Pain Management
defines pain as “an unpleasant sensory and emotional experience
associated with actual or potential tissue damage or described in terms
of such damage.” Two important points can be taken from this
definition. First, pain is subjective and thus, the gold standard for
pain measurement is the patient’s self-report. In children, one must
consider their developmental level when interpreting their pain
behavior. Second, there is always an emotional component to pain. Good
pain management may include treating anxiety and fear.
This can be attributed to a number of factors. These include difficulty
in assessing pain, fear about the side effects of narcotics in
children, and a fear of addiction. In the 1960s, the medical literature
barely refers to pediatric pain and when it does it is only anecdotal.
This likely demonstrates a problem with pain assessment.
studies showing that infants who did not receive good intraoperative
and postoperative pain management had higher incidences of ventilatory
dysfunction, acidosis, cardiovascular complications, infections,
clotting disorders, and ultimately had higher mortality rates. These
articles showed us that not only can pain be treated safely in infants
but that there are dangerous consequences of leaving pain untreated.
stress hormones such as glucagon, cortisol, prostaglandins,
norepinephrine, and substance P. These in turn promote tissue breakdown
and water retention, leading to lactic acidosis and hyperglycemia. This
results in a higher heart rate and blood pressure, increased work of
breathing, impaired bowel function, impaired immune function, and
increased clotting. Together, these factors increase mortality rates in
infants who are left with untreated pain.
pain in childhood can leave a patient more sensitive to painful stimuli
in the future. Since the brain and spinal cord are still forming in
infants and children, experiencing painful stimuli can alter the
development of these structures. N-methyl-D-aspartate
(NMDA) receptors are excitatory amino acid receptors that send messages
to the dorsal horn of the spinal cord. They are activated by painful
stimuli. If a child receives repeated painful stimuli, this may cause
the NMDA receptors to become more plentiful and send an increased
message to the dorsal horn resulting in areas of
hyperalgesia/exaggerated pain responses in the future. Studies show
that undergoing painful procedures, such as circumcision without
analgesic therapy can cause sleep disturbances and behavioral changes
for prolonged periods of time.
receptors begin to appear on the fetus. By 20 weeks, all surfaces are
covered by sensory receptors. Pain can be transmitted by unmyelinated C
fibers and by thinly myelinated A fibers. Myelination occurs between 30
and 37 weeks’ gestation. At 42 weeks’ postconception, pain pathways are
more organized as receptors and transmitters move to positions in the
dorsal horn of the spinal cord. Thus, premature infants may not be able
to localize their pain but they will still feel it. It can compare to
having a “stomachache” in which the pain is diffuse and hard to
localize but is still quite uncomfortable.
Unfortunately, there is no one precise physical test that can be used
to measure pain. A pain assessment scale is a tool used to quantify and
monitor pain. In children, one can measure pain subjectively or
objectively. The gold standard for pain assessment is the
“self-report,” a subjective method in which patients tell caregivers
how much pain that they feel. This is the subjective method of
measuring pain.
-
From birth to 2 years of age, the child
is learning to differentiate self from nonself and is incapable of
reporting pain in words. -
From 7 to 10 years of age, children
become capable of logic and understand cause and effect. They can
understand that reporting pain may lead to treatment. -
From 11 to 14 years of age, children
begin to understand abstract and hypothetical thought and all the
psychosocial aspects of pain come into play.
patients to assess and treat because they can report their pain in
words and can understand why doing this may help them but there aren’t
as many emotional issues tied to the pain as with preteens and teens.
a child’s self-report (subjectively). It may be useful to use the word
“hurt” or “owie” instead of pain for some children. A few of the most
commonly pain scales include the Visual Analog or 0-to-10 scale, the
Oucher scale, and the Wong-Baker FACES scale.
assessment scale that is often used with adults as well as children.
For children to be able to understand how to use this scale, they must
be able to count to 10 and understand the concept of greater than and
less than. The scale can be used with or without the visual aid of a
0-to-10 diagram. Explain to the patient that if 0 means no pain at all,
and 10 is the worst pain (hurt) you can imagine, ask what number the
pain would be. The patient may indicate verbally or by pointing to a
number on the diagram.
 |
|
Figure 33-1 Oucher Pain Assessment Scale.
|
for children 3 years of age and older. Three versions of the Oucher
scale are available (Fig. 33-1). Each has
photos showing Hispanic, Caucasian, or African-American children in
increasing degrees of pain. One can explain to the patient that the
child at the bottom has no pain at all and the child at the top has the
biggest hurt that you could imagine. Ask the patient which picture
shows how much pain that he or she is feeling right now. Once the child
has pointed to a picture, their selection can be converted into a
numeric score. This is meant to be a self-reporting tool, so having the
caregiver compare the patient’s appearance to the pictures on the scale
is not an appropriate use of the tool.
and physiologic changes, rather than self-reporting. Physiologic
changes that occur with pain tend to lessen after the body adjusts and
can be modulated by coping skills. Generally, these signs are
considered less specific and less reliable than patient self-report,
but in noncommunicative patients these signs may be all that is
available. Physiologic parameters associated with pain include elevated
blood pressure, heart rate, respiratory rate, decreased oxygen
saturation, sweating, pupil dilation, muscle tension, and nausea.
Behavioral
observations that may indicate pain include facial grimacing, crying,
posturing, guarding, fatigue, difficulty sleeping and eating, and
shortened attention span. The CRIES Neonatal Postoperative Pain scale (Table 33-1) is an example of a scale that uses physiologic and behavioral measures. The FLACC Pain scale (Table 33-2) uses only behavioral observations.
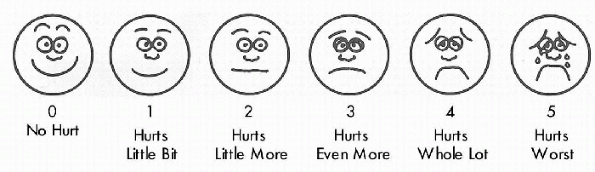 |
|
Figure 33-2
Wong-Baker FACES Pain Rating scale. (From Wong DL, Hockenberry-Eaton M, Wilson D, et al. Wong’s essentials of pediatric nursing, 6th ed. St. Louis: Mosby, 2001.) |
and treatment should be available 24 hours a day. Doctors, nurses,
pharmacists, psychologists, and child life specialists working together
can provide the most comprehensive treatment. Nurses can be taught to
consider pain the fifth vital sign and assess it with every vital sign
check. A physician and a clinical nurse specialist can assess the
patients once or twice daily and make necessary adjustments in
medication (in conjunction with a pharmacist). A child life specialist
can provide and teach distraction techniques from pain and input from a
psychologist can be valuable. An anesthesiologist can provide neural
blockade where helpful.
|
TABLE 33-1 “CRIES” SCALE FOR NEONATAL POSTOPERATIVE PAIN ASSESSMENT
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
need to be in place. Oxygen, naloxone, suction, and an Ambu bag should
be immediately available. A person with airway skills should be
in-house 24 hours a day. For all patients using patient-controlled
analgesia (PCA), opioid drips or epidurals, continuous pulse oximetry
should be considered. Level of consciousness and respiratory rate are
monitored regularly.
a behaviorist but was not used in children until the late 1980s. PCA is
an opioid delivery method that consists of a microprocessor-driven pump
with a button that the child presses to self-administer a bolus of pain
medicine. The bolus is followed by a preset lockout time interval, and
one
can
add a small continuous background infusion of opioid to the PCA. By
delivering small doses of opioids on demand in the confines of lockout
periods, the PCA can better maintain steady levels of pain medication
and therefore consistent pain control, and do it safely.
|
TABLE 33-2 FLACC PAIN SCALE
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
-
The delay associated with delivering
as-needed medications is avoided and the patient is able to maintain a
more constant concentration of pain medication thus, avoiding
oversedation and other narcotic side effects and also painful periods
waiting for more medication. -
It empowers the patient and has been shown to improve the satisfaction of the patient and the family.
-
The ability to self-administer boluses in
anticipation of a painful event (e.g., dressing change, ambulating,
rolling) can improve pain control and reduce the total amount of opioid
needed during the event. -
Because nurses do not have to check and administer every dose of narcotic, nursing time is conserved.
-
When compared with continuous opioid
infusion in children after spine surgery, PCA uses less opioid and has
fewer side effects than the continuous infusion group.
or she will stop pushing the button. As an extra safety precaution, one
can also program a maximum dose of opioid that the child can receive in
4 hours called the 4-hour max. Many studies have shown PCA to enjoy
good patient acceptance, increased family satisfaction with pain
management, and safety. In one large study, patients receiving morphine
PCA (with or without continuous infusion) had lower pain scores, less
somnolence, and better satisfaction than patients receiving
intramuscular morphine injections. In addition, there were no deaths or
major adverse outcomes.
-
To use PCA effectively, a patient must be
able to understand the cause-and-effect relationship between pressing
the button and receiving pain relief and must be physically able to
press the button. -
Children 6 years of age and older
generally can operate the PCA pump. Occasionally, though not reliably,
a 5-year-old child is capable. We have not had success with children
younger than 5 years.
-
Morphine is the most commonly used opioid in PCA. It comes 1 mg per mL, which helps make calculations easier.
-
Hydromorphone and fentanyl are also safe and effective for PCA use.
-
Studies have shown these three opioids to
have similar side-effect profiles though individual patients may
tolerate one opioid better than another. -
Demerol is not a good choice for use in
PCA. It is broken down to an active metabolite normeperidine, which
then must be renally excreted. In large doses or in cases of renal
compromise, normeperidine can accumulate and cause seizures. In
addition, when compared with morphine PCA, patients using demerol PCA
had poorer pain control.
potent than morphine and that fentanyl is approximately 75 times more
potent than morphine in children (Table 33-3).
|
TABLE 33-3 INTRAVENOUS PATIENT-CONTROLLED ANALGESIA IN CHILDREN
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
allow patients to have longer periods of uninterrupted sleep. However,
it has also been shown to increase the incidence of side effects such
as nausea and sedation.
-
Use of continuous infusions with PCA is suggested when the surgery is extensive.
-
Starting at the lower end of the dose range (5 µg/kg/hour of morphine) is recommended.
-
An alternative to using a continuous infusion is the use of Ketoralac every 6 hours.
-
The first step in weaning a PCA is to stop the continuous infusion (if it is present).
-
It is best to wait until the patient can
tolerate a fair bit of oral intake before beginning oral pain
medications due to concerns over nausea and vomiting. -
One hour after the first dose of oral
pain medication is added, the lockout interval on the PCA is increased
to 12 to 20 minutes. -
After the third dose of the oral pain medication, the PCA can be discontinued.
children unable to use the PCA themselves. It has been shown to be
effective pain management and conserve nursing time. However, nurses
tend to underestimate pain and give lower doses of narcotics than a
matched group of patients with PCA gave themselves.
the parent to press the button has been attempted. Although there were
no deaths or permanent adverse events, 9 children of 212 using this
technique required naloxone, a very high rate when compared with
standard PCA patients.
-
If parent-controlled analgesia is used, a lockout interval greater than 20 minutes is recommended.
-
Continuous intravenous infusions of opioids can provide effective pain relief after surgery.
-
Morphine, hydromorphone, and fentanyl are all safe and effective opioid choices.
-
Generally, a range is ordered and the nurses can titrate the infusion to the patient’s level of pain control (Box 33-1).
-
Epidural analgesia can provide excellent postoperative pain relief in infants and children.
-
It can be given as a continuous infusion, single shot, or even as PCEA (patient-controlled epidural analgesia).
-
The epidural space can be accessed at
many levels. Most often the thoracic (T7-11), lumbar (L3-L4), and
caudal (sacrococcygeal ligament) sites are used. -
By placing the analgesics near the nerves
as they emerge from the dural sac, one can produce profound analgesia
while only using very low doses of medicine. -
Children can ambulate with assistance and undergo physical therapy with an epidural in place.
spinal fusion compared intravenous PCA and epidural infusions for
postoperative pain control. Both methods were safe and effective, but
the epidural group tolerated a full diet and was discharged from the
hospital significantly earlier than the PCA group. In infants
undergoing Nissen fundoplication, those with epidurals had shorter
intensive care unit stays and hospitalizations.
postoperative epidural placement is very low, and large series have
reported no cases of permanent sequelae (e.g., nerve injury or death).
infusions made up of a local anesthetic and an opioid. The most common
local anesthetic used is bupivacaine (Box 33-2).
these receptors in the spinal cord, the release of substance P (a
neurotransmitter that facilitates transmission of pain impulses from
the dorsal horn) is blocked, and the perception of pain is altered.
Opioids also cause the brain to release serotonin, a neurotransmitter
that inhibits the transmission of pain impulses.
-
Morphine, 10-40 µg/kg/hr
-
Hydromorphone, 2-8 µg/kg/hr
-
Fentanyl, 0.5-2 µg/kg/hr
-
Infusion rate should not exceed 0.5 mg/kg/h in children >6 mo of age or 0.25 mg/kg/hr in chilren <6 mo of age
-
Concentration can vary from 0.125%-0.05%
-
0.0625% usually provides good pain relief without significant numbness or motor blockade
largely by their lipid solubility. Fentanyl, hydromorphone, and
morphine are all commonly used in the epidural space. Fentanyl is 580
times more lipid-soluble than morphine, while hydromorphone is 1.4
times more lipid-soluble. Increased lipid solubility leads to more
rapid absorption of fentanyl into the epidural fat and also into the
bloodstream causing it to have a shorter duration of action and a
smaller spread through the cerebrospinal fluid.
-
Being more hydrophilic allows morphine to
spread up and down the spinal cord and thus, produce a wider band of
analgesia that spreads across more dermatomes. -
The rostral spread of morphine can be
associated with more opioid-related side effects (pruritus, nausea,
respiratory depression) than with fentanyl. -
Hydromorphone provides more rostral spread than fentanyl and slightly less than morphine.
achieving a level of analgesia above the location of the epidural
catheter so many solutions may be acceptable. A few common epidural
solutions are listed in Box 33-3.
-
An epidural can provide analgesia for infants and children with surgical pain located below the nipple line.
-
Absolute contraindications to placing an
epidural include an infection at the site of placement, a systemic
infection, a coagulopathy, and family refusal of the technique. -
Relative contraindications include
children with preexisting neurologic deficits, spinal abnormalities,
prior laminectomies, and increased intracranial pressure. -
The presence of an indentation or dimple
near the sacral hiatus may indicate an underlying pilonidal cyst and a
caudal should be avoided.
-
Bupivacaine 0.0625% plus hydromorphone 10-20 µg/mL at 0.1-0.4 mL/kg/hr
-
Bupivacaine 0.0625% plus fentanyl, 2-5 µg/mL at 0.15-0.4 mL/kg/hr
-
Bupivacaine 0.0625% plus morphine, 25-50 µg/mL at 0.15-0.4 mL/kg/hr
-
Neonates: bupivacaine 0.05% plus fentanyl, 1 µg/mL at mL/hr
-
Caudal epidural blockade provides
postoperative pain relief for most any surgical procedure within the
distribution of the dermatomes of the L2-S5 dermatomes. -
In children under 8 years of age, the sacral hiatus is incompletely fused.
-
With the patient lying on the side, the
caudal space can be accessed through the sacral hiatus, which is formed
by the nonunion of the S5 vertebral arch. A loss of resistance is felt
as the needle crosses the sacrococcygeal ligament and enters the
epidural space. -
If the patient is an outpatient, 0.75 to
1.0 mL/kg of 0.125% to 0.25% bupivacaine with or without epinephrine or
ropivacaine given caudally in the epidural space can provide hours of
pain relief. -
Adding 2 to 4 µg/kg of clonidine to the solution can prolong the duration of the block.
-
If the patient is an inpatient, morphine,
50 µg/kg, or Dilaudid, 10 µg/kg, can be added to the solution and pain
relief can be expected to last about 12 hours. This technique is
especially useful following repair of a clubfoot.
-
The femoral nerve (L2, L3, and L4)
supplies sensory innervation to the anterior thigh and the periosteum
of the femoral shaft and motor innervation to the quadriceps.-
□ This block can help relieve the pain of femoral shaft fractures/osteotomies and lessen quadriceps spasm.
-
□ One can place 0.6 mL/kg up to a maximum
of 30 mL of 0.25% bupivacaine with epinephrine 1:200,000 lateral to the
femoral artery pulsation below the inguinal ligament.
-
-
The brachial plexus can be blocked using many approaches.
-
□ An axillary block is useful for pain in the forearm and the hand.
-
□ A parascalene approach can be helpful for shoulder and upper arm pain.
-
□ Often these blocks are performed with
the aid of a nerve stimulator as most children need to be asleep and
are unable to detect paresthesias during the application of the block. -
□ One can use 0.5 mL/kg up to a maximum of 20 mL of 0.25% bupivacaine with epinephrine 1:200,000 for these blocks.
-
-
A digital nerve block can be used for finger or toe postoperative pain.
-
□ A dose of 0.5 to 1.0 mL of 0.25% bupivacaine without epinephrine can be placed in a ring at the metacarpophalangeal junction.
-
□ Check the digit’s blood supply prior to block. Using large volumes of local anesthetic may lead to compartment syndrome.
-
-
The sciatic nerve (L4, L5, and S1-3)
supplies sensory innervation to the foot and much of the leg below the
knee. It begins branching 5 to 7 cm above the popliteal fossa and thus,
if one wants to anesthetize the foot, one must perform many distal
nerve blocks or perform a sciatic block more proximal with a nerve
stimulator.-
□ An ankle block is performed by
basically putting a ring of epinephrine-free local anesthetic around
the ankle. Because of the multiple injections, this can be an
uncomfortable block for the patient. -
□ The saphenous nerve supplies sensory
innervation to the foot and can be blocked easily as it comes around
the medial aspect of the patella.
-
-
Ketoralac is the only parenteral nonsteroidal anti-inflammatory drug (NSAID) available in the United States.
-
It can be given by the intravenous, intramuscular, or oral route.
-
The dose is 0.5 mg per kg up to 15 mg if
the patient weighs less than 50 kg and 30 mg if the patient weighs more
than 50 kg, every 6 hours for not more than 5 days. -
It is not necessary to give a loading dose.
-
It is a very potent analgesic (250 times
as potent as aspirin, 25 times as potent as naproxen) that has no
respiratory depressant effects and can reduce opioid usage. -
NSAIDs however may cause gastrointestinal upset and bleeding, platelet dysfunction, and renal impairment.
-
There is also controversy as to whether
Ketoralac significantly inhibits osteoblast growth, and some
practitioners limit its use where this is a concern.
weaned to oral analgesics. The most commonly prescribed oral analgesic
is acetaminophen plus codeine (Box 33-4).
Codeine must be broken down to morphine to have an analgesic effect. Up
to 15% of people lack the enzyme that odemethylates codeine to allow it
to become morphine, rendering the codeine ineffective. Also, codeine is
associated with a significant incidence of nausea and vomiting. Some
pain services prefer Lortab (Box 33-5) over Tylenol #3.
-
Available as a tablet (Tylenol #3 is 300
mg acetaminophen and 30 mg codeine) or an elixir (1 mL = 24 mg
acetaminophen and 2.4 mg codeine) -
Schedule III narcotic—requires no triplicate form and can be called in to a pharmacy
-
Dose is based on the codeine and is 1 mg/kg every 4 hr
-
Children should not receive >75 mg/kg/day up to 4,000 mg/day of acetaminophen
-
Acetaminophen/hydrocodone combination
-
Schedule III narcotic—requires no triplicate form and can be called in to a pharmacy
-
Less nauseating than codeine
-
The tablets come in four strengths:
Lortab 2.5 mg/500 mg, 5 mg/500 mg, 7.5 mg/500 mg, 10 mg/500 mg and the
elixir is 0.5 mg/33.3 mg -
Dose is based on the hydrocodone and is 0.1-0.2 mg/kg every 4 hr
narcotics and do not have any Tylenol added to their preparations. They
may be considered for patients with pain (most likely patients with
chronic pain) not covered by the aforementioned narcotics.
-
Pain is best managed by a combination of pharmacologic and nonpharmacologic therapies.
-
Nonpharmacologic interventions include
acupuncture, art and play, biofeedback, deep breathing, distraction,
guided imagery, heat, hypnosis, massage, comfort positioning, and
transcutaneous electrical nerve stimulation. -
One must assess the patient and the technique for developmental fit.
-
The common side effects of opioids are nausea, pruritus, respiratory depression or oversedation, and inadequately managed pain (Box 33-6).
-
Nausea, pruritus, and oversedation can be treated with the same drugs and methods mentioned in Box 33-6.
-
You can also lower the infusion rate or switch which opioid is used in infusion.
-
Urinary retention is common with epidural
infusions. Some practitioners place indwelling bladder catheters
preemptively, and others wait to see if the patient can void on his or
her own. -
Compartment syndrome is always a concern
in lower extremity trauma. By using more dilute concentrations of local
anesthetic, you can reduce the risk of masking a compartment syndrome,
but a high degree of suspicion should remain.
-
Ondansetron, 0.15 mg/kg up to 4 mg every 6 hr, or
-
Metoclopramide, 0.15 mg/kg up to 10 mg every 6 hr
-
Diphenhydramine, 0.5-1.0 mg/kg every 6 hr, or
-
Hydroxyzine, 0.5-1.0 mg/kg intramuscularly every 4 hr, or
-
Naloxone, 1 µg/kg up to 40 µg every hr
-
If the patient is having excellent pain
control, consider lowering or discontinuing the continuous infusion or
lowering the patient-controlled analgesia bolus dose -
Consider switching opioids, since patients may find one opioid to be less nauseating or to cause less itching than another
-
Stop the opioid for a period and restart administration at a lower dose
-
If the patient cannot be roused or is experiencing respiratory depression, administer oxygen and ventilate
-
Naloxone, started at 2-5 µg/kg and titrated to response
C, Lehn B, Yee J, et al. Patient-controlled analgesia in children and
adolescents: a randomized, prospective comparison with intramuscular
administration of morphine for postoperative analgesia. J Pediatr
1991;118:460-466.
J, Geake J, Grier H, et al. Patient-controlled analgesia for mucositis
pain in children: a three-period crossover study comparing morphine and
hydromorphone. J Pediatr 1996;129;722-728.
E, Dalens B, Gombert A. Epidemiology and morbidity of regional
anesthesia in children: a one-year prospective survey of the French
Language Society of Pediatric Anesthesiologists. Anesth Analg
1996;83:904-912.
M. Comparison of epidural morphine, hydromorphone and fentanyl for
postoperative pain control in children undergoing orthopaedic surgery.
Paediatr Anaesth 1999;9:419-422.
MA, Wilder RT, Berde CB. The risk of infection from epidural analgesia
in children: a review of 1620 cases. Anesth Analg 1995;80:234-238.
A, Katz J, Ilersich A, et al. Effect of neonatal circumcision on pain
response during subsequent routine vaccination. Lancet 1997;349:599.
Boerum DH, Smith JT, Curtin MJ. A comparison of the effects of patient
controlled analgesia with intravenous opioids versus epidural analgesia
on recovery after surgery for idiopathic scoliosis. Spine
2000;25:2355-2357.
