remove both structures to a point where
the edge of the resection contains the visualized physis; this helps
prevent peripheral recurrence. -
Use a small, high-speed burr to carefully
remove the bar in layers; it will have a dense, slightly yellow
appearance that will change into the normal cancellous-bone appearance
as the edge of the bar is reached. Use irrigation to avoid overheating.
Do not stray too far distally; if deeper visualization is required,
burr more proximally. Eventually, the blue-gray cartilage of the physis
will be visible, and with patience the physeal line will be exposed
completely around the cavity of the resected bar (Fig. 164.15). Carefully sweep the burr up and down to smooth the edge of the physis and the contiguous bone. Figure 164.15. A burr is used to remove the dense, yellowish bar material until the physis is visualized throughout the cavity.
Figure 164.15. A burr is used to remove the dense, yellowish bar material until the physis is visualized throughout the cavity. -
When resecting a central bar, remove a large cortical window in the metaphysis through a periosteal window,
P.4193
taking care not to damage the actual physis or perichondrial ring.
Alternatively, perform a transverse osteotomy with a saw, and displace
it by bending to allow visualization from above. -
Using the burr and generous irrigation,
slowly advance until the dense, yellow bony bridge is identified, and
carefully burr in layers to follow the yellowish structure down through
the physeal plane. An image intensifier will help avoid burring too
far. Use a dental mirror to view difficult corners, and enlarge the
cortical window proximally as necessary for exposure. Eventually,
identify the length of the blue-gray cartilage physis completely as it
surrounds the cavity, and smooth it and the attached bone with an
up-and-down motion of the burr. -
At this point, place radiographic markers
such as vascular clips or small K-wire fragments in the epiphyseal and
metaphyseal portions of the bone to allow later measurement of
longitudinal growth (Fig. 164.16).![]() Figure 164.16.
Figure 164.16.
Small radiolucent markers (wires, staples, or vascular clips) help in
the assessment of longitudinal growth after surgery for partial physeal
growth arrest. -
Before deflating the tourniquet, fill the
cavity to prevent blood and eventual fibrous tissue from filling the
space. We prefer Cranioplast, a slow-polymerizing polymethyl
methacrylate (PMMA) that gives off very little heat as it cures. This
material, familiar to orthopaedic surgeons, fully fills the cavity and
leaves very little space for accumulation of organizing fibrous tissue.
Alternatively, autogenous fat may be used, harvested locally or from
the buttock. Fat tends to float out of the wound, and provides no
structural compressive strength, so we no longer recommend it.
Medical-grade Silastic has also been used; however, it is not available
to surgeons for this use and offers no distinct advantages. The object
of the filling is to completely obliterate the cavity without
interlocking with cancellous bone above and below the physis. -
Allow the PMMA to become doughy before
inserting it, and gently push (do not “pressurize”) it while it cures,
irrigating with cool saline to minimize thermal damage. Once the PMMA
is cured, replace the cortical window or fix the osteotomy if one has
been made (see discussion above). Use iliac bone graft as needed for
stability in opening-wedge osteotomies. -
Close the wound, and immobilize the limb, even if internal fixation has been used for an osteotomy.
well healed, usually 6 weeks, and gradually begin increasing protected
weight bearing. PMMA is load-sharing and allows safe weight bearing
once muscle strength has recovered.
often exhibit premature closure after several years of normal growth
after successful bar resection. Patients must be carefully monitored
with periodic clinical, radiographic, and limb-length examinations
until skeletal maturity. Be prepared to reassess late physeal closure
and to carry out prompt treatment by epiphysiodesis, osteotomy, or
other indicated procedure.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
BJ Jr, Kant AP, Emery FE. Displaced Fractures of the Femoral Diaphysis
in Children: Definitive Treatment in a Double Spica Cast. J Trauma 1977;17:8.
SC, Hoffer MM, Aval S. Nonoperative Treatment for Minimally and
Nondisplaced Lateral Humeral Condyle Fractures in Children. J Pediatr Orthop 1998;18:448.
JH, Austin SM, Warner WC, et al. Interlocking Intramedullary Nailing of
Femoral-shaft Fractures in Adolescents: Preliminary Results and
Complications. J Pediatr Orthop 1994;14:178.
NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for Partial Growth
Plate Arrest: Results after Four Years or at Maturity. J Bone Joint Surg Br 1989;71:13.
JV, Kassab MT. Displaced Supracondylar Fractures of the Elbow in
Children: A Report on the Fixation of Extension and Flexion Fractures
by Two Lateral Percutaneous Pins. J Bone Joint Surg Br 1974;56:490.
P, Alvarez-Romera A, Burgos J, et al. Displaced Radial Neck Fractures
in Children Treated by Closed Intramedullary Pinning (Metaizeau
Technique). J Pediatr Orthop 1997;17:325.
P, Lizler J. Magnetic Resonance Imaging in the Evaluation of Partial
Growth Plate Arrest after Physeal Injuries in Children. J Bone Joint Surg Am 1991;73:1234.
SD, Drvaric D, Darr K, MacEwen GD. Stabilization of Pediatric
Diaphyseal Femur Fractures with Flexible Intramedullary Nails (a
Technique Paper). J Orthop Trauma 1992;6:452.
RN, Nicholson JT, Chung SM. Long-term Results in the Treatment of
Femoral Shaft Fractures in Young Children by Immediate Spica
Immobilization. J Bone Joint Surg Am 1976;58:945.
H, Bunnell WP, Duhaime M, Poitras B. Cubitus Varus Deformity Following
Supracondylar Fracture of the Humerus in Children. J Pediatr Orthop 1982;2:539.
IV. Growth and Development of the Acetabulum in the Normal Child:
Anatomical, Histological, and Roentgenographic Studies. J Bone Joint Surg Am 1978;60:575.
SA, Helikson MA, Shorter N, et al. Pelvic Fractures in Children: Review
of 120 Patients with a New Look at General Management. J Pediatr Surg 1980;15:727.
PG, Gehring HW, Iglesias LJ. Open Reduction and Internal Fixation of
Displaced Supracondylar Fractures of the Humerus in Children. Orthop Clin North Am 1976;7:573.
K, Masada K, Tada K, Yamamoto T. Osteosynthesis for the Treatment of
Non-union of the Lateral Humeral Condyle in Children. J Bone Joint Surg Am 1997;79:234.
JG. Techniques for Direct Radiographic Visualization during Closed
Pinning of Supracondylar Humerus Fractures in Children. J Pediatr Orthop 1990;10:555.
AK, Von Laer L. Displaced Fractures of the Radial Neck in Children:
Long-term Results and Prognosis of Conservative Treatment. J Pediatr Orthop B 1998;7:217.
A, Meyer S, Tolo VT, et al. Surgical Treatment of Displaced
Supracondylar Fractures of the Humerus in Children: Analysis of
Fifty-two Cases Followed for Five to Fifteen Years. J Bone Joint Surg Am 1978;60:657.
SH, Lam CY, Choi KY, et al. Percutaneous Intramedullary Kirschner
Wiring for Displaced Diaphyseal Forearm Fractures in Children. J Bone Joint Surg Br 1998;80:91.