SPONDYLOLISTHESIS
spondyloptosis have caused considerable confusion for students of
orthopaedics. This chapter conveys the essentials of what is understood
about these lesions to provide a foundation for rational treatment.
(loosening). Spondylolysis is now specifically used to describe a bony
defect in the pars interarticularis, the portion of the neural arch
just caudal to the confluence of the pedicle and the superior articular
process and at the most cephalad part of the lamina and inferior
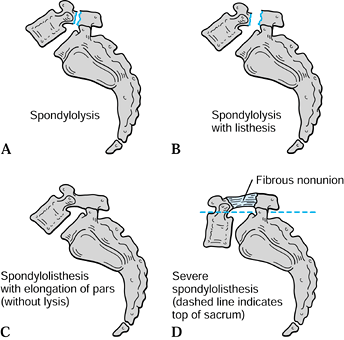
articular process (Fig. 162.1B). Spondylolisthesis can be present with or without spondylolysis (Fig. 162.1C). Spondyloptosis has similar origins, with the same root appended to the Greek word ptosis
(falling). In modern usage, this refers to the most severe form of
spondylolisthesis, when the body of L-5 has slipped into the pelvis and
is positioned directly anterior to the sacrum (Fig. 162.1D).
 |
|
Figure 162.1. A: Spondylolysis. B: Spondylolysis with listhesis. C: Spondylolisthesis and elongation of the pars interarticularis (without lysis). D: Severe spondylolisthesis (dashed line indicates top of sacrum).
|
spondylolisthesis. These can occur simultaneously, but generally one
predominates.
a congenital defect in the bony hook or its catch. The hook is composed
of the pedicle, pars interarticularis, and inferior articular process
of the cephalad vertebra, and the catch is the superior articular
process of the caudal level. Dysplasia of any of these structures sets
the stage for olisthesis when the weight of the trunk is transferred
through the area at the initiation of upright stance and ambulation.
The olisthesis is only potential at birth. Subluxation occurs when the
soft-tissue restraints (intervertebral disc, anterior and posterior
longitudinal ligaments,
ligamentum
flavum, and posterior ligamentous complex) undergo plastic deformation
due to repetitive loading unopposed by bony constraints. If pronounced
subluxation occurs while significant growth still remains, the slippage
will be accompanied by abnormal growth in the involved vertebral bodies
or sacrum. These dysplastic changes form the basis for various
classification schemes. Such changes include a trapezoidal shape of
L-5, rounding of the superoanterior aspect of the sacrum, vertical
orientation of the sacrum, junctional kyphosis at the involved
segments, and a compensatory hyperlordosis at adjacent levels. There is
evidence to support a genetic predisposition to this process, although
no pattern of inheritance has been identified.
repetitive cyclic loading that ultimately results in a stress fracture.
Impingement between the inferior articular process of the cephalad
vertebra and the superior articular process of the caudal vertebra
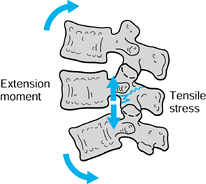
creates a bending moment that must be resisted by the pars (Fig. 162.2).
Repetitive impingement causing loads in excess of the fatigue limit
results in a fatigue (stress) fracture of an otherwise normal pars
interarticularis. This repetitive loading is the same process that
causes stress fractures in other anatomic locations, such as the
femoral neck or the fifth metatarsal. The hard cortical bone of the
pars predisposes it to fatigue fracture, as well as nonunion,
decreasing the likelihood of spontaneous healing. If healing occurs,
the pars often heals in an elongated position. Either outcome (nonunion
or healing with elongation) permits vertebral subluxation. This
fundamental change in bony anatomy exposes the disc to increased shear
load, even though the axial load remains unchanged. The increased shear
load on the disc causes premature disc degeneration. Activities
involving repetitive maximal flexion and extension (e.g., interior line
play in football, pole vaulting, or gymnastics) are notoriously
associated with fatigue fractures of the pars.
 |
|
Figure 162.2. Extension of the lumbar spine causes a tension load in the pars interarticularis.
|
although these are sufficiently different as to be considered distinct
entities. They are included here for completeness.
instability and subluxation caused solely by degenerative change in the
intervertebral disc and facet joints. The degree of subluxation is
necessarily mild because the intact neural arch provides a bony limit
to forward translation. Relatively more sagittal orientation of the
facet joints is associated with degenerative spondylolisthesis (2,22).
defect in the neural arch that can then permit subluxation. This is a
pathologic fracture with resultant translational deformity.
this setting, the spine will have sustained multiple bony and
soft-tissue injuries, which may include a fracture in the pars. Other
skeletal and visceral injuries will typically be present. This
traumatic type of spondylolisthesis is a fracture–dislocation from
high-energy trauma, not from repeated low-energy injuries.
process or more than half of each articular process can functionally
destabilize the spine and permit translational deformity. This is
iatrogenic postsurgical instability. Segments adjacent to previously
fused segments are also at risk for development of degenerative
spondylolisthesis (45). This subluxation is
likely due to resection of the capsular, interspinous, or supraspinous
ligaments at the adjacent level, but the loss of motion of the fused
segment may contribute by increasing the motion demands at the next
open level.
 |
|
Table 162.1. Wiltse–Newman–Mcnab Classification of Spondylolisthesis
|
The principal distinction in their system is between developmental and
acquired forms, which correspond respectively to the dysplastic and
traumatic pathways discussed previously.
 |
|
Table 162.2. Marchetti and Bartolozzi Classification of Spondylolisthesis
|
caused by chronic muscle contraction (spasm) as the body attempts to
limit motion around a painful pseudarthrosis of the pars
interarticularis, by tears in the annulus fibrosus of the degenerating
discs, or by compression of nerve roots. Pain may also derive directly
from impingement at the fibrous pars nonunion, as nerve endings have
been identified there (47). Children and
younger adults who have symptomatic high-grade spondylolisthesis
commonly complain of back fatigue and back pain on movement,
particularly with hyperextension, as well as hamstring fatigue and
pain. On examination, the paraspinal muscles are in chronic reactive
contraction (spasm) to splint the painful underlying motion segment,
and the hamstring muscles are in reactive contraction to stabilize the
pelvis under the painful spinal motion segments. After months of
continuous contraction in a growing child, fixed contractures of the
hamstrings and paraspinal muscles may occur, limiting forward bending
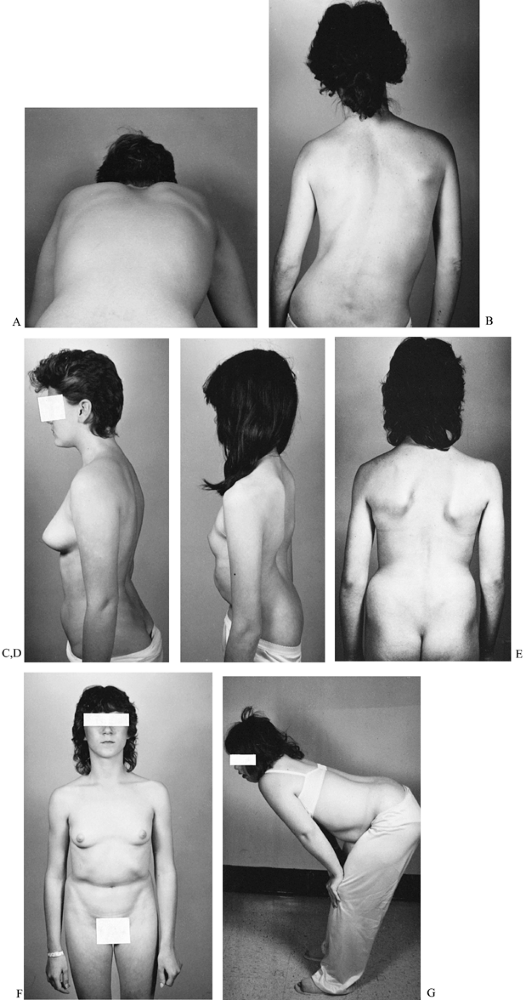
and hip flexion. These may be evident on clinical examination (Fig. 162.3A).
Palpation elicits tenderness over the pars defect when the patient is
lying prone with the spinal muscles relaxed, similar to the tenderness
that exists over any other skeletal nonunion.
 |
|
Figure 162.3. Physical findings in spondylolisthesis. A: Severely limited forward bending in a patient with moderate slippage and paravertebral muscle and hamstring spasm. B:
Sciatic scoliosis in a patient with a disc rupture at the level above a pars defect. This patient’s chief complaint was sciatica with mild back pain. C: Accentuated lordosis in a patient with mild slippage and a low slippage angle. D: Severe posterior tilting of the pelvis and secondary thoracolumbar lordosis are evident in this patient with a high-grade spondylolisthesis and high slippage angle. E: Heart-shaped buttocks and trunk foreshortening are visible in this patient with a high-grade slip. F: The same patient demonstrates an abdominal crease. G: Standing posture of a patient during olisthetic crisis with severe deformity, canal occlusion, and multiple root compression. |
at the level of the slippage or at the level above it), episodes of the
back “giving out” may occur. These episodes may or may not involve
sciatica and vary in severity. As disc degeneration or subluxation
increases, both the spinal canal and the lateral root foramina narrow,
often causing symptoms related to nerve root compression (Fig. 162.3B).
Such compression is manifested by sciatic pain radiating from the
buttock into the posterior thigh and into the calf and foot. It is
associated with numbness in a similar dermatomal distribution and with
positive nerve stretch signs, such as straight-leg raising. Symptoms of
spinal claudication indicate high-level stenosis. Compression of the
central canal is confirmed by one or more of the following:
-
Bowel or bladder symptoms or dysfunction
-
Bilateral leg symptoms
-
Positive straight-leg-raising test bilaterally
-
Positive crossed straight-leg-raising test
spinal deformity. As the amount of subluxation increases, spinal
deformity becomes increasingly visible on inspection of the patient’s
torso. As the spine slides forward, the pelvis rotates posteriorly so
that the top of S-1 becomes progressively more horizontal. This
produces lumbosacral kyphosis and a relative posterior prominence of
the posterior parts of the iliac crests (Fig. 162.3C, Fig. 162.3D).
The gluteal muscles become less prominent. Patients with high-grade
spondylolisthesis are often described as having heart-shaped buttocks (Fig. 162.3E).
As the amount of telescoping approaches total dislocation
(spondyloptosis), foreshortening of the lumbar spine becomes obvious on
physical examination, and a crease appears across the abdomen (Fig. 162.3F).
Patients in olisthetic crisis with total canal occlusion (the most
severe type of spinal stenosis) relieve disc pressure and reduce nerve
root tension by supporting trunk weight with hands on knees (Fig. 162.3G).
the amount of vertebral subluxation; often, they also reveal a pars
interarticularis defect if one is present (Fig. 162.4). Oblique views have also been used to highlight the Scotty-dog sign (Fig. 162.5). In young patients, flexion–extension
views can be used to show excessive movement across the site of
pseudarthrosis in the pars interarticularis and subluxation of the
vertebral body as the patient moves from extension into flexion. This
motion may be more evident if the films are taken with the patient
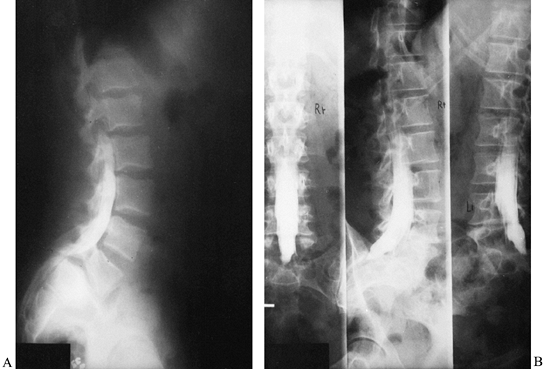
lying in the lateral decubitus position rather than standing (60). Plain standing radiographs are also quite useful for documenting progression of deformity (Fig. 162.6).
 |
|
Figure 162.4.
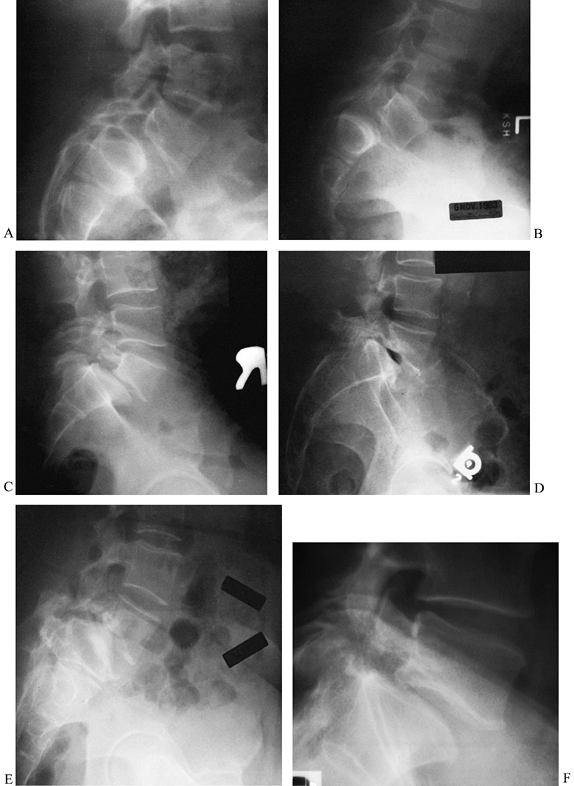
Spondylolisthesis in five patients with varied combinations of slippage, slippage angle, sacral inclination, sacral rounding, and disc degeneration. A: Moderate dysplastic spondylolisthesis. No pars defect is seen, and the slippage angle is low. B: Moderate isthmic spondylolisthesis. A pars defect is seen, and the slippage angle is higher than in the previous image. C: Moderate isthmic slip with moderate disc degeneration. The slippage angle is low, but striking retrolisthesis is present at the level above (L4-5). D: Moderate isthmic slippage with severe disc degeneration and a vacuum disc. E: High-grade slip with a higher slippage angle, vertical sacrum, high lumbar index, and severe disc degeneration. F: Low-grade slip with low slippage angle and minimal disc degeneration. A pars defect is present. |
 |
|
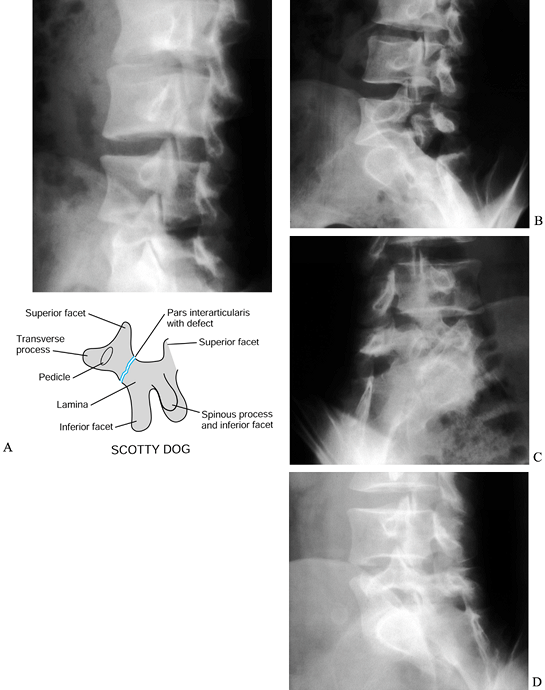
Figure 162.5. Varied radiographic appearance of spondylolysis without olisthesis. A: Oblique radiograph shows lysis at L-3. Note the collar on Scotty dog. B: Oblique radiograph reveals atrophic nonunion at an L-5 pars defect. Oblique (Scotty dog) radiographs reveal a pars defect (C) and an elongated healed pars (D)
in the same patient with a low-grade slip. (Courtesy of Harry Griffiths, MD, University of Missouri Health Sciences Center, Columbia, MO.) |
 |
|
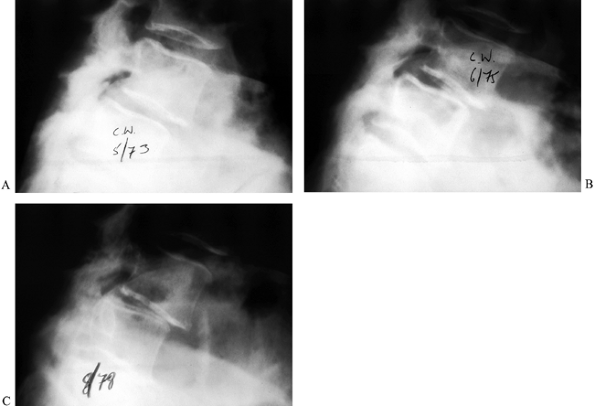
Figure 162.6. Progression of degenerative spondylolisthesis at L4-5 over a 5-year period as seen on lateral radiographs. A: 1973. B: 1975. C: 1978. (Courtesy of Harry Griffiths, MD, University of Missouri Health Sciences Center, Columbia, MO.)
|
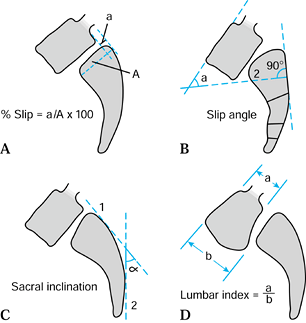
subluxation by the Meyerding classification, which is based only on the
amount of anterior (forward) subluxation of the cephalad vertebra in
reference to the caudal vertebra (Fig. 162.7A). Slippage is graded as a percentage relative to the sagittal diameter of the inferior body:
|
component of the deformity in producing canal narrowing and
sagittal-plane malalignment. Correction of kyphosis is very important
in treating the condition, especially with regard to achieving a solid
fusion (13). The radiographic measurement techniques described by Boxall et al. (5)
emphasize the importance of the slippage angle as the best way to
quantify the degree of kyphosis in a patient with spondylolisthesis.
 |
|
Figure 162.7. A: Calculation of the slippage percentage for spondylolisthesis. B: For a domed sacrum, the slippage angle is calculated from the posterior sacral body cortex and the superior endplate of L-5. C: The sacral inclination is the angle between the posterior sacral body cortex at S-1 and a vertical line (standing radiograph). D: Calculation of the lumbar index.
|
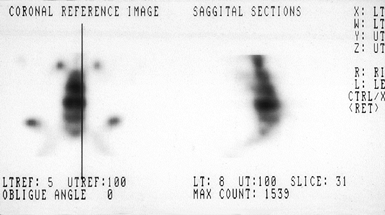
tomography may occasionally be useful in symptomatic patients without
radiographic evidence of spondylolisthesis (Fig. 162.8) (33).
These studies document increased bone metabolic activity in an acutely
injured pars interarticularis; however, they may revert to normal in a
chronic unhealed defect or after a stress fracture heals (33).
 |
|
Figure 162.8.
Bone scan shows increased activity in a patient with bilateral pars defects. (Courtesy of Harry Griffiths, MD, University of Missouri Health Sciences Center, Columbia, MO.) |
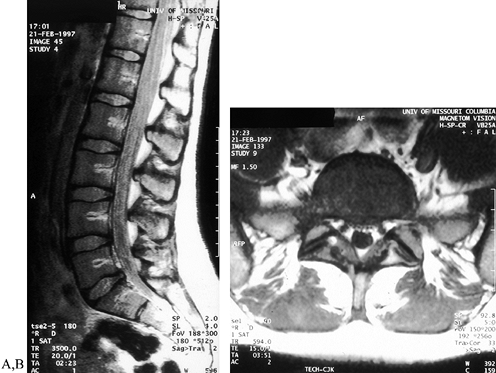
with solely mechanical complaints. If root symptoms, bowel or bladder
complaints, or physical evidence of cord or root compression are
present, then evaluate the soft tissues of the back with magnetic
resonance imaging (MRI) (Fig. 162.9), myelography (Fig. 162.10), computed tomography, or a combination of these studies. MRI findings
have been well correlated with clinical evidence of radiculopathy (28).
Cystometric studies are helpful in patients with bladder dysfunction,
although they are often confirmatory rather than diagnostic.
Somatosensory-evoked cortical or spinal potentials may be of diagnostic
value but are usually only confirmatory.
 |
|
Figure 162.9. Sagittal (A) and axial (B) MR images reveal a herniated nucleus pulposus in a patient with spondylolysis and complaints of sciatica.
|
 |
|
Figure 162.10. A: Myelogram reveals a block as the dural sac is tethered over S-1 in a high-grade slip. B: Lateral disc herniation is evident at L4-5 in a patient with an L-5 pars defect.
|
lumbosacral junction, with decreasing frequency at more cephalad levels
in the lumbar spine. It is seldom seen in the cervical spine and rarely
in the thoracic spine. Pars defects (spondylolysis) have not been
reported at birth. However, the prevalence by early childhood is
between 4% and 5% (17). By adulthood, the prevalence has increased to 6% or 7% (57,31).
The great majority of patients with spondylolysis (radiographic
evidence of a pars defect) are asymptomatic, and substantial slippage
(spondylolisthesis) never develops (11,18,49).
In a long-term study of adolescents with isthmic spondylolisthesis, 90%
of slips occurred at the time of the initial presentation, and the only
factor predictive of progression was the magnitude of the initial
slippage (50). Pronounced vertebral subluxation
(>75%), if it occurs, generally arises during late childhood, at the
time of the adolescent growth spurt, or during pregnancy (1,44).
In patients who manifest a major deformity during adulthood, it is
generally thought that the deformity developed before 20 years of age.
The prevalence of spondylolisthesis is no higher in groups with chronic
debilitating low back pain than in the general population (20). The association between low-back pain and spondylolisthesis is weak but is significant in women (56).
The degree of dysplasia, including spina bifida occulta and small
transverse processes, has been associated with progression but has not
proven to be predictive. Rounding of the sacral promontory, trapezoidal
wedging of L-5, vertical position of the sacrum, and segmental kyphosis
all contribute to the mechanics of progression but are believed to be
secondary changes. Similar to Harrington’s stable zone in scoliosis,
there may be a point of no return, beyond which progression is a
certainty; however, documentation of this has not yet occurred.
(acquired) tend to be more stable, less symptomatic, and less likely to
progress (38). Spondylolisthesis due to a fatigue
fracture of the pars interarticularis will frequently occur in a
high-level athlete. However, participation in athletics has little
effect on progression of symptoms (35).
years of age, and progression is limited by the intact neural arch.
Symptoms are generally due to stenosis, and progression to surgery is
variable. A more sagittal orientation of the facet joints at L4-5 is
strongly associated with the development of degenerative and
postlaminectomy spondylolisthesis (22,43). Oophorectomy has also been identified as a risk factor for degenerative spondylolisthesis (26).
relatively uncommon. Each patient must be evaluated and treated
individually. Nerve root decompression and instrumented spinal fusion
are the fundamentals of treatment.
than leg pain can be managed nonsurgically. In the pediatric
population, symptoms may be controlled by a period of bed rest,
bracing, or cessation of aggravating activities. For adults with any
type of spondylolisthesis, initial nonoperative treatment is the rule.
-
A corset and activity modifications are usually beneficial.
-
For exacerbations, prescribe periods of bed rest.
-
Palliate symptoms with hot or cold
therapy, and use massage to treat the muscle fatigue or spasm resulting
from disproportionate effort to limit movement across a painful motion
segment. -
Initiate a program of aerobic conditioning; specific back exercises have variable effectiveness.
-
Obese patients should lose weight to return to their healthy physiologic range.
-
Nonsteroidal antiinflammatory medications and epidural steroid injections may be of some value.
because fewer than 10% of symptomatic patients eventually require
operative treatment. Surgery should be contemplated only after a trial
of nonoperative care. In adult patients with predominantly sciatic
complaints, nonoperative treatment may be less effective.
adolescents than for adults. For children and adolescents, the
indications for surgery are as follows:
-
Documented progression of a slip beyond 25%
-
Presentation with a high-grade slip (>50%)
-
Intractable pain or neurologic symptoms
-
Progressive postural deformity or gait abnormality
back pain and neurologic or radicular symptoms unresponsive to
nonoperative management. As with other spine surgery, sciatica is more
responsive than back pain to surgery (9).
Patients with more severe symptoms will generally experience greater
benefit from surgery than those with milder symptoms. Poor outcomes
following surgery have been strongly associated with active workers’
compensation claims and smoking (46,55).
technique for direct repair of a pars defect with a screw placed
through the lamina across the defect. There have since been other
direct-repair techniques involving wires, hooks, and pedicle screws (30).
The appropriate patient has spondylolysis but no olisthesis and a
normal disc. Good results have been reported with these techniques, but
because of the simplicity and predictability of fusion in situ, repair is not performed as often as fusion (3).
moderate (<50%) slips can be successfully treated with
posterolateral fusion in situ (typically from L-5 to S-1) (8,19,27). Even patients with radicular symptoms may get good relief with fusion in situ (12). Wiltse et al. (58) advocated a muscle-splitting approach with two paramedian incisions; we favor a midline approach.
although it is commonly used for adolescents and adults. The most
popular systems today use pedicle screws. The biomechanical superiority
of these systems for stabilizing spondylolisthesis has been
demonstrated. Their effect on fusion rates and clinical outcomes is
less clear, although generally beneficial (16,42,53,54,61).
-
Position the patient prone on a Jackson table or blanket rolls without changing the patient’s kyphosis or lordosis.
-
Make a midline incision of sufficient
length, and expose the posterior elements laterally to the tips of the
transverse processes and sacral ala and thoroughly decorticate. -
For most adult-sized patients, we then place pedicle screws across the defect.
-
We now routinely perform a laminectomy of
the loose arch to provide local graft, instead of iliac crest, to
reduce postoperative morbidity. -
Place morcelized autologous bone graft from the laminectomy in the prepared gutter under the plates (between the screws).
-
Place rods or plates.
-
Perform routine closure over a suction drain and epidural catheter.
tolerated, in a corset. Prohibit driving and sitting, except on a
raised toilet seat, for 2 months.
95%. Most children have good or excellent results, with eventual return
to full activity. As in other spine surgery, children do better than
adults. The most common long-term problem is degenerative change at the
level above the fusion (59).
unless there is a cauda equina syndrome. Although foraminal stenosis
with associated root pain is common in adults with isthmic
spondylolisthesis, the indications for decompression are unclear
because the addition of decompression may increase the rate of
postoperative pseudarthrosis (10). Some authors have reported excellent fusion rates and relief of sciatica with fusion in situ (39), whereas others advocate formal decompression (6).
stenosis commonly present with claudication. Pedicle-to-pedicle
posterior decompression is generally accepted, although the addition of
intertransverse fusion has been shown to produce significantly better
results than decompression alone for the treatment of degenerative
spondylolisthesis (23).
long considered adequate decompression, actually fails to decompress
the root in the neural foramen. A thorough decompression must include a
foraminotomy, especially in the patient with radicular complaints. The
best use of the loose laminar arch is as bone graft. We routinely
perform Gill’s procedure to obtain bone graft, not for the purpose of
neural decompression.
primary treatment for low-grade spondylolisthesis. It can be useful for
failed posterior spinal fusion, however. Complications are potentially
severe and include injury of the great vessels, sexual dysfunction, and
retrograde ejaculation (see Chapter 146).
posterolateral fusion in the treatment plan, but the agreement would
stop there. Some authors have reported good results with isolated
posterior fusion in situ (14,27,29).
However, the pseudarthrosis rates are high, and progression is common,
even with a radiographically solid fusion. In addition, fusion in situ fails to correct the clinical deformity and sagittal imbalance that generally accompany these severe deformities (51).
The relative indications for instrumented reduction include olisthetic
crisis, cauda equina syndrome, a slip greater than 50% with a slippage
angle greater than 30°, and major clinical deformity with global
sagittal imbalance. Most intraoperative reduction techniques involve
insertion of pedicle screws into L-4, L-5, and the sacrum. Often, a
second point of pelvic fixation is added (iliac screws, intrasacral
rods, or S-2 screws) to gain mechanical advantage. The forces applied
are distraction, posterior translation of L-5, and sacral flexion. The
terminal portion of the reduction maneuver has been shown to produce a
disproportionate amount of nerve root tension, suggesting that postural
reduction is a reasonable intermediate alternative (40). Many authors stress the importance of sacral flexion for restoring sagittal-plane balance (40).
All series of instrumented reductions have reported nerve root injury,
typically L-5, which manifests as foot drop. For the majority of
affected patients, there is complete or partial recovery. These
procedures are technically demanding and should be attempted only by
experienced surgeons for patients who understand the potential risks.
At long-term follow-up, most series have reported durable correction
and clinical improvement with acceptable complication rates (15,25,37).
spinal fusion, whereas others report higher fusion rates, less
progression, and fewer implant failures with circumferential fusion (4,6).
The relative indications are incomplete reduction, residual kyphotic
slippage angle, and revision for previous pseudarthrosis. We favor the
addition of anterior interbody fusion because of the relative
difficulty of obtaining arthrodesis on the tension side of the
lumbosacral kyphosis. The anterior interbody fusion generally heals
readily on the compression side, but the lower pseudarthrosis rate must
be weighed against the increased morbidity.
Decompression is commonly used in conjunction with fusion for patients
with radicular or neurologic symptoms (52). However, relief of radicular symptoms has been reported with isolated posterior fusion (39). We use the presence of a positive seated straight-leg-raising test as an indication for nerve root exploration.
fortunately rare, clinical challenge. In spondyloptosis, the entire
body of L-5 is caudal to the sacrum. Although the slippage angle varies
widely, the inferior endplate of L-4 is always closer than the inferior
endplate of L-5 to the S-1 endplate. This observation provides the
anatomic rationale for our technique, which is the resection of L-5 and
reduction of L-4 onto the sacrum. The relative paucity of cases and the
variable clinical presentation have led to a plethora of suggested
techniques (4,6,13,27,36,37,39,41,48,52). Treatment options include observation, reduction and casting, fusion in situ,
reduction of L-5 onto the sacrum, and resection of L-5 with reduction
of L-4 onto the sacrum. All options, including nonoperative management,
have been associated with similar complications, including motor and
sensory deficits or a cauda equina syndrome (48).
The two-stage technique described in the following section is designed
to eliminate lumbosacral kyphosis, restore sagittal-plane balance, and
realign the spinal and nerve root canals while avoiding distraction and
potentially devastating iatrogenic cauda equina injury.
resection, instrumentation, and reduction of L-4 onto S-1. As in other
forms of spondylolisthesis, the severity of clinical symptoms in
patients with spondyloptosis does not necessarily correlate with the
degree of subluxation. Thus, L-5 vertebrectomy and reduction are
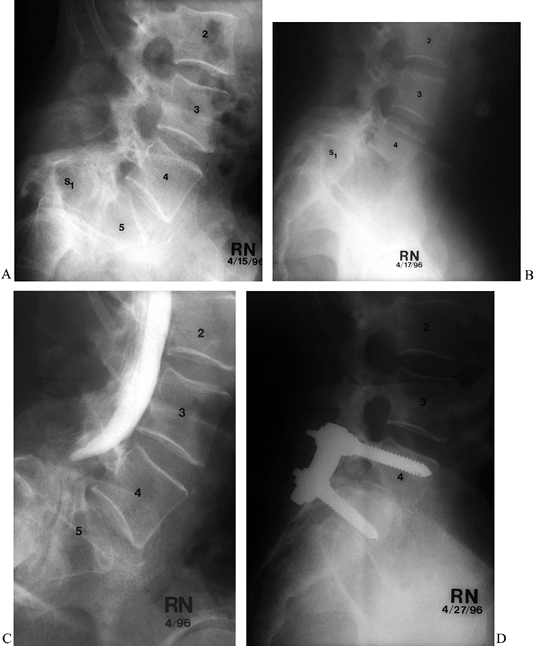
advised only for severely disabled patients (Fig. 162.11).
 |
|
Figure 162.11. A: Preoperative lateral radiograph of a 42-year-old man with spondyloptosis and debilitating back and leg pain. B: Preoperative myelogram shows total blockage. C: Lateral radiograph after first-stage L-5 anterior vertebrectomy. D:
Lateral radiograph after second-stage reduction of L-4 onto the sacrum and pedicle-screw fixation. Alignment and lordosis have been restored. |
-
Perform the first part through an anterior retroperitoneal approach (see Chapter 146).
-
Resect the L4-5 disc, the body of L-5, and the L5–S1 disc.
-
Take the resection back to the base of each pedicle, and take care to avoid injury to the L-5 root.
-
Remove the inferior cartilage endplate of L-4. Retain the bony endplate.
unit for 1 week to await the second stage. Nursing care includes use of
a rotokinetic bed.
-
Perform the second stage through a midline posterior approach.
-
Place Harrington outriggers from L-2 onto
the sacral ala to provide very gentle (1–2 cm) distraction. (This step
may be omitted after the surgeon gains more experience.) -
Remove the loose posterior elements,
transverse processes, and pedicles of L-5. Take special care to avoid
injury to the L-5 root. -
Remove the cartilage endplate of S-1 before reduction. Preserve the bony endplate.
-
Place pedicle screws into L-4 and S-1. Cortical purchase is essential.
-
Accomplish reduction of L-4 onto the
sacrum by removing the outriggers or by gentle distraction and
translation applied to the screws in L-4. Constantly assess the L-4 and
L-5 nerve roots during reduction both visually and with a nerve hook. -
Decorticate the transverse processes of L-4 and the sacral ala.
-
Place autograft retained from the vertebrectomy and from the posterior elements of L-5 in the lateral gutter.
-
Apply rods or plates to the screws. Two
nerve roots (L-4 and L-5) will pass through the reconstructed neural
foramen at L4–S1. Check to ensure that there is no impingement on the
nerve roots before and after application of the rods or plates.
thoracolumbar spinal orthosis for 6 weeks, at which time begin
mobilizing patients.
weakness in dorsiflexion postoperatively. This has been transient for
all but two. All patients with preoperative cauda equina syndrome
recovered postoperatively; no patient has had iatrogenic cauda equina
injury (because the
spine
is not lengthened). When reviewed by an independent observer, patients
reported very high satisfaction, with significant improvements in pain,
function, and appearance (32).
operations in spinal reconstruction. It should be only performed by
surgical teams that are accustomed by experience to performing spinal
osteotomies on a routine basis and are very experienced in handling the
dural tube and nerve roots.
the aorta, vena cava, and both internal and external iliac arteries and
veins. Two to four assistants are regularly required to retract major
vessels, roots, or vertebrae during various portions of the procedure.
vertebra creates the potential for iatrogenic single- or multiple-root
injury or incomplete reduction. Slow rehabilitation of patients is
essential. One month of bed rest is routine before ambulation in a
brace. No work or physical rehabilitation is started until convincing
evidence of union is obvious.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
N, Marchesi D, Zuber K, Aebi M. Treatment of Severe Spondylolisthesis
by Reduction and Pedicular Fixation: A 4–6 Year Follow-up Study. Spine 1993;18:1655.
DS, Boachie-Adjei O. Treatment of Severe Spondylolisthesis by Anterior
and Posterior Reduction and Stabilization: A Long-term Follow-up Study.
J Bone Joint Surg Am 1990;72:1060.
JK, Lonstein JE, Winter RB, Denis F. Long-term Evaluation of
Adolescents Treated Operatively for Spondylolisthesis: A Comparison of in Situ Arthrodesis Only with in Situ Arthrodesis and Reduction Followed by Immobilization in a Cast. J Bone Joint Surg Am 1992;74:693.
EJ. Single Level Posterolateral Arthrodesis, with or without Posterior
Decompression, for the Treatment of Isthmic Spondylolisthesis in
Adults: A Prospective, Randomized Study. J Bone Joint Surg Am 1997;79:1175.
DA, Constantini S, Nena U. Surgical Treatment of Severe L5-S1
Spondylolisthesis in Children and Adolescents: Results of
Intraoperative Reduction, Posterior Interbody Fusion, and Segmental
Pedicle Fixation. Spine 1996;21:728.
JS, Mackay M, Herkowitz HN, et al. Degenerative Lumbar
Spondylolisthesis with Spinal Stenosis: A Prospective, Randomized Study
Comparing Decompressive Laminectomy and Arthordesis with and without
Spinal Instrumentation. Spine 1997;22:2807.
AK, Danielson BI, Nachemson AL. Natural History of Symptomatic Isthmic
Low-grade Spondylolisthesis in Children and Adolescents: A Seven-year
Follow-up Study. J Pediatr Orthop 1991;11:209.
K. Isthmic Spondylolisthesis among Patients Receiving Disability
Pension under the Diagnosis of Chronic Low Back Pain Syndromes. Spine 1994;19:2766.
LJ, Robertson PA, Novotney JE, Pope MH. Etiology of Spondylolisthesis:
Assessment of the Role Played by Lumbar Facet Joint Morphology. Spine 1993;18:80.
HN, Kurz LT. Degenerative Lumbar Spondylolisthesis with Spinal
Stenosis: A Prospective Study Comparing Decompression with
Decompression and Intertransverse Process Arthrodesis. J Bone Joint Surg Am 1991;73:802.
AS, Urquhart AG, Graziano GP, Hensinger RN. Acute Spondylolytic
Spondylolisthesis: Risk of Progression and Neurologic Complications. J Bone Joint Surg Am 1995;77:190.
C, Kimber D, White K. Prevalence of Spondylolisthesis, Transitional
Anomalies and Low Intercrestal Line in a Chiropractic Patient
Population. J Manipulative Physiol Ther 1989;12:200.
SM, Steffee AD, Gaines RW Jr. Treatment of L5-S1 Spondyloptosis by
Staged L5 Resection with Reduction and Fusion of L4 onto S1 (Gaines
Procedure). Spine 1994;19:1916.
JP, Mehdian H, Jaffray D. Reduction of Severe Lumbosacral
Spondylolisthesis: A Report of 22 Cases with a Ten-year Follow-up
Period. Clin Orthop 1994;300:64.
K, Ishida Y, Takatsu T, et al. Vertebral Slip in Lumbar Spondylolysis
and Spondylolisthesis: Long-term Follow-up of 22 Adult Patients. J Bone Joint Surg Br 1995;77:771.
JE, Pflueger PC, Isaza JE, Whitecloud TS 3rd. Transpedicular Fixation
for the Treatment of Isthmic Spondylolisthesis in Adults. Spine 1995;20:1917.
JD, Smith JA, Schleusener RL. Lumbar Motion Segment Pathology Adjacent
to Thoracolumbar, Lumbar, and Lumbosacral Fusions. Spine 1996;21:970.
CL, Freese A, Ansell LV. Outcome Analysis for Adults with
Spondylolisthesis Treated with Posterolateral Fusion and Transpedicular
Screw Fixation. J Neurosurg 1997;86:56.
S, Osterman K, Hyvarinen H, et al. Progression of Spondylolisthesis in
Children and Adolescents: A Long-term Follow-up of 272 Patients. Spine 1991;16:417.
J, Laine T, Pohjolainen T, et al. Spondylodesis Augmented by
Transpedicular Fixation in the Treatment of Olisthetic and Degenerative
Conditions of the Lumbar Spine. Clin Orthop 1993;297:111.
K, Christensen FB, Eiskjaer SP, et al. The Effect of Pedicle Screw
Instrumentation on Functional Outcome and Fusion Rates in
Posterolateral Lumbar Fusion: A Prospective, Randomized Clinical Study.
Spine 1997;22:2813.
L, Ronnemaa T. The Association of Mild–Moderate Isthmic
Spondylolisthesis and Low Back Pain in Middle-aged Patients is Weak and
It Only Occurs in Women. Spine 1993;18:1496.
L, Ronnemaa T, Osterman K, et al. Prevalence of Isthmic Lumbar
Spondylolisthesis in Middle-aged Subjects from Eastern and Western
Finland. J Clin Epidemiol 1992;45:917.
C, Gluch H, Krismer M, et al. AP-translation in the Proximal Disc
Adjacent to Lumbar Spine Fusion: A Retrospective Comparison of Mono-
and Polysegmental Fusion in 120 Patients. Acta Orthop Scand 1997;68:269.
