CONGENITAL SHOULDER AND ELBOW MALFORMATIONS AND DEFORMITIES
IX – PEDIATRIC DISORDERS > CHAPTER 165 – CONGENITAL SHOULDER AND
ELBOW MALFORMATIONS AND DEFORMITIES
Associate Clinical Professor, Department of Orthopaedic Surgery,
University of California–Davis, Shriners Hospital for Children,
Northern California Sacramento, California, 95817.
frequently accompanied by hand malformations or absence; in these
cases, treatment of the shoulder and elbow must be integrated with
treatment of the hand. Surgical reconstruction of the shoulder or
elbow, or both, is not indicated if the hand is absent or
nonfunctional, unless the goal of surgery is to reduce pain. See Chapter 69,
Congenital Hand Malformations, for a discussion of pathophysiology and
principles of treatment of congenital malformations of the upper
extremity.
amyoplasia) is characterized by multiple, symmetric, nonprogressive,
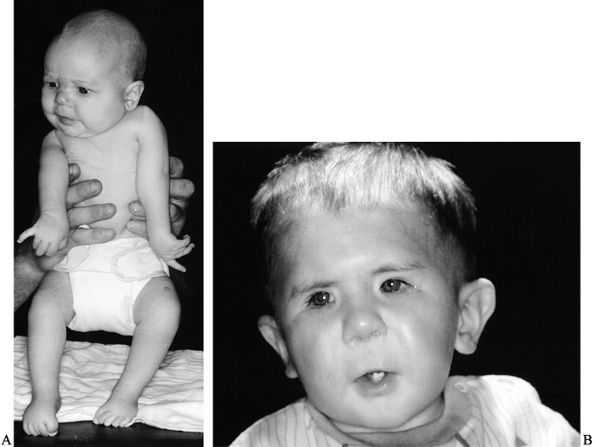
congenital joint contractures (45) (Fig. 165.1A).
Many etiologic factors have been associated with arthrogryposis
(including fetal exposure to mutagens, toxins, hyperthermia,
neuromuscular blocking agents, and mechanical immobilization) (158).
The final common pathway for these etiologies is probably lack of fetal
movement. Because fetal movement is necessary for joint development,
immobile joints fail to develop normally and become contracted (43,108,182).
 |
|
Figure 165.1. A: Infant with arthrogryposis. B: Facial features of child with Freeman–Sheldon syndrome (craniocarpal-tarsal dysplasia).
|
most cases studied show evidence of a neuropathic condition, possibly a
disorder of the anterior horn cells, which partially paralyzes the
fetus. Even though many different fetal exposures to drugs, chemicals,
and mutagens have been associated with arthrogryposis in humans, in
most cases, the cause remains unknown (50,72). Arthrogryposis is not an inherited condition (121), but many other syndromes that feature multiple joint contractures are inherited (72),
and most children with this condition should be seen by a geneticist.
The incidence of arthrogryposis is unknown, although congenital joint
contractures are seen in about 1 in 3,000 live births (72).
child’s ability to perform activities of daily living, especially
eating, toileting, and dressing (30).
birth. Mothers of children with this condition frequently report that
fetal movement was diminished compared with their other pregnancies.
Oligohydramnios is a frequent finding.
usually more severe distally than proximally, and most patients have
upper extremity involvement (58). The shoulder lacks abduction and external rotation (178), and the elbow (one of the most frequently involved joints) (108) may lack flexion or extension (161,167). The wrist is usually flexed and deviated ulnarward (176), the fingers flexed, and the thumb flexed in the palm (184) (see The Fingers: CAMPTODACTYLY and The Thumb: CLASPED THUMB in Chapter 69, Congenital Hand Malformations). Severe clubfoot, knee flexion contracture, dislocated hip, and
scoliosis are frequently associated with arthrogryposis (45,161).
Joint contractures similar to those seen in arthrogryposis are
associated with syndromes such as cranio-carpo-tarsal dysplasia
(Freeman–Sheldon syndrome) (Fig. 165.1B) (53,161).
and are not usually amenable to range-of-motion exercises, splinting,
or surgery. The most limiting deficit, lack of active elbow flexion,
prevents the child from reaching the mouth and head, especially when
accompanied by an elbow extension contracture. Children with only
passive elbow flexion frequently discover “tricks” to help get their
hand to their face, such as leaning the forearm on the edge of a table
or on their leg. Serial casting, splinting, and range-of-motion
exercises may improve elbow range of motion. If less than 90° of
passive elbow flexion is gained after 6 months of supervised elbow
stretching, posterior capsulotomy with triceps lengthening is indicated
(167). In one study of this operation,
postoperative improvement in passive elbow flexion was maintained for
at least 2 years, although range of motion was occasionally limited by
intra-articular incongruity (167).
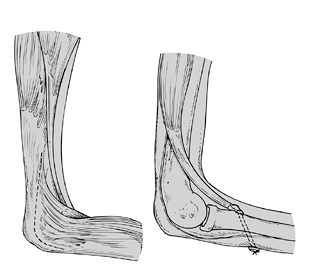
provide active elbow flexion, including pectoralis major, latissimus
dorsi, triceps-to-biceps transfers, or proximal reattachment of the
wrist and finger flexor muscles (Steindler flexorplasty) (12,44,167,178).
Tendon transfer is indicated in children older than 4 years of age who
lack active elbow flexion and have at least 90° of passive elbow
motion, reasonable ipsilateral hand function, absent contralateral
active elbow flexion, and an available donor muscle. Bilateral tendon
transfer is not usually indicated; asymmetric function is usually
desirable, especially when
the
triceps is the best donor available, and elbow extension is sacrificed
for elbow flexion. Potential donor muscles must be carefully assessed
preoperatively because they may be weak and therefore unsuitable for
transfer. Triceps-to-biceps transfer gives the most reliable results (167), although this operation creates an elbow flexion contracture (31)
and is contraindicated in children who need active elbow extension to
ambulate or transfer because of lower extremity contractures. The
pectoralis major is the best donor when the triceps is unavailable, but
the scar left by this operation is large (extending from sternum to
antecubital fossa) and crosses the breast, and transfer of the
pectoralis may cause breast asymmetry. Latissimus dorsi and finger and
wrist flexors are frequently weak in arthrogryposis, so latissimus
dorsi-to-biceps transfer and Steindler flexorplasty are rarely
indicated.
arthrogryposis, including arthrogryposis multiplex congenita, multiple
congenital articular rigidities, amyoplasia congenita, myodystrophia
foetalis deformans, and congenital arthromyodysplasia (162). No classification scheme useful to orthopaedists has been described for this disorder.
elbows and wrists may increase passive range of motion, particularly
for infants younger than 1 year of age. A creative occupational
therapist may help the child improve function with the use of
mechanical aids.
-
Use loupe magnification (for the ulnar
nerve dissection) and perform the operation under general anesthesia
and a tourniquet. A sterile tourniquet may be necessary. -
Through a longitudinal incision on the
posterior elbow, find the ulnar nerve. Open the cubital tunnel and
retract the nerve with a Penrose drain, taking care not to damage
branches to the flexor carpi ulnaris. -
Perform a V-Y lengthening of the triceps
tendon. First, make a V incision through the tendon, with the point of
the V proximal. Expose the humeroulnar joint, leaving the triangular
tail of tendon attached to the olecranon. -
Using scissors, transect the posterior
elbow joint capsule. If necessary to obtain flexion, transect the
lateral and medial capsule, including collateral ligaments. -
Repair the triceps tendon with nonabsorbable suture while holding the elbow in maximum flexion.
-
The ulnar nerve does not usually require transposition.
-
Close the skin with an absorbable, running subcuticular suture. Apply a long-arm cast with the elbow in maximum flexion.
frequent gentle passive elbow flexion exercises, supervised by an
occupational therapist. Supplement with night splinting in maximum
flexion, to be continued indefinitely.
 |
|
Figure 165.2. Triceps-to-biceps transfer (Reprinted from ref. 44, with permission).
|
-
Perform the operation under general anesthesia and a tourniquet. A sterile tourniquet may be required.
-
Through a posterolateral longitudinal
elbow incision, expose the triceps tendon and divide it at its
insertion. Dissect it from the posterior aspect of the distal fourth of
the humerus, and transfer it around the lateral aspect, superficial to
the radial nerve. -
Through a separate anterior zigzag elbow incision, expose the biceps tendon.
-
Pass the triceps tendon through a longitudinal slit in the biceps tendon, and suture it under tension with the elbow in flexion.
-
Close the skin with an absorbable,
running subcuticular suture. Apply a long-arm cast with the elbow in
maximum flexion and neutral rotation.
restrict elbow flexion following posterior elbow capsulotomy. The
outcome of elbow flexion tendon transfer is frequently unsatisfactory,
even when the donor muscle is normal; in arthrogryposis, donor muscles
are likely to be weak and have poor excursion.
better than their strength and range-of-motion measurements would
predict. Surgery should be directed at functional goals shared by the
surgeon, child, and family.
elbow flexion, patients may not like the mandatory elbow flexion
contracture that accompanies this operation.
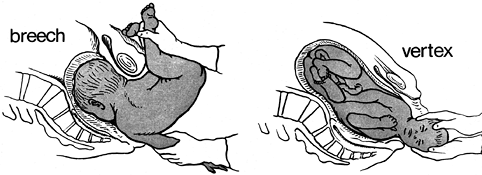
obstetric palsy, occurs when the brachial plexus is injured by traction
during birth. The mechanism of injury is forceful separation of the
head from the shoulder by lateral flexion of the cervical spine and
depression of the shoulder (Fig. 165.3). This
most commonly occurs during a cephalic vaginal birth, owing to shoulder
dystocia. The primary cause of shoulder dystocia is fetal macrosomia,
which may be associated with maternal diabetes or a multiparous mother.
The brachial plexus may also be injured during a breech delivery;
injury during caesarian section is very rare (7,55). Obstetrics literature indicates that injury may occur prenatally (56,130,133), although this finding is controversial (38,60).
 |
|
Figure 165.3. Breech (A) and Vertex births (B), showing wide separation of the head from the downside shoulder.
|
the incidence unchanged or possibly increasing in the past 30 years, in
spite of an increasing rate of caesarian sections (64,75,76,88,103,117,118,134,170).
Although BPBP may often be attributable to poor prenatal care or
obstetrician error, some cases cannot be reliably predicted or
prevented. Macrosomia (fetal weight greater than 4,500 g) is difficult
or impossible to detect with current prenatal diagnostic techniques (62), and shoulder dystocia, which occurs in up to 2% of deliveries, may be difficult to recognize and treat (63,74,80,118). In addition, shoulder dystocia and BPBP frequently occur in normal birth-weight fetuses (64,128).
Other variables, including prolonged gestation, prolonged labor, use of
oxytocin, use of forceps or vacuum suction, and previous maternal
obstetric history of macrosomia are all associated with birth injuries,
but even when combined, these problems fail to predict most birth
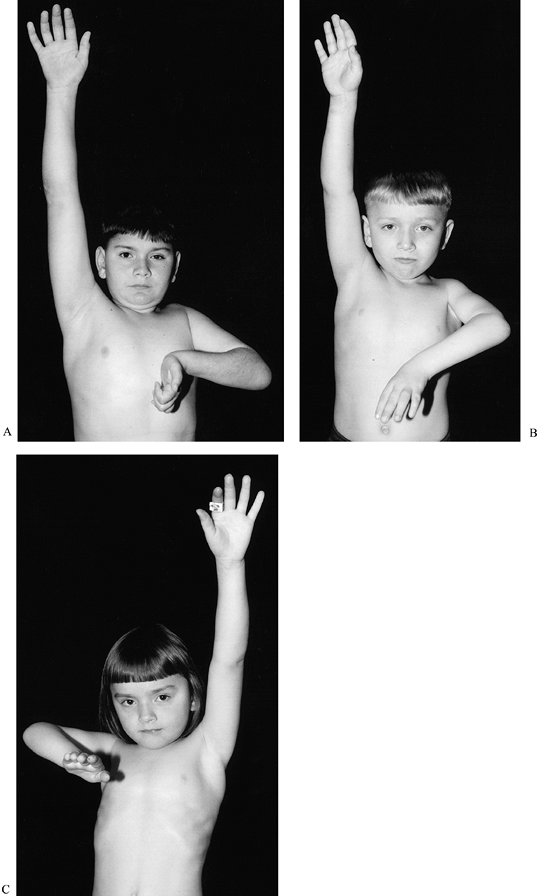
injuries (134). BPBP in an older sibling is the only factor that may reliably predict BPBP (4) (Fig. 165.4).
 |
|
Figure 165.4. A to C: Three siblings with brachial plexus birth palsy. Both boys (A, B) have Horner’s syndrome.
|
and transient upper extremity weakness [approximately 80% of newborns
have full or near-full recovery of function (66,76,88,122)]
to complete permanent upper extremity paralysis. Most of the
improvement gained from nerve recovery is seen in the first 12 months
of life, and the remainder by 2 years of age (5);
recovery of sensation is more complete than recovery of motor function.
Residual disability depends on the severity and location of the plexus
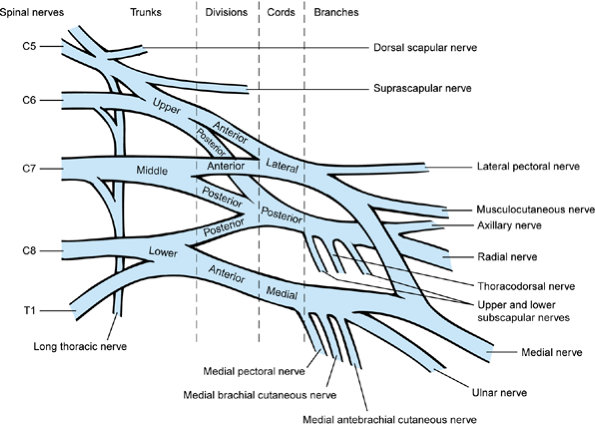
injury (78) (Fig. 165.5). Injury to the upper trunk, where the C5 and C6 nerve roots join and the suprascapular
nerve leaves the plexus, is called Erb’s palsy (49).
This the most common type of plexus injury; the child with this type of
BPBP has weakness or paralysis of shoulder external rotation and
possibly abduction. The next most common type is global plexus palsy.
Isolated injury to the lower trunk (C-8 and T-1 nerve roots), called
Klumpke’s palsy, is the least common type (6,90) and may actually represent partial recovery from global plexus palsy rather than injury to the lower trunk alone (172) (Fig. 165.6).
 |
|
Figure 165.5. Different types of brachial plexus injury (78).
|
 |
|
Figure 165.6. The brachial plexus (172).
|
multiple deformities at the shoulder, including internal rotation
contracture and posterior subluxation or dislocation, as well as at the
elbow, including flexion, supination, or pronation contracture, radial
head dislocation, or complete elbow dislocation (3,13,41,83,104,110,174). The affected arm is smaller than the opposite side, in proportion to the severity of the BPBP (Fig. 165.7).
 |
|
Figure 165.7. Severe brachial plexus birth palsy in a teenager.
|
contractures while recovery is occurring, to restore neurologic
function, to augment weak muscles, and to improve the appearance of
deformities that occur as a result of muscle imbalance. Treatment
depends on age and extent of weakness, and may include passive
range-of-motion exercises (PROM), goal-directed occupational therapy,
brachial plexus exploration and grafting, tendon transfers,
osteotomies,
and arthrodeses. Because of the complexity of their care, children with
BPBP benefit from a multidisciplinary team approach (36).
The BPBP team may include an orthopaedic surgeon, a surgeon with
microneurosurgery expertise, a physiatrist, a pediatric neurologist,
occupational and physical therapists, a social worker, and a
therapeutic recreation specialist. Parents and older children
appreciate team-sponsored activities, such as play groups and peer
contacts (Fig. 165.8).
 |
|
Figure 165.8.
Two children who met through a peer contact program, wearing shoulder abduction splints following shoulder external rotation tendon transfers. |
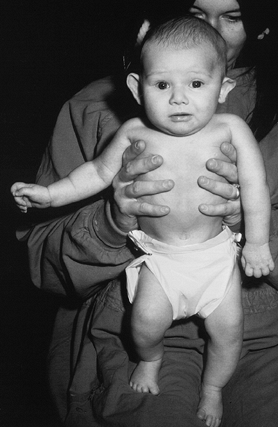
extremity motion. The affected side is typically held in internal
rotation at the shoulder, with the elbow extended and the wrist flexed (Fig. 165.9).
The differential diagnosis includes hemiparesis, a septic glenohumeral
joint, and congenital elevation of the scapula (Sprengel’s deformity).
Clavicle fracture and proximal humeral epiphyseal separation may be
either differential diagnoses or associated lesions (5,59,103).
Although most infants with BPBP recover useful function, those with an
ipsilateral Horner’s syndrome (due to injury to the sympathetic
ganglia, associated with avulsion of the C-8 and T-1 nerve roots off
the spinal cord) or hemidiaphragm paralysis (injury to the phrenic
nerve) have a poor prognosis for recovery (122).
 |
|
Figure 165.9. Infant with left brachial plexus birth palsy.
|
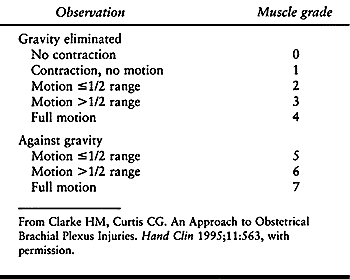
when the infant is 3 to 4 weeks of age. Examine the infant every 1 to 2
months for signs of recovery. The Infant Active
Movement Scale (36) (Table 165.1)
helps the examiner measure muscle recovery more accurately than do
scales devised for adults because it does not require cooperation for
testing muscles against resistance. Brachial plexus exploration with
nerve grafting and transfer may be indicated if the infant does not
recover elbow flexion strength.
 |
|
Table 165.1. Infant Active Movement Scale
|
for three reasons: It is easy to observe; the timing of natural
recovery of elbow flexion may be a prognostic sign for plexus recovery;
and early nerve grafting to the lateral cord of the plexus (Fig. 165.6) may be the best way to restore elbow flexion, which is difficult to replace with tendon transfers (112).
In one often-quoted but unpublished study, children with full recovery
from BPBP at 5 years of age showed antigravity elbow flexion and
shoulder abduction by age 3 months (59,160). Later recovery of elbow flexion (between 3 and 6 months of age) has been associated with residual shoulder weakness (160,171).
In contrast, however, other investigators have reported that most
children with biceps recovery after 3 months of age are likely to have
a good ultimate outcome, but lack of sensation and wrist extension at 3
months are poor prognostic signs (21).
contrast computerized tomography (CT) myelography, magnetic resonance
imaging (MRI), and intraoperative somatosensory-evoked potentials have
all been used to measure and provide images of damage to the neonatal
brachial plexus, with the goal of differentiating nonrepairable
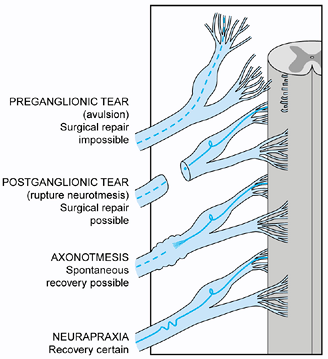
preganglionic rupture from postganglionic injury, which may be
repairable or may recover without surgical treatment (52,77,78,123,153).
EMG and NCS may help differentiate neuropraxia from axonal
degeneration. CT myelography and MRI can identify preganglionic nerve
root injuries (avulsions) if they are associated with traumatic
pseudomeningoceles, as they are in many avulsions; however, root
avulsions may occur without creating a pseudomeningocele, and
pseudomeningoceles may be visualized at the level of a root that has
not been avulsed. All tests require sedation, and CT myelography is
invasive. Testing should be reserved for infants who are candidates for
nerve surgery, to help the surgeon plan the operation.
with nerve grafting and transfer are unclear. Some surgeons recommend
this procedure for all children without full recovery of active elbow
flexion and shoulder abduction by 3 months of age (59); others prefer to allow more time for recovery (35,79,102,137).
Most surgeons agree that complete avulsion of the plexus is rare, so at
least one root has a repairable injury in most cases, and the results
are poor for surgical plexus reconstruction for BPBP performed after 12
months of age. Most surgeons resect the scarred plexus and graft
nonavulsed roots to the lateral cord, suprascapular nerve, and
posterior cord (79) (see Chapter 60). Nerve transfer (neurotization) of the accessory nerve may be helpful (94), but neurolysis of
a neuroma-in-continuity probably is not (35).
The results of plexus surgery vary, and few patients have had long-term
follow-up, but nerve grafting and transfer in infants with poor
prognostic signs probably result in better function than if no surgery
was performed, and complications are unusual in the hands of
experienced surgeons (59,79).
Such a child may have difficulty positioning his or her hand in space,
particularly to reach the head and face, or to throw, climb, turn a
jump rope, or play a musical instrument. Eventually, the child may
develop a shoulder internal rotation contracture, with glenohumeral
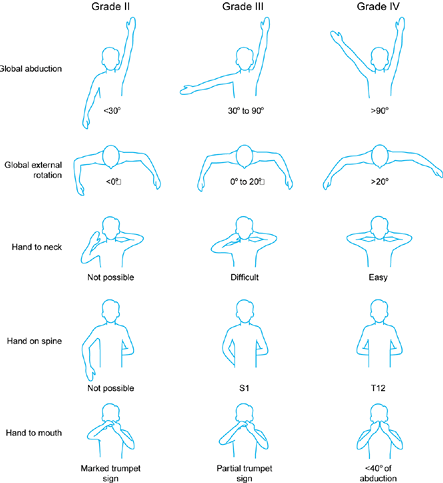
joint changes and eventual posterior dislocation of the humeral head (70,81,174). Shoulder function may be classified using Mallet’s scale (109,172) (Fig. 165.11). To diminish unopposed internal rotation, Sever (150), in 1925, recommended releasing the pectoralis major and subscapularis. In 1934, L’Episcopo (101)
described the results of combining Sever’s anterior release with
transferring the teres major to the proximal humerus in order to
provide active shoulder external rotation. This operation was later
modified by Hoffer and colleagues (19,84,136),
who described latissimus dorsi and teres major transfer to the
supraspinatus, with release of the pectoralis major only (because
release of the subscapularis can cause glenohumeral instability); and
Covey et al. (39), who described latissimus dorsi and teres major rerouting around the proximal humerus.
 |
|
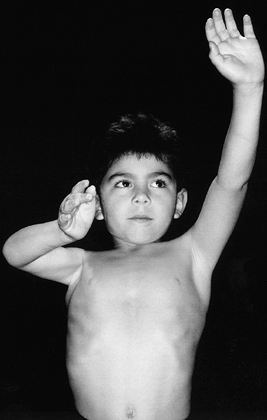
Figure 165.10. Decreased shoulder abduction and external rotation due to right brachial plexus birth palsy.
|
 |
|
Figure 165.11. Modified Mallet’s classification (172). Grade I has no function.
|
both reliably increase abduction, as well as external rotation in
abduction by an average of 20° to 40° each. Abduction is increased
because the transfer helps stabilize the glenohumeral joint, allowing
the deltoid to function more efficiently. The rerouting procedure is
more technically demanding and requires more excursion of the teres and
latissimus muscles but may provide more external rotation than the
transfer procedure (92). Other patient
requirements for these operations include sufficient maturity to work
with a therapist postoperatively to strengthen the transfer (4 years of
age or older), minimal changes in the glenohumeral joint from unopposed
internal rotation (usually 12 years of age or younger), and enough
deltoid strength to abduct to at least 60° against gravity. However,
Hoffer and Phipps (83) have reported that when
posterior shoulder dislocation occurs early (before 4 years of age)
latissimus and teres transfer and pectoralis release can be combined
with closed shoulder reduction with good results and maintenance of
reduction (83).
Elbow flexion contractures can be treated with serial elbow drop-out
casts (long-arm casts applied in maximum elbow extension, with the
posterior above-elbow aspect cut out so that the elbow can extend
farther but cannot flex). Surgical release of elbow flexion
contractures is not usually indicated.
deformity and contracture may make shoulder tendon transfers less
effective. External rotation osteotomy of the humerus improves the
appearance and function of a limb with a shoulder internal rotation
contracture in the older child (61,174).
aside from classification by level of plexus involvement, as described
previously. Shoulder limitation has been classified by Mallet (60,109,172) (Fig. 165.11).
to BPBP. PROM is especially important in the infant, because the
shoulder can become fixed in internal rotation and subluxate or
dislocate before the child is old enough to undergo shoulder external
rotation tendon transfer or rerouting (83,164).
Weekly manipulation by a therapist is not adequate; the child’s
shoulder should undergo PROM several times each day, so the child’s
caregivers must be trained to do shoulder PROM exercises.
brachial plexus exploration and nerve grafting, although the decision
to proceed with this operation is based on lack of clinical recovery,
and the most helpful planning information is gained from
somatosensory-evoked potentials performed intraoperatively.
rerouting, the waist portion of the shoulder spica cast can be applied
with the child standing, to improve fit, then removed and reapplied at
the end of the operation. Postoperative therapy is especially important
following these procedures, so arrangements for this should be made
prior to surgery.
for details on surgical techniques for brachial plexus exploration.
Repair of BPBP differs slightly from repair of traumatic brachial
plexus palsy in adults. The differences stem from the following aspects
of BPBP:
 |
|
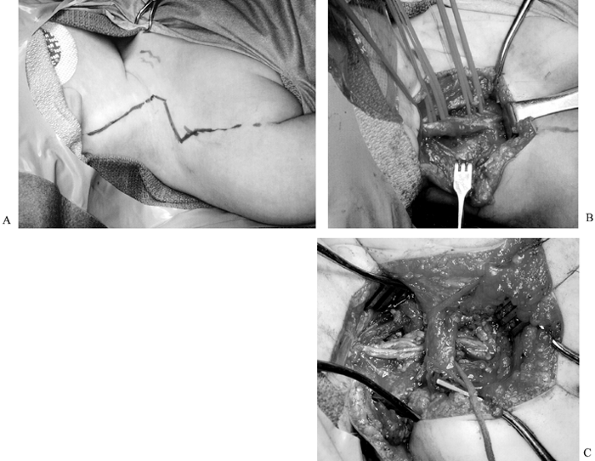
Figure 165.12. A: Marks indicate planned incision for brachial plexus exploration (child is supine, with head to the left). B: Intraoperative view of scarred plexus (same child as in A). The head is to the left, vessel loops surround the roots; the central horizontal structure is the plexus. C: Intraoperative view of clavicle and sural nerve cable grafts (same child as A and B; head is to the left, central vertical structure is clavicle).
|
-
The entire plexus is often shifted
distally, so landmarks such as the clavicle crossing the plexus at the
level of the divisions are not reliable. -
Clavicular osteotomy is not usually necessary.
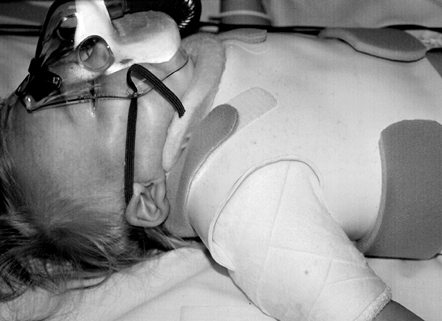
ambulatory, apply short-leg walking casts. Apply a custom-fabricated
chest and neck splint at the end of the operation (Fig. 165.13).
The ipsilateral arm can be placed in a sling or attached to the chest
splint with a Velpeau wrap. Remove the chest and neck splint, sling,
and short-leg walking casts and resume shoulder PROM exercises 3 weeks
after surgery. Return of function may take 6 months to 2 years.
 |
|
Figure 165.13. Infant in custom-fabricated neck brace following brachial plexus exploration and grafting.
|
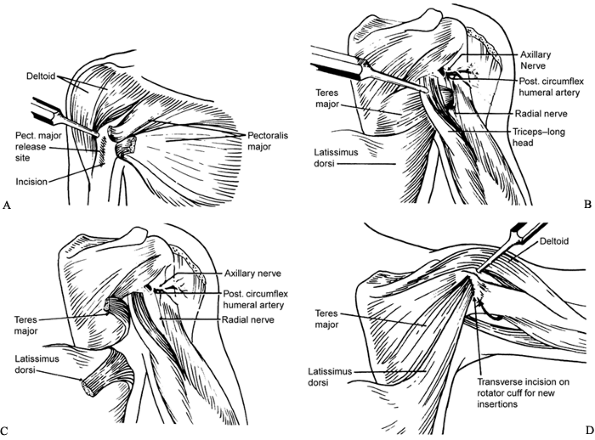 |
|
Figure 165.14. Tendon transfer procedure (84). A: Pectoralis major release. B: Teres major and latissimus dorsi, before attachment from humerus. C: Teres major and latissimus dorsi, after detachment from humerus. D: Teres major and latissimus dorsi transferred to rotator cuff.
|
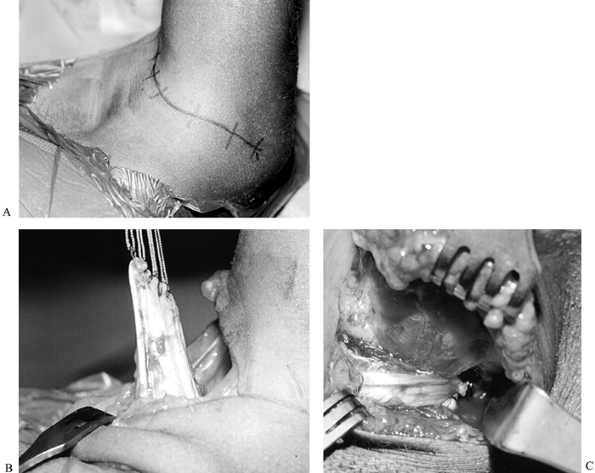 |
|
Figure 165.15. Shoulder external rotation tendon transfer. A: Transverse axillary incision. The patient is in right lateral decubitus position, with left arm abducted to 90°. B: Latissimus dorsi and teres major tendons after detachment from humerus. C: Latissimus dorsi and teres major tendons have been sutured to supraspinatus tendon (at tip of Army-Navy retractor).
|
-
Perform the operation under general anesthesia.
-
Place the patient in a lateral decubitus
position and hold with a beanbag. Isolate the affected axilla and arm
with a U drape. Range the shoulder under anesthesia; if there is less
than 80° passive external rotation when the arm is abducted to 90°,
perform the pectoralis major lengthening as described later. If there
is more than 80° rotation, pectoralis lengthening is not necessary. -
Make a transverse axillary incision after
infiltrating the area with bupivacaine with epinephrine. Extend the
incision posteriorly to the deltoid-triceps interval. -
In the anterior end of the incision,
locate the pectoralis major muscle. The tendon is short and most
prominent on the deep side of the muscle. Using Bovie cautery, transect
the tendinous and fascial portions of the muscle near its insertion on
the humerus. Do not transect the entire muscle. Subscapularis
lengthening is rarely necessary and may destabilize the shoulder. -
In the posterior part of the incision,
locate the teres major and latissimus dorsi muscles. Trace them to
their humeral insertions, using blunt dissection; these tendons are
frequently conjoined (19). The axillary nerve
and posterior humeral circumflex artery cross from anterior to
posterior deep to the latissimus and teres major tendons, just proximal
to their insertions. They are easier to visualize after detaching the
tendons. -
Detach the teres major and latissimus
tendons from their humeral insertions. Bluntly free the muscle bellies
from surrounding attachments, avoiding the circumflex scapular and
thoracodorsal vessels. Tag the tendons with 0 nonabsorbable suture. -
Through the deltoid-triceps interval, locate the rotator cuff tendon. Stay proximal to avoid the axillary nerve.
-
Position the arm in 90° of abduction and
60° to 80° of external rotation, bring the latissimus and teres major
tendons posterior to the triceps muscle, and suture them into the
rotator cuff tendon as high as possible. -
Close the skin incision in two layers.
Apply the prefabricated waist portion of the spica cast, and attach a
long-arm cast to the waist portion (Fig. 165.16). Figure 165.16.
Figure 165.16.
Shoulder spica cast used after shoulder external rotation tendon
transfer or rerouting. (Drawing by Anthony Marotta, with permission).
Prescribe twice-daily occupational therapy (OT) for 2 weeks. The OT
program is designed to strengthen the tendon transfer. Have the child
wear the splint for the first week whenever he or she is not in
therapy; in the second week, the splint can be discontinued during the
day. The child should continue to wear the splint at night for 6
months. Continue OT at less frequent intervals for 6 to 12 months.
follows the same first five steps as those used for tendon transfer
(preceding technique). If the teres major and latissimus tendons are
conjoined, this operation cannot be performed; the tendons must be
transferred instead of rerouted. The surgical technique continues as
follows:
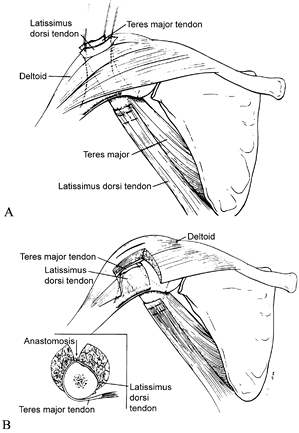 |
|
Figure 165.17. Tendon rerouting procedure. A: Latissimus dorsi tendon and teres major tendon pulled through a split in the deltoid. B: Latissimus dorsi tendon and teres major tendon after anastomosis. Reprinted from ref. 39, with permission.
|
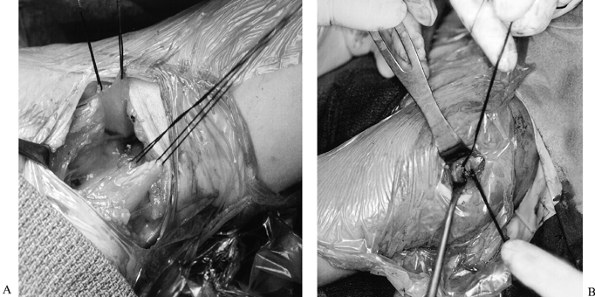 |
|
Figure 165.18. Tendon rerouting procedure. A: Left axilla, upper sutures are in the latissimus dorsi tendon, lower sutures are in the teres major tendon. B: Left shoulder, head is to the right. Latissimus dorsi and teres major tendons pulled through a split in the deltoid muscle.
|
-
Transect the latissimus at the
muscle–tendon junction. Attach the latissimus muscle belly side to side
to the teres major muscle belly; avoid excess tension, or mobilization
of the teres will be difficult. Tag the latissimus tendon, which is
still attached to the humerus at its insertion, with 0 nonabsorbable
suture. -
Detach the teres major from its humeral insertion. Tag it with 0 nonabsorbable suture.
-
Make a 3 cm incision starting at the
acromion, at the posterior one third or anterior two thirds junction of
the deltoid, after infiltrating with bupivacaine and epinephrine. Split
the deltoid fibers bluntly down to the humerus. Stay proximal to the
axillary nerve insertion. -
Bluntly make tunnels around the proximal
humerus, starting at the humeral insertion site of the teres major.
Each tunnel should reach the deltoid incision superiorly. The posterior
tunnel must be large enough to accommodate
P.4212
the teres major muscle; the latissimus tendon will pass through the anterior tunnel. -
Pass the latissimus tendon anteriorly,
and the teres major muscle posteriorly. Both should be visible through
the deltoid incision. -
Attach the latissimus tendon to the teres
major muscle by tying their tag sutures together through the deltoid
incision, with the shoulder in 90° of abduction and 45° to 90° of
external rotation. -
Close the skin incision in two layers.
Apply the prefabricated waist portion of the spica cast, and attach a
long-arm cast to the waist portion (Fig. 165.16).
the rotational osteotomy of the humerus for birth injuries of the
brachial plexus. The surgical technique for osteotomy is as follows:
-
Perform the operation under general anesthesia.
-
Place the patient in supine position with
a small beanbag under the scapula. Examine the arm carefully,
estimating the amount of external rotation necessary for the patient to
reach the face and head. Prepare and drape the affected arm using a U
drape. -
Make a longitudinal anterolateral
shoulder incision after infiltrating with bupivacaine and epinephrine.
Approach the humerus through the deltopectoral and anterior
deltoid–biceps intervals (between the radial and musculocutaneous
nerves, neither of which are usually visualized). The radial nerve will
be more anterior than usual because of the internal rotation
contracture. -
Select the osteotomy site at the deltoid
insertion, with enough exposure proximally and distally to apply a
small six-hole AO plate. Predrill, measure, and tap two holes proximal
to the osteotomy site before making the osteotomy. -
Make the osteotomy after making a longitudinal mark across the osteotomy site.
-
Rotate the distal fragment the amount
previously estimated to allow the patient to reach the face and head.
Insert two screws proximally and one distal to the osteotomy, and
carefully range the shoulder to make sure the new position is optimal.
Remove the distal screw and adjust the rotation if necessary. Insert
the remaining screws, check the position of the fixation on a
radiograph, if necessary, and close the wound in layers. -
Apply a shoulder immobilizer with an abduction pillow.
and check radiographs for bridging callus. If callus is present,
discontinue immobilization. Postoperative rehabilitation is not usually
necessary.
deformity in obstetric palsy. The general surgical technique is as follows:
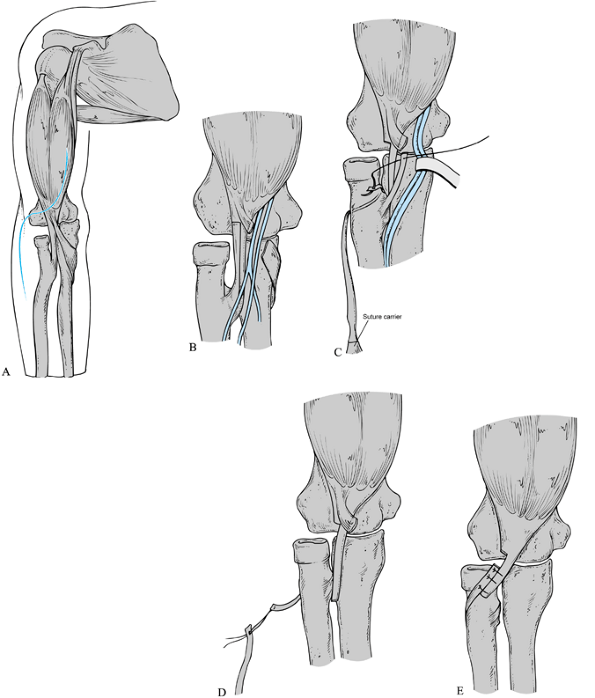 |
|
Figure 165.19.
Rerouting of the biceps tendon. See text for description of the surgical technique. Reprinted with permission from Manske PR, McCarroll HR Jr. Biceps Tendon Re-routing and Percutaneous Osteoclasis in the Treatment of Supination Deformity in Obstetrical Palsy. J Hand Surg 1980;5:153. |
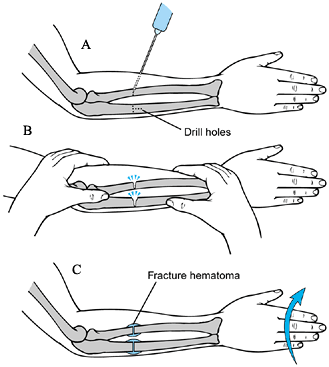 |
|
Figure 165.20. Osteoclasis of the forearm. See text for a description of technique (110).
|
-
Perform the operation under general anesthesia, and use a tourniquet. A sterile tourniquet may be necessary.
-
Approach the biceps tendon through an
anterior zigzag incision, placing the transverse limb of the zigzag
along the elbow flexion crease (Fig. 165.19A). -
Incise the lacertus fibrosis, and retract the median nerve and brachial artery ulnarward (Fig. 165.19B).
-
Expose the biceps tendon to its insertion
on the bicipital tuberosity of the radius. Divide the biceps tendon by
a Z lengthening (the Z should be as long as possible). Protect the
radial artery, which crosses the palmar surface of the tendon near its
insertion. -
Pass a folded wire suture or a curved
suture carrier around the neck of the radius. Reroute the distal tendon
segment so that it has a pronating torque by attaching it to the suture
carrier (Fig. 165.19C). -
Reattach the distal tendon to the
proximal tendon in a side-to-side fashion using nonabsorbable suture.
The biceps tendon usually requires 1.5 cm of lengthening to be repaired
after rerouting (110). Close the wound in layers and apply a long-arm cast in neutral forearm rotation, with the elbow flexed 90° (Fig. 165.19D, Fig. 165.19E). -
If biceps rerouting does not achieve
adequate correction of the supination deformity, perform two-stage
forearm osteoclasis any time after the rerouting has healed (Fig. 165.20). -
Perform the operation under general anesthesia, and use a tourniquet.
-
Through small incisions, use a
one-eighth-inch drill to make three bicortical drill holes in the
middle third of the radius and ulna (Fig. 165.20A). Fracture the bones by manipulating the forearm (Fig. 165.20B). Do not rotate the distal fragment at this time, or the fragments will displace. Close the wounds. -
Place the arm in a long-arm cast, with the elbow at 90°.
anesthesia, remove the cast and manipulate the distal forearm to the
desired position (0° to 40° of pronation) (Fig. 165.20C).
Enough callus will have formed by this time that the fragments will not
displace. Reapply the long-arm cast, with the elbow at 90° and the
forearm in the desired position of rotation. Immobilize for 6 weeks
after biceps rerouting. Focus the postoperative rehabilitation on
active elbow flexion and extension, and forearm rotation. Then
immobilize for 3 to 4 weeks after the second stage of forearm
osteoclasis. Postoperative rehabilitation is not usually necessary.
nerve grafting occur in patient selection. If the surgeon
underestimates the infant’s potential for recovery, resection and
grafting of the plexus may do more harm than good. If the surgeon waits
too long to operate (after 1 year of age), the limited improvement
achieved before surgery will be lost and may not be recovered.
related to shoulder external rotation tendon transfer and rerouting
occur in patient selection. If the deltoid is too weak, the tendon
transfer will not improve range of motion. If the patient is unable to
cooperate with postoperative therapy, the tendon transfer will probably
not function as well. If the shoulder contracture is due to extensive
glenohumeral changes, or if long-standing posterior shoulder
dislocation is present, the tendon transfer will not improve range. The
transverse axillary scar in this procedure is nearly invisible.
elbow flexion contracture is severe (more than 45°), the patient will
probably be unhappy with the position of the forearm after the humerus
is rotated externally. With the shoulder internally rotated, the
forearm can lie across the
front
of the body, but after an external rotation osteotomy, the forearm and
hand will “stick out.” Also, the anterolateral arm scar widens and is
quite noticeable.
the etiology, that is, because it is an injury rather than a congenital
malformation. BPBP is a common cause of malpractice claims against
obstetricians. The well-documented increased risk of recurrent BPBP
with subsequent pregnancies has not been featured in the obstetrics
literature; we advise parents of this risk so they can pass this
information along to their obstetrician.
these data are based on relatively short follow-up. We frequently see
3- to 4-year-old children with functional limitations from BPBP who
were declared fully recovered at 3 to 6 months of age, before they
could cooperate with a thorough active motion and strength examination.
However, many children with slightly limited shoulder range of motion
and strength can function almost normally, and even when function is
limited, older children and parents may be bothered more by the
decreased size of the extremity and length discrepancy than the
decreased function.
termed “congenital amputation,” occurs when the upper limb fails to
form below a certain level. Finger nubbins usually form at the distal
end of the limb, regardless of level (Fig. 165.21);
their presence helps differentiate this condition from congenital
constriction ring syndrome, in which nubbins do not form (see Chapter 69).
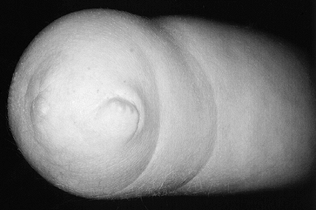 |
|
Figure 165.21. Nubbins at end of below elbow transverse failure of formation.
|
leading hypothesis for the etiology of transverse failure of formation
is the subclavian artery supply disruption sequence theory: disruption
of the embryonic subclavian blood supply causes transverse failure of
formation, Poland anomaly, symbrachydactyly, Mobius syndrome, or other
conditions, depending on the location of the blockage and the timing
and duration of disruption (16,154,175). One study (86)
showed evidence of fetal vascular occlusive disease in the placentas of
infants with transverse failure of formation, and hypothesized that
embolization from placental vascular thrombi caused this condition.
Others (85,115) have
found an increased incidence of transverse failure of formation and
other limb defects in infants whose mothers underwent chorionic villus
sampling before 10 weeks’ gestation.
is proximal forearm (below elbow), followed by transcarpal, distal
forearm, and through humerus (above elbow) (181) (Fig. 165.22). This condition is almost always unilateral (148), and the left side is more commonly affected (91).
Children with this condition have remarkably few functional deficits,
and surgery has not been proven to improve their abilities (18).
A prosthesis enhances prehension and possibly appearance but blocks
sensory feedback. When indicated, goal-directed occupational therapy
may help the school-aged child learn to perform activities of daily
living with and without a prosthesis.
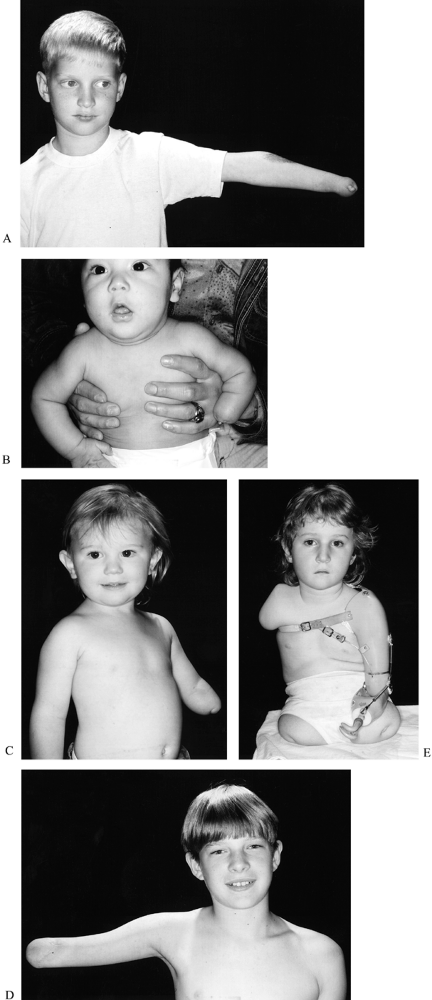 |
|
Figure 165.22. A: Transcarpal transverse failure of formation with thumb nubbin. B: Long below-elbow transverse failure of formation. C: Short below-elbow transverse failure of formation. D: Through elbow amputation. E: Above-elbow transverse failure of formation.
|
anomaly, but children with amelia (failure of formation at the shoulder
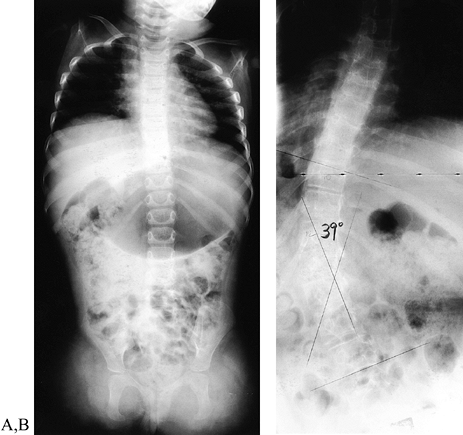
level) have a high incidence of scoliosis (138) (Fig. 165.23). Cognition and developmental milestones are usually normal, except that the child with a very short arm may not crawl.
 |
|
Figure 165.23. Girl with transverse failure of formation of upper (and lower) extremities. A: Radiograph of the spine (the child was 2 years of age). B: Radiograph of the spine (12 years of age) with 39° scoliosis.
|
formation should be assessed by a prosthetic team (which includes a
physician, a prosthetist, and an occupational therapist, and may
include a recreation therapist and social worker) at around 6 months of
age, and fitted with a prosthesis with a passive hand or mitt when able
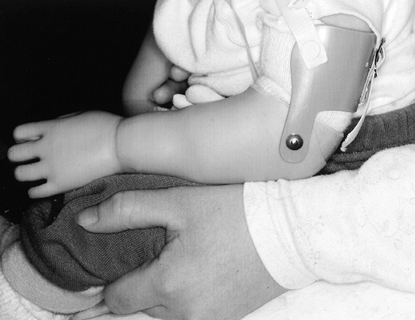
to sit independently (91) (Fig. 165.24). Parents are encouraged to gradually increase wearing time until the child tolerates
the prosthesis for the majority of the waking hours. Children with more
proximal or distal failure of formation are not usually fitted with a
prosthesis in infancy. If the deficiency is at shoulder level, the
prosthesis is too heavy for comfortable wear; if the deficiency is at
the distal forearm or through the carpus, the child functions better
without a prosthesis (148).
 |
|
Figure 165.24. Passive below-elbow upper-extremity prosthesis.
|
times each year) to check prosthesis fit and reinforce the importance
of regular wear. Children who are fitted with a prosthesis before 2
years of age are more likely to continue to use the prosthesis
throughout childhood (148). To enhance
acceptance and use of the prosthesis by the family and child, the
prosthetic team may organize supervised play groups for children who
use prostheses, and the prosthetist can fabricate dolls with prostheses
(Fig. 165.25). At 2 to 3 years of age, evaluate
the child for readiness for an active terminal device (TD). If the
child readily bears weight on the passive prosthesis when crawling and
uses it for pulling up, balance, and two-handed activities, such as
throwing and catching, he or she is probably ready to learn to use an
active TD.
 |
|
Figure 165.25. Doll with prosthesis.
|
a body-powered (cable-operated) prosthesis, which works best with a
hook or similar type TD, and a prosthesis powered by electrical signals
from proximal forearm muscle contractions (myoelectric prosthesis),
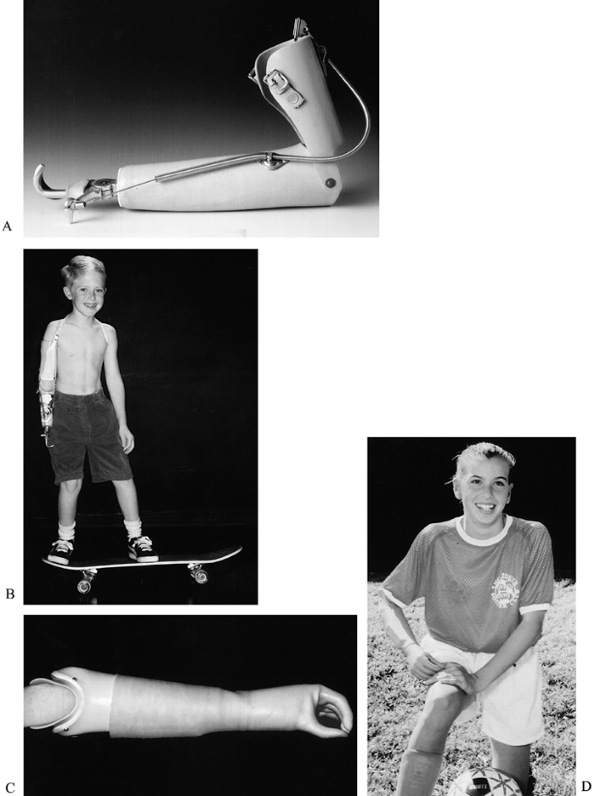
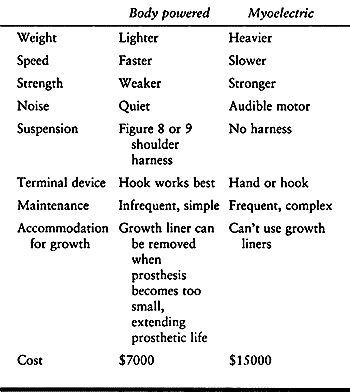
which usually has a TD that looks like a hand and uses a chuck pinch (Fig. 165.26 and Table 165.2).
Each of these prostheses has advantages and disadvantages; fortunately,
because the growing child needs a new prosthesis every 1 to 2 years, no
choice is permanent. The body-powered hook prosthesis is more difficult
to learn to use, but children who learn to use this type of prosthesis
first can usually learn to use a myoelectric prosthesis easily.
Children who learn to use a myoelectric prosthesis first have more
difficulty learning to use a body-powered hook prosthesis. Most studies
comparing performance of body-powered and myoelectric prostheses use a
hand TD on both prostheses. The cosmetic glove used with this TD
increases resistance to opening, which is easily overcome by the
myoelectric motor but makes the body-powered prosthesis very difficult
to use. In spite of this handicap, however, these studies show that
children perform most tasks faster with the body-powered prosthesis (47), and consistent users of a body-powered prostheses are more likely to be pleased with prosthetic function (100). However, parents and older children are often influenced by the
“high-tech” image of the myoelectric prosthesis and the fact that the
TD most commonly used with this prosthesis looks like a hand.
 |
|
Figure 165.26. A and B: Body-powered hook prosthesis. C and D: Myoelectric hand prosthesis.
|
 |
|
Table 165.2. Comparison of Upper-Extremity Prostheses: Body vs. Myoelectric Power
|
this is very rarely indicated. The Krukenberg procedure, in which the
radius and ulna are separated and muscles reattached so that the radius
pinches against the ulna, is most useful for blind bilateral distal
forearm amputees, because it provides unilateral prehension with
sensory feedback. This operation may occasionally be indicated for a
sighted child with above-wrist failure of formation who does not have
access to prosthetic facilities (34,69,157).
However, because children with unilateral above-wrist failure of
formation have very few functional deficits and the appearance of the
Krukenberg forearm is strikingly abnormal, this operation is rarely
indicated for the child with a unilateral deficiency.
transverse failure of formation is helping parents adjust their often
unrealistically high expectations of prosthetic technology. The
importance of good communication with the child and family cannot be
overstressed. We focus on
-
The remarkable abilities of the child
with or without the prosthesis (most children with below-elbow failure
of formation tell us that the only activity they cannot perform is
traveling hand over hand on the monkey bars) -
The opportunity to try a different type of prosthesis when the child outgrows the current one.
-
The need for improved prosthetic technology [well documented in a recent nationwide survey of upper-extremity prosthesis users (11)].
-
The importance of maintaining the
prosthesis so that the child can use it, and the responsibilities of
the child, family, prosthetist, and physician in accomplishing this
task.
family strongly prefers it and has demonstrated that they can keep
their clinic appointments and return promptly if the prosthesis breaks
or if the child outgrows it. Older children who use a body-powered
prosthesis may appreciate interchangeable TDs: a hook for function and
a prosthetic hand when appearance is more important than function.
Although the etiology of CDRH is unknown, it is known to be associated
with dysplasia of the capitellum and proximal radius, as well as
shortening of the ulna (95,125).
In CDRH, the radial head may be dislocated in an anterior, posterior,
or lateral direction; in one series, 47% were anterior, 43% posterior,
and 10% lateral (9).
The presenting complaint is usually posterolateral elbow prominence,
restricted elbow extension and forearm rotation, elbow “popping,” or
pain with activity (1,95),
although pain is uncommon before adolescence. Often, the limitations
due to CDRH are first perceived after an unrelated elbow injury and may
be erroneously attributed to that injury. When the condition is
unilateral, CDRH may be difficult to distinguish from chronic traumatic
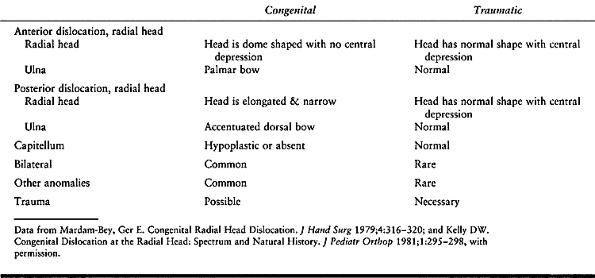
dislocation (29) (Table 165.3).
 |
|
Table 165.3. Congenital vs Traumatic Radial Head Dislocation
|
the cubital fossa in anterior CDRH, and palpable and visible laterally
in posterior CDRH (see Fig. 165.27 and Fig. 165.28).
Elbow motion deficits are often minimal, usually nonprogressive, and
worse in anterior than posterior CDRH. Loss of supination is the most
prominent limitation in both anterior and posterior CDRH. Anterior CDRH
blocks full flexion, and posterior CDRH blocks full extension, causing
a flexion contracture (usually 30° or less). Wrist range of motion may
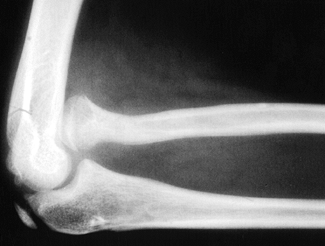
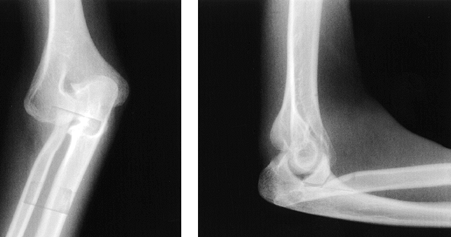
also be limited (1,9,97,111). In the infant, the unossified dislocated radial head may be visualized with diagnostic ultrasound (14), but the diagnosis is most commonly made by plain radiography (Fig. 165.27 and Fig. 165.28).
 |
|
Figure 165.27. Anterior congenital dislocation of the radial head.
|
 |
|
Figure 165.28. AP and lateral views of a posterior dislocation of the radial head.
|
functional limitations (46,125). Radial head resection before skeletal maturity has been associated with several different complications (see Complications,
later). Surgical reduction of the radial head and reconstruction of the
annular ligament or rotational radial and ulnar osteotomies have not
been consistently successful (9,124,125).
In adolescence or adulthood, the laterally or posteriorly dislocated
radial head may become painful owing to degenerative changes at the
contact point between the radial head and the distal humerus; radial
head excision relieves pain, improves appearance, and may improve range
of motion (29,97).
instability, and obtain bilateral wrist radiographs before radial head
resection. If the DRUJ is unstable and the symptomatic side is ulna
positive, resection of the radial head may cause wrist pain.
-
Perform the operation under general anesthesia, and use a tourniquet.
-
Make a longitudinal incision using a
Kocher approach over the dislocated radial head. Develop the interval
between the extensor carpi ulnaris and anconeus muscles. -
Incise the capsule with the forearm in
maximal pronation, and do not extend the capsular incision distal to
the radial neck, to avoid injuring the posterior interosseous nerve.
Preserve the annular ligament to enhance proximal radius stability and
protect the posterior interosseous nerve. Avoid violating the
periosteum of the proximal ulna to prevent radioulnar synostosis. -
Excise the radial head with an
oscillating saw, osteotome, or rongeur perpendicular to the radial
neck. Check elbow flexion and extension and forearm pronation and
supination to make sure that the proximal radius does not contact the
distal humerus; if it does, resect more until it moves freely. Remove
any loose intra-articular osteochondral fragments. -
Close the capsule with nonabsorbable
suture. Close the subcutaneous tissue with interrupted absorbable
suture, and close the skin with running subcuticular suture. -
Immobilize the elbow at 90° of flexion in a long-arm splint.
maturity, several complications can occur, including regrowth of the
proximal radius, postoperative radioulnar synostosis (29),
and cubitus valgus deformity. However, radial head resection for a
variety of indications, including CDRH, did not cause cubitus valgus in
27 elbows of 25 children with an average age of 14 years (range 5 to 18
years) (87). Other potential complications
include injury to the posterior interosseous nerve, radioulnar
synostosis, proximal migration of the radius, valgus elbow instability,
cubitus valgus, and wrist pain (9,29,97,111).
humerus, painful degenerative changes can occur. Radial head resection
reliably relieves pain and is sometimes necessary, even when the DRUJ
is unstable. When wrist pain occurs following radial head resection for
CDRH, it is usually mild and activity related.
failure of the normal caudal migration of the scapula during the fetal
period of development (32,48).
The scapula with this malformation is usually hypoplastic with
decreased vertical length and increased horizontal width-to-height
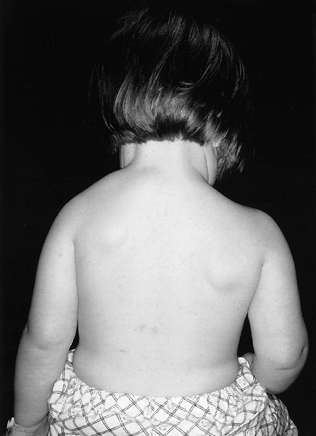
ratio (27), which is 2 to 10 cm more cephalad than normal (Fig. 165.29).
The inferior pole is rotated medially with the glenoid displaced
inferiorly. The periscapular muscles may be hypoplastic or absent,
causing scapular winging (32,33).
In 20% to 30% of cases, the superomedial scapula is connected to the
spinous processes, laminae, or transverse processes by a fibrous
tissue, cartilage, or bone called the omovertebral connection; this
connection is diagnostic of congenital elevation of the scapula (27,32,33). Right and left sides are affected with equal frequency, and bilateral involvement occurs in 10% to 30% of the cases (168).
 |
|
Figure 165.29. Congenital elevation of the scapula.
|
the scapula have associated anomalies, most commonly congenital
scoliosis, Klippel-Feil syndrome, fused or absent ribs, spina bifida,
and VATER association (17,27,32,33).
for resection of the omovertebral connection. Surgical mobilization and
caudal repositioning of the scapula have been recommended, but most
children with congenital elevation of the scapula do not need surgical
treatment (168).
asymmetry, diminished shoulder motion, and fullness at the base of the
neck. The affected scapula appears to be elevated and hypoplastic.
Shoulder abduction is significantly limited in 40% of patients, and
elevation is often limited (33,73). Basilar neck fullness is due to the prominence of the superomedial angle of the scapula (32). Congenital elevation of the scapula is usually not painful.
palsy and isolated scoliosis or Klippel-Feil syndrome (33).
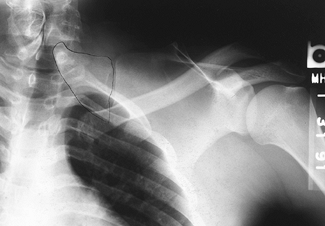
An anteroposterior (AP) radiograph of the shoulder demonstrates
scapular elevation, especially when compared with the contralateral
normal side. A bony omovertebral connection may be seen on the AP view (Fig. 165.30) or on lateral oblique views and CT scan. Examine the child for scoliosis and other associated anomalies.
 |
|
Figure 165.30. Bony omovertebral connection.
|
but many children with this condition have excellent shoulder function.
Passive stretching and abduction early in infancy may improve shoulder
range of motion, and removal of an omovertebral connection may also
improve range of motion.
proportional to the severity of deformity; children with mild grade 1
to grade 2 elevation (see next section, on classifications and terminology) have little functional impairment.
impairment, surgical repositioning of the scapula may be indicated in
addition to resection of the omovertebral connection, with the goal of
improving appearance and range of motion. Various methods of scapular
mobilization have been described. Woodward (180) recommended moving the trapezius and rhomboid muscle origins to a more caudal position along the spine. Borges et al. (27)
modified Woodward’s procedure to include excision of the superomedial
scapular prominence, to improve appearance. Clavicular osteotomy is
recommended to allow further scapular descent and prevent damage to the
brachial plexus (27,33,67).
patients who underwent four different operations for congenital
elevation of the scapula found that the average reduction of elevation
was 2.7 cm, and average improvement in abduction was 19 degrees for
repositioning procedures, with the best results in patients who
underwent Woodward’s procedure. Optimal age for surgery is probably
between 3 and 8 years of age (32,68). Good results of scapular mobilization have been reported in older patients, but with less functional improvement (27). Normal appearance and function should not be expected at any age (33).
called Sprengel’s deformity, it is actually a malformation instead of a
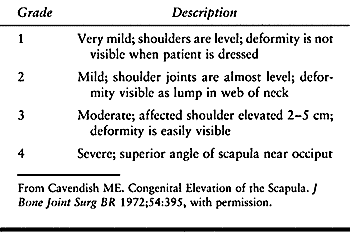
deformity (see Chapter 69). The condition has been classified by severity (Table 165.4). This classification helps the surgeon determine treatment and prognosis and evaluate the postoperative result (33).
 |
|
Table 165.4. Classification of Congenital Elevation of the Scapula
|
 |
|
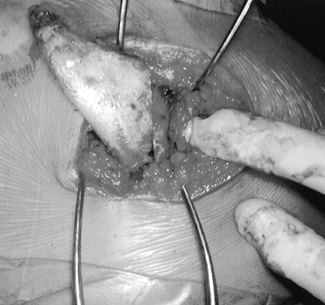
Figure 165.31. Intraoperative view of resection of omovertebral connection (same patient as in Fig. 165.30).
|
-
Place the patient in the prone or lateral decubitus position with the affected side up.
-
Place the incision directly over the
omovertebral connection, in a transverse direction, if possible. Inject
skin and subcutaneous tissue with bupivacaine and epinephrine; this
provides pre-emptive analgesia and improves hemostasis. -
Expose and transect the connection at its scapular and spinous connections, and remove it.
-
Close the incision in layers, and immobilize the arm in a sling or shoulder immobilizer.
Complications of surgical repositioning of the scapula include scar
widening and residual scapular elevation (27,32,33,177,180). Brachial plexus and vascular injury can occur (27); clavicular osteotomy (or morcellation) may prevent neurovascular injury with scapular repositioning (142). In addition, the suprascapular nerve may be injured with resection of the superior scapula.
usually improves appearance and range of motion. Scapular
repositioning, a major operation with considerable risk of
neurovascular injury and a good possibility of inadequate correction,
is rarely indicated.
The etiology is unknown; one hypothesis suggests that the subclavian
artery may compress the developing right clavicle, which might explain
the predominance of right-sided lesions and the occurrence of
left-sided lesions in association with dextrocardia. Bilateral
involvement is rare (57,107,163). Another hypothesis is that the pseudarthrosis is caused by the failure of two ossification centers to fuse (82), but the normal clavicle in the developing embryo has only one ossifcation center (146).
Unlike congenital pseudarthrosis of the tibia and ulna, this condition
is not associated with neurofibromatosis, although it may be inherited
in an autosomal recessive fashion (8,57,146).
treatment, and historically surgery for clavicular pseudarthrosis has
had a high rate of serious complications (8,68,131,163).
Surgical resection of the pseudarthrosis and internal fixation may be
indicated if the pseudarthrosis is very prominent or painful.
have a prominent middle third of the right clavicle at birth or soon
thereafter; the prominence may increase with age (146).
The pseudarthrosis is usually not painful, and shoulder range of motion
is normal. Radiographs of the pseudarthrosis reveal an osseous
separation with enlarged, rounded bone ends, and a distinctive absence
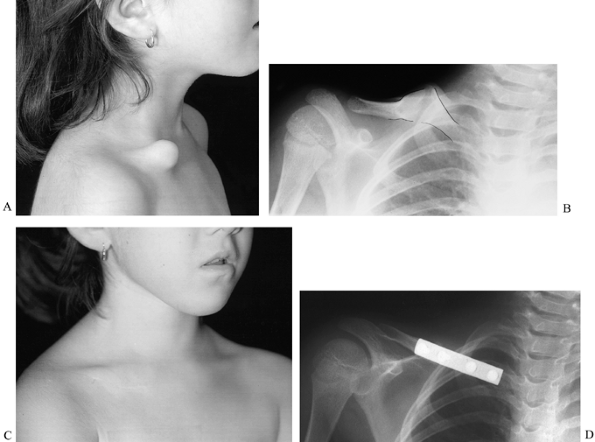
of fracture callus (Fig. 165.32). Although case reports indicate that the natural history of pseudarthrosis of the clavicle is benign (151), no large series of cases has been reported.
 |
|
Figure 165.32. Congenital pseudarthrosis of the clavicle. A: Preoperative photo. B: Preoperative radiograph (pseudarthrosis outlined). C: Postoperative photo. D: Postoperative radiograph.
|
fracture (a common birth injury, which is accompanied by
pseudoparalysis of the involved limb, painful range of motion, and
radiographic callus) and cleidocraniodysostosis (a bilateral inherited
disorder of fetal membranous bone ossification, with clavicle, cranial,
and pelvic dysplasia) (2,8,68,131,146,168).
to obtain union is generally recommended in the postpubertal patient
who wishes to improve appearance. In prepubertal children, most authors
who advocate surgical treatment recommend excision of the
pseudarthrosis, bone grafting, and plating (8,57,68,82,131,146).
-
Position the patient supine, with a pad under the affected scapula.
-
Incise the skin longitudinally along the inferior clavicular border, and expose the pseudarthrosis.
-
Incise the periosteum longitudinally. Resect the pseudarthrosis subperiosteally. Measure the defect.
-
Obtain autologous corticocancellous iliac
crest bone graft to span the clavicular defect created by resection of
the pseudarthrosis, if necessary. -
Internally fix the clavicle with a
semitubular or small low-profile dynamic compression plate. Avoid
inferior placement of the plate or graft, which could cause subclavian
vessel or brachial plexus injury (the subclavian artery passes between
the clavicle and first rib immediately deep to the clavicular defect) (146). -
Close the incision in layers, and immobilize the arm in a sling.
grafting, and plating have been described, including hypertrophic scar
formation, infection, nonunion, neurovascular injury, and bone graft
donor site morbidity (8,131).
Steinmann pin fixation should never be used because it provides
inadequate fixation and pins have been reported to migrate or cause
neurologic injury (163). Simple excision of the pseudarthrosis without bone graft or internal fixation causes the affected shoulder to droop (68,131).
when the malformation is conspicuous and bothersome to the patient or
the patient’s parents. As with any elective operation, the patient and
the parents must fully understand the potential risks. Resection,
grafting, and plating can improve the patient’s appearance considerably
in this condition.
Pseudarthrosis may be present at birth, or it may develop spontaneously
after a fracture or after osteotomy; it has been reported to occur in
the tibia, ulna, femur, clavicle, radius, and humerus (40).
The etiology of congenital pseudarthrosis is unknown; in the majority
of cases, the pseudarthrosis contains fibrous tissue, not neurofibroma (40) (see Chapter 169).
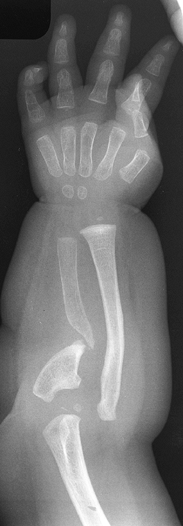
progressive forearm deformity. The forearm is short and bowed, and
eventually the radial head dislocates (114) (Fig. 165.33). The goal of treatment is to obtain union without sacrificing motion.
 |
|
Figure 165.33. Congenital pseudarthrosis of the ulna.
|
instability, weakness, and sometimes pain, although motion is usually
not limited. Like the same condition in the tibia, union can be
achieved with bone grafting and immobilization, but pseudarthrosis
usually recurs. Historically, creation of a one-bone forearm was the
only option available. This operation successfully restores forearm
stability (169) but eliminates forearm rotation. Although the Ilizarov technique may successfully restore union in tibial pseudarthrosis (132),
the use of prolonged immobilization and differential lengthening of the
forearm bones is also likely to result in loss of forearm rotation.
have reported treating ulnar pseudarthrosis with free vascularized
fibular graft; in most cases, union was achieved, length was
maintained, and forearm rotation was diminished but not completely
lost. The indications for vascularized fibular graft, including the
optimal age of the patient, have not yet been established, but this
technique is promising.
preoperative arteriograms of the donor and recipient sites help
identify possible vascular abnormalities (54).
-
Resect the pseudarthrosis and fix the
graft in place using short plates at the proximal and distal ends,
before performing vascular anastomoses. Avoid using intramedullary
fixation or a long plate that spans both ends of the graft because
these fixation methods interfere with graft vascularity. -
Use an end-to-side anastomosis in the forearm, if possible, to minimize reduction of hand perfusion.
-
Use a supplemental cancellous bone graft at each end of the fibular graft to enhance union.
short-leg walking cast, which can be removed after 3 to 4 weeks.
Immobilize the arm with a long-arm cast and check radiographs out of
plaster every 6 weeks. Continue immobilization until graft
incorporation is seen on radiographs; the average time to graft
incorporation is 3 to 6 months.
regrafting with cancellous allograft may be necessary. Even after union
is achieved, pseudarthrosis may recur. If painful pseudarthrosis recurs
and regrafting fails, creation of a one-bone forearm is the only
remaining surgical option.
vascularized fibula grafting are not well established, and follow-up to
skeletal maturity is lacking. The best indications probably are pain
and progressive deformity.
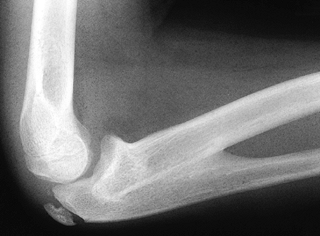
malformation caused by failure of normal prenatal separation of the
radius and ulna. The persistent connection between the two bones is
nearly always proximal; distal radioulnar synostosis is extremely rare (15,96,144,152,173). The connection is initially cartilaginous, but it usually eventually ossifies, forming a bony synostosis (Fig. 165.34). The forearm is usually fixed in pronation, probably because this is the normal fetal position (96,173).
 |
|
Figure 165.34. Proximal radioulnar synostosis.
|
associated with other malformations or syndromes in up to one third of
affected children (152). Other malformations associated with PRUS include thumb hypoplasia, carpal coalition, symphalangism, and clubfoot (124); syndromes associated with PRUS include Apert’s syndrome, arthrogryposis, fetal alcohol syndrome, and Klinefelter’s syndrome (98,152,165,166). Familial occurrence (37,71) with autosomal dominant inheritance (141) has also been reported.
years of age with painless limitation of forearm rotation and a slight
flexion contracture (37,152), although occasionally PRUS is not noted until adolescence (71).
Typically, a parent or teacher notices that the child has difficulty
positioning the hand to catch a ball, drink from a cup, or accept small
objects in an open palm and tends to hold objects in a backhanded, or
hyperpronated, position. Bilateral involvement (60% to 80%) (129,152) and fixation in more than 60° of pronation (up to 40%) (152) are usually noted earlier because they cause more significant impairment.
in addition to a fixed forearm pronation deformity (129,144,152).
Children with PRUS partially compensate for lack of forearm rotation by
developing rotational hypermobility at the wrist and positioning the
shoulder in internal rotation, flexion, and abduction (37,65,129).
96% of patients had mild or no limitations in daily activities
regardless of forearm position, and the authors concluded that
treatment of PRUS is rarely indicated. Other authors (157)
consider fixed pronation between 15° and 60° a relative indication, and
forearm fixation in greater that 60° pronation a definite indication
for surgery, if functional limitations are significant.
although recently a free vascularized fascial flap placed between the
separated forearm bones has been reported to successfully block
postoperative recurrence of the synostosis (93).
At present, however, the generally accepted surgical treatment is
derotation osteotomy through the fusion mass, using K-wires or small
Steinmann pins to fix the osteotomy (65,126,129,152). Others have described gradual correction to neutral following osteotomy, using the Ilizarov method (25), and two-stage osteoclasis without internal fixation (105).
because the best position of rotation varies with the task. Some
surgeons recommend placement of the dominant forearm in 10° to 20° of
pronation, and the nondominant forearm in neutral rotation, if
necessary; and for unilateral PRUS, placement in 0° to 15° of pronation
(152). Others (65,126)
advocate placing the nondominant forearm in 20° to 35° of supination
first, and the dominant forearm in 30° to 45° only, if necessary; and
for unilateral involvement, 0° to 20° supination.
position and the presence of a radioulnar synostosis does not predict
function (37). PRUS is part of a spectrum of
malformations ranging from radial head abnormalities to complete
synostosis with marked forearm shortening and absence of the radial
head (124,152), although these have not been formally divided into types.
helps determine the child’s functional deficits and optimal forearm
position. If possible, surgery should be performed before school age (152),
but this timing precludes considering the child’s future intended
occupation to determine the optimal forearm position, as recommended by
some surgeons (37).
-
Perform the operation under general anesthesia and a tourniquet.
-
Make a dorsal longitudinal incision just
radial to the subcutaneous border of the ulna. Incise the fascia in the
interval between the anconeus and extensor carpi ulnaris muscles
(Kocher approach). Expose the fusion mass subperiosteally. -
Pass a smooth K-wire from the olecranon
apophysis into the intramedullary canal of the ulna under fluoroscopic
guidance. This wire helps maintain longitudinal position while allowing
adjustment of rotation. -
Make a mark across the osteotomy site to
determine rotation. Perform the osteotomy around the wire, using
multiple drill holes and an osteotome or oscillating saw. The osteotomy
should be distal to the coronoid and radial head (if present). -
Rotate the forearm to the desired
position and transfix the osteotomy site with a K-wire, passing
obliquely from proximal ulna to distal radius. Leave the end of this
wire through the skin to facilitate urgent removal for postoperative
derotation if vascular compromise occurs. -
If a large change in forearm rotation is necessary, consider resecting 5 mm of bone at the osteotomy site (129), prophylactic forearm fasciotomy (152), or two-stage derotation to decrease the risk of postoperative neurovascular traction or compartment syndrome.
-
Close the subcutaneous tissues and skin, and place the arm in a splint or long-arm cast with the elbow flexed to 90°.
neurovascular status closely for at least 48 hours postoperatively. If
a long-arm splint was applied, change to a long-arm cast after 1 to 2
weeks. Remove the cast 6 weeks postoperatively to check forearm
radiographs. Remove pins when the forearm bones show signs of healing.
Therapy is not usually necessary.
The most serious complications are vascular compromise and compartment
syndrome, which occur more frequently with rotational position change
of
greater
than 85°. If this much correction is needed, obtain it in two stages.
If either of these complications occur, remove the transfixion pin and
return the forearm to its original position; if forearm compartment
pressures are elevated, perform fasciotomies (see Chapter 13). Rotational correction can be reachieved 5 to 10 days later (152).
Other complications include nerve palsy (due to either intraoperative
damage to the posterior interosseous nerve or traction from rotational
change), wound infection, loss of correction, and nonunion (37,105,129,152).
fetal alcohol syndrome have more severe pronation contractures than
most other children with PRUS. Children with fetal alcohol syndrome are
often mentally retarded, and they may be unable to describe symptoms of
nerve traction following rotational osteotomy.
enchondral bone growth in which cartilaginous exostoses (also called
osteochondromas) grow from the physes of long bones and from the
pelvis, ribs, scapula, and vertebrae (10,135). The exostoses may be sessile or pedunculated (Fig. 165.35).
They grow until the patient is skeletally mature and eventually ossify.
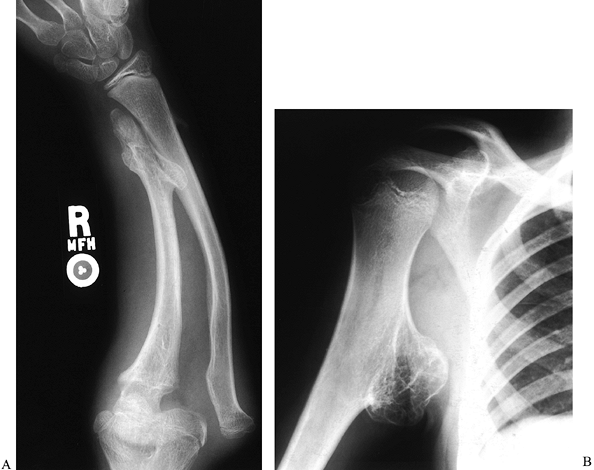
The forearm is affected in 30% to 67% of patients with this condition (22,89,145), and the proximal humerus is also commonly involved (Fig. 165.36).
 |
|
Figure 165.35. Multiple hereditary exostoses. A: Forearm, with distal ulna growth arrest and radial head dislocation. B: Proximal humeral exostosis.
|
 |
|
Figure 165.36. Multiple hereditary exostoses. Forearm deformity associated with radial head dislocation (same patient as in Fig. 165.35A).
|
Although MHE has an equal prevalence in both sexes, boys tend to have more severe involvement (120). Genetic studies of families with this condition have mapped the chromosomal abnormality to at least three different loci (119,120),
indicating that the MHE phenotype can be subdivided into at least three
different genotypes. Multiple exostoses also occur in other conditions,
such as metachondromatosis and the Langer–Giedion syndrome (120).
osteochondromas (secondary chondrosarcoma) in MHE vary from 0.5% to 50%
of patients; the lower incidence is most likely correct. Malignant
degeneration is quite rare in the upper extremity; the pelvis and
proximal femur are the most common locations of secondary
chondrosarcoma (159). Malignant degeneration in children is also very rare (120,135).
surgically removed; exostoses removed before skeletal maturity may
recur. However, exostoses can cause local discomfort, nerve or tendon
impingement, decreased range of motion, and longitudinal and angulatory
growth abnormalities (135). Growth abnormalities may also occur in MHE in the absence of radiologically visible exostoses (24).
decreased range of motion, combined with a clinical examination for
bony bumps of the upper extremity, helps the physician determine which
areas to radiograph. Most exostoses have a cartilage cap and,
therefore, are larger than their radiograph appearance suggests.
impingement, is a frequent indication for exostosis removal.
Subscapular exostoses are common but do not usually cause pain or
require removal. Proximal humeral exostoses may impinge on muscle
tissue, the brachial plexus, or the axillary nerve, causing pain,
paresthesias, or paresis; exostoses of the distal radius and ulna may
impinge on the dorsal radial sensory, median, or ulnar nerves or the
radial or ulnar arteries. These are indications for surgical
removal, which effectively relieves symptoms of impingement (135).
An exostosis in the interosseous space that is blocking forearm
rotation should also be removed. Recurrence is unlikely if the patient
is near skeletal maturity.
MHE are complex, and their interrelationships are not well understood.
Distal ulnar growth arrest is a common sequelae of MHE, even when
osteochondromas of the distal ulna are not visible on radiographs. The
radial articular angle may increase, and the carpus may “slip”
ulnarward, but neither of these findings appears to be associated with
negative ulnar variance (28). Radius bowing, radial head dislocation (179) (Fig. 165.35), and forearm shortening also occur, causing loss of forearm rotation.
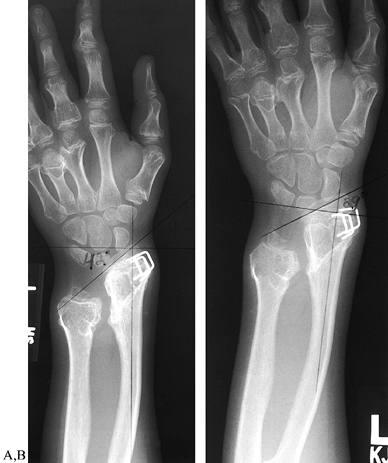
retard these progressive growth disturbances. Hemiepiphyseal stapling
of the radial side of the distal radius retards the growth of that side
while allowing the ulnar side to continue to grow, which corrects the
increased radial articular angle (179) (Fig. 165.37). This procedure may be performed in conjunction with ulnar lengthening (51,135). Resection of a dislocated radial head relieves pain and removes the associated prominence (10,113)
but probably does not improve forearm rotation significantly. This
procedure should be reserved for skeletally mature patients because
removal of the radial head in the growing child may cause cubitus
valgus or proximal radial overgrowth (95,97). Single-stage ulnar lengthening with or without radial osteotomy can be performed (179),
or using the Ilizarov technique, the ulna and radius can be
differentially lengthened, radial bowing corrected, and the dislocated
radial head reduced (42) (Fig. 165.38).
Differential lengthening requires several months of external fixation
and may diminish forearm rotation. Finally, radioulnar fusion may be
performed as a salvage procedure (143).
 |
|
Figure 165.37. Correction of increased radial articulation angle (radial tilt) in multiple hereditary exostoses by hemiepiphyseal stapling. A: Forty-two degree radial tilt, immediately following distal radial stapling. B: Twenty-nine degree tilt, 2 years following distal radial stapling.
|
 |
|
Figure 165.38. Differential radius and ulna lengthening for multiple hereditary exostoses. A: After application of Ilizarov fixator, before initiation of lengthening. B: After lengthening.
|
with untreated forearm deformities due to MHE maintain function are
comfortable with their appearance (10,155).
The authors of these studies recommend a less aggressive approach to
surgical treatment of the forearm in MHE. They point out that
relatively simple procedures, such as removal of symptomatic
osteochondromas and dislocated radial heads, can improve appearance and
relieve pain,
but no surgical treatment has been shown to improve forearm function in MHE.
osteochondroma in a skeletally mature person because this condition may
be a sign of malignant degeneration.
including multiple cartilaginous exostoses, diaphyseal aclasis,
dyschondroplasia, hereditary deforming chondrodysplasia (89,179),
and osteochondromatosis. The individual tumors may be called exostoses
or osteochondromas. MHE is frequently confused with multiple
enchondromatosis (Ollier’s disease), an entirely different condition (89).
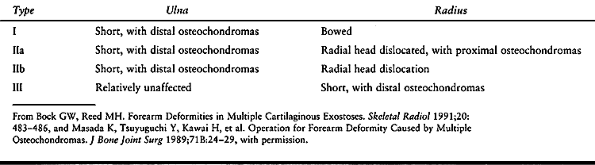
for forearm deformity caused by MHE. In the first, type I forearms are
the most common type (24,113) (Table 165.5).
 |
|
Table 165.5. Classification of the Forearm with Multiple Hereditary Exostoses
|
Group III, with forearm shortening, had earlier initial symptoms, more
frequent lower extremity deformity, a higher number of osteochondromas,
and a shorter height; the authors hypothesize, but do not prove, that
group III patients may be at higher risk of malignant degeneration.
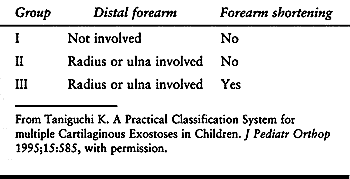 |
|
Table 165.6. Classification of Forearm Involvement as an Index of Overall Disease Severity in Multiple Hereditary Exostoses
|
-
Perform the operation under a tourniquet, if possible.
-
Approach the osteochondroma through a longitudinal incision, centered over the tumor.
-
Use the most readily available anatomic
interval. Usually the osteochondroma splits the surrounding structures,
and approach is not difficult. Watch for nerves or vessels wrapped
around the pedicle of the tumor, or a nerve flattened over its surface. -
Remove the entire tumor, leaving a smooth contour.
-
Perform the operation under a tourniquet, with fluoroscopic guidance.
-
Approach the radial aspect of the distal
radial physis through a longitudinal incision. Find and protect the
dorsal radial sensory nerve. -
Under fluoroscopic guidance, place three
extraperiosteal epiphyseal staples across the distal radial physis: one
directly radial, one slightly palmar, and one slightly dorsal, all
outside of the first dorsal compartment.
postoperatively is adequate immobilization. Obtain radiographs of the
distal radius twice a year, and remove the staples when the desired
amount of radial tilt (usually 20°) is attained.
for a detailed description of planning osteotomies, designing fixation,
ulnar lengthening and angular correction, and radial head reduction.
insertion of hemiepiphyseal staples, permanent physeal arrest and
overcorrection could occur. Hemiepiphyseal staples usually require
removal at the end of growth, if not sooner, because of adequate
correction or local irritation.
and the radial head are simple operations with good results. We measure
forearm rotation twice a year for children with MHE with forearm
involvement, and consider progressive loss of forearm rotation an
indication for osteochondroma removal.
Shriners Hospital Northern California, for her assistance with
references, and Julia Serat, photographer, Shriners Hospital Northern
California, for her assistance with clinical photographs and
radiographic studies.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
MM, Clarke HM, Curtis CG. The Prognostic Value of Concurrent Clavicular
Fractures in Newborns with Obstetrical Brachial Plexus Palsy. J Hand Surg 1994;19B:729.
MM, el-Sayed AA, Al-Kharfy TM, al-Jurayyan NA. Obstetrical Brachial
Plexus Injury in Newborn Babies Delivered by Caesarean Section. J Hand Surg 1996;21B:263.
DJ, Heard DCY, Donovan WH. Epidemiologic Overview of Individuals with
Upper-limb Loss and Their Reported Research Priorities. Journal of Prosthetics and Orthotics 1996;8:2.
JN, Weaver DD. Subclavian Artery Supply Disruption Sequence: Hypothesis
of a Vascular Etiology for Poland, Klippel-Feil, and Mobius anomalies. Am J Med Genet 1986;23:903.
KM, Ma GFY, Cheng JCY, Leung PC. The Krukenberg Procedure: A Method of
Treatment for Unilateral Anomalies of the Upper Limb in Chinese
Children. J Hand Surg 1984;9A:548.
HM, Al-Qattan MM, Curtis CG, Zuker RM. Obstetrical Brachial Plexus
Palsy: Results Following Neurolysis of Conducting
Neuromas-in-Continuity. Plast Reconstr Surg 1996;97:974.
B, Burckhardt A. Isolated Congenital Dislocation of the Radial Head.
Good Function in 4 Untreated Patients after 14–45 Years. Acta Orthop Scand 1997;68:598.
GR, McElfresh EC, Peterson HA, Wicklund PT. Management of Deformities
of the Forearm in Multiple Hereditary Osteochondromas. J Bone Joint Surg 1984;66A:670.
PC, Koby M, Park TS, et al. Fast Spin-echo Magnetic Resonance Imaging
for Radiological Assessment of Neonatal Brachial Plexus Injury. J Neurosurg 1995;83:461.
EM, Forouzan I, Morgan MA. A Retrospective Analysis of Erb’s Palsy
Cases and Their Relation to Birth Weight and Trauma at Delivery. Journal of Maternal-Fetal Medicine 1997;6:1.
B, Rondhuis JJ, Karbowski A. Treatment of Congenital Elevation of the
Scapula. 10 (2–18) Year Follow-up of 37 Cases of Sprengel’s Deformity. Acta Orthop Scand 1993;64:365.
F, Maeder P, Oberson JC, Schnyder P. Magnetic Resonance Imaging of the
Shoulder in Children with Brachial Plexus Birth Palsy. Pediatr Radiol 1995;25:S125.
T, Mitomo M, Hirabuki N, et al. Nerve Root Avulsion of Birth Palsy:
Comparison of Myelography with CT Myelography and Somatosensory Evoked
Potential. Radiology 1991;178:841.
MM, Phipps GJ. Closed Reduction and Tendon Transfer for Treatment of
Dislocation of the Glenohumeral Joint Secondary to Brachial Plexus
Birth Palsy. J Bone Joint Surg 1998;80A:997.
LB. Report of National Institute of Child Health and Human Development
Workshop on Chorionic Villus Sampling and Limb and Other Defects. Teratology 1992;48:7.
MA, McCarroll HR Jr. Shoulder External Rotation Tendon Transfers in
Brachial Plexus Birth Palsy: Results of Two Techniques. Orthopaedic Transactions 1996;20;311.
F, Ibaraki K. Mobilization of a Congenital Proximal Radioulnar
Synostosis with Use of a Free Vascularized Fascio-fat Graft. J Bone Joint Surg 1998;80A:1186.
H, Nogami H, Oki T, et al. Antley-Bixler Syndrome: A Disorder
Characterized by Congenital Synostosis of the Elbow Joint and the
Cranial Suture. J Pediatr Orthop 1996;16:243.
FJ, Tham S, Price A, et al. Outcome of Surgical Treatment for Forearm
Pronation Deformities in Children with Obstetric Brachial Plexus
Injuries. J Hand Surg 1999;24B:43.
HH, Strecker WB, Manske PR, et al. A Surgical Technique of Radioulnar
Osteoclasis to Correct Severe Forearm Rotation Deformities. J Pediatr Orthop 1995;15:53.
J. Paralysie Obstetricale du Plexus Brachial Symposium: Traitement des
Sepuelles: Primaute du Traitement de L’Epaule—Methode d’Expression des
Resultats. Rev Chir Orthop 1972;58:166.
PR, McCarroll HR Jr. Biceps Tendon Re-routing and Percutaneous
Osteoclasis in the Treatment of Supination Deformity in Obstetrical
Palsy. J Hand Surg 1980;5:153.
E, Earley MJ, Stephens MM. Congenital Pseudarthrosis of the Ulna
Treated by Free Vascularized Fibular Graft: A Case Report and Review of
Methods of Treatment. J Hand Surg 1993;18B:285.
P, Tozzi AE, Agosti S, et al. Transverse Limb Reduction Defects after
Chorion Villus Sampling: A Retrospective Cohort Study. GIDEF: Gruppo
Italiano Diagnosi Embrio-Fetali. Prenat Diagn 1993;13:1051.
C, Gilbert A, Azze RG. Congenital Pseudarthrosis of the Forearm:
Treatment of Six Cases with Vascularized Fibular Graft and a Review of
the Literature. Microsurgery 1993;14:252.
LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne’s Palsy: A
Consequence of Fetal Macrosomia and Method of Delivery. Obstet Gynecol 1986;68:784.
JH Jr, Hudson AR, Hoffman HJ. Preliminary Experiences with Brachial
Plexus Exploration in Children: Birth Injury and Vehicular Trauma. Neurosurgery 1988;22:715.
WB, Hall JE. One-bone Forearm as a Salvage Procedure for Recalcitrant
Forearm Deformity in Hereditary Multiple Exostoses. J Pediatr Orthop 1993;13:587.
SB, King JD, Marrero G. Congenital Pseudarthrosis of the Clavicle: A
Review of the Literature and Surgical Results of Six Cases. J Pediatr Orthop 1988;8:316.
Charles S, DiMario FJJ, Grunnet MS. Mobius Sequence: Further In Vivo
Support for the Subclavian Artery Supply Disruption Sequence. Am J Med Genet 1993;47:289.
HH, Piston RW, Clancy M, Betz RR. A Syndrome of Dislocated Hips and
Radial Heads, Carpal Coalition, and Short Stature in Puerto Rican
Children. J Bone Joint Surg 1993;75A:259.
JL. Paralysies Obstetricales du Plexus Brachial. Evolution Spontanee,
Resultats des Interventions Reparatrices Precoces (Thesis). Universite
Paris VII 1983.
JW. Congenital Elevation of the Scapula. Correction by Release and
Transplantation of Muscle Origins: A Preliminary Report. J Bone Joint Surg 1961;43A:219.
R, Lamb DW. Congenital Upper Limb Anomalies: An Etiologic Grouping of
Clinical, Genetic, and Epidemiologic Data from 387 Patients with
“Absence” Defects, Constriction Bands, Polydactylies, and Syndactylies.
J Hand Surg 1985;10A:958.
R, Williams PF, O’Connor JC. The 1960s Epidemic of Arthrogryposis
Multiplex Congenita: A Survey from the United Kingdom, Australia and
the United States of America. J Bone Joint Surg Br 1981;63-B:76.
